At the request of National Institutes of Health (NIH), and in response to congressional inquiry, the Institute of Medicine (IOM) in collaboration with the National Research Council (NRC) convened an ad hoc committee to consider the necessity of the use of chimpanzees in NIH-funded research in support of the advancement of the public’s health.
Specifically, the committee was asked to review the current use of chimpanzees for biomedical and behavioral research and:
• Explore contemporary and anticipated biomedical research questions to determine if chimpanzees are or will be necessary for research discoveries and to determine the safety and efficacy of new prevention or treatment strategies. If biomedical research questions are identified:
ο Describe the unique biological/immunological characteristics of the chimpanzee that make it the necessary animal model for use in the types of research.
ο Provide recommendations for any new or revised scientific parameters to guide how and when to use these animals for research.
• Explore contemporary and anticipated behavioral research questions to determine if chimpanzees are necessary for progress in understanding social, neurological, and behavioral factors that influence the development, prevention, or treatment of disease.
In addressing the task, the committee explored existing and anticipated alternatives to the use of chimpanzees in biomedical and behavioral research. The committee based its findings and recommendations on available scientific evidence, published literature, public testimony, submitted materials by stakeholders, and a commissioned paper, as well as its expert judgment.
To conduct this expert assessment and evaluate the necessity for chimpanzees in research to advance the public’s health, the committee deliberated from May 2011 through November 2011. During this period, the committee held three 2-day meetings and several conference calls, including two public information-gathering sessions on May 26, 2011, and August 11-12, 2011. Each information-gathering session included testimony from individuals and organizations that both supported and opposed the continued use of chimpanzees. The committee also reviewed a number of background documents provided by stakeholder organizations and commissioned a paper, “Comparison of Immunity to Pathogens in Humans, Chimpanzees, and Macaques.”
The committee identified a set of core principles and criteria that were used to assess the necessity of chimpanzees for research now or in the future.
Ethical Considerations
Neither the cost of using chimpanzees in research nor the ethical implications of that use were specifically in the committee’s charge. Rather, the committee was asked for its advice on the scientific necessity of the chimpanzee model for biomedical and behavioral research. The committee agrees that cost should not be a consideration. However, the committee feels strongly that any assessment of the necessity for using chimpanzees as an animal model in research raises ethical issues, and any analysis of necessity must take these ethical issues into account. The committee’s view is that the chimpanzee’s genetic proximity to humans and the resulting biological and behavioral characteristics not only make it a uniquely valuable species for certain types of research, but also demand a greater justification for conducting research using this animal model.
Summary of Chimpanzee Research
The committee was asked, as part of its task, to review the current use of chimpanzees for biomedical and behavioral research. To assess the use of the chimpanzee as an animal model, the committee explored research supported by the NIH and other federally and privately funded research over the past 10 years.
The largest percentage of federally funded chimpanzee research has been supported by the NIH, with additional projects funded by other federal agencies, including the Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), and National Science Foundation. Of the 110 identified projects sponsored by the NIH between 2001 and 2010, 44 were for research on hepatitis; comparative genomics accounted for 13 projects; 11 projects were for neuroscience research; 9 projects were for AIDS/HIV studies; and 7 projects were for behavioral research. The remaining projects funded a limited number of studies in areas such as malaria and respiratory syncytial virus and projects supporting chimpanzee colonies.
Committee analysis of the use of chimpanzees in the private sector was hindered by the proprietary nature of the information. However, based on limited publications and public non-proprietary information, it is clear that the private sector is using the chimpanzee model, especially in areas of drug safety, efficacy, and pharmacokinetics. Although its use appears to be limited and decreasing over the 10 years examined by the committee, the chimpanzee model is being employed by industry in the development of antiviral drugs and vaccines for hepatitis B and C as well as in the development of monoclonal antibody therapeutics.
Principles Guiding the Use of Chimpanzees in Research
The task given to the committee by the NIH asked two questions about the need for chimpanzees in research: (1) Is biomedical research with chimpanzees “necessary for research discoveries and to determine the safety and efficacy of new prevention or treatment strategies?” and (2) Is behavioral research using chimpanzees “necessary for progress in understanding social, neurological, and behavioral factors that influence the development, prevention, or treatment of disease?” In responding to these questions, the committee concluded that the potential reasons for undertaking biomedical and behavioral research as well as the protocols used in each area are different enough to require different sets of criteria.
However, the committee developed both sets of criteria guided by the following three principles:
1. The knowledge gained must be necessary to advance the public’s health;
2. There must be no other research model by which the knowledge could be obtained, and the research cannot be ethically performed on human subjects; and
3. The animals used in the proposed research must be maintained either in ethologically appropriate physical and social environments or in natural habitats.
These principles are the basis for the specific criteria that the committee established to assess current and future use of the chimpanzee in biomedical and behavioral research (see Recommendations 1 and 2).
Conclusions and Recommendations
The committee based the following conclusions and recommendations in large part on the advances that have been made by the scientific community using alternative models to the chimpanzee, such as studies using other non-human primates, genetically modified mice, in vitro systems, and in silico technologies as well as human clinical trials. Having reviewed and analyzed contemporary and anticipated biomedical and behavioral research, the committee concludes that:
• No uniform set of criteria is currently used to assess the necessity of the chimpanzee in NIH-funded biomedical and behavioral research.
• While the chimpanzee has been a valuable animal model in past research, most current use of chimpanzees for biomedical research is unnecessary, based on the criteria established by the committee, except potentially for two current research uses:
ο Development of future monoclonal antibody therapies will not require the chimpanzee, due to currently available technologies. However, there may be a limited number of monoclonal antibodies already in the developmental pipeline that may require the continued use of chimpanzees.
ο The committee was evenly split and unable to reach consensus on the necessity of the chimpanzee for the development of a prophylactic hepatitis C virus (HCV) vaccine. Specifically, the committee could not reach agreement on whether a preclinical challenge study using the chimpanzee model was necessary and if or how much the chimpanzee model would accelerate or improve prophylactic HCV vaccine development.
• The present trajectory indicates a decreasing scientific need for chimpanzee studies due to the emergence of non-chimpanzee models and technologies.
• Development of non-chimpanzee models requires continued support by the NIH.
• A new, emerging, or reemerging disease or disorder may present challenges to treatment, prevention, and/or control that defy nonchimpanzee models and available technologies and therefore may require the future use of the chimpanzee.
• Comparative genomics research may be necessary for understanding human development, disease mechanisms, and susceptibility because of the genetic proximity of the chimpanzee to humans. It poses no risk to the chimpanzee when biological materials are derived from existing samples or minimal risk of pain and distress in instances where samples are collected from living animals.
• Chimpanzees may be necessary for obtaining otherwise unattainable insights to support understanding of social and behavioral factors that include the development, prevention, or treatment of disease.
• Application of the committee’s criteria would provide a framework to assess scientific necessity to guide the future use of chimpanzees in biomedical, comparative genomics, and behavioral research.
Recommendation 1: The National Institutes of Health should limit the use of chimpanzees in biomedical research to those studies that meet the following three criteria:
1. There is no other suitable model available, such as in vitro, nonhuman in vivo, or other models, for the research in question;
2. The research in question cannot be performed ethically on human subjects; and
3. Forgoing the use of chimpanzees for the research in question will significantly slow or prevent important advancements to prevent, control, and/or treat life-threatening or debilitating conditions.
Animals used in the proposed research must be maintained either in ethologically appropriate physical and social environments or in natural habitats. Biomedical research using stored samples is exempt from these criteria.
Recommendation 2: The National Institutes of Health should limit the use of chimpanzees in comparative genomics and behavioral research to those studies that meet the following two criteria:
1. Studies provide otherwise unattainable insight into comparative genomics, normal and abnormal behavior, mental health, emotion, or cognition; and
2. All experiments are performed on acquiescent animals, using techniques that are minimally invasive, and in a manner that minimizes pain and distress.
Animals used in the proposed research must be maintained either in ethologically appropriate physical and social environments or in natural habitats. Comparative genomics and behavioral research using stored samples are exempt from these criteria.
The criteria set forth in the report are intended to guide not only current research policy, but also decisions regarding potential use of the chimpanzee model for future research. The committee acknowledges that imposing an outright and immediate prohibition of funding could cause unacceptable losses to research programs as well as have an impact on the animals. Therefore, although the committee was not asked to consider how its recommended policies should be implemented, it believes that the assessment of the necessity of the chimpanzee in all grant renewals
and future research projects would be strengthened and the process made more credible by establishing an independent oversight committee that builds on the Interagency Animal Model Committee and uses the recommended criteria.
This page intentionally left blank.
The chimpanzee (Pan troglodytes) is a current animal model in biomedical and behavioral research supported by the U.S. government and industry. In fiscal year 2011, of the more than 94,000 active projects sponsored by the National Institutes of Health (NIH), only 53 used the chimpanzee (0.056 percent). However, members of the public, Congress, and some scientists question this use. They argue that research that has relied on chimpanzees could be accomplished using other models, methods, or technologies (Bailey, 2010a, 2010b; Bettauer, 2011) or that chimpanzees are not appropriate models for human disease research (Bailey, 2008; Physicians Committee for Responsible Medicine, 2011).
Ongoing biomedical and behavioral research on chimpanzees is largely conducted at four facilities: the Southwest National Primate Research Center, the New Iberia Research Center at the University of Louisiana-Lafayette, the Michale E. Keeling Center for Comparative Medicine and Research of the University of Texas MD Anderson Cancer Center, and the Yerkes National Primate Research Center at Emory University. Much of the research supported by the first three facilities is focused on proof-of-principle studies for hepatitis C vaccines and therapies, with a lesser amount of research devoted to assessing safety and efficacy of large molecules such as monoclonal antibodies (Watson, 2011). In addition, research supports studies on deriving chimpanzee cell lines, antibodies and other biological materials, as well as comparative genomics research. The Yerkes Center primarily sponsors studies pertaining to developmental and cognitive neuroscience, as well as aging-related comparative neurobiology (Yerkes National Primate Research Center, 2011). In addition to these four centers, the National Center for Research Resources (NCRR) also supports the Alamogordo Primate Facility (APF). Unlike the other facilities, Alamogordo is a research reserve facility that does not have an active chimpanzee research program; no invasive research is conducted on these chimpanzees while on the premises1 (NCRR, 2011a). However, the animals may be used for cardiovascular disease and behavioral studies with data obtained during their annual physicals (Watson, 2011). If these chimpanzees are needed
__________________
1According to solicitation NHLBI-CSB-(RR)-SS-2011-264-KJM (HHS, 2011c), “the current agreements between the National Institutes of Health (NIH) and the U.S. Air Force (USAF) prescribe that no invasive research shall be conducted on chimpanzees currently held at the APF.”
for other types of research, they are relocated to another facility and, once removed, cannot return to Alamogordo Primate Facility (HHS, 2011d).
As of May 2011, 937 chimpanzees, ranging in age from less than 1 year old to greater than 41, were available for biomedical and behavioral research (Tables 1 and 2). The U.S. government currently supports 612 of these animals at four NCRR-supported facilities; the remaining animals are privately owned and supported (HHS, 2011a). The NCRR at the NIH provides programmatic oversight of these facilities and ensures they comply with the Animal Welfare Act, and with policies concerning laboratory animal care and use. Within the NCRR, the Division of Comparative Medicine oversees the NIH Chimpanzee Management Program (ChiMP), which supports the long-term, cost-effective housing and maintenance of chimpanzee facilities (NCRR, 2011a).
In 1995, the NIH instituted a moratorium on the breeding of chimpanzees that they owned or supported (NCRR, 2011b). Soon after, the Chimpanzee Management Plan Working Group was created to periodically assess the need for chimpanzees in research and report its findings to NCRR’s advisory body, the National Advisory Research Resources Council. This Working Group of non-government scientists and nonscientists analyzes relevant issues and drafts proposed position papers. In 2007, this Working Group issued a report2 that “did not make a definitive recommendation as to whether the chimpanzee breeding moratorium should be continued,”3 but the NIH National Advisory Research Resources Council extended the breeding moratorium indefinitely (Cohen, 2007b). Given the life expectancy of chimpanzees in captivity, it is estimated that by 2037 the federally funded chimpanzee research population will “largely cease to exist” in the United States (Cohen, 2007a; NCRR, 2007).
__________________
2Report of the Chimpanzee Management Plan Working Group—March 9, 2007 (NCRR, 2007).
3The 1997 National Research Council report, Chimpanzees in Research: Strategies for their Ethical Care, Management, and Use also recommended a 5-year breeding moratorium (NAS, 1997).
TABLE 1 Number of Chimpanzees Available in the United States for Research
| Total Number of Chimpanzeesa | Number of Chimpanzees Supported by the NCRR, NIHb | |
|
Alamogordo Primate Facility |
176 | 176 |
|
Michale E. Keeling Center for Comparative Medicine and Research |
176 | 159 |
|
New Iberia Research Center |
347 | 124 |
|
Southwest National Primate Research Center |
153 | 153 |
|
Yerkes National Primate Research Centerc |
85 | 0 |
| TOTAL | 937 | 612 |
aNumber of chimpanzees as of October 2011 (Abee, 2011c; Else, 2011; Lammey, 2011; Landry, 2011; Langford, 2011).
bNumber of NIH-supported chimpanzees current as of April 15, 2011 (HHS, 2011a).
cThe Yerkes National Primate Research Center does not use any core funds from the NCRR to support the costs for maintaining humane care and welfare of chimpanzees.
TABLE 2 Ages of Chimpanzees Available in the United States for Researcha,b
| <10 | 10 to 20 | 21 to 30 | 31 to 40 | 41+ | |
|
Alamogordo Primate Facility |
0 | 24 | 99 | 40 | 13 |
|
Michale E. Keeling Center for Comparative Medicine and Research |
0 | 53 | 67 | 27 | 29 |
|
New Iberia Research Center |
100 | 134 | 84 | 6 | 25 |
|
Southwest National Primate Research Center |
4 | 61 | 69 | 13 | 5 |
|
Yerkes National Primate Research Centerc |
1 | 29 | 30 | 12 | 13 |
| TOTAL | 105 | 301 | 349 | 98 | 83 |
aAges of chimpanzees as of October 2011 (Abee, 2011c; Else, 2011; Lammey, 2011; Landry, 2011; Langford, 2011).
bThe committee was unable to match the age of each chimpanzee with the funding source. Numbers represent a mix of federal and other sources of funding.
cThe Yerkes National Primate Research Center does not use any core funds from the NCRR to support the costs for maintaining humane care and welfare of chimpanzees.
