-
to consider transformative strategies for enhancing the way clinical research is organized and conducted.
Through a series of case studies and stakeholder perspectives, workshop participants examined clinical research networks and clinical trials in the four disease areas. Using the presentations and discussion from day one as a starting point, four breakout groups, each focused on one of the four disease areas, produced observations and insights relevant to the workshop objectives.
ORGANIZATION OF THE REPORT
This report is intended to provide a faithful summary of the presentations and discussions that took place during the workshop, although remarks have been substantially abbreviated and reorganized to improve the report’s readability and usefulness. It should be noted that although a number of presenters and participants expressed opinions and recommendations that were summarized in this report, these should in no way be interpreted as attributable to the IOM Drug Forum or the IOM.
The remainder of the report provides a comprehensive summary of the presentations and discussions that occurred during the workshop. Chapter 2 gives an overview of the state of clinical research in the United States today, including new research on the subject commissioned for the workshop. Chapter 3 describes the broad challenges that are faced in conducting clinical trials today. Chapters 4, 5, 6, and 7, respectively, summarize workshop presentations and discussions regarding the strengths and weaknesses of various clinical trial models in the above four disease areas and the usefulness of clinical trial results for informing clinical practice in each area. Chapter 8 describes efforts currently under way to improve clinical trials, summarizes the breakout session discussions regarding strategies for advancing clinical research in the identified disease areas, and presents a vision for a sustainable clinical trials infrastructure in the United States.
It should be noted that, while the Drug Forum conceived the idea for this workshop, its planning was the responsibility of an independently appointed committee. That committee’s role was limited to advance planning; this summary was prepared by the rapporteurs as a factual summary of what occurred at the workshop.
2
The State of Clinical Research in the United States: An Overview
The Institute of Medicine (IOM) reports To Err Is Human: Building a Safer Health System (IOM, 2000) and Crossing the Quality Chasm: A New Health System for the 21st Century (IOM, 2001a), focused the nation’s attention on concerns about the quality of health care in the United States. Since those reports were published, efforts have accelerated to develop a health care system that systematically measures and improves the quality of care delivered. Essential to such a system is a systematic approach for assessing which clinical approaches do and do not work and then ensuring that this knowledge is utilized in clinical decision making. This approach is what is often referred to as a learning health care system.
Many different kinds of evidence can inform the policies and practices of a health care system. Clinical trials, a type of clinical research, are one of the most robust sources of this knowledge. A number of workshop speakers from many backgrounds—clinical investigators, research sponsors, practitioners, and patients—expressed the view that the current clinical research enterprise1 in the United States is unable to produce the high-quality, timely, and actionable evidence needed to support a learning health care system. They identified numerous obstacles to producing this evidence, including the length of time and high financial cost involved in conducting clinical trials, delays associated with navigating the many regulatory and ethical
requirements of studies involving human subjects (e.g., Institutional Review Board [IRB] approval), difficulties in recruiting and retaining the appropriate patient population, and the generally fragmented way clinical research is prioritized and undertaken to advance medical care in the United States.
As noted in Chapter 1, the workshop focused on the randomized controlled trial (RCT), the gold standard in clinical research. Many consider the RCT to be unsustainable as an approach to addressing the large number of research questions that need to be answered because of the time and expense involved. Yet alternative approaches have limitations with respect to producing high-quality data. Christopher Cannon, senior investigator in the Thrombolysis in Myocardial Infarction (TIMI) Study Group, for example, discussed the use of registries, which are large databases that provide extensive observational data on current clinical practice. He commented that while registry data are of good quality and less expensive to obtain compared with data from RCTs, confounding (i.e., why an individual received one therapy versus another) is a significant problem. Because it is difficult to attribute trends in registry data to particular therapies, registries do not provide the conclusive evidence necessary to change clinical practice. Instead, registries generate hypotheses that can then be tested in an RCT. Therefore, while patient registries and other research tools exist, the workshop focused primarily on RCTs.
Results of thousands of RCTs are published each year, yet clinical decision making frequently is not based on the evidence created by these results. A key issue informing the workshop discussions, then, was how RCTs can be conducted in an efficient, timely manner to answer all of the questions and meet all of the needs of a learning health care system. A logical first step in addressing this issue is to examine the clinical research enterprise as it operates in the United States today.
