3
Research Opportunities
Industry and government needs have been the primary drivers of corrosion research, the results of which have often led to new science, followed by further practical improvements in a continuing symbiotic cycle. Government has played many critical roles, including challenging industry with critical problems (such as the need for applications that can function well in extreme environments encountered, for example, during propulsion, and new synthetic-fuel, energy-storage, and fuel-cell concepts)1 and also performing and sponsoring research to address critical gaps in understanding. Future corrosion research priorities should continue to be guided by societal drivers and associated technological needs (top-down drivers), but progress in this area will also benefit from advances enabled by focusing on related areas of fundamental science (bottom-up drivers). The strong interactions between engineering-oriented corrosion grand challenges and the underlying fundamental science as discussed in Chapter 2 are illustrated by the iconic triangle shown there whose foundation is corrosion science.
The impact of corrosion on everyday life is a major issue, given that corrosion and materials reliability affects public infrastructure, industrial complexes, and major areas of governmental endeavor and responsibility. The deleterious effects of corrosion and its societal impact are highlighted by growing concerns about public safety, endangerment of personnel, national security, energy security, national de-
|
1 |
Department of Energy, Basic Research Needs for Materials Under Extreme Environments, Report of the Basic Energy Sciences Workshop on Materials Under Extreme Environments, June 11-13, 2007, available at http://www.sc.doe.gov/bes/reports/files/MUEE_rpt.pdf. |
fense, industrial productivity, economic competitiveness, environmental protection and sustainability, and the standard of living and quality of life. Numerous what-if scenarios suggest ways that fundamental advances in corrosion science could have a positive impact on numerous problems facing society. What if fundamental science uncovered so-called silver bullets in materials or coating designs for mitigation of corrosion that could extend the use of cost-effective materials into more extreme environments or enhance materials capabilities for energy storage? The ability to effectively address many societal and technological challenges could benefit from game-changing advances in corrosion science.
Corrosion science, a truly interdisciplinary field that includes aspects of physics, materials science, surface science, electrochemistry, and fracture mechanics, benefits directly from new developments not only in those associated fields of fundamental science, but also in others. One challenge for the corrosion science community is to pursue strategies to harvest those diverse benefits and apply them to corrosion-related problems.
The multidisciplinary nature of corrosion research requires a balanced portfolio of single investigator and collaborative group activity. Group efforts at various government laboratories have addressed corrosion problems, and some continue at this time. In academia, however, funding for group efforts is difficult to find, particularly for fundamental and applied problems. National Science Foundation (NSF) funding, with the exception of that for large centers, tends to focus on single-investigator projects. A model of what is required is the DOD Multidisciplinary University Research Initiative (MURI) program, which supports research by small teams of investigators from more than one traditional science and engineering discipline in order to accelerate both research progress and the transition of research results to applications. Most MURI efforts involve researchers from multiple academic institutions and academic departments and include support for up to 5 years.
Corrosion science remains a fertile scientific endeavor, poised for advances that will benefit society. As in the past, these advances will be enabled by progress in related fields, particularly in materials characterization and computation. Indeed, an overarching observation is that the amazing recent advances in these areas portend well for the future of corrosion science as capabilities for refining time and length scales allow modeling and experimentation to converge.
While corrosion traditionally has been observed at the macroscale, recent scientific emphasis has shifted to understanding the processes at smaller length (and time) scales. For example, many corrosion processes are now known to be controlled by molecular-, submicrometer-, and micrometer-scale phenomena. Although much has to be learned regarding the nanoscale chemistry, structure, and dynamics at individual grain boundaries or other key material features, progress is also required at the granular scale to understand how networks of boundaries and
arrays of defects behave under corrosive conditions for metallic and nonmetallic materials. Thus multiscale characterization and modeling will enable progress in understanding this phenomenon.
This chapter highlights some of the research opportunities that hold great promise for corrosion mitigation, organized according to the four corrosion grand challenges (CGCs) identified by the committee:
-
CGC I—Development of cost-effective, environment-friendly corrosion-resistant materials and coatings;
-
CGC II—High-fidelity modeling for the prediction of corrosion degradation in actual service environments;
-
CGC III—Accelerated corrosion testing under controlled laboratory conditions that quantitatively correlates to observed long-term behavior in service environments; and
-
CGC IV—Accurate forecasting of remaining service time until major repair, replacement, or overhaul becomes necessary—i.e., corrosion prognosis.
As indicated in each section, high-priority fundamental science issues are at the heart of the ability to predict corrosion damage, design new materials and coatings, and sense as well as predict corrosion. The section below is not intended to be an exhaustive compilation of all corrosion research opportunities. Instead, it highlights some of the challenges in the field of corrosion science and engineering in each of the important high-priority areas identified by the committee. An underlying theme is the need for participation by multidisciplinary and cross-disciplinary teams of researchers, in addition to the individual investigator, to address the above corrosion grand challenges, as well as the need to disseminate the knowledge acquired to the greater community.
This chapter also includes a section on opportunities in instrumentation that briefly describes some of the analytical techniques that have enabled and will continue to enable ongoing advances in corrosion science and mitigation of corrosion.
OPPORTUNITIES FOR RESEARCH
CGC I:
Development of Cost-Effective, Environment-Friendly Corrosion-Resistant Materials and Coatings
Development of superior corrosion-resistant materials and coatings is the ultimate proactive corrosion challenge. While this has long been a goal, it has not been realized in many applications for a number of reasons, including the strategy of using trial-and-error approaches for material development, the high cost of achiev-
ing an ultimate materials solution, the lack of fundamental knowledge about how to design a materials system expressly to resist corrosion while also meeting all the other mechanical and physical property requirements, and inadequate understanding of corrosion processes that degrade materials, including the very definition of the corrosive environment itself.
The research opportunities identified are those that address key needs in the development of:
-
Corrosion-resistant materials,
-
Protective coatings, and
-
Materials for active corrosion protective systems.
Together with factors that impact the recyclability of materials, they suggest the type and scope of effort needed to make progress toward the goal of CGC I. Surface materials science is closely linked to corrosion behavior and should also be a focus of technologies in CGC I. Advances in corrosion mitigation will require better understanding of surface structure and properties.
Development of Corrosion-Resistant Materials
The design stage of a product or system is one of the first lines of defense against corrosion, and a designer should have the ability to prevent the onset of corrosion by choosing materials that are as intrinsically resistant to corrosion and environmental degradation as possible. By carefully considering materials choices, it is possible to affect the thermodynamic stability and/or alter the rate of corrosion kinetics, and thus appreciably impact performance of the system over time. As stated in the 2004 Defense Science Board Task Force report on corrosion,2 “an ounce of prevention is worth a pound of cure.” ICMSE will soon be possible,3 and there is the opportunity to tailor microstructure, composition, and processing to achieve corrosion properties to meet the needs of a design if certain scientific barriers are overcome. Increasingly, environmental concerns are driving the need for engineering materials with intrinsic corrosion resistance,4 but high-performance corrosion-resistant materials are often too expensive to use for applications where
|
2 |
See Defense Science Board, Corrosion Control, Final Report ADA428767, October 2004, available at http://www.acq.osd.mil/dsb/reports2000s.htm. |
|
3 |
National Research Council, Integrated Computational Materials Science and Engineering: A Transformational Discipline for Improved Competitiveness and National Security, The National Academies Press, Washington, D.C., 2008. |
|
4 |
For instance, large amounts zinc, and copper are exposed to the environment as parts of structures such as roofs, facades, and support beams; atmospheric corrosion of these structures results in unintended release of metal ions by run-off and then dispersion into the environment. |
large amounts would be required.5 Clearly one opportunity lies in creating cheaper materials with high performance. Other engineering considerations—such as joining and fabricating—also play important roles in deciding whether a particular material can be used in an application.
As described in Chapter 1, the traditional materials development process is long and drawn out, requiring multiple iterations in chemistry, microstructure, and processing methods to achieve desired material properties. In the past, optimization of corrosion properties has been achieved by trial-and-error, lessons learned, or—at best—by corrosion experts using a mix of empirical experience blended with some scientific intuition. One vision for the inclusion of corrosion in quantitative materials design is the development of focused tool-kits that can be used to optimize the development of materials, coatings, and treatments for mitigation of targeted corrosion processes—such as paint delamination, crevice corrosion, or high temperature selective alloy depletion of coatings. The lack of such a process for rapid, “intelligent” materials development in corrosion has been a major impediment to making significant improvements in the design of new products but also represents a significant opportunity to move this area forward when such tool-kits are developed. Integrated computational materials science and engineering (ICMSE) has shown the potential to optimize a new material relative to its required properties and cost through advanced computational tools and supporting databases.6 Figure 3.1 contrasts the traditional approach with the ICMSE approach.
The ICMSE approach is based on computer modeling and simulations that have a high fidelity to physical experiments. Consequently multiple iterations equivalent to alloy development cycles can be conducted quickly, at low cost, by analysis (with selective physical experiments) compared to the traditional entirely physical materials development approach. This is revolutionizing materials development and reducing the time necessary to do so by more than 50 percent.7 Key to the advance of this process have been advances in other engineering tools and the rapid increase in available computational power. Clearly, development of new materials using this technology is strongly dependent on the availability of good models, which
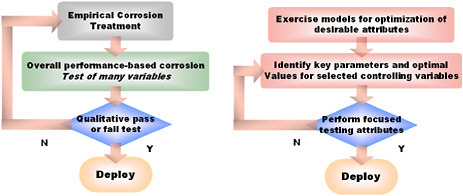
FIGURE 3.1 Comparison of existing or traditional approach (left) and desired approach (right) to the design of corrosion-resistant materials. SOURCE: John R. Scully, Department of Defense Corrosion Conference, 2009.
is discussed later in this chapter as part of CGC II. A significant opportunity for research consists of developing and integrating corrosion models (discussed under Corrosion Grand Challenge II) with other materials models so that high-fidelity predictions can be made regarding corrosion behavior for new materials and new corrosion environments. With this integration, the following types of problems are among those that could be addressed by the ICMSE approach.
-
Identifying the elements that act in synergy with other major alloying elements to enhance the intrinsic effects of corrosion mitigating elements. For instance, additions of molybdenum and minor amounts of nitrogen to stainless steels, copper to weathering steels, and arsenic as well as tin to brass have been found to be incredibly potent strategies to improve aqueous and atmospheric corrosion resistance. The expectation is that other such combinations of elements are soon to be discovered for other materials systems.
-
Improving the properties of ultra-high-strength stainless steels that are desired for critical applications within aerospace, such as highly durable bearings, power transmission shafts, and aircraft landing gear structures. Advanced modeling tools have the potential to guide the selection of an optimum chemistry balance in which general corrosion resistance is improved without increasing the susceptibility to at the expense of other modes of corrosion such as environmental cracking. Because of their ability to form protective chromia layers at elevated temperatures, stainless steels also proffer good high-temperature corrosion resistance—up to a point. As new applications demand higher temperatures and introduce more aggressive reactants, often for energy or process efficiency, conventional stainless steels do not
-
have sufficient strength or corrosion resistance. However, advances in capabilities to do accurate thermochemical modeling and prediction, combined with principles of selective oxidation of alloys and mechanistic knowledge of creep, provide new and unique pathways to producing future stainless steels with higher temperature capabilities.8
-
Developing corrosion-resistant materials for use in concrete reinforced structures often deteriorated by corrosion of the reinforcing steel. Solid stainless steel and stainless steel-clad rebar materials have demonstrated the ability to extend the chloride induced corrosion initiation threshold in concrete to over 100 years when compared with plain carbon steel currently such a material change is quite costly.9 There is a possibility to design new low cost, intrinsically corrosion-resistant reinforcing materials without resorting to the use of expensive alloying elements,10 which will ultimately enable their use not only in concrete but in other environments. Other innovative mitigation strategies that can also be investigated, including developing concrete microstructures that have lower permeability to moisture or contain corrosion inhibitors as part of their intrinsic chemistry.11
-
Developing an affordable, manufacturable, high-strength pipeline steel that is highly corrosion resistant. Pipelines for deep-water oil and gas production and recovery present severe corrosion challenges: hydrogen sulfide, carbon dioxide, oxygen, and mineral salts (especially chlorides) can lead to material degradation by hydrogen embrittlement, sulfide cracking, and localized corrosion. Even though modest progress has been made with corrosion-resistant nickel-based superalloys and supermartensitic stainless steels with Ni and Mo in limited applications the former are currently too expensive for widespread use and thus pose an excellent opportunity for research. The need for material development for pipelines for CO2 sequestration must also be considered as this is a possible solution to excess atmospheric CO2 in global warming control. However there is a shortage of true data and engineering knowledge, especially on potential corrosion problems in such a large undertaking.
The related “add-on” challenge is to optimize materials for conjoint failure modes when conjoint, nonlinear and coupled corrosion processes occur, including mechanically induced modes (wear, fretting, fatigue, and creep). Another need is the ability to handle or anticipate changes in solution or processes with time and transitions in corrosion modes.
The IDEAL Corrosion-Resistant Alloy for Aqueous Environments
Alloys are often designed with properties other than corrosion resistance in mind, such as mechanical strength. In the case of structural materials strength, ductility, toughness and joining issue are often dominant properties. Corrosion resistance is often secondary, although major alloying elements have been incorporated for many years to increase corrosion resistance. The question arises as to the ideal attributes of an alloy for maximizing corrosion resistance whilst keeping its originally intended properties. It should be recognized that there are trade-offs with a range of properties and that all of those below cannot likely be realized simultaneously. A corrosion-resistant alloy ideally would have the following properties:
-
Form a homogeneous solid solution alloy lacking structural and chemical non-uniformities.
-
Contain a critical amount of beneficial alloying elements in homogeneous solid solution that are readily enriched in the passive film, in amounts exceeding the thresholds for passivation.
-
With respect to the key alloying element, be preserved by having the means to avoid interface or surface depletion. The passive films developed on the surface might function in other roles through engineering of the properties of these films (which are, in essence, semi-conducting oxide films) to suppress electron transfer reactions or create ion selective membranes. Avoidance of depassivating alloying elements would be desirable. However, if they cannot be avoided, ideally these elements would not be hydrolysable so that the pH would not be lowered at local corrosion sites.
-
Embody beneficial synergy among the alloying elements listed above.
-
Contain alloying elements that make the passive film a poor substrate for electron transport reactions such that reactions like oxygen reduction are suppressed.
-
Have attributes for maintenance of low interfacial stresses and possess sufficient ductility to avoid cracking and spallation. Perhaps the oxide would be graded to avoid a classical interface.
-
Contain elements that enable fast repassivation rate at scratches and flaws on multiple occasions.
-
Lack negative impurities to the extent possible or otherwise sequester them so that they cannot be swept or collected at surfaces and interfaces (interface engineering).
-
Contain other beneficial trace alloying elements such as bond promoters, passivity promoters, glass formability, or elements that serve as gettering agents to sequester harmful species.
-
Exploit the full capabilities of defect engineering to avoid one-, two-, and three-dimensional defects of critical sizes, and spacings that trigger certain corrosion modes and their spreading. This is especially necessary in classes of alloys where heterogeneity is unavoidable, as in the case of precipitation age-hardened alloys.
