Implementing a
National Cancer Clinical Trials System
for the 21st Century
An American Society of Clinical Oncology and Institute of Medicine Workshop
Clinical trials enable scientific discoveries to advance patient care, and they also inform and guide subsequent research. The National Cancer Institute (NCI) supports the largest U.S. network of clinical trials of any type, of which the largest component is the Clinical Trials Cooperative Group Program (informally known as the Cooperative Group Program). It currently comprises 10 Groups that involve more than 3,100 institutions and 14,000 investigators who enroll more than 25,000 patients in clinical trials each year. Since its inception in the 1950s, the Cooperative Group Program has been instrumental in establishing the standards for cancer patient care and clinical research methods. Research performed by the Cooperative Groups has significantly advanced cancer treatment and prevention (IOM, 2010).
However, despite its many and important accomplishments, the Cooperative Group Program faces several challenges that threaten its ongoing productivity. Stagnant and declining funding, inefficient processes, extensive and complex government oversight, and a lack of resources to pursue cutting-edge research hinder the Cooperative Group Program’s ability to translate research discoveries into timely clinical applications (IOM, 2010).
Recognizing the importance of maintaining an effective publicly funded clinical trials system, the director of NCI at the time, John Niederhuber, requested that the Institute of Medicine (IOM) conduct a consensus study of cancer clinical trials and the Cooperative Group Program and develop
recommendations as to how to improve the current system. In April 2010, the consensus committee’s report, entitled A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program (IOM, 2010) was released to the public.1 The report’s recommendations are summarized in Box 1.
The authoring committee of the report concluded (IOM, 2010):
Collectively, the implementation of [the committee’s] recommendations would reinvigorate the Clinical Trials Cooperative Group Program for the 21st century and strengthen its position as a critical component of the translational pathway from scientific discovery to improved treatment outcomes for patients with cancer. Modifying any particular element of the Program or the clinical trials process will not suffice; changes across the board are urgently needed. All participants and stakeholders, including physicians, patients, and health care insurers, as well as NCI, other federal agencies, academia, foundations, and industry, must reevaluate their current roles and responsibilities in cancer clinical trials and work together to develop a more effective and efficient multidisciplinary trials system.
To discuss how best to achieve the aims underlying the recommendations in the IOM consensus report and to summarize progress to date toward addressing these recommendations, the IOM’s National Cancer Policy Forum and the American Society of Clinical Oncology (ASCO) convened a workshop on March 21, 2011, in Washington, DC. The goals of the workshops were to
1. Establish a venue to promote a collaborative approach by all stakeholders to implement recommended changes;
2. Provide a forum to ensure public involvement;
3. Document changes that take place; and
4. Facilitate progress toward the IOM committee’s goal of ensuring the continued viability and increased productivity of an NCI-funded clinical trials system with widespread academic involvement and community outreach.
This workshop included four panel discussions, which focused on (1) the roles of NCI and the Cooperative Groups; (2) the role of payors; (3) interactions between industry, the Food and Drug Administration, and the publicly funded cancer clinical trials system; and (4) the role of clinical
_______________
1 The Executive Summary from the Institute of Medicine consensus report appears in Appendix B of this workshop summary.
BOX 1
Summary of the IOM Consensus Recommendations
Goal I. Improve the speed and efficiency of the design, launch, and conduct of clinical trials
1. Review and consolidate some front office operationsa of the Cooperative Groups on the basis of peer review
2. Consolidate back office operations of the Cooperative Groups and improve processesb
3. Streamline and harmonize government oversight
4. Improve collaboration among stakeholders
Goal II. Incorporate innovative science and trial design into cancer clinical trials
5. Support and use biorepositories
6. Develop and evaluate novel trial designs
7. Develop standards for new technologies
Goal III. Improve the means of prioritization, selection, support, and completion of cancer clinical trials
8. Reevaluate the role of NCI in the clinical trials system
9. Increase the accrual volume, diversity, and speed of clinical trials
10. Increase funding for the Cooperative Group Program
Goal IV. Incentivize the participation of patients and physicians in clinical trials
11. Support clinical investigators
12. Cover the cost of patient care in clinical trials
_______________
a Front office operations refer primarily to the Cooperative Group scientific committees and statistical offices, which are responsible for activities such as trial design, prioritization, and data analysis.
b Back office operations refer to administrative structures and activities that include such things as data collection and management, data queries and reviews, patient registration, audit functions, case report form processing, image storage and retrieval, drug distribution, credentialing of sites, and funding and reimbursement for patient accrual.
SOURCE: IOM, 2010.
trials investigators and patient advocates. This document summarizes the content of each workshop session, which included presentations by panel members and open discussion. The views expressed in this summary are those of the speakers and discussants, as attributed to them, and are not the consensus views of the workshop participants or members of the National Cancer Policy Forum.
Key Challenges Discussed at the Workshop
• How to effectively and efficiently consolidate the current nine adult Groups into fewer Groups.
• How to monitor ongoing changes and assess outcomes derived from those changes.
• How to overcome technical, structural, and procedural obstacles to conduct cutting edge clinical trials that are likely to advance patient care.
• How to ensure that the full portfolio of NCI-funded clinical trials is adequately evaluated and appropriately allocated.
• How to sufficiently fund the Cooperative Group Program.
• How to provide positive incentives for physicians and patients to participate in clinical trials.
PANEL I: NCI AND THE COOPERATIVE GROUPS
The first session of the workshop opened with accounts from representatives of NCI and leaders of the Cooperative Groups about the responses of their respective organizations to the IOM consensus report. Their five presentations were followed by a panel discussion involving additional Cooperative Group leaders.
NCI Perspective and Current Activities
Dr. James Doroshow, director of NCI’s Division of Cancer Treatment and Diagnosis, opened the session with an account of that agency’s multifaceted efforts to address the IOM consensus report. “NCI is implementing
a comprehensive approach to transforming its clinical trials system to create a highly integrated network that can address rapid advances in cancer biology,” he stated, noting that this process has been informed not only by recommendations from the IOM report, but by several others—most notably those of the Clinical Trials and Operational Efficiency Working Groups (CTWG and OEWG, respectively; NCI, 2005, 2010)—as well as by input from stakeholders. Focusing on the four overarching goals and twelve recommendations put forth in the IOM consensus report (see Box 1), Dr. Doroshow provided detailed documentation of progress in these areas, which is summarized in Table 1.
Efforts to consolidate “front office” operations among the Cooperative Groups (in response to Recommendation 1 of the IOM consensus report) were especially visible at the time of the workshop, which closely followed announcements to voluntarily consolidate the Radiation Therapy Oncology Group (RTOG) and the National Surgical Adjuvant Breast and Bowel Project (NSABP); the Eastern Cooperative Oncology Group (ECOG) and the American College of Radiology Imaging Network (ACRIN); as well as ongoing efforts to consolidate the American College of Surgeons Oncology Group (ACOSOG), the Cancer and Leukemia Group B (CALGB), and the North Central Cancer Treatment Group (NCCTG). NCI’s initial approach has focused on supporting up to four adult Groups and one pediatric Group, according to Dr. Doroshow. He added that NCI intends to implement a new Funding Opportunity Announcement (FOA)2 over the course of the next year that will call for a simultaneous external peer review of all parties, so as to “look at organizations one against another and try to facilitate the allocation of the resources that we have in the most appropriate way.” The timeline for that initiative is shown in Box 2.
The cancer clinical trials network is ripe for transformational, systemic change for the following reasons, Dr. Doroshow noted:
• Requirements for molecular screening of large patient populations to define subgroups for study necessitate that NCI-supported clinical research groups function as a coordinated network.
_______________
2 A Funding Opportunity Announcement is a publicly available document by which a federal agency makes known its intentions to award discretionary grants or cooperative agreements, usually as a result of competition for funds. FOAs may entail program announcements, requests for applications, notices of funding availability, or solicitations, depending on the agency and type of program.
| Goal | Recommendation | NCI Response as of March 2011 |
|
1: Improve speed and efficiency of the design, launch, and conduct of clinical trials |
2: NCI should facilitate some consolidation of Cooperative Group “front office” operations by reviewing and ranking the Groups with defined metrics on a similar timetable and by linking funding to review scores |
Current focus on supporting up to four adult Cooperative Groups with continued funding of one pediatric Cooperative Group Planning for NCI external peer review of all Groups in the same review cycle with new review criteria emphasizing collaboration and evaluating Groups as partners in a National Clinical Trials Network Engaged in ongoing discussion with the Cooperative Group Chairs about potential consolidation activities, with some Groups already taking first steps to consolidate (RTOG-NSABP; ACOSOG-CALGB-NCCTG; ECOG-ACRIN) |
|
2: Require or facilitate consolidation of Group “back office” operations and, working with the extramural community, make process improvement in operations and organizational management a priority |
Instituted comprehensive, centralized 24/7 patient registration for all Group trials, with regulatory and site verification of trial participation by the Cancer Trials Support Unit (CTSU) Implemented Operational Efficiency Working Group (OEWG) timelines for concept evaluation, protocol development, and trial activation Working with Groups on a single, harmonized approach to clinical trial management, including protocol authoring, case report forms, and standardized data collection and management |
|
|
3: The U.S. Department of Health and Human Services (HHS) should lead a trans-agency effort to streamline and harmonize government oversight and regulation of cancer clinical trials |
Established an interagency agreement with the Food and Drug Administration (FDA) for early review of approved Cooperative Group Phase III treatment trials, allowing for 21-day review of a concept if it has been identified as a licensing trial Developed coordinated protocol development and review processes with Groups for Phase III trials developed under FDA Special Protocol Assessment (SPA) Developed adult and pediatric NCI Central Institutional Review Boards with the HHS Office for Human Research Protections (OHRP) for Group trials with recent major improvement in review time lines and plan for accreditation by the Association for the Accreditation of Human Research Protection Programs Working with the Center for Devices and Radiological Health (CDRH) of the FDA to coordinate early review of investigational devices (e.g., biomarker assays, genomic signatures) used in treatment trials |
| Goal | Recommendation | NCI Response as of March 2011 |
|
4: NCI should take steps to facilitate more collaboration among the various stakeholders in cancer clinical trials |
NCI is working across divisions to harmonize guidelines for programs engaged in the conduct of clinical trials so that the appropriate incentives are in place for collaboration among Cooperative Groups, Cancer Centers, and Specialized Programs of Research Excellence (SPOREs) In collaboration with the CEO Roundtable on Cancer, developed Standard Terms of Agreement for Research Trials (START) clauses for company and academic collaborations; accelerated clinical trials negotiations Assessing feasibility of developing standardized Material Transfer Agreements (MTAs) that cover intellectual property (IP) considerations for industry and academic institutions Revised IP option on all Cancer Therapy Evaluation Program (CTEP) Cooperative Research and Development Agreements (CRADAs) relating to drug development and specimen use for correlative science; published in Federal Register March 11, 2011 (CTEP, 2011) |
|
|
5: NCI should mandate submission of annotated biospecimens to high-quality, standardized central biorepositories when samples are collected from patients in the course of Group trials and should implement new funding mechanisms and policies to support the management and use of those resources for retrospective correlative science |
Revising Requests for Application (RFAs) for Resource-Related Research Project-Cooperative Agreement (U24) grants by the National Institutes of Health (NIH) for National Specimen Banks to include common operating procedures for samples collected from patients enrolled in Cooperative Group and other NCI-supported trials and to reflect consolidation of the Group system Working with Groups to develop a common review process and procedures for requests for biospecimens banked from clinical trials Identified the need to develop shared information technology (IT) infrastructure to enhance specimen inventories |
| Goal | Recommendation | NCI Response as of March 2011 |
|
2: Incorporate innovative science and trial design into cancer clinical trials |
6: Cooperative Groups should lead the development and assessment of innovative designs for clinical trials that evaluate cancer therapeutics and biomarkers (including combinations of therapies) |
Initiated the Biomarker, Imaging, and Quality of Life Studies Funding Program (BIQSFP) to ensure that critical correlative studies could be incorporated in a timely manner into Phase III and large, multi-institutional Phase II trials during the process of concept development From mid-2008 to 2010, 14 of 40 concepts incorporating predominantly integral and some integrated markers were supported for a total commitment to date of $22.5 million Children’s Oncology Group Trial AAML0531 incorporating biomarkers completed (>1,000 pts): FLT3/ITD (fms-like tyrosine kinase receptor-3/internal tandem duplication) high allelic ratio (Integral) and CEBP (CCAAT/Enhancer Binding Protein) (Integrated) |
|
7: NCI, in cooperation with other agencies, should establish a consistent, dynamic process to oversee development of national unified standards |
Under auspices of the Clinical and Translational Research Advisory Committee (CTAC), developed definitions of integral and integrated studies for biomarkers, imaging, and quality-of-life investigations associated with Group trials and priorities for support thereof Working with the NIH National Library of Medicine (NLM) and the Association of American Cancer Institutes (AACI) to develop the Cancer Trials Reporting Program database to provide accrual information related to all NCI-supported clinical trials |
|
|
8: NCI should reevaluate its role in the clinical trials system |
Initiated CTAC, the first new federally chartered NCI advisory group in a decade, which has operated for more than three years assuming specific responsibilities for NCI’s clinical trials programs. It is currently engaged in evaluating the implementation of the Clinical Trials Working Group (CTWG) recommendations; developing an extramural group to provide strategic input for clinical trials network, under CTAC guidance Revamped prioritization process for large Phase II and Phase III treatment and control trials by creating disease- and modality-specific steering committees to ensure that the most important trials are given highest priority. Note that • While NCI has a voice on the steering committees, its role is to facilitate trial implementation, rather than to direct the primary review • Steering committees convene clinical trials planning meetings to identify critical clinical trials issues for future studies |
| Goal | Recommendation | NCI Response as of March 2011 |
|
3: Improve prioritization, selection, support, and completion of cancer clinical trials |
9: NCI, Cooperative Groups, and physicians should take steps to increase the speed, volume, and diversity of patient accrual and to ensure high-quality performance at all sites participating in Group trials |
Modernizing the clinical trials IT infrastructure by procuring a clinical trials data management system that can be used across the NCI-supported Cooperative Group System Enhancing trial participant diversity through support for Minority-Based Community Clinical Oncology Programs, Patient Navigator Research Program, and other NCI programs Working with patient advocates in concept development and accrual planning, along with Cooperative Groups, disease steering committees, and Patient Advocate Steering Committee |
|
10: NCI should allocate a larger portion of its research portfolio to the Clinical Trials Cooperative Group Program to ensure that the Program has sufficient resources to achieve its unique mission |
NCI developed targeted initiatives that have increased reimbursement to sites from $2,000 to $5,000 per enrolled patient for large Phase II studies; additional funding provided for select Phase III trials based on complexity, as well as for critical biomarker, imaging, and quality-of-life studies Without an increase in resources, changes in the funding model must be considered in the context of the number of new trials, the total accrual that can be sustained, and the need for supporting correlative science. The need to focus on high-accruing organizations (half of sites accrue approximately 80 percent of patients) and the need for additional infrastructure support are under discussion with Cooperative Group chairs |
|
|
4: Incentivize the participation of patients and physicians in clinical trials |
11: All stakeholders should work to ensure that clinical investigators have adequate training and mentoring, paid protected research time, necessary resources, and recognition |
NCI created the Clinical Investigator Team Leadership Award to promote collaborative science and recognize outstanding clinical investigators; first awarded in 2009 |
|
12: Health care payment policies should value the care provided to patients in clinical trials and adequately compensate that care |
Worked with HHS Centers for Medicare and Medicaid Services (CMS) to establish a pilot program for reimbursement for clinical trials care under a CMS national coverage decision for agents used for colorectal cancer, as well as on data collection to evaluate use of imaging and other clinical modalities Leading new CMS interagency (NIH-FDA-CMS) work groups to assist in the development of approaches to reimbursement for genetic tests used to choose targeted therapy and for the use of helical computed tomography (CT) for lung cancer screening |
|
NOTE: ACOSOG, American College of Surgeons Oncology Group; ACRIN, American College of Radiology Imaging Network; CALGB, Cancer and Leukemia Group B; ECOG, Eastern Cooperative Oncology Group; NCCTG, North Central Cancer Treatment Group; NSABP, National Surgical Adjuvant Breast and Bowel Project; RTOG, Radiation Therapy Oncology Group.
SOURCE: Doroshow presentation, March 21, 2011.
| December 2010–July 2011 | Gather information/input from stakeholders and community for new FOA and Guidelines; develop Concept |
| August 2011 | NCI Divisional/Clinical and Translational Research Operations Committee Concept Review |
| September 2011 | NCI Scientific Program Leadership Concept Review |
| November 2011 | Board of Scientific Advisors Concept Review |
| November 2011–March 2012 | NCI Division of Extramural Activities Review of FOA and Guidelines |
| March 2012–July 2012 | NIH Review of new FOA and Guidelines |
| July 2012 | New FOA Released/Published |
| November 2012 | Receipt of Competing Applications for new FOA |
| February 2013 | Review of Competing Applications by NCI Division of Extramural Activities |
| May 2013 | National Cancer Advisory Board Review |
| After October 2013 | Rollout of Awards in Fiscal Year 2014 |
SOURCE: See http://transformingtrials.cancer.gov/files/NCI-Presentationto-Cooperative-Group-Chairs.pdf.
• Extramural scientific prioritization of the Phase III portfolio across all disease entities is essential to efficiently develop and complete multicenter trials; a smaller number of disease committees are better suited to building such consensus.
• Currently configured Groups have disincentives to study less common cancers because disease committee may wish to avoid taking any risk of accrual failure.
• Shared information technology (IT) infrastructure with a common “front end” for clinical data management and for tissue resource management will require ongoing modification; this will be more manageable if it involves fewer independent Groups.
• Open access to a national cancer clinical trials network for clinical and translational investigators not currently involved in the current Group platform will ensure the best competition of ideas and the movement of high-priority science into the clinical trials arena.
In considering how best to meet these needs, NCI examined a variety of models for consolidating the Groups, including the creation of a single national Group, Dr. Doroshow explained. This structure would have the advantage of being fully integrated, free of operational overlap, and potentially easier to harmonize with respect to biomarker studies and IT, he said; however, he added, NCI ultimately favored a smaller network of Groups, as suggested in the IOM consensus report—and specifically, as previously noted, a network comprised of four adult Groups and a single pediatric Group.
Compared to a single national Group, the small network of Groups provides for greater competition among ideas, makes data management more feasible and less costly, and better facilitates involvement by a broad range of investigators, volunteers, and philanthropic organizations, Dr. Doroshow continued. “I think it’s important, however, to point out that a network, by itself, does not guarantee a coordinated approach across groups or the full integration of this clinical trials activity as a system,” he added. “We have to think long and hard about how to make sure that we don’t end up with five silos rather than ten.”
Similarly, Dr. Doroshow noted that NCI is considering changes in funding for the Cooperative Group Program in order to optimize the use of limited resources. “There is no question but that the resources that we allocate to the Cooperative Group System are inadequate,” he observed. “They are inadequate to provide the per-case reimbursement that actually
pays sites the real costs of doing the studies, and they really are inadequate to pay the real costs of the administrative infrastructure that is supported now by this enormous pro bono effort.” Additional resources—millions of dollars—made available though the auspices of past NCI directors have enabled increased reimbursement for large Phase II trials and also improved reimbursement for more complex Phase III trials, he noted; however, these funds still fall far short of need. “We are probably about a log away from the amounts of additional monies that we actually need to pay the real costs of these trials,” he said.
Therefore, NCI is actively engaged in revising its funding model for cancer clinical trials, Dr. Doroshow reported. “We have to think very seriously about the number of trials we can do, the number of patients we can accrue, so that we allow … [the] 40 to 50 percent of our sites [that] accrue about 75 to 80 percent of our patients … to survive and prosper and have the resources to do the trials that are the kinds of trials that everyone wants to conduct in the 21st century,” he explained. “We have certainly made no decisions in this area,” he continued, “but clearly we need to support what I would view as the critical seed corn of our clinical trials infrastructure by allowing those institutions to receive the support they need to at least pay their costs.”
