Public Health: Protecting the Population
Strategies to promote the health and well-being of an entire population can preserve the health of every individual and family. In diverse ways, ranging from environmental protection to response to natural disasters to nutrition labeling, the field of public health affects the health of people from every walk of life and in every part of the country.
Because of its multiple facets, public health is the most diverse area that the Institute of Medicine (IOM) investigates. An array of government agencies and private organizations concerned with public health regularly depend on the IOM’s findings and recommendations to guide their decisions and actions.
Protecting the nation’s security
Among the broadest challenges the nation faces is protecting its security—and the safety of the population—from both natural disasters and threats linked to hostile human actions. One threat that has emerged in recent years is the possibility that enemies of the nation will attack using biological agents. In response, the Department of Homeland Security (DHS) introduced BioWatch, a federal monitoring system intended to speed detection of specific biological agents in the air. BioWatch air-sampling devices are deployed in more than 30 major cities. The samples typically are tested daily for signs of the agents, and if further laboratory testing confirms their presence, the system generates a signal.
In 2008, Congress directed DHS to ask the IOM and National Research Council (NRC) to convene a committee to evaluate the merits
and costs of both the current BioWatch and the plans for a new generation program, and also to examine more traditional infectious disease surveillance conducted through hospitals and public health agencies.
The current BioWatch system requires better testing to establish its effectiveness and better collaboration with public health systems to improve its usefulness.
In its report, BioWatch and Public Health Surveillance: Evaluating Systems for the Early Detection of Biological Threats (2010), the committee concludes that the current BioWatch system requires better testing to establish its effectiveness and better collaboration with public health systems to improve its usefulness. Also, the proposed enhancements to BioWatch are likely to be possible only if significant and long-standing scientific and technical challenges can be overcome. The report offers a detailed outline of needed actions.
Among actions to improve BioWatch, DHS should lead efforts to systematically test and evaluate current and planned technology to establish a more systematic, scientifically sound, and stakeholder-approved approach to technology acquisition, development, testing, and deployment than was possible when the program began. DHS and other federal agencies also should work to strengthen the relationship between BioWatch and the
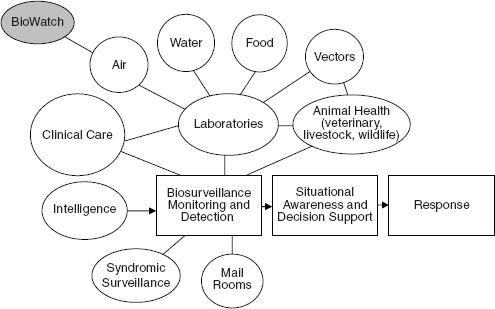
A schematic illustration of the relation between the BioWatch program and other sources of information needed for infectious disease surveillance in the public health and healthcare systems.
SOURCE: BioWatch and Public Health Surveillance: Evaluating Systems for the Early Detection of Biological Threats, p. 174.
state and local jurisdictions in which it operates. Attention should be given as well to making BioWatch more responsive to user needs and better able to aid the timely response to a biological attack, beyond just successfully detecting suspicious materials in the air.
On the public health side, the Department of Health and Human Services (HHS), which has key responsibility in this area, should work with other agencies to develop and evaluate new opportunities in infectious disease surveillance and detection. Also, since early detection of a bio-terrorism event may depend on astute clinicians’ ability to recognize suspicious cases, HHS should promote the development, testing, and evaluation of technologies that strengthen the clinical diagnosis of significant infectious diseases and facilitate timely reporting of outbreaks to public health authorities. At a broader level, HHS and DHS should give high priority to building and sustaining sufficient public health workforce strength and competencies, along with associated laboratory and information management capacities, needed by all states and communities to detect a bioterrorism attack or other public health emergency.
With improvements in both BioWatch and public health infectious disease detection systems, a crucial step will be to link them into a national biosurveillance framework that will provide state and local public health authorities and the healthcare system with the information needed to determine the appropriate response to biological threats.
Preparing for health emergencies
In a major health emergency, whether caused by humans or occurring naturally, thousands or even hundreds of thousands of people across the country may suddenly require and seek medical care. In such emergencies, state and public health officials will turn to crisis standards of care policies that cover how to allocate scarce resources and how care is provided. But standards of care for crisis situations in different jurisdictions tend to be weak, fragmented, or nonexistent. At the request of the Office of the Assistant Secretary for Preparedness and Response in HHS, the IOM convened a committee of experts to consider this problem.
In a major health emergency, whether caused by humans or occurring naturally, thousands or even hundreds of thousands of people across the country may suddenly require and seek medical care.
