2
Transparency and Food Safety and Inspection Service Data-Sharing
TRANSPARENCY AND DISCLOSURE OF DATA
Generally, the release of data like those being contemplated by the Food Safety and Inspection Service (FSIS) is motivated by two broad purposes. The first reflects the principle that public access to information about the activities of government is basic to democratic governance. The Freedom of Information Act (FOIA), enacted in 1966 and amended later, exemplifies the broad aim of transparency and the public’s “right to know”.9 Although the term “right to know” might have various interpretations, the committee uses it as it pertains to the broad public interest in access to information regarding government activities, including its regulatory activities. In this regard, the report is identifying the public interest in providing access to information arising from inspections that can be used broadly by the public for purposes ranging from research by academics to investigative journalism by the media. This is in contrast with the second broad purpose: “targeted transparency” which deals with the release of information to achieve specific outcomes of public benefit (e.g. risk reduction from exposures). As a prime example of targeted transparency, the Bhopal accident led to the passage of the Toxic Release Inventory, which is a disclosure policy directed at the provision of emission information to reduce exposures to potentially dangerous chemicals from manufacturers. In short, this is an example of a response to a public health risk that was addressed through the requirement of disclosure of specific types of information.
Although the vast majority of data collected by FSIS are not made publicly available, some can be accessed through FOIA.10 FSIS data that may be obtained through FOIA requests include the following:
_________________
9The Freedom of Information Act, Pub. L. 89-487, July 4, 1966, 80 Stat. 250 (codified as amended at 5 USC § 552(b) 2000). See Fung et al. (2007), pp. 25–29, for a discussion of the origins of disclosure policies.
10Although strictly speaking these data are already available via FOIA, such a change in policy would represent more than a mere increase in the dissemination of disaggregated data. Making this information readily available in a digital format makes it accessible to a far wider set of users and useful for a potentially broader set of purposes. Throughout this report, therefore, the committee uses the term disclosure (rather than dissemination) in describing the provision of FSIS information via the Internet.
- Microbiological sampling and testing data (for example, testing for Escherichia coli O157:H7, Salmonella and Listeria monocytogenes).
- Residue sampling and testing data (for example, testing for drug, pesticide and other chemical residues).
- Facility-specific noncompliance records (NRs) identified during routine inspection activities.
- Food-safety assessments (FSAs), evaluations of the entirety of a facility’s food-safety program, including the nature and source of raw materials, processes, the environment, and all other aspects included under the Hazard Analysis Critical Control Points (HACCP)11 plan.
- Facility-specific HACCP verifications.
- Foodborne-disease outbreak investigation closeout reports.
Depending on the individual circumstance (case by case), portions of the data listed above may be withheld under one or more FOIA exemptions. The specific reasons for denying FOIA requests for data or for not releasing data in their entirety or original form are given in Box 2-1.12 FSIS has a Web site13 that provides FOIA reports annually, disclosing summary information on the number of initial FOIA requests received, their dispositions, and information on appeals of denials of information. The Web site details the number of requests that were denied and their FOIA exemption categories. For example, in 2004 and 2005, the most common reasons for denying FSIS FOIA requests were (in descending frequency) exemptions 6, 4, 7c, and 2 (see Box 2-1).
_________________
11HACCP is a system for managing the safety of food through the analysis and control of biologic, chemical, and physical hazards (NRC, 2010).
12The Freedom of Information Act 5 USC § 552, As Amended By Public Law 104-231, 110 Stat. 3048. See http://www.justice.gov/oip/foia_updates/Vol_XVII_4/page2.htm (accessed June 18, 2011).
13See http://www.fsis.usda.gov/FOIA/2004_FOIA_Report/index.asp (accessed June 20, 2011).
