Alzheimer’s Diagnostic Guideline Validation:
Exploration of Next Steps:
Workshop Summary
At the request of the Alzheimer’s Association, the Institute of Medicine (IOM) Forum on Neuroscience and Nervous System Disorders planned and hosted a 2-hour public workshop at the 2011 Alzheimer’s Association International Conference (AAIC).1 Held in Paris, France, on July 18, 2011, the session brought together key stakeholders to discuss next steps in the validation of the new diagnostic guidelines for Alzheimer’s disease (AD), specifically, the revised guidelines recently proposed in papers by the International Working Group for New Research Criteria for the Diagnosis of Alzheimer’s Disease, and three working groups under the auspices of the National Institute on Aging (NIA) and the Alzheimer’s Association.
Gabrielle Silver, global strategic marketing leader for neurology at GE Healthcare and co-chair of the session, charged invited panelists and session participants to consider
• the similarities and differences between the two proposed diagnostic guidelines; and
• the lessons learned from the validation of biomarkers for cardiovascular disease that may be relevant to AD, including strategies
![]()
1 The role of the ad hoc planning committee of the IOM Forum on Neuroscience and Nervous System Disorders was limited to developing this session for the AAIC 2011 meeting. This summary has been prepared by the rapporteurs as a factual overview of the presentations at the session. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and are not necessarily endorsed or verified by the IOM or the Forum.
used in the adoption of the new technology, incorporation of biomarker testing in clinical practice, and impact of targeting a surrogate marker of disease incidence; and
• the studies needed (types, design, execution) for validation and standardization of biomarkers for diagnosing pre-dementia and predicting progression to the dementia phase of AD.
This report is limited to a review of workshop speaker presentations and commentary by panelists and participants and is not intended to be a thorough review of all published literature.
Currently, the most widely used AD diagnostic guidelines are those proposed in 1984 by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS, now the National Institute of Neurological Disorders and Stroke [NINDS]) and the Alzheimer’s Disease and Related Disorders Association (ADRDA, now the Alzheimer’s Association). William Thies, chief medical and scientific officer for the Alzheimer’s Association and co-chair of the session, noted that in 1984, there was a sense that AD was a binary condition. However, scientific advances over the past decade now indicate that AD is a continuous, progressive cognitive disease, most likely beginning asymptomatically many years before dementia is apparent. This presents challenges when trying to study the patient population including characterizing patients as normal, at risk, prodromal, or having clinical dementia.
In 2007, the International Working Group for New Research Criteria for the Diagnosis of Alzheimer’s Disease proposed revisions to the long-standing NINCDS-ADRDA guidelines, followed by nomenclature clarifications in 2010 (Dubois et al., 2007, 2010). Phillip Scheltens, director of the Alzheimer Center at the VU University Medical Center, The Netherlands, and a senior author of the 2007 and 2010 International Working Group publications, said that one goal of the group was to update the guidelines to encompass earlier stages of AD, or prodomal AD.
With similar intent, in 2010, three working groups convened by the NIA and Alzheimer’s Association issued revised guidelines for the diagnosis of AD in a set of four publications (Albert et al., 2011; Jack et al., 2011; McKhann et al., 2011; Sperling et al., 2011). Each of these efforts, the International Working Group and the NIA-AA efforts, included international participants and individuals who took part in both efforts. The NIA-AA guidelines are split into three sets of diagnostic criteria: the first
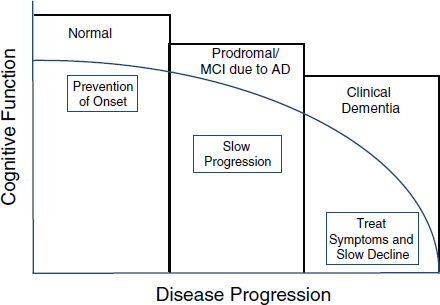
FIGURE 1 Therapeutic implications of Alzheimer’s disease course.
SOURCE: Thies presentation at the AAIC session.
defining preclinical stages of AD, the second focused on diagnosis of mild cognitive impairment (MCI) due to AD, and the third, which is most similar to the original NINCDS-ADRDA criteria, on the diagnosis of dementia due to AD.
