As highlighted throughout this chapter, the benefits of prepositioning strategies will depend on the particular community in which they are implemented. Different communities differ in their likelihood of experiencing an anthrax attack; in their existing surveillance, detection, and dispensing infrastructure; in their population and geographic characteristics; in their values and preferences; and in their available resources. These differences affect which prepositioning strategy or combination of prepositioning strategies, if any, will be most effective in meeting a community’s prophylaxis goals.
To assist state, local, and tribal jurisdictions in evaluating whether their community would benefit from implementing prepositioning, the committee has developed a decision-aiding framework. The key elements of this framework are presented in Box 5-1. The framework is intended to assist each community in evaluating the strategies described in detail in Chapter 4— forward-deployed, cached, and predispensed—according to its own needs, objectives, value trade-offs, and constraints. This chapter details the elements of the framework listed in Box 5-1. It then presents a recommended modeling approach for communities to use in applying the quantitative elements of the framework (evaluation of potential health benefits versus likely costs) to make decisions about the use of prepositioning strategies. The final section presents the committee’s findings and recommendations on the benefits, costs, and sustainability of the various strategies.
BOX 5-1
Key Elements of the Decision-Aiding Framework
Communities across the United States differ in their needs and capabilities. Different communities may benefit most from different strategies for prepositioning antibiotics for anthrax, or may not benefit from prepositioning strategies at all. The committee developed a decision-aiding framework to assist state, local, and tribal jurisdictions in deciding which prepositioning strategies, if any, to implement in their community. The key elements of this framework are:
• Assessment of risk and current capabilities
— Consideration of the risk of an anthrax attack
— Assessment of current capability for timely detection of an attack
— Assessment of current dispensing capability, including (1) overall dispensing capability, and (2) specific gaps in dispensing capability, such as particular subpopulations not well served by current plans
• Incorporation of ethical principles and community values
• Evaluation of potential prepositioning strategies for medical countermeasures for anthrax
— Evaluation of potential health benefits, including evaluation of potential effectiveness in reaching specific populations or filling other specific gaps in dispensing capability
— Evaluation of potential health risks
— Evaluation of likely costs
— Consideration of practicality, including (1) communications needs and expected social behavior and adherence, (2) logistics, and (3) legal and regulatory issues
ASSESSMENT OF RISK AND CURRENT CAPABILITIES
To evaluate whether alternative prepositioning strategies would be appropriate for their communities, jurisdictions must consider the risk of an anthrax attack in their community and assess their current capability to detect such an attack in a timely manner and to distribute and dispense postexposure prophylactic antibiotics to their population. This section describes these assessments.
Risk of an Anthrax Attack
A community’s risk assessment for an anthrax attack is important for prioritizing funding for dispensing capabilities within the context of
overall public health needs, and it helps inform decisions about the specific dispensing strategies that would protect the community most effectively. For example, a community facing a low risk of an anthrax attack might decide to rely on the Strategic National Stockpile (SNS) and not assume the additional costs associated with implementing local prepositioning strategies. Conversely, a community facing a high risk of an anthrax attack might decide that the additional cost was a valuable use of public health resources. This section reviews the factors included in risk assessment, identifies sources of risk information to which jurisdictions already have access and that can be used in decision making about prepositioning, and briefly addresses how this information can be used to inform prepositioning decisions (a topic discussed in greater detail below in the section on evaluation of potential prepositioning strategies).
The Department of Homeland Security (DHS) outlines three components of risk: the threat (the likelihood of an attack), the vulnerability of a community to that attack, and the consequences of a successful attack (DHS, 2008a). For assessment of specific terrorism scenarios, factors that impact the assessment of threat may include intent and capability of adversary, weapon availability, attack simplicity, historical information, and intelligence information (FEMA, 2007). The following factors related to vulnerability and consequences are also taken into account in assessment of risk: “population and population density, the presence of critical infrastructure and key resources, location in high terrorist or high risk natural disaster areas, and capabilities to prevent, protect against, or mitigate a threat” (FEMA, 2007, p. 10).
State, local, and tribal jurisdictions rely primarily on the federal government to provide information about the threat of a terrorist attack. The Secretary of DHS has issued material threat determinations (MTDs) for anthrax and multi-drug-resistant anthrax (DHS, 2008b; GAO, 2009). Many public health officials, particularly those from states or larger cities, have access to additional classified intelligence information that, if available, could be used to inform decisions about prepositioning.
The committee has focused primarily on long-term planning for prepositioning and recognizes that the federal government and public health officials in jurisdictions may not have detailed, long-term information about specific anthrax threats to jurisdictions (beyond what is conveyed by the MTDs), including detailed information about the relative likelihood of specific attack scenarios. Nevertheless, jurisdictions should use this information if they have it (or can make reasonable assumptions about it) to inform policy and decision making about prepositioning. In contrast, the federal government may, on occasion, have specific information about an imminent credible threat to a specific jurisdiction(s). In such circumstances, the risk of an attack might be considered very high, and rapid decisions
might be made to forward-deploy medical countermeasures (MCM) to that jurisdiction(s). The committee focused less on this type of prepositioning in response to a potentially imminent attack.
Vulnerability and consequence are largely independent of specific intelligence information about a threat. Jurisdictions do not need classified, detailed intelligence information about specific threats to consider their vulnerability to an anthrax attack and the potential consequences to the community.
Jurisdictions already have access to several sources of information about risk in their community. Some are provided by the federal government, and jurisdictions generate others as part of the process for applying for federal funding.
First, DHS funding allocations are based on DHS-developed risk assessments (FEMA, 2011). DHS’s Urban Areas Security Initiative (UASI) grant identifies 31 metropolitan areas at the highest risk of a terrorist attack (FEMA, 2011). A designation of UASI Tier 1, assigned to the 11 highest-risk areas, or Tier 2, assigned to the remaining 20 areas, is itself a generalized risk assessment. DHS funding reflects this differential risk, assigning Tier 1 cities 81.6 percent of the program’s total funding.
Jurisdictions funded by DHS’s UASI and State Homeland Security Program (SHSP) grants (another terrorism response-related grant program) have access to the information from their specific Threat and Hazard Identification and Risk Assessment (THIRA), a required part of their grant application (FEMA, 2011). THIRAs assess all threats and hazards facing the jurisdiction, including terrorism threats, and are submitted to DHS as part of the overall State Mitigation Plan, which is intended to foster collaboration among the disaster response plans of all jurisdictions within a state.
Awardees of the Office of the Assistant Secretary for Preparedness and Response’s (ASPR’s) Hospital Preparedness Program (HPP) grant are required to use the results of a Hazard Vulnerability Analysis—which identifies, analyzes, and prioritizes potential threats to a jurisdiction—to inform their planning efforts (ASPR, 2011). This is another existing information source for state and local jurisdictions to use in assessing the value of prepositioning strategies in meeting their prophylaxis goals.
The use of risk-related information in decision making about prepositioning is addressed in greater detail later in the chapter, including how a community’s relative risk of an anthrax attack and the likelihood of specific attack scenarios impact the potential benefits and cost-effectiveness of different prepositioning strategies. The committee recognizes, however, that detailed information about threat and the likelihood of specific attack scenarios may not exist. Jurisdictions should use the best risk assessment information available to inform decision making about prepositioning.
In the absence of such information, jurisdictions can also explore the potential benefits and costs of prepositioning given different assumptions about threat and specific attack scenarios. In some cases, jurisdictions may ultimately rely more heavily on the vulnerability and consequences aspects of risk assessment. For example, a remote county with low population density, no high-profile potential critical infrastructure targets, and a high risk of flooding may decide to rely on the SNS instead of implementing prepositioning strategies, instead devoting more public health resources to flood preparedness. On the other hand, a community with high population density and infrastructure that is thought to be a potential terrorism target might decide to implement several prepositioning strategies. These trade-offs are discussed in greater detail in a later section.
Finally, in further recognition of the fact that some local jurisdictions may lack resources to conduct an in-depth assessment of the risk of an anthrax attack, the committee recommends below partnerships among state, local, and tribal governments and with the federal government in working through the key elements of the decision-aiding framework presented in this chapter. In many cases, federal and state governments may have greater access to classified information and resources for conducting risk assessment and could provide guidance on this element of the framework to local jurisdictions.
Assessment of Current Capability for Timely Detection of an Attack
Along with assessing the risk of an anthrax attack, jurisdictions need to assess their current surveillance and detection capability in order to evaluate their ability to meet prophylaxis goals and the potential usefulness of prepositioning in achieving those goals. The various mechanisms for detecting an attack were discussed in Chapter 2. Minimizing the time between the decision to dispense and antibiotic administration becomes increasingly crucial as the delay in detecting an attack increases. Therefore, prepositioning strategies may be particularly beneficial in jurisdictions that lack robust detection and surveillance systems. Jurisdictions should assess their community’s detection and surveillance capabilities and take into account the possibility of delayed detection of an attack when deciding whether prepositioning strategies would be beneficial.
The committee does not intend to imply that if a jurisdiction’s capability for rapid detection is low (for example, a rural jurisdiction without BioWatch sensors), this should automatically be addressed through the addition of rapid detection technology; many considerations beyond the scope of this report are involved in such a determination. The point is simply that low detection capability is one potential indicator that prepositioning would be beneficial.
Assessment of Current Distribution and Dispensing Capability
Jurisdictions also need to understand the capability of their current distribution and dispensing system in order to evaluate whether prepositioning strategies would benefit the community. If a community’s distribution and dispensing system is already capable of covering the entire population within an appropriate time window, the development of prepositioning strategies is unnecessary. Conversely, if a community has inadequate overall capability or gaps in reaching particular subpopulations, prepositioning strategies may be beneficial. The effort required to gain an accurate understanding of distribution and dispensing capability will likely be more resource-intensive than current practices are, but that understanding is necessary before decisions are made about developing and implementing expensive prepositioning strategies.
Jurisdictions should assess their overall distribution and dispensing capability and determine whether there are any gaps. A gap analysis can reveal whether certain portions of the population, by virtue of socioeconomic status and/or demographic characteristics, are at a systemically increased risk of reduced access to disaster mitigation response. Some people may not be well served by traditional points of dispensing (PODs)—for example, people with low incomes or limited transportation options, people with no or limited English proficiency, historically underserved ethnic/racial groups, people with disabilities (especially the vision impaired, hearing impaired, and mobility impaired), people who are homeless, and people who are homebound. Identifying such gaps is important in determining whether strategies are appropriate to augment current distribution and dispensing mechanisms and, if so, which strategies are likely to be most appropriate.
It is difficult, however, to obtain an accurate assessment of jurisdictions’ current distribution and dispensing capability for three primary reasons. First, the full capability of the SNS has not been demonstrated. Second, the extent to which distribution and dispensing plans have been developed is used as a proxy for understanding how those plans will be implemented. Although this may be a useful first step, it does not capture the realities and potential obstacles associated with implementing a distribution and dispensing plan in a real emergency. Where performance metrics exist, they are scattered across different grant requirements and often simply measure whether a task was performed or not, rather than the quality of performance. Third, full-scale drills have not been required until now, so information on how distribution and dispensing systems function has been obtained from piecemeal exercises. These data cannot provide a complete and accurate picture of the overall capability of a jurisdiction’s distribution and dispensing system.
Distribution from the Strategic National Stockpile
Jurisdictions need to know how quickly SNS assets will reach them so they can calculate their capability to complete dispensing of initial prophylactic MCM to their population within the appropriate time window. SNS Push Packages (described in Chapter 3) are deployed rapidly in response to an ill-defined threat within no more than 12 hours of the federal decision to deploy and are unlikely to include the full quantity of MCM necessary for initial prophylaxis (CDC, 2011a). Current estimates of the time to deliver the initial Push Packages to the Tier 1 UASI cities after the decision to dispense varies between approximately 4 and 8 hours for the first shipment to arrive (Burel, 2011). The committee is unaware of any large-scale exercise of the vendor-managed inventory (VMI) portion of the SNS, which is expected to begin arriving within 24-36 hours and contains the supplies needed beyond the original Push Package quantities. It is also unknown whether the SNS has the capability to distribute MCM to multiple locations simultaneously and over a sustained period of time (as would be necessary in the reload scenario envisioned by Danzig [2003]). The gap in knowledge surrounding distribution from the SNS prevents jurisdictions from understanding the capability and capacity of the current prophylaxis system. If the current system can provide prophylaxis to the exposed population within the specified time window, the disadvantages of prepositioning discussed in Chapter 4 and later in this chapter (e.g., increased cost) may outweigh any marginal benefit from a decrease in time to prophylaxis. If, on the other hand, significant challenges exist in the centralized distribution system, prepositioning at the state and local levels may be highly beneficial.
Existing Performance Measures and Metrics
Four primary sources measure state and local MCM dispensing capability and capacity: the Centers for Disease Control and Prevention’s (CDC’s) Public Health Preparedness Capabilities, the SNS Technical Assistance Review (TAR) tool, the RAND-CDC Performance Metrics Project, and the CDC-administered Public Health Emergency Preparedness (PHEP) grant (CDC, 2009a,b, 2011b; Nelson et al., 2009). This section describes the performance metrics associated with each of these four sources, highlighting the need for more and better measures and metrics for accurately assessing a dispensing system’s performance.
Public Health Preparedness Capabilities: National Standards for State and Local Planning CDC’s Public Health Preparedness Capabilities (CDC, 2011b) catalogs the fundamental capabilities that all jurisdictions should
have to mount a successful mass prophylaxis campaign. These capabilities are broken down into five core dispensing functions:
• identify and initiate MCM dispensing strategies;
• receive MCM;
• activate dispensing modalities;
• dispense MCM to identified populations; and
• report adverse events.
For each function, the document lists associated tasks, performance measure(s), and resource elements required.
This effort is a significant step forward because it highlights the importance of performance measures for each MCM dispensing function and identifies gaps in existing measures. Those gaps remain substantial, however: three of the five core functions as yet have no associated performance measures. Performance measures for specific functions are part of the Medical Countermeasures Distribution and Dispensing (MCMDD) composite measure, introduced in the 2011 PHEP cooperative agreement grant guidance (CDC, 2011c). The composite measure, discussed in greater detail below, was designed to describe comprehensively the capability of a jurisdiction (state or Cities Readiness Initiative [CRI] area) to meet Public Health Preparedness Capability 8: MCM dispensing (CDC, 2011c).
