In its Pathway to Product Safety the U.S. Food and Drug Administration (FDA) emphasizes the importance of operating as a “truly global agency fully prepared for a regulatory environment in which product safety and quality know no borders” (FDA, 2011b, p. 3). To this end, the agency must bridge the many gaps within regulatory systems abroad. In this chapter, the committee recommends actions the FDA and other U.S. government agencies can take to increase the efficiency of their own operations while improving the systems of their counterpart agencies abroad.
USING RISK AS A GUIDING PRINCIPLE
Chapter 4 describes the committee’s strategy in forming its recommendations and emphasizes that the FDA should let risk guide its efforts to build food and medical product regulatory systems abroad. In keeping with its focus on risk, the committee recommends that the FDA divide its limited resources according to risk. An understanding of risk will allow the FDA to choose what problems are its highest priorities.
There are tradeoffs implicit in all decision making. Especially in capacity building, managers need to choose between different risks affecting different populations. When working across many countries, choosing to work with one population means less attention for others. Through the use of an enterprise risk management framework, the FDA can determine which risks are the most serious and have an objective way to rank its priorities.
Recommendation 6-1: FDA should use enterprise risk management1 to inform its inspection, training, regulatory cooperation, and surveillance efforts. Enterprise risk management should apply to the agency’s entire operation, and it should incorporate a number of set criteria such as country of manufacture or production, volume and type of product, facility inspection history, and trends or data shared from other regulatory authorities.
The FDA’s implementation of an enterprise risk management system will be the best measure of this recommendation. The FDA’s allocation of resources in a way that reflects decisions grounded in enterprise risk management will also be a measure of this recommendation. The FDA will also have to select which statistics best measure the impact of its inspections, trainings, and surveillance efforts. Choosing which metrics to monitor most closely will be part of the assessment. The timetable on which the FDA collects these data is up to the agency’s management, but it should be frequent, perhaps every quarter, but at least every 6 months.
Should the results of an enterprise risk management analysis suggest full reorganization of the FDA, such a process would take time. In order to work toward this change promptly, the FDA needs to conduct enterprise-wide risk assessment, analysis, and evaluation. If its results suggest an inefficient or unscientific allocation of resources in the agency’s current operations, as one expects they will, then the FDA will need, at that time, to lobby Congress for permission to revise its operations.
The agency has more freedom in running its capacity building programs. Therefore, an enterprise risk management assessment, analysis, and evaluation can be used to reorganize international programs in the next 3 to 5 years.
Enterprise-wide Risk Management
Multinational food and medical product companies have been using enterprise risk management for some time (see Box 6-1). Even the most profitable business cannot afford to monitor every transaction on its supply chain with the same diligence. Instead, multinational companies develop a hierarchy of risk and devote resources to the highest risks in the hierarchy. These companies may have a broader data set to inform their estimates than the FDA would have. Nevertheless, the FDA has to work with the
![]()
1 Enterprise risk management is a discipline by which an organization “assesses, controls, exploits, finances, and monitors risks from all sources for the purpose of increasing the organization’s short- and long-term value to its stakeholders” (Casualty Actuarial Society-Enterprise Risk Management Committee, 2003, p.8).
BOX 6-1
Enterprise Risk Management
Risk is the potential any action or inaction has to result in an undesir-able outcome. The concept of enterprise risk management comes from the financial services industry, but has been adapted for use in a variety of businesses, as well as in running governments and universities. The Committee of Sponsoring Organizations of the Treadway Commission defined enterprise risk management as “a process, effected by an entity’s board of directors, management and other personnel, applied in strategy setting and across the enterprise, designed to identify potential events that may affect the entity, and manage risk to be within its risk appetite, to provide reasonable assurance regarding the achievement of entity objectives” (COSO, 2004, p. 2).
The principles of enterprise risk management allow any type of organization to assess areas where it has exposure to harm and evaluate the extent of the danger. Assessing mitigation strategies is an important part of enterprise risk management, as is financial and administrative planning against the organization’s risk profile. The advantage of an enterprise-wide risk management assessment (as opposed to a functional or discipline-based assessment) is that the organization’s management gains a framework that presents the connected relationships between decisions and then allows it to integrate their responses to multiple threats (COSO, 2004). The use of enterprise risk management can guide staffing and training decisions. Over time, the use of enterprise risk management can help the organization transition from a culture of responding to crises when they happen to predicting and preventing them (Protiviti Inc., 2006).
data available. Over time the agency may develop data sharing relationships with its counterpart agencies abroad. The FDA may also want to collaborate to develop its own risk assessment tool.
A number of organizations have supported a risk-based approach to food and medical product regulatory strategy. The Pew Health Group encouraged using risk to guide inspections (Pew Health Group, 2011), as have industry spokespeople (Vijay, 2011). The committee’s recommendation is also consistent with the 2010 Institute of Medicine report Enhancing Food Safety that argued for consistency in applying a risk-based food safety system (IOM, 2010).
In understanding the committee’s emphasis on enterprise risk management it is important to consider that this is a way to manage the agency’s enterprise. That is to say, a way to manage everything the agency does. Enterprise risk management is a strategic perspective to set priorities for the
agency, not a tactical perspective applied to any subfunction of the enterprise, such as food safety or medical device safety. Many of the systematic steps in risk management at the enterprise level and at the subordinate levels can be described in similar terms. At the level of the organization’s leadership, concerns over specific product lines or countries need to be reconciled with the entire risk and opportunity profile the FDA needs to address. Food, drug, vaccine, and medical device safety must be reconciled with each other and with other FDA responsibilities. Enterprise risk management can reconcile an array of risks at the agency level. The goal is for the FDA to optimally balance its limited resources with the full array of risks the agency needs to control.
This committee recommends an enterprise-wide risk assessment be used to inform the FDA’s capacity building projects and all its routine work. An enterprise-wide assessment will help the FDA allocate its staffing, trainings, and operations to the highest risk, highest priority activities, not just the inspections. The FDA has, for some time, been working to base inspections on a risk assessment paradigm. In 2007 the FDA was already using a risk-based process to rank foreign manufactures according to the urgency of the need for inspection (GAO, 2007). In a speech to the Partnership for Safe Medicines, the FDA commissioner explained that the agency has systematically ranked more than 1,000 active pharmaceutical ingredients according to respective risk of economically motivated adulteration (Hamburg, 2010). Clearly, the FDA has a strong foundation on which to build its enterprise risk management system. Its use of risk to guide foreign inspections is exemplary. The challenge to the agency now is to persuade others that it can better protect consumers if it allocates more of its resources, not just inspections, based on modern risk management.
The use of enterprise risk management will be especially valuable to the FDA given the poor economy and fiscal austerity. The agency has been underfunded for years. The fiscal year 2010 budget was relatively generous to the FDA. This, combined with modest increases in fiscal years 2008 and 2009, brought the agency’s budget back to 1994 levels (see Figure 6-1) (McCain, 2011). At the same time, the agency’s responsibilities have increased dramatically. The increasing number of foreign food facility inspections demanded by the Food Safety Modernization Act (about 19,200 by 2016) cannot be reasonably managed by an agency that, according to a 2007 GAO report, operates on about one-seventh of its required budget (GAO, 2007; McCain, 2011). The committee believes that enterprise risk management will help the FDA triage its funding, which, especially during the 2012 election cycle, will likely be “hijacked and delayed by political maneuvering” (Semeniuk, 2011).
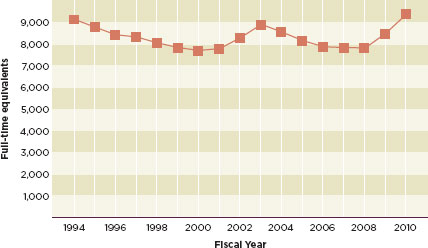
FIGURE 6-1
Full-time equivalents supported by congressional appropriations, from fiscal year 1994 to fiscal year 2010.
SOURCE: McCain, 2011.
Implementing Enterprise Risk Management at the FDA
The committee recognizes that implementing an enterprise-wide risk management program is challenging for any large organization. Fortunately, the International Organization of Standardization and other enterprise risk management experts publish guidance on implementing enterprise risk management strategies (COSO, 2004; ISO, 2009; Protiviti Inc., 2006).
These sources all emphasize that the risk management framework is different for every organization. The committee agrees; this report does not dictate what the FDA’s strategy will be. Such a level of prescription would be inappropriate and impossible: it would require analysis of the agency’s internal data and consideration of internal contextual factors of which expert committees have no knowledge. Instead of dictating the agency’s plan, the committee recommends that the FDA undertake an enterprise-wide risk assessment in keeping with its objectives and the Department of Health and Human Services’ (HHS’) goals as explained in its Global Health Strategy (HHS, 2011). The Global Health Strategy provides a framework in which to evaluate all of the FDA’s activities and consider their risks. The department’s goals are protecting the health of Americans through global health action; advancing American interests in diplomacy, development,
and security through global health action; and leading in science, policy, and programs that advance global health (HHS, 2011). The FDA’s enterprise risk assessment will need to consider these goals and identify where the biggest risks are in relation to meeting them.
The FDA’s risk management framework will define the processes, staffing, timelines, and budgeting needed to manage its risks. First, the FDA will undertake a thorough risk assessment. This will include identifying the risks it faces and evaluating its response options. In this phase the FDA will need to define how risks will be measured and when, and also how it will determine the level of the risks identified (ISO, 2009). Input from all stakeholders will be important to this process, especially as the FDA tries to determine if there are likely combinations of risk (ISO, 2009). The framework will mandate the schedule on which the FDA revisits its priorities to keep pace with changing risks.
The next step will be a risk analysis that accounts for the sources and causes of risk as well as their consequences and likelihood of reoccurring. The risk analysis step may include analyzing internal data and running simulations of different crises. The last step is risk evaluation, which analyzes the identified risks against pre-determined criteria to guide decisions. In risk evaluation, the FDA will consider the costs, effort, and benefits of all actions. A well-executed risk evaluation will provide the FDA’s leaders with the information they need to develop their capacity building priorities.
Implementation in the Short-Term Should Focus on FDA Activities Outside the United States
There are many restrictions on the FDA’s authority to allocate its resources domestically. Therefore, especially in the next 3 to 5 years, the committee sees promise in using enterprise-wide risk management to organize the FDA’s foreign operations.
Enterprise risk management depends on ongoing assessment of current and potential future risks. The FDA can use its data and, when confidentiality agreements allow, reliable data from its counterpart agencies abroad to inform its understanding of product risks. These risks are always changing; the product lines and suppliers considered highest risk a decade ago are different from those that are highest risk today. The FDA is best positioned to know which countries are increasing their exports to the United States. It should better define which countries are increasing their high-risk exports or which product lines are increasing in risk. Once it has identified these trends, it can allocate its resources accordingly. The FDA should also target its capacity building efforts to the countries and regions that export the highest risk products. The committee recommends that the FDA focus its resources on high-risk suppliers abroad for the next 3 to 5 years.