Publication of the International Working Group and NIA-AA guidelines, Thies said, reflects a consensus in the field that new guidelines are needed, incorporating the spectrum of cognitive function associated with AD pathophysiological processes (normal, mild impairment, and dementia). Most significantly, both the International Working Group and NIA-AA guidelines present a framework for the incorporation of biomarkers in the diagnosis of AD; however, many researchers feel that more work is needed to determine the validity of these guidelines and their potential utility in both the research and clinical setting.
Thies noted that there are therapeutic implications of validated predictive and diagnostic biomarkers of AD, including the potential ability to not only treat symptoms and slow cognitive decline of patients with clinical dementia, but also to slow disease progression of those in the prodromal stage, and even perhaps to prevent disease onset in the normal population (Figure 1).
Scheltens provided a brief comparison of the International Working Group and NIA-AA guidelines on the basis of terminology, cognitive guidelines, biomarker guidelines, and potential implementation challenges.
Although there are minor differences in terminology between the two guidelines, the main difference is that for the symptomatic predementia stage, the NIA-AA retained the term “MCI due to AD,” while the International Working Group chose the term “prodromal AD” to refer to this stage.
Regarding the cognitive guidelines, the International Working Group chose memory impairment as the starting point. The NIA-AA criteria took a more liberal standpoint, Scheltens said, in that they kept the term mild cognitive impairment, and also included dementia.
The biomarker guidelines differ more significantly. The International Working Group considered cerebrospinal fluid (CSF) amyloid β (Aβ) or tau protein biomarkers, structural magnetic resonance imaging (sMRI), and positron emission tomography (PET) all as suitable evidence of brain amyloid or neuronal injury, without any hierarchy (Dubois et al., 2007). In contrast, the NIA-AA guidelines make a distinct separation between neuronal injury markers (e.g., CSF tau, hippocampal injury, and MRI evidence of neurodegeneration) and brain amyloid markers (e.g., PET and CSF Aβ). The NIA-AA guidelines use the two sets of markers together to define certainty of the diagnosis as high (both markers positive), intermediate (one marker positive and the other absent), low (both markers negative), or uninformative (one marker positive and the other negative).
The strength of the International Working Group criteria, Scheltens said, is that it is disease-driven, focusing on the disease in the brain. A limitation is the lack of a specific hierarchy of biomarkers, which was not possible given the knowledge at the time, Scheltens noted. Also, the new term “prodromal AD” led to some confusion, which is why the lexicon paper was subsequently published (Dubois et al., 2010).
The strengths of the NIA-AA guidelines included retention of the MCI definition, taking into account the additional evidence on the use of biomarkers available at the time (targeting the two stages, amyloid deposition and neuronal injury), and recognition of the hierarchy of the biomarkers. A limitation, Scheltens said, is the publication of three sets of guidelines to address, in effect, the same issues.
Challenges to Implementation of the Revised AD Diagnostic Criteria
One issue not yet resolved, Scheltens said, is how to deal with conflicting biomarker results (e.g., low Aβ with normal tau, elevated phospho-tau
with a normal Aβ, or normal PET scan and abnormal Aβ). Standardization and validation of each of the marker methodologies is also needed, especially as the focus moves from diagnostic to prognostic (i.e., assessing the biomarker profile of preclinical patients to predict progression to dementia).
While the guidelines were developed specifically for research use (i.e. identification of patients with AD, or those with the potential to progress to AD, for enrollment in clinical trials), Scheltens noted that these guidelines will inevitably be used in clinical practice. Advancing the diagnosis of AD raises a variety of questions. For example, how will biomarkers be incorporated into clinical practice? Are there ethical issues? For example, will patients labeled as having the potential for AD be treated earlier? What is the practical impact of asymptomatic patients being labeled as having AD based on a biomarker (e.g., driving, insurance)? In addition, implementation of the guidelines requires that Alzheimer’s centers may have to develop a higher level of sophistication with regard to technologies (e.g., PET scanners) and methodology (e.g. biomarker testing), as well as staff understanding and expertise. There may be costs associated with the acquisition and use of these platforms and the education of staff.
