6
Case Study of a Targeting Decision That Spans Food and Drug Administration Centers
This final case study focuses on the use of the proposed framework to set priorities for work that could affect more than one center when choices must be made to allocate agency resources to several pressing needs that arise simultaneously. It is based loosely on two decision scenarios provided by the Food and Drug Administration (FDA), one of which described the need to set priorities for laboratory resources and the second described the need to balance a flood of laboratory testing requests associated with the 2007 melamine-contamination threat with other demands on laboratory resources. As with the other case studies, the data used were gleaned from publicly available Web sites or publications or were provided by FDA. The committee did not conduct exhaustive literature searches or reviews, and all information is illustrative. The case study simply provides an illustration of how the committee’s framework might be used for a targeting decision that spans FDA centers.
FRAMING THE ISSUE: MELAMINE CONTAMINATION
This case study considers a hypothetical situation in which several products are considered to be at risk for melamine contamination, and a series of decisions must be made about how to set testing priorities to understand the extent of contamination. The case study is built on events that occurred in 2007 and 2008. Some of the key issues associated with potential melamine contamination and the chronology of events that took place are described below.
Melamine and Melamine Contamination in Human Food and Animal Feed
Melamine is a common stable chemical used to produce a variety of materials, including resins, laminates, glues, adhesives, coatings, and flame retardants (WHO 2008). It is a residual byproduct of metabolism of the pesticide cyromazine in plant and animal tissues (Lim et al. 1990; FAO/WHO 2010). Cyromazine is not approved for use in the United States but has approval in other countries that might export plant or animal products. Melamine contains relatively high nitrogen content (66% by mass), and this property has been exploited by some to raise nitrogen concentrations in products tested with conventional nitrogen-detection methods. Specifically, standardized protein-test methods rely on nitrogen concentration as a proxy for protein content, so the high nitrogen content of melamine can be used to increase the tested protein content of a food artificially.
Melamine and its analogues have been found to have low oral toxicity in laboratory animals. Short-term exposures to melamine require high doses (LD50, about 3 g/kg in rats) to cause acute toxic effects (WHO 2009). The toxic effects appear to be restricted primarily to the kidney and bladder. Despite its low toxicity in laboratory animals, melamine has been associated with several outbreaks of toxicity in humans and companion animals. In animals, the consumption of feed that contains melamine and cyanuric acid has been shown to cause nephrolithiasis (kidney stones) and renal failure at much lower doses than would occur with either chemical alone (Puschner et al. 2007).
China is recognized as the leading manufacturer of industrial melamine in the world and uses it in a wide array of products. One of the byproducts of industrial melamine production is a less concentrated version known as melamine scrap. Melamine scrap is reported to contain impurities, including cyanuric acid, which may increase its potential toxicity in humans and animals (Bradley 2008a,b). Recently, it has been reported that melamine scrap has been used in abundant quantities for years as a “protein powder” to boost the nitrogen values of livestock feedstuffs produced in China (Reuters 2008). Furthermore, similar protein powder concoctions have allegedly been added directly to liquid milk to ensure that the milk passes protein-quality standards (Ma 2008).
Timeline of Events
Melamine made headlines in the United States in 2007 when numerous pets presented with illnesses related to renal malfunction or failure and sudden death. Initially, the widespread occurrences were sufficiently disparate in location, food sources, and presentation as not to manifest a singular clinical picture of food toxicity. However, as the number of affected dogs and cats rose above an estimated 1,000, veterinary diagnostic laboratories and FDA became involved in investigating the root cause of the clinical presentations. As the search for causation narrowed, melamine and cyanuric acid were identified in pet-food
samples received from owners of the animals that had toxicosis. Multiple pet-food manufacturers determined that pet food that they produced and marketed was adulterated with melamine and cyanuric acid, and they instituted recalls. The contamination was judged to be the cause of the clinical manifestations in affected dogs and cats and was consistent with an earlier outbreak in Asian dogs and cats reported in the literature (Brown et al. 2007). Ultimately, over 150 brands of dog and cat foods were associated with the contamination.
Further investigation of the event found that wheat gluten and rice-protein concentrate that originated in China were the sources of pet-food adulterants. Food-microscopy analyses indicated that melamine crystals had been used to spike the protein concentrations to acceptable commercial standards. As a result of publicity about the investigation, a separate source of melamine was found in selected domestically produced animal and aquatic feeds (Itchmo 2007; Tembec BTLSR Inc. 2007). Two companies were selling a pellet binder in aquatic feeds that contained low melamine concentrations, both domestically and internationally, and products were withdrawn from U.S. markets on discovery of the contamination. That event demonstrated that potential contamination was not limited to products directly from China. Therefore, investigations of other feed ingredients, both imported and domestic, were appropriate to ensure the safety of pet foods and food-animal feeds that could be manufactured with such ingredients.
In September 2008, news from China indicated foodborne toxicosis in young children caused by melamine contamination of infant formula. On December 1, 2008, the Chinese Ministry of Health reported that over 290,000 children were being treated for clinical signs of toxicosis; 51,900 children were hospitalized, and six deaths were confirmed (Gossner et al. 2009). An investigation by the Centre for Food Safety in Hong Kong indicated that 99% of children who exhibited clinical signs were less than 3 years old (CFS 2008). Investigations indicated that likely sources of contamination were dried dairy products made from milk produced in China. It was determined that milk was being diluted with water and that melamine was being added to increase the nitrogen (protein) concentration to hide this diversion. Because the practice was reported to be relatively widespread in rural China (an estimated 20% of dairies in the country were involved), large quantities of milk and milk products were potentially contaminated. Baby formula was the first product to be identified because of the toxic effects observed in children exposed to contaminated brands of formula. However, a wide array of other foods—including cookies, biscuits, confections, milk-flavored instant coffee and tea, and other products—that contain powdered milk as an ingredient were suspected of melamine contamination.
DECISION CONTEXT FOR THE CASE STUDY
For this case study, the committee assumed that an analytic laboratory receives many demands from field investigators for melamine testing in two prod-
ucts: infant formula and animal-feed ingredients. Extensive testing of any one of the product types, using the testing methods available, would overwhelm available laboratory resources. There is strong circumstantial evidence that one or more products may be at risk for contamination, but the sources and extent of contamination are unknown at the outset. Before other FDA resources are redirected or outside laboratories are engaged, some sense of the magnitude of the problem must be ascertained. This is an example of a targeting decision: FDA must decide where, among competing demands, it should allocate resources when all priorities cannot be addressed simultaneously. Follow-up decisions could include both mitigation-selection decisions, in which FDA decides which (if any) actions the agency will take to reduce or mitigate the identified risks, and, in the longer term, strategic-investment decisions, in which FDA decides whether to invest resources in improved testing methods, increased surveillance, or similar efforts devoted to understanding the identified risks.
