Summary
Formaldehyde is ubiquitous in indoor and outdoor air, and everyone is exposed to formaldehyde at some concentration daily. Formaldehyde is used to produce a wide array of products, particularly building materials; it is emitted from many sources, including power plants, cars, gas and wood stoves, and cigarettes; it is a natural product in some foods; and it is naturally present in the human body as a metabolic intermediate. Much research has been conducted on the health effects of exposure to formaldehyde, including effects on the upper airway, where formaldehyde is deposited when inhaled, and effects on tissues distant from the site of initial contact.
For more than a decade, the U.S. Environmental Protection Agency (EPA) has been in the process of re-evaluating the health effects of formaldehyde; in June 2010, it released its draft health assessment of formaldehyde for EPA’s Integrated Risk Information System (IRIS). Given the complex nature of the assessment and recognition that the assessment will be used as a basis of risk calculations and regulatory decisions, EPA asked the National Research Council (NRC) to conduct an independent scientific review of the draft IRIS assessment and to answer questions related specifically to its derivation of reference concentrations (RfCs) for noncancer effects and unit risk estimates for cancer. In response to EPA’s request, NRC convened the Committee to Review EPA’s Draft IRIS Assessment of Formaldehyde, which prepared this report.
In addressing its charge,1 the committee reviewed the draft IRIS assessment provided. It did not perform its own assessment, which would have been beyond its charge. Accordingly, the committee did not conduct its own literature searches, review all relevant evidence, systematically formulate its own conclusions regarding causality, or recommend values for the RfC and unit risk. The committee reviewed the draft IRIS assessment and key literature and determined whether EPA’s conclusions were supported on the basis of that assessment and literature.
|
1 |
See Chapter 1 for the committee’s verbatim statement of task. |
THE DRAFT IRIS ASSESSMENT
Overall, the committee noted some recurring methodologic problems in the draft IRIS assessment of formaldehyde. Many of the problems are similar to those which have been reported over the last decade by other NRC committees tasked with reviewing EPA’s IRIS assessments for other chemicals. Problems with clarity and transparency of the methods appear to be a repeating theme over the years, even though the documents appear to have grown considerably in length. In the roughly 1,000-page draft reviewed by the present committee, little beyond a brief introductory chapter could be found on the methods for conducting the assessment. Numerous EPA guidelines are cited, but their role in the preparation of the assessment is not clear. In general, the committee found that the draft was not prepared in a consistent fashion; it lacks clear links to an underlying conceptual framework; and it does not contain sufficient documentation on methods and criteria for identifying evidence from epidemiologic and experimental studies, for critically evaluating individual studies, for assessing the weight of evidence, and for selecting studies for derivation of the RfCs and unit risk estimates. This summary highlights the committee’s substantive comments and recommendations that should be considered in revision of the draft IRIS assessment; more detailed comments and recommendations can be found at the conclusions of individual chapters or following the discussions on individual health outcomes.
Toxicokinetics
The committee reviewed the extensive discussion on toxicokinetics of formaldehyde in the draft IRIS assessment and focused on several key issues: the implications of endogenous formaldehyde, the fate of inhaled formaldehyde, the systemic availability of formaldehyde, the ability of formaldehyde to cause systemic genotoxic effects, and the usefulness of various models.
Endogenous formaldehyde. Humans and other animals produce formaldehyde through various biologic pathways as part of normal metabolism. Thus, formaldehyde is normally present at low concentrations in all tissues, cells, and bodily fluids. Although there is some debate regarding interpretation of the analytic measurements, formaldehyde has been measured in exhaled breath and is most likely present normally at a concentration of a few parts per billion. The endogenous production of formaldehyde complicates the assessment of the risk associated with formaldehyde inhalation and remains an important uncertainty in assessing the additional dose received by inhalation, particularly at sites beyond the respiratory tract.
Fate of inhaled formaldehyde. Formaldehyde is a highly water-soluble, reactive chemical that has a short biologic half-life. Despite species differences in uptake due to differences in breathing patterns and nasal structures, formaldehyde is absorbed primarily at the site of first contact where it undergoes exten-
sive local metabolism and reactions with macromolecules. Thus, the net result is that inhaled formaldehyde remains predominantly in the respiratory epithelium that lines the airways.