Technical Assistance Review (TAR) tool for states and localities The SNS TAR entails a detailed evaluation of state and local jurisdictions’ planning efforts for receiving, distributing, and dispensing SNS MCM (CDC, 2009a,c). At both the state and local levels, the TAR assesses 12 core distribution and dispensing functions, similar to but more detailed than the five core functions of CDC’s Public Health Preparedness Capabilities (the state TAR measures the additional function of capability to repackage). The SNS TAR does not emphasize performance measures: of the local TAR tool’s 85 metrics, only 7 are performance measures. TAR scores are based primarily on an “all or nothing” scale: a full score is awarded if an item can be identified in the plan or if an exercise has been conducted. The score does not depend on the quality of performance during the exercise.
The TAR, like Public Health Preparedness Capabilities, represents progress in assessing state and local preparedness to mount a mass prophylaxis campaign, but it evaluates jurisdictions primarily by how well they plan. Adding more performance metrics and measures of the ability to achieve preset prophylaxis goals to the SNS TAR would enable jurisdictions to chart their progress quantitatively and determine persistent weaknesses.
RAND-CDC Performance Metrics Project (Nelson et al., 2009) The 2009 RAND report New Tools for Assessing State and Local Capabilities for Countermeasure Delivery is intended to provide detailed performance metrics that build on and are compatible with the TAR (Nelson et al., 2009). RAND’s ongoing project has yielded the most detailed performance metrics to date. They measure the capability of system elements to meet their goal, as well as the time required to complete various tasks. Because a primary motivation for prepositioning is to decrease the time to prophylaxis, measurement of the time to completion is critical for determining the potential benefit of prepositioning strategies. If the current distribution and dispensing system were capable of providing prophylaxis to a population within the appropriate time frame, prepositioning strategies would be redundant.
On the other hand, like the performance measures in the Public Health Preparedness Capabilities and the TAR, the RAND metrics are not comprehensive. Specifically, they do not assess the distribution system (SNS to states), a potentially rate-limiting process in a mass prophylaxis campaign. There also are no performance metrics designed to collect data from realistic full-scale exercises rather than piecemeal drills.
Public Health Emergency Preparedness (PHEP) cooperative agreement program Beginning in fiscal year 2011, jurisdictions receiving funding through the CDC-administered 2011-2016 cooperative agreement program are required to report on the MCMDD composite measure described above and must achieve a minimum benchmark score that will increase gradually over time (CDC, 2011c). The measure is meant “to serve as a collective indicator of [MCM distribution and dispensing] preparedness and operational capability” (CDC, 2011c, p. 24). In the first year (i.e., required by July 15, 2012), a jurisdiction’s composite score will comprise the state and local 2011 TAR scores and the results of a minimum of three different drills (from the eight described by previous PHEP guidance) (CDC, 2009b, 2011c). Within the 5-year grant cycle (by 2016), each jurisdiction must participate in a full-scale exercise, the results of which will then contribute to its composite score. These requirements apply to both states and CRI areas, with potentially different requirements for each (specific guidance on conducting and reporting the results of full-scale exercises is expected at a later date). Adoption of this composite measure will enhance intrastate planning and will represent a significant step toward standardizing performance measurement across jurisdictions. Beyond the composite measure, the 2011 PHEP grant will require funded jurisdictions to provide more detailed reporting on capability-based performance measures for MCM dispensing; those details are also forthcoming (CDC, 2011c).
Data from Full-Scale Exercises and Real Events
Along with performance metrics, data on a distribution and dispensing system’s performance are needed to assess how the system will function during a real event. If the data identify system inadequacies, prepositioning of antibiotics may be a means to fill identified gaps. Performance data may be gathered in two ways: from exercises and drills and from real-world experiences.
Data from full-scale exercises Full-scale exercises have been included as an acceptable method of collecting data for fulfilling PHEP reporting requirements in previous years, although these exercises often have not been performed. Instead, other acceptable exercises and drills have been used in their place, including tabletop exercises, drills, and functional exercises (CDC, 2009b). As noted above, the most recent PHEP cooperative agreement (2011-2016) requires each state and CRI area to participate in at least one full-scale exercise over the course of the 5-year grant period (CDC, 2011c). While the details of the exercise requirements are forthcoming, current guidance explains that a CRI area’s full-scale exercises “must include all pertinent jurisdictional leadership and emergency support function leads,” along with all planning and operational staff who will have a response role in a crisis (CDC, 2011c, p. 33).
However, the cost and resources required to conduct a full-scale exercise remain significant barriers for public health departments, which already face limited funding. Full-scale exercises also require partnership and significant coordination with non–public health elements of the distribution and dispensing system, such as schools, community centers, and private businesses. These entities may not be willing or able to interrupt their daily operations to participate in such exercises. Yet without the data from full-scale exercises, jurisdictions are left to extrapolate how quickly the entire system would work in a real emergency, what obstacles they might face (e.g., logistics or communications), and what mechanisms could be used to circumvent those obstacles.
In addition, the data collected in other types of exercises are not standardized, limiting the ability to compare neighboring distribution and dispensing systems and, in turn, making it difficult to identify and apply best practices across a region. Recognizing these issues, CDC has continued to develop and refine templates and guidance, making significant strides with the 2011 PHEP grant requirements for collecting data and interpreting metrics more efficiently and cost-effectively at the state and local levels (Neff, 2011). Ongoing efforts by CDC to facilitate large-scale exercises, whether through funding or through technical support and guidance, will
enable state and local jurisdictions to assess the performance of their MCM distribution and dispensing system.
In addition to large-scale exercises conducted in the context of the PHEP program, other efforts to assess large-scale performance could be useful. DHS, for example, sponsors an annual nationwide exercise that could be a potentially useful venue for assessing the performance of the entire system—from the federal government to state and local entities. In addition, the use of computer simulations or models could be explored to assess a community’s ability to dispense MCM in a timely manner without having to conduct large-scale exercises in each high-risk jurisdiction. Such models would need to be anchored in real-world data to the maximum extent possible and be flexible enough to represent the unique attributes of a wide range of different communities with reasonable fidelity.
Data from real events: the 2009 H1N1 vaccination campaign The 2009 H1N1 influenza pandemic was the most recent real-world test of emergency preparedness plans, offering insights into the working of the entire emergency response system (ASTHO, 2010; FICEMS, 2009; HHS and DHS, 2009; IOM, 2010; NACCHO, 2010). However, the differences between pandemic influenza and anthrax limit the utility of these data for informing anthrax preparedness policy. A response to pandemic influenza takes place over many weeks or even months, whereas the current goal for initial antibiotic prophylaxis after an anthrax attack is 48 hours after the decision to begin dispensing is made (see Chapter 2). Additionally, the 2009 H1N1 influenza pandemic saw an ebb and flow of the number of patients seeking immediate treatment, whereas an anthrax attack could necessitate offering medication to an entire city at once, creating a sudden massive demand on the public health system. The performance of the distribution and dispensing system can also be assessed using more routine real-life distribution events, such as annual influenza vaccination campaigns.
Finding 5-1: To determine the potential benefits of prepositioning strategies, it is critical that jurisdictions accurately assess their distribution and dispensing capability. The few performance measures available for assessing prophylaxis capability are still nascent in their development. Existing performance data often have come from small-scale drills rather than full-scale exercises because of limited financial resources and personnel and the infeasibility of interrupting the functioning of non–public health entities such as schools, community centers, and private businesses. This fact, coupled with limited standardization and comparability of measurement across jurisdictions, makes it difficult to evaluate the current capability of a distribution and dispensing system and, in turn, the value of adopting prepositioning strategies to augment that capability.
Recommendation 5-1: Enhance assessment of performance in implementing distribution and dispensing plans for medical countermeasures. The Centers for Disease Control and Prevention should continue to facilitate assessment of state, local, and tribal jurisdictions’ performance in implementing dispensing plans for medical countermeasures, in addition to assessing planning efforts. More specifically, the Centers for Disease Control and Prevention, in collaboration with state, local, and tribal jurisdictions, should facilitate assessment of the entire distribution and dispensing system by:
• demonstrating Strategic National Stockpile distribution capabilities to high-risk jurisdictions;
• facilitating large-scale, realistic exercises in high-risk jurisdictions to test dispensing capability; and
• continuing efforts to identify objective criteria and metrics for evaluating the performance of jurisdictions in implementing mass dispensing.
INCORPORATION OF ETHICAL PRINCIPLES AND COMMUNITY VALUES
Many authors have addressed the question of which values and principles ought properly to serve as the basis for policies in public health, both in general and for the prevention of and response to disasters (Childress et al., 2002). Addressing ethical principles specifically for the public health emergency of a pandemic, the University of Toronto Joint Centre for Bioethics produced a list of substantive and procedural ethical principles to guide policy making for disasters (Joint Centre for Bioethics, 2005). Similarly, in its letter report on crisis standards of care, the Institute of Medicine (IOM) proposed an ethical framework that included such elements as fairness, the duty to care and to steward resources, transparency, and proportionality (IOM, 2009).
In these reviews of public health ethical principles, distinguishing features include a concern with both outcomes and processes. Utilitarian goals, such as saving the greatest number of lives, clearly play a part in shaping ethical priorities. Just as important to note, however, is that utilitarian goals never supply the entire ethical framework for public health policy. Rather, utilitarian goals within democratic societies are balanced by the need to uphold additional substantive and process principles. A desirable outcome, such as saving many lives, if attained by unethical means, such as discrimination against a vulnerable class within the population, does not reflect an ethically viable policy.
Ethical Framework
Among the various possible ethical frameworks, which substantive and procedural principles are most relevant for the current context of providing MCM for an anthrax attack? The committee believes an ethically sound policy for this context should include the following elements:
• Promotion of public health—Strive for the most favorable balance of public health benefits and harms based on the best available research and data.
• Stewardship—Demonstrate stewardship of public health resources.
• Distributive justice—Distribute benefits and harms fairly, without unduly imposing burdens on any one group in the population.
• Reciprocal obligations—Recognize the professional’s duty to serve and the reciprocal obligation to protect those who serve.
• Transparency and accountability—Maintain public accountability and transparency so that community members grasp relevant policies and know from whom they may request explanation, information, or revision.
• Proportionality—Use burdensome measures, such as those that restrict liberty, only when they offer a commensurate gain in public health, and no less onerous alternatives are both available and feasible.
• Community engagement—Engage the public in the development of ethically robust MCM dispensing plans, including plans for prepositioning antibiotics, to ensure the incorporation of community values.
This section addresses each of the above elements of an ethical framework in turn, starting with the need for promotion of public health. First, it is necessary to acknowledge that sound ethical policies are based on the best available evidence regarding public health interventions. To promote public health effectively, public health authorities must assess a wide range of possible interventions and carefully weigh their possible impact. Good policy rests on good science. Thus the most challenging aspect of evaluating different prepositioning strategies may well be the lack of conclusive data. Acknowledging this uncertainty and working to obtain better data are ethical obligations that emerge from this first element of the framework. Failure to adhere to established public health practices and to acknowledge uncertainty can have dire consequences, such as when the Environmental Protection Agency (EPA) too speedily pronounced air quality safe in the immediate vicinity of the World Trade Center after the terrorist attack of
September 11, 2001, arguably increasing both exposure and health consequences for workers and residents of the area (EPA, 2003; Schorn, 2009).
Stewardship, the next element of the framework, is especially crucial now, when public health dollars are severely limited in many jurisdictions. Any expenditure or intervention will mean that other possible public health measures can no longer be funded. Communities today face significant health challenges from threats as diverse as obesity, diabetes, E. coli in the food supply, tobacco use, and influenza. Public health authorities must scrutinize the level of every risk and the benefit of every dollar. Public health measures against terrorism cannot be viewed in isolation, but they form part of the total context of public health priorities and interventions.
The next element in the framework calls for distributive justice. Health care access and health outcomes are highly variable within communities and often are unevenly distributed according to levels of income and education, insurance status, and ethnicity. We cannot hope to correct these inequities in the moment of a public health disaster, but policies for such events should not exacerbate existing inequities. Ethically sound public health policies must provide equivalent benefits to different groups within the population, while harms such as the inconvenience and risk of picking up MCM at a distant site should not be borne disproportionately by vulnerable groups.
The next element addresses the reciprocal obligations of professionals and those they serve. Professionals have an ethical obligation to use their skills and training in times of crisis, while communities in turn have an obligation to offer appropriate protection to those who take risks on their behalf. In the context of prepositioning MCM for anthrax, this principle is relevant to options that ensure that families of first responders have access to MCM when the first responders must work and cannot avail themselves of standard means of obtaining MCM used by the general public (see the discussion of the postal model in Chapter 3 and 4).
The next three elements of the ethical framework focus on the role of ethics in relation to the community. Transparency and accountability are crucial elements of any important public policy in a democracy, especially policies related to disasters. A natural disaster or terrorist attack creates fear and turmoil, which, in turn, place extraordinary pressure on the bonds of trust between public servants and communities. A perception that authorities are less than candid or inadequately responsive can further erode a community’s ability to function just when full and efficient cooperation is most needed.
The principle of proportionality instructs us to preserve liberty and human rights as crucial aspects of our democracy even when under severe duress. To some extent, a command-and-control orientation must exist in the early stage of a disaster while damage is being assessed and first
responders are caring for those in acute need. It is equally important, however, to preserve the legal structure of democracy in a way that does not impede disaster response yet limits incursions on liberty to those necessary to protect public health and safety. Any limit to individual freedom that is imposed must be the only viable means of promoting an important public health goal.
The final element of the ethical framework calls for community engagement. This crucial step can mean the success or failure of attempts to create viable public health policy. When disaster strikes, if people are either uninformed or unwilling to accept disaster plans, they will not carry them out effectively. Expert opinion and review of data are important components of planning but never can be the sole determinants of what is appropriate for an individual community. During planning for pandemic influenza, for example, experts gave priority to the needs of medically vulnerable adults. A community engagement project revealed that the public believed that children should be given higher priority than those vulnerable adults (Keystone Center and University of Nebraska, 2008). Indeed, a consistent message that flows from public engagement processes is that the needs of children should receive greater attention than is currently the case in public health planning (Keystone Center and University of Nebraska, 2008; Li-Vollmer, 2010; Vawter et al., 2010). Public engagement is necessary to ground planning in public values.
At the same time, however, community values that may serve as the basis for ethically sound policy are not without limits. One could imagine, and perhaps find, a community that wished to prevent members of a locally reviled group from receiving scarce benefits in a disaster. Various laws enshrine ethical principles that prevent discrimination and would likely render unworkable a plan that prevented access to emergency resources for vulnerable groups. For instance, a community could not legally enact a preference to exclude prisoners in the local jail from access to scarce resources, even though doing so might accurately reflect the community’s values. A responsible process for community engagement will include adequate transparency and educational information to make clear that not all options are ethically and/or legally viable. Nonetheless, there is room for flexibility and for addressing specific concerns related to values and preferences. The demand for greater emphasis on the needs of children is one example of how successful community engagement can improve alignment between community values and public policy. An effective community engagement process will facilitate deliberation of ethically and legally viable choices, building consensus and transparency in the process.
