2
Travel, Conflict, Trade, and Disease
OVERVIEW
The essays collected in this chapter examine how travel, armed conflict, and trade move people—as well as animals, plants, and products made from them—and how these movements influence patterns of infectious disease transmission. The discussion begins with an exploration of the role of the global traveler in the emergence of infectious disease by workshop speaker Mary Wilson of Harvard, who illustrates the profound impact of recent increases in the volume, speed, and reach of global travel. “Humans can reach almost any part of the earth today within the incubation period for most microbes that cause disease in humans,” Wilson writes. “Travel is also discontinuous, often including many stops and layovers along the way. This means that travelers are part of the dynamic global process of moving biota, along with trade, which moves plants, animals, and other materials.” Moreover, she observes, several other trends—including growth in human and food animal populations and urbanization—further contribute to infectious disease emergence.
Travelers can serve as sentinels for disease, and thereby contribute to the global disease surveillance system, as Wilson demonstrates through key findings by the decade-old GeoSentinel Surveillance Network regarding transmission of falciparum malaria and dengue fever. The network gathers information on ill international travelers and migrants from 42 travel and tropical medicine clinics on six continents in order to provide early alerts about unusual infections or infections in unusual locations or populations. Studying travelers can help characterize “global microbial traffic,” Wilson concludes.
Travel, migration, and displacement are significant characteristics of armed
conflict that contribute to increased risk of infectious disease. Certain categories of infectious diseases tend to increase during war, according to workshop speaker Barry Levy of Tufts University; these include diarrheal diseases and acute respiratory infections, as well as measles, malaria, meningococcal disease, and tuberculosis. In the chapter’s second paper, Levy discusses major causes—apart from injury—that contribute to the increased incidence of infectious diseases during wartime: reduced availability of health services, environmental damage, and forced migration. Interestingly, Levy notes that whereas one might expect HIV transmission to increase during war due to concomitant increases in several risk factors for its transmission, “several studies have demonstrated that HIV incidence has generally decreased during war—only to increase again after conflict has ended.” Moreover, he adds, “there have been many successful HIV/AIDS prevention and treatment programs during armed conflict.”
Absent the cessation of armed conflict, the war-related burden of infectious disease can be addressed through attention to specific war-associated risk factors, as well as through a host of measures (e.g., surveillance, preparedness) that apply to any high-risk situation, Levy explains. He also notes the importance of protecting health care workers and preserving health-supporting infrastructure, which may be supported by maintaining their neutrality both during war and in its aftermath.
Like travel, globalized trade is vast, rapid, on the rise, and a significant risk factor for infectious disease emergence. In the chapter’s third essay, workshop speaker Ann Marie Kimball, of the University of Washington, and co-author Jill Hodges present case studies of several emerging infectious diseases, including H5N1 influenza and bovine spongiform encephalopathy (BSE), and their relationship to “risky” trade practices in food production and medicine. “While microbial risks have been globalized along with commerce, the corresponding health and protective measures for the most part have not,” the authors observe. The International Health Regulations (IHR) 2005 “provides some important safeguards to help limit the international spread of infectious disease,” they note, but these regulations require support for both capacity building and community building if their intent is to be fulfilled.
Responding to some of the disease threats described in the previous three essays is the daunting task taken on by workshop speaker David Acheson of the Food and Drug Administration (FDA). He describes the agency’s response to two recent challenges to the security of the U.S. food supply—the 2008 outbreak of Salmonella Saintpaul and the deliberate contamination of imported wheat gluten with melamine—in his contribution to this chapter. He also discusses changes in FDA’s food security efforts to respond to such threats by seeking to understand where and when they arise, to anticipate their potential to spread globally, and to use risk-based inspections to detect them before an outbreak occurs in the United States; these include efforts under way to increase the FDA’s presence in foreign countries, to develop model systems for risk-based inspections, and to make use
of inspection and testing data generated by industry or other “third parties” to increase the breadth and depth of their surveillance.
GLOBAL TRAVEL AND EMERGING INFECTIONS
Mary E. Wilson, M.D., F.A.C.P., F.I.S.D.A.1
Harvard University
Humans travel in numbers and at speeds unprecedented in history (IOM, 2003; Wilson and Chen, 2008). Travelers visit remote areas as well as major population centers. Humans may be displaced because of social, economic, or political upheavals or extreme events and environmental disasters (IOM, 2008). The elimination of spatial and temporal barriers, especially by long-distance air transport, means that humans can reach almost any part of the Earth today within the incubation period for most microbes that cause disease in humans. Travel is also discontinuous, often including many stops and layovers along the way. This means that travelers are part of the dynamic global process of moving biota, along with trade, which moves plants, animals, and other materials (Wilson, 1995b). Natural movement of animals via migration, and transport of seeds, microbes, and other materials via water and air currents, is the backdrop against which massive travel and trade are occurring in today’s world (Wilson, 1995a). One consequence of this movement is the juxtaposition of species that have never before had physical proximity. The contact between microbes, humans, and animals may result in infection, which may or may not be expressed in disease or death.
Another potential consequence of the movement of species, such as arthropods, mammals and other animals, and plants, whether intentional or inadvertent, is the establishment of species in new geographic areas (Tatem et al., 2006). These introductions may cause major changes in the existing ecosystem, including marine ecosystems. Many examples exist of the harmful effects of invasive species, though many species of well-regarded plants and animals in the Americas were not native to the Americas (Crosby, 1972).
Characteristics of Global Travel
Global travel has increased as reflected in Figure 2-1, showing numbers of international tourist arrivals from 1950 though 2005 and the projections until 2020. In addition to the marked increase in the overall number, there has also been a shift in areas visited by travelers, especially to areas in Asia. The 2006 figures from the World Tourism Organization showed the most rapid relative increase was to sub-Saharan Africa (UNWTO, 2008b). Travel between regions
|
1 |
Associate Professor of Global Health and Population, Harvard School of Public Health; Associate Clinical Professor, Harvard Medical School. E-mail: mewilson@hsph.harvard.edu. |
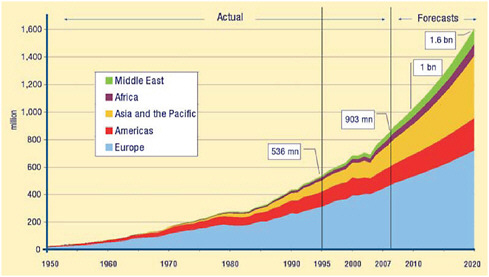
FIGURE 2-1 International tourist arrivals by region (in millions), 1950-2020.
SOURCE: Reprinted with permission from UNWTO (2008a).
was increasing faster than travel within regions, and air transport was growing at a faster pace than ground and water transport. Figure 2-2 shows the breakdown by means of transport, with air travel accounting for 46 percent of transport (UNWTO, 2008). The shifts in destination mean more people will be traveling to low-latitude countries—areas with greater species richness (Guernier et al., 2004) and often characterized by poor sanitation and limited infrastructure, a milieu where risk for exposure to common and previously unidentified microbes may be higher. In looking ahead, it is unclear to what extent the current dramatic changes in the global economy will affect numbers of travelers or favored destinations. Political instability and disease outbreaks can also influence travel destinations, sometimes abruptly.
A vivid example of the rapid increase in travel is the outline of lifetime tracks by David Bradley (1989; figure also reproduced in Cliff and Haggett, 2004), who recorded the life travel over four male generations in his own family (Figure 2-3). The linear scale for the spatial movement increases by a factor of 10 for each generation (Cliff and Haggett, 2004). Similar findings were noted in a study which showed that spatial mobility, taking into account all forms of transportation, of the French population between 1800 and 2000 increased 1,000-fold (Grubler and Nakicenovic, 1991). Using numbers from the U.S. Census Bureau and the United Nations World Tourism Organization (UNWTO, 2008c), one can calculate that the world population between 1950 and 2007 increased 2.6-fold, whereas the international tourist arrivals increased 35-fold. Although individuals
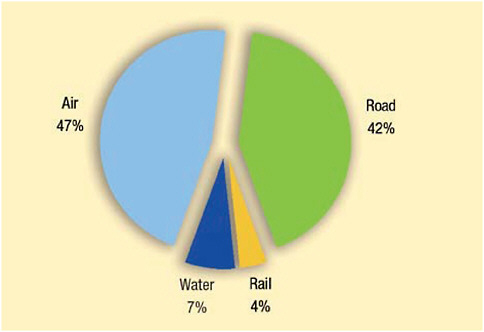
FIGURE 2-2 Inbound tourism by means of transport.
SOURCE: Reprinted with permission from UNWTO (2008a).
on average are traveling much greater distances, González and colleagues found that individual human trajectories showed high degrees of temporal and spatial regularity, with individuals returning to a few highly frequented locations (González et al., 2008).
Population Size, Density, Location, and Proximity to Animals
This increase in human movement is not occurring as an isolated event. Today, the global human population is the largest ever recorded—and so is the population of food animals. About half of the people on Earth live in urban areas, the largest fraction ever (Wilcox et al., 2008). These human hosts provide expanded opportunities for viral replication and mutation events. Most of the population and projected growth are in low-latitude urban areas—regions that are home to sprawling megacities, many surrounded by vast slum areas that lack clean water and sanitary facilities (Figure 2-4). Animals such as dogs, chickens, cows, and rats live in and near human living quarters, which have been assembled from whatever materials can be found. Individuals who inhabit these areas often work in major metropolitan areas, so they have regular contact with large, dense human populations in high-rise buildings and other built environments. Residents
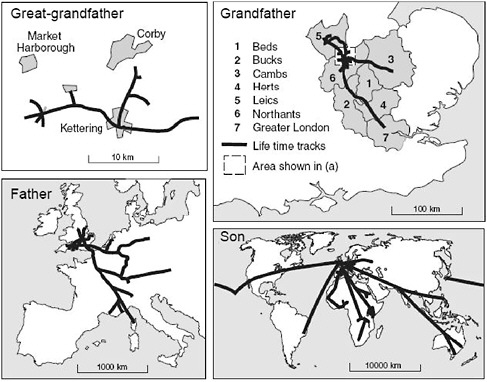
FIGURE 2-3 Life travel over four male generations in the same family.
SOURCE: Adapted from Bradley (1989) and reprinted from Cliff and Haggett (2004) with permission from Oxford University Press.
of periurban slums may also visit family in rural areas, thus potentially providing a link from rural to urban populations and to the rest of the world. Changes in the environment, including extreme weather events, can favor the appearance of some infections and can also displace populations (IOM, 2008).
Animals have been the origin of many of the recently identified emerging infectious diseases, including HIV/AIDS, H5N1 avian influenza, severe acute respiratory syndrome (SARS) (Jones et al., 2008; Wang et al., 2008), and swine-origin H1N1 influenza A (Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team, 2009; Smith et al., 2009a). Populations of animals have expanded rapidly, in large part to accommodate the desire for more animal protein in the diet, which has coincided with the economic resources to buy it. In China, for example, at the time of the 1968 influenza pandemic, the size of the human population was 790 million, the pig population 5.2 million, and the poultry population 12.3 million. By 2005, while the human population in China increased less than two-fold, the pig population increased about 100-fold to 503 million and the poultry population increased 1,000-fold to 13 billion (Osterholm, 2005).

FIGURE 2-4 Juxtaposition of urban slums and modern buildings in São Paulo, Brazil.
SOURCE: Image courtesy of Zema Fontoura.
Concentrated animal feeding operations, where large numbers of genetically similar animals are raised in concentrated areas—so-called factory farms—are becoming increasingly common in the United States and other countries (Pew Commission, 2008). Unfortunately, little systematic surveillance of influenza and other potential pathogens in swine populations is routinely done, a shortcoming highlighted by the emergence and spread of a reassortant influenza in 2009 (Smith et al., 2009a).
