7
Research on the Global Control of TB: Understanding the Role of Drugs, Vaccines, and Funding
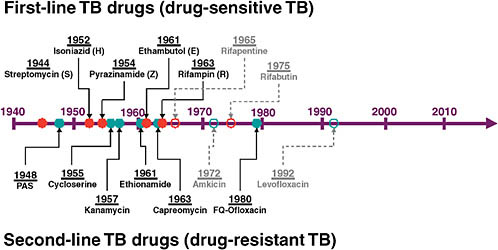
The lack of new drugs is a major gap in the global fight against TB. Harrington observed that there has been a long period of stagnation in TB drug development: As noted in Chapter 1, the last new drug approved for TB was rifampin in the 1970s.
Currently, five new compounds are in Phase I or II clinical development, and two “repurposed” drugs are in Phase III development. Progress has been made, particularly with the drug candidate TMC207, which has shown preliminary efficacy in patients with MDR TB in South Africa. Off-label use of drugs such as the quinolones has played an important role in the management of drug-resistant TB.
Harrington compared the aggressive development of drugs for HIV with the lack of serious progress on drugs for TB. He cited the lack of biomarkers and surrogate end points as one of the major obstacles to TB drug development. As a result, getting drugs into the field is difficult for companies because they do not understand the critical path by which drugs can be approved quickly. In addition, Harrington suggested that TB drug manufacturers should take advantage of the U.S. Food and Drug Administration’s (FDA’s) accelerated approval mechanism, which was used effectively for HIV drug development in the 1990s. Further, research is focusing on only few classes of drugs. Harrington expressed his view that the number of drugs in the pipeline is inadequate to revolutionize treatment of TB, whether drug-sensitive or drug-resistant. He suggested further that “neither the background regimen nor how to use the new drugs with it is yet well defined, and there is no clinical trials infrastructure available to carry out the needed strategy trials, even as industry carries out its own
registrational studies.” Finally, Harrington said that even as work on developing biomarkers proceeds, it may be possible to use indicators such as time to sputum smear conversion or culture conversion as surrogate markers to accelerate approval of new drugs for drug-resistant TB, given the unmet medical need.
THE PIPELINE FOR NEW DRUGS
Ann Ginsberg of the TB Alliance gave an overview of the pipeline for new TB drugs. The current drug classes—both first- and second-line drugs—were all discovered between the 1940s and the 1970s (see Figure 7-1). From then until 5 years ago, there was little work on TB drug development.
To find effective treatments for MDR and XDR TB, it is important to establish treatment regimens that are better tolerated, more efficacious, and more affordable. The root of the drug resistance problem is the complexity and length of drug-sensitive regimens. Thus it is critical to have shorter, simpler regimens for drug-sensitive TB. To meet this need, it will be necessary to develop new drugs that will shorten and simplify treatment. They must be effective against those mycobacteria that persist now in the face of drugs to which they are genetically susceptible. Ideally, one wants drugs with novel mechanisms of action that are equally effective against MDR and XDR and drug-sensitive strains of TB. They must also be effective and have minimal drug–drug interactions for both HIV-positive and HIV-negative patients. Additionally, they should be able to be delivered orally once a day or less frequently if possible, and obviously be low cost.
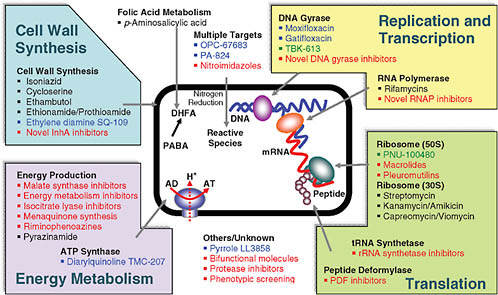
Ginsberg discussed the strategies that are being explored to achieve these goals. Figure 7-2 shows the variety of targets being pursued. One can see that the bulk of current drugs—listed in black—are cell wall active. This means they work well against the most rapidly replicating mycobacteria, but are probably not effective against persistent organisms that are replicating slowly or not at all. Cell wall active drugs are consequently unlikely to shorten therapy, an objective that requires drugs acting against other kinds of targets. Many new discovery projects—listed in red—are focused on energy metabolism. TMC207 from Tibotec (a subsidiary of Johnson & Johnson) is the most developed drug candidate that has targeted that pathway successfully and will likely contribute to shortening therapy.
The ideal is to find new drugs that simultaneously will be effective in drug-resistant TB using novel mechanisms of action and will shorten treatment. Current drugs that shorten treatment include rifampin, which combined with pyrazinamide is the most effective of the current drugs in shortening therapy and which inhibits RNA polymerase. The fluoroquinolones, which have the potential to shorten therapy, work against DNA gyrase. TMC207 works against adenosine triphosphate (ATP) synthase.
