4
Supporting the NIH AIDS Research Program
The success of the NIH AIDS research program depends not only on identifying the most important scientific questions and promising research opportunities and having an effective structure for managing the research effort, but also on adequate levels of high-quality resources to support the research effort. These resources include funds for the research itself, in the form of grants, contracts, and intramural projects, and for research training programs and facilities and equipment grants. They also include NIH's review apparatus for research grant and contract applications and proposals. Finally, NIH's own staff and facilities are important resources, because it takes people in offices and laboratories to plan AIDS research activities, conduct intramural research, award extramural grants and contracts, evaluate results, and determine new areas of research opportunities and needs.
AIDS research funding has expanded greatly, especially during 1986 –1990, and it is slated to increase another 8.7 percent in fiscal year 1991. Some observers point to this expansion as adequate and call for maintenance, if not actual reduction, of AIDS funding levels. Yet the epidemic is still growing and spreading, and it threatens to persist for years to come. Important research advances have been and will continue to be made. Nevertheless, some research areas are underdeveloped, and scientific progress in others calls for an expansion of effort. This situation calls for a careful assessment of the adequacy of AIDS research funding. Among areas of particular concern for NIH are the inadequate facilities and staffing limits that have created an imbalance between the size of programs and the size of the staff to plan, implement, coordinate, and evaluate them. These limits have been eased, but it will take careful planning to bring staffing in line with program requirements. This chapter reviews the status of research funding, grant review resources, and NIH staffing and facilities; assesses their adequacy; and makes recommendations for strengthening NIH support of the AIDS research effort.
FUNDING AIDS RESEARCH
The committee does not believe that increased funding alone is a panacea for all of the problems noted in the NIH research effort. For this reason, the recommendations for increasing the effectiveness of management of the AIDS research program were presented first, in Chapter 2, and issues concerning appropriate balance and coordination among research areas were addressed in Chapter 3, before funding levels were considered. Funding is an input measure of research effort that is only indirectly related to the variables of most interest, which are research output and the quality and significance of that output. NIH's primary mechanism for assuring research quality, the
peer-review system for ranking research applications, is discussed in a later section of this chapter. If not a sufficient condition for high-quality research results, however, adequate funding surely is a necessary one, especially in a completely new area of research that lacks an existing body of researchers, ongoing studies, appropriate facilities and equipment, and training programs. This section reviews the history of AIDS research funding at NIH, in total and by institute, mechanism, and category of research; it also assesses its adequacy, its impact on non-AIDS research funding and progress, and the appropriateness of its allocation among categories of research, types of research support, and research mechanisms.
History of AIDS Funding
NIH and Its Institutes
The AIDS epidemic was first recognized in early 1981, a time of severe fiscal stringency in the federal budget that constrained the initial federal response (Office of Technology Assessment, 1985; Lee and Arno, 1986; Panem, 1988) and continues to affect federal action. The NIH budget for fiscal year 1981 was $3.57 billion, just 4.2 percent higher than the previous year's, and it increased only 2 percent–to $3.64 billion–in fiscal year 1982. After inflation, NIH's research purchasing power, using the biomedical research and development price index, actually declined by 5.6 percent in 1981 and by 6.1 percent in 1982, regaining its 1980 level only in 1984 (NIH, 1989a:Table 7). Yet despite the constraints on funding, some researchers in NIH's intramural programs and extramural projects and centers found AIDS to be an urgent medical problem, as well as an interesting scientific puzzle, and they began to study it.
The syndrome was first recognized and described by NIH grantees in mid-1981, and the first AIDS patient was admitted to the NIH clinical center in September 1981. Before the end of that year, NCI viral epidemiologists began studies; NCI held a national conference on Kaposi's sarcoma and AIDS-related opportunistic infections; general clinical research centers supported by the National Center for Research Resources became involved in AIDS studies; and NIAID supplemented grants to its extramural sexually transmitted disease centers and other researchers to study AIDS. In the first several years, support for AIDS research had to be reprogrammed from other areas of research. Congress first appropriated additional funding for AIDS research at NIH in a supplemental appropriations bill in July 1983, which provided about $9 million of the $21.7 million that NIH spent on AIDS in fiscal year 1983. The recent substantial growth in AIDS funding started in 1986 when Congress began “earmarking” AIDS funding in the regular appropriations to the institutes (Figure 4.1). As a result, AIDS funding began to increase more quickly than funding for non-AIDS activities (Table 4.1)–between 81 and 111 percent per year in fiscal years 1986 through 1988, and about 25 percent per year in fiscal years 1989 and 1990. In part because of the AIDS funding, NIH's budget went from $3.6 billion in 1981 to $7.6 billion in 1990, an increase of 112 percent. After inflation, the increase was 51 percent (using the gross national product deflator), or 28.8 percent (using the biomedical research and development price index). AIDS funding accounted for 18.5 percent of the overall NIH increase ($741 million of the $4 billion).
AIDS activities have grown steadily as a proportion of NIH spending, from less than 0.1 percent in fiscal year 1982 to 9.8 percent in fiscal year 1990 (Figure 4.2 and Table 4.2).1 Most of
|
1 |
Although the fiscal year 1991 appropriation for AIDS represented an increase of 8.7 percent, the appropriation for non-AIDS research was also larger. Consequently, the AIDS share of the NIH budget dropped slightly, to 9.7 percent. |
the NIH AIDS funding (53.1 percent in fiscal year 1990) goes to NIAID; much of the rest goes to four other institutes: NCI (20.3 percent), National Center for Research Resources (NCRR; 6 percent), NHLBI (5.7 percent), and NICHD (3.6 percent). The remaining 11.3 percent is spread among the remaining NIH units (Table 4.3).
Because it receives more than half the AIDS funding at NIH, nearly half (47.3 percent in fiscal year 1990) of NIAID's overall budget is devoted to AIDS. AIDS funding amounts to 9.1 percent of NCI's budget, 6.1 percent of NICHD's, and less than 4 percent of most other units. It is a larger set of activities for the Fogarty International Center and the units providing intramural and extramural research support (NCRR, Buildings and Facilities, and the OD; Table 4.4). Because of AIDS funding, NIAID went from being the sixth largest institute in 1981 (23 percent the size of NCI, the largest institute at that time) to the third largest institute in 1990 (51 percent the size of NCI, still the largest NIH component).
Research Support Mechanisms
Early in the NIH response to the AIDS epidemic, intramural research accounted for a large proportion of the agency's AIDS effort (47 percent in 1982, 31 percent in 1983, 27 percent in 1984, and 25 percent in 1985; Table 4.5). In comparison, intramural research accounted for only about 12 percent of NIH's non-AIDS budget during that time. The use of contracts was prominent in the early years, reaching 53 percent and 43 percent of the AIDS budget in the 1986–1987 fiscal years when the large extramural programs, such as the AIDS clinical trials units, were being launched. In those years contracts accounted for about 6 percent of the non-AIDS budget (Table 4.6).
The AIDS effort has relied less on grants, especially on research project grants, than has non-AIDS research. The proportion of AIDS funding going to research project grants (RPG) reached a low of 19 percent in 1986, during a time when the proportion of non-AIDS funding for such grants was increasing steadily (from 50 percent in 1982 to 56 percent in 1986). This trend in AIDS funding was reversed in 1987 when the large contracts for ACTUs were converted to cooperative agreements, which are classified as RPGs. In 1990 RPGs account for only 39 percent of the AIDS budget (compared with 59.5 percent of the non-AIDS budget; Table 4.5 and Table 4.62). In addition, compared with non-AIDS programs, relatively more of the AIDS RPG dollars go to RFA-initiated cooperative agreements with ACTUs, national cooperative groups for drug and vaccine development, and other large programs in which NIH staff play a role in decision making. About 60 percent of RPGs have been individual investigator-initiated R01s (Table 4.7), but the bulk of RPG funding goes to cooperative agreements (U01s) and research project grants (P01s), most of which are solicited by NIH through RFAs. This is because most of the non-R01 grants are very large. U01s for AIDS clinical trials units and national cooperative drug and vaccine discovery groups, for example, are in the range of $0.5 to $1 million. As Table 4.7 shows, however, the proportion of research grants solicited by RFAs has decreased.
NIH's standard planning mode has been, first, to let the public, through Congress, indicate broad priorities among health problems by appropriating a certain amount for each categorical entity of NIH (institutes, centers, and divisions), and, second, to allow NIH and the scientific community to identify the research efforts that are needed to address priority problems. These
|
2 |
These figures refer to overall AIDS expenditures, including extramural research, intramural research, and program support activities. In 1990 RPGs accounted for 51 percent of the AIDS budget for extramural grants and contracts, compared with 71 percent of the non-AIDS extramural budget. |
efforts rely on grants, especially individual investigator-initiated grants, as the mechanism of support, and on the peer-review system, in which research applications are rated for scientific merit by disciplinary study sections of experts. Through these processes NIH supports a high proportion of basic research initiated by individual investigators as well as more directed efforts to apply the results of basic research in clinical practice and public health prevention and control programs. Thus, in fiscal year 1990, more than 60 percent of NIH's non-AIDS budget supported basic research (Figure 4.3), and 59.5 percent of the non-AIDS budget supported investigator-initiated grants (research project grants) rather than research centers, contracts, intramural research, or other research mechanisms (Table 4.6). Some of the larger institutes with a more explicit disease focus, such as NCI and NHLBI, have substantial applied efforts (e.g., drug screening and development, clinical trials) and prevention and control programs (antismoking, cholesterol and blood pressure control), but they still devote half of their resources to basic research.
NIH's emphasis on basic research and its traditional posture of waiting for high-quality research proposals turned out to be too slow in the case of AIDS. As public and congressional pressure mounted in the mid-1980s to expand AIDS research, NIH pursued several routes: it quickly expanded its intramural efforts and, extramurally, expedited grant review, used RFAs and RFPs to stimulate research in specific areas, funded some applications with relatively lower peer-review scores, and used directive mechanisms (e.g., cooperative agreements, contracts) to support specific approaches. It is for these reasons that less of the NIH AIDS budget than the non-AIDS budget goes to research project grants. The share of AIDS research funds for basic research is also comparatively lower, although the exact figure is unknown. The large-scale programs initiated through RFAs and supported by cooperative agreements–AIDS clinical trials units, the national cooperative drug and vaccine development groups, and so forth–are classified as research project grants; on the other hand, some fundamental research in immunology and virology of relevance to AIDS research is funded through the non-AIDS budget. Although the share of the AIDS budget going to research and development contracts has been greatly reduced (down from 53 percent in 1986), it still accounts for more than 26 percent of the AIDS budget in 1990 (compared with 5.5 percent of the non-AIDS budget3). Reliance in the AIDS program on intramural research is also higher –16.5 percent of AIDS funding compared with 10.8 percent of non-AIDS funding–although this figure includes a substantial clinical treatment program mandated by Congress.