Roles of the Traveler
Human travelers can easily carry person-to-person transmitted infections to any part of the world. An example is the human immunodeficiency virus (HIV), which was introduced to all areas of the world almost exclusively by travelers (Perrin et al., 2003). Recently, swine-origin H1N1 has spread globally, its movement hastened by global air travel. Although drug-resistant forms of tuberculosis can emerge in settings with inadequate and inappropriate treatment regimens, humans also transport and transmit tuberculosis, including multidrug resistant (MDR) and now extensively resistant (XDR) forms of tuberculosis, in geographic areas far from the point of acquisition (Jassal and Bishai, 2009; Oeltmann et al., 2008). Although tuberculosis is an old disease and is present worldwide,
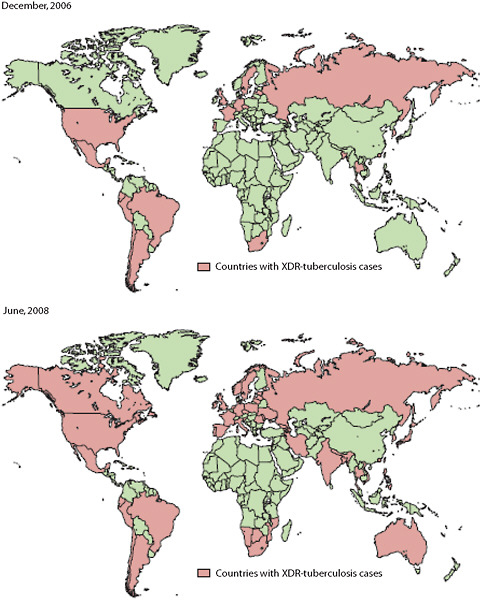
the incidence of infection and the levels of drug resistance vary enormously by population and geographic area. In 2008, the overall incidence of tuberculosis in the United States was 4.2 cases per 100,000 population (CDC, 2009). The rate in persons who were foreign born was 10 times higher than the rate in persons who were U.S. born, reflecting the vast differences in rates of tuberculosis around the world. A number of countries have annual incidence rates exceeding 300 per 100,000. Figure 2-5 displays the geographic spread of XDR tuberculosis between December 2006 and June 2008.
The traveler can serve in many roles (Wilson, 2003). Nonimmune travelers are at risk for a number of infections that exist primarily in tropical areas. Vaccines and drugs are available today to reduce the risk for many of these infections, such as yellow fever and malaria. Travelers were important historically in the spread of infections and remain so today, perhaps to an even greater extent (Colizza et al., 2006). They can also carry microbes with resistance genes, even if they are unaware of it. Travelers today continue to spark outbreaks of measles in populations that do not have high levels of immunity. It is easy to see how travelers could play a key role in the global epidemiology of infections that are transmitted from person to person, such as HIV, SARS, tuberculosis, influenza, and measles (Hufnagel et al., 2004), but they are also important in the spread of some vector-borne infections, as will be discussed below.
Receptivity to Introductions
Geographic areas and populations vary in their receptivity to introductions of potential pathogens that can cause human disease. Multiple factors are in play. The physicochemical environment may preclude the presence of a necessary mosquito vector or essential intermediate host. The physical environment may also influence transmission dynamics. For example, influenza has a strong seasonal pattern, especially in temperate regions. This seasonality is influenced by the humidity; recent studies suggest that absolute humidity is a more useful measure than relative humidity. The absolute humidity affects influenza virus transmission and virus survival. Absolute humidity can explain 90 percent of the variability of influenza virus survival, whereas relative humidity can explain only 36 percent of variation (Shaman and Kohn, 2009). Hence, travelers with influenza returning to temperate areas during hot, humid months are unlikely to spark epidemic spread (Lowen et al., 2008), though focal outbreaks in contained, air-conditioned spaces (e.g., air-conditioned nursing homes and barges) have been reported during hot, humid weather. Influenza outbreaks in the Southern Hemisphere occur during hot-weather months in the Northern Hemisphere; in the tropics influenza can circulate throughout the year. Analysis of H3N2 epidemics worldwide between 2002 and 2007, including those in temperate regions, suggested that they were seeded annually by viruses that had first appeared in East and Southeast Asia (Russell et al., 2008). These viral strains appeared to have evolved from other Asian strains.
Other local factors that affect the receptivity of individuals and a population to introduction of a new infection include housing, sanitation, and living conditions. Good nutrition can reduce the vulnerability to some infections or diminish their severity. Populations may be immune because of vaccination or prior infection. Human behavior and activities influence exposure to a number of infections (e.g., sexually transmitted infections). And finally, good surveillance and wider access to good medical care may reduce the burden of an infection in a population and allow it to be brought under control.
In addressing the question of how controllable an infection is that is directly transmitted from person to person, a key factor is the proportion of transmission that occurs before onset of symptoms or during asymptomatic infection (Fraser et al., 2004). Public health measures are most likely to be effective when little or no infection is transmitted during asymptomatic infection. Figure 2-6 displays four
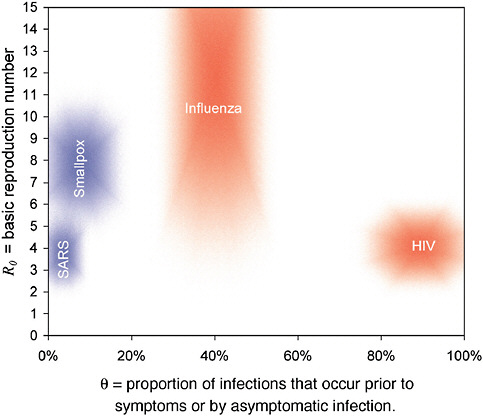
FIGURE 2-6 How controllable is an infection? Plausible ranges for the key parameters R0 and θ for four viral infections of public concern are shown as shaded regions. The size of the shaded area reflects the uncertainties in the parameter estimates.
SOURCE: Fraser et al. (2004).
infections that are transmitted from person to person: SARS, smallpox, influenza, and HIV. Fortunately, the fever caused by infection with the SARS coronavirus preceded onset of transmissibility, meaning that with strict surveillance for symptoms and isolation of those with symptoms, it became possible to interrupt the transmission of this infection. Influenza, on the other hand, is more difficult to contain because transmission may begin before onset of symptoms, and infected patients may have little or no fever. Based on epidemiological analyses, Fraser et al. (2009) estimated the basic reproductive number of the swine-origin H1N1 influenza A virus in the range of 1.4 to 1.6.
Vector-borne Infections
Vector-borne infections can also be introduced into new geographic areas by travelers, though the vulnerable areas are restricted to those with competent mosquito or other arthropod vectors. Because important vectors, such as Aedes aegypti and Aedes albopictus, can be dispersed by trade (especially via ships), more human-inhabited areas of the world are infested with these potential vectors than ever before (Tatem et al., 2006). Aedes aegypti thrives in an urban environment, and today about 2.5 to 3 billion people live in tropical and subtropical areas infested with this mosquito.
Two vector-borne infections, dengue fever and chikungunya infection, which have expanded in distribution in recent years, illustrate multiple contributions to this dynamic process and spread. Dengue virus is causing more infections, including more cases of severe and complicated dengue fever, than ever before (Wilder-Smith and Gubler, 2008). Although multiple factors contribute, three forces described above are especially important: urbanization with major expansion of populations living in tropical and subtropical areas; population size; and rapid, frequent travel of viremic humans to areas infested with competent vectors. Lax vector control programs and urban settings that lack piped water (so residents must store water in their homes) and are littered with used tires, discarded plastic cups, and other trash with standing water that allow breeding of mosquito vectors exacerbate the problem. Today, the dengue viruses have a much larger host population in which to replicate, recombine, and mutate than ever before, given the size of the human population. Zanotto and colleagues (1996) found that the number of dengue lineages has increased roughly in parallel with the size of the human population over the past 200 years (Figure 2-7). More urban areas in tropical and subtropical regions now have a population size large enough (estimated to be between 150,000 and 1 million) to allow the ongoing circulation of dengue viruses. An increasing number of geographic areas are experiencing cocirculation of more than one dengue serotype, setting the stage for secondary infections and more severe disease.
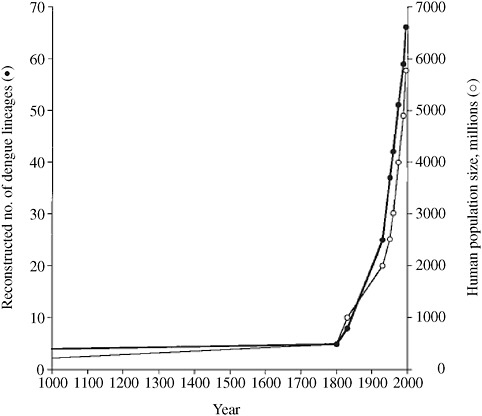
FIGURE 2-7 Global population size and lineages of dengue virus over time.
SOURCE: Zanotto et al. (1996).
Spread of Chikungunya Virus Infections
Although the mosquito-transmitted alphavirus, chikungunya2 (family Togaviridae), was first identified as a cause of outbreaks in Africa in 1953 (Lumsden, 1955) and has caused outbreaks at irregular intervals since then in Africa and Asia, the virus has caused multiple outbreaks in new geographic areas since 2004. Outbreaks in coastal Kenya in 2004 were followed by multiple explosive outbreaks on the islands of the Indian Ocean beginning in 2005 (Charrel et al., 2007; Josseran et al., 2006; Renault et al., 2007; Sergon et al., 2008). Subsequent outbreaks occurred in India, Thailand, Indonesia, Malaysia, and other Asian countries (AbuBakar et al., 2007; Mavalankar et al., 2008). In India alone, more than a million cases were reported. In some areas, extremely
high attack rates of infection were observed, affecting more than half of the population (Sergon et al., 2008). Because the virus causes a severe, frequently incapacitating polyarthralgia3 as part of the illness, the infection has been devastating in areas with high attack rates (Simon et al., 2008). Unlike dengue fever, another acute febrile illness, chikungunya infection is frequently followed by persistent joint symptoms that can continue for months or even years.
More than 1,000 cases have occurred in travelers who have returned to non-endemic regions (Hochedez et al., 2006; Panning et al., 2007; Parola et al., 2006; Simon et al., 2008). A high number of travelers with chikungunya fever have been diagnosed in Europe, reflecting frequent travel destinations in Indian Ocean islands. Chikungunya fever has always been considered a disease of the tropics, but in the summer of 2007, 175 cases were laboratory confirmed as the cause of an outbreak of an acute illness in two villages in northeastern Italy (Rezza et al., 2007). The clinical attack rate increased with increasing age in this outbreak, as has been observed in other outbreaks as well. Investigation identified a visitor from India as the index case. Transmission occurred during the hottest months of the year and stopped when temperatures cooled. Chikungunya virus was found in Aedes albopictus, the presumed mosquito vector in this outbreak.
Textbooks describe chikungunya fever as a self-limited illness with fever, polyarthralgia, headache, and rash, but in the recent outbreaks excess mortality was also reported (Figure 2-8; Mavalankar et al., 2008). Deaths have been concentrated in older persons.
Because virus is present in high concentration in the blood of infected humans (may exceed 109 RNA copies/mL of plasma), potential risk exists for nosocomial transmission. At least one instance is described of transmission to a health care provider in France after blood exposure (Parola et al., 2006). The virus could also be transmitted by transfusion of blood donated during the period of viremia. Investigators have modeled the potential risk for transfusion in an area with an outbreak (Brouard et al., 2008). In Reunion, where a massive outbreak occurred in 2005-2007, blood collection was interrupted for several months during the epidemic. Based on sentinel surveillance, knowledge of the duration of viremia in chikungunya infections, and the frequency of asymptomatic infections, the authors estimated that the risk of viremic blood donation was 1,500 per 100,000 donations during the peak of the epidemic and was a mean of 132 per 100,000 donations over the entire course of the outbreak. Returning travelers who donated blood during asymptomatic viremia could also be a potential source of infection in new geographic areas.
