3
Taste and Flavor Roles of Sodium in Foods: A Unique Challenge to Reducing Sodium Intake
From a culinary perspective, salt has many desirable properties. Added salt improves the sensory properties of virtually every food that humans consume, and it is cheap. There are many reasons for adding salt to foods. The main reason is that, in many cases, added salt enhances the positive sensory attributes of foods, even some otherwise unpalatable foods; it makes them “taste” better. For people who are accustomed to high levels of salt in their food, its abrupt absence can make foods “taste” bad. If we are to successfully lower salt consumption in the population as a whole, it will be necessary to reduce salt levels in the human food supply with careful attention to their flavor-enhancing properties. Consideration of what is known about the effects of salt on food and flavor perception and why people like foods with added salt can help to inform efforts to lower salt consumption. Further, knowledge of how salt is detected by sensory receptors may aid in developing salt substitutes or enhancers that could contribute to an overall reduction of salt in the food supply.
SALT THROUGH ANCIENT TIMES
It is first important to set salt consumption in historical context. Adding salt to food is a specific human trait (although Kawai [1965] wrote about an apparently learned behavior of Japanese macaques that involved dipping potatoes in salt water rather than fresh water, presumably to improve the flavor). It is believed that the relatively high salt usage of virtually all
societies today became common beginning between 5,000–10,000 years ago (He and MacGregor, 2007; MacGregor and de Wardener, 1998; Man, 2007). Most commentators believe that the reason for early salt use was food preservation (MacGregor and de Wardener, 1998; Multhauf, 1978) and that this early use was the origin of the current high consumption. Nevertheless, with the advent of extensive salt mining and improved transportation beginning in China more than 4,000 years ago (Adshead, 1992), the characteristic taste of salted food became widely expected and accepted (Multhauf, 1978). Indeed, it has been argued that many distinguishing characteristics of human society and culture owe their origins to the desire for salt and the salt trade (Beauchamp, 1987; Bloch, 1963; Fregley, 1980).
It is difficult to know how much salt was consumed by humans prior to recent times, since the only good way to estimate intake is to determine 24-hour urinary excretion (for the most part, excess salt is not stored in the body; therefore salt balance under most normal conditions is reflected by equal input and output). Nevertheless, estimates based on historical records have been made. In an estimate of early usage, the average daily sodium intake in certain parts of China in 300 B.C. was reported to be nearly 3,000 mg/d for women and 5,000 mg/d for men (Adshead, 1992). Multhauf (1978) estimated that, in France and Britain in 1850, the human culinary intake of sodium was 4,000–5,000 mg/d. These numbers, if reliable, are within the range of the amounts consumed in many societies today (INTERSALT Cooperative Research Group, 1988). Thus, high salt intake by humans does not have its origins in twentieth-century food processing, but instead likely reflects food processing needs, especially preservation of food, that originated thousands of years ago. It should also be acknowledged that similarities in intake over time and across many different ethnic groups have led to speculation that there may be some as-yet-unknown physiological or nutritional factor that predisposes humans to desire a high salt intake (Fessler, 2003; Kaunitz, 1956; McCarron et al., 2009; Michell, 1978), but there is little experimental support for this hypothesis (Luft, 2009), and some limited data are inconsistent with it (Beauchamp et al., 1987). Further experimental evaluation about whether human sodium intake at levels far above any known physiological need is under metabolic regulation will be of interest.
TASTE VERSUS FLAVOR
Taste and flavor are terms that are often confused. The word “taste” has two meanings, one technical and the other as commonly used in the English language, which encompasses the larger concept of flavor. In this chapter, the word taste is used in its technical sense, but in other chapters of this document, it is often used in its more generic sense.
Taste as a Technical Term
The sense of taste, one of the five major senses, is defined based on anatomy. In mammals, it is the sense subserved by taste receptor cells located primarily on taste buds in the oral cavity. These taste receptor cells are innervated by branches of the seventh, ninth, and tenth cranial nerves that synapse first in the brainstem prior to sending messages to other parts of the brain (Breslin and Spector, 2008).
Most investigators agree that the sense of taste is composed of a small number of primary or basic taste qualities, usually consisting of sweet, sour, salty, bitter, and savory or umami (Bachmanov and Beauchamp, 2007). It is thought that these specific classes or categories of taste evolved to help the animal solve two of its most primary problems: the identification and ingestion of nutrients and the avoidance of poisons. As a presumed consequence of these dedicated critical functions, positive or negative responses to taste compounds (tastants) are often genetically programmed. For example, sweet tastants are generally innately liked and ingested by animals that consume plants (herbivores and omnivores—some carnivores, such as cats, do not detect sweet compounds) (Li et al., 2005). In contrast, bitter tastants are generally disliked and avoided, since many are toxic (Breslin and Spector, 2008).
Common Use of the Word Taste as a Synonym for Flavor
Virtually all foods and beverages impart sensations in addition to taste. For example, a complex food such as soup not only has taste properties (e.g., it is salty, sour, or sweet) but also has volatile compounds that give it its specific identity (e.g., pea soup compared to potato soup), and it may also have burning properties, such as those caused by hot peppers. These sensory properties are conveyed by the sense of smell (cranial nerve 1), experienced mainly through the retronasal route—from the throat up through the nasal passages and up to the olfactory receptors in the upper regions of the nasal cavity—and the sense of chemesthesis (Green et al., 1990) or irritation (cranial nerve 5), respectively. In common parlance, the entire sensation elicited by this food is called its “taste.” However, most scientists would instead use the term “flavor” to refer to this total sensation, and that is how it will be used here. It should be noted that many also include the texture of a food as a component of flavor. Taste molecules such as salt can influence flavor in many ways, some of which are described below.
Importance of Flavor in Food Acceptance
Although this chapter focuses on how the taste imparted by salt influences food palatability, it needs to be emphasized that the other chemi-
cal sensory systems (smell, chemesthesis) that contribute to overall flavor perception play a crucial role in food acceptance and thus may be useful to take into account in developing strategies to successfully reduce overall sodium in the diet (Koza et al., 2005). For example, certain volatiles detected by smell receptors are often judged as “sweet” and may contribute to judgments of a substance’s overall taste of sweetness and acceptability (Schifferstein and Verlegh, 1996). An analogous phenomenon may also occur for saltiness (e.g., Manabe et al., 2009). Recent studies imaging the human brain (e.g., using functional magnetic resonance imaging) have shown that flavor information from these separate sensory systems comes together in several parts of the brain, most prominently in the orbitofrontal cortex (Rolls et al., 2010). This leads to a unitary percept of flavor despite its being made up of anatomically independent sensory systems and emphasizes the prominent role that overall flavor perception plays in judgments of a food’s pleasantness.
