Core Elements of Regulatory Systems
Having safe food and medical products is a cornerstone of public health around the world. The U.S. Food and Drug Administration (FDA) protects U.S. consumers from tainted products, and increasingly it works with its counterpart agencies abroad to the same ends. Before identifying the common gaps in developing country food and medical product regulatory systems, and before making a strategy to bridge these gaps, the committee identified the core elements of a functional regulatory system. The committee concluded that the most basic elements of a regulatory system are the same around the world. Therefore, the core elements of regulatory systems are the same in developed and developing countries. This chapter describes these elements as well as the minimal pieces necessary for an effective food and medical product regulatory system.
There is more than one right way to organize a regulatory system. In its analysis, the committee considered a number of different policy and administrative tools governments can use to ensure the safe manufacture, labeling, distribution, and marketing of food and medical products. While the mechanisms employed can vary between countries, effective regulatory programs have a number of common characteristics.
This chapter lays out the characteristics and practical elements of a good regulatory system. It also describes the minimal elements necessary to ensure food and medical product safety. It provides an overview of the organization of food and medical product regulatory systems in developing countries, paying special attention to the importance of harmonized standards in these countries. The importance of international cooperation among regulators is introduced; the committee will concentrate on this
theme throughout the report. This chapter ends with a discussion of two important points of international cooperation: the use of risk and hazard analysis in food safety and the regulation of active pharmaceutical ingredients (APIs).
COMMON ATTRIBUTES OF EFFECTIVE REGULATORY PROGRAMS
The first step in understanding the core elements of food and medical product regulatory systems is identifying the underlying attributes of successful systems. The committee identified five main characteristics of good systems: they should be responsive, outcome-oriented, predictable, risk-proportionate, and independent. These attributes are consistent with those outlined in the World Trade Organization (WTO) Agreement on the Application of Sanitary and Phytosanitary Measures (SPS), especially in their emphasis on the protection of human, plant, and animal health without the application of regulations that would “arbitrarily or unjustifiably discriminate between countries where identical or similar conditions prevail” (WTO, 1998). Similarly, the WTO relies on a scientific evidence base for decision making. Its preference is to use international standards whenever possible, but does allow countries to set their own standards so long as their standards comply with the basic tenets of the WTO rules.
The major attributes the committee identified are common to all highly functioning regulatory systems. These attributes are not the system’s main duties, which will be discussed later, but are scientific and philosophical underpinnings of a robust system.
Responsive
The responsiveness of a regulatory system involves two related functions. The first is the ability to respond rapidly to a crisis. The regulatory system should be able to contain and correct any product safety lapse that has occurred, minimizing the health effects. Responsiveness also includes the ability of the system to promptly modify its policies. Responsive regulation keeps pace with the emergence of new hazards, changes in technology, expanding evidence base, and evolving consumer expectations. This attribute also includes the ability of the system to stay up-to-date and knowledgeable about new science. Responsiveness refers to the ability of the regulatory agency to continually expand its knowledge base, to be a learning organization that has internal scientific depth and effective collaboration with academics, and to draw on the technical and business expertise of regulated industry.
Outcome Orientation
A robust regulatory system focuses on product safety outcomes, not on the details of how to arrive at the outcomes. That is not to say that a strong system is not concerned with process. On the contrary, strong regulatory systems often stipulate manufacturing standards and inspection processes. Rather, the outcome-oriented system issues regulations that do not get in the way of innovation. Furthermore, in an outcome-oriented system, industry has a clear avenue to petition the regulatory authority to use alternative processes, and this process is not unduly onerous. An outcome-oriented regulatory agency has the scientific expertise to be abreast of changes in food and medical product technology and the modern equipment to analyze it.
Predictable
The regulatory agency has a clear framework guaranteeing that the regulators’ decisions are neither arbitrary nor capricious. Predictable regulatory systems make their procedures readily available to the public. The rules are applied consistently, enforced fairly, and are based on the best scientific evidence available at the time of the decision. Predictability assumes a level playing field and describes a function in the regulatory system that is vigilant against bias. A fair and predictable system does not work for or against large industry or small industry; regulations are applied the same way to imported and domestic products.
Proportional
A proportional or risk-based system allocates controls based on threat to public health: product lapses with serious health consequences are monitored stringently, while those with few or insignificant risks receive less attention. Products with similar risks are regulated in similar ways. Proportionality depends first and foremost on the ability of the regulatory agency to assess risk. It also assumes that the agency will consider a cost-benefit analysis when measuring the impact of potential risk management options. A proportional regulatory system actively sets priorities ensuring that the agencies’ programs give the most attention to the most pressing public health threats.
Independent
Regulatory policies are the combination of scientific decisions and societal expectations. This is especially true of the system’s legislative oversight. However, once its legal authority is set, the agency functions best when it
is independent of the political process. The predictability of a regulatory system also relies on independence: regulated industry cannot predict how and when regulations will be enforced if the enforcer changes every time the political regime does. Consumer trust depends on independence. The public needs to know that the agency is devoted to its best interests and is not unduly influenced by politics or money.
Approaches to Regulation
Good food and medical product regulation strikes a balance between protecting public health and not unfairly restricting market access. To this end, governments need to ensure that companies comply with regulations, but governments do not function alone. Industry, academia, and consumers give important feedback to the regulatory system, which includes the legal and health systems. The best regulatory model is one that engages all stakeholders. Box 2-1 describes approaches to food and medical product regulation that emphasize the role of consumers and regulated industry.
CORE ELEMENTS OF A STRONG REGULATORY SYSTEM
Responsiveness, outcome orientation, predictability, proportionality, and independence are the underpinnings of strong food and medical product regulatory systems. A regulatory system grounded in these values will be able to execute its core responsibilities. The main duties of a medical product regulatory system are: the registering of medicines; the publishing of clear requirements for licensure; the provision of unbiased information; market entry notification; safety and effectiveness surveillance; quality control testing; the inspecting of manufacturers for compliance with good manufacturing practices; inspection of distributors; and the evaluation of performance through authorized trials (WHO, 1999, 2001, 2003a). In countries that manufacture vaccines, the regulatory authority is also responsible for the systematic lot release of the vaccine (WHO, 1999). The main duties of a food regulatory system are: providing unbiased education and advice to all stakeholders; inspecting food production and processing plants; evaluating hazard analysis and critical control point (HACCP) plans; physical, chemical, and microbiological analysis of food and food additives; and epidemiological surveillance (FAO/WHO, 2003). Essentially, the regulatory system is an important piece of the public health system. By providing manufacturers and producers with unbiased information, guaranteeing the use of best practices, and inspecting producers and manufacturers, the regulatory authority protects the safety of food and medical products.
The committee also identified the core elements of a food and medical product regulatory authority system, as described in Box 2-2. Whether
BOX 2-1
Approaches to Regulation
Informational Approaches
One technique for ensuring strong product safety regulation is education. An informational approach directly educates the consumer on product safety and assumes that consumers will use this information to make the best decisions for themselves. This approach assumes that having more information, particularly about the concealed characteristics of products, will cause people to change their behavior and buy safe products (IOM, 2006). Governments, industry, academia, or other organizations can provide this information, although the British government’s Department for Business, Innovation, and Skills explains that consumer education is best done by non-governmental organizations, leaving the government free to enforce regulations (BIS, 2011a). Labeling, warnings, and safety rating systems are important tools in informational regulation (BIS, 2011a; Sunstein, 2011).
Market Approaches
Market approaches to regulation use monetary rewards and punishments to modify behavior. Market regulation gives government control of the final regulatory action while putting industry in charge of the route there. Proponents of the market approach maintain that market controls also empower consumers to make their own cost-benefit estimates and choose products based on these decisions. Taxes on products with negative externalities, such as soft drinks, are a market approach to regulation. Bonded warranties are another market tool sometimes used in food and drug regulation to ensure product safety (BIS, 2011b; Office of Information and Regulatory Affairs, 2003).
Regulated Standards
Government regulatory authorities choose and enforce product standards based on scientific evidence. They use either performance standards or design standards. Performance standards set the final product requirements, but do not mandate the techniques industry must use to meet these standards. Design standards dictate the means as well as the end product of the product requirements. Proponents of performance standards explain that they encourage innovation, while advocates of design standards emphasize the process as much as the product (IOM, 2006; Office of Information and Regulatory Affairs, 2003).
BOX 2-2
Elements of a Food and Medical Product Regulatory System
Government is the foundation for a strong regulatory system. As the national standard-setting body, governments:
• use science and risk as a basis for developing policy;
• participate in international cooperation and harmonization of standards;
• make ethical decisions; and
• recognize, collect, and transmit evidence when breaches of law occur.
A food and medical product regulatory system integrates:
• product safety through good manufacturing, clinical, laboratory, and agricultural practices;
• staff development and training for employees;
• monitoring and evaluation of product quality using laboratories;
• inspection and surveillance of products throughout the supply chain;
• risk assessment, analysis, and management; and
• emergency response.
Protecting the public’s health is crucial in a food and medical product regulatory system. The system needs to quickly communicate information to the public in emergencies to ensure the public’s safety.
SOURCES: Ratanawijitrasin and Wondemagegnehu, 2002; WHO, 2003a; WHO Regional Committee for Africa, 2006.
some or all of the core elements noted in Box 2-2 are part of a country’s regulatory system depends heavily on wealth and infrastructure. It is difficult for companies to manage their supply chains without reliable transportation systems, for example. Political will to enforce product safety laws can also be variable. A more detailed analysis of the issues developing country regulators face in implementing safety controls follows in Chapter 3.
MINIMAL ELEMENTS OF A REGULATORY SYSTEM
The ideal regulatory system described above depends on funding, infrastructure, workforce, and political commitment. One or more of these is usually missing in low- and middle-income countries (Brown et al., 2006). With this in mind, the committee also identified the minimal elements of a regulatory system that protects public health and ensures product safety.
The minimal elements of regulation should be the top priority for developing countries trying to build product safety systems.
A Rule-Making System
The minimal requirements outlined in Box 2-3 focus on the process regulators use to make regulations and on the data they use to enforce them. An open rule-making system ensures that people governed by a new regulation have a chance to publically comment on it (U.S. Department of State, 2011). Through an open rule-making process, consumers and industry are informed of proposed food and medical product regulations before they take effect. An open system for rule making involves stakeholders in a regulatory dialogue and lays the groundwork for risk communication.
For example, a rule-making process is critical to establishing effective food safety laws. In many developing countries there are food safety rules in place, but there is a lack of processes to ensure their implementation and effectiveness. In Canada, the European Union (EU), and the United States, rule processes have been established to assess risks, analyze cost-benefit and cost-effectiveness, and assess the environmental impact of food safety regulations (European Commission, 2011; Federal Crop Insurance Reform and Department of Agriculture Reorganization Act of 1994, P.L. 103-354;
BOX 2-3
Minimal Elements of a Regulatory System
At a minimum, a food and medical product regulatory system should include:
• an established process for rule making;
• a protocol to coordinate work within and across the agencies responsible for regulation, especially during a crisis;
• a system for stakeholder public comment on regulations and the review process;
• a way to identify when a regulatory action is necessary; and
• a means to enforce its regulations.
