4
Portal-of-Entry Health Effects
The Environmental Protection Agency (EPA) evaluated an array of health effects associated with formaldehyde exposure. The health effects can be characterized as portal-of-entry effects or systemic effects. The committee defined portal-of-entry effects as those that arise from direct interaction of inhaled formaldehyde with the airways or from the direct contact of airborne formaldehyde with the eyes or other tissue. It defined systemic effects as effects that occur outside those systems.
EPA’s evaluation of portal-of-entry health effects—which are irritation, decreased pulmonary function, respiratory tract pathology, asthma, and respiratory tract cancers—is reviewed in this chapter. The committee determined whether EPA identified the appropriate studies, whether the studies were thoroughly evaluated, whether hazard identification was conducted appropriately according to EPA guidelines, and whether the best studies were advanced for calculation of the reference concentration (RfC) or unit risk.
For two portal-of-entry effects (irritation and decreased lung function), evidence was available from chamber studies that used brief, controlled exposures to assess acute responses and from epidemiologic studies that evaluated chronic exposures primarily in a residential setting and prevalence of symptoms or diseases or the degree of lung-function impairment. Both types of studies have strengths and weaknesses for serving as the basis of candidate RfCs. The chamber studies involve exposures to known concentrations of formaldehyde without the presence of other air contaminants, and outcome measures can be rigorously measured. However, the study populations are selected groups of volunteers, more sensitive people may avoid participation, and the numbers of participants in the studies are generally small, leading to inadequate statistical power to detect biologically significant changes in many of the studies. Furthermore, there is uncertainty about extrapolating from an acute exposure to a chronic exposure, which would be required for derivation of an RfC; and for irritant responses, observations made for a single, brief exposure may not reflect
the consequences of sustained exposure. There is some indication from acute and short-terms studies that irritant responses to formaldehyde are lessened by acclimatization.
The epidemiologic studies considered in the draft IRIS assessment are primarily cross-sectional studies and subject to the general weaknesses that affect studies of this design, including the simultaneous measurement of exposure and outcome. Many of the studies involved exposure in residences, and the exposure-assessment protocols covered only a brief time window, leaving the possibility that exposures were misclassified. Furthermore, few of the studies took into account potentially confounding exposures, such as secondhand smoke or other air pollutants. The epidemiologic studies do have the advantage of assessing the risks of formaldehyde exposures as they are experienced on a chronic basis. The study populations cover the range of susceptibility and, to the extent that the effects of formaldehyde exposure are modified by interactions with other agents, the exposure to formaldehyde is experienced along with exposure to the many other contaminants in indoor air.
Given the quite different strengths and weaknesses of the two lines of research, the findings from chamber studies and epidemiologic studies should be considered as complementary. The draft IRIS assessment sets aside the chamber studies as less relevant to derivation of candidate RfCs, but the findings from the studies could be useful, and the committee does not concur with EPA's decision to set them aside. Specific recommendations are provided below for the individual health outcomes.
IRRITATION
Formaldehyde is a reactive gas that has been consistently shown to be an eye, nose, and throat irritant. Sensory irritants act at the sensory fibers of the trigeminal nerve in the nose and upper respiratory tract. Sensory-irritation end points include self-reported sensations of pain, burning, or itching and objective measures of eye-blink counts and lacrimation (Doty et al. 2004). Although EPA’s review focuses on eye, nose, and throat irritation, other types of irritation, such as dermal irritation, have been reported. EPA selected sensory irritation as a candidate critical effect on the basis of concentration-response relationships between formaldehyde and eye irritation observed in three epidemiologic studies of residential populations.
Study Identification
EPA identified many studies that evaluated sensory irritation in response to formaldehyde exposure in residential, occupational, and clinical settings in humans and in experimental animal studies. Human studies evaluated sensory irritation responses in the eyes, nose, and throat after exposure to formaldehyde at 100-3,000 ppb and for durations ranging from 90 sec in chamber studies to
chronic residential exposure. They included potentially sensitive members of the population: children less than 4 years old, adults over 65 years old, and people who have asthma.
EPA appears to have identified all appropriate exposure-response studies in humans and animals, but the literature review of studies related to the mode of action of sensory irritation associated with formaldehyde exposure should be expanded. The literature on the biologic basis of sensory irritation is more extensive than that included in the draft IRIS assessment and includes studies relevant for evaluating the mode of action of formaldehyde in the respiratory system.
Study Evaluation
EPA summarized human and animal studies that were identified as having data on formaldehyde concentrations and sensory irritation responses in the eyes, nose, and throat. Population characteristics, exposure assessment, exposure-response relationships, and data analysis presented by the study authors were discussed by EPA. However, the committee found that study details (such as age ranges of study participants, sampling durations, and participant-selection processes) and study weaknesses (such as the limitations of the exposure assessments performed in the residential and occupational epidemiologic studies) were not thoroughly presented or critically evaluated in a consistent manner by EPA. In some cases, EPA did not give sufficient weight to study weaknesses, such as bias in the selection of participants and the possibility of confounding by other pollutants.
Hazard Identification and Use of EPA Guidelines
Formaldehyde is a well-recognized reactive and irritant gas. EPA does not have a separate guidance document for evaluating sensory irritation responses. However, the assessment of the available human and animal studies for development of an RfC for sensory irritation was consistent with the guidance for evaluation of studies in the RfC guidelines (EPA 1994).
EPA’s discussion of sensory irritation included direct sensory responses and reflex responses observed in humans and animals. The draft IRIS assessment cites the Arts et al. (2006) analysis to support its conclusion that the onset and severity of irritant responses to formaldehyde were observed to be time-dependent and concentration-dependent. However, the results of the Arts et al. (2006) analysis are incorrectly characterized and do not provide strong support for that conclusion.1 The committee evaluated several recent reviews of formal-
dehyde sensory irritation and did not identify any studies explicitly designed to characterize the relationship of response, concentration, and exposure duration for sensory irritation for either acute or chronic exposures.2 As noted by EPA, the chamber studies demonstrate that formaldehyde exposure causes sensory irritation in humans; this finding supports the association of increased sensory irritation with increased formaldehyde concentration observed in the residential epidemiologic studies. The potential contribution of sensory irritation to other respiratory health effects was acknowledged during the discussion of other effects, such as lung function, respiratory tract pathology, sensitization, and asthma.
Chapter 4.4.1 of the draft IRIS assessment provides a possible mode of action for sensory irritation: “formaldehyde-induced stimulation of the trigeminal nerve (though whether formaldehyde acts as a direct agonist is unknown)” (EPA 2010a, p. 4-458). Chapter 6 of the draft assessment makes a stronger statement about the same mode of action. Both sections, however, omit discussion of activity related to the transient receptor potential (TRP) and its association with sensory irritation. Several papers have identified the TRP family of ion channels in sensory neurons as important mediators of response to chemical irritants (Bautista et al. 2006; Macpherson et al. 2007; Bessac and Jordt 2008; Caceres et al. 2009; Bessac and Jordt 2010). Formaldehyde has been shown to activate the TRPA1 ion channel irreversibly by covalent modification—the same as the activation mechanism of other known sensory irritants, such as mustard oil and cinnamaldehyde (Macpherson et al. 2007)—and to act on TRPA1 channels to elicit pain (Macpherson et al. 2007; McNamara et al. 2007). Work by Caceres et al. (2009) provides evidence that TRPA1 plays a critical role in allergic asthmatic responses as a major neuronal mediator of allergic airway inflammation. Other environmental irritants—including the metabolites of naphthalene and styrene, ozone, acrolein, and products of lipid peroxidation resulting from oxidative stress—have been shown to activate TRPA1 (Bautista et al. 2006; Macpherson et al. 2007; Taylor-Clark et al. 2008; Taylor-Clark and Undem 2010; Lanosa et al. 2010). Multiple endogenous and exogenous agents may activate the TRPA1 ion channel simultaneously (Macpherson et al. 2007; Bessac and Jordt 2008). The committee suggests that EPA review this research and consider its potential for improving understanding of the mode of action underlying the irritant effects associated with formaldehyde exposure.
Study Selection for Calculation of Reference Concentration and Identification of Point of Departure
Three epidemiologic studies—Hanrahan et al. (1984), Ritchie and Lehnen (1987), and Liu et al. (1991)—that evaluated sensory irritation in residents of mobile and conventional homes were advanced as a group by EPA and considered adequate for calculation of candidate RfCs. The studies provided concentration-response data on several sensory irritation responses, including irritation of the eyes, nose, and throat, of which eye irritation was identified by EPA as the most sensitive and best characterized. The committee agrees with EPA’s decision to advance the eye irritation effects observed in the residential epidemiologic studies in spite of their limitations. However, it found that EPA set aside the chamber and occupational studies too soon in the process. Although the chamber studies are of acute duration (5-hr maximum single exposure), they are complementary with the residential studies and provide controlled measures of exposure and response. Therefore, the committee strongly recommends that EPA also present the concentration-response data from the occupational, chamber, and residential studies on the same graph and include the point estimate and measures of variability in the exposure concentrations and responses. The concentration-response relationship for eye irritation among the different types of human studies would strengthen EPA’s argument for selection of residential studies for development of candidate RfCs.