BOX 2-1
Types of Information that Cannot Be Released through the Freedom of Information Act
1. Specifically authorized under criteria established by an Executive order to be kept secret in the interest of national defense or foreign policy and are in fact properly classified pursuant to such Executive order;
2. Related solely to the internal personnel rules and practices of an agency;
3. Specifically exempted from disclosure by statute (other than section 552b of this title), provided that such statute
a. requires that the matters be withheld from the public in such a manner as to leave no discretion on the issue, or
b. establishes particular criteria for withholding or refers to particular types of matters to be withheld;
4. Trade secrets and commercial or financial information obtained from a person and considered privileged or confidential;
5. Inter-agency or intra-agency memoranda or letters which would not be available by law to a party other than an agency in litigation with the agency;
6. Personnel and medical information, and similar files the disclosure of which would constitute a clearly unwarranted invasion of personal privacy;
7. Records or information compiled for law enforcement purposes, but only to the extent that the production of such law enforcement records or information
a. could reasonably be expected to interfere with enforcement proceedings
b. would deprive a person of a right to a fair trial or an impartial adjudication
c. could reasonably be expected to constitute an unwarranted invasion of personal privacy
d. could reasonably be expected to disclose the identity of a confidential source (including a state, local, or foreign agency or authority or any private institution) which furnished information on a confidential basis, and, in the case of a record or information compiled by a criminal law enforcement authority in the course of a criminal investigation or by an agency conducting a lawful national security intelligence investigation, information furnished by a confidential source
e. would disclose techniques and procedures for law enforcement investigations or prosecutions, or would disclose guidelines for law enforcement investigations or prosecutions if such disclosure could reasonably be expected to risk circumvention of the law or could reasonably be expected to endanger the life or physical safety of any individual;
8. Contained in or related to examination, operating, or condition reports prepared by, on behalf of, or for the use of an agency responsible for the regulation or supervision of financial institutions; or
9. Geological and geophysical information and data, including maps, concerning wells.
The second broad purpose of information release is to achieve specific public-policy objectives. Such disclosure is a form of “targeted transparency”. Lessons can be drawn from a wide variety of targeted-transparency policies that have been enacted in the last 3 decades (Fung et al., 2007). The drivers that make disclosure or transparency effective or not are influenced by the behavior of three sets of actors: the parties disclosing information either voluntarily or because of mandated requirements (usually private businesses), the parties that use the disclosed information (consumers, workers, investors, and academic researchers), and the parties that act as the providers, aggregators, or conduits of information (such as the disclosers themselves, the government, and third-party providers). Whether disclosure will ultimately improve the public outcomes of concern depends in large part on the behavior of those three sets of parties, which in turn depends on the specific problem under consideration.
Fung et al. (2007) describe the critical interactions between users and disclosers as constituting an “action cycle”.14 The action cycle is driven by how embedded the information is in the decision-making processes of users and disclosers. As Figure 2-1 illustrates, the effect of a targeted transparency policy (or information disclosure in general) depends first on how users understand and integrate information into their decisions, which translate into changes in their behavior (such as the products that they buy or the activities that they undertake). Actions taken by users, in turn, have effects if they are perceived and then acted on by the disclosers. That depends heavily on whether disclosers are able to discern changes caused by disclosure and how much those changes alter business performance.15 Finally, effectiveness is determined by the degree to which changes in discloser behavior lead to improved social outcomes of initial concern (as opposed to fostering gaming behavior or shifting of activities from those whose disclosure is required to others that might have undesirable outcomes).
_________________
14Dranove and Jin (2010) cover similar ground on the drivers of transparency effectiveness but bring in a great deal of additional research (theoretical and empirical) published in the last 3–4 years. Although they classify the drivers of the effects of quality disclosure in somewhat different terms that are rooted in more formal economic theory, they identify similar explanatory factors that affect when disclosure policies (voluntary or mandatory) are most likely to improve social outcomes.
15This step of the action cycle is in many respects similar to firms’ expected response to any form of regulation—that is, it is driven by the perceived benefits of responding relative to the costs of doing so. The difference is that behavior change arises from a more complex chain of events under transparency policies than under traditional standards-based regulation. See Levin et al. (2009) for a related discussion.
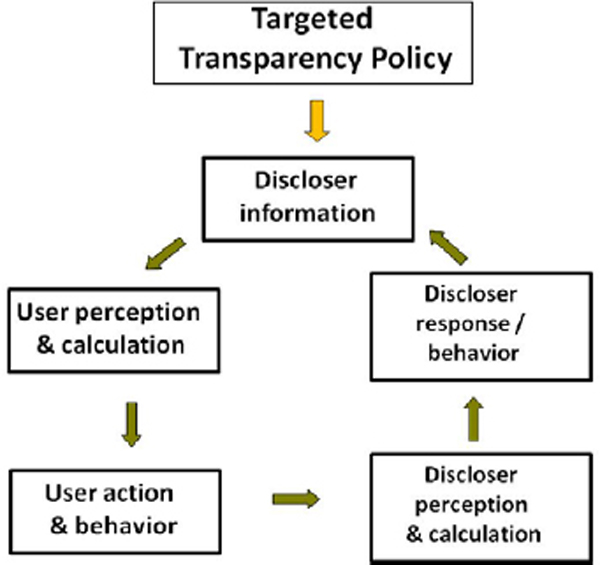
Figure 2-1 Transparency action cycle.