To this end, regulators need to have surveillance data and understand their data sources. They should also have a strong enough understanding of their system’s weaknesses that they can identify data gaps and know what assumptions to make about unknowable data and when to rely on the private sector for additional information. Crisis early warning systems are invaluable tools to make the most of limited surveillance data.
Health Canada and Canadian Food Inspection Agency, 2007; National Environmental Policy Act of 1969, P.L. 91-190). Many developing countries including China, India, the Philippines, Vietnam, and Thailand are in the process of reorganizing their food safety systems (Fairclough, 2009; FAO, 2004; Ramos and Oblepias, 2002; Smart, 2011; WHO, 2009). Box 2-4 discusses the changes India has made to its food safety rules to develop a farm-to-table approach.
Openness in rule making is not part of the political tradition in some countries, particularly those with one-party governments or an authoritarian history (Dalpino, 2000). This is a challenge to the regulation of food and medical products. By definition, food and medical product safety laws govern regulated industry; it is imperative that the regulated understand
BOX 2-4
Rule Making in India
In 2006, the Indian government established the Food Safety and Standards Act. The act “aims to establish a single reference point for all matters relating to food safety and standards” (Palthur et al., 2009). This act formed the Food Safety and Standards Authority of India (FSSAI). FSSAI’s mandate is to consolidate previous food laws, make science-based food standards, and regulate and monitor the manufacturing, processing, storage, distribution, sale, and import of foods to ensure safe food for human consumption (FSSAI, 2011; Palthur et al., 2009).
Although India has made strides to improve its food safety rules, implementing the rules is difficult. Small-scale producers, who do not know how to make the changes required by law, cannot comply with the act (Palthur et al., 2009). Some producers are unclear about the terms of the act; others simply cannot implement HACCP and good manufacturing processes. In a 2010 survey of the Indian food processing sector, 28.51 percent of respondents identified bottlenecks in the implementation of food safety laws as a concern (FICCI, 2010). Many of the quality-control laboratories lack proper equipment and reliable power, complicating the task of surveillance. There are also not nearly enough of them (Palthur et al., 2009).
In January 2011, the FSSAI published a final draft of the Food Safety and Standards rules. In the draft, it specifies how to implement the rules established by the 2006 Food Safety and Standards Act. The new rules replace the country’s outdated food adulteration rules from 1955 (Singh, 2011). Other changes include the establishment of state-level licensing authorities, the addition of batch numbers to processed foods for easy product recalls, laboratory expansion, and the requirement for producers to have a surveillance plan (Smart, 2011). India is clearly making progress toward a farm-to-table approach.
the rules governing them. Without a public comment period it is doubtful manufacturers are even aware of the regulations affecting them. Regulators are also held back if they cannot receive feedback on their practices from a range of stakeholders. In the United States, for example, there is an open comment period where all stakeholders can submit comments on proposed rules, including those of the FDA (Regulations.gov, 2012).
Involving All Stakeholders
Part of the problem in developing countries is that the food and, to a lesser extent, the medical products industry are made up of many small producers. In China, 80 percent of food producers have fewer than 10 employees (Roth et al., 2008); India has more than 20,000 drug manufacturers (KPMG International, 2006). Involving so many stakeholders in public forums is challenging, especially when the communication system is not strong and the tradition of two-way comment on law making is not entrenched. Chapter 3 will discuss these gaps in more detail.
Legal Basis to Enforce Regulations
The end goal of an orderly rule-making process that involves industry, government, consumers, and academics, is to have a set of enforceable regulations. The committee believes that, at a minimum, the regulatory agency needs to have the legal authority to enforce its rules. To this end, the regulations must be enforceable by the national regulatory authority. Box 2-5 describes the goals of food and medical product legislation. As Chapter 3 describes, many developing countries have problems with enforcing or even developing coherent product safety laws.
FOOD AND MEDICAL PRODUCT REGULATION IN DEVELOPING COUNTRIES
There is a continuum of regulatory capacity in the world. On one end, there are the so-called stringent regulatory agencies of the United States, Canada, Western Europe, Australia, New Zealand, and Japan, with high standards and consistent enforcement. These agencies may struggle to monitor all of their regulated products, especially their imports, but they are nonetheless considered gold standards for product safety. At the other end, there are the regulatory agencies of the least developed countries, many in sub-Saharan Africa and in South and Southeast Asia that may not have a single food control laboratory or a system for medicines registration, one of the most basic functions of a drug regulatory authority. In the middle there are many emerging manufacturing nations including India, China, Brazil,
BOX 2-5
Food and Medical Product Legislation
The World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) give good guidance on what a food and medical product legal framework should include. The following summary of the role of food and medical product safety legislation comes from the WHO’s Effective Medicines Regulation: Ensuring Safety, Efficacy and Quality, and the FAO and WHO’s Assuring Food Quality and Safety: Guidelines for Strengthening National Food Control Systems.
• State the purpose of regulation;
• Define the categories of products and activities to be regulated;
• Ensure legal provision for the creation of a regulatory authority;
• Coordinate responsibilities when the regulatory authority includes more than one agency;
• Create mechanisms for ensuring transparency and accountability of regulation;
• Define the roles, responsibilities, rights, and functions of all parties involved in the manufacture and trade of food and medical products, and also in the use of medicines;
• Set the qualifications and standards required for all those who handle medicines and biologics;
• Define the norms, standards, and specifications to be applied in assessing product quality, safety, and (in the case of medicines and biologics) efficacy;
• Include clear provisions that the primary responsibility for product safety and quality lies with the producers and processors;
• State the terms and conditions for suspending, revoking, or canceling activity and product licenses;
• Define prohibitions, offenses, penalties, and legal sanctions;
• Create mechanisms for government oversight to assess implementation of regulations;
• Recognize the country’s international obligations in relation to trade; and
• Include provisions for the rights of consumers to have access to accurate information.
SOURCES: FAO/WHO, 2003; WHO, 2003b.
South Africa, Mexico, and Thailand, representatives of which the committee heard from during this study. These countries are leaders in regional harmonization efforts, but they have problems with training and regulatory infrastructure. Across low- and middle-income countries there are some common gaps in the ability to enforce standards, monitor producers for use
of best practices, run surveillance systems, issue product recalls, or respond to emergencies. Chapter 3 will describe these gaps.
Medical Products Regulatory Oversight
The Organisation for Economic Co-operation and Development (OECD) Regulatory Policy Committee stressed the importance of regulatory oversight bodies in government to ensure fair and quality regulation (OECD, 2009).
Drug and Vaccine Regulation and WHO Prequalification
Oversight bodies are a core element of proper food and medical products regulation, yet according to the WHO, less than 20 percent of its 191 member states have well-developed drug regulatory agencies (Ratanawijitrasin and Wondemagegnehu, 2002). In the African region, the WHO found that only 10 percent of medicines regulatory authorities have a full system in place (WHO Regional Committee for Africa, 2006). Box 2-6 describes how South Africa restructured its drug regulatory authority.
In some countries, the WHO Prequalification Programme is an important piece of the drug and vaccine regulatory oversight. This program was established in 2001 to ensure that the medicines supplied by United Nations (UN) agencies were safe and efficacious (WHO, 2010). The prequalification process has five steps. First, the WHO or another UN agency invites drug or vaccine companies to submit a product for prequalification. Products considered are either on the WHO essential medicines list, applying for inclusion on the WHO essential medicines list, or recommended for use in a WHO treatment guideline (WHO, 2010). The manufacturer submits a dossier on the product’s safety and efficacy, and a team of experts from regulatory agencies around the world reviews the dossier. Next, inspectors verify that the factory, laboratories, and research mentioned in the dossier all meet international best practices. If the manufacturer passes all inspections, the WHO grants prequalification (WHO, 2010).
Originally, WHO prequalification was granted only to HIV, tuberculosis, and malaria drugs (WHO, 2010). Now UN agencies procure more than 240 medicines, vaccines, and contraceptives through the prequalification program (WHO, 2010, 2011e). As part of the program, the WHO provides training for national regulators and for manufacturers from private companies, and improves quality control laboratories in developing countries (WHO, 2010). In places where the regulatory authority lacks technical depth, this program is a welcome guarantee of medicines quality.
In countries that manufacture vaccines, WHO prequalification depends on the country having a competent national regulatory authority (Brhlikova
BOX 2-6
Restructuring the Drug Regulatory Authority in South Africa
For years, the Medicines Control Council (MCC) of South Africa has worked under cumbersome legislation. Because of a poor legislative framework and complex emerging health products, the council had become inefficient and ineffective, with extensive backlogs delaying regulatory decisions on vital medicines (Report of the ministerial task team, 2008). Thus, to significantly improve drug regulation in South Africa, the government is restructuring the council. A rigorous review of other national models and global regulatory trends guided the formation of the South African Health Products Regulatory Authority (Ministry of Health of South Africa, 2008).
The new regulatory authority is different from the MCC in many ways. Consistent with other mature and credible national regulatory authorities, it will regulate medical devices and in vitro diagnostics as well as medicines. The agency will be a national public entity, meaning that it is outside of any specific department but still part of the government. This will help ensure the agency’s independence and foster public confidence in its objectivity. Its staff will inspect all plants and enforce regulations on all health products, but it will not be involved in pricing, procurement, distribution, wholesaling, or logistics (Crisp, 2011a).
The restructuring process is ongoing. The parliament passed the Medicines Amendment Act in 2008, and President Motlanthe signed it into law in 2009. However, the implementation team is still working out practical details about the body’s management. The transition is expected to begin in late 2011 (Crisp, 2011b).
The large-scale restructuring of the medicines regulatory system in South Africa has far-reaching benefits. In addition to the great benefits within South Africa, the development of the new agency is a great opportunity for sharing training, information technologies, inspection, and enforcement within the Southern African region (Crisp, 2011a). Still, growing pains come with the transition. The new agency now has far too few staff and relies heavily on part-time consultants. It will also take time to get the electronic medicines management system working. Most importantly, the agency’s registrar is a subordinate employee in the health ministry and lacks the authority of a chief executive.
et al., 2007). The United Nations Children’s Fund (UNICEF) procures vaccines for 80-100 countries in the world; 64 of the poorest receive all of their vaccines from UNICEF (Rosnbom, 2010; UNICEF, 2011). The WHO has programs to strengthen oversight from national regulatory authorities, and it gives highest priority to those emerging manufacturing nations that supply vaccines to many other countries; their second priority is building the
systems of countries that procure directly from manufacturers without going through UN procurement (WHO, 2011b). Countries that rely fully on UN procurement are lower priority. Though vaccines make up only about 3 percent of the global pharmaceutical market (Wilson, 2010), they require disproportionate regulatory oversight, especially for postmarketing surveillance. Inadequate regulatory oversight of vaccines is a common problem in low- and middle-income countries, and one discussed more in Chapter 3.