Systemic availability of formaldehyde. The issue of whether inhaled formaldehyde can reach the systemic circulation is important in assessing the risk of adverse effects at nonrespiratory sites. The draft IRIS assessment provides divergent statements regarding systemic delivery of formaldehyde that need to be resolved. Specifically, some parts of the draft assume that the high reactivity and extensive nasal absorption of formaldehyde restrict systemic delivery of inhaled formaldehyde so that formaldehyde does not go beyond the upper respiratory tract, and other parts of the draft assume that systemic delivery accounts in part for the systemic effects attributed to formaldehyde exposure.
The committee concludes that the weight of evidence suggests that formaldehyde is unlikely to appear in the blood as an intact molecule except perhaps at concentrations high enough to transiently overwhelm the metabolic capability of the tissue at the site of exposure. Thus, direct evidence of systemic delivery of formaldehyde is generally lacking. Furthermore, it is unlikely that formaldehyde reaches distal sites via its hydrated form, methanediol. Although equilibrium dynamics indicate that methanediol would constitute more than 99.9% of the total free and hydrated formaldehyde, experimental data provide compelling evidence that hydration of formaldehyde does not enhance delivery beyond the portal of entry to distal tissues. Pharmacokinetic modeling also supports that conclusion.
Systemic genotoxic effects of formaldehyde exposure. The draft IRIS assessment correctly concludes that formaldehyde is a genotoxic (DNA-reactive) chemical that causes cytogenetic effects, such as mutations. Furthermore, the overall body of evidence suggests that inhaled formaldehyde has a cytogenetic effect that can be detected in peripheral (circulating) blood lymphocytes. However, the committee concludes that data are insufficient to conclude definitively that formaldehyde is causing cytogenetic effects at distant sites. First, the observed effects have occurred in highly exposed people, and extrapolating to more typical environmental exposures is difficult given the uncertainty surrounding the form of the dose-response curve for cytogenetic changes. Second, a mechanism that would explain the occurrence of cytogenetic effects in circulating blood cells has not been established. That gap in mechanistic understanding is particularly problematic because the data strongly suggest that formaldehyde is not available systemically in any reactive form. Thus, the committee can only hypothesize that the observed effects result from an unproven mechanism in portal-of-entry tissues.
Usefulness of various models. Computational fluid dynamics (CFD) models have been developed to help to predict the dose to nasal tissues from inhaled formaldehyde. EPA fairly evaluated the models and sources of uncertainty but did not use the models to extrapolate to low concentrations. The committee concludes that the models would be useful for that purpose and recommends that EPA use the CFD models to extrapolate to low concentrations, include the re-
sults in the revised IRIS assessment, and explain clearly its use of CFD modeling approaches.
A biologically based dose-response (BBDR) model that has been developed for formaldehyde could be used in the derivation of the unit risk estimates. EPA explored the uncertainties associated with the model and sensitivities of various model components to changes in key parameters and assumptions and, on the basis of those extrapolations, decided not to use the BBDR model in its assessment. Although the committee agrees that EPA’s evaluation of the model yielded some important findings on model sensitivity, some of the manipulations are extreme, may not be scientifically justified, and should not have been used as the basis of rejection of the use of the BBDR model in its assessment. Model manipulations that yield results that are implausible or inconsistent with available data should be rejected as a basis for judging the utility of the model.
The primary purposes of a BBDR model are to predict as accurately as possible a response to a given exposure, to provide a rational framework for extrapolations outside the range of experimental data (that is, across doses, species, and exposure routes), and to assess the effect of variability and uncertainty on model parameters. In developing a BBDR model, a model structure and parameter values should be chosen to constrain model predictions within biologic and physical limits, all relevant data should be reconciled with the model, and model predictions should be reconciled with credible outcomes. Thus, it provides a valuable method for predicting the range of plausible responses in a given exposure scenario. Given that the BBDR model for formaldehyde is one of the best-developed BBDR models to date, the positive attributes of BBDR models generally, and the limitations of the human data, the committee recommends that EPA use the BBDR model for formaldehyde in its cancer assessment, compare the results with those described in the draft assessment, and discuss the strengths and weaknesses of each approach.
Mode of Action for Formaldehyde Carcinogenesis
Mode of action is defined as a sequence of key events that describe the biologic pathway from exposure to adverse outcome. Understanding the mode of action is important because it can provide support for conclusions regarding causality, and it can affect how unit risk estimates are calculated. Potential modes of action for formaldehyde carcinogenesis have been debated. EPA based its approach to its cancer assessment primarily on the conclusion that formaldehyde is a genotoxic chemical that causes mutations (a mutagenic mode of action). However, for nasal tumors attributed to formaldehyde exposure, animal data also support a mode of action characterized by regenerative cellular proliferation that results from cytotoxicity. Because multiple modes of action may be operational, the committee recommends that EPA provide additional calculations that factor in regenerative cellular proliferation as a mode of action, com-
pare the results with those presented in the draft assessment, and assess the strengths and weaknesses of each approach.
