A
Commissioned Papers
The Committee on Lyme Disease and Other Tick-Borne Diseases: The State of the Science commissioned 10 papers on range of topics that were not covered in depth at the workshop. The committee felt these papers were necessary for the discussion at the workshop. These papers are reproduced in their entirety in this appendix.
A1
THROUGH A GLASS, DARKLY: THE GLOBAL INCIDENCE OF TICK-BORNE DISEASES
Christopher D. Paddock, M.D., M.P.H.T.M., and Sam R. Telford III, Sc.D.
Infectious Diseases Pathology Branch, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA
Division of Infectious Diseases, Department of Biomedical Sciences, Tufts University Cummings School of Veterinary Medicine
Corresponding author: Christopher D. Paddock
Infectious Diseases Pathology Branch, Bldg 18, Rm. SB 109, Mailstop G-32, Centers for Disease Control and Prevention
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Introduction
Several events that occurred during the final decades of the 20th Century, and at the cusp of the 21st Century, suggest that increases in the scope and magnitude of tick-borne infections have occurred worldwide. These include recent national and regional epidemics of historically recognized diseases, including tick-borne encephalitis (TBE) in Central and Eastern Europe, Kyasanur forest disease (KFD) in Karnataka state in India, Crimean-Congo hemorrhagic fever (CCHF) in northern Turkey and the southwestern regions of the Russian Federation, and Rocky Mountain spotted fever (RMSF) in Arizona and Baja California (Randolph, 2008; Pattnaik, 2006; Maltezou et al., 2010; McQuiston et al., 2010; Bustamente Moreno and Pon Méndez, 2010a). Globally, the recognized number of distinct and epidemiologically important diseases transmitted by ticks has increased considerably during the last 30 years. By example, >10 newly recognized spotted fever rickettsioses have been identified since 1984 (Raoult et al., 1996; Parola et al., 2005; Paddock et al., 2008; Shapiro et al., 2010). In the United States, only 2 tick-borne diseases, RMSF and tularemia, were nationally notifiable in 1990; by 1998, this list included 3 newly recognized
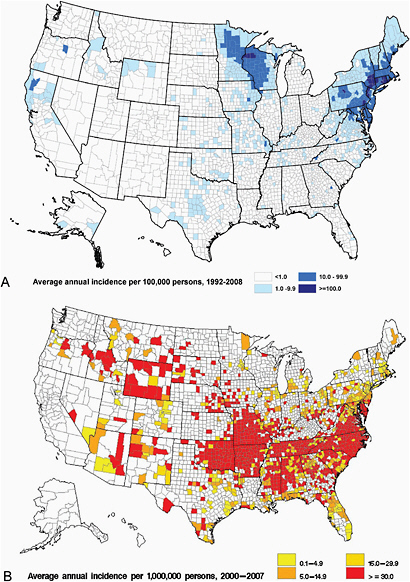
infections: Lyme disease, human granulocytic ehrlichiosis [anaplasmosis] (Anaplasma phagocytophilum infection), and human monocytic ehrlichiosis (Ehrlichia chaffeensis infection), each of which has increased steadily in average annual incidence. Lyme disease is now the most commonly reported vector-borne illness in the United States, with the number of reported cases increasing 101% (from 9,908 to 19,931) during 1992-2006. (Bacon et al., 2008). During 2000-2008, the annual reported incidence of RMSF in the United States also increased dramatically, from 1.7 to 9.4 cases per million persons (Figure A1-1), representing the steepest rise to the highest rate ever recorded (Openshaw et al., 2010). From 2000-2007, the incidence of infections caused by A. phagocytophilum and E. chaffeensis also increased linearly, from 0.80 to 3.0, and 1.4 to 3.0, cases per million population, respectively (Dahlgren et al., in press).
Against this background of rapidly expanding pathogen recognition and escalating incidence have been concerns about the accuracy of case counts that form the basis for these statistics (Mantke et al., 2008; Raoult and Parola, 2008; Paddock, 2009). Many of these agents were catapulted into the realm of human recognition by extraordinary advances in molecular technology; however, epidemiologic tools for capturing cases and calculating incidence have not undergone similar transformative changes. Paradoxically, the discoveries of new pathogens made possible by contemporary diagnostic methods have cast suspicion on certain aspects of the
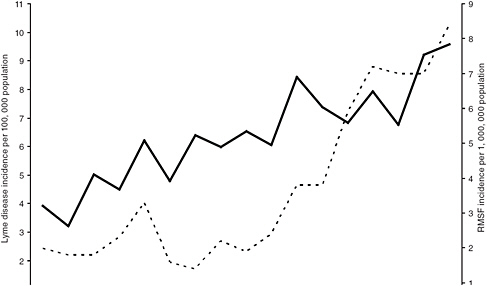
FIGURE A1-1 Average annual incidence of Rocky Mountain spotted fever and Lyme disease in the United States, 1992-2008 (Bacon et al., 2008; Openshaw et al., 2010).
distribution, frequency, and clinical heterogeneity of some older, historically recognized, tick-borne diseases. In essence, the pace of pathogen discovery has eclipsed fundamental epidemiologic knowledge of many of the diseases caused by these agents.
Incidence rates of tick-borne infections pale in comparison with those of many other arthropod-borne diseases, including malaria, dengue, Chagas’ disease, onchocerciasis, and leishmaniasis. Only Lyme disease, with tens of thousands of new cases each year, distributed across several continents, can be considered as prevalent across a wide distribution (Table A1-1). Lyme
TABLE A1-1 Estimated Global Incidence and Distribution of Major Tick-Borne Infections
|
Global Incidence and Distribution of Major Tick-Transmitted Infections |
|
Very common (>10,000 new cases each year) |
|
Lyme disease – Holarctic |
|
(Bacon et al., 2008) |
|
Common (1000-10,000 new cases each year) |
|
Tick-borne encephalitis – Holarctic |
|
(www.isw-tbe.info/upload/medialibrary/12th_ISW-TBE_Newsletter.pdf) |
|
Tick-borne relapsing fever – tropical Africa; western United States |
|
(Felsenfeld, 1971; Trape et al., 1996; Vial et al., 2006) |
|
Tick-borne spotted fever group rickettsioses – global |
|
(Rovery et al. 2008; Openshaw et al., 2010) |
|
Ehrlichiosis and anaplasmosis – global |
|
(Demma et al., 2005b) |
|
Masters’ disease – eastern, central, and south-central United States |
|
(CDC, 1990) |
|
Crimean-Congo hemorrhagic fever–southern Europe, Africa, western and central |
|
Russian Federation, North Asia |
|
Moderately common (100-1,000 new cases each year) |
|
Colorado tick fever and other other coltivirus infections–western United States; central Europe |
|
(http://www.cdphe.state.co.us/dc/zoonosis/tick/Colorado_tick_diseases.pdf) |
|
Babesiosis – northeastern United States; Europe |
|
(Telford et al., in press) |
|
Omsk hemorrhagic fever – eastern Russia and Siberia |
|
(Lvov, 1988) |
|
Tick-borne tularemia – eastern and central United States; central Europe; Russian Federation |
|
(CDC, 2002) |
|
Kyasanur forest disease – Karnataka and adjacent states in India; Saudi Arabia; Egypt |
|
(Dandawate et al., 1994; Pattnaik, 2006; Carletti et al., 2010) |
|
Rare (sporadic cases) |
|
Powassan/deer tick virus –Canada; northeastern and north central United States |
|
(Ebel, 2010) |
disease is still less common, by an order of magnitude, than leishmaniasis, represented by 1 million new cases a year among a population at risk of 350 million persons (Anonymous, 1994). Nonetheless, in some regions of the world, such as Europe, tick-borne diseases are the most widespread and medically important of all vector-borne infectious diseases (Randolph, 2010). In addition, some tick-borne diseases are associated with high case-fatality rates or long-term morbidity, and frequently generate considerable fear among the population who reside in areas where these pathogens are endemic; in this context, public health concerns may far exceed actual disease burden. By example, the average annual incidence of Brazilian spotted fever in São Paulo State, Brazil, during 2000-2008 ranged from 0.2 to 1.1 cases per million population, comprising only 285 total cases; however, 89 of these resulted in death, for an average case-fatality rate of 31% (www.cve.saude.sp.gov.br/htm/zoo/fm_i8503.htm). Other tick-borne diseases, including CCHF and KFD, are associated with high case-fatality rates that rival or exceed those of many of the most severe infectious diseases (Hoogstraal, 1979; Swanepoel et al., 1987; Pattnaik, 2006).
This discussion compares the perceived and actual burden of various tick-borne infections suggested by existing surveillance data, evaluates some of the strengths and limitations of current systems that measure incidence, and suggests several approaches for improving the accuracy of incidence determinations for these diseases. While tick-borne infections also pose important veterinary health problems around the world, this synopsis focuses on the occurrence of these diseases in human populations. Although this discussion also incorporates some information that is anecdotal, inferred, or derived from non-controlled circumstances, we hope that a contemporary synthesis of all observations may serve as a guide for subsequent epidemiologic approaches to this remarkably diverse and important collection of zoonotic diseases.
Case Counts, Reporting, and Incidence of Tick-Borne Diseases
Are the global rises in incidence reflective of true events or greater levels of reporting? Simplistically, increased reporting is indeed responsible for these trends; however, this question is somewhat circular, because incidence statistics are obtained principally from reported cases of disease. Incidence rates are dependent directly on the size of the population at risk during a specific interval of time and the number of identified cases of disease; however, from most of the scientific literature, it is difficult to determine whether a change in incidence reflects increased transmission, better reporting, or a change in the population at risk. Ideally, surveillance systems for tick-borne diseases accurately identify rises or declines of the disease in question; however, any of a number of variables may change
over time, including ecologic, climatologic, or social variables, case definitions, diagnostic assays, or the appearance or emigration of cognizant and enthusiastic clinicians who actively search for cases and specifically pursue confirmatory tests.
Incidence and Regional Context
Incidence statistics of tick-borne infections, when interpreted flatly as national rates, characteristically lose impact and meaning. These zoonoses are influenced profoundly by a complex mixture of predictable and unpredictable factors that include landscape, climate, wildlife hosts, and tick distributions that coalesce to create regional pockets of intensified risk (Pavlovskey, 1966); in this context, incidence rates for these diseases assume far greater impact when viewed regionally. Because of marked differences in population sizes across regions, it is axiomatic that high incidence does necessarily equate to a large number of reported cases. By example, sparsely populated Cameron County, Pennsylvania, reported only 14 cases of Lyme disease during 2002-2006; however, the county’s average annual incidence rate was greater than the incidence of the more populous Windham County, Connecticut, where approximately 18 × as many cases were reported during the same interval (Bacon et al., 2008). Nantucket County in Massachusetts, reported 151 cases of Lyme disease during 1992-2006, representing only 0.061% of 248,074 total reports received by CDC during this interval; however, it ranked highest in incidence of all U.S. counties during 1992-2001, and third during 2002-2006, with rates of 361 to 755 per 100,000 population (Figure A1-2A). By comparison, the average annual rate of Lyme disease in the entire state of Massachusetts was 14.5 per 100,000 population during the same study period (Bacon et al., 2008).
During 1989-2000, Portugal reported the highest country-wide incidence of Mediterranean spotted fever (MSF) in the Mediterranean basin (9.8 per 100,000 persons); however, the regional incidence in this country ranged markedly, from 3.1 per 100,000 in Lisboa and Vale do Teja, to 31 per 100,000 in the nearby region of Alentejo (de Sousa et al., 2003). During 2000-2007, 11,531 cases of RMSF were reported from 46 states and the District of Colombia; however, approximately two-thirds of these cases originated from only 5 states (Arkansas, Missouri, North Carolina, Oklahoma, and Tennessee), where the incidence ranged from 20.3 to 52.6 per million persons (Figure A1-2B). By comparison, the national incidence of RMSF during the study period was 4.9 per million (Openshaw et al., 2010). These statistics are magnified further when foci of infected ticks overlap rural or undeveloped regions with relatively low population density. During 2003-2009, 88 cases of RMSF were reported from 3 Apache Indian communities in Eastern Arizona that resulted in an average annual incidence of
437 /million persons for this 5,000 square mile region, more than 62 times greater than the national average (McQuiston et al., 2010). In some circumstances, regional variation develops when cultural, racial or socioeconomic homogeneity exists among the population at risk. By example, the incidence of RMSF among American Indians has risen dramatically (Figure A1-3), when compared with other racial groups in the United States: during 2001-2005, the average annual incidence among American Indians was 16.8 per 1,000,000 population, compared with rates of 4.2 and 2.6 among white and black racial groups, respectively (Holman et al., 2009).
Trends in Drequency and Distribution
Dramatic shifts in numbers of reported cases of tick-borne diseases over time and space are well-recognized; indeed, such shifts are epidemiologic hallmarks of many of these infections. National or regional trends are best characterized by surveillance systems with sufficient maturity and camber to accommodate for input that might otherwise immediately confound interpretation. The incidence of TBE in the Czech Republic has exhibited at least 4 cycles of rising and declining incidence since 1971, with the greatest upsurge occurring during 1990-1995, when the incidence climbed steadily from approximately 1.7 to 7.2 /100,000 population (Kriz et al., 2004). Similar increases were witnessed in several other eastern European countries
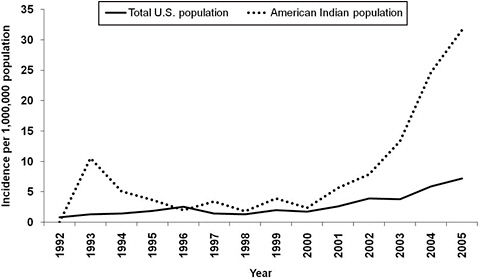
FIGURE A1-3 Annual incidence rates of Rocky Mountain spotted fever, per 1 million population, among American Indians, and the total U.S. population, 1992-2005 (Holman et al., 2009).
during this same interval (Figure A1-4) (Šumilo et al., 2007; Randolph, 2008) and more recently, has extended across several countries of Western Europe, including Italy, Germany, and Switzerland, where the incidence of TBE in 2006 exceeded average levels for the previous decade by as much as 183% (Zimmerman, 2005; Randolph et al., 2008; Rizzoli et al., 2009).
In the United States, the annual incidence of RMSF has undergone 3 major shifts (Figure A1-5) since national surveillance for this disease was initiated in 1920 (Childs and Paddock, 2003; Openshaw et al., 2010). While average annual incidence rates of Lyme disease in the United States increased steadily during 1992-2006 (Figure A1-1), at least 88% of all U.S. cases reported in any given year, and 229,782 (92.6%) of the 248,074 cases reported cumulatively during this interval, originated consistently from the 10 states in which Lyme disease is highly endemic (Bacon et al., 2008). During the mid-1970s through the early 1980s, increases in the case numbers
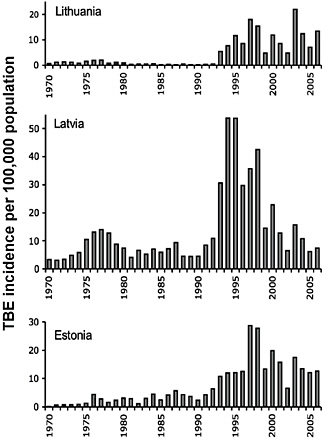
FIGURE A1-4 Incidence of tick-borne encephalitis, per 100,000 population, in Lithuania, Latvia, and Estonia, 1970-2006 (Šumilo et al., 2007).
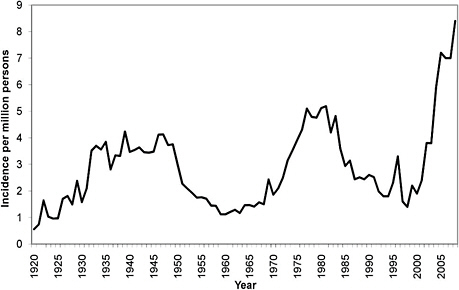
FIGURE A1-5 Average annual incidence of Rocky Mountain spotted fever, per 1 million population in the United States, 1920-2008 (Childs and Paddock, 2002; Openshaw et al., 2010).
of reported spotted fever group rickettsioses were documented in several countries bordering the Mediterranean Sea, including Israel, Italy, and Spain (Piras et al., 1982; Segura and Font, 1982; Otero et al., 1982; Gross et al., 1982; Mansueto et al. 1986). Approximately 30 cases of MSF were reported in Italy each year during 1962-1973; however, during the next 6 years, the number of cases identified rose dramatically, to >800 annually by 1979 (Scaffidi, 1981). In the area of the Vallés Occidental near Barcelona, Spain, the incidence of MSF, per 100,000 persons, rose from 3.28 cases in 1979 to 19.05 cases in 1984 (Espejo Arenas et al., 1986). During the mid-1980s through the early 1990s, <20 cases of Japanese spotted fever were reported annually; during the subsequent 15 years, reports climbed steadily to 129 cases in 2009 (Anonymous, 1999; Anonymous, 2006; Anonymous, 2010).
Drivers of Incidence
Unfortunately, the reasons suggested for major periods of increased or diminished incidence of tick-borne diseases have, with few exceptions, been difficult to investigate and even more difficult to corroborate. These infections have circulated dynamically in nature for many thousands of years, and biological equilibria among the pathogen, tick, and vertebrate hosts parasitized by the tick or infected by the pathogen characteristically exist in
the absence of humans. Nonetheless, the emergence and flux of tick-borne diseases can most often be traced to specific human activities and behaviours that create disequilibrium in these cycles and position greater numbers of persons into disrupted ecosystems. Outbreaks of tick-borne disease are often linked to ecologic and social upheavals, resulting directly from human influence, that create circumstances advantageous for large numbers of ticks and reservoir hosts. During World War II, following the occupation of Crimea by Axis forces, there was abandonment of agricultural lands and diminished hunting of European hares (Lepus europaeus) because of combat activities. When Soviet troops reoccupied the Crimean steppes in 1944, pastures and farms had become overgrown by weeds, and hares had become extremely abundant and were heavily parasitized with Hyalomma ticks. The combination of these factors is believed to have contributed to an epidemic of CCHF among military personnel during 1944-1945, involving especially signalmen and surveyors, who frequented brushy areas (Hoogstraal, 1979).
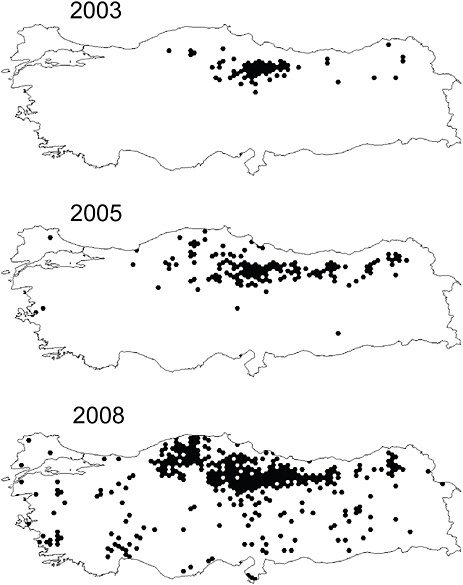
A careful analysis of climatic and vegetation features with georeferenced cases of CCHF in Turkey during 2003-2008 identified a recent expansion of extensively fragmented habitats in the Anatolia region as the most important factor for the CCHF epidemic in this region. This process resulted from the loss of mature forests to farming activities, and the reversion of farms to dense undergrowth and subsequent to second growth forest with the emigration of persons from rural to urban areas (Estrada-Peña et al., 2010). In a similar manner, whole-scale clearing of primary old-growth forests by inhabitants in the northeastern United States in the late 18th and early 19th Centuries, followed by the abandonment of farms during the westward expansion of the late 19th Century, and subsequent deciduous successional growth that provided ideal habitats for white-tailed deer (Odocoileus virginianus) and deer ticks (Ixodes scapularis (dammini), fueled the emergence of Lyme disease in the second half of the 20th Century (Spielman et al., 1985). In Italy, changes in forest management practices during the last several decades of the 20th Century transitioned a greater percentage of coppice cover (small areas of broad-leaved forest harvested regularly for firewood) to high-stand forests and improving habitat suitability for small reservoir hosts of Ixodes ricinus ticks. This manipulation of forest structure is believed to have contributed to the steadily increasing incidence of TBE observed in 17 northern alpine provinces since the early 1990s (Rizzoli et al., 2009).
Tick Abundance and Distribution
Environmental disturbance is a frequent trigger for outbreaks of tick-borne infection. The most extensively studied example, Lyme disease in
the northeastern United States, resulted from reforestation, increased deer density, and increased development and use of forested sites by humans (Spielman et al., 1985). It is likely that the deer tick vector and its microbial guild survived in relict sites during and after glaciation and through Colonial times (Telford et al., 1993; Hoen et al., 2009). Infestations of the deer tick were first recognized from the terminal moraine areas of southern New England and Long Island as well as northwest Wisconsin; these were also the sites where the first cases of Lyme disease or babesiosis were identified in the United States (Spielman et al., 1985; Scrimenti, 1970; Western et al., 1970). In the mid-1980s, Ipswich, Massachusetts represented the northernmost established infestation in the Northeast (Lastavica et al., 1989). Within a decade, the distribution of the deer tick expanded on a northsouth axis to the Bar Harbor region in Maine and the coastal peninsula of Delaware, Maryland, and Virginia (Rand et al., 1998). Infestation of migratory birds by deer tick larvae and nymphs served as the primary mode of introduction (Battaly et al., 1993; Ginsberg, 1993). Transport of adult ticks by deer along major waterways also contributed to a rapid spread, particularly in the Hudson River Valley (Chen et al., 2005).
Other recent examples of range expansions of medically important tick species include the establishment of Amblyomma americanum (a vector of E. chaffeensis and Ehrlichia ewingii) in the northeastern United States (Paddock and Yabsley, 2007) Amblyomma maculatum (a vector of Rickettsia parkeri) throughout Arkansas (Trout et al., 2010), I. scapularis across the lower peninsula of Michigan (Hamer et al., 2010), and Dermacentor reticularis (a vector of Rickettsia raoultii) in western Germany and the Netherlands (Dautel et al., 2006; Nijhof et al., 2007).
Conversely, loss of habitat or a host species may reduce the abundance of a historically dominant tick species. From a tick surveillance program in Ohio during 1984-1989 D. variabilis ticks accounted for 13,351 (97%) of 13,764 ticks submitted to the Vector-Borne Disease Unit of the Ohio Department of Health by the general public, physicians, and local health departments from every county in the state; fewer than 1% of the ticks submitted during this period were A. americanum (Pretzman et al., 1990). However, during 1994-1999, only 3,841 (62%) of 6,234 of the submitted ticks were D. variabilis, with 1,351 (22%) now comprising A. americanum (Scott Odee, Richard Gary, pers. comm.). From 1978-1981, 1,342 adult D. variabilis ticks were collected during a mark-and-release survey of ticks at Panola Mountain State Park in Georgia (Newhouse, 1983); however, D. variabilis was only rarely encountered when extensive tick surveys were conducted at this same park approximately 20 years later (Michael Levin, pers. comm.). The causes of these apparent shifts remain speculative; however, a hypothesis to explore is the effect of periodic scarcity of keystone hosts for D. variabilis, i.e., skunks and raccoons, from repeated
rabies epizootics in certain areas of the eastern United States (Anthony et al., 1990; Guerra et al., 2003).
Changes in Vertebrate Host Abundance and Distribution
The anthropogenic nature of tick-borne infections is considerable and sustained human activities that deplete or amplify the vertebrate host populations can manifest as surges of disease incidence in human populations. In Brazil, capybara (Hydochoerus hydrochaeris) are important hosts to the tick Amblyomma cajennense, a vector of RMSF, and an effective amplifying host for R. rickettsii (Souza et al., 2009). The resurgence of RMSF in many areas of São Paulo State in Brazil during 1998-2007 coincides with explosive increases in the numbers of capybara, and a broadening distribution of these rodents into urban areas of this region (Verdade and Ferraz, 2006). Collectively, these data suggest that dramatic increases in the numbers of RMSF in São Paulo State (www.cve.saude.sp.gov.br/htm/zoo/fm_i8503.htm), and other areas of southeastern Brazil, may be linked closely to a rapidly expanding population of a tick host species that is well adapted to anthropogenic habitats (Labruna et al., 2004; Angerami et al., 2006).
Several arguments document the role of white-tailed deer in the emergence and expansion of Lyme disease, babesiosis, ehrlichiosis, and anaplasmosis (Piesman et al., 1979; Spielman et al., 1993; Paddock and Yabsley, 2007). These 4 diseases were identified and characterized during the last 3 decades of the 20th century, following a period of near-exponential growth of white-tailed deer populations in multiple regions of the eastern United States. At the end of the 19th Century, following several decades of overhunting and habitat loss, an estimated 300,000-500,000 deer existed in the United States. Intensive conservation efforts, coupled with expansive environmental changes that inadvertently provided ideal habitats for these animals to proliferate, caused an eruptive increase of the numbers of deer to approximately 18 million animals by 1992. Because these animals serve as keystone hosts for I. scapularis and A. americanum ticks, the extraordinary increase in range and numbers of white-tailed deer also contributed to increases in these vector tick populations. Because O. virginianus is also an important reservoir host for E. chaffeensis and E. ewingii, this remarkable increase in numbers also expanded considerably the reservoir pool of these pathogens (Paddock and Yabsley, 2007). In a similar manner, roe deer (Capreolus capreolus) were nearly extirpated from the Italian Alps by the end of World War II; however, changes in wildlife practices during the last 50 years enabled a dramatic rebound of this species, by as much as 2000% in some provinces. Because roe deer are also considered crucial in maintaining and amplifying I. ricinus tick populations, an upsurge in deer denisty in northern Italy is likely to have contributed to the rapid and
steady rise in TBE incidence witnessed in this same region since the early 1990s (Rizzoli et al., 2009).
Mediterranean spotted fever all but disappeared from the Côte Varoise of France during 1952-1966, a period that closely approximated the disappearance wild rabbits (Oryctolagus cuniculus) following an epizootic of myxomatosis along the Mediterranean coast. In 1967, a dramatic resurgence of MSF in the region occurred simultaneously with the recovery of the wild rabbit population, suggesting to some investigators that these two events were linked ecologically and epidemiologically (Le Gac et al., 1969). Disequilibrium among domesticated animals may create drastic changes as well. More than 90 cases of RMSF, including 11 deaths, have been reported from several small communities in the White Mountain area of eastern Arizona since 2003. This outbreak appears to be linked directly to enormous numbers of R. rickettsii-infected R. sanguineus ticks in the peridomestic environment that resulted from unchecked populations of stray and free-ranging dogs in the community (Demma et al., 2005; Nicholson et al., 2006). During the 1940s, investigators in Mexico reported a similar occurrence in states of Sonora, Coahuila, Durango, Nuevo León, and San Luis Potosí (Bustamente and Varela, 1947).
Climate Change
Direct effects of climate change on the incidence of tick-borne infections remain largely speculative; (Šumilo et al., 2007; Rizzoli et al. 2009; Randolph, 2009a; Randolph, 2010; Randolph et al., 2008). Many transmission models have been developed, but the lacuna in virtually all of these systems is a quantitative assessment of the “zoonotic bridge,” i.e, biological events that introduce the pathogen from the natural enzootic cycle into the realm of human health (Spielman and Rossignol, 1984). Risk factors for human exposure to vectors, and human-associated factors that modify this risk, including activity patterns and the use of personal protection, remain poorly studied. In addition, incidence data of sufficient duration and at the appropriate temporal and spatial scales are often not available to validate existing quantitative models. Accordingly, if one cannot accurately predict incidence for a site over a short interval of time, despite readily measured surveillance variables, then any long-term prediction for the results of climate change remain conjectural. Nonetheless, climate change has been implicated frequently as an important driver of incidence. The spread of tick-borne borreliosis in West Africa is possibly linked to a sub-Saharan drought that allowed the tick vector, Alectorobius sonrai, to colonize new savannah areas (Trape et al., 1996). It has been suggested that warmer weather increases the frequency with which R. sanguineus will bite humans and thereby transmit its associated pathogens (Parola et al., 2008).
Of the tick-borne diseases, TBE perhaps has the best incidence data across a range of scales, as well as ecological data, that permit detailed examination for causality. For several years, rising incidence of TBE throughout central Europe was attributed by many investigators to climate change (http://www.ecologyandsociety.org/vol2/iss1/art5/). During the mid-1980s, the incidence of TBE in Sweden increased from 2 to 5 per 100,000 population, and two opposing factors confounded epidemiologic analyses: an increase in roe deer density, to suggest a greater abundance of I. ricinus ticks and increased transmission, and the introduction of a TBE vaccine, suggesting greatly diminished risk. Even with confounding, a multiple regression analysis of meteorological data and TBE incidence suggested that a milder winter in the previous year, with 2 consecutive mild spring and fall seasons, predicted increased incidence.
However, climate change alone does not adequately explain the remarkably rapid increase in the incidence of TBE across much of Europe during the last few decades, particularly in the Baltic States (Figure A1-4); the factors that influence changes in TBE transmission, and ultimately human risk, appear to be more numerous and complex. What is known is that the risk of TBE in humans is dependent on the frequency of exposure to bites by infected ticks, which is dependent on human behaviour and on various biotic and abiotic factors, including climate (Rizzoli et al., 2009). Starting in 1989, mean springtime temperatures increased across the Baltics; however, the change in TBE incidence among these counties was spatiotemporally heterogeneous and inconsistent with regional weather phenomena (Sumilo et al., 2007). Simultaneously, a decline of collective farming in the post-communism Baltic States conceivably induced successional growth that promoted landscape changes, altering the fauna associated with I. ricinus ticks. In addition, berry picking, mushroom gathering, and other socioeconomically related food-seeking activities placing individuals in more frequent contact with tick-infested habitats are believed to have increased during this same period, as a result of economic changes associated with the fall of the Soviet Union. In this context, short-term climate changes that provided optimal growing conditions for mushrooms and berries in Baltic forests may indeed have been a driver for risk, but only in direct association with human behaviors that resulted in increased exposure to tick-infested habitats (Figure A1-6) (Randolph, 2008; Randolph et al., 2010).
Changes in Funding and Scientific Interest
Scientific, medical, or veterinary interest in a particular pathogen, or changes in the epidemiologic programs or organizational frameworks used to survey for a particular disease, may have enormous impact on the recorded incidence. By example, only 27 cases of Powassan encephalitis were
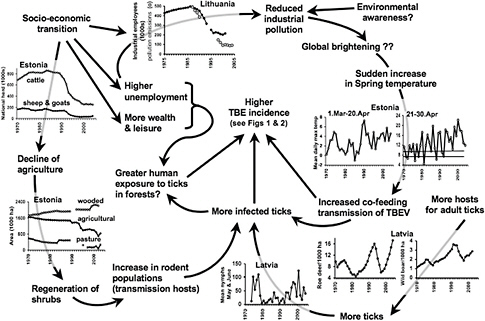
FIGURE A1-6 Hypothetical explanation for the upsurge in cases of tick-borne encephalitis in Estonia, Latvia, and Lithuania, following the end of Soviet rule (Šumilo et al., 2007)
reported in North America during the 3 decades after its discovery in 1958; however, the introduction of West Nile virus to the continent in the late 1990s stimulated enhanced surveillance for arthropod-borne encephalitis by state and local health departments, and is believed to be the major factor in the recognition of 20 U.S. cases of this disease during 1999-2009, including identifications from 4 northeastern and upper Midwestern states that had never previously reported the disease (Hinten et al., 2008; Hoang Johnson et al., 2010).
Prior to the early 1980s, research in tick-borne infections focused largely on RMSF, TBE, and diseases associated directly with animal health, such as babesiosis and theileriosis. The emergence of Lyme disease in the northeastern United States during the late 1970s, particularly at sites where an affluent population lived or vacationed, stimulated a renaissance in tick biology and ecology that was driven, in part, by increased availability of state and federal funds for Lyme disease research. A single individual with interest in a specific disease, particularly one that is otherwise infrequently diagnosed and seldom reported, can have tremendous impact on the incidence of that disease. By example, no cases of MSF from Algeria were documented in the medical literature until 1 clinician identified 93 cases during
a 4-month interval in 2004 (Mouffok et al., 2006). Austria and Slovenia have among the highest reported rates of Lyme disease in Europe, with an average annual incidence of 135 and 206 per 100,000 population, respectively (Smith and Takkinen, 2006); however, reporting in these countries is likely enhanced because of particularly energetic Lyme disease researchers who work in this region (Stanek and Strle, 2009). Conversely, it has been suggested that the precipitous drop in reported cases of RMSF witnessed in the United States during the 1950s might be attributable, in part, to the death in 1949 of R. R. Parker, who had been the driving force behind U.S. RMSF surveillance activities for more than 3 decades (Burgdorfer, 1975).
Active and Passive Surveillance
In the United States, surveillance data for tick-borne diseases are acquired voluntarily through separate but complementary reporting instruments that collectively comprise a national system of passive surveillance. The National Electronic Disease Surveillance System (NEDSS), (formerly the National Electronic Telecommunications System for Surveillance [NETSS]) represents the primary reporting instrument. Incidence statistics for all U.S. tick-borne diseases are calculated from electronically reported data; however, these systems acquire relatively limited supplemental information, so that detailed clinical data must be gathered by using other collection instruments, including an extended NEDSS record for Lyme disease, and a standardized case report form (CRF) for RMSF, ehrlichiosis, and anaplasmosis. State or local health departments are responsible for ensuring that cases reported to CDC through NEDSS meet the case definition for these diseases, while CRF data are generally screened at CDC for accuracy and consistency. Understandably, compliance with requests to physicians and state and local health department staff to provide supplemental data is problematic. By example, only 61% of Lyme disease cases reported to CDC during 1992-2006 contained data for reported signs and symptoms (Bacon et al., 2008). During 2000-2007, the number of CRFs submitted for RMSF was approximately 68% of the 11,531 cases reported through NETSS (Openshaw et al., 2010). Submitted CRFs often lack requested information necessary to confirm the diagnosis approved by the Council of State and Territorial Epidemiologists (CSTE) and CDC. Indeed, only 6% of RMSF CRF cases could be classified as confirmed during 2000-2007 (Openshaw et al., 2010). The non-submission of CRFs and the incompleteness of supplementary data collection suggest that a considerable percentage of cases tick-borne disease in the United States are unavailable for detailed analyses, including analysis of risk factors that determine severity (Childs and Paddock, 2002).
Case Definitions
Establishing an accurate and specific case definition that couples well-defined clinical characteristics with specific laboratory confirmation is fundamentally important in all forms of surveillance and provides the foundation from which subsequent epidemiologic parameters are derived. The absence of standardized case definitions for tick-borne diseases that cross regional or national borders remains a crucial problem in the accumulation of broad scale incidence data for many of these infections. Despite a system of mandatory reporting for TBE in Poland since 1970, no standardized case classification for this disease existed until 2005, creating a mix of confirmed, probable, or possible cases that were defined by clinical or laboratory criteria that differed among the county’s multiple provinces. When a national working group created a uniform case definition and retrospectively evaluated data from 1999-2002, they determined that only 25% of cases reported during this interval had sufficient laboratory testing to be classified as confirmed (Stefanoff et al., 2005).
Case definitions are not immutable, and as clinical knowledge about a particular disease expands and evolves, the case definition may be remodeled to include clinical or laboratory data specific to the disease. Because so many tick-borne infections are relatively new to science and medicine, it is not surprising that case definitions and surveillance systems for several of these diseases have required years or decades of refinement (Table A1-2). In this context, incidence statistics, particularly early in the evolution of these systems, should be interpreted cautiously, and comparisons of data from year-to-year, or even decade-to-decade, may be misleading. While passive surveillance requires a reasonably high level of stability to maintain its effectiveness, it must also remain malleable, and responsive to new information gained about a particular disease. By example, the nationally notifiable disease category of “Rocky Mountain spotted fever” was modified in 2010 to a less specific, but more inclusive and more accurate designation of “Spotted fever rickettsiosis, including Rocky Mountain spotted fever,” to adjust to recent data identifying causes of spotted fever group rickettsioses in the United States other than R. rickettsii (Council of State and Territorial Epidemiologists, 2009a).
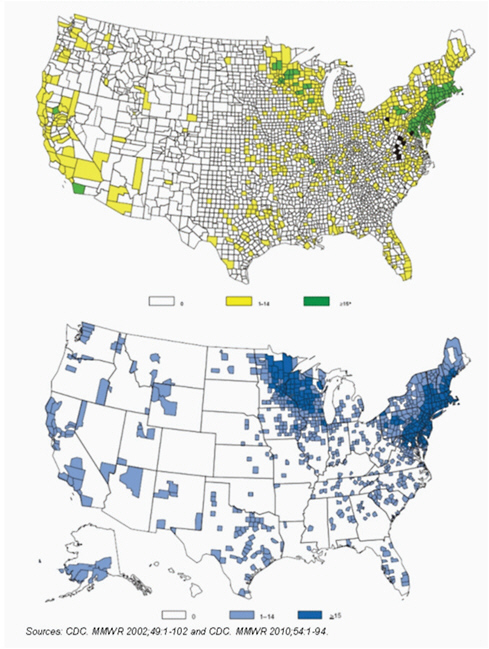
A well-crafted case definition provides more robust surveillance for the recognized disease and better positions investigators to detect clinically or epidemiologically similar diseases that otherwise might be embedded in data gathered by using a non-specific case definition. Erythema migrans rashes were noted on many patients in the southern and southcentral United States during the late 1980s (Masters et al., 1998), and in 1989, 715 cases of ‘Lyme disease’ were reported from Georgia (CDC, 1990), placing this state among the top 7 in the country for reporting Lyme disease. In the
TABLE A1-2 Changes in Case Definitions of Selected Nationally Notifiable Tick-Borne Diseases in the United States, 1996-2008
|
Disease (year of first case definition) |
Year of change(s) in case definition |
Change(s) |
Reference |
|
Lyme disease (1990) |
1996 |
Recommendation of 2-tiered approach for serologic confirmation. |
CDC, 1996a |
|
2008 |
Inclusion of western blot testing as a single confirmatory assay; addition of probable and suspect categories to case classification. |
CDC, 2008a |
|
|
Ehrlichiosis (1996) |
2000 |
Inclusion of Ehrlichia ewingii as an agent of ehrlichiosis; distinction between laboratory assays for Ehrlichia chaffeensis and Ehrlichia phagocytophila (Anaplasma phagocytophilum); formation of new reporting category, ehrlichiosis/anaplasmosis, human, undetermined. |
CDC, 2000 |
|
2008 |
Nomenclature change from human monocytic ehrlichiosis to E. chaffeensis infection; from ehrlichiosis (unspecified, or other agent) to E. ewingii infection; from human granulocytic ehrlichiosis to Anaplasma phagocytophilum infection; inclusion of ELISA format as laboratory criterion for diagnosis of probable infection. |
CSTE, 2009a |
|
|
Rocky Mountain spotted fever (1990) |
1996 |
Inclusion of PCR testing for laboratory confirmation; single titer of greater than or equal to 64 defined as laboratory criterion for a probable case. |
CDC, 1996b |
|
2004 |
Inclusion of ELISA format as laboratory criterion for diagnosis of confirmed or probable case; inclusion of immunohistochemical staining of tissue specimen as confirmatory laboratory criterion; cutoff titers used to determine probable cases defined by individual laboratories; elimination of Proteus OX-2 and OX-19 agglutinin titers as supportive laboratory criteria for a probable case. |
CDC, 2004 |
|
|
2008 |
Confirmation by serologic testing defined as fourfold change in IgG antibody titer; single elevated IgG or IgM titer sufficient for a probable case; ELISA format sufficient only to determine probable cases; inclusion of suspect case classification. |
CDC, 2008b |
|
|
2010 |
Change of disease category from “Rocky Mountain spotted fever” to “Spotted fever rickettsiosis”; inclusion of an eschar as clinical evidence of disease; requirement of PCR or cell culture isolation as laboratory confirmation of Rocky Mountain spotted fever. |
CSTE, 2009b |
following year, however, only 161 cases were reported from Georgia (CDC, 1991), and even fewer cases in subsequent years. The decline in reported cases reflected the adoption of the CDC surveillance case definition in 1990 by the Georgia Department of Public Health. The abundance of cases reported during 1989 most likely reflected a Lyme disease mimic, associated with bites of lone star ticks, now recognized as Masters’ disease, or southern tick-associated rash illness (Felz et al., 1999; Wormser et al., 2005).
In a similar scenario, investigators in southern Spain identified several patients with atypical ‘Lyme borreliosis,’ who were serologically reactive with Borrelia burgdoreferi antigens, but who lacked classical erythema migrans skin lesions and who originated from a region of the country where the recognized tick vector of Lyme borreliosis was distributed sparsely. Indeed, blood cultures subsequently revealed a relapsing fever Borrelia sp., genetically distinct from B. burgdorferi and transmitted by an entirely different tick species. In this case, discovery of a novel disease agent occurred because these patients did not meet the established case definition for Lyme borreliosis (Anda, et al., 1996; Guy, 1996). Recent discoveries of novel rickettsioses in the United States caused by R. parkeri and Rickettsia sp. 364D were precipitated by reports of atypical ‘RMSF,’ associated prominently with eschars, which are characteristically absent in the great majority of cases of classical RMSF (Paddock, 2008; Shapiro et al., 2010).
The efforts required to verify that reported data comply with an established case definition are considerable and are magnified further when clinical data and exposure history are uncoupled from laboratory results. Electronic laboratory reporting, used increasingly by states to expand case identification of Lyme disease, captures positive test results, but does not provide supportive information about clinical findings or exposure history. Instead, these data must be collected by public health personnel, creating added burden to surveillance endeavors that often exceeds investigative capacity (Kudish et al., 2007; CDC, 2008c). In response, CSTE Epidemiologists modified the national surveillance case definition for Lyme disease in 2007 to allow reporting of probable cases, i.e., those diagnosed by a health care provider and supported with laboratory evidence of infection (CDC, 2008a).
Strengths and Limitations of Passive and Active Surveillance
The most accurate incidence rates are obtained through active surveillance, but these apply to relatively small catchment areas defined for the investigation, and typically provide a snapshot of incidence in a specified region during a relatively short interval of time. This process allows greater control in the selection of clinical specimens and data collected, but is labor-intensive and requires a level of funding that is prohibitive to sustain
indefinitely. Indeed, most contemporary active surveillance endeavors are supported by federal grants and have a defined period of patient enrollment that spans, at most, only a few years (IJdo et al., 2000; Olano et al., 2003). In contrast, passive surveillance systems provide data that define endemicity and provide long-term trends over larger geographic regions; however, there is generally less control over the quality and quantity of the acquired data, and this activity requires sustained commitment and appropriate infrastructure at local, state, and national levels to collect, collate, and analyze data collected over broad intervals of time and space.
Inherent differences between these systems preclude direct comparisons of the data generated by each method. By example, prospective active surveillance for ehrlichiosis in southeast Missouri identified 29 confirmed and probable cases from 1997-1999, for a calculated average annual incidence of 3.2 cases per 100,000 population during this 3-year interval (Olano et al., 2003). By comparison, the average annual incidence of ehrlichiosis for the entire state of Missouri, determined by passive surveillance, was only 0.52 cases per 100,000 during 1997-2001 (Gardner et al., 2003), and 0.68 cases per 100,000 during 2001-2002 (Demma et al., 2005b). The nature of active and passive surveillance explains these marked differences in incidence rates. Catchment areas for active surveillance are not chosen at random; rather, these are selected by investigators using passive surveillance estimates that indicate the disease exists in relative abundance in that region (Wilfert et al., 1984; IJdo et al., 2000). In the case of the Missouri investigation, patient ascertainment was facilitated by a motivated clinician who was skilled at identifying potential cases of ehrlichiosis. Additionally, national surveillance for ehrlichiosis was initiated only in 1997, and several years of maturation may be required before passive surveillance systems reach a level of familiarity and frequent use by clinicians and epidemiologists.
Underreporting of true cases is a problem inherent to all passive surveillance systems and can be substantive: in the Marshfield Clinic Epidemiologic Research Study Area, only 34% of the identified Lyme disease cases were reported to the Wisconsin Department of Public Health during 1992-1998 (Naleway et al., 2002). Even for epidemiological statistics that document outcomes as important as death, there is considerable underreporting to public health authorities. From a capture-recapture study evaluating deaths caused by RMSF in the United States during 1983-1998, approximately 64% of fatal RMSF cases identified by death certificate data were not reported to state health departments or to CDC (Paddock et al., 2002)
Prospective cohort studies provide the best estimates of incidence but the resources that are needed to undertake such research preclude their use over larger scales. A good example of the value of a prospective study is that of the clinical trials for SmithKline Beecham’s Lymerix Lyme disease vaccine during 1994-1998. Phase II dose-ranging studies were done during
1994-1995 in coastal New England with 353 enrolled subjects. A conservative case definition was adopted, with definite Lyme disease comprising compatible clinical manifestations and laboratory confirmation. Cases of suspected symptomatic Lyme disease comprised erythema migrans without laboratory confirmation and compatible clinical manifestations without other plausible explanation. Such active case finding demonstrated an incidence of 3.4% (95% CI, 1.2-9.5) for either confirmed or suspected Lyme disease among the placebo subjects. This study cost $250,000 (Telford et al., unpublished). Starting in 1995, 10,936 subjects were enrolled in a controlled, double-blind, multicenter Phase III trial in 31 sites in 10 northeastern and mid-Atlantic states from Maine to Maryland (Steere et al., 1998). In the placebo group, the incidence of Lyme disease was 1.5% (95% CI, 1.2-1.9) during the first year, and 2.0% (1.6-2.4) in the second year. Of particular interest were the percentages of asymptomatic seroconversion (16% and 14%, respectively) (Smith et al., 2002). This expensive (>$5,000,000) trial provided our best estimates for Lyme disease incidence, as well as data on the asymptomatic to symptomatic ratio, a critical statistic to help define burden of disease.
Accuracy of Surveillance Data
Underreporting is invariably cited as a limitation to surveillance activities, but over-reporting may be even more damaging to epidemiologic assessments. One of the greatest obstacles to surveillance is ensuring that the data collected represent the disease under consideration. Blended data arise when a single common diagnosis is used to identify multiple related diseases caused by distinct pathogens. This process ultimately creates epidemiological havoc by producing incorrect distributions, hospitalization rates, and case-fatality rates, based erroneously on amalgamated characteristics of several individual diseases.
Most diagnoses of tick-borne infections are diagnosed, and subsequently categorized as ‘confirmed cases’ for epidemiologic analyses, by detecting antibodies in the serum of patients in whom the disease was suspected. Unfortunately, the antibodies generated by humans to specific pathogens often react with other closely related agents that are similar antigenically, but may cause illnesses that differ considerably in disease severity and clinical outcome. Overreliance on serologic methods is also the basis for many non-confirmed cases of tick-borne diseases. It has been demonstrated repeatedly that antibody responses for many of these diseases, including ehrlichiosis and RMSF, often require 7-10 days before a diagnostic titer is detected. Most patients may appear for care during the first few days of the illness, and may never return for subsequent evaluation. From 1 study, approximately two-thirds of culture-confirmed patients infected with
E. chaffeensis lacked diagnostic IgG titers, as measured by IFA, when they initially presented for care (Childs et al., 1999). Because diagnostic levels of IgG and IgM antibodies are frequently absent from the serum of patients who die from RMSF, fatal cases of this disease are often not confirmed if appropriate samples are not collected for immunohistochemical, molecular, or culture-based diagnotics (Paddock et al., 1999).
Diagnostically relevant levels of antibodies reactive with R. rickettsii have been detected in approximately 5%-10% of the U.S. population (Wilfert et al., 1985; Graf et al., 2008; Marshall et al., 2003; Taylor et al., 1985; Hilton et al., 1999). Even more troubling are the frequent descriptions of seroconversions to spotted fever group (SFG) Rickettsia and Ehrlichia spp. antigens that occur among as many as a 33% of healthy asymptomatic individuals following exposure to tick bites and tick-infected habitats. (Sanchez et al., 1992; Yevich et al., 1995; Hilton et al., 1999; McCall et al., 2001). Nonetheless, serology is used increasingly to diagnose cases of RMSF; as a result, fewer cases are confirmed and a far greater percentage of cases are considered probable (Figure A1-7). The impact of diagnostic inaccuracy upon epidemiologic observations may be considerable. During 2000-2007, the reported case fatality rate for RMSF in the United States was 0.5%, based on CRF denominator data comprising 7,796 cases, or approximately 1000 cases each year (Openshaw, 2010). The validity of this statistic is unreasonable when considered with historical U.S. case-fatality rates of RMSF that typically approach 10%. One explanation
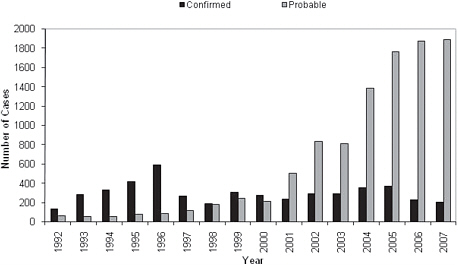
FIGURE A1-7 Reported cases of Rocky Mountain spotted fever in the United States, by case classification status, 1992-2007 (Openshaw et al., 2010).
for this estimate lies in the composition of the denominator, which is likely populated with patients with milder infections, caused by SFG Rickettsia species other than Rickettsia rickettsii (Paddock, 2009).
Under some circumstances, serological methods may produce results that divert attention from the true arthropod vector, creating a false portrait of the disease ecology. In this situation, a ‘tick-borne’ disease may in fact be something altogether different. By example, patients infected with Rickettsia felis, a flea-borne pathogen, generate antibodies that react with various SFG Rickettsia species. Because R. felis has a cosmopolitan distribution and commonly infects multiple species of wild and commensal human-biting fleas, (Reif and Macaluso, 2009), the potential for human infections is enormous. Recent studies in Kenya and Senegal revealed a prevalence of infection with R. felis in approximately 4% of 297 febrile patients from rural areas of these countries; only 1 patient from either series was infected with a tick-borne Rickettsia sp. (Socolovschi et al., 2010; Richards et al., 2010). To place these results in context, it is important to recognize that at least 7 pathogenic, tick-borne SFG Rickettsia species have been detected in ticks or human patients from the immense and ecologically diverse continent of Africa (Cazorla et al., 2008). If these researchers had not used molecular techniques to correctly identify R. felis as the causative agent, and relied only on serologic methods (as many investigators have done for >50 years), the etiology of the disease in these patients could have been ascribed erroneously to any of multiple tick-borne SFG rickettsial pathogens endemic to this continent. Broader use of similar techniques around the world might change considerably existing notions about the ecology, epidemiology, and clinical presentations of tick-borne rickettsioses.
Network Approaches for Detecting Shifts in Incidence and Disease Severity: Challenges, Prospects, and New Rubrics for Surveillance
Any form of surveillance requires sustained and coordinated efforts to provide meaningful data. As with all regional and national services, these programs require continuous funding and prospective governmental commitment, based on public health priorities defined by that state or country. Coordinated national efforts that track these diseases are notably absent in many developed countries. In Germany, an estimated 60,000 new cases of Lyme borreliosis occur each year; however, Lyme borreliosis is not a nationally notifiable disease in this country and precise incidence data do not exist (Mehnert and Krause, 2005). Many countries with active, internationally recognized research programs in rickettsioses, including Australia and France, lack formal national surveillance for tick-borne rickettsioses (Stephen Graves and Philippe Parola, pers. comms). Even in countries
where national surveillance exists for one or more of these diseases, the systems are often porous or lack the discriminatory power needed to epidemiologically characterize individual infections. In Italy, tick-borne rickettsioses are lumped in the general category of “rickettsiosis,” without respect to specific disease or arthropod vector species (Ciceroni et al., 2006). In Norway, only cases of disseminated or chronic Lyme borreliosis are notifiable to the Norwegian Institute of Public Health; the majority of infections, represented by erythema migrans, are not tabulated (Nygård et al., 2005).
Not surprisingly, tick-borne diseases are seldom included as nationally notifiable conditions in developing countries with considerable and diverse infectious disease burdens. In the southern Indian state of Karnataka, approximately 400-900 cases of KFD were reported annually during 2001-2004 (Pattnaik, 2006); however, the lack of national surveillance for this potentially lethal hemorrhagic fever has contributed to a belief that this disease is confined to a few small districts of a single state in this vast country. This seems highly improbable, considering that the virus as been isolated from or transmitted experimentally by at least 16 species of hard and soft ticks (Boshell, 1969; Pattnaik, 2006), and strains of the flavivirus responsible for the disease have been isolated from patients as far away as Saudi Arabia and Egypt (Mehla et al., 2009; Carletti et al., 2010), suggesting that variants of KFD might be found in other parts of India or other countries.
Expanding and Unifying Epidemiologic Coverage
More countries need to adopt national surveillance for tick-borne infections, and work to better harmonize and coordinate case definitions of diseases that cross national borders. Meaningful comparisons of incidence across broad geographical expanses are compromised when a melange of independent and varied case definitions exist for the same disease. Currently there are no standardized case definitions for CCHF notification or contact tracing in European countries, despite a vector tick (Hyalomma marginatum) that is distributed broadly across southern and southeastern Europe, and epidemic disease occurring in neighboring parts of Turkey (Figure A1-8), and in several Territories and Republics of the Russian Federation (Maltezou et al., 2010). From a recent survey of 21 European countries that compared national surveillance efforts for TBE, it was determined that case definitions differed widely across these countries, and for 6 countries where the disease is endemic, there was no officially or clearly formulated case definition (Mantke et al., 2008).
The European Union Concerted Action on Lyme Borreliosis (EUCALB) was established in 1997 to provide information on all aspects of Lyme disease, obtained from peer-reviewed literature and edited by a committee of experienced researchers and clinicians. Despite the intent to provide
guidance for obtaining and disseminating the highest standard of information, EUCALB has not been able to standardize surveillance across the European Union, and the compilation of European Union case definitions (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:159:0046:0090:EN:PDF) does not have an entry for Lyme borreliosis. Few European countries have made Lyme disease a compulsorily notifiable condition. Estimates of incidence for this and other tick-borne diseases in many countries are derived from laboratory-based reporting which typically lacks sufficient clinical information to integrate into a robust case definition. Accordingly, many of the same problems for Lyme disease surveillance in the United States are also seen for the European Union as a whole and for most of the member states.
Applying Species-Specific Diagnostics
Robust and predictive epidemiology is predicated on the use of sensitive and specific diagnostic assays. Currently, molecular techniques represent the most widely available standard for species-specific diagnosis. Whenever possible, culture isolation, the microbiological reference standard of diagnosis, should also be attempted to compliment molecular assays. Repeatedly, these methods have leveraged the discovery of novel tick-borne infections and clarified long-standing epidemiologic concerns regarding atypical clinical manifestations, unusual geographic distributions, or exaggerated or diminished severity of diseases that were incorrectly diagnosed. Diligent use of molecular and culture-based diagnostics during a study of 140 Portuguese patients with MSF enabled investigators to identify specifically a strain of R. conorii that was more frequently associated with severe disease in this patient population (de Sousa et al., 2008). The case-fatality rate in this series, determined by using accurate and specific diagnostic assays, was 21%, more than 8 times greater than the previously recognized lethality of this disease (Parola et al., 2005). Molecular techniques were used recently to identify infections with E. ewingii and E. chaffeensis in dogs and R. sanguineus ticks in Cameroon (Ndip et al., 2005: Ndip et al., 2010). These findings provided impetus to search for cases of ehrlichiosis in humans; surprisingly, 12 (10%) of 118 Cameroonian patients with undifferentiated febrile illnesses in whom malaria and typhoid were excluded showed PCR evidence of infection with E. chaffeensis (Ndip et al., 2009).
In Missouri, investigators used molecular methods to discriminate infections caused by E. ewingii from those caused by E. chaffeensis (Buller et al., 1999). Their discovery unveiled a second, clinically and ecologically similar illness, the identity of which was previously obscured because of sufficient overlap of disease manifestations and a shared tick vector. Indeed, surveys examining the relative prevalence of Ehrlichia spp. in reservoir
hosts and lone star ticks in the United States suggest that E. ewingii occurs in these species at frequencies similar to, or in some cases greater than, infection with E. chaffeensis (Paddock et al., 2005). However, E. ewingii appears to cause a milder illness, and most commonly causes disease in immmunosuppressed patients. Without molecular methods, these infections would have remained submerged among those caused by E. chaffeensis, contributing to a falsely heterogeneous portrait of E. chaffeensis ehrlichiosis. More recently, an Ehrlichia muris-like agent, identified by molecular methods from the blood of 4 patients in Minnesota and Wisconsin, is likely responsible for many other serologically diagnosed cases of ehrlichiosis in the upper Midwestern United States, where neither E. chaffeensis nor E. ewingii are endemic (McFadden et al., 2010).
Repeatedly, patients with antibodies reactive to multiple SFG Rickettsia spp. are found ultimately to be infected with unexpected agents when PCR and cell culture methods are used. In these circumstances, the diagnosis sheds light on clinical or epidemiologic characteristics not conventionally associated with the presumed pathogen, including occurrence of disease in a different geographical region, greater or lesser severity of illness, or presence or absence of prominent cutaneous manifestations. Just a few of the recent discoveries that exemplify the utility of these assays include Rickettsia heilongjiangensis in Japan, R. parkeri in the southeastern United States, Rickettsia sp. 364D in California, Rickettsia massiliae in Argentina, and Rickettsia sibirica mongolotimonae in southern France (Raoult et al., 1996; Paddock, 2009; Shapiro et al., 2010; Ando et al., 2010; Garciá-Garciá et al., 2010). Each of these diagnoses provides a foundation to explain more accurately the epidemiology of the historically recognized SFG rickettsioses for which these were initially confused.
Improved Integration of Entomologic and Veterinary Sciences
For decades, epidemiologists have integrated surveillance of non-human sources, such as mosquitoes, birds, and domesticated animals, in the detection of arboviruses. Similar efforts must be considered with tick-borne infections. Entomologic expertise ensures correct species identification of tick vectors, defines appropriate ecologic associations, and most importantly, provides data otherwise missing that establish the zoonotic bridge and better define incidence.
Epidemiological Entomology
Robust programs in medical entomology at state and national levels are essential to predict and respond to issues relating to tick-borne infections. In 2003, investigators in Mexicali, Mexico, conducted an entomologic
survey to determine the prevalence of R. sanguineus among 94 stray and privately owned dogs in the city, and determined that 60% of these animals were infested with R. sanguineus, a prevalence far greater than reported in other areas of Mexico and other parts of the world (Tinoco-Gracia et al., 2009). In retrospect, these results served as a tocsin to an epidemic of RMSF that occurred in Mexicali and other areas of Baja California during 2009, resulting in 275 confirmed and 734 probable infections (Bustamente Moreno and Pon Méndez, 2010a). In 2009, surveys for R. sanguineus identified these ticks in all 14 districts of Mexicali, where 96% of the cases occurred (Sanchez et al., 2009; Bustamente Moreno and Pon Méndez, 2010b). A survey for ticks on dogs from a small community in São Paulo, Brazil, in 2005, identified R. sanguineus-infested animals at approximately one-third of the households, including specimens infected with R. rickettsii. A canine serosurvey conducted in the same community one year later revealed that 70% of the sampled dogs showed high levels of antibodies reactive with R. rickettsii, and that human cases were also occurring (Morares-Filho et al., 2008).
Even in the absence of accurate incidence data, entomologic studies can provide objective and quantifiable data to reconcile a real or perceived public health burden. By example, a coastal Maryland community determined by questionnaire that Lyme disease afflicted more than 15% of their residents each year, creating apprehension among residents and local public health officials. Entomologic surveillance, in conjunction with a cross-sectional epidemiologic study, provided objective evidence that Lyme disease was actually rare, and disproportional to the perceived risk. In fact, the community did not know that it was plagued by an infestation of A. americanum ticks, that accounted for >90% of all ticks saved by residents, and the majority of ticks collected from vegetation (Armstrong et al., 2001).
Tick-borne infections may be thought of as guilds, or a group of species, not necessarily related, that utilize a common resource. The best example of a tick microbial guild may be found within deer ticks, comprising B. burgdorferi, Babesia microti, A. phagocytophilum, and deer tick virus (Telford et al. 1997). Globally, wherever there are related ticks from the Ixodes persulcatus complex, Lyme disease spirochetes, babesiae, Anaplasma sp., and a TBE- group virus may be found. Indeed, detecting one member of the guild is cause for search for all of the others (Telford and Goethert, 2008). Because concurrent or sequential infection with more than one agent is not infrequent (Krause et al., 1996) the guild model has epidemiologic implications. Accordingly, when a patient is diagnosed with Lyme disease, evidence of babesiosis and anaplasmosis should also be sought. When Nantucket Cottage Hospital started using a clinical laboratory
that automatically tested for direct molecular evidence of infection with B. burgdorferi, B. microti, and A. phagocytophilum of all patients with a suspected tick-borne illness, the number of confirmed cases of babesiosis and anaplasmosis immediately doubled (T. J. Lepore, pers. comm.).
Natural cycles of known pathogens do not necessarily imply a zoonotic risk to humans. By example, Ixodes dentatus feeds primarily on rabbits, and only rarely bites humans, yet maintains a diverse guild of pathogens and potential pathogens comprising Borrelia andersoni, B. microti, a Babesia divergens-like parasite, A. phagocytophilum, Anaplasma bovis, and a Kemerovo-group orbivirus in northeastern U.S. sites (Telford and Spielman 1989; Goethert and Telford, 2003 a, b, c). However, this guild has minimal epidemiological importance, even when sympatric with human-biting deer ticks, because deer ticks rarely feed on rabbits. Ticks, similar to all multicellular organisms, harbor a diverse array of microbes, and novel high-throughput DNA amplification and sequencing techniques are unveiling the complex microbiome of ticks (Benson et al., 2004; Clay et al., 2008); however, and public health relevance of an agent detected in a tick should never be assumed a priori.
Currently applied entomological risk indices include only the prevalence of infection in host-seeking ticks and the number of ticks collected in an hour (Piesman et al., 1987). However, an epidemiologically predictive risk index will require information on the species of ticks infesting humans, how frequently these attach, and the actual duration of attachment. Although B. burgdorferi typically infects 20% of host-seeking nymphal deer ticks in northeastern United States, the median annual incidence of Lyme disease from 9 prospective studies in the most intensely enzootic communities is only 2% (range, 0.4%-4.0%) (Hanrahan et al., 1984; Steere et al., 1986; Lastavica et al., 1989; Alpert et al., 1992; Shapiro et al., 1992; Krause and Telford, unpublished; Wormser et al., 1998; Steere et al., 1998; Sigal et al., 1998). In addition, fewer than 10% of all Lyme disease cases in the northeastern United States are reported during the fall or winter months when adult deer ticks are most active, even though as many as 50%-75% of host-seeking adult ticks contain spirochetes (Piesman et al., 1986). These apparent paradoxes can be answered by entomology: most tick-borne bacterial pathogens require a period of ‘reactivation’ before they attain infectivity (Spencer and Parker, 1923; Piesman et al. 1987; Katavolos et al., 1997). The probability of infection is directly proportional to the duration of feeding and may differ by life stage of the tick, so that 24-48 hours of attachment are generally required for B. burgdorferi to be transmitted by nymphal deer ticks; in contrast, adult deer ticks require approximately 3-5 days of attachment to transmit B. burgdorferi to a susceptible host (Telford, unpublished).
Sentinel Species for Tick-Borne Disease
Despite repeated successes using wildlife and domesticated animals as sentinel species for tick-borne infections, these resources remains under-utilized in formal surveillance programs around the world. Because domestic dogs are frequently parasitized by human-biting ticks, develop robust antibody titers to most agents, are closely associated with human habitation, and are easily sampled, these animals represent exceptional sentinel species for tick-borne pathogens. This technique has been used effectively to predict or corroborate the occurrence, or in some cases, relative absence, of disease burden in human populations, including spotted fever group rickettsiosis in southeastern Australia (Sexton et al., 1991), and Lyme disease and anaplasmosis in the United States (Guerra et al., 2001; Hinrichsen et al., 2001; Duncan et al., 2004; Bowman et al., 2009).
With respect to infections caused by SFG rickettsiae, canine antibodies typically show greater specificity to the infecting agent than to other, antigenically related Rickettsia spp. (Nicholson et al., 2006; Demma et al., 2006; Piranda et al., 2008), and may provide greater accuracy than results of human antibody assessments in serologic surveys. Retrospective analysis of 329 archival canine serum specimens, collected from dogs in a community in the White Mountain region of eastern Arizona during 1996, revealed epidemiologically relevant titers of antibodies reactive with R. rickettsii in only 2 (0.6%). When dogs were tested 7 years later, following recognition of an RMSF epidemic in this community, 70 (72%) of 97 animals demonstrated epidemiologically relevant antibody titers, and the geometric mean titer was > 84 times higher than observed in 1996 (Demma et al., 2006). Of even greater interest, high antibody titers were also detected in 8 (57%) of 14 dogs sampled at a second community, 60 miles distant, where no cases of RMSF had been reported; however, the following year, an outbreak of RMSF occurred in the second community, involving 9 residents and causing 2 deaths. This approach was later applied to serosurvey of dogs in counties adjacent to the outbreak communities, where approximately 6% of animals demonstrated antibodies to R. rickettsii, primarily among R. sanguineus-infected dogs, suggesting future cases of human disease outside of the recognized boundaries established during the initial outbreak (McQuiston et al., 2009).
Because white-tailed deer are important hosts for several species of ticks that transmit infectious agents to humans, these animals have been used effectively in the United States as sentinels for multiple tick-borne infections, including E. chaffeensis ehrlichiosis and Lyme disease. Among the advantages cited for this species include an extensive and inclusive distribution throughout the range of these diseases, the relatively sedentary nature of deer, rates of exposure to ticks that far exceed those of human
exposures, and regulated harvests in all states that facilitates collection of samples from hunter-killed animals (Yabsley et al., 2003; Gill et al., 1994).
Prospecting Other Databases
Tick-borne infections can mimic many other infectious diseases, including meningococcemia, leptospirosis, hantavirus pulmonary syndrome, dengue, and malaria. Recent investigations in Senegal and Togo suggest that tick-borne relapsing fever may be a common cause of fever in many parts of West Africa; however, the diagnosis is seldom considered, because many cases are misdiagnosed with malaria. Indeed, several studies have identified infections with Borrelia spp. in as many as 10% of febrile patients (Vial et al., 2006; Nordstrand et al., 2007).
From a 1993 study of dengue fever in Yucatan and Jalisco states of Mexico, 50 patients with a recent compatible illness had no serologic evidence of infection with dengue virus. Of these, 20 had high levels of antibody reactive to SFG rickettsiae (Zavala-Velazquez et al., 1996). A similar approach was used for a study in Colombia, when 158 serum samples collected from febrile patients during 2000-2004 as part of national or regional surveillance for malaria, dengue, or yellow fever were evaluated for SFG rickettsiae and 21% showed antibodies suggesting recent infection with a Rickettsia species (Hildago et al., 2007).
Evaluations of databases comprising those patients negative for a particular tick-borne disease of interest may be especially fruitful, as many of these patients have been bitten by ticks, reside in tick-infested areas, or present during periods of peak tick activity. From a study conducted by investigators at CDC, paired serum samples collected from 3 (10%) of 29 patients for whom RMSF was suspected, but subsequently showed no serologic evidence of acute disease, were determined retrospectively to have seroconversions to Ehrlichia antigens, and corroborative clinical evidence of ehrlichiosis (Fishbein et al., 1987). A novel bunyavirus, transmitted by Haemaphysalis longicornis ticks, was recently identified as the cause of a life-threatening disease (severe fever with thrombocytopenia syndrome), confirmed in 171 patients from six provinces in Central and Northeast China. Clinical similarities with human anaplasmosis, and an assosciation with tick bites, focused initial investigations on A. phagocytophilum as the suspect pathogen; however, when molecular and serologic assays failed to identify this agent with the outbreak, culture of clinical specimens in multiple permissive cell lines, eventually yielded isolates of a previously undescribed phlebovirus (Yu et al., 2011).
Every year, spider bite statistics are collected by poison control centers across the United States; however, the accuracy of these data have been questioned by prominent arachnologists, who argue that hundreds
of reports of necrotic arachnidism attributed to the brown recluse spider (Loxosceles reclusa) originate from areas where these spiders are absent (Vetter and Furbee, 2006), including much of Georgia, Florida, and South Carolina (Vetter et al., 2004; Vetter et al. 2009). Because necrotic arachnidism is occasionally considered by physicians as a diagnosis for patients with eschar-associated rickettsioses (Paddock et al., 2008) and Lyme disease (Rosenstein and Kramer, 1987; Osteroudt et al., 2002), closer inspection of these databases could uncover many previously undiagnosed and uncounted cases of tick-borne disease. This approach could also yield interesting results in other parts of the world, including Australia and Brazil, where necrotic skin lesions are also often attributed to bites from spiders that do not exist in the reporting area (Ibister, 2004).
Extracting New Data from Existing Surveillance Systems
Increasingly, physicians and scientists are identifying specific genetic characteristics and co-morbid or infectious conditions in patients that predispose these individuals to particularly severe forms of certain tick-borne infection. These include life-threatening disease in HIV-infected patients who become co-infected with E. chaffeensis, fulminant R. rickettsii and R. conorii infections in patients with glucose-6-phosphate dehydrogenase deficiency, fatal MSF in alcoholics, and increased severity of TBE in patients with a specific deletion in the chemokine receptor CCR5 (Walker et al., 1983; Raoult et al., 1986; Paddock et al., 2001; Kindberg et al., 2008; de Sousa et al., 2008). These observations have been identified in relatively small cohorts of patients who have been evaluated by relatively few clinicians who become cognizant of a specific characteristic that places these patients at increased risk for more severe infection; to identify similar characteristics among the total number of counted cases in a state or country poses a great challenge for future surveillance efforts (Childs and Paddock, 2002).
Age-specific incidence data gained from the first several years of national surveillance (Demma et al., 2005b; CDC, unpublished data) for ehrlichiosis and anaplasmosis show a striking age-related increase in frequency of these infections among older persons (Figure A1-9). One plausible hypothesis for this observation suggests that cholesterol dependence by E. chaffeensis and A. phagocytophilum may correlate with greater disease severity in older patients, because cholesterol levels typically rise with increasing age, and these bacteria lack all of the genes necessary for the biosynthesis of lipid A (Lin and Rikihisa, 2003). A direct association between cholesterol levels and the clinical severity of other forms of gram-negative sepsis add support to this hypothesis (Ayyadurai et al., 2010); in this context, serum cholesterol levels, collected as supplemental data on CRFs, could ultimately provide important clues to the pathogenesis of these diseases.
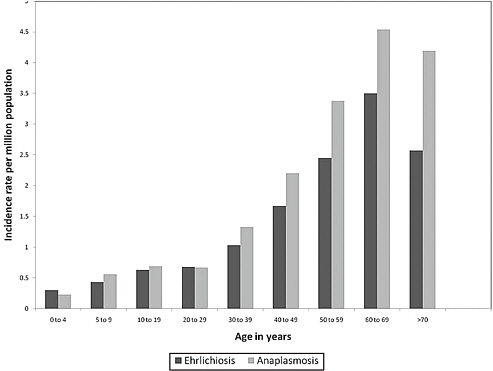
FIGURE A1-9 Age-specific incidence of human ehrlichiosis (n = 3,104) and human anaplasmosis (n = 4,134) in the United States, 2000-2007 (Dahlgren et al., in press).
In Silico Analytic Methods
Molecular phylogenetic tools have refined our understanding of the etiology of tick-borne infections. Advances in data analysis, made possible by tremendous computing power, should provide similar refinement of our understanding of transmission dynamics and environmental correlates of risk. Predictive models for guiding intervention and proactively studying new zoonotic sites are currently untenable. Validation of these models would require incidence data at the same scale as that for enzootic parameters; it is not axiomatic that the case counts for an entire state would be predicted by regressors, such as an entomological risk index, estimated from solely one town in that state. Although some analyses demonstrate correlations between measured tick density and Lyme disease case reports (Stafford et al., 1998; Mather et al., 1996), a priori predictions are not currently possible.
Vector-borne infections are complex to model. Sir Ronald Ross initiated mathematical modeling for malaria risk in 1909, but it was not until coordinated efforts in the 1950s (MacDonald, 1957) promoted enough quantitative understanding of malaria epidemiology that the effects of
interventions such as vaccination could be predicted (Molineaux, 1985). In this respect, considerably more attention needs to be directed toward studies that provide data for quantitative models of tick-borne diseases. These efforts could also help develop testable hypotheses on critical aspects of tick life cycles or pathogen transmission cycles. Given the difficulty of finding study sites for longitudinal ecology study of R. rickettsii, for example, the role of transovarial transmission in perpetuating this agent in nature could be explored by modeling (Telford, 2009).
Great advances in remote sensing of environmental parameters and data analysis algorithms such as Geographic Information Systems (GIS) may allow prediction of the distribution of infection, or even incidence. However, given the extremely focal nature of transmission for most tick-borne infections, the utility of GIS would be a function of the scale of the analyses, which would depend on the availability of satellite data of a useful scale. For GIS analyses, as with mathematical models, the bridge from nature to human risk requires the filter of human behaviors; in the absence of this filter, associations of environmental variables with incidence data, even when available at the correct scale and duration, are less robust. In this context, GIS analyses are potentially powerful tools, whose practical utility are currently limited by our understanding of the basis for and measurement of human risk.
Summary and Perspective
Tick-borne diseases are the Cinderella of vector-borne disease (VBD) systems: always beautiful, but for so long over-shadowed by the big ugly sisters of insect-borne malaria, trypanosomiasis, dengue, etc. In traditional happy-ending style, they are now emerging as the princess of VBDs, their full significance appreciated: as important in temperate as tropical regions, as much a veterinary as a medical problem to fit neatly into the ‘one world, one health’ maxim, and with an exquisite complexity to keep many a scientist fully occupied over a lifetime.
S. E. Randolph, 2009b
Predicting the future is an imprecise science; nonetheless, a clear association exists between human disturbance of natural habitats and the emergence and expansion of tick-borne diseases, and it is precisely this link that portends important increases in the scope and range of these zoonoses in the years to come. Remarkable scientific and medical discoveries during the last several decades have expanded considerably the known spectrum of tick-borne infections. Fundamental surveillance efforts for these infections, curiously, have not made similar strides. There are many practical reasons
to explain the paucity of effective surveillance systems for tick-borne diseases around the world, including perceived need, long-term interest, and monetary commitments necessary to sustain these programs. These factors affect the longevity necessary for fine-tuning of case definitions and collection instruments, and ultimately, meaningful interpretations of the collected data; however, as more unique pathogens are identified, distinctions among the diseases caused by these agents may become increasingly subtle. In the end, the effort required to discover a new disease is generally far less than the subsequent endeavors required to establish and maintain long-lasting and accurate surveillance for that disease.
The measurement of incidence is important because it can be used to quantify and rank the causes of ill health so that scarce resources may be allocated. Incidence is critical to refine and validate predictive or explanatory models, which help develop new testable hypotheses or identify critical breakpoints. Changes in incidence may point to fundamental alterations of ecology or human behavior. Measuring incidence accurately, however, can be prohibitively expensive, as seen with the Lymerix Phase III trials. Indeed, surveillance will become increasingly important in the places that have the least existent infrastructure and the fewest resources to devote to establishing such programs. In Africa, scientific and medical concerns for tick-borne diseases are vastly overshadowed by other arthropod-borne infections; nonetheless, the unrecognized burden of these infections could be considerable. By example, the incidence of tick-borne relapsing fever in Senegal has been estimated at 5.1% (Trape et al., 1996), and the spread of infections with Ehrlichia spp. among an expanding and highly vulnerable HIV-infected cohort on this continent could have devastating consequences (Ndip et al., 2009).
Our challenge is to improve the measurement of incidence within the limits of available resources and capacities. With technological advances, global disparities in the capacity to collect this information may lessen; today, even the poorest countries are rapidly gaining access to thermal cyclers and the Internet, and the coverage of such critical technology will only increase. In the interim, our use of the currently available data—a dark glass—can be enhanced if we understand its limitations and frame our interpretations accordingly.
Acknowledgments
We thank the following individuals who generously provided data and input to this work: Marcelo Labruna (Cidade Universitária São Paulo, São Paulo, Brazil); Elba de Lemos (Instituto Oswaldo Cruz, Rio de Janeiro, Brazil); Rita de Sousa (Instituto Nacional de Saúde Dr Ricardo Jorge, Lisboa, Portugal); José Oteo (Hospital San Pedro, Logroño, Spain); Shuji
Ando (National Institute of Infectious Diseases, Tokyo, Japan); Agustín Estrada-Pena (University of Zaragoza, Zaragoza, Spain); Claudia Colomba (Universita di Palermo, Palermo, Italy); Philippe Parola (Université de la Mediterannée, Marseille, France); Robert Swanepoel (National Institute for Communicable Diseases, Sandringham, South Africa); Stephen Graves (Hunter Area Pathology Service, New South Wales, Australia); Scott O’Dee and Richard Gary (Zoonotic Disease Program, Ohio Department of Health), and; Scott Dahlgren and Robert Holman (Division of High Consequence Pathogens and Pathology, CDC). We are indebted to Paul Mead and Jennifer McQuiston (Division of Vector-borne Diseases, CDC) for their thoughtful review and invaluable comments to the manuscript. SRT is supported by NIH R01 AI 064218.
References
Alpert, B., J. Esin, S. L. Sivak, and G. P. Wormser. 1992. Incidence and prevalence of Lyme disease in a suburban Westchester County community. New York State Journal of Medicine 92: 5-8.
Anda, P., W. Sánchez-Yebra, M. del Mar Vitutia, et al. 1996. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet 348: 162-141.
Ando, S., M. Kurosawa, A. Sakata, et al. 2010. Human Rickettsia heilongjiangensis infection, Japan. Emerging Infectious Diseases 16: 1306-1308.
Angerami, R. N., M. R. Resende, A. F. C. Feltran, et al. 2006. Brazilian spotted fever: a case series from an endemic area in southeastern Brazil. Epidemiologic aspects. Annals of the New York Academy of Sciences 1078: 170-172.
Anonymous. 1994. Leishmaniasis. Pan American Health Organization Epidemiological Bulletin 15: 8-11.
Anonymous. 1999. Japanese spotted fever. Infectious Agents Surveillance Report 20: 211-212.
Anonymous. 2006. Scrub typhus and Japanese spotted fever in Japan, as of December 2005. Infectious Agents Surveillance Report 27: 27-29.
Anonymous. 2010. Scrub typhus and Japanese spotted fever in Japan 2006-2009. Infectious Agents Surveillance Report 31: 120-122.
Anthony, J. A., J. E. Childs, G. E. Glass, G. W. Korch, L. Ross, and J. K. Grigor. 1990. Land use associations and changes in population indices of urban raccoons during a rabies epizootic. Journal of Wildlife Diseases 26: 170-179.
Armstrong, P. M., L. R. Brunet, A. Spielman, and S.R. Telford III. 2001. Risk of Lyme disease: perceptions of residents of a lone star tick-infected community. Bulletin of the World Health Organization 79: 916-925.
Ayyadurai, S., H. Lepidi, C. Nappez, D. Raoult, and M. Drancourt. 2010. Lovastatin protects against experimental plague in mice. PLoS One 5: e10926.
Bacon, R. M., K. J. Kugler, and P. S. Mead. 2008. Surveillance for Lyme disease—United States, 1992-2006. MMWR 57(No. SS-10): 1-9.
Battaly, G. R. and D. Fish. 1993. Relative importance of bird species as hosts for immature Ixodes dammini (Acari: Ixodidae) in a suburban residential landscape of southern New York. Journal of Medical Entomology 30: 740-747.
Benson, M. J., J. D. Gawronski, D. E. Eveleigh, and D. R. Benson. 2004. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket
and Wellfleet, Cape Cod, Massachusetts. Applied and Environmental Microbiology 70: 616-620.
Boshell, J. M. 1969. Kyasanur forest disease: ecologic considerations. American Journal of-Tropical Medicine and Hygiene 18: 67-80.
Bowman, D., S. E. Little, L. Lorentzen, J. Shields, M. P. Sullivan, and E. P. Carlin. 2009. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Veterinary Parasitology 160: 138-148.
Buller, R. S., M. Arens, S. P. Himmel, et al. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. New England Journal of Medicine 341: 148-155.
Burgdorfer, W. 1975. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. Journal of Medical Entomology 12: 269-278.
Bustamente, M. E., and G. Varela. 1947. IV. Estudios de fiebre manchada en Mexico. Papel del Rhipicephalus sanguineus en la transmisión de la fiebre manchada en la República Mexicana. Revista del Instituto de Salubridad y Enfermadades Tropicales 8: 138-141.
Bustamente Moreno, J. G., and A. Pon Méndez. 2010a. Actualización en la vigilancia epidemiológica de “rickettsiosis”. Part I. Epidemiológia Boletin 6: 1-4.
Bustamente Moreno, J. G., and A. Pon Méndez. 2010b. Actualización en la vigilancia epidemiológica de “rickettsiosis”. Part II. Epidemiológia Boletin 7: 1-3.
Carletti, F., C. Castilletti, A. Di Caro, et al. 2010. Alkhurma hemorrhagic fever in travelers returning from Egypt, 2010. Emerging Infectious Diseases 16: 1979-1982.
Cazorla, C., C. Socolovschi, M. Jensenius, and P. Parola. 2008. Tick-borne diseases: tickborne spotted fever rickettsioses in Africa. Infectious Disease Clinics of North America 22: 531-544.
Centers for Disease Control and Prevention. 1990. Lyme disease surveillance—United States, 1989-1990. MMWR 39:397-399.
Centers for Disease Control and Prevention. 1991. Lyme disease surveillance—United States, 1990-1991. MMWR 40: 417-421.
Centers for Disease Control and Prevention. 1996a. Lyme disease (Borrelia burgdorferi) 1996 case definition. http://www.cdc.gov/ncphi/disss/nndss/casedef/lyme_disease_1996.htm.
Centers for Disease Control and Prevention. 1996b. Rocky Mountain spotted fever (Rickettsia rickettsii) (RMSF) 1996 case definition. http://www.cdc.gov/ncphi/disss/nndss/casedef/rocky_mountain_spotted_fever_1996.htm.
Centers for Disease Control and Prevention. 2000. Ehrlichiosis (HGE, HME, other or unspecified) 2000 case definition. http://www.cdc.gov/ncphi/disss/nndss/casedef/ehrlichiosis_2000.htm.
Centers for Disease Control and Prevention. 2002. Tularemia—United States, 1990-2002. MMWR 51:182-184.
Centers for Disease Control and Prevention. 2004. Rocky Mountain spotted fever (Rickettsia rickettsii) (RMSF) 2004 case definition. http://www.cdc.gov/ncphi/disss/nndss/casedef/rocky_mountain_spotted_fever_2004.htm.
Centers for Disease Control and Prevention. 2008a. Lyme disease (Borrelia burgdorferi) 2008 case definition http://www.cdc.gov/ncphi/disss/nndss/casedef/lyme_disease_2008.htm.
Centers for Disease Control and Prevention. 2008b. Rocky Mountain spotted fever (RMSF) (Rickettsia rickettsii) 2008 case definition. http://www.cdc.gov/ncphi/disss/nndss/casedef/rocky_mountain_spotted_fever_2008.htm.
Centers for Disease Control and Prevention. 2008c. Effect of electronic reporting on the burden of Lyme disease surveillance—New Jersey, 2001-2006. MMWR 57: 42-45.
Charrel, R. N., H. Attoui, and A. M. Butenko. 2004. Tick borne virus diseases of human interest in Europe. Clinical Microbiology and Infection 10: 1040-1055.
Chen, H., D. J. White J, T. B. Caraco, and H. H. Stratton . 2005. Epidemic and spatial dynamics of Lyme disease in New York State, 1990-2000. Journal of Medical Entomology 42:899-908.
Childs, J. E., and C. D. Paddock. 2002. Passive surveillance for Rocky Mountain spotted fever: is there more to learn? American Journal of Tropical Medicine and Hygiene 66: 450-457.
Childs, J. E., J. W. Sumner, W. L. Nicholson, R. F. Massung, S. M. Standaert, and C. D. Paddock. 1999. Outcome of diagnostic tests using samples from patients with culture-proven human monocytic ehrlichiosis: implications for surveillance. Journal of Clinical Microbiology 37: 2997-3000.
Ciceroni, L., A. Pinto, S. Ciarrochi, and A. Ciervo. 2006. Current knowledge of rickettsial diseases in Italy. Annals of the New York Academy of Sciences 1078: 143-149.
Clay, K., N. Klyachko, D. Grindle, et al. 2008. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Molecular Ecology 17: 4371-4381.
Council of State and Territorial Epidemiologists. 2009a. Position statement 09-ID-15. Public health reporting and national notification for human ehrlichioses and anaplasmosis. http://www.cste.org/ps2009/09-id-15.pdf.
Council of State and Territorial Epidemiologists. 2009b. Position statement 09-ID-16. Public health reporting and national notification for spotted fever rickettsiosis (including Rocky Mountain spotted fever). http://www.cste.org/ps2009/09-id-16.pdf.
Dahlgren, F. S., E. Mandel, J. Krebs, R. Massung, and J. H. McQuiston. Surveillance for infections with Ehrlichia chaffeensis and Anaplasma phagocyophilum in the United States, 2000-2007. In press. American Journal of Tropical Medicine and Hygiene.
Dandawate, C. N., G. B. Desai, T. R. Achar, and K. Banerjee. 1994. Field evaluation of formalin inactivated Kyasanur forest disease virus tissue culture vaccine in three districts of Karnataka state. Indian Journal of Medical Research 99: 152-158.
Dautel, H., C. Dippel, R. Oehme, K. Hartlet, and E. Schettler. 2006. Evidence for an increased geographical distribution of Dermacentor reticularis in Germany and detection of Rickettsia sp. RpA4. International Journal of Medical Microbiology 296: 149-156.
de Sousa, R., S. Dória Nóbrega, F. Bacellar, and J. Torgal. 2003. Sobre a realidade da febre escaro-nodular em Portugal. Acta Médica Portuguesa 16: 429-436.
de Sousa, R., A. França, S. D. Nòbrega, et al. 2008. Host- and microbe-related risk factors for and pathophysiology of fatal Rickettsia conorii infection in Portuguese patients. Journal of Infectious Diseases 198: 576-585.
Demma, L. J., M. S. Traeger, W. L. Nicholson, et al. 2005a. Rocky Mountain spotted fever from an unexpected vector in Arizona. New England Journal of Medicine 353: 587-594.
Demma, L. J., R. C. Holman, J. H. McQuiston, J. W. Krebs, and D. L. Swerdlow. 2005b. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001-2002. American Journal of Tropical Medicine and Hygiene 73: 400-409.
Demma, L. J., M. Traeger, D. Blau, et al. 2006. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector-Borne and Zoonotic Diseases 6: 423-429.
Duncan, A. W., M. T. Correa, J. F. Levine, and E. B. Breitschwerdt. 2004. The dog as a sentinel for human infection: prevalence of Borrelia burgdorferi C6 antibodies in dogs from the southeastern and mid-Atlantic states. Vector-Borne and Zoonotic Diseases 4: 221-229.
