2
Surveillance
Public-health surveillance is an essential tool in the prevention and control of infectious and chronic diseases and the medical management of people who have the diseases. Surveillance data are used to estimate the magnitude of a health problem, to describe the natural history of a disease, to detect epidemics, to document the distribution and spread of a health event or disease, to evaluate control and prevention measures, and to aid in public-health planning (Thacker, 2000). Public-health surveillance requires standardized, systematic, continuing collection and management of data. In addition, surveillance should encompass timely analysis and dissemination to allow public-health action (CDC, 2001a; Thacker, 2000). Through those steps, federal agencies and state and local health departments are able to inform stakeholders by providing reliable information that can be used to reduce morbidity and mortality through public policy, appropriate resource distribution, and programmatic and educational interventions. The committee has defined (see Box 2-1) the role of surveillance for hepatitis B virus (HBV) and hepatitis C virus (HCV) that is within the scope of its study.
This chapter describes how surveillance data are used or could be used to determine the focus and scope of viral hepatitis prevention and control efforts. The committee reviewed the weaknesses of the current surveillance system for hepatitis B and hepatitis C, including the timeliness, accuracy, and completeness of data collection, analysis, and dissemination. It found that there were few published sources of information about viral hepatitis surveillance. To obtain a clearer picture of the activities that were taking place at state and local levels, the committee gathered information from
|
BOX 2-1 Role of Disease Surveillance
|
various sources. Its findings are based on its review of the literature and on information gathered through surveys of and direct contact with professionals working in this field.
Much of the information gathered through surveys involved state-level and city-level public-health department staff who were working on programs funded by the Centers for Disease Control and Prevention (CDC). Forty-nine states have a cooperative agreement with CDC that funds a coordinator who conducts viral-hepatitis prevention activities, such as health-care provider and consumer education, integration of viral-hepatitis prevention services into health-care and public-health settings, and development of state viral-hepatitis prevention plans. Although the cooperative agreements do not include funds for viral-hepatitis surveillance, the coordinators are good sources of information about surveillance activities being conducted in each jurisdiction. CDC’s Division of Viral Hepatitis (DVH) performed a brief survey of the CDC-funded hepatitis C coordinators in 2006 to gather information about viral-hepatitis surveillance activities. At the request of the committee, CDC again surveyed the coordinators (now called adult viral-hepatitis prevention coordinators, AVHPCs) in April 2009. As part of a national assessment of viral-hepatitis surveillance initiatives, the National
Alliance of State and Territorial AIDS Directors interviewed staff involved in enhanced viral-hepatitis surveillance projects funded through CDC’s Emerging Infections Programs early in 2009 (the programs are described in more detail later in this chapter). Committee members also contacted several AVHPCs directly in April and May 2009 to discuss their work.
The recommendations for surveillance based on the committee’s findings focus on the development of a model designed to improve the quality and accuracy of information by developing systems to collect, analyze, and disseminate data on acute and chronic HBV and HCV infections. The recommendations call for a two-part system: core surveillance activities, building the capacity of state and local health departments to conduct standard disease surveillance on newly diagnosed acute and chronic HBV and HCV infections, and targeted surveillance to obtain data on specific populations that are not represented fully in the collection of core surveillance data. Core surveillance means those activities in which all jurisdictions must engage to provide accurate, complete, and timely information to monitor incidence, prevalence, and trends in disease diagnoses. Data from other activities, such as targeted surveillance, supplement information from core surveillance, and are necessary to provide accurate incidence estimates, given the challenges of conducting hepatitis B and C surveillance, as detailed in this chapter. The recommendations also include guidance regarding the interpretation and dissemination of surveillance data.
APPLICATIONS OF SURVEILLANCE DATA
Surveillance data are used in a variety of ways by a broad base of state health-department staff, researchers, clinicians, policy-makers, and private industry. Federal and state health-department surveillance systems provide population-based information that can be used to improve the public’s health. They also offer an opportunity for public-health intervention at the individual level by linking infected people to appropriate care and support services (Klevens et al., 2009). Overall, surveillance data are critical in estimating incidence and prevalence of HBV and HCV infections (CDC, 2008c), and they provide a basis for studying and understanding the mechanisms of diverse outcomes in the natural history of these infections (Thacker, 2000).
Public health surveillance generally involves name-based reporting of cases of specified diseases to state and local health departments. As such, it requires the gathering of information that some people consider private. Public health officials and state legislatures have weighed the costs and benefits of public health surveillance and have required name-based reporting of specific diseases with confidentiality safeguards in place to protect private information (Fairchild et al., 2008). Confidential name-based re-
porting is standard practice for infectious diseases surveillance, including HIV surveillance (CDC, 2008d). Acute HBV infections are reportable in all states and acute HCV infections are reportable in all but one state. All states report the cases to CDC. Chronic HBV infections are reportable in all but six states and chronic HCV infections are reportable in all but seven states (CSTE, 2009).
Outbreak Detection and Control
Accurate and timely surveillance data are necessary to identify out-breaks of acute HBV and HCV infection in the health-care and community settings. The data can assist in recognizing and addressing breaches in infection control, and they can help to mitigate the size of outbreaks. There have been several outbreaks of hepatitis B and hepatitis C in health-care settings in recent years (CDC, 2003b, 2003d, 2005b, 2008a, 2009c; Fabrizi et al., 2008; Thompson et al., 2009). Research on those outbreaks has shown that they typically occurred in dialysis units, medical wards, nursing homes, surgery wards, and outpatient clinics and resulted from breaches in infection control (Lanini et al., 2009). In a 2009 study, researchers found evidence of 33 outbreaks in nonhospital health-care settings in the United States in the last 10 years. Transmission was primarily patient to patient and was caused by lapses in infection control and aseptic techniques that allowed contamination of shared medical devices, such as dialysis machines. The authors stated that successful outbreak control depended on systematic case identification and investigation, but most health departments did not have the time, funds, personnel resources, or legal authority to investigate health-care–associated outbreaks (Thompson et al., 2009).
Hepatitis B and hepatitis C surveillance data can be used to identify or quantify new trends in the transmission of HBV and HCV. For example, surveillance data can help epidemiologists to determine whether sexual transmission of HCV reported among some cohorts of HIV-positive men who have sex with men (Matthews et al., 2007; van de Laar et al., 2009) is statistically significant on a population level. Surveillance data have also been used to identify clusters of newly acquired cases of hepatitis C in adolescents and young adults and to direct appropriate interventions to persons in the clusters (CDC, 2008f). Those findings can help public-health officials to target their resources at emerging populations being affected by HBV and HCV, such as racial and ethnic populations or geographically linked active injection-drug users (IDUs).
Resource Allocation
Surveillance data are often used to determine how to use resources most effectively. For example, estimates of disease burden are commonly used to provide guidance to policy-makers on the level of funding required for disease-related programs. If surveillance data are not available or understate the disease burden, legislators and public-health officials will not allocate sufficient resources to mount an appropriate public-health response.
Information on disease burden is only one factor that guides policy-makers in allocating public-health resources. Priorities in public funding are also driven by public awareness and advocacy. Therefore, it is important to communicate surveillance trends and disease burden clearly to policy-makers and community advocates. For example, estimates of trends indicate that mortality from HCV may soon exceed that from HIV (Deuffic-Burban et al., 2007). However, despite the large number of individuals and communities affected by hepatitis B and hepatitis C, the resources available for addressing viral hepatitis are only a small fraction of those available for addressing HIV. CDC’s National Center for HIV/AIDS, Viral Hepatitis, Sexually Transmitted Diseases, and Tuberculosis Prevention had a budget of almost $1 billion for 2008, and only 2% of it was allocated to hepatitis B and hepatitis C (Ward, 2008). Sixty-nine percent of the budget was allocated for HIV, 15% for sexually transmitted diseases (STDs), and 14% for tuberculosis.
Programmatic Design and Evaluation
Public-health organizations use surveillance data to design programs that target appropriate populations. For example, CDC requires states to set priorities among populations for HIV prevention according to data generated by HIV/AIDS surveillance programs and community-services assessments (CDC, 2003a). Surveillance data can also be used to evaluate systems for delivery of prevention and care service. A key potential role of hepatitis surveillance programs is to evaluate the effect of HBV vaccination programs (Wasley et al., 2007).
Linking Patients to Care
For some diseases, it is desirable to have a surveillance system closely involved in ensuring the linkage of persons who have new diagnoses to health-care services, often called case management (Fleming et al., 2006). For viral-hepatitis surveillance, linking patients who have recent diagnoses to comprehensive viral-hepatitis programs may be indicated to ensure access to appropriate services, including clinical evaluation, regular followup
visits, referral to drug-treatment and harm-reduction programs, education about liver health, and prevention of transmission to others. Chapter 5 will discuss the components of viral-hepatitis services.
DISEASE-SPECIFIC ISSUES RELATED TO VIRAL-HEPATITIS SURVEILLANCE
Many of the difficulties that surveillance systems face in identifying and tracking cases of hepatitis B and hepatitis C are related to the complexity of the infections and their associated progression (see Figures 2-1 and 2-2). This section highlights some of those challenges. Chapter 5 will provide more detail on issues related to screening and identification.
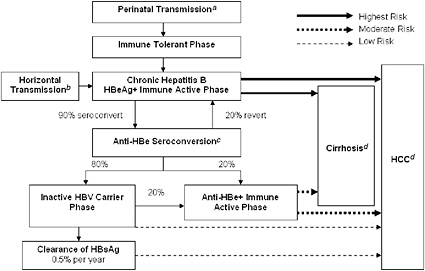
FIGURE 2-1 Natural progression of hepatitis B infection.
Abbreviations: HBeAg, hepatitis B e antigen; anti-HBe, antibody to hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
aTransmission occurs in 90% of infants of HBsAg+/HBeAg+ mothers and 15% of infants of HBsAg+/anti-HBe+ mothers.
b30% of those infected from the age of 1–5 years and under 7% of those infected at the age of 6 years or older.
cAbout 50% of patients by 5 years and 70% of patients by 10 years will seroconvert to anti-HBe.
d15-25% risk of premature death from cirrhosis and HCC.
