Leila dos Santos Macedo, Ph.D.
Brazilian Biosafety Association
Background and Disclaimers:
1- The Brazilian Biosafety Association (ANBio), whose main objective is to improve biosafety and biosecurity in Brazil, prepared this report in response to a 2 April 2009 letter from the United States National Academy’s Committee on International Security and Arms Control (CISAC). The Brazilian Government has no responsibility for this report or for the information in it.
2- ANBio recognizes the importance of CISAC’s project on the risks and responsibilities associated with the international expansion of high-containment laboratory accidents, as well as the importance of following international guidance and agreements to reduce biological threats.
3- This report was produced from survey data collected by ANBio as part of their capacity building program on biosecurity (Figure E1.1). The survey took place from May 2008 through February 2009. Overall, 237 questionnaires were received, which included responses from two institutions in other South American countries (Peru and Equator). The data presented reflects that submitted by survey responders; as such, ANBio is not responsible for any misleading or incomprehensive information.
4- In producing this report, ANBio also considered presentations exhibited by representatives of the Brazilian Government during ANBio’s capacity building program.
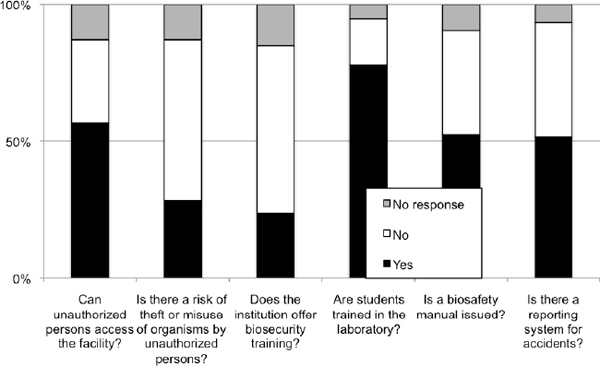
Figure E1-1 The results of a survey conducted by ANBio about the state of biosafety and biosecurity in biological research institutions across Brazil (including two from Peru and Equator). The survey took place from May 2008 to February 2009, and 237 questionnaires were received.
SOURCE: Data are the result of a survey conducted by ANBio.
1- What high-containment biological research facilities exist in your country?
In Brazil, only those laboratories conducting research related to public health, agriculture, and genetically modified organisms (GMOs) are subject to government regulation. There is no oversight or evaluative mechanism for laboratories, high-containment or otherwise, that are established at public or private universities for research activities with biological agents (other than GMOs). So, the answer here refers to public health, agriculture, and GMO laboratories only.
There are a total of 12 BSL-3 laboratories under the responsibility of the Ministry of Health and 8 BSL-3 laboratories under the responsibility of the Ministry of Agriculture (Table E1-1). Brazil currently has no BSL-4 laboratories, although there has been ongoing discussion about building one.
Table E1-1 List of known BSL-3-capable laboratories and microorganisms in Brazil.
| Item | Institution | Microorganism | Risk Classa | Lab BSL |
| 1 | Universidade Federal do Amazonas Laboratório de Genética Animal |
Aspergillus M. tuberculosis MDR |
2 3 |
BSL2 BSL-3 |
| 2 | Fiocruz – IOC Laboratorio de AIDS e Imunologia Molecular |
HIV | 3 | BSL-3 |
| 3 | LACEN – CE Laboratório de Microbiologia |
Micobacterium tuberculosis MDR Yersinia pestis Burkholderia pseudomallei |
3 3 3 |
BSL-3 |
| 4 | Embrapa Suínos e Aves - SC Lab. Virologia/ Laboratório de Sanidade |
Avian Flu virus, New Castle virus, virus of respiratory and reproductive syndrome in swine (PRRS), Mycobacteria |
3,4 | BSL1 BSL2 BSL-3b |
| 5 | Merial Saúde Animal LTDA - SP Departamento Qualidade - Segurança Biológica |
Brucella abortus FMDV |
3 4 |
BSL-3 |
| 6 | FIOCRUZ – IOC Laboratório de Biologia e Parasitologia de Mamíferos Silvestres Laboratórios |
T. cruzi Leishmanias |
2 3 |
BSL-3 |