The most desirable drugs act against targets that are new to the TB drug arsenal so the drugs will be active against drug-resistant TB and targets that are in pathways essential in persistent bacilli so the drugs will also shorten therapy.
Figure 7-3 shows the portfolio of drugs for TB currently in clinical trials, aimed toward registration. Ginsberg said that the first two, gatifloxacin and moxifloxacin, belong to the same chemical class; they are 8-methoxyfluoroquinolones. Because they are so similar and have exactly the same mechanism of action, it will make sense for only one of them to be incorporated into any regimen. TMC207 brings a completely novel mechanism of action to the TB armamentarium; as noted, it is an ATP synthase inhibitor. The next two drugs in Figure 7-3 are both nitroimidazoles—OPC67683 from Otsuka Pharmaceutical Group, which is currently in Phase II trials in MDR TB patients, and PA-824, which is being developed by the TB Alliance. Again, only one of these would ultimately be incorporated into any given regimen because they have very similar if not identical mechanisms of action. Finally there are two compounds, SQ109 from Sequella, Inc., and LL-3858 from Lupin Limited, which are both in Phase I development.
PROBABILITY OF SUCCESS
While promising drug development efforts are under way, and there are far more drugs in the pipeline than was the case even in 2000, it is important to keep in mind both the time frames for such efforts and the probabilities of success. A typical drug takes at least 10–15 years to be developed from discovery to registration. Drugs for TB may take even longer than this average because the clinical trials for these agents can be very lengthy as a result of the efficacy end points that are currently required. The Phase III timelines could be shortened for MDR TB if regulators were to accept surrogate end points, such as sputum smear conversion rates at 4 or 6 months.
In general, however, the probability of ultimate success for any given candidate is discouraging. A compound that has progressed to preclinical development out of the thousands of compounds that enter the discovery phase has about a 1 in 10 chance of making it to registration and therefore to patients. Only in Phase III development do the odds become fairly good. About two-thirds of drugs that make it all the way to pivotal clinical trials will ultimately be registered.
Ginsberg identified a number of strategies for addressing the challenges facing TB drug development:
-
A focus on developing multidrug regimens rather than individual drug candidates is needed. Combating drug resistance requires
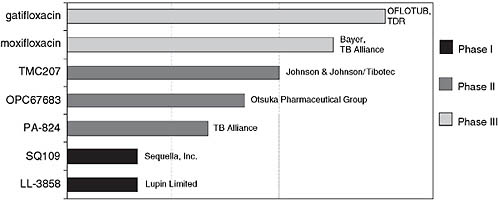
FIGURE 7-3 Global clinical portfolio of TB drugs in development.
NOTE: The OFLOTUB project (Ofloxacin-containing, short-course regimen for the treatment of pulmonary TB) is an ongoing study designed to simplify and shorten TB treatment from 6 to 4 months. TDR is an independent global program for research and training in tropical diseases.
SOURCE: Ginsberg, 2008.
-
cocktails of drugs with different mechanisms of action administered together. None of the current first-line TB drugs were developed in the modern regulatory era, and none have an ideal safety, pharmacokinetic, and efficacy profile for TB treatment, so entirely novel regimens will likely be required to markedly shorten and simplify TB treatment.
-
Improved biomarkers and validated surrogate end points are essential to streamline clinical development, and although research is progressing in this area, it will be a multiyear process.
-
Validated animal models that are predictive of drug efficacy and pharmacokinetic–pharmacodynamic relations in humans are needed.
-
POC trial designs that can predict not only which drugs are likely to be efficacious but also which ones will help shorten therapy would also streamline the development process.
-
Clinical trial capacity needs to be strengthened, including the development of sites, staff, and investigators who can work to current global registration standards.
-
Clear, harmonized regulatory guidance for TB drug development in both drug-sensitive and drug-resistant TB are needed. Both the EMEA and the FDA are working to meet this need, and significant advances may soon be achieved. Globally harmonized guidance would significantly simplify the challenge of development as registration for TB drugs is required not only by the EMEA and the FDA, but also by regulatory authorities in high-burden countries.
-
For any given drug candidate with a novel mechanism of action, simultaneous clinical development programs should be carried out to evaluate the drug for treatment of drug-sensitive and drug-resistant TB. Clinical trials for these two indications involve very different patient populations and study designs, so the resources required to do both are essentially double those required to pursue either indication alone.