Little is known about a potential mode of action for hematopoietic cancers, such as leukemias, that have been attributed to formaldehyde exposure and that are assumed to arise from sites distant from the portal of entry. The draft IRIS assessment speculates that formaldehyde could reach the bone marrow and cause the mutagenic effects that lead to the cancers noted. However, despite the use of sensitive and selective analytic methods that are capable of differentiating endogenous exposures from exogenous ones, numerous studies have demonstrated that systemic delivery of formaldehyde is unlikely at concentrations that do not overwhelm metabolism. The draft assessment further speculates that circulating hematopoietic stem cells that percolate the nasal capillary bed or nasal-associated lymphoid tissues may be the target cells for the mutagenic effects that eventually lead to the cancers noted. However, experimental evidence supporting that mechanism is lacking.
Portal-of-Entry Health Effects
EPA evaluated a wide array of outcomes that the committee chose to characterize as portal-of-entry health effects or systemic health effects.2 The portal-of-entry effects include irritation, decreased pulmonary function, respiratory tract pathology, asthma, and respiratory tract cancers. Overall, the committee found that the noted outcomes were appropriate to evaluate. EPA identified relevant studies for its assessment, and on the basis of the committee’s familiarity with the scientific literature, it does not appear to have overlooked any important study. For a few outcomes, however, as noted below, EPA did not discuss or evaluate literature on mode of action that could have supported its conclusions. Although EPA adequately described the studies, critical evaluations of the strengths and weaknesses of the studies were generally deficient, and clear rationales for many conclusions were not provided. In several cases, the committee would not have advanced a particular study or would have advanced other studies to calculate the candidate RfCs. Comments on the specific outcomes are provided below.
Irritation. Formaldehyde has been consistently shown to be an eye, nose, and throat irritant, and EPA used several studies of residential exposure to calculate candidate RfCs. However, the favorable attributes of one particular selected study (Richie and Lehnen 1987)3 were outweighed by the potential for participant-selection bias, and EPA should not have used it to calculate an RfC. Fur-
thermore, EPA set aside the chamber and occupational studies too soon in the process. Although the chamber studies are of acute duration, they are complementary with the residential studies and provide controlled measures of exposure and response. Therefore, the committee recommends that EPA present the concentration-response data from the occupational, chamber, and residential studies on the same graph and include the point estimate and measures of variability in the exposure concentrations and responses. The committee notes that EPA did not (but should) review research findings on transient-receptor-potential ion channels and evaluate the utility of this evidence for improving understanding of the mode of action for sensory irritation and respiratory effects attributed to formaldehyde exposure.
Decreased pulmonary function. The committee agrees with EPA that formaldehyde exposure may cause a decrease in pulmonary function, but EPA should provide a clear rationale to support that conclusion. Furthermore, although the committee supports the use of the study by Kryzanowski et al. (1990)4 to calculate a candidate RfC, EPA should provide a clear description of how the study was used to estimate a point of departure and should also consider the studies conducted by Kriebel et al. (1993, 2001)5 and the chamber studies for possible derivation of candidate RfCs.
Respiratory tract pathology. Animal studies in mice, rats, and nonhuman primates clearly show that inhaled formaldehyde at 2 ppm or greater causes cytotoxicity that increases epithelial-cell proliferation and that after prolonged inhalation can lead to nasal tumors. Although the committee agrees with EPA that the human studies that assessed upper respiratory tract pathology were insufficient to derive a candidate RfC, it disagrees with EPA’s decision not to use the animal data. The animal studies offer one of the most extensive datasets on an inhaled chemical, and EPA should use the data to derive a candidate RfC for this outcome.
Asthma. Asthma is a term applied to a broad phenotype of respiratory disease that comprises an array of symptoms resulting from underlying airway inflammation and associated airway hyper-reactivity. In infants and children, wheezing illnesses that are the result of lower respiratory tract infections are often labeled as asthma, and in adults, the symptoms can overlap with those of other chronic diseases, such as chronic obstructive pulmonary disease. Thus, a critical review of the literature is essential to ensure that what is being evaluated is asthma. The committee notes that this issue is not adequately addressed in the
draft IRIS assessment and that EPA advanced a study (Rumchev et al. 2002)6 that most likely suffers from misclassification of infection-associated wheezing in young children as asthma. The draft IRIS assessment also provides little discussion of the current understanding of the mechanisms of asthma causation and exacerbation. Given the abundant research available, the committee recommends that EPA strengthen its discussion of asthma to reflect current understanding of this complex disease and its pathogenesis. Although the committee agrees that the study by Garrett et al. (1999)7 should be used to calculate a candidate RfC, the approach taken to identifying the point of departure needs further justification.