The case study examines the first type of decision (a targeting decision) in two steps. The first step is to characterize and compare the public-health consequences associated with potential contamination of the two products. That example is fully developed through the evaluation and completion of the attribute table. The second step is to consider how laboratory resources should be allocated given the understanding of risk magnitudes from the comparison. The approach that one could take to addressing the second step is described but is not fully developed.
CHARACTERIZING THE PUBLIC-HEALTH CONSEQUENCES
For this case study, the committee attempted to rely on information that would have been available to FDA laboratory personnel at the time of the historical event in 2007-2008. The products discussed in this case study—infant formula and animal-feed ingredients—are credible sources of melamine in 2008; at that time, the potential for melamine in liquid milk was not known. In characterizing the public-health consequences, the committee relied on general, publicly available information about the population size and food consumption patterns, as described below. The melamine-specific information that was needed to develop the case study included estimates of the probability and concentration of melamine in each food type and the human dose-response relationship. Those factors were highly uncertain, and the committee struggled to find descriptions of the data that would have been available to FDA in 2008. In developing the detailed estimates described below, the committee consulted two primary sources. First, melamine concentrations found in infant formula in China in 2008, as reported in Gossner et al. (2009), were used as a basis of estimates of the potential concentrations in formula. Second, the study Interim Safety and Risk Assessment of Melamine and Its Analogues in Food for Humans (FDA 2008) provided the basis of most of the assumptions about the human risk asso-
ciated with contaminated animal feeds and the information to hypothesize a dose-response relationship.
The lack of data and the complexity of the situation being evaluated required the committee to make informed judgments, assumptions, and a variety of calculations to characterize the public-health consequences of potential contamination of the two products. Those judgments, assumptions, and calculations are described below. Table 6-1 summarizes the risk characterization in terms of the attributes.
Infant Formula
Exposed Population
About 4 million children are born in the United States each year, and most of them consume at least some formula during the first year of life. Those younger than 6 months old are at greatest risk from contaminated formula because their primary dietary sources are exclusively baby formula or baby formula and breast milk.1 After 6 months, most children begin to reduce formula consumption as their diet shifts to cereals and other solid foods; after 1 year, most children have transitioned away from formula. Table 6-2 shows the number of children in an annual birth cohort that fall within various age groups, the percentage of children in each age group who consume infant formula at least once a day, and the resulting number of children annually who consume infant formula at least once a day.
However, the committee defined the exposed population for this case study as consisting of children consuming infant formula that is potentially contaminated with melamine, not simply all children consuming baby formula. U.S. baby formula is produced by five prominent manufacturers (over 90% of the market) that, when surveyed by FDA, reported exclusive use of domestic milk products in their formulations. None reported use of imported milk-protein sources in their formulations. Imported baby formula and possibly baby formula from nonsurveyed domestic producers would have been suspect. On the basis of that information, the committee estimated that 2-10% of the total U.S. infant formula supply would have had the potential for some level of melamine contamination; the best estimate was 5% of the supply.
Assuming that the market and consumption patterns are such that an infant’s formula source does not vary substantially over time (that is, there is high brand loyalty), the best estimate of the number of infants exposed to potentially contaminated formula is about 161,000 (5% of 3.23 million) with a range of about 62,000-320,000.
TABLE 6-1 Risk Attributes for Targeting Decision that Spans Food and Drug Administration Centers
|
Attribute |
Metric |
Product type |
|
|
Infant Formula |
Hog-Feed Ingredients |
||
|
Exposed population |
Number exposed to potential melamine contamination |
160,000 (60,000-320,000) |
500,000 (240,000-1,700,000) |
|
|
Populations of concern |
Entire exposed population consists of children under the age of 1 year |
No populations of special concern identified |
|
Mortality |
Number of deaths per year |
6×10−5 (0-0.04) |
0 |
|
Morbidity |
Number experiencing severe adverse health effects per year |
1 (0–630) |
0 |
|
|
Number experiencing less severe adverse health effects per year |
4.5 (0-2,900) |
0 |
|
Number per year experiencing adverse health effects that affect only quality of life |
5.5 (0-3,500) |
0 |
|
|
Personal controllability |
Degree to which a person can eliminate or reduce his or her own risks through voluntary actions |
Before any determination that melamine might be present in infant formula, parents have no ability to control risks to their infants; once aware of the potential problems, 90-95% of parents could avoid potentially contaminated formula. |
Before any determination that melamine might be present in pork products, consumers have no ability to control those risks; once aware of the potential problem, 80-90% of consumers could avoid potentially contaminated food products. |
|
Ability to detect adverse health effects |
Ability to detect unexpected population-level adverse effects |
Adverse health effect rates would have to be 10-100 times higher than expected to be detected and correctly attributed. |
Not detectable |
|
Ability to mitigate adverse health effects |
Probability that an informed institution will be able to reduce or mitigate any adverse health effects associated with the product being evaluated if such a problem is known to exist |
90-100% |
10-50% of effects associated with contaminated pork products could be mitigated before the contamination source is identified; 90-100% of future problems could be mitigated once the contamination source is known. |
TABLE 6-2 Formula Consumption by Age
Mortality and Morbidity
Although melamine is not regarded as highly toxic, continuous exposure to melamine-contaminated formula could result in nephrolithiasis in a portion of the exposed population. On the basis of the Chinese experience in 2008, the mortality rate is likely to be low. However, any estimate is highly uncertain because of the lack of historical data and a poor understanding of the dose-response relationship, the effect that other contaminants (such as cyanuric acid) might have on overall toxicity,2 consumption patterns, and other factors, such as cultural practices, that might affect overall toxicity. Rough estimates of the number of deaths that could result from melamine contamination in infant formula can be calculated as follows:
(6-1)
where d represents dose, and the integration is over the entire range of potential doses; Pr[death|d] represents the dose-response relationship as the probability that a person who receives dose d will die; and Nd is the number of infants who receive that dose. The number who would experience each type of health effect (severe adverse effects, less severe effects, or adverse quality-of-life effects) can be estimated similarly by substituting the probability of that effect as a function of dose for Pr[death|d] in Equation 6-1.
The critical factors in the calculation are the number of children who receive various doses and the dose-response relationship. Each factor is highly uncertain, but the uncertainty can be estimated or modeled. The discussion below illustrates how a relatively quick analysis of the factors might be conducted; more rigorous analyses could be conducted with more time, effort, and data.
Dose Estimates
Estimating the number of children who receive various doses of melamine through contaminated formula requires estimates of each of the following:
-
Total number of infants who consume potentially contaminated infant formula (Nd as shown above).
-
Level of contamination in the formula.
-
Daily intake of formula relative to body weight.
