FUNGAL DISEASES: AN EMERGING THREAT TO HUMAN, ANIMAL, AND PLANT HEALTH
Will the blight end the chestnut?
The farmers rather guess not.
It keeps smouldering at the roots
And sending up new shoots
Till another parasite
Shall come and end the blight.
—Robert Frost (1936)
Fungi are the only group of organisms that have been convincingly shown to cause extinction.
—Arturo Casadevall (2010)
At the beginning of the 20th century, the American chestnut population counted nearly 4 billion trees. The American chestnut tree, once dominant in the forests of the Eastern United States, was decimated by an accidentally introduced and previously unknown fungal pathogen. Within a span of 40 years, this once abundant, iconic forest tree was all but annihilated by this microscopic fungus. In the middle of the 20th century, an epidemic of Dutch elm disease—a vector-borne fungal disease, also unknown to science at the time—ravaged the elm trees of North America, Europe, and England (Brasier and Buck, 2001). Together, these diseases rapidly and radically transformed the landscape of America’s cities and forests (Money, 2007).
Fungal diseases of plants, animals, and humans have altered tree population diversity and forest ecosystem dynamics, devastated agricultural crops, triggered global population declines and extinctions in wildlife, and contributed to death and disability in humans. Cryptococcus gattii (C. gattii), a pathogenic fungus that emerged in 1999 on Vancouver Island, British Columbia, Canada, is causing a growing epidemic of human and animal infections and deaths (Galanis and MacDougall, 2010). Since its initial recognition, the pathogen has spread from Vancouver Island to mainland British Columbia and south into the Pacific Northwest of the United States. This fungal pathogen has been associated with 338 confirmed human infections and 40 deaths1 in these regions, which represents the largest documented population of C. gattii infected people in the world (Datta et al., 2009a; Galanis and MacDougall, 2010). Bat white-nose syndrome (WNS) and amphibian chytridiomycosis2 have caused massive population declines and threaten local extinctions of New World bat and amphibian species, respectively (Frick et al., 2010; Skerratt et al., 2007). By 2009, the geographic range of two virulent and highly aggressive strains3 of yellow “stripe” rust—first detected in North America in 2000—expanded to include major wheat-producing areas on five continents, threatening the global wheat supply (Hovmøller et al., 2010). The recent observation that a fungus (Nosema spp.), in combination with a DNA virus, might be associated with “colony collapse” disorder—a disease that has destroyed 20–40 percent of the honeybee colonies in the United States since 2006—underscores the direct and indirect impacts and ecosystem dynamics of fungal diseases in human, plant, and animal communities (Bromenshenk et al., 2010).
Fungal organisms interact with humans, animals, and plants in beneficial as well as pathogenic ways. A dozen fungal diseases are considered “life threatening” to humans. At the same time, human health has benefited immensely from fungal-derived antibiotics, such as penicillin (Blackwell et al., 2009; Buckley, 2008; Casadevall, 2007). Indeed, fungi are indispensible to life on this planet through their ability to break down complex organic matter and recycle essential nutrients back into the environment (Wainwright, 1992).
The fungal kingdom is among the most diverse kingdoms in the Tree of Life (Blackwell, 2011). Yet, fewer than 10 percent of fungal organisms have been formally described (Hawksworth, 1991, 2001). For the purposes of this chapter, the terms fungi, fungal, and fungus are used inclusively to describe all organisms traditionally studied by mycologists—including species that are now excluded from Kingdom Fungi (e.g., Phytophthora spp. which are members of Oomycota) or whose relationship to the fungal kingdom have yet to be determined (e.g., the
______________
1 As of December 2010.
2 In this chapter, we will refer to this disease as amphibian chytridiomycosis and to the associated pathogen (Batrachochytrium dendrobatidis) as Bd.
3Puccinia striiformis Westend. f.sp. tritici Eriksson.
microsporidia Nosema spp. and the newly discovered cryptomycota) (see Jones et al., 2011; Stajich et al., 2009).
Despite the extensive influence of fungi on economic well-being, as well as on human, animal, plant, and ecosystem health, the threats posed by emerging fungal pathogens are often unappreciated and poorly understood. On December 14 and 15, 2010, the Institute of Medicine’s (IOM’s) Forum on Microbial Threats hosted a public workshop on this topic in order to explore the scientific and policy dimensions associated with the causes and consequences of emerging fungal diseases. Through invited presentations and discussions, the workshop explored the environmental, host (plant, animal, and human), and pathogen-related factors influencing the emergence, establishment, and spread of fungal pathogens, as well as the impacts of these diseases on human and animal health, agriculture, and biodiversity. Workshop participants also considered and discussed opportunities to improve surveillance, detection, and response strategies for identifying and mitigating the impacts of these diseases in order to better prepare for future outbreaks. Convened in response to the perceived threat posed by emerging fungal diseases to human, animal, and plant health, this was the first workshop in the Forum’s 15-year history that focused exclusively on fungal pathogens.
Organization of the Workshop Summary
This workshop summary was prepared by the rapporteurs for the Forum’s members and includes a collection of individually authored papers and commentary. Sections of the workshop summary not specifically attributed to an individual reflect the views of the rapporteurs and not those of the Forum on Microbial Threats, its sponsors, or the IOM. The contents of the unattributed sections are based on presentations and discussions at the workshop.
The summary is organized into sections as a topic-by-topic description of the presentations and discussions that took place at the workshop. Its purpose is to present lessons from relevant experience, to delineate a range of pivotal issues and their respective challenges, and to offer potential responses as discussed and described by the workshop participants. Manuscripts and reprinted articles submitted by some, but not all, of the workshop’s participants may be found, in alphabetical order, in Appendix A.
Although this workshop summary provides a description of the individual presentations, it also reflects an important aspect of the Forum’s philosophy. The workshop functions as a dialogue among representatives from different sectors and allows them to present their beliefs about which areas merit further attention. This report only summarizes the statements of workshop participants. This workshop summary report is not intended to be an exhaustive exploration of the subject matter nor does it represent the findings, conclusions, or recommendations of a consensus committee process.
THE HIDDEN KINGDOM
Fungi are among the most evolutionarily and ecologically diverse organisms on the planet, comprising a kingdom of organisms that provide valuable ecosystem services through their decomposition of organic matter, symbiotic associations with numerous plant and animal species, and as food sources (Blackwell, 2011; Taylor et al., 2004). Initially thought by early taxonomists to be members of the plant kingdom, fungi are actually more closely related to animals than plants (Figure WO-1) (McLaughlin et al., 2009).
According to keynote speaker Arturo Casadevall, of the Albert Einstein College of Medicine, fungal organisms—in terms of sheer numbers of species—constitute the most successful kingdom in the tree of life. (Dr. Casadevall’s contribution to the workshop summary report can be found in Appendix A, pages 177–188.) Yet fewer than 10 percent of the estimated 1.5 million species of fungi have been formally identified and described4 (Blackwell, 2011; Hawksworth, 2001). Forum Chair David Relman, of Stanford University, observed that, “We are blind to a lot of their biology and what it is that they spend most of their time doing and why and for whom. I think many in this room would agree that fungi are ignored and underappreciated.” This “blindspot,” he continued, “leaves us with fairly poor situational awareness: a relatively poor understanding of fungal biogeography—meaning their spatial distribution patterns—the factors that determine their distribution in space and time, and the factors that underlie their evolution, especially within short time-frames.”
Fungal Diversity
Existing as single-celled organisms, such as yeasts, or complex communities of filamentous mycelial networks covering hundreds of acres, fungi are ubiquitous in nature and display a dazzling array of sizes, shapes, and colors, including many that are bioluminescent (Figure WO-2) (Blackwell, 2011; Desjardin et al., 2010; Lutzoni et al., 2004).
The fungal life cycle is equally varied. Fungi can reproduce asexually or sexually through life cycles that range from simple to complex—including “dimorphic” switching between yeast and filamentous forms and the use of multiple host species (Blackwell et al., 2009). Spores5 are produced during the fungal life cycle and may be passively or actively dispersed through a variety of environmental media including air, water, wind, animals, and materials (Blackwell et al., 2009). Fungal growth, reproduction, spore production, and dispersal are also exquisitely sensitive to environmental conditions including temperature, humidity,
______________
4 This number is considered by many to be an underestimate of the actual number of fungal species; see contributed manuscripts by Blackwell in Appendix A (pages 116–167).
5 Spores are well-protected structures that can survive in adverse environmental conditions, such as freezing or drying (better than mycelia and yeast cells), for months and even years.
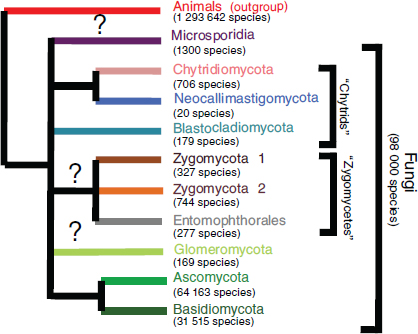
Figure WO-1 The fungal kingdom. The classification of species within kingdom Fungi continues to evolve. The diagram above provides an overview of some of the primary lineages of fungal organisms and the estimated number of species for each lineage.
SOURCE: Blackwell (2010).
winds, and water (Bahn et al., 2007; Judelson and Blanco, 2005; Kauserud et al., 2008; Kumamoto, 2008).
Fungi are highly adaptable to new environmental niches including what might be considered “extreme” environments (Gostinčar et al., 2010; Le Calvez et al., 2009). Some have suggested the ability of fungi to access multiple strategies for reproduction contributes to why fungi are so “adept at adaptation.” Under different environmental conditions, fungal reproduction can maintain characteristics adapted to a particular environmental niche or generate genetically diverse offspring that can quickly respond to changing host or environmental factors (Heitman, 2006). (Dr. Blackwell’s contribution to the workshop summary report can be found in Appendix A, pages 116–167.) Keynote speaker Meredith Blackwell, of Louisiana State University, noted that scientists continue to find new species of fungi in a wide range of environments—from tropical and temperate forests to the guts of insects (e.g., Arnold et al., 2003; Gostinčar et al., 2010; Miller et al., 2001; Suh and Blackwell, 2006). These discoveries often reveal the unique capabilities of these microorganisms. As observed by Casadevall, some fungal species can survive and thrive in high radiation and other extreme environments. Zhdanova et al. (2000) reported extensive fungal growth on the walls
Figure WO-2 Diversity of fungal morphology. (A) Two flagellated fungal cells from the recently discovered group of fungi known as cryptomycota. This ancient group of organisms is thought to be distinct from other fungi because of the absence of a cell wall made of chitin; (B) asexual, spore-producing culture of Cryphonectria parasitica (chestnut blight fungus); (C–F) multicellular, spore-producing structures (fruiting bodies) are produced during the sexual phase of the fungal life cycle. Many fruiting bodies are familiar as mushrooms—including species that are consumed by humans as food: (C) Morchella conica (morel) and (D) Crucibulum laeve (bird’s nest fungus). Mushrooms of some species are known to be toxic or poisonous to humans: (E) Amanita muscaria. Fungal fruiting bodies can exhibit a wide range of shapes and sizes, including (F) the bioluminescent “shelf” fungus, Panellus stipticus; (G) Micrograph of Phytophthora ramorum chlamydospores; (H) SEM photomicrograph prepared from G. destructans culture isolated from bat tissue samples collected from Williams Hotel Mine; note curved conidia borne in whorls on septate hyphae; bar is 2 µm. All images are pseudo-colored in Adobe Photoshop 9.0; (I) “fairy rings” in which mushrooms sprout along the outer edge of a sprawling, underground mycelial network. These networks (mycelia) have been known to cover several hundred acres. One of largest known mycelia has been estimated to encircle 900 hectares (3.4 square miles).
SOURCE: (A) Micrograph kindly provided by Meredith Jones, Exeter University; (B) photo by Kent Loeffler, provided by Alice C.L. Churchill, Cornell University; (C–F) Wikimedia Commons; (G) photo provided courtesy of Paul Reeser, Oregon State University; (H) Chaturvedi et al. (2010); (I) Wikimedia Commons.
and other areas of the shelter installed around the damaged unit of the Chernobyl nuclear power plant, including 37 species among 19 genera6; fungi are also known to inhabit high-radiation space environments and have even colonized the International Space Station (Dadachova and Casadevall, 2008).
The fungal pathogen responsible for sudden oak death and ramorum blight, Phytophthora ramorum, was only identified as a new species in 2000. Since then, according to speaker David Rizzo of the University of California at Davis, researchers have identified an additional 50 Phytophthora7 species. (Dr. Rizzo’s contribution to the workshop summary report can be found in Appendix A, pages 312–324.) As Rizzo observed, these new discoveries do not reflect recent fungal evolution, but are a reflection of the fact that “we just haven’t really been looking
______________
6 Many of the species inhabiting the most heavily contaminated sites of the Chernobyl nuclear power plant were rich in melanin (a high molecular weight pigment). Dadachova et al. (2007) reported that radiation enhances the growth of melanized Wangiella dermatitidis, Cryptococcus neoformans, and Cladosporium sphaerospermum cells.
7Phytophthora (“plant destroyer”) is a genus of approximately 100 species that includes several notorious plant pathogens, including Phytophthora infestans, which caused the Irish Potato Famine. Phytophthora species are oomycetes, which are fungus-like organisms in the kingdom Stramenopila.
for them.” Several other forest fungi that have caused major damage in the past, including the fungi responsible for chestnut blight and Dutch elm disease, were unknown to science until they started causing noticeable damage and die-off of forest and urban trees (Brasier and Webber, 2010).
Ecosystem Services8 and Interactions
The ability of fungi to process complex organic matter into essential nutrients (e.g., nitrogen, phosphorus) makes them indispensible members of virtually all ecosystems and “invisible” shapers of the world around us (Wainwright, 1992). The vast majority of described fungal species are saprophytic,9 surviving on dead plant matter and animal tissue (Blackwell et al., 2000). Fungi can be “free living”10 or form mutualistic, commensalistic, or parasitic relationships with plants, animals, and microbes—deriving benefits from and contributing to their living hosts (Blackwell et al., 2009).
Humans have used fungi as a direct source of food (e.g., truffles, mushrooms), as a leavening agent for bread, and in the fermentation of various food products, including, but not limited to, beer, wine, and soy products (Buckley, 2008). Some fungi contain psychotropic compounds that may be consumed recreationally or in traditional spiritual ceremonies, and they have been used for millennia for medicinal purposes (Capasso, 1998). The fruiting structures of a few species are highly valued in China for their purported medicinal benefits including as a “libido booster”11 (Roach, 2011). Blackwell stated that since the early 1940s, fungi have been exploited for their life-saving antibiotics.12 More recently, various enzymes and pigments produced by fungi have been used industrially and in the manufacture of a wide variety of products, including furniture, musical instruments, and clothing (Blanchette et al., 1992; Buckley, 2008; Keller et al., 2005). These organisms have been used extensively as biological pesticides to control weeds, plant diseases, and insect pests (Buckley, 2008). Blackwell observed that biomedical researchers have used certain species of fungi extensively as model organisms for genetic and other scientific research for decades.
Many fungi maintain close associations with their insect hosts. Blackwell discussed the symbiotic fungi that inhabit insect guts and are essential to the
______________
8 Services provided by ecosystems that benefit humans and are necessary for a healthy planet like oxygen production, water purification, pollination, soil formation, and nutrient recycling. See www.conservation.org/resources/glossary/Pages/e.aspx (accessed on June 13, 2011).
9 Deriving nutrients from dead organic matter.
10 Not dependent on a host for survival.
11 For example: A parasitic fungus, Ophiocordyceps sinensis, grows in the Tibetan Plateau in China and is highly valued for its “purported medicinal benefits,” including uses as “a treatment for cancer and aging and as a libido booster.” The nutty-tasting fungus is considered “fungal gold” because it can be sold for high prices in Chinese markets (see Roach, 2011).
12 Other medicines such as the immunosuppressant cyclosporine A and statin drugs also are derived from fungi.
nutrition of many insects (e.g., Nardi et al., 2006; Suh et al., 2003, 2005). Fungi also are cultivated by fungus-farming termites and ants (Aanen et al., 2002; Currie et al., 2003; Dentinger et al., 2009; Munkacsi et al., 2004) (Box WO-1).
Not all fungal–insect associations are mutualistic. Blackwell described the parasitic but not usually pathogenic fungi in the order Laboulbeniales. She noted the reports of extreme host specificity exhibited by different species in this order—sometimes inhabiting only certain parts of the host insect (Weir and Beakes, 1995). Most laboulbenialean species are associated with beetles (Coleoptera), and flies (Diptera), but they are also associated with a diverse array of host species in other insect orders, mites and millipedes (Weir and Beakes, 1995).
Blackwell discussed a number of fungal–plant symbioses. She estimated that:
- Half of all ascomycetes (Phylum Ascomycota) are lichens [symbiotic associations between fungi and photosynthetic partners (algae)] (Lutzoni et al., 2001; Schoch et al., 2009);
- 90 percent of all photosynthetic plants have mycorrhizal associates (Ruehle and Marx, 1979); and
- 95 percent of all plants have fungal endophytes (Arnold, 2007; Rodriguez et al., 2009).
Endophytes—fungi that live inside the plant tissue but without causing any obvious negative effects—are less well known than other plant–fungal associations, but mycologists find them wherever they look (Arnold et al., 2003; Rodriguez et al., 2009). Numerous endophytic fungal infections have been observed in cocoa trees (Theobroma cacao) and they may play an important role in host defense by decreasing the damage associated with Phytophthora spp. infections (Arnold et al., 2003). To illustrate the complexity of these relationships, Blackwell noted interactions among the fungus Curvularia protuberata, the grass Dichanthelium lanuginosum,13 and a fungal virus. The grass infected with the fungus infected with “Curvularia thermal tolerance virus” provides thermal resistance benefits for the host plant. This tripartite relationship allows the grass to grow in the high-temperature soils of Yellowstone National Park (Márquez et al., 2007). Blackwell pointed to the red-cockaded woodpecker (Picoides borealis) as just one example of the many ways that fungi confer benefits to the health of ecosystems. These woodpeckers usually nest in trees infected with red heart rot (Phellinus pini) (Hooper et al., 1991).
______________
13 Commonly referred to as Panic Grass.
BOX WO-1
The Fungal Gardens of Leafcutter Ants
Over the past 50 million years, a unique symbiosis has developed between attine (fungal growing) “leafcutter” ants and fungi in the Lepiotacea family. In what biologists consider the earliest form of agriculture, leafcutter ant colonies grow and meticulously maintain a specific fungal cultivar for food (Schultz and Brady, 2008; Wade, 1999).
Inhabiting forest ecosystems throughout Mexico and Central and South America, these ant colonies can number more than 8 million individuals. Foraging ants bring cut pieces of leaves back to the colony where they are broken down and fed to the fungus by worker ants (see Figure WO-1-1).
A second symbiotic relationship protects these fungal gardens. Pseudonocardia bacteria, which grow on the bodies of the worker ants, produce antibiotic compounds that prevent the growth of parasitic molds (Currie et al., 1999).

Figure WO-1-1 Leafcutter ants tending their fungal garden.
SOURCE: © Alex Wild.
For more information on leafcutter ants, visit the PBS video segment: “Ancient Farmers of the Amazon,” © WGBH Educational Foundation and Clear Blue Sky Productions, Inc., 2001, available at: http://www.youtube.com/watch_popup?v=RH3KYBMpxOU&vq=medium#t=11.
Or, use your smart phone to link directly to the video using the QR code at right:

Fungi as Pathogens
The longstanding utility of fungi to all life on earth has often been matched by their ability to directly or indirectly cause devastating disease in human, animal, and plant hosts. Fungi are the predominant pathogen species in plants, remarked Casadevall, and fungi can also cause disease in healthy humans and animals. Described by several workshop participants as “formidable pathogens,” many fungi can also endure adverse environmental conditions and thrive outside of their host (Casadevall, 2007).
Fungal pathogens in general execute a series of sequential steps in order to cause disease, remarked speaker Barbara Howlett of the University of Melbourne. (Dr. Howlett’s contribution to the workshop summary report can be found in Appendix A, pages 264–273.) These pathogens must:
- Recognize and attach to the host;
- Germinate, colonize, and derive nutrition from the host;
- Subvert host defense responses;
- Reproduce, exit, and disperse; and
- Find another host14 (Sexton and Howlett, 2006).
Very few fungal pathogens are able to cause disease in hosts from the plant and animal kingdoms; those that do are referred to as trans-kingdom pathogens (De Lucca, 2007).15 Fungi can also form different associations with different host types. For example, the fungus Cryptococcus gattii is pathogenic in animals including humans, but forms non-pathogenic associations with plants –which play an essential role in the maintenance of C. gattii spores in certain environmental niches (Bartlett et al., 2007; Xue et al., 2007).Once outside of a host, fungal pathogens of animals and plants often have different requirements for survival. Animal pathogens, noted Howlett, are often soil saprophytes that are free-living rather than obligate.16 In contrast, some plant pathogens can only survive on the tissue of a specific plant host(s).
______________
14 For more information, see contributed manuscript by Barbara Howlett in Appendix A (pages 264–273).
15 Howlett noted two trans-kingdom pathogens during her remarks: Fusarium oxysporum f. sp. lycopersici, which causes vascular wilt in plants and is an emerging human pathogen (Ortoneda et al., 2004); and Aspergillus flavus, which infects corn and is an emerging pathogen in immunocompromised humans (Krishnan et al., 2009).
16 Capable of existing only in a particular environment; an obligate parasite cannot survive independently of its host (Science dictionary).
Fungal Pathogens of Plants
In addition to contributing heavily to annual losses in global crop production,17 fungal plant pathogens are associated with many notable episodes of human suffering and economic and ecological loss, including:
- Irish Potato Famine: The mid-19th-century epidemic of potato late blight in Ireland led to the Irish Potato Famine, which caused or contributed to the starvation and death of well over 1 million people and the emigration of another 1 million (Money, 2007; Vurro et al., 2010). At the time of the Potato Famine, one-third of Ireland’s population of eight million was dependent upon the potato as a primary food source (Money, 2007) (Figure WO-3). See also Large (1965) and Woodham-Smith (1962).
- Southern corn leaf blight: The 1970 southern corn leaf blight epidemic led to the loss of 710 million bushels of corn—valued at more than $1 billion at the time, or about $5.6 billion in 2009 dollars (Tatum, 1971).
- Dutch elm disease: The impact of Dutch elm disease extends well beyond the death of 100 million mature elm trees in the midle of the 20th century. It not only transformed the landscape of cities and forests, but it has continued to alter associated ecosystem dynamics to this day through reduced food sources and nesting sites for wildlife, altered tree composition, and density (Loo, 2009; Money, 2007).
Fungal plant diseases have far-reaching health impacts that extend beyond the infected plant species—including, but not limited to, negative impacts on associated flora and fauna (Giraud et al., 2010; Loo, 2009). As the Irish Potato Famine illustrated, crop losses can have devastating impacts on populations that are heavily, or solely, dependent on a single food source for their caloric needs. Speaker Jim Stack of Kansas State University observed that with 59 percent of calories consumed by humans being derived from just four plant species (rice, wheat, maize, and potatoes), fungal diseases in these staple crops may catastrophically threaten local and global food security (Strange and Scott, 2005; Vurro et al., 2010). (Dr. Stack’s contribution to the workshop summary report can be found in Appendix A, pages 273–296.)
Fungal Pathogens of Humans and Animals
Given the ubiquity and diversity of fungi, it is perhaps surprising that, of the nearly 1,400 recognized human pathogens, a little more than 20 percent (~ 325) are fungal, and fewer than a dozen are associated with “life-threatening” disease (Casadevall, 2007; Woolhouse and Gaunt, 2007). Historically, fungal diseases
______________
17 Crop losses due to all pathogens (1988–1990) totaled $33 billion for rice, $14 billion for wheat, $7.8 billion for maize, and $9.8 billion for potatoes (Oerke et al., 1995; Rosenzweig et al., 2001).

Figure WO-3 Depiction of starving Irish children in 1847 potato famine; by Cork artist James Mahony (1810–1879).
SOURCE: Wikimedia Commons.
of humans have had a lower disease burden than bacterial, viral, or parasitic infections, although this disease burden may be changing. It has been noted that fungal diseases are increasing in incidence in the growing populations of immunocompromised human hosts (Romani, 2004). Once established, fungal diseases are often difficult to treat (Casadevall, 2007; Romani, 2004).
Disease in humans most often results from opportunistic18 infections (Shoham and Levitz, 2005). Only a few fungal diseases (e.g., coccidioidomycosis, histoplasmosis) are caused by “primary” fungal pathogens19 that induce symptomatic disease in otherwise healthy people (Casadevall, 2007; Cutler et al., 2007). The “apparent” resistance of humans to fungal disease may be a reflection of the host immune response, coupled with the high basal temperature of mammals, which often exceeds the thermotolerance20 range for many fungi (Casadevall, 2005; Garcia-Solache and Casadevall, 2010; Robert and Casadevall, 2009).
Primary fungal pathogens of humans can also infect other mammals, such as domesticated livestock and companion animals. These diseases are generally not considered contagious and are acquired via inhalation of aerosolized infectious propagules21 from environmental reservoirs, such as soil or trees (Casadevall and Pirofski, 2007). According to speaker Luis Padilla of the Smithsonian Conservation Biology Institute, wildlife are also affected by opportunistic and primary fungal pathogens, but the epidemiology of these diseases in wildlife is not well understood. (Dr. Padilla’s contribution to the workshop summary report can be found in Appendix A, pages 296–312.) Two fungal diseases of wildlife, amphibian chytridiomycosis and bat white-nose syndrome, emerged rapidly and unexpectedly over the past several decades. These diseases are associated with unprecedented local and global population declines of amphibian and bat species, and pose serious threats to biodiversity and ecosystem stability (Frick et al., 2010; Wake and Vredenburg, 2008).
Fungal Pathogens as “Invasive Species”
“Fungi are the only group of organisms that have been convincingly shown to cause extinction,” Casadevall remarked, referring to the extinction of the land snail Partula turgida by a parasitic microsporidian fungus (Cunningham and Daszak, 1998). As Casadevall observed, this capacity for destruction may be
______________
18 Resulting from pathogen entry via wounds or weakened state of the host, or as a disturbance of a normally benign host–fungus relationship.
19 Medically important fungi can be categorized as opportunists or primary pathogens. The opportunists rarely cause disease in an immunocompetent host whereas the primary pathogens do. For more information see: Cutler et al. (2007).
20 Garcia-Solache and Casadevall (2010) define thermotolerance as the ability to grow at mammalian (37°C) and higher temperatures. Most fungi thrive in the range of 12°C to 30°C, but there are wide temperature tolerances among species, with some growing at temperatures as low as –10°C or as high as 65°C. See contributed manuscript by Casadevall in Appendix A (pages 181–188).
21 Spores or encapsulated yeast cells.
due, in part, to the fact that “when [fungal pathogens] get into an ecosystem—a vertebrate host, for example—they simply don’t care. They have no need for that host in order to go forward. They will take down every last member of the species.” In contrast, most newly introduced viral and bacterial pathogens in a naïve host eventually attenuate their virulence such that infection does not kill the host. Such adaptations are beneficial to both the host and pathogen in that the host survives and the pathogen avoids an “evolutionary dead end” (IOM, 2009). As noted by Casadevall and Pirofski (2007), the host independence of “environmental” microbes,22 including many fungi, may confer advantages that promote survival and virulence in other niches, including new ecosystems and novel host species.
The term “invasive species” is used to describe “non-native”23 plants and animals that, when introduced to new environments, reproduce or spread so aggressively that they harm their adopted ecosystems (Carlton, 2004; Dybas, 2004). They compete with native organisms for food and habitat, act as predators or parasites of native species, and cause or carry diseases, often with devastating ecological and economic consequences (Pimentel et al., 2005). As observed by Morse (2004), infectious diseases represent another form of biological invasion—often arising “out of nowhere” with devastating effects.
Discussions during the workshop illuminated the capacity of many fungal pathogens to persist in environmental reservoirs and to readily adapt to new environmental niches and host species. Like invasive species, these fungal pathogens have been able to thrive in new environments and are changing the ecosystem in ways that are difficult to anticipate and even more daunting to prevent (Desprez-Loustau et al., 2007; Giraud et al., 2010; Rizzo, 2005). Given both the links and similarities between invasive species and many pathogenic fungi, it may be useful to view the origins of disease emergence, and the strategies deployed to prevent or mitigate the threats associated with fungal pathogens, through the larger lens of biological invasiveness.
FACTORS OF EMERGENCE
Diseases are categorized as “emerging” if their incidence24 or virulence25 has recently increased or if they begin to infect a novel host or population (WHO, 2010). As illustrated in Figure WO-4, disease26 results from a complex interplay of interactions among the pathogen, host, and environment.
______________
22 Microbes acquired from the environment (in contrast to acquisition from other living hosts) (Casadevall and Pirofski, 2007).
23 Also called “exotic,” “alien,” and “nonindigenous” species.
24 As used in epidemiology, the number of new cases of a disease that occur in a defined population within a specified time period; the rate of occurrence (IOM, 1992).
25 The degree of pathogenicity of an organism as evidenced by the severity of resulting disease and the organism’s ability to invade the host tissues (IOM, 1992).
26 A situation in which infection has elicited signs and symptoms in the infected individual; the infection has become clinically apparent (IOM, 1992). Some exposures to infectious disease-causing agents can also produce asymptomatic illnesses that can be spread to others.
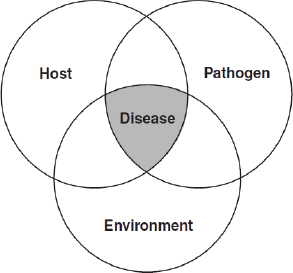
Figure WO-4 The epidemiological triad. The familiar “epidemiological triad” concept (host–pathogen–environment), as illustrated in the famous diagram of Snieszko (1974), neatly illustrates the complex interplay of factors that result in disease at the individual and population levels. The presence of a pathogen is a necessary, but not sufficient, cause of a particular disease (IOM, 2008b).