More broadly, the addition of certain ingredients with high flavor impact to the cooking or manufacturing process may assist in reducing the need for added salt. For example, the addition of fresh herbs and spices, citrus, mustards, and vinegars that impart distinctive flavorings may sometimes be used instead of or in conjunction with added salt, as has been suggested by many authors writing about strategies for lowering sodium in the diet (e.g., Beard, 2004; MacGregor and de Wardener, 1998; Ram, 2008). Some cooking techniques (e.g., searing) may also help reduce the need for added salt in many foodservice operations and in home cooking, in part because they result in the production of new flavors (Ram, 2008). Whether these techniques are applicable to foods prepared by manufacturers and large foodservice operators requires study. Many foods prepared by manufacturers and in foodservice operations are necessarily highly processed; they are cooked at high temperatures for relatively long periods of time, and they must remain acceptable for extended periods. These contingencies may work against using certain flavoring techniques and fresh ingredients to reduce salt in some parts of the food supply. Further work to find alternative approaches is required.
Beyond the consideration of optimal sodium levels in a single manufactured food product, flavor issues need to be considered when evaluating the palatability of sodium levels in composite dishes, whole meals, and entire diets. The food supply contains a vast array of commercially successful products and ingredients—fresh, prepared, and manufactured—whose sodium levels range from very high to moderate to very low. The fact that the same individual, for example, might be fully satisfied with two snacks of widely varying sodium levels—one a fresh apple and the other a handful of salted pretzels—reminds us how dependent the sodium taste issue is on wider flavor contexts. The opportunities to successfully combine higher-
sodium foods with other foods that are naturally low in sodium (e.g., fresh fruits and vegetables) in dishes or meals in ways that meet consumer taste demands suggest a set of flavor questions that have not been adequately studied. However, at least for foodservice and home cooking—if to a lesser extent for food manufacturing—the salt taste challenge might be as much a matter of reconsidering flavor options in recipe selection and menu development (e.g., less aggregation of high-sodium ingredients in a single dish) as needing to overcome technical challenges with salt substitutions.
SALT TASTE: HUMAN PERCEPTION AND PREFERENCE
Tastes have several sensory attributes that can be distinguished (Breslin and Spector, 2008). Each molecule detected by the sense of taste is characterized by one or more qualities—for example, salty, sweet, and bitter. Sodium chloride, the prototypical salt taste molecule, imparts an almost pure salt taste, whereas potassium chloride, often used in lowered-sodium formulations, tastes both salty and bitter (this bitterness is one reason it is often not fully successful in replacing the sensory effects of salt).
In addition to their qualities, taste molecules impart intensity: as concentration is increased, the saltiness also increases, up to some maximum above which no further saltiness is perceived. Tastants also can be evaluated for their time course or persistence. In the case of salt, taste intensity increases within a few hundred milliseconds and then rapidly falls. This very sharp time course is generally valued by the consumer. Tastes can also be localized in the oral cavity. Salt taste can be identified by receptors throughout the oral cavity, although there is evidence that the front and sides of the tongue are more sensitive than the back (Collings, 1974).
A critical attribute of salt taste is its hedonic or pleasantness dimension. For many foods, adding salt increases the liking for that food up to a certain point, after which more salt reduces its pleasantness (palatability). This inverted “U” function of added salt can be used in formulating foods, by testing the acceptance of different salt concentrations with many consumers. For any one food, there are substantial individual differences in where the optimal point (which has been termed the “bliss point”) resides (McBride, 1994). Some of these differences are most likely due to differences in experience with salt in that food and other foods. That is, the optimal level (the bliss point) can be shifted by altering one’s salt exposure. As described later in this chapter, this theory provides a sensory basis for the committee’s recommendations. Additionally, the term “bliss point” seems to imply that the optimal level is a very precise point, when in fact there may be a fairly wide range of concentrations of added salt that are judged fully acceptable. For this reason, there may be a wide range of sodium levels within seemingly similar food categories (Figure 3-1). Moreover, this phenomenon may
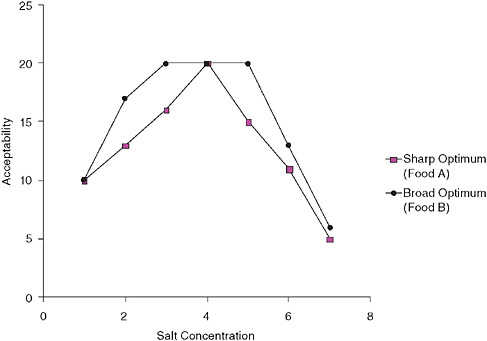
FIGURE 3-1 Hypothetical analysis of optimal salt levels in two foods, A and B. For food A, with a sharp optimum, it may be difficult to reduce salt levels quickly if it is now manufactured or served at concentration level 4. For food B, if it is currently manufactured or sold at level 4, it may be relatively easy to reduce it to level 3, since this is equally acceptable.
help to explain why it is relatively easy in some instances to substantially reduce salt in foods without reducing perceived pleasantness.
SALT FLAVOR EFFECTS
Salt imparts more than just a salt taste to overall food flavor. In work with a variety of foods (soups, rice, eggs, and potato chips), salt was found to improve the perception of product thickness, enhance sweetness, mask metallic or chemical off-notes, and round out overall flavor while improving flavor intensity (Gillette, 1985). These effects are illustrated in Figure 3-2, using soup as an example. In the figure, the distance of each of the points (e.g., “thickness,” “saltiness”) from the center point represents the intensity of that particular attribute. This figure shows that when salt is added to a soup, not only does it increase the saltiness of that soup (compare closed circles with open triangles and open circles for saltiness), but it also increases other positive attributes, such as thickness, fullness, and overall balance.
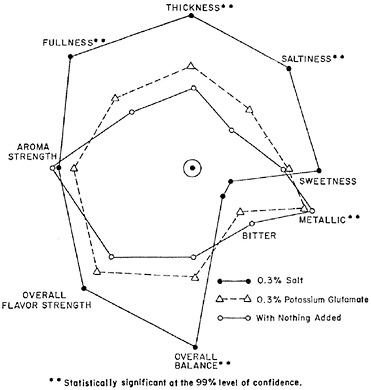
FIGURE 3-2 Aroma and flavor profiles for split pea soup with 0.3 percent salt, 0.3 percent potassium glutamate, or nothing added.
SOURCE: Gillette, 1985. Reprinted with permission.
The mechanisms underlying these varied sensory effects of salt in foods are not well understood. In particular, how salt increases the perceived body or thickness of liquids such as soups is a mystery. It is conceivable that in addition to interacting with salt taste receptor(s), salt could also activate somatosensory (touch) neural systems.
One understood mechanism by which sodium-containing compounds may improve overall flavor is by the suppression of bitter tastes. Various sodium-containing ingredients have been known to reduce the bitterness of certain compounds found in foods, including quinine hydrochloride, caffeine, magnesium sulfate, and potassium chloride (Breslin and Beauchamp, 1995). Further, the suppression of bitter compounds may enhance the taste attributes of other food components. For example, the addition of sodium acetate (which is only mildly salty itself) to mixtures of sugar and the bitter compound urea enhanced the perceived sweetness of this mixture as a consequence of sodium suppressing bitterness and thereby releasing sweetness, as illustrated in Figure 3-3. No change in sweetness was found when
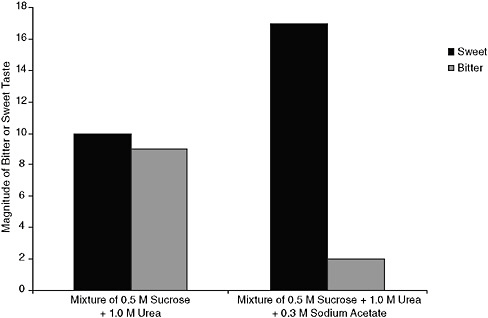
FIGURE 3-3 Magnitude of bitter or sweet taste of various solution mixtures. Adding sodium acetate to a mixture of sucrose and urea increases the sweet, sucrose taste while decreasing the bitter urea taste.