At the time of this hypothetical study, little was known about the extent of melamine contamination in U.S. infant formula, that is, the amount of formula contaminated or the melamine concentration in any contaminated formula. (For this case study, it is assumed that all concentrations shown in parts per million are for the formula powder that is added to water, not the liquid that is consumed. Once mixed with water, formula constitutes about 10% of the liquid.) The World Health Organization (WHO 2009) reported concentrations up to several thousand parts per million in some of the Chinese formulas in 2008. Gossner et al. (2009) reported data that showed that the melamine-contaminated formula in 18 of 22 cases had concentrations less than 100 ppm. For the purpose of this study, the concentrations in contaminated dry formula in the United States are uncertain and assumed to be 0-200 ppm with 10 ppm as a most likely value. The committee notes that this analysis could be easily re-run for different assumptions as to the range of possible values.
Formula consumption varies with age, body weight, and infant eating habits, as shown in the first four columns of Table 6-3 (Family Education 2010). It is important to note that the amount of formula consumed begins to decrease after the age of 3-6 months as other foods are added to children’s diets. Calculating the melamine dose to the exposed population requires combining the estimated concentration of melamine in the formula and the amount of formula consumed relative to body weight. Table 6-3 shows an example calculation of the
dose in milligrams per kilogram of body weight per day based on an assumed concentration of 100 ppm in dry formula (diluted to 10 ppm in liquid formula) and the body weight and formula consumption estimates shown.
Table 6-3 shows, for example, that a 1-week-old consuming formula contaminated at 100 ppm in the dry formula will most likely ingest melamine at 1.5 mg/kg per day, but it could be as high as 2.6 or as low as 0.5 mg/kg per day depending on eating habits and infant weight. After the time that this hypothetical study would have been conducted, WHO set the tolerable daily intake (TDI) at 0.2 mg/kg per day (WHO 2009). The committee assumed that the information used to support that determination would have been available at the time of the study.
Table 6-3 shows the range of dose for a single concentration of melamine in dry formula (100 ppm) for illustrative purposes; the actual concentration is uncertain. Including uncertainty about the concentration increases the uncertainty of the estimated dose received by the exposed group. For example, for a single exposed child 1-3 months old, assumed to be of average weight (6 kg) and consuming an average amount of formula per day (31 oz), a concentration of 100 ppm in dry formula leads to a dose of 1.46 mg/kg per day, as shown in Table 6-3. However, if uncertainty in the concentration in formula is included in the calculation (that is, a distribution with 10 ppm as the best estimate and a range of 0-200 ppm), the estimated dose for that child is now a distribution with a median of 0.15 mg/kg per day and a range of 0-2.9 mg/kg per day.
TABLE 6-3 Estimated Dose for Infants Consuming Formula with Melamine at 100 ppm in Dry Formula
|
Age |
Formula per day, oz |
Average infant weight, kg |
Melamine consumed per day, mg |
Daily dose of melamine, mg/kg |
||||
|
Min |
Max |
Min formula consump |
Max formula consump |
Min formula consump |
Max formula consump |
Median |
||
|
1 week |
6 |
30 |
3.3 |
1.70 |
8.51 |
0.52 |
2.58 |
1.55 |
|
1 week to 1 month |
14 |
32 |
5 |
3.97 |
9.07 |
0.79 |
1.81 |
1.30 |
|
1-3 months |
20 |
42 |
6 |
5.67 |
11.91 |
0.95 |
1.98 |
1.46 |
|
3-6 months |
24 |
35 |
7.8 |
6.80 |
9.92 |
0.87 |
1.27 |
1.07 |
|
6-9 months |
21 |
32 |
9.2 |
5.95 |
9.07 |
0.65 |
0.99 |
0.82 |
|
9-12 months |
21 |
24 |
10.2 |
5.95 |
6.80 |
0.58 |
0.67 |
0.63 |
Repeating that calculation for all the uncertainties described above is easily done with a simple Monte Carlo simulation model. Such a model3 was developed to generate an estimate of the daily intake of melamine by an exposed infant. Figures 6-1 and 6-2 illustrate the results of the calculation. Figure 6-1 shows the median and 5th and 95th percentiles of the estimated intake by infants in each of six age groups, incorporating uncertainties in melamine concentration in formula and amount of formula consumed in each age group. The uncertainty is greatest for the youngest children, and both the median intake and the uncertainty in the intake decrease for children over 3 months old as their formula consumption decreases and body weight increases. For comparative purposes, the figure also shows the WHO TDI of 0.2 mg/kg per day. The median intake estimated for all age ranges is below the TDI, but the 95th percentile estimate is significantly higher. Figure 6-2 provides the full distribution of intake, displayed as an inverse cumulative distribution function (CDF). The inverse CDFs for various infant ages show the probability of exceeding different doses. For example, looking at a dose of 1 mg/kg per day on the x axis and tracing up to the line for children from birth to the age of 3 months, one can see that there is a 0.2 probability (20% chance) that a child in that age range consuming contaminated formula will have a dose that exceeds 1 mg/kg per day. The probability that a child 9-12 months old (the lowest line on the figure) would exceed 1 mg/kg per day is lower, about 0.05.
The final step in estimating the number of infants receiving each dose is to combine the number of exposed children in each age group (which is also uncertain) with the distribution of dose by age shown above and then aggregate across the age groups. Figure 6-3 shows the distribution of the total number of infants exceeding specified doses measured in milligrams per kilogram per day. For example, as shown in the figure, about 70,000 infants every day consume melamine at 0.1 mg/kg or more; the number could be as low as 0 or as high as 290,000.
Dose-Response Relationship
At the time this study would have been conducted, little was known about the dose-response relationship of the effects observed in infants and melamine consumption. In China, where melamine concentrations ranged from less than 1 ppm to over 1,000 ppm, Gossner et al. (2009) stated that there were 294,000 cases of renal illness, including 51,900 that required hospitalization, and at least
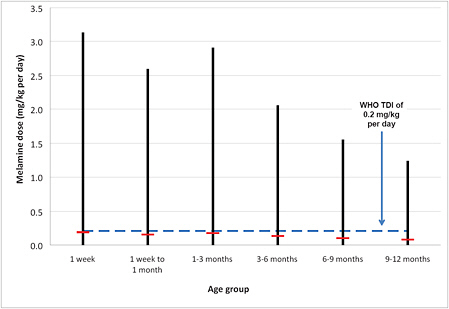
FIGURE 6-1 Median (red dash) and range (black vertical line) of estimated melamine dose for an exposed infant of various ages given uncertainties in melamine concentration in formula, infant weight, and formula consumption. Blue dashed line represents tolerable daily intake (TDI) from the World Health Organization (WHO).