SOURCE: Snieszko (1974, Figure 1).
The range of factors identified as influencing the interactions between these elements (see Box WO-2) underscores the fact that exposure to a potential pathogenic agent is a necessary but insufficient condition for infectious disease emergence (IOM, 2003).
Significant factors for infectious disease emergence include the introduction of a pathogen into a new ecosystem or the disruption of an established ecosystem (IOM, 1992, 2003, 2010; Woolhouse and Gaunt, 2007). Such changes often expose immunologically naïve hosts to potential disease-causing organisms that have been released from the constraints imposed on them in their native environments (Woolhouse and Gaunt, 2007). Important catalysts for such disruptions and subsequent disease emergence include human activity, weather, and climate (Anderson et al., 2004; Daszak et al., 2000; Harvell et al., 2002; IOM, 2008a, 2010).
Anthropogenic and environmental factors play integral roles in the introduction and spread of many emerging fungal diseases. In recent years, the emergence of new plant diseases has been attributed to the evolution of hybrid pathogen species (Brasier, 2000). These hybrid species are thought to result from trade-mediated geographic redistribution of plants infected with the parental
BOX WO-2
Factors in the Emergence of Infectious Diseases
Thirteen factors of emergence of infectious diseases were elucidated in a 2003 Institute of Medicine report, Microbial Threats to Health: Emergence, Detection, and Response:
- Microbial adaptation and change
- Human susceptibility to infection
- Climate and weather
- Changing ecosystems
- Human demographics and behavior
- Economic development and land use
- International travel and commerce
- Technology and industry
- Breakdown of public health measures
- Poverty and social inequality
- War and famine
- Lack of political will
- Intent to harm
__________________
SOURCE: IOM (2003).
pathogens27 (Brasier, 2000). As Rizzo noted, “for some of these tree pathogens, I don’t think there is anything extraordinary about the pathogens themselves.” Rather, “it is the movement of pathogens from one environment to another that seems to be driving much of the destruction.”
As described below, discussion at the workshop considered the influence of human activity and behavior, winds and weather, host susceptibility, and pathogen adaptation and change on fungal disease emergence.
Human Activity and Behavior
During the past century, human activities have dramatically influenced local environments and ecosystems, breaking down natural habitats and exposing new
______________
27 Stack explained that interspecific hybridization is another unexpected outcome of pathogen globalization. Two different (and previously isolated) fungal species mate and produce novel “hybrid” offspring. Fungal pathogens of plants produced by interspecific hybridization are more aggressive than either parental phenotype and may occupy a new host range (see Brasier, 2000). Stack noted that even in a single nursery, the normal process of taking care of plants, which includes watering, can result in a water splash that brings two different fungal species together in a single pot, where interspecific hybridization can occur.
hosts to infectious disease agents (Anderson et al., 2004; Brasier, 2008; Daszak et al., 2000). Travel, trade, migration, agricultural practices, and land use patterns have all contributed to increased opportunities for contact between introduced pathogens and naïve and susceptible host populations (IOM, 2010, and references within).
Speaker Matthew Fisher of the Imperial College London, remarked that migrating humans have been globalizing pathogens for thousands of years. (Dr. Fisher’s contribution to the workshop summary report can be found in Appendix A, pages 355–367.) He pointed to the spread of Coccidioides posadasii that accompanied human migration between 5,000 and 10,000 years ago through North, Central, and South America as an example (Fisher et al., 2001). Stack agreed, noting that “global trade is not new; we have had 3,000 years of global trade.” He went on to state that, “what is new is the magnitude of trade in plants and plant products and the speed at which they move around the world” [emphasis added].
Trade, Travel, and Tourism
The increase in international transportation, travel, and trade associated with globalization in the 20th century has amplified the frequency of interactions between people, plants, animals, and microbes—providing novel opportunities for the rapid introduction, emergence, and spread of infectious diseases (IOM, 2010). The explosive growth of globalization—with dramatic increases in both the quantity and diversity of goods—has been enabled by a simultaneous decrease in travel time (IOM, 2010). Goods can be transported between most places in the world in less time than the incubation period for most infectious diseases (Cliff and Haggett, 2004; IOM, 2010). A study of factors associated with the emergence of diseases in crop plants demonstrated that the majority were spread via trade and travel (Anderson et al., 2004). Stack said this should not be surprising: In 2007 alone, the United States imported more than 48 million tons of agricultural products, only 1–2 percent of which were inspected for possible pathogens and other pests (Becker, 2009; Stack, 2010).28
Local and global transportation of ornamental plants, trees, and timber also contribute to the introduction and spread of fungal diseases. The pathogens responsible for Dutch elm disease and chestnut blight were transported to America in shipments of beetle-infested timber imported from Asia and live chestnut trees imported from Japan, respectively (Money, 2007). Molecular epidemiological analyses of many P. ramorum isolates support the hypothesis that nursery plants infected with Phytophthora ramorum were the initial “source” for the epidemic of sudden oak death that began in California in 1995 (Mascheretti et al., 2008). P. ramorum has since emerged in the United Kingdom and Europe and now infects
______________
28 According to Stack (2010), each year, 12,000–14,000 potential pathogen and pest problems are intercepted during these inspections.
more than 100 plant species (Grünwald et al., 2008). Stack noted that potential pathogens can be transported in plants, plant associated material (e.g., soil), seeds, and objects manufactured using plant products, such as wooden instruments and packing materials.
Human spatial mobility has increased at least 1,000-fold in the past 200 years, with more people traveling faster, farther, and less expensively than ever (Figure WO-5) (Cliff and Haggett, 2004; Hufnagel et al., 2004).
Travelers are now able to easily explore once-remote areas that serve as both sources and sinks for emerging infectious diseases (Choffnes, 2008; IOM, 2010). Adventure travelers intrude on once-remote environments and often make contact with exotic wildlife, encountering microbes that have never before been recognized as human pathogens in the “developed” world (IOM, 2010). These ecotourists become unwitting vectors of disease when they bring these exotic infectious diseases back with them—on their person/clothing/luggage, etc.—when they return to their home countries. If the conditions are favorable, an introduced pathogen may persist and spread (Wilson, 2003). White-nose syndrome, which is currently decimating New World bat populations in the United States, may have been accidentally introduced by recreational cavers from Europe29 (Wibbelt et al., 2010).
Infectious disease pandemics have also been associated with the legal and illegal trade in and transportation of animals (IOM, 2010; Karesh et al., 2005; Smith et al., 2009). Between 2000 and 2006, the United States traded approximately 1.5 billion animals, according to speaker and Forum member Peter Daszak of EcoHealth Alliance. (Dr. Daszak’s contribution to the workshop summary report can be found in Appendix A, pages 188-196.) These animals come from a wide range of species, and most animal imports into the United States come from emerging infectious disease “hot spots” (see Table WO-1) (Jones et al., 2008; Smith et al., 2009).
The international amphibian trade is thought to have contributed to the emergence and global spread of amphibian chytridiomycosis (Catenazzi et al., 2010; Daszak et al., 2003; Fisher and Garner, 2007; Schloegel et al., 2010; Weldon et al., 2004). Since the 1990s, this fungal disease has been implicated in the widespread population declines—including some local extinction events—of more than 200 species of frogs, toads, and salamanders (Fisher et al., 2009; Kilpatrick et al., 2009; Lips et al., 2006; Schloegel et al., 2006; Skerratt et al., 2007).
Speaker Ché Weldon of North-West University of South Africa noted the many points in the global amphibian trade pathway where traded species from different origins come into contact including collector-supplier facilities, breeding facilities, end-user facilities, etc. (Dr. Weldon’s contribution to the workshop summary report can be found in Appendix A, pages 355–367.) Weldon discussed two widely traded amphibian species—the African clawed frog, Xenopus laevis,
______________
29 Other possible explanations include the importation of horticultural soils from Europe.
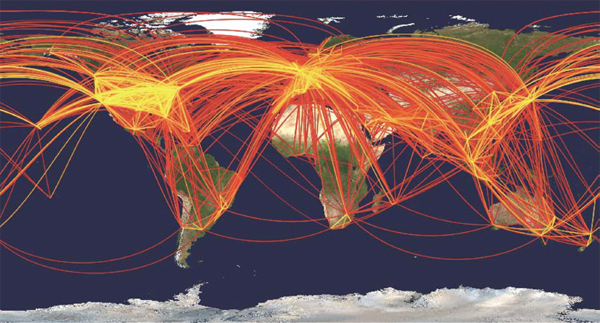
Figure WO-5 Global aviation network. A geographical representation of the civil aviation traffic among the 500 largest international airports in 100 countries is shown. Each line represents a direct connection between airports. The color reflects the number of passengers per day traveling between two airports, with the most intense traffic (25,000) noted in yellow.
SOURCE: Hufnagel et al. (2004).
TABLE WO-1 Number of Individual Animals Traded by the United States (2000–2006)
| Class | Import | Export |
| Amphibia | 27,631,172 | 1,594,961 |
| Annelida | 485,011 | 76,737 |
| Arachnida | 1,175,483 | 208,553 |
| Aves | 1,195,014 | 48,117 |
| Chilopoda | 4,358 | 274 |
| Cnidaria | 3,265,622 | 59,699 |
| Crustacea | 80,275,054 | 2,752,200 |
| Diplopoda | 13,926 | 1,218 |
| Echinodermata | 53,351 | 634 |
| Insecta | 469,606 | 88,686 |
| Mammalia | 184,682 | 32,879 |
| Merostomata | 60 | 0 |
| Miscellaneous | 5,430,083 | 154,195 |
| Mollusca | 3,187,671 | 555,829 |
| Null | 4,017,720 | 244,026 |
| Pisces | 1,316,977,591 | 138,404,653 |
| Polychaeta | 437 | 0 |
| Reptilia | 10,211,806 | 35984,895 |
| TOTAL | 1,458,805,947 | 180,207,556 |
| SOURCE: Daszak (2010). | ||
and the American bullfrog, Rana catesbeiana—that are “asymptomatic” carriers of the causative agent of amphibian chytridiomycosis Bd. X. laevis has been traded internationally since the 1930s (Weldon et al., 2007). Between 1998 and 2004 alone, more than 10,000 specimens of X. laevis were exported from South Africa to over 100 institutions in more than 30 countries worldwide (Weldon et al., 2007). Rana catesbeiana is one of more than 200 amphibian species in the international food trade, which altogether moves tens of millions of individual amphibians around the globe every year (Schloegel et al., 2010). He added that both of these species have now established feral populations in import countries, placing “native” species at risk of exposure to Bd (Weldon et al., 2007).
Winds and Weather
Along with anthropogenic introductions, wind and weather—including extreme weather events30—are associated with the introduction, establishment, and spread of fungal diseases (Anderson et al., 2004). Because many fungal patho-
______________
30 Includes weather phenomena that are at the extremes of the historical distribution, especially severe or unseasonable weather (e.g., extreme heat or cold, tropical cyclones, tornadoes). http://en.wikipedia.org/wiki/Extreme_weather.
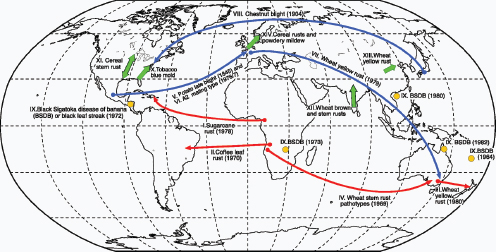
Figure WO-6 Selected dispersal events of fungal pathogens. Red and blue arrows indicate invasions of new territories (first year recorded in brackets). Red arrows indicate dispersal that probably occurred by direct movements of airborne spores (I, II, III, and IV). Blue arrows indicate pathogens that were probably transported to the new territory in infected plant material or by people and spread thereafter as airborne spores (V, VI, VII, and VIII). Orange circles indicate the worldwide spread of black Sigatoka disease of banana; the first outbreak on each continent is marked (IX). Green arrows indicate periodic migrations of airborne spores in extinction-recolonization cycles (X, XI, XII, XIII, XIV). SOURCE: From J. K. M. Brown and M. S. Hovmøller. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 29(5581):537–541, reprinted with permission from AAAS. Background map provided courtesy of Christopher Lukinbeal, University of Arizona.
gens are soil-associated, wind and other factors associated with soil disturbances can disperse spore-associated dusts into the air. Once airborne, spores may passively travel on the wind over great distances—often hundreds or thousands of miles—to new geographic areas and new host environments (Figure WO-6 [red]) (Brown and Hovmøller, 2002).
Aerial Dispersal—Winds and Extreme Weather Events
Limited dispersal of fungal spores carried by the wind is common and is considered a key factor in the local spread of some fungal diseases. Sporadic outbreaks of valley fever have occurred when spores of Coccidioides spp. are swept up from the soil and carried by winds to be inhaled by susceptible hosts. Long recognized as a threat to the health of military personnel stationed in arid regions of California, Valley Fever outbreaks have also been associated with land use changes and occupational or recreational exposures to dust (Chiller
et al., 2003; Crum-Canflone, 2007; Warnock, 2006). In 1994, the magnitude 6.7 Northridge earthquake led to an outbreak of valley fever in southern California (Figure WO-7A) (Schneider et al., 1997). More recently, the massive dust storm that swept through Arizona on July 5, 2011 is predicted to cause a similar increase in cases of valley fever (Chan, 2011) (Figure WO-7B). Speaker John Galgiani,31 of the University of Arizona, explained that even small winds or soil disturbances can easily loft spore-laden dusts into the air. (Dr. Galgiani’s contribution to the workshop summary report can be found in Appendix A, pages 196–207.) Galgiani further observed that inhalation of a single spore “at the right time” can cause disease. Approximately 40 percent of infected persons develop symptoms, which initially manifest as pneumonia (i.e., cough, chest pain, fever, and weight loss); fatigue; bone and joint pains (“desert rheumatism”); or skin rashes (Hector and Laniado-Laborin, 2005; Tsang et al., 2010). While dust storms and environmental disturbances are clearly an important driver of the spread of Coccidioides spp., Galgiani said that simply living in an endemic region,32 without any direct contact with the soil, puts one at risk of exposure. Yet the fungus is only sparsely distributed. Galgiani noted that, “you can do a lot of desert digging and disrupting and not even be close to the fungus.”33
Airborne spore dispersal may also synergize with intercontinental trade and travel to rapidly spread diseases between and within continents (Figure WO-6 [blue]). Yellow rust (Puccinia striiformis f. sp. tritici) is believed to have been introduced into Western Australia from southern Europe, in 1979, as an adherent spore on an air traveler’s clothing (Wellings, 2007). Once introduced into Australia, the pathogen spread across Australia’s wheat belt and into New Zealand via wind dispersal (Brown and Hovmøller, 2002). Indeed, winds allow many agriculturally important fungal plant diseases to gradually expand their geographic range (Brown and Hovmøller, 2002).
Pandemics caused by intercontinental aerial dispersal of spores can and do occur—often facilitated by hurricanes and other extreme weather events. Examples include:
- Sugarcane rust (Puccinia melanocphala) is believed to have been introduced from West Africa into America by cyclonic winds (Figure WO-6 [red]) (Brown and Hovmøller, 2002).
______________
31 Dr. Galgiani is also Chief Medical Officer at Valley Fever Solutions, Inc. which has licensed the development of nikkomycin Z as a treatment for valley fever from the University of Arizona.
32 Although widely perceived as endemic to the southwestern United States, Galgiani observed that the endemicity of the disease extends through Mexico into Central and some parts of South America (Tsang et al., 2010).
33 It has been suggested that this spotty distribution is a result of the abundant fungal sporulation that may accompany fungal decomposition of infected animal remains (whether or not fungal infection was responsible for an animal’s death) (Sharpton et al., 2009).

Figure WO-7 Environmental disturbances and dust storms contribute to the dispersal of fungal spores. (A) Dust from landslides caused by a 5.6 magnitude aftershock of the 1994 Northridge earthquake blows out of the Santa Susana Mountains into the Simi Valley. An outbreak of valley fever occurred in the Simi Valley following the January 17, 1994, 6.7 magnitude Northridge earthquake. (B) The leading edge of a violent dust and sand storm (known as a haboob). The wall of dust and sand that swept through Arizona on July 5, 2011, was estimated to be more than 50 miles wide. The storm travelled over 150 miles and reached peak heights of 8,000 to 10,000 feet.
SOURCE: Photos courtesy of Tom Freeman; National Oceanic and Atmospheric Administration.
- Coffee leaf rust (Hemileia vastatrix) may have been transported via transatlantic winds between Angola to Bahia in Brazil in 1970 (Figure WO-6 [red]) (Brown and Hovmøller, 2002).
- Asian soybean rust was brought into the United States from South America by Hurricane Ivan in 2004 (Schneider et al., 2005).
Some scientists are concerned that the frequency and duration of such extreme weather events could increase with global climate change, which in turn could influence the incidence and intensity of fungal disease outbreaks (Garrett et al., 2006; Greer et al., 2008).
Temperature, Humidity, and Climate Change
Like most microorganisms, fungi are highly sensitive to changes in weather and climate34—particularly temperature, humidity, and wind—that can directly influence their growth, spread, and survival (Harvell et al., 2002). One of the most tragic outcomes of a weather-induced fungal disease outbreak was the Irish Potato Famine, in which a sustained pattern of cool, rainy weather enabled the emergence and spread of the “fungus-like” oomycete,35Phytophthora infestans, the causative agent of potato late blight (Fry and Goodwin, 1997; Large, 1965; Woodham-Smith, 1962). In 1845 and 1846, late blight led to yield reductions of 40 and 90 percent, respectively, in the potato—at that time Ireland’s staple food crop (Money, 2007). As previously noted, the resulting “Great Famine” led to the death of more than 1 million and the emigration of over 1 million more Irish people, primarily to the United States (Strange and Scott, 2005; Vurro et al., 2010).
When combined with reduced genetic diversity in the host plant, weather can contribute to a “perfect storm” for a devastating agricultural disease epidemic (Rosenzweig et al., 2001; Vurro et al., 2010). Unusually warm, moist weather, coupled with a wholly susceptible host, provided the ideal conditions for the emergence and spread of Helminthosporium maydis (also known as Cochliobolus heterostrophu and Bipolaris maydis), the causative agent of Southern corn leaf blight (SCLB) (Rosenzweig et al., 2001). Over the course of the 1970–1971 growing season, the SCLB epidemic spread from the tip of Florida up to Alberta, Canada, destroying a significant proportion of the corn crop in its path (Ullstrup, 1972). Yield reductions were most severe in the southern states, with many farms
______________
34 As explained on the National Aeronautics and Space Administration website (www.nasa.gov), the difference between weather and climate is a measure of time. Weather is the state of the atmosphere over a short period of time; climate is how the atmosphere “behaves” over relatively long periods of time.
35 As noted by speaker David Rizzo, Phytophthora spp. is not a “true fungus”; it is an oomycete or “water mold” that belongs to the Kingdom Stramenopila (a major eukaryotic group that includes diatoms and brown algae, and is distinct from plants, fungi, and animals). Like fungi, oomycetes “exhibit filamentous growth, produce sexual and asexual spores, and can feed on decaying matter or be obligate parasites of plants” (Kliejunas, 2010).
experiencing total crop loss. Average yield loss in the Corn Belt states36 was 20–30 percent, with some parts of Illinois and Indiana reporting yield losses of 50–100 percent (Ullstrup, 1972). In the 1970 season alone, the SCLB epidemic led to the loss of 710 million bushels of corn—valued at more than $1 billion at the time (or about $5.6 billion in 2009 dollars) (Tatum, 1971).
Compton Tucker, of the National Aeronautics and Space Administration (NASA) Goddard Space Flight Center, presented data from a variety of satellite and ground sources37 documenting increases in global temperatures worldwide, as well as changes in the atmospheric concentration of carbon dioxide. (Dr. Tucker’s contribution to the workshop summary report can be found in Appendix A, pages 324-342.) He also explained how general circulation models, which simulate the atmosphere, accounting for wind, humidity, clouds, temperature, composition of the atmosphere (e.g., presence of trace gases), and other weather-related variables, can be used to predict where on the surface of the earth (both land and water) temperature and precipitation levels are likely to change.38 According to Tucker, these models predict that over the next century, average surface temperatures will increase by 2–5°C, and regions of the world will get wetter or drier (Figure WO-8).
Fungal diseases are influenced by weather fluctuations and display “seasonality”—suggesting the possible influence of long-term climate changes (IOM, 2003, 2008a; Rosenzweig et al., 2001). Stack noted that the onset of potato late blight has been occurring earlier and earlier over the past 20 years in some regions of the world and has resulted in more severe losses and greater mitigation challenges (Hannukkala et al., 2007). In part, this is due to changing temperatures and increased frequency of precipitation (Hannukkala et al., 2007).
Stack observed that modeling studies predict many negative impacts on plant health in response to climate change, including shifts in the range, timing, and severity of fungal diseases of plants39 (Jeger and Pautasso, 2008; Pautasso et al., 2010). A 3°C increase in temperature, for example, is anticipated to alter the phenology40 and conditions of the host species enough to result in expansion of the geographic range of Phytophthora cinnamomi, which has already decimated forests across southeastern Australia (Lonsdale and Gibbs, 1996). An enormous effect is predicted for the severity of phoma stem canker (Leptosphaeria macu-
______________
36 The area in the Midwestern United States, roughly covering western Indiana, Illinois, Iowa, Missouri, eastern Nebraska, and Eastern Kansas, in which corn (maize) and soybeans are the predominant field crops (Encyclopedia Britannica: eb.com).
37 These data include NASA satellite data on solar irradiance (i.e., energy output of the sun); National Oceanic and Atmospheric Administration, NASA, and other surface data on land and ocean temperatures worldwide; U.S. military and other satellite and ground data on sea ice; sea-level data; NASA gravity data; and data on the atmospheric concentration of carbon dioxide and other components of the atmosphere.
38 See also contributed manuscript by Tucker in Appendix A (pages 324–342).
39 See contributed manuscript by Jeger in Appendix A (page 273–296).
40 The scientific study of cyclical biological events, such as flowering, breeding, and migration.
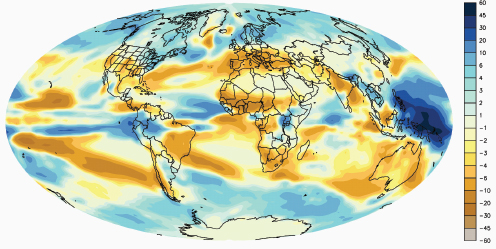
Figure WO-8 Change in precipitation between the 1971–2000 average and the 2091–2100 average in inches of liquid water/year.
SOURCE: Geophysical Fluid Dynamics Laboratory, National Oceanic and Atmospheric Administration.
lans) on oilseed rape, with many regions of the United Kingdom expected to experience a 40–50 percent yield loss by 2050 (Butterworth et al., 2010).
Modeling studies predict that it is not just the plant pathogens themselves that are likely to be impacted by continued climate change, Stack observed, but host species as well (Loustau, 2006; Pautasso et al., 2010). Stack remarked that while modeling studies forecast climate change effects on the distribution or severity of many fungal plant pathogens, for most crop plants the future is uncertain—both with regard to plant disease occurrence and the associated impacts on food security.
Host and Pathogen Characteristics
Whether caused by anthropogenic or natural forces, the mere introduction of a fungal organism is a necessary but insufficient condition for infectious disease emergence. Or, as viewed through the lens of biological invasion: Not all introduced species become “invasive.” Indeed, when introduced into new environments, invasive species become quickly established and spread in a new environment, while many other introduced organisms do not. As discussed at the workshop and summarized below, host and pathogen characteristics are important determinants for whether fungal pathogens will “thrive” in a new host or environment. For many emerging fungal pathogens, environmental factors have the greatest influence on the interactions between a naïve host and an introduced pathogen.
Host Defenses in Plants and Animals
As discussed previously, fungal diseases of plant or animal hosts involve several common steps (see “Fungi as Pathogens”). When it comes to host defenses, remarked Howlett, animals and plants have several important similarities and differences. Basal innate immunity41 is an important defense system shared by organisms that infect plants, animals, and insects, with immunity activated by recognition of pathogen-associated molecular patterns (PAMPs) (Nürnberger et al., 2004). Other plant defense systems include a complex physical barrier (a thick and impervious cuticle and cell wall), a repertoire of pathogen-specific resistance genes, and systemic acquired resistance (i.e., if one leaf is infected and the plant does not die, the plant mounts a strong immune defense in the event that another leaf is infected later). By contrast, animals have a less complex physical barrier (i.e., the skin and respiratory surface) and distinct innate immune system components (with the complement system and phagocytes and other circulating cells) as well as a battery of adaptive,42 antibody-mediated, immune system defenses (i.e., T and B cells) (Sexton and Howlett, 2006). “Most serious human fungal diseases occur in immunocompromised hosts,” noted Howlett, suggesting that “the mammalian immune defense system is very effective.”
Fungal Disease and the Mammalian Immune System
Fungal disease in humans usually reflects some underlying immune dysfunction (Holland and Vinh, 2009). Speaker Steven Holland of the National Institute of Allergy and Infectious Diseases noted several examples of fungal diseases in otherwise healthy individuals that were ultimately associated with previously unknown primary immune disorders.43 (Dr. Holland’s contribution to the workshop summary report can be found in Appendix A, pages 248–252.) Holland described a healthy and young individual with no previously recognized immunodeficiency who presented to an emergency department with acute shortness of breath that rapidly progressed to severe respiratory distress. The woman was eventually diagnosed with chronic granulomatous disease (CGD).44 Infection by the ubiquitous fungus, Aspergillus fumigatus, probably occurred when she was handling soil and plant debris and led to the onset of symptoms (Siddiqui et al., 2007). Holland also reviewed the discovery of rare genetic immune deficiencies that
______________
41 Immune response (of both vertebrates and invertebrates) to a pathogen that involves the preexisting defenses of the body (e.g., barriers formed by skin and mucosa, antimicrobial molecules and phagocytes). Such a response is not specific for the pathogen (Alberts et al., 2002).
42 Response of the vertebrate immune system to a specific antigen that typically generates immunological memory (Alberts et al., 2002).
43 See contributed manuscript by Holland in Appendix A (pages 248–252).
44 CGD is characterized by recurrent bacterial and fungal infections and inflammatory complications (Holland, 2010; Holland and Vinh, 2009).
underlie two serious diseases associated with fungal infection: Job’s syndrome45 and severe coccidioidiomycosis (Buckley et al., 1972; Davis et al., 1996; Holland et al., 2007; Vinh et al., 2009).
The incidence of opportunistic fungal infections46 has increased recently and is associated with the growing populations of vulnerable, immunocompromised individuals (e.g., people living with HIV/AIDS, recent organ transplant recipients) (Romani, 2004). In Casadevall’s opinion, the period since the 1950s should be viewed as a transition decade in which “fungi become more important to human health” (Figure WO-9).
In the 1950s, only about 100 reported cases of disease were caused by Cryptococcus neoformans, Casadevall observed; today, there are about 1 million cases worldwide, mostly among persons with HIV/AIDS (Park et al., 2009). The yeast infection caused by Candida spp. was also uncommon until the 1950s. Many have associated the increase in Candida infections to the increased number of immunocompromised individuals (Dixon et al., 1996). Casadevall speculated that this may also be linked to the introduction of antibiotics, which altered the microbial flora in the human host.
Forum member Fred Sparling of the University of North Carolina, Chapel Hill, remarked “that there was significant cryptococcal disease47 in the pre-HIV era,” and that he continued to observe the disease in apparently healthy individuals. Holland agreed and noted that he expected that “we may find new mechanisms for susceptibility that might not be ‘Mendelian,’48 because it is not familial, but something that comes on, typically, in adulthood.”
Casadevall considers host immune status in humans so important in the development of fungal disease that, in his opinion, fungal virulence can only be properly defined as a function of it. Casadevall went on to explain that pathogenicity is not an invariant, absolute quality in an infectious disease agent, but that the pathogenicity of a microorganism varies depending on the host and over time (Casadevall, 2007). He reviewed the “damage response framework”—illustrated in Figure WO-10—that was developed by Pirofski and Casadevall as a way to illustrate these concepts (Casadevall and Pirofski, 2003; Pirofski and Casadevall, 2008).
Host damage can derive from either the pathogen (e.g., among immuno-
_______________
45 A rare, inherited disease associated with abnormalities of the skin, sinuses, lungs, bones, and teeth. People with this condition have chronic and severe skin infections (also known as hyper immunoglobulin E [IgE] syndrome). MedlinePlus: http://www.nlm.nih.gov/medlineplus/ency/article/001311.htm.
46 These opportunistic fungal diseases include invasive aspergillosis and aspergilloma (Aspergillus spp.), invasive fusariosis (Fusarium spp.), Pneumocystis pneumonia (Pneumocystis jirovecii), and invasive candidiasis (Candida sp.) (Nucci and Marr, 2005; Pfaller and Diekema, 2010).
47 Disease caused by Cryptococcus neoformans or gattii infection.
48 A single gene disorder caused by a defect in one particular gene, and characterized by how they are passed down in families. MedlinePlus: http://www.nlm.nih.gov/medlineplus/ency/article/002048.htm.
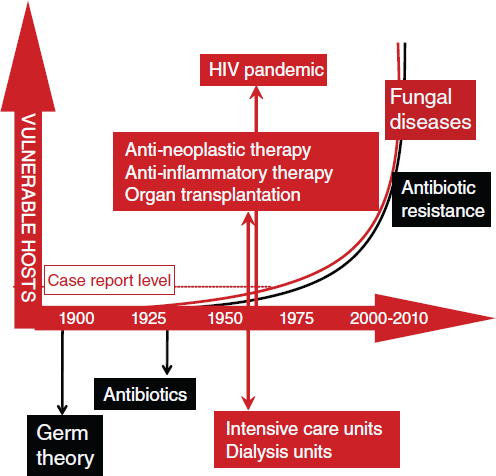
Figure WO-9 Incidence of systemic fungal disease has increased since the 1950s. Over the same period, the use of medical technology and the HIV/AIDS pandemic have led to an increased number of immunocompromised individuals. The emergence of systemic fungal disease in humans is considered by many to be a 20th-century phenomenon.