The WHO prequalification of vaccine manufacturers in China was not granted easily. In March 2011 the WHO recognized China’s regulatory system for complying with its vaccine production standards. During an approval period that took 19 months, China’s regulators worked to develop a plan that followed the WHO’s advice on how to strengthen vaccine regulation. A team of experts from various countries assessed China’s regulatory system against WHO indicators. Meeting international standards created opportunities for China to apply for WHO prequalification and in the next few years supply vaccines to UN agencies (WHO, 2011d).
Medical Device Regulatory Oversight
The regulation of medical devices is more variable in low- and middle-income countries. The regulation of devices is often in the purview of the drug regulatory authority. In the United States and Europe, the national drug regulatory authorities have monitored device safety since the mid-1970s; Australia, Canada, and Japan followed course in 1989, 1998, and 2002, respectively (Gropp, 2011). Today, 85 countries regulate devices (Gropp, 2011). Figure 2-1 describes the varying levels of comprehensiveness and international consistency or harmonization in different countries’ device regulation (Gropp, 2011). In many parts of the world, devices are overwhelmingly imported; Latin American countries import more than 80 percent of their medical devices (PAHO, 2010a). Even countries with a strong manufacturing base import most medical devices: India imports about 75 percent of them; Malaysia imports 90 percent (Chigullapalli and Tandulwadikar, 2011). Marketing and postmarket oversight of medical devices is important nonetheless, yet often neglected.
Food Regulatory Oversight
Food safety regulatory oversight is historically more complicated than medical product regulation. In the United States, as in other countries, the government constructed a food safety system in pieces as its understanding of foodborne hazards grew (Dyckman, 1999). Around the world, the food regulatory authority often rests with both the departments of health and agriculture. There is a trend in many parts of the world to combine
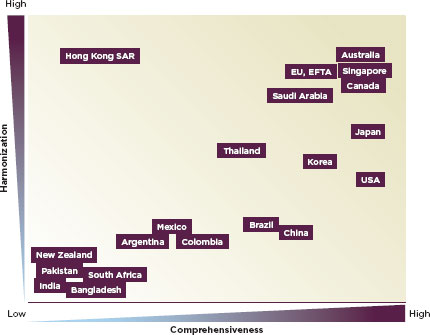
FIGURE 2-1
Conceptual qualitative overview of current national medical device regulatory systems trends.
SOURCE: Gropp, 2011.
all aspects of food regulation into the purview of one agency. Canada, Denmark, Ireland, the United Kingdom, Germany, the Netherlands, and New Zealand have all consolidated their regulatory authority in the last decade (GAO, 2005). Nevertheless, regulatory oversight for food is commonly split between health and agriculture sectors.
HARMONIZATION
Harmonization of food and medicines regulations can both increase product safety and promote trade. When countries harmonize their systems, they eliminate the need for redundant testing and reduce registration times, allowing products to enter the market more quickly (Lelieveld and Keener, 2007). Harmonization facilitates fair competition, thereby promoting trade. It can also ensure that imports meet internationally recognized standards for quality and safety (Anand et al., 2010). Because of globalization, inter-
national harmonization of product standards is increasingly important (ICDRA, 2010).
Harmonization is of particular value in developing countries where there are infrastructure problems and deficits in regulatory laws. Harmonized and simplified requirements for medicines registration can ensure life-saving medicines are more quickly available in poor countries (Ndomondo-Sigonda and Ambali, 2011). Figure 2-2 describes the value of harmonizing medicines regulations to different stakeholders. Many regional economic communities are active in harmonizing their food and medical product regulations.
Since 1980, the WHO has provided a forum for drug regulatory authorities to come together and discuss strengthening their collaborations (WHO, 2011c). The Pan American Health Organization (PAHO) is also a leader in the Americas in medicines regulatory harmonization. PAHO is conducting technical cooperation projects in conjunction with national regulatory authorities and national control laboratories in the regulation and control of pharmaceutical products, vaccines, and other biologics (PAHO, 2010b).
The Association of Southeast Asian Nations (ASEAN) is active in harmonization in Southeast Asia. In 1992, the ASEAN economic ministers formed the Consultative Committee for Standards and Quality with the goal of harmonizing regulation across multiple sectors, including food and medical products (Ramesh and WG3 Chair). ASEAN’s regulatory harmonization focuses on four areas: quality, efficacy, safety, and administration. The committee has established guidelines for many aspects of food and medicines regulation, including labeling, pesticide control, and traditional
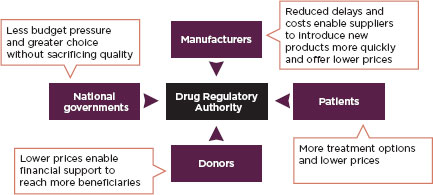
FIGURE 2-2
Benefits of harmonization by stakeholders.
SOURCE: Ndomondo-Sigonda and Ambali, 2011.
medicines. It can be difficult to put the guidelines into practice, however. ASEAN member countries range from some of world’s least developed (Cambodia and Laos) to some of the most developed (Singapore). ASEAN standards are more stringent than the national standards of some of its member states. However, through collaboration and capacity building, the member states have developed a system of using different timelines for implementing different standards based on national readiness (ASEAN, 2005; Ratanawijitrasin and Wondemagegnehu, 2002).
The African Medicines Regulatory Harmonization Initiative (AMRHI) is an effort to strengthen regulatory capacity in Africa (Ndomondo-Sigonda and Ambali, 2011). Within Africa, there are several ongoing harmonization efforts. The Southern African Development Community, the East African Community, the Economic and Monetary Union of West Africa, the Economic Community of West African States, and the Economic Community of Central African States all work on harmonization. The AMRHI coordinates the process (AMRH, 2010). Regional partners in Africa work around different languages, varying levels of development, and a lack of an information-sharing system. To further complicate the matter, some countries are active in multiple international harmonization efforts. The countries that have made the best progress on harmonization have political will, legal frameworks for cooperation, and common language or currency or both (WHO Regional Office for Africa, 2005).
The International Conference on Harmonization of Technical Requirements for Regulation of Pharmaceuticals for Human Use (ICH) brings together drug regulatory authorities and pharmaceutical trade associations for the harmonization of standards (ICH, 2010). Regulatory authorities that participate in the ICH, either as observers or participants, or regulatory authorities that have legally binding recognition agreements with a conference member are considered stringent regulatory authorities (WHO, 2011a). The United States, Japan, EU member counties, Switzerland, Canada, Australia, Norway, Iceland, and Liechtenstein are all stringent regulatory authorities (Stop TB, 2009). ICH guidelines are widely recognized as high-quality and scientifically sound, though some object to an organization with a relatively narrow membership setting standards used internationally (Abraham, 2002; Molzon et al., 2011). Through its Global Cooperation Group, the ICH involves stakeholders from countries outside of the membership (ICH, 2011).
COOPERATION AMONG REGULATORY AGENCIES
Because of the international trade and multi-country distribution systems described in Chapter 1, the committee concluded that cooperation with other regulators, both within county and among neighboring coun-
tries, is a core element of a modern regulatory system in any country. Cooperation is critical for protecting the health and safety of consumers and for consistent enforcement of national policies (Kraemer et al., 2011).
Cooperation Within a Country
The best regulatory systems work at different levels of government (the national, provincial, and municipal levels, for example), but this does not happen without cooperation and coordination. When coordination is lacking, multi-level engagement can lead to duplicating work and overlapping responsibilities (Ratanawijitrasin and Wondemagegnehu, 2002). Poor coordination is a common problem in many low- and middle-income countries. For example, in China, food safety regulation falls to multiple agencies including the Ministry of Agriculture; the State Administration for Quality Supervision, Inspection, and Quarantine; the State Administration of Industry and Commerce; the Ministry of Health; and the State Food and Drug Administration. This lack of regulatory coordination for food safety is not unique to China and can be found in developed and developing countries around the world (Otsuki and Wilson, 2001; Stewart and Gostin, 2011). Chapter 3 discusses this problem in more detail.
Regulation of medical products is often simpler, but still requires extensive intergovernmental coordination. For example, many Asian nations have more than one regulatory body overseeing the safety and registration of drugs and other medical products (Pacific Bridge Medical, 2011). China is particularly complicated with an overall agency, the State Food and Drug Administration, housing 10 departments responsible for various aspects of medical product regulation and a decentralization of authority that grants relative independence to provincial authorities (SFDA, 2011; Tsoi, 2007). A particularly stark example of troubled coordination is Pakistan, which, in June 2011, abolished its national health ministry, leaving drug regulatory responsibility to the provinces without any apparent system of coordination (Punjab refuses to accept drug regulatory authority, 2011; Seventeen federal ministries devolved to provinces, 2011).
It is not uncommon to have drug regulatory powers divided between the federal and state levels, as in India. Each Indian state has its own drug control organization that is responsible for the quality of the drugs as well as licensing the manufacture, sale, and distribution of drugs within that state (CDSCO, 2011). Federal agencies are responsible for coordinating states’ activities, as in Australia, Malaysia, and Venezuela, for example. Both Australia and Malaysia have a coordination mechanism in place to ensure that federal and state regulatory agencies communicate (Ratanawijitrasin and
Wondemagegnehu, 2002). One way to improve coordination is to bring agencies together. Taiwan recently consolidated its Bureaus of Pharmaceutical Affairs, Food and Drug Analysis, and Controlled Drugs into one agency known as the Taiwan Food and Drug Administration that now regulates drugs, medical devices, and other health products (Pacific Bridge Medical, 2011).
The Canadian government is an uncommon example of coordinating multiple agencies in a country efficiently. The Canadian Food Inspection Agency coordinates regulators from the federal, provincial or territorial, and municipal authorities (Health Canada and Canadian Food Inspection Agency, 2007). The federal government agencies (Health Canada and the Canadian Food Inspection Agency) work with the provincial and territorial agencies to facilitate national harmonization, streamline the inspection process, and reduce regulatory pressures on industry (FAO/WHO, 2003).
Evaluating the Regulatory System
The WHO, FAO, and other international organizations have tools that allow regulators to evaluate their agencies’ effectiveness and identify weak spots in their systems. For example, the World Organization for Animal Health, known as the OIE,1 has developed a tool for assessing the veterinary services in a country against international standards (OIE, 2010). The Phytosanitary Capacity Evaluation, an International Plant Protection Convention management tool, aids countries in identifying the strengths and weaknesses in their phytosanitary systems. Table 2-1 lists other capacity evaluation tools; the WTO has compiled an extensive list of the same (Standards and Trade Development Facility, 2011).
Cooperation Among Countries
Ensuring safety and quality are the main goals of food and medical product regulation. Globalization and international trade have made the world smaller; countries can no longer expect their national regulatory authority to guarantee product safety. The modern food and medical product supply is shared among many countries, and protecting it requires global action (Guenther and McCormick, 2011). Table 2-2 and 2-3 give information about international programs building food and medical product safety capacity.