Overall, Scheltens commented, the field is moving in a direction of diagnosing AD before the onset of dementia. The advent of clinical biomarkers to the field will enable opportunities for earlier definitive diagnosis and intervention. Current recommendations for revisions to AD diagnostic guidelines call for biomarkers as evidence of AD, but there is still work to be done (e.g., hierarchy, standards, validation, normal values) and issues to be addressed (e.g., ethics, practicality).
Over the years, better prevention and earlier diagnosis and treatment of cardiovascular disease has been made possible, in part, by testing for markers of risk such as increased serum cholesterol. Robert Mahley, senior investigator at the J. David Gladstone Institutes of Cardiovascular Disease and Neurological Disease and professor of pathology and medicine and at the University of California, San Francisco, described some of the lessons learned from studies of cardiovascular disease, cholesterol, and treatment approaches that could help inform strategy development for the validation and use of biomarkers in the diagnosis of AD.
Coronary Heart Disease
Coronary heart disease (CHD) deaths have decreased significantly in the United States over the past several decades; over the 20-year period from 1980 to 2000, the CHD death rate per 100,000 individuals was reduced by nearly half (Ford et al., 2007). Mahley attributed this reduction to two major causes: advances in evidence-based medical therapies (e.g., better initial treatment, more effective revascularization techniques such as angioplasty and bypass surgery, and secondary prevention following a myocardial infarction), and change in risk factors associated with heart disease (e.g., reduction in total plasma cholesterol levels, better control of blood pressure, decreased smoking, and an increased understanding of the importance of exercise).
Despite the overall reduction in cardiac-related deaths, heart disease remains the number one cause of death in the United States, and in almost all other industrialized countries of the world, accounting for more than 30 percent of deaths. It is estimated that in 2011 there will be 785,000 new myocardial infarctions (MIs) in the United States and 470,000 recurrent MIs (Roger et al., 2011).
Success Factors in Reducing Cardiac Deaths
Mahley offered three key factors contributing to the downward trend in cardiac deaths.
• Strong basic science foundation. There is a large body of knowledge on cholesterol including origin, control of synthesis, transport via lipoproteins, importance of apolipoproteins, metabolic function, arterial wall accumulation, effect of diet, effect of genetic mutations, and development of relevant animal models.
• Successful drug trial reporting reductions in cardiovascular events. Results reported in 1984 from the Coronary Primary Prevention Trial showed a 1 to 2 percent reduction in CHD for every 1 percent reduction in low-density lipoprotein (LDL) cholesterol, and that greater adherence to the drug therapy regimen resulted in greater reduction.
• Strong expert consensus. An NIH consensus conference, held in 1984 and reported in 1985, issued conclusions based on a strong scientific foundation and the results of the Coronary Primary Prevention Trial. The experts’ primary conclusion was that cholesterol is a causative factor in CHD, and that levels must be reduced (Consensus Conference, 1985).
Lipid Research Coronary Primary Prevention Trial
The Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT) was the first trial to demonstrate that lowering cholesterol prevents MIs (Lipid Research Clinics Program, 1984a,b). The randomized, double-blind study, which began in the 1970s, enrolled 3,800 men with no history of heart disease, but with very high cholesterol levels, in excess of 260 mg/dl. Participants received cholestyramine, a bile acid sequestrant, and were followed for 7 years.
The overall results indicated that total cholesterol and LDL were decreased by 13 percent and 20 percent, respectively, and there was a 19 percent decrease in cardiac events (definite CHD; non-fatal MI). Participants who adhered to the protocol fully, taking all doses of cholestyramine, had larger reductions in cholesterol (35 percent decrease) and cardiac events (49 percent decrease).