SOURCE: Adapted from Casadevall (2010).
compromised individuals with weak immune systems) or the host (e.g., among healthy host individuals whose microbial flora has been disturbed by antibiotic use, triggering a disproportionately strong immune response) (Casadevall and Pirofski, 2003). This model predicts that not just immunocompromised individuals are at risk of disease from fungal infection(s), but also healthy hosts who mount a disproportionately strong immune response (Casadevall and Pirofski, 2003).
Casadevall also suggested that there may be additional “subtle” effects of fungal infection that we are just beginning to observe on a population level.
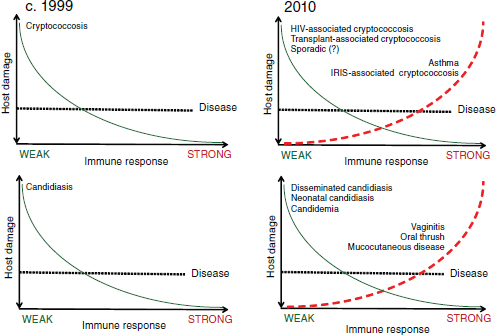
Figure WO-10 Damage response framework. When the framework was proposed in the late 1990s, conventional thinking was that the stronger the immune system response, the less damage to the host, as depicted in the two lefthand graphs (Cryptococcus spp. on the top, Candida spp. on the bottom). Since then, it has become clear that this is not the case and that people with very strong immune systems can also become sick, as depicted in the two righthand graphs. On the top righthand graph, notice that HIV-positive individuals with weak immune systems are at risk of HIV-associated cryptococcosis, but HIV-positive individuals with reconstituted immune systems (i.e., via antiretroviral therapy) are at risk of immune reconstitution inflammatory syndrome (IRIS)–associated cryptococcosis. On the bottom righthand graph, notice that candida vaginitis is believed to be associated with an overreactive immune system.
SOURCE: Casadevall (2010).
He noted that “we are dealing with things now that we never saw 30–40 years ago. The elimination of many viral and bacterial exposures, especially early in life, without the concomitant elimination of fungal diseases could be a factor in asthma and other atopic diseases.” Relman added, “We are not good at measuring subtle damage. If there are fundamentally important but less obvious forms of damage going on in the environment due to the emergence of fungi, we are not going to be very swift at detecting them, or insightful about understanding their implications.” Infectious propagules49 of C. neoformans spp. are everywhere, Casadevall stated, and “we are all exposed to them.” Despite this high level of
_______________
49 Spores or desiccated, encapsulated yeast cells.
exposure, Casadevall observed, C. neoformans infection rarely causes illness in non-immunocompromised individuals. Casadevall went on to suggest that asthma in children may be linked to an immune system that has been thrown out of immunological balance by chronic exposure to C. neoformans. To support this hypothesis, Casedevall pointed to two studies: Goldman et al. (2001) reported that C. neoformans infects the majority of immunocompetent children ages 2 and older,50 and animal studies that demonstrated that even when asymptomatic, chronic cryptococcal infection predisposes an individual to asthma (Goldman et al., 2006).
Microbial Flora
Host immune defenses against fungal disease extend to their microbial flora. As speaker Vance Vredenburg of San Francisco State University explained, amphibians “wear their defenses on their skin” (e.g., glands produce defensive toxins). (Dr. Vredenburg’s contribution to the workshop summary report can be found in Appendix A, pages 342–355.) Indeed, Brucker et al. (2008) isolated a strain of bacteria (Janthinobacterium lividum) from the skin of the red-backed salamander (Plethodon cinereus) and demonstrated that the bacteria produced antifungal metabolites at concentrations lethal to the causative agent of amphibian chytridiomycosis (Bd) (Figure WO-11).
Host Behavior and Thermal Tolerance
Other host characteristics including individual or group behavior can contribute in unexpected ways to disease establishment and spread. Several participants noted that behaviors such as clustering for warmth (amphibians) or during hibernation (bats) may increase opportunities for pathogen transmission between animals. According to Casadevall, having a body temperature that exceeds the thermal tolerance of fungi51 may also be a significant host defense (Robert and Casadevall, 2009). Casadevall explained that most fungi thrive in the temperature range of 12°C to 30°C. The mammalian body temperature of 37°C, he speculated, may represent a balance between warding off fungal infection (not too cold, or too close to ambient) and keeping metabolic costs down (not too hot) (Bergman and Casadevall, 2010).
_______________
50 Based on sera obtained from individuals who were being evaluated in an urban emergency department.
51 Robert and Casadevall (2009) found that of the 4,802 fungal strains examined (from 144 genera), most could not grow at mammalian temperatures, and that every “1°C increase in the 30°C–40°C range excluded an additional 6 percent of fungal isolates,” implying that fever could significantly increase the thermal exclusion zone. This led them to conclude that, “Mammalian endothermy and homeothermy are potent nonspecific defenses against most fungi that could have provided a strong evolutionary survival advantage against fungal diseases.” See contributed manuscripts by Casadevall in Appendix A (pages 177–188).
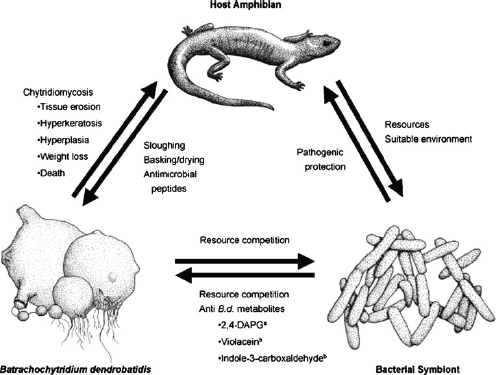
Figure WO-11 Microbial flora as a host defense. Components of a host’s microbial flora can be beneficial to the host by competing for resources or by secreting compounds that affect the survival of other, potentially harmful microbial components of the flora.
SOURCE: With kind permission from Springer Science+Business Media: Journal of Chemical Ecology, Amphibian Chemical Defense: Antifungal Metabolites of the Micro-symbiont Janthinobacterium lividum on the Salamander Plethodon cinereus, 34, 2008, 1422–1429, R.M. Brucker, Figure 1.
Pathogen Adaptation
Many fungi do not need a living host to survive. Fungi are well adapted to exploit winds and water as a means for their dispersal. Moreover, a variety of “environmental” cues trigger fungal growth, sexual and asexual reproduction, sporulation, and continued existence during adverse environmental conditions (Bahn et al., 2007; Judelson and Blanco, 2005; Kauserud et al., 2008; Kumamoto, 2008). In response to environmental stimuli, such as heat or drought, fungal organisms can become “dormant”—an inactive state during which growth and development cease but from which the organisms can be revived—or transform into forms that are resilient to heat, drought, and winds. As discussed at the meeting, the environment and environmental stimuli may also serve as a reservoir and
trigger for fungal pathogen adaptation and evolution (Casadevall, 2007; Lin and Heitman, 2006; Stukenbrock and McDonald, 2008).
Sexual reproduction in fungi typically requires the presence of two different mating types (Heitman, 2006). Two signals that regulate the sexual cycle of C. gattii are interactions with plants and extreme desiccation (Lin and Heitman, 2006; Xue et al., 2007). According to Heitman, evidence suggests that when only one mating type is present in an environment, C. gattii will adopt a “same-sex” mating strategy for reproduction (Fraser et al., 2005; Lin et al., 2005; Saul et al., 2008). This adaptability may be a widespread phenomenon, one that enables recombination, the generation of genetic diversity, and the geographic expansion of fungi (Heitman, 2006, 2009).52
Same-sex mating may have contributed to the expansion of C. gattii’s geographical range to Vancouver Island and the U.S. Pacific Northwest (Fraser et al., 2005). Heitman also discussed how recombination between C. gattii lineages of the same “sex” may have resulted in a “hypervirulent” recombinant genotype associated with the outbreak. Two of the three pathogen genotypes associated with the C. gattii outbreak (VGIIa and VGIIc) are considered highly virulent (Byrnes et al., 2010; Fraser et al., 2005). Moreover, VGIIa is considerably more virulent than VGIIa isolates from other parts of the world (Fraser et al., 2005). Although the reason why the VGIIa and VGIIc genotypes are so virulent is unclear, there may be a link between the capacity for mating and production of spores and virulence (Byrnes et al., 2010; Fraser et al., 2005; Lin and Heitman, 2006).
Howlett explained that plant breeders consider fungal pathogens to have a “high evolutionary potential’’ if organisms undergo prolific sexual reproduction and produce large numbers of genetically diverse spores that then act as inoculum. This capacity leads to frequent breakdowns in a host plant’s resistance to infection by particular strains of a fungal pathogen. Howlett also explained how the agricultural environment plays a role in the breakdown of resistance.
Agricultural crops are large swaths of genetically identical plants that “exert high levels of selection pressure” on populations of fungal strains produced during sexual reproduction. Of the billions of offspring produced, the few fungal strains that can infect these “resistant” plant strains will be amplified with each subsequent disease cycle. As the frequency of virulent pathogens increases, host resistance to disease eventually breaks down. Howlett noted that this is exactly what happened with Leptosphaeria maculans, the causative agent of blackleg in canola. In 2000, a new cultivar53 of L. maculans with a major resistance gene was released on the Eyre Peninsula, Australia. Within 3 years, the fungal pathogen had developed the capacity to overcome the host species’ genetic resistance resulting in yield losses of more than 90 percent (Sprague et al., 2006).
The environment can also serve as a reservoir for pathogen adaptation and
_______________
52 See contributed manuscript by Heitman in Appendix A (pages 226–248).
53 A variety of a plant that has been created or selected intentionally and maintained through cultivation.
evolution. Fungal pathogens that are free living in the environment may acquire what Casadevall called “accidental virulence.” He noted that the soil can be an extreme environment and that soil-dwelling microbes must adapt to rapidly changing, often harsh, conditions (Casadevall and Pirofski, 2007). Traits acquired in this environment, which allow fungal species to survive predation from amoeba and other protozoan organisms, may also contribute to virulence capabilities in hosts never before encountered by fungal pathogens. Casadevall suggested that the concept of “accidental virulence” might best describe how environmentally acquired fungi can be so virulent in “new” mammalian and other host organisms (Casadevall, 2007; Casadevall and Pirofski, 2007).
EMERGING FUNGAL DISEASES OF
HUMANS, ANIMALS, AND PLANTS
Several case studies of emerging fungal disease were discussed at the workshop. These case studies illustrate the many factors that influence disease emergence, the myriad direct and indirect impacts of fungal diseases on human and ecosystem health, and the challenges of detecting and responding to these infectious diseases.
Cryptococcus gattii
C. gattii already had the ability to survive in a wide range of environmental variations, but the Western North America outbreak teaches us that it may exploit hitherto unrecognized but clement environments and provide a wider exposure, and thereby, risk of infection to the human and animal populations.
—Datta et al. (2009b, p. 5)
Cryptococcus gattii (C. gattii) is a pathogenic, environmental fungus that emerged in humans and domestic animals on Vancouver Island, British Columbia, Canada, in 1999, causing a growing epidemic of human and animal infections and deaths. The fungus, which causes deadly infections of the lung and brain, had been previously restricted to the tropical or subtropical regions of Australia, the South Pacific, Southeast Asia, and Africa (Datta et al., 2009a,b). Since its initial recognition in 1999 as an emerging disease, the outbreak has spread from Vancouver Island to the British Columbia mainland and south into the Pacific Northwest of the United States (Datta et al., 2009b) (Figure WO-12).
According to speakers Julie Harris, of the Centers for Disease Control and Prevention, and Karen Bartlett, from the University of British Columbia, as of December 2010, this fungal pathogen has been associated with approximately 338 confirmed human infections and at least 40 deaths. (Dr. Harris’ contribution to the workshop summary report can be found in Appendix A, pages 207–225; Dr. Bartlett’s contribution to the workshop summary report can be found in
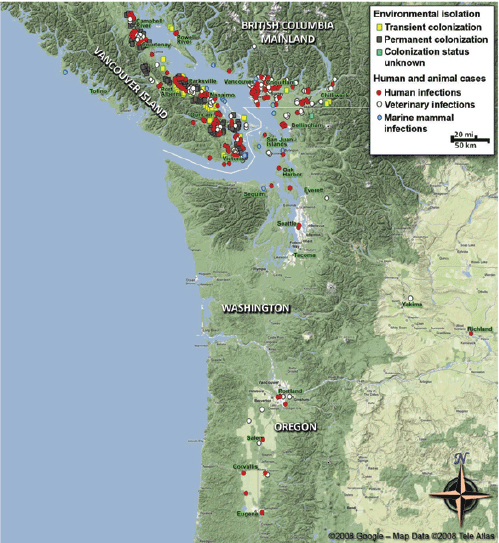
Figure WO-12 Map of the Pacific Northwest, comprising parts of British Columbia, Canada, and the states of Washington and Oregon in the United States, showing human and veterinary Cryptococcus gattii cases (including marine mammals) by place of residence or detection, and locations of environmental isolation of C. gattii during 1999–2008 (strain NIH444 [Seattle] or CBS7750 [San Francisco] not included). Data were collected from various state health departments and published reports referenced in the text. The map and icons have been used at a scale that shows gross geographic areas, effectively masking any personally identifiable patient locality information. Use of the map is courtesy of exclusive permission from Google Maps: ©2008 Google, map data ©2008 NAVTEQ.
SOURCE: Datta et al. (2009a).
Appendix A, pages 101–116.) Investigators still do not know the origins of the current epidemic, how C. gattii was introduced into the Pacific Northwest, or how this invasive fungal pathogen is spreading (Datta et al., 2009a).
Phenomenology
C. gattii is a basidiomycetous yeast that colonizes tree bark, decaying wood, and nearby soil and is a cause of cryptococcosis, a potentially fatal infection in humans and animals (Galanis and MacDougall, 2010; Levitz, 1991; Lin and Heitman, 2006; MacDougall et al., 2007). Before the current outbreak in British Columbia, Canada and the Pacific Northwest, the environmental source with which C. gattii had been most often associated was the wood, bark, and detritus of eucalyptus trees (Levitz, 1991). More recent and widespread global surveillance has established that the fungus also colonizes other tree species (Lin and Heitman, 2006).54
Individuals become exposed to C. gattii by inhaling the organism or its spores from soils or trees that have been colonized by the fungus (Lin and Heitman, 2006; Sorrell, 2001). Once inhaled, C. gattii can cause severe infection of the lungs and brain, including pneumonia, meningoencephalitis, and cryptococcomas. Unlike C. neoformans, which has become a major cause of death in HIV-infected individuals around the world, C. gattii also infects apparently healthy, immunocompetent individuals (Galanis and MacDougall, 2010). The disease affects a wide variety of humans and animals, but no case of transmission between animals and/or humans has ever been documented (CDC, 2010; Datta et al., 2009a).
Timely diagnosis of a C. gattii infection can be difficult. Patients infected with this fungal pathogen often remain asymptomatic for 6 months or more. When symptoms do present, fungal agents are not commonly considered by physicians when evaluating pulmonary disease in an otherwise healthy patient (Knox, 2010). Treating infected individuals can also be challenging because the disease tends to require prolonged antifungal therapy, sometimes with multiple drug courses (Iqbal et al., 2010; Sorrell, 2001). Some have suggested that when compared with C. neoformans, C. gattii infections tend to require more prolonged and invasive treatment (Sorrell, 2001). Harris remarked that “existing data suggest that not all cryptococcal infections are alike” and “it is not clear which factors are the most influential on the patient’s presentation—the species, subtype, host immune status, or host genetics, or some combination of factors.”55
_______________
54 See contributed manuscript by Bartlett in Appendix A (pages 101–116).
55 For more information, see contributed manuscripts by Harris and Heitman in Appendix A (pages 207–225 and 226–248).
Discovery and Spread
Veterinarians and clinicians first observed cases of C. gattii infections in animals and humans on Vancouver Island in 1999 (Datta et al., 2009a). Until 2004, all known human cases of C. gattii infection in the region occurred in individuals who either resided on or visited the island, specifically its eastern coast (Bartlett et al., 2007). In 2004, cases of C. gattii infections emerged on the British Columbia mainland in humans and animals that had not visited Vancouver Island, suggesting an expansion of the endemic zone of the fungus (Datta et al., 2009a; MacDougall et al., 2007). Also in 2004, cases of humans infected with C. gattii who had not traveled to British Columbia emerged in Washington and Oregon, marking the southern expansion of the fungus into the United States (CDC, 2010; Datta et al., 2009a; MacDougall et al., 2007). The emergence and recognition of a new, more virulent strain of the fungus accompanied C. gattii’s expansion into Oregon (Byrnes et al., 2009, 2010). Recently, investigators found evidence that the outbreak may have also expanded into other states, as researchers have collected isolates from humans and animals in California and Idaho (Iqbal et al., 2010).
Animal sentinels were instrumental to the study of C. gattii emergence and spread in British Columbia (Bartlett et al., 2007). Bartlett noted that “human cases, in all cases, were preceded by veterinary cases.” There are at least three to four times as many pet cases as human cases, she continued, and “it was a veterinarian that tipped us off that we had an outbreak.” Harris remarked that C. gattii “is not a picky pathogen,” infecting a wide variety of animals, including, but not limited to dogs, cats, dolphins, porpoises, elks, llamas, Bactrian camels, alpacas, horses, and sheep.
The exact means by which C. gattii spread from Vancouver Island to the British Columbia mainland, and south to the United States, remains unknown. Researchers suspect that a number of factors may be responsible, including human-mediated dispersal. A 2007 sampling study in British Columbia found C. gattii in areas of its endemic zone that were subject to high foot and vehicle traffic. These observations, combined with the finding of positive fungal samples on peoples’ shoes and in the wheel wells of vehicles that had been driven in fungal endemic regions, support the contention that dispersal may be partially anthropogenic (Kidd et al., 2007). Forestry activities could also facilitate C. gattii dispersal by aerosolizing fungus particles during tree cutting and/or mechanically “seeding” the fungus during the transfer of cut tree products, such as mulch, to new areas (Kidd et al., 2007). In addition to these human-mediated dispersal methods, Kidd and colleagues (2007) suggested that birds and animals might also play a role by passively transporting the fungus during migration.
As reviewed by Bartlett, environmental sampling of endemic areas has helped to describe how C. gattii is distributed in the environment and how easily it might spread. Sampling revealed high levels of C. gattii in the soil as well as its presence in the air, freshwater, saltwater, trees, and even dead wood, such as
fence posts, Bartlett remarked. Sampling results have also illustrated the effects of forestry activities on the abundance of C. gattii in the air (measured in colony-forming units,56 or CFUs). According to Bartlett, air samples taken in endemic areas, where trees were being removed, revealed a baseline concentration on the order of 100 CFU/m3, compared to 10,000 CFU/m3 during tree chainsawing and wood chipping in the same area (Kidd et al., 2007). Once it is in the air, Bartlett observed “the organism can travel 10 kilometers, easily, probably further than that.” The organism is also resilient: Bartlett noted that she can still isolate viable propagules from sawdust samples taken in 2001.
Origins of the Outbreak in the Pacific Northwest
The origins of the Pacific Northwest outbreak of C. gattii remain a mystery (Datta et al., 2009a). Some investigators have suggested that the fungus was introduced through the importation of contaminated trees, shoes, wooden pallets, or shipping crates (Kidd et al., 2007). Supporting this hypothesis is the finding that the VGIIb minor subtype found in British Columbia and the Pacific Northwest is similar and may be related to VGIIb strains found in Australia (Byrnes et al., 2010). Another origin hypothesis suggests that the VGIIa subtype has existed in the Pacific Northwest for some time. The latter hypothesis is supported by a case reported in 1971 of a patient in Seattle, Washington, who was infected with a VGIIa strain of C. gattii similar to the strain in the current outbreak (Byrnes et al., 2010; Datta et al., 2009a). However C. gattii was introduced, it is clear that the fungus is now established in the region and appears to be evolving into new, more virulent strains, as evidenced by the newly discovered and highly virulent VGIIc strain (Byrnes et al., 2010; Knox, 2010).
Perhaps more interesting than the question of how C. gattii was introduced to British Columbia and the Pacific Northwest is why it has now colonized the region. Bartlett emphasized that the fungus previously was endemic only in areas with tropical or subtropical climates—never in a temperate rainforest. Researchers have speculated that global warming may play a role, with the temperature in the region having increased enough for the fungus to become established (Bartlett et al., 2007; Kidd et al., 2004). Indeed, between 1998 and 2004, British Columbia experienced six consecutive seasons of above-average temperatures, with increases of more than 3°C in some seasons (Kidd et al., 2004).
Another possible explanation for C. gattii’s establishment in British Columbia and the U.S. Pacific Northwest is that the organism itself has adapted such that it can now successfully colonize a “novel” environment. Investigators have found environmental isolates of C. gattii in trees that had never previously been found to harbor the fungus, such as the Douglas fir and Western hemlock (Datta
_______________
56 CFUs are a standard unit of measurement for environmental sampling. Colonies reflect the number of “viable” organisms (i.e., organism capable of forming colonies when provided with nutritional elements necessary for growth).
et al., 2009a). Bartlett remarked that the environmental sampling data may help to define C. gattii’s ecological niche in British Columbia. The distribution of C. gattii in the environment, thus far, appears heterogeneous, with colonization levels differing significantly in regions such as the west and east coasts of Vancouver Island, which, observed Bartlett, are “dramatically different in terms of rainfall, soil type and vegetation” (Figure WO-13).
Further research into the reasons why C. gattii emerged in the temperate rainforest of the Pacific Northwest is needed because it could help researchers predict the fungus’s future spread and further the scientific community’s understanding of how environmental pathogens establish themselves in new environmental niches.
Molecular Epidemiology, Virulence, and Drug Resistance
Heitman explained that C. gattii spans four genetically isolated species groups: VGI, VGII, VGIII, and VGIV. Examination of the molecular genotypes of fungal isolates from infected patients reveals that nearly all of the observed infections in British Columbia and the U.S. Pacific Northwest have been caused
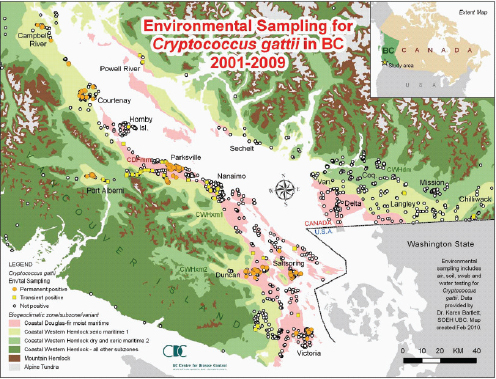
Figure WO-13 Environmental sampling for Cryptococcus gattii in British Columbia (2001–2009). Colonization levels of C. gattii reflect a heterogeneous distribution of C. gattii in the environment. Biogeoclimatic zones are also indicated.
SOURCE: Bartlett (2010).
by one molecular subtype of C. gattii—the VGII type (Byrnes et al., 2009). In other regions of the world where C. gattii is endemic, two other molecular subtypes predominate—VGI and VGIII (Byrnes et al., 2009; Kidd et al., 2004). The VGII genotype is further subdivided into three subtypes: the majority genotype VGIIa, which is unique to the Pacific Northwest region and not found in other endemic regions; the less common VGIIb genotype; and the VGIIc subtype, which has appeared in Oregon within the past several years (Byrnes et al., 2010; Kidd et al., 2004).
Some researchers have suggested that the predominant VGIIa and the newly discovered VGIIc C. gattii subtypes are more virulent than strains found in other endemic countries such as Australia (Byrnes et al., 2010). This is supported by the high rate of C. gattii infections in the current outbreak, which, at 25.1 cases/million people on Vancouver Island, is among the highest in the world (Galanis and MacDougall, 2010). Bartlett cautioned, however, that the high rate of C. gattii infection could be a result of increased surveillance or exposure, not increased virulence.
Recently, researchers have compared the drug susceptibility of the three VGII subtypes found in British Columbia and the Pacific Northwest to the more common VGI and VGIII genotypes. The VGIIc strain was found to be significantly more drug resistant to nearly all of the tested antifungal compounds (voriconazole, fluconazole, flucytosine, and amphotericin B) than the VGI or VGIII genotypes. The VGIIa and VGIIb strains were also observed to be more resistant to some antifungal drugs (fluconazole, flucytosine, and amphotericin B for VGIIa; fluconazole for VGIIb) than the VGI and VGIII strains, though their levels of resistance were lower than those of the VGIIc strain (Iqbal et al., 2010).
White-Nose Syndrome in Bats
Last year we estimate that we found between 10,000 and 20,000 dead bats on the cave floor…and to be honest the mortality is so disturbing.…We just can’t crawl through so many piles of dead bats.
—Scott Darling, Vermont Fish and Wildlife Department (Buchen, 2010, p. 144)
Since the winter of 2006, a mysterious and previously unknown disease—bat white-nose syndrome (WNS)—has decimated hibernating bat populations in the eastern and central United States. Named after the visually distinctive white fungus—Geomyces destructans—that grows on the muzzles, ears, and wings of affected bats, the disease has spread rapidly across the United States and Canada. Since it was first discovered, WNS has killed more than 1 million bats in the United States, with some hibernation sites (hibernacula) losing 90–100 percent of their bat populations (Figure WO-14) (FWS, 2011).
The population declines have been so rapid and dramatic that bat biologists

Figure WO-14 Signs of bat white-nose syndrome (WNS). (A) Mortality caused by WNS. (B) Geomyces destructans fungus, forming the visually distinctive white growth on the muzzle, ears, and wings of an infected bat.
SOURCE: Photos provided courtesy of Alan C. Hicks, New York State Department of Environmental Conservation.
at the U.S. Fish and Wildlife Service fear the extinction of entire New World57 bat populations in the United States and Canada (FWS, 2011).
Such extinctions could have devastating ecological and economic consequences. Bats play a critical role in plant pollination, seed dissemination, and the control of flying insects, including mosquitoes, moths, beetles, and other night-flying insect populations (Blehert et al., 2009; Boyles et al., 2011). Large-scale declines or complete disappearances of bat populations could result in reduced plant pollination (which is already under siege by colony collapse disorder in honeybees), significant increases in “nuisance” insect populations, and increased insect damage to agricultural and forestry resources (Blehert et al., 2009; Boyles et al., 2011; FWS, 2011). Because bats have very low reproductive rates—producing one pup a year, sometimes two, in a single litter—WNS is predicted to have long-lasting effects (Barclay et al., 2004; FWS, 2011). The value of bats to the agricultural industry has been estimated to be roughly $22.9 billion/year, with a range per year of $3.7 billion to $53 billion (Boyles et al., 2011).
_______________
57 Refers to the Western Hemisphere; in a biological context, New World species are those from the Nearctic Neotropic ecological zones, versus Old World species from the Palearctic and Afrotropic ecological zones.
Phenomenology
Geomyces destructans hyphae58 and conidia59 invade the hair follicles and sebaceous and sweat glands of bats hibernating in caves and mines with seasonal temperature ranges between 2°C and 14°C (Blehert et al., 2009). The skin of affected bats does not typically show signs of inflammation or an immune response at the site of fungal invasion (Meteyer et al., 2009). Hibernating bats infected with WNS often have severely depleted fat reserves, which are critical for successful hibernation (Blehert et al., 2009).60
Researchers have not yet confirmed whether Geomyces destructans is the primary pathogen that causes WNS and the eventual death of affected bats, or if it is an opportunistic infection that invades animals already immunocompromised by some other, yet to be defined pathogen (Puechmaille et al., 2010). How WNS kills bats is also unclear. Infection by the pathogen may irritate the animals, rousing them from hibernation, and causing them to deplete their fat reserves to such an extent that they are unable to survive through the winter (Blehert et al., 2009; Cryan et al., 2010; FWS, 2011). Speaker David Blehert of the National Wildlife Health Center at the U.S. Geological Survey presented recent research suggesting that the fungus causes damage to the wing epidermis and skin structures that help protect against water loss, causing bats to lose too much water to survive their winter hibernation (Cryan et al., 2010). (Dr. Blehert’s contribution to the workshop summary report can be found in Appendix A, pages 167–176.)
Discovery and Spread
WNS was first documented in February 2006 in a single cave near Albany, New York (FWS, 2011). Since then, WNS has spread rapidly across the United States and Canada, killing more than a million bats of six different species, making it the “worst wildlife health crisis in memory” (FWS, 2011). WNS is thought to spread primarily through bat-to-bat contact. However, human cavers and tourists may also be contributing to the spread of this pathogen by inadvertently transporting spores of Geomyces destructans from cave to cave on their clothing and equipment (Frick et al., 2010; FWS, 2011).
During routine hibernacula surveys in 2006–2007, biologists with the New York Department of Environmental Conservation discovered bats exhibiting signs of WNS in five caves, all within a 15-km radius of what is now recognized as the likely “index” site near Albany, New York, recounted Blehert. The following winter (2007–2008), researchers reported the discovery of WNS at 33 sites across
_______________
58 Slender tubes that develop from germinated spores and form the structural parts of the body of a fungus. A large mass of hyphae is known as a mycelium, which is the growing form of most fungi.
59 Asexually produced fungal spore. Most conidia are dispersed by the wind and can endure extremes of cold, heat, and dryness. When conditions are favorable, they germinate and grow into hyphae.
