Introduction: From Smallpox
to SMART Vaccines
Edward Jenner was an impatient man. He firmly grasped the importance of vaccination. In a pamphlet, On the Origin of the Vaccine Inoculation, published in 1801, Jenner famously articulated the vision of immunizing people against smallpox:
An hundred thousand persons, upon the smallest computation, have been inoculated in these realms. The numbers who have partaken of its benefits throughout Europe and other parts of the Globe are incalculable: and it now becomes too manifest to admit of controversy, that the annihilation of the Small Pox, the most dreadful scourge of the human species, must be the final result of this practice. (Jenner, 1801)
An 1806 letter to Jenner from fellow experimentalist Thomas Jefferson—then the president of the United States—illustrates the reach and impact of Jenner’s efforts:
I have received a copy of the evidence at large respecting the discovery of the vaccine inoculation which you have been pleased to send me, and for which I return you my thanks. Having been among the early converts, in this part of the globe, to its efficiency, I took an early part in recommending it to my countrymen. I avail myself of this occasion of rendering you a portion of the tribute of gratitude due to you from the whole human family…. You have erased from the calendar of human afflictions one of its greatest. Yours is the comfortable reflection that mankind can never forget that you have lived. Future nations will know by history only that the loathsome small-pox has existed and by you has been extirpated. (Jefferson, 1806)
We now know far more about how human immune systems work and about ways to create immunity against diseases than Jenner did. And
as science and technology continue to grow in knowledge and capabilities, a major challenge now is to select from among many options the most important disease targets and to develop new or improved vaccines against them—that is, to prioritize, a task that Jenner did not face since he had the means to conquer only one disease, smallpox.
In the last two centuries, vaccines—in conjunction with antibiotics, clean water, and good hygiene—have served to eliminate or significantly mitigate many infectious diseases that used to kill hundreds of millions of people. Even though the vaccine enterprise has seen great strides since the 1800s, the basic research and development challenges in vaccinology have remained essentially the same (Stern and Markel, 2005). Additionally, the sluggish and fragile nature of the global economy is stressing the need for prioritized investments across the board, and especially in the realm of health care.
Private industrial and philanthropic forces have begun to play a far more prominent role in the push for new and improved preventive vaccines for diseases. Consider, for example, the $10 billion investment from Bill Gates announced at the 2010 meeting of the World Economic Forum to help fund the research, development, and delivery of vaccines for the world’s poorest countries. Gates said then, “I see the next 10 years as the Decade of Vaccines—a time when we will make more progress than ever on immunizations that save lives in the developing world…. This work will make it possible to save more than 8 million lives by 2020” (Gates, 2010).
More recently, Gates’ additional $750 million donation toward funding the Global Fund to Fight AIDS, Tuberculosis, and Malaria, in association with other philanthropic and operational partners, has highlighted the serious commitment of multinational alliances in tackling vaccine-preventable diseases of domestic and global importance (McNeil, 2012).
The U.S. government launched the 2010 National Vaccine Plan to enhance efforts in the development and delivery of vaccines (HHS, 2011). This plan, released as a living document by the Department of Health and Human Services, lists goals and priorities primarily directed toward developing new and improved vaccines and the related safety, communication, and surveillance systems (see Box 1-1).
Similarly, other countries are designing their own national vaccine plans. For example, in 2011 the Indian government released its national vaccine policy aimed at strengthening the country’s framework, infrastructure, and decision-making practices for immunization policies and programs (Government of India, 2011).
Large-scale efforts that began in the early 1970s resulted in the World Health Organization’s Expanded Program on Immunization (EPI), created
BOX 1-1
The 2010 National Vaccine Plan
U.S. Department of Health and Human Services
Goals
1. Develop new and improved vaccines.
2. Enhance the vaccine safety system.
3. Support communications to enhance informed vaccine decision making.
4. Ensure a stable supply of, access to, and better use of recommended vaccines in the United States.
Priorities
A. Develop a catalogue of priority vaccine targets of domestic and global health importance.
B. Strengthen the science base for the development and licensure of new vaccines.
C. Enhance timely detection and verification of vaccine safety signals and develop a vaccine safety scientific agenda.
D. Increase awareness of vaccines, vaccine-preventable diseases, and the benefits/risks of immunization among the public, providers, and other stakeholders.