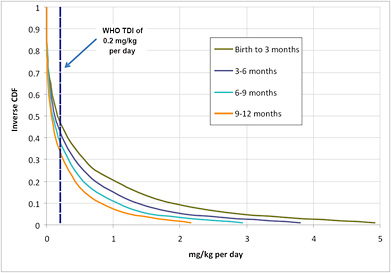
FIGURE 6-2 Distribution of estimated melamine dose for an exposed infant of various ages given uncertainties in melamine concentration in formula, infant weight, and formula consumption. CDF = cumulative distribution function. Blue dashed line represents tolerable daily intake (TDI) from the World Health Organization (WHO).
six deaths. The number of infants that were exposed to the high concentrations is not known, so precise risk calculations are impossible, but the ratios of deaths to hospitalizations to less severe renal disease is useful. Lack of historical and scientific data on the long-term effects of infant morbidity and on lesion severity makes characterizing the health effects of the renal illnesses equally difficult and highly uncertain. Depending on the melamine dose, the frequency of exposure, and the percentage of formula affected, a substantial part of the cohort might be involved. However, if high concentrations were present in formula in the United States, sick infants would soon start appearing in doctors’ offices and emergency rooms, and there would be a quick response, so repeated exposure to large amounts is not expected; this is described in following sections that discuss mitigation opportunities.
A hypothesized dose-response relationship between melamine dose and the percentage who would experience adverse health effects at that dose is shown in Figure 6-4. The actual values are highly uncertain, and the hypothesized relationship is intended only to provide a starting point for further analyses. The estimated distribution of the types of effects (that is, the ratios of deaths to severe adverse effects to less severe adverse effects) is based loosely on the data from China, assuming that the ratios are essentially constant over exposure and that for every infant who experiences clinical signs of renal disease another suffers from discomfort or some other effect that diminishes quality of life. The overall estimate of the rate of any adverse effects is consistent with an assumption that if exposure is 10 times the WHO TDI of 0.2 mg/kg per day, less than 5% of the population would experience any adverse effects. The dose-response
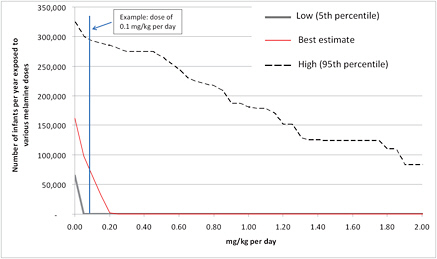
FIGURE 6-3 Distribution of the number of infants exposed to various melamine doses. Red line shows the median estimate of the number of infants who receive at least the indicated dose. Dashed and gray lines show the high and low estimates, respectively.
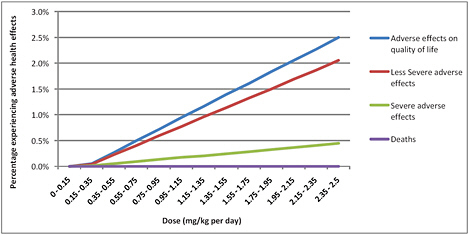
FIGURE 6-4 Dose-response relationship for health effects of melamine consumption used for illustrative calculations.
relationship shown here—given the assumption that response can be extrapolated linearly as a function of dose—produces the same magnitude of adverse effects as occurred in China if 4 million infants consumed formula adulterated with melamine at 200 ppm in liquid form, a concentration substantially higher than the concentrations hypothesized in this case study.
Number of Deaths and Other Adverse Health Effects
The distribution of health outcomes can be estimated by using the logic of Equation 6-1 and the estimated dose distribution described above with the dose-response relationship. Table 6-4 shows the results of that calculation. The best estimates are that the total number of adverse health effects of any sort would be around 10, and that deaths would be extremely unlikely. At the high end, it is possible that thousands of cases of renal disease requiring medical attention would occur; deaths would still be very rare.
TABLE 6-4 Estimates of Mortality and Morbidity from Melamine Contamination in Infant Formula for the Hypothesized Dose-Response Relationship Incorporating Uncertainty in Exposure and Dose
|
Type of Effect |
Low |
Median |
High |
|
Affecting quality of life only |
0 |
5.5 |
3,500 |
|
Less severe adverse effects |
0 |
4.5 |
2,900 |
|
Severe adverse effects |
0 |
1.0 |
630 |
|
Death |
0 |
6 × 10−5 |
0.04 |
Personal Controllablity
To be able to control the risks to their children from potentially contaminated formula, parents must be aware that risks exist and must have the ability to exercise options to avoid or reduce the risks. In this case study, the main issue is whether parents are aware that a small portion of the total supply of infant formula is potentially contaminated. There is no way to detect contamination on the basis of a physical examination of the product itself, so such awareness would have to come from a determination and an announcement of potential contamination from a public-health agency. At the time this study would have been conducted, it was not known whether such contamination existed, so parents of the potentially exposed children would have had virtually no ability to control the risks. Once aware of the potential health consequences, parents could take steps to avoid potentially contaminated formula relatively easily. If the problem were isolated to a small number of identified formula manufacturers, the availability of formula from other manufacturers would allow virtually all parents to select uncontaminated formula.
Ability to Detect Adverse Health Effects
The probability that a single case of nephrolithiasis caused by melamine-contaminated infant formula would be detected depends on the severity of the illness. According to the American Society of Pediatric Nephrology, signs and symptoms of melamine poisoning in infants include the following:
-
Unexplained crying in infants, especially when urinating.
-
Vomiting.
-
Unexplained fever due to urinary tract infection caused by urinary obstruction.
-
Blood in the urine.
-
Stones discharged while urinating.
-
Signs of renal infection.
-
High blood pressure.
-
Elicitable flank pain.
Detecting population-level effects resulting from melamine contamination of infant formula at the hypothesized level and correctly attributing the cause of the effects would be difficult. The presence of kidney stones in infants would not necessarily trigger concern about melamine contamination, but if multiple children developed kidney stones with no other risk factors, the problem would probably be detected. However, the ability of institutions to detect a problem, assuming that one exists, would probably be somewhat hindered because the contaminated formula would likely be geographically dispersed and the vast majority of adverse health outcomes would be minor.
In the case of the melamine-contaminated pet food in 2007, it was estimated that by the time the problem was identified, about 100 pets had died and about 500 had suffered kidney failure (AP 2007); some estimates of the number of deaths exceeded 4,000 (Weise and Schmit 2007). The committee assumed that unexpected cases of kidney stones in infants would be recognized as a problem much sooner than adverse health effects in pets. Thus, the committee estimated that adverse effects occurring 10-100 times more frequently than expected would be detected (for example, 50-500 children with kidney stones serious enough to require medical attention would be a sufficiently strong signal to health authorities that there was a systematic problem).