60 See contributed manuscript by Blehert in Appendix A (pages 167–176).
Connecticut, Massachusetts, New York, and Vermont—all within a 210 km radius of the index site (Blehert et al., 2009). By the end of the 2010–2011 hibernation season, the disease had been confirmed in bats in 18 U.S. states (FWS, 2011). The disease has also spread north into Ontario, Quebec, New Brunswick, and Nova Scotia in Canada (FWS, 2011) (Figure WO-15).
Blehert remarked that the magnitude of the mortality events being observed is not only unprecedented among U.S. bat species but also among the 1,100-plus bat species worldwide. Indeed, a recent modeling study predicted that for one of the most common bat species in North America—the little brown bat (Myotis lucifugus)—there is a 99 percent chance of regional extinction within the next 16 years as a result of mortality from white-nose syndrome (Frick et al., 2010).
Affected New World bat species include the big brown bat (Eptesicus fuscus), Eastern small-footed bat (Myotis leibii), little brown bat (M. lucifugus), Northern long-eared bat (M. septentrionalis), endangered Indiana bat (M. sodalis), and tricolored bat (Perimyotis subflavus) (Figure WO-16). Although disease pathology has not been confirmed, DNA from G. destructans has been detected
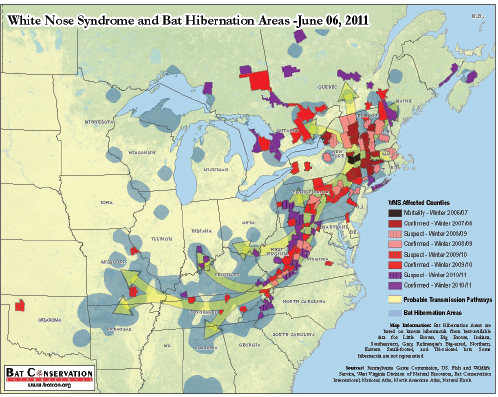
Figure WO-15 Spread of bat white-nose syndrome (WNS) in North America as of June 6, 2011.
SOURCE: Figure courtesy of Bat Conservation International, www.batcon.org.

Figure WO-16 Species affected by bat white-nose syndrome (WNS).
SOURCE: Merlin D. Tuttle, Bat Conservation International, www.batcon.org.
on skin samples from the endangered grey bat (M. grisescens), the southeastern bat (M. austroriparius) and the cave bat (M. veilfer) (FWS, 2011). Investigators are concerned that the endangered Virginia big-eared bat (Corynorhinus townsendii virginianus) may also be “at risk.” Although the fungus has been detected on other bat species that share their hibernacula, there has yet to be confirmed case of an infected animal (FWS, 2011).
Investigators are concerned that WNS could eventually spread to infect all 25 of the hibernating bat species native to the United States, threatening more than 50 percent of the native U.S. bat populations (Bat Conservation International, 2010). In late May 2010, FWS officials reported that a live bat from Oklahoma was PCR-positive for G. destructans DNA. This finding alarmed many, because the infected species, the cave bat M. velifer, frequently shares hibernacula with other bat species with migratory ranges that extend across the western United States into Mexico, increasing the potential for further spread of the disease to the west and south (Bat Conservation International, 2010; Oklahoma Department of Wildlife Conservation and FWS, 2011). This finding, however, was not confirmed by fungal culture or histopathology; and, to date, WNS has not been confirmed in states west of the Mississippi River.
Bat researchers and human cavers may also be contributing to the rapid spread of this pathogen within and across states. Blehert noted that the index site for WNS, Howes Cave, is connected to Howe Caverns, a commercial tourist cave that entertains up to a quarter-million visitors per year. Although there are no data supporting (or refuting) the hypothesis that humans are serving as transmission
“vectors,” Blehert noted that the U.S. Fish and Wildlife Service recommends that all people who enter caves (e.g., researchers and speleologists) employ decontamination protocols for potentially contaminated clothes and equipment.
Origins
The origins of WNS and its relationship to G. destructans remain unknown (Qaummen, 2010). However, recent evidence from Europe and characterization of the newly described pathogen may provide clues. In early 2010, researchers published several reports confirming that a number of bats (all Myotis spp.) in France, Hungary, Germany, and Switzerland, while infected with G. destructans, remained healthy (Puechmaille et al., 2010; Wibbelt et al., 2010). Speaker Gudrun Wibbelt, of the Leibniz Institute for Zoo and Wildlife Research, reported that not all of the European hibernacula surveyed have infected bats, and of those that do, often only a small number of bats within the colony are infected. (Dr. Wibbelt’s contribution to the workshop summary report can be found in Appendix A, pages 368–403.) Researchers have now confirmed the presence of G. destructans on 8 species of Old World bats in 12 countries in Europe (Puechmaille et al., 2011).61 In addition, photographic evidence suggests that the fungus was present on bats in Europe at least as early as the 1980s (Wibbelt et al., 2010). These findings, which have important implications for future WNS research, could help explain the origins of the disease and may also provide clues for understanding the mechanisms of the infection.
One possible interpretation of these data is that G. destructans may have originated in Europe and that Old World bats and this pathogen may have coevolved (Puechmaille et al., 2011; Wibbelt et al., 2010). This hypothesis is supported anecdotally by reports from routine winter bat surveys in Europe from the past 30 years. The surveys occasionally noted the presence of a white fungus similar in appearance to G. destructans on otherwise healthy bats, Wibbelt noted. If European bats have coevolved with the fungus, they might be able to muster a sufficient immune response to control and survive infection by G. destructans (Puechmaille et al., 2010). Some have proposed the possibility that the microbial flora of bat skin or other abiotic surfaces in European hibernacula “may have also coevolved to incorporate G. destructans as a non pathogenic component of the microbial community” (Wibbelt et al., 2010). Wibbelt also reported the possibility that Old World bats may only be colonized in a superficial fashion on the outer epidermis without any invasion into deeper tissues.
A second possible interpretation of the discovery of healthy European bats infected with G. destructans is that disease transmission in Old World bat populations may be affected for some biological or behavioral reason (Puechmaille et al., 2010, 2011; Wibbelt et al., 2010). European bats tend to hibernate in
_______________
61 See contributed manuscripts by Wibbelt in Appendix A (pages 368–403).
relatively small groups (rarely more than 100 individuals per cluster), Wibbelt explained. This might make it more difficult for the disease to spread. In the United States, bats hibernate in groups that can reach into the hundreds of thousands (Wibbelt et al., 2010).
Additional explanations for why European bats infected with G. destructans do not succumb to WNS include the possibility that the fungal strain in the United States is more virulent than the strain in Europe or that G. destructans is not the primary cause of death in WNS. However, Blehert did note that his team’s and others’ diagnostic investigations of infected New World bats had ruled out toxins, parasites, and known viral and bacterial pathogens as associated with WNS (Blehert et al., 2009; Chaturvedi et al., 2010; Gargas et al., 2009). Wibbelt observed that G. destructans isolates from North America and Europe appear identical in morphology and in the sequence of two genes (ITS and SSU) commonly used as a marker to distinguish between different species (Puechmaille et al., 2011). Further research will be necessary to determine the true cause of differences between North American and European bats infected with G. destructans.
Amphibian Chytridiomycosis
The effect of chytridiomycosis on amphibians has been described as the greatest loss of vertebrate biodiversity attributable to disease in recorded history.
—Vredenburg et al. (2010, p. 9689)
Amphibians are currently the most threatened class of vertebrates on the planet (Stuart et al., 2004). Researchers classify approximately one third of the more than 6,500 known amphibian species as threatened and more than 40 percent of species have experienced population declines in recent decades (Lips et al., 2006; Lötters et al., 2010; Stuart et al., 2004). The proximate cause of many of these declines is a recently described disease associated with the fungus, Batrachochytrium dendrobatidis (Bd), which infects more than 350 species of frogs, toads, and salamanders on every continent except Antarctica (Fisher et al., 2009) (Figure WO-17). In susceptible animals, Bd infection causes the deadly disease chytridiomycosis, which has been implicated as the catalyst for the global decline of more than 200 amphibian species, including local extinctions of several species in the wild (Fisher et al., 2009; Kilpatrick et al., 2009; Lips et al., 2006; Lötters et al., 2010; Schloegel et al., 2006; Skerratt et al., 2007).
Until effective control measures are in place, investigators expect Bd to continue to threaten more species with extinction, making the fungus a candidate for the most destructive emergent, infectious epizootic disease ever recorded (Fisher, 2008). Despite its widespread impact, little is known about Bd’s origins, how it has spread across the globe, the specific mechanism by which it causes death, and why it is so devastating to some amphibian species while others are appar-
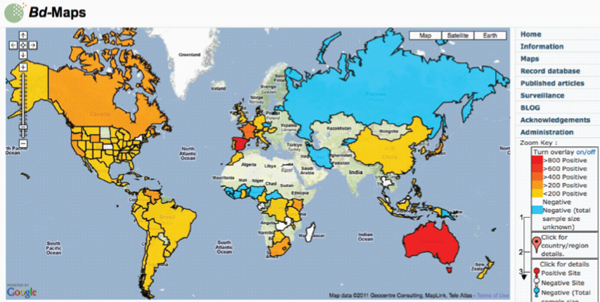
Figure WO-17 Global distribution of Bd.
SOURCE: The Global Bd-Mapping Project, http://www.bd-maps.net/.
ently able to control infections without significant morbidity or to resist infection entirely (Rosenblum et al., 2009).
Phenomenology
B. dendrobatidis is an aquatic chytrid fungus—an early diverging class of fungi—that infects keratinized epidermal cells of amphibians, causing rapidly progressing and deadly chytridiomycosis in susceptible species (Fisher et al., 2009). First identified in 1998 and characterized in 1999, Bd is unique among other chytrids (Berger et al., 1998; James et al., 2006; Longcore et al., 1999). It is one of only two known chytrid fungi to parasitize vertebrates and the only known species to infect the keratinized skin of living amphibians (Berger et al., 1998; Fisher et al., 2009). Bd’s asexual spore is a “free living” and motile zoospore, possessing a single flagellum that allows the spore to travel small distances (usually less than 2 cm) and thrive in aquatic habitats such as streams and ponds (Fisher et al., 2009; Kilpatrick et al., 2009; Kriger and Hero, 2007; Rosenblum et al., 2010).
According to speaker Vance Vredenburg, from San Francisco State University, investigators believe that amphibians become infected with Bd through both casual contacts with zoospores in the water as well as through direct animal-to-animal transmission. Once infected, the susceptible animals carry Bd for 24 to 220 days before they succumb to chytridiomycosis (Lips et al., 2006). Vredenburg remarked that the susceptibility to Bd colonization and subsequent development of chytridiomycosis varies widely across species, populations, and individuals. Indeed, laboratory experiments have found mortality rates from 0 to 100 percent, depending on temperature, species, and age of the infected animals (Berger et al., 2005; Daszak et al., 2004; Kilpatrick et al., 2009; Lamirande and Nichols, 2002; Woodhams et al., 2003).
Many of the most notable and rapid declines in amphibia have occurred among those populations living at high altitudes in mountainous regions, leading some to associate outbreaks of fatal chytridiomycosis with cooler climates and high altitudes (see Fisher et al., 2009). Several amphibian species that live near sea level also have experienced notable population declines from this fungal disease, however, suggesting that this association may be an “oversimplification of a complex host–pathogen relationship” (Fisher et al., 2009). The preponderance of the evidence supports the observation that Bd does prefer cooler temperatures, growing and reproducing between 4°C and 25°C (Berger et al., 2004; Drew et al., 2006; Kilpatrick et al., 2009; Kriger and Hero, 2007). Indeed, the available evidence suggests that the virulence of Bd is inversely related to temperature, perhaps in a species-dependent manner (Fisher et al., 2009; Walker et al., 2010).
The specific mechanisms by which some species suffer rapid declines when Bd is introduced while others are able to tolerate varying levels of infection without the development of disease—or even resist infection entirely—remain

Figure WO-18 A chytridiomycosis outbreak in southern mountain yellow-legged frogs (Rana muscosa), Sixty Lake Basin, Kings Canyon National Park, CA, USA.
SOURCE: Photo kindly provided by V. Vredenburg (August 15, 2006).
unknown. Nevertheless, chytridiomycosis apparently results from the complex interplay of pathogen, host, and environmental factors (Briggs et al., 2010; Rosenblum et al., 2010; Vredenburg et al., 2010; Walker et al., 2010). Recounting his investigations of disease dynamics in multiple populations of Bd-infected amphibians (Rana muscosa and Rana sierrae) in Sequoia and Kings Canyon National Parks in the Californian Sierra Nevada, Vredenburg noted that even populations of the same species can have “very different outcomes of infection, depending on where you are in the Sierra Nevada.”62
Although numerous studies have established that Bd is the proximate cause of the observed declines in amphibian populations around the world, the specific ways by which chytridiomycosis causes death remains a mystery (Figure WO-18).
In susceptible animals, chytridiomycosis causes a thickening of the skin (hyperkeratosis), abnormal proliferation of epidermal cells (hyperplasia), and sometimes increased skin shedding (Berger et al., 1998, 2005). Only rarely do infected animals have visible skin lesions or other pathologies generally associ-
_______________
62 See contributed manuscript by Vredenburg in Appendix A (pages 342–355).
ated with lethal infections. Bd may disrupt the normal regulatory functions of amphibian skin, causing osmotic imbalances, electrolyte depletion, and ultimately death (Rosenblum et al., 2010). Although some investigators have suggested that Bd might release lethal toxins, no specific toxin has been identified (Fisher et al., 2009; Rosenblum et al., 2010). As discussed by Vredenburg, investigators are also exploring the possibility that other microbial components of the amphibian skin microbiome can contribute to disease mitigation (Harris et al., 2009).
Is Bd a Newly Emergent or Previously Endemic Pathogen?
One of the many unresolved debates among scientists who study this disease is whether Bd is a newly emerging pathogen that recently spread across the globe (known as the novel pathogen hypothesis) or if it has existed for some time as a commensal or symbiont of amphibians, and only recently became more virulent as a result of environmental changes that altered its relationship with its hosts (the endemic pathogen hypothesis) (Fisher and Garner, 2007; Fisher et al., 2009; Rachowicz et al., 2005; Rosenblum et al., 2009, 2010).
What is known is that Bd has existed at a low prevalence in some populations of amphibians since at least the 1930s, but massive species declines were not reported until recently (Weldon et al., 2004). A number of studies established significant associations between Bd, declines in amphibian populations, and global warming (Fisher et al., 2009; Pounds et al., 2006). Fisher remarked that more research is needed to illuminate the specific mechanisms through which climate change or other environmental factors might influence the host–pathogen dynamic between Bd and certain species of amphibians (Fisher et al., 2009). The preponderance of the evidence generated to date supports the novel pathogen hypothesis—Bd appears to be a newly emergent disease (Fisher et al., 2009; James et al., 2009; Rosenblum et al., 2009, 2010).
The most compelling evidence in support of the novel pathogen hypothesis comes from recent studies mapping the genome of Bd isolates from around the world (Rosenblum et al., 2009). All global diversity of Bd can be explained by a single ancestral diploid strain that subsequently spread across the world, diversifying through mitotic and meiotic recombination (James et al., 2009; Morgan et al., 2007). According to Fisher, investigators still do not know the origin of this single diploid strain; however, all known Bd strains most likely came from a small, genetically “bottlenecked” ancestral population (James et al., 2009). These data provide strong, inferential evidence in support of the theory that Bd is a recently emergent fungal pathogen that rapidly expanded its geographic range across the globe in the first half of the 20th century (Fisher et al., 2009; Rosenblum et al., 2009).
Origins and Spread
Despite its widespread “footprint,” little is known about Bd’s origins and how it has spread across the planet (Fisher et al., 2009; Kilpatrick et al., 2009; Rosenblum et al., 2009, 2010). Bd clearly is widely distributed, but its distribution is not homogeneous (Fisher et al., 2009). Indeed, a number of areas around the world—most notably Madagascar—have a rich diversity of amphibian species, but Bd has not spread there yet (Fisher et al., 2009; Weldon et al., 2008).
Skin samples from the African clawed frog (Xenopus laevis) collected in 1938 on the Western Cape of South Africa provide the earliest evidence of Bdassociated amphibian skin infections (Weldon et al., 2004). As noted by Weldon, X. laevis is able to asymptomatically carry the fungus without developing chytridiomycosis and has not experienced any population declines as a result of Bd infections. In the early 20th century (between the 1930s and 1960s), the frogs were globally marketed and used in human pregnancy assays (Weldon et al., 2004). X. laevis is also widely used as a model organism in developmental biology because its metamorphosis from zygote to tadpole can be easily observed in the laboratory (Weldon et al., 2004). The global trade in African clawed frogs has led investigators to suggest that Bd originated in Africa and that X. laevis served as the natural reservoir host for this fungal pathogen (Weldon et al., 2007). The fungus then spread to new hosts and environmental niches as a result of the human-mediated movement of amphibians, globally and locally (Weldon et al., 2004).
Some studies have cast doubt on the hypothesis that the fungus originated in Africa (Fisher et al., 2009; Rosenblum et al., 2009, 2010). As noted by Fisher, studies of the genetic diversity of Bd in different species found that fungal isolates from North American bullfrogs (Rana catesbeiana), another species that is widely traded and is able to carry Bd without any associated morbidity, are significantly more genetically diverse than isolates from African clawed frogs (James et al., 2009). This is contrary to what researchers would expect if African clawed frogs were the original reservoir for Bd and suggests that the fungus origins may be outside of Africa (Fisher et al., 2009; Rosenblum et al., 2010). Fisher observed that genomic sequencing of global Bd isolates reveals that “what we have been calling Bd actually consists of at least three highly divergent lineages.”
A number of theories have been put forward to explain Bd’s spread around the globe (Fisher and Garner, 2007). As Weldon observed, many believe the spread was at least partially human-mediated, the result of international trading and transportation of amphibians that were infected with the fungus, but did not show signs of obvious illness. Anthropogenic spread through the amphibian trade, however, cannot explain how the fungus was introduced into and spread across environments where there has been minimal human activity.
Phytophthora ramorum in Europe and North America
In the USA, the economic impact of losses due to P. ramorum is estimated to be in the tens of millions of dollars due to the direct loss of nursery and ornamental crops, the decrease of property values due to dead/dying trees, the cost of monitoring, tracking, and eradicating the disease, the societal impact through loss of recreational value and cultural value, and the ecological impact through loss of food resources for native fauna.
—Grünwald et al. (2008, p. 2)
The first reports of sudden oak death occurred in California forests in 1994–1995 (Rizzo and Garbelotto, 2003). While the origins of the associated pathogen, Phytophthora ramorum (P. ramorum), remain unknown, investigators believe the “source” of the subsequent epidemic in California was an infected ornamental Rhododendron plant(s) (Kliejunas, 2010; Mascheretti et al., 2009). Now known to cause disease (commonly known as ramorum blight) in more than 100 plant species, P. ramorum has also emerged as a novel plant pathogen in the United Kingdom and Europe (Grünwald et al., 2008). Collectively, these diseases have led to the rapid decline of oak forests on the west coast of the United States and have led to widespread disease in trees and woody ornamental plants throughout the United Kingdom and Europe (Grünwald et al., 2008).
P. ramorum is just one of the many invasive tree diseases that were introduced into the forests of North America in the 20th century. As in the case of chestnut blight and Dutch elm disease, the transportation and trade of plants and plant materials contributed to the movement of this pathogen across oceans and continents, as well as between suburban and forest ecosystems (Brasier and Webber, 2010; Goss et al., 2009; IOM, 2010). As reviewed by speaker Rizzo, once P. ramorum is established in the landscape, treatment options are extremely limited. The discovery in 2009 that P. ramorum was reproducing in Japanese larch trees in the United Kingdom led to the immediate clear cutting of 4 million larch trees—more than 10,000 acres of forest—in a heroic effort to slow the spread of the disease (Hardman, 2011).
Phenomenology
P. ramorum is a fungus-like oomycete63 or “water mold” that thrives in the cool, wet climate of California coastal forests (Kliejunas, 2010). In contrast to most species of Phytophthora, P. ramorum exhibits a remarkably broad
_______________
63 As noted by speaker Rizzo, Phytophthora spp. is not a “true fungus.” It is an oomycete or “water mold” that belongs to the Kingdom Stramenopila (a major eukaryotic group that includes diatoms and brown algae, and is distinct from plants, fungi, and animals). Like fungi, oomycetes “exhibit filamentous growth, produce sexual and asexual spores, and can feed on decaying matter or be obligate parasites of plants” (Kliejunas, 2010).
host range, infecting a variety of tree and non-tree species—ranging from hardwood and conifer trees, to shrubs and leafy plants64 (Brasier and Webber, 2010; Grünwald et al., 2008). P. ramorum colonizes the leaves of many plants and the inner bark and sapwood of trees. The fungus can also survive in a dormant state in decaying matter, such as leaf litter on the forest floor, and in the soil (Parke and Lucas, 2008). Rizzo remarked that within forest ecosystems in California, “once we started looking, we started finding it on just about every plant we looked at, ranging from ferns to redwood trees.”
Infection occurs when spores and zoospores—dispersed by winds or water—come into contact with susceptible plants. Moisture is not only essential for the production of infectious propagules, but free water on plants—from fog, dew, or rainfall—enhances infection and dispersal (Kliejunas, 2010; Rizzo and Garbelotto, 2003). Indeed, monitoring streams that are “baited” with Rhododendron leaves, Rizzo said, has been an effective method for early detection of this pathogen in forest ecosystems. Disease manifests differently depending on the plant species and the part of the host plant that is infected (Grünwald et al., 2008) (Figure WO-19 and Table WO-2). Bleeding lesions and stem cankers develop in forest trees, followed by rapid declines and “sudden” death. Ramorum blight causes shoot-tip dieback and leaf spots in woody shrubs and ornamental trees. Unlike sudden oak death, ramorum blight rarely kills its host (Grünwald et al., 2008).
Discovery and Spread
Observers first spotted symptoms of sudden oak death and ramorum blight in the mid-1990s in Marin County, California, and in European nurseries (Mascheretti et al., 2009). In 2000, researchers identified the common causative fungal pathogen for these diseases, P. ramorum (Garbelotto and Rizzo, 2005; Kliejunas, 2010). Trade and transportation of ornamental plants enabled the introduction of this novel pathogen into the United States and Europe. P. ramorum has since spread north to southern Oregon and south to Big Sur in California, where tree mortality rates are among the highest (Kliejunas, 2010). In 2009, symptoms of the disease were first detected in Japanese larch trees in the English counties of Devon, Cornwall, and Somerset (Forestry Commission, 2010). By August 2010, the disease had spread to Japanese larch trees in the counties of Waterford and Tipperary in Ireland (Brasier and Webber, 2010; Forestry Commission, 2010). Many now consider this disease to be a serious threat to Japanese larch and possibly other tree species in Europe (Brasier and Weber, 2010).
The ornamental trade and other anthropogenic factors continue to serve as a source for global and local spread of P. ramorum. In 2004, “pre-symptomatic” Rhododendron plants infected with the fungal pathogen were discovered in a
_______________
64 See contributed manuscript by Rizzo in Appendix A (pages 312–324).
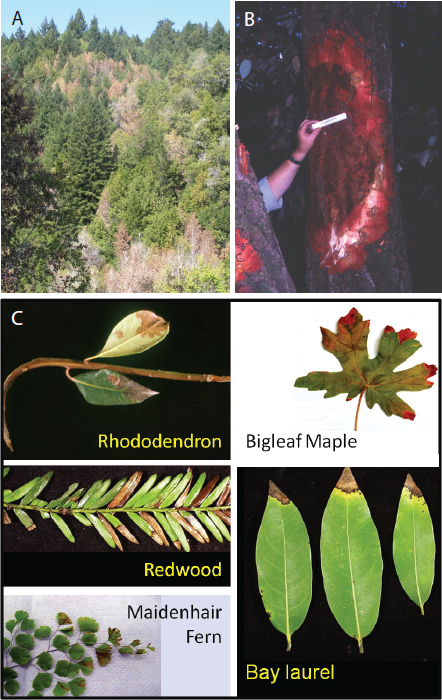
Figure WO-19 Sudden oak death and ramorum blight. (A) Aerial view of a forest in Humbolt County with patches of trees dying of sudden oak death; (B) canker on tanoak; (C) signs of ramorum blight on a variety of host plant species.
SOURCE: Rizzo (2010).
TABLE WO-2 Disease Types and Associated Symptoms Caused by P. ramorum
| Disease | Symptoms | Host Categories | Typical Hosts* | Geography and Environment |
| Sudden oak death | Stem cankers; bleeding cankers | Forest trees; garden trees | Coast live oak, tanoak, European beech | North American forests; European gardens |
| Ramorum blight | Foliar and twig blight; tip and shoot dieback; leaf blight | Ornamental trees and woody shrubs; forest understory plants | Viburnum, rhododendron, pieris, lilac; coast redwood, Douglas fir; tanoak, California bay laurel | European nurseries and gardens; North American nurseries and forest |
*Only a small selection of typical hosts is presented. For a complete list of hosts refer to Grünwald et al., 2008, and references within.
SOURCE: Adapted from Grünwald et al. (2008).
Southern California plant nursery, but not before the nursery had shipped potentially infected plants to more than 40 states (Figure WO-20) (Goss et al., 2009).
As Rizzo noted, many of the fungicides used in the nursery trade do not kill the pathogen, but rather suppress symptoms; this is probably how P. ramorum spreads over very long distances via the plant trade. Leaves, flowers, and stems of infected plants carry the pathogen, which is also spread by the transportation of plant or associated plant materials, including soil (Kliejunas, 2010). Waterways are an effective means of spreading P. ramorum. Investigators have detected the pathogen in streams contaminated with run-off from infected nurseries (Kliejunas, 2010). P. ramorum may also provide an interesting case study of humans acting as vectors for fungal disease, as sudden oak death “has potentially been spread to new areas by hikers, mountain bikers, and equestrians” (IOM, 2010). Rizzo also pointed to the movement of “green waste,” that is, compost, firewood, mulch, and other plant matter, as an another possible means of spreading the fungus within and across ecosystems.
As of 2009, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service reported detection of P. ramorum in 11 states (Alabama, California, Georgia, Maryland, Mississippi, New Jersey, North Carolina, Oregon, Pennsylvania, South Carolina, and Washington) at 30 sites (24 nurseries and 6 in the landscape) (Kliejunas, 2010). The pathogen’s eastward spread has placed Eastern native forests at risk and has led many experts to worry that P. ramorum could have ecological consequences comparable to those of chestnut blight and Dutch elm disease (Goss et al., 2009).
The distribution of sudden oak death in California forests is heterogeneous, Rizzo noted. Weather—including winds, temperature, humidity—contribute to the pathogen’s establishment and spread within a new environment (Kliejunas
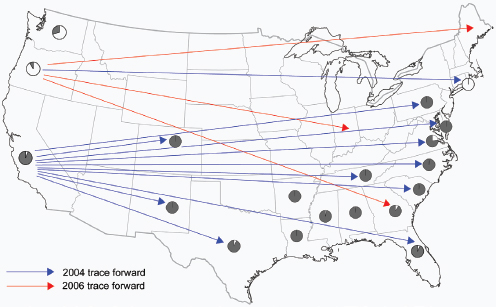
Figure WO-20 P. ramorum “migration” pathways. The ornamental plant trade continues to serve as a major pathway for the spread of P. ramorum. Arrows indicate confirmed P. ramorum-positive nursery trace forwards. Blue arrows are 2004 trace forwards and red arrows are 2006 trace forwards. There were no confirmed trace forwards in 2005 or 2007. Pie charts show the distribution of the two groups of isolates (of NA 1 lineage) among sampled states.
SOURCE: Goss et al. (2009).
, 2010). Infected tanoaks do not contribute significantly to disease spread, but, as Rizzo observed, many other plants in forested areas (e.g., understory and canopy trees) are also host species for P. ramorum. The pathogen’s very broad host range, therefore, is also an important transmission factor for disease. In California forests, the California bay laurel (Umbellularia californica) drives transmission of the pathogen (Kliejunas, 2010; Rizzo and Garbelotto, 2003). Leaf tips of these trees serve as a prolific source of inocula. Dissemination of spores can occur via wind and rainsplash (Rizzo and Garbelotto, 2003). In the United Kingdom, Japanese larch trees not only developed canker and died but the leaves also served as foliar hosts and the source of massive amounts of inocula (Brasier and Weber, 2010).
Origins
The lack of reports of P. ramorum in the United States before the mid-1990s, combined with its aggressiveness and limited geographic range relative to its hosts’ distribution, suggests that P. ramorum was only recently introduced
into the United States (Grünwald et al., 2008). Genetic evidence supports the hypothesis that the U.S. and European strains are distinct and that both strains likely originated from a third, as yet unknown, source (Grünwald et al., 2008). Molecular studies have identified three main lineages: (1) EU1—found in both North American and European nurseries and some European woodlands; (2) NA2—found in California and Washington nurseries; and (3) NA1—found in North American nurseries and in California and Oregon forests (Grünwald et al., 2008).
An alternative explanation is that P. ramorum may have existed in California for many years, but only recently emerged because of changes in the environment (e.g., increased temperatures, fire suppression, modifications in land use patterns) that have led to an increased prevalence and aggressiveness of the pathogen (Rizzo and Garbelotto, 2003). Native P. ramorum or other Phytophthora sp. may also have evolved into a more virulent form, may have undergone a change in host specificity or preference, or may represent an entirely new hybrid species (Rizzo and Garbelotto, 2003). It has been reported that a novel Phytophthora hybrid—a cross between P. cambivora, an oak pathogen, and P. fragariae-like isolates, a strawberry pathogen—emerged in Europe in the 1990s and has killed thousands of alder trees (Alnus spp.) (Brasier et al., 1999). Given the large number of Phytophthora spp. in California agricultural and horticultural environments, a hybrid origin for P. ramorum is certainly a possibility; although other explanations are also possible (Rizzo and Garbelotto, 2003; Tyler et al., 2006).
