5
Systemic Health Effects
As noted in Chapter 4, the health effects of exposure to formaldehyde evaluated by the Environmental Protection Agency (EPA) can be characterized as portal-of-entry effects or systemic effects. In this chapter, the committee reviews EPA’s evaluation of systemic health effects, including immunotoxicity, neurotoxicity, reproductive and developmental toxicity, and lymphohematopoietic cancers. The committee determined whether EPA identified the appropriate studies, whether the studies were thoroughly evaluated, whether hazard identification was conducted appropriately in light of EPA guidelines, and whether the best studies were advanced for calculation of the reference concentration (RfC) or unit risk.
Chapter 3 of the present report addresses the question of systemic bioavailability of inhaled formaldehyde. High reactivity and extensive nasal absorption of formaldehyde restrict systemic delivery of inhaled formaldehyde beyond the upper respiratory tract and major conducting airways of the lung. Indeed, the weight of evidence suggests that it is unlikely for formaldehyde to appear in the blood as an intact molecule, except perhaps when exposure doses are high enough to overwhelm the metabolic capability of the tissue at the site of exposure. Thus, systemic responses are unlikely to arise from the direct delivery of formaldehyde (or its hydrated form methanediol) to a distant site in the body. However, it is important to distinguish between systemic delivery of formaldehyde and systemic effects. The possibility remains that systemic delivery of formaldehyde is not a prerequisite for some of the reported systemic effects seen after formaldehyde exposure. Those effects may result from indirect modes of action associated with local effects, such as irritation, inflammation, and stress. Therefore, the committee reviewed EPA’s evaluation of the systemic effects and determined whether the evidence presented supported EPA’s conclusions.
IMMUNOTOXICITY
Immunomodulation or immunotoxicity occurs when environmental factors (such as stress, health status, and chemical exposure) change the homeostatic processes that regulate the immune system in susceptible populations. The consequences of immunotoxicity can be highly divergent and depend on the environmental factor, the duration and timing of the exposure, the overall health status of the exposed person, and the route of exposure. Immunotoxicity may occur from direct effects on immune cells or from indirect effects on various cell components, such as altered endocrine function. It may also occur at an anatomic site that is distant from the point of entry. Indeed, the systemic nature of the immune system may mean that an exposure at one site causes damaged or modified cells to move to another location in the body where they may mediate the effects of the toxicant.
Adverse health effects associated with immunotoxicity may include higher infection rate; alterations in lymphocyte cell populations; hyperactivity of immune cells, such as increased respiratory activity (increased production of reactive oxygen and reactive nitrogen species) and cytokine production; autoimmunity; altered immune-cell trafficking throughout the body; increased allergy or atopy; and susceptibility to cancer. Research to determine the immunotoxicity of an agent focuses on those and other responses. EPA has developed a health-effects test guideline for immunotoxicity (EPA 1998a).
In the case of formaldehyde, as has been discussed elsewhere in the present report, most of or all the direct effects occur at the point of entry in the upper respiratory tract. Immune cells in the bronchial and nasal associated lymphoid tissue (BALT and NALT) are most likely proximal targets of formaldehyde. Understanding the potential immunotoxicity of formaldehyde is therefore of critical importance.
Adverse effects of formaldehyde on BALT and NALT may be manifested systemically because these lymphoid cells migrate to the lymph nodes, spleen, liver, peripheral blood, and other immune tissues. Specifically, most BALT and NALT cells belong to the arm of the immune system referred to as the innate immune system. The role of the innate immune cells is to recognize and respond to tissue damage, apoptotic cells, and evolutionarily conserved protein and glycoprotein patterns expressed on bacteria, viruses, parasites, and other pathogens. The consequences of innate immune-cell recognition of pathogen-associated molecular patterns (PAMPs) are to increase production of reactive oxygen species, to engulf the particles expressing PAMPs, and to migrate systemically where the potential infectious agent is presented to the adaptive arm of the immune system. The role of the adaptive arm of the immune system is to produce cytokines, which activate antibody production, increase inflammation, and recruit lymphocytes to the site of infection.
Consequently, the systemic nature of the immune system and interplay between the innate and adaptive arms of the immune system suggest a plausible potential target of formaldehyde despite its limited distribution beyond the point of entry. Moreover, potential alterations of the innate immune cells mediated by formaldehyde may have profound effects on the adaptive and peripheral immune system. The draft IRIS assessment summarizes a number of human and animal studies that describe formaldehyde-induced immunotoxicity. Although many of the appropriate studies were identified, a more careful evaluation of the relative strengths and weaknesses of the key studies should have been provided. Moreover, additional weight could have been given to animal studies in which exposure assessment was more rigorously controlled and a diverse set of end points was examined. The committee recognizes that differences exist in leukemia sensitivities between animals and humans. However, the differences in responses may not be relevant for other immunotoxicities, such as respiratory burst activity, sensitivity, and atopy.
Study Identification
The draft IRIS assessment discusses immunologic end points affected by formaldehyde exposure on the basis of human and animal studies in the compiled database of published studies. The committee did not perform an additional literature search, but it appears that, in general, the appropriate studies were identified and adequately discussed. Specifically, the draft IRIS assessment presents studies designed to address the following questions:
-
Does formaldehyde exposure increase upper respiratory tract infections?
-
Does formaldehyde induce lymphocyte associated respiratory burst activity and inflammation?
-
Is formaldehyde exposure associated with allergic sensitivity or atopy?
-
What is the toxicologic significance of antibody responses directed against formaldehyde or formaldehyde-protein complexes?
The structure of the draft assessment is such that the research questions regarding human and animal end points are addressed separately. As will be discussed below, the committee finds that a more integrated approach in which the human and animal studies of a given immunologic end point are discussed and evaluated together would result in a more concise and transparent report.
Study Evaluation
In addressing the questions above, the draft IRIS assessment presents numerous studies that provide data that suggest that formaldehyde is immuno-
modulatory (EPA 2010). Specifically, in addressing the question of what effect formaldehyde has on susceptibility to upper respiratory tract infections, the draft cites Holness and Nethercott (1989), Krzyzanowski et al. (1990), and Lyapina et al. (2004). The Holness and Nethercott (1989) and Lyapina et al. (2004) studies were conducted in occupational settings, and the Kyzyzanowski et al. (1990) study was conducted in homes in which formaldehyde concentrations were measured. The concentrations in the occupational settings were 0.71-1.55 ppm, and the average concentration in the home study was 26 ppb. As reported in the draft assessment, all three studies showed an association between formaldehyde exposure and increased incidence of upper respiratory tract infections. No directly comparable animal studies that would have strengthened or weakened those findings are cited. However, formaldehyde exposure at higher exposure concentrations (2 ppm or higher) has been shown to reduce mucociliary apparatus function in the rodent (Morgan et al. 1986). Similar effects, such as slowed mucociliary clearance, have been seen in occupationally exposed people (Holmström and Wilhelmsson 1988). The mucociliary apparatus is an important barrier to infection and other exogenous agents, so the finding is supportive of the human studies. The three key studies used by EPA reflect the state of the science with respect to formaldehyde and virally induced upper respiratory tract infections. Given the small number of studies, this section of the draft IRIS assessment would have been greatly improved by a critical evaluation of those studies.
Regarding the question of whether formaldehyde affects lymphocyte respiratory burst activity or inflammation, several studies in humans and animals are listed. Specifically, Lyapina et al. (2004) used flow cytometry to measure changes in neutrophil respiratory burst activity in occupationally exposed workers who had chronic bronchitis. A weakness of the study is that the assay used to measure respiratory burst activity is not specific. Moreover, the details of the study preclude concluding whether formaldehyde exposure or a chronic bronchial condition in the selected subjects was the cause of the changed cellular activity. However, Dean et al. (1984) and Adams et al. (1987) performed animal studies using 3-week exposures to formaldehyde at 15 ppm and measured changes in peritoneal macrophage hydrogen peroxide production. As shown in Table 4-47 of the draft IRIS assessment, peroxide production was increased in response to macrophage activation. If the results of the studies were synthesized, EPA could strengthen its conclusion that formaldehyde exposure affects respiratory burst activity in the immune system. Moreover, the animal studies demonstrate effects on the innate immune system at a distant site (for example, the peritoneum); this lends credence to the biologic plausibility of systemic effects caused by formaldehyde exposure.