Ray Woosley of The Critical Path Institute described recent discussions between the FDA and NIH, in association with the IOM, regarding formulation of a critical path for TB drug development. He also described the new TB data standards being developed by the Clinical Data Interchange Standards Consortium1 for use in drug development. In addition to new drug development, the codevelopment of drugs and diagnostics can be facilitated by sharing data to create quantitative disease progression models and biomarker assays that evolve to become FDA-approved diagnostic
|
1 |
For more information on these standards, visit http://www.cdisc.org/standards/. |
tests. In this case the goal is not just approval of a new drug or diagnostic but a therapeutic strategy that includes both, as well as how they can be used together effectively.
Cassell discussed the broad strategy for drug development. Based on data from Tomsk and Peru, it is apparent that only 60–70 percent of patients with XDR TB can be cured. Consequently, an urgent concern in the development of new drugs is treatment of MDR and XDR TB. Echoing a point made by Ginsberg, Cassell noted that, unlike MDR staph infections or malaria, which in some cases can be treated effectively with one antibiotic, TB requires a cocktail of drugs to be treated effectively. Treating and stopping the spread of MDR and XDR TB will require a minimum of three to four new antibiotics immediately.
Fauci spoke about research needs for TB. He suggested that there are important lessons to be drawn from the experience with AIDS research—a discipline that did not exist prior to 1981 and yet became one of the best funded in the history of biomedical research.
An estimated 1.7 million deaths annually are attributed to TB, which ranks fourth among the highest-burden infectious diseases globally. In terms of mortality, TB follows diarrheal and respiratory diseases, which cause approximately 4.3 and 2.2 million deaths, respectively, and HIV/AIDS, which causes approximately 2 million deaths. Fauci stated that one of the reasons for the extraordinary response to HIV/AIDS and the large increase in funding was the impact of the disease on the U.S. population. Although federal funding for HIV/AIDS increased slowly at first, it eventually began to grow exponentially (see Figure 7-4). The U.S. federal government as a whole has spent a total of $233 billion on HIV/AIDS, and in fiscal year 2008 alone spent approximately $23 billion. The NIH research component has shown a similar increase, reaching a cumulative total of $36 billion and nearly $2.9 billion per year. While the overall NIH budget has not increased for several years, HIV/AIDS funding constitutes a stable 11 percent of the entire NIH research budget. Solid funding and the resulting research efforts have led to a number of extraordinary advances over the 27 years since AIDS was first recognized. There are more antiretroviral drugs for HIV/AIDS than the total of all drugs available for all other viral diseases combined. The extent of product development that took place in HIV/AIDS was possible because of a serious investment in biomedical research, partnerships with industry, and the pharmaceutical industry’s realization that the development of antiretroviral drugs promises a large return on investment and significantly impacts the lives of patients in the United States and globally.
In addition to a significant pharmaceutical industry investment in the development of antiretroviral drugs, there has been a profusion of governmental and privately funded global programs directed at the prevention of
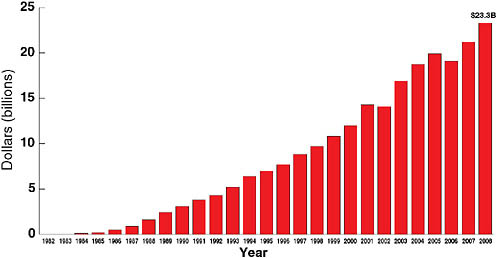
FIGURE 7-4 Federal funding for HIV/AIDS, 1982–2008.
NOTE: Through fiscal year 2008, more than $233 billion in federal funding has been spent on HIV/AIDS.
SOURCE: Fauci, 2008 (based on data from the Kaiser Family Foundation [KFF], unpublished analysis of data from the Office of Management and Budget, Congressional Research Service, federal agency documents, and congressional legislation; used with permission from KFF).
HIV/AIDS and treatment and care of HIV/AIDS patients (e.g., PEPFAR and the Global Fund for AIDS, Tuberculosis, and Malaria). A variety of other philanthropic organizations have also proven critical to helping HIV/AIDS patients worldwide. Since 2002, the number of HIV/AIDS patients in the developing world receiving antiretroviral drugs has grown from about 200,000 to more than 3 million.
Fauci noted that many of the challenges faced in combating HIV are similar to those for TB, and that lessons learned from biomedical, clinical, and operational research in HIV/AIDS may apply to TB. These lessons include the need to commit substantial financial and human resources, to enlist the best and brightest investigators in basic and clinical research, to engage the affected communities, to foster cross-collaboration with industry and global organizations, and to garner the support of leaders and policy makers.