NOTE: M = molarity of solution.
SOURCE: Breslin and Beauchamp, 1997. Adapted by permission from Macmillan Publishers Ltd: Nature 387(6633):563, copyright 1997.
sodium acetate was added to sugar solutions without urea, indicating that it is the suppression of bitterness by sodium acetate that is responsible for the improved taste of those solutions (Breslin and Beauchamp, 1997).
Influence on water activity (the amount of unbound water) is another proposed reason that salt may potentiate flavors in foods. Use of salt decreases water activity, which can lead to an effective increase in the concentration of flavors and improve the volatility of flavor components (Delahunty and Piggott, 1995; Hutton, 2002). Higher volatility of flavor components improves the aroma of food and contributes greatly to flavor.
In short, salt plays a role in enhancing the palatability of food flavor beyond imparting a desirable salt taste. This non-salty sensory role may be magnified in products that have reduced amounts of other positive sensory properties (e.g., low-fat products) or increased amounts of non-preferred flavors (e.g., foods fortified with often bitter antioxidants). Consequently, in reducing salt in the food supply, it may often be necessary to identify ways to replace the flavor-modifying effects of salt. This illustrates the technological challenges that have to be met in successfully reducing salt in complex foods while maintaining their palatability. Further research is needed to understand all of the perceptual attributes of salt in foods.
MECHANISMS OF SALT TASTE
Sodium chloride—once dissociated into ions (individual atoms that carry an electrical charge)—imparts salt taste. It is now widely accepted that it is the sodium ion (Na+) that is primarily responsible for saltiness, although the chloride ion (Cl−) plays a modulatory role (Bartoshuk, 1980). For example, as the negatively charged ion (anion) increases in size (e.g., from chloride to acetate or gluconate), the saltiness declines. Many sodium compounds are not only salty but also bitter; with some anions, the bitterness predominates to such a degree that all saltiness disappears (Murphy et al., 1981).
It is believed that there are two or more types of receptors in the oral cavity, primarily on the tongue, that are responsible for triggering salt tastes (Bachmanov and Beauchamp, 2007), but major gaps in the understanding of salt taste reception remain. The most prominent hypothesis, which has been demonstrated in mice and rats, is that one set of receptors playing a role in salt taste perception involves ion channels or pores (Epithelial sodium [Na] Channels: ENaCs). ENaCs allow primarily sodium (and lithium) to move from outside the taste receptor cell, where it has been dissolved in saliva, into the taste cell. The resulting increase in Na+ inside the taste cell causes the release of neurotransmitters that eventually signal salt taste to the brain (Chandrashekar et al., 2010; McCaughey, 2007; McCaughey and Scott, 1998) (Figure 3-4). Because sodium and lithium are the only ions known to produce a purely salt taste, it is believed that these sodium-and lithium-specific channel receptors play a major role in sensing saltiness (Beauchamp and Stein, 2008; McCaughey, 2007).
The body of evidence supporting sodium channel receptors as salt taste receptors is based largely on animal models, primarily rodents. These findings indicate that the diuretic compound amiloride, a molecule that blocks sodium channels, reduces salt taste perception in these animals. In humans, however, amiloride is much less effective in blocking salt taste perception (Halpern, 1998). Nevertheless, since human salt taste mechanisms are highly unlikely to differ in fundamental ways from those of rodents, most investigators are convinced that an ENaC is the most likely receptor in humans as well. If this hypothesis is correct, it has profound implications for the search for salt substitutes. Given the specificity of this channel for sodium, it is highly unlikely that any substance could fully replace sodium (with the exception of lithium, which is unacceptable because it is highly toxic).
At least one other type of taste receptor that detects sodium chloride and some other salts is thought to exist. The hypothesis for a second receptor is based in part on work showing that some salt taste is perceived even when cations that cannot fit into the ENaC (potassium, calcium, am-
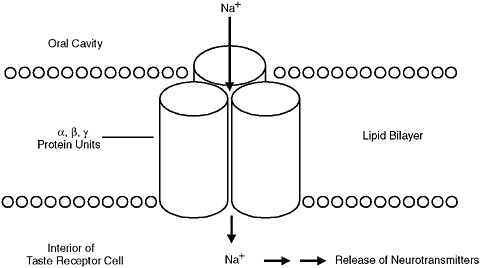
FIGURE 3-4 An epithelial sodium channel (ENaC). The epithelium is represented as a lipid (fat) bilayer (round circles), the area above the lipid bilayer (oral cavity) represents the outside of the taste receptor cell, and the area below the lipid bilayer is the interior of the taste receptor cell. The channel itself is made up of three protein units (alpha, beta, and gamma) that are represented by the cylindrical structures. This channel is thought to form a tunnel through the taste receptor cell that allows Na+ ions outside the cell to move inside the cell. This channel is quite specific to sodium, which may explain why few compounds are purely salty. Once sodium is inside the taste receptor cell it causes a cascade of biochemical reactions that result in the release of neurotransmitters that signal salt taste to the brain.
monium) are present, rather than sodium or lithium. In addition, salt still elicits a taste in animal model studies, although to a lesser extent and with less specificity, when the ENaC is blocked by amiloride (DeSimone and Lyall, 2006; McCaughey, 2007). A full understanding of how salt taste is recognized by humans, a major gap in our understanding, could facilitate the discovery of effective and economically feasible salt taste enhancers.
EVOLUTION OF SALT TASTE PERCEPTION AND PREFERENCE
It is widely assumed that the ability to detect salt—hence, salt taste perception—arose in response to the need by plant-eating organisms to ensure an adequate intake of sodium (Denton, 1982; Geerling and Loewy, 2008). Sodium is crucial to many physiological processes, and the body cannot store large amounts. Moreover, outside the sea, salt is often hard to find or in low levels in the environment (Bloch, 1963).
There are two conditions under which animals, including humans, choose to consume salt. The first, which has been widely studied in experimental animals, occurs when there is a true sodium need, such as experienced by many plant-eating animals that live in low-sodium environments. This is called salt need (Denton, 1982; Geerling and Loewy, 2008). A number of hormonal, central nervous, and behavioral systems are engaged when an animal is truly deficient in sodium, which motivates it to search for sodium salts, avidly consume them based on their salt taste, and thereby restore sodium balance (Morris et al., 2008). Sodium-depleted animals have an innate ability to recognize, by its distinct taste, the needed nutrient. Although true sodium need may be experienced by humans under some conditions and has been studied experimentally (Beauchamp et al., 1990; McCance, 1936), it is a very rare occurrence under most circumstances. It thus cannot explain why humans consume as much salt as they do (Beauchamp and Stein, 2008; Leshem, 2009). A marginal deficiency of other minerals, particularly calcium, may play a role in stimulating human salt intake (Tordoff, 1992). If this proposed relationship is supported in further studies, it would suggest that one strategy to reduce salt liking and perhaps intake would be to encourage increased calcium consumption, which is already strongly recommended for bone health (HHS, 2000).
