Discussion Paper1
Developing a Robust Clinical Trials Workforce
Ann Bonham, Association of American Medical Colleges; Robert Califf, Duke Translational Medicine Institute; Elaine Gallin, QE Philanthropic Advisors; and Michael Lauer, National Heart, Lung, and Blood Institute2
INTRODUCTION
The gap between the growing demand for evidence to inform practice and our current state of knowledge calls for serious consideration about the best approach to developing a workforce capable of meeting those demands. As referenced in the discussion paper The Clinical Trials Enterprise in the United States: A Call for Disruptive Innovation (Califf et al., 2012), the clinical trials enterprise (CTE) is falling behind in the need for evidence, especially in the United States. We envision a need for a clinical research workforce organized in several dimensions that reflect the broad missions of the CTE, the specific disciplines involved, and the level of desirable expertise. The latter could range from broad participation in the CTE to expert participation, and to conceptualization and research on the methods employed by the CTE.
In addition, this workforce must be broadened tremendously to meet the goal implicit in the creation of a true learning health system: the inte
______________________
1 The views expressed in this discussion paper are those of the authors and not necessarily of the authors’ organizations or of the Institute of Medicine. The paper is intended to help inform and stimulate discussion. It has not been subjected to the review procedures of the Institute of Medicine and is not a report of the Institute of Medicine or of the National Research Council.
2 Participants in the activities of the IOM Forum on Drug Discovery, Development, and Translation. This discussion paper was presented in draft form at the Forum’s November 2011 workshop, Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020, and finalized by the authors following the workshop.
gration of research and continuous learning into practice. However, the more traditional arenas of mechanistic research and efficacy trials call for specialized workforces that until now have all too often depended on ad hoc, “on-the-job” learning, as opposed to the prospective training and education that defines a mature discipline. Finally, the new field of community engagement requires special skills that blend traditional clinical trials knowledge with social and organizational constructs.
The challenge is considerable, because most roles in clinical research at their core involve human experimentation and require specific expertise in a clinical or scientific discipline. Moreover, this expertise must be augmented by focused training in the standards and principles that undergird the conduct of particular types of clinical trials, which range from small, intensive mechanistic studies to very large, community-based interventions.
A Salutary Example: The Occluded Artery Trial (OAT)
One example of the need for broadening the discipline to include medical practitioners is the recent OAT Trial, a cardiology study funded by the National Institutes of Health (NIH). In the 1990s, cardiologists sought to understand how best to manage patients who survived the first few days of ST-segment-elevation acute myocardial infarction (STEMI), but with persistent total occlusions of their infarct-related artery. The enormous benefits of rapid reperfusion in the acute phase of STEMI had already been established in well-controlled clinical trials. Although the detection of an occluded artery several days after the onset of the STEMI would come too late for treatment to be effective in salvaging damaged heart muscle, cardiologists wondered whether there might still be benefit in deploying stents to open up the occluded arteries; the newly opened arteries might supply blood to “watershed” areas at the edge of the infarct zone, thereby reducing the risk of life-threatening arrhythmias and other major events. Further, experimental evidence suggested that the infarction might heal more effectively with perfusion.
Some investigators analyzed observational cohorts and found that patients who were discharged with patent arteries did indeed fare better. The “open artery hypothesis” was alive and supported by observational evidence, so much so that for many cardiologists it became standard practice to ensure that their STEMI patients went home with an open artery.
However, some academic cardiologists questioned whether routine post-STEMI stenting of persistently occluded arteries was of clinical value: Did this practice actually improve clinical outcomes? With support from the National Heart, Lung, and Blood Institute (NHLBI), they organized OAT, a multicenter clinical trial in which patients would be randomly
assigned to optimal medical therapy alone or to optimal medical therapy combined with stent placement.
Although STEMI is hardly a rare condition, the investigators experienced enormous difficulty enrolling patients. Most of the American hospitals they approached refused to participate, because domestic cardiologists were convinced of the value of routine post-STEMI stenting. By the time the trial was complete, nearly three-quarters of the study participants enrolled were from research sites outside the United States.
And the results of OAT were surprising. Contrary to expectations, patients who were randomly assigned to stenting seemed to fare slightly worse than those assigned to medical therapy alone. The results, which were published in the New England Journal of Medicine (Hochman, 2006), led to rapid changes in cardiology practice guidelines. Yet, 5 years after the OAT study was published, there has been little change in practice. Many cardiologists continue to routinely deploy stents in stable post-infarct STEMI patients, despite the absence of evidence of benefit (and despite the evidence of absence of benefit), and in direct contradiction to the clear recommendations of their own professional societies.