Categories of Research
NIH has used several sets of research categories for tracking AIDS funding over the course of the epidemic. Currently, NIH uses the so-called Mason categories, which are also used by the PHS to track AIDS activities in all its agencies (see Table 4.8 for a breakdown of NIH spending in fiscal years 1989–1991). Yet none of the sets of functional categories used to report the content of the PHS AIDS program have been especially suited to a scientific characterization of NIH's program. It is not possible to determine how much of the AIDS budget goes to such basic science areas as immunology, virology, molecular biology, and microbiology, or to categories of biomedical research, such as epidemiology and natural history, etiology, pathogenesis, therapeutics, and vaccine development. From 1984 to 1989, NIH used the Charlottesville functional categories, which came closest to those used by the PHS; the agency used projected rather than actual data for 1989 and 1990, however (Table 4.9). The committee was unable to obtain a parallel breakdown of non-AIDS categories for comparison.
|
3 |
Looking just at the extramural part of the budget, 35 percent of the AIDS extramural budget went for contracts, compared with 7 percent of the non-AIDS extramural budget. |
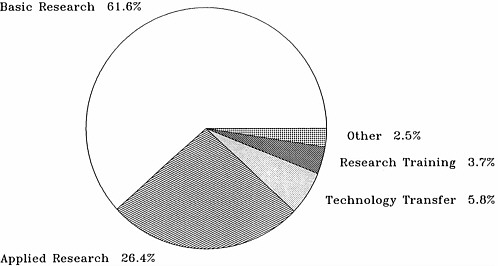
Figure 4.4a, based on data in Table 4.9, shows that research on pathogenesis and clinical manifestations and therapeutic studies (preclinical drug and vaccine development and clinical trials research) have dominated AIDS funding at NIH. Figure 4.4b, which presents the same data but as a percentage of total funding, reveals that budget allocations among the broad categories of research have been relatively stable since 1988, with about 40 percent of the effort devoted to the development of therapies ($305.8 million in fiscal year 1990), a third to epidemiology and pathogenesis ($254 million), 10 percent to vaccine development and testing ($83.9 million), and about 3 percent to public health-oriented activities, especially public information, blood supply protection, and HIV test development ($21.1 million). Breakdowns of the therapeutics category into preclinical and clinical phases or into anti-HIV and other AIDS drugs are not available. The approximately 33 percent for pathogenesis and clinical manifestations breaks down into about 13 percent epidemiology (natural history and surveillance) and 20 percent pathogenesis.
By way of comparison, NCI obligations for preclinical and clinical treatment research accounted for 33.5 percent of NCI's budget in fiscal year 1990 ($548 million of $1.6 billion; NCI, 1990:16). Epidemiology accounted for 5.7 percent ($93.2 million) and cancer prevention and control for 4.9 percent ($80.2 million; NCI, 1990:19).
Adequacy
A major part of the committee's charge was to consider the adequacy of the AIDS research effort at NIH. As noted earlier, the AIDS program has become the third largest NIH research program, after cancer and heart disease, involving nearly 1,200 staff (out of NIH's total employment of 14,000 FTEs in fiscal year 1991) and nearly 10 percent of the total NIH budget. Indeed, federal spending on HIV/AIDS research is approaching that for cancer and heart disease and exceeds spending for other diseases that cause more deaths–cerebrovascular disease and chronic obstructive pulmonary disease, for example (Winkenwerder et al., 1989). HIV is not a chronic disease, however; it is a fatal infectious disease that will continue to spread unless steps are taken to prevent transmission. The effectiveness of prevention efforts depends in turn on behavioral and biomedical knowledge about how the virus spreads and causes disease. Moreover, because deaths from AIDS primarily affect young persons, the ratio of research spending to burden of illness looks more equal when years of potential life lost before age 65 are used as a comparison measure. In 1987 the YPLL for AIDS was 432,000, compared with 1.8 million for cancer, 1.5 million for heart disease, 246,000 for stroke, and 131,000 for bronchitis and emphysema (CDC, 1990:10). Unlike the rates for chronic diseases, however, the YPLL for HIV/AIDS will increase steeply, as the number of deaths per year continues to grow, to between 1.2 and 1.4 million in 1991 and between 1.5 and 2.1 million in 1993 (Buehler, 1990). The PHS estimates that, by 1991, HIV/AIDS could rank third in YPLL from disease (PHS, 1990b).
The committee reviewed carefully the size and composition of the budget for AIDS-related research, aware of a general perception that the problem is receding and that the current level of effort is adequate. The committee finds, however, that the epidemic of HIV infection and AIDS is a severe global public health emergency that is growing and spreading, increasing still further the burden of illness and death and placing severe stresses on the nation's health care system. Containing this disease must be a high national priority. More effective interventions are urgently needed.
The NIH AIDS program must respond in a balanced way to gaps in needed knowledge, emerging scientific opportunities, changes in the epidemic (for example, as the virus moves into new populations or responds to improved treatments), and other, unforeseen contingencies. The
question of program balance is thus an evolving one that should be addressed through the planning and priority-setting process recommended in Chapter 2. At this time, for instance, promising scientific developments in vaccine research urge additional research, which will require substantially more resources than the $79 million devoted to it last year (fiscal year 1990).
The committee reviewed the adequacy of investment in fiscal year 19904 in each program area discussed in Chapter 3, taking into account (1) the state of current knowledge, (2) scientific opportunities in each area, and (3) the overall balance desired in a comprehensive long-term research program. As noted above, recent advances in vaccine research should be exploited, which not only calls for increased support of research grants but also of such research resources as reagents, research animals, and animal and laboratory facilities and equipment. The committee also concluded that a balanced long-range program should invest more in undirected individual investigator-initiated research, given the lack of fundamental knowledge about HIV, its transmission and pathogenesis, and clinical manifestations in and immune response of the host. A number of unfunded scientific opportunities exist. Although about 37 percent of the research grant applications are rated as outstanding or excellent by scientific peer-review groups, 5 funding will be available for only about 25 percent of the nearly 1,000 AIDS grant applications expected to be approved for fiscal year 1991. Progress in treating and preventing HIV infection and AIDS would probably be accelerated if these highly rated projects were funded and all awarded grants–new, renewal, and continuing–were funded fully.
Other areas of AIDS-related research are relatively underdeveloped and should be expanded. The committee believes that behavioral research, nursing research, development and testing of therapies for AIDS-related opportunistic infections and cancers, and research training are examples of fields that have received relatively little support and deserve a much greater investment by NIH as part of a long-range effort to reduce HIV infection and deal with its consequences. The committee has recommended that about 3 percent of the AIDS budget go to the support of research training, triple the current level. Small, beginning programs such as behavioral research and nursing research on patient care will require large percentage increases for several years to reach an adequate level of effort. NIH has placed increased emphasis on OI drug development and testing in the last two years, and further increases are needed. Also of importance is a balanced emphasis on training and facilities as well as on research project funding (IOM/NAS, 1990). Training was addressed in Chapter 3; greater attention should also be paid to maintaining the other aspects of the research program–facilities and equipment–that make good research possible.
There are also possibilities of greater efficiency in some of the large-scale programs that have been running for at least several years, which could result either in greater effort for the same budget or in freeing up dollars for other, higher-priority programs. The committee has recommended, for example, that epidemiology studies be evaluated to ensure that each is worthwhile, given potential alternative uses of the funds at this time. The committee is also aware that NIH expects to improve the performance of the ACTG within its current budget, in part by
|
4 |
1990 is used as the base because it is the last year for which there are figures on actual program obligations, by funding mechanism, by institute and program, and by functional area. The fiscal year 1991 budget was under consideration during the time of the study. The administration had asked for $800.2 million for AIDS research, an increase of 8.1 percent (2.1 percent after inflation using the biomedical research and development price index). On October 20, 1990, after the last committee meeting, Congress appropriated an overall increase of $700 million for NIH as a whole, compared with the $354 million requested by the administration. At the time this report went to press in late December 1990, NIH planned to use about $4 million of the additional increase for AIDS, for a total AIDS budget of $804.6 million in fiscal year 1991, an increase of 8.7 percent (2.7 percent after inflation). |
|
5 |
Applications with scores between 100 and 150 are considered “outstanding”; those between 150 and 200 are considered “excellent” (see NIH, 1989b). |
redefining its mission to focus on studies that are not likely to be undertaken by the private sector but also by increasing efficiencies in protocol development, laboratory services, and patient accrual and retention.
In the opinion of the committee, increased management efforts and program activity in a number of areas would not be adequately accommodated within the present level of effort ($740.5 million in 1990 dollars). 6 The committee estimated that the net effect of its recommendations could increase costs on the order of magnitude of 25 percent over the current level of effort. This figure is admittedly a very rough estimate; it would be less if there were significant savings in existing activities and more if there were major breakthroughs that needed to be exploited. The committee believes that an increase of this magnitude could be productively absorbed at once in most areas, although some underdeveloped areas may take several years to build up. The detailed phasing in of any increases that occur should be an integral part of the long-range planning effort recommended in Chapter 2.
Many people believe that the budget for NIH as a whole is inadequate and that there is an immediate crisis in funding a sufficient number of competing grants this fiscal year and next to maintain the nation 's biomedical research momentum (NAS/IOM, 1990). The committee is acutely aware that many other areas of biomedical research could justify larger budgets in an absolute sense. Advances in containing and controlling HIV infection and AIDS rest on the overall strength of the institutes of NIH. Taking resources from its other components to expand the AIDS research program would impede progress in biomedical research and the AIDS program itself, which is an integral part of NIH and dependent on a wide range of its activities.
Recommendation 4.1: Implementing the long-term AIDS research program recommended by this committee will require a larger budget to ensure that the most promising basic science opportunities are supported, that underdeveloped areas of research are expanded, and that research resources are adequate to support the planned level of research effort. These opportunities and needs could justify an immediate increase of as much as 25 percent in NIH's budget for AIDS research; the exact timing of the increase should be an integral part of the long-range plan recommended by the committee. It is essential that any such budget increases be new funds and that they not be derived at the expense of ongoing NIH programs.
Impact on Non-AIDS Research
It is impossible to know what NIH's research budget would be today if AIDS had never happened. Although it is very clear that NIH appropriations for AIDS grew much more quickly than non-AIDS research, it does not necessarily follow that non-AIDS research funding suffered because of the increases in AIDS monies. In comparison with funding for the research programs of other non-defense agencies, NIH has done comparatively well, achieving real growth most years except in 1982 and 1986 (Figure 4.5). A definite benefit for the slower-growing non-AIDS programs is that AIDS funds have supported some immunology, molecular biology, and other basic research that might otherwise not have been possible, although, as already noted, the share of the AIDS budget going to basic research is relatively small.
|
6 |
It would take $783.5 million to sustain this level of expenditures in fiscal year 1991 after inflation (using the biomedical research and development price index). |
Interviews at NIH and on Capitol Hill indicate that from at least 1985 through fiscal year 1989, the AIDS budget was considered separately from the non-AIDS budget at NIH, and there is little reason to believe that the funds appropriated to AIDS would have gone to non-AIDS activities if there had not been a separate AIDS budget. Today, however, the situation is changing. A perception at the appropriations committee level that AIDS and non-AIDS funding shares were out of balance and that AIDS research should compete with non-AIDS studies in priority setting at the institutes resulted in no formal AIDS earmark in fiscal year 1990 for the first time since 1984. Although the institutes are expected to maintain the detailed budgets submitted in the congressional justification, including those for AIDS, lack of an earmark means that “the precise amount expended is determined by the institutes based on the quality of applications submitted and competing research priorities,” according to the House appropriations committee in its report (U.S. Congress, 1989:22–23). “This process relies on the judgment of the peer review system and scientific advisory boards which are the backbone of NIH's quality control system. Use of this process could result in a somewhat higher or lower final figure for AIDS. ”
During fiscal year 1990 NIH basically allocated funding according to the amounts in its original budget submission but used the flexibility of not being restricted to a certain budget amount for AIDS to include closely related basic research in immunology and microbiology in the AIDS program (U.S. Congress, 1990a:61, 1156). The House appropriations committee report on the 1991 appropriations bill stated the committee 's preference of continuing to suggest an approximate target figure for AIDS activities in the report stage rather than setting a precise amount in statutory language (U.S. Congress, 1990c:25).7
The committee believes that the basic knowledge base for understanding and controlling AIDS is inadequate and has already recommended an expansion of basic research as part of a balanced long-term AIDS research program. In the past, NIH has defined AIDS research narrowly to encourage well-established researchers to shift emphasis from ongoing research in other areas. This goal has been met, and the artificial distinction between AIDS research and AIDS-related basic research has outlived its usefulness.