-
Exhibit avoidance of grain boundaries or incorporate clean grain boundaries with controlled application of a number of boundaries with low CSL.
-
Avoid segregation or depletion of the alloying element during heating.
-
Exhibit diffuse, not co-planar, plastic deformation that occurs in grain interiors and is not focused at grain boundaries.
-
Have alloying elements that, once the oxide film was broken down or penetrated, would have a slow dissolution rate, resist noncongruent dissolution that is detrimental, and collect beneficial alloying elements at easy low coordination dissolution sites. The alloying element might also be engineered to alter surface diffusion rates.
Inspection of this list suggests that amorphous metallic alloys satisfy many of these criteria. This is partially true. Amorphous materials are a possible choice in some applications, and this list is growing with the advent of bulk metallic glasses. However, in many cases where conventional crystalline alloys must serve as the best available choice, they are either unavailable in the product form needed or lack some other desired properties. A slightly different set of attributes might be desired for resistance of hydrogen embrittlement.
The ideal corrosion allowance material might:
-
Exploit species in the solution to make an insoluble tenacious oxide or corrosion product that slows the corrosion rate,
-
Dissolve in a extremely predictable manner,
-
Avoid stress buildup that can spall or crack oxide, or
-
Corrode in a uniform, predictable manner.
Protective Coatings
The ICMSE approach is also applicable to the development of corrosion-resistant coatings. An advantage of coatings is that they offer the potential of a hybrid structure in which the function of the coating can be specialized for corrosion resistance while not affecting the key properties of the underlying substrate material. (However, this is a particular challenge at high temperatures, where inter-
diffusion puts additional requirements on the coating-substrate system.) Coatings can also provide a lower cost solution than using a higher grade substrate material. Such coatings are often solely physical and chemical protective barriers between the corrodible substrate and its environment. Barrier protection from corrosion occurs when a physical layer is constructed to prevent damaging environmental species from reaching an object or system. In the simplest sense, humans use buildings as barriers between the contents of the building and the outside environment, thus preventing weathering attack on the interior objects. This form of barrier is passive, because the barrier material does not depend on a chemical process to provide protection, but rather simply stops the passage of environmental threats—such as acid rain—to the object to be protected. Passive barriers, coatings, and barrier layers include the following:
-
Electro-deposited metal layers such as gold, silver, chromium, and others that put a relatively inert material between the corrodible substrate and its environment;
-
Vapor-phase-deposited metals, alloys, oxides, or other materials for protection of reactive metal substrates;
-
Spray-applied layers, including flame-sprayed metals, reactive sprays, high velocity sprays, cold sprays, and plasma-deposited layers of all sorts;
-
Zinc- and zinc/aluminum-based galvanized layers for steel that combine a cathodic protection layer and a barrier layer; and
-
Organic coatings (paints) that insulate reactive metal surfaces from aggressive environments.
The following is a selection of areas focusing on coatings that the committee identified as high-priority opportunities:
-
Coatings possessing high adhesion, mechanical property matching, interfacial compatibility, and low interfacial impurities. This is applicable to a wide range of films from high-temperature ones of the type used on turbine blades to those on electro-coated metal connectors. As an example, for coatings designed to proffer high temperature oxidation resistance, it is often critical to tightly control rare earth (RE) and light element (e.g., carbon and silicon) concentrations at the less than 0.1 atomic percentage level, both respectively and with respect to the RE-C and RE-S ratios.12 These concentrations can be quite difficult to achieve without advances in understanding and controlling processing approaches. If there were
-
new deposition monitoring tools capable of accurately determining composition, direct feedback could achieve the needed accuracy in the composition.
-
Robust, defect-free cost-effective barrier films, available for large surface areas. Nanoscale thin films for aqueous corrosion resistance show much promise; however, the results have been based on films produced in the laboratory. Further assessing the feasibility of these barrier films’ properties requires considerable process scale-up. Recently, paints containing zinc oxide powder, which have long been utilized as anticorrosive coatings for various metals and alloys, have been seen in a new light. These paints are especially effective for galvanized steel, which is difficult to protect because its reactivity with organic coatings leads to brittleness and lack of adhesion. Zinc oxide paints retain their flexibility and adherence on such surfaces for many years. Progress has been made recently13 with ZnO highly n-type doped with Al, Ga, or In which is transparent and conductive and can be used as heat protection if applied to windows. Another exciting ongoing development is the incorporation of additives such as Mg in the zinc oxide. This Mg addition renders the oxide inactive by a change of semiconductor type from n-type to p-type for the reduction of oxygen. This inactivation of oxygen-reduction capability is a necessary step in mitigating corrosion and coating degradation. The n-type oxide is detrimental because it reduces oxygen too easily, whereas conversion to the p-type makes that process less probable and as such improves the protective capability of an already-flexible and highly adherent sacrificial coating.14
-
Self-sensing and self-healing films and coatings. These smart films can be externally interrogated or can self-sense their own health and respond by either self-healing or actuating an external healing response.15 A number of strategies that can be used to trigger healing and release.16 However, more research is needed to determine what approaches works best. One example is a material that contains small spheres with a sealant; if a crack forms, some spheres will break open and release the sealant into the crack. This sealant can be designed so that it reacts upon release and solidifies, effectively repairing the crack.
-
Thorough understanding of corrosion protection mechanisms for nontoxic corrosion inhibitors and conversion-coating procedures. Over the past several years, considerable effort has been spent in replacing chromate coatings, which pose a
-
health and environmental hazard due to the chromium VI ion. This issue is especially important with respect to protecting aluminum structural materials, including those used in commercial and military aircraft. Researchers have examined a range of new potentially corrosion-inhibiting compounds as well as radically different approaches to protective coatings. None of these approaches has yet achieved the broad and effective performance of chromates. Unlike chromates, many current-generation, nonchromate inhibitors have not shown consistent performance for aluminum alloys; herein lies a very important area for additional research.
-
Taking advantage of—and exploiting—mineral scales that can naturally develop on materials’ surfaces. Limiting the corrosion of copper and copper-based alloys, for example, often depends on the formation of mineral scales (e.g., atacamite, brochantite, malachite, and other) that can increase their corrosion resistance. Magnesium offers even greater opportunities in this regard because phosphate- and carbonate-based mineral scales may significantly alter corrosion behavior. Here, clever design of materials17 in combination with exploiting the environment can generate extremely low rates of corrosion18 owing to the formation of corrosion-resistant compounds. This would be especially true for materials where damage to the protective film (as a result of wear, pinhole formation, and so on) would be repaired by the regeneration of new scale. The development of such scale should incorporate detailed understanding at the molecular scale in order to fine-tune properties appropriately.19
Corrosion Environments—Properties of the Ideal Coating for Aqueous Corrosion Resistance
An ideal coating (Figure 3.2) should have a very hydrophobic surface and should form high barriers to water and electrolytes. This property normally requires a polymer coating that is highly cross-linked and that contains silicone or fluorocarbon monomer, has reduced polar groups on the polymer, and maintains desirable thermo-mechanical properties. The ideal coating should also have controllable gradients in chemical composition to minimize interfacial discontinuities and create a covalent bonded structure from the substrate through to all of the coating layers.
In the substrate immediate interfacial region, one needs a gradient in properties from bulk to interface that maximizes the desirable mechanical and physical prop-
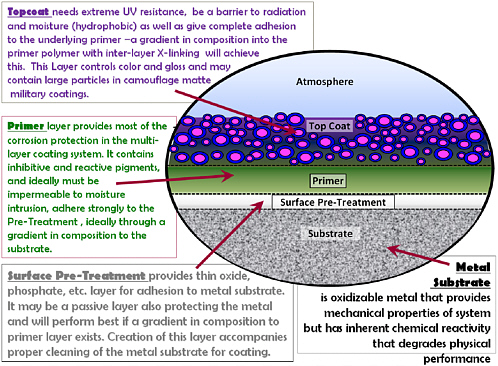
FIGURE 3.2 The ideal coating system—technical issues.
erties of the substrate but minimizes its corrosion/degradation susceptibility and provides a proper composition for interaction with the protective coating system. This would be a coarse gradient on the atomic scale from bulk to several nanometers of interface. Then there should be a tightly controlled interfacial region that can be chemically matched to the coating system and provide a basis for water-resistant adhesion and mechanical property matching with the coating layer to avoid excess stress concentration in the interface and subsequent coating mechanical delamination. This may include control of surface oxide formation with residual reactivity for the matrix of the pretreatment (which may be simply oxide formation) or substrate enrichment of minor elements in an alloy substrate. If one can get cross-diffusion across an interface without creating excess chemical reactivity or mechanical stress, one gets true improvements in adhesion.
If the coating is applied as a liquid layer, no defects in the application and film formation processes are acceptable (this is why electro-deposited paints such as those used in automobiles are so excellent at corrosion protection)—this is a true ongoing challenge in quality engineering.
If it were possible to create composite structures within the films described above while still maintaining the prerequisite environmentally resistant adhesion, this would potentially improve film properties considerably, because the composite structures would offer significant improvements in layer chemical, mechanical, and barrier properties.
A multicoat system provides a higher probability of ensuring complete, uniform coverage of the corrodible substrate. Maintenance of interlayer adhesion is crucial. The multilayer structure might allow incorporation of many desirable properties in the outer coatings system, such as:
-
Water resistance,
-
Controlled or minimal oxygen permeability,
-
Electrolyte resistance,
-
Chemical resistance,
-
Capability of sequestration of undesirable reactive materials permeating in the film to extend diffusion lag times by orders of magnitude, and
-
Pigments with shape factors (such as platelets or needle-like shapes) that orient in the film to give desirable improve resistance of electrical connection properties.
A new emphasis in the design of coating systems that needs field demonstration to deliver its considerable promise is the so-called smart coating. Coatings in this category respond to stresses from their environments in a designed, intelligent manner. This includes triggered release of corrosion inhibitors when the possibility of corrosion occurs due to external threats, self-repair of mechanical damage, local sequestration of corrosion-inducing impurities such as chloride ions, local color or spectral changes that allow external sensing of corrosion, and even alteration in local cross-link density in polymer matrices. However, many issues remain in achieving these attributes, such as simultaneous optimization of properties and their translation into exact lifetime extensions by other than trial-and-error approaches.
Improved Materials for Active Corrosion Protection Systems
A common mode of corrosion protection under aqueous or atmospheric conditions is to actively protect a metal object by electrochemical means. Two basic types of this protection are in use: anodic and cathodic.20 Anodic protec-
tion consists of electrochemically driving a metal to a passive state it would not spontaneously achieve as an electrochemical anode.21 This form of protection requires very good potentiostatic control and a robust, accurate reference electrode. Unfortunately, expanding the use of active protection mechanisms requires more accurate and more robust reference electrodes to ensure precise control of the anodic protection potential. Hence, research in developing a new robust, accurate reference electrode is needed in order to extend active corrosion protection to areas such high-temperature systems.22
Another research need for anodic protection is alloys designed to be effectively self-driven to a stable, self-healing passive state under ambient conditions. This is the current mechanism for the corrosion resistance of stainless steels in oxygenated near-neutral water. The development of alloy compositions that could achieve the same effect in more aggressive environments is a major driver for further research.
Cathodic protection, currently one of the most widely used forms of corrosion protection, includes applying an external potential to an object that might corrode, driving it to a potential where the corrosion rate will be decreased, or—for passive alloys—driving it to a potential below a critical potential for localized corrosion.23 Some advances needed in cathodic protection include:
-
Improving methods for controlling imposed cathodic (impressed-current) protection. Currently most modes use galvanostatic control with a strong tendency to overprotect the substrate because of uncontrollable potential fluctuations.24 Shifting to potentiostatic control would reduce this, but no methods are commonly available to bring a reference electrode close enough to the object being protected to give accurate control of potential. The usual problem with cathodic protection is that objects are extended and cannot be controlled from one spot. Another issue is the need for self-sensing, self-correcting anodes that are on and aware of changes
-
in environment or of the need for protection. These anodes could then change or adapt to the conditions in order to optimize protection of corrosion-prone materials without causing embrittlement or coating damage. They could also enhance anode lifetime if they did not operate when conditions were more benign. While tunable resistors are currently available to limit current output, there is the need for set-and-forget smart anodes requiring minimal monitoring that can self-correct themselves. There is also the need for tunability, but this requires electrochemical interface control. Clearly, additional research efforts in this area represent a good opportunity to advance active protective methods.
-
Increasing the range of metals and alloys that can be cathodically protected. There are indications that a broader range of metals and alloys (other than steels) can benefit from this form of protection. Magnesium particles in coatings have been shown to be effective in protecting aluminum without the need for chromates. However, there is a need for additional research to determine promising materials combinations.
CGC II:
High-Fidelity Modeling for the Prediction of Corrosion Degradation in Actual Service Environments
Corrosion grand challenge II entails developing a better understanding of corrosion mechanisms and morphologies, coupled with the environments in which they operate, and the fusion of the knowledge, data, models, and tools available into more accurate—and widely descriptive—models of materials and environments for all types of corrosion.
This capability will greatly accelerate the development of corrosion-resistant materials, as discussed above in connection with CGC I. Additionally, it will provide insight into the feasibility and benefits of various corrosion mitigation approaches. However, modeling has several challenges besides the usual computational limitations, including lack of fundamental mechanistic information, lack of understanding of the environment, and the large statistical distributions often seen in corrosion damage processes and metrics that represent real effects, not instrument errors, which are often limited by shortcomings in mechanistic knowledge in the corrosion field. It is often said the best models are possible when the mechanism is well understood. Two key areas for future work in this field are (1) increasing the available knowledge of fundamental aspects of corrosion the lack of which thwart modeling and (2) connecting the current islands of knowledge to provide more complete models, such as linking the governing nanoscale processes across many time and length scales to meaningful engineering-scale metrics, depicted in Figure 3.3. These needs are complementary to the larger, multifaceted modeling efforts directed at predicting materials performance as part of the integrated product development process. It is also clear that even more
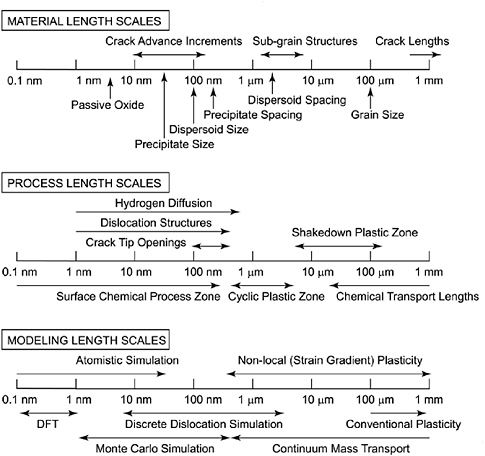
FIGURE 3.3 The wide span of different length scales with relation to the material, the processes, and the models needed to correctly predict corrosion degradation. SOURCE: University of Virginia Center for Electrochemical Science and Engineering, courtesy of Matthew R. Begley and Richard P. Gangloff.
overarching hierarchies of models might be required at three levels: (1) materials behavior (ICMSE); (2) degradation mode models; and (3) system-level models for equipment or infrastructure systems.