This chapter describes various aspects of clinical research in the United States, beginning with clinical research networks (CRNs). Research commissioned for the workshop from Ronald Krall, former Chief Medical Officer, GlaxoSmithKline, is then presented, addressing tools available for assessing clinical research in the United States; volume and type of clinical trials conducted; the clinical investigator workforce; and the overall capacity of the clinical research enterprise.
CLINICAL RESEARCH NETWORKS
CRNs have been developed to pool resources and expertise in conducting clinical research. They include clinical sites and investigators usually organized around a specific disease area and can be accessed by many different research stakeholders for the conduct of clinical research.
The National Institutes of Health’s (NIH’s) Roadmap for Medical
Research points specifically to CRNs and their ability to rapidly conduct high-quality studies as a way to improve the efficiency and productivity of the clinical research enterprise. In this vein, NIH’s National Center for Research Resources (NCRR) manages the Inventory and Evaluation of Clinical Research Networks (IECRN) project to survey active networks and characterize best practices that could potentially be implemented in other networks or clinical trial settings. Although the exact structures vary, the NIH project defines a CRN as an organization of clinical sites and investigators that conducts or intends to conduct multiple collaborative research protocols. CRNs can carry out a number of different types of studies, including clinical trials, and the organization of sites and investigators can be formal or informal as long as the collaborative accomplishments of the group are clear. For instance, a group of researchers that conducts a single trial and subsequently disbands is not considered to be a network (NCRR, 2006).
By pooling the resources of multiple entities, CRNs can realize efficiencies in implementing and conducting clinical trials. They create a supportive infrastructure for investigators and can facilitate the rapid conduct of trials to answer important research questions. For instance, CRNs organized around a particular disease often have access to patients with that disease who can serve as study participants. The in-house scientific leadership of CRNs can also streamline the protocol development process and create uniformity in clinical trials across the network or disease area. When clinical trials from a particular network generate consistent results, this can also accelerate the drug development pipeline for the disease studied.
TOOLS FOR ASSESSING CLINICAL RESEARCH IN THE UNITED STATES2
Krall obtained information on the current state of clinical trials in the United States from various public and private sources. A key source was data on submissions to clinicaltrials.gov, a federally sponsored, publicly available registry of clinical trials. Information was also obtained from the Tufts Center for the Study of Drug Development, KMR Group, Citeline, and individual pharmaceutical companies. The Tufts Center and KMR collect data from pharmaceutical companies for the purpose of providing benchmarking data and proprietary analyses. Citeline is a proprietary data source that draws from a number of resources (literature, advertising, and clinicaltrials.gov) to create a comprehensive database of clinical research and the global investigator workforce.
Clinicaltrials.gov
The Food and Drug Administration Modernization Act (FDAMA) of 1997 mandated the creation of the clinicaltrials.gov registry for efficacy trials in serious and life-threatening conditions and interventions regulated by the FDA. Developed by NIH’s National Library of Medicine (NLM) in 2000, it allows interested parties to find information on both completed and ongoing clinical trials. The database includes federally and privately supported clinical trials, and study sponsors are responsible for submitting timely and accurate information about their studies.
The database registered a modest number of clinical trials in its initial years (Figure 2-1). A dramatic increase in trial registration came in 2005 in response to the newly introduced International Committee of Medical Journal Editors’ (ICMJE’s) requirement that studies published in their journals be registered in clinicaltrials.gov or other equivalent publicly available registries. The Food and Drug Administration Amendments Act (FDAAA) of 2007 created a legal requirement for the registration of trials of drugs, biologics, and devices, generating a modest increase in the registration of
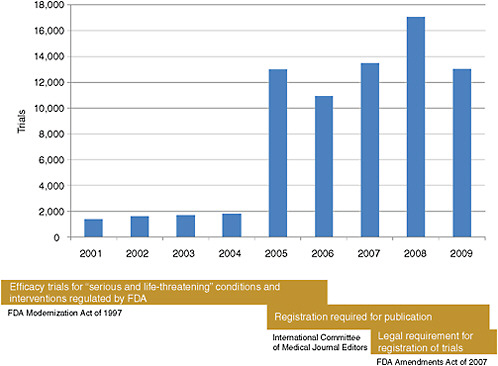
FIGURE 2-1 Timeline reflecting the number of clinical trials registered on clinicaltrials.gov and regulatory changes affecting the database registration from 2001 to 2009.
SOURCE: Krall, 2009. Reprinted with permission from Ronald Krall 2009.
trials over what had been seen in 2005. Given the increasing number of trials registered on clinicaltrials.gov over time, the database encompasses a broad spectrum of research organized by study sponsor (industry, government, and nonprofit), disease and treatment being studied, and trial design.