International agreement on minimum product standards is an important piece of international cooperation. International collaboration can lead
![]()
1 An acronym for its earlier name, Office International des Epizooties.
TABLE 2-1
Overview of Capacity Evaluation Tools
| Tool | Developed by | Focus |
| Strengthening National Food Control Systems: Guidelines to Assess Capacity Building Needs | FAO | Food safety |
| Food Safety Toolkit (IFC, 2011) | IFC | Food safety and food hygiene |
| Performance, Vision and Strategy (PVS) for FoodSafety | IICA | Food safety |
| Performance of Veterinary Services (PVS) Pathway | OIE | Animal health |
| Performance, Vision and Strategy (PVS) for National Veterinary Services | IICA | Animal health |
| Phytosanitary Capacity Evaluation (PCE) Tool | IPPC | Plant health |
| Performance, Vision and Strategy (PVS) for National Plant Protection Organizations | IICA | Plant health |
| Guide to Assess Biosecurity Capacity | FAO | Biosecurity |
| Performance, Vision and Strategy (PVS) Tool for SPS | IICA | Food safety and agricultural health |
| Food safety and agricultural health assessments and action plans | World Bank | Food safety and agricultural health |
| Approach to Evaluate Conformity Assurance Infrastructure | UNIDO | Conformity assessment |
| National capacity self assessment tool for the Convention on Biological Diversity (CBD) | CBD | Global environmental commitments |
| Diagnostic tool for analysis and assessment of trade and health | WHO | Trade and health |
| Data collection tool for the review of drug regulatory systems (WHO, 2007) | WHO | Data collection |
| Computer-Assisted Drug Registration (WHO, 1998) | WHO | Drug regulation efficiency |
| Strengthening National Regulatory Authorities (WHO, 2003) | WHO | Capacity building |
SOURCE: Adapted from Standards and Trade Development Facility, 2011.
to more timely detection of problems, promote cross-fertilization of ideas, and eliminate redundant expenses.
A good example of collaboration is the One Health Initiative. This worldwide movement works toward expanding collaboration and communication among specialists in human, animal, and environmental health. This initiative involves individual clinicians and researchers and has the endorsements of the American Medical Association, American Veterinary Medical Association, the American Society of Tropical Medicine and Hygiene, the Centers for Disease Control and Prevention (CDC), the U.S. Department of Agriculture (USDA), and the U.S. National Environmental Health Association (One Health Initiative, 2011).
A growing international interest in food defense, the prevention of intentional food supply contamination, has encouraged international cooperation among food regulators (Guenther and McCormick, 2011). The Asia-Pacific Economic Cooperation (APEC) convenes regulators from 21 countries that account for 43.7 percent of the world’s trade (Guenther and McCormick, 2011). APEC member countries include representatives of every level of regulatory system capacity, ranging from some of the most developed (the United States, Japan, and Canada) to some of least developed (Papua New Guinea and Indonesia). Technical cooperation is integral to the APEC mission. APEC symposia bring together regulators from many nations to meet and exchange knowledge (APEC, 2012).
Much as work against fraud has encouraged international collaboration among food regulators, preventing pharmaceutical fraud demands the cooperation of medicines regulators from around the world. Building “coordinated networks across and between countries” to stop drug counterfeiting was the goal of the WHO’s International Medical Products Anti-Counterfeiting Taskforce (WHO, 2012). However, the fight against pharmaceutical fraud has been held back by, among other things, the inability of stakeholder governments to agree on common definitions of substandard, counterfeit, and falsified drugs (Shepherd, 2010). The need for international cooperation is no less pressing among drug regulators than among food regulators, but in this case communication has drawn to a halt.
The committee believes that international cooperation and communication will be an even more important piece of regulatory agencies’ responsibilities in the future. Sometimes the venues to bring together regulators can be hard to find. Centers of excellence and national science academies can bring academia, industry, and government together. The Department of Homeland Security Science & Technology Directorate Centers of Excellence (COE) network is a good example of this. The centers of excellence are comprised of 12 centers, each directed or co-directed by a university in collaboration with partners from other agencies, laboratories, or the private sector (DHS, 2011). National academies can convene meetings and bring
together differing perspectives. Ultimately, the science academy is a useful neutral forum to come to consensus on controversial problems facing food and medical product regulators.
RISK AND SHARED REGULATORY RESPONSIBILITY
This chapter described some differences in the food and medical product regulatory systems in developed and developing countries. International trade and modern manufacturing bring these systems together, however. Regulators from many different countries debate microbial contamination risks in food and how to best manage the regulation of pharmaceutical ingredients. An overview of these two issues is important to understanding this report.
Risk, Hazard, and Food Regulatory Philosophy
During the past 5 years there has been increasing discussion about differentiating food safety systems based on whether they are hazard-based or risk-based. Before defining these two systems, it is important to note that there are two primary ways to manage food safety hazards and risks: good hygienic practices and Hazard Analysis Critical Control Point (HACCP) (Juneja and Sofos, 2010). The best systems rely on a combination of both, as it is impossible to implement HACCP without strong underlying good hygienic practices. Box 2-7 describes the basic principles of HACCP.
Good hygienic practices are the methods that ensure hygienic manufacturing in any food manufacturing plant. This includes sanitary practices, facility design, and employee hygiene. HACCP is a supplemental management tool loosely based on systems engineering, failure mode, and effect analysis. HACCP stresses attention to the critical steps in production, processing, distribution, marketing, and preparation that have a reasonable likelihood of failing, as well as the likelihood that the failure causes harm. These points are identified and control measures are put into place to prevent such failures. HACCP programs are divided into two phases: hazard analysis and hazard management. The application of good hygienic practices is a prerequisite to the use of HACCP (NACMCF, 1997).
There are no universally accepted definitions of hazard-based and risk-based safety systems. Though Codex publications such as Guidelines for the Control of Campylobacter and Salmonella in Chicken Meat and Principles and Guidelines for the Conduct of Microbiological Risk Management offer commentary on these terms, they give no formal definitions (CAC, 2007). In general, hazard-based systems consider the mere presence of a hazard sufficient reason to conclude contamination in the end products. A risk-based system considers the extent of exposure in relation to the likelihood
BOX 2-7
HACCP
Hazard Analysis and Critical Control Points (HACCP) is a systematic approach to controlling food safety hazards as food moves through supply chains. Pillsbury developed HACCP in the 1960’s while working on the food supply for the space program. There are seven principles in this system, listed below. The goal is to prevent hazards from occurring rather than removing them after the fact.
In 2005, the International Standards Organization developed the ISO 22000 standard for food safety management. ISO 22000 integrates into HACCP auditable requirements. Suppliers identify and assess the hazards as they move along the supply chain. ISO 22000 also requires that supply chains have a system management program and interactive communication program to ensure the foods pass through the supply chain with minimal risk. The seven principles of HACCP are:
• Principle 1: Conduct a hazard analysis.
• Principle 2: Identify critical control points.
• Principle 3: Establish critical limits for each critical control point.
• Principle 4: Establish critical control point monitoring requirements.
• Principle 5: Establish corrective actions.
• Principle 6: Establish procedures for ensuring the HACCP system is working as intended.
• Principle 7: Establish record keeping procedures.
SOURCES: Faergemand, 2008; NACMCF, 1997.
that the exposure causes harm. Some experts go further, suggesting that a hazard-based system is grounded in the application of good hygienic practices or HACCP or both. In their estimation, only food safety programs that have undergone a formal risk assessment should be considered hazard-based. Some food safety experts consider the hazard analysis conducted as the first step in developing a HACCP program distinctly different from a risk assessment (Wallace et al., 2011).
There is wide consensus, however, that HACCP hazard analysis is qualitative or semi-quantitative risk assessment. This idea is further evidenced by the guidance describing HACCP’s focus on significant hazards (HACCP principles and application guidelines, 1998). Codex gives similar guidance (CAC, 1999). The FDA’s National Advisory Committee on Microbiological Criteria for Foods explains, “The HACCP team conducts a hazard analysis and identifies appropriate control measures. The purpose of the hazard analysis is to develop a list of hazards which are of such
significance that they are reasonably likely to cause injury or illness if not effectively controlled…. Hazards that are not reasonably likely to occur would not require further consideration within a HACCP plan” (emphasis added) (NACMCF, 1997).
Clearly, the developers of HACCP plans need to consider risk—the probability and severity—of different threats. Most developed countries have effectively adopted HACCP as the primary risk management system for essentially all foods (Satin, 2005; Unnevehr and Jensen, 1999). The European Union requires food companies to implement HACCP principles. It is required in the United States, Australia, New Zealand, and Canada. As an outcome of HACCP’s adoption in developed countries, some developing countries have also adopted HACCP principles in order to export their products to developed countries (Satin, 2005; Unnevehr and Jensen, 1999).
There are differences in the ways that countries use HACCP and even differences among regulatory agencies within a country. For example, both the United States and the EU use risk analysis for managing food safety concerns. Both have premarket and postmarket safety protocols. The EU recognizes the precautionary principle (Box 2-8), however. It withholds premarket approval from food additives and novel ingredients when there is no absolute proof of safety, a logical impossibility. In the United States, regulatory agencies build an appropriate caution into their standards based on the degree of uncertainty in the science. When there is evidence that a risk to human health exists but scientific data are insufficient to understand it, the Codex Alimentarius Commission should not proceed to elaborate a standard, but should consider elaborating a related text, such as a code of practice, provided that such a text is supported by the available scientific evidence (CAC, 2003). However, the difficulty in all these cases is that there is often a lack of agreement on when scientific data are sufficient and complete.
The United States and the EU also differ on their standards for foodborne pathogenic microorganisms. The EU has moved to define microbiological standards for a number of pathogens, thereby establishing clear, non-zero requirements that recognize the inherent residual risk in all food safety plans (Huss et al., 2004). The equivalent requirements in the United States are established through relatively vague, non-numerical standards. Instead of detailed end-product standards, the U.S. regulators publish official sampling plans and the analytical methods they will use when testing for pathogenic microorganisms (Domesle and O’Keefe, 2011). This allows them to achieve a similar level of control as their European counterparts without having to officially recognize the trace contamination allowed even when the system is operating well.
However, the U.S. food industry generally relies much more on
BOX 2-8
The Precautionary Principle
The precautionary principle has roots in a philosophy of risk management that stresses anticipation of, preparedness for, and prevention of harm. Most applicable in the fields of environmental safety and human health, the precautionary principle states that scientific evidence of a threat, rather than indisputable proof, is sufficient grounds for action to prevent harm. The precautionary principle has been invoked to justify the discontinuation of certain pesticides that present a possible health hazard (SEHN, 1998). The general philosophy of the principle is widely accepted among national and international organizations, but its applications and the degree of precaution expected vary greatly. The precautionary principle can take several forms, from a weak, triply negative statement that a lack of proof of danger does not necessitate inaction, to the much stronger version that requires action in the case of suspected hazard. Used wisely, the principle can facilitate rapid and effective response to hazards without the burden of scientific proof (Europa, 2011).