E. Use evidence-based science to enhance vaccine-preventable disease surveillance, measurement of vaccine coverage, and measurement of vaccine effectiveness.
F. Eliminate financial barriers for providers and consumers to facilitate access to routinely recommended vaccines.
G. Create an adequate and stable supply of routinely recommended vaccines and vaccines for public health preparedness.
H. Increase and improve the use of interoperable health information technology and electronic health records.
I. Improve global surveillance for vaccine-preventable diseases and strengthen global health information systems to monitor vaccine coverage, effectiveness, and safety.
J. Support global introduction and availability of new and underutilized vaccines to prevent diseases of public health importance.
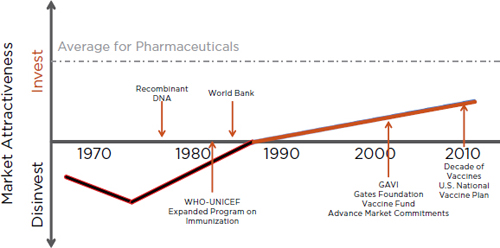
FIGURE 1-1 Historical attractiveness of investments in vaccines. Since the late 1980s significant increases in the efforts and funding relating to vaccine-preventable diseases have made the enterprise attractive for more investments.
SOURCE: Adapted from Rappuoli et al., 2002.
to reduce mortality caused by vaccine-preventable diseases. By partnering with the United Nations Children’s Fund (UNICEF) and other organizations, EPI has played a central role in making vaccines available and increasing coverage for children around the world (Keja et al., 1988).
Jenner and his colleagues mostly self-financed their vaccination regimens, as the concept of multinational partnerships was virtually absent during their time. Today, however, there are scores of collaborative ventures that are helping to drive the vaccine enterprise in many countries. The GAVI Alliance, for example, has had multinational support in its efforts to increase access to vaccines in developing countries (GAVI Alliance, 2010).
The explosion of interest, efforts, and new collaborations relating to vaccine-preventable diseases is reflected in the growing attractiveness of investments in vaccines (see Figure 1-1). Coupled with the momentum of the “decade of vaccines,” a renewed focus on developing new priority-setting strategies for new vaccine development is timely and critical.
The first goal of the 2010 National Vaccine Plan is to “develop new and improved vaccines,” and the first implementation priority is “to develop a catalogue of priority vaccine targets of domestic and global health importance.” To accomplish this task the National Vaccine Program Office (NVPO)
of the Department of Health and Human Services envisions a three-step strategy: The first step is devoted to creating and validating a prioritization model; the second step is focused on populating the model with data; and the third step is to evaluate the model and prioritize vaccines against a catalog of attributes (see Figure 1-2). The current study pertains to Phase I of the first step within NVPO’s strategy to help create a model. (The committee’s task is presented in Box S-1.) Immediately following its completion of Phase I, the committee is expected to carry out the Phase II work. which will be focused on enhancing and refining the model and adding to its utility and effectiveness.
In early 2011 a 16-member committee was appointed by the Institute of Medicine to conduct this study. (Appendix D contains the biographical information of the committee members.) The committee met five times in 2011 and organized an international stakeholder session as well as a public workshop during its first two meetings. (See Appendix C for a list of speakers.)
In addition to the five committee meetings, four modeling subgroup meetings were also held. The committee engaged several consultants to assist in achieving its goal: two consultants to help with modeling, two for software development, and eleven experts for evaluating the concept of SMART Vaccines Beta.