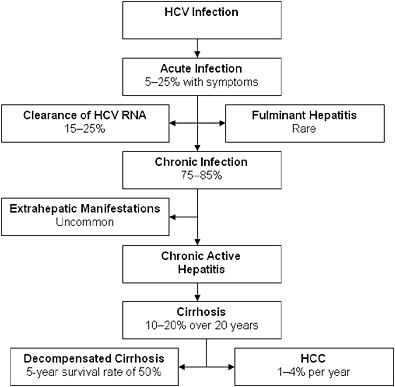
FIGURE 2-2 Natural progression of hepatitis C infection.
Abbreviations: HCV, hepatitis C virus; RNA, ribonucleic acid; HCC, hepatocellular carcinoma.
SOURCE: Adapted from Chen and Morgan, 2006. Reprinted with permission from Ivyspring International Publisher, copyright 2006.
Identifying Acute Infections
Several factors contribute to the difficulty in identifying acute HBV and HCV infections. Many newly acquired cases are asymptomatic, or they may have symptoms similar to those of other common illnesses and so do not prompt health-care providers to conduct serologic testing for HBV and HCV, or the serologic tests that are conducted are inadequate to distinguish between acute and chronic cases. About 90% of acute HBV infections in children under 5 years of age and 70% of HBV infections in adults are asymptomatic (McMahon et al., 1985); 75–95% of acute HCV infections are asymptomatic (Chen and Morgan, 2006; Guerrant et al., 2001), so few infected patients seek care for the acute illness; and there is a very high probability of underreporting even when care is obtained (Chen and Morgan, 2006; Cox et al., 2005; Hagan et al., 2002). Clinicians
often are not fully aware of reporting requirements in connection with other reportable diseases and do not initiate reports routinely (Allen and Ferson, 2000). In addition, some persons with chronic HBV infection can experience sudden increases in alanine aminotransferase (ALT) that may be associated with jaundice or liver decompensation. That change may have a variety of causes, including infection with another hepatitis virus; alcohol, drug, or medication use; or sudden hepatitis B disease reactivation that can be associated with the period of seroconversion from a hepatitis B e antigen (HBeAg) state to an antibody to hepatitis B e (anti-HBe) state or reversion from an anti-HBe state back to an HBeAg-positive state (Koff, 2004). Therefore, in investigating acute symptomatic infections, it is important to identify outbreaks so that preventive measures can be undertaken and, in the case of hepatitis B, to identify and screen close contacts who might benefit from the hepatitis B vaccine. Such information is needed if surveillance staff is to determine which cases are newly diagnosed, the result of recent exposure, or chronic (Fleming et al., 2006).
Classifying acute cases of hepatitis B and hepatitis C requires a complex integration of clinical data, positive and negative laboratory data, and prior or repeat testing (see Boxes 2-2 and 2-3). Many of the test results—for
|
BOX 2-2 CDC Acute Hepatitis B Case Definition Clinical case definition: An acute illness with
and
Laboratory criteria for diagnosis:
or
Case classification: Confirmed: a case that meets the clinical case definition and is laboratory confirmed Abbreviations: CDC, Centers for Disease Control and Prevention; HAV, hepatitis A virus; HBV, hepatitis B virus. SOURCE: CDC, 2009a. |
|
BOX 2-3 CDC Acute Hepatitis C Case Definition Clinical case definition: An acute illness with a discrete onset of any sign or symptom consistent with acute viral hepatitis (e.g., anorexia, abdominal discomfort, nausea, vomiting), and either jaundice or serum alanine aminotransferase (ALT) levels >400 IU/L. Laboratory criteria for diagnosis: One or more of the following three criteria:
and, meets the following two criteria:
Case classification: Confirmed: A case that meets the clinical case definition, is laboratory confirmed, and is not known to have chronic hepatitis C. Abbreviations: CDC, Centers for Disease Control and Prevention; HAV, hepatitis A virus; HCV, hepatitis C virus; RIBA, recombinant immunoblot assay; RNA, ribonucleic acid. NOTE: URL for the signal-to-cutoff ratios: http://www.cdc.gov/ncidod/diseases/hepatitis/c/sc_ratios.htm. SOURCE: CDC, 2009a. |
example, for ALT, aspartate transaminase, immunoglobulin M (IgM) antibody to the hepatitis A virus, and IgM antibody to the hepatitis B core antigen (HBcAg)—are difficult for health departments to obtain, particularly because negative test results often are not automatically reported to health departments (Fleming et al., 2006). Because auxiliary test results are not systematically reported to health departments, surveillance staff must actively follow up with health-care providers to obtain them and other clinical indicators of acute disease. If the data cannot be obtained, either because the proper tests were not ordered or because there is insufficient staff to conduct followup, cases will be classified ambiguously as nonacute infections.
Furthermore, current CDC case definitions may miss a substantial fraction of clinically apparent acute cases because they lack clinical markers that could improve case identification and help to distinguish between acute and chronic cases. Using data from electronic medical records, Klompas et al. (2008) found that CDC’s case definition of acute HBV had a positive predictive value of only 47.2% (that is, out of 1,000 people identified as having acute hepatitis B with the CDC case definition, only 472 of them were found to truly have acute hepatitis B). When patients with prior positive tests for HBV infection (or International Classification of Diseases, revision 9, codes for chronic HBV infection) were excluded, the positive predictive value increased to 68.4%. However, the positive predictive value was raised to above 96% by adding the requirement for peak ALT over 1,000 or total bilirubin over 1.5. Most important, when applying the most sensitive algorithm (the algorithm that detected the greatest number of cases of acute hepatitis B), the study found that only four of the eight cases of acute hepatitis B were in the state’s surveillance system and only one of the four was correctly classified as acute; this suggests that 88% of acute hepatitis B cases may be missed if current reporting algorithms are used (Klompas et al., 2008).
Similarly, detection of acute hepatitis C can be challenging because no single case definition is either sensitive or specific for it. HCV seroconversion may be missed, and there is no IgM-based assay that reliably distinguishes acute hepatitis C from chronic hepatitis C, unlike the situation with hepatitis A virus or HBV infection. Relatively low HCV ribonucleic acid (RNA) concentrations and more than one log fluctuation in HCV RNA concentration are features of acute HCV infection that may be useful for the development of more dynamic diagnostic algorithms, but the accuracy of these algorithms has not been validated (Cox et al., 2005; McGovern et al., 2009; Villano et al., 1999).
In summary, the identification of acute hepatitis infection is inherently flawed because the vast majority of cases are asymptomatic and patients do not seek medical care or testing. Such persons would be identified only in prospective studies that include routine serial testing of liver enzyme concentrations, such as those previously conducted to identify the incidence of transfusion-associated hepatitis. Underreporting of diagnosed cases and misclassification of reported cases seriously limit the accuracy of data on cases of acute viral hepatitis collected by state and territorial surveillance programs and transmitted to CDC. Thus, the estimates of the incidence of acute hepatitis in the United States are based solely on symptomatic cases. The majority of those cases may be missing from the surveillance system because of poor access to health care, underreporting, and misclassification. Taken together, published surveillance summaries of reported cases of acute viral hepatitis substantially underestimate the number of cases; these
summaries may give misleading impressions of the incidence of disease to policy-makers and program planners.
Identifying Chronic Infections
Given that both hepatitis B and hepatitis C infections are largely asymptomatic, most people do not receive a diagnosis until the infection is chronic. For hepatitis B, the chance of developing a chronic infection varies with age at the time of infection.
In persons over 6 years old, the vast majority of acute HBV infections are self-limited (Hyams, 1995). However, hepatitis B infections become chronic in over 90% of infants who are infected at birth or in the first year of life and in 30% of children who are infected at the age of 1–5 years (Pungpapong et al., 2007). Although hepatitis B surface antigen (HBsAg) is detectable within 4–10 weeks after infection, it is indicative of chronic HBV infection only if it persists for more than 6 months (Koff, 2004). An accurate diagnosis of chronic hepatitis B may therefore require the reporting of multiple serologic markers at more than one time (Koff, 2004).
For disease-surveillance purposes, it can be challenging for health departments to obtain the complete laboratory results that are necessary to classify a chronic hepatitis B case according to CDC’s case definitions (see Box 2-4). In general, a full hepatitis B panel (including any negative results for IgM anti-HBc) is required or two HBsAg results at least 6 months apart. Although states govern laboratory-reporting requirements in their jurisdictions, negative test results are generally not reportable and must be actively obtained. CDC’s Guidelines for Viral Hepatitis Surveillance and Case Management recommend that only positive HBsAg-test results be reported, but this test alone is inadequate to distinguish acute from chronic infection. Automated systems attached to electronic medical records may help to address surveillance for chronic HBV cases in the future, but in the meantime many diagnoses of chronic HBV infection probably will not be correctly captured and classified as confirmed cases (CDC, 2005a).
Surveillance for chronic HCV infection also presents challenges (see Box 2-5). In adults, about 15–25% of acute hepatitis C infections resolve spontaneously (Villano et al., 1999). That may increase to about 45% in children and young adults (Vogt et al., 1999). The presence of HCV RNA is generally detected within 1 week of infection (Mosley et al., 2005), but antibodies to HCV (anti-HCV) can be detected in only 50–70% of infected persons at the onset of symptoms; this increases to more than 90% after 3 months (NIH, 2002). A chronic infection is characterized by the persistent presence of HCV RNA for at least 6 months (NIH, 2002).