In Guidance for Establishing Crisis Standards of Care for Use in Disaster Situations: A Letter Report (2009), the committee first defines the conditions under which “crisis standards would be used.” Crisis standards of care represent a substantial change in usual healthcare operations made necessary by a pervasive or catastrophic disaster. A state government must declare that crisis standards of care are in effect, and likely to be effect for a long period, thereby enabling specific legal and regulatory powers and protections for healthcare providers in allocating and using scarce medical resources and implementing alternate care facility operations.
Crisis standards of care represent a substantial change in usual healthcare operations made necessary by a pervasive or catastrophic disaster.
With this foundation, the committee proposes a national framework for developing crisis standards of care, identifies key elements that should be included in the standards, and provides detailed guidance that state and local public health officials can use to establish and implement crisis standards of care. The proposed roadmap encompasses the full spectrum of the health system, including emergency medical services and dispatch, public health, hospital-based care, home care, primary care, palliative care, mental health, and public health.

To meet public health needs in large-scale emergencies, the nation also requires adequate amounts of safe and effective chemical, biological, radiological, and nuclear medical countermeasures (MCM)—including vaccines, drugs, and diagnostics. But the public health enterprise has inadequate research, development, and production of needed countermeasures. Recognizing this, HHS directed its Office of the Assistant Secretary for Preparedness and Response to lead a review of its public health emergency medical countermeasures enterprise, and the office turned to the IOM to assist with this task. The IOM convened a workshop in 2009, bringing together representatives from government agencies and the private sector to examine the federal policies and activities that affect MCM discovery, development, and approval and to explore potential opportunities to enhance the medical countermeasures enterprise. The discussions, reported in The Public Health
Emergency Medical Countermeasures Enterprise: Innovative Strategies to Enhance Products from Discovery Through Approval: Workshop Summary (2010), highlighted a number of key points, among them how to achieve the greatest health impact in the face of diminishing resources, regulatory aspects of the enterprise, and product discovery and development.
In addition to the research, development, and production of MCM, it is critical to be able to deliver them to those in need. As part of efforts under way nationwide to improve the nation’s ability to rapidly distribute and dispense MCM, the Office of the Assistant Secretary for Preparedness and Response (ASPR) within HHS commissioned the IOM to examine the potential uses, benefits, and disadvantages of strategies for prepositioning antibiotics. Prepositioning involves storing antibiotics close to or in the possession of the people who would need rapid access to them should an attack occur. These prepositioning strategies—intended to help ensure timely access to antibiotics in the event of an attack—would complement existing plans that rely heavily on more centralized stockpiles.

In Prepositioning Antibiotics for Anthrax (2011), the IOM committee appointed to this task finds that the earliest sign of inhalational anthrax symptoms will likely occur four days or later after an attack. It may take a day or two—if not more—to detect that an attack has occurred and for public health officials to decide that antibiotics should be dispensed to people who may have been exposed. Since antibiotics are most effective at preventing anthrax if taken before symptoms begin to occur, to prevent illness, public health officials must act quickly to distribute and dispense antibiotics in the remaining time.
The Centers for Disease Control and Prevention (CDC) and state and local jurisdictions currently aim to dispense antibiotics to the entire population within 48 hours after the decision is made to dispense antibiotics. The committee finds—given the limited evidence available—that this goal appears to be appropriate as long as the attack is detected soon after it occurs.
The backbone of current distribution plans is the Strategic National Stockpile (SNS)—a national repository of medicine and medical supplies
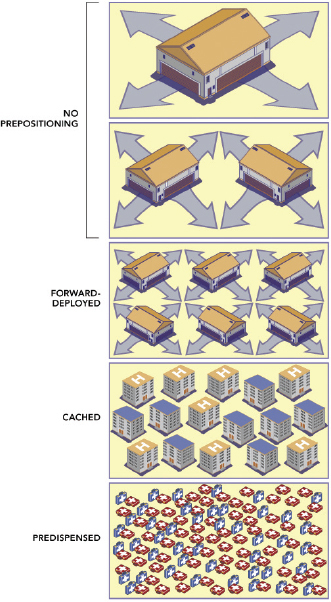
Medical countermeasures storage locations needed to provide eqivalent coverage.
SOURCE: Prepositioning Antibiotics for Anthrax.
maintained by the CDC—which can be deployed rapidly around the country as a supplement to state and local antibiotic stockpiles. State and local public health authorities dispense antibiotics from all of these stockpiles to the public primarily via points of dispensing that are set up throughout the community. The committee defines three categories of prepositioning strategies that could complement more centralized stockpiling strategies:
• Forward-Deployed MCM: MCM stored near the locations from which they will be dispensed.
• Cached MCM: MCM stored at the locations from which they will be dispensed, such as workplaces and healthcare facilities.
• Predispensed MCM: MCM stored by the intended users or by heads of households or other nonmedical caregivers for use by those in their care.
Further, because communities differ widely in their needs and capabilities, the committee offers a framework to help state, local, and tribal public health officials determine which prepositioning strategies could benefit their communities, if any.