The proposed organizational structure for NCI’s clinical trials program is shown in Figure 1. This model provides for a system in which all Groups interact to develop a national agenda for clinical trials and increase efficiencies of accrual, initiation, and completion of all trials, Dr. Doroshow said. It also encourages input from Cancer Centers3 throughout the system and permits greater integration of investigators who participate in Specialized Programs of Research Excellence (SPOREs)4 and in Program Project
_______________
3 NCI-designated Cancer Centers are recognized for their scientific excellence. They are a major source of discovery and development of more effective approaches to cancer prevention, diagnosis, and treatment. They also deliver medical advances to patients and their families, educate health care professionals and the public, and reach out to underserved populations. An NCI-designated Cancer Center may be a freestanding organization, a center within an academic institution, or part of a consortium of institutions.
4 A SPORE grant is a specialized center grant to support interdisciplinary teams of investigators who conduct translational research focused on an organ-specific human cancer (e.g., breast cancer) or a highly related group of human cancer types (e.g., gastrointestinal cancers).
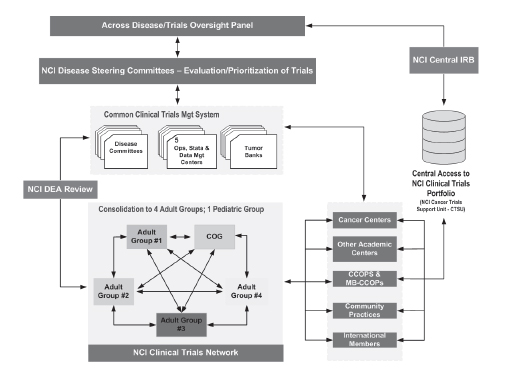
FIGURE 1 NCI’s proposed new organizational structure for the Cooperative Group Program.
SOURCE: Doroshow Presentation, March 21, 2011.
Grants.5 Another important feature of this model is the existence of an oversight body to guide NCI’s management of the clinical trials system, which he characterized as “something that we have woefully lacked.”
The remainder of Dr. Doroshow’s presentation focused on measures NCI has taken in recent years as they relate to specific goals and recommendations of the IOM consensus report, as summarized in Table 1. For example, he reported that NCI has implemented time lines for concept evaluation, protocol development, and trial activation recommended by the OEWG (NCI, 2010) and further endorsed by the IOM committee (IOM, 2010). NCI is also pursuing the OEWG’s recommendation to adopt a single, harmonized approach to clinical trials management, includ-
_______________
5 A Program Project Grant, or P01, is an assistance award for the support of a broadly based multidisciplinary research program that has a well-defined central research focus or objective. It may also include support for common resources (cores) required for conduct of the P01 research projects. Interrelationships between projects are expected to result in a greater contribution to program goals than if each project were pursued separately.
ing protocol authoring, case report forms, and standardized data collection and management.
Additional actions taken by NCI in response to the IOM consensus report include working with the Center for Devices and Radiological Health (CDRH) of the U.S. Food and Drug Administration (FDA) to coordinate early review of biomarkers and other investigational devices used in treatment trials; revising the intellectual property (IP) option on all Cancer Therapy and Evaluation Program (CTEP) Cooperative Research and Development Agreements (CRADAs) relating to drug development and specimen use for correlative science (CTEP, 2011); and improving review time lines for the NCI Central Institutional Review Boards (CIRBs; one for pediatric trials and one for adult trials).
Several participants in the subsequent panel discussion noted that the expansion of CIRB usage could further increase efficiency gains afforded by Cooperative Group consolidation. However, as Dr. Roy Herbst, of Yale Cancer Center, observed, “That will only work if the local IRBs actually recognize that central IRB.” Dr. Doroshow reported that the U.S. Department of Health and Human Services (HHS) is likely to make a rule change that will mandate reliance on a single IRB for any multisite clinical trial.6 In that case, a trial approved by a single IRB (not necessarily NCI’s CIRB) could determine approval for a clinical trial on a national basis. Even then, however, the local IRB would have to be willing to accept a decision made by another IRB. The potential expansion of CIRB usage was further discussed during Panel III (see subsection “Central IRB and Informed Consent”).
NCI has also participated in “significant ongoing efforts” to improve the clinical trials IT infrastructure of the Cooperative Groups by procuring a clinical trials data management system that can be used across the NCI-supported Cooperative Group System, Dr. Doroshow said. This process was discussed in a subsequent presentation by Dr. Robert Gray of the Dana-Farber Cancer Institute and lead statistician for ECOG (see page 26).
“Developing a national clinical trials network is an ongoing process,” Dr. Doroshow observed in conclusion. “We need to hear from every stakeholder, to think about all the issues … [and at] the same time, we need to continue the process of [improving] efficiency, enhancing the coordination
_______________
6 On July 26, 2011, the U.S. Department of Health and Human Services announced that the federal government is contemplating various ways of enhancing the regulations overseeing research on human subjects, as described in an Advance Notice of Proposed Rule-making (Federal Register, 2011).
activities in the system, and conducting the evaluations that are an ongoing process of this activity overall.”
Response of the NCI Community Clinical Oncology Program (CCOP)
Following Dr. Doroshow’s overview of NCI’s efforts toward the comprehensive revision of the national cancer clinical trials system, Dr. Lori Minasian, director of CCOP and acting director of the Division of Cancer Prevention at NCI, discussed CCOP’s role in the clinical trials system and described how the program has begun to address the goals and recommendations of the IOM consensus report. She explained that CCOP has three components, each of which supports its own Request for Applications (RFA)7: the Community Consortium to Accrue; the Minority-Based CCOPs; and the CCOP Research Base, which is comprised of Cooperative Groups and Cancer Centers funded to design, develop, and conduct clinical trials in cancer prevention and treatment.
The CCOP Research Base is NCI’s primary mechanism for funding Cooperative Groups and certain Cancer Centers that conduct clinical trials for interventions other than treatment, Dr. Minasian pointed out. “Under the CCOP Research Base, the scope of the research has grown over the last 20 years,” she said; highlights of this expansion included the launch of large, significant, cancer prevention trials and the accumulation of a growing portfolio of cancer control studies focused on methods for early detection and improvements in quality of life, continuing care, and palliative care. She emphasized that cancer prevention and quality-of-life trials require different strategies and produce different types of data than cancer treatment trials and that these differences need to be addressed in attempts to harmonize and standardize data collection and management in cancer clinical trials, as recommended in the IOM consensus report.
The planned consolidation of Cooperative Groups into four adult groups and one pediatric group presents an unusual opportunity to review and redefine cancer prevention and treatment agendas among CCOPs that are funded as Research Bases, Dr. Minasian observed. The CCOP releases FOAs on an annual basis, she explained; with the release of the CCOP
_______________
7 A Request For Applications (RFA) is the official statement that invites grant or cooperative agreement applications to accomplish a specific program purpose. RFAs indicate the amount of funds set aside for the competition and generally identify a single application receipt date.
Research Base RFA in the spring of 2011, competing Cooperative Groups were required to describe their current process in the transition to consolidation and examine how their cancer prevention and control agendas may evolve. “We are not expecting them in their applications to be able to foresee the next five years, but this will be a development over time,” she acknowledged.
In the meantime, Dr. Minasian reported, the Clinical Trials Support Unit (CTSU8) has already begun to accept cancer control trials, and some Cooperative Groups have been using the Regulatory Support System for cancer control studies—even those that have not involved the CTSU for accrual. She also noted that the audit guidelines for the CCOP Research Bases have been incorporated into NCI guidelines for all Cooperative Groups and that all of the CCOP Research Base studies are now part of CTEP. “There is a CTEP and CCOP team working together, meeting about every two weeks now, to help coordinate and facilitate the transition so that the systems and the processes, if not the same, at least are in parallel and complementary, so that we are implementing things in the same spirit,” she said.
On the other hand, Dr. Minasian continued, it is important to recognize certain unique needs of cancer control studies; for example, because few interventions studied for cancer control are drugs, there are few partnerships with pharmaceutical companies. By contrast, cancer control studies are often chaired by Ph.D.s or investigators outside the field of oncology, she said; in those cases, CCOP has encouraged collaborative funding from external sources (e.g., the National Institutes of Health other than NCI, the American Cancer Society). However, she added, these circumstances also make it more difficult to involve investigators that are not routinely part of the treatment clinical trials program. Seeking such additional funding automatically lengthens trial development, because even after a concept is approved, protocol development cannot move forward without external funding, she explained.
Dr. Minasian noted that the IOM recommendation to incorporate innovative science and trial design is particularly appropriate for cancer control studies, and she also noted the need for translational studies on cancer prevention and control trials. “Clearly cancer control endpoints are not the same as cancer treatment endpoints, so we absolutely encourage novel trial design,” she said. Two representatives of the Division of Cancer Prevention
_______________
are members of the Investigational Drug Steering Committee, she reported, and are therefore well placed to identify candidate drugs for cancer control.
Dr. Minasian emphasized that cancer control assessments often hinge on criteria that differ from treatment studies, particularly with regard to neuropathy or pain. Obtaining consensus for a non-treatment concept often takes extra time, she observed; however, in cases where no treatment option exists for an indication, protocol development tends to proceed more quickly.
The development of steering committees has improved the review process for cancer control studies, Dr. Minasian said. “The advantage with the steering committee right now is that we are allowed to call on extramural individuals with expertise in neuropathy, in CAM [complementary and alternative medicine], in other topics of cancer control interest,” she explained; by contrast, a large prevention trial once would have had to be submitted to independent peer review by a study section specifically developed for that trial. However, she added, there is no current need to develop a standing prevention steering committee, due to the relatively low volume of prevention studies; ad hoc groups can be assembled to draw on appropriate expertise. “If this area develops and becomes larger, we would consider a prevention steering committee at that time,” she stated.
With regard to the goal of improving the diversity of patient populations in clinical trials, Dr. Minasian observed that the Minority-Based CCOP program has been instrumental in accruing minority patients onto cancer clinical trials. The Minority-Based CCOPs account for about a third of the minority accrual onto NCI clinical trials, and about 60 percent of patients accrued through Minority-Based CCOP programs are members of a minority group, she reported. She added that the CCOP strategic plan identifies the underserved population as a core issue, which has in turn sparked efforts to identify relevant research questions and to develop a transdisciplinary working group.
A related project, jointly administered by NCI and ASCO, is aimed at eliminating cancer disparities (the ultimate goal of increasing the diversity of clinical trial patient populations), according to Dr. Minasian. The ASCO-NCI Cancer Disparities Research Group is examining ways to increase collaboration among academic and community and public institutions, both by developing a consensus statement with recommendations and by promoting specific research projects to be undertaken by the Cooperative Groups, she said.
In terms of funding, Dr. Minasian reported that CCOP is exploring
ways to incorporate multiple principal investigators in the structure of a CCOP grant. “Our usual grantees are community hospitals, and now we are seeing more health systems consolidate, and so the health systems are looking to become the CCOP grantees,” she observed. “That is creating both some unique opportunities and some unique strains on the system in terms of keeping the CCOP program primarily as a doctor- or physician-run program.”
In closing, Dr. Minasian described the results of a recent survey of more than 1,500 specialty physicians who cared for colorectal and lung cancer patients (Klabunde et al., 2011), which suggest that many physicians at CCOPs and Cancer Centers do not participate in clinical trials. In addition, a recent patient survey by Research!America found that more than 70 percent of those polled would be willing to participate in clinical trials, but that only 6 percent reported that their physicians had ever suggested doing so (Research!America, 2010), she said; another survey by the Mayo Clinic indicates that most patients expect their physicians to inform them of clinical trials (Sood et al., 2009). Together, these results suggest the need for improved and enhanced outreach to physicians, so that they engage and accrue more patients to cancer clinical trials, she concluded.
Cooperative Group Leadership Perspective and Current Activities
Cooperative Group Chairs’ Perspective
Dr. Jan Buckner of the Mayo Clinic, chair of the NCCTG and also of the Cooperative Group Chairs, introduced his presentation to the workshop with a summary of the many advances in cancer prevention, diagnosis, and treatment identified through clinical trials conducted by Cooperative Groups; these were described in the IOM consensus report and also in a recent issue of Seminars in Oncology (Perry et al., 2008). He also noted that an editorial published just prior to the workshop in the New England Journal of Medicine (Moss et al., 2011) called for an organizational structure similar to that proposed in the IOM consensus report for the conduct of clinical trials across all disease groups, not just cancer.
Reporting on the scientific and operational accomplishments of the Cooperative Groups, and on the challenges they face, Dr. Buckner observed that the Groups “have been and will continue to be vital engines to conduct multidisciplinary, practice-changing, biologically driven clinical trials in the academic and community setting” and that the Groups have demonstrated
both the will and the capacity to respond to the recommendations of the IOM consensus report.
Among recent scientific accomplishments of the Cooperative Groups, Dr. Buckner noted the collection of tumor and normal tissue samples from hundreds of thousands of cancer patients and those at risk for cancer; these biospecimens are linked to clinical outcomes and treatment protocols, as well as to follow-up data and laboratory observations. Examples of the latter include such high-impact translational research as the prospective clinical trial (TAILORx9) to assess the clinical utility of the 21-gene assay Oncotype DX, which is used to predict the risk of disease recurrence in women with early-stage node-negative, estrogen receptor-positive breast cancer, identifying those who are at high risk for recurrence and thus more likely to benefit from adjuvant chemotherapy and those who are at low risk for recurrence and thus can safely avoid additional treatment.
“As a result of the clinical data and the biospecimens, the Cooperative Groups have been collaborating with Cancer Center investigators for many, many years,” Dr. Buckner observed. In recent years, he reported, Cooperative Group clinical data and biospecimens have been used in more than 60 collaborations with Cancer Centers, 6 program project grants, and more than 10 SPORE collaborations, among others projects—including non-federally funded studies. He added that he expects this collaborative trend to continue and accelerate.
Such collaborations have led to a number of biomarker-driven trials by Cooperative Groups that have produced definitive results, according to Dr. Buckner. He provided several examples of such achievements, including the following:
• Identification of trastuzumab as an active adjuvant therapy for HER2-positive breast cancer in combination with chemotherapy;
• Evaluation of cetuximab as adjuvant therapy for patients undergoing therapy for KRAS wild-type stage III colon cancer;
• Examination of imaging as a biomarker to guide treatment decisions in non-Hodgkin’s lymphoma; and
• Assessment of risk of recurrence in stage II colon cancer for patients with deletion of 18q and microsatellite instability.
_______________
9 Trial Assigning IndividuaLized Options for Treatment; see http://www.cancer.gov/clinicaltrials/noteworthy-trials/tailorx.
TABLE 2 Overall Cooperative Group Funding Structure, Fiscal Year 2007
| Funding Component | Total Costsa | |
| Cooperative Group awards | $161 million (45%) | |
| CCOP accrual support | $ 10 million (3%) | |
| CTSU contract | $ 18 million (5%) | |
| Accrual cost sharing | $ 88 million (24%) | |
| Pro–bono investigator time | $ 28 million (8%) | |
| Industry support | $ 41 million (11%) | |
| Philanthropic support | $ 6 million (1.5%) | |
| Other support | $ 9 million (2.5%) | |
| Total | $361 million | |
a Direct and indirect costs.
SOURCES: Judith Hautala, 2010; Buckner presentation, March 21, 2011.
These trials have benefited not only from NCI’s financial support, but also from countless volunteer hours from members of the Cooperative Groups, Dr. Buckner pointed out. As shown in Table 2, nearly half of the funds necessary to conduct such trials have been provided from volunteer hours and from sources apart from NCI.
Several recent operational accomplishments by the Cooperative Groups echo the IOM consensus report recommendations, Dr. Buckner continued. In 2005, the Groups initiated a collaboration to develop detailed user-needs assessments and technical specifications for a single remote data capture system that would be utilized for all trials, he reported; system implementation has now begun. Through participation in the OEWG (as previously noted by Dr. Doroshow), each Group has developed internal processes and metrics to meet protocol-development milestones in a timely manner. The Groups’ statistics and data-management units have contributed to the development of innovative clinical trial designs to accommodate the increasing complexity of integrating biomarkers into design and interpretation, Dr. Buckner said. They have also partnered with clinical investigators to develop valid endpoints, reflecting the changing nature of clinical research and practice, and have worked to streamline clinical trial conduct
by evaluating components of standardized clinical outcome assessment systems, such as RECIST10 and the Common Toxicity Criteria.11
Among Cooperative Groups that are consolidating their front and back office operations, three (ACOSOG, CALGB, and NCCTG) have already begun integration of a single statistics and data center to support all three existing Groups, Dr. Buckner stated. These Groups have also agreed to complete integration of scientific and operational functions to create a new group.12 Such combined Groups will have to address several outstanding issues, he said; the most pressing of these is to develop a rational and stakeholder-informed system for setting research priorities. “The voice of the investigator community and the patient community must be paramount if [clinical] trials are to succeed,” he observed. “Central control often stifles innovation.”
Stakeholders must be offered concrete incentives for scientific collaboration, Dr. Buckner continued. “Academic and community investigators should be rewarded in their grant awards for participating in collaborative research,” he said; therefore, grant guidelines and terms of awards should have specific language outlining the rewards for contributing to collaborative science.
Coordinated review and support of translational science must occur in order to better integrate the aims of clinical trials and correlative science, Dr. Buckner observed. While acknowledging that BIQSFP (Biomarker, Imaging, and Quality of Life Studies Funding Program) funds have been helpful to this end, he encouraged support of additional efforts to optimize clinical and scientific collaborations, such as providing preliminary data for the next Phase III trials.
Enhancements of the systems that the Cooperative Groups have devel-
_______________
10 Since the year 2000, an international committee has promulgated unified, easily applicable criteria for measuring tumor response using X-ray, CT, and MRI, which are known as Response Evaluation Criteria in Solid Tumors (RECIST). The technique is recommended but not mandatory for NCI-sponsored trials and involves formalized rules for measurement of tumor target lesions. See http://imaging.cancer.gov/clinicaltrials/imaging/.
11 These scales and criteria are used by doctors and researchers to assess how a patient’s disease is progressing, to assess how the disease affects the daily living abilities of the patient, and to determine appropriate treatment and prognosis. See http://ecog.dfci.harvard.edu/general/common_tox.html.
12 The three Groups have since announced a new name for the merged organization: the Alliance for Clinical Trials in Oncology (or “Alliance”). See http://www.alliance-website.org/site/.
oped for multisite conduct of clinical trials are also needed, along with improved biospecimen annotation and informatics support, implementation of remote data capture systems across all groups, and assistance with overall operational management (e.g., membership concerns, regulatory affairs, finances), Dr. Buckner said. “The process of scientific prioritization, collaboration among laboratory and clinical investigators from multiple venues, and modernization of informatics support remain key issues to address in the future,” he concluded.
Group Statisticians’ Perspective
Speaking on behalf of the Cooperative Group statistical leaders, Dr. Robert Gray of the Dana-Farber Cancer Institute, who leads the Eastern Cooperative Oncology Group Statistical Center, considered the role of statistical centers in a changing Cooperative Group System and their contribution toward meeting goals and recommendations stated in the IOM consensus report. In approaching these challenges, Group statisticians adhered to two basic principles, he said: (1) biostatistics is an essential component of Group science, and (2) the complexity of the research performed by the Groups requires independent, academically based statistical leadership.
Group science needs to be integrated with the biostatistical leadership in order to encourage collaboration between statisticians, scientists, and clinical researchers, Dr. Gray continued. “The statisticians need to understand the scientific issues, the issues that are required for the research in an area, and that’s best done as an ongoing collaboration over a period of time,” he said. He also stressed the importance of integrating data management with biostatistics, due to the difficulty and expense of the data collection process. “There needs to be substantial interaction among the statistical analysts, data managers, and study chairs throughout the life of a study,” he observed. “The prioritization of data management work needs to be driven by the needs of statistical analyses and the timetables for those analyses as well.”
Most Cooperative Group statistical centers combine biostatistics and data management under a separate grant within the Cooperative Agreement, Dr. Gray explained. Group statisticians support this structure for several reasons, he noted: having a separate grant attracts leadership from top academic centers, providing them with stable support, while offering incentives for institutions to share the cost of research at statistical centers. In addition, he said, such “semi-independent” statistical centers help ensure
that research is conducted properly, while at the same time being well integrated into Group science.