NIH Consensus Conference
The subsequent 1984 NIH Consensus Conference launched the National Cholesterol Education Program to educate both physicians and the public about heart disease and cholesterol. Around the time of the conference, Mahley said, total serum cholesterol of greater than 260 mg/dl was considered too high. By 1995, it was thought that cholesterol in excess of 220 mg/dl was probably too high. More recently, levels in excess of 200 mg/dl are considered too high.
A survey in 1986 showed that only 34 percent of physicians actually considered LDL cholesterol as important in heart disease. In 1995, 75 percent of physicians surveyed accepted the importance of cholesterol in CHD (Cleeman and Lenfant, 1998). Mahley noted that the general public came to recognize the importance of cholesterol in heart disease through NIH publications and through popular magazines such as Woman’s Day.
The NIH Consensus Conference also helped to establish Adult Treatment Panels (ATP) that meet periodically to establish treatment guidelines. Each successive panel report has issued a lower target LDL level, such that levels of less than 100 mg/dl are now considered to be most protective.
The Lipid Hypothesis and Drug Development
Despite the clinical evidence, the “lipid hypothesis” of CHD was somewhat controversial, and Mahley said that the national media coverage of whether the role of cholesterol was fact or fiction resulted in much unwarranted confusion (Steinberg, 2006).
The lipid hypothesis proposed that hypercholesterolemia, or high LDL,
was a causative factor but not the only causative factor, of heart disease. Additional factors include other dyslipidemias; high blood pressure; metabolic syndrome, diabetes, and insulin resistance; obesity; smoking; lack of exercise; and a pro-coagulant state involved in thrombosis. The lipid hypothesis also proposed that correction of LDL cholesterol would reduce progression of CHD. Dietary studies and clinical trials were conducted, and statins, HMG CoA reductase inhibitors, ultimately became the gold standard to test the hypothesis. Based on the combined results of clinical trials of various statins, it is now generally accepted that there is a 20 percent reduction in major vascular events for every 40 mg/dl LDL cholesterol lowered.
There are now data demonstrating pleiotropic effects that may have both direct and indirect impacts on CHD risk. Statins have been shown to also directly affect inflammation, endothelial cell function, plaque stability, and thrombosis; changes in these have been shown to indirectly decrease CHD (Robinson, 2008). Lowering cholesterol with statins is a major mechanism responsible for the decrease in coronary heart disease which has lead to statins becoming the “standard of care.” Statins are now used in higher doses, with wider age groups, and in low-risk patients. This widespread use and acceptance of the value of statins may have the unintended effect of decreasing research into new mechanisms of action for lowering cholesterol and therefore the development of new treatments. This may be of particular concern in light of the growing number of statin-intolerant patients.
Mahley described the Framingham Heart Study, which assessed lifetime risk for CHD associated with multiple risk factors. For example, an individual with an optimal risk burden at age 50 (normal blood pressure, low total cholesterol, absence of diabetes, nonsmoker) would be anticipated to have a 5 to 8 percent risk of CHD-related events over the rest of their life-span. However, having any two or more risk factors at age 50 increases the predicted risk to 50 to 70 percent. Those with an optimal risk profile have a mean life expectancy of at least 10 years longer than those with a risk burden. Together these studies confirm that atherosclerosis and CHD are complex, involving multiple risk factors and multiple pathways to disease, and thus require multiple therapeutic approaches.
Reasons for Partial Success
In clinical trials statins reduced cardiovascular events relative to placebo by 25-35 percent, but this means that drug therapy did not prevent two-thirds to three-fourths of CHD events (Kastelein, 2005). Mahley suggests several reasons for this partial success.
Too Late
A major issue facing the cardiovascular field is “how early is early?” When should cardiac assessment and intervention begin? Atherosclerosis begins in childhood or young adulthood, but the practice is to wait until there are signs of CHD to treat. This, Mahley said, is too late. In studies of Korean War casualties (average age 22 years), 33 percent had fatty streaks in their coronary arteries, which in some cases is a precursor of atherosclerosis (Enos et al., 1953). In another study, healthy organ donors in their 20s showed that 37 percent had raised lesions in their coronary arteries, another indication of early atherosclerosis (Tuzcu et al., 2001). Of individuals who survive the first 2 days following an MI and receive the best current standard of care, 20 to 30 percent will die from CHD within 1 year and 40 to 50 percent of individuals will die will die within 5 years after the first MI.