Ebel, G. 2010. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annual Review of Entomology 55: 95-110.
Espejo Arenas, E., B. Font Creus, F. Bella Cueto, and F. Segura Porta. 1986. Climatic factors in resurgence of Mediterranean spotted fever. Lancet 1: 1333.
Estrada-Peña, A., Z. Vatansever, A. Gargili, and Ö. Ergönul. 2010. The trend towards habitat fragmentation is the key factor driving the spread of Crimean-Congo haemorrhagic fever. Epidemiology and Infection 138: 1194-1203.
Felsenfeld, O. 1971. Borrelia: strains, vectors, human and animal borreliosis. W. H. Green, St. Louis, MO.
Felz, M. W., F. W. Chandler Jr, J. H. Oliver Jr. 1999. Solitary erythema migrans in Georgia and South Carolina. Archives of Dermatology 135: 1317-1326.
Fishbein, D. B., L. A. Sawyer, C. J. Holland, et al. 1987. Unexplained febrile illnesses after exposure to ticks: infection with an Ehrlichia? Journal of the American Medical Association 257: 3100-3104.
Garciá-Garciá, J. C., A. Portillo, M. J. Núñez, et al. 2010. Case report: a patient from Argentina infected with Rickettsia massiliae. American Journal of Tropical Medicine and Hygiene 82: 691-692.
Gardner, S. L., R. C. Holman, J. W. Krebs, R. Berkelman, and J. E. Childs. 2003. National surveillance for the human ehrlichioses in the United States, 1997-2001, and proposed methods for evaluation of data quality. Annals of the New York Academy of Sciences 990: 80-89.
Gill, J. S., R. G. McLean, R. B. Shriner and R. C. Johnson. 1994. Serologic surveillance for the Lyme disease spirochete, Borrelia burgdorferi, in Minnesota by using white-tailed deer as sentinel animals. Journal of Clinical Microbiology 32: 444-451.
Ginsberg, H. S. 1993. Geographical spread of Ixodes dammini and Borrelia burgdorferi. In Ginsberg, H.S. (ed.) Ecology and environmental management of Lyme disease. Rutgers University Press, New Brunswick, NJ. pp. 63-82.
Goethert, H. K., and S. R. Telford III. 2003a. Enzootic transmission of the agent of human granulocytic ehrlichiosis among cottontail rabbits. American Journal of Tropical Medicineand Hygiene 68: 633-637.
Goethert, H. K., and S. R. Telford III. 2003b. Enzootic transmission of Babesia divergens among cottontail rabbits on Nantucket Island, Massachusetts. American Journal of Tropical Medicine and Hygiene 69: 455-460.
Goethert, H. K., and S. R. Telford III. 2003c. Enzootic transmission of Anaplasma bovis in Nantucket cottontail rabbits. Journal of Clinical Microbiology 41: 3744-3747.
Graf, P. C. F., J. P. Chretien, L. Ung, J. C. Gaydos, and A. L. Richards. 2008. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse U.S. sample. Clinical Infectious Diseases 46: 70-77.
Gross, E. M., P. Yagupsky, V. Torok, and R. A. Goldwasser. 1982. Resurgence of Mediterranean spotted fever. Lancet 2: 1107.
Guerra, M. A., E. D. Walker, and U. Kitron. 2001. Canine surveillance system for Lyme borreliosis in Wisconsin and northern Illinois: geographic distribution and risk factor analysis. American Journal of Tropical Medicine and Hygiene 65: 546-552.
Guerra, M. A., A. T. Curns, C. E. Rupprecht, C. A. Hanlon, J. W. Krebs, and J. E. Childs. 2003. Skunk and raccoon rabies in the eastern United States: temporal and spatial analysis. Emerging Infectious Diseases 9: 1143-1150.
Guy, E. 1996. Epidemiological surveillance for detecting atypical Lyme disease. Lancet 348: 141-142.
Hamer, S. A., J. I. Tsiao, E. D. Walker, and G. J. Hickling. 2010. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicty. EcoHealth doi: 10.1007/s10393-010-0287-0.
Hanrahan, J. P., J. L. Benach, J.L. Coleman, et al. 1984. Incidence and cumulative frequency of endemic Lyme disease in a community. Journal of Infectious Diseases 150: 489-496.
Hildago, M., L. Orejuela , P. Fuya, et al. 2007. Rocky Mountain spotted fever, Colombia. Emerging Infectious Diseases 13: 1058-1060.
Hilton, E., J. DeVoti, J. L. Benach, et al. 1999. Seroprevalence and seroconversion for tickborne diseases in a high-risk population in the Northeast United States. American Journal of Medicine 106: 404-409.
Hinrichsen, V. L., U. G. Whitworth, E. B. Breitschwerdt, B. C. Hegarty, and T. N. Mather. 2001. Assessing the association between the geographic distribution of deer ticks and seropositivity rates to various tick-transmitted disease organisms in dogs. Journal of the American Veterinary Medicine Association 218: 1092-1097.
Hinten, S. R., G. A. Beckett, K. F. Gensheimer, et al. 2008. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector-borne and Zoonotic Diseases 8: 733-740.
Hoang Johnson, D. K., J. E. Staples, M. J. Sotir, D. M. Warshauer, and J. P. Davis. 2010. Tickborne Powassan virus infections among Wisconsin residents. Wisconsin Medical Journal 109: 91-97.
Hoen, A. G., G. Margos, S. J. Bent, et al. 2009. Phylogeography of Borrelia burgdorferi in the eastern United Staes reflects multiple independent Lyme disease emergence events. Proceedings of the National Academy of Sciences U.S.A. 106: 15013-15018.
Holman, R. C., J. H. McQuiston, D. L. Haberling, and J. E. Cheek. 2009. Increasing incidence of Rocky Mountain spotted fever among the American Indian population of the United States. American Journal of Tropical Medicine and Hygiene 80: 601-605.
Hoogstraal, H. 1979. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. Journal of Medical Entomology 15: 307-417.
Ibister, G. K. 2004. Necrotic arachnidism: the mythology of a modern plague. Lancet 364: 549-553.
IJdo, J. W., J. I. Meek, M. L. Carter, et al. 2000. The emergence of another tickborne infection in the 12-town area around Lyme, Connecticut: human granulocytic ehrlichiosis. Journal of Infectious Diseases 181: 1388-1393.
Katavolos, P., P. M. Armstrong, J. E. Dawson, and S. R. Telford III. 1998. Duration of tick attachment required for transmission of human granulocytic ehrlichiosis. Journal of Infectious Diseases 177: 1422-1425.
Kindberg, E., A. Mickieneċ, C. Ax., et al. 2008. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tickborne encephalitis. Journal of Infectious Diseases 197: 266-269.
Krause, P. J., S. R. Telford III, A. Spielman, et al. 1996. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. Journal of the American Medical Association 275: 1657-1660.
Kudish, K., W. Sleavin, and L. Hathcock. 2007. Lyme disease trends: Delaware, 2000-2004. Delaware Medical Journal 79: 51-58.
Labruna, M. B., T. Whitworth, M. C. Horta, et al. 2004. Rickettsia species infecting Amblyomma cooperi ticks from an area in the State of São Paulo, Brazil, where Brazilian spotted fever is endemic. Journal of Clinical Microbiology 42: 90-98.
Lastavica, C. C., M. L. Wilson, V. P. Berardi, et al. 1989. Rapid emergence of a focal epidemic of Lyme disease in coastal Massachusetts. New England Journal of Medicine 320: 133-137.
Le Gac, P., E. Gouyer, and P. Barisain-Monrose. 1969. Réapparition de la fiévre exanthématique boutonneuse sur le littoral méditerranéen. Comps Rendues de l’Academie de Sciences Paris 269: 1723-1725.
Lin, M., and Y. Rikihisa. 2003. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infection and Immunity 71: 5324-5331.
Lvov, D. K. 1988. Omsk hemorrhagic fever. In Monath, T. P. (ed). The arboviruses: epidemiology and ecology. Volume III. CRC Press, Boca Raton, FL. pp 205-216.
MacDonald, G. 1957. The epidemiology and control of malaria. Oxford University Press, Cambridge. 201 pp.
Maltezou, H. C., L. Andonova, R. Andraghetti, et al. 2010. Crimean-Congo hemorrhagic fever in Europe: current situation calls for preparedness. Eurosurveillance 15: pii=19504.
Mansueto, S., G. Tringali and D. H. Walker. 1986. Widespread, simultaneous increase in the incidence of spotted fever rickettsioses. Journal of Infectious Diseases 154: 539-540.
Mantke, O. D., R. Schädler, and M. Niedrig. 2008. A survey on cases of tick-borne encephalitis in European countries. Eurosurveillance 13: pii=18848.
Marshall, G. S., G. G. Stout, R. F. Jacobs, et al. 2003. Antibodies reactive to Rickettsia rickettsii among children living in the southeast and south central regions of the United States. Archives of Pediatric and Adolescent Medicine 157: 443-448.
Masters, E., S. Granter, P. Duray, and P. Cordes. 1998 . Physician-diagnosed erythema migrans and erythema migrans-like rashes following lone star tick bites. Archives of Dermatology 134: 955-960.
Mather, T. N., M. C. Nicholson, E. F. Donnelly, and B. T. Matyas. 1996. Entomologic index for human risk of Lyme disease. American Journal of Epidemiology 144:1066-1069.
McCall, C. L., A. T. Curns, L. D. Rotz, et al. 2001. Fort Chaffee revisited: the epidemiology of tick-borne rickettsial and ehrlichial diseases at a natural focus. Vector-Borne and Zoonotic Diseases 1: 119-127.
McFadden, J. D., K. M. McElroy, D. K. Hoang, et al. 2010. Ehrlichia infection previously unidentified in North America—Wisconsin and Minnesota, 2009. Paper presented at the 2010 International Conference on Emerging Infectious Diseases, Atlanta, Georgia.
McQuiston, J. H., R. C. Holman, A. V. groom, S. F. Kaufman, J. E. Cheek, and J. E. Childs. 2000. Incidence of Rocky Mountain spotted fever among American Indians in Oklahoma. Public Health Reports 115: 469-475.
McQuiston, J. H., M. A. Guerra, M.R. Watts, et al. 2009. Evidence of exposure to spotted fever group rickettsiae among Arizona dogs outside of a previously documented outbreak area. Zoonoses and Public Health doi:10.1111/j.1863-2378.2009.01300.x.
McQuiston, J., C. Levy, M. Traeger, et al. 2010. Rocky Mountain spotted fever associated with Rhipicephalus sanguineus ticks: from emergence to establishment of an enzootic focus in the United States. Paper presented at the 2010 International Conference on Emerging Infectious Diseases, Atlanta, Georgia.
Mehla, R., S. R. P. Kumar, P. Yadav, et al. 2009. Recent ancestry of Kyasanur forest disease virus. Emerging Infectious Diseases 15: 1431-1437.
Mehnert, W. H., and G. Krause. 2005. Surveillance of Lyme borreliosis in Germany, 2002 and 2003. Eurosurveillance 10: pii=531.
Molineaux, L. 1985. The pros and cons of modeling malaria transmission. Transactions of the Royal Society of Tropical Medicine and Hygiene 79: 743-747.
Moraes-Filho, M., A. Pinter, R. C. Pacheco, et al. 2009. New epidemiological data on Brazilian spotted fever in an endemic area in the state of São Paulo, Brazil. Vector-Borne and Zoonotic Diseases 9: 73-78.
Mouffok, N., A. Benabdellah, H. Richet, et al. 2006. Reemergence of rickettsiosis in Oran, Algeria. Annals of New York Academy of Sciences 1078: 180-184.
Naleway, A. L., E. A. Belongia, J. J. Kazmierczak, et al. 2002. Lyme disease incidence in Wisconsin: a comparison of state-reported rates and rates from a population-based cohort. American Journal of Epidemiology 155: 1120-1127.
Ndip, L. M., R. N. Ndip, S. N. Esemu, et al. 2005. Ehrlichial infection in Cameroonian canines by Ehrlichia canis and Ehrlichia ewingii. Veterinary Microbiology 111: 59-66.
Ndip, L. M., M. Labruna, R. N. Ndip, D. H. Walker, and J. W. McBride. 2009. Molecular and clinical evidence of Ehrlichia chaffeensis infection in Cameroonian patients with undifferentiated febrile illness. Annals of Tropical Medicine and Parasitology 103: 719-725.
Ndip, L. M., R. N. Ndip, S. N. Esemu, D. H. Walker, and J. W. McBride. 2010. Predominance of Ehrlichia chaffeensis in Rhipicephalus sanguineus ticks from kennel-confined dogs in Limbe, Cameroon. Experimental and Applied Acarology 50: 163-168.
Newhouse, V. F. 1983. Variations in population density, movement, and rickettsial infection rates in a local population of Dermacentor variabilis (Acarina: Ixodidae) ticks in the Piedmont of Georgia. Environmental Entomology 12: 1737-1746.
Nicholson, W. L., C. D. Paddock, L. Demma, et al. 2006. Rocky Mountain spotted fever in Arizona: documentation of heavy environmental infestations of Rhipicephalus sanguineusat an endemic site. Annals of the New York Academy of Sciences 1078: 338-341.
Nijhof, A. M., C. Bodaan, M. Postigo, et al. 2007. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector-Borne and Zoonotic Diseases 7: 585-595.
Nordstrand, A., I. Bunilis, C. Larsson, et al. 2007. Tickborne relapsing fever diagnosis obscured by malaria, Togo. Emerging Infectious Diseases 13: 117-123.
Nygård, K., A. Broch Branstæter, and R. Mehl. 2005. Disseminated and chronic Lyme borreliosis in Norway, 1995-2004. Eurosurveillance 10: pii=568.
Olano, J. P., E. Masters, W. Hogerfe, and D. H. Walker. 2003. Human monocytotropic ehrlichiosis, Missouri. Emerging Infectious Diseases 9: 1579-1586.
Openshaw, J. J., D. L. Swerdlow, J. W. Krebs, et al. 2010. Rocky Mountain spotted fever in the United States: interpreting contemporary increases in incidence. American Journal of Tropical Medicine and Hygiene 83: 174-182.
Osterhoudt, K. C., T. Zaoutis, and J. J. Zorc. 2002. Lyme disease masquerading as brown recluse spider bite. Annals of Emergency Medicine 39: 558-561.
Otero, R., A. Fenoll, and J. Casal. 1982. Resurgence of Mediterranean spotted fever. Lancet 2: 1107.
Paddock, C. D. 2009. The science and fiction of emerging rickettsioses. Annals of the New York Academy of Sciences 1166: 133-143.
Paddock, C. D., and M. J. Yabsley. 2007. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Current Topics in Microbiology and Immunology 315: 289-324.
Paddock, C. D., P. W. Greer, T. L. Ferebee, et al. 1999. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. Journal of Infectious Diseases 179: 1469-1476.
Paddock, C. D., S. M. Folk, G. M. Shore, et al. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clinical Infectious Diseases 33: 1586-1594.
Paddock, C. D., R. C. Holman, J. W. Krebs, and J. E. Childs. 2002. Assessing the magnitude of fatal Rocky Mountain spotted fever in the United States: comparison of two national data sources. American Journal of Tropical Medicine and Hygiene 67: 349-354.
Paddock, C. D., A. M. Liddell, and G. A. Storch. 2005. Other causes of tick-borne ehrlichioses, including Ehrlichia ewingii. In Tick-borne diseases of humans, edited by J. L. Goodman, D. T. Dennis, and D. E. Sonenshine. Washington, D.C.: ASM Press. Pp. 258-267.
Paddock, C. D., R. W. Finley, C. S. Wright, et al. 2008. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clinical Infectious Diseases 47: 1188-1196.
Parola, P., C. D. Paddock, and D. Raoult. 2005. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clinical Microbiology Reviews 18: 719-756.
Parola, P., C. Socolovschi, L. Jeanjean, et al. 2008. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Neglected Tropical Diseases 2: e338.
Pattnaik, P. 2006. Kyasanur forest disease: an epidemiological view in India. Reviews in Medical Virology 16: 151-165.
Pavlovsky, E. N. 1966. Natural nidality of transmissible diseases. University of Illinois Press, Urbana, IL.
Philip, R. N. 2000. Rocky Mountain spotted fever in Western Montana. Anatomy of a pestilence. Bitter Root Valley Historical Society. Hamilton, MT.
Piesman, J., A. Spielman, P. Etkind, T. K. Ruebush, and D. D. Juranek. 1979. Role of deer in the epizootiology of Babesia microti in Massachusetts USA. Journal of Medical Entomology 15: 537-540.
Piesman, J., T. N. Mather, J. G. Donahue, et al. 1986. Comparative prevalence of Babesia microti and Borrelia burgdorferi in four populations of Ixodes dammini in eastern Massachusetts. Acta Tropica 43: 263-70.
Piesman, J., T. N. Mather, G. J. Dammin, et al. 1987. Seasonal variation of transmission risk of Lyme disease and human babesiosis. American Journal of Epidemiology 126: 1187-1189.
Piesman, J., T. N. Mather, R. J. Sinsky, and A. Spielman. 1987. Duration of tick attachment and Borrelia burgdorferi transmission. Journal of Clinical Microbiology 25: 557-558.
Piranda, E. M., J. L. H. Faccini, A. Pinter, et al. 2008. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: clinical and laboratory findings. Memorias de Intstituto de Oswaldo Cruz, Rio de Janeiro 103: 696-701.
Pretorius, A. M., and R. J. Birtles. 2002. Rickettsia aeschlimannii: a new pathogenic spotted fever group rickettsia, South Africa. Emerging Infectious Diseases 8: 874.
Pretzman, C., N. Daugerty, K. Poetter , and D. Ralph. 1990 The distribution and dynamics of rickettsia in the tick population of Ohio. Annals of the New York Academy of Sciences 590: 227-236.
Rand, P. W., E. H. LaCombe, R. P. Smith, and J. Ficker. 1998. Participation of birds (Aves) in the emergence of Lyme disease in southern Maine. Journal of Medical Entomology 35: 270–276.
Randolph, S. E. 2008. Tick-borne encephalitis in Central and Eastern Europe: consequences of political transition. Microbes and Infection 10: 209-216.
Randolph, S. E. 2009a. Perspectives on climate change impacts on infectious diseases. Ecology 90: 927-931.
Randolph, S. E. 2009b. Tick-borne disease systems emerge from the shadows: the beauty lies in molecular detail, the messsage in epidemiology. Parasitology 136: 1403-1413.
Randolph, S. E. 2010. To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Veterinary Parasitology 167: 92-94.
Randolph, S. E., and D. J. Rogers. 2000. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proceedings of the Royal Society of London B 267: 1741-1744.
Randolph, S. E., L. Asokliene, T. Avsic-Zupanc, et al. 2008. Variable spikes in tick-borne encephalitis incidence in 2006 independent of variable tick abundance but related to weather. Parasites and Vectors 1: 44.
Randolph, S. E., on behalf of the EDEN-TBD sub-project team. 2010. Human activities predominate in determining changing incidence of tick-borne encephalitis in Europe. Eurosurveillance 15: pii=19606.
Raoult, D., D. Lena, H. Perrimont, H. Gallais, D. H. Walker, and P. Cassanova. 1986. Haemolysis with Mediterranean spotted fever and glucose-6-phosphate dehydrogenase deficiency. Transactions of the Royal Society of Tropical Medicine and Hygiene 80: 961-962.
Raoult, D., and P. Parola. 2008. Rocky Mountain spotted fever in the USA: a benign disease or a common diagnostic error? Lancet 8: 587-589.
Raoult, D., P. Broqui, and V. Roux. 1996. A new spotted fever group rickettsiosis. Lancet 348: 412.
Reif, K. E., and K. R. Macaluso. 2009. Ecology of Rickettsia felis: a review. Journal of Medical Entomology46: 723-736.
Richards, A. L., J. Jiang, S. Omulo, et al. 2010. Human infection with Rickettsia felis, Kenya. Emerging Infectious Diseases 16: 1081-1086.
Rizzoli, A., H. C. Hauffe, V. Tagliapietra, M. Neteler, and R. Rosa. 2009. Forest structure and roe deer density abundance predict tick-borne encephalitis risk in Italy. PLoS ONE 4: e4336.
Rosenstein, E. D., and N. Kramer. 1987. Lyme disease misdiagnosed as a brown recluse spider bite. Annals of Internal Medicine 107: 782.
Rovery, C., P. Brouqui, and D. Raoult. 2008. Questions on Mediterranean spotted fever a century after its discovery. Emerging Infectious Diseases 14:1360-1367
Sanchez, J. L., W. H. Candler, D. B. Fishbein, et al. 1992. A cluster of tick-borne infections: association with military training and asymptomatic infections due to Rickettsia rickettsii. Transactions of the Royal Society of Tropical Medicine and Hygiene 86: 321-325.
Sanchez, R., C. Alpuche, H. Lopez-Gatell, et al. 2009. Rhipicephalus sanguineus–associated Rocky Mountain spotted fever in Mexicali, Mexico: observations from an outbreak in 2008-2009. Paper presented at the 23rd Annual meeting of the American Society for Rickettsiology, Hilton Head, SC (abstract # 75).
Scaffidi, V. 1981. Attuale espansione endemo-epidemica della febbre bottonosa in Italia. Minerva Medica 72:2063-2070.
Scrimenti, R. J. 1970. Erythema chronicum migrans. Archives of Dermatology 102: 104-105.
Segura, F., and B. Font. 1982. Resurgence of Mediterranean spotted fever in Spain. Lancet 2: 280.
Sexton, D. J., J. Banks, S. Graves, K. Hughes, and B. Dwyer. 1991. Prevalence of antibodies to spotted fever group rickettsiae in dogs from southeastern Australia. American Journal of Tropical Medicine and Hygiene 45: 243-248.
Shapiro, E. D., M. A. Gerber, N. B. Holabird, et al. 1992. A controlled trial of antimicrobial prophylaxis for Lyme disease after deer-tick bites. New England Journal of Medicine 327: 1769-1773
Shapiro, M. R., C. L. Fritz, K. Tait, et al. 2010. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clinical Infectious Diseases 50: 541-8.
Sigal, L. H., J. M. Zahradnik, P. Lavin, et al. 1998. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. New England Journal of Medicine. 339: 216-222.
Smith, R. P., R. T. Schoen, D. W. Rahn, et al. 2002. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Annals of Internal Medicine 136: 421-428.
Smith, R., and J. Takkinen, and the Editorial team. 2006. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Eurosurveillance 11: pii=2977.
Socolovschi, C., Mediannikov O., Sokhna C., et al. 2010. Rickettsia felis-associated uneruptive fever, Senegal. Emerging Infectious Diseases 16: 1140-1142.
Souza, C. E., J. Morares-Filho, M. Ogrzewalska, et al. 2009. Experimental infection of capybaras Hydrochoerus hydrochaeris by Rickettsia rickettsii and evaluation of the tranmission of the infection to ticks Amblyomma cajennense. Veterinary Parasitology 161: 116-121.
Spencer, R. R., and R. R. Parker. 1923. Rocky Mountain spotted fever: infectivity of fasting and recently fed ticks. Public Health Reports 38:333-339.
Spielman, A., C. M. Clifford, J. Piesman, et al. 1979. Human babesiosis on Nantucket Island, USA: description of the vector, Ixodes (Ixodes) dammini, n.sp. (Acarina:Ixodidae). Journal of Medical Entomology 15: 218-234.
Spielman, A., and P. Rossignol. 1984. Insect vectors and scalars. In Tropical and geographic medicine, edited by K. S Warren and A. A. F. Mahmoud. McGraw Hill, New York. Pp. 167-183.
Spielman, A., M. L. Wilson, J. F. Levine, and J. Piesman. 1985. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annual Review of Entomology 30: 439-460.
Spielman, A., S. R. Telford III, and R. J. Pollak. 1993. The origins and course of the present outbreak of Lyme disease. In Ecology and environmental management of Lyme disease, edited by H. S. Ginsberg. New Brunswick, NJ: Rutgers University Press. Pp. 83-96.
Stafford III, K. C., M. L. Cartter, L. A. Magnarelli, et al. 1998. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. Journal of Clinical Microbiology 36: 1240-1244.
Stanek, G., and F. Strle. 2009. Lyme borreliosis: a European perspective on diagnosis and clinical management. Current Opinions in Infectious Diseases 22: 450-454.
Steere, A. C., E. Taylor, M. L. Wilson, et al. 1986. Longitudinal assessment of the clinical and epidemiological features of Lyme disease in a defined population. Journal of Infectious Diseases 154: 295-300.
Steere, A. C, V. K. Sikand, F. Meurice, et al. 1998. Vaccination against Lyme disease withrecombinant Borrelia burgdorferi outer surface lipoprotein A with adjuvant. New England Journal of Medicine 339: 209-215.
Stefanoff, P., M. Eidson, D. L. Morse, and Z. Zielinski. 2005. Evaluation of tickborne encephalitis case classification in Poland. Eurosurveillance 10: pii=514.
Šumilo, D., L. Asokliene, A. Bormane, et al. 2007. Climate change cannot explain the upsurge of tick-borne encephalitis in the Baltics. PLoS One 2: e500.
Swanepoel, R., A. J. Shepherd, P. A. Leman, et al. 1987. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in Southern Africa. American Journal of Tropical Medicine and Hygiene 36: 120-132.
Taylor, J. P., W. B. Tanner, J. A. Rawlings, et al. 1985. Serological evidence of subclinical Rocky Mountain spotted fever infections in Texas. Journal of Infectious Diseases 151: 367-369.
Telford, S. R. III, and A. Spielman. 1989. Enzootic transmission of the agent of Lyme disease in rabbits. American Journal of Tropical Medicine and Hygiene 41: 482-490.
Telford, S. R. III., A. Gorenflot, P. Brasseur, and A. Spielman. 1993. Babesial infections in humans and wildlife. In Parasitic protozoa, Vol. 5, edited by J. P. Kreier. San Diego Academic Press. Pp. 1-47.
Telford, S. R. III, P. M. Armstrong, P. Katavolos, et al. 1997. A new tick-borne encephalitis-like virus infecting deer ticks, Ixodes dammini. Emerging Infectious Diseases 3: 165-170.
Telford, S. R. III, and H. K. Goethert. 2008. Emerging and emergent tickborne infections. In Ticks: biology, disease, and control, edited by L. H Chappell, A. S. Bowman, and P. A. Nuttall. Cambridge University Press. Pp. 344-376.
Telford, S. R. 2009. Status of the “East side hypothesis” (transovarial interference) 25 years later. Annals of the New York Academy of Sciences 1166: 144-150.
Telford, S. R. III, P.F. Weller, and J.H. Maguire. In press. Babesiosis. In Tropical infectious diseases: principles, pathogens, practice, 3rd edition, edited by R. Guerrant, D. H. Walker, and P. F. Weller. Churchill Livingstone.
Tinoco-Gracia, L., H. Quiroz-Romero, M. T. Quintero-Martínez, et al. 2009. Prevalence of Rhipicephalus sanguineus ticks on dogs in a region on the Mexico-USA border. Veterinary Record 164: 59-61.
Trape, J. F., B. Godeluck, G. Diatta, et al. 1996. The spread of tick-borne borreliosis in West Africa and its relationship to sub-Saharan drought. American Journal of Tropical Medicine and Hygiene 54: 289-293.
Trout, R. T., C. D. Steelman, A. L. Szalanski, and K. Loftin. 2010. Establishment of Amblyomma maculatum (Gulf Coast tick) in Arkansas, U.S.A. Florida Entomologist 93: 120-122.
Verdade, L. M., and K. M. P. M. B. Ferraz. 2006. Capybaras in an anthropogenic habitat in southeastern Brazil. Brazilian Journal of Biology 66: 371-378.
Vetter, R. S., G. B. Edwards, and L. F. James. 2004. Reports of envenomization by brown recluse spiders (Araneae: Sicariidae) outnumber verifications of Loxosceles spiders in Florida. Journal of Medical Entomology 41: 593-597.
Vetter, R. S., and R. B. Furbee. 2006. Caveats in interpreting poison control centre data in spider bite epidemiology studies. Public Health 120: 179-181.
Vetter, R. S., N. C. Hinckle, and L. M. Ames. 2009. Distribution of the brown recluse spider (Araneae: Sicariidae) in Georgia with comparison to poison center reports of envenomations. Journal of Medical Entomology 46: 15-20.
Vial, L., G. Diatta, A. Tall, et al. 2006. Incidence of tick-borne relapsing fever in West Africa: longitudinal study. Lancet 368: 37-43.
Walker, D. H., H. K. Hawkins, and P. Hudson. 1983. Fulminant Rocky Mountain spotted fever. Its pathologic characteristics associated with glucose-6-phosphatate dehydrogenase deficiency. Archives of Pathology and Laboratory Medicine 107: 121-125.
Western, K. A., G. D. Benson, N. N. Gleason, et al. 1970. Babesiosis in a Massachusetts resident. New England Journal of Medicine 283: 854-856.
Wilfert, C. M., J. N. MacCormack, K. Kleeman, et al. 1984. Epidemiology of Rocky Mountain spotted fever as determined by active surveillance. Journal of Infectious Diseases 150: 469-479.
Wilfert, C. M., J. N. MacCormack, K. Kleeman, et al. 1985. The prevalence of antibodies to Rickettsia rickettsii in an endemic area for Rocky Mountain spotted fever. Journal of Infectious Diseases 151: 823-831.
Wormser, G. P., J. Nowakowski, R. B. Nadelman, et al. 1998. Efficacy of an OspA vaccine preparation for prevention of Lyme disease in New York State. Infection 26: 208-212.
Wormser, G. P., E. Masters, J. Nowakowski, et al. 2005. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clinical Infectious Diseases 41:958-965.
Yabsley, M. J., V. G. Dugan, D. E. Stallknecht, et al. 2003. Evaluation of a prototype Ehrlichia chaffeensis surveillance system using white-tailed deer (Odocoileus virginianus) as natural sentinels. Vector-Borne and Zoonotic Diseases 3: 195-207.
Yevich, S. J., J. L. Sánchez, R. F. DeFraites, et al. 1995. Seroepidemiology of infections due to spotted fever group rickettsiae and Ehrlichia species in military personnel exposed in areas of the United States where such infections are endemic. Journal of Infectious Diseases 171: 1266-1273.
Yilmaz, G. R., T. Buzgan, H. Irmak, et al. 2009. The epidemiology of Crimean-Congo hemorrhagic fever in Turkey, 2002-2007. International Journal of Infectious Diseases 13: 380-386.
Yu, X. J., M. F. Liang, S. Y. Zhang, et al. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. New England Journal of Medicine 10.1056/NEJMoa1010095.
Zavala-Velazquez, J. E., X. J. Yu, P. D. Teel, and D. H. Walker. 1996. Unrecognized spotted fever group rickettsiosis masquerading as dengue fever in Mexico. American Journal of Tropical Medicine and Hygiene 55: 157-159.
Zimmerman, H. 2005, Tickborne encephalitis in Switzerland: significant increase in notified cases, 2005. Eurosurveillance 10: pii=2806.
A2
GLOBAL ENVIRONMENTAL CHANGE AND TICK-BORNE DISEASE INCIDENCE
Sarah H. Olson, Ph.D., and Jonathan A. Patz, M.D., M.P.H.
Introduction
The diverse and complex ecology of tick-borne diseases (TBDs) is exceptional among vector-borne diseases. Today it is estimated that tick species exceed 850 and inhabit every continent (Dennis and Piesman 2005, Benoit et al 2007). Their resilience and persistence in the environment can be traced back in the fossil record, which suggests that they originated 65-146 million years ago (Fuente 2003). The Swiss Army knife of disease vectors, ticks generate a neurotoxin and they can host bacterial, viral, and protozoan pathogens as well, a greater variety of disease agents than any other arthropod vector (Sonnenshine 1991).
Grouped into Ixodidæ and Argasidæ families, or hard- and soft-bodied ticks respectively, all ticks are obligate blood-suckers and undergo three stages of development: larval, nymphal, and adult. The life cycle of ticks starts when, “larvæ seek hosts, attach, feed, detach, and develop in sheltered microenvironments where they molt to nymphs; nymphs follow the same pattern and molt to adults (except argasids, which first molt into further nymphal stages); adults seek hosts, mate, feed, and in the case of engorged females, drop off to deposit eggs” (Dennis and Piesman 2005). Tick hosts are terrestrial vertebrates, and host-seeking activity is called questing. Ticks may have vastly different host preferences, among species and at different developmental stages, as well as seasonal questing that depends on climate and environmental conditions (Dennis and Piesman 2005). See Table A2-1 for an overview of major tick-borne diseases, their vectors, endemic areas, and studies relevant to global environmental change.
Global environmental change is the cumulative effect of climate change, the changing built environment and landscape, and evolving biodiversity (MEA 2005). This review will emphasize these three elements. In the context of disease ecology, climate change encompasses abiotic risk factors of temperature, precipitation, and the intensity and frequency of extreme weather events. Globally these events are shifting climatic zones and influencing seasonality. Landscape risk factors include observations of the amount and arrangement of land cover types and uses, from native land cover to urban development. Landscapes provide both the habitat for host and tick vector populations and set the stage for human behavioral risk factors. The presence of host and vector populations are key biodiversity risk factors in TBD ecology, but a less obvious risk factor is the biodiversity of
TABLE A2-1 Major Tick-Borne Diseases, Their Vectors, Endemic Areas, and Studies Relevant to Global Environmental Change
|
Disease |
Vector |
Research |
Effects |
|
Tick-borne paths |
[Anaplasma phagocytophilum, Ehrlichia caffeensis |
Wimberly et al 2008 |
Climate: Habitat (bkwrds finding) biodiversity |
|
TBE |
Ixodes ricinus |
Perkins et al 2006 |
Biodiversity |
|
TBE |
Ixodes ricinus |
Gilbert et al 2010 |
Climate: habitat |
|
TBE |
Ixodes ricinus |
Ogden et al 2008 |
Climate: Habitat |
|
TBE |
Ixodes ricinus |
Randolph and Rogers 2000 |
Climate: Habitat |
|
TBE |
Ixodes ricinus |
Lindgren and Gustafson 2001 |
Climate: Seasonality |
|
TBE |
Ixodes ricinus |
Lindgren et al 2000 |
Climate: Seasonality |
|
TBE |
Ixodes ricinus |
Carpi et al 2008 |
Climate: Seasonality and habitat |
|
TBE |
Ixodes ricinus |
Estrada-Peña et al 2003 |
Habitat |
|
TBE |
Ixodes ricinus |
Halos et al 2010 |
No climate habitat/built enviro |
|
TBE |
Ixodes ricinus |
Vanwambeke et al 2009 |
No climate habitat/built enviro |
|
Lyme |
Ixodes ricinus TBEV B. burgdorferi sensu lato |
Danielová et al 2010 |
Climate: Habitat |
|
Lyme |
Ixodes scapularis |
Brisson et al 2008 |
Biodiversity |
|
Lyme |
Ixodes scapularis |
Brunner et al 2008 |
Biodiversity |
|
Lyme |
Ixodes scapularis |
Giardina et al 2000 |
Biodiversity |
|
Lyme |
Ixodes scapularis |
Keesing et al 2009 |
Biodiversity |
|
Lyme |
Ixodes scapularis |
Ostfeld and Keesing 2000 |
Biodiversity |
|
Lyme |
Ixodes scapularis |
Ostfeld and Keesing 2000 |
Biodiversity |
|
Lyme |
Ixodes scapularis |
Ostfeld and Keesing 2006 |
Biodiversity |
|
Lyme |
Ixodes scapularis |
Schauber et al 2005 |
Biodiversity/Climate-weather mice acorns |
|
Lyme |
Ixodes scapularis |
Brownstein et al 2003 |
Climate: Habitat |
|
Lyme |
Ixodes scapularis |
Ogden et al 2005 |
Climate: Habitat |
|
Lyme |
Ixodes scapularis |
Jones and Kitron 2000 |
Climate: Seasonality |
|
Lyme |
Ixodes scapularis |
Kitrone and Kazmierczak 1997 |
Climate: Seasonality |
|
Lyme |
Ixodes scapularis |
Prusinski et al 2006 |
Habitat |
|
Lyme |
Ixodes scapularis |
Wilder and Meikle 2004 |
Habitat (bkwrds finding)/biodiversity |
|
Lyme |
Ixodes scapularis |
Bunnell et al 2003 |
No climate habitat |
|
Lyme |
Ixodes scapularis |
Allan et al 2003 |
No climate habitat/built enviro |
|
Disease |
Vector |
Research |
Effects |
|
Lyme |
Ixodes scapularis |
Brownstein et al 2005 |
No climate habitat/built enviro |
|
Lyme |
Ixodes scapularis |
Cromley et al 1998 |
No climate habitat/built enviro |
|
Lyme |
Ixodes scapularis |
Das et al 2002 |
No climate habitat/built enviro |
|
Lyme |
Ixodes scapularis |
Frante et al 1998 |
No climate habitat/built enviro |
|
Lyme |
Ixodes scapularis |
Horobik et al 2006 |
No climate habitat/built enviro |
|
|
Mix |
Cumming and Van Vuuren 2006 |
Climate: Habitat |
|
|
Multiple |
Altizer et al 2006 |
Climate: Seasonality |
|
Rickettsioses |
Rhipicephalus sanguineus |
Parola et al 2008 |
Climate: Seasonality |
|
|
Dermacentor andersoni, Ixodes ricinus |
Eisen et al 2008 |
Climate: Seasonality |
other vertebrate species in the community. Predation and competition may affect host densities, depending on the species composition of biodiversity changes. Lastly, just as the elements of global environmental change range from local to worldwide (Patz and Olson 2006, Patz et al 2008), temporal and spatial scale effects of global environmental change on vector-borne diseases are anticipated (Kovats et al 2001, Patz et al 2005, Plowright et al 2008).
Tick vector species are often vectors for multiple disease-causing microorganisms (Table A2-1), so our approach allows generalization of changing environmental effects on pathogens not yet studied. Other important elements of disease emergence, including genetic, biological, social, political and economic factors, are included when available, but in-depth analysis of these risk factors is beyond the scope of this paper (IOM 2003).
Climate Change
Research on climate change and TBD is directed toward three areas of investigation. First, the fundamental question is how climate change will impact the tick vector and microbial agent. In vitro observations allow us to understand precisely how temperature and humidity affect tick and pathogen development, behavior, and survival. The second question is where will climate change affect the transmission of TBD. Use of information from in-vitro studies can be used (directly or in simulation models) to identify current and future projected climate-based geographic ranges (Ogden et al
2005, 2006). Alternatively (and particularly useful where detailed information on climate effects on vector biology are not available) correlations between observed current distributions of vectors and climate (accounting for other important variable such as habitat) are generalized to large areas using simulations of projected climate change. Finally, when may TBD be altered under future climate change scenarios, or more specifically changes to transmission intensity and duration, including patterns of tick, host, and microbe seasonality and synchrony? For this question, data-rich longitudinal field studies are particularly useful to sort out the integrated impact of climate change on disease ecology.
Methodological approaches are implicit to the framing of each question and the different approaches provide varied perspectives of climate change impacts on TBD. A severe lack of long-term data on coupled disease and environmental systems restricts integrated assessment of all three questions (Pascual and Bouma 2009). So uncertainty grows between the research gaps, but taken together, a mosaic is slowly taking shape of how, where and when TBD will be modulated by climate change.
How Will Climate Affect Tick-Borne Diseases?
Many dimensions of tick development and behavior and TBD transmission are directly linked to climate cues. Higher temperatures yield faster development rates of larvae, nymphs, and adults, with the precise rate of development varying between stages and species. Diapause, or a period of rest between stages, has latitudinal relationships corresponding to photoperiod (tropical species) and temperature-linked physiological aging (temperate species). Together photoperiod and temperature also influence host questing. “At the end of each year, decreasing day length reduces the probability of questing and low temperatures may inhibit activity altogether while at the start of each year, increasing day length is permissive, but only if temperatures are high enough” (Randolph 2004). Of particular note are species and stage specific temperature thresholds for tick activity (3.9–9.8°C), coordinating host seeking activity (7.2–13.9°C), and cold temperature survival (−18.5 – −11.6°C) (Clark 1995, Vandyk et al 1996, Schulze et al 2001). Slight temperature changes around thresholds can have a large impact on tick survival and host-seeking behavior. Beyond temperature in the tropics, Rhipicephalus appendiculatus female to larvae survival drops under conditions of high rainfall but low humidity (Randolph 1994, 1997). At local scales, there are exceptions to these climate maxims (Schulze et al 2009, Hubalek et al 2003). Lastly, there is some evidence for direct effects of climate on the pathogen. The rate of parva transmission to cattle increases at higher temperatures but the development of Theileria parva
requires a specific temperature range (18–28°C) (Ochanda et al 1988, 2003; Young and Leitch 1981).
Dichotomous habitat and host-seeking behavior define two distinct groups of ticks. Exophilic or nonnidicolous species live in the open environment, whereas nidicolous ticks live in the nests and burrows of host species. Since non-nidiculous ticks spend nearly 90% of the life off the host, they are consequently sensitive to external conditions caused by climate change. In contrast, nidiculous ticks are dependent on the presence of host-related microhabitats (e.g., nests, burrows) and are thus sensitive to species distributions, which may change as a result of climate change.
“Nidicolous ticks exhibit behavioral patterns that restrict their distribution to these cryptic microhabitats, avoiding bright sunlight and low humidity. Under these conditions nidicolous ticks can wait for months or even years until hosts arrive and take up residence in these shelters. For the nonnidicolous ticks, survival in the open environment depends upon many factors, among the most important of which is tolerance to desiccation. Ticks adapted to the cool, humid arboreal and deciduous forests of northern Europe and North America typically have only limited tolerance of desiccation. After brief periods of questing on vegetation for passing hosts where they are exposed to subsaturated air, the loss of body water overwhelms their hunger and induces them to retreat to saturated atmospheres in the rotting meadow vegetation or damp leaf litter on the forest floor.” (Sonenshine 2005)
Climate, along with other biotic factors such as host availability, is strongly connected to the population density of nonnidicoulous ticks, which transmit the majority of human TBDs. Primarily, this is because climate factors, such as cumulative degree-days and humidity, affect tick survival and longevity (McEnroe 1977, McEnroe 1984, Lindsay et al 1995). In Illinois, Jones and Kitron report severe drought in the previous year significantly lowers Ixodes scapularis larval density on Peromyscus leucopus mice. There are also positive correlations between larval density and cumulative rainfall and degree-days (2000). In fact, climate is thought to be the major factor controlling Lyme disease occurrence, stronger even than host factors (Randolph 2000). And the importance of climate extends from macro down to micro scale, as survival strongly depends on narrow microclimatic conditions found on the ground, which may be very different even from local ambient conditions (Daniel and Dusbabek 1994, Bertrand and Wilson 1996). When ambient air temperature (measured 50cm above ground) is substituted for ground air temperature, a model of I. ricinus development erroneously predicts nymphs emerge 2–3 weeks earlier than observed in southern UK (Randolph 2004). Sensitivity analyses of a population-based
land use and land cover physiological tick model of the cattle-tick species Boophilus spp confirmed bi-hourly temperature and relative humidity strongly affect tick population dynamics (Corson et al 2004). Landscape and soil conditions will need to be integrated with high-resolution climate projections in order to model tick emergence effectively.
Altitudinal gradients are useful to study the specific mechanisms of climate change on TBDs (Eisen 2008). An altitude gradient creates a linear temperature gradient that represents different climate scenarios while holding other environmental factors relatively constant. Across a difference of 380m and a temperature loss of 0.72°C per 100m gained in elevation, Jouda and colleagues show annual questing nymph and adult I. ricinus density and the spring onset of questing is negatively associated with changes in elevation or positively associated with warmer temperatures (2004). A follow-up study between earlier years and more recent times on these same slopes attributed a significant increase in tick density and phenology at higher elevations to recent warmer summers and falls in the higher elevations as measured by saturation deficit (the drying ability of the atmosphere). They also observed an increase in the density of nymphs infected with Borrelia at the highest elevation, but overall Borrelia infection prevalence dropped with altitude (Cadenas et al 2007, Burri et al 2007). While this study was not able to adjust for differences in host populations across the elevation gradient, another study in Scotland showed Ixodes ricinus tick abundance decreases with altitude, again as higher elevations are cooler, even after controlling host (deer) abundance. The authors conclude, “It could be inferred that ticks may become more abundant at higher altitudes in response to climate warming. This has potential implications for pathogen prevalence such as louping ill virus if tick numbers increase at elevations where competent transmission hosts (red grouse Lagopus lagopus scoticus and mountain hares Lepus timidus) occur in higher numbers” (Gilbert 2010). However, the authors fail to consider how climate change may shift the abundance of grouse and hare hosts at the higher elevations. In Colorado, Eisen observed Dermacentor andersoni (Stiles) along an altitudinal gradient and projected a 1 to 2°C increase in mean maximum April-September temperature would generate the same peak tick abundances at 2390 to 2480m that currently occur at 2290m (2008). Adaptation to specific local conditions may also influence future patterns of TBD, as Eisen also observed a site-specific positive correlation between daily maximum temperatures during peak tick host-seeking activity and mean daily maximum temperatures (2007). In sum, the literature consistently shows warmer temperatures increase tick abundance but cautions against assumptions that host distributions will remain relatively constant.
Just as climate influences overall tick population dynamics, evidence suggests biting rates of ticks also are affected by climate. A fascinating epidemiological detective story began in May 2007 when two patients
presented with Rickettsia massiliae and R. conorii infections in southern France. In April, both patients had visited the residence of a mutual friend. An entomological survey of the residence in July found 218 adult Rhipicephalus sanguineus ticks in just one hour, of which roughly one-fourth were positive for rickettsia. The authors point out that April 2007 was the warmest since 1950, and they also performed a healthy volunteer human biting study with pathogen-free Rh. sanguineus to test affiliations between aggressive tick biting behavior and temperature. Surprisingly, between 27-67 percent of larvae incubated at 40°C attached to the skin where only 0-6 percent of larvae incubated at 25°C attached successfully. Known commonly as the dog tick, Rh. sanguineus transmits dog pathogens but also vectors important and emerging human rickettsioses (Parola et al 2008). Few studies have investigated temperature and I. scapularis biting affinity, but McCabe and Bunnell attribute greater numbers of Lyme disease cases to increased survival and tick activity in the northeastern United States brought about by above average rainfall in May and June (2004).
How climate change will impact TBDs is further complicated by the way it alters host diversity, abundance, dispersal, the development cycle of the pathogen, and the suitability of habitat for both tick and hosts (Schulze and Jordan 1996, Gubler et al 2001). Using structural equation modeling, Cumming and Guegan show climate will have a greater direct influence on community richness of the tick vectors than on the pathogens themselves, but that alterations in tick biodiversity will then affect pathogen diversity (2006). In addition to direct effects of climate change on tick and pathogen populations, routes and species of migratory birds transporting Crimean-Congo haemorrhagic fever virus (CCHFV) may change under future climate scenarios. In fact, climate is interwoven into multiple elements of CCHFV zoonotic transmission, from the risk of dispersal by changing migration patterns and bird species to shifting livestock and small vertebrate reservoirs (Gale et al 2009). There may be complicated interactions between temperature, host behavior, and tick population dynamics (Craine et al 1995). A study found when temperatures exceeded 40°C, radio-tracked Australian lizards were more likely to seek out microhabitats or dens where they became infested with high numbers of ticks (Kerr and Bull 2006). Researchers anticipate weather will also affect human behavior in TBD transmission. Randolph et al point to climate influences on cultural practices and socioeconomic conditions as a more critical pathway than climate influences on tick abundance for a tick-borne encephalitis (TBE) epidemic in Europe in 2006. “An alternative explanation, supported by qualitative reports and some data, involves human behavioural responses to weather favourable for outdoor recreational activities, including wild mushroom and berry harvest, differentially influenced by national cultural practices and economic constraints” (Randolf 2008).
Where Might Climate Change Affect Tick-Borne Diseases?
Just as there are multiple factors that contribute to TBD risk, there may be more than one factor that limits the geographic range of a tick-borne disease. At regional and national scales the spatial area of Lyme disease risk has been documented to correlate with annual precipitation, temperature, and drought events (Estrada-Peña 2002; McCabe and Bunnell 2004; Jones and Kitron 2000). Therefore, why is Lyme disease not more prevalent in the southern United States where ideal climate conditions already occur? Ostfeld and Keesing suggest that higher species richness, and especially those of ground-dwelling birds, reduces transmission probabilities, or in other words, creates a protective dilution effect (2000a). Therefore, in the south Lyme disease is restricted by biodiversity and species assemblages that limit its ability to spread. Other tick-borne pathogens reveal similar limitations from other factors. The joint distribution of Ehrlichia chaffeensis and Anaplasma phagocytophilum, the agents of human monocytotropic ehrlichiosis and human granulocytic anaplasmosis, respectively, is bounded in range by climate in the southeastern United States and by the forest fragmentation and cover of the Great Plains (Wimberly et al 2007). While the likelihood of whether climate or land use has a greater effect on tick abundance is an interesting question, it overlooks the dynamic balance of joined climate and land use systems. Land use may be a dominant factor in one year and one location, followed by climate the next. More likely both may limit the geographic extent of TBDs.
The relative dominance of climate or land use on the tick vector of Lyme disease, Ixodes scapularis, will depend on the location, the rate of change of climate, the rate of change of land-use conditions, and scale. To forecast tick distribution 40 years hence requires an understanding of both current and projected trends of land use and climate. Climate projections for 2050 from B1-low and A2-high scenarios estimate 1.0-3.7°C warming for mean temperatures in Dec/Jan/Feb and June/July/Aug over the present range of Lyme disease transmission (Hulme and Sheard 1999). Generally, earlier spring and warmer winters are positively associated with tick abundance and Lyme disease incidence. Land-use patterns in the last 50 years in the United States reveal exurban development leading to habitat fragmentation changed 20-60 percent for much of the range of Lyme disease (Brown et al 2005) (see Figure A2-1).
Recent research shows that at present climate is limiting the migration of Ixodes ticks into eastern Canada and that under several climate change scenarios this migration is anticipated to spread to the southeastern corner of Alberta and across to the eastern tip of Newfoundland by 2050 or 2080 (Ogden et al 2005; Ogden et al 2006) (see Figure A2-2). The rate of climate change is relatively assured, compared to the rate of land-use change, which
FIGURE A2-2 Land-use change in rural America.
varies with economic, social, and political institutions. As Ixodes is new to this environment all predictions are uncertain. Knowledge of land use and tick habitat suitability is still emerging. Unlike climate, land use is not readily quantifiable. For example, it is difficult to state that 10 ha of type A landcover relates to a given degree of risk. Over the next century, climate is a good bet to be the dominant driver that spreads Lyme disease into Canada (Ogden et al 2010). Land-use change may influence the intensity of the initial epidemic and persistence of endemicity that follows this expansion. In the Northeast, to the extent that climate and forests are linked ecosystems,
there may be synergistic effects of climate and land-use change. Deforested and fragmented lands may not be able to support historical tick densities under different climate regimes. We are only beginning to quantify the consequences of land-use and climate patterns on biodiversity, vector-borne diseases and human health (Hansen 2005).
The complex dynamics of climate and land use patterns are underscored by a debate on the rising incidence of tick-borne encephalitis (TBE) in Central Europe that began in the mid-1990s. A survey of dogs carrying ticks and tick collections were performed in 2001 and 2002 and compared to a 1957 survey of small terrestrial vertebrates. The authors conclude I. ricinus tick habitat encroached up to around 1000m from 700m in elevation as a result of climate change since the 1950s (Daniel et al 2003, 2006). Another research group that included landscape in a more comprehensive analysis of risk factors found that “there was little evidence for the role of climate change in the increase in infections … in northern Italy from 1996 to 2006. However, support was found for the influence on TBE increases due to changing forest landscapes and that both red and roe deer, essential hosts in maintaining and amplifying tick populations, had increased 10% from 1996 to 2000 in the region and over 300% in the last 50 years” (Rizzoli et al 2009). The large influence of the host population in this case study underscores the importance of gestalt in disease ecology systems.
Despite this cautionary tale, geographic areas of TBD risk are projected to change globally. An expert panel concluded climate change would have the greatest impact on the distribution of ticks that transmit two of the top three vector-borne viruses in the EU, African horse sickness virus (AHSV) and Crimean-Congo haemorrhagic fever virus (CCHFV) (Gale et al 2010). Increases in the density and geographic range of I. ricinus in the mid-1990s is attributed to milder winters compared to the early 1980s (Lindgren 2000). Further research in Sweden found a correlation between increased TBE, transmitted by I. ricinus ticks and global warming trends that led to earlier onset of spring and milder winters (Lindgren and Gustafson 2001). It remains unclear why the UK is experiencing a large increase in the distribution and abundance of this tick (Kirby et al 2004; Scharlemann et al 2008). A rising number of TBE cases reported in Slovakia since 1980 appears to reflect a migration of the disease to submountainous habitat as a response to a warming climate (Lukan et al 2010). Yet this relationship may not directly translate to entomological risk (Zeman and Bene 2004, Danielová et al 2010). Scientists using a global habitat suitability model for 73 African tick species predict that over the next 100 years the habitat area will increase 1-9 million square kilometers. “If this is also typical of other invertebrates, then climate change will disrupt not only the geographic location of [their] communities but also their structure. Changes in tick
communities are also likely to influence tick-borne pathogens” (Cumming and Van Vuuren 2006).
When Will Climate Change Affect Tick-Borne Diseases?
The significance of climate change on TBDs goes beyond the debate of shifting distributions of ticks (Pascual and Bouma 2009). “Seasonal patterns are one major pathway for the subtle but potentially drastic effects of climate change on disease dynamics” (Pascual and Dobson 2005). Further, “empirical evidence points to several biologically distinct mechanisms by which seasonality can impact host-pathogen interactions, including seasonal changes in host social behaviour and contact rates, variation in encounters with infective stages in the environment, annual pulses of host births and deaths and changes in host immune defenses” (Alitzer et al 2006). Seasonal patterns of host pathogen prevalence also need to be considered. Sero prevalence of Anaplasma phagocytophilum, an emerging pathogen in roe deer in Denmark, was nearly two times higher in the summer versus the fall hunting season (Skarphédinsson et al 2005). The challenge of monitoring these dynamics is further complicated by adaptation and evolution (Tabachnick 2010). Simulations done by Ogden and colleagues demonstrate different seasonal patterns predicted by climate change will select for I. scapularis-borne pathogens that are “shorter-lived, less efficiently transmitted, and more pathogenic to their natural hosts, i.e. altered frequency distribution and evolution trajectories” (Ogden et al 2008a).
Climate Caveats
The narrow focus of this section on climate change (or the focus of the following section) should not dismiss the critical role of human behavioral factors along with social and political changes (Randolph 2008, 2010; Vanwambeke et al 2010).
Biodiversity, the Landscape, and Built Environment
“Epidemiological models should consider not only the demographic structure of the host population, but its spatial structure as well, and this can in some cases be effectively inferred from landscape structure.” (Langlois et al 2001)
The complex interwoven ties between disease ecology and biodiversity, landscape, and the built environment were first elucidated for Lyme borreliosis in the northeastern United States. It was observed that small mammal reservoir competency, or the ability of a species to infect a biting larva,
varies from species to species. In the northeast, white-footed mice (Peromyscus leucopus), eastern chipmunks (Tamias striatus), and short-tailed or masked shrews (Blarina brevicauda or Sorex cinereus) are the reservoir for 80-90 percent of infected nymphs (Brisson et al 2008). Because the tick is a nonspecific feeder, diverse community assemblages effectively decrease disease spread by reducing the number of blood meals taken from more competent reservoirs. So in high biodiversity settings, this phenomenon, called the “dilution effect,” decreases the likelihood that larval blood meals are taken from mice, an animal with high reservoir competence, while increasing the likelihood that larval blood meals are taken from species with lower reservoir competence, such as raccoons or squirrels (Norman et al 1999; Schmidt and Ostfeld 2001; LoGuidice et al 2003; Brisson et al 2008).
Research suggests the dilution effect is an ecological disease process related to forest fragmentation and that landscape metrics can detect this process. Landscape metrics quantify the amount and arrangement of landscape components. Following the theory, in smaller forest patches the population density of white-footed mice, a highly competent reservoir of Lyme disease, nymphal infection prevalence (NIP), and density of infected nymphs increase exponentially (Nupp and Swihart 1998; Allan et al 2003). Diverse communities of small mammals and birds (lacking in small forest fragments) limit disease spread by reducing the number of blood meals taken from mice, which have the highest reservoir competency (Ostfeld and Keesing 2000a, b). Landscape patterning is important because the disease-buffering ability of an ecosystem may decline with reduced species richness, which often results from decreasing habitat patch size.
Notably, the universality of the dilution effect is being challenged. Recent modeling suggests changing host abundance may also amplify pathogen transmission, “with the outcome depending precisely on mechanisms of competition, host contact rates with ticks, and acquired host resistance to ticks” (Ogden and Tsao 2009; Salkeld et al 2010). Further, the emerging infectious disease, enzootic granulocytic anaplasmosis seroprevalence was greatest in habitats with high biodiversity (ticks, rodents and vegetation) (Foley et al 2009). Bottom line, depending on locality, biodiversity can increase or decrease TBD transmission.
In addition to the dilution effect, other environmental and ecological processes may relate Lyme disease to specific landscapes. Habitat fragmentation can alter host movements and disease transmission dynamics. Micro-infestations or dense patches of I. ricinus in a habitat mosaic landscape are critical nodes in a host movement network that sustain the permanent tick population on 1,400 km2 in northern Spain (Estrada-Peña et al 2003). Lyme disease is linked to numerous environmental and ecological systems. Soil characteristics (including order and texture), bedrock, land cover and forest type, sandy soil, low elevation, and deciduous forests have
all been implicated (Bunnell et al 2003; Jones and Kitron 2000; McCabe and Bunnell 2004). Deciduous forests have edaphic characteristics that support higher levels of ticks than coniferous forests (Guerra et al 2001; Bunnell et al 2003). In townships surrounding Lyme, Connecticut, landscape metrics of fragmentation are positively associated with tick density and NIP (Brownstein et al 2005). Smaller deer exclosures (less than 2.5 ha) had greater tick densities and within those exclosures, greater densities in the center (Perkins et al 2006). These varied findings suggest that there may be multiple forest fragmentation-linked ecological processes related to Lyme disease.
Tick habitat suitability, a common surrogate estimate of Lyme borrelosis risk, is defined by landscape characteristics across spatial scales. In the North Central United States, Guerra et al (2002) demonstrate tick presence or absence depends on soil order and texture, bedrock, land cover and forest type. In Maryland, agricultural land use has lower tick abundance than forested land cover (Das et al 2002). And along the East Coast, climate, sandy soil, low elevation, deciduous forests, soil characteristics and water availability are all related to elevated abundance of adult ticks (Bunnell et al 2003). In Wisconsin, vegetation detected by satellite—the spring and autumn normalized difference vegetation index (NDVI)—helped distinguish agricultural from forested areas and was significantly associated with human disease cases and tick distributions (Kitron and Kazmierczak 1997). In between this patchwork of regional scale analyses, a lack of uniform surveillance and environmental data limit the understanding of landscape characteristics and tick habitat suitability. Even the fine scale structure of understory vegetation may drive tick density and infection prevalence (Prusinski et al 2006).
Landscape ecology studies provide a powerful reminder that in addition to landscape composition, its configuration plays an important roll in biodiversity and TBD risk. Indices of landscape configuration suggest underlying biological relationships depend on the arrangement of the natural and built environments (Turner 2005). Fragmentation, edge and patch landscape metrics can help describe risks in the built environment, host abundance and distribution, vector abundance, and pathogen prevalence. Jackson et al show high incidence rates of Lyme borrelosis in Maryland are best explained by the amount of edge between forest and herbaceous land cover (2006). The configuration of the built environment, specifically the density and size of residential settings, defines the greatest variation of Lyme cases in a hyperendemic community in Connecticut (Cromley et al 1998). The dilution effect, noted above, is directly connected to the arrangement of the forest; the density of a highly competent Lyme reservoir, the white-footed mouse, is closely associated with the size of forest fragments (Nupp and Swihart 1998). Yet, fragmentation-related entomological risk may or may
not necessarily translate into human risk (Brownstein et al 2005, Wilder and Meikle 2004, Horobik et al 2006).
The survivability and stability of ticks in a world of declining biodiversity is not well understood. Biodiversity loss is a silent endpoint of global environmental change. “For the past 300 years, recorded extinctions for a few groups of organisms reveal rates of extinction at least several hundred times the rate expected on the basis of the geological record. The loss of biodiversity is the only truly irreversible global environmental change the Earth faces today” (Dirzo and Raven 2003). Studies of the dilution effect show higher rates of TBD transmission occur when vertebrate biodiversity that effectively buffers pathogen prevalence is reduced (Schmidt and Ostfeld 2001, LoGuidice et al 2003). However, TBD transmission may decline if just the highly competent pathogen reservoirs are absent (Giardina et al 2000). Kessing et al recently proposed another perspective on biodiversity in an ecological disease system. They find hosts, by killing ticks, can act as “ecological traps,” and the removal of these hosts may elevate TBD risk, “by increasing both vector numbers and vector infection rates with a zoonotic pathogen” (2009).
Biodiversity plays an important but unassuming role in controlling ticks. There are numerous “natural enemies” of ticks, including mammals and birds, parasitoid wasps, nematodes, bacteria, and fungi proposed to be used as bio-control agents (Ostfeld et al 2006a, Samish et al 2004). For instance, “fire ants” (Solenopsis invecta) are thought to have reduced populations of the tick Amblyomma americanum in southern US, while spiders are thought to have reduced populations of the tick Rhipicephalus sanguineus in Corsica (Wilson 1994). Another biodiversity-linked method of tick control is the exploitation of host pheromones and immunology. These natural organic compounds stimulate tick assembly, dispersal, attraction, attachment and mating behavior (Sonenshine 2006). Micro-infestations of ticks can emerge from pheromonal feedback loops (Yoder et al 2008). And natural systems of biodiversity are critical in identification of pheromones that may support future tick control. For instance, recently penguins in the Antarctic Peninsula were found to provide an assembly pheromone (via guano) of the seabird tick, Ixodes uriae (Benoit et al 2008). Another discovery shows the blood of a lizard host of I. pacificus blood contains an anti-bacterial agent that effectively eliminates Borrelia burgdorferi s.l. from its blood stream (Lane and Quistad 1998).
Given the historical resilience and persistence of ticks through the millennium, their expanding geographic range at the very least introduces a powerful vector of infectious disease and the impact of that introduction depends on local biodiversity. Tick distributions and tick-borne disease transmission potential have localized dependencies on biotic diversity (Ogden and Tsao 2009). In alpine regions in Italy the increased abundance
of roe deer is a strong predictor of resurgent TBE, along with changes in forest structure (Rizzoli et al 2009). But locally in the same region deer exclosures were found to have greater nymphal tick abundance on rodents (Perkins et al 2006). As another example, when deer density in a New Jersey township with endemic Lyme borrelosis was reduced, transmission appeared to increase the population of host-seeking ticks (Jordan et al 2007). Anticipating future transmission risk is challenging in a static environment, and even more so under scenarios of tick migration, as the spatial habitats of key reservoir species, which may also vary by locality, are also shifting (Jannette et al 2007, Brisson et al 2009, Surgeoner et al 1997, Sutherst et al 2007). The gross impact of tick expansion into new areas will be mitigated by local relationships of species richness and diversity and new adaptations within the community (Ogden et al 2008b, Frank et al 1998).
Integration on Environmental Determinants
The study of TBDs is uncovering dynamic ecological disease processes. One classic and elegant study tells the story of how Lyme disease risk in Dutchess County, New York, is embedded in an oak forest system, and connected to masting and rodent, deer, and gypsy moth abundance (Ostfeld et al 1996). But ecological disease processes cannot always be isolated from anthropogenic processes. Vanwambeke et al tracked TBE incidence in Latvia from 1999–2003, and found it was related to land cover, but also patterns of land use and ownership (2009). Further, at a regional scale the TBE increase in Central and Eastern Europe is attributed in part to “human behaviour determined by socio-economic conditions” that followed the collapse of the Soviet Union (Randolph 2007).
Scientists continue to move ahead with studies of global environmental change and the complex disease ecology of TBDs turning research gaps into challenges:
“The emerging research field focusing on climate-driven change in spatial and temporal patterns of arthropod vectors, vector-borne pathogens, or incidence of vector-borne diseases is characterized by a plethora of models based on empirical data of variable quality and a disturbing lack of empirical long-term studies that will allow us to demonstrate that future change was in fact driven by climate factors.” (Eisen 2008)
“[There is] a need for a more standardized and comprehensive approach to studying the spatial dynamics of the Lyme disease (LD) system…. Significant progress in identifying the determinants of spatial variation in LD risk and incidence requires that: (1) existing knowledge of the biology of the individual components of each LD system is utilized in the development of spatial models; (2) spatial data are collected over longer periods of time;
(3) data collection and analysis among regions are more standardized; and (4) the effect of the same environmental variables is tested at multiple spatial scales.” (Killilea et al 2008)
“Improved methods for collection and presentation of spatial epidemiologic data are needed for vectorborne diseases in the United States. Lack of reliable data for probable pathogen exposure sites has emerged as a major obstacle to the development of predictive spatial risk models…. New methods are urgently needed to determine probable pathogen exposure sites that will yield reliable results while taking into account economic and time constraints of the public health system and attending physicians. Recent data demonstrate the need for a change from use of the county spatial unit for presentation of incidence of vectorborne diseases to more precise ZIP code or census tract scales.” (Eisen and Eisen 2007)
Added to these challenges is the need for comprehensive transdisciplinary approaches to risk assessment, disease surveillance, and treatments (Patz et al 2004). This need requires collaboration among academics and practitioners of public health to join epidemiological, environmental and social determinants, and it must cross political boundaries (AagaardHansen et al 2009, Ahmed et al 2009, Hui 2006). The best science on global environmental change TBD ecology will need to reflect the complex and dynamic ecological connections driving them.
Acknowledgements
The authors greatly appreciate the comments and feedback from Dr. Nicholas Odgen and Dr. Jack Teng, as well as editing by George Allez. We also thank the Robert Wood Johnson Working Group on Interdisciplinary Perspectives on Health and Society (based at the University of Wisconsin) for a Graduate Research Award to Dr. Olson to address land-use change and Lyme disease risk.
References
Aagaard-Hansen, J., B. H. Sorensen, and C. L. Chaignat. 2009. A comprehensive approach to risk assessment and surveillance guiding public health interventions. Tropical Medicine & International Health 14(9): 1034-1039.
Ahmed, J., M. Bouloy, O. Ergonul, A. R. Fooks, J. Paweska, V. Chevalier, C. Drosten, and R. Moormannet. 2009. International network for capacity building for the control of emerging viral vector-borne zoonotic diseases: Arbo-zoonet. Eurosurveillance 14(12): 12-15.
Allan, B.F., F. Keesing and R.S. Ostfeld. 2003. Effect of forest fragmentation on lyme disease risk. Conservation Biology 17(1): 267-272. doi:10.1046/j.1523-1739.2003.01260.x.
Altizer, S., A. Dobson, P. Hosseini, P. Hudson, M. Pascual and P. Rohani. 2006. Seasonality and the dynamics of infectious diseases. Ecology Letters 9(4): 467-484. doi:10.1111/j.1461-0248.2005.00879.x.
Benoit, J.B., J. A. Yoder, G. Lopez-Martinez, M. A. Elnitsky, R. E. Lee and D. L. Denlinger. 2007. Habitat requirements of the seabird tick, Ixodes uriae (Acari: Ixodidae), from the Antarctic Peninsula in relation to water balance characteristics of eggs, nonfed and engorged stages. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 177(2): 205-215, DOI: 10.1007/s00360-006-0122-7.
Benoit, J.B., G. Lopez-Martinez, S.A. Philips, M. A. Elnitsky, J. A. Yoder, R.E. Lee and D.L. Denlinger. 2008. The seabird tick, Ixodes uriae, uses uric acid in penguin guano as a kairomone and guanine in tick feces as an assembly pheromone on the Antarctic Peninsula. Polar Biology 31(12): 1445-1451. doi:10.1007/s00300-008-0485-1. http://www.springerlink.com/index/10.1007/s00300-008-0485-1.
Brisson, D., D. E. Dykhuizen and R. S. Ostfeld. 2008. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proceedings Of The Royal Society B-Biological Sciences 275(1631): 227-235.
Brown, D.G., K.M. Johnson, T.R. Loveland and D.M. Theobald. 2005. Rural land-use trends in the conterminous United States, 1950-2000. Ecological Applications 15(6): 1851-1863.
Brown, R.N., R.S. Lane and D.T. Dennis. 2005. Geographic distributions of tick-borne diseases and their vectors. In tick-borne diseases of humans, J.L. Goodman, D.T. Dennis, and D.E. Sonenshine, 363-391. ASM Press.
Brownstein, J. S., D. K. Skelly, T. R. Holford and D. Fish. 2005. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia 146: 469-475.
Bunnell, J.E., S.D. Price, A. Das, T.M. Shields and G E Glass.. 2003. Geographic Information Systems and spatial analysis of adult Ixodes scapularis (Acari : Ixodidae) in the Middle Atlantic region of the USA. Journal of Medical Entomology 40(4): 570-576.
Burri, C., F.M. Cadenas, V. Douet, J. Moret and L. Gern. 2007. Ixodes ricinus density and infection prevalence of Borrelia burgdorferi sensu lato along a North-facing altitudinal gradient in the Rhône Valley (Switzerland). Vector Borne and Zoonotic Diseases (Larchmont, N.Y.) 7(1): 50-58. doi:10.1089/vbz.2006.0569. http://www.ncbi.nlm.nih.gov/pubmed/17417957.
Cadenas, F.M., O. Rais, F. Jouda, V. Douet, P.F. Humair, J. Moret and L. Gern. 2007. Phenology of Ixodes ricinus and infection with Borrelia burgdorferi sensu lato along a north-and south-facing altitudinal gradient on Chaumont Mountain, Switzerland. Journal of Medical Entomology 44(4): 683-693. http://www.ncbi.nlm.nih.gov/pubmed/17695026.
Carey, J.R. 2001. Insect biodemography. Ann. Rev. Entomol 46: 79-110.
Clark, D.D. 1995. Lower temperature limits for activity of several Ixodid ticks (Acari: Ixodidae): effects of body size and rate of temperature change. Journal of Medical Entomology 32, no. 4: 449-452. http://www.ncbi.nlm.nih.gov/pubmed/7650705.
Corson, M.S., P.D. Teel and W.E. Grant 2004. Microclimate influence in a physiological model of cattle-fever tick (Boophilus spp.) population dynamics. Ecological Modeling 180(4): 487-514. doi:10.1016/j.ecolmodel.2004.04.034.
Craine, N.G., S.E. Randolph and P.A. Nuttall. 1995. Seasonal variation in the role of grey squirrels as hosts of Ixodes ricinus, the tick vector of the Lyme disease spirochaete, in a British woodland. Folia Parasitologica 42: 73-80.
Cromley, E.K., M.L. Cartter, R.D. Mrozinski and S.H. Ertel. 1998. Residential setting as a risk factor for Lyme disease in a hyperendemic region. American Journal of Epidemiology 147(5): 472-477.
Cumming, G.S. and D.P. Van Vuuren. 2006. Will climate change affect ectoparasite species ranges?. Global Ecology and Biogeography 15(5): 486-497.
Cumming, G.S. and J.F. Guegan. 2006. Food webs and disease: is pathogen diversity limited by vector diversity? EcoHealth 3:163-170.
Daniel, M. and F. Dusbabek. 1994. Micrometeorological and micro-habitat factors affecting maintenance and dissemination of tick-borne diseases in the environment. In: Sonenshine, DE, Mather, TN, eds. Ecological Dynamics of Tick-borne Zoonoses. Oxford, UK: Oxford University Press; 91-138.
Danielová, V., M. Daniel, L. Schwarzová, J. Materna, N. Rudenko, M. Golovchenko, J. Holubová, L. Grubhoffer and P. Kilián 2010. Integration of a tick-borne encephalitis virus and Borrelia burgdorferi sensu lato into mountain ecosystems, following a shift in the altitudinal limit of distribution of their vector, Ixodes ricinus (Krkonoše Mountains, Czech Republic). Vector-borne and Zoonotic Diseases 10(3): 223-230.
Das, A., S.R. Lele, G.E. Glass, T. Shields and J. Patz. 2002. Modeling a discrete spatial response using generalized linear mixed models: application to lyme disease vectors. International Journal of Geographical Information Science 16(2): 151-166.
Dennis, D.T. and J.E. Piesman. 2005. Overview of tick-borne infections of humans. In tickborne diseases of humans, Jesse L. Goodman, David Tappen Dennis, and Daniel E. Sonenshine, 3-11. ASM Press.
Dirzo, R. and P.H. Raven. 2003. Global State of Biodiversity and Loss. Annual Review of Environment and Resources 28(1): 137-167. doi:10.1146/annurev.energy.28.050302.105532. http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.energy.28.050302.105532.
Eisen, L. and R.J. Eisen. 2007. Need for improved methods to collect and present spatial epidemiologic data for vectorborne diseases. Emerging Infectious Diseases 13(12): 1816-1820.
Eisen, L. 2008. Climate change and tick-borne diseases: A research field in need of long-term empirical field studies. International Journal of Medical Microbiology 298: 12-18. doi:10.1016/j.ijmm.2007.10.004.
Estrada-Peña, A. 2002. Increasing habitat suitability in the United States for the tick that transmits Lyme disease: A remote sensing approach. Environmental Health Perspectives 110(7): 635-640.
Estrada-Peña, A. 2003. The relationships between habitat topology, critical scales of connectivity and tick abundance Ixodes ricinus in a heterogeneous landscape in northern Spain. Ecography 26(5): 661-671. doi:10.1034/j.1600-0587.2003.03530.x.
Foley, J.E., N.C. Nieto and P. Foley. 2009. Emergence of tick-borne granulocytic anaplasmosis associated with habitat type and forest change in northern California. The American Journal of Tropical Medicine and Hygiene 81(6): 1132-1140. doi:10.4269/ajtmh.2009.09-0372. http://www.ncbi.nlm.nih.gov/pubmed/19996448.
Frank, C., A.D. Fix, C.A. Pena and G.T. Strickland.. 2002. Mapping Lyme disease incidence for diagnostic and preventive decisions, Maryland. Emerging Infectious Diseases 8(4): 427-429.
Fuente, J.D.L. 2003. The fossil record and the origin of ticks (Acari: Parasitiformes: Ixodida). Vector-borne and Zoonotic Diseases 29: 331-344.
Gale, P., A. Estrada-Peña, M. Martinez, R.G. Ulrich, A. Wilson, G. Capelli and P. Phipps. 2009. The feasibility of developing a risk assessment for the impact of climate change on the emergence of Crimean-Congo hemorrhagic fever in livestock in Europe: a Review. Journal of Applied Microbiology 108: 1859-1870. doi:10.1111/j.1365-2672.2009.04638.x.
Gale, P., A. Brouwer, V. Ramnial, L. Kelly, R. Kosmider, A.R. Fooks and E.L. Snary. 2010. Assessing the impact of climate change on vector-borne viruses in the EU through the elicitation of expert opinion. Epidemiology and Infection 138(2): 214-225. doi:10.1017/S0950268809990367.
Giardina, A.R., K.A. Schmidt, E.M. Schauber and R.S. Ostfeld. 2000. Modeling the role of songbirds and rodents in the ecology of Lyme disease. Canadian Journal of Zoology 78(12): 2184-2197. doi:10.1139/cjz-78-12-2184.
Gilbert, L. 2010. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases?. Oecologia 162(1): 217-225. doi:10.1007/s00442-009-1430-x. http://www.ncbi.nlm.nih.gov/pubmed/19685082.
Gubler, D., J. Reiter and Ebi, K.L. 2001. Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environmental Health Perspectives, 109: 223-233.
Guerra, M.A., E.D. Walker and U. Kitron. 2001. Canine surveillance system for Lyme borreliosis in Wisconsin and northern Illinois: Geographic distribution and risk factor analysis. The American Journal of Tropical Medicine and Hygiene 65(5): 546-552.
Guerra, M., E. Walker, C. Jones, S. Paskewitz, M.R. Cortinas and A. Stancil. 2002. Predicting the risk of Lyme disease: Habitat suitability for Ixodes scapularis in the north central United States. Emerging Infectious Diseases 8(3): 289-297.
Hansen, A.J., R.L. Knight, J.M. Marzluff, S. Powell, K. Brown and P.H. Gude, 2005. Effects of exurban development on biodiversity: patterns, mechanisms, and research needs. Ecological Applications 15(6): 1893-1905.
Horobik, V., F. Keesing and R.S. Ostfeld. 2006. Abundance and Borrelia burgdorferi-infection prevalence of nymphal Ixodes scapularis ticks along forest–field edges. EcoHealth 3(4): 262-268. doi:10.1007/s10393-006-0065-1.
Hubalek, Z., J. Halouzka and Z. Juricova. 2003. Host-seeking activity of Ixodid ticks in relation to weather variables. Journal of Vector Ecology 28(2): 159-165.
Hui, E.K.W. 2006. Reasons for the increase in emerging and re-emerging viral infectious diseases. Microbes and Infection 8(3): 905-916.
Hulme, M. and N. Sheard. 1999. Climate change scenarios for the United States of America. Norwich, UK: Climatic Research Unit.
IOM (Institute of Medicine). 2003. Microbial threats to health: emergence, detection, and response. By Mark S. Smolinski, Margaret A. Hamburg, Joshua Lederberg, Institute of Medicine (U.S.). Committee on Emerging Microbial Threats to Health in the 21st Century. The National Academies Press, Washington, DC.
Jackson, L.E., E.D. Hilborn and J.C. Thomas. 2006. Towards landscape design guidelines for reducing Lyme disease risk. International Journal of Epidemiology 35(2): 315-322. doi:10.1093/ije/dyi284.
Jannett, F.J., M.R. Broschart, L.H. Grim and J.P. Schaberl. 2007. Northerly range extensions of mammalian species in Minnesota. The American Midland Naturalist 158(1): 168-176. doi:10.1674/0003-0031.
Jones, C.J. and U.D. Kitron. 2000. Populations of Ixodes scapularis (Acari: Ixodidae) are modulated by drought at a Lyme disease focus in Illinois. Journal of Medical Entomology 37(3): 408-415. http://www.ncbi.nlm.nih.gov/pubmed/15535585.
Jordan, R.A., T.L. Schulze and M.B. Jahn. 2007. Effects of reduced deer density on the abundance of Ixodes scapularis (Acari: Ixodidae) and Lyme disease incidence in a northern New Jersey endemic area. Journal of Medical Entomology 44(5): 752-757. http://www.ncbi.nlm.nih.gov/pubmed/17915504.
Jouda, F., J.L. Perret and L. Gern 2004. Ixodes ricinus density, and distribution and prevalence of Borrelia burgdorferi sensu lato infection along an altitudinal gradient. Journal of Medical Entomology 41(2): 162-169. http://www.ncbi.nlm.nih.gov/pubmed/15061274.
Keesing, F., J. Brunner, S. Duerr, M. Killilea, K. Logiudice, K. Schmidt, H. Vuong and R.S. Ostfeld. 2009. Hosts as ecological traps for the vector of Lyme disease. Proceedings of the Royal Society B: Biological Sciences 276(1675): 3911-3919. doi:10.1098/rspb.2009.1159. http://www.ncbi.nlm.nih.gov/pubmed/19692412.
Kerr, G.D. and C.M. Bull. 2006. Interactions between climate, host refuge use, and tick population dynamics. Parasitology Research 99(3): 214-222. doi:10.1007/s00436-005-0110-y. http://www.ncbi.nlm.nih.gov/pubmed/16541265.
Killilea, M.E., A. Swei, R.S. Lane, C.J. Briggs and R.S. Ostfeld. 2008. Spatial dynamics of Lyme disease: a review. EcoHealth 5(2): 167-195. doi:10.1007/s10393-008-0171-3. http://www.ncbi.nlm.nih.gov/pubmed/18787920.
Kirby, A.D., A.A. Smith, T.G. Benton and P.J. Hudson. 2004. Rising burden of immature sheep ticks (Ixodes ricinus) on red grouse (Lagopus lagopus scoticus) chicks in the Scottish uplands. Medecal Veterinary Entomology 18: 67-70.
Kitron, U. and J.J. Kazmierczak. 1997. Spatial analysis of the distribution of Lyme disease in Wisconsin. American Journal of Epidemiology 145(6): 558-566. http://www.ncbi.nlm.nih.gov/pubmed/9063347.
Kovats, R.S., D. H. Campbell-Lendrum, A.J. McMichel, A.Woodward and J.S.H. Cox. 2001. Early effects of climate change: do they include changes in vector-borne disease?. Philosophical Transactions of the Royal Society B: Biological Sciences 356(1411): 1057-1068. doi:10.1098/rstb.2001.0894.
Langlois, J.P., L. Fahrig, G. Merriam and H. Artsob. 2001. Landscape structure influences continental distribution of hantavirus in deer mice. Landscape Ecology 16(3): 255-266.
Lindgren, E., L. Talleklint and T. Polfeldt. 2000. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environmental Health Perspective. 108(2): 119-123. http://www.jstor.org/stable/3454509.
Lindgren, E. and R. Gustafson. 2001. Tick-borne encephalitis in Sweden and climate change. Lancet358(9275): 16-18.
Lindsay, L.R., I.K. Barker, G.A. Surgeoner, S.A. McEwen, T.J. Gillespie and J.T. Robinson. 1995. Survival and development of Ixodes scapularis (Acari: Ixodidae) under various climatic conditions in Ontario, Canada. Journal of Medical Entomology 32(2): 143-152. http://www.ncbi.nlm.nih.gov/pubmed/7608920.
LoGiudice, K., R.S. Ostfeld, K.A. Schmidt and F. Keesing. 2003. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences of the United States of America 100(2): 567-571.
Lukan, M., E. Bullova and B. Petko. 2010. Climate warming and tick-borne encephalitis, Slovakia. Emerging Infectious Diseases 16(3): 524-526. http://www.ncbi.nlm.nih.gov/pubmed/20202437.
McCabe, G.J. and J.E. Bunnell. 2004. Precipitation and the occurrence of Lyme disease in the northeastern United States. Vector-borne and Zoonotic Diseases 4(2): 143-148.
McEnroe, W.D. 1997. The restriction of the species range of Ixodes scapularis, say, in Massachusetts by fall and winter temperature. Acarologia (18): 618-625.
McEnroe, W.D. 1984. Winter survival and spring breeding by the fall tick, Ixodes dammini, in Massachusetts (Acarina: Ixodidae). Acarologia (25): 233-229.
MEA (Millennium Ecosystem Assessment). 2005. Ecosystems and human well-being: Synthesis. Washington, DC: World Resources Institute.
Norman, R., R.G. Bowers, M. Begon and P.J. Hudson. 1999. Persistence of tick-borne virus in the presence of multiple host species: tick reservoirs and parasite-mediated competition. J. Theoretical. Biology. 200: 111-118.
Nupp, T.E. and R.K. Swihart. 1998. Effects of forest fragmentation on population attributes of white-footed mice and eastern chipmunks. Journal of Mammalogy 79(4): 1234-1243.
Ochanda, H., A.S. Young, J.J. Mutugi, J. Mumo, P.L. Omwoyo. 1998. The effect of temperature on the rate of transmission of Theileria parva parva infection to cattle by its tick vector, Rhipicephalus appendiculatus. Parasitology 97(2): 239-245.
Ochanda, H., A.S. Young and M. Graham. 2003. Survival of Theileria parva in its nymphal tick vector Rhipicephalus appendiculatus under laboratory and quasi natural conditions. Parasitology 126(6): 571-576.
Ogden, N.H., M. Bigras-Poulin, C.J. O’Callaghan, I.K. Barker, L.R. Lindsay, A. Maarouf, K.E. Smoyer-Tomic, D. Waltner-Toews and D. Charron. 2005. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. International Journal for Parasitology 35(4): 375-389.
Ogden, N.H., H.A. Maarouf, I.K. Barker, M. Bigras-Poulin, L.R. Lindsay, M.G. Morshed, C.J. O’Callaghan, F. Ramay, D. Waltner-Toews and D.F. Charron. 2006. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. International Journal for Parasitology 36(1): 63-70. http://www.ncbi.nlm.nih.gov/pubmed/16229849.
Ogden, N.H., M. Bigras-Poulin, K. Hanincová, A. Maarouf, C.J. O’Callaghan and K. Kurtenbach. 2008a. Projected effects of climate change on tick phenology and fitness of pathogens transmitted by the North American tick Ixodes scapularis. Journal of Theoretical Biology 254(3): 621-632. http://www.ncbi.nlm.nih.gov/pubmed/18634803.
Ogden, N.H., L.R. Lindsay, K. Hanincová, I.K. Barker, M. Bigras-Poulin, D.F. Charron and A. Heagy. 2008b. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Applied and Environmental Microbiology 74(6): 1780-1790. doi:10.1128/AEM.01982-07. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2268299&tool=pmcentrez&rendertype=abstract.
Ogden, N.H. and J.I. Tsao. 2009. Biodiversity and Lyme disease: Dilution or amplification?. Epidemics 1(3): 196-206. doi:10.1016/j.epidem.2009.06.002. http://linkinghub.elsevier.com/retrieve/pii/S1755436509000322.
Ostfeld, R.S. and C.G. May 1996. Of mice and mast. Bioscience 46(5).
Ostfeld, R.S. and F. Keesing. 2000a. Biodiversity and disease risk: The case of Lyme disease. Conservation Biology 14(3): 722-728.
Ostfeld, R. and F. Keesing. 2000b. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Canadian Journal of Zoology-Revue Canadienne De Zoologie 78(12): 2061-2078. ://000165450400002 .
Ostfeld, R.S., A. Price, V.L. Hornbostel, M.A. Benjamin and F. Keesing. 2006. Ticks controlling and tick-borne and zoonoses with biological chemical agents. Sciences-New York 56(5): 383-394.
Parola, P., C. Socolovschi, L. Jeanjean, I. Bitam, P.E. Fournier, A. Sotto, P. Labauge and D. Raoult. 2008. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS neglected tropical diseases 2(11): e338. doi:10.1371/journal.pntd.0000338. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2581602&tool=pmcentrez&rendertype=abstract.
Pascual, M. and A. Dobson. 2005. Seasonal patterns of infectious diseases. PLoS Medicine 2(1): 18-20.
Pascual, M. and M.J. Bouma. 2009. Do rising temperatures matter?. Ecology 90(4): 906-912. http://www.ncbi.nlm.nih.gov/pubmed/19449684.
Patz, J.A., P. Daszak, G.M. Tabor, A.A. Aguirre, M. Pearl, J. Epstein, N.D. Wolfe, A.M. Kilpatrick, J. Foufopoulos, D. Molyneux, D.J. Bradley and Members of the Working Group on Land Use Change and Disease Emergence. 2004. Unhealthy Landscapes: Policy Recommendations on Land Use Change and Infectious Disease Emergence. Environ Health Perspect 101: 1092-1098.
Patz, J.A., D. Campbell-Lendrum, T. Holloway and J.A. Foley. 2005. Impact of regional climate change on human health. Nature 438: 310-317.
