Integrating Community Practice and Clinical Trials
The workshop’s first session, “Framing the Need for Change: Envisioning a Clinical Trials Enterprise in the Health Care System of 2020,” considered how the CTE in the United States can complement the health care system of 2020. Several speakers in this session emphasized that a CTE integrated into the future health care system would look far different from today’s CTE. They outlined the case for disruptive innovation by pointing out current challenges and problems, proposed ways to link the CTE more effectively with the evolving U.S. health care system, and discussed how a transformed CTE can help build a learning health system.
A CALL FOR DISRUPTIVE INNOVATION IN THE CLINICAL TRIALS ENTERPRISE1
Participation in research is an essential dimension of the social compact among the health care delivery system, health care providers, the public, and the scientific enterprises that serve them.
—Robert Califf, Duke University Medical Center;
Gary Filerman, Atlas Health Foundation; Richard Murray,
Merck & Co., Inc.; and Michael Rosenblatt, Merck & Co., Inc.
______________________
1 This section is based on the presentations and Discussion Paper by Robert Califf, Vice Chancellor for Clinical Research, Director of the Duke Translational Medicine Institute, Professor of Medicine in the Division of Cardiology, Duke University School of Medicine;
There has been a “widening separation” in the relationship between the CTE and the health care system, according to the Discussion Paper prepared by Robert Califf, Vice Chancellor for Clinical Research, Director of the Duke Translational Medicine Institute, and Professor of Medicine in the Division of Cardiology at the Duke University Medical Center; Gary Filerman, President, Atlas Health Foundation; Richard Murray, Head of the Global Center for Scientific Affairs, Merck & Co., Inc.; and Michael Rosenblatt, Executive Vice President and Chief Medical Officer, Merck & Co., Inc. (See “The Clinical Trials Enterprise in the United States: A Call for Disruptive Innovation” in Appendix D.) Coupled with CTE problems such as lengthy delays in mounting trials and low recruitment of patients, this divergence deprives clinicians, patients, and policy makers of scientific evidence justifying the selection of one treatment (or clinical intervention) over another.
According to the authors, the CTE appears to be neither satisfying current health care needs nor keeping pace with changes in the organization of health care. Data now available, as a result of the mandatory registration of clinical trials at the ClinicalTrials.gov website, required under recent legislation,2 reveal that most studies are very small, enrolling fewer than 100 people, while large, industry-sponsored studies are increasingly global, or conducted “offshore,” where costs are lower. Few studies address critical clinical decision points, and most take place in dedicated research centers rather than normal clinical settings. The medical community lacks definitive evidence of the relative risks and benefits of most treatments; as just one example, Califf noted that the majority of clinical guidelines for cardiovascular care are based merely on expert opinion or low-quality data (Tricoci et al., 2009). Meanwhile, health care is moving toward integrated delivery systems, lower rates of hospitalization, expanded utilization of pharmacists and other nonphysicians, and the widespread use of EHRs, the latter of which—if designed to produce useful data on outcomes across patients, and bolstered by adequate decision support—could be mined for broader clinical research efforts on the effectiveness of treatments.
The authors suggested that the transformed CTE may be perceived as consisting of four overlapping “laboratories”: Innovation, Traditional Clinical Research, Health Care Delivery, and Community Engagement.
______________________
2 FDA Amendments Act of 2007, Public Law 110-85.
Gary Filerman, President, Atlas Health Foundation; Richard Murray, Head of the Global Center for Scientific Affairs, Merck & Co., Inc.; and Michael Rosenblatt (unable to attend workshop), Executive Vice President and Chief Medical Officer, Merck & Co., Inc. (See Appendix D for the Discussion Paper “The Clinical Trials Enterprise in the United States: A Call for Disruptive Innovation.”) Table 2-1 portrays these components, which marshal the efforts of different types of stakeholders. Systemic invigoration is called for in order for each of the laboratories to reach its full potential and contribute to a CTE that informs health care decisions.