Mahley noted that a clinical trial to prove that early treatment could reduce CHD will probably never be done; the event rate in young men and women is too low, and such a primary prevention study would likely require hundreds of thousands of subjects, and 20 or more years of follow-up.
Too Short
Most clinical trials are about 5 years long, but cholesterol and other risk factors can be lifelong problems. Thus, the typical study period is too short to be fully informative. There is now evidence that lifelong low cholesterol confers protection. The liver protein, proprotein convertase subtilisin/kexin type 9 (PCSK9), functions as a regulator of LDL receptors on the cell surface. In a small portion of the population, less than 2 percent, there is a loss-of-function mutation that causes low LDL from birth. Levels of LDL in these individuals ranges from 70 to 110 mg/dl, 15 to 28 percent lower than that seen in the general population. These individuals have a 47 to 88 percent reduction in CHD risk (Cohen et al., 2006).
Based on 5-year clinical trials, a 28 percent reduction in cholesterol would result in a 25 to 35 percent drop in CHD. How does this short study translate to the lifelong impact of reduced LDL? Maximal time is needed to achieve a maximal effect.
Too Little
Perhaps the current recommendations for target cholesterol levels are not aggressive enough to protect the majority of individuals, Mahley said. An LDL of 100 to 130 mg/dl may still be too high. Optimal LDL levels are less than 100 mg/dl, and Mahley opined, perhaps closer to 70 mg/dl. Humans tend to have LDL levels of 140 to 150 mg/dl, where lower species
(e.g., mouse, rat, hamster, rabbit, monkey) have LDL cholesterol levels of 20 to 50 mg/dl. Perhaps optimal levels for humans are similar to levels seen in animals.
Too Narrow
Finally, the approach to reducing CHD may be too narrow. As CHD is a complex disease with multiple mechanisms, LDL cholesterol-lowering drugs (i.e., statins) alone may not be enough. High-risk patients may benefit from combination therapy such as aspirin, beta-blockers, and cholesterol lowering drugs (“ABC therapy”). There are also other dyslipidemias, such as low high-density-lipoprotein (HDL) cholesterol or high triglycerides, that warrant intervention. Metabolic syndrome is a management challenge for providers, involving the control of obesity, insulin resistance, coagulation disorders, high triglycerides, and low HDL cholesterol. Hypertension, Mahley said, is one of the best examples of a multi-factorial disease requiring multiple drugs for treatment. In addressing CHD, lifestyle modification is also important (e.g., smoking cessation, increased exercise, improved diet) (Libby et al., 2011).
Lessons for AD Research
Mahley suggested that the barriers to progress for reducing CHD offer lessons for AD research. The same questions will need to be answered for AD.
Too late: When should treatment be started? Both CHD and AD are typically late-onset diseases. Although CHD begins in young adulthood, the average age of the first MI is 65 to 70 years. Clinical symptoms of AD are most commonly diagnosed after the age of 65, however there are predictive signs that could be detected earlier. For example, studies suggest that those who carry the apolipoprotein variant gene, ApoE4, are at higher risk for AD and begin cognitive decline earlier than those with the wildtype ApoE3 gene (Caselli et al., 2009).
Too short: How long should patients be followed in AD clinical trials? As Mahley noted earlier, clinical trials of CHD interventions typically run for 5 years, and require several thousand patients for adequate statistical power, and are likely too short to really see an effect. For AD, he said, there is not yet enough clinical data to be able to predict how many patients are needed and how long the studies must continue, to observe the effects of an intervention.
Too little: How low (or high) should target values for key AD biomarkers be set? For CHD, no lower detrimental level for cholesterol has yet been
established and perhaps “the lower the better.” Ultimately, cells can make what they need. In AD, however, the function of Aβ is not fully understood. What should the target level of Aβ be for amyloid reduction clinical trials? Some studies suggest that high levels of Aβ impair long-term potentiation (LTP) and memory (Cleary et al., 2005; Lambert et al., 1998), and other studies show that low Aβ levels impair LTP, synaptic plasticity, and memory (Puzzo et al., 2011; Weyer et al., 2011).