Patz, J.A. and S.H. Olson. 2006. Climate change and health: global to local influences on disease risk. Annals of Tropical Medicine and Parasitology 100(5-6): 535-549.
Patz, J.A., S.H. Olson, C.K. Uejio and H.K. Gibbs. 2008. Disease emergence from climate and land use change. Medical Clinics of North America 92: 1473-1491.
Perkins, S.E., I.M. Cattadori, V. Tagliapietra, A.P. Rizzoli and P.J. Hudson. 2006. Localized deer absence leads to tick amplification. Ecology 87(8): 1981-1986. http://www.ncbi.nlm.nih.gov/pubmed/16937637.
Plowright, R.K., S.H. Sokolow, M.E. Gorman, P. Daszak and J.E. Foley. 2008. Causal inference in disease ecology: investigating ecological drivers of disease emergence. Frontiers in Ecology and the Environment 6(8): 420-429. doi:10.1890/070086. http://www.esajournals.org/doi/abs/10.1890/070086.
Prusinski, M.A., H.Y. Chen, J.M. Drobnack, S.J. Kogut, R.G. Means, J.J. Howard, J. Oliver, G. Lukacik, P.B. Backenson and D.J. White. 2006. Habitat structure associated with Borrelia burgdorferi prevalence in small mammals in New York State. Environmental Entomology 35(2): 308-319. ://000236865500016.
Randolph, S.E. 1994. Population dynamics and density-dependent seasonal mortality indices of the tick Rhipicephalus appendiculatus in east and southern Africa. Medical and Veterinary Entomology 8: 351-368.
Randolph, S.E. 1997. Abiotic and biotic determinants of the seasonal dynamics of the tick Rhipicephalus appendiculatus in South Africa. Medical and Veterinary Entomology 11: 25-37.
Randolph, S.E. and D.J. Rogers. 2000. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proceedings Of The Royal Society B-Biological Sciences 267(1454): 1741-1744. doi:10.1098/rspb.2000.1204. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1690733&tool=pmcentrez&rendertype=abstract.
Randolph, S.E. 2004. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 129(7): S37-S65. doi:10.1017/S0031182004004925.
Randolph, S.E., L. Asokliene, T. Avsic-Zupanc, A. Bormane, C. Burri, L. Gern and I. Golovljova. 2008. Variable spikes in tick-borne encephalitis incidence in 2006 independent of variable tick abundance but related to weather. Parasites & Vectors 1(1): 44. doi:10.1186/1756-3305-1-44.
Randolph, S.E. 2008. Tick-borne encephalitis incidence in Central and Eastern Europe: consequences of political transition. Microbes and Infection / Institut Pasteur 10(3): 209-216. doi:10.1016/j.micinf.2007.12.005. http://www.ncbi.nlm.nih.gov/pubmed/18316221.
Randolph, S.E. 2010. To what extent has climate change contributed to the recent epidemiology of tick-borne diseases?. Veterinary parasitology 167(2-4): 92-94. doi:10.1016/j. vetpar.2009.09.011. http://www.ncbi.nlm.nih.gov/pubmed/19833440.
Rizzoli, A., H.C. Hauffe, V. Tagliapietra, M. Neteler and R. Rosa. 2009. Forest structure and roe deer abundance predict tick-borne encephalitis risk in Italy. PLoS ONE 4, e4336. doi:10.1371/journal.pone.0004336.
Salkeld, D.J. and R.S. Lane. 2010. Community ecology and disease risk: lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology 91(1): 293-298. http://www.ncbi.nlm.nih.gov/pubmed/20380218.
Samish, M., H. Ginsberg and I. Glazer. 2004. Biological control of ticks. Parasitology 129: S389-S403.
Scharlemann, J.P.W., P.J. Johnson, A.A. Smith, D.W. Macdonald and S.E. Randolph. 2008. Trends in ixodid tick abundance and distribution in Great Britain. Medical Veterinary Entomology 22: 238-247.
Schmidt, K.A. and R.S. Ostfeld. 2001. Biodiversity and the dilution effect in disease ecology. Ecological Society of America 82(3): 609-619.
Schulze, A.L. and R.A. Jordan. 1996. Seasonal and long-term variations in abundance of adult Ixodes scapularis (Acari: Ixodidae) in different coastal plain habitats of New Jersey. Journal of Medical Entomology 33: 963-970.
Schulze, T.L., R.A. Jordan and R.W. Hung. 2001. Effects of Selected Meteorological Factors on Diurnal Questing of Ixodes scapularis and Amblyomma americanum (Acari :Ixodidae). Journal of Medical Entomology 38(2): 318-324.
Schulze, T.L., R.A. Jordan, C.J. Schulze and R.W. Hung. 2009. Precipitation and temperature as predictors of the local abundance of Ixodes scapularis (Acari: Ixodidae) nymphs. Journal of Medical Entomology 46(5): 1025-9. http://www.ncbi.nlm.nih.gov/pubmed/19769032.
Skarphédinsson, S., P.M. Jensen and K. Kristiansen. 2005. Infectious Diseases 11(7): 1055-1061.
Sonenshine, D. 1991. Biology of Ticks. Vol. 1. Oxford: Oxford University Press.
Sonenshine, D. 2005. The Biology of Tick Vectors of Human Disease. In Tick-Borne Diseases of Humans, by Jesse L. Goodman, David Tappen Dennis, and Daniel E. Sonenshine, 12-36. ASM Press.
Sonenshine, D.E. 2006. Tick pheromones and their use in tick control. Annual Review of Entomology 51(21): 557-580. doi:10.1146/annurev.ento.51.110104.151150. http://www.ncbi.nlm.nih.gov/pubmed/16332223.
Surgeoner, A., A. McEwen and I.K. Barker. 1997. Duration of Borrelia burgdorferi infectivity in white-footed mice for the tick vector Ixodes scapularis under laboratory and field conditions in Ontario. Journal of Wildlife Diseases 33(4): 766-775.
Sutherst, R. W., G. F. Maywald and A. S. Bourne. 2007. Including species interactions in risk assessments for global change. Global Change Biology 13(9): 1843-1859. doi:10.1111/j.1365-2486.2007.01396.x. http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2486.2007.01396.x.
Tabachnick, W.J. 2010. Challenges in predicting climate and environmental effects on vectorborne disease episystems in a changing world. Journal of Experimental Biology 213(6): 946-954. ://000275002600016.
Turner, M.G. 2005. Landscape ecology: What is the state of the science? Annual Review of Ecology, Evoution, and Systematics 36: 319-344.
Vandyk, J.K., D.M. Bartholomew, W.A. Rowley and K.B. Platt. 1996. Survival of Ixodes scapularis (Acari: Ixodidae) exposed to cold. Journal of Medical Entomology 33(1): 6-10. http://www.ncbi.nlm.nih.gov/pubmed/8906898.
Vanwambeke, S.O., D. Sumilo, A. Bormane, E.F. Lambin and S.E. Randolph. 2010. Landscape predictors of tick-borne encephalitis in Latvia: land cover, land use, and land ownership. Vector Borne and Zoonotic Diseases 10(5): 497-506. doi:10.1089/vbz.2009.0116. http://www.ncbi.nlm.nih.gov/pubmed/19877818.
Wilder, S.M. and D.B. Meikle. 2004. Prevalence of deer ticks (Ixodes Scapularis) on white-footed mice (Peromyscus Leucopus) in forest fragments. Journal of Mammalogy 85(5): 1015-1018. doi:10.1644/020. http://www.bioone.org/doi/abs/10.1644/020.
Wilson, M.L. 1994. Population ecology of tick vectors: Interaction, measurement, and analysis. In Ecological Dynamics of Tick-Borne Zoonoses, ed. T.N. Sonenshine and D.E. Mather, pp. 20-44. Oxford: Oxford University Press.
Wimberly, M.C., M.J. Yabsley, A.D. Baer, V.G. Dugan and W.R. Davidson. 2007. Spatial heterogeneity of climate and land-cover constraints on distributions of tick-borne pathogens. Global Ecology and Biogeography. http://www.blackwell-synergy.com/doi/abs/10.1111/j.1466-8238.2007.00353.x.
Yoder, J.A., J.T. Ark and A.C. Farrell. 2008. Failure by engorged stages of the lone star tick, Amblyomma americanum, to react to assembly pheromone, guanine and uric acid. Medical and Veterinary Entomology 22(2): 135-139. doi:10.1111/j.1365-2915.2008.00729.x. http://www.ncbi.nlm.nih.gov/pubmed/18498612.
Young, A.S. and B.L. Leitch. 1981. Epidemiology of East Coast fever: some effects of temperature on the development of Theileria parva in the tick vector, Rhipicephalus appendiculatus. Parasitology 83(1): 199-211. http://www.ncbi.nlm.nih.gov/pubmed/6791119.
Zeman, P. and C. Bene. 2004. A tick-borne encephalitis ceiling in central Europe has moved upwards during the last 30 years: possible impact of global warming? International Journal of Medical Microbiology 293: 48.
A3
THE HUMAN DIMENSION OF LYME AND OTHER TICK-BORNE DISEASES: THE PATIENT PERSPECTIVE
Submitted by: The National Capital Lyme & Tick-Borne Disease Association
“Courage is what it takes to stand up and speak; courage is what it takes to sit down and listen.”
Winston Churchill
When one family member is infected with Borrelia burgdorferi (Bb), the entire family is affected. Life as previously lived comes to a screeching halt. The focus of the family turns to survival of a disease that drains the energy, ability and hope from the patient and replaces it with pain, weakness and helplessness.
The unfortunate patients who fail the 2-4 week course of antibiotics are often reassured by their doctor they need no further treatment. They fall further into the progressive illness that includes facial paralysis, cognitive processing delays, difficulty walking, slurred speech, pain, and the loss of their former life and self. Prescription bottles and IV poles become the norm in the home. The roles of parent and spouse disappear as the victim of the disease can no longer fulfill responsibilities that once had been second-nature. Child patients often require individualized education plans, accommodations and special education. Some children must be home-schooled because they cannot get out of bed to participate in a regular classroom curriculum.
Months and years of illness march on and the patient grows wearier of any possibility of recovery. While the medical community spends its time arguing over whether to provide promising additional treatment, the patient faces a daily battle alone with the medical safety net stretched thin. Doctors, family and friends, who long since have had no viable answers, continue to be at a loss to know how to help and stop communicating. The fortunate patients who respond to aggressive antibiotic treatment face a slow climb back to their former life and health. Extensive treatment, however, comes with a price tag that for most patients means the obliteration of their retirement plans and, for some, becoming a financial burden to their children. When the IV pole has been moved from the bedroom to the garage, the question remains: “Do we give it away, or might we need it again?” Tick populations and their related illnesses are increasingly creating epidemics in neighborhood after neighborhood. Each year, more and more new cases are tabulated, documented and stored somewhere … and the band plays on.
Overview
Lyme borreliosis, commonly referred to as Lyme disease, alone or concurrent with other tick-borne infections, can be a debilitating illness that severely diminishes a person’s health and quality of life. The more inclusive term “Lyme borreliosis” is used in this paper, rather than Lyme disease, to include patients with persistent and relapsing infections of the Borrelia burgdorferi bacterium after initial therapy. This paper documents the impact of tick-borne infections on patients and their families. It draws on survey data, patient testimonies, and scientific research to examine symptom manifestation, chronicity of illness, and the human and economic costs of tick-borne infections. Children account for a significant number of Lyme borreliosis cases, and this paper examines how tick-borne infections disrupt their development into productive adults. Despite years of research on Lyme borreliosis, understanding of this infection and its impact on patients remains inadequate. This paper identifies gaps in research and medical care. The overwhelming consensus of Lyme patients and their families is that neither the government research community nor the medical community gives sufficient credibility to this disease or devotes adequate resources to combating it. The paper concludes with recommendations that include future research and policy changes needed to combat the serious and growing problem of tick-borne diseases in the United States.
Onset of Illness and Problems of Diagnosis
The beginning of patients’ struggle with Lyme borreliosis can be confusing and frightening. Misperception about tick bites, tick-related rashes, and the variety of symptoms leaves the patient feeling bewildered with a medical system that does not produce answers. As the mother of a 15-year-old patient from New Jersey described the situation, “The most worrisome thing was not knowing what was afflicting my daughter—not knowing who the enemy was for lack of a diagnosis.” Worry returns when doctors offer no explanation for the recurrence of symptoms after a short course of treatment.
Patients are fortunate if they observe the telltale erythema migrans (EM) or bull’s-eye rash at the time of their tick bite, which alerts them to seek immediate antibiotic treatment. As few as 44 percent of Lyme patients with chronic illness recall seeing a rash and only 29 percent recall seeing a tick bite (Donta, 1997). Unfortunately, many of those patients who do seek care for a rash do not always receive accurate or appropriate medical advice. Physicians unfamiliar with the many variations of EM rashes may miss the diagnosis of Lyme disease. Only 19 percent of rashes resemble a classic bull’s-eye rash (Tibbles and Edlow, 2007), and as many as 15 percent
of EM rashes were misdiagnosed, most often as spider bites but also as cellulitis and shingles (Aucott et al, 2009).
Sister of Patient A from Virginia, ill for 6 years:1
“She told the doctor of her fear of Lyme and showed him the bull’s-eye rash and she was ignored. We went to doctors located in Virginia where my sister resides and was bitten who said, ‘Lyme doesn’t exist here. We will not treat.’ My beloved twin began a horrible spiral that has gone downhill ever since. She, a woman who worked in healthcare 17 years helping people with HIV, became a victim of a system she used to believe in.”
One of the most common patient experiences is the misuse of the Centers for Disease Control and Prevention’s (CDC) Lyme disease surveillance case definition for diagnostic purposes. Many doctors are not aware that the CDC’s case definition opens with the warning, “This surveillance case definition was developed for national reporting of Lyme disease; it is not intended to be used in clinical diagnosis” (CDC, 2010a). Despite such admonitions, many doctors refuse to diagnose and treat unless the patient meets the CDC’s recommended two-tier testing criterion which requires a positive enzyme-linked immunosorbent assay (ELISA) or IFA before use of the Western blot. When the ELISA is negative, patients are routinely refused the more specific Western blot test. As a patient from New Hampshire reports, “All of my five Elisa tests were negative. I was sick with fatigue, headaches, and cognitive issues for a year and a half and missed a whole year of school. When a Western blot was finally done it was CDC positive for Lyme disease.”
Moreover, the tests for Lyme disease are notoriously unreliable due to the lack of sensitivity and specificity. Studies have found the sensitivity of the ELISA test for Bb infection to vary from 59 to 95 percent (Depietropaolo et al, 2005), meaning that tests can miss up to two-in-five of all cases. The authors found that two-tier testing further decreases test sensitivity, resulting in more missed diagnoses. Unreliable testing hampers both surveillance and patient care when the CDC criteria, designed for patient surveillance purposes, are being misused to deny treatment to patients.
The two-tier testing for Bb infection is not recommended since testing is unreliable until several weeks after the onset of illness as the test reflects antibodies in the blood that are not produced until that time (Aguero-Rosenfeld et al, 2005; Depietropaolo et al, 2005). Many patients report that they are tested prior to 4-6 weeks; and when their ELISA comes back
negative, they are not re-tested, and their infection goes undiagnosed. Since early diagnosis and treatment greatly improve outcomes, it is a tragedy that so many patients do not receive a timely diagnosis.
Patient B from North Carolina, sick for unknown years:
“I continue to deteriorate, now so weak I couldn’t go any real distance without a wheelchair … I experienced violent muscle jerks that left me sore afterwards, as if I had been beaten up. I went into long coma-like sleep lasting 20 hours a day, waking up lethargic, as if I never slept. I started to have impaired short term memory loss, difficulty multitasking or concentrating and forgetting how to do the simplest tasks, like how to take off my seatbelt. As I’m knee deep in this hell, my dogs start to get sick one right after the other. Their tick titers came back positive for ehrlichiosis, Lyme, and Rocky Mountain spotted fever. It was then I researched Lyme for myself and found all my seemingly unrelated symptoms. Excited I had found the answer, I went back to my doctor and told him to test me for Lyme. We did the Elisa which was negative, so he confidently proclaimed, ‘You do not have Lyme. We do not have it in North Carolina.’”
Even positive test results are sometimes dismissed by doctors as irrelevant without additional investigation into the cause of their illness. As one Virginia patient states, “Even with a positive ELISA and Western Blot and 23 symptoms I am being told by Infectious Disease [doctor] I am negative. I am being told we are not sure what you have had for 7 years but it is not Lyme.” This common experience indicates a perception of unreliability of Bb tests in the medical community, and therefore Lyme borreliosis should remain a clinical diagnosis. Far too many patients never hear about the possibility of Lyme borreliosis. A Johns Hopkins study found that 54 percent of patients were misdiagnosed when they did not present with a rash (Aucott et al, 2009). Other patients see a number of specialists who are unable to determine the proper diagnosis.
Mother on behalf of Patient C from Illinois, sick for 10 years:
“He saw 21 doctors who ran numerous tests. He was getting sicker, missing school and no one was putting it all together … the contact with friends diminished. At times he wondered what he has to live for.”
Lyme borreliosis is often not considered in the differential diagnosis of a patient’s worsening symptoms. Rather, patients are offered a diagnosis such as chronic fatigue syndrome or fibromyalgia, conditions for which there is no known cause or cure. Eventually, depression may also be diagnosed and antidepressants prescribed. Rarely do antidepressants relieve symptoms that Lyme patients experience.
Patient D, sick for more than 5 years:
“Sometimes I wonder if things would have been different had I been diagnosed earlier and received treatment at a younger age. This illness has destroyed my quality of life. Before my illness, I was a very active person capable of working long hours, jogging, tennis, running a household, and active in my church and community activities. It destroyed 2 marriages and I was not able to have children. It is not only costly, it is demoralizing to not be able to take care of yourself on a daily basis.”
Some patients with enough observable signs and symptoms are diagnosed with multiple sclerosis, lupus, or amyotrophic lateral sclerosis (ALS). Steroids are prescribed, which have the effect of worsening the Lyme patient’s infection through immune suppression, a finding reflected in rhesus monkey studies (Pachner et al, 2001). While the possibility of Lyme borreliosis is overlooked or outright rejected, patients are given seemingly unrelated diagnoses as varied as postural orthostatic tachycardia syndrome (POTS), gastroparesis, autonomic dysfunction disorder, hypoadrenalism, sleep apnea, obsessive compulsive disorder, Aspergers, Tourette syndrome, blood clotting disorders or cyclic vomiting syndrome.
Patient E from California, sick for 7 years:
“My father had unknowingly contracted Lyme, Babesia, and Bartonella. Six years later, he began to lose strength and felt like he was losing muscle. His HMO doctor ignored his complaint for over a year. A neurologist diagnosed him with ALS. My brother urged our father to pursue Lyme testing and a Western blot came back positive. The HMO agreed to put him on IV antibiotics for one month. After thirty days, Dad could stand up again but [the HMO] refused to continue IV antibiotics citing IDSA treatment guidelines. The HMO insisted Dad had ALS not Lyme Disease, ignoring the fact Dad gained back the use of his legs. [The HMO] also insisted that he had tongue atrophy and put him on a feeding tube. We were told to take Dad home to die and call Hospice. We chose to take our father out of [the HMO hospital] and put him in a nursing home under the care of a physician who would treat him for advanced Lyme Disease. An occupational therapist found that our father did not have tongue atrophy and he began to eat three meals a day. In the next eight months, my father was walking, off the ventilator, and off the feeding tube.”
Reaching the correct diagnosis of Lyme borreliosis can be a long and arduous process. A survey of patients conducted by the California Lyme Disease Association (CALDA) in 2009 found that 35 percent of patients consulted 10 or more doctors before receiving the diagnosis of Lyme borreliosis and 36 percent reported a delay of six years or more between onset of illness
and diagnosis. Lack of public and physician awareness leads to significant diagnostic delays. Like the correct diagnosis itself, the search for a knowledgeable physician proves extremely difficult for 58 percent of patients surveyed.
Mother on behalf of Patient F from Virginia, sick for 13 years:
“My son was diagnosed with chronic Lyme at age 18 after being misdiagnosed with chronic sinus infections and Chronic Fatigue Syndrome. He started having chronic headaches, fatigue and low fevers at age 9, and would improve on antibiotics, but then relapse a couple months after discontinuing them. He saw a CFS/FM specialist and was treated symptomatically for 5 years. He had to be home schooled the whole time due to his severe fatigue. He also acquired a severe sleep disorder, severe POTS, tremors, sound and light sensitivity, and severe cognitive issues (primarily brain fog, concentration problems and word retrieval difficulty.) He lost 35 lbs. At age 18, he was diagnosed with chronic Lyme disease. At age 22 after long-term Lyme treatment, he is improved enough to attend college part-time.”
Other Tick-Borne Infections
Joseph Piesman, who oversees the tick-borne disease program at the CDC, notes that, “The more people study ticks, the more new pathogens are discovered” (Landro, 2010). More than a dozen tick-borne diseases have been documented to cause serious illness in humans. “Ticks can be infected with bacteria, viruses, or parasites. Some of the most common tick-borne diseases in the United States include: Lyme disease, babesiosis, ehrlichiosis, Rocky Mountain Spotted Fever, anaplasmosis, Southern Tick-Associated Rash Illness, Tick-Borne Relapsing Fever, and tularemia. Other tick-borne diseases in the United States include Colorado tick fever, Powassan encephalitis, and Q-fever” (CDC, 2010b). A tick study from southern Connecticut found that 20 percent of the 230 Ixodes scapularis ticks collected were infected with Bb, and of these ticks 68 percent were co-infected with one or more additional pathogens (Sapi et al, 2009). Consequently, a person bitten by a tick is at risk for being infected with multiple tick-borne diseases.
The National Capital Lyme and Tick-borne Disease Association (NatCapLyme) conducted an online survey in July 2010 on Lyme disease for 10 days.2 A total of 1,438 subjects, elicited via e-mail from patient
support groups across the country, participated in the survey. Such a large response in so short a time demonstrates that those affected with tick-borne infections want serious consideration and recognition of the impact of the disease.
The NatCapLyme survey found that 46 percent of the respondents had been diagnosed with two or more tick-borne infections. Babesiosis is the most common co-infection, with 41 percent of respondents afflicted. Patients who are co-infected with Lyme borreliosis and other tick-borne infections experience more symptoms and more persistent illness than those with only Bb infection (Krause et al, 1996). Because symptoms for other tick-borne infections can be similar to those of Lyme borreliosis, these infections may go undiagnosed, contributing to ongoing illness despite treatment.
Patient G from Virginia, sick for 13 years:
“After my initial two and a half years of ‘lyme’ treatment, it was believed the lyme was ‘cured.’ Unfortunately, after a couple months, not only did all my symptoms come back, but, I had developed cardiac issues. Sadly, at this time, I was also diagnosed with Babesia and Bartonella by a specialty lab which verified IgG and IgM for Babesia. The physician believed the initial regional test he had performed earlier for Babesia did not include the strain I had, which was WA-1. If the co-infection testing had been accurate back in early 2004, I may not have had the struggle I still face with cardiac issues due to years of infection with Babesia and Bartonella.”
Lyme patients frequently suffer from other infections. The NatCapLyme survey found that fully 39 percent of respondents had been diagnosed with bartonellosis in addition to Lyme borreliosis. The tick study from southern Connecticut found the Bartonella henselae bacterium to be present in 30 percent of ticks, suggesting an association with ticks as a vector.
The exact role that other tick-borne diseases and opportunistic illnesses play in the disease course is poorly understood, suggesting the need for more research. Researchers have found that spirochete DNA remains in the circulation longer in subjects co-infected with both Lyme disease and babesiosis, compared to patients with Lyme disease alone, leading them to speculate that “babesial infection may impair human host defense mechanisms, as it does in cattle and mice” (Krause et al, 1996). Tick-borne infections can overwhelm the immune system, making the patient vulnerable to other infections, such as mycoplasma, Epstein Barr virus, yeast, H. pylori and Chlamydia pneumoniae, etc. Consequently, a downward spiral of health problems may consume the patient’s life and resources.
Ongoing Illness
Some patients report a full or nearly complete recovery following a month or less of antibiotic treatment for Lyme borreliosis. Receiving early treatment is critical, because left untreated, Lyme borreliosis can become a debilitating disease. “When ticks transmit B. burgdorferi to humans, a rash develops where the spirochetes enter the skin. Several days to weeks later, there may be symptoms elsewhere in the body, where the spirochetes have spread. The symptoms are more likely to appear in the joints, the nervous system, and the heart than anywhere else in the body. When the symptoms of Lyme disease appear in these parts of the body, the person may be considerably disabled. It is this potential for disability that, understandably, makes people so afraid of the disease” (Barbour, 1996).
Early treatment often fails when medication is discontinued prior to full recovery. Some patients experience a slow, insidious deterioration, while others report an abrupt return of former symptoms as well as new, disturbing ones. A literature review on treatment failure of antibiotic therapy found that after a 2-4 week course of treatment, 10 to 61 percent of patients relapse with debilitating symptoms that are indistinguishable from those of late Lyme borreliosis (Green, 2009). Further delay in treatment comes when patients wait to call their doctors during the initial relapse because they do not want to complain after being treated with what they have been told should be sufficient treatment. They are naturally bewildered that the therapy ceases before they feel better, since it is common for doctors to repeat a course of antibiotics when a throat or ear infection persists. In the case of Lyme disease, the end of treatment is often dictated by treatment guidelines rather than the resolution of symptoms.
It is well-documented that Bb can infect most parts of the body, producing different symptoms at different times (Steere, 1989; Duray, 1989). Early symptoms are easily mistaken as aches and pains attributable to the common flu, stress at work, over-exercising, or simply the natural aging process. The initial dismissal of possible Bb infection allows an otherwise more easily treated infection to develop into a full-blown central nervous system infection which is difficult to treat.
One of the more perplexing dimensions of untreated or under-treated infection is the multiplicity of symptoms that seem to change, appear and disappear suddenly, only to reappear. In patients with Lyme meningitis, Pachner (1995) notes that, “Symptoms can be surprisingly variable, so that days of near normality can alternate with days of profound debility.” Symptoms can include, but are not limited to, fever, chills, fatigue, body aches, headaches, rash, swollen lymph nodes, stiff neck, pain, meningitis, neurological problems, poor motor coordination, cognitive impairment, heart problems, eye inflammation, skin disorders, gastrointestinal issues,
and general weakness. The great range of symptoms can make disease recognition difficult.
Mother of Patient H from Florida, sick for 15 years:
“One of her doctors told us that if one doesn’t want to be bored in the medical profession, one should focus on Lyme disease since every organ in the body is affected. Our daughter has had brain encephalitis, arthritis, vision problems, sporadic rashes, extreme fatigue, a headache that has lasted 11 years, photosensitivity, hyperacusis, tinnitus, skin sensitivity, carditis, autonomic nervous system dysautonomia and much, much more.”
Patients choosing to continue their search for a treating physician often report great difficulties finding a doctor who has experience in treating persisting infection. Many patients report that the specialists they consult are focused on their chosen field of medicine and miss the multi-system nature of the disease. When this multi-system concept is understood and patients receive long-term antibiotic therapy, they report benefits. A patient survey found that between 72 and 78 percent of respondents experienced improvement in neuropathy, joint pain, concentration difficulties and fatigue with additional treatment beyond 2-4 weeks (CALDA, 2009).3 Also, two NIH-funded studies on re-treatment of patients with persistent Lyme borreliosis found a statistically significant improvement in symptoms. A study found that 64 percent of patients in the treatment group, versus 19 percent in the placebo group, showed substantial and sustained improvement in fatigue (Krupp et al, 2003). Another study found that patients with more severe symptoms who received an additional 10 weeks of antibiotics reported sustained improvement in pain and physical functioning (Fallon et al, 2008). Although there are treatment failures, long-term, combination antibiotic therapies do return many patients to functional and rewarding lives.
Patient I:
“After 10 weeks of Rocephin therapy, I had regained about 80% of my previous health, and most significantly, the return of my intellect and termination of my depression…. Now after 6 weeks on oral antibiotics, I’m approximately at 90% recovered…. And the big question: will my improvement hold with the eventual ending of antibiotics?”
A common theme in the patient stories we collected was that patients asked for more effective treatment. More research is needed regarding treatment failures in order to meet the needs of those Lyme borreliosis patients who continue to be ill. Lyme borreliosis can lower the quality of
life dramatically. Chronic Lyme borreliosis patients experience deficits in health status of someone with congestive heart failure or osteoarthritis and suffer more impairment than someone living with type 2 diabetes or a recent heart attack (Klempner et al, 2001). A more recent study found the fatigue level in patients with chronic Lyme disease equals that of multiple sclerosis (Fallon et al, 2008).
Impact on Work
Quality of life for adults with Lyme borreliosis is marked by major declines in vocational, social and recreational functioning, as well as overall deterioration in cognitive and neuropsychiatric impairment. Persistent Lyme borreliosis severely impacts patients’ abilities to function in the workplace. A James Madison University survey (Wilcox and Uram, 2009) on the impact of persistent Lyme disease on workplace performance found that patients experience difficulties working. Prior to their illness, all the respondents had been working full-time (a selection criterion), whereas only 28 percent were still working full-time at the time of the survey.
Cognitive impairment, memory, attention deficiencies, and lack of word fluency interfere with Lyme disease patients’ work performance. Eightynine percent of the 315 respondents said that their symptoms had either a “moderate impact” or a “severe impact” on their ability to remember facts and details. Moreover, 75 percent said that Lyme disease impacted their ability to understand complex concepts and analyze information. More than half of the respondents reported an adverse impact on their basic skills of reading, writing, and math. Such impaired employees are not the only ones affected. When they cannot fulfill their duties, their colleagues are forced to pick up the slack, which has a complex, negative impact on the workplace itself.
Fatigue, pain and dizziness also negatively affect Lyme patients’ ability to work. A study of Lyme patients with persistent symptoms found that 90 percent suffered from fatigue and malaise (Klempner et al, 2001); and nearly all of the respondents (95%) in the work performance survey reported that fatigue made it difficult, and in many cases impossible, to maintain a full-time workload (Wilcox and Uram, 2009). Connecticut biologist Joe Dowhan conducted tick research and brought the first Ixodes scapularis ticks to Dr. Allen Steere in 1976. Dowhan struggled to keep up with his work as he developed chronic Lyme disease:
“By the beginning of July (of 1976), I was crawling up the stairs to get home. The other biologists were finally telling me to just stay in the car because it was slowing everyone down. I was incapacitated with the disease…. In 1990, I started feeling a generalized malaise, a real fatigue,
headaches, neck aches … I just had overwhelming fatigue, unrelated to exercise. I would find myself at two in the afternoon absolutely needing to drop my head on the desk. I just couldn’t move. I’d come home. My pockets would be stuffed with notes (to myself); I was having a difficult time remembering things and getting dinged by my bosses for not following through with things, not even remembering phone conversations with them” (Edlow, 2003).
Dowhan’s story is repeated in workplaces across the country. Many Lyme patients struggle to adequately fulfill the requirements and responsibilities of their jobs. More than half (52%) of the respondents in the work performance survey changed jobs, usually for a less challenging and less stressful job (Wilcox and Uram, 2009). Many Lyme patients eventually became too disabled to work as their health deteriorated. Half (51%) of the respondents had left the workforce, and 55 percent of those who stopped working were receiving disability benefits.
Patient J from Maryland, sick for 6 years:
“In 2004, I developed severe left-sided head pain and pressure, and eventually migraine headaches. In November 2006 I collapsed at my job where I was working as a surgical nurse in open heart surgery. I had no feeling from the neck down. I have been on disability since. I had a constant ‘buzzing’ or ringing in my ears, with occasional loud blasts of sounds. Extreme fatigue, blurred vision, floaters in my eyes were next. Tingling and numbness in my legs and eventually my arms. Balance problems were next, stumbling and falling also. I eventually walked with a cane…. I could have avoided all of this if I had been treated with oral Doxycyline for 28 days. I would still be working as a registered nurse, a job that I loved. I have been disabled since I was 52…. After 3 years of treatment for Lyme Disease, I am better, but very different from a high functioning nurse that I was. I am mentally challenged and every day is a struggle, but I am thankful that I am alive.”
Functionality at Home
Many Lyme patients have to cut back on usual everyday activities. Cooking, cleaning, and other household tasks become arduous, if not impossible, to perform due to cognitive problems, pain and fatigue. Fifty-six percent of respondents to the NatCapLyme survey said that their ability to cook a meal was “moderately impacted” or “severely impacted” by their illness, and 70 percent said that cleaning the house was nearly impossible.
Patient K from Maryland, sick for more than 10 years:
“Despite [my husband’s] long work days, he was now in charge of meals and most everything else. I was rarely able to do the shopping. I no longer cooked. One morning I decided I wanted scrambled eggs for breakfast. I remember standing at the stove, spatula in hand, looking down at these yellow things in a pan, and very clearly thinking: I DON’T KNOW WHAT THESE ARE.”
Many patients have to curtail driving due to dizziness, vision problems and/or cognitive problems. Forty-six percent of the respondents in NatCapLyme’s survey said that their driving was impaired. Lyme patients have reported that they have become lost just blocks from their own home, as they are unable to recognize familiar landmarks. One Lyme patient from Maryland wrote, “Cognitive function declined rapidly … I would get lost less than a mile from my home that I lived at for thirty years.” Consequently, patients become dependent on family and friends for transportation or otherwise find themselves homebound and isolated.
Social Impact
Interpersonal relationships are strained to the breaking point by the challenges of Lyme borreliosis. Sometimes family and friends are sympathetic to the initial illness, assuming that it will pass like the flu. Patience wears thin, however, when a patient cannot resume normal vocational and recreational activities. Being part of a school, work, sports or social event requires accommodations for the patient. Only the immediate family members, who witness the planning required to get ready for simple daily activities, understand the extent of the efforts required to maintain some normalcy. Everyday grooming requires effort. Acting normal requires stamina. Showing up requires mobility. Patients must adopt a “one day at a time” philosophy and have to cancel vacations and other important events at the last minute due to flaring symptoms. Patients feel that repeated comments from family and friends ranging from “You look fine” and “Do you still have Lyme?” to “Why don’t you take some energy pills and get back into life?” invalidate their illness.
Patient L from Virginia, sick for unknown years:
“At first my children told me that I didn’t have Lyme disease, that it was all in my head, and then they began to make fun of me, and finally, now that I have a doctor and have been under treatment, they tell me I spend too much time focusing on my Lyme disease, that I need to stop thinking about it…. I have learned, over the years, to keep it hidden as much as
possible, to say nothing, to never ask for help, because when and if I try to talk to them about it, I am quickly silenced or humiliated. Their lack of compassion and understanding is what hurts the most.”
The patients’ testimonies frequently speak about broken engagements, divorces and abandonment by loved ones who have difficulty accepting that the person they once knew has unnaturally changed. Meanwhile, patients suffer a loss of self-esteem due to the burden of their illness being imposed on the family. Marriages are understandably strained. Spouses may be frustrated by their partner’s sudden inability or unwillingness to have children. Disagreements erupt over the patient’s inability to work and care for their children as well as the drain of life savings to pay for treatment. Parents may differ about how to treat an ailing child, and adult children can underestimate the depth of their parents’ suffering.
Mother on behalf of Patient M from Virginia, sick for 8 years:
“It’s rough on a marriage to have a child sick without a diagnosis for a long period of time. And, it’s even more difficult once there’s a controversial diagnosis that is very expensive to treat. Add to that a sick spouse and the opportunities for implosion are everywhere. Our life turned from activities, work, vacations, individual pursuits, hobbies and pleasures to pain, isolation, pressure to make treatment decisions based on limited understanding, and total exhaustion.”
Many times Lyme borreliosis infects multiple family members. If one or both parents are affected, children reverse roles and become caretakers to their parents at the expense of their own social lives and with undue stress on their personal development. The focus of the entire family is redirected toward daily survival of new and variable symptoms, the reduction of pain, and the potential for emergency room visits. The healthy family members feel the full weight of being the caregivers. Some have to start working multiple jobs to compensate for the patient’s lost income and the added financial burden.
The patient’s personality may undergo significant changes due to the illness. Previously vibrant people become confused, scared, and angry. A mother from New Jersey wrote: “In his teen years his health and personality changed. He was having problems with anger that were uncharacteristic for him. Over several years he became homicidal, suicidal, and was in jail.” Patients who were independent before their illness become dependent on family and friends for everything. Friendships are hard to maintain, and many patients feel isolated and alone. Feeling they are a burden to their loved ones makes it difficult to face another day of suffering. Seven percent of the respondents in the NatCapLyme survey said they had attempted suicide after contracting Lyme disease and 42 percent had suicidal thoughts.
Patient N from Kansas, sick for 9 years:
“Last September, I tried to commit suicide. I ended up in the hospital due to my wounds for two weeks. Everyone thought I just had a bad case of post-partum depression. But I knew the truth, I wasn’t depressed, I was absolutely terrified about how lyme was destroying my body, and I just could not take the pain anymore. Slowly I have gotten better on antibiotics. It has almost been a year … I have come a long way from where I was, and I am able to function as wife and mother again.”
As patients seek understanding on how to deal with the multi-faceted issues related to having Lyme disease, support groups can play an important role in helping patients regain their health. Struggling with bewildered family members and the lack of support from physicians and insurance companies, they turn to Lyme disease support groups to learn more about their illness and how to cope. Support groups provide life skills in how to deal with daily challenges, illness-related social problems, and disability and insurance issues. As former U.S. Surgeon General, C. Everett Koop, MD, explained: “I believe in self-help as an effective way of dealing with problems, stress, hardship and pain…. Mending people … is no longer enough; it is only part of the total health care that most people require.”
Financial Impact
As the illness stretches into months and then years, health care costs accumulate. NatCapLyme’s survey asked respondents for their “best estimate” of the total costs of their Lyme-related treatments. Forty-six percent said that they and their insurance company paid $50,000 or more for treatment. Many patients find their insurance coverage is inadequate to cover their growing health care needs (Jorgensen, 2010). Patients also report that their health insurance company has denied coverage of antibiotic therapy for persistent Lyme borreliosis based on the Infectious Diseases Society of America’s (IDSA) 2006 treatment guidelines on Lyme disease. A patient from Virginia, sick for over a year, explains, “Not only do Lyme patients have to battle the medical community for a proper diagnosis, but they are forced to battle the insurance companies for treatment.” Treatment that falls outside the guidelines is deemed “to exceed the standard of care” or is considered “experimental” and therefore is not covered. Thirty-five percent in the NatCapLyme survey responded that they were denied either insurance coverage or treatment due to IDSA treatment guidelines. A husband of a patient from Virginia who has been sick for 3 years states, “I have paid medical insurance premiums for more than 50 years but today’s medical system allows my insurance carrier to deny coverage of lyme treatment. Big insurance is allowed to practice medicine without a license.” Insurance
denial leaves Lyme patients with the choice of either paying for treatment out of pocket or being condemned to a life of persistent illness.
Patient O from Iowa, sick for 6 years:
“Our insurance company to this date refuses to pay for the treatment and antibiotics citing them to be experimental and not medically necessary. We exhausted all appeals with the insurance company, put a mortgage on our home and cashed out our retirement funds to pay for the medical bills. We currently have a case pending before the Supreme Court of Iowa pertaining to medical coverage denied by our health insurer.”
Most health care costs could be avoided if the infection were diagnosed in a timely manner and treated aggressively. According to a CDC study on the economic impact of Lyme disease, the estimated average annual costs, both medical and non-medical, per patient with late-stage Lyme disease are $18,880 (in 2009 dollars). That amount is 10 times higher than the per patient costs for early-stage Lyme disease (Zhang et al, 2006).
Lyme borreliosis poses a serious financial strain on families. Numerous patients with persistent Lyme borreliosis are forced to cut back on work, modify their career goals, or stop working altogether. Parents may have to curtail work hours to care for a sick child. According to the JMU work performance survey, 37 percent of the respondents had lost $100,000 or more in income and wages from onset of illness to the time of the survey. Job loss usually means health insurance coverage is lost as well. Families often deplete savings accounts, run out of personal resources, and lose their homes to pay for medical care. Many patients must depend on government assistance. Twenty-five percent of respondents in the CALDA survey had been on public support or received disability benefits for their illness.
The loss of income has a staggering effect on Lyme patients and represents a burden on our economy. The CDC estimates the national annual economic impact of Lyme disease to be $295 million (in 2009 dollars) (Zhang et al, 2006).4 This figure underestimates the true economic impact for several reasons. First, the CDC acknowledges significant underreporting (by a factor of 6 to 12) in the number of Lyme disease cases under their case definition (CDC, 2004). This means that the actual number of new Lyme disease cases could be over 300,000 per year. Secondly, the CDC’s economic impact study looked at a narrow set of economic variables. It excluded the costs of loss in productivity resulting from spouses and parents taking time
off from work to care for a family member. Other economic costs, such as those associated with childcare services and adults needing in-home or assisted living services also were not included. A 2007 study on the economic burden of persistent disease found that caregivers’ lost productivity can add fully 7 percent to economic costs (DeVol and Bedroussian, 2007). Not only does the disease affect patients’ financial stability, it has the potential to impact the financial security of generations to come.
Patient P from Maryland, sick for more than 20 years:
“If the doctors had met their most basic obligations, or cared at all, I would have been cured decades ago…. They have decided I do not have value as a human being and am not worthy of a healthy, happy life.”
Youth
According to the CDC, children 5 to 14 years of age face one of the highest risks of Bb infection (CDC, 2006). This risk is likely due to the amount of time children spend playing outdoors, and a lack of awareness of the importance of finding and removing ticks. While children experience the same range of symptoms as adults, they often do not have the capacity to express and understand what they are feeling. Parents and doctors often dismiss non-specific symptoms of headaches, fatigue, gastrointestinal issues, and behavioral changes as a part of growing up. Such dismissal often results in delayed diagnosis, thereby allowing dissemination of the infection and consequent long-term illness.
Studies have revealed a long-term impact of Lyme borreliosis on a child’s physical and intellectual functioning. Researchers at Columbia University found “deficits in visual and auditory attention, or in working memory and mental tracking” in children with cognitive problems associated with Lyme disease. They concluded that their study demonstrates that children whose diagnosis and treatment are delayed may suffer considerable impairment (Tager et al, 2001).
The education of children with persistent Lyme borreliosis becomes compromised, leading to inconsistent school attendance and performance. According to the NatCapLyme survey, 45 percent reported that their children with Lyme borreliosis missed school more than one day a week, and 42 percent reported that their children were tardy more than once a week. Studies on the long-term impact of Lyme borreliosis on children’s physical and intellectual functioning have documented behavioral changes, forgetfulness, cognitive deficits, and partial complex seizure disorder (Bloom et al, 1998; Tager et al, 2001). Teachers notice changes in play behavior, declining school performance, excessive sleepiness and frequent trips to the school
nurse. At home, parents notice that some children present with cognitive and behavioral issues, nightmares, trouble falling asleep, difficulty concentrating and learning difficulties.
Adolescents present special diagnostic and treatment challenges, since their new-found desire for privacy, normalcy and independence may prevent full-body tick checks by parents and compliance with keeping doctor appointments and taking prescribed medications. Lyme borreliosis can cause symptoms including fatigue, slurred speech, and confusion which can be misinterpreted as resulting from illegal drug or alcohol use. Although some school districts offer generous support through Individualized Educational Plans and home instruction, others resist. Sadly, some teenagers do turn to street drugs and alcohol to self-medicate unmanaged neurologic and rheumatologic pain.
Many teenagers, overwhelmed by the illness and socially isolated, find it increasingly difficult to cope with life. Parents of children 8 to 16 years old reported in the Columbia University study that 41 percent of their children had expressed suicidal thoughts and 11 percent had made a suicidal gesture (Tager et al, 2001). Among the most disturbing results of the NatCapLyme survey were that 54 percent of the respondents reported that their children with Lyme borreliosis suffered from depression and 13 percent reported that their children had attempted suicide. Such suicidal ideation arises from an understandable weariness from chronic illness. Most poignant is the simple statement from a Lyme-infected child, “I just don’t want to live anymore.”
Patient Q, age 13 from Missouri, sick for 4 years:
“The kids at school pick on me because I am now in a wheelchair. I have a pic line in my arm … I am very sad. I can’t play sports and I am very tired all the time. Sometimes I wish I were not alive. I really have no friends. Who wants to be a friend of someone in a wheelchair or with an illness like mine?”
Relationships with friends and family become strained for some young people with Lyme borreliosis. Some children are confined at home by their illness. Their participation in normal activities, such as athletics or hobbies, becomes a vague memory. Persistent illness affects youths’ ability to participate in normal rites of passage.
Mother on behalf of Patient R from Florida, sick for more than 10 years:
“Since R was not able to attend school for so many years, receiving home-bound tutoring through the school, she missed out on the normal social growth of a teenager. I think one of the most difficult moments for
me was the night her only two friends brought their dates over to show off their prom finery, with the limo waiting outside. I thought my heart would break when my Cinderella ran upstairs after the kids left.”
Parents understandably suffer along with their children. During periods when symptoms abate, hope of recovery rises, only to be dashed when symptoms return. Parents must make unimaginable decisions about putting their children on powerful painkillers or pursuing experimental treatments. They spend sleepless nights weighing the risks and benefits of committing their own child’s life to one of the opposing treatment philosophies.
Mother of Patient S from Virginia, sick for more than 8 years:
“The treatment decisions often boil down to the lesser of two evils. I worry constantly about the potential long-term effects of all of these medications on her developing body, her reproductive capacity.”
Parents wonder why there is a narrow focus of concern from physicians regarding the dangers of curative antibiotics while little concern is expressed regarding the dangers of all the other medications including painkillers and “black-box” medications for the treatment of symptoms. As the mother of an 8-year-old Maryland patient states, “There is also, as a parent, guilt … tremendous guilt if we make what later turns out to be the wrong choice.”
Most parents share the goal of guiding their children to a happy, healthy, productive adulthood. To that end, many parents of children suffering from Lyme borreliosis stretch their resources of time, energy and money to the limit. When young adults expect to launch their independence via college and career, ongoing illness interferes with this natural process. As the years of illness accumulate, hopes of becoming a productive adult and having a family fade. Too many of these young people are forced to create new life plans, but how does one plan for life under these circumstances?
Patient T, young adult from Virginia, sick for more than 10 years:
“Lyme has taken what was supposed to be my decade of life, promise, and opportunities and turned it into a decade of intense struggle, betrayals, and constant disappointment. While I once celebrated the highest academic achievements, strove to draw that perfect sound from my instrument, and worried which boys’ words rang true, I now celebrate making it to the bathroom, strive for ways to diminish my ever growing medical costs, and worry this time the insurance company will reject my claim because I’m too expensive and growing too weak to fight back. And when Lyme traps me in a twitching body that gasps for air, tries to vomit and feels crushed by an elephant all at the same time, I remember my past filled with promise,
ponder my present filled with doctors who say if they can’t figure out what’s wrong it doesn’t exist and family that says if you just try hard enough all problems will disappear, and then contemplate my future—wondering if there is still any hope left in it. They say that adversity builds character, but Lyme weaves darker paths in life that I hope my friends will never need to travel.”
Conclusion
Lyme borreliosis erodes every facet of an individual’s life, decimates marriages, and causes children to leave the educational system. Life never returns to normal for many patients, as they must accommodate activity restrictions and ongoing health concerns. Recovery is further complicated by physicians who do not acknowledge the possibility of ongoing illness, and medical policymakers who do not consider additional avenues worthy of scientific exploration, in spite of mounting evidence of persistent infection.
Research remains inconclusive about optimal treatment. Yet state medical boards routinely penalize doctors for deviating from the Infectious Diseases Society of America’s (IDSA) guidelines for treatment of Lyme disease (Wormser et al, 2006) by subjecting them to investigations and disciplinary procedures rarely visited on physicians treating other diseases. Consequently, a growing numbers of patients lose their doctors, who stop treating Lyme disease in fear of losing their licensing. Physicians are reluctant to treat Lyme and other tick-borne infections in a manner that contradicts the IDSA guidelines. This phenomenon appears to be unique to Lyme borreliosis. The government-sanctioned medical society favoring one treatment philosophy over another places an unnecessary burden on patients who are left to fend for themselves. In an ironic twist of the medical axiom “First do no harm,” patients are left to wonder why the harm they endure from failure to receive treatment is not a rejection of that cherished standard.
The patient testimonies submitted illustrate that the conventional medical wisdom is seriously disserving Americans with tick-borne infections. They also support the frequent allegation that Lyme and associated tick-borne infections are major life-debilitating diseases that should be taken very seriously. Disease manifestations and treatment responses vary among patients. Some patients who are diagnosed and treated early respond well to IDSA-recommended treatment. Patients, whose diagnosis and treatment are delayed, often benefit from long-term or intermittent antibiotic therapy, which is why patients should have the right to pursue all treatment options.
Lyme patient support groups are encountering a growing number of people who were healthy prior to contracting Lyme borreliosis and have not returned to their former level of functioning after treatment. Patients across the United States report innumerable treatment failures. Worse yet, some
people never receive treatment. It is unconscionable that so many people suffer for lack of an accurate diagnosis or effective treatment.
A recent study on the use of guidelines in medical negligence litigation in England and Wales concluded that, “there is a danger in applying the generalized prescription of guidelines in a rigid fashion to every patient” (Samanta et al, 2006). The authors noted that this interference with clinical freedom can result in “cookbook medicine.” “In medical practice, many situations arise where the art of identifying patient problems and the application of clinical acumen to individual patient’s needs remain removed from the science and technological advances of the discipline. Evidence-based medicine cannot fully capture the art of medical practice, and there remains a need for clinical judgment and discretion” (Samanta et al, 2006). Weak research data undermine the validity of treatment guidelines.
Clearly a serious gap between the conventional medical community’s acknowledgment and patient reality is evident. Harvard Medical School professor Dr. Jonathan Edlow states in his book, Bull’s-Eye, “We still have a lot to learn about Lyme disease, and more importantly, we still have a lot to learn about the scientific process” (Edlow, 2003). Patients hope that the Institute of Medicine’s (IOM) Workshop will lay the foundation by bringing university and private-sector scientists and practicing physicians from different viewpoints together to collaborate on solving the problems of treatment failures and persistent illness. We must utilize the scarce resources available to reach out to new thinkers, to explore fresh approaches that will give us the answers to find a cure for all victims of Borrelia burgdorferi.
“The controversy in Lyme Disease research is a shameful affair. And I say that because the whole thing is politically tainted. Money goes to people who have, for the past 30 years produced the same thing…. Nothing.”
Willy Burgdorfer, Entomologist who first identified the bacterial spirochete responsible for causing Lyme disease (Wilson, 2009).
Recommendations
The National Capital Lyme and Tick-borne Disease Association recommends:
-
Improve surveillance by effective, thorough national oversight of tick-borne infections. Tick-borne infections are a serious health concern, and effective national surveillance of these diseases is needed in order to improve public awareness, prevention and diagnosis. Develop a more inclusive surveillance case definition that reflects the actual experiences of practicing physicians confronted with patients presenting with symptoms consistent with Lyme borreliosis. Suggestions to accomplish this are:
-
Broaden the case definition for surveillance to include advanced late-manifestations of Lyme disease.
-
Abandon the two-tier testing approach by eliminating the ELISA as a prescreening requirement before advancing to the Western Blot. The ELISA is too unreliable to be used as a preliminary screening test, given its extremely low sensitivity.
-
Standardize national reporting forms by issuing brief, automated forms that will enable treating physicians to report cases easily. Reports should be held confidential and not disclosed to state medical boards or insurance companies and their service organizations in order to remove the physicians’ fear of adverse consequences from treating patients with Lyme borreliosis.
-
Differentiate between the criteria for surveillance and clinical diagnosis. Once sufficient data have been gathered to form valid conclusions with respect to symptoms of Lyme borreliosis, develop a working clinical case definition for broad application within the medical community. Immediately, the CDC should inform all health service providers and health departments that the surveillance case definition is not intended to be used in clinical diagnosis.
-
Improve diagnostics by developing definitive, reproducible tests with a sensitivity of 95% or higher, that can detect active and latent infection of Bb and other tick-borne infections. The test should be reliable enough to be part of an annual physical examination.
-
Design a national survey. While both NatCapLyme and CALDA have conducted informal surveys which yielded useful information, their results point to the need for an unbiased national scientifically valid survey that collects reliable data on the experience of those suffering from Lyme and other tick-borne infections. Include the patient community in designing the survey.
-
Broaden clinical trials to include patients with persistent Lyme borreliosis. These patients are at the heart of the Lyme controversy. Broaden the entrance criteria for government-funded clinical trials to include entire classes of Lyme patients, whose disease expression and treatment response are poorly understood.
-
Base research grants for tick-borne infections on a non-biased approach. Given the recognized controversy over treatment of Lyme and other tick-borne infections, priority should be given to fund new researchers with innovative ideas and methods that would help to settle this controversy.
-
Many in the Lyme community believe that the Bayh-Dole Act of 1980 ultimately hindered advancement in Lyme disease research. Notably, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) researchers recently discovered significant new biomarkers. Under
-
-
-
the ADNI, researchers agree to share all data, make every finding public immediately, and renounce ownership and patent rights accruing to any individual researcher. According to Dr. John Trojanowski, a researcher at the University of Pennsylvania, “… we all realized that we would never get biomarkers unless all of us parked our egos and intellectual-property noses outside the door” (Kolata, 2010). NatCapLyme strongly advocates adoption of this model in the field of Lyme disease research.
-
Conduct treatment trials that better mirror the variety of treatment regimens actually used by treating physicians.
-
Research the unique attributes of Bb including multiple life forms and the incredible survivability of this organism in the host, genetic complexity, cell-wall deficient forms, its capacity for intercellular sequestration, antigenic variation, immune suppression, and the possible role of borrelial colonies and biofilms.
-
Research the complex disease resulting from multiple tick-borne infections.
-
-
Create dialogue among the scientific research community, health agencies, medical societies, and the full spectrum of treating physicians, including those who view Lyme borreliosis and tick-borne diseases as a potential persistent and infectious process. NatCapLyme encourages all parties interested in Lyme and tick-borne diseases to work together to find solutions. NatCapLyme believes that true solutions to the dilemma of Lyme and tick-borne infections may be found only when all parties are willing to consider the views that each seeks to contribute.
-
Respect patients’ right to choose treatment. Medical guidelines are designed to provide recommendations and not mandates. No monolithic treatment solution should be offered for such an unresolved area of medical research. While the overuse of antibiotics is a concern to all, some Lyme patients are reporting benefit from longer-term and combination antibiotic therapy.
-
Design a National Informed Consent Form to protect the patients’ right to choose as well as protect the doctors’ right to treat. The consent form should reflect fair and balanced treatment options and the benefits and risks of both short-term and long-term treatment choices. The committee that writes this should include medical and legal professionals who hold varied and conflicting points of view. This consent form must not favor any single treatment protocol. It should only include information necessary for fully informed patients to exercise their right to choose treatment.
-
Conduct Institute of Medicine Workshop on tick-borne infections, Part II. This workshop should address effective treatments for persistent and assimilated Lyme disease.
Our committee would be happy to discuss further these recommendations and the experiences of Lyme and tick-borne disease patients that led to our conclusions. We were asked to provide the IOM committee with insight into the patient experience. We concluded that a simple cathartic expression of the angst and suffering of patients and their families would be useless, unless it was combined with insights into the research and science that further illuminates that experience. There is an urgent need to broaden our understanding of tick-borne infections. It is our hope that the scientific community will address the chronic forms seriously and effectively, so that many more of Lyme victims will be able to return to useful, productive lives and the pursuit of happiness.
The National Capital Lyme and Tick-borne Disease Association thanks the Institute of Medicine committee members for allowing us to offer you the experience, thoughts, concerns, and needs of Lyme patients. Our hope is that the work of this committee will result in better care for patients and continued research to find a cure for this disease. We look forward to continuing to work with all governmental agencies and the medical community in hopes of finding successful treatment for all patients with tick-borne diseases.
The NatCapLyme Working Group: Monte Skall, Executive Director, NatCapLyme; Don Boileau, Professor; Barbara Cohen, Psychologist; Cindy Eisenhart, School Librarian; Susan Green, Attorney; Janis Ivicic, School Teacher; Helene Jorgensen, Economist; Linda Lobes, Advocate; Bill Merrigan, Legislative Attorney; Mimi Segal, Clinical Social Worker; Gregg Skall, Attorney; Lisa Torrey, Tick-Borne Disease Advocate; Joy Walker, Law Student; Judith Weeg, former CDC Health Educator; Sharon Whitehouse, Editor; Diane Wilcox, Professor.
Acknowledgment: We would like to thank the following organizations for their contributions to this paper: Lyme Disease United Coalition, Judith Weeg, President; Michigan Lyme Disease Association, Linda Lobes, President; National Tick-borne Disease Advocates, Lisa Torrey, President; and Parents of Children with Lyme.
Correspondence: Monte Skall, Executive Director, National Capital Lyme and Tick-borne Disease Association; phone: 703-821-8833; e-mail: Natcaplyme@natcaplyme.org.
References
Aguero-Rosenfeld, M. E., G. Wang, I. Schwartz, and G. P. Wormser. 2005. Diagnosis of Lyme borreliosis. Clinical Microbiology Reviews 18(3):484-509.
Aucott, J., C. Morrison, B. Munoz, P. C. Rowe, A. K. Schwarzwalder, and S. West. 2009. Diagnostic challenges of early Lyme disease: Lessons from a community case series. BMC Infectious Diseases 9(79):1-8.
Barbour, A. 1996. Lyme disease: The cause, the cure, the controversy. Baltimore and London: The Johns Hopkins University Press. xiii.
Bloom, B. J., P. M. Wyckoff, H. C. Meissner, and A. C. Steere. 1998. Neurocognitive abnormalities in children after classic manifestations of Lyme disease. Pediatric Infectious Disease Journal 17(3):189-96.
CALDA (California Lyme Disease Association). 2009. Lyme Policy Wonk: CALDA survey results are in! (Attachment titled Surveysummary_09102009_blog_attach_872443128. pdf). http://www.lymedisease.org/news/lymepolicywonk/198.html (accessed July 15, 2010).
CDC (Centers for Disease Control and Prevention). 2004. Lyme disease-United States, 2001-2002. Morbidity and Mortality Weekly Report 53(17):365-9.
CDC, Division of Vector-Borne Infectious Diseases. 2006. Average annual incidence of reported cases of Lyme disease by age group and sex, United States, 1999-2004. http://www.cdc.gov/ncidod/dvbid/lyme/ld_MeanAnnualIncidence.htm (accessed July 2, 2010).
CDC, National Center for Public Health Informatics. 2010a. Lyme disease (Borrelia burgdorferi). 2008 case definition. http://www.casedef/lyme_disease_2008.htm (accessed July 17, 2010).
CDC, National Institute for Occupational Safety and Health. 2010b. NIOSH Safety and Health Topics: Tick-borne diseases. http://www.cdc.gov/niosh/topics/tick-borne/ (accessed July 28, 2010).
Depietropaolo, D. L., J. H. Powers, J. M. Gill, and A. Foy. 2005. Diagnosis of Lyme disease. American Family Physician 72(2):297-304.
DeVol, R., and A. Bedroussian. 2007. An unhealthy America: The economic burden of chronic disease. Santa Monica, CA: Milken Institute.
Donta, S. T. 1997.Tetracycline therapy for chronic Lyme disease. Clinical Infectious Diseases 25(Suppl 1):S52–6.
Duray, P. H. 1989. Clinical pathologic correlations of Lyme disease by stage. Reviews of Infectious Diseases 11(Suppl.6):S1487-93.
Edlow, J. A. 2003. Bull’s-eye: Unraveling the medical mystery of Lyme disease. New Haven and London: Yale University Press. 197-8, 253.
Fallon, B. A., J. G. Keilp, K. M. Corbera, E. Petkova, C. B. Britton, E. Dwyer, I. Slavov, J. Cheng, J. Dobkin, D. R. Nelson, and H. A. Sackeim. 2008. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 70(13):992-1003.
Green, C. 2009. Challenge to the clinical definition of late Lyme disease and post-Lyme disease syndrome. Unpublished paper. Los Altos, CA: Green Oaks Medical Center.
Jorgensen, H. 2010. Sick and tired: How America’s health care system fails its patients. Sausalito, CA: Polipoint Press.
Kolata, G. 2010. Sharing of data leads to progress on Alzheimer’s. The New York Times, August 13. http://www.nytimes.com/2010/08/13/health/research/13alzheimer.html (accessed August 13, 2010).
Klempner, M. S., L. T. Hu, J. Evans, C. H. Schmid, G. M. Johnson, R. P. Trevino, D. Norton, L. Levy, D. Wall, J. McCall, M. Kosinski, and A. Weinstein. 2001. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. New England Journal of Medicine 345(2):85-92.
Krause, P. J., S. R. Telford, A. Spielman, V. Sikand, R. Ryan, D. Christianson, G. Burke, P. Brassard, R. Pollack, J. Peck, and D. H. Persing. 1996. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 275(2):1657-60.
Krupp, L. B., L. G. Hyman, R. Grimson, P. K. Coyle, P. Melville, S. Ahnn, R. Dattwyler, and B. Chandler. 2003. Study and treatment of post Lyme disease (STOP-LD): A randomized double masked clinical trial. Neurology60(12):1923-30.
Landro, L. 2010, More tick infections begin at home. The Wall Street Journal, August 2. http://online.wsj.com/article/SB10001424052748703999304575399234058978228.html?KEYWORDS=Landro (accessed August 2, 2010).
Pachner, A. R. 1995. Early disseminated Lyme disease: Lyme meningitis. American Journal of Medicine 98(4A):30S-43S.
Pachner, A. R., K. Amemiya, M. Bartlett, H. Schaefer, K. Reddy, and W. F. Zhang. 2001. Lyme borreliosis in rhesus macaques: effects of corticosteroids on spirochetal load and isotype switching of anti-borrelia burgdorferi antibody. Clinical and Diagnostic Laboratory Immunology 8(2):225-32.
Samanta, A., M. M. Mello, C. Foster, J. Tingle, and J. Samanta. 2006. The role of clinical guidelines in medical negligence litigation: A shift from the Bolam standard? Medical Law Review 14(3):321-66.
Sapi, E., J. Bober, M. Montagna, M. Raman, M. Reddy, M. Hessberger, and A. Melillo. 2009. Prevalence of tick-borne pathogens in Ixodes scapularis ticks from southern Connecticut. Unpublished paper. New Haven, CT: New Haven University, CT.
Steere, A. C. 1989. Lyme disease. New England Journal of Medicine 321(9):586-96.
Tager, F. A., B. A. Fallon, J. Keilp, M. Rissenberg, C. R. Jones, and M. R. Liebowitz. 2001. A controlled study of cognitive deficits in children with chronic Lyme disease. Journal Neuropsychiatry and Clinical Neurosciences 13(4):500-7.
Tibbles, C. D., and J. A. Edlow. 2007. Does this patient have erythema migrans? JAMA 297(23):2617-27.
Wilcox, D. M., and C. Uram. 2009. The impact of chronic Lyme disease on workplace performance: A survey of chronic Lyme patients. (Addendum of tables and charts). Paper presented at the International Lyme and Associated Diseases Society Conference 2009, National Harbor, MD.
Wilson, A. A. 2009. Under Our Skin. Open Eye Pictures, Inc. http://underourskin.com/blog/?p=191 (Accessed August 5, 2010).
Wormser, G. P., R. J. Dattwyler, E. D. Shapiro, J. J. Halperin, A. C. Steere, M. S. Klempner, P. J. Krause, J. S. Bakken, F. Strle, G. Stanek, L. Bockenstedt, D. Fish, J. S. Dumler, and R. B. Nadelman. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases 43:1089-134.
Zhang, X., M. I. Meltzer, C. A. Pena, A. B. Hopkins, L. Wroth, and A.D. Fix. 2006. Economic impact of Lyme disease. Emerging Infectious Diseases 12(4):653-60.
A4
DIAGNOSIS OF TICK-BORNE DISEASES: BORRELIA BURGDORFERI, BABESIA MICROTI, AND ANAPLASMA PHAGOCYTOPHILUM
David H. Persing, M.D., Ph.D.
Chief Medical and Technology Officer
Cepheid
Sunnyvale, CA
Consulting Professor of Pathology
Department of Pathology, Stanford University School of Medicine
Abstract
Lyme disease is the most common tick-borne zoonotic infection in the northern hemisphere. It is a complex multisystem disorder that may involve single or multiple organ systems. Infection with the causative agent, the spirochete Borrelia burgdorferi, may involve the skin, central and peripheral nervous systems, as well as targets within the cardiovascular and musculoskeletal systems. Patients at risk for Lyme disease are also at risk of acquiring infection or coinfection with other agents transmitted by the same tick vector, including Babesia microti and Anaplasma phagocytophilum. Since infection with all three agents can manifest initially as a nonspecific febrile illness with or without specific organ system involvement, the clinical diagnosis of a presenting patient can be challenging. Laboratory methods for detection of and discrimination among these infections are of significant value in the clinical evaluation of such patients. This review will describe the curious convergence of these tick-borne infections and the diagnostic challenges they pose, and will detail the laboratory procedures that can be used to decipher the complex array of diagnostic possibilities.
Introduction
Although significant progress has been made over the past several decades in understanding the immunobiology of Lyme disease and in increasing awareness that this disease is an important public health problem, much about the disease still remains puzzling. Its pathogenesis is still poorly understood, and the interdependent problems of diagnosing the disease accurately and assessing therapeutic outcomes confound one another. One of the most curious aspects of Lyme disease in humans has been its inconsistent presentation, in terms of both disease severity and organ system involvement (Malawista and Steere, 1986; Rahn, 1991; Steere, 2001). By far, the most consistent finding is erythema migrans, often accompanied by
a nonspecific febrile illness. However, even these findings may be absent or unrecognized in many cases, and subclinical or self-limiting infections may occur in a substantial proportion of exposed persons. Within a few weeks to months of infection, a wide array of signs may appear that affect certain subsets of infected patients. These signs (in roughly descending order) include arthritis, lymphadenopathy, meningitis, cranial neuritis (including Bell’s palsy), myopericarditis, nonexudative sore throat, mild transient or recurrent hepatitis, and, less commonly, pancarditis, ocular involvement, and adult respiratory distress syndrome. Years after the onset of disease, occasional patients may develop migratory musculoskeletal disorders along with persistent malaise and fatigue or chronic encephalomyelitis (Logigian et al., 1990). These complications usually respond to therapies directed against Borrelia burgdorferi, but some patients have delayed responses and others do not respond at all.
In the United States, the apicomplexan blood parasite Babesia microti and the more recently recognized agent of human granulocytic anaplasmosis (HGA) are also transmitted by the deer tick (Pancholi et al., 1995; Spielman, 1976; Spielman et al., 1979; Telford et al., 1996). Both infections appear to be most intensely enzootic in established Lyme disease-endemic habitats, and Lyme disease coinfection with one or both of these organisms has been described in most of these areas in the upper Midwest and northeastern United States. In general, cardinal symptoms of babesiosis and anaplasmosis are often absent in co-infected patients, such that coinfected patients can be difficult to distinguish objectively from patients with uncomplicated Lyme disease. However, because the three infections may require different approaches to treatment, an understanding of the natural history of infection with Babesia and Anaplasma may become critical for understanding the biological variation of human Lyme disease and determining optimal medical management for affected patients. For these and other reasons, considerable effort in several laboratories has gone into further defining the transmission cycles of these agents, developing serological and molecular markers of infection for all three pathogens, propagating all three pathogens from human sources, and defining the extent of human exposure to these pathogens (Reviewed in Persing, 1997).
Several other tick-borne pathogens have been described in recent years, including the agent of human monocytic ehrlichiosis (reviewed in Bakken and Dumler, 2000) (Dumler et al., 2007; Thomas et al., 2009) and a novel babesia species (B. duncani) which is found primarily in the western U.S. (Persing et al., 1995; Quick et al., 1993). However, these organisms do not appear to be enzootic with Lyme disease and are not part of the Lyme disease transmission cycle. As a result, coinfection of these organisms with B. burgdorferi, though not impossible, is much less likely. The diagnosis of infection with these organisms should be considered on the basis of
likelihood within their respective endemic areas, but the focus of this review will be on agents that are proven to be part of the Lyme disease ecology.
B. microti Infection
B. microti infection has been diagnosed in residents of many areas in the northeastern and northern midwestern United States where Lyme disease is prevalent (Homer et al., 2000a; Krause et al., 2003; Pruthi et al., 1995; Vannier and Krause, 2009). It is interesting to note that B. microti was once considered to be a possible agent of Lyme disease, since many patients with babesiosis presented with an erythema migrans-like rash at the site of a deer tick bite. The initial symptoms of both illnesses somewhat overlap: like Lyme disease, babesiosis in humans often presents with nonspecific symptoms including fever, fatigue, and other flu-like symptoms. Hemolytic anemia, which lasts from several days to a few months, may occur in patients with clinically severe cases, most commonly asplenic or elderly patients. However, most cases of human babesiosis in normosplenic, immunocompetent patients are probably subclinical and occur as a self-limiting illness (Krause et al., 1996d; Ruebush et al., 1977).
Seroepidemiological data suggest that ~10% of Lyme disease patients in Connecticut and perhaps even higher proportions of such patients in other areas have been exposed to B, microti (Filstein et al., 1980; Krause et al., 1991). However, these two studies did not provide proof of coinfection (vs. sequential infection) with both agents, because they were conducted retrospectively, and specimens for the direct demonstration of both pathogens were not collected. A prospective study by Krause et al. (Krause et al., 1996d) provided more convincing evidence of simultaneous infection with B. burgdorferi and B. microti in substantial numbers of patients with presumptive Lyme disease from coastal New England. Although not usually recognized as coinfected on clinical grounds, patients harboring both organisms often had more severe disease and a higher likelihood of persistent post-infectious fatigue.
Since antibiotic therapy for early Lyme disease is unlikely to be effective against coinfection with B. microti, it is easy to envision a scenario in which underlying babesiosis is responsible for the persistence of symptoms after therapy for early Lyme disease. However, since the clinical presentation is nonspecific, it will be difficult to know at the time of presentation whether a patient would benefit from therapy targeting babesial coinfection. The risks associated with antibabesial therapy such as atovaquone plus clindamycin are not insignificant, and given a low probability of coinfection, presumptive diagnosis and therapy would not appear to be warranted even in the most heavily endemic areas. It is important to note
that the converse scenario does not appear to be true: among patients with case defined chronic fatigue syndrome and no history of antecedent Lyme disease, there is no evidence supporting a major role of coinfection with B. burgdorferi and/or B. microti (Macdonald et al., 1996). A more conservative approach would employ selective testing of patients, who based on clinical, epidemiological, and initial laboratory evaluations, may be at higher risk of coinfection (see below).
Human Granulocytic Anaplasmosis
In 1994, Bakken et al. (Bakken et al., 1994) described a nonspecific febrile illness among patients in the Upper Midwest that was characterized by, thrombocytopenia, and neutrophilic inclusions (morulae). Genetic and serological analyses of patients’ blood samples indicated that the agent of human granulocytic ehrlichiosis was closely related to Ehrlichia equi and Ehrlichia phagocytophila. Infections caused by a similar or identical agent were subsequently described in many states including New York, Connecticut, Massachusetts, Rhode Island, Minnesota, Wisconsin, and California (reviewed in Bakken and Dumler, 2000) (Dumler et al., 2007; Thomas et al., 2009). Since then, phylogenetic analyses have determined that the agent of human granulocytic ehrlichiosis is more closely related to the genus Anaplasma and the infection is now referred to as human granulocytic anaplasmosis (HGA) (Dumler et al., 2007).
The report of a case of HGA that occurred following a deer tick bite (Pancholi et al., 1995), together with recognition of the apparent overlap between areas where Lyme disease and HGA are endemic, prompted an investigation into whether the deer tick could also transmit HGA. Ixodes ticks collected from fields in several locations in Wisconsin where HGA cases have been described were analyzed retrospectively; the collection obtained from Wisconsin in 1993 and an earlier collection stored in alcohol since 1982 both contained A. phagocytophilum-infected PCR-positive specimens. Just as retrospective epidemiological studies of B. burgdorferi demonstrated the presence of pathogen DNA in suitable vectors (Persing et al., 1990b) or reservoirs (Marshall et al., 1994a) from historic specimens, the existence of the agent of HGA clearly preceded the first descriptions of the disease itself. Indeed, the increased incidence of HGA is likely to be due, in part, to the emergence of its recognition. In a subsequent study, Telford et al. (Telford et al., 1996) showed that the deer tick is competent to transmit an agent of HGA that was recovered from a patient on Nantucket Island. Ixodes pacificus may be the vector of HGA in the western United States; this tick is the primary vector of E. equi, which is the agent of HGA.
Immunomodulatory Effects of Babesia and Anaplasma: Impact on Diagnostic Markers?
The elucidation of the immunologic interplay of the microbial agents that exist with the transmission cycle of Lyme disease is likely to yield a better understanding of what can be expected in occasional human cases. In the case of multiple pathogens that coexist within a rodent reservoir involved in overlapping transmission cycles (as is the case for B. burgdorferi, B. microti, the HGA agent, and perhaps other organisms), it is reasonable to hypothesize that immune responses to one organism may have an impact on concurrent infections, especially if the underlying infection is associated with immune suppression. Infections by both Babesia and Anaplasma species are associated with substantial immunosuppressive or immunomodulatory effects (reviewed in Persing, 1997). Coinfection by the agent of HGA and B. burgdorferi or by B. microti and B. burgdorferi could have substantial theoretical effects on the immune response, but the practical questions are these: Are disease outcomes altered, and are immunologic effects severe enough to alter immune responses so that they can no longer be used as reliable indicators of infection?
Animal models are helpful in answering these questions. Consistent with the above predictions, experimental coinfection of a mouse model with B. burgdorferi and A. phagocytophilum increased the number of CD4+ cells and drove the cytokine responses toward a Th1 lymphocyte response (Zeidner et al., 2000). In another animal model, coinfection with B. burgdorferi and A. phagocytophilum led to increased pathogen burden in blood and tissue, and to more severe Borrelia-induced arthritis than single infection with B. burgdorferi alone (Thomas et al., 2001). However, during coinfection, levels of IL-12, gamma interferon, and tumor necrosis factor in serum were paradoxically decreased whereas levels of IL-6 were elevated. A similar study of coinfection in C3H/HeN mice evaluated the tissue distribution of tick-transmitted B. burgdorferi and A. phagocytophilum infection by quantitative PCR (Holden et al., 2005). Coinfected animals had increased spirochetal burdens in multiple tissues but Anaplasma numbers (found primarily in blood) remained constant. Although antibody responses were diminished somewhat for A. phagocytophilum, levels of antibody that developed against B. burgdorferi were not affected.
Although coinfection with B. burgdorferi and B. microti was found to be associated with increased disease severity and greater likelihood of spirochetemia in human subjects enrolled in a blinded clinical evaluation, experimental evidence of increased severity in animal models has been mixed. Initial studies showed immunologic effects of coinfection in some mouse strains but not others. C3H mice showed no evidence of increased severity, but normally Lyme disease-resistant Balb/c mice showed an increase in arthritis severity
at day 30, along with reductions in levels of immunosuppressive cytokines IL-10 and IL-13 (Moro et al., 2006). Another mouse model showed that dual infection with B. burgdorferi and B. microti appeared to follow independent courses, with no apparent increase in Lyme disease severity (Coleman et al., 2005), although mouse strains used and inoculation times differed between the two studies. In both studies, serological responses to both agents appeared to be largely unaffected by coinfection.
Taken together, the data from animal models and human clinical studies suggest that coinfection with A. phagocytophilum and/or B. microti may have immunological effects during the course of B. burgdorferi infection, but these effects are not in themselves sufficient to completely abrogate cellular or humoral immune responses to the point of complicating the serodiagnosis of Lyme disease. Likewise, the presence of concomitant infection with B. burgdorferi does not appear to suppress immune responses to either the HGA agent or B. microti to the point that infection cannot be detected in diagnostic assays. A. phagocytophilum infection may be associated with false positive serologic responses to B. burgdorferi, (Hofmeister et al., 1996; Wormser et al., 1996), but not false negative responses.
Clinical and Initial Laboratory Evaluation of Tick-Borne Diseases
For patients exposed to ticks in areas where multiple tick-borne pathogens are endemic, it seems reasonable for clinicians to be aware of clinical signs that may be consistent with each infection alone or in combination, especially when patients with Lyme disease fail to respond promptly to antibiotic therapy. Patients with uncomplicated erythema migrans, without systemic symptoms, may require no further laboratory-based evaluation and can be treated presumptively for Lyme disease in many cases. More severe presentations may merit closer scrutiny. Symptoms including nausea and/or vomiting, fever, chills, sweats, severe malaise, and a delayed clinical response to antibiotic therapy for presumptive Lyme disease were characteristic of Babesia-co infected patients (Krause et al., 1996d). Fever, chills, myalgias, and severe headache are characteristic of granulocytic anaplasmosis (Thomas et al., 2009). Carditis has been described as a complication of Lyme disease, but has also been described in patients with HGA without evidence of B. burgdorferi infection (Jahangir et al., 1998).
Initial laboratory evaluations to be considered include examination of blood smears for the presence of intraerythrocytic inclusions (merozoites) typical of babesial infection and granulocytic morulae typical of HGA. However, the sensitivity of blood smear evaluations for immunocompetent, normosplenic patients has not been firmly established and may be relatively low for both diseases (see below). The presence of elevated liver enzymes or hematologic abnormalities may be especially useful in identifying coinfected
patients, since both babesiosis and anaplasmosis have been associated with increases in the levels of alanine aminotransferase. Thromocytopenia may also be present in patients with either disease, but it is rarely a feature of uncomplicated Lyme disease. Thus, the presence of thromobocytopenia in patients presenting with early Lyme disease should trigger a suspicion of coinfection with one or the other agent.
Laboratory Diagnosis of Tick-Borne Zoonoses
In general terms, the available laboratory methods for the diagnosis of the tick-borne diseases described here fall into two categories: 1) Direct methods (microscopy, culture, or PCR, depending on the agent), and 2) indirect methods (detection of organism-specific immune responses). With some notable exceptions, direct methods are generally useful for characterization of acute or active infections, whereas detection of antibody responses is most useful for confirmation of clinical suspicion in post-acute and convalescent phases.
Irrespective of the diagnostic method used, an important consideration is the pre-test probability of infection. Since Lyme disease, HGA and human babesiosis are all characterized initially by seasonal occurrence of a nonspecific febrile illness, the likelihood of tick-bite or tick exposure, guided by prevalence information for each of the infectious agents, should be a critical gating item for physician test ordering. Unfortunately, I. scapularis tick exposure is fairly common, and all three agents have been described in most of the areas endemic for Lyme disease, so laboratory testing plays an unusually prominent role in the diagnosis. However, since all of the laboratory methods currently in use have been shown to give rise to false-positive results, the positive predictive values of each of these methods may reach unacceptable levels, particularly when a multiplicity of tests is ordered. According to a recent treatment guideline from the American College of Physicians and the IDSA, patients with vague subjective complaints (headache, fatigue, and myalgia in the absence of respiratory symptoms) apart from other risk factors are at low risk for Lyme disease. They recommend against routine testing of such patients because the odds of a false positive result are greater than for obtaining a true positive result (Tugwell et al., 1997; Wormser et al., 2000b). Likewise, for patients with nonspecific persistent fatigue without a history of Lyme disease, even if living in or near endemic areas, seroprevalence rates for HGA and B. microti are low and these organisms are unlikely to be implicated (Macdonald et al., 1996).
Hematologic Evaluation
The complete blood count (CBC) with a manual differential (direct blood smear evaluation by a medical technologist) can be an extremely
useful tool in the initial evaluation of patients with nonspecific presentations. blood counts and peripheral blood smears along with tests of liver function. Leukopenia, lymphopenia, granulocytopenia, and especially thrombocytopenia are found in HGA. Granulocytopenia is less commonly associated with uncomplicated Lyme disease but has been described in some cases. Anemia and hemolysis are more common in babesiosis, and especially thrombocytopenia is frequently evident in babesiosis and HGA infections.
Babesiosis and HGA can diagnosed directly by observing organisms on Giemsa-stained smears of peripheral blood. For patients with intact spleens, erythrocytes may show B. microti ring forms on thin blood smears; this proportion may be as high as 80% for asplenic patients (Homer et al., 2000a; Meldrum et al., 1992; Vannier and Krause, 2009). In one study (Krause et al., 1996b), most patients coinfected with Borrelia and Babesia species were smear negative for babesiosis at the time of presentation and at all time points thereafter.
Blood smear evaluation has been advocated for the diagnosis of HGA when the index of suspicion is high (Bakken and Dumler, 2006; Dumler et al., 2007; Thomas et al., 2009), but detection by this route is unlikely unless the examining technologist is alerted to the possibility of HGA. Moreover, visual inspection of blood smears for the length of time required to find a single morula may be impractical. In one study, a small subset of HGA patients with mild infection had intragranulocytic morulae detected on smears (Belongia et al., 2001), in contrast with symptomatic, untreated patients whose smear results were evaluated after several days of fever (Bakken et al., 1994). The blood smear evaluation for HGA should be carried out within a week of disease onset, as sensitivity is highest at this time (Bakken and Dumler, 2006). Blood samples should be collected prior to administering doxycycline therapy since morulae are eliminated from the blood within 24–72 h after the start of therapy (Thomas et al., 2009). However, the since the sensitivity for detecting A. phagocytophilum is only 25–75%, blood smear evaluation is useful if the results are positive but not particularly helpful if the results are negative.
False-positive results are possible; artifacts such as platelets superimposed on red cells or Howell-Jolly bodies can appear like Babesia (Matthews et al., 2003); and to an untrained eye, a well-separated nuclear segment within a neutrophil may give the appearance of a morula. Acridine orange stain, which intercalates into double stranded DNA, can help enhance detection of B. microti inclusions in red cells and avoid detection of platelet artifacts.
Direct Detection Methods
Direct Detection of B. burgdorferi
For direct detection of B. burgdorferi, a variety of approaches have been used in the clinical or research laboratories, including microscopic evaluation of tissues, detection of B. burgdorferi-specific proteins (by EIA or by immunohistochemistry) or nucleic acids (by PCR), and in vitro cultivation. Direct microscopic detection of B. burgdorferi has limited usefulness because of low throughput, low organism abundance in tissues (such as skin biopsies) that are confirmed positive by other methods, and the requirement for silver staining and a skilled operator. Antigen detection assays for detection of B. burdorferi have suffered similar limitations; although B. burgdorferi antigens can be detected by immunohistochemistry in tissues and by EIA within specimens from confined anatomical sites such as cerebrospinal fluid (CSF) and synovial fluid, where clearance of antigens is limited. Their presence in other samples such as urine has been more controversial (Dorward et al., 1991) (Hyde et al., 1989) (Klempner et al., 2001b) and has not stood up well to additional scrutiny. In contrast, culture and PCR techniques do appear to be diagnostically useful in certain circumstances, and we will limit the remainder of this section to these methods.
Culture-based methods for detection of B. burgdorferi were used for the initial establishment of the etiologic basis of Lyme disease, and have been used on and off for decades in the evaluation of various clinical samples for research protocols. However, their use for clinical diagnosis has been hampered by long incubation times, poor sensitivity (with the possible exception of skin biopsies from EM), and limited availability of the specialized growth medium for routine use.
The liquid media currently used for recovery of B. burgdorferi are modified versions of the original Kelly medium (Kelly, 1971) through various modifications made over time (Barbour, 1984) (Stoenner et al., 1982) (Barbour, 1986). The most commonly used medium (Barbour-Stoenner-Kelly II) is commercially available and is used for direct recovery of spirochetes from clinical specimens including plasma, CSF, whole blood and skin biopsies. Since serum supplementation is required for this medium, one source of variability in the efficiency of spirochete recovery has been the presence of anti-spirochetal antibodies in the serum (Pollack et al., 1993). Cultures are visually inspected by darkfield microscopy or after staining with specific antibodies or intercalating dyes. With newer modified media protocols, spirochetes can be detected within 5-7 days, but they may require incubation for up to three months at 30-34°C.
Recent studies of high-volume inoculation of whole blood or plasma have been surprisingly successful at recovering circulating spirochetes. For many years, rates of recovery of B. burgdorferi from blood samples collected
from untreated patients with EM had been less than 5-10% (Wallach et al., 1993) (Steere et al., 1983). However, the inoculum volumes in these studies were generally small. Based on previous studies which showed that circulating bacteria in patients with sepsis are often rare, and that the volume of cultured blood is directly related to culture yield, a team of investigators at New York Medical College investigated the use of high volume inocula of blood or blood fractions. They showed the following: 1) recovery of B. burgdorferi was superior from serum or plasma compared to the same volume of whole blood (Wormser et al., 1998) and 2) the culture yields from plasma were significantly greater than that from serum (Wormser et al., 2000a). Recovery of B. burgdorferi from high-volume cultures (9 or more ml of plasma) inoculated into a modified BSK II medium was above 40%. However, increasing the volume of plasma from 9 ml to 18 ml for adult patients with EM met with diminishing returns; a mere 10% increase in culture yield was observed after doubling the inoculum volume (Wormser et al., 2000a).
PCR protocols for detection of B. burgdorferi have been in use since a few years after the description of PCR itself. PCR was initially promising as a study tool in studies of the retrospective epidemiology of B. burgdorferi, including detection of the organism in suitable tick vectors 30 years prior to the formal recognition of Lyme disease in the U.S. (Persing et al., 1990a) and in suitable animal reservoirs nearly a century earlier (Marshall et al., 1994b). However, its track record for dramatically improving the general lot of Lyme disease diagnostics has been more spotty, and in general disappointing (reviewed in Dumler, 2001), (Schmidt, 1997), (Aguero-Rosenfeld, 2003, 2008). A variety of chromosomal and/or plasmid targets have been used in the various PCR protocols with varying levels of sensitivity (Dumler, 2001), (Schmidt, 1997), (Aguero-Rosenfeld, 2003, 2008).
A number of studies have compared PCR to culture for detection of spirochetes in skin biopsies. Culture has proven to be roughly equivalent to PCR in several studies with a few exceptions (Moter et al., 1994) (Nowakowski et al., 2001) (Steere et al., 1998) as reviewed in (AgueroRosenfeld et al., 2005). Widely disparate results are likely to be attributable to differences in the various studies in the PCR protocols employed, including type of PCR and/or primer and target selection and/or method of tissue preservation, as well as differences in culture techniques, including size of the skin biopsy sample cultured and/or choice of culture medium. In one study of skin biopsy samples of 47 untreated patients with EM lesions, culture sensitivity was 51%, compared 81% by using a Taqman-based realtime PCR (Nowakowski et al., 2001).
