7
The Regulatory Framework: A Powerful and Adaptable Tool for Sodium Intake Reduction
Salt and other sodium-containing compounds—like other substances added to food—are subject to federal food safety and labeling laws. Sodium content has been a mandatory declaration in the Nutrition Facts panel on packaged foods since 1993. The manner in which these laws are used and implemented can significantly impact sodium intake by the U.S. population.
As a general matter, federal laws enacted by Congress have evolved over time to ensure the safety and adequacy of the food supply, protect public health, and require that the food industry provide information needed by consumers, including information with which to make healthful food choices. These laws are administered by various agencies of the federal government, and the “rules” or regulations needed to implement the laws are put in place commonly as part of a public rulemaking process. Details about the federal rulemaking process can be found in Appendix H.
The federal laws and regulations that relate to sodium in foods may appear complex to those unfamiliar with food law and regulations. However, once the framework is understood, it becomes evident that regulatory approaches can offer a powerful and adaptable tool for reduction of sodium intake. The options in the current regulatory framework are diverse and could be used in creative ways to facilitate a meaningful reduction of sodium intake. Further, regulations are developed through a public process, thereby enabling stakeholders to provide information on making them realistic and well matched to the reality of the world in which they will be applied.
This chapter addresses first the regulatory requirements surrounding
salt and other sodium-containing compounds in the context of the safety of substances added to foods. Next, the regulatory requirements that pertain to nutrition information provided to the consumer and the requirements related to making claims about food products are outlined. In addition, the application of federal regulations to restaurant/foodservice operations is discussed.
REGULATION TO ENSURE SAFETY OF SUBSTANCES ADDED TO FOODS BY MANUFACTURERS
Protecting, enhancing, and preserving food by using “food additives” began in ancient times, undoubtedly long before documented history. The Romans added sulfites to wine as a preservative and Europeans sought spices not only to flavor but also to preserve foods. There is evidence that many cultures and geographic regions used salt as a preservative, especially for meats (Folkenberg, 1988). In the absence of a scientific understanding of the effects of such substances, it was assumed that they were safe unless they poisoned the consumer.
Today, one role of government is to ensure that the food sold to its citizens is safe to eat. The history for such authority in the United States begins with the Food and Drugs Act of 1906,1 but the key provisions for the purposes of this report rest within the Federal Food, Drug, and Cosmetic Act of 1938.2 This act was passed after a legally marketed elixir killed 107 people. Thus, the act was specifically intended to overhaul the public health system in the United States.3 Among other provisions, the law authorized the government agency now housed in the U.S. Food and Drug Administration (FDA) to issue standards for foods and to demand evidence of safety for new drugs.
Concerns about the possible long-term harmful effects of food chemicals on health led Congress in 1958 to enact the Food Additives Amendment,4 which became Section 409 of the 1938 act (hereafter referred to as the 1958 Amendment) to ensure the safety of substances added to foods.5 Salt—sodium chloride—is a substance intentionally added to food by manufacturers; therefore the provisions of this amendment apply to salt as well as to any other sodium-containing compound added to foods.
As illustrated in Figure 7-1, the 1958 Amendment specified that sub-
|
1 |
Food and Drugs Act of 1906, Public Law 59-384, 34 Stat 768; 21 USC § 1-15 (1934); repealed in 1938 by 21 USC § 329(a). |
|
2 |
Federal Food, Drug, and Cosmetic Act of 1938, Public Law 75-717; 52 Stat 1040. |
|
3 |
Food and Drug Administration (FDA), 2009. Available online: http://www.fda.gov/regulatoryinformation/legislation (accessed October 2, 2009). |
|
4 |
Food Additives Amendment of 1958, Public Law 85-929; 72 Stat 1784. |
|
5 |
21 USC 348 and 342(a)(2)(C), and 21 CFR 170-179. |
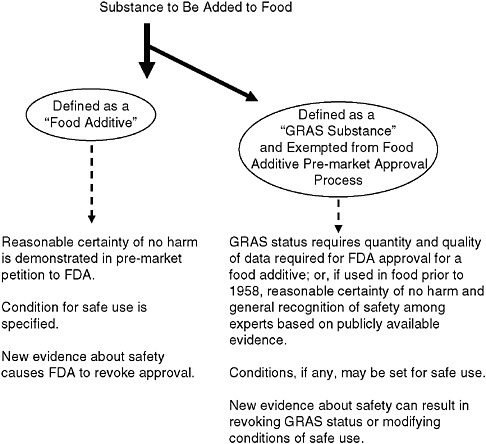
FIGURE 7-1 Pathways for a substance to gain approval for addition to food.
NOTE: FDA = U.S. Food and Drug Administration; GRAS = generally recognized as safe.
stances intentionally added to food are defined as “food additives” and, in turn, that food additives must be approved by FDA before they are added to foods. The process of approval requires scientific evidence gathered by the petitioner to demonstrate that the substance—under the conditions of its intended use—meets the safety standard of a reasonable certainty of no harm.6 This standard recognizes both that safety cannot be proven with absolute scientific certainty and that a substance may be safe for one use or under certain conditions, yet possibly unsafe for other uses or under other conditions.
At the same time that Congress set in place the food additives framework, it also concluded that many substances intentionally added to foods—
such as vinegar, baking powder, and pepper—had a long history of use in food and were commonly accepted ingredients that should not require a formal pre-market review by FDA to ensure their safety. It made little sense for these substances to undergo review or to burden FDA with the process of doing so. Therefore, a classification of substances that were regarded as generally recognized as safe (GRAS) was excluded from the definition of food additive and is not subject to the requirement of pre-market review.