SMART Vaccines Beta is a result of a modeling effort intended ultimately to help users in making decisions relating to setting vaccine priorities. Potential users of and audiences for this model include decision makers from the institutions funding and pursuing basic research, vaccine manufacturers, and philanthropic organizations with interests in improv-
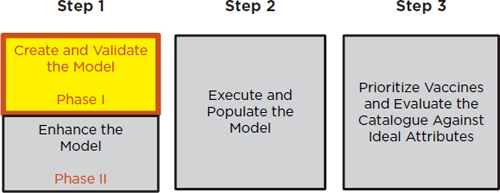
FIGURE 1-2
A three-step vaccine prioritization strategy envisioned by the National Vaccine Program Office. The study described in the current report pertains to Phase I in Step 1.
ing global health; ministers of health, commerce, and finance and other high-level government officials at the country, state, and regional levels; international health agencies; and nongovernmental alliances of interested parties.
Until the 1990s vaccines were not considered to be commercially attractive investments, as highlighted in Figure 1-1. Pharmaceutical companies typically considered vaccines as a commodity with high liability risks (or with potentially high costs of litigation), and their business strategy was to exit or to stay away from vaccine development.
A 2008 estimate suggested that the vaccine market had grown 20-fold in the previous two decades and had come to exceed $14 billion, with its sales accounting for between 2 and 3 percent of the global pharmaceutical market, for 40 percent of the market existing in North America, and for 30 percent of the markets in Europe and the rest of the world (Greco and Hessel, 2008). Industrial sources have recently suggested that in 2011 the worldwide vaccine market was around $23 billion, with continued sale growth expected between 5 and 15 percent annually, with the total market possibly reaching $32 billion by 2017.
As the vaccine companies in industrialized countries were consolidating into large pharmaceutical firms, a similar evolution was taking place among the manufacturers in low- and middle-income countries that have begun to obtain World Health Organization (WHO) prequalification and to supply vaccines to UNICEF and GAVI.
Despite the robust growth trends in the vaccine market mentioned above, the fundamental challenges in vaccinology have largely remained unchanged. On the other hand, disease profiles have begun to change, mainly because of major demographic shifts in society. For example, the people for whom vaccines were developed between 1750 and 1850 had an average life expectancy of 35 to 45 years; by contrast, the people for whom most of the current vaccines were developed had a life expectancy of 60 to 65 years, and by 2050 vaccines may need to be developed for people with a life expectancy of as much as 90 to 95 years (see Figure 1-3). Representative vaccines for the 21st century demographics, segmented by age and target population, are shown in Figures 1-4 and 1-5, respectively.
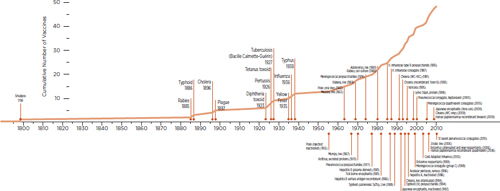
Almost all currently used vaccines were developed in the latter half of the 20th century, with the exception of a few older vaccines such as those developed for such conditions as smallpox, rabies, and typhoid. Figure 1-6, which provides a timeline of vaccine development, shows that
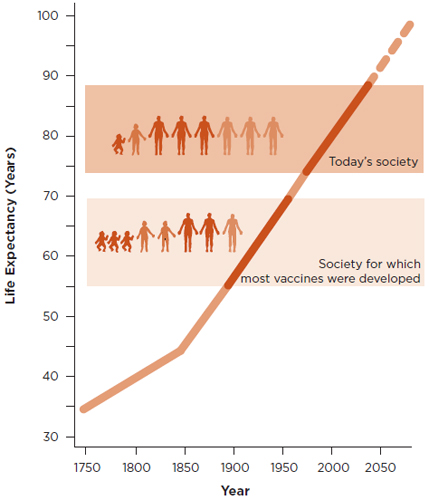
FIGURE 1-3
Increases in life expectancy and longevity since 1750, with projections for the rest of the 21st century. The steady increase clearly shows that the demographic nature of society has changed significantly. The society for which most currently available vaccines were developed had an average life expectancy of 60 to 65 years and was characterized by large numbers of children and young people, which is notably different from today’s society, which is characterized by a high proportion of senior citizens and a life expectancy at birth that is more than 80 years in many countries.
