Over millions of years, the structure and function of organisms have evolved under the influence of a constant gravity stimulus, which consists of the natural force of attraction exerted by celestial bodies such as Earth. To fully understand this influence of gravity, living systems must be studied by essentially eliminating the gravity variable. This task can be daunting because organisms must live for a sufficient time outside the effects of Earth’s gravity, in a state of free-fall. In the United States, the agency designated by Congress to develop a space research program involving the life and physical sciences is the National Aeronautics and Space Administration (NASA). During its 50 years of existence, NASA has continued to evolve such a program, which, at the present time, is centered primarily on operational medicine objectives being pursued on the National Space Laboratory, a key component of the International Space Station (ISS).
It is now recognized that habitation of the microgravity environment poses potential deleterious consequences for essentially all the organ systems of the body, even though it is routine for human astronauts and cosmonauts to spend 180 days or longer living and performing a number of challenging tasks on the ISS. Given the typical lifespan of humans, 180 days in space may seem trivial. However, in the case of rodents, the animal model most scientists have used to study fundamental biological processes in space, such a time frame represents approximately one-fourth to one-third of the species’ adult life. Thus, studies on these rodents in space have the potential to extrapolate important implications for humans living in space well beyond 6 months.
As part of the decadal survey process, the Committee for the Decadal Survey on Biological and Physical Sciences in Space formed the advisory Animal and Human Biology (AHB) Panel and tasked it to address the research needed to (1) enable humans to carry out long-term space exploration and (2) ascertain opportunities provided by the space environment that enable a greater understanding of how gravity shapes fundamental biological processes of various organisms.
To meet its objectives, the AHB Panel focused on the following topics: (1) what is known about the risk and deleterious effects of spaceflight (and ground-based analogs) on the structure and function of the musculoskeletal (bone and muscle), sensory-motor, cardiovascular, pulmonary, endocrine, and immune systems, as well as how animals develop in the absence of gravity; (2) the effectiveness of the countermeasures currently used to maintain organ system homeostasis in the face of microgravity; (3) the knowledge gaps in understanding of the above topics that need to be addressed; (4) the research platforms needed to undertake new research initiatives in the next decade; (5) the overarching issues that have to be addressed in fostering cutting-edge, integrative research in humans and animals, and spanning multiple physiological systems, to generate future countermeasure strategies; and (6) the
specific high-priority research initiatives that are needed to sharpen and advance the science knowledge necessary for progress in the next decade. (It is important to note that certain topics that have a major impact across multiple physiological systems, such as nutrition, are in most cases not covered in this chapter, but rather in Chapter 7, which focuses on crosscutting issues.) Finally, in examining programmatic activities relevant to this chapter, and as discussed in the committee’s interim report to NASA,1 the AHB Panel was deeply concerned that NASA had severely reduced research initiatives in the life and physical sciences in the latter half of the past decade. In the panel’s view, this action has effectively paralyzed research initiatives previously recommended by National Research Council (NRC) study committees (as reflected by the relative paucity of publications since 2005 in recommended subject areas such as bone) and poses a daunting challenge to future administrations attempting to reverse the neglect and to accomplish the life and physical sciences research initiatives recommended in this report.
Risks for Bone Loss During Long-Duration Space Missions
The skeletal (bone) system provides the solid framework for humans and mammals to oppose gravity, and its fidelity in accomplishing this fundamental process has evolved over millions of years. Given this evolutionary role, it is not surprising that bone loss occurs in astronauts at a rate that is both substantial and progressive with time spent in microgravity.2-5 Accordingly, without appropriate countermeasures, spaceflight of 2 years or longer will present serious risks due to progressive bone fragility. Therefore, there is a need to adopt effective countermeasures that have been appropriately tested in relevant human and animal models. The 1998 NRC report A Strategy for Research in Space Biology and Medicine in the New Century6 recommended several experiments to address the problem of bone loss during spaceflight. At present, several key issues raised in the 1998 NRC report have not been addressed. For instance, the report recommended that genetically altered mice be used in flight experiments to investigate the molecular mechanisms of bone loss, yet these experiments have not been completed. The report also recommended that in-flight animal facilities should house 30 adult rats or mice, but the ISS can currently house only 6 mice (in the Mice Drawer System on the Italian Space Agency investigation). These recommendations should be implemented, and additional steps should be taken to advance research into bone loss in microgravity for the development of effective countermeasures.
Effects of Spaceflight Environment on the Structure and Function of Bone
Bone loss during spaceflight appears to be due primarily to increased resorption in load-bearing regions of the skeleton.7,8,9 There is also some evidence of a decrease in bone formation. The rate of bone loss in microgravity is roughly 10 times greater than the bone mineral density (BMD) loss per month that occurs in postmenopausal women on Earth who are not on estrogen therapy.10-13 Results from Skylab,14 Mir,15,16 the space shuttle,17 and the ISS18,19,20 missions have shown substantial areal and volumetric bone loss in critical regions such as the proximal femur and spine. The most accurate data, derived from quantitative computed tomography, have shown that spinal volumetric BMD was lost at a rate of 0.9 percent per month and total hip volumetric BMD was lost at rate of 1.4 percent per month; there was, however, considerable variability between individuals.21 Changes in bone strength (expressed as percentage loss) were much greater than changes in BMD.22
BMD lost in 6-month missions appears to be mostly reversible by 1,000 days after return to normal gravity (1 g).23,24 However, changes in bone structure are not reversible and seem to mimic changes in the elderly.25
An important question that remains unanswered is whether any loading that is performed by simply living and working in partial gravity—such as the 1/6 g of the Moon or the 1/3 g of Mars—will provide any protection from the bone loss that occurs in microgravity. Expert opinion as presented in a recent symposia is that it will not,26 although data from a partial-gravity mouse model is just becoming available.27
Animal Studies
Rodents have been flown on the Cosmos biosatellite28-33 and on space shuttle missions34-46 to measure bone loss. The most consistent finding was the striking decrease in bone formation with spaceflight, which stopped
completely at some skeletal sites.47 In contrast to what occurs in humans, bone resorption in rats was not substantially changed in one flight experiment.48 whereas it was increased in another.49 Most rodents flown in space have been immature and undergoing rapid bone growth.50 In these studies, bone formation dominates during growth, and so it is not surprising that greater changes were observed in bone formation than in bone mineral resorption. It is difficult to extrapolate from these data to the expected changes in the mature skeleton during long-term spaceflight. A few studies have used adult animals, and in these studies bone formation was suppressed at the periosteal surface.51,52,53 In contrast, longitudinal growth was minimally affected by either spaceflight or hindlimb unloading (HU).54 One study has shown bone loss in spaceflight to be greater than that in ground-based models, such as HU,55 although it is important to distinguish between bone loss, the removal of existing bone, and failure to gain bone in growing animals. In this model, traction applied to the tail of rodents elevates the lower extremities and eliminates generation of ground reaction forces.
In Vitro Studies
Bone cell culture experiments have been performed on space shuttle missions,56,57 on Skylab,58 and on the free-flyer Foton-M.59 These studies demonstrated differences in gene expression and growth factor production by osteoblasts.60 Osteoclasts were also affected by spaceflight.61 Interpretation of these experiments is a challenge, because cells in culture behave much differently than cells embedded in bone. Furthermore, the vibration during launch can confound cell culture experiments, particularly for short-term (1 to 2 weeks) experiments.62 The experimental complications during spaceflight and the difficulty with interpretation make bone cell experiments of lower priority compared with animal or human spaceflight studies. There is, however, a potential use of cultured bone cells in biotechnology applications.
Status of Countermeasures
Exercise Countermeasures
To date, NASA and the Russian space program have relied primarily on exercise countermeasures to attenuate bone loss,63,64,65 but no exercise has yet proven to be uniformly effective for maintaining bone mass66,67 during flight or bed rest. Similarly, low-magnitude, low-frequency mechanical signals were not effective in prolonged best rest.68 There is evidence that the external loading on previous exercise devices used in space has been insufficient to provide the required stimulus to bone.69,70 Recent (fall 2009) additions to the exercise devices on the ISS now offer the possibility for greater loading and for definitive research to examine the efficacy of exercise countermeasures. The capacity to measure loads was also added to these new devices, but interaction with NASA personnel indicates that further refinements are required to produce accurate load estimates. The ISS provides an excellent research platform for studies that are relevant to missions outside low Earth orbit because the microgravity environment on the ISS presents a greater challenge to the musculoskeletal system than does a partial-gravity environment.71
Ground-based research using bed rest (head-down for microgravity or head-up for lunar simulation) provides another important research platform for exercise countermeasures.72-81 For example, bed rest studies suggest that bone may be somewhat protected with sufficient loads and exercise time. Results from Vernikos et al.82 showed that intermittent upright posture and exercise reduce the increased blood calcium levels observed in bed rest, and data from Smith et al.,83,84 and Zwart et al.85 showed positive benefits of supine treadmill running within lower-body negative pressure during 30 and 60 days of bed rest. There are no studies indicating positive effects on bone in passive intermittent rotational artificial gravity.86 It is possible that lower-body negative pressure or centrifugation coupled with exercise may be more effective than exercise alone, perhaps through modulation of some other necessary physiological factor (e.g., improved blood flow, fluid shifts, or circulating hormones).
Pharmaceutical and Nutritional Countermeasures
Vitamin D supplementation has been used by NASA during spaceflight,87,88,89 but one report90 showed that serum 25-hydroxycholecalciferol was decreased after flight despite supplementation with vitamin D.
Short-term calcium supplementation has not been effective in reducing bone loss during spaceflight or head-down bed rest.91,92
Over the past 15 years, several drugs have been developed to prevent bone loss associated with osteoporosis
(e.g., bisphosphonates, selective estrogen receptor modulators, parathyroid hormone). Some of these drugs may be useful for preventing bone loss in astronauts during long-term spaceflight. As with exercise countermeasures, the ISS is ideal for testing the effectiveness of drugs. However, at the time of the writing of this report only one experiment involving oral bisphosphonates had been flown on the ISS, and only two astronauts had participated. Bisphosphonates substantially reduce bone resorption in Earth-bound patients and can be given by mouth daily or weekly, or yearly by infusion. A new potent antiresorptive bisphosphonate, zolendronic acid, effectively preserves bone mass in osteoporotic patients when given by infusion once per year.93
Bisphosphonates have shown promise in ground-based studies. As an example, a single injection of pamidronate maintained a slightly increased BMD in the spine and hip in a 90-day bed rest study.94 These drugs have been shown to attenuate bone loss in hindlimb unloaded rodents.95 In addition, excess urinary calcium excretion was reversed by pamidronate. This dual action, blocking bone loss and reducing urinary calcium excess, suggests there is potential utility for bisphosphonates in space.
A theoretical concern with use of bisphosphonates is that suppressing of resorption will also suppress bone formation, but data from spaceflight are lacking to address this issue. Should a fracture occur in space, use of bisphosphonates might slow healing. Consequently, it will be important to study fracture healing in space with antiresorptives to provide assurance that fractures will heal. Further, the long-acting nature of some bisphosphonates means that the suppression of bone turnover could persist upon return to normal gravity. The suppression of bone turnover in people who maintain vigorous levels of activity could have deleterious effects on bone quality. Other potential issues with the use of bisphosphonates include osteonecrosis of the jaw96 and atypical sub-trochanteric fractures after prolonged bisphosphonate use.97
Another drug recently approved for use in postmenopausal women with osteoporosis, Denosumab, blocks an important orthoclase-stimulating peptide called RANKL and stops bone loss in osteoporotic patients when given by injection every 6 months.98 A bone anabolic drug, teriparatide, is approved by the Food and Drug Administration for the treatment of patients at high risk for fracture,99 but there are ethical concerns in the treatment of a person with a normal skeleton with this drug because of the possibility of undesirable side effects.
Challenges for the use of drugs in space include storage and packaging that prevent degradation in the space environment. Additionally, it is not known whether drugs will have the same bioactivity when taken in a weightless environment.100,101 Bone-acting drugs have not yet been tested to determine the length of time they remain active in space, where, for example, they are exposed to higher radiation levels than on the ground. If bioactivity is compromised, long-acting drugs might be given pre-flight to avoid the need for in-flight dosing, although currently the longest interval between dosing of any appropriate drug is 12 months. Treatment of astronauts upon return to Earth with therapeutic drugs also needs to be explored.
An animal experiment using a myostatin inhibitor was tested on the space shuttle (STS-118). Myostatin is an antigrowth factor protein that blocks muscle growth, and so researchers expected the use of myostatin inhibitors to prevent muscle loss. In addition to preventing muscle loss during the 13-day mission, there are initial indications that the drug also preserved bone mass and strength.102,103 In another important recent study, sclerostin knockout mice did not lose bone during ground-based disuse.104,105 In addition, a sclerostin antibody improved bone mass in an animal model of colitis-induced bone loss.106
Gaps in Knowledge
Mechanism of Bone Loss
An animal research program offers many vitally needed tools to better understand bone loss during weightlessness—both to better define risks to the skeletal health of humans in prolonged spaceflight and to provide models to rigorously test recently developed pharmacologic strategies to control bone loss. However, past animal studies in spaceflight have been confusing. As discussed previously, many studies of rodents found that bone loss was due primarily to cessation of bone formation. Studies of astronauts using biomarkers have identified increased bone breakdown by resorption as the predominant mechanism, although the role of suppressed bone formation should be further investigated. These findings are complicated by the fact that most rodents studied in space were immature and growing, whereas all astronauts are mature and have stopped growing. Further experiments with
mature animals will help determine the relative contributions of decreased bone formation and increased bone mineral resorption in bone loss in microgravity. This will be critical in identifying effective pharmacologic countermeasures, which target either bone mineral resorption or bone formation.
Molecular and Cellular Mechanisms
There are many unanswered questions about bone loss during spaceflight. For instance, the molecular mechanisms by which bone senses gravitational forces remain unknown. Currently, it is thought that osteocytes within the bone sense mechanical perturbations and signal to osteoblasts and osteoclasts, but the biochemical nature of these signals among cells is poorly understood. Bone cell factors that have been identified as therapeutic targets include sclerostin (an inhibitor of bone formation), RANKL (a stimulator of bone resorption), and osteoprotegerin (an inhibitor of bone resorption); others will likely emerge in the coming decade.107 These targets are ideal for continued studies using animal models.
Fracture Repair
Without an effective countermeasure during spaceflight, bone loss occurs at an alarming rate and fracture risk is increased. Any fracture sustained by an astronaut during a long-duration mission must heal in a microgravity or partial-gravity environment. Fracture healing was studied on shuttle flight STS-29, and deficiencies in angiogenesis were noted.108 However, the shuttle flight was too short in duration (5 days) to fully evaluate fracture healing. A subsequent 5-week ground-based study showed that fracture healing was impaired in rats subjected to HU.109
Effects of Radiation
A major obstacle to long-term spaceflight is the effects of space radiation on astronauts. The effects of radiation on bone during spaceflight are currently unknown. However, ground-based studies demonstrate that space-like radiation causes bone loss.110 Further studies of the combined effects of radiation and unloading on bone structure are urgently needed.
Hormonal Issues
Endocrine hormones, such as parathyroid hormone, calcitonin, glucocorticoids, and insulin-like growth factor, influence bone homeostasis on Earth. Estrogen deficiency plays a major role in the pathogenesis of bone loss and fracture in both women and men on Earth.111 It has also recently been shown that the leptin receptor plays a key role in mechano-signal transduction.112 There is no direct evidence that these endocrine hormones play a major role in bone loss in space, as evidenced by the site-specific, rather than systemic, nature of bone loss in the skeleton due to microgravity. However, experiments with the rodent model have shown that estrogen status alters the skeletal response to spaceflight and HU.113,114 Recent evidence suggests that local (autocrine or paracrine) effects are more important in regulating bone lost as a result of disuse,115,116 and local expression of autocrine and paracrine factors have been investigated in the rat.117,118,119 However, traditional systemic endocrine factors (e.g., estrogen, cortisol) can also act in a paracrine manner because bone cells contain the enzymatic machinery to produce these factors locally.
Fluid Shifts
Bone loss in human volunteers subjected to bed rest is greatest in the lower extremities, particularly the calcaneus. In contrast, the upper extremities do not lose bone, and there is a net gain in bone mass in the cranial bones.120 This pattern of bone loss matches the expected changes in fluid pressures caused by change in body position during bed rest, relative to the gravitational vector. A similar pattern in bone loss occurs in rats subjected to HU.121 Because fluid shifts are an important physiological adaptation to spaceflight, further studies of animals and humans during spaceflight are warranted to allow a better understanding of the mechanisms of bone loss and their possible associations with fluid shifts.
McCarthy122 postulated that the shear forces created by interstitial fluid flow influence bone loss in microgravity. He has created a pneumatic venous tourniquet that can modulate fluid flow within tissues when placed around the ankle of an astronaut or a rat. More recently Yokota and colleagues123 have shown that lateral loading
of joints increases both fluid flow within bone and bone formation. These findings demonstrate the link between bone fluid flow and the maintenance of bone mass. Further research using tourniquets or other means will help to determine to what extent fluid shifts are responsible for bone loss.
Calcium Metabolism and Kidney Stones
Risk factors for renal stone formation (high urinary calcium excretion, low urinary volume) are exaggerated in spaceflight.124,125 Urinary calcium excretion was highly variable both before and during flight, but impressively elevated calcium excretions were noted in several individuals,126,127 and the source was likely increased bone resorption.128 Calcium oxalate and calcium phosphate concentrations during flight increased to levels that favored crystallization.129 Countermeasures should include increased fluid intake to increase urine volumes, but such efforts are complicated by fluid shifts in microgravity, reduced plasma volume, fluid retention from hormonal adjustments, and practical constraints encountered in spaceflight. Studies on agents to prevent reductions in urinary pH and citrate in flight130 and during bed rest131 have been explored, but their effectiveness is uncertain. The key logical countermeasure would be to reduce bone breakdown and thereby achieve a dual benefit. For example, pamidronate both blocked bone resorption and reduced excessive urinary calcium excretion in a bed rest study.132 Nutrition plays a role in calcium metabolism and bone health during long-duration spaceflight that needs further investigation. Astronauts have been reported to consume only 80 percent of the recommended energy intake during long-duration spaceflight, and food restriction has been shown to exaggerate the effects on bone of unloading in animals and humans.133,134,135 Nutrition, a crosscutting issue affecting multiple systems, is discussed in detail in Chapter 7.
Research Models and Platforms
Animal Experiments
An active animal research program is critical both to better understand the adaptive response of bone to weightlessness and to better define risks to the skeletal health of humans in prolonged spaceflight. In addition, animal experiments are necessary to rigorously test pharmacological strategies to control bone loss. Studies of genetically modified mice, such as the sclerostin knockout mouse, provide a means of isolating the importance of specific signaling factors in bone.136,137 Further studies of genetically altered mice subjected to weightlessness, both as ground-based models and on the ISS, are urgently needed.
The sclerostin knockout mouse is an example of the wide variety of highly informative and newly available138 genetically modified animals. In fact, there are several hundred genetically modified mouse strains that selectively delete (gene knockout or replacement) or overexpress (transgenic animals) specific genes and gene products that are important in bone biology.139-144 The rapid pace of advances in molecular biology pertinent to bone metabolism provides the basis for breakthroughs in space biology, thus emphasizing the importance of reinvigorating basic research on bone biology in altered gravity. In ground-based studies, rodent HU is a proven model for disuse and fluid shift caused by spaceflight,145 and this model should continue to be exploited and supported. The ISS would be an excellent platform for spaceflight studies of rodents, if adequate rodent housing facilities were added. Given the breadth of this field, a rigorous selection process will be needed to prioritize the use of particular genetically modified mice, including conditional knockouts, which are best suited to answer the research questions. The limitations in comparing effects in the mouse to those in humans should be carefully considered, and the recent breakthroughs in gene technology in rats may provide alternatives to mice for genetic studies.146,147
Human Experiments
The six-degree head-down bed rest model has been successfully exploited in a number of U.S. and European studies.148,149,150 In human bed rest studies, the rate of bone resorption increased two-fold and bone formation was significantly reduced.151 However, changes in bone formation markers did not reflect the histologic evidence of reduced bone formation.152 This finding calls attention to the relative insensitivity of bone formation markers to detect reduced bone formation. More research on bone cell turnover is needed.
Research Recommendations
Need for More Basic Research
The severe drop in NASA funding between 2005 and 2009 has had a chilling effect on basic research to explain the changes seen in physiological systems during spaceflight. In the ISS era (2000 to the present), the emphasis of NASA research has been on exercise countermeasures to bone loss in humans (see relevant NASA Research Announcements).153
Basic research into the mechanisms of bone adaptation in altered gravity using animal and human models needs to be reinvigorated. The agreement between, and the joint solicitations from, the National Institutes of Health and NASA could be exploited to expand the research focus from primarily operational issues to include fundamental science that will inform future missions. Maximizing the possibilities offered by the ISS National Laboratory over the next decade will also be critical to the future success and safety of long-duration missions.
Recommended Experiments
Human Studies. All experiments listed below can be completed within the next decade.
1. Ongoing human research on the efficacy of exercise to preserve bone during ISS missions should continue. There is a need for studies with adequate statistical power of all the available ISS exercise devices (including the latest devices: the advanced resistive exercise device and T2 treadmill), with accurate quantification of external loads and compliance with prescribed exercise in order to determine whether exercise is an effective countermeasure for bone loss.
2. The efficacy of bisphosphonates and other anti-osteoporosis drugs should be tested on the ISS during approximately 6-month missions in an adequate population of astronauts. The interaction of pharmacological and exercise countermeasures also needs to be studied, since prior use on Earth has invariably involved weight bearing, and only a single study has examined the use of bisphosphonates in the bed rest analog, and the subjects in this study did not exercise.154
3. Use of any drug as a countermeasure during long-term spaceflight will require that the drug can be stored in space without losing its effectiveness. Whether bone-active drugs can be stored in space is currently not known, and this question should be studied further using the ISS.
4. The possibility exists that neither exercise nor currently available pharmaceutical countermeasures will stop bone loss completely during spaceflight. In this case, studies that include exercise and existing or new pharmacological therapies should be undertaken to test the synergy between these countermeasures. These studies should include the evaluation of changes in bone structure and strength.155,156,157
5. The bone health of women during long-duration spaceflight requires further study. The practice of inhibiting menstrual periods during flights of up to 6 months is likely to contribute to marked, and possibly irreversible, bone loss in longer missions. These issues are further addressed in the section of Chapter 7 titled “Biological Sex/Gender Considerations.”
6. Interventions to accelerate skeletal recovery following long-duration spaceflight should be conducted.
7. More studies are required on the lifetime bone health of astronauts who have flown on long-duration missions. In particular, the risks of fracture and renal stones need to be examined and, if found to be elevated, addressed.
8. Future studies should address issues of bone quality and not just bone mineral density, because the former is more relevant to performance and fracture resistance.
Animal Studies. All ground-based studies listed below can be completed within the next decade, but flight studies will be limited by the inadequate animal housing on the ISS.
1. Animal experiments should be conducted on rodents that are skeletally mature for relevance to adult organisms.
2. Studies of genetically altered mice exposed to weightlessness in space and to the newly developed partial-gravity analogs on Earth are strongly recommended.
3. The efficacy of existing and new osteoporosis drugs under clinical development should be tested in animal models of weightlessness (both ground-based and in spaceflight).
4. Fracture healing, methods to improve fracture healing, and effects of antiresorptive drugs on fracture healing should be further evaluated in animal models of weightlessness (both ground-based and in spaceflight).
5. The combined effects on bone of space radiation and altered-gravity should be evaluated in ground-based animal models.
6. The precise cellular signaling mechanisms responsible for initiating increased bone resorption and reduced bone formation in weightlessness should be studied further in animal models of weightlessness.
Consideration should be given to using the rat model because of its successful track record in predicting the actions of pharmacological interventions on human bone and because of new technology to genetically manipulate rats.
Obstacles to Progress
Progress in identifying countermeasures to bone loss during long-duration spaceflight has been hindered by a number of factors that need to be addressed in the next decade.
Historically, the exercise devices that have been flown on the ISS (with the exception of the advanced resistive exercise device158) have undergone limited pre-flight testing to establish their efficacy. This is evidenced by the lack of published studies in the literature. In addition, conversations and direct interaction with NASA personnel indicate that the devices have not had the longevity required to survive programmed use by crew members without large investments of crew time in maintenance. For example, large maintenance and redesign costs are known to have been incurred for repairs of the interim resistive exercise device (iRED) and TVIS. A further issue is whether or not the stimuli provided by the exercise devices are sufficient to generate the required responses to preserve musculoskeletal homeostasis. This has not been the case in the past.159,160
NASA should develop a larger bed rest facility that will allow more rapid evaluation of ground-based simulations of countermeasures with adequate statistical power. As indicated a number of times elsewhere in this chapter, having appropriate facilities on the ISS for conducting animal studies is also an important need.
Pharmaceutical countermeasures include bisphosphonates, but two rare potential problems with this class of drugs have received much negative public attention and may have prevented their more widespread use. Atypical subtrochanteric femoral fractures have been reported after long-term use.161 Osteonecrosis of the jaw has also been observed, but the frequency from bisphosphonate use is generally agreed to be quite low, estimated to be 0.7 per 100,000 person-years of exposure.162,163 The American Dental Association has published guidelines that propose careful examination of patients for underlying dental conditions. Despite this recommendation, community dental care has been denied to individuals (including astronauts) who have used bisphosphonates. Thus the negative perception of some of the rare side effects of bisphosphonate use has prevented more widespread use of this class of drugs among astronauts. NASA should help allay concerns by assisting with careful selection of dentists who do needed dental work for astronauts in advance of bisphosphonate dosing and agree to take on dental care later if required. Further exploration of the effects of artificial gravity on bone is also warranted. A specific discussion of the use of artificial gravity as an integrated countermeasure for a wide range of systems can be found in Chapter 7.
Risks for Skeletal Muscle During Long-Duration Spaceflight
While the skeletal (bone) system evolved to provide a solid foundation in animals and humans in opposing the force of gravity during weight bearing, the skeletal muscle system, which is the largest organ system of the body, also evolved in response to gravity. The skeletal system developed the capacity for generating high-force contraction processes not only to synergize with bone in opposing gravity but also to enable individuals to perform a wide range of activity patterns under normal gravity loading conditions such as running, jumping, lifting, and moving heavy objects. Hence, over millions of years mammalian skeletal muscle fibers evolved into two general
functional types, referred to as motor units. A motor unit consists of a group of fibers of relatively similar structural and functional properties that is innervated by a common neuron. Motor units can be classified as either slow contracting (type I) or fast contracting (type II).164 These contrasting fibers types, under the influence of the nervous system, account for the great diversity in activity pattern that humans and animals can achieve in transitioning from the physiological state of inactivity to activity of varying intensity.
Similar to the bone system, both the slow and fast muscle fiber types are negatively affected by reduced gravitational loading, which occurs during spaceflight, as well as along the long axis of the skeleton in ground-based analogs such as prolonged bed rest. Therefore, the goal of this section is to summarize what is known and not known about the risks of spaceflight for the skeletal muscle system.165 The concern is that when human and animal subjects are exposed to microgravity, their lower limb and core trunk muscles atrophy and lose strength and stamina, thereby reducing the fidelity of movement spanning a wide range of activities.166,167,168 This, in turn, can negatively affect the overall fitness of the astronaut when functioning in gravity environments, whether on Earth or other celestial bodies.169 These alterations in muscle structure and function were clearly identified by the 1998 NRC report A Strategy for Research in Space Biology and Medicine in the New Century170 and are elaborated further in this section.
Effects of the Spaceflight Environment on the Structure and Function of the Skeletal Muscle System
Muscle Mass
Rodent Studies. Exposure to microgravity during the Russian Cosmos Program and NASA Space Lab missions showed that skeletal muscle fibers rapidly atrophy. This alteration occurs principally in the soleus (ankle plantar flexor), the vastus intermedius (deep quadriceps knee extensor), and the adductor longus (femur adductor) muscles, all of which predominantly express slow type I fibers.171,172 The muscle atrophy of these slow muscles is greater than that of their fast type synergists such as the gastrocnemius and vastus lateralis muscles.173,174 As much as a 40 to 45 percent loss in muscle fiber mass/size can occur in the soleus muscle, depending on the duration of the unloading state.175 As a result, both slow and fast muscle fibers shrink in size.176 The ground-based analog for spaceflight involving rodents is the HU model.177-180 This analog is described in the previous section. Interestingly, this model mimics the muscle loss seen in response to spaceflight, suggesting that HU is a good model to undertake studies on the rodent skeletal muscle system, given the current lack of opportunities to study animal subjects in space.
Human Studies. A similar response of muscle wasting has been reported in humans for muscles such as the soleus and the vastus, thereby resulting in loss of muscle volume and muscle fiber size.181-184 However, in humans the reduction in fast fiber cross-sectional area can equal or even exceed the loss in slow fiber size.185 Since the fast fibers are larger than the slow fibers in humans, it appears that the larger fibers may be more susceptible to the unloading stimulus. In bed rest studies, which are the primary analog to mimic spaceflight microgravity conditions in humans, losses in muscle mass (volume) and reductions in the size of the individual fibers closely resemble the responses seen in both short-duration spaceflight on the space shuttle and long-duration missions on the ISS.186 These losses in muscle fiber mass are the signature alteration affecting muscle fiber homeostasis.
Alterations in Protein Balance, Expression, and Contractile Phenotype
Rodent Studies. The Cosmos and shuttle spaceflight animal studies have provided insight on alterations in the subcellular muscle protein milieu.187-190 The myofibril fraction, which accounts for more than 50 percent of a muscle’s total protein pool, is the primary target for degradation, especially of the key proteins such as myosin heavy chain (referred to as MHC) and actin,191 which govern force development and hence the strength of the contraction.192,193,194 Also, there are shifts in MHC isoform gene expression,195-198 showing that the slow MHCs become repressed while the fast type II MHCs are turned on, which indicates a significant shift from a slow antigravity to a faster contractile phenotype. Moreover, such studies in spaceflight conditions were corroborated by
studies using the rodent HU model.199 Taken together, it is apparent that the HU model is an important analog to the spaceflight environment in terms of altering muscle mass, strength, and contractile phenotype.200-203 Unfortunately after the completion of the NASA flight program in 1998 involving animals, there has been little further progress in ascertaining the effects of long-duration spaceflight on the homeostasis not only of the skeletal muscle system but also of other important systems such as bone, cardiovascular, pulmonary, sensory-motor, and immune systems. This has left a tremendous void in understanding the biological processes governing muscle atrophy and phenotype plasticity in response to long-duration spaceflight missions.
Human Studies. In the early 1990s, Edgerton and colleagues were the first to obtain biopsy samples from astronauts before and after short-duration shuttle spaceflight missions (5 and 11 days).204 Their findings suggest that shifts in slow to fast MHC gene expression also occur in humans. Additional studies obtained from missions of longer duration revealed that individual fibers demonstrated lower force per cross-sectional area as well as shifts to fast type IIa and IIx MHC expression.205,206 Such losses in muscle mass and shifts in contractile phenotype have important functional consequences as presented below.
Functional Alterations in Skeletal Muscle
Rodent Studies. While only a few studies have been performed to examine the functional properties of rodent muscle immediately following spaceflight, these studies clearly show that there are alterations in the contractile processes as delineated by force-velocity tests involving the antigravity soleus muscle.207,208 These alterations involve (1) a reduction in force output for any given velocity of contraction, (2) a reduction in power output, and (3) a decrease in the resistance to fatigue in response to repetitive contraction output. These observations of reduced function are consistent with the atrophy process and the transformation from a slow to faster contractile phenotype as discussed above. In additional studies, there is strong evidence that the slow muscle fibers show evidence of susceptibility to injury as a result of initially readapting to the normal gravity environment.209,210 Collectively, these observations suggest that the performance of individuals undertaking physical activity in a gravity environment could be compromised and that the muscle could be prone to further injury in performing tasks demanding high functional output. Such deficits are illustrated by marked changes in rodent posture (low center of gravity), as well as the extensive use of the tail for support. Also, there is an inability to move quickly while pushing off from the balls of the feet for locomotion.211 Thus, these observations point to deficits in the sensory-motor system of rodents following spaceflight that warrant further investigation, especially in response to long-term spaceflight.
Human Studies. Studies on humans following both spaceflight and ground-based bed rest exposure demonstrate alterations similar to those reported in rodents. The signature response involves a reduction in absolute strength of the target muscle group and decrements in the torque-velocity relationship.212-215 These functional alterations appear to be greater than the deficits in muscle mass, especially early on in the time course of spaceflight.216 The differential responses in muscle strength could be due, in part, to sensory-motor alterations, which impair the nervous system’s ability to recruit motor units in response to high loading stimuli. Individual-fiber analyses further suggest that the loss in force capability could also be due to deficits in the intrinsic properties of the myofibers.217,218 Also, there appears to be a wide range of response in such muscle function deficits among human subjects.219,220 Whether such diversity is due to the responsiveness of astronaut subjects to the unloading state or to differences in countermeasure strategies that are being employed among the astronaut subjects remains to be determined. (Note: astronauts do not perform a prescribed exercise routine.) In humans, little information is available as to whether skeletal muscle is prone to injury during early recovery from spaceflight. However, significant soreness has been reported anecdotally by astronauts; such soreness could impact high-intensity emergency egress capability during the early recovery period.221 In a previous report on astronauts and cosmonauts following spaceflight of varying duration, evidence based on magnetic resonance T2 analyses suggested that muscle injury was probably occurring in some of the subjects during the early stages (days) of recovery.222
Key Synergies with Other Systems
Bone. It is well recognized that both skeletal muscle and bone homeostasis are negatively affected by prolonged exposure to spaceflight as well as to ground-based simulations of spaceflight.223,224 As noted in the bone section, pre- and post-flight quantitative computed tomography analysis has shown that long-duration spaceflight missions induced average volumetric BMD losses of about 0.9 percent per month and 1.4 percent per month in the spine and hip, respectively.225,226 Findings on skeletal muscles similarly suggest a range of atrophy averaging about 6 percent to 8 percent per month, also with greater losses in the lower extremities compared with the upper extremities.227 These respective deficit profiles for bone and muscle actually exceed what is observed during the aging-induced disorders of osteoporosis of bone and muscle sarcopenia. Hence, the question arises as to whether the structural and functional integrity of the two systems are physiologically linked.
Recent bed rest study findings provide evidence that the mechanical stress strategically imposed on skeletal muscles by physical exercise during spaceflight or ground-based analogs can have a positive impact on the homeostasis of bone. However, pharmacological strategies specifically targeting bone homeostasis do not synergistically affect skeletal muscle.228 These findings suggest that while both resistance exercise (RE) and bisphosphonate treatment (an inhibitor of bone resorption) have a positive effect on bone homeostasis, only the RE treatment has a positive impact on both skeletal muscle and bone, particularly in those regions where the mechanical stress on the muscle system is enhanced.
Similar findings were provided by Shackelford et al.,229 who compared bed rest plus RE with bed rest alone. The RE consisted of a vigorous loading program targeting multiple muscle groups for a period of 17 weeks. Volitional strength increased significantly compared to pretraining values in the RE group, whereas it declined in the bed rest control group. Losses in muscle mass across the muscle groups were significantly less than that which occurred for the control group, indicating that muscle atrophy was markedly retarded by the RE program. Interestingly, losses in BMD were significantly less in the RE group than in the controls. In fact, in the calcaneal region the BMD was actually increased somewhat over the pre-exposure values, indicating that RE can have a powerful impact on bone even under unloading states. These studies point to the potential positive value of RE programs, when carried out under appropriate training conditions, in reducing the deleterious effects of chronic unloading on muscle strength and muscle and bone mass.
Sensory-Motor. As noted above, during the early stages of unloading (as seen in microgravity and bed rest), muscle group strength is compromised before significant muscle atrophy occurs, providing evidence that the ability to recruit motor units likely is compromised during the early stages of unloading states. In animal studies, locomotor patterns are compromised, as reported above. These observations indicate that the combined skeletal muscle and sensory-motor systems are highly integrated; dysfunction in either system has deleterious consequences when the systems are challenged following spaceflight. In the future, the two systems should be studied as a functional entity. Such research not only should examine muscle structure and performance but also should examine function originating from different areas of the cortex, the activation of muscle motor units, and the properties of the neuromuscular junction, in order to dissect the complete pathways in the control of movement.
Status of Countermeasures
Animal Studies. Studies on animals have used a variety of manipulations to counteract atrophy responses induced by HU. The most physiologically relevant to the human resistance exercise program involves two different resistance loading paradigms: one employed a paradigm of repetitive isometric contractions;230 the second used a sequenced combination of isometric, concentric, and eccentric muscular actions during each contraction cycle.231 Both studies used an experimental strategy of studying the effectiveness of the training paradigm during the rapid state of atrophy, which occurs during the first 7 to 10 days of HU, during which the rodent gastrocnemius muscles atrophy by approximately 25 percent. The isometric-only paradigm was not fully successful in maintaining muscle mass. This result was attributed to imposing an insufficient amount of loading stimuli on the muscle. This interpretation was supported by the inability of the muscle to maintain sufficient signaling pathway stimuli to optimize protein synthesis capability.232 However, in the second study involving the integrated contraction mode
paradigm,233 muscle mass and contractile protein concentration and content were maintained. This response was consistent with the muscle maintaining a normal protein translational signaling cascade. These studies point to the potential for using RE loading that targets different muscle groups in order to mitigate muscle atrophy.
Human Studies. During spaceflight on the ISS typically spanning 6 months, NASA astronauts are instructed to perform an activity regimen of their own choosing, typically consisting of combinations of treadmill exercise with a loading support system, cycle ergometry, and RE. As noted by Trappe et al., there is no specific prescription that each subject follows.234 Also, no control group is used as a reference. Thus, it is difficult to compare responses among the subjects as to which exercise combinations are most effective. Given these caveats, the general consensus indicates that the current paradigms being used are not fully successful in preventing muscle strength loss, muscle fiber atrophy, and the contractile phenotype shift from slow to fast properties, e.g., the muscle system alterations that are well documented.235,236 However, there is evidence that a combination of endurance and resistance loading does have a significant positive effect on some of the subjects.237,238
Bed Rest Studies. Recent bed rest studies have provided important information concerning the effects of RE on skeletal muscle homeostasis. The importance of these studies is that (1) bed rest results in muscle alterations that are similar in scope to that seen in spaceflight;239-243 (2) control bed rest groups were used routinely to serve as a reference to any countermeasure paradigm imposed; (3) the studies mimicked the time frame that has been examined on the ISS; and (4) novel RE equipment and paradigms were used, including a flywheel device that imposes high concentric/eccentric forces on the target muscle groups, e.g., imposed loads that are greater than those apparently attained with the equipment currently used on the ISS. For example, on the ISS an interim resistance exercise device (iRED) is used for loading skeletal muscle. Its loading unit consists of two canisters capable of producing loads up to about 68 kg per canister (a loading amount insufficient for loading large muscle groups). A known limitation of the iRED is the inability to precisely set the load and quantify the workloads.244 Overall, the results show that the quadriceps muscles can be maintained at normal volume (size) with the training paradigms imposed.245 However, with regard to calf muscle, especially the slow soleus muscle, the findings indicate that this muscle cannot be maintained at both normal mass and functional capacity.246 This result suggests that a more robust training program is needed to protect the calf muscles. On the other hand, there is encouraging evidence that current paradigms are effective in maintaining the slow contractile phenotype in soleus muscle.247
Experiments Using Alternative Loading Countermeasures
A new type of countermeasure, explored recently by NASA in a pilot study, employs the principle of artificial gravity (AG) or hypergravity. This pilot study used a short-arm centrifuge to expose bed rest subjects for 1 hour per day to a dose of acceleration force vector that was directed from the head to the feet (2 g at the foot).248 A separate control group of bed rest subjects was given a simulated posture without the applied 2 g force. While this study focused on several systems including bone, cardiovascular, sensory-motor/vestibular, and skeletal muscle, this section discusses only the analyses that focused on the skeletal muscle system. Post/pre-torque-velocity determinations revealed greater decrements in knee extensor performance in the control group than in the bed rest plus AG group. Also, muscle strength in the AG group was preserved, especially in the calf muscle groups, because the plantar flexors of the AG group actually produced a net gain in torque-velocity properties, whereas the control group showed a significant decrement. While these findings provide evidence that AG has potential as a countermeasure, it should be noted that other devices also may have beneficial effects on skeletal muscle and other organ systems (see the section “Interaction of Muscle and Other Systems”). While AG suggests promising opportunities for sustaining skeletal muscle homeostasis, more research is needed regarding the potential impact of AG on other organ systems such as bone, immune, and cardiovascular systems. Further, it remains to be seen how AG paradigms applied to animal subjects affect both developmental processes and other fundamental processes across the organ systems.
Gaps in Knowledge
High-Priority Gaps in Fundamental Knowledge: Animal Studies
Contractile Protein Turnover. It is well known that maintenance of muscle mass depends on the balance between processes regulating the synthesis of contractile proteins and processes governing their degradation. This is normally a continuous balancing process, referred to as “protein turnover.” Although great strides have been made in understanding protein turnover, more research is critical to unravel the cascade from the perspective of identifying (1) the mechanoreceptor system(s) that are sensitive to gravity stimuli and (2) the downstream signaling pathways and transcription factors that control the expression of the contractile protein genes comprising the contractile muscle synthesis apparatus. Recent findings on atrophy of rodent muscle in response to unloading suggest that transcriptional regulation of both myosin and actin (the two most abundant proteins expressed in muscle) may be the pivotal link, because these genes are rapidly turned off within several hours of initiating unloading stimuli. Figure 6.1 shows the rapidity with which the two most abundantly expressed genes in slow antigravity muscle fibers, that is, slow type I myosin and actin, are markedly inhibited within 24 hours of unloading. These two proteins are the drivers of the muscle contraction process. This loss in expression causes a reduction in the ability of the muscle fibers to maintain muscle size, which is also shown in Figure 6.1. These findings thus provide new insight concerning target areas that must be regulated in order to maintain skeletal muscle homeostasis.
To degrade the contractile proteins, each protein must be separated from its intact cohesive myofibril structure and targeted and labeled for degradation,249 but very little information is known about these latter critical steps of the degradation process. Thus, additional studies on protein turnover are pivotal to understanding the homeostasis of muscle in response to altered loading states.
Substrate Energy Turnover. Studies on rodent skeletal muscle metabolic pathways in the context of spaceflight and other states of unloading have revealed a variety of responses with no clear-cut adaptive responses in the oxidative enzyme systems.250,251,252 For example, Baldwin et al.253 observed that in rodent skeletal muscle immediately following spaceflight, no reduction occurred in the capacity of skeletal muscle mitochondria to metabolize pyruvate, a derivative substrate of carbohydrate. However, they found a reduction in the capacity of different muscle types to oxidize a long-chain fatty acid, palmitate. This latter finding is in agreement with an observed increase in the accumulation of lipid in the fibers of skeletal muscles exposed to spaceflight.254 Also, the metabolic pathway
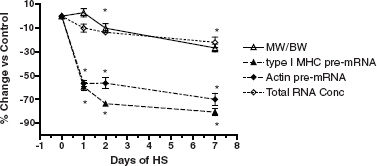
FIGURE 6.1 Time course of the change in the soleus after 1, 2, and 7 days of hindlimb unloading (HU) in rodents. Percentage change versus control for soleus muscle weight (relative to body weight), total RNA concentration, type I MHC pre-mRNA, and skeletal α actin pre-mRNA expression. Asterisk (*) is for P < 0.05 versus control. SOURCE: Data from J.M. Giger, P.W. Bodell, M. Zeng, K.M. Baldwin, and F. Haddad, Rapid muscle atrophy response to unloading: Pretranslational processes involving MHC and actin, Journal of Applied Physiology 107:1204-1212, 2009.
for glucose uptake is increased in muscles exposed to reduced gravity.255 Although data on enzyme activity are equivocal, it is possible that as a result of a shift in substrate preference in response to reduced gravity, carbohydrates are used preferentially to provide energy to support muscle contraction. Further studies are needed that focus on organismal metabolic processes in humans during long-duration spaceflight and/or bed rest to ascertain (1) if there are intrinsic lesions in the mitochondrial system limiting metabolism of fatty acids and (2) if alterations in the substrate preference for sources of energy production during exercise in spaceflight under varying loading conditions affect the utilization of carbohydrates during prolonged exercise, such as during extravehicular activity on different planets with reduced-gravity conditions.
The Role of Reactive Oxygen Species, Satellite Cells, and Growth Factors in Protein Balance Regulation. Recent findings suggest that treatment with antioxidants can slow the atrophy of unloaded respiratory muscles involved in breathing.256 Further, any stimuli to improve protein balance appear to be predicated on expressing Insulin-Like Growth Factor-1, which functions as an autocrine-paracrine regulator within skeletal muscle and plays a regulatory role in muscle growth, even in the absence of the pituitary-growth hormone axis.257,258 This growth factor is thought to regulate satellite cell proliferation and differentiation, which play a role in maintaining protein balance in muscle tissue during both hypertrophy and atrophy.259,260 These observations suggest that this factor, along with others such as reactive oxygen species and other growth regulators such as myostatin, may play an integrative role in buffering muscle atrophy stimuli if such target genes are fully activated. Studies on this topic in animal models using the HU model could be of value, as they would focus on limb muscles that are sensitive to gravity.
Maintaining the Slow Contractile Phenotype. One of the key questions in muscle biology concerns the mechanism of contractile phenotype switching that occurs in response to muscle unloading. New findings on this topic in the evolving area of epigenetics may provide keys to understanding such mechanisms of gene switching.261 Epigenetic gene regulation phenomena constitute an evolving field in the study of transcription regulation that involves altering the function of DNA without changing its nucleotide sequence. Instead, alterations are generated in the chromatin, histone, and DNA structural properties by a variety of processes that respond to environmental stimuli. These alterations in turn result in alterations in the transcriptional activity of the target gene. Thus, the field of epigenetics may provide insights into understanding contractile protein expression. Studies on this topic could be easily accomplished using the HU model.
High-Priority Gaps in Applied Knowledge: Human Studies
Highly loaded resistance exercise is a promising countermeasure for ameliorating loss in muscle mass and strength, but additional research is needed to develop (1) the appropriate training devices and (2) the optimal prescriptions to maintain muscle homeostasis and sensory-motor function. These latter properties are pivotal for complex movement paradigms such as performing a variety of extravehicular activities in space, as well as performing emergency egress from the spacecraft on landing. Further, it is most probable that those exercise paradigms that benefit the skeletal muscle system will also affect the functional capacity of other systems in the context of integrated organ system function. It is imperative to design modalities and exercise protocols that can condition the body for cardiovascular/aerobic fitness, skeletal muscle strength and endurance, and sensory-motor fidelity, as well as bone and connective tissue homeostasis. Such broadly effective exercise modalities would further the strategy of designing optimal exercise prescriptions for improving the widest range of physiological systems. To NASA’s credit, since 2007 NASA/NSBRI has indicated, in its Research Focus Announcements262 soliciting ground-based research studies for human health in space, a keen interest concerning applications related to new exercise devices that can affect multiple physiological systems. This is an important direction for advancing knowledge of how to enhance countermeasures mitigating the deleterious effects of long-duration spaceflights on astronaut health and performance.
Research Models and Platforms
Animal Research
Ground-based research on animal models such as rats and mice have played a major role in generating fundamental knowledge concerning the effects of microgravity on muscle alterations and in developing countermeasures to microgravity-induced alterations in muscle mass, phenotype, and function. This fundamental research can be continued using the HU model.263,264,265 As discussed above, new avenues of animal research are unfolding in the fields of epigenetics of gene expression and protein turnover in response to unloading stimuli.
Equally important is the capacity to expose adult mammals such as rodents to spaceflight for long durations, because this is the only model in which to study long-term effects on the physiology of the organism without any interference from the countermeasures that are obligatory when human subjects are tested. Furthermore, normal adult mice, as well as mice with either knockout or overexpressed genes, can provide fundamental information about skeletal muscle function in microgravity (see discussion of this topic in the section above, “Risks for Bone Loss During Long-Duration Space Missions”). For example, one can examine the time course of the alterations and compare such deleterious effects with effects on ground-based control animals of the same age and sex. Also, given a capability to bring test animals back to Earth following long-term exposure to microgravity, their recovery process across several physiological systems can be studied to ascertain whether there are changes in response to long-term microgravity that are not reversible with re-exposure to a normal 1-g environment. Currently, the lack of an animal facility for rodents on the ISS suitable for long-duration studies on adult animals is a major research impediment that will hamper the ability to obtain information important for maintaining astronaut health and fitness for duty. Furthermore, research on animal models will be constrained without the ability to manipulate the gravity variable as a factor modulating the fundamental processes underlying organ system homeostasis.
Human Research
Currently, appropriate flight opportunities on the ISS, as well as ground-based analogs (bed rest and other models of unloading) are available to pursue both basic biological science and translational science initiatives. The key is to take greater advantage of the ISS as a platform for conducting long-duration studies on the effects of countermeasures and interactions of different countermeasures in terms of human health and performance. For example, exercise equipment with a greater potential to improve the functional capacity of more than one physiological system needs to be tested. The key is to design facilities that can accommodate a variety of exercise modalities and to perform integrative studies that simultaneously monitor the functions of multiple organ systems. Mechanistic studies on humans are essential to ascertain how the results of mechanistic research in animal models translate to human organ systems.
High-Priority Research Recommendations
Animal Studies
1. Studies should be conducted to identify the underlying mechanism(s) regulating net protein balance and protein turnover in skeletal muscle during states of unloading and recovery. These studies are essential to understanding the process of muscle wasting. Such studies should examine the roles of growth factors, hormones, signaling pathways, protease and myostatin inhibitors, possible pharmacological interventions such as antioxidants, and nitric oxide signaling. This research could potentially be concluded in a 10-year time frame and is not dependent on access to space if the HU model is used.
2. Studies should be conducted to ascertain the regulatory processes controlling expression of the contractile protein genes in response to states of unloading. The focus should be activity-induced transcriptional mechanisms with a thrust toward the evolving field of epigenetics. These studies are needed because the slow contractile phenotype is repressed during exposure to both spaceflight and chronic bed rest, thereby jeopardizing muscle function and movement fidelity. Such research, using the HU model, could be conducted on rodents within an 8-year time frame.
3. Research should be pursued on small animal systems that can delve into the developmental biology of
the undifferentiated skeletal muscle system, as well as other organ systems.266,267 Early studies during the Neurolab project clearly demonstrated that skeletal muscles fail to grow and develop when infant rodents are flown in space.268-272 The same impediment is seen for other systems such as bone and sensory-motor development. A major gap in developmental biology studies on microgravity/spaceflight is that there are no suitable mammalian models to fill in the evolutionary gap currently linking very small living creatures (e.g., worms) to the human species. This is a major flaw that needs correction.
Human Studies
Studies should be undertaken to (1) develop and test new prototype exercise devices; (2) optimize physical activity paradigms/prescriptions targeting multisystem countermeasures, preferably with the same training device; and (3) assess substrate energy turnover during exercises of different intensities as a function of prolonged spaceflight. Also, NASA should continue to explore the possibility of alternative devices (e.g., flywheel, lower-body negative-pressure/running, rowing, and artificial gravity) that have the potential to affect multiple organ systems, in addition to more recent conventional exercise equipment (such as the advanced resistive exercise device (ARED) and combined operation load bearing external resistance treadmill (COLBERT)). As discussed in the preceding sections, these studies are important because the current exercise devices and corresponding physical activity countermeasure prescriptions are reported to be insufficient for optimally maintaining physical fitness and organ system homeostasis. Depending on how rapidly such new devices come onboard, this research could be completed within 15 years.
Risks for Sensory-Motor and Vestibular Deficits During Long-Duration Spaceflight
While this section is titled “sensory-motor and vestibular function,” the sensory-motor functions covered encompass all sensory systems, e.g., vision, proprioception, pain, and even odor and taste. The central nervous system continuously monitors environmental elements that provide the responses needed to survive, such as gathering food and eating, reproducing, and so on. Traveling safely for extended times in different gravitational environments depends on the successful integration of multiple sensory and motor systems so that movement can be controlled effectively and safely. Precision in movement is critical in that even small errors in control of movement during flight maneuvers, extravehicular activities, controlling robotic devices, and traversing the surface of the Moon while in a spacesuit can have serious consequences. Thus from a safety perspective preservation of well-controlled movements within these variable environments is of utmost importance.273 For example, neural control of movement in the spaceflight environment must take into account not only the vestibular sensory system but also information related to the dynamics of the interaction of head/neck, trunk, and limb muscle positions, as well as control of eye position.274,275 Full awareness of the integrated function of the sensory-motor system is likely to provide a clearer perception into the problems and solutions related to the performance of humans in space and to their postflight recovery. A well-quantified illustration of this integrative function in postural control is reported by Speers et al.,276 who showed that the instabilities of posture and gait following spaceflight resulted at least in part from highly interactive adaptations of each of the sensory inputs associated with vestibular, proprioceptive, and visual systems.