To be GRAS, the intended uses of a substance must satisfy the same “reasonable certainty of no harm” safety standard that is applicable to food additives, based on the same quantity and quality of data (“scientific procedures”) required for FDA approval of a food additive, except that for substances used in food prior to 1958, the safety can be satisfied on the basis of such data or “experience based on common use in food.”7
While a substance that is GRAS is exempted from the food additive requirement of pre-approval by FDA, it must still be safe and, with reasonable certainty, cause no harm. Further, as with a food additive, a GRAS substance is considered safe only under the conditions of use for which it is recognized as safe. Therefore, importantly, a substance may be GRAS under one condition of use and not GRAS under a different condition of use.8 This concept of conditions of use can apply to the amounts added to food and is therefore important for the purposes of this report. Additionally, conclusions about the safety of a substance can be revised as warranted by new and evolving science. Therefore, the ability to retain GRAS status and the conditions of use associated with that status likewise can be changed as needed.
Salt as a GRAS Substance
FDA first issued a list of GRAS substances in December 1958, but the agency underscored that the list could not be considered complete and provided examples of GRAS substances only. The current GRAS list appears as Parts 182, 184, and 186 of the Code of Federal Regulations (CFR) and contains hundreds of substances, including salt.9 Beginning in 1969, FDA worked to review the safety of the substances included within the GRAS
list. At that time and continuing through today, no conditions of use for salt have been incorporated into the GRAS status determination, and therefore its addition to food is not limited or prescribed.
As part of its 1969 review, FDA requested the assistance of the Life Sciences Research Office, established by the Federation of American Societies for Experimental Biology. As a result, a Select Committee on GRAS Substances (SCOGS) was designated and worked to conduct a full safety review of 235 GRAS substances during the 1970s. One of the substances was salt.
In 1979, SCOGS delivered its safety review of salt to FDA. The report (SCOGS, 1979) concluded that “the evidence on sodium chloride is insufficient to determine that the adverse effects reported are not deleterious to the public health when it is used at levels that are now current and in the manner now practiced.” This conclusion raises questions about the GRAS status of added salt in light of the safety standard of “reasonable certainty of no harm” and the requirement that there be “general recognition” of safety to achieve and maintain GRAS status. Such a conclusion would typically trigger FDA action to revise or revoke the GRAS status of a substance, but the agency at that time did not begin such activities.
In early 1978, FDA was petitioned (CSPI, 2005) to reclassify salt from a GRAS substance to a food additive, which would make salt subject to pre-market approval for its use. In addition, the petitions encouraged FDA to implement other aspects of existing regulations and require a warning label on high-sodium foods and salt packets. The petitions did not request that FDA modify the conditions under which salt could be used in foods as a GRAS substance, only that salt no longer be classified as GRAS.
Based on the SCOGS report and its own analysis of regulatory options, FDA responded to the petitions by publishing a “Policy Notice” in 1982 (HHS/FDA, 1982), stating that it would not act “at this time” to revise the GRAS status of salt. The FDA called on industry to voluntarily reduce the levels of added salt in processed food based on concerns about hypertension. Furthermore, public education was emphasized, as well as a new FDA effort to expand disclosure of sodium content on product labels. FDA further stated: “The agency wishes to emphasize that if there is no substantial reduction in the sodium content of processed foods and if information [sic] sodium labeling is not adopted after a reasonable period, FDA will consider additional regulatory actions, including proposing a change in salt’s GRAS status” (HHS/FDA, 1982, p. 26593).
In 2005, the House of Representatives’ Committee on Appropriations issued a statement that encouraged the Secretary of Health and Human Services “to focus on ways—including both voluntary actions by the food industry and regulatory actions by FDA and the Department of Agri-
culture—to reduce salt in processed and restaurant foods.”10 A petition submitted to FDA in 2005 (CSPI, 2005) cited, among other reports, the congressional committee’s statement and requested FDA to (1) revoke the GRAS status of salt, (2) amend any prior sanctions for salt, (3) require food manufacturers to reduce the amount of sodium in all processed foods, (4) require health messages on retail packages of salt (≥ 0.5 oz.), and (5) reduce the Daily Value (DV)11 for sodium from its current level of 2,400 mg to 1,500 mg. In response to the petition, FDA held a public hearing in November 2007 to discuss the regulatory status of salt. The comment period for additional responses to the public hearing and the petition closed in August 2008 (HHS/FDA, 2008). To date, no further FDA action on this petition has taken place.
The Government Accountability Office (GAO) has also undertaken a study related to GRAS, which was released in February 2010 (GAO, 2010). The objectives of this work included determining the extent to which FDA’s oversight of GRAS determinations helps ensure the safety of food ingredients and the extent to which FDA reconsiders GRAS determinations. This study found that FDA does not systematically reconsider the safety of GRAS substances as new information becomes available and that the agency has acted slowly on petitions regarding GRAS status of substances including salt due to resource constraints and other priorities. As a result, one of the recommendations of the GAO report was that FDA “develop a strategy to conduct reconsiderations of the safety of GRAS substances in a more systematic manner, including taking steps such as allocating sufficient resources to respond to citizen petitions in a timely manner, [and] developing criteria for the circumstances under which the agency will reconsider the safety of a GRAS substance.”