Dr. Gray identified several areas in which the statistical centers have addressed the IOM consensus report goals and recommendations. Patient registration has been improved through the development of a common web-based system known as OPEN (Oncology Patient Enrollment Network). Significant progress has also been made toward adoption of a common remote data entry system, Medidata Rave®,13 which is currently being implemented and is expected to be applied to studies by late 2011. Through the Cancer Biomedical Informatics Grid (caBIG),14 the statistical centers have been developing standardized case report forms (CRFs) that can address the complex data collection requirements of diverse Groups and trials.
The merger of Cooperative Groups raises several issues for the Groups’ statistical centers, Dr. Gray observed. For example, legacy databases, which include some 100,000 patients, must continue to be managed and maintained. He predicted that the combined statistical centers are likely to continue, with largely the same personnel and in the same locations, and many will operate using a multiple-principal-investigator model. He added that economies of scale do not apply to operations in the existing statistical centers, because statisticians can only work on so many projects, and the projects still take largely the same amount of time, regardless of how many people staff the operation. Thus, he concluded, efficiency gains will continue to come primarily from the use of common information systems infrastructure and from greater standardization of processes and data across groups.
Experience from the Consolidation of the Children’s Oncology Group (COG)
The consolidation in 2000 of four pediatric oncology Cooperative Groups—the Children’s Cancer Group, the Pediatric Oncology Group, the Intergroup Rhabdomyosarcoma Study Group, and the National Wilms’ Tumor Study Group—to form COG offers a model for present-
_______________
13 A single platform system for capturing, managing, and reporting clinical research data. Source: http://www.mdsol.com/products/rave_overview.htm.
14 The Cancer Biomedical Informatics Grid (caBIG) is an NCI-sponsored collaborative information network that includes more than 50 Cancer Centers, other NCI-supported research endeavors, and a variety of federal, academic, not-for-profit, and industry organizations. Source: https://cabig.nci.nih.gov/overview/.
day Cooperative Group mergers. In his presentation to the workshop, Dr. Gregory Reaman of George Washington University and past chair of COG, described the rationale for undertaking this process, the challenges it presented, and the results it produced.
The creation of COG was driven primarily by the need to develop adequate study populations, Dr. Reaman explained. “Much of the work that we did in pediatric cancer outside of the acute leukemias and neuroblastoma, where we had relatively sizable patient populations for study, necessitated collaborative efforts and an intergroup process,” he said. “We saw that we were currently, and certainly in the future, going to fail in achieving our mission to cure and prevent childhood cancer as competing entities.”
Despite this urgency, several issues had to be resolved to move the consolidation forward, he recalled. Timing was complicated by the need to continue a large number of open studies while planning new initiatives, some of which depended on results of the ongoing studies, Dr. Reaman noted; additional hurdles involved resolving differences among the legacy Groups regarding investigator and institutional membership designations, redistributing funds, and choosing which of the existing administrative operation and data centers would serve the entire COG. In light of the complexity and importance of biostatistics in clinical research and in order to preserve the crucial knowledge base developed by biostatisticians whose work had focused on pediatric cancer for decades (as Dr. Gray had previously noted), COG also established a distributed statistics department comprised of biostatisticians at legacy Group locations, as well as some independent biostatisticians at other academic institutions, he explained. A remote data entry system, originally developed by the legacy Pediatric Oncology Group, was adapted in order to handle the larger volume of studies undertaken by COG.
“What transpired as a result of the consolidation was the world’s largest childhood cancer research organization, which still encompasses more than 200 pediatric cancer programs in North America, Australia and New Zealand, Switzerland, and the Netherlands,” Dr. Reaman observed. COG, he continued, is a multidisciplinary research enterprise incorporating diverse specialties including pediatric oncology, surgery, radiation therapy, biostatistics, laboratory investigation, and epidemiology, among others.
COG’s single biopathology center is a national resource for pediatric cancer specimen banking, Dr. Reaman said; it has enabled a large number of correlative studies and unique translational research opportunities. The Group employs a system of centralized reference and resource laboratories
to manage its cytogenetic and molecular genetic studies of risk-adjusted approaches to therapy and plans to implement a similar model for radiology, he added. COG has also developed a national childhood cancer registry for North American sites, the Childhood Cancer Research Network, in order to develop a research database for future epidemiologic and molecular epidemiology studies. The network enables all patients with cancer diagnoses at COG member institutions to be registered and the resulting database to be available for research, he explained. More than 98 percent of the families of the nearly 20,000 patients currently registered have consented to be contacted in the future for nontherapeutic epidemiological studies, he reported. Indeed, he added, a dramatic increase in accrual to nontherapeutic studies resulted from the formation of COG, while therapeutic study accrual has grown less consistently since consolidation.
Dr. Reaman recounted numerous accomplishments by COG to date in three main areas: (1) organization and administration; (2) clinical practice-changing research; and (3) translational science. Organizational and administrative advances included establishment of the NCI Pediatric Central IRB, which has in turn reduced time lines for opening studies; participation in an international collaboration for osteosarcoma; development of an interoperable infrastructure for clinical research between COG and the Pediatric Blood and Marrow Transplant Consortium and the Bone Marrow Transplant Clinical Trials Network of the National Heart, Lung, and Blood Institute (NHLBI); and the performance of clinical trials for rare tumors, including retinoblastoma (which is, however, endemic in some countries).
Practice-changing accomplishments by COG include the development of a clinical and biological risk-based classification scheme for acute lymphoblastic leukemia, myeloid leukemia, neuroblastoma, and Wilms’ tumor, which Dr. Reaman said would not have been possible without the collaborative efforts of the consolidated Groups. Similarly, increased patient population sizes enabled COG to demonstrate the prognostic significance of minimal residual disease in acute lymphoblastic and myeloid leukemia (ALL and AML), in neuroblastoma, and in non-Hodgkin’s lymphoma. Based on the collective results of patients treated in legacy studies by the consolidated Groups, COG has also developed exposure-related surveillance recommendations for childhood cancer survivors.
COG’s achievements in translational science include the use of gene expression and microarrays to develop cancer signatures for diagnosis and prognosis in ALL, AML, and rhabdomyosarcoma, according to Dr. Reaman. Through NCI’s Therapeutically Applicable Research to Gen-
erate Effective Treatments (TARGET) initiative,15 COG researchers have genomically characterized and investigated potential therapeutic targets in ALL and neuroblastoma and will soon extend these studies to Ewing’s sarcoma and rhabdomyosarcoma, he reported. COG also participates in NCI’s Cancer Discovery and Development Network,16 which employs new scientific approaches to accelerate the translation of genomic discoveries into new treatments.
Summing up the process of consolidating four pediatric oncology Cooperative Groups to form COG, Dr. Reaman recalled that it was not easy, particularly with regard to merging data systems among the constituent Groups. However, he continued, “the results have clearly indicated that it was something that we don’t regret doing … [and] we are a much stronger group for having done so.”
Following their presentations, Drs. Doroshow, Minasian, Buckner, Gray, and Reaman were joined by 16 Cooperative Group chairs and statistical leaders for a panel discussion. It focused on three main subjects: (1) the logistics and potential consequences of Cooperative Group consolidation; (2) opportunities for encouraging collaboration among the new Cooperative Groups and other institutions, both within and beyond NCI; and (3) various mechanisms for shifting the role of NCI from oversight to support of the Cooperative Groups.
Consolidation of Cooperative Groups
Moderator and workshop chair Dr. Richard Schilsky of the University of Chicago opened the discussion by soliciting brief statements from panel participants regarding their organizations’ specific plans to address the IOM consensus report recommendations. Dr. Robert Comis, of ECOG, attributed the consolidation of ECOG with ACRIN—announced just prior to the workshop—to the IOM consensus report. “I don’t think this would have happened if the IOM report hadn’t come about, but I think that clearly we have brought together two organizations that are extremely complementary. Bringing together our biomarker programs from the
_______________
genetic and proteomic side, and combining that with imaging, will make us all stronger,” he observed.
By contrast, Philip DeSaia, of the Gynecological Oncology Group (GOG), asserted that this group has thrived as a singular entity. “The backbone of the Gynecological Oncology Group is the gynecological oncologists, just like the pediatric oncologists are to the Pediatric Group,” he said, adding that most of the approximately 1,000 such specialists in the United States participate in GOG, largely on a volunteer basis. “It’s hard for my executive committee to figure out how we are going to merge,” he continued. “Would you cut us up into five pieces, four pieces, and put us in each [adult Cooperative] Group?”
Dr. Norman Wolmark of NSABP responded by asking GOG (in the form of a laughter-provoking marriage proposal) to join the NSABP-RTOG alliance.
In a related discussion, Dr. Sharon Murphy, IOM scholar-in-residence, noted that the IOM consensus report did not recommend a specific number of consolidated Cooperative Groups, and wondered why five, not four, adult groups might not be possible. “I think what we have seen is a rather hasty rush to the altar and some arranged marriages, and this was not what the IOM suggested,” she observed.
In fact, the formal IOM recommendation did not specify a particular number of groups. Rather, a hypothetical example of four multidisciplinary groups was described as just one possible approach to consolidation in Chapter 3 of the report.17 Dr. Mendelsohn said that, in theory, its recommendations could be fulfilled with one group, four, or ten, but he also deemed the current approach leading to the “four plus one” model “excellent.”
Dr. Walter Curran of RTOG noted that alliances such as that between RTOG and NASBP represent only one model of consolidation. “Some of the newly created relationships will look different from one another,” he observed. “Some will be one entity; some will be a confederation, or alliance, of many entities. My hope is that the federal guidelines for review will allow such flexibility.” Moreover, he continued, the relationships between these new groups and Cancer Centers or other federally funded entities are likely to vary. Several participants in this discussion shared similar hopes for flexibility in the structure of Groups and in their interactions with each other and with other institutions, particularly the Cancer Centers.
_______________
17 See p. 148 of the IOM (2010) consensus report.
Dr. William Dalton, director of the Moffitt Cancer Center in Tampa, Florida, emphasized that each Group must be multidisciplinary, in addition to encompassing expertise in specific diseases. Rather than merely consolidate, he said, the Cooperative Groups should reorganize so as to bring together experts with specific interests across broad areas of knowledge—as currently occurs now in COG. “We have been talking about an anatomical change to the groups,” Dr. Schilsky observed, but he said that the real goal is functional reorganization.
“Working together and having flexibility … about how we come together and how we interact is very, very important,” Dr. Comis added. He emphasized that the hybrid Cooperative Group System lies at the heart of translational clinical research and relies not only on NCI and industry funds, but also on commitment and in-kind support from the entire cancer research community.
Dr. Schilsky, who has served as both a Cancer Center director and a Cooperative Group chair, observed that nearly every Cancer Center participates in more than one Cooperative Group. “In most cases, it’s Cancer Center members who provide the lion’s share, if not all, of the scientific leadership of the Cooperative Group Program,” he said.
Recalling that the Cooperative Groups, SPOREs, and Cancer Centers emerged as separate entities, Dr. Monica Bertagnolli, of CALGB, reiterated the message in the IOM consensus report that these “siloed” institutions need to work together. That NCI has begun to mandate collaboration through peer review is healthy and beneficial to the overall scientific agenda, she said.
This mandate is not without its challenges, however, as several discussants pointed out. Dr. Constantine Gatsonis, of ACRIN, wondered how existing scientific expertise within Cooperative Groups could be preserved and enhanced as the Groups are consolidated. “I speak with experience from the imaging group, where we had a pretty hard time figuring out how we all fit into this [therapy-driven] system … and how we preserve and enhance the expertise in imaging,” he said. “How do we move, for instance, toward some kind of an imaging hub that would be available for the entire network?” He therefore suggested that the RFAs defining the new groups allow for the creation of a network of strengths and expertise, rather than a reduced number of similar, competing Cooperative Groups.
Dr. Buckner asked whether guidelines for the various NCI programs are being revised so that the Cancer Centers and Cooperative Groups will have specific review criteria for collaboration. Dr. Doroshow responded that substantive changes “are actually now going to be sprinkled throughout the guidelines for Cancer Center Support Grants (CCSGs), giving substantially more weight to the role of the Cancer Centers and their collaborations with the groups.” He added that the SPORE guidelines have been revised quite extensively to emphasize collaboration with Cooperative Groups. “There is going to be a whole new section of the grant that requires the clear delineation of what the aspects of those collaborations are and a specific review criterion with a score,” he explained. “Those guidelines have not yet been approved, but they have been, after a very long period of time, finalized. They will go in the relatively near future to the scientific group, the former executive committee at NCI, and then on to NIH for their purview.”
However, he stressed that guidelines also have to “be translated into the ethos of the review committee.” “There will be now another step, once [the guidelines] are approved, to really think about and help to educate the cultures of these very different review committees.” Dr. Schilsky added that the Clinical and Translational Research Advisory Committee also has a subcommittee looking at ways to harmonize guidelines for CCSGs, the Cooperative Groups, and SPOREs to incentivize more collaboration.
In the meantime, Drs. Buckner and Bertagnolli emphasized, significant collaboration is already under way, as evidenced by numerous joint grants involving combinations of Cooperative Groups, Cancer Centers, and SPOREs. “I think there is a huge level of involvement and engagement,” Dr. Bertagnolli said, particularly given the low rate of project funding. “It requires intense engagement on the part of the scientific community in the groups to even put forward these proposals,” she observed.
Cancer Center and SPORE investigators need to be better educated about the kinds of studies that lend themselves to collaboration with Cooperative Groups, how such studies are developed, and the various mechanisms by which they might be funded, Dr. Buckner said. Dr. Bertagnolli noted that extramural investigators might become discouraged if they fail to understand that a study accepted by a Cancer Center may not be approved by a steering committee.
Extramural investigators also need to be educated about the existence and availability of specimens from Cooperative Group tissue banks, Dr. Schilsky pointed out. “All of the groups have experience operating those
tissue banks now for quite a long time and engaging the investigator community broadly,” he said, “but there still are large segments of the scientific community that either don’t know how to access those specimens or don’t believe that they are available for the broader scientific community.”
Another form of collaboration was raised by audience member Dr. Jeffrey Humphrey of Bristol-Myers Squibb, Inc.: the participation of newly consolidated Cooperative Groups in public-private partnerships. He observed that the pharmaceutical industry has created several high-level positions to support more functional partnerships with the Cooperative Groups in order to take advantage of their investigators’ superior expertise in disease management. “There is an increasing understanding in pharma (the pharmaceutical industry), particularly under its own financial pressures, that there has to be a selective integration with people who do things truly well, and [since] the true disease expertise resides in many of these Cooperative Groups … there is a need for public-private partnerships,” he said. Further discussion of such partnerships occurred in the Panel III discussion on interactions between industry, the FDA, and the publicly funded cancer clinical trials system (see “Partnership Between Industry and the Cooperative Groups”).
Reducing NCI Oversight of the Cooperative Groups
Noting that the recommendations in the IOM consensus report addressed the theme of shifting the role of NCI from oversight of the Cooperative Groups to facilitation of their work, Dr. Schilsky raised this issue for discussion. Dr. John Crowley of the Southwest Oncology Group (SWOG) observed that while cooperative agreements once resembled grants, many are now more like contracts. He urged a return to agreements that are more investigator-initiated, rather than being controlled by NCI.
Dr. Doroshow said that defining the role of NCI in the clinical trials system is a very important issue. He pointed out that original program “that went into operation in 1956 was a system in which all the trials and all the review were, for many, many years—decades, done exclusively by the NCI.” “That’s not the way a system should work. We should utilize the best available evidence that is discussed and evaluated by the experts,” he said, but he added that the Cooperative Groups have come a long way since 2004, when NCI conducted every review, entirely without extramural oversight. NCI has revised the prioritization process for large Phase II and Phase III treatment trials by creating steering committees in specific diseases
and across modalities. “The NCI clearly has a voice in these committees, but by no means does it have the dominant voice … roughly 3 or 4 votes out of 20 or 25 in any of these committees,” he said. Currently, NCI is focused on enlisting the help of “investigator experts” in prioritizing types of studies to be done, he said. However, he added that the institute is “just in the beginning phase” of establishing a much-needed extramural group that would represent a spectrum of constituencies in discussions of national strategic priorities across diseases.
Dr. Mendelsohn stated that disease site-oriented scientific steering committees should be charged with reducing redundancy among Cooperative Group studies and with improving both the quality and the completion rate of clinical trials. Also, in times of restricted funding for trials, scientific steering committees should be well equipped to prioritize the most cost-effective trials, he said.
“Just to be clear, the scientific steering committees have been going on for some time already, even before our report,” Dr. Schlisky noted. However, it’s important to assess whether and how such committees may encumber the approval process for clinical trials, particularly in light of the possibility that an “overarching oversight committee” might be added to the chain of approval, he added. Dr. Doroshow responded that the purpose of such an oversight committee would not be to add another layer of review to the approval process, but instead “to take a look several years down the line, to say where there are scientific opportunities and provide input to NCI about where the priorities ought to be.”
“I certainly applaud the concept of the NCI steering committees,” Dr. Curran stated, “but what I don’t want to see is a trend for the scientific core and the development process of new and exciting trials to shift [away] from the Group committees, where there is true expertise … [as well as] information and content and trials and translational research to interrogate as the beginning of the hypothesis generation.” The scientific development process should reside in the Group committees, in conjunction with Cancer Centers, SPOREs, and other colleagues, he insisted; steering committees should review, rather than generate, trials.
Since Cooperative Groups, SPOREs, and Cancer Centers compete for the same pot of money, steering committees should be established to review the entire cancer research portfolio, not just the Cooperative Groups, Dr. Comis asserted. “Whenever CTEP or DCTD [the Division of Cancer Treatment and Diagnosis] spends a dollar on clinical research … you ought to have a steering committee review everything and make sure every dollar is spent right,” he said.
Reflecting on the process of transformation of the cancer clinical trials system in light of the IOM consensus report recommendations, Dr. Peter Adamson of the Children’s Hospital of Philadelphia, a member of COG, noted that it remains to be determined which among the many changes underway will produce significant improvements. “Right now my overarching concern is, How are we going to attract the best talent?” he said, adding that a more cumbersome and complex clinical trials system will surely deter the participation of the best scientists.
“Ideas will fail late in this system, [which is] understandable, because it’s a matrix organization that is very difficult to manage,” Dr. Adamson explained. Such a system is geared to lose talent, because investigators become frustrated after committing two or three years of their lives to an idea, only to have it “blow up three years later,” he observed. Therefore, he said, “if we really don’t know what’s going to work, let’s incentivize innovation and lead to flexibility. I don’t think any of us, including COG, have the perfect model.”
Mr. Michael Katz, a Cooperative Group advocate, urged consideration of the possible advantages of some redundancy in the cancer clinical trials system. “We tend to paint with a very broad brush and we say things like ‘redundancy is bad,’” he observed. “When we are manufacturing Toyotas and we are buying PCs, scale is a black-and-white thing; it’s a good-and-evil thing. But when we are doing things like research, sometimes we explicitly have competing efforts,” he said. Steering committees are often plagued by competing conflicts of interest among their members, he added, so “it is very possible that we will now set the system to fail [too] early instead of failing too late.”
“I think we all have a great concern that research by committee tends toward the safe and not the brave and the innovative,” Dr. Bertagnolli replied. “We cannot forget that our work will greatly suffer if we stifle innovation.”
Because the IOM consensus report included recommendations directed toward health care insurers and others who set health care payment policies, the second session of the workshop focused on the relationship between clinical health care professionals and the payors who cover all or part of the costs of patient care within cancer clinical trials. It included presentations by representatives of two large insurance companies, the HHS Center for
Medicare and Medicaid Services (CMS), a major academic research center, and ASCO. The presenters then joined a panel discussion moderated by Dr. Lee Newcomer of United HealthCare.
Dr. James Cross of Aetna, Dr. Sharon Levine and Dr. Louis Fehrenbacher of Kaiser Permanente, and Dr. Louis Jacques of CMS outlined their employers’ payment policies for patients participating in clinical trials.