Effects of the Spaceflight Environment on the Function of the Sensory-Motor System
Mismatch of Functional Integration of Sensory and Motor Circuits
In travels from 1 g to microgravity and back to 1 g, disturbances in occulomotor control, vestibular function, pain sensitivity, muscle stretch sensitivity, joint position sense, and cutaneous sensitivity to vibration have been observed.277-287 All of these sensory systems must be integrated effectively to successfully control movement and position.288 The numerous and consistently observed changes to the responses in gravitational fields altered from 1 g demonstrate that the sensory-motor system is essentially calibrated for a 1-g environment. Illusions of movement of self relative to the environment reflect a mismatch between the expected and real patterns of sensory information.289,290,291
Upon withdrawal of a vertically oriented gravitational force on the whole body, it has been observed on Mir, space shuttle missions, and the ISS that a general state of flexion mechanically and neurophysiologically is assumed, with the exception of the ankle.292,293 This change in posture undoubtedly involves a new state of activity of muscle spindles, Golgi tendon organs, and other proprioception afferents that would occur in conjunction with altered vestibular control in the absence of the gravitational loading that normally occurs at 1 g. While the ankle position tends to become more plantarflexed mechanically in the 1-g environment, the neural response is one of active dorsiflexion; that is, upon removal of weight bearing, the tibialis anterior, the primary dorsiflexor of the ankle, becomes much more active than when the individual is in a standing position in a 1-g environment. Thus, the flexors, including the ankle—i.e., dorsiflexors, not plantar flexors—become hyperactive, and remain so for prolonged periods during spaceflight.294 The most logical explanation for this relatively plantar-flexed mechanical position in the ankle in spite of the hyperactivity of the dorsiflexors involves the balance of force generated passively by dorsiflexors versus plantar flexors of the ankle.
Numerous experiments have demonstrated disturbances in balance capability for several days or more following return to 1 g. Postural responses before and after spaceflight reflect changes in vestibular function and its interactions with vision when traveling from 1 g to microgravity and back to 1 g.295,296,297 The variability in the magnitude of these disturbances in different crew members has been substantial, and the differences are not readily attributable to the length of duration of the spaceflight.298 Perhaps such variability in response to gravity changes is related to the consistency of adhering to a given type of countermeasure.299
Extensive attention has been paid to the role of vestibular dysfunction in microgravity as having a key role in causing motion sickness.300,301 It has been hypothesized that this sensory mismatch compared to 1 g is the reason for motion sickness. Although this is an appealing concept, it has never been tested stringently. Many unanswered questions remain about the role of the vestibular system in the process of adaptation to variable gravity, but future priorities related to neuroscience must be considered from a broader perspective: not only how the vestibular system responds to the challenges of space missions but also how understanding this system could provide insights into fundamental issues regarding other organ systems.
Functional Recalibration of Sensory-Motor Circuits Controlling Flexion Versus Extension
Modulation of monosynaptic and polysynaptic reflexes has been observed both during and following flight.302,303 There have been shifts in the relative activation of motor unit pools associated with flexion versus extension and with fast versus predominantly slow motor pools when tested at 1 g after returning from a microgravity environment lasting for days to weeks.304,305 For example, when a rhesus monkey walked on a treadmill after only 12 days of spaceflight, the relative recruitment of fast muscles was significantly elevated compared to preflight, while that of slow muscles was depressed (Figure 6.2). There was a marked decrease in the maximum force that could be generated voluntarily, and most of this decrease appeared to be neural in nature rather than muscular.306,307 Also, in rats, monkeys, and humans, loss of extensor muscle function significantly exceeds that of the flexors. As observed in crew members, the susceptibility to the activation of sensory-motor circuits becomes more biased toward flexion compared to extension and more biased to fast versus slow muscles and motor units within a period of only 2 weeks of exposure to a microgravity environment. This means that the neural circuitry for controlling locomotion has adapted by selectively changing the translation of sensory input to motor output and therefore must require some combination of rapid re-adaptation and compensatory modulations that can correct for the acquired changes that occurred in microgravity.
Gaps in Knowledge
The chronic patterns and levels of activity of sensory-motor circuits define to a large degree the efficacy of neuronal pathways and the interlinking of these different circuits. These chronic patterns shape the ability to perform any routine or specialized motor task with some well-defined level of predictability of success.308 These patterns of activity are also known to modulate nerve growth factors and endocrine responses, both of which play supportive roles in neural reorganization of circuits and the homeostasis of multiple tissues.309 Clearly, the pattern and amount of activity play an important role in the homeostasis of the metabolic and contractile properties of skel-
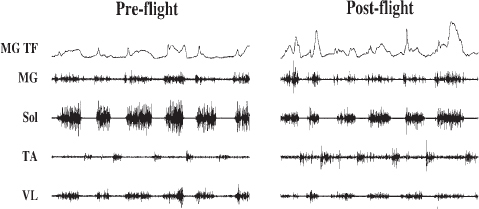
FIGURE 6.2 Medial gastrocnemius tendon force (TF) and raw electromyographic signals of the medial gastrocnemius (MG), soleus (Sol), tibialis anterior (TA), and vastus lateralis (VL) muscles from a Rhesus monkey are shown during pre-flight and post-flight stepping after 12 days of spaceflight. SOURCE: M.R. Recktenwald, J.A. Hodgson, R.R. Roy, S. Riazanski, G.E. McCall, I. Kozlovskaya, D.A. Washburn, J.W. Fanton, and V.R. Edgerton, Effects of spaceflight on rhesus quadrupedal locomotion after return to 1G, Journal of Neurophysiology 81:2451-2463, 1999, reproduced with permission.
etal muscles.310 These neuromuscular activity patterns in turn modulate the homeostasis of the connective tissues that transmit stresses and strains associated with muscle function, which, in turn, affect intra- and extra-muscular connective tissues such as aponeuroses and tendons, as well as bone. Therefore, some “dose” of neuromuscular activity-exercise of the neural pathways affected in spaceflight is one of multiple potential solutions for maintaining homeostasis and function within and among multiple gravitational environments.
The critical information needed to formulate an activity-based strategy for spaceflight is to know the dose-response features of alterations of these tissues to unloading stimuli. Important progress has been made in some ground-based models, particularly for muscles. But there has been little or no opportunity to systematically define this dose-response relationship for different tissues in the space environment, primarily because of the lack of programmatic synergies involving bone, muscle, and sensory-motor integrative research, as noted previously.
Careful documentation of changes in the activity patterns of specific muscle groups is important for any countermeasure. For example, the levels of muscular activity of a major flexor, the tibialis anterior (TA), and an extensor, the soleus, throughout a 17-day spaceflight were markedly elevated compared with either pre- or post-flight (Figure 6.3). In a given 24-hour period the soleus muscle was more than twice as active during flight compared to pre-or post-flight, and the activity of the tibialis anterior was as much as 50 times greater. These results illustrate why one cannot assume or even expect that spaceflight is associated simply with a reduction in activity level. They suggest that the mechanisms underlying the common changes in sensory-motor performance are unlikely to be attributable simply to low levels of activity of the neural control circuits when exposed to microgravity or other features of the space environment. From a general perspective, one can reasonably claim that an integrative approach has been the mode of operation throughout spaceflight endeavors. However, when evaluating the details, critical failures at multiple levels have persisted, and these shortcomings have precluded a reasonable rate of progress toward solving the problems at hand.
Thus, a critical need is better understanding of the amount and patterns of activity that can maintain reasonably normal properties of the sensory-motor circuits, muscle, connective tissue, and hormonal and growth factor components known to play a role in the homeostasis of these tissues.311,312 Research to answer these questions should incorporate experiments ranging from the molecular control of specific proteins, transcription factors, etc., to in vivo experiments on mammals in the space environment. Significant progress toward this goal can be made most rapidly with a systematic, sustained, and well-planned effort.
All of the modulations of sensory-motor function that have been documented demonstrate that the neuromus-
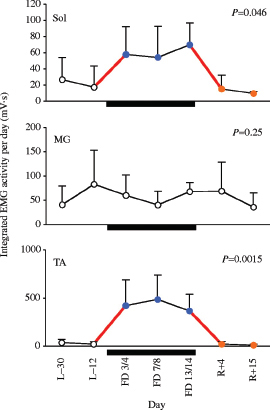
FIGURE 6.3 Mean integrated electromyographic activity (millivolt-seconds, mV·s) observed over the entire recording day for the soleus (Sol), medial gastrocnemius (MG), and tibialis anterior (TA) muscles for test days pre-flight (L-30 and L-12), in-flight (FD 3/4, FD 7/8, and FD 13/14), and post-flight (R+4 and R+15). The filled bar on the x axis indicates flight days. Values are mean values and ± standard error of the mean. SOURCE: V.R. Edgerton, G.E. McCall, J.A. Hodgson, J. Gotto, C. Goulet, K. Fleischmann, and R.R. Roy, Sensorimotor adaptations to microgravity in humans, Journal of Experimental Biology 204:3217-3224, 2001; available at http://jeb.biologists.org/cgi/content/full/204/18/3217, reproduced with permission.
cular system can adapt rapidly to varying gravitational environments. Nevertheless, it is necessary to determine the magnitude and pattern of adaptations that undoubtedly reflect the duration of exposure to a given gravitational environment and the activities that are performed while in a novel gravitational environment.313,314,315 Similarly the rate and magnitude of these modulations will vary in duration following return to a 1-g environment.
Based on the experience to date on the ISS, it appears that all of the adaptations in the sensory-motor system to microgravity and back to 1 g can be accommodated safely, if appropriate accommodations are made in the transition to different gravitational environments, at least for the duration of the flights experienced thus far.316 Whether these adaptations become more critical in more prolonged flights is completely unknown.
Consequently, for very long-duration flights, as would occur on a trip to and from Mars, it seems prudent to accept the ability of the sensory-motor system to adapt to the new gravitational environments encountered, but to develop countermeasure strategies to adapt to these new environments. To do this, one needs to know the time course and the specific changes that occur, as well as ascertaining the countermeasure strategies needed to modulate the specific sensory-motor functions that are so responsive to changes in the gravitational environment. It seems unlikely that a single or even a few countermeasure interventions can address each of the adaptations that must occur among the different organ systems.
Status of Countermeasures
While the technical capability and scientific expertise exist to understand important mechanisms of sensory-motor function under different gravity states, there has been little integration between the study of the skeletal muscle and sensory-motor systems to unravel how each system impacts the other. As a result of this lack of integrated research, minimum progress has been made toward developing countermeasures to avoid dysfunctional control of movement.317,318 A combination of different models of spaceflight as well as data from spaceflight using activity-based interventions appears to have shown some effectiveness at least in preserving muscle mass and some physiological properties of muscle. While crew members on the Mir space station exercised for up to 3 hours per day,319 it seems unlikely that this volume of exercise is necessary to maintain skeletal muscle volume and function. The more critical variable is likely to be more related to the type of activity, for example, activity in which high forces are generated.320 More than 3 percent of the astronaut’s time in space has been reported to be involved in exercise. Astonishingly, there has been no comprehensive reporting of activity patterns prior to this recent account of the activities of space patient crew members. There remain no prescribed exercise programs, and this has limited the potential for identifying a scientifically based program of activity with known consequences for different physiological systems, including those involved in the neural control of movement.321 A number of studies have demonstrated pre- and post-flight changes in movement performance, but there has been little attention to countermeasures that might prevent a loss in gross and fine motor skills when astronauts return to Earth.
Some of these programmatic barriers are as follows:
1. There have been inadequate opportunities to form the multidisciplinary teams needed to conduct well-designed and comprehensive studies. Instead, scientists have worked as single-discipline teams. For instance, there has been minimal interaction between experts in neurophysiology and muscle physiology.
2. There have been inadequate opportunities to address any one problem in a consistent, systematic manner.322 A researcher might have an opportunity to conduct an experiment on one flight, but rarely is there an opportunity for follow-up experiments, thereby precluding systematic efforts to resolve a given issue.
3. While the technology is theoretically available for answering many of the questions at hand, resources are needed to develop these technologies so that they can be used effectively in the space environment. This will require significant support for cross-discipline teams drawn from both the biological and the engineering communities.
4. After effective multidisciplinary teams have been developed, a concerted effort is needed to redefine the operational challenges related to safety, health, and productivity while performing routine and challenging motor tasks during and after arriving at different gravitational environments. For example, the focus within the muscle is frequently expressed as a problem related to strength and endurance (fatigability) and as problems related to maintaining muscle mass.
Are these really the key problems? Or are the key problems related more to how to maximize accurate and safe performance of a specific or even routine motor task, most of which demand neither maximum strength nor maximum endurance? How do the problems of accuracy and precision of relatively fine movements relate to changes in vestibular function, ocular motor function, and the interaction of these control systems with the spinal neural control mechanisms? Is the aerobic capacity of a crew member a critical factor for performance, health, and safety? Is it important to maintain levels of cardiopulmonary function similar to that at Earth’s gravity so as to maintain general body metabolic homeostasis and to avoid, for example, the reduction in insulin sensitivity associated with chronic deconditioning? These issues illustrate the importance of cross-disciplinary solutions, particularly when considering the potential role of exercise countermeasures. The issues raised in this section are relevant as well to the areas covered in Chapter 7, which discusses integration and translational strategies.
Research Models and Platforms
Animals
Multiple models and platforms should be employed to address the highest-priority questions. While one of the most popular ground-based models has been hindlimb unloading of rats and mice, critical answers can be derived for specific questions using a wide range of models. While research can proceed most rapidly by taking advantage of multiple platforms on Earth, the ISS is an essential platform. Further, its value would be greatly enhanced with a centrifuge facility. As in the past, access to biosatellites will also provide a very effective platform.
Humans
There should be a greater focus on performing comprehensive in vivo in-flight experiments using animals, but such in vivo experiments are even more important for studies of humans. With proper biotechnical development, major improvements can be made in measuring a vast number of interrelated variables, which are likely to provide more insight than has been possible to date into the critical mechanisms that control the homeostasis of multiple tissues.
Research Recommendations
Problems related to sensory-motor function in the spaceflight environment require a highly multidisciplinary approach from concept to solution. Although some significant experiments, both ground-based and during spaceflight, have provided novel insights and have given a better understanding of the possibility of successful long-duration flights and what the potential problems are likely to be,323 the sparseness of sensory-motor knowledge precludes confidently establishing a comprehensive strategy that will maximize the probability of successful mobility in space environments and after the ultimate return to Earth. To close these gaps, there will need to be fundamental changes in programmatic policies that determine distribution of resources and means of decision making.
More specifically, the following prototype studies are needed:
1. Determine the daily levels and pattern of recruitment of flexor and extensor muscles of the neck, trunk, arms, and legs at 1 g and after being in a novel gravitational environment with accommodating life support systems for up to 180 days. These changed patterns of neuromuscular activity over different time periods need to be carefully examined with respect to changes in the accuracy of movements and the type and severity of functional disruption to other tissues, particularly muscle, connective tissue, and cardiovascular and hormonal systems.
2. Identify the neuromuscular mechanisms that underlie the loss of accuracy in controlling movement with respect to (1) changes in neural control at the cortical, subcortical, and spinal levels; (2) changes in muscle properties; and (3) changes in visual, vestibular, and/or proprioceptive perception.
3. Determine whether changes in the accuracy of movement of the head, trunk, and limbs due to changing gravitational environments can be prevented or corrected with an exercise countermeasure designed to preserve aerobic function using a treadmill or stationary bike or other exercise device that maintains muscle strength and increases resistance to fatigue.
Effects of the Spaceflight Environment on Fluid Shifts
Fluid shifts due to the elimination of gravitational gradients are a fundamental consequence of the entrance into and existence in microgravity. Such shifts are particularly prominent in humans because of their predominantly upright posture. Although the 1998 NRC report did not recommend fluid shifts for future study,324 the uncertainty of the effects of cephalic fluid shifts during extended flights in microgravity indicates that this subject does need to be reviewed. For decades, the loss in plasma volume with fluid shifts was cited as the reason for the decrease in body mass experienced during the early stages of spaceflight; however, careful research by Drummer and associates demonstrated that this explanation was incomplete in that the decrease in body mass was due, in part, to inadequate hydration and salt intake coupled with a reduced caloric intake.325 Furthermore, Leach et al. reported
that with short spaceflights not every astronaut who exhibited a decline in plasma volume had a reduction in total body water.326 However, the astronauts did demonstrate sodium retention.327
Cardiovascular system performance is closely tied to fluid shifts because the latter can result in decreased plasma, cardiac, and stroke volume in microgravity. Fluids shifts can be associated with the risks of (1) orthostatic hypotension,328 (2) inadequate physical performance for egress or for vigorous activities, and (3) a loss in visual acuity with flights of extended durations.329
To understand the acute and chronic effects of fluid shifts in microgravity, it is essential to specify the reference point to which comparisons are being made. Thus, when compared to the fully adapted upright standing position in a 1-g environment, the transition to microgravity is dramatic and predictable, with hydrostatic changes becoming the dominating stimulus. Prior to entering space, astronauts often dehydrate themselves to avoid urination during launch, then spend many hours on their backs with their legs elevated in a prelaunch position, and frequently experience the nausea and vomiting of “space sickness”330 during the early hours in space. These circumstances compromise the initial fluid measurements made in microgravity and raise questions concerning their relevance.
Observed Effects of the Spaceflight Environment
Human Studies
Entry into space is associated with diminution of hydrostatic forces331 and a fluid volume translocation of approximately 2 liters332 from the lower body to the thorax and cephalic regions. The first invasive measures of central venous pressures (CVP), referenced to the atmosphere, were quite surprising in that CVP decreased to near 0 mm Hg in the acute transition to space.333,334 However, left ventricular end-diastolic volume and stroke volume clearly increased at the same time,335,336 which argued strongly for an increase in transmural pressure (intracardiac pressure minus pericardial pressure).337 This hypothesis was supported by studies during parabolic flight, which demonstrated that esophageal pressure decreased more than CVP referenced to the atmosphere.338 Thus spaceflight altered the external constraining forces of the chest and thorax, leading to increased cardiac distending pressure despite an apparently reduced CVP.
These fluid shifts were associated not only with an increased cardiac transmural pressure339,340,341 but also with reduced intravascular volumes and pressures in the legs342 and elevated transcapillary fluid filtrations into regions of the upper body.343,344 Ultrasound measurements of the thickness of facial and tibial tissues of a cosmonaut early in flight demonstrated that fluids had shifted into and remained within facial tissues for 3 days (+7 percent), while leaving the tissues located over the tibia (−17 percent).345 After landing, the fluids shifted from the head region but exhibited no changes at the tibia level.
Intraocular pressure was measured on the D-2 mission (N = 4). After an initial increase of 114 percent, the pressures rapidly returned to within pre-flight values (10 mm Hg) where they remained during the 10-day mission.346 Concerns have recently been raised by an expert panel on papilledema about the clinical possibility of visual acuity being affected not only by elevated intraocular pressures during long-duration missions but also by optic disc edema, choroidal folds, cotton wool spots, and intracranial hyptertension.347
A ground-based head-down analog study was conducted by Hargens and associates, who used wick catheters inserted into the lower legs of subjects. They found significant decreases in interstitial pressures located in muscle (4.6 mm Hg to −2.8 mm Hg) and subcutaneous (8.5 mm Hg to 6.7 mm Hg) tissues with no significant changes in capillary and in interstitial colloid osmotic pressures.348 On the other hand, measurement of Starling forces in the head and neck regions in a similar analog by Parazynski et al. revealed an increased capillary hydrostatic pressure of approximately 7 mm Hg in the lower lip with a decreased colloid osmotic pressure of 3 mm Hg.349 Plasma colloid osmotic pressure and plasma volume were measured in subjects participating in a head-down bed rest study, and after 16 days, colloid osmotic pressure had significantly increased (29 percent), while plasma volume was significantly reduced, by 18 percent. Although no measurements were made, the authors attributed these changes to a reduction in extracellular volume.350 From the results of the SLS-1 and SLS-2 space shuttle missions, Leach et al. hypothesized that an increased permeability of the capillary membrane was an important mechanism for the reduction of plasma volume.351 However, there were no measurements that directly supported this hypothesis.
Fluid shifts can also alter intracranial pressures (ICPs), which can influence cerebrospinal fluids, intraocular
pressures, the activation of the space adaptation syndrome, and possibly, visual acuity.352 Using a non-invasive tympanic membrane displacement technique based on a sigmoidal relationship between membrane displacement and ICP, Murthy and colleagues reported that 6° head-down tilt was associated with a significant displacement that corresponded to an estimated ICP value of 17 mm Hg, an estimate of some clinical importance.353
Animal Studies
In Cosmos 1229, ICP was evaluated in monkeys using the pulse waveform and was found to be significantly increased on the first day but to have decreased to within normal values at the end of flight.354 Histological assessment of brains from rats suspended for 93 days exhibited changes that indicated to the investigators that the animals had experienced increased ICP and brain edema.355 Kawai concluded from his direct measurements of ICP in rats and rabbits that larger animals had higher ICP values, that it was unlikely that brain edema would occur in 24 hours but likely after 84 hours, and that the initial increase in ICP was related to the shift of cerebrospinal fluid to the head region and to the increased volume from intracranial veins. For decreases in ICP to occur with head-down tilt, increased absorption of cerebrospinal fluid by the arachnoidal villi was expected.356
Specific Effects of Fluid Shifts on Plasma, Cardiac, and Stroke Volumes of Humans
Associated with the intravascular fluid shifts into the interstitial and intracellular spaces357,358 have been the following volume changes.
Plasma Volume. As summarized in the first row of Table 6.1, for both spaceflight and head-down bed rest analogs, fluid and protein shifts from the plasma were predominately responsible for the acute response, which represented a 17 percent reduction in plasma volume.359-362 Although Leach et al. proposed that the set point for plasma volume is re-established by microgravity, this concept has yet to be verified. Among the numerous factors interacting to regulate plasma volume in microgravity, which is consistently reduced by 10 to 15 percent, select hormones363 must be considered.
Cardiac Volume. As summarized in the second row of Table 6.1, after 48 hours of head-down bed rest, presumably because of a loss in plasma volume, left ventricle end diastolic volume decreased when compared to the supine position.364-370 However, the bed rest value was higher than recorded for the standing position.371 Entrance into microgravity significantly influenced diastolic filling volumes so that the acute stage represented an approximate increase of 20 percent.372 With continued existence in microgravity or bed rest, and in the absence of countermeasures, the left ventricle atrophied, resulting in a decrease in left ventricle end diastolic volume (even at the same filling pressure)373 at a rate that approximated 1 percent per week for both men and women.374,375 Ultimately, the combination of a reduced plasma volume and progressive atrophy will result in a reduced left ventricle end diastolic volume (compared to the supine position) being available for perfusion purposes.
Stroke Volume. As summarized in the third row of Table 6.1, the changes with both analogs and spaceflight followed the pattern exhibited by cardiac volumes, resulting in a significantly lower stroke volume being available for situations that require maximal performance in an upright posture (e.g., astronaut egress) or for returning to Earth and facing conditions associated with orthostatic hypotension.376-380 Because spaceflight can result in cardiac atrophy and hypovolemia, the supine stroke volume value measured after landing is approximately halfway between the standing and supine values recorded prior to flight.
Sodium Retention. Sodium balance data from space shuttle flights SLS-1 and SLS-2, Mir 97, and EuroMIR 94 missions have effectively demonstrated that sodium retention occurs with an existence in microgravity presumably because of a dissociation between sodium and water.381,382 However, it is not known at what stage (early or chronic) in microgravity an equilibrium is established.
TABLE 6.1 Fluid Shifts and Select Volume Changes
| Measurement | Analog Results | Spaceflight Results | |||||
| Acute Responses | Early Adaptive Response | Chronic Adaptive Response | Parabolic Response | Acute Responses | Early Adaptive Response | Chronic Adaptive Response | |
| Plasma volume | ▼ | ▼a | ▼a | Not measured | ▼a | ▼ | ▼ |
| Cardiac volume | ▲ | ▼a | ▼a | ▲a | ▲a | ▼a | ▼a |
| Stroke volume | ▲a | ▼a | ▲ | ▲a | ▲a | ▼a | ▼a |
NOTE: Acute, responses in 24 hours or less; early, responses in 2 to 18 days; chronic, responses in 84 to 180 days; cardiac volume, left ventricle end diastolic volume, reference body position was supine. Either decreased or increased volumes are indicated.
aResponse was statistically significant.
SOURCE: Plasma volume: Data from C.S. Leach, C.P. Alfrey, W.N. Suki, J.L. Leonard, P.C. Rambaut, L.D. Inners, S.M. Smith, H.W. Lane, and J.M. Krauhs, Regulation of body fluid compartments during short-term space flight, Journal of Applied Physiology 81:105-116, 1996; M.A. Perhonen, F. Franco, L.D. Lane, J.C. Buckey, G.C. Blomqvist, R.M. Zerwekh, R.M. Peshock, P.T. Weatherall, and B.D. Levine, Cardiac atrophy after bed rest and space flight, Journal of Applied Physiology 91:645-653, 2001; S.M. Smith, J.M. Krauh, and C.S. Leach, Regulation of body fluid volume and electrolyte concentration in spaceflight, Advances in Space Biology and Medicine 6:123-165, 1997; S.H. Platts, D.S. Martin, M.B. Stenger, and S.A. Perez, Cardiovascular adaptations to long-duration head-down bed rest, Aviation, Space, and Environmental Medicine 80(5 Suppl.):A29-A36., 2009.
Cardiac volume: Data from P. Arbeille, G. Fomina, J. Roumy, I. Alferova, N. Tobal, and S. Herault, Adaptation of the left heart, cerebral arteries and jugular and femoral veins during short-and long-term head-down tilt and space flights, European Journal of Applied Physiology 86:157-168, 2001; B.D. Levine, J.H. Zuckerman, and J.A. Pawelczyk, Cardiac atrophy after bed-rest deconditioning: A nonneural mechanism for orthostatic intolerance, Circulation 96:517-525, 1997; M.A. Perhonen, J.H. Zuckerman, and B.D. Levine, Deterioration of left ventricular performance after bed rest, Circulation 103:1851-1857, 2001; M.A. Perhonen, F. Franco, L.D. Lane, J.C. Buckey, G.C. Blomqvist, R.M. Zerwekh, R.M. Peshock, P.T. Weatherall, and B.D. Levine, Cardiac atrophy after bed rest and space flight, Journal of Applied Physiology 91:645-653, 2001; E.G. Caini, L. Weinert, R.M. Lang, and P. Vaida, The role of echocardiology in the assessment of cardiac function in weightlessness—Our experience during parabolic flights, Respiratory Physiology and Neurobiology 169(Suppl. 1):S6-S9, 2009; J.B. Charles and C.M. Lathers, Cardiovascular adaptation to spaceflight, Journal of Clinical Pharmacology 31:1010-1023, 1991; D.S. Martin, D.A. South, M.I. Wood, M.W. Bungo, and J.V. Meck, Comparison of echocardiographic changes after short-and long-duration spaceflight, Aviation, Space, and Environmental Medicine 73:532-536, 2002.
Stroke volume: Data from P. Arbeille, G. Fomina, J. Roumy, I. Alferova, N. Tobal, and S. Herault, Adaptation of the left heart, cerebral arteries and jugular and femoral veins during short-and long-term head-down tilt and space flights, European Journal of Applied Physiology 86:157-168, 2001; B.D. Levine, J.H. Zuckerman, and J.A. Pawelczyk, Cardiac atrophy after bed-rest deconditioning: A nonneural mechanism for orthostatic intolerance, Circulation 96:517-525, 1997; E.G. Caini, L. Weinert, R.M. Lang, and P. Vaida, The role of echocardiology in the assessment of cardiac function in weightlessness—Our experience during parabolic flights, Respiratory Physiology and Neurobiology 169(Suppl. 1):S6-S9, 2009; J.B. Charles and C.M. Lathers, Cardiovascular adaptation to spaceflight, Journal of Clinical Pharmacology 31:1010-1023, 1991; D.S. Martin, D.A. South, M.I. Wood, M.W. Bungo, and J.V. Meck, Comparison of echocardiographic changes after short-and long-duration spaceflight, Aviation, Space, and Environmental Medicine 73:532-536, 2002.
Fluid Shifts and Hormonal Influences in a Spaceflight Environment
The inability to secure numerous in-flight hormonal measurements from subjects is a condition that needs to be corrected in future experiments. This is especially true for animals because most, if not all, measurements have been obtained after landing. The following hormones are of interest because of their role in restoring fluid volumes.
Vasopressin. In the SLS-1 and SLS-2 flights, urinary concentrations of arginine vasopressin (AVP) were found to have increased when compared to pre-flight results.383 In the important metabolic studies conducted on Mir, extensive blood measurements from one cosmonaut demonstrated that AVP was significantly elevated.384 Mean post-flight blood AVP levels from 27 cosmonauts returning from missions lasting from 120 to 366 days were increased by 135 percent compared to pre-flight values.385 In contrast, two cosmonauts on days 216-219 in space had AVP blood levels that were reduced by 21 percent and 40 percent, respectively; these changes were attributed to a possible change in sensitivity of the kidneys.386 Vasopressin or ADH was measured in nine subjects after a 90-day head-down bed rest study, and no significant changes were reported. The authors suggested that the decrease in plasma volume of approximately 5 percent was insufficient to alter plasma osmolality.387 Among available animal
studies, measurements of vasopressin concentration per unit of protein in posterior pituitary tissues from rats on Cosmos 2044 found significantly lower concentrations than in ground-based controls.388
Plasma Renin Activity (PRA). During the early stages of SLS-1 and SLS-2 missions, PRA values were variable but, near the end of the flights, they remained elevated over pre-flight values.389 A summary of PRA data from Spacelab D-2, Mir, and EuroMIR 97 missions reported increased values that ranged from 69 percent to 472 percent when compared to pre-flight supine conditions.390 The percentage changes were markedly lower when crew members were in a supine position as opposed to seated, demonstrating the importance of position when securing a pre-flight PRA measure. Serial PRA results from the MIR 97 metabolic mission also showed increased levels from pre-flight measurements.391
Aldosterone. Summarized plasma and urinary results from short- and long-term flights showed both increased and decreased levels of aldosterone, as well as combinations of increases and decreases.392 Values from SLS-1 and SLS-2 showed that aldosterone decreased,393 while plasma results from the Mir metabolic study found that aldosterone levels were significantly increased.394 However, data from both flights demonstrated positive sodium balances. Post-flight results from 23 cosmonauts who had been in microgravity between 120 to 366 days demonstrated a 61 percent average increase.395 As with PRA levels, prolonged bed rest was associated with a marked increase (87 percent) and extensive variability.396
Atrial Natriuretic Peptide (ANP). In-flight plasma ANP levels were measured in two astronauts during an 8-day flight, and marked reductions were noted.397 Similar plasma results were obtained with one astronaut after 26 days in microgravity,398 whereas no changes were noted for a single cosmonaut after 20 days.399 Hinghofer-Szalkay et al. reported that an astronaut in microgravity for 438 days had post-flight plasma ANP values that were lower (12 percent) than baseline values; additionally, when an astronaut was subjected to conditions of lower body negative pressure (LBNP) throughout the flight, ANP levels were consistently reduced.400 After a 90-day head-down bed rest study, ANP levels were associated with a 33 percent reduction in ANP, which was attributed to a reduction in plasma volume.401
Norepinephrine (NE). In the 16-day Neurolab mission, NE spillover and clearance values were elevated over pre-flight values, as was muscle sympathetic nerve activity (MSNA).402 Compared to pre-flight values, plasma NE levels from three cosmonauts measured after 217 to 219 days in microgravity were markedly increased but were regarded to be within normal limits. Their urinary NE levels were more variable and revealed no observable trend.403 Goldstein and coworkers reported that 14 days of head-down bed rest was associated with a nonsignificant 27 percent decrease in plasma NE and a significant 32 percent decrease in urinary NE levels.404 In a 42-day bed rest study, plasma NE levels decreased by 14 percent after 21 days but increased by 33 percent at the end.405 Christensen et al. utilized platelet NE concentrations to assess sympathetic activity in cosmonauts and in ground-based subjects in a head-down bed rest study; they found increased platelet NE values in the cosmonauts and significant decreases in the bed rest subjects.406 Shoemaker and colleagues investigated the changes in MSNA in subjects participating in 14-day head-down bed rest studies and reported that a significant decrease occurred in baseline MSNA.407 Furthermore, in subjects prone to hypotension an inadequate increase in sympathetic discharge had occurred.408 However, subjects who were not prone to hypotension experienced an increase in supine baseline MSNA.409 A similar result was reported by Pawelczyk et al. for subjects participating in an 18-day head-down bed rest study.410 MSNA measurements throughout a long-duration head-down bed rest study would help clarify the uncertainties associated with the previous findings.
Commentary
Entry into microgravity is associated with a marked central fluid shift, leading to cardiac distension, compared with the standing upright position on Earth. If all other factors were unchanged, this central hypervolemia would likely lead to a neurohumoral environment that would result in a volume loss, with most astronauts exhibiting reductions in total body water. However, this response is variable and seldom achieves statistical significance.
Because of confounding variables and limited access to crew members, this acute neurohumoral response has been difficult to characterize in microgravity, making it hard to determine what afferent or efferent pathways have been altered by a central fluid shift. However, after the early stage of spaceflight (see Table 6.1), it appears that cardiac volumes, fluid hemodynamics, and the neural and hormonal adaptive responses reach an equilibrium that is approximately halfway between the values associated with the supine and upright positions on Earth and close to the values seen in the seated upright position.411,412
Status of Countermeasures for Fluid Shifts
A previous NASA task force recommended that astronauts consume a liter of isotonic saline solution 2 hours before re-entry to enhance plasma volume.413 To prevent blood pooling in the legs and to minimize the loss of plasma volume, LBNP at 30 mm Hg has been advocated.414 Russian authorities promote the utilization of the “Penguin suit,” an elastic loading suit that enhances venous return during the activity of extensor muscles.415 In addition, thigh occlusion cuffs known as “Brasselyets” have been combined with exercise and are regarded as an effective countermeasure. Prior to descent, the use of an antigravity suit is recommended to protect astronauts or cosmonauts from negative hypergravity effects of landing.416 Although systematic centrifugation in a small-arm centrifuge has potential as an effective countermeasure for use on the ISS or possibly in a lunar module, its implementation remains uncertain. Animals that were centrifuged in Cosmos 936 exhibited fewer deleterious effects of microgravity than did their flight controls.417 With the increasing number of female astronauts, it should be noted that ground-based research has indicated that estrogen and progesterone supplementation has promise as a countermeasure against loss of plasma volume that occurs in microgravity (see Table 6.1).418,419 However, because these hormone supplements have many other systemic effects, their utility as a fluid countermeasure is uncertain.
Gaps in Fundamental and Applied Knowledge Concerning Fluid Shifts
The longitudinal effects of the influence of microgravity on the Starling forces in human and animals warrant investigation, since, to date, there is incomplete information pertaining to humans and none from animals on this fundamental topic. Inherent in this recommendation is the need to develop and maintain a database that includes fluid shift data that incorporate the information on volume change listed in Table 6.1.
Gaps in applied knowledge pertain to whether the loss of tissue weight combined with changes in blood pressure shift the Starling-Landis equation toward greater filtration into tissues,420 whether lymphatic function is compromised by simulated and actual gravitational conditions,421 and whether fluid shifts are a primary or a secondary reason for the decrease in visual acuity associated with long-duration flights. Systematic investigations into the efficacy of mechanical countermeasures (e.g., Brasselyets, LBNP, centrifugation) that restore Earth-normal head-to-foot hydrostatic gradients are important because inhibiting this fundamental response to microgravity may provide no meaningful benefit during prolonged existence in space. Tests of new quantitative strategies to restore plasma volume prior to landing, such as augmentation of venous return, increased salt intake several days earlier, or hormonal manipulation with or without salt consumption and with careful attention to the timing of interventions, should be undertaken before long-duration flights are initiated.
Research Models (Analogs) and Platforms
For research in the next decade, the head-down bed rest model has recently been selected by NASA Johnson Space Center in conjunction with the University of Texas Medical Branch to become the analog for testing human countermeasures for lunar and Mars missions.422 For rats and mice, the HU model is endorsed by the AHB Panel.423 The adoption of the bed rest model incorporates the essence of the 6° head-down position of Kakurin et al.,424 which has been an analog for simulated microgravity experiments for more than three decades because of its effectiveness in producing cephalic fluid shifts. An equally effective analog in producing the cephalic fluid shifts of microgravity has been the immersion model advocated by Epstein.425 During the past decade, these two models have been challenged because they have been unable to duplicate the results that have been predicted by
the Henry-Gauer reflex,426,427 namely the increased central venous pressure,428,429 diuresis,430 and natriuresis431 that occur during the acute and early stages of spaceflight. In a white paper submitted to the study committee, Norsk and Christensen argue that a limitation of prevailing ground-based analogs has been their inability to simultaneously reproduce the increased functional residual capacity (compared to the supine position in 1 g) of thoracic expansion and the cephalic shifts.432 Elsewhere, these authorities on the subject have suggested perfecting an analog with thoracic compression for assessing entry into space or a dry immersion analog for oral water loads.433
Numerous scientists believe that the Henry-Gauer fluid shift model for humans fails because it is too simplistic in defining the afferent and efferent pathways that mediate the responses to microgravity. Moreover, it is unable to account for changes in capillary permeability, increased upper-body capillary pressures, hydration, and elevated feet posture or for the changes associated with motion sickness. For the reasons noted previously, it is necessary to secure meaningful measurements of fluid shifts (as shown in Table 6.1) during the acute and early stages of spaceflight; thus modifications of existing analogs are in order. However, for flights longer than the early stages, and especially for long-duration flights on the ISS and beyond, the long-duration head-down bed rest analog adopted by NASA434 should be utilized.
Besides ground-based platforms, it is essential that investigators have access to the facilities of the ISS for basic animal and human studies. Access to a free-flyer platform is not recommended because the fluid equilibrium in the subject would be disrupted after landing.
Recommendations for Fundamental and Applied Research Studies
1. Investigations are needed to determine the basic mechanisms, adaptations, and clinical significance of changes in regional vascular/interstitial pressures (Starling forces) during long-duration space missions. Fundamental studies should be done with both animals and humans that characterize the effect of microgravity on Starling forces and test the hypothesis that microgravity will increase capillary permeability. Investigations should be undertaken to determine whether a set point for plasma volume exists and to determine the mechanisms responsible for its existence. Cellular and molecular studies should be conducted on the suppressional influences of simulated and actual microgravity on the secretion of vasopressin by cells in the posterior pituitary. Related investigations should be undertaken to determine whether microgravity will alter the sensitivity of kidney tubules to the presence of vasopressin.
2. Applied studies are recommended that use invasive procedures with animals and non-invasive techniques to determine the early and late adaptive changes in response to microgravity on intracranial pressure, intraocular pressure, and cerebral edema. Systematic investigations should be conducted on the efficacy of mechanical countermeasures (e.g., Brasselyets, LBNP, centrifugation) that restore Earth-normal head-to-foot hydrostatic gradients. Such studies are important because inhibiting this fundamental response to microgravity may provide no meaningful benefit during prolonged existence in space. Tests of new quantitative strategies to restore plasma volume in the period before landing, such as augmentation of venous return, increased salt intake several days earlier, or hormonal manipulation with or without salt consumption, should be undertaken before long-duration flights are initiated.
Interactions should begin between vision researchers and flight surgeons to conduct long-term simulated and real microgravity research on the influences of fluid shifts on visual acuity and relevant cardiovascular parameters.
Risks for the Cardiovascular System During Long-Duration Spaceflight
The cardiovascular system is particularly affected by gravity and weightlessness because of its dynamic nature and dependence on hydrostatic pressure for the delivery of blood flow to all organs at an optimal perfusion pressure. The adaptation and adjustments that characterize the responses to postural changes and to the metabolic demands of activities on Earth are altered significantly under microgravity conditions and the associated reductions in mechanical forces during physical activities. Chronic reductions in metabolic demands and oxygen uptake reduce
the demands on cardiac output and tissue perfusion. The magnitude of the neurohumoral responses to activity and posture and of cardiac and vascular structural changes will likely be a function of the duration of spaceflights.
Such changes may result in significant loss of essential enabling cardiovascular functions and thus present a clinically significant risk. Specifically, (1) the capacity for physical stress (oxygen transport and aerobic power) and thermal regulation in space or during landing may be severely limited at times of greatest need for increased systemic perfusion; (2) the “adaptive” structural and functional changes in space over prolonged periods may result in debilitating orthostatic intolerance upon return to partial or full gravity, with potential failure to reverse structural changes that could become pathological; and (3) inadequate screening for subclinical cardiovascular disease, or acceleration of the atherosclerotic process by prolonged (or high-energy bursts of) total body irradiation, may lead to potentially catastrophic coronary events in space that could be life- or mission-threatening. In the new paradigm of very prolonged exposures to space (i.e., 6 months or more), cardiovascular pathobiology will need to be better defined not just at the integrated systems level but also at the molecular, cellular, and genetic levels in order to adopt definitive countermeasures or therapeutic strategies.
Comprehensive reviews of this topic include one by Blomqvist and Stone in the Handbook of Physiology in 1983 titled “Cardiovascular Adjustments to Gravitational Stress”435 and more recently one by Aubert et al. in Acta Cardiologica in 2005 titled “Cardiovascular Function and Basics of Physiology in Microgravity.”436 An important workshop on cardiovascular research in space was held at the International Space University in Strasbourg, France, in 2008. Its proceedings, published in Respiratory Physiology and Neurobiology (2009),437 cover the spectrum of cardiovascular adjustments in microgravity and provide an excellent baseline for future direction of research using the resources of the ISS.438-443
Effects of the Spaceflight Environment on the Structure and Function of the Cardiovascular System
Increased Cardiopulmonary Volume
Entry into microgravity elicits acute physiological responses, some of which become chronic adaptations. The most obvious response, which is a direct effect of altered gravitational and hydrostatic gradients, is the central and cephalic fluid shift from the lower extremities and abdomen, estimated to be approximately 2 liters.
Because of the central role of fluid shifts in the multisystem adjustments to microgravity, a separate, previous section of this report is devoted to fluid shifts. Acute consequences have included facial puffiness, headaches, and “bird legs,” with increased capillary hydrostatic pressure and transcapillary fluid shifts in tissues of the head and neck444 as a result of an increase in venular pressure.445 It is not known whether the cephalic fluid shift contributes to a chronic increase in intracranial pressure446,447 or to the observed increase in cephalic bone mass.448
A direct consequence of the redistribution of blood volume with a central shift is an increase in cardiopulmonary volume compared to upright standing on Earth. With that increase in central volume, stroke volume increases as well as cardiac output, since the decline in heart rate is minimal.
Despite the increase in cardiopulmonary volume, central venous and atrial pressures are decreased when referenced to atmospheric pressure, as indicated by direct invasive measurements in space.449,450 This reduction is not due to reduced cardiac filling, since left ventricular end diastolic volume and stroke volume also increase451 (see Table 6.1). Thus the transmural ventricular diastolic filling pressure must actually increase.452,453 This conclusion is supported by measurements during parabolic flight of esophageal pressure (an estimate of intrathoracic pressure and an index of the directional change in pericardial pressure), which decreased more than central venous pressure.454 It is not likely that myocardial compliance changes within a few seconds of exposure to microgravity; however, extracardiac constraining forces, generated predominantly by compression from the lungs and thorax,455 also likely decrease due to expansion of the rib cage in space, thereby decreasing extracardiac pressure and allowing expansion of the cardiac chambers. Arterial systolic pressure and pulse pressure at heart level both increase.
Decreased Total Blood Volume
These early adjustments to the increase in central blood volume last for a few hours or days. Subsequently, stroke volume and cardiac output return to levels that are less than those associated with a supine position, greater than those in a standing position, and equivalent to a head-up tilt of about 30° on Earth.456 This gradual reduction
in stroke volume is associated with a clear loss of plasma volume457 without any evidence that systolic cardiac function deteriorates. These early rapid hemodynamic changes do not seem to present a problem during flight and for the most part are within the range of changes in the cardiovascular system observed during postural adjustments in daily life.
Compounding the acute reduction in plasma volume are reductions in red cell mass458-464 and hence in total blood volume.465-475 These reductions take place more slowly over time and certainly contribute to post-flight orthostatic intolerance by further impairing cardiac filling when returning astronauts stand upright on Earth.476-486 While there is no simple explanation for the decrease in blood volume, the fact remains that in microgravity total body water may not change consistently in all astronauts, whereas in-flight measurements of plasma volume consistently show reductions of 10 to 17 percent. Neurohumoral and hormonal factors regulate fluid intake, diuresis, natriuresis, pre- and post-capillary resistances, capillary filtration, and permeability, thereby causing this reduction in plasma volume. These factors include sympathetic nerve activity, renin/angiotensin/aldosterone, vasopressin, and atrial natriuretic factors. A reduction in erythropoietin may be a factor in the reduction of red cell mass.487-489 (See details in the “Fluid Shifts” section above.)
Decreased Cardiac Work and Cardiac Atrophy
There is evidence of cardiac atrophy and reduced cardiac distensibility (reduced volume at the same transmural distending pressure), which further impair cardiac filling in the upright position.490-497 Magnetic resonance imaging has shown a 7 to 10 percent decrease in cardiac muscle mass following 10 days of spaceflight, although only four astronauts were examined in these studies.498 Diastolic function was further compromised by reduced left ventricular untwisting, leading to diminished diastolic filling, which compounded the structural changes and together with them resulted in a substantially reduced upright stroke volume on return to Earth.499 Numerous studies involving 2 to 12 weeks of bed rest, including both men and women as subjects, suggest that without countermeasures the heart atrophies at a rate of about 1 percent per week.500-503 The mechanism of this atrophy is unknown, but it is likely due to reductions in cardiac work from a combination of reduced gravitational loading and decreased physical activity and metabolic demands.504-508
Cardiac mass is highly regulated in response to changes in loading conditions. When unloading is marked, cardiac atrophy is rapid (within 7 days) and dramatic (40 percent decrease in heart weight and myocyte volume).509 These adaptations appear to be localized exclusively to cardiac myocytes.510 According to McGowan et al., “As a function of decreased myocyte volume, the relative concentration of collagen is increased, which contributes to increased chamber stiffness.”511 As reported recently by Lisy et al., a dog model of cardiac atrophy (matching quite closely the human bed rest deconditioning model) confirmed that chronic reduction in left ventricular volume (from inferior venocaval banding) causes a significant reduction in left ventricular mass and myocyte volume “despite marked neurohumoral stimulation by the growth promoters endothelin and antiotensin II.”512 The molecular mechanisms underlying cardiac atrophy and remodeling, which have been reviewed recently, suggest novel targets for this process such as autophagy.513
Cardiac atrophy can be prevented when bed rest—and presumably spaceflight—are accompanied by exercise training and significant increases in cardiac work.514,515 Intriguingly, protein supplementation during bed rest, even without exercise training, also appears to minimize cardiac atrophy, though the mechanism underlying this observation is unknown.516 When combined with skeletal muscle atrophy, the reduction in cardiac mass and blood volume limits maximal cardiac output, decreases aerobic exercise capacity, and may limit performance. If cardiac atrophy during bed rest is prevented (by exercise) and hypovolemia is corrected (by volume infusion), orthostatic intolerance can be eliminated,517 highlighting the importance of these factors in mediating orthostatic intolerance after bed rest.
At present, there are limited data from long-duration spaceflight lasting 6 months or longer, which are the durations of a typical ISS sojourn. It is likely that, with prolonged flight, cardiac morphological changes may be accentuated and could even limit in-flight performance, although such an in-flight limitation has not been identified to date.
Cardiac Arrhythmias
Cardiac rhythm irregularities including premature ventricular contractions, atrial fibrillation, and a short run of ventricular tachycardia have been reported, especially during long-duration flights,518,519 and could have been, at least theoretically, associated with the known changes in cardiac structure. However the arrhythmias reported do not appear to have been life threatening or even clearly induced by spaceflight.520-523 There have been reports of minor prolongations in the corrected QT interval (longest reported, 420 ms),524 which never reached durations in the clinically worrisome range (i.e., >500 ms),525,526 and there are no reports of torsade de pointes, the arrhythmia that would likely be induced by clinically significant QT prolongation. It is not known whether cardiac atrophy is a substrate for electrical remodeling in space. Although changes in autonomic control have been implicated as potential contributing factors to arrhythmias, the sympathetic activation associated with spaceflight is quite modest527,528 and well within the normal adjustments that occur during daily life. Nonetheless, rigorous quantification of arrhythmia frequency and its variability pre-flight as well as in-flight, along with non-invasive assessments of cardiac electrophysiological properties, will be necessary to determine the magnitude and significance of these observations. Recovery of left ventricular mass and the time needed for it are uncertain, and this aspect has been poorly studied, especially after long-duration flight.
Cardiovascular Control Systems
The regulation of the cardiovascular system is defined by intrinsic properties of the heart and blood vessels as well as extrinsic neurohumoral influences.
• Intrinsic properties are related to cardiac or vascular muscle behavior and include the force of contraction, relaxation, vascular tone, resistance, volume distribution, protein expression, myosin heavy or light chains, and cytoskeletal structures.
• Extrinsic regulation refers specifically to autonomic neurohumoral and hormonal influences, including efferent innervation, renal and adrenal hormones, central neuronal nuclei, sensory receptors, and both autocrine and paracrine factors.
The intrinsic properties of the heart and blood vessels, including their contractile properties, are closely linked to the neurohumoral regulatory process by the presence of a rich afferent sensory network within the heart itself529 and by the distortion of arterial baroreceptors that are influenced by both flow530 and pulsatility.531,532
Data from spaceflights suggest that there are modest reductions in the vagally mediated regulation of heart rate that explain the mild tachycardia seen in space relative to the supine position on Earth.533,534 The sympathetic nervous system seems to be slightly upregulated.535-539 Direct measurements of sympathetic nerve activity indicate appropriate increases in response to reductions in central blood volume with lower-body negative pressure540 and with upright tilt,541 to the fall in arterial pressure with the Valsalva maneuver,542 and to other autonomic stimuli such as nociception or static exercise.543 After 2 weeks of spaceflight, the magnitude of sympathetic activation associated with upright posture appears to be precisely calibrated to the degree of reduction in stroke volume.544,545
Most ground-based bed rest studies are similar in reporting mild increases in muscle sympathetic nerve activity after 2 to 3 weeks of 6° head-down rest; these increases also appear appropriate for the reduction in cardiac filling with either lower-body negative pressure546 or head-up tilt.547 Of interest is the observation that the increase in sympathetic nerve activity with mental stress is augmented following the period of head-down bed rest.548 Despite the increases in sympathetic nerve activity, orthostatic intolerance has been reported in some individuals following 14 days of head-down bed rest and in some astronauts after spaceflights lasting 16 days.549,550 In the former, increases in nerve activity were significantly less during 10 to 15 minutes of 60° head-up tilt in subjects with orthostatic intolerance, compared with those who were tolerant to the tilt.551 Similarly, the astronauts who were intolerant to 10 minutes of standing without assistance on landing day had significantly lower plasma NE levels than those who were tolerant,552 although their NE release in response to tyramine was normal.553 Thus, the extensive studies on cardiovascular adjustments primarily to bed rest but also to microgravity, which have been reviewed recently,554-561 indicate that the reflex responses of the sympathetic nervous system to changes in cardiac filling, stroke volume, and arterial pressure are preserved,562-569 while vagal responses are reduced.570,571 However,
in individuals who may be susceptible to orthostatic intolerance,572,573 the magnitude of the sympathetic response and the subsequent cardiovascular adjustment may be insufficient to offset the effects of reductions in blood volume and stroke volume. Susceptible individuals may therefore be unable to maintain an adequate increase in peripheral vascular resistance574-578 to support cerebral perfusion in the face of this prominent hemodynamic compromise.579
Animal Studies. There is an extensive literature investigating the mechanisms of animal cardiovascular adaptation to adjustments in hydrostatic gradients, predominantly using the hindlimb unloading (HU) rat model.580 Because quadrupeds are fundamentally different from bipedal upright humans,581 the relevance of this model for human orthostatic tolerance can be questioned, and indeed HU rats do not develop cardiac atrophy in short-duration studies.582 However, they do if the hindlimb unloading is continued for at least 28 days.583 Key findings have included differential atrophy/hypertrophy and vascular responsiveness of hindlimb/mesenteric and cerebral vasculature, to a large extent because of directionally opposite changes in local vascular perfusion pressure when normal hydrostatic gradients are reversed by hindlimb unloading.584,585 Additional observations have included altered mesenteric vasoconstriction through changes in ryanodine receptor function,586 reduced aortic contractile function587,588 and increased aortic stiffness,589,590 and altered central nervous system processing of baroreceptor inputs at the rostral ventrolateral medulla (RVLM).591 High-resolution studies in the HU model have suggested that tonic GABA-mediated inhibition of the RVLM is enhanced after hindlimb unloading, which could restrain sympathetic activation.592
Risks of Cardiovascular Events
During a prolonged space mission to Mars, astronauts will not have access to comprehensive health care services for periods of 2 to 3 years or more. Since the majority of experienced astronauts are middle aged (the average age of the current astronaut corps is 46 years, with a range 33 to 58 years), they are at risk for developing serious cardiovascular events such as a myocardial infarction or sudden cardiac death, especially during high-intensity exertion. Such events are of course life-threatening for the astronaut and mission-threatening for NASA.
One of the greatest challenges facing the cardiovascular community is termed the “sudden death paradox”: although the patients at highest relative risk for sudden cardiac death can be clearly identified, based on known risk factors, the greatest number of sudden deaths on an absolute basis occur in patients not previously determined to be at high risk.593 Thus, the ability to identify at-risk individuals who are currently asymptomatic is a topic of intense research within the cardiovascular community that is relevant for both NASA and public health.
Although astronauts are now carefully screened prior to selection, they often must wait a decade or longer to fly select missions. NASA invests considerable resources in training astronauts, and so screening and monitoring strategies should be implemented to follow astronauts from selection to flight, identify individuals whose short-term (i.e., 2- to 3-year) risk for a cardiovascular event may have increased, and develop risk mitigation strategies, either pharmacological (such as administration of statins)594 or physiological (training for high levels of fitness),595 that will keep the risk sufficiently low.