The Rapid Global Spread of Yellow “Stripe” Rust on Wheat
The presence of two virulent and highly aggressive yellow rust strains at high frequencies at epidemic sites on five continents may represent the most rapid and expansive spread ever of an important crop pathogen. This epidemic trend may continue because the aggressive strains, which can tolerate higher temperatures, are still evolving.
—Hovmøller et al. (2010, p. 369)
Yellow “stripe” rust on wheat has recently reemerged as a major threat to global food security (Hovmøller et al., 2010). This destructive, cooler climate, wheat disease can spread rapidly via wind and human activities locally and globally. Between 2000 and 2002, two new and highly aggressive65 strains of the associated fungus (Puccinia striiformis f. sp. tritici) appeared on three continents—North America, Australia, and Europe—causing record wheat crop losses (Hovmøller et al., 2010; Milus et al., 2009). As of 2009 these new strains of yellow rust had spread to major wheat-growing areas in the Middle East, North
_______________
65 Speaker Mogens Hovmøller defined “aggressiveness” as the quantitative ability to cause more disease, more quickly, on a susceptible host.
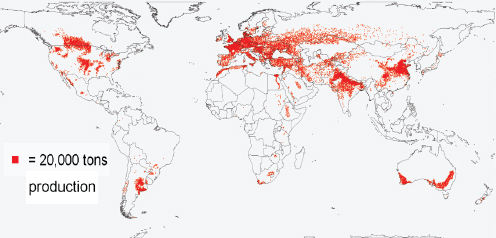
Figure WO-21 Wheat production regions worldwide. Each red dot represents 20,000 tons of wheat production.
SOURCE: Trethowan et al. (2005).
and Eastern Africa, Western and Central Asia, and China (Hovmøller et al., 2010). According to Hovmøller et al. (2010), this may be the most rapid and expansive spread of an important crop pathogen ever documented.
Wheat is the most widely grown cereal crop, produced as food for humans and as feed for livestock (Figure WO-21). Worldwide, wheat accounts for one fifth of the total human caloric intake. In regions such as Western Asia, it can account for as much as half of the daily calorie intake (Hovmøller, this volume; Stone, 2010). Epidemics of rust disease have been held in check by rust-resistant wheat cultivars developed in the mid-20th century, and other agricultural practices (Koerner, 2010). Since 2000, however, the natural history66 of yellow rust appears to have changed. Disease is now emerging in regions previously considered inhospitable to this fungal pathogen (Hovmøller et al., 2008; Milus et al., 2009).
Phenomenology
Puccinia striiformis f. sp. tritici (hereafter, P. striiformis) is an obligate, basidomycetous, pathogen of wheat. It infects the green tissues of host plants, causing damage to leaf blades, and reducing the yield and quality of produced grains and seeds (Chen, 2005). Severe infections of yellow rust also stunt the growth of wheat plants. Average reported yield losses due to yellow rust range from 10 to 70 percent; yield losses in highly susceptible cultivars can reach 100 percent (Chen, 2005). Weather—including humidity, rainfall, temperature, and
_______________
66 The natural development of something (as an organism or disease) over a period of time.
wind—is critical for developing favorable conditions for fungal infection and growth (Chen, 2005).
Named for the yellow pustules of powdery spores (urediniospores) that appear as “stripes” on the leaf blades of infected plants, yellow “stripe” rust is an important “cooler climate” wheat disease (Chen, 2005) (Figure WO-22). Disease can occur early in the growing season, when temperatures are low. Areas affected by this disease tend to be in temperate regions and high-elevation areas in the tropics (Chen, 2005). P. striiformis depends on a living host for survival and produces huge numbers of airborne spores that are carried by wind from one susceptible host to another (Brown and Hovmøller, 2002).
Discovery and Spread
The most widespread yellow rust epidemic in the United States occurred in 2000 (Milus et al., 2006). Before that time, yellow rust in the United States was restricted to the temperate regions of California and the Pacific Northwest, where cool and moist weather patterns prevail (Chen, 2005). Suddenly, “overnight, more or less,” remarked Mogens Støvring Hovmøller of Aarhus University, the disease appeared in the warmer and drier wheat belt. (Dr. Hovmøller’s contribution to the workshop summary report can be found in Appendix A, pages 252–263.) For the first time, severe epidemics were reported east of the Rocky Mountains—in South Dakota, Nebraska, Kansas, Oklahoma, and Texas (Figure WO-23) (Milus et al., 2009).
Similarly, between 2002 and 2003, yellow rust swept across Western Australia, where yellow rust was absent until 2002—an area once considered too warm for severe epidemics of yellow rust (Milus et al., 2009). In central and northern Europe, epidemics on wheat at that time were less pronounced, the majority remaining resistant to the disease. According to Hovmøller, two P. striiformis strains of almost identical DNA-fingerprints were responsible for these epidemics (Hovmøller et al., 2008). Human activity was likely the main driver for pathogen introduction in North America and Australia, Hovmøller remarked, given the rapid spread of the pathogen to distant continents in less than 3 years and the indistinguishable DNA fingerprints of prevalent pathogen isolates. Once the pathogen arrived, the disease spread rapidly via airborne spores, he said. According to Hovmøller, the presence of large areas of susceptible crop varieties likely amplified and accelerated the spread of these yellow rust strains.
The emergence of yellow rust in new warmer regions concerns researchers because it suggests a change in its epidemiology. Indeed, the strains of P. striiformis associated with recent outbreaks of disease are more aggressive,67 more heat tolerant, and able to produce two to three times more spores in less time than other strains (Milus et al., 2009). These adaptations appear to improve the fitness
_______________
67 Able to cause more disease more quickly on susceptible host plants.
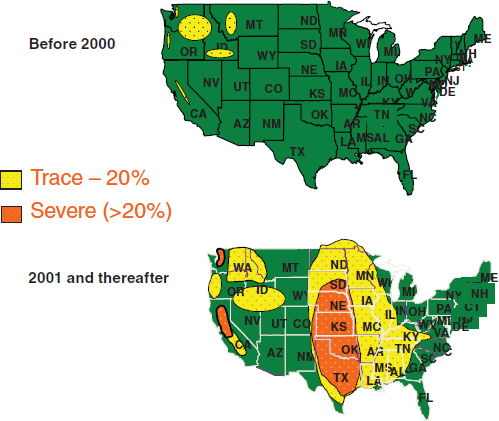
Figure WO-23 Presence of “trace” and “severe” levels of yellow rust in North America since 2000.
SOURCE: Adapted from Chen (2005).
of these strains and may explain their rapid spread on a global scale (Hovmøller et al., 2008). In Australia and the United States, these newer, more aggressive strains appear to have replaced older strains and have continued to thrive in subsequent seasons (Milus et al., 2009). According to Hovmøller, while the epidemic has apparently slowed down in North America from 2006 to 2009, major yellow rust epidemics occurred in northern and eastern Africa, Western and Central Asia, China, and the Middle East (Hovmøller et al., 2010). In 2010, according to Hovmøller, observers reported disease outbreaks in northern and eastern Africa, Asia, and the Middle East, and the epidemics reappeared in the United States.
Origins
The exact geographic origin of these more aggressive strains of P. striiformis is unknown, although phylogenetic analyses reveal that West and Central Asia may be the evolutionary origin. Hovmøller remarked that the dramatic change in
phenotype (e.g., spore production, heat tolerance), coupled with the sudden appearance of these strains suggests a recombination event somewhere, rather than evolution through a series of mutations.
Unlike many of the fungal diseases discussed at the workshop, yellow rust is well known. Epidemics of this disease have plagued the world’s farmers for centuries, and P. striiformis is familiar to plant breeders. In the 1940s, Norman Borlaug68 developed new “rust-resistant” wheat strains that also dramatically increased global crop yields (Rust in the bread basket, 2010). These advances inspired the “Green Revolution” that brought these techniques and disease-resistant wheat into widespread use. According to Brown and Hovmøller (2002), these techniques have helped to keep many crop diseases under control, but now, relatively few crop varieties (with specific resistance genes) are sometimes used across large areas at “continental scales.” A reduced crop diversity in modern agriculture, according to Hovmøller, increases the potential impact posed by the global movement of new, more virulent forms of plant pathogens (Brown and Hovmøller, 2002; Stukenbrock and McDonald, 2008).69
SURVEILLANCE, DETECTION, AND RESPONSE
Fungal diseases are an emerging threat to human, animal, and plant health—not simply because of the morbidity and mortality associated with these infections, but also because of the limited means and capabilities to rapidly detect and diagnose these diseases and the lack of effective tools for disease mitigation and treatment. Detection of many emerging fungal diseases—such as amphibian chytridiomycosis, WNS, and C. gattii—has relied on the astute observer in the field. However, once these diseases have become established in a new environment, the spread of fungal pathogens is limited by the environmental constraints imposed on them, such as temperature, humidity, drought, or moisture. Therapeutic interventions and management strategies for these diseases remain limited—underscoring the urgent need for active disease surveillance and additional research to better understand and address fungal disease threats. As observed by Forum member Kevin Russell of the Department of Defense’s Global Emerging Infections Surveillance and Response System, the fungal world
_______________
68 Norman Borlaug was an American agronomist. His work to develop disease-resistant crop strains earned him the titles of Nobel laureate and “the father of the Green Revolution.” Borlaug was one of only six people to have won the Nobel Peace Prize, the Presidential Medal of Freedom, and the Congressional Gold Medal. He was also a recipient of the Padma Vibhushan, India’s second highest civilian honor.
69 In 1999 a new, highly virulent, strain of wheat stem “black” rust, Ug99, emerged in Uganda. This pathogen was able to circumvent the genetic resistance of wheat hybrids developed during the Green Revolution. This pathogen has now spread to Kenya, Ethiopia, Sudan, Yemen, and Iran (Vurro et al., 2010). Researchers worry that Ug99 will soon spread to the major wheat growing regions of Pakistan and India, which account for ~20 percent of the world’s wheat supply (Vurro et al., 2010).
is too huge, too unknown, and too threatening not to develop improved capacity for detection, diagnosis, and response to emerging fungal pathogens and diseases.
David Blehert, joined by Forum member Russell and fellow Forum member Jacqueline Fletcher of Oklahoma State University, emphasized the importance of cross-disciplinary communication and a “One Health”70 approach for developing a more robust capacity for global disease surveillance, detection, and response. As noted by Bartlett, the detection and response to the emergence of C. gattii in British Columbia and the U.S. Pacific Northwest in 1999 ultimately involved professionals and expertise from the veterinary, medical, public health, and plant and wildlife communities. Indeed, because of its close interactions with plants and ability to cause disease in humans and animals, C. gattii was called the “poster child” for a One Health approach.
The plant health community was considered by many at the meeting to be an equal partner in a One Health approach to infectious disease detection and response. Fungi can form associations with and, in some cases, be pathogenic to humans, animals, and plants, Fletcher observed. She described the need to gain a better understanding of how fungal pathogens might “jump” between plant and animal or human systems. Others remarked on the indirect, but potentially significant, impacts of plant pathogens on human health and well-being, including threats to ecosystem stability or global food security. As Fletcher noted, however, “plant pathogens have only been incorporated into current One Health initiatives [in] a very minor way.”
Surveillance Networks
Over the past several decades, various systems for passive and active surveillance for emerging and reemerging diseases of humans, animals, and plants have been developed at regional, national, and international levels (GAO, 2010). Systems supporting the surveillance and detection of emerging fungal plant pathogens are the most sophisticated, possibly due to the historical importance of fungal diseases on economically important foodstuffs, crops, and plants (IOM, 2007; Rossman, 2009).
Disease surveillance and detection in the United States is a shared responsibility of various state and federal programs (Figure WO-24) (GAO, 2010). The Centers for Disease Control and Prevention (CDC) and other federal agencies, including the USDA, Department of Defense (DoD), and Department of the Interior (DOI), independently gather and analyze national infectious disease surveillance reports as well as morbidity and mortality data for humans, plants, livestock, and
_______________
70 One World, One Health® is a registered trademark of the Wildlife Conservation Society and reflects the need to establish a more holistic approach to preventing epizootic disease and for maintaining ecosystem integrity for the benefit of humans, their domesticated animals, and the foundation biodiversity that supports us all. For more information, see http://www.oneworldonehealth.org/ (accessed April 11, 2011).
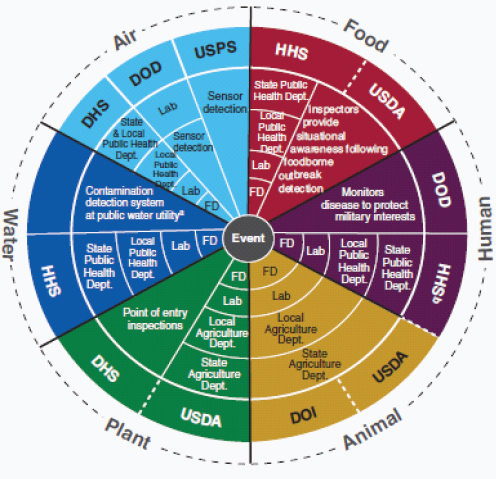
Figure WO-24 Roles and responsibilities for monitoring for pathogens in humans, animals, plants, food, and the environment in the United States.
SOURCE: GAO (2010).
wildlife. The CDC, USDA, DoD, and DOI independently fund and maintain both domestic and international laboratory networks for infectious disease diagnostics (Choffnes, 2008; GAO, 2010). Reporting and verification of outbreaks of specific diseases of concern or of unusual symptoms or health disturbances takes place at the state level. The findings are sent to federal agencies for further investigation, and if appropriate, the coordination of a response to the potential disease threat (GAO, 2010).
The DOI includes the U.S. Geological Survey (USGS) National Wildlife Health Center (NWHC), which is tasked with providing information, technical assistance, research, education, and leadership on national and international wildlife health issues. According to Blehert, WNS surveillance is currently largely opportunistic, based upon people making unusual observations in the field, then
sending bats to the NWHC or other laboratory for diagnostic investigation. Federal, state, and local government officials have taken several steps to try to curb the spread of WNS and prevent additional bat deaths. In 2009, the U.S Fish and Wildlife Service issued a cave advisory that established guidelines for entering bat hibernacula, that issued recommendations for decontamination of caving gear, and that asked researchers and spelunkers not to bring clothing or equipment that has been used in caves from affected areas to caves in unaffected areas (FWS, 2011). Federal and state agencies have also closed caves on public lands in order to prevent people from inadvertently spreading the fungus to new areas (FWS, 2011). Total funding for WNS research from federal and state agencies increased from approximately $1.8 million to $10 million between fiscal year 2007 and fiscal year 2010.
Formal global surveillance programs—tracking the emergence and reemergence of microbial threats to human, animal, and plant health—are coordinated by the World Health Organization (WHO), the World Organisation for Animal Health (OIE), and the Food and Agriculture Organization (IOM, 2007). WHO manages the Global Outbreak Alert and Response Network which partners with 120 “informal” (as discussed below) and “formal” (e.g., regional WHO offices, government, military, or university research centers) information sources to identify and respond to disease outbreaks (Heymann and Rodier, 2004). OIE manages an international reporting system on animal disease that includes reporting of “exceptional epidemiological events” and periodic gathering of animal health information (Jebara, 2004). Forum member Peter Daszak of the EcoHealth Alliance noted that the responsibility for infectious disease surveillance of wildlife could be undertaken by the United Nation’s International Union for the Conservation of Nature (IUCN). The IUCN has developed a working group of 400 wildlife specialists from around the world.71
International Regulations and Coordination
International regulations that support infectious disease surveillance and detection activities include: the International Health Regulations (IHRs) and the Sanitary and Phytosanitary Measures (Baker and Fidler, 2006; Cash and Narasimhan, 2000; MacLeod et al., 2010; WHO, 2008). In 2008, the OIE added amphibian chytridiomycosis to its list of “notifiable”72 aquatic animal diseases, (Schloegel et al., 2010). As several participants observed, however, this report-
_______________
71 For more information, see http://www.iucn.org/about/work/programmes/species/about_ssc/specialist_groups/specialist_group_pprofiles/veterinary_sg_profile/.
72 Within the OIE, a notifiable disease is one whose “detection must, by mandate, be notified by the competent veterinary authority to the OIE as required under Chapter 1.1 of the Aquatic Code. OIE members are also required to report the presence or absence of each disease in their territory on a semi-annual basis, and ensure disease surveillance programs are implemented to support any claims of freedom from one or both diseases” (Schloegel et al., 2010, p. 4).
ing requirement only applies to OIE member states, and only to animals traded internationally. Indeed, the effectiveness of these formal and informal reporting regimes has yet to be demonstrated, and many have suggested that fear of adverse economic consequences (e.g., trade and tourism restrictions) will limit their usefulness as an early warning disease reporting network (Cash and Narasimhan, 2000; Fidler, in IOM, 2010; Hueston, in IOM, 2007; Perrings et al., 2010).
Guidelines on hygiene and quarantine procedures for captive and wild animals have also been developed by the conservation community to reduce the spread of zoonotic diseases, but these guidelines are considered underused or difficult to enforce (Daszak et al., 2000). Two conventions developed to address the international wildlife trade and the conservation of biodiversity, the Convention for International Trade of Endangered Species and the Convention on Biological Diversity are based entirely on voluntary agreement, noted speaker Weldon. While these agreements reflect noble aspirational goals, according to Weldon, there is limited opportunity to actually implement the measures.
Several participants emphasized the limitations of current capacity to detect emerging pathogenic fungi. As speaker Fisher observed, national strategies are limited by their focus on known threats to humans and agriculturally important species, and international strategies are nearly nonexistent or very slow moving. This is particularly true of wildlife surveillance, which Fisher said is “completely under the radar.” Forum member Roger Breeze of Lawrence Livermore National Laboratory agreed, noting that “we are not very good at looking for things we know about, even those diseases that are economically important, such as foot and mouth disease.” Breeze continued that “what we are talking about over the last few days is broadening the number of organisms involved and the number of areas of economic life that are involved.” He went on to note that many organisms discussed during the workshop, such as ornamental plants, do not currently fall under any one organization’s regulatory responsibility. “We have a huge international failure in biosecurity,” according to Breeze, and the problem “needs to be approached in a different manner.”
No single agency or multilateral organization is solely focused on infectious diseases in humans, plants, and animals. Several workshop participants observed that the creation of a single entity that was responsible for collecting and analyzing data from across the “threat” spectrum and ensuring that disease interventions are based on the input of professionals working with humans, domestic animals, and wildlife could significantly enhance current disease surveillance and response capabilities (Choffnes, 2008; GAO, 2010; Hueston, in IOM, 2007; Perrings et al., 2010).
Improved coordination of disease surveillance and response activities, Daszak noted, would “benefit all sectors—whether it is food production, travel and trade, or human and environmental health.” Moreover, sectors may benefit in unanticipated ways. Blehert remarked that “wildlife health is important to world health. Not just with regard to disease surveillance, but also with regard to basic
research. There is much that we can learn from emerging diseases of wildlife such as WNS or amphibian chytridiomycosis that likely have significant implications with regard to ecosystem integrity and function. Only by incorporating domestic animal health, wildlife health, and human health into the same model can we fully understand the ecology of infectious disease.”
Several participants suggested that within the United States, an interagency task force could link together the plant, animal, and human health communities. Forum member Russell added that the Department of Defense is now one of several interagency partners involved in a forum on emerging pandemic threats as a sub-Interagency Policy Committee (IPC) of the U.S. government’s Global Health Initiative (GHI). Among its other activities, this sub-IPC assembled an interagency working group that developed a document detailing the U.S. response to the revised IHRs. He suggested that this interagency forum could serve as an effective model for coordinating the U.S. government activities in areas of common concern.
Forum member Edward McSweegan, from the National Institutes of Allergy and Infectious Diseases, added that a previous interagency program that was focused on international infectious diseases was orchestrated by the U.S. Department of State and the Office of Science and Technology Policy (OSTP). Forum Vice-Chair, James Hughes of Emory University, noted that this effort was established under the aegis of the Committee on International Science, Engineering, and Technology Policy of President Clinton’s National Science and Technology Council and involved many agencies: the National Institutes of Health (NIH), CDC, Food and Drug Administration, U.S. Agency for International Development, and DoD, among others. He said that many of the recommendations from their 1995 report are “still relevant to today’s world.”73 McSweegan also suggested that the funding of cross-disciplinary research on emerging fungal diseases might be modeled after the success of the NIH–National Science Foundation (NSF) Ecology of Infectious Diseases Initiative,74 perhaps as a collaboration of the NIH, USGS, and USDA.
Informal Disease Reporting Networks
Informal disease reporting networks are an increasingly important component of global disease surveillance. Examples include ProMED-mail, which is administered by the International Society for Infectious Diseases, and Bd-Maps, which was developed at Imperial College London. Both are platforms that allow
_______________
73 For more information see http://clinton1.nara.gov/White_House/EOP/OSTP/CISET/html/exsum.html#top.
74 For more information on the joint NIH–NSF Ecology of Infectious Diseases Initiative, visit the website http://www.fic.nih.gov/programs/research_grants/ecology/index.htm.
contributors anywhere in the world to report and access disease observations—even via cell phone applications (Brownstein et al., 2009; Fisher et al., 2009).
Since its founding in 1994, ProMED-mail75 (Program for Monitoring Emerging Diseases, PMM) has served as an important platform for rapid communication about emerging infectious diseases of humans, animals, and plants (IOM, 2007). Speaker Larry Madoff, of the Massachusetts Department of Public Health and University of Massachusetts Medical School, explained that in contrast to the traditional, hierarchical approach to public health reporting, ProMED collates information from a wide variety of unofficial or informal sources76 and distributes reports to members in near real time (Brownstein et al., 2009; Madoff, 2004; Morse et al., 1996). A recent quantitative assessment of the effect of informal source reporting on the global capacity for infectious disease detection concluded that ProMED-mail and other informal disease reporting resources improve the timeliness of detection and reporting, although the effect varies geographically (Chan et al., 2010).
ProMED-mail is a free service, with all reports screened by a panel of expert moderators before being posted to over 54,000 subscribers from more than 180 countries. ProMED-mail emphasizes a One Health approach to disease surveillance, Madoff remarked, by reporting on plant (mostly threats to food crops), animal (both agricultural and zoonotic threats), and human pathogens to all subscribers. Pointing to C. gattii as an example of a recently emerged fungal human pathogen with many non-human hosts, Madoff reminded the audience that the risk of disease emergence in humans is greater among pathogens with many non-human hosts (Woolhouse and Gowtage-Sequeria, 2005).
Informal efforts to aggregate information on the presence or absence of disease in the field also contribute to improving the speed and broadening the scope of current disease surveillance. Fisher described the Bd Global Mapping Project77 (Bd-Maps) and its associated activity, RACE (Risk Assessment of Chytridiomycosis to European amphibian biodiversity). Bd-Maps collects information from groups of researchers in the field and national surveillance data from several European countries, including the Netherlands, Spain, Switzerland, and the United Kingdom, to create a shared database that provides information on where Bd has been detected, globally and locally. Fisher hopes this information will not only aid in the prediction of where Bd will likely emerge in the future, but also encourage at-risk areas to implement appropriate biosecurity controls.
The Bd-Maps project has a public website with a map detailing the incidence of positive Bd reports and, for each report, links to data. There is also an embargoed (private) website to encourage participation of scientists who prefer that their data remain private. As of December 2010, the publicly available database
_______________
75 See http://www.promedmail.org.
76 Including clinician reports, blogs, chat rooms, websites, news media, YouTube videos, and other Internet sources.
77 See at www.bd-maps.net.
reported about 6,500 Bd-positive animals out of 30,000 sampled among 3,500 sites worldwide, with 49 of 74 countries and 440 species of amphibians with known Bd infections. The data come from multiple sources, including contributions directly from the field using a smart phone application called EpiCollect.78 Fisher observed that these data may be used to assess either global or country-level trends and to detect broad-level associations (e.g., the data show that Bd is present in many areas where species richness has declined without any [other] explanation). Fisher noted that the project has provided a means for communicating important information rapidly among interested parties.
Predictive Modeling
Surveillance and response efforts could be better targeted to at-risk populations or circumstances through the use of mathematical models and Geographical Information Systems (Weinberg, 2005). Several workshop participants described the use of predictive modeling as a way to “get ahead” of the spread of invasive fungal diseases into new and highly susceptible regions:
- Rödder et al. (2009) developed a model based on taxa susceptibility to Bd, biogeographic, basic biology, environmental, and demographic data to illustrate which regions of the world are more at risk for Bd among amphibians than others.
- Meentemeyer et al. (2004) and Václavík et al. (2010) identified a number of areas in California and Oregon, respectively, currently unaffected by sudden oak death, but that are at high risk based on host species distributions, climate suitability for pathogen transmission (e.g., rain), and other factors.
- Kelly et al. (2005) used the agreement of multiple models to develop a risk map for the development of sudden oak death in the United States based on information on nationwide vegetation/host (hardwood diversity and hardwood density), topography, and climate (e.g., precipitation, frost days, temperature, and many other layers) (Figure WO-25).
- Mak et al. (2010) demonstrated that data derived from environmental sampling (in native vegetation, soil, air, and water), combined with animal and human surveillance data, could be used to predict C. gattii occurrence. The methodology employed, ecological niche modeling, yielded very accurate predictions for C. gattii in British Columbia, with animal surveillance data in particular being a good indicator of C. gattii in an area (Mak et al., 2010).
_______________
78 EpiCollect allows global positioning systems-localized data to be submitted by phone to a common web database (see Aanensen et al., 2009).
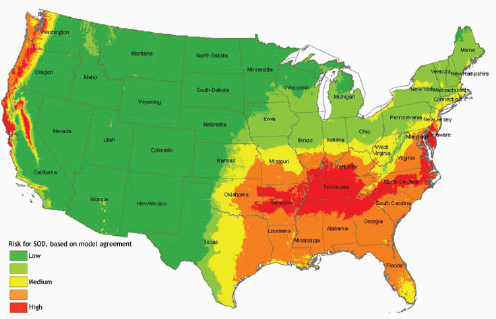
Figure WO-25 Risk for sudden oak death in the continental United States based on agreement among five spatially referenced models.
SOURCE: U.S. Department of Agriculture, Forest Service (Kelly et al., 2005).
Daszak discussed the use of predictive models to get ahead of disease emergence entirely by anticipating where viral pathogens of zoonotic origin are most likely to emerge in the future. The PREDICT project is part of the U.S. Agency for International Development Emerging Pandemic Threat program.79 PREDICT uses wildlife surveillance data and models to identify (1) geographic hot spots for the emergence of infectious disease, and (2) species that may serve as reservoirs of disease.80 Daszak noted that the prediction models developed for the PREDICT project were grounded in wildlife surveillance data that included the active collection of tens of thousands of samples from wildlife among 24 countries. Any newly discovered viruses in these samples are deemed “high priority” if they appear to be closely related to other known viral pathogens. High-priority pathogens are further characterized and, if appropriate, people who interact with the wildlife that may be affected by these pathogens are educated and advised to avoid contact. While PREDICT is currently focused only on viral pathogens, Daszak observed that the same approach could be used for fungi and fungal pathogens.
_______________
79 For more information, see: http://www.usaid.gov/our_work/global_health/home/News/ai_docs/emerging_threats.pdf.
80 See contributed manuscript by Daszak in Appendix A (pages 188–196).
Detection and Diagnosis
As many participants noted, improving the capacity for detecting fungal disease threats relies on having trained and acute observers in the field as well as a better understanding of the “baseline” of fungal diversity and distribution. Methods for the “discovery” of fungi and fungal pathogens are also needed. As speaker Rizzo noted, “We can put things on lists, but those are the things we know about. The big problems are the ones we don’t know about.” Similar challenges were identified for disease diagnosis.
Astute Observers in the Field
Human capacity is needed for more effective surveillance and detection of fungal and other infectious diseases of humans, animals, and plants, remarked many participants. Weldon noted the importance of field biologists in discovering the accelerated loss of amphibian biodiversity and in initiating investigations on the possible causes—from climate change and habitat destruction to chemical pollutants. Blackwell remarked that the causative agent of amphibian chytridiomycosis (Bd) was recognized as a fungus rather late in the epidemic by one of the few mycologists who study the fungal phylum Chytridiomycota. Howlett urged more training in classical mycology: “While molecular systematics and phylogenomics has helped to advance understanding of mycology, these methods need to be complemented by field studies and identification of the causative agent of a disease by symptoms or pathogen morphology.” Forum member Fletcher agreed, adding that more classically trained plant pathologists are needed: “These are the scientists who can go out into the field and identify pathogens of any type, fungal or otherwise.” Many senior plant pathologists are near retirement, but not many younger pathologists have the skills and knowledge to take their place, she said. Rizzo agreed, noting that in the 1980s, seven researchers in California specialized in forest diseases; by the time that sudden oak death emerged as a major problem in California, there were none. He further observed that agricultural departments in colleges across the United States continue to be downsized.
Speakers Galgiani and Holland emphasized the importance of improved education of physicians and other front-line healthcare workers in the diagnosis and treatment of fungal diseases. Holland noted that fungal diseases due to previously undiagnosed primary immune deficiencies are not frequent, but that a few cases happen “every year, in every country.” He further cautioned that, “if you don’t think about them and you don’t recognize these diseases as fungal, the patients don’t survive.” Even in areas with endemic fungal disease, the correct differential diagnosis is often missed, commented Galgiani. In the case of symptomatic Valley Fever, which often initially presents as a community-acquired pneumonia, specific antibody testing is required to discriminate Coccidioides infections from other causes of pneumonias. Even then, Galgiani noted, with early infections conventional serological testing produces false positives in an estimated one third to two thirds of all infected patients.