In another examination of major medical emergencies, the IOM explored ways to improve the nation’s ability to respond to mass casualty incidents (MCIs) that occur in rural settings, where healthcare resources often are limited. One incident occurred in January 2008 in Utah when a chartered bus carrying 56 passengers crashed, killing 9 passengers and injuring 43 others, as well as the driver. The crash crystallized the need for an integrated infrastructure capable of responding to MCIs that occur in rural areas. In response, the Federal Interagency Committee on Emergency Medical Services, with support from the National Highway Traffic Safety Administration, asked the IOM’s Forum on Medical and Public Health Preparedness for Catastrophic Events to hold a workshop to explore the issue.
Many rural areas are medically underserved with regard to both prehospital services, including emergency medical service units, and hospital services, which often face day-to-day shortages of equipment, supplies, or personnel.
The workshop brought together experts from a variety of fields and included an open panel discussion. Preparedness and Response to a Rural Mass Casualty Incident: Workshop Summary (2011) captured the discussions, which revealed a number of common themes. For example, MCIs are common in rural areas and likely will become more frequent in coming decades as more people use trains, buses, and airplanes to traverse these regions. Many rural areas, however, are medically underserved with regard to both prehospital services, including emergency medical service (EMS) units, and hospital services, which often face day-to-day shortages of equipment, supplies, or personnel.
Responding to MCIs in these areas also is hampered by limited 9-1-1 access and broadband services. In addition, the vast distances often involved can delay response to the scene and transport of patients to care facilities. Once on the scene, rural EMS providers may have radios for communication, but there are numerous “dead areas,” particularly in mountainous regions and expansive land areas with limited communication towers. When multiple EMS teams respond, their radio systems are not necessarily compatible.
Common themes also emerged on possible improvements. Developing mechanisms to identify and share best practices in planning for and responding to MCIs will help federal, state, and local governments. Better communications and patient tracking also can be a tremendous asset to everyone involved in a disaster response. Leveraging existing federal programs can provide an opportunity to improve access to broadband technologies. For all responders, providing communications interoperability, including across state lines, can be critically helpful.
Developing mechanisms to identify and share best practices in planning for and responding to mass casualty incidents will help federal, state, and local governments
Promoting regional cooperation among stakeholders, including governments, the private sector, and local military bases, may help in efforts to better assess the risks in various areas and their response capabilities. Although some concerns arose about possible loss of local control, there was considerable agreement that regionalization, especially when supported by increased federal funding support, can facilitate partnerships and sharing of resources that result in greater flexibility to plan and respond at the local level.
Revitalizing public health laws and policy
In national efforts to protect and improve the health of the population, public policies have a major role to play. Sound laws and regulations are particularly important in a time of scarce resources, because they can diminish or preclude the need for other, more costly and potentially less efficient interventions. As part of a 2.5-year study requested and supported by the Robert Wood Johnson Foundation (RWJF), the IOM convened a committee of experts to examine the legal and regulatory authority for public health activities, identify past efforts to develop model public health legislation, and describe the implications of the changing social and policy
context for public health laws and regulations. This analysis comprises the second phase of a three-part study, which also will examine measurement and funding issues that are critical to public health.
In its report, For the Public’s Health: Revitalizing Law and Policy to Meet New Challenges (2011), the IOM finds that public health law warrants systematic review and revision, given the enormous transformations in the practice, context, science, and goals of public health agencies and changes in society as a whole, especially in the past 2 to 3 decades. Both federal and state policy makers should takes steps to ensure that public health laws adequately meet current health needs. State laws should provide health agencies with broad authority to deal with chronic diseases and conditions such as obesity, inju-
The 10 Essential Public Health Services
1. Monitor health status to identify and solve community health problems.
2. Diagnose and investigate health problems and health hazards in the community.
3. Inform, educate, and empower people about health issues.
4. Mobilize community partnerships and action to identify and solve health problems.
5. Develop policies and plans that support individual and community health efforts.
6. Enforce laws and regulations that protect health and ensure safety.
7. Link people to needed personal health services and assure the provision of health care when otherwise unavailable.
8. Assure a competent public and personal healthcare workforce.
9. Evaluate effectiveness, accessibility, and quality of personal and population-based health services.
10. Research for new insights and innovative solutions to health problems.
SOURCE: For the Public’s Health: Revitalizing Law and Policy to Meet New Challenges, p. 4.
ries, and substance abuse, and to develop immunization registries and surveillance systems that could help detect bioterrorist attacks or disease outbreaks. Whenever possible, federal and state governments should set minimum public health standards and develop regulations to allow lower levels of government to enact further restrictions when necessary.