Typically, when a patient presents for HCV testing, the first test that is conducted is for the presence of anti-HCV. This test is generally an en-
|
BOX 2-4 CDC Chronic Hepatitis B Case Definition Clinical description Persons with chronic HBV infection may have no evidence of liver disease or may have a spectrum of disease ranging from chronic hepatitis to cirrhosis or liver cancer. Persons with chronic infection may be asymptomatic. Laboratory criteria
and
or
Case classification Confirmed: a case that meets either laboratory criteria for diagnosis Probable: a case with a single HBsAg positive or HBV DNA positive or HBeAg positive lab result when no IgM anti-HBc results are available Comment Multiple laboratory tests indicative of chronic HBV infection may be performed simultaneously on the same patient specimen as part of a “hepatitis panel.” Testing performed in this manner may lead to seemingly discordant results, e.g., HBsAg-negative and HBV DNA-positive. For the purposes of this case definition, any positive result among the three laboratory tests mentioned above is acceptable, regardless of other testing results. Negative HBeAg results and HBV DNA levels below positive cutoff level do not confirm the absence of HBV infection. Abbreviations: CDC, Centers for Disease Control and Prevention; anti-HBc, hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; DNA, deoxyribonucleic acid. SOURCE: CDC, 2009a. |
|
BOX 2-5 CDC Hepatitis C Virus Infection Case Definition (Past or Present) Clinical description: Most HCV-infected persons are asymptomatic. However, many have chronic liver disease, which can range from mild to severe including cirrhosis and liver cancer. Laboratory criteria for diagnosis:
or
or
or
or
Case classification: Confirmed: a case that is laboratory confirmed and that does not meet the case definition for acute hepatitis C. Probable: a case that is anti-HCV positive (repeat reactive) by EIA and has ALT or SGPT values above the upper limit of normal, but the anti-HCV EIA result has not been verified by an additional more specific assay or the signal to cutoff ratio is unknown. Abbreviations: CDC, Centers for Disease Control and Prevention; HCV, hepatitis C virus; EIA, enzyme immunoassay; RIBA, recombinant immunoblot assay; RNA, ribonucleic acid; ALT or SGPT, alanine aminotranferase. SOURCE: CDC, 2009a. |
zyme immunoassay (EIA). A repeatedly reactive EIA is followed by a more specific assay to detect viremia, such as the recombinant immunoblot assay (RIBA) for anti-HCV, or by nucleic acid testing for HCV RNA. In some cases, an EIA with a high signal-to-cutoff ratio predictive of a true positive will be used in the place of a confirmatory RIBA. However, all confirmed anti-HCV test results should be followed by a test for the presence of HCV
RNA (Alter et al., 2003; Ghany et al., 2009). The difficulty in identifying chronic cases often revolves around the need for two separate tests (or other supplemental antibody tests) and the 6-month timeframe required for a diagnosis of chronic HCV infection. Many infected people are tested at public or nonclinical testing sites—such as drug-treatment facilities, sites for testing for HIV or STDs, or community-based organizations—that conduct only the less expensive anti-HCV tests. Persons tested at those sites might not have access to an HCV RNA test, or the laboratory conducting the initial EIA test might not routinely test for HCV RNA when an EIA has been positive. The process leads to incomplete diagnoses and inaccurate reporting of the number of chronic cases.
The CDC-recommended case definition of nonacute HCV infection also poses some problems in interpreting the collected data. Although it is assumed that the majority of nonacute hepatitis C cases represent chronic infections, this case definition also includes acute cases that do not meet the confirmed acute case classification (CDC, 2005a). However, a case defined only by the presence of anti-HCV could be a late acute infection, a chronic infection, a resolved infection, or a false-positive assay result. It is therefore essential that anti-HCV testing be supplemented by testing for HCV RNA and by followup samples to classify cases correctly and to use the data for program-planning purposes.
Identifying Perinatal Hepatitis B
Since 1992, CDC has awarded funds to 64 grantees to support perinatal hepatitis B prevention programs through its Immunization Services Division’s cooperative agreements with health departments. The funds support perinatal hepatitis B coordinators, who are charged with identifying all HBsAg-positive pregnant women, ensuring the administration of appropriate immunoprophylaxis to all infants born to these women, ensuring the completion of postvaccination serologic testing of the infants, and ensuring the completion of the hepatitis B vaccine series. Most coordinator programs also include ensuring vaccination of household contacts and sexual partners of HBsAg-positive women in their mission (CDC, 2009g).
In an economic analysis of immunization strategies to prevent hepatitis B transmission in the United States, Margolis et al. (1995) found that prevention of perinatal infection and routine infant vaccination would reduce the lifetime risk of HBV infection by at least 68%. They estimated that prevention of perinatal HBV infections would save $41.8 million in medical and work-loss costs. Routine hepatitis B vaccination of infants would provide additional savings of $19.7 million. Both strategies were found to be cost-effective, with estimated costs per year of life saved of $164 for preventing perinatal HBV infection and of $1,522 for infant vaccination.
In 1992, the Connecticut Department of Public Health found that hiring a state-level perinatal coordinator led to significantly better compliance with the recommendation to administer hepatitis B immune globulin (HBIG) than followup by local health departments. They also found that completion of the three-dose vaccination series was higher in cases followed by the state coordinator than in those followed by local public-health officials (CDC, 1996).
Two major problems occur in the identification and management of possible cases (see Box 2-6). The first involves linking pregnancy status with positive HBsAg laboratory reports on females of child-bearing age in a timely manner. In 2005, the Advisory Committee on Immunization Practices called for improving prevention of perinatal and early childhood HBV transmission by improving laws and regulations to improve identification of HBsAg-positive and HBsAg-undetermined mothers (Mast et al., 2005).
|
BOX 2-6 CDC Perinatal Hepatitis B Virus Infection Case Definition Clinical description Perinatal hepatitis B in the newborn may range from asymptomatic to fulminant hepatitis. Laboratory criteria HBsAg positive Case classification HBsAg positivity in any infant aged >1–24 months who was born in the United States or in US territories to an HBsAg-positive mother Comment: Infants born to HBsAg-positive mothers should receive hepatitis B immune globulin (HBIG) and the first dose of hepatitis B vaccine within 12 hours of birth, followed by the second and third doses of vaccine at 1 and 6 months of age, respectively. Postvaccination testing for HBsAg and anti-HBs (antibody to HBsAg) is recommended from 3 to 6 months following completion of the vaccine series. If HBIG and the initial dose of vaccine are delayed for >1 month after birth, testing for HBsAg may determine if the infant is already infected. Abbreviations: CDC, Centers for Disease Control and Prevention; HBsAg, hepatitis B surface antigen; anti-HBs, antibody to HBsAg; HBIG, hepatitis B immune globulin. SOURCE: CDC, 2009a. |
Implementation of automated electronic systems can greatly increase the speed with which cases can be identified (LaPorte et al., 2008), but they are not available in most states. The second problem involves a lack of resources to follow up on all potential and known cases and their contacts in a timely manner. Followup of an infant can take up to 2 years. In addition, a substantial number of HBsAg-positive mothers are not identified in time to ensure the required followup of the mothers and their infants (see Chapter 4).
Other Challenges for Hepatitis B and Hepatitis C Surveillance Systems
Repeat testing in high-risk populations can confuse the number of suspected acute versus chronic infections. Members of some populations, such as IDUs, may repeatedly incur HCV infection that resolves spontaneously without ever becoming chronic (Mehta et al., 2002). Those cases could mistakenly be classified as chronic infections based on antibody results alone.
Many of the people affected by hepatitis B and hepatitis C have limited access to health care (for example, active IDUs, homeless people, some Pacific Islanders, legal immigrants living in poverty, and undocumented immigrants) and are less likely to be diagnosed appropriately, to provide complete and accurate demographic and behavioral information, or to access followup care. Structural and political barriers, stigma, and fear of legal repercussions contribute to the limitations on their access. Each HBV-infected or HCV-infected person who does not enter into appropriate medical care represents a missed opportunity for secondary prevention and may contribute to the collection of inaccurate and less detailed surveillance data. Finding ways to ensure that patients receive comprehensive and culturally appropriate care and referrals not only would increase the likelihood of improving their health outcomes but is likely to affect surveillance-data collection favorably.
Finally, because of the chronic nature of viral hepatitis, it is important that surveillance staff communicate well between jurisdictions. Persons with chronic disease can be misclassified as having acute cases if earlier diagnoses made in other jurisdictions are not identified. Not infrequently, a previous diagnosis has been reported in another state or jurisdiction. The ability of state and local surveillance-program staff to track cases across jurisdictions is hampered by various factors, including inadequacy of staff resources, nonstandardized surveillance software systems, and the lack of a national database that could be used to identify potential matches in other jurisdictions.
INFRASTRUCTURE AND PROCESS-SPECIFIC ISSUES WITH SURVEILLANCE
Current public-health surveillance systems for hepatitis B and hepatitis C are poorly developed and are inconsistent among jurisdictions. As a result, surveillance data do not provide accurate estimates of the current burden of disease, are insufficient for program planning and evaluation, and do not provide the information that would allow policy-makers to allocate sufficient resources to address the problem. The AVHPCs, funded by CDC in state and territorial health departments, are tasked with identifying mechanisms for educating the public, at-risk populations, and medical-service and social-service providers about viral hepatitis; for managing and coordinating viral-hepatitis prevention activities; and for integrating viral-hepatitis screening programs and related services into health-care settings and public-health programs that serve at-risk adults. In most cases, however, CDC funding covers only the AVHPC’s salary. No funding is provided for viral-hepatitis testing, hepatitis B immunizations, or other services. Most important, AVHPCs are not funded to conduct surveillance activities, although many of them provide technical assistance for such programs.
In addition, CDC’s DVH has scant resources for providing funding and guidance to local and state health departments to perform surveillance for viral hepatitis. The resources provided for viral-hepatitis surveillance contrast sharply with the resources that CDC provides for HIV surveillance. For example, CDC has specific cooperative agreements with the states and territories to conduct core HIV/AIDS surveillance activities. The cooperative agreements are accompanied by dedicated funding, specific CDC project officers and epidemiologists, regular technical-assistance meetings and training, and a help desk that has trained staff to answer database-related questions. The guidance for HIV/AIDS surveillance is a three-volume set containing more than 500 pages of detailed instructions, standards, and guidelines. In contrast, CDC’s cooperative agreements with state and territorial health departments for viral hepatitis do not include surveillance activities. In addition, although CDC’s DVH has produced guidelines for viral-hepatitis surveillance for state and territorial health departments, they are presented in fewer than 50 pages (CDC, 2005a). Given that the guidelines cover three distinct and complex diseases (hepatitis A, hepatitis B, and hepatitis C), they lack the detail necessary to create surveillance practices that are consistent among jurisdictions. As a result of the deficiency of resources dedicated to hepatitis surveillance, data are incomplete, variable, and inaccurate. Inconsistency between jurisdictions seriously undermines the validity of the data provided at the state, regional, and national levels.
