3
Clinical Data as a Public Good for Discovery
INTRODUCTION
Clinical data have immense potential to drive progress in health care by providing the means to measure and track care processes and outcomes, to develop and refine best practices, and to enable rapid discovery and innovation. Over the past decade, the amount of data captured in electronic form has increased exponentially. Given the federal government’s encouragement of the adoption of electronic health records (EHRs), these data will increasingly be augmented by clinically rich course-of-care data. With the potential to be easily stored, aggregated, and shared, these data can enable rapid learning and continuous improvement in the efficiency and effectiveness of care practices (Blumenthal, 2010; IOM, 2007). Harnessing the power of these data, however, will require efforts to address barriers to their access and use.
The papers in this chapter explore the potential of clinical data to improve research and health care, strategies to enhance access to health data and information, and key challenges to ensure data integrity (e.g., privacy, security, and proprietary concerns). Additionally, this chapter discusses opportunities to better inform and engage patients and the public as advocates.
The first paper, by Farzad Mostashari of the Office of the National Coordinator for Health Information Technology (ONC), argues that as the nation works to develop a unified health information technology (HIT) infrastructure, efforts will be needed to identify a limited set of core data that can meet the basic needs of multiple functions. As ONC continues its efforts to encourage and support data capture and use, it is considering how
to create data requirements that are relevant and not burdensome, how to reward patients and providers for the creation and documentation of structured data, and the merits of distributed versus centralized approaches to information exchange.
Todd Park of the Department of Health and Human Services (HHS) highlights the significant amount of data currently held by the various agencies of HHS and how these data could improve the value, science base, and patient experience of health care. He describes HHS’s efforts to open access to high-value data sets and to encourage public participation in the use of these data for socially beneficial purposes.
The quality and accuracy of research results depend on the availability and integrity of data. Don Detmer of the University of Virginia discusses opportunities to increase the quantity and quality of data for health care and research. To achieve this end, he proposes several options for national policy that address security and privacy concerns while empowering citizens to allow their health data to be used for learning and discovery.
INFORMATION NEEDS FOR A LEARNING HEALTH SYSTEM
Farzad Mostashari, M.D., Sc.M.
Office of the National Coordinator for Health Information Technology
The mission and goal of the Office of the National Coordinator for Health Information Technology is to improve health and health care for all Americans through the appropriate use of HIT. ONC is therefore focused on characterizing key system needs and outcomes in addition to determining how technology can be a means to that end.
Meaningful Use and a Learning Healthcare System
“Meaningful use” is the term coined by Congress to connect technology to desired outcomes, and ONC’s proposed regulations represent our best guess as to how technology should be used to achieve these outcomes. If providers—eligible professionals or hospitals—use HIT systems in a meaningful way, they will be able to qualify for payments from CMS.
It is our goal, however, that meaningful use be applied for more than just qualifying for payments. Rather, it should be used to ensure that measurable improvements are made in health and in the quality, safety, and efficiency of health care. A secondary goal is to move beyond improving care for an individual patient at the point of care to creating a learning healthcare system. This is an outcome to which ONC aspires and a worthy endpoint toward which to build.
The next decade will witness a fundamental transformation of the
healthcare delivery system, including changes that in many ways will be more profound than those that transpired during the previous decades of American medicine. HIT will certainly help providers take better care of patients, but there is also the prospect that, in the aggregate, electronic health systems will contribute to a learning healthcare system and enable providers to understand and influence healthcare improvement—whether in public health, quality improvement, drug discovery, or clinical effectiveness research. The HIT infrastructure of the future should not just be the eyes and ears but also be the action arm of population health.
Key Considerations for Developing a National Unified HIT Infrastructure
A number of projects now under way focus on developing HIT infrastructure for many different functions, including clinical effectiveness research, drug discovery, quality measurement, and public health surveillance. Because these projects are taking different approaches and building different architectures, it is temping to call for a halt to work on these siloed activities. Indeed, if investment in HIT continues to create and support a multitude of data islands, the nation will not achieve a unified HIT infrastructure.
This is the critical challenge faced by ONC in its work to implement a national system, to define meaningful use, and to develop grant programs: How can we work to develop a common, national HIT infrastructure while not creating additional network and system silos? Because projects now under way will not be put on hold, this is a critical time to bring key stakeholders and HHS together to begin a discussion on laying the groundwork for a unified HIT infrastructure.
Clinical Data Needs and the 80/20 Rule
One approach to clarifying key data needs for a learning health system is to agree on a core data set—sufficient, if not perfect, for a number of information needs. Known as the 80/20 rule, this approach advocates starting with something simple that can be achieved now and developing clever approaches over time. The American Recovery and Reinvestment Act (ARRA) presented a significant opportunity for HIT in this respect, as it gave patients a right to their records in electronic format. Even if only a small fraction of the patients in any given system choose to exercise this right, every healthcare provider and EHR vendor must produce a patient summary document in a common format. This could be a key opportunity to use the clinical care summary—which includes the medications list, the problem list, the allergies, the lab values, and the patient encounters—as
the foundational 80 percent, the common data core on which to build a unified HIT infrastructure.
As data users have a wide variety of needs and will require additional information based on these needs, some users are likely to believe that starting with the clinical care summary is inadequate. For quality reporting, for example, detailed information with which to calculate exclusions may be needed; for public health case reporting, information on whether a certain infection was central line associated may be required; for drug safety, the first date of prescribing of the medication may be needed. Although this may be true in each instance, the nation will never achieve a unified HIT infrastructure if the starting point is to identify a data set that is perfect for each user. An alternative approach is to leverage the 80/20 rule and supplement it with targeted additional data collection where needed.
Creating Infrastructure for Targeted Data Collection
The example of clinical trials recruitment at a storefront doctor’s office illustrates this approach. One cannot expect a provider to use an EHR in the routine delivery of care while also collecting all the information needed to determine patient eligibility for a multitude of clinical trials. As one example, an individual’s occupation may be a data element necessary to determine eligibility for a clinical trial. Although those with an interest in this information may think that requiring it as a data element should be elementary, a provider’s front-office staff is not going to determine and select each patient’s occupation from among the hundreds of potential Census Bureau categories as a routine part of delivering care.
