4
Vaccine Supply and Use
Goal 4 in the draft National Vaccine Plan—ensure a stable supply of recommended vaccines and achieve better use of existing vaccines to prevent disease, disability, and death in the United States—covers an extraordinarily broad set of issues. Objectives include topics related to every point along the journey from the manufacturer’s production facility to the prospective recipient of the vaccine: supply; purchase, financing, and reimbursement for vaccines; vaccine management and administration; availability of and access to services; compensation for vaccine injuries; and data and information technology needs (from provider-level information technology to disease surveillance, immunization coverage, and safety surveillance capabilities). Also, vaccine supply and use issues are intertwined with safety (covered under Goal 2 of the plan and discussed in Chapter 2), research and development (covered under Goal 1 and discussed in Chapter 1), and communication issues (covered under Goal 3 and discussed in Chapter 3).
Goal 4 illustrates a characteristic of the entire plan: the absence of an explicit vision statement and an extremely broad range of objectives and strategies without explanation of (1) why certain items were included in the plan and what remained on the “cutting room floor,” (2) which items represent activities that are budgeted agency strategic priorities and expected to take place regardless of the National Vaccine Plan, and (3) which items represent novel contributions of the plan that are not explicitly part of other existing (agency) plans.
When formulating its recommendations on priority actions in Goal 4, the committee considered the implications of current efforts to reorganize the U.S. health care delivery system to support payment systems and ensure
delivery of vaccines and to make concrete advances in the use of health information technology (HIT) to improve health care performance and effectiveness. Although the fate of health care reform is uncertain at the time of this writing, considerable progress has been made with regard to HIT by building on the foundation set in 2004 by the President’s Executive Order 13335, establishing the Office of the National Coordinator for Health Information Technology (ONCHIT) in the Department of Health and Human Services (HHS), whose role is to lead the implementation of a nationwide HIT infrastructure that is interoperable and safeguards privacy (GAO, 2009). Changes in the ways health information is recorded, stored, and used can have enormous implications for the delivery of immunization services.
Vaccination is a cost-effective, high-value component of preventive health care and is a good indicator of how well a health care delivery system functions. Under ideal circumstances, a health information system would indicate a patient’s immunization status, remind a provider of needed vaccines for a given patient, record and facilitate the reporting of potential adverse events following immunization, help a provider obtain reimbursement for delivery of immunization services, allow public health officials and researchers to measure vaccine coverage, monitor rates of vaccine-preventable diseases, and facilitate studies of the relationship between vaccines and suspected adverse events. In reality, neither the delivery of health care nor the relevant information technology systems are constituted in ways that optimize the delivery of immunization among other preventive services.
OVERVIEW OF THE NATION’S IMMUNIZATION SERVICES
As noted in the Introduction, the terms vaccination and immunization are sometimes used interchangeably. The committee uses vaccination to refer to the delivery of the vaccine to an individual, and immunization services to refer to the range of activities (e.g., storage and management of vaccine stocks, communication) that lead to vaccine administration. The Introduction also describes the large network of federal, state, and local public health agencies that play important roles in implementing the use of vaccines routinely to prevent infectious diseases and to respond to public health emergencies such as disease outbreaks and the 2009 H1N1 influenza pandemic. Although the federal government provides advice, support, and funding, most immunization policy is made at the state level, thus stakeholders in this area include organizations such as the Association of State and Territorial Health Officials and National Conference of State Legislatures.
As described in Chapter 2, after vaccines are licensed by the Food and Drug Administration (FDA), they can be used in the population according to the recommendations of the Advisory Committee on Immunization Prac-
tices (ACIP), which is authorized by the Public Health Service Act to provide advice and guidance to the Secretary of Health and Human Services, the Assistant Secretary for Health, and the director of the Centers for Disease Control and Prevention (CDC).
FRAMING OF GOAL 4
The nine objectives (see Box 4-1) in this goal range widely from ensuring consistent and adequate availability of vaccines to maintaining “a strong, science-based process for developing and evaluating immunization recommendations” (see Chapter 3, which discusses the importance of better communication of how immunization policies are made).
This chapter offers discussion and recommendations intended to help focus Goal 4 on addressing a narrower set of challenges and on a priority action pertaining to each major challenge to the effective use of vaccines for children, adolescents, and adults. Major types of challenges are described below.
Some barriers to effective use of vaccines stem from the lack of affordability of certain newer vaccines (e.g., HPV [human papilloma virus] vaccines recommended for young women, varicella zoster vaccine recommended for older adults) for significant numbers of patients. Not all private insurers cover such vaccines, and patients may be unwilling or unable to incur an out-of-pocket cost.
There are challenges that stem from the failure of health care financing (whether via public and private insurance or through direct grant financing by the federal government) to ensure that health care providers are adequately reimbursed for the purchase and provision of vaccines (Freed et al., 2008a,b). Related to these challenges are problems associated with vaccine production and interruption of the supply of vaccines available (Hinman et al., 2006; IOM, 2003). Another Institute of Medicine (IOM) committee has described the “tensions [that] exist between the need to control public and private expenditures on vaccines and the need to encourage investment in their development” (IOM, 2003). In other words, inadequate financing for vaccines and related costs have played a role in decreasing the attractiveness of the vaccine market to companies and investors.
Another category of challenges relates to system performance in the delivery of immunization services. Health plans have had some success using pay-for-performance approaches to incentivize provider practices that led to increased immunization rates (AHIP, 2009). However, incentives for high performance in immunization within the health care system are lacking (Berman, 2005), as is clear evidence about what works to motivate high performance. The Centers for Medicare & Medicaid Services (CMS) require managed care organizations to submit Healthcare Effectiveness Data
|
BOX 4-1 Goal 4 Objectives in the 2008 Draft National Vaccine Plan
SOURCE: HHS, 2008. |
and Information Set (HEDIS)1 data for Medicare enrollees (i.e., Medicare Advantage). HEDIS contains several measures of immunization status.
Challenges pertaining to health information systems affect immunization. Local public health agencies cannot adequately measure local-level immunization2 patterns to guide appropriate targeted supplemental population interventions. Gaps in local-level data are currently due to incompleteness of immunization registries and the expense of sample surveys; in the future widespread use of electronic health records and adequate national health information network infrastructure should facilitate monitoring coverage of immunization services. Exceptions that point the way forward may be found in the Veterans’ Health Administration health information system and
Louisiana state immunization information systems that survived Hurricane Katrina and facilitated the delivery of care including enabling providers to determine a patient’s immunization status and provide needed vaccinations (Bristol, 2005; Urquhart et al., 2007).