Public engagement should not be limited to a focus on planning strategies, but should also include attempts to provide an overall picture of the bioterrorism and other public health threats to the community and the op-
tions for protective measures. Thorough efforts at education and dialogue are critical for building community resilience.
Ethical Issues Specific to Prepositioning of Medical Countermeasures
Various ethical challenges arise in planning for different sorts of public health responses. In the case of a pandemic, the tension between isolation/ quarantine and individual liberty may be of primary concern. Since inhalational anthrax is noncommunicable, isolation is not a useful response and thus is not a relevant ethical challenge in this context. In the case of an anthrax attack involving a well-defined area of known contamination with anthrax spores, public health and public safety authorities might consider quarantining the area or confining people to their homes so they would not come in contact with the contaminated area.
One question relevant to prepositioning of anthrax MCM is whether public health resources should function as the primary source for distribution of emergency MCM or as a safety net for individuals unable to meet their needs in another way. In areas prone to natural disasters, all community members are encouraged to maintain supplies such as drinking water, emergency food rations, and means to provide warmth and light. Public shelters also supply these critical items and will offer them to any member of the community, but there is no ethical objection to individuals’ maintaining private stores of these items in case of disaster. Indeed, those who are able to access their own supplies during a disaster lessen the strain of providing for those in desperate need.
Antibiotics likely to be of use in response to an anthrax attack, including doxycycline and ciprofloxacin, are widely available and not prohibitively expensive. In the event of an anthrax attack, these antibiotics could nevertheless be temporarily scarce if exposed citizens needed rapid access to greater supplies than were immediately available. Still, this scarcity would be temporary and related to the disaster context rather than absolute, as is the case, for example, with solid organs for transplantation.
The lack of absolute scarcity changes the ethical viability of certain proposals for the distribution of anthrax antibiotics. For instance, if some people secured their own supply of antibiotics in advance of an attack, this would not diminish the overall supply of antibiotics available for the rest of the community. When there is an absolute limit to a scarce resource, such as kidneys for transplant, distribution to any one individual precludes distribution to someone else. In the case of rapid distribution of MCM after a disaster, prior distribution to some individuals would potentially reduce wait times for others and thus could provide a benefit to both groups (win/win) rather than a gain for one group at the expense of the other (win/ lose). Community members who wished to maintain their own supplies of
antibiotics might argue that they were merely keeping emergency supplies at hand, much as others might wish to keep a supply of clean water or canned goods ready in case of a flood or power outage. Further, such individuals could argue that the state should have a compelling reason to prevent them from taking this step to protect their families. Of course, families could not simply purchase antibiotics as they would emergency food supplies; they would first need to obtain a prescription from a physician who supported their plan. As discussed above, the procedural principle of proportionality indicates that public health policy should not limit individual freedom unless compelling and proportionate public health goals cannot be met in a less restrictive fashion. This line of argument results in a favorable appraisal of the ethical aspect of proposals to permit individual citizens to keep initial supplies of anthrax antibiotics in their home, independently from publicly held supplies. A final decision on whether individuals should maintain home stockpiles requires a full assessment of the other factors discussed in this report, such as the local risk of attack, the well-documented risks associated with taking antibiotics in inappropriate doses or for the wrong indication, cost, effectiveness, and flexibility (see Chapter 4 and below).
At the same time, while there is no strong ethical argument to prevent community members from obtaining MCM in advance of an anthrax attack, neither is there an ethical basis for public authorities relying on this method in lieu of other modes of distribution, particularly if individuals, not public health entities, are intended to bear the costs. Many community members lack sufficient funds to meet their current daily medical needs. If individuals bore the cost of home stockpiling, the impoverished, uninsured, and underinsured would effectively be prevented from relying on this strategy. A lack of stable housing or of knowledge of the danger of terrorist attack, or a host of other factors, would likely prevent large groups of individuals within the community from purchasing MCM privately in advance. For these vulnerable groups, home stockpiling poses excessive challenges, and additional distribution measures are needed.
Other features specific to distribution of MCM in an anthrax attack have ethical implications. For instance, the POD system requires that people arrive at a distribution center to receive antibiotics. For those with substantial cognitive or mobility impairments, finding and getting to a POD, let alone waiting in a lengthy line, may prove an insurmountable burden. For these vulnerable individuals, different distribution systems may be more viable and therefore ethically appropriate. Current proposals include a head-of-household model in which a single person collects MCM for a family or for groups of vulnerable community members, such as residents in a group home. Community engagement should include specific efforts to engage members of vulnerable populations. Such efforts are important to maintain transparency and earn trust, but they are also critical to help en-
sure that strategies developed for MCM dispensing will function effectively for vulnerable members of the community. This idea has been expressed by the disability community, and has since adopted by others, as “nothing about us without us” (e.g., Carlin, 2011; Charlton, 1998).
Taking into consideration both the general ethical considerations that should be involved in drafting public health policy and issues specific to the question of prepositioning anthrax MCM, the committee makes the following recommendation.
Recommendation 5-2: Integrate ethical principles and public engagement into the development of prepositioning strategies within the overall context of public health planning for bioterrorism response. State, local, and tribal governments should use the following principles as an ethical framework for public health planning of prepositioning strategies:
• Promotion of public health—Strive for the most favorable balance of public health benefits and harms based on the best available research and data.
• Stewardship—Demonstrate stewardship of public health resources.
• Distributive justice—Distribute benefits and harms fairly, without unduly imposing burdens on any one population group.
• Reciprocal obligations—Recognize the professional’s duty to serve and the reciprocal obligation to protect those who serve.
• Transparency and accountability—Maintain public accountability and transparency so that community members grasp relevant policies and know from whom they may request explanation, information, or revision.
• Proportionality—Use burdensome measures, such as those that restrict liberty, only when they offer a commensurate gain in public health and when no less onerous alternatives are both available and feasible.
• Community engagement—Engage the public in the development of ethically sound dispensing plans for medical countermeasures, including plans to preposition antibiotics, so as to ensure the incorporation of community values.
EVALUATION OF POTENTIAL MCM PREPOSITIONING STRATEGIES FOR ANTHRAX
As discussed in the preceding chapter, various strategies for prepositioning MCM are available, including
• forward-deployed MCM (e.g., local stockpiles),
• cached MCM (e.g., hospital caches, workplace caches),
• predispensed MCM in all or some homes in a community, and
• combinations of these.
The committee defines a prepositioning strategy as the specification of locations where MCM are stored and, for each location, the amount of antibiotics stored, associated dispensing methods, and protocols for their use in the event of a confirmed or suspected attack (e.g., for general use at public PODs, for use at a specific closed POD, for home use).
The primary purpose of prepositioning is to provide individuals who have been exposed or potentially exposed to anthrax more rapid access to MCM. (Potentially exposed individuals include those who are not known to have inhaled enough spores to become sick but who nonetheless require antibiotics.) All of these individuals are referred to here as members of the population at risk.
Each potential prepositioning strategy can have advantages and disadvantages, depending on where and how it is implemented and the characteristics of potential anthrax attacks. This section refers to the characteristics of an attack (the type of locale, number and concentrations of spores released, method of release, weather conditions, location indoors or outdoors, local population density, etc., combined with the nature and distribution of the exposure within the population at risk) as an attack scenario, or scenario.
This section focuses on two of the most critical factors impacting the ultimate decision about whether and how to preposition: the potential health benefits and likely economic costs of prepositioning strategies. It describes a general approach that can be taken by local communities to estimate these factors and explains how the results could be used to support informed decision making. In particular, this section presents a modeling approach that is recommended for use in evaluating potential health benefits and the likely economic costs of alternative prepositioning strategies. Thus instead of recommending any particular strategy, the committee recommends that each community use this approach to identify and evaluate strategies appropriate to its own needs, objectives, value trade-offs, and constraints. This section also provides a synthesis of the existing evidence regarding the potential health benefits and likely costs of the various strategies. Note that, referring to the decision-aiding framework presented earlier in Box 5-1, two of the elements of evaluation of potential MCM prepositioning strategies for anthrax—evaluation of potential health risks and consideration of practicality—were discussed in detail in Chapter 4, and that discussion is not repeated here (although health risks are touched on briefly in the context of evaluating potential health benefits).
Evaluation of Potential Health Benefits
Prepositioning has two potential health benefits. First, prepositioning can directly reduce time to prophylaxis, defined here as the time from when an individual is exposed to anthrax (which is assumed for purposes of this discussion to be the same as the time of the release) until the time he or she receives antibiotics for anthrax. Because the intent of prepositioning is to place the MCM close to the point of dispensing, the MCM are expected to be available for dispensing sooner. Second, prepositioning can potentially reduce the overall time to prophylaxis for the community indirectly by reducing demand on some PODs, thus allowing those individuals seeking prophylaxis from these PODs to receive antibiotics sooner. Ultimately, both of these benefits, if achieved, could translate into reductions in mortality (as well as morbidity), compared with a base case of no prepositioning, if an attack occurred. Conversely, if a prepositioning strategy delayed time to prophylaxis (e.g., by creating additional traffic jams, queuing delays, and travel time requirements), it could result in increased mortality.
Health benefits are defined here, then, as reduced mortality compared with the base case of no prepositioning. The value of any health benefit, of course, depends strongly on both the size and the type of the affected community and the attack scenario. For example, in the case of a small anthrax attack with known exposure (e.g., a known attack in a specific building), predispensing in homes might provide little benefit, whereas in the case of a large and diffuse attack (e.g., aerosol dispersal of anthrax spores over a large metropolitan region), predispensing might have the potential to provide significant health benefits. Similarly, health benefits will depend on the intensity of spore dispersal and inhalation. For example, individuals who have inhaled few spores may become sick later than individuals who have inhaled many spores, or not at all (Baccam and Boechler, 2007; Brookmeyer et al., 2003; Coleman et al., 2008). As discussed below, health benefits also will depend on the time between the release and when the decision is made to order mass dispensing and use of MCM. For example, if an alarm were declared relatively early (e.g., within a couple of days after the attack occurred) compared with a typical 4- to 8-day incubation period for anthrax (Chapter 2), reductions in time to prophylaxis due to prepositioning might have little effect on mortality.
As indicated by the decision-aiding framework presented earlier in Box 5-1, prepositioning also entails potential health risks that must be weighed against the above benefits. These risks were discussed in detail in Chapter 4. In sum, forward-deployed MCM would not be associated with additional health risks relative to more centralized MCM storage strategies. Cached MCM also would not be associated with additional health risks if appropriate secure storage were used. Predispensed MCM, however, would
pose potential health risks. The most extensive body of relevant evidence (statistics on the misuse of antibiotics prescribed for routine medical care) suggests that inappropriate use would be high if predispensing were implemented broadly for the general public. Inappropriate use could result in adverse reactions, increased community and individual antibiotic resistance, and other concerns related to storage conditions and medication disposal. For groups and individuals lacking timely access to MCM through other dispensing mechanisms, however, the risk of not receiving postexposure prophylaxis following an anthrax attack may outweigh the potential health risks associated with inappropriate use. In addition, with a more focused strategy targeting vulnerable subpopulations, it might be easier to develop systems to decrease inappropriate use.
Evaluation of Likely Economic Costs
A range of costs are associated with prepositioning, including not only the quantifiable economic costs of creating and maintaining antibiotic stockpiles but also potentially nonquantifiable social costs that might result from prepositioning (over and above those that might exist without prepositioning). The latter might include costs due to possible inequities among different groups of people in the population at risk created by the existence of prepositioning; medical consequences of misuse of prepositioned MCM; social costs due to possible civil unrest, black market activities, and the like that might be engendered by the existence of prepositioned MCM; or costs arising from false confidence, moral hazard effects, or other distortions of incentives to manage risks created by prepositioning. Without downplaying the importance of such nonfinancial costs to society, the present discussion focuses on quantifiable economic costs.
The economic costs of prepositioning include the costs of purchasing the antibiotics; transporting them to their storage locations; maintaining the stockpiles, including the costs of labor, storage facilities, inventory tracking and replacement, and security; and dispensing, which may vary according to the type and amount of prepositioning.
Many of the economic costs associated with prepositioning are fixed: they are incurred regardless of whether an attack occurs. These include, for example, the costs to purchase, store, and maintain stockpiles of prepositioned antibiotics. Other economic costs may be incurred only in the event of an attack. These include, for example, the cost of lost time for people who must wait in line for antibiotics and wages for those who will manage and dispense the MCM. A well-designed approach for determining an appropriate prepositioning strategy (if any) must take into account all of these different economic costs within the context of different types and sizes of communities and alternative attack scenarios.
A Recommended Modeling Approach
As noted above, benefits and costs will depend on the prepositioning strategy, the size and type of community, and the attack scenario. Because of the great variability in communities across the United States (e.g., in size, population density, geographic area, transportation network, and risk of an attack), different communities may benefit most from different prepositioning strategies, including the possibility of no prepositioning. Thus, “one size does not fit all,” and the decision to use (and design) a prepositioning strategy must be based in the context of the particular community. This section describes a modeling approach that can—and the committee believes should—be used by individual communities to inform their decisions regarding prepositioning of antibiotics for anthrax. The committee recommends that each community seek to populate a table with the following conceptual scheme:
| Attack Scenario (and Modeling assumptions) | Prepositioning Strategy | Expected Deaths Averted* | Costs* | ||
| Initial | Annual | Only in event of an attack | |||
*Compared with the base case of no incremental prepositioning.
A range of potential attack scenarios should be considered in evaluating any potential prepositioning strategy. For example, the first column of the above table should list community-relevant attack scenarios. These might include such scenarios as the following:
• Scenario A—small attack with known exposure (e.g., anthrax enclosed in a letter to a member of Congress);
• Scenario B—medium attack with known exposure (e.g., release at a known subway station);
• Scenario C—large attack with unknown exposure (e.g., a crop duster flying over a large metropolitan area); and
• Scenario D—a “reload” multicity attack scenario (three cities attacked, and two additional cities attacked 2 weeks later) (Danzig, 2003; HHS, 2011).
The above are only examples; other scenarios could be envisioned. As discussed above, if information about the likelihood of different attack scenarios is available, it should be used. If not, the model can be used to explore the likely benefits and costs of different prepositioning strategies given different assumptions about the likelihood of these scenarios.