Source: Fung et al. (2007).
For transparency policies to be effective in changing regulated actors’ behavior in the direction specified by public policies, the empirical research summarized by Fung et al. (2007) and Dranove and Jin (2010) points to a set of stringent conditions regarding how information is presented, interpreted, and incorporated in decision-making. Not surprisingly, many transparency policies fall short in that the various requirements of the action cycle fail to be met, either because of poor policy design or because of the poor fit between the identified policy problem and the use of disclosure as a tool to address it. Disclosure or transparency initiatives must be crafted with a clear understanding of who the users of information are and how they will respond to information; the profile of the disclosers, the markets in which they operate, and their incentives to respond to the provision of more information about them; and the part that the government and other third-party actors may play in providing the information or aggregating it into a form most useful to consumers or other users.
TYPES OF DATA COLLECTED BY THE FOOD SAFETY AND INSPECTION SERVICE
Government agencies can disclose many types of data. For purposes of general overview, the committee identified three general data categories:
Category 1: Data arising from the activities of agencies as part of their normal enforcement and compliance efforts.
Category 2: Data arising from the outcomes of enforcement and compliance efforts that have been interpreted by others for use by end users.
Category 3: Data collected by agencies from voluntary programs that are not associated with normal enforcement and compliance efforts but are nonetheless intended to provide information.
In general, decisions regarding public data release will depend on which data type is at issue. Data are collected by FSIS in association with its mission to monitor the safety of domestic and imported meat, poultry, and processed egg products and in the process of its routine inspection, sampling or testing, and enforcement activities. FSIS uses its data as the basis for determining the effectiveness of its oversight activities, primarily through the implementation of Pathogen Reduction (PR) HACCP programs. In consultation with FSIS, the committee identified two major FSIS data types to be considered for public release:
- Inspection and enforcement data, which are collected by inspectors whenever a facility is in operation. These data are collected to ensure that performance standards are being met and that an establishment is controlling its processes in an appropriate manner. Data in this category include NRs and Food Safety Administrative Actions.
- Analytical data, also called sampling or testing data, which are collected in support of verification and enforcement and include the incidence of key foodborne pathogens—such as Salmonella, E. coli O157:H7, and L. monocytogenes. Data on residue sampling and testing are also in this category (See Box 2-2 for more details on specific FSIS pathogen sampling and testing programs that provide these data).
According to the broad data categories presented above, all the data that FSIS is considering for establishment-specific public access arise from current activities of the agency as parts of its normal enforcement and compliance efforts. That is, the data are being collected as part of FSIS’s mandated inspection requirements. The vast majority of data are microbiological. The data are detailed further in Tables 2-1 and 2-2. Tables 2-3 and 2-4 are examples of establishment-specific microbiological data that FSIS is considering for public release.
Note that FSIS is not considering the release of establishment-specific data from baseline studies, which constitute a form of microbiological sampling and testing intended to provide background information to inform future regulations or to evaluate the efficacy of existing regulation. Likewise, release of establishment-specific molecular typing data, which would require collaboration with other food-safety agencies, is not being considered by FSIS. FSIS recognizes that these data types might be considered in the future, but release of establishment-specific data from baseline studies and molecular typing would pose a different set of issues; by agency request, they were considered outside the committee’s deliberations.
Box 2-2
FSIS Pathogen Sampling and Testing Programs
Escherichia coli O157:H7 testing program. For regulatory purposes, FSIS initiated a microbiological testing program in 1994 for detecting E. coli O157:H7 in raw ground beef. The program’s original objective was to stimulate industry testing and other actions to reduce the presence of E. coli 0157:H7 in raw ground beef. At present, product sampling is among several activities conducted by FSIS for verifying the effectiveness of HACCP systems. E. coli O157:H7 is classified as an adulterant by FSIS, so finding it in a food product has specific regulatory consequences. If it is found, the implicated lot of ground beef must be segregated and then sent to a renderer, a landfill, or an establishment that will cook it in compliance with FSIS regulations. Data from this program include results of testing of verification samples and followup samples (taken after a positive finding) taken from federal, retail, and import establishments. Details of the program can be found at http://www.fsis.usda.gov/Science/Ground_Beef_E.Coli_Testing_Results/index.asp.