The second condition responsible for salt intake occurs in many species, including humans, even when there is no apparent need for salt—that is, when sufficient sodium for all bodily needs has been consumed. This has been termed salt preference (Denton, 1982), even though the desire does not reflect a conscious preference. Taste preference for salt (in the absence of need) has been identified in many animals. Humans generally consume far more salt than is actually necessary and continue to enjoy salty foods even when physiological needs are met. Thus, it appears that salt preference rather than a true physiological need drives salt intake in human populations. Why people consume so much more salt than they need is a concept that is not fully understood and needs explanation.
It has been argued that a preference for salt beyond physiological need is due primarily or exclusively to learning, particularly early learning, or even that it is an addiction (Dahl, 1972; MacGregor and de Wardener, 1998; Multhauf, 1978). In contrast, other investigators have argued that while learning may play a role, evolutionary pressures to consume salt have shaped people and some other animals to have an innate liking for its taste, even when sodium is not needed (Beauchamp, 1991; Denton, 1982). Denton (1982) noted that merely because salt is consumed in excess of contemporaneous need in no way mitigates against such consumption being driven by innate propensities, just as sexual activity occurs in the absence of intent to increase numbers of the species. Even under the first hypothesis, which proposes that high salt intake is due to powerful learning, salt
consumption beyond need must necessarily provide some kind of strong reward. People generally do not become highly attracted to substances unless these substances have powerful positive physiological effects. Greater understanding of the basis for high salt preference would help guide efforts to reduce that preference. Thus, there is a need to examine the existing knowledge about the origin of preference during human development.
EARLY DEVELOPMENT OF HUMAN SALT TASTE
Although human infants need sodium in moderation (IOM, 2005), at birth, they are indifferent to salt or reject it, particularly at concentrations higher than found in human blood (hypertonic). By approximately 4–6 months of age, infants show a preference (relative to plain water) for saline solutions around the level found in blood (isotonic) or even higher (Cowart et al., 2004). This age-related hedonic shift may represent in part the maturation of the salt taste receptor cell. Some rodent studies have shown that the ability to detect salt taste matures after birth (Hill and Mistretta, 1990); this may also be the case for humans.
The amount of salt an infant consumes can influence the infant’s salt taste preference (Harris and Booth, 1985). In a study by Geleijnse et al. (1997) it was reported that children who had been randomized to either a low or normal sodium diet during the first 6 months of life exhibited differences in blood pressure when tested after 15 years of follow-up, with the low sodium group having lower blood pressures. These data are consistent with the hypothesis that lowered exposure to salt in infancy results in lower preference and intake later in life. Unfortunately data were not collected to specifically test this hypothesis.
The most dramatic effects of early environmental variation on later salt preference and intake have been observed following large sodium loss (true sodium depletion, which is very rare in adulthood) during late fetal life or early infancy. Clinical observations (Beauchamp, 1991) and studies of clinical populations (Leshem, 2009) indicate that true sodium depletion during this period may enhance later salt liking, perhaps permanently. These human studies are consistent with a large body of experimental rodent studies indicating that early depletion causes permanent changes in neural circuits that mediate salt intake. Since there is very little evidence that adult salt depletion has comparable long-term effects on salt liking (Beauchamp et al., 1990; Leshem, 2009), one may speculate that variation in salt exposure during a critical period of maturation permanently alters peripheral or central structures or both and is thereby particularly potent in establishing childhood and perhaps even adult patterns of sodium intake.
Children have been reported to have higher preference for salt than do adults (Beauchamp and Cowart, 1990; Beauchamp et al., 1990; Desor
et al., 1975). The behavioral and physiological basis for this age-related difference is not understood. It could reflect cohort effects if, for example, children were exposed to higher salt levels than adults, or it could reflect some underlying difference in the sensory or metabolic properties of salt for individuals of different ages.
Taken together, these data highlight the importance of understanding salt taste and salt taste preference in children and how early experiences modulate these sensory responses. It is likely that during infancy and childhood, the salt environment—and any changes in it that result from lowering the overall salt level in the food environment—will have the most profound effects. However, since research in this area has been limited, it is highly important that studies be conducted to evaluate how changes in salt exposure (while maintaining adequate intake) during this crucial period influence later liking.
MAINTAINING FOOD ACCEPTABILITY WHILE REDUCING SODIUM IN FOODS
In light of the considerable role that salt taste plays in food choice, it is necessary that sodium intake reduction focus on approaches that rely on modification or manipulation of salt taste along with the search for salt substitutes. Several approaches may be relevant to strategies to reduce intake.
Changes in Salt Taste Preference in Adulthood: A Potential Model for Population-Wide Reductions
Anecdotal reports, clinical impressions, and a limited body of experimental evidence suggest that when people assume a lower-sodium diet, they will gradually come to appreciate the lowered sodium and acclimate to it. For example, the Arctic explorer Stefansson (1946) reported that while he was living with Inuit groups who do not add salt to their food, he first found the foods insipid and craved salt; within a few months, however, he lost desire for added salt, and when he tasted food with it, he found it unpalatable.
Experimental evidence, albeit limited, supports these anecdotes and suggests that the preference for salt is a malleable trait. These studies reveal that when people undertake a low-sodium diet, the immediate response is to strongly dislike the foods with less salt (Beauchamp, 1991). However, the lower-sodium diet eventually becomes accepted, and in fact, foods containing the previous amount of salt may be perceived as too salty (Beauchamp et al., 1983; Blais et al., 1986; Elmer, 1988; Mattes, 1997; Teow et al., 1986). For example, one study that examined a very small number of individuals (Bertino et al., 1982) reported that after consuming
a diet with a 30–50 percent overall reduction in sodium content for 2 to 3 months, volunteers gradually developed a preference for foods with lower salt levels. In other words, they acclimated to the lower-salt diet. In a study with many more subjects, Elmer (1988) reported very similar results, as shown in Figure 3-5.
This shift in preference may also be moved in the other direction: when people were placed on a higher-salt diet, they shifted preference upward to like more salt in their foods (Bertino et al., 1986). A number of lines of evidence suggest that these shifts are due to the actual sensory experience with salt rather than some sort of physiological regulatory process (Leshem, 2009).