There are challenges stemming from gaps in knowledge regarding the balance of vaccine risks and benefits for individuals and for society. These challenges affect the behavior of both providers and patients (or parents) within the system, and include failure to provide immunizations in clinical practice settings, and avoiding age-appropriate vaccinations. These challenges are exacerbated by a health care delivery system that not only lacks incentives for providers but also in fact does not even reimburse providers for conversations with patients or parents on the topic of immunization and vaccines.
Finally, there are challenges having to do with preparedness for naturally occurring or deliberately introduced infectious disease threats. In a public health emergency, such as a disease outbreak, the capabilities of public health agencies at all levels are tested, including all aspects of their ability to mount mass vaccination efforts, such as the availability of vaccine, distribution of vaccine, identification of unvaccinated individuals, administration to appropriate populations, and monitoring potential adverse events and the spread of disease. Not only are vaccine shortages a concern in a response to an outbreak, but a shortage may itself precipitate a potential public health crisis. A recent example may be found in the response to the 2004-2005 influenza vaccine shortage, and the decision making at different levels of government and in the private sector regarding allocation of scarce vaccine. This is also an area in which coordination among all public health agencies is essential, and the influenza vaccine shortage highlighted both positive aspects and areas in need of improvement.
It is important that Goal 4 address these challenges faced by our nation’s immunization system with a clear and coherent vision of what is needed to ensure the effective use of vaccines to prevent and control infectious diseases. A comprehensive reframing of Goal 4 to focus on priority areas germane to the range of challenges presented above would strengthen the plan. Such clarity is necessary to develop measurable performance standards that can be translated into action. The committee suggests key elements (shown in Figure 4-1) needed to achieve the vision of enabling effective use of vaccines.
This suggested reframing endeavors to address all dimensions of the problems relating to the effective use of vaccines, refers to the evidence presented to this committee as well as the findings of previous IOM studies (2000, 2003), and reflects the modernization of health information technology as applied to immunization services and the integration of immunization into broad health care reforms currently in progress.
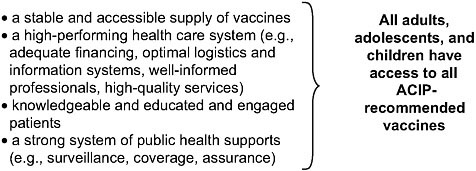
FIGURE 4-1 Prerequisites to achieve a vision for vaccine use.
To set strategic direction in Goal 4 of the National Vaccine Plan and ensure translation into effective action over time, the goal could be restructured into a smaller set of broad sub-goals, each of which would have its own set of objectives. The following five sub-goals are based on the committee’s information-gathering activities and a review of the literature, including past IOM reports.
|
Sub-goal 1. |
Assure a stable and adequate vaccine supply for public health preparedness and for recommended routine use purposes. |
|
Sub-goal 2. |
Eliminate financial barriers to vaccination. |
|
Sub-goal 3. |
Eliminate barriers related to access (for consumers) and to medical practice and delivery system functioning (for health professionals). Note that one specific non-financial3 barrier, knowledge regarding the safety and effectiveness of vaccination, is discussed in Chapter 3. |
|
Sub-goal 4. |
Develop and adopt health information technology systems that can advance clinical and public health immunization practice, measure clinical and system performance, advance knowledge about immunization status and system effectiveness in achieving high immunization rates and reducing immunization disparities, and support research on alleged adverse events and the potential link to immunization (see discussion of vaccine safety in Chapter 2). This sub-goal aims to assure better alignment between the National |
|
|
Vaccine Plan and existing planning efforts within HHS regarding HIT adoption, stemming from the American Reinvestment and Recovery Act (ARRA). |
|
Sub-goal 5. |
Strengthen the public health infrastructure to measure system performance, support high quality clinical practice that maintains or improves rates of disease and vaccine coverage, facilitate study of alleged adverse events (discussed in Chapter 2) and intervene to address disparities in health and health care, as well as emerging naturally occurring and intentional public health threats. |
SUB-GOAL 1:
SUPPLY
Vaccine shortages are, surprisingly, a predictably perennial problem of the U.S. immunization program. As an example, Coleman et al. (2005) found that “between 2000 and 2004 there were nationwide shortages of six recommended, childhood vaccines that prevent nine diseases, and the supply of adult influenza vaccine was interrupted three times.” More recently, the 2004-2005 influenza season coincided with a much-publicized serious shortage of influenza vaccine that required close public-private collaboration and coordination to ensure the best allocation of limited vaccine. Despite that coordination, there were problems with allocating available vaccine, and the season ended with 5 million doses of unused vaccine. In 2007, a recall of certain lots of Haemophilus influenzae b (Hib) conjugate vaccines (both as a single antigen and as a combination vaccine with meningitis and hepatitis B vaccines) led to a shortage, and CDC recommended deferral of a booster dose of Hib vaccine in most children who had already received the three-dose primary series (CDC, 2007b). The recommendation for a fourth (booster) dose was reinstated in June 2009, although supply was still not back to normal levels (AAFP, 2009).
Shortages occur for a complex set of reasons, such as the nature of the product and market (e.g., single manufacturers for some vaccines); manufacturing challenges with regulatory implications (e.g., contamination of vaccine lots); demand for a newly ACIP-recommended vaccine outstripping supply; and uncertain demand for seasonal influenza vaccine. Each shortage may have a somewhat different etiology (Hinman et al., 2006; Santoli et al., 2003) but all present communication and practice challenges for providers, confuse consumers, complicate the work of public health agencies at all levels, and place people at risk for contracting and spreading disease.
The committee recognizes that the draft plan contains an objective that addresses supply issues, and the recommendation below represents the committee’s agreement that this is an area that rises to the level of a priority.
Recommendation 4-1: The National Vaccine Plan should include the development and implementation of strategies to assure a stable and adequate vaccine supply for public health preparedness and recommended routine use purposes.