Recommendation 4.2 NIH should adopt NIAID's recent redefinition of AIDS research (to include closely related basic research in immunology, virology, molecular biology, cellular biology, and other related areas) for use throughout its institutes.
Spillover Effects of AIDS Research on Non-AIDS Efforts
AIDS research has depended heavily on earlier national investments in studies in such fields as retrovirology, cellular immunology, clinical trials, and infectious disease epidemiology. Indeed, without this earlier research, the progress already experienced in identifying and characterizing the causal agent of AIDS and in developing several efficacious therapies would have been impossible. For example, the “War on Cancer” in the 1970s supported a greatly expanded research program on the virology and immunology of retroviruses. When it was suspected that AIDS was a retrovirus, the investigators and facilities involved in work on retroviruses were quickly mobilized to work on HIV, which allowed scientists in only a short time to learn a great deal about the virus and how it causes disease. In addition, the techniques developed to screen for anticancer drugs were used for anti-HIV drugs, which is how zidovudine, or AZT, was originally identified.
|
7 |
Subsequently, the December 1990 conference report on NIH funding for fiscal year 1991 did not contain specific earmarks for AIDS. |
Conversely, the intense scientific work on HIV and the disease it causes has contributed important new information to the basic and clinical research knowledge bases. Investigation of the molecular biology of HIV will soon make it the best understood of all retroviruses, and that knowledge can add understanding to work on viruses in general and on other retroviruses in particular. The discovery that CD4 is the receptor for HIV has contributed to research progress on the interaction of viruses with their specific receptors. A major effort is under way to understand the molecular controls that determine the level and timing of viral replication. These studies will provide important insights into the control of latency and of replication of other viruses, as well as better understanding of the control of normal cellular genes. In addition, as a result of AIDS research, the CD4 molecule has been cloned and sequenced and its crystal structure is under analysis, which will add to knowledge about the role of CD4 in the function of the immune system.
In general, AIDS studies are making important contributions to resolving basic problems in immunology. Not surprisingly, a survey of scientists and clinicians by the congressional Office of Technology Assessment (OTA) found that most of those interviewed believed that federally funded research on HIV/AIDS had already contributed substantially to the basic science fields of virology, immunology, microbiology, and molecular biology. Specialists in a number of clinical medicine areas, especially the disciplines of infectious disease, oncology, neurology, hematology, and pulmonary medicine, reported substantial contributions of HIV/AIDS research to their areas as well. Experts in drug and vaccine development, diagnostics, epidemiology, and behavioral sciences also cited substantial benefits from AIDS/HIV research (OTA, 1990:7–12).
GRANTS POLICY AND ADMINISTRATION
One of NIH's key resources is a large-scale process for identifying high-quality research ideas and productive investigators worthy of funding support. NIH's grant review system maintains a pipeline of research proposals deemed to have high scientific merit by other scientists. At the beginning of the epidemic, AIDS, as a new disease, did not have a community of dedicated researchers or such a pipeline. Consequently, NIH resorted to ad hoc arrangements to expedite the review and award of AIDS research proposals before developing a permanent set of review groups and procedures.
Problems in Responding to the AIDS Epidemic
Under NIH's grant review process, an investigator proposes a well-designed research project that addresses an important scientific question. Review and, if the application is successful, approval occur in two stages. A scientific peer-review group or study section conducts an initial review of the application and decides whether to approve and recommend it for funding; the group then scores it on a scale from 100 (outstanding) to 500 (acceptable; NIH, 1989b:11). The application then goes to the appropriate institute or institutes for review and approval for funding by the institute's national advisory council. Most institutes fund a few applications with scores below the cutoff point (the lowest score normally funded) to address areas of “high program relevance.” If an institute determines more research is needed in an area, it can use a variety of devices to stimulate investigator interest and applications, ranging from workshops to program announcements (stating the institute's interest in receiving applications on a particular topic) to RFAs that state the number of grants and level of funding the institute will devote to that set of applications. If an institute has a strong programmatic interest in a particular area of study or type of research, it can offer program project or center grants to support larger-scale research efforts,
or it can use a cooperative agreement. In these latter cases, and in the case of most RFAs, institutes rather than Division of Research Grants (DRG) groups review the applications.
Funding extramural research usually takes nine months from receipt of a grant application to award, but the process can take longer if, as with AIDS in the early years of the epidemic, the research area is new and investigators must be encouraged to apply. In those first years NIH was criticized for neglecting extramural research on AIDS, for taking too long to review research proposals, and for funding low-quality applications (OTA, 1985:41). For example, NCI, after cosponsoring with CDC a national conference on Kaposi's sarcoma and opportunistic infections in September 1981, began to develop an RFA for studies of AIDS. It was nearly a year before the RFA was issued in August 1982. At that time, however, NIH took various steps to expedite the review and award process. A large ad hoc review committee was formed, and mail ballots were used to make the first awards beginning in March 1983 (Stoolmiller, 1990). NIH also expedited extramural research by supplementing ongoing grants for research on sexually transmitted diseases (through NIAID) and Kaposi's sarcoma (through NCI). Subsequently, AIDS research grant applications were reviewed by the DRG in the usual manner–by regular chartered study sections or by ad hoc review study sections–although arrangements were often made to add ad hoc members with AIDS expertise to regular study sections (Maurer, 1990).
As noted earlier, NIH in those years was criticized for funding studies with relatively poor peer-review scores (see Table 4.10; OTA, 1985:42), a problem that has continued to be a concern of the scientific community. These concerns have not been mitigated by the fact that institute review groups of AIDS experts rather than disciplinary DRG study sections review many AIDS grant applications. Concerns remain because some of the studies are large, complicated projects, often solicited by the institute with an RFA, and funded through cooperative agreements, which involve institute staff in the direction of a project.
By 1986 NIAID's AIDS grant application review workload had become so large that the institute chartered a 51-member AIDS research review committee. Its work was carried out by four subcommittees: (1) basic research I (immunology); (2) basic research II (virology); (3) clinical applications, prevention, and treatment; and (4) epidemiology and technology transfer (NIH, 1990b:38). Meanwhile, DRG continued to review individual investigator-initiated grant applications.
By 1987 AIDS applications in the areas of virology and immunology were overloading the DRG study sections to which they were assigned. In response NIH established a special review committee, Special Study Section A, to handle AIDS virology and immunology applications. This arrangement, which was first used for the January 1987 round of grant reviews (Maurer, 1990), remained in place until the initiation of the expedited review of all AIDS research grant applications that began with the February/March 1988 receipt deadline. Until then, other nonvirology, nonimmunology AIDS applications were reviewed by regular chartered study sections.
The Current System
Division of Research Grants Capacity
Because of concerns about the workload and speed of AIDS grant review, in 1988 Congress began to appropriate funds designated for staff positions (FTEs) for grant review work; it also mandated an expedited review process in which reviews and awards were to be made within six (rather than nine) months of receipt of the grant application. By this time, the number and quality of unsolicited applications had begun to improve. At NIAID, for example, from fiscal year 1987
to fiscal year 1988, the number of solicited applications (e.g., stimulated by an RFA) dropped from 250 to 130, and the number of unsolicited applications increased from 150 to more than 400. As a result, the ratio of solicited to unsolicited grant awards dropped from more than 2 to 0.5, and most of the solicited awards were for the large NIAID AIDS programs–for example, the national cooperative drug discovery groups, the AIDS research centers, Programs of Excellence for Basic Research in AIDS, and AIDS clinical trial units. The priority scores of unsolicited grants improved, whereas the number of solicited grant awards with priority scores greater than 175 fell sharply (NIH, 1989a).
With the advent of the six-month expedited award policy, DRG designated separate dates for the receipt of AIDS proposals, and AIDS grant review sections proliferated, from three in 1988 (immunology, virology, epidemiology/behavior) to five in 1989 (sections for preclinical drug discovery/development and clinical research were added). In 1990, seven sections were formally chartered (the epidemiology and behavior section was split in two and neuroscience was pulled out of the clinical section). By the January 1990 review round, the study sections were averaging about 50 applications (from 40 to 70), compared with the 75 or 80 handled by the regular virology and immunology sections during each round (Meier, 1990). DRG's successful management of this process indicates that it has the capacity to handle an increased number of basic science and other individual investigator-initiated grants as recommended by this committee.
Quality of AIDS Research Applications
As noted earlier, the average priority scores and priority score distributions for AIDS and non-AIDS grants began to converge by fiscal year 1988. In fiscal year 1989, priority scores at the 50th percentile for AIDS and non-AIDS grant applications and awards were comparable for both individual investigator-initiated grants (R01s) and for all research project grants (including R01s; Table 4.11); this was true for each institute (Figure 4.6). The distribution of priority scores for AIDS and non-AIDS grant applications–as measured by the mean priority score in each decile, for example (Table 4.12)–is also similar.
Success of AIDS Research Applications
Until recently AIDS grants have had higher award rates (the percentage of approved applications that are funded) than non-AIDS grants. The award rate for all AIDS research project grants was 34 percent in fiscal year 1989, compared with 27 percent for non-AIDS grants ( Table 4.13); by fiscal year 1991 both will be about 25 percent.
Conclusion
NIH was not well prepared at first to speed the review and award of grants to meet the urgency of the epidemic of HIV infection and AIDS. Review and award procedures were expedited but on an ad hoc basis, and the strain on already busy DRG and institute staff was heavy. Initially, NIH relied on contracts and then on RFA-solicited cooperative agreements and program project grants rather than on traditional investigator-initiated grants to launch studies quickly and attract productive researchers. High-quality proposals were scarce, and some awards went to applications with relatively poor peer-review ratings.
Over the past four years, AIDS research project grant applications and awards have increased in number and improved in quality, as measured by peer-review scores. The distribution of priority scores for AIDS applications has become quite similar to that for non-AIDS applications; the average scores of funded applications are also similar. The share of the AIDS budget allocated to research project grants has increased, from a low of 19 percent in 1986 to about 40 percent currently. This share is still smaller than in other NIH-supported research areas, however, where research project grants constitute nearly 60 percent of the budget. The proportion of research project grant awards solicited by RFAs has decreased relative to the number of individual investigator-initiated grants. The committee recognizes the need for solicited research and directive mechanisms in building a fast response to a public health emergency but believes that greater reliance on grants, especially individual investigator-initiated grants, is warranted in a long-range research program on AIDS.
