Workshop Summary
Recent research suggests that obesity and excess weight can play a prominent role in the incidence and progression of various cancers. Obesity results from an energy imbalance—that is, energy intake that is higher than energy expenditure—that could also influence the growth of cancers. In addition, by generating hormones and growth factors, and by fostering inflammation, fat tissue could directly fuel the growth of tumors, thereby affecting cancer incidence, progression, recurrence, and survival rates. Given the current obesity epidemic and an aging population more susceptible to cancer, there is mounting concern about the role that obesity plays in malignancies. There is also interest in exploring possible interventions to break the obesity–cancer link, especially in patients already diagnosed with cancer, who are at risk for cancer progression and recurrence and are also more susceptible to developing new cancers. Cancer survivors currently number 12 million in the United States and are rapidly increasing in number.
Recognizing the impact that current findings on obesity and cancer could have on future cancer prevention and care, the National Cancer Policy Forum (NCPF) of the Institute of Medicine (IOM) held a 2-day workshop1 on “The Role of Obesity in Cancer Survival and Recurrence,”
_________________
1 This workshop was organized by an independent planning committee whose role was
in Washington, DC, on October 31 and November 1, 2011. At the workshop, experts presented the latest clinical evidence on the obesity–cancer link and the molecular mechanisms that might explain that link. Clinicians, researchers, cancer survivors, and policy makers also discussed potential interventions to counter the effects of obesity on cancer, and research and policy measures needed to stem the rising tide of cancer mortality predicted by an increasingly overweight and older population worldwide. More specifically, the workshop explored:
• The complex web of molecular mechanisms that underlie the obesity–cancer link and whether it is obesity itself, the energy imbalance that leads to obesity, or the molecular pathways that are deregulated due to obesity, that increases the risk of cancer initiation or progression;
• Clinical evidence of the obesity link to cancer incidence and outcomes and study design issues that may affect the strength of that evidence and its interpretation, as well as ways to design future studies to acquire the information needed to guide patient care;
• Potential interventions to counter or prevent obesity effects and/or restore energy balance, including lifestyle measures, as well as drug and surgical therapies;
• What to advise cancer patients about weight loss, diet, exercise, and other measures to reduce their risk of cancer progression or recurrence, and the challenges in inducing healthy behaviors; and
• Policy suggestions related to research, education, and dissemination of the findings on obesity and cancer, as well as what the private and public sectors can do to help break the obesity–cancer link.
This document is a summary of the workshop. The views expressed in this summary are those of the speakers and discussants, as attributed to them, and are not the consensus views of workshop participants or the members of the NCPF.
limited to identification of topics and speakers. This workshop summary was prepared by the rapporteurs as a factual summary of the presentations and discussions that took place at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and are not necessarily endorsed or verified by the Forums or the National Academies, and they should not be construed as reflecting any group consensus.
OVERVIEW OF THE OBESITY–CANCER LINK
The ancient Greek medical practitioner Hippocrates anecdotally reported the observation that a healthy balance of food intake and physical activity promotes good health. But it was not until the early part of the 20th century that researchers showed tumor cells transplanted into underfed mice did not grow as rapidly as in those fed more abundantly, noted Wendy Demark-Wahnefried, chair of nutrition sciences at the University of Alabama at Birmingham and chair of the workshop planning committee.
More recently, there has been an explosion of research on obesity and cancer, with more than 2,000 papers on the topic published in the scientific literature. An extensive 2007 review of published research found convincing evidence to link obesity to the risk of endometrial, colorectal, esophageal, kidney, and pancreatic cancer as well as to postmenopausal breast cancer (World Cancer Reasearch Fund and American Institute for Cancer Research, 2007). This review found more limited evidence linking obesity to gallbladder or liver cancer, as well as accumulating evidence that obesity is linked to the risk of non-Hodgkin’s lymphoma, ovarian cancer, and aggressive prostate cancer. “There are important public health implications in these findings. Obesity is the second leading risk factor for cancer,” stressed Dr. Susan Gapstur, vice president of epidemiology at the American Cancer Society.
This is especially alarming considering that since the 1970s, the number of Americans becoming obese or overweight has steadily risen; they now comprise more than two-thirds of the population. The obesity epidemic is also spreading internationally; the World Health Organization estimates that more than 1.5 billion adults worldwide are overweight or obese. “The obesity epidemic is not just a U.S. problem—it’s a worldwide problem,” noted Dr. Gapstur. However, there is debate over why obesity is increasing in prevalence, with several reasons posited by experts, including the rising consumption of sugar-sweetened beverages, an increase in portion size, and the decline in physical activity, in part due to increased automation. “I don’t think we can say there is any one sole factor that has led to that change,” said Dr. Rachel Ballard-Barbash, who serves as the associate director of applied research in the Division of Cancer Control and Population Sciences at the National Cancer Institute. Dr. Gapstur agreed, noting “there’s not one simple answer because it gets back to that concept of energy balance.” (See Box 1 for a discussion of energy balance.)
The increasing incidence of breast cancer in developing countries
BOX 1
Energy Balance and Cancer Risk
Dr. Wendy Demark-Wahnefried gave a brief overview of energy balance and what factors influence it and how it influences cancer risk. She noted that the energy balance in a person’s body is determined by energy intake from the diet, which can be modified by ingestion and absorption, balanced by the total calories expended. Typically, about 60 to 75 percent of energy is expended by maintaining the resting metabolic rate, 5 to 10 percent is expended as the energy needed to digest food, and 15 to 30 percent is expended through physical exercise (Figure 1).
Dr. Demark-Wahnefried also noted that a gastric bypass or a lap band will induce a shift in energy balance by reducing calorie intake. The main dispeller of energy is resting metabolic rate, which is driven, in part, by lean body mass. As people age, she noted, they expend less energy because they do not have as much muscle mass. Chemotherapy and other cancer treatments can also decrease lean body mass. Resting metabolic rate is also driven by the balance of the external temperature with the body’s internal temperature, with more energy expended when the external temperature drops below 78 degrees Fahrenheit. Disease can affect body temperature as well.
is thought to reflect the increase in obesity in these nations, added Dr. Ballard-Barbash. Dr. Nathan Berger, a professor of medicine and director of the Center for Science, Health, and Society at the Case Western Reserve University School of Medicine, added that “the convergence of obesity and aging is the perfect storm or tsunami in terms of increasing the overall incidence of cancer.”
But obesity does not appear to have a uniform effect on all types of cancers, nor to affect cancer risk the same in men and women. One study found that obesity increases the risk of dying from all cancers by about 52 percent in men, but nearly doubled the risk of dying from any type of cancer in women (Calle et al., 2003). For some cancers, such as liver cancer, obesity was linked to about a five-fold increased risk of cancer mortality in both sexes. In contrast, the association between obesity and colon cancer mortality is not equally strong in women and men, perhaps because body mass index (BMI) is a better measure of abdominal fat in men than women,
Several researchers have attempted to assess how the various components of energy balance affect cancer risk. One study showed that calorie restriction consistently lowers the risk of cancer mortality across several species, from the mouse to the cow (Hursting et al., 2003). Studies on the effects of physical exercise on cancer risk are less consistent, Dr. Demark-Wahnefried said, although a few studies suggest that when energy intake is kept constant, animals that exercise more have less cancer progression. However, one study found that expression of several genes is altered when mice are placed on caloric restriction, but there are relatively few changes in gene expression when caloric intake is kept stable while mice are exercised (Padovani et al., 2009).
Dr. Demark-Wahnefried stressed “the need to disentangle effects of caloric restriction and increased physical activity, as well as obesity. Cancer is complex and energy balance is complex. It’s really difficult to make a change in one factor without impacting another.” As she noted when people exercise a lot, their appetite tends to increase and leads them to eat more, whereas there is some evidence that moderate amounts of physical activity can actually suppress appetite. “So if you are going to conduct a physical activity intervention, then it is important to measure and control for dietary intake,” Dr. Demark-Wahnefried said.
or because of hormonal factors that are protective, Dr. Gapstur pointed out (Box 2).
Obesity-related breast cancer risk also varies by menopausal status. Increasing BMI levels are linked to a lower incidence of breast cancer in premenopausal women, but a greater incidence of breast cancer in postmenopausal women, Dr. Gapstur said, for reasons that are not yet clearly defined. Obesity’s influence on prostate cancer risk also varies. Although obesity is associated with a lower incidence of prostate cancer, studies suggest that obesity is linked to a greater risk of being diagnosed with a more aggressive form of prostate cancer, and studies have consistently shown that obesity substantially increases the risk of dying from prostate cancer. “These data suggest that one shoe doesn’t fit all, and it may be very important to separate the different disease types,” she said.
Growing evidence also indicates that obesity during childhood can increase the risk of childhood cancers, such as leukemia, and young-onset brain tumors, Dr. Ballard-Barbash noted. “This is an issue that is urgent
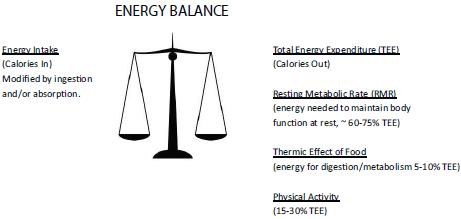
FIGURE 1 Energy balance. Weight maintenance occurs when energy input equals energy expenditure, with gains and losses occurring when there is an imbalance. A gain of one pound occurs when approximately 3,500 calories are consumed in excess of energy needs. For most individuals, resting metabolic rate (RMR) comprises the major component of energy expenditure, accounting for 60-75 percent of energy needs (IOM, 2005; Pi-Sunyer, 2000). Resting metabolic rate is largely governed by lean tissue, which has a higher metabolic rate than adipose tissue. Neoplastic tissue also may have a higher metabolic rate, though a study of 200 cancer patients compared to 200 healthy controls found that metabolic rate was highly variable among cancer patients (50-175 percent of predicted values) and greatly influenced by tumor stage and site (Knox et al., 1983). Speculation also exists that RMR may be influenced by various cytokines, though more research is necessary (Pi-Sunyer, 2000). The Thermic Effect of Food, also known as Specific Dynamic Action, is the energy needed for digestion and metabolism of food—transient energy needs that go above and beyond normal metabolism. The Thermic Effect of Food accounts for only 5-10 percent of energy needs and is dependent upon the magnitude of dietary intake, and also may be influenced by the consumption of specific foods or food-related substances. For example, tea, capsaicin, and caffeine may increase metabolism even further (Bell and Goodrick, 2002), though more studies are needed to determine if these transient increases are clinically meaningful. Physical activity comprises the third component of energy expenditure and for individuals living in the developed world, usually accounts for 15-30 percent of energy needs. The energy expended for physical activity is the most modifiable component of energy expenditure. Furthermore, given the potential of exercise (especially resistance training) to increase lean body mass, physical activity also may act indirectly to increase RMR.
SOURCE: Demark-Wahnefried presentation (October 31, 2011). Reprinted with permission from Wendy Demark-Wahnefried.
There are several assessments of overweight and obesity; the most common is body mass index (BMI). BMI is weight (kg)/height squared (m2). It is frequently used in studies because it is a single measure that can be determined easily, Dr. Susan Gapstur pointed out, and is comparable among studies. Below are the World Health Organization criteria for overweight and obesity:
• Underweight = BMI < 18.5 kg/m2
• Normal weight = BMI 18.5-24.9 kg/m2
• Overweight = BMI 25.0-29.9 kg/m2
• Obese = BMI 30+ kg/m2
An individual’s body fat distribution can also be assessed with anthropometric methods such as measurements of waist and hip circumference or the ratio of the two, or techniques such as air displacement or bioelectrical impedance to determine percentage of body fat. BMI correlates well with percentage of body fat in the average population, according to Dr. Gapstur.
for our children, even beyond what the effect will be in their adult lives,” she said.
The variability in how obesity affects the incidence, progression, or mortality of various cancers suggests that these effects derive from multiple mechanisms, which animal research supports. This research implies a web of interacting hormones, growth factors, cytokines, and inflammation mediators that promote tumor initation and growth.
As Dr. Berger noted, an excess of nutrients causes an imbalance in energy. This imbalance causes oxidative stress and abnormalities of fatty acid metabolism that foster inflammation and insulin resistance (Figure 2). This results in a number of processes that underlie cancer initiation and promotion, including DNA damage, cell division, delayed cell death, an increase in blood vessel formation, and cell migration (Figure 3).
The complex interactions between all the obesity-activated hormones
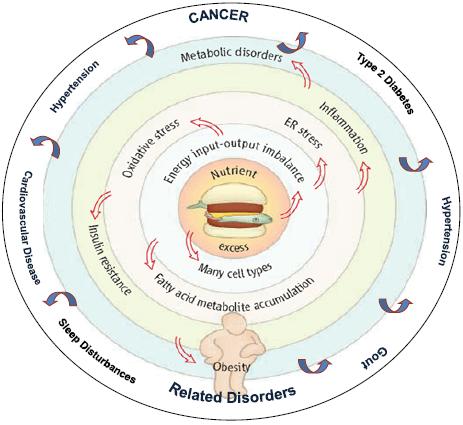
FIGURE 2 An integrative view of obesity.
SOURCE: Berger presentation (October 31, 2011). Adapted from Wisse et al. (2007). An Integrative View of Obesity. Science 318:928-929. Reprinted with permission from AAAS and Nathan Berger.
and growth factors make it difficult to pinpoint targets for interventions. “It’s important to remember that if you are directing your therapy at leptin, for example, or at reducing insulin levels, all these other factors are going on simultaneously. If we really are going to attack the obesity–cancer link, it’s going to require attacking many of these components, if not all, simultaneously,” Dr. Berger said.
Dr. Berger stressed the crosstalk that occurs among several different obesity-activated pathways leading to cancers, as well as the cellular signaling that occurs among fat tissue, the immune system, and cancer cells. Dr. Derek LeRoith, director of the Metabolism Institute and chief of Endocrinology, Diabetes, and Bone Diseases at the Mount Sinai School of Medicine, agreed, pointing out that animal models can help researchers delineate
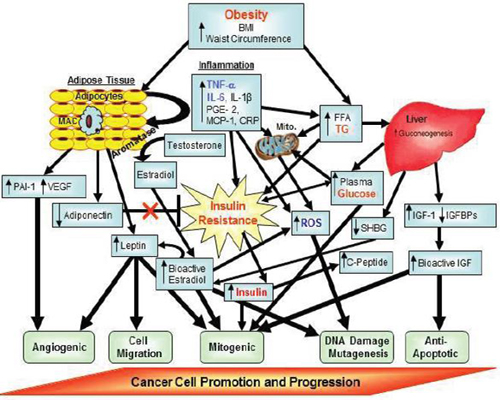
FIGURE 3 Putative factors involved in obesity-related carcinogenesis. Factors denoted in red text are core features in the Metabolic Syndrome.
Factors denoted in blue text are additional features that may also be components of the Metabolic Syndrome.
NOTE: BMI = body mass index; CRP = C-reactive protein; FFA = free fatty acid; IGF = insulin-like growth factor; IGFBPs = insulin-like growth factor binding proteins; IL = interleukin; MAC = macrophages; MCP = monocyte chemotactic protein; mito = mitochondria; PAI = plasminogen activator inhibitor; PGE = prostaglandin; ROS = reactive oxygen species; SHBG = sex hormone binding globulin; TG = triglycerides; TNF = tumor necrosis factor; VEGF = vascular epithelial growth factor.
SOURCE: Berger presentation (October 31, 2011). Adapted from Nock and Berger, 2010. Reprinted with permission from Nathan Berger.
which molecules and pathways underlie the obesity–cancer link, but that those individual compounds or pathways need to be kept in context. “It’s all of them, and hitting one may be insufficient,” he said, adding that many pharmaceutical companies are trying to develop combination therapies that target two or more key molecules in different pathways simultaneously.
Some of these molecular pathways could foster cancer initiation or promotion, or both. For example, Dr. Pamela Goodwin, senior scientist and Marvelle Koffler Chair in Breast Cancer Research at Mount Sinai Hospital, noted that obesity is linked to an increased risk of breast cancer progression, but not initiation in premenopausal women. Obese premenopausal women actually have a lower incidence of breast cancer than premenopausal women of normal weight, perhaps because of abnormal estrogen metabolism and/or other factors related to having an energy imbalance, Dr. Gapstur pointed out. Dr. LeRoith suggested the low testosterone of obese men may protect them from developing prostate cancer, but once they develop this tumor, obesity and the excessive insulin production it causes could foster the aggressive growth and metastasis of prostate cancer. Dr. Stephen Hursting, professor at the University of Texas at Austin, and MD Anderson Cancer Center, pointed out that studies using a variety of animal models support the premise that obesity fosters cancer promotion, but the link between obesity and cancer initiation is more tenuous.
Dr. Berger noted that the heightened metabolism of fatty acids that occurs in obese individuals might increase DNA damage due to oxidation. But little to no evidence shows that this DNA damage triggers a malignant transformation, even though DNA damage in general has been shown to cause cancer. He questioned whether obesity actually fosters the initiation of cancer, or rather causes cancer promotion so that the cancer is diagnosed earlier in life.
“Data exist to support the latter for pancreatic cancer (Harvey, 2011), but for none of the other cancers. This is a really important question, because if obesity is causing one of the initial mutations, then we need to treat it early and think about how to block the mutations. But if obesity is really causing progression, then there will be whole different strategy to deal with it,” Dr. Berger said. Dr. John DiGiovanni, professor of pharmacology, toxicology, and nutrition sciences at the University of Texas at Austin, noted that the AKT-mTOR pathway that is activated in obese animals is linked to increased risk of developing a cancer, as well as to the progression of many cancers.
There was general agreement, however, that cancer is a disorder whose
hallmark is abnormal regulation of the growth and survival of cells, and that fat cells generate many hormones, growth factors, and cytokines that can disrupt regulation of cell growth and survival. The key mechanistic molecular factors discussed at the workshop were estrogen, insulin, insulin-like growth factor 1 (IGF-1), leptin, adiponectin, and adipokinase, as well as several mediators of inflammation.
Seventy percent of all breast cancer patients are postmenopausal women. Most of the estrogen produced in postmenopausal women is derived from fat tissue via the enzyme aromatase, which converts adrenal androgens into estrogen. The more fat tissue there is, the greater the levels of estrogen produced and in circulation. Such estrogen can fuel the growth of estrogen receptor–positive breast cancers. Studies show that mice made obese by being fed a high-fat diet and then inoculated with breast cancer cells had significantly greater tumor growth rates than mice similarly inoculated, but fed a normal diet (Sabnis et al., 2009). When inoculated obese mice were given an aromatase inhibitor, the tumor growth rate was markedly inhibited, reported Dr. Angela Brodie, professor of pharmacology and experimental therapeutics at the Marlene and Stewart Greenbaum Cancer Center of the University of Maryland School of Medicine.
Clinical studies confirm that circulating estradiol levels are linked to risk of recurrence of breast cancer, but Dr. Goodwin pointed out this mechanism does not explain the association of obesity with premenopausal breast cancer outcomes or with estrogen receptor–negative breast cancer outcomes (Pierce et al., 2007a). Dr. Gapstur added that it also does not explain why estrogen is linked to both pre- and postmenopausal endometrial cancer risk (Reeves et al., 2007).
Dr. Goodwin and others have data to suggest that another factor making obese women more susceptible to breast cancer recurrence and death is the higher than normal insulin level that is often linked to obesity. Increases in BMI correlate closely with increases in fasting insulin levels in the non-diabetic population, Dr. Goodwin noted, and greater levels of insulin are linked to increased risk of distant recurrence and death in breast cancer patients (Gallagher and LeRoith, 2011; Goodwin et al., 2002).
She pointed out that a fetal version of the insulin receptor is overexpressed in breast cancer cells and can combine with itself or with IGF-1 to turn on the PI3K or Ras/Raf signaling pathways known to foster the growth of several types of cancers. In women with early-stage breast cancer, total expression of the fetal insulin receptor is linked to worse survival rates, as is activation of the receptor by IGF-1.
Insulin effects on breast cancer prognosis often are not apparent 5 years after diagnosis, suggesting that “insulin may be an early mediator of the prognostic effects of obesity in breast cancer, but other factors are going to be important later on,” said Dr. Goodwin. One of those factors appears to be leptin. She recently showed that higher levels of leptin are linked to an increased risk of distant recurrence and death from breast cancer—an effect that persists beyond 5 years postdiagnosis (Goodwin et al., 2012).
Dr. LeRoith added that “insulin could be a major player, not just as an indicator of insulin resistance, but actually as a hormone that is driving cancer growth.” He and his research partners genetically engineered mice with severe muscle, liver, and fat cell insulin resistance, which resulted in elevated insulin levels and an inability to metabolize their diet properly, akin to what occurs in people with type 2 diabetes (LeRoith, 2010). The mice were highly susceptible to developing breast cancer. Unlike most people who have insulin resistance, the experimental mice were not obese, giving the researchers the opportunity to assess the role that high levels of insulin without concurrent obesity plays in fueling breast cancer growth.