The OAT experience encapsulates what is wrong with how clinical trials are perceived, implemented, and interpreted in the United States. Relatively few U.S. patients participated, in large part because their American cardiologists were not engaged. As was discussed at a 2009 Institute of Medicine (IOM) workshop, OAT also highlighted misaligned financial incentives because physicians are focused on performing a high volume of procedures, which creates a disincentive for them to refer patients to a clinical trial to receive a procedure (IOM, 2009). Also, the lengthy timeline to conduct a large, multicenter trial hinders medical learning and potential benefits to patients. The OAT trial took 10 years from hypothesis generation to completion. Even though the trial has been completed and its results communicated, many American patients are not benefitting from the findings, because their cardiologists have not changed their practices. Nor is the OAT experience unique or even unusual; the difficulty experienced across fields by American clinical trialists seeking to enroll patients and engage practitioners is well documented.
Clinical Research: A Public Good?
Two years ago, Schaefer, Emanuel, and Wertheimer (Schaefer et al., 2009) argued for a “cultural shift in the moral framework that is brought to participation in research.” They suggested that clinical research should be regarded as a “public good” in which all people stand to benefit, whether they actively participate or not. But if research is a public good,
people should then aim to participate in research unless there is a compelling reason not to, “participation being a moral obligation for everyone to do his part.”
Such a view may seem extreme. Should we go so far as to expect, not just encourage, participation in research? But, in fact, evidence suggests that the majority of Americans would be willing to participate if asked. However, only 7 percent of American adults say that their doctors have ever suggested that they participate in a clinical study (Charlton Research Company, 2007). In one study of specialty physicians, one-third of physicians affiliated with an organization designed to support clinical trial participation were not actively engaged in the research process (Klabunde et al., 2011). In other words, many Americans would gladly participate, but their doctors aren’t asking.
Some commentators have lamented that American doctors seem to have little interest in science as a way to drive their practices. Even doctors’ professional societies, which have some degree of scientific orientation, routinely issue guidelines of which only a small proportion are based on evidence from high-quality randomized trials, a point reinforced by recent publications examining guidelines for cardiology (Tricoci et al., 2009) and infectious diseases (Lee and Vielemeyer, 2011). Thus, the paucity of physician and public engagement cannot be ascribed to our knowing all the answers—we aren’t even a fraction of the way there.
In our view, the largest segment of the clinical trials workforce comprises all individuals—the general public—including the diverse communities and neighborhoods across the United States. Individuals should not only participate in trials as volunteers but should be viewed as partners in the clinical trials enterprise with the ability to mobilize their friends, families, and communities to drive change. This segment of the workforce has the important responsibility of working in partnership with clinicians to identify the important questions about care that need answers through a clinical trial. The future of research might also be headed in the direction of e-trials, where there are no investigative sites or physicians/middlemen. Individuals with a smart phone or a computer can enroll in an e-trial directly through a website. In this sense, individual patients could truly become the largest segment of the clinical trials workforce. In terms of training, the public should be educated as to the value of clinical research and the link between clinical trials and improvements in care. This broad-based appreciation and understanding of the value of clinical research could be instilled via national public service announcements, key opinion leaders and policy makers, and the greater integration of science education, and specifically clinical research, in K-12 programs.
In partnership with the public, the next largest segment of the clinical trials workforce would be, in some respects, no different from the clinical
care workforce. In a true learning health system, every patient with an incurable disease—and that is eventually most of us—would present an opportunity for active learning. Each practicing clinician and his or her consenting patients would be engaged in a massive tapestry of well-designed trials that would enable all of us to answer real-world questions in the most robust way possible: through randomization.
Some have argued that routine implementation of electronic health records (EHRs) and large-scale registries would suffice. While these tools would make observational research easier, they cannot by themselves enable rigorous scientific evaluation of the most important clinical questions, which can only be properly addressed with randomization.
We view the ideal clinical trials workforce as one in which all clinicians think scientifically. When confronted with a clinical problem for which definitive evidence is lacking, the clinician’s first thought should be, “What good trial would apply here? How can my patient and I be part of the solution?” Of course, effective deployment of EHRs with standardized data elements would allow randomized controlled trials (RCTs) to be done at a much lower cost by reducing or eliminating redundant data collection, but unless knowledge generation is embraced as a fundamental component of practice, the gap between existing evidence and practice needs will remain cavernous.
The ideal clinical trials workforce will be orders of magnitude larger than its present size, because it will include nearly all of the 90 percent of clinicians who currently are not part of the clinical trials enterprise. These clinicians will embrace research as a critical, indeed morally imperative, component of their work as medical professionals. They will work closely with professional clinical trialists who implement clinical research in the context of practice and will collaborate with them to prioritize, design, analyze, and interpret the trials that will be embedded into their practices. Of course, with a much larger proportion of American clinical practitioners and patients participating in the trials, there will also be an acute need for a larger workforce of professional trialists and clinical research professionals (see Table 1: Core Competencies in Clinical and Translational Research).