The second column of the table could include the following general categories:
• no prepositioning—no prepositioning of antibiotics for members of the public (referred to here as the base case);
• prepositioning in local warehouses—prepositioning in local warehouses for shipment to PODs (not stockpiled for closed PODs);
• prepositioning in health care settings—prepositioning in local hospital/ pharmacy/health provider stockpiles or institutional facilities for older adults;
• prepositioning in workplace caches—prepositioning in government and private workplaces (e.g., state and local government infrastructure, Fortune 500 companies, small businesses); and
• predispensing in homes, of two types (either of which could be done with home MedKits or personal stockpiles dispensed via normal prescribing routes):
—mass predispensing to the general public, or
—targeted predispensing to specific individuals or groups.
The following sections first present models for estimating the information in the third column of the above table: the health benefits (expected deaths averted) associated with various prepositioning strategies under different attack scenarios and in different types of communities. Two possible models are described: a simple analytical model that can be used to estimate the distribution of time to prophylaxis and the resulting fraction of exposed individuals saved in a community, and a more detailed simulation-based model that estimates these quantities. Next, methods for estimating economic costs associated with prepositioning strategies are discussed. The final section describes how these estimates of benefits and costs can be used to inform decisions about prepositioning strategies.
Modeling to Evaluate Health Benefits
A first-order model This section presents a simplified first-order mathematical model that can be used to estimate health benefits for a given anthrax attack scenario in a given community. The committee does not argue for the accuracy (or even the general form) of this particular model. Rather, given the magnitude of the uncertainties in the various components
of the model—such as delays between release and the decision to dispense, the nature and shape of the anthrax incubation curve, and the ability of a community to achieve its planned POD dispensing time goals—any model that is more detailed than this first-order model will have enough uncertainty in its outputs to make claims of greater accuracy irrelevant.
The underlying approach is straightforward. To determine the health benefits that might accrue from a particular prepositioning strategy, the committee posits some likely parameters describing release/exposure scenarios and then develops a model for computing and comparing estimates of health outcomes for that strategy. The model is described briefly here; full details are provided in Appendix C.
Let ![]() denote the time between anthrax release (and, by assumption, exposure) and the decision to dispense. Assume that all individuals are exposed at the moment of release. For any community, estimates of
denote the time between anthrax release (and, by assumption, exposure) and the decision to dispense. Assume that all individuals are exposed at the moment of release. For any community, estimates of ![]() should ideally be informed by submodels that incorporate the capabilities of currently used (or planned) monitoring and surveillance systems—as well as data from past BioWatch Actionable Results, accidental releases, and exercises—to estimate the various times contributing to the value of
should ideally be informed by submodels that incorporate the capabilities of currently used (or planned) monitoring and surveillance systems—as well as data from past BioWatch Actionable Results, accidental releases, and exercises—to estimate the various times contributing to the value of ![]() . These include the time required to determine clinically that at least one individual has been infected and the time between positive diagnosis and the decision by the responsible public health authority to issue an order to dispense MCM to the population at risk.
. These include the time required to determine clinically that at least one individual has been infected and the time between positive diagnosis and the decision by the responsible public health authority to issue an order to dispense MCM to the population at risk.
Define g as the required time to deliver prophylaxis, from the decision to dispense to completion of prophylaxis for all exposed individuals. Assume that, once dispensing begins, PODs work at full capacity with no idle dispensing staff and that prepositioning of MCM can reduce g: stockpiles located in the community can be available for dispensing sooner than inventories from the SNS, thus enabling prophylaxis to begin—and end—sooner than if there are no local stockpiles.
Define a function f(t) that represents, for any particular release scenario, “the fraction of potential victims that can, in principle, be saved as a function of the time at which medical intervention begins” (Wilkening, 2006, p. 7593), where t is the time since exposure. The curve f(t) is based on data and values for the incubation period, as discussed in Chapter 2. As pointed out in Chapter 2, data with which to compute this survival function are either suspect or limited, and the function will depend on many unknown scenario variables. Nevertheless, to obtain insights into the potential health benefits of prepositioning, the committee has taken the liberty of fitting f(t) to the survival data (based on the Sverdlovsk release) presented by Wilkening (2006, 2008) and Brookmeyer et al. (2001, 2005). Using these data, the curve f(t) can be well fit, for values of t up to about 200 hours, by f(t)=e-(.004)2 . (Note that although this curve does not have a fixed “minimum incubation period,” for t = 24 hours the fraction surviving
is higher than .99.) This function can in turn be approximated (for t up to around 150 hours) by f(t)=1-(.004t)2.
Finally, define S as the expected fraction of the population that will be saved for any prepositioning strategy and any assumed time ![]() after release at which the decision to dispense is made. Given the above assumptions, the quantity S can be calculated (for
after release at which the decision to dispense is made. Given the above assumptions, the quantity S can be calculated (for ![]() + g <150 hours) using the formula below (details are given in Appendix C):
+ g <150 hours) using the formula below (details are given in Appendix C):
![]()
This equation is valid for g >0; since the practical realities of even the most ideal strategy for predispensing to individuals will involve some finite delay, for all practical purposes g will never be exactly equal to 0. In this first-order model, the estimated fraction of individuals who survive a release is a simple function of the time between anthrax release and the decision to dispense (![]() ) and the time from the start of dispensing MCM to completion (g).
) and the time from the start of dispensing MCM to completion (g).
Insights from the first-order model A great deal of insight can be obtained from using the above first-order model, although this simple model is not meant to be the sole basis for quantitative decision making; much more detailed models would need to be used by any jurisdiction to determine precise prepositioning strategies. Given different values of ![]() —the time until the decision to dispense—one can evaluate the fraction of exposed individuals who will be saved, S, for different times until completion of dispensing, g. This latter quantity can be reduced by prepositioning.
—the time until the decision to dispense—one can evaluate the fraction of exposed individuals who will be saved, S, for different times until completion of dispensing, g. This latter quantity can be reduced by prepositioning.
Table 5-1 shows results of an illustrative example. In this example, three prepositioning strategies are considered: no prepositioning, prepositioned caches, and predispensed antibiotics. For the case of no prepositioning, it is assumed that prophylaxis is completed within 48 hours of the decision to dispense (g = 48 hours); this is the current goal of PODs (CDC, 2010). For the case of caches, it is assumed that prophylaxis is completed within 12 hours of the decision to dispense (g = 12). For the case of predispensed MCM, it is assumed that prophylaxis occurs immediately when the decision to dispense is made (g = 0).
In this example, it is also assumed that a BioWatch Actionable Result occurs 24 hours after the anthrax event, an additional 24 hours is required until the first positive anthrax diagnosis is made, and an additional 12 hours is required to confirm that diagnosis (IOM, 2011). Four scenarios are considered. In the first, prophylaxis begins as soon as the BioWatch Actionable Result occurs (24 hours after the anthrax event). In the second, prophylaxis begins as soon as the first positive clinical anthrax diagnosis is
TABLE 5-1 Example Results Using the First-Order Model: Fraction of Exposed Individuals Saved (S) for Different Times Until the Decision to Dispense Is Made (d) and Different Times for Completion of Prophylaxis After the Decision to Dispense (g)
| Prepositioning Strategy | Time Between Decision to Dispense and Completion of Prophylaxis (g, hours) | Fraction of Exposed Individuals Saved (S) | ||||
| SCENARIO 1 Prophylaxis Starts at BioWatch Actionable Result ( |
SCENARIO 2 Prophylaxis Starts at Time of First Clinical Positive Diagnosis ( |
SCENARIO 3 Prophylaxis Starts After Laboratory Confirmation of First Positive Diagnosis ( |
SCENARIO 4 Prophylaxis Starts Ater Delayed Detection and Diagnosis* ( |
|||
| No Prepositioning | 48 | 0.96 | 0.91 | 0.88 | 0.67 | |
| Prepositioned Caches | 12 | 0.99 | 0.95 | 0.93 | 0.75 | |
| Predispensed MCM | 0 | 0.99 | 0.96 | 0.94 | 0.77 | |
* This corresponds to a scenario in which, for example, there is no warning of an attack (e.g., no terrorist announcement), no environmental detection of the attack, and a delay in clinical diagnosis.
made (48 hours after the anthrax event).1 In the third, prophylaxis begins as soon as the first positive diagnosis is confirmed through laboratory testing (60 hours after the anthrax event). In the fourth scenario, the initiation of prophylaxis occurs after delayed detection and diagnosis (120 hours after the anthrax event). For each of these scenarios, the first-order model was used to calculate the expected fraction of exposed individuals who will be saved (the quantity S in the first-order model) for each of the three prepositioning strategies.
Table 5-1 quantifies the increase in the fraction of individuals who will be saved as the time to complete dispensing decreases. In the first scenario (prophylaxis begins 24 hours after attack detection), for example, an expected 96 percent of individuals will be saved if prophylaxis is completed within 48 hours. If local stockpiles enable completion of prophylaxis within 12 hours, or if prophylaxis is completed immediately, the expected fraction of lives saved increases to 99 percent. As another example, in the third scenario (prophylaxis begins 60 hours after attack detection), if prophylaxis is completed within 48 hours of the decision to dispense, the result is an 88 percent expected fraction saved. This can be compared with the 93 percent fraction saved for those who take an average of 12 hours to receive prophylaxis, or the 94 percent fraction saved for those who can receive prophylaxis immediately. In the fourth scenario, where the decision to dispense is not made until 120 hours after the event, if prophylaxis is completed in 48 hours, only 67 percent of the exposed population will be saved, whereas 75 percent of exposed individuals who can receive prophylaxis within 12 hours and 77 percent of individuals with home stockpiles will be saved.
One critical assumption of this analysis is that the MCM is essentially 100 percent effective when dispensed before an exposed individual becomes symptomatic. If data are available that allow calculating the percent effectiveness for a particular MCM and target population, the numbers in Table 5-1 can simply be multiplied by that percentage. This provides the opportunity to introduce into the assessment of the value of a prepositioning strategy the possibility that the prepositioned MCM might have a lower percent efficacy due to such factors as improper storage, wrong dosage, or lower patient adherence.
![]()
1As described in Chapter 2, symptoms of inhalational anthrax do not appear until 4-8 days postexposure or longer. However, symptoms of cutaneous anthrax may appear as quickly as 1-3 days following exposure and could result in detection of the attack following a clinical anthrax diagnosis.
Another insight from this simple model is of interest. If:
• the decision to dispense is approximately the time at which the first case can be clinically and positively diagnosed;
• the survival function is 1.0 for t less than some time period tm, and f( t – tm) for t >tm(tm can be interpreted to be a “minimum incubation period”); and
• the shape of the exponential “drop-off” of f(t) is the same as that given in the example above;
then the health benefits of any prepositioning strategy will essentially be those shown in Table 5-1. In other words, the health benefits depend more on the shape of the survival function than on the minimum incubation period.
Whether the increase in the fraction saved among the exposed population who have access to prepositioned MCM is outweighed by the costs and inequities of the policy providing that access is, of course, the major question that must be answered. The model, however, provides a basis for framing this question in specific terms.
These illustrative analyses show how the simple first-order model could be used to estimate the fraction of exposed individuals saved for different times to decision to dispense, different times until all exposed individuals can be dispensed an initial supply of prophylactic antibiotics, and different assumptions about the anthrax survival curve. As has been emphasized, communities should derive their own estimates of these quantities using data and assumptions specific to their own locale. A copy of the Excel spreadsheet containing the first-order model can be downloaded from www.iom.edu/anthraxreadiness.
More detailed modeling of health benefits Bravata and colleagues (Brandeau et al., 2008; Bravata et al., 2006; Zaric et al., 2008) developed a more detailed logistics model combined with a population-level model of anthrax disease to evaluate the likely impact, in terms of the distribution of time to prophylaxis and thus the fraction of lives saved, for different prepositioning strategies. This spreadsheet-based model numerically projects relevant logistical and disease factors in a population at risk after an anthrax attack. The logistics model captures the flow of antibiotics to PODs and to people, local dispensing capacity, local hospital capacity, and queues for prophylaxis and treatment. The disease model captures the progression of anthrax in the population at risk given the logistical constraints.
For different attack scenarios and different levels and types of prepositioning, the combined model estimates the distribution of time to prophy-
laxis for the population. The time to prophylaxis calculated by the model can be used to estimate mortality in the population and thus deaths averted compared with the base case of no prepositioning. This calculation is made by combining the curve describing the distribution of time to prophylaxis in the population with a curve describing the probability of anthrax survival as a function of how long after exposure the antibiotics are received. Several such curves have been estimated from data for both human and animal populations (Brookmeyer et al., 2001, 2005; Wilkening, 2008), as described in Chapter 2.
Behavior of the public is an important factor affecting the effectiveness of response to an anthrax attack in general and prepositioning strategies in particular (see Chapter 4 for a detailed discussion). Relevant behavioral factors include the rate at which unexposed and exposed people seek prophylaxis, adherence to prophylaxis, and load balancing at the PODs (which may affect the distribution of time to prophylaxis). The model can be used to evaluate changes in these factors and, thus, to evaluate the potential health benefits of prepositioning strategies in light of different assumptions about the behavior of the public, as well as strategies of public health and other officials for communicating with the public. (See Brandeau et al., 2008.)
The analyses of Bravata and colleagues (Brandeau et al., 2008; Bravata et al., 2006; Zaric et al., 2008) were intended to provide general insights into the logistics of anthrax preparation and response, but they were not tailored to specific communities. Communities must perform their own analyses to evaluate the likely benefits and costs of prepositioning strategies in their locale.
Determining Likely Economic Costs
The committee commissioned a paper from PRTM Management Consultants (Appendix D) to evaluate likely costs and time to response for the following prepositioning strategies:
• no incremental prepositioning (e.g., public PODs supplied by the SNS);
• workplace caches, along with workplace PODs, that could serve 20 percent of the population, used to augment inventories supplied by the SNS and public PODs;
• hospital and pharmacy caches, along with associated PODs, that could serve 20 percent of the population, used to augment inventories supplied by the SNS and public PODs; and
• predispensed MCM using home MedKits.
The paper estimates the likely costs of each prepositioning strategy, using as a case study data for Minneapolis-St. Paul. The specific results of the authors’ cost estimates are summarized and discussed below in the section on the committee’s findings and recommendations. The authors estimated costs of three types:
• initial costs of purchasing and stockpiling antibiotics,
• annual costs associated with managing and replacing the antibiotics and with ongoing training of dispensing personnel, and
• costs incurred only in the event of an attack (the costs of dispensing the antibiotics from PODs).