| 7 | Instituto Nacional de Salud - Peru | M. tuberculosis MDR Leishmania Yellow fever virus Hepatitis, A,B,C virus |
3 3 2 2 |
BSL-3 |
| 8 | Universidade Federal do Rio de Janeiro Departamento de Diagnóstico Oral e Patologia |
HIV | 3 | BSL-3 |
| 9 | Embrapa Gado de Corte - MS Lab. Sanidade Animal e Virologia |
FMDV Brucella spp. Mycobacterium bovis |
4 3 3 |
BSL1 BSL2 BSL-3b |
| 10 | Universidade de Sᾶo Paulo Núcleo de Pesquisas em Raiva do Lab. Virologia Clínica e Molecular do Depto Microbiologia |
Arbovirus Hantavirus |
3 3 |
BSL-3 BSL2 |
| 11 | LANAGRO/SP Setor de Sanidade Aviária |
Avian Influenza virus Newcastle virus |
4 4 |
BSL-3 |
| 12 | Universidade Federal de Pernambuco Departamento de Antibióticos/Laboratórios de Fármacos e Processos microbianos e laboratório de Processos Fermentativos |
Escherichia coli Chlostridium botulinum Coccidioides immitis Penicillium spp. Aspergillus spp. Candida spp Salmonella spp. |
2 2 3 2 2 2 2 |
BSL1 BSL2 BSL-3 |
| 13 | Fundaçᾶo de Medicina Tropical do Amazonas Lab. Virologia |
M. tuberculosis MDR Hepatitis virus Dengue virus Oropoche- and Mayaro virus |
3 2 2 4 |
BSL2 BSL-3 |
| 14 | Fiocruz Centro de Pesquisas Aggeu Magalhᾶes Biotério Central |
Yersinia pestis Hantavirus |
3 3 |
BSL2 BSL-3 |
| 15 | Fiocruz - Centro de Pesquisas Aggeu Magalhᾶes Lab. serviço de referencia em peste |
Yersinia pestis | 3 | BSL-3 |
| 16 | UNESP - Faculdade de Ciências Farmacêuticas Araraquara Depto Análises Clínicas |
HIV M. tuberculosis MDR Hepatitis virus |
3 3 2 |
BSL2 BSL-3 |
| Depto Análises Clínicas | Hepatitis virus | 2 | ||
| 17 | Ouro Fino Saúde Animal | FMDV | 4 | BSL-3 |
| 18 | FIOCRUZ – Centro de Pesquisas Aggeu Magalhᾶes, Lab. IMUNOLOGIA |
Hantavirus | 3 | BSL-3 |
| 19 | Universidade Federal de Pernambuco Lab. Virologia |
HIV HTLV Chamydia trachomatis |
3 3 2 |
BSL-3 |
| 20 | Universidade Federal de Pernambuco Lab. Microbiologia |
E. coli Salmonella Listeria monocytogenes Vibrio parahemoliticus Vibrio cholera |
2 2 2 2 2 |
BSL-3 |
SOURCE: Data was a result of a survey conducted by ANBio from June 2008 to March, 2009. MDR: multi-drug resistant
a according to Brazilian rule
b undergoing validation and commissioning
2- What government organizations are responsible for safety and security of high-containment biological (high BSL) laboratories?
The Ministry of Health is responsible for public health laboratories; the Ministry of Agriculture is responsible for agriculture laboratories. For university labs (except GMO labs), there is no oversight body. For laboratories working with GMOs, the National Biosafety Committee (CTNBio) is the responsible organization.
In March 2008, the Brazilian Ministry of Science and Technology published a resolution with a list of selected agents. The definition of “selected agent” is the same used by the United States Centers for Disease Control and Prevention: “selected agents are those considered by the Health and Human Services to pose a severe threat to human and/or animal health.” With the exception of camel pox virus, Phoma glycinicola, and Rathayibacter toxicus, the Brazilian selected agent list includes all of the agents and toxins on the United States Health and Human Services and United States Department of Agriculture lists. The Brazilian list also includes additional microorganisms, toxins, and some equipment used in BSL-2 and BSL-3 laboratories (Table E1-2). Labs using select agents may voluntarily notify the government. Notification results in informal government visits and additional training.
Table E1-2 Brazilian selected agents that are not also on the United States selected agents list.
