6
Reference Concentrations for Noncancer Effects and Unit Risks for Cancers
Chapter 5 of the draft IRIS assessment discusses the derivation of reference concentrations (RfCs) for noncancer effects and unit risks for cancers. Because estimates of RfCs and unit risks are subject to uncertainty and variation at every stage of the computational process, the committee conducted a thorough appraisal of the Environmental Protection Agency (EPA) process and analysis for calculating the estimates. In this chapter, the committee provides its review of EPA’s derivation of RfCs and unit risks and offers its conclusions and recommendations regarding these two key products of the IRIS assessment.
The committee notes that EPA’s dose-response assessments for cancer and noncancer effects have evaluated some end points for which there may not be adequate evidence to support the conclusion of a causal relationship between that end point and formaldehyde exposure. For example, modes of action for leukemia and Hodgkin lymphoma remain questionable, as noted by the present committee at various places in this report (Chapters 3 and 5). The committee recognizes, however, that EPA has followed its various risk-assessment guidelines (EPA1991, 1998, 2005) in conducting the dose-response assessments. In cancer risk assessment, for example, “dose-response assessments are generally completed for agents considered ‘carcinogenic to humans’ and ‘likely to be carcinogenic to humans’” (EPA 2005). Dose-response assessments include an analysis of all tumor types on the basis of potential causality of the agent and may be conducted to provide a sense of the magnitude and uncertainty of potential risks, especially when the evidence is provided from a well-conducted study (EPA 2005). It is within that framework that the present committee reviewed EPA’s calculation of RfCs for noncancer effects and unit risks for cancer. The review is partly geared toward an analysis of uncertainties associated with the
underlying risk estimates and is not necessarily an endorsement, for example, of using a specific cancer, such as leukemia, for a consensus risk estimate. The committee’s opinions on mode of action and weight of evidence concerning specific health outcomes are given in Chapters 3-5 of the present report.
FORMALDEHYDE REFERENCE CONCENTRATIONS
EPA defines an RfC as “an estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime” (EPA 2010a). That is, an RfC is a concentration at which exposures would be allowed to occur with sufficient certainty, taking into account susceptibility and variability, that adverse outcomes would not result. RfCs are used by EPA, state agencies, various regulatory agencies, and other entities to develop allowable ambient air concentrations and to evaluate risks posed by current and potential exposures.
The draft IRIS assessment proposes several RfCs for formaldehyde that are based on “three studies of related health effects: asthma, allergic sensitization, pulmonary function, and symptoms of respiratory disease in children from in-home exposure to formaldehyde” (Rumchev et al. 2002; Garrett et al. 1999; Krzyzanowski et al. 1990) (EPA 2010b, p. 5-66). The discussion concludes by presenting a range (1-9 ppb), rather than a specific value, for the RfC. The committee was asked to comment on values of the uncertainty factors used to derive the RfCs that account for human population variability and for deficiencies in the overall database (see Box 1-1).
Chapters 4 and 5 of the present report addressed the health effects associated with formaldehyde exposure and reviewed the candidate critical effects, relevant studies, and points of departure identified by EPA. EPA’s process for developing the RfC for formaldehyde is illustrated in Figure 6-1. The following sections briefly summarize EPA’s selection of critical effects and key studies and identification of points of departure for derivation of candidate RfCs. Information that is relevant to evaluating the uncertainty factors proposed by EPA is then presented, and the committee provides its recommendations for those factors. Finally, the committee comments on the IRIS process for derivation of RfCs and provides suggestions for improving the process of selecting a final RfC.
Selection of Candidate Noncancer Effects
Health effects associated with formaldehyde exposure have been studied extensively in people, laboratory animals, and in vitro systems with a variety of study designs. EPA evaluated a broad array of health effects that the committee
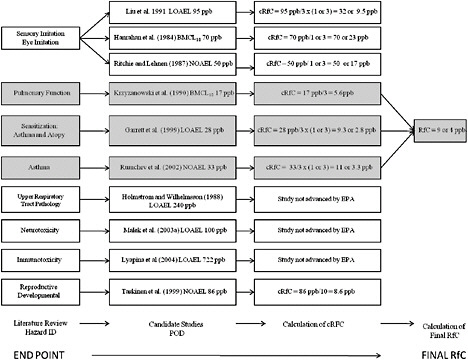
FIGURE 6-1 Illustration of EPA’s process for deriving a reference concentration for formaldehyde. Abbreviations: ID, identification; RfC, reference concentration; cRFC, candidate reference concentration; POD, point of departure; EPA, Environmental Protection Agency; LOAEL, lowest observed adverse effect-level; NOAEL, no observed adverse effect level; and BMCL10, lower 95% confidence limit on the benchmark concentration corresponding to a 10% response level.
characterized as portal-of-entry or systemic. For portal-of-entry noncancer effects, the draft IRIS assessment concludes that formaldehyde causes sensory irritation, decreased pulmonary function, histopathologic lesions of the upper respiratory tract, and asthma and allergic sensitization. The committee agrees with EPA’s assessment of a causal relationship between formaldehyde and those effects with the exception of incident asthma, which was based on the study by Rumchev et al. (2002). As noted in Chapter 4, the draft IRIS assessment does not sufficiently consider the complexities of the asthma phenotype or the potential role of formaldehyde in causing new cases of asthma as opposed to exacerbating existing asthma.
For systemic noncancer effects, the draft IRIS assessment identifies associations of formaldehyde exposure with effects on the immune system, the nervous system, the reproductive system, and development. The committee does not find the evidence to be sufficient to support a causal relationship between
formaldehyde exposure and those effects, given the weaknesses of the available evidence. First, the committee agrees that there is evidence indicating possible immune effects, including increased incidence of upper respiratory tract infections, respiratory burst activity in the immune system, and modulation of responses to known allergens, but the evidence is insufficient to conclude that these relationships are causal. Second, the committee finds that the draft IRIS assessment overstates the evidence in concluding that formaldehyde is neurotoxic; the selected studies are not sufficient for neurotoxicity-hazard identification, given deficiencies in study design. Third, although the draft IRIS assessment concludes that the epidemiologic studies provide evidence of a convincing relationship between formaldehyde exposure and reproductive and developmental effects, the committee concludes that the evidence indicates a suggestive, rather than convincing, relationship.
The committee supports EPA’s selection of the following health effects on which to base a candidate RfC: sensory irritation (eye, nose, and throat), upper respiratory tract pathology, decreased pulmonary function, increased asthma and allergic sensitization, and, despite the weak evidence of causality, reproductive and developmental toxicity. However, as described in Chapters 4 and 5 of the present report, the draft IRIS assessment has substantive problems that weaken the arguments related to those outcomes.
Selection of Critical Studies
The draft IRIS assessment characterizes the approach for study selection for noncancer outcomes as follows: “in general, studies are included where study quality and ability to define exposures are considered adequate for RfC derivation. Whenever possible, greater consideration is typically given to human data from observational epidemiology studies for derivation of an RfC” (EPA 2010b, p. 5-3). The committee views the stated overall approach as reasonable but found no explicit criteria for its application. Similarly, the concept of “adequate,” which appears central in decision-making, is left undefined. The draft IRIS assessment offers six general points that were used to evaluate studies: study size, whether the study evaluated humans or animals, whether an epidemiologic study was in a residential or occupational setting, whether children were included in the study population in a human study, the accuracy of formaldehyde concentration measurements, and whether the study evaluated low formaldehyde concentrations and sensitive end points. The committee agrees that those criteria are appropriate for study selection but notes that no explicit judgments are identified in the draft assessment about how well the individual studies met the criteria. The effects, studies, and points of departure advanced by EPA for candidate RfCs are summarized in Table 6-1. The committee’s comments on the studies selected for the specific outcomes are provided in Chapters 4 and 5 of the present report.
TABLE 6-1 Derivation of Candidate RfCs by EPAa
|
End Point |
Study |
Selected POD (range of POD) |
UFL |
UFS |
UFH |
|
Respiratory Effects, Asthma, and Sensitizations |
|||||
|
Asthma incidence |
Rumchev et al. (2002) |
NOAEL, 33 ppb (24-39 ppb) |
1 |
3 |
1 or 3 |
|
Increased asthma |
Garrett et al. (1999) |
LOAEL, 28 ppb (16-41 ppb) |
3 |
1 |
1 or 3 |
|
Pulmonary function—reduction in PEFR in children (10%) |
Krzyzanowski et al. (1990) |
BMCL10, 17 ppb (BMC10, 27 ppb) |
1 |
1 |
3 |
|
Sensory Irritation |
|||||
|
Eye irritation, burning eyes |
Hanrahan et al. (1984) |
BMCL10, 70 ppb (LOAEL, >100 ppb) |
1 |
1 |
1 or 3 |
|
|
Liu et al. (1991) |
LOAEL, 95 ppb (70-120 ppb) |
3 |
1 |
1 or 3 |
|
|
Ritchie and Lehnen (1987) |
NOAEL, 50 ppb (0-100 ppb) |
1 |
1 |
1 or 3 |
|
Reproductive and Developmental Toxicity |
|||||
|
Decreased fecundability density ratiob |
Taskinen et al. (1999) |
NOAEL, 86 ppb (estimated 8-hr TWA) |
1 |
1 |
10 |
|
aAll uncertainty factor values are those assigned by EPA. Source: Adapted from Table 56 in EPA (2010b). bDecreased fecundability density ratio is estimated as “the conception rate for exposed women relative to that for unexposed women in each menstrual cycle of unprotected intercourse” (Rowland et al. 1992). Abbreviations: UFL, uncertainty factor for adjustment of LOAEL to NOAEL; UFS, uncertainty factor for adjustment of less than chronic study to chronic duration; UFH, uncertainty factor that accounts for human population variability; RfC, reference concentration; POD, point of departure; EPA, Environmental Protection Agency; PEFR, peak expiratory flow rate; LOAEL, lowest observed adverse effect level; NOAEL, no-observed-adverse-effect-level; BMCL10, lower 95% confidence limit on the benchmark concentration corresponding to a 10% response level; and TWA, time-weighted average. |
|||||
Candidate RfCs were derived for the related group of effects occurring in the respiratory system by using three observational epidemiologic studies of children exposed in their homes. The committee agrees with EPA’s assessment that two of the selected studies are sufficient to support derivation of candidate RfCs for decreased pulmonary function (Krzyzanowski et al. 1990) and prevalence and severity of allergic sensitization and respiratory symptoms (Garrett et al. 1999). However, the committee does not support the selection of the Rumchev et al. (2002) study because the end point of “incident asthma” is not supported by an understanding of the phenotype of asthma in the age range of participants in the study.