Recommendation 4.3: NIH should continue to increase its use of research grants, especially traditional individual investigator-initiated and related grant mechanisms, to carry out the expanded research effort recommended by the committee, in particular, the increased effort in basic research.
At NIH, RFAs and RFPs are usually initiated “from the bottom up,” that is, by program staff who identify gaps in research and propose grant solicitations to address those gaps. After approval at the institute and council levels, proposed RFAs and RFPs receive an administrative review in the Office of the NIH Director and are circulated to the other institutes for comment to minimize duplication of effort. The committee believes that central review of RFAs and RFPs should be coordinated with the research planning effort recommended in Chapter 2 to identify and remedy gaps in AIDS research.
Recommendation 4.4: The NIH associate director for AIDS research and the AIDS Program Advisory Committee should review all RFAs and RFPs for AIDS research to ensure coordination and avoid duplication. They should also have the authority to recommend RFAs and RFPs in the case of gaps in the NIH AIDS research program that are not being addressed by individual institutes.
ADMINISTRATIVE SUPPORT
The effectiveness and success of the NIH AIDS research program depend in part on the adequacy of the administrative support the program receives in the form of staffing and facilities, a statement that applies to both the extramural and intramural programs. Like the funding of research projects, more personnel and facilities may not be sufficient to guarantee high-quality, productive research or swift scientific progress, but a minimum of such resources is necessary for NIH to carry out its mission. At NIH, these resources are provided through centralized processes somewhat separate from those for planning and funding research projects, which makes it a challenge to ensure that resources are matched with program needs, especially in a program that has grown as quickly as AIDS research.
Staffing
It takes people to award grants and contracts, evaluate the results, and determine new areas of research. It also takes people to staff the intramural research laboratories of the institutes and the research units of the NIH clinical center. Much concern has been expressed about the
adequacy of the number of FTE staff at NIH for both AIDS and non-AIDS research. AIDS FTEs increased from 27 in fiscal year 1982 to 763 in fiscal year 1989 (Table 4.14a and Table 4.14b). The administration proposed 887 AIDS FTEs in the fiscal year 1990 budget, but a year later, after allowing the Department of Health and Human Services and NIH to set staffing levels (within the overall NIH budget), the Office of Management and Budget raised the ceiling for 1990 to 1,072. The administration proposed 1,183 AIDS FTEs in its budget request for fiscal year 1991. Non-AIDS FTEs increased from 12,662 in 1982 to a high of 13,493 in 1984, but fell by 1,188 to 12,305 in 1986. The non-AIDS FTE level reached 12,712 two years later (fiscal year 1988) and was originally slated to decline to 12,327 in fiscal year 1990. The administration later revised the 1990 level upward to 12,707 and is proposing a total of 12,950 non-AIDS FTEs in its budget request for fiscal year 1991.
In 1985, OTA's study of the PHS response to AIDS found that the administration had consistently suggested decreases in personnel ceilings for NIH institutes conducting AIDS research, even as AIDS funding increased. The OTA report concluded that personnel ceilings had been a “special problem” affecting AIDS and non-AIDS research (OTA, 1985:6). In late 1986, an NIH advisory group reported to Congress that there was a “clear-cut need for additional FTEs, both scientific and administrative, at the NIH,” rather than redeployment of existing FTEs from other NIH programs. The advisory group concluded that “the allocation of new monies for AIDS research will not achieve the desired goal without a parallel and proportionate increase in scientific and administrative FTEs” (Ad Hoc Consultants, 1986:29). In 1988, the Presidential Commission on the Human Immunodeficiency Virus Epidemic concluded that OMB was inappropriately trying to micromanage AIDS research at the NIH institute level through staffing ceilings and other regulations. The commission said these practices “prevent the deployment of a sufficient number of researchers to deal with pressing problems,” force an incremental loss of personnel from other NIH research areas, and deny NIH the management flexibility called for in a scientific research effort (Presidential Commission, 1988:41–43). In 1988, the IOM committee to study the NIH intramural program criticized OMB-imposed FTE ceilings for inhibiting effective management: “The overall effect of FTE ceilings that grow more slowly than budgets is that managers who are best placed to make decisions about how to allocate money to fulfill congressional mandates are prevented from making the most productive decisions ” (IOM/NAS, 1988:67).
The NIH AIDS effort has experienced two types of personnel problems that have hampered efforts to recruit and retain staff and thus hindered program managers from making the most effective use of public resources. One type is the traditional set of staffing problems faced by all organizations: having enough money to hire people, being able to find and hire people with the needed skills, motivating people to do their best for the program, and retaining the people who are hired by providing adequate working conditions and a competitive salary structure. These problems also include having an appropriate organizational structure, competent leadership, and a balance between the amount of work to be done and the staff to do it. NIH, however, has also faced a second set of problems caused by external limits on the number of people it can employ. These two types of problems are discussed below.
Traditional Personnel Problems
NIH and its AIDS research program have been constrained by government-wide personnel policies and procedures that have made it difficult for the agency to recruit and retain senior scientists, especially physicians, and some types of support personnel–for example, nurses, allied health workers, and secretaries (IOM/NAS, 1988:3–4; PHS, 1990a:61–62). NIH has not been able to offer competitive salaries for M.D. or Ph.D. senior-level scientists and science administrators or
for mid-level physicians, and there have been significant pay problems with regard to research support personnel (IOM/NAS, 1988:64–65). In addition, personnel hiring procedures make timely appointments quite difficult: in fiscal year 1988, it took an average of 8.5 months to process senior-level appointments at NIH (IOM/NAS, 1988:65). Historically, good scientists have been willing to forego higher pay to enjoy the distinctive research environment at NIH, which they find conducive to scientific productivity and creativity. Others want to participate in an extramural program they believe makes a difference in addressing a major health problem. Increasingly, however, widening pay differentials, cumbersome personnel procedures, and other “barriers to a productive work environment” (IOM/NAS, 1988:68–72), such as inadequate space and equipment (discussed below), threaten NIH's ability to recruit and retain high-quality staff.
These problems have been especially severe for the AIDS program because of its fast growth, long work hours, and intense pressures associated with implementing AIDS research programs with inadequate numbers of staff. For example, congressionally mandated plans to double the AIDS outpatient and inpatient capacity of the NIH clinical center were slowed by problems in recruiting nurses and other support staff. The Division of AIDS at NIAID has experienced major problems in filling high-level positions and recruiting physicians as medical officers in its treatment research program. Staff turnover rates have been high at DAIDS–about 33 percent a year. Thus, NIH has had problems filling positions, and keeping them filled, even when they are made available. The AIDS FTE ceiling for fiscal year 1988 was 544, but NIH finished the year with 537; the ceiling for fiscal year 1989 was 780 positions, of which 763 were filled. Current plans call for an increase of 309 AIDS FTEs in fiscal year 1990, bringing the total to 1,072, and another 111 in fiscal year 1991, for a total of 1,183 slots. Both increases will pose additional challenges for the NIH personnel office.
Several steps have been taken to address personnel problems at NIH generally and for the AIDS program in particular.
-
Legislation passed in July 1986 permits NIH to offer more competitive salaries for nurses and (since September 1988) for 10 allied health personnel categories, a policy that has reduced position vacancies and turnover rates. Thus, the number of nurses at the NIH clinical center increased by 25 percent, vacancy rates decreased from 10 percent in early 1987 to 7 percent in January 1990, and the turnover rate fell from 25 percent in 1986 to 13 percent in 1989 (U.S. Congress, 1990a:93).
-
A pay raise in January 1990 and one scheduled for January 1991 will reduce much of the pay differential for Ph.D. scientists and some of the differential for physician researchers. According to NIH, its physicians in the Senior Executive Service are paid an average of 49 percent less than their peers in U.S. medical schools; NIH Ph.D. scientists average 19 percent less in pay than comparable scientists in research-intensive universities (U.S. Congress, 1990c:698).8
-
Physical working conditions will improve considerably if current construction and renovation plans are carried out (see the discussion later in this chapter).
-
In June 1988 NIH and the Office of Personnel Management (OPM) agreed that OPM would process and certify eligible candidates within 21 days; the Health Omnibus Programs
|
8 |
For example, in 1988 a physician at the highest level of the Senior Executive Service (SES) received $77,500 plus a physician's comparability allowance (PCA) of up to $20,000 plus possible performance bonuses, subject to a total-salary statutory limit of $99,500; the average pay for NIH M.D.s in the SES in 1988 was about $89,000 (IOM/NAS, 1988:84, 100) and $94,400 in 1989 (U.S. Congress, 1989:87). As of January 1991, SES pay levels will increase between 22.2 percent (level 1) and 29.5 percent (level 6; Balz, 1990:A1). If the PCA is reauthorized, a physician scientist at the highest SES level could make up to $128,300 (base pay of $108,300 plus PCA of up to $20,000), as well as any performance bonuses, although a statutory salary cap of $120,000 is likely (U.S. Congress, 1990c:697). Only one NIH employee is at the highest SES level, level 6; most are at level 4 (for which the base pay will increase to $100,500 in January 1991; U.S. Congress, 1990a:64). |
-
Extension Act of 1988 (P.L. 100-607) subsequently granted authority to NIH to request expedited approval of requests for AIDS personnel and administrative support or space. Such requests are deemed to be approved if not denied by the director of OPM or administrator of the General Services Administration within 21 days.
Constraints of Federal Personnel Ceilings
In addition to the more traditional types of staffing issues discussed above, NIH has also faced a second set of problems caused by external limits on the number of people who can be employed. The limits set on the number of FTE positions an agency may support are a peculiarly governmental problem that is not directly related to sufficient funds for the positions or the ability to recruit and retain qualified staff. OMB sets overall FTE ceilings for each federal department and independent agency. The Department of Health and Human Services (DHHS) then allocates positions under its ceiling to the Public Health Service, which in turn subdivides its allocation among the various PHS agencies, including NIH. The NIH positions are allocated among its institutes and other organizational components by the NIH director, based on the recommendations of the Resource Allocation Group (RAG). The RAG is composed of senior staff in the Office of the Director and several institute directors.
The FTE ceilings have caused significant personnel problems for the AIDS research effort in several ways.
-
Although Congress controls appropriations and specifies them in some detail for each NIH component, OMB controls the allocation of FTEs. Until recently at least, OMB was unwilling to raise the NIH FTE ceiling to keep pace with the needs of the AIDS research program.
-
Cuts in its overall FTE ceiling since the early 1980s have left DHHS with little latitude to provide NIH with extra positions. Total FTE employment at DHHS has declined steadily, from 138,480 in fiscal year 1982 to 115,045 in fiscal year 1989. Although the majority of the cuts were in the Social Security Administration, every major component, including the PHS, lost FTEs. FTE employment in the PHS fell from 42,904 FTEs in 1982 to less than 40,000 in 1989.
-
NIH, together with CDC and ADAMHA, has maintained and even increased its FTE employment during the 1980s. Because the FTEs for AIDS research have come from the overall ceiling authorized for NIH, however, increasing AIDS FTEs has reduced the number of personnel available for non-AIDS research. In the early years, when the NIH AIDS effort was mostly intramural, most AIDS FTEs were shifted from other work. In 1986, for example, just 9 of the 235 AIDS FTEs were additions rather than transfers (U.S. Congress, 1988a:351). Since 1986, most but not all increases in AIDS FTEs have been additions. Nevertheless, as the number of FTEs related to AIDS increased from 0 in fiscal year 1981 to 780 in fiscal year 1989, the net number of non-AIDS FTEs declined by 165–from 12,637 to 12,472 (Table 4.15). (The decreases in non-AIDS staffing occurred while NIH's non-AIDS budget grew in constant dollars.)