A more feasible approach would be to collect a limited amount of information in the routine delivery of care, which could also serve as an opportunity to screen for the need for additional data collection. Instead of asking each provider to record each patient’s occupation and expecting these data to be collected in a structured form, a system could be developed to trigger deeper data collection when appropriate (i.e., manual rather than routine data collection on a small subset of patients). If a patient is diagnosed with hepatitis A, for example, it is entirely appropriate to prompt the clinician to inquire about the patient’s occupation—specifically, whether the patient is a healthcare worker, daycare worker, or food worker. This type of approach makes sense to providers, as the information requested is relevant to the person’s care and is limited in scope. For clinical trials, minimal information can also serve as an initial screen, with follow-up questions about trial participation asked only as appropriate.
This example clearly illustrates that despite the broad data needs of a learning health system, the nation should start with something simple—perhaps the humble clinical care summary. Additional work could expand
on this data core to create infrastructure that supports additional and targeted data collection only as needed.
Adding Value for Providers and Patients
To ensure data quality, it is also important to consider the value proposition for data capture and use. The provider is doing work that involves both the care of the patient and the creation and documentation of the structured data needed for other purposes. A business case needs to be made to ensure that the latter work is done well. For recruitment of clinical trial participants, for example, it would be beneficial for all if recruiting appropriate patients for trials was not just the right thing to do, but also a source of income for the primary care provider. As the owner of the data, this notion extends to the patient as well. Perhaps patients should also benefit financially from the use of their health information.
Choosing the Best Approach for Critical Infrastructure
ONC continues to identify the key principles that should underlie the creation of a national HIT infrastructure and develop supporting policies and incentives. A particularly important principle to consider as we work toward achieving our goals is to use a distributed rather than centralized infrastructure. Although a centralized infrastructure works well for some purposes, it is not particularly effective for supporting the participation of individual physicians in advancing a wide range of population health missions.
A centralized infrastructure requires separation of the data producer from the data, centralization of the data, and central analysis. In today’s fragmented and heterogeneous system, this approach typically does not yield the answers people expect in a timely way. The costs are always higher, the points of failure are always greater, the system is more fragile, and the quality and cost of cleaning the data (once separated from the source) become prohibitive. My view is that a decentralized system whereby the questions go out to the data and the answers come back is a much more resilient, feasible, cost-effective, and privacy-protective approach.
Part of ONC’s mission is to think about how such distributed queries can be expressed in a standardized way. Again, the 80/20 rule can be applied to these issues—whether to develop a standardized data model across spheres of activity or a standardized way of expressing the question or receiving responses. Formulating a strategy across fields, whether public health surveillance, quality measurement, or effectiveness research, will require simple case definitions tailored to the needs of the field—perhaps
one case definition for public health, two (numerator and denominator and maybe exclusions) for quality reporting, and four (i.e., a 2 × 2 table) for much of clinical effectiveness research and drug discovery.
Next Steps
Throughout its work, ONC needs to consider opportunities to streamline data needs and create limited but multifunctional shared elements of a unified HIT infrastructure. These actions can include identification of overriding policies and principles, such as those discussed above (“architecture”); establishment of basic, shared infrastructure (standards, services, directories, terminologies, value sets); development and deployment of common tools (e.g., CONNECT, National Health Information Network [NHIN] Direct, popHealth); demonstrations (e.g., Beacon Community Program); research (e.g., the Safety and Health Achievement Recognition Program [SHARP]); and coordination across federal agencies. ONC looks forward to receiving the input of all stakeholders and to making progress on these critical issues. How much progress the nation will make depends on our collective ability to start simple and work together.
OPENING ACCESS TO HIGH-VALUE DATA SETS
Todd Park
Department of Health and Human Services
Across many industries, opening access to and encouraging the use of quality data has driven dramatic innovation and performance improvement. Recognition of data’s transformative potential is reflected in the Open Government initiative launched by the Obama Administration in 2009, which seeks to enhance the efficiency and effectiveness of the public sector through improved transparency, public participation, and collaboration. The vast stores of data captured by HHS agencies, for example, are an important national resource for enhancing the value, science base, and patient experience of health care. The creation within HHS of the position of Chief Technology Officer—whose mission is to leverage the power of data, technology, and innovation to improve health—and the development of an HHS Open Government Plan signal HHS’s commitment to the principles of open government and to playing a catalytic role in the development of a learning health system.
Open Government
Fostering the development of open government is an important means of improving government efficiency and effectiveness and ensuring public trust, and is an ongoing priority of the Obama Administration. Almost immediately following his inauguration, the President issued a memorandum to heads of Executive departments and agencies outlining key principles of open government—transparency, public participation, and collaboration.1
- Transparency means publishing data with the intent of improving government accountability and enabling citizens to access and use data in ways that are socially beneficial.
- Public participation means welcoming and accessing widely dispersed national interests and expertise to help the government better accomplish its mission of improving the well-being of the American people.
- Collaboration means the federal government will work effectively across agencies; with state and local governments; and with others outside of government, such as nonprofits, advocacy organizations, business, and academia.
Collectively these principles reflect the reality that the challenges and opportunities facing the United States are so complex that no one organization, regardless of how capable, ingenious, or well funded, can possibly address them alone. Instead, efforts are needed to improve the availability of data; to engage a broad spectrum of talent and expertise in the use of these data; and to support the work, across all sectors, needed for meaningful progress.
A Presidential memorandum led to an Open Government Directive issued by the Office of Management and Budget (OMB),2 which required all federal departments and agencies to take steps to better support and implement these principles. Key opportunities outlined by the directive include publishing government information online, improving the quality of government information, creating and institutionalizing a culture of open government, and establishing an enabling policy framework for open government.
In response to the OMB directive, HHS developed an Open Government Plan, which outlines immediate opportunities to improve access to data and to provide a means for their use in accomplishing HHS’s mission
________________
1 See http://www.whitehouse.gov/the_press_office/TransparencyandOpenGovernment/ (accessed October 11, 2010).
2 See http://www.whitehouse.gov/sites/default/files/microsites/ogi-directive.pdf (accessed October 11, 2010).
more effectively. The plan represents a sharp break from past approaches to HHS data and is an initial step toward better leveraging these data to accomplish the following objectives: “to help citizens understand what (HHS) does and hold (HHS) accountable; help the public hold the private sector accountable; increase awareness of health and human services issues; generate insights into how to improve health and well-being; spark public and private sector innovation and action; and provide the basis for new products and services that can benefit the American people” (HHS, 2010).