SOURCE: Rappuoli et al., 2011.
development activity increased steadily between 1950 and 2010 and that it has now reached a level higher than at any time in history.
Prioritization efforts in the vaccine enterprise
Investment in vaccination as well as in the associated research and production aspects has grown rapidly. The investments are expected to increase
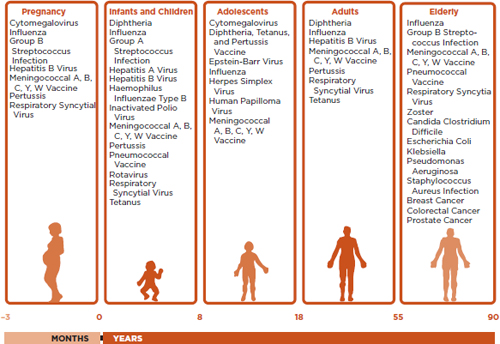
FIGURE 1-4
Target population for vaccines in the 21st century with a listing of representative vaccines for each population segment.
SOURCE: Rappuoli et al., 2011.
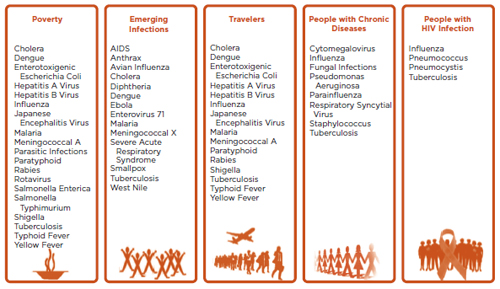
FIGURE 1-5 Special target groups for vaccination in the 21st century with a listing of important representative vaccines for each group.
SOURCE: Rappuoli et al., 2011.
even further in response to the economic growth of transitional and developing countries. However, prioritization exercises have not kept up with the pace of spending. Very few published prioritization efforts exist, and organizations’ internal mechanisms to set priorities are not well known or publicized. Yet, given the vibrancy of the vaccine enterprise, the need for prioritization plans is tremendous.
The cost of making the best decision on developing a new vaccine may be only a small part of the total health expenditure associated with that vaccine, but significantly large health and economic benefits can be derived. In addition to these health and economic benefits, vaccines also generate broad intangible social benefits that are often underappreciated. All too often health financing and policy decisions are guided mainly by narrowly construed “cost–benefit”1 analyses which are extended to assessing the introduction and use of vaccines.
In theory, cost–benefit analysis can accommodate a full spectrum of benefits, both monetary and subjective, but the complexities of incorporating non-monetized values often overwhelm the efforts. Since cost–benefit analysis values every benefit in dollars, methods must be devised to estimate these values in situations where no market prices exist. Common approaches to this seek ways to elicit willingness-to-pay measures from appropriate populations and then use these willingness-to-pay estimates to complete the cost–benefit calculations. Thus, for example, willingness-to-pay measures have been used in environmental studies to estimate the value of things such as pollution reduction, reduced traffic congestion, preservation of endangered species, agricultural land, and unique geologic settings. Because these willingness-to-pay approaches are expensive and complicated, many cost–benefit analyses omit such considerations and center more on measurable benefits, such as the value of enhanced water
___________________
1 “Cost–benefit” and “cost-effectiveness are many times used synonymously, but there is a technical difference between these terms. Cost–benefit analysis—derived from economic models to maximize social welfare—is used to compute the net dollar amount represented by the difference in dollar costs and dollar-valued benefits of an investment. The focus is on whether this difference is positive or negative. In contrast, cost-effectiveness refers to an analysis in which net dollar costs of a health care investment are used in the numerator and benefits are measured in the denominator using natural units of health and health benefit, yielding a price per unit of benefit as output. This can be derived from basic principles of maximizing individual utility, subject to a budget constraint (Garber and Phelps, 1997). The price per unit is a measure of how efficient an investment in health care is. The two approaches converge once a decision maker has chosen a critical cutoff value for the cost-effectiveness analysis (Phelps and Mushlin, 1988), although some users object to specifying an exact cutoff and hence report cost-effectiveness ratios without making specific judgments about a specific cutoff.
supply in agriculture, leaving out those facets of a decision that are more difficult to measure, such as the effect on spawning salmon.