Origin of Study and Committee Statement of Task
The formation of the present committee activity and subsequent report was precipitated by events that took place in 2010, when the NIH announced its decision to transfer the chimpanzees located at the Alamogordo Primate Facility to the Southwest National Primate Research Center, where they would be consolidated with the chimpanzee colony that was already maintained and available for research (HHS, 2011b, 2011d). As the NIH’s 10-year contract with Charles River Laboratories to manage the Alamogordo Primate Facility neared its completion, the NIH stated that consolidating the chimpanzees into a single colony at the Southwest National Primate Research Center facility would save $2 million a year and make the animals available for future research (HHS, 2011a; Korte, 2010). This decision stirred controversy. Animal rights activists and primate experts objected to returning the Alamogordo chimpanzees to a location where research is allowed, advocating instead for their permanent retirement (The Humane Society of the United States, 2010). Then–New Mexico Governor Bill Richardson also objected to closing the facility, which employs about 35 people (Korte, 2010). He asked the NIH to reverse its plans and requested that the U.S. Department of Agriculture (USDA) formally evaluate the way in which relocation plans were made. Governor Richardson requested that the Alamogordo Primate Facility be converted to an official sanctuary4 or be operated by local universities for non-invasive behavioral research (Ledford, 2010).
In December 2010, amid increasing attention to the issue,5 U.S. Senators Jeff Bingaman (D-NM), Tom Harkin (D-IA), and Tom Udall (D-NM) requested the National Academies conduct an in-depth analysis of the current and future need for chimpanzee use in biomedical research, an analysis they anticipated would consider the “great progress the science
__________________
4The U.S. Chimpanzee Health Improvement, Maintenance, and Protection Act of 2000 (106th Cong., 2nd sess.) required sanctuaries to house chimpanzees no longer needed for medical research.
5While not directly related to this study, it is of historical interest that bills were introduced in the U.S. Congress in 2008, 2009, 2010, and 2011 to ban research using chimpanzees and other great apes. Legislation included the Great Ape Protection Act of 2008, 110th Cong., 2d sess.; Great Ape Protection Act of 2009, 111th Cong., 1st sess.; Great Ape Protection Act of 2010, 111th Cong., 2d sess.; and Great Ape Protection and Cost Savings Act of 2011, 112th Cong., 1st sess. To date, the bills have not been adopted into law; however, activities related to the proposed legislation have also contributed to the national discussion about the necessity of chimpanzees for research.
community has made in research techniques” and “allow our nation’s research institutions to make long-range decisions about the merits of continued invasive research using chimpanzees.” In January 2011, the NIH announced it would suspend transfer of the Alamogordo colony and that it had tasked the Institute of Medicine (IOM) to study this issue (HHS, 2011b). Upon completion of this study, the NIH will revisit its decision regarding the Alamogordo colony.
In response to the request from the NIH, the IOM, in collaboration with the National Research Council, assembled the Committee on the Use of Chimpanzees in Biomedical and Behavioral Research to conduct a study and issue a report on the use of chimpanzees in NIH-funded research that is needed for the advancement of the public’s health. The committee’s statement of task is in Box 1.
BOX 1
Statement of Task
In response to a request from the National Institutes of Health (NIH), the Institute of Medicine, in collaboration with the National Research Council, will assemble an ad hoc expert committee that will conduct a study and issue a letter report on the use of chimpanzees in NIH-funded research that is needed for the advancement of the public’s health. The primary focus will be animals owned by the National Institutes of Health, but will also include consideration of privately owned animals that are currently financially supported by the NIH.
Specifically, the committee will review the current use of chimpanzees for biomedical and behavioral research and:
• Explore contemporary and anticipated biomedical research questions to determine if chimpanzees are or will be necessary for research discoveries and to determine the safety and efficacy of new prevention or treatment strategies. If biomedical research questions are identified:
ο Describe the unique biological/immunological characteristics of the chimpanzee that make it the necessary animal model for use in the types of research.
ο Provide recommendations for any new or revised scientific parameters to guide how and when to use these animals for research.
• Explore contemporary and anticipated behavioral research questions to determine if chimpanzees are necessary for progress in understanding social, neurological, and behavioral factors that influence the development, prevention, or treatment of disease.
In addressing the task, the committee will explore contemporary and anticipated future alternatives to the use of chimpanzees in biomedical and behavioral
research that will be needed for the advancement of the public’s health. The committee will base its findings and recommendations on currently available protocols, published literature, and scientific evidence, as well as its expert judgment.
This report is based on the committee’s evaluation of the ongoing chimpanzee research and its expert judgment and assessment of the needs for chimpanzee research. Neither the cost of using chimpanzees in research nor the ethical implications of that use were specifically in the committee’s charge. Rather, the committee was asked for its advice on the scientific necessity of the chimpanzee as a human model for biomedical and behavioral research. The committee agrees that cost should not be a consideration. However, it recognizes that any assessment of the necessity for using chimpanzees as an animal model in research raises ethical issues, and any analysis must take these ethical issues into account. The committee’s view is that the chimpanzee’s genetic proximity to humans and the resulting biological and behavioral characteristics not only make it a uniquely valuable species for certain types of research, but also demand a greater justification for their use in research than is the case with other animals. Reports over many decades have established the principles and guidelines dictating that animal subjects must be used in studies only where the risk to the health and welfare of humans is too great (European Union, 2010; NAS, 2010; Parliament of the United Kingdom, 1987). Chimpanzees share biological, physiological, behavioral, and social characteristics with humans, and these commonalities may make chimpanzees a unique model for use in research. However, this relatedness—the closeness of chimpanzees to humans biologically and physiologically—is also the source of ethical concerns that are not as prominent when considering the use of other species in research. This is consistent with the 2010 European Union Directive, which notes that ethical issues are raised by the genetic proximity to human beings (European Union, 2010).
In simplest terms and following the committee’s focus on necessity, the research use of animals that are so closely related to humans must offer insights not possible when using other animal models. In addition, the research must be of sufficient scientific or health value to offset these moral costs. There are many ethical approaches to analyze and either
justify or proscribe the use of animals in research, and the committee was neither tasked nor appropriately composed to evaluate and reach consensus on a particular approach or to apply it to research on chimpanzees. However, in animal research policy, utilitarian justifications form part of the rationale for continued research in animals; that is, animals are subjected to risk for the benefit of humans, and justification relies on assessments that the benefits gained from research on animals are sufficient to outweigh the harms caused in the process. Purely utilitarian justifications are tempered in animal research through policy requirements for humane treatment and the use of appropriate species and minimal number of animals. Furthermore, imposing requirements for justifying the use of higher species is an implicit recognition that the use of higher animals comes at higher moral costs. Thus, the use of chimpanzees should face the most stringent requirements for justification, and constraints that acknowledge the characteristics that make chimpanzees unique among animal research subjects. For the committee, this ethical context is reflected in its assessment of when, if ever, the use of chimpanzees in biomedical research is necessary.
METHODS AND ORGANIZATION OF THE REPORT
To conduct this expert assessment and evaluate the need for chimpanzees in research to advance the public’s health, the committee deliberated from May through November 2011. During this time, the committee held three 2-day meetings and several conference calls, including two public information-gathering sessions on May 26, 2011, and August 11-12, 2011 (see Appendix C for meeting agendas). Each information-gathering session included testimony from individuals and organizations that both supported and opposed the continued use of chimpanzees. The objectives of the information-gathering sessions were to:
• Obtain background data on the current use of chimpanzees in biomedical and behavioral research;
• Explore potential alternative models to chimpanzees; and
• Seek public comment about the scientific need for chimpanzees in biomedical and behavioral research.
In addition, during the course of the study the committee solicited and received over 5,700 comments via the Internet.
The committee examined the current availability of chimpanzees and use of the chimpanzee as an animal model. The committee also reviewed the use of chimpanzees in the peer-reviewed scientific literature, as described later in the section titled “Summary of Chimpanzee Research.” In addition, it reviewed NIH projects that supported chimpanzee research from 2001 to 2010. The committee reviewed a number of background documents provided by stakeholder organizations. The committee also commissioned a paper titled “Comparison of Immunity to Pathogens in Humans, Chimpanzees, and Macaques” (see Appendix B).
The committee completed its task by identifying a set of core principles to guide current and future use of the chimpanzee, and based on these principles derived a set of criteria used to assess whether chimpanzees are necessary for research now or in the future.
INTERNATIONAL POLICIES GUIDING CHIMPANZEE USE
Many countries have legislation banning the use of great apes, and therefore chimpanzees.6 Legal action may have been deemed unnecessary in countries where chimpanzee biomedical and behavioral research no longer occurs. The most recent legislative action around great ape use took place within the European Union (EU), with its 27 member states. In November 2010, following an eight-year political process, the EU adopted Directive 2010/63 outlining the protection of animals used for research purposes (European Union, 2010). This directive bans the use of great apes in research (Article 8), except for a specific safeguard clause that is described below (Article 55). Limitation of the ban to great apes, but not other non-human primates, and inclusion of the safeguard clause were based on political compromise that occurred over several years. Factors in the development of this compromise may have included
• No research using chimpanzees has been conducted at an EU facility since 1999 (European Parliament, 2007; Vogel, 2001);
• The last facility to house chimpanzees stopped all research in 2004 (BPRC, 2011);
__________________
6As will be discussed later in the “Summary of Chimpanzee Research” section, the committee did find that investigators from countries outside the United States have supported limited use of chimpanzees in the United States.
• Support by the European Commissions’ Scientific Committee on Health and Environmental Risks (SCHER) for the continued use of non-human primates (NHPs) (Bateson, 2011; SCHER, 2009); and
• Recognition of the claims by the research community that the direction of new research is by definition unpredictable, as are the development of epidemics and emergence of new diseases.
The safeguard clause states that the use of great apes is permitted only for the purposes of research aimed at the preservation of those species or where action in relation to a potentially life-threatening, debilitating condition endangering human beings is warranted, and no other species or alternative method would suffice in order to achieve the aims of the procedure. While this clause was already in place in the previous version of the directive (European Communities and Office for Official Publications, 1986; Hartung, 2010), further details in the new directive (European Union, 2010) stipulate that in order for a member state to authorize a study involving great apes the member state must obtain approval from the European Commission in consultation with a relevant Committee (European Communities and Office for Official Publications, 1986) and (European Union, 2010). At the time of this report, Directive 2010/63 is still to be implemented in all European Union member states.
A number of countries, including EU member states, have specific laws or regulations involving the use of great apes and in some cases other NHPs (Table 3). The committee was unable to find any official policies guiding the use of chimpanzees in biomedical and behavioral research in other countries with large research investments, such as China and India, or to determine whether these countries maintain research populations of chimpanzees.
TABLE 3 International Policies on the Use of Great Apes in Scientific Research
| Country or Entity (Year) | Policy or Statement |
|
Australia (2003) |
Restricts research and stipulates that “great apes may only be used for scientific purposes if the following conditions are met: Resources, including staff and house, are available to ensure high standards of care for the animals; the use would potentially benefit the individual animal and the species to which the animal belongs; the potential benefits of the scientific knowledge gained will outweigh harm to the animal” (Australian Government National Health and Medical Research Council, 2003). |
|
Netherlands (2003) |
The principal law on animal experimentation was amended with the insertion of a new Sec. 10e, which prohibits experimentation on chimpanzees, bonobos, orangutans, and gorillas. An exception was made in the case of experiments commenced before January 1, 2003, in which chimpanzees were used with a view to developing a vaccine against hepatitis C (WHO, 2003). |
|
New Zealand (1999) |
The Animal Welfare Act stipulates that the Director-General must not give approval unless he or she is satisfied that the use of the non-human hominid in that research, testing, or teaching either (1) it is in the best interests of the non-human hominid; or (2) it is in the interests of the species to which the non-human hominid belongs and that the benefits to be derived from the use of the non-human hominid in the research, testing, or teaching are not outweighed by the likely harm to the non-human hominid (Animal Welfare Act 1999 [New Zealand], 1999). |
|
Spain (2008) |
The Commission on Environment, Agriculture and Fishing submitted a proposal to the Spanish Parliament to approve a resolution urging the country to comply with the Great Apes Project, founded in 1993, which argues that nonhuman great primates—chimpanzees, gorillas, orangutans and bonobos, should have the right to life, the protection of individual liberty, and the prohibition of torture (Congress of Spain, 2008). |
|
United Kingdom (1997) |
In November 1997, the government issued a supplementary note to its response to an interim report in which it published a policy statement on the use of animals in scientific procedures. It promised: the use of great apes in scientific procedures would not be allowed. While such animals have never been used under the 1986 Act, the government decided that it would be unethical to use such animals for research purposes due to their cognitive and behavioral characteristics and qualities. In the Home Office “News Release” accompanying the publication of the Interim Report, Lord Williams is quoted as follows: “Although these proposed bans cannot be statutory under current legislation, I do not foresee any circumstances in which the Home Office would issue licenses in such cases” (Reynolds and CEECE, 2001; Secretary of State for the Home Department and Parliament of the United Kingdom, 1998). |
SUMMARY OF CHIMPANZEE RESEARCH
The committee was asked, as part of its task, to review the current use of chimpanzees for biomedical and behavioral research. To assess the use of the chimpanzee as an animal model, the committee explored research supported by the NIH and other federally and privately funded research over the past 5 years, and where possible, 10 years. A summary of this analysis is presented in the following section.
Analysis of Federally Supported Research
The largest percentage of federally funded chimpanzee research over the past 10 years has been supported by the NIH, with additional projects funded by other federal agencies, including the Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), and National Science Foundation (NSF).
NIH-Supported Research
To explore NIH-supported research, the committee used the Research Portfolio Reporting Tools Expenditures and Results (RePORTER) system to search for projects that included the terms “chimpanzee(s)” or “Pan troglodyte(s).” The search, conducted on July 6, 2011, was refined to exclude projects that were found to not use chimpanzees. Finally, the projects were categorized. From 2001 to 2010, the NIH funded 110 projects that used chimpanzees, chimpanzee genomic sequences, or other chimpanzee-derived compounds (Table 4). Hepatitis research,7 the largest category with 44 projects, has included projects that range from molecular studies of the virus to immune responses in chimpanzees chronically infected with hepatitis C. In addition, studies have examined the pathogenesis of acute and chronic liver disease following infection. Comparative genomics studies included analysis of human and chimpanzee polymorphism rates. Some of the 11 neuroscience research projects focused on studies of neurodevelopment, while behavioral research studies examined task engagement and sociocommunicative development.8
__________________
7The term “hepatitis” is inclusive of all types of hepatitis, including A, B, C, D, and E.
8Behavioral research studies may also fall under additional categories, such as neuroscience.
Additional research areas included acquired immune deficiency syndrome (AIDS)/human immunodeficiency virus (HIV), malaria, and immunology. Of the remaining 22 projects, a portion was for research on a variety of topics, including studies of respiratory syncytial virus (RSV) and vaccines against anthrax toxin, while the remaining group of projects supported chimpanzee colonies, including the care and maintenance of the animals. Because each project varied in the number of years of funding, a breakdown of the number of research projects ongoing in each year in each disease category was performed (Figure 1). The number of annually funded NIH projects varied from 38 projects in 2002 to 52 in 2007.