The commissioned paper provides a detailed description of the many different factors and assumptions that would go into estimating likely costs associated with prepositioning strategies. Specific estimates for likely costs would have to be determined by each community using data specific to that community and appropriate assumptions about prepositioning (e.g., the proportion of the population that workplace caches would be expected to cover), but the cost analysis presented in the paper could serve as a practical model for jurisdictions to use in developing their own cost estimates.
Using Estimates of Health Benefits and Economic Costs to Inform Decisions
The above sections have described ways in which the health benefits and economic costs of various prepositioning strategies can be estimated for given attack scenarios and in given communities. This section describes how these estimated quantities can be used to inform decision making.
Evaluating prepositioning strategies for given attack scenarios Given estimates of the health benefits and economic costs of alternative prepositioning strategies, one can consider several measures of cost-benefit. In general, the cost-benefit ratio of an intervention strategy is defined as follows:
| Cost-benefit ratio | = | Incremental cost of a strategy Incremental benefit of a strategy |
Similarly, if both costs and benefits of strategies are monetized, their difference provides a monetary estimate of the net benefit (positive or negative) of implementing the strategy:
Net benefit = Incremental benefit of a strategy – Incremental cost of a strategy
(If cost and benefit estimates are uncertain, and especially if the uncertainties are correlated, their ratio or difference will have an associated probability distribution. If the cumulative distribution of net benefits for one strategy lies to the right of the cumulative distribution of net benefits for another, the first may be said to have greater net benefits even though the actual quantity of those benefits may be quite uncertain.) The measure of net benefits should be determined by the user (examples are given below). Whatever measure is used, the committee does not recommend its monetization.
To evaluate prepositioning strategies, one can compare the costs and benefits of any of the prepositioning strategies incremental to the base case of no prepositioning:
Cost-benefit ratio = C0/Cp
where:
| C0 | = | incremental cost of prepositioning compared with the base case of no prepositioning, and | |
| Cp | = | incremental benefits of prepositioning compared with the base case of no prepositioning. |
To evaluate the cost-effectiveness of strategies aimed at improving health, benefit typically is measured in terms of the incremental health benefits generated by a strategy (Gold et al., 1996). For the case of response to an anthrax attack, an intuitive measure of health benefits is deaths averted. However, one could also use average time to prophylaxis, a benefit measure that is not explicitly a health measure. Considering average time to prophylaxis as a measure of benefit could be useful because of the uncertainty surrounding the time to respond to an anthrax attack and resulting survival. Thus, two cost-benefit measures are considered here: incremental cost per death averted and incremental cost per reduction in average time to prophylaxis. Note that average time to prophylaxis may not be very relevant for predicting health outcomes, which may depend more on the tails of the time-to-prophylaxis distribution than on its mean; that is, if some members of the population do not receive medication for an especially long time, they are far more likely to fall ill. Also, the relationship between average time to prophylaxis and health consequences may be unknown or ambiguous, depending on the rest of the time-to-prophylaxis distribution. The evaluation of alternative strategies will differ with different measures of benefit. In particular, deaths averted and average time to prophylaxis may yield different results.
For each attack scenario, the estimated costs and benefits can be used
to create a figure showing cost on the x-axis; average time to prophylaxis on the y-axis; and a vertical bar for each of the prepositioning strategies, including the base case of no prepositioning. The vertical bar for each strategy reflects the variability in estimated deaths. (If costs are variable as well,
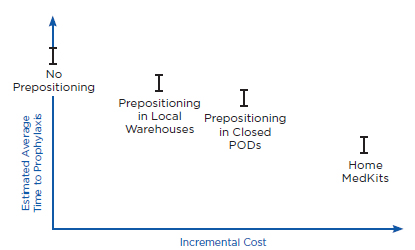
(a) Incremental costs and estimated average time to prophylaxis
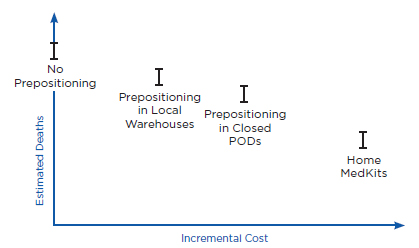
(b) Incremental costs and estimated deaths
FIGURE 5-1
Envisioned model output: incremental costs and absolute benefits, assuming the occurrence of a given attack scenario.
NOTES: These figures are designed to illustrate an example of the envisioned model output; actual results will vary for each jurisdiction.
POD = point of dispensing.
the vertical bar will become a rectangle.) A similar figure can be created showing deaths on the y-axis. Examples are shown in Figure 5-1.
Similarly, it is useful to create figures showing costs and benefits incremental to the base case of no prepositioning (Figure 5-2). From such a
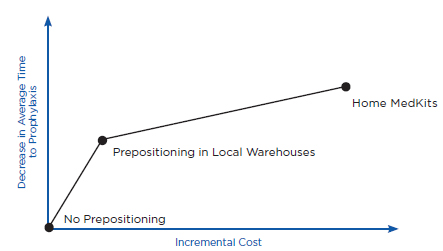
(a) Incremental costs and changes in average time to prophylaxis
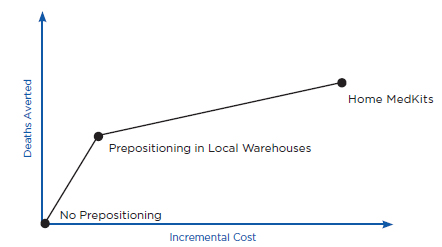
(b) Incremental costs and deaths averted
FIGURE 5-2
Envisioned model
Envisioned model output: incremental costs and incremental benefits, assuming the occurrence of a given attack scenario.
NOTE: These figures are designed to illustrate an example of the envisioned model output; actual results will vary for each jurisdiction.
figure, one can determine the cost-benefit frontier: these are strategies for which no other strategy or linear combination of strategies achieves greater benefits for less cost. (Note that in this example, it is assumed that the costs and benefits of strategies scale linearly, making it possible to draw straight lines between strategies in creating the cost-benefit frontier.) Strategies on the cost-benefit frontier are said to be undominated. The approach suggested here presents both costs and benefits for each prepositioning strategy and attack scenario so that decision makers can make their own value judgments about how to trade off these opposing attributes.
Evaluating expected costs and benefits of prepositioning strategies The expected cost-benefit ratio for any prepositioning strategy (measured either as cost per death averted or cost per hour of decrease in average time to prophylaxis) depends not only on the attack scenario (e.g., size, diffusion, time to detect) but also on the probability that an attack will occur. For example, the expected cost-effectiveness of prepositioning strategies that yield significant benefits primarily in the case of a relatively unlikely attack scenario may be lower than the expected cost-effectiveness of strategies that yield significant benefits for more likely attack scenarios. For a given probability of an attack of a given type (or assumptions about a given probability of a specific type of attack), one can calculate the cost-effectiveness of each prepositioning strategy relative to the base case strategy of no prepositioning (see Bravata et al., 2006; Zaric et al., 2008).
It should be emphasized that “point estimates” of benefits from prepositioning can be misleading. Prepositioning strategies must be evaluated in the context of the many potential attack scenarios that could occur, ranging from no attack to a large, diffuse attack that potentially would affect many people.
This section presents the committee’s findings and recommendations regarding the benefits, costs, and suitability of alternative prepositioning strategies. The goal is to help inform decision making in communities as part of a comprehensive evaluation of the benefits and costs of alternative prepositioning strategies.
Findings
Importance of Adequate Dispensing Capability and Timely Decision to Dispense
The committee reviewed evidence regarding the effect on population survival of dispensing capability and the time until the decision to dispense is made. These factors are important in determining the effectiveness of any response to an anthrax attack, regardless of whether MCM are prepositioned. The committee’s findings establish the importance of these factors in influencing the effectiveness of potential MCM prepositioning strategies.
Bravata and colleagues (Bravata et al., 2006; Zaric et al., 2008) developed a model, described above, that can be used to evaluate the likely impact, in terms of the distribution of time to prophylaxis, of different prepositioningstrategies for anthrax MCM, assuming that all locally prepositioned MCM would be used in public PODs. The authors analyzed time to prophylaxis as well as the number of deaths that might occur for a variety of attack scenarios (e.g., large and small attacks with different numbers of individuals exposed and potentially exposed, and different times until attack detection) and a variety of logistical scenarios (e.g., different times to receive MCM from the SNS, different levels of local inventories of MCM, different levels of local dispensing capacity). They implemented their model using data for a representative metropolitan area with 5 million people.
The analyses of Bravata and colleagues showed that local dispensing capability, not local anthrax antibiotic inventories, is likely to be the rate-limiting factor in response to an anthrax attack: “because of the reportedly rapid availability of regional inventories, the critical determinant of mortality following anthrax bioterrorism is local dispensing capacity. Bioterrorism preparedness efforts directed at improving local dispensing capacity are required before benefits can be reaped from enhancing local inventories” (Bravata et al., 2006, p. 244). The analyses also showed the importance of timely attack detection in preventing deaths from anthrax. Zaric and colleagues (2008, p. 332) conclude that “improved surveillance systems can significantly reduce deaths from such an attack, but only if the local community has sufficient antibiotic dispensing capacity.” In the model presented above, both dispensing capacity and population requiring prophylaxis are reflected in the parameter g, defined as the time from decision to dispense to completion of prophylaxis.
A community’s dispensing capability does not impact the effectiveness of predispensing strategies because predispensed MCM are already dispensed to anticipated users. Some might see this as an argument in favor of widely implementing predispensing strategies in communities in which dispensing capability is low. In fact, the committee instead recommends
that resources be dedicated to enhancing dispensing capability in those communities. The findings that led to this conclusion are discussed in more detail later in this chapter.
Finding 5-2: In the event of an attack, forward-deployed stockpiles and caches will have the potential to decrease morbidity and mortality only if there is adequate dispensing capability, and the time from release to the decision to dispense is short compared with the minimum incubation time. Analytical models of existing distribution strategies show that in the event of a large-scale attack, dispensing capability—not antibiotic inventories—is likely to be the rate-limiting step in getting antibiotics to the potentially exposed population.
In another analysis using the same model, Brandeau and colleagues (2008) show that the behavior of the public is an important factor that can affect the ability of local public health officials to dispense antibiotics in a timely fashion. Depending on the type and amount of communication regarding an anthrax attack that has occurred, members of the public may have different levels of trust in—and understanding of—the messages issued by public health authorities. The level of trust may affect the rate at which exposed individuals seek and receive prophylaxis, the number of unexposed people seeking prophylaxis, and the number of individuals who report to different PODs requesting prophylaxis (thus potentially creating workload imbalances across PODs). The analyses of Brandeau and colleagues (2008) show that each of these factors can have a significant impact on effective dispensing capacity and consequently on mortality. Behavior of the public was also mentioned numerous times in expert testimony to the committee as an important factor affecting dispensing capacity and effectiveness (e.g., Bernier, 2011).
Finding 5-3: Behavior of the public is an important factor affecting dispensing capacity and must be considered in evaluating a community’s likely dispensing capability and thus in evaluating the potential benefits of prepositioned MCM for anthrax.
Potential Effectiveness of Prepositioning Strategies
As mentioned above, the committee commissioned a paper from PRTM Management Consultants (Appendix D) to evaluate likely costs and time to response for the following prepositioning strategies:
• no incremental prepositioning (e.g., public PODs supplied by the SNS);
• workplace caches, along with workplace PODs, that could serve 20 percent of the population, used to augment inventories supplied by the SNS and public PODs;
• hospital and pharmacy caches, along with associated PODs, that could serve 20 percent of the population, used to augment inventories supplied by the SNS and public PODs; and
• predispensed MCM using home MedKits.
The authors developed estimates of cost and time to response for the Minneapolis-St. Paul metropolitan statistical area.
As expected, and consistent with other analyses (e.g., Bravata et al., 2006; Herrmann and Houck, 2011; Zaric et al., 2008), the analysis by PRTM showed that time to prophylaxis decreases as more inventory is forward-deployed, assuming sufficient local capacity for dispensing that inventory. Using specific assumptions about the population (e.g., 1.7 million people needing prophylaxis, 745,000 households), local dispensing capacity (e.g., 20 public PODs that could each provide prophylaxis for 1,000 people per hour), and local dispensing strategy (e.g., one household member could obtain antibiotics for all members of his/her household at a public POD), the authors derived the following estimates:
• If there were no prepositioning, dispensing could be completed in just over 48 hours from the decision to dispense (assuming 745,000 households needing prophylaxis and 20 PODs with dispensing capacity of 1,000 people per hour).
• Dispensing from public PODs, along with hospital/pharmacy caches and associated PODs that could serve 20 percent of the population, could be completed in approximately 43 hours (assuming 20 PODs with dispensing capacity of 1,000 people per hour; 19 hospitals with caches, each having a dispensing capacity of 100 people per hour and serving approximately 29,000 individuals; and 310 pharmacies with caches, each having a dispensing capacity of 100 people per hour and serving 120,000 individuals).
• Dispensing from public PODs, along with workplace caches and associated PODs that could serve 20 percent of the population, could be completed in approximately 43 hours (assuming 20 PODs with dispensing capacity of 1,000 people per hour; 17 workplaces serving approximately 170,000 individuals and each having a dispensing capacity of 1,000 people per hour; and 566 small workplaces serving a total of 170,000 individuals and each having a dispensing capacity of 100 people per hour).
• Dispensing from home MedKits could be accomplished almost instantaneously.
Although these numbers are based on specific assumptions for a specific community, they show the potential decreases in time to prophylaxis associated with different levels and types of prepositioning.
It should be noted that the PRTM analysis assumes that dispensing from home MedKits could be accomplished almost instantaneously. In practice, such dispensing might not be completed for several hours or more because people might not learn immediately of the need to take the antibiotics, and when they did learn, might not be at home. Delays in the time required to complete home dispensing would reduce the time benefit accruing from home prepositioning relative to other prepositioning strategies.
It should also be noted that the PRTM analysis assumes that the hospital/pharmacy and workplace caches and PODs could each serve 20 percent of the population. If these new PODs served a larger fraction of the population, the overall time to complete prophylaxis in the population would be less than that estimated above; conversely, if the new PODs served a smaller fraction of the population, the time to complete prophylaxis would be longer than that estimated.
