MARCH 2-3, 2011
MEETING 1—AGENDA
Keck Building
500 Fifth Street NW
Washington, DC 20001
Day 1 Goals:
1. Introduce the National Academies’ study process
2. Discuss bias and conflict of interest
3. Fully understand this study’s statement of task
4. Learn about the capacity and priorities of the FDA
DAY ONE: WEDNESDAY, MARCH 2, 2011
KECK BUILDING, ROOM 109
| 8:30 | Breakfast Available |
9:00-11:00
SESSION 1—CLOSED
IOM COMMITTEE PROCESS AND CHARGE TO COMMITTEE
Objectives: To review the National Academies’ study process that includes a bias and conflict-of-interest discussion; to discuss the role of the committee in addressing the statement of task; and to ensure the committee understands its statement of task.
| 11:00-11:10 | Break |
SESSION 2—OPEN
QUESTIONS ON STATEMENT OF TASK
| 11:10-11:30 | Project Timeline and Statement of Task Sponsor Representative Introductions Jim Riviere, Committee Chair |
| 11:30-12:15 | Questions to Sponsor |
|
Mary Lou Valdez, Associate Commissioner for International Programs, FDA |
|
|
Kate Bond, Associate Director for Technical Cooperation/Capacity-Building, FDA |
|
| 12:15 | Lunch |
SESSION 3—OPEN
THE FDA PERSPECTIVE
Objective: To learn about the FDA’s current capacity and its international work.
| 12:45 | Welcome the Public and Introduce Commissioner Hamburg Jim Riviere, Committee Chair |
| 12:45-1:05 | Keynote Address: Why is this study important to the FDA? Margaret Hamburg, Commissioner, FDA |
| 1:05-1:25 | Questions |
| 1:25-2:30 | What is the capacity of the FDA Centers? What are the key issues they face in international work? |
|
Deb Autor, Director, FDA Center for Drug Evaluation and Research |
|
|
Karen Midthun, Director, FDA Center for Biologics Evaluation and Research |
|
|
Lillian Gill, Senior Associate Director, FDA Center for Devices and Radiological Health |
|
|
Don Kraemer, Acting Deputy Director for Operations, FDA Center for Food Safety and Applied Nutrition |
| 2:30-2:50 |
How is the FDA already working to build regulatory systems abroad? |
|
Mac Lumpkin, FDA Deputy Commissioner for International Programs |
|
| 2:50-3:20 | Panel discussion with presenters |
| Jane Henney, Moderator | |
| 3:20-3:35 | Break |
SESSION 4—OPEN
CORE ELEMENTS OF REGULATORY SYSTEMS
Objective: To identify the core elements of regulatory systems in developing countries and what gaps exist in these systems.
| 3:35-4:00 | Core Elements of Medical Device Regulatory Systems in Developing Countries |
|
Michael Gropp, Vice President, Global Regulatory Strategy, Medtronic |
|
|
Greg Kalbaugh, Director and Counsel, U.S. Chamber of Commerce, U.S.-India Business Council |
|
| 4:00-4:25 | Core Elements of Food Regulatory Systems in Developing Countries |
|
Ernesto Enriquez, Ministry of Health, Mexico |
|
|
Paul B. Young, Director, Chemical Analysis Operations, Waters Corporation |
|
| 4:25-4:55 |
Core Elements of Drug and Biologics Regulatory Systems in Developing Countries |
|
Jose Luis Di Fabio, Area Manager, PAHO |
|
|
Ekopimo Okon Ibia, Director and U.S. Regulatory Policy Lead, Global Regulatory Strategy, Policy, and Safety, Merck & Co., Inc. |
|
| 4:55-5:45 |
What are the gaps in the systems? A panel discussion with presenters |
|
Martha Brumfield, Moderator |
|
| 5:45 | Adjourn |
DAY TWO: THURSDAY, MARCH 3, 2011
KECK BUILDING, ROOM 110
Day 2 Goals:
1. Learn about existing recommendations and the obstacles to implementing them
2. Make a strategy for how to tackle the statement of task
3. Discuss how to structure the final report
4. Begin considering possible recommendation topics
| 8:00 | Breakfast Available |
SESSION 5—CLOSED
REACTIONS TO PRESENTATIONS AND PLANNING TRAVEL
Objective: To discuss the presentations and plan the travel meetings.
