Why Combinations and Collaborations Are Necessary
Presenters highlighted several reasons for supporting combination strategies to develop more effective cancer therapies, including
• The current high failure and relapse rate for single-agent targeted therapies;
• The mounting evidence that combination targeted or immunotherapies will be more effective than single agents; and
• The need to counter the heterogeneity and evolution of tumors.
Participants also stressed the importance of collaboration to develop combination therapies because of the inability of a single drug company to have the resources to effectively and expediently counter the complex mechanisms by which cancer cells become resistant to treatment. Over the past decade, the scientific complexity and skyrocketing costs (Booth and Zemmel, 2004; Munos, 2009) of drug development have increased the incentives for more collaborative approaches. Dr. Bernard Munos, founder of the Innothink Center for Research in Biomedical Innovation, stressed the explosion of data on cancer due to the rise of genomics, metabolomics, proteomics, and the combinatorial expansion of treatment options. “It’s bigger than we can handle in any single pharmaceutical company, in any single organization given our limited resources. We’re running out of patients, money, and scientists. The only way to really make much progress is to join hands in order to be more effective,” he said. Dr. Rachel Sherman, associate director for Medical Policy at the
FDA Center for Drug Evaluation and Research (CDER), agreed, adding, “Companies can no longer successfully develop groundbreaking therapy in isolation. The era of doing this solo is over.”
Perhaps the most important reason for collaboration is to speed up the process of drug development so that effective treatments are delivered sooner to cancer patients, who may not have time on their sides. “Patients have a real sense of urgency. We can’t wait,” noted Dr. Jane Perlmutter, patient advocate and founder of the Gemini Group.
“We have been developing targeted agents for one pathway or target at a time, and that hasn’t necessarily yielded the type of breakthrough therapies for patients that we are looking for,” said Dr. Stuart Lutzker, vice president of Oncology Exploratory Clinical Development at Genentech. “From a sponsor’s perspective, there has been an extremely high failure rate.”
Several speakers elaborated on that theme by pointing out different reasons why most patients do not respond or eventually become resistant to targeted therapies. Many of these treatments target a single biochemical pathway to inhibit the activity of a kinase enzyme that fuels tumor growth, but as Dr. Jeffrey Engelman, assistant professor of medicine at Harvard Medical School and director of Thoracic Oncology at Massachusetts General Hospital, noted, “Most cancers are not really that sensitive to a perturbation of a single kinase pathway.” He described how certain breast and gastric cancers are “addicted” to receptor tyrosine kinases,1 such as epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2/neu), and a kinase called MET.2 In some cases, when these receptors are blocked with targeted therapies, tumor cells die and patients go into remission. Two major downstream signaling pathways emanate from these receptor tyrosine kinases—the PI3K (phosphatidylinositol 3-kinase)-AKT3 pathway and the MAPK (mitogenactivated protein kinase) pathway. These pathways foster tumor growth by promoting cell division and inhibiting cell death. Consequently, when
![]()
1 Kinases are a type of enzyme that can activate molecules in a cell, and some cancer treatments target certain kinases that are linked to cancer. Receptor tyrosine kinases are a type of cell-surface receptor important in normal cellular processes and the development and progression of certain types of cancer.
2 EGFR binds to epidermal growth factor, causing cell division. In some cancers, EGFR is found at abnormally high levels on cells. The HER2/neu protein is a tyrosine kinase receptor involved in normal cell growth and is abnormally active in some types of cancer. MET is a tyrosine kinase receptor protein involved in wound repair that is abnormally activated in some cancers.
3 AKT is a kinase that is involved in cell growth and proliferation, survival, and motility. It has been implicated as a major factor in many types of cancer.
drugs block the EGF, HER2/neu, or MET kinases, both these major downstream pathways that fuel tumor growth also are blunted (see Figure 2-1).
Research suggests that both the PI3K-AKT pathway and the MAPK pathway have to be blocked to counter a tumor’s progression, and single-agent targeted therapies that only block one of these pathways are
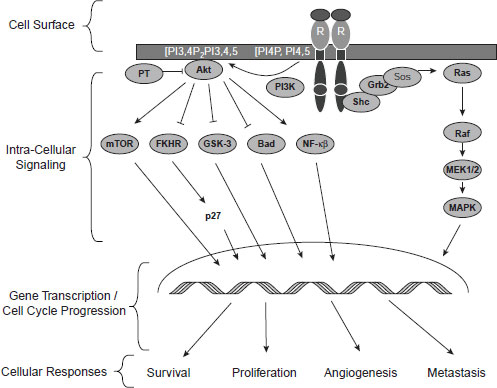
NOTE: FKHR = forkhead in human rhabdomyosarcoma; Grb2 = growth factor receptor-bound protein; GSK-3 = glycogen synthase kinase 3; MAPK = mitogenactivated protein kinase; MEK1/2 = MAPK kinase; mTOR = mammalian target of rapamycin; NF–κB = nuclear factor kappa B; PI3K = phosphatidylinositol 3-kinase; Sos = son of sevenless.