The strength of the selected epidemiologic studies lies in their evaluation of responses in the general human population who are chronically exposed, their measurement of formaldehyde concentrations in residences, and their assessment of effects during or soon after sampling. EPA concluded that potential weaknesses of the studies—use of subjective surveys to collect response information, short sampling duration, and potential bias in selection of homes—were sufficiently controlled for by the study authors. However, the committee has concerns about the potential weaknesses, especially of the study conducted by Ritchie and Lehnen (1987). There are several general concerns that are relevant to each study: they are cross-sectional in design, the formaldehyde concentration measurements were taken during brief intervals and may not accurately represent usual exposure concentrations, and the investigators considered potential confounding by other pollutants to a varying extent.
The committee identified the most serious problems in the study by Ritchie and Lehnen (1987). The committee concluded that the positive attributes of that study—the large sample (2,007) and administration of the survey assessing health effects by a technician at the time of sampling—did not outweigh the potential for selection bias in self-selection of participants, who before participation in the study had to meet with a physician for prescreening and have a written request from the physician to the Minnesota Department of Health (MDH).3
That process is likely to have resulted in enrichment of the sample with people who were symptomatic and concerned about formaldehyde exposure. At that time, there was substantial controversy concerning formaldehyde exposure, and people who experienced symptoms and had knowledge of formaldehyde sources in their homes would have been more likely to have sought a test from a physician and have a referral to the MDH. The very high rate (86-93%) of participants who reported eye irritation at concentrations of 300 ppb or greater, particularly in comparison with the prevalence estimates for the middle exposure category, suggests considerable participant selection bias. The draft IRIS assessment does not address that issue but comments on recall bias, noting that participants were not aware of formaldehyde concentration when the questionnaires were completed. The committee further notes that mothers responded for their children and that the analytic strategy did not account for the data structure (that is, household was the unit of assignment for exposure, but the data were analyzed as though the data from individuals within a household were independent). Thus, the committee recommends that the Ritchie and Lehnen (1987) study not be used to estimate a point of departure for a candidate RfC.
Although the contribution of cigarette smoke to sensory irritation was controlled for in the residential epidemiologic studies, the absence of evaluation of chemicals other than formaldehyde in the indoor air samples and their potential to confound the association of formaldehyde and sensory irritation is not directly addressed in the draft IRIS assessment.
EPA identified a point of departure for each study that was selected for derivation of a candidate RfC for eye irritation: a no-observed-adverse-effect level (NOAEL) of 50 ppb (Ritchie and Lehnen 1987), a lower 95% confidence limit on the benchmark concentration corresponding to a 10% response level (BMCL10) of 70 ppb (Hanrahan et al. 1984), and a lowest observed-adverse-effect level (LOAEL) of 95 ppb (Liu et al. 1991). The committee supports the points of departure selected by EPA for the Hanrahan et al. (1984) and Liu et al. (1991) studies. Although the committee does not recommend that EPA advance the Ritchie and Lehnen (1987) study for calculating a candidate RfC, it is included in its comments on the point of departure.
The draft IRIS assessment appears to use an inconsistent approach for identifying points of departure from studies that present exposure as categories or ranges of concentrations. For example, the Ritchie and Lehnen (1987) and Liu et al. (1991) studies determined points of departure on the basis of the results of three exposure categories. Neither study had a nonexposed reference group for evaluating background response rate. Ritchie and Lehnen reported a 1-2% prevalence of eye irritation in the lowest exposure group (exposed to formaldehyde at less than 100 ppb) and a 12-32% prevalence in the middle expo-
sure group (exposed at 100-300 ppb). EPA identified less than 100 ppb as the NOAEL and assigned 50 ppb (the midpoint between 0 ppb and 100 ppb) as the NOAEL for calculation of the candidate RfC. In the other case, Liu and co-workers reported a prevalence of eye irritation of 11-13% in the lowest exposure group (exposed at less than 70 ppb; detection limit for a 7-day passive air sample was 10 ppb) and a prevalence of 15-17% in the middle exposure group (exposed at 70-120 ppb). EPA selected 70-120 ppb as the LOAEL and 95 ppb (the midpoint of the range) as the LOAEL for calculation of the candidate RfC. The uncertainty associated with the process for selecting a point of departure is not explicitly discussed in the draft assessment.
The discussions of uncertainty associated with the points of departure for individual critical studies in Sections 5.1.4.1 and 6.2.1.4.1 of the draft IRIS assessment are too limited. For example, the draft assessment does not discuss uncertainty in the points of departure contributed by sources specific to the formaldehyde database, such as differences in methods used by the critical studies to adjust exposures (such as exposure estimated from samples collected for 7 days vs one or two sample collections of 30 or 60 min each) to reflect chronic exposure and differences in methods of characterizing exposure-response relationships (such as using regression coefficients to estimate a BMC and BMCL for a specific study’s response rate vs using the midpoint of an exposure category as the estimate of the exposure concentration associated with the study’s response).
Conclusions and Recommendations
The committee agrees with EPA’s selection of eye irritation as a critical sensory-irritation effect caused by formaldehyde exposure because residential, occupational, and chamber studies have demonstrated that the eyes are more sensitive to irritation from formaldehyde than the nose and throat. The committee supports EPA’s advancement of the residential studies by Liu et al. (1991) and Hanrahan et al. (1984) for derivation of candidate RfCs as adequately conducted studies of a randomly selected general population and agrees with the points of departure identified by EPA from these studies:
LOAEL = 95 ppb (Liu et al. 1991)
BMCL10 = 70 ppb (Hanrahan et al. 1984)
The committee recommends that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Strengthen its critical evaluation of the studies.
-
Re-evaluate the chamber and occupational studies for calculation of candidate RfCs.
-
Not advance the Ritchie and Lehnen (1987) study for calculation of a candidate RfC.
-
Review research on the TRPA1 and TRPV1 ion channels and use the information to strengthen discussion of the mode of action underlying the sensory irritation and respiratory effects associated with formaldehyde exposure.
-
Add a figure that contains all the studies that evaluated eye irritation, include for each study the mean concentration, the concentration range, and the participant response rate, and organize the data by study population (residential, occupational, and chamber).
DECREASED PULMONARY FUNCTION
Pulmonary function is assessed with spirometry, which measures the amount of air and the speed at which the air is exhaled during a forced exhalation after a maximum inhalation. Commonly used measures of pulmonary function include the total amount of air exhaled (forced vital capacity, FVC), the amount of air exhaled in the first second of exhalation (forced expiratory volume in 1 sec, FEV1), the ratio of FEV1 to FVC (FEV1/FVC ratio), and the peak expiratory flow rate (PEFR). Pulmonary-function testing is an important tool for the assessment of both asthma and chronic obstructive pulmonary disease. The mode of action of formaldehyde’s effect on pulmonary function may be direct irritation of the airways that result in an inflammatory response or in an asthmatic response in sensitive people.
Study Identification
Acute and chronic adverse effects of occupational and residential exposures to formaldehyde on measures of pulmonary function have been investigated in several epidemiologic studies. The study populations have included occupational groups exposed to formaldehyde in various trades and industries, medical students exposed to formaldehyde in anatomy laboratories, and children and adults exposed to formaldehyde in indoor air coming from residential sources. The research approaches have included cross-sectional studies that involved testing workers’ lung function before and after work shifts and cohort studies with follow-up ranging from weeks to years. The studies have used standard methods of pulmonary-function testing, including spirometry and peak-flow measurement. There is substantial literature on standardizing the testing methods and guidance on interpreting the findings (Miller et al. 2005).
Chamber studies involving brief exposures of volunteers to formaldehyde have also been conducted and have provided mixed findings. The draft IRIS assessment discusses those studies in a descriptive fashion. Deficits in pulmonary function due to formaldehyde exposure have been demonstrated in some human experimental studies that included exercise. An adverse effect of formal-
dehyde on pulmonary function has generally not been observed in studies of healthy volunteers who were not exercising. The lack of evidence of an effect of formaldehyde on pulmonary function in many of the chamber studies might be explained by their small samples and by the acute nature of the exposure in the experiments. Because of those issues, the chamber studies are of limited use for estimating an RfC for pulmonary-function loss. However, given the small samples, a more formal analysis that includes a display of the data with appropriate forest plots might be helpful.