Ability to Mitigate Adverse Effects
In this framework, the ability to mitigate refers to the ability to manage, reduce, or otherwise control any adverse health effects of the products being evaluated, assuming that such effects occur and are detected. The manufacture of infant formula is well understood and controlled, although its distribution and consumption are less so. As discussed in Chapter 4, the effectiveness of a recall depends on several factors. However, the committee assumes that the recall of infant formula given the population potentially affected would be much more effective than, for example, the recall of canned foods. There would most likely be a highly visible media campaign to alert parents. Furthermore, because the potentially exposed population receives frequent medical check-ups, pediatricians would certainly alert parents to the potential dangers. Therefore, the committee assumes that recall procedures would be quick and effective and that the prompt notification of wholesale and retail outlets and of the general public would lead to a high probability of mitigation (90-100%).
Animal-Feed Ingredients
The second product of interest for this case study is animal-feed ingredients. There is potential for melamine to enter the human food supply through contaminated animal feeds. That is, if animals raised for human consumption eat feed that is contaminated with melamine, melamine may accumulate in the animal’s flesh and ultimately be eaten by humans. The potential exposure pathway for animals is a feed ingredient that is adulterated with melamine to disguise protein deficiencies; this was the case with some pet foods. Hogs are sometimes fed excess (scrap) pet food as an inexpensive addition to their diets. Because melamine contamination of pet food was known to have occurred, the exposure pathway considered most likely and evaluated here is from contaminated pet foods being fed to hogs raised for human consumption.
When melamine contamination in pork was first suspected, each step in the pathway from contaminated animal feed to human consumption involved great speculation and uncertainty. To clarify the situation, a series of studies and
laboratory tests were conduced that resulted in a 2007 report, Interim Melamine and Analogues Safety/Risk Assessment, that greatly reduced the concerns associated with this pathway (FDA 2007). The discussion presented here is based on what was known and believed at the time that report was written.
Exposed Population
The exposed population is defined as the number of people in the United States who could consume pork that is contaminated with detectable melamine. That calculation requires estimating the size of the population that consumes pork and the percentage of the total pork supply that could be contaminated with melamine.
The U.S. per capita pork consumption has been steady, averaging 1 lb/week, and about 80% of the U.S. population regularly consume some form of pork products (FDA 2007). Only a very small portion of the U.S. hog supply is fed excess pet food, the only hypothesized route to melamine contamination. In 2010, the U.S. hog and pig inventory was 64 million head (USDA 2010). Over the last 2 decades, the number of hog farms has been sharply reduced—from over 650,000 farms in 1980 (NHF 2005) to 74,000 in 2009 (USDA 2009)—as the size of the farms has dramatically increased. Table 6-5 shows the concentration of production on large farms (over 40% of hogs are produced on 0.1% of the farms). The large-scale operations are highly efficient and tightly controlled operations and are unlikely to supplement their animal feed with excess (scrap) pet food. For this analysis, the problem focuses on the numerous smaller farms that make up a few percent of the market where operations are less regimented and some low-cost excess pet food could be added to the animals’ diet.
On the basis of expert judgment, the committee estimated that less than 1% of the pork that makes it to market would come from hogs that were fed pet food contaminated with melamine (0.001% of U.S. hogs is the low estimate, 0.2% is the median, and 0.7% is the high estimate). If 80% of the U.S. population of 310 million is assumed to eat pork, and 0.2% of the market consists of hogs that may have been exposed to melamine through consumption of excess pet food, hundreds of thousands of people have the potential to be exposed to melamine-contaminated pork (240,000-1,730,000, with a best estimate of 496,000).
Mortality and Morbidity
Estimating the potential extent of the problem in terms of deaths and other adverse health effects that might result from contaminated animal feeds is a multistep process. It follows the same logic used to estimate the potential health effects of melamine in infant formula described above. Estimating the mortality and morbidity requires estimates of the amount of melamine that would be consumed by members of the exposed population and the long-term health effects
of melamine in humans (the dose-response relationship). However, several extra steps are also necessary. For example, to estimate the amount of melamine consumed by one person who is assumed to consume pork that has been fed melamine-contaminated scrap pet food requires the following estimates:
-
Amount of melamine present in the animal feed.
 Melamine concentration in scrap pet food.
Melamine concentration in scrap pet food. Fraction of animal diet that is scrap pet food.
Fraction of animal diet that is scrap pet food. -
How melamine from pet food accumulates in animal flesh.
-
Amount of contaminated pork consumed.
Each of those factors is uncertain; they are explored in order below.
The committee estimated the melamine concentration in the diet of a single hog by multiplying the melamine concentration in the pet food consumed by the fraction of the diet that was assumed to be pet food. For this case study, the committee estimated that the melamine concentration in the contaminated pet food could range from 100 ppm to 15,000 ppm with a median of 1,000 ppm. The diet of hogs that receive any contaminated scrap pet food would vary considerably. The committee estimated that most would receive the contaminated pet-food supplement as a small portion of their total lifetime diet: 2% of diet is the low estimate, 35% is the median, and 100% is the high estimate. So, for example, a hog consuming 35% of its diet as pet food that is contaminated at 1,000 ppm would have a net concentration of 350 ppm melamine in its diet. The committee combined the uncertainties in diet composition and melamine concentrations in the feed with uncertainty in the number of hogs consuming any contaminated pet food in a simple Monte Carlo simulation model to estimate a distribution of the number of hogs consuming feed with different net concentrations of melamine. All factors were assumed to be uncorrelated.
TABLE 6-5 Concentration of Hog Production in 2006
|
Number of Head Marketed |
Number of Farms |
Percent of Farms |
Percent of Market |
|
Under 1,000 |
48,434 |
86.1 |
1 |
|
1,000-2,999 |
4,025 |
7.1 |
5 |
|
3,000-4,999 |
1,150 |
2 |
3 |
|
5,000-9,999 |
1,100 |
1.9 |
6 |
|
10,000-49,999 |
1,450 |
2.6 |
21 |
|
50,000-499,999 |
164 |
0.3 |
21 |
|
500,000+ |
27 |
0.1 |
43 |
|
TOTAL |
56,350 |
100 |
100 |
|
Source: Adapted from Pork Checkoff 2009, p. 87. |
|||
Figure 6-5 illustrates the resulting distribution of the number of hogs consuming a diet with various net concentrations of melamine. It shows the number of hogs consuming diets with melamine exceeding different concentrations. For example, looking at a concentration of about 5,000 ppm on the x axis and tracing up to the “best estimate” line, one can see that about 65,000 hogs will be consuming melamine at over 5,000 ppm in their diets. The 5th and 95th percentile lines indicate that the number of hogs can range from 20,000 to 150,000.