Data Limitations
The information gathered by Krall to inform the workshop discussions of the state of the U.S. clinical research enterprise was not intended to provide an exhaustive analysis of the impact of every role and action of the broad range of research stakeholders involved. Rather, the goal was to highlight the productivity of one aspect of the clinical research enterprise—clinical trials. The data gathered reflect not the “effectiveness” of trials in terms of how well they answer the study questions, but how efficiently they are conducted. The commissioned research was designed to meet the needs of the workshop, however, the topics covered and issues raised by Krall’s analysis could be informative for other areas of the clinical research enterprise as well.
The data collected have some limitations. With respect to certain industry information, individual pharmaceutical company data can vary significantly depending on how the various elements and costs of clinical trials are measured. Also, although NLM reviews information submitted to the clinicaltrials.gov database, neither the accuracy of the data nor the scientific relevance of the study is guaranteed. Thus, while the information gathered on the number and type of clinical trials being conducted today is revealing, it would be incorrect to assume that it reflects the quality or relevance of those trials. Krall also noted that some types of clinical trials do not need to be reported to the database, and that there are concerns about the timeliness and accuracy of the data that are submitted. Variability in the reporting and classification of certain data elements in clinicaltrials.gov (e.g., drugs vs. biologics, phases of research, reporting no funding source, and currency of investigator site information) is another concern. Yet while clinicaltrials.gov is not without limitations, Krall suggested that its creation is undoubtedly a positive step toward developing a clearer picture of the state of clinical research in the United States.
VOLUME AND TYPE OF CLINICAL TRIALS CONDUCTED
In RCTs, investigators control which participants receive the study treatment by assigning them at random to a particular experimental study group. Observational, non-experimental studies occur in natural settings and involve no manipulation of the interventions or treatments study par-
ticipants receive. Because RCTs were the focus of the workshop, observational studies were excluded from Krall’s analysis.
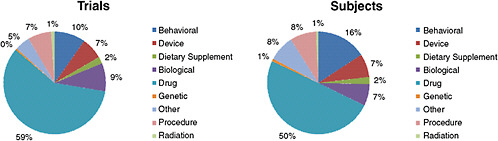
Krall reported that as of August 16, 2009, there were 10,974 ongoing, interventional clinical trials with at least one U.S. center. The 10,974 ongoing trials collectively are seeking to enroll 2.8 million subjects. As Figure 2-2 indicates, the majority of trials (59 percent) are testing drugs. A distant second and third to drug interventions are behavioral trials (10 percent) and those testing biologics (9 percent), respectively.
Clinical Trials by Phase of Research
The phase of clinical trials (i.e., phases 0–IV; see Chapter 1) is considered by some to be a marker of innovation, reported Krall. An analysis of clinical research by phase of experimental clinical trials can indicate the degree to which innovative new therapies are being developed and tested. It takes 10–15 years for a typical drug to be developed successfully from discovery to registration with the FDA. In the earlier phases of research, the chance of a drug reaching patients is small—approximately 1 in 10. In phase III research, however, the odds of registering a new product improve. About two-thirds of drugs that reach pivotal phase III trials will make it to the market (IOM, 2009c, p. 85).
To characterize trials by phase more precisely, Krall narrowed the focus of his research to trials for FDA-regulated interventions (drugs, biologics, devices, and dietary supplements). In these FDA-regulated categories, there are 8,386 trials recruiting 1.9 million subjects. As shown in Figure 2-3, among clinical trials for FDA-regulated products, phase II research is the largest category, followed closely by phase IV. Also referring to Figure 2-3, although there are larger numbers of phase II and III trials, phase III trials by design involve the largest number of participants; thus it makes sense that 52 percent of all subjects are enrolled in these pivotal trials.
Clinical Trials by Disease
Krall described ongoing clinical trials in the four disease areas of focus at the workshop—cardiovascular disease, depression, cancer, and diabetes. Figure 2-4 indicates that approximately half of the 10,974 trials being conducted today are in cancer; however, each such trial involves a relatively small number of participants. Figure 2-4 also reveals that cardiovascular disease trials are seeking more than 300,000 participants—10 percent of all clinical trial participants being recruited and far more than the number of participants sought for cancer, diabetes, or depression trials. Recruiting a large number of subjects per trial is a trademark of cardiovascular disease studies: on average, 275 patients are sought per cardiovascular trial, as