These personnel constraints have acted to redirect intramural staff from non-AIDS to AIDS research and to hold down the number of staff available to plan, implement, and monitor the extramural AIDS research programs. Initially, NIH institutes responded to the AIDS epidemic with their most flexible resource, the intramural program; FTE ceilings subsequently hampered needed adjustments to compensate for the shifts of intramural staff to AIDS work. This situation can be seen most clearly at NCI. In 1989, half of the 188 FTEs allocated to AIDS research had come from NCI's non-AIDS FTE allocation. That year the institute received 42 additional AIDS FTEs over its 1988 level, but instead of using them to free up staff to return to non-AIDS work, they were assigned to expanded AIDS activities–primarily the large-scale drug screening and development
program initiated in 1988 (NIH, 1989b). Not surprisingly, the institutes are using much of the new flexibility allowed them in fiscal year 1990 to set personnel levels for “a restoration of the previous losses ” (U.S. Congress, 1990a:63).
The constraints on FTE levels and problems in filling available positions were also acute on the extramural side, where funding growth has outstripped the available staff's ability to administer it. This problem has been most apparent at NIAID's Division of AIDS, which administers 60 percent of the extramural AIDS research dollars at NIH. DAIDS was established in January 1986 to develop and manage a large extramural AIDS research program, but it has been chronically understaffed and has had difficulty planning, implementing, and monitoring its many fast-growing activities. Most of its activities are supported by such mechanisms as cooperative agreements and contracts that involve more staff effort to develop, fund, monitor, and evaluate than is normally required to administer a portfolio of traditional individual investigator-initiated grants. For example, DAIDS took over the MACS and launched the multicenter ACTG and NCDDG programs. Funding for these efforts totaled $52.3 million in fiscal year 1986 and was administered by only a 4-person staff. In 1987, with a staff of 13, DAIDS expanded the ACTG greatly, added more NCDDGs, developed the NCVDG program, and put out a number of animal-model and other contracts totaling $129.6 million; in 1988 it launched additional programs, studies, clinical trials, and associated contracts for total expenditures of $203.2 million with 42 FTEs. To accomplish the work, 80-hour work weeks were typical–as was staff burnout and turnover of 33 percent a year.
In response to a congressional hearing on AIDS research needs, NIAID estimated it would need 116 more FTEs through fiscal year 1989, 63 for extramural research management and support (DAIDS), 40 for expedited review and award of AIDS research grants and contracts, and 13 for intramural research. Its projections also included a need for additional FTEs in fiscal years 1990 and 1991 (U.S. Congress, 1988a:329).9 Internally, NIAID was arguing that it was not receiving enough additional FTEs to manage its AIDS research activities, especially the ACTG program and the vaccine research and testing effort. Although it received an increase of 74 FTEs (for a total of 232) in fiscal year 1989, the institute estimated that it was 83 FTEs short of meeting its needs (NIH, 1989a). NIAID was given a further 31 FTEs (for a total of 263) in fiscal year 1990, but expanded activities as well as new responsibilities imposed by the Health Omnibus Extension Act of 1988 led NIAID to estimate that it was still 89 FTEs short at the beginning of the 1990 fiscal year (NIH, 1990a).
Faced with a health emergency, the small staff of DAIDS worked long hours to develop and issue RFAs and RFPs, usher them through the review and award process, help successful applicants set up and begin work on their projects, and coordinate the activities of multiple sites. The heavy workload took a heavy toll on staff members, however, at the same time causing delays and other implementation problems for the research projects.
OMB and DHHS recently gave the NIH director increased authority to determine NIH staffing ceilings, which will allow the agency to bring its staffing levels into line with program requirements. As a result of more realistic FTE planning, NIH increased substantially NIAID 's allocation for fiscal year 1990 in January 1990, from 263 to 306 (3 more slots than were requested
|
9 |
Overall, NIH estimated it would need 955 FTEs in fiscal year 1989 (it was actually allocated 780), 1,143 in 1990 (the original allocation of 887 was increased to 1,072 in January 1990), and 1,311 in 1991 (the administration proposed 1,183 in the 1991 budget request for NIH; U.S. Congress, 1988a:326). |
by the institute in its initial budget request for the 1990 fiscal year). The DAIDS ceiling increased from 95 to 115. The overall 1990 FTE ceiling for NIH, originally 38 FTEs smaller than in 1989, increased 527 FTEs; an additional increase of 354 positions has been requested for fiscal year 1991. A majority (292 of 527) of the additional FTEs in fiscal year 1990 are for AIDS research; a third (111 of 354) of the additional FTEs proposed for fiscal year 1991 would be for AIDS. These increases must be achieved, however, within NIH's overall budget total because NIH is not slated to receive additional funds for personnel and must pay for staffing increases out of program funds. Determining appropriate staffing levels for all NIH programs will require careful analysis of program needs and difficult choices. It will also require closer integration of program planning, budgeting, and personnel planning in the future.
In conclusion, OMB's imposition of arbitrary personnel ceilings for NIH as a whole has constrained the AIDS research program because the number of scientists and science administrators could not increase as quickly as the scientific opportunities for intramural AIDS studies or the number of extramural AIDS grants and contracts that had to be reviewed, awarded, and managed. This constraint in turn hampered NIH's ability to conduct AIDS research and adequately plan, administer, and evaluate its extramural AIDS programs.
Recommendation 4.5: The committee strongly opposes arbitrary restrictions on NIH staffing levels that are established without regard to program requirements because they hamper effective, efficient management Personnel ceilings should be abandoned permanently, and future staffing decisions should be part of the strengthened program planning and budgeting processes as recommended in Chapter 2 and coordinated by the Office of the NIH Director. Adjustments in staffing levels should be made carefully over several years to achieve appropriate balances between AIDS and non-AIDS programs, between the elimination of past deficits and the needs of new initiatives, and between the budgets for extramural grants and contracts and for staff to administer those grants and contracts.
The committee also supports broader efforts to maintain the excellence of the NIH staff by addressing personnel problems relating to compensation and to inflexible or cumbersome policies and procedures of government personnel systems. It endorses as well special efforts to resolve problems specific to the AIDS program, such as recruitment and retention of medical officers in the NIH AIDS treatment research (clinical trials) program.
Facilities
Over the years, NIH more than once has delayed construction of new facilities and maintenance and structural improvements in old buildings in the face of constrained budgets (U.S. Congress, 1990c:7). The resulting problems with inadequate space and deteriorated and obsolete facilities have hindered the agency's research efforts and added to problems in recruiting and retaining high-quality staff. Indeed, the IOM committee studying the NIH intramural program found that space was inadequate for a number of institutes, that facilities had deteriorated in recent years, and that scheduled improvements had been delayed (e.g., renovations of the six oldest laboratory buildings that were scheduled for completion in 1991 are now projected to take until 1997; IOM/NAS, 1988:69). Facilities problems have become quite serious in terms of obsolete laboratory space, deteriorated heating and cooling systems, substandard research animal facilities, and unsafe patient and research areas, as well as sheer lack of space for AIDS and other growing research areas.
These NIH-wide space limitations and inadequacies have affected the AIDS research program disproportionately because it is a new, fast-growing set of activities. Also, because of the general delays in renovating, modernizing, and expanding laboratory facilities on campus and in constructing a consolidated office building for 2,700 extramural program administrators and support staff now located outside NIH 's main Bethesda campus, much of the AIDS space is scattered in off-campus sites. These dispersed locations hamper communication and collaboration between AIDS basic and clinical researchers and between AIDS and non-AIDS researchers involved in related studies; they also impede coordination among administrators of extramural AIDS and AIDS-related research programs in the different institutes, centers, and divisions.
To address these problems, NIH is preparing a comprehensive facilities plan for Congress and has made facilities modernization and improvement a high priority in its 1991 budget request. According to acting NIH director William Raub, NIH requires a building program that could cost up to $1 billion over the next 10 years (U.S. Congress, 1990a:51). The agency's 1991 budget request includes $88.6 million for buildings and facilities, including $16.5 million for AIDS facilities.10
The need for more–and more appropriate–facilities specifically for AIDS work was acutely apparent in early 1988 when NIH director James Wyngaarden and NIH AIDS coordinator Anthony Fauci testified before several congressional committees (U.S. Congress, 1988a:259, 1988c:331). Their concerns were echoed in the June 1988 report of the Presidential Commission on the HIV Epidemic. The commission noted that plans for AIDS office and lab space were seriously delayed, and recommended that intramural construction and instrumentation needs be assessed and made a high priority in future budget requests. They also called for expedited approval of the proposed consolidated office building (Presidential Commission, 1988:40–41, 44). In 1989, NIH outlined its AIDS facilities problems, noting that rapid scientific developments might require adjustments in these priorities:
The most critical concerns at the NIH are the provision of high-level biocontainment facilities to enhance safety in the workplace; the consolidation of currently dispersed laboratories to improve communications and efficiency among the many scientists involved in the program; the alleviation in scientific areas of overcrowding; the creation of a modern, consolidated facility for the primates so essential in vaccine and drug development; and the accommodation of increasing numbers of inpatients requiring intensive care. (U.S. Congress, 1990b:1285)
In the past several years, Congress has appropriated funds for AIDS research and office space that have greatly reduced the backlog of needs. Congress appropriated $19.2 million for AIDS buildings and facilities in fiscal year 1988 to build a three-story addition to the clinical center for lab facilities and offices, to lease and renovate space for AIDS labs and animal facilities off campus (the Twinbrook II facility), and to construct a new biocontainment facility for producing HIV at NCI's Frederick Cancer Research Facility (U.S. Congress, 1988b:63). Part of the appropriation was used to lease 16,000 square feet of off-campus office space for extramural program administration; in addition, the General Services Administration was asked to find 25,000 square feet (U.S. Congress, 1988a:270). Outpatient examining rooms and a new 26-bed unit in the NIH clinical
|
10 |
The remainder is for initial funding ($19.2 million) of a $150 million program to modernize the utility infrastructure of the Bethesda campus (boilers, chillers, steam lines, etc.), continued renovation of the clinical center (which eventually could take between $400 million and $800 million to complete), rehabilitation of the older laboratory buildings, and renovation of animal facilities, as well as routine repairs (PHS, 1989:234). In addition, initial construction of a new Child Health and Neurosciences Building and planning and design of a consolidated office building will be supported by carryover funds. |
center were assigned to AIDS research (although at first it was only possible to staff 14 of the beds; U.S. Congress, 1988a:268).
Congress appropriated $4.9 million in 1989 and $14.9 million in 1990 for AIDS facilities at NIH. In its budget request for fiscal year 1991, NIH reported that the addition to the clinical center was under construction, as were a new building at the Frederick Cancer Research Facility and renovations of NIAID lab space in the Twinbrook II facility. NIH's 1991 request of $16.5 million would allow completion of the renovations of lab space in Twinbrook II, construction of a new primate facility, and renovation and expansion of NCI AIDS labs at the Frederick facility (PHS, 1989:244,246). If the 1991 request is approved, appropriations since 1988 for AIDS buildings and facilities will total $55.3 million.