Compared with the NIH budget for HIV/AIDS, the budget for TB is relatively modest. The current budget, shown in Figure 7-5, is roughly $152 million, $131 of which is NIAID funding. In fiscal year 2007, NIAID funding for biomedical TB research amounted to approximately $60million for fundamental research; approximately $47 million for drug development; and approximately $8.5 and $15 million for vaccine and diagnostic
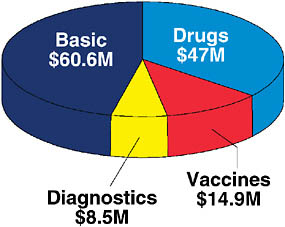
FIGURE 7-5 Funding for TB from the National Institute of Allergy and Infectious Diseases in fiscal year 2007.
NOTE: Total funding in 2007 was $131.1 million.
SOURCE: Fauci, 2008.
research, respectively. NIAID published a research agenda for MDR and XDR TB in June 2008 (Fauci, 2008). Fauci highlighted five areas of the research agenda that, in NIAID’s view, require major consideration:
-
Development of rapid and reliable diagnostic methods that can be used at the point of care;
-
Investment in the pipeline of new drugs, as well as proper use of existing first- and second-line therapies;
-
Investment in research to understand the epidemiology that contributes to the spread of drug-resistant and drug-sensitive strains of TB;
-
Understanding of the relationship between and comorbidities of HIV/AIDS and TB; and
-
Development of effective vaccine and chemotherapy prevention strategies for all forms of TB (see Table 7-1 for a list of the most advanced international vaccine development efforts).
NIH and several nongovernment organizations have launched initiatives in these areas.
Fauci reflected on the steps that must be taken to move the science forward. He first noted the extraordinary importance of coordinating research efforts among government agencies such as NIH, CDC, and the
TABLE 7-1 Four of the Eight TB Vaccine Candidates in Clinical Trials That Have Moved into Phase II Studies
U.S. Agency for International Development (USAID) and global partners such as other international government agencies, international development entities such as PEPFAR, philanthropic organizations such as the Bill and Melinda Gates Foundation, pharmaceutical and biotechnology companies, public–private partnerships and research consortia such as those at Eli Lilly, and a variety of others.
In addition, Fauci noted that it is essential not to treat TB in isolation because it almost invariably occurs in the context of other diseases. An obvious connection is seen in the coepidemics of HIV and TB in many areas of sub-Saharan Africa and other low- and middle-income regions of the world.
Finally, Fauci emphasized the need to balance fundamental research efforts with product development to ensure proper integration of scientific disciplines within infectious disease research, immunology, and state-of-the-art technological approaches. Multidisciplinary research must also include robust partnerships with various product developers and sustainable investment in the development and retention of human capital.
Cassell asked Fauci to share his experience with advances in accelerated review and licensure of new antiretroviral drugs and whether they offer any lessons applicable to product development for TB. Fauci explained that, while the concept of accelerated review was a paradigm shift for regulatory authorities in the 1990s, regulators are now amenable to this strategy. Researchers need to engage the FDA early in discussing the possibility of and requirements for expedited review as well as expedited or conditional approval. The same could be said of the parallel-track concept for clinical trial design (i.e., those who cannot be enrolled because of geographic
constraints or enrollment criteria could still receive a drug contemporaneously with the clinical trial). Regulators have now embraced this concept, which has proven to be a productive mechanism for clinical evaluation of experimental therapies while making therapies available to approved patients who lack other therapeutic options. As for moving forward without animal models, Fauci noted that there is a much better possibility of gaining important research and preliminary efficacy data from current and emerging models of TB infection and disease than was the case with HIV since HIV/AIDS is a uniquely human disease.
Woosley asked whether a national clinical research infrastructure comparable to the AIDS Clinical Trial Group (ACTG) network would be effective for TB.2 Castro replied that the ACTGs were in fact used for TB research in the context of HIV/AIDS coinfection, specifically to study the efficacy of rifampin and pyrazinamide for latent TB infection in HIV-infected patients.
Drazen asked Fauci what he would consider the highest priorities if new resources became available for TB research. Fauci replied that the development of quick, sensitive, and affordable point-of-care diagnostics is paramount, as are translational studies to discover and evaluate new drug and vaccine candidates.
Citing the significant advances in HIV once validated surrogate end points were available to aid in drug evaluation and regulatory approval, Castro asked Fauci to comment on immune correlates of protection for TB. Fauci responded that for HIV/AIDS, as is the case for TB, this was a difficult effort that took years and required the involvement of investigators from a variety of disciplines.