The inability of health departments to track all diagnosed cases also seriously undermines case-management and prevention efforts. For example,
studies have shown that vaccinating close contacts of persons chronically infected with HBV (that is, ring vaccination) is cost-effective (Hutton et al., 2007). The strategy remains cost-effective even in populations in which the prevalence of chronic HBV infection is as low as 2%. However, without funding and staffing for surveillance and identification of new cases of HBV, ring vaccination is not a public-health activity that is typically supported by most health departments.
CDC has funded seven enhanced projects through the Viral Hepatitis Surveillance Emerging Infections Programs (EIPs). The projects began in 2004 and were scheduled to end in 2009. CDC plans to extend the program for 2 more years (personal communication, J. Efird, CDC, May 18, 2009). They are in Colorado, Connecticut, Minnesota, New York state, New York City, Oregon, and San Francisco. Although the projects focus on surveillance for hepatitis A, hepatitis B, and hepatitis C, they all take different approaches, including multiple approaches in individual jurisdictions. Project funding supports epidemiologic and data-entry staffing. Methods used in the seven programs include verification of diagnoses with medical providers, chart review, followup calls to infected persons that focus on education or data collection, educational mailings (for example, letters and booklets), followup with persons that are household or close contacts (especially acute HBV cases), sampling for followup of cases of chronic HBV and HCV, review of all data, and matching with HIV and STD programs. There is no uniform evaluation of the projects.
In February and March 2009, staff of the National Alliance of State and Territorial AIDS Directors (NASTAD) interviewed the coordinators of the seven EIPs. From those interviews, staff identified additional programmatic issues that affect reporting. They include resource issues, such as the varied capacity of county and city health departments (which leads to inconsistencies in data collection and data systems, in some instances in the same state); the staffing requirements needed to collect, process, and manage data; and the staff and time needed to investigate health-care–related outbreaks adequately. Other issues are the need to educate medical providers better on which laboratory tests are needed for appropriate diagnosis (also noted by Fleming et al., [2006]), and the difficulty that staff face in obtaining demographic data (including data on race, ethnicity, and country of origin) and data on risk history (Klevens et al., 2009; NASTAD, 2009).
Funding Sources
Funding for hepatitis surveillance is highly fragmented. No federal funds are dedicated to chronic-hepatitis surveillance except for the seven jurisdictions that receive funds from CDC’s DVH to perform enhanced surveillance activities. State, territorial, or city health-department viral-
hepatitis surveillance units, where they exist, often receive no dedicated federal funding for this activity. Others may receive funding directly from their state or city, or they may be integrated into or receive funding from other programs or units that receive federal funding from CDC programs whose missions are related to epidemiology or viral hepatitis. The CDC programs include the Immunization Services Division (related to perinatal hepatitis B), the Epidemiology and Laboratory Capacity for Infectious Diseases program (acute hepatitis B), EIPs, and the DVH. Funding may also be available through private organizations or foundations. Some surveillance units receive funding from multiple sources. Each funding source may require different activities and may provide varied guidance on the receiving unit’s activities. The recent survey of AVHPCs conducted by NASTAD found that fewer than one-fourth of the 43 responding jurisdictions reported receiving funding for surveillance for either chronic HBV or chronic HCV infection (NASTAD, 2009).
Program Design
Variability among jurisdictions is also due to a wide array of program structures. In a 2006 survey, 33% of the 52 hepatitis C coordinators funded by CDC reported being in their jurisdictions’ communicable-diseases or epidemiology programs, 25% in HIV–STD programs, 14% in HIV programs, and the remainder in the immunization or STD programs (CDC, 2006). The hepatitis C coordinators’ locations within public health departments may or may not correspond with the health department program responsible for conducting surveillance, which can lead to reduced involvement and oversight by the coordinator of viral hepatitis surveillance activities.
In a later survey of the (renamed) AVHPCs by CDC in April 2009, only 32 states reported having state viral-hepatitis plans. Of the 32, 29 included surveillance as a component. However, fewer than two-thirds of the program coordinators reported being able to implement the surveillance components. The reasons listed for not implementing plan components were lack of staff and lack of funding (CDC, 2009h).
Reporting Systems and Requirements
Reporting of surveillance data to CDC by state and territorial health departments is voluntary, and in general little federal funding is provided for HBV and HCV surveillance activities (Klein et al., 2008). Chronic hepatitis B is reportable in 42 states, but only 38 states conduct surveillance and maintain systems, and only 20 report cases to CDC (George, 2004). Chronic hepatitis C is reportable in 40 states, but only 20 report cases to CDC (George, 2004). CDC collects data from states that report
chronic HBV and HCV infections (and received data on 133,520 cases of HCV infection in 2007 alone), but these data are not presented routinely in Morbidity and Mortality Weekly Report, which is published by CDC, or elsewhere (Klevens et al., 2009). Although all states but one perform some degree of surveillance for acute HBV and HCV (Daniels et al., 2009b), much of this surveillance is passive at best.
There are significant barriers to implementing more comprehensive surveillance activities. In the previously mentioned survey conducted by NASTAD in 2009, it was reported that of the 43 responding jurisdictions, almost half received between 1,000 and 10,000 HBV laboratory results annually, and over 70% reported the same range for the number of HCV laboratory results received annually (NASTAD, 2009). Many states do not have the staffing or systems to keep up with such a high volume of information received and are often unable to follow up with medical providers to address underreporting or to obtain demographic and risk-history information, such as race, ethnicity, and drug-use details (Klein et al., 2008). The lack of funding to hire adequate staff is the fundamental barrier to complete and accurate surveillance.
Although CDC provides case-reporting forms for the collection of viral-hepatitis surveillance data, the forms do not have required elements, and they ask for data that are often difficult to obtain. Moreover, the use of the forms is inconsistent among states and local jurisdictions. Of the 43 health departments that responded to inquiries from the present committee, 26 have developed their own HBV case-reporting form, and 21 have developed an HCV case-reporting form. The forms were created to capture behavioral-risk information not included on CDC’s form or to improve data collection and entry into the separate jurisdictions’ specific software systems. For example, the CDC case-reporting form does not collect risk-behavior information specific to chronic HBV. Finally, 8 states do not use a case-reporting form at all for reporting HBV, and 12 states do not use one for HCV; these jurisdictions rely solely on the reporting of laboratory-test results.
Paradoxically, efforts to modernize and enhance public-health surveillance systems have led to greater inconsistency in data collection. In 1984, CDC began work on the Epidemiologic Surveillance Project. The goal of the project was to develop computer-based transmission of public-health surveillance data between states and CDC. By 1989, all 50 states were participating in the reporting system for certain acute infectious diseases, and the system was renamed the National Electronic Telecommunications System for Surveillance (NETSS) (CDC, 2009f). Data were transmitted weekly to CDC in a standard record format. However, the system quickly became dated with advances in information and surveillance technology, such as electronic laboratory reporting and electronic medical records.
Moreover, other surveillance systems and software were used for reporting of HIV/AIDS, STDs, tuberculosis, and some vaccine-preventable diseases.
In 1999, CDC developed the concept of the National Electronic Disease Surveillance System (NEDSS), which was designed to promote the development of interoperable surveillance systems (that is, the Public Health Information Network, or PHIN) at federal, state, and local levels (CDC, 2009e). The NEDSS initiative describes information-system standards to which all systems must adhere but does not require use of CDC-produced software. CDC provides the NEDSS Base System, a software system that may be used, but only 16 jurisdictions have opted to use it. Most jurisdictions use PHIN-compliant systems, which are either purchased from a commercial vendor or developed specifically for a particular jurisdiction. A few jurisdictions continue to use the NETSS system while their PHIN-compliant systems are being developed (personal communication, J. Efird, CDC, April 1, 2009). The result is that CDC no longer provides a standardized database for inputting and reporting data on viral hepatitis. Consequently, there is a wide array of state systems with an even wider array of capabilities. The lack of standardization makes it difficult for states to share information efficiently. In addition, creating and modifying their systems can lead to substantial expenses for states and jurisdictions (CDC, 2009i).
Even states and jurisdictions that have PHIN-compliant systems in place may not have the staff to enter the high volume of viral-hepatitis data received. Four of the 43 states that responded to the recent questionnaire for this committee reported not having any staff to enter data. They do not include states that may not be able to enter all received data fully. In the 2009 NASTAD survey of AVHPCs, it was reported that 27 of the 43 reporting jurisdictions had backlogs of HCV data, with an average of 6,200 cases that needed to have data entered (NASTAD, 2009).
Capturing Data on At-Risk Populations
As discussed previously, current surveillance systems do not adequately capture cases of acute and chronic HBV and HCV infections. That is particularly true for members of marginalized populations. IDUs are at high risk for both HBV and HCV infections. The incidence of HCV infection in IDUs ranges from 2% to 40% per year, with most rates in the range of 15–30% per year1 (Maher et al., 2006; Mathei et al., 2005; van den Berg et al., 2007), and the incidence of HBV infection from 10% to 12% per year (Ruan et al., 2007). A study of IDUs in Seattle looked at those who became infected with HBV or HCV during the 12-month study period. The study matched study participants who had become infected to those identified in
the health department’s surveillance database. Of the 113 study participants who became infected, only two cases of those identified in the study were picked up by the state’s surveillance system (Hagan et al., 2002). None of the 65 acute HCV infections in IDUs was reported.
Enhanced sero-surveillance studies, such as the National Health and Nutrition Examination Survey (NHANES), which are often used to provide supplemental data and prevalence estimates when surveillance systems are inadequate or incomplete, have serious limitations when addressing viral hepatitis. For example, NHANES, the study most commonly used to estimate the disease burden of chronic HBV and HCV infections, excludes or underrepresents populations that are most at risk for HBV and HCV infections. Those populations include homeless persons, institutionalized and incarcerated persons, and persons of Asian and Pacific Island descent. About 1.5 million people are in state or federal prisons in the United States (Department of Justice, 2009). CDC estimated chronic HBV prevalence in the adult prison population to be around 1–3.7%, and the prevalence of chronic HCV infection has been reported to vary from 12–35% (CDC, 2003c). Thus, the national estimate of 2.7–3.9 million people chronically infected with HCV (CDC, 2009b; Daniels et al., 2009a) potentially excludes several hundred thousand cases. Similarly, Asians, Pacific Islanders, American Indian, and Alaska Native people are undersampled among NHANES participants (Coleman et al., 1998). Given the higher prevalence of HBV in those populations, NHANES underreports the number of chronic HBV cases in the national estimates (Kim, 2007).
