3
Current Dispensing Strategies for
Medical Countermeasures for Anthrax
All levels of government (federal, state, and local) and the private sector are involved in the distribution and dispensing of medical countermeasures (MCM) to the public in an emergency.1 This chapter reviews current plans and existing infrastructure for the distribution and dispensing of MCM necessary for public protection against a terrorist attack with Bacillus anthracis (anthrax). The first section provides an overview of the current MCM distribution and dispensing system, from the national to the local level. The chapter then reviews concerns about the current dispensing system, as well as salient legal and regulatory issues.
CURRENT MCM DISTRIBUTION AND DISPENSING SYSTEM
Figure 3-1 depicts the basic MCM distribution and dispensing system currently in place in the United States. In practice, distribution and dispensing activities will vary depending on the type of public health emergency and on state and local resources, infrastructure, and needs. Stores of MCM currently are housed around the country in the federally managed Strategic National Stockpile (SNS), state stockpiles, and smaller local caches (CDC, 2010a). Stocks of MCM also are maintained by the manufacturers. In the event of a public health emergency, these stockpiles and caches are accessed as appropriate. If state and local resources are insufficient or if a specific
![]()
1Distribution, dispensing, and other key terms used in the report are defined in Box 1-2 in Chapter 1.
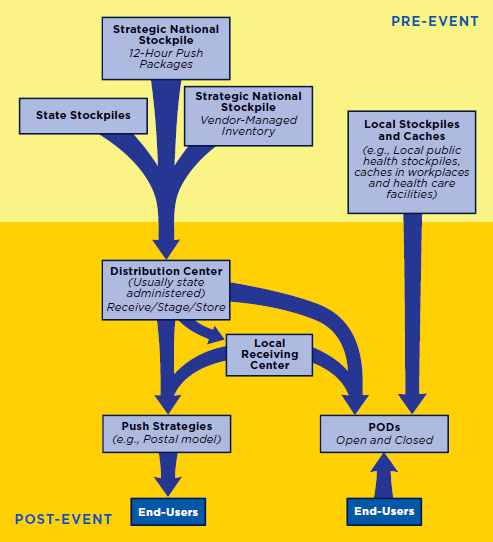
FIGURE 3-1
Basic medical countermeasures (MCM) distribution anddispensing strategy currently in place in the United States.
NOTES: Actual pathways may vary depending on the location and nature of the public health emergency. MCM are released from secure stockpiles around the country to regional or state centers, which facilitate the distribution of MCM to where they will be dispensed to the public. Note that caches may also be the sites of points of dispensing (PODs) (e.g., workplace caches). Vendor-managed inventory may be shipped to distribution sites or directly to PODs.
pharmaceutical is needed, the state governor may request supplementary supplies from the SNS or directly from preidentified vendors.
Supplies released from the stockpiles generally are sent to a state-administered regional distribution center, which receives the shipment, breaks down the packages, stages the MCM in a fashion that allows for rapid distribution, and inventories and stores them as appropriate (CDC, 2010a).2 From this central point, the MCM are distributed to where they will be dispensed to the public, either directly or through intermediate receiving, staging, and storage (RSS) centers. These intermediate RSS centers can be at the state, county, and/or local level depending on the state, but they are referred to collectively as the RSS stage through the report. Currently, the primary distribution model is for the public to receive MCM at points of dispensing (PODs) located throughout the community. Efforts also are under way to develop plans to use the U.S. Postal Service (USPS) to dispense MCM to individual homes (HHS et al., 2011). In addition, many states and localities have plans to dispense MCM via “closed PODs,” in which the MCM are dispensed to preidentified groups—such as employees, their families, and patients—rather than to the public at large. Not shown in Figure 3-1 is prepositioning of MCM for anthrax in homes since there have been only pilot studies of this strategy.
Strategic National Stockpile
The SNS, managed by the Centers for Disease Control and Prevention (CDC), is a national repository of medicine and medical supplies that can be rapidly deployed in the event of a public health emergency that is severe enough to exhaust local supplies (CDC, 2010a). The repository is intended to provide a minimum level of federal coverage as a supplement to state and local resources, and it could be called upon during such events as a natural disaster (e.g., earthquake), an infectious disease outbreak (e.g., influenza), or an act of terrorism (e.g., biological attack with anthrax). SNS resources were deployed, for example, to New York State during the September 11, 2001, terrorist attacks and to most states during the 2009 H1N1 influenza pandemic (CDC, 2009; TFAH, 2005). Established in 1999 as the National Pharmaceutical Stockpile and renamed the SNS in 2003, the repository now contains antibiotics, chemical antidotes, antitoxins, life-support medications, intravenous (IV) catheters and administration
![]()
2Some SNS stores of chemical/nerve agent antidotes are an exception to this traditional distribution mechanism. The CHEMPACK Program, discussed in greater detail in Chapter 4, forward-deploys SNS materiel to state and local warehouses (HHS, 2009). The Centers for Disease Control and Prevention retains control of the materiel until release, while the participating state is responsible for materiel security, the storage facility, and distribution after an attack.
sets, airway maintenance supplies, and medical/surgical supplies (CDC, 2010a). The SNS contains products that have been approved or cleared by the U.S. Food and Drug Administration (FDA) for use as MCM, as well as investigational products that can be used only as specified under an Investigational New Drug (IND) application (or Investigational Device Exemption, as applicable) or under an Emergency Use Authorization (EUA) issued by the FDA (HHS, 2007).3
In response to an emergency, the state governor’s office can make a request for SNS resources to the Department of Health and Human Services (HHS) or CDC (CDC, 2010a). Once federal and local authorities have decided to deploy resources from the SNS, the supplies can be delivered to a designated RSS site in any state within 12 hours. To facilitate this rapid response, Push Packages containing a predefined set of pharmaceuticals, antidotes, and medical supplies are housed in secure warehouses in (undisclosed) strategic locations around the country, ready for immediate release. The packages are stored in such a way that they can be loaded immediately onto trucks or aircraft. These Push Packages are designed to deliver a broad range of supplies that would be most useful during the early stages of an event when a specific threat to health might not yet be well defined.
The contents of the SNS are determined by HHS and CDC based on such factors as current threats, the medical vulnerability of the civilian population to those threats, currently available medical products, and the ability to disseminate those products (CDC, 2010a). Since many, if not most, medical products have a defined shelf life, SNS stock routinely is rotated and replenished, and required quarterly quality assurance/quality control (QA/QC) checks and annual content inventories of all Push Packages are conducted.