Post-Flight Maladjustments
Optimal performance of astronauts upon return to partial or full gravity has to be a primary goal of research in the next decade.
Orthostatic Intolerance. Orthostatic intolerance even after a few hours of spaceflight has been a significant problem596-602 for NASA since the early days of crewed spaceflight. During post-flight orthostasis, the decreased blood volume, even in the absence of excessive pooling, compounded by cardiac atrophy causes a marked fall in cardiac filling pressure and in stroke volume. Indeed, the sine qua non of the cardiovascular maladjustment to spaceflight is a reduced stroke volume in the upright position. A reflex tachycardia and a reflex increase in sympathetic nerve activity do occur.603 However, the reflex increase in vascular resistance known to occur in splanchnic and limb circulation during venous pooling in humans604,605 is insufficient to prevent a fall in blood pressure in the most susceptible astronauts.606-609. This uncoupling of sympathetic activity from the vasoconstrictor response and the reduced vasoconstrictor reserve in the face of a reduced stroke volume are what ultimately result in orthostatic hypotension and hemodynamically mediated collapse.610,611 In some individuals, a true neurally mediated syncopal
reaction may also occur. This may be due to a sudden sympathetic withdrawal, with or without bradycardia,612 or to a reflex neurogenic vasodilation.613-616 The frequency of these different types of responses has not been studied systematically. To provoke syncope or orthostatic intolerance, the fall in pressure must be sufficient to decrease cerebral blood flow, although it has been reported that cerebrovascular autoregulation is preserved after spaceflight.617 There may, however, be a shift in the cerebral autoregulatory curve to a higher pressure, which may be related to a reduction in cerebral vessel wall shear stress with a reduced pulsatile blood flow618 or to a vascular adaptation to microgravity619 or to endothelial damage.620
Orthostatic intolerance has been reported to be more frequent in female, compared to male, astronauts.621 Careful analysis of gender-based differences in blood pressure control points primarily to a smaller heart and blood volume associated with a greater reduction in stroke volume as the primary underlying mechanism for this difference,622,623,624 although women may also have a lower blood pressure and altered sympathetic signaling in the upright posture, especially during the early follicular phase of the menstrual cycle.625
Reduced Exercise Capacity. Exercise capacity is decreased significantly after spaceflight partly because the ability to increase stroke volume during exercise is reduced by more than 30 percent.626-632 Other contributing factors include reduced red cell mass and oxygen-carrying capacity, cardiac muscle atrophy, reduced reflex vasoconstriction in non-exercising vascular beds to appropriately redistribute blood flow, and skeletal muscle atrophy.633,634,635 Microgravity exercise studies conducted after short-duration shuttle missions have shown convincingly that maximal oxygen uptake (VO2 max) during post-flight exercise is reduced by roughly 20 to 25 percent, because of insufficient stroke volume and cardiac output responses.636 Similar observations were made after the longer-duration Skylab missions,637 but VO2 max has not been reported after long-duration flight on the ISS.
The reduction in upright stroke volume and cardiac output is of special concern because of expectations that heavy launch and entry suits will be a future requirement that will present an added physical and heat stress. Improvement in exercise capacity may be rapid after spaceflights of less than 1-month duration, but 1 week is required for full aerobic capacity to recover, even after short-duration missions.638 Longer recovery time would be expected with longer flights, although this has not been proven and may depend on the extent of in-flight exercise training.639 Recovery of autonomic control and blood volume as well as red cell mass may be faster than recovery from cardiac and skeletal muscle atrophy. Both of the latter contribute to the reduction in exercise capacity. There are no data on recovery from these or other unexplored complications after long flights.
Status of Countermeasures
Countermeasures used by the United States and Russia have focused on (1) restoring plasma volume immediately prior to re-entry (fluid loading), (2) reducing peripheral pooling and enhancing central blood volume during the first few hours after landing (compression devices and skin surface cooling), (3) augmenting peripheral vasoconstriction pharmacologically, (4) utilizing LBNP in the Chibis garment, and (5) using a variety of exercise training strategies to limit cardiovascular atrophy and deconditioning during a mission.
Restoring Plasma Volume Immediately Prior to Re-entry
Because of the well-described loss of plasma volume during spaceflight, one of the earliest and most persistent countermeasures used by astronauts has been fluid loading. In 1985, Bungo and Charles described a combination of salt tablets and water drinking that decreased standing heart rate by about 30 percent and ameliorated to some degree the orthostatic fall in blood pressure.640 Since then, fluid loading has been employed by nearly all crew members despite causing nausea and vomiting in some. More recently, this type of countermeasure has been confirmed in bed rest studies to be somewhat effective for restoring plasma volume and improving upright hemodynamics.641 Intriguingly, a recent study showed that the intravenous infusion of dextran sufficient to normalize both plasma volume and cardiac filling pressure did not by itself prevent orthostatic intolerance;642 only when cardiac morphology was preserved with exercise training (supine cycling), in addition to restoration of plasma volume, was orthostatic intolerance completely prevented. Preliminary work by this same group643 has employed 48 hours of a high-salt diet along with fluorocortisone to increase renal salt retention with similar efficacy. Earlier
work had shown that both glucocorticoids and mineralocorticoids increase cardiovascular responses to NE.644,645 An alternative to fluid loading has been a single dose of maximal exercise, which has also been shown to acutely restore plasma volume and improve orthostatic tolerance after bed rest.646 The utility of fluid loading to improve orthostatic tolerance in situations where it is compromised was also confirmed recently by Keller et al., who administered dextran intravenously during heat stress and completely prevented orthostatic intolerance in this setting.647 Although heat activation of thermal receptors suppresses the baroreceptor reflex,648 the primary determinant of orthostatic intolerance in whole-body heat stress is marked expansion of peripheral vascular capacity. This results in reduction of the effective central blood volume and cardiac filling pressure, hence the effectiveness of fluid loading.649
Non-Fluid-Loading Strategies to Restore Central Blood Volume After Landing
Anti-shock garments have been used by astronauts similar to their use by high-performance jet pilots and are part of the standard approach to re-entry. These devices have been shown to increase cardiac filling pressure and stroke volume by redistributing blood from the peripheral veins centrally,650 but they have not been studied systematically during spaceflight. Skin cooling is also used and causes both skin vasoconstriction and a shift in the operating point of the baroreflex control of sympathetic nerve activity, which improves tilt tolerance.651,652 Again, this approach has not been studied systematically and may place a crew at risk for decompensation should the cooling garment fail at a critical time.
Restoring Vasoconstrictor Tone
Despite the increase in sympathetic activity that has been measured in the upright position post-flight, there is a reduced vasoconstrictor reserve that prevents the redistribution of blood flow from the splanchnic and peripheral circulation to the brain during orthostasis. Promising preliminary data exist for adrenergic vasoconstrictor agonists, e.g., midodrine as a countermeasure.653,654 Octreotides are also promising as supplements to compression garments.655
Exercise Training During Bed Rest to Preserve Upright Exercise Capacity
Exercise training has been employed in many different ways and at different levels with the goal of preventing cardiovascular deconditioning.656 Three recent strategies have been particularly effective. The combination of treadmill exercise with lower-body negative pressure to simulate head-to-foot gravitational loads and normal 1-g ground reaction forces has been studied extensively by Hargens and colleagues.657 This strategy is quite effective at preventing cardiac atrophy658 while preserving upright exercise capacity659 and muscle strength, but it is only partially protective against orthostatic intolerance.660 The study by Shibata661 described above prevented cardiac atrophy, preserved upright exercise capacity, and prevented orthostatic intolerance but required 90 minutes of exercise per day and invasive volume restoration. Equally promising is a combination of rowing and strength training.662 A self-powered centrifuge has also been proposed. The fact that at least three different modes, frequencies, and intensities of exercise training have been effective at preventing cardiac atrophy and preserving exercise tolerance is strongly suggestive that cardiovascular loading is the key variable. However, to date none of these interventions have been tested against each other directly or in space. Ultimately, a multidisciplinary approach to exercise countermeasures will be required, as astronauts will likely not do separate exercises for each physiological system. Rather, a single exercise prescription, involving a well-periodized combination of endurance and strength training for different durations and intensities, while allowing for astronaut preference, will likely be most effective. Periodic in-flight testing with feedback to the crew and modification of exercise practices based on clearly defined changes would also improve the practical implementation and efficacy of exercise countermeasures.
Gaps in Knowledge
Is Orthostatic Intolerance Always Due to Orthostatic Hypotension?
Orthostatic intolerance after spaceflight has generally been presumed to be due to hypotension and cerebral hypoperfusion. However, at least some astronauts have been reported to be unable to stand even with a normal
blood pressure.663 To date, virtually all of the studies examining orthostatic tolerance have been conducted under controlled but artificial conditions of either quiet standing or a tilt test. The latter is known to cause false positive results, even in patients who have never had syncope.664 Moreover, simply moving the legs during a tilt study may dramatically improve orthostatic tolerance, as shown after exercise in soldiers more than half a century ago.665 It is also not clear how much other factors such as muscle weakness, neurovestibular abnormalities, a change in cerebrovascular autoregulation, or sympatho-vascular uncoupling with decreased vasoconstriction reserve contribute to symptoms of intolerance. Understanding these factors would be particularly relevant after long-duration spaceflight, when tolerance may be especially low.666 Although orthostatic symptoms can be easily managed under controlled conditions in the supine position with a combination of intravenous fluids and flight surgeon support, it is unclear how severe these symptoms will be under conditions of fractional gravitational loads (i.e., 3/8 g on Mars) when such support will be unavailable or under conditions of emergency egress when the muscle pump is activated, optimal physical performance is needed, and tolerance would be especially critical.
What Is the Best Strategy or Combination of Strategies to Prevent or Manage
Orthostatic Intolerance and Preserve Exercise Capacity After Spaceflight?
As mentioned above, different exercise strategies have been employed. Exercise within a lower-body negative-pressure chamber is effective at preventing cardiac atrophy, preserving upright exercise capacity, and improving—but not eliminating—orthostatic intolerance. Supine cycling combined with acute intravenous volume loading with dextran preserved both exercise capacity and orthostatic tolerance,667 although this exercise model is time-consuming and impractical. Rowing ergometry combined with strength training plus oral volume loading, with a combination of 48 hours of salt loading and fludrocortisone, is also promising.668 It is not clear which of these approaches is most effective.
Augmenting peripheral vasoconstriction pharmacologically with drugs such as midodrine has been beneficial after bed rest669 and may be helpful for reducing orthostatic intolerance after spaceflight.670 This intervention has not been tested rigorously in flight because of possible interactions with other commonly used in-flight drugs.
It is important to know the amount and patterns of activity that can maintain reasonably normal properties of the sensory-motor circuits, muscle, connective tissue, and hormonal and growth factors that are known to play a role in the homeostasis of these tissues. Also needed is information on the reason for the uncoupling of sympathetic activity from the vasoconstrictor response, the changes in vascular function and structure that can influence volume shifts and blood flow redistribution, and the means of enhancing sympathetic nerve activity. The approach to answering these questions should incorporate experiments ranging from the molecular control of specific proteins, transcription factors, etc., to in vivo experiments in the space environment.
Space Radiation and Progression of Atherosclerosis
The optimal strategy to screen and monitor middle-aged astronauts to prevent flying a crew member at increased risk for a cardiovascular event is unknown. Such an objective may be particularly challenging within a population of relatively low-risk individuals such as the astronaut corps. Moreover, although the specific effect of space radiation on the vasculature is unknown, exposure to high-dose radiation (x-ray therapy for cancer, atomic bomb survivors, radiated stents) is well known to accelerate atherosclerosis on Earth671,672 and therefore is considered a potentially serious risk during long-duration spaceflight such as a mission to Mars. Radiation risks are covered in more detail in the radiation biology discussion in Chapter 7.
Integrated Cardiovascular Response to Microgravity and Implications for Astronaut Health
Hundreds of studies have been conducted on animals and humans, on Earth and in space, by hundreds of investigators from many different laboratories in different countries. Much has been learned about the acute and short-term adjustments of the cardiovascular system to microgravity. Most of these studies, however, have been observational and not hypothesis-driven. Some results have led to effective countermeasures, but many more have been controversial, incomplete, and inconclusive.
Of particular concern are the structural changes that would develop after months or years of existence in microgravity. Such changes include reduced cardiac mass; cardiac and skeletal muscle atrophy; reduced bone
density; cardiac and vascular, as well as autonomic and sensory motor, neuronal remodeling; chronic reduction in red cell mass and plasma volume; and changes in vascular myogenic tone, endothelial shear stress and function, and capillary hydrostatic pressure and permeability. The irreversibility of these changes; their potential for accelerating latent pathological processes such as hypertension, atherosclerosis, coronary diseases, or diabetes; and the reduced capacity to respond to demands for increased physical activity and greater cardiovascular stresses are significant risks. Information on the fundamental cellular and molecular mechanisms (such as mechanosensors and metaboreceptors, or protein synthesis and catabolism) responsible for these chronic changes is essential for their prevention or reversibility.
A strategic, coordinated, integrated, research plan and an interdisciplinary approach by international teams of scientists pursuing research programs with specific goals, aims, and hypotheses will be necessary to define and overcome the operational challenges related to the safety, health, and productivity of astronauts while they perform both routine and challenging motor tasks during and after arriving at different gravitational environments.
Is the Aerobic Capacity of a Crew Member a Critical Factor for Performance?
It is important to maintain safe levels of cardiopulmonary function and general body metabolic homeostasis, especially during long-duration flights. NASA must define the physical demands of astronaut tasks and their tolerance levels. For example, many tasks may be relatively unimpeded by a 20 to 25 percent reduction in VO2 max, depending on the fitness of an individual crew member. Such issues again emphasize the importance of cross-disciplinary solutions, particularly when addressing the potential role of exercise countermeasures.
Research Models and Platforms
As discussed at length above in the “Fluid Shifts” section of this chapter, multiple models have been used to simulate the confinement and loss of hydrostatic gradients associated with spaceflight. From the perspective of effects on the cardiovascular system, the highest-resolution model available for humans is the 6° head-down tilt bed rest model.673 The key elements of the physical effects of microgravity—namely, increased cardiac transmural filling pressure and acute increase in left ventricle volume—are reproduced faithfully by the head-down tilt model. Moreover, the ultimate outcome in terms of changes in supine and upright hemodynamics has been demonstrated to be quite similar between head-down tilt and spaceflight.674 There are concerns, however, that no ground-based model can exactly reproduce the changes in external cardiac mechanical constraint experienced in spaceflight. Similarly, the reduced work load and metabolic demands during physical activity in microgravity cannot be reproduced. These may be partly responsible for the observations of cardiac atrophy and the uncoupling of sympathetic nerve activity from vasoconstrictor reserve.
For high-resolution basic science studies involving experimental animals, the HU rodent model is a suitable model for the hormonal factors and for the component of the fluid shifts that deals with peripheral and cephalic shifts. However, concern remains regarding differences between quadrupeds and upright bipedal humans and the lack of cardiac atrophy in the HU model, at least with the most common, short-duration exposures.
The other effect of long-duration spaceflight that cannot be easily reproduced in ground-based models is chronic radiation exposure. The effects of long-term, low-level “spacelike” radiation, accompanied by intermittent high-dose bursts of radiation equivalent to that delivered by solar flares, can at the present time be experienced only in space (although a dedicated effort to reproduce this type of exposure at the NASA Space Radiation Laboratory at Brookhaven National Laboratory is theoretically possible). Therefore only a true space-based platform with access to animal models for periods ranging from months to years—such as a lunar base, for example—can definitively examine the effects of space radiation on the cardiovascular system, as well as explore the mechanisms of observed structural and functional maladjustments and the likelihood of their reversal. (Note that because solar flares are rare and unpredictable events, use of an irradiator on the space platform would allow easier study of the effects of high-dose bursts of radiation.)
Required Platforms
The ground-based platform will be the laboratory of the investigator and is expected to be suitable for both human and animal research. Ground-based laboratories can be (but do not necessarily have to be) supported by
integrated head-down tilt bed rest facilities such as exist at the University of Texas Medical Branch in Galveston, which are coordinated by staff and investigators from Johnson Space Center. For microgravity platforms, the foremost environment will be the National Laboratory on the ISS, which will require both animal and human research facilities. Free-flyer platforms that can house and fly animals for designated periods are better suited for investigations of muscle and bone but could also be useful for cardiovascular investigations.
Research Recommendations
A comprehensive research program in cardiovascular sciences should be carried out at two levels: One, at the system level, would be used to describe and define changes in integrative cardiovascular functions. The second, at the cellular, molecular, or even genetic level, would be used to define basic mechanisms that lead to dysfunction. It will also be essential to determine the integrative effect of these cardiovascular adjustments on other systems, e.g., musculoskeletal, hematopoietic, and renal-endocrine systems.
Human Studies: Enabling Cardiovascular Research Recommendations Targeted to Specific Risks
The following three enabling areas of cardiovascular research are considered essential in the next decade.
Research Area 1. Maintaining an optimal capacity for the level of physical activity required to complete the tasks demanded of astronauts, and for thermal regulation during extravehicular activity (EVA) or unexpectedly high-risk egress from the vehicle.
a. Investigate the effects of prolonged periods of microgravity and partial gravity (3/8 and 1/6 g) on the determinants of task-specific, enabling levels of work capacity. Specifically, are there changes in energy availability, oxygen consumption, tissue perfusion, stroke volume, blood volume, blood flow redistribution to active muscle, or the capacity for heat dissipation that make it impossible to safely meet the excessive demands for physical activity?
• Determine whether microgravity induces a redistribution of blood flow as hydrostatic pressure is eliminated. Changes in tissue perfusion and intravascular pressures may cause changes in vascular structure and vasomotor tone (vascular proliferation or atrophy, secretion of endothelial-derived vasoactive substances, and microcirculatory autoregulatory mechanisms) and changes in organ function (pulmonary gas exchange, renal clearance mechanisms, blood-brain barrier and cerebral pressure including the retina, etc.).
• Determine the effects on the microcirculation and on capillary filtration and permeability as intravascular pressures change in microgravity. Determine the effects of such changes on tissue pressure, edema, intravascular volume loss, and shifts between interstitial, extracellular, and intracellular fluid.
• Determine the changes in oxygen consumption and oxidative and glycolytic metabolism as energy needs are reduced by confinement and loss of gravitational force. Determine consequential changes in cardiac output, blood flow distribution, and perfusion of exchange capillaries.
b. Conduct fundamental studies that are both spaceflight-enabling and enabled by spaceflight on myocardial mass and contractility, capillary filtration, hormonal changes, signaling pathways, and transcriptional regulation of cardiac myosin and actin. Such studies will provide insight into mechanisms that contribute to the decrease in stroke volume and into their future prevention by more specific countermeasures (discussed in more detail below).
Research Area 2. Orthostatic intolerance after restoration of gravitational gradients (both 1 g and 3/8 g): Determining its severity as a function of prolonged microgravity, especially under “real life” task demands such as emergency egress or Mars-like tasks, and the likelihood of functional recovery as well as the time necessary for it.
a. Determine the integrative mechanisms of orthostatic intolerance after restoration of gravitational gradients. Specifically, determine the relative contribution of hypotension and cerebral hypoperfusion, compared with neurovestibular, kinesthetic, or muscular weakness, to orthostatic intolerance following spaceflight.
b. If hypotension is the primary factor in orthostatic intolerance, consider whether reductions in cerebral blood flow, changes in neurovascular control, or fluid shifts and severe loss of central blood volume or cardiac atrophy (or their interaction) cause this hemodynamic compromise.
• Investigate the specific mechanisms underlying the inadequate increase in total peripheral resistance during orthostatic stress observed post-flight, and develop effective countermeasures. Determine whether there is a functional sympatholysis with decreased vascular responses to sympathetic nerve activity. Attempt to identify individuals who may have more limited capacity for orthostatic tolerance, and use the understanding gained about differences in tolerance to help target countermeasures more effectively.
• Determine if the baroreflex-mediated increase in sympathetic nerve activity is blunted or the pattern of sympathetic neural firing is altered, causing inefficient vasoconstriction.
c. Confirm and examine more systematically the efficiency of promising post-flight countermeasures such as use of the pharmacologic agent α1-agonist midodrine, and the use of post-flight gravitational countermeasures such as thigh cuffs, support stockings, or inflation of G-suits (Penguin suit); also determine the efficacy of promising in-flight countermeasures such as exercise of different modes, durations, and intensities; centrifuge-simulated artificial gravity; and novel fluid loading strategies that will more effectively restore blood and plasma volume.
Research Area 3. Alterations in coronary vascular disease with prolonged irradiation and exposure to microgravity. Early undetected coronary pathology may become manifest or be accelerated, with potential catastrophic consequences.
a. Refine and develop more sensitive techniques for detecting early coronary artery disease and its surrogate indicators: e.g., coronary calcification, cardiac CT angiography, intima-media carotid index, and pulse wave analysis and velocity in humans. Because of the small size of the astronaut corps, such studies would have to be undertaken in partnership with the cardiovascular research community.
b. Investigate the influences of total body irradiation in addition to exposure to microgravity on the acceleration of the atherosclerotic process, and determine whether specific medical therapy or other biologic factors (genetics, levels of fitness, etc.) can mitigate this risk over a period (3 years) equivalent to a mission to Mars.
Animal Studies: Basic Research in Microgravity Science Enabled by Simulated Microgravity and Space Studies
In addition to the enabling research areas identified above, which are considered the key areas of research for the next decade necessary to safely support a human presence in space, basic research into critical cardiovascular processes could be enabled by a robust research effort in space involving experimental animals. Areas of interest could include:
Research Area 1. Autonomic neurobiology.
a. Determine alterations in sensory signaling, for example;
• Mechanosensory adaptation, baroreceptor nerve activity, mechanosensitivity, compliance of carotid sinus, neuronal adaptation, resetting, ion channels expression, structural changes, fiber characteristics, and paracrine and autocrine changes; and
• Chemosensory adaptation, pH, pO2, pCO2 responsiveness, carotid body size and structure, glomus cell sensitivity, ion channels, and neuronal changes in petrosal ganglia.
b. Determine alterations in central neuronal nuclei involved in autonomic regulation and circulatory control. These include nucleus tractus solitarius, RVLM, caudal ventrolateral medulla neuronal structures, nucleus ambiguous, dorsal motor vagal nucleus, hypothalamic neuron nuclei, paraventricular nucleus, and others. Assess changes in activation, excitability, neuronal density, and autocrine regulators and receptors.
c. Characterize changes in sympathetic and parasympathetic innervation: density, NE release and turnover, innervation of vascular and cardiac muscle, receptor density, vascular reactivity, and autoregulation (cerebral).
Research Area 2. Cardiac and vascular muscle biology.
a. Heart: areas of interest could include regulators of cardiac mass, apoptosis, hypertrophy, gap junction, compliance, stiffness, contractility, ion channels, pacemaker function, and developmental changes. Identify changes in cardiac proteins, cytoskeleton, matrix, signaling pathways, reactive O2, and gene arrays.
b. Vascular: myogenic tone, innervation, endothelial function, muscle atrophy, proliferation, hypertrophy, protein structure, permeability, and capillary density. Identify cellular and molecular changes as mentioned above.
Research Area 3. Other specific areas relevant to cardiovascular interactions with other systems affected by spaceflight.
a. Circadian rhythm and cardiovascular function (related to sleep disturbances):
• Molecular clocks and cardiometabolic syndrome,
• Circadian proteins and genotoxic stress, and
• Circadian clocks and vascular function.
b. Lipid oxidation and cardiovascular disease (related to immune response alterations):
• Oxidative stress, phospholipid oxidation, and the innate immune system in atherosclerosis; and
• Isoprostanes as biomarkers and effectors in cardiovascular disease.
c. Pathobiology of calcific vasculopathy (related to bone/calcium metabolism):
• Osteogenic Wnt* signaling in valvular and vascular sclerosis,
• Fetuin regulation of calcified matrix metabolism, and
• Molecular imaging of vascular mineral metabolism.
d. Wnt proteins in cardiovascular development (related to developmental biology); Wnt signaling in cardiac hypertrophy/remodeling.
Benefits from the combination of enabling and basic space studies will translate not only to healthier astronauts during space exploration but also to a better understanding of the aging process and the pathophysiology and treatment of patients with syncope, heart failure, and atherosclerosis.
Risks for Pulmonary Function During Long-Duration Space Missions
Effects of the Spaceflight Environment on the Structure and Function of the Pulmonary System
The effects of microgravity on the lungs of crew members were studied extensively on several Space Lab missions in the 1990s, and a few additional measurements have been made on the ISS.675,676 Overall gas exchange
_____________
* Wnt signaling represents a large family of proteins that are involved in the differentiation, proliferation, and maturation of multiple cell types, including bone and cardiac myocytes; such proteins also are active in mediating vascular calcification, a key component of atherosclerosis.
as measured from oxygen uptake and carbon dioxide output during rest and exercise was essentially unchanged compared with the 1-g environment, and the same was true of alveolar pO2 and pCO2 values. The only exception was when the environmental pCO2 was allowed to rise during one of the Space Lab missions. The distributions of ventilation and blood flow became more uniform as expected, although some inequality remained, which is understandable considering the complexity of the airway and blood vessel systems. The diffusing capacity of the lung for carbon monoxide was increased, both because of an increase in the diffusing capacity of the blood-gas barrier and the increased volume of blood in the pulmonary capillaries. This could be explained by the increased thoracic blood volume resulting from the lack of gravity-induced pooling of blood in dependent regions of the body.
They were also minor changes in lung volumes. Functional residual capacity during microgravity was intermediate between that seen in the upright and supine postures at 1 g. This was explained by the influence of the weight of the abdominal contents on the diaphragm and was in fact predicted prior to any spaceflights. An unexpected finding was a reduction in residual volume. This was probably caused by the lung parenchyma being equally expanded in all regions in microgravity, which is not the case at 1 g. All these changes remained during the Space Lab missions, each of which was 9 to 16 days in duration. Total pulmonary blood flow (cardiac output) and cardiac stroke volume increased early in Space Lab flights but then decreased over the duration of the mission. An unexpected and still unexplained finding was an alteration in the intrapulmonary distribution of two gases of very different molecular weights, helium and sulfur hexafluoride, following a single inhalation. The provisional explanation is that there was a change in the topography of the acinar region of the lung, possibly as a result of interstitial edema, but this needs further investigation.
Additional studies were made on the ISS, although these were less sophisticated than those on Space Lab because of the limited equipment and crew time. However, an important finding was that pulmonary function in general returned to the pre-flight state within 2 or 3 days of returning from a 6-month mission.677 The net result of all these measurements suggests that the basic functions of the lung are unlikely to cause a health problem during a 3-year mission, for example to Mars.
Few animal studies of pulmonary function have been made in microgravity partly because much of the important information can be obtained from human investigations. The animal studies that have been made are generally consistent with the human studies. An exception is some invasive studies involving the injection of small microspheres and subsequent cutting of the lung into small pieces. These procedures clearly cannot be carried out in humans, but they show substantial inequality of blood flow at the acinar level. No gender differences in pulmonary function responses to microgravity have been reported.
In the event that pulmonary disease develops during spaceflight—for example, bronchitis or pneumonia—the consequences could be more severe than in the normal 1-g environment if there is an impairment of the immune response. This topic is discussed in the section below on the immune response in microgravity.
Status of Countermeasures
Countermeasures are of limited value in the context of pulmonary function. There is no feasible way of altering the breathing pattern of astronauts to modify the amount and site of aerosol deposition in the lung. The solution to this problem is to prevent exposure to lunar or Mars dust, presumably by appropriate filtering equipment in the breathing circuit. For the denitrogenation problem, it would be valuable to improve understanding of the nitrogen washout rate from the tissues in microgravity or low-gravity fields so that decompression sickness can be avoided. However, in the absence of this information, decompression sickness can be prevented by long nitrogen washout times using oxygen inhalation. The penalty here is loss of working time for the crew member. The occurrence of the presumed subclinical interstitial pulmonary edema resulting from the increase in thoracic blood volume appears to have no measurable effects on pulmonary function for the durations of microgravity encountered so far but conceivably will become an issue for longer flights. The change in chemosensitivity that results in a reduced ventilatory response to hypoxia seems to be of minor importance, at least in the present flight durations.
Gaps in Knowledge
The amount and the site of the deposition of aerosols of different sizes in microgravity and reduced gravitational fields have been investigated to some extent. For example, a few studies made in aircraft flying in parabolic profile patterns have found that, in both microgravity and lunar levels of gravity, dust of 1-micron diameter is deposited in more peripheral parts of the lung compared with 1-g conditions.678 However, much additional research is required. The same is true of denitrogenation rates in microgravity and reduced-gravity fields, for which more information is needed. Note that these rates will depend on the partial pressure of nitrogen to which a crew member has been exposed prior to denitrogenation, and this may be altered in exploration of the Moon and Mars. For example, an astronaut who lives in a reduced-barometric-pressure environment but breathes oxygen at an increased concentration to prevent hypoxia will have a very different denitrogenation rate than someone living in the normal atmospheric pressure of the Earth environment.
Research Models and Platforms
Human studies on the ISS are necessary to obtain more information on the amount and site of inhaled aerosols of different sizes. This can be done using single breath washouts as previously conducted on parabolic profile flights. In addition, animal studies in microgravity and reduced-gravity fields will be valuable for obtaining more information about aerosol behavior in the lung. Measurements in microgravity should be made on the ISS, and appropriate animal holding facilities will be required for this. In addition if a centrifuge for rodents was available on that facility, the measurements could be made at various partial gravitational levels.
Animal studies of the rate of denitrogenation are less applicable to the human situation but nevertheless could elucidate some of the basic biology, such as the rate of nitrogen elimination from organs having different amounts of lipid.
Some information about aerosol deposition in the lung can be obtained from parabolic flights, but the short duration of microgravity or reduced gravity there is a serious limitation. Future suborbital flights may provide additional opportunities.
Research Recommendations
1. Determine the amount and site of deposition of aerosols of different sizes in the lungs of humans and animals in microgravity. The rationale is that deposition is different in microgravity compared with normal gravity, and there is evidence that lunar dust and Mars dust are potentially toxic. The research would be carried out on the ISS and could potentially be concluded in a 10-year time frame.
2. Determine the rate of washout of nitrogen from the body for humans and animals in microgravity—for example, on the ISS. The measurements in humans should be made by recording the nitrogen concentration in expired gas. Measurements in animals could elucidate the washout rate of nitrogen from different organs of the body.
Risks for Specific Endocrine Influences During Long-Duration Space Missions
The endocrine system is essential for normal organism homeostasis on Earth, and its importance in maintaining and in re-establishing homeostasis in response to spaceflight and recovery was effectively documented in the 1998 NRC report A Strategy for Research in Space Biology and Medicine in the New Century.679 That rationale is not repeated in this report. However, the prospect and uncertainties of long-duration exploration and research flights combined with non-endorsed or non-implemented recommendations provided in 1998 indicate that select hormonal influences warrant further review. Thus, the goal of this section is to discuss those hormones whose influences could be enabling within a microgravity environment and those that have the potential to be better understood by study in the same environment. A complete discussion is difficult because existing in-flight data on hormonal influences and reports on humans are limited, and virtually all animal results have been obtained
after landing. As hormones obviously play a key role in many systems, relevant endocrine topics are included in a number of other sections in this chapter.
Alterations and Effects of the Spaceflight Environment on the Endocrine System
Growth Hormone
Human Studies. Growth hormone (GH) is important for bone metabolism, maintaining muscle mass and strength, stimulating afferent neurons, and promotion of growth and development of young animals and humans.680,681,682 However, measurement of GH is complicated by the heterogeneous nature of the hormone and by the presence and heterogeneity of GH-binding protein in the plasma.683 Further, methodological problems have not been resolved. Extensive human GH research has been conducted in Russia, where subjects participating in a long-term bed rest study had 16 percent lower GH levels and altered circadian rhythm profiles after 75 days, compared with the baseline. These changes were attributed to a decrease in motor neuron activity.684 Measurements obtained from 53 cosmonauts participating in Salyut and Mir flights for 114 to 438 days had post-flight GH levels that remained elevated for 10 weeks. The results were complex, given that 32 cosmonauts had values significantly higher than their pre-flight means, whereas 21 had significantly lower results. Additional subgroup investigations (n = 33) indicated an association between resting human GH levels and performing scheduled exercises during flight. Specifically, cosmonauts who did not adhere to prescribed exercise schedules had lower mean values than those with higher compliance records.685 McCall et al.686 reported that the release of bio-assayable GH was suppressed in microgravity, whereas the opposite results were obtained by Macho and associates, who used a similar experimental design but measured isoforms.687 Thus, although results have been mixed, on balance the findings suggest a tendency for reduced GH titers during a prolonged spaceflight, especially in subjects not performing routine physical exercise (see below).
Animal Studies. Hymer and associates reported that the secretory capacity of GH cells from rats flown on three different flights was significantly lower than in control rats, specifically when biologic rather than immunologic assays were used. They also reported similar trends occurring with HU rats.688 Bigbee and associates reported that basal plasma bio-assayable GH levels of HU rats were significantly reduced (51 percent lower after 1 week and 55 percent lower after 8 weeks), compared with those of ambulatory control rats.689 The pituitary cells flown on Cosmos 2044 had reduced secretion of growth hormone, which was attributed to the GH releasing factor peptide.690 Thus, in animals it appears that unloading conditions induced a reduction in GH levels; these results complement those of investigations on the hypothalamic-pituitary axis discussed in the 1998 NRC report.691
Thyroid Hormones
Human Studies. Thyroid hormones are important for metabolism and for the growth of young animals and humans. Slight increases in thyroid stimulating hormone were found for astronauts in the Apollo mission; significant elevations were reported for the astronauts in Skylab692 and for the four astronauts on the D-2 flight.693 In-flight thyroxine (T4) values of Skylab astronauts were significantly increased, whereas triodothyronine (T3) levels were decreased. These observations were thought to result from an inhibitory effect of cortisol on the conversion of T3 to T4.694 Reductions in in-flight urinary T3 and T4 levels were found on SLS flights 1 and 2. In cosmonauts on missions ranging from 115 to 438 days, post-flight plasma levels of T4 were elevated for 7 days before returning to near pre-flight means, while T3 levels were lower than their pre-flight means.695 Thus, it appears that T3, the biologically active thyroid hormone, is slightly reduced during and following spaceflight.
Animal Studies. Post-flight plasma levels of thyroid stimulating hormone were elevated after an 18.5-day flight and decreased after a 7-day mission.696 Summarized results from three Cosmos flights lasting from 14.0 to 19.5 days indicated that the post-flight T3 and T4 concentrations were significantly lower than those in controls.697 In the same report, thyroid gland concentrations of both hormones were significantly lower than those in controls.
Rats suspended for 12 days had significantly lower T4 values after 6 days.698 Surprisingly, there is a paucity of published literature on changes in the pituitary thyroid axis. Adams and colleagues appear to be the first to include thyroid-deficient rats in flight experiments to determine the interactions between thyroid deficiency and MHC gene expression.699 Using neonatal pups, they found that microgravity significantly reduced the expression of the fast type I MHC gene, whereas thyroid deficiency prevented the normal expression of the fast MHC phenotype and prevented the gravity-dependent expression of the embryonic/neonatal isoforms. They concluded that a normal expression of the fast IIb MHC required an intact thyroid state that appeared to be independent of normal weight-bearing activities.
Cortisol and Cortisone
Human Studies. Cortisol changes have been assessed in flight by measurements of blood, urine, and saliva. Stimulated by the secretion of adrenocorticotrophic hormone (ACTH) and known as the “stress hormone,” cortisol becomes an important consideration with long-duration missions because it:
1. Is a major cause of secondary osteoporosis in humans;700
2. Exerts proteolytic actions on both smooth and fast twitch (type II) skeletal muscles;701
3. Stimulates energy expenditure and fat metabolism;702 and
4. Has negative effects on the immune system.
Elevated cortisol levels during flight were characterized in the 1998 NRC report A Strategy for Research in Space Biology and Medicine in the New Century703as a “frequent but not invariable finding.” Data pertaining to short flights (2 to 14 days, N = 182) have indicated that post-flight ACTH and cortisone were significantly decreased, whereas after longer flights (120 to 366 days, N = 21), cortisone was significantly increased, by 171 percent.704 Measurement of plasma cortisol during the Skylab flights revealed an elevation by 20 to 80 percent throughout the flight.705 According to Grigoriev et al.,706 flights of 12-month duration resulted in a 10-fold increase in ACTH levels with lesser changes in cortisol concentrations, which strongly suggested an impact on the hypothalamic-pituitary-adrenal axis. Similar findings were reported for the SLS-1 and SLS-2 flights. Results from flights before 1998 demonstrated that fast twitch (type IIa) muscle fibers had experienced significant atrophy with reduced function.707
Metabolic studies using head-down bed rest conditions for 42 days showed significantly elevated urinary cortisol levels during the study.708 Further, a positive correlation between the change in tibia epiphyseal bone mineral content and elevations in serum cortisol levels was reported in a 90-day bed rest study to evaluate resistive exercise and pamidronate as a countermeasure.709 However, cortisol concentrations did not change in a 7-day bed rest study.710 A longer, 42-day bed rest study of the interactions between body composition, energy expenditure, energy, and water metabolism, along with catabolic and anabolic hormone expression, found marked reductions in body weight, lean body mass, total energy expenditure, energy intake, and the energy available for physical activity.711 The authors suggested that the hormonal profile, including a significant increase in cortisol, interfered with nutrient oxidation, and they proposed nutritional countermeasures. Collectively, these findings suggest that during the stress of spaceflight and bed rest, elevated cortisol can exert a potential confounding effect on protein balance.
Animal Studies. Overall, post-flight plasma corticosteroid results for rats from six missions of durations between 7 and 20 days demonstrated that the flight animals had higher values than did the controls, and in four of the missions, their mean post-flight plasma levels were significantly elevated (approximately four-fold) over the pre-flight mean values.712 Rats tail-suspended (HU model) for 12 days had significant increases in corticosterone at 6 days, which subsequently returned to near the baseline at the end of the study.713
Insulin and Diabetogenic Trends
Human Studies. The prospect of space research and explorations in the future involving long-duration flights merits a re-evaluation of the impact on insulin of prolonged exposure to microgravity. Notably, the 1998 NRC
report underscored the difficulty in interpreting insulin data from flights in isolation without parallel ground-based data.714 That report also noted the presence of conflicting results.
Using a 7-day head-down bed rest analog design that included men and women, Blanc et al. in 2002 demonstrated increased fasting insulin levels and the presence of insulin resistance, which the authors attributed to the magnitude of the skeletal muscle tissue in males and to both skeletal muscles and liver tissue in females.715 Decreased in-flight concentrations of insulin have been reported for crew members of Skylab and of select space shuttle flights,716 whereas nonsignificant elevations of insulin and C-peptide were reported for four astronauts in the SLS-1 mission717 and for the 10-day D-2 flight.718 Post-flight increases in insulin levels have been reported for nearly all Russian and U.S. flights regardless of the duration. It is not clear to what extent an increased dietary intake might be contributory, as suggested by the 1998 NRC report.719 Notably, limited glucose tolerance test results have been reported for astronauts, although flight results show higher insulin and C-peptide levels compared to data collected after landing or during recovery.720,721 Anecdotal findings in two cosmonauts, tested at days 321 and 322, suggest a response of “delayed utilization.”722 Markin et al. observed higher blood glucose concentrations for individuals who had been in microgravity for 6 months or longer.723 These latter findings emphasize the importance of regular, moderate-to-heavy exercise during long-duration flights to minimize increases in insulin resistance and the possibility of type II diabetes-like symptoms. Clearly, more research is needed on this important topic.
Animal Studies. The HU rat model is associated with the presence of insulin resistance.724 A decline in oral glucose tolerance and a decreased insulin-stimulated transport in isolated soleus muscle tissue, associated with an activation of the p38 mitogen-activated protein kinase, was found after 24 hours of head-down tail suspension in juvenile female rats.725 However, the unloading of the soleus muscle did not alter insulin-stimulated activities of various signaling factors. Tobin726,727 perfected a cell rotating culture system for simulating microgravity (high aspect rotation vessel or HARV) for use with islets of Langerhans. Using lipopolysaccharide to stimulate tumor necrosis factor α activity, Tobin and colleagues reported a significant decrease in insulin secretion and an increase in glucose levels. Overall, these findings on rodents support the conclusions derived above for humans.
Testosterone and Gonadal Function
Human Studies. Because the 1998 NRC report was based on information generated between 1977 and 1992, an additional focus on testosterone during long-duration flights is warranted.728 Testosterone influences growth, development, and reproduction, and its secretion is regulated by luteinizing hormone. When measured in the plasma, urine, and saliva during the 10-day D-2 mission (n = 4 astronauts), levels of luteinizing hormone were significantly increased, while levels of urinary and salivary testosterone and testicular androgen 3-α-androstanediol were significantly decreased.729,730
To date, there have been no studies in microgravity involving humans that have pertained to spermatogenesis. In a 120-day Russian bed rest study, Nichiporuk et al. reported that sperm collected after 50 to 60 days and 100 days exhibited a reduction in live spermatozoa with active mobility and an increase in the percentage of morphologically/structurally altered spermatozoa.731
Animal Studies. After 14 days in microgravity, post-flight rat testosterone concentrations were significantly reduced and 60 percent lower than in synchronous ground-based animals.732 A lower basal testosterone secretion rate was seen in rats after an 18.5-day flight.733 Suspended rats, included in the Cosmos 2044 study, exhibited markedly lower testosterone concentrations when compared to synchronous control. Both flight and suspended animals had significantly lighter testes than did controls.734 Because of the possibility of cryptorchism, Deaver et al. ligated the inguinal canal and found significantly lower testosterone levels in serum and intestinal fluid and lower testes mass in HU rats.735 Further, mice suspended for 12 days had significantly lower concentrations of testosterone and less testes mass.736 However, rats centrifuged at 2 g had elevated urinary testosterone levels, suggesting centrifugation as an appropriate countermeasure to restore homeostasis.737
Spermatogenesis research in microgravity began decades ago with two dogs that were in space for 22 days. The main finding was an increase in atypical spermatozoa characterized by tail curling or the absence of a tail.738
However, an extensive spermatogenesis study of rats returning from a 14-day flight reported essentially normal conditions, although the authors appropriately recommended additional studies in longer-duration flights.739 The negative results with the 7- and 14-day Cosmos 1667 and 2044 flights add credence to their statements.740 In 2002, Tash et al. conducted a 6-week HU study in which cryptorchism was prevented and stated “spermatogenesis is severely inhibited by long term HLS [hindlimb suspension].” However, the testosterone concentration was not depressed. Additionally, they reported that spermatogenic cells beyond the round spermatid were not present.741 Motababagni investigated the influence of HU on spermatogenesis in groups of rats that had inguinal canal ligation and observed a severely affected spermatogenesis after 6 weeks, because no late elongated spermatids and spermatozoa were present in the testes.742
Similar findings of impaired testes cell function have also been reported on isolated cell lines studied under simulated microgravity conditions.743 Collectively, both the human and animal studies suggest that gonadal function and spermatogenesis are impaired in response to chronic unloading conditions.744,745 Thus, future research is needed especially in humans to expand on these interesting observations during prolonged spaceflight.
Estrogen and Gonadal Function
In the approximately 5,600 publications listed in MEDLINE since 1948 under the term “weightlessness,” none indicated that estrogen and gonadal function have ever been measured in microgravity or in the recovery period. Moreover, this void is barely improved by simulated microgravity studies, as fewer than five investigations have been reported. Therefore, studies should be undertaken to address female endocrine function during exposure to microgravity and during analog studies such as bed rest.
Status of Countermeasures
The single most important countermeasure for any given hormonal alteration is direct hormonal replacement. Exercise is a likely next countermeasure for both humans and animals, given that exercise intensity affects hormonal responses.746 However, the choice of regimens and implementation of exercise need additional attention to go beyond what is currently advocated by NASA for astronauts scheduled for a 6-month tour on the ISS. Notably, a 2009 assessment of nine astronauts concluded: “Future long-duration space missions should modify the current ISS exercise prescription and/or hardware to better preserve muscle mass and function, thereby reducing the risk imposed to crew members.”747
Astronauts must be cognizant of glucocorticoid-induced type IIa fiber atrophy, which has been demonstrated in human spaceflight748 but can be prevented in fast twitch fibers (in animals) by moderate to heavy dynamic exercise.749 They must also be cognizant of the finding that the inactivity of bed rest will suppress the release of testosterone.750 While the release of insulin is reduced with progressive intensities of dynamic exercise, exercise will facilitate glucose uptake into cells by its action on glucose transporters.751 As to the specifics of the exercise prescription, countermeasures need to be designed in conjunction with the advice of experts in skeletal muscle biology.
In addition to its use as an exercise prescription, centrifugation has the potential to be an effective countermeasure for the endocrine reproductive issues associated with microgravity.752 For human use, a feasible system could be a short-arm centrifuge with the capability of achieving 2-g force vector, similar to one utilized by the University of California, Irvine.753
Gaps in Fundamental Knowledge of High Priority
The Influence of Microgravity on Human Spermatogenesis and Gonadal Function
Although humans have been exposed to space for nearly half a century, there is no published information on the effects of microgravity on human spermatogenesis and gonadal function. In addition, the paucity of simulation studies is striking. Minimal insights can be drawn from animal post-flight data because of the brevity of flights,
the limited number of animals, and the inability to eliminate the possibility of testes abnormalities existing before flight. Consequently, current knowledge of the subject depends on ground-based animal studies with conflicting results. The seriousness of this knowledge gap is emphasized by Tash et al.,754 who wrote concerning their results (spermatogenic cells beyond spermatid were not present), “if these findings hold true in μG [microgravity], it implies that male astronauts may become infertile after long-term exposure to μG.” This knowledge gap is well documented and needs to be addressed to ensure the safety of astronauts participating in future long-duration space missions.
The Influence of Microgravity on Smooth Muscle Atrophy with an Emphasis on the Role of Glucocorticoids
While much remains unknown concerning the effects of microgravity on the mechanisms of atrophy in both skeletal and cardiac muscle, the need for additional research is well recognized. For smooth muscle, assumptions from ground-based models and uterine myometrial results755 associated with parturition form the foundations of current knowledge. Whether smooth muscle not associated with the events of parturition actually exhibits atrophy processes in response to microgravity is unknown. Beyond the fact that glucocorticoids can enhance proteolysis in smooth muscle and are elevated in microgravity, their long-term influences remain to be assessed.
Microgravity and Its Suppressive Influence on Growth Hormone Secretion or Release
As emphasized in the animal studies pertaining to GH (see previous discussion) and when compared to control conditions, the secretory capacities of GH cells or pituitary cells flown in microgravity, as measured by their biological activity, were marked reduced. A similar trend was observed with HU rats.756-759 Since reports indicate that systemic insulin-like growth factor 1 levels were suppressed, mechanistic studies are needed to explain these observations and to determine their influence on the growth and development of neonatal rats or mice in microgravity. A coordinated ground- and flight-based research program is recommended to acquire this needed information. However, the survival, growth, and development of neonatal rats in microgravity will require both the availability and the modification of the existing animal habitat.
Gaps in Applied Knowledge of High Priority
Role of Hormone Changes as Mediators of Other Biological Responses to the Space Environment
It is unclear whether disruptions in multiple hormonal systems (e.g., thyroid, cortisol, GH) have a significant impact on physiologic changes during spaceflight (loss of bone, muscle, etc.), independent of the effects of microgravity. It is also not known whether changes in hormonal systems during spaceflight reduce the effectiveness of countermeasures (e.g., reduced effectiveness of exercise in a low-androgen state to preserve bone). Research in these areas is needed to determine whether hormonal homeostasis represents an important countermeasure target.
Quantifying the Diabetogenic Potential of Microgravity
Beginning in 1977 and continuing today, inferences concerning diabetic tendencies in astronauts continue to be made based on in-flight and/or post-flight hormonal measurements, especially those associated with C-peptide and insulin concentrations. Because analogs for both humans and animals follow profiles similar to those exhibited by flight animals, the significance and risk associated with this gap can be minimized by combining a diabetogenic awareness program with glucose tolerance tests during prolonged durations in simulated and actual microgravity.
Research Models and Platforms
NASA’s recent acceptance of the head-down bed rest model for the testing of potential countermeasures760 will end the debate as to the merits of the head-down bed rest position versus the horizontal bed rest position in maximizing the benefits of fluid shifts, and will standardize the analog for the research needed in the next decade. However, it is essential that the ISS become a platform for securing serial in-flight hormonal measurements to establish an endocrine database. Such a database will serve as a foundation for long-duration investigations pertain-
ing to gap research, countermeasure effectiveness, propensity for diseases, and hormonal interactions with other systems. Access to partial-gravity platforms is recommended for the same reason. Because the free-flyer platform does not allow sufficient time for an equilibrium to be established with regard to fluid shifts, its use is not recommended here for these types of studies.
Results from the HU model for rats and mice761 compare favorably with human data, but extrapolation to flight findings is tenuous because the overwhelming majority of these results were obtained after landing. Thus, it is essential to ensure access to the ISS platform for studies on animal models in order to establish an in-flight hormonal database, initiate long-duration investigations, examine pituitary cell secretion profiles, perform gap-related research, interact with other systems, and become involved with neonatal growth and development studies.
Research Recommendations
Fundamental Science
This section has recommendations for research on specific hormones, given that only the small number of hormones considered relevant to spaceflight are discussed. Because of the absence of in-flight results when post-flight data on hormones have been reported, future investigations (clinical or experimental) should not be endorsed or undertaken unless in-flight measurements are obtained and functional objectives have been defined. In the next decade, endocrine studies should be initiated with females and a database established. Needed areas of research include the following:
• Growth hormone
—Suppression of GH secretion in microgravity. The early reports that GH secretion from anterior pituitary cells was suppressed by microgravity762,763 should be pursued with animals to investigate responsible mechanisms.
—Relationship between GH levels and motor neuron activity. Researchers should explore the relationships between the observations of Russian investigators who reported that reduced basal human GH levels reflected a decreased motor neuron activity764 and reports from Edgerton’s laboratory that indicated the release of the bioassayable form of GH was triggered by muscle spindle afferents.765
—GH influences on growth and development in microgravity require investigation with special attention devoted to neonatal animals.
• Thyroid hormones
—Adaptation of thyroid hormones to microgravity. The variability or absence of free T4 measurements from flight missions indicate that serial thyroid hormones profiles need to be conducted in microgravity to determine the acute, early adaptive, and chronic adaptive stages.
—Animal studies pertaining to the hypothalamic-pituitary-thyroid axis should be conducted in simulated low gravity as well as in microgravity to determine if there is a cellular basis for the reports of reduced secretion in microgravity.
—Neonatal studies with thyroid-deficient rats and the expression of MHC isoforms represent an excellent example of a direction for future research.766
• Cortisol
—Impact of microgravity on smooth muscle. Cortisol is associated with bone loss and with the atrophy of smooth muscle and type IIa skeletal muscle fibers.767 To date, the atrophy of smooth muscle in microgravity
has not been systematically investigated or acknowledged even though its occurrence has been known since before the 1998 NRC report was released.768
—Cortisol and bone loss. With the prospect of long-duration flights, research needs to determine whether cortisol levels are a contributing factor to bone loss.
—Impact of microgravity on hypothalamic-pituitary-adrenal axis. It is important to determine whether ACTH and cortisol will become dissociated during long-duration missions.
• Insulin. Long-duration missions and insulin resistance. In the next decade, it should be determined whether long-duration spaceflight exerts a diabetogenic potential on human and animal subjects.769 Assessment by glucose tolerance tests is recommended. Unfortunately, there is limited relevant scientific data from microgravity research on this topic, with no existing databases and no established guidelines for implementation. Therefore, the recommendation for insulin research in the next decade should be aggressively developed and implemented via a plan that will decrease the possibility of insulin resistance becoming a major risk factor on long-duration spaceflights.
• Testosterone. Impact of microgravity on testosterone production and on spermatogenesis. Research in this area is warranted because of the continuing uncertainty and controversy regarding whether microgravity (1) alters testosterone production or (2) affects the process of spermatogenesis and alters testes mass in humans. Because there is a lack of human data from bed rest or microgravity studies, current concepts on testosterone and spermatogenesis have essentially evolved from animal analog data. This emphasis needs to be refocused on human male gonadal function to address the remote possibility that male astronauts may become infertile after long-term exposure to microgravity.
Effects of the Spaceflight Environment on the Immune System
Human Studies
Most of the studies in this area performed over the past 30 years have used a similar experimental design. Samples are taken from astronauts prior to and upon return from flight. Effects are determined by assessment of changes within an individual over time or compared to ground-based individuals. Investigations of the immune system have addressed whether (1) there was a change in the number and/or percentage of immune cells, (2) the function of the cells was altered, and (3) the changes were reversible or permanent.
Determination of changes in the human immune system has been limited to assessment of peripheral blood. As reviewed in the 1998 NRC report,770 the data reflect a wide range of results; some of the variation can be explained by differences in study parameters (duration of flight, assay conditions, sample treatment). Considering both previously reviewed data and recent space shuttle and ISS missions, the most consistent findings have been an increase in the number of white blood cells, predominately reflected in an increased number of neutrophils and a decreased number of lymphocytes771-774 and natural killer (NK) cells.775,776 Although some studies suggested a shift in CD4 (helper)/CD8 (cytotoxic) T cell ratios, this change has been small in many cases.