Every insurer handles its dealings with clinical trial providers differently, Dr. Cross observed, noting that his remarks would reflect Aetna’s perspective. Most insurance contracts distinguish between goods and services that are medically necessary, which are covered, and those considered experimental or investigational, which are excluded from coverage, he explained. Applying that distinction can be particularly challenging in the context of clinical trials, and he noted that some payors—but not most major insurers—would simply refuse to cover any treatment provided as part of a clinical trial.
First, he noted that Aetna covers off-label cancer treatments that have been shown to be efficacious through peer-reviewed literature and/or have a favorable evaluation from the National Cooperative Cancer Network (NCCN)18 or in other nationally recognized guidelines, Dr. Cross reported. These clinical practice guidelines recommend appropriate treatments based on the level of scientific evidence and consensus supporting their efficacy for particular cancers (see Box 3), and they include the off-label use of drugs in cancer treatment. Aetna covers treatments accorded an NCCN category of evidence of 2B or higher, he reported.
Dr. Cross stated that Aetna also covers routine medical care for patients participating in clinical trials, as well as care for any complications that might arise. He said that traditionally, the company always excluded things
_______________
18 The National Comprehensive Cancer Network®, a not-for-profit alliance of 21 of the world’s leading Cancer Centers, develops information for stakeholders in the health care delivery system. NCCN serves as an arbiter of cancer care by creating clinical practice guidelines appropriate for use by patients, clinicians, and other health care decision makers. Source: http://www.nccn.org/about/default.asp.
BOX 3
NCCN Categories of Evidence and Consensus
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
SOURCE: See http://www.nccn.org/professionals/physician_gls/categories_of_consensus.asp.
that were considered experimental, even for cancer care, but in the early 2000s he worked to change the benefit language so that if a patient has cancer or another life-threatening illness, the experimental treatment will be covered, but only within a clinical trial. He added that most cancer clinical trials involve combinations of multiple drugs and dosages, and Aetna pays for any and all such treatments within the guidelines of its clinical policy bulletins or within its coverage guidelines. This policy encourages patients to enter clinical trials, typically Phase III trials, for treatments otherwise considered to be experimental. “Every once in a while, if it’s a rare situation or a rare disease, we might move into even approving a Phase II trial,” he added.
“I think we are in sync with what the Cooperative Groups are trying to do, which is to try to get patients into Phase III trials, get them into multicenter trials, and get them to the right place in their treatment regimen,” Dr. Cross observed. When Aetna challenges payment for cancer treatment, it typically involves a patient who has received previous treatment and therefore does not qualify for a clinical trial or a patient who has enrolled in a single-institution trial, rather than a multicenter trial, he noted.
Because patients covered by most health care insurance are paying an increasing share of the cost of their drugs, it is important that physicians discuss these costs as they help patients to decide whether or not to join a
clinical trial, Dr. Cross said. These conversations should also address decisions involving palliative or compassionate care at the end of life, he added.
To make better coverage decisions, Aetna needs more and better evidence, Dr. Cross asserted. Therefore, he said, “it’s important for payors to be there for the medical community, to support research, and to do it in the best way that we can afford to do.”
As the largest private integrated delivery system in the United States, Kaiser Permanente acts as both a provider of clinical trials within its hospitals and clinics and as a payor for clinical trials conducted in other facilities, according to Dr. Levine. Kaiser Permanente is well positioned to accrue patients for clinical trials from among its 8.8 million members, through its regional infrastructure for trial enrollment, she said.
About 80 percent of the clinical trials Kaiser Permanente performs are Phase III, and the remainder are Phase II, Dr. Levine reported. She estimated that Kaiser’s patient population largely reflects the diversity of the United States as a whole. “One of the strengths of our clinical oncology trials program is that we have an extremely adhesive, loyal population,” she observed. In 2010, Kaiser Permanente was the largest source of patients for SWOG clinical trials, she reported; about 4,000 members currently participate in oncology trials.
Kaiser Permanente’s coverage policy for clinical trials is variable, because state-legislated mandates set the floor for such coverage, Dr. Levine stated. The determination of whether to refer a patient to a trial, and the extent to which a patient’s care will be covered in a trial outside of Kaiser Permanente, is left to the discretion of the treating oncologist, Dr. Levine said. The company covers routine patient care and will refer a patient to an out-of-plan oncology clinical trial if the following conditions are met:
• The treating physician feels that the trial has reasonable potential to benefit the patient compared with the standard treatment offered;
• The trial is of good quality and is conducted by qualified investigators;
• The primary goal of the trial is not to determine toxicity or dosing; and
• No comparable trial is available through Kaiser Permanente.
Medicare and Medicaid Support of Clinical Trials
In general, Medicare and Medicaid pay for routine services that are furnished in clinical trials—that is, those that the patient would have gotten otherwise, according to Dr. Jacques, of CMS. However, he added, “we probably pay for a lot more investigational items in clinical trials than we are aware of, because our current policy doesn’t actually force people to indicate that something has been furnished in the clinical trial.”
This point was later taken up in the panel discussion by Dr. Jay Bearden, of Upstate Carolina CCOP and Spartanburg NCI Community Cancer Program. He noted that at one time, modifier codes were used to identify all admitted patients in clinical trials. “I think we are still implementing that via regulatory and compliance standards,” he added, as well as comparisons of cost and effect for treatments. Dr. Jacques agreed that modifier codes exist to identify treatments provided through clinical trials but observed that some modifiers have not been applied consistently over time, particularly in outpatient settings.
CMS covers devices or tests with category B investigational device exemptions,19 based on FDA regulation, Dr. Jaques stated. To do so currently requires every local contractor to review conditional approval letters sent out by the FDA, he said, so CMS is attempting to streamline this review process. CMS has also, on a few occasions, provided coverage with evidence development (CED)20 for a Phase I trial, he added.
Demonstrating Utility to Payors
In addition to supporting the costs of conducting cancer clinical trials, payors ultimately must compensate companies that develop cancer treatments, Dr. Jacques observed. “If you want pharma to be enthusiastic about
_______________
19 An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data. An IDE Category B device is a nonexperimental or investigational device for which the incremental risk is the primary risk in question (i.e., underlying questions of safety and effectiveness of that device type have been resolved) or it is known that the device type can be safe and effective because, for example, other manufacturers have obtained FDA approval or clearance for that type. See Medicare Benefit Policy Manual, Chapter 14: Medical Devices, https://www.cms.gov/manuals/Downloads/bp102c14.pdf (accessed May 12, 2011).
20 On July 12, 2006, CMS released a guidance document titled National Coverage Determinations with Data Collection as a Condition of Coverage: Coverage with Evidence Development (CED); see https://www.cms.gov/CoverageGenInfo/03_CED.asp (accessed May 12, 2011).
helping you do trials, one of the things that they may ask is, ‘After we’re done with all this, what’s the likelihood that the payors are actually going to give us some return on this investment?’”
That depends on the treatment’s usefulness in the clinic, Dr. Jacques asserted. “If you want payors to be enthusiastic at the end, we will be much more comfortable if you have demonstrated clinical utility,” he said. Progression-free survival is not a strong criterion for clinical utility, he added; rather, to be successful, a treatment must improve quality of life or overall survival.
Dr. Jacques also expressed hope that biomarkers would eventually provide evidence of clinical utility. “We suspect, in aggregate, that they are probably going to turn out to be good for something,” he speculated; however, he cautioned, we must keep in mind that receptors can be promiscuous and that “the fact that you have found one pathway means that you have found one pathway.”
During the subsequent panel discussion, Dr. Normal Wolmark, of Allegheny General Hospital and NSABP, noted the IOM consensus report’s recommendation to develop a streamlined, integrated clinical trials process that is able to utilize molecular screening and markers, which Dr. Jacques identified as “adolescent technologies.” That perception of the current limited value of biomarkers will affect whether the report’s recommendations can be implemented, Dr. Wolmark asserted.
Regarding the strategic risks of tying drug development to specific biomarkers, Dr. Jacques noted that while this may permit smaller trials with greater effect size, it may also potentially and prematurely limit the eligible population. Biomarkers’ “adolescence” makes them ripe for study, he said, and he advocated integrating them into future cancer clinical trials.
“There is enough there to say that we think there is some promise in this kid,” Dr. Jacques observed. “To the extent that unfettered by any sort of either regulatory oversight or payment oversight, there is always the tendency to maybe claim a little bit more maturity than you have earned,” he continued. “Those of you who have survived child raising, I’m sure, remember those episodes fondly, where you have to say, ‘I’m not saying you can never do it. I’m saying you can’t do it yet.’”
Dr. Jacques expressed hope that in the future, biomarkers would help clinicians target the exposure to dangerous treatments to those patients who have the greatest chance of benefiting from them—a goal that presents an opportunity for collaboration between FDA and CMS, he observed. “The heretofore absence of FDA review on many of these tests certainly causes
us [at CMS] a little bit of anxiety, because we don’t even have basic analytic validity data, other than what might be furnished by the sponsor, who may, obviously, have a bit of a conflict of interest in what we are seeing,” he explained.
The discussion of biomarkers’ potential as cancer diagnostics continued during Panel III (see “Diagnostic Industry Perspective”).
Analysis of Cancer Clinical Trials Coverage
Dr. Charles Rudin, associate director for clinical research at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, presented a formal analysis of insurance clearance for clinical trials at this major cancer research center (Klamerus et al., 2010). First, however, he provided a description of the Cancer Center’s patient demographic, which has also been studied in detail (Kanarek et al., 2010; Su et al., 2010). Hopkins’ large catchment area includes six states and the District of Columbia. Less than one-fifth of patients reside in the city of Baltimore, where Johns Hopkins is located; these patients are largely low-income African Americans, while the majority of the center’s patients are high-income whites from outside the city (Su et al., 2010).
Patients in clinical trials between 2005 and 2007 at the Kimmel Cancer Center included relatively large numbers of older patients (Kanarek et al., 2010), which Dr. Rudin attributed at least in part to coverage provided by Medicare for clinical trial participation. “Women are a little bit more likely to go on therapeutic clinical trials,” he reported. “Whites are more likely than African Americans to go onto a clinical trial at Johns Hopkins, and relatively wealthy people are more likely to go on as well.”
Johns Hopkins’ Access Service Office informs the insurance provider of each patient considered for a therapeutic clinical trial exactly what expenses it will have to cover, which are limited to standard-of-care costs, Dr. Rudin stated. By analyzing data from the Access Service Office, Kimmel Cancer Center fellow Justin Klamerus and coworkers (2010) provided a “snapshot” of insurance coverage offered to clinical trial candidates between 2005 and 2007. During that period, 4,600 patients were referred to a clinical trial, Dr. Rudin said; of those, 490 were denied participation in the clinical trial for reasons related to insurance.
Patients who were accepted into clinical trials tended to be older than those who were eligible for the clinical trial but denied access because of insurance reasons; there were no differences among these populations on
the basis of race, gender, or the type of clinical trial to which the patient was admitted (Kanarek et al., 2010). This surprised the researchers, Dr. Rudin said, because they expected a higher rejection rate for patients considered for Phase I trials.
The researchers were also surprised to find differences in coverage decisions based on the patient’s state of residence, according to Dr. Rudin (Kanarek et al., 2010). Most of these patients came from Maryland, Pennsylvania, and Virginia, he said, but Pennsylvania residents were significantly more likely to be denied access compared with residents of Maryland or Virginia. The apparent reason for this discrepancy is that Pennsylvania, unlike the other two states, does not have a state law that requires insurance carriers to cover standard-of-care costs associated with clinical trials, he reported. Figure 2 depicts such differences nationwide.
The process of approval or denial was found to take an average of five to six working days in either case, Dr. Rudin noted (Kanarek et al., 2010). Rates of approval and time taken to make coverage decisions varied widely among insurers, as shown in Table 3. Time to decision is important, he said, because “a patient who is waiting two weeks to figure out whether [he or she] might go on a clinical trial, as often as not, is going to decide to get some other therapy.”
Dr. Newcomer commented on two aspects of the results of this study. First, he said, he was surprised by the finding that half of federal employees were denied coverage of clinical trial participation. In the past, the Federal Employees Health Benefit Program has not required participating insurers to cover the costs of routine care incurred during participation in a clinical trial (ACS CAN, 2009). However, provisions in the Affordable Care Act of 2010 require (in 2014) all commercial health insurance plans offering group or individual coverage, health plans offered through the Federal Employee Health Benefits Program, employer-sponsored plans that self-insure and operate under the Employee Retirement Income Security Act (ERISA), and state self-insured plans to pay for the routine patient care costs associated with participation in high-quality clinical trials (Phases I to IV) for cancer or another life-threatening disease or condition.21
Second, Dr. Newcomer noted that some clients of large insurance companies, rather than the insurers themselves, assume risk and therefore make coverage decisions. In the case of these “administrative services only” (ASO) accounts, in which the insurer simply administers the employer’s
_______________
21 See http://www.acscan.org/pdf/healthcare/implementation/factsheets/hcr-clinicaltrials.pdf (accessed May 12, 2011).
FIGURE 2 States with laws mandating clinical trials coverage. A total of 29 states (solid colors) plus the District of Columbia have state laws mandating insurance coverage for standard care costs associated with cancer clinical trial participation; black, enacted prior to 2003; light grey, enacted from 2003 to 2007; dark grey, enacted from 2008 to 2010. Diagonal lines, states with comprehensive coverage agreements, for example, coverage of a limited patient population (children or state employees), or voluntary agreements by insurers over clinical trials coverage. An Illinois state law mandating coverage previously in force expired in 2003. New York does not have a law mandating coverage, but has established an expedited independent appeals process for denial of clinical trials coverage.
SOURCE: Klamerus et al., 2010. Adapted and reprinted by permission from the American Association for Cancer Research: Klamerus et al., The Impact of Insurance on Access to Cancer Clinical Trials at a Comprehensive Cancer Center, CCR Focus, 2010, 16(24), page 5999.
plan, denial rates reflect employer decisions. Therefore, he said, “when you see a denial rate … of 6 percent [as is the case for United HealthCare, whose policy for fully insured clients calls for clinical trials coverage for all four phases of trials], that is almost certainly the ASO clients within that environment.”
During the subsequent panel discussion, the topic of insurance coverage of Phase I clinical trials received significant attention. Building on
| Insurance Providera | Cases Reviewed (N) |
Final Approval Rate |
Business Days to Complete Review | |
| Average | SD | |||
| Medicare only | 137 | 100% | 2.8 | 2.6 |
| Medicare and secondary | 769 | 100% | 5.5 | 5.8 |
| Medicare and supplement | 444 | 100% | 3.3 | 2.5 |
| Johns Hopkins EHP | 73 | 100% | 6.1 | 4.0 |
| AETNA | 278 | 98% | 6.5 | 7.7 |
| United Healthcare | 193 | 94% | 6.1 | 5.4 |
| BC/BS DC | 273 | 93% | 6.7 | 4.7 |
| BC/BS Maryland | 287 | 88% | 4.3 | 3.7 |
| Cigna | 110 | 86% | 7.2 | 7.1 |
| Tricare | 120 | 83% | 7.0 | 4.5 |
| UHC Mamsi | 239 | 82% | 7.5 | 5.0 |
| Kaiser | 74 | 81% | 11.2 | 7.2 |
| Out-of-State BC/BS (not DC) | 529 | 76% | 7.6 | 7.3 |
| BC/BS NASCO | 79 | 64% | 4.2 | 2.4 |
| Other PPO | 259 | 55% | 7.8 | 7.1 |
| BC/BS Federal | 250 | 46% | 3.3 | 3.0 |
NOTE: BC/BS, BlueCross/BlueShield; DC, District of Columbia; EHP, Employer Health Programs; NASCO, National Account Service Company; PPO, preferred provider organization; UHC, United Healthcare.
a Only companies reviewing more than 70 cases are listed; approval rates for some carriers have changed over time.
SOURCE: Klamerus et al., 2010. Adapted and reprinted by permission from the American Association for Cancer Research: Klamerus et al., The Impact of Insurance on Access to Cancer Clinical Trials at a Comprehensive Cancer Center, CCR Focus, 2010, 16(24), page 6000.
presentations by Drs. Cross, Levine, Jacques, and Rudin, workshop participants explored what Dr. Newcomer called the “mythology” of Phase I trials, the reality of their diversity, and the decision processes used by payors to determine whether to cover patient expenses associated with such trials.
Dr. Newcomer asked the panel whether—and why or why not—Phase I trials should be approved and covered by an insurance plan. Dr. Cross observed that Phase I or II trials are typically funded by the sponsor of the drug or treatment and that Aetna would be likely to cover general medical care within any phase of such a trial. Dr. Fehrenbacher agreed, adding that many patients have the choice of entering either a Phase I or a
Phase III trial to treat their cancer; Kaiser Permanente tends to “focus more on the evidence-based, established trial,” he said.
Rather than the phase of the trial, the key distinction for coverage approval should be whether the specific expense to be covered is investigational or not, Dr. Rubin observed. As he noted in his presentation, at Johns Hopkins—and at most if not all other major academic Cancer Centers—insurance providers are asked only to cover standard-of-care costs. “In a Phase I clinical trial, almost by definition, the drug is not approved, and we are not charging for it and we are not charging research costs associated with studying that drug’s pharmacokinetics, pharmacodynamics, et cetera,” he explained. However, he added, “within a Phase III clinical trial, it may be that we are comparing two arms involving existing drugs that are actually potentially billable to insurance, in which case they might be asked to pay for those.”
Turning to the issue of treatment of complications, which many payors cover for patients enrolled in clinical trials, Dr. Newcomer noted that such conditions are most likely to arise in Phase I trials. Dr. Rudin replied that current Phase I research focuses on defining biologically effective doses of medications, rather than pushing doses to severe toxicity, as was more common in the past.
Dr. Schilsky agreed. “The Phase I trials of the more targeted therapies, particularly if they are being applied in biomarker-selected populations, which is again increasingly the case—many of those drugs oftentimes don’t have the types of life-threatening toxicities that we associate with cytotoxic chemotherapy,” he said. “It’s not to say that they are nontoxic, but they are, in many ways, less dangerous to patients.” In addition, he noted, the probability of observing an antitumor effect in Phase I trials is substantially greater than it was a decade ago; recent examples of this include the high response rates among biomarker-selected patients treated with a BRAF inhibitor for melanoma (Flaherty et al., 2010) or those treated with crizotinib for non-small cell lung cancer (Kwak et al., 2010). “We are beginning to see a blending of Phase I and Phase II endpoints and a higher probability of a more favorable risk-benefit ratio in contemporary Phase I trials,” he observed.
Moreover, Dr. Schilsky added, “there’s no physician I know who is engaged in Phase I trials who is not offering them to a patient with therapeutic intent,” he said. “I know of very few circumstances where a patient would be eligible for a Phase III trial, but instead goes on a Phase I trial,” he continued. “In almost all cases, the patient is no longer eligible for Phase III
trials…. Therefore, Phase I becomes the best alternative for them, short of some unproven, off-label use of existing therapies.”
“There’s a huge heterogeneity among Phase I trials,” Dr. Fehrenbacher observed. The major factor in determining approval for coverage for such a trial is its potential to benefit the patient, he said. “There are some patients who enter Phase I trials for which we are extremely excited about the agent and think that the patient will definitely benefit, [and] there are other patients who enter Phase I trials where the appropriate alternative and probably the best approach might be palliative care,” he explained.
Noting that patients in Phase I and Phase II trials are usually out of options, Dr. Cross stated that Aetna denies cancer care only on the basis of a corporate committee review, involving the company’s 12 senior national medical directors and three outside consultants. If Aetna approves coverage of a Phase I or a Phase II trial, “there needs to be some evidence that it’s likely to be effective and that it’s truly a promising therapy and it sort of makes intellectual sense, and it’s not just throwing one last thing at somebody,” he said. “If it’s a rare tumor or if it’s a tumor that has been so resistant and the drug has some case studies or something else to put us in the ballpark that it might be promising, then that’s how we try to make that decision.”