In addition, some manufacturers may make available vendor-managed inventory (VMI) that can be called upon to supplement the Push Packages or to provide pharmaceuticals that are specific to a suspected or confirmed agent. VMI is shipped from preselected manufacturers and is expected to begin arriving within 24 to 36 hours (CDC, 2010a).
The federal government oversees distribution of the SNS supplies to the designated RSS sites. State and local public health authorities then assume responsibility for distributing and dispensing the MCM to their populations.
![]()
3As amended by the Project BioShield Act of 2004 (Public Law 108-276), Section 564 of the Federal Food, Drug, and Cosmetic Act “permits the FDA Commissioner to authorize the use of an unapproved medical product or an unapproved use of an approved medical product during a declared emergency involving a heightened risk of attack on the public or U.S. military forces, or a significant potential to affect national security” (FDA, 2007). Such an emergency must be declared by the Secretary of Health and Human Services before an EUA can be issued.
Cities Readiness Initiative
The Cities Readiness Initiative (CRI) is a CDC-administered initiative that provides technical expertise and funding to state and local public health departments to improve their ability to dispense MCM to their entire populations within 48 hours (CDC, 2010b). CRI funding is available to the nation’s largest metropolitan areas. The core CRI planning scenario focuses on distribution and dispensing of antibiotics in response to an aerosolized anthrax attack. When the program first was established under the auspices of the SNS in 2004, a total of 21 cities were funded. Now, 72 metropolitan statistical areas (MSAs) receive funding and technical assistance through the CRI (at least one in each state and the District of Columbia). An MSA is defined by the U.S. Office of Management and Budget (OMB) as a geographic region with “a core urban area of 50,000 or more population . . . and includes the counties containing the core urban area, as well as any adjacent counties that have a high degree of social and economic integration . . . with the urban core” (OMB, 2010).
An evaluation of the CRI conducted by RAND at the request of CDC concluded that the “CRI appears to have improved regions’ readiness to rapidly dispense lifesaving medications and other medical supplies on a large scale” (Willis et al., 2009, p. xiii). This improvement has been achieved through increased staffing, the purchase of key equipment, strengthening of partnerships, development of detailed MCM dispensing plans and streamlined dispensing models, and training and exercising. The RAND report also highlights several factors that impact the effectiveness of programs, including the degree of health system decentralization, state-local relationships, and staff turnover. However, available evidence did not allow for assessment of a jurisdiction’s ability to implement mass dispensing plans under emergency conditions. Actual events are rare, and although some data were available from operational exercises, the lack of standardized performance metrics limited use of those exercises for capacity analysis. Additionally, the RAND evaluation focused on CRI program accomplishments but did not assess the cost-effectiveness of the program.
State and Local Dispensing
States purchase and maintain their own MCM stockpiles in addition to the supplies that come from the SNS or directly from the commercial supply chain. MCM also may be forward-deployed in local community-based stockpiles near planned POD sites or may be cached in the intended POD site itself, such as in a hospital or workplace cache.
Mass dispensing can be accomplished through both pull and push mechanisms (IOM, 2008). Pull mechanisms involve the public coming to
a specified site, such as an open POD, to pick up MCM. Push mechanisms involve delivering the MCM to end-users, such as through the U.S. mail (the postal model, discussed later in this chapter); workplace dispensing; or door-to-door delivery via school buses, as has been tested in Virginia (also discussed below). The more push strategies can be identified in a community and successfully implemented, the more the burden on the open POD system can be alleviated. Many state and local health agencies currently are working to expand the number of push or closed POD sites in their dispensing plans.
Points of Dispensing
Most current state and local strategies rely on the POD model for the dispensing of MCM to the public. This model allows for local customization of dispensing plans to meet the needs of the population and accommodate the local infrastructure. Key features of an effective POD include the ability to accommodate large numbers of people; location in an easy-to-find, accessible site; provisions for secure storage of MCM; areas for each stage in the process (e.g., arrival, triage, dispensing); trained personnel to handle administration and documentation; and support personnel (Lien et al., 2006; Lindner, 2006). MCM generally are distributed to PODs in response to a public health emergency. In some cases, MCM may already be on-site as a result of being forward-deployed for future dispensing (e.g., a workplace cache that is also the site of a POD).
POD Sites
Traditional open POD sites include schools, armories, and other large public facilities, but many other kinds of sites are being explored, including hotels, mobile-home parks, churches, businesses, residential institutions (e.g., nursing homes), and airports (Willis et al., 2009). Certain facilities also lend themselves to a drive-through POD model whereby people do not need to exit their cars, such as in parking lots, underpasses, and fairgrounds. The state of Utah has tested dispensing through the drive-up windows of banks (UDOH, 2009). The use of fast-food restaurant drive-up windows also has been suggested (Lindner, 2006). Results of a 2005 survey of a small set of retail executives indicated that private-sector retail stores that already dispense vaccines and medicines should be considered as potential sites for open PODs (Lien et al., 2006). These would include chain pharmacies, as well as those located in grocery stores and wholesale clubs, which generally have the infrastructure for and expertise with running large influenza vaccination clinics. The survey results suggest that retail leaders are willing to participate, the stores have the physical space for mass distribution, trained
staff are on-site, and the public has existing relationships with the stores that foster trust.
POD Operating Structures
As noted earlier, PODs may be open to the general public or closed, serving only pre-identified individuals. Both types of PODs may be medical or nonmedical. A medical POD is staffed with medical personnel who are able to conduct individualized medical assessments in addition to dispensing MCM. A nonmedical POD is staffed primarily with nonmedical personnel who are trained only to triage and dispense MCM (IOM, 2008). Medical PODs, while offering more individualized dispensing and education, are unlikely to be feasible in an emergency situation. Medical staff are needed to handle incident-related injuries or illness and probably would not be available to staff PODs. In addition, implementation of the medical POD process likely would be too slow to meet the time frame for dispensing required by the CRI. The nonmedical POD offers greater efficiency than the medical POD, but as it does not offer patients individual medical assessments, its use may necessitate altered standards of care and suspension of certain practice requirements. Moreover, while the nonmedical POD model leaves professional medical staff free to tend to victims of an incident, staffing issues still arise as some jurisdictions rely heavily on volunteers.