Using a model derived from that of Bravata and colleagues (Bravata et al., 2006; Zaric et al., 2008), Herrmann and Houck (2011) show that predispensing (e.g., using home MedKits) would reduce time to prophylaxis not only for individuals who had the MedKits but also for other exposed individuals in the population by reducing demand on public PODs, assuming that the capacity of public PODs would not be reduced in light of the availability of MedKits. Because individuals with home MedKits would not have to obtain MCM from public PODs, demand at those PODs would be reduced, and thereby the time needed for the PODs to serve those who did have to use them. Similarly (although not explicitly considered in the analysis of Herrmann and Houck [2011]), the introduction of any incremental closed PODs could reduce demand at public PODs and thereby reduce time to prophylaxis for individuals who used the public PODs to receive MCM.
If the capacity of public PODs were reduced when prepositioning was introduced, these latter benefits would be attenuated, or perhaps even eliminated. Moreover, for the case of home predispensing, people might seek antibiotics from public PODs even if they had a 10-day supply at home. Additionally, if individuals with home stockpiles had used the medication inappropriately before the attack, or if the medication stockpiled in homes were not effective against the strain of anthrax used in the attack, these individuals would need to go to a public POD to receive prophylactic antibiotics. In these cases, the benefits of prepositioning accruing from reduced demand at public PODs would be reduced.
Finding 5-4: Prepositioned MCM have the potential to reduce the expected time until exposed individuals in the population receive prophylaxis. If associated with closed PODs, prepositioned MCM could directly benefit
those receiving MCM from the closed PODs, reducing their time to prophylaxis; by reducing demand at public PODs, they could also benefit those receiving MCM from public PODs, reducing their time to prophylaxis.
The example results estimated in Table 5-1 suggest that prepositioning provides greater benefits when the time from attack until the decision to dispense increases, and conversely, provides lesser benefits for shorter times until the decision to dispense. For example, if the time to the decision to dispense is 48 hours, then with no prepositioning, 91 percent of the exposed population will be saved; with local caches that take an average of 12 hours to complete dispensing of initial doses, 95 percent will be saved; and if dispensing can be accomplished instantaneously from home stockpiles, 96 percent will be saved. In comparison, if the time to the decision to dispense is 120 hours after the event, then with no prepositioning, only 67 percent of the exposed population will be saved; with local caches that take an average of 12 hours to complete dispensing of initial doses, 75 percent will be saved; and if dispensing can be accomplished instantaneously from home stockpiles, 77 percent will be saved. In the first case (48 hours until decision to dispense), if prepositioning can reduce time to prophylaxis to 12 hours, then the fraction saved increases by 4 percent above the baseline fraction of 91 percent. In the second case (120 hours until decision to dispense), the increase in fraction saved for the same prepositioning strategy is 8 percent (calculated as 75-67 percent).
These results occur because of the distribution of the incubation period of anthrax across exposed individuals: reducing time to prophylaxis from 48 to 24 hours after exposure, for example, will likely have little impact on the fraction saved because few individuals will develop prodromal anthrax within that period. On the other hand, reducing time to prophylaxis from, for example, 120 to 96 hours after exposure can significantly improve the fraction saved because many individuals are likely to develop prodromal anthrax between 96 and 120 hours after exposure, as can be seen from the estimated incubation period curves published by various authors (e.g., Brookmeyer et al., 2001, 2003; Wilkening, 2006, 2008).
Finding 5-5: The benefits of prepositioning, measured in terms of time to prophylaxis and resulting fraction of the exposed population saved, increase as the time from attack until decision to dispense increases.
Resources Needed for Prepositioning Strategies
The paper commissioned for this study also estimates the likely costs of each prepositioning strategy, again using data for Minneapolis-St. Paul. The authors’ cost estimates are summarized in Table 5-2. The table summarizes the authors’ estimates for the initial costs of purchasing and stockpiling
TABLE 5-2 Estimated Costs of Alternative Prepositioning Strategies for the Minneapolis-St. Paul Metropolitan Statistical Area
| Strategy | Initial Costs | Annual Costs | Costs Incurred in the Event of an Attack | ||||
| Inventory Purchase/ Stockpiling Cost ($)a | Inventory Replacement Cost ($)b | Inventory Management Cost for Prepositioned MCM ($)c | Costs of Training Dispensing Personnel for SNS-Supplied PODs | Costs of Training Dispensing Personnel for Incremental PODs | Dispensing Costs at SNS-Supplied PODs | Dispensing Costs Associated with Locations Supplied by Prepositioned MCM | |
| No prepositioning (SNS-RSS-PODs) | – | – | – | 895,000 | – | 1,630,000 | – |
| Prepositioning in hospital/pharmacy caches that would serve 20 percent of the population | 718,000 | 0 | 6,000 | 895,000 | 0 | 1,298,000 | 77,000 |
| Prepositioning in workplace caches that would serve 20 percent of the population | 723,000 | 726,000 | 6,000 | 895,000 | 3,683,000 | 1,298,000 | 307,000 |
| Prepositioning in all homes | 16,542,000 | 14,154,000 | 0 | 0 | 0 | 0 | 0 |
NOTES: A population of 1.7 million individuals in 745,000 households is assumed. All estimates are rounded to the nearest $1,000; MCM = medical POD = point of dispensing; RSS = receiving, staging, and storing; SNS = Strategic National Stockpile.
aFor hospital, pharmacy or workplace caches, this includes the cost of purchasing antibiotics and delivering them to the caches. For home prepositioning, this additionally includes the cost of predispensing the medications. The analysis assumes that a 10-day dose stored in a hospital, pharmacy, or workplace cache would cost $2.10, and that a 10-day dose in a home MedKit would cost $5.12. Shipping costs were estimated assuming that a combination of U.S. Postal Service and FedEx shipping would be used.
bIncludes costs to purchase replacement antibiotics and deliver them to the caches (or homes), but does not include costs of disposing of expired inventory.
cFor hospital, pharmacy and workplace caches, a storage/management cost of $14 per 10,000 antibiotic bottles per month is assumed.
SOURCE: PRTM, 2011.
antibiotics; annual costs associated with managing and replacing the antibiotics and with ongoing training of dispensing personnel; and costs that would be incurred only in the event of an attack (the costs of dispensing the antibiotics from PODs). With regard to predispensing strategies, the discussion presented here first focuses on predispensing if implemented as a public health strategy. Individual purchase of personal stockpiles is discussed briefly below.
Initial costs As shown in Table 5-2, the estimated initial purchasing and stockpiling costs for the hospital/pharmacy and workplace prepositioning strategies are quite similar, at approximately $720,000 for this example (based on an estimated cost of $2.10 for a 10-day course of antibiotics, multiplied by 340,000 10-day doses, and a total shipment cost of $3,664 for 340,000 10-day doses), corresponding to an average cost per 10-day dose purchased and delivered of $2.13. The home MedKit strategy would incur estimated initial costs of $16.5 million, an amount that is more than 20 times higher. This amount comprises an estimated $8.7 million to purchase the MedKits (1.7 million at $5.12 per MedKit), plus $5.4 million to ship them (1.7 million shipped at an average cost of $3.18 per kit), plus $2.4 million to dispense them, leading to a total initial cost of $16.5 million—approximately $10 per MedKit.
Annual costs Estimated annual costs of the strategies also vary significantly. Notably, annual inventory replacement costs for the hospital/pharmacy prepositioning strategy are estimated to be negligible (inventories can be rotated into stock when they near their expiration date); annual inventory replacement costs for the workplace prepositioning strategy are estimated to be approximately $726,000; and annual inventory replacement costs for the home prepositioning strategy are estimated to be approximately $14.1 million (comprising $8.7 million to purchase new MedKits plus $5.4 million to ship them to homes). The estimated annual replacement cost of home MedKits is more than 20 times higher than the annual replacement cost for workplace caches for this example (which assumes 100 percent MedKit coverage but 20 percent workplace cache coverage); on a per capita basis, the estimated replacement cost for MedKits is more than four times higher than that for workplace caches.
Note that these estimated annual replacement costs do not include the cost of returning and disposing of expired antibiotics—costs that may be particularly relevant for the case of home MedKits. The vast majority of the MedKits dispensed will reach their expiration date unused. Because of concerns related to improper disposal of antibiotics, it will likely be necessary to include in this strategy a means of enabling people to dispose of their unused MedKits safely. This could involve, for example, includ-
ing a drug disposal mailer (such as the Sharps TakeAway envelope) in the MedKit packaging, with the cost for disposal (estimated at $3 per MedKit2) being incorporated in the product price (Sharps Compliance, Inc., 2011).
The PRTM analysis estimates that annual inventory management costs would be zero for the case of home MedKits and that hospital, pharmacy, and workplace caches would incur very small annual storage/management costs of approximately $6,000 (based on the assumption that storing/managing a pallet of 10,000 bottles of antibiotics would cost $14/month). The committee believes that annual inventory management costs for prepositioned MCM could be higher than this estimate, particularly for workplace caches managed by private-sector entities that lack occupational health programs and infrastructure, because staff time would be needed to monitor storage conditions, oversee disposal and replacement, and ensure compliance with all laws and regulations.
The PRTM estimate of annual costs associated with training dispensing personnel is $895,000 for the case of no prepositioning and for the case of hospital/pharmacy prepositioning (based on the assumption that no incremental dispensing training would be needed for hospital/ personnel); $4,578,000 for workplace prepositioning ($895,000 for training dispensing personnel for SNS-supplied PODs plus $3,683,000 for training dispensing personnel for workplace PODs); and zero for home MedKit prepositioning. This latter value stems from the assumption made in the analysis that, if MedKits are available in all homes, SNS-supplied public PODs will not be needed. To the extent that public PODs are needed, this annual training cost will increase above zero. The analysis assumes that all dispensing staff associated with workplace PODs will have to be trained annually. However, annual costs of training personnel for workplace PODs could vary greatly depending on the workplace. For example, many large corporations have occupational health programs and health personnel on staff who would not need much training, so annual training costs for these workplaces would be lower than those for workplaces without this infrastructure.
Costs incurred in the event of an attack Finally, the PRTM analysis estimates the dispensing costs that would be incurred in the event of an attack for each of the prepositioning strategies. For the case of no prepositioning, the cost of dispensing from public PODs is estimated to be approximately
![]()
2Personal communication to committee member Erin Mullen from Claude Dance, Senior Vice President of Sales and Marketing, Sharps Compliance, Inc. The amount of $3 represents a “ballpark estimate” for the cost of including a self-mailer in the package for a Food and Drug Administration (FDA)-approved MedKit.
$1.6 million. For the case of hospital/pharmacy prepositioning, the cost of dispensing from public PODs is reduced to $1.3 million (as the pharmacy PODs are assumed to serve 20 percent of the population), and the cost of dispensing from hospital/pharmacy PODs is $77,000 (less than the cost of dispensing an equivalent amount of MCM from public PODs because hospital pharmacists and other health care providers at the hospitals could dispense the MCM, whereas some contract staff would likely have to be hired for PODs), leading to a total dispensing cost of approximately $1.4 million. For the case of workplace prepositioning, the cost of dispensing from public PODs is estimated to be $1.3 million (the workplace caches are assumed to serve 20 percent of the population), and the cost of dispensing from workplace PODs is estimated to be $307,000 (slightly less than the cost of dispensing an equivalent amount of MCM from public PODs), leading to a total dispensing cost of approximately $1.6 million. The analysis assumes that the cost of home dispensing in the event of an attack would be zero and that if home MedKits were prepositioned in all homes, public PODs would not be required and thus would incur no dispensing cost. To the extent that public PODs would be needed if home MedKits were available, the cost of dispensing from public PODs would increase above zero.
Relative costs The above cost estimates, although preliminary and specific to the Minneapolis-St. Paul case, highlight some of the cost differentials among the prepositioning strategies. The hospital/pharmacy prepositioning strategy would incur modest incremental initial and ongoing annual costs compared with the strategy of no prepositioning. The workplace prepositioning strategy would incur incremental initial costs similar to those of the hospital/pharmacy prepositioning strategy, but it would likely incur higher annual costs because of the need to replace inventory and train dispensing personnel. Home prepositioning is by far the most costly strategy, with both initial and ongoing annual costs being many times higher than those for the hospital/pharmacy and workplace prepositioning strategies.
Additional costs associated with a Food and Drug Administration (FDA)– approved home MedKit The above estimates of costs associated with home stockpiling do not take into account the costs that would be associated with the development of an FDA-approved home MedKit. The PRTM paper uses the cost per unit of the Emergency Use Authorization (EUA) MedKit provided to postal workers who volunteered in the Minneapolis-St. Paul postal pilot program. Of note, an EUA-approved MedKit does not necessarily require the safety studies and the additional costs associated with standard FDA approval. As noted previously, however, EUA MedKits have been used only for specific targeted groups under carefully limited
conditions, and they have never been used for large groups of the population outside of a research context. The committee observes that the costs of past EUA-approved MedKits do not serve as a good estimate of the cost of an FDA-approved MedKit for sale to the general public, which is likely to be a significantly higher. Use of an FDA-approved MedKit would increase costs over those for either an EUA MedKit or personal stockpiling because of the following additional expenses.
Development cost: Doxycycline is approved for the treatment and prevention of anthrax infection. However, because MedKits would have different packaging and instructions and be dispensed pre-exposure for long-term storage by the ultimate user, FDA approval would be needed for the indication of a prepositioned MCM (NBSB, 2008). While the path forward for FDA approval of a MedKit for anthrax postexposure prophylaxis is not clear, it may be reasonable to assume that the required studies would be similar to those for an antiviral MedKit intended for treatment or postexposure prophylaxis of pandemic influenza. The manufacturers of the antivirals oseltamivir (Roche’s Tamiflu®) and zanamivir (GlaxoSmithKline’s Relenza®) submitted proposals for pandemic influenza MedKits that each proposed four studies to examine labeling comprehension; compliance; and other issues, such as mixing and dosing for pediatric populations (FDA, 2008). Average costs for such studies can range from $610,000 each for nonclinical studies to $5.3 million each for clinical studies (Tufts Center for the Study of Drug Development, 2007). Additionally, as a condition for approval, there may be requirements for continued postmarketing studies and risk evaluation and mitigation strategies (REMS), which, depending on how comprehensive the REMS requirements are, can cost up to $1 million for start-up costs and $100,000/month for ongoing operational maintenance (Morel and Murphy, 2009; Shelley, 2009). If predispensing strategies targeted specific individuals or groups, and not the general public, development costs per capita would be higher.
Packaging costs: Because of different labeling requirements and unit-of-use packaging, MedKits would have higher costs for packaging relative to personal stockpiling.
Insurance coverage: Given their commitment to keep health care costs down, insurers may not cover the increased cost of a MedKit given that a low-cost generic form of doxycycline already is available in the marketplace. Thus, the full cost of a MedKit is likely to be borne by individuals.