Too narrow: What are the causative factors of AD? What are the treatment targets? There is strong genetic data supporting the role of the amyloid pathway in AD, however, clinical trials seeking to change the course of the disease with amyloid-targeting drugs have been inconclusive. Tau protein is part of the AD pathogenesis, and studies have shown that reduction of tau is beneficial in amyloid animal models. Inflammation may play a direct role in the central nervous system pathology in AD, and microglial activation and cytokine generation may also be therapeutic targets. Finally, ApoE4 is a major risk factor for AD (a major gene and causative factor); 65 to 80 percent of patients with AD have at least one copy of the ApoE4 allele (Mahley et al., 2006).
In summary, Mahley said, AD, like CHD, is a complex disease requiring multiple therapeutic approaches. A strong basic science foundation and integration of data from multiple types of studies is essential to understand the potentially multiple causes, interacting pathways, and risk factors.
MOVING FORWARD:
VALIDATION OF THE NEW DIAGNOSTIC GUIDELINES
Following the presentations, Mahley was joined for a panel discussion by David Brooks, professor at Imperial College and medical advisor to GE Healthcare; Charles DeCarli, director of the Alzheimer’s Disease Center at the University of California, Davis; and Monique Breteler, professor at the German Center for Neurodegenerative Diseases (DZNE), Germany. Panelists discussed the role of the new guidelines in both clinical trials and in practice, and the importance of standardization as the first step to validation.
Standardization
The focus of AD research has long been diagnosis and treatment, but the availability of biomarkers is moving research into the presymptomatic, preclinical phases. It was clear throughout the discussion that a key challenge to validation and implementation of the new guidelines is the lack of standardization of biomarker tests. Approaches to measuring Aβ, for example, are not standardized and different groups use different cutoff
points and ranges. It was also noted that there are not yet validated neuropsychological measures sensitive enough to detect cognitive effects of drugs in asymptomatic patients.
The first step, Brooks said, to developing biomarkers that are cheaper to standardize is to better define what should be measured. Is it beta-amyloids? If so, in what form? Soluble Aβ in spinal fluid? What do the results mean? Should inflammatory markers that are elevated in MCI and AD be measured? Proteomic profiles? The more that can be understood about the disease, the easier it will be to develop and test for a “fingerprint” of AD, to assess risk and identify those who would benefit from early treatment.
Mahley noted that the cardiovascular field spent a lot of time early on standardizing methodology. From a sampling standpoint, serum cholesterol may be easier to monitor than Aβ in cerebral spinal fluid, but at the time, cholesterol was not easy to measure. Lipid clinics around the country worked with the Centers for Disease Control and Prevention (CDC) to set up and be qualified as standard reference laboratories, allowing for comparison of results from across the U.S. He suggested that standardization of Aβ methodology should be first on the list of tasks.
A participant pointed out that in the Framingham Heart Study, the cholesterol data was being collected before it was known that cholesterol was important for heart disease. Some participants commented that in moving forward with AD clinical trials and longitudinal, population-based studies it will be important to collect as much data as possible on a broad group of potential markers. The addition of standardized methods during these studies to assess and quantify patient quality of life and cognitive abilities would be valuable.
A participant drew attention to the differences in terminology between the International Working Group and the NIA-AA terminology and suggested that standardization of terminology is also needed. It was noted that MCI is an early feature of many diseases, and the use of the term MCI is not going to disappear because one of the guidelines now uses prodromal AD. It is important to make clear that one is discussing MCI that has evidence of AD pathology.
Validation
If the ultimate goal is to produce affordable, effective, and safe ways of screening elderly patients for abnormal pathology to facilitate early treatment, Brooks said, then biomarker tests must be validated in controlled clinical trials.
However, because the new guidelines also apply to individuals in the prodromal stages of AD when symptoms are not apparent, there is a need to conduct population-level studies of “normal” individuals as well.