As a result of the major effort begun in 1988 to accommodate the space needs of the NIH AIDS research program, space occupied by NIH AIDS activities has doubled between July 1988 and March 1990, from 109,000 to 226,000 square feet, although it is still short of the 309,000 square feet planned. Additional funding will be needed, however, to complete planned renovations, expansions, and new construction of AIDS space at NIH (for example, an on-campus retrovirus lab and full renovation and expansion of one of the buildings at the Frederick facility). NIH's original request for AIDS facilities at NIH was $85.5 million, which was reduced at the PHS and DHHS levels to the $16.5 million included in the President's budget request (see the 1991 AIDS budget chronology in U.S. Congress, 1990a:170).11
Conclusion
As with personnel compensation and problems with the personnel system, NIH-wide space limitations and inadequacies have affected the AIDS research program disproportionately because it is a new and fast-growing set of activities. Similarly, efforts to address the overall problem –in this case by providing adequate amounts and types of modern, safe space and equipment for NIH as a whole–would go far toward solving the problems of inadequate facilities for AIDS research and research administration at NIH. In the meantime, Congress has appropriated funds specifically for AIDS research and office space that have greatly reduced the backlog of needs in the last several years. The next step is to consolidate AIDS activities on campus to promote joint research efforts, the exchange of research information, and program coordination.
Recommendation 4.6: As part of its long-range building and facilities program, NIH should consolidate AIDS research and research administration on the NIH campus. This consolidation will facilitate communication between the intramural and extramural programs and coordination of the multiple institutes, centers, and divisions involved in AIDS research activities. The committee endorses NIH's effort to take a systematic, sustained approach to upgrading and maintaining the campus infrastructure, which will benefit the AIDS program as well as non-AIDS research.
|
11 |
The final 1991 budget, enacted in October 1990, included $9.5 million for AIDS buildings and facilities. |
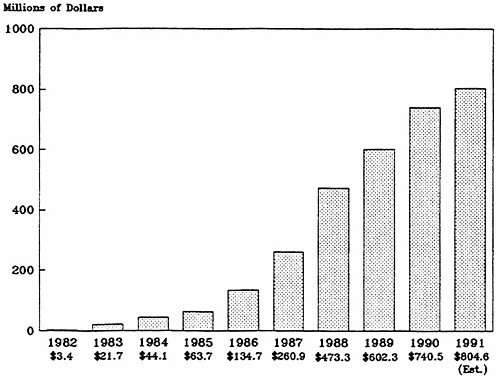
FIGURE 4.1 National Institutes of Health AIDS expenditures, fiscal years 1982–1991.
SOURCE: Institute of Medicine's National Institutes of Health AIDS program data base, which is based on published and unpublished data from NIH's Division of Financial Management and Division of Research Grants and from the National AIDS Program Office.
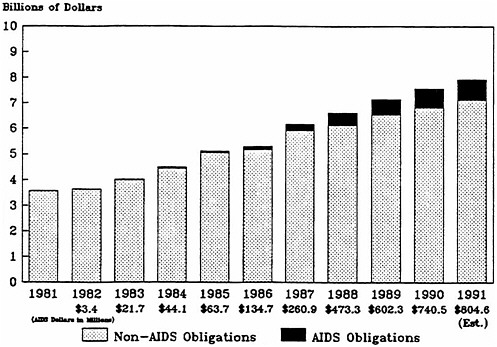
FIGURE 4.2 National Institutes of Health funding: non-AIDS obligations and AIDS obligations (in current dollars).
SOURCE: Institute of Medicine's National Institutes of Health AIDS program data base, which is based on published and unpublished data from NIH's Division of Financial Management and Division of Research Grants and from the National AIDS Program Office.
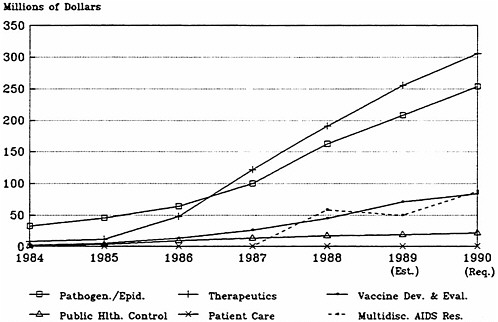
FIGURE 4.4a AIDS funding by functional category, fiscal years 1984 –1990.
SOURCE: Division of Financial Management, National Institutes of Health.
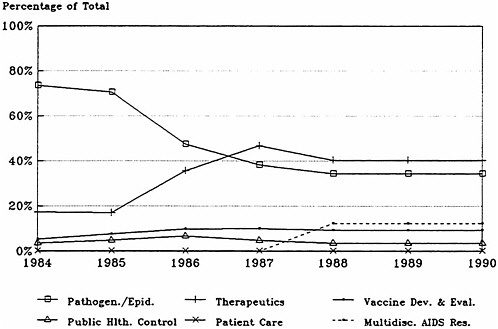
FIGURE 4.4b AIDS funding by functional category as a percent of fiscal year totals, 1984–1990.
SOURCE: Division of Financial Management, National Institutes of Health.
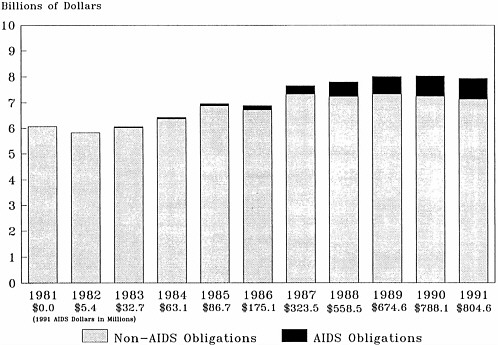
FIGURE 4.5 National Institutes of Health funding: non-AIDS and AIDS obligations (in 1991 dollars). The numbers were deflated using the biomedical research and development price index.
SOURCE: Institute of Medicine's National Institutes of Health AIDS program data base, which is based on published and unpublished data from NIH's Division of Financial Management and Division of Research Grants and from the National AIDS Program Office.
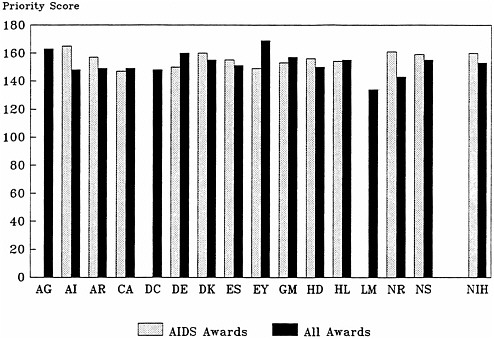
FIGURE 4.6 Priority score at the 50th percentile for National Institutes of Health AIDS R01 and all R01 awards. Abbreviations: AG, National Institute on Aging; AI, National Institute of Allergy and Infectious Diseases; AR, National Institute of Arthritis and Musculoskeletal and Skin Diseases; CA, National Cancer Institute; DC, National Institute on Deafness and Other Communication Disorders; DE, National Institute of Dental Research; DK, National Institute of Diabetes and Digestive and Kidney Diseases; ES, National Institute of Environmental Health Sciences; EY, National Eye Institute; GM, National Institute of General Medical Sciences; HD, National Institute of Child Health and Human Development; HL, National Heart, Lung, and Blood Institute; LM, National Library of Medicine; NR, National Center for Nursing Research; NS, National Institute of Neurological Disorders and Stroke.
Source: Division of Research Grants, National Institutes of Health.
TABLE 4.1 Growth of AIDS, Non-AIDS, and Total National Institutes of Health (NIH) Funding (in thousands of dollars), Fiscal Years 1982 –1990
|
Year |
NIH AIDS Obligations |
Percent Increase |
NIH Non-AIDS Obligations |
Percent Increase |
Total NIH Obligations |
Percent Increase |
|
1981 |
0 |
n.a. |
3,572,506 |
n.a. |
3,572,506 |
n.a. |
|
1982 |
3,355 |
n.a. |
3,640,106 |
1.9 |
3,643,461 |
2.0 |
|
1983 |
21,668 |
545.8 |
3,991,467 |
9.7 |
4,013,135 |
10.1 |
|
1984 |
44,121 |
103.6 |
4,449,432 |
11.5 |
4,493,553 |
12.0 |
|
1985 |
63,737 |
44.5 |
5,057,820 |
13.7 |
5,121,557 |
14.0 |
|
1986 |
134,667 |
111.3 |
5,163,310 |
2.1 |
5,297,977 |
3.4 |
|
1987 |
260,907 |
93.7 |
5,914,131 |
14.5 |
6,175,038 |
16.6 |
|
1988 |
473,285 |
81.4 |
6,137,145 |
3.8 |
6,610,430 |
7.1 |
|
1989 |
602,294 |
27.3 |
6,542,470 |
6.6 |
7,144,764 |
8.1 |
|
1990 |
740,509 |
22.9 |
6,840,975 |
4.6 |
7,581,484 |
6.1 |
|
1991a |
804,567 |
8.7 |
7,472,172 |
9.2 |
8,276,739 |
9.2 |
|
n.a. Not applicable. aAppropriated. SOURCE: Institute of Medicine's NIH AIDS program data base, which is based on published and unpublisheddata from NIH's Divisions of Financial Management and Research Grantsand from the National AIDS Program Office. |
||||||
TABLE 4.2 Growth of AIDS Funding (in thousands of dollars), at the National Institutes of Health (NIH), Fiscal Years 1981–1990
|
Year |
NIH AIDS Obligations |
Total NIH Obligations |
AIDS as Percentage of Total NIH Obligations |
|
1981 |
0 |
3,572,506 |
0.0 |
|
1982 |
3,355 |
3,643,461 |
0.1 |
|
1983 |
21,668 |
4,013,135 |
0.5 |
|
1984 |
44,121 |
4,493,553 |
1.0 |
|
1985 |
63,737 |
5,121,557 |
1.2 |
|
1986 |
134,667 |
5,197,977 |
2.6 |
|
1987 |
260,907 |
6,175,038 |
4.2 |
|
1988 |
473,285 |
6,610,430 |
7.2 |
|
1989 |
602,294 |
7,144,764 |
8.4 |
|
1990 |
740,509 |
7,581,484 |
9.8 |
|
1991a |
804,567 |
8,276,739 |
9.7 |
|
aEstimated. SOURCE: Institute of Medicine's NIH AIDS program data base, which is based on published and unpublisheddata from NIH's Divisions of Financial Management and Research Grantsand from the National AIDS Program Office. |
|||
TABLE 4.3 Percentage of National Institutes of Health AIDS Funding by Institute, Center, and Division, Fiscal Years 1990–1991
|
Unit |
1990 Percent |
1991 Percenta |
|
National Cancer Institute |
20.3 |
20.0 |
|
National Heart, Lung, and Blood Institute |
5.7 |
5.4 |
|
National Institute of Dental Research |
0.6 |
0.8 |
|
National Institute of Diabetes and Digestive and Kidney Diseases |
0.7 |
0.7 |
|
National Institute of Neurological Disorders and Stroke |
2.2 |
2.1 |
|
National Institute of Allergy and Infectious Diseases |
53.1 |
53.8 |
|
National Institute of General Medical Sciences |
2.0 |
2.1 |
|
National Institute of Child Health and Human Development |
3.6 |
3.9 |
|
National Eye Institute |
0.7 |
0.7 |
|
National Institute of Environmental Health Sciences |
0.6 |
0.6 |
|
National Institute on Aging |
0.1 |
0.1 |
|
National Institute of Arthritis and Musculoskeletal and Skin Diseases |
0.2 |
0.2 |
|
National Institute on Deafness and Other Communication Disorders |
– |
0.1 |
|
National Center for Research Resources |
6.0 |
5.9 |
|
National Center for Nursing Research |