Market considerations: It is unclear what the business case would be for industry to develop a product that already is available as a low-cost generic unless a committed market were identified ahead of time. In the current marketplace, once patent protection is lost, conversion to lower-cost generic alternatives reaches 84-94 percent within the first month (Medco, 2009). Given that the costs associated with development of a MedKit and
other related costs would be expected to be recouped through product sales, reduced demand due to generic competition would also require a higher per-MedKit cost.
In sum, FDA-approved MedKits are likely to be substantially more expensive than either EUA-approved MedKits or personal stockpiles attained through the currently available means of prescription given the additional costs of safety studies and packaging. While the costs of disposal of expired drugs may be similar, moreover, costs of replacing the more expensive MedKits would reflect their higher price.
Finding 5-6: Although potentially effective for ensuring that large numbers of people have rapid access to antibiotics, prepositioning strategies will require more resources than strategies that rely on distribution from central locations after an attack. Therefore, prepositioning strategies will provide the greatest value in responding to a large-scale attack in high-risk areas with limited dispensing through the POD system and/or other specific characteristics that would be addressed effectively using prepositioning. Prepositioning strategies may have little added value in areas in which the risk of an attack is low and/or dispensing capacity is sufficient. For these reasons, each jurisdiction should perform its own evaluation of the cost-effectiveness of alternative prepositioning strategies.
As highlighted in the above discussion, predispensing strategies are likely to be much more expensive than other potential prepositioning strategies, probably by at least an order of magnitude (particularly if FDA-approved MedKits are developed and used). Moreover, as highlighted in Chapter 4, they are likely to pose significant safety risks not associated with forms of prepositioning that do not involve home stockpiling. Based on expert testimony provided to the committee, however, there may be cases in which predispensing is appropriate—for example, for individuals who cannot leave their residence or for first responders who do not muster at a workplace.
Based on the available evidence and expert judgment, the committee finds that it is important to consider how to provide antibiotics for those who will be expected to report to work and stay at work during an attack in order to respond and maintain critical functions within the community. These include such individuals as critical infrastructure personnel (e.g., health care workers and power company employees) and first responders. Because these workers would be unable to leave their positions to stand in line at a POD, they would be disadvantaged by a strategy that relied solely on open PODs. Alternative strategies are needed to ensure coverage for such subpopulations. Many jurisdictions also include the families of these workers in their targeted dispensing programs.
The concerns and risks described above for MedKits and personal stockpiles apply equally to the general population and to critical infrastructure personnel, first responders, and their families. Therefore, the committee finds that, where feasible, workplace caches are likely to be a more effective strategy with fewer risks than personal stockpiles in homes. There may be some cases, however, in which workplace caches are not feasible or are not an effective strategy—for example, critical infrastructure personnel and first responders who do not muster at a workplace, and critical infrastructure personnel and first responders for whom it would be infeasible to bring antibiotics from workplace caches back to their families. For this reason, the committee recognizes that communities should retain the flexibility to select various prepositioning strategies, with the suggestion that they select workplace caches over personal stockpiling where possible.
With regard to vulnerable individuals for whom predispensing might be appropriate because of their medical condition and/or social situation, this would likely involve predispensing of a personal stockpile, done through standard prescribing practices and depending on the usual relationship between physician and patient. In this situation, public health would not bear the cost of the predispensed MCM, but similar concerns about risks would exist.
The discussion of ethical principles earlier in this chapter notes that there is no ethical argument against individuals pursuing purchase of personal stockpiles (because these medications are not in short supply, so that purchase by some people does not impose a shortage on others or exacerbate existing inequities in society), and doing so is allowed under current prescribing practices. However, that discussion adds that a final decision on whether individuals should maintain home stockpiles requires a full assessment of the other factors discussed in this report, such as the local risk of attack, the well-documented risks associated with taking antibiotics in inappropriate doses or for the wrong indication, cost, effectiveness, and flexibility. Given this ethical analysis and the findings presented above, the committee does not recommend that individuals pursue personal stockpiles (with the potential exception of those lacking other timely access to MCM). At the same time, with reference to the existing prescription practices, physician-patient relationships, and respect for individual liberties, the committee does not find it advisable to explicitly prohibit this practice either.
Finding 5-7: The use of predispensing as a broad public health strategy for the general population is unlikely to be cost-effective and carries significant risks. Based on a community’s comprehensive risk assessment, however, targeted predispensing may sometimes prove to be an appropriate strategy, particularly for individuals or groups that would lack timely access to anti-
biotics through the existing dispensing system. These might include, for example:
• vulnerable individuals, such as homebound or medically vulnerable individuals for whom physicians and patients agree that predispensing is an appropriate strategy;
• critical infrastructure personnel and first responders who do not muster at a workplace; and
• critical infrastructure personnel and first responders for whom it would be infeasible to bring antibiotics from workplace caches back to their families.
Finding 5-8: The added safety features that might be provided by an FDAapproved MedKit relative to a personal stockpile obtained through regular prescribing practice are unlikely to justify the significant additional cost of developing and purchasing the MedKits. Personal stockpiling currently is allowed under normal prescribing practices, and it could be used to predispense to those targeted groups and individuals for whom predispensing is an appropriate option.
Factors Affecting the Appropriateness of Alternative Prepositioning Strategies
The committee heard testimony from numerous individuals on factors that may affect the appropriateness of different prepositioning strategies, as well as potential health, economic, and other consequences of the strate-gies. Key aspects of this information are synthesized in Table 5-3, which presents, for a range of prepositioning strategies, qualitative characteristics that describe when each strategy would be most likely to be appropriate, along with a qualitative description of the consequences of each strategy.
The first two columns of the table describe, for a continuum of MCM storage locations (first column), associated strategies to consider (second column). The prepositioning strategies are listed in the rows of the table according to increasing levels of forward deployment. The first row, no prepositioning, corresponds to the current situation of inventories held pri-marily in centralized SNS stockpiles, with no incremental prepositioning. The next row, forward-deployed MCM, corresponds to stockpiles held locally in warehouses, either as part of the SNS or other federal deployment (e.g., Department of Defense [DOD] or Department of Veterans Affairs [VA]) or in commercial warehouses. The next row, cached MCM, corresponds to stockpiles held in specific local caches, such as hospitals, pharmacies, or workplaces. Finally, the last row, predispensed MCM, corresponds to per-sonal stockpiles or home MedKits—the maximum level of prepositioning.
TABLE 5-3 Appropriateness and Consequences of Alternative Prepositioning Strategies: Qualitative Summary
| Continuum of MCM Storage Locations | Strategies to Considera | Factors Affecting Appropriateness of Strategies | Consequences of Strategies | |||||
| Risk Statusb | Public Health Dispensing Capabilityc | Gaps in Sub-populations Coveredd | Cost to Public Healthe | Time to Prophylaxisf | Inventory Flexibilityg | Potential for Misuseh | ||
| No Pre-positioning |
– Centralized stockpiles (SNS, other) |
Low | Adequate | None | Limited | Baseline | Greatest | None |
| Forward-Deployed MCM |
– SNS forward-deployed – Other federal forward-deployed (e.g., DOD, VA) – Private forward-deployed |
High | Adequate | n/a | Moderate | Shorter | Medium | None |
| Cached MCM |
– Hospital/pharmacy caches – Workplace caches |
High | Limited | Some | Moderate | Shorter | Less | Some/little |
| Predispensed MCM |
– Personal stockpiles |
Extremely High | Inadequate | Many | Limited | Shortest | Least | Moderate/high |
| – MedKits | High | |||||||
NOTE: DOD = Department of Defense; MCM = medical countermeasures;
n/a = not applicable; SNS = Strategic National Stockpile; VA = Department of Veterans Affairs.
aCombinations of strategies may be appropriate.
bLikelihood of an attack and likelihood of an attack of a given type or size.
cMCM dispensing capability in the event of a large attack.
dSubpopulations that may not be covered by MCM dispensing capacity in the event of an attack.
eThe cost incurred by public health authorities to store and maintain inventories of MCM. Other costs may be borne by other entities, such as private-sector workplaces (e.g., storage, training, and maintenance of workplace caches) and individuals or private insurers (e.g., personal stockpiles). Research and development costs for MedKits may be borne by the federal government, by a private-sector company, or by some combination of these.
fThe time from the decision to dispense until MCM can be delivered to all exposed and potentially exposed individuals.
gInventory flexibility includes the potential for use of multiple drugs, the potential for redeployment of inventories based on need, and the ease with which stockpiles can be rotated.
hPotential for misuse of the prepositioned MCM (e.g., individuals taking the antibiotics for other conditions or not in the event of an anthrax attack).
Factors related to the appropriateness of strategies (the middle columns of Table 5-3) include threat status (the likelihood of an attack and the likelihood of an attack of a given type or size), public health dispensing capacity (capacity to dispense MCM after an anthrax attack), and gaps in coverage of subpopulations (subpopulations that may not be covered by public health MCM dispensing in the event of an attack). These factors would be determined through a local community’s assessment of its anthrax risk and its response capabilities in accordance with the decision-aiding framework presented in this chapter. In Table 5-3, these factors are expressed qualitatively by level (e.g., low versus high versus extremely high threat status).
Consequences of strategies (the rightmost columns of Table 5-3) include the cost to public health (the cost to store and maintain the inventories of MCM), time to prophylaxis (the time from the decision to dispense until MCM can be delivered to all exposed and potentially exposed individuals), inventory flexibility (potential for redeployment of inventories based on need), and potential for misuse (of the prepositioned MCM). Again, these consequences are expressed in Table 5-3 qualitatively (e.g., limited versus moderate versus high cost to public health).
The table consists of a set of suggested “if-then” rules, stored in its rows: if a situation is well described by the entries in a row under “Factors Affecting Appropriateness of Strategies,” then the prepositioning strategy or strategies in the corresponding row might be appropriate to consider. For example, in a community with “some” gaps in covered populations, prepositioning in hospital, pharmacy, or workplace caches might be appropriate. On the other hand, in a community with “low” threat status, “adequate” dispensing capacity, and “no gaps” in covered subpopulations, no prepositioning may be most appropriate.
Similar reasoning can be applied with respect to the consequences of implementing a strategy. As an example, the bottom row (for the MedKits strategy) will lead to the shortest time to prophylaxis, but with a high cost and the least flexibility and the greatest potential for misuse. This strategy is recommended for consideration only if the threat status is “extremely high” (which the committee leaves undefined) and public health dispensing capacity is “inadequate” (also left undefined, but meant to be suggestive). Other strategies in the table represent different trade-offs among cost, flexibility, time to prophylaxis, and potential for misuse, and they are recommended for consideration for different levels of threat status, public health dispensing capacity, and gaps in covered subpopulations.
The rows in Table 5-3 are not meant to be exhaustive—for example, there is no row showing what strategies to consider if threat status is “low” but public health dispensing capacity is “inadequate”—but rather to summarize and synthesize the types of qualitative considerations and trade-offs suggested during discussions for this study of when each strategy most
likely would be appropriate. Although such qualitative summaries can be misleading, as terms such as high and moderate have no precise definitions, and there may be exceptions to the general rules suggested, these summaries do illustrate the types of trade-offs that communities will probably face.
The committee draws several key observations from Table 5-3. First, prepositioning becomes potentially more useful in communities with relatively high threat status, limitations on public health dispensing capacity, and/or gaps in covered subpopulations. Second, prepositioning of MCM is likely to decrease time to prophylaxis but will decrease flexibility, increase costs, and increase the potential for misuse of MCM.
Finding 5-9: Table 5-3 summarizes qualitatively factors related to the appropriateness of various MCM prepositioning strategies and the consequences of implementing these strategies. Prepositioning is potentially useful in communities with relatively high risk status, limitations on public health dispensing capacity, and/or gaps in covered subpopulations. Prepositioning is likely to decrease time to prophylaxis but will decrease flexibility, increase costs, and increase the potential for misuse of MCM.
Recommendations
The above sections have presented an approach for evaluating the benefits and costs of alternative MCM prepositioning strategies, as well as the committee’s findings regarding the benefits, costs, and suitability of these strategies. These findings lead to the following recommendations.
Recommendation 5-3: Consider the risk of attack, assess detection and dispensing capability, and evaluate the use of prepositioning strategies to complement points of dispensing.
State, local, and tribal governments should, in partnership with each other and with the federal government, the private sector, and community organizations:
• Consider their risk of a potential anthrax attack.
• Assess their current detection and surveillance capability.
• Assess the current capability of and gaps in their medical countermeasures dispensing system.
• Based on their risk and capability assessment, evaluate whether specific prepositioning strategies will fill identified gaps and/or improve effectiveness and efficiency. The decision-making framework should include, for a range of anthrax attack scenarios:
— evaluation of the potential health benefits and health risks of alternative prepositioning strategies;
— evaluation of the relative economic costs of alternative prepositioning strategies;
— comparison of the strategies with respect to health benefits, health risks, and costs, taking into account available resources; and
— consideration of ethical principles and incorporation of community values (see Recommendation 5-2).
Recommendation 5-4: Give priority to improving dispensing and developing prepositioning strategies such as forward-deployed or cached medical countermeasures.
In public health planning efforts, state, local, and tribal jurisdictions should give priority to improving the dispensing capability of points of dispensing and push strategies and to developing forward-deployed or cached prepositioning strategies.
The committee does not recommend the development of health strategies that involve broad use of predispensed medical countermeasures for the general population. In some cases, however, targeted predispensed medical countermeasures might be used to address specific gaps in jurisdictions’ dispensing plans for certain subpopulations that lack access to antibiotics via other timely dispensing mechanisms. These might include, for example, some first responders, health care providers, and other workers who support critical infrastructure, as well as their families.
Personal stockpiling might also be used for certain individuals who lack access to antibiotics via other timely dispensing mechanisms (for example, because of their medical condition and/or social situation) and who decide—in conjunction with their physicians—that this is an appropriate personal strategy. This is allowed under current prescribing practice and would usually be done independently of a jurisdiction’s public health strategy for dispensing medical countermeasures.
The following recommendation addresses the development of an FDA-approved MedKit. The committee found that among predispensing strategies, FDA-approved MedKits are likely to be more costly than personal antibiotic stockpiles and EUA-approved MedKits because of the additional costs associated with the rigorous process of FDA approval. Depending on how the MedKit was developed, these costs could be shared between the federal government and the private sector, but consumers and cash-strapped state and local public health agencies might also bear the brunt of these additional costs. There is limited evidence to suggest that FDA-approved MedKits would be less prone to inappropriate use than other forms of
predispensing and, similar to other forms of predispensing, they cannot respond flexibly to different anthrax attack scenarios. The following recommendation does not preclude the use of an EUA MedKit, which would be less costly than an FDA-approved MedKit, but would be appropriate only for targeted use in specific contexts.