PODs may be set up to dispense MCM only to individuals who present at the POD or to heads of households for themselves and their family/ household members. A head-of-household dispensing model was field-tested in Philadelphia in 2005 as part of the city’s CRI planning activities (Box 3-1). The exercise was highly successful in dispensing MCM to a large number of people in a short time using a limited number of medically trained staff (Agócs et al., 2007). A key advantage of dispensing to heads of households is that vulnerable populations (e.g., children, the elderly, the infirm) need not come to the site. Agócs and colleagues suggest that rapid public availability of MCM lessens the tendency for people to seek out medication in desperation or to buy black market (or possibly counterfeit) MCM on the street. Also noted is the rate of adverse events due to drug allergy (4 percent) seen during the exercise, which is a concern for all dispensing strategies given that the time and logistical constraints of a mass prophylaxis campaign make it infeasible to screen individuals rigorously. Finally, the authors note that total dispensing was limited to 15 members per household to curtail hoarding, but such a strategy would not stop people from lying about their total number of household members to obtain extra medication.
A 2005 exercise in Seattle and King County in Washington State was similarly streamlined for timely dispensing and did not include a formal
BOX 3-1
Head-of-Household Point of Dispensing (POD)
Exercise in Philadelphia
Exercise
• The exercise involved an inhalation anthrax exposure scenario.
• Eight pretrained POD staff trained an additional 42 POD staff during the hour prior to the opening of the POD.
• Volunteer heads of households were provided with scenarios to refer to as they filled out intake forms and participated (e.g., children in household, limited English proficiency, acting distressed, trying to steal extra medication).
• Patient education was limited to handouts provided while in line.
• Medical countermeasures (MCM) for a maximum of 15 household members could be obtained by one head of household (to limit the potential for hoarding).
• Six POD staff with medical training reviewed intake forms and referred the head of household to either:
— express dispensing when only adult dosing of ciprofloxacin was needed for all household members, or
— screening to collect further information about household members before dispensing (e.g., children, drug allergies).
• Security was provided by local police.
Results
• MCM were dispensed to 717 heads of households, for a total of 2,120 household members.
• The POD was open for 2 hours, with an average rate of dispensing of 1,060 household members per hour.
• Express line dispensing (median 3 minutes per head of household) was more than twice as fast as dispensing that required screening (median 8 minutes per head of household).
• Ninety-seven percent of people were prescribed antibiotics appropriate for their individual situation.
• Four percent of those with true antibiotic allergies were prescribed a drug to which they were allergic.
SOURCE: Agócs et al., 2007.
health education step (Stergachis et al., 2007). While health educators were available to answer questions as needed, patients who had no questions went directly to triage and dispensing. Exit surveys following the exercise found that 80 percent of volunteer patients felt they knew how to take the medication that had been dispensed. Seventy-three percent of
head-of-household volunteers said they understood the instructions for how the medicine should be used by others in their household. All patients received wallet cards listing an informational website and hotline phone number, yet only 68 percent reported that they knew where to obtain further information.
Closed PODs
Closed PODs may be hosted by private-sector entities, as well as government offices and nonprofit organizations (e.g., hospitals and health care providers). The state of Georgia, for example, has established a collaboration between state and local public health officials and Georgia members of Business Executives for National Security (BENS) to develop and test an SNS dispensing model. BENS is a national, nonpartisan organization working to bring private-sector expertise to bear on national security issues. In this dispensing model, company-managed PODs provide MCM to employees and their families. Once dispensing has been completed at the company, employees volunteer to assist with dispensing at public health PODs. Beyond the Georgia example, data are sparse on the prevalence of closed PODs as a component of state and local plans for dispensing MCM. Closed PODs and workplace caches are discussed in greater detail in Chapter 4.
Following an exercise of the Georgia partners model, Buehler and colleagues (2006) conducted interviews with government, business, and academic participants to identify lessons learned (Buehler et al., 2006).
The review found that such collaboration benefits both sides. Public health PODs face a reduced dispensing burden since large numbers of people are served by company PODs and more volunteers are available (as company volunteers become available once private dispensing has been completed). Companies benefit because they can offer employees and their families access to MCM and are more connected to the community in the event of an emergency.
An initial challenge to the collaboration was the underlying cultural differences between business and government (e.g., values, metrics, resources, constraints, management styles, accountability, terminology). There also were few established relationships between the sectors to call upon. In establishing the model, operational constraints, such as confidentiality, liability, and reliance on volunteerism, were encountered. Buehler and colleagues concluded that the partnership has led to essential new relationships and a sense of trust between partners; engagement of private resources and expertise; and a tested collaborative SNS dispensing model, with a commitment from partners to expand the model.
Push Strategies
In contrast to pull strategies, such as PODs, that require the public to acquire MCM proactively, push strategies take MCM to the public. Many states have been experimenting with potential push strategies; the example from Virginia described below illustrates one potential model. Along with states, the federal government also has explored the use of push strategies through a partnership with the USPS to implement the postal model.
Virginia: Distribution and Dispensing via Push Strategies
A key component of Virginia’s distribution and dispensing plan is contracts with private-sector partners. In particular, Virginia contracts with UPS for distribution of antibiotics in case of an emergency. Virginia already contracts with UPS to deliver office supplies and has made emergency delivery of MCM a required part of that overall agreement. UPS can deliver to PODs for subsequent dispensing or directly to end-users through home delivery (Mauskapf, 2011).
Virginia also has tested a push dispensing model that involves using public school buses carrying Medical Reserve Corps volunteers and city employees to deliver MCM to residences (NACCHO, 2008). Exercises in Chesapeake, Virginia, demonstrated that 90,000 residences (the entire population of 230,000 people) across 350 square miles could be reached within 5 hours. Bags of MCM were hung on front door knobs or, with the approval of the USPS, placed in the mailboxes of rural residents.
Analyses of these exercises suggest that advantages of this push mechanism include rapid dispensing, thus meeting CRI requirements; reduced traffic congestion as there is no need to travel to PODs; enhanced ability to maintain social distancing (recommended during some infectious disease outbreaks, although not relevant for anthrax); and reduced time for which volunteers are needed (one shift for delivery versus multiple shifts to staff PODs). Disadvantages include reliance on vehicles being available and volunteers showing up, the potential need for security, and reduced effectiveness of this dispensing mechanism in densely populated or very rural areas. A review of the exercises also noted that any communications disseminated by the media must clearly convey the areas covered (as those communications may reach residents beyond the covered areas). Costs included purchase of the bags, paper and printing for educational materials, and fuel (NACCHO, 2008).