The breast cancers that developed in the mice with elevated insulin levels were much more aggressive and had greater tumor growth than those that developed in the mice with normal insulin levels. Insulin acting through a fetal insulin receptor was promoting tumor growth, according to Dr. LeRoith, but not initiating tumors. When the receptor was blocked or the levels of insulin in circulation were lowered, the growth of the tumor was similar to that seen in the normal mice. “By bringing down the insulin level or by blocking its receptor, we can show that the hyperinsulinemia is driving the excessive tumor growth,” he said. Other studies he conducted found that high insulin levels also fueled the growth of breast cancer metastases in the genetically engineered mice, and that excessive growth could be blocked by inhibiting the insulin receptor or by bringing down insulin levels.
In addition to insulin, a number of other hormones, enzymes, and growth factors that govern cellular energy balance and growth are thought to play a role in increasing cancer risk in obese individuals, including
• IGF-1, which stimulates cell growth and proliferation and inhibits programmed cell death by activating the AKT signaling pathway. This pathway has been implicated in a number of cancers.
• AMP kinase, an enzyme that acts as a metabolic master switch, using its energy-sensing capabilities to trigger the cellular uptake of sugar and the breakdown of fatty acids when cells need more energy. AMP kinase also modulates insulin levels.
• Leptin, a hormone that is an appetite suppressor. Weight loss decreases leptin levels.
• Adiponectin, a hormone that regulates sugar uptake and the breakdown of fats mainly by upregulating AMP kinase. Adiponectin is linked to greater energy expenditure, and weight loss increases the amount of this hormone.
Animal models have helped researchers understand how all these factors interact in the complex signaling that occurs in response to changes in calorie intake from the diet (Chen, 2011). This research indicates that when mice are put on a calorie-restricted diet (30 percent less than normal), their IGF-1, insulin, and leptin levels decrease and adiponectin levels increase. In some, but not all, tissues, their AMP kinase levels also increase. Under these calorie-restricted conditions, transplanted tumors do not grow, nor do tumors proliferate in response to the tumor promoter TPA. In contrast, mice with diet-induced obesity have elevated levels of IGF-1, insulin, and leptin and lower levels of adiponectin levels, and tumors grow rapidly. These results are seen for several different types of tumors. “This is a fairly consistent story,” Dr. Hursting noted, although he added that some tumor models are more responsive to IGF-1, while others are more responsive to insulin.
To parse out the role IGF-1 in particular plays in obesity and cancer, Dr. LeRoith genetically engineered a mouse to have abnormally low circulating levels of IGF-1, similar to what is seen in mice on a 30 percent calorie-restricted diet (Moore et al., 2008b). The researchers observed the same reduced effects on tumor growth in these mice that they saw in the calorie-restricted mice. These mice with reduced IGF-1 levels had a reduced
tumor proliferation response when given TPA, similar to what was seen with the calorie-restricted animals. This resistance to tumor growth was accompanied by reduced signaling in the IGF-EGF-AKT-mTOR pathway, which is notorious for spurring the growth of several types of tumors. Further studies in Dr. DiGiovanni’s lab suggest that changes in dietary energy modulate crosstalk between IGF-1 and the EGF receptor by reducing circulating IGF-1 levels (Moore et al., 2008a).
In addition, when mice with low levels of IGF-1 were put on a calorie-restricted diet, tumor growth was virtually eliminated, accompanied by a further reduction in IGF-1 levels, in contrast to those mice with genetically engineered low IGF-1 levels that were put on a diet that made them obese. Tumor growth was partially restored in this latter group of obese mice, even though IGF-1 levels were not increased. According to Dr. Hursting, these findings indicate that the leptin/adiponectin ratio is key to tumor growth. This ratio rises in response to diet-induced obesity, but not to further increases in IGF-1 (Hecker et al., 2011; Hursting and Berger, 2010)
The importance of the leptin/adiponectin ratio was confirmed in other studies conducted by Dr. Hursting, which showed that transplanted tumors do not grow in genetically obese mice that have high insulin levels, but low IGF-1 levels and a low leptin/adiponectin ratio (Zheng et al., 2011). This was in contrast to genetically diabetic mice that also are obese and have high insulin levels, but low IGF-1 levels. These mice have very high leptin/adiponectin ratios and when tumors are transplanted into them, they grow exponentially. “The phenotype is not necessarily driving this, but [rather] the consequences of the phenotype,” Dr. Hursting said. He stressed that whether IGF-1, insulin, or leptin is the main driver in the obesity link to cancer may not be as important as the fact that all these molecules affect the same downstream AKT-mTOR pathway. This pathway induces tumor cell growth and staves off tumor cell death and has been implicated in a number of cancers.
Drs. Hursting and DiGiovanni both stressed the importance of mTOR as a major convergence point on these multiple pathways triggered by increased calories in the diet and suppressed by caloric restriction (see Figure 4). AKT-mTOR signaling was also activated in a non-obese diabetic mouse that had high levels of insulin and IGF-1, suggesting that the insulin resistance fostered by obesity is more important in promoting tumor growth than the obesity per se. “It may not be the adiposity, but the consequences of that adiposity, that are key [in promoting tumor growth],” said Dr. Hursting. The importance of mTOR signaling was supported by findings
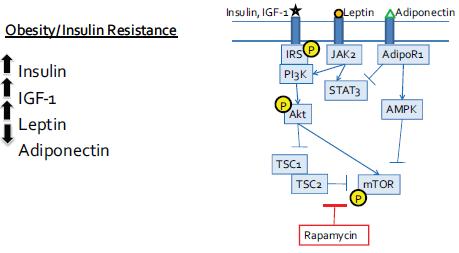
FIGURE 4 Converging signaling pathways.
NOTE: AdiopR = adiponectin receptor; AMPK = 5′ adenosine monophosphate-activated protein kinase; IGF = insulin-like growth factor; IL = interleukin; IRS = insulin receptor substrate, JAK = Janus kinases; mTOR = mammalian target of rapamycin; P = phosphorylated; PI3K = phosphoinositide 3-kinase; STAT = signal transducer and activator of transcription; TSC = tuberous sclerosis protein.
SOURCE: Hursting presentation (October 31, 2011). Reprinted with permission from Stephen Hursting.
that when mice are genetically engineered to have excessive mTOR signaling, caloric restriction does not protect them from the enhanced tumor promotion that signaling provides. “Calorie restriction is restricting the mTOR pathway and obesity is increasing it,” Dr. Hursting stressed. He noted that genetic mutations in this key pathway or upstream or downstream from it will override the effects of calorie restriction.
Dr. Andrew Dannenberg, director of Weill Cornell Cancer Center, showed evidence for his hypothesis that obesity-induced inflammation deregulates pathways previously implicated in the development or progression of breast cancer (Subbaramaiah et al., 2011). The inflammation seen in abdominal and breast fat is induced by the breakdown of lipids into fatty acids, which can activate immune system cells called macrophages, via a receptor called NF-kappaB (NF-kB). Fatty acids latching onto this receptor on macrophages trigger the production of inflammatory mediators such as
prostaglandin E2, IL-1, IL-6, and TNF-alpha. Many of these compounds can induce aromatase to produce more estrogen locally in this breast and abdominal fat. This pathway could explain why obese postmenopausal women are susceptible to an increased risk of breast cancer.
Evidence for this mechanistic pathway comes, in part, from Dr. Dannenberg’s studies in mice. He removed the ovaries from the mice and then fed them a high-fat diet, which caused them to become obese. He then examined their tissues and found numerous crown-like structures—fat cells surrounded by a ring of macrophages. These mice also had increased levels of several inflammatory mediators and, and the fatter the mice, the greater the levels of aromatase activity in their mammary glands or abdominal fat. The same results were seen in genetically obese mice that were leptin deficient.
To see if his animal findings are relevant to women, Dr. Dannenberg examined normal breast tissue removed from women undergoing breast surgery. He found that the crown-like structures seen in the fat tissue of obese mice also occurred in about three-quarters of the breast fat tissue removed from overweight and obese women, but in less than 10 percent of the breast fat tissue removed from women of normal weight. The macrophages in these crown-like structures had activated NF-kB receptors. In addition, the severity of the breast inflammation seen in these tissues strongly correlated with the women’s BMI. Dr. Hursting said that in his studies, he too often sees the crown-like structures of macrophages surrounding fat cells in tumors.
Dr. Dannenberg hypothesizes that when women gain weight and their BMI increases, fat cells in the breast expand. These enlarged fat cells are more likely to die, he speculates, triggering the release of saturated fatty acids that bind to the NF-kB receptors of macrophages and start the cascade of inflammation and subsequent excess production of estrogen in breast tissue. He showed that the severity of inflammation seen in the breast fat correlated with fat cell size, supporting this theory. This led him to develop an inflammation index that correlated more strongly with aromatase activity than the BMI measure, which he claimed is “a crude predictor of physiology.”
The take-home message, according to Dr. Dannenberg, is that “inflammatory mediators are likely to be critical for the induction of aromatase in the context of obesity and teaches us a better way to think about disease pathogenesis and how to evaluate specimens.” He suggested that the crown-like structures of fat cells surrounded by macrophages that he discovered
could be a useful biomarker of breast cancer risk or poor prognosis, and that strategies that disrupt the obesity–inflammation axis may be useful for reducing the risk of breast cancer or its progression. He said the role that inflammation might play in postmenopausal breast cancer is consistent with the documented role of inflammation in several other types of cancers, including esophageal, stomach, liver, and colon cancers.
Dr. Berger’s animal study findings implicate fat in the diet as causing intestinal cancer by modulating inflammatory pathways as well. He found, in mice resistant to obesity, that a high-fat diet led to an increase in the number of intestinal tumors and overall tumor burden, even though the mice remained lean on the high-fat diet. This tumor growth was preceded by an increase in leptin and a decrease in adiponectin, and was linked to an increase in inflammatory mediators such as IL-6 and IL-1 beta. “High-fat diets stimulate cancer progression, independent of obesity or diabetes,” Dr. Berger stressed.
In addition, Dr. DiGiovanni found in obese animals an increase in infiltrating macrophages and lymphocytes, an increased production of inflammatory mediators in their prostates, and an increased production of VEGF, which can support the growth of blood vessels needed to feed tumors. “The mechanisms that are involved in the dietary energy balance effects in prostate cancer are complex and involve many pathways,” Dr. DiGiovanni said. Although these mice also experienced the same changes in growth factor signaling seen in his other diet-induced obesity and skin cancer experiments described previously, there were bigger changes in the degree of inflammation and angiogenesis seen in the prostate of the obese mice. “In this prostate model, inflammation seems to play a much greater role and the diet seems to affect primarily the progression of the prostate lesions in these mice,” he said.
But Dr. Dannenberg postulated that “the obesity–inflammation connection is likely to be relevant for both risk and recurrence of cancer,” and not just to breast cancer but other cancers, such as prostate. He noted that the inflammatory process he discovered in breast cancer is not solely acting through aromatase activation to increase breast cancer risk. “One shouldn’t have blinders on when we make findings on aromatase and recognize that inflammation will have many, many effects locally and systemically, and provides a substrate for thinking about everything from insulin resistance to local effects.”
Dr. Dannenberg summed up the molecular mechanisms related to energy balance by saying, “The take-home message is that cell signaling,
inflammation, and epigenetics are the key collection points for us to really focus on in terms of breaking the obesity–cancer connection. It’s the interface of these areas that is going to be really exciting.”
Recent and mounting evidence implicates cancer stem cells as playing a major role in carcinogenesis and cancer progression. Dr. Madhuri Kakarala, clinical lecturer at the University of Michigan School of Medicine, related this stem cell hypothesis to how obesity might increase cancer risk. As she explained, the stem cell hypothesis posits that not all cells in a particular organ system can become malignant and generate a tumor and metastasis, but rather there is a subpopulation of stem and early progenitor cells that have the capacity for self-renewal and differentiation. Epithelial cells with stem cell properties in breast milk ducts are long lived, and once they accumulate enough mutations, they can develop into breast cancer cells. These cancer cells are supported by surrounding mesenchymal stem cells, which include fat cells. The stem cell hypothesis suggests that the reason why current chemotherapies are often not successful in eradicating cancer in patients is because they target differentiated cells with a rapid turnover, whereas stem cells that are dormant much of the time are relatively resistant to these standard cancer treatments. But when stem cells are stimulated from dormancy by inflammation or other factors, that can lead to metastasis and cancer recurrence.
In the human breast, researchers identify stem and early progenitor cells by aldehyde dehydrogenase staining, and by their distinctive cell surface markers (Kakarala and Wicha, 2008). In invasive breast cancer, cells that are stem cell marker–positive appear on the leading invasive edge. These cells are nursed by inflammatory mediators released by fat cells in the vicinity. Such cytokines can trigger the stem and early progenitor cells to slip into the vascular system and travel to other sites, where they form metastases that are also stimulated to grow by the cytokines and hormones released by fat cells. Dr. Kakarala said several research groups have found that fat cells release several hormones, growth factors, and cytokines that activate signaling pathways that are key to the migration of stem cells and can prompt the “epithelial-mesenchymal transition (EMT)” that is needed for the metastasis of tumors (Thompson and Haviv, 2011). These growth stimulators include IGF-1, leptin, IL-6, and adiponectin (Figure 5).
Dr. Hursting’s lab has also found that the tumors of diet-induced obese
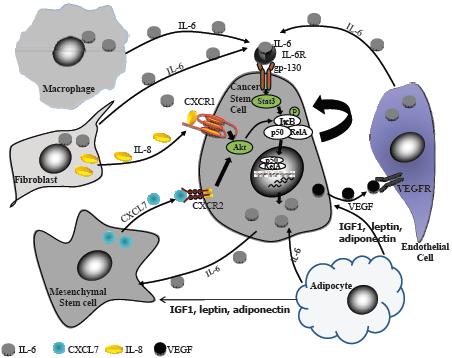
NOTE: CXCL = chemokine (C-X-C motif) ligand; CXCR = chemokine (C-X-C motif) receptor; gp = glycoprotein; IGF = insulin-like growth factor; IKB = inhibitor of kappa B; IL = interleukin; P = phosphorylated; p50 = 50 kD protein subunit of the NF-kappaB protein complex; RelA = reticuloendotheliosis oncogene homolog A; STAT = signal transducer and activator of transcription; VEGF = vascular epithelial growth factor; VEGFR = vascular epithelial growth factor receptor.
SOURCE: Kakarala presentation (October 31, 2011). Reprinted with permission from Madhuri Kakarala.
animals are especially enriched with markers for EMT compared to animals on a calorie-restricted diet. He said this suggests that many EMT regulators are under epigenetic control and influenced by obesity.
There was some discussion at the workshop about whether the type of diet, especially the fat and carbohydrate content, was more important for cancer risk than the total number of calories. This is an important issue, Dr. Berger noted, because of the current popularity of the high-fat, low-
carbohydrate diets. Although such diets may be effective at fostering weight loss, they may be carcinogenic if fats play as significant role in promoting tumors in people as it does in mice, he said. “Every obese person is presumably bathed in all these cytokines and hormones, and yet every cell in their bodies is not becoming cancerous. So there is something, in addition to the obesity, and it may well be whether your obesity is driven by carbohydrates or by fat,” Dr. Berger added.
Dr. Hursting said his studies suggest that it is obesity and its associated metabolic changes, and not the dietary factors causing the obesity that are key to providing the mechanistic pathways to cancer (Harvey et al., 2011). But he also noted recent data suggesting that high fructose intake may be driving the development of fatty livers, which might exacerbate the inflammatory and cell signaling effects he and others have linked to cancer risk (Abdelmalek et al., 2010). Dr. LeRoith said studies show that growth factors such as insulin and IGF-1 stimulate the glycolytic pathway that promotes the DNA and lipid synthesis needed for cell proliferation, suggesting that the breakdown of sugar in the diet combined with these growth factors may be what is driving cancer growth. Dr. Demark-Wahnefried also stressed in her presentation that the source of calories can affect cancer progression just as much as the resultant energy balance disruption, pointing to a study by Dr. Jeffrey Meyerhardt, of the Dana-Farber Institute and Harvard Medical School, that showed patients consuming a Western diet were more likely to experience cancer progression compared to those consuming a prudent diet (Meyerhardt et al., 2007). (A Western diet is characterized by a higher intake of red meat, processed meat, sugary desserts, and refined grains. A prudent diet is characterized by higher intakes of fruits, vegetables, and whole grains.)
There also was some debate on whether obesity is the true cause for increased cancer risk, or rather the most common external face (phenotype) of an inner energy imbalance that more directly heightens cancer risk due to altered metabolism and specific cell signaling pathways. In other words, is obesity a symptom of an underlying long-term energy imbalance that heightens cancer risk, or a more direct cause of that elevated risk? “I think it’s both,” said Dr. Berger. “If you use it as a distinct measurement, then it’s a symptom, but if you consider it a dynamic process that involves all these aspects of inflammation and adipokines and cytokines, then it becomes a prime mover.” Dr. DiGiovanni agreed that obesity is both a symptom and a cause of cancer risk.
In contrast, Dr. Dannenberg argued that obesity is a symptom, and
is an insufficient term for the processes that underlie the condition. He stressed that his work showed there are people with elevated BMI measurements who have no inflammation, although most do. “There’s a disconnect between the phenotype that we have labeled ‘obesity’ and the underlying physiology. Similarly, when we use the term ‘inflammation,’ we are using a very crude term for a very complex process.” He pointed out that inflammation, for example, can encompass the actions of T cells, in addition to the macrophages that seem to play an important role in fostering cancer risk. “The pathobiology of obesity is exceedingly complex, and although specific molecules will be relevant and the medicines that target them will be somewhat helpful, the physiology that underlies obesity and its effects on cancer risk is likely to be a constellation of multiple molecules,” Dr. Dannenberg said.
CLINICAL EVIDENCE OF THE OBESITY LINK TO CANCER
Although findings in animals show strong links between cancer and obesity or its subcomponents, such findings may not translate to what occurs in people. “We all know that things we see in mice don’t always translate into humans, so it’s important to look at the clinical data to determine whether we can help differentiate the impact of these various factors on cancer outcome,” stressed Dr. Jennifer Ligibel, assistant professor and attending physician at Dana-Farber Cancer Institute and Harvard School of Medicine. In addition to the general clinical evidence linking obesity to cancer risk, as previously summarized in the introduction, several presenters elaborated on the evidence linking obesity to the risk of incidence, recurrence, progression, and death for three common cancers: breast, prostate, and colon.
As indicated in the overview, the obesity-related risk of developing breast cancer varies by menopausal status. For patients diagnosed with cancer, a BMI in the normal range is associated with better outcomes for both pre- and post-menopausal women (Protani et al., 2010). Dr. Goodwin noted that the average body size and level of physical activity among women diagnosed with breast cancer differ from the general population. Her research has shown that a BMI between 20 and 25 at diagnosis is linked to the best outcome, with the lowest risk of distant recurrence or death (Goodwin et
al., 2012). There is a gradual increase in risk of recurrence or death as BMI increases. There is also an increased risk of recurrence and death for a BMI under 20, which has been confirmed by other studies and is not explained by the undiagnosed presence of metastases, because many of these women develop their recurrences quite late. A recent meta-analysis of several studies of women diagnosed with breast cancer found that obesity is associated with a one-third increased risk for both breast cancer–specific mortality and all-cause mortality (Protani et al., 2010). Another meta-analysis by Dr. Goodwin’s lab found a 30 percent increased risk of breast cancer–specific death in obese or overweight individuals diagnosed with estrogen receptor (ER)-positive breast cancer, with similar, though non-significant, findings in the studies of women diagnosed with ER/progesterone receptor (PR)-negative breast cancer (Decensi et al., 2010). “There was no evidence that the effect of obesity on breast cancer outcomes differed by ER/PR status,” Dr. Goodwin stressed.
Dr. Goodwin and colleagues found that the risk of developing distant metastases 10 years after a breast cancer diagnosis was significantly increased by 46 percent, and the risk of dying as a result of breast cancer after 30 years was significantly increased by 38 percent for patients with a BMI of 30 or more at the time of diagnosis (Ewertz et al., 2011). “BMI effects persist in the long-term,” Dr. Goodwin stressed.
Recent studies suggest there is little evidence of an adverse prognostic effect of weight gain after breast cancer diagnosis. However, in the cohort of women who developed breast cancer within the Nurses’ Health Study, weight gain after diagnosis was found to be associated with an increased risk of recurrence, disease-specific mortality, and overall mortality (Kroenke et al., 2005). “We have to explore associations like this in greater detail to see if there is a subgroup of women that have a detrimental effect of weight gain,” Dr. Goodwin said.