COMPETENCIES, FUNCTION, AND LOCUS OF NEEDED WORKFORCE
Realizing the vision of a significantly expanded clinical trials workforce will require major training efforts, both for new and veteran health care workers. A core set of competencies, including principles of responsible trials conduct and the fundamentals of study design, should be part of training programs for most of the clinical trials and clinical care
workforce,3 although the level of information provided would vary depending on workers’ backgrounds and responsibilities. However, while identifying core competencies may result in a better-informed workforce, the complex nature of the CTE precludes a “one-size-fits-all” training program. Developing a highly competent workforce will require instruction in two additional dimensions—discipline- and job-specific training and education. In the team-based CTE (and, for that matter, clinical care) environment of the future, training in teamwork and understanding across disciplines will be needed. Facts can be learned interchangeably by experts in different areas, but fundamental knowledge must be transmitted in an effective manner.
Dimension 1: Defining the Workforce
In order to simplify this discussion, we have parsed the clinical trials workforce of the future into five groups, beginning with a very broad-based group and progressing to smaller, more focused workforce groups (see Figure 1 for a schematic of the workforce groups):
• Group 1: Individuals from the community who understand, support, and participate in clinical trials (referred to as the public)
• Group 2: The clinical workforce who participate in trials as part of their clinical practices (referred to as community practitioners)
• Group 3: Clinical trialists who devote specified portions of their professional efforts to serving as principal investigators or collaborating co-investigators at clinical research sites, with primary responsibility for implementing clinical trials at the level of a hospital or research site (referred to as implementers)
• Group 4: Investigators who lead and design clinical trials, as well as scientific experts who develop tools and innovative approaches for conducting trials (referred to as investigators)
• Group 5: Academicians who explore the methodologies of con ducting clinical trials (referred to as methodologists).
______________________
3 “NCATS [National Center for Advancing Translational Sciences], in collaboration with the CTSA [Clinical and Translational Science Awards] Education and Career Development Key Function Committee, formed the Education Core Competency Work Group to define the training standards for core competencies in clinical and translational research. The work group’s final recommendations for core competencies include 14 thematic areas that should shape the training experiences of junior investigators by defining the skills, attributes, and knowledge that can be shared across multidisciplinary teams of clinician-scientists” (https://www.ctsacentral.org/committee/education-and-career-development).
The middle groups—investigators and implementers—will have many overlapping areas of competence, but the level of training (doctoral- versus masters-level training) and scope of the discipline-specific expertise needed to carry out their work is expected to be less for implementers than for investigators (who design and lead trials), or for methodologists (who develop new approaches to the design, conduct, or analysis of trials). Nonetheless, the workforce falling within either of these two groups must be expanded considerably if the promise of universal participation in clinical trials leading to a health system founded on evidence-based medicine is to be met.
The sections below outline the required training, responsibilities, and locations in which the groups work.
Group 1: The Public. This workforce group comprises the nation as a whole and includes all individuals in communities across the country. Individuals consider research a public good and participate in studies unless they have a compelling reason not to do so. Educating the public and inspiring the concept of research as a public good requires strong national leadership.
Group 2: Community Practitioners. This group comprises practitioners who participate in trials as part of their clinical practice. Community practitioners include primary caregivers (physicians, nurses, pharmacists, etc.) and social service employees working at the community level. Their training needs to include an introduction to clinical trials as well as some of the core competencies in translational and clinical research, as defined by many of the Clinical and Translational Science Awards (CTSA) programs (see Table 1: CTSA Core Competencies, p. 13). However, if CTSA-like training materials are used, they will in some cases need to be simplified to align with the skill-set of the targeted workforce. These workers are typically found where primary and secondary health care are delivered.
Group 3: Implementers. This group is primarily responsible for implementing clinical trials at the level of a hospital or research site. Implementers will include physician-scientists, nurse-investigators, operations specialists, data managers, computer specialists, pharmacists, social service personnel, and others vital to the implementation of clinical trials. A major part of the implementation workforce will include clinical research coordinators (CRCs) and professional research-site managers (RSMs) working within academic centers, contract research organizations, industry, hospitals, and large clinics.
As with Group 4 described below, Group 3 implementers receive both discipline-specific specialty training and CTSA-style training in core competencies for translational and clinical research. However, the level of the
training for these personnel is likely to be more pragmatic (less conceptual) and training will likely require fewer years than that for the investigators in Group 4. For example, pharmacists, nurse-investigators, and data managers making up a clinical trials implementation team do not need to hold doctoral-level degrees, and many of these jobs are currently accomplished by individuals with bachelors-level education coupled with discipline-specific training offered by organizations such as the Association of Clinical Research Professionals (ACRP).
There is a critical need to expand this part of the clinical trials workforce and improve its core competencies. In addition, in order to address the need for recruiting more diverse patient populations and to accommodate the trend toward multinational clinical trials, this workforce expansion needs to include an increase in worker diversity.