Postal Model
The USPS has the capability to deliver mail to every residential address in the country. In association with the CRI, the USPS has been evaluating
the potential for using its existing infrastructure to dispense oral antibiotics from the SNS to residences in response to an act of biological terrorism. This postal model is designed to deliver a short-term supply of MCM within hours of an attack, supplementing local capacity and reducing surge at PODs while they are being set up (IOM, 2010). An advantage of this push dispensing mechanism is that a large segment of the population can be served rapidly. Like the Virginia home delivery strategies, moreover, the postal model facilitates social distancing, which as noted is helpful in public health emergencies involving certain infectious diseases, as well as sheltering in place, which is useful in cases of increased environmental risk. Proof-of-concept exercises of the postal model were conducted in 2006 and 2007 in Boston, Philadelphia, and Seattle, where mock medications were delivered to 20,000 to 50,000 residents in each city in 6 to 9 hours (IOM, 2008). A pilot program was subsequently undertaken in Minneapolis-St. Paul (described below), and San Diego is beginning to undertake preparations to implement the postal plan as well (Global Security Newswire, 2011).
Minneapolis-St. Paul pilot program In 2008, a pilot postal model dispensing program was initiated for an estimated 575,000 people in 205,000 residences in the Minneapolis-St. Paul area (ASPR, 2010; IOM, 2010). An analysis by the Minneapolis Postal Service concluded that 179 volunteer carriers, each covering two regular postal delivery routes, could service this population in 8 to 9 hours. Postal carriers volunteered for the program (participation was not required). Under a special EUA,4 all volunteers were provided with home antibiotic kits, or MedKits, containing MCM for anthrax and personal protective equipment (including an N-95 respirator) to keep at home to help ensure that they would be protected should they be called upon to serve the public in an emergency (discussed further in Chapter 4). The MedKits contained enough MCM for family members as well. All the postal volunteers were also provided with a MedKit to maintain at work so they would be able to take their own antibiotics and immediately begin dispensing antibiotics to community members following an attack, regardless of whether they were at home or at work. In the event of an attack, one law enforcement officer would accompany each carrier on the delivery route. (Law enforcement partners are not covered under the postal EUA and were not issued MedKits or personal protective equipment. Instead there is a local MCM cache dedicated to police and emergency
![]()
4An EUA is submitted to the HHS Secretary for approval by the FDA after a declaration of emergency. It specifies the intended use and effective time period of the MCM to be dispensed, the population for which and geographic area in which MCM dispensing is allowed, and which stockpiles of MCM are granted liability protection under the Public Readiness and Emergency Preparedness (PREP) Act. EUAs are discussed in detail in the section on legal and regulatory issues for MCM dispensing later in this chapter.
responders.) Testimony by Jude Plessas of the USPS at a November 18, 2009, workshop of the Institute of Medicine’s (IOM’s) Forum on Medical and Public Health Preparedness for Catastrophic Events indicated that 385 qualified volunteers were part of the program at that time, 80 percent more than the calculated need (IOM, 2010).
Executive order and national postal model The pilot program begun under the auspices of the CRI has now been developed into a national dispensing model as a result of an Executive Order issued by President Obama on December 30, 2009. Addressing the need to supplement the capabilities of local jurisdictions to provide MCM to their populations in a timely fashion, the order states:
The Secretaries of Health and Human Services and Homeland Security, in coordination with the U.S. Postal Service … shall establish a national U.S. Postal Service medical countermeasures dispensing model for U.S. cities to respond to a large-scale biological attack, with anthrax as the primary threat consideration. (Obama, 2010)
The order also calls for the development of a plan to provide security escorts to postal workers as they deliver MCM, including supplementing local law enforcement as necessary, and plans to ensure that MCM are provided to personnel who perform mission-essential federal agency and executive branch functions so those functions would be maintained in the wake of an attack. In response to the Executive Order, HHS, the USPS, and the Departments of Homeland Security, Defense, and Justice have published plans for a National Postal Model for the Delivery of Medical Countermeasures (HHS et al., 2011).
On January 28, 2011, the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR) issued a Funding Opportunity Announcement for postal model proposals (ASPR, 2011). It is expected that six awards of $50,000 each will be made to assist metropolitan areas in developing and testing postal model MCM dispensing programs.
Implementation A 2009 evaluation of the CRI by RAND found that acceptance of the postal model as a dispensing option has been limited. A key challenge has been the security aspect: law enforcement officials have raised concern that the large numbers of officers needed to accompany each postal worker would not be available in an emergency because of the need to fulfill other priority responsibilities (Willis et al., 2009). A bioterrorism attack would exacerbate the limited surge capacity many law enforcement departments face on a daily basis. Other law enforcement concerns include the fact that officers can guard only the carrier, not the MCM supplies; they cannot act on any other criminal activity they observe while escorting the
carrier; and they are not issued MedKits or personal protective equipment (IOM, 2010; Willis et al., 2009). Beyond these security concerns, the postal model as piloted raises logistical overhead issues and concerns about a lack of flexibility that could impede its incorporation into preexisting local MCM dispensing plans and/or divert MCM and planning and response efforts from POD operations.5
The Role of Clinicians and First Responders
Clinicians and first responders are important to many aspects of a response to a bioterrorism attack, from detection to mass prophylaxis. Both professional groups have skills that could be further leveraged through enhanced partnerships with public health and with improved education about their potential roles. This section briefly describes several roles for clinicians and first responders within the overall strategy for responding to an anthrax attack; although further work in this critical area is warranted, it is beyond the scope of the committee’s task. Specific roles for clinicians and first responders in prepositioning strategies are discussed in Chapter 4.
First responders and clinicians could help ensure timely detection of an anthrax attack. Because they would likely see patients on the front line of an attack, providing them with additional training to recognize disease symptoms and to alert appropriate public health officials could help with early detection. As discussed in Chapter 2, the time to detection is crucial: the longer it takes to determine that an attack has occurred, the greater is the time from exposure to prophylaxis.
Clinicians and first responders could also play important roles in the overall MCM dispensing strategy, working alongside nonmedical volunteers in PODs or in other dispensing strategies. Prior education and training for these groups would help ensure that they could effectively participate in a response.
Finally, clinicians could play an important role in counseling their patients on the proper use of antibiotics, in general, and in the context of an anthrax attack, in particular. Additional education and training for clinicians would help them provide appropriate information for their patients, including those who would be worried about how they would receive MCM after an attack. Clinicians may be best positioned to identify some vulnerable individuals who lack timely access to antibiotics through other mechanisms, and to help address this gap. This is discussed in Chapter 4.
![]()
5David Starr, Director of Countermeasure Response, Office of Emergency Preparedness and Response, New York City Department of Health and Mental Hygiene, raised similar concerns in his testimony to the committee on February 28, 2011, focusing specifically on the impracticability of implementing the postal model in New York City.