Among other actions, states should increase the capability and capacity of health departments. States also should require health agencies to provide the 10 essential public health services as the standard of practice and make certain that adequate funding and staffing are in place to provide these services, the report says. Previously developed by a consortium of public health groups, the list of services includes basic functions such as monitoring the health status of communities, diagnosing and investigating community health hazards, mobilizing community action, enforcing laws that protect health, and evaluating population-based services.
States also should require health agencies to provide the 10 essential public health services as the standard of practice and make certain that adequate funding and staffing are in place to provide these services.
Policies and regulations that lie outside the health sector can have a significant impact on people’s health as well. For example, government agricultural subsidies can influence the availability and affordability of certain foods, zoning policies can encourage the creation of green space, and education policies can support the intellectual and physical growth of young people. Governments at all levels should examine the potential positive and negative effects of such cross-sector laws and policies and identify improvements that can boost public health.
The committee’s first report generated in the RWJF-sponsored study, For the Public’s Health: The Role of Measurement in Action and Accountability (2010), is described in more detail earlier in this book, in the chapter on measurement. In its third report in the series, projected for fall 2011, the committee will propose recommendations for funding state and local public health systems within the healthcare landscape expected to evolve as the Affordable Care Act of 2010 is implemented.
One area in which public policy is integral to the health and well-being of the public is the clearance of medical devices. Medical devices play a critical role in the health care of Americans. They can range from simple tools, such as tongue depressors and bandages, to complex or life-saving
equipment, such as pacemakers, magnetic resonance imaging machines, and heart–lung machines. The Federal Food, Drug, and Cosmetic Act (FFDCA) requires a “reasonable assurance of safety and effectiveness” before a device can be marketed, and the U.S. Food and Drug Administration (FDA) is responsible for enforcing this requirement. Devices that are deemed to have a moderate risk to patients generally cannot go on the market until they are cleared through the 510(k) process, named for Section 510(k) of the FFDCA. Devices that are subject to the 510(k) process include such devices as blood pressure cuffs as well as some types of contact lenses and pacemakers. The FDA receives several thousand 510(k) submissions each year.
Some policymakers and patients have expressed concern about the ability of the 510(k) process to ensure that medical devices on the market are safe and effective. Other policy makers and patients, as well as the medical-device industry, have asserted that the process has become too burdensome and time-consuming and that it is delaying important new medical devices from entering the market.

The FDA turned to the IOM to review the 510(k) process and answer two questions:
• Does the current 510(k) process protect patients optimally and promote innovation in support of public health?
• If not, what legislative, regulatory, or administrative changes are recommended to achieve the goals of the 510(k) process optimally?
In its report, Medical Devices and the Public’s Health: The FDA 510(k) Clearance Process at 35 Years (2011), the IOM finds that the current 510(k) process is flawed based on its legislative foundation. Rather than continuing to modify the 35-year-old 510(k) process, the IOM concludes that the FDA’s finite resources would be better invested in developing an integrated premarket and postmarket regulatory framework that provides a reasonable assurance of safety and effectiveness throughout the device life cycle. This new framework should:
• be based on sound science;
• be clear, predictable, straightforward, and fair;
• be self-sustaining and self-improving;
• facilitate innovation that improves public health by making medical devices available in a timely manner and ensuring their safety and effectiveness throughout their lifecycle;
• use relevant and appropriate regulatory authorities and standards throughout the life cycle of devices to ensure safety and effectiveness; and
• be risk-based.
Current information is not adequate to design a new framework, and the FDA should begin to obtain the needed information. Once adequate information is available to design an appropriate medical-device regulatory framework, the report recommends, Congress should enact legislation to do so.
Aiding women, children, and families
Several segments of the population face particular health and healthcare risks and needs. Among these are women, children, and families. Over the years, the IOM has focused on the health needs facing these populations and offered research and policy paths for protecting and improving their health and well-being.
In one study, the IOM examined what changes mandated in the Patient Protection and Affordable Care Act of 2010 will mean for women. The legislation requires that certain preventive services be covered at no cost to patients. HHS is charged with determining precisely what services will be covered.
Given this responsibility, HHS asked the IOM to appoint a committee of experts to comprehensively examine women’s health needs and the potential impact of the new preventive health mandates. In Clinical Preventive Services for Women: Closing the Gaps (2011), the committee reviews the list of currently approved preventive services, examines additional screenings and services that have been shown to be effective in improving women’s health, and recommends services that should be considered for inclusion in the guidelines. The proposed services have the potential to improve the health and well-being of women, and are supported by high-quality scientific evidence.
Women stand to benefit greatly by expanded access to preventive ser-
vices. With longer life expectancies than men, as well as reproductive and gender-specific conditions and a greater burden of chronic diseases and disability, women traditionally pay significant out-of-pocket expenses for preventive services. Also, women have disproportionately lower incomes, often putting the screenings, tests, and treatments that support women’s health financially out of reach.