Given the melamine concentration in the feed, the next step is to estimate the melamine concentration in the animal tissue. On the basis of reports written after the contamination scare, the committee assumed that researchers believed that feed concentrations of less than 500 ppm would result in tissue concentrations less than the limit of detection of 50 ppb, or that tissue concentrations in the pork would be about 0.0001-0.001 net concentration in the food consumed by an animal. Combining the conservative factor of 0.001 with the estimated distribution of the number of hogs consuming foods with various concentrations shown in Figure 6-5 results in estimates of the numbers of hogs with various melamine concentrations in their tissues.
Figure 6-6 illustrates the results of the calculation. Hogs consuming melamine in their diet at a net concentration of less than 500 ppm are assumed not to have any detectable melamine in their tissues. Figure 6-6 groups the hogs that have detectable melamine into 1,000-ppb bins and shows the best estimate (the red marker) and the range of the number of hogs or pounds of pork product with those concentrations that are assumed to reach the market. For example, about 60,000 hogs (12 million pounds of pork) with melamine at 2,000-3,000 ppb would be expected, but the number could range from 20,000 to 120,000.
In the 2007 report on melamine risk, FDA estimated the consumption of pork and pork products that are contaminated with melamine at 100 ppb to deliver a dose of 0.04 μg/kg per day to the average person and 0.10 μg/kg per day to the 90th-percentile person. Extrapolating those values linearly with concentration to the distributions shown in Figure 6-6 yields the estimates shown in Table 6-6.
The last step in the multistep process requires estimating the human-health consequences of the doses described above. In its 2007 report on melamine risk, FDA specified a TDI of 0.63 mg/kg per day (630 μg/kg per day). In 2008, WHO established a lower TDI of 0.2 mg/kg per day (200 μg/kg per day). The TDI is the maximum amount that a human can be exposed to daily over a lifetime without incurring a measurable increase in health risk. The health effects associated with values above the TDI for melamine are kidney stones and renal disease. The highest dose estimated above (and shown in Table 6-6) is 15 μg/kg per day—1/13 the WHO TDI and 1/40 the FDA TDI. On the basis of that comparison, the facts that the TDI incorporates a safety factor of 100 and that a lifetime of contaminated pork consumption is not realistic given the history of the problem, the committee assumed that no adverse health effects would occur. In its preliminary analysis of risk, the committee did not include all the uncertainty, but even adding several layers of uncertainty to the hog or human population
calculations would not push the risk to a level at which adverse health effects would be expected; it would only increase the size of the population exposed to low doses of melamine.
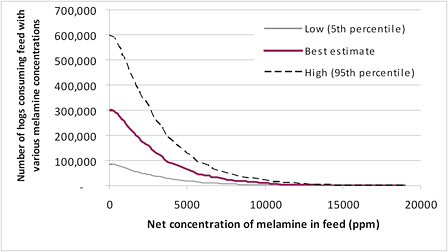
FIGURE 6-5 Distribution of hogs consuming feed with various melamine concentrations.
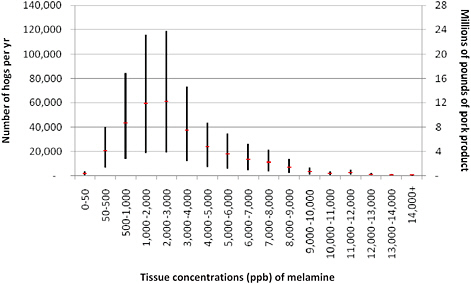
FIGURE 6-6 Distribution of number of hogs (left scale) and pounds of pork product (right scale) with various melamine tissue concentrations. A range and a median value are shown for each concentration.
TABLE 6-6 Melamine Doses to Humans Who Consume Pork with Various Melamine Tissue Concentrationsa
Personal Controllability
As in the case with infant formula, the first element necessary for personal controllability—knowledge that a potential risk exists—is lacking in the case of melamine contamination in pork products. There is no way for a consumer to detect directly that contamination exists, so individual knowledge of a potential problem is possible only after institutions detect and announce that such a problem is possible. At the time of this case study, no such determination had been made. If the possibility of contamination in pork products becomes known, consumers can eliminate the risk of melamine-contaminated food through appropriate decisions concerning shopping and food consumption. Direct consumption of pork could be avoided relatively easily. The problem is more difficult with prepared and processed foods. For some products, the pork content may be discernable only by a close examination of the food label. Nevertheless, a vigilant, knowledgeable consumer could avoid all pork. It is estimated that 80-90% of typical consumers could avoid pork products if they were aware that a contamination problem were possible.
Ability to Detect Adverse Health Effects
Detecting any problems that might occur from human consumption of pork contaminated with melamine as described in this case study would also be extremely difficult. The estimated health effects of such potential contamination are estimated to be so low as to be indistinguishable from the base-rate occurrence of kidney stones and renal disease. The lifetime rate of the occurrence of kidney stones in the United States is 5-9%, and about 2 million doctor or hospital visits per year are related to kidney stones (NIDDK 2010). Even if the effects were much higher than estimated here, detecting them and correctly attributing them to contaminated pet food used as hog feed would be very unlikely. The committee estimated that the annual number of doctor and hospital visits for kidney stones would have to increase by 50-100% (1-2 million additional visits) before such an increase would be noticed and raise questions about what the cause might be. Given the estimated size of the exposed population (500,000, with a range of 240,000 to 1.7 million), even if all the members of the exposed population suffered from kidney stones serious enough to require medical attention, the effect is unlikely to be detected.
Ability to Mitigate Adverse Health Effects
Once a problem is detected, steps can be taken to ensure that contaminated ingredients are no longer used in animal feed. The use of pet food as a supplement is not widespread, and the producers that do use the supplement could be readily identified and notified, so mitigating risks by preventing future use of contaminated animal feed would be relatively easy. Tracking down the contaminated pork products and removing them from market shelves would be more difficult. If the contaminated hogs are mixed with unaffected hogs across a wide geographic area, massive recalls would be required for all the contaminated meat to be removed from the market. If the contaminated hogs are butchered and packaged in isolated batches, a recall could be tightly focused and very effective, but this is not likely to be the case.
In late 2008, dioxin contamination was found in pork fat in Ireland as a result of routine residue testing. In that case, the farm from which the pigs came was known, and follow-up testing quickly identified the source of the problem as contaminated feed. Within a week, all pig and cattle farms that received similar feeds had been identified. However, because they were unable to trace pork products on the market to specific farms, ultimately a full recall of all pork products was issued less than 2 weeks after contamination was found (Casey et al. 2010). The full recall minimized the chance of exposures to dioxin in pork after the problem was identified. The committee assumed that a similar scale of recall might be necessary in the United States to mitigate fully the adverse health effects of melamine in pig feed if such contamination were found.