Candidate RfCs were derived for sensory irritation of the eye by using three residential epidemiologic studies: Hanrahan et al. (1984), Ritchie and Lehnen (1987), and Liu et al. (1991). Although the committee agrees with EPA’s selection of the Hanrahan et al. (1984) and Liu et al. (1991) studies as the best of those available, it disagrees with EPA’s selection of the Ritchie and Lehnen (1987) study because of the high potential for selection bias among the self-selected participants.
A candidate RfC was derived for reproductive effects on the basis of a decreased fecundability density ratio observed in women occupationally exposed in the epidemiologic study by Taskinen et al. (1999). The committee agrees that the choice of that outcome in the study is justifiable for the reasons provided in the draft IRIS assessment.
The committee supports EPA’s decision not to derive candidate RfCs for immunotoxicity and neurotoxicity end points but disagrees with its decision not to calculate a candidate RfC for upper respiratory tract pathology. Many well-documented studies have reported the occurrence of upper respiratory tract pathology in laboratory animals, including nonhuman primates, after inhalation exposure to formaldehyde. The dataset is one of the most extensive available, and the committee therefore recommends that EPA use the animal data to calculate a candidate RfC for this end point.
Uncertainty Factors
As defined by EPA (1994, 2010a), uncertainty factors are used to derive an RfC to account for study limitations, uncertainty in required extrapolations, and variability in response:
-
UFA accounts for uncertainty in animal-to-human extrapolation.
-
UFH accounts for human population variability and uncertainty in estimation of the variability.
-
UFL adjusts a lowest observed-adverse-effect level (LOAEL) to a no-observed-adverse-effect level (NOAEL).
-
UFS adjusts a less than chronic study to a chronic duration.
-
UFD accounts for uncertainty in identifying the critical effect when the database does not evaluate a complete array of health effects.
The default value for each uncertainty factor is 10; a factor of 3 (the approximate square root of 10) is used by convention when there is information to support a partial reduction in the uncertainty factor (EPA 1994). Guidance from EPA on when a specific uncertainty factor might be changed from the default value has been provided by the Toxicology Working Group of the 10X Task Force (EPA 1999) and EPA’s RfD/RfC Technical Panel (EPA 2002). As noted above, EPA requested advice from the committee on determining the values of
the uncertainty factors that account for human population variability and database completeness.
EPA selected study-specific uncertainty factors for each of the candidate RfCs (Table 6-1). All candidate RfCs advanced by EPA are based on observational epidemiologic studies; thus, UFA that accounts for uncertainty in animal-to-human extrapolation is assigned a value of 1. The committee concurs with EPA’s selection of a value of 3 for UFL for the Garrett et al. (1999) and Liu et al. (1991) studies. Although the studies did not report the duration of residence in the homes tested, the exposure period was assumed to correspond to a chronic exposure period of 10% of a lifetime, or 7 years, as defined by EPA. Thus, EPA selected a value of 1 for UFS for all studies except Rumchev et al. (2002) for which a value of 3 was selected because the study participants were exposed for less than 3 years.
Two alternative values (1 and 3) are presented in the draft IRIS assessment for UFH in five of the seven studies for which candidate RfCs were developed (see Table 6-1). In defining UFH, EPA specifically considered susceptible populations, including children, and that is consistent with the NRC (1993) report Pesticides in the Diets of Infants and Children, the Food Quality Protection Act (1996), and the EPA (2006) report A Framework for Assessing Health Risk of Environmental Exposures to Children. As noted previously, the committee does not support the use of the Rumchev et al. (2002) and Ritchie and Lehnen (1987) studies for derivation of candidate RfCs. Thus, the focus of the remainder of this discussion will be on uncertainty factors used to derive candidate RfCs for asthma and allergic sensitization (Garrett et al. 1999) and eye irritation (Harahan et al. 1984; Liu et al. 1991).
Evaluation of Human Population Variability
Variability of the human response to a specific exposure is recognized quantitatively during the development of the RfC through application of the uncertainty factor UFH (EPA 1994). An overarching difficulty in determining the appropriate value for UFH is that the critical underlying parameters—the proportion of the population to be protected by an RfC and the definition of appreciable risk—have not been quantitatively articulated by EPA or other risk managers. In fact, the definition of an appreciable risk is a societal matter, and the selected value might depend on the particular material of concern and the context (Lowrance 1976; NRC 2009). Furthermore, it is often difficult to determine an appropriate value for UFH because chemical-specific information on mode of action and on characteristics of the sensitive populations is typically sparse. Consequently, descriptions of human variability are often highly imprecise and uncertain.
UFH is conceptualized as accounting for population variability that arises from differences in toxicokinetics (variation in the dose to the active site from
the same external exposure) and from differences in toxicodynamics (variation in response to the same dose at the active site) (EPA 1994, 2002). Accordingly, the committee evaluated the data presented in the draft IRIS assessment on toxicokinetics, toxicodynamics, mode of action, and attributes of the key studies to consider how well they represent the dose-response data on susceptible populations. The committee found the discussion of potential sources of population variability and uncertainties related to life stages and mode of action in Section 4.6 of the draft assessment to be generally comprehensive. However, sources of uncertainty and variability identified in that section are not integrated into the discussion of the appropriate value of UFH to use with the key studies; instead, the section focuses primarily on the attributes of the study for the specific candidate RfC. The following sections represent the committee’s synthesis of the available information and the response to its charge question.
Toxicokinetics
The toxicokinetics of inhaled formaldehyde depend on uptake at the portal of entry and metabolism. Total uptake in the upper respiratory tract might vary from person to person because of different physical characteristics of the upper respiratory tract, breathing patterns (oral vs nasal), and ventilation rate. As noted by EPA, modeling of reactive-gas uptake by Ginsberg et al. (2005) suggests that uptake in the upper respiratory tract is similar in 3-month-old children and adults. That relationship was confirmed by Ginsberg et al. (2010) after reanalysis of the models that used the higher ventilation rates in children reported in the updated Child-Specific Exposure Factor Handbook (EPA 2008a). EPA evaluated the computational-fluid-dynamics model of Garcia et al. (2009) that models flux (rate of gas absorbed per unit surface area of the nasal lining) of a generic reactive water-soluble gas, which is representative of formaldehyde, in the individual nasal cavities of five adults and two children, 7 and 8 years old (EPA 2010b, Appendix B). Garcia et al. (2009) report that their simulations of localized flux across the nasal epithelium do not predict differences in nasal dosimetry (uptake) between children and adults; average uptake differed by a factor of 1.6 among the seven subjects. Variability in the local gas flux among different regions of the individual nasal passages of the five adults and the two children was larger (a factor of about 3-5). If the effects associated with formaldehyde exposure are specific to location and cell type in the upper respiratory tract, the variability in local flux could be a contributor to variability in population response. EPA concluded and the committee agrees that the analysis of interindividual flux, although well done, is based on a small sample and involves people whose nasal cavities have a “normal” shape. Consequently, the study probably did not capture the full array of nasal-cavity geometry, and the findings should be generalized with caution. The committee encourages EPA to continue to evaluate the type of data that can aid in characterizing variability in deposited dose in future IRIS assessments.
Ventilation rate is another potential contributor to population variability in toxicokinetics and needs to be evaluated because children have higher ventilation rates in relation to body weight than do adults. Unlike the oral reference dose, the inhalation RfC is typically used directly without adjustment for differences in exposure conditions (EPA 2009a). As noted by EPA, ventilation rate and age-related variation in oral and nasal breathing patterns probably contribute to variability in dose to specific areas of the upper respiratory tract; higher ventilation rates and oral breathing decrease absorption of formaldehyde in the nasal cavity and increase the amount of formaldehyde available to the lower respiratory tract (EPA 2010b).
As described in the draft IRIS assessment, formaldehyde is metabolized primarily by alcohol dehydrogenase (ADH3) (EPA 2010b). ADH3 plays a central role in regulating bronchiole tone and allergen-induced hyperresponsiveness (Gerard 2005; Que et al. 2005) and mediates reduction of S-nitrosoglutathione (GSNO) (Thompson and Grafstrom 2008; Thompson et al. 2010), an endogenous bronchodilator and reservoir of nitric oxide activity (Jensen et al. 1998). The ontogeny and regulation of ADH3 among human life stages is not yet understood (Thompson et al. 2009). ADH3 mRNA transcripts have been detected in the third-trimester human fetus, but the relative expression and activity of ADH3 protein at various life stages are not known (Thompson et al. 2009). Polymorphisms in ADH3 have been reported in members of various ethnic groups (Hedberg et al. 2001), and single-nucleotide polymorphisms in ADH3 have been associated with childhood risk of asthma (Wu et al. 2007). As noted in the draft IRIS assessment, the qualitative and quantitative effects of the interactions of ADH3 and GSNO on the toxicity of formaldehyde and human population variability are not understood.
Toxicodynamics
Toxicodynamics is a potential source of human population variability related to variation in the response to a given dose at the active site. The potential contribution of toxicodynamic differences to population variability is evaluated by considering the mode of action, potential life-stage sensitivities, and the extent to which the study population includes susceptible populations. Although the modes of action of formaldehyde’s effects on the respiratory system are not fully characterized, the committee finds the discussions of the biologic mechanisms underlying sensory irritation, asthma, and immunotoxicity in the draft IRIS assessment to be inadequate and not reflective of current scientific understanding. Formaldehyde has been shown to activate the TRPA1 ion channel irreversibly by covalent modification (Macpherson et al. 2007). The TRPA1 ion channel is associated with sensory irritation responses (Bessac and Jordt 2008) and plays a critical role in allergic asthmatic responses as a major neuronal mediator of allergic airway inflammation (Caceres et al. 2009). The contribution of TRPA1 and the enzymes involved in metabolism or processing of formalde-
hyde—ADH3 (Gerard 2005; Que et al. 2005; Wu et al. 2007; Hedberg et al. 2001) and GSNO (Thompson and Grafstrom 2008)—to population variability in toxicodynamics is not understood.