Because of the inherent complexity of such analyses, cost–benefit analysis too often becomes reductionist in its approach, resulting in an underestimation of the complex factors involved in vaccines. Whereas economic and health benefits can be captured in a cost–benefit analysis, intangible values are often omitted. Therefore, most economically-focused models (which, so far, are most of those that are available) fail to capture the full return of investment in vaccination. The main reason for this is that it is fairly straightforward to identify and calculate costs of vaccination (e.g., vaccine cost, administration cost, and other costs), but determining all the benefits is considerably more difficult.
Capturing the value of vaccination
The overall value of vaccination has not been captured in previous prioritization models for two main reasons. First, the models have looked only at short-term benefits. Second, they do not consider many of the intangible effects related to the long-term benefits of vaccination, including the economic benefits to a country of having a healthier population, the educational benefits due to reduced school absenteeism, the avoidance of potential social disruption caused by a disease with high emotional and political impact (e.g., poliomyelitis or Ebola), and the possibility of preventing or controlling pandemic infections, such as influenza or severe acute respiratory syndrome (SARS). More recent accounts have also called for prioritizing vaccines and other medical interventions according to ethical values and morally just policies (Daniels, 2007; Field and Caplan, 2012; Poland and Marcuse, 2011).
Previous prioritization models have not included the value of vaccination for future generations—that is, getting into the question of “who pays” versus “who benefits.” For instance, the costs and benefits of a vaccine developed today are typically calculated for the next year or two, when the cost of the new vaccine will be at its highest. But the price of the vaccine will typically decrease over the next decade, and future generations will inevitably benefit from the reduction of disease by having lower health costs, better quality of life, and longer life expectancy. One way to think about this scenario is as follows: If a prioritization model had computed the cost–benefit of the smallpox vaccine before the eradication of the disease, it would have included costs saved only during the first few years following the vaccination, whereas the value for future generations of a world without smallpox would not be included. Perhaps the value of a smallpox-free
world is so large that it is difficult to capture it numerically. Nonetheless that value should not be ignored.
When performing cost–benefit analyses, different stakeholders may use different assumptions regarding the costs of vaccine development and introduction. Expert estimates and published literature suggest that manufacturers in the high-income countries typically invest between $500 million and $900 million over a period of 10 to 15 years to develop a new vaccine (Greco and Hessel, 2008). The primary criteria for the manufacturers are commonly medical need, the potential market size, and the probability of technical and commercial success.
Individual countries also use cost–benefit analysis when making decisions about vaccine introduction, comparing the benefits of vaccines with the benefits of other interventions. The World Health Organization usually makes high-level recommendations to assist low- and middle-income countries. Other funding organizations, such as the United Nations, the GAVI Alliance, and philanthropic groups, assign their own priorities using criteria such as cost, medical need, and impact on mortality and morbidity.
Previous IOM efforts in vaccine prioritization
The Institute of Medicine (IOM) has previously undertaken two vaccine prioritization exercises. A study in 1985–1986 resulted in the release of New Vaccine Development: Establishing Priorities, a report containing two individual volumes, Diseases of Importance in the United States (IOM, 1985) and Diseases of Importance in Developing Countries (IOM, 1986). This work helped capture both the domestic and the global perspective on vaccine prioritization, using a decision framework based on the expected health benefits from vaccines.