Respiratory tract cancers. The respiratory tract is considered to be a plausible location of formaldehyde-induced cancers in humans because these cancers occur at the site of first contact and because studies have shown an increased incidence of nasal tumors in rats and mice exposed to formaldehyde. However, the draft IRIS assessment does not present a clear framework for causal determinations and presents several conflicting statements that need to be resolved regarding the evidence of a causal association between formaldehyde and respiratory tract cancers. On the basis of EPA cancer guidelines, the committee agrees that there is sufficient evidence (that is, the combined weight of epidemiologic findings, results of animal studies, and mechanistic data) of a causal association between formaldehyde and cancers of the nose, nasal cavity, and nasopharnyx. It disagrees that the evidence regarding other sites in the respiratory tract is sufficient. The committee agrees with EPA that the study by Hauptmann et al. (2004)8 is the most appropriate for deriving a unit risk value but notes that this study is being updated.
Systemic Health Effects
The systemic effects evaluated by EPA include immunotoxicity, neurotoxicity, reproductive and developmental toxicity, and lymphohematopoietic (LHP) cancers. As noted above, high reactivity and extensive nasal absorption of formaldehyde restrict systemic delivery of inhaled formaldehyde beyond the upper respiratory tract and major conducting airways of the lung, so systemic responses are unlikely to arise from the direct delivery of formaldehyde (or its hydrated form, methanediol) to a distant site in the body. However, a distinction
needs to be made between systemic delivery and systemic effects. The possibility remains that systemic delivery of formaldehyde is not a prerequisite for some of the reported systemic effects seen after formaldehyde exposure. Those effects may result from indirect modes of action associated with local effects, such as irritation, inflammation, and stress. Therefore, the committee reviewed EPA’s evaluation of the systemic effects and determined whether the evidence presented supported EPA’s conclusions.
As in the evaluation of the portal-of-entry effects, the committee concluded that EPA identified relevant literature and adequately described the studies selected; however, critical evaluations of study strengths and weaknesses were generally lacking, and clear rationales for conclusions were often not provided. As a result, some narratives did not support the conclusions stated. Comments on the specific outcomes are provided below.
Immunotoxicity. The draft IRIS assessment presents numerous studies suggesting that formaldehyde has the ability to affect immune functions. However, EPA should conduct a more rigorous evaluation of the strengths and weaknesses of the studies; more integration of the human and animal data would lend support to the conclusions made. The committee agrees with EPA’s decision not to calculate a candidate RfC on the basis of immunotoxicity studies.
Neurotoxicity. The committee found that EPA overstated the evidence in concluding that formaldehyde is neurotoxic; the human data are insufficient, and the candidate animal studies deviate substantially from neurotoxicity-testing guidelines and common practice. Furthermore, the committee does not support EPA’s conclusion that the behavioral changes observed in animals exposed to formaldehyde are not likely to be caused by the irritant properties of formaldehyde. Data indicate that those changes could occur as a result of nasal irritation or other local responses; stress, also an important confounder that can affect the nervous system, was not considered by EPA. The draft IRIS assessment provides conflicting statements that need to be resolved about whether formaldehyde is a direct neurotoxicant. The committee agrees with EPA’s decision not to calculate a candidate RfC on the basis of the neurotoxicity studies.
Reproductive and developmental toxicity. The draft IRIS assessment states that epidemiologic studies provide evidence of a “convincing relationship between occupational exposure to formaldehyde and adverse reproductive outcomes in women.” The committee disagrees and concludes that a small number of studies indicate a suggestive pattern of association rather than a “convincing relationship.” Animal data also suggest an effect, but EPA should weigh the negative and positive results rigorously inasmuch as negative results outnumbered positive ones for some end points, should evaluate study quality critically because some studies of questionable quality were used to support conclusions, and should consider carefully potential confounders, such as maternal toxicity, effects of stress, exposure concentrations above the odor threshold, and potential for oral exposures through licking. Although the epidemiologic studies provide only a suggestive pattern of association, EPA followed its guidelines and chose the best available study to calculate a candidate RfC.