SUB-GOAL 2:
FINANCING BARRIERS IN THE UNITED STATES
There is a high level of consensus among stakeholders on the financial barriers to immunization (e.g., failure by payers and others to acknowledge the full range of costs associated with securing, stocking, managing, and administering vaccines at the practice level) and about plausible solutions. In 2005, Partnership for Prevention issued Strengthening Adult Immunization: A Call to Action, which was widely endorsed by medical and public health organizations (including the American Medical Association and the American Public Health Association) and called for the purchase and distribution of influenza vaccine for uninsured adults, first-dollar coverage for influenza and pneumococcal vaccines in the Federal Employee Health Benefit Program, expansion of Section 317 of the Public Health Service Act to cover adult immunization needs, and the launch of a national education campaign on the value of adult immunization (Hinman and Orenstein, 2007).
Evidence suggests that pediatricians and family practitioners are not adequately reimbursed for providing vaccines to children (Freed et al., 2008a; National Immunization Congress, 2007). As newer, more costly vaccines such as HPV and meningococcal vaccines are recommended for use in children, providers have encountered significant financial barriers including large cash outlays with hundreds of thousands of dollars spent on the purchase of vaccines, potential delays in timely reimbursement by some insurers, and in some cases lower reimbursement for vaccine purchase than the price paid. In a study by Freed et al. (2008b), 11 percent of providers reported they had considered no longer purchasing and providing vaccines for their primary care practice due to these financial barriers. Furthermore, reimbursement of vaccine administration fees has remained extremely low and has not kept pace with the growing financial and administrative burden of practices that provide immunization services, such as the need for providers to carefully monitor vaccine inventories, ensure that vaccines are stored and transported at the correct temperature, purchase immunization supplies (e.g., syringes, needles, alcohol pads), pay for insurance or maintain backup generators in case of power outages, ensure competent administration of vaccines by trained professionals, counsel patients about the risks and benefits of vaccination, and record information in medical records and in many cases with duplicate data entry of information for electronic immunization information systems. For example, vaccine administration costs may range from $5 in public health clinics to $20 in private sector clinics
or according to another source from $20-$40 (NVAC Vaccine Financing Working Group, 2009; Shepard et al., 2005), but some payers reimburse providers far less than the cost incurred by providers. For example, one state Medicaid program reimbursed private sector providers as little as $2 per dose for administration of vaccines given (Freed et al., 2008b). Vaccines for Children (VFC) does not reimburse providers for costs associated with administering the vaccine, but most VFC vaccinations are given to children on Medicaid, which reimburses for vaccine administration. VFC providers can charge an administrative fee to patients without insurance; it is hoped they would not withhold vaccination due to inability to pay.4 Although Section 317 funding may be used for provider reimbursement, there currently is no mechanism for doing so (CDC, 2009c).
Adult health care providers also receive inadequate reimbursement for immunizations. Adults 65 years of age and older are typically covered for vaccines by Medicare, although there are barriers (e.g., the complicated process for receiving Zostavax vaccine, described in this committee’s 2008 letter report in Appendix D). Adults younger than 65 years who are uninsured or underinsured generally do not have an alternative way to finance vaccines other than to pay for them out of pocket.
Rationale for Removing Financial Barriers
Removing financial barriers to immunization could have a considerable impact on access to services, as shown by the 2008 update to the Guide to Community Preventive Services, which found that reducing out-of-pocket costs for immunization services is an effective intervention in increasing access to immunization services (Briss et al., 2000).5 Research also shows that children’s health insurance coverage determines whether they are up-to-date on recommended vaccinations, but gaps in private insurance allow some children to fall through the cracks (Blewett et al., 2008; Santoli et al., 2004). State immunization requirements for school and child care entry also raise an ethical argument for ensuring that children have no financial barriers to receiving needed vaccines.6
Proposed Solutions to Financing Barriers
In April 2008, the National Vaccine Advisory Committee (NVAC) finance workgroup convened a workshop and published a paper on a childhood and adolescent immunization that involved stakeholder input from manufacturers, distributors, insurers (e.g., private, Medicaid), employer groups, providers, state and local public health agencies, and others. While there was general agreement at the workshop that primary care providers are inadequately reimbursed for their role of providing vaccines to children, consensus was not achieved by the NVAC finance work group on the best solutions to the problem at hand. Possible solutions discussed at the NVAC meeting included legal mandates for employers and insurers to provide first-dollar coverage of vaccines; increasing the amount of reimbursement paid for administrative fees to VFC providers by Medicaid programs; and revising the VFC legislation to ensure the purchase and provision of vaccines to underinsured children. These solutions were all challenged by several stakeholder groups at the NVAC vaccine financing meeting. Other potential approaches, such as providing assistance and training to primary care providers on better business practices; facilitating timely reimbursement by insurers; and allowing providers to purchase vaccines on a delayed payment schedule to minimize a practice’s cash outlay, were considered tenable solutions. While many of the solutions put forward by the NVAC financing committee are clearly needed to ensure that children can continue to be vaccinated in their medical home, these solutions should be part of a larger comprehensive approach to solving the overarching problem of how to provide better incentives and remove disincentives for a preventive intervention that is clearly a public health good.
The NVAC finance work group made final recommendations on vaccine purchase and administration reimbursement in the public and private sectors. Recommendations included expanding funding to the Section 317 and Vaccines for Children programs to cover vaccine administration and reimbursement; broadening access to VFC through public health clinics (access is currently allowed only at federally qualified health centers and rural health centers); and expanding VFC to include all underinsured children and adolescents. NVAC also recommended that all states reimburse for Medicaid vaccine administration and fund Medicaid- and SCHIP (State Children’s Health Insurance Program)-managed care plans at a level that provides vaccine administration reimbursement at the CMS-established maximum allowable amount. Another recommendation called for CMS to “update the maximum allowable Medicaid administration reimbursement amounts for each state and include all appropriate non-vaccine related costs as determined by current studies” (NVAC, 2009b).
Although much effort has been devoted to ensuring the gaps in financing
are minimized for children and adolescents, attention to gaps in financing vaccination for adults has lagged (NFID, 2008; NVAC, 2009a). However, the committee is aware of the ongoing activity of the NVAC Adult Immunization Working Group, including draft recommendations (NVAC, 2009b). The financing issues for adults are even more complex than for children. For example, primary care services for the two populations differ considerably—immunization is not a major part of services provided to adults, and there is no adult equivalent of “well child visits” (Orenstein et al., 2007). Although reimbursement for adult vaccination has improved in recent years (compared to data cited in the 2003 IOM report), a majority of providers consider lack of reimbursement a barrier to zoster vaccination, and some providers remain concerned about reimbursement for other vaccines, including influenza, pneumococcal, and hepatitis B.7 In the public sector, there is no VFC-like program for adults who are uninsured. Gaps in funding in the public sector for adult immunization further exacerbate the disparities in access to recommended vaccines for adults.