TABLE 4 Number of Projects and Types of Funding per Disease Area: 2001-2010
| Hepatitis | Projects | Types of Funding | ||||
| R1 | P2 | N3 | Z4 | U5 | ||
| 44 | 14 | 0 | 0 | 25 | 5 | |
| Comparative genomics | 13 | 11 | 1 | 0 | 0 | 1 |
| Neuroscience | 11 | 7 | 1 | 0 | 3 | 0 |
| AIDS/HIV | 9 | 8 | 0 | 0 | 1 | 0 |
| Behavioral | 7 | 7 | 0 | 0 | 0 | 0 |
| Malaria | 2 | 2 | 0 | 0 | 0 | 0 |
| Immunology | 2 | 2 | 0 | 0 | 0 | 0 |
| Other | 11 | 2 | 0 | 0 | 8 | 1 |
| Colony maintenance | 11 | 2 | 1 | 3 | 0 | 5 |
| TOTAL | 110 | 55 | 3 | 3 | 37 | 12 |
1Research project grants.
2Program project/research center grants.
3Research contracts.
4Intramural grants.
5Cooperative agreements.
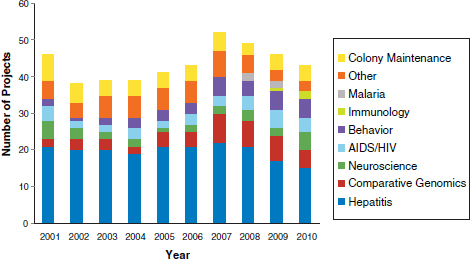
FIGURE 1 Chimpanzee research supported by the NIH: 2001-2010.
Other Federally Supported Research
Over the past decade, the FDA has funded a number of studies using chimpanzees, including the funding of the Laboratory of Hepatitis Research. The research supported by the FDA has focused on understanding the immunobiology and pathogenesis of hepatitis C virus and studying the safety of vaccines under development.
Other government agencies, including the NSF and CDC, have also funded chimpanzee research in the past 10 years, although to a significantly smaller degree than the NIH. During the past 3 years, the NSF has funded nine such studies, ranging from wild female chimpanzee emigration patterns to morphometric analysis of specific neocortical brain regions (NSF, 2011). Overall, the NSF has funded studies that include the use of both captive and wild chimpanzees, imaging data, and chimpanzee genomic information. While the CDC no longer funds chimpanzee research, previous research has included hepatitis vaccine development. Beyond these agencies, the committee did not find any evidence of current chimpanzee work funded by other federal agencies, including the Department of Defense.
Analysis of Private-Sector Supported Research
Animal models are used throughout discovery, development, preclinical testing, and production phases of new medicines and vaccines. Pharmaceutical and biotechnology companies use animals in the research and development of candidate compounds. In addition, regulatory agencies require that all new prescription drugs and biologics be subjected to thorough efficacy and safety testing prior to licensing. These requirements are in place to not only prevent potentially dangerous products from reaching human clinical trials and eventually the market, but also to ensure that only effective medications reach patients. In this context, many pharmaceutical companies state that NHPs are used when no other acceptable alternative exists and that the usual goal of using NHPs is to evaluate efficacy and safety as a final step prior to testing in humans. Several pharmaceutical companies no longer use chimpanzees, including GlaxoSmithKline, which has an official published policy indicating it has voluntarily ended the use of great apes, including chimpanzees, in research and will no longer initiate or fund studies (GlaxoSmithKline, 2011).
Committee analysis of the use of chimpanzees in the private sector was hindered by the proprietary nature of the information. However, based on limited publications and public non-proprietary information, it is clear that the private sector is accessing both the whole-animal model as well as stored biological samples (Carroll et al., 2009; Olsen et al., 2011). In addition, from data provided by the four NCRR-supported centers, the committee learned that from 2006 to 2010, 144 chimpanzees were used for efficacy, safety, and pharmacokinetic (PK) studies, suggesting that chimpanzees have been a part of the process of drug and/or vaccine development. These data do not make clear, however, which of these studies were funded by private companies and which, if any, were funded by the federal government. In addition, between 2005 and 2010, more than 300 requests for biological samples have come from individuals or groups with private funding, but, again, it was not possible to determine what percentage was funded by industry (Abee, 2011b; Langford, 2011; Rowell, 2011).
Use of chimpanzees in the United States is not limited to U.S.-based investigators, agencies, or companies. Between 2005 and 2010, 27 studies were funded by either non-U.S.-based companies or non-U.S.-based academic investigators (Watson, 2011). The majority of these studies were for hepatitis C therapy or vaccine development, with a few additional
studies on monoclonal antibody efficacy and immunogenicity. Eight studies were funded by companies/investigators from Italy, followed by Japan and Denmark (five studies each). In addition, companies from Belgium, Spain, and France funded one study each. The committee hypothesizes that, among other reasons, foreign companies are using U.S. resources because of the EU ban on great ape research, the lack of research facilities in their respective countries capable of supporting chimpanzee research and, for industry, regulatory requirements both in the United States and abroad (Box 2).
While the committee was able to determine that both U.S.- and non-U.S.-based companies conduct limited chimpanzee research in the United States, it was not able to determine if companies independently house chimpanzees, how often the animals are used, and what compounds, if any, currently on the market or in human clinical trials were tested using this model.
BOX 2
Regulatory Requirements
Food and Drug Administration
The FDA regulatory policies regarding the approval of new drugs, vaccines, and other biological products do not specifically refer to chimpanzees. The FDA does provide guidance that safety and toxicology studies must be completed using the most appropriate, or relevant, species prior to preclinical testing. The FDA relies on the sponsor to select the species and demonstrate the usefulness of the model while encouraging dialogue between sponsors and the agency regarding the type of animal models considered for testing. While there are no official policies about the content of these dialogues, the committee was able to learn about internal, unwritten practices of different branches of the FDA. The Center for Drug Evaluation and Research (CDER) does not ask for chimpanzee data and specifically discourages the use of chimpanzees when approached by sponsors. This decision is based on, in part, the availability of other methods for developing the required data, including the use of transgenic and chimeric animals, surrogate antibodies, and the minimal anticipated biological effect level approach. CDER, however, does not turn away applications that contain chimpanzee data, including seven applications in the past 5 years. Like CDER, the Center for Biologics Evaluation and Research (CBER) does not have a specific policy on the use of chimpanzees and does not require their use, if the sponsor is able to demonstrate the relevance or appropriateness of a different animal model. However, in contrast to CDER, CBER does not actively discourage the use of chimpanzees, in particular for use in vaccine development to prove effectiveness or demonstrate safety.
The Special Case of the FDA’s Animal Rule
In some selected circumstances, when it is not possible to conduct human studies, the FDA can grant marketing approval based the Animal Rule (FDA, 2011a, 2011b). The Animal Rule states that approval would require adequate and well-controlled animal studies whose results show that the drug or biologic is reasonably likely to produce clinical benefit in humans (CDER and CBER, 2009).
European Medicines Agency
European Union (EU) regulatory requirements related to marketing authorization of medical products do not specifically refer to chimpanzees, although there is some guidance on the use of the most sensitive and relevant species (EMEA, 2008; 2011a). Within the European Medicines Agency (EMEA), the Committee for Medicinal Products for Human Use (CHMP) is responsible for determining whether or not medicines meet quality, safety, and efficacy requirements (CHMP, 2011). In preclinical safety evaluation guidance, the CHMP defines a relevant species as “one in which the test material is pharmacologically active due to the expression of the receptor or an epitope (in the case of monoclonal antibodies)” (EMEA, 2011a). Additionally, the CHMP recommends that safety evaluation programs should include the use of two relevant species, although one species may be sufficient if justification is provided. The EMEA does not require or recommend the use of chimpanzees for product approval. However, should a marketing authorization application contain results from chimpanzee studies, this does not disqualify the product or data. Between 2004 and 2010, the EMEA has authorized nine products based, in part, on chimpanzee data (European record assessment reports). No marketing ban on medicines or vaccines developed using chimpanzees was provided for in current legislation. Directive 2010/63, which makes the ban of great apes more explicit, does not change anything in EMEA practice as there was no specific requirement for the use of chimpanzees in place before the revision of the previous directive.
Criteria That Guide the Current Use of Chimpanzees
Each chimpanzee research center has individual, but similar, processes by which a researcher has resource requests evaluated (Abee et al., 2011). At each center an ad hoc committee, composed of researchers, veterinarians, behavioral biologists, and other experts, reviews each request using a unique set of questions. These questions are designed to evaluate the study rationale, determine if the chimpanzee is needed, and then assess how many animals are required. The dialogue continues until either it is determined the chimpanzee is no longer required or every member of the advisory committee is convinced that the study will be
conducted appropriately and that all the preliminary studies have been completed.
In addition to the review performed by the Chimpanzee Research Centers, additional reviews occur prior to the start of any chimpanzee study. For all projects, the investigator’s institutional animal care and use committee must approve the study protocol. In addition, the NIH Interagency Animal Model Committee must determine that the chimpanzee is the appropriate model for any project approved by a Chimpanzee Research Center that will use an NIH-owned chimpanzee (Bennett et al., 1995; DHS, 2007). However, as is the case for the reviews performed by the Chimpanzee Research Centers, the Interagency Animal Model Committee does not evaluate protocols against a uniform set of criteria.
Finding
There are currently no uniform set of criteria used to assess the necessity of the chimpanzee in NIH-funded biomedical and behavioral research.
PRINCIPLES GUIDING THE USE OF CHIMPANZEES IN RESEARCH
The task given to the committee by the NIH asked two questions about the need for chimpanzees in research: (1) Is biomedical research using chimpanzees “necessary for research discoveries and to determine the safety and efficacy of new prevention or treatment strategies?” and (2) Is behavioral research with chimpanzees “necessary for progress in understanding social, neurological, and behavioral factors that influence the development, prevention, or treatment of disease?” In responding to these questions, the committee concluded that the potential reasons for undertaking biomedical and behavioral research as well as the protocols used in each area are different enough to require different sets of criteria. However, the committee developed both sets of criteria guided by the following three principles:
1. The knowledge gained must be necessary to advance the public’s health;
2. There must be no other research model by which the knowledge could be obtained, and the research cannot be ethically performed on human subjects; and
3. The animals used in the proposed research must be maintained either in ethologically appropriate physical and social environments or in natural habitats.
Ethologically Appropriate Physical and Social Environments
Chimpanzee research should be permitted only on animals maintained in an ethologically appropriate physical and social environment or in natural habitats. Chimpanzees live in complex social groups characterized by considerable interindividual cooperation, altruism, deception, and cultural transmission of learned behavior (including tool use). Furthermore, laboratory research has demonstrated that chimpanzees can master the rudiments of symbolic language and numericity, that they have the capacity for empathy and self-recognition, and that they have the humanlike ability to attribute mental states to themselves and others (known as the “theory of mind”). Finally, in appropriate circumstances, chimpanzees display grief and signs of depression that are reminiscent of human responses to similar situations. It is generally accepted that all species, including our own, experience a chronic stress response (comprising behavioral as well as physiological signs) when deprived of usual habitats, which for chimpanzees includes the presence of conspecifics and sufficient space and environmental complexity to exhibit species-typical behavior. Therefore, to perform rigorous (replicable and reliable) biomedical and behavioral research, it is critical to minimize potential sources of stress on the chimpanzee. This can be achieved primarily by maintaining animals on protocols either in their natural habitats, or by consistently maintaining with conspecifics in planned, ethologically appropriate physical and social environments in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AZA Ape TAG, 2010; Council of Europe, 2006; NRC, 1997, 2010). Examples of appropriate physical and social environments currently accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International include primadomes or corrals with environmental enrichment, outdoor caging with access to shelter, and indoor caging.
The committee recognizes exceptions to this criterion may be warranted. For example, as a result of previously approved protocols, there are currently a few long-term research projects in which the living conditions and the relationships with humans have been idiosyncratic and integral to the protocols (e.g., studies where a chimpanzee is being taught a symbolic language and lives and/or intensely interacts with a small number of researchers). In addition, current health and prior infectious exposures might prevent social housing for particular animals in potential experiments that may need to be performed in biosafety level (BSL) 3 or 4 facilities. Therefore, while the committee encourages that animals be maintained in planned, ethologically appropriate physical and social settings or natural habitats, existing protocols should be judged on a case-by-case basis, and changes made should impose minimal physiological and psychological harm to the animals and disruption to their existing relationships with people. All future studies should conform to the need for ethologically appropriate housing.
Criteria to Assess the Necessity of the Chimpanzee
for Biomedical Research
As previously discussed, the chimpanzee raises unique considerations due to the ethical issues that arise as a result of the chimpanzee’s genetic proximity to human beings. Therefore, based on the principles previously defined, the committee developed the following criteria to guide its assessment of NIH-funded biomedical research using the chimpanzee:
1. There is no other suitable model available, such as in vitro, nonhuman in vivo, or other models, for the research in question;
2. The research in question cannot be performed ethically on human subjects; and
3. Chimpanzees are necessary to accelerate prevention, control, and/or treatment of potentially life-threatening or debilitating conditions.
Specific and full scientific justification for use of the chimpanzee must meet all three of the above criteria. Assessment of which uses meet these criteria should be done prospectively on a study-by-study basis. It is
important that justification is substantiated and provides adequate evidence; statements such as the following would not be acceptable to the committee:
• “The chimpanzee is immunologically, physiologically, anatomically, and/or metabolically similar to human beings.” This statement is too broad.
• “Chimpanzees have previously been used in safety studies for this class of drug.” This statement is not specific as to the science driving the decision.
It is important to note that the committee focused its task on the type of research supported by the NIH. The committee acknowledges that biomedical research aimed at the preservation and welfare of the chimpanzee species may also necessitate use of the chimpanzee, but this research is not be supported by the NIH unless it has direct application towards advancing human health and so on its own is outside the committee’s task.
Assessing Suitability of Available In Vitro or Non-Human In Vivo Models
Continued advances over the past decade in imaging, genetics, in vitro, and in silico models, and sophisticated rodent disease models have provided scientists with more tools that could be used in place of the chimpanzee. Federal regulations require that animals selected for a protocol should be of an appropriate species and quality and that the minimum number required to obtain valid results should be used (U.S. Office of Laboratory Animal Welfare, 2002). Methods such as mathematical models, computer simulation, and in vitro biological systems should also be considered before chimpanzees are considered for research.
When assessing the necessity of the chimpanzee as a model, a more stringent process of eliminating (“deselecting”) models of species less closely related to human beings should be required, similar to the process adopted by many countries in Europe (European Union, 2010). For example, in the United Kingdom, Section 5 of the Animals Scientific Procedures Act states that the Secretary of State may not authorize any procedures where an alternative exists (Parliament of the United Kingdom, 1987). The rationale for selection of the chimpanzee as the necessary model must be supported by facts and data (Box 3). The process
must be rigorous and principles for deselection must be clearly defined and consistent across institutions.
BOX 3
Deselection Criteria
The following are specific examples of deselection criteria that the committee used to assess the suitability of available in vitro or non-human in vivo models.
In Vitro Culture System
In vitro models must be deselected if specific data required can only be obtained through the use of in vivo models.