Options for the GRAS Status of Salt on the Basis of Safety Concerns
As described above, there are two regulatory options for salt added to foods, other than maintaining the status quo of salt as a GRAS substance with no specified conditions of use. One option is to revoke the GRAS status, requiring a petition process for approval before marketing. The second is to retain salt as a GRAS substance but specify the conditions of use under which the continued addition of salt to foods can be considered safe. This would not require petitions, but food manufacturers would be required to formulate their food in such a manner that the salt levels are consistent with those recognized as safe. Uses or levels of salt in foods that could not meet the new standards could, through a petition process, be put forward as food
additive uses and have the potential for approval. This does not mean that the standard of safety is different between the GRAS approach and the food additive approach, only that there is the option, after the determination that a general recognition of safety does not exist, to permit the substance to be used as a food additive concurrent with whatever restrictions are needed to ensure its safe use.
Key Components of the Process
FDA action to either alter or revoke the GRAS status of salt would involve an established rulemaking process (see Appendix H) that would include, at a minimum, (1) public notice of proposed actions and a justification for the actions based on the available science, (2) an opportunity for public comment, and (3) a reasoned FDA response to the comments. Even prior to publishing such a proposal, FDA could publish an advanced notice of proposed rulemaking, commonly referred to as an ANPR, to outline its initial thinking and to gather information on key issues. These include but are not limited to relevant data ranging from technical processes to consumer behaviors. As part of the process, the agency could also hold public meetings, hearings, scientific consultations, or other dialogue as appropriate to resolve the GRAS status of added salt and determine the agency’s regulatory and policy approaches.
In carrying out activities to alter or revoke salt’s GRAS status, FDA would have to address both scientific and policy questions, including the following:
-
The central question, which is predominantly scientific, is whether the current levels and uses of added salt satisfy the safety standard of “reasonable certainty of no harm” based on today’s science. In addressing this question, FDA would be expected to take into account, among other sources of information and scientific findings, the recommendations of and scientific advisory documents related to the Dietary Guidelines for Americans, which in 2005 established a recommendation of less than 2,300 mg/d of sodium for the general population and no more than 1,500 mg/d for those with hypertension, African Americans, and people middle-aged or older as appropriate upper limits to reduce the risk of elevated blood pressure. Dietary Reference Intakes for sodium as established by the Institute of Medicine (IOM) might also be relevant. Consistent with the provisions established in law (21 USC 348[c][5][B]), the agency would use a total population “exposure” approach for determining the safety of salt or sodium.
-
Further, FDA would have to consider salt’s technical or functional effects in food. In any rulemaking to set standards for the level of added salt in processed food, FDA would have to solicit and analyze food industry information on the intended uses and technical effects of added salt.
Importantly for this report, FDA’s authority under food additives law extends to uses of salt in foods that are under the jurisdiction of other federal agencies. For example, the use of sodium enhancement solutions intended to tenderize raw meat and poultry products that are under the jurisdiction of the U.S. Department of Agriculture (USDA) is subject to the 1958 Amendment.12 Thus, the safety of these solutions (i.e., their risk to human health) is subject to FDA regulation. In turn, these uses of salt-containing compounds would need to be considered by FDA in reviewing the GRAS status of salt.
While FDA is charged with administering the food additives safety provisions that are applicable to meat and poultry products that are under USDA jurisdiction, USDA has its own regulatory authorities that would allow it to implement other suitable and appropriate provisions for meat and poultry products including labeling and the prohibition of deceptive uses of substances and solutions added to meat and poultry. These provisions may have sizeable impact given that USDA is responsible for food products that contain 2 percent or more by weight of meat and poultry which, in turn, constitute about 20 percent of the U.S. food supply. In 2009, USDA issued an advanced notice of proposed rulemaking related to the labeling of meat and poultry products, specifically regarding the use of the voluntary claim “natural” on such products (USDA/FSIS, 2009a). USDA specifically requested comment on whether it should approve a “natural” claim on meat and poultry products that have been enhanced with solutions that contain “natural” ingredients.
Finally, FDA would also have to address the question of so-called prior-sanctioned uses of salt. In its 1982 Policy Notice on salt (HHS/FDA, 1982), FDA noted the possibility that there may be uses of salt in processed food that had received FDA’s approval prior to 1958 and thus could be deemed prior-sanctioned. These particular uses, approved before the 1958 law went into effect, were grandfathered (i.e., not made subject to the food additive law). The 1982 Notice does not identify any prior-sanctioned uses, the agency does not maintain a listing of such uses, and the Committee’s search for prior-sanctioned uses of salt or sodium failed to locate such uses for salt or sodium. Nonetheless, it may be assumed some could exist for specific products. As a practical matter, prior-sanctioned uses are likely to
have been granted on a case-by-case basis to specific companies for specific products, and therefore reflect a very insignificant sodium contribution to the food supply.
However, even if prior-sanctioned uses of substances are identified and are considered by legal authorities to be permanently exempted from the food additive law, they remain subject to review for safety. The authority for this is within the so-called adulterations standards specified in 21 CFR 181.1(b). As FDA itself stated in 1982 and encapsulated in the CFR,13 the safety determinations reflected in those prior sanctions may be reviewed and modified where appropriate, when “scientific data or information… shows that use of a prior-sanctioned food ingredient may be injurious to health.” It should be noted that such activities must meet a higher legal standard compared to those established for the food additive provisions. Moreover, for prior-sanctioned substances, the burden would likely rest on FDA to demonstrate that such regulation is needed to prevent possible harm. In any event, while prior-sanctioned uses would constitute a technical issue for the agency, its impact is expected to be insignificant in terms of sodium contribution to the food supply particularly in contrast to the levels under the GRAS provisions.