Rapid and Accurate Tools for Detection and Diagnosis
The lack of sensitive and specific tools for the diagnosis of emerging diseases limits the effectiveness of disease surveillance and treatment efforts (IOM, 2007). In the ornamental plant trade, surveillance focuses on preventing the introduction of plant pathogens. This occurs at the international, national, and regional levels, Rizzo explained. In Europe and North America, strict controls have been placed on nurseries to quickly contain and eradicate outbreaks of P. ramorum (Kliejunas, 2010). In the United States, plants in nurseries are subject to routine inspections, and plants that are sold across state or county boundaries are closely monitored. In states such as Oregon, Washington, and California, they are quarantined to ensure they are free from infection (Kliejunas, 2010). Despite these measures, P. ramorum continues to spread via ornamental plant trade pathways (see sudden oak death case example). In part, this is due to the lack of effective detection tools. Asymptomatic infections and the use of fungicides can limit the effectiveness of quarantine protocols based only on visual inspection.
To track the spread of the C. gattii outbreak, public health officials in British Columbia and the U.S. Pacific Northwest have listed C. gattii–associated disease as a reportable disease. However, speaker Julie Harris of the CDC remarked that C. gattii surveillance has been limited by overreporting of the most severe cases and underreporting of all cases. In cases where samples are sent to the laboratory for identification, not all labs are using the canavanine-glycine-bromothymol blue (CGB) agar test81 or genetic sequencing82 that are needed to differentiate between C. gattii and C. neoformans infections. Harris noted that underreporting is due to a number of factors including the fact that many of the smaller laboratories across Washington and Oregon:
- may not be aware of the need for culture to make an accurate diagnosis;
- may not be aware that they should be sending isolates to their respective state health departments for confirmatory testing; or
- may lack the capacity for fungal culture and testing.
Harris observed that labs with mycology capacity are not as common as labs with viral or bacterial capacity, and requests for additional training or capacity need to come from the local level. She noted that the C. gattii Public Health Working Group, formed in 2008 by the CDC and state and local public health departments and laboratories and the British Columbian Centre for Disease Control, is working to standardize surveillance by increasing clinician awareness of C.
_______________
81 During a canavanine-glycine-bromothymol blue agar test, C. gattii grows and the medium changes color; C. neoformans does not grow, and the medium remains a light green color.
82 Bartlett pointed out that because of the close evolutionary relationship between the two species, much of the early literature on the outbreak refers to the outbreak pathogen by its former name, C. neoformans var. gattii.
gattii infection and working with global laboratories to characterize genetic and phenotypic variety in C. gattii.
Padilla, from the Smithsonian Conservation Biology Institute (SCBI), identified fungal diagnostic capacity as a particularly challenging area of emerging fungal disease threat management in wild animals and one in need of further research. Too often, new fungal pathogens in wildlife are either misdiagnosed or undiagnosed. The limited diagnostic capacity leads to the “clumping” of information under known fungal disease syndromes, Padilla remarked, and this often precludes the recognition of true emerging fungal diseases and prevents further investigation of true host–pathogen dynamics. Fungal infections are often identified only to the genus, not the species level, making it difficult to understand host–species relationships. The clumping of information could also result in dangerous management decisions—when assumptions about one host are based on what is known about another host. For example, he reported that preliminary findings by researchers working at the SCBI suggested that the bacterium Janthinobacterium lividum does not provide the same anti-chytridiomycosis protection in Bd-infected captive-bred Panamanian golden frogs (Atelopus zeteki), as it does in Bd-infected yellow mountain frogs (Rana muscosa). In fact, J. lividum seems limited in its ability to colonize the skin of A. zeteki and thus is also limited in its ability to play the same anti-fungal role that it does in R. muscosa. However, the understanding of a bacterium–host system conferring anti-fungal protective properties suggests that other species-specific host-adapted bacteria could confer the same protection to A. zeteki. Padilla expressed hope that these findings will lead to more appropriate treatments for this particular frog species.
The Fungal “Background”
Limited understanding of fungal biodiversity and biogeography can impede surveillance, detection, and discovery efforts, noted Blehert. It has been reported that an abundance of closely related Geomyces species have been found in the same soil that harbors G. destructans (Lindner et al., 2010). These related species are currently confounding the ability to conduct routine soil analysis as a mode of surveillance for WNS, Blehert said. Rizzo added that “when we find something new, it’s very difficult to know whether it is exotic and something to be concerned about or an interesting new native organism that we weren’t aware of.”
Russell asked participants for their views on effective techniques for the “discovery” of fungal pathogens (i.e., detection of an unknown disease agent). Heitman suggested that the fungal nuclear ribosomal internal transcribed spacer (ITS83) sequences might be useful for determining if genetic material isolated by researchers is fungal in origin. Blackwell mentioned that mycologists now use
_______________
83 ITS sequences are sections of non-functional RNA that are highly variable even between closely related species and are widely used for taxonomic purposes (e.g., Cendejas-Bueno et al., 2010; Garner et al., 2010; Leaw et al., 2006).
non-culture–based molecular tools coupled with field explorations to identify new fungal species (Jones et al., 2011; Jumpponen and Jones, 2009; Lara et al., 2010; Porter et al., 2008; Schadt et al., 2003) and to characterize the distribution of fungal species (Daughtrey et al., 1996). These techniques are increasingly being used because many fungal species are not easily cultured. Culturing techniques and pathology investigations, however, are still needed to characterize an organism: these are “hard and laborious things to do,” observed Daszak, but the “value of the product is so much better, it is orders of magnitude better, because you can do something with it.” You can “send it to others for consultation or determine an organism’s biology or how it causes disease in host species,” Daszak said.
Blackwell noted that the NSF has had several programs in systematics84 and biodiversity for some years. In 2004, the NSF created the “Assembling the Tree of Life” program with the goal of constructing an evolutionary history for all major lineages of life (See glossary for more information).These and other related programs have increased support for research on the evolution and diversity of Kingdom Fungi, which has been helpful for improving detection, diagnosis, and discovery methods (see Blackwell et al., 2006; Hibbett et al., 2007). A database of 40,000 fungal species (with an emphasis on fungal plant pathogens) developed by the USDA Agricultural Research Service includes information such as host range, geographic distribution, relevant scientific literature, and for some species, descriptions and illustrations (Rossman and Palm-Hernandez, 2008).
Treatment and Response
Active surveillance and early detection of emerging fungal diseases are important and partially effective. However, due to the magnitude of trade in plants and plant products, “[pathogens] are getting through, and they are going to get through; we are not going to stop that” as Stack remarked. Attention is also needed to developing ways to respond to fungal disease threats (e.g., effective and economical treatment options) and to recover from emerging fungal diseases.
Responding to Fungal Diseases of Plants—from Agriculture to Landscapes
In plants, disease eradication strategies include clear-cutting or controlled burning of infected plants (Rizzo et al., 2005). Fungicides are often used to protect high-value plants, but their widespread and frequent use is often not economically feasible (Rizzo et al., 2005; Scheffer et al., 2008). The development of resistant cultivars or strains, which may take years to decades, is currently the most successful disease control strategy in plants, particularly agricultural crops (Vurro et al., 2010).
_______________
84 The study of the general principles of scientific classification, and the classification of organisms according to the presumed, natural, and evolutionary relationships among them.
Developing strains of wheat that are resistant to the newly emerging and more aggressive forms of yellow rust is the primary strategy for limiting the devastating effects of P. striiformis on wheat. In the meantime, early detection is essential to reduce crop yield losses due to yellow rust, Hovmøller said. In the short term, options for control are limited to fungicide sprays which may be unavailable or not affordable to farmers in the developing world. The replacement of susceptible wheat with locally adapted, resistant, or less susceptible varieties can also slow disease spread, he remarked.
As Hovmøller noted, when it comes to wheat rust, “what’s going on in one continent may be your problem the following day.” To prevent long-term damage, intensified international collaboration is needed to build wheat rust surveillance, detection, and response capacity. Several promising developments on the international scale were reported by Hovmøller. In 2008, a Global Rust Reference Center (GRRC) for yellow rust was established to improve yellow rust management in countries where facilities and expertise are scarce. GRRC is supported by Aarhus University in Denmark, the International Center for Agricultural Research in the Dry Area, and the International Maize and Wheat Improvement Center (CIMMYT). In 2011 the activities will be extended to wheat stem rust (Puccinia graminis) via projects facilitated by the Borlaug Global Rust Initiative. GRRC is complementing existing national diagnostic laboratories, which cannot receive rust samples year round from all countries. The primary goals of GRRC are to conduct virulence and race85 analyses, secure isolates for future resistance breeding and research, facilitate research and training, and provide information for a global wheat rust early warning system. The Borlaug Global Rust Initiative, which was established in response to the stem rust Ug99 outbreak in East Africa, now deals with all three wheat rusts.86
Disease management is considerably more difficult when dealing with plants of limited economic value (i.e., non-timber and non-crop plants), despite their significant ecological value, Rizzo noted. Management of sudden oak death and ramorum blight, according to Rizzo, is “scale dependent.” One can manage individual trees, landscapes, or entire regions (Rizzo et al., 2005). At the individual level, fungicides are available that can be injected into a tree or sprayed on the bark to prevent infection (Garbelotto et al., 2002). In forests, containment, including cutting and controlled burnings in areas with infected trees, is the primary means of infection control. When asked whether there is a possibility for developing treatments for sudden oak death that could be applied at the landscape level, Rizzo said the options are limited. In Oregon, there have been attempts to conduct aerial spraying with phosphonate (a chemical fungicide), but it is unlikely that any type of aerial spraying would be acceptable in California. Rizzo also cautioned that it took many decades to breed genetic resistance for Dutch
_______________
85 A subspecies group of pathogens that infect a given set of plant varieties (Cornell University, plant pathology glossary).
86 For more information see www.globalrust.org.
elm disease. The complexity of oak genetics also makes it very challenging to use breeding to develop oaks resistant to P. ramorum infection. Rizzo went on to note that research on potential biocontrol agents, such as viruses, is at a very early stage.
Landscape-level management also uses predictive modeling. For sudden oak death, models based on host species distribution, climate, and other factors identify areas at risk for invasion by the pathogen. These areas can then be surveyed using “aerial imaging, plot-based monitoring, and stream sampling to determine the presence of P. ramorum or signs of infected trees” (IOM, 2008b). Eradication methods are only effective if the disease is detected early enough. For areas where the pathogen is established, management approaches seek to avoid negative ecological consequences, such as the growth of invasive plant species. Ultimately, Rizzo said, we are trying to develop methods to “live with the pathogen.”
Treatment Options for Fungal Diseases of Humans and Animals
Available antifungal therapies are generally of limited value due to toxicity problems (Figure WO-26) (Ostrosky-Zeichner et al., 2010). The lack of accurate diagnostics further limits the effectiveness of existing fungal treatments. Approaches using antibody therapy and vaccines (for certain endemic pathogens) remain challenging due to the ongoing evolution of pathogens (Cox and Magee, 2004; Galgiani, 2007, 2008; Ostrosky-Zeichner et al., 2010). Overall, there are few new therapeutic agents in the development pipeline with the potential for broad antifungal effects (Ostrosky-Zeichner et al., 2010).
The development of new treatments for fungal diseases has also been slowed by an underappreciation for the effects that fungal diseases can have on human health, Galgiani asserted. In endemic areas of Arizona and California, about a third of all coccidioidomycosis cases lead to illnesses requiring medical attention87 (Tsang et al., 2010). While oral therapy with azole antifungal drugs is safe and convenient, many patients do not respond to treatment (20–40 percent failure rate). Moreover, many patients who initially respond to treatment experience relapses after treatment ends (Galgiani, 2007; Hector and Laniado-Laborin, 2005). Galgiani reviewed current efforts at the University of Arizona to develop a new antifungal known as nikkomycin Z, a competitive inhibitor of chitin synthase that interferes with cell wall construction (Galgiani, 2007). Discovered in an antifungal discovery program by Bayer in the 1970s, the compound demonstrated antifungal activity in mice in the 1980s (see Hector et al., 1990). Only after the
_______________
87 Valley fever is often dismissed as a self-resolving mild illness. In fact valley fever can be long-lasting and have a tremendous impact on activity levels (see Galgiani, 2007). Recent surveillance activities conducted by the Arizona Department of Health Services, in collaboration with the CDC, reported that coccidioidal illness lasted an average of 6 months, with 75 percent of workers taking more than one month of sick leave and 40 percent of infected persons requiring at least one night of hospitalization at some point during the course of their illness (Tsang et al., 2010). Annual hospital costs alone amount to nearly $90 million ($86 million in 2007; Tsang et al., 2010).
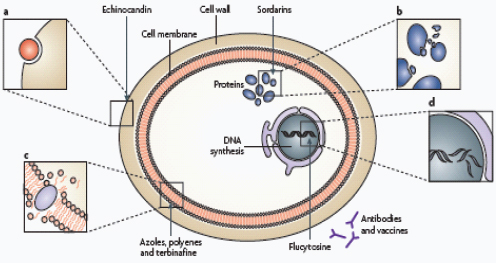
Figure WO-26 Mechanisms of action of selected antifungals. An illustration of the mechanism of action of currently available antifungals as well as selected antifungals under development. (A) Echinocandins and nikkomycin Z inhibit the formation of the fungal cell wall. (B) Sordarins interfere with protein assembly. (C) Azoles, polyenes, and terbinafine disrupt the fungal cell membrane. (D) Flucytosine interferes with DNA synthesis. Antibodies and vaccines prevent fungal infection or block and/or destroy the fungal cells.
SOURCE: Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Drug Discovery (Ostrosky-Zeichner, L., A. Casadevall, J. N. Galgiani, F. C. Odds, and J. H. Rex. 2010. An insight into the antifungal pipeline: Selected new molecules and beyond. Nature Reviews Drug Discovery 9(9):719–727).
University of Arizona acquired the compound in 2005, have clinical trials resumed (Galgiani, 2007).88
In the absence of a vaccine or other preventive measures for C. gattii infection of humans and animals, officials concede the public can do little to protect themselves from infection (Knox, 2010). Additional research is needed to clarify the epidemiology and drug susceptibilities of the various strains of C. gattii present in the region to help inform treatment guidelines. Moreover, researchers need to learn more about the natural history and pathogenicity of the fungus to further prevention, treatment, and intervention efforts (Datta et al., 2009a,b). Heitman noted that research is ongoing to determine the nature of hypervirulence (D’Souza et al., 2011); the differences between immune responses to C. gattii and C. neoformans infections (Cheng et al., 2009); and why C. gattii can so readily invade the cells of immunocompetent individuals (Kronstad et al., 2011; Ma et al., 2009; Voelz and May, 2010).
_______________
88 See conributed manuscript by Galgiani in Appendix A (pages 196–207).

Figure WO-27 Frogs in the Sierra Nevada region, being treated in baths containing a fungicidal bacterium in hopes of eliminating infection by the fungal pathogen (Bd) associated with the deadly disease: amphibian chytridiomycosis.
SOURCE: Photo by Anand Varma.
The challenge of developing effective treatments for fungal diseases is compounded by the scale and complexity of treating diseases of wildlife. Fungicidal treatment protocols are being explored for amphibian chytridiomycosis and include methods to alter the skin microbiome89 (Fisher et al., 2009; Harris et al., 2009) (Figure WO-27). For WNS, some researchers are investigating whether treating bats with antifungal agents might improve their survival (Platt, 2010), while others are exploring the possibility of developing a vaccine against this fungal pathogen (Buchen, 2010). Adapting these protocols to large and dispersed wild animal populations, while minimizing unanticipated ecosystem impacts, is challenging and may continue to limit conservation efforts (Fisher et al., 2009). In addition to captive breeding programs, conservation efforts target habitat preservation, limiting the spread of infected species, and protecting endangered species. Further research is also needed to fill in some gaps in knowledge that still exist. Researchers need to gain a better understanding of the biology of both Bd and G. destructans and their respective hosts to answer questions related to the determinants of virulence, the hallmarks of effective immune response, and the specific
_______________
89 Fungicides, and more recently cutaneous bacteria of amphibians (e.g., J. lividum) known to produce an antifungal metabolite, are applied to the skin of amphibians.
mechanisms by which these pathogens kill their hosts. Added to this is the unique challenge of managing disease in hibernating animals in delicate underground ecosystems. Biology of infectious diseases, however, is not part of the traditional wildlife ecology education curriculum, Blehert remarked. Nor are speleologists, tourists, recreational cavers, or hikers required to have such knowledge.
Buying Time Through the Conservation of Threatened Wildlife Populations
Captive breeding programs at the SCBI90 have helped to rescue species that were on the verge of extinction, endangered by habitat loss or by the introduction of disease into areas with naïve and susceptible host populations. According to Padilla, captive propagation programs can also serve two additional functions in the management and mitigation of emerging fungal diseases91:
- Fungal diseases identified in a captive animal population can be an early indication of an emerging threat in the wild. Based on Padilla’s experience, there is a wide range of opportunistic and primary fungal diseases that have been observed in captive animals.
- Captive populations are also established for the purpose of studying a fungal disease that would otherwise be difficult or impossible to study. An example of this important work is the captive population of Japanese giant salamanders (Andrias japonicus) established by the Smithsonian National Zoological Park, in which the presence of Bd and the efficacy of itraconazole treatment can be studies and monitored over time in ways that could not be possible in their wild counterparts.
SCBI has used captive breeding to save several species from extinction. The endangered black-footed ferret population was revived from just 18 individuals in 1988 to a current population of 800 to 1,000 in the wild (Weidensaul, 2000). Other animal species, including the golden lion tamarin, California condors, Przewalski’s horses, and the scimitar-horned oryx, have also benefited from the SCBI’s captive breeding program’s success.
Despite success with multiple species, establishing and maintaining captive breeding programs is technically challenging. In the fall of 2010, SCBI developed a captive colony of the endangered Virginia big-eared bats (Corynorhinus townsendii virginianus) in response to the threat posed by G. destructans. Specialist insect-eating bats, such as the Virginia big-eared bat, are notoriously difficult to keep in captivity. But, Padilla noted, in light of the possible extinction of this endangered subspecies, SCBI decided to take on the “high risk” project
_______________
90 Formerly known as the National Zoo’s Conservation and Research Center, the SCBI is an umbrella organization for the Smithsonian’s global efforts to conserve species and train future generations of conservationists. See http://nationalzoo.si.edu/scbi/default.cfm.
91 See contributed manuscript by Padilla in Appendix A (pages 296–312).
of developing a captive colony of these bats. However, Padilla said, although the bats did not die of WNS, the colony of 40 bats experienced extremely high mortality (90 percent) in the first 200 days of captivity.
The Amphibian Ark92 is a global network of captive breeding programs working in the short term to protect amphibian species at immediate risk of extinction (IUCN, 2005). The Smithsonian’s National Zoo currently houses a fifth of the world’s Panamanian golden frog populations (Figure WO-28). It is hoped that the Smithsonian’s expertise in captive breeding will contribute to the preservation of amphibians and New World bats that are currently at risk of local or global extinction.
Weldon highlighted a number of successful conservation programs that target amphibian populations outside of captive breeding programs.93 These included:
- The population management of Alytes obstetricans in Peńalara Natural Park, Spain, in response to annual Bd outbreaks;
- Australia’s Bd Threat Abatement Plan, which was initiated in 2006, with the goal of preventing amphibian populations and regions that are currently free of chytridiomycosis from becoming infected; and
- Madagascar’s Early Detection Plan, which monitors high-risk areas (e.g., ports of importation, areas visited by tourists, areas of high biodiversity) and builds facilities for captive breeding and research in the event that Bd does reach the island (Weldon et al., 2008).
Importantly, Weldon noted, these programs include disease prevention as a prioritized conservation measure.
Weldon also recounted one instance in which conservation efforts unintentionally contributed to the spread of diseases. Population decline of amphibians on the island of Mallorca were linked to Bd infection in 2008 (Walker et al., 2008). The “source” of infection was traced to a project designed to boost populations of the island’s midwife toad (Alytes muletensis). Cross-contamination is thought to have occurred between two species that were cohoused at the breeding facility: the midwife toad and imported frogs from South Africa (Xenopus gilli) that were infected with Bd. Captive midwife toads reintroduced into the wild served as vectors that brought the pathogen to other amphibian populations on the island (Rosenblum et al., 2009; Walker et al., 2008). Weldon said, “This illustrates that if we are to proceed with the reintroduction programs, great caution should be taken, because you could potentially be introducing the pathogens with the species that you are trying to conserve.”
_______________
92 The Amphibian Ark carries out the ex situ components of the Amphibian Conservation Action Plan developed by the World Conservation Union. For more information, see http://www.amphibianark.org/pdf/ACAP.pdf and www.amphibianark.org.
93 See contributed manuscript by Weldon and Fisher in Appendix A (pages 355–367).

Figure WO-28 Panamanian golden frog (Atelopus zeteki). The Smithsonian National Zoo has established a captive breeding program to help rescue this critically endangered species.
SOURCE: Photo by Brian Gratwicke, Wikimedia commons.
Prospects for Preventing and Managing Emerging Fungal Diseases
Although participants described a number of challenges in efforts to detect and respond to emerging fungal pathogens, discussion also revealed many opportunities to better prevent and manage these threats. Daszak stressed the need to “focus on the underlying causes, because they cross all the kingdoms. The drivers of plant disease also drive the emergence of disease in wildlife and humans: travel, trade, agriculture, deforestation, and other environmental disturbances.” While improving capacity to prevent and manage disease emergence is an enormous undertaking and a long-term endeavor, Daszak stressed that “the evidence and data on steps that can be taken are there; it is just a matter of turning our knowledge into action.”
To many participants, a better understanding of the ecology of fungi and fungal disease seemed paramount (Rizzo, 2005). To better manage outbreaks of infectious disease, scientists may also benefit from a greater understanding of biological invasions in all their variety and complexity. The incipient “cross-fertilization” of ecology and epidemiology offers support for such investigations, as does the growing recognition of the interdependence of human, animal, and plant health, and of the central importance of the environment in influencing host–pathogen interactions (Scholthof, 2007). The following strategies and areas
of focus for preventing and managing all types of biological invasions, including fungal pathogens, were discussed during the workshop as particularly promising:
- Anticipating invasions based on global and local trade patterns (Dybas, 2004);
- Identifying and interrupting routes of transport that represent high risk for biological invasions of all kinds, rather than focusing on individual species or known diseases (Rossman, 2009);
- Recognizing the importance of human travelers as disease couriers, transmitters, and sentinels and, therefore, a critical target for infectious disease surveillance and detection (Pimentel et al., 2005);
- Recognizing that domestic animals, wildlife, and plants can also serve as important “asymptomatic” carriers or sentinels for disease and are also an important target for disease surveillance and detection efforts;
- Prescreening imported plants and animals that are likely to become problematic invasive species (O’Donnell, 2006);
- Establishing the prevention of the spread of invasive species as an international public good, which requires coordination among nation states (Keller and Perrings, 2010). Because such a system is only as strong as the “weakest link,” efforts are also needed to assist developing nations in establishing capacity for surveillance, detection, and prevention of biological invasions (Keller and Perrings, 2010);
- Educating the public and inspectors at airports and seaports about the environmental and economic threats posed by invasive species, and the role of tourism and travel in biological introductions (Pimentel et al., 2005);
- Focusing efforts on markets (e.g., wildlife markets) to regulate, reduce, or eliminate trade that threatens the health of humans, domestic animals, wildlife, and ecosystems (Karesh et al., 2005);
- Developing bioeconomic models to assess the economic impact of the introduction of invasive species and of alternatives for their prevention and mitigation (Evans, 2003);
- Increasing capacity for the early detection of, and rapid response to, biological invasions (Dybas, 2004); and
- Applying mathematical models to forecast the worldwide spread of infectious diseases, identify endangered regions, and analyze potential control strategies (Hufnagel et al., 2004; Weinberg, 2005).
Each of these approaches supports the overall goal of identifying and exploiting common characteristics of invasive animals, plants, and microbes in order to reduce their impact. To pursue this strategy requires “a new perspective, a new thinking, a consideration of all alien introductions in a deliberate, truly comprehensive system,” ecologist Richard Mack has observed (Dybas, 2004, p. 618). “If we do that, then we will have a sound science-based policy.”
Aanen, D. K., P. Eggleton, C. Rouland-Lefèvre, T. Guldberg-Frøslev, S. Rosendahl, and J. J. Boomsma. 2002. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proceedings of the National Academy of Sciences, USA 99:14887–14892.
Aanensen, D. M., D. M. Huntley, E. J. Feil, F. al-Own, and B. G. Spratt. 2009. EpiCollect: Linking Smartphones to web applications for epidemiology, ecology and community data collection. PLoS ONE 4(9):1–7.
Alberts, B., A. Johnson, J. Lewis, M. Raff, K. Roberts, and P. Walter. 2002. Molecular biology of the cell, 4th ed. New York: Garland Science.
Anderson, P. K., A. A. Cunningham, N. G. Patel, F. J. Morales, P. R. Epstein, and P. Daszak. 2004. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends in Ecology & Evolution 19(10):535–544.
Arnold, A. E. 2007. Understanding the diversity of foliar endophytic fungi: Progress, challenges, and frontiers. Fungal Biology Reviews 21:51–66.
Arnold, A. E., L. C. Mejia, D. Kyllo, E. I. Rojas, Z. Maynard, N. Robbins, and E. A. Herre. 2003. Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences, USA 100:15649–15654.
Bahn, Y. S., C. Xue, A. Idnurm, J. C. Rutherford, J. Heitman, and M. E. Cardenas. 2007. Sensing the environment: Lessons from fungi. Nature Reviews Microbiology 5:57–69.
Baker, M. G., and D. P. Fidler. 2006. Global public health surveillance under new international health regulations. Emerging Infectious Diseases 12(7):1058–1065.
Barclay, R. M. R., J. Ulmer, C. J. A. MacKenzie, M. S. Thompson, L. Olson, J. McCool, E. Cropley, and G. Poll. 2004. Variation in the reproductive rate of bats. Canadian Journal of Zoology 82:688–693.
Bartlett, K. 2010. Knowing where to look—environmental sources of cryptococcal disease in human and animal residents in the Pacific Northwest. Presentation given at the December 14–15, 2010, public workshop, “Fungal Diseases: An Emerging Challenge to Human, Animal, and Plant Health,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Bartlett, K. H., S. E. Kidd, and J.W. Kronstad. 2007. The emergence of Cryptococcus gattii in British Columbia and the Pacific Northwest. Current Fungal Infection Reports 1:108–115.
Bat Conservation International. 2010. What we do: White-nose syndrome. http://www.batcon.org/wns (accessed May 16, 2010).
Becker, G. S. 2009. U.S. food and agricultural imports: Safeguards and selected issues. CRS Report RL34198.
Berger, L., R. Speare, P. Daszak, D. E. Green, A. A. Cunningham, C. L. Goggin, R. Slocombe, M. A. Ragan, A. D. Hyatt, K. R. McDonald, H. B. Hines, K. R. Lips, G. Marantelli, and H. Parkes. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences, USA 95:9031–9036.
Berger, L., R. Speare, H. B. Hines, G. Marantelli, A. D. Hyatt, K. R. McDonald, L. F. Kerratt, V. Olsen, J. M. Clarke, G. Gillespie, M. Mahony, N. Sheppard, C. Williams, and M. Tyler. 2004. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Australian Veterinary Journal 82:434–439.
Berger, L., A. D. Hyatt, R. Speare, and J. E. Longcore. 2005. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 68(1):51–63.
Bergman, A., and A. Casadevall. 2010. Mammalian endothermy optimally restricts fungi and metabolic costs. mBio 1(5):e00212–10.
Blackwell, M. 2010. The good, the bad, and the ugly: Fungi mold your world. Presentation given at the December 14–15, 2010, public workshop, “Fungal Diseases: An Emerging Challenge to Human, Animal, and Plant Health,” Forum on Microbial Threats, Institute of Medicine, Washington D.C.
———. 2011. The fungi: 1, 2, 3...5.1 million species? American Journal of Botany 98(3): 426–438.
Blackwell, M., D. S. Hibbett, J. W. Taylor, and J. W. Spatafora. 2006. Research coordination networks: A phylogeny for kingdom Fungi (Deep Hypha). Mycologia 98:829–837.
Blackwell, M., R. Vigalys, R. James, Y. Timothy, and J.W. Taylor. 2009. Fungi. Eumycota: Mushrooms, sac fungi, yeast, molds, rusts, smuts, etc. (Version 10). http://tolweb.org/Fungi/2377/2009.04.10 (accessed October 4, 2010).
Blanchette, R. A., A. M. Wilmering, and M. Baumeister. 1992. The use of green-stained wood caused by the fungus Chlorociboria in intarsia masterpieces from the 15th century. Holzforschung 46:225–232.
Blehert, D. S., A. C. Hicks, M. Behr, C. U. Meteyer, B. M. Berlowski-Zier, E. L. Buckles, J. T. H. Coleman, S. R. Darling, A. Gargas, R. Niver, J. C. Okoniewski, R. J. Rudd, and W. B. Stone. 2009. Bat white-nose syndrome: An emerging fungal pathogen? Science 323(5911):227.
Boyles, J. G., P. M. Cryan, G. F. McCraken, and H. Kunz. 2011. Conservation: Economic importance of bats in agriculture. Science 332(6025):41–42.
Brasier, C. M. 2000. The rise of the hybrid fungi. Nature 405:134–135.
———. 2008. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathology 57(5):792–808.
Brasier, C. M., and K. W. Buck. 2001. Rapid evolutionary changes in a globally invading fungal pathogen (Dutch elm disease). Biological Invasions 3:223–233.
Brasier, C. M., and J. Webber. 2010. Sudden larch death. Nature 466:824–825.
Brasier, C. M., D. E. L. Cooke, and J. M. Duncan. 1999. Origin of a new Phytophthora pathogen through interspecific hybridization. Proceedings of the National Academy of Sciences, USA 96: 5978–5983.
Briggs, C. J., A. R. Knapp, and V. T. Vredenburg. 2010. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proceedings of the National Academy of Sciences, USA 107 (21):9695–9700.