Recent examination of the function of the innate component of the immune response continues to show a pattern similar to that reported in studies included in the 1998 NRC report:777 (1) the number of studies is limited; (2) although decreases in function are observed, the magnitude of the change is not great and/or the pattern of change across various functions is not consistent.778,779 Most of the studies have focused on the function of lymphocytes. No significant changes have been observed in immunoglobulin levels.780 Although this may suggest no change in B cell function, studies addressing the production of antibodies after immunization during flight have not been performed. Using whole blood or cells isolated from blood, lymphocytes have been stimulated with mitogens (e.g., phytohemagglutinin) or antibodies (anti-CD3 and anti-CD28). Most studies report a decrease in the proliferative responses of the T cells to these stimuli.781 In addition, cytokine production has been consistently altered, although which cytokines are significantly changed varies among studies. Although interleukin 2 (IL-2) is decreased in most studies, leading some investigators to conclude that there is a shift from Th1 (IL-2, IFN-γ) to Th2 (IL-4, IL-10) cytokines, the effect of spaceflight on IFN-γ production has not been as consistent.782,783 More recent studies have addressed the induction of activation markers on the surface of T cells after mitogenic stimulation. Early
activation markers CD69 and IL-2 receptor CD25 appear to be elevated after shorter (9 to 10 day) space shuttle missions, while CD69 appears unchanged but CD25 is decreased upon longer ISS missions. These data suggest that differences in dysregulation of the immune system may depend on flight duration. Interestingly, a similar pattern of decreased proliferation, IL-2 receptor activation, and cytokine dysregulation, including the results with IFN-γ, have been observed in the elderly.784 The major difference between the changes observed in the immune response with aging and with spaceflight is that the effects of spaceflight are reversed days to weeks after flight.
The prevailing study design compares parameters of interest pre-flight with those obtained immediately after return to Earth. Therefore, the differences observed may reflect the forces and stressors of re-entry rather than the effect of flight. Two approaches have been used in humans to study changes in immune response during flight. In the first approach, delayed type hypersensitivity (DTH) responses to the same multi-antigen panel were compared when the antigen challenge was administered 2 days prior to landing and the response evaluated upon landing, and when both antigen challenge and subsequent 48-hour evaluation were performed 2 months prior to flight. In the two studies performed, decreased responses were observed after in-flight antigen administration.785,786 The second approach, which has been used fairly extensively in recent studies, assesses the reactivation of latent herpes viruses.787-790 Because an immune response to the virus is important in maintaining latency, expression of latent herpes viruses has been considered a surrogate for a decrease in virus-specific immune responses. Studies assessing viral DNA in saliva samples every several days have demonstrated expression of varicella zoster virus (VZV, the latent virus causing shingles) during and after flight in 6 of 8 astronauts791 and an increase in both the percentage and the level of Epstein-Barr virus (EBV) DNA in 32 astronauts during and after flight, compared to ground-based controls.792 Both the decreased DTH responses and the increased expression of latent herpes virus (e.g., VZV) are similar to responses observed in the elderly.
Although a significantly decreased immune response will render an individual susceptible to infections and a severely compromised immune system will make normally innocuous organisms life-threatening to individuals, the question is whether the level of change in the immune response that occurs with spaceflight is sufficient to result in increased susceptibilities. The 1998 NRC report concluded that, although initial studies suggested an increase in infection, the number of infections during flight decreased once multiple procedures were implemented to minimize exposure of astronauts to infectious agents prior to flight. Epidemiological review of recent flights indicates 29 incidents of probable infectious diseases during 106 shuttle flights with 742 crew members.793 While the changes in the immune response due to flight may have contributed to the development of infections, other conditions, i.e., confined living spaces or difficulties in maintaining good hygiene, may also have increased crew members’ exposure to potential pathogens, resulting in infection. In addition, because it is difficult to eliminate exposure to agents for which prevention is understood, it seems highly unlikely that it will be possible to develop measures to prevent exposure of astronauts to microbial agents that may develop enhanced potential for disease during flight (see Chapter 4) or that are introduced from the new environment. In these conditions, an immune system that is functioning at an optimum level will be necessary to ensure the health of extended-mission astronauts. Even if no negative event occurs during flight, the question remains whether dysregulation of the immune response during extended missions can have long-term effects on astronauts.
Animal Studies
Studies of animals that were flown had similar design limitations. Most of the studies were performed in rats before 1998, and examinations for effects were performed after the animals returned from flight. The results are similar to those for humans: the most consistent changes occurred in T cell responses.794 However, due to the availability of multiple tissues for examination, it was possible to examine immune responses of various lymphoid organs in both rat and mouse models. Interestingly, although decreases were detected in proliferative responses in lymphocytes isolated from lymph nodes, no changes were observed in the spleen.795,796 In another study, decreases were observed in both spleen and lymph node.797,798 In the latter study, changes in the percentages of white blood cells in both lymph node and spleen paralleled those observed in humans: neutrophils were increased and lymphocytes decreased. The animal studies provide the unique ability, therefore, to determine if spaceflight affects
distribution and recirculation of lymphocytes. For example, if Moon dust affects pulmonary function and immune cells cannot appropriately migrate to the lung, there may be increases in respiratory infections.
Countermeasures
If the change in the immune system in response to microgravity is found to be biologically significant, it is unlikely that there will be a simple countermeasure. However, because the immune system is affected by a number of parameters that are currently being explored as countermeasures for other adverse effects of spaceflight, any or all of those approaches may alter the immune response. For example, exercise is being proposed to counter the loss of bone and muscle. However, intense exercise can decrease the level of immune response.799 Adequate nutrition is a continuing concern during flight. Although long-term caloric restriction can delay the decrease in immune response that occurs with age,800 deficiencies in specific vitamins or minerals or malnutrition can result in significant decreases in immune response, which are reversible upon correction of the defect.801 Because nutritional status has not been correlated to the level of immune dysregulation that has been reported, it is possible that focused nutritional supplementation could minimize some of the decreases observed in immune response. Similarly, alterations in sleep patterns affecting circadian rhythms have also been demonstrated to decrease immune responses.802 These observations, however, must be interpreted in the appropriate context: although both exercise and nutrition have been repeatedly reported to alter immune responses, the effects are often statistically significant but not necessarily biologically important. However, by utilizing multiple approaches (exercise, nutrition, sleep), with each influencing the immune response in small increments, the magnitude of the dysregulation of the immune response may be minimized, thus allowing the immune system to protect the individual adequately.
Gaps in Knowledge
Several major gaps in knowledge identified in the 1998 NRC report803 still have not been addressed. First, most of the studies since then have assessed the changes in immune response upon re-entry to Earth’s atmosphere. Due to the stresses of re-entry, it is unclear to what extent the observed changes were due to re-entry or to exposure to microgravity. Because consistent decreases in lymphocyte function have been observed, it is essential that more studies be done during spaceflight to determine the point of stress. Second, while the reported changes in the immune response are statistically significant and reproducible, it has not been possible to establish if the changes in the immune system would result in increased susceptibility to infection. As indicated in the 1998 NRC report, studies can be performed in humans to address this question: astronauts can be immunized during flight, cells and sera can be collected both prior to and immediately upon re-entry, and results can be compared. T cell and antibody responses specific to the immunogen, as well as the general status of the immune response as defined by number, phenotype, and response to mitogens, not only will provide information on the specific immunization protocol but also will allow better interpretation of the previous data and assessment of potential increased susceptibility to infections. Unfortunately, these studies have not yet been performed.
However, response to immunization remains only a surrogate for the immune response to infection. The ideal situation is immunization and challenge with an infectious agent. That experimental design will never be safe for implementation in humans. The experimental model of choice for comprehensive examination of response to immunization in flight would be animal analogs, and preferably mice, due to the extensive reagents available for characterization of the immune response in mice, the ability to house more animals in a small space, and the high relevance to the human system. Immunization and infectious challenge of mice during flight might not be possible due to concerns about infection spreading to the spacecraft crew. However, as previously recommended in the 1998 NRC report,804 immunization during flight with a collection of immune samples prior to and upon re-entry, with challenge upon re-entry, needs to be performed to definitively establish whether or not the changes observed represent an important risk for astronauts.
In addition to these animal studies, further investigation is needed of the data that are currently available from astronauts. For example, although there is increased activation of latent herpes viruses (e.g., EBV and VZV) during flight as determined by increases in the number of copies of viral DNA, the relationship of these changes
to symptoms is unclear. Symptoms related to reactivation of EBV are difficult to detect, but reactivation of VZV as shingles can be assessed. It was noted that one astronaut developed shingles upon return, but the expression of VZV DNA by this individual was not mentioned.805 In addition, the immunity parameters evaluated before and after flight should be expanded to include more markers reflective of aging. The current profile of changes in immune response after spaceflight reflect those of aging. However, aging is associated with additional changes (e.g., shortening of telomeres, CD28 and Treg expression) that are not currently evaluated in astronauts. If the changes induced by spaceflight reflect the additional parameters that are unique to aging, spaceflight or spaceflight analogs may be useful in exploring the biological underpinnings of the initial stages of aging—a study impossible on Earth because the stressors that induce the aging phenomenon on Earth are not sufficiently defined to allow assessment at initiation of the process.
One of the concerns regarding long-duration spaceflights that has not been addressed is the potential effect of radiation on the immune system. It is well established that radiation associated with x-rays and gamma rays (low-LET radiation; described in Chapter 7) can significantly decrease the immune response: radiation can prevent a primary immune response, with higher doses inhibiting both primary and memory immune responses. While there are fewer studies that have examined immune system effects of the radiation present in space, such as protons and high-LET radiation, a similar decrease in the ability of lymphocytes to proliferate was observed after irradiation with gamma rays or protons.806 More recently, exposure to heavy ions (high LET) resulted in significant loss of telomeres of lymphocytes,807 a situation seen frequently with aging and associated with decreased proliferation. Although these data suggest that the radiation encountered in long-duration spaceflight would decrease immune response, the limited ability to study forms of high-LET radiation and particularly low-dose but long-term exposure to high-LET radiation, makes it difficult to determine the magnitude of the effect radiation would have on immune cells during flight. Studies that involve microgravity in conjunction with radiation would have to be performed in order to definitively assess the impact of these combined stressors on the generation of immune response and the development of abnormalities within the immune system, such as the development of lymphoma and leukemia.
A number of ground-based systems have been developed using both human and animal subjects to allow further exploration of the changes induced by flight.808-813 Although each system may simulate several of the aspects of flight (e.g., stress, alterations in circadian rhythm), none of the systems has the ability to mimic all of the stressors. Until definitive information is developed regarding the parameters that are consistently altered in humans during spaceflight, it is impossible to determine which of the ground-based models should be explored further. In humans, the Antarctic studies appear to have many similarities with flight, including decreased proliferative responses, altered cytokines, and reactivation of latent herpes viruses.814 However, these studies also have significant limitations in the range of experiments that can be performed.
Recommendations
Although no clinically deleterious effects of spaceflight on the immune system have been observed to date, any consistent changes in the immune response due to flight need to be pursued because of the potential for major negative consequences during longer flights or altered external conditions. The panel therefore makes the following recommendations:
1. Existing data from both published studies and longitudinal assessments of astronaut health should be carefully evaluated together to determine whether observed changes in immune response upon re-entry are associated with symptoms related to reactivation of herpes viruses, infections, and autoimmune processes.
2. Multiple parameters of T cell activation should be obtained from astronauts before and after re-entry to establish which parameters are altered during flight. Because many studies were conducted 20 to 30 years ago, the parameters examined need to reflect the current state of the technology for immune assessments.
3. It is essential that studies with mice be performed on the ISS to establish the biological relevance of the changes observed in the immune system. These studies should include immunization and challenge, with samples of lymphocytes and sera acquired both prior to and immediately upon re-entry. The parameters examined need to be aligned with those human immune response components influenced by spaceflight.
4. The changes observed in the immune system may reflect the impact of multiple stressors. To both address the mechanism(s) of the changes in the immune system and develop measures to limit the changes, data from multiple organ/system-based studies need to be integrated. For example, data from cortisol assessments (stress studies), nutritional diaries, weight changes, exercise volume and intensity, and level of activity (e.g., EVA) need to be considered when designing and interpreting immunity data from individuals.
5. The reversibility of the changes that occur during and after flight has to be examined more carefully. Incorporation of parameters used to identify aging (e.g., shortening of telomeres, CD28 and Treg expression) in the expanded evaluation of immune responses in spaceflight may provide insight regarding either how the two systems are different or how the changes in flight might be utilized to identify early aging.
Developmental space biology is the fundamental research discipline concerned with the influence of gravity variations and other factors inherent to the space environment on reproduction, genetic integrity, differentiation, growth development, lifespan, senescence, and subsequent generations.815 Previous NRC study committees816,817 and other advisory groups818,819 have consistently underscored the importance of research on developmental biology for advancing understanding of how gravity shapes life on Earth. These reports have clearly articulated priorities for developmental space biology research, namely: (1) utilizing the space environment to enhance understanding of the nervous system, and (2) understanding effects of the space environment on the complete life cycles of mammals and other vertebrates. The topics discussed in this section focus exclusively on basic research enabled by the unique environment of space.
Prior to 2003, there was a growing emphasis on spaceflight studies of the reproduction and development of insects, fish, amphibians, birds, rats, and mice.820,821 U.S. animal experiments in space have since been dramatically curtailed due to funding shortfalls, retirement of the Spacelab program in 1998, impending loss of the space shuttle, and inadequate animal research capabilities.822 While past recommendations remain important, new vistas have significantly broadened the range of discovery opportunities.
Importance of Developmental Biology Research in Space
Developmental biology is concerned with early determinants of fertilization and embryogenesis and with the subsequent development and maturation of organisms throughout the lifespan and across generations. It is a core discipline within the contemporary life sciences that crosses the boundaries of neurobiology, systems biology, molecular biology, genomics, and epigenetics. Developmental analysis focuses on mechanisms of development, differentiation, growth, and maturation at the molecular, cellular, genetic, and organismal levels, as well as the evolution of ontogenetic mechanisms. Because different organisms solve the problems of development differently, multiple model systems are used to uncover guiding principles and identify molecular mechanisms that are conserved across phylogeny.
In the past decade, major conceptual and technological advances in genetics, molecular conservation, and genome sequencing have considerably expanded the scope and depth of developmental biology in ways that will significantly enhance the ability to uncover fundamental biological principles governing how bodies and brains organize, develop, maintain, and adapt under the constant force of gravity.
• Epigenetics (“above the genome”) refers to gene-environment interactions that establish an individual’s phenotype beginning early in life.823 Epigenetic analysis provides the first mechanistic representation of how environmental factors modulate genetic expression without alterations in DNA sequences. This new approach is increasing basic knowledge of how observable molecular changes (e.g., chromatin remodeling, DNA methylation, and histone modifications of gene products) result in stable, heritable changes in gene expression and later-life phenotypes. Epigenetic modifications can persist across generations, raising important questions regarding the ability of offspring of multigenerational subjects of studies in space to adjust to normal gravity.
• “Omics” systems biology is based on high-throughput gene array technologies that allow for simultaneous examination of multiple, complex changes in DNA, messenger RNA, proteins, metabolites, and methylation state.824,825 These exciting new approaches are rapidly advancing genomics and related fields, thereby significantly advancing perspectives on and understanding of biological subsystems and their functional integration. Next-generation deep sequencing machines promise to yield whole-genome and organ-specific expression profiles at unprecedented speed, thereby enabling impressive scientific achievements and novel biological applications.
In the era of the ISS, developmental biology research is poised to enable new and fundamentally important discovery domains. Developmental studies using model organisms that allow for detailed comparative analyses at cellular, genetic, and molecular levels will provide a wide range of new opportunities for space biology research that were not present in the past decade.
Effects of the Spaceflight Environment on Reproduction and Development
For invertebrates and some vertebrates, fertilization and development can proceed in space. Because of short flight durations, high costs, and limited opportunities for research in space, only brief reproductive phases have been studied in vertebrates. The most promising empirical approaches in developmental biology rely on studies of multiple model systems, comprising key organisms spanning invertebrates to mammals, for which genomic information is available.
Invertebrates
The fruit fly (Drosophila melanogaster) and nematode (Caenorhabditis elegans) are highly organized, multicellular organisms well suited to elucidating certain molecular, genetic, cellular, and physiological responses to the space environment within and across multiple generations.826 Most importantly for mutational analyses, these and other invertebrates are excellent models for studies of DNA damage/repair and programmed cell death (apoptosis)827 and for identifying additive influences of the space environment, particularly microgravity and space radiation828 (important for enabling space habitation rather than fundamental biology). Invertebrates have short life cycles, producing hundreds of offspring within weeks, thereby contributing to the branch of developmental biology concerned with evolution and development—in this case, the adaptive effects of multigenerational exposure to microgravity. Such adaptations could be enhanced using flies with or without gravity sensation. Comparative gene microarray analyses of spaceflight-exposed flies and nematodes can be used to identify gravity-induced changes in expression of orthologous genes.829 No overt structural or functional effects of spaceflight exposure on invertebrate development have been reported, although genes related to embryonic and larval development, gametogenesis, and reproduction are up-regulated in C. elegans.830
Vertebrates
Amphibian embryonic development is adaptive and characterized by a high degree of plasticity. Studies of Medaka fish, Urodele amphibians, and frogs in space have demonstrated successful mating, external fertilization, and hatching.831 Structural abnormalities during early post-fertilization events have not been consistently observed across species, and percentages of fertilization and survival are typical. Space-born fish possessed the normal complement of germ cells and subsequently produced offspring at 1 g.
Mammalian reproduction comprises an intricate and complex series of events, including internal fertilization, implantation, placentation, organogenesis, fetal development, birth, lactation, parental care, and postnatal maturation. Until the first mammal undergoes an entire life cycle in space, it is difficult to specify whether precise developmental phases are gravity-dependent. The minimal existing data, derived from rats, suggest that early reproductive events (i.e., fertilization, implantation, placentation, organogenesis), the transition from prenatal to postnatal life, and maternal-offspring interactions are high-priority research areas. No mammal has given birth in space. It is unclear whether normal vaginal birth of rats or mice can occur in the space environment.832 Cesarean deliveries may be required because of decreased connexin 43, the major gap junction protein in the myometrium that synchronizes and coordinates labor contractions.833
Growth and development of prenatal rats exposed to spaceflight for 4.5 to 11 days and then born on Earth either were normal or the offspring weighed significantly less than controls.834 One-week- but not 2-week-old rat pups flown in a habitat that was suboptimal for supporting nursing litters weighed 25 to 50 percent less than controls.835 The retarded growth of the young postnatal rats was likely due to disrupted nursing and mother-offspring interactions, rather than a direct effect of the space environment on growth and development. These observations attest to the importance of developing space habitats adequate for supporting reproducing and developing rodents.
Vestibular Development. As the primary vertebrate sensory system that detects changes in gravitational force, the vestibular system is responsive to linear accelerations (gravitational and translational) acting on the head. Rotation, or angular acceleration, is detected by the semicircular canals within the inner ear, but also affects the gravistatic receptors through centrifugal force. Information is relayed to the central nervous system via vestibular ganglion cells from the vestibular receptors to vestibular neurons in the brainstem to motor neurons. The vestibular system has widespread interactions throughout the neuraxis, including motoric, autonomic,836 homeostatic,837 circadian,838 affective,839 and spatial navigation systems.840 Studies in the space environment provide the most useful and meaningful approaches to advancing knowledge of how these many systems organize, develop, and become integrated.
Experience transduced into neural activity programs the developing nervous system and determines its ultimate form and function. Early sensory experience plays active, formative roles in shaping both the neural architecture and neurobehavioral function,841 leading to the fundamental biological tenet that specific age-dependent experiences are required for proper development of the central nervous system. The concepts of critical and sensitive periods refer to particular times in development when there is heightened sensitivity to certain environmental stimuli that determine subsequent function. For example, delayed cochlear implants will not restore hearing in deaf-born children when they are past the age to learn to decode sound. In alternating amblyopia, stereovision is lost because of a misalignment of the sensory input (foveation) during the child’s developing years, often because of strabismus. Thus, there is perfect vision in both eyes, but the visual world is viewed by only one eye at a time.
These concepts are broadly applicable to our understanding of vestibular system formation, development, and function. Yet knowledge of vestibular development has progressed far more slowly than knowledge of other sensory systems, in part because of the historic difficulty of depriving young organisms of the Earth-constant stimulus of gravity. Studies of rats that underwent development in space842,843 (Neurolab) provided early indications of the existence and length of the critical period, but additional studies are needed. Mutants without gravity perception can provide some insights but cannot substitute for exposure to microgravity, because such mutants lack all sensation and cannot easily regain it unless vestibular implants are fully functional.
Studies of the effects of different gravitational environments can be an invaluable tool for advancing fundamental understanding of the organizing principles of the vestibular and other neuromotor systems. Gravity deprivation during a critical period in development and maturation is likely to alter each of these systems.844 Progress has been made in specifying molecular and cellular events involved in normal vestibular development,845,846 yet relatively little work has focused on consequences of gravity deprivation. The limited spaceflight data suggest that vestibular system development occurs differently in the absence of gravity. Morphological changes in gravistatic receptors and in central connectivity and/or function have been reported in a variety of species, including tadpoles,847 fish,848 chicks,849 and rats850,851 after varying durations of embryonic or post-hatching/postnatal development during spaceflight. Central vestibular and behavioral changes were noted in perinatal rats that underwent the second half of gestation in space. Studies have also reported changes following development in hypergravity,852 with some effects opposite those observed in microgravity.853 These studies support the importance of dose-response comparisons between gravity load and biological response, using hypergravity to predict changes in microgravity.
Recently, a number of genetically altered mouse strains that fail to develop various elements of the inner ear vestibular apparatus have become available.854 Mice that fail to develop otoconia, thereby lacking gravistatic receptor function,855 and other mutants (e.g., members of the Hmx homeobox gene family) exhibiting depletions of vestibulosensory cells can provide fundamental new information on molecular and cellular bases of vestibular development and evolution. These models should be incorporated into research on space biology platforms, particularly using conditional mutants with defects that allow direct comparison of normal and mutated littermates with little or no other systemic defects and good viability.856 Importantly, while vestibular responses to linear
acceleration are absent in some of these genetically altered mice, they appear to compensate for much of this loss.857 The microgravity of space provides the gold standard for advancing fundamental biological principles of vestibular system development and function.
Motor Development and Sensory-Motor Integration. Neuromuscular development begins in utero and continues throughout the first year of life in humans and throughout the first postnatal month in rats.858 The neuromuscular system requires gravitational loading during early skeletal muscle development. Space-flown neonatal rats exhibited profound changes in development of the weight-bearing soleus muscle, including decreased muscle fiber growth, increased sensitivity to muscle reloading injury, reduced growth of motor neuron terminals, and lowered ability of the muscles to utilize oxygen.859 Changes in the expression of myosin isoforms were observed in neonatal rats flown on the same mission.860 Studies involving hindlimb unloading have recapitulated some of these effects,861 but the definitive tests are studies in space.
The presence of gravity may be as fundamental to the development of posture and movement as images are to the development of the visual system.862 Whereas exposure to spaceflight of prenatal rats had no effect on the development of somatic motor skills, young postnatal rats exposed to spaceflight for either 9 or 16 days displayed a repertoire of motor behaviors different from those of 1-g controls.863
Both size and complexity of the dendritic architecture of the medial spinal motor neurons that receive vestibular input and are involved in postural control were reduced in 1-week-old rats exposed to spaceflight.864 Existing data suggest that these enduring experience-related changes are due to altered numbers and locations of synapses, rather than altered potency of pre-existing stable synapses. Neurons subserving motor function may thus undergo activity-dependent maturation in early postnatal life in a manner analogous to the sensory systems.
Cortical Changes. Studies of prenatal rats flown in space provide preliminary evidence for an aberrant or decelerated schedule of neuronal births in cortical layers, neuronal degeneration, and fewer glial cells and capillaries, compared to controls, indicating possible developmental retardation. Young postnatal rats flown on the space shuttle showed laminar-specific changes in the number and morphology of cortical synapses,865 suggesting that gravity may be an important environmental parameter for normal cortical synaptogenesis.
Cognitive Mapping. A single cognitive mapping study has been done, barely scratching the surface of this important area of inquiry. Young postnatal rats exposed to spaceflight for 16 days showed remarkably normal performance on Morris water maze and radial arm maze tasks,866 suggesting minimal long-term impact of exposure to microgravity, at this particular developmental age and flight duration, on spatial learning and memory.
Gaps in Knowledge
Lifespan and Multigenerational Studies
Lifespan studies of mammals to enable identification of gravity-dependent processes in the organization and development of the central nervous system and other organ systems, including their integration, function and maintenance, and transmission across generations, have not been accomplished. The small amount of existing data is derived from brief (16 days or less) spaceflight studies with post-flight analyses at 1 g. High-priority studies include lifespan and multigeneration studies in the space environment, incorporating developmental programming, epigenetics, and omics systems biology approaches during key reproductive and developmental phases in different model systems, especially mammalian models.
Developmental biology research is needed to fill gaps in knowledge of the effects of the space environment on (1) gene expression changes over time in model organisms; (2) DNA replication and repair processes, and their long-term consequences; (3) changes in single-nucleotide polymorphisms in large populations across multiple generations; (4) replication and reproduction across multiple generations; (5) intracellular molecular changes; and (6) changes in tissues, organs, physiological systems, and whole organisms throughout the lifespan and across generations in space. Throughout these studies, it will be important to determine effects of gravity in relation to those of other space-related factors (e.g., radiation) and their interactions.
Vestibular Development
Gaps exist in knowledge of gravity’s influence on (1) vestibular system development from peripheral end organs to central nervous system targets, including ground-based studies focused on genetically altered models and species in which peripheral organs can be selectively ablated during development (e.g., chick); (2) vestibular interactions with other neural systems (i.e., homeostatic and circadian systems, autonomic system, formation and maintenance of neural maps, affective system); (3) influence of efferent systems on the development and maintenance of peripheral vestibular apparatus; (4) development of postural, gait, and locomotive control systems; (5) mechanisms for vestibular compensation; and (6) role of vestibular experience in complex navigational behavior. There is a major gap in understanding of the organization and development of structural and functional linkages among these diverse systems. Basic research enabled by the unique environment of space and ground-based studies are needed to address this deficiency.
Motor Development and Sensory-Motor Integration
A major gap exists in understanding of how exposure to the space environment from pre-conception throughout adulthood affects the formation, development, function, and maintenance of the motor system. It is important to ascertain whether these effects reflect reorganization of a motor system developing in ways that are appropriate to the environment within which it has developed and the extent to which post-development adaptation to a different environment can occur. It may be that gravitational loading is required for proper development and integration of vestibulomotor function. Understanding the mechanisms underlying these changes will shed important new light on motor development and its controls.
Neuroplasticity
Knowledge is lacking of how experience with gravity influences neural development. Neuroplasticity refers to changes, through new experiences,867 in neurons, neural networks, and their function that occur in target neurons, pathways, or entire sensory systems through apoptosis (programmed cell death) and cell atrophy. Neuroplasticity is not limited to development but persists in adulthood as an inherent feature of everyday brain function; it is critical for learning and memory and the adaptability of primary sensory maps.868 During early life, neuroplasticity is shaped by sensory input during critical periods of development.
Neurotrophins (e.g., nerve growth factor, brain-derived neurotrophic factor, and their Trk receptors) play major roles in central nervous system development, plasticity, and experience, including learning.869 During development, neurotrophins control neuronal survival, target innervation, and synaptogenesis. In the vestibular system, TrkB activation and, to a lesser extent, TrkC activation are important for ganglion neuron survival, innervation, and synaptogenesis.870 Studies in the space environment and ground-based platforms can address this major gap in fundamental understanding of how gravity shapes neural development and plasticity.
Research Models and Platforms
A robust and meaningful research effort in developmental space biology will require access to spaceflight as well as extensive use of ground-based platforms and approaches, including centrifugation, HU, and genetically altered animals. Invertebrate, non-mammalian vertebrate, and mammalian models should be utilized.
• Free-flyers. Free-flyers should be used to conduct shorter-duration missions, optimally with an animal centrifuge to provide proper 1-g controls for mammalian studies, an important experimental control that was eliminated from the ISS.
• Celestial bodies. Beyond 2020, celestial bodies such as Earth’s Moon are important potential research platforms for ongoing studies in partial gravity. They could eventually provide platforms for sustainable research laboratories for developmental biological research across generations.
• Centrifugation. Ground-based centrifugation should be used to apply fractional increments in g-loads exceeding 1 g along a “dose-response” continuum to facilitate understanding of responses in decreased gravity and to help develop a program of research in artificial gravity.
The ISS is a necessary platform for long-duration studies (60+ days). To date, NASA has not added Advanced Animal Habitats (AHHs) to the ISS, and so its current capacity is 6 mice. However, with the addition of the four AHHs recommended in this report (capable of housing 6 rats or 8 to 10 mice) it would be possible to bisect some or all of the AHHs so that long-term developmental studies could be undertaken. Four habitats could be bisected to provide eight compartments for housing a single breeding pair or nursing litter to maintain statistically meaningful numbers of mice for lifespan and multigenerational studies. This plan utilizes a staged approach for mating and for birth and weaning of eight litters within a 30-day time frame, with a second generation produced within 90 days. Offspring can be removed at specific ages for analysis at key developmental time points. This approach serves to limit the overall number of mice to required levels and, once established, is particularly amenable to investigations of genetically altered mouse models.
Research Recommendations
1. Studies should be conducted on transmission across generations of structural and functional changes induced by exposure to space during development. Such research will provide vital fundamental information about how genetic and epigenetic factors interact with the environment to shape gravity-dependent processes and about the penetrating influence of those factors across subsequent generations. Spaceflight experiments offer unique insights into the role of forces omnipresent on Earth (but absent in orbital flight) that can actively shape genomes in ways that are heritable. Such spaceflight experiments would place gravitational biology at the leading edge of modern developmental and evolutionary science.
2. Spaceflight and ground-based (e.g., centrifugation, hindlimb unloading) studies should be conducted to ascertain the role(s) of gravity in the organization, development, and maintenance of the sensory and motor systems in mammalian systems and their functional integration. These studies will yield important new fundamental biological knowledge regarding the development and regulation of gravity-dependent brain, physiological, and behavioral systems. High-priority research should examine structural and functional changes in peripheral and central elements of the sensory and motor systems and their underlying mechanisms, critical periods of development, sensory-motor integration, neural plasticity and modulation of nerve growth factors, and adaptation. Important research in this area could be completed within a 10-year time frame.
3. Model systems offer increasingly valuable insights into basic biology. There should be a coordinated emphasis on utilization of invertebrates, non-mammalian vertebrates, and mammals to identify functional and evolutionary commonalities. Genetically altered models including mice should be used for analysis of key genes, gene products, and signaling pathways. This research should include an organized effort to identify orthologous genes, common changes in gene expression, epigenetic analysis, and key model systems for spaceflight. Experimentation with rats should also be conducted to analyze gravity-based changes in certain systems (e.g., muscle) and to retain continuity with existing developmental space biology research.
Merging of Disciplines to Study Gravity-Dependent Adaptations
The previous sections of this chapter focus on gravity-related alterations occurring in specific organ systems: bone, muscle, sensory-motor, cardiovascular, etc. Each of these systems is affected by alterations in gravity stimuli, especially microgravity. Also, it is apparent that certain systems are structurally and functionally aligned with one another, with muscle and bone and muscle or sensory-motor function as primary examples of such alignment. One of the deficiencies in the evolution of the NASA life sciences research mission concerns the lack of programs designed to foster such integration in terms of addressing specific problems or themes on a more global scale. This deficiency needs to be corrected well beyond the approach of tissue-sharing programs; effective integration can be achieved by sponsoring research initiatives that require the integrated study of multiple systems in the experimental design of individual projects. There should be a strategy to take advantage of research platforms (e.g., hindlimb unloading, centrifugation, bed rest, spaceflight modules/ISS) affecting both animal and human research objectives, whether the focus is on basic science or on more translational research directions. Several examples are given below to amplify the issue of integration.
Integrated Animal Studies on Bone, Microcirculation, and Skeletal Muscle Structure and Function
Rodent studies indicate that musculoskeletal and cardiovascular structure and function depend importantly on weight bearing, as well as gravitational blood pressures and flows within the body.871,872,873 Moreover, Stevens et al.874 have documented that microvascular to interstitial-fluid pressure gradients are important in regulating bone formation in the murine femur during hindlimb unloading. In addition, preservation of neuron-motor control and sensory feedback during exposure to microgravity for normal ambulation on the Moon and Mars may require walking and running against artificial gravity while in transit to these destinations. Such observations are important in understanding why exercise countermeasures used to date have not fully protected the bone, muscle, orthostatic tolerance, and fitness of individuals exposed to prolonged microgravity.
Hargens and Richardson point out that adult humans “spend about two-thirds of their existence in upright, sitting, and standing postures. During upright posture on Earth, blood pressures are greater in the feet than at heart or head levels due to gravity’s effects on columns of blood in the body (hydrostatic or gravitational pressures). For example, mean arterial pressure at heart level is normally about 100 mm Hg, whereas that in the head is slightly lower (e.g., 70 mm Hg) and that in the feet is much greater (e.g., 200 mm Hg).”875 During exposure to microgravity and probably on the Moon as well, arterial, venous, and microcirculatory blood pressure gradients are more or less absent, so that blood immediately shifts to chest and head tissues. According to earlier work, loss during hindlimb unloading of transmural stresses associated with local blood pressures and with maintenance of the structure and function of blood vessels affects musculoskeletal, brain, and cardiovascular structure and function.876,877,878
In ground-based projects involving the rodent HU model, the experimental design can be integrated to examine the time course of simultaneous alterations in bone, connective tissue, microcirculation, and muscle structure and function in normal animals as well as in transgenic models, while targeting specific regulatory pathways that are likely to be involved in microcirculatory, bone, and muscle maladaptations in the lower and upper body. These ground-based approaches are ideal for studies addressing pharmacological interventions such as the use of bisphosphonates, transgenic manipulation, and altered growth factor manipulations such as with myostatin and insulin-like growth factor-1. Based on these results, ground-based approaches can be transformed into similar projects that use the same animal models on the ISS but that can examine long-duration (out to 180 days) time course alterations in space. Additional studies also could be conducted to determine the ability of animals to recover back on Earth from maladaptations to microgravity. Such studies would provide a foundation for subsequent research with humans either during prolonged bed rest or in space.
Integrated Muscle, Bone, Sensory-Motor, Cardiovascular, and Organismic Metabolic Studies: Countermeasure Strategies in Response to Chronic Bed Rest
It is generally accepted within the NASA science community that a variety of exercise paradigms have the potential for maintaining homeostasis of essentially all of the organ systems covered in this report. Thus, it seems reasonable that in formulating an exercise countermeasure in the context of bed rest and/or spaceflight intervention, the primary objective should be to integrate research objectives concerning specific topics or research themes (e.g., control of movement, tissue atrophy/deterioration, regulation of organ system fitness, circulatory/microcirculation alterations, or sensory-motor and skeletal muscle function), across qualified research teams to ensure such integration. These integrative projects could be conducted not only in NASA facilities but also in national or international research centers and within universities or in clinical facilities. For example, it is interesting to note that such cooperation has been achieved by NASA and the National Institutes of Health in areas of common interest on a national level and by NASA, the European Space Agency (ESA), and the French Centre National d’Etudes Spatiales (WISE-2005) on an international level. In this way the knowledge base and effectiveness of such integrative research likely will be much greater than what is learned from the individual projects.
As Hargens and Richardson have pointed out,879
Exercise countermeasures for astronauts in space are still unresolved, although recent calculations suggest that all exercise in space to date has lacked sufficient loads to maintain preflight bone mass.880 Although Russian cosmonauts walk and run on a treadmill for 2 to 3 hours per day in an attempt to prevent bone loss, their bungee-cord loading
apparatus is uncomfortable at loads over 70 percent body weight. Furthermore, blood pressure stimuli at their feet are abnormally low because gravitational blood pressures are absent with their treadmill hardware.
In the context of these integrated studies targeting any given combination of organ systems, it is important to note that certain modality prototypes have begun to evolve for delivering a broader range of exercise/loading stimuli capable of simultaneously affecting the homeostasis of a number of these physiological systems. These prototypes include (1) modification of the flywheel device used in several European bed rest studies summarized above, such that both high resistance-loading stimuli and high aerobic-metabolic stimuli can be achieved with the same instrument, (2) human-powered artificial gravity, which can also provide both total body aerobic-metabolic challenges and high muscle group loading (2 to 3 g body weight), (3) aerobic treadmill exercise within an LBNP chamber combined with resistive flywheel exercise during WISE-2005, and (4) other activity devices, such as rowing machines, designed to challenge the musculoskeletal and cardiovascular systems. These devices, when used in prolonged bed rest studies, could provide a broad range of physiological alterations across the spectrum of organ systems.
Further, as these alternative devices become better characterized, it will be important to compare modality prototypes in order to select the most viable device for inducing alterations that affect the homeostasis of the broadest range of physiological systems being targeted.
Consequently, it is envisioned that integrated studies could simultaneously address skeletal muscle alterations (muscle size, phenotype, and endurance properties) and changes in sensory-motor function (cortical functional magnetic resonance imaging, electromyography, and accelerometer parameters).
In another paradigm, cardiovascular/metabolic alterations (cardiac output regulation, muscle blood flow regulation), as well as microcirculatory function in muscle and skin, could be examined. Further, studies are needed to address the kinetics of oxygen utilization and substrate metabolism turnover (anaerobic threshold, substrate utilization crossover points) as a function of the duration of exposure to chronic bed rest. These alterations would be compared to those in subjects experiencing both bed rest and specific countermeasure stimuli. Obviously, other combinations of studies could be conducted such as studies of bone (quantitative computed tomography, finite element analyses), muscle fitness, and orthostatic and neurosensory interactions. The key is that multiple systems should be studied in the same subject at the same time, particularly organ system combinations that may yield unforeseen outcomes that could lead to new insights—for example, are bone alterations impacted by alterations in the shear stress of blood flow in different anatomical regions?
Thus, in all integrated studies, a multidisciplinary approach is recommended to develop and evaluate integrated exercises that simulate normal daily loads on the cardiovascular, microcirculatory, neuron-motor control, and musculoskeletal systems. In validating the success of the countermeasure program objectives, NASA should define the limits of exercise stress that astronauts are likely to encounter in performing their duties, so that test criteria can be established that address whether the various countermeasures are achieving physiological end points appropriate to the level of fitness needed. Additional information on this important topic can be found in Chapter 7, “Crosscutting Issues for Humans in the Space Environment.”
RESEARCH PRIORITIES AND PLATFORMS
Based on deliberations concerning each of the preceding discipline-specific sections in this chapter, the AHB Panel rank-ordered the following disciplines as high priority, starting with the highest priority: (1) bone and connective tissue, (2) skeletal muscle and sensory-motor performance, and (3) heart and cardiovascular system homeostasis. The high priority given these areas is based on consideration of factors such as the magnitude of the spaceflight-induced changes that have been observed, the potential mission risk posed by spaceflight-induced changes, and the short- and long-term risks to the health of astronauts. Structural and functional deficits in these systems have the potential to severely affect the homeostasis of the organism, as well as cause a significant negative impact on the “fitness for duty” of astronauts, thereby compromising spaceflight missions lasting longer than 6 months. The key high-priority research recommendation for these systems and other ancillary high-priority recommendations are presented below. Very general estimates are given regarding the time that might be needed
to fulfill the individual research recommendations, assuming robust programmatic support and reasonable access to flight opportunities, but the actual length of time will depend on a variety of factors, including the nature of the studies, the complexity of the experimental design, the number of subjects needed for statistical power, and the validity of the findings.
Human Studies
All experiments under this heading can be completed within the next decade.
1. Bisphosphonates have been used to successfully treat bone loss associated with osteoporosis, and bed rest studies suggest that bisphosphonates can reduce bone loss associated with weightlessness. In addition, these drugs may reduce the incidence of kidney stones. The efficacy of bisphosphonates should be tested on the ISS during ~6-month missions in an adequate population of astronauts. This testing should include pre-flight dosing with long-acting bisphosphonates. (AH1)
2. It is known that bone mass decreases during spaceflight, but recent data demonstrate that the structure and strength of bone also deteriorate. The preservation/reversibility of bone structure/strength should be evaluated when assessing countermeasures.881,882,883 These measurements can be made during the countermeasure assessments recommended above. (AH2)
Animal Studies
All ground-based studies under this heading can be completed within the next decade, but flight studies will be limited by the inadequate animal housing on the ISS.
1. More studies of genetically altered mice exposed to weightlessness are strongly recommended. These studies were recommended in the 1998 NRC report884 but not implemented. In particular, further ground-based and spaceflight studies of mice with altered gene expression are warranted. These studies are essential to allow a better understanding of the molecular mechanisms of bone loss and also may allow the development of more effective countermeasures. (AH3)
2. New osteoporosis drugs under clinical development (for example, sclerostin antibody and Denosumab as a candidate) should be tested in animal models of weightlessness (both ground-based and in spaceflight). These studies are essential to identify new pharmacological countermeasures. (AH4)
Animal Studies
Studies should be conducted to identify the underlying mechanism(s) regulating net protein balance and protein turnover in skeletal muscle during states of unloading and recovery. These studies are essential to understanding the process of muscle wasting. Such studies should examine the roles of growth factors, hormones, signaling pathways, protease and myostatin inhibitors, possible pharmacological interventions such as use of antioxidants, and nitric oxide signaling. This research could potentially be concluded in a 10-year time frame and would not depend on access to space if the hindlimb unloading model is utilized. (AH5)
Human Studies
Studies are recommended to (1) develop and test new prototype exercise devices, and (2) optimize physical activity paradigms/prescriptions targeting multisystem countermeasures, in addition to skeletal muscle, preferably
with the same training device(s). Also, consideration should be given to new developed devices, in addition to the conventional exercise equipment onboard the ISS. These studies are needed because the current exercise devices and corresponding physical activity countermeasure prescriptions are ineffective for optimally maintaining physical fitness and organ system homeostasis. Depending on how rapidly such newly improved devices come onboard, this research could be completed within 15 years. (AH6)
How patterns of neuromuscular activity change in the spaceflight environment and upon return to 1 g is not known. Therefore, it is critical to determine the daily levels and pattern of recruitment of flexor and extensor muscles of the neck, trunk, arms, and legs at 1 g and after exposure to a novel gravitational environment for up to 180 days. These altered patterns of neuromuscular activity over different time periods need to be carefully examined with respect to changes in the accuracy of movements and the type and severity of their impact on other tissues, particularly muscle, connective tissue, and cardiovascular and hormonal systems. Studies are needed to:
1. Identify the neuromuscular mechanisms that underlie the loss of accurate control of movement with respect to (1) changes in neural control at the cortical, subcortical, and spinal levels; (2) changes in muscle properties; (3) changes in visual or proprioceptive perception; and (4) vestibular function.
2. Determine whether changes in accuracy of movement due to changing gravitational environments can be prevented or corrected with an exercise countermeasure designed to preserve aerobic function, such as use of a treadmill, stationary bike, or other exercise device designed to maintain muscle strength. (AH7)
A focus on fluid shifts represents a crosscutting research priority that addresses virtually all systems and is particularly relevant for humans because of the large gravitational gradients involved. Studies are needed to determine the basic mechanisms, adaptations, and clinical significance of changes in regional vascular/interstitial pressures (Starling forces) during long-duration space missions. These changes may be especially important in the brain, where chronic and sustained increases in intracranial, intraocular, and retinal pressures may induce clinically important structural and functional abnormalities that alter visual acuity. Studies are recommended of mechanical and/or pharmacologic countermeasures both as interventions to test mechanistic hypotheses regarding the pathophysiology of prolonged, sustained fluid shifts and simultaneously to develop evidence-based clinical treatments. Strategies to restore Earth-like plasma volume and fluid distribution in the period before landing, such as augmentation of venous return, increased salt intake several days earlier, or hormonal manipulation with or without salt consumption, should be studied quantitatively to determine their efficacy in minimizing orthostatic hypotension with restoration of gravitational gradients. (AH8)
Three enabling areas of cardiovascular research are essential in the next decade.
1. Investigate the effect of prolonged periods of microgravity and partial gravity (3/8 or 1/6 g) on the determinants of task-specific enabling levels of work capacity. Specifically, are there changes in energy availability, oxygen consumption, tissue perfusion, stroke volume, blood volume, blood flow redistribution to active muscle, or the capacity for heat dissipation that make it impossible to meet either routine or emergency demands for physical activity? Fundamental studies enabled by this research priority, including basic studies of myocardial mass and contractility, capillary filtration, hormonal changes, signaling pathways, and transcriptional regulation of cardiac structural proteins, will provide insight into mechanisms that contribute to the decrease in stroke volume and their future amelioration by more specific countermeasures. (AH9)
2. Determine the integrative mechanisms of orthostatic intolerance after restoration of gravitational gradients (both 1 g and 3/8 g); examine its severity as a function of prolonged microgravity, especially under real-life conditions of spaceflight such as emergency egress or Mars-like tasks, and the likelihood of as well as the time necessary for functional recovery. Specifically, what must be determined is the relative importance of hypotension and cerebral hypoperfusion, compared with neurovestibular, kinesthetic, or muscular weakness, to the orthostatic intolerance following long-duration spaceflight.
If it is confirmed by systematic examination that orthostatic intolerance after long-duration spaceflight is due to hypotension, determine whether reductions in cerebral blood flow, or neurovascular control, loss of blood volume, or cardiac atrophy (or their interaction) cause this hemodynamic compromise. Use the results from these investigations of mechanism to confirm and examine more systematically the efficiency of promising post-flight countermeasures, such as use of the pharmacologic agent α1-agonist midodrine and the use of post-flight gravitational countermeasures such as thigh cuffs, support stockings, or inflation of G-suits; also determine the efficacy of promising in-flight countermeasures such as exercise of different modes, durations, and intensities, as well as centrifuge-simulated artificial gravity, along with novel fluid loading strategies that will more effectively restore blood and plasma volume. Studying sufficient numbers of astronauts of both genders over a wide age range will help to define the mechanisms underlying individual variability in these responses. (AH10)
3. The primary emergent, mission- and life-threatening medical event (besides trauma) relevant to middle-aged astronauts is likely to be an acute coronary syndrome. Collaborative studies among flight medicine and cardiovascular epidemiologists are recommended to determine the best screening strategies (such as vascular imaging with or without cardiac biomarkers) to avoid flying astronauts with subclinical coronary heart disease that could become manifest during a long-duration exploration-class mission (3 years). It is also recommended that collaborative studies with radiation biologists be conducted at a basic level to determine whether atherosclerotic vascular disease could be accelerated by the chronic exposure to radiation in deep space. (AH11)
Determine the amount and site of the deposition of aerosols of different sizes in the lungs of humans and animals in microgravity. The rationale is that deposition is different in microgravity compared with normal gravity, and there is evidence that lunar dust and Mars dust are potentially toxic. The research can be carried out on the ISS and could potentially be concluded in a 10-year time frame. (AH12)
Although no clinically deleterious effects of spaceflight on the immune system have been observed to date, studies of the acute and chronic effects of the space environment on the immune system have been limited. Further studies should be pursued because of the potential for adverse events during longer flights or in response to altered external conditions. The high-priority recommendations below can be completed in 5 to 10 years, depending on the availability of flight time:
1. Multiple parameters of T cell activation should be obtained from astronauts before and after re-entry to establish which parameters are altered during flight. The parameters examined should represent the current state of technology in assessments of immunity, including those that reflect aging-related changes in the immune system (e.g., shortening of telomeres, expression of CD28 and Treg). (AH13)
2. Changes observed in the immune system may reflect the impact of multiple stressors. To both address the mechanism(s) of changes in the immune system and develop measures to limit the changes, data from multiple organ/system-based studies have to be integrated. For example, data from assessments of cortisol levels (stress studies), nutritional diaries, and logs of weight changes, exercise volume and intensity, and level of activity (e.g., EVA) need to be considered when interpreting data on immune responses from individuals. (AH14)
3. It is essential that studies of mice be performed on the ISS to establish the biological relevance of the changes observed in the immune system. These studies should include immunization and challenge, with samples
acquired both prior to and immediately upon re-entry. The parameters examined need to be aligned with those human immune response components influenced by spaceflight. (AH15)
Studies should be conducted on transmission across generations of structural and functional changes induced by exposure to space during development. Such research will provide vital fundamental information about how genetic and epigenetic factors interact with the environment to shape gravity-dependent processes and about the penetrating influence of these factors across subsequent generations. Spaceflight experiments offer unique insights into the role of forces omnipresent on Earth (but absent in orbital flight) that can actively shape genomes in ways that are heritable. Such spaceflight experiments would place gravitational biology at the leading edge of modern developmental and evolutionary science. Ground-based studies should be conducted to develop specialized habitats to support reproducing and developing rodents in space. This research could be accomplished within 10 years. (AH16)
To address the above recommendations, the following platforms are needed to carry out integrated research projects involving both animal and human subjects.
Animal Platforms
From a practical perspective, the AHB Panel envisions an animal research program centered on rodents (mice and/or rats). The reason is that rodents, especially rats, have been used more extensively than any other animal model for spaceflight studies. Also, the genomic material of rodents is closely aligned to that of humans. Studies utilizing both ground-based analogs and spaceflight are recommended. For ground-based studies, the panel recommends continuing the research foundation generated by the HU model and centrifugation, which have produced extensive data spanning hundreds of studies over the past three decades. As noted in the section above titled “Merging of Disciplines to Study Gravity-Dependent Adaptations,” the HU model can be used to develop time course databases for alterations in the musculoskeletal system, along with generating potential countermeasures (endurance and loading exercise, hormonal and growth factor modulations) through a variety of models in addressing most of the recommendations pertinent to basic science research.
In order to bolster research in more fundamental biology, the panel strongly recommends establishing a flight-based research platform on the ISS. The ISS platform is preferred over other types of free-flyer opportunities because long-duration studies can be carried out on the ISS for approximately 180 days, thus spanning a significant component of an adult rodent’s lifespan. Also, animal research on the ISS provides the only means for establishing the fundamental alterations unfolding during long-duration exposure to microgravity without any countermeasure intervention, which cannot be studied in the humans living on the ISS. This is important because it is only through the physiological responses of animal models that one can ascertain the degree to which microgravity affects the vital systems of the organism. Many of the alterations observed in human physiological homeostasis in response to spaceflight have also been noted in animal models, as delineated in the prior sections of this chapter. Further, the AHB Panel envisions exposing animals to spaceflight for long durations and then returning them to Earth, where their ability to correct the structural and functional deficits that occurred in space can be studied. The findings of the animal flight research program have far-reaching consequences not only for understanding the nuances of spaceflight but also for gaining insight into how humans adapt on Earth in response to lifestyles of chronic inactivity. See Chapter 7 for additional information on this topic.
Human Platforms
Ground-based research opportunities for performing integrated research programs should use the bed rest model extensively, and in certain cases other analogs such as unilateral limb suspension, for studying unloading-
induced deficits in systems such as muscle and bone. For example, it is now routine to study subjects for 90 days or longer during bed rest under controlled experimental conditions. NASA should adopt the model used by ESA, in which such studies use a multicenter approach. Again, the focus should be on integrated approaches for targeting multiple systems with coordinated analyses, as outlined in the section “Integrated Muscle, Bone, Sensory-Motor, Cardiovascular, and Organismic Metabolic Studies: Countermeasure Strategies in Response to Chronic Bed Rest.” These studies should be linked to a variety of countermeasure paradigms centered on exercise approaches challenging bone, skeletal muscle, pulmonary/cardiovascular fitness, and immune system homeostasis. These countermeasures include resistance and aerobic exercise devices (ergometers, treadmill, and rowing modalities), as well as artificial gravity and endurance exercise with lower-body negative pressure, which can also target multiple systems with high loading stimuli. As noted above, it may be necessary to compare different countermeasure modalities for their respective effectiveness in modulating multiple organ systems.
The ISS Platform
Such integrated studies should be extended to the ISS research platform, to validate the science outcomes seen in ground-based analogs. Such studies should heavily involve the science community so that research objectives for understanding underlying mechanisms can be achieved, rather than merely focusing on outcomes from an operational medical perspective. The participants in this decadal survey were aware of situations in which extramural scientists whose projects had been approved were unable to access, or were able to access only with great difficulty, operational medicine data needed for the project. This suggests that mechanisms for engaging the scientific community and for publishing the valuable human research data generated from limited spaceflight opportunities need to be improved. The panel also strongly recommends that any research performed on the ISS should involve systematic studies in which all the subjects perform identical protocols in order to enhance the fidelity of the data that are obtained. This approach is more likely to yield clear insights as to which countermeasure paradigms are optimally successful in maintaining organism homeostasis. Only by establishing a confirmed evidence base can NASA evolve a validated countermeasure program that is truly optimal for astronauts exposed to long-duration space travel.
OVERARCHING AND PROGRAMMATIC ISSUES
As the AHB Panel compared the recommendations and findings from its discipline subgroups, certain overarching issues emerged that have program-wide relevance. These issues are summarized below.
The Need for Animal Research on the ISS and Other Space Platforms
For the past decade, biological research in space has emphasized human countermeasures, as conducted by the operational medicine program. While such research is appropriate, given the dependence of the U.S. space program on a human presence in space, this focus has limited the ability to answer fundamental questions about the response of biological systems to altered gravity. The panel is unanimous in its recommendation that an animal habitat should be incorporated as soon as possible into the ISS.