A supplemental HIV surveillance project funded by CDC’s Division of HIV/AIDS Prevention is the National HIV Behavioral Surveillance System. The system surveys persons at high risk for HIV infection in cities with high rates of AIDS diagnoses to determine their risk behavior, testing behavior, and use of prevention services. Even though IDUs are one of the targeted populations studied through the system (CDC, 2009d), HCV testing has not been included systematically as part of the study design, and this leads to missed opportunities to define the injection and injection-related behaviors as they apply to HCV and HCV prevention.
Case Evaluation, Followup, and Partner Services
The reporting of a case of hepatitis B or hepatitis C by a public test site or private clinic provides an opportunity for public-health followup. Part of the followup generally involves ensuring that the persons with the reported diagnoses and their partners receive proper medical evaluation, counseling, vaccination, and referrals to support services as needed (Fleming et al.,
2006). An inability of a health department to identify a case becomes a missed opportunity to prevent future cases and to ensure that HBV-infected and HCV-infected people receive the care that they need.
In 2007, the Massachusetts Department of Public Health piloted the use of STD disease intervention specialists (DISs) to follow up on cases of HCV infection in people 15–25 years old. The DISs were tasked with collecting risk-history data and providing partner-notification services for drug-sharing partners of the infected people. There was some success in reaching a small sample of the high volume of infected people, but no funding was available to support the staff. In addition, the DISs had competing priorities that kept the department from being able to implement its methods fully so that they could be appropriately evaluated (Onofrey et al., 2008).
Given the demands on staff, most state health-department surveillance units indicated that they were barely able to keep up with the basics of data collection. The 2009 NASTAD survey found that only 18 of the 43 jurisdictions conducted any type of followup; in the ones that did conduct followup, there was wide variation in what that comprised (NASTAD, 2009). Followup can consist of making calls to providers or cases to collect demographic, clinical, or risk-history data and contacting infected people by mail, by telephone, or in person to provide education or referral to medical services. For the most part, even the best resourced surveillance units are able to conduct only very limited case management (Fleming et al., 2006).
Furthermore, the utility and cost effectiveness of conducting partner services for persons who test positive for HBV or HCV is yet to be determined. Currently, states conduct partner notification for HIV and some STDs. Services include notifying sex or needle-sharing partners of exposure to disease and testing, counseling, and referrals for other services. Newly revised CDC guidelines on partner services strongly recommend partner services for persons reported to have HIV or early syphilis; services for gonorrhea and chlamydial infection are recommended for high-priority cases as resources allow (CDC, 2008e). Recommendations for persons who have viral hepatitis were not included in the HIV and STD integrated guidelines.
Recommendations
Recommendation 2-1. The Centers for Disease Control and Prevention should conduct a comprehensive evaluation of the national hepatitis B and hepatitis C public-health surveillance system.
The evaluation should, at a minimum,
-
Include assessment of the system’s attributes, including completeness, data quality and accuracy, timeliness, sensitivity, specificity, predictive value positive, representativeness, and stability.
-
Be consistent with CDC’s Updated Guidelines for Evaluating Public Health Surveillance Systems.
-
Be used to guide the development of detailed technical guidance and standards for viral hepatitis surveillance.
-
Be published in a report.
The committee found little published information on or systematic review of viral-hepatitis surveillance in the United States. Specific information was obtained from a series of surveys of the AVHPCs. In contrast, the history and status of national HIV surveillance is well reviewed and documented (CDC, 1999; Glynn et al., 2007; Nakashima and Fleming, 2003).
In July 2001, CDC published updated guidelines for evaluating public-health surveillance systems (CDC, 2001b). According to the guidelines, the evaluation should “involve an assessment of system attributes, including simplicity, flexibility, data quality, acceptability, sensitivity, predictive value positive, representativeness, timeliness, and stability.” The lack of sensitivity of state hepatitis-surveillance systems is well documented for acute cases, and many states do not perform surveillance for chronic HBV or chronic HCV infections. Moreover, the movement of CDC away from NETSS, a CDC-provided system, and toward a national network of PHIN-compliant systems has left state and territorial health departments with a wide array of software systems and capabilities (CDC, 2009i). A comprehensive review is needed to document the current systems and capacities of public-health jurisdictions. The evaluation should focus on developing guidance to improve consistency of data, guide the development of detailed technical guidance and standards for hepatitis-surveillance programs, and allow CDC to improve understanding and description of the limitations of the data collected. Completion of this task should not delay the implementation of other components of the surveillance-related recommendations in this report.
Recommendation 2-2. The Centers for Disease Control and Prevention should develop specific cooperative viral-hepatitis agreements with all state and territorial health departments to support core surveillance for acute and chronic hepatitis B and hepatitis C.
The agreements should include
-
A funding mechanism and guidance for core surveillance activities.
-
Implementation of performance standards regarding revised and standardized case definitions, specifically through the use of
-
Revised case-reporting forms with required, standardized components.
-
Case evaluation and followup.
-
-
Support for developing and implementing automated data-collection systems, including
-
Electronic laboratory reporting.
-
Electronic medical-record extraction systems.
-
Web-based, PHIN-compliant reporting systems.
-
CDC should provide more comprehensive guidance to states on surveillance for viral hepatitis. The committee suggests that CDC use the HIV surveillance system as a model. The committee focused on that surveillance model as an alternative to the current model because of its organization, availability of technical assistance, and provision of detailed guidelines. The strength of the model is in its centralized guidance, mandatory process and outcome standards, and oversight at a national level, all of which provide consistency in data among jurisdictions (Hall and Mokotoff, 2007).
CDC is able to oversee its national effort through separate cooperative agreements with each state and territory for specific core HIV surveillance activities (CDC, 2007). The agreements not only provide funding for enough dedicated staff to provide followup directly with providers and to conduct active surveillance but commit states and territories to specific methods and performance expectations. States are also provided with detailed guidance on case investigation, classification, and followup requirements (CDC/CSTE, 2006). To ensure consistency, CDC holds required disease-specific technical-assistance meetings for all grantees. Project officers and epidemiologists are assigned to each grantee. As of April 2008, all states and territories had implemented the confidential, name-based reporting method used for all other reportable infectious diseases (CDC, 2008d). The national HIV surveillance system will soon be able to achieve case counts with no duplicates among jurisdictions through an interstate reciprocal notification system wherein CDC provides quarterly reports on cases that might be duplicated between states on the basis of matching soundex codes,2 dates
of birth, race, and sex. There is also guidance and sufficient staffing to be able to investigate cases of public-health importance, including clusters of unusual clinical, laboratory, or geographic occurrences; cases with unusual modes of transmission; cases without detectable antibody response; and cases with unusual strains of HIV, such as HIV-2 and non-B subtypes.
Another aspect of the HIV surveillance model is that all jurisdictions use standardized HIV/AIDS case-report forms. Specific information must be completed before the software system will classify an entry as a case; this information includes laboratory or provider diagnosis confirmation, and patient’s date of birth, race, ethnicity, and sex. The case-report form includes the collection of behavioral-risk information, measures of immunologic function (CD4+ cell count and percentage), and viral load.
Most important, the HIV model includes process and outcome standards that all jurisdictions must strive to achieve (CDC, 1999). The outcome standards include completeness, timeliness, accuracy, risk ascertainment, and collection of first CD4+ cell count. Because the new software system is document-based, it will enable evaluation of the completeness of national case ascertainment with a capture-recapture method (Hall et al., 2006). The resulting information can be used to determine weaknesses in the reporting system and to help interpret data appropriately.
Finally, the process and outcome standards have been incorporated into CDC’s updated framework for evaluating public-health surveillance systems (Hall and Mokotoff, 2007). CDC assesses national HIV surveillance data against the required outcome standards annually. The HIV/AIDS surveillance evaluation framework promotes continuous improvement in the quality of data through technical guidance, measurement of performance, reporting of assessment results to state and local health departments, and adjustments in guidance, training, or technical assistance according to assessment results.
The cooperative agreement and the associated funding have allowed the development of the national HIV surveillance system. Both are imperative for the development of an accurate, timely, and complete hepatitis surveillance system that will provide accurate incidence and prevalence data to inform proper resource allocation, program development and evaluation, and policy-making.
The following section details the committee’s recommended model for structuring surveillance for hepatitis B and hepatitis C.
MODEL FOR SURVEILLANCE
The committee recommends that a two-tiered model be developed: core surveillance and targeted surveillance. The initial focus of the program should be the development and implementation of standardized systems
among all states to maximize their capacity to perform core surveillance for acute and chronic HBV and HCV infection. Standardization will be accomplished through cooperative agreements, improved guidance, and adequate and consistent funding. Systems should be integrated into existing HIV or other disease surveillance infrastructure where feasible. Complementary efforts need to be made in building enhanced supplemental surveillance systems to describe trends in underrepresented at-risk populations better and to address the gaps identified in the current surveillance system. Both types of surveillance activities will provide better information to policymakers and service-delivery systems to improve care for people who are at risk for or living with HBV or HCV infection. Changes should be phased and prioritized, with the first step focused on the development and funding of core surveillance systems for each state.
Core Surveillance
Core surveillance—including collection, processing, analysis, and dissemination of data on cases of acute and chronic HBV and HCV infection—is needed in all states. Because of the public-health importance of quick identification of outbreaks and nosocomial transmission, acute-disease surveillance has had the highest priority in surveillance programs in the past. However, chronic-disease surveillance is also critical in that, if funded appropriately, it will assist in the recognition of acute cases, aid in moving people with recent diagnoses into appropriate care, contribute to an increased understanding of disease burden, allow evaluation of prevention efforts, and, given appropriate case management, save on costs associated with treatment of patients who have cirrhosis, hepatocellular carcinoma, or liver transplantation. Proper chronic-disease surveillance can also improve acute-disease surveillance by enhancing the accuracy and efficiency of related data collection. Evaluation of the core surveillance system should be ongoing to ensure that it is meeting emerging needs.