SOURCE: HHS And USDA Select Agents and Toxins: 7 CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73 (2008) and the Brazilian Ministry of Science and Technology: Office of the Minister: Resolution No. 10 of 13, March 2008. The Interministerial Commission: Export Control: Sensitive goods, CIBES, using the powers granted to it by Article 4. °, II, of Decree No. 4214 of April 30, 2002.
| Microorganisms | ||
| African cassava mosaic virus | Citri spiroplasma | Duck hepatitis virus, types 1, 2 and 3 |
| Agrobacterium ruby | Citrus leper virus | Eastern equine encephalitis virus |
| Amanita muscarina | Clavibacter iranicus | Encephalitis virus of Powassan |
| Amapari virus | Clavibacter michiganensis subspecies | Enteritis virus of Ducks, Geese and |
| Apiosporina morbid | insidiosus | Swans |
| Apple Chat Fruit | Clavibacter michiganensis subspecies | Enzootic encephalomyelitis flu virus |
| Apple proliferation | nebraskensis | Ephemeral fever virus of cattle |
| Aspergillus flavus | Clavibacter michiganensis subspecies | Erwinia amylovora |
| Aspergillus ochraceus | sepedonicus | Erwinia cypripedii |
| Aspergillus parasiticus | Clostridium perfringens epsilon toxin | Erwinia raphontici |
| Aujeszky disease virus | producers | Escherichia coli producing verotoxins |
| Banana bunchy top virus | Cochliobolus miyabeanus | Fusarium graminearum |
| Bartonella sp. | Cocoa swollen shoot virus | Fusarium moniliforme |
| Borna disease virus | Coriomeningite Linfocítica virus | Fusarium oxysporium f. sp. lies |
| Bursaphelenchus sp. | Cowdria ruminatum | lycopersici |
| Cadang-cadang viroids | Coxiella burnetii | Fusarium sporotrichioides |
| Candidatus liberobacter africanus | Crinipellis pernicious | Gibberella fujikuroi |
| Candidatus liberobacter asiaticus | Curtobacterium flaccumfaciens pv. | Gibberella xylarioides |
| Chikungunya fever virus | poinsettiae | Globodera sp. |
| Chlamydia psittaci | Dengue hemorrhagic fever virus | Glomerella manihotis |
| Chondrostereum purpureum | Deuterophoma tracheiphila | Grapevine flavescence pains |
| Guignardia bidwellii | Pantoea stewartii | Rickettsia tsutsugamushi |
| Guignardia citricarpa | Paracoccidioides braziliensis | Rickettsia typhi |
| Guinea grass mosaic virus | Parana virus | Rustic Xanthomonas pv. cassavae |
| Gymnosporangiumm spp. | Pasteurella multocida type B SAMPLE | Rustic Xanthomonas pv. citri biótipos B |
| Haemophilus equigenitalis | Buffalo and other virulent strains | and E |
| Hemileia coffeicola | Peach Rosette | Salmonella enteritidis |
| Hemorrhagic fever virus of Rabbit | Pear decline | Salmonella paratyphi types a, b, c |
| Hepatitis D virus (Delta) | Penicillium verrucosum | Salmonella typhi |
| Herpesvirus including rhadinovírus | Peronosclerospora sorghi | Salmonella typhimurium |
| Herpesvirus of ATEL | Petequial fever virus Infectious Bovine | Severe acute respiratory syndrome |
| Herpesvirus of Saimiri | Phakopsora meibomiae | virus |
| Histoplasma capsulatum all kinds, | Phakopsora pachyrhizi | Shigella dysenteriae |
| including the variety duboisii | Phoma exigua var. foveata | St. Louis encephalitis virus |
| Kahawae Colletotrichum | Phoma tracheiphila | Sugarcane Fiji disease virus |
| (Colletotrichum coffeanum var. | Physopella ampelopsidis (Phakopsora | Thecaphora solani |
| Virulans) | euvitis) | Theileria annulata, bovis, hirci, parva |
| Latino virus | Phytophthora boehmeriae | and related agents |
| Lethal yellowing (lethal yellowing of | Phytophthora erythroseptica | Tilletia controversial |
| coconut palm) | Pichinde virus | Tilletia indicates |
| Linfotróficos virus of adult T cells, | Plamospara halstedii (except strain 2) | Tomato ringspot virus |
| HTLV-1 and HTLV-2 | Pluteus glaucus | Trichodorus sp |
| Longidorus sp. | Potato latent virus Bookshelves | Urocystis agropyri |
| Lyss virus | Potato Spindle Tuber Viroids | Vibrio cholerae |
| Microcyclus ulei | Pseudomonas syringae pv. passiflorae | Viruses of hemorrhagic fever with |
| Monilinia fructigena | Pseudomonas tolaasii | Renal or pulmonary syndrome |
| Moniliophthora roreri | Psilocybe cubensis | (Hantaan, Seoul, Sin Nombre, |