Flight experiments on animals began in 1782 when a duck, a sheep, and a rooster were sent aloft in a hot air balloon by the Montogolfier Brothers of Paris.885 Moreover, animal models have enabled humans to initiate previous exploration and discovery missions by serving as subjects to determine the effects of radiation, establishing biological and safety limits, perfecting life support systems, evaluating countermeasures, and providing insights into anatomical and physiological responses and the mechanism responsible for them.886-889
The human space age began with Gagarin’s epic orbital flight of April 12, 1961, and Shephard’s suborbital flight less than a month later;890 however, each was enabled by Sputnik flights containing dogs, guinea pigs, and mice891 and by the U.S. flight of the chimpanzee Ham.892 The importance of and need for animals in space were officially recognized in the Goldberg report of 1987,893 which listed as the third of its four goals “to understand the role gravity plays in the biological processes of both plants and animals.” This goal, which has yet to be achieved,
has become more important because the ongoing human tasks of exploration, habitation, and discovery require the results of animal research to address the critical human risk factors for human exploration.
From the beginning of the human space age and continuing up to January 16, 2003, the United States and Russia flew 34 missions in which mammalian experiments were conducted on the biological systems mentioned in this report.894,895 However, the average duration of those flights was 11 ± 4 days,896 with the longest being 23 days.897,898 In the overwhelming majority of these flights, the biological data were collected at or after recovery, which means that they are important but are of limited use in determining and interpreting in-flight effects. Meaningful in-flight results have been obtained with monkeys and rodents from the Russian Bion or Cosmos flights899-904 and from monkeys and rodents on Spacelab Life Sciences 1 and 2905,906,907 and from Neurolab or STS-90.908 While these results were obtained from flights lasting only from 9 to 16 days, in-flight measurements enabled investigators to secure molecular, cellular, and systems data encompassing growth and development; motor control; and select structure and functional relationships for the nervous, cardiovascular, musculoskeletal, immune, temperature regulatory, and circadian rhythm systems. Moreover, in-flight studies provided the opportunity to separate gravitational influences from landing effects, secure repeat measurements, secure video recordings of animals performing manual tasks, and demonstrate the biotelemetric effectiveness of surgically implanted electrodes and sensors in animals as a means of acquiring meaningful physiological data on multiple biological systems.909
Recommendation Concerning the National Laboratory
To maximize the contribution of animal experimentation to reducing human risks during space exploration and discovery while enhancing the acquisition of fundamental knowledge, it is essential that single and repeated long-duration in-flight animal experiments be conducted. This can best be accomplished by having NASA make available as soon as possible, within the National Laboratory on the ISS, four Advanced Animal Habitats910 with the capability of accommodating 8 to 10 mice or 4 to 5 rats per habitat for animal investigations devoted to the priorities recommended in this report. It is assumed that animals would be transferred to a clean habitat after each 30-day period. To accommodate longitudinal investigations, including studies of growth, development, and reproduction, while being in accord with current procedures for astronauts, the study time frame would be 6 months.911 The caging infrastructure can be easily modified to create birthing dens to facilitate studies of reproduction. Such an arrangement is consistent with Article 3 of Section 305 in the National Aeronautics and Space Administration Authorization Act of 2005, which directs the NASA administrator to conduct animal research for which it is essential that the United States have an operational laboratory for space-directed animal and human research.912 Finally, despite its awareness that the large centrifuge program has little likelihood of being restarted, the AHB Panel would be remiss if it did not strongly recommend an animal centrifuge capable of accommodating rats/mice at variable gravity levels. This capability would enhance the research potential of the ISS laboratory by creating both partial gravity and hypergravity stimuli to allow analysis of the role of the gravity vector in modulating functional capacity across many organ systems, especially the bone, muscle, neurosensory, and cardiovascular systems.
Research access to the National Laboratory will strengthen existing relationships with U.S. international partners in Russia, Europe, and Japan and promote possible new relationships with China and India. Besides improving existing relationships, research access enhances the outsourcing of animals to international partners for shorter-term landing and recovery experiments. Access will also augment possibilities for commercial transportation to reduce costs and increase durations of parabolic flights and to replace the loss of the space shuttle system for access to the National Laboratory.
Inherent in the recommendation for animal and human experimentation in the National Laboratory is the understanding that the implementation of this decadal survey’s recommended research objectives will have sufficient governmental priority between 2010 and 2020 to supersede existing NASA operational plans.
Animal Research on Other Platforms
Uncrewed flight opportunities on free-flyers should be used to conduct shorter-duration missions than can be accomplished on the ISS, optimally with the availability of an animal centrifuge to provide proper 1-g controls
for animal specimens. Interaction with, and utilization of facilities provided by, international partners should be exploited in this regard.
Improved Access to Biological Samples and Data from Astronauts
The medical and scientific communities interested in human health during long-duration spaceflight have been consistent in their requests for greater access to biological samples and other data collected from astronauts during spaceflight missions. The astronauts’ rights to privacy have, at times, appeared to conflict with the need for access to valuable in-flight data in order to benefit future space travelers. In 2001, the Institute of Medicine published Safe Passage: Astronaut Care for Exploration Missions,913 which included suggestions for resolving this conflict. Among the recommendations were (1) that NASA should establish a comprehensive health care system for astronauts for the purpose of collecting and analyzing data, and (2) that NASA should develop a strategic health care research plan designed to increase the knowledge base about the risks to astronaut health. While some of these goals have been met, much remains to be done to provide more widespread access to data on astronaut health. For example, there is still little information in the public domain about the bone health of women astronauts who have flown on long-duration space missions. Current policies on access to data are limiting progress toward effective countermeasures. NASA is urged to study and take action on this issue.
Limitations of Ground-Based Facilities
Experiments conducted in space are complex and expensive. It is recommended that NASA make an enhanced commitment to ground-based analogs of spaceflight for purposes of human subject research. One example is the bed rest analog.914,915 At the time of writing, NASA has only one facility in the United States for bed rest study, the Flight Analogs Research Project, located at the University of Texas Medical Branch in Galveston, Texas. This unit has a maximum occupancy of 10 subjects and is located in an area that is significantly at risk for hurricane damage. The small number of beds means that progress on the many proposed experiments is slow, and the geographical location has, in the past, led to evacuation of subjects and the premature end of bed rest campaigns. It is thus strongly recommended that NASA consider establishing a bed rest facility allowing study of at least 20 patients simultaneously and that this facility be located in a region of the country that is not at high risk for severe weather that could interrupt experiments. Alternatively, contract arrangements could be established with one or more of the European bed rest facilities to conduct studies for NASA.
Limitations on Sample Delivery Back to Earth
Many biological samples need to be stored under tightly controlled environmental conditions, such as in a −80°C freezer. With the end of the space shuttle era in 2011, an important mechanism for delivery back to Earth of biological samples collected on the ISS will no longer be available. Because much of the up-mass to and down-mass from the ISS will likely be delivered by commercial contractors after 2011, it is essential that the contract specifications for such vehicles include adequate capacity for transporting biological samples. Conditioned down-mass is of particular importance in this regard, because there are facilities on the ISS for storage of samples. Unless suitable down-mass transportation is made available, only the relatively simple analyses that can be conducted onboard the ISS will be feasible.
Space Platform for Research Beyond 2020 Will Be Needed
While most of the recommendations in this report deal with the decade 2010-2019, the AHB Panel recognizes the long time constant inherent in the implementation of some recommendations and thus the importance of planning for the period 2020-2029 when, under current plans, the ISS will have been de-orbited.
The effort in the third decade of this century must begin by extending the findings of research that was conducted in 2010-2019 and filling in the gaps remaining from that work. While these gaps are hard to predict, it seems reasonable to assume that not everything that will be started in the decade to 2019 will reach maturity by 2020.
A major new factor in the third decade will be the presence of new transport vehicles capable of carrying astronauts well beyond low Earth orbit. This will open up at least three new areas of study: (1) Countermeasures will be needed during long voyages in microgravity, in environments that will be drastically smaller than the ISS. This will require small-footprint, low-power yet highly effective devices that will provide countermeasures for changes in multiple systems. (2) It is likely the astronauts will spend time in locations where there is a fraction of Earth’s gravity. The role of partial gravity in preventing deterioration in important physiological systems will need to be clearly understood, and countermeasures to supplement these effects, if necessary, will need to be developed. (3) Current plans appear to call for the design of a new generation of surface rovers, which may not allow for normal conditioning stimuli during EVA. There will, therefore, be a need for exercise facilities for both recreation and countermeasures at a lunar or planetary base.
It is highly likely that, by the end of the present decade, much will remain to be learned about the fundamental molecular mechanisms of the responses to microgravity and altered gravity. NASA should therefore consider a flexible infrastructure of experimental facilities that can be easily upgraded when going from the ISS era to systems used in the next step in human exploration. Currently, genetically modified animals, particularly mice, are the most productive research model on Earth, and it is vital that maximum use of these models occur in space research.
The establishment of a lunar outpost could provide the opportunity for an important research platform for ongoing studies in partial gravity. Among other important uses, this outpost could eventually provide a means for a sustainable research laboratory for developmental biological research on model systems across generations. In such a laboratory, growth, development, maturation, and longevity of animals could be carefully examined. NASA might consider naming such a facility a “National Laboratory” to highlight its importance as a key national scientific resource.
A number of responses of the cardiovascular system to altered gravity will need to be studied in the third decade of this century. Among the important issues left to be answered are likely to be the effects of prolonged weightlessness and irradiation on the pathobiology of the aging process, atherosclerosis, and coronary vascular disease; the regulation of molecular clocks, circadian proteins, and genetic signaling of cardiac and vascular muscle apoptosis and hypertrophy; the autonomic neurobiology of the circulation and cardiac pacemaker function and automaticity; and the genetic profile that determines the risk of accelerated cardiovascular disease and stroke.
If the recommendation to restore NASA’s ground-based and in-flight fundamental biology and non-human life science programs is implemented in the next decade, it is likely to pay dividends in the third decade by opening new vistas for human and animal research.
Relevance of the Report to NASA Fundamental Space Biology Strategic Planning
In June 2010, NASA released the Fundamental Space Biology (FSB) Science Plan for the next decade.916 The FSB Science Plan lists three primary goals: (1) to effectively use microgravity (and an altered-gravity continuum) to enhance understanding of fundamental biological processes; (2) to develop scientific and technological foundations for a safe, productive human presence in space for extended periods in the context of space exploration; and (3) to apply the knowledge gained in science and technology to improve national competitiveness, education, and quality of life on Earth.
The central elements of the plan’s research focus would involve (1) cell, molecular, and microbial biology; (2) organelle, organ, and organismal function; and (3) developmental biology. These scientific inquiries and approaches will continue to utilize ground-based models and facilities as well as space platforms such as the ISS and a variety of free-flyer vehicles.
The driving force for these initiatives includes past reports from the NRC with recommendations presented in 1989 and 1998917 that to date have not been fully implemented.
One of the important thrusts in the FSB Science Plan involves the proposed program’s commitment to enhance research on the ISS that can accommodate animal research opportunities. If pursued, such a thrust could lead to the rejuvenation of NASA’s commitment to animal research for both fundamental science and translational research.
The committee notes that the overall thrust of the FSB Science Plan is aligned with the subject matter, the scientific issues, and the research recommendations presented in this chapter. The key issue that will define the success of the FSB program in the next decade is whether meaningful animal science can unfold on the ISS.
1. National Research Council. 2010. Life and Physical Sciences Research for a New Era of Space Exploration: An Interim Report. The National Academies Press, Washington, D.C.
2. Milstead, J.R., Simske, S.J., and Bateman, T.A. 2004. Spaceflight and hindlimb suspension disuse models in mice. Biomedical Sciences Instrumentation 40:105-110.
3. Frost, H.M., and Jee, W.S. 1992. On the rat model of human osteopenias and osteoporoses. Bone and Mineral 18:227-236.
4. Keyak, J.H., Koyama, A.K., LeBlanc, A., Lu, Y., and Lang, T.F. 2009. Reduction in proximal femoral strength due to long-duration spaceflight. Bone 44:449-453.
5. Smith, S.M., Wastney, M.E., O’Brien, K.O., Morukov, B.V., Larina, I.M., Abrams, S.A., Davis-Street, J.E., Oganov, V., and Shackelford, L.C. 2005. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. Journal of Bone and Mineral Research 20:208-218.
6. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
7. Smith, S.M., Wastney, M.E., O’Brien, K.O., Morukov, B.V., Larina, I.M., Abrams, S.A., Davis-Street, J.E., Oganov, V., and Shackelford, L.C. 2005. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. Journal of Bone and Mineral Research 20:208-218.
8. Smith, S.M., Wastney, M.E., Morukov, B.V., Larina, I.M., Nyquist, L.E., Abrams, S.A., Taran, E.N., Shih, C.Y., Nillen, J.L., Davis-Street, J.E., Rice, B.L., and Lane, H.W. 1999. Calcium metabolism before, during, and after a 3-mo spaceflight: Kinetic and biochemical changes. American Journal of Physiology 277:R1-R10.
9. Zerwekh, J.E., Ruml, L.A., Gottschalk, F., and Pak, C.Y. 1998. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. Journal of Bone and Mineral Research 13:1594-1601.
10. Lang, T.F., LeBlanc, A.D., Evans, H.J., Lu, Y., Genant, H., and Yu, A. 2004. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. Journal of Bone and Mineral Research 19:1006-1012.
11. LeBlanc, A., Lin, C., Shackelford, L., Sinitsyn, V., Evans, H., Belichenko, O., Schenkman, B., Kozlovskaya, I., Oganov, V., Bakulin, A., Hedrick, T., and Feeback, D. 2000. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. Journal of Applied Physiology 89:2158-2164.
12. Iki, M., Kajita, E., Dohi, Y., Nishino, H., Kusaka, Y., Tsuchida, C., Yamamoto, K., and Ishii, Y. 1996. Age, menopause, bone turnover markers and lumbar bone loss in healthy Japanese women. Maturitas 25:59-67.
13. Sirola, J., Kröger, H., Honkanen, R., Jurvelin, J.S., Sandini, L., Tuppurainen, M.T., and Saarikoski, S. 2003. OSTPRE Study Group Factors affecting bone loss around menopause in women without HRT: A prospective study. Maturitas 45:159-167.
14. Whedon, G.D., Lutwak, L., Rambaut, P., Whittle, M., Leach, C., Reid, J., and Smith, M. 1976. Effect of weightlessness on mineral metabolism, metabolic studies on Skylab orbital space flights. Calcified Tissue Research 21(Suppl.):423-430.
15. Smith, S.M., Wastney, M.E., O’Brien, K.O., Morukov, B.V., Larina, I.M., Abrams, S.A., Davis-Street, J.E., Oganov, V., and Shackelford, L.C. 2005. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. Journal of Bone and Mineral Research 20:208-218.
16. LeBlanc, A., Schneider, V., Shackelford, L., West, S., Oganov, V., Bakulin, A., and Voronin, L. 2000. Bone mineral and lean tissue loss after long duration space flight. Journal of Musculoskeletal and Neuronal Interactions 1:157-160.
17. LeBlanc, A.D., Spector, E.R., Evans, H.J., and Sibonga, J.D. 2007. Skeletal responses to space flight and the bed rest analog: A review. Journal of Musculoskeletal and Neuronal Interactions 7:33-47.
18. Keyak, J.H., Koyama, A.K., LeBlanc, A., Lu, Y., and Lang, T.F. 2009. Reduction in proximal femoral strength due to long-duration spaceflight. Bone 44:449-453.
19. Lang, T.F., LeBlanc, A.D., Evans, H.J., Lu, Y., Genant, H., and Yu, A. 2004. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. Journal of Bone and Mineral Research 19:1006-1012.
20. Sibonga, J.D., Evans, H.J., Sung, H.G., Spector, E.R., Lang, T.F., Oganov, V.S., Bakulin, A.V., Shackelford, L.C., and LeBlanc, A.D. 2007. Recovery of spaceflight-induced bone loss: Bone mineral density after long-duration missions as fitted with an exponential function. Bone 41:973-978.
21. Vico, L., Collet, P., Guignandon, A., Lafage-Proust, M.H., Thomas, T., Rehaillia, M., and Alexandre, C. 2000. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355:1607-1611.
22. Keyak, J.H., Koyama, A.K., LeBlanc, A., Lu, Y., and Lang, T.F. 2009. Reduction in proximal femoral strength due to long-duration spaceflight. Bone 44:449-453.
23. Sibonga, J.D., Evans, H.J., Sung, H.G., Spector, E.R., Lang, T.F., Oganov, V.S., Bakulin, A.V., Shackelford, L.C., and LeBlanc, A.D. 2007. Recovery of spaceflight-induced bone loss: Bone mineral density after long-duration missions as fitted with an exponential function. Bone 41:973-978.
24. Lang, T.F., Leblanc, A.D., Evans, H.J., and Lu, Y. 2006. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. Journal of Bone and Mineral Research 21:1224-1230.
25. Lang, T.F., Leblanc, A.D., Evans, H.J., and Lu, Y. 2006. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. Journal of Bone and Mineral Research 21:1224-1230.
26. Cavanagh, P., and Rice, A., eds., 2007. Bone Loss During Spaceflight: Etiology, Countermeasures, and Implications for Bone Health on Earth, Proceedings of the Symposium on Bone Loss During Spaceflight, June 23-24, 2005. Cleveland Clinic Press, Cleveland, Ohio.
27. Wagner, E.B. 2007. Musculoskeletal Adaptation to Partial Weight Suspension: Studies of Lunar and Mars Loading. Ph.D., Massachusetts Institute of Technology.
28. Morey, E.R., and Baylink, D.J. 1978. Inhibition of bone formation during space flight. Science 201:1138-1141.
29. Wronski, T.J., Morey-Holton, E., and Jee, W.S. 1981. Skeletal alterations in rats during space flight. Advances in Space Research 1:135-140.
30. Wronski, T.J., and Morey, E.R. 1983. Effect of spaceflight on periosteal bone formation in rats. American Journal of Physiology 244:R305-R309.
31. Jee, W.S., Wronski, T.J., Morey, E.R., and Kimmel, D.B. 1983. Effects of spaceflight on trabecular bone in rats. American Journal of Physiology 244:R310-R314.
32. Cann, C.E., and Adachi, R.R. 1983. Bone resorption and mineral excretion in rats during spaceflight. American Journal of Physiology 244:R327-R331.
33. Vico, L., Novikov, V.E., Very, J.M., and Alexandre, C. 1991. Bone histomorphometric comparison of rat tibial metaphysis after 7-day tail suspension vs. 7-day spaceflight. Aviation, Space, and Environmental Medicine 62:26-31.
34. Milstead, J.R., Simske, S.J., and Bateman, T.A. 2004. Spaceflight and hindlimb suspension disuse models in mice. Biomedical Sciences Instrumentation 40:105-110.
35. Smith, S.M., Wastney, M.E., Morukov, B.V., Larina, I.M., Nyquist, L.E., Abrams, S.A., Taran, E.N., Shih, C.Y., Nillen, J.L., Davis-Street, J.E., Rice, B.L., and Lane, H.W. 1999. Calcium metabolism before, during, and after a 3-mo spaceflight: Kinetic and biochemical changes. American Journal of Physiology 277:R1-R10.
36. Morey, E.R., and Baylink, D.J. 1978. Inhibition of bone formation during space flight. Science 201:1138-1141.
37. Vico, L., Novikov, V.E., Very, J.M., and Alexandre, C. 1991. Bone histomorphometric comparison of rat tibial metaphysis after 7-day tail suspension vs. 7-day spaceflight. Aviation, Space, and Environmental Medicine 62:26-31.
38. Ortega, M.T., Pecaut, M.J., Gridley, D.S., Stodieck, L.S., Ferguson, V., and Chapes, S.K. 2009. Shifts in bone marrow cell phenotypes caused by spaceflight. Journal of Applied Physiology 106:548-555.
39. Shaw, S.R., Vailas, A.C., Grindeland, R.E., and Zernicke, R.F. 1988. Effects of a 1-wk spaceflight on morphological and mechanical properties of growing bone. American Journal of Physiology 254:R78-R83.
40. Simmons, D.J., Russell, J.E., and Grynpas, M.D. 1986. Bone maturation and quality of bone material in rats flown on the space shuttle ‘Spacelab-3 Mission.’ Bone and Mineral 1:485-493.
41. Bateman, T.A., Zimmerman, R.J., Ayers, R.A., Ferguson, V.L., Chapes, S.K., and Simske, S.J. 1998. Histomorphometric, physical, and mechanical effects of spaceflight and insulin-like growth factor-I on rat long bones. Bone 23:527-535.
42. Wronski, T.J., Morey-Holton, E.R., Doty, S.B., Maese, A.C., and Walsh, C.C. 1987. Histomorphometric analysis of rat skeleton following spaceflight. American Journal of Physiology 252:R252-R255.
43. Kaplanskĭ, A.S., Durnova, G.N., Sakharova, Z.F., and Il’ina-Kakueva, E.I. 1987. Histomorphometric analysis of the bones of rats on board the Kosmos 1667 biosatellite. Kosmicheskaia Biologiia I Aviakosmicheskaia Meditsina 21:25-31.
44. Vico, L., Chappard, D., Alexandre, C., Palle, S., Minaire, P., Riffat, G., Novikov, V.E., and Bakulin, A.V. 1987. Effects of weightlessness on osseous tissue of the rat after a space flight of 5 days (Cosmos 1514). Journal of Physiology (Paris) 82:1-11.
45. Platts, S.H., Martin, D.S., Stenger, M.B., Perez, S.A., Ribeiro, L.C., Summers, R., and Meck, J.V. 2009. Cardiovascular adaptations to long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A29-A36.
46. Reschke, M.F., Bloomberg, J.J., Paloski, W.H., Mulavara, A.P., Feiveson, A.H., and Harm, D.L. 2009. Postural reflexes, balance control, and functional mobility with long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A45-A54.
47. Wronski, T.J., and Morey, E.R. 1983. Effect of spaceflight on periosteal bone formation in rats. American Journal of Physiology 244:R305-R309.
48. Cann, C.E., and Adachi, R.R. 1983. Bone resorption and mineral excretion in rats during spaceflight. American Journal of Physiology 244, R327-R331.
49. Cavolina, J.M., Evans, G.L., Harris, S.A., Zhang, M., Westerlind, K.C., and Turner, R.T. 1997. The effects of orbital spaceflight on bone histomorphometry and messenger ribonucleic acid levels for bone matrix proteins and skeletal signaling peptides in ovariectomized growing rats. Endocrinology 138(4):1567-1576.
50. Turner, R.T. 2000. Invited review: What do we know about the effects of spaceflight on bone? Journal of Applied Physiology 89:840-847.
51. Cavanagh, P., Rice, A. Cavanagh, P., and Rice, A., eds. 2007. Bone Loss During Spaceflight: Etiology, Countermeasures, and Implications for Bone Health on Earth, 1st ed. Cleveland Clinic Press, Cleveland, Ohio.
52. Wronski, T.J., and Morey, E.R. 1983. Effect of spaceflight on periosteal bone formation in rats. American Journal of Physiology 244:R305-R309.
53. Wronski, T.J., Morey-Holton, E.R., Doty, S.B., Maese, A.C., and Walsh, C.C. 1987. Histomorphometric analysis of rat skeleton following spaceflight. American Journal of Physiology 252:R252-R255.
54. Sibonga, J.D., Zhang, M., Evans, G.L., Westerlind, K.C., Cavolina, J.M., Morey-Holton, E., and Turner, R.T. 2000. Effects of spaceflight and simulated weightlessness on longitudinal bone growth. Bone 27(4):535-540.
55. Vico, L., Novikov, V.E., Very, J.M., and Alexandre, C. 1991. Bone histomorphometric comparison of rat tibial metaphysis after 7-day tail suspension vs. 7-day spaceflight. Aviation, Space, and Environmental Medicine 62:26-31.
56. Hughes-Fulford, M., Tjandrawinata, R., Fitzgerald, J., Gasuad, K., and Gilbertson, V. 1998. Effects of microgravity on osteoblast growth. Gravitational and Space Biology Bulletin 11:51-60.
57. Grindeland, R., Hymer, W.C., Framington, M., Fast, T., Hayes, C., Mottler, K., Patil, L., and Vasqyes, M. 1987. Changes in pituitary growth hormone cells prepared from rats flown on Spacelab 3. American Journal of Physiology 252:R209-R215.
58. Hughes-Fulford, M., and Scheld, H.W. 1989. Thin film bioreactors in space. Advances in Space Research 9(11):111-117.
59. Tamma, R., Colaianni, G., Camerino, C., Di Benedetto, A., Greco, G., Strippoli, M., Vergari, R., Grano, A., Mancini, L., Mori, G., Colucci, S., Grano, M., and Zallone, A. 2009. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB Journal 23:2549-2554.
60. Hughes-Fulford, M., Tjandrawinata, R., Fitzgerald, J., Gasuad, K., and Gilbertson, V. 1998. Effects of microgravity on osteoblast growth. Gravitational and Space Biology Bulletin 11:51-60.
61. Tamma, R., Colaianni, G., Camerino, C., Di Benedetto, A., Greco, G., Strippoli, M., Vergari, R., Grano, A., Mancini, L., Mori, G., Colucci, S., Grano, M., and Zallone, A. 2009. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. The FASEB Journal 23:2549-2554.
62. Hughes-Fulford, M. 2004. Lessons learned about spaceflight and cell biology experiments. Journal of Gravitational Physiology 11:105-109.
63. Cavanagh, P.R., Licata, A.A., and Rice, A.J. 2005. Exercise and pharmacological countermeasures for bone loss during long-duration space flight. Gravitational and Space Biology Bulletin 18:39-58.
64. Grigoriev, A.I., Kozlovskaya, I.B., and Potapov, A.N. 2002. Goals of biomedical support of a mission to Mars and possible approaches to achieving them. Aviation Space and Environmental Medicine 73(4):379-384.
65. Kozlovskaya, I.B., and Egorov, A.D. 2003. Some approaches to medical support for martian expedition. Acta Astronautica 53(4-10):269-275.
66. Lang, T.F., LeBlanc, A.D., Evans, H.J., Lu, Y., Genant, H., and Yu, A. 2004. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. Journal of Bone and Mineral Research 19:1006-1012.
67. Cavanagh, P.R., Licata, A.A., and Rice, A.J. 2005. Exercise and pharmacological countermeasures for bone loss during long-duration space flight. Gravitational and Space Biology Bulletin 18:39-58.
68. Rubin, C., Adler, B., Qin, Y., and Judex, S. 2007. Mechanical signals may provide an effective countermeasure to bone and muscle loss during long-term spaceflight. Pp. 175-187 in Bone Loss During Spaceflight: Etiology, Countermeasures, and Implications for Bone Health on Earth (P. Cavanagh and A. Rice, eds.). Cleveland Clinic Press, Cleveland, Ohio.
69. Gopalakrishnan, R., Genc, K.O., Rice, A.J., Lee, S.M.C., Evans, H.J., Maender, C.C., Ilaslan, H., and Cavanagh, P.R. 2010. Muscle volume, strength, endurance, and exercise loads during 6-month missions in space. Aviation, Space, and Environmental Medicine 81(2):91-102.
70. Trappe, S., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
71. Cavanagh, P.R., Gopalakrishnan, R., Rice, A.J., Genc, K.O., Maender, C.C., Nystrom, P.G., Johnson, M.J., Kuklis, M.M., and Humphreys, B.T. 2009. An ambulatory biomechanical data collection system for use in space: Design and validation. Aviation, Space, and Environmental Medicine 80:870-881.
72. LeBlanc, A.D., Spector, E.R., Evans, H.J., and Sibonga, J.D. 2007. Skeletal responses to space flight and the bed rest analog: A review. Journal of Musculoskeletal and Neuronal Interactions 7:33-47.
73. Crucian, B.E., Stowe, R.P., Mehta, S.K., Yetman, D.L., Leal, M.J., Quiriarte, H.D., Pierson, D.L., and Sams, C.F. 2009. Immune status, latent viral reactivation, and stress during long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A37-A44.
74. Inniss, A.M., Rice, B.L., and Smith, S.M. 2009. Dietary support of long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A9-A14.
75. Meck, J.V., Dreyer, S.A., and Warren, L.E. 2009. Long-duration head-down bed rest: Project overview, vital signs, and fluid balance. Aviation, Space, and Environmental Medicine 80:A1-A8.
76. Platts, S.H., Martin, D.S., Stenger, M.B., Perez, S.A., Ribeiro, L.C., Summers, R., and Meck, J.V. 2009. Cardiovascular adaptations to long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A29-A36.
77. Reschke, M.F., Bloomberg, J.J., Paloski, W.H., Mulavara, A.P., Feiveson, A.H., and Harm, D.L. 2009. Postural reflexes, balance control, and functional mobility with long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A45-A54.
78. Seaton, K.A., Bowie, K.E., and Sipes, W.A. 2009. Behavioral and psychological issues in long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A55-A61.
79. Seaton, K.A., Slack, K.J., Sipes, W.A., and Bowie, K.E. 2009. Cognitive functioning in long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A62-A65.
80. Spector, E.R., Smith, S.M., and Sibonga, J.D. 2009. Skeletal effects of long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A23-A28.
81. Zwart, S.R., Oliver, S.A.M., Fesperman, J.V., Kala, G., Krauhs, J., Ericson, K., and Smith, S.M. 2009. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A15-A22.
82. Vernikos, J., Ludwig, D.A., Ertil, A., Wade, C.E., Keil, L., and O’ Hara, D.B. 1996. Effect of standing or walking on physiological changes induced by head down bed rest: Implications for space flight. Aviation, Space, and Environmental Medicine 67(11):1069-1079.
83. Smith, S.M., Davis-Street, J.E., Fesperman, J.V., Calkins, D.S., Bawa, M., Macias, B.R., Meyer, R.S., and Hargens, A.R. 2003. Evaluation of treadmill exercise in a lower body negative pressure chamber as a countermeasure for weightlessness-induced bone loss: A bed rest study with identical twins. Journal of Bone and Mineral Research 18:2223-2230.
84. Smith, S.M., Zwart, S.R., Heer, M., Lee, S.M., Baecker, N., Meuche, S., Macias, B.R., Shackelford, L.C., Schneider, S., and Hargens, A.R. 2008. WISE-2005: Supine treadmill exercise within lower body negative pressure and flywheel resistive exercise as a countermeasure to bed rest-induced bone loss in women during 60-day simulated microgravity. Bone 42:572-581.
85. Zwart, S.R., Hargens, A.R., Lee, S.M., Macias, B.R., Watenpaugh, D.E., Tse, K., and Smith, S.M. 2007. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone 40:529-537.
86. Smith, S.M., Zwart, S.R., Heer, M.A., Baecker, N., Evan, H.J., Feiveson, L., Shackelford, C., and LeBlanc, A.D. 2009. Effects of artificial gravity during bed rest and bone metabolism in humans. Journal of Applied Physiology 107:47-53.
87. Smith, S.M., Zwart, S.R., Block, G., Rice, B.L., and Davis-Street, J.E. 2005. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. Journal of Nutrition 135(3):437-443.
88. Zwart, S.R., Kloeris, V.L., Perchonok, M.H., Braby, L., and Smith, S.M. 2009. Assessment of nutrient stability in foods from the space food system after long-duration spaceflight on the ISS. Journal of Food Science 74(7):H209-H217.
89. Heer, M. 2002. Nutritional interventions related to bone turnover in European space missions and simulation models. Nutrition 18(10):853-856
90. Smith, S.M., Zwart, S.R., Block, G., Rice, B.L., and Davis-Street, J.E. 2005. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. Journal of Nutrition 135(3):437-443.
91. Heer, M. 2002. Nutritional interventions related to bone turnover in European space missions and simulation models. Nutrition 18(10):853-856.
92. Baecker, N., Frings-Meuthen, P., Smith, S.M., and Heer, M. 2010. Short-term high dietary calcium intake during bedrest has no effect on markers of bone turnover in healthy men. Nutrition 26(5):522-527.
93. Black, D.M., Delmas, P.D., Eastell, R., Reid, I.R., Boonen, S., Cauley, J.A., Cosman, F., Lakatos, P., Leung, P.C., Man, Z., Mautalen, C., et al. 2007. HORIZON Pivotal Fracture Trial Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. New England Journal of Medicine 356:1809-1822.
94. Watanabe, Y., Oshina, H., Mizuno, K., Sekiguchi, C., Fukunaga, M., Kohri, K., Rittweger, J., Felsenberg, D., Matsumoto, T., and Nakamura, T. 2004. Intravenous pamidronate prevents femoral bone loss and renal stone during 90 day bed rest. Journal of Bone and Mineral Research 19:1771-1778.
95. Turner, R.T., Evans, G.L., Lotinun, S., Lapke, P.D., Iwaniec, U.T., and Morey-Holton, E. 2007. Dose-response effects of intermittent PTH on cancellous bone in hindlimb unloaded rats. Journal of Bone and Mineral Research 22(1):64-71.
96. American Dental Association Council on Scientific Affairs. 2006. Dental management of patients receiving oral bisphosphonate therapy: Expert panel recommendations. Journal of the American Dental Association 137:1144-1150.
97. Sellmeyer, D.E. 2010. Atypical fractures as a potential complication of long-term bisphosphonate therapy. Journal of the American Medical Association 304(13):1480-1484.
98. Cummings, S.R., San Martin, J., McClung, M.R., Siris, E.S., Eastell, R., Reid, I.R., Delmas, P., Zoog, H.B., Austin, M., Wang, A., Kutilek, S., Adami, S., Zanchetta, J., Libanati, C., Siddhanti, S., and Christiansen, C. 2009. FREEDOM Trial Denosumab for prevention of fractures in postmenopausal women with osteoporosis. New England Journal of Medicine 361:756-765.
99. File, E., and Deal, C. 2009. Clinical update on teriparatide. Current Rheumatology Reports 11(3):169-176.
100. Aronsohn, A., Brazeau, G., and Hughes, J. 1999. The impact of space travel on dosage form design and use. Journal of Gravitational Physiology 6:P169-P172.
101. Czarnik, T.R., and Vernikos, J. 1999. Physiological changes in spaceflight that may affect drug action. Journal of Gravitational Physiology 6:P161-P164.
102. Ferguson, V., Paietta, R., Stodieck, L., Hanson, A., Young, M., Bateman, T., Lemus, M., Kostenuik, P., Jiao, E., Zhou, X., Lu, J., Simonet, W., Lacey, D., and Han, H. 2009. Inhibiting myostatin prevents microgravity associated bone loss in mice. American Society for Bone and Mineral Research 31st Annual Meeting, Denver, Colo., September 15. Abstract A09003077. Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=9901d87a-8a25-4d83-a01e-f891cff99030.
103. Elkasrawy, M.N., and Hamrick, M.W. 2010. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. Journal of Musculoskeletal and Neuronal Interactions 10(1):56-63.
104. Li, X., Ominsky, M.S., Niu, Q.T., Sun, N., Daugherty, B., D’Agostin, D., Kurahara, C., Gao, Y., Cao, J., Gong, J., Asuncion, F., et al. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. Journal of Bone and Mineral Research 23:860-869.
105. Lin, C., Jiang, X., Dai, Z., Guo, X., Weng, T., Wang, J., Li, Y., Feng, G., Gao, X., and He, L. 2009. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. Journal of Bone and Mineral Research 24:1651-1661.
106. Eddleston, A., Marenzana, M., Moore, A.R., Stephens, P., Muzylak, M., Marshall, D., and Robinson, M.K. 2009. A short treatment with an antibody to sclerostin can inhibit bone loss in an ongoing model of colitis. Journal of Bone and Mineral Research 24:1662-1671.
107. Robling, A.G., and Turner, C.H. 2009. Mechanical signaling for bone modeling and remodeling. Critical Reviews in Eukaryotic Gene Expression 19:319-338.
108. Kirchen, M.E., O’Connor, K.M., Gruber, H.E., Sweeney, J.R., Fras, I.A., Stover, S.J., Sarmiento, A., and Marshall, G.J. 1995. Effects of microgravity on bone healing in a rat fibular osteotomy model. Clinical Orthopaedics and Related Research Sep(318):231-242.
109. Midura, R.J., Su, X., and Androjna, C. 2006. A simulated weightlessness state diminishes cortical bone healing responses. Journal of Musculoskeletal and Neuronal Interactions 6:327-328.
110. Bandstra, E.R., Thompson, R.W., Nelson, G.A., Willey, J.S., Judex, S., Cairns, M.A., Benton, E.R., Vazquez, M.E., Carson, J.A., and Bateman, T.A. 2009. Musculoskeletal changes in mice from 20-50 cGy of simulated galactic cosmic rays. Radiation Research 172:21-29.
111. Compston, J. 2009. Clinical and therapeutic aspects of osteoporosis. European Journal of Radiology 71(3):388-391.
112. Kapur, S., Amoui, M., Kesavan, C., Wang, X., Mohan, S., Baylink, D.J., and Lau, K.H. 2010. Leptin receptor (LEPR) is a negative modulator of bone mechanosensitivity and genetic variations in LEPR may contribute to the differential osteogenic response to mechanical stimulation in the C57Bl/6j and C3H/HeJ pair of mouse strains. Journal of Biological Chemistry 285(48):37607-37618.
113. Turner, R.T., Evans, G.L., Cavolina, J.M., Halloran, B., and Morey-Holton, E. 1998. Programmed administration of parathyroid hormone increases bone formation and reduces bone loss in hindlimb-unloaded ovariectomized rats. Endocrinology 139(10):4086-4091.
114. Hefferan, T.E., Evans, G.L., Lotinun, S., Zhang, M., Morey-Holton, E., and Turner, R.T. 2003. Effect of gender on bone turnover in adult rats during simulated weightlessness. Journal of Applied Physiology 95(5):1775-1780.
115. Lerner, U.H., and Persson, E. 2008. Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. Journal of Musculoskeletal and Neuronal Interactions 8(2):154-165.
116. Datta, H.K., Ng, W.F., Walker, J.A., Tuck, S.P., and Varanasi, S.S. 2008. The cell biology of bone metabolism. Journal of Clinical Pathology 61(5):577-587.
117. Zhang, M., and Turner, R.T. 1998. The effects of spaceflight on mRNA levels for cytokines in proximal tibia of ovariectomized rats. Aviation, Space, and Environmental Medicine 69(7):626-629.
118. Cavolina, J.M., Evans, G.L., Harris, S.A., Zhang, M., Westerlind, K.C., and Turner, R.T. 1997. The effects of orbital spaceflight on bone histomorphometry and messenger ribonucleic acid levels for bone matrix proteins and skeletal signaling peptides in ovariectomized growing rats. Endocrinology 138(4):1567-1576.
119. Westerlind, K.C., and Turner, R.T. 1995. The skeletal effects of spaceflight in growing rats: Tissue-specific alterations in mRNA levels for TGF-beta. Journal of Bone and Mineral Research 10(6):843-848.
120. LeBlanc, A., Lin, C., Shackelford, L., Sinitsyn, V., Evans, H., Belichenko, O., Schenkman, B., Kozlovskaya, I., Oganov, V., Bakulin, A., Hedrick, T., and Feeback, D. 2000. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. Journal of Applied Physiology 89:2158-2164.
121. Hefferan, T.E., Evans, G.L., Lotinun, S., Zhang, M., Morey-Holton, E., and Turner, R.T. 2003. Effect of gender on bone turnover in adult rats during simulated weightlessness. Journal of Applied Physiology 95:1775-1780.
122. McCarthy, I.D. 2005. Fluid shifts due to microgravity and their effects on bone: A review of current knowledge. Annals of Biomedical Engineering 33:95-103.
123. Zhang, P., Malacinski, G.M., and Yokota, H. 2008. Joint loading modality: Its application to bone formation and fracture healing. British Journal of Sports Medicine 42:556-560.
124. Whitson, P.A., Pietrzyk, R.A., and Pak, C.Y. 1997. Renal stone risk assessment during space shuttle flights. Journal of Urology 158:2305-2310.
125. Zerwekh, J.E. 2002. Nutrition and renal stone disease in space. Nutrition 18(10):857-863.
126. Smith, S.M., Wastney, M.E., O’Brien, K.O., Morukov, B.V., Larina, I.M., Abrams, S.A., Davis-Street, J.E., Oganov, V., and Shackelford, L.C. 2005. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. Journal of Bone and Mineral Research 20:208-218.
127. Whitson, P.A., Pietrzyk, R.A., and Pak, C.Y. 1997. Renal stone risk assessment during space shuttle flights. Journal of Urology 158:2305-2310.
128. Smith, S.M., Wastney, M.E., O’Brien, K.O., Morukov, B.V., Larina, I.M., Abrams, S.A., Davis-Street, J.E., Oganov, V., and Shackelford, L.C. 2005. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. Journal of Bone and Mineral Research 20:208-218.
129. Whitson, P.A., Pietrzyk, R.A., and Pak, C.Y. 1997. Renal stone risk assessment during space shuttle flights. Journal of Urology 158:2305-2310.
130. Whitson, P.A., Pietrzyk, R.A., and Pak, C.Y. 1997. Renal stone risk assessment during space shuttle flights. Journal of Urology 158:2305-2310.
131. Zerwekh, J.E., Ruml, L.A., Gottschalk, F., and Pak, C.Y. 1998. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. Journal of Bone and Mineral Research 13:1594-1601.
132. Watanabe, Y., Ohshima, H., Mizuno, K., Sekiguchi, C., Fukunaga, M., Kohri, K., Rittweger, J., Felsenberg, D., Matsumoto, T., and Nakamura, T. 2004. Intravenous pamidronate prevents femoral bone loss and renal stone formation during 90-day bed rest. Journal of Bone and Mineral Research 19:1771-1778.
133. Smith, S.M., Zwart, S.R., Block, G., Rice, B.L., and Davis-Street, J.E. 2005. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. Journal of Nutrition 135(3):437-443.
134. Baek, K., Barlow, A.A., Allen, M.R., and Bloomfield, S.A. 2008. Food restriction and simulated microgravity: Effects on bone and serum leptin. Journal of Applied Physiology 104(4):1086-1093.
135. Biolo, G., Ciocchi, B., Stulle, M., Bosutti, A., Barazzoni, R., Zanetti, M., Antonione, R., Lebenstedt, M., Platen, P., Heer, M., and Guarnieri, G. 2007. Calorie restriction accelerates the catabolism of lean body mass during 2 wk of bed rest. American Journal of Clinical Nutrition 86(2):366-372.
136. Li, X., Ominsky, M.S., Niu, Q.T., Sun, N., Daugherty, B., D’Agostin, D., Kurahara, C., Gao, Y., Cao, J., Gong, J., Asuncion, F., Barrero, M., Warmington, K., Dwyer, D., Stolina, M., Morony, S., Sarosi, I., Kostenuik, P.J., Lacey, D.L., Simonet, W.S., Ke, H.Z., and Paszty, C. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. Journal of Bone and Mineral Research 23:860-869.
137. Suva, L. 2009. Commentary: Sclerostin and the unloading of bone. Journal of Bone and Mineral Research 24:1649-1650.
138. National Human Genome Research Institute. Newsroom. 2005. NIH news researchers to gain wider access to knockout mice. October 25. Available at http://www.genome.gov/17015131.
139. Li, X., Ominsky, M.S., Niu, Q.T., Sun, N., Daugherty, B., D’Agostin, D., Kurahara, C., Gao, Y., Cao, J., Gong, J., Asuncion, F., et al. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. Journal of Bone and Mineral Research 23:860-869.
140. Suva, L. 2009. Commentary: Sclerostin and the unloading of bone. Journal of Bone and Mineral Research 24:1649-1650.
141. National Human Genome Research Institute. Newsroom. 2005. NIH news researchers to gain wider access to knockout mice. October 25. Available at http://www.genome.gov/17015131.
142. Han, H.Q., Stodieck, L., Ferguson, V., Zhou, X., Lu, J., Hanson, A., Young, M., Jiao, E., Kwak, K., Rosenfeld, R., Boone, T., et al. 2008. Pharmacological myostatin antagonism effectively mitigates spaceflight-induced muscle atrophy in mice. 24th Annual American Society for Gravitational and Space Biology Joint Life in Space for Life on Earth, June 25. Session 3: Muscle and Metabolism Physiology, Abstract. Available at http://www.congrex.nl/08a09/Sessions/25-06%20Session%203a.htm.
143. Kearns, A.E., Khosla, S., and Kostenuik, P.J. 2008. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocrine Reviews 29:155-192.
144. Ominsky, M.S., Stolina, M., Li, X., Corbin, T.J., Asuncion, F.J., Barrero, M., Niu, Q.T., Dwyer, D., Adamu, S., Warmington, K.S., Grisanti, M., et al. 2009. One year of transgenic overexpression of osteoprotegerin in rats suppressed bone resorption and increased vertebral bone volume, density, and strength. Journal of Bone and Mineral Research 24:1234-1246.
145. Morey-Holton, E., Globus, R.K., Kaplansky, A., and Durnova, G. 2005. The hindlimb unloading rat model: Literature overview, technique update and comparison with space flight data. Advances in Space Biology and Medicine 10:7-40.
146. Iwaniec, U.T., Yuan, D., Power, R.A., and Wronski, T.J. 2006. Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. Journal of Bone and Mineral Research 21(7):1068-1074.
147. Jacob, H.J., Lazar, J., Dwinell, M.R., Moreno, C., and Geurts, A.M. 2010. Gene targeting in the rat: Advances and opportunities. Trends in Genetics September 23.
148. Spector, E.R., Smith, S.M., and Sibonga, J.D. 2009. Skeletal effects of long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80:A23-A28.
149. Pavy-Le Traon, A., Heer, M., Narici, M.V., Rittweger, J., and Vernikos, J. 2007. From space to Earth: Advances in human physiology from 20 years of bed rest studies (1986-2006). European Journal of Applied Physiology 101:143-194.
150. Thomsen, J.S., Morukov, B.V., Vico, L., Alexandre, C., Saparin, P.I., and Gowin, W. 2005. Cancellous bone structure of iliac crest biopsies following 370 days of head-down bed rest. Aviation, Space, and Environmental Medicine 76:915-922.
151. Zerwekh, J.E., Ruml, L.A., Gottschalk, F., and Pak, C.Y. 1998. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. Journal of Bone and Mineral Research 13:1594-1601.
152. Zerwekh, J.E., Ruml, L.A., Gottschalk, F., and Pak, C.Y. 1998. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. Journal of Bone and Mineral Research 13:1594-1601.
153. See NASA Research Opportunities at the NASA Solicitation and Proposal Integrated Review and Evaluation System website, available at http://nspires.nasaprs.com/external/.
154. LeBlanc, A.D., Driscol, T.B., Shackelford, L.C., Evans, H.J., Rianon, N.J., Smith, S.M., Feeback, D.L., and Lai, D. 2002. Alendronate as an effective countermeasure to disuse induced bone loss. Journal of Musculoskeletal and Neuronal Interactions 2(4):335-343.
155. Sibonga, J.D., Evans, H.J., Sung, H.G., Spector, E.R., Lang, T.F., Oganov, V.S., Bakulin, A.V., Shackelford, L.C., and LeBlanc, A.D. 2007. Recovery of spaceflight-induced bone loss: Bone mineral density after long-duration missions as fitted with an exponential function. Bone 41:973-978.
156. Lang, T.F., Leblanc, A.D., Evans, H.J., and Lu, Y. 2006. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. Journal of Bone and Mineral Research 21:1224-1230.
157. Sibonga, J.D., Cavanagh, P.R., Lang, T.F., LeBlanc, A.D., Schneider, V., Shackelford, L.C., Smith, S.M., and Vico, L. 2007. Adaptation of the skeletal system during long-duration spaceflight. Clinical Reviews in Bone and Mineral Metabolism 5(4):249-261.
158. Loehr, J.A., Lee, S.M., English, K.L., Sibonga, J., Smith, S.M., Spiering, B.A., and Hagan, R.D. 2010. Musculoskeletal adaptations to training with the advanced resistive exercise device. Medicine and Science in Sports and Exercise May 13.
159. Genc, K.O., Gopalakrishnan, R., Kuklis, M.M., Maender, C.C., Rice, A.J., Bowersox, K.D., and Cavanagh, P.R. 2010. Foot forces during exercise on the International Space Station. Journal of Biomechanics 43(15):3020-3027.
160. Cavanagh, P.R., Genc, K.O., Gopalakrishnan, R., Kuklis, M.M., Maender, C.C., and Rice, A.J. 2010. Foot forces during typical days on the international space station. Journal of Biomechanics 43(11):2182-2188.
161. Sellmeyer, D.E. 2010. Atypical fractures as a potential complication of long-term bisphosphonate therapy. Journal of the American Medical Association 304(13):1480-1484.
162. American Dental Association Council on Scientific Affairs. 2006. Dental management of patients receiving oral bisphosphonate therapy: Expert panel recommendations. Journal of the American Dental Association 137:1144-1150.
163. Delmas, P.D., Munoz, F., Black, D.M., Cosman, F., Boonen, S., Watts, N.B., Kendler, D., Eriksen, E.F., Mesenbrink, P.G., and Eastell, R. 2009. HORIZON-PFT Research Group Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. Journal of Bone and Mineral Research 24:1544-1551.
164. Adams, G.R., Haddad, F., and Baldwin, K.M. 2003. Gravity plays an important role in muscle development and the differentiation of contractile protein phenotype. Pp. 111-122 in The Neurolab Mission: Neuroscience Research in Space (J.C. Buckley and J.L. Homack, eds.). NASA SP 2003-535. NASA, Washington, D.C.
165. NASA. 2010. Critical Path Roadmap Document. January. Available at http://bioastroroadmap.nasa.gov/index.jsp.
166. Adams, G.R., Caiozzo, V.J., and Baldwin, K.M. 2003. Skeletal muscle unweighting: Spaceflight and ground-based models. Journal of Applied Physiology 95:2185-2201.
167. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
168. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
169. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
170. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
171. Roy, R.R., Baldwin, K.M, and Edgerton, V.R. 1996. Response of the neuromuscular unit to space flight: What have we learned from the rat model. Exercise and Sport Science Reviews 24:399-420.
172. Thomason, D.B., Herrick, R.E., Surdyka, D., and Baldwin, K.M. 1987. Time course of soleus muscle myosin expression during hindlimb suspension and subsequent recovery. Journal of Applied Physiology 63:130-137.
173. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited Review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
174. Shackelford, L.C., LeBlanc, A.D., and Driscoll, T.B. 2004. Resistance exercise as a countermeasure to disuse-induced bone loss. Journal of Applied Physiology 97:119-127.
175. Thomason, D.B., Herrick, R.E., Surdyka, D., and Baldwin, K.M. 1987. Time course of soleus muscle myosin expression during hindlimb suspension and subsequent recovery. Journal of Applied Physiology 63:130-137.
176. Roy, R.R., Baldwin, K.M., and Edgerton, V.R. 1996. Response of the neuromuscular unit to space flight: What have we learned from the rat model. Exercise and Sport Science Reviews 24:399-420.
177. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
178. Giger, J.M., Bodell, P.W., Zeng, M., Baldwin, K.M., and Haddad, F. 2009. Rapid muscle atrophy response to unloading: Pretranslational processes involving MHC and actin. Journal of Applied Physiology 107:1204-1212.
179. Riley, D.A., Ellis, S., Slocum, G.R., Sedlak, F.R., Bain, J.L.W., Krippendorf, B.B., Lehman, C.T., Macias, M.Y., Thompson, J.L., Vijayan, K., and DeBruin, J.A. 1996. In-flight and post flight changes in skeletal muscles of SLS-1 and SLS-2 spaceflown rats. Journal of Applied Physiology 81:133-144.
180. Roy, R.R., Baldwin, K.M., and Edgerton, V.R. 1996. Response of the neuromuscular unit to space flight: What have we learned from the rat model. Exercise and Sport Science Reviews 24:399-420.
181. Adams, G.R., Caiozzo, V.J., and Baldwin, K.M. 2003. Skeletal muscle unweighting: Spaceflight and ground-based models. Journal of Applied Physiology 95:2185-2201.
182. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
183. LeBlanc, A., Schneider, V., and Shackelford, L. 2000. Bone mineral and lean tissue loss after long duration spaceflight. Journal of Musculoskeletal and Neuronal Interactions 1:157-160.
184. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
185. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
186. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
187. Baldwin, K.M., Herrick, R.E., Ilyina-Kakeuva, E., and Oganov, V.S. 1990. Effects of zero gravity on myofibril content and isomyosin distribution in rodent skeletal muscle. The FASEB Journal 4:79-83.
188. Caiozzo, V.J., Baker, M.J., Herrick, R.E., Tao, M., and Baldwin, K.M. 1994. Effect of spaceflight on skeletal muscle: Mechanical properties and myosin isoform content of a slow antigravity muscle. Journal of Applied Physiology 76:1764-1773.
189. Caiozzo, V.J., Haddad, F., Baker, M.J., Herrick, R.E., Prietto, N., and Baldwin, K.M. 1996. Microgravity induced transformations of myosin isoforms and contractile properties of skeletal muscle. Journal of Applied Physiology 81:123-132.
190. Haddad, F., Herrick, R.E., Adams, G.R., and Baldwin, K.M. 1993. Myosin heavy chain expression in rodent skeletal muscle: Effects of exposure to zero gravity. Journal of Applied Physiology 75:2471-2477.
191. Thomason, D.B., Morrison, P.R., Organov, V., Ilyina-Kakueva, E., Booth, F.W., and Baldwin, K.M. 1992. Altered actin and myosin expression in muscle during exposure to microgravity. Journal of Applied Physiology 73(Suppl.):90S-93S.
192. Bottinelli, R., Schiaffino, S., and Reggiani, C. 1991. Force velocity relations and myosin heavy chain isoform compositions of skinned-fibres from rat skeletal muscle. Journal of Physiology 437:655-672.