Key elements of the plan are summarized in Box 3-1. Of particular note are 14 new data sets and three tools that will be released by the end of 2010.3 These new resources are considered “high value” because they can be used to “increase agency accountability and responsiveness, improve public knowledge of the agency and its operations, further the core mission of the agency, create economic opportunity, or respond to need and demand as identified through public consultation” (HHS, 2010). The plan also includes five Flagship Initiatives: the Centers for Medicare & Medicaid Services (CMS) Dashboard; the Food and Drug Administration (FDA) Transparency Initiative; FDA Transparency, Results, Accountability, Credibility, and Knowledge-Sharing; Freedom of Information Act Excellence; and the Community Health Data Initiative.
From Open Government to a Learning Healthcare System
Open government is a paradigm for how HHS can help catalyze a learning health system. HHS has a fiscal year 2010 budget of $840 billion and comprises 27 agencies and offices—including Agency for Healthcare Research and Quality (AHRQ), the Centers for Disease Control and Prevention, CMS, FDA, and National Institutes of Health (NIH). It has assembled vast stores of data that have the power to yield insights into how to improve health. These data stores would be a tremendous public good even if HHS were to invent nothing new and simply make these data more accessible. Yet more is needed to catalyze and foster the development of a national learning healthcare system. In essence, publishing data is necessary, but insufficient, to drive the transformative change needed without support for data use and improvement.
Developing a Data Ecosystem
Developing a system that encourages access to and use of healthcare data will make available important information and guidance for patients, clinicians, and many other health system stakeholders. Perhaps most impor-
________________
3 As of April 2010, HHS has 117 data sets and tools posted on Data.gov.
BOX 3-1
HHS Open Government Plan
Key elements (Flagship Initiatives indicated in bold):
• A multi-faceted new transparency push by the Centers for Medicare & Medicaid Services (CMS) in 2010:
— A new interactive “CMS Dashboard,” debuting in beta mode with the launch of this Open Government Plan, which allows the public to visualize and analyze Medicare spending with unprecedented ease and clarity—starting with inpatient hospital spending. You can visit the Dashboard at http://www.cms.gov/Dashboard/
— Creation of 9 Medicare claim “basic files,” one for each major category of healthcare service, to be released from September to December 2010 for free public download on Data.gov. These files will contain a limited number of variables and be de-identified and configured through a rigorous process, in close consultation with privacy experts, re-identification experts, researchers, and key stakeholders, to ensure the protection of beneficiary privacy and confidentiality
— A significantly improved user interface and analytical tool for viewing existing CMS COMPARE data on quality performance of hospitals, nursing homes, home health agencies, and dialysis centers. This interface and tool is debuting at http://data.medicare.gov with the launch of the Open Government Plan
— Online publication of detailed Medicaid State Plan documents and amendments on the CMS website by 2011
— The release of new national, state, regional, and potentially county-level data on Medicare prevalence of disease, quality, costs, and service utilization, never previously published, as part of HHS’s Community Health Data Initiative by the end of 2010
• A Food and Drug Administration (FDA) Transparency Initiative is being formulated with extensive public input, and focused on (1) providing the public with information regarding how FDA works, (2) proactive disclosure of useful, nonproprietary information in the possession of the agency, and (3) ways in which FDA can become more transparent to regulated industry. FDA is also launching FDA-TRACK (Transparency, Results, Accountability, Credibility, and Knowledge-Sharing), a new agency-wide performance management system, which debuts in beta mode with the launch of the Open Government Plan. FDA-TRACK, when fully implemented, will allow the public to monitor more than 300 performance measures and 80 key projects across 90+ FDA program offices on an ongoing basis
• Other new data sets and tools to be published from across HHS
• Implementation of a new process for the proactive identification, prioritization, publication, and monitoring of data releases—to be coordinated overall by HHS’s Data Council. This process will include an “HHS Apps Challenge”—a public competition for the best applications built utilizing our data
• A major push to assess current HHS operations in support of the Freedom of Information Act (FOIA), identify and prioritize improvement opportunities, and define a roadmap to implement the improvements [FOIA Excellence]
• Expanded opportunities for public participation in HHS activities and for collaboration across HHS and with the world outside HHS—especially via the use of new information and communications technologies. Through a new HHS “Community of Practice” for Participation and Collaboration, Open Government innovators at HHS will be able to network with each other, share learnings and best practices, compile these best practices into an HHS “workplace menu” of participation and collaboration tools, compare the efficacy of different approaches, and work together on common issues. The Community of Practice will focus in particular on the advancement of innovative mechanisms for participation and collaboration at HHS—mechanisms that apply blogging, “crowdsourcing,” group collaboration, idea generation, mobile, and on-line challenge capabilities to key HHS activities:
— Medical research collaborations—e.g., via the application of “crowdsourcing” and innovative patient engagement approaches to research on diabetes and women’s health issues
— Collaboration among HHS employees—e.g., via work by the Office of the Assistant Secretary for Planning and Evaluation to research and pilot advanced collaboration tools
— Better health care through better information—e.g., via the community-driven, highly collaborative
— “Nationwide Health Information Network–Direct” initiative being pursued by the Office of the National Coordinator for Health Information Technology
tant, ongoing data use will drive improvements in data quality and applicability. HHS therefore seeks to catalyze the development of an ecosystem of data by stimulating both data supply and use.
Building on the foundation provided by increased availability of data, HHS will encourage public participation and collaboration by engaging in an ongoing dialogue with potential users of the data. Through such communication, HHS seeks to catalyze learning by advancing understanding of what types of data could be useful, the limitations of currently available data, and means of better supporting data use. This approach is best illustrated by the Community Health Data Initiative, which seeks to help Americans better understand and take action to improve healthcare performance in their communities. The initiative’s initial short-term goal is to build a network of data suppliers and users. In the longer term, this network will
— Innovation in the workplace—e.g., via the piloting of an online employee idea-generation tool and challenge program by the CMS
• Community Health Data Initiative. A major new public–private effort whose goal is help Americans understand health and healthcare performance in their communities relative to others—and to help spark and facilitate action to improve performance. As a core enabler of this initiative, HHS will be providing to the public, free of charge and any intellectual property constraint, a large-scale Community Health Data Set harvested from across HHS—a wealth of easily accessible, downloadable data on public health and health care performance across communities, including a major contribution of Medicare-related data from CMS (i.e., prevalence of disease, quality, cost, and service utilization data at the national, state, regional, and potentially county levels). The initiative is simultaneously working with a growing array of technology companies, researchers, health advocates, consumer advocates, employers, providers, media, etc. to identify and deploy uses of the data that would be most effective at
1. Raising awareness of community health performance,
2. Increasing pressure on decision makers to improve performance, and
3. Helping to facilitate and inform improvement efforts.
Such applications and programs could include interactive health maps, competitions, social networking games that educate people about community health, enhanced web search results for health searches, etc. By leveraging the power of transparency, participation, and collaboration, the Community Health Data Initiative seeks to significantly improve the health of our communities.
develop into a community health data ecosystem in which a broad array of users take data supplied by HHS and others and develop means for their display or application in ways that drive continuous improvement in the health of communities across the United States (Figure 3-1).