EPA’s review covered the relevant body of epidemiologic and experimental literature. The evidence is diverse and comes from multiple exposed populations. Many of the studies were performed several decades ago and reflect the substantial public-health concern at the time. The review does not appear to have missed more recent studies.
Study Evaluation
EPA’s review of epidemiologic studies, toxicologic studies, and experimental-chamber studies of formaldehyde and pulmonary function was thorough and appropriate. Here, EPA used tables to summarize the pulmonary function studies; this aided the committee in its review of the information.
Hazard Identification and Use of EPA Guidelines
EPA concluded that there is extensive evidence that formaldehyde causes decreased pulmonary function in humans (Section 6.1.3.9, EPA 2010a). Although the committee agrees with that conclusion, a clear narrative is needed to provide the rationale for it. There are no specific EPA guidelines for evaluating effects of agents on pulmonary function or other respiratory disease outcomes. The most relevant may be EPA’s RfC guidelines (EPA 1994). Inconsistencies in the approach taken by EPA may reflect the lack of adequate guidance for this domain of health outcomes.
Study Selection for Calculation of Reference Concentration and Identification of Point of Departure
EPA selected the findings in children (6-15 years old) from the Arizona study by Krzyzanowski et al. (1990) as the basis for the development of a candidate RfC for decreased pulmonary function as measured by PEFR. The draft IRIS assessment justifies the choice by stating that “the best single study demonstrating decreased pulmonary function is the moderate residential study by Krzyzanowski et al. (1990)” (EPA 2010a, pp. 5-36 to 5-39). The draft discusses only briefly the possibility of using other studies, such as the Kriebel et al. (1993, 2001) studies of anatomy students exposed to formalin. The committee
notes that the Krzyzanowski et al. (1990) findings are inherently limited by the cross-sectional nature of their study and found that the study design is not sufficiently described in the published report.
Krzyzanowski et al. (1990) found an effect of formaldehyde in children but not in adults. The findings from the studies by Kriebel et al. (1993, 2001) of anatomy students indicate that the effects of formaldehyde on pulmonary function in adults may be more severe in asthmatics. EPA should provide a more thorough analysis and rationale for its choice to advance only the Krzyzanowski et al. (1990) study but also consider the Kriebel et al. (1993, 2001) studies as additional candidates for its assessment.
EPA derived a BMCL10 of 17 ppb as the point of departure on the basis of the study by Krzyzanowski et al. (1990). Regression coefficients were estimated by using a linear mixed-effects regression model, presented in Table 5 in Krzyzanowski et al. (1990), and used by EPA to derive a BMCL10. The PEFR model allowed the effect of formaldehyde exposure to depend on time of day (morning vs bedtime) and asthmatic status. The calculation of a BMCL10 implies the estimation of the dose associated with a particular response level. The model predicts that the dose will vary with the presence of effect modifiers (morning exposure and asthma) of the exposure of interest (formaldehyde). The draft IRIS assessment is unclear about how EPA defined the BMCL10 given the effect modification. Greater elaboration and discussion of how a BMCL10 was based on the model fit are needed.
Conclusions and Recommendations
EPA’s review and evaluation of the literature on the effects of formaldehyde on pulmonary function were thorough and appropriate. Although the committee supports EPA’s determination that exposure to formaldehyde may cause a decrease in pulmonary function, EPA should provide a stronger narrative to support that conclusion. The committee agrees with the choice of the Krzyzanowski et al. (1990) study as the basis of the derivation of a point of departure for a candidate RfC but recommends that other studies also be considered for calculation of an alternative point of departure.
The committee recommends that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Prepare plots of the findings of the chamber studies to assess the utility of pooling their results.
-
Provide further justification for its choice of the study by Krzyzanowski et al. (1990) for estimating the point of departure.
-
Consider estimating an alternative point of departure based on the studies by Kriebel et al. (1993, 2001).
-
Provide a clear description of how the data from the study by Krzyzanowski et al. (1990) were used to estimate the BMCL10.
NONCANCER RESPIRATORY TRACT PATHOLOGY
Formaldehyde-induced effects on the respiratory tract have been studied extensively. Animal studies have clearly shown that inhaled formaldehyde at 2 ppm or higher is cytotoxic and that increases in epithelial cell proliferation occur after chronic formaldehyde inhalation by mice, rats, and nonhuman primates (Kerns et al. 1983; Monticello et al. 1996). The resulting airway lesions include rhinitis, epithelial dysplasia, and squamous metaplasia. Formaldehyde-induced effects on the respiratory tract demonstrate concentration, time, and site dependence, and these lesions exhibit an anterior to posterior severity gradient (Kerns et al. 1983; Monticello et al. 1996). The committee concludes that the effects for which a candidate RfC should be calculated are histopathologic lesions of the nasal epithelium.
Study Identification
The draft IRIS assessment reviews six studies that examined the effects of formaldehyde exposure on the human upper respiratory tract. Two that examined the same worker cohort are identified as the most robust and sensitive and are selected for possible derivation of a candidate RfC (Holmstrom and Wilhelmsson 1988; Holmstrom et al. 1989). The draft assessment also reviews the extensive literature on histopathologic effects in the respiratory tract and effects on mucociliary clearance in laboratory animals that inhaled formaldehyde. There are numerous studies in several species of laboratory animals, including ones using acute, subchronic, and chronic inhalation exposures.
Although the committee did not perform its own literature search, it notes that two papers (Schoenberg and Mitchell 1975; Bracken et al. 1985) directly related to formaldehyde exposure and cited by Holmstrom and Wilhelmsson (1988) are not included in the draft IRIS assessment. Despite that oversight, EPA appears to have identified the appropriate animal and human studies.
Study Evaluation
The review of the literature in the draft IRIS assessment is extensive but is often unfocused and lacks critical evaluation of the studies. The animal studies are presented in detail, and they provide unequivocal evidence that inhalation of formaldehyde by laboratory animals causes histopathologic lesions of the upper respiratory tract. The six studies that evaluated formaldehyde-induced effects in humans all used relatively small samples, and the methods of characterizing exposure were not always discussed. None of the human studies demonstrated that exposure duration was important or that a concentration-response relationship was present. The draft assessment appears to give equal weight to all publications of the human and animal studies, and there is no consideration of study quality, of the validity of the measurement of the exposure concentration, or of
whether a study was conducted under good laboratory practices or their equivalent. A critical analysis of the human study selected for possible derivation of a candidate RfC is lacking, and there is no evidence of a specified format for evaluation of the animal or human studies. The committee concludes that those are all important weaknesses of the draft assessment.
Hazard Identification and Use of EPA Guidelines
EPA has no specific guidelines for evaluating the pathology of the respiratory tract. However, the animal studies provide clear evidence that inhaled formaldehyde causes lesions of the nasal epithelium. Specifically, it causes cell death that is followed by regenerative hyperplasia and metaplasia of the epithelium of the upper respiratory tract (Monticello et al. 1996). Those responses are probably the combined result of overloading of host protective mechanisms, such as mucociliary clearance, detoxification, and DNA repair (Kerns et al. 1983). The draft IRIS assessment provides a summary of respiratory toxicity (see pages 4-467 through 4-469) that adds little to the previous detailed discussion of the human and animal studies provided elsewhere. The mode of action for the development of histopathologic lesions of the respiratory tract in that section is not clearly presented.
The draft IRIS assessment concludes that histopathologic lesions and abnormal mucociliary clearance are equivalent pathologic lesions of the upper respiratory tract. The committee agrees with the statement that “the mucociliary apparatus is an important barrier to infection and exogenous agents and, thus, [a change in mucociliary clearance] is considered as a potential adverse effect” (EPA 2010a, p. 4-67). The committee, however, concludes that the data on animals and humans are not consistent enough to support derivation of a point of departure for mucociliary clearance and that abnormal mucociliary clearance is not equivalent to a histopathologic lesion.
Study Selection for Calculation of Reference Concentration and Identification of Point of Departure
The epidemiologic study (Holmstrom and Wilhelmsson 1988; Holmstrom et al. 1989) that was selected for advancement was the strongest of the six available that studied histopathology of the human upper respiratory tract. However, as discussed in detail below, even that study had substantial weaknesses that limit its use for derivation of a point of departure and calculation of a candidate RfC. In contrast, numerous studies of several species of animals could be used to derive a candidate RfC. The committee recommends that EPA use the animal data to calculate a candidate RfC for respiratory tract lesions in the revised IRIS assessment. That would provide a basis for evaluating the uncertainty associated with the other candidate RfCs that have been calculated.