Salmonella testing programs. FSIS collects Salmonella data as part of a variety of programs (http://www.fsis.usda.gov/Science/Microbiology/index.asp), including its Salmonella verification testing program for raw meat and poultry and its ready-to-eat meat (RTE) and poultry products testing program. Although also pathogenic, Salmonella, unlike E. coli O157:H7, is not classified as an adulterant by FSIS. Therefore, its presence in raw meat does not have lot-specific consequences but is used by FSIS as an indicator of process control. Process control is evaluated by a processing establishment’s performance on “Salmonella sets” or a series of Salmonella tests. The level of performance expected on a Salmonella set is determined for different classes of FSIS-regulated products on the basis of the historical performance of the industry related to those classes. Establishments that “pass” their Salmonella sets are viewed as having their process under control; plants that fail are viewed as having processes that are out of control and are placed under a greater degree of regulatory scrutiny with specific consequences, including a review of their HACCP plans.
Listeria monocytogenes testing programs. Unlike E. coli O157:H7 and Salmonella, L. monocytogenes is an important source of concern not in raw meat and poultry but rather in cooked meat and poultry products that are processed in such a way that its growth is not inhibited. Finished products, food-contact surfaces, and nonfood environments can all be tested for Listeria. Since 1983, FSIS has conducted regulatory microbiological testing programs focused on L. monocytogenes contamination of RTE meat and poultry products. Those programs have evolved; the most recent iterations include the RTE001 project (implemented in 2005), in which establishments are chosen for sampling based on the different risk factors for L. monocytogenes contamination. In 2006, a second project, designated RLm, was initiated on the basis of risk factors referred to as phase 2 of L. monocytogenes risk-based sampling. RLm includes sampling of products, product-contact surfaces, and environmental surfaces in combination with a comprehensive FSA. More details on the L. monocytogenes testing program can be found at http://www.fsis.usda.gov/Science/Micro_Testing_RTE/index.asp.
USES AND USERS OF FOOD SAFETY AND INSPECTION SERVICE DATA AND DATA-SHARING EFFORTS
Some of the data collected by FSIS in establishment-specific form are already publicly available as HTML or PDF documents accessible on the FSIS Web site.16 That is the case, for instance, for some data from verification and laboratory testing programs and for quarterly enforcement-report data (see Tables 2-1 and 2-2). However, the vast majority of FSIS data released to the public are in aggregated form. For example, summary data on FSIS slaughter inspections (such as number of head slaughtered and live and dressed weights) are posted by the National Agricultural Statistics Service (NASS) on its Web site.17 Monthly and annual slaughter-volume data are provided in an aggregated form by class of animal, state, region, or type of facility. Enforcement data are released in aggregated or summarized form in FSIS quarterly enforcement reports. Import and export data collected by other federal agencies can also be found on the FSIS Web site.
Although the data gathered by FSIS from plant inspections and product or environmental testing are used as the basis for ensuring the safety of meat, poultry, and processed egg products that go into general commerce, they serve—or might serve—other public purposes. For instance, the data can be analyzed for trends and anomalies that might indicate current or emerging food-safety problems. FSIS has a Data Analysis and Integration group (DAIG) in the Office of Data Integration and Food Protection.18 The DAIG’s primary role is to “coordinate…the Agency’s data collection, analysis, and integration activities across all program areas”. It is “responsible for evaluating individual FSIS data streams, ensuring data analyses are consistent and of high quality, and conducting data analyses to inform Agency decisions; in addition to processing ad hoc and Freedom of Information Act data requests” (FSIS, 2010c).