Group 4: Investigators. Personnel in this group are charged with leading and designing clinical trials. The rubric “Investigator” also includes scientific experts who develop tools and innovative approaches for conducting trials, and while there is overlap, it is useful to consider investigators of specific projects as different from those who develop new methods (Group 5 below). Investigators comprise MD-, MD/PhD-, and PhD-level investigators from many different disciplines. This highly trained workforce is needed not only to lead and design clinical trials but also to advance the area of “Regulatory Sciences,” defined by the Food and Drug Administration (FDA) as “the science of developing new tools, standards and approaches to assess the safety, efficacy, quality, and performance of FDA-regulated products” (FDA, 2011). Advances in regulatory sciences will require personnel with expertise in new technologies, including imaging, cell and tissue engineering, and nanotechnology. In addition, the current major shortage of biostatisticians and informaticists across academic medicine, industry, and government must be addressed.
We expect that most persons falling within this category will need extensive graduate and postgraduate training in discipline-specific areas such as epidemiology, computational biology, and genomics, and/or disease-specific areas such as cardiology and infectious diseases. In addition, the required scientific/clinical discipline-specific training can be supplemented as needed by core training in translational and clinical research offered through the CTSA. In order to effectively leverage scientific opportunities and stimulate innovative approaches, the traditional departmental training silos must be broken down to encourage investigators to train and work across disciplines.
Most investigators currently can be found within academic health science systems (AHSSs), professional research sites, and community practices that conduct research, as well as in the pharmaceutical and medical
device industries. A smaller number work at government agencies such as NIH, the Centers for Disease Control and Prevention, and FDA; others work with public–private partnerships such as the Medicines for Malaria Initiative. In the future, more AHSSs will be transforming themselves into integrated systems, enabling the efficient bridging of translational gaps between discovery and health care. Such organizations will require many more highly trained clinical trial investigators.
Group 5: Methodologists. This last group includes experts who perform research on the methods and policies pertinent to clinical trials. This relatively small yet substantial cadre of clinical investigators, biostatisticians, epidemiologists, and health services researchers is vital to advancing clinical trials methods. These experts will be located within AHSSs, government, research institutes, and some large industry groups with capacity to fund protected time for research.
Dimension 2: Critical Disciplines
The second dimension of the workforce is the scientific discipline in which the individual works. Our major thesis is that all health care providers (practitioners) should be trained in the fundamentals of participating in research and should be taught that knowledge generation is a fundamental professional responsibility of health care delivery. This extends not only to physicians and nurses, but also to physician assistants, respiratory therapists, physical therapists, pharmacists, and other providers. Any of these professions can produce either implementers or investigators. Many of the investigators and methodologists will be epidemiologists, biostatisticians, and informaticists.
There is currently a critical shortage of biostatisticians and informaticists across academic medicine, industry, and government. Serious efforts are needed in order to develop this segment of the workforce. The costs of training this critical cadre of the workforce are small compared with the overall research budget of the relevant funding organizations, and a modest investment would have a multiplier effect on the quality of clinical trials.
Dimension 3: Critical Competencies and Special Knowledge
The third dimension is competency and special educational knowledge. In the team-based research environment of the future, much of the material can be learned interchangeably by experts in different areas, but the fundamental knowledge remains to be transmitted in an effective manner.
DEMOGRAPHIC TRENDS INFLUENCING WORKFORCE NEEDS
Demographic trends in diverse and aging populations, and the intersections of these trends with systematic health disparities, are significant factors affecting the development of a robust and diverse workforce that will design, perform, and participate in clinical trials, as well as communicate research findings to all relevant populations.
Demographic Shifts
The U.S. Census Bureau projects 334 million people in the United States by 2020, up from 310 million in 2010 (U.S. Census Bureau, 2009). By 2050, this number is expected to grow to nearly 400 million (assuming net-constant immigration). As is well known, the ethnic composition of the U.S. population is also changing, such that by 2050 no single ethnicity will characterize a majority of Americans. Perhaps the most notable shift will be the number of people identified in the U.S. Census as being of Hispanic origin. This demographic group is expected to increase from a current 16 percent of the population (49 million) to 19 percent (55 million) in 2020, on a trajectory to include one in five Americans (70 million) by 2025. All ethnicities tracked by the census will increase in absolute numbers, although this increase will be to varying degrees considered as a percentage of the population.
White persons not identifying as Hispanic or other ethnicities will increase from 201 million to 204 million, but will decline from 65 percent of the population to 61 percent by 2020. Black or African American non-Hispanic persons will increase from 38 million to 41 million by 2020 (a 9 percent increase) but will remain about 12 percent of the total population over the decade. The fastest growth continues to be among Asians and Native Hawaiian/Pacific Islanders (now classified separately), both growing by more than 20 percent from 2010 to 2020, although remaining under 5 percent and under 1 percent of the general population, respectively.
This changing racial and ethnic composition will also be accompanied by the aging of the U.S. population. As underscored in recent news reports, the first cohort of “Baby Boomers”—the generation of Americans born between 1946 and 1964—reaches 65 years of age this year. The number of Americans 65 and older, now 40 million (13 percent of the U.S. population), will increase correspondingly, reaching 16 percent of the population by 2020 and nearly 20 percent of the total population by 2025. According to the Population Reference Bureau, more than 20 percent of this 65-plus cohort will be grouped with the “oldest old”—persons ages 85 and older. Oldest-old persons will number 19 million by 2050—a number greater than the populations of most U.S. states today (Population Reference Bureau, 2011). This aging of the general population is also largely
a global phenomenon, at least for many developed nations. In fact, the U.S. population of persons aged 18 and under will remain nearly stable, decreasing slightly from a current 24.1 percent to 23.8 percent by 2020.