Lymphohematopoietic cancers. EPA evaluated the evidence of a causal relationship between formaldehyde exposure and several groupings of LHP cancers—“all LHP cancers,” “all leukemias,” and “myeloid leukemias.” The committee does not support the grouping of “all LHP cancers” because it combines many diverse cancers that are not closely related in etiology and cells of origin. The committee recommends that EPA focus on the most specific diagnoses available in the epidemiologic data, such as acute myeloblastic leukemia, chronic lymphocytic leukemia, and specific lymphomas.
As with the respiratory tract cancers, the draft IRIS assessment does not provide a clear framework for causal determinations. As a result, the conclusions appear to be based on a subjective view of the overall data, and the absence of a causal framework for these cancers is particularly problematic given the inconsistencies in the epidemiologic data, the weak animal data, and the lack of mechanistic data. Although EPA provided an exhaustive description of the studies and speculated extensively on possible modes of action, the causal determinations are not supported by the narrative provided in the draft IRIS assessment. Accordingly, the committee recommends that EPA revisit arguments that support determinations of causality for specific LHP cancers and in so doing include detailed descriptions of the criteria that were used to weigh evidence and assess causality. That will add needed transparency and validity to its conclusions.
Derivation of Reference Concentrations for Formaldehyde
An RfC is defined by EPA as “an estimate…of a continuous inhalation exposure to the human population…that is likely to be without an appreciable risk of deleterious effects during a lifetime” (EPA 2010).9 It is derived by applying uncertainty factors to a point of departure that is identified in or derived from a study that evaluates a relevant health end point, such as asthma incidence. The committee was asked to comment on specific uncertainty factors used to derive the candidate RfCs in the draft IRIS assessment: the one used to capture variability in response to formaldehyde exposure in the human population (UFH) and the one used to capture the adequacy of the database (UFD). The committee notes that it had some difficulty in commenting on derivation of the RfCs because it would have made some different decisions regarding study selection and calculation of candidate RfCs as indicated above. Accordingly, the committee’s comments here should not be interpreted as a recommendation for any particular RfC as presented in the draft IRIS assessment.
Determining the appropriate value of the UFH involves consideration of possible susceptibility of the human population and the factors that could influ-
|
9 |
EPA (U.S. Environmental Protection Agency). 2010. Glossary, EPA Risk Assessment. U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/risk_assessment/glossary.htm#r [accessed Nov. 29, 2010]. |
ence it. The committee agrees with EPA that available data indicate that there are possible differences in susceptibility to formaldehyde at various life stages and in various disease states. The epidemiologic studies used to calculate the candidate RfCs for respiratory effects and sensory irritation included people in susceptible populations (children and people who have asthma). However, the modes of action for formaldehyde’s effects are not sufficiently understood to ensure that all potential susceptible populations and factors contributing to susceptibility have been identified and adequately described. Thus, the committee supports the use of a UFH of 3 to calculate candidate RfCs for studies identified in the draft IRIS assessment on reduced pulmonary function, asthma, and sensory irritation, noting that the committee does not support the advancement of the studies by Richie and Lehnen (1987)10 and Rumchev et al. (2002).11
Determining the appropriate value of the UFD involves consideration of the breadth and depth of the data available on a specific chemical. The database on formaldehyde is extensive and includes the evaluation of a full array of health outcomes in the human population and laboratory animals. Although there are some gaps in the data on reproductive, developmental, immunologic, and neurotoxic effects, the likelihood that new effects will be observed at concentrations below those at which respiratory effects have been observed is low. Thus, the committee supports the use of a UFD of 1 with the caveat that research of the types noted should be pursued.
Overall, the committee is troubled by the presentation and derivation of the proposed RfC values and strongly recommends the approach illustrated and described in Figure S-1. A similar approach was recommended by the NRC Committee to Review EPA’s Toxicological Assessment of Tetrachloroethylene and used in recent EPA assessments of tetrachloroethylene and trichloroethylene. Appropriate graphic aids that enable the visualization of the concentration ranges of the candidate RfCs may identify a central value, isolate especially low or high RfC values that might not be consistent with the body of literature, and ultimately improve the ability of the assessment to make a compelling case that the RfC proposed is appropriate for the most sensitive end point and protective with regard to other potential health effects.