A mutation in the chikungunya virus may partially explain the intensity of recent outbreaks (Schuffenecker et al., 2006). Ae. albopictus, originally found in Asia, has become widely dispersed beyond Asia and is now found in many parts of the Americas, Europe, Africa, and the Pacific Islands. Although in the past the
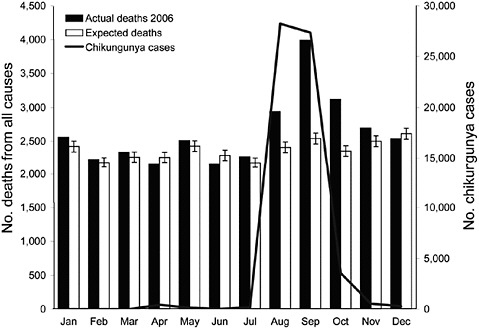
FIGURE 2-8 Increased mortality associated with chikungunya outbreak, India.
SOURCE: Mavalankar et al. (2008).
mosquito had not been considered a particularly efficient vector, it was identified as the primary vector in some of the recent chikungunya virus outbreaks (Reiter et al., 2006). A mutation has been identified in a viral gene encoding the envelope protein of the virus (A226V) and was found in more than 90 percent of viral isolates in the latter part of the outbreak in Reunion and in isolates from the outbreak in Italy. Of note, presence of the mutation is associated with enhanced susceptibility of Ae. albopictus to infection with chikungunya virus and to more rapid dissemination into the mosquito salivary glands. This means that a mosquito can become infected when exposed to a lower level of viremia, which may give the mutant virus enhanced survival benefit (Tsetsarkin et al., 2007). Mosquitoes vary in their susceptibility to infection and competence to transmit viruses (Tesh et al., 1976). Recent studies of Ae. aegypti and Ae. albopictus strains from Florida, using a chikungunya virus isolate from the Reunion outbreak, demonstrated that the mosquitoes were susceptible to infection and capable of transmitting the virus (Reiskind et al., 2008). Large areas of the Americas are infested with Ae. albopictus and Ae. aegypti. The potential for introduction of chikungunya virus is greatest in areas with high temperatures, abundant mosquitoes, housing or work places that allow mosquito-human contact (e.g., absence of window screens and air conditioning), and large volumes of travelers arriving from areas of Africa or Asia experiencing outbreaks (Charrel et al., 2008; Wilson, 2009).
Other Routes of Transmission
Dengue infection (which can lead to dengue hemorrhagic fever) can also be transmitted by blood transfusion (Tambayh et al., 2008) and by blood exposures in health care settings (Chen and Wilson, 2004, 2005). This may occur more frequently than is documented because occasional events of nosocomial and transfusion-related transmission could be difficult to distinguish from background infections in areas where infection is endemic and epidemic. Such events are more likely to be documented in nonendemic regions (Chuang et al., 2008). Recent studies have identified dengue RNA in blood donated by residents in Puerto Rico, Brazil, and Honduras (Linnen et al., 2008; Mohammed et al., 2008).
Medical Tourism
A reason for travel that is cited by increasing numbers of international travelers is medical treatment abroad (Reed, 2008). An estimated four million people travel internationally each year for medical care that often involves surgical procedures. A few countries attract the bulk of these travelers. Thailand, for example, currently receives about a million patients per year, and India, Singapore, and Malaysia are expected to attract similar numbers by 2012 (Smith et al., 2009b).Those who seek medical treatment abroad often undergo major procedures, such as heart bypass graft surgery, heart valve replacement, hip replacements, and bone marrow and liver transplantation, procedures that frequently require support by transfusions of blood and blood products. Some international institutions are seeking accreditation by the Joint Commission International4 to increase patient confidence in the quality of care. Still, in addition to ethical and legal issues, there are concerns about unrecognized risks to these medical tourists because of geographic differences in disease risks. Even if locally donated blood is tested for any evidence of infection, as would be carried out in the United States, other risks may exist in these areas, such as locally endemic and epidemic diseases, like dengue and chikungunya (Wilder-Smith et al., 2009). At present, blood donated in these regions is not screened for these infections.
Travelers as Sentinels
Travelers can serve as sentinels and couriers and should be an integral part of the global surveillance system (Wilson, 2003). Although travelers to an area do not experience all of the same infections seen in a local population, their infections reflect those present in the area. Returned ill travelers often have access to
medical care in facilities where diagnoses can be confirmed, bacteria cultured, malaria species identified, viruses isolated, specific serologic tests carried out, and sensitivity testing done. Under some circumstances, sequencing of an organism or molecular studies carried out in a research laboratory can yield insights that can be helpful in the understanding, prevention, control, or treatment of the disease in the area where it was acquired. Infections in travelers can also provide a global alert about infections in an unexpected location or with an unusual resistance pattern. In some instances, the diagnosis of an infection in a returned traveler can be the first indication of the presence of an infection in a particular geographic area or at a specific site. During their visits, travelers sample the biome of an area simply by staying in the area, and they can be a courier in which the microbes survive, replicate, and can later be sampled and examined.
Study of infections in travelers has also provided new insights about transmission mechanisms that might not have been identified in endemic areas where laboratory support is less robust. Examples have been documented of transmission of dengue to health care workers by exposure to blood of infected travelers in temperate areas where dengue is not endemic (Chen and Wilson, 2004).
The GeoSentinel Surveillance Network,5 a worldwide communication and data collection network of travel and tropical medicine clinics started more than a decade ago, systematically gathers information on ill international travelers and migrants and has been able to provide early alerts about unusual infections or infections in unusual locations or populations. This global network includes staff at 42 travel and tropical medicine clinics with sites on all six continents. As of early 2009, data from more than 100,000 clinical visits had been entered into the database. Despite the limitations of data gathered by this type of surveillance network (Leder et al., 2008), analyses have yielded useful insights, including showing how the spectrum of disease varies depending on the place of exposure among ill returned travelers (Freedman et al., 2006).
Falciparum malaria infections in travelers returned from the Dominican Republic signaled the reappearance of malaria in parts of the island and led to changes in recommendations for chemoprophylaxis (CDC, 2005). The increasing resistance of salmonellae to quinolones documented in returned travelers with typhoid fever has influenced initial treatment choices in patients with severe typhoid fever. An index case of schistosomiasis in a traveler returning to Israel from a luxury safari trip to Tanzania prompted an investigation leading to the identification of 22 cases of acute schistosomiasis (Leshem et al., 2008). Subsequent investigation revealed that 81 percent of those exposed at a specific site became infected. Early diagnosis of the index case enabled clinicians to study the usefulness of different diagnostic tests and observe the clinical course of acute schistosomiasis in nonimmune persons who had been infected during a single short (mean duration, 40 minutes) exposure to an unchlorinated, freshwater pond.
Dissemination of information about the outbreak through electronic networks (ProMED and GeoSentinel) made it possible to inform the global community about the outbreak more than a year before a paper about the outbreak appeared in a peer-reviewed medical journal.
Analysis of dengue cases from the GeoSentinel database over a 10-year period revealed that a surge in dengue infections in returned travelers could herald an increase in dengue-endemic countries in identified regions (Schwartz et al., 2008). In 2002, an increase in cases led GeoSentinel staff to post an alert on ProMed (Freedman et al., 2002). In several instances, an increase had been evident before official surveillance data were available from specific countries, which reinforces the importance of sentinel surveillance in travelers.
A different kind of network, the Boston Area Travel Medicine Network (BATMN), is in the early stages of collecting serum samples on selected travelers before and after travel to begin to assess exposures to infections in different geographic regions.
Conclusion
Travelers play a critical role in the movement of microbes globally. In an increasingly interconnected world with a growing, increasingly urban population in low-latitude areas, new risks exist and disease-causing microbes and resistance genes can move even more rapidly than in past decades. Travelers can also serve as an important sentinel population. Studying them can help to characterize the global microbial traffic.
ARMED CONFLICT AND INFECTIOUS DISEASE
Barry S. Levy, M.D., M.P.H.6
Tufts University School of Medicine
This paper is designed to stimulate discussion on a complex set of issues associated with armed conflict (war), infectious disease, and public health—what we, as a society, do collectively to ensure the conditions in which people can be healthy (IOM, 1988).
The health consequences of war include:
-
War-related injuries and diseases,
-
Adverse effects on medical care and public health services,
-
Damage to health-supporting infrastructure and the environment,
-
Forced migration,
|
6 |
Adjunct Professor of Public Health, 20 North Main Street, Suite 200, P.O. Box 1230, Sherborn, Massachusetts 01770, Telephone: 508-650-1039; Fax: 508-655-4811; E-mail: blevy@igc.org. |
-
Violation of human rights,
-
Diversion of resources, and
-
Promotion of violence (Levy and Sidel, 2008).
Certain categories of infectious diseases are increased in war and other complex emergencies, including diarrheal diseases, acute respiratory infections, measles, malaria, meningococcal disease, and tuberculosis. Two studies from the recent civil war in the Democratic Republic of Congo demonstrated that infectious diseases are frequent causes of death during wartime. One study found that diarrhea, respiratory infections, and suspected malaria were among the most frequent causes of death (Van Herp et al., 2003), and the other study found that fever/malaria and diarrhea were among the most frequent causes of death in young children (Table 2-1; Coghlan et al., 2007).
Causes of Infectious Diseases Due to War
Among the major causes of infectious diseases due to war are:
-
Adverse effects on medical care and public health services,
-
Damage to the health-supporting infrastructure and the environment,
-
Forced migration,
-
Diversion of resources, and
-
Biological weapons.
Each of these causes deserves further discussion.
Adverse Effects on Medical Care and Public Health Services
During war, a variety of factors adversely affect medical care and public health services. Physicians, nurses, and other health workers are injured or killed
TABLE 2-1 Causes of Death in Young Children (0-4 Years of Age), Western Democratic Republic of Congo, January 2006-April 2007
|
Fever/malaria |
35% |
|
Neonatal death |
13% |
|
Diarrhea |
13% |
|
Anemia |
7% |
|
Acute respiratory tract infections |
7% |
|
Measles |
5% |
|
Meningitis |
5% |
|
Malnutrition |
5% |
|
SOURCE: Based on data in Coghlan et al. (2007). |
|
or they flee. Clinics and hospitals may be damaged. Public health services are curtailed. Supplies of medications and vaccines are reduced.
Damage to the Health-Supporting Infrastructure and the Environment
During war and in the immediate aftermath of war, damage to the health-supporting infrastructure is often the main reason for poor health and excessive numbers of deaths. Food safety and supply, sewage treatment, water safety and supply, electrical power, transportation, and communication may be adversely affected. In addition, there may be extensive damage to the physical environment that may, in turn, adversely affect health; for example, bomb craters in Vietnam subsequently filled with stagnant water and became prime breeding sites for malaria-carrying mosquitoes.
Damage to the health-supporting infrastructure and the environment promotes diarrheal disease due to contamination of food and water supplies and inadequate sewage treatment and disposal. It also promotes acute diarrheal disease due to overcrowding, air contamination from indoor fires, and inadequate shelter. Tuberculosis (TB) often increases during war. For example, a study of 36 conflicts found that the TB notification rate before conflicts was 81.9 per 100,000 and after conflicts was 105.1 per 100,000. It also found that the risk of presenting with TB 2.5 years after the outbreak of conflict was the same as that 2.5 years before the conflict, indicating improvement in the post-conflict period (Drobniewski and Verlander, 2000).
Another study found that, in the war in Afghanistan during the 1980s, TB occurrence increased because TB control activities ceased. After the war, the situation improved, but even by 1999 the incidence of active TB cases there was still high (278 per 100,000) and only 10 percent of TB patients received directly observed therapy (Kahn and Laaser, 2002).
A study during the civil war in Guinea-Bissau in 1998 found that those TB patients who received irregular or no treatment had a three-fold increase in mortality, and that HIV-positive patients had an eight-fold increase in mortality (Gustafson et al., 2001).
A study in East Timor found that successful restoration of TB services during the five years after the end of conflict was primarily due to the structure and experience of a local nongovernmental organization, and the commitment and flexibility of local personnel and international advisors (Martins et al., 2006).