Implementation
FDA has great flexibility in adopting regulatory standards. It may determine appropriate implementation periods for new standards and take into account factors such as consumers’ acclimation to changes in the salt taste of products. Other feasibility and related constraints may be considered in implementing new standards, including possible phase-in reductions to acceptable levels.
In considering implementing salt GRAS standards in a stepwise fashion, FDA will benefit from the experience gleaned from the effort to reduce, in a sequential manner over time, the allowable levels of sodium in foods bearing the implied nutrient content claim “healthy.” That experience demonstrates the considerable importance of gathering information and carefully weighing options before making final decisions about an implementation process relative to sodium. It would appear that if the effort results in too rapid an implementation without sufficient regard to the need to make related changes in all food products, the outcome may not be accomplished successfully.
Special Labeling to Ensure Safety
FDA has the option to require special labeling or disclosure statements on the foods containing added substances (whether food additives or GRAS substances) to ensure their safe use. The question of whether to require such labeling has a major policy dimension, as well as a scientific and consumer research dimension, whether the labeling is aimed at the general population or at high-risk subgroups. Such labeling could contribute to a conclusion that certain uses meet the safety standard of “reasonable certainty of no harm” that otherwise might not meet the standard. An example of such labeling that “fixes” a safety issue associated with an added substance is the required statement for aspartame: “Phenylketonurics: Contains phenyalanine.” In this case, there is no safe use of the substance for the subpopulation of phenylketonurics. However, with the safety hazard disclosed by required labeling, the vulnerable subpopulation is alerted, and the rest of the population can consume the substance and benefit from its inclusion in food. FDA could use such labeling as a tool where appropriate in the development of standards for the addition of salt to foods, after the needed exploration of the appropriate nature and impact of such labeling.14
Chapter 6 discusses the available research regarding consumers’ responses to such food label disclosures as well as the labeling associated with the Nutrition Labeling and Education Act (NLEA) of 1990,15 which is discussed below. It suggests the importance of carefully researching consumer response and crafting a framework for such labeling before the provisions are put in place.
Other Sodium-Containing Compounds
The focus on regulatory approaches to reduce sodium intake has centered on salt (sodium chloride) because it is the main contributor of sodium to the American diet. However, as described in Chapter 5, there are a myriad of other compounds that contain sodium and are added to foods. Some of these uses are currently GRAS, and some have been approved as food additives.
Because sodium per se and not just sodium chloride is the concern relative to reducing the risk of elevated blood pressure, the presence of all these
compounds in foods and their total contribution to the diet would need to be factored into FDA efforts to determine safe use. Considerable data gathering will be required to incorporate these compounds into the process.
REGULATION TO REQUIRE NUTRITION INFORMATION AND TO SET STANDARDS FOR LABEL CLAIMS
Background
Nutrition labeling of foods as an activity overseen by FDA began in the 1970s and was initiated, in part, due to concern about nutrient deficiencies. It was a voluntary program unless the food contained any added vitamins, minerals, or protein or a nutritional claim had been made for the food, in which case the food had to display a nutrition label. A nutrition label gave information on calories, protein, carbohydrates, fat, and some vitamins and minerals (Lecos, 1986). Information about sodium was not required in such cases unless a claim was made about sodium content. In 1981, in response to the increasing national concern about sodium intake and elevated blood pressure, FDA began to urge the food industry to voluntarily identify the sodium content of foods on the label. In 1984, FDA issued a sodium labeling regulation (HHS/FDA, 1984), which went into effect in 1986, requiring that sodium content be included on any food that bears a nutrition label. The rule also included definitions for the label claims “sodium free,” “very low sodium,” “low sodium,” and “reduced sodium” and described appropriate use of the terms “without added salt,” “unsalted,” and “no added salt” on food labels (HHS/FDA, 1984, 1985).
By the end of the 1980s, new scientific findings about diet and health were increasingly reported, and consumer interest in diet as a way to improve health was increasing. Food manufacturers were eager to market food products to take advantage of this interest. As a result, the marketplace became crowded with claims about the benefits of foods, and consumers and manufacturers expressed concern about the credibility of the food label and its potential to confuse consumers (Taylor and Wilkening, 2008a). Primarily as a result of these events, the NLEA was passed by Congress in 1990,16 amending the existing 1938 Federal Food, Drug, and Cosmetic Act.
The NLEA was a broad effort not only to reduce consumer confusion, but also to provide information that consumers needed and wanted, by requiring that nutrition labeling rules be put in place by FDA. It further stipulated that declarations of the amounts of certain nutrients would be made mandatory on labels of packaged foods. It required that the government create a framework that allowed manufacturers voluntarily to use
so-called nutrient content claims and health claims on their food labels. In doing so, the NLEA gave FDA the mandate and authority to protect consumers from misleading nutrition claims and to help consumers make more healthful food choices through better access to credible nutrition information. The NLEA was also intended, in part, to establish a level playing field for nutrition information, presumably decreasing both the need and the opportunity for marketing “hype.” Additionally, by providing the opportunity to make positive claims about their products, it sought to encourage manufacturers to formulate foods with improved nutrient profiles, such as foods lower in sodium or saturated fat (Taylor and Wilkening, 2008b). It strengthened FDA’s authority to ensure truthful and non-misleading nutrition information on foods. The NLEA focused on information needed by the general population to follow general dietary recommendations. The result was the Nutrition Facts panel, established in 1993 and now found on most packaged foods, as well as the establishment of a framework for making nutrient-related claims and health claims.