She summed up her talk by stating, “There is an association between obesity and [poorer] breast cancer outcome,” and noted that this link was not due to women who are obese or overweight being diagnosed later with breast cancer than those who are slimmer, because most of the analyses adjusted for stage at presentation.
Studies also show that some of the same factors found to play a role in fostering the link between overweight or obesity and breast cancer in animals might operate in women as well. Several studies show that elevated insulin levels or fasting glucose levels are linked to increased risk of distant
recurrence and death in breast cancer patients and those levels tend to be higher in women with greater BMIs, Dr. Goodwin reported. In addition, studies show that as BMI increases, serum estrogen levels increase, and that increase at higher BMIs is linked to risk of breast cancer recurrence in postmenopausal women, Drs. Goodwin and Brodie both noted. Dr. Ligibel also said one study found that adiponectin levels were linked to breast cancer outcomes, and another study linked levels of C-reactive protein (CRP), a biomarker for inflammation, with breast cancer mortality (Duggan et al., 2011; Pierce et al., 2009).
Dr. Elizabeth Platz, codirector of the Cancer Prevention & Control Program of the Sidney Kimmel Comprehensive Cancer Center and professor of epidemiology in the Johns Hopkins Bloomberg School of Public Health, reiterated the findings on prostate cancer described in the overview, highlighting the results from the NIH-AARP Diet and Health Study, which found that BMI was positively associated with the risk of aggressive disease, while inversely associated with incidence of prostate cancer in general (Wright et al., 2007). “The patterns became more consistent once [investigators] separated more aggressive fatal disease from disease that is more indolent,” she said. She noted that the timing of body fatness might be critical in determining prostate cancer risk. Increased risk of aggressive, deadly prostate cancer may be linked to obesity that occurs before diagnosis when the tumor first develops or after diagnosis and removal of the prostate, when any remaining tumor cells at metastatic sites, such as the bone, can grow.
Dr. Platz said there is emerging support that the extent of body fatness and weight gain before or around the time of diagnosis of prostate cancer can increase the risk of recurrence and death in men with prostate cancer (Cao and Ma, 2011). She reported on one study in which obesity was found to be associated with adverse pathological features and a greater risk of biochemical progression (Freedland et al., 2005; Ma et al., 2008). Another study linked obesity pre-diagnosis to increased risk of death from prostate cancer (Ma et al., 2008). One study also found that men who gain weight in the span from 5 years before to 1 year after prostatectomy have a greater risk of recurrence of their prostate cancer (Joshu et al., 2011). “Evidence is building that obesity and weight gain are risk factors for poor outcome in men diagnosed with prostate cancer, but there are
many knowledge gaps that we need to fill and methodological issues that need to be addressed,” Dr. Platz summed up, adding, “The bottom line is that obesity and weight gain may be something men can do something about to change their risk of poor outcome after the diagnosis of prostate cancer.”
Dr. Meyerhardt stressed that there are consistent data showing a strong association between being overweight or obese and the risk of developing colorectal cancer (World Cancer Reasearch Fund and American Institute for Cancer Research, 2007). There is also more recent evidence that obesity is linked to the risk of cancer progression. The large randomized colon cancer studies conducted by the National Surgical Adjuvant Breast and Bowel Project found that the people with Stage II or III colon cancer who fared the worst, in terms of disease-free and overall survival, were those who were very obese (BMI greater than 36 kg/m2) and the people who were underweight (but increased risk of mortality for underweight patients was dominated by non–colon cancer deaths) (Dignam et al., 2006).
Dr. Meyerhardt also noted a study suggesting that mechanistic factors implicated in animal studies linking obesity to cancer also appear to be operative in people with colorectal cancer. He analyzed data from the Nurses’ Health Study and Health Professionals Follow-up Study and found that those with the greatest circulating levels of C-peptide, a marker of insulin secretion, and lowest levels of IGF binding protein 1 (a carrier protein for IGFs) prior to colon cancer diagnosis had the worst overall mortality (Wolpin et al., 2009).
When conducting clinical studies assessing the link between obesity and cancer and possible interventions to weaken that link, it can be difficult to parse the effects of obesity and other tightly interconnected factors such as energy balance, physical activity, diet, and timing of weight gain or loss. Consequently, researchers have to design studies carefully to avoid confounders, bias, and other factors that might limit the strength of the evidence and accurate interpretation of findings.
Randomized clinical trials, in which each subject is randomly assigned to a treatment group or a control group before the start of the treatment,
represent the “gold standard” for testing interventions, but such studies are often quite expensive and complex. Moreover, it is often not feasible or ethical to assign participants to study groups of interest, such as randomizing patients to eat a high fat diet. Most clinical studies to examine the impact of obesity and related factors have relied on observational designs, in which investigators collect information about the participants and attempt to draw inferences and identify associations from the data.
As noted by Dr. Cheryl Rock, professor of family and preventative medicine at the University of California, San Diego, “the devil is in the details” when it comes to study design and interpretation. Several speakers at the workshop pointed out limitations of clinical studies, including insufficient, inappropriate, or inaccurate study measures; confounders due to how obesity affects treatment and diagnosis of cancer; and accrual, selection, detection, recall, and “healthy person” biases.
Drs. Gapstur and Platz noted that often a single BMI measure used in studies does not reflect the weight gained throughout adulthood. “A single measure of BMI may not provide a complete picture,” Dr. Gapstur said. This is especially true, Dr. Platz added, if the cancer risk linked to obesity differs before or after diagnosis because of differential effects on causing cancer or causing an aggressive cancer subtype before diagnosis versus causing cancer progression after diagnosis. In addition, the effects of weight gain or loss or diet on cancer progression in individuals who are lean when first diagnosed with cancer may differ from that of someone obese at diagnosis. “We want to make sure we evaluate obesity across the entire life course, if we can, so big prospective studies are going to be required for this,” Dr. Platz said. Knowing someone’s dietary patterns both before and after diagnosis is also needed to assess the impact of diet on cancer progression or likelihood of survival, Dr. Rock pointed out. “It’s important to look at your total lifetime dietary exposure,” she stressed.
Another frequent issue that is especially problematic when doing cross-study comparisons is inconsistent or insufficient ways of measuring factors, such as IGF-1, insulin, leptin, or adiponectin levels. Studies typically measure overnight fasting levels of these compounds. But if someone has metabolic syndrome, Dr. Berger pointed out, levels of some of these molecules will return back to normal after an overnight fast, although they may be abnormally high or low during the day. “Sometimes you need to do an
insulin clamp or a glucose clamp2 to really find out what’s happening with someone’s insulin,” Dr. Berger said. He added the importance of measuring phosphorylation of various receptors and other assays that can indicate the activation of PI3K/mTOR downstream from hormonal pathway triggers, such as IGF-1, insulin, or leptin.
Dr. Hursting added that the traditional IGF-1 assay in the human is total IGF-1, but measurements of bioavailable IGF-1 are really needed. He pointed out that there are also multiple forms of adiponectin that can be measured, and the form most important for human studies on obesity and cancer is not certain. “Going forward to really apply the lessons from animals to the human, we need to come to grips with some of these measurements,” Dr. Hursting said.
Dr. LeRoith recommended measuring total IGF-1 in epidemiology studies because he questioned whether bioavailable or free IGF-1 even exists in blood serum. “When you put free IGF-1 into anybody’s serum, it disappears—it gets degraded. It has to be bound to be protected. So trying to measure free IGF-1 in the serum is totally disconnected from free IGF-1 at the target tissue. Total IGF-1 is the measure we should be using to know what IGF-1 is doing in the circulation that we can extrapolate to the tissue in humans because it is the only one that can be measured appropriately and consistently. All the others are very vague,” Dr. LeRoith said. He added, however, that total IGF-1 measures will vary according to recent dietary intakes. For example, in someone well nourished, IGF-1 levels typically go up, but “You can be obese and drop your intake, and your IGF-1 will drop dramatically. All of that has to be balanced and I don’t think they are in many of these studies,” Dr. LeRoith said.
Another issue that frequently clouds the findings of diet studies in people is that small eaters tend to overreport what they eat, and big eaters tend to underreport what they eat, resulting in what Dr. Demark-Wahnefried called “the flat-slope syndrome.” Dr. Rock agreed, noting that the actual calorie intake of obese individuals can be 30 percent greater than what they typically report. Under- and overreporting of exercise also occurs, Dr. Demark-Wahnefried added. Accelerometers can help in physical activity assessments, but they are not perfect, said Dr. Demark-Wahnefried, who noted they occasionally break. Also, physical activity does not necessarily correlate with physical fitness.
_________________
2 Methods for quantifying insulin secretion and resistance through infusion of insulin or glucose.
In addition, the applicability of study findings frequently is hampered by inadequately representing various races,3 ethnicities, and people of different economic standings in the study population, said Dr. Edward Partridge, president of the American Cancer Society National Board of Directors and director of the University of Alabama at Birmingham Comprehensive Cancer Center. “As we approach this obesity pandemic, we need to think about those populations that are most at risk—[a large] percentage of African American women in the Mississippi Delta are obese or overweight, for example,” he said. “And do disparate populations have bariatric surgery available to them or does it cost too much?” he asked. Dr. Ballard-Barbash agreed, noting that nearly all the U.S. data are from white populations. “This is something we need to be paying attention to, particularly given that we know that lack of insurance, low education, and low income are all associated with less appropriate care in the United States for many types of diseases, not just cancer,” she said. Dr. Demark-Wahnefried added that there can be biological differences among ethnic populations that should be assessed. One analysis of data from the HEAL (Health, Eating, Activity, and Lifestyle) study found that the risk of having tumors greater than 1 cm at diagnosis goes up with rising BMI in whites, whereas it goes down for Hispanics (Baumgartner et al., 2004). “We need to be cautious about making sure that our sample population is at least representative,” Dr. Demark-Wahnefried said.
Several speakers noted that obesity can influence cancer diagnosis and treatment. Obese women tend to be diagnosed with breast cancer at a later stage, pointed out Dr. Goodwin. In addition, the types of cancer treatments used and their effectiveness can be different in obese people versus people of normal weight. Dr. Meyerhardt noted that obese men with colorectal cancer are more likely to have colostomies and a slightly higher risk of recurrence than normal-weight men with the cancer because of the difficulties of surgery in the obese pelvis compared to the non-obese pelvis. Dr. Platz noted that extreme obesity can make complete prostate removal technically difficult so obese men diagnosed with prostate cancer may be less likely to have prostatectomies than men of normal weight. “The lower likelihood of
_________________
3 Note that race is not a biologic categorization. It is a complicated grouping that involves culture, similar environmental influences, and usually a common area of geographic origin.
cure in obese men may not be related to the biology of obesity, but merely to the technical problem of removing the prostate in these men,” Dr. Platz said. “Also, obese and lean men may be presented with and/or select different treatment options, which may have different likelihoods of survival irrespective of extent of or change in extent of body fatness We need to think about alternative explanations for what we are seeing,” Dr. Platz stressed.
Dr. Goodwin pointed out that because of toxicity concerns, capping of chemotherapy for breast cancer was common in the past, and thus obese women often did not receive sufficient doses, given their greater size. This capping reduced treatment efficacy, especially in women with ER-negative breast cancer, according to Drs. Goodwin and Ballard-Barbash. Dr. Goodwin noted that current recommendations state that body size should be used to calculate chemotherapy dose. She stressed that studies done within the past 10 to 15 years took this into account, but older studies did not. Chemotherapy in obese leukemia patients also may be less effective, as recent studies suggest fat cells that comprise a large portion of the bone marrow in obese individuals can enable cancer cells to be resistant to the effects of chemotherapy, Dr. Ballard-Barbash said (Behan et al., 2009).
Dr. Goodwin also noted that there is some emerging evidence that the dose and type of hormonal therapy used in obese women with ER-positive breast cancer might also have to be adjusted according to how adequately the therapy blocks the production of estrogen from excessive fat tissue. “These findings have not led to a practice change yet. But we will be examining our dosing of aromatase inhibitors and our use of aromatase inhibitors in overweight and obese individuals,” Dr. Goodwin said, noting that without adjustment, hormonal therapy may be less effective in obese and overweight women, influencing cancer progression risk independent of the more direct effects of obesity on cancer cells. Dr. Brodie concurred, pointing out a subgroup analysis of the ATAC trial (Arimidex, Tamoxifen, Alone or in Combination) showing that when the dose of the aromatase inhibitor Arimidex (Anastrozole) was adjusted according to BMI, there was no increased risk of breast cancer recurrence in obese women compared to normal weight women (Macedo et al., 2008). “For hormonal agents, such as tamoxifen and aromatase inhibitors, adjustment of the dose to consider body mass index is needed to optimize the effect of the treatment,” Dr. Brodie said.
The hormonal effects of obesity may also lead to detection bias in studies on prostate cancer and obesity, Dr. Platz pointed out. Surveillance for early recurrence of prostate cancer is done by assessing blood levels of
the prostate-specific antigen (PSA). But PSA levels are also related to testosterone levels in men, and such levels tend to be lower in obese men. This could result in obese men being diagnosed with a recurrence of prostate cancer at a later point than non-obese men, Dr. Platz suggested. “Will it be the case that it will look like obese men have a delayed time to detection and therefore we would be underestimating how strongly related obesity is to poor outcome in these men?” she asked. In addition, hormonal therapy in men with metastatic prostate cancers can cause weight gain and associated metabolic perturbations. “How will that affect the likelihood of poor outcome—will this environment increase the risk of their metastases growing more and eventually leading to death?” Dr. Platz asked.
People who are obese also tend to have heart disease, diabetes, and other comorbidities that can affect their ability to tolerate cancer treatments and can adversely affect survival. These factors should be considered when assessing the effects of obesity on cancer progression and survival, Dr. Ballard-Barbash pointed out. She cited National Cancer Institute (NCI) studies on patterns of care showing that for many different types of cancers, guideline-based therapy was reduced for patients with one or more comorbidities. Kidney cancer patients were less likely to have surgery to remove the affected kidney, for example, and bladder cancer patients had decreased use of intravesicle chemotherapy.
Other confounders in studies assessing the effects of diet on cancer risk include differences in the baseline diets between the study population and the controls or the general population. As Dr. Ligibel pointed out, the baseline diet in the WHEL (Women’s Healthy Eating and Living) study was seven servings daily of fruits and vegetables, which was greater than what was seen in the average American diet at that time. “It could be that there is a threshold effect with diet, and in WHEL, the patients were too healthy to see an improvement,” Dr. Ligibel said. In contrast, the WINS (Women’s Intervention Nutrition Study) did not include women who were already eating a lower-fat diet at the outset. That was an exclusion criterion, Dr. Rock said. She also said that an increase in fiber in the diet lowers estrogen levels (Rose et al., 1991), so if a high-fiber and lower-fat diet is found to lower the risk of breast cancer, it may be from the effects of fiber and not necessarily due to the lower fat intake.
Dr. Rock also said that for clinical trials assessing the effects of weight loss, researchers frequently fail to make the distinction between intentional weight loss due to diet and/or exercise versus involuntary weight loss that might be due to an advanced cancer. Dr. Sharon Murphy, Scholar in Resi-
dence at the Institute of Medicine, suggested that another potential confounder could be sleep, which affects leptin levels and eating patterns. Dr. Demark-Wahnefried noted that sleep patterns are known to affect energy intake and might affect energy balance. There are also studies showing that certain sleep patterns are correlated with heightened cancer risk, she added. Dr. Ligibel added that there is increased interest in sleep and its effects on cancer risk, and researchers are increasingly collecting sleep behavior information with the aid of accelerometers and other devices or surveys. Dr. Ballard-Barbash noted that at a recent National Institutes of Health (NIH) symposium on sleep and disease, there was interest in how circadian rhythms, especially those that individual organs appear to have, might influence changes in cell cycling and related factors that may influence cancer risk. Dr. Goodwin found in one of her studies that poor sleep was linked to higher levels of insulin and insulin resistance in breast cancer patients, but it was not independently linked to breast cancer outcomes.
Generalizability of Study Results
It can be especially difficult to assess which interventions are feasibly applicable to the general population based on findings from clinical trials. For example, those patients who opt to participate in clinical research in which they must exercise more or make dietary changes tend to be a highly motivated and energetic group not typical of the bulk of the general population, who are overweight or obese, said Dr. Anne McTiernan, director of the Prevention Center of the Fred Hutchinson Cancer Research Center. “The people that you see in the intervention trials are a very select group of people and eventually we are going to need to come up with ideas of how to disseminate [behavioral interventions to] the general population of cancer survivors,” she said. Patient advocate and cancer survivor Kate Murphy, director of research communication for Fight Colorectal Cancer, underlined this point by noting that “I am much more the person that you need to think about.… I have a very poor body image. I have scars from surgery, lumps from radiation and I wobble around on feet that are not quite recovered from cisplatin.… I sit in meetings all day or I’m sitting at an airport or in my home office. My husband and I are early bird eaters that tend to go out for dinner rather than stretch to prepare our own dinner, and the portions are very large. We have found that fat is delicious. Making a change in diet and making a change in exercise is difficult for us.”
Dr. Demark-Wahnefried concurred that there often is accrual or attrition bias resulting in a study population that is not representative of the population to whom the findings will be applied. “To date, there have been few diet and exercise interventions in cancer populations that characterize those who decide to show up and participate versus those who don’t. We really need that information in order to generalize,” she said.
Those who opt for home-based interventions in clinical studies also tend to differ from those who opt for clinic-based interventions, so there is a high likelihood of selection bias, she added. “When you undertake a clinic-based trial you get the Marines showing up at your door and they are much different than the kinds of people who do home-based interventions, so you have to be cautious in assuming that it is the intervention that’s working and not the patient population,” she said.
Dr. Goodwin also discussed the term “healthy person bias,” meaning that those likely to adhere to a healthy diet are also the same people likely to adhere to an exercise plan and other lifestyle measures. Several speakers pointed out that this behavioral clustering makes it difficult to determine from observational data what factors (such as diet, exercise, or weight loss) are associated with the outcome. “You really have to interpret these findings with a grain of salt. You can’t assume intervening will change [the] outcome in unselected patients—we really need to recognize this,” Dr. Goodwin said.
A survey of breast cancer and prostate cancer survivors found overlap in the proportion of people who consumed low-fat diets and the proportion who exercised (Demark-Wahnefried et al., 2000), Dr. Demark-Wahnefried pointed out. She added that an intervention itself—such as bariatric surgery—can cause patients to exercise more because they feel more fit (Bond et al., 2010). “We have to be able to control for those effects,” she said, also stressing the need to control for body composition, which is affected by cancer treatment. Chemotherapy, for example, can cause women with breast cancer to gain fat mass and lose lean body mass over time, unlike radiation therapy.
Dr. Meyerhardt noted that in observational studies of the effects of physical activity on cancer outcomes, researchers should be concerned about what he called reverse causality. “Is the exposure changing the outcome or is the outcome changing the exposure?” he asked. To avoid reverse causality in his own observational studies, he does not measure outcome events until they occur at least 90 days from first measuring physical activity. In other words, he does not include in his analyses patients who develop a cancer recurrence or patients who die within the first 90 days of measuring
their degree of exercising. In addition, “we start the clock at the time of the questionnaire,” he said.
Detection bias can be particularly problematic in studies of obesity and prostate cancer because men who have more body fat tend to have lower PSA levels compared to men of normal weight. So obese men who develop prostate cancer may have PSA levels that are below the levels at which a biopsy would be recommended and thus might be detected at a later stage of cancer, Dr. Platz pointed out. Men who are heavier also tend to have larger prostates, so the same number of cores taken to biopsy the prostate in a man of normal weight may not adequately detect the cancer in a man who is obese. Both factors combined may explain why obese men tend to be diagnosed with more aggressive prostate cancer—the cancer has progressed to a more aggressive type before it is detected in these men, Dr. Platz suggested.
Clustering of unhealthy behaviors and other conditions also occurs, Dr. Platz pointed out, noting that physical inactivity, diabetes, energy intake, and smoking are factors that are highly correlated with body fatness and could be influencing study findings. If not taken into account, factors like smoking that are inversely correlated with obesity, but positively associated with prostate cancer occurrence, would tend to produce an underestimate of how strongly obesity may be associated with recurrence, she said. Dr. Ballard-Barbash added that in the medical literature on cancer incidence, there are few studies on obesity or BMI adjusted for physical activity.