Integrated health care delivery systems—along with other research, clinical, and educational entities—should, in the authors’ view, develop and implement research business plans in order to integrate research into routine clinical practice and thereby change organizational cultures and foster continuous learning. Although research technologies have advanced rapidly, cultural changes in research milieus have lagged. Adoption of research business plans, under the authority of research directors or chief medical officers, can be expected to speed cultural change in organizations.
TABLE 2-1 The Four “Laboratories” of a Transformed Clinical Trials Enterprise
| Laboratory | Research Goal | Usual Site | Researchers (Methods) | Participants |
| Innovation | Develop initial evidence about treatments and biological markers and hypotheses | Academic health and science centers and acute care settings networked with other facilities | Highly trained, with data management expertise (conduct clinical trials) | Small numbers of volunteers per trial, but many trials |
| Traditional Clinical Research | Determine treatment efficacy, or risks and benefits in carefully defined populations | Research centers and clinical settings | Trained researchers (conduct clinical trials and CER) | Larger numbers of volunteers, but fewer studies |
| Health Care Delivery | Evaluate treatment risks and benefits in the context of health care | Clinical settings | Physicians, nurses, other health professionals (CER; use of EHRs and randomization in clinical practice) | Most patients |
| Community Engagement | Assess strategies of disease prevention and wellness (including living with chronic disease) | Communities: voluntary health organizations, schools, churches, etc. | Community-based researchers (use patient-reported outcomes; include cluster randomization) | Ordinary citizens |
SOURCE: Presentations and Discussion Paper by Califf et al., 2012. The Clinical Trials Enterprise in the United States: A Call for Disruptive Innovation. Discussion Paper, Institute of Medicine. (See Appendix D)
United States-based clinical research is increasingly noncompetitive. As Figure 2-1 shows, U.S. costs per clinical trial far exceed costs in India, China, and other increasingly attractive research sites in the developing world.
Califf argued that the loss of research to other countries negatively affects not only U.S. job growth but also the utility of research findings. Studies involving foreign populations may be less applicable to the United States, because Americans may differ from citizens of other nations in both standard treatments, such as aspirin use and various drug combinations, and in the composition of subpopulations (see, e.g., Wallentin et al., 2009). Put another way, globalization, at least as it is currently unfolding, may compound the problem, already observed in the United States, that most scientific evidence is not collected in relevant clinical and cultural contexts.
The integration of patient-centered clinical research into routine health care is a disruptive innovation requiring a national catalytic agent to forge collaborations. This catalytic agent could come in the form of national leadership—several participants suggested that a gap exists in leadership needed to move toward integration of care and research, with many leaders in health policy and health care organization remaining on the sidelines when research is discussed in the policy setting. Bringing health care leadership and policy makers into the conversation about the future of clinical research could lead to development of a concrete, effective, and overarching national strategy.
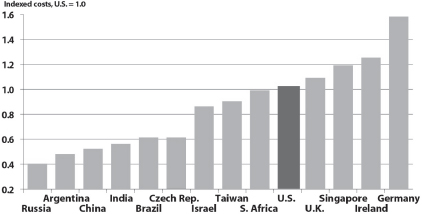
FIGURE 2-1 U.S. costs per clinical trial are noncompetitive.
SOURCE: DeVol et al., 2011. The global biomedical industry: Preserving U.S. leadership. http://www.milkeninstitute.org/pdf/CASMIFullReport.pdf (accessed February 9, 2012). Reprinted with permission from Milken Institute.
A FRAMEWORK FOR THE CLINICAL TRIALS ENTERPRISE IN THE HEALTH CARE SYSTEM OF 2020
Controlled clinical trials are the essential cornerstone of modern evidence-based medical care and health practice.
—Robert Califf, Duke University Medical Center;
Gary Filerman, Atlas Health Foundation; Richard Murray,
Merck & Co., Inc.; and Michael Rosenblatt, Merck & Co., Inc.