In contrast to the experience with skin samples, the sensitivity of PCR for detection of B. burgdorferi in blood samples has been much lower in patients with EM (Goodman et al., 1995) (Oksi et al., 2001). In a prospective
study of U.S. patients with EM, B. burgdorferi sensu stricto DNA was detected by PCR in only 14 of 76 (18.4%) plasma samples (Goodman et al., 1995). Detection of the spirochete in blood by PCR in blood samples for patients with disseminated disease has also been disappointing (Demaerschalck et al., 1995) (Liebling et al., 1993). This is likely due to the same issue that hampered early studies of culture in blood samples of Lyme disease patients, specifically the relative paucity of circulating spirochetes in the small volumes of blood that are typically extracted in a PCR procedure. Most PCR protocols test only 100 to 200 microliter samples, and these may simply be inadequate to capture enough circulating spirochetal DNA for detection, even by as sensitive a method as PCR. So it is no surprise that, given the relative lack of sensitivity of PCR in blood for detection of early, even disseminated disease, it would fare even more poorly in patients with later disease manifestations, despite positive serological findings in many of these patients (Klempner et al., 2001a).
Within anatomically confined compartments, PCR detection methods generally fare better. In a study of 60 U.S. patients with neuroborreliosis (16 with early and 44 with late neuroborreliosis), the sensitivity of PCR in CSF was 38% in early and 25% in late neuroborreliosis, and an inverse correlation was found between duration of antimicrobial treatment and PCR results (Nocton et al., 1996). In this study, four different PCR primer or probe sets were used, three targeting OspA genes and one targeting OspB genes, and concordance between the different assays was relatively poor which suggested low target number within the samples. Other studies from Europe have generated similar findings (Ornstein et al., 2002) (Lebech et al., 2000). Studies of synovial fluid, have provided the greatest clinical utility of PCR testing for the diagnosis of active Lyme arthritis and for monitoring the course of therapy (Aguero-Rosenfeld, 2008; Aguero-Rosenfeld et al., 2005; Bradley et al., 1994; Dumler, 2001; Eiffert et al., 1998; Jaulhac et al., 1996; Malawista et al., 1992; Nocton et al., 1994; Persing et al., 1994a). In a landmark study of 88 patients, B. burgdorferi DNA was detected in synovial fluid of 75 (85%) patients with Lyme arthritis (Nocton et al., 1994). Not surprisingly, the PCR detection rate was lower in patients who had received what should have been effective antibiotic therapy compared to untreated or undertreated patients. Of 73 patients who were untreated or treated with only short courses of oral antibiotics 70 (96%) had DNA detected by PCR in synovial fluid samples. In contrast, 7 of 19 (37%) patients who received either parenteral antibiotics or oral antibiotics for more than 1 month were still PCR positive (Nocton et al., 1994). The observation of higher sensitivity of PCR detection of plasmid-encoded OspA compared to multiple chromosomal loci (the 16S rRNA gene and flagellin) in synovial fluid specimens (Persing et al., 1994b; Nocton et al., 1994; Persing et al., 1994a) was referred to as “target imbalance.” It has been speculated that
B. burgdorferi sensu stricto present in the synovium may selectively shed OspA DNA segments into the synovial fluid (Persing et al., 1994a). With the same or similar reagents, this phenomenon did not appear to occur in CSF specimens (Lebech et al., 1998).
A subsequent meta-analysis has largely confirmed the above assessment; it showed that PCR is a sensitive diagnostic tool for detection of B. burgdorferi DNA in skin biopsy and synovial fluid specimens whereas detection of the organism in blood or blood fractions and in CSF specimens is relatively low (Dumler, 2001). Given the paucity of supporting data for use of PCR as a diagnostic technique in samples other than skin and synovial fluid, extreme caution must be exercised in interpreting PCR test results from samples such as whole blood, urine, and cerebrospinal fluid. Even the best PCR laboratories have had problems with PCR amplicon contamination which, if sporadic, can give rise to undetected rates of false positive results which have been reported in a number of PCR assays, including those for B. burgdorferi. Because of the high credibility assigned to PCR by most clinicians, false positive PCR results can lead to inappropriate treatment and mismanagement, sometimes with fatal results (Molloy et al., 2001; Patel et al., 2000). Laboratories performing PCR should follow Good Laboratory Practices for controlling amplicon contamination, including strict separation of amplified material from areas where clinical samples are being prepared and inclusion of appropriate positive and negative controls in each run.
Direct Detection of Babesia microti
Babesia microti has not been successfully grown in culture, despite multiple attempts, and animal inoculation of whole blood via the intraperitoneal route has been the mainstay of organism propagation for many years. This method is used primarily for organism isolation from patients with obvious parasitemia as demonstrated on Wright-Giemsa stained blood films, and it is limited for practical reasons to research laboratories. Hamster blood, which supports replication of B. microti, begins to show parasitemia at around day 7 after inoculation, and typically reaches peak levels at 2-3 weeks although samples with low numbers of parasites may take longer.
PCR has gained popularity as a diagnostic method, mainly for two reasons: 1) it provides good sensitivity levels and a broad dynamic range, making it suitable for parasitemic patients who are smear negative as well as smear positive, and 2) its turnaround time and general availability as a diagnostic procedure are far superior to comparable methods based on animal inoculation. Initial descriptions of PCR for detection of B. microti were based on conserved elements of the 18 S ribosomal gene, and showed that all strains of B. microti tested were reactive whereas DNA
from Plasmodium and other related parasites were negative (Persing et al., 1992). The 3 hour method was much faster and easily 10- to 100-fold more sensitive than hamster inoculation when tested against a limiting dilution of parasites in whole blood (Persing et al., 1992). The same PCR assay was then used in several studies of babesial infection and co-infection with B. burgdorferi to demonstrate that it was more sensitive than blood smear evaluation for the diagnosis of babesial infection (Krause et al., 1996c) and that it could be used to detect B. microti infection in symptomatic and asymptomatic individuals including blood donors (Krause et al., 1998; Krause et al., 1996c; Krause et al., 1994; Krause et al., 1996b).
The utility of PCR for detection of B. microti was demonstrated in several prospective studies of exposed populations living in Block Island, RI, and Eastern Connecticut. Krause et al showed that patients with convincing evidence of simultaneous infection with B. burgdorferi and B. microti often had more severe disease and a higher likelihood of persistent post-infectious fatigue (Krause et al., 1996b). Coinfected patients also appeared to be more likely to be spirochetemic as determined by detection of B. burgdorferi DNA in their blood. In another landmark study (Krause et al., 1998), Krause et al showed 1) that patients with babesial infection often had prolonged and sometimes persistent parasitemia, as demonstrated by PCR, after primary infection, and 2) that many patients with primary infection with B. microti had infections that were subclinical and/or asymptomatic, and which, even if suspected, would not have been detected by blood smear evaluation (Krause et al., 1998). However, for practical purposes it has been found that virtually all patients who are symptomatic and PCR positive for B. microti are already also seropositive (Homer et al., 2000a; Krause et al., 1994), so the use of serologic methods, even in the initial diagnosis of babesial infection or coinfection with other tick borne organisms, is not unreasonable (see below).
These studies paved the way for a better understanding of the incidence of chronic, subclinical infection and the potential risk to the blood supply of blood donors who may unwittingly donate parasitemic blood to increasingly compromised populations including transplant patients, cancer patients, and other vulnerable recipients. Several studies have been done using serologic testing followed by PCR testing to identify asymptomatic carriers of B. microti in the blood donor population (Leiby et al., 2002; Leiby et al., 2005). In almost all cases, persons identified as asymptomatic carriers are blood smear negative and thus would have escaped detection upon routine examination, yet some of them are positive by hamster inoculation and all are considered potential persistent carriers of B. microti. In part due to the recent rise in the number of transfusion related deaths associated with B. microti infection, several new initiatives by the FDA and CDC are underway to institute screening measures by both serologic methods and PCR
to detect asymptomatic donors in order to exclude infected units of blood from entering the U.S. blood supply (Leiby et al., 2002; Leiby et al., 2005). Because PCR may be able to detect parasitemia before serocoversion, it may play a pivotal role in such screening efforts.
Direct Detection of Anaplasma phagocytophila
Direct cultivation of Anaplasma phagocytophilum was first accomplished by Goodman and colleagues (Goodman et al., 1996) in the few years after the first recognition the disease, by culture of whole blood in the promyelocytic leukemic cell line HL-60. Recovery of A. phagocytophilum from peripheral blood in cell culture can be used to definitively diagnose infection (Horowitz et al., 1998; Thomas et al., 2009). The bacteria develop within vacuoles to form morulae in the cytoplasm of infected cells, which can be detected using Wright or Giemsa staining. Intracellular organisms can be visualized as soon as 5 days postinoculation or can remain undetectable for more than 2 weeks. Culture of A. phagocytophilum in HL-60 cells is arguably the most sensitive method for the direct diagnosis of human anaplasmosis (HA), owing again to the relatively large volume of blood that can be analyzed compared to PCR methods (Aguero-Rosenfeld et al., 2000; Horowitz et al., 1998; Ravyn et al., 2001) However, as a routine diagnostic method, cell culture is rarely or ever used, and detection methods offered by most medical centers rely on demonstration of characteristic inclusion bodies on peripheral smears, immunoserologic methods, and PCR.
PCR is also a sensitive tool for detecting A. phagocytophilum. PCR was used early on to demonstrate the presence of the organism in blood samples and in initial studies that led to the identification of the deer tick as a vector (Pancholi et al., 1995). Several studies and reviews of the diagnostic performance of PCR for the diagnosis of HGA have been published (Aguero-Rosenfeld, 2003; Alberti and Sparagano, 2006; Comer et al., 1999; Krause et al., 2002; Walls et al., 2000). Sensitivity is approximately 67–90% for detecting A. phagocytophilum DNA (Horowitz et al., 1998), but just as for direct smear evaluation, the PCR sensitivity will likely be affected by the phase of infection and antibiotic therapy. After the first week, the bacteremic phase of infection rapidly wanes, thereby limiting the effectiveness of PCR as a diagnostic technique (Bakken and Dumler, 2006). In general, PCR positivity correlates with the concurrent presence of IgM antibodies and eventual IgG seroconversion.
Immunodiagnostic Methods
For all of the tick-borne infections described here, the mainstay of the laboratory diagnosis is the detection of antibody responses in serum of
affected individuals. Initial detection of organism-specific IgM antibodies during acute illness is accompanied by conversion to IgG during the convalescent phase, usually 10 to 14 days later. Serologic testing is more available than direct detection methods based on culture or PCR, and usually less expensive. Given the relative lack of sensitivity of direct detection methods, serologic testing is also usually more sensitive, especially for detection of B. burgdorferi infection. Multiple testing formats are available from commercial sources, including ELISA, IFA, and immunoblotting. This section will provide a summary of immunodiagnostic approaches for each of the three agents addressed by this review.
B. burgdorferi
The regional antigenic variability and complexity of the antigenic components of B. burgdorferi have posed challenges for the serodiagnosis of LB. In Europe, Lyme borreliosis is caused primarily by the three species: Borrelia burgdorferi sensu stricto, B. afzelii and B. garinii. In the U.S., substantial genetic heterogeneity of B. burgdorferi sensu stricto exists, as assessed by pulsed-field gel electrophoresis (PFGE) typing, but the vast majority of human clinical isolates fall into a few closely related PFGE clusters of strains that have similar antigenic composition (Mathiesen et al., 1997). B. burgdorferi strains used in the U.S. for immunodiagnosis generally are drawn from these groups, so it is likely that sufficient serologic cross reactivity exists among these strains to allow for detection of antibody responses irrespective of the geographic location of the infection (Mathiesen et al., 1997). However, to complicate things, some immunodominant antigens or epitopes are expressed exclusively in vivo but not in cultures of organisms that are used to prepare antigens for diagnostic testing (Das et al., 1997) (Fikrig et al., 1997). This has led to the use of serological expression cloning to detect antigens that are expressed in vivo, which in some cases have led to the identification of diagnostically useful reagents.
Several initial studies of the immune response to B. burgdorferi recognized the flagellin protein as an immunodominant antigen that was recognized by IgG and IgM antibody subclasses within a few days of infection (Coleman and Benach, 1987; Craft et al., 1986). Unfortunately, the flagellin gene is conserved among multiple species of bacteria, so crossreactivity and nonspecific immune responses are quite common, especially on immunoblots where the protein is denatured (Fawcett et al., 1992) (Luft et al., 1993). Another immunodominant antigen recognized early during infection with B. burgdorferi sensu lato is the OspC protein which is encoded on a plasmid (Engstrom et al., 1995). OspC is upregulated by the spirochete while is still in the tick midgut and when it begins to migrate to the tick mouthparts prior to transmission. Some strains of B. burgdorferi cultured
in vitro express little or no OspC; indeed, this antigen was unrecognized in early studies that used high-passage strains as the source of antigen. OspC is genetically and antigenically very heterogeneous (Theisen et al., 1993). Recently, conserved epitopes within the OspC protein have been identified comprising the C-terminal 10 amino acids of OspC (pepC10) (Mathiesen et al., 1998). Inclusion of this peptide in immunoassays may be an important step toward developing improved immunodiagnostic assays for Lyme disease (see below).
The so-called Vmp-like sequence expressed (VlsE) protein, a hypervariable immunodominant surface lipoprotein encoded by a linear plasmid, was described in conjunction with expression cloning experiments (Zhang et al., 1997a; Zhang and Norris, 1998a, 1998b). Although subject to extensive antigenic variation, presumably because of immunological pressure, within the variable regions a constant domain was identified called IR6 (or C6). Peptide mapping of this region showed that it is highly immunogenic (Lawrenz et al., 1999). Broad conservation of this region across multiple species of B. burgdorferi sensu lato have suggested that it would make a good immunodiagnostic reagent, and several studies have now been published that support its use alone or in combination with other immunodominant peptides. Several other antigens have been identified for potential immunodiagnostic use, including decorin binding protein (DBP1) (Heikkila et al., 2002) and a fibronectin binding protein (BBK32) (Fikrig et al., 2000).
Testing Formats
Over the years, many commercial immunoassays to detect B. burgdorferi antibodies have been cleared for use in the United States by the Food and Drug Administration (FDA). Most of these assays use the B31 type strain of B. burgdorferi as the source of antigen, but several newer assays have employed recombinant antigens and/or peptides. In general, performance of these tests on blinded proficiency surveys of laboratories across the U.S. has been good, but a significant degree of performance variation still exists between test manufacturers and test methods (unpublished observation from 2009 CAP surveys). One potential source of variability is the method used for antigen preparation. For immunoassays using whole cell preparations, a surprising amount of antigenic variability will be encountered depending on growth conditions, medium used, incubation temperatures, growth phase of the organisms, and methods used to extract antigens, concentrate them and link them to specific substrates for test preparation. Lot-to-lot reproducibility and quality control are major concerns for methods based on whole cell lysates. In contrast, methods based on recombinant proteins or peptides are better defined and may lead to more lot consistency, but the presence of one or a few
epitopes poses a different kind of limitation: the intrinsic variability of the human immune response. Thus, justification of the use of a variety of assay formats continues for a variety of reasons ranging from test performance to cost per test. In general, however, efforts to encourage better standardization should lead to better defined immunologic substrates, and challenging proficiency testing programs are one of the best ways to identify the strengths and weaknesses of individual tests and approaches, whether they are user-developed or commercial methods.
Indirect fluorescent antibody tests look for immunoadsorption of patient antibodies to cultured spirochetes attached to glass slides. A dilution series of patient serum is placed in adjacent wells, incubated on the substrate for a short time, and then detected by fluorescent microscopy after staining with fluorophore-congugated-human IgG or IgM antibody. Antibody titers of 1:64 or above are generally considered positive, though there may be considerable day-to-day and technologist-to-technologist variation in the determination of values because of the subjective nature of the procedure. Automated fluorescence readers have the potential to improve upon this, but variations in the procedural steps such as preparing the dilution series and washing stringency will continue to contribute to variability.
Enzyme immunoassay (EIA) is the most commonly used method for detection of immune responses to B. burgdorferi. Typically, antigen preparations comprising whole-cell sonicates of B. burgdorferi are bound to plastic EIA plates for detection of total Ig response or individual antibody subclasses. With EIAs using well prepared whole cell antigen preparations, about half of patients presenting within a week of the development of erythema migrans (EM) will register positive results. In untreated patients after one week, and in patients with multiple EM lesions or signs of systemic involvement, sensitivities of EIA approach 90% (Dressler et al., 1993). EIA is nearly uniformly positive in sera of patients with late manifestations of disease (Dressler et al., 1993).
False positive EIA reactions can and do occur in some patients with bacterial infections due to other organisms; this is thought to be due to cross reactivity with common bacterial antigens such as heat shock proteins, flagellin, and other conserved bacterial proteins (Fawcett et al., 1992) (Luft et al., 1993). Because all commercially available assays using whole cell sonicates contain the OspA protein, individuals who in the past have received the OspA vaccination will often generate false positive results (Aguero-Rosenfeld et al., 1999) (Molloy et al., 2000). EIAs based on strains that are negative for OspA (by virtue of losing the linear plasmid encoding the protein) or recombinant protein or peptide EIAs devoid of OspA epitopes will be non-reactive in these individuals (Zhang et al., 1997b). Because of the lack of specificity of EIA procedures, and because the pretest probability of having Lyme disease is often lower than the false
positive rate (especially in non-endemic areas), EIA reactive sera should be confirmed by Western immunoblotting according to so-called two-tier testing guidelines.
Western immunoblotting is performed by carrying out electrophoretic separation of spirochetal antigens from whole cell sonicates, followed by transfer to nylon filters and detection of patient antibody reactivity to the denatured proteins on the blot by using labeled anti-IgG or IgM antibodies. Several commercial kits for WIB are available which employ a variety of B. burgdorferi strains. Choice of the strain and the growth conditions used will likely affect expression levels of many immundominant proteins, so significant variation has been seen in WIB performance between laboratories, as determined in proficiency testing surveys (Robertson et al., 2000). Rates of detection of IgM antibodies in patients with EM early in the course of disease have ranged from as low as 3% to as high as 84% (Engstrom et al., 1995; Dressler et al., 1993); some commercially available WIB kits failed to detect IgM responses even after several weeks of infection (Aguero-Rosenfeld et al., 1996; Aguero-Rosenfeld et al., 1993). For detection of IgG antibodies, performance has generally been better, especially for convalescent sera (Aguero-Rosenfeld, 2008; Dressler et al., 1993). Early IgG responses are dominated by antibody to OspC (assuming the strain used makes sufficient levels of OspC) and flagellin. As mentioned, flagellin responses are the least specific of all the IgG responses for reasons cited above. Later in the convalescent phase, additional reactivities are observed against a range of proteins including BmpA (39 kDa) and B31 proteins of 93-, 66-, 45-, 35-, 30-, and 18-kDa.
Interpretive criteria for IgG and IgM WIB have been established for U.S. patients (Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of lyme disease, 1995). The IgM criteria of Engstrom et al. (1995) were adopted based on a study of early Lyme disease patients; they were established for use only within the first 4 weeks of illness. A positive IgM blot was defined by the presence of two of three reactive protein species in strain 297 (OspC, 41 or 39 kDa). The IgG criteria were based on work from Dr. Alan Steere’s group (Dressler et al., 1993); these were intended for use at any time during the course of illness, although they were expected to be more informative and applicable to patients later in their disease course. IgG reactivity against at five of 10 protein species (93, 66, 58, 45, 41, 39, 30, 28, 21, or 18 kDa) in strain 39/40 was considered the minimum number to be considered positive. WIB may be more sensitive than EIA for detection of early Lyme disease, but the two methods are similar in sensitivity for convalescent and late-stage infection. In a study of 46 culture-confirmed patients with EM, IgM WIB was considered reactive in 43% of patients, compared with 33% by EIA (Aguero-Rosenfeld et al., 1996).
This is curiously the opposite of two-tier strategies used for viral serological testing, in which the first test is more sensitive, the second more specific. Operational aspects preclude the use of WIB as a screening test, however, and other factors may influence the performance of the WIB as a screening test. For example, many of the commercial WIB kits use strain B31, which was included in none of the studies that led to the standard interpretive criteria. Although each kit is supposed to have custom criteria that match the standard criteria, this is subject to interpretive error. Subtle differences in molecular weight of antigens, along with antigen degradation can lead to confusing results, even in experienced laboratories. In addition, as with EIA methods, false positives can occur from previous vaccination with the OspA-containing vaccine (Aguero-Rosenfeld et al., 1999; Molloy et al., 2000).
Despite this apparent role reversal of screening and confirmatory testing, the recommended use of two-tier testing has helped to resolve much diagnostic uncertainty in the diagnosis of Lyme disease in the U.S., largely because it improves overall specificity. In most studies of the two-tiered strategy, specificity levels have been 97 to 99%. The low false positive rate has been welcomed, especially in areas where the pre-test probability of infection is low, and among patients presenting with non-specific symptoms in endemic areas. The reduction of sensitivity for detection of early disease was an unfortunate compromise, since non-specific febrile illness can be presenting features of HGA, Lyme disease, and human babesiosis, where diagnostic differentiation is most critical. In 280 patients with various manifestations of Lyme disease, 38% of patients with EM during the acute phase were seroreactive; 67% were positive after antimicrobial treatment. (Bacon et al., 2003). The sensitivity increased in patients with evidence of more invasive disease; 87% of patients with early neuroborreliosis and to 97% of those with Lyme arthritis were seroreactive. Similar results were obtained in another study of 47 patients with clinically defined EM, for whom the sensitivities of the two-tier test were 40.4% in acute-phase sera and 66% during the convalescent phase after treatment (Nowakowski et al., 2001).
Newer antibody tests based on recombinant proteins or immunodominant peptides have the potential of maintaining current levels of specificity without sacrificing sensitivity, especially for detection of early disease. Many studies have been done on recombinant proteins, many of which have been mentioned above, with sensitivities ranging from 30% to nearly 100% depending on the antigen used and the stage of disease being tested reviewed recently in (Aguero-Rosenfeld, 2008). Specificity levels have been somewhat lower than expected (as low as 91%), perhaps because of the presence of small amounts of contaminating protein from the strains of bacteria or yeast used for protein expression. In general, however, no single
recombinant protein has served as the universal solution for immunologic detection of both early and late disease (Aguero-Rosenfeld, 2008). Considerably more promise has been shown in combining immunodominant epitopes, especially those that derive from antigens that are recognized early in the course of disease.
In a different study of the same set of 839 serum samples (including those from 280 Lyme disease patients) cited above, antibody responses to recombinant VlsE1, the C6 peptide, and the conserved C terminal peptide from OspC (pepC10) were evaluated by kinetic enzyme-linked immunoassay (Bacon et al., 2003). At 99% specificity, the overall sensitivities for detecting IgG antibody to rVlsE1 or C6 in samples from patients with diverse manifestations of Lyme disease were equivalent to that of two-tiered testing. When data were considered in parallel, two combinations (IgG responses to either rVlsE1 or C6 in parallel with IgM responses to pepC10) maintained high specificity (98%) and were significantly more sensitive than two-tiered analysis in detecting antibodies to B. burgdorferi in patients with acute erythema migrans. In later stages of Lyme disease, the sensitivities of the in-parallel tests and two-tiered testing were high and statistically equivalent. In established cases of Lyme disease, the C6 ELISA may also be useful for tracking therapeutic responses (Marques et al., 2002; Philipp et al., 2003; Philipp et al., 2005; Wormser et al., 2008a; Wormser et al., 2008b). Although similar observations were made in other studies, some studies have not confirmed these findings (Peltomaa et al., 2003). Operational differences in the ways the assays were performed may account for this. Taken together, these data are supportive of the goal of potentially replacing two-tiered testing while improving the diagnosis for early Lyme disease patients.
Serodiagnosis of Babesiosis
The serological testing methods for B. microti are not as developed as those for Lyme disease, mainly because the organism has not been propagated in vitro. The mainstay of the laboratory diagnosis is the IFA test, in which a standard 1:16 or 1:32 dilution of serum is dispensed onto wells of a microscope slide that has been previously coated with infected red blood cells, usually of hamster origin After a wash step, addition of fluorescent anti-human IgG or IgM, and an additional wash, the microscope wells are examined under a fluorescent microscope for staining of intraerythrocytic merozoite forms of the organism. If the screening serologic testing is positive, further 1:4 serial dilutions are performed to determine the serum titer. Determination of titer end point is somewhat subjective; it is usually associated with a significant, abrupt drop in the level of observed fluorescence. In most laboratories, a 1:64 cutoff titer has been established and is considered to be consistent with exposure to B. microti.
In animal models of infection, IgM antibodies precede IgG responses by 7 to 10 days, but in humans, by the time patients present (usually with nonspecific “flu-like” symptoms) they are already IgG positive (Benach et al., 1985; Homer et al., 2000a; Krause et al., 1996a; Krause et al., 1994). During the acute phase of infection in which patients are PCR positive, antibody titers typically reach 1: 512, 1:1024 or higher and then decline in the weeks and months after therapy. Even with therapy, however, antibody levels typically persist at 1:64 or above for years after the initial infection. Thus, the finding of an antibody titer at 1:64 during acute presentation should not be assumed to be due to active infection; it may simply reflect past exposure, especially if the patient is from an endemic area. PCR testing may be helpful in resolving this issue. On the other hand, titers of greater than 1:512 have been found to be consistently associated with acute infection and correlate with PCR positivity (Krause et al., 1996a; Krause et al., 1998; Krause et al., 1996e).
Enzyme immunoassays based on antigen preparations from infected hamster erythrocytes have been developed, but have been generally hampered by the presence of anti-rodent antibodies in serum of a few percent of patients. These confounding antibodies can to some extent be pre-adsorbed prior to testing to reduce this problem. Immunoblots made from partially purified antigens have also been developed (Houghton et al., 2002).
More recently, recombinant expression cloning efforts, using B. microti expression libraries screened with human serum, led to the discovery of several recombinant proteins that show promise as immunodiagnostic reagents (Homer et al., 2000b; Houghton et al., 2002). From two serocomplementary B. microti-specific antigens, peptide mapping was done to identify immunodominant epitopes that could be combined in a peptide EIA. The prototype peptide EIA was used to detect B. microti-specific antibodies in 15 sera taken before infection and 107 taken after infection from 59 individuals with known tick-borne infections previously confirmed by other methods. The combination peptide detected 98 out of 107 sera taken after infection that were immumnoblot positive; this included 12/12 samples that were PCR positive and six sera from smear-negative patients that were confirmed positive by PCR, immunoblot, or IFA.
In another interesting study, proteins of B. microti that are potentially secreted or surface exposed were identified by serologic expression cloning (Homer et al., 2003). This report described the identification and initial characterization of 27 clones representing seven genes or gene families that were isolated through serological expression cloning by using a technique that was specifically designed to screen for shed antigens. In this screen, sera from B. microti-infected SCID mice, putatively containing secreted or shed antigens from the parasites, were harvested and used to immunize syngeneic immunocompetent mice (BALB/c). After boosting, the sera from
the BALB/c mice, containing antibodies against the immunodominant secreted antigens, were used to screen a B. microti genomic expression library. Analyses of the putative peptides encoded by the novel DNA sequences revealed characteristics indicating that these peptides might be secreted. Initial serological data obtained with recombinant proteins and a patient serum panel demonstrated that several of the proteins could be useful in developing diagnostic tests for detection of B. microti antibodies and antigens in serum. Unfortunately, however, to date none of these recombinant peptides or proteins has been converted into an immunodiagnostic test that is widely available.
Unlike for Lyme disease serology, no proficiency testing programs yet exist for serodiagnosis of B. microti infection, mainly because of lack of demand for such a service. However, this leaves open the possibility that among laboratories performing serodiagnostic methods for this organism, significant inconsistencies may exist between laboratories and within laboratories from run-to-run.
Serodiagnosis of HGA
Like for B. microti, detection of antibody responses in patient serum by the indirect immunofluorescence (IFAT) assay is the most frequently used test for clinical purposes (Walls et al., 1999). However, unlike for B. microti infections, the onset of symptoms after tick bite is fairly abrupt, and symptomatic patients are frequently seronegative. IFA assays typically use HL-60 cells infected with A. phagocytophilum as a substrate, and screening and dilution series are typically performed as described above for B. microti. Sensitivity is high 2–4 weeks following disease onset compared with a few days after onset for PCR, blood smear microscopy and cell culture. A diagnosis of A. phagocytophilum is confirmed by a fourfold increase in antibody titer between acute and convalescent sera or a seroconversion to a titer of 128 or higher (Walls et al., 1999) (Thomas et al., 2009). Like for B. microti, seropositivity for A. phagocytophilum often lasts for months and sometimes years after initial exposure (Aguero-Rosenfeld et al., 2000). Thus, like for B. microti and B. burgdorferi, an HGA antibody titer must be considered in light of other clinical evidence of infection and should not be used as the only criterion for diagnosis. False-positive or cross reactive serologic test results can be seen in cases of Rocky Mountain spotted fever, typhus, Q fever, brucellosis, Lyme disease, Epstein–Barr infection and any variety of autoimmune disorders associated with production of auto antibodies (Dumler et al., 2007). Aguero-Rosenfeld et al evaluated the antibody responses by IFA in the sera of 24 patients with culture-confirmed HGA (Aguero-Rosenfeld et al., 2000). Patients were followed for up to 14 months. Seroconversion was observed in 21 of 23 patients (91.3%)
from whom convalescent-phase sera were obtained. Antibodies were first detected at an average of 11.5 days after onset of symptoms. Peak titers (>/=2,560 for 71.4% of patients and >/=640 for 95.2% of patients) were obtained an average of 14.7 days after onset of symptoms. Most patients were still considered seropositive after 6 months, and half of the patients were still seropositive after 11 months.
Some progress has been made toward development of recombinant serological reagents for the diagnosis of HGA, which might be more amenable to automated testing and or rapid immunodiagnostic assay formats (Lodes et al., 2001). Lodes et al described a panel of seven recombinant antigens, derived from the HGA agent, which were evaluated by class-specific ELISAs for utility in the diagnosis of the infection. Fourteen genomic fragments, obtained by serologic expression screening, contained open reading frames encoding 16 immunodominant antigens. Eleven of these antigens were members of the major surface protein (MSP) multigene family. In addition to two MSP recombinant antigens (rHGE-1 and -3) and a fusion protein of one of these antigens (rErf-1), five further recombinants were evaluated by ELISA. Two of these antigens (rHGE-14 and -15) were novel, while a third (rHGE-2), with no known function, had already been described. The final two recombinant antigens (rHGE-9 and -17) represented overlapping segments of the ankyrin gene. When serologic data for all recombinants were combined, 96.2% (26 of 27) of convalescent-phase patient serum samples and 85.2% (23 of 27) of acute-phase patient serum samples were detected, indicating the potential of these antigens for use in the development of a rapid serologic assay for the diagnoses of HGA.
Conclusions
The three identified infections transmissible by the bite of a deer tick in the United States—Lyme disease, HGA and human babesiosis—have distinct and overlapping clinical features that, in the case of infection by more than one organism, may confound each other. Each may require different treatment approaches, although one can argue that tetracycline derivatives, with activity against B. burgdorferi and HGA, should become the drug of choice for patients with confusing clinical pictures or features of more than one infection. Much of the management of patients with nonspecific presentations will depend on specific diagnostic test results. Fortunately, although babesiosis and HGA have immunosuppressive effects, these effects are not sufficient to confound the specific serologic responses to the cognate infectious agents. In the case of HGA and babesiosis, direct detection by blood smear, culture or PCR (HGA) or smear and PCR (babesiosis) are diagnostic options, but none of the direct detection approaches are very useful for the diagnosis of early Lyme disease. The diagnosis of all of these conditions
may be improved by the use of next generation serologic testing and molecular diagnostic approaches, both of which are likely to make their way into increasing numbers of laboratories over the next decade.
References
Aguero-Rosenfeld, M. E. 2003. Laboratory aspects of tick-borne diseases: Lyme, human granulocytic ehrlichiosis and babesiosis. Mt Sinai J Med 70(3):197-206.
———. 2008. Lyme disease: Laboratory issues. Infect Dis Clin North Am 22(2):301-313, vii.
Aguero-Rosenfeld, M. E., F. Kalantarpour, M. Baluch, H. W. Horowitz, D. F. McKenna, J. T. Raffalli, T. Hsieh, J. Wu, J. S. Dumler, and G. P. Wormser. 2000. Serology of culture-confirmed cases of human granulocytic ehrlichiosis. J Clin Microbiol 38(2):635-638.
Aguero-Rosenfeld, M. E., J. Nowakowski, S. Bittker, D. Cooper, R. B. Nadelman, and G. P. Wormser. 1996. Evolution of the serologic response to borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. Journal of Clinical Microbiology 34(1):1-9.
Aguero-Rosenfeld, M. E., J. Nowakowski, D. F. McKenna, C. A. Carbonaro, and G. P. Wormser. 1993. Serodiagnosis in early lyme disease. J Clin Microbiol 31(12):3090-3095.
Aguero-Rosenfeld, M. E., J. Roberge, C. A. Carbonaro, J. Nowakowski, R. B. Nadelman, and G. P. Wormser. 1999. Effects of ospa vaccination on lyme disease serologic testing. J Clin Microbiol 37(11):3718-3721.
Aguero-Rosenfeld, M. E., G. Wang, I. Schwartz, and G. P. Wormser. 2005. Diagnosis of lyme borreliosis. Clin Microbiol Rev 18(3):484-509.
Alberti, A., and O. A. Sparagano. 2006. Molecular diagnosis of granulocytic anaplasmosis and infectious cyclic thrombocytopenia by pcr-rflp. Ann N Y Acad Sci 1081:371-378.
Bacon, R. M., B. J. Biggerstaff, M. E. Schriefer, R. D. Gilmore, Jr., M. T. Philipp, A. C. Steere, G. P. Wormser, A. R. Marques, and B. J. Johnson. 2003. Serodiagnosis of lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant vlse1 or peptide antigens of borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis 187(8):1187-1199.
Bakken, J. S., and J. S. Dumler. 2000. Human granulocytic ehrlichiosis. Clin Infect Dis 31(2):554-560.
———. 2006. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci 1078:236-247.
Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper midwest United States. A new species emerging? [see comments]. Jama 272(3):212-218.
Barbour, A. G. 1984. Isolation and cultivation of lyme disease spirochetes. Yale Journal of Biology & Medicine 57(4):521-525.
———. 1986. Cultivation of borrelia: A historical overview. Zentralblatt Fur Bakteriologie, Mikrobiologie, Und Hygiene Series A, Medical Microbiology, Infectious Diseases, Virology, Parasitology 263(1-2):11-14.
Belongia, E. A., K. D. Reed, P. D. Mitchell, N. Mueller-Rizner, M. Vandermause, M. F. Finkel, and J. J. Kazmierczak. 2001. Tickborne infections as a cause of nonspecific febrile illness in wisconsin. Clin Infect Dis 32(10):1434-1439.
Benach, J. L., J. L. Coleman, G. S. Habicht, A. MacDonald, E. Grunwaldt, and J. A. Giron. 1985. Serological evidence for simultaneous occurrences of lyme disease and babesiosis. Journal of Infectious Diseases 152(3):473-477.
Bradley, J. F., R. C. Johnson, and J. L. Goodman. 1994. The persistence of spirochetal nucleic acids in active lyme arthritis. Ann Intern Med 120(6):487-489.
Coleman, J. L., and J. L. Benach. 1987. Isolation of antigenic components from the lyme disease spirochete: Their role in early diagnosis. J Infect Dis 155(4):756-765.
Coleman, J. L., D. LeVine, C. Thill, C. Kuhlow, and J. L. Benach. 2005. Babesia microti and borrelia burgdorferi follow independent courses of infection in mice. J Infect Dis 192(9):1634-1641.
Comer, J. A., W. L. Nicholson, J. W. Sumner, J. G. Olson, and J. E. Childs. 1999. Diagnosis of human ehrlichiosis by pcr assay of acute-phase serum. J Clin Microbiol 37(1):31-34.
Craft, J. E., D. K. Fischer, G. T. Shimamoto, and A. C. Steere. 1986. Antigens of borrelia burgdorferi recognized during lyme disease. Appearance of a new immunoglobulin m response and expansion of the immunoglobulin g response late in the illness. Journal of Clinical Investigation 78(4):934-939.
Das, S., S. W. Barthold, S. S. Giles, R. R. Montgomery, S. R. Telford, 3rd, and E. Fikrig. 1997. Temporal pattern of borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Invest 99(5):987-995.
Demaerschalck, I., A. Ben Messaoud, M. De Kesel, B. Hoyois, Y. Lobet, P. Hoet, G. Bigaignon, A. Bollen, and E. Godfroid. 1995. Simultaneous presence of different borrelia burgdorferi genospecies in biological fluids of lyme disease patients. Journal of Clinical Microbiology 33(3):602-608.
Dorward, D. W., T. G. Schwan, and C. F. Garon. 1991. Immune capture and detection of borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. Journal of Clinical Microbiology 29(6):1162-1170.
Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of lyme disease. J. Infect. Dis. 167:392-400.
Dumler, J. S. 2001. Molecular diagnosis of lyme disease: Review and meta-analysis. Mol Diagn 6(1):1-11.
Dumler, J. S., J. E. Madigan, N. Pusterla, and J. S. Bakken. 2007. Ehrlichioses in humans: Epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis 45 Suppl 1:S45-51.
Eiffert, H., A. Karsten, R. Thomssen, and H. J. Christen. 1998. Characterization of borrelia burgdorferi strains in lyme arthritis. Scand J Infect Dis 30(3):265-268.
Engstrom, S. M., E. Shoop, and R. C. Johnson. 1995. Immunoblot interpretation criteria for serodiagnosis of early lyme disease. J. Clin. Microbiol. 33:419-427.
Fawcett, P. T., K. M. Gibney, C. D. Rose, S. B. Dubbs, and R. A. Doughty. 1992. Frequency and specificity of antibodies that crossreact with borrelia burgdorferi antigens. J Rheumatol 19(4):582-587.
Fikrig, E., S. W. Barthold, W. Sun, W. Feng, S. R. Telford, 3rd, and R. A. Flavell. 1997. Borrelia burgdorferi p35 and p37 proteins, expressed in vivo, elicit protective immunity. Immunity 6(5):531-539.
Fikrig, E., W. Feng, S. W. Barthold, S. R. Telford, 3rd, and R. A. Flavell. 2000. Arthropod-and host-specific borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J Immunol 164(10):5344-5351.
Filstein, M. R., J. L. Benach, D. J. White, B. A. Brody, W. D. Goldman, C. W. Bakal, and R. S. Schwartz. 1980. Serosurvey for human babesiosis in new york. Journal of Infectious Diseases 141(4):518-521.
Goodman, J. L., J. F. Bradley, A. E. Ross, P. Goellner, A. Lagus, B. Vitale, B. W. Berger, S. Luger, and R. C. Johnson. 1995. Bloodstream invasion in early lyme disease: Results from a prospective, controlled, blinded study using the polymerase chain reaction. American Journal of Medicine 99(1):6-12.
Goodman, J. L., C. Nelson, B. Vitale, J. E. Madigan, J. S. Dumler, T. J. Kurtti, and U. G. Munderloh. 1996. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. New England Journal of Medicine 334(4):209-215.
Heikkila, T., I. Seppala, H. Saxen, J. Panelius, H. Yrjanainen, and P. Lahdenne. 2002. Species-specific serodiagnosis of lyme arthritis and neuroborreliosis due to borrelia burgdorferi sensu stricto, B. Afzelii, and B. Garinii by using decorin binding protein A. J Clin Microbiol 40(2):453-460.
Hofmeister, E. K., J. Magera, L. Sloan, C. Kolbert, J. Hanson, and D. H. Persing. 1996. Borrelia burgdorferi proteins are recognized by antibodies from mice experimentally infected with the agent of human granulocytic ehrlichiosis. Paper read at Seventh International Congress on Lyme Borreliosis, San Francisco, CA.
Holden, K., E. Hodzic, S. Feng, K. J. Freet, R. B. Lefebvre, and S. W. Barthold. 2005. Coinfection with anaplasma phagocytophilum alters borrelia burgdorferi population distribution in c3h/hen mice. Infect Immun 73(6):3440-3444.
Homer, M. J., I. Aguilar-Delfin, S. R. Telford, 3rd, P. J. Krause, and D. H. Persing. 2000a. Babesiosis. Clin Microbiol Rev 13(3):451-469.
Homer, M. J., E. S. Bruinsma, M. J. Lodes, M. H. Moro, S. Telford, 3rd, P. J. Krause, L. D. Reynolds, R. Mohamath, D. R. Benson, R. L. Houghton, S. G. Reed, and D. H. Persing. 2000b. A polymorphic multigene family encoding an immunodominant protein from babesia microti. J Clin Microbiol 38(1):362-368.
Homer, M. J., M. J. Lodes, L. D. Reynolds, Y. Zhang, J. F. Douglass, P. D. McNeill, R. L. Houghton, and D. H. Persing. 2003. Identification and characterization of putative secreted antigens from babesia microti. J Clin Microbiol 41(2):723-729.
Horowitz, H. W., M. E. Aguero-Rosenfeld, D. F. McKenna, D. Holmgren, T. C. Hsieh, S. A. Varde, S. J. Dumler, J. M. Wu, I. Schwartz, Y. Rikihisa, and G. P. Wormser. 1998. Clinical and laboratory spectrum of culture-proven human granulocytic ehrlichiosis: Comparison with culture-negative cases. Clin Infect Dis 27(5):1314-1317.
Houghton, R. L., M. J. Homer, L. D. Reynolds, P. R. Sleath, M. J. Lodes, V. Berardi, D. A. Leiby, and D. H. Persing. 2002. Identification of babesia microti-specific immunodominant epitopes and development of a peptide eia for detection of antibodies in serum. Transfusion 42(11):1488-1496.
Hyde, F. W., R. C. Johnson, T. J. White, and C. E. Shelburne. 1989. Detection of antigens in urine of mice and humans infected with borrelia burgdorferi, etiologic agent of lyme disease. J Clin Microbiol 27(1):58-61.
Jahangir, A., C. Kolbert, W. Edwards, P. Mitchell, J. S. Dumler, and D. H. Persing. 1998. Fatal pancarditis associated with human granulocytic ehrlichiosis in a 44-year-old man. Clin Infect Dis 27(6):1424-1427.
Jaulhac, B., I. Chary-Valckenaere, J. Sibilia, R. M. Javier, Y. Piemont, J. L. Kuntz, H. Monteil, and J. Pourel. 1996. Detection of borrelia burgdorferi by DNA amplification in synovial tissue samples from patients with lyme arthritis. Arthritis Rheum 39(5):736-745.
Kelly, R. 1971. Cultivation of borrelia hermsi. Science 173(995):443-444.
Klempner, M. S., L. T. Hu, J. Evans, C. H. Schmid, G. M. Johnson, R. P. Trevino, D. Norton, L. Levy, D. Wall, J. McCall, M. Kosinski, and A. Weinstein. 2001a. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of lyme disease. N Engl J Med 345(2):85-92.
Klempner, M. S., C. H. Schmid, L. Hu, A. C. Steere, G. Johnson, B. McCloud, R. Noring, and A. Weinstein. 2001b. Intralaboratory reliability of serologic and urine testing for lyme disease. Am J Med 110(3):217-219.
Krause, P. J., K. McKay, J. Gadbaw, D. Christianson, L. Closter, T. Lepore, S. R. Telford, 3rd, V. Sikand, R. Ryan, D. Persing, J. D. Radolf, and A. Spielman. 2003. Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg 68(4):431-436.
Krause, P. J., K. McKay, C. A. Thompson, V. K. Sikand, R. Lentz, T. Lepore, L. Closter, D. Christianson, S. R. Telford, D. Persing, J. D. Radolf, and A. Spielman. 2002. Disease-specific diagnosis of coinfecting tickborne zoonoses: Babesiosis, human granulocytic ehrlichiosis, and lyme disease. Clin Infect Dis 34(9):1184-1191.
Krause, P. J., R. Ryan, S. Telford, D. Persing, and A. Spielman. 1996a. Efficacy of immunoglobulin m serodiagnostic test for rapid diagnosis of acute babesiosis. Journal of Clinical Microbiology 34(8):2014-2016.
Krause, P. J., A. Spielman, S. R. Telford, 3rd, V. K. Sikand, K. McKay, D. Christianson, R. J. Pollack, P. Brassard, J. Magera, R. Ryan, and D. H. Persing. 1998. Persistent parasitemia after acute babesiosis. N Engl J Med 339(3):160-165.
Krause, P. J., S. R. Telford III, A. Spielman, V. J. Sikand, R. Ryan, D. Christianson, G. Burke, P. Brassard, R. Pollack, J. Peck, and D. H. Persing. 1996b. Concurrent lyme disease and babesiosis: Evidence for increased severity and duration of illness. JAMA 275:1657-1660.
Krause, P. J., S. Telford, 3rd, A. Spielman, R. Ryan, J. Magera, T. V. Rajan, D. Christianson, T. V. Alberghini, L. Bow, and D. Persing. 1996c. Comparison of pcr with blood smear and inoculation of small animals for diagnosis of babesia microti parasitemia. J Clin Microbiol 34(11):2791-2794.
Krause, P. J., S. D. Telford, R. Ryan, A. B. Hurta, I. Kwasnik, S. Luger, J. Niederman, M. Gerber, and A. Spielman. 1991. Geographical and temporal distribution of babesial infection in connecticut. Journal of Clinical Microbiology 29(1):1-4.
Krause, P. J., S. R. Telford, R. Ryan, P. A. Conrad, M. Wilson, J. W. Thomford, and A. Spielman. 1994. Diagnosis of babesiosis: Evaluation of a serologic test for the detection of babesia microti antibody [see comments]. Journal of Infectious Diseases 169(4):923-926.
Krause, P. J., S. R. Telford, A. Spielman, R. Ryan, J. Magera, T. V. Rajan, D. Christianson, T. V. Alberghini, L. Bow, and D. Persing. 1996e. Comparison of pcr with blood smear and inoculation of small animals for diagnosis of babesia microti parasitemia. Journal of Clinical Microbiology 34(11):2791-2794.
Lawrenz, M. B., J. M. Hardham, R. T. Owens, J. Nowakowski, A. C. Steere, G. P. Wormser, and S. J. Norris. 1999. Human antibody responses to vlse antigenic variation protein of borrelia burgdorferi. J Clin Microbiol 37(12):3997-4004.
Lebech, A.-M., K. Hansen, F. Brandrup, O. Clemmensen, and L. Halkier-Sorensen. 2000. Diagnostic value of pcr for detection of borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and lyme neuroborreliosis. Mol Diagn 5(2):139-150.
Lebech, A.-M., K. Hansen, P. Pancholi, L. Sloan, J. Magera, and D. H. Persing. 1998. Direct detection and genotyping of B. Burgdorferi in the cerebrospinal fluid of lyme neuroborreliosis patients. Molecular Diagnosis 3(3):131-141.
Leiby, D. A., A. P. Chung, R. G. Cable, J. Trouern-Trend, J. McCullough, M. J. Homer, L. D. Reynolds, R. L. Houghton, M. J. Lodes, and D. H. Persing. 2002. Relationship between tick bites and the seroprevalence of babesia microti and anaplasma phagocytophila (previously ehrlichia sp.) in blood donors. Transfusion 42(12):1585-1591.
Leiby, D. A., A. P. Chung, J. E. Gill, R. L. Houghton, D. H. Persing, S. Badon, and R. G. Cable. 2005. Demonstrable parasitemia among connecticut blood donors with antibodies to babesia microti. Transfusion 45(11):1804-1810.
Liebling, M. R., M. J. Nishio, A. Rodriguez, L. H. Sigal, T. Jin, and J. S. Louie. 1993. The polymerase chain reaction for the detection of borrelia burgdorferi in human body fluids. Arthritis Rheum 36(5):665-675.
Lodes, M. J., R. Mohamath, L. D. Reynolds, P. McNeill, C. P. Kolbert, E. S. Bruinsma, D. R. Benson, E. Hofmeister, S. G. Reed, R. L. Houghton, and D. H. Persing. 2001. Serodiagnosis of human granulocytic ehrlichiosis by using novel combinations of immunoreactive recombinant proteins. J Clin Microbiol 39(7):2466-2476.
Logigian, E. L., R. F. Kaplan, and A. C. Steere. 1990. Chronic neurologic manifestations of lyme disease [see comments]. New England Journal of Medicine 323(21):1438-1444.
Luft, B. J., J. J. Dunn, R. J. Dattwyler, G. Gorgone, P. D. Gorevic, and W. H. Schubach. 1993. Cross-reactive antigenic domains of the flagellin protein of borrelia burgdorferi. Res Microbiol 144(4):251-257.
Macdonald, K. L., M. T. Osterholm, K. H. Ledell, K. E. White, C. H. Schenck, C. C. Chao, D. H. Persing, R. C. Johnson, J. M. Barker, and P. K. Peterson. 1996. A case-control study to assess possible triggers and cofactors in chronic fatigue syndrome. American Journal of Medicine 100(5):548-554.
Malawista, S. E., R. T. Schoen, T. L. Moore, D. E. Dodge, T. J. White, and D. H. Persing. 1992. Failure of multitarget detection of borrelia burgdorferi-associated DNA sequences in synovial fluids of patients with juvenile rheumatoid arthritis: A cautionary note. Arthritis Rheum 35(2):246-247.
Malawista, S. E., and A. C. Steere. 1986. Lyme disease: Infectious in origin, rheumatic in expression. [review]. Advances in Internal Medicine 31:147-166.
Marques, A. R., D. S. Martin, and M. T. Philipp. 2002. Evaluation of the c6 peptide enzyme-linked immunosorbent assay for individuals vaccinated with the recombinant ospa vaccine. J Clin Microbiol 40(7):2591-2593.
Marshall, W. F., 3rd, S. R. Telford, 3rd, P. N. Rys, B. J. Rutledge, D. Mathiesen, S. E. Malawista, A. Spielman, and D. H. Persing. 1994a. Detection of borrelia burgdorferi DNA in museum specimens of peromyscus leucopus. J Infect Dis 170(4):1027-1032.
Marshall, W. R., S. R. Telford, P. N. Rys, B. J. Rutledge, D. Mathiesen, S. E. Malawista, A. Spielman, and D. H. Persing. 1994b. Detection of borrelia burgdorferi DNA in museum specimens of peromyscus leucopus. Journal of Infectious Diseases 170(4):1027-1032.
Mathiesen, D. A., J. H. Oliver, C. P. Kolbert, E. D. Tullson, B. J. B. Johnson, G. L. Campbell, P. D. Mitchell, K. D. Reed, S. R. Telford, J. F. Anderson, R. S. Lane, and D. H. Persing. 1997. Genetic heterogeneity of borrelia burgdorferi in the United States. Journal of Infectious Diseases 175(1):98-107.
Mathiesen, M. J., A. Holm, M. Christiansen, J. Blom, K. Hansen, S. Ostergaard, and M. Theisen. 1998. The dominant epitope of borrelia garinii outer surface protein C recognized by sera from patients with neuroborreliosis has a surface-exposed conserved structural motif. Infect Immun 66(9):4073-4079.
Matthews, J., E. Rattigan, and H. Yee. 2003. Case 29-2003: A 60-year-old man with fever, rigors, and sweats. N Engl J Med 349(25):2467; author reply 2467.
Meldrum, S. C., G. S. Birkhead, D. J. White, J. L. Benach, and D. L. Morse. 1992. Human babesiosis in new york state: An epidemiological description of 136 cases. Clinical Infectious Diseases 15(6):1019-1023.
Molloy, P. J., V. P. Berardi, D. H. Persing, and L. H. Sigal. 2000. Detection of multiple reactive protein species by immunoblotting after recombinant outer surface protein A lyme disease vaccination [in process citation]. Clin Infect Dis 31(1):42-47.
Molloy, P. J., D. H. Persing, and V. P. Berardi. 2001. False-positive results of pcr testing for lyme disease. Clin Infect Dis 33(3):412-413.
Moro, M. H., O. L. Zegarra-Moro, and D. H. Persing. 2006. Babesia microti and borrelia burgdorferi coinfection associated with increased severity of arthritis. J Infect Dis 194(5):716.
Moter, S. E., H. Hofmann, R. Wallich, M. M. Simon, and M. D. Kramer. 1994. Detection of borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospa-specific pcr. J Clin Microbiol 32(12):2980-2988.
Nocton, J. J., B. J. Bloom, B. J. Rutledge, D. H. Persing, E. L. Logigian, C. H. Schmid, and A. C. Steere. 1996. Detection of borrelia burgdorferi DNA by polymerase chain reaction in cerebrospinal fluid in lyme neuroborreliosis. Journal of Infectious Diseases 174(3):623-627.
Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with lyme arthritis. N Engl J Med 330(4):229-234.
Nowakowski, J., I. Schwartz, D. Liveris, G. Wang, M. E. Aguero-Rosenfeld, G. Girao, D. McKenna, R. B. Nadelman, L. F. Cavaliere, and G. P. Wormser. 2001. Laboratory diagnostic techniques for patients with early lyme disease associated with erythema migrans: A comparison of different techniques. Clin Infect Dis 33(12):2023-2027.
Oksi, J., H. Marttila, H. Soini, H. Aho, J. Uksila, and M. K. Viljanen. 2001. Early dissemination of borrelia burgdorferi without generalized symptoms in patients with erythema migrans. APMIS 109(9):581-588.
Ornstein, K., J. Berglund, S. Bergstrom, R. Norrby, and A. G. Barbour. 2002. Three major lyme borrelia genospecies (borrelia burgdorferi sensu stricto, B. Afzelii and B. Garinii) identified by pcr in cerebrospinal fluid from patients with neuroborreliosis in Sweden. Scand J Infect Dis 34(5):341-346.
Pancholi, P., C. P. Kolbert, P. D. Mitchell, K. D. Reed, J. S. Dumler, J. S. Bakken, S. R. Telford, and D. H. Persing. 1995. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. Journal of Infectious Diseases 172(4):1007-1012.
Patel, R., K. L. Grogg, W. D. Edwards, A. J. Wright, and N. M. Schwenk. 2000. Death from inappropriate therapy for lyme disease. Clin Infect Dis 31(4):1107-1109.
Peltomaa, M., G. McHugh, and A. C. Steere. 2003. Persistence of the antibody response to the vlse sixth invariant region (ir6) peptide of borrelia burgdorferi after successful antibiotic treatment of lyme disease. J Infect Dis 187(8):1178-1186.
Persing, D. H. 1997. The cold zone: A curious convergence of tick-transmitted diseases. Clin Infect Dis 25(Suppl 1):S35-42.
Persing, D. H., B. L. Herwaldt, C. Glaser, R. S. Lane, J. W. Thomford, D. Mathiesen, P. J. Krause, D. F. Phillip, and P. A. Conrad. 1995. Infection with a babesia-like organism in northern California. New England Journal of Medicine 332(5):298-303.
Persing, D. H., D. Mathiesen, W. F. Marshall, S. R. Telford, A. Spielman, J. W. Thomford, and P. A. Conrad. 1992. Detection of babesia microti by polymerase chain reaction. Journal of Clinical Microbiology 30(8):2097-2103.
Persing, D. H., B. J. Rutledge, P. N. Rys, D. S. Podzorski, P. D. Mitchell, K. D. Reed, B. Liu, E. Fikrig, and S. E. Malawista. 1994a. Target imbalance: Disparity of borrelia burgdorferi genetic material in synovial fluid from lyme arthritis patients. Journal of Infectious Diseases 169(3):668-672.
———. 1994b. Target imbalance: Disparity of borrelia burgdorferi genetic material in synovial fluid from lyme arthritis patients. J Infect Dis 169(3):668-672.
Persing, D. H., S. D. Telford, A. Spielman, and S. W. Barthold. 1990a. Detection of borrelia burgdorferi infection in ixodes dammini ticks with the polymerase chain reaction. Journal of Clinical Microbiology 28(3):566-572.
Persing, D. H., S. R. Telford, 3rd, P. N. Rys, D. E. Dodge, T. J. White, S. E. Malawista, and A. Spielman. 1990b. Detection of borrelia burgdorferi DNA in museum specimens of ixodes dammini ticks. Science 249(4975):1420-1423.
Philipp, M. T., A. R. Marques, P. T. Fawcett, L. G. Dally, and D. S. Martin. 2003. C6 test as an indicator of therapy outcome for patients with localized or disseminated lyme borreliosis. J Clin Microbiol 41(11):4955-4960.
Philipp, M. T., G. P. Wormser, A. R. Marques, S. Bittker, D. S. Martin, J. Nowakowski, and L. G. Dally. 2005. A decline in c6 antibody titer occurs in successfully treated patients with culture-confirmed early localized or early disseminated lyme borreliosis. Clin Diagn Lab Immunol 12(9):1069-1074.
Pollack, R. J., S. R. Telford, 3rd, and A. Spielman. 1993. Standardization of medium for culturing lyme disease spirochetes. J Clin Microbiol 31(5):1251-1255.
Pruthi, R. K., W. F. Marshall, J. C. Wiltsie, and D. H. Persing. 1995. Human babesiosis. Mayo Clin Proc 70(9):853-862.
Quick, R. E., B. L. Herwaldt, J. W. Thomford, M. E. Garnett, M. L. Eberhard, M. Wilson, D. H. Spach, J. W. Dickerson, S. R. Telford, K. R. Steingart, R. Pollock, D. H. Persing,
J.M. Kobayashi, D. D. Juranek, and P. A. Conrad. 1993. Babesiosis in washington state - a new species of babesia? Annals of Internal Medicine 119(4):284-290.
Rahn, D. W. 1991. Lyme disease: Clinical manifestations, diagnosis, and treatment. [review]. Seminars in Arthritis & Rheumatism 20(4):201-218.
Ravyn, M. D., C. B. Kodner, S. E. Carter, J. L. Jarnefeld, and R. C. Johnson. 2001. Isolation of the etiologic agent of human granulocytic ehrlichiosis from the white-footed mouse (peromyscus leucopus). J Clin Microbiol 39(1):335-338.
Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of lyme disease. 1995. MMWR Morb Mortal Wkly Rep 44(31):590-591.
Robertson, J., E. Guy, N. Andrews, B. Wilske, P. Anda, M. Granstrom, U. Hauser, Y. Moosmann, V. Sambri, J. Schellekens, G. Stanek, and J. Gray. 2000. A european multicenter study of immunoblotting in serodiagnosis of lyme borreliosis. J Clin Microbiol 38(6):2097-2102.
Ruebush, T. D., D. D. Juranek, E. S. Chisholm, P. C. Snow, G. R. Healy, and A. J. Sulzer. 1977. Human babesiosis on nantucket island. Evidence for self-limited and subclinical infections. New England Journal of Medicine 297(15):825-827.
Schmidt, B. L. 1997. Pcr in laboratory diagnosis of human borrelia burgdorferi infections. Clin Microbiol Rev 10(1):185-201.
Spielman, A. 1976. Human babesiosis on nantucket island: Transmission by nymphal ixodes ticks. Am. J. Trop. Med. Hyg. 25:784-787.
Spielman, A., C. M. Clifford, J. Piesman, and M. D. Corwin. 1979. Human babesiosis on nantucket island, USA: Description of the vector, ixodes (ixodes) dammino, n. Sp. (acarina: Ixodidae).J. Med. Entomol. 15:218-234.
Steere, A. C. 2001. Lyme disease. N Engl J Med 345(2):115-125.
Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of lyme disease. New England Journal of Medicine 308(13):733-740.
Steere, A. C., V. K. Sikand, F. Meurice, D. L. Parenti, E. Fikrig, R. T. Schoen, J. Nowakowski, C. H. Schmid, S. Laukamp, C. Buscarino, and D. S. Krause. 1998. Vaccination against lyme disease with recombinant borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme disease vaccine study group [see comments]. N Engl J Med 339(4):209-215.
Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation of borrelia hermsii. J Exp Med 156(5):1297-1311.
Telford, S. R., J. E. Dawson, P. Katavolos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proceedings of the National Academy of Sciences (USA) 93:6209-6214.
Theisen, M., B. Frederiksen, A. M. Lebech, J. Vuust, and K. Hansen. 1993. Polymorphism in ospc gene of borrelia burgdorferi and immunoreactivity of ospc protein: Implications for taxonomy and for use of ospc protein as a diagnostic antigen. J Clin Microbiol 31(10):2570-2576.
Thomas, R. J., J. S. Dumler, and J. A. Carlyon. 2009. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther 7(6):709-722.
Thomas, V., J. Anguita, S. W. Barthold, and E. Fikrig. 2001. Coinfection with borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of lyme arthritis. Infect Immun 69(5):3359-3371.
Tugwell, P., D. T. Dennis, A. Weinstein, G. Wells, B. Shea, G. Nichol, R. Hayward, R. Lightfoot, P. Baker, and A. C. Steere. 1997. Laboratory evaluation in the diagnosis of lyme disease. Ann Intern Med 127(12):1109-1123.
Vannier, E., and P. J. Krause. 2009. Update on babesiosis. Interdiscip Perspect Infect Dis 2009:984568.
Wallach, F. R., A. L. Forni, J. Hariprashad, M. Y. Stoeckle, C. R. Steinberg, L. Fisher, S. E. Malawista, and H. W. Murray. 1993. Circulating borrelia burgdorferi in patients with acute lyme disease: Results of blood cultures and serum DNA analysis. J Infect Dis 168(6):1541-1543.
Walls, J. J., M. Aguero-Rosenfeld, J. S. Bakken, J. L. Goodman, D. Hossain, R. C. Johnson, and J. S. Dumler. 1999. Inter- and intralaboratory comparison of ehrlichia equi and human granulocytic ehrlichiosis (hge) agent strains for serodiagnosis of hge by the immunofluorescent-antibody test. J Clin Microbiol 37(9):2968-2973.
Walls, J. J., P. Caturegli, J. S. Bakken, K. M. Asanovich, and J. S. Dumler. 2000. Improved sensitivity of pcr for diagnosis of human granulocytic ehrlichiosis using epank1 genes of ehrlichia phagocytophila-group ehrlichiae. J Clin Microbiol 38(1):354-356.
Wormser, G. P., S. Bittker, D. Cooper, J. Nowakowski, R. B. Nadelman, and C. Pavia. 2000a. Comparison of the yields of blood cultures using serum or plasma from patients with early lyme disease. J Clin Microbiol 38(4):1648-1650.
Wormser, G. P., H. W. Horowitz, J. S. Dumler, I. Schwartz, and M. Aguero-Rosenfeld. 1996. False-positive lyme disease serology in human granulocytic ehrlichiosis [letter]. Lancet 347(9006):981-982.
Wormser, G. P., D. Liveris, K. Hanincova, D. Brisson, S. Ludin, V. J. Stracuzzi, M. E. Embers, M. T. Philipp, A. Levin, M. Aguero-Rosenfeld, and I. Schwartz. 2008a. Effect of borrelia burgdorferi genotype on the sensitivity of c6 and 2-tier testing in north American patients with culture-confirmed lyme disease. Clin Infect Dis 47(7):910-914.
Wormser, G. P., R. B. Nadelman, R. J. Dattwyler, D. T. Dennis, E. D. Shapiro, A. C. Steere, T. J. Rush, D. W. Rahn, P. K. Coyle, D. H. Persing, D. Fish, and B. J. Luft. 2000b. Practice guidelines for the treatment of lyme disease. The infectious diseases society of america. Clin Infect Dis 31(Suppl 1):1-14.
Wormser, G. P., J. Nowakowski, R. B. Nadelman, S. Bittker, D. Cooper, and C. Pavia. 1998. Improving the yield of blood cultures for patients with early lyme disease. J Clin Microbiol 36(1):296-298.
Wormser, G. P., J. Nowakowski, R. B. Nadelman, P. Visintainer, A. Levin, and M. E. Aguero-Rosenfeld. 2008b. Impact of clinical variables on borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in north america with culture-confirmed early lyme disease. Clin Vaccine Immunol 15(10):1519-1522.
Zeidner, N. S., M. C. Dolan, R. Massung, J. Piesman, and D. Fish. 2000. Coinfection with borrelia burgdorferi and the agent of human granulocytic ehrlichiosis suppresses IL-2 and ifn gamma production and promotes an IL-4 response in c3h/hej mice. Parasite Immunol 22(11):581-588.
Zhang, J.-R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997a. Antigenic variation in lyme disease borreliae by promiscuous recombination of vmp-like sequence cassettes. Cell Press 89:275-285.
Zhang, J. R., and S. J. Norris. 1998a. Genetic variation of the borrelia burgdorferi gene vlse involves cassette-specific, segmental gene conversion. Infection & Immunity 66(8):3698-3704.
———. 1998b. Kinetics and in vivo induction of genetic variation of vlse in borrelia burgdorferi. Infection & Immunity 66(8):3689-3697.
Zhang, Y. Q., D. Mathiesen, C. P. Kolbert, J. Anderson, R. T. Schoen, E. Fikrig, and D. H. Persing. 1997b. Borrelia burgdorferi enzyme-linked immunosorbent assay for discrimination of ospa vaccination from spirochete infection. Journal of Clinical Microbiology 35(1):233-238.
A5
EHRLICHIOSES, RICKETTSIOSES, AND ANAPLASMOSIS IN THE UNITED STATES: CURRENT STATUS AND OPPORTUNITIES FOR NEW VACCINES
Jere W. McBride,5 Xue-jie Yu,5 and Kelly Brayton6
Ehrlichioses
Introduction
Ehrlichia are obligately intracellular gram-negative bacteria that are associated with emerging, tick-transmitted, life-threatening zoonoses in humans. Human monocytotropic ehrlichiosis (HME) and human ehrlichosis ewingii (HEE) are now well established zoonoses in the United States caused by E. chaffeensis and E. ewingii, respectively. The first recognized case of human ehrlichiosis occurred in 1986 in a patient that acquired the infection in Arkansas (Maeda et al., 1987), and a previously unknown pathogen, E. chaffeensis, was later identified as the etiologic agent (Anderson et al., 1991). As epidemiologic and ecologic understanding of E. chaffeensis biology has developed, it is now considered a prototypical emerging pathogen (Paddock and Childs, 2003). Shortly after the emergence of E. chaffeensis, the canine pathogen, E. ewingii, associated with granulocytic ehrlichiosis in dogs, was molecularly identified in four patients from Missouri presenting with fever, headache and thrombocytopenia (Buller et al., 1999). The emergence of human ehrlichioses has been attributed to changes in biological, demographic and environmental factors, and these factors in addition to increased surveillance and diagnostic capability are likely to result in increasing recognized incidence of human ehrlichiosis in the future (Paddock and Childs, 2003). Thus, there is an immediate need for effective vaccines now for human ehrlichiosis and into the foreseeable future. In this review, we summarize the current status of vaccines and examine the status of new prospects for vaccine development for human ehrlichiosis.
Etiologic agents of the human ehrlichioses
Ehrlichia species are in the subdivision of Proteobacteria and members of the family Anaplasmataceae, which also includes the genera Anaplasma, Wolbachia, and Neorickettsia. Organisms in the genus Ehrlichia include E. chaffeensis, E. ewingii, E. canis, E. ruminantium, and E. muris. Ehrlichia replicate in a membrane-bound cytoplasmic vacuole forming a microcolony called morula. Multiple morulae (1.0 to 6.0 μm) are often present in an infected cell, and by light microscopy they appear as dark blue-to-purple intracytoplasmic inclusions demonstrated by Romanovsky-type stains (Rikihisa, 1991). Morphologically individual ehrlichiae are coccoid and coccobacillary and exhibit two ultrastructural cell types, a larger reticulate cell (RC) (0.4 to 0.6 μm by 0.7 to 1.9 μm) and a smaller dense-cored cell (DC) (0.4 to 0.6 μm in diameter). Both forms have a gram-negative cell wall, characterized by a cytoplasmic membrane and rippled outer membrane separated by a periplasmic space. Reticulate cells are pleomorphic and have uniformly dispersed nucleoid filaments and ribosomes, and DC ehrlichiae are typically coccoid and have centrally condensed nucleoid filaments and ribosomes (Popov et al., 1995; Popov et al., 1998). Small and large morulae containing both RC and DC or exclusively containing DC or RC ehrlichiae usually in loosely packed clusters can be observed within a single infected cell (Popov et al., 1995; Popov et al., 1998). The intramorular space in some morulae contains a fibrillar matrix of ehrlichial origin (Popov et al., 1995). The DC ehrlichiae are infectious and attach to the host cell surface where they are rapidly internalized and transition into a replicating RC forms. RC replicate, doubling every 8 hrs, and then mature to DC within 72 hr after initial cell contact (Zhang et al., 2007).
The intracellular niche occupied by Ehrlichia has resulted in reductive evolutionary processes and corresponding severe loss of genes associated with metabolic processes provided by the host cell. Hence the genome sizes (~1-1.5 Mb) of Ehrlichia are relatively small compared to extracellular bacteria. The genomes of three Ehrlichia species have been sequenced (Collins et al., 2005; Dunning Hotopp et al., 2006; Mavromatis et al., 2006) and exhibit a high degree of genomic synteny, low G+C content (~30%) and one of the smallest genome coding ratios that is attributed to long non-coding regions and numerous long tandemly repeated sequences (TRs) (Frutos et al., 2006). These long non-coding regions and low G+C content in other related Rickettsiales members are speculated to represent degraded genes in the final stages of elimination and excess GC-to-AT mutations (Andersson and Andersson, 1999). Another feature of Ehrlichia genomes is the presence of a large number of long period TRs that appear to have evolved after divergence of the species (Frutos et al., 2007).
The identified agents of human ehrlichosis in the United States include
E. chaffeensis and E. ewingii. E. chaffeensis exhibits tropism for mononuclear phagocytes and causes mild to life-threatening disease in humans and mild to severe disease in dogs (Breitschwerdt et al., 1998b; Dawson and Ewing, 1992). E. chaffeensis is maintained in nature in a zoonotic cycle potentially involving many vertebrate species, and is transmitted primarily by the lone star tick, Amblyomma americanum (Paddock and Childs, 2003).
E. ewingii is an established canine pathogen first described in 1971 (Ewing et al., 1971). E. ewingii exhibits host cell tropism for granulocytes (neutrophils), and is also transmitted by the lone star tick, A. americanum (Anziani et al., 1990). Dogs are a reservoir for E. ewingii, and many individuals with documented infections reported contact with dogs before onset of symptoms (Buller et al., 1999). Most cases of HEE are manifested in immunocompromised patients, and thus, E. ewingii appears to be an opportunistic pathogen (Buller et al., 1999; Paddock et al., 2001).
E. canis is the type strain for the genus Ehrlichia and is the primary etiologic agent of canine monocytic ehrlichosis (CME), a serious and sometimes fatal, globally distributed disease of dogs (Keefe et al., 1982). E. canis is transmitted by the brown dog tick, Rhipicephalus sanguineus (Groves et al., 1975), and infects monocytes/macrophages in dogs. E. canis was initially described in dogs in the United States in 1963 (Ewing, 1963), but received more attention after its identification as the agent responsible for outbreaks of a cryptogenic hemorrhagic disease called tropical canine pancytopenia in American and British military dog units on duty in southeast Asia (Huxsoll et al., 1969; Seamer and Snape, 1970; Wilkins et al., 1967). Human infections with E. canis have been reported in Venuzuela (Perez et al., 1996; Perez et al., 2006). The clinical manifestations of acute infection with E. canis are similar to those observed in humans infected with E. chaffeensis.
Epidemiology and public health importance
Approximately 2,500 cases (passive surveillance) of HME have been formally reported to the Centers for Disease Control from 1999 to 2006, and HME and HEE are Nationally Notifiable Diseases on the public health information network. However, the incidence is likely underestimated since active surveillance studies performed in HME-endemic areas in Missouri, Tennessee and Georgia have revealed an incidence that is 10-100 times higher than reported by passive surveillance (Olano et al., 2003b). HME is a seasonal disease with most reported cases occurring in the spring and summer coinciding with higher tick activity, although cases can occur in the fall in more southern latitudes. The geographic distribution of HME follows the distribution of its vector A. americanum (lone star tick), that
begins in west central Texas and extends east along the Gulf Coast, north through Oklahoma and Missouri, eastward to the Atlantic Coast and proceeds northeast through New Jersey, encompassing all the south central, southeastern and mid-Atlantic states. The main zoonotic reservoir is the white-tailed deer (Odocoileus virginianus), but other potentially important reservoirs are naturally infected with E. chaffeensis including goats, domestic dogs, and coyotes (Breitschwerdt et al., 1998b; Dugan et al., 2000; Kocan et al., 2000). The states with highest incidence include Arkansas, North Carolina, Missouri, Oklahoma, and New Jersey (McQuiston et al., 1999). Outside the USA, HME has been described in Cameroon where confirmation of the diagnosis was based on PCR detection of E. chaffeensis DNA from ill patients and dogs (Ndip et al., 2009a; Ndip et al., 2009b). E. chaffeensis has been found in 5 to 15% of A. americanum ticks collected from at least 15 states in endemic areas in the eastern US (Ijdo et al., 2000; Stromdahl et al., 2001;Whitlock et al., 2000). Human infections with E. chaffeensis or antigenically related ehrlichiae have been reported in Europe (Nuti et al., 1998), Asia (Heppner et al., 1997), South America (Ripoll et al., 1999), and Africa (Uhaa et al., 1992). Most reports of HME from other countries are based on serological studies, and therefore cannot be confirmed as E. chaffeensis infections.
The epidemiology of HEE remains poorly defined due to the lack of a specific serologic assay for this organism and absence of a dedicated reporting system for this disease. Laboratory diagnosis relies on nucleic acid amplification, but new serologic assays to detect E. ewingii antibodies have been recently developed (Zhang et al., 2008). Most cases of HEE have been reported in Tennessee, Missouri, and Oklahoma. However, E. ewingii infection in deer, dogs and ticks have been described throughout the range of the lone star tick, suggesting that human infection with this pathogen might be more widespread than is currently documented. All cases involving tick transmission have been described in immunocompromised patients (Buller et al., 1999; Paddock et al., 2001).
Clinical spectrum and treatment
HME and HEE manifest as undifferentiated febrile illnesses 1 to 3 weeks after the bite of an infected tick. For HME, the most frequent clinical findings reported anytime during the acute illness are fever, malaise, headache, dizziness, chills, and myalgias (Eng et al., 1990; Everett et al., 1994; Fishbein et al., 1989; Fishbein et al., 1994; Olano et al., 2003a; Olano et al., 2003b; Schutze and Jacobs, 1997). HME is more common in male (>2:1) patients >40 years of age; the majority (>80%) report a tick bite (Fishbein et al., 1994; Olano et al., 2003b). Many HME cases are associated with recreational or occupational activities that increase exposure of
humans to tick infested environments (Petersen et al., 1989; Standaert et al., 1995). HME presents as a more severe disease in patients >60 years of age and in immunocompromised patients including persons with HIV/AIDS in whom severe complications can arise such as adult respiratory distress syndrome, acute renal failure, shock and CNS involvement (Paddock et al., 2001). Patients with HEE present with a milder disease with few complications suggesting that E. ewingii is less pathogenic (Buller et al., 1999). Hematologic and biochemical abnormalities usually include leukopenia, thrombocytopenia, anemia, mildly elevated serum hepatic transaminase activities, and hyponatremia (Fishbein et al., 1994; Olano et al., 2003b; Paddock et al., 2001). A high proportion of immunocompetent (41 to 62%) and immunocompromised patients (86%) require hospitalization (Fishbein et al., 1994; Olano et al., 2003b; Paddock et al., 2001) and delays in antibiotic treatment are associated with more pulmonary complications, increased transfer to intensive care, and longer duration of illness (Hamburg et al., 2008). Immunocompromised patients (human immunodeficiency virus-infected persons, transplant recipients, corticosteroid-treated patients) have a high risk of fatal infection associated with overwhelming infection not typically observed in immunocompetent patients (Paddock et al., 2001). No deaths have been reported as a result of infection with E. ewingii (Buller et al., 1999; Paddock et al., 2001).
In vitro susceptibility testing has shown that E. chaffeensis is resistant to representatives of most classes of antibiotics including aminoglycosides (gentamicin), fluoroquinolones (ciprofloxacin), -lactams (penicillin), macrolides and ketolides (erythromycin and telithromycin), and sulfa-containing drugs (co-trimoxazole) (Brouqui and Raoult, 1992). Patients with HME or HEE respond well to tetracyclines, which have bacteriostatic activities against Ehrlichia spp. and other rickettsial agents (Brouqui and Raoult, 1992; Horowitz et al., 2001). Doxycycline is preferred over tetracycline because of its pharmacokinetics and negligible staining of immature teeth. After the first trimester of pregnancy, doxycycline is contraindicated, and successful treatment with rifampin has been reported as an effective alternative (Buitrago et al., 1998).
Overview of Protective Immune Mechanisms
Numerous studies with multiple Ehrlichia spp. indicate that IFN-γ is an essential mediator of protection (Mahan et al., 1994b; Mahan et al., 1996; Mutunga et al., 1998; Totte et al., 1993; Totte et al., 1996). Moreover, CD4+ and CD8+ T cells both contribute to IFN- production (Bitsaktsis et al., 2004; Esteves et al., 2004a; Esteves et al., 2004b; Ismail et al., 2004). Notably, similar conclusions regarding the importance of MHC class I, CD4+ and CD8+ T cells, and the synergistic roles of IFN- and TNF- have
been reported in mice infected with E. muris (Feng and Walker, 2004). An important role for CD4+ T cells in immunity to E. ruminantium and IOE has been suggested (Bitsaktsis et al., 2004; Byrom et al., 2000; Totte et al., 1997). Similarly, mice lacking functional MHC class II genes are unable to clear E. chaffeensis infection, suggesting that CD4+ T cells are essential for ehrlichial clearance (Ganta et al., 2002). The intradermal environment (natural route of inoculation) appears to promote the induction of protective type-1 responses characterized by increased CD4+ and CD8+ T cells and IFN- producing CD4+ T cells (Stevenson et al., 2006).
Antibody mediated immunity appears to play a significant role in protection against E. chaffeensis infection. Infection of SCID mice (B and T cell deficient) with E. chaffeensis results in an overwhelming infection (Li et al., 2001; Li et al., 2002; Winslow et al., 2000). Furthermore, mice lacking B cells or FcRI are unable to resolve an ordinarily sublethal infection by IOE, and passive transfer of antibodies in these mice results in significant reduction in bacterial load (Yager et al., 2005). Similarly, passive transfer of antibodies, but not Fab fragments, also protects mice against lethal infection (Feng and Walker, 2004). The specific anti-ehrlichial antibody-mediated mechanism is not fully understood, but appears to involve binding of antibody to the Fc receptor (Lee and Rikihisa, 1997; Yager et al., 2005) and subsequent generation of a proinflammatory cytokine response (Lee and Rikihisa, 1997) and generation of oxidative defenses (Yager et al., 2005).
Vaccines: Current status and feasibility
Although Ehrlichia are responsible for serious diseases of livestock, companion animals and humans, there are no vaccines available for human ehrlichioses and only one infection-treatment immunization regimen is available for the veterinary ehrlichial disease heartwater, caused by E. ruminantium. Ehrlichia are maintained in nature through subclinical infections of vertebrate hosts (carriers) as well as ticks and have evolved mechanisms to persistently infect mammalian hosts by subverting the innate and adaptive immune responses (Harrus et al., 1998). Effective immune responses leading to the elimination of infections without treatment have been described in E. canis-infected dogs (Breitschwerdt et al., 1998a; Harrus et al., 1998), and infection and treatment strategies in Africa for E. ruminantium have been used for decades to provide protection against challenge (van der Merwe, 1987). Furthermore, experimental E. ruminantium vaccines (inactivated, live attenuated and recombinant) have demonstrated protection against homologous challenge, although less protection has been achieved against natural field challenge (Allsopp, 2009). Cell mediated immune responses and IFN- production correlate with protection against Ehrlichia spp. (Bitsaktsis et al., 2004; Totte et al., 1997), and antibodies also play an
important role in immunity (Feng and Walker, 2004; Winslow et al., 2000; Yager et al., 2005). Thus, vaccines that stimulate humoral and cell mediated immune responses, prevent disease or minimize clinical signs, shorten duration of illness and/or prevent progression to a chronic infection appear to be feasible.
Experimental vaccines
The emergence of human ehrlichioses in the last decade and the risk to public health has elicited interest in the development of vaccines for HME. The use of vaccines to prevent HME may be especially useful for persons who are active outdoors and are at an increased risk level for acquiring the disease. Ehrlichioses, such as heartwater and CME, are important veterinary diseases. Thus, considerable effort has been made to develop vaccines for E. ruminantium, which causes large economic losses to the livestock industry in sub-Saharan Africa, and creates limitations on livestock production and export. Consequently, much of the knowledge base for ehrlichial disease vaccine development has involved E. ruminantium, where experimental vaccine compositions have been tested, including live, attenuated, nucleic acid and recombinant subunit candidates.
A small group of major immunoreactive proteins of E. chaffeensis and E. canis has been identified on the basis of immunoblot reactivity, and most of these proteins contain tandem repeats or ankyrin repeats, and most have been molecularly defined (Doyle et al., 2006; Luo et al., 2008; McBride et al., 2003; McBride et al., 2006; Yu et al., 1996). Moreover, many of these proteins are secreted effector proteins that have major species-specific antibody epitopes (Doyle et al., 2006; Luo et al., 2008; Luo et al., 2009; Luo et al., 2010; McBride et al., 2006; Nethery et al., 2007). However, there is relatively little information regarding the protective efficacy of specific immunoreactive proteins. Major immunoreactive E. chaffeensis proteins are 200-, 120-, 88-, 55-, 47-, 40-, 28- and 23-kDa (Chen et al., 1994; Rikihisa et al., 1994); E. canis, 200-, 140-, 95-, 75-, 47-, 36-, 28-, and 19-kDa (McBride et al., 2003); and E. ruminantium, 160-, 85-, 58-, 46-, 40-, 32-and 21-kDa (Mahan et al., 1994a). E. chaffeensis immunoreactive proteins (Ank200, TRP120, TRP47, TRP32 [VLPT], OMP-1 family [22 genes], and MAP2) have been molecularly characterized as well as the corresponding orthologs in E. canis (Ank200, TRP140, TRP36, TRP19 [VLPT], OMP-1 family [25 genes] and MAP2, respectively). Some of these immunoreactive orthologs have been molecularly identified and characterized in E. ruminantium including (MAP1 family [16 genes], MAP2, and mucin-like protein [clone hw26; TRP36/47 ortholog]) (Jongejan and Thielemans, 1989; Mahan et al., 1994a; Sulsona et al., 1999).
Major immunoreactive proteins identified in Ehrlichia spp. that have
been the primary targets for experimental subunit vaccines include the major outer membrane proteins. Recombinant subunit and nucleic acid vaccines that contain a major surface protein ortholog of Ehrlichia spp. (designated MAP1 in E. ruminantium; p28 in E. chaffeensis and p28/p30 in E. canis), which is a member of a paralogous nonidentical multigene family of outer membrane protein genes (16 to 25 genes) in each respective Ehrlichia species. Partial protection using a recombinant version of the E. chaffeensis P28 protein has been demonstrated in mice after homologous challenge (Ohashi et al., 1998). Moreover, significant protection against homologous challenge using E. ruminantium MAP1 DNA vaccination and recombinant protein boost was demonstrated in a mouse model (Nyika et al., 2002). There is substantial divergence in map1/p28 genes among different isolates of E. ruminantium and E. chaffeensis, and therefore this diversity may complicate development and implementation of vaccines utilizing this protein. Conversely, the p28/p30 genes of E. canis appear to be highly conserved among geographically dispersed strains, and thus may facilitate more rapid development of effective vaccines utilizing this antigen. Most recently, several new vaccine candidates have been identified and molecularly characterized in E. chaffeensis that have major species specific epitopes within tandem repeat regions. These tandem repeat proteins (TRP120, TRP47 and TRP32) from E. chaffeensis are consistently recognized by antibodies in convalescent antisera. The ability of these proteins to protect against homologous challenge is currently under active investigation.
Future prospects
The emergence of human ehrlichioses in the late 20th century has focused new resources and efforts to improve diagnosis and treatment, and to understand pathogenic and protective immune mechanisms that will facilitate vaccine development. The completion of several Ehrlichia genome sequences has provided insight into their evolution, virulence mechanisms, clues to the unique strategies that they utilize to survive in both invertebrate and vertebrate hosts, and their interaction with and dependence on the host cell for survival. Molecular identification and characterization of the majority of the major immunoreactive proteins has been accomplished. These new vaccine prospects, coupled with a more complete understanding of ehrlichial pathobiology and interaction with the innate and adaptive host immune responses, and useful animal models, will undoubtedly stimulate the development of new and more effective nucleic acid or subunit vaccines for human and veterinary use in the future. New technologies including next-generation sequencing will provide researchers with the capability to rapidly and fully explore pathogen gene expression in order to define the dynamics of pathogen phenotype in invertebrate and vertebrate hosts, and
new vaccine strategies will be identified through this exploration. New insights into immunoprotective mechanisms and molecular pathogen-host interactions have marked new areas of progress that have addressed key gaps in our knowledge that are required to make effective vaccines.
Key Points
-
Vaccination is the most cost-effective long-term means of controlling human ehrlichioses, and commercial interest and progress in vaccine development for heartwater and canine ehrlichiosis will enhance prospects for a human ehrlichiosis vaccine.
-
Immunologically well characterized murine models are available for determining vaccine candidate efficacy and defining protective and pathologic immune mechanisms.
-
Defining Ehrlichia proteins that elicit protective humoral and cell mediated immune responses is needed and is an area of active investigation.
-
Understanding innate and adaptive immune evasion strategies of Ehrlichia will improve prospects of effective vaccine development.
-
Effective vaccine development will depend on understanding ehrlichial biology and phenotype in mammalian and arthropod host environments.
-
Major immunoreactive tandem and ankyrin repeat proteins of Ehrlichia have been recently molecularly characterized that contain major continuous species-specific antibody epitopes. Some of these proteins are known to be involved in complex molecular host interactions that may contribute to pathogen survival and may be blocked by the host immune response.
References
Allsopp, B. A. (2009) Trends in the control of heartwater. Onderstepoort J Vet Res 76: 81-88.
Anderson, B. E., Dawson, J. E., Jones, D. C., and Wilson, K. H. (1991) Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol 29: 2838-2842.
Andersson, J. O. and Andersson, S. G. (1999) Genome degradation is an ongoing process in Rickettsia. Mol Biol Evol 16: 1178-1191.
Anziani, O. S., Ewing, S. A., and Barker, R. W. (1990) Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am J Vet Res 51: 929-931.
Bitsaktsis, C., Huntington, J., and Winslow, G. (2004) Production of IFN-gamma by CD4 T cells is essential for resolving ehrlichia infection. J Immunol 172: 6894-6901.
Breitschwerdt, E. B., Hegarty, B. C., and Hancock, S. I. (1998a) Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob Agents Chemother 42: 362-368.
Breitschwerdt, E. B., Hegarty, B. C., and Hancock, S. I. (1998b) Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol 36: 2645-2651.
Brouqui, P. and Raoult, D. (1992) In vitro antibiotic susceptibility of the newly recognized agent of ehrlichiosis in humans, Ehrlichia chaffeensis. Antimicrob Agents Chemother 36: 2799-2803.
Buitrago, M. I., Ijdo, J. W., Rinaudo, P., Simon, H., Copel, J., Gadbaw, J. et al. (1998) Human granulocytic ehrlichiosis during pregnancy treated successfully with rifampin. Clin Infect Dis 27: 213-215.