The human study selected for advancement (reported in two publications) involved 70 workers exposed to formaldehyde in a chemical plant that produced formaldehyde for resins and impregnation of paper for laminate production (Holmstrom and Wilhelmsson 1988; Holmstrom et al. 1989). The study included a second group of 100 workers exposed to wood dust and formaldehyde in a furniture-production facility. The reference group consisted of 36 persons, most of whom were office workers in the same village as the furniture workers. The draft IRIS assessment does not adequately report the exposure concentrations that were reported in the publications and does not adequately discuss the cohort exposed to wood dust and formaldehyde. The mean formaldehyde exposure of the group exposed only to formaldehyde was accurately reported by EPA as 0.210 ppm. However, EPA did not report the exposure range (0.040-0.403 ppm) or the frequent peak short-term exposures (up to 0.810 ppm) provided by the study authors. Thus, there was considerable variability in the exposures that occurred in the occupational study that would not be reflected by the mean exposure data. In addition, 31 of the 70 formaldehyde-exposed workers were potentially exposed to paper dust at up to 1 mg/m3; this exposure is not noted or discussed in the draft IRIS assessment despite being reported by the study authors, and the committee is concerned that the coexposure could be a confounding factor in the study. The furniture workers were exposed to formaldehyde at 0.160-0.243 ppm (mean, 0.202 ppm) and to wood dust at 1-2 mg/m3. The formaldehyde exposure of the office workers was measured at 0.073-0.137 ppm in late summer. The mean exposure was 0.073 ppm, on the basis of four measurements in different seasons. The background formaldehyde exposure of the reference group is not mentioned in the draft IRIS assessment. The group exposed to formaldehyde alone had an increased nasal-resistance score (as measured by rhinomanometry) of 10.2 compared with 6.5 in the reference group; the difference was not statistically significant. The nasal mucociliary clearance was delayed in both exposure groups compared with the reference group but the difference was statistically significant (p < 0.05) only in the formaldehyde group. Participants who were identified with “pathologically slow nasal clearance” amounted to 14 of 69 in the formaldehyde group and 14 of 95 in the formaldehyde and wood group compared with one of 36 in the reference group. That difference may have been statistically significant, but its biologic significance has not been established. Moreover, the outcome was not found to be related to exposure concentration or duration when subgroups were examined. EPA concluded that the study demonstrated a LOAEL of 0.210 ppm on the basis of impairment of mucociliary clearance. On the basis of the available study data, 0.073 ppm (the background concentration for the reference group) might represent a NOAEL.
Many of the subjects in the study also had nasal biopsies taken and evaluated for histopathologic lesions on an 8-point scale in which 0 was normal epithelium, 4 was stratified squamous epithelium with marked horny layer, and 8 was carcinoma. The formaldehyde-exposed group had a mean score of 2.16, the
wood dust and formaldehyde group had a mean score of 2.07, and the control group had a mean score of 1.56. The difference was significant (p < 0.05) only in the formaldehyde group. However, the actual exposure of the 62 members of the formaldehyde group examined histopathologically was reported as 0.240 ppm rather than the 0.210 ppm reported for mucociliary clearance. The higher value was selected by EPA as the point of departure. It is the opinion of the committee that this study has numerous weaknesses, the most important of which is a failure to identify a clear relationship between adverse responses and exposure concentration or exposure duration.
Table 5-4 of the draft IRIS assessment lists the point of departure as 240 ppb as a LOAEL for the upper respiratory tract pathology. As noted above, that is the value reported by Holmstrom et al. (1989) for histopathologic lesions on the basis of nasal biopsy specimens. However, EPA’s analysis does not account for a background exposure of 73 ppb in the reference group (a possible NOAEL). Section 5.1.2.1 goes on to note that the study did not report a concentration-response relationship and states that “this is less exact than other available studies which provide exposure-response relationships” (EPA 2010a, p. 5-36). It is also noted that animal studies support sensory irritation as a more sensitive end point than histopathologic changes in the nasal mucosa. Therefore, a candidate RfC for that end point was not calculated. The committee agrees that this study should not be used for calculation of a candidate RfC. However, the committee does not agree that there are sufficient data from animal studies to support the conclusion that sensory irritation is a more sensitive end point than histopathologic changes in the nasal mucosa. One form of sensory irritation that has been studied in animals and reviewed in the draft IRIS assessment is reflex bradypnea, a response that occurs in mice and rats exposed to formaldehyde vapors. The concentration that causes an acute 50% reduction in the respiratory rate has been reported to be about 3 ppm in mice (Kane and Alarie 1977) and about 10 ppm in rats (Cassee et al. 1996). In contrast, rats exposed to formaldehyde at 2 ppm for 24 months reportedly have respiratory tract lesions (Kerns et al. 1983). Those data suggest that sensory irritant responses seen in animals occur at concentrations associated with airway histopathologic changes.
As noted above, numerous well-documented studies have reported the occurrence of upper respiratory tract pathology in laboratory animals, including nonhuman primates, after inhalation of formaldehyde. The dataset is one of the most extensive available on an inhaled chemical. The computational-fluid-dynamics (CFD) models could be used to predict the dose to the human upper respiratory tract and thereby decrease the need for uncertainty factors associated with animal-to-human extrapolation. The CFD models have been used by EPA for cancer risk assessment and would be applicable to respiratory tract toxicity (see Chapter 3 for a discussion of the CFD models). As stated previously, the committee recommends that EPA use the animal data to calculate an RfC for respiratory tract pathology.
Conclusions and Recommendations
The committee concludes that a candidate RfC should be calculated for noncancer pathology of the respiratory tract (that is, in the nasal epithelium). The committee agrees with EPA that the human studies that include that end point are not sufficiently complete and that they should not be used to calculate a candidate RfC. However, many well-conducted animal studies have reported noncancer histopathologic lesions of the respiratory tract after formaldehyde inhalation.
The committee recommends that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Calculate a candidate RfC from the animal data.
-
Do not calculate a candidate RfC for mucociliary clearance.
ASTHMA
Asthma refers to a broad phenotype of respiratory disease that may differ by age and by causal agent (NHLBI 2007). In the United States, over 22 million people have asthma (NHLBI 2007). Asthma is the most common chronic disease of childhood and affects about 8-10% of children in the United States and a similar percentage of adults (EPA 2010b). Clinically, the phenotype of asthma involves intermittent airflow obstruction with wheezing and shortness of breath, although in some people the airflow obstruction may become persistent. The term is applied to a broad array of phenotypes involving wheezing illnesses, some of which do not correspond directly with the strict definition of asthma. In infants and young children, wheezing illnesses that are the result of lower respiratory tract infections (such as respiratory syncytial virus) are often labeled as asthma (Martinez et al. 1995). Follow-up of children who have had early-life wheezing illnesses has shown that many did not have incident asthma as the cause of the clinical picture. Similarly, in adults, the phenotypes of asthma and chronic obstructive pulmonary disease may overlap (Barnes 2008).
The underlying pathogenetic process involves inflammation of the airways, which occurs in response to multiple environmental triggers (NHLBI 2007; Barnes 2008). The disease aggregates in families, so it is considered to have a genetic basis, although the search for key underlying genes has been difficult and not yet definitive. The committee notes that an environmental agent like formaldehyde potentially could increase the incidence of asthma, lead to a more severe clinical phenotype, or alter the natural history, perhaps by sustaining inflammation.
Study Identification
As acknowledged in the draft IRIS assessment, formaldehyde might have effects on various components of the asthma phenotype: allergic sensitization,
incidence of asthma, the prevalence of asthma, the severity of asthma, increased symptoms and exacerbations, and lower lung function. The committee concurs with the focus on those aspects of asthma occurrence and of the clinical phenotype. However, given the scope of the outcomes of interest, the document should provide the search terms used to ensure that all relevant literature was identified. A broad set of studies are potentially relevant to this set of outcome measures, and consideration is given to the various studies relevant to each phenotype component.
The committee could not identify major studies that were not included but notes that most of the studies considered were completed several decades ago, when there was substantial interest in formaldehyde as an indoor air pollutant. When the studies were conducted, however, the asthma phenotype was not nearly as well characterized as it is now, so although the selected studies were considered by EPA to address asthma, the phenotypic characterizations in children are subject to misclassification when viewed in the context of current understanding. The committee was particularly concerned about the phenotype considered in the study by Rumchev et al. (2002).
Study Evaluation
The relevant studies were observational and, to a lesser extent, experimental. The principal concerns regarding the experimental studies that involved exposure of asthmatic volunteers to formaldehyde are participant characteristics, particularly the severity of their asthma, which affects the magnitude of response and generalizability of findings. In connection with the observational studies, a broader set of methodologic concerns that should have been systematically reviewed can be identified, such as the basis of the characterization of phenotype and the exposure estimation strategy.