Studies covering sensitivity and atopy are similarly listed and described in the draft IRIS assessment with little evaluation of the strengths and weaknesses of the cited work. Moreover, in the section describing human studies, a substantial amount of text is devoted to assessing whether exposed people generate IgE antibodies against formaldehyde. IgE antibodies are generated against allergic agents and chemical haptens (chemicals complexed with endogenous proteins
that elicit an immune response). Although discussion of IgE antibodies against formaldehyde is not a trivial question, this section in the draft assessment could be condensed. Furthermore, an additional question to ask in the section “Sensitivity and Atopy” would be, Does exposure to formaldehyde modulate responses to known allergens, such as dust mites, ragweed, animal dander, and mold spores? If there has been research on that subject, it is not presented in the draft assessment with respect to human exposures.
In comparison, the results of many studies in animal models support a conclusion that formaldehyde exposure modifies allergic responses. Specifically, Tarkowski and Gorski (1995), Riedel et al. (1996), and Lino dos Santos Franco et al. (2009) found increased sensitivity in rodents that were coexposed to a model allergen (ovalbumin) and formaldehyde. Sadakane et al. (2002) and Ohtsuka et al. (2003) found changes in inflammatory cytokine production in the lungs after formaldehyde exposure. Several other studies summarized in Table 4-54 of the draft IRIS assessment showed more modest results or no effect of formaldehyde. The disparate observations may be due partly to the use of different rodent species and strains in the studies. Moreover, the exposure protocols varied widely. In some cases, animals were pre-exposed to formaldehyde and then sensitized; in other studies, sensitization occurred before formaldehyde exposure; and in others, sensitization and exposure occurred simultaneously. Although the committee agrees that each type of protocol appropriately replicates a real-world exposure scenario, the section deserves a robust rubric to evaluate the strengths and weaknesses of the studies presented. That is particularly important given that the section “Sensitization and Atopy” of the draft IRIS assessment concludes with a statement that “taken as a whole, the results support the finding that formaldehyde exposure can aggravate a type I hypersensitivity response” (EPA 2010, p. 4-335). On the basis of the review currently provided, the committee cannot agree with that conclusion because no clear framework for drawing it is presented.
Hazard Identification and Use of EPA Guidelines
Hazard identification for immunotoxicity was conducted and reported in a generally appropriate fashion, given EPA guidelines. However, the language used in the review of some studies could be improved. For example, the discussion of Riedel et al. (1996), which documented airway sensitivity in guinea pigs in response to formaldehyde at 0.13 or 0.25 ppm, uses the term biologically significant (EPA 2010, p. 4-319). The term is used in the absence of a statement of a statistically significant effect by the study authors. Thus, EPA should provide a justification for its conclusion that the effect was biologically significant and indicate whether additional statistical analyses were performed.
In addition, the immune-hazard identification section could have been clearer with a discussion summarizing immune effects of formaldehyde. Specifically, consistencies between human and animal findings regarding inflamma-
tion, target-cell types, and airway responses should be noted. Cells of the innate immune system appear to be targets or mediators of formaldehyde-induced immunotoxicity in animal and human studies, and a concluding statement containing that information would be useful.
Study Selection for Calculation of Reference Concentration and Identification of Point of Departure
The sections on immunotoxicity do not identify any studies for deriving a candidate RfC. Thus, no candidate RfC was calculated for the immunotoxic effects of formaldehyde.
Conclusions and Recommendations
The systemic nature of the immune system and the interplay between the innate and adaptive arms of the immune system provide a plausible potential target of formaldehyde, despite its limited distribution beyond the point of entry. The draft IRIS assessment summarizes many human and animal studies that describe formaldehyde-induced immunotoxicity. The committee agrees with EPA’s decision not to calculate a candidate RfC for immunotoxicity at this time.
The committee recommends, however, that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Provide a more careful evaluation of the relative strengths and weaknesses of the key studies.
-
Consider giving additional weight to animal studies in which exposure assessment was more rigorously controlled.
NEUROTOXICITY
Neurotoxicity is defined as any adverse effect on the chemistry, structure, or function of the nervous system during development or in maturity. Neurotoxicity may be permanent or reversible, and it can be expressed as neuropathologic effects or as neurochemical, electrophysiologic, or behavioral changes. In general, chemical-induced changes in the structure or persistent behavioral, neurochemical, or neurophysiologic changes in the nervous system are regarded as neurotoxic effects. Reversible effects occurring at doses that could endanger performance in the workplace or that are associated with a known neurotoxicologic mode of action are also considered adverse. Formaldehyde exposure via inhalation has been shown to affect nervous-system function adversely in laboratory animals and humans, although there are few data on formaldehyde-induced neurologic effects in humans.
Study Identification
EPA appears to have identified all available relevant literature on formaldehyde neurotoxicology; the committee could not identify any important studies that were not included. The draft IRIS assessment identifies seven neurotoxicity studies that are considered as candidates for RfC development, most notably the epidemiologic studies by Weisskopf et al. (2009) and Kilburn et al. (1985, 1987). Several experimental rat studies are also identified as candidate studies for RfC development. Experimental mouse studies are noted, but they are dismissed because of confounding issues. All studies addressed exposures of short duration, so information regarding the relationship between formaldehyde toxicity and exposure duration is sparse.
Study Evaluation
The evaluation of the epidemiologic studies in the draft IRIS assessment focused on Weisskopf et al. (2009) and Kilburn et al. (1985, 1987). Weisskopf et al. (2009) reported a statistically significant association (relative risk, 2.47; 95% CI, 1.58-3.86) between self-reported years of formaldehyde exposure and death from amyotrophic lateral sclerosis (ALS). The draft assessment concludes that the study supports the “causal association of neuropathological effects in humans following long-term formaldehyde exposure” (EPA 2010, p. 4-476). The committee, however, is not convinced that the study established a causal association. EPA’s conclusion of causality between formaldehyde inhalation and the development of ALS is premature, is supported by an isolated study with limited exposure data, and lacks sufficient evidence of biologic plausibility. Indeed, the study authors stated that “the increased risk attributed to formaldehyde could be the result of exposure to some other unmeasured factor commonly associated with formaldehyde” (Weisskopf et al. 2009). Kilburn et al. (1985) reported that a group of 76 female histology technicians displayed statistically significantly greater frequencies of lack of concentration and loss of memory, disturbed sleep, impaired balance, variations in mood, and irritability than did a control group of 56 unexposed female clerical workers. The technicians had been employed for 2-37 years (mean, 12.8 years). Analysis of workplace air samples indicated the presence of several solvents, including formaldehyde (0.2-1.9 ppm), xylene (3.2-102 ppm), chloroform (2-19.1 ppm), and toluene (8.9-12.6 ppm). Thus, exposure to xylene and other solvents most likely contributed to the observed neurobehavioral effects. Kilburn (1994) also reported that three anatomists and one railroad worker, occupationally exposed to airborne formaldehyde for 14-30 years, showed impaired performance on choice reaction time, abnormal balance, digit symbol, and perceptual motor speed.
EPA’s review of the candidate animal studies is largely descriptive and lacked a systematic or specified format for study evaluation. None of the candidate studies adhered to EPA’s neurotoxicity-testing guidelines (EPA 1998b).
Several studies used designs that deviated substantially from the testing guidelines and common practice. In particular, animal studies performed by Malek and co-workers (2003a) used extremely short-duration (3-min) motor-activity test sessions. EPA neurotoxicity-test guidelines explicitly state that the test session should be “of sufficient duration to allow motor activity to approach steady-state levels during the last 20 percent of the session for control animals” (EPA 1998b, p. 39). There is no indication in the original study that that criterion was reached; indeed most motor-activity test sessions require at least 20 min to reach asymptotic levels (Fitzgerald et al. 1988). That deficiency of the study was not raised by EPA in its review. EPA also largely ignored the absence of exposure-response relationships for some behavioral end points.