In disagreement with Dr. Cross’s initial premise, Dr. Rudin stated that a patient who is truly eligible for Phase I participation—one who has limited comorbidities, for example—often does have other options. If patients are “going to get an anticancer drug, they are either going to get it using an off-label drug, which, as often as not, we would charge to insurance companies, or they are going to participate in a clinical trial,” he said. “I think it’s less true that the Phase I population is … really at the end of life nowadays.”
“I would rather have them on a trial of some type than what we often find, which is no trial at all, just mirroring what treatment somebody else has for a trial,” Dr. Cross responded. “That’s the most common situation.”
Dr. Rudin agreed. “I think the ‘oncologic chef’ choosing drugs off the shelf is really not a great model [for cancer treatment],” he said. On the other hand, Dr. Cross noted, off-label cancer treatment is a reality in the United States. “I don’t think there will be studies, to the FDA’s standards—at the cost that it would take to do those studies—to get everything off-label on label.”
On this point, Dr. Ronald Go, of Gundersen Lutheran Health System, suggested that the rarity of off-label cancer treatments in Europe results in faster accruals for clinical trials there compared to the United States. Dr. Newcomer concurred, noting that off-label cancer treatment presents
an alternative to clinical trials both for biomarkers and for drugs. “It is one of the potential hurdles to enrolling the patient in a clinical trial,” he concluded.
Determining the Standard of Care
Dr. Go also noted that it is sometimes difficult to distinguish between investigational tests and those that represent the standard of care. “When you go on Cooperative Group trials, there is a lot of lab work up front,” he observed, often including tests that would not be performed for other such patients. Dr. Rudin replied that a dedicated office at Johns Hopkins uses the NCCN guidelines to define standards of care for particular disease presentations and that any tests performed that do not meet those criteria would be billed to study budgets, not to insurers. Payors such as Aetna and CMS rely on such information to distinguish between investigational and standard treatments and tests, Dr. Cross said, because they do not perform such analyses themselves.
“There are published data on the costs of routine clinical care within the context of a clinical trial or standard of care,” Dr. Doroshow noted; however, he asked, To what extent do such data influence the decision as to whether to provide coverage for a patient enrolled in a clinical trial? Such decisions largely hinge on the drug or biologic being used in the trial, which tends to be the most expensive aspect of treatment, according to Dr. Cross; diagnostic testing is sometimes considered as well, if it is costly.
Based on his experience in community oncology, Dr. Go suggested to the Cooperative Group chairs that they examine all test criteria currently in place for cancer clinical trials. He illustrated this point with some examples, including that of a patient enrolled in a Cooperative Group trial with diffuse large-cell lymphoma. Now five years in remission, and thus by definition cured, she is nonetheless required by the trial protocol to undergo a yearly positron emission tomography-computed tomography (PET-CT) scan until year 10.
Dr. Karen Hagerty of ASCO noted that discussing the option of entering a clinical trial with patients, and then enrolling and following patients in clinical trials, requires a significant commitment of time on the part of
physicians—time that largely goes uncompensated and therefore represents a barrier to clinical trials enrollment. One option for overcoming this barrier is to create new CPT codes recognizing these activities, thus enabling physicians to bill for time spent offering, enrolling, and following patients in clinical trials.
The Healthcare Common Procedure Coding System (HCPCS), which covers the procedures, services, and items provided by health care professionals, comprises two levels of codes, Dr. Hagerty explained. Level I consists of the American Medical Association CPT, or common procedural terminology, codes; these cover services and procedures furnished by physicians and other health care professionals, but do not include codes for medical items and services that are billed by other suppliers. Level II codes, which are developed and maintained by CMS, comprise a comprehensive and standardized system that is used primarily to identify products, supplies, and services not included in the CPT.
Dr. Hagerty outlined the process by which CPT codes are changed. Such changes are proposed (by medical specialty societies, individual physicians, hospitals, third-party payors, and other interested parties) to the 17-member CPT editorial panel,22 assembled by the American Medical Association (AMA). Coding change requests are reviewed by AMA staff and referred to appropriate medical specialty societies for comment; if they advise action, and the CPT panel concurs, the change is further referred to the AMA’s Relative Value Scale Update Committee (RUC).23 The RUC determines the value of the new code (relative to existing codes) and, thereby, how much a provider will be paid for performing the coded service. If the full RUC votes to approve the valuation for a code, the recommendation is sent to CMS, which may or may not accept it. This process, if uncomplicated, can take between 18 months and 2 years, Dr. Hagerty estimated.
At the time of the workshop, this process had yet to be initiated, Dr. Hagerty said; however, discussions were under way among stakeholders
_______________
22 Of the 17 editorial panel members, 11 are nominated by the national medical specialty societies, and one physician represents each of the following organizations: Blue Cross; the Hospital Association; America’s Health Insurance Plans (AHIP); and CMS. Two seats are reserved for members of the Health Care Professionals Advisory Committee. Source: Workshop presentation by Dr. Karen Hagerty, March 21, 2011.
23 The RUC consists of 29 members, of which 23 are appointed by National Medical Specialty Societies and approved by the AMA Board of Trustees. The AMA Board of Trustees selects the RUC chair and the AMA representative to the RUC. Source: workshop presentation by Dr. Karen Hagerty, March 21, 2011.
regarding proposals for a specific code that would identify time spent enrolling patients in clinical trials. She noted that CMS already has code modifiers for reporting services rendered to patients participating in clinical trials. She also predicted that the new code, as currently envisioned, would be used not only by oncologists, but also by other medical specialists who enroll patients in clinical trials.
During the panel discussion, Dr. Steven Grubbs, of the Helen F. Graham Cancer Center CCOP, asked the payor representatives on the panel whether their organizations were likely to approve payment for a new clinical trial enrollment code. Dr. Jacques responded that although coding doesn’t mandate coverage, such an enrollment-specific code would provide greater certainty as to what the insurer is paying for in clinical trials. Therefore, he concluded, “a code is certainly a reasonable strategy.”
Dr. Cross agreed, but emphasized that this strategy is not without risks. “I think more granularity with coding is helpful, no matter what the actual decision is about reimbursement,” he said. However, he added, if physicians are willing to accept their rate of pay under the existing code structure, it may behoove them not to request a new one, because it could be declined for reimbursement.
Dr. Grubbs reminded workshop participants that the IOM consensus report recommended not only the establishment of such a code, but also enhanced payment by insurers. Dr. Newcomer then observed that enrollment-associated payments “will be minuscule compared to the cost of caring for a cancer patient” and deemed them money well spent, since they encourage physicians to discuss trials in detail with their patients. “I can’t imagine us not paying for it,” he concluded.
The workshop’s third session examined how private and public sectors and government agencies currently interact within the cancer clinical trials system and explored how these relationships could influence, and be influenced by, implementation of recommendations in the IOM consensus report. The session opened with brief remarks from speakers representing pharmaceutical, diagnostics, and informatics companies and the FDA; they were then joined by additional representatives of government agencies in a panel discussion moderated by Dr. John Hohneker, of Novartis.
Pharmaceutical Industry Perspective
Dr. Ira Steinberg, of Sanofi-Aventis, stated that his remarks on the Cooperative Groups would reflect not only his experiences in industry, but also as a member of the CEO Roundtable on Cancer.24 Industry chooses to work with the Cooperative Groups for several well-known reasons, he said; these include access to large numbers of patients, speed of accrual, and working with world-class institutions and physicians—all of which speed the delivery of innovative medicines and treatments to patients.
However, the IOM consensus report identified some challenges in this relationship and proposed ways to address them, Dr. Steinberg noted. These issues include the following:
• Lengthy trial initiation periods;
• Variability in data format and readiness for submission to regulatory agencies;
• Disparate contracting language in multi-institutional agreements; and
• Intellectual property concerns (although he said this was less a problem with Cooperative Groups than with individual institutions).
Plans for consolidating the Cooperative Groups, as previously described by Dr. Doroshow, should address industry’s primary objective of more efficient clinical trials, according to Dr. Steinberg. Proposed efforts by the Groups to develop and implement standardized reporting methods and processes should improve data quality and readiness for submission to the FDA, he added.
The CEO Roundtable on Cancer also hopes to increase clinical trial efficiency through dissemination of the Standard Terms of Agreement for Research Trials (START)25 clauses, which it developed in partnership with NCI, academic cancer systems, Cancer Centers, and the law firm of Hogan Lovells. This series of standardized clauses for both company-sponsored and investigator-sponsored trials is designed to simplify and streamline the contracting process, he explained. The clauses pertain to six key elements of contracts: IP, study data, indemnifications, subject injury, confidentiality, and publication rights. “What this allows us to do is more quickly get to
_______________
24 See http://www.ceoroundtableoncancer.org/default.aspx.
25 See http://transformingtrials.cancer.gov/initiatives/ctwg/standardization/highlights-start.
that point in the contracting process where everyone agrees, which is very often the slowest part of the process,” he said. The START clauses are freely available at the websites of the Life Sciences Consortium26 and the NCI.27
Diagnostic Industry Perspective
Dr. Steven Shak, of Genomic Health, Incorporated, reminded workshop participants that many important advancements in cancer drug development and patient care have been made possible by diagnostics developed as a result of Cooperative Group studies. For example, the potential value of biomarkers to clinical practice was recognized several decades ago, with the finding by Cooperative Group researchers that targeting the estrogen receptor would provide value to a subgroup of breast cancer patients; similarly, the development of tests to identify breast cancer patients who are likely to respond to Herceptin stemmed from trials conducted by NSABP and NCCTG. More recently, he added, as Genomic Health developed its Oncotype DX 21-gene assay that is intended to guide treatment selection for patients with early breast cancer, the company relied on specimens obtained and preserved in previous trials conducted by three Cooperative Groups.
Biomarkers in clinical practice must be “fit for purpose,” defined as proven to offer clinical utility to a specific patient population through consistent results across multiple, high-quality studies of varying design and purpose, according to Dr. Shak. A successful biomarker-based test also offers something of value to patients that is unavailable from existing tests, he said.
Dr. Shak emphasized the importance of standardization for biomarkers and assays. Some earlier studies, such as those aimed at gaining insight into the biological function of a given biomarker, can be performed with less standardized assays, he noted; however, when validating a biomarker and its use in clinical assays, studies must be “standardized and controlled and understood,” he insisted.
Developing a biomarker as a diagnostic requires significant collaboration among several stakeholders, Dr. Shak observed, as well as technical and management expertise—and sufficient funding, which he noted has often been a challenge to obtain. He noted, for example, that while 21 first-generation trials have been conducted to evaluate the potential benefit of
_______________
26 See http://ceo-lsc.org.
27 See http://restructuringtrials.cancer.gov/initiatives/standardization/highlights/start/faqs.
taxane treatment for breast cancer, a study of banked tumor specimens to identify patients who would be most likely to benefit from either taxane or anthracycline has yet to be funded. With regard to the benefits of taxane therapy, he said, “we probably got the answer with fewer than 21 trials.” “Did it really require that many? Did we really need to look at over 38,000 patients?” he asked.
Tumor tissue collection should be funded for every major study, Dr. Shak insisted; he noted that while some Cooperative Groups have followed this practice for years, others are merely “moving in that direction.” To illustrate the importance of tumor tissue banking, he recalled a recent, potentially practice-changing study by U.S. Oncology that compared the benefits of anthracycline- and taxane-containing regimens, in which researchers did not collect tumor blocks that could now be used to search for biomarkers associated with treatment benefit (Gianni, 2009; Jones et al., 2009).
To avoid inefficiencies such as excessive studies addressing the same question, Dr. Shak urged the clinical trials community to develop a portfolio strategy that “would allow us to look at those areas that are most productive and make sure that we are putting our resources in that direction.” This requires not only thinking about the initial questions to be addressed in a research program, but considering the full spectrum of patient needs with regard to a given treatment, he said. Those needs should then be translated into strategies—developed through dialogue among stakeholders including industry, academia, the FDA, and patient advocates—that define treatment effectiveness and adverse events (and specify how to address such events) and identify processes for obtaining data on patient selection, health economics, comparative effectiveness, and clinical utility.
Funding is crucial to the development of biomarker-based diagnostics to guide clinical practice, Dr. Shak observed. A strategy for funding the management and implementation of such studies should include anticipating decisions that will have to be made and identifying the kinds of evidence that will be needed to make them, he said.
Noting that the IOM consensus report highlighted prospects for incorporating translational research on biomarkers into cancer clinical trials, Dr. Hohneker reported that most companies are formulating policies that support comprehensive tumor tissue banking, particularly among small populations defined by disease target or tumor type.
Industry is playing “catch-up” in the use of both biomarkers and tissue banking, Dr. Steinberg observed. “In some cases we haven’t listened
enough, when we work with Cooperative Groups or when we work with individual investigators, about what we need to do to be prospective and to be forward-thinking” with regard to both biomarkers and tissue banking, he said. “Biomarkers are a lot further along than has been indicated [in this discussion],” he asserted, “but I think we have a long way to go.”
Dr. Richard Pazdur, of FDA’s Center for Drug Evaluation and Research (CDER), noted that the increased commitment of the pharmaceutical industry’s resources to the field of oncology over the past two decades, along with the international expansion of cancer clinical trials, raises several key needs. First, he said, it is important to recognize that FDA is no longer the sole regulatory authority for cancer clinical trials. “Trials have to answer to multiple regulatory agencies, including the EMA (European Medicines Agency), Swiss Medica, Health Canada, [and] Health Australia,” he said. “When a trial is being done, whether it’s being done by the Cooperative Groups … [or] for industry, it has to serve multiple masters.”
Another important need is to identify specific types of trials that should be conducted by Cooperative Groups, as opposed to industry—and to understand that this landscape is changeable. “Several years ago, it was said that Cooperative Groups should do adjuvant studies, because industry simply can’t do [them],” Dr. Pazdur recalled. “Well, that’s wrong now.” Industry has also conducted recent trials involving very small groups of patients, orphan diseases, and unique populations—formerly the domain of the Cooperative Groups—that have resulted in FDA approvals, he noted. This situation begs the question of what types of trials are sufficiently important to advancing the field of oncology that Cooperative Groups should spend taxpayer money to undertake them. What compelling questions are not being answered by commercial sponsors of clinical trials?
These issues were examined in a white paper by PhRMA (Pharmaceutical Research and Manufacturers of America) that addressed cooperation and interaction between FDA and other regulatory bodies, the Cooperative Groups, NCI, and commercial sponsors, Dr. Pazdur recalled. That paper was never released, but he said it offered the following four guiding principles on Cooperative Group studies:
1. Accountability for the delivery of quality data to the FDA belongs exclusively to the sponsor. “It’s not just plopping in a clinical trial
and saying, ‘approve it,’” he said. “There are many more issues that need to be addressed here, and the complexity of these relationships for a given drug, even if it is a supplemental indication and not a new molecular agent, might be quite extensive and may not be known to the Cooperative Groups.”
2. Clear lines of communication regarding responsibilities and time lines must be established and maintained between the Cooperative Group and the study sponsor. “We really emphasize that there should be joint meetings between the FDA, CTEP, the Cooperative Group, and the commercial sponsor at every stage,” he said. “In addition, there should be explicit delineation of what types of regulatory documents will be handled by each partner,” such as CTEP, the Cooperative Group, or the company.
3. Whether the intent of the study is for FDA product registration should be defined a priori. This might seem obvious, he said, but often a company will decide that a trial performed by a Cooperative Group is going to be a registration trial after the trial is complete. Instead, he noted, it is far better to know the trial design in advance, what the requirements are for regulatory submission, and which partner is going to submit the documents.
4. Consideration should be given to delegating elements of the Cooperative Group trial conduct to the pharmaceutical sponsor of the trial. For example, he asked, should part of the monitoring of the trial be done by the sponsor if it is going to be submitted to a regulatory authority? “I think there deserves to be at least some discussion of some of the other roles that the pharmaceutical industry can play in this arena,” he said. “It really is a dialogue that has to continue between the sponsor and the pharmaceutical firm.”
Dr. Hohneker asked panelist Dr. Robert Becker, of FDA’s Center for Devices and Radiological Health,28 about the agency’s efforts to advance biomarkers beyond their status as “adolescent technology” (in Dr. Jacques’ words).
_______________
28 FDA’s Center for Devices and Radiological Health is responsible for regulating firms that manufacture, repackage, relabel, and/or import medical devices sold in the United States. In addition, CDRH regulates radiation-emitting electronic products (medical and nonmedical) such as lasers, X-ray systems, ultrasound equipment, microwave ovens, and color televisions. Source: http://www.fda.gov/AboutFDA/CentersOffices/CDRH/default.htm.
“Our efforts are [directed toward] being as clear as we can be, with respect to the developers of those technologies, as to how they can interact with us to put them on a path for proper development and for eventual clinical adoption,” Dr. Becker responded. This is carried out through direct interactions with sponsors of the biomarkers themselves, and also with developers of biomarker-based therapeutic products, through collaborations with [FDA] therapeutic centers including CBER (Center for Biologics Evaluation and Research) and CDER, he said.
CDRH also interacts with agencies that are stakeholders in the development of biomarkers as clinical tests, Dr. Becker continued. “From our perspective as regulators at the end of the device food chain, a biomarker is not the same thing as a clinical test,” he observed. “It’s not the same thing as an in vitro diagnostic that has been analytically, clinically validated and has demonstrated clinical utility.” Instead, biomarkers tend to be validated as companion diagnostics to drugs, which significantly limits their clinical utility.
Dr. Herbst observed that from the perspective of investigators and their institutions, biomarker regulation has become very difficult to understand. For example, he asked, “if you want to use a biomarker to put someone on a clinical trial, one thing that continues to come up is, What sort of laboratory does that test need to be done in?” Also, if patients have sequence data generated outside the clinical trial indicating biomarkers that could guide their treatment, can they be treated on that basis?
“My understanding is that … if the results from testing in the context of a trial will be carried out in a manner that affects patient treatment—for example, in a way [that] is being reported back to patients and explicitly used to modify their treatment—then that does constitute human testing that falls under the [Clinical Laboratory Improvement Amendments (CLIA)29] regulation as one that needs to be carried out in a CLIA-certified laboratory, commensurate with the level of complexity for the testing,” Dr. Becker stated.
For the purposes of cancer clinical trials, biomarkers are considered to be devices if they are used to manage patient care, according to Dr. Becker. This care “could be at the level of accruing to the trial … [or] at the level of deciding what kind of stratification is used in the course of the trial …,
_______________
29 The Clinical Laboratory Improvement Amendments of 1988, finalized in 1992, regulate laboratories, as compared with the Food, Drug, and Cosmetic Act, which regulates tests, Dr. Becker explained.
[or] it could be with respect to deciding which arms of the trial the patient will go into,” he said. In circumstances where those tests are being used in a manner that has not been FDA cleared or approved, they are “investigational-use devices” and are thereby subject to IDE regulations (see footnote 19, page 40). There are possibilities for mitigating or lightening the regulatory load on biomarkers used in cancer clinical trials, Dr. Becker noted, including the ability to handle IDE issues associated with a device in a clinical trial through submission of that information in the Investigational New Drug (IND) application. This is something that has not been done extensively, he said, but it can be explored.
Central IRB and Informed Consent
During the panel discussion, workshop participants explored additional ways to open trials faster and to speed patient accrual: simplifying institutional review and the process for obtaining informed consent. As discussed during Panel I (see “Overview of the NCI Response” in “NCI Perspective and Current Activities”), the expansion of CIRB use could potentially increase the speed and efficiency of Cooperative Group trials. Noting Dr. Doroshow’s previous announcement that HHS is likely to make a rule change allowing a single IRB for any multisite clinical trial, Dr. Hohneker asked panel member Dr. Jerry Menikoff, of the HHS Office for Human Research Protections (OHRP),30 to discuss the general usage of a CIRB, as well as its specific application to Cooperative Group trials.