As discussed in relation to CGC I, advances in ICMSE are making possible the rapid development of new materials based on knowledge gained through the development of better models, as well as through continued empirical inputs. However, the emphasis to date has been on tools to optimize mechanical and physical properties within a target cost range, and corrosion resistance has rarely been directly considered in ICMSE except by extension of physical properties. Figure 3.4 shows
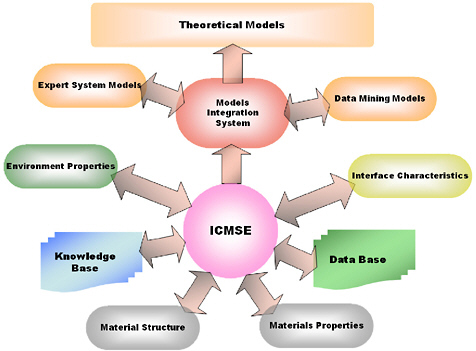
FIGURE 3.4 The flux of materials information required to achieve computational materials design.
some of the multiple databases, knowledge bases, and models that will be required to make ICMSE a reality, with model-integration a critical activity.25
Computational materials design tools are now emerging in physical and mechanical metallurgy but are lagging with respect to corrosion behavior. To parallel these tools for materials degradation modeling would require inputs such as details of alloy chemistry and phase formation, as well as environment definition, mechanistic behavior, potential and pH stability diagrams (for aqueous corrosion), kinetic laws, including the probability of certain events occurring or statistically distributed properties and parameters based on knowledge of the corroding system. The outputs might include initiation, propagation, and arrest of selected corrosion phenomena. Advances in computational methods and enhanced understanding of stages of corrosion processes create an opportunity for solid advancement, especially if gaps in the fundamental knowledge of corrosion are pursued. Filling
these gaps will enable high fidelity models without guesses or assumptions. The subsections below discuss the variety of corrosion models now available.
Corrosion Modeling—General Aspects
Corrosion models incorporate knowledge gained by understanding the details of corrosion mechanisms and through experience with particular materials in specific applications and environments, along with data from corrosion testing to either predict damage or tailor materials to resist it. Prediction is accomplished by capturing the mechanism of time-dependent damage as well as physical, geometric, and environmental factors such that time steps can be accelerated faster than real time to predict damage accumulation. Another goal of modeling, already mentioned above, is computational materials design whereby a range of corrosion behaviors in addition to other materials properties (e.g., mechanical, electrical, and magnetic) can first be understood and then tailored for a given application. Resistance to corrosion can often be achieved by selecting an appropriate material or coating, and such strategies are often a worthy investment.26 For example, empirical formulas such as the pitting resistance equivalency number can be used to predict certain critical parameters (e.g., critical pit and crevice temperatures) of the performance of corrosion-resistant materials in aqueous environments. Such prediction is now possible in a limited number of alloy systems but is in its infancy—for instance, the effects of all alloying elements are not incorporated, and broad applicability to a range of environments in uncertain. Moreover, although some progress has been made in grain boundary engineering using percolation theory and in interface engineering for single species on simple boundaries, the design of materials via tailoring of the composition of major, minor, and trace elements is not yet fully developed. The selection and control of phases and control of defects such as inclusions by interface engineering are also very much in their infancy. By contrast, thermodynamic and phase transformation models are now available to predict the types and amounts of phases in both aqueous and high-temperature, condensed-phase, and gaseous environments. These pockets of progress should be encouraged to continue to grow.
The overarching goal of corrosion modeling is to provide a reasonable estimate of expected corrosion behavior for a material in a particular application. This is inherently a complex undertaking for a number of reasons:
|
26 |
A June 2008 Department of Defense (DOD) report estimates the average return on investment from more than 80 recent corrosion mitigation projects at around 50:1 (DOD, Efforts to Reduce Corrosion of the Military Equipment and Infrastructure of the Department of Defense, 2008, available at http://www.corrdefense.org/CorrDefense%20Magazine/Summer%202007/PDF/2007_DOD_Corrosion_Report.pdf). |
-
Corrosion of even a single form or type often involves multiple stages, each of which has different elementary steps or processes across several length scales.
-
Corroding systems are often multilayered and involve geometric complexities such as physical recesses, crevice, or cracks.
-
Chemical, physical, electrical, electrochemical, metallurgical, and mechanical factors often affect corrosion initiation and kinetics.
-
Chemical, electrical, and material-based gradients are often very steep and can occur over very small length scales.
-
Real environments are often complex, uncontrolled, and incompletely delineated. The critical factors controlling the type and the rate of corrosion are often unknown.
-
Some corrosion parameters are statistically distributed, and the challenge is to properly capture and represent such distributed behaviors.
Because of such complexity, there are scientific barriers to the advancement of lifetime prediction models that forecast damage accumulation, as well as barriers to computational design of materials for obtaining desired corrosion properties and to achieving, at the engineering level, a prognosis of future damage on the basis of physics at the materials science scale. These barriers present opportunities for improvement that include, but are not limited to:
-
Closing the many gaps in understanding of mechanisms of corrosion for improved formulation of scientific laws governing the phenomenon of corrosion damage.
-
Incorporation of environmental parameters into the complex time-dependent laws governing damage of materials. An interface can have a surface composition that may differ from that of the bulk material on both the electrolyte and material sides.
-
Formulation of more corrosion-damage laws that can describe the process in three dimensions or can incorporate necessary combined spatial and temporal aspects of corrosion.
-
Improved definition of the environment affecting corrosion. Corrosion-related properties are not intrinsic to a given material but instead are dependent on the details of the environment to which materials are exposed.
-
Modeling at multiple length scales ranging from atom to component scales, and modeling of the damage from corrosion in three dimensions.
-
Further scientific progress required to completely understand the details of corrosion mechanisms at multiple length scales. Such knowledge will ultimately be useful in engineering applications through their impact on mesoscale or engineering-scale material properties.
Advances in modeling over the last 30 years make predictions of damage evolution a realistic goal for the near future. As increased mechanistic understanding occurs, computational capabilities expand, and the corrosion field follows the progress made in modeling in materials science elsewhere, making this goal achievable. As long as each step in the degradation process can be validated, modeling should improve for use in control of corrosion. Appendix C describes the models in more detail.
Deterministic Modeling
By definition, a deterministic model assumes quantitative, physical-based knowledge of the causal laws that govern corrosion, which is a very complex phenomenon. Accordingly, strong hypotheses simplifying the problem have had to be introduced to allow causal natural laws to be applied. Some of the factors required, and thus research opportunities presented, to perform realistic deterministic modeling are the following:
-
Understanding the key driving factors affecting corrosion, including knowledge of the material (chemistry, defects, processing history, mechanical and physical properties, and so on), the chemistry of the corrosive environment (general and localized), and information about the component (geometry, stress, temperature, pressure, environment, and so on);
-
Accessing reliable information on the kinetic parameters of the corroding system;
-
Understanding the corrosion evolutionary path from initiation through the different phases of the corrosion degradation process; and
-
Understanding and accounting for any inaccuracies introduced by numerical method solutions and by computer system limitations.
Because corrosion phenomena are complex, deterministic models evolve continually as restrictive hypotheses are eased when additional, empirical knowledge is acquired. In essence, it is the scientific method that nudges a model to reality. Hybrid deterministic models have been developed in fracture and fatigue where a particular property or parameter is considered to be statistically distributed. This statistical distribution is carefully chosen for implementation to selected parameters in the deterministic model (a true deterministic model retains its probabilistic aspect as a placeholder until the statistical scatter can be replaced with true mechanistic understanding).
Empirical Modeling
Empirical models, sometimes called “database” models, exist at the other extreme of the modeling spectrum from deterministic models. Empirical (or phenomenological models) are based on the philosophy that everything that can ever be known must have been observed. Accordingly, these models are very restrictive and are not a good choice for exploring the outside limits of the data used to develop the model. For example, if an empirical model is developed based on observation of the corrosion of iron in an acidic medium, the use of that model for the corrosion of the same iron in a basic medium will give inadequate results.
Empirical corrosion models can benefit by adapting techniques from the field of artificial intelligence (AI), especially when the knowledge base is very large. Data itself is not knowledge, but knowledge can be extracted from the data with the proper tools to assist in (1) collecting, formatting, and accessing corrosion data; (2) capturing knowledge in very important research areas; and (3) unifying the best models and making the resulting algorithms readily available. AI tools of interest include:
-
Methods to organize knowledge, such as expert systems;
-
Tools to cluster data by similarities, such as self-organized maps;
-
Tools to extract patterns, such as neural networks;
-
Tools to transform dynamic data, such as wavelets; and
-
Tools to perform optimization and fast search, such as genetic algorithms.
When the underlying knowledge is lacking or poorly defined, data mining is a powerful technique that automates searching for patterns in available data. Data can be ordered, transformed, and mapped and then used to predict results. Data mining also utilizes special algorithms that aid visualization of emergent patterns and trends stored and/or hidden within the data.27 Probability inference can help to estimate the likelihood that the conclusion obtained by analyzing the data is true. Bayesian inference networks—also known as belief networks—are used to estimate the probability distribution of one or more attributes of the data.
Neural networks map variables into results using a black-box approach and can be used as components of analyses designed to build explanatory models, because they explore data sets in a search for relevant variables or groups of variables; the results of such explorations can facilitate the process of model building. Neural networks are capable of approximating any continuous function, and thus a researcher does not need to have a hypothesis about the underlying model. An important
disadvantage is that it can be difficult to interpret the solution in traditional analytic terms such as those used to build theories that explain phenomena. It is clear that these approaches are not used nearly enough in corrosion, and opportunities exist to develop their effective use with dedicated research and the development of relevant corrosion data and testing approaches.
Statistical, Probabilistic, and Stochastic Models
Statistics, the science of making effective use of numerical data relating to experiments, can provide insight into the quality of the data produced experimentally. To apply statistics to a given data set, it is necessary to (1) start with the assumption that the population represented by the data set follows a pre-established behavior or (2) infer a type of population from the data set. In both cases, problems can arise when the data set is small and poorly represents the population or when the data (and the population) are in a nonsteady state or reflect an evolutionary phenomenon. For this last case, when time constants are short and the model evolves within the time record of the collected data, a stochastic approach is more correct mathematically, but it is generally much more difficult to use.
When the phenomenon is known only poorly, the collected data may contain some degree of randomness or even reflect beliefs and bias on the part of the collector. To mitigate this problem, analysts may rely on probability, which relates statistical concepts to stochastic variables by mean of large data sets. An example of the use of one statistical and probabilistic method is the use of extreme value distributions applied to cumulative distribution functions to analyze pitting corrosion as performed by Macdonald and colleagues and shown in Figure 3.5.28
Thus, while statistical analysis of the data is highly recommended, before applying any data mining techniques, it must be understood that the misuse of statistics and/or stochastic models can lead to incorrect predictions. Still, collected data represent a multitude of opportunities for mathematicians and data miners and for researchers dedicated to theoretical electrochemistry. The knowledge obtained from such data can be used to build new and realistic models and thereby expand knowledge of the problem. However, data produced have not always been sufficiently consolidated to reach their full potential. Models are frequently isolated constructs (local models that account for only a limited number of observations), whereas global models that account for all observations are needed and should be sought. Today there are ample opportunities for an overall effort on data consolidation and model evaluation that would greatly benefit corrosion science and engineering.
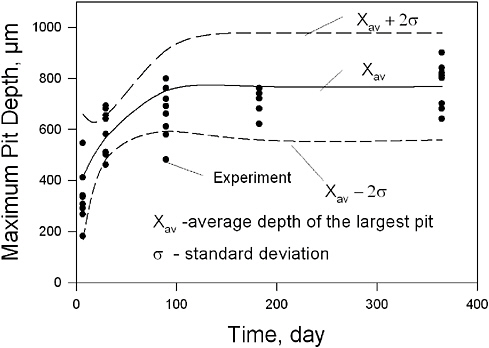
FIGURE 3.5 The mean depth of the deepest pit as a function of time. Experimental data are from Aziz et al. and are for aluminum alloy Alcan 2S-O in Kingston, Ontario, tap water. Note that no calibration was performed on short-term data.
Model Integration
Model integration and multiscale modeling are critical if modeling is to be useful in predicting the behavior of materials in specific environments. Opportunities exist to integrate models across length and time scales, and to describe each step in the corrosion evolutionary path, beginning with initiation of corrosion damage, in order to produce macroscopic predictions from nano- and microscale observations.29 One example of model integration is mixed deterministic-statistical modeling where fundamental laws of corrosion are expressed in the model where they are known. (See deterministic modeling, above, and hybrid modeling.) A statistical spread or distribution may then be applied to a few parameters when that behavior is observed. The statistical spread is best applied when (1) it follows
observations of variability in the lab or field and (2) the modeler can justify the distribution technically but does not know enough details of what controls the statistical distribution to incorporate them deterministically in the model. Such models are attractive because they capture well-understood scientific aspects of realistic behavior but account for observed variations using statistical or probabilistic approaches, the explanations for which may be lacking. Multi-scale modeling has become a reality in other aspects of materials science. In this type of modeling, atomistic information may inform and feed selected properties or phenomena at a higher length and time scales. The notion of multiscale modeling is that not everything is modeled at the atomistic scale, but rather only selected phenomena of great importance whose input at the atomistic scale may influence properties at a coarser length scale and ultimately parameters at the engineering scale. There is no reason why this approach cannot be extended to the corrosion field to benefit both the understanding of material behavior and prognosis. Additionally, because materials degrade through processes other than corrosion, such as fatigue and creep, a complete set of modeling tools would incorporate corrosion modeling with these other degradation models.
Final Thoughts on CGC II
Considerable effort over a substantial period of time will be required to produce fully deterministic models, complete with appropriate boundary conditions. Until then, empirical models will need to be integrated with deterministic models. Mixed models may also provide some utility and become more attractive as the technical basis for statistical distributions becomes understood. Much of the research needed to develop corrosion models requires contributions from many different disciplines, including contributions from theoretical materials scientists and chemists working together on corrosion problems using data and knowledge generated by experimentalists, along with computer scientists and mathematicians, under the guiding hand of real-world corrosion engineering. Indeed, opportunities to apply models to corrosion should not be limited to a particular perspective, and models borrowed from other fields (medical, for example, or other areas, such as math and risk-reliability assessment) may prove very useful. One example is the use of level-set mathematical methods, which are adept at front tracking of corrosion, etching, deposition, or lithography or profile evolution, which can develop sharp changes in profiles and order-of-magnitude changes in speed. These types of mathematical models could be applied to corrosion.30
CGC III:
Accelerated Corrosion Testing Under Controlled Laboratory Conditions That Quantitatively Correlates to Observed Long-Term Behavior in Service Environments
Corrosion grand challenge III attacks the thorny issue of extrapolating—with high fidelity—expected field performance based on laboratory-scale testing. The crux of the challenge is that there is a large mismatch between the time available for laboratory testing, typically on the order of months, and the time that a structural or functional material will be in service, typically many years. Accelerated testing is used for a variety of purposes, including demonstration, validation, and lifetime prediction of new materials, coatings, and environments; early detection of failure mechanisms not foreseen during design; lot acceptance; as well as a tool to assess the return on investment in mitigation and robust design. Unfortunately, accelerated testing in corrosion has many shortcomings, most of which can be traced to either a lack of detailed specific environmental definition or a lack of fundamental understanding of the processes occurring during accelerated testing and field exposure, and how the two are related.