Committee Recommendation for Financing Immunization
The committee believes that Objective 4.2 in the National Vaccine Plan on reducing the financial barriers to immunization is insufficient. The target that is needed is elimination, not reduction of such barriers. No individual should be denied the opportunity to receive ACIP-recommended vaccines due to inability to pay. Innovations in insurance and direct financing arrangements to assure affordability at point of delivery, coupled with system supports that enable efficient practice, such as changes in how practices are supplied with vaccines, are needed.
A gap in the draft Goal 4 is the lack of an objective or strategy on performance measures related to the use of financing to induce and enable providers to seek out, stock, and administer ACIP-recommended vaccines. The committee would like to draw attention to the following matters, which must be addressed in the plan with clear objectives and performance measures:
-
Adoption by public and private insurers and by payers of provider payment mechanisms aimed at assuring that there are incentives for selecting and providing the right care (in this case, ACIP-recommended immunizations) at the right time and in the right setting
-
Acknowledgment of the range of health care providers (e.g., obstetrician-gynecologists and infectious disease specialists) are able to provide vaccination but may not be included in payment systems
-
Integration of immunization in national systems of continuing performance measurement, with data collected by population characteristic, care setting, type of vaccine, and provider type
-
Sufficient funding to cover lapse in public and private coverage
-
The adoption of supply-chain mechanisms across insurer and third-party payer type, similar to the VFC program, so that the cost of stocking and storing vaccines is directly addressed and providers are not discouraged by the cost of carrying fragile and costly inventory that may or may not be used before it expires (this issue is obliquely referenced in strategy 4.2.5 of the draft plan—develop, implement, and evaluate strategies to reduce the financial burden on vaccination providers for purchase of initial and ongoing vaccine inventories).
To realize the full potential of vaccines to prevent costly disease and disability, the committee recommends:
Recommendation 4-2: The National Vaccine Plan should include the development of strategies to eliminate financial barriers such as unreasonable cost-sharing by patients who are unable to afford out-of-pocket costs for vaccines and provider payment mechanisms that discourage full and meaningful participation in the delivery of immunization services.
Strategies could include identifying ways of maximizing the use of public insurance options for the uninsured, as well as efficient use of available funds to aid uninsured and underinsured adult populations, and comparative-effectiveness research of various payment and reimbursement mechanisms for providers.
SUB-GOAL 3:
ACCESS AND PRACTICE
Objective 4.2 in the draft plan is extremely broad, combining financial and non-financial barriers to immunization. The term non-financial barriers is used to refer to a vast array of barriers to both patients (e.g., inconvenient hours of operation, long waits for appointments, limited transportation, knowledge and communication challenges) and barriers to providers (e.g., incomplete information about or failure to review or assess a child’s immunization status) and gaps in provider knowledge that lead to missed opportunities to vaccinate (e.g., not vaccinating when a patient has a mild illness). Some examples of non-financial barriers, such as communication
and information, would seem to fit more appropriately in Goal 3. Aside from organizational matters, the committee has focused on two remaining major non-financial barriers: access to immunization and healthcare provider practice concerns.
In the past decade, non-traditional sites for the delivery of vaccination have become more prominent. These include retail- and other community-based sites (including pharmacies). School-based health centers and senior centers have been part of the immunization delivery landscape for some time, but have also garnered more attention as awareness of adolescent and adult immunization need has increased, owing both to the introduction of new vaccines for these groups and a recognition of poor vaccine uptake, due in part to gaps in access. The dialogue about the optimal sites for delivery of immunization services includes references to the importance of immunizing in the context of a medical home,8 but that may or may not be an appropriate model, depending on the population. Although the emergence of complementary sites for the delivery of health care has been regarded both negatively and positively by health care professionals (CHCF, 2008; Scott, 2007), they offer some advantages for the delivery of immunization services, such as increased access. However, for quality alternative sites to be a useful mechanism to increase access to immunization, they must include financial coverage. The Infectious Diseases Society of America has recommended quality standards for complementary sites of immunization, including “ability to appropriately manage vaccine-related adverse events, proper storage and handling of vaccines, appropriate record keeping, regulatory issues, and provision of education regarding both risks and benefits of immunizations” (Pickering et al., 2009).
Over the past several years, the concept of comparative effectiveness research has been explicitly expanded to include comparisons not only of medical interventions, but also of the ways and settings in which health care is delivered (Brookings Institute, 2009; National Journal Online, 2009; NEHI, 2009). The committee believes that comparative effectiveness research could build on and strengthen the evidence base concerning immunization practices. For example, there are questions about the best settings to deliver immunization services to different populations and age groups, but little research has been done examining the strengths and weaknesses of each delivery setting (e.g., primary care setting versus retail based) for various populations and age groups. For example, how do various settings handle communication about vaccine risks and benefits?
Efforts to improve the delivery and quality of health care include a growing recognition of the value of immunization as a cost-saving and
cost-effective preventive service. Public health and disease prevention objectives are hallmarks of the contemporary policy effort to reform health care. Research indicates that geographic areas with high rates of high-cost health care often have low rates of low-cost preventive services such as influenza and pneumococcal immunization (Fisher and Wennberg, 2003; Fisher et al., 2003). It is possible that the low rates of reimbursement for immunization services are partly to blame, but this example illustrates some of the perverse incentives and disincentives that exist within the U.S. health care system.
In another example of efforts to link immunization with quality measures, the National Committee for Quality Assurance has developed a measure referring to the percentage of Medicare members 65 years of age and older who have received an influenza vaccination. There is increasing recognition that all age groups need access to ACIP-recommended vaccines and that health plans ought to include immunization coverage rates among measures of quality of health systems and communities.
Studies of provider knowledge and practices have indicated both knowledge gaps and systems challenges that range from major hurdles (inadequate or no reimbursement for counseling patients about needed vaccinations) to administrative issues such as lack of effective reminder systems (Davis et al., 2001; Flowers, 2007).