Other notable population factors, although too complex to consider in detail in this discussion, include increasing variations in income distribution in the United States, as reported in recent communications on the increase in the number of Americans living below the federally estimated poverty line. These reports show troubling trends: recent economic stresses have resulted in a 7 percent increase (to 31 million) in the number of children living in low-income families. Childhood poverty may emerge as an increasingly important determinant of clinical research needs in the future.
Health Disparities
The U.S. Department of Health and Human Services (HHS) has defined a health disparity as a particular type of health difference that is closely linked with social, economic, and/or environmental disadvantage. As HHS notes:
Racial and ethnic minorities still lag behind in many health outcome measures. They are less likely to get the preventive care they need to stay healthy, more likely to suffer from serious illnesses, such as diabetes or heart disease, and when they do get sick, are less likely to have access to quality health care. (HHS, 2011)
Disparities identified by HHS relate to infant mortality, asthma, diabetes, flu, cancer, HIV/AIDS, chronic lower-respiratory disease, cardiovascular disease, viral hepatitis, chronic liver disease and cirrhosis, kidney disease, injury deaths, violence, behavioral health, and oral health (HHS, 2011).
The extent of these disparities as a pressing national health issue was recently documented by the IOM (2004), and is the subject of many federal and independent initiatives (AHRQ, 2010), including a 2011 action plan under the Affordable Care Act. One of the plan’s five goals is to advance scientific knowledge and innovation relating to health disparities through data collection and promoting patient-centered outcomes research. A second major goal focuses on improvements to workforce development, including through
a new pipeline program for recruiting undergraduates from underserved communities for public health and biomedical sciences careers, expanding and improving health care interpreting and translation, and supporting more training of community health workers, such as promotoras. (HHS, 2011)
Redressing health disparities will require both a more extensive network for clinical trials and a workforce skilled in community engagement. Clinical trials must be crafted to assess the effects of disparities for separate populations, including by age (discussed below)—aspects that could greatly complicate trial design and analysis. In order to gauge the impact of health disparities on different populations and assess which interventions are truly most effective for diverse communities, the national CTE will need to engage in multisite trials covering broad geographic ranges and including many provider organizations. Along with these challenges, there is enormous potential for such research to improve the overall health of the nation, especially where biological mechanisms supporting an intervention in one segment of the population are already well understood, and may be adapted in a straightforward manner to other populations.
The Burden of Disease Related to an Aging Population
The increasing size and proportion of the national population aged 65 years and older (and the growing subset of the population aged 85 years and older) present new considerations related to the burden of disease. In the United States as elsewhere, a disproportionate share of medical services is provided to older populations. We might thus expect that older persons would be the subject of research aimed at providing the best evidence of which therapies work best in the older population. However, Zulman and colleagues have found that one in five trials examined excluded patients because of age, and nearly half of the remainder included criteria that made participation by older subjects less likely. In addition, fewer than 40 percent of trials examined reported results for different subgroups by age. A further complication is that older patients are more likely to have chronic or other conditions unrelated to a trial’s topic of study, which results in their exclusion. The combined result of these factors is a shortfall in evidence to inform the optimal treatment of older patients with multiple health conditions (Zulman et al., 2011).
For example, in 2011 an estimated 5.4 million Americans of all ages had Alzheimer’s disease, 5.2 million of whom were age 65 or older (Alzheimer’s Association, 2011). Without any breakthrough in preventing or treating the disease and as the population of older persons increases, between 11 million and 16 million people age 65 and older are projected to have Alzheimer’s disease by 2050. Medicare beneficiaries age 65 and older with Alzheimer’s disease and/or other dementias have tripled the average Medicare costs of other beneficiaries. One estimate calculates that in the absence of any new effective treatments, the cumulative costs of care for people with Alzheimer’s disease from 2010 to 2050 will exceed
$20 trillion in 2010 dollars (Alzheimer’s Association, 2010). The impact of advances from clinical trials in this field alone could have enormous economic consequences, and potentially could single-handedly bend the health care cost curve in a more affordable direction.
Implications for a Clinical Trials Workforce
A diverse, inclusive workforce helps to build trust among diverse research participants through outreach and engagement with communities. A diverse workforce can constitute the foundation for increased participation in research by underrepresented groups, and can even change the nature of the questions addressed by clinical trials.
Such a workforce strengthens the design, conduct, or support of clinical trials. A professional commitment to focusing on particular areas of disease, pursuing particular lines of research, or working with different communities or populations is greatly determined by personal experience. A diversity of backgrounds helps ensure a sufficient range of experiences, interests, and corresponding dedication among investigators to address relevant topics for clinical trials in a population as diverse as the United States’.