The review of the studies is largely descriptive and without a specified format for study evaluation. In the case of selected studies, particularly those considered to be more informative, some attention is given to methodologic concerns but in a nonsystematic fashion. The key limitation of cross-sectional data is mentioned but the implications are not sufficiently explored. The key issue of asthma phenotype was not addressed in the draft assessment. Consequently, the committee found the review of studies to be inadequate.
Hazard Identification and Use of EPA Guidelines
The hazard identification discussion (EPA 2010a, pp. 4-462 to 4-467) is largely descriptive and repeats the previous descriptions of the studies, capturing some of the main features and findings. There is also a lack of clarity concerning the health end point considered—that is, incidence, prevalence, or exacerbation of established asthma. The evidence from epidemiologic studies is summarized as follows: “in conclusion, the epidemiologic studies of formaldehyde exposure
among children support the finding that low indoor and outdoor concentrations result in increased asthma incidence and prevalence” (EPA 2010a, p. 4-466). The conclusion is reached that the mode of action has “not been elucidated,” although several studies are indicated as lending “weight of evidence to a neurogenic” mode of action. Much of the discussion of mechanisms is speculative and unreferenced. For example, the draft assessment states that “formaldehyde-induced inflammation of the airways may contribute to observed decreases in measures of pulmonary function. Even short-term inflammatory reactions could reduce the effective diameter of the conductive airways, resulting in lower respiratory volumes in a number of functional tests. Formaldehyde-induced trigeminal nerve stimulation contributes to airway inflammation, which in turn would reduce airway function” (EPA 2010a, p. 4-462). Given the extensive literature on the pathogenesis of asthma, the discussion is inadequate, and any consideration of mode of action should have been better framed in current understanding of the underlying mechanisms of asthma causation and exacerbation. The proposal concerning trigeminal nerve stimulation is not referenced, even though it receives further treatment in the second paragraph of the two that address mode of action. The discussion does not reflect the state of knowledge of asthma pathogenesis. Abundant research and review articles are available and should have been cited (see, for example, Cohn et al. 2004 and Barnes 2008).
Hazard identification is not explicitly based on a guidance document of the agency; the most relevant may be EPA’s RfC guidelines (EPA 1994). The ad hoc approach taken in the draft IRIS assessment may reflect inadequate guidance on asthma. Given the limited discussion of the evidence and the lack of clear criteria for evidence evaluation, the committee did not find sufficient support for the hazard identification.
Study Selection for Calculation of Reference Concentration and Identification of Point of Departure
Few studies were available to evaluate asthma critically; in fact, only the case-control study by Rumchev et al. (2002) addressed the diagnosis of childhood asthma, and the cross-sectional study by Garrett et al. (1999) was one of the few to include assessment of allergen sensitivity. Both included some in-home measurements of formaldehyde concentration, a strong component of the rationale for advancing the studies.
Both studies were labeled as having data of “high quality,” although limitations of both were evident to the committee. The exposure protocols for both included measurements over a short period that may not reflect the biologically relevant period of exposure. The study by Rumchev et al. (2002), although interpreted as addressing the diagnosis and incidence of asthma, involved participants in an age range during which lower respiratory illnesses with wheezing that have an infectious etiology are frequently mislabeled as asthma (Martinez et al. 1995). Consequently, the relevance of this study specifically to childhood
asthma should be questioned because current understanding of wheezing illnesses in this age range indicates that they are transitory and not likely to represent the onset of asthma. A later report by Rumchev et al. (2004) describes higher concentrations of a number of volatile organic compounds in the homes of the cases compared with those of the controls. The potential for confounding by those other pollutants in assessing the effect of formaldehyde was not addressed.
Garrett et al. (1999) carried out a cross-sectional study that was inherently limited with respect to causal inference and establishing the temporality of associations. The cross-sectional findings of the study are used for three outcome measures: prevalence of atopy, prevalence of asthma, and a respiratory symptom score. A study strength is inclusion of four 4-day measurement periods for formaldehyde, but the analyses are cross-sectional. The symptom data, which represented symptoms that occurred during the last year, were collected at one time.
The draft text related to selection of the two studies (EPA 2010a, p. 5-39) does not compare their characteristics and strengths and weaknesses in relation to others that were not selected. The committee finds that the array of studies considered was too narrow and that an expedient choice was made with little additional explanation for the choice. The study by Rumchev et al. (2002) concerned an outcome other than incident asthma and should not have been advanced. The study by Garrett et al. (1999) provides relevant information in spite of its cross-sectional nature. EPA has advanced other cross-sectional studies (for example, Krzyzanowski et al. 1990); thus, that limitation alone does not constitute a sufficient reason not to advance a study for RfC calculation. Consequently, the committee concurs that Garrett et al. can be advanced in the absence of more informative prospective studies.
Two studies are advanced for derivation of candidate RfCs: one for diagnosis of incident asthma based on Rumchev et al. (2002) and the other for allergic sensitization—including critical effects of allergic sensitization, asthma, and respiratory symptoms—based on Garrett et al. (1999). For diagnosis of asthma, two points of departure—a NOAEL of 33 ppb based on Rumchev et al. (2002) and a LOAEL of 28 ppb based on Garrett et al. (1999)—are provided. The committee notes that the Rumchev et al. (2002) study was omitted from Table 5-4 in the draft IRIS assessment that summarizes all studies advanced for candidate RfCs. As noted above, however the study by Rumchev et al. (2002) is inappropriately advanced.
For the Rumchev et al. (2002) study, the committee notes that a key decision made in the calculation is not well documented. Figure 4-1 (Figure 5-5 in EPA 2010a) provides the odds ratios according to measured concentrations of formaldehyde in the residences. Only the odds ratio for the highest category is statistically significant. The NOAEL is taken to be 30-49 μg/m3 with the stated rationale that “the next highest exposure category was considered to be part of an exposure-related trend of increasing asthma risk and, therefore, biologically significant” (EPA 2010a, p. 5-46). Although confidence intervals around the two intermediate concentration points overlap, a linear model with a continuous ex-
posure measure was statistically significant. Regardless, criteria for identifying “an exposure-related trend” are not given.
The candidate RfC values derived from the study by Garrett et al. (1999) are based on EPA's interpretation of the trends observed in the categorical analyses for the three outcomes (allergic sensitization, asthma, and respiratory symptoms). The paper provides outcome data by three levels of highest exposure measured (less than 20, 20-50, and greater than 50 ppb). There appears to be an error in the description of the categories: it refers to a middle exposure range of 16-40 ppb and a high category of greater than 40 ppb. Regardless, the approach used in the draft IRIS assessment for identifying a LOAEL is to use the mid-point of the middle exposure category. That approach is not specifically justified and appears to represent a pragmatic attempt to handle data that are provided in the form of an exposure-response relationship without a nonexposed group and no clear NOAEL.
Conclusions and Recommendations
Asthma is a complex phenotype on whose pathogenesis substantial research has been conducted. The discussion of asthma needs to be strengthened to reflect the extensive literature better. The discussion of mode of action needs to be greatly strengthened and grounded in current understanding of pathogenesis. The current speculative discussion is not satisfactory. In light of the current understanding of wheezing illnesses in early life, the study by Rumchev et al. (2002) cannot be advanced as reflecting “asthma.” The committee agrees that the study by Garrett et al. (1999) can be advanced for calculation of a candidate RfC, but the approach taken for identification of a LOAEL needs better justification.
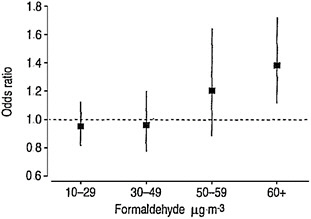
FIGURE 4-1 Odds ratios for physician-diagnosed asthma in children associated with in-home formaldehyde concentrations in air. This is Figure 5-5 in EPA 2010a. Source: Rumchev et al. 2002. Reprinted with permission; copyright 2002, European Respiratory Society.
The committee recommends that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Strengthen the discussion of asthma to reflect current understanding of this complex phenotype and its pathogenesis better. There should be greater clarity regarding the outcomes considered: incident asthma (the occurrence of new cases), prevalent asthma (the presence of asthma at the time of study), or exacerbation of established asthma.
-
Strengthen the discussion of mode of action and cite the extensive relevant literature.
-
Do not advance the study by Rumchev et al. (2002) as pertaining to asthma. That study appears relevant not to the asthma phenotype but rather to early-life wheezing illness.
-
Develop better the approach taken to identifying the LOAEL in the Garret et al. (1999) study.