Populations sensitive to effects of formaldehyde exposure include those who have asthma (Krzyzanowski et al. 1990; Kriebel et al. 1993; Garrett et al. 1999) and atopy (Garrett et al. 1999). They may also include those who have acute and chronic inflammatory airway conditions (such as viral infections, asthma, rhinitis, and chronic obstructive pulmonary disease) (Bessac and Jordt 2008) and those exposed to other respiratory irritants that act through related modes of action (Macpherson et al. 2007; Bessac and Jordt 2008). Children may be a susceptible population, given their developing respiratory tract and nervous system (Pinkerton and Joad 2000; Rice and Barone 2000; Ginsberg et al. 2005).
On the basis of the toxicokinetic and toxicodynamic data, the committee agrees with EPA’s conclusion that the available data are consistent with some life-stage differences in susceptibility to the effects of formaldehyde. However, there is substantial uncertainty regarding the determinants and the distribution of susceptibility in the population.
Values of UFH
The committee considered the appropriate value for UFH for the following studies: Garrett et al. (1999), which evaluated the risk of allergy and asthma-like respiratory symptoms in 148 children 7-14 years old; Liu et al. (1991), which evaluated eye irritation in over 1,000 people 4 to over 65 years old; and Hanrahan et al. (1984), which evaluated eye irritation in 61 teens and adults. Criteria described by the RfD/RfC technical report (EPA 2002) regarding when a value of less than 10 could be assigned to UFH guided the committee in its assessment of the appropriate value for UFH (1 or 3). Specifically, “how completely the susceptible subpopulation has been identified and their sensitivity described (vs. assumed)” and whether “the data set on which the POD [point of departure] is based is representative of the exposure/dose-response data for the susceptible subpopulation(s)” (EPA 2002, p. 4-43, 4-44).
Identification of Sensitive Populations
Children and adults who have asthma and allergic sensitization are susceptible populations on the basis of studies that showed increased exacerbation of respiratory and allergic sensitization responses to formaldehyde exposure in people who have asthma (EPA 2010b, p. 4-543). Increased symptoms of upper airway irritation were observed in study participants that also reported chronic respiratory and allergy symptoms; this finding suggests increased susceptibility to irritation (Liu et al. 1991). Subjects who have acute and chronic inflammatory airway conditions (such as viral infections, asthma, rhinitis, and chronic obstructive pulmonary disease) (Bessac and Jordt 2008) may also be susceptible popu-
lations. However, the mode of action for formaldehyde’s effects is not sufficiently elucidated to understand the influence of such factors as life stage, respiratory tract development, latency, underlying disease status (such as chronic respiratory diseases and allergic symptoms), genetic polymorphisms of ADH3 and aldehyde dehydrogenase, and cumulative effects of exposure to chemicals that affect the same targets as formaldehyde.
To support a value of 1 for UFH, EPA cites the RfD/RfC technical report, which indicates that a UFH of 1 has been applied in cases in which data are very specific “about the particular vulnerability of infants and children within specific age ranges to an agent” (EPA 2002, p. 4-43). To determine how often EPA has used a UFH of 1 in derivation of reference values and its underlying rationale, the committee searched the IRIS database and identified six RfDs with a value of 1 assigned for UFH (EPA 2010c). The RfDs are those for benzoic acid, beryllium, fluorine, manganese, nitrate, and nitrite.1 In contrast with formaldehyde, for example, the RfDs for nitrate and nitrite identified points of departure from studies of the susceptible population (infants) and noted that the duration of susceptibility to the effects of nitrate is short (that is, children are not susceptible after specific points in development are reached). In the view of the committee, the modes of action for formaldehyde effects on the respiratory tract are not sufficiently understood to determine all potential susceptible populations, and the factors contributing to susceptibility are not yet adequately described. Thus, the committee does not support the application of a value of 1 for UFH.
Representativeness of Exposure and Dose-Response Data
For the candidate RfC for asthma and allergic sensitization that was based on the study by Garrett et al. (1999), the draft IRIS assessment assumes that children and adults who have asthma or allergic sensitization are the susceptible populations. As described by EPA, the Garrett et al. (1999) study includes a higher proportion of children that may be predisposed to asthma and allergic sensitization than is found in the general population (53 of the 148 children in the study had a diagnosis of asthma); thus, the study appears to describe responses in susceptible populations (EPA 2010b). Garrett et al. (1999) reported that the children who were most responsive to the effects of formaldehyde had parents or family members who had asthma or atopy; this lends support to the hypothesis that there is a genetic component to the increased sensitivity of these children, but there could also be unrecognized environmental sources that contribute to similarities in responsiveness within families.
For the candidate RfC for sensory irritation, the draft IRIS assessment does not identify a potentially susceptible population but notes that the studies by Liu et al. (1991) and Hanrahan et al. (1984) were population-based. The Liu et al. (1991) study was large; it included children less than 4 years old, the elderly, and both sexes and reported the highest prevalence of eye irritation in participants 20-64 years old (EPA 2010b, p.5-60). Of the study population, 33% reported pre-existing respiratory conditions, including allergy, asthma, chronic bronchitis, and emphysema (Liu et al. 1991). The small number of people (61) and the absence of young children lessen the confidence that the Hanrahan et al. (1984) study is sufficiently representative of a sensitive population. However, as noted in the draft IRIS assessment, the exposure-response (prevalence) relationship is similar in the two studies (EPA 2010b, p.5-60).
The inclusion of potentially susceptible populations in the studies supports a reduction of UFH from the default value of 10 for the candidate RfCs on the basis of the Garrett et al. (1999), Liu et al. (1991), and Hanrahan et al. (1984) studies. EPA has long used the square root of the default value of 10 when reducing uncertainty factors from the default value; thus, the committee supports a value of 3 for UFH for the candidate RfCs on the basis of the Garrett et al. (1999), Liu et al. (1991), and Hanrahan et al. (1984) studies.
Evaluation of Database Completeness
As noted above, the committee was asked to comment on appropriate values for the uncertainty factor that accounts for database completeness, UFD. The draft IRIS assessment presents several options for UFD: (1) apply a UFD of 1 with a qualification that further research on reproductive, developmental, and neurotoxic effects would be valuable; (2) apply a UFD of 1 with a qualification that the RfC is explicitly protective; (3) apply a UFD of 3; or (4) provide two RfCs, one with a UFD of 1 that is protective of the better-studied effects and a second with a UFD of 3 to account for the limitations of the data on reproductive, developmental, and neurotoxic effects (EPA 2010b, p. 5-72).
An RfC is derived on the basis of the critical effect (the effect that occurs at the lowest exposure) and is intended to provide protection from all noncancer effects. The final question in the process is “Do the results of all the studies indicate the possibility of effects on particular systems that have not yet been explored sufficiently or do they indicate that additional studies may reveal effects not yet characterized?” (EPA 2002, p. 4-21). One must consider effects across all life stages from conception to old age, subtle effects that affect a person’s quality of life, and effects that may occur after a long latency period (EPA 2002). The database on formaldehyde is extensive and includes evaluation of health effects in the human population. EPA evaluated a broad array of health effects associated with formaldehyde exposure, including those related to asthma, pulmonary function, sensory irritation, respiratory tract pathology, reproductive toxicity, developmental toxicity, neurotoxicity, and immunotoxicity.
For derivation of the RfC, the draft IRIS assessment selects critical effects related to the respiratory system: 10% reduction in peak expiratory flow rate in children at a BMCL10 of 17 ppb (Krzyzanowski et al. 1990), increased prevalence and severity of allergic sensitization and increased severity of respiratory symptoms in children with a LOAEL of 28 ppb (Garrett et al. 1999), and “incidence of asthma” (Rumchev et al. 2002). As indicated above, the committee does not recommend the use of Rumchev et al. (2002) to derive the RfC. The proposed RfC is thus based on subtle effects observed in children who are expected to be a susceptible population for respiratory effects.
As discussed in the draft IRIS assessment, the principal deficiencies in the database are the lack of studies that provide a full evaluation of the complete spectrum of end points in the reproductive, developmental, nervous, and immune systems and the absence of a multi-generation animal study that evaluates reproductive function. The committee notes a critical need for epidemiologic studies that have high-quality exposure data to examine associations with potential effects. Some information, however, is available for evaluating each system noted by EPA. Reproductive effects were evaluated in women occupationally exposed to formaldehyde; the Taskinen et al. (1999) study provided a NOAEL of 86 ppb (adjusted concentration) for decreased fecundity density ratio. Animal developmental studies and male reproduction studies evaluated effects at concentrations of 10 ppm and higher with little evidence of adverse developmental or reproductive effects in the absence of overt signs of toxicity. EPA considered the rat study of Malek et al. (2003a) with a LOAEL of 100 ppb (adjusted concentration) based on performance of learning tasks to provide evidence of neurotoxicity in animal studies. However, the committee found the quality of the study designs used in this and other available studies to be deficient for neurotoxicity hazard identification. Effects related to the immune system were observed in an occupational cohort and in children. Increased susceptibility to upper respiratory tract infections was observed in the occupational study by Lyapina et al. (2004) with a LOAEL of 722 ppb. Increased prevalence and severity of allergic sensitization in children was observed in the study by Garrett et al. (1999) with a LOAEL of 28 ppb, which was used as the point of departure for the RfC proposed by EPA.
The RfC is based on respiratory effects evaluated in children, including children who may be more susceptible than the general population of children. Although there are gaps in the data, as noted above, the database provides information on effects in various systems (reproductive, developmental, immune, and nervous systems) that are of special concern for identifying effects in sensitive populations. Health effects in those systems were observed at exposures higher than those at which effects were observed in the respiratory system. However, NOAELs were not identified for most effects; as noted by EPA, the lack of clear NOAELs contributes to the uncertainty as to whether the RfC would be protective for those health effects (EPA 2010b, p. 6-29).
Determining the value for UFD is difficult because it is always challenging to predict what is not known. The formaldehyde database is extensive but has
some gaps. However, the breadth of the database suggests that it is unlikely that effects will be observed in organ systems not already identified as affected by formaldehyde. The difficult question to answer is, Do the results of the studies “indicate that additional studies may reveal effects not yet characterized?” (EPA 2002, p. 4-21). A quantitative framing of the question is useful to focus the decision-making process, that is, What is the likelihood that effects not yet studied could occur at exposures lower than the known effects, and if new effects occur, how much lower could those exposures be? (Evans and Baird 1998). As described above, the effects of formaldehyde on the reproductive, developmental, nervous, and immune systems are not completely characterized, and the modes of action are not known. It is possible that better studies could reveal effects that occur at exposures lower than those currently evaluated in those systems. However, the draft RfC is based on effects at the portal of entry on the respiratory system that are observed in children who are thought to be particularly sensitive to the effects of formaldehyde. Thus, the likelihood that as yet unstudied effects occur at exposures lower than those currently used as the basis of the draft RfC appears low. Accordingly, the committee recommends that EPA adopt its first option and apply a UFD of 1 with a qualification that further research on reproductive, developmental, neurotoxic, and immunotoxic effects would be valuable.