In the EU, a strict interpretation of the precautionary principle states that it “applies where scientific evidence is insufficient, inconclusive or uncertain and preliminary scientific evaluation indicates that there are reasonable grounds for concern that the potentially dangerous effects on the environment, human, animal or plant health may be inconsistent with the high level of protection chosen by the EU (UNESCO, 2005).” The EU’s conservative stance on genetically modified organisms (GMOs) is evidence of this. While the United States relies heavily on genetic modification of food crops, including close to 90 percent of all corn, soybeans, and cotton grown in the country, few genetically modified crops are authorized in the EU. Several EU countries, including Austria, Greece, Germany, and France, abide by even stricter guidelines that place a complete or near complete ban on GMOs (European Commission, 2012; GMO Compass, 2009).
In a speech given in 2003 in New York, Tony Van der Haegan, Minister-Counselor of the Agriculture, Fisheries, Food Safety, and Consumer Affairs office of the European Commission Delegation, spoke about the history of the precautionary principle in Europe. He recalled the 1989 U.S. ban on the import of cattle and beef products from the United Kingdom during the Bovine Spongiform Encephalopathy (BSE) crisis as an example of early, effective use of the precautionary principle and suggested that had the United Kingdom been as proactive in its safety measures, it may have prevented the subsequent spread of BSE throughout Europe (EU, 2003). While proponents of the principle insist that it protects human health and encourages development of new, safer products, critics maintain that it stifles innovation.
microbiological testing as a food safety verification tool than the European industry does. During the past decade there has been an increased effort to develop measures of microbial food safety to harmonize the countries’ different approaches to risk management. The International Commission on Microbiological Specification for Foods gives three metrics2 as a means to link the stringency of the food safety system to public health outcomes (ICMSF, 2002). This allows more traditional metrics to be set based on the level of public health protection desired and achievable. Conversely, these risk management measures allow for prediction of the likely change in public health protection brought about by the use of different microbiological criteria. The Codex Alimentarius’ principles on microbial risk management also give guidance on the use of the metric (CAC, 2007).
Regulation of Active Pharmaceutical Ingredients
Most pharmaceutical products are manufactured in two basic steps. The first step converts chemical intermediates and starting materials into an API using chemical synthesis, fermentation, or other synthesis processes. The second step is final formulation, where active ingredients and excipients are mixed to form a drug (pills, tablets, capsules, injectables, etc.). Driven by lower costs and easier environmental compliance standards, more than 80 percent of API manufacture is done in India and China, even for formulations that are manufactured in the United States or Europe (FDA, 2011).
The FDA regulates the manufacture of APIs. It requires the manufacturer of the final drug formulation to provide details on which manufacturer it will buy its API from when it files a New Drug Application, Abbreviated New Drug Application, or a change notification. The FDA also requires manufacturers of APIs to submit a drug master file describing the manufacturing facility, processes, and materials used in the production of the active ingredients. Much of the information in the master file is confidential. When a formulator specifies in an application to the FDA that it will be using API from a certain manufacturer, it refers to that manufacturer’s master file. The FDA then schedules a site inspection for the API manufacturer. This inspection verifies the information in the master file and ensures compliance with good manufacturing practices. Companies must notify the FDA of any change in their API providers. If the active ingredient manufacturer has not been inspected before, the FDA will start the process again.
In addition to the first-time inspection, the FDA also routinely inspects API manufacturing facilities. However, it is not feasible to carry out these routine inspections frequently, especially for manufacturers in India, China, and other foreign countries.
![]()
2 Food Safety Objectives, Performance Objectives, and Performance Criteria.

Before the 2008 Olympic Games, the Chinese government temporarily shut down a large number of API factories around Beijing to improve air quality. This caused API shortages and drove up prices on the international market.
SOURCE: PETER PARKS/AFP/Getty Images/Newscom.
Economies of scale drive the manufacture of APIs. Some companies manufacture both APIs and final formulations, but no firm can manufacture every active ingredient it could need. Therefore, companies that manufacture both APIs and final formulations buy and sell active ingredients on the merchant market. This creates a complex web of buying and selling that becomes difficult to trace.
Often multiple drug applications reference the same API manufacturer because many different companies use the same source for their API. Other times, a drug application references multiple API manufacturers because one manufacturer gets APIs from several sources. In such cases the FDA’s routine inspection covers only the portion of the factory that makes the API in question.
The FDA and other regulatory agencies can regulate API production, but the formulation manufacturers themselves need to audit their API suppliers frequently against international good manufacturing practices. It would also help to coordinate inspections with the European Medicines Agency (EMA) or other stringent regulatory authorities. This would allow for more frequent inspections and better inspection coverage all around,
as was shown by a pilot collaboration among the FDA, the EMA, and the Australian medicines regulatory authority (EMA, 2011).
It is also possible to enable existing manufacturers to set up high-quality GMP manufacturing for drugs for serious infectious diseases, such as HIV, tuberculosis, and malaria, on the same site where cheaper, less complicated drugs like acetaminophen are manufactured. By using existing facilities, developing country manufacturers can work to WHO prequalification without starting from scratch.
CONCLUSION
As Chapter 1 describes, the United States has built its regulatory system in spurts for over 100 years, largely influenced by product safety disasters. Developing countries are now struggling to design systems that ensure food and medical product safety. This chapter describes the characteristic underpinnings governments should consider in designing food and medical product regulatory systems. A good system should be responsive; it should be able to act in a crisis and keep pace with changing technology. It should also be focused on outcomes and not hinder innovation. The system must be predictable; that is, regulatory decisions should be fair, not arbitrary or capricious. The amount of regulatory oversight given to a product should be proportional to the product’s likelihood of causing harm. Finally, the regulatory system should be independent and not unduly influenced by politics or money.
At its core, an effective food and medical product regulatory system uses science and risk to develop policy. Regulators should participate in international harmonization, and they should value international cooperation. The ethical enforcement of laws is a crucial piece of a regulatory system. A strong regulatory system protects against public health emergencies, yet it can communicate promptly and accurately with the public during an emergency. At the very least, food and medical product regulation depends on a system for rule making. All stakeholders should have a way to communicate with the regulatory authority about the rules governing product safety.
International cooperation is an important part of a modern food and medical product regulatory system. There are many examples of countries working together on harmonization, but there is always room for improvement. Differences in understanding of microbiological hazards in the food chain and inconsistencies in the regulation of APIs can present challenges to international cooperation.
TABLE 2-2
Food Safety Capacity Building Programs
| Organization | Project | Objective | bActivities | Where |
| WHO | Global Foodborne Infections Network (GFN | To promote integrated, laboratory-based surveillance and foster collaboration among human health, veterinary, and food-related disciplines, thereby enhancing the capacity of countries to detect, respond, and prevent foodborne and other infection enteric diseases |
• Technical support • Quality control systems for reliable laboratory testing • Training (microbiology, epidemiology, risk assessment) |
Worldwide |
| WHO/FAO | International Food Safety Authorities Network (INFOSAN) | To promote rapid exchange of information during food safety events, share information of global interest, promote partnerships, help countries strengthen their capacity to manage food safety risks, and respond to requests for assistance during food safety emergencies. |
• Improving response capacity of emergency contact points • Training on risk assessment • Guidance and training on developing national food safety emergency response plans • Tools for outbreak investigation |
Worldwide |
| FAO | Food Quality Standards Service (AGNS) | Enhancing food safety and quality along the supply chain at the international, regional, and national levels with the aim of protecting consumers and promoting the production and trade of safe, high-quality food |
• Establish and improve national regulatory frameworks • Provide technical advice on integrated food control systems; independent scientifi c advice; guidelines and tools on food safety risk assessment; and emergency assistance |
Worldwide |
| Organization | Project | Objective | bActivities | Where |
| World Bank | Incorporated Into a wide range of regional and country-level programs | Protecting public health and preventing malnutrition; educating the technical staff necessary for a competitive agriculture sector; and Improving trade and market access for developing countries |
• Partnerships -Part of the WTO’s Standards and Trade Development Facility -Manages the Global Food Safety Fund • Technical advice -Publications such as Food Safety and Agricultural Health Standards: Challenges and Opportunities for Developing Country Exports -Conducts SPS capacity and needs assessments • Larger scale agricultural and development projects -The World Bank’s 2010-2012 Action Pian Includes helping countries develop Infrastructure and institutions for food safety and to better Implement SPS; training, knowledge sharing, and analytical research on food safety standards; and directing money across the supply chain including logistic and advisory services for food safety standards |
Worldwide |
| United Nations | United Nations Conference on Trade and Development (UNCTAD) | Provides food safety capacity building as part of trade promotion. |
• Training in good agricultural and manufacturing practices, ISO 9000, ISO 22000, and HACCP standards for farmers in developing countries • Research on SPS standards in least developed countries to assess the barriers to entering markets and strengthen capacity to bridge these gaps |
Worldwide |
| APEC | Food Safety Cooperation Forum | To Improve and strengthen Information sharing In food safety by enhancing cooperation among member economies; and to Identify, prioritize, and coordinate capacity building In the Asia-Pacific Economic Cooperation region, taking other regional activities Into account. |
• Regulatory frameworks: establish government endorsed food regulatory systems; develop, Implement, and enforce food safety standards • Food Inspection and certification programs: develop legislative frameworks for food management; establish communication systems to support food Inspection Including education and training • Training In food safety management and risk assessment • Establishing relevant forums, encouraging collaboration, and creating surveillance systems for transparent Information sharing |
Asia Pacific |
| Organization | Project | Objective | bActivities | Where |
| ASEAN | Cooperation in Food & Agriculture (under the Ministers of Agriculture) | To coordinate and implement food safety measures and to balance the concerns of food security and market access. |
• Issue guidelines and standards on food handling, quarantine, and testing procedures, GMOs, and pesticide residue limits • Formulate common Codex positions • Harmonize ASEAN standards according to Codex • ASEAN Food Safety Network: an Integrated platform for sharing food safety Information among ASEAN countries, especially related to Codex, OIE, and IPPC |
Southeast Asia |
| Cooperation in Standards & Conformance (under the Ministers of Economics) | To coordinate and Implement food safety measures and to balance the concerns of food security and market access. |
• Implement the ASEAN Framework Agreement on Mutual Recognition Arrangements, moving toward mutually accepted standards and Inspections • Harmonize standards • Ensure transparency of standards, technical regulations, and conformity assessments |
Southeast Asia | |
| Expert Group on Food Safety | To Improve food safety of ASEAN countries, but also to facilitate food trade and formulate a strategic plan to address important food safety Issues for mutual benefits. |
• Provide assistance to ASEAN governments to develop and strengthen food safety Infrastructure and programs • Food Safety Improvement Plan: Identifies 10 priority areas for cooperation Including a center of excellence for Inspection and certification, consumer participation In food safety, education and training, and Increased sharing of food safety Information |
Southeast Asia | |
| Asian Development Bank | Incorporated Into a wide range of regional and country-level programs | Protection of public health through sustainable access to safe foods and promotion of economic development. |
• Technical assistance on developing and Improving national systems, use of food safety technologies • Research - Publications such as: Impact of Food Safety Standards on Processed Food Exports from Developing Countries and Food Safety and ITC TraceabiHty Systems: Lessons from Japan for Developing Countries • Promote regional cooperation In standards |
Asia and the Pacific |
| Organization | Project | Objective | bActivities | Where |
| PAHO | PAHO Regional Program on Food Safety | To build capacity in risk assessment, risk management, risk communication, and education and to mobilize and optimize the use of resources. |
• Providing training courses in surveillance, risk assessment, and the application of good manufacturing practices, HACCP, etc. • Updating and harmonizing legislation • Consolidating regional INFOSAN activities • Improving countries' ability to use systematic risk assessment and develop integrated food safety programs • Supporting active involvement in Codex • Improving national capacity to maintain communication flow between public and private sectors • Improving the technical capability of member states to implement systematic approaches to food safety and to adopt evidence-based decisions • Strengthening farms-to-table links • Maintaining associations and partnerships with UN agencies, Organization of American States, etc. |
South America |
| Mercosur | Incorporated into Its broader goals of regional Integration and Increased market access | To create a food safety and hygiene system and phytosanltary area in the Mercosur region and to increase market access for exports, especially to the EU. |
• Identifies food safety as a priority for its members and sets regional standards • Has a cooperative agreement with the EU (up to €15 million) for cooperation and harmonization of veterinary and phytosanitary procedures. Includes: -Strengthening institutions responsible for food safety -Improving food hygiene control systems -Common food hygiene policies for the region |