No mode of action has been postulated for formaldehyde-induced neurologic effects. EPA concluded that behavioral changes seen in formaldehyde-exposed animals are unlikely to be attributable to the irritant properties of formaldehyde. The committee does not support that conclusion. For example, Sorg and Hochstatter (1999) observed alterations in formaldehyde-exposed rats in an odor-cued test of learning. It is possible that formaldehyde exposure resulted in olfactory epithelial injury sufficient to affect olfaction. Other studies (for example, Sorg et al. 2001) suggest that stress responses, such as altered cortisol concentrations, occur in formaldehyde-exposed animals. It is plausible that those changes occur because of nasal irritation and other local responses. Stress and related alterations in stress hormones are important potential confounders because they are associated with deficits in hippocampal-based memory function, alterations in hippocampal structure, and other neurologic responses (Pavlides et al. 2002; Conrad 2006; McEwen 2008; Zuena et al. 2008). Another concern raised by the committee is that the high reactivity of formaldehyde would not lend itself to substantial delivery to the nervous system.
The draft IRIS assessment indicates that there is some question as to whether formaldehyde should be considered a direct neurotoxicant (EPA 2010). Indeed, some portions of the assessment suggest that systemic effects are unexpected at formaldehyde concentrations less than 20 ppm. That idea is inconsistently presented in other parts of the document. The inconsistency in the document should be resolved.
Hazard Identification and Use of EPA Guidelines
EPA has developed guidelines for neurotoxicity risk assessment (EPA 1998b). One cornerstone of the guidelines is the definition of neurotoxicity as an adverse change in the structure or function of the central or peripheral nervous system after exposure to an agent. Changes in motor activity, learning and memory, and other end points after formaldehyde exposure meet the definition of an adverse response. Although a mode of action for formaldehyde neurotoxicity is lacking, that gap does not preclude drawing a conclusion. The EPA guidelines state that “knowledge of exact mechanisms of action is not,
however, necessary to conclude that a chemically induced change is a neurotoxic effect” (EPA 1998b, p. 10).
The neurotoxicity guidelines state that “the interpretation of data as indicative of a potential neurotoxic effect involves the evaluation of the validity of the database…There are four principal questions that should be addressed: whether the effects result from exposure (content validity); whether the effects are adverse or toxicologically significant (construct validity); whether there are correlative measures among behavioral, physiological, neurochemical, and morphological endpoints (concurrent validity); and whether the effects are predictive of what will happen under various conditions (predictive validity)” (EPA 1998b, p. 10). The draft IRIS assessment does not indicate whether those criteria were considered in the selection of the key studies. Indeed, data supporting concurrent and predictive validity are largely lacking for formaldehyde.
The EPA guidelines also state that “the minimum evidence necessary to judge that a potential hazard exists would be data demonstrating an adverse neurotoxic effect in a single appropriate, well-executed study in a single experimental animal species” (EPA 1998b, p. 53). There is concern that the selected studies are not sufficiently robust in design to be considered “well executed” for the purpose of neurotoxicity hazard identification. For example, motor-activity responses seen by Malek et al. (2003 a,b) in different test sessions in control animals were quite variable. Malek et al. also examined formaldehyde effects on learning and memory using a labyrinth swim maze, a test that could be affected by motor activity. Furthermore, the available human data have important shortcomings—such as limited exposure assessments and coexposures to neurotoxic solvents—that preclude a determination that formaldehyde is neurotoxic to humans.
Study Selection for Calculation of Reference Concentration and Identification of Point of Departure
EPA concluded that the available epidemiologic studies did not provide sufficient exposure information to permit derivation of a point of departure for use in quantitative dose-response assessment. The draft IRIS assessment states that “confounding exposures to other neurotoxic solvents and inconsistent results prevent drawing definitive conclusions concerning the neurotoxicity of formaldehyde from these studies” (EPA 2010, p. 4-97). The committee agrees with EPA’s decision not to use the human studies to calculate a candidate RfC.
Several studies in mice demonstrated dose-related neurotoxic effects after formaldehyde exposure. The studies were not considered for RfC development because the observed results might have been confounded by formaldehyde-induced reflex bradypnea and related physiologic responses. The committee agrees with EPA’s decision not to use the experimental mouse studies for RfC development for the reasons cited.
EPA identified several experimental studies in rats that might be appropriate for candidate RfC development. According to EPA, the selected rat behavioral studies were not confounded by reflex bradypnea inasmuch as the effect occurs in rats only at doses above those at which the neurologic effects of concern were seen. EPA considered the studies by Malek et al. (2003a,c) that reported effects at low exposures to be the most robust. The draft IRIS assessment does not provide criteria that define why the studies were considered “robust” other than that the changes were observed at low concentrations. Malek et al. (2003c) found statistically significant reductions in motor activity after a single 2-hr exposure at 130-5,180 ppb (with testing 2 hr after cessation of exposure). Malek et al. (2003a) also showed a statistically significant reduction in performance on a learning task at similar exposures (100-5,400 ppb) when 2-hr exposures were repeated on 10 consecutive days (p < 0.05); performance was evaluated 2 hr after cessation of exposure, and concentration-related learning deficits were seen at all concentrations. The study was eventually selected as the key study by EPA. As noted earlier, no study was conducted according to existing EPA health-effects test guidelines for the conduct of a neurotoxicity screening battery or for evaluation of neurotoxicity end points (EPA 1998b). Accordingly, the studies have several methodologic shortcomings in how behavior was assessed by the investigators. In addition, neither study assessed subchronic or longer exposures; this draws into question their appropriateness for deriving a chronic RfC. The committee did not identify an alternative study that would be preferred for deriving a candidate RfC.
Malek et al. (2003a) reported a lowest observed-adverse-effect level (LOAEL) of 100 ppb in rats for neurologic and behavioral toxicity (impaired learning) after repeated exposure (2 hr/day over 10 days). A no-observed-adverse-effect level (NOAEL) was not identified for that effect. The committee notes that the point of departure for the study was subject to an exposure adjustment (Table 5-1 in the draft IRIS assessment). Testing was conducted 2 hr after exposure, and the duration was adjusted by EPA to 4 hr to include the entire period between start of exposure and testing. The committee disagrees with the duration adjustment because of the uncertainty in continuous-exposure adjustments for exposure durations as short as that used in the experimental study. The study was not carried forward for derivation of a candidate RfC, partly because of the uncertainty in extrapolating from the exposure conditions in the study to a chronic-exposure scenario; the committee agrees with EPA’s decision in this regard.
Conclusions and Recommendations
The committee concludes that the draft IRIS assessment overstates the evidence that formaldehyde is neurotoxic. The selected studies are not sufficiently robust in design to be considered well executed for the purpose of neurotoxicity-hazard identification. One study of rats by Malek et al. (2003a) was
advanced by EPA for consideration. It was considered to offer information on an outcome relevant to humans at an appropriate concentration. Appropriately, the study was not used to calculate a candidate RfC, partly because of uncertainty in extrapolating from the exposure conditions in the study to a chronic-exposure scenario.
The committee recommends that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Re-evaluate its conclusions that behavioral changes are unlikely to be related to irritant properties of formaldehyde.
-
Resolve inconsistencies regarding the concentration at which systemic effects of formaldehyde exposure are expected. The draft IRIS assessment indicates that there is some question as to whether formaldehyde should be considered a direct neurotoxicant, and some portions of the assessment suggest that systemic effects are unexpected at formaldehyde concentrations less than 20 ppm. That statement is inconsistently made in other parts of the document.