Buller, R. S., Arens, M., Hmiel, S. P., Paddock, C. D., Sumner, J. W., Rikhisa, Y. et al. (1999) Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Eng J Med 341: 148-155.
Byrom, B., Barbet, A. F., Obwolo, M., and Mahan, S. M. (2000) CD8(+) T cell knockout mice are less susceptible to Cowdria ruminantium infection than athymic, CD4(+) T cell knockout, and normal C57BL/6 mice. Vet Parasitol 93: 159-172.
Chen, S. M., Dumler, J. S., Feng, H. M., and Walker, D. H. (1994) Identification of the antigenic constituents of Ehrlichia chaffeensis. Am J Trop Med Hyg 50: 52-58.
Collins, N. E., Liebenberg, J., de Villiers, E. P., Brayton, K. A., Louw, E., Pretorius, A. et al. (2005) The genome of the heartwater agent Ehrlichia ruminantium contains multiple tandem repeats of actively variable copy number. Proc Natl Acad Sci U S A 102: 838-843.
Dawson, J. E. and Ewing, S. A. (1992) Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am J Vet Res 53: 1322-1327.
Doyle, C. K., Nethery, K. A., Popov, V. L., and McBride, J. W. (2006) Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect Immun 74: 711-720.
Dugan, V. G., Little, S. E., Stallknecht, D. E., and Beall, A. D. (2000) Natural infection of domestic goats with Ehrlichia chaffeensis. J Clin Microbiol 38: 448-449.
Dunning Hotopp, J. C., Lin, M., Madupu, R., Crabtree, J., Angiuoli, S. V., Eisen, J. et al. (2006) Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet 2: e21.
Eng, T. R., Harkess, J. R., Fishbein, D. B., Dawson, J. E., Greene, C. N., Redus, M. A. et al. (1990) Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. J Am Med Assoc 264: 2251-2258.
Esteves, I., Vachiery, N., Martinez, D., and Totte, P. (2004a) Analysis of Ehrlichia ruminantium-specific T1/T2 responses during vaccination with a protective killed vaccine and challenge of goats. Parasite Immunol 26: 95-103.
Esteves, I., Walravens, K., Vachiery, N., Martinez, D., Letesson, J. J., and Totte, P. (2004b) Protective killed Ehrlichia ruminantium vaccine elicits IFN-gamma responses by CD4+ and CD8+ T lymphocytes in goats. Vet Immunol Immunopathol 98: 49-57.
Everett, E. D., Evans, K. A., Henry, R. B., and McDonald, G. (1994) Human ehrlichiosis in adults after tick exposure. Diagnosis using polymerase chain reaction. Annals of Internal Medicine 120: 730-735.
Ewing, S. A. (1963) Canine ehrlichiosis. J Am Med Assoc 143: 503-506.
Ewing, S. A., Roberson, W. R., Buckner, R. G., and Hayat, C. S. (1971) A new strain of Ehrlichia canis. J Am Vet Med Assoc 159: 1771-1774.
Feng, H. M. and Walker, D. H. (2004) Mechanisms of immunity to Ehrlichia muris: a model of monocytotropic ehrlichiosis. Infect Immun 72: 966-971.
Fishbein, D. B., Dawson, J. E., and Robinson, L. E. (1994) Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med 120: 736-743.
Fishbein, D. B., Kemp, A., Dawson, J. E., Greene, N. R., Redus, M. A., and Fields, D. H. (1989) Human ehrlichiosis: prospective active surveillance in febrile hospitalized patients. J Infect Dis 160: 803-809.
Frutos, R., Viari, A., Ferraz, C., Morgat, A., Eychenie, S., Kandassamy, Y. et al. (2006) Comparative genomic analysis of three strains of Ehrlichia ruminantium reveals an active process of genome size plasticity. J Bacteriol 188: 2533-2542.
Frutos, R., Viari, A., Vachiery, N., Boyer, F., and Martinez, D. (2007) Ehrlichia ruminantium: genomic and evolutionary features. Trends Parasitol 23: 414-419.
Ganta, R. R., Wilkerson, M. J., Cheng, C., Rokey, A. M., and Chapes, S. K. (2002) Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect Immun 70: 380-388.
Groves, M. G., Dennis, G. L., Amyx, H. L., and Huxsoll, D. L. (1975) Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am J Vet Res 36: 937-940.
Hamburg, B. J., Storch, G. A., Micek, S. T., and Kollef, M. H. (2008) The importance of early treatment with doxycycline in human ehrlichiosis. Medicine (Baltimore) 87: 53-60.
Harrus, S., Waner, T., Aizenberg, I., Foley, J. E., Poland, A. M., and Bark, H. (1998) Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J Clin Microbiol 36: 73-76.
Heppner, D. G., Wongsrichanalai, C., Walsh, D. S., McDaniel, P., Eamsila, C., Hanson, B. et al. (1997) Human ehrlichiosis in Thailand. Lancet 350: 785-786.
Horowitz, H. W., Hsieh, T. C., guero-Rosenfeld, M. E., Kalantarpour, F., Chowdhury, I., Wormser, G. P. et al. (2001) Antimicrobial susceptibility of Ehrlichia phagocytophila. Antimicrob Agents Chemother 45: 786-788.
Huxsoll, D. L., Hildebrandt, P. K., Nims, R. M., Ferguson, J. A., and Walker, J. S. (1969) Ehrlichia canis—the causative agent of a haemorrhagic disease of dogs? Vet Rec 85: 587.
Ijdo, J. W., Wu, C., Magnarelli, L. A., Stafford, K. C., III, Anderson, J. F., and Fikrig, E. (2000) Detection of Ehrlichia chaffeensis DNA in Amblyomma americanum ticks in Connecticut and Rhode Island. J Clin Microbiol 38: 4655-4656.
Ismail, N., Soong, L., McBride, J. W., Valbuena, G., Olano, J. P., Feng, H. M. et al. (2004) Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol 172: 1786-1800.
Jongejan, F. and Thielemans, M. J. (1989) Identification of an immunodominant antigenically conserved 32-kilodalton protein from Cowdria ruminantium. Infect Immun 57: 3243-3246.
Keefe, T. J., Holland, C. J., Salyer, P. E., and Ristic, M. (1982) Distribution of Ehrlichia canis among military working dogs in the world and selected civilian dogs in the United States. J Am Vet Med Assoc 181: 236-238.
Kocan, A. A., Levesque, G. C., Whitworth, L. C., Murphy, G. L., Ewing, S. A., and Barker, R. W. (2000) Naturally occurring Ehrlichia chaffeensis infection in coyotes from Oklahoma. Emerg Infect Dis 6: 477-480.
Lee, E. H. and Rikihisa, Y. (1997) Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IkappaB-alpha and activation of NF-kappaB. Infect Immun 65: 2890-2897.
Li, J. S., Chu, F., Reilly, A., and Winslow, G. M. (2002) Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J Immunol 169: 1419-1425.
Li, J. S., Yager, E., Reilly, M., Freeman, C., Reddy, G. R., Reilly, A. A. et al. (2001) Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J Immunol 166: 1855-1862.
Luo, T., Zhang, X., and McBride, J. W. (2009) Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin Vaccine Immunol 16: 982-990.
Luo, T., Zhang, X., Nicholson, W. L., Zhu, B., and McBride, J. W. (2010) Molecular characterization of antibody epitopes of Ehrlichia chaffeensis ankyrin protein 200 and tandem repeat protein 47 and evaluation of synthetic immunodeterminants for serodiagnosis of human monocytotropic ehrlichiosis. Clin Vaccine Immunol 17: 87-97.
Luo, T., Zhang, X., Wakeel, A., Popov, V. L., and McBride, J. W. (2008) A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect Immun 76: 1572-1580.
Maeda, K., Markowitz, N., Hawley, R. C., Ristic, M., Cox, D., and McDade, J. E. (1987) Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med 316: 853-856.
Mahan, S. M., McGuire, T. C., Semu, S. M., Bowie, M. V., Jongejan, F., Rurangirwa, F. R. et al. (1994a) Molecular cloning of a gene encoding the immunogenic 21 kDa protein of Cowdria ruminantium. Microbiol 140 ( Pt 8): 2135-2142.
Mahan, S. M., Sileghem, M., Smith, G. E., and Byrom, B. (1996) Neutralization of bovine concanavalin-A T cell supernatant-mediated anti-Cowdria ruminantium activity with antibodies specific to interferon gamma but not to tumor necrosis factor. Parasite Immunol 18: 317-324.
Mahan, S. M., Smith, G. E., and Byrom, B. (1994b) Conconavalin A-stimulated bovine T-cell supernatants inhibit growth of Cowdria ruminantium in bovine endothelial cells in vitro. Infect Immun 62: 747-750.
Mavromatis, K., Doyle, C. K., Lykidis, A., Ivanova, N., Francino, M. P., Chain, P. et al. (2006) The genome of the obligately intracellular bacterium Ehrlichia canis reveals themes of complex membrane structure and immune evasion strategies. J Bacteriol 188: 4015-4023.
McBride, J. W., Comer, J. E., and Walker, D. H. (2003) Novel immunoreactive glycoprotein orthologs of Ehrlichia spp. Ann N Y Acad Sci 990: 678-684.
McBride, J. W., Corstvet, R. E., Gaunt, S. D., Boudreaux, C., Guedry, T., and Walker, D. H. (2003) Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect Immun 71: 2516-2524.
McBride, J. W., Doyle, C. K., Zhang, X., Cardenas, A. M., Popov, V. L., Nethery, K. A. et al. (2006) Identification of a glycosylated Ehrlichia canis 19-kDa major immunoreactive protein with a species-specific serine-rich glycopeptide epitope. Infect Immun 75: 74-82.
McQuiston, J. H., Paddock, C. D., Holman, R. C., and Childs, J. E. (1999) Human ehrlichioses in the United States. Emerg Infect Dis 5: 635-642.
Mutunga, M., Preston, P. M., and Sumption, K. J. (1998) Nitric oxide is produced by Cowdria ruminantium-infected bovine pulmonary endothelial cells in vitro and is stimulated by gamma interferon. Infect Immun 66: 2115-2121.
Ndip, L. M., Labruna, M. B., Ndip, R. N., Walker D.H., and McBride J.W. (2009a) Molecular and clinical evidence of Ehrlichia chaffeensis infection in Cameroonian patients with undifferentiated febrile illness. Ann Trop Med Parasit.
Ndip, L. M., Ndip, R. N., Esemu, S. N., Walker, D. H., and McBride, J. W. (2009b) Predominance of Ehrlichia chaffeensis in Rhipicephalus sanguineus ticks from kennel-confined dogs in Limbe, Cameroon. Exp Appl Acarol.
Nethery, K. A., Doyle, C. K., Zhang, X., and McBride, J. W. (2007) Ehrlichia canis gp200 contains dominant species-specific antibody epitopes in terminal acidic domains. Infect Immun 75: 4900-4908.
Nuti, M., Serafini, D. A., Bassetti, D., Ghionni, A., Russino, F., Rombola, P. et al. (1998) Ehrlichia infection in Italy. Emerg Infect Dis 4: 663-665.
Nyika, A., Barbet, A. F., Burridge, M. J., and Mahan, S. M. (2002) DNA vaccination with map1 gene followed by protein boost augments protection against challenge with Cowdria ruminantium, the agent of heartwater. Vaccine 20: 1215-1225.
Ohashi, N., Zhi, N., Zhang, Y., and Rikihisa, Y. (1998) Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun 66: 132-139.
Olano, J. P., Hogrefe, W., Seaton, B., and Walker, D. H. (2003a) Clinical manifestations, epidemiology, and laboratory diagnosis of human monocytotropic ehrlichiosis in a commercial laboratory setting. Clin Diagn Lab Immunol 10: 891-896.
Olano, J. P., Masters, E., Hogrefe, W., and Walker, D. H. (2003b) Human monocytotropic ehrlichiosis, Missouri. Emerg Infect Dis 9: 1579-1586.
Paddock, C. D. and Childs, J. E. (2003) Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev 16: 37-64.
Paddock, C. D., Folk, S. M., Shore, G. M., Machado, L. J., Huycke, M. M., Slater, L. N. et al. (2001) Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis 33: 1586-1594.
Perez, M., Bodor, M., Zhang, C., Xiong, Q., and Rikihisa, Y. (2006) Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci 1078: 110-117.
Perez, M., Rikihisa, Y., and Wen, B. (1996) Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J Clin Microbiol 34: 2133-2139.
Petersen, L. R., Sawyer, L. A., Fishbein, D. B., Kelley, P. W., Thomas, R. J., Magnarelli, L. A. et al. (1989) An outbreak of ehrlichiosis in members of an Army Reserve unit exposed to ticks. J Infect Dis 159: 562-568.
Popov, V. L., Chen, S. M., Feng, H. M., and Walker, D. H. (1995) Ultrastructural variation of cultured Ehrlichia chaffeensis. J Med Microbiol 43: 411-421.
Popov, V. L., Han, V. C., Chen, S. M., Dumler, J. S., Feng, H. M., Andreadis, T. G. et al. (1998) Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol 47: 235-251.
Rikihisa, Y. (1991) The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev 4: 286-308.
Rikihisa, Y., Ewing, S. A., and Fox, J. C. (1994) Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J Clin Microbiol 32: 2107-2112.
Ripoll, C. M., Remondegui, C. E., Ordonez, G., Arazamendi, R., Fusaro, H., Hyman, M. J. et al. (1999) Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am J Trop Med Hyg 61: 350-354.
Schutze, G. E. and Jacobs, R. F. (1997) Human monocytic ehrlichiosis in children. Pediatrics 100: E10.
Seamer, J. and Snape, T. (1970) Tropical canine pancytopaenia and Ehrlichia canis infection. Vet Rec 86: 375.
Standaert, S. M., Dawson, J. E., Schaffner, W., Childs, J. E., Biggie, K. L., Singleton, J. J. et al. (1995) Ehrlichiosis in a golf-oriented retirement community. N Eng J Med 333: 420-425.
Stevenson, H. L., Jordan, J. M., Peerwani, Z., Wang, H. Q., Walker, D. H., and Ismail, N. (2006) An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect Immun 74: 4856-4864.
Stromdahl, E. Y., Evans, S. R., O’Brien, J. J., and Gutierrez, A. G. (2001) Prevalence of infection in ticks submitted to the human tick test kit program of the U.S. Army Center for Health Promotion and Preventive Medicine. J Med Entomol 38: 67-74.
Sulsona, C. R., Mahan, S. M., and Barbet, A. F. (1999) The map1 gene of Cowdria ruminantium is a member of a multigene family containing both conserved and variable genes. Biochem Biophys Res Commun 257: 300-305.
Totte, P., Blankaert, D., Zilimwabagabo, P., and Werenne, J. (1993) Inhibition of Cowdria ruminantium infectious yield by interferons alpha and gamma in endothelial cells. Rev Elev Med Vet Pays Trop 46: 189-194.
Totte, P., McKeever, D., Martinez, D., and Bensaid, A. (1997) Analysis of T-cell responses in cattle immunized against heartwater by vaccination with killed elementary bodies of Cowdria ruminantium. Infect Immun 65: 236-241.
Totte, P., Vachiery, N., Martinez, D., Trap, I., Ballingall, K. T., MacHugh, N. D. et al. (1996) Recombinant bovine interferon gamma inhibits the growth of Cowdria ruminantium but fails to induce major histocompatibility complex class II following infection of endothelial cells. Vet Immunol Immunopathol 53: 61-71.
Uhaa, I. J., MacLean, J. D., Greene, C. R., and Fishbein, D. B. (1992) A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am J Trop Med Hyg 46: 161-164.
van der Merwe, L. (1987) The infection and treatment method of vaccination against heart-water. Onderstepoort J Vet Res 54: 489-491.
Whitlock, J. E., Fang, Q. Q., Durden, L. A., and Oliver, J. H., Jr. (2000) Prevalence of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from the Georgia coast and barrier islands. J Med Entomol 37: 276-280.
Wilkins, J. H., Bowden, R. S., and Wilkinson, G. T. (1967) A new canine disease syndrome. Vet Rec 81: 57-58.
Winslow, G. M., Yager, E., Shilo, K., Volk, E., Reilly, A., and Chu, F. K. (2000) Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect Immun 68: 2187-2195.
Yager, E., Bitsaktsis, C., Nandi, B., McBride, J. W., and Winslow, G. (2005) Essential role for humoral immunity during Ehrlichia infection in immunocompetent mice. Infect Immun 73: 8009-8016.
Yu, X. J., Crocquet-Valdes, P., Cullman, L. C., and Walker, D. H. (1996) The recombinant 120-kilodalton protein of Ehrlichia chaffeensis, a potential diagnostic tool. J Clin Microbiol 34: 2853-2855.
Zhang, C., Xiong, Q., Kikuchi, T., and Rikihisa, Y. (2008) Identification of 19 polymorphic major outer membrane protein genes and their immunogenic peptides in Ehrlichia ewingii for use in a serodiagnostic assay. Clin Vaccine Immunol 15: 402-411.
Zhang, J. Z., Popov, V. L., Gao, S., Walker, D. H., and Yu, X. J. (2007) The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell Microbiol 9: 610-618.
Rickettsiosis
Introduction
Rickettsia are arthropod-borne, gram-negative, obligately intracellular bacteria that reside in the cytosol of host cells. Tick-borne Rickettsia include the most deadly bacterial organism, R. rickettsii, low pathogenic organisms such as R. parkeri and R. sibirica, and nonpathogenic organisms such as R. peacockii, R. bellii, and R. montanensis. Lethality of Rocky Mountain spotted fever (RMSF) caused by R. rickettsii in the pre-antibiotic era was as high as 80%. Even with the availability of effective rickettsiostatic antibiotic treatment, the mortality rate is around 3-5% for RMSF because of late diagnosis and delay in starting appropriate therapy (Paddock et al., 2002; Raoult et al., 2004). R. rickettsii not only causes severe disease naturally, it is also a potential terrorism agent because it is highly infectious at a very low dose (Wike et al., 1972).
Etiologic agents
Rickettsia are small gram negative bacteria (0.3 – 0.5 × 0.8 – 1.0 μm). Rickettsial diseases are transmitted by arthropods including ticks, mites, lice, and fleas. Based on LPS antigens rickettsiae are classified into typhus group (TG) and spotted fever group (SFG). TG rickettsiae are transmitted by lice and fleas. SFG rickettsiae include more than 20 species and most of them are tick-borne except for mite-borne R. akari and flea-borne R. felis. The nontick-borne Rickettsia will not be discussed further. All Rickettsia multiply in the cytoplasm of host cells, but SFG rickettsiae can also multiply in the nuclei of host cells. Rickettsia organisms have undergone genome reduction resulting in a smaller genome (approximately 1Mb), and have lost genes encoding enzymes for sugar metabolism, lipid biosynthesis, nucleotide synthesis and amino acid synthesis (Andersson et al., 1998). SFG rickettsiae spread from cell to cell via actin-based mobility (Teysseire et al., 1992).
Epidemiology/Public health importance
The distribution of tick-borne SFG rickettsioses is restricted to areas where their tick reservoirs are present such as Rocky Mountain spotted fever in the Americas, Mediterranean spotted fever in Europe, Africa and Asia, and Japanese spotted fever in Japan and Korea (Table A5-1).
Tick-borne rickettsiae are maintained in nature largely via transovarian transmission in ticks. Nonvirulent and low virulent tick-borne Rickettsia do not cause adverse effects on their tick vector, but virulent Rickettsia such as R. rickettsii and R. conorii are pathogenic for Dermacentor and Rhipicephalus ticks, respectively (Niebylski et al., 1999; Santos et al., 2002). Thus, virulent rickettsiae such as R. rickettsii need an animal host to amplify the organisms to establish new lines of transovarian rickettsial maintenance (e.g., D. variabilis ticks acquire R. rickettsii while feeding on rickettsemic cotton rats) (Niebylski et al., 1999). The vectors of Rocky Mountain spotted fever are D. variabilis (American dog tick) in the eastern two-thirds of the US and regions of the Pacific coast states, D. andersoni (wood tick) in the Rocky Mountain states, Rhipicephalus sanguineus (brown dog tick) in the southwestern US and northern Mexico, and Amblyomma cajennense and A. aureolatum in South America.
The seasonal and geographic distribution of each rickettsiosis reflects the months of activity of the vector and its contact with humans. Over 90% of cases of Rocky Mountain spotted fever occur during April through September. Approximately 250-1200 cases of Rocky Mountain spotted fever have been reported annually in the past (http://www.cdc.gov/ncidod/dvrd/rmsf/epidemiology.htm), and currently more than 2000 cases are reported each year.
TABLE A5-1 Distribution of Tick-Borne Rickettsioses
|
Disease |
Rickettsia agent |
Geographic Distribution |
|
African tick-bite fever |
Rickettsia africae |
Sub-Saharan Africa, Caribbean islands |
|
Far eastern spotted fever |
Rickettsia heilongjiangensis |
Far East of Russia and China |
|
Flinders Island spotted fever |
Rickettsia honei |
Australia and southeastern Asia |
|
Mediterranean spotted fever |
Rickettsia conorii |
Southern Europe, southern and western Asia, and Africa |
|
North Asian tick typhus Lymphangitis-associated Rickettsiosis |
Rickettisa sibirica Rickettsia sibirica mongolotimonae |
Asia, Europe, and Africa |
|
Oriental spotted fever |
Rickettsia japonica |
Japan and Korea |
|
Queensland tick typhus |
Rickettsia australis |
Australia |
|
Rocky Mountain spotted fever |
Rickettsia rickettsii |
North, Central and South America |
|
Tick-borne lymphadenopathy |
Rickettsia slovaca |
Europe |
|
Unnamed |
Rickettsia parkeri |
North and South America |
|
Unnamed |
Rickettsia massiliae |
Europe and North and South America |
|
Unnamed |
Rickettsia aeschlimannii |
Europe and Africa |
|
Unnamed |
Rickettsia monacensis |
Europe |
|
Unknown |
Rickettsia helvetica |
Europe and Asia |
Clinical spectrum and treatment
Rickettsial diseases are characterized at onset by fever, severe headache, and muscle aches. In Rocky Mountain spotted fever, the characteristic maculopapular rash typically appears 3 to 5 days later. In severe disease, petechiae may appear in the center of the maculopapules. However, up to 10% to 15% of people with RMSF never develop a rash, a condition often referred to as “Rocky Mountain spotless fever” (Sexton and Corey, 1992). Rashes are less frequent in less severe rickettsioses such as African tick bite fever and R. parkeri infection. Focal skin necrosis with a dark scab (an eschar) at the site of tick feeding is a common feature of boutonneuse fever, African tick bite fever, North Asian tick typhus, Queensland tick typhus, Japanese spotted fever, Flinders Island spotted fever, tick-borne lymphadenopathy, and the recently described infections in the US caused by R. parkeri and a novel strain 364 D, but is rare in Rocky Mountain spotted fever.
Tetracyclines are first-line treatment, and doxycycline may be used to avoid tooth staining in children. Tetracyclines are rickettsiostatic, not rickettsicidal. Ciprofloxacin and other fluoroquinolones are effective against certain rickettsiae. Because diagnostic tests can take time and may be
insensitive, antibiotics are usually begun presumptively to prevent significant deterioration, death, and prolonged recovery.
Overview of protective immune mechanisms
Most of our understanding of the immune response against Rickettsia is derived from in vitro studies as well as the murine models of rickettsioses. Proinflammatory cytokines such as IFN- and TNF- are essential for primary defense against rickettsial infection. These cytokines act in concert to activate endothelial cells, the major target cells of rickettsial infections, as well as other minor target cells to kill intracellular organisms via a nitric oxide synthesis-dependent mechanism. The sources of these protective cytokines are hypothesized to be the T lymphocytes and macrophages that infiltrate the perivascular space surrounding the vessels with infected endothelium.
Cell mediated immunity plays a critical role in host defenses against rickettsial infections(Walker et al., 2001). There are two important effector components of acquired immune response against Rickettsia, namely IFN-production by CD4+ and CD8+ type-1 cells, which activates intracellular bactericidal mechanisms of endothelial cells and macrophages, and the generation of Rickettsia-specific cytotoxic CD8+ T cells that lyse infected target cells via pathways involving perforin and/or granzymes. CD8+ T cells are more important in clearance of rickettsial infection against rickettsiae than CD4+ T cells (Walker et al., 2001). Although adoptive transfer of either CD4 or CD8 immune T lymphocytes control the infection and lead to survival, only depletion of CD8 T lymphocytes altered the outcome of infection, and depletion of CD4 cells had no observed effect on the course or outcome of infection (Walker et al., 2001).
Humoral response may play an important role in protection against infection and antibodies against surface protein antigens are very likely critical effectors of vaccines-induce protective immunity. In animal experiments, antibodies to Rickettsia or rickettsial outer membrane proteins can neutralize rickettsial infection (Anacker et al., 1987; Li et al., 1988). However, natural infection does not result in the production of protective antibodies prior to clearance of rickettsiae. Thus, humoral immunity may be more important in preventing reinfection as in vaccine-induced immunity than in clearance of primary infection.
Vaccine feasibility and current status
Currently no commercial vaccine is available for any rickettsial disease. Infection with R. rickettsii and R. conorii is thought to provide long lasting immunity against re-infection. Thus, it is feasible to develop a vaccine against rickettsial diseases. In theory, a subunit vaccine targeting a
conserved rickettsial protein such as OmpB may be developed to prevent all rickettsial diseases. The best rickettsial vaccine may be an attenuated organism that can multiply but does not cause disease in the host. Attenuated Rickettsia has been achieved with gene knockout technology, and more attenuated strains of Rickettsia will become available for vaccine evaluation.
Experimental vaccines and other potential vaccine prospects
Inactivated rickettsial vaccine. The history of development of vaccines against Rocky Mountain spotted fever contains numerous failures and limited success in preventing or ameliorating disease. The first rickettsial vaccine was a killed R. rickettsii preparation from infected ticks (Spencer and Parker, 1925). The method of propagating R. rickettsii in yolk sac of embryonated chicken eggs was adapted soon after the development of this method in 1938 (Cox, 1939). A third killed Rocky Mountain spotted fever vaccine was prepared from cell culture-propagated R. rickettsii by the US Army in the 1970s (Kenyon et al., 1972).
A challenge trial in human volunteers was conducted in 1973. Neither the yolk sac vaccine nor the tick vaccine prevented the illness, which, of course, was treated promptly to prevent severe illness or death. The yolk sac vaccine was withdrawn from the market in 1978. Subsequent challenge trial of the killed-R. rickettsii vaccine prepared from cell culture yielded protection of 25% of the volunteers who received it. Evaluation of the recipients’ immune responses revealed failure to stimulate sustained cellular immunity (Clements et al., 1983).
Subunit vaccine for Rickettsia. Two surface protein antigens of R. rickettsii, OmpA and OmpB, have been identified as major protective antigens and are candidates for use as subunit vaccines. The first evidence that OmpA and OmpB contain protective epitopes came from the studies of monoclonal antibodies to heat sensitive epitopes of OmpA and OmpB, which neutralized R. rickettsii toxicity in mice and infection in guinea pigs (Anacker et al., 1987; Li et al., 1988). Immunization with the E. coli-expressed OmpA N-terminal fragment partially protects guinea pigs against a lethal challenge dose of R. rickettsii (McDonald et al., 1988). A fragment from the N-terminus of R. conorii OmpA protects guinea pigs against experimental infection with R. conorii and partially protects guinea pigs from challenge with the heterologous R. rickettsii (Vishwanath et al., 1990). Fragments of the ompA and ompB genes have been tested as DNA vaccines. In a regime of DNA immunization followed by boosters of the corresponding peptide, mice immunized with one of several R. rickettsii ompA or ompB fragments are partially protected against a lethal challenge with heterologous R. conorii (Diaz-Montero et al., 2001). It is not known
whether the incomplete protection of OmpA and OmpB to the heterologous Rickettsia species challenge in these experiments is caused by the antigenic differences between the rickettsial species, the immunization regime, or the antigen composition.
Attenuation of Rickettsia by gene knockout. Because of the difficulty of transforming Rickettsia, scientists have been unable to knock out rickettsial genes to test their function and to create an attenuated rickettsial vaccine until recently. The phopholipase D (pld) was the first rickettsial gene that was genetically knocked out. The pld- knockout Evir strain is avirulent for guinea pigs at the doses for which the Evir strain is virulent (Driskell et al., 2009). A Sca2 knockout strain of R. rickettsii has lost actin based mobility in cell culture, and in a guinea pig model of infection, the Sca2 mutant did not elicit fever, suggesting that Sca2 is a virulence factor of spotted fever group rickettsiae (Kleba et al., 2010).
Future prospects
The protective immune response to rickettsial infection involves both innate and adaptive immune responses. A concerted action of CD8+ T cells, and CD4+ T cells producing IFN-, and antibodies is required to clear infection and to prevent reinfection. Live attenuated vaccine mimics the natural infection, thus Rickettsia that are genetically attenuated by gene knockout are the strongest future direction for developing a rickettsial vaccine.
Key issues
Rickettsia are obligately intracellular bacteria and are transmitted by arthropods, including ticks.
R. rickettsii cause fatal disease that can be prevented by avoiding tick bites and removing attached ticks promptly.
There is no vaccine for rickettsial diseases despite the fact rickettsial infection stimulates long term immunity. Vaccines are needed for Rocky Mountain spotted fever.
Because diagnostic tests can take time and may be insensitive, antibiotic treatment should be initiated based on clinical and epidemiological information.
References
Anacker,R.L., McDonald,G.A., List,R.H., and Mann,R.E. (1987) Neutralizing activity of monoclonal antibodies to heat-sensitive and heat-resistant epitopes of Rickettsia rickettsii surface proteins. Infect Immun 55: 825-827.
Andersson,S.G., Zomorodipour,A., Andersson,J.O., Sicheritz-Ponten,T., Alsmark,U.C., Podowski,R.M. et al. (1998) The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396: 133-140.
Clements,M.L., Wisseman,C.L., Jr., Woodward,T.E., Fiset,P., Dumler,J.S., McNamee,W. et al. (1983) Reactogenicity, immunogenicity, and efficacy of a chick embryo cell-derived vaccine for Rocky Mountain spotted fever. J Infect Dis 148: 922-930.
Cox,H.R. (1939) Rocky Mountain spotted fever. Protective value for guinea pigs of vaccine prepared from rickettsiae cultivated in embryonic chick tissues. Public Health Rep 54: 1070-1077.
Diaz-Montero,C.M., Feng,H.-M., Crocquet-Valdes,P.A., and Walker,D.H. (2001) Identification of protective components of two major outer membrane proteins of spotted fever group rickettsiae. Am J Trop Med Hyg 65: 371-378.
Driskell,L.O., Yu,X.J., Zhang,L., Liu,Y., Popov,V.L., Walker,D.H. et al. (2009) Directed mutagenesis of the Rickettsia prowazekii pld gene encoding phospholipase D. Infect Immun 77: 3244-3248.
Kenyon,R.H., Acree,W.M., Wright,G.G., and Melchior,F.W., Jr. (1972) Preparation of vaccines for Rocky Mountain spotted fever from rickettsiae propagated in cell culture. J Infect Dis 125: 146-152.
Kleba,B., Clark,T.R., Lutter,E.I., Ellison,D.W., and Hackstadt,T. (2010) Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun 78: 2240-2247.
Li,H., Lenz,B., and Walker,D.H. (1988) Protective monoclonal antibodies recognize heatlabile epitopes on surface proteins of spotted fever group rickettsiae. Infect Immun 56: 2587-2593.
McDonald,G.A., Anacker,R.L., Mann,R.E., and Milch,L.J. (1988) Protection of guinea pigs from experimental Rocky Mountain spotted fever with a cloned antigen of Rickettsia rickettsii. J Infect Dis 158: 228-231.
Niebylski,M.L., Peacock,M.G., and Schwan,T.G. (1999) Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl Environ Microbiol 65: 773-778.
Paddock,C.D., Holman,R.C., Krebs,J.W., and Childs,J.E. (2002) Assessing the magnitude of fatal Rocky Mountain spotted fever in the United States: comparison of two national data sources. Am J Trop Med Hyg 67: 349-354.
Raoult,D., Woodward,T., and Dumler,J.S. (2004) The history of epidemic typhus. Infect Dis Clin North Am 18: 127-140.
Santos,A.S., Bacellar,F., Santos-Silva,M., Formosinho,P., Gracio,A.J., and Franca,S. (2002) Ultrastructural study of the infection process of Rickettsia conorii in the salivary glands of the vector tick Rhipicephalus sanguineus. Vector Borne Zoonotic Dis 2: 165-177.
Sexton,D.J., and Corey,G.R. (1992) Rocky Mountain “spotless” and “almost spotless” fever: a wolf in sheep’s clothing. Clin Infect Dis 15: 439-448.
Spencer,R.R., and Parker,R.R. (1925) Rocky Mountain spotted fever: vaccination of monkeys and man. Public Health Rep 40: 2159-2167.
Teysseire,N., Chiche-Portiche,C., and Raoult,D. (1992) Intracellular movements of Rickettsia conorii and R. typhi based on actin polymerization. Res Microbiol 143: 821-829.
Vishwanath,S., McDonald,G.A., and Watkins,N.G. (1990) A recombinant Rickettsia conorii vaccine protects guinea pigs from experimental boutonneuse fever and Rocky Mountain spotted fever. Infect Immun 58: 646-653.
Walker, D.H., Olano,J.P., and Feng,H.M. (2001) Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect Immun 69: 1841-1846.
Wike, D.A., Tallent,G., Peacock,M.G., and Ormsbee,R.A. (1972) Studies of the rickettsial plaque assay technique. Infect Immun 5: 715-722.
Anaplasmosis
Introduction
Anaplasma are gram-negative -Proteobacteria belonging to the order Rickettsiales, and family Anaplasmataceae (Figure A5-1) (Dumler et al., 2001). Like most Rickettsiales, organisms in the genus Anaplasma are small, ranging from 0.2-0.9 μm. Of the four genera in the family Anaplasmataceae, Anaplasma is most similar in lifestyle and evolutionary history to the closely related Ehrlichia spp. The genus Anaplasma contains five recognized species: A. bovis, A. ovis, A. platys, A. marginale and
![FIGURE A5-1 Phylogenetic tree of order Rickettsiales. [Genera in the families Anaplasmataceae (on yellow background) and Rickettsiaceae (on blue background) are shown. Species of interest are circled or boxed. The Rickettsia are subgrouped according to ancestral group (AG), transitional group (TrG), Typhus group (TG) and spotted fever group (SFG). The tree is based on a clustalW alignment of 16S ribosomal RNA gene sequences using POWER (http://power.nhri.org.tw/power/home.htm).]](/openbook/13134/xhtml/images/p2001dd4dg367001.jpg)
FIGURE A5-1 Phylogenetic tree of order Rickettsiales. [Genera in the families Anaplasmataceae (on yellow background) and Rickettsiaceae (on blue background) are shown. Species of interest are circled or boxed. The Rickettsia are subgrouped according to ancestral group (AG), transitional group (TrG), Typhus group (TG) and spotted fever group (SFG). The tree is based on a clustalW alignment of 16S ribosomal RNA gene sequences using POWER (http://power.nhri.org.tw/power/home.htm).]
A. phagocytophilum, with the first four species infecting animals, and the last being a zoonotic agent. The type species for the genus is A. marginale, a cattle pathogen that was recognized in the early 1900s (Theiler, 1910). Of these species, by far the most research has been done on the latter two species, A. marginale which causes anaplasmosis, and the human pathogen A. phagocytophilum, which causes human granulocytic anaplasmosis. All Anaplasma species are obligate intracellular organisms infecting mature or immature hematopoietic cells where they replicate within membrane bound vacuoles (Dumler et al., 2001). Anaplasma spp. are therefore blood-borne pathogens and are transmitted from host to host by Ixodid ticks. This manuscript will focus on the human pathogen, A. phagocytophilum.
Etiologic agent
A. phagocytophilum was previously classified as an Ehrlichia (the agent of human granulocytic ehrlichiosis). Accumulating genetic information on a number of pathogens originally named as Ehrlichia species drove the reorganization of the families Rickettsiaceae and Anaplasmataceae in 2001 wherein Ehrlichia equi, and Ehrlichia phagocytophila were unified as a single species with the agent of human granulocytic ehrlichiosis, to create the new species Anaplasma phagocytophilum (Dumler et al., 2001). Because anaplasmosis is recognized as a cattle disease, A. phagocytophilum is said to cause human granulocytic anaplasmosis (HGA).
A. phagocytophilum infects polymorphonuclear leucocytes (neutrophils), where it replicates within a membrane bound vacuole forming a morula or microcolony (Chen et al., 1994; Rikihisa, 1991). A. phagocytophilum has a biphasic developmental cycle with two morphologically distinct forms referred to as dense cored cells (DC) and reticulate cells (RC) (Popov et al., 1998). DCs are small electron dense bodies that predominate in early infection, are thought to be metabolically inert and play a role in attachment and invasion of the host cell (Munderloh et al., 1999). RCs are electron lucent larger pleomorphic cells that undergo binary fission and are thought to be the metabolically active form of the organism (Ismail et al., 2010; Troese and Carlyon, 2009).
The Rickettsiales are the closest extant relatives of the bacterial lineage that led to the mitochondria, and have undergone extensive genome reduction with individual species displaying small genome sizes between 0.8 and 1.5 Mb (Sallstrom and Andersson, 2005; Andersson et al., 1998; Viale and Arakaki, 1994). The A. phagocytophilum genome, completed in 2006, is 1,471,282 bp and is reported to contain 1264 protein coding genes as well as 37 tRNA and 3 rRNA genes (Dunning Hotopp et al., 2006). While this number of protein coding genes is greater than many of the closely related organisms (range = 805-1264), this difference is largely due to differences
in annotation style (many small open reading frames were annotated) rather than a real difference in coding capacity (Brayton et al., 2008).
Epidemiology/Public health importance
HGA was first identified as a human pathogen in 1990 when a patient in Wisconsin died after a short acute febrile illness (Dumler et al., 2005). HGA is increasingly recognized as a frequent cause of fever after tick bite in the Upper Midwest, New England, parts of the mid-Atlantic states, northern California (Figure A5-2), many parts of Europe and Asia (Dumler et al., 2005). In the United States, cases of HGA have increased since the CDC started tracking cases in 1999 (Figure A5-3) with 1009 cases reported in 2008 (Hall-Baker et al., 2010).
A. phagocytophilum infects many species including dogs, cats, horses, deer, cattle, sheep, mice, wood rats, bank voles, squirrels, opossums, skunks and raccoons (Foley et al., 2008; Hackett et al., 2006; Lester et al., 2005; Levin et al., 2002; Stuen and Bergstrom, 2001; Stuen et al., 2001a; Stuen et
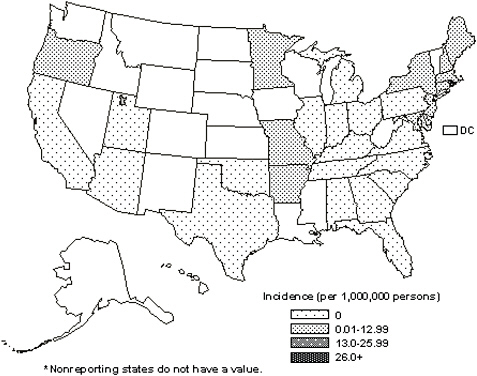
FIGURE A5-2 Average annual incidence of HGA by state as reported in 2001-2002. Data from CDC National Electronic Telecommunications System for Surveillance.
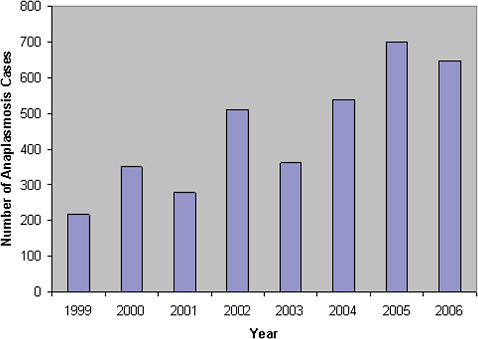
FIGURE A5-3 Number of HGA cases reported by year from 1999-2002. Data from CDC National Electronic Telecommunications System for Surveillance.
al., 2001b; Stuen et al., 2001c; Pusterla et al., 1999). Humans are accidental, dead end hosts for A. phagocytophilum, typically becoming infected when humans encroach on small mammal-tick habitats (Dumler et al., 2005). The major mammalian reservoir is the white-footed mouse, which typically has a transient bacteremia (1-4 weeks). While white tailed deer can be persistently infected, they do not appear to harbor strains of A. phagocytophilum that cause HGA (Massung et al., 2005). Strain definition for A. phagocytophilum is not well defined; however, 1-2 nucleotide differences in the 16S ribosomal RNA gene and 1-3 bp differences in the GroESL gene have been used to identify strain variants (Stuen et al., 2003; Massung et al., 2002).
In the Midwestern and Eastern US Ixodes scapularis is the vector, while I. pacificus vectors A. phagocytophilum in the Western US. I. ricinus is the major vector in Europe, and I. persulcatus transmits disease in Asia. Tick infection is established after an infectious blood meal, and the bacterium is transstadially but not transovarially passed (Dumler et al., 2005). These ticks also transmit agents that cause Lyme disease, babesiosis and tick-borne meningoencephalitis, with about 10% of HGA patients showing serological evidence of co-infection with one of these agents (Ismail et al., 2010; Dumler et al., 2007; Dumler et al., 2005).
Clinical spectrum/Treatment
HGA presents with fever, headache, leukopenia, thrombocytopenia, absence of a skin rash and elevated liver enzymes. Symptoms typically begin a median of 9 days following tick bite, with the majority of patients seeking medical attention within the first 4 days of illness (Ismail et al., 2010; Dumler et al., 2005). Some individuals infected with A. phagocytophilum do not become ill or experience only very mild symptoms and do not seek medical treatment; however, at the other end of the spectrum, the disease can prove fatal, particularly in immunocompromised or elderly individuals (Thomas et al., 2009; Dumler et al., 2005).
Accurate diagnosis of HGA is often difficult due to the non-specific nature of the symptoms. An initial diagnosis is based on the patient’s symptoms and a possible history of tick exposure. Diagnosis is confirmed by laboratory tests: 1) microscopic examination of Wright- or Giemsastained peripheral blood smears looking for dark staining morulae within neutrophils can be used early in infection, 2) a polymerase chain reaction (PCR) assay is the most sensitive test, provides rapid diagnostic evaluation and can discriminate between several tick-borne diseases that present with similar symptoms, 3) A. phagocytophilum can be cultivated within two weeks in the human promyelocytic leukemia cell line HL-60 by direct inoculation of cell cultures with peripheral blood from a potentially infected patient, and 4) serodiagnosis is most commonly used, but is not rapid, as it requires time for antibody to develop which typically takes longer than the onset of clinical symptoms; additionally, nonspecificity of this test may result from cross reactions with Ehrlichia chaffeensis (Ismail et al., 2010; Thomas et al., 2009).
The treatment of choice for both adults and children is a tetracycline antibiotic, usually doxycycline, which should be initiated promptly for improved outcomes and continued for 10-14 days (Hamburg et al., 2008; Chapman et al., 2006).
Overview of protective immune mechanisms
Relatively little is known about protective mechanisms of immunity to A. phagocytophilum infection except that protective immunity is mediated by cellular and humoral immune mechanisms. Development of high titer antibody responses is believed to be indicative of protective immunity (Ismail et al., 2010). Studies in sheep indicate that protection can last from a few months to > 1 year and that a measure of heterologous protection is afforded by some strains but not others (Stuen et al., 2003). Reinfection has been confirmed in some individuals which suggests that long-term immunity was not engendered; however, a lack of cross protection between different
strains of A. phagocytophilum must also be considered (Levin et al., 2004; Horowitz et al., 1998).
Similar to A. marginale, A. phagocytophilum effects immune evasion through antigenic variation of the Msp2/p44 immunodominant surface protein (Granquist et al., 2010; Barbet et al., 2003). There is at least one expression site for msp2/P44 and ~100 functional pseudogenes which can recombine into the expression site to generate variation (Dunning Hotopp et al., 2006; Barbet et al., 2003). The Msp2/P44 protein is characterized by conserved amino- and carboxy-termini flanking a central hypervariable region (Murphy et al., 1998). Msp2/P44 functional pseudogenes are typically truncated at the 5’ and 3’ ends, and recombine into the expression site through a RecF dependent gene conversion mechanism (Lin et al., 2006).
Current vaccine status, experimental vaccines, and potential vaccine prospects
Vaccination against the cattle pathogen A. marginale is effected by infection with an avirulent strain, providing a measure of crossprotection without sterile immunity. This blood-based vaccine is not used in the U.S. due to the threat of transmitting emerging pathogens. There are no vaccines currently available for HGA. The search for vaccine candidates has focused on surface proteins as these are the interface for interactions with the host cell; however, these studies are in their infancy. Little is known about the surface proteins of A. phagocytophilum, aside from Msp2/P44, which due to rapid antigenic variation does not constitute a good vaccine candidate (Ge and Rikihisa, 2007). The genome sequence provides a useful tool to facilitate research to identify vaccine candidates and was recently used in combination with a proteomic approach to identify surface exposed proteins. Two proteins were identified, Asp55 and Asp62, that were also recognized by immune serum from a patient with HGA. Peptide anti-sera for these two proteins were able to partially neutralize A. phagocytophilum infection in the human promyelocytic leukemia cell line, HL-60 cells (Ge and Rikihisa, 2007).
Preliminary studies aimed at understanding the pathogen-tick interface have the long-term goal of developing transmission blocking vaccines, although this research has focused more on interrupting the transmission of the cattle pathogen A. marginale (de la Fuente et al., 2010; Ramabu et al., 2010; Noh et al., 2008). Novel approaches that could reduce the level of human contact with disease agents include the development anti-tick vaccines. An anti-tick vaccine based on the Rhipicephalus microplus gut protein Bm86 has been successful in field trials in Cuba; however, implementation of a similar vaccine was not successful in Australia (de la Fuente et al., 2007; Rodriguez Valle et al., 2004). A successful anti-tick vaccine
would be introduced orally to the wildlife reservoirs in a similar fashion to rabies vaccine, and could eliminate the need for broad scale human vaccination (Slate et al., 2009). This strategy would reduce, but not eliminate risk of transmission. As yet, there are no candidates ready for testing for either of these strategies for ticks that transmit HGA.
Theraupeutics and other biologics for prevention
Preventative antibiotic therapy is contraindicated for individuals who have had recent tick bites but are not ill. Prevention is effected by avoidance of tick bites through the following strategies: 1) avoidance of tick dense areas, 2) wearing of light colored protective clothing (i.e., long pants, closed-toed shoes, etc.), 3) frequent checks for crawling and attached ticks, and 4) application of a repellant such as DEET (N,N-diethyl-m-toluamide). Should a person find an attached tick prompt removal reduces the threat of transmission, as studies have shown that a period of 4 to 24 hours may be necessary before successful transmission from tick to host takes place (Ismail et al., 2010; Bakken and Dumler, 2008; Katavolos et al., 1998).
Key issues
-
Additional effort in identifying surface exposed targets that could illicit protective immune responses is needed. The genome is a key tool in this endeavor and the advent of affordable, widely available proteomic tools should facilitate these efforts. Understanding mechanisms of immune evasion will also help in assessment of vaccine candidates.
-
Understanding the strain composition that makes up the A. phagocytophilum species will facilitate development of appropriate vaccine candidates; i.e., variation in leading vaccine candidate antigens may not be relevant if the variant strain does not infect humans. Representative A. phagocytophilum genome sequences from an array of hosts could assist in exploring the strain composition but this organism does not lend itself to high throughput, next generation sequencing strategies due to the highly repetitive nature of the genome sequence.
-
Understanding of the transmission biology of A. phagocytophilum would facilitate the development of transmission blocking vaccines and potentially help elucidate targets for anti-tick vaccines as well.
-
Understanding host differences in pathogenesis would aid in determining if vaccines tested in sheep, for example, would be relevant for humans.
References
Andersson, S.G., Zomorodipour, A., Andersson, J.O., Sicheritz-Ponten, T., Alsmark, U.C., Podowski, R.M., et al (1998) The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 396: 133-140.
Bakken, J.S. and Dumler, S. (2008) Human granulocytic anaplasmosis. Infect Dis Clin North Am. 22: 433-448, viii.
Barbet, A.F., Meeus, P.F., Belanger, M., Bowie, M.V., Yi, J., Lundgren, A.M., et al (2003) Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect Immun. 71: 1706-1718.
Brayton, K.A., Dark, M.J. and Palmer, G.H. (2008) Anplasma. In Genome Mapping and Genomics in Animal-Associated Microbes. Kole, C., and Nene, V.M. (eds.) Heidelberg, Germany: Springer Life Sciences,, pp. 85-116.
Chapman, A.S., Bakken, J.S., Folk, S.M., Paddock, C.D., Bloch, K.C., Krusell, A., et al (2006) Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 55: 1-27.
Chen, S.M., Dumler, J.S., Bakken, J.S. and Walker, D.H. (1994) Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 32: 589-595.
de la Fuente, J., Almazan, C., Canales, M., Perez de la Lastra, J.M., Kocan, K.M. and Willadsen, P. (2007) A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim Health Res Rev. 8: 23-28.
de la Fuente, J., Kocan, K.M., Blouin, E.F., Zivkovic, Z., Naranjo, V., Almazan, C., et al (2010) Functional genomics and evolution of tick-Anaplasma interactions and vaccine development. Vet Parasitol. 167: 175-186.
Dumler, J.S., Barbet, A.F., Bekker, C.P., Dasch, G.A., Palmer, G.H., Ray, S.C., et al (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 51: 2145-2165.
Dumler, J.S., Choi, K.S., Garcia-Garcia, J.C., Barat, N.S., Scorpio, D.G., Garyu, J.W., et al (2005) Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 11: 1828-1834.
Dumler, J.S., Madigan, J.E., Pusterla, N. and Bakken, J.S. (2007) Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 45 Suppl 1: S45-51.
Dunning Hotopp, J.C., Lin, M., Madupu, R., Crabtree, J., Angiuoli, S.V., Eisen, J., et al (2006) Comparative Genomics of Emerging Human Ehrlichiosis Agents. PLoS Genet. 2: e21.
Foley, J.E., Nieto, N.C., Adjemian, J., Dabritz, H. and Brown, R.N. (2008) Anaplasma phagocytophilum infection in small mammal hosts of Ixodes ticks, Western United States. Emerg Infect Dis. 14: 1147-1150.
Ge, Y. and Rikihisa, Y. (2007) Identification of novel surface proteins of Anaplasma phagocytophilum by affinity purification and proteomics. J Bacteriol. 189: 7819-7828.
Granquist, E.G., Stuen, S., Crosby, L., Lundgren, A.M., Alleman, A.R. and Barbet, A.F. (2010) Variant-specific and diminishing immune responses towards the highly variable MSP2(P44) outer membrane protein of Anaplasma phagocytophilum during persistent infection in lambs. Vet Immunol Immunopathol. 133: 117-124.
Hackett, T.B., Jensen, W.A., Lehman, T.L., Hohenhaus, A.E., Crawford, P.C., Giger, U. and Lappin, M.R. (2006) Prevalence of DNA of Mycoplasma haemofelis, ‘Candidatus Mycoplasma haemominutum,’ Anaplasma phagocytophilum, and species of Bartonella,
Neorickettsia, and Ehrlichia in cats used as blood donors in the United States. J Am Vet Med Assoc. 229: 700-705.
Hall-Baker, P.A., Nieves Jr, E., Jajosky, R.A., Adams, D.A., Sharp, P., Anderson, W.J., et al (2010) Summary of notifiable diseases—United States, 2008. MMWR Morb Mortal Wkly Rep. 57: 1-94.
Hamburg, B.J., Storch, G.A., Micek, S.T. and Kollef, M.H. (2008) The importance of early treatment with doxycycline in human ehrlichiosis. Medicine (Baltimore). 87: 53-60.
Horowitz, H.W., Aguero-Rosenfeld, M., Dumler, J.S., McKenna, D.F., Hsieh, T.C., Wu, J., et al (1998) Reinfection with the agent of human granulocytic ehrlichiosis. Ann Intern Med. 129: 461-463.
Ismail, N., Bloch, K.C. and McBride, J.W. (2010) Human ehrlichiosis and anaplasmosis. Clin Lab Med. 30: 261-292.
Katavolos, P., Armstrong, P.M., Dawson, J.E. and Telford, S.R., 3rd (1998) Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis. 177: 1422-1425.
Lester, S.J., Breitschwerdt, E.B., Collis, C.D. and Hegarty, B.C. (2005) Anaplasma phagocy-tophilum infection (granulocytic anaplasmosis) in a dog from Vancouver Island. Can Vet J. 46: 825-827.
Levin, M.L., Coble, D.J. and Ross, D.E. (2004) Reinfection with Anaplasma phagocytophilum in BALB/c mice and cross-protection between two sympatric isolates. Infect Immun. 72: 4723-4730.
Levin, M.L., Nicholson, W.L., Massung, R.F., Sumner, J.W. and Fish, D. (2002) Comparison of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector Borne Zoonotic Dis. 2: 125-136.
Lin, Q., Zhang, C. and Rikihisa, Y. (2006) Analysis of involvement of the RecF pathway in p44 recombination in Anaplasma phagocytophilum and in Escherichia coli by using a plasmid carrying the p44 expression and p44 donor loci. Infect Immun. 74: 2052-2062.
Massung, R.F., Courtney, J.W., Hiratzka, S.L., Pitzer, V.E., Smith, G. and Dryden, R.L. (2005) Anaplasma phagocytophilum in white-tailed deer. Emerg Infect Dis. 11: 1604-1606.
Massung, R.F., Mauel, M.J., Owens, J.H., Allan, N., Courtney, J.W., Stafford, K.C., 3rd and Mather, T.N. (2002) Genetic variants of Ehrlichia phagocytophila, Rhode Island and Connecticut. Emerg Infect Dis. 8: 467-472.
Munderloh, U.G., Jauron, S.D., Fingerle, V., Leitritz, L., Hayes, S.F., Hautman, J.M., et al (1999) Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J Clin Microbiol. 37: 2518-2524.
Murphy, C.I., Storey, J.R., Recchia, J., Doros-Richert, L.A., Gingrich-Baker, C., Munroe, K., et al (1998) Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect Immun. 66: 3711-3718.
Noh, S.M., Brayton, K.A., Brown, W.C., Norimine, J., Munske, G.R., Davitt, C.M. and Palmer, G.H. (2008) Composition of the surface proteome of Anaplasma marginale and its role in protective immunity induced by outer membrane immunization. Infect Immun.
Popov, V.L., Han, V.C., Chen, S.M., Dumler, J.S., Feng, H.M., Andreadis, T.G., et al (1998) Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol. 47: 235-251.
Pusterla, N., Pusterla, J.B., Braun, U. and Lutz, H. (1999) Experimental cross-infections with Ehrlichia phagocytophila and human granulocytic ehrlichia-like agent in cows and horses. Vet Rec. 145: 311-314.
Ramabu, S.S., Ueti, M.W., Brayton, K.A., Baszler, T.V. and Palmer, G.H. (2010) Identification of Anaplasma marginale proteins specifically upregulated during colonization of the tick vector. Infect Immun. 78: 3047-3052.
Rikihisa, Y. (1991) The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 4: 286-308.
Rodriguez Valle, M., Mendez, L., Valdez, M., Redondo, M., Espinosa, C.M., Vargas, M., et al (2004) Integrated control of Boophilus microplus ticks in Cuba based on vaccination with the anti-tick vaccine Gavac. Exp Appl Acarol. 34: 375-382.
Sallstrom, B. and Andersson, S.G. (2005) Genome reduction in the alpha-Proteobacteria. Curr Opin Microbiol. 8: 579-585.
Slate, D., Algeo, T.P., Nelson, K.M., Chipman, R.B., Donovan, D., Blanton, J.D., et al (2009) Oral rabies vaccination in north america: opportunities, complexities, and challenges. PLoS Negl Trop Dis. 3: e549.
Stuen, S. and Bergstrom, K. (2001) Persistence of Ehrlichia phagocytophila infection in two age groups of lambs. Acta Vet Scand. 42: 453-458.
Stuen, S., Bergstrom, K., Petrovec, M., Van de Pol, I. and Schouls, L.M. (2003) Differences in clinical manifestations and hematological and serological responses after experimental infection with genetic variants of Anaplasma phagocytophilum in sheep. Clin Diagn Lab Immunol. 10: 692-695.
Stuen, S., Djuve, R. and Bergstrom, K. (2001a) Persistence of granulocytic Ehrlichia infection during wintertime in two sheep flocks in Norway. Acta Vet Scand. 42: 347-353.
Stuen, S., Engvall, E.O., van de Poll, I. and Schouls, L.M. (2001b) Granulocytic ehrlichiosis in a roe deer calf in Norway. J Wildl Dis. 37: 614-616.
Stuen, S., Handeland, K., Frammarsvik, T. and Bergstrom, K. (2001c) Experimental Ehrlichia phagocytophila infection in red deer (Cervus elaphus). Vet Rec. 149: 390-392.
Theiler, A. (1910) Anaplasma marginale (gen. spec. nov.). The marginale points in the blood of cattle suffering from a specific disease. In Report of the government veterinary bacteriologist, 1908-1909. Theiler, A. (ed.) Transvaal, South Africa., pp. 7-64.
Thomas, R.J., Dumler, J.S. and Carlyon, J.A. (2009) Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther. 7: 709-722.
Troese, M.J. and Carlyon, J.A. (2009) Anaplasma phagocytophilum dense-cored organisms mediate cellular adherence through recognition of human P-selectin glycoprotein ligand-1. Infect Immun.
Viale, A.M. and Arakaki, A.K. (1994) The chaperone connection to the origins of the eukaryotic organelles. FEBS Lett. 341: 146-151.
A6
EMERGING AND RE-EMERGING TICK-BORNE DISEASES: NEW CHALLENGES AT THE INTERFACE OF HUMAN AND ANIMAL HEALTH
Ulrike G. Munderloh and Timothy J. Kurtti Department of Entomology, University of Minnesota St. Paul, MN 55108
Introduction: Accelerated Increase and Uneven Distribution of Emerging Diseases
This manuscript is meant to be a synthesis of current knowledge about the forces that drive emergence of tick-borne diseases during this era of global change. This is an enormously complex field the components of
which are in constant flux and change dynamically all the time. We therefore do not present here a comprehensive list of all tick-borne pathogens, but rather discuss those that have been researched in sufficient detail to allow assessment of their impact, how they have changed, and how they interact with their environment.
Globally, the great majority of emerging diseases are zoonoses that are predominantly vector-borne (Jones et al., 2008). In temperate climates, tick-borne pathogens are the leading cause of vector-borne diseases, whereas insects dominate the scene as vectors of pathogens in the tropics (Kalluri et al., 2007). The incidence of vector-borne diseases has increased disproportionately in relationship to other emerging diseases, and peaks at times of severe weather events and climate anomalies (Githeko et al., 2000; Gray et al., 2009), a reflection of the sensitivity to and reliance of arthropods on permissive conditions including rain. These effects may be seen relatively quickly, as for pathogens transmitted by mosquitoes, especially those maintained in the insect population transovarially, reducing the lag time before transmission can occur following acquisition. Development of mosquitoes from egg to adult can be completed in two weeks or less, during which larvae feed on microbes suspended in the water. Complete development takes months or years for ticks, but each life stage (except the males of certain species) may transmit pathogens during a blood meal. As for mosquitoes, dynamics of tick-borne disease activity are shaped by climate, though less by rapid weather changes. Availability of suitable larval habitat is of prime importance for maintenance and establishment of mosquito populations, whereas availability of hosts and host behavior are major determinants for ticks. Thus, human activities can shape expansion of different arthropod vectors in different ways, both by habitat modification as well as by altering host populations and their composition. In all, the relationships among factors governing the emergence and spread of vector-borne pathogens, including those that are tick-borne, are very complex. Seasonal and yearly variability determines how ecosystem components interact and contribute to provide habitat suitable for vector arthropods; arthropods, in turn, have evolved behaviors that allow them to take advantage of microclimate niches as needed to “weather” unpredictable conditions (Killilea et al., 2008). These complexities have not been sorted out to date at a global scale, and await a standardized approach to analysis before meaningful conclusions can be drawn.
Climate, weather and temperature directly influence poikilothermic arthropods by dictating periods when important activities, e.g., host seeking, mating, or egg development are possible. Thus climate restricts geographic range to regions with sufficient cumulative degree days to allow completion of these necessary activities and development to the next life stage. Unlike a hot, dry summer that affects ticks when they are normally active,
cold winters are more readily tolerated by ticks that had time to prepare physiologically by seeking suitably protective habitat and accumulating protective anti-freeze compounds (Burks et al., 1996). A recent analysis of gene expression in black-legged ticks subjected to cold revealed that a glycoprotein with homology to a blood protein in cold water fish was more efficiently induced in ticks carrying the zoonotic bacterial pathogen Anaplasma phagocytophilum than in uninfected ones (Neelakanta et al., 2010), presumably providing a selective advantage to overwintering nymphs and adults. This would enhance northward dispersal of “anaplasma-winterized” ticks, and could introduce this pathogen to the mice, chipmunks, squirrels, raccoons and other reservoir hosts that live there (Levin et al., 2002). Davies et al., (2009) proposed that the range of mammals and their ability to expand into new habitats could be predicted by the variability of historic conditions in their range during the Quarternary period. Animals that have evolved to adapt to profound changes in the past are thought to more readily be able to exploit new opportunities. Although a comparable fossil record does not exist for arthropods, ectoparasites that remain on hosts for days at a time, as ticks do, are readily translocated during host movement (Bjöersdorff et al., 2001; McCoy et al., 2003; Ogden et al., 2008; Reed, 2003), thus historic tick host ranges could serve as a proxy for historic ranges of ticks, and their evolved potential to disperse could then be modeled similarly.
Impact of Climate and Global Change Providing New Opportunities
The human population explosion has resulted in dramatic changes of the distribution and composition of natural habitat and land modified to sustain human needs in terms of living space and food production, and this is an ongoing, highly dynamic process. Changes in land use patterns favor establishment and expansion of ticks at the urban/agricultural interface and provide new habitat for highly adaptable wild hosts, as well as new domestic animal hosts for ticks. Even so, advancement of human settlements into virgin land has resulted in a reduction in species diversity and subsequent increase in the risk of tick-borne diseases. Much of the natural vegetation that would presumably cover the earth in the absence of humans (“potential vegetation”), and provide wildlife habitat, has been displaced by cropland and pastures (Foley et al., 2005). These managed agricultural systems lack the rich diversity of plant and animal species characteristic of undisturbed areas, and are more prone to damage from diseases or either natural of human-made disasters such as floods, fires or pressure from invasive species. A rich assembly of plant and animal communities provides a buffer against such events, and enables affected regions to rebound in their wake. Moreover, reduced biodiversity has been linked to increased risk of vector-borne
disease by depletion of natural hosts for vector and pathogen, as well as by provision of new hosts. Domesticated animals living close to farmers and herders, or sharing their dwellings, can act as new reservoirs and bridge hosts in the transfer of emerging diseases to humans (Keesing et al., 2006; LoGiudice et al., 2008; Vora, 2008). A recent example is the discovery of an active transmission focus of Rocky Mountain spotted fever (RMSF) rickettsiae, Rickettsia rickettsii, in Arizona, involving an introduced tick vector, the brown dog tick, Rhipicephalus sanguineus, and domestic dogs acting as reservoirs for the rickettsiae and hosts for the ticks (Demma et al., 2005). Since then, brown dog ticks infected with R. rickettsii have also been detected in California (Wikswo et al., 2007). This tick colonized the Americas along with humans and their dogs arriving from the Old World during the early colonial immigrations (Burlini et al., 2010). It is surprising that spotted fever group rickettsiae endemic to the Mediterranean region, Rickettsia conorii (Mumcuoglu et al., 1993), that are naturally transmitted by brown dog ticks, have thus far not been identified in the New World. The adaptation of R. rickettsii to a new tick vector is a good example of the host switching that can result when human-aided movement of animals and their parasites are introduced into new areas where they intermingle with resident hosts, parasites and disease agents (Hoberg et al., 2008). Such new combinations are more likely to turn up in the multiple host life cycle of tick-borne pathogens, especially when the vectors are non-specialized feeders as is the case with the black-legged tick Ixodes scapularis. In this situation, host switching without the need for subsequent adaptive evolution can occur in pathogens that inherently are equipped to take advantage of new opportunities provided by hosts undergoing range expansion in a process of ecological fitting (Brooks and Ferrao, 2005; Foley et al., 2008).
Human encroachment on wildlife habitat enhances contact with ticks as modern society embraces the concept of living with nature by building homes in natural settings and through engagement in outdoor sports such as hiking or camping. Regional and historical preferences for how and where homes are constructed, and how and where animals are housed or pastured have modified the zoonotic interface between humans and domestic and wild animals in ways that where not anticipated, but were predictable in retrospect. The desire to preserve natural vegetation such as mature stands of trees for aesthetic or practical reasons (e.g., to provide shade) has had the effect to attract wildlife to close proximity of human dwellings, enhancing contact with ticks and other arthropods that may carry disease agents. The increase in Lyme disease cases in residents of affluent housing developments in or near desirable natural wooded areas is a good example of this trend (Barbour and Fish, 1993; Linard et al., 2007). Although much research has been devoted to trying to describe the ecologic/sociologic interface that favors the presence of pathogens, vectors and reservoirs, and has
resulted in large sets of data that do not easily coalesce into a single, well-fitting mosaic, few efforts have been made to systematically incorporate them into urban/suburban/agricultural planning (Ward and Brown, 2004). There is a clear need to apply what has been learned to new and existing urban and suburban as well as agricultural and recreational landscapes. Disease prevention through landscape management must, however, always be in balance with protection of natural habitat, and its meaningful incorporation into managed areas (Foley et al., 2005; Stafford III, 2007). Such decisions must be based on scientific knowledge, and biologists, medical scientists and public health researchers must be included in the planning processes alongside city planners and construction company employees.
Areas that are likely to experience increased or prolonged seasonal tick activity are most likely those located at the current extremes of the current range of distribution, areas where climate change will be felt most acutely. In the northern hemisphere, this will be at the northern edge, and in the southern hemisphere, tick distribution ranges will likely shift further south. A prerequisite is the presence of ecosystems with suitable land cover and hosts to receive the immigrants. Occupation of montane habitat by Ixodes ricinus in Europe has already shifted to greater altitudes (Materna et al., 2005), exposing alpine farming communities to new risk of infection. At these greater latitudes and altitudes, specialized plant communities are utilized by relatively few wild animals that can serve as tick hosts and impose constraints that may limit or curb further spread of the ticks. Domestic animals seasonally introduced into borderline habitat and the people tending them will experience a greater burden of tick bites.
The Domestic Animal/Wildlife Interface
Driving flocks and herds out to pasture on a daily or weekly basis, or even turning live stock out onto range land for entire seasons, has been a tradition for centuries, and animal husbandry is thought to have been a source of human exposure to zoonoses since ancient times (Greger, 2007). A new and worrisome trend is the increasing practice of exotic animal farming and trade in exotic species. Exotic game farms have become popular with hunters seeking the thrill of a chance to shoot an African antelope without having to leave the USA, and offer farmers income from land that may be poorly suitable for traditional farming. Although animals imported from foreign countries must undergo rigorous testing for diseases and quarantine, any as yet unknown pathogens they may harbor may not be detected using existing diagnostics. In addition, tick-borne pathogens, e.g., the bovine anaplasmosis agent, Anaplasma marginale, which is widely present throughout the world, can chronically infect animals at undetectable levels (Eriks et al., 1989; Herrero et al., 1998), and other pathogens may
do the same. There are numerous reports in recent history of accidental introduction of tick-borne animal pathogens or ticks into previously unaffected areas, with economically disastrous results. When imported ticks become established on wild animals, their eradication may be very difficult or impossible, as shown in New Caledonia (Barré et al., 2001) where cattle ticks and bovine babesiosis were accidentally imported. The cattle tick, Rhipicephalus (Boophilus) microplus, is the vector of bovine babesiosis, a disease with major consequences for cattle production wherever it is present. It has spread through most warm regions of the world from its origin in Asia by hitching a ride on imported cattle (Hoogstraal, 1956; Madder et al., 2010). This parasite has adapted well to wild ungulates in infested areas, providing alternate hosts when cattle are intensively treated with acaricides, unraveling control efforts (Cantu-C et al., 2009). After being nearly eradicated in the USA, this tick has recently expanded its range considerably in Texas, in part aided by development of acaricide resistance resulting from intense treatment regimes (George, 2008). There is no reason to believe that R. microplus would not adapt to exotic game on farms, as it has displaced other Rhipicephalus species in West Africa (Madder et al., 2010). Although the development of promising antigens raises the hope that a vaccine could protect cattle against this parasite (Canales et al., 2009), their effectiveness in essentially wild or feral exotic animal species remains unproven.
Much as exotic animals can be a source of exotic pathogens endangering resident fauna, pathogens endemic in areas into which non-endemic species are introduced may prove to be highly infectious for non-indigenous animals. Farming game, e.g., elk (or wapiti, Cervus elaphus canadensis), has been promoted as a sustainable alternative to raising cattle, because these animals are less demanding and are superior in their ability to utilize nutrients from natural pasture. They also produce lean meat that fetches premium prices on the market. In their natural range in the Rocky Mountains of the USA and Canada, elk do not encounter I. scapularis (black-legged ticks), but when raised in the Midwest or Northeast, they are exposed to a protozoan blood parasite, Babesia odocoilei, transmitted by ticks among white-tailed deer who do not show signs of infection (Waldrup et al., 1990). Elk and deer from outside the range of the vector tick may become severely ill, even suffer a fatal infection. Outbreaks of fatal illness have also been documented in a number of animals at zoos, e.g., reindeer (Rangifer tarandus tarandus) and caribou (Rangifer tarandus caribou), bovids such as musk oxen (Ovibos moschatus) and yak (Bos grunniens), as well as other ruminants, e.g., muntjac (Muntiacus reevesi) and markhor goat (Capra falconeri) (Schoelkopf et al., 2005; Bartlett et al., 2009). This list of susceptible species is not inclusive, but serves to demonstrate the enormous infection potential of tick-borne pathogens in animals that have not co-evolved with them. Importation of exotic species for uncontrolled release should be avoided for other reasons as well, because
their impact on natural habitat is not easily predictable, and great environmental harm can result. One need only consider the devastation wrought by domestic goats released by sailors on many islands as a fresh food supply, or the disastrous release of European wild rabbits in Australia (Campbell and Donlan, 2005; Fenner, 2010).
Changes in Climate Favor Establishment and Expansion of Ticks
The spread of human settlement is accompanied by changes in land use that have been linked with increasing risk of disease due to vector-borne pathogens (Hoogstraal, 1981; Harrus and Baneth, 2005). Global change is the sum of largely man-made ecologic disturbances resulting in rising temperatures and altered patterns of precipitation that promote the expansion of the geographic range where conditions are favorable for survival of vector arthropods. Changes in vector distribution and seasonal activity resulting in increased disease incidence are likely to be most pronounced at the geographic extremes of vector distribution (Ogden et al., 2005).
In temperate climates, pathogens transmitted by ticks are the causative agents of the most common vector-borne diseases, far outnumbering those carried by mosquitoes. The incidence of Lyme disease transmitted by Ixodes spp. in North America and Europe has been increasing steadily since the 1970s and 1980s (Gray et al., 2009). In the US Midwest, this steady pace accelerated at the beginning of the new century, with significant deviations from the average rate tied to unusually dry and hot weather, such as during the summer of 2003 that was followed by a rebound in 2004 (Minnesota Department of Health; http://www.health.state.mn.us/divs/idepc/diseases/anaplasmosis/casesyear.html). Human anaplasmosis caused by A. phagocytophilum is now the second most common tick-borne disease in the US, and is also transmitted by I. scapularis. Although still much less common than borreliosis, this disease has paralleled the upward trend of Lyme disease (http://www.cdc.gov/ticks/diseases/anaplasmosis/statistics.html), and may be subject to similar dynamics and constraints of climate and tick biology. Clearly, climate plays a prominent role in the shifting boundaries of tick populations, but it is certainly only one of multiple factors in the equation. Warmer winters with increased precipitation and thus deeper winter snow pack allow enhanced tick overwintering rates by providing critical protection from desiccation and chill injury (Burks et al., 1996), and result in expansion into formerly unsuitable regions—as seen in Canada with I. scapularis (Odgen et al., 2005, 2008). In currently endemic areas, greater humidity and higher temperatures earlier and later in the year extend periods of tick activity into a longer tick season while creating inviting conditions for human outdoor activity. This effectively prolongs risk of exposure and infection.
Planning Ahead: Can Climate Models Predict Public Health Risk?
A number of research teams have attempted to model risk of infection with tick-borne pathogens. Intuitively, one should expect this to be possible in developed countries where there is a wealth of data on human disease cases, distribution of ticks and hosts, and climatologic data collected over decades. Areas that are currently considered to present high risk of encountering ticks can be identified at a regional level, and provide valuable guidance for the management of tick-borne disease risk through rational design of land use, and management of tick hosts and pathogen reservoirs (Ward and Brown, 2004; Stafford III, 2007). Risk assessments indicate that fragmented, patchy forest with a large proportion of edge habitat, support tick and mouse populations well (Allan et al., 2003; Brownstein et al., 2005; Foley et al., 2009; Eisen et al., 2010; Raizman et al., 2010). This agrees with the observation that disturbed ecosystems support larger numbers of ticks than intact ones, and reflects a variety of underlying reasons based in the biology of ecosystem participants. Deer, especially white-tailed deer, are important reproductive hosts for Ixodes ticks, and thrive in second growth forest that characterizes prime tick habitat (Foley et al., 2009). By contrast, intact old growth forests have much lower association with tick-bite risk, probably because they retain higher vertebrate species diversity that dilutes infection risk by interspecies competition and predation on reservoirs. This seeming discrepancy in the association of forests with tick bite risk is an example of the difficulties that analysts face when trying to make sense of the collective data set. The many abiotic and biotic factors that combine to shape the ecology of vector-borne diseases are highly complex, and published studies lack a standardized approach that would make them comparable (Kililea et al., 2008). Models that attempt to span expansive regions or to extend forecasts far ahead are often based on the assumption that current trends will remain continuous over long distances and into the future (Diuk-Wasser et al., 2006; Odgen et al., 2006). These assumptions remain to be validated, but nevertheless present plausible scenarios that have stimulated the debate about interventions and countermeasures. Long-term predictive models could be made more useful if they were continuously updated with new information to reflect the influence of changing populations and land use.
Tick Species with the Greatest Potential for Expansion
Lessons learned from the two globally most widely distributed tick species, the cattle fever tick (pantropical blue tick), Rhipicephalus (Boophilus) microplus (Madder et al., 2010), and the brown dog tick, Rhipicephalus sanguineus (Burlini et al., 2010), indicate that human-facilitated dispersal
of ectoparasites via movement on domestic hosts is by far the most effective mechanism. For ticks, this presents an ideal scenario that ensures a suitable or even preferred host is available at the new location. In the case of R. (Bo.) microplus this success was further enhanced by the fact that this is a one-host tick for which the eggs are the only off-host stage. Notwithstanding the great economic importance of R. (Bo.) microplus as a vector of livestock diseases agents, it does not parasitize humans, and therefore is of no relevance in tick-borne zoonoses.
The brown dog tick has likewise colonized the globe as a parasite of dogs accompanying humans (Burlini et al., 2010). It is found wherever dogs are kept in regions between the latitudes of 50° North and 30° South. It commonly infests kennels and even homes, seeking shelter in cracks, under window sills, and behind furniture. This tick preferentially parasitizes dogs, but may bite humans if dogs are not available, and does so apparently more readily in Europe where it has long been known to transmit Rickettsia conorii, the agent of boutonneuse fever, to people (Péter et al., 1984; Dantas-Torres, 2010). The recent identification of a North American focus of Rocky Mountain spotted fever with transmission of the agent among dogs and children (Demma et al., 2005) reinforces the zoonotic potential of this tick. Notably, brown dog ticks attack alternate hosts including humans more readily when ambient temperatures are high, can complete up to four generations a year, and may become more important vectors of human disease as climate warms in its current range (Dantas-Torres, 2010).
By contrast, tick dispersal on wild hosts is much less efficient, although it can account for increase of tick mobility to hundreds or thousands of miles on hosts such as deer or migratory birds (Klich et al., 1996; Bjöersdorff et al., 2001; Madhav et al., 2004; Odgen et al., 2008). Off host, I. scapularis ticks move at most a distance of a few meters (Carroll and Schmidtmann, 1996). Even though ticks can be carried great distances in these ways, there is no guarantee that their drop-off locations will present them with suitable habitat or hosts for subsequent life stages, but this will change as plant and animal communities respond to a warming climate.
The Interface Between Ticks and Emerging Disease Agents
Tick-borne pathogens of humans causing emerging diseases are primarily reported in temperate climates, but this trend may be a distortion of the true picture, as public health systems and disease reporting are much less accurate and often inconsistent in less developed and tropical countries. As a result, emerging diseases in these countries are under-reported by comparison to those in the Western world (Jones et al., 2008). In Brazil, increasing numbers of suspected tick-borne spotted fever cases previously thought to be of viral origin are being identified (Labruna, 2009), and
result in significant mortality. Brazilian spotted fever caused by a strain of Rickettsia rickettsii is now considered to cause the majority of such cases, but the true extent of its occurrence is not known (Rozental et al., 2006). Amblyomma spp. ticks have been implicated in transmitting the rickettsiae among rodents and opossums, although the presence of more abundant rickettsiae of undetermined pathogenicity has clouded the picture.
Ticks of greatest concern for human health are three-host generalist feeders, commonly utilizing small animals such as birds, rodents, squirrels and hedgehogs during the larval and nymphal stages, and feeding on larger hosts as nymphs and adults. They thus act as bridge vectors between animal reservoirs that are usually not affected by the pathogen, and humans who are dead-end hosts but suffer disease symptoms. A good example are ticks in the genus Ixodes, found around the globe in temperate and subtropical regions. In North America, the black-legged tick, I. scapularis, is probably the most notorious vector of zoonotic pathogens, capable of transmitting viruses (Powassan encephalitis virus; Pesko et al., 2010), bacteria (Borrelia burgdorferi, A. phagocytophilum, and possibly Bartonella spp. as well as a new Ehrlichia muris-like organism; Burgdorfer et al., 1982; Chen et al., 1994; Adelson et al., 2004; Pritt et al., 2009), and protozoa (Babesia microti; Piesman and Spielman, 1980). In Europe, the closely related tick species Ixodes ricinus is involved in a similarly broad spectrum of pathogen transmission, but has a much greater role in viral infections caused by tick-borne encephalitis viruses (Flaviviridae). Certainly, the many different types and species of vertebrates that are suitable hosts for I. scapularis and I. ricinus immature stages contribute significantly to their potential encounters with pathogens that are capable of colonizing and being transmitted by them. It is interesting to note that most zoonotic pathogens vectored by Ixodes species are maintained transstadially in ticks, even when infection rates in vertebrate reservoirs are low and of limited duration. This seems to be the case for A. phagocytophilum in white-footed mice that clear the infection within two weeks (Telford et al., 1996). Anaplasma phagocytophilum has been identified as an emerging human pathogen primarily in the US where it now is responsible for the second most common tick-borne illness (Chen et al., 1994). Serial re-infections of immune-intact laboratory mice (C57BL/6) by inoculation of culture-derived human-infectious A. phagocytophilum carrying different antibiotic and fluorescent marker genes suggests that mice do not develop a protective immune response to re-infection despite the fact that the bacteria are cleared after every inoculation (our unpublished results). If wild-type A. phagocytophilum behaves similarly in wild mice, sufficient levels of infected populations could thus be maintained, as per the susceptible-infected-susceptible model proposed by Kurtenbach et al. (2006).
Although transovarial transmission does occur in pathogens vectored by Ixodes ticks and contributes to viral maintenance in tick populations,
this mechanism appears to be less important than horizontal transmission among co-feeding ticks, at least in European tick-borne encephalitis virus where it has been examined in greatest detail (Labuda et al., 1993; LaSala and Holbrook, 2010). Whether this preference also holds true for Powassan virus (Costero and Grayson, 1996) remains to be determined.
One of the notorious tick-borne diseases in North America has long been Rocky Mountain spotted fever, and as its name implies, it was first described in the Rocky Mountain region in the latter part of the 19th century. There, the agent, R. rickettsii, circulates among small to medium mammals and the Rocky Mountain wood tick, Dermacentor andersoni, in which it is maintained transovarially (Niebylski et al., 1999). Both ticks and mammals can serve the role of reservoir. Over the decades, the greatest disease incidence has shifted south and east, and North Carolina and Oklahoma now account for the highest number of cases (http://www.cdc.gov/ticks/diseases/rocky_mountain_spotted_fever/statistics.html). In these states, the main vector is the American dog tick, Dermacentor variabilis. In both tick vectors, infections are very low at less than 1%, making it hard to predict risk by sampling tick populations. Likewise, this makes it difficult to track how this geographic shift has occurred, if it has occurred, or whether the apparent redistribution of cases is a reflection of better diagnostics and surveillance. Dermacentor and Amblyomma spp. ticks, both of which are present in these states, carry a variety of more abundant related microbes of undetermined or low pathogenicity, e.g., Rickettsia parkeri and Candidatus Rickettsia amblyommii (Paddock, 2009) that are suspected of contributing to Rocky Mountain spotted fever. While R. parkeri is a proven though mild infectious agent, the status of C. R. amblyommi remains undetermined, and it could just as well exclude R. rickettsii from ticks in a manner as Rickettsia peacockii does (Burgdorfer et al., 1981).
Pathogen Evolution Is a Dynamic, Ongoing Process
Anaplasma phagocytophilum, an obligate intracellular bacterium, has been known as a tick-borne pathogen of sheep, goats and cattle in Europe for decades where it was previously named Cytoecetes phagocytophila or Ehrlichia phagocytophila (Dumler et al., 2001; Woldehiwet, 2006). Ruminants remain persistently infected and experience cyclic bacteremia (Stuen, 2007). An organism named Ehrlichia equi was likewise known to infect Californian horses since the mid 1900s (Madigan and Gribble, 1987), but there, as in Europe, human cases are rare (Foley et al., 2009). Notably, A. phagocytophilum variants not found in human patients have been identified in deer in several locations of the US. One can imagine a scenario where European settlers unknowingly introduced infected livestock to North America, and American Ixodes ticks subsequently acquired and
spread the agent which thrived in deer. Whether this strain later developed the ability to infect mice and humans (as well as dogs and horses), or whether the human-infectious strains were introduced separately, or even were already present in North America awaits further phylogenetic analysis.
In Minnesota, I. scapularis may be infected with different variants of A. phagocytophilum (Michalski et al., 2006; Baldridge et al., 2009), not all of which cause human anaplasmosis (HA). We are currently testing the hypothesis that a whole genome comparison of A. phagocytophilum (Ap) isolates that infect humans (Ap-ha) versus those that are pervasively found in ticks and wild animals (Ap-variants) will reveal genetic differences that underlie Ap pathogenicity. Research has focused on Ap-ha and we know little about the biology of the more recently described Ap variants (Massung et al., 2005, 2007; Baldridge et al., 2009). Besides their potential ability to regulate the epidemiology of HA (Massung et al., 2002), the genome sequence of Ap-variants would be a valuable resource to identify mechanisms of host specificity, virulence and tick transmission in Ap-ha. We found that 64% of I. scapularis and 45% of Dermacentor albipictus (the winter or moose tick) collected from whitetail deer (WTD) in the army base at Camp Ripley, MN, carried Ap variants, including two with 16S rRNA gene sequences identical to Ap variants from Wisconsin deer. The D. albipictus variants were transovarially transmitted to F1 larvae at efficiencies of up to 40%, the first evidence for vertical transmission of Ap to tick progeny. These represent the highest Ap prevalence rates reported for any location, notably in the absence of increased numbers of human HA cases, supporting the notion that Camp Ripley Ap variants are truly distinct from Ap-ha. Unlike human-infectious strains, they do not infect mice, and can only be cultured in a cell line from the vector tick, suggesting they are biologically very different (Massung et al., 2005, 2007). Using tick cell line ISE6, we obtained 8 isolates of Ap variants from ticks feeding on WTD hunted in Camp Ripley. One of these, MN-61-2, was infectious for a goat but not mice, similar to Ap-variant 1 from Rhode Island, suggesting that the Northeast and Midwest variants are related (Massung et al., 2007). All our variant Ap isolates have the same 16S rRNA sequences but different ankA gene sequences. Now that Ap-variant isolates are available, their genomes can be sequenced to address the salient differences between Ap-ha and Ap variants: what genes/operons determine infectivity for humans versus ruminants, and what do the genomes reveal about the evolution of this emerging pathogen?
Research with other agents reinforces the notion that genetic population structure of tick-borne pathogens affects the interaction of human-infectious and animal-infectious isolates in endemic areas. There is evidence that multiple genotypes of B. burgdorferi have arisen from multiple, distinct foci (Hoen et al., 2009), and that human infectious borreliae may displace
non-human infectious genotypes in animal populations due to differential transmission by vector ticks (Girard et al., 2009). This suggests that factors affecting pathogen distribution are not limited to climate change, and include fitness determinants that regulate utilization of arthropods.
The Interactive Bacterial Communities of Ticks
The mammalian host and vector tick are two quite divergent environments that tick-transmitted pathogens have adapted to in order to survive and invade new hosts. In addition to the well known pathogens that are acquired and transmitted during the blood meal, ticks are also colonized by symbionts and fortuitous microbes, the latter acquired from contact with animals during the blood meal or from the soil or plants while questing or surviving off the host. The life cycle of tick-borne bacteria is complex and controlled by the requirement for alternating between hosts with vastly different biological characteristics. Most ticks take weeks or months to complete each life stage, and take but a single blood meal each time. Human pathogens transmitted by ticks regulate gene expression to permit successful development in each host, as inappropriate timing of gene expression can abort transmission and infection.
Environmental changes are likely to introduce new environmental challenges and lead to altered tick-microbe and microbe-microbe associations, distributions and interactions. Arguably, the most important vector ticks with the greatest potential for expansion in North America are the Ixodes, Amblyomma and Dermacentor species. These ticks are three host ticks and generalist feeders (feed on different hosts in each of the life stages). Accordingly we focus herein on what is known about the microbial communities of I. scapularis, Amblyomma americanum (Lone Star tick), and D. variabilis. Though a variety of approaches have been used to delineate the bacterial communities of these ticks, we still need to address the question of how symbionts, fortuitous microbes and pathogens interact to affect pathogen acquisition or transmission and the emergence or re-emergence of tick borne disease agents.