SESSION 6—OPEN
EXISTING RECOMMENDATIONS AND OBSTACLES TO IMPLEMENTATION
Objective: To learn what recommendations have already been made to strengthen regulatory systems and what obstacles exist to implementing these recommendations.
| 10:10-10:30 | The Global Harmonization Task Force |
|
Michael Gropp, Vice President, Global Regulatory Strategy, Medtronic |
|
| 10:30-10:50 | Promoting the Quality of Medicines |
|
Patrick Lukulay, Director, Promoting the Quality of Medicines Program, U.S. Pharmacopeia |
|
| 10:50-11:10 | Capacity Building and the Partnership Training Institute Network |
|
Paul B. Young, Director, Chemical Analysis Operations, Waters Corporation |
|
| 11:10-11:30 | The Global Food Safety Initiative |
|
Mike Robach, Vice President Corporate Food Safety and Regulatory Affairs, Cargill |
| 11:30-11:50 |
The International Medical Products Anti-Counterfeiting Taskforce |
|
Howard Zucker, Senior Advisor, Division of Global Health & Human Rights, Massachusetts General Hospital |
|
| 11:50-12:30 | Lunch |
| 12:30-1:15 | What prevents implementing recommendations? A panel discussion with presenters |
| Tom Bollyky, Moderator |
SESSION 7—CLOSED
DISCUSSION AND STRATEGY FOR THE WAY FORWARD
Objective: To review the previous session, begin discussing recommendations, and give feedback on the meeting.
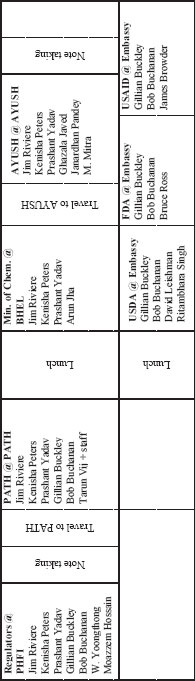
TRAVEL MEETING 1—AGENDA
DAY ONE: WEDNESDAY MAY 11, 2011
CHINESE ACADEMY OF ENGINEERING, BEIJING
SESSION ONE
ORIENTATION
Objective: To explain the study and the purpose of our visit, to exchange introductions with representatives of the Chinese government, and to explain the IOM study process.
| 9:00-9:15 | Welcome |
| Jim Riviere, Committee Chair | |
| 9:15-9:30 | Introductions |
| 9:30-9:50 | Institute of Medicine Process |
| Patrick Kelley, Board Director |
SESSION TWO
FDA’S CHINA PRESENCE
Objective: To learn about the FDA’s work in China.
| 9:50-10:10 | The FDA in China Christopher Hickey, Country Director, FDA |
| 10:10-10:25 | Questions |
SESSION THREE
REGULATOR PANEL
Objective: To how learn about Chinese regulators work and the key issues they face.
| 10:25-12:00 | Panel Discussion, Junshi Chen, Moderator Yinglian Hu, Professor, National Academy of Governance |
|
Ma Yong, Secretary General, China National Food Industry Association |
|
|
Geng Xiao, Director, Columbia Global Center |
|
|
Chen Rui, Deputy Director General, MOH |
|
|
Gao Fang, Deputy Director General, Ministry of Agriculture |
| 12:00-1:00 | Lunch |
SESSION FOUR
REGULATED INDUSTRY PANEL
Objective: To learn how regulated industry works with national, regional, and foreign regulators, how they manage their supply chains, how able they are to comply with standards and harmonization efforts.
| 1:00-2:30 | Panel Discussion, Martha Brumfield, Moderator |
|
Wen Chang, Vice Chairwoman, China Pharmaceutical Quality Association |
|
|
Sun Wei, Director of Scientific and Regulatory Affairs, Coca-Cola China |
|
|
Steve Yang, VP, Head of R&D, Asia and Emerging Markets, AstraZeneca |
|
|
Li Yu, Scientific and Regulatory Affairs, MARS China |
|
|
Penggui Zai, Food Regulatory Affairs Manager, Wahaha Group |
|
|
Libin Zhao, Department of International Regulatory Affairs, Tianjin Tasly Institute |
|
| 2:30-2:40 | Discussion Response Philip Chen, Director, China Health Law Initiative |
| 2:40-2:50 | Break |
SESSION FOUR
DONOR AND INTERNATIONAL ORGANIZATION PANEL
Objective: To explore how international organizations are working on health systems and infrastructure building, and to understand the role of a strong regulatory framework for health, agriculture and economic development.