HIV transmission could increase during war for a variety of reasons, including increased risk-taking behavior, sexual violence, inadequate access to condoms, untreated sexually transmitted infections, commercial sex, HIV-contaminated blood, and inadequate use of universal precautions. However, several studies have demonstrated that HIV incidence has generally decreased during war—only to increase again after conflict has ended. For example, a study of seven countries with long-term civil disorders or wars in sub-Saharan Africa found that
HIV prevalence was relatively low during war. In Sierra Leone and Somalia, adult HIV prevalence was less than 1 percent. In the Democratic Republic of Congo, prevalence stabilized during civil war and disorder after 1991. In Angola and Liberia, there was apparently low HIV prevalence during wartime. And in Mozambique, HIV prevalence was approximately 1 percent immediately after civil war (although there was a dramatic increase in HIV prevalence after the war; Gisselquist, 2004).
There have been many successful HIV/AIDS prevention and treatment programs during armed conflict. In Côte d’Ivoire, this success occurred largely due to the importance of nongovernmental organizations working with regional and international organizations and United Nations agencies (Betsi et al., 2006). In the Democratic Republic of Congo, major factors in successful prevention and treatment of HIV/AIDS were adequate human resources, secure drug storage, decentralization of care, and integration of services (Culbert et al., 2007).
Violation of human rights of women and children adds to vulnerability to HIV infection during wartime. For example, a study in northern Uganda found that mass abduction of children into the resistance army led to increased vulnerability to HIV infection, with boy soldiers being coerced to use rape as a weapon and girls forced to become sexual slaves. It also found that in camps for internally displaced persons in northern Uganda, women were raped and driven to provide sex for money, thus increasing their vulnerability to HIV infection (Westerhaus et al., 2007).
Forced Migration
There are approximately 12 million refugees and 22 to 25 million internally displaced persons globally. They suffer from loss of sociocultural support systems and reduced access to safe food and water, inadequate medical care and public health services, and inadequate clothing and shelter, and they are at increased risk for many infectious diseases. For example, in 1980 at a camp for Cambodians in Thailand, the leading diagnoses in the emergency ward of a 1,000-bed field hospital were predominantly infectious diseases (Table 2-2). In 1994, approximately
TABLE 2-2 Leading Diagnoses, Emergency Ward, Khao-I-Dang Camp for Cambodians, Thailand, 1980
|
Upper respiratory infection and pneumonia |
25% |
|
Gastroenteritis/diarrhea |
13% |
|
Measles |
8% |
|
Otitis media |
5% |
|
Trauma |
5% |
|
Fever of unknown origin |
4% |
|
Meningitis |
4% |
|
Malaria |
2% |
TABLE 2-3 Cause-Specific Mortality Among Internally Displaced Persons in Camps, South Darfur, May-June 2005
|
Diarrhea |
25% |
|
Injuries |
14% |
|
Acute respiratory infections |
7% |
|
Malnutrition |
5% |
|
Tetanus |
5% |
|
Malaria |
5% |
|
Meningitis |
2% |
|
Measles |
2% |
|
Maternal mortality |
2% |
|
Other causes of death |
33% |
|
SOURCE: Reprinted from World Health Organization and Federal Ministry of Health, Sudan (2005) with permission from WHO/EMRO. |
|
1 million refugees from Rwanda fled to Zaire in less than one month; many of them died from cholera or dysentery soon after arrival in refugee camps there. In 2005, mortality among internally displaced persons and others in Darfur, Sudan, was mainly due to infectious diseases (Table 2-3; World Health Organization and Federal Ministry of Health, Sudan, 2005).
Diversion of Resources
War and the preparation for war cause extensive diversion of human and financial resources. For example, in 1990, per capita military expenditures in Ethiopia were $16 compared to $1 per capita for all health expenditures, per capita military expenditures in Sudan were $25 compared to per capita health expenditures of $1, and per capita military expenditures in Angola were $114 compared to per capita health expenditures of $8. Diversion of resources also occurs in developed countries during war and preparation for war. As one relatively small example, the $107 million spent by taxpayers in the District of Columbia for U.S. nuclear weapons programs for fiscal year 2009 could have funded health care for 34,000 children for one year (National Priorities Project, 2008).
Biological Weapons
There is a long history of the sporadic use of biological weapons during war or preparation for war. Examples have included contaminating drinking water with microorganisms, hurling of plague victims into a walled city, infecting blankets with smallpox virus, placing dead animals in water sources, infecting horses with glanders bacteria, and testing anthrax bombs on a deserted island (Metcalf,
2002). In the 1950s and the 1960s, the United States and the Soviet Union each developed a large infrastructure for research and development of both offensive and defensive biological weapons. In 1972, the Biological Weapons Convention was signed, which banned the development, production, stockpiling, or acquisition of biological weapons and their means of delivery, except for peaceful purposes. Although there is no formal verification regime for the Convention, 162 nations have now signed or ratified it.
The Centers for Disease Control and Prevention has developed three categories of diseases caused by biological agents, based on the severity of these diseases and the presumed likelihood that they could be caused by bioweapons. Category A includes anthrax, botulism, plague, smallpox, tularemia, and viral hemorrhagic fevers; category B includes brucellosis, disease caused by epsilon toxin of Clostridium perfringens, food safety threats, glanders, melioidosis, psittacosis, Q fever, ricin toxin, staphylococcal enterotoxin B, typhus fever, viral encephalitis, and water safety threats; and Category C includes emerging infectious diseases, such as those caused by Nipah virus and hantavirus.
Preparedness for bioterrorism should be placed more appropriately among U.S. national priorities for prevention and control of infectious diseases. Table 2-4 shows the incidence and mortality for selected causes of infectious disease in the United States from 2001 to 2004, demonstrating that the numbers of incident cases and deaths for AIDS, hepatitis C, and hospital-associated infections were much higher than those for bioterrorism.
The U.S. National Counterterrorism Center Report for 2007 reported on approximately 14,000 terrorist attacks worldwide, accounting for approximately 22,000 deaths and approximately 44,000 wounded people. Armed attacks and bombings accounted for the vast majority of fatalities. However, none of these reported attacks were due to biological agents (U.S. National Counterterrorism Center Report, 2008).
TABLE 2-4 Incidence and Mortality for Selected Causes, United States, 2001-2004
|
|
Incident Cases |
Deaths |
|
Bioterrorism |
23 |
5 |
|
AIDS |
157,468a |
68,802 |
|
Hepatitis C |
107,000 |
36,000 |
|
Hospital-associated infections |
6,800,000b |
395,948b |
|
aBased on 35 areas with confidential name-based HIV infection reporting. bBased on estimates for 2002. SOURCES: Based on data in CDC (2005), Klevens et al. (2007), Page et al. (2002), and Wasley et al. (2008). |
||
What Needs to Be Done
The following measures need to be better designed and implemented to address the problems related to armed conflict and infectious disease:
-
Surveillance for infectious diseases, and also for factors that are known to increase the risk of armed conflict;
-
Evaluation of prevention and control measures for infectious diseases related to war, and for measures to help prevent armed conflict;
-
Protection of medical care and public health services and maintenance of their neutrality during war and the aftermath of war;
-
Elimination, or the prevention of increases, of disease vectors during war and the aftermath of war;
-
Epidemic preparedness and responsiveness to outbreaks, especially in less-developed countries;
-
Diagnosis, treatment, and prevention of infectious disease;
-
Protection of the health-supporting infrastructure and the environment during war and the immediate aftermath of war;
-
Reduction of forced migration during war and its aftermath, and meeting of the basic needs of refugees and internally displaced persons and protection of their human rights;
-
Control of biological agents and strengthening of the Biological Weapons Convention; and
-
Creation of a world without war by addressing the underlying causes of war, controlling weapons, and strengthening the infrastructure for peace.
Ultimately, eliminating infectious disease caused by armed conflict will require the elimination of armed conflict.
RISKY TRADE AND EMERGING INFECTIONS
Ann Marie Kimball, M.D., M.P.H., F.A.C.P.M.7
University of Washington
Jill Hodges, M.P.H., M.S.L.
Global commerce is rapidly globalizing our food supply, our supply of pharmaceuticals, even our supply of biological sources. It has been well demonstrated that this process entails new microbial threats (Kimball, 2006). The recent outbreak and global spread of a new strain of influenza A (H1N1) that originated in
Mexico provides a compelling illustration of how the dynamics of globalization can contribute to the emergence and spread of microbial disease. As of this writing, the investigation into the source of the H1N1 virus is still under way. But the virus, a blend of strains appearing in wild birds, pigs, and now humans, illustrates the type of reassortment enabled by the intensive farming practices developed to meet the demands of global commerce. These industrial-scale farms, with thousands of animals confined in close quarters, offer ripe breeding grounds for new agents (Weuthrich, 2003). And in the event that these agents jump from animals to humans, nearby population centers present the opportunity for these agents to spread. Indeed, within six weeks of the initial detection of the virus in Mexico in February 2009, the virus had spread to more than 2,000 people in 23 countries across the globe (WHO, 2009) via international travel.
Concern over the spread of the virus virtually shut down Mexico City and led to flight cancellations, school closures, and airport screenings around the globe (Carroll and Branigan, 2009).
Fortunately, the recent H1N1 strain has proven thus far to be relatively mild and the outbreak modest in scale. But it is just one of a growing number of cases that demonstrate how the pressures and incentives of global trade and travel can threaten the biosecurity of the global population. The following discussion will focus on other recent examples that highlight some of the areas of greatest concern, specifically food production, processing, and distribution; the use of anti-microbials in food animals; and xenotransplantation. After examining the risks, this discussion will explore potential solutions. In short, there’s an urgent need to employ a multisector, global approach to enhance the safety web to meet the threats global trade poses for the emergence and spread of microbial diseases.
Cross-Border Trade and the Spread of Infections
In 2008, total global merchandise trade was valued at more than $15.8 trillion (WTO, 2009). Although the annual rate of growth in global trade dropped from 8 percent in 2006 to 6 percent in 2007 and continued its decline in 2008, the international exchange of goods and services has continued to increase, albeit at a slower rate. The following case studies examine the risks that emerge in a world in which people and products are continually crossing borders.
Far-Flung Distribution
The 2006 multistate outbreak of Escherichia coli O157:H7 across the United States (Grant et al., 2008) linked to spinach grown in California aptly demonstrates how cross-border trade can expand the scope and complexity of outbreaks. Between August 5 and September 5, a total of 84 cases were detected in 20 states. Only one of the 84 cases was in California; the other 83 were spread across the country, from Oregon to Wisconsin to New York to Tennessee. Conse-
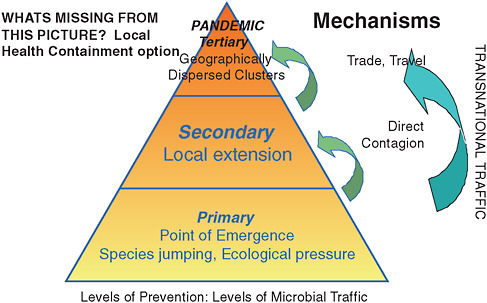
FIGURE 2-9 Trade and travel are key to global dissemination of disease.
quently, there was no ability at the local level to detect the outbreak or identify its source. It was not until investigators conducted the molecular epidemiology across affected states that the cases could be linked and the source identified.
Trade across borders (state or national) creates a new, very direct dissemination of infection and a new challenge for public health, both at the local and the global levels. As Figure 2-9 illustrates, trade and travel are the mechanisms by which local outbreaks become pandemics. And as the blue arrow indicates, it is the growth of transnational trade and travel that enhances the risk of the transnational spread of disease. The E. coli outbreak described above shows that, when agents enter the cross-border trade flow, local public health authorities at the source may not, in fact, be in a position to perceive and address disease clusters.