Funding Mechanism
In the proposed model, the state would be the primary unit of surveillance. Funding should be earmarked for viral-hepatitis surveillance through cooperative agreements with the states. CDC should ensure that all states have sufficient infrastructure to identify and appropriately investigate all suspected cases of acute and chronic HBV and HCV infection. Cooperative agreements should require reporting of standardized viral-hepatitis surveillance data within 3 years of implementation. The agreements should include funding for states to hire staff to process laboratory results, enter data, and follow up cases of acute and chronic HBV and HCV infections.
Case Definitions
CDC should revise and standardize definitions and methods. Revised case definitions should reflect active and resolved hepatitis C infection (for example, a case should not be confirmed if only antibody test results are available). Recommended testing for hepatitis C should include, where possible, HCV RNA tests to determine whether a person is actively infected. The case definition for acute HBV and HCV infection should be revised to remove the need for symptoms for classification as a confirmed case. Classification as a suspected case of acute HCV infection should be used to encourage active followup of likely recent infections (for example, in adolescents and young adults) (CDC, 2008f).
Case-Reporting Form
The case-reporting form should be standardized, and core components of it should be required of all jurisdictions to permit better capture of information on cases of acute and chronic HCV and HBV infection. The required elements should be such that they could reasonably be found in a patient’s medical record. For example, the current CDC form requests the number of sexual partners in a given period. That information is not typically found in a medical record or known by a medical provider. Additional, more comprehensive epidemiologic studies could be funded to provide for patient interviews and a detailed assessment of risk factors (see Recommendation 2-3). Furthermore, the case-reporting form should collect more detailed demographic data on racial and ethnic populations to identify and address disparities among populations. For example, the case-reporting form should include categories for different ethnicities and should disaggregate Asians and Pacific Islanders (for example, Chinese, Vietnamese, Japanese, and Marshallese).
Automated Data-Collection Systems
Automated or passive methods of accessing and processing test results should be supported and improved. Enhancing and expanding automated methods of collecting data (for example, Web-based disease-reporting systems, electronic laboratory reporting, and electronic medical records) reduce staff time, increase timeliness and completeness, and minimize data-entry errors (Klevens et al., 2009; Klompas et al., 2008; Lazarus et al., 2001; Panackal et al., 2002; Vogt et al., 2006; Wurtz and Cameron, 2005). Given the volume of viral-hepatitis data, automated systems clearly are indicated (Hopkins, 2005). However, it has been noted that although electronic laboratory reporting can greatly increase the timeliness and accuracy of
reporting, it does not remove the need for health departments to conduct additional followup to obtain information not contained in laboratory reports, such as symptoms, race and ethnicity, and risk history (Hopkins, 2005; Klevens et al., 2009).
A pilot study of a surveillance system based on electronic medical records in Massachusetts found a 39% increase in reported cases of chlamydia and a 53% increase in reported cases of gonorrhea over a 12-month period compared with cases reported through the existing passive surveillance system. The system was also able to identify 81 instances of pregnancy not identified by passive surveillance in patients with chlamydia or gonorrhea (CDC, 2008b). The system was shown to identify cases of acute HBV infection reliably, including cases that had not yet been reported to state authorities (Klompas et al., 2008). Other studies have found a similar benefit of improving surveillance for infectious diseases via automatic notification with electronic medical records (Allen and Ferson, 2000; Hopkins, 2005). CDC should promote the use of surveillance systems based on electronic medical records and open-source platforms that will enable the extraction and transmission of data to state and local health departments.
Standardized Laboratory Reporting It is essential that laboratory data be standardized and that health departments have automated access to them. Automated electronic laboratory reporting improves the completeness and timeliness of disease surveillance (Effler et al., 1999, 2002; Overhage et al., 2008; Panackal et al., 2002; Ward et al., 2005). Currently, many laboratory-data collection systems do not integrate or link the multiple laboratory tests needed to satisfy a case definition (CDC, 2008b). That could be more easily addressed with electronic laboratory reporting. CDC should work with states and laboratories to develop and standardize electronic systems. In addition, it may be useful for CDC to document and monitor which laboratory tests are reportable in each state, as is done for the HIV surveillance system.
Identifying Pregnant Women There is a strong need to identify pregnant women who have chronic HBV to ensure that appropriate followup of the newborn is conducted with regard to receipt of HBIG and hepatitis B vaccine. Currently, most health departments lack an automated means of determining whether the subject of a reported positive HBsAg test was a pregnant woman. Local health departments have to investigate all positive hepatitis B tests in women of childbearing age, and this creates a substantial workload. CDC should work with national laboratory vendors to identify ways of reporting whether positive HBV tests are linked with prenatal panels. Web-based surveillance systems may be useful for improving capture of data on pregnant women who have HBV infection (LaPorte et al., 2008).
PHIN-Compliant Systems CDC needs to contribute to more timely development of PHIN-compliant systems in all jurisdictions. A review of the literature evaluating the timeliness of reporting of infectious diseases found that reporting lag and the variability among states limit the usefulness of data. The inconsistency in reporting limits CDC’s ability to identify and respond to multistate outbreaks in a timely manner. The review called for a more standardized approach in evaluating and describing surveillance-system timeliness (Jajosky and Groseclose, 2004). Although it did not look specifically at hepatitis B or hepatitis C, its conclusions are relevant to the present report.
Electronic Medical Records The reporting of relevant infectious-disease test results should be a component of electronic medical-record systems. CDC should support state and local jurisdictions in working with clinical and community health-center partners to develop algorithms for automatic viral-hepatitis disease reporting based on electronic medical records. It has already been shown to be effective in enhancing acute-HBV reporting without adding to the burden on medical providers (Klompas et al., 2008).
Case Investigation and Followup
Standards for case investigation and followup should be developed and implemented to ensure that newly diagnosed patients receive adequate information and referrals. An effective surveillance system should identify most of the diagnosed cases of both acute and chronic HBV and HCV infections. Identification of infected people by health departments should be the first step in getting them into appropriate care. Because of resource and system inadequacies, it is not. Most health departments indicated that they were unable to do more than follow up on potentially pregnant HBV-positive women (personal communication, Adult Viral Hepatitis Prevention Coordinators, May 2009). If state health departments had appropriate funding to follow up recently diagnosed cases of HBV and HCV infection directly, more people would be able to receive appropriate education and referral into the array of medical and social-service care that may be indicated.
Analyzing, Reporting, and Disseminating Findings
Once the capacity for state health departments to conduct HBV and HCV surveillance is improved, CDC should report accurate results that are based on the improved data. As discussed earlier in this chapter, there are important concerns about underreporting, particularly of the incidence of
acute HCV infection. Until the quality of the data collected has improved, reports should clearly indicate the limitations of the data. For example,
-
Trends in acute HBV and HCV infections should be interpreted with caution because of systematically missing cases that represent the burden of disease in particular risk groups.
-
Discussions of data on acute HBV and HCV infections should reflect the issue of the large number of chronic infections to ensure appropriate understanding of the scope of the problem.
-
Reported incidences should be presented as ranges rather than single numbers to reflect the uncertainty of the estimates.
Targeted Surveillance
Once core hepatitis B and hepatitis C surveillance activities are well established, supplemental or pilot projects should be tested. CDC should develop and support innovative supplemental surveillance programs.
Recommendation 2-3. The Centers for Disease Control and Prevention should support and conduct targeted active surveillance, including serologic testing, to monitor incidence and prevalence of hepatitis B virus and hepatitis C virus infections in populations not fully captured by core surveillance.
-
Active surveillance should be conducted in specific (sentinel) geographic regions and populations.
-
Appropriate serology, molecular biology, and followup will allow for distinction between acute and chronic hepatitis B and hepatitis C.
Enhanced Surveillance
Supplemental surveillance projects should be funded or conducted by CDC and should include serosurveillance among targeted populations. Serosurveillance projects will provide data for improved estimation of the scope of the problem in underrepresented populations such as certain racial and ethnic groups, and at-risk populations, including institutionalized, homeless, immigrant, and refugee populations. Enhanced surveillance projects should be structured to obtain information in both rural and urban regions of the United States. Serosurveillance programs should be flexible and allow researchers to focus on emerging behavioral risks, for example, in adolescents and young adults and in HIV-positive men who have sex with men (Klevens et al., 2009). Conducting serosurveillance or screening among at-risk populations in correctional facilities may provide opportunities to
collect more detailed data and to refer people directly into appropriate medical care, including treatment for acute HCV infection (McGovern et al., 2006). Other enhanced surveillance projects should include
-
Determining the level of care that patients receive after diagnosis, including medical and social-service referrals and treatment (Fleming et al., 2006).
-
Following subsets of cases to improve understanding of natural history (Global Burden of Hepatitis C Working Group, 2004).
-
Matching data on chronic hepatitis B and hepatitis C with cancer registries (Global Burden of Hepatitis C Working Group, 2004).
-
Matching data on chronic HBV and HCV infections with HIV/AIDS data to determine the burden of coinfection in communities.
-
Measuring the vaccination status of acute HBV infection cases and identifying missed opportunities for vaccination.
-
Ensuring that viral hepatitis is addressed and integrated with appropriate projects for the National HIV Behavioral Surveillance System.
-
Measuring HBV and HCV seroconversion rates in selected populations.
Partner Services
Partner services have been found to be effective in identifying un-diagnosed cases of HIV (Hogben et al., 2007). Similar programs could potentially be useful identifying cases of hepatitis B and hepatitis C (CDC, 2008e; Hogben and Niccolai, 2009; Marcus et al., 2009). State and local health departments should be funded to pilot and evaluate partner-services programs for suspected acute and chronic cases of HBV infection and acute cases of HCV infection, especially in young people. Integration with existing partner service programs should be explored. Evaluation should focus on the efficacy of referral into care services and on screening of exposed partners—sexual partners for hepatitis B and drug-sharing partners for hepatitis B and hepatitis C (CDC, 2007).