193. Caiozzo, V.J., Baker, M.J., Herrick, R.E., Tao, M., and Baldwin, K.M. 1994. Effect of spaceflight on skeletal muscle: Mechanical properties and myosin isoform content of a slow antigravity muscle. Journal of Applied Physiology 76:1764-1773.
194. Caiozzo, V.J., Haddad, F., Baker, M.J., Herrick, R.E., Prietto, N., and Baldwin, K.M. 1996. Microgravity induced transformations of myosin isoforms and contractile properties of skeletal muscle. Journal of Applied Physiology 81:123-132.
195. Caiozzo, V.J., Baker, M.J., Herrick, R.E., Tao, M., and Baldwin, K.M. 1994. Effect of spaceflight on skeletal muscle: Mechanical properties and myosin isoform content of a slow antigravity muscle. Journal of Applied Physiology 76:1764-1773.
196. Caiozzo, V.J., Haddad, F., Baker, M.J., Herrick, R.E., Prietto, N., and Baldwin, K.M. 1996. Microgravity induced transformations of myosin isoforms and contractile properties of skeletal muscle. Journal of Applied Physiology 81:123-132.
197. Haddad, F., Adams, G.R., Bodell, P.W., and Baldwin, K.M. 2006. Isometric resistance exercise fails to counteract skeletal atrophy processes during the early states of unloading. Journal of Applied Physiology 100:433-441.
198. Roy, R.R., Baldwin, K.M., and Edgerton, V.R. 1996. Response of the neuromuscular unit to space flight: What have we learned from the rat model. Exercise and Sport Science Reviews 24:399-420.
199. Roy, R.R., Baldwin, K.M., and Edgerton, V.R. 1996. Response of the neuromuscular unit to space flight: What have we learned from the rat model. Exercise and Sport Science Reviews 24:399-420.
200. Caiozzo, V.J., Baker, M.J., Herrick, R.E., Tao, M., and Baldwin, K.M. 1994. Effect of spaceflight on skeletal muscle: Mechanical properties and myosin isoform content of a slow antigravity muscle. Journal of Applied Physiology 76:1764-1773.
201. Caiozzo, V.J., Haddad, F., Baker, M.J., Herrick, R.E., Prietto, N., and Baldwin, K.M. 1996. Microgravity induced transformations of myosin isoforms and contractile properties of skeletal muscle. Journal of Applied Physiology 81:123-132.
202. Haddad, F., Herrick, R.E., Adams, G.R., and Baldwin, K.M. 1993. Myosin heavy chain expression in rodent skeletal muscle: Effects of exposure to zero gravity. Journal of Applied Physiology 75:2471-2477.
203. Shackelford, L.C., LeBlanc, A.D., and Driscoll, T.B. 2004. Resistance exercise as a countermeasure to disuse-induced bone loss. Journal of Applied Physiology 97:119-127.
204. Edgerton, V.R., Shou, M.-Y., Klitgaard, H., Jiang, B., Bell, G., Harris, B., Saltin, B., Gollnick, P.D., Roy, R.R., Day, M.K., and Greenisen, M. 1995. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. Journal of Applied Physiology 78:1733-1739.
205. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
206. Gallagher, P., Trappe, S., Harber, M., Creer, A., Mazzatti, S., Trappe, T., Alkner, B., and Tesch, P. 2005. Effects of 84-days of bed rest and resistance exercise training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiologica Scandinavica 185:61-69.
207. Caiozzo, V.J., Baker, M.J., Herrick, R.E., Tao, M., and Baldwin, K.M. 1994. Effect of spaceflight on skeletal muscle: Mechanical properties and myosin isoform content of a slow antigravity muscle. Journal of Applied Physiology 76:1764-1773.
208. Caiozzo, V.J., Haddad, F., Baker, M.J., Herrick, R.E., Prietto, N., and Baldwin, K.M. 1996. Microgravity induced transformations of myosin isoforms and contractile properties of skeletal muscle. Journal of Applied Physiology 81:123-132.
209. Riley, D.A., Ellis, S., Slocum, G.R., Sedlak, F.R., Bain, J.L.W., Krippendorf, B.B., Lehman, C.T., Macias, M.Y., Thompson, J.L., Vijayan, K., and DeBruin, J.A. 1996. In-flight and post flight changes in skeletal muscles of SLS-1 and SLS-2 spaceflown rats. Journal of Applied Physiology 81:133-144.
210. Vijayan, K., Thompson, J.L., and Riley, D.A. 1998. Sarcomere lesion damage mainly in slow fibers of reloaded rat adductus longus muscles. Journal of Applied Physiology 85:1017-1023.
211. Riley, D.A., Ellis, S., Slocum, G.R., Sedlak, F.R., Bain, J.L.W., Krippendorf, B.B., Lehman, C.T., Macias, M.Y., Thompson, J.L., Vijayan, K., and DeBruin, J.A. 1996. In-flight and post flight changes in skeletal muscles of SLS-1 and SLS-2 spaceflown rats. Journal of Applied Physiology 81:133-144.
212. Adams, G.R., Caiozzo, V.J., and Baldwin, K.M. 2003. Skeletal muscle unweighting: Spaceflight and ground-based models. Journal of Applied Physiology 95:2185-2201.
213. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
214. Trappe, S., Creer, A., Minchev, K., Slivka, D., Louis, E., Luden, T., and Trappe, T. 2007. Human soleus single muscle fiber function with exercise or nutritional countermeasures during 60 days of bed rest. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294:R939-R347.
215. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
216. Adams, G.R., Caiozzo, V.J., and Baldwin, K.M. 2003. Skeletal muscle unweighting: Spaceflight and ground-based models. Journal of Applied Physiology 95:2185-2201.
217. Adams, G.R., Caiozzo, V.J., and Baldwin, K.M. 2003. Skeletal muscle unweighting: Spaceflight and ground-based models. Journal of Applied Physiology 95:2185-2201.
218. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
219. Trappe, S., Creer, A., Minchev, K., Slivka, D., Louis, E., Luden, T., and Trappe, T. 2007. Human soleus single muscle fiber function with exercise or nutritional countermeasures during 60 days of bed rest. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294:R939-R347.
220. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
221. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
222. LeBlanc, A., Lin, C., Shackelford, L., Sinitsyn, V., Evans, H., Belichenko, O., Schenkman, B., Kozlovskaya, I., Oganov, V., Bakulin, A., Hedrick, T., and Feeback, D. 2000. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. Journal of Applied Physiology 89:2158-2164.
223. LeBlanc, A., Schneider, V., and Shackelford, L. 2000. Bone mineral and lean tissue loss after long duration spaceflight. Journal of Musculoskeletal and Neuronal Interactions 1:157-160.
224. Organov, V.S., Grigoriev, A.I., and Veronin, L.I. 1992. Bone mineral density in cosmonauts after flights lasting 4.5-6 months on the Mir orbital station. Aviakosm Ekolog Med. 26:20-24.
225. LeBlanc, A., Schneider, V., and Shackelford, L. 2000. Bone mineral and lean tissue loss after long duration spaceflight. Journal of Musculoskeletal and Neuronal Interactions 1:157-160.
226. Organov, V.S., Grigoriev, A.I., and Veronin, L.I. 1992. Bone mineral density in cosmonauts after flights lasting 4.5-6 months on the Mir orbital station. Aviakosm Ekolog Med. 26:20-24.
227. LeBlanc, A., Schneider, V., and Shackelford, L. 2000. Bone mineral and lean tissue loss after long duration spaceflight. Journal of Musculoskeletal and Neuronal Interactions 1:157-160.
228. Rittweger, J., Frost, H.M., Schiessl, H., Oshima, H., Alkner, B., Tesch, P., and Felsenberg, D. 2005. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and palmidronate: Results from the LTBR study. Bone 36:1019-1029.
229. Shackelford, L.C., LeBlanc, A.D., and Driscoll, T.B. 2004. Resistance exercise as a countermeasure to disuse-induced bone loss. Journal of Applied Physiology 97:119-127.
230. Haddad, F., Adams, G.R., Bodell, P.W., and Baldwin, K.M. 2006. Isometric resistance exercise fails to counteract skeletal atrophy processes during the early states of unloading. Journal of Applied Physiology 100:433-441.
231. Adams, G.R., Haddad, F., Bodell, P.W., Tran, P.D., and Baldwin, K.M. 2007. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. Journal of Applied Physiology 103:1644-1654.
232. Haddad, F., Adams, G.R., Bodell, P.W., and Baldwin, K.M. 2006. Isometric resistance exercise fails to counteract skeletal atrophy processes during the early states of unloading. Journal of Applied Physiology 100:433-441.
233. Adams, G.R., Haddad, F., Bodell, P.W., Tran, P.D., and Baldwin, K.M. 2007. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. Journal of Applied Physiology 103:1644-1654.
234. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
235. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
236. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
237. Trappe, A., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106:1159-1168.
238. Fitts, R.H., Trappe, S.W., and Costill, D.L., Gallagher, P.M., Creer, A.C., Colloton, P.A., Peters, J.R., Romantowski, J.G., Bain, J.L., and Riley, D.A. 2010. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fiber. Journal of Physiology 588:3567-3592.
239. Adams, G.R., Caiozzo, V.J., and Baldwin, K.M. 2003. Skeletal muscle unweighting: Spaceflight and ground-based models. Journal of Applied Physiology 95:2185-2201.
240. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
241. Gallagher, P., Trappe, S., Harber, M., Creer, A., Mazzatti, S., Trappe, T., Alkner, B., and Tesch, P. 2005. Effects of 84-days of bed rest and resistance exercise training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiologica Scandinavica 185:61-69.
242. Lemoine, J.K., Jaus, J.M., and Trappe, S. 2009. Muscle proteins during 60-day bedrest in woman: Impact of exercise or nutrition. Muscle and Nerve 39:463-471.
243. Trappe, S., Creer, A., Minchev, K., Slivka, D., Louis, E., Luden, T., and Trappe, T. 2007. Human soleus single muscle fiber function with exercise or nutritional countermeasures during 60 days of bed rest. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294:R939-R347.
244. Trappe, S., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106(4):1159-1168.
245. Gallagher, P., Trappe, S., Harber, M., Creer, A., Mazzatti, S., Trappe, T., Alkner, B., and Tesch, P. 2005. Effects of 84-days of bed rest and resistance exercise training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiologica Scandinavica 185:61-69.
246. Trappe, S., Creer, A., Minchev, K., Slivka, D., Louis, E., Luden, T., and Trappe, T. 2007. Human soleus single muscle fiber function with exercise or nutritional countermeasures during 60 days of bed rest. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294:R939-R347.
247. Gallagher, P., Trappe, S., Harber, M., Creer, A., Mazzatti, S., Trappe, T., Alkner, B., and Tesch, P. 2005. Effects of 84-days of bed rest and resistance exercise training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiologica Scandinavica 185:61-69.
248. Caiozzo, V.J., Haddad, F., Lee, S., Baker, M., Paloski, W., and Baldwin, K.M. 2009. Artificial gravity as a countermeasure to microgravity: A pilot study examining the effects on knee extensor and plantar flexor muscle groups. Journal of Applied Physiology 107:39-46.
249. Nakao, R., Hirasaka, K., Goto, J., Ishidoh, K., Yamada, C., Ohno, A., Okumura, Y., Nonaka, I., Yasutomo, K., Baldwin, K.M., Kominami, E., et al. 2009. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Molecular and Cell Biology 29:4798-4811.
250. Baldwin, K.M., Herrick, R.E., and McCue, S.A. 1993. Substrate oxidation capacity in rodent skeletal muscle: Effects of exposure to zero gravity. Journal of Applied Physiology 75:2666-2470.
251. Fitts, R.H., Riley, D.A., and Widrick, J. 2000. Physiology of a microgravity environment. Invited review: Microgravity and skeletal muscle. Journal of Applied Physiology 89:823-839.
252. Jiang, B.Y., Ohira Y., Roy R.R., Nguyen, Q., Ilyina-Kakeuva, E.I., Oganov, V., and Edgerton, V.R. 1992. Adaptation of fibers in fast twitch muscles of rats to spaceflight and hindlimb suspension. Journal of Applied Physiology (Suppl.):58S-65S.
253. Baldwin, K.M., Herrick, R.E., and McCue, S.A. 1993. Substrate oxidation capacity in rodent skeletal muscle: Effects of exposure to zero gravity. Journal of Applied Physiology 75:2666-2470.
254. Musacchia, X.J., Steffen, J.M., Fell, R.D., Dombrowski, M.J., Oganov, V.W., Ilyina-and Kakeuva, E.I. 1992. Skeletal muscle atrophy response to 14 days of weightlessness: Vastus intermedius. Journal of Applied Physiology (Suppl.):44S-50S.
255. Henriksen, E.J., Tischler, M.E., and Johnson, D.G. 1986. Increased response to insulin of glucose metabolism in six day unloaded rat soleus. Journal of Biological Chemistry 261:10708-10712.
256. McClung, J.M., Whidden, M.A., Kavazis, A.N., Falk, D.J., Deruisseau, K.C., and Powers, S.K. 2008. Redox regulation of diaphragm proteolysis during mechanical ventilation. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294:R1608-R1617.
257. Adams, G.R., and Haddad, F. 1996. The relationship among IGF-1, DNA content and protein accumulation during skeletal muscle hypertrophy. Journal of Applied Physiology 81:2509-2516.
258. Adams, G.R., Haddad, F., and Baldwin, K.M. 1999. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. Journal of Applied Physiology 87:1705-1712.
259. Adams, G.R., and Haddad, F. 1996. The relationship among IGF-1, DNA content and protein accumulation during skeletal muscle hypertrophy. Journal of Applied Physiology 81:2509-2516.
260. Adams, G.R., Haddad, F., and Baldwin, K.M. 1999. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. Journal of Applied Physiology 87:1705-1712.
261. Pandorf, C.E., Haddad, F., Wright, C., Bodell, P.W., and Baldwin, K.M. 2009. Differential epigenetic modifications of histones at the myosin heavy chain genes in fast and slow skeletal muscle fibers and in response to muscle unloading. American Journal of Physiology. Cell Physiology 297(1):C6-C16.
262. See Funding Opportunities at the National Space Biomedical Research Insitute website, available at http://www.nsbri.org/funding-opportunities.
263. Giger, J.M., Bodell, P.W., Zeng, M., Baldwin, K.M., and Haddad, F. 2009. Rapid muscle atrophy response to unloading: Pretranslational processes involving MHC and actin. Journal of Applied Physiology 107:1204-1212.
264. Jiang, B.Y., Ohira Y., Roy R.R., Nguyen, Q., Ilyina-Kakeuva, E.I., Oganov, V., and Edgerton, V.R. 1992. Adaptation of fibers in fast twitch muscles of rats to spaceflight and hindlimb suspension. Journal of Applied Physiology (Suppl.):58S-65S.
265. Roy, R.R., Baldwin, K.M., and Edgerton, V.R. 1996. Response of the neuromuscular unit to space flight: What have we learned from the rat model. Exercise and Sport Science Reviews 24:399-420.
266. Adams, G.R., McCue, S.A., Bodell, P.W., Zeng, M., and Baldwin, K.M. 2000. Effects of spaceflight and thyroid deficiency on hindlimb development I: Muscle mass and IGF-I expression. Journal of Applied Physiology 88:894-903.
267. Adams, G.R., Haddad, F., McCue, S.A., Bodell, P.W., Zeng, M., Qin, A.X., and Baldwin, K.M. 2000. Effects of spaceflight and thyroid deficiency on rat hindlimb development II: Expression of MHC isoforms. Journal of Applied Physiology 88:904-916.
268. Adams, G.R., McCue, S.A., Bodell, P.W., Zeng, M., and Baldwin, K.M. 2000. Effects of spaceflight and thyroid deficiency on hindlimb development I: Muscle mass and IGF-I expression. Journal of Applied Physiology 88:894-903.
269. Adams, G.R., Haddad, F., McCue, S.A., Bodell, P.W., Zeng, M., Qin, A.X., and Baldwin, K.M. 2000. Effects of spaceflight and thyroid deficiency on rat hindlimb development II: Expression of MHC isoforms. Journal of Applied Physiology 88:904-916.
270. Ikemoto, M., Nikawa, T., Takeda, S., Watanabe, C., Kitano, T., Baldwin, K., Izumi, R., Nonaka, I., Towatari, T., Teshima, S., Rokutan, K., and Kishi K. 2001. Space shuttle flight (STS 90) enhances degradation of rat myosin heavy chain in association with activation of ubiquitin-proteasome pathway. FASEB Journal 15:1279-1282.
271. Nakao, R., Hirasaka, K., Goto, J., Ishidoh, K., Yamada, C., Ohno, A., Okumura, Y., Nonaka, I., Yasutomo, K., Baldwin, K.M., Kominami, E., et al. 2009. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Molecular and Cellular Biology 29:4798-4811.
272. Nikawa, T., Ishidoh, K., Hirasaka, K., Ishihara, I., Ikemoto, M., Kano, M., Kominami, E., Nonaka, I., Ogawa, T., Adams, G.R., Baldwin, K.M., et al. 2004. Skeletal muscle gene expression in space-flown rats. The FASEB Journal 18:522-524.
273. Paloski, W.H., Oman, C.M., Bloomberg, J.J., Reschke, M.F., Wood, S.J., Harm, D.L., Peters, B.T., Mularvara, A.P., Locke, J.P., and Stone, L.S. 2008. Risk of sensory-motor performance failures affecting vehicle control during space missions: A review of the evidence. Journal of Gravitational Physiology 15(2):1-29.
274. Young, L.R., Yajima, K., and Paloski, W.H., eds. 2009. Artificial Gravity Research to Enable Human Space Exploration. International Academy of Astronautics, Paris, France.
275. Clement, G., and Bukley, A. 2007. Artificial Gravity. Springer, New York, N.Y.
276. Speers, R.A., Paloski, W.H., and Kuo, A.D. 1998. Multivariate changes in coordination of postural control following spaceflight. Journal of Biomechanics 31(10):883-889.
277. Young, L.R., and Shelhamer, M. 1990. Microgravity enhances the relative contribution of visually induced motion sensation. Aviation, Space, and Environmental Medicine 61:525-530.
278. Lackner, J.R. 1992. Spatial orientation in weightless environments. Perception 21:803-812.
279. Lackner, J.R., and DiZio, P. 1992. Gravitoinertial force level affects the appreciation of limb position during muscle vibration. Brain Research 592:175-180.
280. Lackner, J.R., DiZio, P., and Fisk, J. 1992. Tonic vibration reflexes and background force level. Acta Astronautica 26:133-136.
281. Fisk, J., Lackner, J.R., and DiZio, P. 1993. Gravitoinertial force level influences arm movement control. Journal of Neurophysiology 69:504-511.
282. Clement, G., Gurfinkel, V.S., Lestienne, F., Lipshits, M.I., and Popov, K.E. 1984. Adaptation of postural control to weightlessness. Experimental Brain Research 57:61-72.
283. Paloski, W.H., Reschke, M.F., Black, F.O., Doxey, D.D., and Harm, D.L. 1992. Recovery of postural equilibrium control following spaceflight. Annals of the New York Academy of Sciences 656:747-754.
284. Bloomberg, J.J., Peters, B.T., Smith, S.L., Huebner, W.P., and Reschke, M.F. 1997. Locomotor head-trunk coordination strategies following space flight. Journal of Vestibular Research 7:161-177.
285. Jaekl, P., Zikovitz, D.C., Jenkin, M.R., Jenkin, H.L., Zacher, J.E., and Harris, L.R. 2005. Gravity and perceptual stability during translational head movement on Earth and in microgravity. Acta Astronautica 56:1033-1040.
286. Lackner, J.R., and DiZio, P. 2009. Angular displacement perception modulated by force background. Experimental Brain Research 195:335-343.
287. Lackner, J.R., and DiZio, P. 1996. Motor function in microgravity: Movement in weightlessness. Current Opinion in Neurobiology 6:744-750.
288. Speers, R.A., Paloski, W.H., and Kuo, A.D. 1998. Multivariate changes in coordination of postural control following spaceflight. Journal of Biomechanics 31(10):883-889.
289. Paloski, W.H., Reschke, M.F., Doxey, D.D., and Black, F.O. 1992. Neurosensory adaptation associated with postural ataxia following space flight. Pp. 311-315 in Posture and Gait: Control Mechanisms (M. Woolacott and F. Horak, eds.). University of Oregon Press, Eugene, Ore.
290. Reschke, M.F., Bloomberg, J.J., Paloski, W.H., Mulavara, A.P., Feiveson, A.H., and Harm, D.L. 2009. Postural reflexes, balance control, and functional mobility with long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80(5 Suppl.):A45-A54.
291. Jain, V., Wood, S.J., Feiveson, A.H., Black, F.O., and Paloski, W.H. 2010. Diagnostic accuracy of dynamic posturography testing after short-duration spaceflight. Aviation, Space, and Environmental Medicine 81(7):625-631.
292. Clement, G., and Lesienne, F. 1988. Adaptive modifications of postural attitude in condition of weightlessness. Experimental Brain Research 72:381-389.
293. Thornton, W.E. 1978. Anthropometric changes in weightlessness. In Anthropometry for Designers. Anthropometric Source Book, Volume 1 (Anthropology Research Staff, eds.). NASA RP-1024. NASA Johnson Space Center, Houston, Tex.
294. Edgerton, V.R., McCall, G.E., Hodgson, J.A., Grotto, J., Goulet, C., Fleischmann, K., and Roy, R.R. 2001. Sensorimotor adaptations to microgravity in humans. Journal of Experimental Biology 204:3217-3224.
295. Clement, G., and Andre-Deshays, C. 1987. Motor activity and visually induced postural reactions during two-g and zero-g phases of parabolic flight. Neuroscience Letters 79:113-116.
296. Reschke, M.F., Anderson, D.J., and Homick, J.L. 1984. Vestibulospinal reflexes as a function of microgravity. Science 225:212-214.
297. Watt, D.G., K.E. Money, and Tomi, L.M. 1986. M.I.T./Canadian vestibular experiments on the Spacelab-1 mission: 3. Effects of prolonged weightlessness on a human otolith-spinal reflex. Experimental Brain Research 64:308-315.
298. Mulavara, A.P., Cohen, H.S., and Bloomberg, J.J. 2009. Critical features of training that facilitate adaptive generalization of over ground locomotion. Gait and Posture 29(2):242-248.
299. Edgerton, V.R., and Roy, R.R. 1996. Neuromuscular adaptations to actual and simulated spaceflight. Pp. 721-763 in Handbook of Physiology: Environmental Physiology (M.J. Fregley and C.M. Blatteis, eds.). Volume 1. Oxford University Press, New York, N.Y.
300. Oman, C.M. 1998. Sensory conflict theory and space sickness: our changing perspective. Journal of Vestibular Research 8:51-56.
301. Lackner, J.R., and Dizio, P. 2006. Space motion sickness. Experimental Brain Research 175:377-399.
302. Kozlovskaya, I.B., Dmitrieval, I.F., Grigorieva, A., Kirenskaya, A., and Kreidich, Y. 1988. Gravitational mechanisms in the motor systems: Studies in real and simulated weightlessness. Pp. 37-48 in Stance and Motion: Fact and Concepts (V.S. Gurfinkel et al., eds.). Plenum, New York, N.Y.
303. Kozlovskaya, I.B., Kreidich, Yu.V., Oganov, V.S., and Koserenko, O.P. 1981. Pathophysiology of motor functions in prolonged manned space flights. Acta Astronautica 8:1059-1072.
304. Roy, R.R., Hodgson, J.A., Aragon, J., Day, M.K., Kozlovskaya, I., and Edgerton, V.R. 1996. Recruitment of the Rhesus soleus and medial gastrocnemius before, during and after spaceflight. Journal of Gravitational Physiology 3:11-15.
305. Recktenwald, M.R., Hodgson, J.A., Roy, R.R., Riazanski, S., McCall, G.E., Kozlovskaya, I., Washburn, D.A., Fanton, J.W., and Edgerton, V.R. 1999. Effects of spaceflight on rhesus quadrupedal locomotion after return to 1 G. Journal of Neurophysiology 81:2451-2463.
306. Edgerton, V.R., and Roy, R.R. 1996. Neuromuscular adaptations to actual and simulated spaceflight. Pp. 721-763 in Handbook of Physiology: Environmental Physiology (M.J. Fregley and C.M. Blatteis, eds.). Volume 1. Oxford University Press, New York, N.Y.
307. Kozlovskaya, I.B., Kreidich, Yu.V., Oganov, V.S., and Koserenko, O.P. 1981. Pathophysiology of motor functions in prolonged space flights. Acta Astronautica 8:1059-1072.
308. Fong, A.J., Roy, R.R., Ichiyama, R.M., Lavrov, I., Courtine, G., Gerasimenko, Y., Tai, Y.C., Burdick, J., and Edgerton, V.R. 2009. Recovery of control of posture and locomotion after a spinal cord injury: Solutions staring us in the face. Progress in Brain Research 175:393-418.
309. Neeper, S.A., Gómez-Pinilla, F., Choi, J., and Cotman, C. 1995. Exercise and brain neurotrophins. Nature 373:109.
310. Edgerton, V.R., and Roy, R.R. 1996. Neuromuscular adaptations to actual and simulated spaceflight. Pp. 721-763 in Handbook of Physiology: Environmental Physiology (M.J. Fregley and C.M. Blatteis, eds.). Volume 1. Oxford University Press, New York, N.Y.
311. Alford, E.K., Roy, R.R., Hodgson, J.A., and Edgerton, V.R. 1987. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hind limb suspension. Experimental Neurology 96:635-649.
312. Edgerton, V.R., and Roy, R.R. 1996. Neuromuscular adaptations to actual and simulated spaceflight. Pp. 721-763 in Handbook of Physiology: Environmental Physiology (M.J. Fregley and C.M. Blatteis, eds.). Volume 1. Oxford University Press, New York, N.Y.
313. Edgerton, V.R., and Roy, R.R. 1996. Neuromuscular adaptations to actual and simulated spaceflight. Pp. 721-763 in Handbook of Physiology: Environmental Physiology (M.J. Fregley and C.M. Blatteis, eds.). Volume 1. Oxford University Press, New York, N.Y.
314. Grigoriev, A.I., Bugrov, S.A., Bogomolov, V.V., Yegorov, A.D., Kozlovskaya, I.B., Pestov, I.D., and Tarasov, N.K. 1990. Review of the major medical results of the 1-year flight on space station “Mir.” Kosmicheskaia Biologiia I Aviakosmicheskaia Meditsina 24(5)5:3-10.
315. Layne, C.S., McDonald, P.V., and Bloomberg, J.J. 1997. Neuromuscular activation patterns during treadmill walking after space flight. Experimental Brain Research 113(1):104-116.
316. Grigoriev, A.I., Bugrov, S.A., Bogomolov, V.V., Yegorov, A.D., Kozlovskaya, I.B., Pestov, I.D., and Tarasov, N.K. 1990. Review of the major medical results of the 1-year flight on space station “Mir.” Kosmicheskaia Biologiia I Aviakosmicheskaia Meditsina 24(5)5:3-10.
317. Edgerton, V.R., and Roy, R.R. 1996. Neuromuscular adaptations to actual and simulated spaceflight. Pp. 721-763 in Handbook of Physiology: Environmental Physiology (M.J. Fregley and C.M. Blatteis, eds.). Volume 1. Oxford University Press, New York, N.Y.
318. Lackner, J.R., and DiZio, P. 2000. Artificial gravity as a countermeasure in long-duration space flight. Journal of Neuroscience Research 62:169-176.
319. Edgerton, V.R., and Roy, R.R. 1996. Neuromuscular adaptations to actual and simulated spaceflight. Pp. 721-763 in Handbook of Physiology: Environmental Physiology (M.J. Fregley and C.M. Blatteis, eds.). Volume 1. Oxford University Press, New York, N.Y.
320. Trappe, S., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106(4):1159-1168.
321. Trappe, S., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106(4):1159-1168.
322. Cohen, H.S. 2003. Update on the status of rehabilitative countermeasures to ameliorate the effects of long-duration exposure to microgravity on vestibular and sensorimotor function. Journal of Vestibular Research 13:405-409.
323. Grigoriev, A.I., Bugrov, S.A., Bogomolov, V.V., Yegorov, A.D., Kozlovskaya, I.B., Pestov, I.D., and Tarasov, N.K. 1990. Review of the major medical results of the 1-year flight on space station “Mir.” Kosmicheskaia Biologiia I Aviakosmicheskaia Meditsina 24(5)5:3-10.
324. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C., p. 139.
325. Drummer, C., Hesse, C., Baisch, F., Norsk, P., Elmann-Larsen, B., Gerzer, R., and Heer, M. 2000. Water and sodium balances and their relation to body mass changes in microgravity. European Journal of Clinical Investigation 30:1066-1075.
326. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.L., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term flight. Journal of Applied Physiology 81:105-116.
327. Drummer, C., Norsk, P., and Heer, M. 2001. Water and sodium balance in space. American Journal of Kidney Diseases 38:684-690.
328. Blomqvist, C.G., Buckey, J.C., Gaffney, F.A., Lane, L.D., Levine, B.D., and Watenpaugh, D.E. 1994. Mechanisms of post-flight orthostatic intolerance. Journal of Gravitational Physiology 1:122-124.
329. Polk, J.D. 2009. “Flight Surgeon Perspective: Gaps in Human Health, Performance, and Safety,” presentation to Committee of the Decadal Survey on Biological and Physical Sciences in Space, August. National Research Council, Washington, D.C.
330. Heer, M., and Paloski, W.H. 2009. Space motion sickness: Incidence, etiology and countermeasures. Autonomic Neuroscience: Basic and Clinical 129:77-79.
331. Smith, S.M., Krauh, J.M., and Leach, C.S. 1997. Regulation of body fluid volume and electrolyte concentration in spaceflight. Advances in Space Biology and Medicine 6:123-165.
332. Moore, T.P., and Thornton, W.E. 1987. Space shuttle inflight and postflight fluid shifts measured by leg volume changes. Aviation, Space, and Environmental Medicine 58(9 Suppl.):A91-A96.
333. Buckey, J.C., Gaffney, F.A., Lane, L.D., Levine, B.D., Watenpaugh, D.E., Wright, S.J., Yancy, C.W., Jr., Meyer, D.M., and Blomqvist, C.G. 1995. Central venous pressure in space. Journal of Applied Physiology 81:19-25.
334. Foldager, N.T., Anderson, A.E., Jensen, F.B., Ellegaard, P., Stadeager, C., Videbaek, R., and Norsk, P. 1996. Central venous pressure in humans during microgravity. Journal of Applied Physiology 81:408-412.
335. Buckey, J.C., Gaffney, F.A., Lane, L.D., Levine, B.D., Watenpaugh, D.E., Wright, S.J., Yancy, C.W., Jr., Meyer, D.M., and Blomqvist, C.G. 1995. Central venous pressure in space. Journal of Applied Physiology 81:19-25.
336. Prisk, G.M., Guy, H.G.B., Elliott, A.R., Deutschman III, R.A., and West, J.B. 1993. Pulmonary diffusing capacity, capillary blood volume, and cardiac output during sustained microgravity. Journal of Applied Physiology 75:15-26.
337. White, R.G., and Blomqvist, C.G. 1998. Central venous pressure and cardiac function during spaceflight. Journal of Applied Physiology 85:738-746.
338. Videbaek, R., and Norsk, P. 1997. Atrial distension in humans during microgravity induced by parabolic flights. Journal of Applied Physiology 83:1862-1966.
339. Tyberb, J.V., and Hamilton, D.R. 1996. Orthostatic hypotension and the role of changes in venous capacitance. Medicine and Science in Sports and Exercise 28(Suppl.):S29-S31.
340. White, R.G., and Blomqvist, C.G. 1998. Central venous pressure and cardiac function during spaceflight. Journal of Applied Physiology 85:738-746.
341. Videbaek, R., and Norsk, P. 1997. Atrial distension in humans during microgravity induced by parabolic flights. Journal of Applied Physiology 83:1862-1966.
342. Watenpaugh, D.E. 2001. Fluid volume control during short-term space flight and implications for human performance. Journal of Experimental Biology 204:3209-3215.
343. Diedrich, A.S., Paranjape, Y., and Robertson, D. 2007. Plasma and blood volume in space. American Journal of the Medical Sciences 334:80-85.
344. Watenpaugh, D.E. 2001. Fluid volume control during short-term space flight and implications for human performance. Journal of Experimental Biology 204:3209-3215.
345. Kirsch, K.A., Baartz, F.-J., Gunga, H.C., and Rocker, L.C. 1993. Fluid shifts in and out of superficial tissues under microgravity and terrestrial conditions. Clinical Investigation 71:687-689.
346. Kuipers, A. 1996. First results from experiments performed with the ESA Anthrorack rack during the D-2 Spacelab mission. Acta Astronautica 38:865-875.
347. Wakins, S.D., and Barr, Y.R. 2010. Papilledema Summit: Summary Report. NASA/TM-2010-2I6114. March. NASA, Washington, D.C., pp. 1-12.
348. Hargens, A.R., Tipton, C.M., Gollnik, P.D., Murbarak, S.J., et al. 1983. Fluid shifts and muscle function in humans during acute simulated weightlessness. Journal of Applied Physiology 54:1003-1009.
349. Parazynski, S.E., Hargens, A.R., Tucker, B., Aratow, M.F., and Crenshaw, A. 1991. Transcapillary fluid shifts in tissues of the head and neck and after simulated microgravity. Journal of Applied Physiology 71:2469-2475.
350. Shi-Tong, T., Ballard, R.E., Murthy, G.E., Hargens, A.R., Convertino, V.A. 1998. Plasma colloid osmotic pressure in humans during simulated microgravity. Aviation, Space, and Environmental Medicine 69:23-26.
351. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.L., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term space flight. Journal of Applied Physiology 81:105-116.
352. Polk, J.D. 2009. “Flight Surgeon Perspective: Gaps in Human Health, Performance, and Safety,” presentation to the Committee on Decadal Survey on Biological and Physical Sciences in Space, August. National Research Council, Washington, D.C.
353. Murthy, G., Marchbanks, R.J., Watenpaugh, D.E., Meyer, J.-U., Eliashberg, N., and Hargens, A.R. 1992. Increased intracranial pressure in humans during simulated microgravity. The Physiologist 35(Suppl.):S184-S185.
354. Krotov, V.P., Trambovetski, E.V., and Korolkov, V.I. 1994. [Intracranial pressure in monkeys under microgravity.] [Russian]. Fziol. Zh. Im. I. M. Sechenova 80:1-8.
355. Krasnov, I.B., Gulevskaia, T.S., and Morgunov, V.A. 2005. [Morphology of vessels and vascular plexus in the rat’s brain following 93-day simulation of the effects of microgravity.] [Russian]. Aviakosmicheskaia i Ekologicheskaia Meditsina 39:32-36.
356. Kawai, Y., Doi, M., and Rimoyama, R. 1998. Intracranial pressure in rabbits exposed to head-down tilt. Journal of Gravitational Physiology 5:P23-P24.
357. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.L., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term space flight. Journal of Applied Physiology 81:105-116.
358. Drummer, C., Norsk, P., and Heer, M. 2001. Water and sodium balance in space. American Journal of Kidney Diseases 38:684-690.
359. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.L., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term space flight. Journal of Applied Physiology 81:105-116.
360. Perhonen, M.A., Franco, F., Lane, L.D., Buckey, J.C., Blomqvist, G.C., Zerwekh, R.M., Peshock, R.M., Weatherall, P.T., and Levine, B.D. 2001. Cardiac atrophy after bed rest and space flight. Journal of Applied Physiology 91:645-653.
361. Smith, S.M., Krauh, J.M., and Leach, C.S. 1997. Regulation of body fluid volume and electrolyte concentration in spaceflight. Advances in Space Biology and Medicine 6:123-165.
362. Platts, S.H., Martin, D.S., Stenger, M.B., and Perez, S.A. 2009. Cardiovascular adaptations to long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80(5 Suppl.):A29-A36.
363. Diedrich, A., Paranjape, S.Y., and Robertson, D. 2007. Plasma and blood volume in space. American Journal of the Medical Sciences 334:80-85.
364. Arbeille, P., Fomina, G., Roumy, J., Alferova, I., Tobal, N., and Herault, S. 2001. Adaptation of the left heart, cerebral arteries and jugular and femoral veins during short-and long-term head-down tilt and space flights. European Journal of Applied Physiology 86:157-168.
365. Levine, B.D., Zuckerman, J.H., and Pawelczyk, J.A. 1997. Cardiac atrophy after bed-rest deconditioning: A nonneural mechanism for orthostatic intolerance. Circulation 96:517-525.
366. Perhonen, M.A., Zuckerman, J.H., and Levine, B.D. 2001. Deterioration of left ventricular performance after bed rest. Circulation 103:1851-1857.
367. Perhonen, M.A., Franco, F., Lane, L.D., Buckey, J.C., Blomqvist, G.C., Zerwekh, R.M., Peshock, R.M., Weatherall, P.T., and Levine, B.D. 2001. Cardiac atrophy after bed rest and space flight. Journal of Applied Physiology 91:645-653.
368. Caini, E.G., Weinert, L., Lang, R.M., and Vaida, P. 2009. The role of echocardiology in the assessment of cardiac function in weightlessness-our experience during parabolic flights. Respiratory Physiology and Neurobiology 169(Suppl. 1):S6-S9.
369. Charles, J.B., and Lathers, C.M. 1991. Cardiovascular adaptation to spaceflight. Journal of Clinical Pharmacology 31:1010-1023.
370. Martin, D.S., South, D.A., Wood, M.I., Bungo, M.W., and Meck, J.V. 2002. Comparison of echocardiographic changes after short-and long-duration spaceflight. Aviation, Space, and Environmental Medicine 73:532-536.
371. Levine, B.D., Zuckerman, J.H., and Pawelczyk, J.A. 1997. Cardiac atrophy after bed-rest deconditioning: A nonneural mechanism for orthostatic intolerance. Circulation 96:517-525.
372. Caini, E.G., Weinert, L., Lang, R.M., and Vaida, P. 2009. The role of echocardiology in the assessment of cardiac function in weightlessness—Our experience during parabolic flights. Respiratory Physiology and Neurobiology 169(Suppl. 1):S6-S9.
373. Levine, B.D., Zuckerman, J.H., and Pawelczyk, J.A. 1997. Cardiac atrophy after bed-rest deconditioning: A nonneural mechanism for orthostatic intolerance. Circulation 96:517-525.
374. Perhonen, M.A., Franco, F., Lane, L.D., Buckey, J.C., Blomqvist, G.C., Zerwekh, R.M., Peshock, R.M., Weatherall, P.T., and Levine, B.D. 2001. Cardiac atrophy after bed rest and space flight. Journal of Applied Physiology 91:645-653.
375. Dorfman, T.A., Levine, B.D., Tillery, T., Peshock, R.M., Hastings, J.L., Schneider, S.M., Macias, B.R., Biolo, G., and Hargens, A.R. 2007. Cardiac atrophy in women following bed rest. Journal of Applied Physiology 103:8-16.
376. Arbeille, P., Fomina, G., Roumy, J., Alferova, I., Tobal, N., and Herault, S. 2001. Adaptation of the left heart, cerebral arteries and jugular and femoral veins during short-and long-term head-down tilt and space flights. European Journal of Applied Physiology 86:157-168.
377. Levine, B.D., Zuckerman, J.H., and Pawelczyk, J.A. 1997. Cardiac atrophy after bed-rest deconditioning: A nonneural mechanism for orthostatic intolerance. Circulation 96:517-525.
378. Caini, E.G., Weinert, L., Lang, R.M., and Vaida, P. 2009. The role of echocardiology in the assessment of cardiac function in weightlessness—Our experience during parabolic flights. Respiratory Physiology and Neurobiology 169(Suppl. 1):S6-S9.
379. Charles, J.B., and Lathers, C.M. 1991. Cardiovascular adaptation to spaceflight. Journal of Clinical Pharmacology 31:1010-1023.
380. Martin, D.S., South, D.A., Wood, M.I., Bungo, M.W., and Meck, J.V. 2002. Comparison of echocardiographic changes after short-and long-duration spaceflight. Aviation, Space, and Environmental Medicine 73:532-536.
381. Drummer, C., Hesse, C., Baisch, F., Norsk, P., Elmann-Larsen, B., Gerzer, R., and Heer, M. 2000. Water and sodium balances and their relation to body mass changes in microgravity. European Journal of Clinical Investigation 30:1066-1075.
382. Drummer, C., Norsk, P., and Heer, M. 2001. Water and sodium balance in space. American Journal of Kidney Diseases 38:684-690.
383. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.L., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term space flight. Journal of Applied Physiology 81:105-116.
384. Drummer, C., Hesse, C., Baisch, F., Norsk, P., Elmann-Larsen, B., Gerzer, R., and Heer, M. 2000. Water and sodium balances and their relation to body mass changes in microgravity. European Journal of Clinical Investigation 30:1066-1075.
385. Grigoriev, A.I., Huntoon, C., Natochin, Yu.V. 1995. On the correlation between individual biochemical parameters of human blood serum following space flight and their basal values. Acta Astronautica 36:639-648.
386. Grigoriev, A.I., Morukov, B.V., and Vorobiev, D.V. 1994. Water and electrolyte studies during long-term missions on board the space stations SALYUT and MIR. Clinical Investigation 72:169-189.
387. Custaud, M.-A., de Chantemele, E.B., Blanc, S., Gauquelin-Koch, G., and Gharib, G. 2005. Regulation de la volemie au cours d’une stimulation d’impesanteur de longue duree. Canadian Journal of Physiology and Pharmacology 83:1145-1153.
388. Keil, L., Evans, J., Grindeland, R., and Krasnov, I. 1992. Pituitary oxytocin and vasopressin content of rats flown on COSMOS 2044. Journal of Applied Physiology 73(Suppl.):166S-168S.
389. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.L., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term space flight. Journal of Applied Physiology 81:105-116.
390. Drummer, C., Norsk, P., and Heer, M. 2001. Water and sodium balance in space. American Journal of Kidney Diseases 38:684-690.
391. Drummer, C., Norsk, P., and Heer, M. 2001. Water and sodium balance in space. American Journal of Kidney Diseases 38:684-690.
392. Huntoon, C.S., Clintron, N.W., and Whitson, P.A. 1994. Endocrine and biochemical functions. Pp. 334-350 in Space Physiology and Medicine (A.E. Nicogossian, C.L. Huntoon, and S.L. Pool, eds.). Lea and Febiger, Philadelphia, Pa.
393. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.L., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term space flight. Journal of Applied Physiology 81:105-116.
394. Drummer, C., Hesse, C., Baisch, F., Norsk, P., Elmann-Larsen, B., Gerzer, R., and Heer, M. 2000. Water and sodium balances and their relation to body mass changes in microgravity. European Journal of Clinical Investigation 30:1066-1075.
395. Grigoriev, A.I., Huntoon, C., Natochin, Yu.V. 1995. On the correlation between individual biochemical parameters of human blood serum following space flight and their basal values. Acta Astronautica 36:639-648.
396. Custaud, M.-A., de Chantemele, E.B., Blanc, S., Gauquelin-Koch, G., and Gharib, G. 2005. Regulation de la volemie au cours d’une stimulation d’impesanteur de longue duree. Canadian Journal of Clinical Pharmacology 83:1145-1153.
397. Grigoriev, A.I., Huntoon, C.L., Morukov, B.V., and Lane, H.W. 1996. Endocrine, renal and circulatory influences on fluid and electrolyte homeostasis during weightlessness: A joint Russian-U.S. project. Journal of Gravitational Physiology 3:83-86.
398. Drummer, C., Gerzer, R., Baisch, F., and Heer, M. 2000. Body fluid regulation in μ-gravity differs from that on Earth. Pflügers Archiv: European Journal of Physiology 441(Suppl):R66-R72.
399. Gauqelin, G., Geelen, E., Gharib, C., Grigoriev, A.I., Guell, A., Kvetnansky, R. Macho, L., Noskov, V., Pasi, P., Soukanov, J., and Patricot, M., et al. 1990. Volume regulating hormones, fluid and electrolyte modification during the Aragatz mission (Mir Station). Pp. 603-608 in Proceedings of the Fourth European Symposium on Life Science Research in Space, Trieste, Italy, May 28-June 1, 1990. ESA SP-30 7. European Space Agency, Paris, France.
400. Hinghofer-Szalkay, H.G., Noskov, V.K., Rossler, A., Ggrigoriev, A.I., Kvetnansky, R., and Polyakow, V.V. 1999. Endocrine status and LBNP-induced hormone changes during a 438-day spaceflight: a case study. Aviation, Space, and Environmental Medicine 70:1-5.
401. Custaud, M.-A., de Chantemele, E.B., Blanc, S., Gauquelin-Koch, G., and Gharib, C. 2005. Regulation de la volemie au cours d’une stimulation d’impesanteur de longue duree. Canadian Journal of Clinical Pharmacology 83:1145-1153.
402. Ertl, A.C., Diedrich, A., Biaggioni, I., Levine, B.D., Robertson, R.M., Cox, J.F., Zuckerman, J.H., Pawelczyk, J.A., Ray, C.A., Buckey, J.C., Jr., Lane, L.D., et al. 2002. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. Journal of Physiology 538(Pt 1):321-329.
403. Kvetnansky, R., Ddavydova, N.A., Noskov, V.B., Vigas, M., Popova, I.A., Usakov, A.C., Macho, L., and Grigoriev, A.I. 1988. Plasma and urine catecholamine levels in cosmonauts during long-term stay on Space Station Salyut-7. Acta Astronautica 17:181-186.
404. Goldstein, D.S., Vernikos, J., Holmes, C., and Convertion, V.A. 1995. Catecholaminergic effects of prolonged head-down bed rest. Journal of Applied Physiology 78:1023-1029.
405. Sigaudo, D., Fortrat, J.-O., Allevard, A.-M., Maillet, A., Cottet-Emard, J.M., Vouillarmet, A., Hughson, R.L., Gauquelin-Koch, G., and Gharib, C. 1998. Changes is the sympathetic nervous system induced by 42 days of head-down bed rest. American Journal of Physiology 274 (6 Pt 2):H1875-H1884.
406. Christensen, N.J., Heer, M., Ivanova, K., and Norsk, P. 2005. Sympathetic nervous activity decreases during head-down bed rest but not during microgravity. Journal of Applied Physiology 99:1552-1557.
407. Shoemaker, J.K., Hogeman, C.S., Leuenberger, U.A., and Herr, M.D., Gray, K., Silber, D.H., and Sinoway, L.I. 1998. Sympathetic discharge and vascular resistance after bed rest. Journal of Applied Physiology 84:612-617.
408. Shoemaker, J.K., Hogeman, C.S., and Sinoway, L.T. 1999. Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology 277(4 Pt 2):R1084-R1090.
409. Shoemaker, J.K., Hogeman, C.S., and Sinoway, L.T. 1999. Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology 277(4 Pt 2):R1084-R1090.
410. Pawelczyk, J.A., Zuckerman, J.H., Blomqvist, C.G., and Levine, B.D. 2001. Regulation of muscle sympathetic nerve activity after bed rest deconditioning. American Journal of Physiology. Heart and Circulatory Physiology 280:H2230-H2239.
411. Pancheva, M.V., Panchev, V.S., and Suvandjieva, A.V. 2006. Lower body negative pressure vs. lower body positive pressure to prevent cardiac atrophy after bed rest and spaceflight. What caused the controversy? Journal of Applied Physiology 100:1090-1093.
412. Norsk, P., Drummer, C., Rocker, L., Strollo, F., Christensen, N.J., Warberg, J., Bie, P., Stadeager, C., Johansen, L.B., Heer, M., Gunga, H.C., and Gerzer, R. 1995. Renal and endocrine responses in humans to isotonic saline infusion during microgravity. Journal of Applied Physiology 78:2253-2259.
413. Baldwin, K.M. 1997. Task Force on Countermeasures. Report to NASA Headquarters, Washington, D.C., pp. 12-15.
414. Traon, A.P., Sigaudo, D., Vasseur, P., Maillet, A., Fortrat, J.O., Hughson, R.L., Gauquelin-Koch, G., and Gharib, C. 1998. Cardiovascular responses to orthostatic tests after a 42-day head-down bed rest. 1998. European Journal of Applied Physiology and Occupational Physiology 77:50-59.
415. Kozlovskaya, I.B., and Grigoriev, A.I. 2004. Russian system of countermeasures on board of the International Space Station (ISS): The first results. Acta Astronautica 55:2233-2237.
416. Kozlovskaya, I.B., and Grigoriev, A.I. 2004. Russian system of countermeasures on board of the International Space Station (ISS): The first results. Acta Astronautica 55:2233-2237.
417. Gurovsky, N.N., Gazenko, O.G., Adamovich, B.A., Ilyin, E.A., Genin, A.M., Korolkov, V.I., Shipov, A.A., Kotovskaya, A.R., Kondratyeva, V.A., Serova, L.V., and Kondratyev, Yu. I. 1980. Study of physiological effects of weightlessness and artificial gravity in the light of the biosattelite Cosmos-936. Acta Astronautica 7:113-121.
418. Fortney, S.M., Beckett, W.S., Carpenter, A.J., Davis, J., Drew, H., LaFrance, N.D., Rock, J.A., Tankersley, C.G., and Vroman, N.B. 1988. Changes in plasma volume during bed rest, effects of menstrual cycle and estrogen administration. Journal of Applied Physiology 65:525-533.
419. Stachenfeld, N.S., and Taylor, H.S. 2005. Progesterone increases plasma volume independent of estradiol. Journal of Applied Physiology 98:1991-1997.
420. Hargens, A.R., Richardson, S. 2009. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respiratory Physiology and Neurobiology 169S:S30-S33.
421. Hargens, A.R., and Richardson, S. 2009. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respiratory Physiology and Neurobiology 169S:S30-S33.
422. Meck, J.V., Dreyer, S.A., Warren, L.E. 2009. Long-duration head-down bed rest: Project overview, vital signs, and fluid balance. Aviation, Space, and Environmental Medicine 80(Suppl.):A1-A8.
423. Morey-Holton, E., and Globus, K. 2002. Hindlimb unloading rodent model. Technical aspects. Journal of Applied Physiology 92:1367-1377.
424. Kakurin, L.I., Lobachik, V.I., Mikhailov, V.M., and Senkevich, Y.A. 1976. Antiorthostatic hypokinesia as a method of weightlessness simulation. Aviation, Space, and Environmental Medicine 47:1083-1086.
425. Epstein, M. 1992. Renal effects of head-out water immersion in humans, a 15-year update. Physiological Reviews 81:563-621.
426. Drummer, C., Gerzer, R., Baisch, F., and Heer, M. 2000. Body fluid regulation in μ-gravity differs from that on Earth: An overview. Pflügers Archiv: European Journal of Physiology 441:R66-R72.
427. DeSanto, N.G., Christensen, N.J., Drummer, C., Kramer, H.J., Regnard, J., Heer, M., Cirillo, M., and Norsk, P. 2001. Fluid balance and kidney function in space: Introduction. American Journal of Kidney Diseases 38(3):664-667.
428. Buckey, J.C., Gaffney, F.A., Lane, L.D., Levine, B.D., Watenpaugh, D.E., Wright, S.J., Yancy, C.W., Jr., Meyer, D.M., and Blomqvist, C.G. 1995. Central venous pressure in space. Journal of Applied Physiology 81:19-25.
429. Foldager, N.T., Anderson, A., Jensen, F.B., Ellegaard, P., Stadeager, C., Videbaek, R., and Norsk, P. 1996. Central venous pressure in humans during microgravity. Journal of Applied Physiology 82:408-412.
430. Norsk, P., Christensen, N.J, Gabrielsen, A., Heer, M., and Drummer, C. 2000. Unexpected renal responses in space. Lancet 357:146.
431. Drummer, C., Heer, M., Dressendorfer, R.A., Strasburger, C.J., and Gerzer, R. 1993. Reduced natriuresis during weightlessness. Clinical Investigation 71:678-686.
432. Norsk, P., and Christensen, N.J. 2009. “Adaptation of the human cardiovascular system to prolonged spaceflight: Health aspects and questions to be addressed,” white paper submitted to the Committee of the Decadal Survey on Biological and Physical Sciences in Space, National Research Council, Washington, D.C.
433. Norsk, P., Christensen, N.J., Gabrielsen, A., Heer, M., and Drummer, C. 2000. Unexpected renal responses in space. Lancet 357:146.
434. Meck, J.V., Dreyer, S.A., and Warren, E. 2009. Long-duration head-down bed rest: Project overview, vital signs, and fluid balance. Aviation, Space, and Environmental Medicine 80(Suppl.):A1-A8.
435. Blomqvist, C.G., and Stone, H.L. 1983. Cardiovascular adjustments to gravitational stress. Pp. 1025-1063 in Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow (J.T. Shepherd, F.M. Abboud, and S.R. Geiger, eds.). American Physiological Society, Bethesda, Md.
436. Aubert, A.E., Beckers, F., and Verheyden, B. 2005. Cardiovascular function and basics of physiology in microgravity. Acta Cardiology 60:129-151.
437. Norsk, P., and Linnarsson, D., eds. 2009. Cardio-respiratory physiology in space. Respiratory Physiology and Neurobiology 169 (Suppl.). Elsevier, Paris, France.
438. Norsk, P. 2009. Cardiovascular research in space. Respiratory Physiology and Neurobiology 169(S1):S2-S3.
439. Hoffmann, U. 2009. Results from recent spaceflight experiments. Respiratory Physiology and Neurobiology 169(S1):S4-S5.