Comments on the IRIS Process for Deriving Reference Concentrations
The draft IRIS assessment develops several candidate RfCs in accordance with the recommendations of EPA’s guidelines (2002). However, there is little synthesis of the relationships among the health effects identified in the respiratory tract and among target organs in the draft IRIS assessment until the final summary after RfC derivation in Section 6.1.3. Each respiratory tract end point (sensory irritation, upper respiratory tract pathology, decreased pulmonary function, increased asthma, and allergic sensitization) and its associated dose-response information are considered individually, although the draft assessment acknowledges that they are etiologically and clinically related. Thus, the draft assessment appears excessively driven by the need to identify the best study to represent each end point at the cost of overlooking studies that identify related effects at slightly higher concentrations or animal studies that would inform the biologic exposure-response story.
A clearer presentation of information with more tables that summarize available studies, figures that synthesize related effects from multiple studies (see Figure 6-2), and greater integration of information about mode of action and potentially susceptible populations during study selection and assignment of uncertainty factors would improve the assessment’s ability to make a compelling case for the RfC ultimately put forward.
The approach described and illustrated in Figure 6-2 has been used in recent EPA assessments of tetrachloroethylene (EPA 2008b) and trichloroethylene
(EPA 2009b). The Science Advisory Board, which conducted a peer review of EPA’s draft IRIS assessment titled “Toxicological Review of Trichloroethylene,” commented that “the Panel supported the selection of an RfC and an RfD based on multiple candidate reference values in a narrow range, rather than basing these values on the single most sensitive critical endpoint. This approach was supported by the Panel because it was a very robust approach that increases confidence in the final RfC and RfD” (EPASAB 2011, p. 39). The National Research Council (NRC) Committee to Review EPA’s Toxicological Assessment of Tetrachloroethylene also offered advice on the graphic presentation of reference values as part of the noncancer assessment. It was noted that “the committee strongly supports the use of … graphical aids… to make it clear which uncertainty factors were applied, to which studies they were applied, and the effects of particular assumptions” (NRC 2010, p. 93).
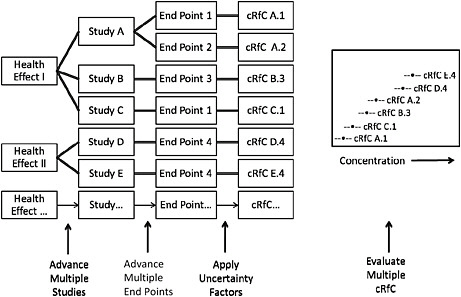
FIGURE 6-2 Illustration of a potential process for identifying an RfC from a full database. Health effects in organ systems associated with exposure to the chemical are identified. For each health effect, studies that meet criteria for inclusion are advanced. From each study, one or more end points that meet specified criteria are advanced, and the point of departure is identified and adjusted to a human-equivalent concentration. Uncertainty factors are selected on the basis of study and end-point attributes and applied to the point of departure to yield candidate RfCs (cRfCs). All cRfCs are evaluated together with the aid of graphic displays that incorporate selected information on attributes relevant to the database and the decision to be made. A final RfC is selected from the distribution after consideration of all critical end points from studies that met the criteria for inclusion.
The committee concurs that appropriate graphic aids that enable the visualization of the range of concentrations evaluated in each published study selected for quantitative assessment may help to identify clusters of studies and especially low or high reference values that may not agree well with the body of literature. The NRC Committee to Review EPA’s Toxicological Assessment of Tetrachloroethylene, argued that “the convergence of sample reference values into clusters would confer confidence on the use of a critical study if other studies led to similar conclusions” and that “convergence of estimated values from studies that are methodologically sound, even if they are not listed as key, would support the RfC proposed by EPA” (NRC 2010, p. 93). That committee’s arguments are highly relevant to the risk assessment of formaldehyde.
FORMALDEHYDE UNIT RISKS FOR CANCER
Sections 5.2 and 5.3 of the draft IRIS assessment provide detailed accounts of the derivation of unit risks for formaldehyde inhalation and also discuss sources of uncertainty and variation in the estimates. Briefly, EPA chose as the primary study the National Cancer Institute (NCI) occupational cohort of U.S. workers involved in the production or use of formaldehyde (Blair et al. 1986). Within the cohort, EPA identified nasopharyngeal cancer (NPC) as the primary cancer associated with formaldehyde in a follow-up of the NCI study to 1994 (Hauptmann et al. 2004) and also selected leukemia and Hodgkin lymphoma as additional cancers to evaluate on the basis of an extended follow-up of the same cohort to 2004 (Beane-Freeman et al. 2009). Relying on the Poisson regression models for cancer mortality reported in Hauptmann et al. (2004) and Beane-Freeman et al. (2009), which used a detailed workplace exposure inventory, EPA estimated life time risks (probabilities) of cancer mortality by using a life-table analysis in conjunction with mortality and cancer incidence in the U.S. population and estimated effective concentration (EC) exposure to formaldehyde corresponding to 0.5-0.05% extra risk. It then used linear extrapolation from the upper confidence limit of the EC to derive cancer unit risk estimates for the three cancers separately. It concluded that the unit risk is in the range of 1.1 × 10−2 to 5.7 × 10−2 ppm−1 for a single cancer group and has an upper bound of 8.1 × 10−2 ppm−1, which combines the risks of the three cancers. Analyses conducted by EPA that used animal data on the incidence of nasal squamous cell carcinoma in F344 rats yielded a human-equivalent unit risk of 1.2 × 10−2 to 2.2 × 10−2 ppm−1. The draft IRIS assessment concludes that 8.1 × 10−2 ppm−1 is a reasonable estimate of unit risk of total cancer.
Many sources at various stages of the risk-estimation process contribute to the overarching uncertainty and variation in the final risk estimates. The effect of the uncertainties in aggregation is complex and difficult to quantify. The draft IRIS assessment makes commendable efforts in discussing a number of sources of uncertainties. The committee’s appraisal of EPA’s analyses follows and focuses on some key factors that determine the overarching uncertainty in cancer
unit risk estimates, including the choice of study, cancer end point, dose metric, dose-response model, point of departure, and extrapolation to low doses.
Selection of Studies
EPA reviewed a number of cohort studies for the purpose of estimating a unit risk for formaldehyde inhalation and chose the studies of the NCI cohort (Hauptmann et al. 2004; Beane-Freeman et al. 2009). EPA’s choice has the following basis: the NCI studies used the largest known cohort, had detailed individual exposure estimates to support dose-response assessment, and used an internal comparison group that is less likely to be confounded by the “healthy-worker” effect than is an external reference group. Results from two follow-ups of the NCI cohort were selected. The first (Hauptmann et al. 2004) went up to 1994 and included a total of 865,708 person-years in 10 U.S. plants, and the second (Beane-Freeman et al. 2009) went through 2004 and had a total of 998,106 person-years of follow-up. More important, the NCI studies exhibited positive and statistically significant exposure-response relationships for some exposure metrics and selected cancers, notably NPC, Hodgkin lymphoma, and leukemia. EPA also reviewed other epidemiologic studies but chose not to use them for unit risk estimation. EPA judged the results from the other studies to be consistent with those of the NCI studies but concluded that various limitations prevented them from being used for quantitative risk estimation, such as a lack of sufficient quantitative exposure data to permit dose-response assessment. Chapters 4 and 5 of the present report provide further discussion of EPA’s study selection.
The committee agrees that the NCI studies are a reasonable choice because they are the only ones with sufficient exposure and dose-response data for risk estimation. However, the NCI studies have limitations. The committee is concerned about the clustering of seven of nine NPC deaths in a single plant (Hauptman et al. 2004) and missing death reports (Beane-Freeman et al. 2009). The committee strongly encourages EPA to state its inclusion and exclusion criteria clearly for its systematic review, analysis, and selection of studies. Systematic use of such criteria enhances the transparency of risk assessment.
In principle, identifying the “best” study for general risk-assessment purposes is neither feasible nor necessary. Inclusion of multiple studies that meet the selection criteria will enhance EPA’s ability to examine variability and uncertainty attributable to, for example, different study designs, populations, and exposure conditions.
Selection of Cancer End Points
NPC was the only respiratory cancer in Hauptmann et al. (2004) that exhibited a positive exposure-response relationship for cancer mortality under all four dose metrics (peak, average intensity, duration, and cumulative exposure).
The exposure-response relationship was statistically significant under the metric of peak exposure, was marginally significant under cumulative exposure and average intensity, and not significant for duration. The draft IRIS assessment notes that prostate cancer showed a statistically significant trend with peak exposure and that bone cancer was associated with peak and cumulative exposure. However, EPA decided to exclude those two cancers from further assessment, and the committee agrees with that decision.
On the basis of the findings from the extended 2004 follow-up of the NCI cohort, EPA identified Hodgkin lymphoma and leukemia as two additional cancers for which to estimate unit risk (Beane-Freeman et al. 2009). Hodgkin lymphoma (27 deaths) showed a positive, statistically significant exposure-response relationship in cancer mortality for peak exposure but was only marginally significant for average intensity and cumulative exposure. The exposure-response trend of leukemia mortality (123 deaths) was only marginally significant for peak and cumulative exposure and was not significant for average intensity (Beane-Freeman et al. 2009).
The lack of consistency in exposure-response relationships between various exposure metrics and the three types of cancer is of concern. The inconsistency may simply be a result of applying multiple metrics, some of which are not highly valid or precise or are perhaps less relevant to the underlying mechanisms. It could also reflect the absence of causal mechanisms associating, for example, leukemia with formaldehyde exposure.