Most of the research on the sensory effects of lowering sodium intake was conducted more than 20 years ago, and many important questions were never fully explored. For example, it is not known whether it is necessary to reduce total sodium intake to obtain sensory accommodation or whether it would occur if salt were reduced in a single product category, such as soup or bread. That is, would the consumer begin to prefer lower-sodium soup or bread if his or her overall sodium intake was not reduced at the same time? Also, would judicious consumption of very salty food items (e.g., olives, anchovies, certain cheeses, processed meats) in the context of an overall lower-salt diet inhibit these sensory changes? Furthermore, it is also not known how long such sensory changes persist or how resistant they
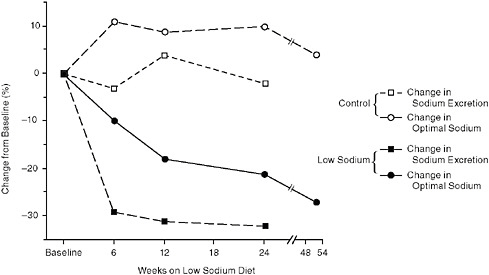
FIGURE 3-5 Shifting of salt taste preference in response to a lower-salt diet. Change in salt content of the diet indicated by the change in urinary sodium excretion.
SOURCE: Elmer, 1988.
would be to shifts back upward when an individual temporarily goes off the low-sodium diet. Finally, and perhaps most importantly, this mechanism of decreasing the desire for salt has not been tested in young children for whom, based on the arguments above, it might be particularly effective in reducing this desire. In this regard, it might have been expected that the elimination of added salt in virtually all commercially prepared baby food, which occurred more than 30 years ago (Barness et al., 1981), would have reduced salt preference in children. Unfortunately, there are no data available by which this hypothesis could be tested. And because many parents use table foods during weaning, the sensory effects of elimination of added salt to baby foods may not be easy to detect even if appropriate data were collected.
Despite these outstanding questions, it seems likely that if salt intake from foods could be reduced on a population-wide basis, consumers’ preference for salty foods would also shift downward. It will be critical to monitor this proposed shift in preference along with monitoring changes in overall consumption in any nationwide salt reduction program.
Potential Sensory Approaches for Successful Reduction of Salt in the Food Supply
Gradual Reduction Without Consumers’ Knowledge
One approach to changing ingredients in foods without the consumer noticing is to make the change gradually (Dubow and Childs, 1998). Perceptual studies with taste show that people are generally unable to detect differences between two concentrations of a taste substance when the difference is less than approximately 10 percent (called a Just Noticeable Difference [JND]; Pfaffmann et al., 1971). However, it may be the case that this estimate is misleading because it is based on sensory tests with pure taste solutions, not real foods. Foods are much more chemically complex and this complexity could make it more difficult to identify changes in individual ingredients. For example, M. Gillette1 has suggested that the JND in foods is more likely 20 percent and thus a change of 15 percent would not be noticed. However, a representative at the committee’s public information-gathering workshop (March 30, 2009) reported the opposite in some cases. Reductions in sodium content well below 10 percent in some food systems resulted in significant loss of palatability, indicating that these small changes could be perceived. A possible explanation for this is that, as discussed above, the other sensory actions of salt may be characterized by smaller JNDs. Apparently, for each food, this is an empirical question
that will require data to determine the size of a detectable salt reduction. More research in salt-flavor interactions may, however, reveal general principles that will permit predictions in different food systems. Based on this reasoning, it has been suggested that a gradual reduction of salt in food, in incremental steps, would be unnoticed by the consumer. According to this argument, if incremental reductions were instituted regularly (e.g., once each year or even more frequently), it would be possible to substantially reduce the salt content of foods over the course of several years without the consumer noticing. For example, Girgis et al. (2003) reported that 25 percent of the salt in bread could be eliminated, over a cumulative series of small decreases, without people recognizing a taste change (see also, Cauvain, 2007). All sellers of bread would have to make this reduction; otherwise, the changes would be noticed, and the reduced sodium version would be less preferred.
This is an attractive strategy for reducing salt in foods while maintaining their acceptability, and several food manufacturers are reported to have already undertaken it. However, advancements in several research areas may optimize the implementation of such a strategy. First, industry has not undertaken reduction of sodium across all foods, so there may be some individual products for which reductions may be limited. Second, it is likely that there will be a limit to reductions that can be achieved by simply lowering sodium content without additional reformulation and taste changes, but there are no published data testing the limits of this strategy. It seems likely for many foods that at some point further reductions may not be possible while maintaining consumer palatability. Determination of where the point of limited reductions resides will vary by food item and is a focus of industry research during the reformation process. Third, since salt has many sensory functions in foods in addition to making it taste salty, it is unclear whether changes in these other functions would go unnoticed following small reductions or whether additional changes in food formulations would be required.
Use of Low-Sodium Foods and Ad Libitum Salt Use
Reduction of sodium intake may be achieved by reducing salt in food and permitting people to use a salt shaker to add back to the food as much salt as desired (i.e., ad libitum salt use). For example, in one study (Figure 3-6), sodium intake from clinically prepared foods decreased from an average of 3,100 mg/d to an average of 1,600 mg/d over a 13-week period, and participants were permitted unlimited use of a salt shaker to salt their food to taste. Importantly, less than 20 percent of the overall sodium removed during food preparation was replaced by increased use of table salt—the use of which was measured without participants’ knowledge (Beauchamp et al., 1987).
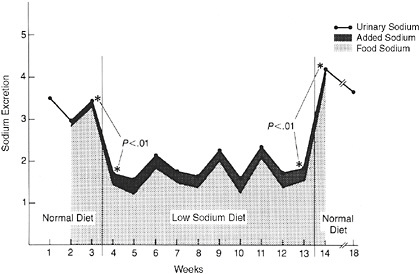
FIGURE 3-6 Failure to compensate decreased dietary sodium with increased table salt use in participants on a low-sodium diet. Sodium intake as measured by 24-hour urinary excretion is presented on the vertical axes. Participants in this study consumed approximately 3,100 mg of sodium per day (weeks 1–3, horizontal axis), a typical amount, prior to going on a low-sodium diet (1,600 mg/d on average) in a hospital (weeks 3–13). In week 14, 24-hour urines were again collected after the subjects were permitted regular foods in the hospital. The gray shaded area represents the total sodium consumed in food. The black shaded area represents the amount of sodium added by the participants from their ad libitum use of salt shakers.
SOURCE: Adapted from Beauchamp et al., 1987. Journal of the American Medical Association 258(22):3275–3278. Copyright © 1987 American Medical Association. All rights reserved.
In another study, a similar lack of salt replacement by use of table salt was found when students were fed regular or reduced-sodium beef stew. When the lower-sodium stew was served, only 22 percent of the removed sodium was replaced by use of table salt (Shepherd et al., 1989).
In both of these studies, the failure to compensate was likely due in large measure to the fact that salt was added to the surface of the food and not suffused throughout it, thereby requiring less to obtain a sufficient salt taste. Because such a low percentage of salt in the U.S. diet comes from use of the salt shaker (see Chapter 5), it may be counterproductive to recommend, as some do (MacGregor and de Wardener, 1998), that the first step in salt reduction should be to cease using salt at the table. A better approach may be to use lower-sodium foods but permit judicious use of added salt when needed to reach a sufficiently salty and flavorful sensory profile.
Use of Other Flavors or Flavoring Techniques to Reduce the Need for Added Salt
It is possible to replace some of the salt in foods with other taste or flavor compounds or through other flavor strategies or techniques. Some of these compounds or strategic elements may be added by the processor, chef, or consumer, whereas others may be created during food preparation, such as cooking.