Enterprise risk management assessments should inform the FDA’s decisions on where to put its overseas staff, which overseas offices to scale up, and the best use of its overseas staff’s time. The FDA should devote the most energy to training people in countries that are exporting high-risk products. For example, holding workshops on food safety for regulators in the Middle East and North Africa, as the FDA did in 2010, does not appear to be a decision grounded in risk management (FDA, 2011a). Arab countries export little food to the United States or anywhere else; they are net food importers (World Bank, 2009). The implementation of a risk management system to all FDA work might better empower the staff of the Office of Technical Cooperation and Capacity Building to choose more useful topics and audiences for capacity building programs.
Implementation in the Longer-Term Should Be FDA Activities in Both Domestic and International Markets
The results of these assessments should also inform the FDA’s inspections in the United States. There are some firms in the United States that have never failed inspection, yet Congress demands that the FDA revisit the sites every 2 years. The FDA is surely better aware than anyone that this is not an efficient use of its inspectors’ time, yet it is bound by dated laws. An enterprise risk management system would allow the FDA to reallocate its resources to give more attention to inspections abroad.
The committee recognizes that the FDA will need to work with Congress to change the laws governing it if it is to fully revise its domestic work based on risk. It is important to remember that the existing laws were designed for a time when most foods and medical products were produced domestically. Nowadays, much of the food and pharmaceutical supply comes from abroad. This shift demands a complementary shift in the allocation of fixed resources to ensure product safety.
The Food Safety Modernization Act requires the FDA to inspect at least 600 foreign facilities in 2011 and double those inspections every year until 2016.2 The FDA will struggle to meet these requirements, especially if Congress does not increase its funding (Stewart and Gostin, 2011). If the agency were able to reallocate its domestic staffing, then it could give more attention to needs overseas. This does not mean that the FDA should neglect inspection and product safety responsibilities in the United States. The Salmonella-tainted egg crisis of 2010 and persistent quality control problems at Johnson & Johnson are a reminder that American companies can also prove to be high risk (Kavilanz, 2010; Silverman, 2011; Un oeuf is enough, 2010).
![]()
2 21 USC 350(j)(a)(2)(D).
USING INFORMATION TECHNOLOGY
The committee’s recommendation of a modern systemic risk management system depends on upgrades to the FDA’s information technology system. A series of recent Government Accountability Office (GAO) reports have highlighted inaccuracies in the FDA’s foreign supplier database and problems with its data management system (GAO, 2008, 2010a, b, 2011). The FDA science review committee recommended in 2007 that the agency “enhance the program to monitor performance metrics and put the appropriate [information technology] infrastructure in place to track the evolution of those metrics” (FDA, 2007, p. 42). The committee sees efficient use of modern information technology as indispensible to a risk-based regulatory system. The proper use of such a system could improve cooperation and communication among regulatory agencies. Information technology holds great promise to enhance surveillance. The results drawn from reliable data management systems will give the public and legislators a better understanding of product safety threats.
Recommendation 6-2: FDA should develop an information and informatics strategy that will allow it to do risk-based analysis, monitor performance metrics, and move toward paperless systems. In the next 3 to 5 years, the FDA should propose, in all its international harmonization activities, a standardized vocabulary, a minimum data set to be collected, and the frequency of data collection.
This recommendation can be measured when the FDA releases a standardized vocabulary for data collection, a codebook of the minimum data required from all points on the supply chain, and a timetable explaining how often these data should be collected.
As with the implementation of enterprise risk management, there are aspects of this recommendation that will take a decade to execute. A full overhaul of the FDA informatics and information strategy will take at least 10 years. However, in the next 3 to 5 years, the FDA can work out a standardized vocabulary and data collection protocols to propose for international use.
Information and Informatics Strategies
For the purposes of this report, information strategies are ways to ensure that all data about food and medical products are accurately collected, well annotated, recorded in permanent electronic media, and securely shared with authorized personnel for better information management. The
committee uses the term informatics strategies to describe how the data are cleaned of errors, transformed to proper formats, analyzed, and shared promptly with regulatory agents for better decision making.
Modern information management uses networked computing infrastructure across national borders. Information management lays the foundation for computer-assisted automated information extraction. Without such data, risk modeling would be impossible. The strategy outlined in this report also emphasizes the need to share information across international borders. The committee feels that the FDA should promote the development of secure and open protocols for electronic data capture, computerized data management, electronic data sharing, and decentralized data exchange. It is suitable that the FDA provide leadership in this international endeavor.
A good architecture for storing, collecting, and exchanging information is key to reliable, modern food and medical product regulation, although the importance of such a system, especially in developing countries, is not always obvious. A regulatory agency has to access data and information about drug registration, facility inspections, and surveillance from disparate data sources and in varying formats on an ongoing basis. In developing countries much of these data is still stored as paper documents. This severely decreases the productivity of the already thinly staffed regulatory agencies. A functional informatics system thus has the ability to enhance the productivity of regulatory agencies in developing countries. Well-defined data architectures and topologies can allow the multiple agencies regulating food and medical products to coordinate their work and reduce redundancy. In addition to sharing across different agencies within a country, common data architectures and good systems for information sharing can also facilitate better international harmonization. For example, the use of a reliable information system can allow the FDA to share inspection reports with its counterpart agencies in developing countries and vice versa.
Collecting data in common formats can also lay the groundwork for developing tools that will make regulatory agencies more productive. Pharmacovigilance and postmarket surveillance are two areas that could greatly improve with informatics tools that allow for simpler data collection and analysis. The path to having modern informatics tools in developing country regulatory agencies depends on the FDA leading in defining common standards, which will enable data collection, sharing, and (at some time in the more distant future) advanced decision support. The committee believes that moderate investments in informatics and information technology will yield significant long-term benefits in the quality of food and drug regulation.

Used with the permission of Dwayne Powell and Creators Syndicate. All rights reserved.
Challenges in Implementation
The committee recognizes that implementing an information and informatics strategy will be difficult, especially in developing countries. First of all, the data sharing that it recommends requires collecting information from many sources at different agencies. For example, border rejection data, public safety breakout events, regulated product safety recall records, assessment reports of export and import companies, and product ingredient tracking information all need to be collected and linked. There is also room for misunderstanding when many people are responsible for data collection. Field staff may have different interpretations of questions that can lead to inconsistencies in the data. Attempts to work with the European Union (EU) border rejection data, described in Appendix G, made it clear that worker inconsistency is a threat to data quality. Additionally, all large data analysis projects must deal with missing data and attempt to control for human error as much as possible.
International survey and data collection experts in academia have expertise in standardizing data collection across many different languages. Standardizing questions and response categories are challenging, but not impossible, especially for people who have worked on similar problems before. Programmers can easily adapt decision-making algorithms to tablet
computers and mobile phones, tools that would be accessible to field staff. Using such an algorithm, even minimally educated staff could be trained to collect and transmit standard data.
The FDA’s informatics strategy should aim to produce reports that will be compatible with reports produced by its counterpart agencies abroad. As Chapter 2 explains, the committee sees collaboration with the FDA’s counterpart agencies abroad as fundamental to ensuring product safety. In a report on drug safety the GAO also encouraged such collaborations. The FDA should ensure that its information strategy uses the same measurement conventions or has an accurate way to adjust for different conventions.
Most of all, the FDA’s information and informatics strategy will need to protect confidential information. The committee understands that willingness to share data may be minimal at first, especially in developing countries with a history of deficits in this area. De-identified or aggregated data can still be useful, however.
Models for Implementation
The biomedical research and financial services industries use modern information sharing, and the FDA might do well to study lessons learned from these sectors. All of them define a minimal set of XML-based files for information sharing. The data elements, semantics, and structure of such data sharing reports can be jointly determined in international standard setting committees. These committees would do well to draw on expertise at the World Wide Web Consortium and the Institute of Electrical and Electronics Engineers. Participating countries should agree to minimal data reporting elements. Experts need to identify the key minimal data set that will allow for the best communication among regulators and the best product safety assurances. Information management tools such as Protégé are based on ontologies, explicit definitions categories, and sub-categories of information and the relationships between them (Noy and Mcguinness, 2000). The use of Protégé and systems like it promotes a common understanding of the concepts and data being captured among different organizations. Over time, a shared ontology can facilitate adherence to basic standards and improve standards (Pisanelli et al., 1999).
The committee proposes that the FDA encourage its counterparts in developed countries to develop similar information sharing and informatics strategies. Ideally, all agencies can agree on standardized data collection and information sharing practices from the start. Eventually, developed country regulatory authorities can expand the system to include developing country regulatory authorities (GAO, 2011). The FDA should demonstrate how information and informatics strategies can improve its logistics and risk management in both domestic and export markets.
The FDA has made a good start at modernizing its informatics strategy by implementing the PREDICT system, a dynamic, integrated, risk-based evaluation method. The FDA uses PREDICT to target its inspections. The system aims to expedite entry of products that meet American market standards while vigilantly screening products likely to be adulterated or misbranded (FDA, 2011c).
The PREDICT system uses web-based technology to prevent dangerous products from passing through customs. At the port, inspectors check each product for a code that links to an FDA database on the product manufacturer, country of origin, history of recalls, and import alerts. Inspectors scrutinize the products that the system flags as possible threats. This makes the most of a limited number of inspectors. PREDICT uses automated data mining and pattern recognition algorithms to identify patterns that humans would miss, such as ratings of inherent product risk, results of field exams, and analyses from facility inspections (FDA, 2011c). This committee believes systems like PREDICT are a step in the right direction. Collecting data from more sources and using a standard data format would improve PREDICT and allow inspectors to cross-reference disparate databases.
BRIDGING TRAINING GAPS AT HOME AND ABROAD
Training deficits are at the root of many product safety problems in emerging economies. Chapter 3 describes the consequences of poor training in some detail. The committee sees training regulators abroad as an invaluable piece of the strategy to build capacity for food and medical product regulation in emerging economies.
Recommendation 6-3: The FDA should facilitate training for regulators in developing countries. The purpose is workforce training and professional development through an ongoing, standing regulatory science and policy curriculum. In the next 3 to 5 years, the FDA should broaden the scope of FDA University to educate FDA staffers on international compliance with its regulations. In the long term, the FDA should consider the options the committee puts forth in this chapter.
The first measure of this recommendation is revisions to the curriculum at the FDA staff college and the creation of an international curriculum. The number of countries participating in the international trainings, the number of people trained, and the number of people passing certification tests will also be useful indicators. A more capable workforce and a credentialing system can help improve morale at regulatory agencies. Therefore, the percentage of staff staying in government service after 3 years, 5 years, etc., will be long-term indicators of the trainings’ success.
The training that the committee recommends should take three forms: the training of regulators from abroad, the training of trainers, and the training of FDA staff. The following justification explains these options in more detail.
As with the earlier recommendations in this chapter, some aspects of this recommendation will take 10 years to achieve. The committee understands that training regulators at an international regulatory college and developing an apprenticeship program akin to the Centers for Disease Control and Prevention’s (CDC’s) Epidemic Intelligence Service (EIS) program will take about a decade. However, revising the FDA staff college curriculum for a more international focus should happen in the next 3 to 5 years.