SOURCE: Tabernero, J., T. Macarulla, F. J. Ramos, and J. Baselga. 2005. Novel targeted therapies in the treatment of gastric and esophageal cancer. Annals of Oncology 16(11):1740–1748, by permission of the European Society for Medical Oncology.
often not effective. Based on preclinical modeling that showed improved efficacy of concurrent administration of MAPK kinase (MEK) and PI3K inhibitors compared to either agent alone, a Phase I dose escalation study of the combination therapy was initiated in patients with solid tumors. Results from this study suggest that combination therapy of MEK and PI3K inhibitors demonstrated some antitumor activity and was generally well tolerated, with side effects similar to Phase I studies involving the single agents (Shapiro et al., 2011).
Combination targeted therapy can be beneficial even when the initial single-agent therapy is effective, Dr. Engelman added, because of the development of drug resistance. Such resistance usually comes in two types. One type is due to a mutation or genetic event affecting the target of the drug itself so that the kinase is still able to drive the growth of the tumor, despite the continued presence of the drug. Another type of resistance occurs when the cancer uses pathways that bypass the blocked kinase. These bypasses activate the same key downstream tumor growth-promoting signaling pathways so that the tumor no longer needs the kinase the drug inhibits in order to grow. Research suggests that most cancers have multiple drivers—multiple inputs into the PI3K-AKT and MAPK pathways that can serve as bypasses, according to Dr. Engelman. When researchers perturb one signaling pathway with a kinase inhibitor, it often causes rebound activation of these bypass backup pathways such that the effectiveness of the inhibitor is muted (Engelman et al., 2007; Hsieh and Moasser, 2007; Nagata et al., 2004; Zhang and Yu, 2010). Consequently, combination treatments are needed that target both the kinase and the bypass pathway the kinase inhibitor activates.
For example, if a compound inhibits mTORC1 (mammalian target of rapamycin complex-1), which is downstream from AKT, it triggers the activation of AKT by lifting the negative feedback on the insulin-like growth factor (IGF) receptor, which normally suppresses AKT activation. However, early phase clinical trials suggest that if both a TORC1 inhibitor and an IGF inhibitor are used in combination, the rebound activation of AKT is effectively blocked. Such an approach has shown impressive activity in estrogen receptor (ER)–positive breast cancers, said Dr. Engelman (Cosimo et al., 2010; Rathkopf et al., 2010).
Due to the emergence of bypass pathways or mutations in drug targets, “these great responses that make the cover of Time magazine are really modest because the time to progression, on average, is a year. When you are talking to a patient, that is not even close to being something to celebrate,” Dr. Engelman said. “We now know that we are going to need to employ combination therapies to deal with this resistance that’s emerging.”
Combinations with immunotherapies are also needed to fully provide
the complexity of an antitumor immune response, and to make such a response more likely to be effective by combining it with chemotherapy or radiation, several participants pointed out. For example, several studies show that T cells that are removed from a patient’s body and genetically engineered and/or treated with immune stimulants to boost their numbers and/or tumor-killing abilities will be more likely to shrink the patient’s tumor if, prior to receiving that treatment, the patient receives “host conditioning” with chemotherapy or radiation, said Dr. Carl June, professor of pathology and laboratory medicine at University of Pennsylvania School of Medicine and director of Translational Research at Abramson Cancer Center (see Figure 2-2).
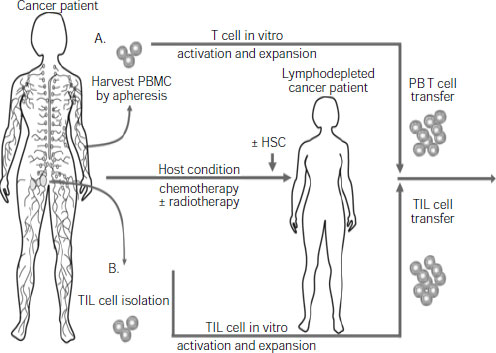
NOTE: HSC = hematopoietic stem cells; PBMC = peripheral blood mononuclear cell; TIL = tumor infiltrating lymphocytes.