REFERENCES
Allen, C. J., and M. J. Ferson. 2000. Notification of infectious diseases by general practitioners: A quantitative and qualitative study. Medical Journal of Australia 172(7):325-328.
Alter, M. J., W. L. Kuhnert, and L. Finelli. 2003. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. Morbidity and Morality Weekly: Recommendations and Reports 52(RR-3):1-13, 15; quiz CE11-CE14.
CDC (Centers for Disease Control and Prevention). 1996. Prevention of perinatal hepatitis B through enhanced case management—Connecticut, 1994-95, and the United States, 1994. Morbidity and Mortality Weekly Report 45(27):584-587.
———. 1999. Guidelines for national human immunodeficiency virus case surveillance, including monitoring for human immunodeficiency virus infection and acquired immunodeficiency syndrome. Centers for Disease Control and Prevention. Morbidity and Morality Weekly: Recommendations and Reports 48(RR-13):1-27, 29-31.
———. 2001a. National hepatitis C prevention strategy: A comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences. Atlanta, GA: CDC. http://www.cdc.gov/hepatitis/HCV/Strategy/PDFs/NatHepCPrevStrategy.pdf (accessed August 21, 2009).
———. 2001b. Updated guidelines for evaluating public health surveillance systems: Recommendations from the guidelines working group. Morbidity and Mortality Weekly Report 50(RR-13):1-36.
———. 2003a. 2003-2008 HIV prevention community planning guidance. http://www.cdc.gov/hiv/topics/cba/resources/guidelines/hiv-cp/pdf/hiv-cp.pdf (accessed May 18, 2009).
———. 2003b. Hepatitis C virus transmission from an antibody-negative organ and tissue donor—United States, 2000-2002. Morbidity and Mortality Weekly Report 52(13):273-274, 276.
———. 2003c. Prevention and control of infections with hepatitis viruses in correctional settings. Morbidity and Mortality Weekly Report 52(RR01):1-33.
———. 2003d. Transmission of hepatitis B and C viruses in outpatient settings—New York, Oklahoma, and Nebraska, 2000-2002. Morbidity and Mortality Weekly Report 52(38): 901-906.
———. 2005a. Guidelines for viral hepatitis surveillance and case management. Centers for Disease Control and Prevention.
———. 2005b. Transmission of hepatitis B virus among persons undergoing blood glucose monitoring in long-term-care facilities—Mississippi, North Carolina, and Los Angeles county, California, 2003-2004. Morbidity and Mortality Weekly Report 54(9):220-223.
———. 2006 (unpublished). 2006 assessment tool: Hepatitis C coordination and integration activities.
———. 2007. Program announcement ps08-802: HIV/AIDS surveillance. http://www.cdc.gov/od/pgo/funding/PS08-802.htm (accessed August 19, 2009).
———. 2008a. Acute hepatitis C virus infections attributed to unsafe injection practices at an endoscopy clinic—Nevada, 2007. Morbidity and Mortality Weekly Report 57(19): 513-517.
———. 2008b. Automated detection and reporting of notifiable diseases using electronic medical records versus passive surveillance—Massachusetts, June 2006-July 2007. Morbidity and Mortality Weekly Report 57(14):373-376.
———. 2008c. Disease burden from hepatitis A, B, and C in the United States.
———. 2008d. HIV infection reporting. http://www.cdc.gov/hiv/topics/surveillance/reporting.htm (accessed July 28, 2009).
———. 2008e. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. Morbidity and Morality Weekly: Recommendations and Reports 57(RR-9):1-83; quiz CE81-84.
———. 2008f. Use of enhanced surveillance for hepatitis C virus infection to detect a cluster among young injection-drug users—New York, November 2004-April 2007. Morbidity and Mortality Weekly Report 57(19):517-521.
———. 2009a. Case definitions. http://www.cdc.gov/ncphi/disss/nndss/casedef/case_definitions.htm (accessed August 21, 2009).
———. 2009b. FAQs for health professionals: Hepatitis C. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm (accessed August 21, 2009).
———. 2009c. Hepatitis C virus transmission at an outpatient hemodialysis unit—New York, 2001-2008. Morbidity and Mortality Weekly Report 58(8):189-194.
———. 2009d. HIV-associated behaviors among injecting-drug users—23 cities, United States, May 2005-February 2006. Morbidity and Mortality Weekly Report 58(13):329-332.
———. 2009e. National electronic disease surveillance system. http://www.cdc.gov/nedss/ (accessed July 28, 2009).
———. 2009f. National electronic telecommunications system for surveillance. http://www.cdc.gov/ncphi/disss/nndss/netss.htm (accessed July 28, 2009).
———. 2009g. Perinatal hepatitis B prevention coordinators. http://www.cdc.gov/hepatitis/Partners/PeriHepBCoord.htm (accessed August 21, 2009).
———. 2009h. (unpublished) Report on the status of state viral hepatitis plans for the Institute of Medicine.
———. 2009i. Status of state electronic disease surveillance systems—United States, 2007. Morbidity and Mortality Weekly Report 58(29):804-807.
CDC/CSTE. 2006. Technical guidance for HIV/AIDS surveillance programs, volumes I-III Centers for Disease Control and Prevention.
Chen, S. L., and T. R. Morgan. 2006. The natural history of hepatitis C virus (HCV) infection. International Journal of Medical Sciences 3(2):47-52.
Coleman, P. J., G. M. McQuillan, L. A. Moyer, S. B. Lambert, and H. S. Margolis. 1998. Incidence of hepatitis B virus infection in the United States, 1976-1994: Estimates from the national health and nutrition examination surveys. Journal of Infectious Diseases 178(4):954-959.
Cox, A. L., D. M. Netski, T. Mosbruger, S. G. Sherman, S. Strathdee, D. Ompad, D. Vlahov, D. Chien, V. Shyamala, S. C. Ray, and D. L. Thomas. 2005. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clinical Infectious Diseases 40(7):951-958.
CSTE (Council of State and Territorial Epidemiologists). 2009. State reportable conditions assessment (SRCA). http://www.cste.org/dnn/ProgramsandActivities/PublicHealthInformaticsOLD/StateReportableConditionsQueryResults/tabid/261/Default.aspx (accessed December 15, 2009).
Daniels, D., S. Grytdal, and A. Wasley. 2009a. Surveillance for acute viral hepatitis—United States, 2007. Morbidity and Mortality Weekly Report: Surveillance Summaries 58(3):1-27.
———. 2009b. Surveillance for acute viral hepatitis—United States, 2007. Morbidity and Mortality Weekly Report: Surveillance Summaries 58(3):1-27.
Department of Justice. 2009. Office of Justice Programs, Bureau of Justice Statistics. http://www.ojp.usdoj.gov/bjs/ (accessed August 30, 2009).
Deuffic-Burban S., T. Poynard, M. S. Sulkowski, and J. B. Wong. 2007. Estimating the future health burden of chronic hepatitis C and human immunodeficiency virus infections in the United States. Journal of Viral Hepatitis 14(2):107-115.
Effler, P., M. Ching-Lee, A. Bogard, M.-C. Ieong, T. Nekomoto, and D. Jernigan. 1999. Statewide system of electronic notifiable disease reporting from clinical laboratories: Comparing automated reporting with conventional methods. Journal of the American Medical Association 282(19):1845-1850.
Effler, P. V., M. C. Ieong, T. Tom, and M. Nakata. 2002. Enhancing public health surveillance for influenza virus by incorporating newly available rapid diagnostic tests. Emerging Infectious Diseases 8(1):23-28.
Fabrizi, F., A. Marzano, P. Messa, P. Martin, and P. Lampertico. 2008. Hepatitis B virus infection in the dialysis population: Current perspectives. International Journal of Artificial Organs 31(5):386-394.
Fairchild, A. L., R. Bayer, and J. Colgrove. 2008. Privacy, democracy and the politics of disease surveillance. Public Health Ethics 1(1):30-38.
Fleming, D. T., A. Zambrowski, F. Fong, A. Lombard, L. Mercedes, C. Miller, J. Poujade, A. Roome, A. Sullivan, and L. Finelli. 2006. Surveillance programs for chronic viral hepatitis in three health departments. Public Health Reports 121(1):23-35.
George, P. 2004 (unpublished). New directions for hepatitis surveillance. Division of Viral Hepatitis, CDC.
Ghany, M. G., D. B. Strader, D. L. Thomas, and L. Seeff. 2009. Diagnosis, management, and treatment of hepatitis c: An update. Hepatology 49(4):1335-1374.
Global Burden of Hepatitis C Working Group, GBHCWG. 2004. Global burden of disease (GBD) for hepatitis C. Journal of Clinical Pharmacology 44(1):20-29.
Glynn, M. K., L. M. Lee, and M. T. McKenna. 2007. The status of national HIV case surveillance, United States 2006. Public Health Reports 122(Suppl 1):63-71.
Guerrant, R. L., D. H. Walker, and P. F. Weller, eds. 2001. Essentials of tropical infectious diseases. Philadelphia, PA: Churchill Livingstone.
Hagan, H., N. Snyder, E. Hough, T. Yu, S. McKeirnan, J. Boase, and J. Duchin. 2002. Case-reporting of acute hepatitis B and C among injection drug users. Journal of Urban Health 79(4):579-585.
Hall, H. I., and E. D. Mokotoff. 2007. Setting standards and an evaluation framework for human immunodeficiency virus/acquired immunodeficiency syndrome surveillance. Journal of Public Health Management and Practice 13(5):519-523.
Hall, H. I., R. Song, J. E. Gerstle, 3rd, and L. M. Lee. 2006. Assessing the completeness of reporting of human immunodeficiency virus diagnoses in 2002-2003: Capture-recapture methods. American Journal of Epidemiology 164(4):391-397.
Hogben, M., and L. M. Niccolai. 2009. Innovations in sexually transmitted disease partner services. Current Infectious Disease Reports 11(2):148-154.