Bromenshenk, J. J., C. B. Henderson, C. H. Wick, M. F. Stanford, and A. W. Zulich. 2010. Iridovirus and microsporidian linked to honey bee colony decline. PLoS ONE 5(10):e13181.
Brown, J. K. M., and M. S. Hovmøller. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297(5581):537–541.
Brownstein, J. S., C. C. Freifeld, and L. C. Madoff. 2009. Digital disease detection—harnessing the web for public health surveillance. New England Journal of Medicine 360(21):2153–2157.
Brucker, R. M., R. N. Harris, C. R. Schwantes, T. N. Gallaher, D. C. Flaherty, B. A. Lam, and K. P. Minbiole. 2008. Amphibian chemical defense: Antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. Journal of Chemical Ecology 34:1422–1429.
Buchen, L. 2010. Disease epidemic killing only U.S. bats. Nature 463(7278):144–145.
Buckley, M. 2008. The Fungal Kingdom: A report from the American Academy of Microbiology. Washington, DC: American Academy of Microbiology.
Buckley, R. H., B. B. Wray, and E. Z. Belmaker. 1972. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics 49(1):59–70.
Butterworth, M. H., M. A. Semenov, A. Barnes, D. Moran, J. S. West, and B. D. L. Fitt. 2010. North-south divide: Contrasting impacts of climate change on crop yields in Scotland and England. Journal of the Royal Society Interface 7:123–130.
Byrnes, E. J., R. J. Bildfell, S. A. Frank, T. G. Mitchell, K. A. Marr, and J. Heitman. 2009. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. The Journal of Infectious Diseases 199:1081–1086.
Byrnes, E. J., W. Li, Y. Lewit, H. Ma, K. Voelz, P. Ren, D. A. Carter, V. Chaturvedi, R. J. Bildfell, R. C. May, and J. Heitman. 2010. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathogens 6(4):e1000850.
Capasso, L. 1998. 5300 years ago, the ice man used natural laxatives and antibiotics. The Lancet 352:1864.
Carlton, J. 2004. Invasions in the world’s oceans: How much do we know, and what does the future hold? Presentations to the annual meeting of the American Institute of Biological Sciences, 2004. Available at: http://www.aibs.org/media-library
Casadevall, A. 2005. Fungal virulence, vertebrate endothermy, and dinosaur extinction: Is there a connection? Fungal Genetics and Biology 42:98–106.
———. 2007. Determinants of virulence in the pathogenic fungi. Fungal Biology Reviews 21:130–132.
———. 2010. Emerging fungal pathogens—past, present, and future. Presentation given at the December 14–15, 2010, public workshop, “Fungal Diseases: An Emerging Challenge to Human, Animal, and Plant Health,” Forum on Microbial Threats, Institute of Medicine, Washington, D.C.
Casadevall, A., and L. A. Pirofski. 2003. The damage response framework of microbial pathogenesis. Nature Reviews, Microbiology 1(1):17–24.
———. 2007. Accidental virulence, cryptic pathogenesis, martians, lost hosts, and the pathogenicity of environmental microbes. Eukaryotic Cell 6:2169–2174.
Cash, R. A., and V. Narasimhan. 2000. Impediments to global surveillance of infectious diseases: Consequences of open reporting in a global economy. Bulletin of the World Health Organization 78(11):10.
Catenazzi, A., V. T. Vredenburg, and E. Lehr. 2010. Batrachochytrium dendrobatidis in the live frog trade of Telmatobius (Anura: Ceratophyryidae) in the tropical Andes. Diseases of Aquatic Organisms Preprint, 2010. http://web.me.com/vancevredenburg/Vances_site/Publications_files/CatenazziVredenburgLehr2010.pdf.
CDC (Centers for Disease Control and Prevention). 2010. Emergence of Cryptococcus gattii—Pacific Northwest, 2004–2010. Morbidity and Mortality Weekly Report 59(28):865–868.
Cendejas-Bueno, E. A., E. Gomez-Lopez, E. Mellado J. L. Rodriguez-Tudela, and M. Cuenca-Estrella. 2010. Identification of pathogenic rare yeast species in clinical samples: Comparison between phenotypical and molecular methods. Journal of Clinical Microbiology 48:1895–1899.
Chan, C. 2011. Valley fever cases likely to increase after Phoenix dust storm. The Arizona Republic. July 18.
Chan, E. H., T. F. Brewer, L. C. Madoff, M. P. Pollack, A. L. Sonricker, M. Keller, C. C. Freifeld, M. Blench, A. Mawudeku, and J. S. Brownstein. 2010. Global capacity for emerging infectious disease detection. Proceedings of the National Academy of Sciences, USA 107:1–6.
Chaturvedi, V., D J. Springer, M. J. Behr, R. Ramani, X. Li, M. K. Peck, P. Ren, D. J. Bopp, B. Wood, W. A. Samsonoff, C. M. Butchkoski, A. C. Hicks, W. B. Stone, R. J. Rudd, and S. Chaturvedi. 2010. Morphological and molecular characterizations of psychrophilic fungus Geomyces destructans from New York bats with white nose syndrome (WNS). PLoS ONE 5(5):e10783.
Chen, X. M. 2005. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Canadian Journal of Plant Pathology 27:314–337.
Cheng, P. Y., A. Sham, and J. W. Kronstadt. 2009. Cryptococcus gattii isolates from the British Columbia Cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infectious Immunity 77:4284–4294.
Chiller, T. M., J. N. Galgiani, and D. A. Stevens. 2003. Coccidioidomycosis. Infectious Disease Clinics of North America 17:41–57.
Choffnes, E. R. 2008. Improving infectious disease surveillance. Bulletin of the Atomic Scientists http://www.thebulletin.org/web-edition/op-eds/improving-infectious-disease-surveillance (accessed October 26, 2010).
Cliff, A., and P. Haggett. 2004. Time, travel and infection. British Medical Bulletin 69(1):87–99.
Cox, R. A., and D. M. Magee. 2004. Coccidioidomycosis: Host response vaccine development. Clinical Microbiology Reviews 17:804–839.
Crum-Canflone, N. F. 2007. Coccidioidomycosis in the U.S. military: A review. Annals of the New York Academy of Sciences 1111:112–121.
Cryan, P. M., C. Uphoff Meteyer, J. G. Boyles, and D. S. Blehert. 2010. Wing pathology of whitenose syndrome in bats suggests life-threatening disruption of physiology. BMC Biology 8(135).
Cunningham, A. A., and P. Daszak. 1998. Extinction of a species of land snail due to infection with a microsporidian parasite. Conservation Biology 12:1523–1739.
Currie, C. R., U. G. Mueller, and D. Malloch. 1999. The agricultural pathology of ant fungus gardens. Proceedings of the National Academy of Sciences, USA 96(14):7998–8002.
Currie, C. R., B. Wong, A. E. Stuart, T. R. Schultz, S. A. Rehner, U. G. Mueller, G. H. Sung, J. W. Spatafora, and N. A. Straus. 2003. Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299:386–388.
Cutler, J. E., S. G. Deepe, Jr., and B. S. Klein. 2007. Advances in combating fungal diseases: Vaccines on the threshold. Nature Reviews Microbiology 5:13–28.
Dadachova, E., and A. Casadevall. 2008. Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Current Opinions in Microbiology 11(6):525–531.
Dadachova, E., R. A. Bryan, X. Huang, T. Moadel, A. D. Schweitzer, P. Aisen, J. D. Nosanchuk, A. Casadevall. 2007. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2(5):e457.
Daszak, P. 2010. Global capacity for coordinated surveillance, detection, and response to emerging diseases of wildlife. Presentation given at the December 14–15, 2010, public workshop, “Fungal Diseases: An Emerging Challenge to Human, Animal, and Plant Health,” Forum on Microbial Threats, Institute of Medicine, Washington D.C.
Daszak, P., A. A. Cunningham, and A. D. Hyatt. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287(5452):443–449.
———. 2003. Infectious disease and amphibian population declines. Diversity and Distributions 9:141–150.
Daszak, P., A. Strieby, A. A. Cunningham, J. E. Longcore, C. C. Brown, and D. Porter. 2004. Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetological Journal 14:201–207.
Datta, K., K. H. Bartlett, R. Baer, E. Byrnes, E. Galanis, J. Heitman, L. Hoang, M. J. Leslie, L. MacDougall, S. S. Magill, M. G. Morshed, and K. A. Marr. 2009a. Spread of Cryptococcus gattii: Into Pacific Northwest region of the United States. Emerging Infectious Diseases 15(8):1185–1191.
Datta, K., K. H. Bartlett, and K. A. Marr. 2009b. Cryptococcus gattii: Emergence in western North America: Exploitation of a novel ecological niche. Interdisciplinary Perspectives on Infectious Diseases. Article ID 176532, 8 pages doi:10.1155/2009/176532.
Daughtrey, M. L., C. R. Hibben, K. O. Britton, M. T. Windham, and S. C. Redlin. 1996. Dogwood anthracnose: Understanding a disease new to North America. Plant Disease 80(4):349–358.
Davis, S. D., J. Schaller, and R. J. Wedgwood. 1996. Job’s syndrome: Recurrent, “cold,” staphylococcal abscesses. The Lancet 1(7445):1013–1015.
De Lucca, A. J. 2007. Harmful fungi in both agriculture and medicine. Revista iberoamericana de micología 24:11.
Dentinger, B. T. M., D. J. Lodge, A. B. Munkacsi, D. E. Desjardin, and D. J. McLaughlin. 2009. Phylogenetic placement of an unusual coral mushroom challenges the classic hypothesis of strict coevolution in the Apterostigma pilosium group ant-fungus mutualism. Evolution 63:2172–2178.
Desjardin, D. E., B. A. Perry, D. J. Lodge, C. V. Stevani, and E. Nagasawa. 2010. Luminescent mycena: New and noteworthy species. Mycologia 102(2):459–477.
Desprez-Loustau, M. L., C. Robin, M. Buee, R. Courtecuisse, J. Garbaye, F. Suffert, I. Sache, and D. M. Rizzo. 2007. The fungal dimension of biological invasions. Trends in Ecology and Evolution 22(9):472–480.
Dixon, D. M., M. M. McNeil, M. L. Cohen, B. G. Gellin, and J. R. La Montagne. 1996. Fungal infections: A growing threat. Public Health Reports 111(3):226–235.
Drew, A., E. J. Allen, and L. J. Allen. 2006. Analysis of climatic and geographic factors affecting the presence of chyridiomycosis in Australia. Diseases of Aquatic Organisms 68:245–250.
D’Souza, C. A., J. W. Kronstad, G. Taylor, R. Warren, M. Yuen, G. Hu, W. H. Jung, A. Sham, S. E. Kidd, K. Tangen, N. Lee, T. Zeilmaker, J. Sawkins, G. McVicker, S. Shah, S. Gnerre, A. Griggs, Q. Zeng, K. Bartlett, W. Li, X. Wang, J. Heitman, J. E. Stajich, J. A. Fraser, W. Meyer, D. Carter, J. Schein, M. Krzywinski, K. J. Kwon-Chung, A. Varma, J. Wang, R. Brunham, M. Fyfe, B. F. F. Ouellette, A. Siddiqui, M. Marra, S. Jones, R. Holt, B. W. Birren, J. E. Glagan, and C. A. Cuomo. 2011. Genome variation in Cryptococcus gattii, an emerging pathogen of immunocompetent hosts. mBio 2;e00342–10.
Dybas, C. L. 2004. Invasive species: The search for solutions. BioScience 54(7):615–621.
Erwin, D. C., and Ribeiro, O. K. 1996. Phytophthora diseases worldwide. St. Paul, MN: APS Press.
Evans, E. A. 2003. Economic dimensions of invasive species. Choices—Second quarter 2003: United States Department of Agriculture Animal and Plant Health Inspection Service [APHIS]. 2001. APHIS strategic plan 2000–2005.
Fisher, M. C. 2008. Molecular toolkit unlocks life cycle of the panzootic amphibian pathogen Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences, USA 105: 17209–17210.
Fisher, M. C., and T. W. J. Garner. 2007. The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biology Reviews 21(1):2–9.
Fisher, M. C., G. L. Koenig, T. J. White, G. San-Blas, R. Negroni, I. G. Alvarez, B. Wanke, and J. W. Taylor. 2001. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proceedings of the National Academy of Sciences, USA 8:8.
Fisher, M. C., T. W. J. Garner, and S. F. Walker. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annual Review of Microbiology 63:291–310.
Forestry Commission (Great Britain). 2010. Phytophthora ramorum. http://www.forestry.gov.uk/ pramorum (accessed October 27, 2010).
Fraser, J. A., S. S. Giles, E. C. Wenink, S. G. Geunes-Boyer, J. R. Wright, S. Diezmann, A. Allen, J. E. Stajich, F. S. Dietrich, J. R. Perfect, and J. Heitman. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360–1364.
Frick, W. F., J. F. Pollock, A. C. Hicks, K. E. Langwig, D. S. Reynolds, G. G. Turner, C. M. Butchkoski, and T. H. Kunz. 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329(5992):679–682.
Frost, R. 1936. Evil Tendencies Cancel. In The Poetry of Robert Frost, edited by E. C. Lathem. New York: Henry Holt and Company. P. 308.
Fry, E. E., and S. B. Goodwin. 1997. Resurgence of the Irish potato famine fungus. BioScience 47(6):363–371.
FWS (U.S. Fish and Wildlife Service). 2011. The bat white-nose syndrome mystery: Something is killing our bats. Hadley, MA.
Galanis, E., and L. MacDougall. 2010. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerging Infectious Diseases 16(2):251–257.
Galgiani, J. N. 2007. Coccidioidomycosis: Changing perceptions and creating opportunities for its control. Annals of the New York Academy of Sciences 1111:1–18.
———. 2008. Vaccines to prevent systemic mycoses: Holy grails meet translational realities. The Journal of Infectious Diseases 197:938–940.
GAO (Government Accountability Office). 2010. Biosurveillance: Efforts to develop a national biosurveillance capability need a national strategy and a designated leader. Washington, DC: GAO.
Garbelotto, M., and D. M. Rizzo. 2005. A California-based chronological review (1995–2004) of research on Phytophthora ramorum, the causal agent of sudden oak death. Phytopathologia Mediterranea 44:1-17.
Garbelotto, M., D. M. Rizzo, and L. Marais. 2002. Phytophthora ramorum and sudden oak death in California. Chemical control. In: Proceedings of the 5th Symposium on California Oak Woodlands, edited by R. Standiford and D. McCreary. U.S. Department of Agriculture, Forest Service, pp. 811–818.
Garcia-Solache, M. A., and A. Casadevall. 2010. Global warming will bring new fungal diseases for mammals. mBio 1:e00061–10.
Gargas, A. M., T. Trest, M. Christensen, T. J. Volk, and D. S. Blehert. 2009. Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotaxon 108:147–154.
Garner, C. D., J. K. Starr, P. L. McDonough, and C. Altier. 2010. Molecular identification of veterinary yeast isolates by use of sequence-based analysis of the D1/D2 region of the large ribosomal subunite. Journal of Clinical Microbiology 48:2140–2146.
Garrett, K. A., S. P. Dendy, E. E. Frank, M. N. Rouse, and S. E. Travers. 2006. Climate change effects on plant disease: Genomes to ecosystems. Annual Reviews in Phytopathology 44:489–509.
Giraud, T., P. Gladieux, and S. Gavrilets. 2010. Linking the emergence of fungal plant diseases with ecological speciation. Trends in Ecology and Evolution 30:101–109.
Goddard, M. R., H. C. Godfray, and J. A. Burt. 2005. Sex increases the efficacy of natural selection in experimental yeast populations. Nature. 434:636–640.
Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, L. Pirofski, R. Niang, and A. Casadevall. 2001. Serological evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:e66.
Goldman, D. L., J. Davis, F. Bommarito, X. Shao, and A. Casadevall. 2006. Enhanced allergic inflammation and airway responsiveness in rats with chronic Cryptococcus neoformans infection: Potential role for fungal pulmonary infection in the pathogenesis of asthma. Journal of Infectious Diseases 193:1178–1186.
Goss, E. M., M. Larsen, G. A. Chastagner, D. R. Givens, and N. J. Grünwald. 2009. Population genetic analysis infers migration pathways of Phytophthora ramorum in U.S. nurseries. PLoS Pathogenics 5(9):e1000583.
Gostinčar, C., M. Grube, S. de Hoog, P. Zalar, and N. Gunde-Cimerman. 2010. Extremotolerance in fungi: Evolution on the edge. FEMS Microbiology Ecology 71:2–11.
Greer, A., N. Victoria, and D. Fisman. 2008. Climate change and infectious diseases in North America: The road ahead. Canadian Medical Association Journal 178:6.
Grünwald, N. J., E. M. Gross, and C. M. Press. 2008. Phytophthora ramorum: A pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Molecular Plant Pathology 9(5):1–11.
Hannukkala, A., O. Kaukoranta, T. Lehtinen, and A. Rahkonen. 2007. Late-blight epidemics on potato in Finland, 1933–2002: Increased and earlier occurrence of epidemics associated with climate change and lack of rotation. Plant Pathology 56:167–176.
Hardman, R. 2011. Britain’s forests: 10k acres of trees cut down to stop pathogen. Dailymail, January 27.
Harris, R. N., R. M. Brucker, J. B. Walke, M. H. Becker, C. R. Schwantes, D. C. Flaherty, B. A. Lam, D. C. Woodhams, C. J. Briggs, V. T. Vredenburg, and K. P. C. Minbiole. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. The ISME Journal 2009:1–7.
Harvell, C. D., C. E. Mitchell, J. R. Ward, S. Altizer, A. P. Dobson, R. S. Ostfeld, and M. D. Samuel. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296(5576):2158–2162.
Hawksworth, D. L. 1991. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycology Research 6:641–655.
———. 2001. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycological Research 105(12):1422–1432.
Hector, R. F., and R. Laniado-Laborin. 2005. Coccidioidomycosis—a fungal disease of the Americas. PLoS Medicine 2:0015–0018.
Hector, R. F., B. L. Zimmer, and D. Pappagianis. 1990. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrobial Agents and Chemotherapy 34:587–593.
Heitman, J. 2006. Sexual reproduction and the evolution of microbial pathogens. Current Biology 16:R711–R725.
———. 2009. Love the one you’re with. Nature 460(13):807–808.
Heymann, D. L., and G. Rodier. 2004. Global surveillance, national surveillance, and SARS. Emerging Infectious Diseases 10(2):3.
Hibbett, D. M., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. Eriksson, S. Huhndorf, T. Y. James, P. M. Kirk, R. Lücking, T. Lumbsch, F. Lutzoni, P. B. Matheny, D. J. McLaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y.-C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, D. L. Hawksworth, V. Hofstetter, K. Hosaka, R. A. Humber, K. Hyde, U. Kõljalg, C. P. Kurtzman, K.-H. Larsson, R. Lichtwardt, J. Longcore, A. Miller, J.-M. Moncalvo, S. Mozley Standridge, F. Oberwinkler, E. Parmasto, J. D. Rogers, L. Ryvarden, J. P. Sampaio, A. Schuessler, J. Sugiyama, J. W. Taylor, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiss, M. White, K. Winka, Y.-J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509–547.
Holland, S. M. 2010. Chronic granulomatous disease. Clinical Reviews in Allergy and Immunology 38(1):3–10.
Holland, S. M., and D. C. Vinh. 2009. Yeast infections—human genetics on the rise. New England Journal of Medicine 361:1798–1801.
Holland, S. M., F. R. DeLeo, H. Z. Elloumi, A. P. Hsu, G. Uzel, N. Brodsky, A. F. Freeman, A. Demidowich, J. Davis, M. L. Turner, V. L. Anderson, D. N. Darnell, P. A. Welch, D. B. Kuhns, D. M. Frucht, H. L. Malech, J. I. Gallin, S. D. Kobayashi, A. R. Whitney, J. M. Voyich, J. M. Musser, C. Woellner, A. A. Schäffer, J. M. Puck, and B. Grimbacher. 2007. STAT3 mutations in the hyper-IgE syndrome. New England Journal of Medicine 357:1608–1619.
Hooper, R. G., M. R. Lennartz, and D. H. Muse. 1991. Heart rot and cavity tree selection by red-cockaded woodpeckers. Journal of Wildlife Management 55(2):323–327.
Hovmøller, M. 2010. Rapid global spread of aggressive strains of Puccinia striiformis on wheat—origins, causes, and consequences. Presentation given at the December 14–15, 2010, public workshop, “Fungal Diseases: An Emerging Challenge to Human, Animal, and Plant Health,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Hovmøller, M. S., A. H. Yahyaoui, and E. A. Milus. 2008. Rapid global spread of two aggressive strains of a wheat rust fungus. Molecular Ecology 17:3818–3826.
Hovmøller, M. S., S. Walter, and A. F. Justesen. 2010. Escalating threat of wheat rusts. Science 329:369.
Hufnagel, L., D. Brockmann, and T. Geisel. 2004. Forecast and control of epidemics in a globalized world. Proceedings of the National Academy of Sciences, USA 101(42):15124–15129.
IOM (Institute of Medicine) 1992. Emerging infections. Washington, DC: National Academy Press.
———. 2003. Microbial threats to heath. Washington, DC: The National Academies Press.
———. 2007. Global infectious disease surveillance and detection: Assessing the challenges. Workshop summary. Washington, DC: The National Academies Press.
———. 2008a. Global climate change and extreme weather events: Understanding the contributions to infectious disease emergence: Workshop summary. Washington, DC: The National Academies Press.
———. 2008b. Vector borne diseases: Understanding the environmental, human health, and ecological connections. Washington, DC: The National Academies Press.
———. 2009. Microbial evolution and co-adaptation: A tribute to the life and scientific legacies of Joshua Lederberg. Workshop summary. Washington, DC: The National Academies Press.
———. 2010. Infectious disease movement in a borderless world. Washington, DC: The National Academies Press.
Iqbal, N., E. E. DeBess, R. Wohrle, B. Sun, R. J. Nett, A. M. Ahlquist, T. Chiller, and S. R. Lockhart. 2010. Correlation of genotype and in vitro susceptibilities of Cryptococcus gattii strains from the Pacific Northwest of the United States. Journal of Clinical Microbiology 48(2):539–544.
IUCN (The World Conservation Union). 2005. Amphibian Conservation Action Plan. http://www.amphibianark.org/pdf/ACAP.pdf (accessed November 20, 2010).
James, T. Y., K. Kauff, C. L. Schoch, P. B. Matheny, V. Hofstetter, C. J. Cox, G. Celio, C. Gueidan, E. Fraker, J. Miadlikowska, H. T. Lumbsch, A. Rauhut, V. Reeb, A. E. Arnold, A. Amtoft, J. E. Stajich, K. Hosaka, G. H. Sung, D. Johnson, B. O’Rourke, M. Crockett, M. Binder, J. M. Curtis, J. C. Slot, Z. Wang, A. W. Wilson, A. Schüssler, J. E. Longcore, K. O’Donnell, S. Mozley-Standridge, D. Porter, P. M. Letcher, M. J. Powell, J. W. Taylor, M. M. White, G. W. Griffith, D. R. Davies, R. A. Humber, J. B. Morton, J. Sugiyama, A. Y. Rossman, J. D. Rogers, D. H. Pfister, D. Hewitt, K. Hansen, S. Hambleton, R. A. Shoemaker, J. Kohlmeyer, B. Volkmann-Kohlmeyer, R. A. Spotts, M. Serdani, P. W. Crous, K. W. Hughes, K. Matsuura, E. Langer, G. Langer, W. A. Untereiner, R. Lücking, B. Büdel, D. M. Geiser, A. Aptroot, P. Diederich, I. Schmitt, M. Schultz, R. Yahr, D. S. Hibbett, F. Lutzoni, D. J. McLaughlin, J. W. Spatafora, and R. Vilgalys. 2006. Restructuring the early evolution of Fungi using a six gene phylogeny. Nature 443:818–822.
James, T. Y., A. P. Litvintseva, R. Vilgalys, J. A. Morgan, J. W. Taylor, M. C. Fisher, L. Berger, C. Weldon, L. du Preez, and J. E. Longcore. 2009. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathogens 5(5):e1000458 1–12.
Jebara, K. B. 2004. Surveillance, detection and response: Managing emerging diseases at national and international levels. OIE Revue Scientifique et Technique 23(2):709–715.
Jeger, M. J., and M. Pautasso. 2008. Plant disease and global change—the importance of long-term data sets. New Phytologist 177:8–11.
Jones, K. E., N. G. Patel, M. A. Levy, A. Storeygard, D. Balk, J. L. Gittleman, and P. Daszak. 2008. Global trends in emerging infectious diseases. Nature 451:990–993.
Jones, M. D. M., I. Forn, C. Gadelha, M. J. Egan, D. Bass, R. Massana, T. A. Richards. 2011. Discovery of novel intermediate forms redefines the fungal tree of life. Nature Published online May 11, 2011. doi:10.1038/nature09984.
Judelson, H. S., and F. A. Blanco. 2005. The spores of phytophthora: Weapons of the plant destroyer. Nature Reviews/Microbiology 3:47–58.
Jumpponen, A., and K. L. Jones. 2009. Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytologist 184:438–448.
Karesh, W. B., R. A. Cook, E. L. Bennet, and J. Newcomb. 2005. Wildlife trade and global disease emergence. In Emerging Infectious Diseases.
Kauserud, H., L. C. Stige, J. O. Vik, R. H. Økland, K. Høiland, N. C. Stenseth. 2008. Mushroom fruiting and climate change. Proceedings of the National Academy of Sciences, USA 105(10):3811–3814.
Keller, N. P., G. Turner, and J. W. Bennett. 2005. Fungal secondary metabolism from biochemistry to genomics. Nature Reviews/Microbiology 3:937–947.
Keller, R., and C. Perrings. 2010. International policy options to reduce the harmful impacts of alien invasive species. UNEP Ecosystem Services Economics Working Papers, Nairobi, UNEP
Kelly, M., D. Shaari, Q. Guo, and D. Liu. Spatial modeling of sudden oak death nationwide. 2005. U.S. Department of Agriculture, Forest Service Gen. Tech. Rep. PSW-GTR-196-006-063. http://www.suddenoakdeath.org/pdf/KellyetalSOD2-22-05.pdf (accessed May 3, 2011).
Kidd, S. E., F. Hagen, R. L. Tscharke, M. Huynh, K. H. Bartlett, M. Fyfe, L. MacDougall, T. Boekhout, K. J. Kwon-Chung, and W. Meyer. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proceedings of the National Academy of Sciences, USA 101(49):17258–17263.
Kidd, S. E., P. J. Bach, A. O. Hingston, S. Mak, Y. Chow, L. MacDougall, J. W. Kronstad, and K. H. Bartlett. 2007. Cryptococcus gattii dispersal mechanisms, British Columbia, Canada. Emerging Infectious Diseases 13(1):51–57.
Kilpatrick, A. M., C. J. Briggs, and P. Daszak. 2009. The ecology and impact of chytridiomycosis, an emerging disease of amphibians. Trends in Ecology and Evolution 25:109–118.
Kliejunas, J. T. 2010. Sudden oak death and Phytophthora ramorum: A summary of the literature. 2010 edition. Gen. Tech. Rep. PSW-GTR-234. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station.
Knox, R. 2010. Fungal disease spreads through Pacific Northwest. National Public Radio News (April 23, 2010), http://www.npr.org/templates/story/story.php?storyId=126198896 (accessed May 4, 2010).
Koerner, B. I. 2010. Red menace: Stop the ug99 fungus before its spores bring starvation. Wired March 2010.
Kriger, K. M., and J. M. Hero. 2007. The chytrid fungus Batrachochytrium dendrobatidis is non-randomly distributed across amphibian breeding habitats. Diversity and Distributions 13(6): 781–788.
Krishnan, S., E. K. Manavathu, and P. H. Chandrasekar. 2009. Aspergillus flavus: An emerging non-fumigatus Aspergillus species of significance. Mycoses 52:206–222.
Kronstad, J. W., R. Attarian , B. Cadieux, J. Choi, C. A. D’Souza, E. J. Griffiths, J. M. Geddes, G. Hu, W. H. Jung, M. Kretschmer, S. Saikia, and J. Wang. 2011. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nature Reviews Microbiology 9(3):193–203.
Kumamoto, C. A. 2008. Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nature Reviews 6.
Lamirande, E. W., and D. K. Nichols. 2002. Effects of host age on susceptibility to cutaneous chytridiomycosis in blue-and-yellow poison dart frogs (Dendrobates tinclorius). In: Proceedings of the Sixth International Symposium on the Pathology of Reptiles and Amphibians, St. Paul, MN, April 18–19, 2001. Pp. 3–13.
Lara, E., D. Moreira, and P. Lopez-Garcia. 2010. The environmental clade LKM11 and Rozella form the deepest branching clade of fungi. Protist 161:116–121.
Large, E. C. 1965. Advance of the Fungi. New York: Dover Publications, Inc.
Le Calvez, T., G. Burgaud, S. Mahe, G. Barbier, and P. VandenKoornhuyse. 2009. Fungal diversity in deep-sea hydrothermal ecosystems. Applied and Environmental Microbiology 75(20): 6415–6420.
Leaw, S. N., H. C. Chang, H. F. Sun, R. Barton, J.-P. Bouchara, and T. C. Chang. 2006. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. Journal of Clinical Microbiology 44:693–699.
Levitz, S. M. 1991. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Reviews of Infectious Diseases 13(6):1163–1169.
Lin, X., and J. Heitman. 2006. The biology of the Cryptococcus neoformans species complex. Annual Review of Microbiology 60(1):69–105.
Lin, X., C. M. Hull, and J. Heitman. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434:1017–1021.