Three high-priority research needs for advancing accelerated testing are described in the subsections that follow. The list is not meant to be inclusive, but rather to indicate the type and scope of effort that must be made to achieve the goals of CGC III. Although many of the examples relate to metals, accelerated testing is also needed that yields meaningful prediction of long-term corrosion behavior for all material types (see Figure 3.6 for one example).
Corrosion Intensity Factor
A critical research gap in corrosion science is the absence of the corrosion equivalent for the stress intensity factor (K) that has been the mainstay of structural mechanics for the past several decades.31 The stress intensity factor was developed to predict the behavior of pre-existing flaws in structural materials and the eventual life of a component under conditions in which the flaw develops into stable cracks. The power of K is in the concept of similitude: well-defined cracks and crack tips that are different in size or shape but possess the same K (as determined by geometry, loading, and the theories of linear-elastic fracture mechanics) will experience the same mechanical driving force for crack growth. Thus, similitude allows small, well-defined samples to be tested in the laboratory to determine the conditions of crack growth and fracture and the results to be quantitatively extended to more complicated real-world structures containing cracks. Virtually
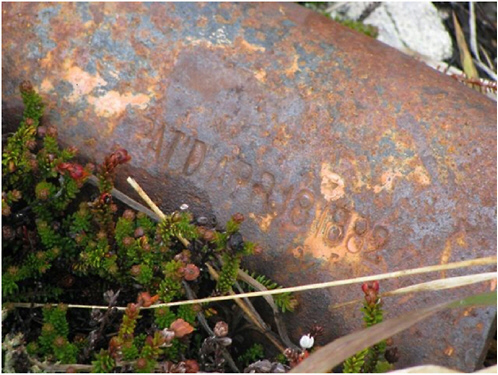
FIGURE 3.6 A ferrous based alloy exposed to the environment since 1882 (note the date on the part). With a cold and dry environment the corrosion process is very slow. SOURCE: University of Virginia Center for Electrochemical Science and Engineering, courtesy of John R. Scully and UVA MSE 3080 class participant.
all structural integrity approaches rely on similitude for their predictions of a component’s mechanical performance.
Although there continues to be extensive research on K and on the limitations in the concept of similitude, the creation of the field of fracture mechanics in the 1960s was a breakthrough for the understanding and prediction of crack growth and eventual fracture. Many industries now regularly use fracture mechanics in the design and maintenance of their engineering structures. Moreover, damage-tolerant design can specify the size and shape of defects, plus load levels that can or cannot be tolerated according to a specification of critical stress intensity factors for crack growth or sudden fracture. In many ways the safety of modern air travel and of power-generating equipment such as nuclear reactors is possible only through the application of fracture mechanics in the design and maintenance of aircraft structural components and reactors. On the small end of the scale, the same is true
for the conductors used in the leads that deliver electrical energy to the heart from implantable defibrillators and defibrillators in general.
The field of corrosion science could make very good use of an analogous corrosion intensity factor32 if one could be developed to help define equivalent corrosive situations in a more quantitative manner. This would allow much needed predictions from accelerated tests toward field exposure of components as well as facilitate comparisons between one condition and another. One example is the use of an exposure factor in sulfidation, whereby the product of H2S concentration and exposure time yields the same “corrosion intensity” or “exposure” factor. This means that the same thickness of copper sulfide film could be produced on copper exposed to H2S at parts-per-million concentrations in a thousandth of the time needed when exposure to the same gas occurs at parts-per-billion. However, rarely is it the case in corrosion that the concentration of the corroding agent can be traded for time so simply.33 One reason is that all the driving forces that control the rate of corrosion are not completely understood; another is that there is currently no way to equate them or even describe them in common terms. If a corrosion intensity factor could be realized, then the driving force for corrosion in a laboratory test, a field test, and a component in service could be equated or the differences quantified. Therefore, it is possible to foresee a time when an environmental or corrosion intensity factor, Cenvir, akin to K, based on similitude would quantify the acceleration provided by test conditions and allow predicting the performance of equipment based on equivalent exposures or CIFs between field and accelerated environments. With such a tool, corrosion conditions could be manipulated to control and dial in the driving force and hence the rate of various corrosion processes as well as forces controlling transitions in corrosion mode or triggering changes in corrosion mode. A science-based parameter would allow quantitative connections between corrosion rates observed in laboratory exposures and those found in field environments and might also allow databases on materials in one environment to be translated into equivalent corrosion behavior in another environment, such as one with lower concentrations of aggressive species. The
scientific and technological impact of a viable environmental corrosion intensity factor would be of great significance.
However, as stated above, there are many scientific barriers to the development of an environmental corrosion intensity factor, including the complexity of many field environments, lack of accurate knowledge of many exposure environments, and the highly nonlinear nature of corrosion rates with certain environmental species attacking metals (e.g., ammonia, sulfur, and chloride). As such, even when precise environmental definitions are known, the highly negative and positive synergies created between various combinations of factors and the lack of quantitative information in many environments make this an extremely challenging goal. It should also be pointed out that a corrosion intensity factor is not a replacement for deterministic or some other form of modeling to predict behavior and performance. In fact, deterministic models of sulfidation exist, but there is still the acute need for relevant accelerated testing.
Quantitative Connections Between Laboratory Exposures and Field Environments
Another issue affecting the ability to perform accelerated testing is replication of the actual environment in crevices or local occluded sites in metals formed by complex geometries or deposits (see Figure 3.7) when that environment differs from the nominal bulk environment, whether it is submersed in liquid or exposed to the atmosphere. A classic example is that of steam generators in nuclear reactors. These structures were originally fabricated from nominally corrosion-resistant nickel-based alloys; however, crevices formed between the tubes and tube sheets used in these heat exchangers led to environments in which a variety of species were concentrated owing to the local heating and boil off of water.
Through the years, as corrosion problems have persisted, increasingly corrosion-resistant alloys and new environmental chemistries have been used. Accelerated testing clearly requires knowledge of all environments to which a structure will be exposed.
There are numerous electrochemical approaches to quantitatively assessing the corrosion resistance of bare and painted metallic materials immersed in conductive electrolytes, and they can be very sensitive and relatively easy and rapid to perform (Figure 3.8), either in the laboratory or in the field for corrosion rate monitoring. However, in some environments such as those with high-temperature gases or high-resistivity electrolytes that do not follow classic electrolyte behavior, standard electrochemical approaches either are extremely difficult or fail completely. An important research opportunity is to develop sensitive, quantitative, and accurate methods for evaluating corrosion resistance in these environments.
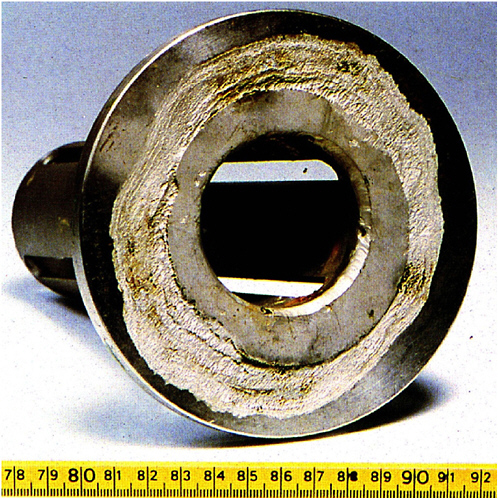
FIGURE 3.7 Crevice corrosion of a corrosion-resistant alloy flange. SOURCE: Corrosion Atlas (E.D.D. During, ed.) 1997, copyright 1997, Elsevier.
Understanding the Corrosion Processes That Occur During Accelerated Testing
Current accelerated testing methods are empirically correlated with field performance, and acceleration factors are often deduced from relative differences in the evolution of damage over time. However, there is often little understanding of why a given method succeeds or whether it will be effective on another coating, material, or in another environment, because the rules that determine its applicability to other situations are not understood. The uncertainty surrounding
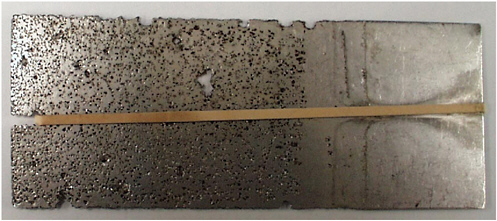
FIGURE 3.8 Acid-induced pitting and crevice corrosion of a stainless steel alloy exposed to ferric chloride (note the corrosion under the rubber band at left—left side dipped). SOURCE: University of Virginia Center for Electrochemical Science and Engineering, courtesy of John R. Scully.
accelerated testing could be reduced if research were done to better understand how to accelerate the mechanisms and modes of corrosion that prevail in the field, and how to avoid introducing spurious mechanisms that do not occur under the conditions of use. If these were better understood, it would then be possible to compare various environments as well as materials, and even coatings. Eventually this would lead to improved rules for conducting and interpreting accelerated tests. In the nuclear industry, a common concept is the material improvement factor. For example, much information has been gained about alloy-600 failures over the years, but only a few have occurred in alloy-800 and none in alloy-690. Scientists are currently working on accelerated laboratory tests that do crack these latter materials and are developing statistical methods to predict when and how they will eventually fail in service.
CGC IV:
Accurate Forecasting of Remaining Service Time Until Major Repair, Replacement, or Overhaul Becomes Necessary—i.e., Corrosion Prognosis
Corrosion grand challenge IV addresses the critical need for better methods to monitor the actual deterioration of a component once it is placed in service in a corrosive environment, analyze that information, and, based on decision-making algorithms (or “reasoners”), provide a reasonable forecast of the time remaining before maintenance or replacement becomes necessary.
As noted elsewhere in this report, corrosion is ubiquitous, quite complex, and driven by a multitude of variables. Many properties of materials are directly,
and most often negatively, affected by corrosion. As a consequence, much effort is expended in mitigating corrosion by providing effective protection and ensuring proper inspection, assessment, and maintenance. Monitoring is needed to verify modeling and accelerated test predictions and to provide information on corrosion occurring in high-value or critical equipment.
There are two high-priority research needs required to advance the real-time, accurate determination of the extent of in-service corrosion degradation. The technology required to address CGC IV draws heavily on the modeling work described under CGC II, which involves basic understanding of the corrosion types and of damage accumulation, as well as corrosion modeling algorithms. Two key research opportunities are highlighted below.
Improved and Automatic Sensing Devices for Quality Inspection
An essential aspect of corrosion mitigation involves inspection of engineered structures, in order to avoid costly unscheduled repairs and to ensure safe and reliable materials performance. Extensive inspection and maintenance/repair programs have been established in a multitude of sectors concerned with corrosion and its costly impact—for example, in oil transmission pipelines, bridges, naval vessels, refineries, and chemical plants. These programs typical rely on traditional inspection methods, among them visual methods using magnifying glasses, borescopes, liquid penetrants, and magnetic particle testing), and electromagnetic, ultrasonic, and radiographic techniques.
Traditional inspection methods have known limitations (such as lack of accessibility to difficult- to-reach areas), can involve personnel safety concerns, and are not always reliable (such as operational inaccuracies due to scale formation, noise, or vibration); all of these add a measure of uncertainty to the effectiveness of the inspection and raise concern about the possibility of undetected corrosion problems in areas that cannot be inspected. For a comparison of monitoring techniques, see Table 3.1.
Effective corrosion mitigation and control require appropriate action at the correct time. Since many engineered structures include multiple components and corrosion processes are cumulative, periodic inspection is often not the optimal procedure for identifying areas requiring timely action to mitigate corrosion. Thus there is an urgent need to change from time-based inspection to a more effective condition-based inspection. This change can be achieved through continuous monitoring with advanced sensor technologies that generate meaningful data, provide accurate assessment of materials condition, detect corrosion, analyze its impact, and determine the risk of further degradation. The importance of condition-based, continuous monitoring programs is being recognized in various sectors affected by corrosion, such as shipboard tanks.
Interest in sensors as reliable evaluation tools is increasing, based on expressed needs for online, nondestructive, accurate, and cost-effective evaluation of the condition of materials and the integrity of equipment. Figure 3.9 illustrates how sensors might be utilized at various stages in the lifetime of a component or device.34
Currently, continuous monitoring methods are aimed at generating on-demand data for determining the condition of materials and for detecting the presence of corrosion. These monitoring methods for metallic systems rely on sensors that fall into several categories:
-
Sensing electrochemical measurements of instantaneous rates of corrosion of metals;
-
Sensing of environmental reactivity, including upset conditions, which does not measure corrosion rates but instead indicates the propensity or risk for corrosion based on environmental severity; and
-
Sensing a material’s accumulated damage at some location, often using surrogate material for damage assessment but preferably based on the accumulated damage of the actual component.
Overall, the information provided by these sensor technologies tends to include:
-
Mechanical data, such as strain field;
-
Chemical data, such as pH, oxygen, and dissolved ions; and
-
Electrochemical data for metallic systems, such as current, potential, and impedance.
Experience with monitoring methods based on generating data from samples or coupons (such as damage sensors) indicates the need to ensure relevant correlation to the condition of the system’s component itself. Electrochemical methods of monitoring suffer drawbacks and limitations related to deposition of corrosion products and changes in a corrosion medium’s conductivity that may affect the sensor output. Furthermore, most corrosion sensor technologies are not capable of accurately identifying localized corrosion or clearly evaluating cumulative corrosion processes.