In Vivo Models
Other species—such as monkeys, dogs, mini-pigs, and rodents, including transgenic and chimeric animals modified to mimic specific disease attributes—must be deselected prior to determining that data required from a specific experiment can only be obtained through the use of a chimpanzee. Non-chimpanzee models in most cases sufficiently mimic the aspect of the disease (e.g., susceptibility, sustainability, progression) or disease pathways or targets, to the extent that they will provide sufficient data for the question being asked. The model system chosen does not need to replicate the complete pathophysiology of the disease/disorder being studied.
Species Differences in Absorption, Distribution, Metabolism, and Excretion (ADME)
Other species—such as monkeys, dogs, mini-pigs, and rodents, including transgenic and chimeric animals modified to mimic specific disease attributes—must be deselected by determining that ADME profiles do not adequately match the profile generated by humans.
Other species—such as monkeys, dogs, mini-pigs, and rodents, including transgenic animals modified to mimic specific disease attributes—must be deselected prior to determining that pharmacokinetic data (bioavailability, distribution, or metabolic data) obtainable from these species are significantly less suitable than data that are expected to be obtained from chimpanzees. For example, if a species fails to convert a pro-drug (inactive drug) to the active moiety, that species would be unsuitable as a toxicology species.
The standard in vitro (e.g., microsomal) model must be deselected when metabolism and pharmacokinetic data must show qualitative or substantial quantitative differences, and incremental differences are not considered sufficient.
Species Differences in Vehicle Tolerability
Other species—such as monkeys, dogs, mini-pigs, and rodents, including transgenic and chimeric animals modified to mimic specific disease attributes—must be deselected by determining that the test article is unable to be formulated in a vehicle tolerated by these models. In these limited cases, the chimpanzee may be justified if the formulation is tolerated in the chimpanzee and if testing in humans is not ethically possible (see below).
Species Differences in Response to Test Article Tolerability
Deselecting other species—such as monkeys, dogs, mini-pigs, and rodents, including transgenic and chimeric animals modified to mimic specific disease attributes—must be data driven. These data can be derived from in vitro studies (e.g., test articles demonstrated to be potent COX2 inhibitors or SSRIs are contraindicated in dogs, and some antimicrobials are contraindicated in rabbits and guinea pigs) if there is strong historical evidence of compound class intolerability.
Poor tolerability is justification for not using other species only if it precludes assessment of other relevant toxicities (e.g., if emesis precludes achieving adequate systemic exposure). If the basis for intolerability of a test article is clinically relevant, it may be a reason for selection rather than deselection of a nonrodent or non-human primate (NHP) species.
Pharmacology
The standard non-rodent and NHP species must be deselected if there is a lack of pharmacologic response (demonstrated inactivity) in these animals. In these cases, the chimpanzee may be justified if there is scientific evidence that pharmacological activity will occur in chimpanzees and those specific safety concerns of exaggerated pharmacology need to be characterized in animal toxicity studies. If other species—such as monkeys, dogs, mini-pigs, and rodents, including transgenic animals modified to mimic specific disease attributes—have pharmacological sensitivity that precludes testing at adequate multiples of clinical exposure, use of chimpanzees may be justified if toxicity studies in chimpanzees could achieve significantly greater exposure. However, if safety concerns of exaggerated pharmacology can be adequately characterized in other species—such as monkeys, dogs, mini-pigs, and rodents, including transgenic animals modified to mimic specific disease attributes—pharmacological responsiveness of chimpanzees is not necessarily a factor in species selection.
Immunogenicity
Other species—such as monkeys, dogs, mini-pigs, and rodents, including transgenic animals modified to mimic specific disease attributes—must be deselected if there is a scientifically based expectation for significant antigenicity for test articles not intended to be immunogenic. Vaccine research and development requires an appropriate immunogenic response to the vaccine and/or to an adjuvant, which in some cases may necessitate the use of chimpanzees, if human experiments cannot be ethically performed (see below).
Availability of Test Article or Cost of Species
A limited supply of the most suitable experimental animal or individual cost of the proposed species is not a justification for deselecting the standard nonrodent or NHP species.
Assessing Whether the Research Can Be Performed on Human Subjects
As the criteria regarding necessity outline, chimpanzee research is not necessary if it can be ethically performed on humans. Standard arguments about protection of human subjects require that there be an acceptable balance of the risks and potential benefits of proposed research, that the distribution of the risks and benefits are equitable (higher risk research can be justified when the potential therapeutic benefits accrue to the subjects themselves), and that the subjects are voluntary and informed of potential liabilities during their decision making. Relevant examples of critical human health-related research that would not meet human subjects’ protection standards include trials that intentionally expose subjects to untreatable infectious diseases and exposure trials to hazardous substances that pose significant health risks without prospect of benefit.
When research on humans is justified, federal policies on protection of human subjects impose limits, including for research on subjects who cannot consent for themselves. Subparts of the federal regulations concerning research on human subjects also impose clear limits on acceptable research on children and prisoners (HHS, 2005). These include restrictions on research that poses greater than minimal risk to subjects; such research cannot be approved unless it has the potential for offsetting therapeutic benefit to the subjects themselves.
These standards and additional protective restrictions mean that more research may take place using animal models than would otherwise be the case if additional risks to human subjects were deemed acceptable.
Assessing Advancements to Treat Potentially Life-Threatening or Debilitating Conditions
The standard non-rodent and NHP species may be deselected if it can be demonstrated that forgoing the use of chimpanzees for the research in question will significantly slow or prevent important advancements to treat potentially life-threatening conditions in humans or debilitating conditions that have a significant impact on a person’s health, and thus slow or prevent important advancements for the public’s health. This assessment is based on the potential impact on human health and potential to improve well-being, which can be partially assessed by the burden of the disease or disorder. The committee notes that for emerging infectious
diseases and biodefense-related threats, this information may not exist for low-probability, high-consequence threats.
Criteria for Use of the Chimpanzee in Comparative Genomics
and Behavioral Research
As previously discussed, research using the chimpanzee raises unique ethical issues because of its genetic proximity to human beings and highly developed cognitive and social skills. Therefore, based on the principles previously defined, the committee developed the following criteria to guide its assessment of NIH-funded comparative genomics and behavioral research using the chimpanzee:
1. Studies provide otherwise unattainable insight into comparative genomics, normal and abnormal behavior, mental health, emotion, or cognition; and
2. All experiments are performed on acquiescent animals, in a manner that minimizes pain and distress, and is minimally invasive.
Specific and full scientific justification for the continued and future use of the chimpanzee must meet the above criteria, as well as the housing/ maintenance requirements described earlier in the document. This assessment should be applied prospectively on a study-by-study basis.
Assessing the Objectives of the Project
The review of research projects on a study-by-study basis must demonstrate that the primary objective of the research is to provide otherwise unattainable, specific insight into human evolution, normal and abnormal behavior, mental health, emotion, or cognition. Research may be either basic or applied, but must be consistent with the mission of the NIH “to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce the burdens of illness and disability” (NIH, 2011).
The committee recognizes that most behavioral research differs fundamentally from biomedical research in the sense that mental or behavioral disorders (with few exceptions) cannot be modeled explicitly using
chimpanzees. This is because the naturally occurring prevalence of such disease is likely to be low if compared to what is observed in human populations, thus precluding reasonably sized studies using chimpanzees. Some conditions (e.g., depression or post-traumatic stress syndrome) may be inducible in chimpanzees, but likely only using procedures that would be judged unacceptably invasive. This is especially true inasmuch as other animals, including other nonhuman primates, have been used to model these disorders. It is for the forgoing reasons that the majority of comparative genomics or behavioral studies using chimpanzees have focused on continua of behavioral and developmental phenomena from normal to abnormal, taking advantage of similarities in behavioral and brain complexity that mark chimpanzees and humans apart from virtually all other species.
Assessing Animal Acquiescence and Distress
Comparative genomics and behavioral research should only be performed on acquiescent animals and in a manner that minimizes distress to the animal. Evidence of acquiescence includes situations in which animals do not refuse or resist research-related interventions and that do not require physical or psychological threats for participation. In addition, only minimally invasive protocols should be performed. Examples of minimally invasive procedures include behavioral observation and the introduction of novel objects to the living area. In performing some comparative genomics or behavioral research, it also may be necessary to temporarily isolate an animal from its social group to perform behavioral tasks or for anesthesia. It is anticipated that anesthesia may be necessary for noninvasive imaging studies, the collection of biological samples (including blood, skin, adipose, or muscle) that do not involve surgical invasion of body cavities, the implantation of radio transmitters to measure autonomic nervous system function or physical activity, and the use of biosensors for recording central nervous system responses in freely moving animals. Whenever possible, anesthesia for comparative genomics or behavioral purposes should coincide with scheduled veterinary examination. Research on elderly or infirm animals in particular should take full advantage of anesthesia performed as part of routine veterinary care. It is recognized, however, that some study protocols may require that animals be anesthetized apart from veterinary examinations. The annual occurrence of such episodes of anesthesia should be minimized in number and the length of time the animals are sedated, consistent with accepted veterinary
practice, including post-procedure analgesia as required. In all instances, anesthesia protocols should be designed to ensure that effects on the central nervous system or other organs are transient, and anesthesia for research purposes only should be avoided when possible in elderly or infirm animals. When animal protocols for anesthesia are not available, protocols used for human patients under similar circumstances may guide the choice of procedures.
Finally, when temporary removal from the social group is required for behavioral manipulation or anesthesia, animals must be handled in a manner that minimizes stress. Successful strategies have included positive reinforcement training that allows animals to be called by name or otherwise enticed to leave their habitual setting to engage in research procedures.
REVIEWING THE NECESSITY OF CURRENT
CHIMPANZEE RESEARCH
The following case studies are meant to demonstrate how the committee envisions its criteria for the use of chimpanzees in research might be employed. In each case, the committee reviews the current use of the chimpanzee against the criteria and makes a determination of whether or not the research should be continued or prohibited. It is important to note that the committee is not reviewing any specific research grant, but rather the larger body of research in each area. As reviewed previously in the report, chimpanzees are used in multiple research areas (see Figure 1). Based on the propensity of current research, the committee chose to assess the necessity of the chimpanzee in areas of research where there is significant on-going research or a potential for significant research. The committee assessed the following research areas: monoclonal antibodies, RSV, hepatitis C virus (HCV) antiviral drug development, HCV vaccine development, comparative genomics, cognition, and neurobehavioral function. Other areas, for example, malaria research, have limited ongoing studies using the chimpanzee. From 2001-2010 there were only two studies that were done in the field of malaria, both currently still funded. For this reason, the committee chose not to use this and similar areas for case studies. However, the use of the chimpanzee in this and other research areas not reviewed by the committee can be assessed by using the same criteria.
Background
Currently, two separate uses of monoclonal antibodies rely on the chimpanzee. These are the production of chimpanzee monoclonal antibodies and preclinical safety testing of monoclonal antibodies prior to their introduction into humans. The development of monoclonal antibodies for use in any laboratory or clinical application follows the groundbreaking methods pioneered by Georges Köhler and César Milstein in the mid-1970s (Köhler and Milstein, 1975). Köhler and Milstein developed robust cell culture methods to immortalize individual B cells and thus create clonal cell lines that produce one type of antibody, hence the term “monoclonal antibody.” The ability to produce essentially unlimited supplies of a unique monoclonal antibody provides a powerful technological platform for the generation and use of a wide range of affinity reagents in a myriad of applications.
In recent years the utility of having antibodies that bind to a single site on a molecule of interest has been expanded by the ability to produce affinity reagents using any of a series of in vitro molecular cloning methods (reviewed extensively over the years, but see de Marco, 2011; Demarest and Glaser, 2008; and Kneteman and Mercer, 2005, for recent comprehensive reviews). These approaches range from simple cloning of cDNA copies of the antibody mRNAs from immortal B cells, which allows the production of the monoclonal antibody in other cells and in vitro systems, to complete synthetic methods that identify individual binding domains from pools of expression vectors. The sequences that encode the binding domains can be expressed to produce a wide range of affinity reagents. It is now common to place the antigen interaction domains in antibody sequences from any organism, including humans, or in any antibody subtype, allowing the functional activities to be selected to achieve the best results. The antigen binding sites can be fused to other domains to make chimeric molecules that allow the production of reagents that bind to an antigen of choice and bring essentially any functional activity to the location of the antigen. These methods allow researchers to tailor affinity reagents to fulfill a wide range of desired activities. While monoclonal antibodies are still most commonly made by immunization of animals and immortalization of their B cells, synthetic or semi-synthetic methods are gaining increasing application.
Development of Chimpanzee Monoclonal Antibodies
For slightly over a decade researchers have been using the chimpanzee for the production of monoclonal antibodies (Altaweel et al., 2011; Chen et al., 2006a, 2006b, 2007b, 2009; 2011b, 2011c; Goncalvez et al., 2004a, 2004b, 2007, 2008; Men et al., 2004; Schofield et al., 2000, 2002, 2003). Typically these monoclonal antibodies are prepared by cloning antibody-encoding cDNAs from immunized chimpanzees. In brief, one or a small number of chimpanzees are injected with an immunogen of interest. Immunogens that have been used for successful monoclonal antibody production have included such agents as inactivated human viruses or bacterial toxins. At a chosen interval after the final boost, a bone marrow sample is collected from the chimpanzee. Lymphocytes are purified from the bone marrow samples, RNA is isolated, and cDNA is prepared for cloning in various expression vectors. Coding sequences that express protein fragments can bind to the desired immunogen and are then isolated. In most procedures the chimpanzee-coding region for antigen-binding domains are cloned as chimpanzee/human chimeric antibodies and used for subsequent experiments.
It has been suggested that this approach provides two potential advantages over monoclonal antibody production in other species. First, because the antibody protein sequences between the chimpanzee and the human are so similar (Ehrlich et al., 1990), further subcloning and humanization of the chimpanzee antibody sequences are not needed, and the resulting antibodies can be used directly in humans without further work. Second, because the immune responses of the chimpanzee and the human are so similar, it is likely that chimpanzees would mount immune responses that are similar to analogous immune challenges seen in humans. The chimpanzee/human chimeric monoclonal antibodies produced in these manners have proven to be effective in both in vitro and in vivo assays to neutralize infectious viruses or to block the action of bacterial toxins.
Criteria 1: Alternative Models
It is possible to develop monoclonal antibodies with these types of binding specificities in species other than chimpanzees. As is commonly done, these binding domains can readily be converted into fully humanized antibodies (see Nelson et al., 2010, and the references within for a review of this procedure and its common use in antibody therapeutics).
Monoclonal antibodies prepared in other species with properties similar to the chimpanzee antibodies are already described in the literature (reviewed by Marasco and Sui, 2007). Further, genetic humanization of the immunoglobulin locus in mice allows for rapid and high throughput production of fully human antibodies. For example, Regeneron Pharmaceuticals has created the so-called VelocImmune mouse by directly replacing mouse antibody gene segments with their human counterparts at the same location (Valenzuela et al., 2003). Alternatively, human antibodies can be induced in human xenotransplantation models (Becker et al., 2010). While the chimpanzee is clearly capable of making an effective humoral response to these immunogens, there seems to be no unique properties to the resultant antibodies to suggest that the continued use of the chimpanzee is required.