Latin America |
| African Development Bank | Incorporated Into a wide range of regional and country-level programs | To support improved health and nutrition through access to safe foods and to promote economic growth through market access and Income generation. |
• Providing training in SPS measures, handling and processing techniques, quality standards • Improving infrastructure such as hygienic meat slaughtering facilities • Improving supply chains with roads and cold chains • Promoting regional standards harmonization |
Africa |
| Organization | Project | Objective | bActivities | Where |
| WHO Quality and Safety of Medicines Program (continued) | The International Pharmacopeia | To provide the source material for reference or adaptation by any member state who wants to establish pharmaceutical requirements. |
Outlines quality specifications for:
• active ingredients and excipients • dosage • general methods of supporting analysis |
Worldwide |
| WHO Department of Immunization, Vaccines and Biologicals | Vaccine Safety | To identify and consolidate consensus opinions on key vaccine regulatory issues; and to communicate these opinions to national authorities and manufacturers through guidance documents. | WHO Technical Report Series (TRS) informs members and manufacturers of up-to-date methods; releases international reference materials for the standardization of assays and testing | Worldwide |
| Regulatory Pathway Initiative | To improve developing countries' ability to regulate new vaccines by supporting the development and implementation of regulatory strategies for assessing clinical trial applications and establishing regulatory mechanisms for licensing new vaccines that are not registered in the country of manufacture. |
• Developing Countries' Vaccine Regulators Network -The WHO established the network and plays a secretariat role -Forum for discussion of and exposure to policies and procedures for the evaluation of clinical trial proposals and clinical trial data • Collaboration with the European Medicines Agency, the FDA, and other developed regulatory authorities to develop new regulatory strategies for licensing of novel vaccines • Establishment of regional regulatory authority and research center networks to address the short-term need for review of clinical protocols, monitoring trials, and evaluation of trial data • Training and technical assistance for national regulatory authorities (NRAs) that have not developed the expertise to review license applications |
Worldwide | |
| Organization | Project | Objective | bActivities | Where |
| USDA | Foreign Agriculture Service | Leads USDA’s efforts to help developing countries improve their agricultural systems and trade capacity and develop market-institutions and science-based regulatory frameworks. |
• Trade and scientific exchanges - i.e., Cochran Fellowship Program: provides participants from middle-income countries, emerging markets, and emerging democracies with high-quality training to improve local agricultural systems and strengthen and enhance trade links with the United States |
Worldwide |
| UK Department for International Development (DFID) | Incorporated into country-level development projects and research | Safe food is part of DFID’s overall mission to promote health and economic development in low-income countries. Access to safe food is critical for health, and compliance with safety regulations is necessary to export food to wealthy countries. |
• Research: funds a wide range of research on improving food safety systems in developing countries • Country-level training and technical assistance -Food control laboratory capacity building -Training African trainers to implement food safety programs based on HACCP |
Worldwide |
| GIZ | Food Quality and Food Safety | To advise public and private actors on the process of setting national and international standards and integrating international standards into their own legislation and quality operations management. |
• Advise governments on WTO food standards like SPS • Training for governments to develop food safety systems • Support countries in developing risk assessment and risk management by extending food monitoring programs • Training laboratory personnel |
Worldwide |
TABLE 2-3
Medical Product Capacity Building Programs
| Organization | Project | Objective | Activities | Where |
| WHO Quality and Safety of Medicines Program | Regulatory Support | To develop internationally recognized norms, standards, and guidelines; and to provide guidance, technical assistance, and training to support national medical regulatory agencies. |
• Assessment of national medicines regulatory systems • Regulatory information and practical manuals • Training • Model website for regulatory authorities • Model system for computer-assisted medicines registration • Certification scheme on quality of pharmaceutical products • International Conference of Drug Regulatory Authorities |
Worldwide |
| Safety, Efficacy and Utilization | To develop norms and standards for pharmacovigilance, promote information exchange on medicines safety, and provide country support. |
• WHO Advisory Committee on Safety of Medicinal Products • Monitoring medicines (FP-7): 42-month project launched in 2010 including the WHO, the Uppsala Monitoring Centre, and other partners. The project has four key components: 1. Strengthening of consumer reporting 2. Identification of problems by national pharmacovigilance centers 3. Better use of existing global data 4. Development of active and focused systems to address urgent safety questions in priority diseases Projects to improve pharmacovigilance in drugs for Chagas, TB, HIV, and malaria |
Worldwide | |
| Quality Assurance | To develop norms, standards, and guidelines for quality assurance including quality control, production, distribution, and inspections. |
Publications:
• WHO good manufacturing principles: main principles for pharmaceutical products (2011) • Good distribution practices for pharmaceutical products (2010) • Quality system requirements for national GMP inspectorates (2003) |
Worldwide | |
| Organization | Project | Objective | Activities | Where |
| WHO Quality and Safety of Medicines Program (continued) | The International Pharmacopeia | To provide the source material for reference or adaptation by any member state who wants to establish pharmaceutical requirements. |
Outlines quality specifications for:
• active ingredients and excipients • dosage • general methods of supporting analysis |
Worldwide |
| WHO Department of Immunization, Vaccines and Biologicals | Vaccine Safety | To identify and consolidate consensus opinions on key vaccine regulatory issues; and to communicate these opinions to national authorities and manufacturers through guidance documents. | WHO Technical Report Series (TRS) informs members and manufacturers of up-to-date methods; releases international reference materials for the standardization of assays and testing | Worldwide |
| Regulatory Pathway Initiative | To improve developing countries' ability to regulate new vaccines by supporting the development and implementation of regulatory strategies for assessing clinical trial applications and establishing regulatory mechanisms for licensing new vaccines that are not registered in the country of manufacture. |
• Developing Countries' Vaccine Regulators Network -The WHO established the network and plays a secretariat role -Forum for discussion of and exposure to policies and procedures for the evaluation of clinical trial proposals and clinical trial data • Collaboration with the European Medicines Agency, the FDA, and other developed regulatory authorities to develop new regulatory strategies for licensing of novel vaccines • Establishment of regional regulatory authority and research center networks to address the short-term need for review of clinical protocols, monitoring trials, and evaluation of trial data • Training and technical assistance for national regulatory authorities (NRAs) that have not developed the expertise to review license applications |
Worldwide | |
| Organization | Project | Objective | Activities | Where |
| WHO Department of Essential Health Technologies | Global initiative on health technologies | To establish a framework for the development of national health technology programs; and to support World Health Assembly resolution 60.29, which urges member states to have guidelines for good manufacturing and regulatory practices, to establish surveillance systems, and to participate in international harmonization (other major objectives not related to safety or regulatory capacity building). |
• Establish guidelines for national health technology programs • Develop methodologies to help member states conduct assessments • Identify national, international, and global standards for countries to identify their gaps and future needs • Create tools to implement gaps in policies • Publishes WHO documents on designing and implementing effective medical device regulatory systems |
Worldwide |
| World Bank | Incorporated into a wide range of projects, primarily in its Health, Nutrition and Population (HNP) sector | To assist countries in improving the health, nutrition, and population outcomes of poor people via strengthening the health care systems and securing sustainable health financing. To protect the most vulnerable from the impoverishing effects of illness, malnutrition, and high fertility by developing health policies that enhance the knowledge, skills, and values leading to equitable economic and human development. |
• Partnerships - Partner in the African Medicines Regulatory Harmonization Initiative • Research • Overall HNP strategy includes improving health governance, health systems strengthening, supply chain management, access to medicines • Country-level investment |
Worldwide |
| Organization | Project | Objective | Activities | Where |
| PAHO | Pan American Network for Drug Regulatory harmonization | To maintain a constructive dialogue among drug regulatory authorities and other stakeholders, adopt guidelines on specific aspects of regulation, and promote technical cooperation among countries. |
Harmonization and capacity building efforts in:
• Bioequivalence • Biotechnological products • Counterfeit medicines • Good clinical, laboratory, and manufacturing practices • Medicine manufacturing plants • Medicines classification and registration • Pharmacopeia • Pharmacovigilance • Vaccines |
The Americas |
| APEC | APEC Harmonization Center | Increase regulatory harmonization among member states with the goals of supporting access to best practices, information exchange, and clinical trials that meet international standards and to improve the quality, safety, and efficacy of medical products to enhance health outcomes and facilitate international trade. |
• Research on harmonization policies and best practices • Education and training, including fellowship programs • Establish strong information exchange networks • Maintain online publications • Develop and disseminate harmonization models • Support international cooperation |
Asia Pacific |
| ASEAN | ACCSQ Pharmaceutical Product Working Group | The harmonization of pharmaceutical regulations to facilitate the goals of the ASEAN Free Trade Area, particularly eliminating technical barriers to trade, without compromising the quality or safety of medicines. |
• GMP training • Implementation of common technical requirements • Mutual recognition agreements for GMP inspections • Shared postmarket surveillance information • Vaccine regulation capacity building |
Southeast Asia |
| Organization | Project | Objective | Activities | Where |
| NEPAD, WHO, World Bank, Gates Foundation, DFID, Clinton Health Access Initiative | African Medicines Regulatory Harmonization Initiative | To Improve health by Increasing access to safe and effective medicines of good quality through strengthening the technical and administrative capacity of national medicines regulatory authorities. |
• Create a collaborative network of regional regulatory authorities • Harmonize technical requirements of medical products and build confidence so that standards are respected by participating authorities • Establish a framework for joint evaluations of applications and inspections of manufacturing sites • Strengthen the capacity for regulatory oversight • Develop information management systems and promote the exchange of regulatory information |
Sub-Saharan Africa |
| South African Development Community (SADC; with support from the African Development Bank) | SADC Pharmaceutical Programme | To strengthen the capacity of member states and to mitigate the threat of diseases that are major public health concerns by Increasing access to quality medicines. |
• Harmonizing regional regulations • Strengthening regulations and enforcement infrastructure • Educating and retaining competent pharmaceutical staff; strengthening regional training centers • Promoting joint procurement of quality essential medicines • Maximizing research and production capacity of quality, generic essential medicines • Assessing gaps in national regulatory authorities and laboratories |
Southern Africa |
| FDA | Office of International Programs, Technical Cooperation and Capacity Building | Defines regulatory capacity, the ability of national regulatory authorities to perform regularly their core functions to ensure the availability of high-quality and safe food and medical products, as part of the FDA mission to ensure safe products In the United States. |
• Strengthen information and evidence so the FDA can make informed decisions about how to use its resources • Transfer its expertise and identify training efforts globally that do not require the use of FDA resources • Encourage global information networks to strengthen detection, surveillance, and assessment systems • Support surveillance and tracking efforts for global supply chains • Support pharmacovigilance capacity |
Worldwide |
| Organization | Project | Objective | Activities | Where |
| USAID and Management Sciences for Health | Strengthening Pharmaceutical Systems | To build capacity within poor countries to effectively manage pharmaceutical systems, successfully Implement USAID priority services, and protect public health by Improving access to and use of quality-assured medicines. |
• Improving governance in the pharmaceutical sector • Strengthening pharmaceutical and laboratory management systems • Containing the mergence and spread of antimicrobial resistance • Expanding access to medicines |
Worldwide |
| USP | Promoting Quality of Medicines | A 5-year, USAID-funded project to address poor-quality medicines In developing countries and to assure the quality, safety, and efficacy of medicines for USAID priority diseases. |
• Strengthen national quality control programs: -Build the capacity of national quality control laboratories -Support medicines registration, inspections, laboratory testing, and postmarket surveillance • Increase supply of quality-assured medicines by offering technical assistance to manufacturers on WHO prequalification • Combat substandard and counterfeit medicines by developing testing mechanisms, conducting quality research on essential medicines, and providing technical support for detecting and monitoring substandard medicines • Provide technical leadership and global advocacy on the dangers of substandard medicines |
Worldwide |
| Brighton Collaboration | A non-profit, scientifically Independent partnership with the goal of conducting and promoting high-quality vaccine safety research. |
• Set vaccine safety research standards and provide common terminology • Build research capacity • Joined with the WHO and Gates Foundation to build vaccine safety monitoring • Developed network of local vaccine experts to serve as a global vaccine safety resource • Developed pilot infrastructure (with the European Center for Disease Prevention and Control) to link electronic records and an online classification tool • Established a virtual online research institute to facilitate communication • Plans to develop a vaccine training program |
Worldwide | |
REFERENCES
Abraham, J. 2002. The pharmaceutical industry as a political player. Lancet 360(9344):1498-1502.