Additional corrosion sensor issues that must be considered include probe life, sensitivity range, response time, flexibility, and detection of prior damage. Table 3.1 lists key characterizations for several corrosion sensors for monitoring
TABLE 3.1 Comparison of Techniques for Monitoring Bridge Health
|
EIS |
EN |
Galvanic |
Multi-elctrode Array |
Oxidation Power |
|
Instant |
Instant |
Instant |
Instant |
Instant |
|
Yes |
No |
Yes |
No |
|
|
No |
No |
Yes |
No |
|
|
Yes |
Yes |
For corrosivity |
Yes |
For corrosivity |
|
Yes |
Yes |
Yes |
Yes |
Yes |
|
Maybe if use structure used as one electrode |
Maybe if use structure used as one electrode |
Maybe if use structure used as one electrode |
No |
Yes (for estimation, if coupled to structure) |
|
High |
High |
High |
High |
|
|
No |
No |
No |
No |
No |
|
Long (structure as one electrode) |
Can be long if imbedded |
Short for thin film, long for imbedded |
Long for imbedded type |
Unlimited for single noble electrode type |
|
Maybe |
Yes |
Yes |
Yes |
Yes |
|
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Maybe |
Maybe |
Yes |
Yes |
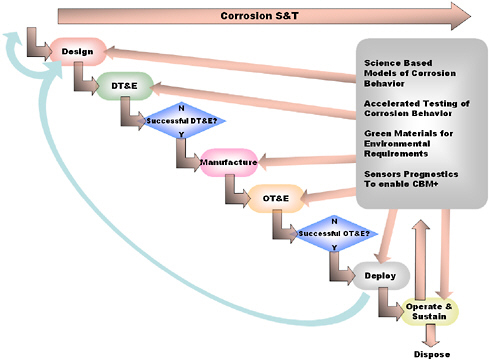
FIGURE 3.9 The cradle-to-grave development of an engineering system requiring corrosion protection and mitigation of corrosion and the impact of corrosion modeling, accelerated testing, and sensors. CBM+ is an enhanced form of condition-based maintenance that uses advanced hardware and software tools to support decision making. Adapted from Defense Science Board, Corrosion Control, Final Report ADA428767, October 2004, available at http://www.acq.osd.mil/dsb/reports2000s.htm, and based on information in Ammtiac Quarterly, Vol. 4, No. 2, 2009.
bridge health. The reader will note that each existing sensor has shortcomings and cannot be used at all with certain electrolytes or in challenging situations such as hidden corrosion and corrosion under coatings. Another strategy is the vast use of surrogate coupons or materials instead of interrogation of the actual structure or component of interest. The actual component is always preferred for analysis, and the surrogate coupon always represents a proxy that may or may not replicate the actual situation. There is an urgent need to improve these capabilities.
For corrosion in environments with high-temperature gases, the ability to detect corrosion in real time suffers from the lack of a good (sufficiently conductive) electrolyte for electrochemical measurements. However, electrochemical methods, including noise and impedance measurements, have been and are being
pursued for these environments, albeit with poor or mixed results.35 More extensive and innovative studies in this area would prove extremely valuable for real-time quality-control sensing applications as well as for condition monitoring as part of prognosis. Such measurements would have to be compared with those obtained by the traditional, cumbersome approach of probing for corrosion by inserting actual materials of construction that are removed at intervals for examination, and with measurements of corrosion rates by gravimetric or (more accurately) metallographic/ceramographic methods. Although electrochemical methods can be used reliably for measurements of corrosion rates in molten salts (hot corrosion),36 techniques of greater sophistication, such as are used in aqueous environments, would be worth developing.
Researchers continue to make progress in corrosion monitoring and inspection. Recent developments include the use of fiber optic sensors to monitor pipeline corrosion and stress corrosion cracking, the development of both surface and penetrating sensors aimed at detecting cracks and corrosion in bridges, laser scanner systems that are being tested for evaluating urban structures’ integrity and fluorescing nanomaterial coatings to reveal when and where corrosion is occurring in the substrate. Needless to say, there are many more advances that will be required in order to develop reliable sensor technologies.
Numerous challenges still face personnel engaged in inspection and monitoring, as well as those entrusted with accurate assessment of corrosion and prediction of materials performance. Opportunities include:
-
Correlating data from surrogate sensors with actual performance of structural components;
-
Gaining increasingly accurate of measurements, automation, and usefulness in inaccessible areas;
-
Developing sensors that generate data to identify chronological and cumulative corrosion processes;
-
Developing smart materials with embedded capability to indicate the onset and rates of degradation;
-
Developing sensors that can sense remaining barrier properties, inhibitor or sacrificial anode capacity; and
-
Developing sensors that interrogate, use, or consist of the actual structure or component.
Ability to Track and Monitor Corrosion Protection Systems
Currently there is no commercially available electrochemical sensor or sensor suite that has been proven to yield the corrosion rate of a metal under a protective coating. The instantaneous detection and measurement of corrosion under paint in atmospheric exposure is a challenge that has eluded a solution. Although in principle damage sensors should work under paint, they typically employ a surrogate material under a new coating. Thus, past performance and degradation of the coating up to the point of installation is challenging to match, and the rate of corrosion of the sensor surrogate material must be correlated with the corrosion rate of the structure. Work to determine atmospheric corrosion rates of metallic materials under coatings in the atmosphere is thus a high priority.
There is also the need for distributed, non-hard-wired sensor systems that assess damage modes, corrosivity, instantaneous corrosion rates, and other information. The ideal distributed sensor system of choice for many applications should provide a suite of remotely situated field sensors that can simultaneously measure instantaneous corrosion rate, corrodent concentration, time of wetness, and possibly, accumulated damage. A sensor that has the ability to characterize pre-existing coating damage is also attractive because a freshly coated sensor on a surrogate material is not representative of this situation.
Condition-monitoring technology has evolved to the point that sensors and data mining modeling research areas might be mature enough to produce a monitoring system that will be reliable, robust, and cost-effective. However, those systems are not yet widely available because of several remaining hurdles: u Existing wireless sensor systems are not robust, or affordable, or workable for many applications.
-
Existing data-mining software is not tailored to work with wireless sensors, nor can it manage the large amounts of data collected continuously by the sensors.
-
Decision-making algorithms are lacking or are in their infancy and not proven in widespread systems.
-
When success is achieved with sensing, the conditions under which the success can be extended to other systems without extensive reproving are unknown.
Because advances in modeling and prediction of damage will need verification, there is an urgent need to bring monitoring to the state where it can operate in a range of environments to sense corrosion, not just in the easiest environments. Moreover, sensors in the chemical process stream can be used for real-time moni-
toring and feedback for process control. In some cases, monitoring will be the only strategy available other than careful initial selection of materials. Finally, there is a need for further advancement of distributed sensors and sensors that can detect different corrosion modes.
The Base—Corrosion Science
Although the fundamental principles of corrosion may be fairly simple, the details of how corrosion actually occurs are complex and often are not known. The goal of fundamental research in the field of corrosion is to better understand the detailed mechanisms by which corrosion occurs. Multiple length and time scales must be considered, because information at each scale helps feed into and produce an assessment of the risk of corrosion, such as the risk of pitting as shown in the example in Figure 3.10.
These research opportunities will lead not only to better materials but also to better models of corrosion processes for a variety of materials. Another key opportunity lies is developing knowledge of the real environmental variables to which a component is subjected, as well as the actual localized corrosive environment in occluded regions such as pits, cracks, or crevices or under films or deposits of various types. Until researchers investigate these opportunities and materials scientists apply the results to design better materials with the needed corrosion resistance, it will never be possible to control or eliminate corrosion simply by selecting the right material. Theories from other fields of science and exciting new investigative tools can also have a great impact on the corrosion field, as the advances described in Box 3.1 and some of the success stories in Chapter 1 indicate.
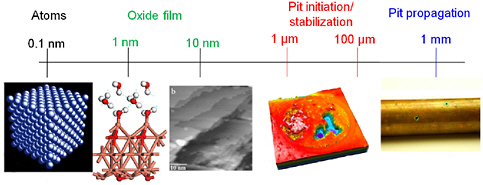
FIGURE 3.10 The length scales over which pitting corrosion is active. SOURCE: University of Virginia Center for Electrochemical Science and Engineering, courtesy of Hongbo Cong.
|
BOX 3.1 Advances in Science Theories and Tools in Related Fields and Subsequent Impact on the Corrosion Field Advances in corrosion research have been facilitated over the years by theoretical developments in other fields as well as by new experimental methods. Of the numerous examples of such advances, only a few are mentioned here. For instance, when Tafel1 observed in 1905 that electrochemical current was proportional to the exponential of electrochemical potential (where the electrochemical reaction rate is described by the current density), absolute-reaction rate theory (transition state theory) had not yet been developed. However, once developed in 1935 by Eyring and Polanyi in chemistry,2,3 it was rapidly adapted to the corrosion field by Wagner and Traud4 and used to explain Tafel’s observation. Measurement of corrosion reaction kinetics based on these original electrochemical theories is now a mature aspect of corrosion science and engineering with a strong scientific underpinning. Field measurements of corrosion rate based on the polarization method and associated current sensing are made routinely with some accuracy using motes and telemetry; such measurements also rely on these original theories. Similarly, advances in chemical thermodynamics based on pioneering work of J.W. Gibbs5 and others in the late 1800s rapidly spread to solid state engineered materials systems through the efforts of Darken6 and Smith and were nearly simultaneously adapted to the subspecialties of corrosion and high-temperature oxidation. Tafel, Pourbaix, and Ellingham diagrams are examples of the routine use of a thermodynamic approach in studies of corrosion and oxidation. The state of the art is so advanced that commercial software toolkits are available to users7 and enable thermodynamic modeling in technological applications involving corrosion and oxidation. Neither of these advances would have been possible without fundamental research in corrosion that capitalized on related scientific discoveries in thermodynamics and kinetics. Major advances in materials characterization and in materials modeling and simulation in recent years also have provided new tools with impressive capabilities for addressing the most perplexing problems in corrosion science. For instance, there was once an ongoing debate over the very basic nature of the passivity (films versus adsorbed layer), and various theories were advanced. Development of techniques in surface science such as optical and later photoelectric and synchrotron radiation methods to characterize valence state, thicknesses, and composition produced a leap forward in the understanding of passive films.
|
One of the tools that is rapidly adding new knowledge is the synchrotron, as discussed in the first subsection below. A number of additional areas, beyond synchrotron work, present opportunities for pushing the frontier in corrosion science in three primary areas that are also discussed below: corrosion mechanisms, corrosion morphologies, and corrosion environments. Advances in understanding in these areas are needed to achieve the goals of all the corrosion grand challenges.
Synchrotron Radiation
An irony of the study of corrosion is how remarkably difficult it is to directly measure the rate of corrosion since it is often highly heterogeneous and takes place in cavities such as pits shielded by metal in wet environments. Electrochemical measurements cannot give spatial information. The method traditionally used is serial sectioning, but this destroys the pit, so that it is not possible to obtain time-dependent information. By far the most straightforward approach is to use highly intense X-rays (particularly those from synchrotron sources) to make microtomographic measurements that provide three-dimensional images in situ in wet environments and in real time. This approach has already been successfully pioneered to examine pits and pit-to-crack transitions in stainless steel; intergranular corrosion and pitting in Al alloys; and localized attack of Mg. The corrosion rate can be monitored by counting voxels (three-dimensional pixels) of metal that have disappeared between one frame and the next, which is the most direct method possible for quantifying corrosion.
The technology is advancing rapidly, with the time required to obtain a single tomogram (three-dimensional image) decreasing from hours to seconds in the last few years. The spatial resolution is routinely just submicron using synchrotron radiation, with developments moving in the direction of resolutions of tens of nanometers at various beamlines. Absorption contrast gives images based on the difference in electron density. For elements with characteristic absorption edges at energies appropriate for imaging, it is possible to make measurements above and below the edges to give three-dimensional elemental maps, and this could potentially be extended to discriminate between different oxidation states of the same element. Phase contrast imaging (which is easily carried out by increasing the distance between the sample and the detector) can pick up interfaces between phases of similar atomic number and is therefore effective for imaging corrosion products and water droplets. It is also possible to see the location of hydrogen bubbles. Corrosion on metal pins under microelectrochemical cells has already been achieved, so that electrochemical data can be correlated with the evolution of corrosion.
Some examples of areas where microtomography might make a valuable contribution to the understanding of corrosion include the following:
-
Search for pit initiation sites. It is straightforward to take an image of a metal microstructure prior to pit initiation, and a further series of images as localized corrosion develops. This allows working backward in time to locate the initiation site prior to pitting.
-
Corrosion under paint films and atmospheric corrosion. The loss of metal due to corrosion can be observed with absorption contrast, and any changes in the paint film (e.g., cracks, swelling with water) could be observed with phase contrast imaging. If suitable ions are introduced, their distribution could be tracked with energy difference imaging, described above. For atmospheric corrosion, salt droplets and crystallization of salts and corrosion products could be observed and correlated with pit development.
-
Corrosion of heavily corroded delicate objects such as archaeological artifacts or failure analysis without the need to remove corrosion products.
-
Long-term corrosion rates—for example, metals in concrete or in clays used in nuclear waste storage. Measurements could be carried out in sealed glass vessels to control the environment, and these samples measured at monthly or even yearly intervals. Current methods for measuring the evolution of corrosion for nuclear waste samples of this type include the indirect measurement of hydrogen evolution, which is not straightforward.
-
Crystallographic effects in stress corrosion cracking. It is possible to use microdiffraction to obtain the crystallographic orientation of individual grains in a tomography sample.37 This could be correlated with discontinuous advances in stress corrosion cracks.
One of the current limitations of tomography is that, for reasons connected with image processing, the sample size is limited to 2000 times the resolution required. However, “region-of-interest” imaging is under development, so that an entire object can be imaged at low resolution and the resulting tomogram used to reconstruct a region of interest that is subsequently imaged at high resolution.
While three-dimensional imaging is likely to have the most dramatic impact, there are opportunities to carry out fast radiography to image two-dimensional processes in thin foils at submillisecond rates. Such imaging can also be correlated with chemical information, including X-ray fluorescence maps, X-ray absorption spectroscopy, microdiffraction, and small-angle scattering. The spatial distribution of different chemical species is important for the development of robust corrosion
propagation models, and the ability to carry this out in a time-dependent fashion under electrochemical control gives a route for direct measurement of diffusion and electromigration coefficients in realistic environments. This can be done on localized corrosion sites themselves and also to evaluate the distribution of corrosion products surrounding corrosion objects. For example, two groups are currently working on the distribution of metal ions and corrosion products in human tissue around failed implants.
There is vigorous development in the technology of beamlines in synchrotrons around the world (one example is the NSLS, shown in Figure 3.11), and so possibilities are continually opening up to characterize corrosion processes more rapidly, at higher resolution, and in more hostile environments. X-rays can easily pass through diamond windows, and many synchrotron measurements are currently made at elevated temperatures and pressures, making the prospect of future opportunities extremely exciting.
Synchrotrons are only one example of how advanced tools can be used effectively in corrosion science. Other tools with great potential include, but are not limited to, neutron scattering, advanced scanning transmission electron microscopy (particularly in situ efforts), scanning probe techniques, and low-voltage electron microscopy. Advances in first-principles theory, modeling approaches, and simulation provide new opportunities to generate needed understanding over important time and length scales consistent with those being accessed experimentally. Not only do individual tools have great promise, but the fusion of tool output data or tool output integrated both spatially and temporally also promises significant impact. Tool fusion involves taking and considering the output of several tools that interrogate a material or its corrosion processes simultaneously using several different approaches. In this manner it would be desirable to map, for instance, electrochemical, electronic oxide properties, physical damage, and crystallographic texture simultaneously. This kind of unprecedented information gathering would help identify what about a given grain boundary makes it prone to corrosion initiation and propagation. This has already been done with respect to microdeformation and IGSCC, but additional information would provide a rather complete picture of the corrosion process.