Thus, the NLEA was directed to the labeling of foods, primarily packaged foods, regulated by FDA. In addition, FDA established a voluntary labeling program for raw fruits, vegetables, and fish.17 However, the NLEA exempted nutrition labeling for restaurant foods as well as packaged foods products sold only to restaurant/foodservice operations.18 Despite the exemption, and in light of the growing proportion of American meals consumed outside the home, FDA has sought to enlist the assistance and support of restaurants in addressing national obesity concerns by urging them to provide point-of-purchase nutrition information to consumers (HHS/FDA, 2004). As signed into law in March 2010, the Patient Protection and Affordable Care Act19 contains provisions to address nutrition labeling of menu items. Restaurants with 20 or more outlets are required to post calories on menus, menu boards (including drive-thrus) and food display tags, with additional information (fat, saturated fat, carbohydrates, sodium, protein and fiber) available in writing upon request.
Further, the NLEA did not address advertising, which is under the authority of the Federal Trade Commission, nor did it cover foods regulated by USDA, which are primarily meat and poultry products. However, USDA voluntarily put in place nutrition labeling regulations consistent with those adopted by FDA.20 USDA has in place guidelines for the voluntary nutrition labeling of single-ingredient, raw products and ground or chopped meat and
poultry products,21 and it has proposed but not yet finalized regulations requiring nutrition information for these products on labels or at point of purchase (USDA/FSIS, 2009b). In order to establish comparable nutrition labeling requirements for meat and poultry products, USDA in 1993 acting under its own authorities made mandatory the nutrition labeling of meat and poultry products, other than single-ingredient, raw products. Voluntary guidelines were set in place for nutrition labeling of single-ingredient, raw meat and poultry products. In 2001, USDA proposed to make these voluntary guidelines mandatory (USDA/FSIS, 2001). This proposal was not finalized, but in December of 2009 USDA announced it would solicit further public comments on the proposed rule (USDA/FSIS, 2009b).
Sodium and the Nutrition Facts Panel
The Nutrition Facts panel, an example of which is shown in Appendix I, provides nutrient information in amounts per serving and as a percentage of the DV for certain required nutrients. Sodium is one of the required nutrients, and its declarations are expressed both as a milligram amount and as a percentage of the recommended DV, which currently is established as 2,400 mg.22 FDA regulations provide a procedure for food producers to analyze the sodium content and determine quantitative sodium levels in their products. To be in compliance with labeling requirements, the actual nutrient content must not differ from the amounts declared in the panel by more than 20 percent.23 For sodium, the actual amount can not be more than 20 percent above the declared value.24
Establishing the Daily Value for Sodium
One of the goals of the NLEA was to allow consumers to quickly and easily view and understand the nutrition information on food labels. Consumers were to be able to understand the nutrients’relative significance “in the context of the total daily diet”25—to tell at a glance whether the nutrients in a product represented a large or small amount of a “desirable” intake or an intake associated with better health. The DV information not only allows consumers to make choices about the foods they consume, but also it allows them to make trade-offs. By observing that a particular product may contribute, for example, 75 percent of the amount of sodium considered
appropriate for a daily diet while only contributing perhaps 3 percent of a desirable nutrient from the consumer’s perspective (for example, calcium), consumers can better balance their food choices. Moreover, the presence of this information on the packaged food label could incentivize the food industry overall to develop foods with better “nutrition profiles.”
At the time of NLEA implementation, FDA explored approaches to set the quantitative nutrition information within the context of a total daily diet (Levy et al., 1996; Lewis and Yetley, 1992). No single format proved best for all tasks, but the use of a percentage of a reference intake scored the highest. So, for each nutrient to be declared within the Nutrition Facts panel, FDA developed a DV. Generally, for essential nutrients, a reference value for adequate intake is used as the basis for the DV. For non-essential nutrients, such as total fat, saturated fat, and cholesterol, a reference value related to intake above which there may be harm to health is used (Taylor and Wilkening, 2008a). The current DVs were issued through notice-and-comment rulemaking and finalized in 1993.
A challenge occurred in 1993, however, in that the National Academy of Sciences (NAS) had not provided reference values for a number of nutrients and food components that the NLEA required be listed on the food label, sodium among them. Accordingly, FDA turned to the available authoritative consensus documents and extracted from them reference intakes that could form the basis for DVs for nutrients and food components without Recommended Dietary Allowances (RDAs) from the NAS. In the case of sodium, the NAS 1989 consensus report known as Diet and Health: Implications for Reducing Chronic Disease Risk was used because it suggested that an intake of more than 6 g of salt (2,400 mg of sodium) per day was associated with elevated blood pressure (NRC, 1989). The value 2,400 mg became the DV used in the Nutrition Facts panel, and the levels of sodium in a serving of food have been expressed as percentage of this DV (i.e., a percentage of 2,400 mg) since that time.