Studies to determine the bacterial communities of ticks have generally focused on a given geographical region or tick developmental stage. The microbial community of I. scapularis is best described for ticks collected in areas endemic for Lyme disease, e.g., New York state (Moreno et al., 2006) or Massachusetts (Benson et al., 2004). Moreno et al. (2006) used temporal temperature gradient gel electrophoresis separation and sequencing of 16S DNA PCR-amplified products to detect specific bacteria in I. scapularis larvae, nymphs and adults, engorged and unfed. The most abundant were Rickettsia, Pseudomonas and Borrelia, whereas Ralstonia, Anaplasma, Enterobacteria, Moraxella, Rhodococcus, and “uncultured proteobacteria”
were less common. There was considerable stage and fed/unfed variation, but in general, engorged nymphs and females harbored the most diverse bacteria, suggesting that the blood meal exerted major impact on the microbial diversity of the tick. The rickettsial endosymbiont of I. scapularis (REIS; Baldridge et al., 2010) was found in all ticks and stages, whether fed or not, and no correlations between REIS and presence or absence of B. burgdorferi, A. phagocytophilum or other microbes associated with I. scapularis have been found (Moreno et al., 2006; Steiner et al., 2008). REIS has also been referred to as “Rickettsia cooleyi” (Billings et al., 1998) or “Rickettsia midichlorii” (Parola et al., 2005). In contrast, four different genera of intracellular bacteria were detected in I. scapularis nymphs collected in Massachusetts: Rickettsia, Anaplasma, Wolbachia and Cardinium (Benson et al., 2004). The Cardinium species is closely related to a bacterium isolated from I. scapularis (Kurtti et al., 1996) that itself is closely related to Cardinium hertigii from mites and insects (Nakamura et al., 2009). Identification of Wolbachia and Cardinium, known to be involved in reproductive alterations in insects and mites, is intriguing, but no such effects have been reported for I. scapularis. Several of the nymphs were coinfected with two intracellular bacteria, but Arsenophonus spp. endosymbionts, found in a wide range of arthropods including Amblyomma and Dermacentor ticks (Novakova et al., 2009), were absent from I. scapularis.
The microbial communities of A. americanum and D. variabilis diverge from those reported for I. scapularis (Grindle et al., 2003; Clay et al., 2008; Dergouseff et al., 2010). Rickettsia spp. are associated with both ticks but unlike I. scapularis, they also harbor Coxiella- or Francisella-like endosymbionts that are members of the gammaproteobacteria. A Coxiella sp. is highly prevalent (100%) and Rickettsia sp. less so in A. americanum, but not D. variabilis, from several different states in the US (Jasinkas et al., 2007; Clay et al., 2008) (MD, OK, IN, MO, KY, GA, SC, and MS). Most of the A. americanum were infected with two to three microbes, and all ticks at all locations were infected with the Coxiella sp. endosymbiont that appears to have undergone genome reduction (Jasinskas et al., 2007). In contrast, a Rickettsia sp. with 99% similarity to Candidatus Rickettsia amblyommii was present in 45-61% of ticks, while prevalence of an Arsenophonus sp. was geographically spotty and varied from 0-90%. The Coxiella endosymbiont and Pseudomonas spp. were detected in larvae suggesting that both were transmitted transovarially. Coinfections involved the Coxiella endosymbiont and Arsenophonus or C. R. amblyommii and 26% of the ticks were infected with all three microbes. No sex ratio distortion was detected but a negative correlation between infection by Arsenophonus and Rickettsia sp. was noted, suggesting that one endosymbiont could potentially interfere with infection by another. In contrast to the wide presence of endosymbionts, pathogens, i.e., the monocytic ehrlichiosis agent, Ehrlichia
chaffeensis, and Borrelia lonestari, were rare. The microbial community of D. variabilis ticks is less well characterized, though the most prevalent microbe in Dermacentor ticks is the symbiotic Francisella sp. (Scoles, 2004). Canadian D. variabilis also harbor Arsenophonus similar to that found in D. variabilis in eastern US (Dergouseff et al., 2010).
Most microbial surveys currently rely on the use of PCR technology to detect and identify microorganisms in ticks. Few culture isolates of tick-associated microorganisms are available which makes it difficult to characterize traits such as vertebrate infectivity and pathogenicity and hinders genome sequencing. An obligate intracellular gammaproteobacterium has recently been culture isolated in vertebrate cells from I. ricinus collected in Slovakia (Mediannikov et al., 2010). A polyphasic taxonomic approach showed it is most closely related to Rickettsiella spp, in the family Coxiellaceae. Bacteria belonging to this group are sometimes detected in ticks (Noda et al., 1997; Kurtti et al., 2002) but their influence on tick physiology or ability to cause human disease is unknown. Uncharacterized microbes isolated during an attempt to isolate pathogens in cell cultures may turn out to be potentially important regulators of tick biology and vectorial capacity. We isolated a bacterium from ticks collected in Connecticut that was later found to belong to the genus Cardinium, a group known to cause reproductive disorders in insects and mites (Nakamura et al., 2009). Our culture isolate from I. scapularis remains the only one for this important group of bacteria.
The Dynamic Microbiomes of Vector Ticks
Studies outlined above suggest that tick-associate bacteria other than vertebrate pathogens modulate the vectorial capacity of ticks either by competition or possibly by exchange of genetic elements, and might provide tools to manipulate pathogen transmission. To exploit the microbial communities interacting with ticks, we propose that the microbiomes of major vector ticks be characterized. A microbiome is defined as “the totality of microbes, their genetic elements (genomes), and environmental interactions in a defined environment” (http://en.wikipedia.org/wiki/Microbiome). The microbiome of I. scapularis it is incomplete and weighted towards human pathogens transmitted by this tick (Table A6-1, completed and in progress). The only other microbial genome from I. scapularis is that of the REIS obtained during genome sequencing of the host tick (Van Zee et al., 2007). Pathogenic ehrlichiae and rickettsiae are so far the only characterized members of the microbiomes ofA. amblyommii and D. variabilis, but other prominent microbes associated with these ticks should be considered for genome sequencing (Table A6-1, proposed). Because of its importance as a vector of Lyme disease and human
TABLE A6-1 Prokaryotic Microbiomes of Ixodes scapularis, Dermacentor variabilis, and Amblyomma americanum
|
|
Microbe |
Classification |
Isolate |
Reference |
|
Ixodes scapularis |
|
|
|
|
|
COMPLETED |
||||
|
|
Borrelia burgdorferi |
Spirochaetes |
B31 |
Fraser et al., 1997 |
|
|
Anaplasma phagocyophilum |
Alphaproteobacteria Anaplasmataceae |
HZ |
Dunning Hotopp et al., 2006 |
|
IN PROGRESS |
||||
|
|
Borrelia burgdorferi |
Spirochaetes |
297, CA8, DN127, JD1, N40 |
unpublished (see NCBI Genome Project web page) |
|
|
REIS et al.a |
Alphaproteobacter Rickettsieai |
Wikel |
Joardar et al., unpublished |
|
|
Cardinium sp. |
Bacteroidetes |
IsCLO |
Noda et al., (unpublished, personal commun.) |
|
PROPOSED |
||||
|
|
REIS |
Alphaproteobacteria Rickettsieae |
ISO-7 |
Kurtti, unpublished |
|
|
Anaplasma phagocytophilum |
Alphaproteobacteria Anaplasmataceae |
Ap-variant 1 |
Massung et al., 2007 |
|
|
Pseudomonas spp (Symbiont) |
Gammaproteobacteria Pseudomonadaceae |
nab |
Moreno et al., 2006 |
|
Dermacentor variabilis |
|
|
|
|
|
COMPLETED |
||||
|
|
Rickettsia rickettsii |
Alphaproteobacteria Rickettsieae |
Iowa |
Ellison et al., 2008 |
|
PROPOSED |
||||
|
|
Francisella sp endosymbiont |
Gammaproteobacteria Francisellaceae |
na |
Niebylski et al., 1997a Scoles, 2004 |
|
|
Arsenophonus sp |
Gammaproteobacteria Enterobacteriales |
na |
Grindle et al., 2003 |
anaplasmosis, comparing the microbiome of I. scapularis from different geographical regions should be a priority.
Tick-associated bacteria contain genes that encode molecular chaperones responsive to a wide range of stress conditions (Feder and Hofman, 1999), such as the small heat-shock protein genes (Hsps) found in Rickettsia and Anaplasma species. In most non-pathogenic rickettsiae hsp2 is localized to plasmids and hsp1 to the chromosome (Baldridge et al., 2008), suggesting expression may be controlled differently. Indeed, transcriptional regulation of host adaptive genes is facilitated by their location on plasmids as has been described in B. burgdorferi (Stewart et al., 2005). Hsps respond to a variety of stress effectors (pH, osmotic pressure, etc) and help to stabilize membrane proteins and nucleic acids. In the tick, intracellular bacteria face significant changes in temperature, pH, osmotic pressure, metabolite concentrations, and CO2 and O2 levels during the alternating periods of starvation and blood feeding (Munderloh et al., 2005), and the observed differential expression of Hsps in A. phagocytophilum growing in human versus tick cells implies a role in mitigating deleterious effects (Nelson et al., 2008). GenBank data derived from the I. scapularis genome project indicate that REIS carries at least three plasmids (pREIS1, 2 and 3) which is supported by pulsed field electrophoresis results of our REIS culture isolate (Baldridge et al., 2010). The presence of multiple plasmids in REIS is an
enigma but may compensate for functional gene loss resulting in impaired ability to respond to environmental stressors such as elevated temperature and oxidation. The NCBI database for REIS indicates loss of a heat shock induced serine protease, HtrA, that degrades misfolded proteins. REIS also has a frame-shift in the poly-beta-hydroxybutyrate polymerase gene (phbC) that is upregulated in R. conorii in response to stress encountered in the skin of patients infected with Mediterranean spotted fever (Renesto et al., 2008). Diverse bacteria are associated with I. scapularis, and coinfections of a single tick with the Cardinium endosymbiont and REIS have been reported (Benson et al., 2004). This could facilitate horizontal gene transfer (hgt) between them, and is supported by the presence of closely related transposons in the Dermacentor tick symbiont Rickettsia peacockii and the Cardinium endosymbiont from I. scapularis. This transposon, likely acquired by hgt, is associated with extensive genomic reorganization and deletions in the R. peacockii genome (Felsheim et al., 2009). The potential for rickettsial plasmid mobility and hgt between intracellular bacteria cohabiting the same intracellular arena should be examined.
Cohabitation and Horizontal Gene Transfer
Hgt has shaped the genomes of tick-transmitted pathogens and has played an important role in the acquisition of environmental adaptive traits and virulence determinants. There are two prominent hypotheses related to intracellular bacteria that can potentially infect the same host cell in a tick. The “intracellular arena hypothesis” posits that the coinhabitants can coexist, interact and exchange genetic material (Blanc et al., 2007). The “interference hypothesis” posits that interspecific competition between closely related species interferes with their ability to cohabit the same intracellular environment (Burgdorfer, 1981; Macaluso et al., 2002). There is considerable evidence for cohabitation of dissimilar intracellular microbes within the same host cell, especially among the symbionts that infect the ovarian cells of ticks. Rickettsia peacockii and a Francisella-like symbiont are present together in the interstitial ovarian cells of D. andersoni (Niebylski et al., 1997a). A Coxiella-like symbiont is found together with C. Rickettsia amblyommii in the ovarian cells of A. americanum. The evidence for interference between two closely related species derives mainly from research with Rickettsia and Anaplasma. On the other hand, the presence of mobile genetic elements (plasmids and transposons) suggests that Rickettsia spp. coinfecting the same host cell have the potential for the generation of genetic diversity, but it needs to be demonstrated that these genetic elements are indeed mobile. The ability to interact and acquire novel adaptive traits and virulence determinants is clearly germane to generation of new tick borne pathogens. The research tools to test these hypotheses have recently become available.
Discovered only recently (Ogata et al., 2005), plasmids appear to be surprisingly common in rickettsiae (Blanc et al., 2007; Baldridge et al., 2010), and several rickettsiae carry tra genes encoding type IV secretion system (T4SS) that may mediate rickettsial acquisition of foreign DNA, possibly via pili formation and conjugation (Ogata et al., 2005; Ogata et al., 2006; Blanc et al., 2007; Felsheim et al., 2009). Gillespie et al. (2010) proposed that the RvhB6 proteins (comparable to VirB6 in other bacterial species) in Rickettsia and Anaplasma play a role in DNA import and export in congener bacteria and create the potential for hgt. Tiling microarrays have detected host cell specific transcription patterns in the rvhB6 genes of A. phagocytophilum during growth in human and I. scapularis cells in vitro (Nelson et al., 2008). Given the diversity of animals that I. scapularis feeds on and the temporal scale available for microbe-microbe interactions, hgt is most likely to take place in ticks. The experimental tools to examine genetic exchange between congeners of Rickettsia and Anaplasma have recently been developed (Felsheim et al., 2006; Baldridge et al., 2005).
Mobile Genetic Elements: Future Directions in Laboratory Research on Tick-Borne Pathogens
Research in rickettsiology has lagged far behind that on diseases caused by bacteria that can be propagated on axenic media, despite the pressing needs created by emergence and re-emergence of severe illnesses such as RMSF, anaplasmosis and ehlichiosis. Transformation of obligate intracellular bacteria has been a challenge that researchers have only recently been able to address, but this important tool is still in need of refinement (Baldridge et al., 2005; Felsheim et al., 2006; Liu et al., 2007). Original methods developed for rickettsial transformation, including homologous recombination (Rachek et al., 1998) and use of selectable markers with EZ:TN transposon vectors (Qin et al, 2004; Baldridge et al., 2005), had low efficiency, fueling our efforts to find better systems. The mariner class transposase, Himar1, which has shown broad activity in bacteria, proved useful in transforming A. phagocytophilum (Felsheim et al., 2006), enabling us to create mutants with defective phenotypes that can now be functionally characterized. This system is equally suited for rickettsial mutagenesis, but is still quite inefficient, yielding one or a few transformants at each electroporation. Nevertheless, an advantage of is the stability of resulting mutants when transposition occurs into the chromosome, making them well suited for in vivo tracking by live imaging applications such as time-lapse microscopy and tracking in vectors and vertebrate animals.
Making the Most of Rickettsial Plasmids
With the discovery of plasmids in many Rickettsi spp. came the realization that they could be fashioned into an efficient transformation tool to facilitate studies on rickettsial functional genomics. Till now, analysis of rickettsial gene function has relied on cloning genes of interest into E. coli in the hope they would perform in this artificial system as they would “at home.” To overcome this drawback, we set out to utilize rickettsial plasmids for direct analysis of rickettsial genes in rickettsiae themselves. To start we cloned the Rickettsia monacensis plasmid pRM (Baldridge et al, 2007) and R. amblyommii plasmids pRAM18 and pRAM23 (Baldridge et al., 2010) with the aim to design shuttle vectors that could be used as effective transformation systems. We modified pRAM18 to express a fluorescent (GFPuv) marker to successfully transform the nonpathogenic R. bellii. To create a more efficient plasmid we transferred the parA and dnaA genes of pRAM18 that regulate plasmid replication and partitioning into smaller (8.7 and 10.3 kbp) constructs that were efficiently transformed into three species, R. montanensis, R. monacensis and R. bellii (Burkhardt et al., 2010). While these initial results provide a good start towards eventual production of a shuttle vector system for efficient transformation of a wide range of rickettsiae, problems of incompatibility remain to be sorted out.
Can the Paradigm of Paratransgenesis Be Realized in Rickettsiology?
The results of testing the “intracellular arena” and “interference” hypotheses have important implications for the potential application of paratransgenesis in the control of tick-borne diseases. The paratransgenesis paradigm involves the replacement or supplementation of an indigenous symbiont with a genetically altered (transformed) congener that interferes with the ability of the arthropod to transmit a pathogen without killing the arthropod. Manipulation of tick populations by subversion of their indigenous endosymbionts is an attractive concept because it targets a vehicle naturally restricted to the tick population. Systems for genetic modification of REIS could be applied to interfere with the transmission of B. burgdorferi or A. phagocytophilum by I. scapulris.
The relationship between ticks and their symbionts is not clear, and at this time, bacteria that could be regarded as “primary tick symbionts” analogous to those in insects (Dale and Moran, 2006) have not been identified. REIS and R. peacockii are regarded as the closest to being mutualistic endosymbionts among the known, non-pathogenic rickettsiae, because of their apparent inability to invade vertebrate cells. This is likely due to the disruption of rickettsial genes involved in mammalian cell invasion, such as rompA and rickA (Niebylski et al., 1997b; Simser et al., 2001, 2005).
The high prevalence of REIS in widely distributed I. scapularis populations indicates that it is essential to I. scapularis survival. The sequenced genomes of Rickettsiales, including pathogens, have revealed a significant capacity to produce cofactors such as lipoate, protoheme, ubiquinone, and several amino acids, e.g., glutamine, glycine, diaminopimelate and aspartate (Dunning Hotopp et al., 2006). This suggests that even tick-borne pathogens may supply their tick hosts with some needed nutrients, acting like symbionts for their vector. In essence, there is much that remains to be learned about tick symbionts before they can be considered for paratransgenic tick control. Clearly, there is a need to elucidate the relationships between ticks and the symbiotic and pathogenic microorganisms they carry and how the interactions are affected by environmental changes.
Conclusions
The reasons for the accelerated increase, expansion and uneven distribution of tick-borne emerging and re-emerging diseases are complex but several conclusions can be drawn. Human activities that modify habitats to support available hosts for maintaining tick populations while at the same time reducing species richness that could act to dilute risk. Weather and temperature regimes restricts the current range of tick populations, but global climate changes will provide new opportunities for the expansion of ticks into currently uncolonized regions. In addition, domestic animals can act as reservoirs for tick-borne pathogens and act as bridge hosts in the transfer of emerging and re-emerging pathogens to humans. Fragmentation of wildlife habitats by human encroachment increases the contact with ticks and exposure to zoonotic disease agents (Allan et al., 2003). The areas likely to experience increased or prolonged seasonal tick activity are most likely located at the extremes of the current range of distribution. Long-term predictive models are complex and in need of refinement in order to predict public health risks associated with ticks and tick-borne pathogens. The expansion of three host ticks that feed on wild and domestic animals and humans show the greatest potential for expansion and acquisition and transmission of emerging pathogens. Introduction of ticks to new habitats or importation of exotic hosts are likely to increase the exposure of ticks to novel microbial communities. More information is needed about the potential for horizontal genetic exchange and interaction between the microorganisms within the tick’s microbial community. Characterizing the microbiomes of major vector ticks from different geographical regions would assist in detecting and monitoring these interactions and determine their role in the generation of emerging and re-emerging tick-borne pathogens. Characterizing the microbial communities would also assist in the
identification of microbes that could complement the biological control of tick populations.
References
Adelson, M. E., R. V. Rao, R. C. Tilton, K. Cabets, E. Eskow, L. Fein, J. L. Occi, and E. Mordechai. 2004. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in Northern New Jersey. Journal of Clinical Microbiology 42(6):2799-801.
Allan, B. F., F. Keesing, and R. S. Ostfeld. 2003. The effect of habitat fragmentation on Lyme disease risk. Conservation Biology 17:267-272.
Baldridge, G. D., N. Y. Burkhardt, M. J. Herron, T. J. Kurtti, and U. G. Munderloh. 2005. Analysis of fluorescent protein expression in transformants of Rickettsia monacensis, an obligate intracellular tick symbiont. Applied and Environmental Microbiology 71(4):2095-2105.
Baldridge, G. D., N. Y. Burkhardt, R. F. Felsheim, T. J. Kurtti, and U. G. Munderloh. 2008. Plasmids of the pRM/pRF family occur in diverse Rickettsia species. Applied and Environmental Microbiology 74(3):645-652.
Baldridge, G. D., G. A. Scoles, N. Burkhardt, B. Schloeder, T. J. Kurtti, and U. G. Munderloh. 2009. Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: Ixodidae). Journal of Medical Entomology 46:625-632.
Baldridge, G. D., N. Y. Burkhardt, M. B. Labruna, R. C. Pacheco, C. D. Paddock, P. C. Williamson, P. M. Billingsley, R. F. Felsheim, T. J. Kurtti, and U. G. Munderloh. 2010. Wide dispersal and possible multiple origins of low-copy-number plasmids in Rickettsia species associated with blood-feeding arthropods. Applied and Environmental Microbiology 76(6):1718-1731.
Barbour, A. G. and D. Fish. 1993. The biological and social phenomenon of Lyme disease. Science 260(5114):1610-1616.
Barré, N., M. Bianchi, and L. Chardonnet. 2001. Role of Rusa deer Cervus timorensis russa in the cycle of the cattle tick Boophilus microplus in New Caledonia. Experimental and Applied Acarology 25(1):79-96.
Bartlett, S. L., N. Abou-Madi, J. B. Messick, A. Birkenheuer, and G. V. Kollias. 2009. Diagnosis and treatment of Babesia odocoilei in captive reindeer (Rangifer tarandus tarandus) and recognition of three novel host species. Journal of Zoo and Wildlife Medicine 40(1):152-159.
Benson, M. J., J. D. Gawronski, D. E. Eveleigh, and D. R. Benson. 2004. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Applied and Environmental Microbiology 70(1):616-620.
Billings, A. N., Tetlow, G. J., Weaver, S. C., and D. H. Walker. 1998. Molecular characterization of a novel Rickettsia species from Ixodes scapularis in Texas. Emerging Infectious Diseases 4(2):305-309.
Bjöersdorff, A., S. Bergström, R. F. Massung, P. D. Haemig, and B. Olsen. 2001. Ehrlichia-infected ticks on migrating birds. Emerging Infectious Diseases 7(5):877-879.
Blanc, G., H. Ogata, C. Robert, S. Audic, J.-M. Claverie, and D. Raoult. 2007. Lateral gene transfer between obligate intracellular bacteria: evidence from the Rickettsia massiliae genome. Genome Research 17:1657-1664.
Brooks, D., and A. Ferrao. 2005. The historical biogeography of co-evolution: emerging infectious diseases are evolutionary accidents waiting to happen. Journal of Biogeography 32:1291-1299.
Brownstein, J. S., D. K. Skelly, T. R. Holford, and D. Fish. 2005. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia. 146(3):469-475.
Burgdorfer, W., S. F. Hayes, and A. J. Mavros. 1981. Non-pathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii. In Rickettsiae and Rickettsial Diseases, edited by W. Burdorfer and R. L. Anacker. Academic Press, New York, NY. Pp. 585-594, 650.
Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease-a tick-borne spirochetosis? Science. 216(4552):1317-1319.
Burkhardt, N. Y., G. D. Baldridge, P. C. Williamson, T. J. Kurtti, and U. G. Munderloh, 2010. Development of a shuttle vector for transformation of diverse Rickettsia species. Abstract of poster presented at the 24th meeting of the American Society for Rickettsiology. Stevenson, WA, July 31 to August 3rd.
Burks, C. S., R. L. Stewart Jr., G. R. Needham, and R. E. Lee Jr.. 1996. Cold hardiness in the ixodid ticks (Ixodidae). In Acarology IX, Proceedings, edited by R. Mitchell, D.J. Horn, G.R. Needham, and W.C. Wellbourn. Ohio Biological Survey, Columbus, Ohio. Vol. 1, Pp. 85-87.
Burlini, L., K. R. Teixeira, M. P. Szabó, and K. M. Famadas. 2010. Molecular dissimilarities of Rhipicephalus sanguineus (Acari: Ixodidae) in Brazil and its relation with samples throughout the world: is there a geographical pattern? Experimental and Applied Acarology. 50(4):361-374.
Campbell, K., and C. J. Donlan. 2005. Feral goat eradications on islands. Conservation Biology 19:1362-1374.
Canales, M., C. Almazán, V. Naranjo, F. Jongejan, and J. de la Fuente. 2009. Vaccination with recombinant Boophilus annulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnology 9:29. http://www.biomedcentral.com/1472-6750/9/29
Cantu-C, A., J. A. Ortega-S, Z. García-Vázquez, J. Mosqueda, S. E. Henke, and J. E. George. 2009. Epizootiology of Babesia bovis and Babesia bigemina in free-ranging white-tailed deer in northeastern Mexico. Journal of Parasitology 95(3):536-542.
Carroll, J. F., and E. T. Schmidtmann. 1996. Dispersal of blacklegged tick (Acari:Ixodidae) nymphs and adults at the woods-pasture interface. Journal of Medical Entomology 33(4):554-558.
Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulo-cytotropic Ehrlichia species as the etiologic agent of human disease. Journal of Clinical Microbiology 32(3):589-595.
Clay, K., O. Klyachko, N. Grindle, D. Civitello, D. Oleske, and C. Fuqua. 2008. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Molecular Ecology 17:4371-4381.
Costero, A., and M. A. Grayson. 1996. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari:Ixodidae). American Journal of Tropical Medicine and Hygiene 55(5):536-546.
Dale, C., and N. A. Moran. 2006. Molecular interactions between bacterial symbionts and their hosts. Cell 126:453-464.
Dantas-Torres, F. 2010. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites and Vectors 3:26. http://www.parasitesandvectors.com/content/3/1/26
Davies, T. J., A. Purvis, amd J. L. Gittleman. 2009. Quaternary climate change and the geographic ranges of mammals. American Naturalist 174(3):297-307.
Demma, L. J., M. S. Traeger, W. L. Nicholson, C. D. Paddock, D. M. Blau, M. E. Eremeeva, G. A. Dasch, M. L. Levin, J. Singleton Jr, S. R. Zaki, J. E. Cheek, D. L. Swerdlow, and J. H. McQuiston. 2005. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. New England Journal of Medicine 353(6):587-594.
Diuk-Wasser, M. A., A. G. Gatewood, M. R. Cortinas, S. Yaremych-Hamer, J. Tsao, U. Kitron, G. Hickling, J. S. Brownstein, E. Walker, J. Piesman, and D. Fish. 2006. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. Journal of Medical Entomology 43(2):166-176.
Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. International Journal of Systematic and Evolutionary Microbiology 51(6):2145-65.
Dunning Hotopp, J. C., M. Lin, R. Madupu, Crabtree, J., Angiuoli, and 35 others. 2006. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genetics 2(2):e21.
Eisen, R. J., L. Eisen, Y. A. Girard, N. Fedorova, J. Mun, B. Slikas, S. Leonhard, U. Kitron, and R. S. Lane. 2010. A spatially-explicit model of acarological risk of exposure to Borrelia burgdorferi-infected Ixodes pacificus nymphs in northwestern California based on woodland type, temperature, and water vapor. Ticks and Tick Borne Diseases 1(1):35-43.
Ellison, D. W., T. R. Clark, D. E. Sturdevant, K. Virtaneva, S. F. Porcella, and T. Hackstadt. 2008. Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa. Infection and Immunity 76(2):542-550.
Eriks, I. S., G. H. Palmer, T. C. McGuire, D. R. Allred, and A. F. Barbet. 1989. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. Journal of Clinical Microbiology 27(2):279-284.
Feder, M. E., and G. E. Hofman. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology 61:243-282.
Felsheim, R. F., M. J. Herron, C. M. Nelson, N. Y. Burkhardt, A. F. Barbet, T. J. Kurtti, and U. G. Munderloh. 2006. Transformation of Anaplasma phagocytophilum. BMC Biotechnology 6:42. Doi:10.1186/1472-6750/6/42.
Felsheim, R. F., T. J. Kurtti, and U. G. Munderloh. 2009. Genome sequence of the endosymbiont Rickettsia peacockii and comparison with virulent Rickettsia rickettsii: identification of virulence factors. PLoS ONE 4(12): e8361. Doi:10.1371/journal.pone.0008361.
Fenner, F. 2010. Deliberate introduction of the European rabbit, Oryctolagus cuniculus, into Australia. Revue scientifique et technique 29(1):103-111.
Foley, J. A., R. Defries, G. P. Asner, C. Barford, G. Bonan, S. R. Carpenter, F. S. Chapin, M. T. Coe, G. C. Daily, H. K. Gibbs, J. H. Helkowski, T. Holloway, E. A. Howard, C. J. Kucharik, C. Monfreda, J. A. Patz, I. C. Prentice, N. Ramankutty, and P. K. Snyder. 2005. Global consequences of land use. Science 309(5734):570-4.
Foley, J., N. C. Nieto, P. Foley, and M. B. Teglas. 2008. Co-phylogenetic analysis of Anaplasma phagocytophilum and its vectors, Ixodes spp. ticks. Experimental and Applied Acarology 45(3-4):155-170.
Foley, J. E., N. C. Nieto, and P. Foley. 2009. Emergence of tick-borne granulocytic anaplasmosis associated with habitat type and forest change in northern California. American Journal of Tropical Medicine and Hygiene 81(6):1132-1140.
Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, and 33 others. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586.
George, J. E. 2008. The effects of global change on the threat of exotic arthropods and arthropod-borne pathogens to livestock in the United States. Annals of the New York Academy of Sciences 1149:249-54.
Gillespie, J. J., K. A. Brayton, K. P. Williams, M. A. Quevedo Diaz, W. C. Brown, A. F. Azad, and B. W. Sobral. 2010. Phylogenomics reveals a diverse Rickettsiales type IV secretion system. Infection and Immunity 78(5):1809-1823.
Girard, Y. A., B. Travinsky, A. Schotthoefer, N. Fedorova, R. J. Eisen, L. Eisen L, A. G. Barbour AG, and R. S. Lane. 2009. Population structure of the Lyme disease spirochete Borrelia burgdorferi in the western black-legged tick (Ixodes pacificus) in Northern California. Applied and Environmental Microbiology 75(22): 7243-7252.
Githeko, A. K., Lindsay, S. W., Confalonieri, U.E., and J.A. Patz. 2000. Climate change and vector-borne diseases: a regional analysis. Bulletin of the World Health Organization 78(9):1136-1147
Gray, J. S., H. Dautel, A. Estrada-Peña, O. Kahl, and E. Lindgren. 2009. Effects of climate change on ticks and tick-borne diseases in Europe. Interdisciplinary Perspectives on Infectious Diseases. 2009:593232.
Greer, A., V. Ng, and D. Fisman. 2008. Climate change and infectious diseases in North America: the road ahead. Canadian Medial Association Journal 178(6):715-22.
Greger, M. 2007. The human/animal interface: emergence and resurgence of zoonotic infectious diseases. Critical Reviews in Microbiology 33(4):243-99.
Grindle, N., J. J. Tyner, K. Clay, and C. Fuqua. 2003. Identification of Arsenophonus-type bacteria from the dog tick Dermacentor variabilis. Journal of Invertebrate Pathology 83:264-266.
Harrus, S., and G. Baneth. 2005. Drivers for the emergence and re-emergence of vectorborne protozoal and bacterial diseases. International Journal of Parasitology 35(11-12): 1309-1318.
Herrero, M. V., E. Perez, W. L. Goff, S. Torioni de Echaide, D. P. Knowles, T. F. McElwain, V. Alvarez, A. Alvarez, and G. M. Buening. 1998. Prospective study for the detection of Anaplasma marginale Theiler, 1911 (Rickettsiales: Anaplasmataceae) in Costa Rica. Annals of the New York Academy of Sciences 849:226-233.
Hoberg, E. P., L. Polley, E. J. Jenkins, and S. J. Kutz. 2008. Pathogens of domestic and free-ranging ungulates: global climate change in temperate to boreal latitudes across North America. Revue scientifique et technique 27(2):511-28.
Hoen, A. G., G. Margos, S. J. Bent, M. A. Diuk-Wasser, A. Barbour, K. Kurtenbach, and D. Fish. 2009. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events Proceedings of the National Academy of Sciences of the USA 106(35): 15013-8.
Hoogstraal, H. 1956. African ixodoidea. In. Ticks of the Sudan. (With special reference to Equatoria Province and with preliminary reviews of the genera Boophilus, Margaropus and Hyalomma). Research report NM 005 050. 29.07. Department of the Navy, Bureau of Medicine and Surgery, Washington. Vol. 1.
Hoogstraal, H. 1981. Changing patterns of tickborne diseases in modern society. Annual Review of Entomology 26:75-99.
Jasinskas, A., J. Zhong, and A. G. Barbour. 2007. Highly prevalent Coxiella sp. Bacterium in the tick vector Amblyomma americanum. Applied and Environmental Microbiology 73(1):334-336.
Jones, K. E., N. G. Patel, M. A. Levy, A. Storeygard, D. Balk, J. L. Gittleman, and P. Daszak. 2008. Global trends in emerging infectious diseases. Nature 451:990-994.
Kalluri, S., P. Gilruth, D. Rogers, and M. Szczur. 2007. Surveillance of arthropod vectorborne infectious diseases using remote sensing techniques: a review. PLoS Pathogens 3(10):1361-71.
Keesing, F., R. D. Holt, and R. S. Ostfeld. 2006. Effects of species diversity on disease risk. Ecology Letters 9(4):485-498.
Killilea, M. E., A. Swei, R. S. Lane, C. J. Briggs, and R. S. Ostfeld. 2008. Spatial dynamics of Lyme disease: a review. EcoHealth 5(2):167-95.
Klich, M., M. W. Lankester, and K. W. Wu. 1996. Spring migratory birds (Aves) extend the northern occurrence of blacklegged tick (Acari: Ixodidae). Journal of Medical Entomology 33:581-5.
Kurtenbach, K., K. Hanincová, J. I. Tsao, G. Margos, D. Fish D, and N. H. Ogden. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nature Reviews Microbiology 4(9):660-669.
Kurtti, T. J., U. G. Munderloh, T. G. Andreadis, L. A. Magnarelli, and T. N. Mather. 1996. Tick cell culture isolation of an intracellular prokaryote from the tick Ixodes scapularis. Journal of Invertebrate Pathology 67(3):318-321.
Kurtti, T. J., A. T. Palmer, and J. H. Oliver, Jr. 2002. Rickettsiella-like bacteria in Ixodes woodi (Acari: Ixodidae). Journal of Medical Entomology 39(3):534-540.
Labruna, M. B. 2009. Ecology of Rickettsia in South America. Annals of the New York Academy of Sciences 1166:156-166.
Labuda, M., V. Danielova, L. D. Jones, and P. A. Nuttall. 1993. Amplification of tick-borne encephalitis virus infection during co-feeding of ticks. Medical and Veterinary Entomology 7(4):339-342.
LaSala, P. R., and M. Holbrook. 2010. Tick-borne flaviviruses. Clinics in Laboratory Medicine 30(1):221-235.
Levin, M. L., W. L. Nicholson, R. F. Massung, J. W. Sumner, and D. Fish. 2002. Comparison of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector-Borne and Zoonotic Diseases 2(3):125-136.
Linard, C., P. Lamarque, P. Heyman, G. Ducoffre, V. Luyasu, K. Tersago, S. O. Vanwambeke, and E. F. Lambin. 2007. Determinants of the geographic distribution of Puumala virus and Lyme borreliosis infections in Belgium. International Journal of Health Geographics 26:15.
Liu, Z.-M., A. M. Ticker, L. O. Driskell, and D. O. Wood. 2007. mariner-based transposon mutagenesis of Rickettsia prowazekii. Applied and Environmental Microbiology 73(20):6644-6649.
LoGiudice, K., S. T. Duerr, M. J. Newhouse, K. A. Schmidt, M. E. Killilea, and R. S. Ostfeld. 2008. Impact of host community composition on Lyme disease risk. Ecology 89(10):2841-9.
Macaluso, K. R., D. E. Sonenshine, S. M. Ceraul, and A. Azad. 2002. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhbits transovarial transmission of a second Rickettsia. Journal of Medical Entomology 39(6):809-813.
Madder, M., E. Thys, L. Achi, A. Touré, and R. De Deken. 2010. Rhipicephalus (Boophilus) microplus: a most successful invasive tick species in West-Africa. Experimental and Applied Acarology Aug 15, 2010 [Epub ahead of print] http/www.springerlink.com/content/w812r4t206207135/fulltext.pdf.
Madhav, N. K., J. S. Brownstein, J. I. Tsao, and D. Fish. 2004. A dispersal model for the range expansion of blacklegged tick (Acari: Ixodidae). Journal of Medical Entomology 41(5):842-852.
Madigan, J. E., and D. Gribble. 1987. Equine ehrlichiosis in northern California: 49 cases (1968-1981). Journal of the American Veterinary Medical Association 190(4):445-448.
Massung, R. F., M. J. Mauel, J. H. Owens, N. Allan, J. W. Courtney, K. C. Stafford III, and T. N. Mather. 2002. Genetic variants of Ehrlichia phagocytophila, Rhode Island and Connecticut. Emerging Infectious Diseases 8:467-472.
Massung, R. F., J. W. Courtney, S. L. Hiratzka, V. E. Pitzer, G. Smith, and R. L. Dryden. 2005. Anaplasma phagocytophilum in white-tailed deer. Emerging Infectious Diseases 11(10):1604-1606.
Massung, R. F., M. L. Levin, U. G. Munderloh, D. J. Silverman, M. J. Lynch, J. K. Gaywee, and T. J. Kurtti. 2007. Isolation and propagation of the Ap-variant 1 strain of Anaplasma phagocytophilum in a tick cell line. Journal of Clinical Microbiology 45(7):2138-2143.
Materna, J., M. Daniel, and V. Danielová. 2005. Altitudinal distribution limit of the tick Ixodes ricinus shifted considerably towards higher altitudes in central Europe: results of three years monitoring in the Krkonose Mts. (Czech Republic). Central European Journal of Public Health 13(1):24-8.
McCoy, K. D., T. Boulinier, C. Tirard, and Y. Michalakis. 2003. Host-dependent genetic structure of parasite populations: differential dispersal of seabird tick host races. Evolution 57(2):288-96.
Mediannikov, O., S. Sekeyova, M.-L. Birg, and D. Raoult. 2010. A novel obligate intracellular gamma-proteobacterium associated with ixodid ticks, Diplorickettsia massiliensis, Gen. Nov., Sp. Nov. PLoS ONE 5(7): e11478. doi:10.1371/journal.pone.0011478.
Michalski, M. C., C. Rosenfeld, M. Erickson, R. Selle, K. Bates, D. Essar, and R. Massung. 2006. Anaplasma phagocytophilum in central and western Wisconsin: a molecular survey. Parasitology Research 99:694-699.
Moreno, C. X., F. Moy, T. J. Daniels, H. P. Godfrey, and F. C. Cabello. 2006. Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess counties, New York. Environmental Microbiology 8(5):761-772.
Mumcuoglu, K. Y., K. Frish, B. Sarov, E. Manor, E. Gross, Z. Gat Z, and R. Galun. 1993. Ecological studies on the brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) in southern Israel and its relationship to spotted fever group rickettsiae. Journal of Medical Entomology 30(1):114-121.
Munderloh, U. G., S. D. Jauron, and T. J. Kurtti. 2005. The tick: a different kind of host for human pathogens. . In Tick-Borne Diseases of Humans, edited by J. L. Goodman, D. T. Dennis and D. E. Sonenshine. ASM Press, Washington, D.C. Pp 37-64.
Nakamura, Y., S. Kawai, F. Yukuhiro, S. Ito, T. Gotoh, R. Kisimoto, T. Yanase, Y. Matsumoto, D. Kageyama, and H. Noda. 2009. Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Applied and Environmental Microbiology 75(21):6757-6763.
Neelakanta, G., H. Sultana, D. Fish, J. F. Anderson, and E. Fikrig. 2010. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. Journal of Clinical Investigation 120(9):3179-3190.
Nelson, C. M., M. J. Herron, R. F. Felsheim, B. R. Schloeder, S. M. Grindle, A. O. Chavez, T. J. Kurtti, and U. G. Munderloh. 2008. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics 2008, 9:364 doi:10.1186/1471-2164-9-364.
Niebylski, M. L., M. G. Peacock, E. R. Fischer, S. F. Porcella, and T. G. Schwan. 1997a. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Applied and Environmental Microbiology 63(10):3933-3940.
Niebylski, M. L., M. E. Schrumpf, W. Burgdorfer, E. R. Fischer, E. R. Gage, and T. G. Schwan. 1997b. Rickettsia peacockii sp. nov., a new species infecfting wood ticks, Dermacentor andersoni, in western Montana. International Journal Systematic Bacteriology 47:446-452.
Niebylski, M. L., M. G. Peacock, and T. G. Schwan. 1999. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoniI). Applied and Environmental Microbiology 65(2):773-778.
Noda, H., U. G. Munderloh, and T. J. Kurtti. 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Applied and Environmental Microbiology 63(10):3926-3932.
Novakova, E., V. Hypsa, and N. A. Moran. 2009. Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC Microbiology 2009, 9:143doi:10.1186/1471-2180-9-143.
Ogata, H., P. Renesto, S. Audic, C. Robert, G. Blanc, P.E. Fournier, H Parinello, J.-M. Claverie, and D. Raoult. 2005. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biology 3(8):e248.
Ogata, H., B. La Scola, S. Audic, P. Renesto, G. Blanc, C. Robert, , P.-E. Fournier, J.-M. Claverie, and D. Raoult. 2006. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genetics 2(5): e76. DOI: 10:1371/journal.pgen.0020076.
Ogden, N. H., A. Maarouf, I. K. Barker, M. Bigras-Poulin, L. R. Lindsay, M. G. Morshed, C. J. O’Callaghan, F. Ramay, D. Waltner-Toews, and D. F. Charron. 2005. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. International Journal for Parasitology 36:63-70.
Ogden, N. H., L. R. Lindsay, K. Hanincová, I. K. Barker, M. Bigras-Poulin, D. F. Charron, A. Heagy, C. M. Francis, C. J. O’Callaghan, I. Schwartz, and R. A. Thompson. 2008. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Applied and Environmental Microbiology 74(6):1780-1790.
Paddock, C. D. 2009. The science and fiction of emerging rickettsioses. Annals of the New York Acadmies of Science 1166:133-143.
Parola, P., C. D. Paddock, and D. Raoult. 2005. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clinical Microbiology Reviews 18:710-756.
Pesko, K., F. Torres-Perez, B. Hjelle, and G. D. Ebel. 2010. Molecular epidemiology of Powassan virus in North America. Journal of General Virology Jul 14. [Epub ahead of print] http://vir.sgmjournals.org/cgi/rapidpdf/vir.0.024232-0v1.pdf
Péter, O., W. Burgdorfer, A. Aeschlimann, and P. Chatelanat. 1984. Rickettsia conorii isolated from Rhipicephalus sanguineus introduced into Switzerland on a pet dog. Zeitschrift für Parasitenkunde 70(2):265-70.
Piesman, J., and A. Spielman. 1980. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. American Journal of Tropical Medicine and Hygiene 29(5):742-6.
Pritt, B. S., L. M. Sloan, S. A. Cunningham, J. J. Franson, R. Patel, M. P. Wilhelm, D. F. Neitzel, U. G. Munderloh, C. M. Nelson, D. K. Hoang Johnson, J. McQuiston, K. M. McElroy, J. D. McFadden, C. R. Steward, K. Bogumill, M. E. Bjorgaard, J. P. Davis, D. M. Warshauer, and M. E. Eremeeva. 2009. Emergence of novel human ehrlichiosis in the Mid West United States, 2009. Late-breaker abstract; poster presented at the ASTMH Annual Meeting, Washington D.C., Nov. 18-22, 2009.
Qin, A., A. M. Tucker, A. Hines, and D. O. Wood. 2004. Transposon mutagenesis of the obligate intracellular pathogen Rickettsia prowazekii. Applied and Environmental Microbiology 70(5):2816-2822.
Rachek, L. I., A. M. Tucker, H. H. Winkler, and D. O. Wood. 1998. Transformation of Rickettsia prowazekii to rifampin resistance. Journal of Bacteriology 180(8):2118-2124.
Raizman, E. A., J. D. Holland, L. M. Keefe, and M. H. Moro. 2010. Forest and surface water as predictors of Borrelia burgdorferi and its vector Ixodes scapularis (Acari: Ixodidae) in Indiana. Journal of Medical Entomology 47(3):458-65.
Reed, K. D. 2003. Birds, Migration and Emerging Zoonoses: West Nile Virus, Lyme Disease, Influenza A and Enteropathogens. Clinical Medicine & Research 1:5-12.
Renesto, P., C. Rovery, J. Schrenzel, Q. Leroy, A. Huyghe, W. Li, H. Lepidi, P. Francois, and D. Raoult. 2008. Rickettsia conorii transcriptional response within inoculation eschar. PLoS ONE 3(11): e3681. doi:10.1371/journal.pone.0003681.
Rozental, T., M. E. Eremeeva, C. D. Paddock, S. R. Zaki, G. A. Dasch, and E. R. Lemos. 2006. Fatal case of Brazilian spotted fever confirmed by immunohistochemical staining and sequencing methods on fixed tissues. Annals of the New York Academy of Sciences 1078:257-259.
Schoelkopf, L., C. E. Hutchinson, K. G. Bendele, W. L. Goff, M. Willette, J. M. Rasmussen, and P. J. Holman. 2005. New ruminant hosts and wider geographic range identified for Babesia odocoilei (Emerson and Wright 1970). Journal of Wildlife Diseases 41(4):683-90.
Scoles, G. A. 2004. Phylogenetic analysis of the Francisella-like endosymbionts of Dermacentor ticks Journal of Medical Entomology 41(3):277-286.
Simser, J. A., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 2001. Isolation of a spotted fever group rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Applied and Environmental Microbiology 67(2)546-552.
Simser, J. A., M. S. Rahman, S. M. Dreher-Lesnick, and A. Azad. 2005. A novel and naturally occurring transposon, ISRpe1 in Rickettsia peacockii genome disrupting the rickA gene involved in actin-based motility. Molecular Microbiology 58(1):71-79.
Stafford, K. C. III. 2007. Tick Control Handbook. Bulletin 1010; The Connecticut Agricultural Experiment Station. http://www.cdc.gov/ncidod/dvbid/lyme/resources/handbook.pdf
Steiner, F. E., R. R. Pinger, C. N. Vann, N. Grindle, D. Civitello, K. Clay, and C. Fuqua. 2008. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. 2008. Journal of Medical Entomology 45(2):289-297.
Stuen, S. 2007. Anaplasma phagocytophilum - the most widespread tick-borne infection in animals in Europe. Veterinary Research Communications. 31:79-84, Supplement 1.
Telford, S. R. 3rd, J. E. Dawson, P. Katavolos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proceedings of the National Academy of Sciences of the USA. 93(12):6209-6214.
Uspensky, I., and I. Ioffe-Uspensky. 2002. The dog factor in brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) infestations in and near human dwellings. International Journal of Medical Microbiology 291:156-63, Suppl 33.
Van Zee, J. P., N. S. Geraci, F. D. Guerrero, S. K. Wikel, J. J. Stuart, V. M. Nene, and C. A. Hill. 2007. Tick genomics: the Ixodes genome project and beyond. International Journal for Parasitology 37:1297-1305.
Vora, N. 2008. Impact of anthropogenic environmental alterations on vector-borne diseases. Medscape Journal of Medicine 10(10):238.
Waldrup, K. A., A. A. Kocan, R. W. Barker, and G. G. Wagner. 1990. Transmission of Babesia odocoilei in white-tailed deer (Odocoileus virginianus) by Ixodes scapularis (Acari: Ixodidae). Journal of Wildlife Diseases 26(3):390-391
Ward, S. E., and R. D. Brown. 2004. A framework for incorporating the prevention of Lyme disease transmission into the landscape planning and design process. Landscape and Urban Planning 66:91-106.
Wikswo, M. E., R. Hu, M. E. Metzger, and M. E. Eremeeva. 2007. Detection of Rickettsia rickettsii and Bartonella henselae in Rhipicephalus sanguineus ticks from California. Journal of Medical Entomology 44(1):158-62.
Woldehiwet, Z. 2006. Anaplasma phagocytophilum in ruminants in Europe. Annals of the New York Academy of Sciences 1078:446-60.
A7
LYME BORRELIOSIS AND OTHER IXODID TICK-BORNE DISEASES—A EUROPEAN PERSPECTIVE
Susan O’Connell, M.D. Consultant Medical Microbiologist Head, Lyme Borreliosis Unit Health Protection Agency Southampton, UK
Ixodid Ticks in Europe, Their Geographic Distribution and Ecological Requirements
Ticks of the Ixodes ricinus complex are vectors of Borrelia burgdorferi and several other bacterial and viral infectious agents. In Europe the main tick vector for these organisms is Ixodes ricinus, commonly called the sheep tick or castor bean tick, and in Asia it is Ixodes persulcatus, the taiga tick. There is an area of overlap in the range of these species in parts of eastern Europe including the Baltic republics and western regions of Russia (See Figure A7-1). Ixodes ricinus is widely distributed, from countries on the western seaboard eastwards to Russia and it overlaps with I persulcatus in western Russia, the Baltic republics and eastern Europe.
Ixodes ricinus ticks have three active stages in their life-cycle, usually over two to three years, and at each stage they take a single blood meal lasting from about three to seven days (See Figure A7-2; European Union Concerted Action on Lyme Borreliosis [EUCALB], 2010). There is a high mortality throughout the process, with few ticks surviving to complete the life-cycle from an initial egg batch of about 2,000. The essential habitat requirements for tick survival are high humidity to maintain water balance and presence of suitable animal species as feeding hosts. Ticks survive only in areas where there is good vegetation cover, with a mat of decaying vegetation (leaf litter etc) that will maintain a relative humidity of 80-85% during the driest periods, providing protection against desiccation during the long interstadial development periods. Immature ticks can feed on a wide variety of mammalian and ground-feeding avian hosts which may be reservoir-competent for Borrelia burgdorferi and other potential human pathogens. Adult female ticks usually feed successfully only on large mammals such as deer, sheep, cattle and horses, underlining the importance of those hosts to the reproductive stage of the tick life-cycle.
These essential requirements are optimally provided in mixed deciduous woodland. They can also occur in coniferous forests provided that there is enough vegetation litter and a moist microclimate. Some heathland,
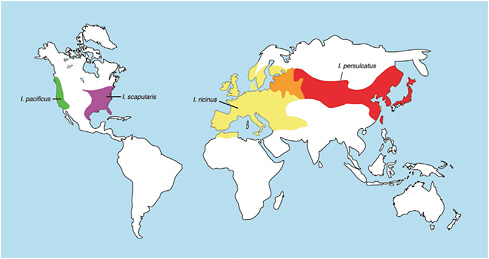
FIGURE A7-1 Distribution of Ixodes ricinus complex ticks Courtesy of Professor J Gray and Mr B Kaye and taken with permission from the European Union Concerted Action on Lyme Borreliosis (EUCALB) website.
moorland and pastureland habitats of regions with mild, damp climates, such as the British Isles, also provide suitable conditions. In these environments large animals are likely to be feeding hosts for all three stages of the ticklife-cycle (Gray, 1991, 1998). Many areas in southern Europe are too hot and dry for survival of Ixodes ricinus. Areas experiencing repeated droughts or episodes of severe flooding are also less favourable for tick survival.
There is evidence for changing distribution of ixodid tick populations in some parts of Europe, which may be related to changing climate. This is demonstrated most significantly at the geographic distribution limits of Ixodes ricinus.Few ticks are found in altitudes greater than about 1100 metres, but this altitude limit has risen significantly over 30 years from an earlier maximal altitude of 700 metres, as shown by well-documented studies in the Czech Republic and Switzerland (Lindgren and Jaenson, 2006). An extension in the northerly distribution of ixodid ticks into higher latitudes has occurred in Scandinavia in the past 20 years, associated with less severe winter temperatures and a greater number of days with temperature >10C.
More generally in Europe, changing climate has led to milder and shorter winters in many regions with earlier onset of spring (on average two weeks earlier than seen before the 1980s) and longer autumns, leading to earlier start of tick feeding activity and potentially greater tick survival. Conversely, conditions will become less favourable for ixodid ticks if areas
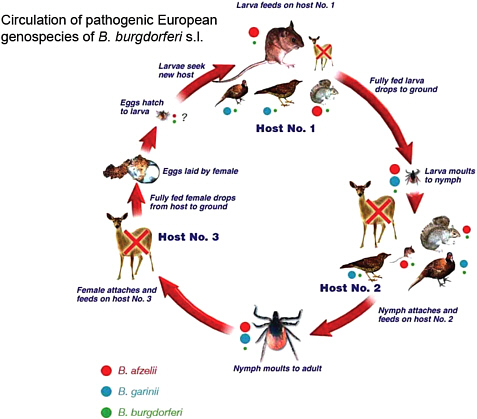
FIGURE A7-2 Ixodes ricinus lifecycle Courtesy of Professor J Gray and Mr B Kaye and taken with permission from the EUCALB website. http://meduni09.edis.at/eucalb/cms/index.php?option=com_content&task=view&id=53&Itemid=84. The relative size of the animals approximates their significance as hosts for the different tick life-cycle stages in a typical woodland habitat.
of hotter, more arid conditions expand in southern Europe. Tick survival and activity is also affected by more localized and short-term weather conditions. Other ecological aspects include changes in biodiversity. The possible effects of these and other factors on Ixodes ricinus and Lyme borreliosis in Europe were addressed in a World Health Organisation (WHO) publication in 2006, and this continues to be an important area of research (Lindgren and Jaenson, 2006). The summary of findings and recommendations of a 2007 workshop on environmental change and infectious diseases disease burden in Europe, organised by the European Centre for Disease Control (ECDC) is another valuable data source, as is a review published in 2009 (ECDC, 2007; Gray et al., 2009).
Other factors contributing to increased tick populations include changes in land use, particularly agriculture and forestry practice, with reforestation projects and pine monoculture being replaced by mixed for-placed by mixed forestry in many parts of Europe (see Figure A7-3 and A7-3a). Increased deer population densities are reported from many areas, which can also promote

tick reproductive success, as deer are the most important feeding hosts for ticks’ reproductive life-cycle stage. Wider geographic range of deer caused by deer population pressure has also resulted in expansion of tick populations into new areas in many parts of Europe. A report from the WHO Regional Office for Europe published in 2004 provided a comprehensive survey of vector-borne diseases, including tick-transmitted infections, and ecological, environmental and human behavioural factors influencing their incidence (WHO, 2004).
Human factors must be considered when assessing risks of tick-transmitted infections. These include residential, occupational and recreational factors. People living and working in tick habitats are at obvious risk
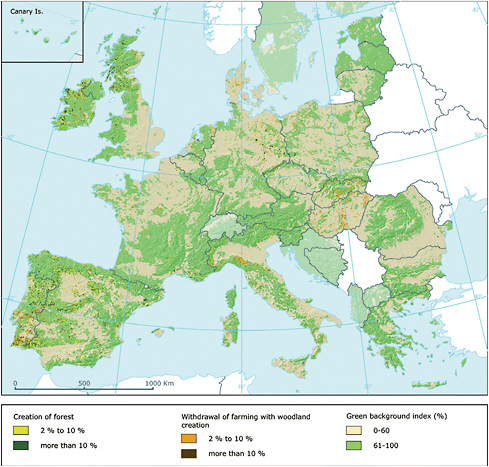
FIGURE A7-3 and A7-3A Dominant landscape types of Europe and changes. (European Environment Agency). http://www.eea.europa.eu/data-and-maps/figures/dominant-landscape-types-of-europe-based-on-corine-land-cover-2000-1.
of acquiring tick-transmitted infections. Housing developments in previously semi-rural and rural environments can expose new populations to these risks, as has been seen in many parts of Europe. Occupational risks include forestry and game management, and there have been significant changes in these industries in the past fifty years. Recreational aspects are important, with the high and increasing popularity of outdoor activities in tick-permissive environments, which can expose participants with little previous awareness of ticks to risk of tick-transmitted infections. Epidemiological studies from various European countries suggest that recreational activities, including those undertaken on vacation in other countries, are major factors for acquisition of Lyme borreliosis. This can have important economic implications for tourism in endemic areas.
Human beings can be incidental hosts for all three stages of the Ixodes ricinus life-cycle, although in practice the nymphal stage feed is the most likely to result in transmission of Borrelia burgdorferi, which is the most common tick-transmitted infection, as the organism rarely infects larval ticks transovarially. Few ticks survive to adulthood and in general only female adults take significant feeds. Because of their larger size adults are more likely to be noticed and removed earlier in the feeding period than earlier-stage ticks. The immature stages of Ixodes persulcatus appear not to feed readily on human beings and most transmission of infectious agents from this Ixodes species results from adult feeds (Korenberg et al., 2001). Human behavioural factors related to tick-transmitted diseases are addressed in more detail in a later section.
Ixodid feeding activity is affected by several factors, including diapause (dormancy) mechanisms, day-length, temperature and availability of hosts (see Figures A7-4, A7-5, and A7-6; EUCALB, 2010). These latter features produce some variations throughout Europe. In general Ixodes ricinus ticks feed between March and October, peaking in May to July, with a smaller secondary peak in the early autumn, but in countries with mild winters there can be a low level of feeding activity and potential risk of infection on warmer winter days. Ixodes persulcatus appears to have a similar level of activity in spring and early summer but is rarely active in autumn. Tick feeding seasonality affects the epidemiology of tick-transmitted infections, with peak incidence of tick-borne encephalitis in the late spring and early summer months. Lyme borreliosis presentations peak slightly later, reflecting the longer incubation period.
Ixodid Ticks and Micro-Organism Carriage
A variety of micro-organisms have been identified in Ixodes ricinus and I persulcatus ticks but organism carriage or DNA positivity must be distinguished from vector-competence. To establish vector-competence a
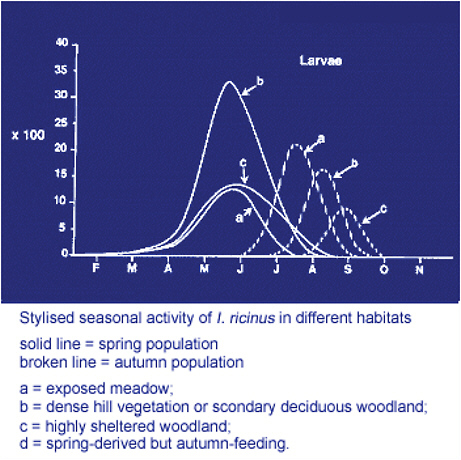
FIGURE A7-4 Stylized seasonal activity of I. ricinus in different habitats: Larvae. Courtesy of Professor Jeremy Gray and Mr Bernard Kaye and taken, with permission, from the EUCALB website. http://meduni09.edis.at/eucalb/cms/index.php?option=com_content&task=view&id=54&Itemid=89.
tick must be capable of maintaining organisms obtained at an earlier feed or transovarially and able to transmit them during a subsequent feed.
Ixodes ricinus and I persulcatus are known to be vector-competent for the flavivirus agents of tick-borne encephalitis virus and louping-ill. The latter is a well-recognised pathogen of sheep, cattle, goats and grouse, and also causes rare cases of human disease in the UK and Ireland. Several other viruses have been identified in Ixodes ricinus, including Tribec, Tettnang and Eyach viruses, but the public health importance of these agents seems to be very limited (WHO, 2004).
Tick-borne encephalitis is focally endemic in many parts of western, central and eastern Europe and in southern Scandinavia. It is mandatorily
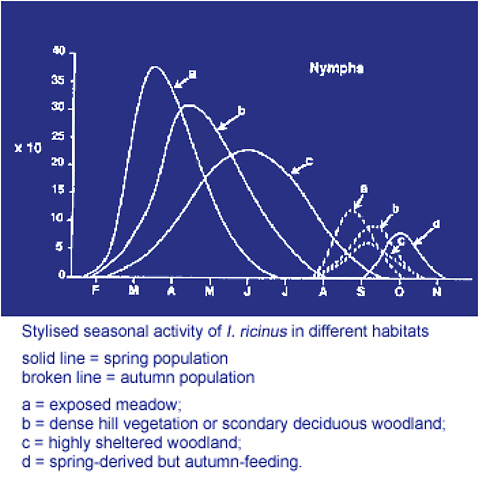
FIGURE A7-5 Stylized seasonal activity of I. ricinus in different habitats: Nymphs. Courtesy of Professor Jeremy Gray and Mr Bernard Kaye and taken, with permission, from the EUCALB website. http://meduni09.edis.at/eucalb/cms/index.php?option=com_content&task=view&id=54&Itemid=89.
notifiable in most states in which it occurs and between 2,000 and 3,000 cases are reported annually from European countries, including the Baltic states. It has an estimated mortality of between 0.5% and 2%, and significant long-term morbidity following meningoencephalitis, especially in older people. There is evidence of increased range and incidence into higher latitudes and altitudes in some regions, which may in part be due to changing climate, but other biological and human behavioural factors also play significant roles. Studies suggest that in the long-term the incidence may decrease in the more southerly regions as climate change alters tick seasonal dynamics, disrupting synchrony of larval and nymph co-feeding on rodent reservoirs. Co-feeding
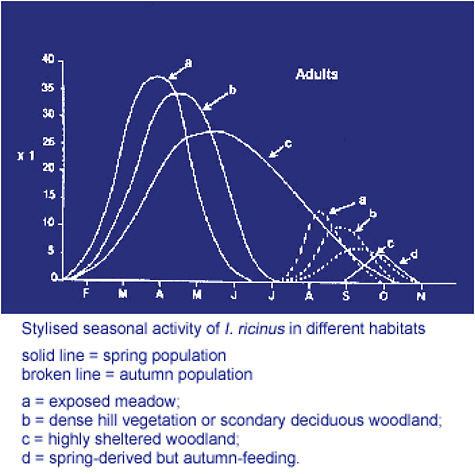
FIGURE A7-6 Stylized seasonal activity of I. ricinus in different habitats: Adults. Courtesy of Professor Jeremy Gray and Mr Bernard Kaye and taken, with permission, from the EUCALB website. http://meduni09.edis.at/eucalb/cms/index.php?option=com_content&task=view&id=54&Itemid=89.
appears to be an important factor in maintaining enzootic cycles of TBE (ECDC, 2007; Randolph, 2001). An effective TBE vaccine is available.
Ixodes ricinus is vector-competent for Borrelia burgdorferi sensu lato, the causes of Lyme borreliosis, which is by far the most common tick-transmitted disease in Europe. European Lyme borreliosis will be described in more detail later. It is also a vector for Borrelia miyamotoi, a member of the relapsing fever group of borreliae, which was first identified in Japan in 1995 and has been found in tick populations in many parts of Russia and Europe. At present it is unclear if this organism has human pathogenic potential (Karan et al., 2007).
Anaplasma phagocytophilum and rickettsioses, including Rickettsia helvetica are also tick-transmitted. Anaplasma phagocytophilum is widely distributed in Europe and livestock infections are common, causing a significant financial burden (Bown et al., 2009). Fewer than 100 human cases have been reported since the first European case was identified in Slovenia in 1997. Seroprevalence studies have found antibodies in 1.5-21% of forestry workers and other people exposed to ticks in northern and central Europe but significant systemic disease appears to be very uncommon (Parola, Davoust, et al., 2005).
Rickettsia helvetica was first isolated from Ixodes ricinus ticks in Switzerland in 1979 and subsequently identified in many European countries. Few human cases of clinical disease have been serologically confirmed, mainly presenting with relatively mild, self-limited illnesses with headache and myalgias and less frequently with a rash and/or an eschar. A condition characterised by eschar, usually on the scalp and accompanied by regional lymphadenopathy following bites from Dermacenter spp ticks have been documented from France and Hungary, where the condition has been termed tick-borne lymphdenopathy (TIBOLA). It is caused by R slovaca, which is widely distributed in Dermacenter spp ticks in Europe, and was first isolated in 1968. With molecular methods allowing more sensitive detection and refinement of speciation the range of rickettsial agents associated with Ixodes spp and other tick species is likely to increase, as extensively reviewed in 2005 (Parola, Paddock, et al., 2005).
Ixodes ricinus can also transmit Babesia spp, which are intraerythrocytic protozoa. Babesia divergens is the cause of redwater fever in cattle and also causes occasional cases of human disease, which can be overwhelming in asplenic or otherwise immunocompromised patients. About 40 cases have been reported in Europe in the past ten years, and numbers are likely to rise with increased awareness of tick-transmitted infections and rising numbers of potentially susceptible individuals. A few cases of Babesia venatorum (previously termed Babesia-EU1) infection have been reported in splenectomised patients, causing less severe clinical presentations than those seen with B divergens. This organism and several Babesia divergens-like strains have been identified in European deer. A human case of European-acquired Babesia microti infection was reported in 2007 and the species has been identified in ticks and animal reservoirs in several regions of Europe. Data on babesial infection has recently been reviewed and it is likely that other babesial species and reservoir hosts will be identified (Gray et al., 2010).
Francisella tularensis, the agent of tularaemia, and Coxiella burnetii, which causes Q fever, can also be transmitted to human beings through tick bites, but other transmission routes are more important for these organisms. The authors of the 2004 WHO report on vector-borne diseases
in Europe concluded that a large outbreak of tularaemia in Kosovo that occurred in 1999-2000 was associated with food or water contamination from a rodent source, related to post-war disruption and poor living conditions. Another outbreak in north-central Sweden in 1981 was thought to be related mainly to transmission by mosquitoes. Sporadic cases of tick-transmitted tularaemia are well-documented, particularly in eastern Europe and Scandinavia, highlighting the importance of raising public and healthcare worker awareness of this risk to enable early recognition and treatment. Tick transmission may be significant in maintaining enzootic infection cycles of both tularaemia and Q fever.
There is debate about the vector-competence of ixodid ticks for Bartonella spp including Bartonella henselae, the agent of catscratch disease. Bartonellae are common mammalian haemoparasites and there is increasing appreciation of the range of clinical presentations found in human infections. Bartonella DNA has been identified in many tick species, including I ricinus. This is not surprising, given the significant prevalence of Bartonella spp infection in rodent tick-feeding hosts, but transmission to human beings from I ricinus or I scapularis has not been proven. Only one study, published in 1996, showed successful culture of a Bartonella species from an I ricinus tick, suggesting that the organisms may not easily remain viable in ticks. The issue was recently reviewed in some detail and on currently available evidence it appears that Ixodes spp ticks are unlikely to be significant vectors of bartonellosis (Angelakis et al., 2010).
Borrelia Burgdorferi Sensu Lato in Europe
At least fifteen genospecies of Borrelia burgdorferi sensu lato have been identified and three cause the bulk of Lyme borreliosis in Europe: Borrelia afzelii, Borrelia garinii and Borrelia burgdorferi sensu stricto (EUCALB website). Borrelia garinii OspA serotype 4 has recently been designated B bavariensis. Another genospecies, B spielmanii, has been isolated occasionally from erythema migrans lesions but seems to cause little systemic ill-effects. Borrelia valaisiana appears to be non-pathogenic and is found in many parts of Europe, including the UK and Ireland, where it has been identified as the major infecting borrelia in some tick populations. This may account in part for the lower incidence of Lyme borreliosis in these countries by comparison to other European regions, where most infected ticks carry more pathogenic genospecies. Borrelia lusitaniae is rare, with foci mainly on the Iberian peninsula, and there have been only a few reports of associated human disease.
Many mammalian species are reservoir-competent for Borrelia burgdorferi sensu lato. The most important are rodents, particularly Apodemus spp mice, voles and squirrels. Borrelia afzelii is strongly associated with
these species, and B bavariensis (previously B garinii OspA serotype 4) with Apodemus spp. Birds, particularly ground-feeding species such as thrushes, blackbirds and pheasants are potential reservoirs of B valaisiana and B garinii. Some lizard species appear to be reservoir-competent for B lusitaniae (EUCALB, 2010). Ungulates (deer, sheep, goats, cattle and pigs) are crucially involved in the eco-epidemiology of Lyme borreliosis as maintenance hosts for ticks, but they are not significant as reservoir hosts.
The mammalian and avian reservoir host differences for B afzelii and B garinii are thought to be linked to differences in sensitivity of these genospecies to the host serum complement. Borrelial complement regulator-acquiring surface proteins (CRASPs) bind host immune regulators that protect spirochaetes from complement lysis. The CRASP repertoire of B garinii protects the spirochaete from avian complement lysis, whereas the CRASPs of B afzelii and B bavariensis protect these organisms from lysis by rodent sera (Piesman and Schwan, 2010).
Geographic distribution of different European genospecies has some effect on incidence and distribution of various clinical presentations of Lyme borreliosis in different parts of the continent. A useful meta-analysis based on publications between 1986 and 2003 summarised tick infection rates and genospecies identified in studies from 24 European countries (Rauter and Hartung, 2005). All pathogenic genospecies can cause erythema migrans. B burgdorferi sensu stricto is arthritogenic and causes disease presentations similar to those found in the USA, but is the least common of the major pathogenic genospecies in Europe. Lyme arthritis is a less frequent European complication than neuroborreliosis, predominantly caused by B garinii, the most neurotropic genospecies, which is widespread particularly in western Europe. The most common genospecies in central and eastern European countries and Scandinavia is B afzelii, which causes erythema migrans lesions that are less rapidly progressive and have less evidence of inflammatory response than those caused by B burgdorferi sensu stricto or B garinii. (Strle et al., 1999) They are also less likely to have extracutaneous manifestations, but can cause acrodermatitis chronica atrophicans, an indolent gradually progressive skin condition which may persist for years if left untreated, and occasional cases of neuroborreliosis.
Epidemiology of Lyme Borreliosis in Europe
There is no centralised reporting or surveillance system for Lyme borreliosis or tick-borne encephalitis in Europe. A ECDC-funded initiative is underway to collate all currently available data on Lyme borreliosis and will report during 2011. It aims to provide a pan-European assessment of the epidemiological patterns, laboratory diagnostic and reporting criteria and the overall impact of Lyme borreliosis on human populations throughout
the EU and EFTA countries. Data is also being sought from the current EU Candidate countries and from a number of European Neighbourhood Policy countries. A similar programme is underway for tick-borne encephalitis.
Epidemiological evidence for Lyme borreliosis is available piecemeal from numerous sources, including national or regional mandatory notification schemes in a few countries, surveillance schemes in some endemic regions, primary care surveys, seroprevalence studies and reporting systems based on laboratory-confirmed cases. About 85,000 cases are reported annually in Europe but this is a considerable underestimate, both because of inconsistent case reporting mechanisms and under-recognition of disease manifestations, particularly erythema migrans (Lindgren and Jaenson, 2006). In 2002 it was estimated that at least 60,000 cases are likely to occur annually in Germany alone, giving an approximate incidence rate of 75/100,000 in that country (Mehnert and Krause, 2005). A more recent study estimated the incidence in Germany as about 32/100,000 in 2009 (Poggensee and Adlhoch, 2010). Reviewing data from various sources it is likely that there are over 200,000 cases annually in Europe, with a bimodal age incidence, peaking in the 5-15 and 45-65 age groups.
Overall national figures have only limited value, especially in the larger, more industrialised countries where most of the population is urban-dwelling, as they do not indicate regional and sub-regional variations in risk, which can be very marked. Regional and local data analysis is important for the appropriate targeting of public health and clinical interventions.
Mandatory Notification Schemes
Few countries have mandatory notification schemes for Lyme borreliosis. Erythema migrans and other manifestations of the disease are mandatorily notifiable in Slovenia, with a reported incidence rate in 2005 of 206/100,000 (Smith and Takkinen, 2006) and 312/100,000 in 2009. Notifications are incomplete, especially for erythema migrans, but data related to disseminated and late complications are more accurate because most Slovenian patients with these presentations are managed within a few research-orientated institutions. Neuroborreliosis has been notifiable in Denmark since 1994; with an annual average of 83 cases (1.5/100,000), ranging from 41 in 3002 to 104 in 2006 (Christiansen and Mølbak, 2005) and 61 cases (1.1/100,000) in 2009 (EpiNorthData, 2011). Cases of dis-. Cases of disseminated and late borreliosis have been notifiable in Norway since 1995. Annual incidence of neuroborreliosis varied from 75 to 200 cases in the ten years 1995-2004 (average 3/100,000), with a marked increase of nearly 100 cases between 2003 and 2004 (Nygard et al., 2005). There were 273 notifications in 2009, a rate of 5.6 /100,000 (EpiNorthData, 2011). As
neurological complications are the most significant manifestations of disseminated and late Lyme borreliosis in Europe data on neuroborreliosis obtained from the Slovenian, Danish and Norwegian notification schemes can give useful information on epidemiological trends in widely geographically separated areas of Europe. Pan-European monitoring methods for neuroborreliosis would be a welcome epidemiological initiative.
Regional Clinical Surveillance and Prospective Studies
In some countries case surveillance is regionally focussed on areas of known high endemicity, e.g. Alsace and Limousin in France and in six eastern states of Germany (La maladie de Lyme. Données du réseau de surveillance de la maladie en Alsace, Institut Veille Sanitaire, 2008; Fülöp and Poggensee, 2008; Mehnert and Krause, 2005). A French national primary care-based prospective study estimated an overall national incidence rate of 9.4/100,000, (Letrilliart et al., 2005), whereas data from the Alsace study suggested a regional rate of 180-232/100,000, which varied from 30 to 511/100,000 between individual cantons in the region. Erythema migrans was the only manifestation of disease in 90% of the cases; a further 5% had evidence of neuroborreliosis. Similar detailed and useful study reports are available for several other regions of France from L’Institut Veille Sanitaire. A prospective study performed in the Wurzburg region of Germany in 1996 followed an extensive awareness campaign and reported an incidence rate of 111/100,000 (313 cases). Erythema migrans was the only manifestation in 89% of cases. (Huppertz et al., 1999) It is notable that in these and other recent prospective studies erythema migrans was the presenting feature in around 90% of cases. In an earlier primary care-based prospective study performed in endemic counties of southern Sweden in 1992-1993 the overall annual incidence was 69/100,000 (1471 cases) and ranged focally from 26 to 160/100,000 (Berglund et al., 1995). Erythema migrans was the presenting feature in 77% of patients; 16% had neuroborreliosis and 7% had arthritis. Prospective community-based studies can provide longer-term benefits in addition to their epidemiological value, through raising awareness of the condition, its clinical features, management and prevention within primary and secondary care health care providers and the general community.
Laboratory-Based Surveillance
Some countries use laboratory-based surveillance, and erythema migrans cases are certainly under-reported in these schemes. Variability in test requesting patterns and diagnostic methods limit the validity of direct comparisons of laboratory-based surveillance findings between countries.
Nevertheless, some useful demographic, geographic and seasonality data can be obtained and year-on-year data compared in stable data collection systems, especially in schemes that approach referring clinicians and patients for additional clinical and tick exposure risk information such as the enhanced surveillance system in England and Wales, where the great majority of specialised laboratory tests for Lyme borreliosis are performed in a single reference facility. Annual incidence of laboratory-confirmed cases rose from 268 (0.5/100,000) in 2001 to 973 (1.79/100,000) in 2009, with a rate of 15/100,000 in one focal area. At least 18% of reported cases in 2009 had been acquired in other countries. Neuroborreliosis accounts for between 10% and 20% of laboratory-confirmed cases each year and appears to be a useful sentinel for year-on-year comparison. It has been estimated that there may be 2,000-3,000 cases of Lyme borreliosis annually in the UK (Health Protection Agency, 2011).
Seroprevalence studies have been performed in many parts of Europe. Population groups studied include healthy blood donors and people whose residence, occupation or recreational interests place them at higher risk of acquiring Borrelia burgdorferi infection. The overall picture shows a trend of increasing seroprevalence from west to east in Europe, which is consistent with findings from prospective studies and other surveillance methods. The findings from some of these studies and the prospective studies also suggest significant incidence of asymptomatic infections.
Clinical Presentations of Lyme Borreliosis in Europe
Clinical case definitions for use in Europe were published in 1996 by the European Union Concerted Action on Lyme Borreliosis (EUCALB, 2010; Stanek et al., 1996). A recent review by the EUCALB group has affirmed the robust nature of the 1996 definitions, as more recently published evidence has necessitated only minor additions to the definitions, which were updated in 2010 (Stanek et al., 2011). The 1996 case definitions have been cited in various diagnosis and treatment guidelines and recommendations from European specialist societies and national groups, which were summarised in a presentation at the 2010 European Conference on Clinical Microbiology and Infectious Diseases (ECCMID) (O’Connell, 2010).
The EUCALB case definitions acknowledge similarities between the major manifestations of Lyme borreliosis and North America, including erythema migrans, early neuroborreliosis and Lyme arthritis. They also recognise the broader spectrum of clinical presentations seen in Europe, eg borrelial lymphocytoma, acrodermatitis chronica atrophicans and late encephalomyelitis, all of which are rarely reported in association with American-acquired infections. The 2011 case definitions also describe rare ocular manifestations, including conjunctivitis, uveitis and papillitis and
discuss objective and subjective long-term sequelae of Borrelia burgdorferi infection. They describe the requirement for laboratory supporting evidence for the diagnosis of all manifestations other than erythema migrans. A brief resume of the principal features is given here.
Erythema Migrans in Europe
The variety of pathogenic borrelial genospecies in Europe can cause some variation in presentations of erythema migrans. For example, a rash caused by B afzelii usually expands more slowly and is more likely to have central clearing than one caused by B burgdorferi sensu stricto, and less likely to be accompanied by significant systemic symptoms (Strle et al., 1999). Erythema migrans caused by B garinii is usually more homogeneous than that caused by B afzelii, and it is more frequently accompanied by systemic symptoms. Overall the clinical picture of B garinii infection suggests greater acute pathogenicity than caused by B afzelii.
Other Skin Manifestations of European Lyme Borreliosis
Borrelial lymphocytoma is an uncommon early manifestation, presenting as a bluish-red nodule or plaque, usually on the earlobe, ear helix, nipple or scrotum, occurring more frequently in children than adults. It has a distinctive histological appearance, with an intense B-lymphocytic infiltrate and has occasionally been misdiagnosed as cutaneous B-cell lymphoma.
Acrodermatitis chronica atrophicans (ACA) is an uncommon later manifestation of active infection, which is usually seen in older adults, predominantly women. It presents with bluish-red discolouration, usually on the extensor surfaces of one or more limbs. There can be doughy swelling and atrophic changes developing later. Local involvement of peripheral nerves can cause an axonal polyneuropathy, usually presenting with predominantly mild sensory symptoms. B afzelii causes the great majority of ACA presentations, which occur more frequently in Scandinavia and central Europe than in the west of the continent.
Neuroborreliosis in Europe
Neuroborreliosis is the most common complication of European Lyme borreliosis and most cases appear to be caused by B garinii, which is the most neurotropic of the pathogenic genospecies. The European Federation of Neurological Societies (EFNS) recently published guidelines for diagnosis and treatment, giving detailed descriptions of presentations in adults and children (Mygland et al., 2010). About 95% of European neuroborreliosis cases present acutely, usually within twelve weeks of infection, and early
neuroborreliosis is often self-limiting. The most common manifestation in adults is a painful meningoradiculitis (Garin-Bujadoux-Bannwarth syndrome). Pain may be very severe and paresis can affect muscles innervated by the facial (unilateral or bilateral) or other cranial nerves and those of the trunk and limbs. In children the most common presentations of acute neuroborreliosis are facial palsy, which may be an isolated clinical feature, other cranial nerve palsies and lymphocytic meningitis, and headache can be a prominent feature. Painful radiculopathy is very uncommon in children.
Although the differences between presentations of European and American Lyme neuroborreliosis have been stressed over the years, they may have been overemphasised in the case of early neuroborreliosis (Halperin, 2008). This is also supported by clinical experience in the UK, where between 10 and 20% of patients with serologically confirmed Lyme borreliosis acquired infections abroad, in mainland Europe or USA (HPA, 2011). Clinicians in the UK have noted marked similarities in acute neurological presentations of patients with USA-acquired infection and those acquired in the UK and other parts of Europe (Dillon et al., 2010).
Less than 5% of European neuroborreliosis patients present with late neuroborreliosis, with duration of symptoms from six months to several years (Mygland et al., 2010). This condition is likely to have a chronic course if left untreated and can affect the central and peripheral nervous systems.
Central nervous system manifestations of late neuroborreliosis include encephalitis or encephalomyelitis with tetraspastic syndrome, spastic-ataxic gait disorder and disturbed micturition, which may lead to misdiagnosis with other conditions such as multiple sclerosis if the possibility of neuroborreliosis is overlooked. Clinical awareness of this possibility is crucial, as antibiotic treatment will arrest progression. The degree of clinical recovery following microbiological cure depends on the severity of tissue damage. Recovery may be slow, especially in older patients, and can be incomplete, particularly in those who had been severely affected prior to treatment.
Peripheral nervous system manifestations include radiculopathy and mononeuropathy. Occasional patients, mainly in the older age groups, present with radiculopathy of gradual onset, progressing over many months and resulting in severe debilitating pain. This most commonly affects a lower limb and can be misdiagnosed as nerve entrapment conditions such as sciatica. The patient may not be aware of, or may have forgotten an earlier tick bite or erythema migrans. It is important that clinicians are aware of this condition, as antibiotic treatment usually brings rapid reduction in pain. It has been suggested that this more slowly evolving manifestation of radiculopathy may be related to direct spread of borreliae from the inoculation site along nerves to the nerve roots (Rupprecht et al.,
2008). A polyneuropathy can also occur in association with acrodermatitis chronica atrophicans, which is an uncommon late manifestation of cutaneous B afzelii infection.
Lyme Arthritis in Europe
Lyme arthritis is less prominent a feature of Lyme borreliosis in Europe than in the USA although myalgias and arthralgias frequently occur in early disease. Borrelia burgdorferi sensu stricto, which is less prevalent in Europe than B afzelii or B garinii, appears to be the predominant cause of Lyme arthritis, which occurs most frequently in areas of Europe where this genospecies is most prevalent. The clinical and laboratory findings and outcomes are similar to those seen in North American-acquired infection, where it is caused exclusively by the same genospecies.
Diagnostic Tests for Lyme Borreliosis in Europe
The EUCALB case definitions, EFNS guidelines for neuroborreliosis and other European guidelines and consensus documents recommend that laboratory support should be sought for the clinical diagnosis of all manifestations of Lyme borreliosis other than erythema migrans, as clinical features of later stage presentations are not unique to Borrelia burgdorferi infection. (Stanek et al., 2011; Mygland et al., 2010; O’Connell, 2010). In all cases the clinical presentation and tick exposure risk should be carefully evaluated and tests performed only on patients in whom there is a significant likelihood of Lyme borreliosis, i.e., the pre-test likelihood of infection should be evaluated. In recent years there has been a tendency for “tests for Lyme disease” to be included as part of a broad serological investigation panel for patients with a wide range of clinical presentations, without adequate consideration of its appropriateness in the individual patient’s case. Indiscriminate testing without significant clinical indications can lead to misleading results, as the positive predictive value in such circumstances is low.
The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) published guidelines for the laboratory diagnosis of bacterial tick borne diseases in Europe, including Lyme borreliosis, in 2004 (Brouqui et al., 2004). The German Society of Hygiene published recommendations for test use and performance in 2000 (Wilske et al., 2000). These have been widely used in Europe. Testing for Lyme borreliosis in Europe as recommended by these authorities has many similarities to standard practices recommended in North America and a recent publication gives an excellent overview of the issues in European and American infections (Wang et al., 2010).
Antibody detection is the most widely available and useful method and
there have been significant improvements in both sensitivity and specificity of tests in recent years, particularly with developments in recombinant antigens derived from the major pathogenic genospecies. Direct testing methods using culture or DNA detection have more limited practical value, similar to the situation in North America (Aguero-Rosenfeld et al., 2005; Wilske et al., 2007).
The greater heterogeneity of pathogenic genospecies in Europe must be considered when evaluating test methods. In the case of DNA detection, borrelial DNA targets should be capable of detecting all pathogenic genospecies. A variety of target sequences are currently used in Europe, including those based on OspA, flagellin, 16s RNA and 5S-23S rRNA intergenic spacer region. Sensitivity of the method is similar to that of culture on tissues (about 70% overall for erythema migrans and as high as 90% for ACA). In neuroborreliosis only about 10-30% of DNA detection tests on CSF are positive, and highest rates are obtained on samples taken within the first two weeks of a clinical presentation. It is considerably more sensitive than culture for synovial tissue and fluid, for which culture has rarely been successful (Wilske et al., 2007). Borrelial DNA detection in blood culture samples from European erythema migrans patients has a lower yield than those taken in American-acquired infections, most likely because B afzelii, the most common infecting organism, has a lower frequency of haematogenous dissemination than B burgdorferi sensu stricto and because smaller sample volumes have been used in European studies.
Several factors are significant in relation to antibody testing for European Lyme borreliosis. These include genospecies variation and also variations within genospecies; heterogeneity of immunodominant epitopes, speed of immune response development to individual infecting genospecies and duration of infection prior to testing. Generally, Borrelia burgdorferi sensu stricto seems to cause the most acute infection presentations of the three major infecting genospecies, and immune response development is brisker than that seen in most B afzelii infections, which have slower development of rash, and lower incidence of significant systemic symptoms. The immune response to early B garinii infection also seems to be detectable earlier than that of B afzelii in many cases.
Patients with prolonged infection prior to antibody testing usually exhibit a broad expansion of immune response. Patients with ACA and late neuroborreliosis are usually strongly seropositive, with reactions on IgG immunoblot to many borrelial antigens, similar to findings in European and American patients with well-established Lyme arthritis. In response to concerns regarding seronegativity in patients with late stage infection the EUCALB case definition revision group reviewed published case reports of suspected seronegative late Lyme borreliosis. They concluded: “The diagnosis of so called ‘seronegative chronic Lyme disease’ in supposed
long-standing infections is highly unsatisfactory, requiring further clinical and laboratory investigations Seronegative late LB, if it occurs at all, is extremely rare and there have been only two reported cases of apparently seronegative ACA and one of seronegative Lyme arthritis in immunocompetent patients. There are no reliable reports of seronegative late-stage Lyme neuroborreliosis” (Stanek et al., 2011).
Most European countries follow a two-tier antibody testing approach, similar to the American system, with a first-stage test using a sensitive screening immunoassay and second-tier test to assess specificity, usually immunoblot. There is increasing interest in using more highly specific immunoassays such as those based on C6 synthetic peptide or recombinant VlsE antigens rather than immunoblots as second tests (Nyman et al., 2006). Assessments of this simpler approach are underway in several European centres. It would be a significant gain if this approach were found to be equivalent or superior to the traditional second-tier immunoblot system, as it involves less complex laboratory procedures and interpretation is objective, although immunoblots would still be necessary in some situations. Development of a highly sensitive and specific single tier system remains the ultimate aim.
Developments in antibody test formats, incorporating recombinant antigens (including homologous proteins from different genospecies) in immunoassays and immunoblots have increased the sensitivity of new-generation tests. These are now widely used in European laboratories and some are available on automated test platforms. Some specificity problems remain, particularly with IgM tests, including immunoblots, and false-positive IgM results frequently lead to misdiagnosis if the results are not critically evaluated in the light of the patient’s clinical presentation. Generally, IgM test use should be restricted to patients with short duration of illness and later follow-up samples tested if there is diagnostic uncertainty as to the specificity of an IgM result.
European criteria for IgG immunoblot positivity require fewer reactions to be present than the CDC criteria (i.e., two or three out of eight to ten candidate bands compared to five of ten in the CDC criteria) but European candidate bands exclude someless specific antigens such as p41 and p60 that are included in the CDC candidates. The European criteria also reflect the slightly slower evolution of antibody response generally seen in European infections. Experts emphasise the need for strict attention to performance and interpretation of reaction (cut-off) controls, to avoid inappropriate scoring of very weak non-specific reactions, which is a frequent cause of false-positive immunoblots and potential misdiagnosis. High background seropositivity (between 5% and 20% in many European endemic regions) can also cause confusion and potential misdiagnosis if the clinical significance of a result is
not carefully assessed in the circumstances of the patient’s history and clinical findings (Stanek et al., 2011; Wilske et al., 2007).