RESPIRATORY TRACT CANCERS
The respiratory tract has been considered to include plausible locations for formaldehyde-induced cancer in humans because it is a site of first contact and because of the observed increased incidence of nasal tumors in laboratory animals exposed to formaldehyde. It is particularly true for cancers of the nose and nasal cavity (International Classification of Diseases, Revision 8 [ICD8] 160) and nasopharynx (ICD8 147) because the dose of formaldehyde is expected to be the greatest at these upper respiratory sites. In contrast, lung cancer (ICD8 162) is a less plausible site because the delivered dose for formaldehyde is expected to be much less in the lower respiratory tract than in the upper respiratory tract. There is extensive evidence from human and experimental studies that the mode of action of the induction of upper respiratory tract tumors by formaldehyde involves a genotoxic mechanism. It is also likely that the mode of action involves induction of cell proliferation by formaldehyde toxicity. Chapter 3 presents a more extensive discussion of the mode of action of formaldehyde induction of respiratory cancers.
Study Identification
The draft IRIS assessment appears to have identified all the pertinent studies of formaldehyde and respiratory cancers available at the time of its release. Thus, the draft assessment presents and discusses findings on lung cancer, nasopharyngeal cancer, nasal cancer, and other respiratory cancers from a large number of occupational cohort studies and several population-based case-control studies of adults. The committee is aware that an update of the National Cancer Institute (NCI) cohort for solid tumors is in progress, and the formaldehyde IRIS
assessment will need to include the update when it becomes available. However, the committee is not recommending that EPA wait until release of the update to complete its assessment.
Study Evaluation
The draft assessment presents an extensive evaluation of the pertinent studies published before EPA’s review. Particular attention was appropriately given to discussion of the findings of an excess of nasopharyngeal cancer (NPC) in the NCI study of workers employed in formaldehyde industries (Hauptmann et al. 2004). The draft assessment also considers alternative analyses and criticisms of the study by Marsh and colleagues (Marsh et al. 1996, 2002, 2007a,b; Marsh and Youk 2005). A primary finding of the NCI study is that the excess of NPC in the cohort was attributable almost entirely to one of the 10 study facilities (Marsh et al. 2007a). That facility was one of the largest; this would translate into greater statistical power to detect an increase in NPC mortality than some of the smaller facilities, which had fewer cases of this very rare cancer and thus much lower statistical power. The draft assessment suggests that there was also an excess of NPC in a second plant, but this finding was too unstable to be useful given that it was based on only one case and had a very wide confidence interval (standardized mortality ratio, 5.35; 95% confidence interval, 0.13-29.83). The pooling of results across plants translates into even greater power to detect a formaldehyde-associated excess of NPC. Plant differences other than statistical power may explain differences in observed cancer rates and are worth noting as limitations in interpreting risk estimates based on this study. For example, a reanalysis of the NCI cohort by Marsh et al. (2007b) provides evidence that the excess of NPC might be explained by other employment in silversmithing or other metal-working industries in Connecticut. However, there is no evidence from other studies that those industries are associated with an increased risk of NPC.
The study evaluation would be improved substantially if the EPA framework for causal determinations were stated explicitly because it would help to provide structure for the study evaluations and clarify which findings were most relevant to future causal determinations. The committee finds that the review regarding all the other respiratory cancer sites is thorough and appropriate.
Hazard Identification and Use of EPA Guidelines
The draft IRIS assessment draws inconsistent conclusions about hazard identification in four sections. In the summary of the section “Respiratory Tract Cancer,” the draft concludes that there is sufficient epidemiologic evidence that formaldehyde is causally associated with NPC and sinonasal cancer (EPA 2010a, section 4.1.2.1.5.4.). In the section “Summary: Carcinogenic Hazards in Humans,” the draft concludes that “the weight of the epidemiologic evidence at
this time supports a link between formaldehyde exposure and NPC in humans” but does not mention sinonasal cancer (EPA 2010a, section 4.1.2.3., p. 4-188). In the section “Synthesis and Evaluation of Carcinogenicity: Cancers of the Respiratory Tract,” the text offers the conclusion that “formaldehyde is causally related to cancers of the upper respiratory tract as a group” (EPA 2010a, section 4.5.1., p. 4-486). In the section “Hazard Characterization for Formaldehyde Carcinogenicity,” the draft states that “human epidemiological evidence is sufficient to conclude a causal association between formaldehyde exposure and nasopharyngeal cancer, nasal and paranasal cancer” (EPA 2010a, section 4.5.4., p. 4-535). The inconsistencies in the conclusions obviously need to be resolved.
Because the draft IRIS assessment presents no causal framework explicitly, the committee considered the appropriateness of EPA’s conclusions in the context of EPA’s Guidelines for Carcinogen Risk Assessment (EPA 2005). The guidelines state that for a substance to be a known human carcinogen, there should be “convincing epidemiologic evidence of a causal association between human exposure and cancer” or, exceptionally, if all the following conditions are met: “(a) there is strong evidence of an association between human exposure and either cancer or the key precursor events of the agent’s mode of action but not enough for a causal association, and (b) there is extensive evidence of carcinogenicity in animals, and (c) the mode(s) of carcinogenic action and associated key precursor events have been identified in animals, and (d) there is strong evidence that the key precursor events that precede the cancer response in animals are anticipated to occur in humans and progress to tumors, based on available biological information” (EPA 2005, p. 2-54).
EPA’s conclusion that NPC is causally related to formaldehyde was based on the positive findings of the NCI cohort study (Hauptmann et al. 2004) and on several case-control studies (Olsen et al. 1984; Rousch et al. 1987; West et al. 1993; Vaughan et al. 2000). Although an excess of NPC was not observed in other cohort studies of formaldehyde-exposed workers (Coggon et al. 2003; Pinkerton et al. 2004), the negative findings might be explained by the low statistical power of the studies for these rare tumors. There was a consensus in the committee that it would be consistent with EPA guidelines to draw a causal conclusion for NPC and formaldehyde on the basis of the combination of the epidemiologic findings with experimental data and mechanistic data on formaldehyde.
For nasal and paranasal (that is, sinonasal) cancers, EPA found a causal relationship with formaldehyde exposure on the basis of three factors: positive findings of several case-control studies (Hayes et al. 1986; Olsen and Asnaes 1986; Luce et al. 2002), stronger associations between formaldehyde and cancer in a neighboring tissue (NPC), and an excess of nasal cancer in rats exposed to formaldehyde (EPA 2010a). The committee concluded that EPA’s causal determination regarding sinonasal cancer is consistent with its cancer guidelines.
In Section 4.5.1 of the draft IRIS assessment (EPA 2010a), EPA extends its determination of causality to include all upper respiratory cancers and formaldehyde. EPA does not define what it means by “all upper respiratory can-
cers,” but it might be taken to include cancers of the oral cavity and larynx, as well as nasopharyngeal and sinonasal cancers. That determination was made even though little evidence about any upper respiratory cancer site other than NPC or sinonasal cancer was offered. The committee does not find that determination to be consistent with EPA’s cancer guidelines.
The draft IRIS assessment does not conclude that there is a causal association between exposure to formaldehyde and cancers of the lung. Only one of the major cohort studies of formaldehyde-exposed workers has reported a significant excess of lung cancer (Coggon et al. 2003). In addition, studies of deposition of formaldehyde in the respiratory tract have demonstrated clearly that the amount of formaldehyde deposited in the lower respiratory tract would be low. The committee concurs with EPA that there is a lack of sufficient evidence of an increased risk of lung cancer in humans exposed to formaldehyde.
Study Selection for Calculation of Cancer Unit Risk
EPA selected the study by Hauptmann et al. (2004) as the basis of its exposure-response assessment of formaldehyde and NPC. That was the only possible choice because the Hauptmann et al. (2004) study was the only study from which an exposure-response relationship could be derived. Furthermore, that study has a number of strengths for conducting an exposure-response assessment. In addition to having individual estimates of formaldehyde exposure, the study had a long period of follow-up, controlled for a number of potential confounding variables, and used internal comparisons that minimized biases related to the healthy-worker effect.