Changes to the Daily Value for Sodium
Reference values for nutrients have been established beginning in the 1940s under the auspices of the National Research Council (NRC) of the NAS. In 1994, the IOM of the National Academies began a process to expand the reference values in that instead of providing a single number meant to be a recommended intake for each of the more than 25 age, gender, or life stage groups, a set of reference values is given for each nutrient for each group. The reference values are listed in Box 7-1 where it should be noted that an Adequate Intake (AI) is established when it is not possible to determine an Estimated Average Requirement (EAR) (and in turn an RDA).
|
BOX 7-1 Current Dietary Reference Intake (DRI) Components Estimated Average Requirement (EAR): Reflects the estimated median requirement. Recommended Dietary Allowance (RDA): Derived from the EAR; covers the requirements for 97 percent of the population. Adequate Intake (AI): Used when an EAR or RDA cannot be developed; reflects an average intake level based on observed or experimental intakes or on other scientific judgments. Tolerable Upper Intake Level (UL): Highest average intake that is likely to pose no risk. |
A DRI reference value was established for sodium for the first time in 2005. The sodium reference value is now established as an AI of 1,500 mg (approximately, varying somewhat by age group) and a Tolerable Upper Intake Level (UL) of 2,300 mg (approximately, varying somewhat by age group) (IOM, 2005).26
FDA is in the process of preparing to update all DVs based on the 1997–2005 IOM effort to establish DRIs and has issued an Announcement of Proposed Rulemaking (HHS/FDA, 2007). In that announcement the agency asked the following question: “Should the Daily Reference Value (DRV) [note: basis for the DV for sodium] be based on the UL (2,300 mg/d) as suggested by the 2005 Dietary Guidelines for Americans or should it be based on the AI (1,500 mg/d using the population-coverage approach)?”
If the DV were changed to the lower AI value from the current value, which is closer to the current UL, the quantitative amount of a nutrient (500 mg, for example) per serving for a particular food would still be listed in the Nutrition Facts panel and would not change as a result of the DV change. However, the percentage of the DV as listed would change. Currently, if there were 500 mg of sodium in a serving, the label would reflect that a serving of the food contains about 20 percent of the DV, while an updated DV of 1,500 mg would result in the label indicating that a serv-
ing of the food contains 33 percent of the DV. As outlined earlier in this discussion, the DV declarations within the Nutrition Facts panel play an important role in informing consumers about the nutritional content of the packaged foods they purchase by placing the food’s nutritional contribution within the context of a total daily diet, the general target toward which consumers should strive. Therefore, the expectation is that the DV declarations will be consistent with the best thinking about the desirable composition of a daily diet.
Sodium Claims
Sodium Content Claims
As described above, the NLEA also directed FDA to establish the standards for which manufacturers could make claims on food labels. In 1993, FDA, in implementing the NLEA, made provisions for nutrient content claims, which specify how much sodium packaged foods may contain in order to bear declarations such as “sodium free,” “low sodium,” and “reduced sodium.”27 The thresholds for these claims are summarized in Table 7-1. Again, USDA made similar provisions for meat and meat products (USDA, 1993).
“Healthy” Claim
While the claim that a food is “healthy” is an implied nutrient content claim, it is in a slightly separate category, because the levels of certain nutrients besides sodium (specifically fat, saturated fat, fiber, cholesterol, vitamins A and C, calcium, iron, protein, and fiber) are also taken into account in determining whether a food can be labeled as “healthy.” As mentioned previously, the history of “healthy” claims provides a lesson for strategies to reduce sodium intake.
The term “healthy” for label claims was defined and regulated beginning in 1994 (HHS/FDA, 1994). When the rules were first issued in 1994, foods making the claim were to contain no more than 480 mg of sodium per serving or, in the case of packaged meals and main dishes, no more than 600 mg. In response to the recognized need to allow for a stepwise reduction in sodium to foster consumer acceptance and allow time for technological adjustments, the rules stipulated that after January 1, 1998, the levels of sodium permitted for a “healthy” claim were to drop to 360 mg and 480 mg, respectively.
TABLE 7-1 Definitions of Nutrient Content Claims for Sodium
|
Nutrient |
Free |
Low |
Reduced or Less |
Comments |
|
Sodium |
Less than 5 mg per serving |
140 mg or less per servinga (140 mg or less per 50 g if serving is small) |
At least 25% less sodium per serving than an appropriate reference foodb |
“Light” (for sodium-reduced products): if food is “Low Calorie” and “Low Fat” and sodium is reduced by at least 50% |
|
(21 CFR 101.61 and 9 CFR 317.361) |
||||
|
Contains no ingredient that is sodium chloride or generally understood to contain sodiumc |
||||
|
“Light in Sodium”: if sodium is reduced by at least 50% per servinge |
||||
|
“Very Low Sodium”: 35 mg or less per servingd (35 mg or less per 50 g if serving is small) |
Reference food may not be “Low Sodium” |
|||
|
“Lightly Salted”: 50% less sodium than normally added to reference food and if not “Low Sodium,” so labeled on information panel |
||||
|
“Salt Free” must meet criterion for “Sodium Free” |
||||
|
NOTES: g = gram; mg = milligram. aMeals and main dishes: 140 mg or less per 100 g. bFor meals and main dishes: at least 25% less sodium per 100 g. cExcept if the ingredient listed in the ingredient statement has an asterisk that refers to a footnote clarifying that the presence of the ingredient adds only a trivial amount of the nutrient in question. dFor meals and main dishes: 35 mg or less per 100 g. eNutrition Labeling and Education Act of 1990. Public Law 101-535, 104 Stat 2353. SOURCE: Adapted from FDA, 2008a. |
||||
This level of reduction reflects about a one-third decrease in a single step within 4 years. Yet it appears to have been problematic. In 1997, FDA was persuaded to postpone the reduction in sodium requirements based on comments that indicated technical difficulties in finding suitable alternatives for sodium and claimed that consumers would reject certain so-called “healthy” products made with lower levels of sodium or salt substitutes. The comments also voiced technological concerns with reducing sodium in food products, such as impacts on microbial safety, changes in texture and water-binding capacities, and effects on flavor characteristics of other ingredients (HHS/FDA, 1997, 2005). These comments concluded that the more stringent sodium thresholds would risk substantially eliminating existing “healthy” products from the market because of unattainable nutrient requirements or unmarketable flavor profiles. Thus, the sodium limits of 480 mg per serving and 600 mg for meals or main dishes remain in effect today, and plans to revise or reinstitute the stepwise process have not been announced.
