Appendix D
Environmental Considerations for Photovoltaics
Life Cyle of Photovoltaic Technology
In the past five years, solar photovoltaic industries have continuously increased manufacturing capacity, decreased costs, and achieved remarkable growth. Conventional flat plate silicon PV occupies greater than an 80 percent share of the global solar cell market. However, the industry presently suffers from high energy consumption and serious pollution, such as the treatment and recycling of large amounts of wastewater containing hydrofluoric acid (HF), nitric acid (HNO3), and other metal ions. As such, the development of environment-friendly technologies is urgently needed (Gu, 2008; Hu, 2008).
Environmental Problems in the Preparation of Polysilicon
The factories under construction use a modified Siemens process to manufacture polysilicon. Currently, the process is the most advantageous process available, and it produces 70 to 80 percent of the total production in the world. Figure D-1 shows the modified Siemens process (Long et al., 2008).
However, some of the key technologies for the modified Siemens process are owned by several companies in the United States, Germany, and Japan. It is very difficult for Chinese companies to acquire these key technologies, which is a major reason for the high energy consumption and high pollution of existing polysilicon producers. Table D-1 shows the economic factors for an annual production of 1,000 tons of polysilicon in China (Long et al., 2008; Su, 2008; Zeng, 2008).
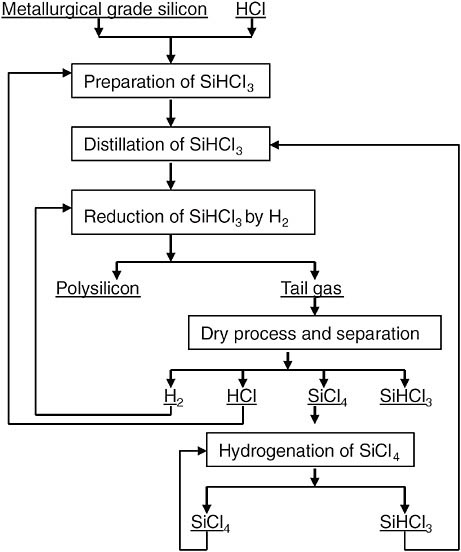
FIGURE D-1 Flow sheet of the modified Siemens process.
Preparation and Purification of Trichlorosilane (SiHCl3)
High purity SiHCl3 is prepared from quartz sand. The steps are as follows.
-
Preparation of industrial silicon: SiO2 + C → Si + CO2↑.
-
Preparation of SiHCl3: Si + HCl → SiHCl3 + H2↑, which produces a gas mixture of H2, HCl, SiHCl3, SiCl4, and Si.
-
Purification of SiHCl3 from the gas mixture.
TABLE D-1 Economic Factors for an Annual Production of 1,000 Tons of Polysilicon in China
|
Items |
Economic costs/benefits |
|
Yield of polysilicon |
1,000 tons per annum |
|
Total investment |
U.S.$ 0.17 billion |
|
Production cost |
U.S.$ 70–80/kg (China) U.S.$ 25/kg (Europe and U.S.) |
|
Power consumption |
0.6 billion kWH per annum |
|
Power produced by solar cells |
200 MW per annum |
|
Power regeneration ratio (over 20 years) |
About 8 |
|
Power generation cost |
7–12 times biomass power generation, 6–10 times wind power generation, 11–18 times traditional coal power generation. |
|
By-product SiCl4 |
8,000 tons per annum |
In the above steps, there are two pollution problems that need to be solved: the capture of by-product CO2 in the preparation of industrial silicon, and the recycling of the tail gas in the purification of SiHCl3 from the gas mixture.
Reduction of SiHCl3
High purity polysilicon is obtained from the reduction of SiHCl3. The reactions are as follows.
-
Main reaction: SiHCl3 + H2 = Si + 3HCl↑,
-
Secondary reaction: 4SiHCl3 = Si + 3SiCl4 + 2H2↑.
Si obtained from reactions (1) and (2) is deposited to form high purity polysilicon. In this process, about 25 percent of trichlorosilane is converted into polysilicon, and the remainder into tail gas. A large amount of tail gas containing many useful materials such as H2, HCl, SiHCl3, and SiCl4 is emitted from the reduction furnace. Moreover, the tail gas includes many erosive and toxic substances. The tail gas must be recycled, or else it will cause serious pollution and also increase costs.
Silicon tetrachloride (SiCl4) is the main component in the tail gas. It is a highly corrosive and toxic liquid. About 8,000 tons of by-product SiCl4 are produced in the production of 1,000 tons of polysilicon. Due to the expensive treatment cost of SiCl4, most Chinese companies do not have such treatment equipments, which then makes tail gas treatment become the bottleneck in the manufacture of polysilicon.
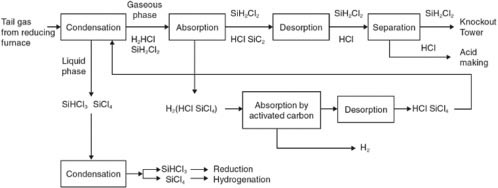
Separation and Recycling of Tail Gas
The components of the tail gas are very complex. These often include H2, HCl, SiHCl3, and SiCl4, whose separation and recycling are difficult. It is necessary to separate H2, HCl, SiHCl3, SiH2Cl2, and SiCl4 individually from the tail gas before they can be recycled. A multistage separation technology that couples pressure condensation, absorption, and desorption is needed. Figure D-2 shows the process of separation and recycling of the tail gas.