CONCERNS ABOUT THE CURRENT DISPENSING SYSTEM
Concerns about the current system for dispensing MCM include the dispensing capacity of state and local jurisdictions, security, workforce issues, the need for effective communication and public education, adherence, and transportation and site selection issues. These concerns have led to increased interest in prepositioning strategies. The costs associated with the current POD model of dispensing are discussed in detail in Chapter 5.
Dispensing Capability of State and Local Jurisdictions
As noted earlier, initial supplies are delivered from the SNS to designated RSS sites within 12 hours of the decision to deploy (CDC, 2010a). The timing of distribution from the RSS sites to the PODs and end-users, however, is dependent on the local jurisdictions and is highly variable (Burel, 2011). Although evidence and metrics are lacking, the scope of the challenge and the resources required have raised concern that most U.S. communities still lack adequate capability to dispense MCM quickly to all exposed and potentially exposed populations (see, for example, HSPD-21, 2007). This concern is amplified by recent and ongoing cuts to funding for state and local public health departments (TFAH, 2010). The Executive Order mandating a national USPS MCM dispensing model was issued based on the need to supplement state and local capabilities (Obama, 2010).
Security
Although all PODs have security plans that anticipate the participation of law enforcement, there is concern that during a terrorist attack, local law enforcement personnel would be unable to guarantee the safety of stockpiles and staff because of other priorities during and after the attack (IOM, 2010). In a field test of a head-of-household POD dispensing model in Philadelphia, discussed above, participants who were scripted to try to steal extra antibiotics were successful in doing so (Agócs et al., 2007). While anecdotal evidence from recent disasters provides a spectrum of potential population reactions to a crisis, from relative calm to concentrated looting and potential rioting, there nonetheless exists a perception that safety is a significant concern for MCM dispensing plans. As discussed below, personal safety at potentially overcrowded PODs was one reason respondents in a survey gave for choosing not to go to a POD when advised to do so by public health officials (SteelFisher et al., 2011). Concern also has been raised specifically with respect to the security requirements of the postal model, as discussed previously (Willis et al., 2009).
Workforce Issues
Participants at a 2008 workshop of the IOM Forum on Medical and Public Health Preparedness for Catastrophic Events raised several potential workforce issues associated with the POD dispensing approach, including large numbers of staff required to operate the PODs, the need for training of volunteer staff pre-event and supervision during an event, and protection of volunteers’ health while working at the PODs (IOM, 2008). Some jurisdictions have opted to redirect government employees to staff the PODs, reducing the reliance on volunteers. Use of community health centers and hospitals as dispensing sites could disrupt the provision of both routine and critical medical services and draw medical staff away from caring for patients (although hospitals and other health care facilities could serve as closed PODs for staff and existing patients; see Chapter 4). While many jurisdictions developed more sophisticated staffing plans during the response to the 2009 H1N1 influenza pandemic, concerns may remain (particularly in jurisdictions with fewer resources) about staffing during a more sudden response, such as would be required for an anthrax attack.
Communication/Public Education
In the event of a public health emergency, effective communication is critical to ensure that the public knows when and where to go to obtain MCM, regardless of which dispensing mechanisms are employed. Communications are likely to be one of the major challenges following an anthrax attack because of uncertainties and because of how quickly the attack and response are likely to unfold. Although official direction and information can influence individual decision making, the main determinants of behavior include risk perceptions and appraisals, trust and concerns about the safety and effectiveness of MCM, and the ease of implementing the recommended behavior (Vaughan, 2011).
State and local officials could use existing knowledge from both emergency and nonemergency public health messaging campaigns to develop a plan tailored to their population and response strategies. Tailored plans are needed because responses to a public health emergency are inconsistent across vulnerable populations and are not related exclusively to health literacy (Vaughan, 2011). Effective risk communication to a sociodemographically diverse audience will need to involve the use of multiple communication strategies (e.g., traditional media, unofficial Internet sites, social media, social interactions). Social media, with their ability to inform millions of people instantly, can be a viable source of communication in disasters, but they are unlikely to reach the entire population.
Public engagement also can inform communication plans. During a
potassium iodide prepositioning campaign in a jurisdiction within 10 miles of a New Jersey nuclear reactor, for example, researchers were able to determine which communication channels (Internet, television, radio) the public was most likely to use to obtain information and directions (Blando et al., 2008).
Adherence
Adherence to the recommended course of prophylactic antibiotics following an anthrax attack is a major concern. Survey data and evidence from the 2001 anthrax attack suggest adherence is likely to be quite poor. Following that attack, a mass anthrax postexposure prophylaxis campaign was implemented in six areas where exposures had been confirmed. Approximately 10,000 people were recommended to undergo at least 60 days of antibiotic treatment. Follow-up interviews with more than 6,000 of these individuals revealed that while 97 percent had obtained their initial supply of antibiotics, 10 percent had not initiated therapy (Shepard et al., 2002). Only 44 percent of those obtaining the antibiotics had completed the 60-day regimen. Adherence was highest at the Brentwood mail facility in Washington, DC (64 percent), and lowest at the Morgan postal facility in New York City (21 percent). A variety of reasons for nonadherence were cited, including experience with adverse reactions and a perceived low risk of having been exposed. In a separate survey of 245 of the more than 2,000 workers exposed at the Brentwood facility, only 40 percent reported full adherence to their 60-day antibiotic regimen, while 18 percent had completely discontinued the antibiotic at some point, and 42 percent reported stopping and restarting therapy one or more times, skipping days, reducing dosage, or otherwise deviating from the prescribed regimen (Jefferds et al., 2002).
A national opinion poll conducted by researchers at the Harvard School of Public Health raised the concern that while people may obtain MCM at a POD, they may delay starting therapy (SteelFisher et al., 2011). In response to a fictional anthrax attack in their own town, 89 percent of respondents said they would follow recommendations from public health authorities to obtain antibiotics from a local POD within 48 hours. However, 34 percent of individuals who said they would obtain the MCM said they would most likely wait to take them until they knew whether they really had been exposed to anthrax, and 6 percent would wait “for the foreseeable future.” Of those who would most likely not go to the POD, primary reasons included concerns about public officials not being able to control crowds, exposure to anthrax while going to the POD, insufficient supply of antibiotics, and safety of the antibiotics.