Training of FDA Staff
First of all, the committee recommends that the FDA staff college include more emphasis on the application of FDA regulations abroad. Domestic FDA staffers should be more aware of international compliance with their regulations and the challenges of adherence to standards, enforcement, and quality assurance in developing countries. Learning about international compliance with the FDA’s regulations would also guarantee the same kind of international focus in the training for American regulators that the committee recommends for those in low- and middle-income countries.
FDA also faces challenges in employing scientists who speak foreign languages. The committee recommends that FDA consider incentive programs to encourage learning foreign languages among its technical staff or hire scientists already fluent in foreign languages.
More importantly, the FDA needs to encourage an institutional shift whereby taking an overseas posting for 2 to 5 years is not seen as a way to de-rail a career. The committee believes that the FDA should reward personnel who complete foreign rotations. The FDA could consider an advancement system used at the United Nations Children’s Fund, for example, whereby serving on a field mission accelerates an employee’s promotions.
The FDA could revise the curriculum at its staff college in the next 3 to 5 years. Changing the institutional culture to reward service in foreign offices will take much longer, probably about a decade. Nevertheless, the committee believes that with an attitudinal shift from senior leadership, the effects of the FDA’s new global outlook could begin to be realized in 5 years.
Training Foreign Regulators
The committee sees some training gaps as particularly problematic. First, regulators abroad are desperate for better training in risk assessment. As Chapter 4 explains, meeting this need is in the best interest of the FDA
as much as it is to the benefit of its counterparts abroad. A full curriculum in risk assessment can include training in risk management and risk communication. Mid-career professionals may also be rusty on the mathematics and basic science that underlie risk assessment or food hygiene. There is also a need for training in laboratory science and protocols, probabilities, and other fundamentals. The committee also sees, for example, a need for general training in food safety concepts and procedures, the training that would qualify a food safety inspector. Regulators in all sectors need a formal credentialing system that complements a clear career progression at their agencies. Respected credentialing could also do much to improve the professionalism and esprit de corps of the regulatory workforce.
In-service training is core to staffing a modern regulatory agency, and the committee sees the FDA’s staff college as a gold-standard training institution in the emerging field of regulatory science and policy. The committee also recognizes that an international fixed curriculum of regulatory procedures and regulatory science cannot and should not be the purview of the FDA or any one country’s regulatory authority. The FDA, like its counterpart agencies around the world, is charged with protecting health and product safety among its country’s citizens. Regulatory agencies are not primarily training or international development agencies. The committee is also sensitive to the fact that the FDA’s mandate already far exceeds its modest budget. The Food Safety Modernization Act of 2011 and the road map outlined in the agency’s Pathway to Global Product Safety and Quality will also require expensive changes to the status quo (FDA, 2011b).
There was consensus from all the foreign guests at the study meetings that having the FDA lead an international training institution would not be wise anyway. Perceptions of imperialism, political tensions, and the Cold War era’s lingering resentments could sabotage the U.S. government’s best intentions to train on regulatory science. This is why the committee believes that the FDA should use its diplomatic staff abroad and its gravity in international forums to facilitate training for foreign regulators, not necessarily to host it, and to expand the concept of the regulatory staff college to an international forum. The FDA would also do well to work with existing training networks such as the Asia Pacific Economic Cooperation Partnership Training Institute Network (APEC PTIN) to expand its trainings and make them standing.
Models for Training
A hodge-podge of inconsistent donor trainings is part of the problem, after all. The development banks, the World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations, donor organizations, and nongovernmental organizations (NGOs) are all willing
to host the occasional training on rational drug use or post-harvest storage. These trainings are a useful service, but, as Chapter 3 explains, developing country regulators cannot rely on these trainings or plan their professional development around them. Sometimes the European Medicines Agency or the FDA hosts one-time trainings on good manufacturing practices or good clinical practices, but they do not revisit the same topic again on any schedule; their budgets and mandates do not allow them to do so. The training institution needed is one that offers a predictable, standing curriculum. This would also help ensure that training meets the proper audience. As mentioned in Chapter 3, the participants in donor trainings are too often senior staff who are close to retirement. This is understandable as long as trainings are seen as a diversion and a chance to collect per diem payments. It would be difficult to maintain such misconceptions about a formal college where students work through a credentialing program.
A Standing Regulatory Science College
The committee recommends that the FDA use its authority to facilitate the creation of a standing international regulatory science college. Ideally, the costs of this college will be shared among many donor countries, foundations, and development banks. The center would not necessarily have to be a brick-and-mortar college, although that is a possibility, but it should rely on adjunct faculty to teach a standing, predictable curriculum of regulatory science. Students should complete credentialing examinations and earn universally accepted certifications in regulatory science.
Training of Trainers
It will take more than a decade to implement the type of college the committee envisions. However, in the next 3 to 5 years the FDA can facilitate the training of trainers. Training trainers is both cost-effective and conducive to building a technical infrastructure in developing countries. Trainers can learn the regulatory science material in English or Spanish or another major world language, but return to their home countries and train others in local languages.
The committee recommends that the FDA partner with existing training networks for training of trainers. The APEC PTIN would be an excellent organization for the FDA to work with on training trainers, as would the U.S. Agency for International Development (USAID). Trainings in the United States offer students from emerging economies a chance to observe the practices of a robust regulatory system at close range. The committee believes that information on the regulatory requirements of the American
regulatory system and other stringent regulatory authorities will be an important piece of the curriculum in all trainings.
Another advantage of training trainers through existing networks is that the training remains relevant and avoids the pitfalls described in Chapter 3 of introducing scientists to equipment and protocols that they will never have access to at home.
An Apprenticeship Program
Another different, but complementary, approach would be establishing a training pilot project based on the Epidemic Intelligence Service at the U.S. Centers for Disease Control and Prevention (Bollyky, 2010). The pilot project could put mid-career FDA officials into developing country national regulatory authorities or the WHO on 1- or 2-year rotations. In these rotations the FDA staff would work closely with their counterparts in foreign agencies to identify product safety problems before they affect consumers.
The FDA should consider basing this training program off the Field Epidemiology Training Program (FETP), another international capacity building program inspired by the EIS program. The first program was in Canada in 1975 (PHAC, 2010), and USAID funded expansion to Thailand in 1980 (CDC, 2010). A Central American FETP started in 2000 as part of the Hurricanes Mitch and Georges Reconstruction with funding from USAID and input from the American Association of Public Health Laboratories, the Pan American Health Organization, and ministries of health throughout Central American and the Caribbean (López and Cáceres, 2008). Initially the CDC ran the program as a 2-year master’s degree-accredited, in-service training for epidemiologists, but eventually the program expanded to include credentialing at the basic and intermediate levels as well (López and Cáceres, 2008). At each level, the training emphasizes field work over classroom work and relies on mentors to train students in an apprentice-like method (López and Cáceres, 2008). In the beginning of the program, CDC staff were the mentors and the University of North Carolina designed much of the curriculum (Figure 6-2). Over time, Central American and Caribbean universities have taken over the classroom training. Graduates of this program have gone on to reorganize their countries’ national epidemiology offices (López and Cáceres, 2008).
The committee recommends that the FDA study the Field Epidemiology Training Program and use it as a model for training foreign food and medical product regulators. The program should begin with a 1-month intensive training program for FDA regulators in regulatory science, the role of international institutions in supporting food and drug safety regulation, and the role of food and drug safety in global health, international trade, and development. This training program should be followed by field deployment.
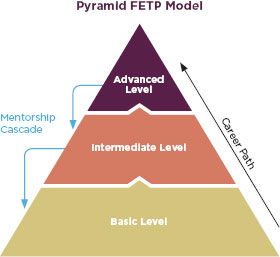
FIGURE 6-2
Conceptual model of the pyramid training approach used in the FETP.
SOURCE: López and Cáceres, 2008.
The focus of the program, as in the Field Epidemiology Training Program, should be learning by doing and public service. The alumni of the program could form the foundation of a more globally oriented FDA. If successful, this program could expand to include mid-career professionals from foreign regulatory authorities. Program alumni could eventually establish a global training program as in the EIS alumni program model (Pendergrast, 2010).
The committee realizes that the FDA and other regulatory agencies may be reluctant to allow foreign nationals to work in their agencies even on a brief rotation. There is precedent, however, for such international collaborations. The U.S. military routinely trains foreign officers at their staff colleges, for example. The Joint Forces Staff College in Norfolk has trained 171 officers from 46 countries (Joint Forces Staff College, 2011). The Army’s Command and General Staff College recently inducted four of its foreign graduates into the school’s hall of fame (Foreign officers chosen for hall of fame, 2011). Foreign students are active at the War College in Carlisle and the Industrial College of the Armed Forces in Washington, DC. Holding seats for foreign students in its staff colleges benefits the U.S. military as much as it does the foreign officers they train. These schools enroll officers from countries where the military controls the government and expose them to a system in which civilians control the military.
A Role for Academic Partnerships
Because of its limited budget, the FDA should also partner with academic programs that train foreign regulators. The University of Maryland’s Joint Institute for Food Safety and Applied Nutrition has experience training regulators from abroad. Its U.S.-China SPS Leadership Development Program is an example of an excellent training program. The U.S. Department of Agriculture (USDA), FDA, CDC, Environmental Protection Agency, HHS, and the Executive Office of the President all participated in the program; the U.S. Meat Export Federation and the USA Poultry and Egg Export Council funded it (Final agenda: 2007 U.S.-China SPS leadership development program, 2007). This program was a 2-month immersion for Chinese regulators exposing them to how the American system works, how Congress passes laws, how the different agencies involved in regulation work together, and how the American government works with industry and academia (Final agenda: 2007 U.S.-China SPS leadership development program, 2007).
Another noteworthy training program is Purdue University and Kilimanjaro School of Pharmacy’s Sustainable Medicine Program in Tanzania. The program trains manufacturing scientists in an effort to alleviate Tanzania’s dependence on other countries for lifesaving medicines, especially drugs for pediatric HIV, malaria, tuberculosis, and parasitic disease. Students are trained in good manufacturing practices and pharmaceutical science at the program’s laboratory and factory. The laboratory, operated by Howard University and Purdue University graduate students, allows trainees to receive hands-on experience in pharmaceutical good manufacturing practices. With German government funds, the program is building a pharmaceutical factory that will meet international manufacturing standards, only the second such facility in sub-Saharan Africa (Purdue University, 2011, 2012). This program and the University of Maryland’s U.S.-China SPS Leadership Development Program are exemplary programs, and ones that the FDA and other government agencies should consider as models.
Involvement of Industry and Academia
In developed countries, product safety depends on the regulatory authority, industry, and academia. In the weakest developing country regulatory systems, the regulatory authority works in isolation. It has no means to communicate with regulated industry and no input from academia. Especially in India and China, academics maintain a distance from both government service and private-sector consulting. An international regulatory science college would enlist academic and industry experts from around
the world to contribute. This could help drive an attitudinal change long overdue in low- and middle-income countries.