The Perfect Storm: H5N1
The influences of the global economy, in particular increased urbanization and intensified agricultural processes, have contributed to the brewing threat of an avian influenza pandemic. These dynamics are particularly evident in areas of Southeast Asia, where poor families are moving, along with their animals, from rural areas to crowded periurban settings in pursuit of economic opportunity. The families maintain their food animals in backyard farms with poor sanitation and water supply— an opportune blend for the emergence of bird flu. Further compounding the risk
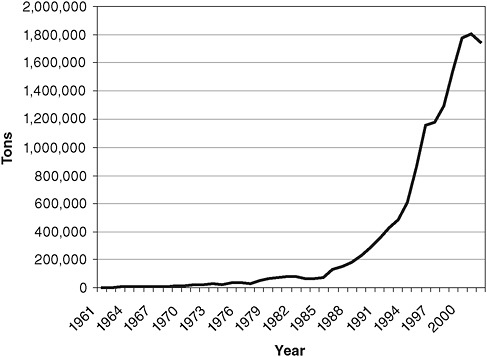
FIGURE 2-10 Poultry exports from Far East Asian countries from 1961 to 2002.
SOURCE: Based on data in FAOSTAT 2003 and reprinted from Kimball (2006) with permission from Ashgate Publishing.
of spread is the nearby introduction of intensive poultry agriculture operations that raise thousands of birds from hatching through slaughter. Between 1980 and 2000, poultry exports from Far East Asian countries leapt from less than 200,000 tons of exports to nearly 1,800,000 tons (Figure 2-10; Kimball, 2006). These burgeoning poultry operations generate a variety of potential biosecurity risks such as crowded coops with large volumes of waste—risks that they do not always have the resources or incentive to mitigate. Added to the mix are free-ranging ducks that serve as asymptomatic reservoirs for the H5NI virus.
Ultimately, these communities crowded with people and poultry are approaching an ecological tipping point that could someday result in an influenza A (H5N1) pandemic among humans. There are, in fact, two distinct opinions on this issue: (1) large industrial operations provide enhanced biosecurity and backyard poultry should be eliminated to reduce risk, and (2) the large-scale operations are themselves risky. While definitive studies are lacking, a careful review of the Thai experience suggests that the second opinion is more aligned with the evidence (Graham et al., 2008). Already, H5N1 outbreaks have occurred in poultry throughout Asia. As of September 2009, 442 cases of human H5N1 and 262 deaths had been
documented in 15 countries, including China, Hong Kong, Thailand, Vietnam, Cambodia, and Indonesia (WHO Global Alert and Response, 2009). To date, the H5N1 virus has had limited ability to move from poultry to humans and even less ability to move from person to person. The rapid global spread of the recent H1N1 virus linked to pigs provides sobering evidence of the pandemic potential once an agent that originates in animals develops the capacity to spread among humans.
Bovine Spongiform Encephalopathy and New-Variant Creutzfeldt-Jakob Disease
The emergence of BSE and new-variant Creutzfeldt-Jakob disease (nvCJD) provides another example of the potential consequences of the pressures that result from global trade. In this case, the chain of events arguably began when the United Kingdom entered the World Trade Organization (WTO) in 1993 (Gibbs and Shaw, 1996). As a condition of joining the WTO, the United Kingdom lifted some 13 percent of the tariffs charged for beef imports, thus opening their beef market to greater global competition. This in turn resulted in a consolidation of the previously fragmented beef industry. As part of that transition, beef producers changed their rendering practice and, to some extent, their animal husbandry practices.8 Specifically, they changed their rendering process from a so-called batch procedure to a lower-temperature vacuum-extraction process. This process cost less and enabled continuous production, but unfortunately it had a hidden downside as well—it did not deactivate the prion, a new pathogenic agent with a 10-year incubation period. This long incubation period enabled extensive circulation before any problems were detected. During this period, meat and bone meal that had been rendered in Great Britain from cattle infected with BSE were shipped around the world in animal feed.
In the mid-1990s, a new series of cases of vCJD began to appear in the United Kingdom. The frightening disease, which claims victims’ lives in 18 months and has no known treatment, was linked through case-control studies to consumption of beef from the United Kingdom. While scientists continue to debate the causal link between prion disease in cattle and prion disease in humans, the global markets reacted swiftly to the possibility. UK beef exports plummeted in 1996 (Figure 2-11) after trading partners began embargoing beef shipments under the WTO’s Sanitary and Phytosanitary (SPS) agreement. The SPS agreement allows importers to issue “urgent notifications” that they will suspend import of a particular product when human safety is at risk (WTO, 2000). Thus, in the world of global food trade, where safety concerns can amount to economic devastation, incentives run against reporting potential risks.
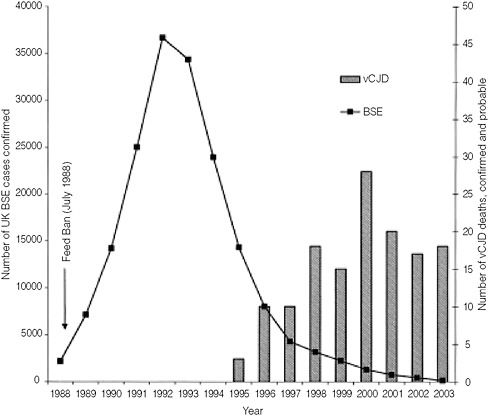
FIGURE 2-11 Superimposed epidemics. Long incubation period of up to 10 years allowed extensive circulation of meat and bone meal-infected product in the global market prior to identification of risk.
SOURCE: Reprinted from Beisel and Morens (2004).
Antimicrobials in Farm Animals
Global economic pressures have contributed to other risky practices in animal husbandry, including the use of antibiotics as growth promoters, which fosters the development of antibiotic-resistant pathogens in both animals and humans. It is estimated that, in the United States alone, some 12 to 70 million kg of antibiotics are administered annually in this way (Aarestrup and Pires, 2008). Evidence suggests that the use of antibiotics in animal feed may be even higher in developing economies that carry heavier disease burdens. In some parts of Asia, integrated fish farming facilities feed fish farm-animal waste that is laced with antibiotics to suppress the level of microbes. Humans in turn consume the fish, along with the antibiotics, potentially perpetuating the spread of antibiotic-resistant pathogens
The European Union has banned the use of antibiotics as growth promoters in food animals (Silbergeld et al., 2008). A similar global ban prohibiting the application of antibiotics for farm animals outside of therapeutic use would be well advised.
The use of antimicrobials as growth hormones and in feed is another illustration that underscores the fact that incentives in global trade often are misaligned with the interests of biosecurity. Add to that the far-reaching and rapid distribution system for food products and the result is a pandemic risk that cannot be ignored.
Beyond Food: Xenotransplantation
Another growing facet of global trade in services that poses a significant microbial risk is xenotransplantation, or the transplantation of animal parts into humans. Implanting animal organs or valves in humans introduces yet another level of cross-species exposure to pathogens. A variety of global pressures are driving this trade, including increases in diabetes and other chronic conditions detrimental to organ health, a lack of effective therapies for degenerative conditions, and, in countries such as the United States, an upcoming surge of baby boomers entering old age. As a result, the number of people seeking organs far exceeds the number of organs available for transplant and in many countries, people seeking transplants face long waiting lists.
Some players in the global marketplace have responded to the growing demand for organs and alternative therapies by venturing into experimental areas such as xenotransplantation and stem cell therapies that are strictly regulated or even prohibited in other countries. Stem cell procedures and “precursor” stem cell procedures are promoted, often via the Internet, on the global medical marketplace. They include various donor species, such as rabbits and pigs.9 The majority of these procedures are offered without systematic protocols, primate studies, or regulation.
In the United States, the transplantation of tissues from other mammalian species is highly regulated. For instance, although the pig has been extensively explored as a potentially promising donor source, the United States has prevented the licensure of pig organs (with the exception of heart valves) because pigs carry in their genetic material endogenous retrovirus known as “PERV” (Porcine Endogenous Retrovirus), which potentially could infect its human host post-transplantation. In light of such concerns, the World Health Assembly in 2004 passed a resolution on xenogenic transplantation that urges member states to establish regulation and surveillance mechanisms and to collaborate on global strategies to prevent infections (Resolution 57-18 2004, section II). Although the United States was a key promoter of this resolution, it did not invest in World Health Organization (WHO) programming to support the initiative. Consequently,
|
9 |
For example, see http://www.bcro-stemcells.com. |
xenotransplantation regulatory progress has lagged on a global level. The WHO collaborating center at Geneva University attempts to keep a dataset on xenotransplantation therapies;10 however, the information is not exhaustive.
Global Problem: Global Solutions
The foregoing cases have demonstrated how our increasingly global economy, with growing international travel and trade (including trade in services such as transplantation), has ultimately made virtually any emerging microbial risk global in nature. In the examples of foodborne E. coli and BSE, we see the globalization of direct infectious risk. In the instance of the overuse of antimicrobials in food animals, we see the globalization of antimicrobial resistance. With medical travel for organ transplants, we see traveling patients become potential vectors for the spread of disease.
While microbial risks have been globalized along with commerce, the corresponding health and protective measures for the most part have not. The second edition of the IHR (2005), which took effect in 2007, provides some important safeguards to help limit the international spread of infectious disease. The IHR require countries to conduct surveillance for and report to the WHO a “public health emergency of international concern,” that is, an event “that may cause international disease spread.” If WHO determines such a threat exists, as it did with the recent H1N1 outbreak, it may issue recommendations to curb the spread of disease, such as quarantine or travel restrictions for affected or potentially affected individuals. As the experience with H1N1 demonstrated, WHO must carefully balance the threat of disease spread with the potential economic consequences of any travel or trade restrictions in order to minimize disincentives for countries to report potential threats. While WHO Director-General Dr. Margaret Chan raised the “Pandemic Alert” level to 6 (the highest), WHO actively discouraged trade and travel restrictions after determining that they would not be effective in curbing the spread of the influenza virus and could needlessly result in significant economic repercussions. Instead, WHO focused on identifying and treating individuals with infection and urged those individuals with illness or symptoms to avoid travel and contact with others. This did not stop some countries from instituting their own travel restrictions. Several nations banned flights to Mexico, and China quarantined more than 70 travelers from Mexico (Browne, 2009). Despite the moderated response, Mexican authorities estimated $2.2 billion losses to the nation’s economy as a result of the outbreak, including more than a 40 percent drop in tourism revenue (Llana, 2009).
The revised IHR represent an important step toward a more coherent global response to microbial threats. However, the full implementation of the core capacities for public health competency will not be in place until 2011, and resources will
be needed to achieve that benchmark. Another promising initiative has been the efforts of the Asia Pacific Economic Cooperation (APEC) to create a community of interest in health across the 21 APEC economies, including a communications platform to enable members to collaborate in the event of public health emergencies. The APEC effort actively seeks to bring in the vibrant private sector through collaborative projects in biopreparedness led by its Health Working Group. The full engagement of the private sector as a stakeholder would bring a key “driver” of global trade into the discussion and implementation of public health protection.
In conclusion, there are some key questions raised by these case studies for which critical information is missing: (1) What exactly is the nature of emergent influenza risk of industrialized poultry and swine practices in poor and medium-income settings and can these be mitigated? and (2) What surveillance of trade product and practice would be useful to inform global public health? Moreover, at a global level, the IHR process requires support to ensure capacity building to enhance global access to surveillance, laboratory, and epidemiological investigation capabilities; and community building to improve cross-border communications and collaboration.
Our discussion of xenotransplantation highlights some of the risks entailed in the growing practice of medical tourism, or the movement of patients across international borders, issues that we explore in greater depth elsewhere (Hodges and Kimball, in preparation).
Finally, as we move into the pandemic phase of H1N1 and the Northern Hemisphere moves into its next influenza season, it is timely to reflect seriously on the risk our ever-globalizing trade, travel, and food production poses to population health and biosecurity.
GLOBALIZATION OF THE FOOD SUPPLY: TIME FOR CHANGE IN APPROACH
David W. K. Acheson, M.D., F.R.C.P.11
Food and Drug Administration
The globalization of the food supply is causing changes in regulatory thinking at the FDA and moving us toward a new approach. In this paper, I share with you some of the challenges our agency currently faces, illustrated with examples of recent episodes of food contamination.