AMRH (African Medicines Registration Harmonisation). 2010. Issue 3. African Medicines Registration Harmonisation Newsletter (April). http://www.amrh.org/download/eng_amrh_newsletter_03.pdf.
Anand, K., K. Saini, S. Binod, and Y. Chopra. 2010. To recognize the use of international standards for making harmonized regulation of medical devices in Asia-Pacific. Journal of Young Pharmacists 2(3):321-325.
APEC (Asia-Pacific Economic Cooperation). 2012. Agricultural technical cooperation. http://www.apec.org/Home/Groups/SOM-Steering-Committee-on-Economic-and-Technical-Cooperation/Working-Groups/Agricultural-Technical-Cooperation.aspx (accessed February 1, 2012).
ASEAN (Association of Southeast Asian Nations). 2005. ASEAN policy guideline on standards and conformance. Manila, Philippines: ASEAN.
BIS (Business Innovation and Skills). 2011a. Choose the alternative. http://www.bis.gov.uk/policies/bre/better-regulation-framework/alternatives-to-regulation/choose-the-alternative (accessed October 5, 2011).
———. 2011b. Market/economic instruments. http://www.bis.gov.uk/policies/bre/better-regulation-framework/alternatives-to-regulation/choose-the-alternative/market-economic-instruments (accessed December 7, 2011).
Brhlikova, P., I. Harper, and A. Pollock. 2007. Good manufacturing practice in the pharmaceutical industry. Working Paper 3. Prepared for Workshop on “Tracing Pharmaceuticals in South Asia,” University of Edinburgh, Scotland, UK, July 2-3.
Brown, A. C., J. Stern, B. Tenenbaum, and D. Gencer. 2006. Handbook for evaluating infrastructure regulatory systems. Washington, DC: The International Bank for Reconstruction and Development/The World Bank.
CAC (Codex Alimentarius Commission). 1999. Codex alimentarius food hygiene basic texts- Second edition. Rome: WHO and FAO.
———. 2003. Principles for the risk analysis of foods derived from modern biotechnology. Rome: WHO and FAO.
———. 2007. Principles and guidelines for the conduct of microbiological risk management (MRM). Rome: WHO and FAO.
———. 2011. Guidelines for the control of campylobacter and salmonella in chicken meat. Rome: WHO and FAO.
CDSCO (Central Drugs Standard Control Organization). 2011. List of state drugs controllers. http://cdsco.nic.in/html/state%20drugs1.htm (accessed November 11, 2011).
Chigullapalli, R., and A. Tandulwadikar. 2011. Asian medical device markets—Emerging regulations. Asian Hospital and Healthcare Management. http://www.asianhhm.com/Knowledge_bank/articles/asian-medical-device-markets.htm (accessed November 11, 2011).
Crisp, N. 2011a. Changes to South African medicines regulation body. Strengthening South Africa’s Response to HIV and Health. http://www.sarrahsouthafrica.org/LinkClick.aspx?fileticket=tfjZOGSkMMU%3D&tabid=2067 (accessed November 18, 2011).
———. 2011b. SAHPRA: Progress regarding establishing the entity and impact/relevance for medical device industry. Paper presented at 2nd Annual South African Medical Devices Industry Association (SAMED) Conference, Midrand, South Africa, May 31.
Dalpino, C. E. 2000. Deferring democracy: Promoting openness in authoritarian regimes. Washington, DC: The Brookings Institution.
DHS (Department of Homeland Security). 2011. Homeland security centers of excellence. http://www.dhs.gov/files/programs/editorial_0498.shtm (accessed November 9, 2011).
Domesle, A., and M. O’Keefe. 2011. United States national residue program: 2011 scheduled sampling plans. Washington, DC: USDA/FSIS/OPHS.
Dyckman, L. J. 1999. U.S. needs a single agency to administer a unified, risk-based inspection system: Statement of Lawrence J. Dyckman. Washington, DC: Government Accountability Office.
EMA (European Medicines Agency). 2011. Final report on the international API inspection pilot programme. London: EMA, Australian Government, and FDA.
EU (European Union). 2003. EU view of precautionary principle in food safety. http://www.eurunion.org/news/speeches/2003/031023tvdh.htm (accessed February 14, 2012).
Europa. 2011. Summaries of EU legislation: The precautionary principle. http://europa.eu/legislation_summaries/consumers/consumer_safety/l32042_en.htm (accessed February 14, 2012).
European Commission. 2011. General food law—Principles. http://ec.europa.eu/food/food/foodlaw/principles/index_en.htm (accessed November 21, 2011).
———. 2012. Rules on GMOs in the EU—ban on GMOs cultivation. http://ec.europa.eu/food/food/biotechnology/gmo_ban_cultivation_en.htm (accessed February 14, 2012).
Faergemand, J. 2008. The ISO 22000 series: Global standards for safe food supply chains. Geneva, Switzerland: International Organization for Standardization.
Fairclough, G. 2009. Beijing tightens food-safety laws: New measures call for tougher penalties and more oversight. http://online.wsj.com/article/SB123591044294303161.html (accessed November 21, 2011).
FAO (Food and Agriculture Organization of the United Nations). 2004. Foodborne diseases: Situation of diarrheal diseases in Thailand. ftp://ftp.fao.org/docrep/fao/meeting/006/ad703e/ad703e00.pdf (accessed November 18, 2011).
FAO and WHO (World Health Organization). 2003. Assuring food safety and quality: Guidelines for strengthening national food control systems. Rome, Italy: FAO and WHO.
FDA (U.S. Food and Drug Administration). 2011. Pathway to global product safety and quality. Washington, DC: FDA.
FICCI (Federation of Indian Chambers of Commerce and Industry). 2010. Bottlenecks in Indian food processing industry. New Delhi, India: FICCI.
FSSAI (Food Safety and Standards Authority of India). 2011. About FSSAI. http://www.fssai.gov.in/AboutFSSAI/introduction.aspx (accessed November 17, 2011).
GAO (Government Accountability Office). 2005. Food safety: Experiences of seven countries in consolidating their food safety systems. Washington, DC: GAO.
GMO Compass. 2009. USA: Cultivation of GM plants. http://www.gmo-compass.org/eng/agri_biotechnology/gmo_planting/506.usa_cultivation_gm_plants_2009.html (accessed February 14, 2012).
Gropp, M. 2011. Core elements of medical device regulatory systems in developing countries. Paper presented at Strengthening Core Elements of Regulatory Systems in Developing Countries: Meeting One, Institute of Medicine, Washington, DC, March 2-3.
Guenther, J. C., and K. J. McCormick. 2011. U.S. government efforts to build global food defense capacity. Paper presented at Strategies for Achieving Food Security in Central Asia, Bishkek, Kyrgyzstan.
HACCP (Hazard Analysis and Critical Control Point) principles and application guidelines. 1998. Journal of Food Protection 61(6):762-775.
Health Canada and Canadian Food Inspection Agency. 2007. The food regulatory system in Canada. Ottowa, Ontario: Health Canada and Canadian Food Inspection Agency.
Huss, H. H., L. Ababouch, and L. Gram. 2004. Assessment of seafood safety and quality. Rome, Italy: FAO.
ICDRA (International Conference of Drug Regulatory Authorities). 2010. 14 ICDRA recommendations. Paper presented at the Fourteenth International Conference of Drug Regulatory Authorities, Singapore, November 30-December 3.
ICH (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use). 2010. The value and benefits of ICH to drug regulatory authorities: Advancing harmonization for better health. Geneva, Switzerland: ICH.
———. 2011. ICH harmonisation for better health: Official website. http://www.ich.org/ (accessed October 4, 2011).
ICMSF (International Commission on Microbiological Specifications for Foods). 2002. Microorganisms in foods 7: Microbiological testing in food safety management. New York, NY: Kluwer Academic/Plenum Publishers.