SOURCES: June presentation (June 13, 2011) and Grupp and June, 2011. With kind permission from Springer Science+Business Media: Cancer Immunology and Immunotherapy, Adoptive cellular therapy, 2011, 151, S. A. Grupp and C. H. June, Figure 1.
Studies also suggest that patients treated with such T cell therapy and a tumor vaccine will have a greater response than either alone, Dr. June added. “There are a number of studies that show these immunotherapies can be synergistic, in terms of serologic antibody responses and cellular immune responses,” he said. Dr. Renzo Canetta, vice president of Oncology Global Clinical Research at Bristol-Myers Squibb, and Dr. Jeffrey Schlom, chief of the Laboratory of Tumor Immunology and Biology and head of the Immunotherapeutics Group at the National Cancer Institute (NCI), added that many immunotherapies are cocktails of immune stimulants, costimulatory molecules, immune checkpoint suppressors, and other effectors of the immune system that singly are not effective, but in concert have a synergistic antitumor effect, as shown in studies, including some clinical studies.
Dr. Schlom stressed that vaccines used in combination with standard chemotherapy, radiation, or hormonal therapy induce minimal added toxicity and can act independently of concomitant therapy. Certain chemotherapeutic agents or radiation can alter tumor cells so they are more susceptible to killing by T cells, his preclinical models show, and these T cells can continue to inhibit tumor growth even after the tumor has become resistant to the chemotherapy used. This may explain why, in a study of a tumor vaccine combined with docetaxel in metastatic breast cancer patients, preliminary analyses suggest that those patients who received docetaxel alone had a median time to progression of 84 days, whereas those who received the vaccine combined with docetaxel had a time to progression of 265 days (NCI, 2011b). “We firmly believe that vaccines should be part of an immune-oncology platform,” Dr. Schlom said.
Dr. Canetta and Dr. Keith Flaherty, associate professor of medicine and director of Developmental Therapeutics at the Massachusetts General Hospital Cancer Center, also noted several studies showing that various immunotherapies combined with targeted cancer therapies can be more effective than either modality used alone. Dr. Flaherty cautioned that there can be antagonistic effects with some targeted therapies and immunotherapies, however. MEK inhibitors, for example, appear to also inhibit T cell proliferation, unlike BRAF (rapidly accelerated fibrosarcoma (B family)) inhibitors, which appear to increase the influx of CD8 T cells into tumors, he said. Dr. Canetta added that not all cytotoxic agents may affect the immune system equally, and those interactions need to be studied more.
Another reason combination cancer therapies are needed is because of the heterogeneity of the cancer cells within individual patients, particularly in advanced tumors, in which the genetic instability of tumor cells fosters the emergence of multiple metastatic clones, each with a different genetic profile and varying sensitivity to specific treatments. As Dr. Michael Barrett, associate professor and head of the Oncogenom-
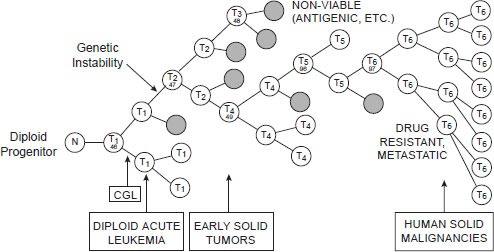
NOTE: CGL = chronic granulocytic leukemia; N = normal cell; T = tumor cell.
SOURCE: Barrett presentation (June 13, 2011). From Nowell, P. C. 1976. The clonal evolution of tumor cell populations. Science 194(4260):23–28. Reprinted with permission from AAAS.
ics Laboratory at the Translational Genomics Research Institute, pointed out, such sensitivity will vary over time because a specific treatment applies selective pressure on some genetic subtypes of cancer cells, such that those lacking the genetic variant the treatment targets will expand in number (see Figure 2-3).
As Dr. David Stern, professor of pathology at Yale Medical School and associate director of the Shared Resources for the Yale Comprehensive Cancer Center, summarized, “There are rapid routes through genetic and epigenetic plasticity, through on-target and bypass mutations, and through tumor cell population heterogeneity. This is the landscape we’re all working in.” Such a landscape is too complex for one company to tackle alone, said Dr. Munos. “The challenges are too big because the science is very complex,” he said. “I’ve been in meetings in industry where people sat around the table and looked at a cluster of pathways and tried to pick targets. You can do that, but figuring out what will happen once you modulate those targets is basically next to impossible, because you cannot grasp the network effects that lie outside this cluster you’re looking at.”
This page intentionally left blank.