A prominent example of an added compound involves glutamic acid (an amino acid). Combining glutamic acid with sodium creates the wellknown flavoring compound monosodium glutamate, or MSG. MSG imparts a savory taste (called “umami”) as well as a salt taste to food. Some studies have shown that it is possible to maintain food palatability with a lowered overall sodium level in a food when MSG is substituted for some of the salt (Ball et al., 2002; Roininen et al., 1996; Yamaguchi, 1987). In these cases, less MSG is added back to the food than is removed by using less salt. Other possibilities for the use of glutamates are included in Appendix D, Table D-2. It should be noted that although the use of MSG is controversial (Fernstrom, 2007), it is a generally recognized as safe (GRAS) substance.2 Beyond MSG, quite a wide number of naturally occurring or traditionally prepared foods exhibit these same “umami” qualities (e.g., mushrooms, tomatoes, vegetable extracts) that might displace some of the need for added sodium in food preparation or manufacturing (Marcus, 2005).
Potential Technological Approaches for Reduction of Salt in the Food Supply
Modification of the Size and Structure of Salt Particles
For surface applications of salt to foods (e.g., on potato chips), changing the size of salt particles can make it possible to provide the same salt taste with a lower amount of salt. Dissolution of salt in the mouth is needed to impart a salt taste, but ordinary salt particles often do not dissolve completely. Changing the size of salt particles can help improve dissolution and thereby increase the salt taste of the salt (Kilcast, 2007).
Changing the crystal structure of salt may also produce the same salt taste from reduced amounts of salt in the product (Beeren, 2009). Additional technologies being investigated to provide salt taste with less salt include mock salts and multiple emulsions. Mock salts are starch particles coated in a thin layer of salt. For topically applied salt applications, these particles can create surface coverage with less salt (Kilcast, 2007).
Multiple emulsions are also being investigated as a way to maintain salt taste in sodium- and fat-reduced emulsion products. These emulsions consist of water droplets dispersed in fat droplets that are then dispersed in another outer layer of water that contains salt. The inner layer of water dispersed in the fat droplets can be sodium-free and can replace some of the volume of the product, requiring less of the outer, salted aqueous phase (Figure 3-7). As a result, consumers of these products will continue to enjoy the salt taste of the outer aqueous phase while consuming less total sodium.3
Use of Different Salt Sources: Sea Salt
It is possible that the crystal structure of sea salt may be responsible for its pleasing taste profile when used on the surface of foods (Kilcast, 2007). Sea salt usually contains minerals in addition to sodium that impart a variety of tastes that may be desirable in some cases, but may also impart bitter aftertastes. While unsubstantiated reports from trade journals suggest that sea salt may contain as little as 41 percent sodium chloride (Pszczola, 2007), sodium chloride is the main component of most sea salt and thus its composition is similar to table salt.
Use of Substitutes and Enhancers
One approach to reducing salt in the food system would be the development of salt substitutes with the same sensory properties as salt but without the sodium—a sort of aspartame or sucralose but for salt. Alternatively, one might develop a salt taste enhancer, a compound that magnifies the taste of low levels of salt. Adequate substitutes and enhancers for many uses do not yet exist, but one way to attempt to identify such molecules is to use the salt taste receptor to assay for such effects. Unfortunately, the molecular and cellular mechanisms underlying salt taste perception are not fully understood, and this represents a major gap in both our understanding and our ability to efficiently search for salt substitutes and enhancers.
The hypothesized specificity of the salt taste mechanism makes the existence of a true salt taste substitute unlikely, although not impossible. Thus, this differs in principle from a sweet taste, where the receptor mechanisms are more easily mimicked by other molecules; as a consequence, there exist many alternative sweeteners (Beauchamp and Stein, 2008). Many of the alternative sweeteners now used were discovered serendipitously, but no non-sodium, primarily salty-tasting molecule has ever been identified, with perhaps the single exception of potassium chloride.
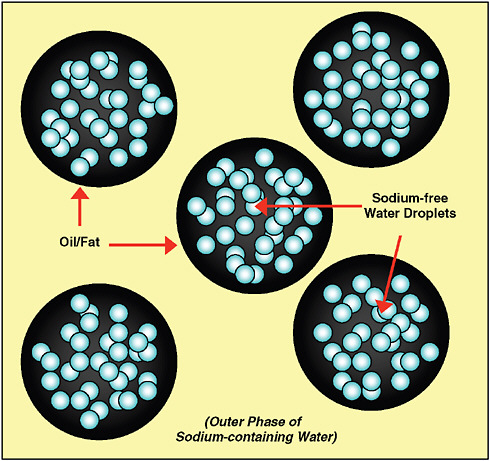
FIGURE 3-7 Multiple emulsion consisting of fat droplets dispersed in the outer phase of sodium-containing water and other water-soluble components. To expand the size of the fat droplets and create less need for the sodium-containing outer phase, sodium-free water droplets are dispersed within the fat.
SOURCE: Adapted from Beeren, 2009.
Potassium chloride has been proposed as a salt substitute either alone or in combination with table salt. However, in addition to tasting salty, many people find potassium chloride bitter (Beauchamp and Stein, 2008). Nonetheless, the interest in increasing potassium consumption among Americans has resulted in considerable interest in pursuing potassium chloride as a salt substitute. As shown in Appendix D, Table D-1, many foods use potassium chloride mixed with sodium chloride in up to a 50:50 ratio; a significant increase in bitterness is observed when a higher ratio is used (Desmond, 2006; Gou et al., 1996). Other salt substitutes have been proposed, but most of the claims remain scientifically unverified (see Appendix D, Table D-1).
Although identification of a salt substitute analogous to artificial sweeteners is thus unlikely, a salt enhancer—that is, a compound that does not taste salty itself but increases the taste intensity of a low amount of salt—is more likely. Indeed, the patent literature contains proposed examples, and recently some of the patent claims have been supported in peer-reviewed papers (Stähler et al., 2008). A concerted effort to identify salt taste enhancers could provide additional tools for overall reduction of salt in the food supply. Examples of putative salt enhancers are listed in Appendix D, Table D-2.
REFERENCES
Adshead, S. A. M. 1992. Salt and civilization. Christchurch, New Zealand: Canterbury University Press.
Bachmanov, A. A., and G. K. Beauchamp. 2007. Taste receptor genes. Annual Review of Nutrition 27:389-414.
Ball, P., D. Woodward, T. Beard, A. Shoobridge, and M. Ferrier. 2002. Calcium diglutamate improves taste characteristics of lower-salt soup. European Journal of Clinical Nutrition 56(6):519-523.
Barness, L. A., P. R. Dallman, and H. Anderson. 1981. Sodium intake of infants in the United States. Pediatrics 68(3):444-445.
Bartoshuk, L. M. 1980. Sensory analysis of the taste of NaCl. In Biological and behavioral aspects of salt intake, edited by M. R. Kare, M. J. Fregly, and R. A. Bernard. New York: Academic Press. Pp. 83-98.