Many of these regulatory challenges result from the fact that American consumers want all kinds of food, and they expect it to be available year-round. Their demand drives global food trade, which is in turn influenced by the relative cheapness of growing and producing food in countries other than the United States, and
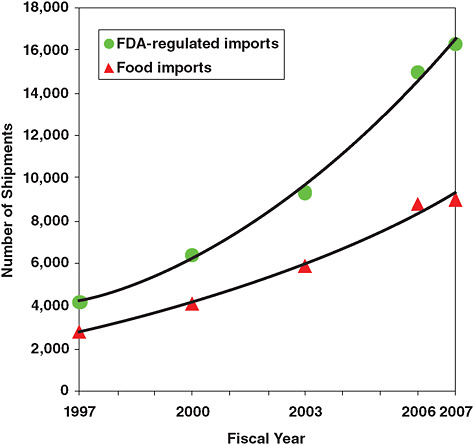
FIGURE 2-12 Food and other FDA-regulated imports to the United States.
SOURCE: Food and Drug Administration Food Protection Plan.
shipping it to its destination. One challenge is that many of those foreign food-producing countries may lack food safety standards on a par with ours.
Approximately 15 percent of all food consumed in the United States is imported. This includes more than 80 percent of aquacultured seafood, and more than 50 percent of fresh produce; other items, such as dairy, are imported in relatively small amounts. Foods imported to the United States come from more than 200,000 foreign registered facilities (each of which is registered with the FDA) in more than 200 countries and territories. Imported food enters the United States through more than 300 ports.
There has been a steady increase in food importation into the United States over the past 10 years (Figure 2-12). The global economic crisis may have an impact on this trend, but it is difficult to predict whether it will cause food imports to increase or decrease. If imports remain substantially cheaper than domestically-produced foods, demand for imports may grow.
Real-World Challenges
The FDA has encountered several issues linked to the globalization of the food supply. The most pervasive is the nondeliberate or accidental contamination of food. For example, in 2003, a massive hepatitis-A outbreak of more than 1,000 cases across multiple states was linked to green onions imported from Mexico. In 2008, an outbreak of Salmonella Saintpaul caused by contaminated hot peppers imported from Mexico resulted in 1,450 documented cases, which probably represents a fraction of the actual number of people who got sick.
We have also dealt with the deliberate contamination of food for the purpose of economic gain. This opened a new arena for regulatory consideration, because, unlike bioterrorism, these deliberate acts are not intended to cause harm. One such case occurred in 2007, when it was discovered that the chemical melamine had been added to wheat gluten by Chinese manufacturers (FDA, 2008a). Because melamine is high in nitrogen, it increased the apparent protein content of the wheat gluten (which is gauged by nitrogen content), and therefore its price. The use of this adulterated wheat gluten in pet foods manufactured in the United States resulted in animal deaths due to kidney failure. Unfortunately, lessons were not learned from this experience, and within a year, the FDA was investigating melamine-contaminated dairy products from China, where some infants allegedly died after ingesting formula containing melamine, and more than 50,000 were reportedly hospitalized in China for urinary problems (FDA, 2007; WHO, 2009).
The following descriptions of the investigations of foodborne Salmonella Saintpaul in 2008 and melamine in pet food in 2007 illustrate the complexities involved in determining the sources of contaminants once they enter the globalized food system.
Salmonella Saintpaul in Peppers
The 1,450 cases confirmed in the 2008 Salmonella Saintpaul outbreak occurred in 43 states and the District of Columbia, and they were linked to multiple food types. The outbreak came to the attention of the FDA via an alert from the Centers for Disease Control and Prevention (CDC) in early May 2008. Within two weeks, tomatoes had emerged as the likely vehicle, so we initiated a traceback to determine the origin of the contamination. This led us to Florida and Mexico, where we began inspecting tomato farms and their supply chains.
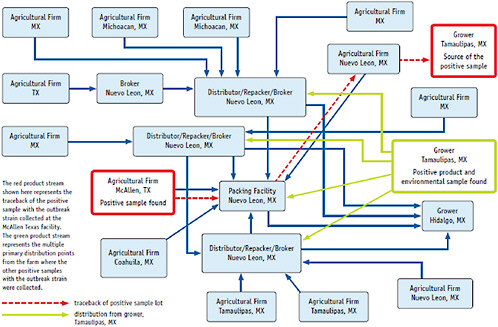
When we approached the Mexican government about this issue, they were very cooperative and met with us daily. The FDA visited several farms in the state of Sinaloa, Mexico, but failed to find tomatoes contaminated with Salmonella, and as the epidemiological investigation continued, other food sources were implicated. In July, we began tracing back peppers, as illustrated in Figure 2-13.
As Figure 2-13 illustrates, this was an extremely complicated process, and it begs the question as to how such episodes of contamination, which are occurring
with increasing frequency, can best be prevented and addressed. The investigation led us to a distributor in McAllen, Texas, where we found positive pepper samples. They had received their jalapeno peppers from a packing facility in Nueva Leone, Mexico, so we returned to an entirely different part of that country to inspect pepper farms. On July 30, we found the outbreak strain, Salmonella Saintpaul, in irrigation water and on remaining peppers on a farm in Tamaulipas, Mexico.
Melamine in Pet Food
This investigation began with reports of sick pets from consumers, and also from a pet food company whose research animals developed kidney failure following routine taste tests (FDA, 2008a). The only associated change in pet food formulation was the source of the wheat gluten it contained.
Once this was recognized, we soon determined that scraps from affected pet food manufacturers were being used to produce food for livestock, which could in turn introduce the adulterant into foods consumed by humans. Thus, melamine was traveling up the food chain, beginning with food ingredient manufacturers, and onward to feed mills, to poultry farms and hog farms, and from there to chicken and pork in supermarkets. A joint risk assessment was conducted between the FDA and the U.S. Department of Agriculture (USDA) to determine if the levels in poultry and pork were of a concern to public health. The assessment concluded the levels were not a public health concern. We also discovered that not only was this wheat gluten coming into the United States, but a U.S. company was also importing it to Canada. We alerted the Canadian authorities, who found that the gluten was being used there to make fish feed that was being shipped back into the United States.
The complete U.S. distribution chain of the melamine-contaminated wheat gluten is shown in Figure 2-14. It makes the point that contaminants—including pathogens—connected to food may be disseminated through vast and complex systems that profoundly affect international trade and economic relationships, as other workshop participants noted.
Time for a New Approach
In light of these complexities, the FDA is attempting to change its approach by becoming more proactive in addressing food safety by addressing the whole supply chain. One important route to this goal is to conduct targeted, risk-based inspections, but these may be difficult to identify. Risk-based inspections are dependent on having adequate data, analytical capabilities, and inspectors. Currently such inspections are conducted at ports of entry, where it is decided whether a given product made in a foreign country and shipped to the United States will be inspected or tested for chemical or microbiological contamination, based on the various factors such as the nature of the product, its origin and
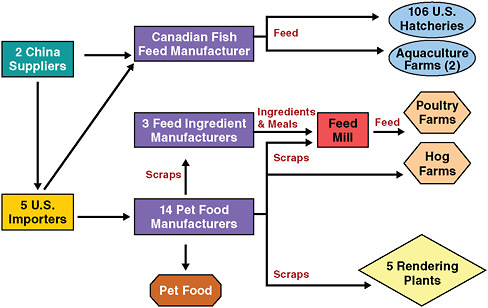
FIGURE 2-14 Sequence of events—different routes of melamine-contaminated wheat gluten into the United States.
SOURCE: Food and Drug Administration.
destination, and the past inspection history of similar products. This procedure, euphemistically called a “snapshot,” examines only one point in the distribution chain: importation. In the future, our goal is to gain greater assurance for the complete supply chain—from grower to manufacturer to shipper to importer to distributor to retailer—of imported food products. This is going to require a multifaceted approach that includes more inspections.
There are significant challenges and scarce resources for pursuing inspections of overseas growers and manufacturers, among them the sheer numbers (approximately 200,000, as previously noted) of registered foreign food facilities. There is no way, despite some peoples’ wishes, that we could inspect and sample every import at the port of entry. Rather, we must try to optimize inspection based on risk. We cannot simply test or inspect our way to safe food and having appropriate preventive controls throughout is a key step.
Risk-Based Inspections
To pursue a risk-based approach to food safety, we need leverage with foreign governments and with industry in order to gather information on the status of foreign growers, manufacturers, and foreign governments. Using these data, we must conduct effective analyses to inform risk-based decisions at the port of
entry. Finally, rather than determining whether to examine food products on a case-by-case basis, we need to build a decision-making system.
We are currently using a prototype of such a system, which we call PREDICT, to maximize the use of FDA data in making risk-based decisions. PREDICT software allows the FDA to make risk-based decisions for import inspections and has the capacity to receive new datasets as other data streams come online. A pilot project, conducted in the Port of Los Angeles, employed PREDICT to make inspection decisions regarding seafood, for which clear inspection standards had already been established. In this trial, PREDICT prompted inspectors to detect contaminated foods more frequently than did the existing inspection system (known as OASIS). The FDA is currently attempting to expand PREDICT, recognizing that this system is only as good as the data provided to it.
Increased Foreign Presence
Another way the FDA is supporting a more proactive stance on addressing threats to the globalized food supply is by establishing a greater foreign presence. To that end, we recently opened three offices in China—in Beijing, Shanghai, and Guangzhou—staffed by about a dozen permanent FDA employees. Although their staffs are too small to conduct significant numbers of inspections, these foreign offices will be able to strengthen relationships with the Chinese government that will improve our ability to exchange information and deal with contamination issues as they arise, and potentially before they become problems for the United States. In order to situate FDA personnel throughout the world, we are opening similar outreach offices in New Delhi and Mumbai, India; in central South America; and in Europe.
Increased Inspections
Currently the FDA performs between 100 and 155 inspections of foreign food manufacturers per year. We plan to perform 1,000 such inspections per year by 2011. While we recognize that we cannot inspect our way to safe food, we think that targeted inspections associated with the most potentially risky products are an important move forward.
Use of Third-Party Data
The food industry extensively inspects and tests foods that are imported into the United States. Can the FDA make use of this information? Recognizing that this is a potentially contentious issue (see below), our goal is to examine the process surrounding third-party certifications and determine how the FDA can use that information to better protect the public and make maximal use of resources. One aspect of this is to use standards for third parties that provide information
supplied to the FDA, whether it is derived from state agencies, foreign governments, or private third parties. We then want to be able to use that information to inform the risk-based inspection process. Currently, the PREDICT model at ports of entry operates primarily on U.S. compliance data. Incorporating inspection data and information from overseas and from industry into our analyses should improve our ability to make risk-based decisions.
Ensuring Confidence
Any risk-based decision-making system that employs third-party data must be completely transparent, and it must be clear that providing data to the FDA will not enable industry or importers to bypass inspections at the port of entry.
In order to increase confidence in such a system by all stakeholders—which include companies, consumers, regulators, and Congress—the FDA issued a guidance document in January 200912 describing attributes that a third-party certification program should have in order for the FDA to have confidence in the quality of the audit conducted by the program. It also provides information on the certification process, including guidance on application, certification, recertification, and withdrawal of certification. In order to determine how such a third-party certification program might operate, the FDA has established a pilot program focused on aquacultured shrimp. We issued a Federal Register Notice13 asking for volunteers among companies that import shrimp into the United States to submit an application to this certification program. By processing these applications, we hope to determine infrastructure needs for handling these kinds of data, find out whether importers can meet our data standards, and establish processes for evaluating third-party certification programs.
This pilot program does not guarantee entry into the U.S. market by participating shrimp importers. We are not using the data they provide to make importation decisions; rather, this pilot study should identify strengths and weaknesses in our developing third-party certification program and help us learn how to make such a program transparent and credible.
Conclusion
The regulatory challenges involved in providing safe food to the United States will increase as globalization of the food system continues. The complexities of the food supply are enormous, and there is considerable economic benefit in making food distribution as rapid and efficient as possible. It is therefore
|
12 |
Guidance for Industry Voluntary Third Party Certification Programs for Foods and Feeds. See http://www.fda.gov/oc/guidance/thirdpartycert.html. |
|
13 |
See http://www.regulations.gov/fdmspublic/component/main?main=DocumentDetail&o=090000648066388c; Docket No.: FDA-2008-N-0382. |
critical that we abandon the practice of focusing our efforts on ports of entry and instead embrace a new approach focused on the whole supply chain that attempts to understand foodborne threats in foreign countries, anticipates their potential to spread globally, and uses risk-based inspections to detect them before an outbreak occurs in the United States.