OHRP has been encouraging the use of CIRBs, Dr. Menikoff replied. The agency now registers about 6,000 IRBs, both domestically and internationally, representing more than 10,000 institutions that have federal-wide assurances, he reported; however, he noted, there is little evidence to justify the money and man-hours spent in those dispersed efforts. “I think there are good reasons to think that by having fewer IRBs, we would be saving a lot of time on behalf of researchers, subjects, [and] administrators,” he observed. Perhaps more significantly, he added, the use of CIRBs might improve the ethics of approved studies, since the review of a study by multiple IRBs
_______________
30 OHRP provides leadership in the protection of the rights, welfare, and well-being of subjects involved in research conducted or supported by HHS. OHRP helps ensure this by providing clarification and guidance, developing educational programs and materials, maintaining regulatory oversight, and providing advice on ethical and regulatory issues in biomedical and social-behavioral research. Source: http://www.hhs.gov/ohrp/.
diffuses responsibility. Therefore, he said, “OHRP has officially endorsed the notion that we think there are benefits to moving from so many IRBs to fewer IRBs and, perhaps ideally, one IRB per study.”
In 2009, OHRP issued an advance notice of proposed rulemaking to determine whether the agency had clear authority to compel the use of CIRBs. Based on the response, it appears that the effect of tweaking the liability rules may be limited, because “a lot of institutions seem very wedded to having their own IRBs,” he said. However, he added, sponsors and funding agencies actually already have the authority to simplify the system a great deal by insisting on CIRB use as a condition of participation in a study. “That is totally consistent with our rules,” he said.
In response to this statement, Maria Gonzalez, of St. Joseph Hospital, in Orange, California, noted that hospitals instructed to use a CIRB by a sponsor might still decide to review studies, which could result in studies approved by a CIRB being subsequently declined by a hospital IRB.
“There’s nothing forcing these institutions that have their own IRBs,” Dr. Menikoff observed; however, hospitals presumably want to be part of clinical trials, and if so, they’ll have to decide if they want to accept CIRB decisions. Moreover, he added, hospitals might benefit from outsourcing IRBs. “A lot of institutions know they are not going to get many pharma studies unless they agree to get one of the bigger, private [for-profit] IRBs,” which are capable of reviewing a study within days of its proposal. A site that will accept a decision from such an IRB can move quickly to initiate studies, he concluded. Dr. Hohneker agreed and noted that some institutions use commercial IRBs for industry-sponsored studies and their own IRBs for investigator-initiated trials.
Because the proposed changes will not lock any institution into using a particular IRB, it is important to educate them as to the benefits of using CIRBs, Dr. Menikoff observed. “There is actually not a lot of evidence that we are getting huge benefits from having 50 or 100 IRBs reviewing each study,” he said.
Dr. Adamson disagreed. “If the majority of adult centers are not using a central IRB—in the Children’s Oncology Group, we have, I think, 70 percent—that means you have a systems problem,” he observed; in this case, it’s important to find out why an institution is not using a CIRB, as well as whether the institution is right or wrong to do so. His remarks elicited applause from the audience.
OHRP is also working to simplify the process of obtaining informed consent from patients participating in clinical trials—a need noted in the
IOM consensus report, Dr. Menikoff said. Many stakeholders would say that the part of the cancer clinical trials system most in need of improvement is consent, he observed. “Consent forms are long and complicated and could benefit from some simplification,” he explained. “The Secretary’s Advisory Committee on Human Research Protections (SACHRP)31 is actively looking into it and has a subcommittee looking into ways to improve consent forms,” he reported.
However, Dr. Menikoff continued, it remains to be determined whether the length and complication of consent forms is really the major barrier to patient accrual. When the Bioethics Department of NIH’s Clinical Center studied this question by randomizing patients to a standard consent form and a consent form that is approximately one-third the length of the original one, it found that this didn’t make a significant difference in patient accrual (Stunkel et al., 2010). This suggests that instead of simplifying the form, it might be better to highlight the most important information it contains and perhaps provide more detailed information on some topics, he concluded. “A lot of the people who are pushing simplification might suddenly not be as eager to rewrite consent forms when … the first thing it will say is … why you might think about not being in this study,” he observed—particularly since many patients have the option of receiving off-label treatments from their own physicians.
OHRP is “very much willing to partner with IOM and others in terms of improving consent,” Dr. Menikoff concluded. “It’s just a question of knowing what the problem really is. I think it might be too simple to say it’s just that the consent forms are complicated and use too much high-level verbiage.”
Partnerships Between Industry and the Cooperative Groups
“We heard an eloquent description of the changes [under way at NCI relevant to recommendations in the IOM consensus report] from
_______________
31 SACHRP is governed by the Federal Advisory Committee Act and provides expert advice and recommendations to the Secretary on issues and topics pertaining to the protection of human research subjects. The committee was created by Secretary Thompson in 2001 after dissolution of the prior National Human Research Protections Advisory Committee (NHRPAC). To date SACHRP has focused its attention on areas such as research involving children, prisoners, and individuals with impaired decision-making capacity; informed consent and the use of biospecimens; harmonization of human subjects regulations and guidance; the reduction of regulatory burden; the HIPAA Privacy Rule; community-engaged research; and accreditation. Source: http://www.hhs.gov/ohrp/sachrp/.
Dr. Doroshow in the first moments of the meeting,” Dr. Hohneker observed, referring to the Panel I presentation summarized in “Overview of the NCI Response” in “NCI Perspectives and Current Activities.” “Any thoughts about how you think that will improve partnership between industry and the Cooperative Groups?” he asked the panel.
There are multiple layers of complexity to working relationships between the Cooperative Groups and industry, Dr. Steinberg noted; anything that can be done to simplify those relationships—such as streamlining the opening of trials and collecting good-quality data that can be readily submitted for review—is a good thing, he added, and Dr. Hohneker agreed. “If we are doing a Phase II trial that would support a registration, [receiving] a nice robust-quality data package … [would mean] less work on our side to prepare that for submission, particularly on a key trial,” he said.
It’s clear that the Cooperative Groups must work with the pharmaceutical industry in order to gain access to the most interesting drugs in development, as well as to resources for conducting trials, Dr. Schilsky observed. The Groups, in turn, possess unique technical capabilities and access to patient populations. However, the necessity for NCI review stands as a significant barrier to mutually beneficial collaborations between industry and the Cooperative Groups, he said; the IOM consensus report addressed this concern with its recommendation to streamline and harmonize government oversight and regulation of cancer clinical trials. He therefore asked the panel’s industry representatives, “If that recommendation or something like it were to be implemented, would that actually enhance, do you think, the potential for collaboration between the pharmaceutical industry and the Cooperative Groups, at least in circumstances where the Group was the IND holder for the drug under study?”
If by implementing that recommendation, clinical trials could open more quickly without compromising patient safety, it would encourage pharmaceutical companies to work with the Cooperative Groups, Dr. Steinberg said. Dr. Hohneker agreed, noting that time to opening trials represents not only a barrier to patients’ receiving improved treatments, but a competitive disadvantage in the increasingly crowded field of oncology drug development. Industry is likely to welcome partnerships with more efficient Cooperative Groups, much as companies have partnered with institutions in other countries, he observed. “It may take some time to go through some of the regulatory agencies in some other countries,” he noted, “but once you have done that, the pathway is very robust to patient accruals.”
Dr. Steinberg noted “a tremendous disparity” between the conduct of clinical trials in the United States compared to China and other parts of the world and wondered whether “best practices” from clinical trials systems in other nations could be incorporated into the U.S. system. There is a lot of investment in China (as well as in Singapore and other countries) to develop infrastructure that will compete with the U.S. clinical trials system, Dr. Hohneker reported.
Similarly, Dr. Schilsky noted that some of the Cooperative Groups have established mechanisms to conduct clinical trials in collaboration with pharmaceutical companies that are not officially NCI-sponsored studies. “If you can get a study up and running by working with the company and working with the FDA, and essentially leave the NCI out of it, you can get the study going oftentimes more quickly and without having the whole issue of multiple masters,” he said. Dr. Hohneker noted that his company, Novartis, partners with private clinical trials networks such as U.S. Oncology (now part of McKesson Corporation); such partnerships have streamlined decision making and expedited trials, he reported.
“We [at Sanofi-Aventis] have also looked at those networks,” Dr. Steinberg stated. “In some [but not most] cases the issues there are not that different from the issues with some of the Cooperative Groups in terms of the data, in terms of the complexity,” he observed.
Characterizing the cancer clinical trials system as a hybrid of NCI and industry, Dr. Comis emphasized the importance of “foundation structures” that enable the Cooperative Groups to work directly with industry without involvement with NCI. “One of the consistent requests from the steering committee is more preliminary data,” he observed. “It’s much easier, I think, for us to develop preliminary data working in our foundation structures; [then] … we can bring [them] to the steering committee and see if they want to bite on a Phase III study or not … and if not, we can do it through another structure.”
Dr. Shak noted that clinical trials, and their objectives, are diverse. For large simple trials, it’s best to have all the Cooperative Groups work together, but other studies are more appropriately conducted through Cancer Centers and their networks or even a single center or Cooperative Group, he explained. Therefore, he concluded, a robust cancer clinical trials system is one of appropriately managed flexibility that takes advantage of diversity and different strengths among its component institutions.
The pharmaceutical industry faces another key deterrent, in addition to lengthy time lines, to forging public-private partnerships with the Coopera-
tive Groups, Dr. Humphrey pointed out: the regulatory acceptability of the submission. When Bristol-Myers Squibb approaches a Cooperative Group partner about a trial that, if positive, could be submitted for a supplemental indication, it is concerned about several aspects of the submission, he said; these include statistical analysis, data presentation, and decisions regarding the collection of evidence of adverse events. This presents an opportunity for the Cooperative Groups to focus on developing regulatory expertise—in discussion with the FDA—that would make it easier for pharmaceutical companies to work with the Groups, he continued. The object of such a discussion should be to clarify exactly what is required to prepare a trial for regulatory submission, he said.
The designation of a clinical trial as “registrational” simply indicates that if the trial is positive, it will be submitted to the FDA, Dr. Pazdur replied. The FDA has no hidden agenda in this regard, he said; the designation indicates the intention of placing a study with a Cooperative Group and specifies subsequent regulatory actions. “Some of these issues can be clarified by earlier meetings with the FDA, with all of the partners present,” he observed. “Please come in with the Cooperative Group, with the industry [partner]. Let’s outline the program. Let’s outline a statistical plan. How many looks will be taken at the data? When will they be looked at? When will the data be submitted? Who is going to be responsible [for them]? … It’s basically communication and upfront planning, rather than waiting and saying, ‘Oh, my God, this is a positive study. Let’s send it to the FDA.’ Let’s have an honest discussion up front and then work on the plan.”
Given the Cooperative Groups’ limited budget, questions about how much data to collect and how many supplemental new drug applications (sNDAs)32 to submit have important implications for the number of trials that can be conducted, former CTEP director Dr. Michaele Christian pointed out. Because regulatory submissions can divert dollars
_______________
32 When the sponsor of a new drug believes that enough evidence of the drug’s safety and effectiveness has been obtained to meet FDA’s requirements for marketing approval, the sponsor submits to FDA a new drug application (NDA). The application must contain data from specific technical viewpoints for review, including chemistry, pharmacology, medical, biopharmaceutics, and statistics. If the NDA is approved, the product may be marketed in the United States. For internal tracking purposes, all NDAs are assigned an NDA number. A supplemental NDA is an application to allow a company to make changes in the label for a product that already has an approved NDA. FDA must approve all important NDA changes to ensure that the conditions originally set for the product are still met. Source: http://www.fda.gov/Drugs/informationondrugs/ucm079436.htm#N.
from research studies, it’s important to consider the balance between new scientific observations and regulatory documentation, she said. “We are looking at a situation where we are probably going to do fewer studies across the system because of limitations of funds, so the question is, How do we find the most important ones and get the most out of the data that we do collect?”
Dr. Hohneker in turn offered an industry perspective on managing the costs of clinical trials, which requires a good, upfront estimate of the cost of a trial, specifying what the company will contribute in terms of resources (e.g., drug, funding). Recognizing that Cooperative Groups may not be able to predict a trial budget until the protocol is established, he noted that in the past “there have been successful collaborations with industry on Phase III trials, where we have had to ante up and provide support.” However, he added, “we like to know early on, as opposed to later on in the process.” Dr. Pazdur stated that discussions regarding support for Cooperative Groups conducting clinical trials in partnership with pharmaceutical companies should take place during early deliberations with the FDA.
There is a fundamental difference between a Cooperative Group and a company with regard to responsibility and accountability, Dr. Adamson asserted. “We have a bizarre system. We can in a Cooperative Group be as successful as anyone in the world, but if the NCI falls down on the job, it [the Cooperative Group] fails. The NCI knows that.” Similarly, he added, under other circumstances the Cooperative Group can fail the NCI.
Dr. Adamson noted that his Cooperative Group, COG, has conducted studies that led to NDAs and sNDAs with funding from NCI, which he characterized as “not ideal, but ultimately the FDA has been able to act on the data presented [to it].” These NCI-funded studies cost an order of magnitude less to conduct than would a comparable industry study, he said, “so we have a disconnect. How much data is really needed? Industry’s approach, I believe, is, ‘We don’t really know, so we will do everything.’ And the Cooperative Group’s is, ‘We have no money, so we will do as little as possible to get by.’ The truth has got to be somewhere between those two.”
These efficiencies can be accomplished by early planning, much as Dr. Pazdur had suggested, Dr. Hohneker replied. He made note of a recent conference hosted by the Brookings Institution, Friends of Cancer Research, and ASCO, which recommended minimum data standards and guidelines for determining how much data would have to be collected in a clinical trial (Abrams et al., 2009). He said these standards and guidelines should be considered as all stakeholders in a clinical trial come together to
decide, early on, what is needed, leading to the prospective design of the required dataset, he said.
Blame for excessive monitoring of clinical trials tends to be unfairly directed at the FDA, Dr. Pazdur observed. “We do not set monitoring practices,” he insisted; rather, representatives of pharmaceutical companies “come out to your institution on a monthly basis because they want to come out, not because it’s FDA mandated,” he told the Cooperative Group representatives. “Pharma likes to control what they can control,” he concluded.
Responding to these remarks, Dr. Hohneker said, “If industry is going to undertake a Phase III trial that has thousands of subjects, it’s costly. It’s going to be millions and millions of dollars. One can never anticipate all the questions you are going to get from various regulators around the world on the dataset when you submit.” Therefore, he continued, “we tend to err on completeness of data.” However, he added, Novartis is trying to reduce the burden to investigators associated with completing case report forms (CRFs),33 which he characterized as long and complicated, particularly those associated with Phase III trials.
Defining a clinical trial protocol encourages stakeholders to specify its objectives and the prospective hypotheses to be tested, in order to design data collection to accomplish those goals, Dr. Shak observed. Stakeholders should also be aware that secondary objectives could be explored in the course of a clinical trial, he added; for example, he said, “it seems ironic that we would spend a lot of time in an oncology trial collecting blood urea nitrogen concentrations (BUNs)34 for a biologic and then not collect the tumor block at study entry … for future studies [aimed at] better understanding the biology of the drugs that we use and how to use them.”
Mr. Glen de Vries, of Medidata Solutions, Incorporated, summarized his perspective on informatics needs of the Cooperative Groups as follows:
_______________
33 The form (paper or electronic) on which data about each trail participant are recorded, including adverse events.
• An emphasis on infrastructure and research processes, not systems;
• Interoperability;
• Standards design;
• Common sources of authority for all stakeholders—sites, users, subjects—that reside above the level of the study;
• Application of best practices from outside of clinical research;
• Approaching planning and execution as a single problem;
• Design processes that overcome organizational barriers (at both departmental and Cooperative Group levels) and create measurable management metrics;
• Adopting a metrics-based approach to defining success and creating operational standards; and
• Leveraging commercial platforms.
Mr. de Vries then expanded on each point. Over the course of the last decade, the adoption of business processes to manage clinical trials has revolutionized their conduct, he observed. “A business process can be all about finding the patients you need, making them healthier, making them live longer,” he explained, “but we have to do certain things, like have the right accrual rate, to get there…. A lot of that thinking is necessary to put the right foundational layer of systems in place.”
Interoperability applies to clinical trials not only in the sense of making multiple institutions and systems work together, but also in terms of their relevance over time. To illustrate this point, Mr. de Vries speculated that every researcher of a certain age in the room had written a worthwhile paper in Word Perfect that now resides on a floppy disk in a closet, rendering it totally useless. Researchers have an operational and an ethical obligation to make sure that the data assets they create are leveraged as much as they can be—but this requires standards, which are difficult to achieve, he said. “If you look at the [informatics] industry, we spent about 10 years trying to define standards, and we’re still not that good at it,” he observed. “Standards design is not something that just happens on its own. It requires a proactive, focused set of visionary people to really define them and get everybody else to come along with them.”
It’s somewhat easier to establish a common source of authority with regard to clinical trials than to define standards, Mr. de Vries continued. “We need to create a way that we can find the source of authority for a person—maybe … an investigator or a nurse; maybe … a patient. We need to find the common definition for a trial, so that people working on it
can have a common source of information, and so everybody else can find it the right way as well.”
Planning is critical to the success of clinical trials, Mr. de Vries insisted. “We have a kernel of an idea, and we need to really build out a whole set of … systems to make it successful from the beginning. This needs to be done in a cross-functional way. Anytime we are revolutionizing a process, whether it’s an oncology trial process or a larger process in research or a process in business, you need to think about how to blur the lines between, not just Cooperative Groups, but maybe departments in a Cooperative Group. Thus, in addition to consolidating front office and back office operations, he wondered, “Are we challenging enough what the front office does versus the back office? Maybe some of those lines don’t need to be there in the future as well.”
The Cooperative Groups would benefit by defining metrics for goals, rather than setting goals based on process steps, according to Mr. de Vries. A goal (e.g., increased data sharing between institutions, a particular rate of patient accrual, the appropriate reuse of study data) should be given to the person, or people, whose job is to achieve that goal by any means they can, he said. “Let people really take on the mission of making the numbers better and then measure their success by that,” he advised.
Finally, Mr. de Vries said, the Cooperative Groups need to leverage commercial platforms, so that more resources are available to conduct research. “There are commercial things that one can leverage in terms of having centralized infrastructure and communications—for example, the Internet, the best example in the world—to make these business processes that we need to underpin all the things that we do,” he concluded.
Completeness Versus Flexibility
During the subsequent panel discussion, Dr. Murphy noted that the need for common data systems for the Cooperative Groups had been recognized for years, but is far from realization, and that without such a common base, the hoped-for efficiencies of consolidation of the Groups could not be achieved. “If we can’t have significant progress in achieving these standards and interoperability and data capture forms and clinical trials management systems, where are we?” she asked the panelists.
“Informatics typically doesn’t solve anything,” Mr. de Vries observed. “You cannot increase the speed of something and maintain quality and lower the cost unless you change what it is that you are doing,” he contin-
ued. Moreover, he said, “I don’t think anybody has successfully plotted out an informatics revolution and known exactly what was going to happen step by step.” Instead, he said, the focus needs to be on finding addressable problems and making metrics-based goals to solve them.
Dr. Buckner noted that informatics can be confused with standards, and he agreed with the notion, expressed by Mr. de Vries during his presentation, that developing common standards is crucial to the success of a common data system for the Cooperative Groups. The importance of calling “the same item by the same name … across a variety of domains—the clinical data domains, the biospecimen domains, the protocol, the patient level, the site—is woefully underappreciated,” he said; getting to that goal requires considerable time and thought. He noted that the Cooperative Groups have adopted the same scale for measuring toxicity—an achievement that should serve as an example for similar efforts.
Dr. Gray asserted that progress has been made to establish a common data system for the Cooperative Groups, as described in his presentation to Panel I. “There are, I believe, contracts set up for having that system hosted by Medidata,” he said, and he reported that all of the Groups are now engaged in a variety of activities to implement this system, with the goal of using it for studies by the end of 2011. “The legacy systems are going to be with us for many years as well,” he acknowledged, “but we are planning to put all new studies beyond some point into this new system. That work is proceeding.”
“There’s no question that in terms of developing genomic assays, the fact that we didn’t have a legacy system to begin with was actually a tremendous advantage,” Dr. Shak recalled; this situation allowed Genomic Health to tailor the development of its informatics tools to their specific needs and uses. It’s a far larger challenge to merge data systems while data-generating research is ongoing, he observed. Understanding that systems changes take time and effort, and providing the resources to support that process, will pay off in the long run, he said.