Ginsberg asked whether the market incentives for antiretroviral drugs and the extent of activism leveraged for HIV could make lessons learned less relevant to TB. Fauci asserted that there is potential for growth of TB activism. Because TB is a health crisis that is still unfolding, growing awareness of issues surrounding the global TB epidemic will likely lead to increased activism.
With respect to financial incentives for the development of health care interventions, there is a greater difference between HIV and TB. With HIV, there is a large patient population in the developed world willing and able to pay $17,000–18,000 a year for effective therapies; this is not the case with TB. TB occurs primarily in low- and middle-income countries, where patients are less likely to be able to afford expensive drugs. This disparity
calls for a greater role for government involvement in the development and distribution of affordable curative and preventive measures. A large, multilateral initiative may be the appropriate way to share risks and financial returns to provide the financial incentive for the development of new drugs (see the discussion of financial incentives below). Friedland asked about operational research strategies, such as cost-effectiveness studies, which enhance clinical and public health decision making. Fauci replied that for HIV/AIDS and TB alike, many studies in this area have yet to be conducted. Both infrastructure and operational research often receive less attention since they are peripheral to but highly integrated with basic and clinical science. The importance of operational research—of learning whether what is being done is the right thing—is still underappreciated. However, PEPFAR programs recently have become involved in several operational research projects for HIV, and similar attention is warranted for TB.
ECONOMIC INCENTIVES FOR DRUG DEVELOPMENT
Jeffrey Moe of Duke University described the various incentives that influence the decisions of companies to develop new drugs for TB. There are two important types of incentives in global health—push and pull mechanisms. Some examples of each are listed in Box 7-1. Push mechanisms stimulate the supply or production side of the market, while pull mechanisms stimulate the demand side.
The Orphan Drug Act of 1983 is an example of a push mechanism because it is aimed at making it easier, less costly, or less risky for a company to develop an orphan product.3 The specific incentives include the use of research and development (R&D) tax credits and grant support. BioShield4 represents another form of push mechanism that involves directly funding R&D for terrorism countermeasures. A third approach is the development of a public–private partnership such as the TB Alliance.
These partnerships are effective because they organize strategies within the field and facilitate the sharing of scientific knowledge and effort.
Pull mechanisms include the market exclusivity provisions of the Orphan Drug Act. However, market exclusivity for a TB drug in the United States would not be particularly attractive because the incidence of the disease, and therefore the market, are very limited. Another pull mechanism is the use of advance market commitments, through which market demand—e.g., a price and a certain number of units to be purchased—is guaranteed (typically by government or a philanthropic organization) in advance. A third pull mechanism is patent extension for an existing medicine as a reward for developing a drug to treat a disease of the developing world or a bioterrorism agent. The benefit to a company is clear: Extended patent life and therefore increased profitability of a drug constitute a highly tangible reward, which if applied to a blockbuster drug would be worth billions to a manufacturer. However, such an incentive singles out a group of patients and their insurers, by disease state, to bear the burden of the incentive. When such an incentive was discussed for inclusion in BioShield II, significant objections were raised by the generic pharmaceutical industry and patient advocacy groups.
Another type of incentive has actually been instituted in the form of the priority review voucher (PRV) concept included as Sec. 524 of the 2007 Food
and Drug Administration Amendments Act. In exchange for developing a new treatment for a neglected disease, such as TB or malaria (there are 16 qualifying diseases), a company receives a voucher that can be used to receive priority review by the FDA for another drug that is being developed or can be sold to another company.5 In 2006, Moe and colleagues published their estimate that priority review is accomplished on average 12.6 months more quickly than standard review (Ridley et al., 2006). If this speed to market is applied to a blockbuster drug for the period studied, the value, on average, is $322million to the manufacturer.
Moe cited five criteria that must be met for such incentives to be effective (Towse and Kettler, 2005):
-
The incentive must be efficient. For this reason, a program for an individual disease may not be as valuable as one that targets multiple diseases.
-
Target diseases must be carefully specified in advance.
-
The incentive must be credible in the eyes of potential developers, as drug development is a long and risky process that may span multiple administrations and regulatory regimes. If there is any question about the incentive’s being honored over a long time period, its value will be negated.
-
The definition and treatment of new chemical entities as opposed to follow-on drugs or new combinations of existing drugs must be carefully specified to avoid any concern that the rules of the game may change or be enforced capriciously.
-
There must be some assurance that the product will be used by patients.
Moe argued that, if the incentive is to be effective, the application of these five criteria is critical to motivate private industry to direct R&D monies toward highly risky development efforts for neglected diseases, including TB.