This is not to say that investigators and health workers should confine their interests to communities where they live or grew up; in academic settings, it appears to be the norm that clinical investigators and health professionals cross broad cultural, social, and geographic boundaries to conduct their research. But researchers uniformly cite their personal backgrounds, experiences, and early motivations as critical to the later development of their careers and as influential in their choice of the areas of medicine they practice.
A key requirement, which is common to the scientific and medical research enterprise at large, is to ensure that talent and skills are drawn from across all segments of the U.S. population. Despite a record of rapid growth in public and private investment in medical research up to the current decade, despite growing interests from minority and international students in medical and health care fields, and despite many notable instances of accomplishment by prominent minority scientists, academic research has not succeeded in expanding the participation of underrepresented groups, including African Americans and Hispanic or Latino Americans in the research career pipeline. Nor has academic medicine been notably successful in advancing or retaining minority scientists within research careers. There are also notable disparities by gender in recruitment, advancement, and retention of MD or PhD scientists, which in one sense can exacerbate underrepresentation by some minorities for whom women are more likely than men to earn baccalaureate degrees—a
preliminary requirement for careers in science. Especially troubling is a recent NIH analysis showing that even well-established minority scientists are 10 percent less likely than other applicants to receive R01 research grants, raising concerns that similar disparities might plague minority scientists in applications for clinical trials (Ginther et al., 2011).
The failure to engage sufficient involvement of minorities in clinical trials is exemplified in FDA’s recent approval of belimumab, the first new drug for the treatment of systemic lupus erythematosus to be approved in more than 50 years. In trials, belimumab relieved symptoms of lupus in about 43 perecnt of patients receiving the active drug, compared with 34 percent of those receiving placebo. However, the trial did not demonstrate significant benefit for African Americans, who have a higher incidence of lupus than whites. According to press reports, FDA said that there were “too few African Americans in the trials to draw a definitive conclusion” (Pollack, 2011).
Finally, discussions related to the definition of the clinical trials workforce could prompt examination of the question of “who” is the workforce. Imagine, for instance, a future in which all citizens— including all providers—view themselves as working hand-in-glove with the clinical trials research workforce as part of a collective commitment to improving the health of the nation.
REFERENCES
Agency for Healthcare Research and Quality (AHRQ). 2010. National Healthcare Disparities Report. http://www.ahrq.gov/qual/nhdr10/nhdr10.pdf (accessed October 13, 2011).
Alzheimer’s Association. 2010. Changing the Trajectory of Alzheimer’s Disease: A National Imperative. http://www.alz.org/alzheimers_disease_trajectory.asp (accessed October 13, 2011).
Alzheimer’s Association. 2011. 2011 Alzheimer’s Disease Facts and Figures. http://www.alz.org/downloads/Facts_Figures_2011.pdf (accessed October 13, 2011).
Califf, R., G. Filerman, R. Murray, and M. Rosenblatt. 2012. The Clinical Trials Enterprise in the United States: A Call for Disruptive Innovation. Discussion Paper, Institute of Medicine, Washington, DC. http://www.iom.edu/Global/Perspectives/2012/CallforCTE.aspx (accessed April 23, 2012).
Charlton Research Company. 2007. A Public Opinion Study for Research!America. http://www.researchamerica.org/uploads/poll.report.2007.transforminghealth.pdf (accessed October 12, 2011).
FDA (Food and Drug Administration). 2011. Advancing Regulatory Sciences at the FDA: Strategic Plan 2011. http://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/ucm268095.htm (accessed October 13, 2011).
Ginther D. K., W. T. Schaffer, J. Schnell, B. Masimore, F. Liu, L. Haak, and R. Kington. 2011. Race, ethnicity, and NIH research awards. Science 333:1015-1019.
HHS (Department of Health and Human Services). 2011. HHS Action Plan to Reduce Health Disparities. http://www.hhs.gov/news/press/2011pres/04/20110408a.html (accessed October 13, 2011).
Hochman, J. S., et al. 2006. Coronary intervention for persistent occlusion after myocardial infarction. New England Journal of Medicine 355:2395-2407.
IOM (Institute of Medicine). 2004. In the Nation’s Compelling Interest: Ensuring Diversity in the Health-Care Workforce. Washington, DC: The National Academies Press.
IOM. 2009. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington, DC: The National Academies Press.
Klabunde, C. N., N. L. Keating, A. L. Potosky, A. Ambs, Y. He, M. C. Hornbrook, and P. A. Ganz. 2011. A population-based assessment of specialty physician involvement in
cancer clinical trials. Journal of the National Cancer Institute 103:384-397.
Lee, D. H., and O. Vielemeyer. 2011. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Annals of Internal Medicine 171:18-22.
Pollack, A. 2011. FDA approves drug for Lupus, an innovation after 50 years. New York Times, March 9. http://www.nytimes.com/2011/03/10/health/10drug.html (accessed October 13, 2011).