The data can also be used in support of food-attribution estimates (estimates of the proportion of cases of particular diseases that are associated with specific food products). Food attribution is one of the objectives of the Foodborne Diseases Active Surveillance Network (FoodNet),19 a program that involves the Centers for Disease Control and Prevention, 10 state health departments, FSIS, and the US Food and Drug Administration. The US Department of Agriculture (USDA) Agricultural Marketing Service (AMS), which procures meat for various federal food and nutrition programs, uses FSIS data in its vendor-evaluation process to ensure that it contracts only with establishments that can produce or process safe and wholesome products (M. E. O’Connor, USDA AMS, Washington, DC, personal communication, June 2, 2011). The NASS uses data collected through the FSIS Electronic Animal Disposition Report System (eADRS) for estimating total red-meat production in the United States; these data are posted on its Web site. Production estimates for the various classes of livestock are used by USDA and the livestock industry in determining future meat supplies and producer prices. Estimates are also used by agricultural economists in their analysis and research programs (NASS, 2009).
_________________
16See http://www.fsis.usda.gov/science/Data_Collection_&_Reports/index.asp (accessed June 12, 2011).
17See http://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Livestock_Slaughter/index.asp (accessed June 14, 2011).
18See http://www.fsis.usda.gov/about/ODIFP/index.asp (accessed August 17, 2011).
19See http://www.cdc.gov/foodnet/ (accessed June 20, 2011).
The main users of FSIS data are consumer-advocacy groups, companies and industry associations, the news media, and academics. Individual consumers may not be willing or able to invest the time and effort necessary to analyze FSIS data, but consumer groups or others can perform this function on their behalf and disseminate FSIS data and analyses of data to the public. Consumer-advocacy groups have used FSIS data to educate consumers about the safety of meat and poultry products and to inform public-policy decision-making (see CSPI, 2002; FWW, 2006; FWW, 2010). In most cases reviewed by the committee, the information used for those purposes arose from FOIA requests.
Food processors and retailers could potentially use FSIS data to inform sourcing decisions and to manage risks associated with their supply chains. Industry groups might use them in a similar manner and serve as collective agents in analyzing information for members and potentially for education or even for self-regulation. Companies may seek to use the information to determine how they rank relative to their peers and for competitive advantage.
Both the traditional news media and emerging Internet news organizations (such as ProPublica) may draw on disaggregated data in developing stories about food safety. This source of disaggregated data may be of particular importance given the contraction in the number of traditional local news reporters.
Finally, academic researchers are an important user group. For example, FSIS data obtained through FOIA have been used in peer-reviewed publications (see Nelson, 2009; White and Moore, 2009) and meeting presentations (M. Ellis, University of Illinois, Urbana, IL, personal communication, August 12, 2011). Academicians have perhaps the broadest interest in establishment-specific data, analyzing it in ways to discern patterns, distributions, and data complexity that would not be possible with aggregated data. Such analyses can support risk-assessment efforts, epidemiological attribution, and public-policy decision-making.
THE ROLE OF THE FOOD SAFETY AND INSPECTION SERVICE PUBLIC HEALTH INFORMATION SYSTEM
With so many data being produced daily, it is important for FSIS to have a means of archiving its data, preferably in a form that can be readily updated and is searchable. FSIS has recently embarked on an effort to develop a data analytics system called the Public Health Information System (PHIS) (FSIS, 2010a). The PHIS was created in response to a 2007 recommendation from the USDA Office of the Inspector General for the purpose of improving FSIS’s inspection systems and developing an integrated data infrastructure (FSIS, 2010b).
The PHIS was designed to
- Serve as a repository for data gathered from all domestic inspections and import and export inspections.
- Help FSIS to have a consistent, data-driven inspection, auditing, and scheduling system.
- Support predictive analytics by facilitating timely analysis of data from multiple sources, thereby enhancing FSIS’s ability to determine trends, patterns, and anomalies for the purpose of identifying emerging food-safety problems.
- Facilitate more effective coordination within FSIS, between FSIS and other federal agencies, and between FSIS and industry to improve investigations and contaminant trace-back activities.
The PHIS is not accessible to the general public. It can currently be accessed by FSIS personnel. FSIS plans to provide access, on a restricted basis, to other federal agencies (only after authorization through a memorandum of understanding) and to private entities that have been granted authorization by FSIS to view data about their own establishments. Data in the PHIS that are accessible to other federal agencies and private establishments may also be obtained by the public through FOIA requests. FSIS was clear that any public data-sharing efforts would not be designed through direct interface with the PHIS but rather that data would be accessible through export to a portal, such as data.gov.
In summary, FSIS releases large amounts of data, usually in aggregated form, in periodic releases or as summaries. The question is whether the benefits of augmenting existing disclosure to include establishment-specific data would outweigh the potential costs.