440. Navasiolava, N., and Custaud, M.A. 2009. What are the future top priority questions in cardiovascular research and what new hardware needs to be developed? Respiratory Physiology and Neurobiology 169(S1):S73-S74.
441. Coupé, M., Fortrat, J.O., Larina, I., Gauquelin-Koch, G., Gharib, C., and Custaud, M.A. 2009. Cardiovascular deconditioning: From autonomic nervous system to microvascular dysfunctions. Respiratory Physiology and Neurobiology 169(S1):S10-S12.
442. Furlan, R., Barbic, F., Casella, F., Severgnini, G., Zenoni, L., Mercieri, A., Mangili, R., Costantino, G., and Porta, A. 2009. Neural autonomic control in orthostatic intolerance. Respiratory Physiology and Neurobiology 169(S1):S17-S20.
443. Verheyden, B., Liu, J., Beckers, F., and Aubert, A.E. 2009. Adaptation of heart rate and blood pressure to short and long duration space missions. Respiratory Physiology and Neurobiology 169(S1):S13-S16.
444. Parazynski, S.E., Hargens, A.R., Tucker, B., Aratow, M.F., and Crenshaw, A. 1991. Transcapillary fluid shifts in tissues of the head and neck and after simulated microgravity. Journal of Applied Physiology 71:2469-2475.
445. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.L., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term spaceflight. Journal of Applied Physiology 81:105-116.
446. Krotov, V.P., Trambovetskii, E.V., and Korokov, V.I. 1994. [Intracranial pressure in monkeys under microgravity.] [Russian]. Fziolog Zhurnal Imeni M Sechenova 80(10):1-8.
447. Steinbach, G.C., Macias, B.R., Tanaka, K., Yost, W.T., and Hargens, A.R. 2005. Intracranial pressure dynamics assessed by noninvasive ultrasound during 30 days of bed rest. Aviation, Space, and Environmental Medicine 76(2):85-90.
448. LeBlanc, A., Lin, C., Shackelford, L., Sinitsyn, V., Evans, H., Belichenko, O., Schenkman, B., Kozlovskaya, I., Oganov, V., Bakulin, A., Hedrick, T., and Feeback, D. 2000. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. Journal of Applied Physiology 89:2158-2164.
449. Buckey, J.C., Gaffney, F.A., Lane, L.D., Levine, B.D., Watenpaugh, D.E., Wright, S.J., Yancy, C.W., Jr., Meyer, D.M., and Blomqvist, C.G. 1995. Central venous pressure in space. Journal of Applied Physiology 81:19-25.
450. Foldager, N., Andersen, T.A., Jessen, F.B., Ellegaard, P., Stadeager, C., Videbaek, R., and Norsk, P. 1996. Central venous pressure in humans during microgravity. Journal of Applied Physiology 81:408-412.
451. Buckey, J.C., Gaffney, F.A., Lane, L.D., Levine, B.D., Watenpaugh, D.E., Wright, S.J., Yancy, C.W., Jr., Meyer, D.M., and Blomqvist, C.G. 1995. Central venous pressure in space. Journal of Applied Physiology 81:19-25.
452. Tyberg, J.V., Belenkie, I., Manyari, D.E., and Smith, E.R. 1996. Ventricular interaction and venous capacitance modulate left ventricular preload. Canadian Journal of Cardiology 2(10):1058-1064.
453. White, R.J., and Blomqvist, C.G. 1998. Central venous pressure and cardiac function during spaceflight. Journal of Applied Physiology 85(2):738-746.
454. Videbaek, R., and Norsk, P. 1997. Atrial distension in humans during microgravity induced by parabolic flights. Journal of Applied Physiology 83:1862-1966.
455. Hsia, C.C., Herazo, L.F., and Johnson, R.L., Jr. 1992. Cardiopulmonary adaptations to pneumonectomy in dogs. I. Maximal exercise performance. Journal of Applied Physiology 73(1):362-367.
456. Pancheva, M.V., Panchev, V.S., and Suvandjieva, A.V. 2006. Lower body negative pressure vs. lower body positive pressure to prevent cardiac atrophy after bed rest and spaceflight. What caused the controversy? Journal of Applied Physiology 100:1090-1093.
457. Diedrich, A., Paranjape, S.Y., and Robertson, D. 2007. Plasma and blood volume in space. American Journal of Medical Science 334:80-85.
458. Alfrey, C.P., Udden, M.M., Leach-Huntoon, C., Driscoll, T., and Pickett, M.H. 1996. Control of red cell mass in spaceflight. Journal of Applied Physiology 81:98-104.
459. Smith, S.M. 2002. Red cell and iron metabolism during space flight. Nutrition 18:864-866.
460. Rice, L., and Alfrey, C.P. 2000. Modulation of red cell mass by neocytolysis in space and on Earth. Pflügers Archiv: European Journal of Physiology 441(Suppl.):R91-R94.
461. Allebban, Z., Gibson, L.A., Lange, R.D., Jago, T.L., Strickland, K.M., Johnson, D.M., and Ichki, A.T. 1996. Effect of spaceflight on rat erythroid parameters. Journal of Applied Physiology 81:117-122.
462. Leach, C.S., and Johnson, P.C. 1984. Influence of space flight on erythrokinetics in man. Science 225:216-218.
463. Gunga, H.-C., Kirsch, K., Baartz, F., Maillet, A., Gharib, C., Nalishiti, W., Rich, I., and Röcker, L. 1996. Erythropoietin under real and simulated conditions in humans. Journal of Applied Physiology 81:761-773.
464. De Santo, N.G., Cirillo, M., Kirsch, K.A., Correale, G., Drummer, C., Frassl, W., Perna, A.F., Di Stazio, E., Bellini, L., and Gunga, H.-C. 2005. Anemia and erythropoietin in space flights. Seminars in Nephrology 25:379-387.
465. Parazynski, S.E., Hargens, A.R., Tucker, B., Aratow, M.F., and Crenshaw, A. 1991. Transcapillary fluid shifts in tissues of the head and neck and after simulated microgravity. Journal of Applied Physiology 71:2469-2475.
466. Parazynski, S.E., Hargens, A.R., Tucker, B., Aratow, M.F., and Crenshaw, A. 1991. Transcapillary fluid shifts in tissues of the head and neck and after simulated microgravity. Journal of Applied Physiology 71:2469-2475.
467. Leach, C.S., Alfrey, C.P., Suki, W.N., Leonard, J.I., Rambaut, P.C., Inners, L.D., Smith, S.M., Lane, H.W., and Krauhs, J.M. 1996. Regulation of body fluid compartments during short-term spaceflight. Journal of Applied Physiology 81:105-116.
468. Diedrich, A., Paranjape, S.Y., and Robertson, D. 2007. Plasma and blood volume in space. American Journal of the Medical Sciences 334:80-85.
469. Convertino, V.A. 2002. Mechanisms of microgravity induced orthostatic tolerance: Implications for effective countermeasures. Journal of Gravitational Physiology 9:1-14.
470. Drummer, C., Valenti, G., Cirillo, M., Perna, A., Bellini, L., Nenov, V., De Santo, N.G. 2002. Vasopressin, hypercalciuria and aquaporin-the key elements for impaired renal water handling in astronauts? Nephron 92:503-514.
471. Grigoriev, A.I., Huntoon, C.L., Morukov, B.V., and Lane, H.W. 1996. Endocrine, renal and circulatory influences on fluid and electrolyte homeostasis during weightlessness: A joint Russian-U.S. project. Journal of Gravitational Physiology 3:83-86.
472. Norsk, P., Christensen, N.J., Bie, P., Gabrielsen, A., Heer, M., and Drummer, C. 2000. Unexpected renal responses in space. Lancet 356:1577-1578.
473. Drummer, C., Heer, M., Dressendorfer, R.A., Strasburger, C.J., and Gerzer, R. 1993. Reduced natriuresis during weightlessness. Clinical Investigation 71:678-686.
474. Drummer, C., Norsk, P., and Heer, M. 2001. Water and sodium balance in space. American Journal of Kidney Diseases 38:684-690.
475. Lenz, W., Herten, M., Gerzer, R., and Drummer, C. 1999. Regulation of natriuretic peptide (urodilatin) release in a human kidney line. Kidney International 55:91-99.
476. Convertino, V.A. 2002. Mechanisms of microgravity induced orthostatic tolerance: Implications for effective countermeasures. Journal of Gravitational Physiology 9:1-14.
477. Meck, J.V., Reyes, C.J., Perez, S.A., Goldberger, A.L., and Ziegler, M.G. 2001. Marked exacerbation of orthostatic intolerance after long- vs. short-duration spaceflight in veteran astronauts. Psychosomatic Medicine 63(6):865-873.
478. Fritsch-Yelle, J.M., Whitson, P.A., Bondar, R.L., and Brown, T.E. 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology 81:2134-2141.
479. Waters, W.W., Ziegler, M.G., and Meck, J.V. 2002. Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. Journal of Applied Physiology 92:586-594.
480. Meck, J.V., Waters, W.W., Ziegler, M.G., deBlock, H.F., Mills, P.J., Robertson, D., and Huang, P.L. 2004. Mechanisms of postspaceflight orthostatic hypotension: Low alpha1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. American Journal of Physiology. Heart and Circulatory Physiology 286(4):H1486-H1495.
481. Goldstein, D.S., Pechnik, S., Holmes, C., Eldadah, B., and Sharabi, Y. 2003. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 42(2)136-142.
482. Traon, A.P., Vasseur, P., Sigaudo, D., Maillet, A., Fortrat, J.O., Hughson, R.L., Gauquelin-Koch, G., and Gharib, C. 1998. Cardiovascular responses to orthostatic tests after a 42-day head-down bed rest. European Journal of Applied Physiology and Occupational Physiology 77:50-59.
483. Bleeker, M.W., De Groot, P.C., Pawelczyk, J.A., Hopman, M.T.E., and Levine, B.D. 2004. Effects of 18 days of bed rest on leg and arm venous properties. Journal of Applied Physiology 96:840-847.
484. Fu, Q., Witkowski, S., and Levine, B.D. 2004. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation 110:2931-2937.
485. Moore, A.D., Jr., Lee, S.M., Charles, J.B., Greenisen, M.C., and Schneider, S.M. 2001. Maximal exercise as a countermeasure to orthostatic intolerance after spaceflight. Medicine and Science in Sports and Exercise 33:75-80.
486. Cui, J., Durand, S., Levine, B.D., and Crandall, C.G. 2005. Effect of skin surface cooling on central venous pressure during orthostatic challenge. American Journal of Physiology. Heart and Circulatory Physiology 289:2429-2433.
487. Leach, C.S., and Johnson, P.C. 1984. Influence of space flight on erythrokinetics in man. Science 225:216-218.
488. Gunga, H.-C., Kirsch, K., Baartz, F., Maillet, A., Gharib, C., Nalishiti, W., Rich, I., and Röcker, L. 1996. Erythropoietin under real and simulated conditions in humans. Journal of Applied Physiology 81:761-773.
489. De Santo, N.G., Cirillo, M., Kirsch, K.A., Correale, G., Drummer, C., Frassl, W., Perna, A.F., Di Stazio, E., Bellini, L., and Gunga, H.-C. 2005. Anemia and erythropoietin in space flights. Seminars in Nephrology 25:379-387.
490. Rokhlenko, K.D., and Mul’diiarov, P. 1981. [Myocardial ultrastructure of rats exposed aboard “Cosmos-936.”] [Russian]. Kosmich Biol Aviak Meditsa 15:77-82.
491. Bungo, M.W., Goldwater, D.J., Popp, R.L., and Sandler, H. 1987. Echocardiographic evaluation of space shuttle crewmembers. Journal of Applied Physiology 62:278-283.
492. Goldstein, M.A., Edwards, R.J., and Schroeter, J.P. 1992. Cardiac morphology after conditions of microgravity during Cosmos 2044. Journal of Applied Physiology 73(Suppl.):94S-100S.
493. Perhonen, M.A., Franco, F., Lane, L.D., Buckey, J.C., Blomqvist, G.C., Zerwekh, R.M., Peshock, R.M., Weatherall, P.T., and Levine, B.D. 2001. Cardiac atrophy after bed rest and space flight. Journal of Applied Physiology 91:645-653.
494. Levine, B.D., Zuckerman, J.H., and Pawelczyk, J.A. 1997. Cardiac atrophy after bed-rest deconditioning: A nonneural mechanism for orthostatic intolerance. Circulation 96:517-525.
495. Perhonen, M.A., Zuckerman, J.H., and Levine, B.D. 2001. Deterioration of left ventricular chamber performance after bed rest: “Cardiovascular deconditioning” or hypovolemia? Circulation 103:1851-1857.
496. Pancheva, M.V., Panchev, V.S., and Suvandjieva, A.V. 2006. Lower body negative pressure vs. lower body positive pressure to prevent cardiac atrophy after bed rest and spaceflight. What caused the controversy? Journal of Applied Physiology 100:1090-1093.
497. Dorfman, T.A., Levine, B.D., Tillery, T., Peshock, R.M., Hastings, J.L., Schneider, S.M., Macias, B.R., Biolo, G., and Hargens, A.R. 2007. Cardiac atrophy in women following bed rest. Journal of Applied Physiology 103:8-16.
498. Perhonen, M.A., Franco, F., Lane, L.D., Buckey, J.C., Blomqvist, G.C., Zerwekh, R.M., Peshock, R.M., Weatherall, P.T., and Levine, B.D. 2001. Cardiac atrophy after bed rest and space flight. Journal of Applied Physiology 91:645-653.
499. Dorfman, T.A., Rosen, B.D., Perhonen, M.A., Tillery, T., McColl, R., Peshock, R.M., and Levine B.D. 2008. Diastolic suction is impaired by bed rest: MRI tagging studies of diastolic untwisting. Journal of Applied Physiology 104:1037-1044.
500. Perhonen, M.A., Franco, F., Lane, L.D., Buckey, J.C., Blomqvist, G.C., Zerwekh, R.M., Peshock, R.M., Weatherall, P.T., and Levine, B.D. 2001. Cardiac atrophy after bed rest and space flight. Journal of Applied Physiology 91:645-653.
501. Dorfman, T.A., Levine, B.D., Tillery, T., Peshock, R.M., Hastings, J.L., Schneider, S.M., Macias, B.R., Biolo, G., and Hargens, A.R. 2007. Cardiac atrophy in women following bed rest. Journal of Applied Physiology 103:8-16.
502. Hastings, J., Pacini, E., Krainski, F., Shibata, S., Jain, M., Snell, P.G., Fu, Q., Palmer, M.D., Creson, D., and Levine, B.D. 2008. The multi-system effect of exercise training during prolonged bed rest deconditioning: An integrated approach to countermeasure development for the heart, lungs, muscle and bones. Circulation 118:S682. Abstract.
503. Shibata, S., Perhonen, M., and Levine, B.D. 2010. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. Journal of Applied Physiology 108(5):1177-1186.
504. Marcus, M.L., Eckberg, D.L., Braxmeier, J.L., and Abboud, F.M. 1977. Effects of intermittent pressure loading on the development of ventricular hypertrophy in the cat. Circulation Research 40(5):484-488.
505. Cooper, G.I.V., Kent, R.L., and Mann, D.L. 1989. Load induction of cardiac hypertrophy. Journal of Molecular and Cellular Cardiology 21(Suppl. 5):11-30.
506. Zile, M.R., Tomita, M., Ishihara, K., Nakano, K., Lindroth, J., Spinale, F., Swindle, M., and Carabello, B.A. 1993. Changes in diastolic function during development and correction of chronic LV volume overload produced by mitral regurgitation. Circulation 87:1378-1388.
507. Lisy, O., Redfield, M.M., Jovanovic, S., Jougasaki, M., Jovanovic, A., Leskinen, H., Terzic, A., and Burnett, J.C., Jr. 2000. Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function in vivo. Circulation 102(3):338-343.
508. Perhonen, M.A., Franco, F., Lane, L.D., Buckey, J.C., Blomqvist, G.C., Zerwekh, R.M., Peshock, R.M., Weatherall, P.T., and Levine, B.D. 2001. Cardiac atrophy after bed rest and space flight. Journal of Applied Physiology 91:645-653.
509. Frenzel, H., Schartzkopff, B., Holtermann, W., Schnurch, H.G., Novi, A., and Hort, W. 1988. Regression of cardiac hypertrophy: Morphometric and biochemical studies in rat heart after swimming training. Journal of Molecular and Cellular Cardiology 20:737-751.
510. Urabe, Y., Mann, D.L., Kent, R.L., Nakano, K., Tomanek, R.J., Carabello, B.A., and Cooper, G.I.V. 1992. Cellular and ventricular contractile dysfunction in experimental canine mitral regurgitation. Circulation Research 70:131-147.
511. McGowan, B.S., Scott, C.B., Mu, A., McCormick, R.J., Thomas, D.P., and Margulies, K.B. 2003. Unloading-induced remodeling in the normal and hypertrophic left ventricle. American Journal of Physiology. Heart and Circulatory Physiology 284:H2061-H2068.
512. Lisy, O., Redfield, M.M., Jovanovic, S., Jougasaki, M., Jovanovic, A., Leskinen, H., Terzic, A., and Burnett, J.C., Jr. 2000. Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function in vivo. Circulation 102(3):338-343.
513. Hill, J.A., and Olson, E.N. 2008. Cardiac plasticity. New England Journal of Medicine 358(13):1370-1380.
514. Shibata, S., Perhonen, M., and Levine, B.D. 2010. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. Journal of Applied Physiology 108(5):1177-1186.
515. Hastings, J., Pacini, E., Krainski, F., Shibata, S., Jain, M., Snell, P.G., Fu, Q., Palmer, M.D., Creson, D., and Levine, B.D. 2008. The multi-system effect of exercise training during prolonged bed rest deconditioning: An integrated approach to countermeasure development for the heart, lungs, muscle and bones. Circulation 118:S682. Abstract.
516. Dorfman, T.A., Levine, B.D., Tillery, T., Peshock, R.M., Hastings, J.L., Schneider, S.M., Macias, B.R., Biolo, G., and Hargens, A.R. 2007. Cardiac atrophy in women following bed rest. Journal of Applied Physiology 103:8-16.
517. Shibata, S., Perhonen, M., and Levine, B.D. 2010. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. Journal of Applied Physiology 108(5):1177-1186.
518. Fritsch-Yelle, J. M., Leuenberger, U.A., D’Aunno, D.S., Rossum, A.C., Brown, T.E., Wood, M.T., Josephson, M.E., and Goldberger, A.L. 1998. An episode of ventricular tachycardia during long-duration spaceflight. American Journal of Cardiology 81:1391-1392.
519. D’Aunno, D.C., Dougherty, A.H., deBlock, H.F., and Meck, J.V. 2003. Effect of short- and long-duration spaceflight on QTc intervals in healthy astronauts. American Journal of Cardiology 91(4):494-497.
520. Fritsch-Yelle, J.M., Charles, J.B., Jones, M.M., and Wood, M.L. 1996. Microgravity decreases heart rate and arterial pressure in humans. Journal of Applied Physiology 80(3):910-914.
521. Rossum, A.C., Wood, M.L., Bishop, S.L., Deblock, H., Charles, J.B. 1997. Evaluation of cardiac rhythm disturbances during extravehicular activity. American Journal of Cardiology 79:1153-1155.
522. Goldberger, A.L., Bungo, M.W., Baevsky, R.M., Bennett, B.S., Rigney, D.R., Mietus, J.E., Nikulina, G.A., and Charles, J.B. 1994. Heart rate dynamics during long-term spaceflight: Report on Mir cosmonauts. American Heart Journal 128(1):202-204.
523. Berry, C.A., and Catterson, A.D. 1967. Pre-Gemini medical predictions versus Gemini flight results. Pp. 197-199 in Gemini Summary Conference. NASA SP-138. NASA, Washington, D.C.
524. D’Aunno, D.C., Dougherty, A.H., deBlock, H.F., and Meck, J.V. 2003. Effect of short- and long-duration spaceflight on QTc intervals in healthy astronauts. American Journal of Cardiology 91(4):494-497.
525. Hobbs, J.B., Peterson, D.R., Moss, A.J., McNitt, S., Zareba, W., Goldenberg, I., Qi, M., Robinson, J.L., Sauer, A.J., Ackerman, M.J., Benhorin, J., Kaufman, E.S., Locati, E.H., Napolitano, C., Priori, S.G., Towbin, J.A., Vincent, G.M., and Zhang, L. 2006. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. Journal of the American Medical Association 296:1249-1254.
526. Basavarajaiah, S., Wilson, M., Whyte, G., Shah, A., Behr, E., and Sharma, S. Prevalence and significance of an isolated long QT interval in elite athletes. 2007. European Heart Journal 28:2944-2949.
527. Ertl, A.C., Diedrich, A., Biaggioni, I., Levine, B.D., Robertson, R.M., Cox, J.F., Zuckerman, J.H., Pawelczyk, J.A., Ray, C.A., Buckey, J.C., Jr., Lane, L.D., et al. 2002. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. Journal of Physiology 538(Pt 1):321-329.
528. Cox, J.F., Tahvanainen, K.U.O., Kuusela, T.A., Levine, B.D., Cooke, W.H., Mano, T., Iwase, S., Saito, M., Sugiyama, Y., Ertl, A.C., Biaggioni, I., et al. 2002. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva’s manoeuvre. Journal of Physiology 538(Pt 1):309-320.
529. Abboud, F.M. 1989. Ventricular syncope: Is the heart a sensory organ? (Editorial). New England Journal of Medicine 320:390-392.
530. Hajduczok, G., Chapleau, M.W., and Abboud, F.M. 1988. Rheoreceptors in the carotid sinus of dog. Proceedings of the National Academy of Sciences U.S.A. 85:7399-7403.
531. Chapleau, M.W., and Abboud, F.M. 1987. Contrasting effects of static and pulsatile pressure on carotid baroreceptor activity in dogs. Circulation Research 61(5):648-658.
532. Abboud, F.M., and Chapleau, M.W. 1988. Effects of pulse frequency on single unit baroreceptor activity during sine-wave and natural pulses in dogs. Journal of Physiology 401:295-308.
533. Cox, J.F., Tahvanainen, K.U.O., Kuusela, T.A., Levine, B.D., Cooke, W.H., Mano, T., Iwase, S., Saito, M., Sugiyama, Y., Ertl, A.C., Biaggioni, I., et al. 2002. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva’s manoeuvre. Journal of Physiology 538(Pt 1):309-320.
534. Eckberg, D.L., Halliwill, J.R., Beightol, L.A., Brown, T.E., Taylor, J.A., and Goble, R. 2010. Human vagal baroreflex mechanisms in space. Journal of Physiology 588(7):1129-1138.
535. Ertl, A.C., Diedrich, A., Biaggioni, I., Levine, B.D., Robertson, R.M., Cox, J.F., Zuckerman, J.H., Pawelczyk, J.A., Ray, C.A., Buckey, J.C., Jr., Lane, L.D., et al. 2002. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. Journal of Physiology 538(Pt 1):321-329.
536. Cox, J.F., Tahvanainen, K.U.O., Kuusela, T.A., Levine, B.D., Cooke, W.H., Mano, T., Iwase, S., Saito, M., Sugiyama, Y., Ertl, A.C., Biaggioni, I., et al. 2002. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva’s manoeuvre. Journal of Physiology 538(Pt 1):309-320.
537. Levine, B.D., Pawelczyk, J.A., Ertl, A.C., Cox, J.F., Zuckerman, J.H., Diedrich, A., Biaggioni, I., Ray, C.A., Smith, M.L., Iwase, S., Saito, M., et al. 2002. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. Journal of Physiology 538(Pt 1):331-340.
538. Norsk, P., and Christensen, N.J. 2009. The paradox of systemic vasodilation and sympathetic nervous stimulation in space. Respiratory Physiology and Neurobiology 169(Suppl. 1):S26-S29.
539. Christensen, N.J., Heer, M., Ivanova, K., and Norsk, P. 2005. Sympathetic nervous activity decreases during head-down bed rest but not during microgravity. Journal of Applied Physiology 99:1552-1557.
540. Ertl, A.C., Diedrich, A., Biaggioni, I., Levine, B.D., Robertson, R.M., Cox, J.F., Zuckerman, J.H., Pawelczyk, J.A., Ray, C.A., Buckey, J.C., Jr., Lane, L.D., et al. 2002. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. Journal of Physiology 538(Pt 1):321-329.
541. Levine, B.D., Pawelczyk, J.A., Ertl, A.C., Cox, J.F., Zuckerman, J.H., Diedrich, A., Biaggioni, I., Ray, C.A., Smith, M.L., Iwase, S., Saito, M., et al. 2002. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. Journal of Physiology 538(Pt 1):331-340.
542. Cox, J.F., Tahvanainen, K.U.O., Kuusela, T.A., Levine, B.D., Cooke, W.H., Mano, T., Iwase, S., Saito, M., Sugiyama, Y., Ertl, A.C., Biaggioni, I., et al. 2002. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva’s manoeuvre. Journal of Physiology 538(Pt 1):309-320.
543. Fu, Q., Levine, B.D., Pawelczyk, J.A., Ertl, A.C., Diedrich, A., Cox, J.F., Zuckerman, J.H., Raya, C.A., Smith, M.L., Iwase, S., Saito, M., et al. 2002. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. Journal of Physiology 544(Pt 2):653-664.
544. Levine, B.D., Pawelczyk, J.A., Ertl, A.C., Cox, J.F., Zuckerman, J.H., Diedrich, A., Biaggioni, I., Ray, C.A., Smith, M.L., Iwase, S., Saito, M., et al. 2002. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. Journal of Physiology 538(Pt 1):331-340.
545. Levine, B.D., Pawelczyk, J.A., Zuckerman, J.H., Zhang, R., Iwasaki, K., and Blomqvist, C.G. 2003. Neural control of the cardiovascular system in space. Pp. 175-185 in The Neurolab Spacelab Mission: Neuroscience Research in Space: Results from the STS-90, Neurolab Spacelab Mission (J.C. Buckey and J.L. Homick, eds.). NASA 1-11. NASA Johnson Space Center, Houston, Tex. January.
546. Pawelczyk, J.A., Zuckerman, J.H., Blomqvist, C.G., and Levine, B.D. 2001. Regulation of muscle sympathetic nerve activity (MSNA) after bed rest deconditioning. American Journal of Physiology. Heart and Circulatory Physiology 280:H2230-H2239.
547. Shoemaker, J.K., Hogeman, C.S., and Sinoway, L.T. 1999. Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology 277(4 Pt 2):R1084-R1090.
548. Kamiya, A., Iwase, S., Michikami, D., Fu, Q., and Mano, T. 2000. Head-down bed rest alters sympathetic and cardiovascular responses to mental stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 279:R440-R447.
549. Shoemaker, J.K., Hogeman, C.S., and Sinoway, L.T. 1999. Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology 277(4 Pt 2):R1084-R1090.
550. Fritsch-Yelle, J.M., Whitson, P.A., Bondar, R.L., and Brown, T.E. 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology 81:2134-2141.
551. Shoemaker, J.K., Hogeman, C.S., and Sinoway, L.T. 1999. Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology 277(4 Pt 2):R1084-R1090.
552. Fritsch-Yelle, J.M., Whitson, P.A., Bondar, R.L., and Brown, T.E. 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology 81:2134-2141.
553. Meck, J.V., Waters, W.W., Ziegler, M.G., deBlock, H.F., Mills, P.J., Robertson, D., and Huang, P.L. 2004. Mechanisms or postspaceflight orthostatic hypotension: Low alpha 1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. American Journal of Physiology. Heart and Circulatory Physiology 286:H1486-H1495.
554. Blomqvist, C.G., and Stone, H.L. 1983. Cardiovascular adjustments to gravitational stress. Pp. 1025-1063 in Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow (J.T. Shepherd, F.M. Abboud, and S.R. Geiger, eds.). American Physiological Society, Bethesda, Md.
555. Aubert, A.E., Beckers, F., and Verheyden, B. 2005. Cardiovascular function and basics of physiology in microgravity. Acta Cardiologica 60:129-151.
556. Norsk, P. 2009. Cardiovascular research in space. Respiratory Physiology and Neurobiology 169(S1):S2-S3.
557. Hoffmann, U. 2009. Results from recent spaceflight experiments. Respiratory Physiology and Neurobiology 169(S1)S4-S5.
558. Navasiolava, N., and Custaud, M.A. 2009. What are the future top priority questions in cardiovascular research and what new hardware needs to be developed? Respiratory Physiology and Neurobiology 169(S1):S73-S74.
559. Coupé, M., Fortrat, J.O., Larina, I., Gauquelin-Koch, G., Gharib, C., and Custaud, M.A. 2009. Cardiovascular deconditioning: From autonomic nervous system to microvascular dysfunctions. Respiratory Physiology and Neurobiology 169(S1):S10-S12.
560. Furlan, R., Barbic, F., Casella, F., Severgnini, G., Zenoni, L., Mercieri, A., Mangili, R., Costantino, G., and Porta, A. 2009. Neural autonomic control in orthostatic intolerance. Respiratory Physiology and Neurobiology 169(S1):S17-S20.
561. Verheyden, B., Liu, J., Beckers, F., and Aubert, A.E. 2009. Adaptation of heart rate and blood pressure to short and long duration space missions. Respiratory Physiology and Neurobiology 169(S1):S13-S16.
562. Ertl, A.C., Diedrich, A., Biaggioni, I., Levine, B.D., Robertson, R.M., Cox, J.F., Zuckerman, J.H., Pawelczyk, J.A., Ray, C.A., Buckey, J.C., Jr., Lane, L.D., et al. 2002. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. Journal of Physiology 538(Pt 1):321-329.
563. Cox, J.F., Tahvanainen, K.U.O., Kuusela, T.A., Levine, B.D., Cooke, W.H., Mano, T., Iwase, S., Saito, M., Sugiyama, Y., Ertl, A.C., Biaggioni, I., et al. 2002. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva’s manoeuvre. Journal of Physiology 538(Pt 1):309-320.
564. Levine, B.D., Pawelczyk, J.A., Ertl, A.C., Cox, J.F., Zuckerman, J.H., Diedrich, A., Biaggioni, I., Ray, C.A., Smith, M.L., Iwase, S., Saito, M., et al. 2002. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. Journal of Physiology 538(Pt 1):331-340.
565. Norsk, P., and Christensen, N.J. 2009. The paradox of systemic vasodilation and sympathetic nervous stimulation in space. Respiratory Physiology and Neurobiology 169(Suppl. 1):S26-S29.
566. Levine, B.D., Pawelczyk, J.A., Zuckerman, J.H., Zhang, R., Iwasaki, K., and Blomqvist, C.G. 2003. Neural control of the cardiovascular system in space. Pp. 175-185 in The Neurolab Spacelab Mission: Neuroscience Research in Space: Results from the STS-90, Neurolab Spacelab Mission (J.C. Buckey and J.L. Homick, eds.). NASA 1-11. NASA Johnson Space Center, Houston, Tex. January.
567. Pawelczyk, J.A., Zuckerman, J.H., Blomqvist, C.G., and Levine, B.D. 2001. Regulation of muscle sympathetic nerve activity after bed rest deconditioning. American Journal of Physiology. Heart and Circulatory Physiology 280:H2230-H2239.
568. Shoemaker, J.K., Hogeman, C.S., and Sinoway, L.T. 1999. Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology 277(4 Pt 2):R1084-R1090.
569. Fritsch-Yelle, J.M., Whitson, P.A., Bondar, R.L., and Brown, T.E. 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology 81:2134-2141.
570. Cox, J.F., Tahvanainen, K.U.O., Kuusela, T.A., Levine, B.D., Cooke, W.H., Mano, T., Iwase, S., Saito, M., Sugiyama, Y., Ertl, A.C., Biaggioni, I., et al. 2002. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva’s manoeuvre. Journal of Physiology 538(Pt 1):309-320.
571. Eckberg, D.L., Halliwill, J.R., Beightol, L.A., Brown, T.E., Taylor, J.A., and Goble, R. 2010. Human vagal baroreflex mechanisms in space. Journal of Physiology 588(7):1129-1138.
572. Shoemaker, J.K., Hogeman, C.S., and Sinoway, L.T. 1999. Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology 277(4 Pt 2):R1084-R1090.
573. Fritsch-Yelle, J.M., Whitson, P.A., Bondar, R.L., and Brown, T.E. 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology 81:2134-2141.
574. Norsk, P., and Christensen, N.J. 2009. The paradox of systemic vasodilation and sympathetic nervous stimulation in space. Respiratory Physiology and Neurobiology 169(Suppl. 1):S26-S29.
575. Fritsch-Yelle, J.M., Whitson, P.A., Bondar, R.L., and Brown, T.E. 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology 81:2134-2141.
576. Shoemaker, J.K., Hogeman, C.S., Silber, D.H., Gray, K., Herr, M., and Sinoway, L.I. 1998. Head-down-tilt bed rest alters forearm vasodilator and vasoconstrictor responses. Journal of Applied Physiology 84:1756-1762.
577. Meck, J.V., Waters, W.W., Ziegler, M.G., deBlock, H.F., Mills, P.J., Robertson, D., and Huang, P.L. 2004. Mechanisms or postspaceflight orthostatic hypotension: Low alpha 1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. American Journal of Physiology. Heart and Circulatory Physiology 286:H1486-H1495.
578. Fu, Q., Witkowski, S., and Levine, B.D. 2004. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation 110:2931-2937.
579. Kamiya, A., Iwase, S., Michikami, D., Fu, Q., and Mano, T. 2000. Head-down bed rest alters sympathetic and cardiovascular responses to mental stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 279:R440-R447.
580. Hasser, E.M., and Moffitt, J.A. 2001. Regulation of sympathetic nervous system function after cardiovascular deconditioning. Annals of the New York Academy of Sciences 940:454-468.
581. Rowell, L.B. 1993. Human Cardiovascular Control. Oxford University Press, New York, N.Y.
582. Ray, C.A., Vasques, M., Miller, T.A., Wilkerson, M.K., and Delp, M.D. 2001. Effect of short-term microgravity and long-term hindlimb unloading on rat cardiac mass and function. Journal of Applied Physiology 91(3):1207-1213.
583. Zhang, L.F., Yu, Z.B., Ma, J., and Mao, Q.W. 2001. Peripheral effector mechanism hypothesis of postflight cardiovascular dysfunction. Aviation, Space, and Environmental Medicine 72:567-575.
584. Delp, M.D. 1999. Myogenic and vasoconstrictor responsiveness of skeletal muscle arterioles is diminished by hindlimb unloading. Journal of Applied Physiology 86(4):1178-1184.
585. Lin, L.J., Gao, F., Bai, Y.G., Bao, J.X., Huang, X.F., Ma, J., and Zhang, L.F. 2009. Contrasting effects of simulated microgravity with and without daily -Gx gravitation on structure and function of cerebral and mesenteric small arteries in rats. Journal of Applied Physiology 107:1710-1721.
586. Colleran P.N., Behnke, B.J., Wilkerson, M.K., Donato, A.J., and Delp, M.D. 2008. Simulated microgravity alters rat mesenteric artery vasoconstrictor dynamics through an intracellular Ca(2+) release mechanism. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294:R1577-R1585.
587. Purdy, R.E., Duckles, S.P., Krause, D.N., Rubera, K.M., and Sara, D. 1998. Effect of simulated microgravity on vascular contractility. Journal of Applied Physiology 85(4):1307-1315.
588. Summers, S.M., Hayashi, Y., Nguyen, S.V., Nguyen, T.M., and Purdy, R.E. 2009. Hindlimb unweighting induces changes in the p38MAPK contractile pathway of the rat abdominal aorta. Journal of Applied Physiology 107(1):121-127.
589. Tuday, E.C., Meck, J.V., Nyhan, D., Shoukas, A.A., and Berkowitz, D.E. 2007. Microgravity-induced changes in aortic stiffness and their role in orthostatic intolerance. Journal of Applied Physiology 102(3):853-858.
590. Tuday, E.C., Nyhan, D., Shoukas, A.A., and Berkowitz, D.E. 2009. Simulated microgravity-induced aortic remodeling. Journal of Applied Physiology 106(6):2002-2008.
591. Hasser, E.M., and Moffitt, J.A. 2001. Regulation of sympathetic nervous system function after cardiovascular deconditioning. Annals of the New York Academy of Sciences 940:454-468.
592. Hasser, E.M., and Moffitt, J.A. 2001. Regulation of sympathetic nervous system function after cardiovascular deconditioning. Annals of the New York Academy of Sciences 940:454-468.
593. Myerburg, R.J., and Castellanos, A. 2006. Emerging paradigms of the epidemiology and demographics of sudden cardiac arrest. Heart Rhythm 3:235-239.
594. Ridker, P.M., Danielson, E., Fonseca, F.A., Genest, J., Gotto, A.M., Jr., Kastelein, J.J., Koenig, W., Libby, P., Lorenzatti, A.J., MacFadyen, J.G., Nordestgaard, B.G., et al. 2008. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine 359(21):2195-2207.
595. LaMonte, M.J., Fitzgerald, S.J., Levine, B.D., Church, T.S., Kampert, J.B., Nichaman, M.Z., Gibbons, L.W., and Blair, S.N. 2006. Coronary artery calcium, exercise tolerance, and CHD events in asymptomatic men. Atherosclerosis 189(1):157-162.
596. Convertino, V.A. 2002. Mechanisms of microgravity induced orthostatic tolerance: Implications for effective countermeasures. Journal of Gravitational Physiology 9:1-14.
597. Meck, J.V., Reyes, C.J., Perez, S.A., Goldberger, A.L., and Ziegler, M.G. 2001. Marked exacerbation of orthostatic intolerance after long- vs. short-duration spaceflight in veteran astronauts. Psychosomatic Medicine 63(6):865-873.
598. Fritsch-Yelle, J.M., Whitson, P.A., Bondar, R.L., and Brown, T.E. 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology 81:2134-2141.
599. Waters, W.W., Ziegler, M.G., and Meck, J.V. 2002. Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. Journal of Applied Physiology 92:586-594.
600. Meck, J.V., Waters, W.W., Ziegler, M.G., deBlock, H.F., Mills, P.J., Robertson, D., and Huang, P.L. 2004. Mechanisms or postspaceflight orthostatic hypotension: Low alpha 1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. American Journal of Physiology. Heart and Circulatory Physiology 286:H1486-H1495.
601. Moore, A.D., Jr., Lee, S.M., Charles, J.B., Greenisen, M.C., and Schneider, S.M. 2001. Maximal exercise as a countermeasure to orthostatic intolerance after spaceflight. Medicine and Science in Sports and Exercise 33:75-80.
602. Buckey, J.C., Jr., Lane, L.D., Levine, B.D., Watenpaugh, D.E., Wright, S.J., Moore, W.E., Gaffney, F.A., and Blomqvist, C.G. 1996. Orthostatic intolerance after spaceflight. Journal of Applied Physiology 81(1):7-18.
603. Levine, B.D., Pawelczyk, J.A., Ertl, A.C., Cox, J.F., Zuckerman, J.H., Diedrich, A., Biaggioni, I., Ray, C.A., Smith, M.L., Iwase, S., Saito, M., et al. 2002. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. Journal of Physiology 538(Pt 1):331-340.
604. Abboud, F.M., Eckberg, D.L., Johannsen, U.J., and Mark, A.L. 1979. Carotid and cardiopulmonary baroreceptor control of splanchnic and forearm vascular resistance during venous pooling in man. Journal of Physiology 286:173-184.
605. Pawelczyk, J.A., and Levine, B.D. 2002. Heterogeneous responses of human limbs to infused adrenergic agonists: A gravitational effect? Journal of Applied Physiology 92:2105-2113.
606. Meck, J.V., Waters, W.W., Ziegler, M.G., deBlock, H.F., Mills, P.J., Robertson, D., and Huang, P.L. 2004. Mechanisms or postspaceflight orthostatic hypotension: Low alpha 1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. American Journal of Physiology. Heart and Circulatory Physiology 286:H1486-H1495.
607. Fu, Q., Witkowski, S., and Levine, B.D. 2004. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation 110:2931-2937.
608. Buckey, J.C., Jr., Lane, L.D., Levine, B.D., Watenpaugh, D.E., Wright, S.J., Moore, W.E., Gaffney, F.A., and Blomqvist, C.G. 1996. Orthostatic intolerance after spaceflight. Journal of Applied Physiology 81(1):7-18.
609. Fritsch-Yelle, J.M., Whitson, P.A., Bondar, R.L., and Brown, T.E. 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology 81:2134-2141.
610. Waters, W.W., Ziegler, M.G., and Meck, J.V. 2002. Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. Journal of Applied Physiology 92:586-594.
611. Fu, Q., Witkowski, S., and Levine, B.D. 2004. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation 110:2931-2937.
612. Kamiya, A., Michikami, D., Fu, Q., Iwase, S., Hayano, J., Kawada, T., Mano, T., and Sunagawa, K. 2003. Pathophysiology of orthostatic hypotension after bed rest: Paradoxical sympathetic withdrawal. American Journal of Physiology. Heart and Circulatory Physiology 285(3):H1158-H1167.
613. Abboud, F.M., and Eckstein, J.W. 1966. Reflex vasoconstrictor and vasodilator responses in man. Circulation Research 18/19(Suppl. 1):96-103.
614. Abboud, F.M., and Eckstein, J.W. 1966. Active reflex vasodilatation in man. Federation Proceedings 25(6):1611-1617.
615. Mark, A.L., Kioschos, J.M., Abboud, F.M., Heistad, D.D., and Schmid, P.G. 1973. Abnormal vascular responses to exercise in patients with aortic stenosis. Journal of Clinical Investigation 52(5):1138-1146.
616. Mark, A.L., Abboud, F.M., Schmid, P.G., and Heistad, D.D. 1973. Reflex vascular responses to left ventricular outflow obstruction and activation of ventricular baroreceptors in dogs. Journal of Clinical Investigation 52(5):1147-1153.
617. Iwasaki, K., Levine, B.D., Zhang, R., Zuckerman, J.H., Pawelczyk, J.A., Diedrich, A., Ertl, A.C., Cox, J.F., Cooke, W.H., Giller, C.A., Ray, C.A., et al. 2007. Human cerebral autoregulation before, during and after spaceflight. Journal of Physiology 579 (Pt 3):799-810.
618. Zhang, R., and Levine, B.D. 2007. Autonomic ganglionic blockade does not prevent reduction in cerebral blood flow velocity during orthostasis in humans. Stroke 38:1238.
619. Zhang, L.F. 2001. Vascular adaptation to microgravity: What have we learned? Journal of Applied Physiology 91:2415-2430.
620. Purdy, R.E., and Meck, J.V. 2007. Endothelium in space. Pp. 50-526 in Endothelial Biomedicine (W.C. Aird, ed.). Cambridge University Press, New York, N.Y.
621. Waters, W.W., Ziegler, M.G., and Meck, J.V. 2002. Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. Journal of Applied Physiology 92:586-594.
622. Fu, Q., Arbab-Zadeh, A., Perhonen, M.A., Zhang, R., Zuckerman, J.H., and Levine, B.D. 2004. Hemodynamics of orthostatic intolerance: Implications for gender differences. American Journal of Physiology. Heart and Circulatory Physiology 286(1):H449-H457.
623. Fu, Q., Witkowski, S., Okazaki, K., and Levine, B.D. 2005. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 289(1):R109-R116.
624. Fu, Q., Vangundy, T.B., Galbreath, M.M., Shibata, S., Jain, M., Hastings, J.L., Bhella, P.S., and Levine, B.D. 2010. Cardiac origins of the postural orthostatic tachycardia syndrome. Journal of the American College of Cardiology 55(25):2858-2868.
625. Fu, Q., Okazaki, K., Shibata, S., Shook, R.P., VanGunday, T.B., Galbreath, M.M., Reelick, M.F., and Levine, B.D. 2009. Menstrual cycle effects on sympathetic neural responses to upright tilt. Journal of Physiology 587(Pt 9):2019-2031.
626. Levine, B.D., Lande, L.D., Watenpaugh, D.E., Gaffney, F.A., Buckey, J.C., and Blomqvist, C.Q. 1996. Maximum exercise performance after adaptation to microgravity. Journal of Applied Physiology 81:686-694.
627. Kozlovskaya, I.B., and Grigoriev, A.I. 2004. Russian system of countermeasures on board of the International Space Station (ISS): The first results. Acta Astronautica 55:2233-2237.
628. Macias, B.R., Groppo, E.R., Eastlack, R.K., Watenpaugh, D.E., Lee, S.M., Schneider, S.M., Boda, W.L., Smith, S.M., Cutuk, A., Pedowitz, R.A., Meyer, R.S., and Hargens, A.R. 2005. Space exercise and Earth benefits. Current Pharmaceutical Biotechnology 6:305-317.
629. Convertino, V.A. 1996. Exercise and adaptation to microgravity environments. Pp. 815-843 in Handbook of Physiology, Environmental Physiology. American Physiological Society, Bethesda, Md.
630. Fortney, S.M., Schneider, V.S., and Greenleaf, J.E. 1996. The physiology of bed rest. Pp. 889-939 in Handbook of Physiology, Environmental Physiology. American Physiological Society, Bethesda, Md.
631. Michel, E.L., Rummel, J.A., Sawin, C.F., Buderer, M.C., and Lem, J.D. 1977. Results of Skylab medical experiment 171: Metabolic activity. Pp. 372-387 in Biomedical Results from Skylab (R.S. Johnson and L.F. Dietlein, eds.). NASA, Washington, D.C.
632. Moore, A.D., S.M. Lee, M.S. Laughlin, and R.D. Hagan. 2003. Aerobic deconditioning and recovery following long duration flight onboard the International Space Station. Medicine and Science in Sports and Exercise 35:263.
633. Berg, H.E., Dudley, G.A., Hather, B., and Tesch, P.A. 1993. Work capacity and metabolic and morphologic characteristics of the human quadriceps muscle in response to unloading. Clinical Physiology 13:337-347.
634. Hikida, R.S., Gollnick, P.D., Dudley, G.A., Convertino, V.A., and Buchanan, P. 1989. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviation, Space, and Environmental Medicine 60:664-670.
635. Il’ina-Kakueva, E.I., and Portugalov, V.V. 1979. [Effect of artificial gravitation on the skeletal musculature of rats during space flight.] [Russian] Arkhiv Anat Gistologii Embriologii 76(3):22-27.
636. Levine, B.D., Lande, L.D., Watenpaugh, D.E., Gaffney, F.A., Buckey, J.C., and Blomqvist, C.Q. 1996. Maximum exercise performance after adaptation to microgravity. Journal of Applied Physiology 81:686-694.
637. Michel, E.L., Rummel, J.A., Sawin, C.F., Buderer, M.C., and Lem, J.D. 1977. Results of Skylab medical experiment 171: Metabolic activity. Pp. 372-387 in Biomedical Results from Skylab (R.S. Johnson and L.F. Dietlein, eds.). NASA, Washington, D.C.
638. Levine, B.D., Lande, L.D., Watenpaugh, D.E., Gaffney, F.A., Buckey, J.C., and Blomqvist, C.Q. 1996. Maximum exercise performance after adaptation to microgravity. Journal of Applied Physiology 81:686-694.
639. Moore, A.D., Lee, S.M., Laughlin, M.S., and Hagan, R.D. 2003. Aerobic deconditioning and recovery following long duration flight onboard the International Space Station. Medicine and Science in Sports and Exercise 35:263.
640. Bungo, M.W., Charles, J.B., and Johnson, P.C., Jr. 1985. Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviation, Space, and Environmental Medicine 56(10):985-990.
641. Waters, W.W., Platts, S.H., Mitchell, B.M., Whitson, P.A., and Meck, J.V. 2005. Plasma volume restoration with salt tablets and water after bed rest prevents orthostatic hypotension and changes in supine hemodynamic and endocrine variables. American Journal of Physiology. Heart and Circulatory Physiology 288:H839-H847.
642. Shibata, S., Perhonen, M., and Levine, B.D. 2010. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. Journal of Applied Physiology 108(5):1177-1186.
643. Hastings, J., Pacini, E., Krainski, F., Shibata, S., Jain, M., Snell, P.G., Fu, Q., Palmer, M.D., Creson, D., and Levine, B.D. 2008. The multi-system effect of exercise training during prolonged bed rest deconditioning: An integrated approach to countermeasure development for the heart, lungs, muscle and bones. Circulation 118:S682. Abstract.
644. Schmid, P.G., Eckstein, J.W., and Abboud, F.M. 1966. Effect of 9-α-fluorohydrocortisone on forearm vascular responses to norepinephrine. Circulation 34:620-626.
645. Schmid, P.G., Eckstein, J.W., and Abboud, F.M. 1967. Comparison of effects of deoxycorticosterone and dexamethasone on cardiovascular responses to norepinephrine. Journal of Clinical Investigation 46(4):590-598.
646. Convertino, V.A. 1996. Exercise and adaptation to microgravity environments. Pp. 815-843 in Handbook of Physiology, Environmental Physiology. American Physiological Society, Bethesda, Md.
647. Keller, D.M., Low, D.A., Wingo, J.E., Brothers, R.M., Hastings, J., Davis, S.L., and Crandall, C.G. 2009. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. Journal of Physiology 587(Pt 5):1131-1139.
648. Heistad, D.D., Abboud, F.M., Mark, A.L., and Schmid, P.G. 1973. Interaction of thermal and baroreceptor reflexes in man. Journal of Applied Physiology 35(5):581-586.
649. Keller, D.M., Low, D.A., Wingo, J.E., Brothers, R.M., Hastings, J., Davis, S.L., and Crandall, C.G. 2009. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. Journal of Physiology 587(Pt 5):1131-1139.
650. Gaffney, F.A., Thal, E.R., Taylor, W.F., Bastian, B.C., Weigelt, J.A., Atkins, J.M., and Blomqvist, C.G. 1981. Hemodynamic effects of Medical Anti-Shock Trousers (MAST garment). Journal of Trauma 21(11):931-937.
651. Durand, S., Cui, J.J., Williams, K.D., and Crandall, C.G. 2004. Skin surface cooling improves orthostatic tolerance in normothermic individuals. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 286:R199-R205.
652. Cui, J., Durand, S., and Crandall, C.G. 2007. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. Journal of Applied Physiology 103:1284-1289.
653. Ramsdell, C.D., Mullen, T.J., Sundby, G.H., Rostoft, S., Sheynberg, N., Aljuri, N., Maa, M., Mukkamala, R., Sherman, D., Toska, K., Yelle, J., et al. 2001. Midodrine prevents orthostatic intolerance associated with simulated spaceflight. Applied Physiology 90:2245-2248.
654. Platts, S.H., Ziegler, M.G., Waters, W.W., Mitchell, B.M., and Meck, J.V. 2004. Midodrine prescribed to improve recurrent post-spaceflight orthostatic hypotension. Aviation, Space, and Environmental Medicine 75:554-556.
655. Jarvis, S.S., and Pawelczyk, J.A. 2010. The location of the human volume indifferent point predicts orthostatic tolerance. European Journal of Applied Physiology 109(2):331-341.
656. Greenleaf, J.E., Vernikos, J., Wade, C.E., and Barnes, P.R. 1992. Effect of leg exercise training on vascular volumes during 30 days of 6 degrees head-down bed rest. Journal of Applied Physiology 72:1887-1894.
657. Hargens, A.R., and Richardson, S. 2009. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respiratory, Physiology, and Neurobiology 169(Suppl 1):S30-S33.
658. Dorfman, T.A., Levine, B.D., Tillery, T., Peshock, R.M., Hastings, J.L., Schneider, S.M., Macias, B.R., Biolo, G., and Hargens, A.R. 2007. Cardiac atrophy in women following bed rest. Journal of Applied Physiology 103:8-16.
659. Watenpaugh, D.E., and Hargens, A.R. 1996. The cardiovascular system in microgravity. Pp. 631-674 in Handbook of Physiology, Environmental Physiology. Oxford University Press, New York, N.Y.
660. Watenpaugh, D.E., O’Leary, D.D., Schneider, S.M., Lee, S.M., Macias, B.R., Tanaka, K., Hughson, R.L., and Hargens, A.R. 2007. Lower body negative pressure exercise plus brief postexercise lower body negative pressure improve post-bed rest orthostatic tolerance. Journal of Applied Physiology 103(6):1964-1972.
661. Shibata, S., Perhonen, M., and Levine, B.D. 2010. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. Journal of Applied Physiology 108(5):1177-1186.
662. Hastings, J., Pacini, E., Krainski, F., Shibata, S., Jain, M., Snell, P.G., Fu, Q., Palmer, M.D., Creson, D., and Levine, B.D. 2008. The multi-system effect of exercise training during prolonged bed rest deconditioning: An integrated approach to countermeasure development for the heart, lungs, muscle and bones. Circulation 118:S682. Abstract.
663. Buckey, J.C., Jr., Lane, L.D., Levine, B.D., Watenpaugh, D.E., Wright, S.J., Moore, W.E., Gaffney, F.A., and Blomqvist, C.G. 1996. Orthostatic intolerance after spaceflight. Journal of Applied Physiology 81(1):7-18.
664. Abboud, F.M. 1993. Neurocardiogenic syncope (Editorial Comment). New England Journal of Medicine 328:1117-1120.
665. Eichna, L.W., Horvath, S.M., and Bean, W.B. 1947. Post-exertional orthostatic hypotension. American Journal of the Medical Sciences 213:641-654.
666. Meck, J.V., Reyes, C.J., Perez, S.A., Goldberger, A.L., and Ziegler, M.G. 2001. Marked exacerbation of orthostatic intolerance after long- vs. short-duration spaceflight in veteran astronauts. Psychosomatic Medicine 63(6):865-873.