REPRODUCTIVE AND DEVELOPMENTAL TOXICITY
The reproductive and developmental outcomes considered in the draft IRIS assessment comprise a broad spectrum of specific outcomes, including infertility, low birthweight, spontaneous abortion, birth defects, functional deficits, and other altered health conditions. Each outcome may have a distinct pathogenesis and etiology. A variety of environmental, occupational, lifestyle, and genetic factors have been hypothesized to be associated with an increased risk of those outcomes.
Formaldehyde’s potential mode of action for reproductive and developmental outcomes is uncertain; several modes have been suggested by animal studies, including endocrine disruption, genotoxic effects on gametes, and oxidative stress or damage. Critical questions remain about the association between inhalation exposure and the potential for adverse reproductive and developmental effects. EPA reviewed epidemiologic and animal studies that evaluated formaldehyde exposure in relation to fecundability (the per-cycle probability of conception), spontaneous abortion, birth defects, low birthweight, and reproductive effects (EPA 2010). EPA concluded that the epidemiologic studies provided evidence of a convincing relationship between occupational exposure to formaldehyde and adverse reproductive outcomes in women. EPA selected a single study (Taskinen et al. 1999) that evaluated the association between formaldehyde and fecundability, using time to pregnancy for determination of a candidate RfC.
Study Identification
EPA appears to have conducted a thorough literature search and identified the available and appropriate studies. The epidemiologic literature is mixed in terms of study population, design, exposure conditions, and other factors. In addition to the epidemiologic studies, the draft IRIS assessment identifies 10 animal reproductive studies and 13 developmental studies (and one abstract) that reported reproductive and developmental effects after exposure to formaldehyde. Several animal studies were not described in their original publications in sufficient detail to assess their overall conclusions.
Study Evaluation
The draft IRIS assessment generally follows EPA guidelines for the evaluation of reproductive toxicity (EPA 1996) and developmental toxicity (EPA 1991) regarding the consideration of key factors in the evaluation of epidemiologic studies in risk assessment, including study power, exposure measurement, selection bias, and confounding. Ideally, those factors would be reviewed and presented in an organized and systematic fashion for each study considered, the important biases would be systematically examined, and their potential magnitude and direction would be clearly enumerated. However, the descriptions and evaluation of the individual epidemiologic studies in the draft IRIS assessment are not consistent among studies. The characterization of the strengths and weaknesses of the studies varies; some studies receive a fuller treatment, including a more extensive assessment of bias and its consequences for estimating effect measures, and others receive less attention. For example, the evaluation of the study by Taskinen et al. (1994) of laboratory workers has minimal discussion of study strengths and weaknesses, and the discussion of bias is misleading or incomplete. Specifically, the discussion dismisses potential confounding by xylene exposure because the odds ratio for xylene and spontaneous abortion of 3.1 is less than the formalin odds ratio of 3.5 (EPA 2010, p. 4-88). The committee notes that although the odds ratio is slightly less for xylene, the estimates are the same for all practical purposes, and potential confounding by xylene exposure in the study remains an open question. The draft IRIS assessment also notes that exposure misclassification will not have “impacted the results of the study to any great extent” (EPA 2010, p. 4-88) but does not indicate the possible magnitude or direction of any exposure misclassification bias.
The committee disagrees with EPA’s overall conclusion regarding the totality of the epidemiologic evidence related to the reproductive and developmental effects of formaldehyde. Specifically, the draft IRIS assessment states that “epidemiologic studies suggest a convincing relationship between occupational exposure to formaldehyde and adverse reproductive outcomes in women” (EPA 2010, p. 4-85). The committee, after assessing the literature, finds a suggestive
pattern of association among a small number of studies rather than a convincing relationship. The committee’s assessment is based on the overall pattern of positive association among most of the studies, but the generally limited exposure assessment and concern about other biases leads to the more appropriate descriptor of suggestive rather than convincing.
Many of the conclusions on developmental and reproductive effects in animal studies provided in Section 6.1.3.7 of the draft IRIS assessment are based on studies that are of questionable quality. The following statement seems to over interpret the results:
Nevertheless, a number of animal studies have demonstrated effects of formaldehyde on pre- and postnatal development and on the reproductive system. For example, developmental toxicity was observed in two studies that evaluated a standard battery of developmental endpoints resulting from inhalation exposure on GDs [gestation days] 6-10 [Saillenfait et al. 1989; Martin 1990] (EPA 2010, p. 4-371).
Saillenfait et al. (1989) reported a statistically significant decrease in maternal weight gain at the highest concentration tested (40 ppm); this suggests maternal toxicity, but there were no statistically significant changes in number of implantations, resorptions, stage of resorptions, or live or dead fetuses. There was a statistically significant decrease in mean fetal bodyweights at 20 ppm in male fetuses with no associated maternal toxicity. Martin (1990) found no statistically significant differences in a standard battery of developmental end points, such as live or dead fetuses and implantation sites. There was decreased ossification of pubic bones in the 10-ppm (highest-concentration) group, but the group had a higher number of fetuses per litter associated with an overall lower weight. No other malformations were reported. Other studies were not described in sufficient detail to determine their quality. One study (Kilburn and Moro 1985) was published in abstract form only and probably should not be included in the analysis. In many of the studies, maternal toxicity, litter size, within-litter effects, and other quality-control measures were not discussed; this makes assessment of their quality difficult.
The draft IRIS assessment does not distinguish clearly between studies that are considered of high quality for use in risk assessment and studies that are considered for qualitative assessment only. With respect to the animal studies, it is not clear what weight was given to negative vs positive results. For example, in Section 4.4.9.1 of the draft, preimplantation loss in rats is discussed; two studies are given as supporting evidence (Sheveleva 1971; Kitaev et al. 1984), and eight inhalation studies are given as not reporting treatment-related embryolethality. A clear discussion that weighs both positive and negative results is needed.
Similarly, in the discussion of low birth weight and growth retardation (EPA 2010, Section 4.4.9.3), the effect of maternal toxicity is not discussed in the context of fetal growth retardation even though there is an indication of ma-
ternal toxicity in the effects summarized in Table 4-70. IARC (2006) considered 20 ppm to be a toxic concentration and reported that 10 ppm resulted in a significant decrease in food consumption. That statement suggests that maternal toxicity would occur after exposure at 10 ppm and greater and that effects noted at those doses should probably be attributed to maternal toxicity rather than to direct exposure to formaldehyde; this issue needs to be addressed in the evaluation of the studies. For example, maternal stress that can result from being put into inhalation chambers or from the irritating effects of formaldehyde at high concentrations is not discussed but could be a contributor to maternal toxicity. Because formaldehyde is a natural metabolic intermediate in humans and other animals, some discussion of the endogenous formaldehyde concentrations in the animal models is needed to put the exposures into context.
Hazard Identification and Use of EPA Guidelines
The draft IRIS assessment briefly summarizes the evaluation of key epidemiologic studies and touches on issues of bias (EPA 2010, Section 4.4.9). There is little discussion about the weight of evidence given to the studies. In a conclusion that there was sufficient evidence of fetal toxicity, some animal studies used as supporting evidence are of questionable quality. There is no discussion of whether formaldehyde (or its metabolites) could gain access to the fetus and cause adverse effects or whether the adverse effects were a result of maternal toxicity.
The review of animal studies in the draft IRIS assessment does not discuss the modes of action in sufficient detail to determine the biologic plausibility that formaldehyde adversely affects the fetus. Potential modes of action are endocrine disruption, genotoxic effects on gametes, and oxidative stress or damage (EPA 2010, Section 4.4.9.7). Although more weight is given to studies of animals exposed by inhalation, studies that used other exposure routes are included. A major concern in connection with developmental and reproductive toxicity is whether formaldehyde can penetrate past the portal of entry. That critical question affects the conclusions drawn from the animal studies, particularly those in which exposure was by a route other than inhalation. More emphasis is placed on studies whose route of exposure is inhalation because metabolism and distribution may differ substantially after oral, dermal, and intraperitoneal exposure. If formaldehyde does penetrate past the portal of entry, another important consideration is whether it crosses the placenta and gains entry into the fetus or whether it crosses the blood-testis barrier. There is little information in the draft IRIS assessment or in any of the reviewed studies concerning those points, and no attempts appear to have been made to measure formaldehyde or metabolites in target tissues.