Derivation of Unit Risk Estimates for Formaldehyde
Unit risk for formaldehyde can be defined as the estimate of extra risk caused by inhalation of one unit of formaldehyde, such as 1 ppm or 1 μg/m3, in
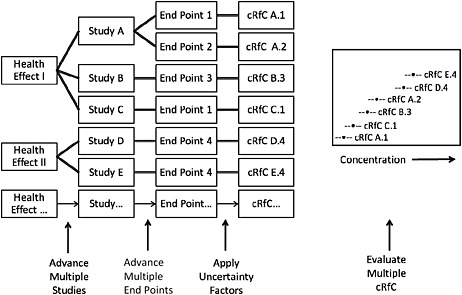
FIGURE S-1 Illustration of potential process for identifying an RfC. Health effects associated with exposure to the chemical are identified. For each health effect, studies that meet inclusion criteria are advanced. From each study, one or more health end points that meet specified criteria are advanced, and a point of departure is identified or derived. Uncertainty factors are selected and applied to the point of departure to yield a candidate RfC (cRfC). All cRfCs are evaluated together with the aid of graphic displays that incorporate selected information relevant to the database and to the decision to be made. A final RfC is selected from the distribution after consideration of all critical data that meet the inclusion criteria.
air. EPA used studies of the National Cancer Institute (NCI) cohort of U.S. workers exposed to formaldehyde through its production or its use (Hauptmann et al. 200412; Beane-Freeman et al. 200913) to estimate unit risk values for three cancers—nasopharyngeal cancer, Hodgkin lymphoma, and leukemia. The committee agrees that the NCI studies are a reasonable choice because they are the only ones with exposure and dose-response data sufficient for calculation of the unit risks; however, the studies are not without their weaknesses, which should be clearly discussed and addressed in the revised IRIS assessment. Although there are uncertainties as discussed above regarding the causal relationship of
formaldehyde exposure and the three kinds of cancer, EPA’s decision to calculate unit risk values for them appears to be defensible on the basis of the agency’s cancer guidelines. However, EPA should provide a clear description of the criteria that it used to select the specific cancers and demonstrate a systematic application of the criteria. The calculation of the unit risk values is a complex process, involves many sources of uncertainty and variability, and is influenced by the low-dose extrapolation used (for example, linear vs threshold). The committee therefore recommends that EPA conduct an independent analysis of the dose-response models to confirm the degree to which the models fit the data appropriately. EPA is encouraged to consider the use of alternative extrapolation models for the analysis of the cancer data; this is especially important given the use of a single study, the inconsistencies in the exposure measures, and the uncertainties associated with the selected cancers.
THE FORMALDEHYDE IRIS ASSESSMENT: THE PATH FORWARD
The committee recognizes that the completion of the formaldehyde IRIS assessment is awaited by diverse stakeholders, and it has tried to be judicious in its recommendations of specific changes noted in its report. However, the committee concludes that the following general recommendations are critical to address in the revision of the draft assessment. First, rigorous editing is needed to reduce the volume of the text substantially and address the redundancies and inconsistencies; reducing the text could greatly enhance the clarity of the document. Second, Chapter 1 of the draft assessment needs to discuss more fully the methods of the assessment. The committee is recommending not the addition of long descriptions of EPA guidelines but rather clear concise statements of criteria used to exclude, include, and advance studies for derivation of the RfCs and unit risk estimates. Third, standardized evidence tables that provide the methods and results of each study are needed for all health outcomes; if appropriate tables were used, long descriptions of the studies could be moved to an appendix or deleted. Fourth, all critical studies need to be thoroughly evaluated for strengths and weaknesses by using uniform approaches; the findings of these evaluations could be summarized in tables to ensure transparency. Fifth, the rationales for selection of studies that are used to calculate RfCs and unit risks need to be articulated clearly. Sixth, the weight-of-evidence descriptions need to indicate the various determinants of “weight.” The reader needs to be able to understand what elements (such as consistency) were emphasized in synthesizing the evidence.
The committee is concerned about the persistence of problems encountered with IRIS assessments over the years, especially given the multiple groups that have highlighted them, and encourages EPA to address the problems with development of the draft assessments that have been identified. The committee recognizes that revision of the approach will involve an extensive effort by EPA staff and others, and it is not recommending that EPA delay the revision of the
formaldehyde assessment to implement a new approach. However, models for conducting IRIS assessments more effectively and efficiently are available, and the committee provides several examples in the present report. Thus, EPA might be able to make changes in its process relatively quickly by selecting and adapting existing approaches. As exemplified by the recent revision of the approach used for the National Ambient Air Quality Standards, this task is not insurmountable. If the methodologic issues are not addressed, future assessments may still have the same general and avoidable problems that are highlighted here.