Recommendation 5-5: Do not pursue development of a Food and Drug Administration-approved MedKit unless this is supported by additional safety and cost research.
The committee does not recommend the development of a Food and Drug Administration-approved MedKit designed for prepositioning for an anthrax attack until and unless research demonstrates that MedKits are significantly less likely to be used inappropriately than a standard prescription and can be produced at costs comparable to those of standard prescription antibiotics.
ASPR (Assistant Secretary for Preparedness and Response). 2011. FY11 Hospital Preparedness Program (HPP) guidance. Washington, DC: HHS, http://www.phe.gov/Preparedness/planning/hpp/Documents/fy2011-hpp-funding-guidance.pdf (accessed August 22, 2011).
ASTHO (Association of State and Territorial Health Officials). 2010. Assessing policy to effective public health response in the H1N1 influenza pandemic. Arlington, VA: ASTHO, http://www.astho.org/Display/AssetDisplay.aspx?id=4933 (accessed May 18, 2011).
Baccam, P., and M. Boechler. 2007. Public health response to an anthrax attack: An evaluation of vaccination policy options. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 5(1):26-34.
Bernier, R. 2011. The need for evidence-AND values-informed policy making on medical countermeasures—the case for public engagement. Slides presented at the Institute of Medicine Public Workshop for the Committee on Prepositioned Medical Countermeasures for the Public, Washington, DC, http://iom.edu/~/media/Session%203-%20Bernier_Public%20Engagement.pdf (accessed August 8, 2011).
Brandeau, M. L., G. S. Zaric, J. Freiesleben, F. L. Edwards, and D. M. Bravata. 2008. An ounce of prevention is worth a pound of cure: Improving communication to reduce mortality during bioterrorism responses. American Journal of Disaster Medicine 3(2):65-78.
Bravata, D. M., G. S. Zaric, J.-E. Cleophas Holty, M. L. Brandeau, E. R. Wilhelm, K. M. McDonald, and D. K. Owens. 2006. Reducing mortality from anthrax bioterrorism: Strategies for stockpiling and dispensing medical and pharmaceutical supplies. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 4(3):244-262.
Brookmeyer, R., N. Blades, M. Hugh-Jones, and D. A. Henderson. 2001. The statistical analysis of truncated data: Application to the Sverdlovsk anthrax outbreak. Biostatistics 2(2):233-247.
Brookmeyer, R., E. Johnson, and S. Barry. 2003. Modeling the optimum duration of antibiotic prophylaxis in an anthrax outbreak. Proceedings of the National Academy of Sciences 100(17):10129-10132.
Brookmeyer, R., E. Johnson, and S. Barry. 2005. Modelling the incubation period of anthrax. Statistics in Medicine 24(4):531-542.
Burel, G. 2011 (February 28). An SNS perspective on pre-positioning medical countermeasures. Slides presented at the Institute of Medicine Public Workshop for the Committee on Prepositioned Medical Countermeasures for the Public, Washington, DC, http://www.iom.edu/~/media/Session%201-%20Burel_Federal%20Perspective.pdf (accessed March 30, 2011).
Carlin, R. 2011 (April 20). Prepositioned medical countermeasures for the public: Prepositioning for at-risk individuals. Slides presented at the third meeting of the Institute of Medicine Committee on Prepositioned Medical Countermeasures for the Public, Irvine, CA, http://www.iom.edu/~/media/Files/Activity%20Files/PublicHealth/PrepositionedCountermeasures/Meeting%203/2%20-%20Carlin%20-%20At-Risk%20Populations.pdf.
CDC (Centers for Disease Control and Prevention). 2009a (April). Local technical assistance review. Atlanta, GA: CDC-SNS, www.odh.ohio.gov/ASSETS/.../pdf (accessed May 17, 2011).
CDC. 2009b (December). Public health emergency preparedness cooperative agreement: Budget period 10 (BP10) performance measures guidance. Atlanta, GA: CDC, http://www.cdc.gov/phpr/documents/FINAL_BP10_PHEP_Performance_Measures_Guidance_May_2010.pdf (accessed May 17, 2011).
CDC. 2009c (March). Strategic National Stockpile state technical assistance review tool user’s guide. Atlanta, GA: CDC, http://nmhealth.org/HEM/sns/documents/DSNSSTATEUSERSGUIDEMarch2009_ac.pdf (accessed August 17, 2011).
CDC. 2010. Cities Readiness Initiative (CRI).Atlanta, GA: CDC, http://www.bt.cdc.gov/cri/ (accessed February 16, 2011).
CDC. 2011a. Strategic National Stockpile. Atlanta, GA: CDC-SNS, http://www.cdc.gov/phpr/stockpile/stockpile.htm (accessed July 17, 2011).
CDC. 2011b. Public health preparedness capabilities: National standards for state and local planning. Atlanta, GA: CDC, http://www.cdc.gov/phpr/capabilities/Capabilities_March_2011.pdf (accessed May 16, 2011).
CDC. 2011c. Public health emergency preparedness cooperative agreement continuation. Atlanta, GA: CDC, http://www.cdc.gov/phpr/documents/PHEP_FY_2011.pdf (accessed August 16, 2011).
Charlton, J. 1998. Nothing about us without us. Berkeley and Los Angeles, CA: University of California Press.
Childress, J. F., R. R. Faden, R. D. Gaare, L. O. Gostin, J. Khan, R. J. Bonnie, N. E. Kass, A. C. Mastroianni, J. D. Moreno, and P. Nieburg. 2002. Public health ethics: Mapping the terrain. Journal of Law, Medicine & Ethics 30:170-178.
Coleman, M. E., B. Thran, S. S. Morse, M. Hugh-Jones, and S. Massulik. 2008. Inhalation anthrax: Dose response and risk analysis. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 6(2):147-160.
Danzig, R. 2003. Catastrophic bioterrorism: What is to be done? Washington, DC: National Defense University.
DHS (Department of Homeland Security). 2008a. DHS risk lexicon. Washington, DC: DHS, http://www.dhs.gov/xlibrary/assets/dhs_risk_lexicon.pdf (accessed June 2, 2011).
DHS. 2008b. Letter from Michael Chertoff to Michael O. Leavitt on determination pursuant to ![]() 564 of the Federal Food, Drug, and Cosmetic Act. Washington, DC: DHS, http://www.dhs.gov/xlibrary/assets/ofsec_signed_determination092308.pdf (accessed July 10, 2011).
564 of the Federal Food, Drug, and Cosmetic Act. Washington, DC: DHS, http://www.dhs.gov/xlibrary/assets/ofsec_signed_determination092308.pdf (accessed July 10, 2011).
EPA (Environmental Protection Agency). 2003. Evaluation report: EPA’s response to the World Trade Center collapse: Supplemental Appendix: EPA September 18, 2001 press release. Report Number 2003-P-00012. Washington, DC: EPA, http://www.epa.gov/oig/reports/2003/wtc/epapr20010918.htm (accessed June 3, 2011).
FDA (Food and Drug Administration). 2008 (October 29). MedKits containing influenza antivirals for the treatment or prophylaxis of influenza during a pandemic. Washington, DC, and Rockville, MD: FDA-Joint Meeting of the Antivirals Drugs Advisory Committee and the Nonprescription Drugs Advisory Committee, http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4385b1-01-FDA.pdf (accessed July 21, 2011).
FEMA (Federal Emergency Management Agency). 2007 (September). Target capabilities list: A companion to the National Preparedness Guidelines. Washington, DC: DHS, http://www.fema.gov/pdf/government/training/tcl.pdf (accessed May 16, 2011).
FEMA. 2011 (May). Homeland Security Grant Program: Guidance and application kit. Washington, DC: DHS, http://www.fema.gov/pdf/government/grant/2011/fy11_hsgp_kit.pdf (accessed May 13, 2011).
FICEMS (Federal Interagency Committee on Emergency Medical Services). 2009. State EMS system pandemic influenza preparedness. Washington, DC: National Highway Traffic Safety Administration, http://www.ems.gov/pdf/State_EMS_System_Pandemic_Influenza_Preparedness.pdf (accessed May 18, 2011).
GAO (Government Accountability Office). 2009. Project BioShield Act: HHS has supported development, procurement, and emergency use of medical countermeasures to address health threats. Washington, DC: GAO, http://www.gao.gov/new.items/d09878r.pdf (accessed August 22, 2011).
Gold, M., J. E. Siegel, L. B. Russell, and M. Weinstein. 1996. Cost-effectiveness in health and medicine. New York: Oxford University Press.
Herrmann, J. W., and M. L. Houck. 2011. Mitigating the risk of an anthrax attack by placing pre-event pharmaceuticals. Institute for Systems Research Technical Report TR_2011-02. http://drum.lib.umd.edu/handle/1903/11066 (accessed July 21, 2011).
HHS (Department of Health and Human Services). 2011. Aerosolized anthrax response playbook: ESF-8 aerosolized anthrax playbook. Washington, DC: ASPR, HHS, http://www.phe.gov/preparedness/planning/playbooks/anthrax/Pages/default.aspx (accessed August 10, 2011).
HHS and DHS (Department of Homeland Security). 2009 (January). Assessment of states’ operating plans to combat pandemic influenza: Report to the Homeland Security Council. Washington, DC: HHS, http://www.flu.gov/professional/states/state_assessment.html (accessed May 18, 2011).
IOM (Institute of Medicine). 2009. Guidance for establishing crisis standards of care for use in disaster situations: A letter report. Washington, DC: The National Academies Press.
IOM. 2010. The 2009 H1N1 influenza vaccination campaign: Summary of a workshop series. Washington, DC: The National Academies Press.
IOM. 2011. BioWatch and public health surveillance: Evaluating systems for the early detection of biological threats, abbreviated version. Washington, DC: The National Academies Press.
Joint Centre for Bioethics. 2005. Stand on guard for thee: Ethical considerations in preparedness planning for pandemic influenza: A report. Toronto, Canada: University of Toronto.
Keystone Center and University of Nebraska. 2008. Public engagement project on pandemic influenza vaccine prioritization. NACCHO Contract #2007-012406. Keystone, CO: The Keystone Center, http://keystone.org/files/file/SPP/health/VaxPPreportdtrev_FINAL_3-25-2010.pdf (accessed July 18, 2011).
Li-Vollmer, M. 2010 (December 14). Health care decisions in disasters: Engaging the public on medical service prioritization during a severe influenza pandemic. Journal of Participatory Medicine 2:e17.
Medco. 2009. The great healthcare debates: Prescriptions for meaningful reform. http://www.medco.com/art/drug_trend/pdf/DT_2009_Drug_Trend_Report.pdf (accessed July 10, 2011).
Morel, H., and R. Murphy. 2009. Dealing with REMS challenges in drug commercialization.
http://www.mckesson.com/static_files/McKesson.com/McKSpecialty/PDFs/REMS%20article%20reprint-8-09.pdf (accessed July 10, 2011).
NACCHO (National Association of County and City Health Officials). 2010. NACCHO H1N1 policy workshop report. Washington, DC: NACCHO, http://www.naccho.org/topics/H1N1/upload/NACCHO-WORKSHOP-REPORT-IN-TEMPLATE-with-chart.pdf (accessed May 18, 2011).
NBSB (National Biodefense Science Board). 2008. Personal preparedness discussion: Excerpted from the summary report of the National Biodefense Science Board June 18, 2008. Washington, DC: NBSB, http://www.phe.gov/Preparedness/legal/boards/nbsb/Documents/nbsb-excrpt-pp-080618.pdf (accessed July 21, 2011).
Neff, L. 2011 (February 23). SNS-related medical countermeasures drills and exercises. Slides presented at the Public Health Preparedness Summit 2011, Atlanta, GA.
Nelson, C., E. W. Chan, C. Fan, D. Lotstein, L. B. Caldarone, S. R. Shelton, A. L. Maletic, A. M. Parker, A. Felton, A. Pomeroy, and E. M. Sloss. 2009. New tools for assessing state and local capabilities for countermeasure delivery. Santa Monica, CA: RAND Corporation, http://www.rand.org/pubs/technical_reports/TR665.html (accessed May 16, 2011).
PRTM. 2011 (unpublished). A cost and speed analysis of strategies for prepositioning antibiotics for anthrax. Washington, DC: PRTM Management Consulting.
Schorn, D. 2009. The Dust at Ground Zero. CBS News, http://www.cbsnews.com/stories/2006/09/07/60minutes/main1982332.shtml?tag=contentMain;contentBody (accessed August 10, 2011).
Sharps Compliance, Inc. 2011. Find your solution: TakeAway™ Environmental Return System. Houston, TX: Sharps Compliance, Inc., http://www.sharpsinc.com/unused-medications.htm (accessed July 10, 2011).
Shelley, S. 2009. Industry tackles the new REMS hurdle. http://pharmaceuticalcommerce.com/frontEnd/1343-Industry_Tackles_the_New_REMS_Hurdle.html (accessed July 10, 2011).
Tufts Center for the Study of Drug Development. 2007. Challenges loom for postmarketing study commitments; benefits unclear. Impact Report 9(3), http://csdd.tufts.edu/files/uploads/05_-_may15,_2007_-_pmcs.pdf (accessed July 10, 2011).
Vawter, D. E., J. E. Garrett, K. G. Gervais, A. W. Prehn, D. A.DeBruin, C. A. Tauer, E. Parilla, J. Liaschenko, and M. F. Marshall. 2010. Minnesota Pandemic Ethics Project Report. Minnesota Department of Health, http://www.health.state.mn.us/divs/idepc/ethics/ethics.pdf (accessed August 9, 2011).
Wilkening, D. A. 2006. Sverdlovsk revisited: Modeling human inhalational anthrax. Proceedings of the National Academy of Sciences 103(20):7589-7594.
Wilkening, D. A. 2008. Modeling the incubation period of inhalational anthrax. Medical Decision Making 28(4):593-605.
Zaric, G. S., D. M. Bravata, J.-E. Cleophas Holty, K. M. McDonald, D. K. Owens, and M. L. Brandeau. 2008. Modeling the logistics of response to anthrax bioterrorism. Medical Decision Making 28(3):332-350.
























