WILSON REFERENCES
AbuBakar, S., I.-C. Sam, P.-F. Wong, N. MatRahim, P.-S. Hooi, and N. Roslan. 2007. Reemergence of endemic chikungunya, Malaysia. Emerging Infectious Diseases 13(1):147-149.
Bradley, D. J. 1989. The scope of travel medicine. In Travel medicine: proceedings of the First Conference on International Travel Medicine, edited by R. Steffen. Berlin: Springer Verlag.
Brouard, C., P. Bernillon, I. Quatresous, J. Pillonel, A. Assal, H. DeValk, and J. C. Desenclos. 2008. Estimated risk of chikungunya viremic blood donation during an epidemic on Reunion Island in the Indian Ocean, 2005 to 2006. Transfusion 48(7):1333-1341.
CDC (Centers for Disease Control and Prevention). 2005. Transmission of malaria in resort areas—Dominican Republic, 2004. Morbidity and Mortality Weekly Report 53(51):1195-1198.
———. 2009. Trends in tuberculosis—United States, 2008. Morbidity and Mortality Weekly Report 58(10):249-253.
Charrel, R. N., X. de Lamballiere, and D. Raoult. 2007. Chikungunya outbreaks—the globalization of vector-borne diseases. New England Journal of Medicine 356(8):769-771.
———. 2008. Seasonality of mosquitoes and chikungunya in Italy. Lancet Infectious Diseases 8(1):5-6.
Chen, L. H., and M. E. Wilson. 2004. Transmission of dengue virus without a mosquito vector: nosocomial mucocutaneous transmission and other routes of dengue transmission. Clinical Infectious Diseases 39(6):e56-e60.
———. 2005. Nosocomial dengue by mucocutaneous transmission. Emerging Infectious Diseases 11(5):775.
Chuang, V. W, T. Y. Wong, Y. H. Leung, E. S. K. Ma, Y. L. Law, O. T. Y. Tsang, K. M. Chan, I. H. L. Tsang, T. L. Que, R. W. H. Yung, and S. H. Liu. 2008. Review of dengue fever cases in Hong Kong during 1998 to 2005. Hong Kong Medical Journal 14(3):170-177.
Cliff, A., and P. Haggett. 2004. Time, travel and infection. British Medical Bulletin 69:87-99.
Colizza, V., A. Barrat, M. Berthelemy, and A. Vespignani. 2006. The role of the airline transportation network in the prediction and predictability of global epidemics. Proceedings of the National Academy of Sciences 103(7):2015-2020.
Crosby, A. W. 1972. The Columbian exchange: biological and cultural consequences of 1492. Westport, CT: Greenwood Press.
Fraser, C., S. Riley, R. M. Anderson, and N. M. Ferguson. 2004. Factors that make an infectious disease outbreak controllable. Proceedings of the National Academy of Sciences 101(16):6146-6151.
Fraser, C., C. A. Donnelly, S. Cauchemez, W. P. Hanage, M. D. Van Kerkhove, T. D. Hollingsworth, J. Griffin, R. F. Baggaley, H. E. Jenkins, E. J. Lhyons, T. Hombart, W. R. Hensley, N. C. Grassly, F. Balloux, A. C. Ghani, and N. M. Ferguson. 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324(5934):1557-1561.
Freedman, D. O., P. Kozarskym, and E. Schwartz. 2002 (April 26). Thailand: out of season dengue outbreak in travellers to Koh Phangan. ProMed.
Freedman, D. O., L. H. Weld, P. E. Kozarsky, T. Fisk, R. Robins, F. von Sonnenburg, J. S. Keystone, P. Pandey, and M. S. Cetron. 2006. Spectrum of disease and relation to place of exposure among ill returned travelers. New England Journal of Medicine 354(2):119-130.
González, M. C., C. A. Hidalgo, and A.-L. Barabási. 2008. Understanding individual human mobility patterns. Nature 453(7196):779-782.
Grubler, A., and N. Nakicenovic. 1991. Evolution of transport systems. Laxenburg, Vienna: ILASA.
Guernier, V., M. E. Hockberg, and J. E. Guegan. 2004. Ecology drives the worldwide distribution of human diseases. Public Library of Science Biology 2(6):740-746.
Hochedez, P., S. Jaureguiberry, M. Debruyne, P. Bossi, P. Hausfater, G. Brucker, F. Bricaire, and E. Caumes. 2006. Chikungunya infections in travelers. Emerging Infectious Diseases 12(10):1565-1567.
Hufnagel, L., D. Brockmann, and T. Geisel. 2004. Forecast and control of epidemics in a globalized world. Proceedings of the National Academy of Sciences 101(42):15124-15129.
IOM (Institute of Medicine). 2003. Microbial threats to health: emergence, detection, and response. Washington, DC: The National Academies Press.
———. 2008. Global climate change and extreme weather events: understanding the contributions to infectious disease emergence. Washington, DC: The National Academies Press.
Jassal, M., and W. R. Bishai. 2009. Extensively drug-resistant tuberculosis. Lancet Infectious Diseases 9(1):19-30.
Jones, K. E., N. G. Patel, M. A. Levy, A. Storeygard, D. Balk, J. L. Gittleman, and P. Daszak. 2008. Global trends in emerging infectious diseases. Nature 451(7181):990-994.
Josseran, L., C. Paquet, A. Zahgnoun, N. Callere, A. Le Tertre, J.-L. Solet, and M. Ledrans. 2006. Chikungunya disease outbreak, Reunion Island. Emerging Infectious Diseases 12(12):1994-1995.
Leder, K., M. E. Wilson, D. O. Freedman, and J. A. Torresi. 2008. A comparative analysis of methodological approaches used for estimating risk in travel medicine. Journal of Travel Medicine 15(4):263-272.
Leshem, E., Y. Maor, E. Meltzer, M. Assous, and E. Schwartz. 2008. Acute schistosomiasis outbreak: clinical features and economic impact. Clinical Infectious Diseases 47(12):1499-1506.
Linnen, J. M., E. Vinelli, E. C. Sabino, L. H. Tobler, C. Hyland, T.-H. Lee, D. P. Kolk, A. S. Broulik, C. S. Collins, R. S. Lanciotti, and M. P. Butsch. 2008. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion 48(7):1355-1362.
Lowen, A. C., J. Steel, S. Mubareka, and P. Palese. 2008. High temperature (30oC) blocks aerosol but not contact transmission of influenza virus. Journal of Virology 82(11):5650-5652.
Lumsden, W. H. 1955. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. II. General description and epidemiology. Transactions of the Royal Society of Tropical Medicine and Hygiene 49(1):33-57.
Mavalankar, D., P. Shastri, T. Bandyopadhyay, J. Parmar, and K. V. Ramani. 2008. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerging Infectious Diseases 14(3):412-415.
Mohammed, H., J. M. Linnen, J. O. Muñoz-Jordán, K. Tomashek, G. Foster, A. S. Broulik, L. Peterson, and S. L. Stramer. 2008. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion 48(7):1348-1354.
Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. New England Journal of Medicine 360(25):2605-2615.
Oeltmann, J. E., J. K. Varma, L. Ortega, Y. Liu, T. O’Rourke, M. Cano, T. Harrington, S. Toney, W. Jones, S. Aaruchit, L. Diem, D. Riethong, J. W. Tappero, K. Ijaz, and S. A. Maloney. 2008. Multidrug-resistant tuberculosis outbreak among U.S.-bound Hmong refugees, Thailand, 2005. Emerging Infectious Diseases 14(11):1715-1721.
Osterholm, M. 2005. Preparing for the next pandemic. New England Journal of Medicine 352(18):1839-1842.
Panning, M., K. Grywna, M. van Esbroeck, P. Emmerich, and C. Drosten. 2007. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerging Infectious Diseases 14(3):416-422.
Parola, P., X. deLamballerie, J. Journda, C. Rovery, V. Vaillant, P. Minodier, P. Brouqui, A. Flahault, D. Raoult, and R. N. Charrel. 2006. Novel chikungunya virus variant in travellers returning from Indian Ocean islands. Emerging Infectious Diseases 12(10):1493-1499.
Perrin, L., L. Kaiser, and S. Yerly. 2003. Travel and the spread of HIV-1 genetic variants. Lancet Infectious Diseases 3(1):22-27.
Pew Commission on Industrial Farm Animal Prodcution. 2008. Putting meat on the table: industrial farm animal production in America. Washington, DC: Pew Research Center.
Reed, C. R. 2008. Medical tourism. Medical Clinics of North America 92(6):1433-1446.
Reiskind, M. H., K. Pesko, C. J. Westbrook, and C. N. Mores. 2008. Susceptibility of Florida mosquitoes to infection with chikungunya virus. American Journal of Tropical Medicine and Hygiene 78(3):422-425.
Reiter, P., D. Fontenille, and C. Paupy. 2006. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infectious Diseases 6(8):463-464.
Renault, P., J. L. Solet, D. Sissoko, E. Balleydier, S. Larrieu, L. Filleul, C. Lassalle, J. Thiria, E. Rachou, H. de Valk, D. Ilef, M. Ledrans, L. Quatresous, P. Quenel, and P. Pierre. 2007. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005-2006. Public Library of Science Biology 77(4):727-731.
Rezza, G., L. Nicoletti, R. Angelini, R. Romi, A. C. Finarelli, M. Panning, P. Cordioli, C. Fortuna, S. Boros, F. Magurano, G. Silvi, P. Angelini, M. Dotori, M. G. Ciufolini, G. C. Majori, and A. Cassone. 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370(9602):1840-1846.
Russell, C. A., T. C. Jones, I. G. Barr, N. J. Cox, R. J. Garten, V. Gregory, I. D. Gust, A. W. Hampson, A. J. Hay, A. C. Hurt, J. C. de Jong, A. Kelso, A. I. Klimov, T. Kageyama, N. Komadina, A. S. Lapedes, Y. P. Lin, A. Mosterin, M. Obuchi, T. Odagiri, A. D. M. E. Osterhaus, G. F. Rimmelzwann, M. W. Shaw, E. Skepner, K. Stohr, M. Tashiro, R. A. M. Fouchier, and D. J. Smith. 2008. The global circulation of seasonal influenza A (H3N2) viruses. Science 320(5874):340-346.
Schuffenecker, I., I. Iteman, A. Michault, S. Murri, L. Frangeul, M.-C. Vancy, R. Lavenir, N. Pardigon, M.-M. Reynes, F. Pettinelli, L. Biscornet, L. Diancourt, S. Michel, S. Duquerroy, G. Guigon, P.-P. Frenkiel, A.-C. Brehin, N. Cubito, P. Despres, F. Junst, F. A. Rey, H. Zeller, and S. Srisse. 2006. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. Public Library of Science Medicine 3(7):1058-1071.
Schwartz, E., L. H. Weld, A. Wilder-Smith, F. von Sonnenburg, J. S. Keystone, K. C. Kain, J. Torresi, and D. O. Freedman. 2008. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997-2006. Emerging Infectious Diseases 14(7):1081-1088.
Sergon, K., C. Njugena, R. Kalani, V. Ofula, C. Onyango, L. A. Konongoi, S. Bedno, H. Burke, A. M. Dumilla, J. Konde, M. K. Njenga, R. Sang, and R. F. Breiman. 2008. Seroprevalence of chikungunya virus (CHIKV) infection on Lamu Island, Kenya, October 2004. American Journal of Tropical Medicine and Hygiene 78(2):333-337.
Shaman, J., and M. Kohn. 2009. Absolute humidity modulates influenza survival, transmission, and seasonality. Proceedings of the National Academy of Sciences 106(9):3243-3248.
Simon, F., H. Savini, and P. Parola. 2008. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Medical Clinics of North America 92(6):1323-1343.