Table 2-1 FSIS Analytical (Sampling and Testing) Data That Are Under Consideration for Establishment-Specific Releasea
| Data Type | Description of Data | Current Status of Public Posting | ||
| Results of microbiological testing program for Escherichia coli O157:H7 |
Data include
|
Data posted only in aggregated form | ||
| Results of RTE meat and poultry testing |
Data include
|
|||
|
Data posted only in aggregated form | |||
| Results of data analysis for routine Listeria monocytogenes (RLm) risk-based sampling program |
The RLm risk-based sampling program covers sampling and testing of products, product-contact surfaces, and environmental surfaces; data include
|
Data posted only in aggregated form | ||
| Analysis of ALLRTE (random verification sampling of all meat and poultry products) and RTE001 (sampling and testing program based on establishment risk factors); sampling results on Salmonella spp. |
Data include
|
Data posted only in aggregated form | ||
| Results from microbiological testing program for pasteurized egg products |
Data includes
|
Data posted only in aggregated form | ||
| Results from Salmonella verification testing program for raw meat and poultry |
Verification that establishments are meeting performance standards for Salmonella; data include
|
Monthly reporting is establishment-specific; quarterly and annual reports are posted only in aggregated form | ||
| Serotyping of salmonellae from meat and poultry products | Serotype profile of Salmonella isolates from meat and poultry products, annual reports (1998–2010) and quarterly reports | Data posted only in aggregated form | ||
| Residue violators alert list | Residue violators repeat list (Part I) contains names of persons or establishments responsible for having more than one residue (drug, pesticide, or other chemical) violation in animals presented at slaughter and tissue, residue, value, and tolerance (to assist FSIS inspection personnel) | Data posted on a per-establishment basis | ||
| Residue violators repeat list (Part II) contains names of producers (listed by state) responsible for having more than one residue (drug, pesticide, or other chemical) violation in animals presented at slaughter (for livestock markets and establishments) | ||||
| Dioxin and dioxin-like chemicals in the US domestic meat and poultry supply | Information gathered through periodic surveys for dioxins, furans, and dioxin-like polychlorinated biphenyls (PCBs); data include toxic equivalent values for dioxins and furans and for dioxin-like PCBs; states where animals were produced are listed for each sample (market hogs, steer or heifer, young chicken, and young turkey) | Data posted only in aggregated form | ||
| FSIS testing results on melamine in retail meat and poultry | Sampling plan (number of samples collected), products analyzed in laboratory, and laboratory test results, 2009 | Data not establishment-specific | ||
aYears are calendar years.
Table 2-2 FSIS Inspection and Enforcement Data That Are Under Consideration for Establishment-Specific Releasea
| Data Type | Description of Data | Current Status of Public Posting | ||
| Quarterly enforcement reports (1998–2011) |
|
Noncompliance records and appeals; product-control actions are aggregated in quarterly enforcement reports Notices of prohibited activity, administrative actions, civil actions, and criminal actions are all posted in establishment-specific form |
||
| Eligible countries, products, and certified establishments |
|
Names of countries and products given Names of eligible establishments given by country Names of audited establishments given by country |
||
| FSIS import data | Volume of imported meat, poultry, and egg products presented for re-inspection by FSIS at port of entry, 2005–2010 | Weight data given by country | ||
aYears are calendar years.
| Form Id | Establishment Number | Establishment Namea | Sample Source Name | Sampling Project | Collect ion Date | Product Name | Analysis | Result |
| 300000001 | M1 | Establishment 1 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/21/11 0:00 | Ground beef patties | E. coli O157:H7 | Negative |
| 300000002 | M2 | Establishment 2 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/21/11 0:00 | Ground beef | E. coli O157:H7 | Negative |
| 300000003 | M3 | Establishment 3 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Ground beef | E. coli O157:H7 | Negative |
| 300000004 | M4 | Establishment 4 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Ground beef | E. coli O157:H7 | Negative |
| 300000005 | M5 | Establishment 5 | Product-Raw-Intact-Beef | MT50 | 10/24/11 0:00 | Beef trimmings | E. coli O157:H7 | Positive |
| 300000006 | M6 | Establishment 6 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Raw ground beef | E. coli O157:H7 | Positive |
| 300000007 | M7 | Establishment 7 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Coarse ground beef | E. coli O157:H7 | Negative |
| 300000008 | M8 | Establishment 8 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Ground beef | E. coli O157:H7 | Negative |
| 300000009 | M9 | Establishment 9 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Ground beef | E. coli O157:H7 | Negative |
| 300000010 | M10 | Establishment 10 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Ground beef | E. coli O157:H7 | Negative |
| 300000011 | M11 | Establishment 11 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Ground beef patties | E. coli O157:H7 | Negative |
| 300000012 | M12 | Establishment 12 | Product-Raw-Intact-Beef | MT50 | 10/24/11 0:00 | Beef trimmings | E. coli O157:H7 | Negative |
| 300000013 | M13 | Establishment 13 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Ground beef | E. coli O157:H7 | Negative |
| 300000014 | M14 | Establishment 14 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43 | 10/24/11 0:00 | Ground beef | E. coli O157:H7 | Negative |
aActual establishment names will appear in this column when FSIS posts the data.