Transportation and Site Selection Issues
Transportation and site selection issues—including ensuring functioning and safe public transportation and a public understanding of what to expect upon arriving at a POD—are prominent for those jurisdictions that utilize primarily pull strategies. A study of traffic and access to PODs found that during an emergency, “it is unrealistic to expect the public to arrive at the PODs in a uniform and steady rate” (Baccam et al., 2011, p. 147). The authors’ model predicted that the total time to process an individual through a POD would be anywhere from 1 to 6 hours under these conditions. Therefore, it is important for state and local jurisdictions to anticipate and mitigate the consequences associated with transportation to and through PODs, especially for vulnerable populations, and to consider their impact on the total time to prophylaxis for the population. Transportation to and from PODs is further complicated when there is a need to shelter in place to avoid traversing highly contaminated areas. Also important to consider is that personnel designated to staff PODs may be overwhelmed simply by providing for the basic needs (e.g., food and water) of a large population.
Depending on the attack scenario (e.g., release at an indoor stadium versus widespread dispersion over a city with a crop duster), the decision about where to establish PODs might depend on environmental sampling results, which could further delay the time between exposure and prophylaxis. Many methods can be used for environmental sampling, depending on the type of environment in which exposure is thought to have taken place. The length of time required for each sampling technique varies: tests can take as little as a few hours or as long as days to be confirmed (CDC, 2006). Communication and public engagement prior to and during an anthrax attack (see above) will play a significant role in identifying potentially exposed populations and directing them to prophylaxis sites (e.g., open PODs).
LEGAL AND REGULATORY ISSUES IN MCM DISPENSING
This section highlights the legal and regulatory issues of primary concern to MCM dispensing, including prescription laws, EUAs, liability and the Public Readiness and Emergency Preparedness (PREP) Act, and expiration of medications and the Shelf Life Extension Program (SLEP). A more detailed discussion of each issue as it pertains to specific prepositioning strategies can be found in Chapter 4.
Prescription Laws
The Federal Food, Drug, and Cosmetic Act (FFDCA) requires that prescription medications be dispensed only with a prescription, and such medications must be appropriately labeled for the individuals for whom they are prescribed. The FDA may deem approved products to be misbranded under the FFDCA if their intended use involves, for example, dispensing without a prescription, absence of required labels, partial dosing, home crushing instructions, SLEP products (see below), or manufacturing that deviates from current good manufacturing practice (cGMP) (Sadove, 2011).
Emergency Use Authorization
As noted earlier, an EUA is submitted to the HHS Secretary for approval by the FDA after a declaration of emergency. It specifies the intended use and effective time period of the MCM to be dispensed, the population for which and geographic area in which MCM dispensing is allowed, and which stockpiles of MCM are granted liability protection under the PREP Act (see below). An EUA is contingent upon the declaration of an emergency by the Secretary of HHS, and such declarations must be renewed yearly. There are currently two EUAs in effect, described below.
The FDA recently issued an EUA for oral formulations of doxycycline products “for the purposes of stakeholder pre-event planning and preparedness activities, and, in a post-event scenario, implementation of post-exposure prophylaxis for inhalational anthrax for individuals who have been exposed, or who may have been exposed, to aerosolized B. anthracis spores” (FDA, 2011, p. 3). This EUA will allow public health authorities to prepare to dispense doxycycline under emergency conditions. Although doxycycline is FDA-approved for anthrax postexposure prophylaxis, the EUA is necessary to allow state and local public health officials to prepare for and implement a mass prophylaxis campaign within an entire community. This is because in the absence of the EUA, dispensing doxycycline through an open POD, for instance, could violate provisions of the FFDCA involving, for example, “[the requirement of distributing and using] emergency use information sheets . . . ; dispensing doxycycline without a prescription and without all of the required information on the prescription label . . . ; dispensing a partial supply of the full 60-day dosage regimen, i.e., initial start-up 10-day supply; pre-event storage or distribution of doxycycline packaged or repackaged for emergency distribution; and waiver of current good manufacturing practice requirements during an event, under certain circumstances” (FDA, 2011, p. 2). While this EUA does provide officials with flexibility in a postattack environment, it does
not allow the pre-event dispensing of doxycycline (as would be required to authorize MedKits).
In 2008, the FDA issued an EUA authorizing distribution of MedKits to the postal carrier volunteers in the pilot postal program in Minneapolis-St. Paul (the EUA was amended in 2009) (FDA, 2009). This EUA does not cover dispensing of MedKits to any individuals or groups beyond postal workers who volunteer to participate in the postal program and the members of their households.
Liability and the PREP Act
The HHS Secretary is authorized to issue a “PREP Act Declaration” that provides immunity from tort liability (except for willful misconduct) for claims of loss associated with the administration or use of MCM for threats that are deemed by the Secretary to constitute a public health emergency to those involved in the development, manufacturing, testing, distribution, administration, and use of covered MCM (Public Law 109-148).6 The statutes of the PREP Act come into effect only after a declaration of emergency by the HHS Secretary. Covered persons include individuals involved in planning and administering the distribution and dispensing of a specified MCM (whether FDA-approved or covered under an event-specific EUA), as well as those individuals authorized under state law to prescribe, administer, and dispense the MCM to end-users. Each MCM required for the response is specified in the emergency declaration, along with the disease the MCM will prevent/treat, the period of time for which the MCM will be used, the populations (demographically and geographically defined) in which it will be used, and the means of its distribution. It is important to note that PREP Act protections are not limited to government officials and programs; the HHS Secretary can expand or limit the groups identified for liability protection. In the PREP Act declaration for anthrax, for instance, the Secretary defines “qualified [or covered] persons” to include a variety of nonmedical individuals operating under the supervision of an authorized person following the declaration of an emergency. This provision extends PREP Act protection to postal carriers, for example, operating under a postal dispensing program (Binzer, 2008; HHS, 2008). Liability continues to be a concern for some private-sector companies that become involved in distributing and dispensing MCM, despite the provisions of the PREP Act, as discussed in Chapter 4.
![]()
6See http://www.hrsa.gov/gethealthcare/conditions/countermeasurescomp/covered_countermeasures_and_prep_act.pdf and http://www.phe.gov/preparedness/legal/prepact/Pages/default.aspx (accessed March 25, 2011).
Expiration of Medications/Shelf Life Extension Program
Expiration of antibiotics could be a considerable issue for pre-event dispensing of MCM, particularly for household MedKits for the public (the manufacturer-defined shelf life of doxycycline is 2 years, and that of ciprofloxacin is 3 years). For instance, in accordance with the EUA that authorized the pilot postal program in Minnesota, the USPS must survey the volunteer participants in the program every 6 months to check on the status of the kits, including expiration of the medications. Expired product must be collected, recorded, disposed of, and replaced. These requirements add substantial costs and logistical challenges to this dispensing mechanism compared with the SNS, which has access to unique mechanisms to decrease replacement costs.