0.1 |
0.4 |
|
Fogarty International Center |
0.7 |
0.7 |
|
National Library of Medicine |
0.1 |
0.1 |
|
Office of the Director |
1.6 |
1.6 |
|
Buildings and Facilities Program |
1.7 |
1.2 |
|
Total |
100.0 |
100.0 |
|
aEstimated. SOURCE: Division of Financial Management, National Institutes ofHealth. |
||
TABLE 4.4 AIDS Funding as a Percentage of Total Funding by Institute, Center, and Division, Fiscal Years 1990–1991
|
Unit |
1990 Percent |
1991 Percenta |
|
National Cancer Institute |
9.1 |
9.4 |
|
National Heart, Lung, and Blood Institute |
3.9 |
3.9 |
|
National Institute of Dental Research |
3.4 |
4.4 |
|
National Institute of Diabetes and Digestive and Kidney Diseases |
0.9 |
1.0 |
|
National Institute of Neurological Disorders and Stroke |
3.3 |
3.0 |
|
National Institute of Allergy and Infectious Diseases |
47.3 |
47.7 |
|
National Institute of General Medical Sciences |
2.1 |
2.1 |
|
National Institute of Child Health and Human Development |
6.1 |
6.5 |
|
National Eye Institute |
2.3 |
2.2 |
|
National Institute of Environmental Health Sciences |
1.9 |
1.3 |
|
National Institute on Aging |
0.4 |
0.3 |
|
National Institute of Arthritis and Musculoskeletal and Skin Diseases |
0.7 |
0.8 |
|
National Institute on Deafness and Other Communication Disorders |
– |
0.5 |
|
National Center for Research Resources |
12.6 |
14.2 |
|
National Center for Nursing Research |
2.9 |
7.2 |
|
Fogarty International Center |
31.6 |
30.5 |
|
National Library of Medicine |
0.6 |
0.6 |
|
Office of the Director |
11.0 |
13.4 |
|
Buildings and Facilities Program |
19.2 |
5.6 |
|
Total |
100.0 |
100.0 |
|
aEstimated. SOURCE: Division of Financial Management, National Institutes ofHealth. |
||
TABLE 4.5 National Institutes of Health (NIH) AIDS Funding (percentage) by Mechanism, Fiscal Years 1982–1991
|
Mechanism |
1982 |
1983 |
1984 |
1985 |
1986 |
1987 |
1988 |
1989 |
1990 |
1991a |
|
Research grants |
||||||||||
|
Projects |
20.1 |
41.5 |
32.6 |
28.0 |
18.8 |
30.9 |
40.5 |
38.3 |
39.0 |
39.3 |
|
Centers |
19.0 |
5.8 |
3.8 |
5.3 |
4.7 |
4.1 |
8.3 |
8.7 |
7.9 |
7.7 |
|
Other |
0.2 |
0.1 |
0.4 |
1.4 |
1.3 |
0.9 |
2.8 |
2.5 |
1.8 |
1.7 |
|
Total |
39.4 |
47.4 |
36.8 |
34.7 |
24.8 |
35.9 |
51.7 |
49.5 |
48.7 |
48.7 |
|
Research training |
–b |
– |
<0.1 |
0.2 |
<0.1 |
0.1 |
0.7 |
1.1 |
1.0 |
1.2 |
|
Research and development contracts |
13.4 |
21.5 |
34.9 |
39.1 |
53.4 |
42.9 |
29.4 |
27.7 |
26.7 |
26.5 |
|
Intramural research |
47.2 |
31.1 |
27.2 |
25.1 |
20.1 |
16.9 |
14.0 |
15.5 |
16.5 |
16.9 |
|
Research management and support |
– |
– |
1.0 |
0.9 |
1.5 |
2.4 |
2.8 |
3.6 |
3.5 |
3.8 |
|
All otherc |
– |
– |
– |
– |
0.1 |
1.8 |
1.4 |
1.8 |
3.5 |
2.9 |
|
Total NIH |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
|
AIDS funding (millions of dollars) |
$3.4 |
$21.7 |
$44.1 |
$63.7 |
$134.7 |
$260.9 |
$430.6 |
$602.3 |
$740.5 |
$804.6 |
|
aEstimated. bNo funds allocated. cAllocations for the National Library of Medicine, Office of the NIH Director, Buildings and Facilities program, and extramural construction grants. SOURCE: U.S. Congress (1989:136–137) for 1982–1988; Division of Financial Management, NIH, for 1989–1991. |
||||||||||
TABLE 4.6 National Institutes of Health (NIH) Non-AIDS Funding (percentage) by Mechanism, Fiscal Years 1982–1991
|
Mechanism |
1982 |
1983 |
1984 |
1985 |
1986 |
1987 |
1988 |
1989 |
1990 |
1991a |
|
Research grants |
||||||||||
|
Projects |
50.3 |
52.3 |
53.4 |
54.4 |
56.0 |
57.2 |
57.7 |
58.3 |
59.5 |
56.0 |
|
Centers |
9.6 |
9.3 |
9.6 |
9.3 |
9.0 |
8.7 |
8.5 |
8.3 |
8.1 |
8.7 |
|
Other |
5.7 |
5.5 |
5.5 |
6.1 |
5.9 |
5.9 |
5.8 |
5.5 |
4.7 |
5.1 |
|
Total |
65.6 |
67.0 |
68.4 |
69.8 |
70.9 |
72.8 |
72.0 |
72.1 |
72.3 |
69.8 |
|
Research training |
4.1 |
4.1 |
3.7 |
4.3 |
4.1 |
3.9 |
3.8 |
3.7 |
3.7 |
4.0 |
|
Research and development contracts |
8.8 |
7.9 |
7.4 |
6.9 |
5.4 |
6.3 |
6.0 |
5.6 |
5.5 |
5.4 |
|
Intramural research |
12.4 |
12.3 |
11.9 |
11.0 |
10.5 |
10.5 |
10.6 |
10.6 |
10.8 |
10.6 |
|
Research management and support |
5.1 |
5.0 |
4.8 |
4.3 |
4.0 |
3.9 |
4.2 |
4.3 |
4.0 |
4.6 |
|
All othera |
3.9 |
3.6 |
3.8 |
3.6 |
5.0 |
3.6 |
3.3 |
3.7 |
3.6 |
5.7 |
|
Total NIH |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
|
Non-AIDS funding (billions of dollars) |
$3.6 |
$4.0 |
4.4 |
$ 5.1 |
$5.2 |
$5.9 |
$6.1 |
$6.5 |
$6.8 |
$7.5 |
|
aEstimated. SOURCE: U.S. Congress (1989:134–135) for 1982–1988; Division of Financial Management, NIH for 1989–1991. |
||||||||||
TABLE 4.7 Number of AIDS Research Project Grants (RPG) by Type and Proportion Solicited by Requests for Applications (RFA), Fiscal Years 1986–1988
|
R01s |
Solicited by RFA |
|||||||
|
Fiscal Year |
Total RPGs |
Number |
Percent |
P01s |
U01s |
Other RPGs |
Number |
Percent |
|
1986 |
182 |
111 |
61 |
17 |
42 |
12 |
57 |
31 |
|
1987 |
292 |
182 |
62 |
24 |
62 |
24 |
142 |
78 |
|
1988 |
501 |
307 |
61 |
45 |
101 |
48 |
90 |
29 |
|
NOTE: Abbreviations: R01, traditional (individual investigator-initiated) research grant; P01, program project grant; U01, cooperative agreement. SOURCE: Division of Research Grants, National Institutes of Health. |
||||||||
TABLE 4.8 National Institutes of Health AIDS Funding (in thousands of dollars) by Mason Functional Categories
|
Category |
Fiscal Year 1989 Budget Authority |
Fiscal Year 1990 Appropriation |
Fiscal Year 1991 President's Request |
|
Basic science research |
|||
|
Biomedical research |
|||
|
HIV and HIV genome |
62,120 |
66,320 |
71,887 |
|
Immunology |
37,954 |
44,911 |
48,075 |
|
Blood/blood products |
11,063 |
12,154 |
8,879 |
|
Diagnostic methods/reagents development |
7,869 |
10,853 |
11,956 |
|
Animal models and related studies |
29,683 |
36,308 |
39,264 |
|
Subtotal |
148,689 |
170,546 |
180,061 |
|
Neuroscience and neuropsychiatric research |
16,645 |
20,324 |
21,669 |
|
Behavioral research |
|||
|
Mechanisms of behavior and behavior change |
3,863 |
4,188 |
4,530 |
|
Prevention of high-risk behaviors |
1,315 |
611 |
642 |
|
Subtotal |
5,178 |
4,799 |
5,172 |
|
Therapeutic agents |
|||
|
Development |
131,421 |
163,712 |
176,082 |
|
Clinical trials |
103,840 |
139,966 |
150,306 |
|
Subtotal |
235,261 |
303,678 |
326,388 |
|
Vaccines |
|||
|
Development |
49,238 |
64,301 |
70,499 |
|
Clinical trials |
10,581 |
14,332 |
15,631 |
|
Subtotal |
59,819 |
78,633 |
86,130 |
|
Research enhancement |
|||
|
Training |
6,473 |
8,253 |
9,445 |
|
Construction (extramural) |
4,940 |
–a |
– |
|
Subtotal |
11,413 |
8,253 |
9,445 |
|
Total, basic science research |
477,005 |
586,233 |
628,865 |
TABLE 4.9 National Institutes of Health AIDS Funding (thousands of dollars), by Charlottesville Functional Categories, Fiscal Years 1984–1990
|
Category |
1984 |
1985 |
1986 |
1987 |
1988a |
1989b |
1990b |
|
Pathogenesis and clinical manifestations |
|||||||
|
Epidemiological studies |
16,202 |
20,468 |
27,848 |
38,964 |
59,869 |
87,896 |
98,598 |
|
Virology |
1,500 |
2,500 |
2,983 |
8,843 |
25,651 |
21,399 |
33,305 |
|
Surveillance |
40 |
600 |
1,979 |
620 |
1,387 |
1,736 |
3,267 |
|
Etiologic agent and co-factors |
5,521 |
9,224 |
12,412 |
22,465 |
30,490 |
45,734 |
53,223 |
|
Immunologic studies |
6,534 |
9,683 |
15,215 |
20,038 |
28,539 |
29,283 |
40,376 |
|
Simian AIDS |
2,589 |
2,351 |
3,541 |
8,057 |
11,627 |
17,564 |
19,248 |
|
Psychosocial factors |
38 |
124 |
39 |
692 |
4,976 |
4,739 |
5,968 |
|
Subtotal |
34,424 |
44,950 |
64,017 |
99,679 |
162,539 |
208,351 |
253,985 |
|
Therapeutics |
|||||||
|
Studies of therapeutic intervention |
7,680 |
10,332 |
38,437 |
105,922 |
174,350 |
232,630 |
278,234 |
|
Drug purchase and distribution |
–c |
500 |
9,564 |
16,132 |
16,284 |
22,755 |
27,519 |
|
Subtotal |
7,680 |
10,832 |
48,001 |
122,054 |
190,634 |
255,385 |
305,753 |
|
Vaccine development and evaluation |
2,379 |
4,839 |
13,300 |
26,174 |
44,333 |
70,926 |
83,886 |
|
Public health control measures |
|||||||
|
Information/education |
573 |
643 |
1,682 |
5,253 |
7,215 |
5,386 |
8,717 |
|
Prevention of transfusion-related AIDS |
22 |
536 |
622 |
1,733 |
3,196 |
4,033 |
4,000 |
|
Development and evaluation of blood tests |
1,015 |
1,879 |
6,866 |
5,782 |
6,548 |
9,684 |
8,419 |
|
Subtotal |