IFC (International Finance Corporation). 2011. International standards and technical regulations program in the Western Balkans: Introduction and overview of the food safety toolkit. Belgrade, Serbia: IFC.
IOM (Institute of Medicine). 2006. Valuing health for regulatory cost-effective analysis. Washington, DC: The National Academies Press.
Juneja, V. K., and J. N. Sofos. 2010. Pathogens and toxins in foods: Challenges and interventions. Washington, DC: American Society for Microbiology Press.
KPMG International. 2006. The Indian pharmaceutical industry: Collaboration for growth. The Netherlands: KPMG International.
Kraemer, D. W. (Deputy Director for Operations, Center for Food Safety and Applied Nutrition) and FDA. 2011. Challenges faced by food safety regulatory systems in developing countries. Paper presented at Strengthening Core Elements of Regulatory Systems in Developing Countries: Meeting One, Institute of Medicine, Washington, DC.
Lelieveld, H., and L. Keener. 2007. Global harmonization of food regulations and legislation—the Global Harmonization Initiative. Trends in Food Science & Technology 18:S15-S19.
Ministry of Health of South Africa. 2008. Executive summary: Report of the ministerial task team on the restructuring of the Medicines Regulatory Affairs and Medicines Control Council and recommendations for the new regulatory authority for health products of South Africa. Pretoria, South Africa: Ministry of Health of South Africa.
Molzon, J. A., A. Giaquinto, L. Lindstrom, T. Tominaga, M. Ward, P. Doerr, L. Hunt, and L. Rago. 2011. The value and benefits of the international conference on harmonisation to drug regulatory authorities: Advancing harmonization for better public health. Clinical Pharmacology and Therapeutics 89(4):503-512.
NACMCF (National Advisory Committee on Microbiological Criteria for Foods). 1997. Hazard analysis and critical control point principles and application guidelines. http://www.fda.gov/food/foodsafety/HazardAnalysisCriticalControlPointsHACCP/ucm114868.htm (accessed December 13, 2011).
Ndomondo-Sigonda, M., and A. Ambali. 2011. The African medicines regulatory harmonization initiative: Rationale and benefits. Clinical Pharmacology and Therapeutics 89(2):176-178.
OECD (Organisation for Economic Co-operation and Development). 2009. Indicators of regulatory management systems: 2009 report. Paris, France: OECD.
Office of Information and Regulatory Affairs. 2003. Regulatory impact analysis: A primer. Washington, DC: Office of Information and Regulatory Affairs.
OIE (World Organisation for Animal Health). 2010. The new tool for the evaluation of performance of veterinary services (pvs tool) using OIE international standards of quality and evaluation. http://web.oie.int/eng/OIE/organisation/en_vet_eval_tool.htm (accessed October 5, 2011).
One Health Initiative. 2011. One Health Initiative will unite human and veterinary medicine. http://www.onehealthinitiative.com/index.php (accessed November 9, 2011).
Otsuki, T., and J. S. Wilson. 2001. Global trade and food safety: Winners and losers in a fragmented system. Washington, DC: The World Bank.
Pacific Bridge Medical. 2011. Asian medical regulatory agencies. http://www.pacificbridgemedical.com/asian-medical-regulatory-agencies.php (accessed November 9, 2011).
PAHO (Pan-American Health Organization). 2010a. Medical devices regulation. http://new.paho.org/hq/index.php?option=com_content&task=view&id=3418&Itemid=1272 (accessed November 11, 2011).
———. 2010b. Quality and regulation. http://new.paho.org/hq/index.php?option=com_?content&task=view&id=2384&Itemid=1179 (accessed October 5, 2011).
Palthur, M. P., S. S. Sajala, and S. K. Chitta. 2009. The Food Safety and Standards Act, 2006: A paradigm shift in Indian regulatory scenario. Pharmainfo 7(5).
Punjab refuses to accept drug regulatory authority. 2011. The Peninsula, October 9.
Ramesh, S. V., and WG3 Chair. ACCSQ working group on standards and technical regulations (WG3)—Standards harmonization and technical regulations. http://www.asean.org/14889.htm (accessed November 21, 2011).
Ramos, A. C., and C. A. Oblepias. 2002. Country report proposed by the Philippines. Marrakech, Morroco: FAO and WHO.
Ratanawijitrasin, S., and E. Wondemagegnehu. 2002. Effective drug regulation: A multicountry study. Geneva, Switzerland: WHO.
Regulations.gov. 2012. http://www.regulations.gov/#!home (accessed January 20, 2012).
Report of the ministerial task team. 2008. Executive summary: Report of the ministerial task team on the restructuring of the Medicine Regulatory Affairs and Medicines Control Council and recommendations for the new regulatory authority for health products of South Africa. Ministry of Health of South Africa.
Rosnbom, K. A. 2010 (February 3). Developments in UNICEF vaccine procurement. Paper presented at Global Immunization Meeting.
Roth, A. V., A. A. Tsay, M. E. Pullman, and J. V. Gray. 2008. Unraveling the food supply chain: Strategic insights from China and the 2007 recalls. Journal of Supply Chain Management 44(1):22-39.
Satin, M. 2005. Quality enhancement in food processing through HACCP. Tokyo, Japan: Asian Productivity Organization.
SEHN (The Science and Environmental Health Network). 1998. Wingspread conference on the precautionary principle. http://www.sehn.org/wing.html (accessed February 14, 2012).
Seventeen federal ministries devolved to provinces. 2011. Pakistan Today, July 1.
SFDA (State Food and Drug Administration). 2011. Internal structure. http://eng.sfda.gov.cn/WS03/CL0764/ (accessed November 15, 2011).
Shepherd, M. 2010. Beef up international cooperation on counterfeits. Nature Medicine 16(4):366-366.
Singh, R. 2011. FSSAI publishes food safety and standards rules 2011. New Delhi, India: USDA Foreign Agricultural Service—Global Agricultural Information Network.
Smart, P. 2011. Features of new rules set by food safety and standards authority. http://www.dnaindia.com/mumbai/report_features-of-new-rules-set-by-food-safety-and-standards-authority_1595836 (accessed November 16, 2011).
Standards and Trade Development Facility. 2011. SPS-related capacity evaluation tools: An overview of tools developed by international organizations. Geneva, Switzerland: World Trade Organization.
Stewart, K., and L. Gostin. 2011. Food and Drug Administration regulation of food safety. Journal of the American Medical Association 306(1):88-89.
Stop TB. 2009. List of countries considered as stringent regulatory authorities. http://www.stoptb.org/assets/documents/gdf/drugsupply/List_of_Countries_SRA.pdf (accessed December 12, 2011).
Sunstein, C. R. 2011. Memorandum for the heads of independent regulatory agencies: Executive order 13579, “Regulation and Independent Regulatory Agencies.” Washington, DC: Office of Information and Regulatory Affairs.
Tsoi, A. 2007. Pharmaceutical policies and regulations in China. http://www.deacons.com.hk/eng/knowledge/knowledge_290.htm (accessed November 14, 2011).
UNESCO (United Nations Educational, Scientific and Cultural Organization). 2005. World Commission on the Ethics of Scientific Knowledge and Technology (COMEST): The precautionary principle. http://unesdoc.unesco.org/images/0013/001395/139578e.pdf (accessed 2012, February 14).
UNICEF (United Nations Children’s Fund). 2011. Supplies and logistics: GAVI. http://www.unicef.org/supply/index_gavi.html (accessed November 11, 2011).
Unnevehr, L. J., and H. H. Jensen. 1999. The economic implications of using HACCP as a food safety regulatory standard. Food Policy 24(6):625-635.
U.S. Department of State. 2011. Rulemaking: What is the rulemaking process? http://www.state.gov/m/a/dir/rulemaking/c42660.htm (accessed October 4, 2011).
Wallace, C. A., W. H. Sperber, and S. E. Mortimore. 2011. Food safety for the 21st century: Managing HACCP and food safety throughout the global supply chain. Oxford, UK: Wiley-Blackwell.
WHO (World Health Organization). 1998. How to implement computer-assisted drug registration: A practical guide for drug regulatory authorities. Geneva, Switzerland: WHO.
———. 1999. Regulation of vaccines: Building on existing drug regulatory authorities. Geneva, Switzerland: WHO Department of Vaccines and Other Biologics.
———. 2001. The impact of implementation of ICH guidelines in non-ICH countries: Report of a WHO meeting. Geneva, Switzerland: WHO.
———. 2003a. Aide-memoire: Strengthening national regulatory authorities. Geneva, Switzerland: WHO.
———. 2003b. Effective medicines regulation: Ensuring safety, efficacy and quality. WHO Policy Perspectives on Medicines 007:1-6.
———. 2007. WHO data collection tool for the review of drug regulatory systems (to be used jointly with the practical guidance for conducting a review). Geneva, Switzerland: WHO.
———. 2009. Food safety background. http://www.wpro.who.int/vietnam/sites/dhp/food_safety/ (accessed November 16, 2011).
———. 2010. Prequalification of medicines by WHO. http://www.who.int/mediacentre/factsheets/fs278/en/index.html (accessed November 14, 2011).
———. 2011a. Guideline on submission of documentation for prequalification of multisource (generic) finished pharmaceutical products (FPPs) approved by stringent regulatory authorities (SRAs). Geneva, Switzerland: WHO.
———. 2011b. Immunization standards: Strengthening national regulatory authorities. http://www.who.int/immunization_standards/national_regulatory_authorities%20/strengthening/en/index.html/ (accessed November 11, 2011).
———. 2011c. International conference of drug regulatory authorities. http://www.who.int/medicines/areas/quality_safety/regulation_legislation/icdra/en/index.html (accessed October 5, 2011).
———. 2011d. National regulatory authority of China meets international standards for a vaccine regulation. http://www.who.int/immunization_standards/vaccine_regulation/nra_china_functional/en/index.html (accessed February 2, 2012).
———. 2011e. WHO list of prequalified medicinal products. http://apps.who.int/prequal/query/ProductRegistry.aspx.
———. 2012. Impact: Frequently asked questions. http://www.who.int/impact/impact_q-a/en/index.html (accessed February 1, 2012).
WHO Regional Office for Africa. 2005. First African medicines regulatory authorities conference: Final report. Paper read at Conference of African Medicine Regulators. Addis Ababa, Ethiopia, October 31-November 3.
———. 2006. Medicines regulatory authorities: Current status and the way forward—report of the regional director. Addis Ababa, Ethiopia: WHO Regional Committee for Africa. Wilson, P. 2010. Giving developing countries the best shot: An overview of vaccine access and R&D. Geneva, Switzerland: Oxfam.
WTO (World Trade Organization). 1998. Understanding the WTO agreement on sanitary and phytosanitary measures. http://www.wto.org/english/tratop_e/sps_e/spsund_e.htm (accessed November 9, 2011).














