The strengths, however, are offset by a number of weaknesses. First, as noted earlier, the excess of NPC in the study was due to an excess in only one of the 10 study facilities. Although that pattern may be explained by the low statistical power of the individual study sites for this rare cancer, it raises concerns about the generalizability of the findings to the other facilities and to other workers exposed to formaldehyde. It also raises the possibility that the results were confounded by other pollutants present at that one facility. Second, the exposure-response findings for NPC were far less significant for cumulative formaldehyde exposure (p = 0.032) than for peak exposures (p < 0.001). Despite that finding, EPA chose to construct a dose-response relationship by using findings on cumulative exposure, given that peak exposures could not be used to estimate risks associated with lifetime exposures to environmental concentrations of formaldehyde. Finally, a serious concern has been raised about an unexplained under ascertainment of deaths in the Hauptmann et al. (2004) study (Marsh et al. 2010). In the update of findings on lymphatic and hematopoietic cancers in the NCI cohort, Beane-Freeman et al. (2009) noted that 1,006 deaths that had occurred before 1995 were missing from earlier analyses of the NCI cohort. The Beane-Freeman analysis also extended follow-up of the cohort to 2004. The additional follow-up period resulted in a total of 7,091 additional
deaths, which is nearly double the number that were included in Hauptmann et al. (2004). The effect of that under ascertainment of deaths and the additional follow-up period has important implications for analyses of the NCI cohort and NPC. Given the importance of the NCI study to the formaldehyde assessment, EPA should make an effort to update its assessment once the NCI study findings on NPC become available.
EPA also conducted an exposure-response analysis based on the combined findings of an increase in nasal squamous cell carcinoma in two long-term bioassays in F344 rats (Kerns et al. 1983; Monticello et al. 1996). The results of the exposure-response models were used to estimate risk of nasal cancer and of cancer of the entire respiratory tract (upper and lower) in humans. The committee concurs with EPA’s decision to use the data as the basis of the development of a model to estimate risk of nasal cancer but has serious doubts about the appropriateness of using the study to estimate risks of all respiratory cancers in humans. Those doubts and the lack of evidence from most of the epidemiologic studies of an excess of respiratory cancers other than sinonasal cancer and NPC support the committee’s conclusion that risks of all respiratory cancers (particularly lower respiratory tract cancers) should not be calculated now.
Conclusions and Recommendations
EPA’s review of the literature on formaldehyde and respiratory cancer was thorough and appropriate. It would be useful if, in the future, EPA could explicitly state its criteria for evaluation of the evidence of causality based on its own cancer guidelines. Several sections of the draft IRIS assessment contain conflicting statements on the evidence of causality that clearly need to be rectified. The committee finds that, on the basis of EPA’s guidelines, there is sufficient evidence of a causal association between formaldehyde and cancers of the nose and nasal cavity (ICD8 160) and nasopharynx (ICD8 147) but not other sites of respiratory tract cancer. The committee agrees that the study by Hauptmann et al. (2004) is an appropriate choice for the derivation of a point of departure and unit risk. Although it is a high-quality study, it is important to recognize some of its deficiencies, such as the apparent inconsistency between the findings in different plants in the study and the weakness of the exposure-response relationship in connection with cumulative exposure. Furthermore, the study was found to be missing deaths in a later update of the cohort for lymphatic and hematopoietic cancers. NCI is updating its cohort for respiratory cancer and other solid tumors. The update not only will include the missing deaths but will extend the follow-up, and this will result in nearly twice the amount of deaths.
The committee recommends that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Revise the document to state clearly the criteria that EPA used to determine the causality for cancer.
-
Resolve the conflicting statements in the document concerning which upper respiratory cancer sites were found to be causally associated with formaldehyde exposure.
-
Update the dose-response analysis in the IRIS assessment when the findings from the update of the NCI cohort on solid cancers become available. However, the committee is not recommending that EPA wait until release of the update to complete its assessment.
REFERENCES
Arts, J.H., M.A. Rennen, and C. de Heer. 2006. Inhaled formaldehyde: Evaluation of sensory irritation in relation to carcinogenicity. Regul. Toxicol. Pharmacol. 44(2):144-160.
Barnes, P.J. 2008. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8(3):183-192.
Bautista, D.M., S.E. Jordt, T. Nikai, P.R. Tsuruda, A.J. Read, J. Poblete, E.N. Yamoah, A.I. Basbaum, and D. Julius. 2006. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124(6):1269-1282.
Bessac, B.F., and S.E. Jordt. 2008. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology 23(6):360-370.
Bessac, B.F., and S.E. Jordt. 2010. Sensory detection and responses to toxic gases: Mechanisms, health effects, and countermeasures. Proc. M. Thorac. Soc. 7(4):269-277.
Beane-Freeman, L.E., A. Blair, J.H. Lubin, P.A. Stewart, R.B. Hayes, R.N. Hoover, and M. Hauptmann. 2009. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: The National Cancer Institute cohort. J. Natl. Cancer Inst. 101(10):751-761.
Bender, JR, L.S. Mullin, G.J. Graepel, and W.E. Wilson. 1983. Eye irritation response of humans to formaldehyde. Am. Ind. Hyg. Assoc. J. 44:463-465.
Bracken, M.J., D.J. Leasa, and W.K. Morgan. 1985. Exposure to formaldehyde: Relationship to respiratory symptoms and function. Can. J. Public Health 76(5):312-316.
Caceres, A.I., M. Brackmann, M.D. Elia, B.F. Bessac, D. del Camino, M. D’Amours, J.S. Witek, C.M. Fanger, J.A. Chong, N.J. Hayward, R.J. Homer, L. Cohn, X. Huang, M.M. Moran, and S.E. Jordt. 2009. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Nat. Acad. Sci. 106(22):9099-9104.
Cassee, F.R., J.H. Arts, J.P. Groten, and V.J. Feron. 1996. Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats. Arch. Toxicol. 70(6):329-337.
Coggon, D., E.C. Harris, J. Poole, and K.T. Palmer. 2003. Extended follow-up of a cohort of British chemical workers exposed to formaldehyde. J. Natl. Cancer Inst. 95(21):1608-1615.
Cohn, L., J.A. Elias, and G.L. Chupp. 2004. Asthma: Mechanisms of disease persistence and progression. Annu. Rev. Immunol. 22:789-815.
Doty, R.L., J.E. Cometto-Muniz, A.A. Jalowayski, P. Dalton, M. Kendal-Reed, and M. Hodgson. 2004. Assessment of upper respiratory tract and ocular irritative effects of volatile chemicals in humans. Crit. Rev. Toxicol. 34(2):85-142.
EPA (U.S. Environmental Protection Agency). 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. EPA/600/8-
90/066F. Office of Health and Environment Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC. October 1994 [online]. Available: http://www.epa.gov/raf/publications/pdfs/RFCMETHODOLOGY.PDF [accessed Nov. 28, 2010].
EPA (U.S. Environmental Protection Agency). 2005. Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. March 2005 [online]. Available: http://www.epa.gov/raf/publications/pdfs/CANCER_GUIDELINES_FINAL_3-25-05.PDF [accessed Nov. 24, 2010].
EPA (U.S. Environmental Protection Agency). 2010a. Toxicological Review of Formaldehyde (CAS No. 50-00-0) – Inhalation Assessment: In Support of Summary Information on the Integrated Risk Information System (IRIS). External Review Draft. EPA/635/R-10/002A. U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=223614 [accessed Nov. 22, 2010].
EPA (U.S. Environmental Protection Agency). 2010b. Asthma Prevalence. U.S. Environmental Protection Agency [online]. Available:
http://cfpub.epa.gov/eroe/index.cfm?fuseaction=detail.viewInd&lv=list.listByAlpha&r=219646&subtop=381 [accessed Nov. 23, 2010].
Garrett, M.H., M.A. Hooper, B.M. Hooper, P.R. Rayment, and M.J. Abramson. 1999. Increased risk of allergy in children due to formaldehyde exposure in homes. Allergy 54(4):330-337 [Erratum-Allergy 54(12):1327].
Hanrahan, L.P., K.A. Dally, H.A. Anderson, M.S. Kanarek, and J. Rankin. 1984. Formaldehyde vapor in mobile homes: A cross sectional survey of concentrations and irritant effects. Am. J. Public Health 74(9):1026-1027.
Hauptmann, M., J.H. Lubin, P.A. Stewart, R.B. Hayes, and A. Blair. 2004. Mortality from solid cancers among workers in formaldehyde industries. Am. J. Epidemiol. 159(12):1117-1130.
Hayes, R.B., J.W. Raatgever, A. de Bruyn, and M. Gerin. 1986. Cancer of the nasal cavity and paranasal sinuses, and formaldehyde exposure. Int. J. Cancer 37(4):487-492.
Holmstom, M., and B. Wilhelmsson. 1988. Respiratory symptoms and pathophysiological effects of occupational exposure to formaldehyde and wood dust. Scand. J. Work Environ. Health 14(5):306-311.
Holmstrom, M., B. Wilhelmsson, H. Hellquist, and G. Rosen. 1989. Histological changes in the nasal mucosa in persons occupationally exposed to formaldehyde alone and in combination with wood dust. Acta Otolayngal. 107(1-2):120-129.
Kane, L.E., and Y. Alarie. 1977. Sensory irritation to formaldehyde and acrolein during single and repeated exposures in mice. Am. Ind. Hyg. Assoc. J. 38(10):509-522.