As discussed in the case study on foods, recalls have limited effectiveness in preventing the recalled product from being consumed; in general, foods with a long shelf-life are easier to recall than those with a short shelf-life, and foods with a well-understood supply chain and those stored at centralized locations for a relatively long time are easier to recall. The distribution of pork products in the United States probably most closely resemble that of shrimp as discussed in Chapter 4. So the committee estimated that 10-50% of the potential adverse effects associated with pork already contaminated at the time that the possibility of adverse effects was recognized could be mitigated through recall.
USING THE RISK CHARACTERIZATION TO SUPPORT DECISION-MAKING
Table 6-1 summarizes the characterization of public-health consequences of potential melamine contamination of infant formula and animal feeds. It shows that, given current understanding and uncertainty, the scenario of contaminated infant formula leads to higher public-health consequences than the one of pork products contaminated from animal feed. Although the likelihood and concentration of melamine in animal feed are higher than those in infant formula, infants consume formula directly, whereas the melamine in animal feed has to be sequentially absorbed in two animal systems before posing a risk. Fewer people are likely to suffer adverse health effects from melamine contamination of animal feeds than from contaminated infant formula, but the ability to detect and mitigate such problems is lower for animal feeds than for infant formula.
The risk characterization described is an illustration of the evaluation that could be used to rank potential risks: it compares the effects of potential contamination in various products by using a consistent set of metrics. A ranking alone, however, is often not sufficient to support decisions about the next step: about what actions should be taken to reduce risks or, in this example, to understand the extent of the risks better. An extension of the analysis above could be conducted, assuming that there is an interest in conducting laboratory tests of infant formula and animal feed to determine whether melamine contamination is present and, if so, at what levels.
For purposes of illustration, the committee assumed that resources are sufficiently limited for laboratory testing to be possible for only one of the products—either infant formula or hog feed but not both. FDA must decide which product to focus testing resources on (an example of a targeting decision). If no testing is conducted on either product, the attribute table developed above provides a summary of the expected consequences on the basis of the current estimates of the likelihood of various melamine concentrations. However, the outcomes of a decision to test either product will need to be evaluated to understand fully the implications of the alternative testing decisions.
Assume that testing will indicate either that melamine is present or that it is not present, and further assume that FDA will intervene with a program to mitigate the associated risk if melamine contamination is detected in either infant formula or hog feed. That next step is illustrated in the decision tree in Figure 6-7. The committee notes that FDA could decide to conduct a risk intervention for either or both products without testing, but for the purposes of this case study, the committee assumed that intervention would occur only after testing.
Finally, if an intervention is conducted, the net outcome depends on the effectiveness of the intervention in reducing or eliminating risks, other public-health effects caused by the intervention itself, and the melamine concentrations in the product not tested. Figure 6-8 depicts a single branch of the decision tree in Figure 6-7—testing infant formula only—with the outcomes delineated. Of the four outcomes, only one would require the construction of a new attribute table, "Outcome given formula intervention." In developing the attribute table, it is important to include not only the reduction in the risk of melamine-induced illnesses, but any additional health effects associated with the intervention. For example, pulling large quantities of baby formula from market shelves will greatly increase anxiety and lead to changes in how babies are fed. The public-health consequences of potential contamination of animal feed have already been evaluated in the sections described above, and the public-health consequences of having no contamination in infant formula can be assumed to be zero.
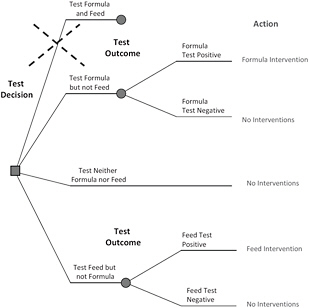
FIGURE 6-7 A decision tree for a testing-with-intervention decision involving infant formula and hog feed.
Actual testing decisions and processes clearly have additional complications. As a final step toward making this description more realistic, one can explicitly incorporate the possibility of a testing error in the decision tree. A false-positive would lead to an intervention for melamine when melamine is not present in the product; a false-negative would lead to no intervention even though melamine is present. Figure 6-9 illustrates that additional consideration for the decision option to test infant formula only.
The upper half of the tree in Figure 6-9 shows the outcomes if the test is positive: a positive test leads to an intervention. If contamination were actually present, the intervention reduces the risks from that contamination. The intervention may carry additional public-health consequences, and it is important to consider all such consequences, including potential adverse effects. For example, the health costs of a formula recall could be large. If no contamination is present, there are no “benefits” (that is, illnesses averted) to balance the increased “costs” of the intervention. The bottom half shows the outcomes if the test is negative; here, the consequences differ, potentially significantly, if the test is accurate or not. In this formulation, a type II testing error (false negative) results in a missed opportunity to mitigate a risk, including all the associated health effects.
There are clearly many decisions associated with the testing procedures that have not been discussed in this simple example. The likelihood of type I and type II errors depends on the parameters of the specific tests conducted, such as sample size and equipment used. Balancing the two types of errors (for example, test sensitivity and specificity) and costs would require an expansion of the decision tree. Ideally a fully developed decision tree with all relevant variables included could be used to complete value-of-information calculations and sensitivity analyses.
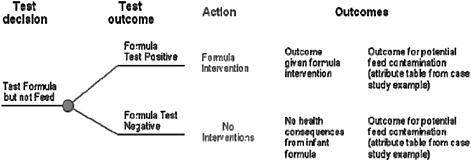
FIGURE 6-8 Potential outcomes of a decision to test infant formula but not animal feed, assuming a test without error, and interventions if the test result is positive.
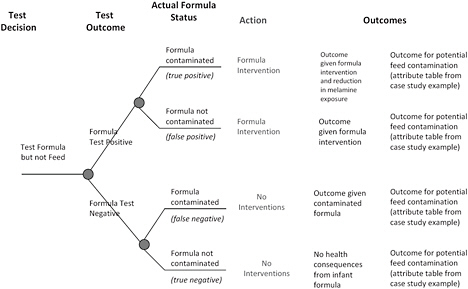
FIGURE 6-9 Tree for the decision to test formula but not feed, including uncertainty about test accuracy.
REFERENCES
AP (Associated Press). 2007. 104 Deaths Reported in Pet Food Recall. New York Times [online]. Available: http://www.nytimes.com/2007/03/28/science/28brfs-pet.html?ex=1176264000&en=8ee0fb91fd221e4b&ei=5070 [accessed March 30, 2011].
Bradley, D. 2008a. Melamine in Milk. Sciencebase.com, September 17, 2008 [online]. Available: http://www.sciencebase.com/science-blog/melamine-in-milk.html [accessed Nov. 8, 2010].
Bradley, D. 2008b. Melamine Scandal Widens. Sciencebase.com, September 22, 2008 [online]. Available: http://www.sciencebase.com/science-blog/melamine-scandal-widens.html [accessed Nov. 8, 2010].