Comparative effectiveness, cost-effectiveness, and other types of research could contribute to determining the best ways to organize immunization services to ensure optimal access in various communities, the best ways to measure quality of services, and the best ways to structure incentives and pay for services (at both the insurer and the provider levels, and to promote the advance purchase and allocation of supply to the point of service).
Recommendation 4-3: The National Vaccine Plan should emphasize the application of research and best practices in the organization and delivery of immunization services to improve patient access (such as location and hours) and service efficiency and quality (such as improved provider knowledge and decrease in missed opportunities for vaccination).
SUB-GOAL 4:
INFORMATION SYSTEMS
There are five purposes for information systems used in immunization services:
-
To track vaccine supply,
-
To assess vaccination coverage at the individual level through immunization information systems or registries,
-
To assess vaccine coverage at the population level through tools such as the National Immunization Survey (NIS) and the Behavioral Risk Factor Surveillance System (BRFSS),
-
To conduct surveillance of disease, and
-
To conduct surveillance of vaccine adverse events.
Tracking Supply
A stable vaccine supply (and distribution of that supply) for ACIP-recommended vaccines is a high priority, and a variety of information systems can provide added intelligence about the movement of vaccine supplies and support decision making. At the national level, CDC is developing a vaccine tracking system (VTrckS) that will be a “fully functional on-line ordering system that supports centralized distribution” and that may help explain some causes of vaccine shortages or excess supply of various vaccines (in both the private and public sector) (CDC, 2009a). If health information technology goals came to fruition, HIT could be used to track data on where vaccines are used and therefore track supply (health care providers, hospitals, retail stores) and identify area’s where certain vaccines have not been administered or if there are pockets of need to determine where excess supply should be sent.
Assessing Vaccination Coverage at the Individual Level
Immunization information systems (IIS) or registries that collect individual-level vaccination coverage data are operated by individual providers, health care organizations, public health agencies, and school systems. Immunization information systems are confidential, computerized systems operated at the state and local level that are intended to record every vaccination given to children; some have additional functions, such as vaccine inventory management and adverse events reporting (CDC, 2007a). In 2006, 64 (70 percent) CDC grantees (i.e., states, territories, several metropolitan areas) reported that their IIS had the ability to track immunizations of people of all ages (CDC, 2008). In 2006, 65 percent of U.S. children under age six were included in an IIS, although the definition of participation is two or more doses recorded, and many records are incomplete (CDC, 2008). IIS permit providers to determine vaccination status of a child seen in their practice and generate the immunization records needed, for example, for school entry or childcare.
Electronic health records (EHRs) are records of “health-related information on an individual that conforms to nationally recognized interoperability standards and that can be created, managed and consulted by authorized clinicians and staff across more than one health care organization” (National
Alliance for Health Information Technology, 2008).9 Ideally, electronic health records would be interoperable with immunization information systems so that the record of immunizations received in a health care setting would automatically be submitted to IIS; practitioners should also be able to search IIS and import a history of previous immunizations received by a specific patient into the EHR at their practice. However, a study conducted in 2007 and 2008 found that only 4 percent of physicians reported having “an extensive, fully functional electronic-records system and 13 percent reported having a basic system” (DesRoches et al., 2008). The 2008 National Ambulatory Medical Care Survey (NAMCS), “an annual nationally representative survey of patient visits to office-based physicians” conducted by the National Center for Health Statistics (NCHS), similarly found that 4 percent of providers use fully functioning electronic medical records systems and 17 percent use basic systems (Hsiao et al., 2008). Denmark is a good example of successful implementation of electronic health records. Denmark has a centralized computer database to which primary care physicians (98 percent), all hospital physicians, and all pharmacists have access to medical records. Patients can also access their own personal records though a secure website. Although it does not have one overarching system, the Danish system is able to link networks established by regional health agencies (Harrell, 2009).
HIT is important in informing providers about patient immunization history. Providers need to be able to obtain information on the vaccination status of their patients quickly and easily (to avoid missed opportunities or duplicate vaccination) both in their practice and remotely; it is also crucial that alternative immunization sites such as schools, workplaces, and pharmacies are able to document vaccinations received and share these data with public health agencies.
Assessing Vaccination Coverage at the Population Level
National, state, and large-city data about vaccination coverage are obtained from the NIS, an annual list-assisted random-digit-dialing telephone survey followed by a mailed survey to children’s immunization providers. The NIS is conducted jointly by the CDC National Center for Immunizations and Respiratory Diseases and NCHS. Levels of coverage in children, and recently adolescents, are assessed through the NIS and for adults through the BRFSS. Since NIS is a phone-based survey with verification through medical records it has small study samples. Local-level data is dif-
ficult to obtain through national surveys such as the NIS and BRFSS, but attempting to expand NIS or similar mechanisms to gather local data for much larger sample sizes would be costly and extremely difficult.
Surveillance of Infectious Diseases
One striking illustration of the complexity of the public health network of information systems is found in the influenza surveillance system (CDC, 2009b), which
… consists of nine complementary surveillance components in five categories. These components include reports from more than 150 laboratories, 2,400 outpatient care sites, vital statistics offices in 122 cities, research and health-care personnel at the NVSN10 and EIP11 sites, and influenza surveillance coordinators and state epidemiologists from all 50 state health departments, and the District of Columbia health department.
Interoperable electronic health records could facilitate surveillance of vaccine-preventable diseases by automating the reporting of notifiable conditions. It would also allow public health workers to measure the impact of vaccines and identify pockets of under-vaccination (and therefore an increased risk of an outbreak) and more effectively distribute resources.
Surveillance of Adverse Events
Surveillance of adverse events is fairly limited at the local and state level. Currently the Vaccine Adverse Events Reporting System, the Vaccine Safety Datalink, and the Clinical Immunization Safety Assessment network described in Chapter 2 are used to identify potential adverse events, but an integrated interoperable system would increase the population studied and would increase the likelihood for a study of an adverse event to have statistical power. Such systems could also be searched systematically for a putative adverse event related to immunization which could accelerate the detection and evaluation of a post-licensure safety problem. Interoperable electronic health records can build on these existing systems to increase the power of studies and evaluation of adverse events following immunization and facilitate research studies (e.g., linkage studies, control groups; see Chapter 2 for a detailed discussion).