| Murray Valley encephalitis virus | Psilocybe Mexican | Puumala, Prospect Hill, Dobrava, |
| Mycobacterium bovis strains except | Puccini graminis | Thailand, and Tottapalayam Muerto |
| BCG strains | Puccini melanocephala | Canyon virus) |
| Mycobacterium tuberculosis | Puccini striiformis | Viruses related to Ganjam and Dugbe |
| Mycoplasma agalacteae (goats and | Pyricularia grisea | Wesselsbron disease virus |
| sheep) | Retroviruses including Human | Western equine encephalitis virus |
| Mycosphaerella fijiensis | immunodeficiency virus HIV-1 and HIV- | White pox viruses |
| Nacobbus sp. | 2 | Xanthomonas albilineans |
| Nairobi sheep disease virus | Rickettsia Akari | Xiphinema sp. |
| Nectria galligena | Rickettsia australis | Xylophillus ampelinus |
| Oncobasidium theobromae | Rickettsia canada | Yellow fever virus |
| Oncornavirus C and D of non-human | Rickettsia conorii | Yellow Peach |
| Primates | Rickettsia montana | Yersinia enterocolitica |
| Oropouche virus | Rickettsia siberica | Yersinia pseudotuberculosis |
| Toxins | ||
| aflatoxins | ||
| Cholera toxin | ||
| microcystin | ||
| Modecina | ||
| ochratoxin A | ||
| Toxins of Clostridium perfringens | ||
| verotoxin | ||
| Viscumina | ||
| Volkensina |
| Genetic Elements | ||
| All genetic elements that contain nucleic acid sequences associated with the pathogenicity or toxin of any selected agent. |
| Equipment | ||
| Class II and Class III Biological safety cabinets | ||
| Chambers designed for aerosol challenge testing with a capacity 1 m3 or greater | ||
| Tangential filtration equipment, except those used for reverse osmosis | ||
| Fermenters for the production of microorganisms (>20 liters) | ||
| Freeze dryers (condenser capacity between 10 and 1000 kgs in 24 hours) | ||
| Sprayer and fumigation aircraft that can generate 50 μm droplets at a rate of 2 liters/ minute | ||
| Units that provide complete BL3 or BL4 containment as specified in the Biosafety Manual of the World Health Organization | ||
| DNA sequencers | ||
| Hybridization ovens | ||
| Equipment for producing genetic probes | ||
| Centrifugal separators with flow rates greather than 100 L / h |
3- If there are BSL laboratories in your country, are there established criteria for deciding:
a. Whether or not to establish such facilities?
The specific criteria are established by the corresponding Ministry as described in the previous answer. For universities, as referenced earlier, it is difficult to evaluate those criteria as they work independently except for laboratories working with GMOs.
b. What criteria are used to select the placement of such facilities? What criteria are used to decide what research will be done in such facilities?
The following are considered: public priorities, prevalence of a specific biological agent, police priorities of the state, institutional support, and institutional capacity to maintain the project.
c. What scientific, technical, and management advice is available to governments when making their decisions.
Each Ministry has advisory groups composed of specialists with a variety of expertise. Those specialists are scientists from the Ministries who can give advice to specific projects when required. Also, the Ministry of Health works closely with the World Health Organization (WHO), Pan-American Health Organization (PAHO) and the Centers for Disease Control (CDC) and can consult these organizations for specific projects when necessary.
4- What standards exist for BSL laboratories?
a. For engineering and construction? For licensing? For safety and security?
Different Ministries have produced some manuals and documentation. The biggest challenge, in our opinion, is to disseminate those materials broadly and make them available to all of the relevant institutions. Again, there is a gap in the case of universities (excluding GMOs labs) for which regulation does not exist. Furthermore, the focus is on biosafety. Biosecurity as a separate issue has only recently been addressed in Brazil.
b. For regular oversight and re-certification?
There is almost no information on these issues and certification is pending for most of the BSL-3 labs within the country.