HHS tested the potential of such a data ecosystem at a workshop, convened with the help of the Institute of Medicine (IOM) on March 11, 2010, featuring leaders from two largely separate worlds—healthcare and information technology applications. About 40 individuals with deep expertise in either healthcare data or how to use data to change people’s lives came together at this meeting, and an amazing thing happened. The information technologists became excited about data already captured by HHS and began a conversation with healthcare experts in a burst of creativity. By the end of the day, ideas had been developed for 19 new applications
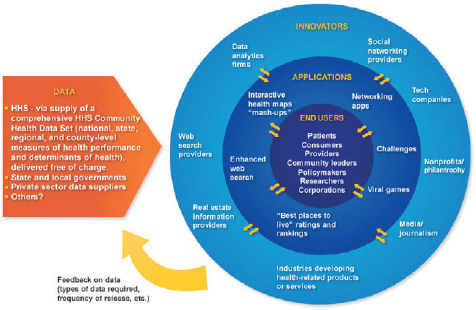
FIGURE 3-1 Design framework for HHS’s Community Health Data Initiative. This initiative is a public–private effort to catalyze the development of a network of community health data suppliers (beginning with HHS) and data “appliers” who will use these data to create a variety of applications aimed at raising awareness of and improving performance on community health issues.
SOURCE: HHS, 2010.
that could help monitor and drive improvements in community health—all of which were plausible and doable in 2010. Participants formed volunteer teams to develop 11 of these applications.
New Data Applications for a Learning Healthcare System
A sample of the proposed applications is presented in Box 3-2. They range from interactive health maps, to means of improving public understanding of national health issues, to community health dashboards that can aid city or county officials in identifying key regional health issues and communicating about these issues with the public.
One application, for example, based on a real estate website that displays school system performance metrics as part of a property’s description, would also include health performance data in home listings. This application alone would probably educate more people about health performance than would be possible with any health-focused interactive map, because the number of people who would seek out such a map is dwarfed by the
BOX 3-2
Selected Examples of Community Health Data Initiative (CHDI) Applications
Palantir—Palantir has integrated several of the CHDI data sets with other publicly and privately available data sets to allow program administrators and other analysts to make data-driven resource allocation decisions. Nontechnical users can identify where at-risk populations are distributed geospatially, determine their demand for services, and evaluate the current supply of services versus this demand. Current providers and government programs in the region can be analyzed to aid the analyst in taking action.
Microsoft Bing—Bing, the decision engine from Microsoft, represents a new generation of search. Finding health information is a mainstream online activity and 60% of people start with a search engine. Unfortunately, 41% of people state that health information found online had no impact on their decisions. Using community health data, Bing has created new features that allow easier selection of hospitals based on patient quality of care ratings and new ways to assess potential areas to live based on a combination of community health measures and access to goods and services.
The Network of Care for Healthy Communities (Trilogy and Health Communities Institute)—Trilogy Integrated Resources and Healthy Communities Institute’s system highlights how existing applications will be able to enable community change. The existing application is a local web portal that brings national, state, and local information to families and policy makers, relative to decisions about health. The site integrates multiple data sources into a Community Dashboard with health and quality of life indicators. The constantly updated dashboard visually presents CHDI and other quality of life data (roughly 150 indicators for a community) and then compares it to other counties via the County Rankings project data as well as allows mapping through a live GIS system from Health Landscape. It also tracks progress toward Healthy People 2010 Goals and automatically ties in “best practice” information from around the nation showing how other communities have tackled similar problems and made positive change in their community.
Asthmapolis: Remote Asthma Monitoring and Collaborative Community Surveillance—Every day, millions of people with asthma use rescue inhalers, inadvertently indicating how well their disease is managed. Knowing when and where these symptoms occur can reveal valuable clues about environmental exposures that trigger asthma. But until now, there has been no way to accurately collect this information. We created a device called the Spiroscout, which uses the global positioning system (GPS) to track inhaler use, automatically capturing the time and geographic location of symptoms. We’ve also developed web and mobile phone applications so that patients and physicians can monitor asthma in daily life, take steps to control the disease, and prevent costly exacerbations. By aggregating this anonymous, crowdsourced data about asthma, we can improve
management and understanding of the disease. Asthmapolis provides people with the latest information about asthma in their communities, and helps scientists and public health agencies target interventions designed to reduce the burden of asthma.
Reach for the Top: Improving Health, Healthcare Delivery, and Cost of Care in Your Community—Reach for the Top is a collaborative, community-based initiative in initial planning stages with support from the Institute for Healthcare Improvement (IHI) and Ingenix. Reach for the Top uses data to provoke communities to improve across three dimensions of care: the health of the population, the individual experience of care, and the cost of care. First, communities will be able to access information from a variety of sources, including both commercial and government data, to gain an understanding of their current status in each of these three dimensions. They will then be able to monitor their improvement as they work to move to the top tenth percentile of each metric. By seeing how their metrics compare to those of other communities, participating communities will be able to seek out top performers and learn from each other. Reach for the Top metrics will expand as more data become available.
Google—To highlight the value of HHS’s Hospital Compare database, public data were imported into Google Fusion Tables so that they can be easily explored and visualized by anyone who wants to ask questions or tell a story about them. Hospitals can be ordered in a given geography by 30-day heart attack mortality, how well they do with pain control, or a combination of these and other measures. The data can be viewed in a table, a graph, or on a map. Most importantly, we can collaborate with others, combine this data with other data, and easily share a given visualization as a link or embed it in a blog or other publication. We expect that more data transparency and tools to explore data will result in a virtuous cycle of better healthcare decisions, new hypotheses, and innovations that will transform health in ways we can’t yet imagine.