| 3:00-4:30 | Panel discussion, Jake Chen, Moderator |
| Gerd Fleischer, Food Safety, GIZ | |
|
Zuo Shuyan, Expanded Program on Immunization, WHO |
|
|
Peter Karim Ben Embarek, Team Leader Food Safety and Nutrition, WHO |
|
|
Jiankang Zhang, Country Program Leader, PATH |
|
| 4:30-4:40 | Discussion Response Geng Xiao, Director, Columbia Global Center |
DAY TWO: THURSDAY, MAY 12, 2011
CHINESE ACADEMY OF ENGINEERING, BEIJING
| 7:30-8:15 | Working breakfast for committee and staff at Intercontinental Hotel |
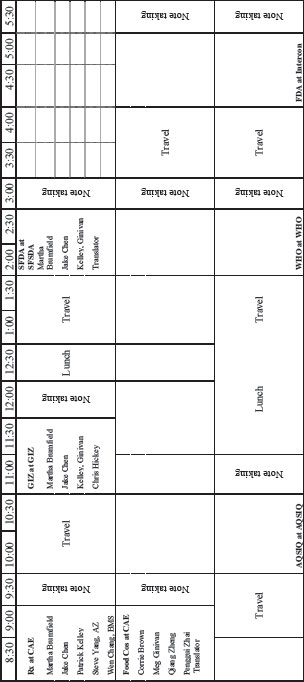
DAY THREE: FRIDAY MAY 13, 2011
CHINA HOTEL, GUANGZHOU
SESSION ONE
ORIENTATION
| 8:30-8:45 | Welcome and introductions Jim Riviere, Committee Chair |
| 8:45-9:00 | Institute of Medicine Process Patrick Kelley, Board Director |
SESSION TWO A
FOOD AND DRUG REGULATION
| 9:00-10:00 | Panel Discussion, Jake Chen, Moderator |
|
Benny Liu, Director Fresh Development, Wal-Mart China |
|
|
Ke Ding, Deputy Director Drug Discovery, Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences |
|
|
Qian Cheng, Deputy Director, South China Center for Innovative Pharmaceuticals |
SESSION TWO B
GUANGDONG FDA
| 9:40-10:00 | Travel to Guangdong FDA |
| 10:00-12:30 | Discussion Chris Hickey, Country Director, FDA Guangdong Provincial Regulators |
SESSION THREE
WRIGLEY FACTORY: SUPPLY CHAIN CASE STUDY
| 10:10-11:00 | Travel to Wrigley Factory |
| 11:00-12:00 | Factory tour |
| 12:00-1:00 | Lunch |
| 1:00-2:00 |
Closing remarks, Thanh Nguyen, Regional Quality Director Asia-Pacific Supply Chain, Wrigley |
SESSION THREE
THE FDA PERSPECTIVE
CHINA HOTEL, GUANGZHOU
| 3:30-5:00 | Panel Discussion, Corrie Brown, Moderator |
| Dennis Doupnik, Investigator, FDA | |
| Dennis Hudson, Consumer Safety Officer, FDA | |
| WeiHua Evid Liu, FDA |
TRAVEL MEETING 2—AGENDA
DAY ONE: MONDAY, JUNE 20, 2011
UNIVERSITY OF SAO PAULO, SCHOOL OF PUBLIC HEALTH, PROFESSOR EDMUNDO JUAREZ ROOM AVENIDA DOUTOR ARNALDO, 715, SAO PAULO
SESSION ONE
ORIENTATION
Objective: To explain the study and the purpose of our visit and to explain the IOM study process.
| 9:00-9:05 | Welcome |
|
Helena Ribeiro, Director, University of Sao Paulo School of Public Health |
|
| 9:05-9:15 | Study Overview Jim Riviere, Committee Chair |
| 9:15-9:25 | IOM Process Gillian Buckley, Study Director |
SESSION TWO
FDA’S LATIN AMERICA PRESENCE
Objective: To learn about the FDA’s work in Latin America.
| 9:25-9:45 | The FDA in Latin America |
|
Ana Maria Osorio, Assistant Regional Director—Latin America, U.S. FDA |
|
| 9:45-10:00 | Questions |
| 10:05-10:20 | Break |
SESSION THREE
REGULATORS’ ROUNDTABLE
Objective: To gain a better understanding of how Latin American regulators work and the key issues they face.