Population Reference Bureau. 2011. America’s Aging Population. http://www.prb.org/pdf11/aging-in-america.pdf (accessed October 13, 2011).
Schaefer, G. O., E. J. Emanuel, and A. Wertheimer. 2009. The obligation to participate in biomedical research. JAMA 302(1):67-72. Tricoci P., J. M. Allen, J. M. Kramer, R. M. Califf, and S. C. Smith Jr. 2009. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 301:831-841.
U.S. Census Bureau. 2009. Table 1-C. Projections of the Population and Components of Change for the United States: 2010 to 2050 Constant Net International Migration Series (NP2009-T1-C). http://www.census.gov/population/www/projections/2009cnmsSumTabs.html (accessed October 13, 2011).
Zulman, D. M., J. B. Sussman, X. Chen, C. T. Cigolle, C. S. Blaum, and R. A. Hayward. 2011. Examining the evidence: A systematic review of the inclusion and analysis of older adults in randomized controlled trials. Journal of General Internal Medicine 26(7):783-790.
TABLE 1 Core Competencies in Clinical and Translational Research from the CTSA Education and Career Development Committee, Education Core Competency Work Group
| Core Thematic Areas | Competencies |
| I. Clinical & translational research questions |
• Identify basic and preclinical studies that are potential testable clinical research hypotheses. • Identify research observations that could be the bases of large clinical trials. • Define the data that formulate research hypotheses. • Derive translational questions from clinical research data. • Prepare the background and significance sections of a research proposal. • Critique clinical and translational research questions using data-based literature searches. • Extract information from the scientific literature that yields scientific insight for research innovation. |
| II. Literature critique |
• Conduct a comprehensive and systematic search of the literature using informatics techniques. • Summarize evidence from the literature on a clinical problem. • Describe the mechanism of a clinical problem reviewed in a manuscript. • Use evidence as the basis of the critique and interpretation of results of published studies. • Identify potential sources of bias and variations in published studies. • Interpret published literature in a causal framework. • Identify gaps in knowledge within a research problem. |
| III. Study design |
• Formulate a well-defined clinical or translational research question to be studied in human or animal models. • Propose study designs for addressing a clinical or translational research question. • Assess the strengths and weaknesses of possible study designs for a given clinical or translational research question. • Design a research study protocol. • Identify a target population for a clinical or translational research project. • Identify measures to be applied to a clinical or translational research project. • Design a research data analysis plan. • Determine resources needed to implement a clinical or translational research plan. • Prepare an application to an institutional review board (IRB). |
| Core Thematic Areas | Competencies |
| IV. Research implementation |
• Compare the feasibility, efficiency, and ability to derive unbiased inferences from different clinical and translational research study designs. • Assess threats to internal validity in any planned or completed clinical or translational study, including selection bias, misclassification, and confounding. • Incorporate regulatory precepts into the design of any clinical or translational study. • Integrate elements of translational research into given study designs that could provide the bases for future research, such as the collection of biological specimens nested studies and the development of community-based interventions. |
| V. Sources of error |
• Describe the concepts and implications of reliability and validity of study measurements. • Evaluate the reliability and validity of measures. • Assess threats to study validity (bias) including problems with sampling, recruitment, randomization, and comparability of study groups. • Differentiate between the analytic problems that can be addressed with standard methods and those requiring input from biostatisticians and other scientific experts. • Implement quality-assurance systems with control procedures for data intake, management, and monitoring for different study designs. • Assess data sources and data quality to answer specific clinical or translational research questions. • Implement quality-assurance and quality-control procedures for different study designs and analysis. |
| Core Thematic Areas | Competencies |
| VI. Statistical approaches |
• Describe the role that biostatistics serves in biomedical and public health research. • Describe the basic principles and practical importance of random variation, systematic error, sampling error, measurement error, hypothesis testing, type I and type II errors, and confidence limits. • Scrutinize the assumptions behind different statistical methods and their corresponding limitations. • Generate simple descriptive and inferential statistics that fit the study design chosen and answer research question. • Compute sample size, power, and precision for comparisons of two independent samples with respect to continuous and binary outcomes. • Describe the uses of meta-analytic methods. • Defend the significance of data- and safety-monitoring plans. • Collaborate with biostatisticians in the design, conduct, and analyses of clinical and translational research. • Evaluate computer output containing the results of statistical procedures and graphics. • Explain the uses, importance, and limitations of early stopping rules in clinical trials. |
| VII. Biomedical informatics |