Community Clash: Healthiest city wins. You play the cards. (MeYou Health—a Healthways company)—Community Clash is an online card game that engages you in a discovery of your community’s health and well-being status and how it compares to other communities in a head-to-head clash. A mash-up of community health indicators, the Gallup-Healthways Well-Being Index and the Healthways Well-Being Assessment, Community Clash lets you pick community indicators you believe give you an edge in the head-to-head game play. Play endless hands, exploring different metrics and locations each time, and share key victories with your social network. After a hand is played, the experience is brought alive with Twitter conversations, filtered by the metric being explored. Finally, Community Clash gets personal, prompting each player to compute his or her own Well-Being Score and encouraging social comparison with friends through Facebook integration. The Community Clash user experience can be enriched in the future by expanding the deck of playing cards to incorporate additional community indicators.
SCVNGR—SCVNGR is an interactive mobile game all about going places, doing challenges, and earning points. This location-based mobile gaming platform helps individuals and communities better understand the relationship between environment, lifestyle choices, and health. The game begins when players check in to rediscover their city and find places where health happens. The “Presidential Trek” will engage players in behavioral health challenges where they can compete with others, create new challenges, earn points and badges, and share their achievements with friends. SCVNGR uses mobile gaming to bridge the gap between information and real-world action. To play in DC after June 2, download the SCVNGR app from the App Store or Android Market. Once in the app, select the Treks Tab and search for PRESIDENTIAL.
Community Health Map—HHS keeps track of a variety of healthcare indicators across the country, resulting in a large geospatially multivariate data set. Community Health Map is a web application that enables users to visualize and analyze healthcare data in multivariate space as well as geospatially. It is designed to aid exploration of this huge data repository and deliver deep insights for policy makers, journalists, and researchers. Users can visualize the geospatial distribution of a given variable on an interactive map, and compare variables using charts and tables. By employing dynamic query filters, visualizations can be narrowed down to specific ranges and regions. The Community Health Map provides a comprehensible and powerful interface for policy makers to visualize healthcare quality, public health outcomes, and access to care in order to help them make informed decisions about health policy.
iTriage—iTriage is a mobile and web-based healthcare platform helping consumers make better healthcare decisions. As a free app for iPhone, Android, Blackberry, and all other Web-enabled devices, iTriage helps consumers process symptoms, explore possible causes, identify the proper level of care and locate the most appropriate treatment facility. iTriage has now integrated more than 7,000 federally-qualified community health centers (FQHC) from the government dataset into its provider database. This helps individuals find the Health Resources and Services Administration (HRSA) centers appropriate for their condition, using GPS. Giving consumers a tool like iTriage empowers Americans to better understand the healthcare system in their communities. By doing this, iTriage changes consumer behavior to reduce unnecessary emergency room (ER) visits, and helps people to find the appropriate level of care, while facilitating a more efficient healthcare delivery system.
GE Healthymagination—GE’s Healthymagination is committed to creating better health for more people by improving the quality of, increasing the access to, and reducing the cost of health care. The world has access to increasing amounts of valuable health data, but the data are frequently complicated or better understood in context. Using advanced data visualization techniques, GE is simplifying
complexity and revealing new insights about important healthcare issues. For example, The County Health Map, created by Ben Fry, allows users to explore County Health Rankings data using key indicators. The resulting maps unveil hot spots and highlight the factors that influence the health of a community. The maps are designed to allow users to see both broad U.S. patterns as well as individual county metrics.
Pillbox—Pillbox, from the National Library of Medicine (NLM) and FDA, has transformed FDA’s drug label data into a platform for innovation. Working with patient, clinical, and HIT communities, the Pillbox team broke through barriers to the creation of applications based on FDA’s drug label data. Opening Pillbox’s data, images, and search function enabled the building of innovative applications that improve the health of citizens. Applications currently in development include voice-activated and instant-messaging pill identification systems, as well as iPhone medication identification and information applications. A group of programmers at the Great American Hackathon used Pillbox’s data to begin development of a Facebook game, writing hundreds of lines of open source code in the process. This fall, as part of the Community Health Data Initiative, the Pillbox team will repeat this process with other government health data sets.
County Health Rankings—The County Health Rankings—a collaboration between the University of Wisconsin and Robert Wood Johnson Foundation (RWJF)—uses a model to describe the overall health of a community, as well as the factors that affect health. This annual “check-up” for each county in the United States allows everyone to compare the health of where they live to that of their neighbors or other counties in their state. Following the February 2010 release of the interactive website (www.countyhealthrankings.org), the Rankings have garnered significant attention throughout the nation. The use of summary measures of health and ranking within states engages the news media and policy makers, leading to community-wide collaborations to “dig deeper” and better understand the problems, as well as to seek solutions.
Quick Health Data Online (HHS Office on Women’s Health)—Quick Health Data Online is an interactive database that provides state- and county-level data for all 50 states, the District of Columbia, and U.S. territories and possessions. Data are available by gender, race, and ethnicity and come from a variety of national and state sources. The system is organized into eleven main categories, including demographics, mortality, natality, reproductive health, violence, prevention, disease, and mental health. Within each main category, there are numerous
subcategories. Users have the flexibility of presenting results in tables, bar graphs, and maps, or exporting results into other processing or display tools.
The MedWatcher Application: Mobile Phones and Crowdsourcing for Drug Safety Surveillance (Children’s Hospital Boston)—The application is designed to engage users (general public and healthcare practitioners) in issues of drug safety. The system has two functions: alerting and reporting. First, the application alerts users about new drug warnings through both official FDA channels (such as MedWatch Alerts) and through informal channels (such as news media). Second, using forms tailored separately to the public and clinicians, we provide a user-friendly tool for reporting information about drug side effects. Serious adverse events from clinicians are automatically submitted to FDA, while public discourse between patients creates a sense of community, as well as a source of information for hypothesis generation for researchers. We believe these early efforts represent an important step in engaging the public as participants in the public health process and empowering individuals to make informed health decisions.