In addition to efforts to obtain safety data described in Chapter 2, government agencies and other institutions are looking at additional opportunities to collect data—for example, FDA’s Sentinel Initiative (intended
to link multiple large databases to enable widespread surveillance of adverse events) and the 2009 Post-Licensure Rapid Immunization Safety Monitoring constructed to conduct active surveillance of adverse events following influenza immunization with the monovalent H1N1 vaccine. However, as programs such as the Sentinel Initiative move forward it will be important for planners to think strategically about what information can be obtained from current systems and what will be the most useful investment of resources. Planners also need to look toward the future (interoperable health records), which will likely obviate the need for these interim solutions. What is learned from working with these interim systems will create a core of analytic and information technology expertise, which will be important to tie into the future HIT activities. State, local, and to a certain extent federal public health agencies must be prepared to participate effectively in HIT by having the technical expertise and the HIT systems to support interfacing with EHRs and the National Health Information Network. Current immunization systems need to be developed using evolving national standards for interoperable HIT to the maximum extent possible. Since many IIS originated in the 1990s and were not based on national standards, it will be necessary to develop strategic approaches to support IIS functions in the evolving NHIN—some functions may be incorporated into clinical EHRs, while some functions will be the responsibility of public health agencies.
Recommendation 4-4: The National Vaccine Plan should encourage the exploration of non-traditional approaches to disease surveillance, monitoring vaccine safety, and assessing vaccine coverage. Such approaches might leverage the increasing ubiquity of the Internet and wireless data services, personal communications devices, and social networking facilities.
The committee believes that the health information technology investments spurred by the American Recovery and Reinvestment Act (ARRA) of 2009 are an extremely important new development, and their implications for immunization deserve careful consideration in the National Vaccine Plan.
Recommendation 4-5: Given the importance placed on the national adoption of certified, interoperable health information technology and electronic health records, the National Vaccine Plan should ensure active involvement of NVPO and relevant partners in the planning and implementation of the national health information initiative.12
This involvement should include:
-
Ensuring the development and adoption of standards necessary for effective immunization clinical practice and population surveillance systems,
-
Ensuring that the definition of “meaningful use” considers immunization practice and reporting,13
-
Facilitating use of vaccine-related data by all public health partners (e.g., state and local health departments),14 and
-
Ensuring that all public health partners have the expertise and resources to participate in the initiative.
SUB-GOAL 5:
PUBLIC HEALTH INFRASTRUCTURE
Public health agencies at all levels play a central role in assuring the best use of vaccines to achieve prevention of infectious diseases. Strengthening and clarifying the draft plan’s strategies for public health agencies would help to reflect the ideal of integrated measurement, monitoring, assurance, and standard setting of public health functions. Specific activities could include assuring that all states have the resources to support immunization services in communities and for populations with inadequate access to immunizations. For example, although ethnic disparities in receipt of recommended vaccines among children have decreased, disparities in receipt of pneumococcal vaccination have increased among Black and Asian adults 65 years or older (AHRQ, 2009). The ability to conduct disease surveillance and registry capacity in all states to ultimately enable identification of under-immunized populations in real time is also important (see earlier discussion about local area monitoring). Review and modernization of health professions licensure statutes to assure that all states have the maximum capacity to deploy all health professionals for immunization practice within their scope of competence is an additional area that warrants attention. For example, only a small number of states have made clear the legal authority of nursing professionals to immunize under standing orders (Smith et al., 2006; Stewart et al., 2005). This could lead to missed opportunities to provide recommended vaccinations. Although standing orders have been shown to
help prevent missed opportunities (CDC, 2000; Daniels et al., 2006) communicating with patients about recommended vaccines and responding to their questions and concerns is an important contributor to decisions about vaccination. Communication is discussed in detail in Chapter 3.
Recommendation 4-6: The National Vaccine Plan should include strengthening the public health infrastructure to support vaccine delivery, measure immunization practice and performance, intervene to address disparities in access to immunization, and respond to emerging infectious disease threats.
Efforts to strengthen the public health infrastructure could include:
-
Development of capacity in all health departments to assure the delivery of immunization services to underserved populations in all communities or during an emergency;15
-
Development of greater public health capacity to identify deficits in access to immunization services;16 and
-
Assistance to states to eliminate barriers to the full use of all appropriate personnel in vaccine administration due to restrictions on licensure and scope of practice.
The final outcome of health care reform efforts will have implications for the delivery of immunization services, and the committee hopes the changes that result will be conducive to improved access and information. Health care reform legislation will ideally include monitoring immunization coverage and achieving targets as a measure of success. At the practice level, measures of health care quality would ideally include the provision of immunization services to adults, adolescents, and children.
Recommendation 4-7: The National Vaccine Plan should incorporate rapid and comprehensive assessment of the outcomes of national health reform and their implications for the nation’s vaccine and immunization priorities.
Specifically, NVPO, as “owner” of the plan, could contribute by:
-
Tracking House and Senate reform proposals and forwarding comments to the Secretary when appropriate;
-
Participating as early as possible in implementation efforts related to the expanded health insurance access for the population;
-
Participating as early as possible in implementation efforts related to the design of health insurance coverage and cost-sharing features, administrative matters affecting the actual provision of vaccines, and standards and procedures governing the measurement and reporting of health plan performance; and
-
Promoting the integration of health plan performance and operations with community public health policy and practice in order to assure (a) the availability of community-wide information about population immunization status, disparities in access, and areas of need; (b) access to immunization services; (c) public health agency analytical, management, and other needed capabilities; and (d) the ability of public health workers, health insurers, and health care providers to mount a joint response to emerging public health threats.
REFERENCES
AAFP (American Academy of Family Physicians). 2009. CDC Reinstates Hib Booster Dose for 12- to 15-Month-olds. Available: http://www.aafp.org/online/en/home/publications/news/news-now/clinical-care-research/20090701hib-reinstated.html [accessed October 6, 2009].
AAFP, American Academy of Pediatrics, American College of Physicians, and American Osteopathic Association. 2007. Joint Principles of the Patient-Centered Medical Home. Available: http://www.medicalhomeinfo.org/joint%20Statement.pdf [accessed January 2009].
AHIP (America’s Health Insurance Plans). 2009. New Health Plan Payment Models are Improving Patient Quality and Safety. Available: http://www.ahip.org/content/pressrelease.aspx?docid=26850 [accessed October 6, 2009].