Industry and academia are indispensible to food and medical product safety. Government regulators in many parts of the world need to acknowledge the expertise of their colleagues in other sectors. Similarly, non-governmental stakeholders should be willing to contribute their expertise to training. Especially among academics in developing countries, there needs to be a cultural shift to encourage occasional consulting for private-sector firms, product research, or time spent in government service. Teaching through an international regulatory science college would provide many academics with a venue to serve. The college would expose faculty and students alike to a variety of different ideas and would allow everyone to see the roles academia and industry play in robust regulatory systems. Academics would come to see this service not as a departure from the academic career track, but as a necessary building of professional creditability.
Encouraging a collaborative yet independent relationship between industry and academia can also advance the economies of low- and middle-income countries. Research and development into new technologies is missing even in emerging manufacturing powerhouses. A robust research sector can create the new technologies that fuel economic development, and industry funding of research can lead to better facilities at universities (Jones-Evans et al., 1999).
LEADERSHIP IN ADOPTING STANDARDS
There are many ways to build a stronger workforce in developing countries. The committee sees value in a training and credentialing system, but training is not the only answer. As Chapter 3 describes, regulators and industry staff in developing countries often fail to observe international safety standards. Sometimes the regulatory authority would welcome better adherence to standards, but the overall political will for such changes is tepid. The FDA has the scientific expertise and the international authority to solve these problems, leading by example in the development and adoption of international standards.
Recommendation 6-4: U.S. policy makers should integrate food and medical product safety objectives into their international economic development, trade, harmonization, and public health work. To this end, the FDA should lead in the development and adoption of international and harmonized standards for food and medical products.
This recommendation can be measured by how many international standards the U.S. adopts. Another measure will be the technical assistance
programs that the FDA and other agencies offer to help low- and middle-income countries adopt international standards.
The integration of trade, development, and health should be judged by the initiatives U.S. government agencies and intergovernmental institutions launch to achieve food and drug regulatory objectives in developing countries. Another measure of the recommendation is the degree to which the FDA participates in and supports these initiatives.
This recommendation has a long time horizon. In the next 3 to 5 years, the FDA can begin adopting harmonized international standards, but the full realization of integrating product safety into the larger U.S. international policy agenda will take a decade.
Using Trade as a Tool to Promote Product Safety
The United States alone cannot ensure the safety of food and medical products produced across the globe. The United States needs partners in this endeavor, including national regulators in emerging economies. The United States must use the broader global health and trade agenda to advance food and medical product safety. By supporting developing country exporters’ economic interests, the United States can gain their cooperation on food and drug safety. The consistent use of harmonized standards is in everyone’s best interest.
Food and drug safety is a matter of domestic consumer protection, but it is also a tool for improving global health, trade facilitation, economic development, and poverty alleviation. Chapter 4 describes how these functions reinforce each other. The sale of high-value agricultural products is a lifeline to many in the world’s poorest nations (IFAD, 2008). Manufacturing jobs are a way out of poverty for millions, especially in Asia (Islam, 2001). The emerging middle classes of Asia, Latin America, and Africa spend more on health care and nutritious foods than the poor do. Healthy workers are productive workers able to fuel their countries’ economic advancement.
The United States should take advantage of the relationships between product safety, health, trade, and development. Many organizations are already working on international health; there are systems and funding infrastructure in place for health and development. The United States can work with the organizations already active and use their systems whenever possible. This approach is more practical than creating a new global food and medical product regulatory architecture. The agenda for these partnerships should be standard setting, information sharing, training for low- and middle-income countries, and improving regulatory cooperation.
A good first step would be for U.S. policymakers to better integrate global food and medical product safety objectives into its own global
health, trade, and development policies. The mandate for that integration already exists. Since the passage of the Food and Drug Administration Modernization and Accountability Act of 1997, one of three mandates for the FDA is reducing regulatory burdens and advancing international harmonization.3 International and U.S. bilateral trade agreements are designed to encourage the transparent adoption of international science-based standards, to develop country capacity building, and to consider the development implications (i.e., the technical and economic feasibility) of standards and regulation.4,5,6 The 2009 U.S. Trade Policy Agenda indicated the intention to pursue product safety as a trade facilitation measure in trade talks (USTR, 2009). USAID has long supported drug quality assurance programs (PSM, 2011), and the USDA’s Foreign Agricultural Service “links U.S. agriculture to the world to enhance export opportunities and global food security” (USDA, 2011).
Food security and nutrition programs are important to the Obama administration (White House, 2012). The Feed the Future program invests in food security and agriculture to improve nutritional status and reduce poverty (Feed the Future, 2012). Through the Feed the Future activities, U.S. policy makers can promote food safety and incorporate it into their programs. USAID’s recent $12 million investment in aflatoxin reduction in Africa is a commendable example of applied nutrition and agriculture programming that promotes food safety (USAID, 2011).
There is precedent for coordinated action as well. In May 2011, the FDA, in collaboration with the Office of the U.S. Trade Representative, signed a memorandum of understanding with the Asia Pacific Economic Cooperation’s food safety forum and the World Bank to collaborate on food safety training programs (USTR, 2011c). This project aims to enhance food safety and to facilitate trade throughout the Asia Pacific region (USTR, 2011c). The FDA has also long worked with other U.S. agencies and the WHO on international drug safety (Carpenter, 2010). U.S. trade officials routinely collaborate with the Department of Labor and the Environmental Protection Agency to encourage the adoption of international environmental and labor regulations through trade negotiations (USTR, 2011a,
![]()
3 21 USC 393(b).
4 Agreement on the Application of Sanitary and Phytosanitary Measures, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1B, THE LEGAL TEXTS: THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS 59 (1999), 1867 U.N.T.S. 493 (1994), art. 3, 5, 9.
5 Agreement on Technical Barriers to Trade, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1B, THE LEGAL TEXTS: THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS 121 (1999), 1868 U.N.T.S. 120 (1994), art. 2.4, 11.
6 19 USC 3802.
2011b). Many of USAID’s greatest successes (oral rehydration therapy, smallpox eradication, and vaccination campaigns), have funded the infrastructure needed to adapt existing technology and ensure its safe distribution in poor countries (Christenson, 2011; HaRP, 2011).
An increasing number of international initiatives are seeking to better integrate trade, development, and regulatory objectives as well. The Organisation on Economic Co-operation and Development (OECD) and the World Bank have adopted the cause of regulatory reform, citing its benefits for trade and development, rule of law, and the achievement of societal objectives (IFC, 2006, 2011; OECD, 2002, 2005, 2011). The World Trade Organization (WTO) has increasingly recognized effective implementation of good regulatory practices as an important means of avoiding and minimizing unnecessary barriers of trade. The most recent triennial review of the WTO Technical Barriers to Trade (TBT) Committee has added regulatory policy coordination to its agenda and stressed the need for more coordination between national regulators, international standard setting bodies, and trade officials (WTO, 2009). Association of Southeast Asian Nations (ASEAN) countries have established a Consultative Committee on Standards and Quality (ACCSQ) and adopted a Framework Agreement on Mutual Recognition Arrangements in order to promote an ambitious agenda on regional cooperation on standards, technical regulations, and conformity assessment (Steger, 2011). Asia Pacific Economic Cooperation (APEC) countries and OECD have launched a cooperative effort to integrate objectives on regulatory quality and market openness (APEC and OECD, 2005).
But more needs to be done. International or harmonized standards and certification regimes provide predictability for exporters and investors. They also simplify compliance with product safety rules and permit economies of scale (Henson and Jaffee, 2008). Intergovernmental institutions, such as Codex Alimentarius, can generate international risk-based standards for foods. These intergovernmental institutions are able to garner support better than bilateral negotiations or memoranda of understanding. USAID, working with other bilateral donors and development banks, should help provide the resources and technical assistance that developing countries require to meaningfully participate in Codex and other international standard setting organizations.
FDA and U.S. trade officials, including, but not limited to, those at the U.S. Trade Representative (USTR), the Department of Treasury, and the USDA, should work in closer collaboration where U.S. trade and regulatory goals overlap. The committee acknowledges that trade and regulatory objectives will not always overlap, but the goal of better product safety will advance the cause of free trade. For example, complex production chains involving food and drug components from multiple suppliers and sourced
from different countries are difficult for the FDA to oversee, leading to redundant inspections and conformity assessments. The unbundled supply chain is a logistical and regulatory problem for the FDA; it is also a failure from the trade perspective because lack of cooperation hinders free trade. Promoting regulatory cooperation and convergence in this context can help advance both U.S. trade and regulatory objectives.
U.S. bilateral and regional trade agreements can establish the structures and incentives necessary to develop and adopt common standards, policies, and assessment procedures for emerging or persistent food and drug regulatory challenges. The WTO SPS and Technical Barries to Trade (TBT) committees convene officials from 157 member countries to discuss regulations, standards, testing, and certification procedures in connection with food and drugs. The mandates of these committees include sharing information, promoting the adoption of international standards, and providing technical assistance to developing country members.,7,8 These committees provide potentially useful venues for building consensus for common regulatory approaches on difficult food and drug safety challenges.
The FDA should work harder to make the adoption of international food and drug safety standards a priority in the United States. The consistent use of standards in the U.S. market could motivate trading partners to do the same (Roberts and Josling, 2011). Even where the FDA cannot adopt an international food safety standard, it should work with other industrialized countries to streamline the means by which low- and middle-income countries can demonstrate conformity or comparability (Horton and Wright, 2008). The USTR should work with the FDA to use trade negotiations and forums such as the WTO TBT and SPS committees to promote the adoption of international, risk-based, commodity-specific performance standards for food and medical products.
EXPANDING ONE-UP, ONE-BACK TRACK AND TRACE
Counterterrorism requires that food companies be able to identify the immediate previous and immediate subsequent recipient of all the products in their supply chains (Gessner et al., 2007). This is called one-up, one-back traceability. The committee recognizes that expanding the
![]()
7 Agreement on the Application of Sanitary and Phytosanitary Measures, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1B, THE LEGAL TEXTS: THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS 121 (1999), 1867 U.N.T.S. 493 (1994), art. 3, 5, 9.
8 Agreement on Technical Barriers to Trade, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1B, THE LEGAL TEXTS: THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS 59 (1999), 1868 U.N.T.S. 120 (1994), art. 5.4, 12.7-.8, 13.1.
one-up, one-back requirements to medical products would be costly and complicated, but is nevertheless something the FDA needs to consider. Not only do one-up, one-back requirements protect American consumers, but they help producers abroad build stronger supply chains.
Recommendation 6-5: FDA, which currently requires one-up, one-back track-and-trace requirements for food, should, in the next year, hold a multi-sector, international, public workshop on applying them to medicines, biologics, and (when appropriate) to devices.
This recommendation can be measured simply by observing whether or not the FDA holds a public consultation on expanding one-up, one-back. The proceedings of this consultation and all the stakeholder input will be useful to FDA and to foreign regulators and producers who struggle with traceability in their supply chains. The immediate goal of this recommendation is to articulate how the FDA can extend one-up, one-back traceability to medical products. For this recommendation, the committee values the process as much as the outcomes. It is not possible for the committee to foresee the outcomes of this meeting, but bringing together all stakeholders to discuss it will be a marked step in the right direction. While implementing one-up, one-back traceability requirements for medical products would take at least 5 years, the process and dialogue about it can begin with a workshop within the next year.