The formal diagnosis of neuroborreliosis in Europe requires CSF evaluation, including white cell count and assessment of intrathecal antibody synthesis, including CSF/serum antibody index, although in practice many clinicians do not perform CSF sampling routinely in patients whose history and clinical examination are strongly indicative of neuroborreliosis and have positive serum antibody tests. In very acute presentations of neuroborreliosis some patients may have antibodies in CSF before seroconversion in peripheral blood (Mygland et al., 2010; Stanek et al., 2011). Lymphocytic pleiocytosis is almost always present in both early and late neuroborreliosis and many patients have raised protein and oligoclonal IgG bands. Patients with ACA-associated neuropathy often have normal CSF as this is essentially a localised peripheral manifestation.
Tests That Are Not Recommended for Diagnosis of Lyme Borreliosis in Europe
The EUCALB case definitions, the EFNS guidelines and numerous other European guidelines and consensus documents do not recommend certain tests that have been marketed as Lyme-diagnostic tests. These include live microscopy of blood, urinary borrelial antigen or PCR tests, unvalidated antibody test methods, immunoblots interpreted using poorly specific criteria, lymphocyte transformation (LTT) tests and CD57 lymphocyte subpopulation typing, as they lack specificity (Duerden, 2006; Duerden et al., 2010; Mygland et al., 2010; Stanek et al., 2011; Wilske et al., 2007).
Outcome Data of Treated Infections
Several recent publications have reviewed outcome data in adults and children treated for various manifestations of Lyme borreliosis. A Norwegian population-based study prospectively enrolled all children in with suspected neuroborreliosis between 1996 and 2006 (Oymar and Tveitnes, 2009). All 143 children received antibiotic treatment (mainly two weeks of ceftriaxone). Following treatment four children had minor residual facial palsy; the remainder had recovered completely. This valuable paper gives an excellent illustration of clinical presentations of paediatric neuroborreliosis and the associated laboratory findings, with high rates of seropositivity and CSF pleiocytosis at presentation.
A recent Swedish prospective paediatric neuroborreliosis study of 177 children also enrolled a healthy control group (Skogman et al., 2008). Outcomes were evaluated at six months after treatment and were good, with no evidence of progressive or recurrent abnormalities. About 10%
of the children had some residual facial weakness, but no other objective findings were present. Non-specific symptoms such as headache and fatigue were reported less frequently by patients than controls. Antibiotic choice (doxycycline or ceftriaxone) did not affect outcomes.
A Slovenian prospective study comparing outcomes of treatment with doxycycline or cefuroxime axetil for erythema migrans in 285 adults also enrolled a healthy control group. Outcomes were good, with no significant differences between the treatment groups, and the incidence of non-specific symptoms at six and twelve months follow-up did not exceed those of the control group. Both of these studies illustrate a significant rate of nonspecific symptoms such as headache and fatigue in non-infected healthy control populations.
A Norwegian prospective double-blind study compared outcomes of oral doxycycline or parenteral ceftriaxone treatment in adults with neuroborreliosis (Ljøstad and Mygland, 2010). Patients with early neuroborreliosis, defined as pre-treatment duration of less than six months, were followed up for one year. Out of 85 patients 41 had remaining complaints (14 with objective findings, 27 with subjective symptoms). Remaining complaints were associated with longer (>6 weeks) pre-treatment duration, higher CSF cell count and female gender. Objective findings, but not subjective symptoms, were associated with pre-treatment duration of >6 weeks, underlying the importance of early diagnosis and treatment. There were no differences in outcomes between the antibiotic treatment groups.
Persisting Symptoms Following Treated Lyme Borreliosis
Further work is required to understand the incidence, causes and best management of persisting symptoms following appropriately treated infection. The two European trials described above that incorporated healthy non-infected controls showed significant incidence of non-specific symptoms in the control groups, and it would be helpful if further studies on patients with a broader range of Lyme borreliosis presentations incorporated healthy control subjects, in order to provide a better assessment of the true incidence of post-treatment non-specific symptoms that are attributable to Lyme borreliosis.
Persisting symptoms are well-documented following other systemic infections, and risk seems to correlate with severity of symptoms during the acute events (Hickie et al., 2006). Studies in patients with continuing symptoms following Lyme borreliosis have not shown evidence of persisting infection nor of sustained benefit from extended antibiotic treatment. Further research is required to review possible causes including immunological mechanisms. A recent publication of a study on samples from patients with persisting symptoms provided some intriguing data on heightened reactivity
of anti-neural antibodies in patients with persisting symptoms compared to healthy post-Lyme borreliosis healthy and normal healthy controls, suggesting the possibility of a differential immune system response in post-Lyme-syndrome patients (Chandra et al., 2010).
Nonstandard Medical Practices in Europe Associated with Lyme Borreliosis
Some European patients have been diagnosed with Lyme borreliosis or chronic Lyme disease on the basis of poorly specific clinical criteria and non-standard laboratory tests, including live blood microscopy, lymphocyte transformation tests or inadequately validated antibody tests including unorthodox immunoblot criteria (Duerden, 2006; Duerden et al., 2010; Stanek et al., 2011). False-positive IgM tests (including immunoblots) appear to be a particularly significant problem leading to misdiagnosis. Unorthodox treatment modalities include multiple or very prolonged courses of oral or parenteral antibiotics and parasitic agents. Some patients have received other agents including arsenicals. Misdiagnosis and inappropriate treatment can cause significant harm to patients, both from potential adverse effects and loss of opportunity for correct diagnosis and appropriate management.
Prevention of Lyme Borreliosis and Other Tick-Transmitted Infections
No vaccine for Lyme borreliosis is currently available in Europe and none is likely to be available in the near future. Antibiotic prophylaxis following tick bites is not routinely recommended, although some European guidelines and consensus documents suggest post-exposure antibiotics could be used under certain very restricted circumstances, for example, in immunodeficient individuals (SPILF, 2007).
An effective vaccine is available for tick-borne encephalitis. A very active immunisation and tick awareness programme in Austria resulted in a marked decline in TBE incidence from a peak of 677 cases in 1979, just prior to the vaccine’s introduction, to 41 in 1999 (WHO, 2004). The vaccine is recommended for residents of TBE-endemic regions throughout Europe and for visitors whose outdoor activities expose them to risk of tick bite. It is now widely promoted in travellers’ health clinics and through outdoor-recreation interest groups and media outlets. The vaccine’s efficacy should not be allowed to distract users from the continuing need for tick bite avoidance strategies, particularly to prevent Lyme borreliosis, which is far more prevalent and widespread in distribution than TBE in Europe.
Primary prevention of tick-borne infections entails awareness of ticks and their potential for transmitting a variety of infections, most commonly
Lyme borreliosis, so public education is an important measure. Many countries’ public health authorities and special interest groups such as sporting associations and voluntary groups have annual publicity campaigns, often timed to coincide with the start of the tick-feeding season. Raising health professionals’ awareness of tickborne infections, their prevention, recognition and management is essential to minimise risk of missed diagnosis or inadequate treatment. ECDC recently issued an educational toolkit on tickborne diseases, with modules for adults, children and healthcare professionals. The toolkit is designed to be modifiable by national public health authorities as appropriate for local circumstances (ECDC, 2010).
People should avoid tick infested areas if possible, but if this is not practicable they should take personal measures to reduce tick bite risk. These include minimising the amount of exposed skin, using DEET-containing insect repellents and frequently checking for attached ticks. People such as forestry workers who have frequent and potentially heavy contact with ticks should consider wearing permethrin-treated clothing.
Lyme borreliosis is unlikely to be transmitted within the first hours of a blood meal, so early removal of attached ticks is a valuable protection measure. There is some experimental evidence in animals to suggest that Borrelia afzelii can be transmitted at a relatively early stage of an I ricinus feed, with a steadily rising risk from about 24 hours of attachment (Crippa et al., 2002; Kahl et al., 1998). Although this differs from the North American situation, where there is a longer lag phase before Ixodes scapularis transmits Borrelia burgdorferi sensu stricto, a thorough search for attached ticks at the end of each day in a tick-infested area remains a very valuable protective measure against Lyme borreliosis in Europe.
Environmental aspects of tickborne disease prevention have been considered by a number of European authorities, including the European Centre for Disease Control and the World Health Organization Regional Office for Europe (ECDC, 2007; Lindgren and Jaenson, 2006). Possible measures included widespread use of acaricides, removal of deer populations and controlled burning of tick-permissive vegetation. None are regarded as feasible or acceptable for large-scale use. Modification of local vegetation by landscaping and removal of leaf litter and undergrowth in gardens and parks may be helpful in reducing tick and host animal abundance in residential settings. Personal protection against tick bites remains the most important measure.
Health Promotion in Relation to Tick-Borne Infections; Presentation of Evidence-Based Medicine and Science to Patients, Support Groups, and the Wider Public
The ECDC educational toolkit is a welcome initiative, particularly if it is taken up by national and regional public health authorities or stimulates more locally based activities, especially in populations with low awareness of ticks. A UK Rural Economy and Land Use (RELU) multidisciplinary research project included a study of educational needs of residents, workers and visitors for prevention of tick-borne infection in Lyme-endemic areas that are heavily used for recreational purposes. It also surveyed Lyme borreliosis awareness and knowledge amongst Lyme borreliosis patients and health professionals in urban and rural practice. The project is due to report in 2011. Preliminary feedback has been useful in assessing the differing educational needs of diverse groups and was presented at the Health Protection Agency Conference 2010 (Marcu et al., 2010).
Much information on tickborne infections available from media sources, including the Internet is of variable quality, ranging from highly accurate, valuable content for raising awareness and disease prevention, to poor quality and misleading, a recent example being the promotion of Lyme borreliosis as an inducer of autism. There is a need to develop methods of presenting the best scientific evidence on conditions such as Lyme borreliosis to the general public, reaching out in ways that are accessible but not condescending to readers and viewers who do not have a scientific background. An important example causing misunderstanding is a frequently-quoted statement that “tests for Lyme are highly inaccurate,” alluding to statistics for antibody positivity in early infection, but implying that these figures are correct for all stages of disease. The work of organisations such as Sense About Science, building understanding and trust between scientists, clinicians and the media and public may be useful in helping to model new approaches to this important aspect of tickborne diseases (Sense About Science, 2008 I’ve Got Nothing to Lose by Trying It).
A proactive approach has already been taken by the ALSUntangled group of clinician/scientists, an international scientific effort to help people with amyotrophic lateral sclerosis investigate alternative and off-label therapies. They reviewed claims of a causal link between ALS and Lyme borreliosis and published a report on Lyme disease testing and treatment in 2009, concluding that there was no convincing evidence to support such a link (ALSUntangled Update 1: Investigating a bug (Lyme Disease) and a drug (Iplex) on behalf of people with ALS, 2009).
Possible Directions for Future Research Related to Lyme Borreliosis and Other Tick-Transmitted Infections
Many further developments are required in the broad range of basic sciences associated with tickborne diseases, including biology of ticks, feeding hosts, infecting agents and ecosystems, in addition to greater understanding of human disease processes. Many areas of basic research are beyond the scope of this paper and are addressed by others, but some important issues already discussed here can be summarised.
Ecology and Epidemiology in Europe
-
Multidisciplinary work on ecological changes affecting tick populations and their distribution, reservoir hosts and human interaction with the environment is ongoing and greater co-ordination of effort should be encouraged.
-
More systematic epidemiological data collection on tickborne diseases is necessary and preliminary work funded by ECDC, due to report in 2011, should lay a firm base for future improvements.
Diagnostic Tests for Lyme Borreliosis
-
Diagnostic tests have improved significantly, particularly through developments in recombinant and synthetic peptide based antigens, but testing algorithms and the two-tier testing approach have not been reviewed to take account of these changes. There is an urgent need to for a Europe-wide (and inter-continental) assessment, with a view to minimising the need for immunoblot tests. The experience of Scandinavian workers would be particularly helpful, as immunoblots are less widely used in that region, without apparent harm. The value of currently available IgM tests should be carefully scrutinised, as experience of many laboratory workers and clinicians suggests that their potential for misleading results may outweigh their benefit.
-
Further developments in antibody tests, aiming for increased sensitivity without loss of specificity would be most welcome, although this may be difficult to achieve because of the relatively slow development of antibody response to B burgdorferi by comparison to many other infectious agents.
-
A reference repository of large volumes of sera with well-defined clinical provenance should be created, for use in development and evaluation of new diagnostic tests and to allow comparison with currently available laboratory assays.
-
Research into development of laboratory markers of response to treatment would be valuable.
-
There is an urgent need for educational efforts to encourage clinicians in the appropriate use of laboratory tests, particularly in the assessment of pre-test probability of disease likelihoodand predic- predictive values of test results.
-
Diagnostic tests for other tickborne infections should be developed further.
Persisting Symptoms Following Treatment of Lyme Borreliosis
-
Further research is urgently required into the incidence and possible mechanisms of persistent post-infection symptoms, which can occur following many systemic infections, including Lyme borreliosis. Lyme borreliosis could be a useful model for studying mechanisms of post-infection syndromes, from a host immune response perspective and other host factors as well as pathogen aspects. This could be a focus for international research collaboration.
-
Development of optimal management strategies for patients affected by persisting symptoms following infections should be a priority.
Prevention
-
Vaccine development against Lyme borreliosis in Europe is an active area of research.
-
A broader approach to education about ticks, infection risks and tick bite prevention should be encouraged.
Communication Issues
-
Further multidisciplinary work is urgently required in this area, as outlined above. This should include patients, support groups and members of the general public in addition to the wide range of professionals working in the field of Lyme borreliosis and other tickborne infections. The diverse needs of different communities should be taken into consideration in assessing needs.
Acknowledgements
I am most grateful to Mr Derek V Nudd, Dr Robert MM Smith, Mrs Anne Southwell, Dr Peter R Hawtin and Dr Adriana Basarab for their valuable and constructive comments and support; to Professor Jeremy Gray for his advice on aspects of tick ecology and biology and to Professor Gray and Mr Bernard Kaye of University College Dublin for their generosity in allowing me to include their illustrations.
References
Aguero-Rosenfeld, Maria E., Guiqing Wang, Ira Schwartz, and Gary P. Wormser. 2005. Diagnosis of Lyme Borreliosis. Clin. Microbiol. Rev. 18 (3):484-509.
ALSUntangled Update 1: Investigating a bug (Lyme Disease) and a drug (Iplex) on behalf of people with ALS. 2009. Amyotrophic Lateral Sclerosis 10 (4):248-250.
Angelakis, E., S. A. Billeter, E. B. Breitschwerdt, B. B. Chomel, and D. Raoult. Potential for Tick-borne Bartonelloses. Emerg Infect Dis 16 (3):385-91.
Berglund, Johan, Rickard Eitrem, Katharina Ornstein, Anders Lindberg, Åke Ringnér, Henrik Elmrud, Mikael Carlsson, Arne Runehagen, Catarina Svanborg, and Ragnar Norrby. 1995. An epidemiologic study of Lyme disease in southern Sweden. New England Journal of Medicine 333 (20):1319-1324.
Bown, K. J., X. Lambin, N. H. Ogden, M. Begon, G. Telford, Z. Woldehiwet, and R. J. Birtles. 2009. Delineating Anaplasma phagocytophilum ecotypes in coexisting, discrete enzootic cycles. Emerg Infect Dis 15 (12):1948-54.
Brouqui, P., F. Bacellar, G. Baranton, R. J. Birtles, A. Bjoersdorff, J. R. Blanco, G. Caruso, M. Cinco, P. E. Fournier, E. Francavilla, M. Jensenius, J. Kazar, H. Laferl, A. Lakos, S. Lotric Furlan, M. Maurin, J. A. Oteo, P. Parola, C. Perez-Eid, O. Peter, D. Postic, D. Raoult, A. Tellez, Y. Tselentis, and B. Wilske. 2004. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect 10 (12):1108-32.
Chandra, A., G. P. Wormser, M. S. Klempner, R. P. Trevino, M. K. Crow, N. Latov, and A. Alaedini. 2010. Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun 24 (6):1018-24.
Christiansen, A. H., and K. Mølbak. 2005. Neuroborreliosis 1994-2004. Statens Serum Institut Report (33):1.
Crippa, M., O. Rais, and L. Gern. 2002. Investigations on the mode and dynamics of transmission and infectivity of Borrelia burgdorferi sensu stricto and Borrelia afzelii in Ixodes ricinus ticks. Vector Borne Zoonotic Dis 2 (1):3-9.
Dillon, R., O’Connell S, and Wright S. 2010. Lyme disease in the UK: clinical and laboratory features and response to treatment. Clin Med 10 (5):454-457.
Duerden, B. I. 2006. Unorthodox and unvalidated laboratory tests in the diagnosis of Lyme Borreliosis and in relation to medically unexplained symptoms. Department of Health, 2006 [cited Oct 8 2010]. Available from http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4138917.pdf.
Duerden, B. I. (Chair) on behalf of the Independent Review Panel. 2010. Independent appraisal and review of the ILADS 2004 “Evidence-based guidelines for the management of Lyme disease”. Health Protection Agency. Available from http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1294739293177 (accessed 16th March 2011)
ECDC. 2007. Meeting Report, Workshop: Environmental Change and Infectious Disease 2007 [accessed Oct 8 2010]. Available from http://www.ecdc.europa.eu/en/publications/Publications/0703_MER_Environmental_Change_and_Infectious_Disease.pdf.
———. 2010. Spotlight: Tick-borne diseases 2010 [accessed Oct 8 2010]. Available from http://www.ecdc.europa.eu/en/healthtopics/spotlight/spotlight_tickborne/Pages/home.aspx.
EpiNorthData. 2011. http://www.epinorth.org/eway/default.aspx?pid=230&trg=Area_5279&MainArea_5260=5279:0:15,2937:1:0:0:::0:0&Area_5279=5291:44530::1:5290:1:::0:0&diseaseid=20 Accessed March 16 2011
EUCALB. 2010. European Union Concerted Action on Lyme Borreliosis - Spirochaete, reservoir hosts 2010 [cited Oct 8 2010]. Available from http://meduni09.edis.at/eucalb/cms/index.php?option=com_content&task=view&id=59&Itemid=93.
Fülöp, Balazs, and Gabriele Poggensee. 2008. Epidemiological situation of Lyme borreliosis in Germany: Surveillance data from six Eastern German States, 2002 to 2006. Parasitology Research 103 (0):117-120.
Gray, J. S. 1991. The development and seasonal activity of the tick Ixodes ricinus: a vector of Lyme borreliosis. Review of Medical and Veterinary Entomology 79 (6):323-333.
———. 1998. Review The ecology of ticks transmitting Lyme borreliosis. Experimental and Applied Acarology 22 (5):249-258.
Gray, J. S., H. Dautel, A. Estrada-Pena, O. Kahl, and E. Lindgren. 2009. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis 2009.
Gray, Jeremy, Annetta Zintl, Anke Hildebrandt, Klaus-Peter Hunfeld, and Louis Weiss. 2010. Zoonotic babesiosis: Overview of the disease and novel aspects of pathogen identity. Ticks and Tick-borne Diseases 1 (1):3-10.
Halperin, John J. 2008. Nervous System Lyme Disease. Infectious disease clinics of North America 22 (2):261-274.
Health Protection Agency. 2011. Lyme borreliosis: epidemiological data. Accessed March 16 2011 at: http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/LymeDisease/EpidemiologicalData/
Hickie, I., T. Davenport, D. Wakefield, U. Vollmer-Conna, B. Cameron, S. D. Vernon, W. C. Reeves, and A. Lloyd. 2006. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 333 (7568):575.
Huppertz, H. I., M. Bohme, S. M. Standaert, H. Karch, and S. A. Plotkin. 1999. Incidence of Lyme borreliosis in the Wurzburg region of Germany. Eur J Clin Microbiol Infect Dis 18 (10):697-703.
I’ve Got Nothing to Lose by Trying It. 2010. Sense about Science 2008 [Accessed March 16 2011]. Available from http://www.senseaboutscience.org.uk/pdf/I%27ve%20got%20nothing%20to%20lose%20by%20trying%20it%20FINAL.pdf.
Kahl, O., C. Janetzki-Mittmann, J. S. Gray, R. Jonas, J. Stein, and R. de Boer. 1998. Risk of infection with Borrelia burgdorferi sensu lato for a host in relation to the duration of nymphal Ixodes ricinus feeding and the method of tick removal. Zentralbl Bakteriol 287 (1-2):41-52.
Karan, L. S., N.A. Rudnikova, A.E. Platonov, and et al. 2007. Ixodes tick-borne borrelioses in Russia. In Abstract book of 5th International Conference on Emerging Zoonoses. Limassol, Cyprus.
Korenberg, E. I., L. Y. Gorban, Y. V. Kovalevskii, V. I. Frizen, and A. S. Karavanov. 2001. Risk for human tick-borne encephalitis, borrelioses, and double infection in the pre-Ural region of Russia. Emerg Infect Dis 7 (3):459-62.
La maladie de Lyme. Données du réseau de surveillance de la maladie en Alsace. 2010. Institut Veille Sanitaire 2008 [accessed September 11 2010]. Available from http://www.invs.sante.fr/publications/2005/lyme_alsace/index.html.
Letrilliart, L., B. Ragon, T. Hanslik, and A. Flahault. 2005. Lyme disease in France: a primary care-based prospective study. Epidemiol Infect 133 (5):935-42.
Lindgren, Elisabet, and Thomas G. T. Jaenson. 2006. Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. Copenhagen, Denmark: WHO Regional Office for Europe. http://www.euro.who.int/document/E89522.pdf.
Ljøstad, U., and Å Mygland. 2010. Remaining complaints 1 year after treatment for acute Lyme neuroborreliosis; frequency, pattern and risk factors. European Journal of Neurology 17 (1):118-123.
Marcu, Afrodita, Julie Barnett, David Uzzell, and Sue O’Connell. 2010. Lyme disease patients’ information needs and their preferences for future precautionary measures. In Health Protection Agency Conference. University of Warwick, Coventry, UK.
Mehnert, W. H., and G. Krause. 2005. Surveillance of Lyme borreliosis in Germany, 2002 and 2003. Euro Surveill 10 (4):83-5.
Mygland, A., U. Ljostad, V. Fingerle, T. Rupprecht, E. Schmutzhard, and I. Steiner. 2010. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 17 (1):8-16, e1-4.
Nygard, K., A. B. Brantsaeter, and R. Mehl. 2005. Disseminated and chronic Lyme borreliosis in Norway, 1995 - 2004. Euro Surveill 10 (10):235-8.
Nyman, D., L. Willen, C. Jansson, S. A. Carlsson, H. Granlund, and P. Wahlberg. 2006. VlsE C6 peptide and IgG ELISA antibody analysis for clinical diagnosis of Lyme borreliosis in an endemic area. Clin Microbiol Infect 12 (5):496-7.
O’Connell, S. 2010. Recommendations for the diagnosis and treatment of Lyme borreliosis: guidelines and consensus papers from specialist societies and expert groups in Europe and North America http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1287144781602 (Accessed 16th March 2011)
Oymar, K., and D. Tveitnes. 2009. Clinical characteristics of childhood Lyme neuroborreliosis in an endemic area of northern Europe. Scand J Infect Dis 41 (2):88-94.
Parola, P., B. Davoust, and D. Raoult. 2005. Tick- and flea-borne rickettsial emerging zoonoses.Vet Res 36 (3):469-92.
Parola, P., C. D. Paddock, and D. Raoult. 2005. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 18 (4):719-56.
Piesman, J., and T. G. Schwan. 2010. Ecology of borreliae and their vectors. In Borrelia, Molecular Biology, Host Interactions and Pathogenesis, edited by D. S. Samuels and J. D. Radolf. Norfolk, UK: Caister Academic Press.
Poggensee, G., and C. Adlhoch. 2010. Lyme-Borreliosis: ein Situationsbericht aus den sechs ostlichen Bundeslanden 2007-2009. UMID 2, 5-8.
Randolph, S. E. 2001. The shifting landscape of tick-borne zoonoses: tick-borne encephalitis and Lyme borreliosis in Europe. Philos Trans R Soc Lond B Biol Sci 356 (1411):1045-56.
Rauter, Carolin, and Thomas Hartung. 2005. Prevalence of Borrelia burgdorferi Sensu Lato Genospecies in Ixodes ricinus Ticks in Europe: a Metaanalysis. Appl. Environ. Microbiol. 71 (11):7203-7216.
Rupprecht, T. A., U. Koedel, V. Fingerle, and H. W. Pfister. 2008. The pathogenesis of lyme neuroborreliosis: from infection to inflammation. Mol Med 14 (3-4):205-12.
Sense About Science (UK Registered Charity 11101114) http://www.senseaboutscience.org.uk/index.php (Accessed 16th March 2011).
Skogman, Barbro Hedin, Stefan Croner, Maria Nordwall, Mattias Eknefelt, Jan Ernerudh, and Pia Forsberg. 2008. Lyme Neuroborreliosis in Children: A prospective study of clinical features, prognosis, and outcome. The Pediatric Infectious Disease Journal 27 (12):1089-1094 10.1097/INF.0b013e31817fd423.
Smith, R., and J. Takkinen. 2006. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Euro Surveill 11 (6):E060622 1.
SPILF. 2007. Borréliose de Lyme : démarches diagnostiques, thérapeutiques et préventives. Texte court. Médecine et Maladies Infectieuses 37 (4):187-193.
Stanek, G., V. Fingerle, K. P. Hunfeld, B. Jaulhac, R. Kaiser, A. Krause, W. Kristoferitsch, S. O’Connell, K. Ornstein, F. Strle, and J. Gray. 2011. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 17(1):69-79.
Stanek, G., S. O’Connell, M. Cimmino, E. Aberer, W. Kristoferitsch, M. Granstrom, E. Guy, and J. Gray. 1996. European Union Concerted Action on Risk Assessment in Lyme Borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr 108 (23):741-7.
Strle, F., R. B. Nadelman, J. Cimperman, J. Nowakowski, R. N. Picken, I. Schwartz, V. Maraspin, M. E. Aguero-Rosenfeld, S. Varde, S. Lotric-Furlan, and G. P. Wormser. 1999. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann Intern Med 130 (1):32-6.
Wang, G., M. E. Aguero-Rosenfeld, G.P. Wormser, and I. Schwarz. 2010. Detection of Borrelia burgdorferi. In Borrelia, Molecular Biology, Host Interactions and Pathogenesis, edited by D. S. Samuels and J. D. Radolf. Norfolk, UK: Caister Academic Press.
WHO. 2004. The vector-borne human infections of Europe: their distribution and burden on public health. WHO Regional Office for Europe 2004 [accessed October, 8 2010]. Available from http://www.euro.who.int/__data/assets/pdf_file/0008/98765/e82481.pdf.
Wilske, B., V. Fingerle, and U. Schulte-Spechtel. 2007. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol 49 (1):13-21.
Wilske, B., L Zöller, V. Brade, H. Eiffert, U.B. Gobel, G. Stanek, and H.W. Pfister. 2000. Lyme-Borreliosis. In MiQ: mikrobiologisch-infektiologische Qualitätstandards(MiQ); Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik, edited by H. Mauch and R. Lutticken. Munich: Urban und Fischer.
A8
THE TICK MICROBIOME: DIVERSITY, DISTRIBUTION, AND INFLUENCE OF THE INTERNAL MICROBIAL COMMUNITY FOR A BLOOD-FEEDING DISEASE VECTOR
Authors: Keith Clay and Clay Fuqua Department of Biology Indiana University
Abstract
Ticks are well established as important vectors for human disease, accounting for a growing number of zoonotic infections. Certain primary pathogens such as the Lyme disease agent, have received great attention. Less well understood is the overall microbial community that is harbored within ticks, in addition to human pathogens. A variety of powerful molecular detection approaches have revealed a constrained but significant microbial community associated with ticks, including vertically-transmitted symbionts, opportunistic pathogens, and more transient guest commensals, which include viruses, bacteria, protozoans, and fungi. Ticks join a growing number of arthropod and filarial systems in which microbial symbionts can have profound and extensive effects on the activity of their host and in certain cases, a direct impact on human disease. The recognized human pathogens are in fact vastly outnumbered by these other microorganisms,
and pathogens represent a relatively small fraction of the total microbial community in ticks. The tick-borne microbial community affords the opportunity for functional interactions between microorganisms, which can have significant influence on the relative population sizes of the different resident microbial taxa. In ticks, limited evidence suggests that specific microbes or the overall microbial community can influence the acquisition, transmission and virulence of known pathogens such as Borrelia burgdorferi, Anaplasma phagocytophilum, or Babesia microti, as well as newly emerging pathogens. This area remains understudied at this point and represents a current gap in our knowledge. Future research efforts are required in light of recent results from other arthropod systems such as aphids and Drosophila, and will greatly benefit from new technologies for in-depth profiling of the tick microbiome, allowing high sampling depth for ecological investigations and for experimental laboratory approaches.
Introduction
Ticks (Class Arachnida, Order Acari) are blood-feeding arthropods that feed on terrestrial vertebrates and vector a diverse group of human and wildlife pathogens, including viral, bacterial, and protozoan disease agents (Sonenshine and Mather 1994; Goodman, Dennis, and Sonenshine 2005). Ticks vector more human pathogens than any other arthropod, and are the primary source of vector-borne infectious disease in many temperate areas (Asia, Europe, North America). Unlike other blood-feeding arthropods such as mosquitos, fleas and lice, ticks exhibit extended time periods between blood meals of up to a year or more. Ticks can acquire pathogens during blood meals but transmission of pathogens to susceptible vertebrate hosts depends on ticks maintaining their infections during transstadial molts (from larvae to nymphs and from nymphs to adults) (Sonenshine 1991). Most hard ticks (Ixodidae) have a three-stage life cycle (larvae, nymph, adult) and each blood meal may be from a different host species. As a result, pathogens are potentially spread widely among vertebrate species, making ticks important sources of zoonotic disease.
Ticks have been well-studied because of their human health impacts but new microbial associations continue to be described (Jasinskas, Zhong, and Barbour 2007; Grindle et al. 2003; Morimoto, Kurtti, and Noda 2006) and new emerging diseases are being recognized (e.g. Paddock and Yabsley 2007, STARI, Loftis et al. 2008, Panola Mountain Ehrlichia, LaSala and Holbrook 2010, viral haemorrhagic fevers). In addition to pathogens, ticks serve as hosts for a variety of endosymbiotic, vertically-transmitted bacteria, including Coxiella-, Francisella- and Rickettsia-like organisms (Perotti et al. 2006; Noda, Munderloh, and Kurtti 1997; Sun et al. 2000; Morimoto, Kurtti, and Noda 2006), and
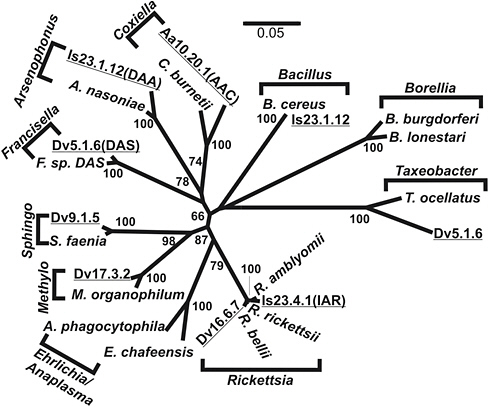
FIGURE A8-1 Phylogram of representative a subset of 16s-rDNA phylotypes from ticks. Brackets indicate genera. Underlined entries from recent tick isolates. Dv, D. variabilis; Aa, A. americanum; Is, I. scapularis. Numbers at nodes are boot strap support.
SOURCE: Clay and Fuqua, unpublished.
newly described symbionts of tick mitochondria (Sassera et al. 2006; Epis et al. 2008). Tick endosymbionts are often closely related to virulent human pathogens (Figure A8-1). It is likely that ticks are host to a larger diversity of, as yet undiscovered, microbes.
Changing environmental conditions, including climate change, land-use patterns, wildlife populations and agricultural practices, are acting to alter host and tick ecology and their geographical distributions, leading to new regions of tick activity, overlapping distributions and emerging disease (Childs and Paddock 2003; McDiarmid et al. 2000; Masuzawa et al. 2008; Sun et al. 2008; van Overbeek et al. 2008; Randolph 2010). These dynamic changes are providing new opportunities for pathogen host shifts
and mixed infections, including new microbial community associations (Eisen, Meyer, and Eisen 2007; Eisen 2008; Randolph and Rogers 2010).
Methodologies for Identifying and Enumerating Microbes in Ticks
The field of microbiology has relied for over a century on the ability to cultivate microorganisms derived from natural environments. Although this approach remains one of the most commonly employed and useful means of identifying microbes, it excludes the detection of a potentially vast range of microorganisms. Estimates from soil environments suggest that greater than 99% of active microorganisms are not detectable by conventional cultivation methods (Rondon et al. 2000; Hugenholz, Goebel, and Pace 1998). It is particularly clear that ticks and other arthropods frequently harbor microbes that have obligate intracellular life histories, either as commensals or pathogens, or are very difficult to cultivate (Dale and Moran 2006). Although traditional microscopy and histological staining can provide presumptive identifications of tick-associated microbes, the information is often ambiguous and of limited utility.
Powerful molecular approaches now enable the detection of microorganisms independent of the limitations of cultivation. Microbes can be identified and phylogenetically characterized, often to the level of genus and species, using nucleic acid or antibody probes directed towards highly conserved macromolecules (Clements and Bullivant 1991). Although a range of conserved proteins, fatty acids and nucleic acids have been used as targets for this purpose, small subunit ribosomal RNAs (SSUs) such as bacterial 16S rRNA, are the most generally applied, and often the most informative (Stahl 1995). No area of investigation has benefited more from these approaches than arthropod-microbe interactions. Molecular analyses have revealed diverse arthropod-associated microbes for a variety of systems (Dale and Moran 2006). Many of these microbes have not yet been cultured and their identification would be virtually impossible without cultivation-independent methods. Likewise, the study of tick-borne pathogens increasingly relies on molecular detection approaches to identify, distinguish and compare pathogens among different tick species and populations (Sun et al. 2000; Schabereiter-Gurtner, Lubitz, and Rölleke 2003; Burkot et al. 2001).
A number of studies have utilized Polymerase Chain Reaction (PCR) to amplify conserved microbial sequences, such as 16S rRNA gene sequences from total DNA extracts isolated from ticks, either as individuals or in small pools (Heise, Elshahed, and Little 2010; Clay et al. 2008; Benson et al. 2004). In the gene library sequence approach, these amplicons are ligated en masse into standard PCR cloning vectors, transformed into a cloning host such as Escherichia coli, and the plasmids are isolated from the initial transformants. The 16S rRNA amplicons carried on these plasmids
are then sequenced and the source microorganism is deduced by comparison with rRNA gene sequence databases, such as the Ribosomal Database Project (http://rdp.cme.msu.edu/). This cultivation-independent approach has been tremendously informative, and has revealed a number of tick-associated microbes that would have never been identified otherwise (Adar, Simaan, and Ulitzur 1992; Grindle et al. 2003; Heise, Elshahed, and Little 2010; Jasinskas, Zhong, and Barbour 2007; Schabereiter-Gurtner, Lubitz, and Rölleke 2003). A major limitation is however numerical—each clone must be sequenced individually. Hundreds of plasmids may be generated from a single tick, and sequencing to significant depth per tick is extremely expensive and time consuming. In depth analysis of large numbers of individual ticks becomes prohibitive. This problem is exacerbated by the trend for there to be a single, highly abundant type of microbe that colonizes each tick to high density, and therefore a large fraction the 16 S rRNA gene sequences determined are from this one taxon.
Molecular community fingerprinting techniques such as denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphisms (T-RFLP) analysis, ostensibly provide efficient snapshots of microbial community composition (Muyzer and Smalla 1998; Osborn, Moore, and Timmis 2000). These approaches also utilize PCR to amplify diagnostic sequences from samples, again most typically 16S rRNA genes. The underlying microbial diversity in any given community is revealed by electrophoretic separation of amplicons with different sequences, providing a microbial fingerprint. Specific microbial identification is also possible with both DGGE and T-RFLP approaches, but in practice this is considerably less reliable than the library sequencing approach for microbial identification. These techniques have proven useful in analyzing certain microbial communities, but have only been employed sparingly to analyze tick microbiota (Schabereiter-Gurtner, Lubitz, and Rölleke 2003). They provide somewhat course resolution on microbial diversity, revealing the major trends in composition, and therefore the numerical dominance of a single symbiont taxon cited above also creates problems for this approach.
The molecular approaches described above provide a way to gauge diversity in a microbial community, but are not an efficient way to determine the presence or absence of specific microbes in a sample. Nor do they provide robust information on relative abundances of a given microbe. Once specific microbial taxa of interest have been identified, these microbes can be targeted directly. Direct and nested PCR based assays with primer sets specific to diagnostic sequences (often, but not always 16S rRNA genes) for targeted microbial groups allows highly sensitive detection (Clay et al. 2008). Fluorescent in situ hybridization (FISH) analysis of sectioned ticks with specific oligonucleotide probes allows visualization of the site(s) of colonization for specific microbial taxa (Figure A8-2; Klyachko et al.
FIGURE A8-2 Visualization of Coxiella-type symbiont from A. americanum. (A) Transmission electron microscopy of thin sectioned ovarian tissue from a engorged female. Arrows demarcate presumptive outer and inner membrane structures (B) FISH microscopy of tissue section from a dissected ovary A. americanum show oocytes stained with a Coxiella-specific probe labeled with Cy5. Arrows highlight fluorescent punta resulting from probing and indicate of the Coxiella-type symbiont.
SOURCE: Klyachko et al. 2007.
2007; Hammer et al. 2001). These taxon-specific assays are able to detect microbes at very low relative abundance that often escape the more general community approaches. Furthermore, quantitative PCR allows the relative abundance of specific microbes to be determined (Jasinskas, Zhong, and Barbour 2007). The directed approaches, however, also suffer from several limitations and complexities. Investigators must know precisely which microbe they aim to detect, and they must have high confidence that the specific PCR primer set they employ is not confounded by cross-amplification of other microbes. Even minor divergence in the targeted sequences can lead to loss of detection, and it is difficult to trust PCR failures of a single target sequence as evidence for the absence of specific pathogens or other symbionts. Multilocus sequence typing (MLST) approaches target multiple conserved genes (usually 5-10) in a targeted microbe, again using PCR, but following this by sequencing of the amplicons (Maiden et al. 1998). Specific microbial lineages, or sequence types, are defined by the complete set of sequences obtained. This technique generally relies on the physical isolation of the targeted microbe from the sample, however, most typically by cultivation, and has been used only to analyze closely related tick microbiota (Margos et al. 2008).
The advent of high-throughput, next generation sequencing techniques promises to surmount many of the limitations in current molecular
approaches to microbial diversity studies described above. The ability to obtain hundreds of thousands of individual sequences in a single run provides tremendous power to probe the depth and breadth of a wide range of microbial communities. In the analysis of microbial communities, short segments of the 16S rRNA gene are PCR amplified using specific primer sets to generate 16S “tags” (Sogin et al. 2006). These amplified tags are then subjected to high-throughput sequencing such as pyrosequencing using a 454 sequence analyzer (http://www.454.com/). Current 454 technology provides from 400-600 bp sequence reads per fragment, allowing complete coverage of each amplicon. Upwards of 100,000 individual sequences can be obtained from a single 454 experiment. The primers used to perform the initial tag amplification contain specific identifier sequencers or “bar codes” outside of the region of the primer that anneals to the target sequence. These bar codes allow correlation of the sequences, obtained en masse, back to an original sample. For example, bar coding allows the simultaneous sequencing of greater than 100 individual ticks in a single experiment, generating hundreds of individual 16S rRNA tag sequences that can be correlated back to each specific source tick. Each sequence is then analyzed using the sequence databases (such as the RDP described above) to provide phylogenetic information about each microbe. Because of the depth of sequencing afforded, the number of sequences matching a specific taxon also provides information on the relative abundance of the microbe within the original sample. In our own studies we have found good agreement between abundances determined by tag sequencing and those determined by more direct assays such as targeted Q-PCR (Silvanose et al. in preparation).
All of these molecular approaches depend on PCR to amplify targeted genes and thus are all subject to any biases introduced by the PCR reaction itself. It is clear that there are no truly universal primer sets, and that in any given experiment it is possible to miss an important community member simply because of poor amplification. Conversely, some microbes may be overrepresented due to aberrantly efficient PCR. High-throughput sequencing may offer the answer here as well. New sequencing technologies, such as that provided by Solexa sequencing on Illumina instruments (http://www.illumina.com/technology/sequencing_technology.ilmn), can generate staggeringly high numbers of sequences, now up to 2 × 1011 bp in a single experiment. At a read length of roughly 100 bp this represents greater than 109 individual sequences (generally much shorter in length than those obtained by 454). With Illumina sequencing and other emerging technologies the ability to acquire sequence information from samples will no longer be the rate-limiting issue (Morozova and Marra 2008). With this sequencing power it may be possible to analyze total DNA from ticks directly, obtaining the tick genome and its microbial colonist’s genomes, without the need for PCR, and avoiding the bias described above. As with many of these
sequence-based technologies, the true challenge will be analyzing the bioinformatic data to obtain reliable information on the microbiome.
Molecular approaches have also begun to show great utility in studying tick host-microbiome interactions. Several groups have generated expressed sequence tag (EST) cDNA libraries from ticks that provide information not only on gene content, but also gene activity at the time of sampling (Wang et al. 2007; Hill and Gutierrez 2000). These libraries can also provide information about the microbiome. Our own analysis of several A. americanum EST libraries revealed a strikingly large percentage of cDNAs derived from a bacterial symbiont, indicating active expression of symbiont genes (Smith et al., in preparation). The sequence information gained from such libraries also facilitates construction of DNA microarrays to analyze gene expression (Colbourne et al. 2007). Whole genomic sequence projects for Ixodes scapularis and Boophilus microplus are well underway (Pagel Van Zee et al. 2007; Guerrero et al. 2006), and this information should provide even more comprehensive information for construction of DNA microarrays. With these microarrays, the expression patterns induced in ticks under a variety of conditions, including variable composition of the microbiome, may be monitored readily (Rodriguez-Valle et al. 2010). Even more powerful for these purposes may be massively parallel sequencing of transcripts, or RNASeq, using next generation sequencing technology to generate information on the genes being expressed and their level of expression (Ronning et al. 2010). This is as yet a new approach and we are not aware of its application to ticks or tick-borne disease, but the technique has great potential.
Microbial Communities of Ticks
Over the past two decades, research has revealed unsuspected microbial diversity in arthropods. For example, it is estimated that Wolbachia occurs in over 65% of all insect species (Hilgenboecker et al. 2008) and other prokaryotes (e.g., Cardinium, Arsenophonus, Rickettsia) are also highly represented (Perlman, Hunter, and Zchori-Fein 2006; Duron, Wilkes, and Hurst 2010; Duron et al. 2008; Weinert et al. 2009; Novakova, Hypsa, and Moran 2009). These microbes are often associated with reproductive alterations in hosts such as feminization, induced parthenogenesis or reproductive incompatibilities (Werren, Baldo, and Clark 2008). More specific arthropod families or genera are often associated with other bacterial endosymbionts such as Buchnera in aphids that play a role in nutrition of their hosts by provisioning critical amino acids (Douglas 1998; Oliver et al. 2010). Other insect groups (e.g., beetles, cockroaches, termites) are also associated with specific groups of microbes with enzymatic capabilities for the digestion of cellulose-rich food materials (Dillon and Dillon 2004; Vasanthakumar et al. 2008; Sabree, Kambhampati, and Moran 2009). A growing literature
suggests that bacterial symbionts can also play an important role in host defense against biotic enemies (Oliver, Moran, and Hunter 2005; Oliver et al. 2009; Jaenike et al. 2010), and also against abiotic stresses such as heat and cold (Montllor, Maxmen, and Purcell 2002; Neelakanta et al. 2010). Except for pathogens of humans and domestic animals, the functional role and impact of most microbial associations in ticks is unknown. It is likely that some endosymbionts play a nutritional role during blood feeding.
Most attention has been given to pathogenic bacteria vectored by ticks but they are also capable of transmitting pathogenic piroplasms (e.g., Babesia and Theileria) (Florin-Christensen and Schnittger 2009; Bishop et al. 2004) and a variety of viral pathogens. For example, tick-borne encephalitis is major human health threat worldwide (LaSala and Holbrook 2010; Charrel et al. 2004) and there is growing concern over deer tick or Powassan virus (Flavivirus) in Ixodes-endemic areas (Tokarz et al. 2010; Ebel 2010). In addition to tick-borne Flaviviruses, Colorado tick fever is caused by a Coltivirus transmitted by Dermacentor andersoni in the western United States and Canada (Brackney et al. 2010). Other pathogenic viruses could potentially be transmitted by ticks but standard methodologies for detecting bacteria would not detect them. The panviral Virochip approach would represent one possible method for quickly screening tick samples for viruses. Their blood-feeding lifestyle makes ticks potential vectors for a wide range of blood-borne pathogens.
Ticks may be co-infected by multiple pathogens (Schouls et al. 1999; Mixson et al. 2006; Moreno et al. 2006; Tokarz et al. 2010). Moreover, because of the high prevalence of vertically-transmitted endosymbionts in ticks, including multiple endosymbionts within the same tick (Scoles 2004; Goethert and Telford 2005; Carmichael and Fuerst 2006; Clay et al. 2008), pathogen infections almost always co-occur with resident endosymbionts (Yabsley et al. 2009; Jasinskas, Zhong, and Barbour 2007; Sun et al. 2000; Niebylski et al. 1997; Noda, Munderloh, and Kurtti 1997). Prior studies of the relationships among ticks, vertebrate hosts and pathogens have generally given little consideration to how microbial interactions and the entire microbial community within ticks, including endosymbionts and other microbes of unknown function, impact tick-borne disease (Table A8-1). These associations might affect the colonization, transmission and virulence of human or animal pathogens.
Ticks could become co-infected by pathogens while consuming a single blood meal containing multiple pathogens, or by transfer of pathogens between co-feeding ticks (Piesman and Happ 2001). It is less likely that ticks become infected by a diversity of pathogens from sequential feeding on multiple animals given that the hard tick life cycle includes only three blood meals that are well-separated in time. In contrast, co-infections of vertebrates by tick-borne pathogens could easily result from sequential
TABLE A8-1 Tick-Borne Bacteria and Human Diseases
|
Tick Genus |
Species |
Bacteria |
Human Disease |
Reference |
|
Ixodesa |
Is, Ir |
Borrelia burgdorferi |
Lyme Disease |
Burgdorfer et al. 1982 |
|
|
Is |
Anaplasma phagocytophila |
Anaplasmosis |
Belongia et al. 1997 |
|
|
Is, Ir |
Rickettsia symbiont |
None recognized |
Noda, Munderloh, and Kurtti 1997 |
|
|
Is, Ip |
Arsenophonus symbiont |
None recognized |
Grindle et al. (Unpublished) |
|
|
Ir |
Cytophaga symbiont |
None recognized |
Morimoto, Kurtti, and Noda 2006 |
|
|
Ir |
Midichloria mitochondrii |
None recognized |
Beninati et al. 2004 |
|
|
Ir |
Diplorickettsia massiliensis |
None recognized |
Mediannikov et al. 2010 |
|
Dermacentorb |
Dv |
Rickettsia rickettsii |
Rocky Mountain Spotted Fever |
Shepard and Goldwasser 1960 |
|
|
Dv, Da |
Rickettsia montana |
None recognized |
Steiner et al. (Unpublished) |
|
|
Dv, Da |
Francisella symbiontc |
None recognized |
Sun et al. 2000 |
|
|
Dv, Da |
Arsenophonus symbiont |
None recognized |
Grindle et al. 2003 |
|
Amblyommad |
Aa |
Borrelia lonestari |
Southern Tick-Associated Rash Illness (STARI) |
Varela et al. 2004 |
|
|
Aa |
Ehrlichia chafeensis |
Ehrlichiosis |
Anderson et al. 1991 |
|
|
Aa |
Rickettsia amblyommii |
None recognized |
Clay et al. 2008 |
|
|
Aa |
Arsenophonus symbiont |
None recognized |
Clay et al. 2008 |
|
|
Aa |
Coxiella symbiontc |
None recognized |
Jasinskas, Zhong, and Barbour 2007; Klyachko et al. 2007 |
|
aIs, I. scapularis; Ir, I. ricinus; Ip, I. Pacificus. bDv, D. variabilis; Da, D. andersoni. cThe mammalian pathogens Coxiella burnettii (Q-Fever) and Francisella tularensis (Tularemia) can be occasionally harbored and transmitted by multiple tick species. dAa, A. americanum. |
||||
and independent tick bites given that a large animal host could have a tick burden in the hundreds or thousands (Ginsberg 2008). Moreover, hosts may be bitten by ticks co-infected with multiple pathogens as described above. Human co-infections are most likely to arise from the bite of a single co-infected tick. Simultaneous infections by multiple tick-borne pathogens occur frequently in mammalian hosts, including humans. For example, of 96 patients in Wisconsin and Minnesota infected with Borrelia burgdorferi, five were co-infected with Anaplasma phagocytophilum, two with Babesia microti and two with all three pathogens (Mitchell, Reed, and Hofkes 1996). In New York, 60-90% of patients diagnosed with Human Granulocytic Anaplasmosis (A. phagocytophilum) tested positive for B. burgdorferi, a higher than expected rate based on pathogen prevalence (Wormser et al. 1997, see also Mitchell, Reed, and Hofkes 1996). Tick-borne co-infections may result in increased severity and duration of illness (Alekseev et al. 2001; Nyarko, Grab, and Dumler 2006) and misdiagnosis resulting from symptom overlap (Belongia et al. 1997). Co-infections with tick-borne pathogens have also been reported from domestic and wild animals including dogs (Kordick et al. 1999), deer (Little et al. 1998), rodents (Zeidner et al. 2000), cattle (Marufu et al. 2010) and horses (Parola, Davoust, and Raoult 2005).
Co-infections within ticks and competitive or facilitative interactions among microbes can affect the colonization and transmission of other tick-borne pathogens (Lively et al. 2005; Burgdorfer, Hayes, and Mavros 1981; Macaluso et al. 2002; de la Fuente, Blouin, and Kocan 2003; Ginsberg 2008) and the severity of ensuing disease (Korenberg 2004). For example, Ixodes ticks may be simultaneously infected by B. burgdorferi and other Borrelia species, B. microti, A. phagocytophilum, Bartonella henselae and Powassan virus (Tokarz et al. 2010; Goodman, Dennis, and Sionenshine 2005). Similarly, Amblyomma ticks may simultaneously harbor Borrelia lonestari, Ehrlichia spp., and Rickettsia amblyommii (Heise, Elshahed, and Little 2010; Clay et al. 2008; Castellaw et al. 2010). If colonization of ticks by a particular microbe leads to the exclusion or facilitation of other microbes, this would be manifested as a significant statistical deviation from random co-occurrence. For example, Mather et al. (Mather, Riberiro, and Spielman 1987) suggested that the agents of Lyme Disease and Babesiosis occurred together in ticks more frequently than expected. In contrast, Schauber et al. (Schauber et al. 1998) found that infection of I. scapularis by B. burgdorferi and A. phagocytophilum were independent of each other. Likewise, A. phagocytophilum and B. burgdorferi were acquired by mice regardless of their prior infection status by the opposite agent and were transmitted independently (Levin and Fish 2000).
Analysis of microbial exclusion or facilitation requires explicit reporting of co-infection rates. In a recent meta-analysis, 44% of the Ixodes tick populations (8 of 18) meeting criteria for inclusion significantly
deviated from expected co-infection frequencies under the assumption of independent infection of A. phagocytophilum and B. burgdorferi (Civitello, Rynkiewicz, and Clay 2010; Ginsberg 2008). In contrast, there was no evidence of deviation from expected rates of co-occurrence of five microbial taxa in Amblyomma americanum (Clay et al. 2008). However, the Coxiella endosymbiont occurred at 100% prevalence and two recognized human pathogens (E. chaffeensis and B. lonestari) occurred at very low frequencies, leading to limited statistical power to detect deviations from independent association.
Competition and crossover of vertebrate host immune response may be greatest between closely related strains (Barthold 1999; Pal et al. 2001). For example, infection by some Spotted Fever Group Rickettsia in Dermacentor variabilis prevents establishment and vertical transmission of related Rickettsia (Macaluso et al. 2002) see also (Burgdorfer, Hayes, and Mavros 1981). Price (Price 1953) described a different form of interaction between virulent and non-virulent rickettsiae where guinea pigs injected with both forms were protected from the effects of the virulent rickettsiae, possibly as a result of immunological cross-protection. We expect that vertically-transmitted tick endosymbionts should inhibit or exclude pathogens if those pathogens cause some harm to tick hosts (e.g., Niebylski, Peacock, and Schwan 1999). It is to the evolutionary benefit of vertically-transmitted endosymbionts to exclude pathogens from the tick microbial community because infection by a virulent pathogen condemns that endosymbiont community to extinction (Lively et al. 2005). It is clear that complex communities of microorganisms can coordinate activities within hosts or interfere with other microbes via cell-cell communication, and such mechanisms may be relevant to the interactions between tick-borne endosymbionts and pathogens (Fuqua and Greenberg 2002).
Overall, these studies demonstrate that ticks harbor a diversity of pathogens and symbionts, potentially allowing for ecological interactions among microbes within ticks. Microbial interactions could affect pathogen prevalence and transmission within tick populations. The role of microbial interactions in the organization of microbial communities within vectors and hosts needs further critical evaluation. An important first step is the evaluation and enumeration of microbial diversity within ticks.
Diverse Microbiome of Ticks
In preliminary studies of eastern North American ticks, we have examined the prokaryotic diversity of ticks by 16S rRNA tag sequencing using a 454 approach for amplicons from DNA extracts of A. americanum, D. variabilis and I. scapularis collected from the wild. All individuals were adult, questing ticks that were rigorously surface sterilized before DNA
extraction and sample preparation. The proportion of annotated sequences corresponding to the 10 most frequent taxa are presented in Figure A8-3 for each species. The number of sequences from a given taxon is presumed to reflect the density of that microbe within the tick. For A. americanum,the most abundant sequences were from the Coxiella endosymbiont (approx. 40% of all sequences) with Rickettsia being the second most common (approx. 5% of total), in good agreement with our direct probing data (Clay et al. 2008). We did not distinguish species of Rickettsia (and other groups) with accuracy so only present generic classifications. More than 40% of the identified sequences were from a large variety of other microbes. For D. variabilis, an Arsenophonus endosymbiont was the most frequently identified prokaryote followed by Methylobacterium and Francisella. Nearly 40% of the identified sequences were from a large number of rare taxa. Finally, for I. scapularis, Rickettsia represented nearly 75% of the total sequences with Bacillus making up approximately 10% of the total. The “other” category was relatively small in Ixodes.
The prevalence of prokaryotes across individual ticks provides another measure of the tick microbiome (Figure A8-4). 100% of the sampled A. americanum ticks were host to Coxiella with over 90% host also to Methylobacterium and Sphingomonas. 75% were infected by Rickettsia. Notably, Rhizobium, usually associated with nitrogen fixation, was also detected in 75% of the sampled ticks. For D. variabilis, the three most prevalent microbes were Methylobacterium, Francisella and Sphingomonas, each found from 19 of 22 (86%) sampled ticks. Three-quarters of I. scapularis hosted Rickettsia with no other microbe found in greater than 53% of the samples. Bradyrhizobium, another known N-fixing group, was found in 37% of the sampled Ixodes ticks. Although these findings are preliminary and subject to modification based on additional experiments, they do clearly indicate the potentially significant microbial diversity in ticks.
Known human pathogens were occasionally detected (data not shown because of their low density and prevalence) including Ehrlichia from Amblyomma and Borrelia from Ixodes. In Amblyomma we also occasionally detected sequences from Cardinium, another commonly-reported arthropod endosymbiont (Duron et al. 2008) that has never before been reported from ticks. Arsenophonus is another widespread insect endosymbiont (Novakova, Hypsa, and Moran 2009) that has recently been reported from several tick species (Dergousoff and Chilton 2010; Grindle et al. 2003; Clay et al. 2008). While our results are preliminary and need to be repeated with a larger sample of tick species and individuals, they clearly point to the fact that the dominant members of the tick microbiome are endosymbionts and/or microbes of unknown specificity and function. A similar result was recently obtained by Andreotti et al. (2011), who used 16S pyrosequencing to enumerate bacterial diversity in
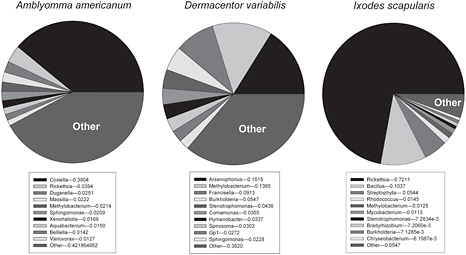
FIGURE A8-3 Density of annotated bacterial sequences in Amblyomma americanum (N=32), Dermacentor variabilis (N=22) and Ixodes scapularis (N=19) based on 454 sequencing. All ticks were collected from various sites in Indiana. The top 10 most abundant sequences are given for each species; other indicates all remaining sequences.
SOURCE: Clay and Fuqua, unpublished.
the cattle tick, Rhipicephalus (Boophilus) microplus. They found from 53–61 bacterial genera in adult males, eggs and females, respectively, with the very large majority not typically recognized as tick-borne pathogens. It is likely that some of these microbes play a nutritional role by helping to provision critical amino acids, vitamins, or otherwise help ticks survive on a limited diet of blood. Parallel microbiome studies of sapsucking insects point to a critical role for nutritional endosymbionts, and a recurrent theme of convergent evolution for these symbionts (Sabree, Kambhampati, and Moran 2009; Oliver et al. 2010).
Future Directions
Ticks represent a compelling yet challenging system for the study of microbiomes and microbial interactions. They require blood meals prior to molting, their symbionts are difficult to cure and to deliberately inoculate, and their genomes are highly complex. The microbes that colonize ticks also can be very difficult to work with since many have not yet been cultivated or are obligate intracellular symbionts. Little is currently known about the
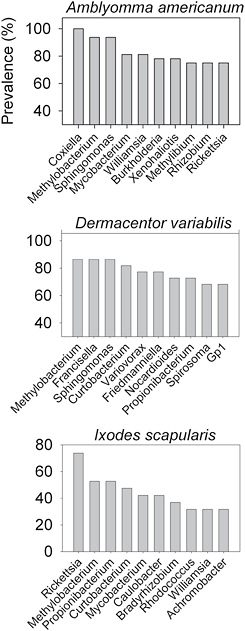
FIGURE A8-4 Density of annotated bacterial sequences in Amblyomma americanum (N=32), Dermacentor variabilis (N=22) and Ixodes scapularis (N=19) based on 454 sequencing. All ticks were collected from various sites in Indiana. The top 10 most abundant sequences are given for each species; other indicates all remaining sequences.
SOURCE: Clay and Fuqua, unpublished.
roles and activities of many of these microorganisms. Additionally, they can have plastic genetic content, and the features which separate a benign commensal from a significant pathogen are not always clear or well understood. Now that it is clear that there can be multiple microorganisms colonizing the same tick and even the same tissues, the prospects for genetic exchange between these microbes are quite distinct, and it is conceivable that an otherwise benign commensal microbe might acquire virulence functions through this route. Likewise, synergistic or antagonistic interactions between microbes may be manifested by the emergence of new polymicrobial diseases or, alternatively, the decline of a current disease agent.
Although more efficient arthropod systems exist for fundamental investigations into microbial-host interactions, few have the human health impact of ticks and the microbes they vector. Ultimately, it is the importance of ticks to human health that drives active research in this area rather than their utility as a model system. The increasing availability of genomic information for ticks and tick-associated microbes creates significant opportunities to broaden the range of analyses that can be performed. Several tick-vectored pathogens have had their genomes sequenced (Seshadri et al. 2003) and several whole tick genome sequences should be forthcoming (Guerrero et al. 2006; Pagel Van Zee et al. 2007). Genomic sequencing of non-pathogenic symbionts is thus far less common, but would add to the understanding of the tick-borne community, affording opportunities for comparative genomics between related pathogens and non-pathogens, and yielding insights into acquisition and transmission processes. In addition, the ability to simultaneously evaluate tick gene expression as well as those of their resident microbiota, through DNA microarrays and RNA-Seq, should begin to unravel these tight and stable arthropod-microbe interactions.
As with other efforts rooted in genomic science a major hurdle lies within the bioinformatics. Acquisition of sequence or expression data is no longer rate limiting, but rather the ability to distill the potentially massive amount of data down to manageable segments or significant patterns is very challenging, and efficient progress will require interdisciplinary teams of microbiologists, epidemiologists and bioinformaticians.
While technological advances and deep sequencing has revealed unsuspected microbial diversity, many basic questions remain unanswered. What are the evolutionary origins and means of spread of these microbes? What is their functional role or are they simply highly abundant guest commensals? For example, Methylobacterium was highly represented in all tick sequences and occurred at very high prevalence in all tick species examined (Figures A8-3 and A8-4). What is the functional role of the highly abundant endosymbionts such as Arsenophonus, Coxiella, Francisella and Rickettsia? What distinguishes hereditary endosymbionts from virulent human
pathogens (e.g.. Coxiella endosymbiont of A. americanum vs. C. burnetti (Jasinskas, Zhong, and Barbour 2007; Klyachko et al. 2007; Seshadri et al. 2003)? Could horizontal gene exchange between related pathogens and endosymbionts give rise to new virulent pathogens? More generally, are there characteristic microbial communities associated with different tick species and what regulates the structure of these tick-associated microbial communities? Addressing these questions will require more genomic data from non-pathogens combined with efficient inoculation and disinfection strategies. Greater understanding of the dynamics and organization of tick-associated microbial communities may also contribute to the development of more accurate epidemiological and disease risk models.
The increasing homogenization of Earth’s biota and human domination of terrestrial ecosystems may be increasing, rather than decreasing human health risks. Ticks and their pathogens are highly dispersible and thrive in many human-dominated habitats. Increasing wildlife populations, such as deer and turkey, may also contribute increasing risk of tick encounters (Childs and Paddock 2003). For example, annual incidence of Lyme disease is increasing despite greater awareness and prompt medical responses. Climate change may further alter geographical ranges of ticks, pathogens and vertebrate hosts (Randolph 2010), potentially leading to host and vector shifts of tick-associated microbes and the composition of their microbial communities. Tick-borne pathogens are just one component of larger, diverse microbial communities. Genetic exchange between pathogens and symbionts, exchange of virulence factors, new mechanisms for contagious transmission and new host associations all need to considered in light of larger scale ecological and environmental changes.
References
Adar, Y. Y., M. Simaan, and S. Ulitzur. 1992. Formation of the LuxR protein in the Vibrio fischeri lux system is controlled by HtpR through the GroESL proteins. Journal of Bacteriology 174 (22):7138-7143.
Alekseev, A. N., H. V. Dubinina, I. Van De Pol, and L. M. Schouls. 2001. Identification of Ehrlichia spp, and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. Journal of Clinical Microbiology 39 (6):2237-2242.
Anderson, B. E., J. E. Dawson, D. C. Jones, and K. H. Wilson. 1991. Ehrlichia chaffeensis, a new species associated with Human Ehrlichiosis. Journal of Clinical Microbiology 29 (12):2838-2842.
Andreotti, R., A. A. P. de Leon, S. E. Dowd, F. D. Guerrero, K. G. Bendele, and G. A. Scoles. 2011. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiology 11:6.
Barthold, S. W. 1999. Specificity of infection-induced immunity among Borrelia burgdorferi sensu late species. Infection and Immunity 67 (1):36-42.
Belongia, E. A., K. D. Reed, P. D. Mitchell, C. P. Kolbert, D. H. Persing, J. S. Gill, and J. J. Kazmierczak. 1997. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. Journal of Clinical Microbiology 35:1465-1468.
Beninati, T., N. Lo, L. Sacchi, C. Genchi, H. Noda, and C. Bandi. 2004. A novel alpha-proteobacterium resides in the mitochondria of ovarian cells of the tick Ixodes ricinus. Applied and Environmental Microbiology 70 (5):2596-2602.
Benson, M. J., J. D. Gawronski, D. E. Eveleigh, and D. R. Benson. 2004. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Applied and Environmental Microbiology 70 (1):616-20.
Bishop, R., A. Musoke, S. Morzaria, M. Gardner, and V. Nene. 2004. Theileria: intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 129:S271-S283.
Brackney, M. M., A. A. Marfin, J. E. Staples, L. Stallones, T. Keefe, W. C. Black, and G. L. Campbell. 2010. Epidemiology of Colorado Tick Fever in Montana, Utah, and Wyoming, 1995-2003.Vector-Borne and Zoonotic Diseases 10 (4):381-385.
Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme Disease - A tick-borne spirochetosis. Science 216 (4552):1317-1319.
Burgdorfer, W., S. F. Hayes, and A. J. Mavros. 1981. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii. In Rickettsiae and Rickettsial Diseases, edited by W. Burgdorfer and R. L. Anacker. New York: Academic Press.
Burkot, T. R., G. R. Mullen, R. Anderson, B. S. Schneider, C. M. Happ, and N. S. Zeidner. 2001. Borrellia lonestari DNA in adult Amblyomma americanum ticks, Alabama. Emerging Infectious Disease 7:471-473.
Carmichael, J. R., and P. A. Fuerst. 2006. A rickettsial mixed infection in a Dermacentor variabilis tick from Ohio. In Century of Rickettsiology: Emerging, Reemerging Rickettsioses, Molecular Diagnostics, and Emerging Veterinary Rickettsioses, edited by K. E. Hechemy, J. A. Oteo, D. Raoult, D. Silverman and J. R. Blanco.
Castellaw, A. H., J. Showers, J. Goddard, E. F. Chenney, and A. S. Varela-Stokes. 2010. Detection of Vector-Borne Agents in Lone Star Ticks, Amblyomma americanum (Acari: Ixodidae), From Mississippi. Journal of Medical Entomology 47 (3):473-476.
Charrel, R. N., H. Attoui, A. M. Butenko, J. C. Clegg, V. Deubel, T. V. Frolova, E. A. Gould, T. S. Gritsun, F. X. Heinz, M. Labuda, V. A. Lashkevich, V. Loktev, A. Lundkvist, D. V. Lvov, C. W. Mandl, M. Niedrig, A. Papa, V. S. Petrov, A. Plyusnin, S. Randolph, J. Suss, V. I. Zlobin, and X. de Lamballerie. 2004. Tick-borne virus diseases of human interest in Europe. Clinical Microbiology and Infection 10 (12):1040-1055.
Childs, J. E., and C. D. Paddock. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annual Review of Entomology 48:307-37.
Civitello, D. J., E. Rynkiewicz, and K. Clay. 2010. Meta-analysis of co-infections in ticks. Israel Journal of Ecology and Evolution (in press).
Clay, K., O. Klyachko, N. Grindle, D. Civitello, D. Oleske, and C. Fuqua. 2008. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Molecular Ecology17:4371-4381.
Clements, K. D., and S. Bullivant. 1991. An unusual symbiont from the gut of surgeonfishes may be the largest known prokaryote. Journal of Bacteriology 173:5359-5362.
Colbourne, J. K., B. D. Eads, J. Shaw, E. Bohuski, D. J. Bauer, and J. Andrews. 2007. Sampling Daphnia’s expressed genes: preservation, expansion and invention of crustacean genes with reference to insect genomes. BMC Genomics 8:217.
Dale, C., and N. A. Moran. 2006. Molecular interactions between bacterial symbionts and their hosts. Cell 126 (3):453-65.
de la Fuente, J., E. F. Blouin, and K. M. Kocan. 2003. Infection exclusion of the rickettsial pathogen anaplasma marginale in the tick vector Dermacentor variabilis. Clinical and Diagnostic Laboratory Immunology 10 (1):182-4.
Dergousoff, S. J., and N. B. Chilton. 2010. Detection of a new Arsenophonus-type bacterium in Canadian populations of the Rocky Mountain wood tick, Dermacentor andersoni. Experimental and Applied Acarology 52 (1):85-91.
Dillon, R. J., and V. M. Dillon. 2004. The gut bacteria of insects: Nonpathogenic interactions. Annual Review of Entomology 49:71-92.
Douglas, A. E. 1998. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annual Review of Entomology 43:17-37.
Duron, O., D. Bouchon, S. Boutin, L. Bellamy, L. Q. Zhou, J. Engelstadter, and G. D. Hurst. 2008. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. Bmc Biology 6.
Duron, O., T. E. Wilkes, and G. D. D. Hurst. 2010. Interspecific transmission of a male-killing bacterium on an ecological timescale. Ecology Letters 13 (9):1139-1148.
Ebel, G. D. 2010. Update on Powassan Virus: Emergence of a North American Tick-Borne Flavivirus. Annual Review of Entomology 55:95-110.
Eisen, L. 2008. Climate change and tick-borne diseases: A research field in need of long-term empirical field studies. International Journal of Medical Microbiology 298:12-18.
Eisen, L., A. M. Meyer, and R. J. Eisen. 2007. Climate-based model predicting acarological risk of encountering the human-biting adult life stage of Dermacentor andersoni (Acari : Ixodidae) in a key habitat type in Colorado. Journal of Medical Entomology 44 (4):694-704.
Epis, S., D. Sassera, T. Beninati, N. Lo, L. Beati, J. Piesman, L. Rinaldi, K. D. McCoy, A. Torina, L. Sacchi, E. Clementi, M. Genchi, S. Magnino, and C. Bandi. 2008. Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse species. Parasitology 135 (4):485-494.
Florin-Christensen, M., and L. Schnittger. 2009. Piroplasmids and ticks: a long-lasting intimate relationship. Frontiers in Bioscience 14:3064-3073.
Fuqua, C., and E. P. Greenberg. 2002. Listening in on bacteria: Acyl-homoserine lactone signalling. Nature Reviews Molecular Cell Biology 3 (9):685-695.
Ginsberg, H. S. 2008. Potential effects of mixed infections in ticks on transmission dynamics of pathogens: comparative analysis of published records. Experimental and Applied Acarology 46 (1-4):29-41.
Goethert, H. K., and S. R. Telford. 2005. A new Francisella (Beggiatiales : Francisellaceae) inquiline within Dermacentor variabilis say (Acari : Ixodidae). Journal of Medical Entomology 42 (3):502-505.
Goodman, J. L., D. T. Dennis, and D. E. Sionenshine, eds. 2005. Tick-borne disease of humans. Washington, D.C.: ASM Press.
Grindle, N., J.J. Tyner, K. Clay, and C. Fuqua. 2003. Identification of Arsenophonus-type bacteria from the dog tick Dermacentor variabilis. Journal of Invertebrate Pathology 83:264-266.
Guerrero, F. D., V. M. Nene, J. E. George, S. C. Barker, and P. Willadsen. 2006. Sequencing a new target genome: the Boophilus microplus (Acari: Ixodidae) genome project. Journal of Medical Entomology 43 (1):9-16.
Hammer, B., A. Moter, O. Kahl, G. Alberti, and U. B. Gobel. 2001. Visualization of Borrelia burgdorferi sensu lato by fluorescence in situ hybridization (FISH) on whole-body sections of Ixodes ricinus ticks and gerbil skin biopsies. Microbiology 147 (Pt 6):1425-36.
Heise, S. R., M. S. Elshahed, and S. E. Little. 2010. Bacterial diversity in Amblyomma americanum (Acari: Ixodidae) with a focus on members of the genus Rickettsia. Journal of Medical Entomology 47 (2):258-268.
Hilgenboecker, K., P. Hammerstein, P. Schlattmann, A. Telschow, and J. H. Werren. 2008. How many species are infected with Wolbachia? - a statistical analysis of current data. FEMS Microbiology Letters 281 (2):215-220.
Hill, C. A., and J. A. Gutierrez. 2000. Analysis of the expressed genome of the Lone Star Tick, Amblyomma americanum (Acari: Ixodidae) using an expressed sequence tag approach. Micro. Comp. Genomics 5:89-101.
Hugenholz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. Journal of Bacteriology 180:4765-4774.
Jaenike, J., R. Unckless, S. N. Cockburn, L. M. Boelio, and S. J. Perlman. 2010. Adaptation via symbiosis: Recent spread of a Drosophila defensive symbiont. Science 329 (5988):212-215.
Jasinskas, A., J. Zhong, and A. G. Barbour. 2007. Highly prevalent Coxiella sp. bacterium in the tick vector Amblyomma americanum. Applied and Environmental Microbiology 73 (1):334-6.
Klyachko, O., B. D. Stein, N. Grindle, K. Clay, and C. Fuqua. 2007. Localization and visualization of a coxiella-type symbiont within the lone star tick, Amblyomma americanum. Applied and Environmental Microbiology 73 (20):6584-94.
Kordick, S. K., E. B. Breitschwerdt, B. C. Hegarty, K. L. Southwick, C. M. Colitz, S. I. Hancock, J. M. Bradley, R. Rumbough, J. T. McPherson, and J. N. MacCormack. 1999. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. Journal of Clinical Microbiology 37:2631-2638.
Korenberg, E. I. 2004. Problems in the study and prophylaxis of mixed infections transmitted by ixodid ticks. International Journal of Medical Microbiology 293:80-85.
LaSala, P. R., and M. Holbrook. 2010. Tick-Borne Flaviviruses. Clinics in Laboratory Medicine 30 (1):221-+.
Levin, M. L., and D. Fish. 2000. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infection and Immunity 68:2183-2186.
Little, S. E., D. E. Stallknecht, J. M. Lockhart, J. E. Dawson, and W. R. Davidson. 1998. Natural coinfection of a white-tailed deer (Odocoileus virginianus) population with three Ehrlichia spp. Journal of Parasitology 84:897-901.
Lively, C. M., K. Clay, W. J. Wade, and C. Fuqua. 2005. Competitive coexistence of vertically and horizontally transmitted parasites. Evolutionary Ecology Research 7:1183-1190.
Loftis, A. D., T. R. Mixson, E. Y. Stromdahl, M. J. Yabsley, L. E. Garrison, P. C. Williamson, R. R. Fitak, P. A. Fuerst, D. J. Kelly, and K. W. Blount. 2008. Geographic distribution and genetic diversity of the Ehrlichia sp from Panola Mountain in Amblyomma americanum. Bmc Infectious Diseases 8.
Macaluso, K. R., D. E. Sonenshine, S. M. Ceraul, and A. F. Azad. 2002. Rickettsial infection in Dermocentor variabilis (Acari:Ixodidae) inhibits transovarial transmission of a second Rickettsia. Journal of Medical Entomology 39:809-813.
Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proceedings of the National Academy of Sciences, USA 95 (6):3140-5.
Margos, G., A. G. Gatewood, D. M. Aanensen, K. Hanincova, D. Terekhova, S. A. Vollmer, M. Cornet, J. Piesman, M. Donaghy, A. Bormane, M. A. Hurn, E. J. Feil, D. Fish, S. Casjens, G. P. Wormser, I. Schwartz, and K. Kurtenbach. 2008. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proceedings of the National Academy of Sciences, USA 105 (25):8730-5.
Marufu, M. C., M. Chimonyo, K. Dzama, and C. Mapiye. 2010. Seroprevalence of tick-borne diseases in communal cattle reared on sweet and sour rangelands in a semi-arid area of South Africa. Veterinary Journal 184 (1):71-76.
Masuzawa, T., I. G. Kharitonenkov, Y. Okamoto, T. Fukui, and N. Ohashi. 2008. Prevalence ofAnaplasma phagocytophilum and its coinfection with Borrellia afzefii in Ixodes ricinus and Ixodes persulcatus ticks inhabiting Tver Province (Russia) - a sympatric region for both tick species. Journal of Medical Microbiology 57 (8):986-991.
Mather, T. N., J. M. C. Riberiro, and A. Spielman. 1987. Lyme disease and babesiosis: acaricide focused on potentially infected ticks. American Journal of Tropical Medicine Hygene 36:609-614.
McDiarmid, L., T. Petney, B. Dixon, and R. Andrews. 2000. Range expansion of the tick Amblyomma triguttatum, an Australian vector for Q fever. International Journal of Parasitology 30:791-793.
Mediannikov, O., Z. Sekeyova, M. L. Birg, and D. Raoult. 2010. A Novel Obligate Intracellular Gamma-Proteobacterium Associated with Ixodid Ticks, Diplorickettsia massiliensis, Gen. Nov., Sp Nov. Plos One 5 (7).
Mitchell, P. D., K. D. Reed, and J. M. Hofkes. 1996. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. Journal of Clinical Microbiology 34:724-727.
Mixson, T. R., S. R. Campbell, J. S. Gill, H. S. Ginsberg, M. V. Reichard, T. L. Schulze, and G. A. Dasch. 2006. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. Journal of Medical Entomology 43 (6):1261-8.
Montllor, C. B., A. Maxmen, and A. H. Purcell. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecological Entomology 27 (2):189-195.
Moreno, C. X., F. Moy, T. J. Daniels, H. P. Godfrey, and F. C. Cabello. 2006. Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess Counties, New York. Environmental Microbiology 8 (5):761-772.
Morimoto, S., T. J. Kurtti, and H. Noda. 2006. In vitro cultivation and antibiotic susceptibility of a Cytophaga-like intracellular symbiote isolated from the tick Ixodes scapularis. Current Microbiology 52 (4):324-329.
Morozova, O., and M. A. Marra. 2008. Applications of next-generation sequencing technologies in functional genomics. Genomics 92 (5):255-64.
Muyzer, G., and K. Smalla. 1998. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie van Leeuwenhoek 73:127-141.
Neelakanta, G., H. Sultana, D. Fish, J. F. Anderson, and E. Fikrig. 2010.Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. Journal of Clinical Investigation 120 (9):3179-3190.
Niebylski, M. L., M. G. Peacock, and T. G. Schwan. 1999. Lethal effect of Rickettsia rickettsii on its tick vector Dermacentor andersoni. Applied and Environmental Microbiology 65:773-778.
Niebylski, M. L., M. G. Peacock, E. R. Fischer, S. F. Porcella, and T. G. Schwan. 1997. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Applied and Environmental Microbiology 63:3933-3940.
Noda, H., U. G. Munderloh, and T. J. Kurtti. 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Applied and Environmental Microbiology 63:3926-3932.
Novakova, E., V. Hypsa, and N. A. Moran. 2009. Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. Bmc Microbiology 9.
Nyarko, E., D. J. Grab, and J. S. Dumler. 2006. Anaplasma phagocytophilum - infected neutrophils enhance transmigration of Borrelia burgdorferi across the human blood brain barrier in vitro. International Journal for Parasitology 36 (5):601-605.
Oliver, K. M., P. H. Degnan, G. R. Burke, and N. A. Moran. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annual Review of Entomology 55:247-266.
Oliver, K. M., P. H. Degnan, M. S. Hunter, and N. A. Moran. 2009. Bacteriophages Encode Factors Required for Protection in a Symbiotic Mutualism. Science 325 (5943):992-994.
Oliver, K. M., N. A. Moran, and M. S. Hunter. 2005. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proceedings of the National Academy of Sciences U. S. A.102 (36):12795-12800.
Osborn, A. M., E. R. B. Moore, and K. N. Timmis. 2000. An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environmental Microbiology 2:39-50.
Paddock, C. D., and M. J. Yabsley. 2007. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum - Associated zoonoses in the United States. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission.
Pagel Van Zee, J., N. S. Geraci, F. D. Guerrero, S. K. Wikel, J. J. Stuart, V. M. Nene, and C. A. Hill. 2007. Tick genomics: the Ixodes genome project and beyond. International Journal of Parasitology 37 (12):1297-305.
Pal, U., R. R. Montgomery, D. Lusitani, P. Voet, V. Weynants, S. E. Malawista, Y. Lobet, and E. Fikrig. 2001. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. Journal of Immunology 166 (12):7398-7403.
Parola, P., B. Davoust, and D. Raoult. 2005. Tick- and flea-borne rickettsial emerging zoonoses.Veterinary Research 36 (3):469-492.
Perlman, S. J., M. S. Hunter, and E. Zchori-Fein. 2006. The emerging diversity of Rickettsia. Proceedings of the Royal Society B-Biological Sciences 273 (1598):2097-2106.
Perotti, M. A., H. K. Clarke, B. D. Turner, and H. R. Braig. 2006. Rickettsia as obligate and mycetomic bacteria. Faseb Journal 20 (13):2372-+.
Piesman, J., and C. M. Happ. 2001. The efficacy of co-feeding as a means of maintaining Borrelia burgdorferi: a North American model system. Journal of Vector Ecology 26 (2):216-220.
Price, W. H. 1953. Interference phenomenon in animal infections with rickettsiae of Rocky Mountain spotted fever. Proceedings of the Society for Experimental Biology and Medicine 82:180-184.
Randolph, S. E. 2010. To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Veterinary Parasitology 167 (2-4):92-94.
Randolph, S. E., and D. J. Rogers. 2010. The arrival, establishment and spread of exotic diseases: patterns and predictions. Nature Reviews Microbiology 8 (5):361-371.
Rodriguez-Valle, M., A. Lew-Tabor, C. Gondro, P. Moolhuijzen, M. Vance, F. D. Guerrero, M. Bellgard, and W. Jorgensen. 2010. Comparative microarray analysis of Rhipicephalus (Boophilus) microplus expression profiles of larvae pre-attachment and feeding adult female stages on Bos indicus and Bos taurus cattle. 11:437.
Rondon, M. R., P. R. August, A. D. Bettermann, S. F. Brady, T. H. Grossman, M. R. Liles, K. A. Loiacono, B. A. Lynch, I. A. MacNeil, C. Minor, C. L. Tiong, M. Gilman, M. S. Osburne, J. Clardy, J. Handelsman, and R. M. Goodman. 2000. Cloning the soil metagenome: a strategy for accessing the genetic and functional diversity of uncultured microorganisms. Applied and Environmental Microbiology 66:2541-2547.
Ronning, C. M., L. Losada, L. Brinkac, J. Inman, R. L. Ulrich, M. Schell, W. C. Nierman, and D. Deshazer. 2010. Genetic and phenotypic diversity in Burkholderia: contributions by prophage and phage-like elements. BMC Microbiology 10:202.
Sabree, Z. L., S. Kambhampati, and N. A. Moran. 2009. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proceedings of the National Academy of Sciences U. S. A. 106 (46):19521-19526.
Sassera, D., T. Beninati, C. Bandi, E. A. P. Bouman, L. Sacchi, M. Fabbi, and N. Lo. 2006. ’Candidatus Midichloria mitochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. International Journal of Systematic and Evolutionary Microbiology 56:2535-2540.
Schabereiter-Gurtner, C., W. Lubitz, and S. Rölleke. 2003. Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. Journal of Microbiological Methods 52:251-260.
Schauber, E. M., S. J. Gertz, W. T. Maple, and R. S. Ostfeld. 1998. Coinfection of blacklegged ticks (Acari : Ixodidae) in Dutchess County, New York, with the agents of Lyme disease and human granulocytic ehrlichiosis. Journal of Medical Entomology 35 (5):901-903.
Schouls, L.M., I. van De Pol, G.T. Rijpkema, and C.S. Schot. 1999. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. Journal of Clinical Microbiology 37:2215-2222.
Scoles, G. A. 2004. Phylogenetic analysis of the Francisella-like endosymbionts of Dermacentor ticks. Journal of Medical Entomology 41 (3):277-286.
Seshadri, R., I. T. Paulsen, J. A. Eisen, T. D. Read, K. E. Nelson, W. C. Nelson, N. L. Ward, H. Tettelin, T. M. Davidsen, M. J. Beanan, R. T. Deboy, S. C. Daugherty, L. M. Brinkac, R. Madupu, R. J. Dodson, H. M. Khouri, K. H. Lee, H. A. Carty, D. Scanlan, R. A. Heinzen, H. A. Thompson, J. E. Samuel, C. M. Fraser, and J. F. Heidelberg. 2003. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proceedings of the National Academy of Sciences U.S.A. 100 (9):5455-5460.
Sogin, M. L., H. G. Morrison, J. A. Huber, D. Mark Welch, S. M. Huse, P. R. Neal, J. M. Arrieta, and G. J. Herndl. 2006. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proceedings of the National Academy of Sciences U. S. A. 103 (32):12115-20.
Sonenshine, D. E. 1991. Biology of Ticks. Vol. 1. New York: Oxford University Press. Sonenshine, D. E., and T. N. Mather. 1994. Ecological Dynamics of Tick-Borne Zoonoses. New York: Oxford University Press.
Stahl, D. A. 1995. Application of phylogentically base hybridization probes to microbial ecology. Molecular Ecology 4:535-542.
Sun, J. M., Q. Y. Liu, L. Lu, G. Q. Ding, J. Q. Guo, G. M. Fu, J. B. Zhang, F. X. Meng, H. X. Wu, X. P. Song, D. S. Ren, D. M. Li, Y. H. Guo, J. Wang, G. C. Li, J. L. Liu, and H. L. Lin. 2008. Coinfection with four genera of bacteria (Borrelia, Bartonella, Anaplasma, and Ehrlichia) in Haemaphysalis longicornis and Ixodes sinensis Ticks from China. Vector-Borne and Zoonotic Diseases 8 (6):791-795.
Sun, L. V., G. A. Scoles, D. Fish, and S. L. O’Neill. 2000. Francisella-like endosymbionts of ticks. Journal of Invertebrate Pathology 76:301-303.
Tokarz, R., K. Jain, A. Bennett, T. Briese, and W. I. Lipkin. 2010. Assessment of polymicrobial infections in ticks in New York State. Vector-Borne and Zoonotic Diseases 10 (3):217-221.
van Overbeek, L., F. Gassner, C. L. van der Plas, P. Kastelein, U. N. D. Rocha, and W. Takken. 2008. Diversity of Ixodes ricinus tick-associated bacterial communities from different forests. Fems Microbiology Ecology 66 (1):72-84.
Varela, A. S., M. P. Luttrell, E. W. Howerth, V. A. Moore, W. R. Davidson, D. E. Stallknecht, and S. E. Little. 2004. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. Journal of Clinical Microbiology 42 (3):1163-1169.
Vasanthakumar, A., J. Handelsman, P. D. Schloss, L. S. Bauer, and K. F. Raffa. 2008. Gut microbiota of an invasive subcortical beetle, Agrilus planipennis Fairmarine, across various life stages. Environmental Entomology 37 (5):1344-1353.
Wang, M., F. D. Guerrero, G. Pertea, and V. M. Nene. 2007. Global comparative analysis of ESTs from the southern cattle tick, Rhipicephalus (Boophilus)microplus. BMC Genomics 8:368.
Weinert, L. A., J. H. Werren, A. Aebi, G. N. Stone, and F. M. Jiggins. 2009. Evolution and diversity of Rickettsia bacteria. Bmc Biology 7.
Werren, J. H., L. Baldo, and M. E. Clark. 2008. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology 6 (10):741-751.
Wormser, G. P., H. W. Horowitz, J. Nowakowski, D. McKenna, J. S. Dumler, S. Varde, I. Schwartz, C. Carbano, and M. AgueroRosenfeld. 1997. Positive Lyme disease serology in patients with clinical and laboratory evidence of human granulocytic ehrlichiosis. American Journal of Clinical Pathology 107:142-147.
Yabsley, M. J., T. N. Nims, M. Y. Savage, and L. A. Durden. 2009. Ticks and tick-borne pathogens and putative symbionts of black bears (Ursus americanus floridanus) from Georgia and Florida. Journal of Parasitology 95 (5):1125-1128.