AHRQ (Agency for Healthcare Research and Quality). 2009. National Healthcare Disparities Report (Publication No. 09-0002). Rockville, MD: AHRQ.
Berman, S. 2005. Challenge of transforming our private and public pediatric health care systems to emphasize value. Pediatrics 115(4):1068-1070.
Blewett, L.A., G. Davidson, M.D. Bramlett, H. Rodin, and M.L. Messonnier. 2008. The impact of gaps in health insurance coverage on immunization status for young children. Health Serv Res 43(5p1):1619-1636.
Briss, P.A., L.E. Rodewald, A.R. Hinman, A.M. Shefer, R.A. Strikas, R.R. Bernier, V.G. Carande-Kulis, H.R. Yusuf, S.M. Ndiaye, and S.M. Williams. 2000. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The task force on community preventive services. Am J Prev Med 18(1 Suppl):97-140.
Bristol, M. 2005. “Preparedness Pays Off: Department Shines During Major Test of Emergency Capabilities.” In Vanguard September/October 2005(7). Washington, DC: Department of Veterans Affairs.
Brookings Institute. 2009 (June 9). Implementing Comparative Effectiveness Research: Priorities, Methods, and Impact. Available: http://www.brookings.edu/~/media/Files/events/2009/0609_health_care_cer/20090609_health_care_cer.pdf [accessed August 2009].
CDC (Centers for Disease Control and Prevention). 2000 (March 24). Use of standing orders programs to increase adult vaccination rates. MMWR 49(RR01):15-26.
CDC. 2006 (December 15). Immunization information systems progress—United States, 2005. MMWR 55(49):1327-1329.
CDC. 2007a. Developing Immunization Information Systems. Available: http://www.cdc.gov/NCIRD/progbriefs/downloads/iis.pdf [accessed October 6, 2009].
CDC. 2007b. Questions and Answers about Hib Recall. Available: http://www.cdc.gov/vaccines/recs/recalls/hib-recall-faqs-12-12-07.htm [accessed August 12, 2009].
CDC. 2008. Immunization information systems progress—United States, 2006. MMWR 57(11):289-291.
CDC. 2009a. External Information Systems (ExIS)) and the Vaccine Tracking System (VTrckS). Available: http://www.cdc.gov/vaccines/programs/vmbip/downloads/newsletters/2009/exis-aira-final.doc [accessed October 6, 2009].
CDC. 2009b. Flu Activity and Surveillance. Available: http://www.cdc.gov/flu/weekly/fluactivity.htm [accessed August 13, 2009].
CDC. 2009c. VFC: Frequently Asked Questions. Available: www.cdc.gov/vaccines/programs/vfc/projects/faqs-doc.htm [accessed October 2009].
CHCF (California Healthcare Foundation). 2008. Retail Clinics: Disruptive Innovation in Primary Care? Proceedings from California HealthCare Foundation/Health Affairs Roundtable. Oakland, CA: CHFC.
Coleman, M.S., N. Sangrujee, F. Zhou, and S. Chu. 2005. The infrastructure for vaccine development factors affecting U.S. manufacturers’ decisions to produce vaccines. Health Aff 24(3):635-642.
Daley, M.F., K.A. Hennessey, C.M. Weinbaum, S. Stokley, L.P. Hurley, L.A. Crane, B.L. Beaty, J.C. Barrow, C.I. Babbel, L.M. Dickinson, and A. Kempe. 2009. Physician practices regarding adult hepatitis B vaccination: a national survey. Am J Prev Med 36(6):491-496.
Daniels, N.A., S. Gouveia, D. Null, G.L. Gildengorin, and C.A. Winston. 2006. Acceptance of pneumococcal vaccine under standing orders by race and ethnicity. J Natl Med Assoc 98(7):1089-1094.
Davis, T.C., D.D. Fredrickson, C.L. Arnold, J.T. Cross, S.G. Humiston, K.W. Green, and J.A. Bocchini, Jr. 2001. Childhood vaccine risk/benefit communication in private practice office settings: A national survey. Pediatrics 107(2):e17.
DesRoches, C.M., E.G. Campbell, S.R. Rao, K. Donelan, T.G. Ferris, A. Jha, R. Kaushal, D.E. Levy, S. Rosenbaum, A.E. Shields, and D. Blumenthal. 2008. Electronic health records in ambulatory care—a national survey of physicians. NEJM 359(1):50-60.
Fisher, E.S., and J.E. Wennberg. 2003. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med 46(1):69-79.
Fisher, E.S., D.E. Wennberg, T.A. Stukel, D.J. Gottlieb, F.L. Lucas, and E.L. Pinder. 2003. The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Ann Intern Med 138(4):273-287.
Flowers, L. 2007. Racial and ethnic disparities in influenza and pneumococcal immunization rates among medicare beneficiaries. Issue Brief (Public Policy Inst (Am Assoc Retired Pers)) (IB83):1-6.
Foxhall, F. 2009. Expensive Vaccines May Wipe Out the Wrong Target—the Doctor. Available: http://www.acpinternist.org/archives/2009/04/vaccine.htm [accessed August 13, 2009].
Freed, G.L., A.E. Cowan, and S.J. Clark. 2008a. Primary care physician perspectives on reimbursement for childhood immunizations. Pediatrics 122(6):1319-1324.
Freed, G.L., A.E. Cowan, S. Gregory, and S.J. Clark. 2008b. Variation in provider vaccine purchase prices and payer reimbursement. Pediatrics 122(6):1325-1331.
GAO (Government Accountability Office). 2009. Health Information Technology: Federal Agencies’ Experiences Demonstrate Challenges to Successful Implementation. Washington, DC: GAO.
Harrell, E. 2009. In Denmark’s Electronic Health Records Program, a Lesson for the U.S. Available: http://www.time.com/time/health/article/0,8599,1891209,00.html [accessed August 13, 2009].
HHS (Department of Health and Human Services). 2008 (November). Draft Strategic National Vaccine Plan. Available: http://www.hhs.gov/nvpo/vacc_plan/2008plan/draftvaccineplan.pdf [accessed December 2008].
Hinman, A.R., and A.J. Davidson. 2009. Linking children’s health information systems: Clinical care, public health, emergency medical systems, and schools. Pediatrics 123(Suppl 2): S67-S73.