There is a tendency in any highly regulated environment to develop and implement a fully specified data standard or system, Mr. de Vries observed—only then do you find out if you realized your goal. By contrast, he said, most systems that are developed today, outside of the world of clinical trials, are created through a much more agile approach, which evolves with time and provides incremental value every time its standards are improved. In fact, Dr. Steinberg noted, sometimes value is added by end users who adapt systems to meet particular and unanticipated needs.
Nevertheless, Dr. Comis responded, there has to be a timetable for making this transition. For example, he noted, the development of an informatics framework is crucial to the proposed reorganization of the Cooperative Groups’ tumor tissue banks and the creation of a “virtual tumor bank,” enabling access to all samples archived by individual Groups. “I think we need a formal process and a formal approach and a time line to when we are going to get the virtual tumor bank set up … [and] we need to have clear-cut guidelines and an approach to that IT problem,” he concluded.
Dr. Amy Abernethy, of Duke University, urged the Cooperative Groups to develop a common vision and culture of information sharing that would guide decision making related to informatics. The Cooperative Groups also need to align themselves with national trends in informatics, such as the use of electronic health records, she added.
“I think the Cooperative Groups have agreed in principle on what we need to do,” Dr. Buckner observed; what remains to be determined is who defines the standards—and whether industry can be persuaded to adopt them. Speaking as an individual who works in the pharmaceutical industry, Dr. Steinberg replied, “if we have a seat at the table and we can be part of that discussion, my sense is that, yes, we’ll follow along with the standards. I think it’s in everybody’s best interest.”
Dr. Hohneker agreed and noted that pharmaceutical companies have faced similar challenges during frequent recent mergers. “We are all looking at developing standard case report forms … [and] learning about new technologies and what kind of data we need to collect. I think we just have to put a stake in the ground and establish a data standard today, with the ability to be flexible in the future, to adapt to changes that may come out through innovation or what we know about diseases,” he concluded.
Describing himself as a patient advocate whose “day job has a lot of systems content,” Mr. Katz commented that major systems transitions in all sorts of corporate environments require companies to address their legacy systems, whether those are 40-year-old life insurance policies or “music contracts that go back to Caruso.” “To carry forward all of the systems that take care of all of the data that’s relevant until it’s not relevant anymore, we are never going to get rid of those systems,” he said. On the other hand, “if an institution needs to change the process from A to Z and the systems from A to Z all at once, it never happens. So it might be that we have to scale back the aggressiveness on the vision to get it to be more modular,” he concluded. “If there is no migration scenario, if there is no way to implement a vision, then the vision, no matter how perfect, is worthless.”
“I think you hit the nail on the head,” Mr. de Vries responded. Informatics can solve the relatively easy problem of making systems talk with each other, he said, but the real challenge is defining standards—the “master record” upon which all users of the system agree, and which serves as its foundation. “We are never going to be able to build the be-all and end-all system for everybody at the same time,” he concluded.
PANEL IV: CLINICAL TRIALS INVESTIGATORS AND PATIENT ADVOCATES
Speakers in the workshop’s final session included investigators who volunteer their time to participate in Cooperative Group clinical trials, as well as patient advocates who support clinical trials and the Groups’ work. Participants in the subsequent panel discussion expanded on several ideas raised by these speakers and in prior sessions.
Dr. Stephen Grubbs, of the Helen F. Graham Cancer Center, discussed the IOM consensus report’s findings and recommendations from the point of view of the Community Clinical Oncology Program. Figure 3 shows the distribution of the 63 CCOP and Minority CCOP sites across the United States and in Puerto Rico, as of June 2010.
Dr. Grubbs, who served on the IOM committee that prepared the consensus report, said that CCOP principal investigators and administrators, along with NCI representatives, convened in 2010 to create a new strategic plan, which reflects several of the consensus report’s recommendations. Box 4 lists the strategic goals identified by the CCOP committee and highlights common elements present in the IOM consensus report recommendations.
As he presented the IOM consensus report to his colleagues in the CCOP community, Dr. Grubbs made note of topics that generated particular interest, other than the consolidation of the Cooperative Groups. “Physicians were most anxious to hear about broad patient education regarding availability, payment coverage, and the value of the clinical trials,” he reported. Ideally, CCOP physicians would like patients to be familiar with the concept of clinical trials, so that when they are asked to consider joining one, the question doesn’t come as a surprise. Many physicians felt
that patient eligibility criteria for trials tend to be too narrow, he stated, and he noted that this issue would likely become more prominent with the increased use of biomarkers to identify patient candidates. Some physicians suggested that they could enroll more patients in clinical trials if they had a tool to alert them as to the availability of trials for individual patients, based on their electronic health records.
Dr. Grubbs remarked that CCOP physicians would like to see more patient advocates participate in cancer clinical trial design and recruitment. Physicians also expressed interest in developing site portfolios that draw from all Cooperative Group trials. “If I’m in Cooperative Group A, I certainly want to be able to pick a C, D, or E Cooperative Group that has a trial that fits my patient population and my interest and my clinicians in my community,” he said.
CCOP physicians want care coverage policies to reflect the value of clinical trials, Dr. Grubb observed. “We need to have federal and state benefit programs, as well as private insurers, to cover the patient care costs within the realm of standard of care in NCI trials,” he said, noting that the federal Patient Protection and Affordable Care Act of 2010 mandates that all individual and group health plans must pay such costs.
Unfortunately, some health insurers have vastly complicated the fulfillment of this mandate, Dr. Grubbs added. To make this point, he displayed a policy posted on an insurer’s website, which states what a physician must do to get a patient approved for treatment on a clinical trial with insurance coverage. In addition to providing exhaustive documentation of the patient’s needs and all aspects of anticipated care, it requires physicians to “attest” that their patient was eligible for the trial. “When I hear ‘attestation,’ my legal antennas go up, because it seems to me that if they are found ineligible later on, I might be responsible for all their health care costs,” Dr. Grubb explained. “I’m not sure this has taken effect,” he added; if so, it represents a significant barrier to accrual. “It fits the law,” he concluded. “They will cover a clinical trial, but the hoops you are going to have to jump through to do this look astronomical.”
Academic centers should recognize and reward clinical trials team research in their promotion and tenure decisions, Dr. Grubbs asserted. “I have heard this over and over again—that my colleagues who are junior faculty members really don’t get any credit for this for promotion and that gives them no incentive [to become involved in clinical trials],” he said. However, he noted, CCOP physicians were divided as to the value of a possible NCI clinical investigator certification program and registry. “Some
SOURCE: Grubbs presentation, March 21, 2011; Lori Minasian, CCOP director.
BOX 4
CCOP Strategic Goals, 2010
1. Incorporate Emerging Science and Novel Trial Designs into Cancer Prevention and Control (CPC) Research
• Develop Survivorship Research
• Focused on Symptom and Toxicity Research
• Foster Research on Risk Assessment and Risk Modeling for Cancer Prevention and Early Detection
• Explore Funding Mechanisms for Correlative Studies in Association with CPC studies
• Foster Relationships with Basic Science Researchers
• Foster Training for Investigators in CPC
• Facilitate Drug Distribution in Cancer Control Trials
2. Maximize Community Resources to Conduct Complex Clinical Trials (Treatment & CPC)
• Develop a Mechanism for Biospecimen Collection
• Develop a Flexible Funding Model
• Encourage Standardization Across the Network
• Monitor the Changing Business Model
• Foster Mentoring Community Investigators
• Enhance Communication Efforts to Educate the Public on Clinical Trials and Provide Tools
• Address Overlap with Other Programs
3. Use Epidemiological and Biological Data from Underserved Populations to Address Disparate Health Outcomes
• Identify Relevant Research Questions in Underserved Populations for Study by Research Bases
• Develop a Transdisciplinary Working Group to Design Nested (or Stand Alone) Pilot Studies to Evaluate the Effect of Relaxing Eligibility for Some Studies
• Promote Cancer Risk Assessment in Underserved Populations
4. Improve Clinical Trials Access and Participation Among Populations Underrepresented in Clinical Research
• Consider Broadening Eligibility of Minority-Based (MB)-CCOP
• Facilitate Translations of Informed Consents and Patient Information for Studies
• Develop Plan for Assigning Credit for Screening Patients and At-Risk People for Trials
• Develop Guidelines for Publications
• Review Accrual Requirements (in conjunction with flexible funding model) for MB-CCOPs
• Incorporate Patient Navigation
• Foster Development of Mentorship
5. Build on CCOP or MB-CCOP Success to Improve Ability of Community Groups to Accrue Patients
• Develop Best Practices
• Collect Data on Screening Patients for Trials
• Develop Process to Rapidly Identify Clinical Trials with Accrual Issues
• Encourage Studies That Address Accrual Specifically
NOTE: Underlined elements are common to the IOM consensus report.
SOURCE: Grubbs presentation, March 21, 2011.
are not in favor of this [because] they don’t want another layer of certification and all the hassles,” he acknowledged. On the other hand, Dr. Grubbs noted, such certification would distinguish community trialists from other community physicians; therefore, he continued, “I am certainly in favor of carrying that title.”
Conversely, while recognizing investigators is likely to provide a “carrot” to encourage greater involvement of researchers in clinical trials, sometimes a “stick” is also needed, Dr. Grubbs observed. At his CCOP, researchers who do not enroll enough patients in clinical trials are removed as investigators. “That has prompted physicians to all increase their accrual,” he said.
Another request of CCOP physicians is for a centralized credentialing and audit system across the Cooperative Groups. “I’m in enough Cooperative Groups that we have an audit team in about every four to five months, year after year after year,” Dr. Grubbs stated. “I would like one audit every three years, and do it all.”
CCOP physicians’ work is inadequately supported, Dr. Grubbs observed. Per-case reimbursement is generally too low, he argued, so NCI should fund principal investigators (PIs) involved with clinical trials. Some progress has been made to increase funding for PIs at the academic centers, he noted, but not for those at CCOP and community cancer centers. “We need enhanced levels of compensation for physicians for offering, enrolling, managing, and following clinical trials patients,” he said. Under the CPT code system (see Panel II, “Establishing New Common Procedural Terminology (CPT) Codes for Care of Patients Enrolled in Clinical Trials”), he observed, “we are charging for face-to-face, nose-to-nose time with our patients, unlike our legal colleagues, who charge by the hour,” he remarked; physicians are not compensated for the hours they spend on paperwork, preparing for the patient, and reviewing information. He noted that a survey conducted in 2003 and 2004 determined median costs for Phase II and Phase III patients to be about $6,000, and $3,500, respectively (C-Change and the Coalition for Cancer Cooperative Groups, 2006), yet baseline per-case compensation from NCI remains at $2,000. “The Cooperative Groups have pharma money they give us for several trials,” he acknowledged, but it still fails to meet the costs of many trials. “We do need more money to do this,” he concluded.
Without the volunteer contributions of its physicians, the CCOP network “grinds to a halt very quickly,” Dr. Grubbs observed. He listed the reasons he and other CCOP physicians participate in the cancer clinical trials system and how their contributions could be further encouraged and
supported. He stressed the importance of Cooperative Groups as a “home team” for physicians, providing a sense of belonging and identity. The groups also offer collegiality in an age where it is fading elsewhere, he said. Through the Cooperative Groups, CCOP physicians forge relationships they might not otherwise have with academic scientists and clinicians. “It gives us an ability to rub elbows and be part of the process,” he explained. “It gives us publication opportunities.”
However, Dr. Grubbs noted, there is a limit to how much time and expense CCOP physicians can contribute to the cancer clinical trials system. “Time and expense versus best patient care options: this is an issue we are going to have to deal with,” he said. “There is nothing wrong with treating a patient with the standard of care … [but] on the other hand, the time and the expense of doing a clinical trial has to get worked out with us.”
“Finally, we need to optimize the size [of the Cooperative Groups],” Dr. Grubbs concluded. “Please, let’s not make them too large or too amorphous, because we are going to lose this connection. I think that’s very important for us in the community.”
Academic Clinical Investigator Perspective
Dr. Melanie Thomas, of the Medical University of South Carolina, offered a dual perspective on the Cooperative Groups, recalling her experience as an academic researcher at M.D. Anderson Cancer Center prior to her current position, as associate director for clinical investigation for an institution she called “a very small but feisty cancer center.” Dr. Thomas was the recipient of a 2009 NCI Clinical Team Leadership Award. She praised the Cooperative Groups as serving an important role as an “incubator” for new investigators, especially those (unlike herself) who do not come from elite training programs, for whom the groups provide “a great opportunity for training and mentorship.”
Posed in the form of a question, Dr. Thomas’ first comment echoed a point raised by Dr. Grubbs: Is participation in the Cooperative Groups valued? She too admonished academic centers to consider the large amount of time and effort required of investigators involved in clinical trials. She also noted that just as clinical trials investigators face barriers to academic advancement, they are also hindered from professional advancement within the Cooperative Groups, in which many committee and subcommittee members hold their positions for decades, making it difficult for young investigators to move into positions of responsibility.
Responding to this comment during the panel discussion, Dr. Larry Baker, of the University of Michigan Comprehensive Cancer Center and SWOG, noted that the issue of leadership turnover among the Cooperative Groups had been raised in a recent white paper35 expressing the consensus opinion of physicians at the Case Comprehensive Cancer Center at Case Western Reserve University, and authored by its director, Dr. Stanton Gerson. It states that term limits should be considered for Cooperative Group committee chairs, and perhaps also for senior leadership positions, in order to improve opportunities for advancement, to encourage broader participation of early-career and mid-level investigators in the Groups, and to reward their participation with meaningful and funded leadership positions.
Dr. Baker said that upon reading this paper, “we immediately turned inward to begin the process [of reconsidering leadership turnover] at SWOG.” He said that he expected a “firm resolution” to change leadership practices to be made in April 2011, specifying criteria by which disease chairs, group chairs, group statisticians, and other officers can maintain their positions.
Every Cancer Center must strike a balance between industry trials, Cooperative Group trials, and investigator-initiated trials, Dr. Thomas noted. However, she observed, “When it comes time for NCI review [of the Cancer Center], the Cooperative Group trials don’t mean anything. I’m sure that I’m not the only person who has ever been told that or heard that or said that. Is that true or is it not true? That’s very important for the Cancer Centers, because we are subsidizing this process with money, with time, with effort, with our patients.”
Dr. Thomas concluded her remarks by summarizing a “tremendous set of opportunities” afforded by the proposed re-invigoration of the Cooperative Groups, as described in the IOM consensus report. These included the following:
• Making room for new investigators;
• Increased collaboration among the leadership of the existing Cooperative Groups;
• Examination of the national clinical trials portfolio to reduce waste of resources on low-accruing trials and to emphasize scientific advancement;
_______________
35 See http://cancer.case.edu/notices/files/CooperativeGroupWhitePaper.pdf.
• Leveraging existing intellectual capital, in the form of cancer specialists now spread across the Cooperative Groups, who upon consolidation can work more as a team; and
• Identifying what it is that the Cooperative Groups can do best, such as trials that cannot otherwise be undertaken. “There will never be trials done by industry for a great number of tumor sites,” she noted.
Community Cancer Center Perspective
Speaker Maria Gonzalez represented St. Joseph Hospital, Orange (SJO), California, a recipient of the NCI Community Cancer Centers Program (NCCCP) grant, where she is cancer research manager. She described the 525-bed hospital, with approximately 1,000 physicians on staff, as serving a highly competitive environment. SJO has the highest number of emergency department visits in Orange County, with more than 1,600 analytic cases per year. Its primary service area is 53 percent Hispanic, 17 percent Asian, and 25 percent Caucasian, with a relatively high percentage of undocumented immigrants, she reported. “Our hospital is a community benefit-focused hospital, so we take all comers,” she stated.
SJO is among 30 sites to obtain an NCCCP grant, which funds formal research programs that foster interactions between basic laboratory, clinical, and population scientists; access for investigators to shared services and technologies that are necessary to their research efforts; and other scientific infrastructure.36 “NCCCP sites are research-ready community hospitals with the ability to be dynamic, [be] supportive of community physicians, and provide infrastructure for clinical trials and biospecimens in a diverse setting,” Ms. Gonzalez explained. “This is a public-private partnership. It enables improved infrastructure. We all have our administrative commitment from the top down. Our physicians are highly engaged. We are a complementary network. I think that’s what really stands out about the NCCCP. We work together. We leverage each other’s resources … [and] we provide synergy to NCI programs.”
SJO was awarded its NCCCP grant in 2010, which encouraged the hospital’s ongoing efforts to increase clinical trials accrual. Since then, she said, “we have made a complete culture shift to clinical trials—so much so that all of our cancer conferences provide CME [continuing medical education] credits based on clinical trials metrics.” SJO has also established
_______________
a diversity team consisting of a nurse practitioner, a financial counselor, expanded navigation, and a research team that conducts a multidisciplinary clinic for the underinsured and the uninsured, she added.
The NCCCP grant has also enabled SJO to increase its collaborations with the Cooperative Groups, including RTOG, SWOG, GOG, and NSABP. “All of those collaborations came from a grassroots effort,” Ms. Gonzalez observed. “They came from me calling the PI at Pittsburgh and saying, ‘Hey, I have a great community hospital. Will you let me be part of your trial that I found on clinicaltrials.gov?’” In the continuum of cancer care, she noted, “the community hospital is kind of at the end of it, so we have to really reach out.” SJO has also engaged in collaborations with other NCCCP sites, including several that mentored the hospital’s establishment of a biospecimen repository in February 2011, she said.
Ms. Gonzalez described several potential opportunities that a revitalized Cooperative Group System might offer community hospitals such as SJO. Community hospital physicians should be included in the planning of clinical trials and incentivized to participate in them, she said. Links between academic and community hospitals should be strengthened, she added; this could be achieved by requiring NCI Comprehensive Cancer Centers to work with the community hospitals in order to obtain funding and to use some of their grant money to fund their community hospital partners.
Increasing clinical trials advocacy education and physician training is another important goal, Ms. Gonzalez said. In particular, she added, “we are really trying to start from the first point of entry to work with our community network partners to increase minority and rural enrollment.”
The streamlining of the Cooperative Group process could be a boon to SJO and sites like it, Ms. Gonzalez said. As a member of four different Cooperative Groups, “all of my quotas are different, all of my regulations are different, my reporting, and my audits,” she explained. “Minimize that duplication, collaborate with local biospecimen repositories, improve communication, and educate the local IRBs,” she requested. In conclusion, she suggested that the Cooperative Groups develop ongoing internal and external assessment of their community Cancer Center partners.
Cooperative Group Patient Advocate Perspective
Michael Katz, a retired management consultant who volunteers as a patient advocate for the Cooperative Groups, said that while he derives no income from cancer care or clinical trials, he nonetheless has a large stake in the success of the Groups. “In my view and in the view of most patient advocates … the Cooperative Group system is a national treasure,” he observed. “We need to be both bold and careful as we move forward to make it better, because breaking it would be a tragedy for all cancer patients, present and future.”
Mr. Katz noted that previous attempts to reform the Cooperative Groups have been “fraught with false starts, with unintended consequences, and with stillborn constructs.” However, he said, “We are all, I think, older and wiser, and we have all learned a lot.” This effort will not be without problems, he continued, and it will be important to stay open-minded and ready to fine-tune strategy as it is implemented. “There are a lot of unresolved issues about exactly how this is going to happen and what the best way is to do it,” he observed.
Several “hopeful signs” suggest that the current revitalization effort will be productive, according to Mr. Katz. These include new rules establishing protocol-development time frames and tools to monitor these time frames as described by Dr. Doroshow in Panel I; likelihood of expanded use of CIRBs; and more stringent data monitoring, along with increased willingness to terminate unproductive trials at earlier stages. “The IOM, to its credit, has taken a zero-based approach that makes bold recommendations for structural change,” Mr. Katz stated. “It has driven clear mandates for change from the NCI.” Nevertheless, he added, the NCI has acknowledged that these changes are going to cost more before they cost less.