The committee agrees that EPA’s choice of NPC, Hodgkin lymphoma, and leukemia to estimate the unit risk is appropriate given that the use of Hodgkin lymphoma and leukemia primarily supports the assessment of uncertainty and the magnitude of cancer risk where there is a lack of evidence to support the biologic plausibility of a relationship between formaldehyde exposure and the two cancers. The committee also notes that the positive exposure-response findings from the NCI studies support the use of the three cancers for unit risk estimation. However, there are major uncertainties in using the cancers for risk estimation. As discussed in Chapter 5 of the present report, there is a noticeable lack of evidence of a causal relationship of formaldehyde exposure and Hodgkin lymphoma or leukemia. In contrast, there is strong epidemiologic evidence of a causal relationship of formaldehyde exposure and NPC. However, that seven of nine NPC deaths occurred in the Wallingford, Connecticut, factory in the NCI cohort is intriguing. Marsh et al. (2002) investigated the Wallingford cohort, 7,328 workers who were employed in the factory during 1941-1984. Follow-up continued until 1998, and exposure history was reconstructed independently of Marsh et al. (2002). Although the analysis of Marsh et al. (2002) appears to support an increased standardized mortality ratio (SMR) in association with categories of increased formaldehyde exposure, the fact that the SMR was similar in workers with exposure history of less than 1 year (SMR, 5.35; 95% CI, 1.46-14; 4 deaths) and exposure history of greater than 1 year (SMR, 4.59; 95% CI, 0.95-13; 3 deaths) led the authors to question the association with formaldehyde. Marsh and Youk (2005) reanalyzed the NPC data reported in Hauptmann et al.
(2004) and argued that the exposure-response relationship in the NCI cohort was driven largely by the Wallingford, Connecticut, plant. Marsh and colleagues (Marsh et al. 2007a,b) further speculated, through analysis of a case-control study nested within the NCI cohort, that the exposure-response relationship in NPC mortality could be due to the workers whose prior occupation was silversmithing. The committee notes that although data are insufficient to substantiate the confounding argument of Marsh et al. (2007a,b), uncertainties about the causal relationship between formaldehyde exposure and NPC mortality also exist and cannot be eliminated on the basis of the NCI-cohort studies alone even when the data are pooled over all 10 plants. The negative findings in the nine other plants (that is, the plants other than the one in Wallingford, Connecticut) need to be considered.
Although EPA’s choice of NPC, Hodgkin lymphoma, and leukemia for its cancer assessment seems defensible in light of dose-response data requirements described in its carcinogenicity risk-assessment guidelines (EPA 2005), it needs to provide a clear description of its criteria for selecting cancers for its assessment and clearly demonstrate a systematic application of the criteria. For example, how strong a dose-response relationship is required for inclusion? How strong does the evidence of a causal relationship need to be? For leukemia mortality, the Poisson regression-based trend test yielded p values of 0.08 and 0.12 for all and exposed person-years only, respectively, in the case of cumulative exposure (EPA 2010b, Table 5-12). However, the draft IRIS assessment states that “only all leukemias combined and Hodgkin lymphoma were judged to have exposure-response data adequate for the derivation for unit risk estimates” (EPA 2010b, p. 5-75). In that case, did the preferred metric of cumulative exposure outweigh the less significant dose-response relationship? Whereas the committee supports the use of multiple cancers to demonstrate sensitivity, variability, and uncertainty in cancer risk estimation, it recommends that EPA describe and systematically apply a set of selection criteria for cancer end points.
Selection of Dose Metrics
The NCI cohort studies explored four exposure metrics: peak, average intensity, duration, and cumulative exposure. EPA reasoned that cumulative exposure is preferable for dose-response assessment. In the NCI cohort, peak exposure is based on frequency of periods, typically less than 15 min, in which concentrations were above an 8-hr time-weighted average (TWA). The frequency of such periods of increased concentrations led to the ranking of peak exposure as low, medium, and high. Thus, peak exposure does not fully account for the actual concentrations during the peaks, nor does it account for exposure duration. Average intensity derived from an 8-hr TWA also fails to account for exposure duration. Exposure duration does not account for concentrations that vary over time and work place. Therefore, cumulative exposure that combines duration and average intensity into one measure is a more reasonable and ac-
ceptable choice for quantitative exposure-response assessment. However, contrary to the draft IRIS assessment (EPA 2010b, p. 5-79), a statistically significant trend test with cumulative exposure does not necessarily indicate a good fit for the cumulative exposure. The committee agrees with EPA about the choice of cumulative exposure as the metric for risk estimation but notes that other dose metrics can be biologically applicable in various situations. Peak exposure, for example, can be relevant for effects more likely to be induced by acute and high exposure to formaldehyde.
Choice of Dose-Response Models
EPA relied on Poisson regression to model exposure-response associations in mortality related to the selected cancers. Poisson regression is commonly used to analyze mortality in follow-up studies (Breslow and Day 1987; Hauptmann et al. 2004). Its strengths include using person-years at risk as a sample unit of risk, using individual workers’ data (death and risk profile), the ability to stratify by population characteristics (such as sex, ethnicity, and age), and the ability to incorporate time from initial exposure to death or cancer occurrence. The Poisson regression models were initially reported by Hauptmann et al. (2004) for NPC mortality and by Beane-Freeman et al. (2009) for leukemia and Hodgkin lymphoma mortality. The logarithm of mortality rate or SMR is modeled as a linear function of exposure and other risk factors, and the incidence of death is assumed to follow the Poisson distribution. The draft IRIS assessment calls it a “log-linear model,” which in statistics literature commonly refers to methods designed for analyzing cross-tabulated frequency data. EPA used the rate ratio (RR) that results from the Poisson regression models to evaluate the exposure-response relationship in cancer mortality. To conduct risk assessment with the models directly, EPA obtained the regression coefficients of the models from the authors through personal communications because the coefficients were not reported in the published papers. The draft IRIS assessment should provide more details on the models, including, for example, the degree to which the model fits the data.
Conducting Poisson regression for mortality or incidence in follow-up studies may require extensive data manipulation to create person-years at risk according to patterns of all covariates that are being considered. It is difficult for the committee to confirm to what extent EPA tried to validate and verify the published models before using them for risk assessment. Whereas the original models may inform a significant association between formaldehyde exposure and NPC mortality, for example, they may result in a point of departure that is unreliable for low-dose extrapolation if they do not fit the data adequately well at low exposures and thus become inappropriate for risk-assessment purposes. That issue is a concern especially for events that have extremely low rates at which Poisson assumptions may not hold. One may also wonder whether there were any covariates (such as sex) that interacted with formaldehyde exposure.
The presence of any interactions that indicate effect modification will make the extra-risk formula (Rx − R0)/(1 − R0) (EPA 2010b, p. 5-81) depend on the covariates involved rather than independent, as assumed in the draft IRIS assessment. Also of concern is whether the log-linear model reflects the true underlying shape of the exposure-response relationship. Therefore, the committee recommends that EPA conduct its own analysis to confirm the degree to which the Poisson models fit the data appropriately, including an evaluation of goodness of fit, potential interactions between covariates and exposure, and nonlinearity. That analysis is essential because dose-response models used for risk estimation must fit the data well in the low-dose range; this may not necessarily be required for an analysis of the dose-response relationship to establish an association between formaldehyde exposure and a selected cancer. EPA is encouraged to consider the use of alternative extrapolation models, including Cox regression models and nonlinear model forms. The details of such modeling activities should be included in an appendix to the IRIS assessment in sufficient detail that the results can be reproduced.
Callas et al. (1998) conducted extensive analyses and simulations, using the 1994 follow-up data of the same NCI cohort. They reported that Poisson regression models can substantially underestimate RR, hence relative risk of deaths from rare cancers, especially at low exposures. For example, they showed that the relative risk estimated with the Poisson model was 22% less than with Cox regression models at exposures of 0.05- 0.5 ppm-year. Consequently, using the Poisson model may lead to considerable underestimation of unit risk. The authors suggested that Cox regression be used when confounding cannot be well controlled or when age at cancer death does not follow an exponential distribution.
Because seven of the nine NPC deaths were from the Wallingford plant alone, a subgroup analysis of the Wallingford plant with Poisson regression would afford a valuable opportunity to understand better the uncertainty associated with the clustering of the deaths. That would require EPA to conduct Poisson regression on the raw data.
The committee recognizes the substantial resources required to conduct alternative and independent analyses of such data as the follow-up of the NCI cohort. However, having alternative models and analyses enhances EPA’s ability to quantify variability and uncertainty attributable to models. EPA’s carcinogenicity risk-assessment guidelines recommend considering alternative models, especially when biologically based dose-response models are unavailable (EPA 2005). That exercise is especially important when a single study with uncertainties associated with selected cancers and inconsistency with exposure metrics is used.
Lifetime Risk of Cancer Mortality or Incidence
EPA’s process of estimating unit risk appears to consist of the following steps:
-
Obtain the Poisson regression model parameter estimates that assume no effect modification due to interactions between exposure and other factors and a log-linear relationship.
-
Convert the RR estimates from the Poisson model into a lifetime (up to 85 years) mortality risk (probability), R(EC), for NPC, leukemia, or Hodgkin lymphoma for any given formaldehyde exposure, EC. That conversion requires the use of the life-table method (EPA 2010b, Appendix C) in conjunction with the Poisson model mortality risk, age-specific all-cause mortality rates in the U.S. population, and NPC mortality rates derived from the NCI Surveillance Epidemiology and End Results (SEER) database. ECs are converted from occupational exposures to continuous environmental exposures to account for differences in the number of days exposed per year (240 vs 365) and the amount of air inhaled per day (10 vs 20 m3).
-
Compute lifetime risk of NPC death, R0, in an unexposed reference population, using the U.S. age-specific all-cause mortality data and NPC incidence data from SEER.
-
Use the equation
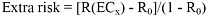
to determine ECx and its lower confidence limit, LECx. The extra risk was 0.05% for NPC and Hodgkin lymphoma and 0.5% for leukemia. Extra risk is typically 1-10%.
-
Use ECx corresponding to x% increase in risk as a point of departure. The unit risk is equal to x/LECx.
Steps (b) and (c) are complicated, but the draft IRIS assessment provides only the following brief explanation:
Extra risk estimates were calculated using the μ regression coefficients and a life-table program that accounts for competing causes of death. U.S. age-specific 1999 all-cause mortality rates for all race and gender groups combined (National Center for Health Statistics [NCHS], 2002) were used to specify the all-cause background mortality rates in the life-table program. NCHS 1996-2000 age-specific background mortality rates for NPC were provided by Dr. Eisner of NCI’s Surveillance Epidemiology and End Results (SEER) program (EPA 2010b, p. 5-81).