In a panel discussion, the session chair, panel presenters, Discussion Paper co-authors, and audience members reacted to and built upon the ideas contained in the Discussion Paper. Participants included session chair, Alastair Wood, Professor of Medicine and Pharmacology, Weill Cornell Medical College, and Partner, Symphony Capital LLC; and panelists Neil Weissman, President of MedStar Health Research Institute, Professor of Medicine at Georgetown University School of Medicine, and Director of the Cardiovascular Core Laboratories at Washington Hospital Center, Washington, DC; and Ihor Rak, Vice President, Global Clinical Development, Neuroscience Therapy Area, AstraZeneca. This section provides an integrated summary of their remarks and should not be construed as reflecting consensus or endorsement by the workshop participants, the planning committee, the Forum, or the National Academies.
In 2020, said Wood, the CTE and health care system should serve, inform, and use each other, thereby constituting a learning system. Table 2-2 displays potentially beneficial features of both a transformed CTE and a more mature health care system. Increased productivity of the health care system is a particularly salient goal, given that productivity of the U.S. health care system fell 0.6 percent during the past two decades, while productivity in the four U.S. economic sectors of manufacturing, retail trade, finance/insurance/real estate, and professional/scientific/technical/legal services grew at rates ranging from 4.7 to 2.2 percent—and productivity of the U.S. economy overall climbed 1.7 percent (Kocher and Sahni, 2011).
One integrated delivery system, MedStar Health, is striving to develop a research orientation, described by Weissman. MedStar Health enrolls one-fifth of residents in the Baltimore-Washington, DC, area and
TABLE 2-2 Potential Features of the Clinical Trials Enterprise and Health Care System of 2020
| Clinical Trials Enterprise | Health Care System | |
| Organization and structure in relation to health care delivery | Integration into health care system | Less fragmentation of care, as integrated delivery systems become more prominent |
| Objective to facilitate clinical trials | Larger, more numerous, longer, and more relevant studies | Greater use of health IT, with interoperability and standardization |
| Relationship between the CTE and health care system | Investigates treatment effectiveness, not just drug efficacy; treatments must show net benefit and cost-effectiveness, not just novelty | Payment driven by outcomes, not procedures; expectation that care will match evidence |
| Ultimate desired outcome | Efficiency | Higher productivity |
SOURCE: Adapted from Wood, 2011. Presentation at IOM workshop on Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020.
controls 9 hospitals as well as 20 other health care entities. At MedStar, research is integrated into clinical practice, with EHRs used, among other things, to recruit study participants and apply research findings to clinical decisions. Some clinicians receive enhanced payment for participating in research projects and enjoy access to MedStar’s research facilities. Still, only 1 percent of approximately $4 billion in annual MedStar expenditures goes toward research, partly because the value of integrating research into practice is yet to be proved to many hospital administrators and chief medical officers, especially while hospitals are increasingly hard-pressed financially. To be convincing, proof of the value of such integration is best expressed in terms that are not only relevant to academic or health policy goals, but also relevant to a hospital or health care delivery system perspective. Furthermore, there is little incentive, even for integrated delivery systems, to use EHRs for research, when federal requirements for “meaningful use”3 of EHRs do not mandate that EHRs include a research function.
______________________
3 The Health Information Technology for Economic and Clinical Health (HITECH) Act, part of the American Recovery and Reinvestment Act of 2009, allowed CMS to provide financial incentives to health providers for the adoption and “meaningful use” of certified EHR technology. Meaningful use entails three components: (1) the use of certified EHR technology in a meaningful manner, such as e-prescribing; (2) the use of certified EHR technology for electronic exchange of health information to improve quality of health care; and
Rak expressed optimism that we are close to a tipping point for integration of research and care. He noted that the full spectrum of clinical research could be integrated with “real-world” care, and not just clinical trials. Factors supporting this optimism include
• inclusion of all major stakeholders in recent conversations about the need to incorporate evidence into clinical decisions,
• growing recognition that quality care requires scientific evidence, the rapid pace of scientific breakthroughs, growing economic incentives for gathering clinical evidence, and
• incentives for consolidation of private-sector organizations that sponsor research.