| 10:20-11:20 | Roundtable Discussion, Carlos Morel, Moderator |
|
Renato Spindel, Director, Scult Health Planning and Consultancy Ltda. |
|
|
Amelia Villar, Consultant in Essential Medicine and Biologicals, PAHO |
|
| 11:20-11:30 | Discussion Response |
|
Terezinha de Jesus Andreolli Pinto, Professor, University of Sao Paulo School of Pharmaceutical Sciences |
|
| 11:30-12:40 | Roundtable Discussion, Andy Stergachis, Moderator |
|
Adriana Valenzuela, Head of International Affairs, Division of Livestock Service, Chile Ministry of Agriculture |
|
|
Marta H. Taniwaki, Science Researcher, State Food Technology Institute |
|
|
Claudio Poblete, Professor of Livestock Legislation, Universidad Mayor School of Veterinary Medicine |
|
|
Hector Lazaneo, Division Director, Ministry of Livestock, Agriculture and Fisheries, Uruguay |
|
| 12:40-1:40 | Lunch |
SESSION FOUR
REGULATED INDUSTRY ROUNDTABLE
Objective: To learn how regulated industry works with national, regional, and foreign regulators, how they manage their supply chains, how able they are to comply with standards and harmonization efforts, and what incentives could help them comply with standards and harmonization efforts.
| 1:40-2:45 | Roundtable Discussion, Clare Narrod, Moderator |
|
Rosane Cuber Guimaraes, Good Practices Manager, Department of Quality Assurance, Bio-Manguinhos/Fiocruz |
|
Lauro Moretto, Executive Vice-President, Association of the Pharmaceutical Industry in the State of Sao Paulo |
|
|
Carlos Alberto Goulart, Executive President, Brazilian Association for Importers of Medical Equipment, Products and Supplies |
|
|
Debora Germano, Associate Director of Regulatory Affairs, Pfizer Brazil |
|
| 2:45-3:05 |
Discussion Response |
|
Silvia Storpirtis, Associate Professor, University of Sao Paulo School of Pharmaceutical Sciences |
|
|
Marco Antonio Stephano, Professor, University of Sao Paulo School of Pharmaceutical Sciences |
|
| 3:05-3:20 |
Break |
SESSION FIVE
INTERNATIONAL AND DONOR ORGANIZATION ROUNDTABLE
Objective: To explore how international organizations are working on health systems and infrastructure building, and to understand the role of a strong regulatory framework for health, agriculture, and economic development.
| 3:20-4:30 | Roundtable Discussion, Tom Bollyky, Moderator Raymond Dugas, Regional Food Safety Advisor, PAHO |
|
Sergio Nishioka, Scientist, WHO, Department of Immunization, Vaccines and Biologicals |
|
|
Ana Marisa Cordero Pena, Agricultural Health and Food Safety Specialist, Inter-American Institute for Cooperation on Agriculture |
|
| 5:00 |
Adjourn |
| 6:30-8:00 |
Working dinner for committee members and staff |
DAY TWO: TUESDAY, JUNE 21, 2011
UNIVERSITY OF SAO PAULO, CHOOL OF PBULIC HEALTH—SAO PAULO BRAZIL
| 7:30-8:15 | Working breakfast for committee and staff at the Sofitel |
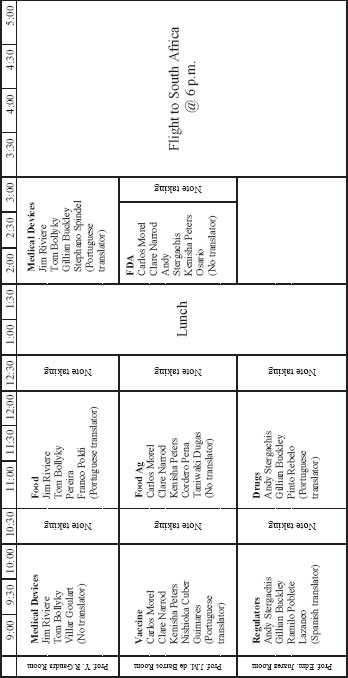
TRAVEL MEETING 3—AGENDA
THURSDAY, JUNE 23, 2011
ACADEMY OF SCIENCE OF SOUTH AFRICA FIRST FLOOR, BLOCK A, THE WOODS, 41 DEHAVILLAND CRESCENT, PERSEQUOR PARK, PRETORIA
SESSION ONE
ORIENTATION
Objective: To explain the study and the purpose of our visit to the participants and to explain the IOM study process.