Smith, G. J. D., D Vijakrishna, J. Bahl, S. J. Lycett, M. Worobey, O. G. Pybus, S. K. Ma, C. L. Cheung, J. Raghwani, S. Bhatt S, J. S. M. Peiris, Y. Guan, and A. Rambaut. 2009a. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459(7250):1122-1125.
Smith, R. D., R. Chanda, and V. Tangcharoensathien. 2009b. Trade in health-related services. Lancet 373(9663):593-601.
Tambayh, P. A., E. S. C. Koay, M. L. M. Poon, R. V. T. P. Lin, and B. K. C. Ong. 2008. Dengue hemorrhagic fever transmitted by blood transfusion. New England Journal of Medicine 359(14):1526-1527.
Tatem, A. J., S. I. Hay, and D. J. Rogers. 2006. Global traffic and disease vector dispersal. Proceedings of the National Academy of Sciences 103(16):6242-6247.
Tesh, R. B., D. J. Gubler, and L. Rosen. 1976. Variation among geographic stains of Aedes albopictus in susceptibility to infection with chikungunya virus. American Journal of Tropical Medicine and Hygiene 25(2):326-335.
Tsetsarkin, K. A., D. L. Vanlandingham, C. E. McGee, and S. A. Higgs. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. Public Library of Science Pathogens 3(12):1895-1906.
UNWTO (United Nations World Tourism Organization). 2008a. Tourism highlights, 2008 edition, http://www.unwto.org/facts/eng/highlights.htm (accessed July 22, 2009).
———. 2008b. Tourism indicators, http://www.world-tourism.org/facts/menu.html (accessed February 8, 2008).
U.S. Census Bureau. 2008. International data base, http://www.census.gov/ipc/www/idb (accessed March 1, 2008).
Wang, L., Y. Wang, S. Jin, Z. Wu, D. P. Chin, J. P. Koplan, and M. E. Wilson. 2008. Emergence and control of infectious diseases in China. Lancet 372(9649):1598-1605.
Wilcox, B. A., D. J. Gubler, and H. F. Pizer. 2008. Urbanization and the social ecology of emerging infectious diseases. In Social ecology of infectious diseases, edited by K. Mayer and H. F. Pizer. London: Elsevier, Academic Press. Pp. 113-137.
Wilder-Smith, A., and D. J. Gubler. 2008. Geographic expansion of dengue: the impact of international travel. Medical Clinics of North America 92(6):1377-1390.
Wilder-Smith, A., L. Chen, E. Massad, and M. E. Wilson. 2009. Threat of dengue to blood safety in dengue-endemic countries. Emerging Infectious Diseases 15(1):8-11.
Wilson, M. E. 1995a. Infectious diseases: an ecological perspective. British Medical Journal 311(7021):1681-1684.
———. 1995b. Travel and the emergence of infectious diseases. Emerging Infectious Diseases 1(2):39-46.
———. 2003. The traveller and emerging infections: sentinel, courier, transmitter. Journal of Applied Microbiology 94(Suppl):1S-11S.
———. 2009. Chikungunya continues to expand: what lies ahead? Infectious Diseases in Clinical Practice 17(1):1-2.
Wilson, M. E., and L. H. Chen. 2008. Travel. In Social Ecology of Infectious Diseases, edited by K. Mayer and H. F. Pizer. London, UK: Elsevier.
Zanotto, P. M., E. A. Gould, D. F. Gao, P. H. Harvey, and E. D. Holmes. 1996. Population dynamics of flaviviruses revealed by molecular phylogenies. Proceedings of the National Academy of Sciences 93(2):548-553.
LEVY REFERENCES
Betsi, N. A., B. G. Koudou, G. Cissé, A. B. Tschannen, A. M. Pignol, Y. Ouattara, Z. Madougou, M. Tanner, and J. Utzinger. 2006. Effects of an armed conflict on human resources and health systems in Côte d’Ivoire: Prevention of and care for people with HIV/AIDS. AIDS Care 18(4):356-365.
CDC (Centers for Disease Control and Prevention). 2005. HIV/AIDS surveillance report, 2004. Vol. 16. Atlanta, GA: CDC. Tables 1 and 7.
Coghlan, B., V. N. Bemo, P. Ngoy, T. Stewart, F. Mulumba, J. Lewis, C. Hardy, and R. Brennan. 2007. Mortality in the Democratic Republic of Congo: an ongoing crisis. New York: International Rescue Committee, and Melbourne, Australia: Burnet Institute.
Culbert, H., D. Tu, D. P. O’Brien, T. Ellman, C. Mills, N. Ford, T. Amisi, K. Chan, S. Venis, and Médecins Sans Frontière. 2007. HIV treatment in a conflict setting: outcomes and experiences from Bukavu, Democratic Republic of the Congo. Public Library of Science Medicine 4(5):794-798.
Drobniewski, F. A., and N. Q. Verlander. 2000. Tuberculosis and the role of war in the modern era. International Journal of Tuberculosis and Lung Disease 4(12):1120-1125.
Gisselquist, D. 2004. Impact of long-term civil disorders and wars on the trajectory of HIV epidemics in sub-Saharan Africa. Journal of Social Aspects of HIV/AIDS 1(2):114-127.
Gustafson, P., V. F. Gomes, C. S. Vieira, H. Jensen, R. Seng, R. Norberg, B. Samb, A. Nauclér, and P. Aaby. 2001. Tuberculosis mortality during a civil war in Guinea-Bissau. Journal of the American Medical Association 286(5):599-603.
IOM (Institute of Medicine). 1988. The future of public health. Washington, DC: National Academy Press.
Kahn, I. M., and U. Laaser. 2002. Burden of tuberculosis in Afghanistan: update on a war-stricken country. Croatian Medical Journal 43(2):245-247.
Klevens, R. M., J. R. Edwards, C. L. Richard, Jr., T. C. Horan, R. P. Gaynes, D. A. Pollock, and D. M. Cardo. 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Reports 122:160-166.
Levy, B. S., and V. W. Sidel, eds. 2008. War and public health (second edition). New York: Oxford University Press.
Martins, N., E. Heldal, J. Sarmento, R. M. Araujo, E. B. Rolandsen, and P. M. Kelly. 2006. Tuberculosis control in conflict-affected East Timor, 1996-2004. International Journal of Tuberculosis and Lung Disease 10(9):975-981.
Metcalf, N. 2002. A short history of biological warfare. Medicine, Conflict and Survival 18:271-282.
National Priorities Project. 2008. National Priorities Project, www.nationalpriorities.org (accessed December 15, 2008).
Page, E. H., K. F. Martinez, T. A. Seitz, B. P. Bernard, A. L. Tepper, R. S. Weyant, C. P. Quinn, N. E. Rosenstein, B. A. Perkins, T. Popovic, H. T. Holmes, and C. W. Shepard. 2002. Public health dispatch: update: cutaneous anthrax in a laboratory worker—Texas, 2002. Morbidity and Mortality Weekly Report 51(22):482.
U.S. National Counterterrorism Center. 2008. 2007 Report on terrorism, http://wits.nctc.gov/reports/crot2007nctcannexfinal.pdf (accessed January 5, 2009).
Van Herp, M., V. Parqué, E. Rackley, and N. Ford. 2003. Mortality, violence and lack of access to health-care in the Democratic Republic of Congo. Disasters 27(2):141-153.
Wasley, A., S. Grytdal, and K. Gallagher. 2008. Surveillance for acute viral hepatitis—United States, 2006. Morbidity and Mortality Weekly Report 57(SS22):1-24.
Westerhaus, M. J., A. C. Finnegan, Y. Zabulon, and J. S. Mukherjee. 2007. Framing HIV prevention discourse to encompass the complexities of war in northern Uganda. American Journal of Public Health 97(7):1184-1186.
World Health Organization and Federal Ministry of Health, Sudan. 2005. Mortality survey among internally displaced persons and other affected populations in greater Darfur, Sudan. Geneva: World Health Organization, and Khartoum: Federal Ministry of Health, Sudan.
KIMBALL AND HODGES REFERENCES
Aarestrup, F. M., and S. M. Pires. 2008. Comment on: causal regulations vs. political will: why human zoonotic infections increase despite precautionary bans on animal antibiotics. Environment International 34(4):459-475.
Beisel, C. E., and D. M. Morens. 2004. Variant Creutzfeldt Jakob disease and the acquired and transmissible spongiform encephalopathies. Clinical Infectious Diseases 38(5):697-704.
Browne, A. 2009 (May 4). China forces dozens of Mexican travelers into quarantine. The Wall Street Journal, http://online.wsj.com/article/SB124137876507580987.html (accessed October 21, 2009).
Carroll, R., and T. Branigan. 2009 (May 3). Mexico complains of swine flu backlash. The Guardian, http://www.Guardian.co.uk (accessed May 20, 2009).
Gibbs, J., and S. Shaw. 1996. Implications of changes in GATT for the marketing strategies of British beef producers. British Food Journal 97(1):3-10.
Graham, J. P., J. H. Leibler, L. Price, J. M. Otte, D. U. Pfeiffer, T. Tiensen., and E. K. Silbergeld. 2008. The animal human interface and infectious disease in industrial food animal production: rethinking biosecurity and biocontainment. Public Health Reports 123:282-299.
Grant, J., A. M. Wendelboe, A. Wendel, B. Jepson, P. Torres, and C. Smelser. 2008. Spinach-associated Escherichia coli O157:H7, Utah and New Mexico, 2006. Emerging Infectious Diseases 14(10):1633-1636.
Hodges, J. R., and A. M. Kimball. In preparation. Emerging challenges and opportunities for health care systems: risks of transnational infections. WHO workshop on the movement of patients across international borders, Kobe, Japan, February 24-25, 2009.
Kimball, A. M. 2006. Risky trade: infectious diseases in an era of global trade. London: Ashgate Publisher. P. 59.
Llana, S. M. 2009 (May 6). Mexico City returns to normal as swine flu restrictions fade. The Christian Science Monitor, http://www.csmonitor.com/2009/0506/p90s01-woam.html (accessed May 20, 2009).
Silbergeld, E. K., J. Graham, and L. B. Price. 2008. Industrial food animal production, antimicrobial resistance and human health. Annual Review of Pubic Health 29:151-169.
Weuthrich, B. 2003. Chasing the fickle swine flu. Science 299(5612):1502-1505.
WHO (World Health Organization). 2009. WHO influenza A (H1N1)—update 19, http://www.who.int/csr/don/2009_05_07/en/index.html (accessed May 7, 2009).
WHO Global Alert and Response. 2009. Confirmed human cases of avian influenza A (H5N1), http://www.who.int/csr/disease/avian_influenza/country/en/ (accessed October 20, 2009).
WTO (World Trade Organization). 2000. The WTO agreement on the application of sanitary and phytosanitary measures (SPS Agreement), Article 5.7, http://www.wto.org/english/tratop_e/sps_e/spsagr_e.htm#Article5 (accessed May 22, 2009).
———. 2009. World trade 2008, prospects for 2009: WTO sees 9% global trade decline in 2009 as recession strike, http://www.wto.org/english/news_e/pres09_e/pr554_e.htm (accessed April 21, 2009).
ACHESON REFERENCES
FDA (Food and Drug Administration). 2007. Melamine pet food recall of 2007, http://www.fda.gov/AnimalVeterinary/SafetyHealth/RecallsWithdrawals/ucm129575.htm (accessed July 1, 2009).
———. 2008a. FDA investigation leads to several indictments for importing contaminated ingredients used in pet food. FDA News, http://www.fda.gov/bbs/topics/news/2008/new01792.html (accessed April 1, 2009).
———. 2008b. Salmonella Saintpaul outbreak investigations, http://www.fda.gov/AboutFDA/CentersOffices/OC/OfficeofOperations/ucm120882.htm (accessed July 1, 2009).
WHO (World Health Organization). 2009. Expert meeting to review toxicological aspects of melamine and cyanuric acid, 1-4 December 2008, http://www.who.int/foodsafety/fs_management/infosan_events/en/index.html (accessed April 1, 2009).