A lack of consideration of the confounders previously described not only hampers the accuracy of the study outcomes, but also influences the accuracy of meta-analyses done using the study data, said Dr. Martin Murphy, chief executive officer of the CEO Roundtable on Cancer. “Confounding and comorbidity factors play such a vital role in the real interpretation of what’s going on. We could not only pay homage to science, but do a great service going forward if we were to reflect on what the appropriate tools are for doing these very important epidemiological studies,” he said. Dr. Platz agreed, noting that because of the diversity in the designs and populations of epidemiologic studies, it may not be appropriate to combine their results via a meta-analysis to generate a single measure of association. A scientific review that uses a consensus approach on the evidence often may be more appropriate, she said.
Dr. Ballard-Barbash also cautioned about combining datasets that are not similar, such as combining data collected from patients diagnosed with cancer 20 to 30 years ago with data from patients recently diagnosed. The treatments these two groups of patients received are likely to be quite different, she noted, “and there is no way we can control for those differences in treatment if we are talking about a 30-year time window.” Cohorts of different ages also probably should not be combined in meta-analyses, she added.
Several speakers and participants provided suggestions for how to improve the designs and accuracy of clinical studies aimed at assessing the effects of obesity on cancer or lifestyle measures to lessen those effects (Box 3). Given the mounting mechanistic evidence for how obesity increases cancer risk in certain subtypes of people, several experts suggested designing studies to include subset analyses and including large enough numbers of those subtypes so that studies are adequately powered to do subtype analyses. “When we look in a survivorship cohort or in population-based studies, we have to sort out the subtypes. We have to sort out ER-positive and ER-negative breast cancer because they are completely different diseases,” stressed Dr. Jo Anne Zujewski, head of the NCI Breast Cancer Therapeutics Clinical Investigation Branch.
Dr. Rock agreed, adding that women should be stratified according to their estrogen levels or degree of hot flashes. In her subgroup analysis of the WINS data, she found that the diet intervention appeared to be more effective in women who had hot flashes versus those who did not. A lack of funding prevented collecting and analyzing estrogen levels on all women, but women who were not having hot flashes had higher estrogen levels at the beginning of the study, she pointed out. “This suggests there is a subgroup of women who might benefit from this kind of dietary pattern—greater intake of fruits, vegetables, and fiber,” Dr. Rock said.
Dr. McTiernan also stressed basing interventions on proposed mechanisms. For example, she suggested testing interventions that might reduce hyperinsulinemia, inflammation, or sex hormones, or other factors thought to be driving the relationship between obesity and prognosis. She suggested looking at and testing interventions that have been proven to reduce one or more of these factors in people with diabetes or other patient populations. Dr. Berger added that the level of exercise needed to improve quality of life may be different than that required to lower the levels of insulin, leptin, or
BOX 3
Suggestions from Individual Participants on How to Improve Study Designs
• Include a diverse study population.
• Adequately power studies for subgroup analyses.
• Include participants with comorbidities and concurrent medications, with appropriate analyses.
• Stratify women with breast cancer according to estrogen levels.
• Base interventions on proposed biological mechanisms.
• Employ more electronically based interventions.
• Test interventions with documented efficacy in other disease populations, such as those with diabetes.
• Use stratified or individualized exercise programs.
• Include forms of exercise not based in the gym.
• Engage dietitians and other relevant non-medical professionals.
• Facilitate ongoing contact with health care or other professionals to maintain weight loss.
• Include biomarkers for energy balance and undertake correlative science.
• Use a body adiposity index rather than weight measure.
• Use age at diagnosis as a time metric.
• Collect measures of weight, alcohol and tobacco use, and other factors that might influence long-term survival in cancer patients.
• Monitor for possible adverse effects of interventions.
• Screen for cancer treatment toxicity that might make exercise dangerous.
• Focus recruitment primarily on patients who are post cancer treatment.
other key biomarkers. “If you really want to prolong survival, you are going to have to lower some of these cytokines,” he said. Dr. Ligibel suggested that cancer clinical trials include biomarkers for energy balance, such as caloric intake, BMI, and physical activity. “We need more studies showing that there is a direct effect of these energy-balance changes on cancer outcomes in people, as well as in mice. As we design these trials, we need to include correlative science so we learn more about these pathways to determine how these relationships are mediated,” she said.
Dr. Robin Blackstone, president of the American Society for Metabolic and Bariatric Surgery, suggested using a body adiposity index rather than weight measures when studying obesity and cancer, as it is the fat mass in the body that is likely to be the causative factor. The body adiposity index may be more useful in places in the world where scales are not widely available, and easier to use on severely obese individuals in whom it can be difficult to measure waist circumference. “That’s something people might want to make sure to collect as we go forward so that we can sort out the difference between adiposity and BMI,” she said. Dr. Platz agreed, adding, “We really need to figure out what it is we’re trying to measure and then the right tools to measure it.”
Dr. Patricia Ganz, professor at the University of California, Los Angeles (UCLA), School of Medicine and Public Health, suggested that researchers make an effort to include a diverse group of participants so that different races and ethnic groups are adequately represented. This is especially important, she stressed, given the growing numbers of cancer survivors who are Hispanics, African Americans, and members of other minority populations. “We have very little information on these groups even though they very often do have obesity as a major risk factor,” Dr. Ganz said. Dr. Demark-Wahnefried agreed that more studies need to be done on ethnic minorities because their risk may differ from that of the general population. “We need to make sure our sample population is at least representative,” she said. Dr. Platz noted that using representative populations may not be sufficient to draw conclusions in subgroups of studies. She said that we need to oversample racial/ethnic groups to ensure that we have adequate power to test the effectiveness of the interventions in each racial/ethnic group.
To avoid having a study population that is fitter than the average cancer survivor, several speakers stressed the need to include study participants with comorbidities and on concurrent medications for those conditions. But Dr. Ballard-Barbash said that investigators need to do the appropriate analyses to account for a large degree of concurrent illnesses with cancer. She suggested considering competing risk when analyzing data to sort out the heightened risk of cancer mortality in obese patients from the heightened risk of mortality from the various comorbidities that obese cancer patients, especially those who are older, tend to have. Such an analysis is especially important for studies that include a wide age range of patients, she said. She also suggested using age at diagnosis as a time metric to control for similar age groups with similar comorbidities.
Dr. Ganz commented on the importance of accounting for the effects
of medications that cancer survivors might be taking for other conditions. The diabetes medicine metformin, for example, could help lessen the effects of obesity on cancer risk, but some drugs used to treat other conditions might contribute to heightened cancer risk. For clinical trials enrolling cancer survivors, Dr. Ganz also stressed the importance of collecting the list of medications the participants are taking, as well as measures of weight, alcohol and tobacco use, and other factors that might influence their long-term survival. Although some people balk at the added expense and effort of collecting such information, Dr. Ganz noted that the largest expense involved in a clinical trial is recruiting subjects and obtaining consent. “Once you have that cohort, asking these few additional questions will elucidate so much more.”
In addition to measuring the possible benefits of exercise and diet interventions, Dr. McTiernan suggested monitoring for possible adverse effects. She conducted a study in non-cancer patients that linked weight loss to a decrease in white blood cells, which might be problematic for patients recovering from chemotherapy who typically have depressed immune function (Imayama et al., 2011). She also noted another study showing that breast cancer survivors who lost weight after diagnosis had a greater risk of developing recurrences or dying (Caan et al., 2008). She also stressed the importance of determining long-term effects of interventions, such as weight loss or exercise, in addition to their short-term effects. Dr. Demark-Wahnefried added, “Most of the studies that have been done in diet and in exercise only last 12 to 24 weeks. If we are going to really make an impact on cancer survival or cancer progression, we have to keep people on the diet for a long time.”
Ms. Murphy, who has a genetic disorder (Lynch syndrome) that makes her especially prone to developing cancer, suggested conducting intervention trials in similar high-risk populations. It might be easier to assess the effects of an intervention in such populations, she suggested, because often the effects will be seen sooner than in a general population. Another participant suggested conducting animal studies that explore epigenetic effects, such as the microbiome (aggregate of microorganisms that reside on or within the human body) and its effects on cancer risk. Dr. Hursting noted such studies are important, but difficult to conduct. But he added that the Microbiome Project4 is developing tools to help researchers in this
_________________
4 The NIH Common Fund’s Human Microbiome Project aims to characterize the microbial communities found at several different sites on the human body, including nasal
regard. Given all the limitations of observational trials, several workshop participants stressed the need to study the effects of obesity on cancer risk as well as the potential benefits and harms of various interventions within randomized clinical trials. “An association is not the same as causation, and the way we are going to prove causation is through randomized trials where we change things,” said Dr. Goodwin. “We need to view these lifestyle interventions as we would any other cancer intervention and require the same level of rigor if we want to focus on cancer outcomes.”
Dr. Berger expressed concern about conducting a study of an exercise intervention that might be harmful to volunteers with cardiovascular or other comorbidities. In the study he described, a stress test was conducted on participants within 24 hours of stopping their medications to assess baseline fitness. But many people who wanted to participate in the study were unable to stop their medicines without their blood pressure shooting up to dangerous levels. “Is our study going to wind up just taking people who are very fit and don’t have any cardiac and hypertensive abnormalities?” he asked, adding, “How do you deal with people who have cardiac or hypertensive comorbidities?”
Dr. Kerry Courneya, Canada Research chair in physical activity and cancer at the University of Alberta, responded that the American College of Sports Medicine has recommendations for patient risk and a risk stratification approach that stipulates, for example, that patients at high risk may need medical supervision or electrocardiogram monitoring. “Based on these guidelines, a lot of patients would be at high risk,” he said, for high-intensity exercise. But if participants will be doing a low- to moderate-intensity exercise intervention, most recommendations do not require stress test screening or monitoring while exercising, he added. Dr. Courneya suggested that the best approach is to have stratified or individualized exercise programs. “We have an individualized exercise program that starts patients off where they are at, and they progress to more exercise over time. Some may never progress to the higher levels, but they progress enough to show benefits,” he said.
Dr. Rock noted that because of safety concerns, most exercise inter-
passages, oral cavities, skin, gastrointestinal tract, and urogenital tract, and to analyze the role of these microbes in human health and disease.
vention studies in the obese exclude participants with BMIs greater than 40, which limits the applicability of the findings to people in that weight category. “There is increasing evidence now that we have been overly conservative in having that cutpoint at 40. In most of our studies now, we include people with a BMI up to 45,” she said. Dr. Goodwin added that she conducted a weight loss study that excluded participants with BMIs greater than 40 because of the supposition that the intervention would not be sufficient for women with higher BMIs, who should be referred to more focused interventions that might include bariatric surgery. But Dr. Thomas Wadden, professor of psychology and director of the Center for Weight and Eating Disorders at the University of Pennsylvania Perlman School of Medicine, countered that although researchers often have the concern that lifestyle interventions are not going to be useful for people with BMIs over 40, when these individuals are included in studies and subanalyses are done, they discover that people with BMIs of 40 or greater respond as well as people with BMIs of 30 to 35.
Workshop participants made several other suggestions related to studying the effects of exercise. Dr. Demark-Wahnefried suggested exercise interventions include other forms of physical activity besides traditional gym-based activities. “We have to be more creative, particularly since not all cancer survivors are going to be living near a gym. We really have to offer interventions in different flavors,” she said, adding that in one of her studies, gardening was the physical activity intervention. She found that this intervention improved participant’s physical performance measures. Dr. Wadden added that if the goal of physical activity is to lose weight, it does not have to be intense aerobic activity, but anything that burns extra calories. This includes lifestyle measures such as parking further away from a store or not taking an elevator, so the participant walks more steps. “Lifestyle activities seem to be as beneficial for weight loss as more intensive planned and structured activity. Since the patients that we see hate to exercise, we just give them pedometers and try to get them to take 5,000 to 10,000 steps a day,” he said. Dr. Ligibel noted that there are accelerometers that patients can wear all the time to collect more accurate information on their physical activity and sleep habits.
Dr. McTiernan added that many older people do not feel comfortable increasing their heart rate with vigorous activity. “If you are constantly pushing participants to get their heart rate up, they might get really fed up and not want to come back,” she said and pointed out that if patients are taking beta-blockers, they will be unable to have their heart rate increase
into the range required for some studies. But she added that if people on cardiovascular medications are excluded from participating in such studies, “then you will lose half your population. I would definitely include them and have them on their medications in the morning. If you are going to include diabetics in your sample, then I would have their doctor recommend what to take that morning because if they take their diabetes medication, then food is an issue—that is the only one I would be a little bit more careful about,” Dr. McTiernan said. She also suggested screening for cancer treatment toxicity that might make exercise dangerous for some participants. Some chemotherapies, for example, can damage the heart in ways that might make exercise interventions more dangerous.
POTENTIAL TREATMENTS TO COUNTER EFFECTS OF OBESITY
Many workshop participants suggested potential interventions to counter the effects of obesity on cancer risk and recurrence, and reported on studies that had tested some of those interventions. These potential interventions included lifestyle or behavioral changes (Box 4) such as diet modifications or exercise, bariatric surgery, or drug therapy such as the diabetes drug metformin and other drugs that target the factors thought to play a role in the cancer risk–increasing mechanisms of obesity.
Clinical studies in cancer patients have not yet firmly established if or how much weight loss is necessary to reduce cancer risk. However, studies in other patient populations suggest just a 10 percent weight loss, regardless of starting weight, is enough to generate health benefits, including lower insulin levels and fewer diabetes complications, Dr. Wadden reported. Larger weight losses generate greater benefit, he added. “If you can get a larger weight loss of 15 percent or greater, you will have even greater improvements in your comorbid conditions,” he said. However, these larger weight losses are more difficult to achieve with lifestyle modification and drug therapy, and they are also more difficult to sustain over time, according to Dr. Wadden. “That’s how 10 percent has gotten to be the golden rule,” he said. He added that one large study found it is better to lose weight and regain it than to never lose weight, at least with regard to lowering the risk of developing type 2 diabetes (Knowler et al., 2002).
BOX 4
Lifestyle Modifications to Reduce Weight
As reported by Dr. Thomas Wadden, the most basic components of lifestyle measures to foster weight loss are to:
• Set realistic goals for weight loss and behavior change;
• Reduce energy intake by 500-1,000 kcal/day (by reducing portion size, fat, and sugar) while increasing intake of fruits and vegetables to induce a weight loss of 1 or 2 pounds per week;
• Exercise—brisk walking or various types of aerobic exercise and resistance training—for more than 150 min/week; and
• Record food intake, physical activity, and weight, and receive feedback on weight loss progress.
A diet plan in which meals and snacks are consumed at regular, established intervals is generally recommended. “So many of our patients come in as grazers. We just want to get them on a structured meal plan of having a breakfast, lunch, and dinner with a couple of planned snacks,” Dr. Wadden said. He added that the calorie limit for women should be between 1,200-1,500 kcal/day and for men 1,500-1,800 kcal/day. Ideally, the diet should consist of 12-15 percent protein and should have a fat content equal to or less than 30 percent, including saturated fat that comprises less than 10 percent of calories. But as long as caloric restriction is maintained at a constant level, the amount of fat versus carbohydrate versus protein does not affect weight loss, studies show (Foster et al., 2010; Sacks et al., 2009).
Dr. Cheryl Rock agreed that it is the total amount of calories consumed that is critical, but added that emerging evidence shows that people who are more insulin resistant have better metabolic responses to eating less carbohydrates—on the lower end of the 45 to 60 percent of calories from carbohydrates that the Institute of Medicine recommends in its dietary guidelines (IOM, 2005).
Dr. Wadden pointed out that the U.S. Preventive Services Task Force recommends that clinicians screen all patients for obesity, and then offer obese patients intensive lifestyle counseling (at least one visit or session per month) and behavioral interventions to promote sustained weight loss (USPSTF, 2003). “There’s a fair likelihood that you will have a beneficial outcome if you implement this,” he said.
NIH guidelines recommend lifestyle measures, such as dieting, physical exercise, or behavior modification therapy, for all overweight individuals who wish to lose weight. Those who cannot lose 10 percent of their weight with these efforts are eligible for pharmacotherapy if they have a BMI of 30 or higher or a BMI of 27 or higher with comorbidities (NIH, 2000). Those who cannot lose weight with a combination of lifestyle measures and pharmacotherapy are eligible for bariatric surgery with a BMI of 40 or higher, or a BMI of 35 or higher with comorbidities. The Food and Drug Administration (FDA) recently approved the lap band for individuals with a BMI of 30 and the presence of type 2 diabetes so “surgery is going down to lower BMI levels,” Dr. Wadden noted.
No highly effective drugs are currently approved for weight loss, Dr. Wadden pointed out. The weight loss drug sibutramine was taken off the market in 2010 because it was shown to increase cardiovascular events in patients with a pre-existing history of cardiovascular disease. The only other drug approved for long-term use by the FDA is Orlistat, a lipase inhibitor that blocks the absorption of about one-third of the calories consumed from fat. This drug produces a minor weight loss (3 to 4 kg above placebo) and has unpleasant side effects, including oily stools and diarrhea, Dr. Wadden reported.
Dr. McTiernan noted that in non-cancer populations, weight loss is minimal with exercise alone and no caloric restriction. Studies indicate that one can attain only about a 2- to 3-kg weight loss over a year with just exercise, and similar findings have been seen in cancer patients and survivors, she said.
The large, randomized Women’s Intervention Nutrition Study found that postmenopausal women with early-stage breast cancer who were assigned to a low-fat diet had a significantly reduced risk of relapse compared to control women who did not change their diets. The greatest increase in relapse-free survival occurred in women with ER-negative breast tumors, especially in those women with the highest BMI, Dr. Goodwin reported.
However, a similarly large and randomized study of both pre- and postmenopausal women diagnosed with breast cancer, the WHEL study, found that when women both reduced the amount of fat in their diets as well as increased their fruit, vegetable, and fiber intakes, their disease-free survival or overall survival was no different than controls. Dr. Goodwin said the different findings in the WHEL study and WINS may be because the women in the WHEL study did not lose weight, unlike the women on the diet intervention in the WINS, who lost an average of 2.5 kg compared to
control women. Dr. Ligibel added that the dietary fat was not lowered as much in the WHEL study as in the WINS, which required that patients have a certain amount of fat in their diet to be eligible, unlike WHEL.
“It could be there is a threshold effect with diet and in WHEL the patients were too healthy to see this improvement,” Dr. Ligibel said. She added, “What is really important about WINS is that it suggests that a weight loss or dietary change after diagnosis could influence the risk of recurrence.” Most other studies assessed how weight at diagnosis affected outcomes. “It’s important to know that it’s not too late to intervene, and we can approach people after diagnosis, change these behaviors, and potentially improve their cancer outcomes,” Dr. Ligibel said.
Data from intentional weight loss studies, mostly due to bariatric surgery, showed decreases in some of the markers thought to mediate the link between obesity and breast cancer outcomes. These markers included insulin, IL-6, estradiol, and IGF-1 levels. “There is some biologic basis for a beneficial effect of weight loss in breast cancer,” Dr. Goodwin stressed. She also pointed out that bariatric surgery can cause weight loss that is three times what can be typically achieved due to lifestyle changes. “We need to have an open mind when it comes to bariatric surgery,” she said. “Many of our markedly obese women may require a much more aggressive approach to weight loss than lifestyle interventions. We have to carefully consider the pros and cons of bariatric surgery in cancer survivors.”
Diet also appears to affect colon cancer outcomes. Colon cancer patients who tended to eat more meats, processed meats and grains, and sugary foods had significantly worse disease-free survival compared to those who ate less of these foods, a study by Dr. Meyerhardt showed (Meyerhardt et al., 2007).
As detailed by Dr. Courneya, exercise interventions used in clinical trials may be supervised or unsupervised. Most exercise research typically involves three supervised exercise sessions per week. Unsupervised interventions use printed materials and/or telephone counseling to encourage people to exercise more. Although supervised interventions tend to be more effective, they are expensive to conduct and have limited reach into the population of interest, Dr. Courneya said.
However, Dr. Courneya said it is more feasible to conduct a large study using a hybrid approach than to conduct traditional supervised trials, not-
ing that the Diabetes Prevention Program (DPP) and the Look AHEAD (Action for HEAlth in Diabetes) trials employed effective hybrids that involved both supervised and unsupervised sessions. The key components of the DPP model trial included face-to-face sessions with qualified staff that incorporated some supervised physical activity. Participants were given clear and challenging physical activity goals that were tailored to their needs with a “toolbox” approach. Researchers gave participants some written materials to support the advice given to them in person. In addition, researchers applied behavioral modification techniques based on a theoretical behavioral model. There was intensive, ongoing contact that could be tapered over time, but as Dr. Courneya noted, “Eliminating contact altogether tends to cause the behavior to revert back to baseline.”