First, the SNS has an extensive QA/QC and stock rotation/replacement program to ensure that the medications in 12-hour Push Packages have not expired. This type of large-scale rotation may not be available to smaller local or private-sector stockpiles. Second, the SNS can participate in the federal SLEP, which extends the expiration of some of its MCM. The expiration of pharmaceutical products is specified by the manufacturer based on the results of stability testing. However, many medications may have a considerably longer shelf life under ideal storage conditions. Prior to the advent of the SNS, expiration of stockpiled medications had been a particular concern for the military given its need to stockpile very large quantities of certain products or to have highly specialized products with limited commercial use (e.g., antidotes for nerve agents). To address this issue, the SLEP was established in 1986 though an interagency agreement between the FDA and the Department of Defense (Courtney et al., 2009). Under the program, the FDA tests samples from individual lots of an expiring stockpiled drug to determine stability and quality. Ninety-five percent of the product must still be chemically available if the expiration date is to be extended. Shelf-life-extended products are retested every 6 months to 1 year. Although the SLEP originally pertained primarily to military stockpiles, it also has been used for the SNS since 2002. At this time, the SLEP cannot be applied to nonfederal antibiotic stockpiles or caches, resulting in significant replacement costs for state, local, and private-sector (i.e., workplace) stockpiles.
Current MCM distribution plans rely on distribution from the federally managed SNS and state and local stockpiles. Following an attack, MCM generally are sent to state-administered regional distribution centers and from there to the locations from which they will be dispensed. State, local, and tribal dispensing plans rely primarily on dispensing to the public
at open PODs. In many cases, the open PODs are supplemented by other strategies, such as closed PODs at workplaces and hospital caches. Concerns about the current dispensing system have led to the exploration and development of other dispensing strategies, including the postal model; enhanced involvement by the private sector; and other novel strategies, such as Virginia’s efforts to use school buses to dispense MCM to the public. These concerns also have led to increased interest in prepositioning strategies, which are examined in the next chapter. Finally, in considering any dispensing strategy, including those that involve prepositioning, it is important to take into account the legal and regulatory issues involved.
Agócs, M., S. Fitzgerald, S. Alles, G. J. Sale, V. Spain, E. Jasper, T. L. Grace, and E. Chernak. 2007. Field testing a head-of-household method to dispense antibiotics. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 5(3):255-267.
ASPR (Assistant Secretary for Preparedness and Response). 2010. National postal model for the delivery of medical countermeasures. Washington, DC: Department of Health and Human Services, http://www.phe.gov/Preparedness/planning/postal/Documents/eo13527-section2.pdf (accessed February 23, 2011).
ASPR. 2011. Postal model for medical countermeasures delivery and distribution grant announcement. Funding opportunity number EP-PMC-11-001. Washington, DC: HHS, http://www.grants.gov/search/search.do?mode=VIEW&oppId=67033 (accessed June 21, 2011).
Baccam, P., D. Willauer, J. Krometis, Y. Ma, A. Sen, and M. Boechler. 2011. Mass prophylaxis dispensing concerns: Traffic and public access to PODs. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 9(2):139-151.
Binzer, P. 2008. The PREP Act: Liability protection for medical countermeasure development, distribution, and administration. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 6(4):1-6.
Blando, J., C. Robertson, and E. Bresnitz. 2008. Communicating information in an emergency preparedness pill distribution campaign. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 6(1):57-65.
Buehler, J. W., E. A. Whitney, and R. L. Berkelman. 2006. Business and public health collaboration for emergency preparedness in Georgia: A case study. BMC Public Health 6:285-297.
Burel, G. 2011 (February 28). An SNS perspective on pre-positioning medical countermeasures. Slides presented at the Institute of Medicine Public Workshop for the Committee on Prepositioned Medical Countermeasures for the Public, Washington, DC, http://www.iom.edu/~/media/Session%201-%20Burel_Federal%20Perspective.pdf (accessed March 30, 2011).
CDC (Centers for Disease Control and Prevention). 2006. Anthrax Q&A: Laboratory testing. Atlanta, GA: CDC, http://www.bt.cdc.gov/agent/anthrax/faq/labtesting.asp (accessed September 2, 2011).
CDC. 2009. CDC health update: Swine influenza A (H1N1) update: New interim recommendations and guidance for health directors about Strategic National Stockpile material. Atlanta, GA: CDC, http://www.cdc.gov/h1n1flu/HAN/042609.htm (accessed August 3, 2011).
CDC. 2010a. Strategic National Stockpile (SNS). Atlanta, GA: CDC-SNS, http://www.bt.cdc.gov/stockpile/ (accessed February 16, 2011).
CDC. 2010b. Cities Readiness Initiative (CRI). Atlanta, GA: CDC, http://www.bt.cdc.gov/cri/ (accessed February 16, 2011).
Courtney, B., J. Easton, T.V. Inglesby, and C. SooHoo. 2009. Maximizing state and local medical countermeasure stockpile investments through the Shelf-Life Extension Program. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 7(1):101-107, http://www.upmc-biosecurity.org/website/resources/publications/2009/2009-03-27-max_st_local_med_cntr.html (accessed February 24, 2011).
FDA (Food and Drug Administration). 2007. Emergency Use Authorization of Medical Products Guidance—Emergency Use Authorization of Medical Products. Rockville, MD: FDA, http://www.fda.gov/RegulatoryInformation/Guidances/ucm125127.htm#eligibility (accessed August 22, 2011).
FDA. 2009. Letter from Randall W. Lutter to Robin Robsinson regarding the BiomedicalAdvanced Research and Development Authority (BARDA) request for Emergency Use Authorization of Postal Worker Medkits. Silver Spring, MD: FDA, http://www.fda.gov/downloads/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/UCM107309.pdf (accessed February 23, 2011).
FDA. 2011. Letter from Margaret A. Hamburg to Thomas R. Frieden regarding the Centers for Disease Control and Prevention (CDC) request for Emergency Use Authorization of oral formulations of doxycycline products for the post-exposure prophylaxis (PEP) of inhalational anthrax. Silver Spring, MD: FDA, http://www.fda.gov/downloads/EmergencyPreparedness/Counterterrorism/UCM264068.pdf (accessed July 25, 2011).