Briefel, R.R., K. Reidy, V. Karwe, and B. Devaney. 2004. Feeding infants and toddlers study: Improvements needed in meeting infant feeding recommendations. J. Am. Diet. Assoc. 104(Suppl. 1):S31-S37.
Brown, C.A., K.S. Jeong, R.H. Poppenga, B. Puschner, D.M. Miller, A.E. Ellis, K.I. Kang, S. Sum, A.M. Cistola, and S.A. Brown. 2007. Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J. Vet. Diagn. Invest. 5(5):525-531.
Casey, D.K., J.S. Lawless, and P.G. Wall. 2010. A tale of two crises: the Belgian and Irish dioxin contamination incidents. British Food Journal. 112(10):1077-1091.
CDC (Centers for Disease Control and Prevention). 2010. Breastfeeding. Frequently Asked Questions. Centers for Disease Control and Prevention [online]. Available: http://www.cdc.gov/breastfeeding/faq/index.htm [accessed Nov.8, 2010].
CFS (Centre for Food Safety). 2008. Melamine. Frequently Asked Questions. Centre for Food Safety, Hong Kong [online]. Available: http://www.cfs.gov.hk/english/whatsnew/whatsnew_fstr/whatsnew_fstr_Test_dairy_product_FAQ.html
[accessed Nov.8, 2010].
Family Education. 2010. How Much and How Often to Formula Feed. Family Education [online]. Available: http://life.familyeducation.com/formula-feeding/baby/39374.html [accessed Nov. 9, 2010].
FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization). 2010. Proposed Draft Maximum Levels for Melamine in Food and Feed (N13-2009). CX/CF 10/4/5. Joint FAO/WHO Food Standards Program, Codex Committee on Contaminants in Foods, 4th Session, Izmir, Turkey, 26-30 April 2010 [online]. Available: http://www.cclac.org/english/committees/cf_e.php [accessed Nov. 9, 2010].
FDA (U.S. Food and Drug Administration). 2007. Interim Melamine and Analogues Safety/Risk Assessment. U.S. Food and Drug Administration [online]. Available: http://www.fda.gov/ScienceResearch/SpecialTopics/PeerReviewofScientificInformationandAssessments/ucm155012.htm [accessed Nov. 9, 2010].
FDA (U.S. Food and Drug Administration). 2008. Interim Safety and Risk Assessment of Melamine and Its Analogues in Food for Humans. U.S. Food and Drug Administration [online]. Available: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Melamine/ucm164522.htm [accessed Nov. 9, 2010].
Gossner, C.M., J. Schlundt, P. Ben Embarek, S. Hird, D. Lo-Fo-Wong, J.J. Beltran, K.N. Teoh, and A. Tritscher. 2009. The melamine incident: Implications for international food and feed safety. Environ. Health Perspect. 117(2):1803-1808.
Itchmo. 2007. FDA Warns Tembec About Violations at Animal Feed Plant. Itchmo: News for Dogs and Cats [online]. Available: http://www.itchmo.com/fda-warns-tembec-about-violations-at-animal-feed-plant-3061 [accessed Nov. 9, 2010].
Lim, L.O., S.J. Scherer, K.D. Shuler, and J.P. Toth. 1990. Disposition of cyromazine in plants under environmental conditions. J. Agric. Food Chem. 38(3):860-864.
Ma, J. 2008. Adding Chemicals to Milk Common: Insiders. South China Morning Post A3, September 29, 2008.
NHF (National Hog Farmer). 2005. Hog Farm Numbers Continue to Fall. National Hog Farmer [online]. Available: http://nationalhogfarmer.com/ar/numbers-fall/ [accessed Jan. 25, 2011].
NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases). 2010. Kidney and Urologic Diseases Statistics for the United States. National Kidney and Urologic Diseases Information Clearinghouse (NKUDIC), National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Health, Bethesda, MD [online]. Available: http://kidney.niddk.nih.gov/kudiseases/pubs/kustats/ [accessed Nov. 9, 2010].
Pork Checkoff. 2009. Quick Facts: The Pork Industry at a Glance. National Pork Board, Des Moines, IA [online]. Available: http://www.pork.org/MediaLibrary/FlipBooks/QuickFacts2010/document.pdf [accessed Nov. 9, 2010].
Puschner, B., R.H. Poppenga, L.J. Lowenstine, M.S. Filigenzi, and P.A. Pesavento. 2007. Assessment of melamine and cyanuric acid toxicity in cats. J. Vet. Diagn. Invest. 19(6):616-624.
Reuters. 2008. Melamine Use “Rampant” in China Feed Business. Reuters, September 25, 2008 [online]. Available: http://www.reuters.com/article/idUSPEK166968 [accessed Nov. 9, 2010].
Tembec BTLSR Inc. 2007. Re: Warning Letter CIN-DO 07-33572-18. Response letter to Carol A Heppe, Director, Cincinnati District, U.S. Food and Drug Administration,
Cincinnati, OH, from Randy Fournier, President, Tembec BTLSR Inc., Toledo, OH. October 3, 2007 [online]. Available: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2007/ucm171217.htm [accessed Nov. 9, 2010].
USDA (U.S. Department of Agriculture). 2009. Overview of the U.S. Hog Industry. National Agricultural Statistics Service, Agricultural Statistics Board, United States Department of Agriculture [online]. Available: http://usda.mannlib.cornell.edu/usda/nass/hogview//2000s/2009/hogview-10-30-2009.pdf [accessed Jan. 25. 2011].
USDA (U.S. Department of Agriculture). 2010. Quarterly Hogs and Pigs. National Agricultural Statistics Service, Agricultural Statistics Board, United States Department of Agriculture [online]. Available: http://usda.mannlib.cornell.edu/usda/nass/HogsPigs/2010s/2010/HogsPigs-12-27-2010.pdf [accessed Jan. 25, 2011].
Weise, E. and J. Schmit 2007. FDA limits Chinese food additive imports. USA Today [online]. Available: http://www.usatoday.com/money/industries/2007-04-30-chinese-imports-usat_N.htm [accessed March 30, 2011].
WHO (World Health Organization). 2008. Melamine and Cyanuric Acid: Toxicity, Preliminary Risk Assessment and Guidance on Levels in Food. World Health Organization [online]. Available: http://www.who.int/foodsafety/fs_management/Melamine.pdf [accessed Nov. 9, 2010].
WHO (World Health Organization). 2009. Toxicological and Health Aspects of Melamine and Cyanuric Acid. Report of a WHO expert meeting in collaboration with FAO supported by Health Canada Geneva: World Health Organization [online]. Available: http://www.who.int/foodsafety/publications/chem/Melamine_report09.pdf [accessed Nov. 9, 2010].




