Despite an illustration given in Appendix C of the draft IRIS assessment for the computation process of R(EC), the committee finds that additional detail and explanation would be helpful in making the underlying assumptions clear, the process transparent, and uncertainties better understood. For example, EPA should clarify that the hazard rate for NPC mortality in a given age group is the product of the population NPC mortality that is derived from the SEER database
and the probability (as approximated by RR) derived from the Poisson model of observing any NPC death in the occupational cohort. Moreover, EPA appears to have used age-specific NPC incidence from SEER to replace age-specific NPC mortality (column D of Appendix C, EPA 2010b). The implication could be an upward inflation of cancer risk because NPC survival rate is high (Lee and Ko 2005). EPA’s computation for R0 and R included all groups under 30 years old. The NCI cohort workers were at least 16 years old when occupational exposure to formaldehyde began, and there was a 15-year lag for NPC mortality in the Poisson regression model. Therefore, the risk of NPC death in the NCI cohort before the age of 30 years is essentially ignored in EPA’s model; nonetheless, EPA includes groups less than 30 years old in computing R0 and R. Some explanation of the conversion of cumulative exposure (ppm-year) used in the dose-response model and extra risk to average intensity for EC in R also would be helpful.
The committee recognizes the complexity of dose-response and risk-quantification processes for the occupational cohort mortality data and therefore recommends inclusion of adequate description and interpretation to ensure transparency and readability. Specifically, sufficient detail about data, models, methods, and software should be provided in an appendix to any IRIS assessment to allow independent replication and verification.
Selection of Point of Departure
EPA’s carcinogenicity risk-assessment guidelines (EPA 2005) recommend the use of an extra risk of 1-10% for deriving effective concentration, ECx. The recommended range of risk increase is expected to be within the available data range. The draft IRIS assessment makes an unusual choice of 0.05% for NPC and Hodgkin lymphoma. EPA justified the choice on the grounds that NPC death is rare in the general population (background lifetime risk, 2.2 × 10−4), so a 1% increase would be well above the observed range of the NCI data and would result in upward extrapolation. The extra risk of 0.05% corresponds to an RR within the model-based RR range for the cohort. If a higher extra risk were used, the uncertainty of low-dose extrapolation would be greater. Given the extreme rarity of NPC and Hodgkin-lymphoma death, EPA’s choice of point of departure is reasonable.
Derivation of Unit Risks by Using Linear Extrapolation
To derive unit risk estimates for formaldehyde inhalation, EPA relied on the default option of low-dose linear extrapolation. EPA justified its choice on several grounds. First, there is a plausible mutagenic mode of action for NPC and other upper respiratory track cancers. Second, the extra risk appeared linear with exposure below 0.01 ppm on the basis of a comparison of risks that were taken directly from the fitted dose-response models at various exposures. How-
ever, the committee notes that the computation was driven entirely by the fitted Poisson model; the degree to which the model fits the data on NPC, Hodgkin lymphoma, or leukemia is not verified or documented. Third, there is no well-established mechanistic dose-response model.
Linear extrapolation entails three steps. First, a dose-response model, often a mathematical function in the absence of reliable information on mode of action, that fits the observed data appropriately well within the available data range must be identified. In the present case, it is the Poisson models fitted to NPC, leukemia, and Hodgkin lymphoma mortality rates. It is less clear how the model fits the datasets. Second, a point of departure is determined from the fitted dose-response model that corresponds to an exposure concentration (ECx) that induces a specified risk increase (x) above that of a reference population. EPA chose a point of departure that corresponds to 0.05% extra risk in lifetime NPC mortality with the risk R(ECx) derived from the Poisson models and the life-table method. Third, the extra risk level is divided by the point of departure (ECx or LECx) to yield a unit risk or slope factor.
Applying linear extrapolation to NPC mortality data on exposed workers yields only a unit risk of 5.5 × 10−3 based on 0.05% extra risk and LEC0005 = 0.091 ppm (EC0005 = 0.15 ppm). Adding unexposed workers into the calculation changes the unit risk estimate only slightly. Recognizing the high survival rate of NPC patients, EPA also calculated unit risk by using NPC incidence from the NCI SEER database to replace NPC mortality (that is, replace columns D and I with the SEER NPC incidence data). To be consistent, the calculation would also require the use of cancer-incidence data from the NCI cohort in the Poisson dose-response modeling. However, that was not feasible because cancer-incidence data on the NCI cohort (that is, when new cases were first diagnosed) were not available. Nonetheless, EPA’s exercise resulted in new estimates of unit risk that are twice those based on mortality data. EPA correctly pointed out that the correction was attributable to substantial survivorship after NPC onset but was based on the assumption that the exposure-response relationship between formaldehyde exposure and cancer mortality was the same as the relationship between exposure and cancer incidence. That assumption is practical but untestable. EPA also reported unit risk estimates based on Hodgkin lymphoma and leukemia mortality obtained from the extended follow-up of the NCI cohort (Beane-Freeman et al. 2009). The analyses followed the same methods that were used for NPC except that a 2-year lag was used instead of 15 years. The extra risk level at the point of departure was 0.05% for Hodgkin lymphoma mortality but 0.5% for leukemia because of the relatively high leukemia mortality observed in the NCI cohort. Unit risk estimates are summarized below in Table 6-2 for the three cancers, using mortality or incidence, including all person-years vs exposed workers only to demonstrate uncertainties and variability as influenced by these factors.
EPA’s unit risk estimate for leukemia is greater than that for NPC or Hodgkin lymphoma and reflects the choice of point of departure and the high
background leukemia mortality. Unit risk estimates based on cancer incidence are universally greater than those based on mortality because of the substantial survivorship. Although the range of variation in the unit risk estimates does not incorporate the effect of all sources of variation, EPA’s estimation is consistent with the principle of variability and uncertainty analysis in risk assessment.
TABLE 6-2 Cancer Unit Risk Estimates for Formaldehyde
|
Cancer |
Data |
Person-Years |
ECa (ppm) |
LECa (95%) (ppm) |
Unit Riskb (ppm−1) |
|
NPC |
Mortality |
Exposed only |
0.15 |
0.091 |
5.5 × 10−3 |
|
|
|
All |
0.15 |
0.093 |
5.4 × 10−3 |
|
|
Incidence |
Exposed only |
0.072 |
0.045 |
1.1 × 10−2 |
|
|
|
All |
0.074 |
0.046 |
1.1 × 10−2 |
|
Hodgkin lymphoma |
Mortality |
Exposed only |
0.155 |
0.088 |
5.7 × 10−3 |
|
|
|
All |
0.151 |
0.088 |
5.7 × 10−3 |
|
|
Incidence |
Exposed only |
0.053 |
0.030 |
1.7 × 10−2 |
|
|
|
All |
0.052 |
0.030 |
1.7 × 10−2 |
|
Leukemia |
Mortality |
Exposed only |
0.246 |
0.126 |
4.0 × 10−2 |
|
|
|
All |
0.224 |
0.121 |
4.1 × 10−2 |
|
|
Incidence |
Exposed only |
0.178 |
0.091 |
5.5 × 10−2 |
|
|
|
All |
0.162 |
0.088 |
5.7 × 10−2 |
|
Total cancerc |
Mortality |
All |
0.1 |
|
4.5 × 10−2 |
|
|
Incidence |
All |
0.1 |
|
8.1 × 10−2 |
|
aExtra risk level = 0.0005 for NPC and Hodgkin lymphoma, 0.005 for leukemia. bUnit risk = extra risk/LEC cRisk associated with total cancer is based on the sum of estimated extra risk of each cancer at an exposure of 0.1 ppm. Abbreviations: NPC, nasopharyngeal cancer; EC, effective concentration; and LEC, lower confidence limit on the effective concentration. Source: EPA 2010a. |
|||||
EPA further derived an estimate of “total cancer” risk by combining risk of the three cancers. First, EPA estimated the lifetime extra risk of each cancer separately at 0.1 ppm and then added the three estimates and computed the upper confidence limit of the sum. The upper confidence limit is reported as the unit total cancer risk: 4.5 × 10−2 and 8.1 × 10−2 ppm−1 for mortality and incidence, respectively (see Table 6-3). EPA’s computation amounts to using the sum of risk of each cancer as a conservative approximation of the risk of any (total) cancer and relies on the assumption that the maximum likelihood estimates of the three cancer risks are independent, an assumption that is convenient but needs justification because the estimates were derived from the same sample of person-years of exposure. A statistically sound alternative would be to consider incidence or mortality of any cancer and then follow the same methods for NPC incidence or mortality. That would be a preferred approach but would require EPA to fit a Poisson regression to the total cancer incidence or mortality.
Sources of Uncertainty
A unit risk estimate is subject to uncertainty and variability attributable to many sources at various stages of the derivation process. Moreover, it is difficult to determine the degree to which each source affects the overall uncertainty and variation in the final estimate. EPA discussed many potential sources of uncertainty involved in the derivation of the final unit risk estimates. It not only qualitatively identified important sources of uncertainty but quantitatively explored the variability and uncertainty with respect to different cancers, points of departure, all person-years vs only exposed person-years, and mortality vs incidence. It also adjusted for susceptibility in earlier-life exposure. Although EPA did a commendable job in evaluating some of the underlying uncertainties, the committee finds that there is room for further improvement, especially in describing and applying systematic inclusion and exclusion criteria for selecting studies and cancer end points and in using alternative dose-response models.
Estimating Unit Risks by Using Animal Studies
To validate and supplement the unit risk estimates using human data, EPA reanalyzed the nasal squamous cell carcinoma (SCC) incidence data from two long-term bioassays that used F344 rats (Kerns et al. 1983; Monticello et al. 1996). The two bioassays were combined in EPA’s reanalysis to achieve a set of robust dose-response data. The combined dataset has SCC incidences of 0% (n = 341), 0% (n = 107), 0% (n = 353), 0.87% (n = 343), 21.4% (n = 103), and 42% (n = 386) in dose groups of 0, 0.7, 2, 6.01, 9.93, and 14.96 ppm, respectively. EPA conducted a dose-response assessment by using a clonal growth model of the nasal tumor with formaldehyde flux to tissue as the dose metric. The analysis resulted in a unit risk of 1.2 × 10−2 ppm−1 (extra risk, 0.005) and 2.2 × 10−2 ppm−1 (extra risk, 0.01) for humans after interspecies scaling. The estimates are rela-
tively consistent with the risk estimates derived from human data from the NCI studies. Moreover, EPA characterized uncertainties attributable to dose-response models (Weibull model with threshold, multistage model for time to tumor, and clonal growth model), extra risk level (1%, 5%, or 10%), and dose metric (flux, DPX). The resulting unit risk estimates are in the range of 1.4 × 10−2 to 1.9 × 10−1 ppm−1. The variation confirms increasing unit risk with increasing extra risk level. Uncertainty remained within less than a factor of 3 between various dose-response models. EPA’s efforts to conduct independent dose-response assessment are valuable.