The preventive services recommended by the committee cover a range of needs and health conditions. They include a number of services and screenings that support and improve women’s sexual, reproductive, and emotional health. For example, women should have access to contraceptive education, counseling, and services so they can better avoid unwanted pregnancies and space their pregnancies to promote optimal birth outcomes. Screening also should be available for gestational diabetes in early pregnancy for women at high risk of diabetes, and all pregnant and postpartum women should receive lactation counseling and equipment to ensure successful breastfeeding, which benefits the mother, the child, and society. Over time, HHS should regularly review and update the approved preventive services to stay current with science and changing health needs.
Protecting and improving the health of children presents another major challenge to the nation generally and the public health community in particular. Child health is important not only in its own right but to future health as well; the quality of health and health behaviors laid prenatally and in early childhood contribute significantly to lifelong health. Research from a variety of fields demonstrates that the social and economic conditions under which children live greatly influence their health in ways that continue through their life spans. Further, disparities in socioeconomic conditions among children contribute to the health disparities observed among some racial and ethnic adult populations across the nation. These factors raise the importance of using community-level activities and interventions to positively affect key determinants of health.
The quality of health and health behaviors laid prenatally and in early childhood contribute significantly to lifelong health.
Recommendations for Preventive Healthcare Services for Women That Should Be Considered by the U.S. Department of Health and Human Services
| Recommendation 5.1: Screening for gestational diabetes in pregnant women between 24 and 28 weeks of gestation and at the first prenatal visit for pregnant women identified to be at high risk for diabetes. | Recommendation 5.6: Comprehensive lactation support and counseling and costs of renting breastfeeding equipment. A trained provider should provide counseling services to all pregnant women and to those in the postpartum period to ensure the successful initiation and duration of breastfeeding. (The ACA ensures that breastfeeding counseling is covered; however, the committee recognizes that interpretation of this varies.) |
| Recommendation 5.2: The addition of high-risk human papillomavirus DNA testing in addition to cytology testing in women with normal cytology results. Screening should begin at 30 years of age and should occur no more frequently than every 3 years. | Recommendation 5.7: Screening and counseling for interpersonal and domestic violence. Screening and counseling involve elicitation of information from women and adolescents about current and past violence and abuse in a culturally sensitive and supportive manner to address current health concerns about safety and other current or future health problems. |
| Recommendation 5.3: Annual counseling on sexually transmitted infections for sexually active women. | Recommendation 5.8: At least one well-woman preventive care visit annually for adult women to obtain the recommended preventive services, including preconception and prenatal care. The committee also recognizes that several visits may be needed to obtain all necessary recommended preventive services, depending on a woman’s health status, health needs, and other risk factors. |
| Recommendation 5.4: Counseling and screening for human immunodeficiency virus infection on an annual basis for sexually active women. | |
| Recommendation 5.5: The full range of Food and Drug Administration-approved contraceptive methods, sterilization procedures, and patient education and counseling for women with reproductive capacity. |
SOURCE: Clinical Preventive Services for Women: Closing the Gaps.
At a workshop convened by the IOM’s Roundtable on Health Disparities, experts from a variety of fields, including academia, community development, health care, business, and philanthropy, examined the relationship between socioeconomic conditions early in life and later health outcomes, discussed how community approaches can help, and explored a number of successful models that engage both community factors and health care to affect life course development.
Focusing on Children’s Health: Community Approaches to Addressing Health Disparities: Workshop Summary (2009) describes a number of common threads that emerged. Participants generally agreed, for example, that it is time for action. Although more can be learned about the roles of social, racial, and economic determinants of health, the success of model programs shows that disparities in health are not insurmountable. Successful interventions involve the community as a full partner in the process. Identification of health challenges and priority concerns, and development of intervention strategies suited to a community’s needs and culture, cannot be done without the input of the community members.

Families, of course, also greatly influence children’s health. The American family is a complicated institution—and rapidly becoming more so. Demographic changes, immigration, economic upheavals, and changing societal mores are creating new and altered structures, processes, and relationships in families. As a result, the lives of infants, children, and adolescents differ in fundamental ways from those of past generations.
As families undergo rapid change, so is family science, which is spawning a large and growing body of findings from various disciplines. The science of family research cuts across demography, anthropology, psychology, sociology, economics, education, genetics, neuroscience, and developmental biology. Researchers, often working in multidisciplinary teams, are generating a better understanding of how to improve the health and well-being of children.
The American family is a complicated institution—and rapidly becoming more so.
In light of advances and ongoing changes in family science, the IOM
and the NRC convened a workshop where researchers, funders, and users of research results on families explored current and emerging issues, and their discussions and presentations were presented in Toward an Integrated Science of Research on Families: Workshop Report (2011). Among areas of common agreement, participants suggested that advances in family research will require approaches that can move beyond problem-oriented studies to identify positive family strengths and functioning that contribute to the well-being of family members, especially during times of social disruption and adversity. Also, more efforts need to be devoted to clarifying the structures, processes, and relationships within families that contribute to the resilience, well-being, school readiness, and healthy development of children, in order to inform the next generation of programs and policies to support children and families.