Similar components comprise the innovative Colon Health and Life-Long Exercise Change (CHALLENGE) trial, which will be the first randomized control trial that will have cancer disease outcomes—improved survival and reduced risk of recurrence—as primary endpoints (Courneya et al., 2008). This trial, which is currently accruing patients, is a 3-year multinational exercise intervention study. It would not be feasible to have a supervised intervention for the entire duration of such a long trial, Dr. Courneya noted, so instead it provides a combination of supervised exercise sessions, face-to-face behavioral support sessions, and telephone counseling sessions, which are intensively applied during the first 6 months of the trial and then tapered off to a limited degree for the rest of the trial. By the second year, monthly behavioral support sessions are mandatory, although they can be by telephone or face-to-face, and monthly supervised physical activity sessions are recommended. So far, most participants prefer to have monthly supervised exercise sessions, and monthly face-to-face behavioral support sessions, according to Dr. Courneya (Box 5).
Participants are given toolbox money ($400) to buy running shoes or pedometers, memberships at fitness centers, or anything else that will facilitate their adherence to the exercise intervention. All the centers participating in the trial were also asked to provide free or low-cost access to a fitness facility for the volunteers. “This is especially important during the winter months. Most people are willing and able to exercise outside during the summer, but once the winter comes, if they don’t have access to a fitness facility, you are going to struggle in delivering the intervention,” Dr. Courneya said. Researchers also give participants a guidebook that covers topics such as goal setting and planning, pedometers, barriers to physical activity, assessing exercise opportunities in the neighborhood, social sup-
BOX 5
Colon Health and Life-Long Exercise Change (CHALLENGE) Trial
• Randomized controlled trial comparing 3-year physical activity intervention to general health education on disease-free survival in colon cancer survivors, enrolled 2-6 months after treatment.
• First exercise trial with disease outcome as the primary endpoint.
• Multinational trial with 962 planned participants.
• Trial opened to accrual in May 2009.
SOURCE: Courneya presentation (November 1, 2011).
port, having fun with physical activity, stimulus control, self-monitoring, and time management. Behavioral sessions are based on this guidebook, and supportive spouses or other support people are asked to come in for a session with the trial participant. Dr. Courneya stressed that making sure participants are enjoying their physical activity is particularly important. “We know from the data looking at the determinants of exercise that the perceived enjoyment of the activity is the strongest correlate of whether or not they are going to continue,” he said, adding that he has done some preliminary research about what makes exercise fun for patients and has applied his findings in the CHALLENGE guidebook and behavioral session designs.
Several observational studies have shown the benefits of exercise in reducing colorectal cancer risk, consistent with experimental results in an animal model of colon cancer in which exercise, especially voluntary exercise, reduced polyps by 25 percent, Dr. Hursting reported (Colbert et al., 2003, 2006; Mai et al., 2003). These clinical studies showed that those individuals who exercised the most at or before diagnosis had the best disease-free and overall survival rates, Drs. Meyerhardt and Ligibel reported (Haydon et al., 2006; Meyerhardt et al., 2006, 2010; Saltz et al., 2007). However, these studies were not designed to assess the effects of exercise as an intervention.
No randomized studies have explored the effects of physical exercise on outcomes for patients with breast cancer, but several observational studies
have found that women who are the most physically active pre- or postdiagnosis have the best breast cancer outcomes (Chen et al., 2011; Holick et al., 2008; Holmes et al., 2005; Irwin et al., 2008). But one study found that this effect is present only in individuals with a BMI above 25 at the time of diagnosis (Abrahamson et al., 2006). Dr. Ligibel noted that most studies find only moderate exercise (at least 3 hours a week) is needed to substantially reduce risk of breast cancer recurrence or breast cancer–related death by as much as 50 percent (Table 1).
Dr. Courneya said that systematic reviews and meta analyses indicate that exercise in cancer survivors is effective for improving health-related fitness, cardiorespiratory fitness, muscular strength and body composition and for some patient-reported measures, such as quality of life and fatigue (Brown et al., 2011; Craft et al., 2012; Ferrer et al., 2011; Speck et al., 2010). Most exercise effects are small to moderate, he added, with
TABLE 1 Observational Evidence Suggests a Link Between Physical Activity and Breast Cancer Prognosis
| Study | N | Patient Population | Timing of Exercise | Results |
| NHS | 2.987 | Pre- and postdiagnosis | 2+ years postdiagnosis | Breast cancer death RR=0.50 (95% CI, 0.31-0.32) |
| CWLS | 4.482 | Pre- and postdiagnosis | 5+ years postdiagnosis | Breast cancer death: RR=0.61 (95% CI, 0.36-1.05) Death from any cause: RR=0.53 (95% CI, 0.40-0.71) |
| HEAL | 933 | Pre- and postdiagnosis | Pre- and postdiagnosis | Death from any cause: RR=0.33 (95% CI, 0.15-0.73) |
| Shanghai | 4.626 | Pre- and postdiagnosis | Postdiagnosis | Breast cancer recurrence and/or death RR=0.59 (95% CI, 0.45-0.76) Death from any cause: RR=0.65 (95% CI, 0.51-0.84) |
NOTE: CWLS = Collaborative Women’s Longevity Study; HEAL = Health, Eating, Activity, and Lifestyle; NHS = Nurses’ Health Study.
SOURCES: Adapted from Ligibel presentation (October, 31, 2011) (Chen et al., 2011; Holick et al., 2008; Holmes et al., 2005; Irwin et al., 2008; Ligibel, 2011).
effects tending to be larger and more consistent posttreatment than during treatment. Dr. Courneya added that the effects seem to be largest for supervised, facility-based exercise lasting at least 30 minutes per session, and for moderate- versus light-intensity exercise. Resistance exercise appears particularly helpful, especially for older prostate cancer survivors. Many of these patients are on androgen-deprivation therapy so exercise provides “a very good fit for that intervention and the issues and challenges it creates,” Dr. Courneya said. He concluded that “Exercise is safe and feasible in cancer patients, even during treatment, and does not seem to interfere with treatment completion or treatment response outcomes, or cause adverse events.”
Dr. Ligibel reported on other studies that found exercise was linked to more favorable measures of the molecular factors thought to play a role in increasing cancer risk in overweight or obese individuals. She and another research group found that when overweight women with a history of breast cancer increased their physical activity, they had significantly reduced insulin levels as well as favorable changes in IGF-1 (Irwin et al., 2009, 2011; Ligibel et al., 2008). Larger studies in women have also found that the more physically active they are, the lower their insulin and leptin levels, and the higher their IGF-1 levels, she added (Chlebowski et al., 2004; Irwin et al., 2005). But Dr. Goodwin noted some conflicting evidence as to whether exercise modifies insulin levels in women who have been diagnosed with breast cancer.
Dr. Demark-Wahnefried noted that there are different types of exercise, including aerobic and strength training exercise, as well as different intensities of exercise. She pointed out that the literature shows that different types of exercise affect hormonal factors differently. For example, men who run marathons have lower testosterone levels than men who weight-train. “Can we infer that all exercise is the same if we are looking at prostate cancer, for example?” Dr. Demark-Wahnefried asked.
Exercise Versus or in Combination with Diet and/or Weight Loss
Some debate took place at the workshop on which lifestyle interventions or combinations of lifestyle measures, such as diet, exercise, and weight loss, were most important in lowering cancer risk. Dr. Hursting pointed out that in animal studies, calorie restriction appears to have more powerful effects on hindering cancer risk than exercise. He noted that the two interventions seem to operate via different mechanisms, with calorie restriction having more effects on IGF-Akt/mTOR signaling than exercise.
But those findings may not translate to humans, Dr. Hursting cautioned, because animals forced to exercise are also subjected to greater psychological stress that might be influencing study results. A subanalysis of the WHEL data found that those women who had at least three 30-minute bouts of aerobic exercise per week, in addition to eating at least five or more servings of vegetables and fruits a day, had a lower risk of breast cancer recurrence, unlike those who just adhered to the diet alone without exercising aerobically (Pierce et al., 2007b).
There seemed to be general agreement that what energy balance factor works best at decreasing cancer risk depends on which mechanistic factors one is trying to counter with the intervention. Studies suggest that if the goal is to reduce inflammation, as indicated by levels of CRP, cancer patients need to reduce weight or change their diet, but exercise will have a minimal effect, according to Dr. McTiernan. As much as a 45 percent reduction in CRP levels can be attained by losing weight and exercising, one study found.
Other studies have strived to reduce insulin levels or IGF levels and have had mixed results. One study in postmenopausal women found that a diet resulting in about a 10 percent weight loss was more effective at reducing insulin levels than exercise (Mason et al., 2011). But this study found that changes in diet without any change in weight did not affect fasting insulin levels. However, Dr. Ligibel noted a study she conducted found that a combination of aerobic and strength-training exercise in sedentary, overweight breast cancer survivors decreased insulin levels by 30 percent with no change in weight (Ligibel et al., 2008). Similar findings were reported in another study, which also found that exercise significantly decreased levels of IGF-1 (Irwin et al., 2009, 2011).
If the goal is to reduce levels of serum estradiol or free estradiol, that can be done effectively with a 5 to 10 percent or greater weight loss. The weight loss can be due to diet alone, or due to diet and exercise, according to Dr. McTiernan.
Dr. Ligibel summed up the evidence on diet, exercise, weight changes, and cancer risk by concluding, “There is evidence, at least in some malignancies, that diet, exercise, and weight are related to the risk of cancer recurrence. We really don’t have the randomized data to determine which of these factors are more important. The closest we have to that randomized data are WINS and WHEL, which suggest that weight loss or a low-fat diet may be important in breast cancer survivors.”
Surgery for weight loss involves gastric banding and gastric bypass. Dr. Bruce M. Wolfe, professor of surgery at the Oregon Health & Science University, reported that a long-term Swedish study found that a 15 to 30 percent weight loss due to bariatric surgery can be maintained over a period of many years (Sjostrom et al., 2007). In contrast, lifestyle measures tend to foster only about a 5 percent weight loss at 4 years, the Look AHEAD trial found (Pi-Sunyer et al., 2007). Three studies have found that the weight loss induced by bariatric surgery was linked to a substantial reduction in cancer incidence and mortality, Dr. Wolfe noted (Adams et al., 2007; Christou et al., 2008; Sjostrom et al., 2007). One study found the reduction in cancer mortality was as high as 60 percent over a period of 10 years or more (Adams et al., 2007). The Swedish study found that the effect on reducing the incidence of cancer was almost entirely limited to females, Dr. Wolfe noted. The Utah study found a dramatic difference (0.62 relative risk of distant disease) in the staging of cancer at time of diagnosis in those who had bariatric surgery versus controls. “Weight loss following surgery reduces cancer incidence and mortality,” Dr. Wolfe concluded. “It’s reasonable to hypothesize that the molecular phenomena responsible for these changes also contribute to survival among cancer patients,” he added. Dr. Wadden concurred that bariatric surgery for patients who are extremely obese is the most effective intervention for weight loss and for improving comorbid conditions, including cardiovascular and cancer conditions.
But Dr. Wolfe pointed out that only 1 percent or less of the obese people who are eligible for bariatric surgery undergo the procedure each year. One reason more patients do not undergo this type of surgery may be due to concerns about its risks. Dr. Wolfe said that “the risks of bariatric surgery have been dramatically reduced in the last 5 to 10 years, despite it being conducted in a high-risk population.” An NIH consortium that Dr. Wolfe chaired reported a 0.2 percent mortality for laparoscopic gastric bypass, and a 4 percent complication rate (Flum et al., 2009), which compares favorably to what is seen in other types of surgery, he added, noting that the mortality rate for open-heart surgery is 10 times higher (Ranucci et al., 2011).
Drugs Targeting Cancer-Causing or Cancer-Promoting Obesity Mechanisms
Several participants suggested potential drugs for targeting cancer-causing or cancer-promoting obesity mechanisms and reported on studies
done to date on these drugs, most of which were conducted in animals. These potential drugs that might mitigate cancer risk include the diabetes drug metformin, mTOR inhibitors, anti-inflammatory drugs, compounds that block pathways used by stem cells to regulate proliferation, Akt inhibitors, and endocrine therapies such as aromatase inhibitors and tamoxifen that reduce estrogen or block its effects.
Metformin attracted cancer researchers’ attention when it was found to be associated with a lower risk of breast cancer risk in observational studies of diabetic patients, Dr. Goodwin reported (Bodmer et al., 2010; Bosco et al., 2011; Currie et al., 2009; Decensi et al., 2010; Libby et al., 2009). Studies suggest that in addition to lowering blood sugar and insulin levels, this widely used type II diabetes drug lowers CRP, reduces systemic inflammation, and alters the leptin-adiponectin ratio favorably. Dr. Goodwin said metformin might act systemically by reducing gluconeogenesis in the liver and enhancing skeletal muscle insulin sensitivity, thereby reducing insulin levels and signaling through the PI3K pathway. Metformin may also act through an insulin-independent mechanism directly on the tumor cell (Figure 6).
Dr. Kakarala also cited evidence that metformin inhibits the epithelial to mesenchymal transition required for metastasis (Cufi et al., 2010; Menendez et al., 2011).
A few studies have found that neoadjuvant metformin (given before surgery to remove a breast cancer tumor) is linked to higher pathological complete response rates or lower levels of a marker of cell proliferation (Ki-67) (Decensi et al., 2010; Jiralerspong et al., 2009). Dr. Goodwin is currently testing metformin in an adjuvant setting for breast cancer patients.
Dr. DiGiovanni found metformin effective at reducing tumor development in response to a carcinogen in a skin cancer animal model (Checkley et al., 2011). The reduced tumor promotion was linked to an increase in phosphorylation of AMP kinase and inhibition of the mTORC1 signaling pathway.
Rapamycin and Other mTOR Inhibitors
Rapamycin is a drug known to block mTOR, which is thought to be overactive in obese animals, and Drs. DiGiovanni and Hursting reported on
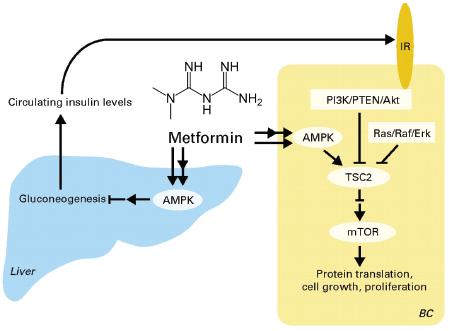
FIGURE 6 Mechanism of metformin action in the clinical setting.
NOTE: AMPK = 5 ’ adenosine monophosphate-activated protein kinase; BC = breast cancer cell; ERK = extracellular-signal-regulated kinase; IR = insulin receptor; mTOR = mammalian target of rapamycin; PI3K = phosphoinositide 3-kinase; PTEN = phosphatase and tensin homolog, RAF = Rapidly Accelerated Fibrosarcoma oncogene; RAS = Rat Sarcoma oncogene; TSC = tuberous sclerosis protein.
SOURCE: Goodwin et al., 2009. Reprinted with permission from Journal of Clinical Oncology.
the findings from their labs and others that suggest low doses of rapamycin are as effective as calorie restriction for lifespan extension in several different strains of mice, and can mitigate the effects of obesity on cancer risk (Checkley et al., 2011; Harrison et al., 2009). Rapamycin combined with calorie restriction provided the most benefits, in terms of preventing cancer development in obese mice, than either intervention alone. “We’re pretty excited about those data showing that we may be able to block the effects of obesity, at least in animal models, using rapamycin,” Dr. DiGiovanni said. His lab is currently studying plant compounds that have mTOR-inhibiting activity, including some that cause dramatic upregulation of AMP kinase. “Because mTOR signaling is downstream from both the IGF-1 and the EGF receptor, I think it is a potential target for offsetting the effects of obesity-related cancer promotion,” Dr. DiGiovanni said. Dr. Hursting added, “The animals are still obese and metabolically still a mess, but [using
rapamycin] we were able to uncouple that obesity from the tumor growth. I find that exciting.”
As Dr. Kakarala elucidated previously, stem cell growth is regulated via key molecular pathways (Figure 7). Research has uncovered several compounds that appear to block NF-kB (nuclear factor kappa-light-chainenhancer of activated B cells), which plays a key role in these pathways. These compounds include curcumin, which is derived from the spice turmeric, Akt inhibitors, and certain anti-inflammatory drugs. Dr. Kakarala is especially interested in pursuing curcumin because in transformed cell line models, it also inhibits other key breast stem cell signaling molecules called Wnt/beta-catenin, notch, tumor necrosis factor alpha, and hedgehog in addition to blocking NF-kB activity (Aggarwal et al., 2003).
But cancer stem cells are able to pump out chemotherapy compounds
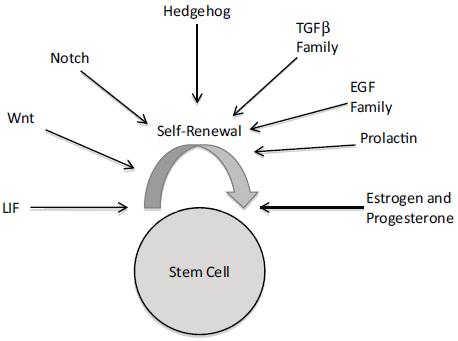
FIGURE 7 Regulation of stem cell self-renewal and clonal expansion.
NOTE: EGF = epidermal growth factor; LIF = leukemia inhibitory factor; TGFβ = transforming growth factor β; Wnt = wingless integration oncogene.
SOURCE: Kakarala presentation (October 31, 2011). Reprinted with permission from Madhuri Kakarala.
and other substrates, including curcumin. To enhance sensitivity to curcumin, Dr. Kakarala combined it with piperine, a derivative from black peppers and hot peppers that blocks the molecular cell pump (p-glycoprotein) cancer stem cells use to pump out curcumin. Piperine also inhibits NF-kB, Dr. Kakarala reported, so it also is likely to decrease cancer risk independent of its p-glycoprotein-blocking abilities (Shoba et al., 1998). Her in vitro study found that the combination of curcumin and piperine caused a decrease in the percentage of ALDH-positive cells (ALDH is a marker for stem cells and early progenitor cells) (Kakarala et al., 2010a).
Piperine and curcumin are relatively nontoxic compounds, Dr. Kakarala noted, and might be acquired from one’s diet. Her studies found that piperine has good bioavailability from the diet, and she continues to explore it and curcumin as anticancer compounds (Kakarala et al., 2010b).
Drugs That Target Estrogen or Its Receptor
Several speakers noted the benefits of tamoxifen and aromatase inhibitors that block the growth-promoting effects of estrogen on breast cancer cells. These drugs have already been shown in randomized clinical trials to extend breast cancer survival and help prevent breast cancer recurrence.
CHALLENGES IN STUDYING OR INDUCING LIFESTYLE CHANGES IN PATIENTS
Many speakers and workshop participants noted obstacles to inducing potential cancer risk-reducing lifestyle changes in patients, as well as ways to overcome those obstacles. The challenges described included the physical limitations or time constraints of cancer survivors, difficulties in adhering to a diet or exercise plan for the long length of the trials, and the costs of frequent sessions with participants.
Physical Limitations or Time Constraints of Cancer Survivors
Dr. McTiernan noted that some cancer patients have physical effects from surgery, such as lymphedema, loss of a limb, or bowel removal, that may make physical activity difficult, and chemotherapy may damage the heart or nerves. In addition, chemotherapy and radiation therapy can cause appetite and taste changes that can make diet changes difficult. The scheduling demands of chemotherapy, radiation, and other cancer therapies can
hamper patients’ enthusiasm about adding an exercise routine or taking the time to make healthful meals. “They spend a tremendous amount of time going to medical appointments and many of them are continuing to work or raise families or have caretaking responsibilities,” Dr. McTiernan said.
Dr. Rock added that many cancer patients have fatigue after treatment, as well as lingering psychosocial and body image issues that can make it hard for them to adopt lifestyle measures aimed at stemming their risk of recurrence or the development of a new cancer. In addition, because many cancer patients are older, they tend to be heavier. “Many of the people coming to a cancer diagnosis are already obese or overweight and if they weren’t sedentary before they got their diagnosis, they are certainly going to be when going through their treatment,” Dr. McTiernan said.
Despite the physical and time challenges that breast cancer survivors face, they can be recruited to exercise and diet intervention trials, and their adherence to the interventions and retention in the trials are adequate, Dr. McTiernan said, although it appears that breast cancer patients are easier to recruit than other cancer patients. She added that postprimary treatment recruitment seems to be easier than recruitment during primary treatment.
Long-Term Adherence to Diet or Exercise Plans
A major challenge is having patients maintain a diet or exercise program for a long period of time, Dr. Demark-Wahnefried noted. Participation generally lasts for 12 to 24 months, and it can be challenging for volunteers to adhere to a diet or exercise plan for that long. But it is feasible, studies show, with the right degree of follow-up, Dr. Demark-Wahnefried stressed. “You need to keep up with the participants and follow them through the phone, the computer, etc.”