Hinman, A.R., and W.A. Orenstein. 2007. Adult immunization: What can we learn from the childhood immunization program? Clin Infect Dis 44(15 June):1532-1535.
Hinman, A.R., W.A. Orenstein, J.M. Santoli, L.E. Rodewald, and S.L. Cochi. 2006. Vaccine shortages: History, impact, and prospects for the future. Annu Rev Publ Health 27:235-259.
Hsiao, C-J., C.W. Burt, E. Rechtsteiner, E. Hing, D. Woodwel, J.E. Sisk, and the National Center for Health Statistics, Division of Health Care Statistics. 2008. Preliminary Estimates of Electronic Medical Record Use by Office-based Physicians: United States, 2008. Available: http://www.cdc.gov/nchs/products/pubs/pubd/hestats/hestats.htm [accessed August 2009].
Hurley, L.P., R. Harpaz, M.F. Daley, L.A. Crane, B.L. Beaty, J. Barrow, C. Babbel, M. Marin, J.F. Steiner, A. Davidson, L.M. Dickinson, and A. Kempe. 2008. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 197(Suppl 2):S216-S223.
IOM (Institute of Medicine). 2000. Calling the Shots: Immunization Finance Policies and Practices. Washington, DC: National Academy Press.
IOM. 2003. Financing Vaccines in the 21st Century: Assuring Access and Availability. Washington, DC: The National Academies Press.
Kempe, A., L. Hurley, S. Stokley, M.F. Daley, L.A. Crane, B.L. Beaty, L.M. Dickinson, C. Babbel, J. Barrow, and J.F. Steiner. 2008. Pneumococcal vaccination in general internal medicine practice: current practice and future possibilities. J Gen Intern Med 23(12):2010-2013.
National Alliance for Health Information Technology. 2008. Defining Key Health Information Technology Terms. Available: http://healthit.hhs.gov/portal/server.pt/gateway/PTARGS_0_10741_848133_0_0_18/10_2_hit_terms.pdf [accessed October 2009].
National Immunization Congress. 2007. Adult and Adolescent Immunization Summary. Available: http://www.izta.org/PDF%20Presentations/2007%20National%20Immunization%20Congress.pdf [accessed August 13, 2009].
National Journal Online. 2009. Comparative Effectiveness: Where to Begin? Available: http://healthcare.nationaljournal.com/2009/04/comparative-effectiveness-wher.php [accessed August 13, 2009].
NEHI (New England Healthcare Institute). 2009. Balancing Act: Comparative Effectiveness Research and Innovation in U.S. Health Care, a White Paper. New England Healthcare Institute.
NFID (National Foundation for Infectious Diseases). 2008. Saving Lives: Integrating Vaccines for Adults into Routine Care. Bethesda, MD: NFID.
NVAC (National Vaccine Advisory Committee). 2009a. Appendix 1: Children and Adolescents Vaccine Financing Recommendations Adopted by NVAC—September 2008. Available: http://www.hhs.gov/nvpo/nvac/CAVFRecommendationsSept08.html [accessed October 7, 2009].
NVAC. 2009b. Recommendations for Federal Adult Immunization Programs Regarding Immunization Delivery, Assessment, Research, and Safety Monitoring. Available: http://www.hhs.gov/nvpo/nvac/NVACAdultImmunizationsWorkingGroupJune2009.html [accessed October 7, 2009].
NVAC Vaccine Financing Working Group. 2009. Assuring Vaccination of Children and Adolescents without Financial Barriers: Recommendations from the National Vaccine Advisory Committee (NVAC). Washington, DC: Department of Health and Human Services.
Orenstein, W.A., G.T. Mootrey, K. Pazol, and A.R. Hinman. 2007. Financing immunization of adults in the United States. Clin Pharmacol Ther 82(6):764-768.
Partnership for Prevention. 2005. Strengthening Adult Immunization: A Call to Action. Available: http://www.prevent.org/images/stories/calltoaction.pdf [accessed August 13, 2009].
Pickering, L.K., C.J. Baker, G.L. Freed, S.A. Gall, S.E. Grogg, G.A. Poland, L.E. Rodewald, W. Schaffner, P. Stinchfield, L. Tan, R.K. Zimmerman, W.A. Orenstein, and the Infectious Diseases Society of America. 2009. Immunization programs for infants, children, adolescents, and adults: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 49(6):817-840.
Santoli, J.M., G. Peter, A.M. Arvin, J.P. Davis, M.D. Decker, P. Fast, F.A. Guerra, C.M. Helms, A.R. Hinman, R. Katz, J.O. Klein, M.B. Koslap-Petraco, P.R. Paradiso, W. Schaffner, P.N. Whitley-Williams, D.E. Williamson, B. Gellin, and National Vaccine Advisory Committee. 2003. Strengthening the supply of routinely recommended vaccines in the United States. JAMA 290(23):3122-3128.
Santoli, J.M., N.J. Huet, P.J. Smith, L.E. Barker, L.E. Rodewald, M. Inkelas, L.M. Olson, and N. Halfon. 2004. Insurance status and vaccination coverage among US preschool children. Pediatrics 113(6):1959-1964.
Scott, M.K. 2007. Health Care in the Express Lane: Retail Clinics Go Mainstream. Oakland: California HealthCare Foundation.
Shepard, C.W., I.R. Ortega-Sanchez, R.D. Scott, 2nd, and N.E. Rosenstein. 2005. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 115(5):1220-1232.
Smith, N.M, J.S. Bresee, D.K. Shay, T.M. Uyeki, N.J. Cox, and R.A. Strikas. 2006. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 55(RR10):1-42.
Stewart, A., M. Cox, and S. Rosenbaum. 2005. The Epidemiology of U.S. Immunization Law: Immunization requirements for Staff and Residents of Long-Term Care Facilities Under State Laws/Regulations. Washington, DC: Department of Health Policy of the George Washington University School of Public Health and Health Services.
Szilagyi, P.G., L.P. Shone, R. Barth, R.W. Kouides, C. Long, S.G. Humiston, J. Jennings, and N.M. Bennett. 2005. Physician practices and attitudes regarding adult immunizations. Prev Med 40(2):152-161.
Urquhart, G.A., W. Williams, J. Tobias, F.J. Welch. 2007. Immunization information systems use during a public health emergency in the United States. J Public Health Man 13(5):481-485.
