Sodium-Related Health Claims
The provisions for health claims, as provided for by the NLEA, were intended to encourage consumption of foods with potential to improve nutrient intake and reduce the risk of chronic disease and to create standards for such claims in order to “level the playing field” for food manufacturers (Taylor and Wilkening, 2008b). Further, sodium levels in foods below a certain amount serve as one of the criteria that must be met before any health claim can be placed on the label of a food. One of the allowed health claims is that diets low in sodium are associated with a low prevalence of hypertension or high blood pressure.28 This claim has been permitted for use since 1993. To bear this claim, foods must meet the criteria for “low sodium” nutrient content claims described above. Table 7-2 provides additional information on language requirements for this claim and model claim statements. More recently, under the Food and Drug Administration Modernization Act29 a health claim was approved that states: “Diets containing foods that are good sources of potassium and low in sodium may reduce the risk of high blood pressure and stroke.” Foods bearing this claim must meet all the health claim provisions as established in earlier provisions.
APPLICATION OF FEDERAL REGULATIONS TO RESTAURANT/FOODSERVICE OPERATIONS
Application of the Food Safety Provisions and GRAS Status to Restaurant/Foodservice Menu Items
The food additive provisions of the Federal Food, Drug, and Cosmetic Act apply to substances the intended use of which results in their becoming a component of any food.30 Because the act applies to all foods that have moved in interstate commerce, FDA regulations limiting the amount of a substance in a food (in this case, salt or sodium) apply whether the substance has been added before or after the food has moved in interstate commerce.31 In essence, the thrust of the provisions are that it is unlawful to add an unapproved food additive to food before, during, or after the food’s passage in interstate commerce. Therefore, menu items are within the purview of the FDA authorities, even if they have undergone further onsite processing or are assembled onsite in restaurant/foodservice operations. This conclusion is based on the plain language of the statute, and is consis-
TABLE 7-2 Definitions of Health Claims for Sodium and Hypertension
|
Approved Claim |
Requirements for the Food |
Claim Requirements |
Model Claim, Statements |
|
Sodium and hypertension |
Must be “low sodium” as defined by 21 CFR 101.61 |
Required terms: “sodium,” “high blood pressure” |
Diets low in sodium may reduce the risk of high blood pressure, a disease associated with many factors |
|
(21 CFR 101.74) |
Includes physician statement (individuals with high blood pressure should consult their physicians) if claim defines high or normal blood pressure |
||
|
SOURCE: Adapted from FDA, 2008b. |
|||
tent with the discussions held during the committee’s open public workshop (March 30, 2009). The decision to use these authorities and the manner in which they could be implemented have the potential to be controversial, but the legal authorities in the committee’s opinion are clear.
On this basis, FDA may establish safe levels for the intended use of sodium in items prepared solely for restaurant/foodservice use and shipped in interstate (as commonly done by major restaurant/foodservice operations), just as it may for foods sold directly to consumers. Likewise, as is the case for packaged food, the agency could provide for disclosures or special labeling statements on such items as appropriate to assist restaurant/foodservice customers in achieving safe intake of sodium. Its implementation would require detailed preliminary analysis so as to ensure its success given the diverse and complicated nature of restaurant/foodservice operations.
Application of the Nutrition Labeling and Education Act to Restaurant/Foodservice Operations
Under the 1990 NLEA, the requirement for declarations about the nutritional content of a food product is limited to packaged foods, with some exceptions.32 Thus, the mandatory use of a Nutrition Facts panel type of declaration is not currently applicable to restaurant/foodservice menu items. However, the act did not limit nutrient content and health claims to packaged foods, and therefore FDA could, under its existing misbranding and enforcement authorities, regulate the voluntary use of such claims by restaurant/foodservice operators on foods at least some component of which has moved in interstate commerce. In developing its 1993 regulations, FDA exempted menu claims from the requirements
applicable to such claims on packaged foods, but notice-and-comment rulemaking could be used to expand or adjust these regulations to include restaurant/foodservice menu items as appropriate. It is important to note that such rulemaking would relate only to the voluntarily use of such claims by restaurant/foodservice operations, as they do now to packaged foods. The advantage of such provisions is that they could be considered specifically for the unique characteristics of restaurant/foodservice operations and would offer the opportunity for a consistent approach and format across this industry.