HealthLandscape (Health Foundation of Greater Cincinnati)—HealthLandscape is a web-based data mapping platform developed in collaboration by the Robert Graham Center and the Health Foundation of Greater Cincinnati that helps Americans understand health and healthcare system performance in their communities—and to help spark and facilitate action to improve performance and value. It allows users to create and display maps of community health and health resource data. It also allows secure access to data for limited sharing and for secure uploading for display in combination with the other public data. The goal is to help users add a spatial context to data in order to promote understanding and improvement of health and health care. Tools include
- Community HealthView: A home for data relevant to health in communities.
- Primary Care Atlas: A place to explore physician workforce data and federally designated shortage area data.
- Health Center Mapping Tool: A Health Insurance Portability and Accountability Act-compliant, secure home for uploading, geocoding, and mapping patient data to understand service areas, neighborhood penetration, and relationships to local and regional populations.
- My HealthLandscape: A secure environment for users to upload and geocode their own health-relevant data.
SOURCE: IOM Workshop, Community Health Data Initiative, June 3, 2010.
number who will buy homes in the next 5 years. Such an application also has the potential to create a new business case for investing in health at the community level, as it transforms health improvement from something that is a good thing to do into something that also affects property values, a principal revenue source for communities.
This innovation—just one of the many possibilities with increased availability of data—underscores the need for public participation and collaboration to develop creative applications that serve local needs. Because creating applications requires users to gain familiarity with the data, such work also helps HHS identify opportunities to improve the quality and utility of the data to support learning and health system and health improvement. The ecosystem approach therefore solves two problems. First, data that were previously collected but not used are being applied in a timely way to generate a discernable social return and a means to improve data quality. Second, because of these returns, a business case is developed for HHS to produce and deliver more data as a vehicle to engender social change.
At a follow-up meeting hosted by the IOM on June 3, 2010, the 11 volunteer teams showcased their results to an audience that included individuals interested in joining the work under way or starting new projects, organizations that could supply data beyond those captured by HHS, and community leaders who could use the applications to improve health.
As a result of these initial discussions, the potential impact of these data has dramatically expanded. For example, Clay Johnson of the Sunlight Foundation has proposed launching a great American hack-a-thon for health, in which advocacy organizations and nonprofits would compete to develop innovative ideas for applications that leverage community health data to advance their mission. The best ideas would then be handed over to hundreds of hackers in 30 different cities who would volunteer for a weekend out of the year to build these applications. Other participants, such as Ingenix, are considering contributing data to help make some of the proposed applications more robust.
Concluding Thoughts
The spirit of commonwealth and of “all hands on deck” emerging from efforts to foster open government is essential to a data ecosystem and to a learning health system. Initial results of HHS’s Open Government Plan also illustrate the important catalytic role government can play in health system and health improvement. Instead of developing a centralized system to control data access and use, HHS can supply data to all and help consolidate and catalog ecosystem information so that data use can be driven by and serve the needs of the community.
Opening access to health-related data from the public and private sectors could be transformative for improving the health of Americans and the effectiveness of the healthcare system. However, people of all talents, capabilities, and resources must come together to realize this potential. HHS needs guidance from the public about what data it should capture and release and how the data can be used to advance social missions. As good as the HHS data are now, they will become much better over time with public input. The dialogue should be about how HHS and the public, in the spirit of open government, can collaborate on an ongoing basis to achieve the promise of a learning health system.
ENSURING DATA INTEGRITY: ADDRESSING PRIVACY PROTECTION AND PROPRIETARY CONCERNS
Don E. Detmer, M.D., M.A.
University of Virginia
In the American context, a worthy society is exemplified by a healthy citizenry pursuing happiness through the beneficence of sound governance and positive expressions of personal freedom. Key to those positive expressions of personal freedoms is the exploration of “the illimitable expressions of the human mind.”4 The nation’s origins were suffused with the optimism and courage characteristic of the Enlightenment. Discovery and support of progress across all dimensions of human activity were central themes, as characterized by efforts as diverse as the pursuit of copyright law and the rapid spread of smallpox vaccination (Sheldon, 2010). As Ralph Waldo Emerson noted, however, “The first wealth is health.”5
These early emphases on discovery and improvements in health and health care were underscored 150 years later when, in the midst of a great world war, Franklin Delano Roosevelt dedicated the NIH for the discovery of cures for the maladies plaguing humanity. Over the years, this national investment has benefited the care of Americans and people everywhere, while also leading to major improvements in the health of domesticated and even wild animals.
The Blue Ridge Academic Health Group has advocated for some years for a value-driven healthcare system (Blue Ridge Academic Health Group, 1998, 2004) that continually improves the quality of care and practices evidence-based management of illnesses. To secure such a system requires
________________
4 See http://wiki.monticello.org/mediawiki/index.php/Illimitable_freedom_of_the_human_mind_(Quotation) (accessed October 11, 2010).
5 See http://www.notable-quotes.com/e/emerson_ralph_waldo.html (accessed October 11, 2010).
that the entire enterprise focus on learning and become a learning health system (IOM, 2007). Requirements for a learning health system include (1) widespread access to patient information to better understand the comparative effectiveness of treatments and foster ongoing research into less understood conditions; (2) policies and procedures that do not waste scarce resources, including money and the valuable time of health professionals; (3) active engagement of patients in their care to improve their understanding of and commitment to treatments of known effectiveness; and (4) ongoing, credible systems that preserve privacy and manage patient information securely to achieve data integrity for both healthcare operations and research of all types (e.g., biomedical, public health, and health services research).
Recently, Secretary of Health and Human Services Kathleen Sebelius noted: “The key to benefiting from change will be in ensuring that healthcare decision makers are guided by science and research” (Sebelius, 2010). With the passage of the ACA, access to basic healthcare services was affirmed as worthy of the investment in terms of national health status and social productivity. The absence of prior legislation providing all citizens with access to basic healthcare services gave rise to fears that unauthorized distribution of personal health data would lead to healthcare uninsurability. With the passage of ARRA, the Genetic Information Nondiscrimination Act of 2008, and the ACA, we have now entered a new era where this concern is moot.