In 2000 the IOM released another consensus study report which contained an updated vaccine prioritization framework and also assessed the barriers to vaccine research and development and recounted the progress that had been made since the 1985–1986 report. That study’s focus was on vaccine candidates that had the potential to be developed and used within the next two decades within the United States, so it did not consider the global burden of diseases. The report’s analytical criterion was cost-effectiveness, using quality-adjusted life years (QALYs) as the measure of health benefits.
Moreover, the 2000 model used cost-effectiveness analysis only from a societal perspective, hence making the prioritization of limited value to such stakeholders as industry and international vaccine suppliers. Furthermore, the model does not calculate costs (which could be interpreted
differently by different stakeholders) from multiple perspectives (e.g., vaccine development costs, costs of immunization, and vaccine administration costs). While the cost–benefit framework emphasizes the economic impact of vaccines, it often neglects to consider their social impacts, such as possible intergenerational benefits gained when immunizing a pregnant woman.
Previous WHO efforts in vaccine-related prioritization
The World Health Organization’s Initiative for Vaccine Research (IVR) recently published Strategic Plan 2010–2020, which outlined four high-level strategies in order to highlight the importance of vaccine research in public health practice (WHO, 2010). Of relevance here is the strategy oriented toward the identification of vaccine and vaccination research priorities over the next 10 years, with a special focus on low- and middle-income countries.
The WHO strategy for prioritizing vaccines began by categorizing diseases and then assessing vaccine characteristics. The diseases for which vaccines are still needed were classified into two groups: (1) diseases that have ongoing vaccine development efforts but no license, and (2) diseases with underutilized vaccines. IVR’s efforts have principally focused on identifying diseases that are of highest public health importance for vaccine development. Together with disease impact and burden, IVR has also considered economic restraints on developing and using vaccines as well as the ability to contain or prevent diseases through alternative measures.
WHO’s Global Immunization Vision and Strategy (GIVS) goes hand in hand with the IVR approach of establishing priorities for vaccines in low- and middle-income countries. The 10-year plan of GIVS, which was released in 2006 jointly by WHO and UNICEF, outlines specific objectives for the control of mortality and morbidity caused by vaccine-preventable conditions (WHO, 2006). As a part of its larger objective to immunize more people with more vaccines, GIVS also aims to strengthen country-level capacity to determine and set policies and priorities for new vaccines. Many countries have adopted GIVS to serve as the framework for their immunization programs and to advance the use of high-priority new vaccines.
The Pan American Health Organization (PAHO) also recently developed a decision-making framework to assist countries in the introduction of new and underused vaccines. PAHO’s ProVac Initiative is structured to promote and strengthen evidence-based decision-making capacity for the introduction of new vaccines in WHO’s region of the Americas. While the lack of economic analyses and the subsequent absence of successful
immunization policies at the country level is a lingering concern, the ProVac Initiative not only intends to strengthen the economic basis for decision making but also to assess other broad factors that should be considered in making decisions (Andrus et al., 2007). ProVac includes technical, programmatic, operational, and social criteria to help set priorities for the introduction of vaccines in a given region or a country (Andrus et al., 2006, 2007). Finally, PAHO recognizes that the necessary analytical tools and collection of data are needed from the WHO region of the Americas in order to properly evaluate and assess priorities for vaccine introduction in each country.
New technologies and development strategies
As science and engineering progressed in the 21st century, vaccine development methods also evolved. In particular, vaccine development received a boost from the application of state-of-the-art technologies, which led to new and improved products for changing populations. While Pasteur’s injunction to “isolate, inactivate, and inject” the microorganism causing the disease is still the mainstay of vaccine development, modern vaccines are also being developed through a number of novel techniques.
The combination of genomics, systems biology, the structure-based design and optimization of immunogens, small molecule adjuvants targeting specific receptors, and sophisticated assays to monitor the immune response is transforming the traditional field of vaccinology into one of today’s most dynamic areas of research. Using these methods there is now the real possibility of developing vaccines for diseases that were regarded as not “vaccinable” in the past.