Finding
The committee finds that the continued use of chimpanzees for the production of monoclonal antibodies does not meet the suggested criteria for the use of the chimpanzee in biomedical research. Production of monoclonal antibodies following immunization in other species or through in vitro synthetic methods is equally powerful for the generation of such reagents. There appear to be no obvious reasons to suggest that the immunogenic regions of the antigens used for monoclonal antibody production in the chimpanzee are unique to this species. Neutralizing antibodies appear in other species in high frequency, and therefore it seems likely that antigen-binding domains seen in species other than the chimpanzee can be identified and used for the production of these reagents. The humanization of these antibodies should be similar in scope and difficulty to the approaches used with the chimpanzee, and the resulting reagents should be equally useful in humans. No added time savings are inherent in approaches compared to work in other species.
Safety Testing of Monoclonal Antibody Therapies
Monoclonal antibodies used in treatment of human disease bind to a carefully chosen antigen, often a protein, and through this interaction interfere with a cellular process that underlies disease development. Therapeutic monoclonal antibodies have become important front-line treatments for a wide range of human diseases and clinical procedures,
including inflammation, autoimmunity, cardiovascular disease, cancer, macular degeneration, and transplantation. The first monoclonal antibodies approved for clinical use were introduced in the mid-1980s. The pace of FDA approval of monoclonal antibody-based therapies continues to increase—one treatment was approved in the 1980s (FDA, 1986), 7 in the 1990s, and 18 in the 2000s (An, 2010; Beck et al., 2010; Nelson et al., 2010; Reichert et al., 2005; Reynolds, 2011). Given the number of current clinical trials that are exploring new uses of monoclonal antibodies, it is likely that the introduction of novel therapies that rely on monoclonal antibodies will continue at least at this level in the future, and it is reasonable to speculate that the rate of FDA approval for new therapies may increase significantly over time.
Criteria 1: Alternative Models
When developing monoclonal antibody therapies for human clinical use, it is important to determine what, if any, unexpected effects these treatments might provoke in humans (see ICH Harmonized Tripartite Guideline, 2011, for regulatory practices and Chapman et al., 2009, and Tabrizi et al., 2009, for discussions of the process and needs for preclinical safety testing). Good preclinical models should mimic the biological effects of introducing the monoclonal antibody into humans and thus would provide predictions of any unexpected effects in humans. Issues that are important for the measurement of safety include the display of target molecules with analogous binding sites for the monoclonal antibody therapeutics, immune responses that are as similar to the human as possible, similar kinetics of monoclonal antibody presentation and clearance, and minimal immune response to the monoclonal antibody. The chimpanzee provides this close relationship, and has often been used as a model (Chapman et al., 2007).
Preclinical tests in the chimpanzee may lead to adverse events, and these adverse events may arise from three sources. First, the antibody could bind with the intended targeted protein, but the target may have unknown roles in the body that are unrelated to its disease-causing effects, thus giving on-target toxicity. Second, the monoclonal antibody may bind to proteins other than the intended target, and these interactions could give rise to unwanted side effects, yielding off-target toxicity. Third, analogous types of on- or off-target toxicities could arise from functional domains on the monoclonal antibody other than the antigenbinding domain. Other models, such as other monkey species, have not
proven to be as effective for detecting such toxicities, and over time it has become common to test for such unwanted effects using appropriately monitored and carefully sized trials in the chimpanzee. Undesired results in chimpanzee safety tests have led to the termination of a number of monoclonal antibody programs before they have advanced to clinical tests in humans, presumably saving unwarranted human suffering in the process (Abee, 2011a; Reynolds, 2011). In addition, there are also rare examples of monoclonal antibodies that have been tested directly in humans without previous chimpanzee safety tests and that have caused severe and undesired responses in humans (see Eastwood et al., 2010, for a potential biological explanation of one such undesired response). Therefore, the use of the chimpanzee for safety tests has proven to be valuable, and such studies have been used to protect human health.
Although safety trials for monoclonal antibody therapies continue to be performed in the chimpanzee, the committee also has noted that in recent years there is a trend in many groups to avoid its use. This trend is driven both by advances in monoclonal antibody technology and by changes in how potential monoclonal antibody treatments are first introduced into humans.
There are currently four methods in use that lessen the need for safety tests in the chimpanzee: (1) genetic engineering of the target protein in rodents; (2) selection of antibodies that recognize target epitopes shared across species; (3) selection of multiple antibodies that can serve as surrogates for responses; and (4) microdosing in humans (Chapman et al., 2007; Reynolds, 2011).
The first of these approaches relies on expressing the target protein in a rodent, expressing the target epitope’s ortholog in the rodent, or developing mice with xenotransplanted human tissue. This is an approach that offers some benefits, but there is considerable worry that the target protein may not function identically in the rodent, and other functional domains on the monoclonal antibody may not be recognized in an identical fashion in the rodent compared to the human. Since much of the potential response to monoclonal antibody treatment cannot be mimicked by this method, it has a limited potential to change the necessity for chimpanzee use. Nonetheless, this is a useful experimental approach and can help guide researchers to potential problems. By itself, however, this approach does not significantly change the need for chimpanzee research.
Two other approaches to lower the need for chimpanzees in safety testing rely on changes in how monoclonal antibodies are chosen for potential clinical development. As mentioned above, the development of
various recombinant antibody methodologies has dramatically expanded the range of properties that can be selected or developed during antibody creation. In one useful approach, researchers select monoclonal antibodies that bind to the target antigen at sites found both in the human and in other species beyond the chimpanzee, often in another NHP (Reynolds, 2011). In these cases, the safety of interfering with the activity of a disease-specific protein can be tested in species other than the chimpanzee. If this species has other features that mimic the human, confidence in the safety profile of a preclinical candidate being considered for human studies is raised. There is still some considerable concern about how well the preclinical model mimics the human, and it is commonly argued that even good results in such tests cannot ensure how human tests will proceed. Here, as in other cases, safety studies in more than one species raise confidence about prediction of response in the human.
In a third approach, researchers choose two or more monoclonal antibodies that bind to the same target protein. These antibodies are frequently called surrogate antibodies. With two or more antibodies that bind to the same target antigen, on-target effects can be established by comparing the responses in dose-escalating safety studies. These studies are performed in preclinical safety models. On-target effects can be identified as those that are common to all antibodies, while undesired effects are specific to one of the agents. While surrogate antibodies may not have all of the best properties of a true clinical development candidate, studying the responses to multiple agents can increase the confidence of the potential safety profiles of monoclonal antibodies prior to introduction into humans.
Criteria 2: Testing on Human Subjects
A fourth approach that may lower the dependence on safety testing in the chimpanzee relies on microdosing in humans. Monoclonal antibody treatments, which have previously shown good Pharmacokinetics/Pharmacodynamies (PK/PD) and toxicology results in preclinical studies in other models, can be tested for safety directly in humans using microdosing schedules, such as using minimal anticipated biological effect level strategies (see Muller et al., 2009, for a careful review of the use of microdosing strategies). Starting with very low doses enables clinical researchers to carefully monitor for any unexpected side effects in settings where adverse events can be detected before serious harm is done to the patient. These microdosing approaches can be teamed with
the introduction of radiolabeled tracer preparations of the monoclonal antibody to follow the in vivo localization of the antibodies and thus potentially link side effects to particular organ sites for further studies.
Finding
These approaches—use of genetically engineered rodents, directed strategies to select monoclonal antibodies with broader binding specificities across species, use of surrogate antibodies, and different methods to introduce antibodies into humans—combined with the recognition that the FDA does not require safety testing of new monoclonal antibody therapies in chimpanzees, promise to provide a series of methods that can be used to protect human safety while avoiding use of the chimpanzee.9 Therefore, the committee finds that use of these methods, often in combination, can make the chimpanzee largely unnecessary in the development of future monoclonal antibodies therapies.
Not all companies and few academic laboratories have fully adopted monoclonal antibody approaches, such as recombinant antibody production, that allow the selection of monoclonal antibody therapeutic agents that meet these more defined criteria. Therefore, there may be a limited number of monoclonal antibodies currently in the development pipeline that may require the continued use of chimpanzees. For these specific cases, the use of the chimpanzee should be assessed against the committee’s criteria for biomedical research. In addition, the NIH should be expeditious in supporting the development of broadly accessible recombinant technologies for development of novel therapeutic monoclonal antibodies.
Prevalence and Treatment Options
Respiratory syncytial virus is a pneumovirus initially described as chimpanzee coryza agent in 1955 and renamed “respiratory syncytial virus” in 1956 following virus isolation from infants with bronchiolitis
__________________
9It is important to note that if data from safety testing in the chimpanzee are presented as part of an investigational new drug application, the FDA requires that these studies reach appropriately high standards to contribute to the prediction of eventual safety in humans.
and pneumonia (Blanco et al., 2010; Chin et al., 1969; Wright and Piedimonte, 2011). Overall, RSV is the most common cause of acute lower respiratory tract infections and bronchiolitis in children under the age of 5 years (Krilov, 2011; Nair et al., 2011). Nearly all children have been infected with RSV at least once by age 2, with a large percentage of infants infected during their first RSV season (Fulginiti et al., 1969; Weisman, 2009; Wright and Piedimonte, 2011). RSV is believed to account for 85 percent of bronchiolitis and 20 percent of pneumonia cases globally, with 1 in 200 infants requiring hospitalization (Nair et al., 2011). Today, RSV is the leading cause of hospitalizations in U.S. children less than 1 year old, with an estimated 100,000 to 126,000 infants hospitalized each year due to bronchiolitis (Krilov, 2011; Wright and Piedimonte, 2011). In addition to children, RSV is reported to have similar rates of hospitalization and mortality in the elderly (Shadman and Wald, 2011). Another at-risk population includes patients who have undergone hematopoietic stem cell transplantation, with RSV affecting approximately 2-17 percent of these transplant recipients (Shah and Chemaly, 2011).
Current treatment and prevention options for RSV are very limited. Treatment for hospitalized infants and children primarily includes supportive care, but may also include administration of α-adrenergics or corticosteroids (Krilov, 2011). For severe RSV-induced lower respiratory tract infections or where there is a high risk of mortality, Ribavirin is the only licensed antiviral currently on the market. Its use is limited due to factors such as minimal clinical benefits and high cost (Krilov, 2011; Wright and Piedimonte, 2011). Palivizumab, the only approved prophylactic RSV drug, is a humanized monoclonal antibody that is administered intramuscularly every 30 days during RSV season. It is only used to reduce severity and morbidity in high-risk populations, including premature infants less than 35 weeks of gestation, infants with chronic lung or congenital heart disease, or infants and children with lung abnormalities such as cystic fibrosis (Nair et al., 2011; Shadman and Wald, 2011). Palivizumab is not commonly used in low-risk populations due to the high cost of treatments and limited evidence of clear benefit in these populations (Krilov, 2011; Prescott et al., 2010; Shadman and Wald, 2011).
Criteria 1: Alternative Models
Multiple cell lines from human and animal sources are currently used in preclinical research of RSV, including differentiated normal human
bronchial epithelial (NHBE) cells (DeVincenzo et al., 2010; Tayyari et al., 2011). Recently bronchial epithelial cells derived from human lung adenocarcinoma were found to be susceptible to RSV infection and release infectious virus similar to NHBE cells, indicating a new cell line for potential use (Harcourt et al., 2011). The use of cell culture has limitations, including the inability to replicate tissue organization and disease manifestations, such as respiratory infection in the case of RSV. Therefore, animal models of human RSV (hRSV) provide an important link between mechanistic cell culture research and human clinical trials.
No single animal model, including the chimpanzee, reproduces all aspects of RSV infection. Chimpanzees are susceptible to infection with hRSV with replication of the virus in high titers in the upper and lower respiratory tracts (Murphy et al., 1992). The chimpanzee also has the same body temperature as humans, unlike other animal models, which is important when investigating the degree of attenuation of candidate temperature-sensitive vaccine strains (Murphy et al., 1992). Seronegative chimpanzees can serve as surrogates for seronegative infants, the target population for vaccines. However, identification of animals that are seronegative can be difficult since the infection commonly spreads between animal handlers and chimpanzees (Murphy et al., 1992). The inability of chimpanzees to develop lower respiratory diseases such as bronchiolitis and pneumonia, the limited availability of specific reagents, and large size are noted disadvantages of this model (Bem et al., 2011; Graham, 2011). Alternative models for RSV research, including sheep, cotton rats, and mice, have significantly reduced the use of chimpanzees in RSV basic research; the last published research paper to use the chimpanzee appeared in 2000.
Sheep are susceptible to hRSV infection; neonatal lambs develop upper respiratory tract disease, bronchiolitis, and mild pneumonia. In addition to showing clinical signs of the disease, the respiratory tracts of sheep share many structural and developmental features with humans, unlike rodents (Bem et al., 2011). The limited availability of immunological reagents and other molecular tools, along with animal size, are two disadvantages of this model. Cattle provide another useful model of RSV infection because bovine RSV (bRSV) shares many common characteristics with hRSV, including respiratory tract infections, increased susceptibility of the young, and seasonal outbreaks (Byrd and Prince, 1997). Like sheep, however, the limited availability of reagents and animal size are disadvantages of this model. Currently the most commonly used animal models for hRSV are mice and cotton rats. The cotton rat is a semi-permissive
model of hRSV replication that develops mild to moderate bronchiolitis or pneumonia following exposure. Studies have demonstrated that this model can develop vaccine-enhanced disease at all ages and that the disease is similar to that seen in humans (Mohapatra and Boyapalle, 2008). However, cotton rats do not show clinical signs of respiratory tract disease (Bem et al., 2011; Graham, 2011). Mice, specifically the BALB/c inbred strain, develop lower respiratory tract disease symptoms following infection along with signs of illness such as weight loss (Bem et al., 2011; Domachowske et al., 2004; Nair et al., 2011). In addition, the immunohistopathology of RSV infection in mice resembles that of human infection (Mohapatra and Boyapalle, 2008). The ability to develop transgenic mouse lines offers a distinct advantage to the mouse model in comparison to other models. However, the difference in innate and adaptive immune responses between mice and humans and the inability for hRSV to robustly replicate in mouse lung tissue and spread between the upper and lower airway are disadvantages of this model (Bem et al., 2011; Mohapatra and Boyapalle, 2008).
Together these alternative models to the chimpanzee for RSV research demonstrate both susceptibility to the human form of the virus and the ability to develop clinical signs of the virus, including bronchiolitis and pneumonia; therefore, they cannot be deselected from use. Their availability and suitability, along with increasing number of cell culture systems, indicates that the first criteria for the use of chimpanzees are not met in the case of RSV research.