Lindner, D. L., A. Gargas, J. M. Lorch, M. T. Banik, J. Glaeser, T. H. Kunz, and D. S. Blehert. 2010. DNA-based detection of the fungal pathogen Geomyces destructans in soil from bat hibernacula. Mycologia doi:10.3852/10-262.
Lips, K. R., F. Brem, R. Brenes. J. D. Reeve, R. A. Alford, J. Voyles, C. Carey, L. Livo, A. P. Pessier, and J. P. Collins. 2006. Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proceedings of the National Academy of Sciences, USA 103(9):3165–3170.
Longcore, J. E., A. P. Pessier, and D. K. Nichols. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91(2):219–227.
Lonsdale, D., and J. N. Gibbs. 1996. Effects of climate change on fungal diseases of trees. In: Fungi and environmental change, edited by J. E. Frankland, N. Magan, and G. M. Gadd. British Mycological Society, Symp vol. XX. Cambridge, UK: Cambridge University Press, Pp. 1–19.
Loo, J. 2009. Ecological impacts of non-indigenous invasive fungi as forest pathogens. Biological Invasions 11(1):81–96.
Lötters, S., J. Kielgast, J. Bielby, S. Schmidtlein, J. Bosch, M. Veith, S. F. Walker, M. C. Fisher, and D. Rödder. 2010. The link between rapid enigmatic amphibian decline and the globally emerging chytrid fungus. EcoHealth 1–15.
Loustau, D. 2006. Climate change impacts on extensively managed forest: a modelling approach, Wilton Park Conference. See: http://www.forestry.gov.uk/forestry/INFD-6VKDVB (accessed June 22, 2011).
Lutzoni, F., M. Pagel, and V. Reeb. 2001. Major fungal lineages are derived from lichen symbiotic ancestors. Nature 411:937–940.
Lutzoni, F., F. Kauff, C. J. Cox, D. McLaughlin, G. Celio, B. Dentinger, M. Padamsee, D. Hibbett, T. Y. James, E. Baloch, M. Grube, V. Reeb, V. Hofstetter, C. Schoch, A. E. Arnold, J. Miadlikowska, J. Spatafora, D. Johnson, S. Hambleton, M. Crockett, R. Shoemaker, G. H. Sung, R. Lucking, T. Lumbsch, K. O’Donnell, M. Binder, P. Diederich, D. Ertz, C. Gueidan, K. Hansen, R. C. Harris, K. Hosaka, Y. W. Lim, B. Matheny, H. Nishida, D. Pfister, J. Rogers, A. Rossman, I. Schmitt, H. Sipman, J. Stone, J. Sugiyama, R. Yahr, and R. Vilgalys. 2004. Assembling the fungal tree of life: Progress, classification, and evolution of subcellular traits. American Journal of Botany 91(10):1446–1480.
Ma, H., F. Hagen, D. J. Stekel, S. A. Johnston, E. Sionov, R. Falk, I. Polacheck, T. Boekhout, and R. C. May. 2009. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proceedings of the National Academy Sciences, USA 106(31):12980–12985.
MacDougall, L., S. E. Kidd, E. Galanis, S. Mak, M. J. Leslie, P. R. Cieslak, J. W. Kronstad, M. G. Morshed, and K. H. Bartlett. 2007. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerging Infectious Diseases 13(1):42–50.
MacLeod, A. M. Pautasso, M. J. Jeger, and R. Haines-Young. 2010. Evolution of the international regulation of plant pests and challenges for future plant health. Food Security 2:49–70.
Madoff, L. C. 2004. ProMED-mail: An early warning system for emerging diseases. Clinical Infectious Diseases 39(2):227–232.
Mak, S., B. Klinkenberg, K. Bartlett, and M. Fyfe. 2010. Ecological niche modeling of Cryptococcus gattii in British Columbia, Canada. Environmental Health Perspectives 118:653–658.
Márquez, L. M., R. S. Redman, R. J. Rodriguez, and M. J. Roossinck. 2007. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science 315:513–515.
Mascheretti, S. P., J. P. Croucher, A. Vettraino, S. Prospero, and M. Garbelotto. 2008. Reconstruction of the sudden oak death epidemic in California through microsatellite analysis of the pathogen Phytophthora ramorum. Molecular Ecology 17:2755–2768.
Mascheretti, S. P., J. P. Croucher, M. Kozanitas, L. Baker, and M. Garbelotto. 2009. Genetic epidemiology of the sudden oak death pathogen Phytophthora ramorum in California. Molecular Ecology 18(22):4577–4590.
McCullough, D. G., T. T. Work, J. F. Cavey, A. M. Liebhold, and D. Marshall. 2006. Interceptions of nonindigenous plant pests at U.S. ports of entry and border crossings over a 17-year period. Biological Invasions 8:611–630.
McLaughlin, D. J., D. S. Hibbett, F. Lutzoni, J. W. Spatafora, and R. Vilgalys. 2009. The search for the fungal tree of life. Trends in Microbiology 17(11):488–497.
Meentemeyer, R., D. Rizzo, W. Mark, and E. Lotz. 2004. Mapping the risk of establishment and spread of sudden oak death in California. Forest Ecology and Management 200(1–3):195–214.
Meteyer, C. U., E. L. Buckles, D. S. Blehert, A. C. Hicks, D. E. Green, V. Shearn-Bochsler, N. J. Thomas, A. Gargas, and M. J. Behr. 2009. Histopathologic criteria to confirm white-nose syndrome in bats. Journal of Veterinary Diagnostic Evaluation 21(4):411–414.
Miller, O. K., Jr., T. Henkel, T. Y. James, and S. L. Miller. 2001. Pseudotulostoma, a remarkable new volvategenus in the Elaphomycetaceae from Guyana. Mycological Research 105:1268–1272.
Milus, E. A., E. Seyran, and R. McNew. 2006. Aggressiveness of Puccinia striiformis f. sp. tritici isolates in the South-Central United States. Plant Disease 90:847–852.
Milus, E. A., K. Kristensen, and M. S. Hovmøller. 2009. Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp. tritici causing stripe rust wheat. Phytopathology 99:89–94.
Money, N. P. 2007. The triumph of the fungi: A rotten history. New York: Oxford University Press.
Morgan, J. A. T., V. T. Vredenburg, L. J. Rachowicz, R. A. Knapp, M. J. Stice, T. Tunstall, R. E. Bingham, J. M. Parker, J. E. Longcore, C. Moritz, C. J. Briggs, and J. W. Taylor. 2007. Population genetics of the frog-killing fungus Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences, USA 104:13845–13850.
Morse, S. 2004. Emerging infections: Microbial invaders discover new territory. Presentation to the annual meeting of the American Institute of Biological Sciences, 2004. http://www.aibs.org/media-library/ (accessed June 22, 2011).
Morse, S., S. B. Hatch, Rosenberg, and J. Woodall. 1996. Global monitoring of emerging diseases: Design for a demonstration program. Health Policy 38:135–153.
Munkacsi, A. B., J. J. Pan, P. Villesen, U. G. Mueller, M. Blackwell, and D. J. McLaughlin. 2004. Convergent coevolution in the domestication of coral mushrooms by fungus-growing ants. Proceedings of the Royal Society of London, B 271:1777–1782.
Nardi, J. B., C. M. Bee, L. A. Miller, N. H. Nguyen, S.-O. Suh, and M. Blackwell. 2006. Communities of microbes that inhabit the changing hindgut landscape of a subsocial beetles. Arthropod Structure & Development 35:57–68.
Nucci, M., and K. A. Marr. 2005. Emerging fungal diseases. Clinical Infectious Diseases 41(4): 521–526.
Nürnberger, T., F. Brunner, B. Kemmerling, and L. Piater. 2004. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunology Reviews 198:249–266.
O’Donnell, A. 2006. Invasive species: More aggressive import screening is cost-effective, says study. Land Letter: Natural Resources Weekly Report.
Oerke, E. C., H. W. Dehne, F. Schohnbeck, and A. Weber. 1995. Crop production and crop protection: Estimated losses in major food and cash crops. Amsterdam, The Netherlands and New York: Elsevier.
Oklahoma Department of Wildlife Conservation and U.S. Fish and Wildlife Service. 2010. Bat fungus documented in Oklahoma, www.wildlifedepartment.com/newsreleasearchive/05-10nr. htm#Bat_fungus_documented_in_Oklahoma (accessed May 4, 2011).
Ortoneda, M., J. Guarro, M. P. Madrid, Z. Caracuel, M. I. Roncero, E. Mayayo, and A. Di Pietro. 2004. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infection and Immunity 72:1760–1766.
Ostrosky-Zeichner, L., A. Casadevall, J. N. Galgiani, F. C. Odds, and J. H. Rex. 2010. An insight into the antifungal pipeline: Selected new molecules and beyond. Nature Reviews Drug Discovery 9(9):719–727.
Park, B. J., K. A. Wannemuehler, B. J. Marston, N. Govender, P. G. Pappas, and T. M. Chiller. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530.
Parke, J. L., and S. Lucas. 2008. Sudden oak death and ramorum blight. The Plant Health Instructor. doi:10.1094/PH-I-2008-0227-01.
Pautasso, M., K. Dehnen-Schmutz, O. Holdenrieder, S. Pietravalle, N. Salama, M. Jeger, E. Lange, and S. Hehl-Lange. 2010. Plant health and global change—some implications for landscape management. Biological Reviews 85(4):729–755.
Perrings, C., S. Burgiel, M. Lonsdale, H. Mooney, and M. Williamson. 2010. International cooperation in the solution to trade-related invasive species risks. Annals of the New York Academy of Sciences 1195:198–212.
Pfaller, M. A., and D. J. Diekema. 2010. Epidemiology of invasive mycoses in North America. Critical Reviews in Microbiology 36(1):1–53.
Pimentel, D., R. Zuniga, and D. Morrison. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52(3):273–288.
Pirofski, L. A., and A. Casadevall. 2008. The damage-response framework of microbial pathogenesis and infectious diseases. Experimental Biology and Medicine 635:135–146.
Platt, J. 2010. Bad news for bats: Deadly white-nose syndrome still spreading. http://www.scientificamerican.com/blog/post.cfm?id=bad-news-for-bats-deadly-white-nose-2010-02-20 (accessed May 12, 2010).
Porter, T. M., C. W. Schadt, L. Rizvi, A. P. Martin, S. K. Schmidt, L. Scott-Denton, R. Vilgalys, and J. M. Moncalvo. 2008. Widespread occurrence and phylogenetic placement of a soil clone group adds a prominent new branch to the fungal tree of life. Molecular Phylogenetics and Evolution 46:635–664.
Pounds, J. A., M. R. Bustamante, L. A. Coloma, J. A. Consuegra, M. P. L. Fogden, P. N. Foster, E. La Marca, K. L. Masters, A. Merino-Viteri, R. Puschendorf, S. R. Ron, G. A. Sánchez-Azofeifa, C. J. Still, and B. E. Young. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439(7073):161–167.
Puechmaille, S. J., P. Verdeyroux, H. Fuller, M. Ar Gouilh, M. Bekaert, and E. C. Teeling. 2010. Whitenose syndrome fungus (Geomyces destructans) in bat, France. Emerging Infectious Diseases 16(2):290–293.
Puechmaille, S. J., G. Wibbelt, V. Korn, H. Fuller, F. Forget, K. Muhldorfer, A. Kurth, B. Wieslaw, C. Borel, T. Bosch, T. Cherezy, M. Drebet, T. Gorfol, A. J. Haarsma, F. Herhaus, G. Hallart, M. Hammer, C. Jungmann, Y. Le Bris, L. Lutsar, M. Masing, B. Mulkens, K. Passior, M. Starrach, M. Wojtaszewski, U. Zophel, and E. C. Teeling. 2011. Pan-European distribution of white-nose syndrome fungus (Geomyces destructans) not associated with mass mortality. PLoS Pathogens 6(4):e19167.
Qaummen, D. 2010. Bat crash. National Geographic Magazine, December, Pp. 126–137.
Rachowicz, L. J., J. M. Hero, R. A. Alford, J. W. Taylor, and J. A. T. Morgan. 2005. The novel and endemic pathogen hypotheses: Competing explanations for the origin of emerging infectious diseases of wildlife. Conservation Biology 19:1441–1448.
Rizzo, D. M. 2005. Exotic species and fungi: Interactions with fungal, plant and animal communities. In: The fungal community, 3rd ed., edited by J. Dighton, P. Oudemans, and J. White. CRC Press, Pp. 857–877.
———. 2010. Emergence of Phytophthora ramorum in Europe and North America. Presentation given at the December 14–15, 2010, public workshop, “Fungal Diseases: An Emerging Challenge to Human, Animal, and Plant Health,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Rizzo, D. M., and M. Garbelotto. 2003. Sudden oak death: Endangering California and Oregon forest ecosystems. Frontiers in Ecology and the Environment 1(5):197–204.
Rizzo, D. M., M. Garbelotto, and E. M. Hansen. 2005. Phytophthora ramorum: Integrative research and management of an emerging pathogen in California and Oregon forests. Annual Review of Phytopathology 43(1):309–335.
Roach, J. 2011. Caterpillar fungus making Tibetan herders rich. National Geographic News. http://news.nationalgeographic.com/news/2011/04/110427-fungus-caterpillars-tibet-china-herdersscience (accessed June 22, 2011).
Robert, V. A., and A. Casadevall. 2009. Vertebrate endothermy restricts most fungi as potential pathogens. Journal of Infectious Disease 200:1623–1626.
Rödder, D., J. Kielgast, J. Bielby, J. Bosch, T. J. W. Garner, S. Schmidtlein, M. Veith, S. Walker, M. C. Fisher, and S. Lötters. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 1:52–66.
Rodriguez, R. J., J. F. White, Jr., A. E. Arnold, and R. S. Redman. 2009. Fungal endophytes: Diversity and functional roles. New Phytologist 182:314–330.
Romani, L. 2004. Immunity to fungal infections. Nature Reviews Immunology 4:1–13.
Rosenblum, E. B., M. C. Fisher, T. Y. James, J. E. Stajich, J. E. Longcore, L. R. Gentry, and T. J. Poorten. 2009. A molecular perspective: Biology of the emerging pathogen Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms.
Rosenblum, E. B., J. Voyles, T. J. Poorten, and J. E. Stajich. 2010. The deadly chytrid fungus: A story of an emerging pathogen. PLoS Pathogens 6(1).
Rosenzweig, C., A. Iglesias, X. B. Yang, P. R. Epstein, and E. Chivian. 2001. Climate change and extreme weather events. Global Change and Human Health 2(2):90–104.
Rossman, A. Y. 2009. The impact of invasive fungi on agricultural ecosystems in the United States. Biological Invasions 11:11.
Rossman, A. Y., and M. E. Palm-Hernandez. 2008. Systematics of plant pathogenic fungi: Why it matters. Plant Disease 92(10):1376–1386.
Ruehle, J. L., and D. H. Marx. 1979. Fiber, food, fuel, and fungal symbionts. Science 206(4417): 419–422.
Rust in the bread basket. 2010. The Economist.
Saul, N., M. Krockenberger, and D. Carter. 2008. Evidence of recombination in mixed-mating-type and alpha-only populations of Cryptococcus gattii sourced from single Eucalyptus hollows. Eukaryotic Cell 7:727–734.
Schadt, C. W., A. P. Martin, D. A. Lipson, and S. K. Schmidt. 2003. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science 301:1359–1361.
Scheffer, R., J. G. Voeten, and R. P. Guries. 2008. Biological control of Dutch elm disease. Plant Disease 92(2):192–200.
Schloegel, L. M., J. M. Hero, L. Berger, R. Speare, K. McDonald, and P. Daszak. 2006. The decline of the sharp-snouted day frog (Taudactylus acutirostris): The first documented case of extinction by infection in a free-ranging wildlife species? EcoHealth 3:35–40.
Schloegel, L. M., P. Daszak, A. A. Cunningham, R. Speare, and B. Hill. 2010. Two amphibian diseases, chytridiomycosis and ranaviral disease, are now globally notifiable to the World Organization for Animal Health (OIE): An assessment. Diseases of Aquatic Organisms 92:101–108.
Schneider, E., R. A. Hajjeh, R. A. Spiegel, R. W. Jibson, E. L. Harp, G. A. Marshall, R. A. Gunn, M. M. McNeil, R. W. Pinner, R. C. Baron, R. C. Burger, L. C. Hutwagner, C. Crump, L. Kaufman, S. E. Reef, G. M. Feldman, D. Pappagianis, and S. B. Werner. 1997. A coccidioidomycosis outbreak following the Northridge, Calif., earthquake. Journal of the American Medical Association 277(11):904–908.
Schneider, W., C. A. Hollier, H. K. Whitam, M. E. Palm, J. M. Mckemy, J. Hernandez, L. Levy, and R. Devries-Paterson. 2005. First report of soybean rust caused by phakopsora pachyrhizi in the continental United States. Plant Disease 89:774.
Schoch, C. L., G. H. Sung, F. L. López-Giráldez, J. P. Townsend, J. Miadlikowska, V. Rie Hofstetter, B. Robbertse, P. B. Matheny, F. Kauff, Z. Wang, C. Gueidan, R. M. Andrie, K. Trippe, L. M. Ciufetti, A. Wynns, E. Fraker, B. P. Hodkinson, G. Bonito, J. Z. Groenewald, M. Arsanlou, G. S. De Hoog, P. W. Crous, D. Hewitt, D. H. Pfister, K. Peterson, M. Grysenhout, M. J. Wingfield, A. Aptroot, S. O. Suh, M. Blackwell, D. M. Hillis, G. W. Griffith, L. A. Castlebury, A. Y. Rossman, H. T. Lumbsch, R. L. Lücking, B. Büdel, A. Rauhut, P. Diederich, D. Ertz, D. M. Geiser, K. Hosaka, P. Inderbitzin, J. Kohlmeyer, B. Volkmann-Kohlmeyer, L. Mostert, K. O’Donnell, H. Sipman, J. D. Rogers, R. A. Shoemaker, J. Sugiyama, R. C. Summerbell, W. Untereiner, P. R. Johnston, S. Stenroos, A. Zuccaro, P. S. Dyer, P. D. Crittenden, M. S. Cole, K. Hansen, J. M. Trappe, R. Yahr, F. Lutzoni, and J. W. Spatafora. 2009. The Ascomycota tree of life: A phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58:224–239.
Scholthof, K. B. G. 2007. The disease triangle: pathogens, the environment, and society. Nature Reviews Microbiology 5:152–156.
Schultz, T. R., and S. G. Brady. 2008. Major evolutionary transitions in ant agriculture. Proceedings of the National Academy of Sciences, USA 105(14):5435–5440.
Sexton, A. C., and B. J. Howlett. 2006. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryotic Cell 5:1941–1949.
Sharpton, T. J., J. E. Stajich, S. D. Rounsley, M. J. Gardner, J. R. Wortman, V. S. Jordar, R. Maiti, C. D. Kodira, D. E. Neafsey, Q. D. Zeng, C. Y. Hung, C. McMahan, A. Muszewska, M. Grynberg, M. A. Mandel, E. M. Kellner, B. M. Barker, J. N. Galgiani, M. J. Orbach, T. N. Kirkland, G. T. Cole, M. R. Henn, B. W. Birren, and J. W. Taylor. 2009. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Research 19:1722–1731.
Shoham, S., and S. M. Levitz. 2005. The immune response to fungal infections. British Journal of Haematology 129:569–582.
Siddiqui, S., V. L. Anderson, D. M. Hilligoss, M. Abinun, T. W. Kuijpers, H. Masur, F. G. Witebsky, Y. R. Shea, J. I. Gallin, H. L. Malech, and S. M. Holland. 2007. Fulminant mulch pneumonitis: An emergency presentation of chronic granulomatous disease. Clinical Infectious Diseases 45:673–681.
Skerratt, L. F., L. Berger, R. Speare, S. Cashins, K. R. McDonald, A. D. Phillott, H. B. Hines, and N. Kenyon. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134.
Smith, K., F. M. Behrens, L. M. Schloegel, N. Marano, S. Burgiel, and P. Daszak. 2009. Reducing the risks of the wildlife trade. Science 324(5927):594–595.
Snieszko, S. F. 1974. The effects of environmental stress on outbreaks of infectious diseases of fishes. Journal of Fish Biology 6(2):197–208.
Sorrell, T. C. 2001. Cryptococcus neoformans variety gattii. Medical Mycology 39(2):155–168.
Sprague, S. J., S. J. Marcroft, H. L. Hayden, and B. J. Howlett. 2006. Major gene resistance to blackleg in Brassica napus overcome within three years of commercial production in southeastern Australia. Plant Disease 90:190–198.
Stajich, J. E., M. L. Berbee, M. Blackwell, D. S. Hibbett, T. Y. James, J. W. Spatafora, and J. W. Taylor. 2009. The fungi. Current Biology 19:R840–R845.
Stone, M., 2010. Virulent new strains of rust fungus endanger world wheat. Microbe 5(10):423–428.
Strange, R. N., and P. R. Scott. 2005. Plant disease: A threat to global food security. Annual Review of Phytopathology 43:83–116.
Stuart, S. N., J. S. Chanson, N. A. Cox, B. E. Young, A. Rodrigues, D. L. Fischman, and R. W. Waller. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786.
Stuckenbrock, E. H., and B. A. McDonald. 2008. The origins of plant pathogens in agro-ecosystems. Annual Review of Phytopathology 46:75–100.
Suh, S. O., and M. Blackwell. 2006. Three new asexual arthroconidial yeasts, Geotrichum carabidarum sp. nov., Geotrichum histeridarum sp. nov., and Geotrichum cucujoidarum sp. nov., isolated from the gut of insects. Mycological Research 110:220–228.
Suh, S. O., C. J. Marshall, J. V. McHugh, and M. Blackwell. 2003. Wood ingestion by passalid beetles in the presence of xylose-fermenting gut yeasts. Molecular Ecology 12:3137–3145.
Suh, S. O., J. V. McHugh, D. D. Pollock, and M. Blackwell. 2005. The beetle gut: A hyperdiverse source of novel yeasts. Mycology Research 3:261–265.
Tatum, L. A. 1971. The Southern corn leaf blight epidemic. Science 171:1113–1116.
Taylor, J. W., J. Spatafora, K. O’Donnell, F. Lutzoni, T. James, D. S. Hibbett, D. Geiser, T. D. Bruns, M. Blackwell. 2004. The Fungi. In Assembling the Tree of Life, edited by J. Cracraft and M. J. Donoghue. Oxford University Press. Pp. 171–194.
Trethowan, R. M., D. Hodson, H.-J. Braun, W. H. Pfeiffer, and M. Van Ginkel. 2005. Wheat breeding environments. In Impacts of international wheat breeding research in the developing world, 1988–2002, edited by M. A. Lantican, H. J. Dubin, and M. L. Morris. Mexico. D.F.: CIMMYT. P. 5.
Tsang, C. A., S. M. Anderson, S. B. Imholte, L. M. Erhardt, S. Chen, B. J. Park, C. Christ, K. K. Komatsu, T. Chiller, and R. H. Sunenshine. 2010. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007–2008. Emerging Infectious Diseases 16(11):1738–1744.
Tyler, B. M., S. Tripathy, X. Zhang, P. Dehal, R.H. Jiang, A. Aerts, F. D. Arredondo, L. Baxter, D. Bensasson, J. L. Beynon, J. Chapman, C. M. Damasceno, A. E. Dorrance, D. Dou, A. W. Dickerman, I. L. Dubchak, M. Garbelotto, M. Gijzen, S. G. Gordon, F. Govers, N.J. Grunwald, W. Huang, K. L Ivors, R.W. Jones, S. Kamoun, K. Krampis, K. H. Lamour, M. K. Lee, W. H. McDonald, M. Medina, H. J. Meijer, E.K. Nordberg, D. J. Maclean, M. D. Ospina-Giraldo, P. F. Morris, V. Phuntumart, N. H. Putnam, S. Rash, J. K. Rose, Y. Sakihama, A. A. Salamov, A. Savidor, C. F. Scheuring, B. M. Smith, B. W. Sobral, A. Terry, T. A. Torto-Alalibo, J. Win, Z. Xu, H. Zhang, I. V. Grigoriev, D. S. Rokhsar, and J. L. Boore. 2006, Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313(5791):1261–1266.
Ullstrup, A. J. 1972. The impacts of the Southern corn leaf blight epidemics of 1970–1971. Annual Reviews in Phytopathology 10:37–50.
Václavík, T. A., E. M. Kanaskie, J. L. Hansen, J. L. Ohmann, and R. K. Meentemeyer. 2010. Predicting potential and actual distribution of sudden oak death in Oregon: Prioritizing landscape contexts for early detection and eradication of disease outbreaks. Forest Ecology and Management 260:1026–1035.
Vinh, D. C., F. Masannat, R. B. Dzioba, J. N. Galgiani, and S. M. Holland. 2009. Refractory disseminated coccidioidomycosis and mycobacteriosis in inteferon-gamma receptor 1 deficiency. Clinical Infectious Diseases 49:e62–e65.
Voelz, K. and R. C. May. 2010. Cryptococcal interactions with the host immune system. Eukaryotic Cell 9:835–846.
Vredenburg, V. T., R. A. Knapp, T. S. Tunstall, and C. J. Briggs. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proceedings of the National Academy of Sciences, USA (published ahead of print May 10, 2010).
Vurro, M., B. Bonciani, and G. Vannacci. 2010. Emerging infectious diseases of crop plants in developing countries: Impact on agriculture and socio-economic consequences. Food Security 2(2):113–132.
Wade, N. 1999. For leaf-cutter ants, farm life isn’t so simple. The New York Times, August 3.
Wainwright, M. 1992. The impact of fungi on environmental biogeochemistry. In The Fungal Community: Its Organization and Role in the Ecosystem, edited by G. C. Carroll and D. T. Wicklow. New York: Marcel Decker, Inc. Pp. 601-616.
Wake, D. B., and V. T. Vredenburg. 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proceedings of the National Academy of Sciences, USA 105(Suppl 1):11466–11473.
Walker, S. F., J. Bosch, T. Y. James, A. P. Litvintseva, J. A. Oliver Valls, S. Piña, G. García, G. A. Rosa, A. A. Cunningham, S. Hole, R. Griffiths, and M. C. Fisher. 2008. Invasive pathogens threaten species recovery programs. Current Biology 18(18):R853–854.
Walker, S. F., J. Bosch, V. Gomez, T. Garner, A. A. Cunningham, D. S. Schmeller, M. Ninyerola, D. A. Henk, C. G. Christian-Phillipe Arthur, and M. C. Fisher. 2010. Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecology Letters 2–11.
Warnock, D. W. 2006. Fungal diseases: An evolving public health challenge. Medical Mycology 44(8):697–705.
Weidensaul, S. 2000. The rarest of the rare. Smithsonian 31(8):118–127.
Weinberg, J. 2005. Surveillance and control of infectious diseases at local, national and international levels. Clinical Microbiology and Infection 11:12–14.
Weir, A., and G. W. Beakes. 1995. An introduction to the Laboulbeniales: A fascinating group of entomogenous fungi. Mycologist 9:6–10.
Weldon, C., L. H. Du Preez, A. D. Hyatt, R. Muller, and R. Speare. 2004. Origin of the amphibian chytrid fungus. Emerging Infectious Diseases 10(12):2100–2105.
Weldon, C., A. De Villiers, and L. H. Du Preez. 2007. Quantification of the trade in Xenopus laevis from South Africa, with implications for biodiversity conservation. African Journal of Herpetology 56(1):77–83.
Weldon, C., L. D. Preez, and M. Vences. 2008. Lack of detection of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) in Madagascar. Monografie del Museo Regionale di Scienze Naturali di Torino, XLV (2008):95–106.
Wellings, C. R. 2007. Puccinia striiformis in Australia: A review of the incursion, evolution, and adaptation of stripe rust in the period 1979–2006. Australian Journal of Agricultural Research 58(6):567–575.
WHO (World Health Organization). 2008. International health regulations (2005), 2nd ed. http://whqlibdoc.who.int/publications/2008/9789241580410_eng.pdf (accessed, May 3, 2011).
_____. 2010. Emerging diseases. http://www.who.int/topics/emerging_diseases/en. (accessed March 9, 2011).
Wibbelt, G., A. Kurth, D. Hellmann, M. Weishaar, A. Barlow, M. Veith, J. Pruger, T. Gorfol, L. Grosche, F. Bontadina, U. Zophel, H. P. Seidl, P. M. Cryan, and D. S. Blehert. 2010. Whitenose syndrome fungus (Geomyces destructans) in bats, Europe. Emerging Infectious Diseases 16(8):1237–1243.
Wilson, M. E. 2003. The traveler and emerging infections: Sentinel, courier, transmitter. Journal of Applied Microbiology 94(Suppl):1S–11S.
Woodham-Smith, C. 1962. The great hunger. New York: Harper & Row.
Woodhams, D. C, R. A. Alford, and G. Marantelli. 2003. Emerging disease of amphibians cured by elevated body temperature. Diseases of Aquatic Organisms 55:65–67.
Woolhouse, M., and E. Gaunt. 2007. Ecological origins of novel human pathogens. Critical Reviews in Microbiology 33(4):231–242.
Woolhouse, M. E. J., and S. Gowtage-Sequeria. 2005. Host range and emerging and reemerging pathogens. Emerging Infectious Diseases 11(12):1842–1847.
Xue, C., Y. Tada, X. Dong, and J. Heitman. 2007. The human fungal pathogen cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell and Microbe 1:263–273.
Zhdanova, N. N., V. A. Zakharchenkoa, V. V. Vembera, and L. T. Nakonechnaya. 2000. Fungi from Chernobyl: Mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycological Research 104 (12):1421–1426.






































































