| Form Id | Establishment Number | Establishment Namea | Sample Source Name | Sampling Project | Collect ion Date | Product Name | Analysis | Result |
| 300000015 | M16 | Establishment 16 | Product-RTE-Other Fully Cooked, Not Sliced-Beef | RTE001 | 10/26/11 0:00 | Brisket | Salmonella | Negative |
| 300000016 | M17 | Establishment 17 | Product-RTE-Fully Cooked, Sausage Products-Combination species | ALLRTE | 10/26/11 0:00 | Chili | Salmonella | Negative |
| 300000017 | M18 | Establishment 18 | Product-RTE-Fully Cooked, Sausage Products-Pork | ALLRTE | 10/26/11 0:00 | Sausage | Salmonella | Negative |
| 300000018 | M19 | Establishment 19 | Product-RTE-Fully Cooked, Hot Dog Products-Combination species | RTE001 | 10/26/11 0:00 | Wieners | Salmonella | Negative |
| 300000019 | M20 | Establishment 20 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000020 | M21 | Establishment 21 | Product-RTE-Fully Cooked, Sausage Products-Beef | ALLRTE | 10/26/11 0:00 | Beef salami | Salmonella | Negative |
| 300000021 | M22 | Establishment 22 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000022 | M23 | Establishment 23 | Product-Raw-Ground, Comminuted or | HC01_GB | 10/26/11 0:00 | Raw ground | Salmonella | Positive |
| Otherwise Nonintact-Beef | veal | |||||||
| 300000023 | M24 | Establishment 24 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000024 | M25 | Establishment 25 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000025 | M26 | Establishment 26 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000026 | M27 | Establishment 27 | Product-RTE-Fully Cooked, Meat+Nonmeat MulticomponentChicken | RTE001 | 10/26/11 0:00 | Caesar wrap | Salmonella | Negative |
| 300000027 | M28 | Establishment 28 | Product-RTE-Other Fully Cooked, Not Sliced-Combination species | ALLRTE | 10/26/11 0:00 | Cooked salami | Salmonella | Negative |
| 300000028 | M29 | Establishment 29 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000029 | M30 | Establishment 30 | Product-RTE-Fully Cooked, Sausage Products-Pork | ALLRTE | 10/26/11 0:00 | Sausage | Salmonella | Negative |
| 300000030 | M31 | Establishment 31 | Product-RTE-Fully Cooked, Hot Dog | RTE001 | 10/26/11 0:00 | Wieners | Salmonella | Negative |
| Products-Turkey | ||||||||
| 300000031 | M32 | Establishment 32 | Product-RTE-Fully Cooked, Meat+Nonmeat Multicomponent-Beef | ALLRTE | 10/26/11 0:00 | Cooked beef patty | Salmonella | Negative |
| 300000032 | M33 | Establishment 33 | Product-RTE-Fully Cooked, Sausage Products-Combination species | RTE001 | 10/26/11 0:00 | Smoked sausage | Salmonella | Negative |
| 300000033 | M34 | Establishment 34 | Product-RTE | RTE001 | 10/26/11 0:00 | Patties | Salmonella | Negative |
| 300000034 | M35 | Establishment 35 | Product-RTE-Fully Cooked, Sausage Products-Pork | ALLRTE | 10/26/11 0:00 | Sausage gravy | Salmonella | Negative |
| 300000035 | M36 | Establishment 36 | Product-RTE | ALLRTE | 10/26/11 0:00 | Beef chili | Salmonella | Negative |
| 300000036 | M37 | Establishment 37 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000037 | M38 | Establishment 38 | Product-RTE-Other Fully Cooked, Not Sliced-Combination species | ALLRTE | 10/26/11 0:00 | Meatballs | Salmonella | Negative |
| 300000038 | M39 | Establishment 39 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000039 | M40 | Establishment 40 | Product-RTE-Fully Cooked, Sausage Products-Combination species | RTE001 | 10/26/11 0:00 | Smoked sausage | Salmonella | Negative |