Several conclusions are stated regarding the reproductive and developmental effects of inhalation exposure to formaldehyde (EPA 2010, Section 6.1.3.7). References are given to support the conclusions; however, the overall
quality of the database is not discussed. For example, it is stated that “exposure of rat dams to formaldehyde during pregnancy has been shown to result in significantly decreased fetal weight gain” (EPA 2010, p. 6-13); but a statistically significant decrease in fetal weight, which was not associated with maternal toxicity, was reported in only one study in which male, but not female, fetuses were affected (Saillenfait et al. 1989).
Conclusions concerning male reproduction are similar; supporting studies generally used concentrations that result in significant weight loss and overt toxicity (Sarsilmaz et al. 1999; Ozen et al. 2002). The conclusions need to be placed into the context of potential confounders, such as maternal toxicity, stress from being placed in inhalation chambers, irritant concentrations above the odor threshold, and potential oral exposures by licking. The quality of the supporting studies needs to be stated clearly.
The overall database on developmental and reproductive effects of inhalation exposure to formaldehyde in animal studies is suggestive of an effect but not conclusive. When given by oral exposure or by injection, formaldehyde or its metabolites are capable of reaching reproductive tissues and the fetus. However, whether inhaled formaldehyde passes the portal of entry to access distant tissues—such as the gonads, hypothalamus, or the fetus—remains unresolved. In evaluating the animal data for reproductive effects, the draft IRIS assessment notes that there are no multigenerational tests for reproductive function (EPA 2010, Section 4.4.9.8). The committee agrees that that constitutes a data gap; particularly for male reproductive effects, such information is needed.
Study Selection for Calculation of Reference Concentration and Identification of Point of Departure
Regarding RfC derivation, EPA guidelines discuss methods to determine the adequacy of individual studies and the completeness of the overall database (EPA 2002). As noted above, the information on many health outcomes is not complete and does not contain critical details, such as duration and timing of exposure. Furthermore, in reviewing critical studies, the draft IRIS assessment does not adequately address important aspects of its guidance, such as the following:
-
Is there sufficient description of the protocol, statistical analyses, and results to make an evaluation?
-
Were appropriate statistical techniques applied for each end point, and was the power of the study adequate to detect effects?
-
Did the study establish dose-response relationships?
-
Are the results of the study biologically plausible?
The epidemiologic studies provide only a suggestive pattern of association. However, to be consistent with EPA guidelines, an RfC can be calculated by using the best available study evidence. EPA chose the study by Taskinen et al. (1999) to derive a candidate RfC. That study of female Finnish wood workers examined estimated workplace formaldehyde exposure primarily in relation to time to pregnancy but secondarily to other outcomes, including endometriosis and spontaneous abortion. The study had multiple strengths, including the national identification of workers and birth outcomes; industrial-hygienist assessment of potential exposures, including workplace measurements; and adjustment for multiple potential confounders, including other exposures. The study weaknesses included the use of a mailed questionnaire for exposure and covariate information; potential recall bias as to work tasks; no consideration of work accidents; inadequate description of exposure sources, such as the number of measurements taken; and the use of measurements from workplaces other than their own specific workplace that varied by exposure category. Furthermore, participant response to the questionnaire was less than outstanding.
EPA chose that study from the available epidemiologic studies of reproductive effects because of its overall strengths, the low likelihood of an important effect of selection bias, and consistency with other epidemiologic studies and animal evidence on fetal loss (EPA 2010). Furthermore, the draft IRIS assessment notes that the study population was well defined and adequately selected to allow examination of health effects in people who had different exposures. The committee agrees that it has a number of important strengths compared with the other reproductive epidemiologic studies evaluated. Notable strengths include exposure assessment, a relatively easily measured outcome (time to pregnancy), and assessment of confounding, such as by occupational exposures.
The draft IRIS assessment indicates that the study could be used for three outcomes: miscarriage, endometriosis, and decreased fecundity density ratio (FDR). However, because of the concerns about the miscarriage and endometriosis analyses, the FDR results were chosen as the critical effect for a candidate RfC. For example, the spontaneous-abortion analysis was not the primary aim, and the exposure and response pattern was not consistent with the increased risk found in the low-exposure group. In addition, the spontaneous-abortion analysis did not adjust for all covariates used in the FDR analysis. EPA also noted that the endometriosis results may be confounded by other solvents. The committee agrees that the choice of outcome from Taskinen et al. (1999) is appropriate for the reasons provided in the draft IRIS assessment.
However, the committee is concerned that basing an RfC on a single human study in a minimal human database is problematic. EPA guidelines state that “a reference value based on a single study would likely have a high degree of uncertainty” (EPA 2002, p. 4-20). Although multiple studies of varied quality have assessed spontaneous abortions, the study by Taskinen et al. (1999) is the only one that measured time to pregnancy.
Conclusions and Recommendations
The review of the reproductive and developmental outcomes in the draft IRIS assessment includes relevant outcomes and literature. It does not consistently provide a critical evaluation of the quality of publications and data presented or note strengths and weaknesses of each study. That is especially the case with the animal studies. The rationale for the assessment of the body of the epidemiologic evidence as convincing is not well articulated. Issues regarding the potential portal of entry and mode of action in relation to reproductive and developmental outcomes are not integrated into the weight-of-evidence discussion. Nonetheless, despite the shortcomings in the database and aspects of the review, the most relevant epidemiologic study and specific outcome are advanced for derivation of a candidate RfC. The point of departure is appropriately selected.
The committee recommends that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Provide a consistent critical evaluation of the study quality and data presented, particularly strengths and weaknesses of each study. That is especially needed for the animal studies.
-
Articulate better the basis of the assessment of the epidemiologic evidence.
-
Integrate better the issues surrounding systemic delivery and mode of action for reproductive and developmental outcomes into the weight-of-evidence discussion.
LYMPHOHEMATOPOIETIC CANCERS
Lymphohematopoietic (LHP) cancers are a heterogeneous group of cancers that encompass a wide variety of leukemias and lymphomas. Although they all arise from the hematopoietic system, these cancers are often derived from cells of different origin, can demonstrate unique genetic abnormalities, and may arise in different tissues (Figure 5-1). Those differences indicate that their etiologic bases may be distinct.
Although the draft IRIS assessment explores specific diagnoses—such as acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and Hodgkin lymphoma and multiple myeloma (see, for example, EPA 2010, Table 4-92)—the determinations of causality are made for the heterogeneous groupings “all LHP cancers,” “all leukemias,” and “myeloid leukemias.” The grouping “all LHP cancers” includes at least 14 biologically distinct diagnoses in humans (Figure 5-1) and should not be used in determinations of causality. The draft IRIS assessment should include information about the relative incidence of the
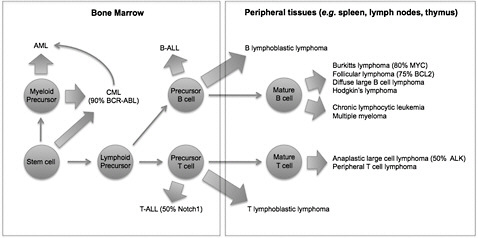
FIGURE 5-1 Origins of lymphohematopoietic cancers. Cells of origin, common genetic abnormalities, and tissues of origin are indicated for diverse hematopoietic malignancies. Compared with Figure 4-32 in the draft IRIS assessment, this figure clarifies the distinct cells of origin of acute myeloblastic leukemia (AML), T-cell acute lymphoblastic leukemia (T-ALL), B-cell acute lymphoblastic leukemia (B-ALL), and most mature leukemias and lymphomas. Abbreviations: ALK, anaplastic lymphoma kinase; BCL2, B-cell leukemia 2; BCR-ABL, breakpoint cluster region-Abelson murine leukemia; and MYC, myelocytomatosis viral oncogene homolog.
leukemia subtypes because the contribution of AML, CML, “myeloid leukemias,” and “all leukemias” to the grouping “all LHP” may not be obvious to readers and may help with interpretation (Figure 5-2).