Corrosion Mechanisms—Fundamental Advances in Micro- and Nanoelectrochemical Theory and Methods
In the past, electrochemical measurements of corrosion as well as processes in batteries or other electrochemical processes were averaged over many atoms and molecules. Atomic details of electron- and ion-transfer reactions associated with dissolution and passivation are now ripe for further progress given nanoscale experimental and computational advances. Recent advances such as scanning electro-
FIGURE 3.11 Aerial view of the National Synchrotron Light Source (top) and the vacuum ultraviolet, 200-10 nm, floor (bottom). SOURCE: Courtesy of NSLS, Brookhaven National Laboratory, available at http://www.nsls.bnl.gov/about/imagelibrary/.
chemical microscopy; electrochemical capillary probe microscopy; and electrochemistry mapping combined with other techniques such as micro-Raman and atomic force microscopy, scanning Kelvin probe, or scanning tunneling microscopy will allow understanding beyond that enabled by global electrochemical methods traditionally used. Societies such as the Electrochemical Society and the Interna-
tional Society of Electrochemistry regularly conduct symposia on these topics. Even spatial radiotracer methods (chemical interrogation) combined with electrochemistry may be possible.38 Micro- or nanoscale electrochemistry in combination with other methods will advance the field toward new discoveries in both corrosion and electrochemical research. Two key factors are increased spatial resolution and concurrent combinations of mapping.
Corrosion Mechanisms—The Chemistry at a Variety of Occluded Sites
As mentioned above, progress in understanding localized corrosion under aqueous conditions could be gained through further understanding of the chemistry at occluded sites (e.g., pits, crevices, cracks, and delaminated sites under blisters in coatings) in three dimensions. Although there has been substantial progress in rigorously defining the chemical compositions and electrochemical conditions in pits and crevices, there are a number of opportunities for further research in areas such as ionic and transport properties of films, precipitates, and corrosion product as well as their colloidal properties. There is a need to understand conductivity and transport in concentrated solutions at low water contents as well as other aspects of physical chemistry such as activity coefficients and pH, how to make measurements in concentrated solutions, and how measurements change with concentration and other such variables.
The properties needed to either verify, or serve as inputs to, existing models remain elusive, and because of their dimensions and steep chemical concentration gradients, experimental probes of crack tips are challenging. Hence, reliable predictions, even for well-behaved materials, can be difficult to produce without the use of hypothesized, estimated, or reverse-engineered parameters.
Moreover, lack of understanding of processing and heat-to-heat variations or the effects of trace alloying elements further limits the predictability of the onset or growth of stress corrosion cracks. Similar considerations can apply to environmental effects on high-temperature crack growth in gaseous environments.
Corrosion Mechanisms—Forms of Localized Corrosion Controlled by Defects
There is a lack of atomistic-scale understanding of the role of various specific chemical species in the initiation of corrosion processes. For example, the role of ions on the precise atomic scale in processes such as pitting or cracking represents one opportunity for research. Moreover, the hierarchy of important defects across the length scales has been established only in a rudimentary way, and understand-
ing of the effects of arrays or distributions of defects on corrosion is in its infancy (Figure 3.12). Consequently, defect engineering39 or damage-tolerant approaches as championed in fracture mechanics is almost totally lacking in the study of corrosion but could easily be developed. In contrast, much progress has been made in understanding the role(s) of micrometer-scale defects, propagation and stabilization. The field is well positioned for much additional progress in this area given the development of a number of imaging tools now available.
There is also a need to understand water absorption, passivity, dissolution, and cathodic reaction rates at the nanolength scale such as on nanoscale materials, where the Gibbs-Thompson effect may be large, quantum size effects may occur, and a greater percentage of atoms are on the surface and outnumber the bulk atoms, and also where the number of nearest neighbors is low and there are profound effects of strain and defects. Nanoparticles produce conditions where concentration gradients, the double layer, and ohmic voltage fields all exist on the same length scale. An additional opportunity exists to improve understanding of the reactivity of patterned surfaces, including superlattices and piezoelectric oxide supported metal.40 Hence, nanoscale material applications may require new fundamental understandings of surface and electrochemical phenomena at the nanometer and subnanometer scales. This affects a range of applications, from catalysts to nanoelectronics. Tools such as scanning tunneling microscopy and atomic force microscopy as well as first-principles modeling methods make progress in this area possible.
Corrosion Mechanisms—Stress and Strain Effects
There is a lack of fundamental understanding of the effect of elastic tensile stress or strain and plastic strain on dissolution by either thermodynamic or kinetic interpretations. The roles of stress and/or strain in aqueous corrosion reactions occurring at the atomistic level close to room temperature have been modeled for micrometer-scale descriptions of stress corrosion cracking (SCC) and hydrogen
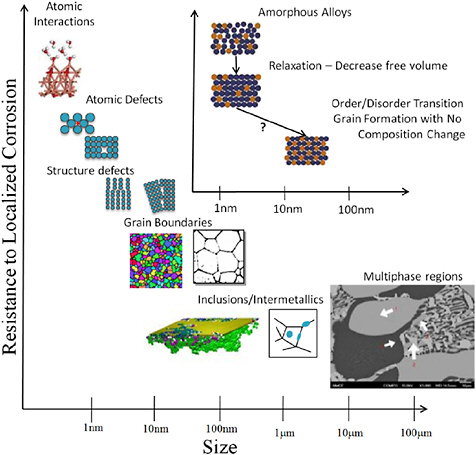
FIGURE 3.12 Hypothetical variation in resistance to localized corrosion with the type of defect and its approximate size (size on the x-axis denotes the defect size). SOURCE: University of Virginia Center for Electrochemical Science and Engineering, courtesy of Derek Horton.
embrittlement for both short and long cracks. However, this has been accomplished only for a limited number of cases, and an atomistic- or molecular-level understanding of these phenomena is still lacking.
In particular, there is no quantified understanding of how stress affects some well-known anodic dissolution SCC mechanisms, such as for pre-existing, active-path corrosion. For example, the distinction between stress-assisted intergranular corrosion and intergranular stress corrosion cracking (IGSCC) with a mix of mechanical and electrochemical driving forces versus intergranular corrosion (IGC) by purely electrochemical driving forces, is blurred (Figure 3.13). The effects of tensile stresses may involve the aggravation of IGC by opening the fissures that
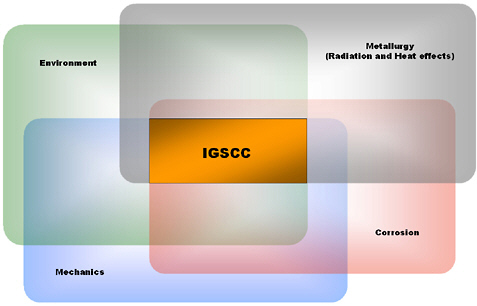
FIGURE 3.13 Areas that are related in intergranular stress corrosion cracking (IGSCC).
develop along grain boundaries facilitating diffusion and electrochemical current flow within the fissures.
The specific role of stress and strain in hydrogen embrittlement and cracking mechanisms has been established in the case of only a few models such as hydrogen-induced decohesion (HIDE) and hydrogen-induced local plasticity (HELP).
Stress also plays a role in localized corrosion owing to its connection with elastic-plastic incompatibilities between particles and matrices. For example, it has been proposed that stress or strain creates a crevice between a hard particle and a soft matrix where a special chemistry may subsequently develop. Stress effects are more complicated in many observed cases of SCC emanating from pits in complex alloys where the oxide film has already been ruptured. Because these driving forces are not well understood, the concept of accumulation of damage under stressed conditions cannot be used as a basis for quantitative life prediction.
Another area that is not well understood is the role played by dynamic plastic strain in the case of hydrogen embrittlement that occurs during hydrogen production taking place in either aqueous or gaseous hydrogen environments. Many engineering alloys are susceptible to this form of attack, yet the role of dynamic strain is unresolved. Many intermediate- and low-strength alloy systems exhibit relative immunity to this form of degradation under purely static loading but become sus-
ceptible to environmentally assisted cracking when subjected over time to slowly rising temperature (dK/dt) or to ripple, cyclic, or slow-strain-rate loading.
Although this phenomenon has been studied for years, cracking susceptibility, hydrogen interactions, and deformation substructures have rarely been systematically studied in pre-strained, actively strained, or under film-free or plastic straining conditions. The alloy systems of interest are those with oxide films that can be ruptured to produce clean surfaces prone to hydrogen entry and consequently prone to hydrogen effects such as cleavage, interface cracking, and/or hydriding, Other factors that contribute to a metal’s susceptibility deserve further attention, such as dynamic trap state creation within highly deformed crack or notch structures, enhanced surface uptake on atomically clean surfaces, and dislocation transport. Finally, tight cracks and atomically sharp crack tips require additional investigation. Progress is possible given techniques, theories, and modeling. For instance tomographic transmission electron microscopy methods offer the possibility of mapping dislocation structures at crack tips, and diffraction contrast tomography and three-dimensional atom probe methods can help to advance understanding of cracking phenomena inside materials.
Corrosion Mechanisms—Self-stress in the Breakdown of Passive Films and Protective Scales
Although externally applied stresses are certainly important, many corrosion processes generate in situ local stresses. The role that this self-stress or “electrostriction” plays in the breakdown of passive films has often been discussed but is still not understood. In particular, corrosion product formation of films, and passive films, may generate either tensile or compressive stresses—for example magnetite films formed on steel in hot water or steam are in strong compression, resulting in tensile stresses in the underlying steel. Because many of these effects are not well understood, the concept of damage accumulation under stressed conditions cannot be used as a basis for quantitative life prediction.
While some of the effects of, and issues with, stress under aqueous corrosion conditions are fairly well recognized, those associated with high temperatures have received less attention. However, these stress effects play a critical role in determining whether a scale is truly protective with respect to the substrate on which it has grown.
Stress development in oxide scales has been the subject of study, both fundamentally and in terms of its consequences (spallation and its effect in accelerating corrosion) for many years.41 By far the greatest contribution to stress arises from
the cooling down from the reaction temperature that occurs because of the differences in the respective coefficients of thermal expansion between the reaction product and the material on which it is grown. These stresses can be on the order of gigapascals for certain oxides on metals and as a consequence dominate the ultimate rate of degradation.42 While the source of thermal stress is predictable, its consequences, in terms of mechanisms of damage accumulation and cracking in the scale and along its interface with the substrate, are still being debated. These mechanisms are quite important because often the rate of spallation ultimately determines corrosion-limited lifetimes.43 Unlike thermal stresses, the mechanism(s) underlying the source(s) of growth stresses in scales (those that arise during isothermal exposure) are still a subject of great contention. Although debates about mechanisms of growth stresses have been ongoing for decades,44 interest in this and related issues has been reinvigorated by the relatively recent introduction of synchrotron sources to capture the earliest stages of their development via in situ experiments.45 While steady-state growth stresses are just a small fraction of thermally generated ones, they can be much higher initially and thus can influence subsequent development of the scale (defect structure and associated transport rates across it). Indeed, the effects of stress (both transient and steady-state) on reaction kinetics, and vice-versa, constitute an understudied area in high-temperature corrosion and offer a presumably fascinating route to better understanding of the atomistic and structural/chemical factors associated with reaction dynamics.
Corrosion Mechanisms—Cracks
An understanding of the chemistry at crack tips and crack surfaces and in the fracture process zone remains elusive. Although there has been substantial progress in rigorously defining the chemical compositions and electrochemical conditions of crack tip solutions, there are a number of opportunities for further development of the model, including a lack of understanding of the exact mechanical properties of passive films at crack tips, uncertainty over bare area created by straining, and the inability to properly inventory bare surface dissolution rates once the

FIGURE 3.14 Large magnification of environmental stress cracking from biological stress cracking of sample under mechanical stress (left) and metal ion oxidation on inner insulation of pacing lead (right). Note impression from outer coils on polymer. SOURCE: Courtesy of Medtronic.
passive film is ruptured. See Figure 3.14 for an example. Another research opportunity lies in the fact that specific effects of trace alloying elements on hydrogen embrittlement in a variety of alloying systems cannot be anticipated or predicted, and that emerging quantitative models of hydrogen embrittlement can only loosely predict material behavior, or must be calibrated by limited experiments to define damage parameters. As with stress corrosion cracking, the effects of heat-to-heat variations cannot be predicted. Consequently, there are no quantitative models or fundamental knowledge to guide the design of high-performance engineering alloys with good fracture toughness and slow crack growth rates for use in extreme environments. Finally, the effects of reactive agents on high-temperature cracking are not as well documented as those associated with aqueous environments and as such represent an additional opportunity for research. An example of this is the processes by which high-temperature corrosion results in preferential reactions along grain boundaries and other extended microstructural features that then serve as paths for cracking and failure. Of greater interest, but less frequently studied, is the direct role of oxygen and other elements in high-temperature crack growth, as exemplified by the seminal work of Woodford and others on nickel-based alloys.46 Issues that are in some ways similar to the competition between passivity and film breakdown at the crack tip have been discussed,47 but they have not been studied in any detail, particularly compared to what has been done under aqueous conditions. This is clearly an area for further study given the potentially catastrophic
nature of such cracking, the obvious role of stress, the important scientific issues at play, and recent advancements in both modeling and simulation and experimental techniques that can be used to analyze the phenomena.
Corrosion Morphologies—Passivity and Protective Films
A metal in contact with an environment can develop—or not develop—a protective passive film comprising a defective barrier layer and a precipitated, porous outer layer of an oxide, hydroxide, or oxyhydroxide, or even a metal compound containing an anion present in the solution (e.g., siderite in the case of iron corrosion in carbon dioxide-acidified brine). Both films, if present, play important roles in determining the extent of metal passivity and hence metal resistance to corrosion. Passivity breakdown (when passivity exists) is the origin of localized corrosion. Several models exist to explain passivity. Those models based on fundamental principles are still under continual development; i.e., they often contain strong hypotheses, in order to simplify the complexity of passivity. The models evolve by relaxing the underlying assumptions and hypotheses, one at a time, as new information on the mechanism becomes available. The roles of oxide structure, disorder, devitrification, defects, dehydration, adsorbate and water adsorption, and other phenomena must be studied at the molecular scale. As elsewhere, computational and experimental methods are emerging to help produce significant advances in this area.
One model approach is that of examining other alloy systems for transitions in passivity with alloy composition analogous to those seen in stainless steel, but that approach will not lead to breakthroughs in terms of the amount of alloying element required, and there seems to be a fundamental percolation-like law that dictates the need for at least 10 to 12 percent of the beneficial element(s).
A key property of a passive film that may be manipulated in the future is its semiconductive nature in the context of the cathodic reaction that necessarily occurs on top of the film. It is not always appreciated that chromium oxide is a very good electrical insulator, so that in theory one could enrich chromium to such an extent that the reduction of oxygen on top of the passive film on stainless steel would cease, thus eliminating localized corrosion in salt water. In practice, not much iron content is required to make the film rather conductive, but still, manipulation of cathodic reaction kinetics on passive films has to be considered an important challenge for the future. Many tools and theoretical underpinnings are available for further progress in this area, including a variety of in situ probes, surface-science tools, and classical electrochemistry methods.