Criteria 2: Testing on Human Subjects
RSV antiviral drug and prophylactic vaccine clinical trials progress from Phase I studies in adults to trials with seropositive children and then seronegative infants (Nair et al., 2011). While time consuming, clinical testing is possible on human subjects, but the development of novel vaccines may be limited by an inability to predict adverse reactions to vaccines, something that can be accomplished in chimpanzees. This obstacle may be overcome with the recent development of a human experimental infection model (DeVincenzo et al., 2010). Healthy adult volunteers were infected with RSV, causing a self-limited upper respiratory illness. The researchers then tested the safety and efficacy of a small interfering RNA (siRNA) antiviral, ALN-RSV01. This proof-of-concept trial suggests a mechanism for development of a challenge model for testing vaccines against RSV in the future. While this human infection model is new and
has only been examined in one study so far, it suggests future avenues for immunogenicity, efficacy, and safety testing in human subjects. The development of a human challenge model suggests that RSV research can be performed ethically on human subjects; therefore, the use of chimpanzees does not meet the second criteria for biomedical research.
Criteria 3: Impact of Forgoing Chimpanzee Use
Forgoing the use of the chimpanzee will not significantly slow or prevent advancement of either therapeutic or prophylactic drugs for RSV and therefore, chimpanzee use does not meet the third criteria. This finding is based, in part, on both the availability of multiple non-human animal models that recapitulate several aspects of RSV disease and the ability to conduct proof-of-concept trials in a human model of infection. Currently three vaccines and two antiviral compounds are in clinical trials. MicroDose Therapeutx is in a Phase I trial of MDT-637, an inhalable small molecule antiviral (MicroDose Therapeutx, 2011). Alnylam is conducting a Phase II efficacy and safety trial of the siRNA antiviral ALN-RSV01 in RSV-infected lung transplant patients (Alnylam Pharmaceuticals, 2011). Novavax and MedImmune are in Phase I and I/IIa clinical trials of potential vaccines, respectively. Novavax is testing four different recombinant RSV-F formulations of NVX 757 01 in healthy adults (Novavax, 2011). MedImmune is testing two attenuated intranasal vaccines (MEDI-534 and MEDI-559) in children and infants (MedImmune LLC, 2011a, 2011b). In addition to these, at least seven other pharmaceutical companies have preclinical RSV programs in development for either therapeutics or prophylactics, including a trivalent anti-RSV-F nanobody (Nb ALX-0171) derived from immunized camels (Pharmaceutical Business Review, 2011).
Finding
The committee finds that currently, chimpanzee use for RSV research is not necessary. This finding is based on the inability of the use of the chimpanzee to meet the three criteria for biomedical research, the current state of research, availability of alternative models, and the large number of drug development efforts. However, the committee cannot completely eliminate the potential for a future need of this animal model. The development of a safe and effective vaccine would confer the greatest benefit on the most vulnerable of populations, infants under 6 months
of age. The committee acknowledges that there are still barriers in the development of a prophylactic vaccine for RSV, including the need to immunize young infants who potentially may not respond to vaccines or have adverse reactions, possible interactions with other pediatric vaccines, or enhanced reactivity (Blanco et al., 2010). The chimpanzee may be required in the future for testing of novel vaccines because of the ability of the chimpanzee to serve as an early surrogate model for seronegative infants (Mohapatra and Boyapalle, 2008; Pollack and Groothuis, 2002; Weisman, 2009).
Prevalence and Treatment Options
Hepatitis C virus currently infects 130-170 million people worldwide (WHO, 2011). In the United States, 3.2 million people are chronically infected with hepatitis C virus (Williams et al., 2011). More than 350,000 individuals die each year due to HCV-induced cirrhosis, endstage liver disease, or hepatocellular carcinoma (Klevens and Tohme, 2010). Current therapy for patients chronically infected with HCV includes pegylated interferon α and ribovarin plus the recent addition of an HCV protease inhibitor, telaprivir (Incivek) or boceprivir (Victrelis). This regimen leads to viral cures in a high percentage of HCV-infected subjects, including those with more-difficult-to-treat genotype 1 infections common in the United States and Europe (Ilyas and Vierling, 2011). The two new NS3/NS4A protease inhibitors, telaprevir and boceprevir, were approved by the FDA in 2011 based on their effectiveness in in vitro culture systems (Sheehy et al., 2007) and then in large controlled clinical trials (Jacobson et al., 2011; Jensen, 2011; Kwo et al., 2010). Additional inhibitors of HCV’s NS5A replication complex assembly factor and the NS5B RNA polymerase are currently in advanced clinical development, offering future hope for a highly effective, completely oral, and interferon-free therapeutic regimen for patients chronically infected with HCV (Ilyas and Vierling, 2011). The current HCV pipeline now includes four drugs in Phase III and 22 in Phase II of development.
Criteria 1: Alternative Models
Because of their unique susceptibility to HCV infection and the initial lack of in vitro culture systems, chimpanzees were particularly valuable during the early phases of HCV research. For example, molecular clones of HCV were first isolated from a cDNA expression library prepared with mRNA from a chimpanzee infected with non-A, non-B hepatitis virus (Choo et al., 1989). More recently, the use of chimpanzees has declined as both HCV replicons (Lohmann et al., 1999) and fully infectious HCV molecular clones (Lindenbach et al., 2005; Wu et al., 2005; Zhong et al., 2005) were identified, enabling the establishment of in vitro culture systems. Various animal models (reviewed in Boonstra et al., 2009) have also emerged, including immunotolerized rats containing human hepatocytes (Wu et al., 2005); immunodeficient mice with heterotopic human liver grafts (Galun et al., 1995); SCID mice expressing the urokinase plasminogen activator transgene that destroys the endogenous mouse liver, permitting xenotransplantation of human hepatocytes (Mercer et al., 2001; Meuleman et al., 2005); and genetically humanized, immunocompetent mice containing human surface receptors required for HCV entry (Dorner et al., 2011). While culture systems are not yet available for genotypes 3, 4 and 6, the replicon system could be used to screen inhibitors against the protease and polymerase, but not NS5A. However, it is very likely that infectious molecular clones will soon emerge for these genotypes—a blueprint for their development now exists—and further alternative small animal models supporting the growth of these viruses will also likely progress rapidly.
The currently available experimental systems, coupled with the challenges inherent to chimpanzee experiments, including limited numbers of animals and high costs, have resulted in a steady deemphasis of the chimpanzee model in HCV antiviral drug design and development. For example, both boceprevir and telaprevir were developed and approved without the use of chimpanzees; instead, preclinical experiments were conducted in mice, rats, rabbits, dogs, and cynomolgus monkeys (EMEA, 2011b; Vertex Pharmaceuticals Incorporated, 2011). However, a few companies continue to use previously HCV-infected chimpanzees (Chen et al., 2007a; Olsen et al., 2011).
Criteria 2: Testing on Human Subjects
Chimpanzees have been used to establish PK/PD relationships of candidate drugs and to assess antiviral activity in vivo. An ethical alternative to performing PK studies in chimpanzees is now available. Specifically, Phase 0 studies can be performed in consenting humans involving microdosing of a drug candidate (Ings, 2009). Such microdosing studies involve the administration of the drug at very low, subtherapeutic amounts that are unlikely to produce toxic side effects, followed by monitoring of drug distribution and clearance using highly sensitive bioanalytical methods. Drugs with unacceptable pharmacokinetic profiles can be rapidly excluded from further development. For Phase I toxicity studies or more advanced efficacy studies, consenting individuals chronically infected with HCV could be recruited. The use of humans for evaluation of these HCV antivirals is further supported by the fact that HCV infection in chimpanzees only partially recapitulates the clinical and laboratory features of HCV infection in humans. Specifically, hepatic disease is milder in chimpanzees (Bukh et al., 2001) and a greater fraction of these animals spontaneously clear the virus (Bassett et al., 1998). Chronic HCV infection in chimpanzees also does not generally result in hepatic fibrosis or cirrhosis (Bukh et al., 2001), and chimpanzees, unlike humans, fail to transmit HCV vertically from mother to infant (Zanetti et al., 1995). Finally, chimpanzees mount weaker neutralizing antibody responses to HCV than humans (Su et al., 2002; Thimme et al., 2002). The current pace of HCV drug development is testimony to the adequacy of human subjects for most of this work.
Criteria 3: Impact of Forgoing Chimpanzee Use
Forgoing the use of chimpanzees will not significantly slow the development of new HCV antivirals. Many new classes of HCV antivirals are already approved or in advanced clinical trials (Ilyas and Vierling, 2011). Progress in their development has been driven not by the availability of a chimpanzee model, but rather by the emergence of powerful in vitro culture systems supporting production and spread of fully infectious HCV virions and by the large number of HCV-infected patients who are willing to participate in clinical trials. Additionally, new small animal models are further reducing the need for chimpanzees in HCV antiviral drug development (Dorner et al., 2011; Galun et al., 1995; Mercer et al., 2001; Wu et al., 2005). As noted, the pharmaceutical industry is steadily
moving away from the chimpanzee model for HCV drug discovery and development.
Finding
The committee finds that chimpanzees are not necessary for HCV antiviral drug discovery and development and does not foresee the future necessity of the chimpanzee model in this area.
Prevalence and Treatment Options
Approximately one out of every 30-50 persons in the world is chronically infected with HCV (WHO, 2011) but, encouragingly, 15-30 percent of humans acutely infected with HCV succeed in clearing the virus (Wang et al., 2007). Many individuals exhibiting chronic HCV viremia lack specific T cell responses to the virus, including production of interferon-γ (Cooper et al., 1999). This finding suggests it may be possible to create a therapeutic HCV vaccine eliciting the type of missing immune response required for a sustained viral response and viral clearance (Houghton and Abrignani, 2005). A therapeutic vaccine would be given to individuals already infected with HCV in contrast to a prophylactic or preventive HCV vaccine that would be given to uninfected individuals who are at risk for infection. By redirecting the immune response and clearing the virus, a therapeutic vaccine could halt and potentially reverse progression of hepatic disease. A therapeutic vaccine also represents an attractive and cost-effective alternative to antiviral drugs in the management of patients with chronic hepatitis C infection. It could be particularly useful in patients who either are unable to tolerate interferon-α or fail to respond to this cytokine. The intrinsic sequence variation of HCV within each of its 6 recognized genotypes and more than 50 subtypes poses a major challenge to the successful development of a therapeutic HCV vaccine (Kurosaki et al., 1993).
Criteria 1: Alternative Models
Because of its tropism and growth requirements, HCV infection in vivo is limited to chimpanzees and humans. Small animal models involving implantation of human liver into immunodeficient mice (Galun et al., 1995) or engineering wild-type mice to express HCV entry receptors on hepatocytes (Dorner et al., 2011) have been developed. However, these mouse models are currently not appropriate and will require additional improvements for testing a therapeutic HCV vaccine where high-titer HCV infection in an immunocompetent host is required. Currently, chimpanzees and humans represent the only acceptable options for testing of an HCV therapeutic vaccine.
Criteria 2: Testing on Human Subjects
Subjects chronically infected with HCV are frequently used to test therapeutic HCV vaccine candidates. Therapeutic vaccine candidates are now being tested in humans without prior testing in the chimpanzee model (Halliday et al., 2011). The fact that chimpanzees produce weaker neutralizing antibody responses than humans (Thimme et al., 2002) and fail to respond to interferon like many humans (Lanford et al., 2007) argues that humans might represent a better system for testing therapeutic HCV vaccines.
Criteria 3: Impact of Forgoing Chimpanzee Use
The fact that therapeutic vaccine testing can be performed in consenting human subjects chronically infected with HCV without prior experimentation in chimpanzees indicates that forgoing the use of chimpanzees would have little or no impact. It is possible that direct testing in humans might accelerate development of an efficacious therapeutic vaccine for HCV.
Finding
The committee finds that chimpanzees are not necessary for development and testing of a therapeutic HCV vaccine.
Prevalence and Treatment Options
HCV is an important cause of human disease—about 3.2 million Americans are chronically infected with hepatitis C virus, mostly from initial infections occurring years ago; however, there are about 17,000 new infections each year, according to the CDC (Williams et al., 2011). The U.S. incidence has fallen dramatically over recent years, but the disease remains a major problem worldwide, with 130-170 million infected. Persistent infection is common and can lead to liver fibrosis, cirrhosis, and hepatocellular carcinoma; hepatitis C has become the most common cause of liver failure and liver transplantation.
Rapid advances are being in made in the development of new therapeutics for subjects chronically infected with HCV, but an efficacious prophylactic vaccine against this virus has not yet been produced. Creation of such a vaccine will be especially challenging because of the great genetic and antigenic diversity manifested within HCV’s multiple genotypes, subtypes, and quasi-species (Halliday et al., 2011).
Chimpanzees are highly susceptible to experimental HCV infection—in fact, the virus was initially identified by its transmission to chimpanzees, followed by molecular methods to detect viral RNA in infected chimpanzee plasma. The unique tropism of HCV for chimpanzee and human hepatocytes makes the chimpanzee model of experimental infection valuable for studies of pathogenesis, including mechanisms of persistence, and for development and testing of prophylactic HCV vaccine candidates by helping to identify those that are safe and efficacious.
The chimpanzee model could also provide important insights into the correlates of immune protection (Strickland et al., 2008); however, research is proceeding on prophylactic HCV vaccine development in the absence of testing in chimpanzees (Catanese et al., 2010; Garrone et al., 2011). Although the determinants of protective immunity against persistent infection with multiple strains have not yet been defined, studies of the outcome of vaccination and challenge of chimpanzees suggest that immune responses to envelop glycoproteins are important for protection, while responses to nonstructural proteins may be detrimental (Dahari et al., 2010; Houghton, 2011). Both neutralizing antibodies and T cell responses are likely to be important (Choo et al., 1994; Folgori et al., 2006; Meunier et al., 2011; Shoukry et al., 2003; Verstrepen et al., 2011). Of note, interpretation of many of these studies is complicated by the use of
small numbers of animals (Bettauer, 2010), coupled with the fact that chimpanzees more effectively clear HCV infections than humans and are less likely to develop hepatic fibrosis, cirrhosis, or hepatocellular carcinoma than humans (Bassett et al., 1998, 1999; Boonstra et al., 2009; De Vos et al., 2002; Erickson et al., 2001; Thomson et al., 2003).
Criteria 1: Alternative Models
Chimpanzees and humans are the only two species that are susceptible to HCV infection. Currently, no other suitable animal models exist for evaluation of a prophylactic HCV vaccine. Although progress is being made in the development of various mouse models that can be infected with HCV, these do not allow evaluation of the human protective immune response against HCV. One model developed requires engraftment of human hepatocytes into the injured liver of an immunodeficient mouse, so HCV infection can be established, but the mouse is not capable of an adaptive immune response (Bissig et al., 2010). A second model involves ectopic implantation of human liver tissue into an immunocompetent mouse, so infection could theoretically occur, but any immune response would be murine in origin (Chen et al., 2011a). In the most recent model, transgenic mice have been engineered to express human HCV entry receptors, so infection can be established in an immunocompetent mouse and immune-mediated protection evaluated, but again, the immune response is murine (Dorner et al., 2011; Gewin, 2011). Likewise, no in vitro systems currently exist that display both HCV infectivity and the capability of an effective anti-HCV adaptive immune response. It is not known whether the recent identification of a canine hepacivirus, which is closely related to HCV and causes respiratory disease (Kapoor et al., 2011), will provide an additional relevant animal model system for vaccine testing and development.