Global Security Newswire. 2011 (August 25). San Diego preps postal workers to deliver anthrax antibiotics. Washington, DC: Nuclear Threat Initiative, http://gsn.nti.org/gsn/nw_20110825_9801.php (accessed August 31, 2011).
HHS (Department of Health and Human Services). 2007. Public health emergency medical countermeasure enterprise implementation plan for chemical, biological, radiological and nuclear threats. Washington, DC: HHS, http://www.pswrce.uci.edu/pdf/phemce_implplan_041607final.pdf (accessed February 19, 2011).
HHS. 2008. Declaration under the Public Readiness and Emergency Preparedness Act (Bacillus anthracis), October 1, 2008. Federal Register 73(194):58239-58242, http://edocket.access.gpo.gov/2008/E8-23547.htm (accessed February 24, 2011).
HHS. 2009 (December). CDC’s CHEMPACK Project: Nerve agent antidote storage. Washington, DC: HHS, http://oig.hhs.gov/oei/reports/oei-04-08-00040.pdf (accessed July 15, 2011).
HHS, DHS (Department of Homeland Security), DOD (Department of Defense), DOJ (Department of Justice), and USPS (U.S. Postal Service). 2011. National postal model for the delivery of medical countermeasures. Washington, DC: HHS, http://www.phe.gov/Preparedness/planning/postal/Documents/eo13527-section2.pdf (accessed July 8, 2011).
HSPD-21 (Homeland Security Presidential Directive). 2007 (October 18). HSPD-21: Public health and medical preparedness. Washington, DC: White House, http://www.fas.org/irp/offdocs/nspd/hspd-21.htm (accessed June 24, 2011).
IOM (Institute of Medicine). 2008. Dispensing medical countermeasures for public health emergencies: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2010. Medical countermeasures dispensing: Emergency use authorization and the postal model: Workshop summary. Washington, DC: The National Academies Press.
Jefferds, M. D., K. Laserson, A. M. Fry, S. Roy, J. Hayslett, L. Grummer-Strawn, L. Kettel-Khan, and A. Schuchat. 2002. Adherence to antimicrobial inhalational anthrax prophylaxis among postal workers, Washington, DC, 2001. Emerging Infectious Diseases 8(10):1138-1144.
Lien, O., B. Maldin, C. Franco, and G. K. Gronvall. 2006. Getting medicine to millions: New strategies for mass distribution. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 4(2):176-182.
Lindner, P. 2006. CRI alternative dispensing guide: A collection of model practices and pilot projects. Washington, DC: NACCHO, http://www.naccho.org/toolbox/tool.cfm?id=508&program_id=6 (accessed February 20, 2011).
Mauskapf, R. 2011 (January 24). Remarks presented to the Institute of Medicine Committee on Prepositioned Medical Countermeasures for the Public Meeting One, Washington, DC.
NACCHO (National Association of County and City Health Officials). 2008. Alternative methods of dispensing: Model highlights. Washington, DC: NACCHO, http://www.naccho.org/topics/emergency/SNS/upload/POD-Article-2_utilizing-school-busses.pdf (accessed February 21, 2011).
Obama, B. 2010. Establishing federal capability for the timely provision of medical countermeasures following a biological attack. Executive Order 13527 of December 30, 2009. Federal Register 75(3):737-738.
OMB (Office of Management and Budget). 2010. Metropolitan and Micropolitan Statistical Areas. Washington, DC: OMB, http://www.census.gov/population/www/metroareas/metroarea.html (accessed February 18, 2011).
Sadove, E. 2011. Prepositioned antibiotics. Slides presented at the Institute of Medicine Public Workshop for the Committee on Prepositioned Medical Countermeasures for the Public, Washington, DC, http://iom.edu/~/media/Session%206%20SadoveLegalRegulatory%20Perspective.pdf (accessed March 23, 2011).
Shepard, C. W., M. Soriano-Gabarro, E. R. Zell, J. Hayslett, S. Lukacs, S. Goldstein, S. Factor, J. Jones, R. Ridzon, I. Williams, N. Rosenstein, and the CDC Adverse Events Working Group. 2002. Antimicrobial postexposure prophylaxis for anthrax: adverse events and adherence. Emerging Infectious Diseases 8(10):1124-1132.
SteelFisher, G., R. Blendon, L. J. Ross, B. C. Collins, E. N. Ben-Porath, M. M. Bekheit, and J. R. Mailhot. 2011. Public response to an anthrax attack: Reactions to mass prophylaxis in a scenario involving inhalation anthrax from an unidentified source. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 9(3):1-12.
Stergachis, A., C. M. Wetmore, M. Pennylegion, R. D. Beaton, B. T. Karras, D. Webb, D. Young, and M. Loehr. 2007. Evaluation of a mass dispensing exercise in a Cities Readiness Initiative setting. American Journal of Health-System Pharmacy 64(3):285-293.
TFAH (Trust for America’s Health). 2005. Ready or not 2005: Protecting the public’s health from diseases, disasters, and bioterrorism. Washington, DC: TFAH, http://healthyamericans.org/reports/bioterror05/bioterror05Report.pdf (accessed August 2, 2011).
TFAH. 2010. Ready or not 2010: Protecting the public’s health from diseases, disasters, and bioterrorism. Washington, DC: TFAH, http://healthyamericans.org/assets/files/TFAH2010ReadyorNot%20FINAL.pdf (accessed February 16, 2011).
UDOH (Utah Department of Health). 2009. Unique drive-through medication distribution strategy practiced in Utah. The Preparedness Post 2(2):5, http://health.utah.gov/epi/newsletter/Preparedness/Oct_09.pdf (accessed February 20, 2011).
Vaughan, E. 2011 (March 1). Risk communication and household prepositioning strategies for diverse urban populations. Slides presented at the Institute of Medicine Public Workshop for the Committee on Prepositioned Medical Countermeasures for the Public, Washington, DC, http://iom.edu/~/media/Session%2011A-%20Vaughan_Risk%20Communication%20Home%20MedKits.pdf (accessed March 23, 2011).
Willis, H. H., C. Nelson, S. Shelton, A. M. Parker, J. A. Zambrano, E. W. Chan, J. Wasserman, and B. A. Jackson. 2009. Initial evaluation of the Cities Readiness Initiative. Technical Report TR-640-CDC. Santa Monica, CA: RAND Corporation, http://www.rand.org/pubs/technical_reports/TR640.html (accessed February 16, 2011).
