Secretary Sebelius wants decisions to be based on evidence and research. To this end, public policy must support a learning health system. Further, citizens should be actively engaged in the support of legitimate healthcare research and institutional review boards (IRBs). The nation now has stringent policies and regulations, including both civil and criminal policies, relating to the use and protection of person-specific health information. Indeed, in 2009, ARRA added so many new protections that many experts consider this law to have created a second incarnation of the privacy provisions of the Health Insurance Portability and Accountability Act (HIPAA) of 1997. Even before ARRA was passed, multiple expert groups involved in the nation’s research enterprise had examined the impact of HIPAA on a range of biomedical, public health, and health services research. An IOM report reviewed all such studies and offered the conclusion that improvements to HIPAA were needed to facilitate better privacy protections (some of which are included in ARRA), as well as better access to data for research (IOM, 2009).
Another component of ARRA is a mandate that the government assess the comparative effectiveness of treatments to develop greater understanding of which treatments are most effective. This mandate specifically calls for health services research that will require access to person-specific health-
care data. The time has come to put before the American people the opportunity to support the discovery of new treatments and help ensure that only those resources needed to support research and protect data integrity and security are expended. At the same time, the research enterprise should be constructed so as to offer the greatest levels of patient safety and highest quality of care for standard practices.
To address these needs, Congress should introduce and pass a law that will facilitate the achievement of a number of important objectives with respect to biomedical, public health, and health services research. This law should include a number of features (Box 3-3). First, it should enable Americans to have a unique personal health identifier, which will facilitate the integrity of research data. The identifier should also be allowed to be used for authentication of routine care unless the individual wishes to formally opt out of such usage. Second, the law should mandate that all research studies meeting HIPAA requirements and having been approved by an IRB have access to nonanonymized health record data for legitimate research purposes. Further, citizens should explicitly be given the opportunity to opt out of the accessibility of any personal genetic information for research that meets HIPAA requirements and has been approved by an IRB to use nonanonymized health record data for legitimate research purposes. Any breaches of data security relating to research use would fall within
BOX 3-3
Components for a “Health Research and Safe Care Act of 2011”
Citizens have the opportunity to
- opt out of selection of a unique personal health identifier for use in research databases (including an option for the identifier to be used for routine personal health care);
- opt out of consent to share their personal health data for IRB-approved research that complies fully with HIPAA security regulations and requirements without any data anonymization; and
- opt out of consent to share their personal genetic data, if available, for IRB-approved research that complies fully with HIPAA security regulations and requirements without any data anonymization.
In addition:
- use of anonymized data would continue to be allowed without explicit personal consent; and
- public–private partnerships would be able to allow researchers to identify potential research participants, who might then be approached to take part in clinical studies for which consent is needed.
the current boundaries of HIPAA and ARRA as defined above. Finally, a public–private collaboration should be established to develop a process by which citizens interested in becoming involved in a research protocol that may apply to them as a result of preliminary data screening could be located and offered information on potential inclusion in the study.
Some further explanations are appropriate to better understand the logic behind the above policy proposal. The above proposal flows from the 2008 policy conference of the American Medical Informatics Association (AMIA). AMIA held a conference on “Informatics, Evidence-based Care, and Research: Implications for National Policy” (Bloomrosen and Detmer, 2010). A diverse group including federal regulators, citizens, researchers, and informaticians, concluded that national policy must be revised to secure a learning health system, improve and ensure data integrity, attend to data security and privacy, and facilitate research. The group concluded that only through a refocusing of public policy with respect to data access could these objectives be met. It concluded that a learning health system requires broad access to personal health data. Further, citizen support for legitimate biomedical and health research should be facilitated because today the entire burden of engaging citizens in research falls not on society but on researchers and IRBs, thereby creating significant disincentives for caregivers and researchers to pursue many promising lines of inquiry. In short, at this time, federal policy adversely impacts legitimate biomedical and health research by restricting data access, and there is no evidence that this is the will of most Americans.
The perspective of clinicians is important to data integrity as well. With the drive to make EHRs the norm for clinical practice settings, it is important to motivate the clinical community to be highly committed to data integrity. Payment for “meaningful use” holds promise to at least encourage recording data to enhance payment rather than spending the time to capture the exact clinical situation. There is a real possibility that data quality will benefit from clinicians learning that their data will be reviewed for both research purposes and for assessing their clinical practice behavior for quality assurance and maintenance of licensure. EHRs have the potential to reduce the quality of clinical notes as well as improve care through better data access, and it is wise not to overlook the role that support for professionalism can play in a contractual regulatory world.
At the broader policy level, instead of supporting research, current policy focuses overwhelmingly on privacy protection of personal health information, and such federally enforced protections continue to mount with neither the ability nor support by citizens to alter this policy course. Further, with Americans strongly in favor of legitimate biomedical, public health, and health services research, no policy exists to encourage the system to
favor access to patient data for such research (Allcott and Mullainathan, 2010; Thaler and Sunstein, 2008).
Equally disturbing in the context of a learning healthcare system, current policies and regulations limit data integrity through a variety of mechanisms. The most prominent of these is the absence of unique personal health identifiers, which is a threat to privacy and safety in day-to-day care and a major threat to data integrity because data (especially those relating to people with common names) can be misfiled quite easily. Another issue with major implications in terms of cost and wasted time relates to the gap between the use of health data for quality management and for clinical and health services research. There is a substantial risk that ARRA will further limit data access through the “minimum necessary” requirement reviewed in summer 2010.
Other nations are ahead of the United States in addressing this problem. For example, the Research Capability Program of the Department of Health of England and Wales,6 passed in 2007, is explicitly establishing mechanisms to allow researchers full access to 21 formal data sets created by the Department. The goal is to facilitate access to such data by researchers, including the pharmaceutical industry. England is convinced that this can be good law while remaining consistent with patient privacy protections. The National Health Service (NHS) data sets include NHS Care Records Service (CRS) Personal Demographics Service, Death Registration, Birth Notification Dataset, Commissioning Data Set/Hospital Episode Statistics, Cancer Register, NHS CRS Electronic Prescribing Service and National Prescribing Service, General Practice Research Database, Health Improvement Network, EMIS Primary Care (QResearch), IMS-Mediplus, General Practice Extraction Service, Cardiac Register; National Diabetes Audit, National Congenital Anomaly System, UK Renal Registry, Townsend Material Deprivation Score, Jarman Underprivileged Area Score, Office for National Statistics Demographic indexes, National Statistics Postcode Directory, NHS HealthSpace, and Mental Health Minimum Data Set.
Specific recommendations of the United Kingdom Research Capability Program include “safe havens” for population-based research in which the protection of confidentiality is paramount; systems for approving and accrediting researchers allowed to work in such environments; involvement of academic and other partners in safe havens; and development of systems to allow researchers to identify potential participants, who may then be approached to take part in clinical studies for which consent is needed.