CONCLUSIONS AND RECOMMENDATIONS
The committee reviewed EPA’s approach to derivation of the RfCs and unit risks for formaldehyde as described in the draft IRIS assessment. The committee’s general conclusions and recommendations to be considered in revision of the draft assessment are provided below.
-
The committee supports EPA’s selection of effects on which it based candidate RfCs but does not support the advancement of two studies selected by EPA: Ritchie and Lehnen (1987) and Rumchev et al. (2002). Furthermore, the lack of clear selection criteria, inadequate discussion of some modes of action, little synthesis of responses in animal and human studies, and lack of clear rationales for many conclusions weaken EPA’s arguments as presented in the draft IRIS assessment.
-
The committee disagrees with EPA’s decision not to calculate a candidate RfC for upper respiratory tract pathology. Many well-documented studies have reported the occurrence of upper respiratory tract pathology in laboratory animals, including nonhuman primates, after inhalation exposure to formaldehyde, and the committee recommends that EPA use the animal data to calculate a candidate RfC for this end point.
-
The committee found that EPA dismissed the results of the exposure chamber and other nonresidential studies too readily. Although the exposure durations for the chamber studies are short relative to the chronic duration of the RfC, the studies provide compliementary information that could be used for deriving a candidate RfC.
-
Regarding the uncertainty factor that accounts for variability in response of the human population, the committee suggests application of a value of 3 to calculate the candidate RfCs on the basis of the work of Garrett et al. (1999), Hanrahan et al. (1984), and Liu et al. (1991). Those studies included potentially susceptible populations, so the default value of 10 is not necessary. However, uncertainties remain regarding susceptible populations and factors that affect susceptibility, so a value of 1 is not recommended.
-
Regarding the uncertainty factor that accounts for database completeness, the committee suggests that EPA apply its first option as described in the
-
draft IRIS assessment; that is, apply a value of 1 with the qualification that further research on reproductive, developmental, neurotoxic, and immunotoxic effects would be valuable.
-
Overall, the committee found little synthesis of the relationships among the identified noncancer health effects; it appeared that EPA was driven by the need to identify the best study for each health effect rather than trying to integrate all the information. The committee strongly recommends the use of appropriate graphic aids that better display the range of concentrations evaluated in each published study selected for quantitative assessment; the figures may help to identify how findings of studies cluster and especially identify low or high reference values that may be inconsistent with the body of literature. Ultimately, such graphics will improve the ability of the assessment and make a compelling case for the RfC ultimately put forward.
-
Regarding calculation of unit risks, the committee agrees that the NCI studies and the findings of the two follow-ups are a reasonable choice because they are the only ones with sufficient exposure and dose-response data for risk estimation. However, the studies are not without their weaknesses, and these need to be clearly articulated in the revised IRIS assessment.
-
The committee agrees that EPA’s choice of NPC, Hodgkin lymphoma, and leukemia data from the NCI studies to estimate a unit risk is appropriate given that the analysis of Hodgkin lymphoma and leukemia primarily supports the assessment of uncertainty and the magnitude of potential cancer risk. However, the mode of action for formaldehyde-induced Hodgkin lymphoma and leukemia has not been clearly established. Moreover, the highly limited systemic delivery of formaldehyde draws into question the biologic feasibility of causality between formaldehyde exposure and the two cancers. Thus, substantial uncertainties in using Hodgkin lymphoma and leukemia for consensus cancer risk estimation remain.
-
Overall, the committee finds EPA’s approach to calculating the unit risks reasonable. However, EPA should validate the Poisson dose-response models for NPC, leukemia, and Hodgkin lymphoma mortality with respect to adequacy of model fit, including goodness of fit in the low-dose range, (log) linearity, and absence of interactions of covariates with formaldehyde exposure. Furthermore, EPA is strongly encouraged to conduct alternative dose-response modeling by using Cox regression or alternative nonlinear function forms.
-
The draft IRIS assessment does not provide adequate narratives regarding selection of studies and end points for derivation of unit risks. The committee strongly recommends that EPA develop, state, and systematically apply a set of selection criteria for studies and cancer end points.
The committee recognizes that uncertainty and variability remain critical issues as EPA continues to promote quantitative assessment to improve environmental regulation. There are still technical gaps in developing and applying quantitative analysis of uncertainty and variability, especially to incorporate
from all sources and at all stages into an overall summary. The NRC Committee to Review EPA's Toxicological Assessment of Tetrachloroethylene (NRC 2010) made several recommendations for advancing methodology and promoting applications. Further research is needed to study various approaches. Small (2008) discussed a probabilistic framework. Given a set of options related to a key assumption (such as mode of action) or a key choice (such as cancer end point), a preference score (or prior probability) may be assigned to each option. The final risk estimate thus also has a weight or probability attached that combines the preference on all options over each assumption or choice. The overarching weight is the result of propagation of uncertainty in each assumption or choice and aggregation of all assumptions over the risk assessment process tree. The collection of final risk estimates for all permissible combinations of assumption and choice forms an empirical distribution. That distribution quantifies the full range of variation and uncertainty in the risk estimate. With the full range of variation of risk estimates and other information on preference of key assumptions and choices, regulatory policy can depend less on a single principal study, a single principal dataset, or a principal end point. The risk-management process may use the distributional properties of the risk estimate to choose a final risk estimate in the context of all feasible assumptions and choices. The committee concludes that further development of systematic approaches to quantifying uncertainty and variation will enable EPA to conduct IRIS assessments in a more transparent and objective fashion.
REFERENCES
Beane-Freeman, L.E., A. Blair, J.H. Lubin, P.A. Stewart, R.B. Hayes, R.N. Hoover, and M. Hauptmann. 2009. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: The National Cancer Institute cohort. J. Natl. Cancer Inst. 101(10):751-761.
Bessac, B.F. and S.E. Jordt. 2008. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda). 23:360-370.
Blair, A., P. Stewart, M. O’Berg, W. Gaffey, J. Walrath, J. Ward, R. Bales, S. Kaplan, and D. Cubit. 1986. Mortality among industrial workers exposed to formaldehyde. J. Natl. Cancer Inst. 76(6):1071-1084.
Breslow, N.E., and N.E. Day. 1987. Statistical Methods in Cancer Research, Vol. 2. The Design and Analysis of Cohort Study. IARC Scientific Publications No. 82. Lyon: International Agency for Research on Cancer [online]. Available: http://www.iarc.fr/en/publications/pdfs-online/stat/sp82/SP82.pdf [accessed Jan. 25, 2011].
Caceres, A.I., M. Brackmann, M.D. Elia, B.F. Bessac, D. del Camino, M. D’Amours, J.S. Witek, C.M. Fanger, J.A. Chong, N.J. Hayward, R.J. Homer, L. Cohn, X. Huang, M.M. Moran, and S.E. Jordt. 2009. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Nat. Acad. Sci. 106(22): 9099-9104.
Callas, P.W., H. Pastides, and D.W. Hosmer. 1998. Empirical comparisons of proportional hazards, poisson, and logistic regression modeling of occupational cohort data. Am. J. Ind. Med. 33(1):33-47.
EPA (U.S. Environmental Protection Agency). 1991. Guidelines for Developmental Toxicity Risk Assessment. EPA/600/FR-91/001. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. December 1991 [online]. Available: http://iccvam.niehs.nih.gov/SuppDocs/FedDocs/EPA/EPA-devtox.pdf [accessed Feb. 28, 2011].
EPA (U.S. Environmental Protection Agency). 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. EPA/600/B-90/066F. Office of Health and Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC [online]. Available: http://www.epa.gov/raf/publications/pdfs/RFCMETHODOLOGY.PDF [accessed Nov. 28, 2010].
EPA (U.S. Environmental Protection Agency). 1998. Guidelines for Neurotoxicity Risk Assessment. EPA/630/R-95/001F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. April 1998 [online]. Available: http://www.epa.gov/raf/publications/pdfs/NEUROTOX.PDF [accessed Feb. 28, 2011].
EPA (U.S. Environmental Protection Agency). 1999. Toxicology Data Requirements for Assessing Risks of Pesticide Exposure to Children’s Health: Report of the Toxicology Working Group of the 10X Task Force. Draft Report, April 28, 1999. U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/scipoly/sap/meetings/1999/may/10xtx428.pdf [accessed Jan. 25, 2011].
EPA (U.S. Environmental Protection Agency). 2002. A Review of the Reference Dose and Reference Concentration Processes. External Review Draft. EPA/630/P-02/002A. Reference Dose/Reference Concentration (RfD/RfC) Technical Panel, Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/raf/publications/pdfs/rfdrfcextrevdrft.pdf [accessed Jan. 6, 2010].
EPA (U.S. Environmental Protection Agency). 2005. Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. March 2005 [online]. Available: http://www.epa.gov/raf/publications/pdfs/CANCER_GUIDELINES_FINAL_3-25-05.PDF [accessed Nov. 24, 2010].
EPA (U.S. Environmental Protection Agency). 2006. A Framework for Assessing Health Risk of Environmental Exposures to Children. EPA/600/R-05/093F. National Center for Environmrntal Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC. September 2006 [online]. Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=158363 [accessed Mar. 10, 2010].
EPA (U.S. Environmental Protection Agency). 2008a. Child-Specific Exposure Factors Handbook (Final Report) 2008. EPA/600/R-06/096F. National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=199243 [accessed Jan. 25, 2011].
EPA (U.S. Environmental Protection Agency). 2008b. Toxicological Review of Tetrachloroethylene (Perchloroethylene) (CAS No. 127-18-4) In Support of Summary Information on the Integrated Risk Information System (IRIS). External Review Draft. EPA/635/R-08/011A. U.S. Environmental Protection Agency, Washington, DC. June 2008 [online]. Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=192423 [accessed Jan.25, 2011].
EPA (U.S. Environmental Protection Agency). 2009a. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for
Inhalation Risk Assessment). EPA-540-R-070-002. OSWER 9285.7-82. Office of Superfund Remediation and Technology Innovation, U.S. Environmental Protection Agency, Washington, DC. January 2009 [online]. Available: http://www.epa.gov/oswer/riskassessment/ragsf/pdf/partf_200901_final.pdf [accessed Nov. 29, 2010].