| 300000040 | M41 | Establishment 41 | Product-RTE-Other Fully Cooked, Not Sliced-Pork | RTE001 | 10/26/11 0:00 | Canadian bacon | Salmonella | Negative |
| 300000042 | M43 | Establishment 43 | Product-RTE-Fully Cooked, Hot Dog Products-Beef | RTE001 | 10/26/11 0:00 | Beef frankfurters | Salmonella | Negative |
| 300000043 | M44 | Establishment 44 | Product-RTE-Fully Cooked, Hot Dog Products-Combination species | RTE001 | 10/26/11 0:00 | Pork and beef frankfurters | Salmonella | Negative |
| 300000044 | M45 | Establishment 45 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000045 | M46 | Establishment 46 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | HC01_GB | 10/26/11 0:00 | Ground beef | Salmonella | Negative |
| 300000046 | M47 | Establishment 47 | Product-Raw-Ground, Comminuted or Otherwise Nonintact-Beef | MT43S | 10/27/11 0:00 | Ground beef | Salmonella | Negative |
aActual establishment names will appear in this column when FSIS posts the data.
CSPI (Center for Science in the Public Interest). 2002. Field Guide to Safer Turkeys. Available at http://www.cspinet.org/new/pdf/turkeyguide-black_white.pdf. Accessed June 9, 2011.
Dranove, D., and G.Z. Jin. 2010. Quality disclosure and certification: Theory and practice. Journal of Economic Literature 48(4):935-963.
FSIS (Food Safety and Inspection Service). 2010a. Food Safety and Inspection Service’s Public Health Information System: Improving the Safety of Imported and Exported Meat, Poultry, and Processed Egg Products. Available at http://www.fsis.usda.gov/PDF/FSIS_PHIS_Improving_the_Safety_of_Imports_&_Exports.pdf. Accessed May 30, 2011
FSIS. 2010b. Public Health Information System: An Overview for Stakeholders. Available at www.fsis.usda.gov/PPT/PHIS_Stakeholder_Briefing.ppt. Accessed May 30, 2011.
FSIS. 2010c. Office of Data Integration and Food Protection. Available at http://www.fsis.usda.gov/About_FSIS/ODIFP/index.asp#intro. Accessed October 31, 2011.
FWW (Food and Water Watch). 2006. Foul Fowl: An Analysis of Salmonella Contamination in Broiler Chickens, July 2006. Available at http://documents.foodandwaterwatch.org/salmonella%20report%20final%20.pdf. Accessed June 10, 2011.
FWW. 2010. Poison Free Poultry: Why Arsenic Doesn’t Belong in Chicken Feed October 2010. Available at http://www.foodandwaterwatch.org/tools-and-resources/poison-freepoultry/. Accessed June 10, 2011.
Fung, A., Graham, M., and D. Weil. 2007. Full Disclosure: The Perils and Promise of Transparency. New York: Cambridge University Press.
Levin, D., Peck, J., and Y. Lixin. 2009. Quality Disclosure and Competition. Journal of Industrial Economics 57(1):167-196.
NASS (National Agricultural Statistics Service). 2009. Available at http://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Livestock_Slaughter/index.asp. Accessed June 21, 2011.
National Research Council (NRC). 2010. An Evaluation of the Food Safety Requirements of the Federal Purchase Ground Beef Program. Available at http://www.nap.edu/catalog.php?record_id=13069. Accessed June 20, 2011.
Nelson, M.L. 2009. Utilization and application of wet potato processing coproducts for finishing cattle. Available at http://jas.fass.org/content/88/13_electronic_suppl/E133.full.pdf. Accessed August 15, 2011.
White, T.L., and D.A. Moore. 2009. Reasons for whole carcass condemnations of cattle in the United States and implications for producer education and veterinary intervention. Journal of the American Veterinary Medical Association 235(8):937-941.






