Oral health: A silent epidemic
For the nation as a whole, one major—and often overlooked—challenge to public health is the poor oral health of much of the population. Contributing to the problem, millions of people lack routine access to basic oral health care. This gap is particularly acute among racial and ethnic minorities, older adults, children, pregnant women, people with special needs, people of lower socioeconomic status, and people living in medically underserved rural and urban areas, among others. At the request of the Health Resources and Services Administration (HRSA) and the California HealthCare Foundation, the IOM and the NRC convened a committee of experts to examine these issues.
Millions of people lack routine access to basic oral health care.
In Improving Access to Oral Health Care for Vulnerable and Underserved Populations (2011), the committee describes current levels of access to oral health care for vulnerable and undeserved populations, identified problem areas, and proposed a sweeping new vision for oral health care. In this vision, everyone has access to quality oral health care across their life spans. Further, the oral healthcare system will be based on sound scientific evidence; eliminate barriers that contribute to oral health disparities; prioritize disease prevention and health promotion; provide oral health services in a variety of settings; make use of a diverse and expanded array of providers who are competent, compensated, and authorized to provide
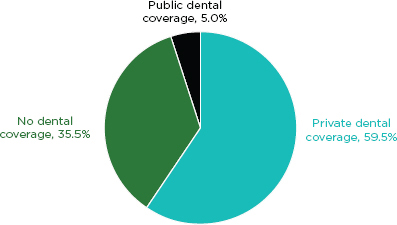
Percentage of adults 21–64 according to dental coverage status: U.S. civilian noninstitutionalized population, 2007.
SOURCE: Manski and Brown, 2010.
SOURCE: Improving Access to Oral Health Care for Vulnerable and Underserved Populations.
evidence-based care; include collaborative and interdisciplinary teams working across the healthcare system; and foster continuous improvement and innovation.
The system also must ensure that oral health care is incorporated as an integral component of comprehensive health care. With proper training, nondental healthcare professionals, such as nurses, pharmacists, physician assistants, and physicians, could screen for oral diseases and deliver preventive care services. While several nondental healthcare education programs have made great strides in improving the oral health education and training of their students, these efforts have not spread widely through the professions.

Other efforts to improve access to oral health care should include developing and promulgating optimal regulations and policies that determine who may provide care and how it may be provided. Also, the dental education system should be improved to ensure that current and future generations of dental professionals can deliver quality care to diverse populations, in various settings. Financial and administrative barriers to oral health care should be modified as well, including barriers within public programs such
as Medicaid and the Children’s Health Insurance Program, which are the primary funders of healthcare coverage for underserved and vulnerable individuals.
Given the lingering—and pervasive—problems among many segments of the population, HRSA also asked the IOM to convene a committee of experts to assess the current oral healthcare system and recommend strategic improvements in light of the role that HHS can play.
In Advancing Oral Health in America (2011), the committee found that HHS’s efforts to improve oral health and oral health care have been wide ranging, but the priority placed on these endeavors, including financial support, has been inconsistent. Areas needing expanded support and attention include research on best practices in oral health care and behavior change, oral health literacy, quality measurement, integration and standardization of data, oral healthcare financing, and workforce innovations.
Areas needing expanded support and attention include research on best practices in oral health care and behavior change, oral health literacy, quality measurement, integration and standardization of data oral healthcare financing, and workforce innovations.
HHS already had recognized that its programs needed improving, and in 2010 the department launched an Oral Health, Initiative—a cross-agency effort to improve oral health care nationwide. As part of its deliberations, the IOM committee examined the plans and recommended improvements in a number of areas. The recommendations collectively comprise what the committee called a New Oral Health Initiative (NOHI), to distinguish it from and build on the current initiative.
The proposed agenda rests on a set of core organizing principles based on the areas in greatest need of attention and the approaches that have the most potential for creating improvements. The principles include establishing high-level accountability within HHS, emphasizing disease prevention and oral health promotion, improving oral health literacy and cultural competence, reducing oral health disparities among underserved populations, exploring new models for payment and delivery of care, enhancing the role of nondental healthcare professionals, expanding oral health research and improving data collection, promoting collaboration among private and public stakeholders, and measuring progress toward short-term and long-term goals and objectives. A final principle, address-
ing evaluation, calls on HHS to use the goals of Healthy People 2020—an existing set of benchmarks for achieving better health for the country—rather than creating new goals that would be redundant.