At the same time, Mr. Katz expressed concern that management of the Cooperative Group revitalization “lacks the rigor that is the glory of our trials.” Drawing an analogy between a clinical trial protocol and the Cooperative Group reorganization plans, he asserted that the latter effort fails to fulfill the criteria used to judge the quality of a clinical trial. Characterizing the consolidation of the adult Cooperative Groups from ten to four as a “single-armed [trial with an] ambiguous schema,” he wondered, “Do we know [it should be] four instead of five? Are we doing any comparison to the standard of care? Are there alternative ways to do this? Are we going to compare those?”
Like a dose modification schedule in a clinical trial, the Cooperative Group revitalization process needs to have backup plans in place to correct for unforeseen problems, Mr. Katz asserted. “I think also there is an overabundance of surrogate endpoints in the discussions that we are having,” he continued; for example, the change to four groups instead of ten, and to single committees from multiple ones, makes theoretical sense, but remains to be proven useful. “What constitutes success?” he asked. “If we were running a trial, we would hold the investigator’s feet to the fire to document these things.” For example, he said, he questioned the composition of the oversight committees as not having people with the experience and skills necessary to manage the consolidation and diagnose and address problems as they arise.
Further extending his analogy, Mr. Katz noted the apparent lack of a “data-monitoring plan” as part of the Cooperative Group revitalization. “I’m not saying that the intent is not there to do it,” he added, “but I sure hope it’s going to happen before … we take steps that are irreversible.” Comparing the IOM consensus report to a molecule that could, in theory, cure all cancers, he observed that such a drug would never be distributed solely on the basis of its potential. Similarly, he said, implementing recommendations in the IOM report requires “some of the same rigor that we apply to the clinical trials enterprise,” he concluded. Therefore, on behalf of cancer patients, he requested that efforts be made to
• Define what constitutes success in terms of concrete endpoints and time frames;
• Include some tangible patient and scientific outcomes as endpoints;
• Put in place clear metrics, with targets and time frames for achieving them, and alternative plans if they prove unworkable; and
• Rapidly course correct if necessary.
In closing, Mr. Katz wondered aloud why he—among the more than 100 volunteer advocates working for the Cooperative Groups—was the lone Cooperative Group patient advocate speaking at the workshop.37 “On behalf of the patient community, I ask that this not be the model for the implementation,” he said. “There should be more patients in the room, more patient advocates. We volunteer along with everybody else. So please let us be involved as we move this enterprise forward.”
_______________
37 In the subsequent panel discussion, Dr. Go noted that while only one “official” Cooperative Group patient advocate was invited to speak at the workshop, one could argue that all 40 speakers, and indeed, all workshop participants, advocate for cancer patients.
Research Foundation Perspective
Shelley Fuld Nasso, of the Susan G. Komen for the Cure Foundation, concurred with Mr. Katz’s comments. Ms. Nasso directs patient advocates, many of whom are involved with the Cooperative Groups, who volunteer within the foundation’s science program. These advocates—who are passionate and more than willing to dedicate their time to advancing cancer research—want to be part of the discussion of revitalizing cancer clinical trials, she observed. “They have so much to offer,” she said. “We work with them to train them and place them in peer review and IRBs and working in the Cooperative Groups and other avenues for research advocacy.”
Ms. Nasso also pointed out that patient volunteers for Komen raise significant funds to support cancer research. “We have invested $610 million cumulatively, $65 million last year … [in] Cooperative Group efforts,” she stated. “We know we have benefited from the Cooperative Groups in terms of practice-changing results for breast cancer patients.”
In the last several years, the Komen Foundation has shifted its research portfolio away from basic studies and toward more translational research, Ms. Nasso reported. The foundation is “really trying to look at the key challenges in breast cancer and prioritizing our research to focus on some of those key issues, giving larger grants for collaborative efforts,” she said. “There has been a lot of talk about prioritization in the Cooperative Group trials … but not a lot of discussion about how that’s going to happen,” she observed. “I think it’s really important to make sure that patients are at the table when that prioritization happens.”
Building on the point made by Mr. Katz about the importance of metrics to judging the success of revitalization efforts, Ms. Nasso noted that the Komen Foundation is developing metrics to evaluate its own research portfolio, “to see that we are making a difference and that we are funding research that really changes clinical practice and isn’t just validating something that we already knew.” For example, she said, “we have made a goal in our research to decrease incidence or mortality within ten years. That’s a criterion that we look at in funding research.”
Regarding the issue of educating patients—and potential patients—about cancer trials, Ms. Nasso observed that “when you are faced with that diagnosis, it really doesn’t matter what you know before that; it’s what your doctor says to you.” She offered the example of a physician, recently diagnosed with stage IV renal cell carcinoma, who—despite his education and experience—was overwhelmed by the treatment decisions that confronted
him. “No matter how much you may know beforehand, it’s when you are faced with that diagnosis and you are talking to your doctor that you need to hear the message that clinical trials are there for you to participate in,” she said.
It’s important to recognize, as part of any attempt to conserve resources allocated to clinical trials, that patients be regarded as a particularly precious resource, Ms. Nasso added. “When a patient agrees to participate in a clinical trial, it’s really a gift that they are giving to science,” she said. “We have to make sure that we are using that effectively.”
Maintaining Engagement of Cooperative Group Investigators
Noting that the question of how to keep investigators at the academic and community levels engaged in the Cooperative Group process had been raised in this and in prior sessions, Dr. Buckner turned the question over to the panel. Dr. Michael Caligiuri, of Ohio State University (OSU), who described the OSU Cancer Center as “very, very active in the Cooperative Groups,” focused on the importance of leadership. He concurred with the points made earlier by Drs. Thomas and Baker regarding the importance of recognition for participation in the Cooperative Groups and rewarding researchers through promotion, tenure, and pay. “It’s very important that we promote them into, first, committee and then leadership participation in the Cooperative Groups,” he said.
However, Dr. Caligiuri added, it is the Cancer Center that currently foots the bill for advancing and rewarding Cooperative Group researchers. “We at the Cancer Center pay for a lot of people, for travel, for their participation, for opening trials where there is insufficient funding,” he said. During his opening remarks in the panel discussion, in his capacity as president of the Association of American Cancer Institutes (AACI),38 Dr. Caligiuri noted that Cancer Centers provide faculty, staff, infrastructure for early
_______________
38 The Association of American Cancer Institutes comprises 95 leading cancer research centers in the United States. AACI’s membership roster includes NCI-designated centers and academic-based cancer research programs that receive NCI support. AACI is dedicated to promoting the nation’s leading research institutions’ efforts to eradicate cancer through a comprehensive and multidisciplinary program of cancer research, treatment, patient care, prevention, education, and community outreach. See http://www.aaci-cancer.org/about.asp, http://www.aaci-cancer.org/mission.asp.
discovery, clinical scientific review of early concepts, early-phase clinical trials, core labs for correlative science, and funding for patient accrual to the Cooperative Groups, as well as much of their leadership. He also observed that some institutional hosts provide “extraordinary” financial support to Cancer Centers, while others can offer little more than a “paltry sum.”
Dr. Grubbs stressed the importance to clinical trials physicians of establishing—through institutional leadership—an environment in which treating a patient on a clinical trial is considered a standard of care above and beyond the usual evidence-based medicine we have available today. “You just have to instill in your physicians that you are better physicians and your patients are getting better care [for doing clinical trials],” he said. This attitude has a positive ripple effect that affects even those patients that are not in trials, he added.
One participant emphasized Dr. Grubbs’ earlier point that participating in the Cooperative Groups enables community physicians to collaborate with their academic counterparts, noting that academic Cancer Centers have been encouraged to apply for joint grants to increase accruals of minorities to surgical clinical trials. These funds facilitate increased collaboration between academic and community clinical centers, which in turn encourages clinical investigators by allowing them to publish their research and attend academic meetings, the participant said.
Noting that regulatory “red tape” frustrates community physicians involved in clinical trials, Ms. Gonzalez urged simplification of the IRB process as a key incentive for their continued participation. Community physicians would also welcome the implementation of previously suggested improvements in CPT codes, recognition, and external research collaborations, she said.
Mr. Katz suggested that investigators’ engagement in the Cooperative Groups be defined in terms of specific, measurable outcomes. “We don’t really know, I think, where we are today in terms of [how many] young investigators are participating [in the Cooperative Groups] and how many are opting out and how many might come in,” he stated. “I think if we are serious about this, we ought to figure out where we are, set a target, and figure out what we need to do to get there.”
Echoing the suggestion (by Mr. Katz and Ms. Nasso) that metrics for the success of Cooperative Group consolidation be defined and monitored,
Dr. Deborah Bruner, of the University of Pennsylvania, suggested that such measurements be conducted on a single consolidation, before other Groups follow suit. For example, she asked, do rates of accrual rise for a new Group formed from three former Groups? “We have no articulation or plan for that kind of pilot testing,” she noted with concern.
“Accruals are critical and important, and we can’t do trials without getting patients,” Dr. Murphy acknowledged. “But if you will remember from Dr. Reaman’s presentation this morning, immediately after we merged the four pediatric groups, we actually had a dip in accruals. We were so busy finishing the legacy trials, trying to get our act together, writing new grants, getting the finances in order, that we didn’t have the uninterrupted scientific progress, because we were also merging the science.” She therefore predicted that it will be difficult to maintain even current accrual levels in the adult groups until consolidation is truly complete. Dr. Hohneker agreed with this point, and Dr. Buckner noted that since April 2010, only five Phase III trials have been approved throughout the Cooperative Groups.
Moreover, Dr. Murphy observed, “suppose we could double accruals in the Cooperative Groups. We don’t have the capacity for that … so be careful what you wish for.” Nevertheless, she continued, it is important to define what consolidation is meant to accomplish and ways to measure progress toward such goals.
Adopting a business model to describe consolidation of the Cooperative Groups, Dr. Hohneker stated that corporate mergers tend to be defined as successful within specific time frames. He said you have to get to the so-called 80-20 rule,39 which states that for many events, about 80 percent of the effects come from 20 percent of the causes, and move expediently for change to work. “The idea is to improve speed and efficiencies and to look at metrics for that and targets for that that you would expect,” he stated.
Such time frames must be appropriate to the disease being studied, Dr. Thomas observed. For example, she said, “in pancreatic cancer, there have been 10 years of negative clinical trials, but we only have those because the Cooperative Group pushed to get them done. Many of those studies wouldn’t have been done and completed if it weren’t for the Cooperative Group.” She explained that all the Groups that study gastrointestinal cancers convene twice a year to examine their collective clinical trials portfolio, which offers the possibility of judging the quality of the portfolio that emerges from that process, rather than the number of accruals or number of
_______________
trials. In fact, the number of trials for such intractable diseases may decline, but the quality of studies may improve as a result of Group consolidation, she concluded.
An NCI committee is examining metrics to be used to judge future Cooperative Group performance, Dr. Grubbs reported; he added that many workshop participants have been involved with this effort. While objective measurements such as numbers of people enrolled in trials, or length of time to begin and conduct a trial, are easy to obtain, measurements of quality—such as whether a study significantly changed patient care—are very difficult to define, he observed.
“There aren’t that many events to look at when it comes to the completion of trials,” Mr. Katz responded. “It’s not like trying to look at every patient. You can review all the trials that actually changed the standard of care, and you wouldn’t have a desk full of papers.”
“What’s very important in terms of resource utilization is, Did the study answer the question?” Dr. Buckner asserted. “Too often in the cancer field, the study was well done; unfortunately, it didn’t turn out to be an advance, but that still enhances our collective knowledge of what works and what doesn’t work,” he continued. Thereafter, translational research can address the question of why something didn’t work, or only sometimes works, he added. “To expand on the metric, it’s not only what changed practice, but what we learned and, importantly, whether we learned anything,” he concluded. “The worst trial is one in which we don’t get an answer of any kind.”
Dr. James Dignam, of the University of Chicago and RTOG, asserted that the 40 percent trial failure rate due to low accrual in cancer clinical trials, which was cited in the IOM consensus report (Cheng et al., 2009; Schroen et al., 2009), is not the most accurate measurement available. He called participants’ attention to a recent article by Korn and colleagues (2010), published after the IOM report, which estimates that rate at 22 percent and concludes that only about 2 percent of patients will be enrolled in such failed trials. “Often these trials that are smaller than we like are still orders of magnitude larger than other studies and serve as the best information about a given disease situation,” he observed.
Based on a question from Dr. Go, who was unable to enroll an interested patient in a trial conducted by a Cooperative Group of which he
was not a member, participants discussed whether the cancer clinical trials system should be more open in order to allow any Cooperative Group investigator to enroll patients into any Group’s trials. Most Phase III trials accrue many patients and are available across the country, Dr. Buckner explained, but even large Phase II trials accrue no more than 250 patients, and they tend to be relatively popular with patients. Therefore, he asked, What is gained by opening such a trial—at greater expense—to accrue patients at multiple sites?
An institution that enrolls lots of people in clinical trials, but does not belong to a particular Group, should nonetheless have access to that Group’s trials, Mr. Katz responded. Some institutions accrue very few patients to trials, he noted; nevertheless they incur costs associated with having trials open. Dr. Grubbs agreed and noted that individual sites can determine whether it makes sense to assume the cost of opening a given trial. “I’m not going to open a trial that costs money and not accrue…. If institutions are seriously participating in the research, then they ought to have access to all the trials,” he asserted. Moreover, he added, “if you have a great trial, people are going to want to do it and it will get done fast. Isn’t that the final point here, that we want to get trials done quickly and get the answer and move on?”
Following a question from Dr. Cross, the panel considered which types of trials should be opened broadly across the country at all institutions and which should be opened primarily at referral centers (e.g., community hospitals). Dr. Cross said that as a payor, he would prefer to have as many trials as possible conducted in community settings, where routine costs are lower. (Patients who lack insurance are often unable to enroll in trials conducted in tertiary centers, Ms. Gonzalez pointed out.)
Mr. Katz remarked that this issue has recently been discussed at a steering committee meeting and that it may be an accrual barrier when experimental agents must be given in a tertiary center. In some cases, he said physicians are not even allowed to give the pills to patients at the local community cancer center; they have to be certified as investigators and there are regulatory issues. He suggested that a hybrid approach could solve this issue.
“The trial should be done where it can be done,” Dr. Caligiuri insisted. In a given trial, there may be a device or an agent or a treatment for a particular tumor type that might be most efficiently administered in a tertiary
center, but might also be provided at certain community sites, he said. “It’s not a black-and-white issue.”
“This is a perfect question for comparative effectiveness research,” Dr. Murphy observed. “Are the results in Cancer Centers better than in the communities, or vice versa? The groups and the CCOPs are positioned to answer those kinds of questions,” she said. “It’s a matter of delivery of cancer services, basically, and optimum outcomes…. These are important issues for payors, as well as patients—convenience, impact on quality of life,” she continued. “These could all be studied.” Dr. Grubbs responded that CCOPs had excellent audit reports, just as the Cancer Centers did. “So I think we have shown there is equivalency there…. It’s not unusual that in the Phase II trial, the leaders in accruals are the CCOPs, not the main member institutions,” he said.
Dr. Bertagnolli remarked that clinical trials offer the best way to disseminate novel findings to the community, and therefore research should not be confined within tertiary centers if at all possible. When a clinical trial is performed in a research center, she said, “it allows the quality of the entire health care system to be expanded in probably the most efficient and best-controlled way.”
Dr. Buckner noted that while critical evaluations of some patients may require expertise and technology available only at tertiary centers, they often can still receive care at community centers close to their homes. “It depends on what the disease is, what the comfort level is of the patient, what the comfort level is of the physician or provider who is treating the patient, and the expertise available at a convenient referral center,” he explained.
Dr. Schilsky noted that an analysis presented at the March 2011 CTAC meeting determined that annual funding for the entire NCI clinical trials portfolio amounts to nearly $1 billion, of which approximately $250 million supports the Cooperative Groups and the CCOPs. “Where is the other $750 million going?” he asked. “What are we getting for that investment of resources?”
Dr. Doroshow said that the analysis40 of the Fiscal Year 2006 NCI budget was undertaken by Judy Hautala of the Science and Technology Policy Institute. A total of nearly $975,000,000 was devoted to interventional
_______________
40 See http://deainfo.nci.nih.gov/advisory/ctac/0311/Hautala.pdf.
clinical trials that year, with about 17 percent going to intramural studies, about 82 percent going toward extramural studies, and the remaining 1 percent going to research management support. The extramural portion includes many different programs, but only three constitute more than 10 percent of the total: the Cooperative Group Program (21 percent), the Cancer Centers (13 percent), and the Clinical Grants and Contracts Branch of the NCI Division of Cancer Treatment and Diagnosis (13 percent). As a result, Dr. Doroshow said, “it’s easy to look at the Group budgets because they are the biggest line, but it is much harder to evaluate all of these other pieces broken up across a large playing field. One of the things that I think is very clear from the last CTAC meeting is that we should do such an analysis.” We need to know “what we get for that amount of money,” he added.
“I would suggest that you could double the $250 million spent on the Cooperative Groups, which would be transformative for the Cooperative Group Program, and still have $500 million to spend on other clinical trials sponsored by the NCI,” Dr. Schlisky suggested. “If there’s $1 billion being invested by NCI in clinical trials every year, we really need to look at how to make sure that that money is being really well spent.”
As the workshop drew to a close, Dr. Schilsky offered the following reflections, based on the day’s presentations and discussions.
“Surely everybody here would agree that it’s vitally important to this country that we have a publicly funded clinical trials system,” he began. “It’s really only a question of how much we invest in it and what it looks like, to make it optimally functional.” He noted several ways to improve the current clinical trials system as expressed by workshop participants:
• Support innovation;
• Encourage greater collaboration across all elements of the system;
• Enhance system efficiency, thereby reducing development time for new treatments; and
• Increase communication among all stakeholders.
As emphasized in the IOM consensus report and reflected in workshop discussions among diverse stakeholders, everyone can do something to improve the cancer clinical trials system, Dr. Schilsky observed. “The system will not improve if only the Cooperative Groups change, or if only
the NCI changes, or if only pharma develops a new attitude, or if only the FDA puts in place different approval criteria,” he said.
Clearly, increased funding for the Cooperative Group Program is crucial to its revitalization, Dr. Shilsky asserted. “The budget for the Cooperative Groups today is essentially the same, in real dollars, as it was more than a decade ago, yet the work is more complicated and the challenges are at least as great, and the opportunities are greater than they have ever been,” he stated. “If more money is not put into the system, the only possible result is that there will be fewer trials and lower accrual,” he continued. “That’s not necessarily a bad thing, if the trials can be launched more quickly, completed more quickly, and ask and answer high-impact questions. But I think that’s the inevitable result of a system that is essentially being strangled by inadequate funding.” Under such circumstances, priority setting is critical, he added—and again, something all stakeholders must face and to which they should contribute.
It will also be important—particularly given such resource limitations—to preserve volunteerism in the Cooperative Groups, Dr. Schilsky noted. Recalling Dr. Grubbs’ comment that his Cooperative Group is his professional “home,” Dr. Schilsky observed that many people in the Cooperative Group system feel similarly and, because they do, invest considerable time, energy, and money in the work of their Group. He reminded participants of the previously discussed NCI portfolio analysis showing that NCI supports only about half of the Cooperative Groups’ total operating budget and that most of the remainder is provided by institutions that value their participation in the Cooperative Group Program.
The Cooperative Groups play an important role in developing the next generation of clinical researchers, Dr. Schilsky added. Many leaders in clinical cancer research “grew up, made their reputations, and learned how to do clinical trials in the Cooperative Group Program,” he said. “There really is no other venue anywhere in the world where young investigators can get the experience of working with experienced clinical trialists, disease experts, laboratory scientists, expert biostatisticians, people who know how to construct databases, and so on like in the Cooperative Group program,” he continued. “If the program downsizes and the younger generation perceives fewer opportunities to participate in the Cooperative Group program, I fear that we are going to lose some of them as time goes by.”
Dr. Schilsky closed by reiterating Mr. Katz’s remarks: “Let’s remember to be bold, but to be careful in our attempts to make the system better, and let’s be sure we know what constitutes success before we start making whole-


























































