EPA (U.S. Environmental Protection Agency). 2009b. Toxicological Review of Trichloroethylene (CAS No. 79-01-6) In Support of Summary Information on the Integrated Risk Information System (IRIS). External Review Draft. EPA/635/R-09/011A. U.S. Environmental Protection Agency, Washington, DC. October 2009 [online]. Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=215006 [accessed Nov. 22, 2010].
EPA (U.S. Environmental Protection Agency). 2010a. Glossary, EPA Risk Assessment. U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/risk_assessment/glossary.htm#r [accessed Nov. 29, 2010].
EPA (U.S. Environmental Protection Agency). 2010b. Toxicological Review of Formaldehyde (CAS No. 50-00-0) – Inhalation Assessment: In Support of Summary Information on the Integrated Risk Information System (IRIS). External Review Draft. EPA/635/R-10/002A. U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=223614 [accessed Nov. 22, 2010].
EPA (U.S. Environmental Protection Agency). 2010c. Integrated Risk information (IRIS). U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/iris/ [accessed Dec. 8, 2010].
EPA SAB (U.S. Environmental Protection Agency Science Advisory Board). 2011. Review of EPA’s Draft Assessment Entitled “Toxicological Review of Trichloroethylene” (October 2009). EPA-SAB-11-02. Science Advisory Board, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://yosemite.epa.gov/sab/sabproduct.nsf/B73D5D39A8F184BD85257817004A1988/$File/EPA-SAB-11-002-unsigned.pdf [accessed Jan. 13, 2011].
Evans, J.S., and S.J.S. Baird. 1998. Accounting for missing data in noncancer risk assessment. Hum. Ecol. Risk Assess. 4(2):291-317.
Garcia, C.L., M. Mechilli, L.P. De Santis, A. Schinoppi, K. Katarzyna, and F. Palitti. 2009. Relationship between DNA lesions, DNA repair and chromosomal damage induced by acetaldehyde. Mutat. Res. 662(1-2):3-9.
Garrett, M.H., M.A. Hooper, B.M. Hooper, P.R. Rayment, and M.J. Abramson. 1999. Increased risk of allergy in children due to formaldehyde exposure in homes. Allergy 54(4):330-337 [Erratum-Allergy 54(12):1327].
Gerard, C. 2005. Biomedicine. Asthmatics breathe easier when it’s SNO-ing. Science 308(5728):1560-1561.
Ginsberg, G., B.P. Foos, and M.P. Firestone. 2005. Review and analysis of inhalation dosimetry methods for application to children’s risk assessment. J. Toxicol. Environ. Health A. 68(8):573-615.
Ginsberg, G., B. Foos, R.B. Dzubow, and M. Firestone. 2010. Options for incorporating children’s inhaled dose into human health risk assessment. Inhal. Toxicol. 22(8):627-647.
Hanrahan, L.P., K.A. Dally, H.A. Anderson, M.S. Kanarek, and J. Rankin. 1984. Formaldehyde vapor in mobile homes: A cross sectional survey of concentrations and irritant effects. Am. J. Public Health 74(9):1026-1027.
Hauptmann, M., J.H. Lubin, P.A. Stewart, R.B. Hayes, and A. Blair. 2004. Mortality from solid cancers among workers in formaldehyde industries. Am. J. Epidemiol. 159(12):1117-1130.
Hedberg, J.J., M. Backlund, P. Strömberg, S. Lönn, M.L. Dahl, M. Ingelman-Sundberg, and J.O. Höög. 2001. Functional polymorphism in the alcohol dehydrogenase 3 (ADH3) promoter. Pharmacogenetics. 11(9): 815-824.
Holmström, M., and B. Wilhelmsson. 1988. Respiratory symptoms and pathophysiological effects of occupational exposure to formaldehyde and wood dust. Scand. J. Work Environ. Health 14(5):306-311.
Jensen, D.E., G.K. Belka, and G.C. du Bois. 1998. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 331(Pt. 2):659-668.
Kerns, W.D., K.L. Pavkov, D.J. Donofrio, E.J. Gralla, J.A. Swenberg. 1983. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 43(9):4382-4392.
Kriebel, D., S.R. Sama, and B. Cocanour. 1993. Reversible pulmonary responses to formaldehyde. A study of clinical anatomy students. Am. Rev. Respir. Dis. 148(6 Pt 1):1509-1515.
Krzyzanowski, M., J.J. Quackenboss, and M.D. Lebowitz. 1990. Chronic respiratory effects of indoor formaldehyde exposure. Environ. Res. 52(2):117-125.
Lee, J.T., and C.Y. Ko. 2005. Has survival improved for nasopharyngeal carcinoma in the United States? Otolaryngol. Head Neck Surg. 132(2):303-308.
Liu, K.S., F.Y. Huang, S.B. Hayward, J. Wesolowski, and K. Sexton. 1991. Irritant effects of formaldehyde exposure in mobile homes. Environ. Health Perspect. 94:91-94.
Lowrance, W.W. 1976. Of Acceptable Risk: Science and the Determination of Safety. Los Altos, CA: W. Kaufmann.
Lyapina, M., G. Zhelezova, E. Petrova, and M. Boev. 2004. Flow cytometric determination of neutrophil burst activity in workers exposed to formaldehyde. Int. Arch. Occup. Environ. Health 77(5):335-340.
Macpherson, L.J., B. Xiao, K.Y. Kwan, M.J. Petrus, A.E. Dubin, S. Hwang, B. Cravatt, D.P. Corey, and A. Patapoutian. 2007. An ion channel essential for sensing chemical damage. J. Neurosci. 27(42):11412-11415.
Malek, F.A., K.U. Möritz, and J. Fanghänel. 2003a. A study on the effect of inhalative formaldehyde exposure on water labyrinth test performance in rats. Ann. Anat. 185(3):277-285.
Marsh, G.M., and A.O. Youk. 2005. Reevaluation of mortality risks for nasopharyngeal cancer in the formaldehyde cohort study of the National Cancer Institute. Regul. Toxicol. Pharmacol. 42(3):275-283.
Marsh, G.M., A.O. Youk, J.M. Buchanich, L.D. Cassidy, L.J. Lucas, N.A. Esmen, and I.M. Gathuru. 2002. Pharyngeal cancer mortality among chemical plant workers exposed to formaldehyde. Toxicol. Ind. Health 18(6):257-268.
Marsh, G.M., A.O. Youk, J.M. Buchanich, S. Erdal, and N.A. Esmen. 2007a. Work in the metal industry and nasopharyngeal cancer mortality among formaldehyde-exposed workers. Regul. Toxicol. Pharmacol. 48(3):308-319.
Marsh, G.M., A.O. Youk, and P. Morfeld. 2007b. Mis-specified and non-robust mortality risk models for nasopharyngeal cancer in the National Cancer Institute formaldehyde worker cohort study. Regul. Toxicol. Pharmacol. 47(1):59-67.
Monticello, T.M., J.A. Swenberg, E.A. Gross, J.R. Leininger, J.S. Kimbell, S. Seilkop, T.B. Starr, J.E. Gibson, and K.T. Morgan. 1996. Correlation of regional and nonlinear formaldehyde-induced nasal cancer with proliferating populations of cells. Cancer Res. 56(5):1012-1022.
National Center for Health Statistics. 2002. United States Life Tables, 1999. National Vital Statistics Reports, vol. 50, no. 6. National Center for Health Statistics
[online]. Available: http://www.cdc.gov/nchs/data/nvsr/nvsr50/nvsr50_06.pdf [accessed Feb. 1, 2011].
NRC (National Research Council). 1993. Pesticides in the Diets of Infants and Children. Washington, DC: National Academy Press.
NRC (National Research Council). 2009. Science and Decisions: Advancing Risk Assessment. Washington, DC: National Academies Press.
NRC (National Research Council). 2010. Review of the Environmental Protection Agency's Draft IRIS Assessment of Tetrachloroethylene. Washington, DC: National Academies Press.
Pinkerton, K.E. and J.P. Joad. 2000. The mammalian respiratory system and critical windows of exposure for children's health. Environ. Health Perspect. 108(Suppl 3):457-462.
Que, L.G., L. Liu, Y. Yan, G.S. Whitehead, S.H. Gavett, D.A. Schwartz, and J.S. Stamler. 2005. Protection from experimental asthma by an endogenous bronchodilator. Science 308(5728):1618-1621.
Rice, D. and S. Barone Jr. 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 108(Suppl 3):511-533.
Ritchie, I.M., and R.G. Lehnen. 1987. Formaldehyde-related health complaints of residents living in mobile and conventional homes. Am. J. Public Health 77(3):323-328.
Rowland, A.S., D.D. Baird, C.R. Weinberg, D.L. Shore, C.M. Shy, and A.J. Wilcox. 1992. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N. Engl. J. Med. 327(14):993-997.
Rumchev, K.B., J.T. Spickett, M.K. Bulsara, M.R. Phillips, and S.M. Stick. 2002. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur. Respir. J. 20(2):403-408.
Small, M.J. 2008. Methods for assessing uncertainty in fundamental assumptions and associated models for cancer risk assessment. Risk Anal. 28(5):1289-1308.
Taskinen, H.K., P. Kyyronen, M. Sallmen, S.V. Virtanen, T.A. Liukkonen, O. Huida, M.L. Lindbohm, and A. Anttila. 1999. Reduced fertility among female wood workers exposed to formaldehyde. Am. J. Ind. Med. 36(1):206-212.
Thompson, C.M., and R.C. Grafstrom. 2008. Mechanistic considerations for formaldehyde-induced bronchoconstriction involving S-nitroglutathione reductase. J. Toxicol. Environ. Health A. 71(3):244-248.
Thompson, C.M., B. Sonawane, and R.C. Grafstrom. 2009. The ontogeny, distribution, and regulation of alcohol dehydrogenase 3: Implications for pulmonary physiology. Drug Metab. Dispos. 37(8):1565-1571.
Thompson, C.M., R. Ceder, and R.C. Grafström. 2010. Formaldehyde dehydrogenase: beyond phase I metabolism. Toxicol Lett. 193(1):1-3.
Wu, H., I. Romieu, J.J. Sienra-Monge, B.E. Del Rio-Navarro, D.M. Anderson, C.A. Jenchura, H. Li, M. Ramirez-Aguilar, I. Del Carmen Lara-Sanchez, and S.J. London. 2007. Genetic variation in S-nitrosoglutathione reductase (GSNOR) and childhood asthma. J. Allergy Clin. Immunol. 120(2):322-328.

