Vaccinations aiding public health
A fundamental component in the nation’s public health and preventive care system, vaccinations prevent the spread of infectious and potentially deadly diseases. In recognition of their importance, the federal government in 1994 established a National Vaccine Plan, and in 2008 the government proposed an updated national plan. The National Vaccine Program Office (NVPO), created by Congress and located in HHS, oversees the plan, and the NVPO asked the IOM to review the proposed new plan and identify priority areas for improvement.
In Priorities for the National Vaccine Plan (2009), the IOM committee that conducted the review concludes that the NVPO has not functioned as intended and that the proposed plan has numerous shortcomings. The NVPO falls short, in particular, in meeting a key responsibility, that of coordinating vaccine activities within government agencies and across the larger immunization system. The system engages many partners—including multiple government agencies and departments, vaccine researchers, manufacturers, public health officials, healthcare providers, and the public—in identifying vaccine needs, researching and developing new products, assessing safety, and getting people immunized. But the stakeholders do not regularly or effectively work together to advance collective goals. HHS can take an important step forward by clarifying the NVPO’s role as the central coordinator for critical immunization activities and giving it the necessary funding to fulfill this mission.

Among recommended improvements for the proposed plan update, a greater proportion of vaccine research and development should be directed at specific goals, such as producing vaccines against diseases for which there are none or developing a single vaccine that would work against all influenza viruses. The majority of vaccine research and development currently stems from
the interests of individual researchers rather than a set of priority targets identified through a centralized planning process.
The plan should set a more aggressive agenda for research on vaccine safety, with sufficient funding, and should include establishing a permanent group to advise the government on safety issues. A new vaccine safety advisory group could guide efforts to address potential safety concerns and the development of a research agenda with clear priorities. The plan should include a strategy to eliminate financial barriers to immunization, such as lack of health plan coverage for all recommended vaccines and insufficient reimbursements that do not cover all of a clinic’s costs of providing vaccines, as well as the development of a national communications strategy that engages the latest techniques and methods, such as social networking. In addition, it should promote the use of health information technology, including electronic health records, to monitor disease incidence, rapidly detect potential safety signals, and measure vaccine coverage.
One of the key aims of developing a National Vaccine Plan is to prevent adverse reactions to vaccines. Though generally very rare or minor, there are side effects, or “adverse effects,” associated with some vaccines. Importantly, some adverse events following a vaccine may be due to coincidence and are not caused by the vaccine. To make this distinction, researchers use evidence to determine if adverse events following vaccination are causally linked to a specific vaccine; if so, these events are referred to as adverse effects. HRSA asked the IOM to review a list of adverse events associated with eight vaccines—varicella zoster, influenza (except 2009 H1N1), hepatitis B, HPV, MMR, hepatitis A, meningococcal, and those that contain tetanus—and evaluate the scientific evidence about the vaccine–adverse effect relationship. The IOM committee appointed to this task was not asked to assess the benefits or effectiveness of vaccines but only the risk of specific adverse events.
In its report, Adverse Effects of Vaccines: Evidence and Causality, the committee presents 158 causality conclusions, one for each pair of vaccine–adverse effect pairing. The committee finds that evidence convincingly supports a causal relationship between some vaccines and some adverse events. However, for the majority of cases—135 vaccine–adverse event pairs—the evidence was inadequate to accept or reject a causal relationship. Overall, the committee concludes that few health problems are caused by or clearly associated with vaccines.
Few health problems are caused by or clearly associated with vaccines.
Summary of Causality Conclusions
| Vaccine | Adverse Event | Causality Conclusion |
| Varicella | Disseminated varicella infection (widespread chickenpox rash shortly after vaccination) | Convincingly Supports |
| Varicella | Disseminated varicella infection with subsequent infection resulting in pneumonia, meningitis, or hepatitis | Convincingly Supportsa |
| Varicella | Vaccine strain viral reactivation (appearance of chickenpox rash months to years after vaccination) | Convincingly Supports |
| Varicella | Vaccine strain viral reactivation with subsequent infection resulting in meningitis or encephalitis (inflammation of the brain) | Convincingly Supports |
| MMR | Measles inclusion body encephalitis | Convincingly Supportsa, b |
| MMR | Febrile seizures (a type of seizure that occurs in association with fever and is generally regarded as benign) | Convincingly Supports |
| MMR | Anaphylaxis (a very rare but sudden allergic reaction) | Convincingly Supports |
| Varicella | Anaphylaxis | Convincingly Supports |
| Influenza | Anaphylaxis | Convincingly Supports |
| Hepatitis B | Anaphylaxis | Convincingly Supportsc |
| Tetanus Toxoid | Anaphylaxis | Convincingly Supports |
| Meningococcal | Anaphylaxis | Convincingly Supports |
| Injection-Related Event | Deltoid bursitis (frozen shoulder, characterized by shoulder pain and loss of motion) | Convincingly Supports |
| Injection-Related Event | Syncope (fainting) | Convincingly Supports |
| HPV | Anaphylaxis | Favors Acceptance |
| MMR | Transient arthralgia (temporary joint pain) in women | Favors Acceptanced |
| MMR | Transient arthralgia in children | Favors Acceptance |
| Influenza | Oculorespiratory syndrome (a mild and temporary syndrome characterized by conjunctivitis, facial swelling, and upper respiratory symptoms) | Favors Acceptancee |
| MMR | Autism | Favors Rejection |
| Influenza | Inactivated influenza vaccine and Bell’s palsy (weakness or paralysis of the facial nerve) | Favors Rejection |
| Influenza | Inactivated influenza vaccine and asthma exacerbation or reactive airway disease episodes in children and adults | Favors Rejection |
| MMR | Type 1 diabetes | Favors Rejection |
| DT, TT, or aP containing | Type 1 diabetes | Favors Rejection |
a The committee attributes causation to individuals with demonstrated immunodeficiencies.