Another important topic of discussion in the draft IRIS assessment is that of potential modes of action of formaldehyde as a cause of diverse LHP cancers. As discussed in Chapter 3 of the present report, the available experimental data indicate that formaldehyde itself does not penetrate beyond the superficial layer of the portal of entry, the epithelium of the nasopharynx. Therefore, long-used models of chemical leukemogenesis in which there is direct toxicity to hematopoietic cells in the bone marrow are unlikely to explain the proposed distal effect of formaldehyde on hematopoietic precursors. However, evidence of formaldehyde-induced DNA adducts and DNA damage in circulating lymphocytes suggests that hematopoietic cells might be affected by inhaled formaldehyde, presumably at the nasal epithelium or nasal-associated lymphoid tissue (NALT). EPA and others propose a model in which lymphoid precursors or hematopoietic stem cells circulate or migrate to the nasal epithelium, where they are directly exposed to formaldehyde and ultimately result in diverse LHP cancers. As the draft IRIS assessment states, that hypothesis seems plausible for Hodgkin lymphoma and multiple myeloma, which arise from precursors in the peripheral
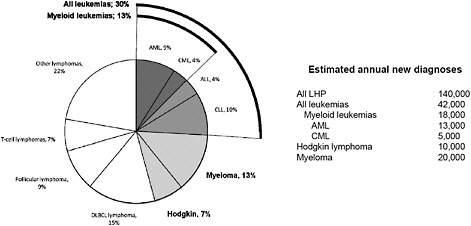
FIGURE 5-2 Relative incidence and estimated annual new diagnoses of common lymphohematopoietic cancer subtypes in the United States. Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphoblastic leukemia; CML, chronic myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; and LHP, lymphohematopoietic. Figure based on data from ACS (2010), LLS (2011), and SEER (2010).
tissues. However, inasmuch as experimental evidence is absent, there is no direct support for the hypothesis. Similarly, although recent evidence demonstrates that normal hematopoietic precursors do indeed leave the bone marrow and circulate as part of a daily circadian rhythm (Mendez-Ferrer et al. 2009), studies have not shown that the cells are present in the nasal epithelium or NALT, nor have they shown formaldehyde-induced effects in vivo. An additional hypothesis is that formaldehyde exposure at the port of entry induces secondary systemic effects, such as immune modulation or systemic inflammation, both of which are associated with LHP cancers. However, given the lack of direct data that could support those hypothetical modes of action, EPA could shorten those sections of the draft substantially and note that the modes of action remain uncertain.
Similarly, there is a paucity of evidence of formaldehyde-induced LHP cancers in animal models. EPA’s unpublished re-analysis of the Battelle chronic inhaled formaldehyde experiments in mice and rats (Battelle Columbus Laboratories 1981), although intriguing, provides the only positive findings and thus does not contribute to the weight of evidence of causality.
Study Identification
The draft IRIS assessment comprehensively presents studies available through late 2009 that evaluate formaldehyde exposure and risk of LHP cancers. The draft provides commentary on multiple studies that had negative and posi-
tive findings, cohorts that were the subject of multiple analyses or publications, and meta-analyses. The emphasis on studies of occupational cohorts is appropriate, given that they provide the most specific and detailed exposure assessment that can be applied in risk assessments. The committee is not aware of any important studies that are missing from the analysis, although several relevant studies have been published since the draft was released (for example, Andersen et al. 2010; Bachand et al. 2010; Lu et al. 2010; Schwilk et al. 2010). To make the IRIS assessment as timely as possible, inclusion of the recent studies seems warranted in the revision.
Study Evaluation
EPA’s review is extensive and covers in considerable detail a substantial body of pertinent epidemiologic and toxicologic literature. The study evaluations tend to be long narratives that provide substantial detail on reported findings. The heterogeneity of LHP cancers is acknowledged as a complicating factor in assessing causation and ultimately in the cancer assessment.
However, there is no clearly articulated framework for establishing causation on the basis of the weight and strength of evidence. An a priori presentation of the study selection criteria (for example, quality of exposure assessment, control of confounding variables, and statistical power) is also missing. Both the framework and study selection criteria are critical for any determination of causation. For example, the concept of consistency of findings within and among studies is not defined, so it is difficult to determine how studies with different study populations, cancer incidence, and exposure measures were combined to provide a consistent and conclusive determination of causality. As a result, the conclusion of causation appears to be based on a subjective view of the overall data. Given the limitations of the epidemiologic studies (particularly uncertainties of exposure assessment, possible confounding by other pollutants, and reliance on mortality rather than incidence data), a clear statement and consistent use of the weight-of-evidence criteria would strengthen the conclusions. Additional explicit reference to the EPA Guidelines for Carcinogen Risk Assessment (EPA 2005a) and the Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens (EPA 2005b) or other guidelines or precedent for assessing the quality and importance of studies is recommended.
The absence of a causation framework is especially problematic for the individual LHP cancers, given the highly variable epidemiologic literature and the high uncertainty of mode of action. Important differences exist in the reported findings of the most influential studies—of UK (Coggon et al. 2003) and U.S. (Beane-Freeman et al. 2009) industrial cohorts, the National Institute for Occupational Safety and Health garment workers cohort (Pinkerton et al. 2004), and U.S. embalmers (Hauptmann et al. 2004). The differences should be discussed and weighed, specifically as to how they were taken into account in EPA’s determinations of causality. For example, in the highly influential Na-
tional Cancer Institute (NCI) cohort study (Beane-Freeman et al. 2009), the strength and specificity of the exposure-response associations varied considerably over the period in which the cohort was followed. In addition, the reliance on the peak-exposure metric to determine causality in that study rather than the more conventional dose metric of cumulative exposure should be further justified, particularly in the absence of established modes of action.
Hazard Identification and Use of EPA Guidelines
The hazard identification concluded that there is a causal association between formaldehyde exposure and mortality from all LHP cancers, all leukemias as a group, myeloid leukemias, Hodgkin lymphoma, and multiple myeloma. As noted earlier, the committee strongly discourages the use of the grouping “all LHP cancers,” given the biologic heterogeneity within this group. For the other groupings, the committee finds that the conclusions of causality are not adequately supported by the current narrative. Further discussion of LHP subtype diagnosis in various studies would aid in comparison of findings. Sections covering all leukemias and myeloid leukemias are adequate in depth but would benefit from a clearer synthesis of the data and more explicit reference to guidelines or precedents for evaluating evidence when there are notable differences in data quality and conflicting results among studies. The committee recommends caution on EPA’s part in using meta-analyses performed by others to assess causality or to quantify effects. Meta-analysis can be a valuable method for summarizing evidence but can also be subject to variable interpretations depending on how literature is selected and reviewed and data analyzed. Given the conflicting conclusions of published meta-analyses of formaldehyde and LHP cancers (see, for example, Zhang et al. 2009; Bachand et al. 2010; Schwilk et al. 2010), EPA is encouraged to perform its own meta-analysis if the agency chooses to use meta-analysis as a tool to assess causation.
Study Selection for Calculation of Unit Risk
As articulated by EPA, few studies can be used to calculate risk estimates. Regardless, the selection and use of the NCI cohort (Beane-Freeman et al. 2009) should be further justified. Indeed, interpretation of the study results is not straightforward given that the findings differ from those of earlier analyses of the same cohort and differ for peak, average, and cumulative formaldehyde exposures. In the absence of evidence regarding exposure-disease mechanisms, as in the case of formaldehyde and LHP cancers, cumulative exposure is typically the default dose metric applied in epidemiologic analyses and risk assessment. But the most significant results were found for peak exposures, which have the greatest associated uncertainty. In view of the importance of this study, EPA should clarify the basis of its interpretations of the results regarding the various
dose metrics and the various LHP cancers. Despite those concerns, the committee agrees that the NCI study is the most appropriate available to carry forward for calculation of the unit risk.