Figure D-2 shows that the components of the tail gas can be well separated and recycled, which would reduce tail gas emission and environmental pollution. Silicon tetrachloride can also be used as the raw material for the preparation of white carbon black by the gas phase method. However, due to the backward technologies and equipments, the costs of separation and recycling of the tail gas are very expensive and cannot be borne by Chinese companies.
The environmental pollution from the preparation of polysilicon has attracted the attention of the Chinese government, especially the separation and recycling of tail gas. Research on key technologies for comprehensive utilization of byproducts of the polysilicon production process has been supported by 863 program of China (CNCIC, 2009).
However, the effort has just begun. Further research on efficient and low cost technologies and equipments for the separation and recycling of the tail gas is urgently needed.
Wastewater from the Texturing of Polysilicon
The application flow sheet of chemicals used for the texture preparation of polysilicon wafers are shown in Figure D-3. The first trough uses most of the acid and alkali for the texture preparation (nitric acid, hydrofluoric acid, hydrochloric acid, and potassium hydroxide).
In 2008, Solarbuzz (2009) reported that the Chinese annual production of solar cells had a capacity for 3,000 MW. This consumed a lot of hydrofluoric acid and nitric acid. With the rapid growth of the solar industry, it is apparent that even more of these substances will be used in the future.
Currently, Chinese companies follow the principle of respective discharging and treating of acid wastewater and alkali wastewater. Too much fluoride in drinking water will lead to a variety of diseases, so the discharging and treating of wastewater with hydrofluoric acid is controlled, and the standard for the discharging of fluoride wastewater is very strict. Table D-2 shows the effects of excessive fluoride in drinking water on health, based on Shanxi Province’s guidelines issued in 2006.
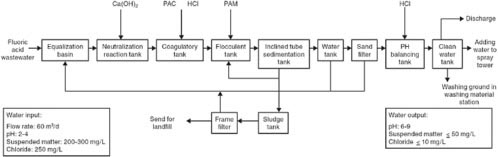
The treatment process in the discharging and treating of hydrofluoric acid wastewater couples chemical precipitation, flocculent precipitation, and filtration. The fluoride is changed into calcium fluoride and separated from the wastewater
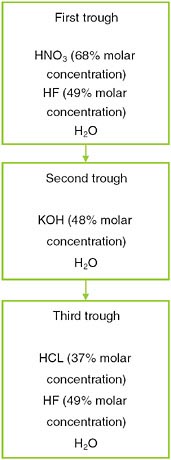
FIGURE D-3 Flow sheet of chemicals used for the texture preparation (based on discussions with polysilicon production plants in China).
by flocculent precipitation and filtration. The filtered mud is abandoned and buried. The process is shown in Figure D-4.
However, if the environment has acid rain or acidic soil, the fluoride ion of the calcium fluoride in the filtered mud will be displaced and will enter the groundwater with the rainwater and pollute the soil and water. This is a very serious problem. Thus, a better technology is urgently needed, such as the recycling of hydrofluoric acid, in order to reduce costs and solve the pollution problem.
It is currently difficult to separate hydrofluoric acid from nitric acid. Advanced membrane separation technologies, such as a modified polytetrafluoroethylene membrane and ceramic membrane, can be used for the purification of the acid wastewater by the removal of metal and nonmetal compounds in the acid wastewater. The purified acid wastewater would only have hydrofluoric acid and nitric acid, which can be easily recycled for reuse by adjusting the concentrations of the two acids.
TABLE D-2 Effect of Excessive Fluoride in Drinking Water on Health
|
Fluoride content (mg/L) |
Body parts |
Effect on health |
|
0.5–1.0 |
Tooth |
There is an anti-caries effect, in which the tooth forms a protective surface layer with a hard mass density. |
|
1.1–2.0 |
Tooth and skeleton |
The incidence of dental fluorosis is 30 percent, with a small amount of skeletal fluorosis. |
|
2.1–4.0 |
Tooth and skeleton |
The incidence of dental fluorosis is 80 percent, with a certain amount of skeletal fluorosis. |
|
> 4.1 |
Tooth and skeleton |
The incidence of dental fluorosis is 90 percent, with much skeletal fluorosis. |
Urgent Problems
In China, the production of high purity polysilicon and solar photovoltaic panels causes many serious environmental problems. These problems have become the bottleneck in the development of the solar energy industry. The following environment-friendly technologies with low energy consumption are urgently needed:
-
In the process of making polysilicon advanced capture technologies for the CO2 by-product produced in making industrial silicon is urgently needed. Efficient technologies and low cost equipment for the separation and recycling of the tail gas are also urgently needed.
-
In the process of manufacturing solar cells from polysilicon, a treatment process for the acidic wastewater that contains hydrofluoric acid and nitric acid is urgently needed. Because it is difficult to separate hydrofluoric acid and nitric acid, the simultaneous recycling of hydrofluoric acid and nitric acid should be used.
-
Advanced membrane separation technologies, such as the modified polytetrafluoroethylene membrane and ceramic membrane, can be used for the purification of the acidic wastewater to remove metal and nonmetal compounds in the acidic wastewater. The purified acid wastewater then only contains hydrofluoric acid and nitric acid, which can be easily recycled for reuse by adjusting the concentrations of the two acids.
-
Due to their low cost, material abundance, and material non-toxicity, thin-film silicon solar cells are also promising for producing electricity cleanly. And amorphous silicon solar cells have been much studied and have reached large-scale production. However, the low conversion efficiency and photo-induced attenuation effects of amorphous silicon solar cells also urgently need to be solved.