• Describe trends and best practices in informatics for the organization of biomedical and health information. • Develop protocols utilizing management of information using computer technology. • Describe the effects of technology on medical research, education, and patient care. • Describe the essential functions of the EHR and the barriers to its use. • Explain the role that health information technology (IT) standards have on the interoperability of clinical systems, including health IT messaging. • Access patient information using quality checks via EHR systems. • Retrieve medical knowledge through literature searches using advanced electronic techniques. • Discuss the role of bioinformatics in the study design and analyses of high-dimensional data in areas such as genotypic and phenotypic genomics. • Collaborate with bioinformatics specialists in the design, development, and implementation of research projects |
| Core Thematic Areas | Competencies |
| VIII. Responsible conduct of research |
VIII.a. Clinical Research Ethics Competencies • Summarize the history of research abuses and the rationale for creating codes, regulations, and systems for protecting participants in clinical research that requires community input. • Critique a clinical or translational research proposal for risks to human subjects. • Explain the special issues that arise in research with vulnerable participants and the need for additional safeguards. • Determine the need for a risk-benefit ratio that is in balance with the outcomes in clinical and translational research. • Describe the elements of voluntary informed consent, including increasing knowledge about research, avoiding undue influence or coercion, and assuring the decision-making capacity of participants. • Assure the need for privacy protection throughout all phases of a study. • Assure the need for fairness in recruiting participants and in distributing the benefits and burdens of clinical research. • Adhere to IRB application procedures. • Explain how the structural arrangement of science and the research industry may influence the behavior of scientists and the production of scientific knowledge. VIII.b. Responsible Conduct of Research Competencies • Apply the main rules, guidelines, codes, and professional standards for the conduct of clinical and translational research. • Adhere to the procedures to report unprofessional behavior by colleagues who engage in misconduct in research. • Implement procedures for the identification, prevention, and management of financial, intellectual, and employment conflicts of interest. • Apply the rules and professional standards that govern data collection, sharing, and protection throughout all phases of clinical and translational research. • Apply elements of voluntary informed consent, of fostering understanding of information about clinical research, and for avoiding undue influence or coercion, and taking into consideration the decision-making capacity of participants. • Explain the need for privacy protection and best practices for protecting privacy throughout all phases of a study. • Explain the need for fairness in recruiting participants and in distributing the benefits and burdens of clinical research. • Explain the function of the IRB. |
| Core Thematic Areas | Competencies |
| IX. Scientific communication |
• Communicate clinical and translational research findings to different groups of individuals, including colleagues, students, the lay public, and the media. • Translate the implications of clinical and translational research findings for clinical practice, advocacy, and governmental groups. • Write summaries of scientific information for use in the development of clinical health care policy. • Translate clinical and translational research findings into national health strategies or guidelines for use by the general public. • Explain the utility and mechanism of commercialization for clinical and translational research findings, the patent process, and technology transfer. |
| X. Cultural diversity |
• Differentiate between cultural competency and cultural sensitivity principles. • Recognize the demographic, geographic, and ethnographic features within communities and populations when designing a clinical study. • Describe the relevance of cultural and population diversity in clinical research design. • Describe cultural and social variation in standards of research integrity. • Critique studies for evidence of health disparities, such as disproportional health effects on select populations (e.g., gender, age, ethnicity, race). |
| XI. Translational teamwork |
• Build an interdisciplinary/intradisciplinary/multidisciplinary team that matches the objectives of the research problem. • Manage an interdisciplinary team of scientists. • Advocate for multiple points of view. • Clarify language differences across disciplines. • Demonstrate group decision-making techniques. • Manage conflict. • Manage a clinical and/or translational research study. |
| XII. Leadership |
• Work as a leader of a multidisciplinary research team. • Manage a multidisciplinary team across its fiscal, personnel, regulatory-compliance, and problem-solving requirements. • Maintain skills as mentor and mentee. • Validate others as a mentor. • Foster innovation and creativity. |
| Core Thematic Areas | Competencies |
| XIII. Cross-disciplinary training |
• Apply principles of adult learning and competency-based instruction to educational activities. • Provide clinical and translational science instruction to beginning scientists. • Incorporate adult learning principles and mentoring strategies into interactions with beginning scientists and scholars in order to engage them in clinical and translational research. • Develop strategies for overcoming the unique curricular challenges associated with merging scholars from diverse backgrounds. |
| XIV. Community engagement |
• Examine the characteristics that bind people together as a community, including social ties, common perspectives or interests, and geography. • Appraise the role of community engagement as a strategy for identifying community health issues, translating health research to communities, and reducing health disparities. • Summarize the principles and practices of the spectrum of community-engaged research. • Analyze the ethical complexities of conducting community-engaged research. • Specify how cultural and linguistic competence and health literacy have an impact on the conduct of community-engaged research. |
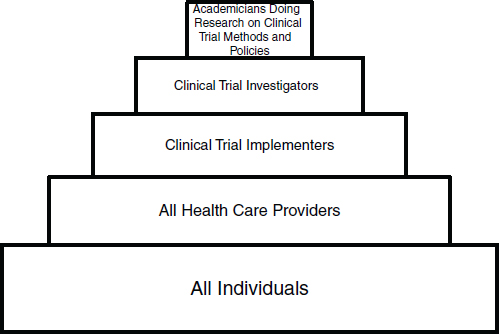
FIGURE 1 Workforce for a transformed clinical trials enterprise.






