667. Shibata, S., Perhonen, M., and Levine, B.D. 2010. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. Journal of Applied Physiology 108(5):1177-1186.
668. Hastings, J., Pacini, E., Krainski, F., Shibata, S., Jain, M., Snell, P.G., Fu, Q., Palmer, M.D., Creson, D., and Levine, B.D. 2008. The multi-system effect of exercise training during prolonged bed rest deconditioning: An integrated approach to countermeasure development for the heart, lungs, muscle and bones. Circulation 118:S682. Abstract.
669. Ramsdell, C.D., Mullen, T.J., Sundby, G.H., Rostoft, S., Sheynberg, N., Aljuri, N., Maa, M., Mukkamala, R., Sherman, D., Toska, K., Yelle, J., Bloomfield, D., Williams, G.H., and Cohen, R.J. 2001. Midodrine prevents orthostatic intolerance associated with simulated spaceflight. Journal of Applied Physiology 90(6):2245-2248.
670. Platts, S.H., Ziegler, M.G., Waters, W.W., Mitchell, B.M., and Meck, J.V. 2004. Midodrine prescribed to improve recurrent post-spaceflight orthostatic hypotension. Aviation, Space, and Environmental Medicine 75:554-556.
671. Lee, P.H., and Malik, R. 2005. Cardiovascular effects of radiation therapy: Practical approach to radiation therapy-induced heart disease. Cardiology in Review 13:80-86.
672. Andersen, R., Wethal, T., Gunther, A., Fossa, A., Edvardsen, T., Fossa, S.D., and Kjekshus, J. 2010. Relation of coronary artery calcium score to premature coronary artery disease in survivors >15 years of Hodgkin’s lymphoma. American Journal of Cardiolology 105:149-152.
673. Meck, J.V., Dreyer, S.A., and Warren, E. 2009. Long-duration head-down bed rest: Project overview, vital signs, and fluid balance. Aviation, Space, and Environmental Medicine 80(Suppl):A1-A8.
674. Pancheva, M.V., Panchev, V.S., and Suvandjieva, A.V. 2006. Lower body negative pressure vs. lower body positive pressure to prevent cardiac atrophy after bed rest and spaceflight. What caused the controversy? Journal of Applied Physiology 100:1090-1093.
675. Prisk, G.K., Paiva, M., and West, J.B., eds. 2001. Gravity and the Lung: Lessons from Microgravity. Dekker, New York, N.Y.
676. West, J.B., Elliott, A.R., Guy, H.J.B., and Prisk, G.K. 1997. Pulmonary function in space. Journal of the American Medical Association 277:1957-1961.
677. Prisk, G.K., Fine, J.M., Cooper, T.K., and West, J.B. 2008. Lung function is unchanged in the 1 G environment following 6-months exposure to microgravity. European Journal of Applied Physiology 103:617-623.
678. Darquenne, C., and Prisk, G.K. 2008. Deposition of inhaled particles in the human lung is more peripheral in lunar than in normal gravity. European Journal of Applied Physiology 103:687-695.
679. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C., pp. 132-155.
680. Ohlsson, C., Bengtsson, B.A., Isaksson, O.G., Andreassen, T.T., and Slootweg, M.C. 1998. Growth hormone and bone. Endocrine Reviews 19:55-79.
681. Welle, S., Thornton, S., Stalt, M., and McHenry, B. 1996. Growth hormone increases muscle mass but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years. Journal of Clinical Endocrinology and Metabolism 81:3239-3243.
682. Gooselink, K.L., Grindeland, R.E., Roy, R.R., Zhong, H., Bigbee, A.J., Grossman, E.J., and Edgerton, V.R. 1998. Skeletal muscle regulatin of bioassayable growth hormone in the rat pituitary. Journal of Applied Physiology 84(4):1425-1430.
683. Popil, V., and Baumann, G. 2004. Laboratory measurement of growth hormone. Clinica Chimica Acta 350(1-2):1-16.
684. Grigoriev, A.I., and Larina, I.M. 1999. Translated 2009. Growth hormone and other regulators of muscle metabolism in blood of humans exposed to prolonged space missions and hypokinesia. Fiziol. Cheloveka. 25:89-96.
685. Grigoriev, A.I., and Larina, I.M. 1999. Translated 2009. Growth hormone and other regulators of muscle metabolism in blood of humans exposed to prolonged space missions and hypokinesia. Fiziol. Cheloveka. 25:89-96.
686. McCall, G.E., Goulet, C., Roy, R.R., Grindeland, R.E., Boorman, G.I., Bigbee, A.J., Hodgson, J.A., Greenisen, M.C., and Edgerton, V.R. 1999. Spaceflight suppresses exercise-induced release of bioassayable growth hormone. Journal of Applied Physiology 87:1207-1212.
687. Macho, L., Koska, J., Ksinantova, L., Vigas, M., Noskov, V.B., Grigoriev, A.I., and Kvetnansky, R. 2001. Plasma hormone levels in human subjects during stress loads in microgravity and at readaptation to Earth’s gravity. Journal of Gravitational Physiology 8:P131-P132.
688. Hymer, W.C., Grindeland, R.E., Kransov, I., Victorov, K., Motter, K., Mukherjee, P., Shellenberger, K., and Vasques, M. 1992. Effect of space flight on rat pituitary cell function. Journal of Applied Physiology 73(2 Suppl.):151S-157S.
689. Bigbee, A.J., Grindeland, R.E., Roy, R.R., Zhong, H., Gosselink, K.L., Arnaud, S., and Edgerton, V.R. 2006. Basal and evoked levels of bioassayable growth hormone are altered by hindlimb unloading. Journal of Applied Physiology 100:1037-1042.
690. Sawachenko, P.E., Arias, C., Kransnov, I., Grindeland, R.E. and Vale, W. 1992. Effects of spaceflight on hypothalamic peptide systems controlling growth hormone dynamics. Journal of Applied Physiology 73(2 Suppl.):158S-165S.
691. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
692. Leach, C.L., and Rambaut, P.C. 1977. Biochemical responses of the Skylab crewman: An overview. Pp. 204-216 in Biomedical Results from Skylab (R.S. Johnson and L.E. Dietlein, eds.). NASA SP-377. NASA, Washington, D.C.
693. Kuipers, A. 1996. First results from experiments performed with the ESA Anthrorack during the D-2 Spacelab mission. Acta Astronautica 38:865-875.
694. Leach, C.S., Johnson, P.C. and Driscoll, T.B. 1977. Prolong weightlessness effect on post flight plasma hormones. Aviation, Space, and Environmental Medicine 48(7):595-597.
695. Grigoriev, A.I., and Larina, I.M. 1999. Translated 2009. Growth hormone and other regulators of muscle metabolism in blood of humans exposed to prolonged space missions and hypokinesia. Fiziol. Cheloveka. 25:89-96.
696. Macho, L., Kvetnansky, R., Fickova, M., Kolena, J., Knopp, J., Tigranian, R.A., Popova, I.A., and Grogoriev, A.I. 2001. Endocrine responses to space flight. Journal of Gravitational Physiology 8:P117-P120.
697. Macho, L., Kvetnansky, R., Fickova, M., Kolena, J., Knopp, J., Tigranian, R.A., Popova, I.A., and Grogoriev, A.I. 2001. Endocrine responses to space flight. Journal of Gravitational Physiology 8:P117-P120.
698. Wimalawansa, S.M., and Wimalawansa, S.J. 1999. Simulated weightlessness-induced attenuation of testosterone production may be responsible for bone loss.1999. Endocrine 10:253-260.
699. Adams, G.R., Haddad, F., McCue, S.A., Bodell, P.W., Zeng, M., Qin, A.X., and Baldwin, K.M. 2000. Effects of spaceflight and thyroid deficiency on rat hindlimb development II: Expression of MHC isoforms. Journal of Applied Physiology 88:904-916.
700. Mazziotti, G., Angeli, A., Bilezikian, J.P., Canalis, E. and Guistina, A. 2006. Glucocorticoid-induced osteoporosis: An update. Trends in Endocrinology and Metabolism 17:144-149.
701. Kelley, F.J., and Goldspink, D.F. 1982. The differing response of four muscle types to desamethasone treatment in the rat. Biochemical Journal 208:147-151.
702. Blanc, S., Normand, S., Pachiaudi, C., Duvareille, C., and Gharib, C. 2000. Leptin responses to physical inactivity induced by simulated weightlessness. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 279:R891-R898.
703. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
704. Grigoriev, A.I., Huntoon, C., and Natochin, Yu.V. 1995. On the correlation between individual biochemical parameters of human blood serum following space flight and their basal values. Acta Astronautica 36:639-648.
705. Leach, C.S., Atchuler, S.I., and Clintron-Trevino, N.M. 1983. The endocrine and metabolic responses to space flight. Medicine and Science in Sports and Exercise 15:432-440.
706. Grigoriev, A.I., Bugrov, A.A., Bogomolov, V.V., Egorad, A.D., Polyakov, V.V., Tarasov, I.K., and Shulzhenko, E.B. 1993. Main medical results of extended flights on Space Station Mir in 1986-1990. Acta Astronautica 29:581-585.
707. Fitts, R.H., Riley, D.R., and Widrik, J.J. 2001. Functional and structural adaptations of skeletal muscle to microgravity. Journal of Experimental Biology 204:3201-3208.
708. Blanc, S., Normand, S., Rotz, P., Pachiaudi C., Vico, L., Gharib, C., and Gauquelin-Koch, G. 1998. Energy and water metabolism, body composition, and hormonal changes induced by 42 day of enforced inactivity and simulated weightlessness. Journal of Clinical Endocrinology and Metabolism 83:4289-4297.
709. Rittweger, J.H., Frost, M., Schiessl, H., Ohshima, H., et al. 2005. Muscle atrophy and bone loss after 90 days’ bed rest and effects of flywheel resistive exercise and pamidronate: Results from the LTBR study. Bone 36:1019-1029.
710. Blanc, S., Normand, S., Pachiaudi, C., Duvareille, C. and Gharib, C. 2000. Leptin responses to physical inactivity induced by simulated weightlessness. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 279:R891-R890.
711. Blanc, S., Normand, S., Rotz, P., Pachiaudi C., et. al. 1998. Energy and water metabolism, body composition, and hormonal changes induced by 42 day of enforced inactivity and simulated weightlessness. Journal of Clinical Endocrinology and Metabolism 83:4289-4297.
712. Macho, L., Kvetnansky, R., Fickova, M., Kolena, J., et al. 2001. Endocrine responses to space flight. Journal of Gravitational Physiology 8:P117-P120.
713. Wimalawansa, S.M., and Wimalawansa, S.J. 1999. Simulated weightlessness-induced attenuation of testosterone production may be responsible for bone loss.1999. Endocrine 10:253-260.
714. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
715. Blanc, S., Normand, S., Pachiaudi, C., Fortrat, J.-O., et al. 2000. Fuel homeostasis during physical inactivity induced by bed rest. Journal of Clinical Endocrinology and Metabolism 85:2223-2233.
716. Leach, C.S., Atchuler, S.I., and Clintron-Trevino, N.M. 1983. The endocrine and metabolic responses to space flight. Medicine and Science in Sports and Exercise 15:432-440.
717. Stein, T.P., Schluter, M.D., and Boden, B. 1994. Development of insulin resistance by astronauts during spaceflight. Aviation, Space, and Environmental Medicine 65:1091-1096.
718. Kuipers, A. First results from experiments performed with the ESA Anthrorack during the D-2 Spacelab mission. 1996. Acta Astronautica 38:865-875.
719. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C., p. 142.
720. Kuipers, A. 1996. First results from experiments performed with the ESA Anthrorack during the D-2 Spacelab mission. Acta Astronautica 38:865-875.
721. Leach, C.L., and Rambaut, P.C. 1977. Biochemical responses of the Skylab crewman: An overview. Pp. 204-216 in Biomedical Results from Skylab (R.S. Johnson and L.E. Dietlein, eds.). NASA SP-377. NASA, Washington, D.C.
722. Grigoriev, A.I., Bugrov, A.A., Bogomolov, V.V., Egorad, A.D. et al. 1993. Main medical result of extended flights on Space Station Mir in 1986-1990. Acta Astronautica 29:581-585.
723. Markin, A., Balashov, O., Polyakov, V., and Tigner, T. 1998. The dynamics of blood biochemical parameters in cosmonauts during long-term space flights. Acta Astronautica 42:247-253.
724. Mondon, C.E., Rodnick, K.J., Dolkas, C.B., Azhar, S., and Reaven, G.M. 1992. Alterations in glucose and protein metabolism in animals subjected to simulated microgravity. Advances in Space Research 12:169-177.
725. O’Keefe, M.P., Perez, F.R., Kinnick, T.R., Tischler, M.E., and Hendriksen, E.J. 2004. Development of whole-body and skeletal muscle insulin resistance after one day of hindlimb suspension. Metabolism 53:1215-1522.
726. Tobin, B.W., Tobin, W.W., Uchakin, P.N., and Leeper-Woodford, S.K. 2002. Insulin secretion and sensitivity in space flight: Diabetogenic effects. Nutrition 18:842-848.
727. Tobin, B.W., Leeper-Woodfford, S.K., Hashemi, B.B., Smith, S.M., et al. 2001. Altered TNF-α, glucose, insulin, and amino acids in islets of Langerhans cultered in a microgravity model system. American Journal of Physiology. Endocrinology and Metabolism 280:E92-E102.
728. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C., p. 137.
729. Strollo F., Riondino, G., Harris, B., Strollo, G., et al. 1998. The effect of microgravity on testicular androgen secretion. Aviation, Space, and Environmental Medicine 69:133-136.
730. Strollo, F., Strollo, G., More, M., Ferretti, C., et al. 1994. Changes in human adrenal and gonadal function onboard Spacelab. Journal of Gravitational Physiology 4:P103-P104.
731. Nichiporuk, I.A., Evdokimov, V.V., Erasova, V.I., Smirov, O.A., et. al. 1998. Male reproductive system in conditions of bed-rest in a head down tilt. Journal of Gravitational Physiology 5:P101-P102.
732. Merrill, A.H., Jr., Wang, E., Mullins, R.E., Grindeland, R.E., et al. 1992. Analysis of plasma for metabolic and hormonal changes in rats flown aboard COSMOS 2044. Journal of Applied Physiology 73(Suppl.):132S-135S.
733. Macho, L., Kvetnansky, R., Fickova, M., Kolena, J., et al. 2001. Endocrine responses to space flight. Journal of Gravitational Physiology 8:P117-P120.
734. Merrill, A.H., Jr., Wang, E., Mullins, R.E., Grindeland, R.E., et al. 1992. Analysis of plasma for metabolic and hormonal changes in rats flown aboard COSMOS 2044. Journal of Applied Physiology 73(Suppl.):132S-135S.
735. Deaver, D.R., Amann, R.P., Hammerstedt, H., Ball, K., et al. 1992. Effects of caudal elevation on testicular function in rats, separation of effects on spermatogenesis and steroidogenesis. Journal of Andrology 13:224-231.
736. Sharma, C.S., Sarka, S., Periyakaruppan, A., Ravichandran, P., et al. 2008. Simulated microgravity activates apoptosis and NF-kappaB in mice testis. Molecular and Cellular Biochemistry. 313:71-78.
737. Oritz, R.M., Wade, C.E., and Morey-Holton, E. 2000. Urinary excretion of LH and testosterone from male rats during exposure to increased gravity: Post-spaceflight and centrifugation. Proceedings of the Society for Experimental Biology and Medicine 225:98-102.
738. Fedotova, N.L. 1967. [Spermatogenesis of the dogs Ugolyok and Veterok after their flight on board the Satellite Kosmos 110]. [Russian]. Kosmicheskaya Biol. Med. 1:28.
739. Amann, R.P., Deaver, D.R, Zirkin, B.R., Grills, S., et al.1992. Effects of microgravity or simulated launch on testicular function in rats. Journal of Applied Physiology 73(2 Suppl.):S174-S185.
740. Serova, L.V., Denisova, L.A., and Baikova, C. V. 1989. The effect of microgravity on the reproductive function of male rats. The Physiologist 32(Suppl. 1):S29-S30.
741. Tash, J.S., Johnson, D.C., and Enders, G.C. 2001.Long term (6-wk) hindlimb suspension inhibits spermatogenesis in adult male rats. Journal of Applied Physiology 92:1191-1198.
742. Motabagani, M.A.H. 2007. Morphological and morphometric study on the effect of simulated microgravity on rat testis. Chinese Journal of Physiology 50:199-209.
743. Strollo, F., Masini, M.A., Pastorino, M., Ricci, F., et al. 2004. Microgravity induced alterations in cultured testicular cells. Journal of Gravitational Physiology 11:P187.
744. Strollo, F., Masini, M.A., Pastorino, M., Ricci, F., et al. 2004. Microgravity induced alterations in cultured testicular cells. Journal of Gravitational Physiology 11:P187.
745. Uva, B.M., Strollo, F., Ricci, F., Pastorino, M., et al. 2005. Morpho-functional alterations in testicular and nervous cells submitted to modeled microgravity. Journal of Endocrinological Investigation 28(11 Suppl. Proceedings):84-91.
746. Borer, K. 2003. Exercise Endocrinology. Human Kinetics Champaign, Ill., pp. 1-259.
747. Trappe, S., Costill, D., Gallagher, P., Creer, A., Peters, J.R., Evans, H., Riley, D.A., and Fitts, R.H. 2009. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. Journal of Applied Physiology 106(4):1159-1168.
748. Fitts, R.H., Riley, D.R., and Widrik, J.J. 2001. Functional and structural adaptations of skeletal muscle to microgravity. Journal of Experimental Biolology 204:3201-3208.
749. Falduto, M.T., Czerwinski, S.M., and Hickson, R.C. 1990. Glucocorticoid-induced muscle atrophy prevention by exercise in fast-twitch fibers. Journal of Applied Physiology 69:1058-1062.
750. Wade, C.E., Stanford, K.I., Stein, T.P., and Greenleaf, J.G. 2005. Intensive exercise training suppresses testosterone during bed rest. Journal of Applied Physiology 99:59-63.
751. Borer, K.T. 2003. Exercise Endocrinology. Human Kinetics, Champaign, Ill., pp. 106-108.
752. Tou, J., Ronca, A., Grindeland, R., and Wade, C. 2002. Models to study gravitational biology of mammalian reproduction. Biology of Reproduction 67:1681-1687.
753. Caiozzo, V.J., Haddad, F., Lee, S., Baker, M., et al. 2009. Artificial gravity as a countermeasure to microgravity: A pilot study examining the effects on knee extensor and plantar flexor muscle groups. Journal of Applied Physiology 107:39-46.
754. Tash, J.S., Johnson, D.C., and Enders, G.C. 2001. Long term (6-wk) hindlimb suspension inhibits spermatogenesis in adult male rats. Journal of Applied Physiology 92:1191-1198.
755. Burden, H.W., Poole, M.C., Zary, J., Jeansonne, B., et al. 1998. The effects of space flight during gestation on rat uterine, Journal of Gravitational Physiology 5:23-29.
756. Hymer, W.C., Grindeland, R.E., Kransov, I., Victorov, K., et al. 1992. Effect of space flight on rat pituitary function. Journal of Applied Physiology 73(Suppl.):151S-157S.
757. Bigbee, A.J., Grindeland, R.E., Roy, R.R., Zhong, H., et al. 2006. Basal and evoked levels of bioassayable growth hormone are altered by hindlimb unloading. Journal of Applied Physiology 100:1037-1042.
758. Sawachenko, P.E., Arias, C., Kransnov, I., Grindeland, R.E., and Vale, W. 1992. Effects of spaceflight on hypothalamic peptide systems controlling growth hormone dynamics. Journal of Applied Physiology 73(Suppl.):158S-165S.
759. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
760. Meck, J.V., Dreyer, S.A., and Warren, E. 2009. Long-duration head-down bed rest: Project overview, vital signs, and fluid balance. Aviation, Space, and Environmental Medicine 80(Suppl.):A1-A8.
761. Morey-Holton, E., and Globus, K. 2002. Hindlimb unloading rodent model. Technical aspects. Journal of Applied Physiology 92:1367-1377.
762. Hymer, W.C., Grindeland, R.E., Kransov, I., Victorov, K., et al. 1992. Effect of space flight on rat pituitary function. Journal of Applied Physiology 73(Suppl.):151S-157S.
763. Hymer, W.C., Grindeland, R.E., Salada, T., Nye, P., et al. 1996. Experimental modification of rat pituitary growth hormone cell function after space flight. Journal of Applied Physiology 80:955-970.
764. Grigoriev, A.I., Huntoon, C., and Larina, I.M. 1999. Translated 2009. Growth hormone and other regulators of metabolism in blood of humans exposed to prolonged space missions and hypokinesia. Fizol. Cheloveka. 25:89-96.
765. Gooselink, K.L., Grindeland, R.E., Roy, R.R., Zhong, H., Bigbee, A.J., Grossman, E.J., and Edgerton, V.R. 1998. Skeletal muscle regulatin of bioassayable growth hormone in the rat pituitary. Journal of Applied Physiology 84(4):1425-1430.
766. Adams, G.R., Haddad, F., McCue, S.A., Bodell, P.W., Zeng, M., Qin, A.X., and Baldwin, K.M. 2000. Effects of spaceflight and thyroid deficiency on rat hindlimb development II: Expression of MHC isoforms. Journal of Applied Physiology 88:904-916.
767. Kelley, F.J., and Goldspink, D.F. 1982. The differing response of four muscle types to dexamethasone treatment in the rat. Biochemical Journal 208:147-151.
768. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
769. Tobin, B.W., Leeper-Woodfford, S.K., Hashemi, B.B., Smith, S.M., et al. 2001. Altered TNF-α, glucose, insulin, and amino acids in islets of Langerhans cultered in a microgravity model system. American Journal of Physiology. Endocrinology and Metabolism 280:E92-E102.
770. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C., pp. 155-168.
771. Taylor, G.R., Neale, L.S., and Dardano, J.R. 1986. Immunological analyses of U.S. space shuttle crewmembers. Aviation, Space, and Environmental Medicine 57(3):213-217.
772. Meehan, R.T., Neale, L.S., Kraus, E.T., et al. 1992. Alteration in human mononuclear leucocytes following space flight. Immunology 76:491.
773. Stowe, R.P., Sams, C.F., Mehta, S.K., et al. 1999. Leukocyte subsets and neutrophil function after short-term spaceflight. Journal of Leukocyte Biology 65:179.
774. Crucian, B.E., Stowe, R.P., Pierson, D.L., and Sams, C.F. 2008. Immune system dysregulation following short- vs long-duration spaceflight. Aviation, Space, and Environmental Medicine 79(9):835-843.
775. Mehta, S.K., Kaur, I., Grimm, E.A., Smid, C., Feeback, D.L., and Pierson, D.L. 2001. Decreased non-MHC-restricted (CD56+) killer cell cytotoxicity after spaceflight. Journal of Applied Physiology 91:1814-1818.
776. Meshkov, D., and Rykova, M. 1995. The natural cytotoxicity in cosmonauts on board space stations. Acta Astronautica 36(8-12):719-726.
777. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
778. Kaur, I., Simons, E.R., Castro, V.A., Ott, C.M., and Pierson, D.L. 2004. Changes in neutrophil functions in astronauts. Brain, Behavior, and Immunology 18:443-450.
779. Kaur, I., Simons, E.R., Castro, V.A., Ott, C.M., and Pierson, D.L. 2005. Changes in monocyte functions of astronauts. Brain, Behavior, and Immunology 19:547-554.
780. Voss, E.W. 1984. Prolonged weightlessness and humoral immunity. Science 225:214-215.
781. Konstantinova, I.V., Sonnenfeld, G., Lesnyak, A.T., Shaffar, L., Mandel, A., Rykova, M.P., Antropova, E.N., and Ferrua, B. 1991. Cellular immunity and lymphokine production during spaceflights. Physiologist 34:S52-S56.
782. Manié, S., Konstantinova, I., Breittmayer, J.P., Ferrua, B., and Schaffar, L. 1991. Effects of a long duration spaceflight on human T lymphocyte and monocyte activity. Aviation, Space, and Environmental Medicine 62:1153-1158.
783. Crucian, B.E., Cubbage, M.L., and Sams, C.F. 2000. Altered cytokine production by specific human peripheral blood cell subsets immediately following space flight. Journal of Interferon and Cytokine Research 20:547.
784. Gardner, E.M., and Murasko D.M. 2002. Age-related changes in type 1 and type 2 cytokine production in humans. Biogerontology 3:271-290.
785. Taylor, G.R., and Janney, R.P. 1992. In vivo testing confirms a blunting of the human cell-mediated immune mechanism during space flight. Journal of Leukocyte Biology 51:129-132.
786. Gmünder, F.K., Konstantinove, I., Cogoli, A., et al. 1994. Cellular immunity in cosmonauts during long duration spaceflight on board the orbital MIR station. Aviation, Space, and Environmental Medicine 65:419.
787. Mehta, S.K., Cohrs, R.J., Forghani, B., Zerbe, G., Gilden, D.H., and Pierson, D.L. 2004. Stress-induced subclinical reactivation of Varicella Zoster Virus in astronauts. Journal of Medical Virology 72:174-179.
788. Pierson, D.L., Stowe, R.P., Phillips, T.M., Lugg, D.J., and Mehta, S.K. 2005. Epstein-Barr virus shedding by astronauts during space flight. Brain, Behavior, and Immunology 19:235-242.
789. Mehta, S.K., Stowe, R.P., Leiveson, A.H., Tyring, S.K., and Pierson, D.L. 2000. Reactivation and shedding of cytomegalovirus in astronauts. Journal of Infection 182:1761-1764.
790. Stowe, R.P., Mehta, S.K., Ferrando, A.A., Feeback, D.L., and Pierson, D.L. 2001. Immune responses and latent herpevirus reactivation in spaceflight. Aviation, Space, and Environmental Medicine 72:884.
791. Mehta, S.K., Cohrs, R.J., Forghani, B., Zerbe, G., Gilden, D.H., and Pierson, D.L. 2004. Stress-induced subclinical reactivation of Varicella Zoster Virus in astronauts. Journal of Medical Virology 72:174-179.
792. Pierson, D.L., Stowe, R.P., Phillips, T.M., Lugg, D.J., and Mehta, S.K. 2005. Epstein-Barr virus shedding by astronauts during space flight. Brain, Behavior, and Immunology 19:235-242.
793. NASA. 2009. Risk of Crew Adverse Health Event Due to Altered Immune Response. HRP-47060. Human Health Countermeasures Element Evidence Book. Human Research Program, NASA Johnson Space Center, Houston, Tex., June, p. 13-8.
794. Sonnenfeld, G. 2005. Use of animal models for space flight physiology studies, with special focus on the immune system. Gravitational and Space Biology Bulletin 18(2):31-35.
795. Nash, P., and Mastro, A. 1992. Variable lymphocyte responses in rats after space flight. Experimental Cell Research 202:125-131.
796. Grove, D., Pishak, S., and Mastro, A. 1995. The effect of a 10-day space flight on the function, phenotype, and adhesion molecule expression of splenocytes and lymph node lymphocytes. Experimental Cell Research 219:102-109.
797. Baqai, F.P., Gridley, D.S., Slater, J.M., Luo-Owen, X., Stodieck, L.S., Ferguson, V., Chapes, S.K., and Pecaut, M.J. 2009. Effects of spaceflight on innate immune function and antioxidant gene expression. Journal of Applied Physiology 106:1935-1942.
798. Gridley, D.S., Slater, J.M., Luo-Owen, X., Rizvi, A., Chapes, S.K., Stodieck, L.S., Ferguson, V.L., and Pecaut, M.J. 2008. Spaceflight effects on T lymphocyte distribution, function and gene expression. Journal of Applied Physiology 106:194-202.
799. Pahlavani, M.A. 2004. Influences of caloric restriction on aging immune system. Journal of Nutrition, Health, and Aging 8:38-47.
800. Gleesen, M., Bishop, N.C. 2005. The T cell and NK cell immune response to exercise. Annals of Transplantation 10(4):43-48.
801. Field, C.J., Johnson, I.R., and Schley, P.D. 2002. Nutrients and their role in host resistance to infection. Journal of Leukocyte Biology 71:16.
802. Dinges, D.F., Douglas, S.D., Zaugg, L., Campbell, D.E., McMann, J.M., Whitehouse, W.G., Orne, E.C., Kapoor, S.C., Icaza, E., and Orne, M.T. 1994. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. Journal of Clinical Investigation 93:1930-1939.
803. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C., pp. 55-168.
804. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
805. Pierson, D.L., Stowe, R.P., Phillips, T.M., Lugg, D.J., and Mehta, S.K. 2005. Epstein-Barr virus shedding by astronauts during space flight. Brain, Behavior, and Immunity 19:235-242.
806. Pecaut, M.J., Gridley, D.S., Smith, A.L., and Nelson, G.A. 2002. Dose and dose rate effects of whole-body proton irradiation on lymphocyte blastogenesis and hematological variables: Part II. Immunology Letters 80(1):67-73.
807. Durante, M., George, K., and Cucinotta, F.A. 2006. Chromosomes lacking telomeres are present in the progeny of human lymphocytes exposed to heavy ions. Radiation Research 165:51-58.
808. Sonnenfeld, G. 2005. Use of animal models for space flight physiology studies, with special focus on the immune system. Gravitational and Space Biology Bulletin 18(2):31-35.
809. Wei, L.X., Zhou, J.N., Roberts, R.I., and Shi, Y.F. 2003. Lymphocyte reduction induced by hindlimb unloading: Distinct mechanisms in the spleen and thymus. Cell Research 13(6):465-471.
810. Nash, P.V., Bour, B.A., and Mastro, A.M. 1991. Effect of hindlimb suspension simulation of microgravity on in vitro immunological responses. Experimental Cell Research 195:353-360.
811. Crucian, B.E., Stowe, R.P., Mehta, S.K., Yetman, D.L., Leal, M.J., Quiriarte, H.D., Pierson, D.L., and Sams C.F. 2009. Immune status, latent viral reactivation, and stress during long-duration head-down bed rest. Aviation, Space, and Environmental Medicine 80(5):A37-A44.
812. Crucian, B., Lee, P., Stowe, R., Jones, J., Effenhauser, R., Widen, R., and Sams, C. 2007. Immune system changes during simulated planetary exploration on Devon Island, high arctic. BMC Immunology 8(7):1471-2172.
813. Tingate, T., Lugg, D.J., Muller, H.K., Stowe, R.P., and Pierson, D.L. 1997. Antarctic isolation: Immune and viral studies. Immunology and Cell Biology 75:275-283.
814. Tingate, T., Lugg, D.J., Muller, H.K., Stowe, R.P., and Pierson, D.L. 1997. Antarctic isolation: Immune and viral studies. Immunology and Cell Biology 75:275-283.
815. International Space Life Sciences Working Group (ISLSWG). 1999. Developmental Biology Workshop, Marine Biological Laboratory, Woods Hole, Mass., September 27-30.
816. National Research Council. 1987. A Strategy for Space Biology and Medical Science for the 1980 s and 1990s. National Academy Press, Washington, D.C.
817. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
818. Moody, S.A., and Golden, C. 2000. Developmental biology research in space: Issues and directions in the era of the International Space Station. Developmental Biology 228:1-5.
819. NASA Developmental Biology Review Panel Report. 1999. NASA Ames Research Center, August 10-12, 1998.
820. Buckey, J.C., and Homick, J.L. 2000. The Neurolab Spacelab Mission: Neuroscience Research in Space. NASA Lyndon B. Johnson Space Center, Houston, Tex.
821. Ronca, A.E. 2003. Mammalian development in space. In Development in Space (H.J. Marthy, ed.). Advances in Space Biology and Medicine, Volume 9. Elsevier, The Netherlands.
822. Morey-Holton, E.R., Hill, E.L., and Souza, K.A. 2007. Animals and spaceflight: From survival to understanding. Journal of Musculoskeletal and Neuronal Interactions 7:17-25.
823. Goldberg, A.D., Allis, C.D., and Bernstein, E. 2007. Epigenetics: A landscape takes shape. Cell 128:635-638.
824. Meaney, M.J., Szyf, M., and Seckl, J.R. 2007. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends in Molecular Medicine 13(7):269-277.
825. Kandpal, R., Saviola, B., and Felton, J. 2009. The era of ’omics unlimited. Biotechniques 46:351-352, 354-355.
826. Marthy, H.J., ed. 2003. Development in Space. Advances in Space Biology and Medicine, Volume 9. Elsevier, The Netherlands.
827. Ikenaga, M., Yoshikawa, I., Kojo, M., Ayaki, T., Ryo, H., Ishizaki, K., Kato, T., Yamamoto, H., and Hara, R. 1997. Mutations induced in Drosophila during space flight. Biological Sciences in Space 11:346-350.
828. Reitz, G., Bucker, H., Facius, R., Hornck, G., Graul, E.H., Berger, H., Ruther, W., Heinrich, W., Beaujean, R., Enge, W., Alpatov, A.M., Ushakov, I.A., Zachvatkin, Yu.A., and Mesland, D.A.. 1989. Influence of cosmic radiation and/or microgravity on development of Carausius morosus. Advances in Space Research 9:161-173.
829. Leandro, L.J., Szewczyk, N.J., Benguría, A., Herranz, R., Laván, D., Medina, F.J., Gasset, G., Loon, J.V., Conley, C.A., and Marco, R. 2007. Comparative analysis of Drosophila melanogaster and Caenorhabditis elegans gene expression experiments in the European Soyuz flights to the International Space Station. Advances in Space Research 40:506-512.
830. Higashibata, A., Higashitani, A., Adachi, R., Kagawa, H., Honda, S., Honda, Y., Higashitani, N., Sasagawa, Miyazawa, Y., Szewczyk, N.J., Conley, C.A., Fujimoto, N., Fukui, K., Shimazu, T., Kuriyama, K., and Ishioka, N. 2007. Biochemical and molecular biological analyses of space-flown nematodes in Japan, the First International Caenorhabditis elegans Experiment (ICEFirst). Microgravity Science and Technology 19:159-163.
831. Marthy, H.J., ed. 2003. Development in Space. Advances in Space Biology and Medicine, Volume 9. Elsevier, The Netherlands.
832. Ronca, A.E., and Alberts, J.R. 2000. Physiology of a microgravity environment selected contribution: Effects of spaceflight during pregnancy on labor and birth at 1 G. Journal of Applied Physiology 89:849-854.
833. Burden, H.W., Zary, J., and Alberts, J.R. 1999. Effects of spaceflight on the immunohistochemical demonstration of connexin 26 and 43 in the postpartum uterus in rats. Journal of Reproduction and Fertility 116(2):229-234.
834. Ronca, A.E. 2003. Mammalian development in space. In Development in Space (H.J. Marthy, ed.). Advances in Space Biology and Medicine, Volume 9. Elsevier, The Netherlands.
835. Buckey, J.C., and Homick, J.L. 2000. The Neurolab Spacelab Mission: Neuroscience Research in Space. NASA Lyndon B. Johnson Space Center, Houston, Tex.
836. Kerman, I.A., McAllen, R.M., and Yates, B.J. 2000. Patterning of sympathetic nerve activity in response to vestibular stimulation. Brain Research Bulletin 53:11-16.
837. Fuller, P.M., Jones, T.A., Jones, S.M., and Fuller, C.A. 2004. Evidence for macular gravity receptor modulation of hypothalamic, limbic and autonomic nuclei. Neuroscience 129:461-471.
838. Fuller, P.M., Jones, T.A., Jones, S.M., and Fuller, C.A. 2002. Neurovestibular modulation of circadian and homeostatic regulation: Vestibulohypothalamic connection? Proceedings of the National Academy of Sciences U.S.A. 99:15723-15728.
839. Balaban, C.D. 2002. Neural substrates linking balance control and anxiety. Physiology and Behavior 77:469-475.
840. Knierim, J.J., McNaughton, B.L., and Poe, G.R. 2000. Three-dimensional spatial selectivity of hippocampal neurons during space flight. Nature Neuroscience 3:209-210.
841. Hubel, D.H., and Wiesel, T.N. 1982. Ferrier lecture: Functional architecture of the macaque monkey visual cortex. Proceedings of the Royal Society London (Biology) 198:1-59.
842. Ronca, A.E., Fritzsch, B., Bruce, L.L., and Alberts, J.R. 2008. Orbital spaceflight during pregnancy shapes function of mammalian vestibular system. Behavioral Neuroscience 122(1):224-232.
843. Walton, K.D., Harding, S., Anschel, D., Harris, Y.T., and Llinás, R. 2005. The effects of microgravity on the development of surface righting in rats. Journal of Physiology 565(Pt 2):593-608.
844. Walton, K.D., Harding, S., Anschel, D., Harris, Y.T., and Llinás, R. 2005. The effects of microgravity on the development of surface righting in rats. Journal of Physiology 565(Pt 2):593-608.
845. Beisel, K.W., Wang-Lundberg, Y., Maklad, A., and Fritzsch, B. 2005. Development and evolution of the vestibular sensory apparatus of the mammalian ear. Journal of Vestibular Research 15:225-241.
846. Fritzsch, B., Beisel, K.W., and Hansen, L.A. 2006. The molecular basis of neurosensory cell formation in ear development: A blueprint for hair cell and sensory neuron regeneration? Bioessays 28(12):1181-1193.
847. Böser, S., Dournon, C., Gualandris-Parisot, L., and Horn, E. 2008. Altered gravity affects ventral root activity during fictive swimming and the static vestibuloocular reflex in young tadpoles (Xenopus laevis). Archives Italiennes de Biologie 146(1):1-20.
848. Wiederhold, M.L., Harrison, J.L., and Gao, W. 2003. A critical period for gravitational effects on otolith formation. Journal of Vestibular Research 13(4-6):205-214.
849. Jones, T.A., Fermin, C., Hester, P.Y., Vellinger, J., Kenyon, R.V., Kerschmann, R., Sgarioto, R., Jun, S., and Vellinger, J. 1993. Effects of microgravity on vestibular ontogeny: Direct physiological and anatomical measurements following space flight (STS-29). Acta Veterinaria Brno 62(6 Suppl.):S35-S42.
850. Raymond, J., Demêmes, D., Blanc, E., Sans, N., Ventéo, S., and Dechesne, C.J. 2000. In The Neurolab Spacelab Mission: Neuroscience Research in Space: Results from the STS-90, Neurolab Spacelab Mission (J.C. Buckey and J.L. Homick, eds.). NASA 1-11. NASA Johnson Space Center, Houston, Tex. January.
851. Ronca, A.E., Fritzsch, B., Bruce, L.L., and Alberts, J.R. 2008. Orbital spaceflight during pregnancy shapes function of mammalian vestibular system. Behavioral Neuroscience 122(1):224-232.
852. Bouët, V., Wubbels, R.J., de Jong, H.A., and Gramsbergen, A. 2004. Behavioural consequences of hypergravity in developing rats. Brain Research. Developmental Brain Research 153(1):69-78.
853. Wade, C.E. 2005. Responses across the gravity continuum: hypergravity to microgravity. Advances in Space Biology and Medicine 10:225-245.
854. Fritzsch, B., Pauley, S., Matei, V., Katz, D.M., Xiang, M., and Tessarollo, L. 2005. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hearing Research 206(1-2):52-63.
855. Bergstrom, R.A., You, Y., Erway, L.C., Lyon, M.F., and Schimenti, J.C. 1998. Deletion mapping of the head tilt (het) gene in mice: A vestibular mutation causing specific absence of otoliths. Genetics 150(2):815-822.
856. Soukup, G.A., Fritzsch, B., Pierce, M.L., Weston, M.D., Jahan, I., McManus, M.T., and Harfe, B.D. 2009. Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Developmental Biology 328(2):328-341.
857. de Caprona, M.D., Beisel, K.W., Nichols, D.H., and Fritzsch, B. 2004. Partial behavioral compensation is revealed in balance tasked mutant mice lacking otoconia. Brain Research Bulletin 64:289-301.
858. Riley, D., and Wong-Riley, M.T.T. 2000. Neuromuscular development is altered by spaceflight. In The Neurolab Spacelab Mission: Neuroscience Research in Space: Results from the STS-90, Neurolab Spacelab Mission (J.C. Buckey and J.L. Homick, eds.). NASA 1-11. NASA Johnson Space Center, Houston, Tex. January.
859. Riley, D., and Wong-Riley, M.T.T. 2000. Neuromuscular development is altered by spaceflight. In The Neurolab Spacelab Mission: Neuroscience Research in Space: Results from the STS-90, Neurolab Spacelab Mission (J.C. Buckey and J.L. Homick, eds.). NASA 1-11. NASA Johnson Space Center, Houston, Tex. January.
860. Adams, G.R., McCue, S.A., Zeng, M., and Baldwin, K.M. 1999. Time course of myosin heavy chain transitions in neonatal rats: Importance of innervation and thyroid state. American Journal of Physiology 276(4 Pt 2):R954-R961.
861. Huckstorf, B.L., Slocum, G.R., Bain, J.L., Reiser, P.M., Sedlak, F.R., Wong-Riley, M.T., and Riley, D.A. 2000. Effects of hindlimb unloading on neuromuscular development of neonatal rats. Brain Research. Developmental Brain Research 119(2):169-178.
862. Walton, K.D., Harding, S., Anschel, D., Harris, Y.T., and Llinás, R. 2005. The effects of microgravity on the development of surface righting in rats. Journal of Physiology 565(Pt 2):593-608.
863. Walton, K.D., Benavides, L., Singh, N., and Hatoum, N. 2005. Long-term effects of microgravity on the swimming behaviour of young rats. Journal of Physiology 565(Pt 2):609-626.
864. Inglis, F.M., Zuckerman, K.E., and Kalb, R.G. 2000. Experience-dependent development of spinal motor neurons. Neuron 26(2):299-305.
865. DeFelipe, J., Arellano, J.I., Merchán-Pérez, A., González-Albo, M.C., Walton, K., and Llinás, R. 2002. Spaceflight induces changes in the synaptic circuitry of the postnatal developing neocortex. Cerebral Cortex 12(8):883-891.
866. Temple, M.D., Kosik, K.S., and Stewart, O. 2002. Spatial learning and memory is preserved in rats after early development in a microgravity environment. Neurobiology of Learning and Memory 78:199-216.
867. Kandel, E.R., Kupferson, I., and Iverson, S. 2000. Learning and memory. Pp. 1227-1246 in Principals of Neural Science (E.R. Kandel, J.H. Schwartz, and T.M. Jessell, eds.). 4th Edition. McGraw Hill.
868. Sanes, J.R., and Jessell, T.M. 2000. Pp. 1087-1113. in Principals of Neural Science (E.R. Kandel, J.H. Schwartz, and T.M. Jessell, eds.). 4th Edition. McGraw Hill.
869. Minichiello, L. 2009. TrkB signalling pathways in LTP and learning. Review. Nature Reviews Neuroscience 10(12):850-860.
870. Agerman, K., Hjerling-Leffler, J., Blanchard, M.P., Scarfone, E., Canlon, B., Nosrat, C., and Ernfors, P. 2003. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development 130(8):1479-1491.
871. Hargens, A.R., and Watenpaugh, D.E. 1996. Cardiovascular adaptations to spaceflight. Medicine and Science in Sports and Exercise 28:877-882.
872. Colleran, P.N., Wilkerson, M.K., Bloomfield, S.A., Suva, R.T., and Delp, M.D. 2000. Alterations in skeletal perfusion with simulated microgravity: A possible mechanism for bone remodeling. Journal of Applied Physiology 89:1046-1054.
873. Hwang, S., Shelkovinkov, S.A., and Purdy, R.E. 2007. Simulated microgravity effects on the rat carotid and femoral arteries: Role of contractile protein expression and mechanical properties of the vessel wall. Journal of Applied Physiology 102:1595-15603.
874. Stevens, H.Y., Meays, D.R., and Frangos, J.A. 2006. Pressure gradients and transport in the murine femur upon hindlimb suspension. Bone 39:565-572.
875. Hargens, A.R., and Richardson, S. 2009. Cardiovascular adaptations, fluid shifts, and countermeasures related to spaceflight. Respiratory Physiology and Neurobiology 169(Suppl. 1):S30-S33.
876. Hargens, A.R., and Watenpaugh, D.E. 1996. Cardiovascular adaptations to spaceflight. Medicine and Science in Sports and Exercise 28:877-882.
877. Colleran, P.N., Wilkerson, M.K., Bloomfield, S.A., Suva, R.T., and Delp, M.D. 2000. Alterations in skeletal perfusion with simulated microgravity: A possible mechanism for bone remodeling. Journal of Applied Physiology 89:1046-1054.
878. Hargens, A.R., and Richardson, S. 2009. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respiratory Physiology and Neurobiology 169(Suppl. 1):S30-S33.
879. Hargens, A.R., and Richardson, S. 2009. Cardiovascular adaptations, fluid shifts, and countermeasures related to spaceflight. Respiratory Physiology and Neurobiology 169(Suppl. 1):S30-S33.
880. McCrory, J.L., Derr, J., and Cavanagh, P.R. 2004. Locomotion in simulated zero gravity: Ground reaction forces. Aviation, Space, and Environmental Medicine 75:203-210.
881. Sibonga, J.D., Evans, H.J., Sung, H.G., Spector, E.R., Lang, T.F., Oganov, V.S., Bakulin, A.V., Shackelford, L.C., and LeBlanc, A.D. 2007. Recovery of spaceflight-induced bone loss: Bone mineral density after long-duration missions as fitted with an exponential function. Bone 41:973-978.
882. Lang, T.F., Leblanc, A.D., Evans, H.J., Lu, Y. 2006. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. Journal of Bone and Mineral Research 21:1224-1230.
883. Sibonga, J.D., Cavanagh, P.R., Lang, T.F., LeBlanc, A.D., Schneider, V., Shackelford, L.C., Smith, S.M., Vico, L. 2007. Adaptation of the skeletal system during long-duration spaceflight. Clinical Reviews in Bone and Mineral Metabolism 5(4):249-261.
884. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
885. Gillispie, C.C. 1983. The Montgolfier Brothers and the Invention of Aviation, 1783-1784. Princeton University Press, New Jersey, p. 15.
886. Sonnenfeld, G. 2005. Overview. Pp. 1-5 in Experimentation with Animals in Space (G. Sonnenfeld, ed.). Advances in Biology and Medicine, Volume 10. Elsevier, Amsterdam.
887. Il’in, YeA. 1989. The Cosmos satellites: Some conclusions and prospects. USSR Space Life Sciences Digest 22:109-114. NASA-CR-3922(26). Available at http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19900002837_1990002837.pdf.
888. Pishcik, V., and Faybishenko, Yu. 1986. The hard road to the stars. in commemoration of the 25th Anniversary of the first manned space flight. USSR Life Science Digest 7:91-98. NASA-CR-3922(08). Available at http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19860022611_1986022611.pdf.
889. Morey-Holton, E.R., Hill, E.L., and Souza, K.A. 2007. Animals and spaceflight: From survival to understanding. Journal of Musculoskeletal and Neuronal Interactions 7:17-25.
890. Nicogossian, A.E., Pool, S.L., and Uri, J.J. 1994. Historical perspectives. Pp. 3-29 in Space Physiology and Medicine (A.E. Nicogossian, C.L. Leach, and S.L. Pool, eds.). 3rd Edition. Lea and Febiger, Philadelphia.
891. Pishcik, V., and Faybishenko, Yu. 1986. The hard road to the stars. in commemoration of the 25th Anniversary of the first manned space flight. USSR Life Science Digest 7:91-98. NASA-CR-3922(08). Available at http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19860022611_1986022611.pdf.
892. Pace, N., Rahlmann, D.F., Kodama, A.M., Mains, R.C., and Grunbaun, B.W. 1977. Physiological studies in space with nonhuman primates using the monkey pod. Pp 23-33 in The Use of Nonhuman Primates in Space (R.C. Simmonds and G.H. Bourne, eds.). NASA Conference Publication 005. NASA National Technical Office, Springfield, Va.
893. National Research Council. 1987. A Strategy for Space Biology and Medical Sciences for the 1980s and 1990s. National Academy Press, Washington, D.C., p. 4.
894. Grindeland, R.E., Ilyn, E.A., Holley, D.C., and Skidmore, M.G. 2005. International collaboration on Russian spacecraft and the case for free flyer biosatellites. Pp. 41-80 in Experimentation with Animal Models in Space (G. Sonnenfeld, ed.). Advances in Space Biology and Medicine, Volume 10. Elsevier, Amsterdam.
895. Tipton, C.M. 1996. Animal model and their importance to human physiological responses in microgravity. Medicine and Science in Sports and Exercise 28(10 Suppl.):S94-S100.
896. Committee calculation on November 5, 2009, of the data listed in reference 9.
897. Il’in, YeA. 1989. The Cosmos satellites: Some conclusions and prospects. USSR Space Life Sciences Digest 22:109-114. NASA-CR-3922(26). Available at http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19900002837_1990002837.pdf.
898. Grindeland, R.E., Ilyn, E.A., Holley, D.C., and Skidmore, M.G. 2005. International collaboration on Russian spacecraft and the case for free flyer biosatellites. Pp. 41-80 in Experimentation with Animal Models in Space (G. Sonnenfeld, ed.). Advances in Space Biology and Medicine, Volume 10. Elsevier, Amsterdam.
899. Gazenko, O.G., Genin, A.M., Ilyin, E.A., Oganov, V.S., and Serova, L.V. 1980. Adapation to weightlessness and it physiological mechanisms (Results of animal experiments aboard biosatellites). The Physiologist 23(Suppl. 6):S11-S15.
900. Cherniack, N.S., ed. 1992. Cosmos 2044 mission. Journal of Applied Physiology 73:1S-200S.
901. Korolkov, V., Helwig, D., Viso, M., and Connolly, J. 1996. Cosmos 2229 mission. Overview. Journal of Applied Physiology 81(1):186-208.
902. Kozlovskaya, I.B., Grindeland, R.E., Visco, M., and Korolkov, V.I. 2000. Bion 11 science objectives and results. Journal of Gravitational Physiology 7:S19-S26.
903. Tipton, C.M. 2003. Animals and biosatellites in space. Journal of Gravitational Physiology 10:1-3.
904. Cohen, B., Yakushin, S.B., Holestin, G.R., Dai, M., Tomko, D.L., Badakva, A.M., and Kozlovskaya, I.B. 2005. Vestibular experiments in space. Pp. 41-80 in Experimentation with Animal Models in Space (G. Sonnenfeld, ed.). Advances in Space Biology and Medicine, Volume 10. Elsevier, Amsterdam.
905. Riley, D.A, Ellis, S., Slocum, G.R., Sedlak, F.R., Bain, J.L.W., Krippendorf, B.B., Lehman, C.T., Macias, M.Y., Thompson, J.L., Vijayan, K., and DeBruin, J.A. 1996. In-flight and post flight changes in skeletal muscles of SLS-1 and SLS-2 spaceflown rats. Journal of Applied Physiology 81:133-144.
906. Durvova, G., Kaplansky, A., and Morey-Holton, E. 1996. Histomorphometric study of tibia of rats exposed aboard American Spacelab Life Sciences 2 Shuttle Mission. Journal of Gravitational Physiology 3:80-81.
907. National Aeronautics and Space Administration. 1993. Mission Information, Space Flight Mission STS-58. Life Sciences Data Archive. NASA Johnson Space Center, Houston, Tex. Available at http://Lsda.jsc.nasa.gov/scripts/mission/miss.cfm?mis_index=7.
908. Buckey, J.C., and Homick, J.L. 2000. The Neurolab Spacelab Mission: Neuroscience Research in Space. NASA Lyndon B. Johnson Space Center, Houston, Tex.
909. Golov, V.K., Magedov, V.S., Skidmore, M.G., Hines, J.W., Kozlovskaya, I.B., and Korololkov, V.I. 2000. Bion 11 mission hardware. Journal of Gravitational Physiology 7:S27-S36.
910. Tomko, D., and Souza, K. 2009. Advanced Animal Habitat (AAH). P. 2 in Flight and Specialized Ground Equipment for Animal, Cell and Microbial Experiments: Status and Availability. October 26.
911. Ground, D. 2009. Six month ISS missions have greatly expanded our knowledge of human adaptation. In Human Research Program ISS Research Opportunities. October 26.
912. National Aeronautics and Space Administration Authorization Act of 2005. P. L. 109-15 119 Stat. 2895, December 30, 2005.
913. Institute of Medicine. 2001. Safe Passage: Astronaut Care for Exploration Missions (J.R. Ball and C.H. Evans, Jr., eds.). National Academy Press, Washington, D.C.
914. LeBlanc, A.D., Spector, E.R., Evans, H.J., and Sibonga, J.D. 2007. Skeletal responses to space flight and the bed rest analog: A review. Journal of Musculoskeletal and Neuronal Interactions 7(1):33-47.
915. Pavy-Le Traon, A., Heer, M., Narici, M.V., Rittweger, J., and Vernikos, J. 2007. From space to Earth: Advances in human physiology from 20 years of bed rest studies (1986-2006). European Journal of Applied Physiology 101(2):143-194.
916. National Aeronautics and Space Administration. 2010. The NASA Fundamental Space Biology Science Plan 2010-2020. Advanced Capabilities Division. NASA Headquarters, Washington, D.C. June.
917. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
This page intentionally left blank.










































































