Speakers compared the United States’ ability to integrate research and care with that of other countries. One comparative disadvantage faced by the United States is that its pluralistic, fragmented, and still mostly fee-for-service health care system impedes several conditions of transformation:
• implementation of a common clinical strategy across all providers, even within a single geographic market area;
• routine and smooth information exchanges among health care providers, with standard data formats;
• systematic evaluation of treatments; and
• use of global reimbursement and regulatory controls to effect change.
By contrast, countries with a unified health care system—notably, China— can apply mandates and industrial processes to create change. Another speaker cautioned, however, that although a more top-down system might be more susceptible to broad policy changes than the United States’ more fragmented approach, China has many systems that do not work in practice and may not be a model to emulate.
Some workshop participants discussed the challenge of recruiting clinicians to participate directly in the development and implementation of a clinical trial as an investigator or refer their patients to clinical trials:
• There are many challenges associated with use of Medicare funds for research in clinical settings. Moreover, congressional resistance
_________________________________
(3) the use of certified EHR technology to submit clinical quality and other measures. For more information, visit http://www.cms.gov/EHRIncentivePrograms/30_Meaningful_Use.asp (accessed March 28, 2012).
to using research results for reimbursement purposes has emerged. The political controversy could limit the potential utility of findings obtained through studies sponsored by the new, comparative effectiveness-oriented Patient-Centered Outcomes Research Institute (PCORI).
• Many clinicians who might be interested in research are otherwise being compelled to increase clinical productivity and so may be less receptive to additional demands on their time.
• Cultural barriers have also been identified, to the extent that clinicians may not welcome research findings that cast doubt on the effectiveness of procedures that make up a substantial portion of their practices. Califf and Wood suggested that negative research results often are not used by practitioners; for example, it is questionable whether most physicians treating dyspnea in patients with acute heart failure are changing their prescribing patterns to conform with results of a recent study that concluded that nesiritide cannot be recommended for early relief of dyspnea for the broad population of patients with acute heart failure (O’Connor et al., 2011; see also Topol, 2005).
• Medical schools and students tend to see research as a separate track from clinical practice (although some schools now require students to conduct a research project). Box 2-1 displays these and other aspects of the challenge of persuading clinicians to contribute to research efforts, as noted by individual workshop participants.
To build momentum for change in this environment, a participant suggested, success stories, or “small wins,” are needed, for example, progress in interoperability or data standardization. Oncologists were described as pointing the way toward a future of smaller, faster, more targeted trials, accompanied by genetic screening. Similarly, New York State’s Partnership to Advance Clinical electronic Research (PACeR) was described as an example of an industry-academic collaborative established to use EHR data on a continuing, sustainable basis. Many of the comments included the observation that a cultural shift is called for to achieve transformative change. One participant stated
that cultural shift has to be that it is unacceptable for a patient encounter to occur without a question being asked about whether this patient at this point in time can contribute to our bigger knowledge, our greater knowledge about health care.
BOX 2-1a
Some Aspects of the Challenge of Persuading Clinicians to Engage in Research
Issues Affecting Clinician Incentives to Enroll Patients
• Lack of demand from patients to participate in research
• Inadequate, unsystematic methods of informing clinicians about ongoing or new research studies and clinical trials
• Information overload; a physician might receive hundreds of emails per day concerning clinical trials but none coming from an entity or individual the physician knows or trusts
• If a clinician is not participating in the trial as an investigator, there is concern over the failure of communication with researchers throughout the lifecycle of a study, including concern by clinicians that they will be unaware of treatments and side effects their patients experience as part of the study
• Need for consideration of patients’ insurance coverage, or lack thereof, which could determine whether a research study would make financial sense for a patient under a particular clinician’s care
• Heterogeneous patient mix and a diversity of patient medical needs that may or may not be solved or improved through care received in a research study
Issues Affecting Clinician Willingness to Incorporate Clinical Research into Their Clinical Practice
• Absence of cultural awareness and commitment to the principle that physician- patient encounters should generally contribute to the body of scientific evidence
• Rising productivity pressures and other demands on physicians’ time
• Prospective income loss for physicians performing procedures that are found ineffective
• Lack of reimbursement incentives by Medicare and other third-party payers for physicians to devote time to clinical research, including clinical trials
• Patient information in a physician’s office is collected both for direct patient care purposes and to support administrative claims requirements. In many cases, new IT tools and increased staff time will be needed to collect the additional information necessary for a research study
Issues Affecting Clinician Use of Medical Evidence in Decision Making
• Medical schools’ separation of research from practice
• Lack of clinician knowledge as to the array of clinical trials available and how participation in a research study might benefit their patients
_____________________
a This box provides a list of suggestions from the workshop audience, themes contained in a summary of the previous IOM workshop on clinical trials and public engagement (IOM, 2012a), and speaker presentations by Alastair Wood, Professor of Medicine and Pharmacology, Weill Cornell Medical College, and Partner, Symphony Capital LLC; Neil Weissman, President of MedStar Health Research Institute, Professor of Medicine at Georgetown University School of Medicine, and Director of the Cardiovascular Core Laboratories at Washington Hospital Center, Washington, DC; and Ihor Rak, Vice President, Global Clinical Development, Neuroscience Therapy Area, AstraZeneca.