Planning for Recalls
The ability to intervene quickly in an emergency is the essence of response to a product safety emergency. The faster the regulatory authority and companies move to remove an unsafe product from the market, the fewer the consumers harmed. Product recalls are used to this end, both voluntary recalls from industry and mandatory ones from the FDA. Identifying the source of the contamination is usually the rate limiting step in the response to a product safety crisis. The past 20 years have seen substantial efforts to increase the speed at which outbreak investigations and product trace-back or trace-forward investigations take place. Several collaborative programs have received worldwide attention. The PulseNet program described in Chapter 3 is an example of such a program. PulseNet was established during the 1990s; it has a worldwide network of participating laboratories that provide genetic fingerprints of pathogen microorganisms from patients and foods (CDC, 2011).
A number of national and international foodborne outbreaks have stimulated the search for more efficient tracing systems. Regulatory requirements for food and medical products also increasingly emphasize the
importance of traceability. In the United States, the 2002 Bioterrorism Act required the FDA to register all food manufacturers, producers, and warehouses whose products are on the U.S. market.9 It also requires that food producers, excluding farmers and restaurateurs, have information on immediate previous sources of foods (one-up) and the immediate subsequent recipients (one-back).10 These records need to be available in the event that “FDA has a reasonable belief that an article of food is adulterated and presents a threat of serious adverse consequences or death to humans or animals.”11 The Food Safety Modernization Act of 2011 further strengthened traceability by requiring the FDA to develop and implement enhanced tracking systems for high risk foods.12 Congress has established certain recordkeeping requirements:
• “they must relate only to information that is reasonably available and appropriate;
• they must be science-based;
• they may not prescribe specific technologies to maintain records;
• the public health benefits must outweigh the cost of complying with the requirements;
• they must be practical for facilities of varying sizes and capabilities;
• to the extent practical, they may not require a facility to change business systems to comply;
• they must allow for the maintenance of records at a reasonably accessible location, provided that the records can be made available to FDA within 24 hours of a request; and
• they may not require a full pedigree, or a record of the complete previous distribution history of the food from the point of origin” (FDA, 2011d).
Traceability is a common requirement among developed countries, although the specifics of different traceability programs can vary substantially. Tracing back the sources of imported products is a common problem, particularly if the exporting country has no traceability requirement. Increasingly, U.S. importers are requiring enhanced traceability in their contracts as a way to manage risk and to comply with the law. Although there is still room for improvement, traceability requirements have been credited with decreasing the response times to food safety emergencies (Agriculture and Agri-Food Canada, 2012; Food Standards Agency, 2002).
![]()
9 21 USC 350(d).
10 21 USC 350(c).
11 21 USC 350(c).
12 21 USC 2223.
They also improve inventory management and can allow for more targeted (and therefore less wasteful) recalls (Mejia et al., 2010).
Medical product traceability lags behind food traceability. The requirements appear to be largely limited to finished product lot or unit identification or both, particularly for medical devices (GS1, 2009; ISO, 2003). Although the FDA has articulated a need for enhanced programs, particularly in relation to counterfeit drugs, traceability has not risen to the point of a regulatory requirement. Tracing the supply chain is no less essential in the production of medical products than food, however. Counterfeit drugs are a growing problem, especially in developing countries.
By demanding traceability in the medical products market, the FDA could improve supply chain management in developing countries. If all producers are required to maintain one-up, one-back traceability for their export products, economies of scale will make it attractive to extend the same standards to products for the domestic market. Such requirements would be evidence of a commitment from the FDA to tighten the drug supply chain around the world. This would be most valuable in the poorest countries, the ones most devastated by fake drugs.
RESEARCHING INEXPENSIVE TECHNOLOGY
The human capital, research infrastructure, and creativity at American and foreign universities needs to be better harnessed for global food and medical product safety. Groundbreaking research can also come from government labs and from industry. The committee’s concern is that the U.S. government should be encouraging research into frugal technologies that would be useful in low- and middle-income countries. The committee values a collaborative research model that would build the private sector and academia in developing countries and involve them in the regulatory system. Cooperative Research and Development Agreements (CRADAs) are especially useful tools for technology transfers; the committee encourages the FDA and the USDA to enter into these useful partnerships.
Recommendation 6-6: Starting in the next 2 years, the FDA and USDA should implement Cooperative Research and Development Agreements and other programs to encourage businesses and academia to research and develop innovations for low-cost, appropriate fraud prevention, intervention, tracking, and verification technologies along the supply chain.
The number of requests for proposals that the FDA and the USDA issue will be the main measure of this recommendation. Eventually the number of patents issued and publications about low-cost appropriate technologies will also reflect the impact of this recommendation.
The time frame on this recommendation is fluid: the first CRADAs can be made in the next 2 years, and renewed over time, becoming an ongoing piece of the FDA’s and the USDA’s capacity building operations. The agencies should also explore other programs to involve industry and academia in research that benefits producers in developing countries. This too should begin in the next 3 to 5 years and continue.
Determinants of Research Investments
There are three key determinants of agricultural research investments by for-profit companies. The first is the size of the potential market for new technology. Second is the ease of improving the technology relative to the research investment. Last is the ability of a firm to capture the returns on its investments and protect intellectual property (Pray and Fuglie, 2008). Since the mid-19th century, much agricultural research has been carried out by the public sector because the knowledge produced from agricultural research has the non-rivalness and non-excludability characteristics of a public good. Without public-sector investment, research would suffer.
It can be argued that food safety research is an impure public good because it has benefits that are both private (i.e., product liability) and public (i.e., health). The appropriability of benefits of new technology and the costs associated with a recall may affect a firm’s decision to invest in food safety research. However, some technology developed through applied research will not be appropriable and thus not covered by the private sector. To date, much of the food safety research has been directed at supply-side questions, such as technologies to ensure proper detection of pathogens. The public sector has done more of the basic research, the original investigation that advances the science but has no immediate, commercial value. The private sector has focused on applied market research where it finds justifiable economic returns.
Research for the Small-scale Producer
For the most part, the focus of research on pathogen-reducing technologies has been on the needs of the big players on the supply chain. The technologies developed exhibit economies of scale on a per unit basis. In the case of beef, for example, following the 1993 E. coli O157:H7 outbreak associated with Jack-in-the-Box hamburgers, Frigoscandia Equipment developed a steam pasteurization technology that reduced 90-99 percent of the pathogen loads on beef carcasses (Corantin et al., 2005; Golan et al., 2004). The cost of steam pasteurization varies from $0.28-$0.46 per cattle head for large slaughterhouses to $3.58-$7.05 for small slaughterhouses. Clearly, steam pasteurization is not cost-effective for most small slaughter-
houses, even in the United States, unless a smaller-scale version requiring less throughput is produced (Malcolm et al., 2004). Technologies appropriate for small-scale and medium-scale producers for the most part have not been addressed. Currently there is a need to either adapt this technology so that it is cost-effective for small-scale slaughterhouses or find an equally effective cheaper technology. Similar examples abound in hazard-reducing food technologies. To date there has been little incentive for the private sector to direct research and development efforts toward tackling problems for small- and medium-scale producers.
A substantial portion of food and medical products production in developing countries is done by small-scale producers, either acting alone or by providing larger companies with key components and ingredients. There is therefore a need to give incentives to private-sector actors to develop hazard-reduction technologies for small- and medium-scale producers. The food industry in developed countries increasingly requires suppliers to implement hazard-reduction measures with limited knowledge of whether these standards are more effective than the control measure given the size of the producer. Although the private sector is increasingly implementing traceability schemes, this does not solve the problem of finding appropriate and cost-effective solutions to ensure an acceptable level of risk. Research efforts need to be directed at finding appropriate cost-effective technologies for reducing risk for small- and medium-scale producers in both developed and developing countries, rather than looking at prescriptive solutions that may not be scaled neutrally. Based on the experience gained in the United States and other developed countries, small- and medium-sized companies have less money to invest in research or use to buy expensive equipment. Targeted funding of size-appropriate technologies would allow such companies to address product safety concerns.
The public sector has difficulty testing its research outside of the laboratory setting. It also struggles to bring its innovations to market quickly. Therefore, it might not be efficient to rely on the public sector for the research that would help ensure safety for small- and medium-scale producers. Rather, the FDA and the USDA, in conjunction with research funding agencies, should advance research and development programs that would encourage small- and medium-sized companies in developing countries to meet product safety goals. The FDA and the USDA should encourage private-sector participation in this research.
In recent years, the financial crisis has constrained public-sector research, leading to greater collaboration in agricultural and food safety research under the CRADA system. A CRADA is a written agreement between a private company and a government agency to work together on project development (USGS, 2009). Such agreements optimize resources and share research costs. They also improve technology transfer, providing
incentives to the private sector to aid in the commercialization of federally developed technology (USGS, 2009).
The committee recommends similar collaborations with developing countries. This would enhance the academic infrastructure in developing countries. The technologies developed by these collaborations would also help the small- and medium-sized companies in the United States. The FDA should facilitate research collaborations by providing guidance to new technology providers in developing countries about the requirements for premarket approval. FDA should also consider funding the advancement of promising technologies.
GIVING MARKET INCENTIVES FOR SUPPLY CHAIN MANAGEMENT
The FDA’s charge to protect the American consumer can also work to the advantage of consumers in emerging economies. Chapter 4 describes how market incentives can help importers and exporters of American and foreign products. Market incentives are a useful means to encourage adherence to standards and help control supply chains in developing countries. Economic incentives could also do much to increase political will for product safety in developing countries. The proper incentives would eliminate the false dichotomy that pits product safety against economic development.
Recommendation 6-7: FDA should ensure an adequate mix of incentives to importers of food and medical products that are confirmed to meet U.S. regulatory standards. One such promising initiative is the 2-year FDA Secure Supply Chain pilot program. The FDA should evaluate this program immediately after its pilot phase (scheduled to end in 2014). The program should be expanded, if successful, to include a greater number of importers and food.
The number of incentive programs FDA proposes and the volume of imports going through these programs will be a simple measure of this recommendation’s effectiveness.
The evaluation of the Secure Supply Chain program should take place immediately after the program is finished. As of February 2012, the FDA is working out the logistics of the program start-up, so the evaluation should happen in the spring of 2014. The scale-up of the program is contingent on the results of this evaluation and should begin in the next 3 to 5 years.
A View of the Entire Supply Chain
Food, pharmaceuticals, vaccines, biologics, and medical devices move through a complex supply chain before entering the United States. The FDA does not have regulatory authority over all of the upstream activities in this supply chain. It is difficult to re-create this chain even for domestically manufactured products. Safety risks in manufacturing of both food and medical products are not just limited to the final manufacturing stage, or to the active ingredient manufacturing stage. Problems may arise anywhere in the supply chain, from inadequate raw material to user errors. Although strengthening food and drug regulatory authorities in developing countries would help in better regulation of the upstream supply chain in the medium to long term, currently many of the developing country regulatory authorities regulate only the final stages of the food and medical product production.