There is also a lack of understanding concerning nonpassivating, but protective, films, as well as gaps in knowledge concerning how these form and what controls their degree of protection. This is really a complex microscopic chemical engineering system with reaction, transport, pH change, and precipitation of metastable
and stable phases. Wetting and drying, as in atmospheric corrosion, add another level of complexity. The length scale of the porosity, for a good protective film, approaches the atomic scale, leading to a whole range of poorly understood transport phenomena, and the physics of such processes are a major challenge in corrosion science. Magnesium and magnesium-aluminum would be a good basis for a systematic study, with an easier metal, such as zinc, as a possible model system.
Corrosion Morphologies—Protective Layers Formed at High Temperature
The key to achieving high-temperature corrosion resistance is the development of a continuous, protective surface layer. (See Box 3.2.) Many of the fundamental mechanisms underlying the phenomena of passive and active oxidation and corrosion at high temperature are quite different from those in low-temperature, aqueous environments, because atoms are more mobile, substrate processes are intimately involved, and gaseous products may form. Rate-limiting mechanisms may include gaseous diffusion through a static gas boundary layer, surface chemical reactions, solid-state diffusion through a surface film or substrate, or dissolution of a subsurface phase. Methods to interrogate buried interfaces and subsurface
|
BOX 3.2 High-Temperature Protective Scales The formation and maintenance of protective scales at high temperatures involve the interplay of reactive subsurface diffusive processes influenced by both materials (composition, microstructure) and environmental factors. Once a layer of this type is established, the substrate’s access to the environment is effectively isolated, and henceforth controlled by the transport properties and stability of the surface scale as long as its integrity is maintained.1 A number of possible mechanisms can limit or control the rate of corrosion of a material undergoing a reaction with a fluid, and, consequently, the ability to produce this protective scale. Assume, for example, that a metal (e.g., chromium) reacts with dry oxygen to form a protective scale, chromia, to form a “surface film.” In loose analogy with aqueous corrosion, this scale can be considered a passivation layer, but it is much more dynamic than the passive film formed at low temperatures in aqueous environments in that it has a finite but, by definition of a protective layer, low growth rate. Under conditions of very low oxygen partial pressure, a surface oxide may be absent and instead a gaseous, volatile oxide may form, resulting in so-called “active” oxidation. Passive and active oxidation may also occur in parallel. This leads to the formation of a film that grows to a steady state thickness.2 |
structural changes in situ are therefore of great and particular interest to the study of high-temperature corrosion phenomena and complement information gleaned from surface analytical techniques.
Wider study using sensitive in situ and ex situ probes of protective scale breakdown and subsurface processes, especially for multiphase materials and under multi-reactant conditions, is needed to develop a comprehensive, generic understanding of factors controlling terminal protective scale breakdown in order to predict corrosion lifetimes and to guide mitigation strategies related to materials/coating design for extended component life.
The effects of water vapor on high-temperature oxidation have always been of scientific interest for many of the reasons described earlier in this section. However, within the past 10 to 15 years, this area of study has received increasingly greater attention because many important applications (e.g., gas turbines, steam generation systems, and solid-oxide fuel cells) involve environments with relatively high levels of water that scale, in many cases, with efforts to enhance efficiency (e.g., ultrasupercritical steam or higher-turbine-pressure ratios). The presence of water vapor in the environment often promotes higher oxidation rates—particularly for chromia- or silica-formers where water can dramatically enhance oxide volatization; impact oxide structure, stability, and/or solid-state transport properties in a negative way; and decrease corrosion lifetimes.48 While there has been substantial progress in finding alloys that are more resistant to water vapor or coatings to protect silicon-bearing ceramics in water-bearing environments,49 there is still much to be learned about effects on protective scale stability and the underlying defect mechanisms controlling transport, particularly for the more extreme environments envisioned for future technologies.50
|
48 |
E.J. Opila, N.S. Jacobson, D.L. Myers, and E.H. Copland, Predicting oxide stability in high-temperature water vapor, Journal of the Minerals, Metals, and Materials Society 58:22-28, 2006; I. Kvernes, M. Oliveira, and P. Kofstad, High temperature oxidation of Fe–13Cr–xAl alloys in air/water vapor mixtures, Corrosion Science 17:237-52, 1977; H. Asteman, J.-E. Svensson, M. Norrell, and L.-G. Johansson, Influence of water vapor and flow rate on the high-temperature oxidation of 304L: Effect of chromium oxide hydroxide evaporation, Oxidation of Metals 54:11-26, 2000; J.M. Rakowski and B.A. Pint, Observations on the effect of water vapor on the elevated temperature oxidation of austenitic stainless steel foil, Proceedings of Corrosion 2000, NACE Paper 00-517, NACE International, Houston, Texas, 2000; E. Essuman, G.H. Meier, J. Zurek, M. Hänsel, and W.J. Quadakkers, The effect of water vapor on selective oxidation of Fe–Cr Alloys, Oxidation of Metals 69:143-162, 2008; E.J. Opila, Oxidation and volatilization of silica formers in water vapor, Journal of the American Ceramic Society 86(8):1238-1248, 2003. |
|
49 |
See, for example, K.N. Lee, D..S. Fox, and N.P. Bansal, Rare earth silicate environmental barrier coatings for SiC/SiC composites and Si3N4 ceramics, Journal of the European Ceramic Society 25:1705-1715, 2005. |
|
50 |
Department of Energy, Basic Research Needs for Materials Under Extreme Environments, Report of the Basic Energy Sciences Workshop on Materials Under Extreme Environments, June 11-13, 2007, available at http://www.sc.doe.gov/bes/reports/files/MUEE_rpt.pdf. |
Even seemingly simple, well-studied, high-temperature corrosion and oxidation phenomena continue to offer research challenges, mainly related to the clear advantages of making direct observations of atomic-level processes at high temperatures and studying product and phase evolution, reaction kinetics, and other developments (such as stress and morphology) in real time. To date, most mechanistic studies have required the analysis of many pieces of data, which are typically derived from indirect measurements of atomic-level processes—for example, measurements of weight change, film thickness, or characterization of microstructural features. Emerging capabilities to probe atomic- and molecular-level phenomena in situ and with high time, spatial, and chemical resolution—and to model such over equivalent time and length scales—offer great opportunities to make substantial progress in high-temperature corrosion science over the next few years.
Corrosion Morphologies—Phase/Phase Morphologies and Microstructural Defects
Defect engineering is a concept that has been relegated primarily to serendipity and is not yet well developed; however, defect engineering involving control via manipulation of surface defects (e.g., undesirable intermetallic compounds that serve as pit initiation sites) is a possible cost-effective and novel strategy. Analogous to damage tolerant design in the fracture community, defect engineering incorporates flaw (crack) size and shape into fracture mechanics analysis, as well as mechanical load and global geometry. The purpose is to assess the risk of cracking. Thus corrosion damage is already incorporated on a very limited basis in defect engineering, which is also referred to as grain boundary and interface engineering. Types of defect engineering include changing the intrinsic properties of susceptible pit-forming defects or replacing them with more benign defects, changing the critical spacing of such defects, and changing the size or other attributes of critical defects. Defect engineering of grain boundaries is also possible where the distribution and percentage of corrosion-prone grain boundaries (i.e., highly misoriented grain boundaries) are controlled. Models for defect engineering may be expanded to include the effects of defect size, spacing, and intrinsic defect properties or extended to the case of grain boundaries where the most corrosion-prone boundaries, their distribution, connectivity, and grain shape can all be explored computationally and experimentally to optimize resistance to intergranular corrosion. Materials design and processing can be guided by these insights.
Corrosion Environments—Predicting Corrosion Properties of Materials in a Wide Range of Environments
There is often a great deal of corrosion data on a number of engineered materials. However, much of the available data is clustered in a limited number of
environments, particularly full immersion environments. This has a number of implications, including the inability to create a meaningful national database of corrosion data useful to industry, government, and academia. Not only is there the issue of full immersion, atmospheric, and alternate immersion aqueous environments, but there are also radically different environments such as nonaqueous and high-temperature environments.
Ethanol is an example of the sort of environment for which a better ability to predict its influence on various forms of corrosion is paramount, because planned widespread use of ethanol will require an efficient and reliable transport system. Steel pipelines are by far the most cost-effective means of transporting large quantities of fuel over long distances. However, it is critical to assure that pipelines transporting ethanol are reliable and safe. Recently, SCC in the presence of fuel-grade ethanol (FGE) has been recognized and identified as a phenomenon in enduser storage and blending facilities. Investigation of the mechanisms of corrosion and cracking of steel in biofuels is still in the early stages, and the electrochemical techniques that have been applied successfully to evaluate SCC in aqueous systems are a challenge in the high-resistivity FGE environment. Bioethanol derived from sugar cane is used extensively in Brazil. Brazilian ethanol is hydrous, containing about 6 percent water (compared to the bioethanol used in the United States, which has <1 percent), which does not cause SCC. Other nonaqueous environments of interest in corrosion include a wide range of environments in batteries and fuel cells as well as a variety of biological environments. In general, environments with low water content produce different behavior, especially when an insufficient level of water is available for passivation.
Corrosion Environments—Connection Between Nominal Environmental Conditions and the Actual Chemistry of the Environment
A critical but poorly understood factor related to atmospheric exposure is the connection between nominal environmental conditions (e.g., humidity, ultraviolet radiation, pollution, atmospheric particles, and so on) and the actual chemistry of the environment on the material’s surface. Time-of-wetness is known to be an important parameter in outdoor exposure, given that water associated with precipitation or condensation is critical to the corrosion processes. However, accurate prediction of corrosion rates depends on knowing how the water on the surface affects the concentration of all the important corrosive species.
TECHNIQUES AND TOOLS FOR RESEARCH
The committee highlights below some of the analytical techniques that have enabled, and will continue to enable, ongoing advances in corrosion science and in
mitigation of corrosion. Steady progress in improving sensitivity, area and volume sampled, acquisition speed, and quantification makes it worthwhile to look not only toward new research efforts but also back at questions previously examined. A smaller probe size might finally enable insights about the evolution of a particular material’s degradation, or the enhanced discrimination of species might shed light on the chemical environment around a corrosion pit. Steady improvement in capabilities is occurring across a wide breadth of techniques, only a few of which are highlighted below to indicate the current exciting possibilities. The committee felt strongly that it should not try to indicate which techniques and approaches hold the greatest potential for addressing the corrosion grand challenges, not only because of its inability to cover every one of the vast array of experimental and computational advances, but also, and more importantly, because the research community has historically proved adept at taking advantage of such advances when and where scientifically and technically appropriate.
Examples of Relevant Techniques and Tools
Atom Probe Tomography
Atom probe tomography and its ability to reveal the three-dimensional atomic structure of materials has become a very powerful tool in modern materials science.51 However, its application to corrosion science has been quite limited despite the ability to finely resolve phenomena such as elemental segregation that can play such an important role in sensitization and associated stress corrosion cracking or in internal oxidation. Furthermore, until the recent advent of the laser-induced local-electrode atom probe (LEAP) technique,52 ceramics materials could not be analyzed because of the inability of such brittle materials to withstand the high fields necessary to release atoms for subsequent mass analysis. However, using a laser-LEAP, it has been recently demonstrated that bulk alumina can be imaged with quantitative concentration determinations.53 This opens up the possibility for detailed examination of protective oxide scales, such as alumina, to probe the disposition of minor species, which can profoundly influence how effective such surface oxides are at proffering oxidation at high temperatures. For corrosion effects at more moderate temperatures, a recent study has used the laser-LEAP to
resolve fine microstructural and compositional details of an oxide film on stainless steel formed under pressurized-water-reactor conditions (see Figure 3.15).54 Such findings indicate the potential of state-of-the-art atom probe techniques to advance basic corrosion science knowledge and help understand origins of failures due to environmental degradation.
Time-of-Flight SIMS
Secondary ion mass spectrometry (SIMS) has had an important role in corrosion science (as shown, for example, in the work of Bishop et al. 55 and Marriott et al.56). For high-temperature oxidation, it has been particularly powerful in determining transport mechanisms in protective oxide scales grown on metallic specimens using O18 exposures.57 Recently, time-of-flight SIMS has been used to generate detailed images through sections of protective oxide scales, revealing details of the distribution of various alloying elements/phases (Figure 3.16).58 Further application of this technique to corrosion science and engineering seems well justified.
Advanced Electron Microscopy
For many years, transmission electron microscopy has been of great benefit to corrosion science and engineering by revealing the relationships among microstructure (and, now, nanostructure), composition, and different corrosion behaviors (dissolution, crack growth, scaling, and so on)—see, for example, Przybylski et al.59 and Gertsman and Bruemmer.60 Advances in analytical election microscopy for high-resolution chemical analysis and focused ion beam techniques to provide high-quality thinned sections of oxidized material from precise areas of a corroded specimen (for example, Haynes61) have truly made electron microscopy an essential
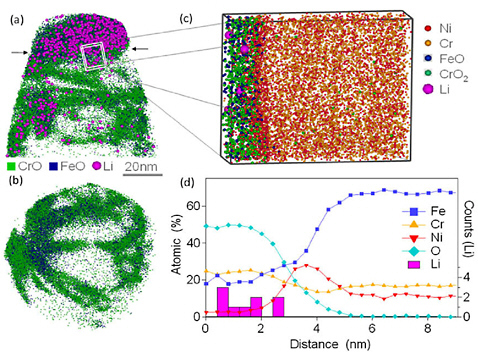
FIGURE 3.15 (a) Atom probe tomography reconstruction showing the presence of Li atoms within the cap and sub-interface oxides. The arrows indicate the location of the cap-oxide-to-metal interface. The Li atom distribution is superimposed on the oxide atom maps. (b) Top view of the sub-interface region showing the distribution of oxides (cap oxide removed). The oxide regions beneath the cap are interconnected. (c) Sub-volume (5 × 15 × 18 nm; ~40,000 detected atoms) taken from the cap-oxide-to-metal interface showing selected species. (d) Concentration profile across the oxide–metal interface generated using the proxigram technique. The presence of Li is represented by an atom count because its concentration is very low. Uncertainties in the data points are comparable to the marker size. SOURCE: Reproduced from S. Lozano-Perez, D.W. Saxey, T. Yamada, and T. Terachi, Atom-probe tomography characterization of the oxidation of stainless steel, Scripta Materialia 62:855-858, 2010.
part of corrosion research and development and failure analysis. Currently, there are numerous efforts to develop ways to conduct in situ experiments inside the columns of high-resolution electron microscopes, including the introduction of liquids (see Figure 3.17).62 Such developments have obvious relevance to advancing the state of fundamental knowledge about corrosion processes and how such processes are controlled by structure and composition.
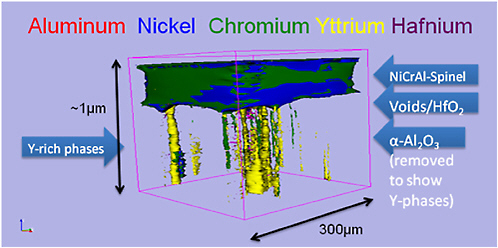
FIGURE 3.16 Cross-sectional view of multilayer scale formed on an oxidized NICrAl (YHf ) alloy (spinel overlaying α-alumina). The α-alumina data have been removed to show the morphology of the embedded Y-rich oxide phases. SOURCE: D.B. Hovis and A.H. Heuer, unpublished data.