The technologies developed over the past two decades have improved our understanding of the immune system, making it possible to produce vaccines through novel means, and have helped in the development of novel adjuvants. For example, hepatitis B vaccine was developed using recombinant DNA technology, and genomics has made it possible to discover new vaccine candidates through reverse vaccinology, leading to the development of a vaccine against N. meningitidis B (Rappuoli et al., 2011).
The development and testing of vaccines requires significant time, money, and effort. The story of vaccine discovery and development proceeded differently in the 1980s than it does today. Then, development was fast, and clinical trials required only a few hundred subjects. Timelines have shifted, however, and it now takes about a decade to develop and commercialize a vaccine. Vaccine licensure itself typically requires tens of thousands of people in clinical trials, with Phase I, Phase II, and Phase III
TABLE 1-1
Representative Stakeholder Priority Areas
|
Stakeholders |
Representative Priorities or Interest Areas |
|
Public Sector: Health agencies; preparedness and response units; public health units; regulatory agencies; basic research divisions; domestic and foreign policy agencies; and military. |
• Disease burden and health impact. • Non-market and non-economic benefits of vaccines. • Costs relating to vaccine development and delivery. • Long-term benefits to the vaccine development enterprise, including (a) effective combination vaccines and (b) strategies to optimize existing or new production and delivery platforms. • Ability to produce and administer a vaccine promptly for novel threats. • Innovative methods for administration, including self-administration. • Vaccines with long shelf life and ease of storage and management. • Low number of doses and longevity of protection. • Development of tracking systems from manufacturing plant to recipients in the field. • Development of a scientific base for a new vaccine. • Budgetary constraints for new vaccine research and development. • Programmatic and operational aspects of administering new vaccines. • Fitting new vaccines into existing vaccination schedules. • Building harmony among general public, medical community, public health community, research community, manufacturers, and other international partners. • Identification of disease and vaccine candidates that should not be prioritized. |
|
Private Sector: Vaccine and biopharmaceutical industry. |
• Development of desired product profiles that clearly describe target population and subpopulation segments, potential indications, and key product attributes. • Consideration of uncertainty around licensure and identify clinical endpoints that will be used by regulators to assess vaccine efficacy, adjuvants, and key product attributes. • Global need for certain vaccines with volume and price considerations. • Financial burden due to clinical trials and barriers to successful licensing of a vaccine. • Cost of development and projected time to economic return. • Status of competition for a vaccine in developing and developed country markets. |
|
Stakeholders |
Representative Priorities or Interest Areas |
|
Nongovernmental and Other Organizations: International vaccine initiatives, private foundations, and multinational groups. |
• Cost-effectiveness and effective implementation for each vaccine. • Special attention for countries with poor resources. • Logistics of vaccine delivery with a regional and local resolution than a national focus. • Consideration of operational criteria for vaccines—availability of cold chain and trained human resources. • Consideration of public perception of risk and the acceptance of vaccines. • Availability of effective surveillance strategies and technologies. • Alternative methods to prevent the disease. • Availability of sufficient number of doses of quality vaccines for distribution. |
trials performed sequentially. The requirement for sequential trials further extends the development period and cost.
With the newest technologies, however, the hope is that the development of new vaccines can be accelerated. Systems biology and the adaptive design of clinical trials may help reduce development time by allowing more rapid identification of vaccine candidates and making it possible to conduct the exhaustive and monitored Phase I and Phase II trials in parallel (Rappuoli and Aderem, 2011).
Various stakeholders are involved in the development and deployment of vaccines. To better understand the different priorities of the public sector, private sector, and nongovernmental groups, the committee organized information-gathering sessions in meetings I and II. Representative priorities and interest areas are listed in Table 1-1.
It became clear to the committee that in order to create a broad-based decision framework that would be relevant to multiple stakeholders and communities, the committee needed to consider not only the vibrancy of the vaccine enterprise but also the specific needs and interests of these stakeholders. The modeling strategy of the committee is discussed in Chapter 2.