DeCarli added that, for various reasons, the healthy control population in a clinical trial is not reliably representative of the broader general population, as has been observed on some of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) clinical trials.
A participant noted that in CHD, the endpoints (MI or death) are clear-cut and easily counted. In AD, the progression from MCI to severe cognitive impairment is a slope, and cognitive decline also occurs in individuals who do not have AD. What endpoints will be used to validate the surrogate markers of AD? Research in this area, including careful measures of trajectories of cognitive decline, could be valuable.
It was also noted that conversion to dementia is the major outcome in MCI clinical trials. The precision of that diagnosis, and the differentiation of MCI from dementia, is absolutely fundamental. Currently, the diagnosis hinges entirely on the judgment of the skilled clinician. The draft DSM-V criteria and the NIA-AA guidelines focus on independence of function. One can have mild functional impairment with mild MCI. Dementia is the point when one loses independence in those functions. However, whether a patient remains independent in function is highly dependent on the level of support they have. There is a need for a clear consensus on what this functional impairment has to look like before it constitutes a diagnosis of dementia. Breteler said that rather than defining an absolute cutoff, it may be more important to agree on what rate of decline, or increased rate of decline, constitutes the progression to dementia. While participants and speakers frequently noted that cognition is a continuum, some felt that a binary outcome may still be needed to operationalize change. DeCarli pointed out that there have been more than five clinical trials looking at the conversion of MCI to dementia using adjudication committees to reliably establish the diagnosis. Many suggested, however, that as the guidelines move into clinical practice, the definitions and endpoints will have to be crisper, and that clarity will only come from successful clinical trials.
The New Guidelines in Clinical Research and in Practice
The new guidelines will facilitate measurement of some of the early biomarkers of AD in people who are cognitively normal, DeCarli said. Measuring biomarkers of amyloid pathology in people who are otherwise asymptomatic could help expand the understanding of the role and mechanisms of amyloid abnormalities in the brain. It may also help determine at what point in the process, and how aggressively, to attempt to alter amyloid deposition in the brain to have a meaningful effect on disease and clinical profile. Implementing new diagnostic criteria in clinical trials will also provide data that can help in establishing the risk of AD for people who are asymptomatic.
Breteler suggested that biomarker data can be used to define more homogeneous groups within the very heterogeneous population of AD patients. This could help facilitate more specific, targeted clinical trials and help to identify effects in specific subgroups.
Another benefit of having validated biomarkers of prodromal AD will be the ability to conduct epidemiological studies of Alzheimer’s disease versus Alzheimer’s dementia. Breteler noted that measurement of cholesterol has been very successfully used in studies because it is a relatively inexpensive test requiring only a blood sample. To better understand prodromal AD in the population at large and develop preventive interventions, Breteler suggested that there is a need for easier, cheaper, standardized, more accessible biomarker tests for the early stages of AD.
A participant from industry discussed the development and recruitment of the first prodromal AD clinical trial based on the International Working Group guidelines, and the specific challenges of operationalizing the guidelines. He opined that even with the methodology standardization issues, there is still overwhelming evidence that Alzheimer’s pathology reflected through CSF biomarkers predicts progression to Alzheimer’s dementia. His view is that these studies can be done now, and will improve incrementally as better, standardized assays are available. The available assays for these biomarkers can be carefully used, in parallel to the other mechanisms, to identify those at risk and enrich the study population of a trial.
Although the new guidelines geared toward defining preclinical AD are clearly defined as being for research purposes only, some participants acknowledged that there will be great interest in applying these guidelines in the clinic as well. Specifically, biomarkers may be a useful diagnostic tool when incorporated into the examination by skilled physicians. Individuals in their 50s and 60s are eager to know their risk, often because a parent or grandparent has AD.
Overall, the panel agreed that the new guidelines are not an end point, and it will be important to continue to incorporate new insights and challenge the existing thinking. Moving forward, revisiting the guidelines on a regular basis as new data becomes available will be important, as well as maintaining a multidisciplinary global dialogue.