Neutron Scattering
Neutron scattering has not found wide applicability in corrosion science but is proving scientifically powerful in unraveling the structure and dynamics of oxide-H2O interfacial regions of relevance to geochemistry and the fundamental science of aqueous solutions in contact with solids.63,64 The greater penetrating power of neutrons, compared to other energetic beams used for analysis, can allow subsurface (or undercoating) structures and processes to be probed and could prove to be of particular value for in situ experiments. Defect distributions, structural fluctuations, and film growth can be monitored as a function of depth into a solid using various neutron scattering techniques, and the sensitivity of neutrons to protonic species is of particular relevance to many corrosion processes.
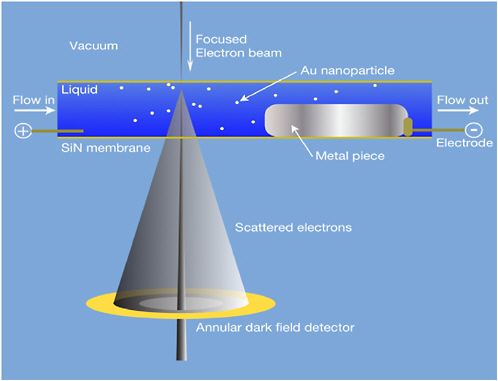
FIGURE 3.17 Schematic of scanning transmission electron imaging of metallic nanoparticles in a liquid. SOURCE: Reproduced from N. de Jonge, D.B. Peckys, G.J. Kremers, and D.W. Piston, Electron microscopy of whole cells in liquid with nanometer resolution, Proceedings of the National Academy of Sciences 106:2159-2164, 2009.
X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) is a surface analytical approach that provides detailed chemical information from the top 1 to 10 nm of a sample surface. The surface is irradiated with an x-ray beam and the kinetic energy of the emitted electrons is analyzed. This technique is well established in the corrosion field because it has great utility to measure thin protective films and corrosion product layers. The latest technical developments in XPS instrumentation enhance the usefulness of the technique. One trend is a decreasing x-ray beam size. X-ray beams are much more difficult to focus than electron beams, which are used in many other analytical techniques. As a result, XPS has relatively poor lateral resolution. However, XPS tools now provide x-ray beams less than 10 micrometers in diameter, which allows for the analysis of surfaces on small microstructural features
such as second phases and inclusions. Another development in XPS technology involves the ion beams used to sputter samples for depth profiling. Standard ion beams such as Ar ion beams can damage samples, particularly organic samples such as paint coatings, which is relevant to corrosion. In recent years beams of large ion clusters have been offered for use in sample sputtering. Ions of C60, so-called buckyballs, allow for gentler sputtering. The large ions impact the surface with a lower speed so that there is much less penetration and much less damage compared to beams of single ions. Very recent reports have shown that large gas cluster ions caused very little damage during sputtering of polyimide, which is very sensitive to ion irradiation damage.65 In this work the gas cluster ion gun generated an Ar ion cluster distribution centered at Ar2500. The polyimide XPS spectra before and after sputtering with this beam exhibited very little change.
Electron Backscatter Diffraction
Although the fundamentals of the technique can be traced back to the work of Kikuchi in the late 1920s, electron backscatter diffraction (EBSD) emerged in the early 1980s as a method for analysis of local crystallographic texture in materials.66 Automation and computerization of the analysis of backscattered electron diffraction patterns led by Adams and co-workers in the 1990s further led to the development of commercial software and hardware that has enabled EBSD to become a relatively mature technique today for characterizing microtexture.67 It is well known that the crystallographic texture of a material influences its thermophysical properties. Manipulating texture through heat treatment or forming techniques such as rolling or pilgering may lead to improvements in corrosion resistance or resistance to environmentally influenced cracking if applied properly. A few groups have used EBSD to inspect growth processes at crack tips during stress corrosion cracking and other crack-growth processes in structural alloys68
FIGURE 3.18 Electron backscatter diffraction image of a fatigue crack in alloy X. Bar is 200 µm. Colors represent specific crystal orientations. SOURCE: Courtesy of J. Burns and M. Frary, Boise State University, from J. Burns, “High Temperature Fatigue Crack Growth Behavior and Microstructural Evolution in Alloy 230,” M.S. thesis, Boise State University, 2010.
(see Figure 3.18). Although only a small number of materials have been analyzed under a narrow range of conditions, studies suggest that the propensity for environmentally induced crack growth can be strongly affected by changes in the orientation from one grain to another. For example, Arafin and Szpunar found that low-angle and special coincident site lattice boundaries in API X-65 pipeline steel were more resistant to crack propagation compared to high-angle boundaries.69 The use of EBSD, particularly when combined with other tools such as FIB and
microstructural modeling, may lead to the discovery of textured, low-cost materials resistant to SCC and other environmentally induced crack processes.
Terahertz Acoustical and Electromagnetic Spectroscopy
One of the real measurement issues facing the corrosion scientist and engineer involves detecting and characterizing “hidden” corrosion, which ranges from corrosion in structures underlying the tiles on the space shuttle and corrosion of the steel reinforcement bars used in concrete bridges, roads and structures, to corrosion and blistering under paints and other types of protective and decorative coatings. An approach whose use has been growing recently is the use of both electromagnetic (EM) methods in the terahertz frequency (often between 300 GHz and 3 THz) range and acoustic spectroscopy/microscopy in related frequency ranges, as well as mixed-mode methods sometimes identified as pulsed laser acoustics or photoacoustic near-infrared spectroscopy. Reviewed in several references,70 the EM methods occupy a niche in EM nondestructive evaluation (NDE) used in studies of corrosion under tiles in the space shuttle.71 Acoustic methods, often described under acoustic microscopy,72 have been used recently in studies of corrosion-related blistering in organic coatings.73 Laser-induced acoustics to study corrosion at interfaces has also been developing quite rapidly.74 All of these methods seek to identify hidden sites of corrosion, characterize the events and processes occurring at these hidden interfaces, and provide images, numerical characterization, or mechanistic interpretation of results. Blistering, adhesion loss, and degradation of protective coatings have been studied in detail by these methods.
Combined Techniques and Tools
One of the great hurdles in the study of corrosion is how remarkably difficult it is to make direct measurements of the rate of corrosion. Direct measurement is especially problematic because corrosion is often highly heterogeneous and sometimes takes place in cavities such as pits shielded by metal in wet environments. Electrochemical measurements cannot give spatial information: the traditional method is serial sectioning, but this causes the pit or intergranular corrosion site to cease to exist, so that it is not possible to obtain time-dependent information. The second greatest need beyond the need for information on aspects of hidden corrosion is the acute need to acquire information linking the electrochemical properties and other damage-related phenomena—such as cracking, pitting, locally dissolving, dealloying, and even hydriding—to the chemical and structural characteristics of the metallic alloy in question with spatial resolution. Traditionally this has been done with separate yet similar sites or separate specimens analyzed using separate techniques. In other words, no one corrosion site can be simultaneously subjected to multiple characterization probes. Another way to state this need is that there is an acute need for several types or channels of information (structural, chemical, electrochemical, hydrogen, and information from electrical and chemical spectroscopy) at the same time and at the same location on a surface with nanometer-scale resolution in situ. Connected to this need is the desire to look at more than one corrosion site, such as several grain boundaries, so that results from one isolated boundary or crack tip are not accidentally taken to represent the average or most prevalent behavior in the entire material. This has been a traditional shortcoming of STEM work capable of structural and chemical characterization of a slice of a crack tip. The effort expended is enormous to section and characterize just a few crack tips.
Recent advances in three-dimensional microtomography as discussed below present the opportunity to map hidden damage in three dimensions and in real time.75 Advances in the three-dimensional atom probe tomography, three-dimensional secondary ion mass spectroscopy, and three-dimensional techniques focused ion beam sectioning are all helpful, but the need exists to combine these approaches into a supertool than can raster over large areas and then focus on sites of interests with high resolution.
Electrochemical Impedance
In the last several decades there has been an explosion of techniques that can probe instantaneous corrosion rates, including electrochemical impedance, har-
monic and electrochemical frequency modulation methods, and electrochemical noise methods. One approach to determining rates of corrosion and gaining mechanistic insight about corrosion of a metal involves the electrochemical impedance (sometimes known as AC impedance) method. Over the past 30 years this method has received much attention in corrosion and has shed a good deal of additional light on corrosive processes. It also has applicability to corrosion sensors. In this technique, typically a small-amplitude sinusoidal potential perturbation is applied to the subject alloy at a number of discrete frequencies, ω. At each one of these frequencies, the resulting current waveform will exhibit a sinusoidal response that is out of phase with the applied potential signal by a certain amount (Φ) and has a current-amplitude that is inversely proportional to the impedance of the interface. The electrochemical impedance, Z(ω), is the frequency-dependent proportionality factor that acts as a transfer function by establishing a relationship between the excitation voltage signal and the current response of the system. The electrochemical impedance is a fundamental characteristic of the electrochemical system it describes and contains information on the resistance to charge transfer, mass transfer, and ohmic resistive processes. Knowledge of the frequency dependence of impedance for a corroding system allows a determination of an appropriate equivalent electrical circuit describing that system. The method has application to organic coatings, bare metals, passive films, and other corrosion-related applications. Challenges still remain, however, such as determination of corrosion rates under coatings and at defects.
Both harmonic and electrochemical frequency modulation (EFM) methods takes advantage of nonlinearity in the E-I response of electrochemical interfaces to determine corrosion rate. A special application of harmonic methods involves harmonic impedance spectroscopy. The EFM method uses one or more AC voltage perturbations in order to extract corrosion rate. In the most often used EFM method, a potential perturbation by two sine waves of different frequency is applied across a corroding metal interface. The E-I behavior of corroding interfaces is typically nonlinear such that such a potential perturbation in the form of a sine wave at one or more frequencies can result in a current response at the same and at other frequencies. The result of such a potential perturbation is various AC current responses at various frequencies such as zero, harmonic, and inter-modulation. The magnitude of these current responses can be used to extract information on the corrosion rate of the electrochemical interface or conversely the reduction-oxidation rate of an interface dominated by redox reactions, as well as the Tafel parameters. This is an advantage over polarization resistance and electrochemical impedance spectroscopy (EIS) methods. A special extension of the method involves harmonic impedance spectroscopy whereby the harmonic currents are converted to harmonic impedance values at various frequencies through knowledge of the magnitude of the AC perturbation. Electrochemical noise analy-
sis can provide a parameter called the electrochemical noise resistance, Rn. It is desirable to utilize this parameter in an analogous fashion as the polarization resistance or EIS. This method also has application in sensors, but interpretation is often controversial. The future direction in these techniques is to extend them to heterogeneous corrosion processes on smaller length scales, and in combination with other spectroscopies such as local atom and molecular spectroscopies in such a way that spatial and temporal information is given from the same surface at once. Still, hidden corrosion remains a challenge, as do sensor interpretation and subsequent decision-making algorithms.
Near-Field Scanning Optical Microscopy76
Near-field scanning optical microscopy (NSOM) is a technique for nano-structure investigation that breaks the far-field resolution limit by exploiting the properties of evanescent waves. This is done by placing the detector very close (at a distance much smaller than wavelength λ) to the specimen surface, thus allowing for inspection of the surface with high spatial, spectral, and temporal resolving power. With this technique, the resolution of the image is limited by the size of the detector aperture and not by the wavelength of the illuminating light in the traditional wave optic limit. In particular, lateral resolution of 20 nm and vertical resolution of 2 to 5 nm have been demonstrated. As in optical microscopy, the contrast mechanism can be easily adapted to study different properties, such as refractive index, chemical structure, and local stress. Dynamic properties can also be studied at a subwavelength scale using this technique.
It is possible to take advantage of the various contrast techniques available to optical microscopy through NSOM but with much higher resolution. By using the change in the polarization of light or the intensity of light as a function of the incident wavelength, it is possible to make use of contrast-enhancing techniques such as staining, fluorescence, phase contrast, and differential interference contrast. Staining and fluorescence have large applications in corrosion, especially if the staining or fluorescence indicates key chemicals that are significant to corrosion reactions. These methods have been applied to corrosion already but seldom are coupled to an array of methods. It is also possible to provide contrast using the change in refractive index, reflectivity, local stress, and magnetic properties, among others.
Instead of performing imaging of a surface, various near-field spectroscopy methods can be applied to the study of corrosion, such as micro-Raman and surface-enhanced micro-Raman or other atomic and molecular microscopy techniques.
|
76 |
Paragraphs one and two reproduced from http://en.wikipedia.org/wiki/Near-field_scanning_optical_microscope. |
Scanning Probe Microscopy
Scanning probe microscopy (SPM) makes it possible to form images of surfaces using a physical probe that scans the specimen. An image of the surface is obtained by mechanically moving the probe in a raster scan of the specimen, line by line, and recording the probe-surface interaction as a function of position. SPM began with the invention of the scanning tunneling microscope in 1981. Many scanning probe microscopy techniques can image several interactions simultaneously. The manner of using these interactions to obtain an image is generally called a mode. The resolution varies somewhat from technique to technique, but some probe techniques reach a rather impressive atomic resolution, owing largely to the ability of piezoelectric actuators to execute motions with a precision and accuracy at the atomic level or better on electronic command. One could rightly call this family of techniques “piezoelectric techniques.” The other common denominator is that the data are typically obtained as a two-dimensional grid of data points, visualized in false color as a computer image.77
A number of methods have applications in studies of corrosion, such as Kelvin probe atomic force and chemical force and scanning tunneling methods. Of these techniques, atomic force microscopy and scanning tunneling microscopy are the most commonly used for roughness measurements. NSOM and SNOM are scanning probe methods used to obtain optical imaging or some form of contrast.
Summary Observations on Instrumentation
Some of the techniques and tools outlined above have been used in corrosion research over the past 20 years. The future direction for these techniques is extension to heterogeneous corrosion processes on smaller and smaller length scales, and combination with other spectroscopies such as local atom and molecular spectroscopies in ways such that spatial and temporal information is given from the same surfaces at once. Hidden corrosion remains a challenge, as do sensor interpretation and subsequent decision-making algorithms. Pan and Leygraf78 have combined atomic force microscopy with scanning electrochemical methods to obtain a coordinated x-y surface view of metrology and electrochemical reactivity. The future for instrumentation is to expand the number of channels of information and to ensure sufficient dynamic range to sample large enough areas so as to obtain a clear picture about corrosion in complex materials.
|
77 |
Paragraph reproduced from http://en.wikipedia.org/wiki/Scanning_probe_microscopy. |
|
78 |
A. Davoodi, J. Pan, C. Leygraf, and S. Norgren, Integrated AFM and SECM for in situ studies of localized corrosion of Al alloys, Electrochimica Acta 52(27):7697-7705, 2007. |




































