Dr. Wadden suggested several measures to improve adherence to weight loss interventions, including portion control, group treatment, and ongoing contact with health care providers or other professionals. He said diets that provide a fixed amount of food with known calorie content are critical because, for example, “If you consume a Slim Fast or Boost shake, you know you are getting 200 to 220 calories and when the can is empty, your lunch is over and it is time to stop eating.” Similar results can be achieved by using frozen food entrees, such as Lean Cuisine, he added. “You also can avoid problem foods in the short term by knowing precisely what you are going to eat, and by having convenient foods to eat. Many dietitians are upset by things like Slim Fast. But I always say, ‘What would you prefer—a breakfast
of Slim Fast or a ham and egg and cheese sandwich and hot chocolate?’” Dr. Wadden cited a study that compared conventional reducing diets of 1,200 to 1,500 calories to those that were portion-controlled meal replacements and found that those on the meal replacements lost significantly more weight in the short and long terms (Heymsfield et al., 2003).
He also stressed the importance of people having ongoing contact with their health care provider once they have lost weight, so they do not gain it back. One study found that a group that had no provider contact after a 5-month period of weekly sessions regained about 40 percent of their weight 1 year later. Another group that continued to meet with their health care provider every other week for a year after the first 5-month period fared better in maintaining weight loss (Perri et al., 1988). He suggested teaching patients to maintain their weight loss by weighing themselves regularly, engaging in high levels of physical activity, and continuing to eat a low-calorie diet. “Physical activity is crucial,” he stressed. “The greater the physical activity the better, and strength training is a very beneficial part of this physical activity.” Dr. Wadden noted studies that found those individuals who exercised 200 or more minutes per week were more likely to maintain their weight loss than those who exercised less than 150 minutes per week (Jakicic et al., 1999). “The recommendation now is to engage in about 200 minutes of exercise per week and 275 minutes or more per week to maintain weight loss,” Dr. Wadden said.
High-intensity interventions, in which patients meet regularly with providers, generate better results (Wadden et al., 2011), he noted, and mentioned that a systematic review by the U.S. Preventive Services Task Force showed that if the intensity of treatment is decreased by dropping down to fewer than 12 sessions over a year, patients only lose 3 percent of their weight at the end of the year (Leblanc et al., 2011). If patients participate in a high-intensity treatment that includes between 12 and 26 sessions, by contrast, they lose twice as much weight during the same 1-year period.
Dr. Wadden noted that the Centers for Medicare & Medicaid Services (CMS) had proposed paying for high-intensity lifestyle counseling for seniors. CMS defines high intensity as weekly sessions the first month, and twice-monthly or every-other-week sessions for the next 4 months. If participants lose 3 kg during that first 6-month period, then they would be allowed to have six monthly sessions after that. “What is striking is that
the interventions that CMS is going to reimburse are done by physicians, nurse practitioners, and physician assistants and not dietitians and related professionals. That means you are going to end up with extremely expensive weight control interventions provided by primary care practitioners who don’t specialize in this area,” Dr. Wadden said.
He suggested that a more efficacious and cost-effective approach to providing high-intensity weight loss treatment for people who are obese would be to refer patients to community programs, such as those offered by the YMCA or by commercial providers, such as Weight Watchers or Jenny Craig. One study found that wellness instructors at the YMCA induced a 6 percent weight loss in participants at 1 year, which is near what the DPP trial was able to achieve. But the YMCA treatment cost $300 per patient, compared to $1,400 per patient treated similarly at academic medical centers (Ackermann et al., 2008).
Research reveals that commercial weight loss programs are also effective. One study found participants in a Weight Watchers program lost more than twice as much weight as those treated in their primary care practice (Jebb et al., 2011). Another study found that participants on a Jenny Craig program that were provided portion-controlled meals and center-based therapy lost more than 7 kg at 2 years, compared to the 2 kg lost by those who received standard dietary advice (Rock et al., 2010). Participants who received their behavioral modification therapy over the phone while receiving the portion-controlled meals lost about 6 kg. “There is loss of very little efficacy by doing telephone-based therapy,” Dr. Wadden stressed. “This has been shown in two or three obesity trials. That is very exciting in terms of extending the reach of treatment.” He added, “Clearly we need an economic analysis of these studies. It is very possible that patients, by eating less food and making healthier food choices, can pay for the cost of these commercial programs just by changing their diet and the cost of the diet they are consuming. So many of my patients when they come in are spending $100 to $200 a week eating at restaurants and eating lots of things they shouldn’t be eating.”
Dr. Wadden also suggested researchers employ more electronically based interventions. “You can deliver Internet-based programs on computer, smartphone, iPad, and you can develop cell phones to provide texting programs. We are seeing a lot of research going into that right now, and clearly the greatest benefit is that people always have a phone in their hand and can use it to check and see how many calories they can eat for dinner. If you don’t know how many calories are in the food, you can take a picture
of the food, transmit the image, and get a read back on it. It is remarkable what these mobile phones will be able to do for us,” he said.
He noted one study that compared a 24-week behavioral program in which everyone got identical behavioral weight loss coaching, but one group of participants received that counseling in person, another group received it via the Internet, and a third hybrid group received a combination of Internet-based and onsite counseling (Harvey-Berino et al., 2010). This study found that onsite treatment led to a greater weight loss than the other two programs, but that the Internet and hybrid programs led to about a 6-kg weight loss within 6 months. All three groups slowly regained some of the lost weight over the following year. “Increasingly we are going to see Internet-based, mobile phone delivery of weight loss, which is going to open up all sorts of parameters for us,” Dr. Wadden said.
Dr. McTiernan noted several studies that found that group plus individual diet and/or exercise interventions had greater effectiveness, in terms of weight loss, in breast cancer survivors than individual interventions alone, and the least effective were home-based interventions (Table 2).
WHAT TO ADVISE CANCER PATIENTS
Several participants pointed out that once people are diagnosed with cancer, especially after they have completed their cancer therapy, they are especially open to practicing interventions that can promote good health and emotional well-being, and possibly prevent a recurrence. “Cancer survivors want to do anything they can to prevent a recurrence or progression of the disease,” stressed Dr. Ganz. This point was brought home by cancer survivor Kate Murphy, who said, “When you reach the end of your treatment, you are just sitting there shivering with fear that the next shoe is going to fall and you are going to be hit with a cancer recurrence.” Dr. Dean Brenner, professor of internal medicine at the University of Michigan Cancer Center, added, “The cancer diagnosis is a teachable moment and the oncologists should and do serve the role of educator in dealing with issues of cancer prevention.” He also noted that family members of cancer patients often ask what they can do, with regard to diet or exercise, to help prevent a recurrence in a loved one.
Dr. Rock noted that studies show breast cancer patients are motivated to make dietary and other lifestyle changes, and counseling on these topics prompts them to make behavioral changes that are well maintained, at least during the first 4 years following their participation in a study. Diana Dyer,
TABLE 2 Weight Loss Interventions in Breast Cancer Survivors
| Study | Type of Intervention | Mean Baseline Body Mass Index | N | %Weighr Lora; 6 Month | %Weighr Lora; 12 Month |
| Djurk | Individual + group | 35 | 48 | 9.8 | |
| Rock (HVM5) | Group + phone | 31 | 85 | 7 (4 mos.) | 8 |
| Rock (SHAPE) | Group | unknown | 259 | 5.5 | 4.5 (18 mos.) |
| Rock (ENERGY, ongoing) | Group + phone/e-mail | unknown | first 103 | 4.3 | |
| Goodwin (LISA) | Individual phone, Diabetes Prevention Program based | 31.3 | 338 | 5.6 | 6.1 |
SOURCE: McTiernan presentation (November 1, 2011).
a cancer survivor, added, “Please think of this as an opportunity to help your patients help themselves and help patients take control of their lives back.” Ms. Murphy agreed, noting, “Weight loss or physical activity programs give you a sense that you have some control, and self-efficacy is tremendously important to cancer survivors. I like counseling programs that emphasize what will make you a stronger person, rather than those that emphasize what you should do, because that can induce a tremendous amount of guilt that is counterproductive. Think about the emotional balance, and not just the energy balance, when you see a cancer survivor.”
But as Ms. Dyer noted, her oncologist had no advice on lifestyle interventions to prevent a cancer recurrence when she asked him. Dr. Brenner concurred that this frequently happens and suggested the American Society of Clinical Oncology (ASCO) provide educational materials to oncologists that will aid them in advising patients on lifestyle changes or other measures they can take to help lower their cancer risk. “Just about every new patient we see asks these questions,” he said. Dr. Ganz added, “We tell patients about their diagnosis and treatment, but we don’t do a very good job of telling them how they can promote health and well-being and prevent recurrence going forward.”
Dr. Ganz pointed out that most cancer patients are older, overweight, and with comorbidities that would benefit from weight loss and/or increased exercise. Consequently, there is a valid need to encourage health promotion in cancer survivors. Such promotion would involve counseling on weight loss if they are overweight, and how to be more physically active if they are too sedentary. “Avoidance of weight gain is very important just as it is in the general population as people age. If one is overweight in the general population, physical activity certainly is not controversial,” Dr. Ganz said, adding, “Shouldn’t we be making the same recommendations to cancer survivors that we would be doing to an older adult facing many of the same comorbid conditions? We cannot promise this is going to extend their survival, but we do know there are general health benefits just from maintaining or losing weight.” Dr. Meyerhardt made a similar comment in his presentation, “Those who survive colorectal cancer will be at risk for other diseases, many of which are associated with some of the factors that impacted their risk of developing colorectal cancer. So a message of being physically active and avoiding obesity over time obviously raises other survivorship issues, in terms of impacting other diseases.”
Drs. Ganz and Rock added another reason to recommend exercise to cancer survivors; physical activity has been shown to be important for main-
taining weight. “Even if you don’t want to talk about weight loss, weight maintenance and not gaining weight is an achievable goal in survivors,” Dr. Ganz said. She also noted that physical training can prevent lymphedema in breast cancer survivors, and she and several other speakers stressed the quality of life benefits of weight loss and/or exercise that several studies on cancer patients have shown. There is also the potential that weight loss and/or physical activity might prevent the occurrence of secondary cancers for which cancer survivors are at increased risk, especially if they have certain hereditary forms of cancers, Dr. Ganz pointed out.
Ms. Murphy said she thought it was sensible to advise cancer patients to either maintain or lose weight, and she was encouraged by the finding that moderate weight loss is effective in lowering a number of health risks, and that exercise can lower fatigue and possibly depression. “That is good information to offer patients,” she said, and pointed out that one way to encourage cancer survivors to exercise more is to encourage them to have a pet that requires daily walks. “My animals encourage me to walk because they want to walk,” she said. Ms. Dyer added, “I am not a gym rat and nothing you could tell me as a cancer survivor would probably get me into a gym. There are other forms of exercise, such as yoga, tai chi, and gardening that can have a spiritual component, let alone the aerobic, the strengthening, and the flexibility aspect for exercise. We need to think outside the box.”
Ms. Dyer also stressed that equally, if not more, important to cancer survivors than extension of life is improvement of quality of life, which exercise and weight loss can foster. “I can stand here and tell you as a long-term cancer survivor that if push came to shove and I had to choose length of life over quality of life, I would choose quality of life, and would appreciate you offering me suggestions for that.”
Ms. Dyer also stressed the importance of dietary advice, preferably from a knowledgeable dietitian. “It is simply not enough to tell our cancer survivors to eat right and exercise, and hand them a pamphlet, and send them out into the nether land. I hope that dietitians will be valued for what they bring, the benefit they can provide to the patients, and that all survivors have access to a survivorship program that looks at their life holistically and considers the nutritional components to make it as good as it can be,” she said. Dr. Rock added that more than just giving general health advice, dietitians are trained in how to use motivational interviewing to foster behavioral changes.
Some debate took place over whether there was enough evidence to recommend that overweight cancer patients reduce their weight, exercise more, and/or take other measures to stem the effects of obesity on their risk of cancer progression or recurrence. Although Dr. Meyerhardt noted that most of the evidence for the impact of obesity on cancer outcomes is observational and there are insufficient data from randomized, controlled trials, Drs. Hursting and Goodwin pointed out the consistency in mechanistic targets indicated by both animal and human studies. “We are beginning to see some congruence between the human studies and the animal studies, and we are starting to hone down on specific mechanisms,” Dr. Goodwin said. Dr. Ligibel added that because of their expense, length, and complexity, randomized trials are unlikely to be completed soon.
Drs. Zujewski and McTiernan stressed the the need for more age-, high-risk-, comorbidity- or race-specific information on obesity and cancer, information on alternative weight loss diets and which diets are most appropriate for the molecular changes one wishes to induce, the timing of the weight loss relative to diagnosis and treatment, and the effects of weight loss medications in cancer populations. Dr. McTiernan also said it is unknown whether cancer patients will get their needs met in non-cancer settings, such as Weight Watchers or general exercise programs. In addition, she noted not enough is known about combination therapy to counter obesity effects, such as weight loss combined with metformin or hormonal therapy, nor is enough known about the additive effects of physical activity in addition to losing weight.
But Dr. Kathryn Schmitz, senior scholar and epidemiologist at the University of Pennsylvania Perelman School of Medicine, noted that there have been decades of advice on guidelines for exercise as well as prescriptions and dietary interventions that could apply to cancer patients and should be worked into cancer survivorship care plans. Dr. Partridge added, “We have enough information now [supporting the role of] obesity, unhealthy eating, and lack of physical activity [in] cancer and cancer recurrence in survivors—the epidemiological evidence is there and there are a lot of molecular mechanisms that explain the epidemiology. We need to continue to tease those out. But it is time now to figure out what we can do to change the tsunami. We have to stabilize and reverse it.” Tom Kean, CEO of C-Change, agreed, adding “There is certainly a lot still to be learned. But the time to act is now because we know enough to say something very definitive about the issue
that we have in hand.” Dr. Ganz pointed out that many cancer patients are already primed to receive health and recovery advice that is not certain, but is supported by a large body of evidence and data in the general population.
Research and Clinical Tools—Needs and Opportunities
Several workshop participants suggested tools for use in research and clinical care that could enhance our understanding of the connection between obesity and cancer. Some of the tools are on researchers’ “wish lists” but do not actually exist or are not readily available at this point. These tools include obese rodents that are immunosuppressed so they will not reject transplanted human cancer stem cells. Dr. Kakarala noted that such animals would be useful to study how cancer stem cells act differently in obese versus non-obese rodents. Alternatively, researchers could develop better markers for rodent stem cells so they could be injected into obese mice, she added. “This would help us elucidate the links between obesity and stem cell biology,” she said.
Dr. Kakarala also noted that because most women who elect for breast reduction surgery are overweight or obese, the tissue removed from that surgery would be useful to study whether, for example, it is in a more inflamed state than normal tissue. She also stressed the need for large quantities of fresh or frozen tissue to assess dynamic molecular endpoints. Such tissue might be acquired from mastectomies, although that would not be feasible for clinical trials. Dr. Kakarala currently is using tissue removed from core breast biopsies. “This represents a leap forward in our ability to look at the [molecular biology effects of ] clinical trial interventions,” she said. She also suggested researchers could use fixed tissue to do DNA profiling for stem cell–specific genes, such as Oct4, Sox, and Nanog, or to explore epigenetics. In situ imaging is another new research tool that enables multiplex imaging of up to 20 different markers in a single cell, Dr. Kakarala pointed out.
Dr. Ballard-Barbash noted the current availability of innovative accelerometers that not only are water resistant, so they can be worn while swimming, but also can collect acceleration data through three vectors, as opposed to just one. The NIH is currently working with software developers to translate that extensive data so researchers can assess what individuals are doing simply by assessing how they are moving through space. “That’s sort of a frontier area, where we may not need to rely just on self-report for some of that information,” she said.
A few participants also noted new health monitoring apps and hand-
held devices that can monitor and boost adherence to an exercise or diet program. Dr. Demark-Wahnefried noted that researchers who are pioneering the study of this technology’s impact on cancer survivors are Deborah Mayer at the University of North Carolina, David Gustafson at the University of Wisconsin, and Karen Basen-Engquist at the MD Anderson Cancer Center. Dr. Wadden agreed that electronic weight loss aids and monitors “are just beginning to take off in the past 5 years.” But Dr. Schmitz noted that a lack of reliable Internet access can impede the use of these devices, especially in the typically older population who have cancer, and who tend not to gravitate toward such new technologies.
To assess dietary habits and behaviors as well as physical activity, Dr. Ganz recommended the REAP (Rapid Eating and Activity Assessment for Patients) questionnaire (Gans et al., 2003), which was designed for primary care physicians and nurses to use in the clinic. Just two pages long, the REAP questionnaire can be filled out within a single clinical visit.
Workshop participants made numerous policy suggestions related to research, education, and dissemination, and directed at both private and public sectors (Box 6).
Dr. Linda Nebeling, chief of the Health Promotion Research Branch at NCI, suggested more policy-specific research that addresses diet, physical activity, and other energy balance behaviors, and how such behaviors can be influenced by manipulating the environment to support lifestyles less likely to lead to obesity. She also suggested supporting more comprehensive behavioral and health outcomes research that has greater integration of biomarkers to reveal the effectiveness of certain interventions. She noted that if research can document the benefits of structural changes to clinical or prevention modes of practice, that will aid efforts to have congressionally mandated financial support for those types of changes. She also suggested strengthening resources and infrastructures that enable researchers from diverse fields to work together. “We continue to work with the research community to expand the comprehensiveness of common measures across datasets that can be used and shared by agencies, researchers,” Dr. Nebeling said. She also encouraged transdisciplinary experimentation (Box 7).
BOX 6
Potential Policy Actions Suggested by Individual Workshop Participants
• Consider the influence of the built environment on behavior and lifestyle.
• Support more comprehensive behavioral and health outcomes research.
• Enable collaborations by teams of investigators from diverse fields.
• Enable more partnerships between the National Cancer Institute and other parts of the National Institutes of Health.
• Collect more measures of energy balance in clinical trials.
• Collect, store, and share more blood and tissue samples from clinical studies.
• Examine the potential role of obesity as a barrier to cancer screening.
• Engage the private sector in measures to counter obesity.
• Develop cancer rehabilitation programs to help cancer survivors stay physically active.
• Encourage health insurers to cover weight loss programs and cancer rehabilitation.
• Educate clinical oncologists and primary care providers about lifestyle interventions.
• Develop guidelines for recommending lifestyle interventions to patients.
• Improve coordination of patient care after cancer treatment.
Dr. Brenner stressed that in the current era of budget cuts, there is greater need to advocate for NIH/NCI funding so more funds can be devoted to the long-term, expensive clinical trials of obesity interventions and their effect on cancer risk. “We need to exercise all the opportunities we have with regards to policy and the importance of funding for our National Cancer Institute,” he said.
Many workshop participants called for having researchers routinely collect more energy balance measures, such as BMI, height, weight, waist circumference, or physical activity, when studying the link between cancer and other diseases, such as diabetes. Some participants suggested such data be collected for all NCI Cooperative Group clinical trials. Dr. Nebeling
BOX 7
Transdisciplinary Research on Energetics and Cancer (TREC)
The National Cancer Institute (NCI) established the TREC initiative in 2005 in response to growing public health concern with overweight and obesity in the United States and mounting evidence that obesity plays a role in the development of many types of cancer (TREC, 2012). The initiative supports interdisciplinary research aimed at discerning the complex relationships among obesity, energy balance, nutrition, physical activity, and cancer. The TREC initiative also helps train new and established scientists to carry out this kind of integrated research. In 2005, the NCI funded the first four TREC Research Centers and one Coordination Center. The centers included scientists from multiple disciplines and encompassed projects spanning the basic biology and genetics of behavioral, sociocultural, and environmental influences on nutrition, physical activity, weight, energy balance, energetics, and cancer risk. The Coordination Center facilitated interactions across and between the Research Centers and the NCI. In the first 5 years of funding, 14 research projects and several developmental pilot projects were supported, and a transdisciplinary infrastructure to promote research collaborations in the area of energy balance and cancer was established.
In 2011, the NCI announced the four newly awarded TREC Research Centers and continuation of the Coordination Center. As in the first 5 years of funding, the reissued TREC initiative continues to emphasize collaboration across diverse disciplines such as behavior
said attempts have been made to collect more data on cancer survivors in Cooperative Group studies, stating that “that is a place where there is an infrastructure that exists that we could potentially do things like waist circumference or collect accelerometer data from a subgroup. It’s important to provide the specific evidence so we can make our messages evidence based.” Dr. Demark-Wahnefried added, “If weight is that important, we should be collecting it every time and adjust for it within all of our pharmacologic trials. Height and weight aren’t really difficult to collect—how hard can that be?”