5- Have there been any BSL accidents in your country?
Yes. Some are reported in master’s and doctoral theses and Brazilian journals, but there is no regular system for notification from which to produce a comprehensive report. Thus, the available data significantly underestimates the actual cases. For hospital workers, however, there is a formal system for accident notification. Thus, most published data on accidents are related to hospital professionals (mainly nurses). For health care workers, some studies have shown that most of the accidents are due to overwork, inadequate personnel protective devices, and working with sharp materials.
a. If yes, how and why did accidents at high-containment facilities occur?
About 55 percent of the reports mention absent or non-fitted personnel protective equipment. Overwork was the second most common contributing factor.
b. How, to whom, and when are they reported?
In some cases, accidents are reported to the Institutional Biosafety Committees or to the Commission on Prevention of Hospital Infections. There are no specific guidelines for labs not working with GMOs, and there is no regular and mandatory system for accident notification or for notification of liability measures. However, some voluntary guidance is available (Ministry of Health publication in 2010).
c. Who has authority to investigate accidents?
For GMO work, authority rests in the Institutional Biosafety Committee and/or CTNBio (Law 11.105/2005). For other types of work with biological agents, there are no provisions.
d. What disciplinary or legal actions can be taken?
There are only provisions for working with GMOs.
6- Have any steps been taken to minimize BSL laboratory accidents?
Since its foundation in 1999, ANBio, a non-profit society, has dedicated the bulk of its work to training researchers and biological science students on biosafety measures and procedures.
As part of ANBio’s effort, the Brazilian Government has taken a step forward in establishing a biosafety program at public health laboratories and added biosafety university courses to the graduate and post-graduate curriculum. The courses concentrate on biosafety for GMOs but discuss biosafety generally and last between 40 and 420 hours. The courses are multidisciplinary and have attracted pharmacy, biology, medicine, veterinary science, chemistry, agronomy, and engineering students. The courses include the following topics:
• A review of microbiology, biochemistry, immunology, and plant physiology
• Risk classification, assessment, and management
• Personal protective clothing and equipment
• Biosafety regulations
• Biosafety of GMOs
• Animal biosafety
• Waste treatment and transport
• Bioethics, intellectual property rights, and biosecurity
• Design of facilities and commissioning
• Case studies
Most recently, the Brazilian Ministry of Education (Coordination of Post-Graduation Courses-CAPES), the U.S Biosecurity Engagement Program, and the Oswaldo Cruz Foundation (Fiocruz) supported ANBio’s development of a new post-graduation course on biosafety and biosecurity (specialization with a total of 180 hours). Forty students took the course the first time it was offered.
Additionally, in 1999, the Ministry of Health started a project to enhance the capacities of BSL-3 public health laboratories. Since then, 12 facilities have been reviewed and more than 4,000 professionals have been trained. In our opinion, however, an important challenge is the lack of regular equipment maintenance and facility certification. Also, training should be regular and frequent. In particular, regular training should be included in the biosafety program of all laboratories working with selected agents.
7- Have any steps been taken to increase security at BSL facilities?
In 2004, Brazil established the National Program for Sensible Goods (PRONABENS) in order to meet Resolution 1540/2004 of the UN Security Council. Since then, the PRONABENS team has conducted site visits, which included presenting talks on biosecurity. ANBio has worked together with this team, and they have participated in ANBio’s capacity-building program on biosecurity since 2007.
In 2007, ANBio, with the support of the United States Biosecurity Engagement Program, offered Brazil’s first biosecurity course.
Currently, ANBio is organizing our VII Brazilian Biosafety Congress and a “Biosafety and Biosecurity Conference for Latin America and the Caribbean” to be held from September 19-23, 2011 in Joinville, Brazil.
Additionally, ANBio is starting to plan and seek support for a project on “Virtual and in situ training on procedures for detection and control of biological threats” in order to support the 2014 World Cup and the 2016 International Olympiad, both which will be held in Brazil.
Even in light of these actions, Brazil should still create a long-term biosafety and biosecurity program with a dedicated budget, in which all stakeholders should take part. The main gaps that should be addressed are maintenance, certification, waste management, and the lack of a global inventory of samples. Brazil also needs to decide how and whether to destroy its polio samples post-eradication.
This page intentionally left blank.