Hogben, M., T. McNally, M. McPheeters, and A. B. Hutchinson. 2007. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals a systematic review. American Journal of Preventive Medicine 33(2 Suppl): S89-S100.
Hopkins, R. S. 2005. Design and operation of state and local infectious disease surveillance systems. Journal of Public Health Management and Practice 11(3):184-190.
Hutton, D. W., D. Tan, S. K. So, and M. L. Brandeau. 2007. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Annals of Internal Medicine 147(7):460-469.
Hyams, K. C. 1995. Risks of chronicity following acute hepatitis B virus infection: A review. Clinical Infectious Diseases 20(4):992-1000.
Jajosky, R. A., and S. L. Groseclose. 2004. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BMC Public Health 4:29.
Kim, W. R. 2007. Epidemiology of hepatitis B in the United States. Current Hepatitis Reports 6(1):3-8.
Klein, S. J., C. A. Flanigan, J. G. Cooper, D. R. Holtgrave, A. F. Carrascal, and G. S. Birkhead. 2008. Wanted: An effective public health response to hepatitis C virus in the United States. Journal of Public Health Management and Practice 14(5):471-475.
Klevens, R. M., C. Vonderwahl, S. Speers, K. Alelis, K. Sweet, E. Rocchio, T. Poissant, T. Vogt, and K. Gallagher. 2009 (unpublished). Hepatitis C virus infection from population-based surveillance in six U.S. sites, 2006-2007.
Klompas, M., G. Haney, D. Church, R. Lazarus, X. Hou, and R. Platt. 2008. Automated identification of acute hepatitis b using electronic medical record data to facilitate public health surveillance. PLoS ONE 3(7):e2626.
Koff, R. S. 2004. Hepatitis B and hepatitis D. In Infectious diseases, edited by S. L. Gorbach, J. G. Bartlett, and N. R. Blacklow. Philadelphia, PA: Lippincott, Williams & Wilkins. Pp. 765-784.
Lanini, S., V. Puro, F. Lauria, F. Fusco, C. Nisii, and G. Ippolito. 2009. Patient to patient transmission of hepatitis B virus: A systematic review of reports on outbreaks between 1992 and 2007. BMC Medicine 7(1):15.
LaPorte, T., M. Conant, M. McGarty, S. Troppy, S. Barrus, S. Lett, M. O’Donnell, G. Haney, and A. DeMaria. 2008 (March). Improved case finding of hepatitis B positive women of child-bearing age through implementation of a web-based surveillance system. Paper presented at National Immunization Conference, Atlanta, Georgia.
Lazarus, R., K. P. Kleinman, I. Dashevsky, A. DeMaria, and R. Platt. 2001. Using automated medical records for rapid identification of illness syndromes (syndromic surveillance): The example of lower respiratory infection. BMC Public Health 1:9.
Maher, L., B. Jalaludin, K. G. Chant, R. Jayasuriya, T. Sladden, J. M. Kaldor, and P. L. Sargent. 2006. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction 101(10):1499-1508.
Marcus, J. L., K. T. Bernstein, and J. D. Klausner. 2009. Updated outcomes of partner notification for human immunodeficiency virus, San Francisco, 2004-2008. AIDS 23(8): 1024-1026.
Margolis, H. S., P. J. Coleman, R. E. Brown, E. E. Mast, S. H. Sheingold, and J. A. Arevalo. 1995. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. Journal of the American Medical Association 274(15):1201-1208.
Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer, B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices (ACIP) part 1: Immunization of infants, children, and adolescents. Morbidity and Morality Weekly: Recommendations and Reports 54(RR-16):1-31.
Mathei, C., G. Robaeys, P. van Damme, F. Buntinx, and R. Verrando. 2005. Prevalence of hepatitis C in drug users in Flanders: Determinants and geographic differences. Epidemiology and Infection 133(1):127-136.
Matthews, G. V., M. Hellard, J. Kaldor, A. Lloyd, and G. J. Dore. 2007. Further evidence of HCV sexual transmission among HIV-positive men who have sex with men: Response to Danta et al. AIDS 21(15):2112-2113.
McGovern, B. H., C. E. Birch, M. J. Bowen, L. L. Reyor, E. H. Nagami, R. T. Chung, and A. Y. Kim. 2009. Improving the diagnosis of acute hepatitis C infection using expanded viral load criteria. Clinical Infectious Diseases 49.
McGovern, B. H., A. Wurcel, A. Y. Kim, J. Schulze zur Wiesch, I. Bica, M. T. Zaman, J. Timm, B. D. Walker, and G. M. Lauer. 2006. Acute hepatitis C virus infection in incarcerated injection drug users. Clinical Infectious Diseases 42(12):1663-1670.
McMahon, B. J., W. L. M. Alward, D. B. Hall, W. L. Heyward, T. R. Bender, D. P. Francis, and J. E. Maynard. 1985. Acute hepatitis B virus infection: Relation of age to the clinical expression of disease and subsequent development of the carrier state. Journal of Infectious Diseases 151(4):599-603.
Mehta, S. H., A. Cox, D. R. Hoover, X. H. Wang, Q. Mao, S. Ray, S. A. Strathdee, D. Vlahov, and D. L. Thomas. 2002. Protection against persistence of hepatitis C. Lancet 359(9316):1478-1483.
Mosley, J. W., E. A. Operskalski, L. H. Tobler, W. W. Andrews, B. Phelps, J. Dockter, C. Giachetti, M. P. Busch, f. t. T.-t. V. Study, and R. E. D. S. Groups. 2005. Viral and host factors in early hepatitis C virus infection. Hepatology 42(1):86-92.
Nakashima, A. K., and P. L. Fleming. 2003. HIV/AIDS surveillance in the United States, 1981-2001. Journal of Acquired Immune Deficiency Syndromes 32(Suppl 1):S68-S85.
NASTAD (National Alliance of State and Territorial AIDS Directors). 2009 (unpublished). Viral hepatitis surveillance survey.
NIH (National Institutes of Health). 2002. NIH consensus statement on management of hepatitis C: 2002. NIH Consensus and State-of-the-Science Statements 19(3):1-46.
Onofrey, S., D. Church, D. Heisey-Grove, P. Briggs, T. Bertrand, and A. J. DeMaria. March 10-13, 2008. Utilizing disease intervention specialist for follow-up on hepatitis C in individuals between the ages of 15 and 25 years: A 3-month pilot program. Paper presented at 2008 National STD Prevention Conference, Chicago, IL.
Overhage, J. M., S. Grannis, and C. J. McDonald. 2008. A comparison of the completeness and timeliness of automated electronic laboratory reporting and spontaneous reporting of notifiable conditions. American Journal of Public Health 98(2):344-350.
Panackal, A. A., M. M’Ikanatha N, F. C. Tsui, J. McMahon, M. M. Wagner, B. W. Dixon, J. Zubieta, M. Phelan, S. Mirza, J. Morgan, D. Jernigan, A. W. Pasculle, J. T. Rankin, Jr., R. A. Hajjeh, and L. H. Harrison. 2002. Automatic electronic laboratory-based reporting of notifiable infectious diseases at a large health system. Emerging Infectious Diseases 8(7):685-691.
Pungpapong, S., W. R. Kim, and J. J. Poterucha. 2007. Natural history of hepatitis B virus infection: An update for clinicians. Mayo Clinic Proceedings 82(8):967-975.
Ruan, Y., G. Qin, L. Yin, K. Chen, H. Z. Qian, C. Hao, S. Liang, J. Zhu, H. Xing, K. Hong, and Y. Shao. 2007. Incidence of HIV, hepatitis C and hepatitis B viruses among injection drug users in southwestern China: A 3-year follow-up study. AIDS 21(Suppl 8): S39-S46.
Thacker, S. B. 2000. Historical development. In Principles and Practice of Public Health Surveillance, edited by S. Teutsch and R. E. Churchill. Oxford, United Kingdom: Oxford University Press.
Thompson, N. D., J. F. Perz, A. C. Moorman, and S. D. Holmberg. 2009. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998-2008. Annals of Internal Medicine 150(1):33-39.
van de Laar, T., O. Pybus, S. Bruisten, D. Brown, M. Nelson, S. Bhagani, M. Vogel, A. Baumgarten, M. L. Chaix, M. Fisher, H. Gotz, G. V. Matthews, S. Neifer, P. White, W. Rawlinson, S. Pol, J. Rockstroh, R. Coutinho, G. J. Dore, G. M. Dusheiko, and M. Danta. 2009. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 136(5):1609-1617.
van den Berg, C. H., C. Smit, M. Bakker, R. B. Geskus, B. Berkhout, S. Jurriaans, R. A. Coutinho, K. C. Wolthers, and M. Prins. 2007. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. European Journal of Epidemiology 22(3):183-193.
Villano, S. A., D. Vlahov, K. E. Nelson, S. Cohn, and D. L. Thomas. 1999. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 29(3):908-914.
Vogt, M., T. Lang, G. Frosner, C. Klingler, A. F. Sendl, A. Zeller, B. Wiebecke, B. Langer, H. Meisner, and J. Hess. 1999. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. New England Journal of Medicine 341(12):866-870.
Vogt, R. L., R. Spittle, A. Cronquist, and J. L. Patnaik. 2006. Evaluation of the timeliness and completeness of a web-based notifiable disease reporting system by a local health department. Journal of Public Health Management and Practice 12(6):540-544.
Ward, J. 2008. FY 2008 domestic enacted funds. Presentation to the committee: December 4, 2008.
Ward, M., P. Brandsema, E. van Straten, and A. Bosman. 2005. Electronic reporting improves timeliness and completeness of infectious disease notification, the Netherlands, 2003. Euro Surveillance 10(1):27-30.
Wasley, A., J. T. Miller, and L. Finelli. 2007. Surveillance for acute viral hepatitis—United States, 2005. Morbidity and Mortality Weekly Report: Surveillance Summaries 56(3):1-24.
Wurtz, R., and B. J. Cameron. 2005. Electronic laboratory reporting for the infectious diseases physician and clinical microbiologist. Clinical Infectious Diseases 40(11):1638-1643.






