Criteria 2: Testing on Human Subjects
Studies in consenting humans at high risk for HCV infection can be ethically performed to evaluate prophylactic HCV vaccine candidates, provided these vaccines are first shown to be safe and immunogenic in experimental animals such as mice and nonhuman primates. This type of human study will ultimately be required for any prophylactic HCV vaccine to gain licensure for widespread use. These studies require large numbers of subjects that are at increased risk of HCV infection. In developed
countries, most new infections occur in people who inject drugs, a population that presents biological, methodological, and ethical challenges for vaccine trials (Maher et al., 2010).
One clear advantage offered by the chimpanzee model is the ability to infect the animals at a precise time following administration of a vaccine candidate. This could facilitate identification of promising vaccines and help define correlates of immunity and determine the durability of protection. However, a truly efficacious HCV vaccine must provide long-lasting protection within the human population, making such timing less important.
Criteria 3: Impact of Forgoing Chimpanzee Use
While ethical prophylactic vaccine studies in high-risk human populations can and ultimately must be performed, such trials are likely to prove challenging and time-consuming. Use of the chimpanzee HCV model of experimental infection could potentially speed identification of promising prophylactic HCV vaccine candidates for testing in humans, though the FDA does not have policies requiring data from chimpanzees for the development of any compound or vaccine (though will accept such data if submitted). However, differences in the pathogenesis of HCV infection in chimpanzees and humans with respect to immune responses, including weaker neutralizing antibody responses and higher rates of spontaneous viral clearance in chimpanzees, must be considered in judging the various vaccines. In addition, preclinical experiments using chimpanzees must be designed to include adequate numbers of animals for the generation of statistically meaningful results. Ongoing research that is proceeding without using chimpanzees may avoid these weaknesses, though such efforts are in their early stages.
Finding
The committee finds that while there are limitations to the current chimpanzee preclinical model, it has provided valuable knowledge for the development of prophylactic HCV vaccines. The committee is aware of progress on non-chimpanzee models that can be infected with HCV. Such models, if further improved, may reduce or obviate the need for the continued use of the chimpanzee for prophylactic HCV vaccine research. Moving directly to human trials in high-risk populations, without prior testing in chimpanzees, can be ethically performed and could lead to the
development of an HCV prophylactic vaccine. After consideration of all of these facts, the committee was evenly split and unable to reach consensus on the necessity of the chimpanzee model, and on whether or how much the chimpanzee model would accelerate or improve prophylactic HCV vaccine development. Specifically, the committee could not reach agreement on whether a preclinical challenge study using the chimpanzee model was necessary and if or how much the chimpanzee model would accelerate or improve prophylactic HCV vaccine development.10
Molecular genetics and comparative genomics hold enormous potential for developing biomedical therapies as well as for a more basic understanding of the origins of our own species. However, true genomic advances require two components beyond genetic material: (1) phenotypes
__________________
10As elaborated in the case study discussion, the committee could not agree on the necessity of the chimpanzee for research involving the development of a prophylactic HCV vaccine. In summary, the disagreement centered on whether chimpanzee testing is a necessary step in the path to human trials of candidate vaccines.
Some members of the committee held the view that chimpanzees provide the only available challenge model for testing a candidate vaccine and that without the use of chimpanzees (1) important data regarding the immunogenicity, protective efficacy, and safety of candidate vaccines would be foregone; (2) studies in populations of humans at high risk of HCV infection are likely to be difficult based on population demographics and currently available HCV treatment options; and (3) some candidate vaccines of limited promise might make their way into human trials, at the cost of additional time and resources.
An equal number of committee members held the view that (1) rodent and other rapidly-developing alternative models can provide sufficient immunogenicity and safety data to proceed to human efficacy trials without the need for prior studies in chimpanzees, that chimpanzee data is not always predictive of vaccine toxicity or efficacy in humans, and that use of the chimpanzee model is frequently complicated by the lack of a sufficient number of animals to generate statistically significant results; these committee members felt that foregoing chimpanzee models may actually spur greater attention to developing more tractable experimental alternatives; (2) studies in populations of humans at high risk of HCV infection studies can be designed and carried out successfully; and (3) the likelihood and length of any possible delay in vaccine development caused by foregoing chimpanzee research is difficult to assess, and human trials are required whether or not research proceeds using the chimpanzee during the course of vaccine development.
It is important to note that there was a consensus among the committee that human trials of candidate vaccines could be designed and performed ethically with or without data from chimpanzee research.
that can be linked to underlying genes, gene expression, or genetic processes; and (2) comparative studies across species to elucidate the origin and potential impact of genetic variation. The sequencing of the chimpanzee genome (Mikkelsen et al., 2005) in addition to the mouse (Waterston et al., 2002), the macaque (Gibbs et al., 2007), and other species, has shown that changes that set human beings apart from other species not only involve amino acid substitutions, but also to a large extent relate to minor alterations in regulatory regions, gene transcription, and gene expression (Marques-Bonet et al., 2009). However, the ability to apply the new wealth of genomic and genetic information to health and behavioral problems is impeded to the extent that developmental, physiological, behavioral, cognitive, and other phenotypic information is absent for comparator species, especially the closest and in some ways most informative taxon, the chimpanzee.
The development of advanced sequencing techniques provides access to another tool that investigators can use to further examine tissues that are obtained either from biopsy or necropsy. For example, transcriptional assessment can be applied to tissues in chimpanzees with different life histories and disease experience as well as to multiple tissues from the same chimpanzee. Analysis of the resulting gene expression profiles could greatly enhance our understanding of the biological pathways that are activated among individuals that have been subjected to specific life experiences and disease states. While such information is slowly becoming available from humans and other species, the systematic study of gene expression in the current NIH-supported population of chimpanzees may comprise an important source of biomedical and behavioral knowledge. Moreover, the mechanisms underlying environmentally induced alterations in gene expression are also becoming better understood and applied to chimpanzees. Specifically, epigenetic regulation of gene expression through DNA methylation or histone modification can now be readily evaluated in a wide variety of tissues and in blood. The general understanding of functional similarities and differences between chimpanzees and human proteins will be informed by better understanding of the factors affecting epigenetic regulation of genes and the resulting patterns of gene expression in chimpanzees, as compared to humans. Finally, insight through the examination of alternative splicing may also yield valuable information. For example, 6-8 percent of the proteins they examined exhibited profound differences in splicing between chimpanzees and humans and hypothesized that alternative splicing is an important source of the differences between humans and chimpanzees.
Forkhead Box P2 Gene Function in Chimpanzees and Humans
Chosen here for case evaluation in comparative genomics is an investigation that uses material from chimpanzees and humans to evaluate transcriptional regulation of the area of the central nervous system (CNS) that appears to encode language development—the preeminent human characteristic (Konopka et al., 2009). The human capacity to generate spoken language and the ability to understand and apply language to complex problems is a hallmark human feature, the origins of which remain relatively poorly known. Over the past several years, a number of investigators have produced various types of data demonstrating that the “forkhead box P2” or FOXP2 gene has important effects on the development of language skill (Enard et al., 2002). FOXP2 is a transcription factor, a gene that codes for a protein that binds to DNA and functions by turning on and turning off numerous other genes.
Furthermore, comparisons of the FOXP2 gene sequence in humans, chimpanzees, and other primates indicate that the human FOXP2 gene has undergone significant, rapid, and recent evolutionary change (Enard et al., 2002). This pattern has led researchers to infer that changes occurring in FOXP2 in human ancestors—after their divergence from the ancestors of modern chimpanzees—may help explain the evolution of the human capacity for language. Equally important from a biomedical perspective, mutations in FOXP2 have been associated with speech and language disorders (Lai et al., 2001; MacDermot et al., 2005).
Criteria 1: Studies Provide Otherwise Unattainable Insight
Using human neuronal cell lines that express either the human or chimpanzee forms of FOXP2, the investigators examined the function of the FOXP2 protein (Konopka et al., 2009). The results indicated that the human form of the protein had significantly different effects on the expression of many other brain-expressed genes, compared with the chimpanzee form of FOXP2. In addition, the investigators examined RNA isolated from both human and chimpanzee brains and showed that many of the genes that were differentially regulated by human vs. chimpanzee FOXP2 in the neuronal cell line were also differentially expressed in normal intact brains. These data demonstrate that changes in the FOXP2 gene that occurred during human evolution (subsequent to our divergence from chimpanzees) significantly affect gene expression in the human brain, potentially underlying the obligatory nature of human
symbolic abilities and language in comparison to the rudimentary abilities and opportunistic expression of the homologous skills in chimpanzees. In parallel, other investigators continue to examine the relationship between FOXP2 and clinical speech and language disorders. Results confirm the hypothesis that the differences between chimpanzees and humans derive less from DNA sequence (i.e., amino acid substitutions) than from differences in gene expression and regulation.
The study by Konopka et al. (2009) provides an informative example of the unique insights that access to captive chimpanzee phenotypes, genotypes, and tissue can provide on the gamut of research from comparative genomics to behavior and biomedical. No living animals were required for this study, but it did require the following:
• Well-defined genetic sequences from both humans and chimpanzees, which of course requires access to DNA from both species as well as relatively complete information concerning the type and source of genetic variation in each species;
• Access to high-quality RNA samples from fresh chimpanzee brains, which can then be compared with similar RNA samples from human brains; and
• Detailed information about the behavioral and cognitive capacities of chimpanzees.
This type of study fulfills the general requirement to produce fundamental knowledge. Moreover, it is clear that this type of study is only possible because of the close phylogenetic relationship between humans and chimpanzees, indicating that the material from the chimpanzees provides unique, otherwise unattainable information that not only elucidates the origin of human symbolic communication, but sheds light on the mechanisms that may contribute to developmental abnormalities in this domain.
Criteria 2: All Experiments are Performed on Acquiescent Animals and in a Manner that Minimizes Distress
Inasmuch as the study required access to resources that were originally collected from living animals (genetic material, behavioral and cognitive phenotypes) or that required animals to have been alive (brain tissue harvested appropriately from deceased animals for reasons unrelated to the study), the general criteria for species-appropriate housing,
acquiescence to procedures, and minimal distress for manipulations would have to be fulfilled by all animals while still alive. Importantly, maintenance in complex, species-appropriate environments would be particularly important for this type of investigation in order to maintain the species-typical (“normal”) pattern of gene expression and gene regulation across the lifespan. Only in such circumstances is it likely that the derived brain and ancillary tissue will comprise the appropriate controls for the human tissues used in the same studies.
Finding
Given the information provided in the publication regarding the collection of material, the chimpanzee study used as this case example meets the committee’s criteria regarding unattainable insight, acquiescence, and the minimization of pain and distress. Other examples of the application of genomic tools to behavioral or neurobehavioral investigations include the collection of tissues (including blood) that can be sequenced to provide transcriptomes (gene expression profiles). These transcriptomes, in turn, could be studied in relation to rearing experiences, temperamental characteristics, neurobehavioral traits, and other biobehavioral phenotypes to help characterize the relationship between the chimpanzee genome and the life histories of individual animals. Each such study would have to be assessed to determine whether it meets the proposed criteria.
Studying behavior of individual chimpanzees as well as groups of animals provides insights into human behavior, thus informing scientific understanding about the nature of humans. Through their investigation of specific aspects of behavior, scientists are identifying characteristics in chimpanzees that were once thought to be unique to humans, such as certain facets of intelligence and communication, and patterns of social relationships.
Altruism is among the most contentious areas in human behavioral research. Although it has long been observed that people help each other, sometimes at great risk to their own health or well-being, there is little agreement concerning the evolutionary origin of this behavior and whether it can be expected to occur in the absence of clear self-interest
(Okasha, 2010). It is argued that studies of chimpanzees may be particularly relevant for addressing complex behaviors such as altruism because of our shared evolutionary history and recent common ancestry. Even for chimpanzees, however, disagreement remains over the degree to which the animals are sensitive to the needs of conspecifics (Horner et al., 2011).
Chosen here for a case evaluation is one study that attempts to determine whether chimpanzees actively choose to help others, and whether such help is spontaneous—and thus could be interpreted as reflecting sensitivity to the needs of another animal—or is triggered by a solicitation from the partner (Horner et al., 2011). The study design involves allowing chimpanzees to choose between two different tokens, a “selfish” token that provides a reward for the actor only, and a “prosocial” token that rewards both the actor and a partner. Seven females were tested, each with three different partners. The actors demonstrated a significant overall bias for the prosocial token, but more so when the partner either showed no reaction or engaged in neutral attention getting (not directed at the actor); attempts to pressure the actor resulted in reduced prosocial choice (Horner et al., 2011).
Criteria 1: Studies Provide Otherwise Unattainable Insight
The research objectives of the study question addressed clearly fall within the broad NIH mission to “seek fundamental knowledge about the nature and behavior of living systems” (NIH, 2011) and more specifically, meets the first criteria: to provide “otherwise unattainable insight into evolution, normal and abnormal behavior, mental health, emotion, or cognition.” The insights derive from the arguably close genetic relationship between chimpanzees and human beings and the substantial similarity in brain structure and complexity—a similarity that is much less pronounced in monkeys (Sakai et al., 2011). The less complex brain structures in other old worlds of monkeys result in a tendency toward a simpler pattern of signaling and signal recognition. It was of particular interest that, in this study, actors behaved prosocially toward their partners irrespective of relative social status, genetic relationship, or expectation of reciprocity. These results imply that human beings may have a tendency to help other individuals unconditionally, at least when the help can be given at no cost.
Criteria 2: All Experiments Are Performed on Acquiescent Animals and in a Manner That Minimizes Distress
In assessing the degree of acquiescence and distress on the part of the subjects, the study reported that the seven adult female chimpanzees “volunteered to participate and were willing to exchange tokens with an investigator.” However, no details regarding the definition of “volunteer” were given in the manuscript or the associated methodology. The study did describe the conditions under which the animals were maintained. Specifically, they were housed in a large outdoor grass enclosure with climbing structures as part of a long-established social group comprising 11 females and one male. There were two associated buildings, one with indoor sleeping quarters and a second building designed for cognitive research testing. No details were provided on the dimensions of these buildings.
Finding
This study involved temporary removal of animals from their usual housing and social group to engage in a cognitive task paired with other chimpanzees. The information provided suggests that chimpanzee use in this study could meet all criteria if more complete descriptions of the handling and housing were provided. Specifically, the investigators would have to substantiate the statement that the animals “volunteered” for the procedures, confirm that the indoor sleeping quarters were of sufficient size for the species, and demonstrate that the cognitive testing apparatus would meet all enrichment requirements for this species.