Conclusions and Recommendations
The committee recommends that EPA address the following in the revision of the formaldehyde draft IRIS assessment:
-
Focus on the most specific diagnoses available in the epidemiologic data, such as acute myeloblastic leukemia (International Classification of Diseases [ICD] 205.0), chronic lymphocytic leukemia (ICD 204.1), and specific lymphomas, such as Burkitt (ICD 200.2), Hodgkin (ICD 201), anaplastic large-cell (ICD 200.6), and peripheral T-cell lymphoma (ICD 202.7). The committee does not support consideration of the grouping “all LHP cancers” because this grouping combines diverse cancers that are not closely related in cells of origin and in other characteristics.
-
Evaluate existing studies and data with concise discussions of background and speculative hypotheses. The narratives in the draft IRIS assessments are sometimes too long and unfocused.
-
Clarify how EPA determined weight and strength of evidence. The draft assessment should be revised to discuss the benefits, limitations, and justifications of using one exposure metric to determine causality and another to calculate cancer unit risk. Because the draft assessment relies solely on epidemiologic studies to determine causality, further discussion of the specific strengths, weaknesses, and inconsistencies in several key studies is needed. As stated in EPA’s cancer guidelines, EPA’s approach to weight of evidence should include “a single integrative step after assessing all of the individual lines of evidence” (EPA 2005a, Section 1.3.3, p. 1-11). Although a synthesis and summary are provided, the process that EPA used to weigh different lines of evidence and how that evidence was integrated into a final conclusion are not apparent in the draft assessment and should be made clear in the final version.
-
Revisit arguments that support determinations of causality of specific LHP cancers and in so doing include detailed descriptions of the criteria that were used to weigh evidence and assess causality. That will add needed transparency and validity to the conclusions.
-
If EPA decides to rely on meta-analysis as a tool to assess causation, it should perform its own meta-analysis with particular attention to specific diagnoses and to variables selected and combined for analysis. The contrasting conclusions of the published meta-analyses make it difficult to rely on conclusions from any one analysis (see, for example, Zhang et al. 2009; Bachand et al. 2010; Schwilk et al. 2010).
REFERENCES
ACS (American Cancer Society). 2010. Cancer Facts & Figures 2010. American Cancer Society [online]. Available: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf [accessed March 2, 2011].
Adams, D.O., T.A. Hamilton, L.D. Lauer, and J.H. Dean. 1987. The effect of formaldehyde exposure upon the mononuclear phagocyte system of mice. Toxicol. Appl. Pharmacol. 88(2):165-174.
Andersen, M.E., H.J. Clewell III, E. Bermudez, D.E. Dodd, G.A. Willson, J.L. Campbell, and R.S. Thomas. 2010. Formaldehyde: Integrating dosimetry, cytotoxicity, and genomics to understand dose-dependent transitions for an endogenous compound. Toxicol. Sci. 118(2):716-731.
Bachand, A.M., K.A. Mundt, D.J. Mundt, and R.R. Montgomery. 2010. Epidemiological studies of formaldehyde exposure and risk of leukemia and nasophryngeal cancer: A meta-analysis. Crit. Rev. Toxicol. 40(2):85-100.
Battelle Columbus Laboratories. 1981. Final Report on a Chronic Inhalation Toxicology Study in Rats and Mice Exposed to Formaldehyde. Prepared by Battelle Columbus Laboratories, Columbus, OH, for the Chemical Industry Institute of Toxicology (CIIT), Research Triangle Park, NC. CIIT Docket No. 10922.
Beane-Freeman, L.E., A. Blair, J.H. Lubin, P.A. Stewart, R.B. Hayes, R.N. Hoover, and M. Hauptmann. 2009. Mortality from Lymphohematopoietic malignancies among workers in formaldehyde industries: The National Cancer Institute cohort. J. Natl. Cancer Inst. 101(10):751-761.
Coggon, D., E.C. Harris, J. Poole, and K.T. Palmer. 2003. Extended follow-up of a cohort of British chemical workers exposed to formaldehyde. J. Natl. Cancer Inst. 95(21):1608-1615.Conrad, C.D. 2006. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav. Cogn. Neurosci. 5(1):41-60.
Dean, J.H., L.D. Lauer, R.V. House, M.J. Murray, W.S. Stillman, R.D. Irons, W.H. Steinhagen, M.C. Phelps, and D.O. Adams. 1984. Studies of immune function and host resistance in B6C3F1 mice exposed to formaldehyde. Toxicol. Appl. Pharmacol. 72(3):519-529.
EPA (U.S. Environmental Protection Agency). 1991. Guidelines for Developmental Toxicity Risk Assessment. EPA/600/FR-91/001. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. December 1991 [online]. Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=23162 [accessed Nov. 24, 2010].
EPA (U.S. Environmental Protection Agency). 1996. Guidelines for Reproductive Toxicity Risk Assessment. EPA/630/R-96/009. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. October 1996 [online]. Available: http://www.epa.gov/raf/publications/pdfs/REPRO51.PDF [accessed Nov. 24, 2010].
EPA (U.S. Environmental Protection Agency). 1998a. Health Effects Test Guidelines OPPTS 870.7800 Immunotoxicity. U.S. Environmental Protection Agency [online]. Available: http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2009-0156-0049 [accessed Feb. 22, 2011].
EPA (U.S. Environmental Protection Agency). 1998b. Guidelines for Neurotoxicity Risk Assessment. EPA/630-95/001F. Risk Assessment Forum, U.S. Environmental
Protection Agency, Washington, DC. April 1998 [online]. Available: http://www.epa.gov/raf/publications/pdfs/NEUROTOX.PDF [accessed Nov. 24, 2010].
EPA (U.S. Environmental Protection Agency). 2002. A Review of the Reference Dose and Reference Concentration Processes. External Review Draft. EPA/630/P-02/002A. Reference Dose/Reference Concentration (RfD/RfC) Technical Panel, Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/raf/publications/pdfs/rfdrfcextrevdrft.pdf [accessed Jan. 6, 2010].
EPA (U.S. Environmental Protection Agency). 2005a. Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. March 2005 [online]. Available: http://www.epa.gov/raf/publications/pdfs/CANCER_GUIDELINES_FINAL_3-25-05.PDF [accessed Nov. 24, 2010].
EPA (U.S. Environmental Protection Agency). 2005b. Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens. EPA/630/R-03/003F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. March 2005 [online]. Available: http://www.epa.gov/ttn/atw/childrens_supplement_final.pdf [accessed Nov. 24, 2010].
EPA (U.S. Environmental Protection Agency). 2010. Toxicological Review of Formaldehyde (CAS No. 50-00-0) – Inhalation Assessment: In Support of Summary Information on the Integrated Risk Information System (IRIS). External Review Draft. EPA/635/R-10/002A. U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=223614 [accessed Nov. 22, 2010].
Fitzgerald, R.E., M. Berres, and U. Schaeppi. 1988. Validation of a photobeam system for assessment of motor activity in rats. Toxicology 49(2-3):433-439.
Hauptmann, M., J.H. Lubin, P.A. Stewart, R.B. Hayes, and A. Blair. 2004. Mortality from solid cancers among workers in formaldehyde industries. Am. J. Epidemiol. 159(12):1117-1130.
Holmström, M., and B. Wilhelmsson. 1988. Respiratory symptoms and pathophysiological effects of occupational exposure to formaldehyde and wood dust. Scand. J. Work Environ. Health 14(5):306-311.
Holness, D.L., and J.R. Nethercott. 1989. Health status of funeral service workers exposed to formaldehyde. Arch. Environ. Health 44(4):222-228.
IARC (International Agency for Research on Cancer). 2006. Formaldehyde. Pp. 39-325 in Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 88. Lyon, France: World Health Organization, International Agency for Research on Cancer.