We seek the development of a learning health system which generates and applies the best evidence for the collaborative health care choices of each patient and provider; drives the process of discovery as a natural outgrowth of patient care; and ensures innovation, quality, safety, and value in health care.
—IOM Roundtable on Value and Science-Driven Health Care, Roundtable Charter5
There is abundant reason to be sanguine about the prospects for transformation of the CTE, said Richard Platt, Professor and Chair of the Department of Population Medicine at Harvard Medical School and Executive Director of the Harvard Pilgrim Health Care Institute, because this requires not only developing but, equally important, applying scientific evidence. According to Platt, participants in the IOM’s Roundtable on Value and Science-Driven Health Care seek a health care system in which 9 of every 10 clinical decisions are evidence based by 2020. Round-table efforts include five Innovation Collaboratives: Best Practices, Value Incentives, Evidence Communication, Clinical Effectiveness Research, and Digital Learning. The Roundtable has recognized in meetings and reports that evidence-based clinical decision making depends on the establishment of a health system that learns both what care to deliver and how to deliver it. This knowledge can be developed through the secondary use of clinical data, provided there is adequate governance and coordination of health information.
Multiple public–private partnerships are building platforms for secondary use of clinical data, Platt noted. First, the Office of the National Coordinator for Health Information Technology has launched a Query Health initiative—an effort to distribute “population health queries.” As envisioned, researchers’ and health care providers’ requests for information about health care outcomes in large populations will be answered with information assembled from EHRs and other data sets maintained by integrated delivery systems and other sources. In voluntarily sharing their data in response to a query, health care organizations maintain control of their data behind their firewalls, allowing them to maintain security and
______________________
4 This section is based on a keynote address by Richard Platt, Professor and Chair of the
5 For additional information on the work of the Roundtable, visit http://iom.edu/Activities/Quality/VSRT.aspx (accessed March 28, 2012).
patient privacy. This and similar approaches require a standard clinical information model, such as Informatics for Integrating Biology and the Bedside (i2b2).6 Second, the FDA’s Mini-Sentinel distributed database initiative is exploring ways to develop evidence about the postmarketing safety of drugs, vaccines, and other products through data obtained from multiple sources.7 As of July 2011, Mini-Sentinel contains quality-checked data from 17 partner organizations, mostly large insurers and integrated delivery systems, covering nearly 100 million people (Platt et al., 2012). Its features include an operations center, secure portal, and opportunities by the partners to examine the queries that are distributed and review their own results before submitting them for pooling with results from other partners.
Third and perhaps most significant, CER is gaining strength. Currently, only 0.1 percent of health expenditures are devoted to this important research and development activity, but it is expanding. Steps involved in building a mature CER system might include the following:
• Build the research base—weave CER into the fabric of care, so that clinicians do not have to choose to participate or perform extra steps in its conduct;
• Build the business case—align financial incentives for providers with quality of care;
• Address regulatory barriers—relax or harmonize requirements for informed consent, patient privacy, and other mandated protections, when appropriate;
• Build demand for scientific evidence from patients, providers, payers, and purchasers—involve everyone in the transformation.