Zeidner, N. S., T. R. Burkot, R. Massung, W. L. Nicholson, M. C. Dolan, J. S. Rutherford, B. J. Biggerstaff, and G. O. Maupin. 2000. Transmission of the agent of human granulocytic ehrlichiosis by Ixodes spinipalpis ticks: Evidence of an enzootic cycle of dual infection with Borrelia burgdorferi in northern Colorado. Journal of Infectious Diseases 182:616-619.
A9
MANAGING SUSPECTED, ATYPICAL LYME DISEASE IN NON-REFERRAL PRACTICES
Matthew H. Liang, M.D., M.P.H., F.A.C.P., F.A.C.R. Massachusetts Veterans Epidemiology and Research Center, Section of Rheumatology Boston VA Healthcare System, Boston, MA Division of Rheumatology, Immunology and Allergy, Brigham & Women’s Hospital, Boston, MA
Summary
Lyme disease is the most common vector borne disease in Europe and North America. In the U.S. alone, over 50,000 cases have been reported to the U.S. Centers for Disease Control since 1988 from the Northeast, upper Mideast and far West with some reported incidence rates as high as 1192 cases/100,000 (Nantucket Island, Massachusetts).
Based on actual cost data from the Maryland Eastern Shore from 1997
to 2000, the mean per patient direct medical costs of early-stage LD decreased from $1,609 to $464, and the mean per patient direct medical cost of late-stage LD decreased from $4,240 to $1,380 (Zhang et al., 2006). The estimated median of all costs (direct medical cost, indirect medical cost, nonmedical cost, and productivity loss) aggregated across all patients, was ~$281 per patient; as with many cost of illness studies, a small number of LD patients accounted for the majority of the costs.
To approximate the annual economic impact of LD nationwide, these results can be extrapolated to the total number of LD cases reported nationwide, 23,763 LD cases in 2002 corresponds to ~$203 million (in 2002 dollars). LD cases reported using the CDC surveillance case definition underreport the true incidence; therefore, the estimate is likely to be low. The decline in average cost per LD case is observed in all cost categories, drug costs, hospital days, diagnostic testing, and may be related to successful adoption of personal protection measures and/or prompt consultation and treatment after exposure or tick bite. It may also be reporting bias.
The description of Lyme disease in the U.S. in 1976 and subsequent characterization of its mode of transmission, causative organism and treatment is an important saga in the history of medicine. In theory, Lyme disease could be prevented and eradicated but in practice it continues to grow as a public health problem and many biological and clinical questions remain unanswered. Typical acute Lyme Disease, by definition, is fairly straightforward to diagnose and treat. However, acute Lyme may not present typically and acute Lyme with persistent symptoms respond to recommended antibiotic regimens. Both forms in practice are major challenges in non-referral practices.
My frame of reference
I live and work in Massachusetts where Lyme Disease can be endemic. Nevertheless, I missed diagnosing chronic Lyme in a physician’s wife from Vermont. Her husband and I did internship and residency together, and it has haunted me since. This was an object lesson again that Lyme Disease is always in the differential.
I am a salaried general internist and primary care physician and board-eligible rheumatologist seeing patients since 1969. I am a general internist for patients at the Brigham and Women’s Hospital, an academic health center, and in the Veterans Administration. As a clinician-scientist, I study health services, the epidemiology of systemic rheumatic disease, health disparities, determinants of health outcomes, and have done more than 25 investigator-initiated clinical trials.
I also bring the view of a patient with chronic illness having had a six-vessel coronary by-pass operation in 2000 and a strept millerei lung abscess
and empyema in 2003 from a dental procedure. I am doing very well, thank you. I am inevitably drawn in as a coach for my extended family when they have a health problem, doing telephone consultations for my three 30+ year old children who work harder than I but are underinsured, internet inhabitants, and use alternative and over the counter therapies whenever they can, as does my bride. I am happy coaching.
Atypical acute Lyme Disease
A major challenge of ministering to patients in non-referral practices is not missing treatable illnesses that might present atypically. A study of consecutive patients with possible early Lyme disease either self- or physician-referred to a general internist with infectious disease training in a region with endemic Lyme disease (Aucott et al., 2009) suggests that about 39% of such patients do not meet CDC criteria nor alternative diagnostic criteria.
Of these nearly 40% had negative Lyme serology and an acute, viral-like illness without objective findings. Many of these patients had already been treated with antibiotics. In a quarter, another diagnosis could be made, including parvovirus, Ramsay Hunt syndrome or varicella zoster virus. About a third had a rash which did not meet criteria for EM and were thought to be local hypersensitivity reactions to tick bites or nonspecific or non-diagnosable lesions.
EM was the most common presentation of early Lyme disease in this case-series. However, prior misdiagnosis were common, similar to experiences reported from other endemic areas. While 80% of EM in the United States are uniformly red, only 19% have the stereotypical bull’s-eye appearance. Typically circular or oval, it can also be triangular, rectangular or distorted in other ways when occurring in areas such as the neck. Atypical EM may appear erythematous with central induration, urticarial-like, confluent red-blue lesions, vesicles, and with central necrosis.
If erythema migrans goes unnoticed, the disease may present months after the initial tick bite when the spirochete has disseminated. Disseminated Lyme disease often has symptoms of malaise, fatigue, or generalized or regional lymphadenopathy. Patients may have multiple, slow-growing erythema migrans lesions. Joint inflammation occurs in up to 70% of untreated patients with dessiminated disease and is typically mono- or oligoarticular, migratory, and involves the large joints and often recurring over several years.
Neurological Lyme involvement manifests as meningitis, cranial neuritis or radiculoneuritis. The most pronounced symptom is painful radiculoneuritis involving the chest or abdomen and like most neuropathies,
prominent at night. The facial nerve is the cranial nerve most commonly involved and can be bilateral.
Diagnosis (Corapi et al., 2008)
Early Lyme disease is best diagnosed by recognizing an erythema migrans lesion, which is present in 70-90% of cases. Laboratory testing should be used to confirm a clinician’s suspicion of Lyme disease rather than to be the sole basis of diagnosis. Serology is often negative early in the disease and may take three to four weeks for IgM antibodies to borrelia to appear and four to six weeks for IgG to be present. The American College of Physicians guidelines indicates that a patient with a high index of suspicion for Lyme disease in an endemic area for the disease may require no testing and that a diagnosis can be made solely on the clinical picture.
Serological testing is used to confirm Lyme disease in patients with disseminated disease with arthritis, carditis, or neurological involvement. Unfortunately the options for testing are not ideal and the results can be unreliable. One study demonstrated that 14-21% of laboratories failed to correctly identify positive samples (Bakken et al., 1997).
A two-test algorithm for active disease and for previous infection using a sensitive enzyme immunoassay (EIA) or immunofluorescent assay (IFA) followed by a Western immunoblot is recommended by the CDC (CDC, 1995). Specimens positive or equivocal by the EIA or IFA should be tested by a standardized Western immunoblot. Specimens negative by a sensitive EIA or IFA need not be tested further. When Western immunoblot is used during the first 4 weeks of disease (early LD), both IgM and IgG procedures should be used. A positive IgM test result alone is not recommended for determining active disease in persons with illness greater than 1 month’s duration because the likelihood of a false-positive test result for a current infection is high for these persons. If a patient with suspected early LD has a negative serology, serologic evidence of infection is best sought by obtaining acute and convalescent serum samples. Serum samples from persons with disseminated or late-stage LD almost always have a strong IgG response to Borrelia burgdorferi antigens.
It was recommended that IgM immunoblots be considered positive if two of the following three bands are present: 24 kDa (OspC), 39 kDa (BmpA), and 41 kDa (Fla) (1). It was further recommended that an that IgG immunoblot be considered positive if five of the following 10 bands are present: 18 kDa, 21 kDa (OspC), 28 kDa, 30 kDa, 39 kDa (BmpA), 41 kDa (Fla), 45 kDa, 58 kDa (not GroEL), 66 kDa, and 93 kDa. This serial testing has a specificity of 99-100%, but low sensitivity due to variable interpretation of results across laboratories.
Culture is labor-intensive, expensive, and time-consuming. Polymerase
chain reaction (PCR) detects the genetic material of the spirochete; positive results do not neccesarily indicate active infections. PCR may be helpful in suspected cases of co-infection or to confirm a clinical suspicion of Lyme disease. While PCR is useful for identifying the spirochete in skin, the technique is of marginal usefulness, as these cases can be diagnosed clinically.
Therefore, clinicians have to work with the limitations of the technology available. In most situations, as with most diagnostic testing, if the test result will not change how one treats or follows a patient or what they tell them, it should not be done. Serodiagnostics indicate exposures and whether the exposure has been recent or remote. Re-exposure and/or treatment can alter the results. The diagnostic test performance characteristics (i.e., its sensitivity, specificity, predictive value positive, and predictive value negative) of any test or testing algorithm is determined by the prior probability of the disease given a particular combination of symptoms and signs.
Treatment of early Lyme Disease
The aims in treating Lyme disease are to relieve symptoms and prevent the late stage complications. Delay in treatment increases a patient’s risk for treatment failure. In patients who present with or shortly after a tick bite, the question of antibiotic prophylaxis arises. A randomized, double-blind, placebo-controlled trial in a hyper-endemic area showed that Doxycycline as a single 200 mg dose was associated with fewer cases of subsequent erythema migrans; the primary endpoint of the study (Nadelman et al., 2001). While these results are significant, it is important to note that only a small number of subjects in the control group developed Lyme disease, resulting in a wide confidence interval. Also important is that 30% of patients treated with doxycyline experienced a drug adverse event such as nausea, vomiting, and diarrhea.
The 2006 IDSA guidelines suggest antibiotic prophylaxis only if all of the following four conditions are met: the attached tick is positively identified as an adult or nymphal Ixodes scapularis tick which has been attached for more than 36 hours, prophylaxis can be started within 72 hours of tick removal, local ecologic information demonstrates that local ticks have a borrelia burgdorferi infection rate of greater than 20%, and there are no contraindications to doxycycline use (Wormser et al., 2006). For pregnant or lactating women and children less than 8, in whom doxycycline should not be used, the guidelines do not recommend the use of substitute prophylaxis. Whether or not patients meet the criteria for antibiotic prophylaxis, the guidelines recommend that all patients who remove a tick should be observed for thirty days and treated promptly Lyme disease, human granulocytic anaplasmosis, or babesia.
When a patient presents with signs and symptoms suggestive of early
Lyme disease, whether localized or disseminated, doxycycline, amoxicillin, and cefuroxime axetil are effective. The IDSA recommends 10-21 days of oral doxycycline (100mg twice daily), 14-21 days of amoxicillin (500mg three times daily), or 14-21 days of cefuroxime (500mg twice per day) for the treatment of early, localized or disseminated disease. While 10 days may be a sufficient course of doxycycline, at least two weeks is needed for betalactam antibiotics because of their shorter half-lives (Wormser et al., 2003).
Doxycycline is the drug of choice as it is also effective against human granulocytic anaplasmosis, which may occur as a co-infection with Lyme disease. Amoxicillin is used when there is a contra-indication to using doxycycline. When a patient is unable to take both doxycycline and amoxicillin, cefuroxime or erythromycin may be used. Cefuroxime is as efficacious as doxycycline but is more expensive.
A randomized, double-blind, placebo-controlled study of one hundred and eighty patients with erythema migrans compared the efficacy of 10 days of oral doxycycline versus 20 days of oral treatment and combination oral doxycycline and intravenous ceftriaxone. The study concluded that treating patients for 10 days with oral doxycycline was as efficacious as the two other regimens studied (Wormser et al., 2003).More than 83% of patients in each treatment group described complete resolution of symptoms at the 30 month evaluation.
While antibiotics for the treatment of early Lyme disease are effective, 10-17% of patients continue to have problems(Wormser et al., 2003)and it is not clear as to why some patients with early Lyme disease improve and others do not.
Some patients with early Lyme disease have central nervous system involvement, such as radiculopathy, neuropathy, meningitis, or facial nerve palsy. Such patients require treatment with intravenous ceftriaxone (2g once daily) for 10-28 days. Alternatives to ceftriaxone include intravenous penicillin G or intravenous cefotaxime. Ceftriaxone has the advantage of once-daily dosing, making it the preferred agent. Intravenous administration is preferred to ensure adequate penetration of the blood-brain barrier. Data from Europe show that oral regimens, particularly doxycycline are also efficacious in neuroborreliosis. There is no definitive data to establish the superiority of either oral or parenteral therapy in the treatment patients with CNS involvement. Patients with evidence of increased intracranial pressure (papilledema, sixth cranial nerve palsy), may benefit from the addition of steroids, serial lumbar punctures, or CSF shunting. Although antibiotic treatment may not hasten the resolution of facial nerve palsy, treatment is recommended to prevent further sequelae such as Lyme arthritis.
Patients with cardiac involvement, namely first or second-degree atrioventricular block, should be treated with either oral or parenteral antibiotics for 14 days. Patients presenting with syncope, chest pain, or other cardiac
symptoms, require hospitalization to allow for continuous monitoring. The degree of heart block associated with Lyme disease is known to fluctuate and therefore careful monitoring is required. Intravenous ceftriaxone is felt to be useful in the management of hospitalized patients with cardiac involvement, but there are no studies addressing this. The placement of a cardiac pacemaker may be indicated in cases of severe heart block. Patients may switched to the standard oral antibiotic treatment for early Lyme disease once they are stable enough to be followed as outpatients.
Lyme arthritis can be treated with either oral or intravenous antibiotics. The majority of patients improve with one month of oral antibiotics, either doxycycline or amoxicillin. If arthritis persists after the initial course of treatment, then a second course should be tried. The improvement may be slow. A minority of patients continue to have arthritic symptoms, and non-steroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroid injections, and hydroxychloroquine, methotrexate, or infliximab have been used successfully.
The optimal length of intravenous antibiotics in late Lyme disease has not been determined. A prospective, open-label, randomized, multi-center study with one hundred and forty-three participants compared a 14- to 28-day regimen. All participants had a history of erythema migrans at least 3 months, dermatological, rheumatological, and neurological manifestations of disease, and no prior treatment for Lyme disease. The 14 days of ceftriaxone relieved the symptoms of late Lyme disease in 70% of patients; the same improvement rate was observed in the 28 day treatment group. Patients in both groups had higher cure rates at 12 months than when evaluated at 3 months; demonstrating that patients continue to improve even after completion of treatment (Wormser et al., 2003).Of note, 30% of patients remained symptomatic at the end of this study.
Approximately 15% of patients experience a Jarisch Herxheimer-like reaction in the first 24 hours of starting treatment. This involves worsening of systemic symptoms and an increase in the size and intensity of skin lesions. The majority of patients notice improvement by the end of the course of treatment. Erythema migrans lesions usually respond first and typically resolve within one to two weeks. Systemic symptoms, however, take longer to resolve. Three months after treatment, one in four patients may still have systemic complaints. Patients should be forewarned that they may still experience symptoms at the end of treatment and be reassured that in most cases they will improve steadily with time.
Treatment of persons with persistent symptoms after treatment for Lyme Disease
Some patients experience symptoms following treatment with appropriate antibiotic therapy for Lyme disease, a phenomenon known as post-Lyme syndrome (PLS). While the IDSA guidelines used the term PLS (Wormser et al., 2006), the International Lyme and Associated Diseases Society (ILADS) uses the term “chronic Lyme disease” (Cameron et al., 2004). The IDSA proposes a definition of the post-Lyme syndrome as persons who develop subjective symptoms within 6 months of their Lyme disease diagnosis, which last at least a further 6 months. Symptoms include cognitive dysfunction, fatigue, or persistent musculoskeletal complaints. The ILADS lists similar symptoms under their discussion of chronic Lyme disease.
The IDSA definition points out the importance of excluding pre-existing or concomitant disease that may account for the symptoms. Some of these may occur in “healthy” persons. For example, chronic fatigue occurs in up to 20-30% of the population. When a patient fails to respond to accepted antibiotic therapy it is important to consider the possibility of co-infection with another tick borne illness. Controversy exists as to whether PLS reflects persistent infection or not, and this has implications in the debate over how to manage such patients.
Two randomized controlled trials examined whether antibiotics are efficacious in PLS. One study examined fatigue and cognitive impairment as endpoints; the other improvement in quality of life. The first compared 28 days of placebo or intravenous ceftriaxone in 55 patients with PLS. While ceftriaxone improved fatigue, there was no improvement in either group in cognitive function. The authors concluded that antibiotics had no role in the treatment of PLS and pointed out that 7% of patients receiving ceftriaxone experienced serious side effects requiring hospitalization (Krupp et al., 2003).
The second study, of one hundred and twenty-nine patients, received either one month intravenous ceftriaxone followed by 60 days of oral doxycycline or placebo. Of note, none of the participants had evidence of persistent infection with borrelia burgdorferi by culture and PCR. The study was stopped after a planned interim analysis revealed no difference between the two groups in terms of quality of life (Klempner et al., 2001). While the authors conclude that antibiotics do not improve health related quality of life in patients with PLS, the findings could be interpreted more specifically that the antibiotic regimen of intravenous ceftriaxone and oral doxycycline does not benefit PLS patients.
The IDSA guidelines present the case against persistent infection in PLS while the ILADS guidelines support the idea. One expert guideline
states that patients with chronic symptoms of Lyme disease do not benefit from antibiotics, while the other expert group advocates their use. That two expert panels differ underscores the need to elucidate the pathogenesis of PLS and to develop improved treatment. Crucial to this effort will to develop a universally accepted definition of PLS to provide a framework in which to work.
Chronic previously unexplained symptoms attributed to Lyme Disease
As problematic as post-Lyme syndrome is the association of chronic previously unexplained symptoms (often cognitive difficulties and fatigue) with Lyme Disease. These persons have usually had exhaustive unproductive evaluations eventually having serologic testing for Lyme Disease which may be “positive.” One school of thought maintains that this is a subset of Lyme; the other believe that chronic Lyme disease is only the latest syndrome postulated to attribute previously unexplained symptoms to particular infections—other examples that have lost credibility being “chronic candida syndrome” and “chronic Epstein–Barr virus infection.” A review stated in no uncertain terms:
“The assumption that chronic, subjective symptoms are caused by persistent infection with B. burgdorferi is not supported by carefully conducted laboratory studies or by controlled treatment trials. Chronic Lyme disease, which is equated with chronic B. burgdorferi infection, is a misnomer, and the use of prolonged, dangerous, and expensive antibiotic treatments for it is not warranted.” (Feder et al., 2007)
When there is no answer
For some individuals, this declaration, although technically correct and the dominant opinion, provides no comfort nor acknowledgment of their suffering. Furthermore it may be heard or felt as being dismissive or disbelief in their symptoms.
I schedule an open-ended appointment, try to review all previous records before the visit or shortly after the encounter, and write my summary of what has been done. I take a careful review of systems and “park” anything that needs more detail to complete or check later., Some patients have found me by word of mouth or Googling and these patients don’t walk into my practice but I have screened them … so they come prepared. I ask them to keep a 24 diary of their routine and symptoms noting what has been tried, helped a little or not at all, and any side effects in case some treatments might be recycled or gradually increased to tolerance. It’s important
as one marches through trials of therapy that options are not abandoned if they have not had a chance to work.
I use my network of senior doctors for help. Nowadays I might even ask the question of Google. If there is any inkling, I have no reticence to refer the patient. When I don’t know or I don’t have any ideas, I think I am secure enough to tell the patient. I follow the adage, primum non nocere, and deconstruct what I am looking for and why and ask them to help me help them. Reassurance is not about saying something is ruled out but saying
Unexplained fatigue, cognitive dysfunction, lancinating pain without focal abnormalities occur but when they persist are a source of consternation for both the person and the physician asked to sort it out and treat. The exhaustive differential diagnosis is in theory long but after a year or 2 in diagnostic pursuit of a label, and the symptoms unchanged is unlikely to be related to life-threatening disease but it is a major source of suffering.
The longer the symptoms and the higher the number of previous unsuccessful therapy, the less probable is it that I will come up with a new possibility (although I have) or that all symptoms will vanish with treatment. It’s important to be honest in establishing expectations that they be attainable.
I try never start a treatment with potential side effects if I cannot state à priori what the criteria of success will be. It’s essential that the criteria for success be discussed with the person affected and that they agree and believe it (Daltroy, 1993). I give my rationale as to why the medication might be helpful, its mode of action, and guess how likely it is to work, all conceivable side effects. I might increase the dose to what is a therapeutic dose by increasing the medication to tolerance and/or effect. Cost is almost never a consideration unless the patient is paying for it. Interestingly, some patients elect the most expensive option because they believe it to be better (“you pay for what you get”). The decision should never be made on one visit.
When I can find no objective evidence of Lyme exposure nor make an alternative diagnosis to explain the symptoms, there is no book on what to do next. Being older, acknowledging my inability to make a specific diagnosis doesn’t make me feel anxious or less competent. I am also comfortable asking, “Could this be due to depression or stress?” It may help validate and legitimize their feelings and give them permission to discuss difficulties instead of a medical problem.
I explain that we are not always able to find a reason for many symptoms but this does not prevent us from trying things that might help balancing harms and benefits for the most troubling symptoms while providing support (Aronowitz, 2001). Every person and patient is slightly different but the trials of therapy have a patient chart and rate their symptoms and moves from realistic assessment of what we can do, revising expectations
often, to life style modification (“working and living within their ability”) before trials of medications directed at sleep disturbance, pain management.
Prevention of Lyme Disease
Not emphasized enough and unfortunately not covered in the reimbursement of health care is the teachable moment or opportunity that is afforded when a person comes in with suspected Lyme Disease in an endemic area. Prevention strategies directed at the environment, ticks and/or the vector, reviewed elsewhere, theoretically could put a stop to Lyme disease (Corapi et al., 2007; Hayes and Piesman, 2003). Protective behaviors, tick-avoidance or tick checking and removal, can be highly effective, voluntary, economical, and suitable for residents and visitors to endemic areas. Avoidance involves recognition and reduction of time spent in high-risk areas (woods, brush, and tall grass). Protective clothing, such as long-sleeved shirts and long pants, should be light colored so that ticks can be easily detected. Tick repellents can be applied to skin or clothing. The repellant DEET (N,N-diethyl-meta-toluamide, Morflex® Inc. Greensboro, NC) may be used on the skin; however, it is harmful to children in large doses, is neurotoxic, and must be reapplied every few hours for maximal effect. Permethrin, a repellant applied to clothing, kills ticks upon exposure, but should not come into contact with skin.
Effective tick check and removal behaviors are truly “green” approaches and take advantage of the fact that an infected tick has to be attached and feed for anywhere from 24 to 72 hours to transmit infection. The messages must be combined with an appreciation of the barriers to their practice (Shadick et al., 1997). A daily visual and manual search of exposed skin after visiting tick-infested areas provides an opportunity to identify and remove feeding ticks. A novel effective theory-based public education intervention demonstrating tick avoidance and removal health behaviors directed towards travelers to an epidemic area reduced the incidence of disease and is a model that could be implemented elsewhere (Daltroy et al., 2007).
In 1998 a recombinant vaccine against Lyme disease was approved by the FDA but was withdrawn after 4 years due to poor sales. This probably occurred due to lingering concerns about its long-term safety and because its immunization schedule was inconvenient requiring 3 injections before transmission season started to ensure optimum antibody levels and boosters because protective antibody titers declined rapidly.
Vaccination is cost effective where the incidence of Lyme disease is greater than 1% (Hsia et al., 2002; Shadick et al., 2001; Sigal, 2002) and would only be recommended for persons who reside, work, or do recreational activities in high-risk areas. A vaccine is unlikely to be 100%
effective, and would not protect against other tick-borne illnesses; therefore, efforts to avoid contact with ticks would still be required.
Conclusion
Lyme disease continues to be a problem and even grow as hosts, vectors and man live closer together with the reduction of the forest habitat. While effective antibiotics have been identified for the early localized and disseminated stages of Lyme disease considerable uncertainty surrounds the management of patients with post-Lyme syndrome. More research needs to be done to understand the pathophysiology of persistent symptoms.
Educating patients on prevention strategies is essential in decreasing the annual incidence. Health educational programs in endemic areas beginning in the school and others focused on vacationers in these areas should be a priority. Another vaccine may be developed but is likely to face the same problems in gaining acceptance and reinforce complacency with tick avoidance and tick removal behaviors.
Acknowledgments
I am indebted to Dr. Lynn Gerber who made suggestions on an earlier draft.
References
Aronowitz, R. A. 2001. When do symptoms become a disease? Ann Intern Med 134 (9 Pt 2):803-8.
Aucott, J., C. Morrison, B. Munoz, P. C. Rowe, A. Schwarzwalder, and S. K. West. 2009. Diagnostic challenges of early Lyme disease: lessons from a community case series. BMC Infect Dis 9:79.
Bakken, L. L., S. M. Callister, P. J. Wand, and R. F. Schell. 1997. Interlaboratory comparison of test results for detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists Proficiency Testing Program. J Clin Microbiol 35 (3):537-43.
Cameron, D., A. Gaito, N. Harris, G. Bach, S. Bellovin, K. Bock, S. Bock, J. Burrascano, C. Dickey, R. Horowitz, S. Phillips, L. Meer-Scherrer, B. Raxlen, V. Sherr, H. Smith, P. Smith, and R. Stricker. 2004. Evidence-based guidelines for the management of Lyme disease. Expert Rev Anti Infect Ther 2 (1 Suppl):S1-13.
CDC. 1995. Recommendations for test performance and interpretation from the Second National Conference of. MMWR: Morbidity & Mortality Weekly Report 44 (31):590.
Corapi, K. M., M. I. White, C. B. Phillips, L. H. Daltroy, N. A. Shadick, and M. H. Liang. 2007. Strategies for primary and secondary prevention of Lyme disease. Nat Clin Pract Rheumatol 3 (1):20-5.
Corapi, Kristin M, Samardeep Gupta, and Matthew H Liang. 2008. Management of Lyme disease. Expert Review of Anti-infective Therapy 6 (2):241-250.
Daltroy, L. H. 1993. Doctor-patient communication in rheumatological disorders. Baillieres Clin Rheumatol 7 (2):221-39.
Daltroy, L. H., C. Phillips, R. Lew, E. Wright, N. A. Shadick, and M. H. Liang. 2007. A controlled trial of a novel primary prevention program for Lyme disease and other tick-borne illnesses. Health Educ Behav 34 (3):531-42.
Feder, H. M., Jr., B. J. Johnson, S. O’Connell, E. D. Shapiro, A. C. Steere, G. P. Wormser, W. A. Agger, H. Artsob, P. Auwaerter, J. S. Dumler, J. S. Bakken, L. K. Bockenstedt, J. Green, R. J. Dattwyler, J. Munoz, R. B. Nadelman, I. Schwartz, T. Draper, E. McSweegan, J. J. Halperin, M. S. Klempner, P. J. Krause, P. Mead, M. Morshed, R. Porwancher, J. D. Radolf, R. P. Smith, Jr., S. Sood, A. Weinstein, S. J. Wong, and L. Zemel. 2007. A critical appraisal of “chronic Lyme disease”. N Engl J Med 357 (14):1422-30.
Hayes, Edward B., and Joseph Piesman. 2003. How Can We Prevent Lyme Disease? New England Journal of Medicine 348 (24):2424-2430.
Hsia, E. C., J. B. Chung, J. S. Schwartz, and D. A. Albert. 2002. Cost-effectiveness analysis of the Lyme disease vaccine. Arthritis Rheum 46 (6):1651-60.
Klempner, M. S., L. T. Hu, J. Evans, C. H. Schmid, G. M. Johnson, R. P. Trevino, D. Norton, L. Levy, D. Wall, J. McCall, M. Kosinski, and A. Weinstein. 2001. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 345 (2):85-92.
Krupp, L. B., L. G. Hyman, R. Grimson, P. K. Coyle, P. Melville, S. Ahnn, R. Dattwyler, and B. Chandler. 2003. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology 60 (12):1923-30.
Nadelman, R. B., J. Nowakowski, D. Fish, R. C. Falco, K. Freeman, D. McKenna, P. Welch, R. Marcus, M. E. Aguero-Rosenfeld, D. T. Dennis, and G. P. Wormser. 2001. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med 345 (2):79-84.
Shadick, N. A., L. H. Daltroy, C. B. Phillips, U. S. Liang, and M. H. Liang. 1997. Predictors of tick avoidance behaviors in an endemic area for Lyme Disease. American Journal of Preventive Medicine 13:265-270.
Shadick, N. A., M. H. Liang, C. B. Phillips, K. Fossel, and K. M. Kuntz. 2001. The cost-effectiveness of vaccination against Lyme disease. Arch Intern Med 161 (4):554-61.
Sigal, L. H. 2002. Vaccination for lyme disease: Cost-effectiveness versus cost and value. Arthritis & Rheumatism 46 (6):1439-1442.
Wormser, G. P., R. J. Dattwyler, E. D. Shapiro, J. J. Halperin, A. C. Steere, M. S. Klempner, P. J. Krause, J. S. Bakken, F. Strle, G. Stanek, L. Bockenstedt, D. Fish, J. S. Dumler, and R. B. Nadelman. 2006. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43 (9):1089-134.
Wormser, G. P., R. Ramanathan, J. Nowakowski, D. McKenna, D. Holmgren, P. Visintainer, R. Dornbush, B. Singh, and R. B. Nadelman. 2003. Duration of antibiotic therapy for early Lyme disease. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138 (9):697-704.
Zhang, X., M. I. Meltzer, C. A. Pena, A. B. Hopkins, L. Wroth, and A. D. Fix. 2006. Economic impact of Lyme disease. Emerg Infect Dis 12 (4):653-60.
A10
DISEASE SURVEILLANCE AND CASE DEFINITIONS IN TICK-BORNE DISEASES
Prepared for the IOM Committee on Lyme Disease and Other Tick-Borne Diseases: The State of the Science James L. Hadler, M.D., M.P.H. September 20, 2010
This briefing on case definitions and surveillance for tick-borne disease is presented in three main sections: Background on surveillance and methods, particularly as they relate to tickborne diseases and nationally notifiable diseases; Lyme disease surveillance and case-definitions; and Public Health Surveillance for other tick-borne diseases in the United States.
Background
Public health surveillance is the ongoing, systematic collection, analysis, interpretation, and dissemination of data regarding a health-related event for use in public health action to reduce morbidity and mortality and to improve health (CDC, 2001). For any given public health surveillance activity, it is critical to define the purpose of surveillance, use surveillance methods that are efficient and appropriate to achieving that purpose, and subsequently evaluate whether surveillance efforts are meeting the surveillance objectives (CDC, 2001; Meriwether 1996).
Depending on the objectives, a variety of methods can be used to conduct vector-borne public health surveillance (Hadler and Petersen, 2007). For example, to evaluate potential and emerging tickborne diseases, ongoing systematic efforts can be done to capture vector ticks, monitor their population size and determine infection rates. In addition, if appropriate serologic tests are available, serosurveys can be done to monitor the percentage of the population that has been infected with the disease agent. To determine the annual burden of human illness and its epidemiology, surveillance for human illness using provider and/or laboratory reporting to public health authorities, analysis of hospital discharge and death data, and for high incidence diseases population surveys can be done. To determine and monitor the prevalence of risk factors for tickborne disease (e.g., spending time outdoors, tick bites) and prevention practices (e.g., daily tick checks, wearing long light-colored pants tucked into socks, use of insect repellants), regular telephone and/or community surveys can be done.
Each method of surveillance has its particular limitations, however. Surveillance for ticks and tick infection rates is limited in part by the need to sample, the uneven distribution of vectors and infection rates
geographically, and the need to confirm human risk by obtaining human infection data. Human disease reporting is limited by the need for laboratory and/or explicit symptom confirmation for diseases such as Lyme disease in which many other diseases may present with similar symptoms, and underreporting by healthcare providers who do not take the time needed to report. Reporting of laboratory findings alone, while less subject to underreporting than clinician reporting, is limited in part by the fact that positive tests may indicate infection long in the past or be falsely positive, necessitating the need to get clinical information to back up the laboratory report. In addition, they can take weeks to turn positive, so that persons in the early stages of infection may not have positive laboratory tests. Telephone and community surveys may have limited and non-representative response rates, and the results of community surveys are only clearly applicable to the communities in which they are done. In addition, each of these methods of surveillance has substantial costs to conduct and maintain.
Human Disease Surveillance and Nationally Notifiable Diseases
Constitutionally, local public health is a responsibility of state rather than federal government (Moulton et al., 2007). Correspondingly, surveillance for human disease using mandatory reporting of cases and laboratory findings to public health authorities is generally a state and local health department function rather than a federal one. State and local health departments have the legal authority, spelled out in statute in each state, to collect personally identifiable data on persons with selected diseases from laboratories and clinicians. Each state conducts surveillance for human disease according to its needs and resources. There is no standard list of diseases for which all states have reporting, and each has its own legislatively specified means for adding diseases to its state-specific list. The federal Centers for Disease Control and Prevention (CDC) conducts national surveillance for diseases reportable at the state level through collaboration with states via the Council of State and Territorial Epidemiologists (CSTE). Through resolutions passed by the majority of state representatives attending the annual CSTE meeting each June (a quorum is required, one vote per state present), a list of nationally notifiable diseases reportable to the CDC has been established, known as the National Notifiable Disease Surveillance System (NNDSS). For a disease to be included in the NNDSS, the purpose of national surveillance and a case definition to be used to count cases for national purposes must be agreed upon. Placement of a disease in the NNDSS does not obligate each state to conduct surveillance for it or to conduct surveillance in a standardized manner. The NNDSS is simply an agreement that states that conduct surveillance will de-identify and share their information with CDC using standard case definitions. Placement of
a disease in the NNDSS also does not guarantee that resources are or will be available to each state to conduct surveillance. However, to the extent that CDC provides funding through cooperative agreements with states, CDC can require recipients to conduct surveillance for a specific disease and specify surveillance methods within the limits of funding provided.
Principles of Surveillance Based on Case Reporting—Case Definitions
There are at least four important principles of public health surveillance for human illness through disease reporting. First, surveillance for human disease based on clinician or laboratory reporting generally requires that a suspected case be confirmed. Cause-specific diagnoses based on a physician’s best guess may be wrong, especially for persons with symptoms that can be caused by a number of different microbial agents or mechanisms other than infection (e.g., fever, malaise, skin rash, arthritis, headache, cough, diarrhea). Positive laboratory tests alone may reflect past disease (e.g., serologic tests for antibodies) or a carrier state without disease (e.g., bacterial colonization of the intestinal tract). Thus, case definitions are needed to define relevant symptoms in combination with relevant laboratory results that make it highly likely that a “case” really has the disease under surveillance, or, when laboratory confirmation is not possible, to define symptoms and findings that are characteristic of only of the disease under surveillance (e.g., erythema migrans for Lyme disease).
Second, because of the need for laboratory confirmation to make sure that only real cases of disease due to any given microbe are being counted, surveillance based on reporting is likely to underestimate the true magnitude of a disease. Cases for which it is technically difficult or too costly to confirm will not be counted, nor will cases that go unreported. Despite the legal requirements for reporting, some clinicians never get around to reporting patients they suspect of having a disease. To determine the true number of people with a given disease (e.g., Lyme disease), it may be necessary to conduct population and/or provider surveys.
Third, it is not critical for most surveillance purposes to count every possible case of a disease. For purposes of monitoring a disease over time to determine whether its epidemiology (which groups are most affected) is changing and its occurrence is stable, increasing or decreasing, it is only necessary to count cases in the same way and to invest the same effort over time. If one monitors a consistent part of the “iceberg” of disease, then changes in it will reflect what is happening to the whole iceberg.
Finally, consistency of the means of surveillance is important. It can be expected that there will be under-reporting and/or inability to follow-up every reported clinical case and laboratory finding because of resource restraints. If no funding is appropriated for surveillance, it may be impossible
for a health department to conduct follow-up on thousands of laboratory reports to find out if a person had symptoms consistent with recent disease.
Lyme Disease Surveillance and Case Definitions
Of the five tickborne diseases under national surveillance in the U.S., Lyme disease is by far the most common and has had the most public interest and dynamic surveillance history. Lyme disease was first recognized as a distinct entity in 1975 by epidemiologists investigating an apparent cluster of juvenile arthritis cases in Lyme, Connecticut. Informal national surveillance for human illness via annual surveys of states conducted by the CDC began in 1980 (CDC, 1981), and Lyme disease was formally added to the National Notifiable Disease Surveillance System in 1991 (CSTE, 1990). The causative infectious agent, Borrelia burgdorferi, was recognized in 1982, after which time laboratory tests were developed and gradually over several years began to be available for surveillance and clinical purposes.
Features of Lyme Disease Relevant to Surveillance
Lyme disease has a number of clinical, laboratory and epidemiologic features that make conducting surveillance for human illness a challenge. These include: 1) erythema migrans (EM), an early stage disease manifestation and the most common one, a spreading skin lesion that begins as a papule or macule and over the course of days to weeks becomes a red, expanding lesion that must be diagnosed clinically because supportive laboratory tests are often negative, and only begins to be readily distinguishable from insect bite reactions or local skin infections when it gets to a substantial size and has a characteristic “target” pattern; 2) later clinical manifestations such as arthritis, neurologic involvement (lymphocytic meningitis, Bells’ palsy, radiculoneuropathy) and cardiac complications (transient, high grade atrioventricular conduction defects sometimes accompanied by myocarditis) that are not unique to Lyme disease and need laboratory confirmation of B. burgdorferi infection; 3) confirmatory laboratory test methods which produce results that can be falsely positive and result in mistaken diagnosis of Lyme disease, especially in geographic areas where neither a competent tick vector (Ixodes ticks) nor B. burgdoreri are present; 4) positive serologic tests occurring after true infection that can remain positive indefinitely, making it essential that there be corroborating clinical data to back up laboratory findings (i.e., laboratory findings alone cannot be used for surveillance); 5) transmission from a tick that is small enough that attachment and feeding (i.e., “bites”) often go unnoticed, making a history of having an antecedent tick bite an insensitive way to conduct surveillance; and 6) limited geographic areas in which infected, competent
tick vectors are present, making it important for epidemiologic and public health purposes to distinguish between human disease in “endemic” areas and disease diagnosed in residents of geographic areas without a previous history of Lyme disease.
Case Definitions of Lyme Disease for Public Health Surveillance
The objectives of formal national public health surveillance for human Lyme disease were agreed upon at the CSTE meeting in 1990 and have not changed since: (1) define the demographic, geographic, and seasonal distribution; (2) consistently monitor disease trends; (3) identify risk factors for transmission in areas where Lyme disease is newly emerging; and (4) develop strategies of prevention and control and evaluate the impact of prevention and control measures (CSTE, 1990, 2007). The recommended methods of surveillance for disease have also not changed: a combination of clinician and laboratory reporting of suspected cases with public health follow-up as needed to obtain detailed clinical information to confirm cases. The above clinical, laboratory and epidemiologic features of Lyme disease have been taken into consideration in the consensus case definitions that have been crafted over time by CSTE and CDC for surveillance to meet these public health surveillance objectives (CDC, 1990, 1997; CSTE, 2007). Importantly, as noted in the publication of each case definition, the surveillance definition for Lyme disease (and for other diseases under public health surveillance) was developed for national reporting of Lyme disease; it is NOT appropriate for clinical diagnosis, including determination of reimbursement by insurers.
To enable comparability between states and over time, public health epidemiologists have favored restriction of clinical manifestations that can be counted to those that are most likely to be Lyme disease rather than counting all possible cases. Thus, measurement of geographic distribution, the descriptive epidemiology and trends in Lyme occurrence within geographic areas have been emphasized over measuring the full magnitude of the problem.
To overcome the lack of specificity of small, evolving erythema migrans (EM) lesions, a lesion must be at least 5 centimeters in diameter to be counted. Annular erythematous lesions occurring within several hours of a tick bite represent hypersensitivity reactions and do not qualify as EM. For later musculoskeletal (joint), neurologic and cardiac manifestations to be counted, there must be laboratory confirmation. In addition, to count arthritis as being due to Lyme disease, the arthritis must be adequately characterized. Recurrent, brief attacks (weeks or months) of objective joint swelling in one or a few joints, sometimes followed by chronic arthritis in one or a few joints is typical of Lyme arthritis. However, manifestations not
considered as criteria for diagnosis include chronic progressive arthritis not preceded by brief attacks and chronic symmetrical polyarthritis. Additionally, arthralgia, myalgia, or fibromyalgia syndromes alone are not criteria for musculoskeletal involvement. To count neurologic manifestations, any of the following, alone or in combination qualify in the absence of another explanation: lymphocytic meningitis; cranial neuritis, particularly facial palsy (may be bilateral); radiculoneuropathy; or, rarely, encephalomyelitis. Encephalomyelitis must be confirmed by demonstration of antibody production against B. burgdorferi in the cerebrospinal fluid (CSF), evidenced by a higher titer of antibody in CSF than in serum. Headache, fatigue, paresthesia, or mildly stiff neck alone are not criteria for neurologic involvement. Similar restrictions apply for cardiovascular manifestations. Acute onset of high-grade (2nd-degree or 3rd-degree) atrioventricular conduction defects that resolve in days to weeks and are sometimes associated with myocarditis can be counted. However, palpitations, bradycardia, bundle branch block, or myocarditis alone are not criteria for cardiovascular involvement.
To increase the probability that a case reported from a county in which Lyme disease has not previously been recognized is truly a case of Lyme disease, persons with suspected EM either should have been in a county in which Lyme disease is known to be endemic some time in the preceding 30 days or have laboratory confirmation. Endemic counties are those in which at least two laboratory confirmed cases meeting the clinical criteria defined above have been acquired and/or in which a known tick vector has been shown to be infected with B. burgdorferi. Of note, having a tick bite is not required for a case to be counted, as only about 20% of cases will report noticing a tick bite in the 3-30 days prior to the onset of EM (CDC, 1982).
There is also a definition for laboratory confirmation to assure the same standards for considering a test positive are used across states and, to the extent possible, over time.
Modification of the Case Definition Over Time
The purpose of public health surveillance for Lyme disease has not changed over time. Thus with one exception (2007), there has not been a particular need to radically change the case definition. The focus has continued to be on specificity (counting only true cases) and on consistency in who is counted in order to monitor trends in geographic distribution and incidence within geographic areas over time.
The original case definition for national public health surveillance published in 1990 and used beginning in 1991 has been modified twice, in 1996 and in 2007 for use beginning the year after modification (CDC, 1990, 1997; CSTE, 1990, 1996, 1997). Most of the modifications have been
to the laboratory criteria for confirming a diagnosis to incorporate new laboratory testing methods and to standardize methods for counting tests as positive for surveillance purposes. The latter has become particularly important as test methods for Lyme disease have proliferated and testing has become more common, and as states have been using laboratory reporting to supplement provider reporting to conduct surveillance for Lyme disease. In the 1990 definition, the laboratory criteria for a positive test were: 1) isolation of Borrelia burgdorferi from a clinical specimen, or 2) demonstration of diagnostic levels of IgM and IgG antibodies to the spirochete in serum or CSF, or 3) a significant change in IgM or IgG antibody response to B. burgdorferi in paired acute- and convalescent-phase serum samples. States were authorized to determine their own criteria for laboratory confirmation and diagnostic levels of antibody (CDC, 1990).
The main change in the case definition in 1996 was a new recommendation to use a two-test approach for laboratory confirmation, using a sensitive enzyme immunoassay or immunofluorescence antibody test followed by immunoblot confirmation (CDC, 1997; CSTE, 1996). In 2007, the laboratory criteria were modified slightly. The criterion: “demonstration of diagnostic levels of IgM and IgG antibodies in serum or CSF” was removed and a requirement made for “single-tier IgG immunoblot seropositivity interpreted using established criteria.” (CSTE, 2007) This completed a shift from dependence on serologic tests using IFA or ELISA methods to only relying on immunoblot methods for confirmation, a positive immunoblot test providing firmer evidence of B. burgdorferi infection than positive tests using the other two methods.
There was another important revision contained in the 2007 case definition. In addition to having a category of “confirmed” cases, two new categories with less stringent criteria were added, “probable” and “suspect” cases. “Probable” cases were defined as “any other case of physician-diagnosed Lyme disease that has laboratory evidence of infection” (as defined above). This means that persons with laboratory criteria for infection who do not meet the strict clinical criteria specified in the confirmed case definition could be counted—for example, persons whose EM diameter is less than 5 centimeters and persons with any disease manifestation that a clinician diagnosed as Lyme disease. “Suspected” cases were defined as “a case of EM where there is no known exposure” (i.e., not having been in an endemic county in the 30 days before EM onset) and no laboratory evidence of infection, or “a case with laboratory evidence of infection but no clinical information available” (i.e., a person with only a positive laboratory report [as defined above]) (CSTE, 2007). The purpose of this change is to enable states to count more cases if they so chose and to better account for the surveillance burden of the huge number of laboratory reports, a burden that some states have been unable to meet (i.e., make efforts to
obtain clinical information), resulting in potentially decreased confirmed case counts. Both confirmed and probable cases are designated as under national as well as state surveillance. The suspect case category is a category designed for optional use by states only. With these changes, it is expected that the national case counts will increase.
Findings from National Surveillance
Taking the national surveillance findings at face value, national public health surveillance for Lyme disease has largely met the major public health surveillance objectives. The demographic, geographic and seasonal distribution of Lyme disease have been defined and trends from 1992 to 2006 have been measured (CDC, 2008). By age, the pattern is similar from year to year with all age groups being affected but incidence being bimodal with 5-9 year olds and 45-49 year olds providing the most cases. The sex distribution has been slowly changing over time, with the percentage of cases that are male gradually increasing, especially among 5-19 year olds and in the 10 states with the highest incidence. Clinically, EM has been present in nearly 70% of reported cases overall and over time, with a fairly wide variation by state, ranging from 87% in Minnesota to 51% in Delaware. Seasonally, new diagnoses of Lyme disease occur throughout the year, with peak occurrence of both early (EM) and later stage (arthritis, neurologic and cardiac) diagnoses during June through August when vector ticks most actively seek mammalian hosts and people spend the most time outdoors. Most importantly, national surveillance has documented the slowly expanding geographic distribution of Lyme disease and its initial intensification in areas as they become endemic, followed by reaching a fluctuating plateau in many endemic areas as, presumably, the ecologic dynamics of the tick, mice, deer and B. burgdorferi populations stabilize.
Figure A10-1 shows the annual number of reported cases to CDC from 1982 to 2008, including the period of informal national surveillance from 1982 to 1990.
There has been a steady upward trend in number of reported confirmed cases. Underlying this trend is an increasing number of states identifying and reporting Lyme disease (from 11 in 1982 to 21 in 1984 to all 50 by 1987) and increasing rates in most states and counties. Figure A10-2 shows maps of Lyme disease incidence by county in the U.S. in 1999 (the first time a county-level map was published by the CDC) and 2007. These illustrate the continually expanding geographic distribution and intensification in counties bordering well-established areas. In addition, using data from human surveillance, risk factor studies have been done (Ley et al., 1995; Orlosky et al., 1998; Cromley et al., 1998), a map of risk of acquiring Lyme disease was produced to guide vaccination recommendations when a
FIGURE A10-1 Annual number of reported cases to CDC 1982 to 2008.
vaccine was transiently available (CDC, 1999), and prevention and control demonstration projects have been conducted in high Lyme disease incidence areas (Vazquez et al., 2008; Connally et al., 2009; Gould et al., 2008).
Impact of Case Definition and Other Factors Affecting Number of Reported Cases
As previously mentioned, counting every single diagnosed case of human Lyme disease has not been the purpose of ongoing national public health surveillance. However, it is important to be able to define the extent to which current surveillance methods may underestimate the magnitude of the problem. With data from special studies conducted by states, often with CDC support, it is possible to crudely estimate the extent of undercounting of Lyme disease cases—or at least those with disease manifestations that are widely accepted as being due to B. burgdorferi infection. Studies done in Connecticut and Maryland in the early 1990s examined underreporting by physicians, and estimated that only 6-12% of EM cases were actually reported (Meek et al., 1996; Coyle et al., 1996). Laboratory reporting, which is particularly important for the 30% of reported cases that do not have EM, tends to be much more complete, but each report needs follow-up to obtain clinical data. States that attempt to follow up on positive laboratory
reports to obtain clinical information that could make a person with a positive laboratory test countable as a case, find that they have success in getting case information back from physicians on only about 40-50% of positive laboratory reports (Connecticut Department of Public Health and New York State Department of Health, personal communication). Assuming these findings apply to all states and are still pertinent, and that EM accounts for 70% of all reported cases, one can estimate that for every counted, reportable case, another 6-12 countable cases occur.
To the extent that the above factors, underreporting of EM and unsuccessful follow-up of positive laboratory reports, are stable, changes in numbers of cases reported and counted should be meaningful. However, to the extent that they are unstable, artifactual increases or decreases could occur. In recent years, several important and interrelated sources of instability of surveillance efforts have been identified, resulting in challenges to interpretation of trends within some states and nationally. Instability of effort has occurred when funding for surveillance has changed and as the work of surveillance has increased with increasing use of laboratory tests for Lyme disease. For example, between 1998 and 2002, Connecticut used CDC funding given to evaluate vaccine impact to support two full-time positions to initiate full scale laboratory result-based surveillance with multiple attempts at follow up of positive laboratory test results to obtain clinical information. This was done in part to be able to evaluate vaccine impact on laboratory test result-defined disease as well as on EM. As a consequence, the number of reported cases more than doubled (Ertel et al., 2006). After the vaccine was withdrawn and CDC switched emphasis from supporting enhanced surveillance to evaluate vaccine impact to other prevention efforts, Connecticut could no longer sustain nor needed such a labor-intensive level of surveillance and ceased laboratory surveillance beginning in 2003 in favor of Lyme disease prevention efforts. The result was a 70% decrease in cases from 4,631 in 2002 to 1,403 in 2003, although physician-reported cases were stable (Ertel et al., 2006).When reporting from laboratories that could submit data electronically was restarted in 2007 but with some lesser efforts at follow-up, the reported case count rose 71% from 1,788 in 2006 to 3,058 in 2007 (Ertel et al., 2008). In 2007, the Connecticut Department of Public Health had 16,799 positive laboratory reports. In New York State (excluding New York City), the number of unique persons with positive laboratory reports needing follow-up increased from 18,420 in 2005 to 38,503 in 2008 (New York State Health Department, personal communication).
These issues need to be taken into account when interpreting trends in national as well as state data. Thus, interpretation of national trends has balanced issues of changes in intensity of surveillance with data from states with stable surveillance. For example, from 2001 to 2002, there was
a 40% increase in the number of reported cases of Lyme disease nationally. The interpretation was “Factors potentially contributing to the increase in reported cases include growing populations of deer that support the Ixodes tick vector, increased residential development of wooded areas, tick dispersal to new areas, improved disease recognition in areas where LD is endemic, and enhanced reporting” (CDC, 2004). When there was no substantial change in incidence from 2003-2005, in part because Connecticut dropped laboratory reporting and other states were also adjusting surveillance methods to resources, the interpretation was more measured: “Since Lyme disease became nationally notifiable in 1991, the annual number of reported cases has more than doubled. This increase likely is the result of several factors, including a true increase in disease incidence and enhanced case detection resulting from implementation of laboratory-based surveillance in several states…. To address this surveillance burden (laboratory reporting) and create more sustainable Lyme disease surveillance systems, some states (e.g., Connecticut) have modified components of their systems, leading to acute reductions in reported cases. However, no evidence exists to suggest a true decrease in Lyme disease incidence in these states” (CDC, 2007).
Other Lyme Disease Surveillance Activities
Although this report focuses on national public health surveillance for human Lyme disease, it should be noted that many states also conduct surveillance for vector tick density and percentage of Ixodes ticks infected with B. burgdorferi. This form of surveillance is used to complement human surveillance, to help define whether a county with reported cases for the first time is becoming endemic, and to help interpret fluctuating incidence in highly endemic areas. It has been well established that infected tick density can vary from year to year and that variations in infected tick density within a county or state correlate well with variation in human incidence (Stafford et al., 1998; Mather et al., 1996).
Current Issues in Human Lyme Disease Surveillance: Questions and Answers
There are a number of Lyme disease human surveillance issues that have been raised recently by various persons and groups including state and local health departments conducting surveillance for Lyme disease. The following is a list of some of the issues with discussion in Issue and Response format.
Issue: Should we be making more of an effort through public health surveillance to fully measure the full magnitude of the Lyme disease problem
annually, in part to call more attention to it and possibly to get more funding devoted to it?
Response: This would require a change in the objectives of public health surveillance and a change in the Lyme disease surveillance case definition to make it inclusive of any clinician-reported case of EM and any clinician-diagnosed case who has a positive laboratory test for B. burgdorferi infection. Such a change would require the consensus of the majority of the official state representatives (usually the State Epidemiologist) at the annual CSTE meeting. While CSTE decided not to change the objectives of surveillance the last time this was considered in 2007, they did partially address the issue of measuring more of the Lyme disease problem by creating new categories of Lyme disease case reports (CSTE, 2007). Beginning in 2008, any person with a positive laboratory test meeting the laboratory criteria who had a physician diagnosis of disease regardless of symptoms could be called a “probable” case. Thus, persons with non-classical manifestations of Lyme disease potentially could be counted as cases. In addition, persons with clinician-diagnosed EM that was less than 5 cm in diameter could be counted if they had a positive laboratory test. Further, persons with a qualifying positive laboratory test but no clinical information could be counted as a “suspect” case. Thus, all persons with qualifying positive laboratory reports can be counted in state-level surveillance. For national surveillance, however, only those with confirmed and probable status will be counted. It will take some years using this system before any additional changes are likely to be considered. Data from 2008 illustrates that it will take time for this system to become fully established. In 2008, there were a total of 28,921 confirmed and 6,277 probable cases reported nationally, a ratio of 0.22 probable cases per confirmed case (CDC, 2010). While 49 states (including Washington, DC) reported at least one confirmed case, only 36 states reported at least one probable case. Neither Pennsylvania nor Delaware, together accounting for 4,590 confirmed cases, reported any probable cases, although at least 1,000 probable cases might have been expected. Given inadequate staffing to initiate follow up or successfully follow up on laboratory reports, suspect cases are likely to provide many more additional reports than probable cases. In 2008, Connecticut reported 2,738 confirmed and 1,158 probable cases, but identified an additional 3,106 suspect cases in state surveillance (Connecticut Department of Public Health, personal communication).
Issue: How can we accurately determine the full magnitude of the human Lyme disease problem in the U.S.?
Response: A definition of what should be included in the full magnitude of the Lyme disease problem is needed. It potentially includes the following: 1) persons truly infected with B. burgdorferi who have widely agreed upon (“classic”) symptoms and disease (currently being partially captured
through confirmed case surveillance); 2) persons truly infected with B. burgdorferi who have non-classical Lyme disease symptoms that some would attribute to B. burgdorferi (e.g., persons with “chronic” Lyme disease, being partially addressed through “probable” case surveillance); 3) persons with a non-qualifying positive laboratory test who have been diagnosed as having Lyme disease (not all laboratories rely on immunoblot testing or a standard interpretation of the pattern found); and 4) persons without documented B. burgdorferi infection by any test or classic EM who are being treated for Lyme disease (includes persons with “seronegative Lyme disease”). In other words, the full impact of Lyme disease on the U.S. healthcare system potentially includes all persons who truly have Lyme disease and all persons getting treated for Lyme disease without standard laboratory confirmation, whether they have Lyme disease or not. There is no easy method to get at this. Given the limitations and challenges of public health surveillance in general and for Lyme disease in particular, conventional reporting of cases to public health departments will not give a complete answer no matter what the case definition, and it is unlikely that there will ever be a surveillance case definition for Lyme disease that is so inclusive. One could conduct a large population-based survey and ask respondents whether or not they have been treated for Lyme disease in the past year and the nature of that disease. However, this is not as easy as it sounds: the sample frame would have to be large, given that it is likely that the expected rate would be somewhere between 1 in a hundred and 1 in a thousand, given that the measured rate through national surveillance is most recently approximately 1.2 per 10,000 population (CDC, 2010). It might also need to be conducted in all states to determine state-specific rates and, subsequently, trends. Thus, a substantial financial investment would be needed to do this.
Issue: Can public health surveillance for human Lyme disease be used to determine whether there is chronic Lyme disease and the magnitude of the problem with it?
Response: Public health surveillance based on case reporting is dependent on having a case definition that can be applied to determine if a suspected case meets the criteria for being counted as a case. Clinically, many diseases can have similar symptoms. Clinicians faced with making a diagnosis of a patient with a given set of symptoms typically make a list of the possibilities (differential diagnoses) and then methodically do testing to determine what disease the patient likely has. It is similar with public health surveillance. At present, there is no standard case definition that could be used, as there is a lack of consensus even for whether there is “chronic Lyme disease.” Until those involved in clinical research establish that there is such an entity and what a standard set of symptoms are, public health surveillance with all its limitations cannot be conducted.
Issue: Given current surveillance objectives and methods, how can we improve physician reporting and the percentage of laboratory reports on which we get clinical information?
Response: There is a hidden question in this issue. Given that clinician reporting of Lyme disease has been well established for a long time and that maintaining a constant level of surveillance is needed to accurately determine trends (a major public health objective of Lyme disease surveillance), do we want to improve physician reporting? Generally, we want to do whatever is necessary to keep it at more or less the same level. In order to maintain a constant level of surveillance, some states have established sentinel provider networks in which more active surveillance is done. Physicians in these networks are actively asked weekly to provide a list of all the persons they have seen with a new Lyme disease diagnosis. In some states, an epidemiologist will visit the practice to extract the necessary clinical information, limiting the work a physician has to do to report. The trends in the numbers of reports from these practices are compared to those from outside the network. As long as both show similar trend results, a state can reasonably assume that reporting levels are constant.
The laboratory question is a newer one. It is only recently that the number of positive laboratory reports in some high incidence states has overwhelmed the scarce public health resources to follow-up on them, threatening the ability to sustain laboratory surveillance for Lyme disease. One proposed solution that has worked well in New York State to address the problem created by the number of laboratory reports on unique individuals increasing from 18,420 in 2005 to 38,503 in 2008, has been to follow up on a random sample of reports (in New York, 1 in 5), then estimate the total number of cases based on the results of follow-up of the sample. In an unpublished validation, the sampling method has been shown to accurately predict what would have been obtained by following up all laboratory reports (New York State Health Department, personal communication). While sampling has not improved the percentage of laboratory reports successfully followed up (40-50% statewide, the percentage usually gotten by a single attempt by mail to contact the provider to complete a case report form), it takes a lot less work and may be a means that could be used by many states to achieve a sustainable, consistent level of Lyme disease laboratory based surveillance in the future. For sampling to be used for national surveillance purposes, it will ultimately be necessary for CSTE to endorse states reporting an estimated case count as a substitute for an exact one when reporting through the National Notifiable Disease Surveillance System.
Issue: Given the challenges of public health surveillance, can one fairly compare rates of reported Lyme disease between states? Within a single state over time?
Response: One cannot reliably compare rates of reported Lyme disease between states unless one knows whether or not they are making the same level of surveillance effort and what the density and infection rates of tick vectors are. Further, most states with high levels of endemic Lyme disease have very different rates from one part of the state to another. Thus, even the overall state incidence of Lyme disease does not reflect the different risk in different parts of the state (see Figure A10-2). The same principles apply to comparison of rates of Lyme disease within a state over time (Ertel, 2006 and 2008). If surveillance methods are stable, then data should accurately reflect changes in Lyme disease risk over time. However, overall state numbers and trends may not reflect risk and trends throughout the state. Some parts of the state may have plateaued and have some years when human disease incidence (and number of infected ticks) decrease while tick populations in other parts of the state and human illness are increasing.
Issue: A number of states and counties report cases of Lyme disease annually but are not known to have a competent tick vector or the presence of B. burgdorferi in tick populations. Do they really have Lyme disease? Are these really cases of Lyme disease?
Response: For purposes of national public health surveillance, a laboratory confirmed case meeting the clinical case definition will be counted from any state that chooses to count it, even if infection cannot be readily attributed to exposure where B burgdorferi and competent tick vectors are known to be well established (i.e., travel to an endemic area, as defined in the case definition). However, such a “case” in a state or county with no known competent tick vector or infected ticks could be a false case. Other diseases can cause similar symptoms and even immunoblot tests are not perfect. When such disease is diagnosed, it is incumbent on the state or county to attempt to look for the tick vector and find infected ticks before announcing that this is a new endemic area and counting persons who only could have been exposed locally. Definitive human risk in any given area is dependent on there being competent tick vectors and infected ticks. Of particular interest in this regard is the recognition of a new disease characterized by EM, Southern Tick-Associated Rash Illness (STARI) following the bite of the lone star tick (not a competent Lyme disease vector) which may be a more common cause of EM in some southern states than Lyme disease (Georgia Department of Human Resources, 2001; CDC, 2010). STARI was recognized during efforts to validate human risk for Lyme disease after EM was diagnosed in some southern states and counties without known B. burgdorferi infected Ixodes species.
Public Health Surveillance for Other Tickborne Diseases in the U.S.
There are at least 6 other recognized tickborne diseases in the United States: Rocky Mountain Spotted Fever, Ehrlichiosis/anaplasmosis, babesiois,
Powassan virus meningoencephalitis, tickborne relapsing fever and STARI. Of these, four, Rocky Mountain Spotted Fever, Ehrlichiosis/anaplasmosis, Powassan virus encephalitis and babesiosis are included in the National Notifiable Disease Surveillance System. A presentation of the public health surveillance objectives for each and their past and current case definitions and history of public health surveillance are discussed below. None has had the public interest that has been generated by Lyme disease nor as complicated a clinical picture with different stages of illness. Thus far, there has been less concern about what surveillance efforts and case definitions for these diseases can and cannot do.
Rocky Mountain Spotted Fever
Rocky Mountain Spotted Fever (RMSF) is an acute, severe and sometimes fatal tickborne illness transmitted in most parts of the country by the bite of Dermacentor species but in Arizona by Rhiphicephalus sanguineus (the brown dog tick). It has been under public health surveillance since at least 1944 (CDC, 1994). The main objective of public health surveillance is to provide information on the temporal, geographic, and demographic occurrence of Rocky Mountain Spotted Fever (and other spotted fever rickettsioses) to facilitate its prevention and control (CSTE, 2009). Recommended surveillance methods are both provider and laboratory reporting. Although under national public health surveillance for a long time, the case definition for national surveillance was first published in 1990 (CDC, 1990). At that time, a case was defined as follows:
“Clinical description—An illness most commonly characterized by acute onset and fever, usually accompanied by myalgia, headache, and petechial rash (on the palms and soles in two-thirds of the cases). To be counted as confirmed, a case needs to be laboratory confirmed. Four different laboratory criteria can independently be used to confirm a diagnosis: a) fourfold or greater rise in antibody titer to the spotted fever group antigen by immunofluorescent antibody (IFA), complement fixation (CF), latex agglutination (LA), microagglutination (MA), or indirect hemagglutination (IHA) test, or a single titer greater than or equal to 64 by IFA or greater than or equal to 16 by CF; or b) demonstration of positive immunofluorescence of skin lesion (biopsy) or organ tissue (autopsy); or c) Isolation of Rickettsia rickettsii from a clinical specimen. In addition to confirmed cases, a “probable” case is: “a clinically compatible case with supportive serology (fourfold rise in titer or a single titer greater than or equal to 320 by Proteus OX-19 or OX-2, or a single titer greater than or equal to 128 by LA, IHA, or MA test).”
Since 1990, the case definition has been revised several times, each time to make modifications based on newer laboratory test methods. In 1996, the laboratory confirmation criteria changed to include having a positive
polymerase chain reaction assay to R. rickettsii as another independent confirmation criterion (CDC, 1997). In 2003, the 4 main confirmatory laboratory test results were reframed in an effort to make them clearer (CSTE, 2003).
In 2007, another revision was made to “clarify misleading or poorly defined laboratory statements, and to improve case classification for reporting” including adding a “suspected” case category (CSTE, 2007). The clinical description of disease was simplified to “any reported fever and one or more of the following: rash, headache, myalgia, anemia, thromobocytopenia, or any hepatic transaminase elevation,” and the description of confirmatory laboratory tests was further clarified. A positive laboratory test became one of the following: a) serological evidence of a fourfold change in immunoglobulin G (IgG)-specific antibody titer reactive with Rickettsia rickettsii antigen by indirect immunofluorescence assay (IFA) between paired serum specimens (one taken in the first week of illness and a second 2-4 weeks later); b) detection of R. rickettsii DNA in a clinical specimen via amplification of a specific target by PCR assay; c) demonstration of spotted fever group antigen in a biopsy/autopsy specimen by IHC; or d) isolation of R. rickettsii from a clinical specimen in cell culture. Laboratory supportive evidence was defined as: has serologic evidence of elevated IgG or IgM antibody reactive with R. rickettsii antigen by IFA, enzyme-linked immunosorbent assay (ELISA), dot-ELISA, or latex agglutination. A confirmed case needed to have both clinical and laboratory confirmation, a probable case needed clinical confirmation in combination with laboratory supportive evidence, while a suspect case only needed laboratory evidence or recent or past infection (no clinical information needed).
The various iterations of surveillance definitions have been used to describe the geographic distribution within the U.S. and trends in occurrence over time. Most recently, without a substantial change in geographic distribution (mostly southeastern and south central U.S. with scattered cases throughout the country), the number of reported cases has increased 300% in the past decade with a trend toward stabilization in the 4 years since 2005 (CDC, 2010). In 2008, a total of 190 confirmed and 2,367 probable cases were reported.
Erhlichiosis/Anaplasmosis
Human ehrlichiosis was recognized as a distinct acute disease entity caused by an intracellular parasitic organism in the Rickettsiae family in the late 1980s, when human monocytic ehrlichiosis (HME) was described (CDC, 1988). Since then, three different species with their own ecology have been identified as causing ehrlichiosis and one has been reclassified as Anaplasma. Despite there being at least three known causes of ehrlichiosis,
they have been grouped together for public health surveillance purposes. After recognition of a second type of ehrlichiosis that resulted in severe, acute disease with a predilection to affect granulocytes (human granulocytic ehrlichiosis, HGE) and which had an apparently different epidemiology than the previously described HME (Bakken et al., 1994; CDC, 1995), CSTE approved a standard case definition for voluntary reporting to CDC, recognizing that this was an emerging infection. However, it did not initially vote to include it in the Nationally Notifiable Disease Surveillance System, in part because it was reportable in only a minority of states (CSTE, 1996). At that time there were two known causes: E. chaffeensis causing HME, apparently transmitted by Lone Star (Amblyomma americanum) ticks, mainly affecting persons in the southeastern and south central U.S., and an E. equi-like agent causing HGE, suspected of being transmitted by Ixodes ticks and mainly affecting persons in the northeastern and north central states.
The provisional case definition included a clinical description of illness and laboratory criteria for diagnosis, a confirmed case being a person with a clinically compatible illness who met the laboratory criteria (CSTE, 1996). The clinical description was “A febrile illness most commonly characterized by acute onset, accompanied by headache, myalgia, rigors and/or malaise; clinical laboratory findings may include: intracytoplasmic microcolonies (morulae) in leukocytes of peripheral smear, cerebrospinal fluid or bone marrow aspirate or biopsy, cytopenias (especially thrombocytopenia and leukopenia), and elevated liver enzymes (especially alanine aminotransferase or aspartate aminotransferase).” Laboratory criteria included any of the following: “a) fourfold or greater change in antibody titer to Ehrlichia spp. antigen by immunofluorescence antibody (IFA) test in acute and convalescent specimens ideally taken four weeks or more apart. HME diagnosis requires E. chaffeensis antigen and HGE diagnosis currently requires E. equi or HGE-agent antigen; b) positive polymerase chain reaction (PCR) assay. Distinct primers are used for the diagnosis of HGE and HME; or c) intracytoplasmic morulae identified in blood, bone marrow or CSF leukocytes and an IFA antibody titer >=1:64.” Probable cases were defined as persons with a compatible illness with a single IFA serologic titer >=1:64 or intracytoplasmic morulae identified in blood, bone marrow or CSF leukocytes.
In 1998, CSTE voted to formally add ehrlichiosis to the NNDSS effective January 1999 (CSTE, 1998). The purpose of public health surveillance was severalfold: 1) to define the epidemiology of ehrlichia infections in the United States; 2) to monitor incidence trends and changes in the geographic distribution of these infections over time; and 3) to identify risk factors for ehrlichia infections. The earlier recommended case definition was approved for national public health surveillance. In 2000, the case definition was revised to account for the recognition that E. phagocytophilum was the cause of HGE, to add a new human disease-causing species, E. ewingii, and to
incorporate newer laboratory test methods (CSTE, 2000). In addition, the reporting classification was modified, “Three categories of confirmed or probable ehrlichiosis should be reported: 1) human ehrlichiosis caused by E. chaffeensis (HME), 2) human ehrlichiosis caused by E. phagocytophilum (HGE), and 3) human ehrlichiosis (other or unspecified agent), which includes cases that cannot be easily classified by available laboratory techniques, and cases caused by novel Ehrlichia species such as E. ewingii.” In addition, the laboratory criteria became ehrlichia category-specific.
Additional changes to the case definition were made in 2007 in part to update taxonomic changes in the pathogens causing ehrlichiosis (CSTE, 2007). E. phagocytophilum was reclassified to Anaplasma phagocytophilum, the specific disease name changed from HGE to human granulocytic anaplasmosis (HGA) and the overall ehrlichiosis reporting classification was further expanded to 4 categories: 1) human ehrlichiosis caused by Ehrlichia chaffeensis, 2) human ehrlichiosis caused by E. ewingii, 3) human anaplasmosis caused by Anaplasma phagocytophilum, and 4) human ehrlichiosis/anaplasmosis—undetermined. Cases reported in the fourth sub-category can only be reported as “probable” because the cases are only weakly supported by ambiguous laboratory test results. Laboratory confirmatory and supportive (probable) criteria were modified to include E. ewingii and “undetermined” categories as follows: E. ewingii: “Because the organism has never been cultured, antigens are not available. Thus, Ehrlichia ewingii infections may only be diagnosed by molecular detection methods: E ewingii DNA detected in a clinical specimen via amplification of a specific target by polymerase chain reaction (PCR) assay.” “Undetermined” infections “can only be reported as ‘probable’ because the cases are only weakly supported by ambiguous laboratory test results.”
In 2008, a total of 2107 confirmed cases of human ehrlichiosis were reported, with disease caused by A. phagocytophilum (1,009 cases) and by E. chaffeensis (957 cases) accounting for 93% of all cases (CDC, 2010). The incidence of both major forms of ehrlichiosis (HGA, HME) has been steadily increasing since 1999, with a 4-5-fold increase since 2001. Surveillance has also confirmed the early findings on geographic distribution of HME and HGA and demonstrated that ehrlichiosis caused by E. ewingii has a similar geographic distribution as HME.
Powassan Virus Encephalitis/Meningitis
Powassan virus encephalitis/meningitis results from central nervous system infection with Powassan virus, a tickborne virus that causes rare cases of arboviral encephalitis in the upper Midwest and northeastern U.S. It was placed under national public health surveillance in 2002 to be included at the same time West Nile virus was added to the list of other domestic
arboviral encephalitis viruses which had been under national public health surveillance since 1995, including California serogroup virus, equine encephalitis, St. Louis encephalitis, and western equine encephalitis (CSTE, 2001). At the time it was described as “an under-recognized tickborne disease.” It was noted that laboratory testing was not widely available, but that 2 cases were diagnosed in New England during evaluation for West Nile virus infection and that there was a case-fatality rate of approximately 10%. The goals of surveillance were multiple: 1) assess the national public health impact of Powassan viral and other arboviral diseases of the CNS and monitor national trends, 2) identify high-risk population groups or geographic areas to target interventions and guide analytic studies, and 3) develop hypotheses leading to analytic studies about risk factors for infection and disease.
The original case definition was the same for all the arboviruses causing central nervous system infection and recognized that infection may result in clinical disease of variable severity and variable CNS involvement. There was no specific definition for Powassan virus infection. However, cases could be classified as “neuroinvasive” or “nonneuroinvasive” depending on symptoms and demonstration of CNS involvement and required laboratory confirmation in one of 4 ways: a) fourfold or greater change in virusspecific serum antibody titer; b) isolation of virus from or demonstration of specific viral antigen or genomic sequences in tissue, blood, cerebrospinal fluid (CSF), or other body fluid; c) virus-specific immunoglobulin M (IgM) antibodies demonstrated in CSF by antibody-capture enzyme immunoassay (EIA); or d) virus-specific IgM antibodies demonstrated in serum by antibody-capture EIA and confirmed by demonstration of virus-specific serum immunoglobulin G (IgG) antibodies in the same or a later specimen by another serologic assay (e.g., neutralization or hemagglutination inhibition).
From 2002-2008, a total of 13 cases of Powassan virus infection were reported with a peak of 7 cases in 2007. All were neuroinvasive, most from upstate New York with several from Maine, Minnesota and Wisconsin (CDC, 2010). In 2009, CSTE voted to continue surveillance for Powassan virus infection with the same objectives (CSTE, 2009). The one change to the case definition was to add a “probable” category. Confirmed cases continue to need clinical criteria for neuroinvasive (any of a variety of central nervous system symptoms plus pleocytosis on lumbar puncture) or nonneuroinvasive (at least fever) and laboratory confirmation. Probable cases need to have a compatible clinical illness plus a lesser degree of laboratory confirmation, either a) stable (less than or equal to a two-fold change) but elevated titer of virus-specific serum antibodies, or b) virus-specific serum IgM antibodies detected by antibody-capture EIA but with no available results of a confirmatory test for virus-specific serum IgG antibodies in the same or a later specimen.
Babesiosis
Babesiosis is a tickborne disease caused by several different species of malaria-like red blood cell infecting parasites of the genus Babesia. B. microti is the most common cause of babesiosis in the U.S., particularly in New England, East coast and Midwestern states, with B. duncani causing disease in California and Washington. Infection ranges from asymptomatic to a life-threatening illness resembling malaria, being most severe in immunosuppressed, asplenic and/or elderly persons. Prior to the 1980s, documented human illness was rare and largely acquired in islands off the coast of New England and New York. In addition to causing disease following tick bites, Babesia can be transmitted by blood transfusion from asymptomatically infected persons, with transfusion-associated disease first described in the U.S. in 1979.
During the 1980s, it was recognized that in some states, babesiosis was a growing problem. In some of those states, babesiosis was made reportable and increases in incidence and geographic range were documented. For example, New York made babesiosis reportable in 1986 following apparent increases in incidence on Long Island. In 1986, 18 cases were reported, all from Long Island. In 2008, 261 cases were reported: 96 from Long Island, 126 from 12 additional counties in New York state and 39 from New York City (New York State Department of Health, 1994 and 2008). In Connecticut, a cluster of 6 cases occurred in 1989 in New London County, near where Lyme disease was first recognized (CDC, 1989). Babesiosis was made reportable in 1991. In 2007, 156 cases were reported from all 8 counties (Connecticut Department of Public Health, 2007).
With the increasing incidence and spread of babesiois, the incidence of blood transfusion-associated disease increased (Stramer et al., 2009). Correspondingly, in 2010, CSTE voted to add babesiosis to the list of notifiable diseases under national public health surveillance (CSTE, 2010). The purpose of surveillance is to provide information on the temporal, geographic, and demographic occurrence of babesiosis, including transfusion-associated babesiosis, to facilitate its prevention and control. It is recommended that states conduct both healthcare provider and laboratory surveillance. The case definition has three categories of disease: confirmed, probable and suspect, with confirmed and probable being under national public health surveillance. The probable definition includes blood donors and recipients without symptoms associated with a transfusion case or a known infected donor, as long as the probable case has either supportive or confirmatory laboratory evidence of infection.
Other Tickborne Illnesses, Coinfection
There are several other tickborne infections known to occur in the US that currently are not under national public health surveillance: STARI and tickborne relapsing fever. Neither disease is known to be common nor widespread enough for CSTE to seriously consider voting it to be part of the National Notifiable Disease Surveillance System. However, individual states in which they are present can choose to make them locally reportable.
Given that Ixodes ticks, especially in the northeast and north-central states, are vectors for Lyme disease, ehrlichiosis/anaplasmosis and babesiosis, it is possible for ticks to carry and transmit more than one agent. In fact, coinfections are not unusual and can result in more severe illness than infection with a single agent (Swanson et al., 2006). At present, there is no systematic effort at national surveillance for coinfection. However, the potential exists in any state to match persons reported with one infection to reports of those with either of the other infections. Thus far, no results of such matching to determine population levels of coinfection have been reported.
References*
Bakken J.S., J.S. Dumler, S.M. Chen, M.R. Eckman, L.L. Van Etta, and D.H. Walker. 1994. Human granulocytic ehrlichiosis in the upper midwest U.S. J Am Med Assoc 272:212–8.
CDC. 1981. Lyme disease—United States. 1980. MMWR Morb Mortal Wkly Rep 30:489-92,497.
CDC. 1982. Lyme disease. MMWR Morb Mortal Wkly Rep 31:367-368. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00015561.htm.
CDC. 1988. Epidemiologic notes and reports human ehrlichiosis – United States. MMWR Morb Mortal Wkly Rep 37:270,275-77. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/00000020.htm.
CDC. 1989. Epidemiologic notes and reports, babesiosis – Connecticut. MMWR Morb Mortal Wkly Rep 38:649-650. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/00001468.htm.
CDC. 1990. Case definitions for public health surveillance. MMWR Morb Mortal Wkly Rep 39(RR-13):1-43. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00025629.htm.
CDC. 1994. Summary of notifiable diseases – United States, 1993. MMWR Morb Mortal Wkly Rep 42:1-91. Available at http://www.cdc.gov/mmwr/PDF/wk/mm4253.pdf.
CDC. 1995. Human granulocytic ehrlichiosis – New York, 1995. MMWR Morb Mortal Wkly Rep 44:593-595. Available at http://www.cdc.gov/mmwr/PDF/wk/mm4432.pdf.
CSTE. 1996. Position statement 1996-17. Ehrlichiosis. Available at http://www.cste.org/ps/1996/1996-17.htm.
CDC. 1997. Case definitions for infectious conditions under public health surveillance. MMWR Morb Mortal Wkly Rep 46(RR-10):1-55. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00047449.htm.
CDC. 1999. Recommendations for the use of Lyme disease vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Appendix: methods used to create a national Lyme disease risk map. MMWR Morb Mortal Wkly Rep 48(RR-07):21-24. Available at: http://www.cdc.gov/mmwr/PDF/rr/rr4807.pdf.
CDC. 2001. Updated guidelines for evaluating public health surveillance systems: recommendations of the guidelines working group. MMWR Morb Mortal Wkly Rep 50(RR-13):1-36. Available at: http://www.cdc.gov/mmwr/PDF/rr/rr5013.pdf.
CDC. 2004. Lyme Disease — United States, 2001—2002 MMWR Morb Mortal Wkly Rep 53:365-369. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5317a4.htm.
CDC. 2007. Lyme Disease — United States, 2003—2005. MMWR Morb Mortal Wkly Rep 56:573-76. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5623a1.htm.
CDC. 2008. Surveillance for Lyme disease – United States, 1992-2006. MMWR Morb Mortal Wkly Rep 57(SS-10):1-9. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5710a1.htm.
CDC. 2010. Southern tick-associated rash illness. Available at http://www.cdc.gov/ncidod/dvbid/stari/.
CDC. 2010. Summary of notifiable diseases – United States, 2008. MMWR Morb Mortal Wkly Rep 54:1-94. Available at http://www.cdc.gov/mmwr/PDF/wk/mm5754.pdf.
Connally N.P., A.J. Durante, K.M. Yousey-Hindes, J.I. Meek, R.S. Nelson and R. Heimer. 2009. Peridomestic Lyme disease prevention: results of a population-based case-control study. Am J PrevMed 37(3):201-6.
Connecticut Department of Public Health. 2010. Reported cases of disease by county, 2007. Available at http://www.ct.gov/dph/lib/dph/infectious_diseases/pdf_forms_/ct_disease_cases_by_county_2007_fnl.pdf.
Coyle B.S., G.T. Strickland, Y.Y. Liang, C. Pena, R. McCarter, and E. Israel. 1996. The public health impact of Lyme disease in Maryland. J Infect Dis 173:1260-62.
Cromley, E.K., M.L. Cartter, R.D. Mrozinski, and S.H. Ertel. 1998. Residential setting as a risk factor for Lyme disease in a hyperendemic region. Am J Epidemiol 147:472-77.
CSTE. 1990. Position statement 1: National surveillance of Lyme disease and resources for Lyme disease and epidemiology. Available at: http://www.cste.org/ps/1990/1990-01.htm.
CSTE. 1996. Position statement 96-18: Revised case definitions for public health surveillance: infectious disease. Available at: http://www.cste.org/dnn/AnnualConference/Position-Statements/tabid/191/Default.aspx.
CSTE. 1998. Position statement 1998-ID-6. Adding Ehrlichiosis as a condition reportable to the National Public Health Surveillance System (NPHSS). Available at http://www.cste.org/ps/1998/1998-id-06.htm.
CSTE. 2000. Position statement 2000-ID-3. Changes in the case definition for human ehrlichiosis, and addition of a new ehrlichiosis category as a condition placed under surveillance according to the National Public Health Surveillance System (NPHSS). Available at http://www.cste.org/ps/2000/2000-id-03.htm.
CSTE. 2001. Position statement 2001-ID-06: Inclusion of West Nile encephalitis/meningitis and Powassan encephalitis/meningitis in the National Public Health Surveillance System (NPHSS), and revision of the national surveillance case definition of arboviral diseases of the central nervous system (CNS). Available at http://www.cste.org/ps/2001/2001id-06.htm.
CSTE. 2003. Position statement 03-ID-08: Rocky Mountain spotted fever. Available at http://www.cste.org/PS/2003pdfs/2003finalpdf/03-ID-08Revised.pdf.
CSTE. 2007. Position statement 07-ID-11: Revised national surveillance case definition for Lyme disease. Available at: http://www.cste.org/PS/2007ps/2007psfinal/ID/07-ID-11.pdf.
CSTE. 2007. Position statement 2007-ID-05: Revision of the surveillance case definitions for Rocky Mountain spotted fever. Available at http://www.cste.org/PS/2007ps/2007psfinal/ID/07-ID-05.pdf.
CSTE. 2007. Position statement 2007-ID-03. Revision of the National Surveillance Case Definition for Ehrlichiosis (Ehrlichiosis/Anaplasmosis). Available at http://www.cste.org/PS/2007ps/2007psfinal/ID/07-ID-03.pdf.
CSTE. 2009. Position statement 2009-ID-16: Public health reporting and national notification for spotted fever rickettsiosis (including Rocky Mountain spotted fever). Available at http://www.cste.org/ps2009/09-ID-16.pdf.
CSTE. 2009. Position statement 09-ID-25: Public health reporting and national notification for Powassan virus disease. Available at http://www.cste.org/ps2009/09-ID-25.pdf.
CSTE. 2010. Position statement 2010-ID-27. Public health reporting and national surveillance for babesiosis. Available at http://www.cste.org/ps2010/10-ID-27.pdf.
Ertel S., B. Esponda, R. Nelson, and M.L. Cartter. 2006. Lyme disease- Connecticut, 2005. Connecticut Epidemiologist 26:13-14. Available at http://www.ct.gov/dph/lib/dph/infectious_diseases/pdf_forms_/vol26no4.pdf.
Ertel S. P. Gacek, R. Nelson, and M.L. Cartter. 2008. Lyme disease – Connecticut, 2007. Connecticut Epidemiologist 28:5-6. Available at http://www.ct.gov/dph/lib/dph/infectious_diseases/ctepinews/vol28no2.pdf.
Georgia Department of Human Resources, Division of Public Health, Epidemiology Branch. 2001. Tick bites and erythema migrans in Georgia: It Might NOT be Lyme disease! Georgia Epidemiology Report 17:1-3. Available at http://health.state.ga.us/pdfs/epi/gers/ger0801.pdf.
Gould H.L., R.S. Nelson, K.S. Griffith, E.B. Hayes, J. Piesman, P.S. Mead and M.L. Cartter. 2008. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999–2004. Vector-borne and Zoonotic Diseases 8:769-776.
Hadler, J.L. and L.R. Petersen. 2007. Surveillance for vector-borne diseases. In Infectious Disease Surveillance, edited by N.M. M’ikanatha, R. Lynfield, C.A. Van Beneden and H. de Valk. Oxford: Blackwell Publishing. Pp. 107-123.
Ley, C., E.M. Olshen, and A.L. Reingold. 1995. Case-control study of risk factors for incident Lyme disease in California. Am J Epidemiol 142:S39-S47.
Mather T.N., M.C. Nicholson, E.F. Donnelly, and B.T. Matyas. 1996 Entomologic index for human risk of Lyme disease. Am J Epidemiol 144:1066--9.
Meek J.I., C.L. Roberts, E.V. Smith, Jr, and M.L. Cartter. 1996. Underreporting of Lyme disease by Connecticut physicians, 1992. J Public Health Manag Pract 2:61-65.
Meriwether, R.A. 1996. Blueprint for a national public health surveillance system for the 21st century. J Public Health Manag Pract 2(4):16-23.
Moulton, A.D., R.A. Goodman and W.E. Permet. 2007. Perspective: law and great public health achievements. In Law and Public Health Practice, 2nd ed, edited by R.A. Goodman, R.E. Hoffman, W. Lopez, G.W. Matthews, M. Rothstein, and K.L. Foster. New York: Oxford University Press. P 13.
New York State Department of Health. 1994. Communicable disease in New York State, reported cases of selected diseases exclusive of New York City, 1984-1994. Available at http://www.nyhealth.gov/nysdoh/cdc/1994/sect5a.pdf
New York State Department of Health. 2008. Reported cases by disease and county, AIDS-dengue fever. Available at http://www.nyhealth.gov/statistics/diseases/communicable/2008/cases/1.htm.
Orloski, K., G. Campbell, C. Genese, J. Beckley, M. Schriefer, K. Spitalny, and D. Dennis. 1998. Emergence of Lyme disease in Hunterdon County, New Jersey, 1993: A case-control study of risk factors and evaluation of reporting patterns. Am J Epidemiol 147:391-7.
Stafford K.C., III, M.L. Cartter, L.A. Magnarelli, S. Ertel, and P.A. Mshar. 1998. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burdorferi and increasing incidence of Lyme disease. J Clin Microbiol 36:1240-4.
Stramer S.L., F.B. Hollinger, L.M. Katz, S. Kleinman, P.S. Metzel, K.R. Gregory, and R.Y. Dodd. 2009. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion 49 Suppl 2:1S-29S.
Swanson S.J., D. Neitzel, K.D. Reed, and E.A. Belongia. 2006. Coinfections acquired from Ixodesticks. Clin Microbiol Rev 19:708-27.
Vázquez M., C. Muehlenbein, M. Cartter, E.B. Hayes, S. Ertel and E.D. Shapiro. 2008. Effectiveness of personal protective measures to prevent Lyme disease. Emerg Infect Dis 14:210-216. Available at: http://www.cdc.gov/eid/content/14/2/210.htm.