Kilburn, K.H. 1994. Neurobehavioral impairment and seizures from formaldehyde. Arch. Environ. Health 49(1):37-44.
Kilburn, K.H., and A. Moro. 1985. Reproductive and maternal effects of formaldehyde (HCHO) in rats. Fed. Proc. 44:535.
Kilburn, K.H., B.C. Seidman, and R. Warshaw. 1985. Neurobehavioral and respiratory symptoms of formaldehyde and xylene exposure in histology technicians. Arch. Environ. Health 40(4):229-233.
Kilburn, K.H., R. Warshaw, and J.C. Thornton. 1987. Formaldehyde impairs memory equilibrium and dexterity in histology technicians: Effects which persist for days after exposure. Arch. Environ. Health 42(2):117-120.
Krzyzanowski, M., J.J. Quackenboss, and M.D. Lebowitz. 1990. Chronic respiratory effects of indoor formaldehyde exposure. Environ. Res. 52(2):117-125.
Lino dos Santos Franco, A., H.V. Domingos, A.S. Damazo, A.C. Breithaupt-Faloppa, A.P. de Oliviera, S.K. Costa, S.M. Oliani, R.M. Oliveira-Filho, B.B. Vargaftig, and W. Tavares-de-Lima. 2009. Reduced allergic lung inflammation in rats following formaldehyde exposure: Long term effects on multiple effector systems. Toxicology 256(3):157-163.
LLS (The Leukemia & Lymphoma Society). 2011. Facts 2010-2011. The Leukemia & Lymphoma Society [online]. Available: http://www.lls.org/content/nationalcontent/resourcecenter/freeeducationmaterials/generalcancer/pdf/facts [accessed March 2, 2011].
Lu, K., L.B. Collins, H. Ru, E. Bermudez, and J.A. Swenburg. 2010. Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol. Sci. 116(2):441-451.
Lyapina, M., G. Zhelezova, E. Petrova, and M. Boev. 2004. Flow cytometric determination of neutrophil burst activity in workers exposed to formaldehyde. Int. Arch. Occup. Environ. Health 77(5):335-340.
Malek, F.A., K.U. Möritz, and J. Fanghänel. 2003a. A study on the effect of inhalative formaldehyde exposure on water labyrinth test performance in rats. Ann. Anat. 185(3):277-285.
Malek, F.A., K.U. Möritz, and J. Fanghänel. 2003b. Formaldehyde inhalation and open field behaviour in rats. Indian J. Med. Res. 118:90-96.
Malek, F.A., K.U. Möritz, and J. Fanghänel. 2003c. A study on specific behavioral effects of formaldehyde in the rat. J. Exp. Anim. Sci. 42(3):160-170.
Martin, W.J. 1990. A teratology study of inhaled formaldehyde in the rat. Reprod. Toxicol. 4(3):237-239.
McEwen, B.S. 2008. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 583(2-3):174-185.
Mendez-Ferrer, S., A. Chow, M. Merad, and P.S. Frenette. 2009. Circadian rhythms influence hematopoietic stem cells. Curr. Opin. Hematol. 16(4):235-242.
Morgan, K.T., D.L. Patterson, and E.A. Gross. 1986. Responses of the nasal mucociliary apparatus of F-344 rats to formaldehyde gas. Toxicol. Appl. Pharmacol. 82(1):1-13.
Ohtsuka, R., Y. Shutoh, H. Fujie, S. Yamahuchi, M. Takeda, T. Harada, and K. Doi. 2003. Rat strain difference in histology and expression of Th1- and Th2- related cytokines in nasal mucosa after short-term formaldehyde inhalation. Exp. Toxicol. Pathol. 54(4):287-291.
Ozen, O.A., M. Yaman, M. Sarsilmaz, A. Songur, and I. Kus. 2002. Testicular zinc, copper and iron concentrations in male rats exposed to subacute and subchronic formaldehyde gas inhalation. J. Trace Elem. Med. Biol. 16(2):119-122.
Pavlides, C., L.G. Nivón, and B.S. McEwen. 2002. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus 12(2):245-257.
Pinkerton, L.E., M.J. Hein, and L.T. Stayner. 2004. Mortality among a cohort of garment workers exposed to formaldehyde: An update. Occup. Environ. Med. 61(3):193-200.
Riedel, F., E. Hasenauer, P.J. Barth, A. Koziorowski, and C.H. Rieger. 1996. Formaldehyde exposure enhances inhalative allergic sensitization in the guinea pig. Allergy 51(2):94-99.
Sadakane, K., H. Takano, T. Ichinose, R. Yanagisawa, and T. Shibamoto. 2002. Formaldehyde enhances mite allergen-induced eosinophilic inflammation in the murine airway. J. Environ. Pathol. Toxicol. Oncol. 21(3):267-276.
Saillenfait, A.M., P. Bonnet, and J. de Ceaurriz. 1989. The effects of maternally inhaled formaldehyde on embryonal and foetal development in rats. Food Chem. Toxicol. 27(8):545-548.
Sarsilmaz, M., O.A. Ozen, N. Akpolat, I. Kus, and A. Songur. 1999. The histopathologic effects of inhaled formaldehyde on Leydig cells of the rats in subacute period [in Turkish]. J. Med. (Firat University Health Science) 13(1):37-40.
Schwilk, E., L. Zhang, M.T. Smith, A.H. Smith, and C. Steinmaus. 2010. Formaldehyde and leukemia: An updated meta-analysis and evaluation of bias. J. Occup. Environ. Med. 52(9):878-886.
SEER (Surveillance Epidemiology and End Results). 2010. Estimated New Cancer Cases and Deaths for 2010: All Races, By Sex. American Cancer Society, Surveillance Epidemiology and End Results [online]. Available: http://seer.cancer.gov/csr/1975_2007/results_single/sect_01_table.01.pdf [accessed March 2, 2011].
Sorg, B.A., and T. Hochstatter. 1999. Behavioral sensitization after repeated formaldehyde exposure in rats. Toxicol. Ind. Health 15(3-4):346-355.
Sorg, B.A., T.M. Bailie, M.L. Tschirgi, N. Li, and W.R. Wu. 2001. Exposure to repeated low-level formaldehyde in rats increases basal corticosterone levels and enhances the corticosterone response to subsequent formaldehyde. Brain Res. 898(2):314-320.
Tarkowski, M., and P. Gorski. 1995. Increased IgE antiovalbumin level in mice exposed to formaldehyde. Int. Arch. Allergy Immunol. 106(4):422-424.
Taskinen, H., P. Kyyronen, K. Hemminki, M. Hoikkala, K. Lajunen, and M.L. Lindbohm. 1994. Laboratory work and pregnancy outcome. J. Occup. Med. 36(3):311-319.
Taskinen, H.K., P. Kyyronen, M. Sallmen, S.V. Virtanen, T.A. Liukkonen, O. Huida, M.L. Lindbohm, and A. Anttila. 1999. Reduced fertility among female wood workers exposed to formaldehyde. Am. J. Ind. Med. 36(1):206-212.
Weisskopf, M., N. Morozova, E.J. O’Reilly, M.L. McCullough, E.E. Calle, M.J. Thun, and A. Ascherio. 2009. Prospective study of chemical exposures and amyotrophic lateral sclerosis mortality. J. Neurol. Neurosurg. Psychiatry 80(5):558-561.
Zuena, A.R., J. Mairesse, P. Casolini, C. Cinque, G.S. Alemà, S. Morley-Fletcher, V. Chiodi, L.G. Spagnoli, R. Gradini, A. Catalani, F. Nicoletti, and S. Maccari. 2008. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One 3(5):e2170.
Zhang, L., C. Steinmaus, D.A. Eastmond, X.K. Xin, and M.T. Smith. 2009. Formaldehyde exposure and leukemia: A new meta-analysis and potential mechanisms. Mutat. Res. 681(2-3):150-168.


