Dr. Brenner pointed out ASCO’s current cancer survivorship initiative and their efforts to capture data from clinical trials that can be used in survivorship studies. Dr. Ganz added that not collecting data on weight, height,
science, physiology and metabolism, sociology, communications, geography, psychology, kinesiology, nutrition, biostatistics, biochemistry, and molecular biology.
The reissued TREC initiative also expands into other research areas, including cancer survivorship, childhood obesity, genomics, and environmental aspects of obesity that include use of tools such as geospatial analysis. The reissued program also includes additional emphasis on testing and integrating behavior change theories, challenges in survivor populations, systems analysis, using animal and human studies in diverse research designs, and expansion of the application of biological markers to inform behavioral-based research. Training new and established scientists to carry out transdisciplinary research continues to be a part of the reissued TREC initiative.
Dr. Jennifer Ligibel pointed out at the workshop that current studies conducted by her and others as part of the TREC initiative should provide information about the impact of various types of energy balance factors on biomarkers linked to cancer prognosis. For example, Drs. Ligibel, Jeffrey Meyerhardt, and others are studying the impact of exercise and/or metformin on insulin levels and other metabolic or inflammatory markers in patients who have been treated for colorectal cancer. Dr. Ruth Patterson at the University of California, San Diego, is conducting a similar study on breast cancer survivors, although the interventions she is studying are weight loss or metformin or the combination of both. In addition, Dr. Kathryn Schmitz at the University of Pennsylvania is studying the impact of exercise, dietary weight loss, or the combination on lymphedema and breast cancer biomarkers in breast cancer patients.
smoking, and comorbidities in clinical trials “is a missed opportunity. To collect that extra bit of data would enlarge our knowledge base about a whole host of other things that have predictive validity in the general population.” Dr. Rock suggested the NCI work in partnership with some of the existing weight loss clinical trials funded by other branches of the NIH to determine cancer-relevant outcomes that they should be collecting.
Several participants also stressed the need to collect, store, and share blood and tumor samples as well as comparable normal tissue with other researchers. Recognizing the expense of doing so, as well as the reluctance to share these resources, Dr. Goodwin said, “We need to get past all of the reasons for people saying no.” She said that for her trial on the effects of metformin on cancer risk, she turned down the participation of centers
that would not provide tumor tissue. “We were very insistent that it had to be done. It meant that some centers that wanted to participate couldn’t. More people have to do that,” Dr. Goodwin said. Dr. Stephen Piantadosi, of the Samuel Oschin Comprehensive Cancer Institute, noted that in his experience, he has found that Institutional Review Boards (IRBs) are major roadblocks to sharing tissue samples, as well as inadequate information systems. “It’s very easy to put tissue into the freezer [but] much harder to retrieve it and connect it to the appropriate information,” he said.
Ms. Murphy stressed that overwhelming percentages of cancer patients are willing to donate tissue, not only to their existing trials, but also for future research. “We are very disturbed when we find the barriers that exist in using that tissue,” she said, adding that the ability to build virtual tissue banks provides researchers with a broader opportunity to find and analyze donated tissues. Dr. Wolfe said, “The key is to get consent for unrestricted use of the samples from the subjects at the start. As long as that consent has been properly done through the IRB and is available, then the specimens can be used for additional [IRB-approved] research over time.”
Dr. Goodwin pointed out that as more small tumors are diagnosed due to screening and as the demand for such tumor tissue increases, pathologists see themselves as the guardians of the tissue and worry that they need to maintain sufficient tissue for some future unforeseen clinical test. “The patient consents and we can’t get the tissue out of the lab. We are going to have to continue to work with the pathology community to [obtain] the tissue. Investigators are also going to have to work out ways to make do with smaller pieces of tissue. This is a hurdle that has to be addressed for us to move forward.”
Ms. Murphy suggested more research on the role of obesity as a barrier to participation in cancer screening. Dr. Partridge concurred that “the research around screening behavior has to concentrate on the 40 percent that are not getting screened to understand what the problem is.” She noted that about 40 percent of women do not have routine mammograms and obesity might be playing a role in their reluctance to have such screening.
Public- and Private-Sector Policies
Dr. Dannenberg suggested the possibility of involving the private sector in efforts to counter obesity and develop policies that counter obesity-promoting practices, much like the private sector was involved in policies developed to counter promotion of tobacco products. Dr. Goodwin noted
that “the private sector is paying a huge price for the obesity problems, in terms of healthcare, time off work, and lost productivity.” Dr. Ballard-Barbash pointed out that the National Collaboration on Childhood Obesity is currently considering policies that might foster obesity prevention, and the Institute of Medicine has also put forward policy suggestions in this regard in previous workshops and consensus reports (IOM, 2011a,b,c, 2012a,b,c). Dr. Rock added that many NIH institutes are funding research related to worksite programs and their influence on obesity and its management. “This is an issue that is not unique to, or only can be solved by, a cancer-focused effort,” she noted, adding that it would be helpful to review the large body of research, and the lessons learned from organizations, such as health maintenance organizations and other insurance companies that have instituted policies aimed at promoting various health behaviors. She added that there have been large advances in this regard internationally.
Dr. Murphy discussed the obesity policy efforts that the CEO Roundtable on Cancer has recently taken. This organization awards the CEO Cancer Gold Standard to organizations, companies, and institutions that promote workplace wellness concepts, of which exercise and nutrition are a part, and collect outcomes data on their interventions. He suggested a willingness on the part of industry to participate in such efforts and advocated bringing them to the table, when obesity policies are crafted. Dr. Partridge agreed, adding, “The private sector has got to be involved. We cannot solve it with government and public support alone.” He has been involved in an organization called CEOs Against Cancer, which is trying to implement worksite interventions related to tobacco control, healthy eating, and exercise. “They are beginning to see that they can play a major role in their communities and they are seeing an even bigger picture because these are global companies now so they are beginning to see that the economic welfare of their company, their community, their nation, and the globe actually is going to require their involvement,” Dr. Partridge said.
Dr. Kakarala suggested focusing obesity prevention efforts in the school food environment. “While we have a lot of regulation about what’s offered in school meal programs, we are reimbursing the schools in terms of high-fat commodities and dairy products, and making it difficult for them to achieve those goals. We also have appallingly little regulation of what’s offered in the vending machines in schools or in school stores. We need to offer healthier food options and encourage children to choose them,” she said.
Dr. Ballard-Barbash noted how researchers at Cornell found that modifying the placement of food in the school cafeteria, such as by putting
the water in the front and the sugar-sweetened beverages in the back and the fruit at the check-out counter, made marked, measurable differences in what the students were buying (Mancino and Guthrie, 2009). “There’s a lot of very interesting innovative research that is looking at how to do this beyond just the point of trying to teach people to choose differently. Instead, they are modifying the environment so that the easier choice is also the healthier choice,” she said. But Dr. Wolfe noted how such efforts are impeded by economic realities, such as the rebates schools receive from the companies that offer sweetened drinks in vending machines, and the support they provide for their sports and non-educational activities. “It’s easier said than done to just remove products from vending machines,” he said.
Dr. Wadden suggested that health insurers pay for weight loss programs. “Ironically, patients can get bariatric surgery paid for, but they can’t get Weight Watchers paid for,” he noted. Dr. Blackstone of the American Society for Metabolic and Bariatric Surgery added that treatment of obesity should be part of the essential health benefits package that the Department of Health and Human Services is currently specifying. Dr. Brenner added that insurers should provide for cancer survivorship care.
Drs. Brenner and Schmitz stressed the need to educate clinical oncologists about lifestyle interventions aimed at lowering cancer risk. “We have to train the future oncologists and educate our current oncologists to support the community in this area, and also to make referrals for family members. Even though perhaps they are not ready for it, oncologists are considered the community resource for cancer genetics, cancer nutrition, etc.,” Dr. Brenner said. He noted that oncologists currently receive no formal training on survivorship care, although they are open to that idea, an ASCO survey revealed. Dr. Schmitz noted that beginning in 2014, the American College of Surgeons (ACS) Commission on Cancer will start to require that hospitals that are certified by the ACS have some kind of survivorship care plan. Dr. Wadden advocated for training dietitians and other health care providers in addition to primary care providers so they can offer weight loss treatment. “I don’t think primary care providers are going to be able to provide obesity treatment because they don’t have the training and the time to do it,” he noted.
Both Drs. Schmitz and Brenner suggested the need for guidelines on lifestyle interventions to recommend to patients, including guidelines on
what to recommend until stronger evidence becomes available. “There is an enormous divide between all these meta-analyses and published studies, and the availability of good guidelines that are actually implementable in the clinic,” Dr. Schmitz said. Dr. Lori Minasian, chief of Community Oncology and Prevention Trials Research Group at the NCI, agreed on the need for guidelines, adding, “In order for there to be dissemination and investment in these kinds of programs, there has to be a proof of principal that the benefit is really worth the investment,” he said.
Dr. Schmitz suggested developing cancer rehabilitation programs focused on monitoring for and assessing how to ameliorate obstacles that cancer survivors have to overcome to be physically active. “Dr. Andrea Cheville at the Mayo Clinic has documented in two separate studies that even when metastatic breast cancer patients have difficulty with ambulation, they do not get referred for rehabilitation [Cheville et al., 2011]. We clearly have a disconnect here—we have the evidence, but we are not getting people to the services that they need,” she said, adding that rehabilitation programs should be part of cancer survivorship care plans. Dr. Ganz agreed and noted the biggest challenge is posttreatment coordination. Another participant stressed the need for such rehabilitation programs that third party payors support, saying, “As a medical oncologist who believes in these interventions deeply, I know I don’t have the ability to cause my patients to increase their activity or to eat better—I don’t see them for long enough periods of time and have to focus on their therapy.”
Dr. Berger also stressed the need for insurers to reimburse for such rehabilitation. “If you break a hip or get a hip replacement, you get the third-party payors to pay for rehabilitation—to get the patients on the treadmill and doing all the other exercises. If you have an MI (myocardial infarction), you get cardiac rehab, which is paid for by the insurance companies. But I don’t think we can get any of the third-party payors to pay for exercise therapy for breast cancer patients undergoing therapy to prevent weight gain or for survivorship care. Convincing third-party payors that they need to compensate the patients for this would not only be great for the patients, but a tremendous asset for those of us doing research and needing to encourage patients to participate in such research.”
Dr. Brenner noted that ASCO has an increased interest in promoting cancer prevention among oncologists and other providers. Over the past year, ASCO developed a new subcommittee in the area of diet, nutrition, obesity, and energy balance. Dr. Brenner stressed that these recent ASCO efforts are likely to have domestic as well as international impact, as the
international cancer community models many of their policies according to what ASCO advocates. Dr. Wadden advocated for more educational and policy efforts being made to prevent obesity in the general population as well. “Treatment alone is clearly not the answer to the obesity epidemic. We have to do more to prevent this disorder,” he said.
Dr. Partridge stressed that obesity is an interesting disease because in addition to its molecular basis, it is impacted by individual behavior, and by family, workplace, or community influences on that behavior. “I can visualize a system’s biomedicine approach to obesity in which you have the basic scientists, the behaviorists, the epidemiologists, and the political scientists working together in transdisciplinary teams with excellent informatics that links all that together, so you can begin to tease out the important factors at each level needed to make some progress,” he said.
Mr. Kean suggested reaching out to experts who can frame the obesity and cancer policy issues within the context of the economy and jobs or other high-priority topics that are more likely to garner the interest of politicians who can influence obesity policies. He also suggested collaborating with the IOM Evidence Communication Innovation Collaborative, which is currently assessing how best to communicate evidence to patients and the public at large.
Dr. Morgan Downey, of the Downey Obesity Report, stressed the genetic and epigenetic components of obesity and cautioned that such factors be considered when devising health or workplace policies related to the disorder. He said that according to obesity researcher Jeffrey Freidman, body weight is as heritable as height (Friedman, 2004), so obesity is different from a behavior such as smoking, which is more likely to be susceptible to financial disincentives or penalties. He said that some workplaces and insurers and even the Affordable Care Act are suggesting placing similar penalties on people who are overweight and obese. “We have to be careful because the types of interventions that were successful in cancer prevention via smoking I don’t think are going to prove very effective in long-term obesity reduction.”
Dr. Wahnefried-Demark provided some closing remarks, noting that growing evidence from both clinical and animal studies shows that obesity increases the risk of cancer incidence, recurrence after treatment, progression, and cancer death for many organ sites. This is a significant public
health concern given that an obesity epidemic is currently spreading worldwide, and obesity is the second leading risk factor for cancer incidence. Hormones, growth factors, and inflammation may underlie the mechanisms by which obesity increases cancer risk, with animal and human study results consistently suggesting specific interacting mechanistic pathways that vary by cancer subtype.
Key molecules in those pathways could serve as targets for drugs aimed at preventing cancer or cancer recurrence or progression. There is growing evidence that lifestyle measures, such as reducing weight, maintaining a healthy diet, and increasing physical activity could also lower cancer risk. Most of that evidence is observational and would be strengthened by randomized clinical trials. However, routine measurements of obesity indicators in clinical trials would aid researchers’ attempts to document the benefits of various interventions in stemming obesity and its impact on cancer risk.
In the meantime, more education of oncologists, dietitians, primary care physicians, and other healthcare practitioners who work with cancer survivors and better dissemination of what is known about obesity’s influence on the risk of cancer recurrence, progression, and mortality, and ways to ameliorate that risk could be beneficial.
Reimbursement by health insurers for obesity treatments, including weight loss programs, and more involvement of industry and schools in promoting policies that help prevent obesity might also have a positive impact, not just on cancer incidence, but on the well-being of cancer patients.
Abdelmalek, M. F., A. Suzuki, C. Guy, A. Unalp-Arida, R. Colvin, R. J. Johnson, and A. M. Diehl. 2010. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51(6):1961-1971.
Abrahamson, P. E., M. D. Gammon, M. J. Lund, J. A. Britton, S. W. Marshall, E. W. Flagg, P. L. Porter, L. A. Brinton, J. W. Eley, and R. J. Coates. 2006. Recreational physical activity and survival among young women with breast cancer. Cancer 107(8):1777-1785.
Ackermann, R. T., E. A. Finch, E. Brizendine, H. Zhou, and D. G. Marrero. 2008. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 35(4):357-363.
Adams, T. D., R. E. Gress, S. C. Smith, R. C. Halverson, S. C. Simper, W. D. Rosamond, M. J. Lamonte, A. M. Stroup, and S. C. Hunt. 2007. Long-term mortality after gastric bypass surgery. N Engl J Med 357(8):753-761.
Aggarwal, B. B., A. Kumar, and A. C. Bharti. 2003. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23(1A):363-398.
Baumgartner, K. B., W. C. Hunt, R. N. Baumgartner, D. D. Crumley, F. D. Gilliland, A. McTiernan, L. Bernstein, and R. Ballard-Barbash. 2004. Association of body composition and weight history with breast cancer prognostic markers: Divergent pattern for Hispanic and non-Hispanic White women. Am J Epidemiol 160(11): 1087-1097.
Behan, J. W., J. P. Yun, M. P. Proektor, E. A. Ehsanipour, A. Arutyunyan, A. S. Moses, V. I. Avramis, S. G. Louie, A. Butturini, N. Heisterkamp, and S. D. Mittelman. 2009. Adipocytes impair leukemia treatment in mice. Cancer Res 69(19):7867-7874.
Bell, S. J., and G. K. Goodrick. 2002. A functional food product for the management of weight. Crit Rev Food Sci Nutr 42(2):163-178.
Bodmer, M., C. Meier, S. Krahenbuhl, S. S. Jick, and C. R. Meier. 2010. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 33(6): 1304-1308.
Bond, D. S., J. M. Jakicic, J. L. Unick, S. Vithiananthan, D. Pohl, G. D. Roye, B. A. Ryder, H. C. Sax, and R. R. Wing. 2010. Pre- to postoperative physical activity changes in bariatric surgery patients: Self report vs. objective measures. Obesity 18(12):2395-2397.
Bosco, J. L., S. Antonsen, H. T. Sorensen, L. Pedersen, and T. L. Lash. 2011. Metformin and incident breast cancer among diabetic women: A population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev 20(1):101-111.
Brown, D. A., K. W. Hance, C. J. Rogers, L. B. Sansbury, P. S. Albert, G. Murphy, A. O. Laiyemo, Z. Wang, A. J. Cross, A. Schatzkin, M. Danta, P. Srasuebkul, J. Amin, M. Law, S. N. Breit, and E. Lanza. 2011. Serum Macrophage Inhibitory Cytokine-1 (MIC-1/GDF15): A potential screening tool for the prevention of colon cancer? Cancer Epidemiol Biomarkers Prev 21(2):337-346.
Caan, B. J., M. L. Kwan, G. Hartzell, A. Castillo, M. L. Slattery, B. Sternfeld, and E. Weltzien. 2008. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control 19(10):1319-1328.
Calle, E. E., C. Rodriguez, K. Walker-Thurmond, and M. J. Thun. 2003. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348(17):1625-1638.
Cao, Y., and J. Ma. 2011. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: A systematic review and meta-analysis. Cancer Prev Res (Phila) 4(4):486-501.
Checkley, L. A., O. Rho, T. Moore, S. Hursting, and J. DiGiovanni. 2011. Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prev Res (Phila) 4(7):1011-1020.
Chen, J. 2011. Multiple signal pathways in obesity-associated cancer. Obes Rev 12(12): 1063-1070.
Chen, X., W. Lu, W. Zheng, K. Gu, C. E. Matthews, Z. Chen, Y. Zheng, and X. O. Shu. 2011. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res (Phila) 4(9):1409-1418.
Cheville, A. L., A. B. Kornblith, and J. R. Basford. 2011. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil 90(5 Suppl 1):S27-S37.
Chlebowski, R. T., M. Pettinger, M. L. Stefanick, B. V. Howard, Y. Mossavar-Rahmani, and A. McTiernan. 2004. Insulin, physical activity, and caloric intake in postmenopausal women: breast cancer implications. J Clin Oncol 22(22):4507-4513.
Christou, N. V., M. Lieberman, F. Sampalis, and J. S. Sampalis. 2008. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis 4(6):691-695.
Colbert, L. H., V. Mai, S. N. Perkins, D. Berrigan, J. A. Lavigne, H. H. Wimbrow, W. G. Alvord, D. C. Haines, P. Srinivas, and S. D. Hursting. 2003. Exercise and intestinal polyp development in APCMin mice. Med Sci Sports Exerc 35(10):1662-1669.
Colbert, L. H., V. Mai, J. A. Tooze, S. N. Perkins, D. Berrigan, and S. D. Hursting. 2006. Negative energy balance induced by voluntary wheel running inhibits polyp development in APCMin mice. Carcinogenesis 27(10):2103-2107.
Courneya, K. S., C. M. Booth, S. Gill, P. O’Brien, J. Vardy, C. M. Friedenreich, H. J. Au, M. D. Brundage, D. Tu, H. Dhillon, and R. M. Meyer. 2008. The Colon Health and Life-Long Exercise Change Trial: A randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol 15(6):279-285.
Craft, L. L., E. H. Vaniterson, I. B. Helenowski, A. W. Rademaker, and K. S. Courneya. 2012. Exercise effects on depressive symptoms in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 21(1):3-19.
Cufi, S., A. Vazquez-Martin, C. Oliveras-Ferraros, B. Martin-Castillo, J. Joven, and J. A. Menendez. 2010. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): From cancer stem cells to aging-associated fibrosis. Cell Cycle 9(22):4461-4468.
Currie, C. J., C. D. Poole, and E. A. Gale. 2009. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52(9):1766-1777.
Decensi, A., M. Puntoni, P. Goodwin, M. Cazzaniga, A. Gennari, B. Bonanni, and S. Gandini. 2010. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev Res (Phila) 3(11):1451-1461.
Demark-Wahnefried, W., B. Peterson, C. McBride, I. Lipkus, and E. Clipp. 2000. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 88(3):674-684.
Dignam, J. J., B. N. Polite, G. Yothers, P. Raich, L. Colangelo, M. J. O’Connell, and N. Wolmark. 2006. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 98(22):1647-1654.
Duggan, C., M. L. Irwin, L. Xiao, K. D. Henderson, A. W. Smith, R. N. Baumgartner, K. B. Baumgartner, L. Bernstein, R. Ballard-Barbash, and A. McTiernan. 2011. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol 29(1):32-39.
Ewertz, M., M. B. Jensen, K. A. Gunnarsdottir, I. Hojris, E. H. Jakobsen, D. Nielsen, L. E. Stenbygaard, U. B. Tange, and S. Cold. 2011. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol 29(1):25-31.