Furthermore, private-sector food and medical product manufacturers have a great deal of freedom in choosing their suppliers. Developing country firms are often attractive suppliers because of their lower prices. In developing countries’ domestic markets there are limited price premiums on better-quality products and usually no widely used certification process. The regulatory authority can encourage upstream quality controls by offering speedy market access to those suppliers that implement quality controls in their suppliers.
Sharing information from nodes along the entire supply chain could greatly reduce the risks of unsafe or falsified products entering the supply chain. Therefore, the FDA should put mechanisms in place to better see the upstream actors on the supply chain for food and medical products entering the United States. Foreign manufacturers should be rewarded for giving detailed information about their sources. These incentives can take various forms such as faster product clearance for import and quicker distribution in the U.S. market. Box 6-2 describes the USDA’s Animal Plant and Health Inspection Service, which works to the benefit of U.S. regulators and their counterparts abroad to facilitate trade in safe foods.
There are flaws in the Food Safety Modernization Act in that its mandates, however well-meaning, are largely unfunded (Ozersky, 2010). Congress should ensure that the FDA has sufficient funding to develop and establish importer incentive programs for both food and medical products. The committee commends the FDA for the Secure Supply Chain pilot program that aims to do just this (FDA, 2009a). The FDA had a public hearing on this 2-year pilot program in January 2009 (Secure supply chain pilot program; notice of pilot program, 2009). As of February 2012 the FDA is still resolving the logistical details of the program; assuming that the pilot starts in 2012, the FDA should promptly evaluate the program in 2014.
BOX 6-2
The Animal Plant and Health Inspection Service
The Animal Plant and Health Inspection Service (APHIS) is a USDA agency established in 1972 to consolidate the USDA’s roles in protecting animal and plant health. Since its founding, APHIS has continued to develop its mission of safeguarding American agriculture (USDA, 2007b). APHIS creates regulations, forms agreements, and implements emergency protocols that maintain the safety standards for food imports into the United States (USDA, 2007a).
Funding for APHIS’ programs comes from a variety of sources including the agency’s user fees. These are charges for APHIS’ programs such as the export certification services and agricultural quarantine and inspection service (USDA, 2010b). The USDA also collects user fees for APHIS services that directly benefit the recipient or are necessary to protect the American public (USDA, 2010c).
The success of APHIS’ mission involves agreements and partnerships with other organizations such as the World Organization for Animal Health and the International Plant Protection Convention, which set standards to guide animal and plant trade. Additionally, APHIS works with the North American Plant Protection Organization, which provides a Phytosanitary Alert System that notifies the authorities of any emerging diseases or pests. These organizations work together to promote the development of practical, risk-based approaches that can reduce and manage the pest and disease risks in agricultural trade (USDA, 2006). APHIS also collaborates with and assists its foreign partners in building their animal and plant health infrastructures. This support reduces the probability that a threat could enter the country undetected and destroy American agriculture (USDA, 2010a).
Several programs within APHIS are effective in helping to reduce the spread of disease and foreign pests to the United States. Preclearance measures, which include thorough offshore inspections and trade facilitation, ensure the safety of food imports (USDA, 2007c). APHIS also has a Plant Protection and Quarantine program that entails the inspection and screening of passenger baggage, mail, and cargo for prohibited agricultural products that may bring unwanted pests into the United States (USDA, 2002).
APHIS’ procedures encourage cooperation not only with other organizations, but also with individual countries. In South America, APHIS partners with countries through bilateral commissions and field programs, in addition to its preclearance processes, as a method to control pests (U.S. Department of State, 2012). A recent example of this partnership is between APHIS and Uruguay’s agriculture department. Uruguay requested market access to export fresh blueberries to the United States. The Uruguayan department submitted a risk assessment in accordance with APHIS preclearance requirements (USDA and APHIS, 2007). Preclearance and surveillance programs like that in Uruguay are conducted in countries throughout South America, as well as in other regions of the world.
This evaluation should be focused on scaling-up to include food producers and more drug firms.
End-to-end supply chain visibility for food and medical products is the best insurance against safety lapses. The committee recommends that FDA evaluate the results from the Secure Supply Chain pilot for the technical and operational feasibility of scaling. A full-scale program should then be instituted in which all interested manufacturers are enthusiastically encouraged to participate. In order to reduce the risk of non-compliance of these incentives programs with WTO law, FDA should develop these food and drug importer incentive programs in close consultation with U.S. trade officials and ensure participation is voluntary and pursuant to objective criteria that do not favor applicants of any one country over those of another.
Promising Initiatives
Globalization has dramatically altered where food and medical products come from. The FDA cannot inspect all of the import lines to the United States or all foreign manufacturers exporting to the United States (GAO, 2008). Even if it could, the GAO pointed out that, “relying solely on inspections is insufficient to secure the drug supply chain” (GAO, 2010a, p. 29). The same is true of relying exclusively on end product testing and food safety inspections to ensure food safety (Young, 2011). There need to be other ways to ensure the safety of import lines.
Market incentives drive food safety in the United States (Thomsen and McKenzie, 2001). The Food Safety Modernization Act requires the traceability of the food supply chain and holds suppliers accountable for ensuring HACCP compliance. The ability to assess the safety of ingredients produced overseas, in places where the FDA has no authority, is a challenge (FDA, 2009a). By expanding incentives programs, the FDA could help encourage foreign producers to adhere to international standards and work to produce higher-quality products. The Food Safety Inspection Service has a program in place to ensure the quality of imported meat. This is not an incentives program, but it is a good example of bilateral coordination to facilitate trade (Box 6-3).
The Secure Supply Chain program promotes drug safety. In this program, foreign firms voluntarily provide the same information FDA requires of American firms (FDA, 2009b). The pilot program includes 100 firms; each firm can include up to five drugs. To be eligible firms must be able to trace their products from manufacture through entry to the United States. They also need a plan for recalling the drug or active ingredient. Qualifying firms receive expedited entry of select products through customs (FDA, 2009b). The FDA and U.S. Customs and Border Protection agency are currently defining how to expedite entry. The solution will make use of the
BOX 6-3
The Food Safety Inspection Service
The Food Safety and Inspection Service (FSIS) is the office within the USDA “responsible for ensuring that domestic and imported meat, poultry, and egg products are safe, wholesome, and accurately labeled” (FSIS, 2009). Meat imported into the United States must meet the same safety, quality, and labeling standards required of domestically produced meat. In order to assure this, the FSIS only allows importation of meat that has gone through its rigorous approval protocol that covers the entire import process (FSIS, 2009).
Any country that wants to export meat to the United States must undergo an FSIS inspection and certification process called an equivalence determination process. This certification evaluates the meat inspection processes of the exporting country. If the export inspection protocol in place is deemed equivalent to U.S. domestic inspection protocol, the country may be granted eligibility. By setting the standard at equivalence, the FSIS ensures products of equal quality without requiring that exporting countries conform to exact U.S. inspection procedures. Within a country, each producer wishing to export meat to the United States must undergo a similar certification. The evaluation process includes both offsite assessment of written protocols and procedures and an onsite inspection by an FSIS team. Both elements stress five primary categories of risk assessment as established by the FSIS: animal disease controls, sanitation controls, residue controls, slaughter and processing controls, and controls for enforcement. Countries and sites are periodically reevaluated to ensure ongoing safety and equivalence. Countries and specific producers may lose eligibility, often temporarily, because of an outbreak of a disease affecting livestock or other adverse health conditions (FSIS, 2009, 2011).
After the appropriate export country inspection paperwork has been filed, meat is re-inspected by an FSIS inspector before it is allowed past the port of entry. Products that are approved during this second inspection are allowed into the United States for sale and are treated no differently than domestically produced products from that point forward (FSIS, 2009).
Equivalent inspection practices, rather than identical ones, must be accepted by importing countries under the guidelines set forth by the WTO, specifically the Sanitary and Phytosanitary Measures (SPS) agreement. The SPS agreement concerns the international trade in food, animal, and plant products. As with all WTO agreements, the SPS agreement seeks to limit barriers to international trade, in part by ensuring that developing countries, often using less technologically advanced equipment, are not unfairly discriminated against. Regardless of the exact measures in place, if exports are of a suitable standard, then they must be treated as such (WTO, 2012).
Customs-Trade Partnership Against Terrorism program, which offers priority processing for products from companies with secure supply chains. The Secure Supply Chain pilot aims to enable FDA to determine the practicality of using a secure supply chain program to prevent importing sub-standard drugs (FDA, 2009b). The committee suggests that this program be evaluated immediately after its pilot phase and implemented and expanded, if successful, to include a greater number of importers and to food.
INCREASING CIVIL LIABILITY
Appendix B describes how the civil liability system, so essential to product safety in the United States, is flawed in low- and middle income countries, and so does not deter faulty manufacturing.
Recommendation 6-8: Over the next 10 years, U.S. government agencies should work to strengthen the ability of those harmed by unsafe food and medical products to hold foreign producers and importers liable in civil lawsuits.
The establishment of mechanisms that increase liability will measure this recommendation. This process will be slow, and it will require major revisions to the status quo that involves multiple government agencies. It is unlikely these changes could be made in less than 10 years.
Product Liability in Developed Countries
The U.S. legal system has two ways to ensure food and medical product safety. The first way is the regulatory framework built around the Federal Food, Drug, and Cosmetic Act; regulatory framework is the primary focus of the current report. Recent legislation has provided the FDA with a variety of new tools with which to enforce this regulatory framework for both foods and medical products. If there is a sufficient likelihood that food is adulterated or misbranded, the FDA may administratively detain products or mandate a recall if there is reasonable probability that food will cause “serious adverse health consequences or death to humans or animals.”13 Food production facilities must register with the FDA in order to introduce their products into U.S. interstate commerce; the FDA can suspend that registration if there is reasonable probability that the food from that facility will harm humans or animals.14 The Food and Drug Administration Amendments Act of 2007 also expanded the legal tools FDA uses to
![]()
13 21 USC 350(l).
14 21 U.S.C. 350(d)(a).
ensure drug and vaccine safety. The FDA may withdraw approval from a marketed drug or biologic,15 order labeling changes or the inclusion of package inserts about new safety information and recommendations,16 mandate postmarket observational studies or clinical trials to assess risks,17 and require sponsors to have strategies to ensure that the benefits of a drug will outweigh its risks.18 In general, most violations of the Federal Food, Drug, and Cosmetic Act are also subject to criminal enforcement, but few are subject to civil enforcement (Hutt et al., 2007).
The second way that the U.S. legal system ensures food and medical product safety in the United States is product liability. Entities involved in the manufacture, distribution, and marketing of a food or drug product to the public are obligated under U.S. common law and, in some cases, U.S. federal and state statutes to ensure the safety of that product. Individuals that suffer harm from that food or drug product in the United States may bring a civil lawsuit against the manufacturers, importers, distributors, and marketers of that product. Any entity with significant contacts with the United States is potentially subject to the jurisdiction of U.S. courts. Redress is typically monetary compensation to cover the damages incurred by the injured party, but it may also involve punitive damages.