b The committee attributes causation to the measles component of the vaccine.
c The committee attributes causation to yeast-sensitive individuals.
d The committee attributes causation to the rubella component of the vaccine.
e The committee attributes causation to two particular vaccines used in three particular years in Canada. All other causality conclusions are the evidence is inadequate to accept or reject a causal relationship.
SOURCE: Adverse Effects of Vaccines: Evidence and Causality.
Emerging genetic technologies
Genetic and genomic technologies also offer broad promise for improving health care and health outcomes for many people. It is well established that alterations in genes—collectively comprising the genome—sometimes can cause any number of diseases or make an individual more prone to particular diseases. A variety of genetic and genomic laboratory tests are now available for a range of purposes, including for disease testing, guiding personalized drug therapy, and screening for markers of increased risk of disease.
These new technologies, however, have not been widely integrated into clinical practice, and questions remain as to how they are valued in the healthcare setting. The IOM, through its Roundtable on Translating Genomic-Based Research for Health, held a workshop that brought together experts from a variety of fields, as well as patients and other laypeople, to explore such questions. As one basis for discussion, participants examined three case examples of current tests that span a range of potential applications.
Their discussions, reported in The Value of Genetic and Genomic Technologies: Workshop Summary (2010), ranged across a number of topics related to the perceived value of genetic and genomic technologies, both present and future, in clinical practice. They considered, for example, how different stakeholders define the value of genetic and genomic technologies; how stakeholders prioritize various aspects of genetic tests when determining value; how people assess the relative value of genetic tests when making personal healthcare decisions; and how perceived values relate, or do not relate, to the monetary cost of the technologies. The aim was not to assess the value of any specific test, but rather, to identify broader issues of how individual stakeholders derive their personal or professional opinions of the value of using these technologies.

Examining the value of cancer care
One challenge facing all parts of the healthcare system centers on costs—and perhaps no area of health care feels more pressure than oncology, or
cancer care. Oncology spending is growing at more than 15 percent annually, faster than total health spending. In oncology, there are certain factors that discourage consideration of evidence concerning safety, effectiveness, and cost-effectiveness. Many cancer patients, for example, have a grim prognosis and are facing imminent death, so patients and healthcare professionals feel a sense of urgency to try every possible treatment in the hopes of at least potentially prolonging life, and patients and physicians in this situation may discount the potential harms of treatments. In addition, the healthcare delivery system’s incentives favor aggressive treatment over many other important steps, such as providing patients with accurate information about prognosis, comfort care measures that can improve the quality of life for patients with cancer and even prolong life, or end-of-life planning, and these may take second place to costly interventions.
Oncology spending is growing at more than 15 percent annually, faster than total health spending.
The IOM’s National Cancer Policy Forum held a workshop to explore subjects related to the value of cancer care from multiple perspectives, including those of patients and patient advocates, providers, insurers, healthcare researchers, federal agencies, and industry. Their discussions, reported in Assessing and Improving Value in Cancer Care: Workshop Summary (2009), ranged across a variety of issues, including the lack of a single description of value on which everyone currently agrees. Many descriptions of value, in general, have been proposed, with descriptions often focusing largely on a return for a cost in terms of goods or services. These descriptions parallel themes at issue for value in oncology—relative worth, fair return, costs, and measures of quality.
In one common theme, participants noted that value in cancer care encompasses numerous complex topics, including evidence for treatment effectiveness, clinical discussions of healthcare costs, and quality end-of-life care, among other factors. Considering these and other issues, participants sought to provide an objective concept of value that could be considered by anyone faced with difficult decisions regarding developing, evaluating, prescribing, and paying for cancer care. A better understanding of value also may help policy makers and other decision makers in developing and using new policy tools that can lead to improvements in the value of cancer services provided to patients.
