Kerns, W.D., K.L. Pavkov, D.J. Donofrio, E.J. Gralla, and J.A. Swenberg. 1983. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 43(9):4382-4392.
Kriebel, D., S.R. Sama, and B. Cocanour. 1993. Reversible pulmonary responses to formaldehyde. A study of clinical anatomy students. Am. Rev. Respir. Dis. 148(6 Pt. 1):1509-1515.
Kriebel, D., D. Myers, M. Cheng, S. Woskie, and B. Cocanour. 2001. Short-term effects of formaldehyde on peak expiratory flow and irritant symptoms. Arch. Environ. Health 56(1):11-18.
Krzyzanowski, M., J.J. Quackenboss, and M.D. Lebowitz. 1990. Chronic respiratory effects of indoor formaldehyde exposure. Environ. Res. 52(2):117-125.
Lanosa, M.J., D.N. Willis, S. Jordt, and J.B. Morris. 2010. Role of metabolic activation and the TRPA1 receptor in the sensory irritation response to styrene and naphthalene. Toxicol. Sci. 115(2):589-595.
Liu, K.S., F.Y. Huang, S.B. Hayward, J. Wesolowski, and K. Sexton. 1991. Irritant effects of formaldehyde exposure in mobile homes. Environ. Health Perspect. 94:91-94.
Luce, D., A. Leclerc, D. Begin, P.A. Demers, M. Gerin, E. Orlowski, M. Kogevinas, S. Belli, I. Bugel, U. Bolm-Audorff, L.A. Brinton, P. Comba, L. Hardell, R.B. Hayes, C. Magnani, E. Merler, S. Preston-Martin, T.L. Vaughan, W. Zheng, and P. Boffetta. 2002. Sinonasal cancer and occupational exposures: A pooled analysis of 12 case-control studies. Cancer Causes Control 13(2):147-157.
Macpherson L.J., B. Xiao, K.Y. Kwan, M.J. Petrus, A.E. Dubin, S. Hwang, B. Cravatt, D.P. Corey, and A. Patapoutian. 2007. An ion channel essential for sensing chemical damage. J. Neurosci. 27(42):11412–11415.
Marsh, G.M., and A.O. Youk. 2005. Reevaluation of mortality risks for nasopharyngeal cancer in the formaldehyde cohort study of the National Cancer Institute. Regul. Toxicol. Pharmacol. 42(3):275-283.
Marsh, G.M., R.A. Stone, N.A. Esmen, V.L. Henderson, and K.Y. Lee. 1996. Mortality among chemical workers in a factory where formaldehyde was used. Occup. Environ. Med. 53(9):613-627.
Marsh, G.M., A.O. Youk, J.M. Buchanich, L.D. Cassidy, L.J. Lucas, N.A. Esmen, and I.M. Gathuru. 2002. Pharyngeal cancer mortality among chemical plant workers exposed to formaldehyde. Toxicol. Ind. Health 18(6):257-268.
Marsh, G.M., A.O. Youk, J.M. Buchanich, S. Erdal, and N.A. Esmen. 2007a. Work in the metal industry and nasophyngeal cancer mortality among formaldehyde-exposed workers. Regul. Toxicol. Pharmacol. 48(3):308-319.
Marsh, G.M., A.O. Youk, and P. Morfeld. 2007b. Mis-specified and non-robust mortality risk models for nasophyngeal cancer in the National Cancer Institute formaldehyde worker cohort study. Regul. Toxicol. Pharmacol. 47(1):59-67.
Marsh, G.M., A.O. Youk, P. Morfeld, J.J. Collins, and J.M. Symons. 2010. Incomplete follow-up in the National Cancer Institute’s formaldehyde worker study and the impact on subsequent reanalyses and causal evaluations. Regul Toxicol Pharmacol 58(2):233-236.
Martinez, F.D., A.L. Wright, L.M. Taussig, C.J. Holberg, M. Halonen, and W.J. Morgan. 1995. Asthma and wheezing in the first six years of life. The Group Health medical Associates. N. Engl. J. Med. 332(3):133-138.
McNamara, C.R., J. Mandel-Brehm, D.M. Bautista, J. Siemens, K.L. Deranian, M. Zhao, N.J. Hayward, J.A. Chong, D. Julius, M.M. Moran, and C.M. Fanger. 2007. TRPA1 mediates formalin-induced pain. Proc. Nat. Acad. Sci. 104(33):13525-13530.
Miller, M.R., R. Crapo, J. Hankinson, V. Brusasco, G. Burgos, R. Casaburi, A. Coates, P. Enright, C.P. van der Grinten, P. Gustaffson, R. Jensen, D.C. Johnson, N. MacIntyre, R. McKay, D. Navajas, O.F. Pedersen, R. Pellegrino, G. Viegi, and J. Wagner. 2005. General considerations for lung function testing. Eur. Respir. J. 26(1):153-161.
Monticello, T.M., J.A. Swenberg, E.A. Gross, J.R. Leininger, J.S. Kimbell, S. Seilkop, T.B. Starr, J.E. Gibson, and K.T. Morgan. 1996. Correlation of regional and nonlinear formaldehyde-induced nasal cancer with proliferating populations of cells. Cancer Res. 56(5):1012-1022.
NHLBI (National Heart Lung and Blood Institute). 2007. Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. U.S. Department of Health and Human Services, National Institute of Health, National Heart Lung and Blood Institute [online]. Available: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm [accessed Nov. 23, 2010].
Olsen, J.H., and S. Asnaes. 1986. Formaldehyde and the risk of squamous cell carcinoma of the sinonasal cavities. Br. J. Ind. Med 43(11):769-774.
Olsen, J.H., S.P. Jensen, M. Hink, K. Faurbo, N.O. Breum, and O.M. Jensen. 1984. Occupational formaldehyde exposure and increased nasal cancer risk in man. Int. J. Cancer 34(5):639-644.
Paustenbach, D., Y. Alarie, T. Kulle, N. Schachter, R. Smith, J. Swenberg, H. Witschi, and S.B. Horowitz. 1997. A recommended occupational exposure limit for formaldehyde based on irritation. J. Toxicol. Environ. Health A 50(3):217-263.
Pinkerton, L.E., M.J. Hein, and L.T. Stayner. 2004. Mortality among a cohort of garment workers exposed to formaldehyde: An update. Occup. Environ. Med. 61(3):193-200.
Ritchie, I.M., and R.G. Lehnen. 1987. Formaldehyde-related health complaints of residents living in mobile and conventional homes. Am. J. Public Health 77(3):323-328.
Rousch, G.C., J. Walrath, L.T. Stayner, S.A. Kaplan, J.T. Flannery, and A. Blair. 1987. Nasopharyngeal cancer, sinonasal cancer, and occupations related to formaldehyde: A case-control study. J. Natl. Cancer Inst. 79(6):1221-1224.
Rumchev, K.B., J.T. Spickett, M.K. Bulsara, M.R. Phillips, and S.M. Stick. 2002. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur. Respir. J. 20(2):403-408.
Rumchev, K, J. Spickett, M. Bulsara, M. Phillips, and S. Stick. 2004. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax 59(9):746-751.
Schoenberg, J.B., and C.A. Mitchell. 1975. Airway disease caused by phenolic (phenol-formaldehyde) exposure. Arch. Environ. Health 30(12):574-577.
Taylor-Clark, T.E., and B.J. Undem. 2010. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J. Physiol. 588(3):423-433.
Taylor-Clark, T.E., M.A. McAlexander, C. Nassenstein, S.A. Sheardown, S. Wilson, J. Thornton, M.J. Carr, and B.J. Undem. 2008. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibers by the endogenous autacoid 4-oxononenal. J. Physiol. 586(14):3447-3459.
Vaughan, T.L., P.A. Stewart, K. Teschke, C.F. Lynch, G.M. Swanson, J.L. Lyon, and M. Berwick. 2000. Occupational exposure to formaldehyde and wood dust and nasopharyngeal carcinoma. Occup. Environ. Med. 57(6):376-384.
Weber-Tschopp, A, T. Fischer, and E. Grandjean, E. 1977. Irritating effects of formaldehyde on man. Int. Arch. Occup. Environ. Health. 39(4):207-218.
West, S., A. Hildesheim, and M. Dosemeci. 1993. Non-viral risk factors for nasopharyngeal carcinoma in the Philippines: Results from a case-control study. Int. J. Cancer 55(5):722-727.
Wolkoff, P., and G.D. Nielsen. 2010. Non-cancer effects of formaldehyde and relevance for setting an indoor air guideline. Environ. Int. 36(7):788-799.




