As noted earlier, while there are no specific provisions for Nutrition Facts panel type of information on menus, FDA has sought to enlist the assistance and support of restaurants in addressing national obesity concerns by urging them to provide point-of-sale nutrition information to consumers (HHS/FDA, 2004). Further, as signed into law in March 2010, the Patient Protection and Affordable Care Act33 contains provisions to address nutrition labeling of menu items. Restaurants with 20 or more outlets are required to post calories on menus, menu boards (including drive-thrus) and food display tags, with additional information (fat, saturated fat, carbohydrates, sodium, protein, and fiber) available in writing upon request. This requires national uniformity, ensuring consistency in information provided. States and localities would not be able to require additional nutrient information on menus.
STATE AND LOCAL MENU LABELING INITIATIVES
The recognition of the contribution that menu items from restaurant/foodservice operations make to the American diet, coupled with growing public health concerns about obesity and other chronic diseases, has increased the focus on point-of-purchase nutrition information within restaurant/foodservice operations. To a large extent, these initiatives are being driven by state and local public health authorities.
Before passage of the Patient Protection and Affordable Care Act, some states and localities considered or passed into law proposals to provide customers with sodium information at the point of purchase. Examples of these initiatives are summarized in Appendix J.
REFERENCES
CSPI (Center for Science in the Public Interest). 2005. Petition to revoke the GRAS status of salt, to set ceilings on the amount of sodium in processed foods, to require a health warning on packaged salt, and to reduce the Daily Value for sodium. http://www.cspinet.org/salt/fda_salt_petition.pdf (accessed September 19, 2009).
FDA (Food and Drug Administration). 2008a. Appendix A: Definitions of nutrient claims. In Guidance for industry: A food labeling guide. College Park, MD: FDA, Center for Food Safety and Applied Nutrition.
FDA. 2008b. Appendix C: Health claims. In Guidance for industry: A food labeling guide. College Park, MD: FDA, Center for Food Safety and Applied Nutrition.
Folkenberg, J. 1988. A primer on food additives. FDA Consumer 22:13-17.
GAO (Government Accountability Office). 2010. FDA should strengthen its oversight of food ingredients determined to be generally recognized as safe (GRAS). GAO-10-246. Washington, DC: Government Accountability Office.
HHS (U.S. Department of Health and Human Services)/FDA. 1982. GRAS safety review of sodium chloride; policy notice; solicitation of views. Federal Register 47(118): 26590-26595.
HHS/FDA. 1984. Food labeling; declaration of sodium content of foods and label claims for foods on the basis of sodium content. Federal Register 49(76):15510-15535.
HHS/FDA. 1985. Food labeling; declaration of sodium content of foods and label claims for foods on the basis of sodium content; extension of effective date. Federal Register 50(126):26984-26987.
HHS/FDA. 1994. Food labeling: Nutrient content claims, definition of term: Healthy; final rule. Federal Register 59(89):24232.
HHS/FDA. 1997. Food labeling: Nutrient content claims, definition of term “healthy”; final rule, partial stay. Federal Register 62(62):15390-15391.
HHS/FDA. 2004. Counting calories: Report of the working group on obesity. College Park, MD: Food and Drug Administration.
HHS/FDA. 2005. Food labeling; nutrient content claims, definition of sodium levels for the term “healthy”; final rule. Federal Register 70(188):56828-56849.
HHS/FDA. 2007. Food labeling: Revision of reference values and mandatory nutrients; advanced notice of proposed rule-making. Federal Register 72(212):62149-62175.
HHS/FDA. 2008. Salt and sodium; petition to revise the regulatory status of salt and establish food labeling requirements regarding salt and sodium; public hearing; reopening of the comment period; proposed rules. Federal Register 73(113):33027.
IOM (Institute of Medicine). 2005. Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: The National Academies Press.
Lecos, C. 1986. New regulation to help sodium-conscious consumers. FDA Consumer, May 3.
Levy, A. S., S. B. Fein, and R. E. Schucker. 1996. Performance characteristics of seven nutrition label formats. Journal of Public Policy & Marketing 15:1-15.
Lewis, C. J., and E. A. Yetley. 1992. Focus group sessions on formats of nutrition labels. Journal of the American Dietetic Association 92:62-66.
NRC (National Research Council). 1989. Diet and health: Implications for reducing chronic disease risk. Washington, DC: National Academy Press.
SCOGS (Select Committee on GRAS Substances). 1979. Evaluation of the health aspects of sodium chloride and potassium chloride as food ingredients. Bethesda, MD: Federation of American Societies for Experimental Biology, Life Sciences Research Office.
Taylor, C. L., and V. L. Wilkening. 2008a. How the nutrition food label was developed, part 1: The nutrition facts panel. Journal of the American Dietetic Association 108(3): 437-442.
Taylor, C. L., and V. L. Wilkening. 2008b. How the nutrition food label was developed, part 2: The purpose and promise of nutrition claims. Journal of the American Dietetic Association 108(4):618-623.
USDA (U.S. Department of Agriculture). 1993. Nutrition labeling of meat and poultry products; final rule. Federal Register 58(3):632-685.
USDA/FSIS (Food Safety and Inspection Service). 2001. Nutrition labeling of ground or chopped meat and poultry products and single-ingredient products; proposed rule. Federal Register 66(12):4970.
USDA/FSIS. 2009a. Product labeling: Use of the voluntary claim “natural” in the labeling of meat and poultry products. Federal Register 74(176):46951.
USDA/FSIS. 2009b. Nutrition labeling of single-ingredient products and ground or chopped meat and poultry products; supplemental proposed rule. Federal Register 74(242): 67736.






