| 9:00-9:05 | Welcome |
|
Nthabiseng Toale, Program Manager, Academy of Science of South Africa |
|
| 9:05-9:15 | Study Overview |
| Jim Riviere, IOM Committee Chair | |
| 9:15-9:25 | IOM Process |
| Patricia Cuff, IOM Senior Program Officer | |
| 9:25-9:35 | Questions |
SESSION TWO
FDA’S AFRICA PRESENCE
Objective: To learn about the U.S. FDA’s work in Africa.
| 9:35-9:45 | The FDA in Africa |
|
Beverly Corey, Senior Regional Advisor for Africa, U.S. FDA |
|
| 9:45-9:55 | Questions |
SESSION THREE
REGULATORS’ ROUNDTABLE
Objective: To understand how African regulators work and the key issues they face.
| 9:55-11:10 | Roundtable Discussion, Andy Stergachis, Moderator |
|
Margareth Ndomondo-Sigonda, Pharmaceutical Coordinator, New Partnership for Africa’s Development, African Union |
|
|
Derek Litthauer, Director, National Control Laboratory for Biological Products, University of the Free State |
|
|
Robert Crookes, Acting Medical Director, South African National Blood Service |
|
| 11:10-11:20 | Discussion Response |
|
Nicholas Crisp, Managing Director, Benguela Health Pty Ltd. |
|
| 11:20-11:35 | Break |
| 11:35-12:35 | Roundtable Discussion, Clare Narrod, Moderator |
|
Malose Daniel Matlala, Deputy Director Food Control, Department of Health |
|
|
Pieter Truter, Technical Specialist, National Regulator for Compulsory Specifications |
|
|
Raymond Wigenge, Director of Food Safety, Tanzania Food and Drugs Authority |
|
|
Sarah Olembo, Technical Expert Sanitary and Phytosanitary Issues and Food Safety, African Union Commission |
|
| 12:35-12:45 | Discussion Response |
| Nick Starke, Chairman, International Life Sciences Institute | |
| 12:45-1:45 | Lunch |
SESSION FOUR
REGULATED INDUSTRY ROUNDTABLE
Objective: To learn how regulated industry works with national, regional, and foreign regulators, how they manage their supply chains, how able they are to comply with standards and harmonization efforts, and what incentives could help them comply with standards and harmonization efforts.
| 1:45-3:00 | Roundtable Discussion, Tom Bollyky, Moderator |
|
Elaine Alexander, Executive Director, South Africa Table Grape Industry |
|
|
Maeve Magner, Chief Executive, RTT |
|
Skhumbuzo Ngozwana, President, South African Generic Manufacturers’ Association |
|
|
Kirti Narsai, Head of Scientific and Regulatory Affairs, Pharmaceutical Industry Association of South Africa |
|
|
Raymonde de Vries, Corporate Quality Assurance, Unilever Foods |
|
| 3:00-3:15 | Break |
SESSION FIVE
INTERNATIONAL AND DONOR ORGANIZATIONS ROUNDTABLE
Objective: To explore how international organizations are working on health systems and infrastructure building, and to understand the role of a strong regulatory framework for health, agriculture, and economic development.
| 3:15-4:30 | Roundtable Discussion, Jim Riviere, Moderator |
|
Gavin Steel, Senior Program Associate, Strengthening Pharmaceutical Systems, Management Sciences for Health |
|
|
Celestine Kumire, Programme Manager, Southern African Regional Programme on Access to Medicines & Diagnostics, John Snow Inc. |
|
|
Henry Leng, Senior Researcher, Accessing Medicines in Africa and South Asia |
|
|
Nick Starke, Chairman, International Life Sciences Institute |
|
|
Sarah Simons, Executive Director, Center for Agriculture and Bioscience International |
|
| 4:40-4:50 | Discussion Response |
|
Sarah Olembo, Technical Expert SPS and Food Safety, African Union Commission |
|
| 5:00 | Adjourn |
FRIDAY, JUNE 24, 2011
ACADEMY OF SCIENCE OF SOUTH AFRICA
| 7:45-8:30 | Working breakfast for committee members and staff at Illyria Hotel |
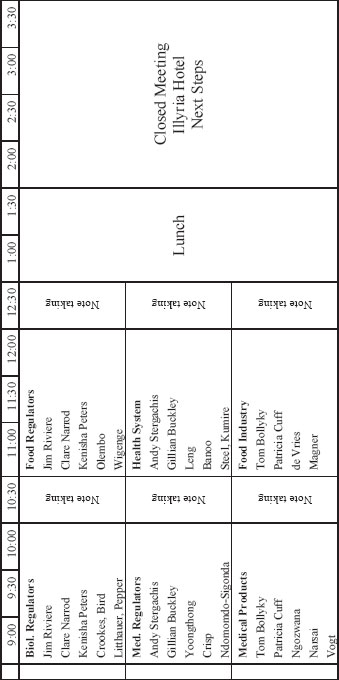
MEETING 2—AGENDA
JULY 27-28, 2011
DAY ONE: WEDNESDAY, JULY 27, 2011
THE KECK BUILDING, ROOM 201
| 8:30 | Breakfast available |
SESSION 1—OPEN THE GLOBAL SYSTEM AND SUPPLY CHAIN
Objectives: To understand the depth and breadth of the publically available enforcement data, and the use of information technology for international surveillance, operations, and supply chain management.