This study exemplifies the numerous cognitive investigations that have been done in chimpanzees. As a group, these studies have demonstrated that human cognitive abilities with respect to the manipulation of the social environment extend to chimpanzees in a variety of domains that include altruism, deception, and grief. Many such studies would be similarly approvable under the proposed guidelines; in other instances they might be limited if, for example, they provided unattainable insights but did not meet the need for acquiescence and minimization of distress.
Joint attention occurs when one animal alerts another to the presence of a stimulus by means of gestural or vocal communication. It is generally thought that a breakdown in the ability to initiate joint attention may be a predictor for autism spectrum disorders or other neurodevelopmental disorders (Mundy et al., 2010). Unfortunately, knowledge of developmental mechanisms of joint attention are poorly understood because some functional imaging techniques used in adults are difficult to administer to children while others, like positron emission tomography (PET), cannot be administered without some risk to normal young subjects. The chimpanzee has been used as a model organism to study the neurodevelopmental basis of joint attention and similar phenomena and to increase the understanding of the development trajectory of human communicative phenomena. This is because, in humans, joint attention (including both gestures and vocalization) is associated with hemispheric lateralization, particularly in the portion of the inferior frontal gyrus (IFG), termed Broca’s area, and is thought to have evolved from a lateralized manual communication system present in the common ancestor of humans and chimpanzees (Corballis, 2002; Kingstone et al., 2000). Chosen here for case evaluation is a PET study designed to determine whether chimpanzees possess a gesture and vocalization-activated brain region homologous with the IFG (Broca’s area), which in humans is most often enlarged on the left side to indicate significant left-lateralized patterns of activation during communication (Taglialatela et al., 2008). While it has been previously shown that chimpanzees engage in joint communication and exhibit structural asymmetry in the brain, it had not been demonstrated that the brain regions underlying joint attention were the same as those underlying homologous communicative behaviors in humans. In particular, it was not known whether the IFG and related cortical and subcortical areas were preferentially activated during gestural or vocal communication in the chimpanzee. The presence of similarly activated underlying brain structures would suggest that chimpanzees could be used to model human communication development.
Criteria 1: Studies Provide Otherwise Unattainable Insight
The forgoing description suggests that the study does fulfill the need to provide fundamental knowledge gain. Moreover, because the chimpanzee and humans both uniquely share a highly convoluted and lateralized
cerebral cortex and the ability to engage in joint attention, it is likely to provide otherwise unattainable insight into the neurodevelopment of communication and, by implication, communicative disorders. Furthermore, while the modern imaging modalities necessary to map neurodevelopment can be applied to both chimpanzees and adult human beings, the application of these techniques to children is often limited by logistical and ethical considerations. Finally, even if all imaging techniques (even those involving unacceptably high radiation exposure) could be applied to children, the study of neurodevelopment would be arguably enhanced by the availability of a comparative model like the chimpanzee.
This study provided the first direct evidence that the neuroanatomical structures underlying communicative signals in chimpanzees are homologous with those present in humans. Furthermore, chimpanzees engaging in communicative gestures—like human beings—activated the left IFG (Broca’s area) in conjunction with other cortical and subcortical brain areas, providing strong evidence in support of the hypothesis that the neurological substrates underlying language production in the human brain were present in the last common ancestor of humans and chimpanzees.
Criteria 2: All Experiments Are Performed on Acquiescent Animals and in a Manner That Minimizes Distress
Chimpanzees were initially separated from groupmates, but maintained within their home enclosure. The animals were then provided with a sweetened drink that contained the radioligand 18F-fluorodeoxyglucose (18F-FDG), which initiated a 40-minute uptake period, during which the radioligand would bind to parts of the brain that were being activated by the chimpanzee’s behavior. During the uptake period, the investigator sitting outside the enclosure placed a favored food item just beyond reach, a situation that predictably elicited both gestural and vocal signaling from subjects. Experimenters would periodically respond to subjects with both vocal communication and small food rewards. At the end of the 40-minute uptake period, chimpanzees received an intramuscular injection of an anesthetic agent and were transported to the PET facility. All animals had been previously trained with positive reinforcement to present for such anesthesia. Following scanning, animals were allowed to fully recover before being reintroduced to their social groups. It should be noted that three chimpanzees were used in the study, and each animal was scanned on two occasions—once following the gesture and vocalization
task and separately following a control task that did not require communicative interaction.
Review of the study indicates that it was conducted in a manner consistent with acquiescence. Animals voluntarily engaged in behavioral testing during the awake part of the procedure (i.e., uptake) and, furthermore, were trained to present for anesthesia. As described in the study, anesthesia persisted for about 50 minutes (transport to and from the PET facility, PET scanning). Animals were allowed to recover (and radioligands allowed to decay) for approximately 18 hours, after which they were returned to their social group. It is important to note that this study likely exposed the animals to more than “minimal” distress in that animals were fasted for 5 hours prior to the procedure, sedated for at least 50 minutes, and then were separated from their social groups to allow recovery. The total time required for the manipulation was probably 28-32 hours. However, the effects of this amount of separation from the social group and handling must be judged against the unique contribution made by the study and the small number of acquiescent animals involved. It should be noted that a complete veterinary examination would involve a similar if not longer period of fasting, social group separation, and anesthesia.
Finding
In view of the scientific benefits compared to the temporary negative impacts on the animal subjects (separation and anesthesia), this study could potentially meet all criteria for approval if sufficient additional assurance were provided that the animals were maintained in species-appropriate housing and groupings and that the number and duration of procedures imposed on individual animals was minimized in a manner consistent with criteria described earlier in this report.
FUTURE USE OF CHIMPANZEES IN BIOMEDICAL
AND BEHAVIORAL RESEARCH
As highlighted throughout this report, over many years scientific advances that have led directly to the development of preventive and therapeutic products for life-threatening or debilitating diseases and disorders have been dependent on scientific knowledge obtained through experiments using the chimpanzee. In addition, many preliminary proof-of-concept
experiments have been carried out in the chimpanzee; for example, development of human and humanized monoclonal antibody therapies have required preclinical testing in the chimpanzee (Iwarson et al., 1985). The same has been the case for early evaluation of therapeutic concepts based on RNAi, microRNA, and antisense RNA (e.g., for treating chronic HCV infection), and for evaluation of TLR7 antagonists (e.g., for treating chronic HBV infection) (Lanford et al., 2011).
The National Institute of Allergy and Infectious Diseases at the NIH has identified eight instances over the past two decades where research on new (or newly recognized), emerging, and reemerging infectious diseases has called for use of the chimpanzee to answer crucial questions pertaining to pathogenesis, prevention, control, or therapy. In five of these, the chimpanzee is still being used.11 At the same time, as has been the case rather often in the past, an important new, emerging, or reemerging disease may present treatment, prevention, and/or control problems that defy available alternative experimental approaches, including the most novel, innovative approaches, and therefore may require use of the chimpanzee—rare as this may be, this possibility cannot be discounted over the long term. The committee recognizes that the limited number of available animals and the potential need to perform experiments under conditions of biocontainment could potentially constrain the value of the chimpanzee during a public health emergency. The similarity in the neuroanatomy between the human and the chimpanzee may make it a model for neuropsychiatric disorders, for example, expressing human risk genes via viral vectors or from optogenetic methods that exploit the chimpanzee functional neuroanotomy.
However, in this case the past is not necessarily prelude—great progress is being made in developing alternatives to the chimpanzee; more studies are using other non-human primates (Ben-Yehudah et al., 2010; Couto and Kolykhalov, 2006; Pan et al., 2010; Suomi, 2006), genetically modified (knock-out, knock-in) mice (Chen et al., 2011a; de Jong et al., 2010; Dorner et al., 2011; Kneteman and Mercer, 2005; Lindenbach et al., 2005; Ma et al., 2010; Ploss and Rice, 2009), and even in silico technologies (Hosea, 2011; Qiu et al., 2011; Valerio, 2011). In some instances, “preclinical studies” in humans, that is, expanded studies carried out
__________________
11Encephalitozoon cuniculi; Helicobacter pylori; Hepatitis C (ongoing); Hepatitis E (ongoing); Human herpesvirus 8 (ongoing); Human herpesvirus 6 (ongoing); Streptococcus, Group A; Staphylococcus aureus (ongoing) (NIAID, 2011).
in the field during disease outbreaks, have served as an alternative to the use of the chimpanzee.
Finding
The committee cannot predict or forecast future need of the chimpanzee animal model and encourages use of the criteria established in this report when assessing the potential necessity of chimpanzees for future research uses.
CONCLUSIONS AND RECOMMENDATIONS
Animal models serve as a critical research tool in facilitating the advancement of the public’s health. The chimpanzee’s genetic proximity to humans and the resulting biological and behavioral characteristics not only make it a uniquely valuable species for certain types of research, but also demand a greater justification for conducting research using this animal model. As this report demonstrates, the committee’s conclusions and recommendations are predicated on the advances that have been made by the scientific community in developing and using alternative models to the chimpanzee, such as studies involving human subjects, other non-human primates, genetically modified mice, in vitro systems, and in silico technologies. Having reviewed and analyzed contemporary and anticipated biomedical and behavioral research, the committee offers the following three conclusions and two recommendations.
Conclusion 1: Assessing the Necessity of the Chimpanzee for Biomedical Research
Having explored and analyzed contemporary and anticipated biomedical research questions, the committee concludes:
• The chimpanzee has been a valuable animal model.
• Based on a set of principles that ensure ethical treatment of chimpanzees, the committee established criteria to determine the necessity for the use of chimpanzees in current biomedical or behavioral research.
• While the chimpanzee has been a valuable animal model in past research, most current use of chimpanzees for biomedical research
is unnecessary, based on the criteria established by the committee, except potentially for two current research uses:
ο Development of future monoclonal antibody therapies will not require the chimpanzee, due to currently available technologies. However, there may be a limited number of monoclonal antibodies already in the developmental pipeline that may require the continued use of chimpanzees.
ο The committee was evenly split and unable to reach consensus on the necessity of the chimpanzee for the development of a prophylactic HCV vaccine. Specifically, the committee could not reach agreement on whether a preclinical challenge study using the chimpanzee model was necessary and if or how much the chimpanzee model would accelerate or improve prophylactic HCV vaccine development.
• The present trajectory indicates a decreasing scientific need for chimpanzee studies due to the emergence of non-chimpanzee models and technologies.
• Development of non-chimpanzee models requires continued support by the NIH.
• A new, emerging, or reemerging disease or disorder may present challenges to treatment, prevention, and/or control that defy nonchimpanzee models and technologies and therefore may require the future use of the chimpanzee.
• Application of the committee’s criteria would provide a framework to assess scientific necessity to guide the future use of chimpanzees in biomedical research.
Recommendation 1: The National Institutes of Health should limit the use of chimpanzees in biomedical research to those studies that meet the following three criteria:
1. There is no other suitable model available, such as in vitro, nonhuman in vivo, or other models, for the research in question; and
2. The research in question cannot be performed ethically on human subjects; and
3. Forgoing the use of chimpanzees for the research in question will significantly slow or prevent important advancements to prevent, control, and/or treat life-threatening or debilitating conditions.
Animals used in the proposed research must be maintained either in ethologically appropriate physical and social environments or in natural habitats. Biomedical research using stored samples is exempt from these criteria.
Conclusion 2: Assessing the Necessity of the Chimpanzee for Comparative Genomics Research
Having reviewed comparative genomics research, the committee concludes the chimpanzee may be necessary for understanding human development, disease mechanisms, and susceptibility because of the genetic proximity of the chimpanzee to humans. Furthermore, comparative genomics research poses minimal risk of pain and distress to the chimpanzee in instances where samples are collected from living animals and poses no risk when biological materials are derived from existing samples. Application of the committee’s criteria would provide a framework to assess scientific necessity to guide the future use of chimpanzees in comparative genomics research that requires samples collected from living animals.
Conclusion 3: Assessing the Necessity of the Chimpanzee for Behavioral Research
Having explored and analyzed contemporary and anticipated behavioral research questions, the committee concludes that chimpanzees may be necessary for obtaining otherwise unattainable insights to support understanding of social, neurological, and behavioral factors that include the development, prevention, or treatment of disease. Application of the committee’s criteria would provide a framework to assess scientific necessity to guide the future use of chimpanzees in behavioral research.
Recommendation 2: The National Institutes of Health should limit the use of chimpanzees in comparative genomics and behavioral research to those studies that meet the following two criteria:
1. Studies provide otherwise unattainable insight into comparative genomics, normal and abnormal behavior, mental health, emotion, or cognition; and
2. All experiments are performed on acquiescent animals, using techniques that are minimally invasive, and in a manner that minimizes pain and distress.
Animals used in the proposed research must be maintained either in ethologically appropriate physical and social environments or in natural habitats. Comparative genomics and behavioral research using stored samples are exempt from these criteria.
The criteria set forth in the report are intended to guide not only current research policy, but also decisions regarding potential use of the chimpanzee model for future research. The committee acknowledges that imposing an outright and immediate prohibition of funding could cause unacceptable losses to research programs as well as have an impact on the animals. Therefore, although the committee was not asked to consider how its recommended policies should be implemented, it believes that the NIH should evaluate the necessity of the chimpanzee in all grant renewals and future research projects using the chimpanzee model based on the committee’s criteria.
In March 1989 the NIH chartered the Interagency Animal Model Committee (IAMC) “to provide oversight of all federally supported biomedical and behavioral research involving chimpanzees” (NIH, unpublished). As indicated in its charter:
The IAMC review mechanism represents a commitment to the public and the U.S. Congress to promote the conservation and care of chimpanzees when this species is the best or possibly the only model for the conduct of research to advance scientific knowledge and to address questions that have significant impact on public health.
The IAMC shall review all federally-supported research protocols involving the use of chimpanzees before the initiation of the study. Prior to this review, the
project must be reviewed and approved by intramural scientific program staff or an extramural initial review group and by the appropriate Animal Care and Use Committee. The IAMC’s evaluation constitutes an additional level of scientific review, focusing on such factors as the appropriateness of the animal model, appropriateness of the numbers of animals, the availability of the animals, the degree of invasiveness of the procedures, and any unnecessary duplication. (NIH, unpublished)
Appointment of the IAMC is evidence that the NIH has determined that the conservation and care of chimpanzees requires additional oversight. Membership on the Interagency Animal Model Committee is restricted to federal employees from the Department of Health and Human Services (including the NIH, CDC, and FDA), Department of Veterans Affairs, and Department of Defense. The committee believes that assessment of potential future use of the chimpanzee would be strengthened and the process made more credible by establishing an independent oversight committee that uses the recommended criteria and includes public representatives as well as individuals with scientific expertise, both in the use of chimpanzees and alternative models, in areas of research that have the potential for chimpanzee use.






































