Beard, T. C. 2004. Salt matters: A consumer guide, edited by M. Stowasser and M. Riley. South Mebourne, Australia: Lothian.
Beauchamp, G. K. 1987. The human preference for excess salt. American Scientist 75(January-February):27-33.
Beauchamp, G. K. 1991. Salt preference in humans. In Encyclopedia of human biology. 1st ed. Vol. 6, edited by R. Dulbecco. New York: Academic Press.
Beauchamp, G. K., and B. J. Cowart. 1990. Preference for high salt concentrations among children. Developmental Psychology 26(4):539-545.
Beauchamp, G. K., and L. J. Stein. 2008. Salt taste. In The senses: A comprehensive reference. 6 vols. Vol. 4, edited by A. I. Basbaum. New York: Elsevier. Pp. 401-408.
Beauchamp, G. K., M. Bertino, and K. Engelman. 1983. Modification of salt taste. Annals of Internal Medicine 98(5, Supplement):763-769.
Beauchamp, G. K., M. Bertino, and K. Engelman. 1987. Failure to compensate decreased dietary sodium with increased table salt usage. Journal of the American Medical Association 258(22):3275-3278.
Beauchamp, G. K., M. Bertino, D. Burke, and K. Engelman. 1990. Experimental sodium depletion and salt taste in normal human volunteers. American Journal of Clinical Nutrition 51(5):881-889.
Beeren, C. 2009. Technological innovations for reducing sodium in foods. Presented at the Institute of Medicine Committee on Strategies to Reduce Sodium Intake’s Public Information-Gathering Workshop, March 30, Washington, DC.
Bertino, M., G. K. Beauchamp, and K. Engelman. 1982. Long-term reduction in dietary sodium alters the taste of salt. American Journal of Clinical Nutrition 36(6):1134-1144.
Bertino, M., G. K. Beauchamp, and K. Engelman. 1986. Increasing dietary salt alters salt taste preference. Physiology and Behavior 38(2):203-213.
Blais, C. A., R. M. Pangborn, and N. O. Borghani. 1986. Effect of dietary sodium restriction on taste responses to sodium chloride: A longitudinal study. American Journal of Clinical Nutrition 44(2):232-243.
Bloch, M. R. 1963. The social influence of salt. Scientific American 209(1):89-98.
Breslin, P. A. S., and G. K. Beauchamp. 1995. Suppression of bitterness by sodium: Variation among bitter taste stimuli. Chemical Senses 20(6):609-623.
Breslin, P. A. S., and G. K. Beauchamp. 1997. Salt enhances flavour by suppressing bitterness. Nature 387(6633):563.
Breslin, P. A. S., and A. C. Spector. 2008. Mammalian taste perception. Current Biology 18(4):148-155.
Cauvain, S. P. 2007. Reduced salt in bread and other baked products. In Reducing salt in foods: Practical strategies, edited by D. Kilcast and F. Angus. Cambridge, UK: Woodhead. Pp. 283-295.
Chandrashekar, J., C. Kuhn, Y. Oka, D. A. David A. Yarmolinsky, E. Hummler, N. J. P. Ryba, and C. Zuker. 2010. The cells and peripheral representation of sodium taste in mice. Nature 464(7286):297-301.
Collings, V. B. 1974. Human taste response as a function of locus of stimulation on the tongue and soft palate. Perception & Psychophysics 16(1):169-174.
Cowart, B. J., G. K. Beauchamp, and J. A. Mennella. 2004. Development of taste and smell in the neonate. In Fetal and neonatal physiology. 3rd ed. Vol. 2, edited by R. A. Polin, W. W. Fox, and S. H. Abman. Philadelphia, PA: W.B. Saunders Co. Pp. 1819-1827.
Dahl, L. K. 1972. Salt and hypertension. American Journal of Clinical Nutrition 25(2): 231-244.
Delahunty, C. M., and J. R. Piggott. 1995. Current methods to evaluate contribution and interactions of components to flavour of solid foods using hard cheese as an example. International Journal of Food Science and Technology 30:555-570.
Denton, D. 1982. The hunger for salt: An anthropological, physiological and medical analysis. New York: Springer-Verlag.
DeSimone, J. A., and V. Lyall. 2006. Taste receptors in the gastrointestinal tract III. Salty and sour taste: Sensing of sodium and protons by the tongue. American Journal of Physiology—Gastrointestinal and Liver Physiology 291(6):G1005-G1010.
Desmond, E. 2006. Reducing salt: A challenge for the meat industry. Meat Science 74(1): 188-196.
Desor, J. A., L. S. Greene, and O. Maller. 1975. Preferences for sweet and salty in 9 to 15 year old and adult humans. Science 190(4215):686-687.
Dubow, J. S., and N. M. Childs. 1998. New coke, mixture perception, and the flavor balance hypothesis. Journal of Business Research 43(3):147-155.
Elmer, P. J. 1988. (unpublished PhD dissertation) The effect of dietary sodium reduction and potassium chloride supplementation on sodium chloride taste perceptions in mild hypertensives. University of Minnesota.
Fernstrom, J. D. 2007. Health issues relating to monosodium glutamate use in the diet. In Reducing salt in foods: Practical strategies, edited by D. Kilcast and F. Angus. Cambridge, UK: Woodhead. Pp. 55-76.
Fessler, D. M. T. 2003. An evolutionary explanation of the plasticity of salt preferences: Prophylaxis against sudden dehydration. Medical Hypotheses 61(3):412-415.
Fregly, M. S. 1980. Salt and social behavior. In Biological and behavioral aspects of salt intake, edited by M. R. Kare, M. J. Fregly and R. A. Bernard. New York: Academic Press.
Geerling, J. C., and A. D. Loewy. 2008. Central regulation of sodium appetite. Experimental Physiology 93(2):177-209.
Geleijnse, J. M., A. Hofman, J. C. M. Witteman, A. A. J. M. Hazebroek, H. A. Valkenburg, and D. E. Grobbee. 1997. Long-term effects of neonatal sodium restriction on blood pressure. Hypertension 29(4):913-917.
Gillette, M. 1985. Flavor effects of sodium chloride. Food Technology 39(6):47-52.
Girgis, S., B. Neal, J. Prescott, J. Prendergast, S. Dumbrell, C. Turner, and M. Woodward. 2003. A one-quarter reduction in the salt content of bread can be made without detection. European Journal of Clinical Nutrition 57(4):616-620.
Gou, P., L. Guerrero, J. Gelabert, and J. Arnau. 1996. Potassium chloride, potassium lactate and glycine as sodium chloride substitutes in fermented sausages and in dry-cured pork loin. Meat Science 42(1):37-48.
Green, B. G., J. R. Maron, and M. R. Kare (eds.). 1990. Chemical senses (Volume 2: Irritation). New York: Marcel Dekker.
Halpern, B. 1998. Amiloride and vertebrate gustatory responses to NaCl. Neuroscience and Biobehavioral Reviews 23:5-47.
Harris, G., and D. A. Booth. 1985. Sodium preference in food and previous dietary exposure in 6-month old infants. IRCS Journal of Medical Sciences 13:1178-1179.