Product liability plays an essential role in ensuring food and drug safety in the United States. The volume of commerce in FDA-regulated food and medical products far exceeds the resources and legal authority that FDA has to monitor and enforce the safety of those products. The threat of product liability suits, high litigation costs, and reputational damage help fill the gap by providing a significant economic incentive to companies to maintain and improve the safety of their products. Ultimately, it is retailers and manufacturers that are best able to ensure the safety of their products.
There are limits to the role of product liability in imports. First of all, the FDA does not have jurisdiction in foreign markets, and even when its staff is permitted to inspect foreign establishments there are practical challenges to working in a new country and in a different language. Furthermore, U.S. plaintiffs have limited ability to litigate and enforce judgments against foreign firms with few U.S. contacts or assets. It is difficult for Americans to bring lawsuits against foreign firms in foreign country courts for injuries that occurred in the United States (Appendix B). U.S.-based importers and distributors may be able to avoid product liability when there is doubt that they knew or should have known about their suppliers’ actions (Bamberger and Guzman, 2008). It may be difficult for consumers
![]()
15 21 USC 355(e).
16 21 USC 355 (o) and 21 USC 355(r).
17 21 USC 355 (k)(3).
18 21 USC 355-1.
to establish accountability for unsafe products when the supply chain is so complicated (Bamberger and Guzman, 2008). With the possibility of more limited liability, manufacturers and distributors of imported products may not have the same market incentives as their U.S. counterparts for continual improvement in consumer safety.
Congress and U.S. government agencies (including, but not limited to, the Department of Justice, the Department of the Treasury, and the U.S. Trade Representative) should consider measures to strengthen the ability of those harmed by unsafe food and medical products to hold foreign producers, exporters to the United States, and U.S. importers of foreign products liable. Congress could increase appropriations for criminal enforcement of the Federal Food, Drug, and Cosmetic Act or amend it to make violations subject to civil penalties. The FDA could issue more stringent guidance for high-risk drugs and foods, such as requiring importers to maintain a staff person at each foreign production facility for high-risk products (Bollyky, 2009). Scholars have made other thoughtful proposals to increase liability. The issuing of bonded warranties would oblige sellers to pay statutory damages to people injured by unsafe products (Baker and Moss, 2009). Another option would be using discriminatory strict liability with damages based on the risk posed by the product and by the importer’s history (Bamberger and Guzman, 2008). Finally, developing country governments seeking to improve the safety of their food and drug production should consider measures to facilitate the ability of domestic consumers to hold firms accountable in civil suits for the harms caused by unsafe products (Appendix B).
CONCLUSION
During its meetings, the committee heard from FDA staff about the agency’s current work in capacity building and the challenges it faces in protecting the U.S. food and medical products supply. The committee commends FDA on its work, but sees some areas where it might improve its operations. First, it should use an enterprise risk management system to identify its most important priorities. That is, it should use risk to inform all its staffing and training decisions, not just its inspections. In the next 3 to 5 years, the FDA should use enterprise risk management to inform its work abroad, but eventually the committee recommends that it use this tool to plan its domestic work as well. The committee recognizes that Congress might need to revise the laws governing FDA for this to happen.
The agency’s use of risk will depend on reliable data collection. A modern information and informatics strategy will allow the agency to collect and analyze data promptly. The FDA’s PREDICT system is a step in the right direction. The committee believes that in the next 10 years the agency should work toward a paperless system in its own operations and
in its dealings with its counterpart agencies abroad. The first step toward this system would be the development of a standard data format and vocabulary, which could be developed in 3 to 5 years.
Regulators around the world need training on how to respond to the challenges of globalization. FDA has the technical depth and international presence to contribute to an ongoing, standing regulatory science and policy college, but developing this college will take a decade. In the meantime, the agency can work with universities and through existing training networks to make better training opportunities available. The FDA’s staff college is an exemplary training center that should be a model for an international regulatory science and policy college. In the next 3 to 5 years, FDA could require their staff to take courses on the international implementation of and compliance with American regulations. In the longer term, that is, in the next decade, FDA could work to develop a training program like the CDC’s Field Epidemiology Training Program. Involving industry and academia in these efforts will also set a valuable example.
The United States could also lead by example in its consistent use of international product standards. Harmonized standards make the market more predictable for foreign investors and exporters. USAID and other agencies can demonstrate U.S. commitment to harmonized standards by empowering scientists in low- and middle-income countries to participate actively in standard setting meetings. Over the next decade, product safety objectives should be fully integrated into U.S. foreign policy.
The United States could also set a powerful example for industry and government around the world by expanding the one-up, one-back, track and trace requirements for food and medical products. The committee realizes that this would be complicated, but believes that the FDA could make progress by holding a public workshop on expanding one-up, one-back in the next year. This workshop should include international stakeholders from government, industry, and academia.
There is great potential for innovation in American universities. The government should, starting in the next 2 years, encourage research into simple and elegant technology that will help small-scale producers prevent fraud and control their supply chains. Similarly, the government can improve supply chain management by giving market incentives that reward supply chain management. The committee is especially impressed by the FDA’s Secure Supply Chain pilot program. The 2014 evaluation for this pilot should consider how to best expand this program to include a greater number of producers.
Market incentives can do much to improve the safety of the food and medical products used around the world. The committee also sees value in increasing civil liabilities on foreign importers over the next 10 years.
REFERENCES
Agriculture and Agri-Food Canada. 2012. Canadian traceability. http://www.ats-sea.agr.gc.ca/trac/trac-eng.htm (accessed March 29, 2012).
APEC (Asia-Pacific Economic Cooperation) and OECD (Organisation for Economic Co-operation and Development). 2005. APEC-OECD integrated checklist on regulatory reform. Singapore and Paris, France: APEC and OECD.
Baker, T., and D. Moss. 2009. Government as a risk manager. In New perspectives on regulation, edited by D. Moss and J. Cisterno. Cambridge, MA: The Tobin Project.
Bamberger, K. A., and A. T. Guzman. 2008. Keeping imports safe: A proposal for discriminatory regulation of international trade. California Law Review 96(6):1405-1447.
Bollyky, T. J. 2009. Global health interventions for U.S. food and drug safety: CSIS Global Health Policy Center. Washington, DC: Center for Strategic and International Studies.
———. 2010. Bridging the gaps: FDA’s role in improving the development pathway for neglected disease therapies. Testimony for the U.S. Senate Appropriations Subcommittee on Agriculture, Rural Development, the Food and Drug Administration, and Related Agencies.
Carpenter, D. 2010. Reputation and power: Organizational image and pharmaceutical regulation at the FDA. Princeton, NJ: Princeton University Press.
Casualty Actuarial Society-Enterprise Risk Management Committee. 2003. Overview of enterprise risk management. Fairfax, VA: Casualty Actuarial Society.
CDC (Centers for Disease Control and Prevention). 2010. Thailand FETP. Center for Global Health.
———. 2011. What is PulseNet? http://www.cdc.gov/pulsenet/whatis.htm#developed (accessed November 21, 2011).
Christenson, K. 2011. USAID celebrates 50 years of saving lives across the globe. http://blog.usaid.gov/2011/11/usaid-celebrates-50-years-of-saving-lives-across-the-globe/#more-8963 (accessed November 21, 2011).
Corantin, H., S. Quessy, M. L. Gaucher, L. Lessard, D. Leblanc, and A. Houde. 2005. Effectiveness of steam pasteurization in controlling microbiological hazards of cull cow carcasses in a commercial plant. Canadian Journal of Veterinary Research 69(3):200-207.
COSO (Committee of Sponsoring Organizations of the Treadway Commission). 2004. Enterprise risk management integrated framework. http://www.coso.org/documents/coso_erm_executivesummary.pdf.
FDA (Food and Drug Administration). 2007. FDA science and mission at risk: Report of the Subcommittee on Science and Technology. Silver Spring, MD: FDA.
———. 2009a. FDA’s approach to medical product supply chain safety. Silver Spring, MD: FDA.
———. 2009b. FDA launches pilot program to improve the safety of drugs and active drug ingredients produced outside the United States. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm109064.htm (accessed November 21, 2011).
———. 2011a. Food safety conference. http://www.fda.gov/InternationalPrograms/WorkshopsandConferences/ucm240912.htm (accessed November 21, 2011).
———. 2011b. Pathway to global product safety and quality. Silver Spring, MD: FDA.
———. 2011c. PREDICT: Briefing slides for importers and entry filers. Silver Spring, MD: FDA Office of Regulatory Affairs and Office of Regulatory Management.
———. 2011d. Product tracing: Information available related to product tracing under the Food Safety Modernization Act (FSMA). http://www.fda.gov/Food/FoodSafety/FSMA/ucm270851.htm (accessed November 18, 2011).
Feed the Future. 2012. About. http://www.feedthefuture.gov/about (accessed January 23, 2012).
Final agenda: 2007 U.S.-China SPS leadership development program. 2007. Paper read at Third Leadership Development Program in the United States for the People’s Republic of China on U.S. Systems and Approaches for Implementation of the World Trade Organization Agreement on the Application of Sanitary and Phytosanitary Measures, College Park, MD, June 4-July 24.
Food Standards Agency. 2002. Traceability in the food chain: A preliminary study. London, UK: Food Standards Agency, Food Chain Strategy Division.
Foreign officers chosen for hall of fame. 2011. Associated Press, October 4. http://cjonline.com/news/2011-10-04/foreign-officers-chosen-hall-fame (accessed Novemeber 30, 2011).
FSIS (Food Safety Inspection Service). 2009. Fact sheets: FSIS import procedures for meat, poultry & egg products. http://www.fsis.usda.gov/Fact_Sheets/FSIS_Import_Procedures/index.asp (accessed January 25, 2012).
———. 2011. Regulations and policies: Checklist for importing meat, poultry and processed egg products. http://www.fsis.usda.gov/regulations_&_policies/Import_Checklist/index.asp (accessed January 25, 2012).
GAO (Government Accountability Office). 2007. Drug safety: Preliminary findings suggest weaknesses in FDA’s inspection program for inspecting foreign drug manufacturers. GAO-08-224T. Washington, DC: GAO.
———. 2008. Drug safety: Better data management and more inspections are needed to strengthen FDA’s foreign drug inspection program. GAO-08-970. Washington, DC: GAO.
———. 2010a. Drug safety: FDA has conducted more foreign inspections and began to improve its information on foreign establishments but more progess is needed. GAO-10-961. Washington, DC: GAO.
———. 2010b. Food and Drug Administration: Response to heparin contamination helped protect public health; controls that were needed for working with external entities were recently added. GAO-11-95. Washington, DC: GAO.
———. 2011. Drug safety: FDA faces challenges overseeing the foreign drug manufacturing supply chain. GAO-11-936T. Washington, DC: GAO.
Gessner, G. H., L. Volonino, and L. A. Fish. 2007. One-up, one-back ERM in the food supply chain. Information Systems Management 24(3):213-222.