The recommendation for an opt-out option relates to recent multiyear experiences in Massachusetts, Minnesota, and Utah (Okwumabua, 2010).
________________
6 See www.connectingforhealth.nhs.uk/systemsandservices/research (accessed October 11, 2010).
Recently, Massachusetts has used an opt-in option for gaining informed consent to share personal data among regional caregivers using a unique health identifier. Upward of 90 percent of patients opt in to data sharing, but the monetary costs to maintain this approach are significant, and substantial time is required by clinicians as well. Meanwhile, in Minnesota, a data sharing system has been in play for some years. It too uses an opt-in option, and experience with this system mirrors that in Massachusetts (e.g., monetary costs and clinician and other administrative costs are substantial). Finally, Utah has had an opt-out program for children’s health data whose original focus was on minimizing under- and overimmunization. Roughly 3 percent of parents opt their children out, and the system is much less expensive than those of Massachusetts and Minnesota. Public acceptance is thus shown by these real-world experiences to approach or exceed 90 percent for sharing of data for care purposes.
With respect to such options and biomedical research, Daniel Masys of Vanderbilt University offered to the author the following personal communication relating to deidentified data:
Vanderbilt has an opt-out model for building a biobank of DNA samples from blood left over from clinical testing linked to de-identified electronic medical record (EMR) data that currently has more than 63,000 samples in it, growing at about 800 per week. The predicted opt-out rate before the project started, based on focus groups and a 5000 person patient survey was five percent. The observed opt-out rate over the past two years, across about one million consent-for-treatment signing events, is about 3.8 percent. Surveys of those who did opt out suggest an “anti-science” phenotype that is suspicious of all organizations, a belief that commercial profit motives are behind any veneer of science altruism. Most are personally convinced to a degree that no additional information would change their minds. These data seem to be a useful validation of the estimated size of an often vocal minority who figure so prominently in all of the public debates about research, uses of clinical data and electronic data systems…. This is not to say that such views are not valid and they clearly must be accommodated in discussions of architecture and policy, but they often seem to occupy a disproportionate amount of public dialog and pedagogy. (Masys, 2009)
If the recommended opt-out option were signed into law, one might predict that, based on available data and with sensible administration, well over 75 percent of the American public would “sign up.” This would benefit research immensely and save billions of dollars and thousands of hours as well.
In conclusion, two quotations from James Madison relate directly to this public policy issue and the challenge facing those seeking to help
Americans find more cures sooner through the passage of legislation such as that suggested here. Madison noted: “A people who mean to be their own Government, must arm themselves with the power to which knowledge gives.”7 Progress comes only when information flows. Madison observed in a letter to Benjamin Rush that he was no stranger to the clash of deeply felt values and how the intensity of belief can warp perception. He stated: “Nothing is so contagious as opinion, especially on questions which, being susceptible of very different glosses, beget in the mind a distrust of itself.”8 One cannot but reflect on this quotation in the context of the continuing health data privacy wars of the past two decades. Within the Beltway, it is commonly taken as fact that patients are more concerned about the privacy of their data than anything else and that trust in health care and EHRs is based overwhelmingly on assurance of this privacy. What facts exist reveal that this is so much finely glossed opinion. According to Mechanic and Meyer (Mechanic and Meyer, 2000), trust in health care is based on three main pillars and a fourth that sometimes is not even mentioned. First and foremost is the ability of the clinician to communicate and connect with the patient. Second is the perceived competence of the clinician. Third is whether the clinician is willing to advocate for the patient’s benefit if necessary. If it is mentioned at all, maintaining privacy and confidentiality of data is fourth.
Americans should be given the option to become full and open participants in a learning health system. To do otherwise is to prevent them from being either Americans in the finest sense of that word or adults with respect to their care and concern for their fellow beings. The right of Americans to be left alone must not be allowed to become a right to be unknown.
REFERENCES
Allcott, H., and S. Mullainathan. 2010. Behavior and energy policy. Science 327:1204-1205.
Bloomrosen, M., and D. Detmer. 2010. Informatics, evidence-based care, and research; implications for national policy: A report of the American Medical Informatics Association’s health policy conference. Journal of the American Medical Informatics Association 17:115-123.
Blue Ridge Academic Health Group. 1998. Academic health centers: Good health is good business. http://whsc.emory.edu/blueridge/_pdf/blue_ridge_report2.pdf (accessed October 11, 2010).
———. 2004. Converging on consensus: Planning the future of health and health care. http://whsc.emory.edu/blueridge/_pdf/blue_ridge_report_8_2004oct.pdf (accessed October 11, 2010).
________________
7 See http://presspubs.uchicago.edu/founders/documents/v1ch18s35.html (accessed October 11, 2010).
8 See http://www.revolutionary-war-and-beyond.com/james-madison-quotes-6.html (accessed October 11, 2010).
Blumenthal, D. 2010. Launching HITECH. New England Journal of Medicine 262(5):382-385.
HHS (Department of Health and Human Services). 2010. HHS open government plan. http://www.hhs.gov/open/plan/opengovernmentplan/index.html (accessed October 11, 2010).
IOM (Institute of Medicine). 2007. The learning healthcare system: Workshop summary. Washington, DC: The National Academies Press.
———. 2009. Beyond the HIPAA Privacy Rule: Enhancing privacy, improving health through research. Washington, DC: The National Academies Press.
Masys, D. 2009. Personal communication, August 14.
Mechanic, D., and S. Meyer. 2000. Concepts of trust among patients with serious illness. Social Science & Medicine 51:657-658.
Okwumabua, E. 2010. Public Health, George Washington University.
Sebelius, K. 2010. Statement of Kathleen Sebelius Secretary U.S. Department of Health and Human Services on the President’s fiscal year 2011 budget before the Subcommittee on Labor, Health and Human Services, Education, and Related Agencies, Committee on Appropriations, U.S. House of Representatives.
Sheldon, G. F. 2010. Hugh Williamson: Physician, poet and founding father. New York: Humanity Books.
Thaler, R. H., and C. R. Sunstein. 2008. Nudge: Improving decisions about health, wealth and happiness. New York: Penguin.


