| 9:00-9:15 | Welcome and orientation Jim Riviere, Committee Chair |
| 9:20-9:50 | Systems Mapping with EU and FDA Enforcement Data |
| Ying Zhang, PhD Candidate, Georgetown University Jake Chen, Committee Member |
|
| 9:50-10:10 | Questions |
| 10:10-10:40 | Global Information Technology Management |
|
Noel Greis, Director, Kenan Institute of Private Enterprise, University of North Carolina at Chapel Hill |
|
| 10:40-11:00 | Questions |
| 11:00-11:15 | Break |
SESSION 2—CLOSED
REPORT OUTLINE
Objective: To approve an outline for the final report, to assign sections.
SESSION 3—CLOSED
TRAVEL MEETING DEBRIEF
Objective: To review the themes that emerged in China, South Africa, and Brazil focusing on statement of task questions 1-5.
DAY TWO: THURSDAY, JULY 28, 2011
THE KECK BUILDING, ROOM 109
| 8:30 | Breakfast available |
SESSION 1—CLOSED
BIAS AND CONFLICT OF INTEREST REVIEW
Objective: To review bias and conflict-of-interest discussion.
SESSION 2—CLOSED
CORE ELEMENTS OF REGULATORY SYSTEMS
Objective: To draft recommendations on statement of task item A and questions 4, 5.
SESSION 3—CLOSED
BRIDGING THE GAPS IN REGULATORY SYSTEMS
Objective: To draft recommendations on statement of task item C and questions 6-9.
SESSION 4—OPEN
TELECONFERENCE WITH ANVISA
Objective: To learn about the Brazilian regulatory system
SESSION 5—CLOSED
A PLAN FOR THE FDA
Objective: To draft recommendations on statement of task items B, D, and F.
SESSION 6—CLOSED
PARTNERSHIPS
Ojective: To draft recommendations on statement of task item E and questions 10-13.
TRAVEL MEETING 3—AGENDA
AUGUST 31-SEPTEMBER 2, 2011
DAY ONE: WEDNESDAY, AUGUST 31, 2011
PUBLIC HEALTH FOUNDATION OF INDIA. VASANT KUNT. NEW DELHI
| 7:45-8:30 | Working breakfast for committee members and staff at the Crowne Plaza Hotel |
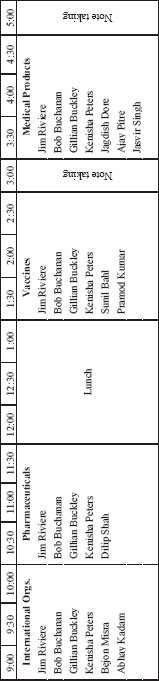
DAY TWO: THURSDAY, SEPTEMBER 1, 2011
TRAVELLING MEETINGS
| 7:15-8:00 | Working breakfast for committee members and staff at the Crowne Plaza Hotel |
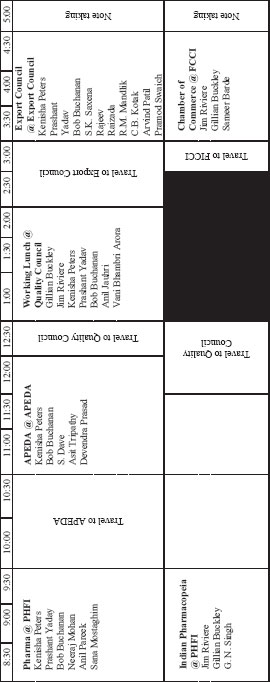
DAY THREE: FRIDAY SEPTEMBER 2. 2011
TRAVELLING MEETINGS
| 7:15-8:00 | Working breakfast for committee members and staff at the Crowne Plaza Hotel |