He, F. J., and G. A. MacGregor. 2007. Dietary salt, high blood pressure and other harmful effects on health. In Reducing salt in foods: Practical strategies, edited by D. Kilcast and F. Angus. Cambridge, UK: Woodhead. Pp. 18-54.
HHS (U.S. Department of Health and Human Services). 2000. Healthy People 2010: Understanding and improving health and objectives for improving health, Volume II, 2nd edition. 2nd ed. Washington, DC: U.S. Government Printing Office.
Hill, D. L., and C. M. Mistretta. 1990. Developmental neurobiology of salt taste sensation. Trends in Neurosciences 13(5):188-195.
Hutton, T. 2002. Sodium: Technological functions of salt in the manufacturing of food and drink products. British Food Journal 104(2):126-152.
INTERSALT Cooperative Research Group (Rose, G., Stamler, J., Stamler, R., Elliott, P., Marmot, M., Pyorala, K., Kesteloot, H., Joossens, J., Hansson, L., Mancia, G., Dyer, A., Kromhout, D., Laaser, U., Sans, S.). 1988. INTERSALT: An international study of electrolyte excretion and blood pressure. Results for 24-hour urinary sodium and potassium excretion. British Medical Journal 297(6644):319-328.
IOM (Institute of Medicine). 2005. Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: The National Academies Press. Pp. 269-423.
Kaunitz, H. 1956. Causes and consequences of salt consumption. Nature 178(4543): 1141-1144.
Kawai, M. 1965. Newly-acquired pre-cultural behavior of the natural troop of Japanese monkeys on Koshima islet. Primates 6(1):1-30.
Kilcast, D. 2007. Cutting sodium. Prepared Foods January:1-5.
Koza, B. J., A. Cilmi, M. Dolese, and D. A. Zellner. 2005. Color enhances orthonasal olfactory intensity and reduces retronasal olfactory intensity. Chemical Senses 30(8):643-649.
Leshem, M. 2009. Biobehavior of the human love of salt. Neuroscience and Biobehavioral Reviews 33(1):1-17.
Li, X., W. Li, H. Wang, J. Cao, K. Maehashi, L. Huang, A. A. Bachmanov, D. R. Reed, V. Legrand-Defretin, G. K. Beauchamp, and J. G. Brand. 2005. Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS Genetics 1(1):1-35.
Luft, F. C. 2009. More mixed messages in terms of salt. Clinical Journal of the American Society of Nephrology 4(11):1699-1700.
MacGregor, G., and H. E. de Wardener. 1998. Salt, diet and health: Neptune’s poisoned chalice: The origins of high blood pressure. Cambridge, UK: Cambridge University Press.
Man, C. M. 2007. Technological functions of salt in food products. In Reducing salt in foods: Practical strategies, edited by D. Kilcast and F. Angus. Cambridge, UK: Woodhead. Pp. 157-173.
Manabe, M., S. Ishizaki, T. Yoshioka, and N. Oginome. 2009. Improving the palatability of salt-reduced food using dried bonito stock. Journal of Food Science 74(7):S315-S321.
Marcus, J. B. 2005. Culinary applications of umami. Food Technology 59(5):24-30.
Mattes, R. D. 1997. The taste for salt in humans. American Journal of Clinical Nutrition 65(2 (Supplement)):692S-697S.
McBride, R. L. 1994. The bliss point as a measure of pleasure. In Pleasure, the politics and the reality, edited by D. M. Warburton. New York: John Wiley & Sons.
McCance, R. A. 1936. Medical problems in mineral metabolism. Lancet 1:832-830.
McCarron, D. A., J. C. Geerling, A. Kazaks, and J. S. Stern. 2009. Can dietary sodium intake be modified by public policy? Clinical Journal of the American Society of Nephrology 4(1):1878-1882.
McCaughey, S. 2007. Dietary salt and flavor: Mechanisms of taste perception and physiological controls. In Reducing salt in foods: Practical strategies, edited by D. Kilcast and F. Angus. Cambridge, UK: Woodhead. Pp. 77-98.
McCaughey, S. A., and T. R. Scott. 1998. The taste of sodium. Neuroscience and Biobehavioral Reviews 22(5):663-676.
Michell, A. R. 1978. Salt appetite, salt intake, and hypertension: A deviation of perspective. Perspectives in Biology and Medicine 21(3):335-347.
Morris, M. J., E. S. Na, and A. K. Johnson. 2008. Salt craving: The psychobiology of pathogenic sodium intake. Physiology and Behavior 94(5):709-721.
Multhauf, R. P. 1978. Neptune’s gift: A history of common salt. Edited by T. P. Hughes, L. Hannah, M. Kranzberg and L. White. Baltimore, MD: The Johns Hopkins University Press.
Murphy, C., A. V. Cardello, and J. G. Brand. 1981. Tastes of fifteen halide salts following water and NaCl: Anion and cation effects. Physiology and Behavior 26(6):1083-1095.
Pfaffmann, C., L. M. Bartoshuk, and D. H. McBurney. 1971. Taste psychophysics. In Handbook of sensory physiology. Vol. IV; Chemical Senses, Part 2, edited by L. M. Beidler. New York: Springer Verlag. Pp. 327-343.
Pszczola, D. 2007. Savoring the possibilities. Food Technology 61(4):55-66.
Ram, C. 2008. Shaking things up: Low-sodium dishes offer flavor without sacrifice. Plate 59-64.
Roininen, K., L. Lahteenmaki, and H. Tuorila. 1996. Effect of umami taste on pleasantness of low-salt soups during repeated testing. Physiology and Behavior 60(3):953-958.
Rolls, E. T., H. D. Critchley, J. V. Verhagen, and M. Kadohisa. 2010. The representation of information about taste and odor in the orbitofrontal cortex. Chemosensory Perception 3(1):16-33.
Schifferstein, H. N. J., and P. W. J. Verlegh. 1996. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychologica 94(1):87-105.
Shepherd, R., C. A. Farleigh, and S. G. Wharf. 1989. Limited compensation by table salt for reduced salt within a meal. Appetite 13(3):193-200.
Stähler, F., K. Riedel, S. Demgensky, K. Neumann, A. Dunkel, A. Täubert, B. Raab, M. Behrens, J. Raguse, T. Hofmann, and W. Meyerhof. 2008. A role of the epithelial sodium channel in human salt taste transduction? Chemosensory Perception 1(1):78-90.
Stefansson, V. 1946. Not by bread alone. New York: The Macmillan Company.
Teow, B. H., R. D. Nicolantonio, and T. O. Morgan. 1986. Sodium chloride preference and recognition threshold in normotensive subjects on high and low salt diet. Clinical and Experimental Hypertension 7(12):1681-1695.
Tordoff, M. G. 1992. Influence of dietary calcium on sodium and calcium intake of spontaneously hypertensive rats. American Journal of Physiology—Regulatory Integrative and Comparative Physiology 262:370-381.
Yamaguchi, S. 1987. Fundamental properties of umami in human taste sensation. In Umami: A basic taste, edited by Y. Kawamura and M. R. Kare. New York: Marcel Dekker.
