An example of effective collaboration in CER is the Hospital Corporation of America (HCA)-based, 42-hospital, 75,000-patient study of methicillin-resistant Staphylococcus Aureus (MRSA), the highly antibiotic-resistant pathogen affecting hospitals nationwide (Platt et al., 2010). The study, called REDUCE (Randomized Evaluation of Decolonization versus Universal Clearance to Eliminate)-MRSA, is comparing routine decolonization
______________________
6 Informatics for Integrating Biology and the Bedside (i2b2), developed by the NIH-
7 Mini-Sentinel is a pilot program for the FDA’s more extensive Sentinel System, now
of critical care patients with contact precautions to evaluate the best way to prevent MRSA and its spread. The study’s cluster (hospital-level) randomization approach, a central IRB, centralized informatics, and a unified data warehouse help keep cost and time commitments down. In addition, HCA’s corporate leadership is committed and the hospitals’ quality assurance staff and infection control practitioners are lending significant expertise and staff time to the study, precluding the need for traditional clinical monitors or dedicated staff. The study was funded with approximately $1 million from HCA and $2 million from federal agencies. HCA is in a position to apply the results consistently throughout its entire system, so the study in effect offers the advantage of determining both efficacy and effectiveness. Platt suggested that the successful marriage of research and delivery of care in this example of a collaborative CER study shows that a business case exists for answering important questions of interest to the health care system.
Bryan Luce, Senior Vice President for Science Policy, United BioSource Corporation, suggested a potentially transformative “learn and confirm” strategy for seamlessly integrating traditional clinical trials for drug development and the generation of evidence to guide clinical guidelines and coverage and reimbursement decisions for a health product. Under this strategy, manufacturers partner with health plans and integrated health systems (IHSs) to engage in a dynamic, adaptive clinical trial process that begins with a tightly controlled RCT that increasingly expands the inclusion criteria for the trial so that eventually the study population approximates the usual care setting. The discreet phases of drug development (Phase I-IV clinical trials) are replaced by an adaptive trial design that facilitates the generation of real-world medical evidence as a product builds toward being available on the market. Potential advantages of this more flexible research strategy include faster and cheaper development of drugs, evidence-based clinical decisions, and payment only for uses that are generally safe and are effective in the relevant population. Registration of a product can take place much earlier and be coordinated with coverage and payment decisions, evidence standards, and thresholds.
The use of adaptive designs and other pragmatic approaches, such as cluster trial designs and virtual trial designs, was also illustrated by Peter Yu, Director of Cancer Research, Palo Alto Medical Foundation. Traditional clinical trials ignore participants who don’t respond to the study drug being tested, Yu explained. However, a sequential trial design can accommodate investigation of multiple drugs, administered in a tiered fashion that allows the initial nonresponder study participant to receive other treatments that might work for them (Figure 2-2).
These more flexible strategies allow for learning from every patient, including nonresponders, about the molecular basis of disease.
According to Yu, these designs will pave the way to advance personalized medicine, in which interventions are tailored to patients based on biologic markers and other individual traits. The strategies also promote a learning system. It is important to investigate primarily those clinical questions for which potential drug prescribers and users—clinicians and patients—actually want answers, or else the research effort will be wasted. In this targeted way, and because more advanced trial designs are expected to result in a trial failure rate well below today’s level of about 85 percent, a learning system is also a “lean” system.
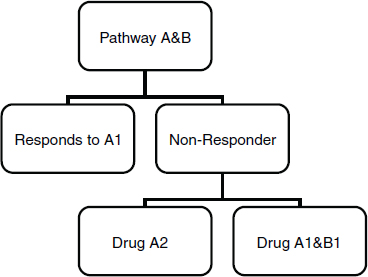
SOURCE: Yu, 2011. Presentation at IOM workshop on Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020.
This page intentionally left blank.