Golan, E., T. Roberts, E. Salay, J. Caswell, M. Ollinger, and D. Moore. 2004. Food safety innovation in the United States: Evidence from the meat industry. Washington, DC: U.S. Department of Agriculture.
GS1. 2009. GS1 Global Traceability Standard for Healthcare (GTSH): Implementation guide. http://www.gs1.org/docs/gsmp/traceability/Global_Traceability_Implementation_Healthcare.pdf.
Hamburg, M. A. 2010. Remarks. Partnership for Safe Medicines Interchange 2010, Washington, DC, October 8.
HaRP (Health Research Program). 2011. Child Health Research (CHR) project achievements. http://www.harpnet.org/chr/chr_achievments.html (accessed November 21, 2011).
Henson, S., and S. Jaffee. 2008. Understanding developing country strategic responses to the enhancement of food safety standards. World Economy 31(4):548-568.
HHS (Department of Health and Human Services). 2011. The global health strategy of the U.S. Department of Health and Human Services. Washington, DC.
Horton, L. R., and E. Wright. 2008. Reconciling food safety with import faciliation objectives: Helping developing country producers meet U.S. and EU food requirements through transatlantic cooperation. Washington, DC: International Food and Agricultural Trade Policy Council.
Hutt, P. B., R. A. Merrill, and L. A. Grossman. 2007. Food and drug law, cases and materials. 3rd ed., University casebook series, Foundation Press.
IFAD (International Fund for Agricultural Development). 2008. The role of high-value crops in rural poverty reduction in the Near East and North Africa. Rome, Italy: IFAD.
IFC (International Finance Corporation). 2006. Simplification of business regulations at the sub-national level: A reform implementation toolkit for project teams. Washington, DC: The World Bank.
———. 2011. About doing business: Measuring for impact. Washington, DC: The World Bank.
IOM (Institute of Medicine). 2010. Enhancing food safety: The role of the Food and Drug Administration. Washington, DC: The National Academies Press.
Islam, R. 2001. Poverty alleviation, employment, and the labor market: Lessons from the asian experience. Paper presented at Asia and Pacific forum on poverty: Reforming Policies and Institutions for Poverty Reduction, Asian Development Bank, Manila, Philippines, February 5-9.
ISO (International Organization for Standardization). 2003. ISO 13485:2003—abstract. Geneva, Switzerland: ISO.
———. 2009. ISO 31000: Risk management—principles and guidelines. Geneva, Switzerland: ISO.
Joint Forces Staff College. 2011. Overview. http://www.jfsc.ndu.edu/schools_programs/if/overview.asp (accessed November 30, 2011).
Jones-Evans, D., M. Klofsten, E. Andersson, and D. Pandya. 1999. Creating a bridge between university and industry in small European countries: The role of the industrial liaison office. R&D Management 29(1):47-56.
Kavilanz, P. 2010. Tylenol plant still plagued by FDA violations. http://money.cnn.com/2010/12/01/news/companies/tylenol_plant_new_problems/index.htm (accessed December 20, 2011).
López, A., and V. M. Cáceres. 2008. Central America field epidemiology training program (CA FETP): A pathway to sustainable public health capacity development. Human Resources for Health 6(27).
Malcolm, S. A., C. A. Narrod, T. Roberts, and M. Ollinger. 2004. Evaluating the economic effectiveness of pathogen reduction technologies in cattle slaughter plants. Agribusiness 20(1):109-123.
McCain, J. 2011. Doing more with less? Past and present challenges for the FDA. Pharmacy and Therapeutics 36(3):145-155.
Mejia, C., J. McEntire, K. Keener, M. Muth, W. Nganje, S. Stinson, and H. Jensen. 2010. Traceability (product tracing) in food systems: An IFT report submitted to the FDA, volume 2: Cost considerations and implications. Comprehensive Reviews in Food Science and Food Safety 9(1):159-175.
Noy, N. F., and D. L. Mcguinness. 2000. Ontology development 101: A guide to creating your first ontology. Stanford University, Stanford, California.
OECD (Organisation for Economic Co-operation and Development). 2002. Regulatory policies in OECD countries: From inverventionism to regulatory governance. Paris: OECD.
———. 2005. OECD guiding principles for regulatory quality and performance. Paris: OECD.
———. 2011. Regulatory policy and governance: Supporting economic growth and serving the public interest. Paris, France: OECD.
Ozersky, J. 2010. The food-safety bill: Flawed, and needed. Time, December 8.
Pendergrast, M. 2010. Inside the outbreaks: The elite medical detectives of the epidemic intelligence service. New York: Houghton Mifflin Harcourt Publishing Company.
Pew Health Group. 2011. After heparin: Protecting consumers from the risks of substandard and counterfeit drugs. Washington, DC: Pew Health Group.
PHAC (Public Health Agency Canada). 2010. Canadian Field Epidemiology Program. http://www.phac-aspc.gc.ca/about_apropos/index-eng.php (accessed July 20, 2012).
Pisanelli, D. M., A. Gangemi, and G. Steve. 1999. Toward a standard for guideline representation: An ontological approach. Proceedings of the AMIA Symposium 906-910.
Pray, C. E., and K. O. Fuglie. 2008. The private sector and international technology transfer in agriculture. In Public-private collaboration in agricultural research, edited by K. O. Fuglie and D. E. Schimmelpfennig. Ames, IA: Iowa State University Press. Pp. 269-299.
Protiviti Inc. 2006. Guide to enterprise risk management: Frequently asked questions. http://www.knowledgeleader.com/KnowledgeLeader/content.nsf/Web+Content/WhitePapers?ArticlesGuidetoEnterpriseRiskManagementFrequentlyAskedQuestions!OpenDocument.
PSM (Partnership for Safe Medicines). 2011. USAID fights fake drugs and helps countries find the fakes. http://www.safemedicines.org/2011/01/usaid-fights-fake-drugs-and-helps-countries-find-the-fakes-126.html (accessed November 21, 2011).
Purdue University. 2011. Making medicine in Sub-Saharan Africa. West Lafayette, IN: Purdue University.
———. 2012. Sustainable medicine program in Tanzania (East & West Africa). http://www.ipph.purdue.edu/sustain/ (accessed January 30, 2012).
Roberts, D., and T. Josling. 2011. Tracking the implementation of internationally agreed standards in food and agricultural production. Washington, DC: International Food and Agricultural Trade Policy Council.
Secure supply chain pilot program; notice of pilot program. 2009. Federal Register 74(10).
Semeniuk, I. 2011. US science agencies dodge deep cuts. Nature 479:455-456.
Silverman, E. 2011. Which drugmaker fails most FDA inspections? http://www.pharmalot.com/2011/03/which-drugmaker-fails-most-fda-inspections/ (accessed November 20, 2011).
Steger, D. 2011. The importance of institutions for regulatory cooperation in comprehensive economic and trade agreements: The Canada-EU CETA. Legal Issues of Economic Integration 39(1).
Stewart, K., and L. Gostin. 2011. Food and Drug Administration regulation of food safety. Journal of the American Medical Association 306(1):88-89.
Thomsen, M. R., and A. M. McKenzie. 2001. Market incentives for safe foods: An examination of shareholder losses from meat and poultry recalls. American Journal of Agricultural Economics 82(3):526-538.
Un oeuf is enough. 2010. Economist, September 2.
U.S. Department of State. 2012. Embassy of the United States, Montevideo, Uruguay: Animal and Plant Health Inspection Service (APHIS). http://uruguay.usembassy.gov/aboutus-offices-aphis.html (accessed April 2, 2012).
USAID (U.S. Agency for International Development). 2011. Press release: U.S. announces support for the Africa-led partnership for aflatoxin control in Africa. Washington, DC: USAID.
USDA. 2002. APHIS factsheet: Safeguarding America’s bounty. Washington, DC: USDA.
———. 2006. International services: International standards setting activities. http://www.aphis.usda.gov/international_safeguarding/international_stand_setting_activities.shtml (accessed September 1, 2011).
———. 2007a. About APHIS. http://www.aphis.usda.gov/about_aphis/ (accessed September 1, 2011).
———. 2007b. History of APHIS. http://www.aphis.usda.gov/about_aphis/history.shtml (accessed September 1, 2011).
———. 2007c. Plant import: Preclearance activities. http://www.aphis.usda.gov/import_export/plants/plant_imports/preclearance_activities.shtml (accessed September 2, 2011).
———. 2010a. International services. http://www.aphis.usda.gov/international_safeguarding/index.shtml (accessed September 2, 2011).
———. 2010b. What happens to the money that APHIS collects through user fees? http://www.aphis.usda.gov/userfees/what_happens.shtml (accessed Septmeber 2, 2011).
———. 2010c. What is a user fee? http://www.aphis.usda.gov/userfees/what_is.shtml (accessed September 1, 2011).
———. 2011. About the Foreign Agricultural Service. http://www.fas.usda.gov/aboutfas.asp (accessed November 18, 2011).
USDA (U.S. Department of Agriculture) and APHIS (Animal and Plant Health Inspection Service). 2007. Importation of fresh blueberry fruit from Uruguay and South Africa into the continental United States (draft). Washington, DC: USDA and APHIS.
USGS (U.S. Geological Survey). 2009. Cooperative Research and Development Agreement (CRADA). http://www.usgs.gov/tech-transfer/what-crada.html (accessed December 22, 2011).
USTR (Office of the United States Trade Representative). 2009. 2009 trade policy agenda and 2008 Annual Report. Washington, DC: USTR.
———. 2011a. Bilateral and regional trade agreements. http://www.ustr.gov/trade-topics/environment/bilateral-and-regional-trade-agreements (accessed November 18, 2011).
———. Cooperative programs. 2011b. http://www.ustr.gov/trade-topics/labor/cooperative-programs (accessed November 21, 2011).
———. 2011c. USTR and FDA welcome collaboration by APEC and the World Bank to enhance food safety and faciliatate food trade. http://www.ustr.gov/about-us/press-office/press-releases/2011/may/ustr-and-fda-welcome-collaboration-apec-and-world-bank (accessed November 18, 2011).
Vijay, N. 2011. Pharma industry needs risk-based approach for plant inspections: Micro labs ed. http://www.pharmabiz.com/ArticleDetails.aspx?aid=65772&sid=1 (accessed October 31, 2011).
White House. 2012. President Obama’s global development policy and global food security. http://www.whitehouse.gov/sites/default/files/Food_Security_Fact_Sheet.pdf (accessed January 23, 2012).
World Bank. 2009. Improving food safety in Arab countries. Washington, DC: The World Bank, FAO, and the International Fund for Agricultural Development.
WTO (World Trade Organization). 2009. Fifth triennial review of the operation and implementation of the Agreement of Technical Barriers to Trade under Article 15.4. Geneva, Switzerland: WTO.
———. 2012. Understanding the WTO: Agreements: Standards and safety. http://www.wto.org/english/news_e/news01_e/sps_oct2001_e.htm (accessed January 26, 2012).
Young, P. B. 2011. Safety first. NGF: Next Generation Food, January 14, 78.










