Ferrer, R. A., T. B. Huedo-Medina, B. T. Johnson, S. Ryan, and L. S. Pescatello. 2011. Exercise interventions for cancer survivors: A meta-analysis of quality of life outcomes. Ann Behav Med 41(1):32-47.
Flum, D. R., S. H. Belle, W. C. King, A. S. Wahed, P. Berk, W. Chapman, W. Pories, A. Courcoulas, C. McCloskey, J. Mitchell, E. Patterson, A. Pomp, M. A. Staten, S. Z. Yanovski, R. Thirlby, and B. Wolfe. 2009. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 361(5):445-454.
Foster, G. D., H. R. Wyatt, J. O. Hill, A. P. Makris, D. L. Rosenbaum, C. Brill, R. I. Stein, B. S. Mohammed, B. Miller, D. J. Rader, B. Zemel, T. A. Wadden, T. Tenhave, C. W. Newcomb, and S. Klein. 2010. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann Intern Med 153(3):147-157.
Freedland, S. J., K. A. Grubb, S. K. Yiu, E. B. Humphreys, M. E. Nielsen, L. A. Mangold, W. B. Isaacs, and A. W. Partin. 2005. Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. J Urol 174(3):919-922.
Friedman, J. M. 2004. Modern science versus the stigma of obesity. Nature Medicine 10(6): 563-569.
Gallagher, E. J., and D. LeRoith. 2011. Minireview: IGF, insulin, and cancer. Endocrinology 152(7):2546-2551.
Gans, K. M., E. Ross, C. W. Barner, J. Wylie-Rosett, J. McMurray, and C. Eaton. 2003. REAP and WAVE: New tools to rapidly assess/discuss nutrition with patients. J Nutr 133(2):556S-562S.
Goodwin, P. J., M. Ennis, K. I. Pritchard, M. E. Trudeau, J. Koo, Y. Madarnas, W. Hartwick, B. Hoffman, and N. Hood. 2002. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol 20(1):42-51.
Goodwin, P. J., J. A. Ligibel, and V. Stambolic. 2009. Metformin in breast cancer: Time for action. J Clin Oncol 27(20):3271-3273.
Goodwin, P. J., M. Ennis, K. I. Pritchard, M. E. Trudeau, J. Koo, S. K. Taylor, and N. Hood. 2012. Insulin- and obesity-related variables in early-stage breast cancer: Correlations and time course of prognostic associations. J Clin Oncol 30(2):164-171.
Harrison, D. E., R. Strong, Z. D. Sharp, J. F. Nelson, C. M. Astle, K. Flurkey, N. L. Nadon, J. E. Wilkinson, K. Frenkel, C. S. Carter, M. Pahor, M. A. Javors, E. Fernandez, and R. A. Miller. 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460(7253):392-395.
Harvey, A. E. 2011. Energy balance, inflammation, and tumor progression: The role of NF-[kappa]B. http://repositories.lib.utexas.edu/handle/2152/ETD-UT-2011-05-3279 (accessed January 30, 2012).
Harvey, A. E., L. M. Lashinger, and S. D. Hursting. 2011. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci 1229:45-52.
Harvey-Berino, J., D. West, R. Krukowski, E. Prewitt, A. VanBiervliet, T. Ashikaga, and J. Skelly. 2010. Internet delivered behavioral obesity treatment. Prev Med 51(2):123-128.
Haydon, A. M., R. J. Macinnis, D. R. English, and G. G. Giles. 2006. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 55(1): 62-67.
Hecker, P. A., K. M. O’Shea, T. F. Galvao, B. H. Brown, and W. C. Stanley. 2011. Role of adiponectin in the development of high fat diet-induced metabolic abnormalities in mice. Horm Metab Res 43(2):100-105.
Heymsfield, S. B., C. A. van Mierlo, H. C. van der Knaap, M. Heo, and H. I. Frier. 2003. Weight management using a meal replacement strategy: Meta and pooling analysis from six studies. Int J Obes Relat Metab Disord 27(5):537-549.
Holick, C. N., P. A. Newcomb, A. Trentham-Dietz, L. Titus-Ernstoff, A. J. Bersch, M. J. Stampfer, J. A. Baron, K. M. Egan, and W. C. Willett. 2008. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev 17(2):379-386.
Holmes, M. D., W. Y. Chen, D. Feskanich, C. H. Kroenke, and G. A. Colditz. 2005. Physical activity and survival after breast cancer diagnosis. JAMA 293(20):2479-2486.
Hursting, S. D., and N. A. Berger. 2010. Energy balance, host-related factors, and cancer progression. J Clin Oncol 28(26):4058-4065.
Hursting, S. D., J. A. Lavigne, D. Berrigan, S. N. Perkins, and J. C. Barrett. 2003. Calorie restriction, aging, and cancer prevention: Mechanisms of action and applicability to humans. Annu Rev Med 54:131-152.
Imayama, I., C. M. Alfano, A. Kong, K. E. Foster-Schubert, C. E. Bain, L. Xiao, C. Duggan, C. Y. Wang, K. L. Campbell, G. L. Blackburn, and A. McTiernan. 2011. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: A randomized controlled trial. Int J Behav Nutr Phys Act 8:118.
IOM (Institute of Medicine). 2005. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: The National Academies Press.
IOM. 2011a. Early childhood obesity prevention policies. Washington, DC: The National Academies Press.
IOM. 2011b. Legal strategies in childhood obesity prevention: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2011c. Leveraging food technology for obesity prevention and reduction efforts: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2012a. Mesuring progress in obesity prevention: Workshop report. Washington, DC: The National Academies Press.
IOM. 2012b. Accelerating progress in obesity prevention: Solving the weight of the nation. Washington, DC: The National Academies Press.
IOM. 2012c. Alliances for obesity prevention: Finding common ground: Workshop summary. Washington, DC: The National Academies Press.
Irwin, M. L., A. McTiernan, L. Bernstein, F. D. Gilliland, R. Baumgartner, K. Baumgartner, and R. Ballard-Barbash. 2005. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev 14(12):2881-2888.
Irwin, M. L., A. W. Smith, A. McTiernan, R. Ballard-Barbash, K. Cronin, F. D. Gilliland, R. N. Baumgartner, K. B. Baumgartner, and L. Bernstein. 2008. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J Clin Oncol 26(24):3958-3964.
Irwin, M. L., K. Varma, M. Alvarez-Reeves, L. Cadmus, A. Wiley, G. G. Chung, L. Dipietro, S. T. Mayne, and H. Yu. 2009. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: The Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev 18(1):306-313.
Irwin, M. L., C. Duggan, C. Y. Wang, A. W. Smith, A. McTiernan, R. N. Baumgartner, K. B. Baumgartner, L. Bernstein, and R. Ballard-Barbash. 2011. Fasting C-peptide levels and death resulting from all causes and breast cancer: The health, eating, activity, and lifestyle study. J Clin Oncol 29(1):47-53.
Jakicic, J. M., C. Winters, W. Lang, and R. R. Wing. 1999. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: A randomized trial. JAMA 282(16):1554-1560.
Jebb, S. A., A. L. Ahern, A. D. Olson, L. M. Aston, C. Holzapfel, J. Stoll, U. Amann-Gassner, A. E. Simpson, N. R. Fuller, S. Pearson, N. S. Lau, A. P. Mander, H. Hauner, and I. D. Caterson. 2011. Primary care referral to a commercial provider for weight loss treatment versus standard care: A randomised controlled trial. Lancet 378(9801):1485-1492.
Jiralerspong, S., S. L. Palla, S. H. Giordano, F. Meric-Bernstam, C. Liedtke, C. M. Barnett, L. Hsu, M. C. Hung, G. N. Hortobagyi, and A. M. Gonzalez-Angulo. 2009. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27(20):3297-3302.
Joshu, C. E., A. M. Mondul, A. Menke, C. Meinhold, M. Han, E. B. Humphreys, S. J. Freedland, P. C. Walsh, and E. A. Platz. 2011. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 4(4):544-551.
Kakarala, M., and M. S. Wicha. 2008. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol 26(17):2813-2820.
Kakarala, M., D. E. Brenner, H. Korkaya, C. Cheng, K. Tazi, C. Ginestier, S. Liu, G. Dontu, and M. S. Wicha. 2010a. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 122(3):777-785.
Kakarala, M., S. K. Dubey, M. Tarnowski, C. Cheng, S. Liyanage, T. Strawder, K. Tazi, A. Sen, Z. Djuric, and D. E. Brenner. 2010b. Ultra-low flow liquid chromatography assay with ultraviolet (UV) detection for piperine quantitation in human plasma. J Agric Food Chem 58(11):6594-6599.
Knowler, W. C., E. Barrett-Connor, S. E. Fowler, R. F. Hamman, J. M. Lachin, E. A. Walker, and D. M. Nathan. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6):393-403.
Knox, L. S., L. O. Crosby, I. D. Feurer, G. P. Buzby, C. L. Miller, and J. L. Mullen. 1983. Energy expenditure in malnourished cancer patients. Ann Surg 197(2):152-162.
Kroenke, C. H., W. Y. Chen, B. Rosner, and M. D. Holmes. 2005. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 23(7):1370-1378.
Leblanc, E. S., E. O’Connor, E. P. Whitlock, C. D. Patnode, and T. Kapka. 2011. Effectiveness of primary care-relevant treatments for obesity in adults: A systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 155(7):434-447.
LeRoith, D. 2010. Can endogenous hyperinsulinaemia explain the increased risk of cancer development and mortality in type 2 diabetes: Evidence from mouse models. Diabetes Metab Res Rev 26(8):599-601.
Libby, G., L. A. Donnelly, P. T. Donnan, D. R. Alessi, A. D. Morris, and J. M. Evans. 2009. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 32(9):1620-1625.
Ligibel, J. 2011. Obesity and breast cancer. Oncology 25(11):994-1000.
Ligibel, J. A., N. Campbell, A. Partridge, W. Y. Chen, T. Salinardi, H. Chen, K. Adloff, A. Keshaviah, and E. P. Winer. 2008. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol 26(6):907-912.
Ma, J., H. Li, E. Giovannucci, L. Mucci, W. Qiu, P. L. Nguyen, J. M. Gaziano, M. Pollak, and M. J. Stampfer. 2008. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: A long-term survival analysis. Lancet Oncol 9(11):1039-1047.
Macedo, L. F., G. J. Sabnis, O. G. Goloubeva, and A. Brodie. 2008. Combination of anastrozole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res 68(9):3516-3522.
Mai, V., L. H. Colbert, D. Berrigan, S. N. Perkins, R. Pfeiffer, J. A. Lavigne, E. Lanza, D. C. Haines, A. Schatzkin, and S. D. Hursting. 2003. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(Min) mice through different mechanisms. Cancer Res 63(8):1752-1755.
Mancino, L., and J. Guthrie. 2009. When nudging in the lunch line might be a good thing. Amber Waves 7(1):32-38.
Mason, C., K. E. Foster-Schubert, I. Imayama, A. Kong, L. Xiao, C. Bain, K. L. Campbell, C. Y. Wang, C. R. Duggan, C. M. Ulrich, C. M. Alfano, G. L. Blackburn, and A. McTiernan. 2011. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med 41(4):366-375.
Menendez, J. A., S. Cufi, C. Oliveras-Ferraros, B. Martin-Castillo, J. Joven, L. Vellon, and A. Vazquez-Martin. 2011. Metformin and the ATM DNA damage response (DDR): Accelerating the onset of stress-induced senescence to boost protection against cancer. Aging 3(11):1063-1077.
Meyerhardt, J. A., D. Heseltine, D. Niedzwiecki, D. Hollis, L. B. Saltz, R. J. Mayer, J. Thomas, H. Nelson, R. Whittom, A. Hantel, R. L. Schilsky, and C. S. Fuchs. 2006. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol 24(22):3535-3541.
Meyerhardt, J. A., D. Niedzwiecki, D. Hollis, L. B. Saltz, F. B. Hu, R. J. Mayer, H. Nelson, R. Whittom, A. Hantel, J. Thomas, and C. S. Fuchs. 2007. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 298(7):754-764.
Meyerhardt, J. A., J. Ma, and K. S. Courneya. 2010. Energetics in colorectal and prostate cancer. J Clin Oncol 28(26):4066-4073.
Moore, T., L. Beltran, S. Carbajal, S. Strom, J. Traag, S. D. Hursting, and J. DiGiovanni. 2008a. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila) 1(1):65-76.
Moore, T., S. Carbajal, L. Beltran, S. N. Perkins, S. Yakar, D. Leroith, S. D. Hursting, and J. DiGiovanni. 2008b. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor I levels. Cancer Res 68(10):3680-3688.
NIH (National Institutes of Health). 2000. The practical guide: Identification, evaluation, and treatment of overweight and obesity in adults. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf (accessed January 10, 2012).
Nock, N. L., and N. A. Berger. 2010. Obesity and cancer: Overview of mechanism, in cancer and energy balance, epidemiology and overview. Edited by N. A. Berger. New York: Springer.
Padovani, M., J. A. Lavigne, G. V. Chandramouli, S. N. Perkins, J. C. Barrett, S. D. Hursting, L. M. Bennett, and D. Berrigan. 2009. Distinct effects of calorie restriction and exercise on mammary gland gene expression in C57BL/6 mice. Cancer Prev Res (Phila) 2(12):1076-1087.
Perri, M. G., D. A. McAllister, J. J. Gange, R. C. Jordan, G. McAdoo, and A. M. Nezu. 1988. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol 56(4):529-534.
Pi-Sunyer, F. X. 2000. Overnutrition and undernutrition as modifiers of metabolic processes in disease states. Am J Clin Nutr 72(2 Suppl):533S-537S.
Pi-Sunyer, X., G. Blackburn, F. L. Brancati, G. A. Bray, R. Bright, J. M. Clark, J. M. Curtis, M. A. Espeland, J. P. Foreyt, K. Graves, S. M. Haffner, B. Harrison, J. O. Hill, E. S. Horton, J. Jakicic, R. W. Jeffery, K. C. Johnson, S. Kahn, D. E. Kelley, A. E. Kitabchi, W. C. Knowler, C. E. Lewis, B. J. Maschak-Carey, B. Montgomery, D. M. Nathan, J. Patricio, A. Peters, J. B. Redmon, R. S. Reeves, D. H. Ryan, M. Safford, B. Van Dorsten, T. A. Wadden, L. Wagenknecht, J. Wesche-Thobaben, R. R. Wing, and S. Z. Yanovski. 2007. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the look AHEAD trial. Diabetes Care 30(6):1374-1383.
Pierce, J. P., L. Natarajan, B. J. Caan, B. A. Parker, E. R. Greenberg, S. W. Flatt, C. L. Rock, S. Kealey, W. K. Al-Delaimy, W. A. Bardwell, R. W. Carlson, J. A. Emond, S. Faerber, E. B. Gold, R. A. Hajek, K. Hollenbach, L. A. Jones, N. Karanja, L. Madlensky, J. Marshall, V. A. Newman, C. Ritenbaugh, C. A. Thomson, L. Wasserman, and M. L. Stefanick. 2007a. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 298(3):289-298.
Pierce, J. P., M. L. Stefanick, S. W. Flatt, L. Natarajan, B. Sternfeld, L. Madlensky, W. K. Al-Delaimy, C. A. Thomson, S. Kealey, R. Hajek, B. A. Parker, V. A. Newman, B. Caan, and C. L. Rock. 2007b. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol 25(17):2345-2351.
Pierce, B. L., M. L. Neuhouser, M. H. Wener, L. Bernstein, R. N. Baumgartner, R. Ballard-Barbash, F. D. Gilliland, K. B. Baumgartner, B. Sorensen, A. McTiernan, and C. M. Ulrich. 2009. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat 114(1):155-167.
Protani, M., M. Coory, and J. H. Martin. 2010. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res Treat 123(3): 627-635.
Ranucci, M., S. Castelvecchio, M. Conte, G. Megliola, G. Speziale, F. Fiore, F. Guarracino, S. Scolletta, R. M. Escobar, M. Falco, E. Bignami, and G. Landoni. 2011. The easier, the better: Age, creatinine, ejection fraction score for operative mortality risk stratification in a series of 29,659 patients undergoing elective cardiac surgery. J Thorac Cardiovasc Surg 142(3):581-586.
Reeves, G. K., K. Pirie, V. Beral, J. Green, E. Spencer, and D. Bull. 2007. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 335(7630):1134.
Rock, C. L., S. W. Flatt, N. E. Sherwood, N. Karanja, B. Pakiz, and C. A. Thomson. 2010. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: A randomized controlled trial. JAMA 304(16):1803-1810.
Rose, D. P., M. Goldman, J. M. Connolly, and L. E. Strong. 1991. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr 54(3): 520-525.
Sabnis, G., A. Schayowitz, O. Goloubeva, L. Macedo, and A. Brodie. 2009. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res 69(4):1416-1428.
Sacks, F. M., G. A. Bray, V. J. Carey, S. R. Smith, D. H. Ryan, S. D. Anton, K. McManus, C. M. Champagne, L. M. Bishop, N. Laranjo, M. S. Leboff, J. C. Rood, L. de Jonge, F. L. Greenway, C. M. Loria, E. Obarzanek, and D. A. Williamson. 2009. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 360(9):859-873.
Saltz, L. B., D. Niedzwiecki, D. Hollis, R. M. Goldberg, A. Hantel, J. P. Thomas, A. L. Fields, and R. J. Mayer. 2007. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol 25(23):3456-3461.
Shoba, G., D. Joy, T. Joseph, M. Majeed, R. Rajendran, and P. S. Srinivas. 1998. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64(4):353-356.
Sjostrom, L., K. Narbro, C. D. Sjostrom, K. Karason, B. Larsson, H. Wedel, T. Lystig, M. Sullivan, C. Bouchard, B. Carlsson, C. Bengtsson, S. Dahlgren, A. Gummesson, P. Jacobson, J. Karlsson, A. K. Lindroos, H. Lonroth, I. Naslund, T. Olbers, K. Stenlof, J. Torgerson, G. Agren, and L. M. Carlsson. 2007. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357(8):741-752.
Speck, R. M., K. S. Courneya, L. C. Masse, S. Duval, and K. H. Schmitz. 2010. An update of controlled physical activity trials in cancer survivors: A systematic review and metaanalysis. J Cancer Surviv 4(2):87-100.
Subbaramaiah, K., L. R. Howe, P. Bhardwaj, B. Du, C. Gravaghi, R. K. Yantiss, X. K. Zhou, V. A. Blaho, T. Hla, P. Yang, L. Kopelovich, C. A. Hudis, and A. J. Dannenberg. 2011. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 4(3):329-346.
Thompson, E. W., and I. Haviv. 2011. The social aspects of EMT-MET plasticity. Nature Medicine 17(9):1048-1049.
TREC (Transdisciplinary Research on Energetics and Cancer). 2012. TREC overview. http://www.trecscience.org/trec/bin/about/overview.aspx?j=21 (accessed January 10, 2012).
USPSTF (U.S. Preventive Services Task Force). 2003. Screening for obesity in adults: Recommendations and rationale. Ann Intern Med 139(11):930-932.
Wadden, T. A, R. H. Neiberg, R. R. Wing, J. M. Clark, L. M. Delahanty, J. O. Hill, J. Krakoff, A. Otto, D. H. Ryan, M. Z. Vitolins; Look AHEAD Research Group. 2011. Four-year weight losses in the Look AHEAD study: Factors associated with long-term success. Obesity 19(10):1987-1998.
Wisse, B. E., F. Kim, and M. W. Schwartz. 2007. An integrative view of obesity. Science 318(5852):928-929.
Wolpin, B. M., J. A. Meyerhardt, A. T. Chan, K. Ng, J. A. Chan, K. Wu, M. N. Pollak, E. L. Giovannucci, and C. S. Fuchs. 2009. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol 27(2):176-185.
World Cancer Reasearch Fund and American Institute for Cancer Research. 2007. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. http://www.dietandcancerreport.org/cancer_resource_center/downloads/summary/english.pdf (accessed January 9, 2012).
Wright, M. E., S. C. Chang, A. Schatzkin, D. Albanes, V. Kipnis, T. Mouw, P. Hurwitz, A. Hollenbeck, and M. F. Leitzmann. 2007. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 109(4):675-684.
Zheng, Q., S. M. Dunlap, J. Zhu, E. Downs-Kelly, J. Rich, S. D. Hursting, N. A. Berger, and O. Reizes. 2011. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer 18(4):491-503.
















































