1,610 |
3,058 |
9,170 |
12,768 |
16,959 |
19,103 |
21,136 |
|
Patient care and health care needs |
|||||||
|
Treatment demonstration project |
– |
– |
95 |
90 |
295 |
– |
50 |
|
Bioethics and safety |
28 |
58 |
84 |
142 |
86 |
337 |
348 |
|
Subtotal |
28 |
58 |
179 |
232 |
381 |
337 |
398 |
|
Multidisciplinary AIDS research |
– |
– |
– |
– |
58,439 |
49,697 |
87,512 |
|
Total, National Institutes of Health |
44,121 |
63,737 |
134,667 |
260,907 |
473,285 |
603,799 |
752,670 |
|
aIncludes $23,935 of no-year extramural construction funds in the National Center for Research Resources and $18,780 in Building and Facilities construction appropriated in fiscal year 1988 that will be obligated in fiscal year 1989. bEstimated. cNo funds budgeted or allocated. SOURCE: Division of Financial Management, National Institutes ofHealth. |
|||||||
TABLE 4.10 Comparison of AIDS and Non-AIDS Priority Scores for Research Grants, Fiscal Years 1982–1985, National Institutes of Health
|
AIDS Research Grants |
|||||||||
|
1982 |
1983 |
1984 |
1985 |
||||||
|
Priority Score Rangea |
NCI |
NCI |
NHLBI |
NIAID |
NCI |
NHLBI |
NIAID |
NCI |
NIAID |
|
100–180 |
13 |
8 |
0 |
12 |
8 |
4 |
20 |
17 |
8 |
|
181–200 |
4 |
5 |
1 |
2 |
1 |
0 |
4 |
0 |
0 |
|
201–250 |
6 |
7 |
0 |
2 |
6 |
3 |
4 |
0 |
1 |
|
251–300 |
1 |
10 |
1 |
1 |
0 |
0 |
0 |
1 |
0 |
|
300+ |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
Total AIDS grants |
24 |
30 |
2 |
17 |
16 |
7 |
28 |
18 |
9 |
|
Paylineb for non-AIDS grants |
183 |
181 |
195 |
166 |
184 |
201 |
167 |
172 |
159 |
|
NOTE: Abbreviations: NCI, National Cancer Institute; NHLBI, National Heart, Lung, and Blood Institute; NIAID, National Institute of Allergy and Infectious Diseases aAll research grants are reviewed for scientific merit and receive a score ranging from 100 (best) to 500 (worst). bThe payline is the score dividing funded and unfunded grants. SOURCE: Division of Financial Management, National Institutes ofHealth, May 16, 1986. |
|||||||||
TABLE 4.11 Priority Scorea at 50th Percentile for Applications and Awards, AIDS and Non-AIDS R01s and All Research Project Grants, Fiscal Year 1989
|
AIDS |
Non-AIDS |
|
|
R01sb |
||
|
Applications |
242 |
235 |
|
Awards |
160 |
153 |
|
Research project grantsc |
||
|
Applications |
240 |
231 |
|
Awards |
161 |
153 |
|
a100 is best score, 500 is worst. bTraditional individual investigator-initiated grants. cIncludes R01, R22, R23, R29, R35, R37, R43, R44, P01, P42, U01, and National Institute of General Medical Sciences P41 (no National Library of Medicine or National Center for Research Resources grants). SOURCE: Division of Research Grants, National Institutes of Health. |
||
TABLE 4.12 Comparison of Peer Review Scores of AIDS Grant Applications with Scores of All Other Grant Applications Reviewed in Division of Research Grants Study Sections, Council Year 1989a
|
Mean Priority Score, by Deciles |
||||||
|
Mean Priority Score |
First Decile |
Second Decile |
Third Decile |
Fourth Decile |
Fifth Decile |
|
|
AIDS applications |
||||||
|
All |
254 |
135 |
155 |
176 |
199 |
225 |
|
Type 1b |
259 |
135 |
155 |
176 |
199 |
225 |
|
Type 2c |
199 |
135 |
154 |
175 |
200 |
224 |
|
All other applications |
||||||
|
All |
240 |
133 |
153 |
170 |
191 |
212 |
|
Type 1b |
251 |
133 |
153 |
170 |
191 |
213 |
|
Type 2c |
210 |
133 |
153 |
170 |
190 |
212 |
|
aCouncil year 1989 includes applications received for consideration at national advisory council meetings held in January, May, and October 1989. bType 1 applications are applications for funding of new research projects. cType 2 applications are applications for continued funding of research projects for which previous grants are running out or expiring. (Also included are type 9 applications, which are also competing renewal applications that are changing institutes.) SOURCE: Division of Research Grants, National Institutes of Health. |
||||||
TABLE 4.13 AIDS and Non-AIDS Individual Investigator-Initiated Grants (R01s) and All Research Project Grants (RPG), Fiscal Year 1989
|
Grant Type |
Reviewed |
Approved |
Awarded |
Success Ratea (percentage) |
Award Rateb (percentage) |
Recommendation Ratec (percentage) |
|
AIDS R01s |
700 |
638 |
205 |
29.0 |
31.8 |
91.1 |
|
Non-AIDS R01s |
15,191 |
14,714 |
3,971 |
27.0 |
26.0 |
96.6 |
|
AIDS RPGs (includes R01s) |
886 |
790 |
273 |
30.5 |
34.2 |
89.2 |
|
Non-AIDS RPGs (includes R01s) |
19,521 |
18,317 |
5,383 |
29.4 |
27.5 |
93.5 |
|
aNumber awarded divided by number reviewed. bNumber awarded divided by number approved. cNumber approved divided by number reviewed. SOURCE: Division of Research Grants, National Institutes of Health. |
||||||
TABLE 4.14a Cumulative Summary of AIDS Staffing (in full-time equivalents [FTE]) by Unit, National Institutes of Health
|
Unit |
1982 |
1983 |
1984 |
1985 |
1986 |
1987 |
1988 |
1989 |
1990a |
1991b |
|
Institute, center, or division |
||||||||||
|
National Cancer Institute |
20 |
31 |
72 |
85 |
98 |
129 |
146 |
188 |
281 |
300 |
|
National Heart, Lung, and Blood Institute |
–c |
– |
– |
1 |
1 |
5 |
9 |
18 |
23 |
35 |
|
National Institute of Dental Research |
– |
– |
1 |
2 |
4 |
8 |
10 |
16 |
18 |
19 |
|
National Institute of Diabetes and Kindney Diseases |
– |
– |
– |
– |
– |
5 |
5 |
10 |
10 |
10 |
|
National Institute of Neurological Disorders and Stroke |
1 |
2 |
10 |
11 |
16 |
21 |
23 |
40 |
40 |
40 |
|
National Institute of Allergy and Infectious Diseases |
– |
12 |
45 |
46 |
57 |
119 |
158 |
229 |
306 |
343 |
|
National Institute of General Medical Sciences |
– |
– |
– |
– |
– |
– |
– |
2 |
2 |
2 |
|
National Institute of Child Health and Human Development |
– |
– |
– |
– |
– |
10 |
12 |
21 |
22 |
25 |
|
National Eye Institute |
– |
– |
– |
– |
1 |
1 |
1 |
4 |
5 |
7 |
|
National Institute of Environmental Health Sciences |
– |
– |
– |
– |
– |
2 |
6 |
7 |
7 |
8 |
|
National Institute on Aging |
– |
– |
– |
– |
– |
1 |
2 |
3 |
5 |
5 |
|
National Institute of Arthritis and Musculoskeletal and Skin Diseases d |
– |
– |
– |
– |
– |
– |
– |
2 |
3 |
4 |
|
National Institute on Deafness and Other Communication Disorderse |
– |
– |
– |
– |
– |
– |
1 |
– |
– |
– |
|
National Center for Research Resources |
– |
– |
– |
– |
– |
3 |
5 |
7 |
7 |
7 |
|
National Center for Nursing Researchf |
– |
– |
– |
– |
– |
– |
– |
2 |
2 |
4 |
|
Fogarty International Center |
– |
– |
– |
– |
– |
– |
2 |
3 |
3 |
3 |
|
Subtotal |
21 |
45 |
128 |
144 |
177 |
304 |
379 |
552 |
735 |
813 |
|
National Library of Medicine |
– |
– |
– |
– |
– |
– |
– |
2 |
7 |
8 |
|
Office of the Director |
– |
– |
– |
– |
– |
2 |
7 |
16 |
35 |
37 |
|
Clinical center |
6 |
14 |
40 |
47 |
58 |
90 |
131 |
– |
– |
– |
|
Division of Research Grants |
– |
– |
– |
– |
– |
3 |
10 |
193 |
295 |
325 |
|
Office of Research Services |
– |
– |
– |
– |
– |
– |
10 |
– |
– |
– |
|
Total |
27 |
59 |
168 |
191 |
235 |
399 |
537 |
763 |
1,072 |
1,183 |
|
aEstimated. bRequested. cNo FTEs allotted. dThe National Institute of Arthritis and Musculoskeletal and Skin Diseases was established in 1986. eThe National Institute on Deafness and Other Communication Disorders was established in 1988. fThe National Center for Nursing Research was established in 1986. SOURCE: Division of Financial Management, National Institutes ofHealth, January 28, 1989. |
||||||||||
TABLE 4.14b Cumulative Summary of AIDS Staffing (in full-time equivalents [FTE]) by Administrative Area, National Institutes of Health
|
Administrative Area |
1982 |
1983 |
1984 |
1985 |
1986 |
1987 |
1988 |
1989 |
1990a |
1991b |
|
Intramural |
21 |
45 |
118 |
129 |
145 |
224 |
275 |
378 |
498 |
555 |
|
Research management and support |
–c |
– |
10 |
15 |
28 |
80 |
104 |
174 |
237 |
258 |
|
Office of the Director and Central Services |
6 |
14 |
40 |
47 |
58 |
95 |
158 |
211 |
337 |
370 |
|
Total |
27 |
59 |
168 |
191 |
235 |
399 |
537 |
763 |
1,072 |
1,183 |
|
aEstimated. bRequested. cNo FTEs allotted. SOURCE: Division of Financial Management, National Institutes ofHealth, January 28, 1989. |
||||||||||
TABLE 4.15 Staffing Levels (in full-time equivalents [FTE]) for the of Health AIDS and Non-AIDS Programs,
|
FTEs |
|||
|
Fiscal Year |
Total |
AIDS |
Non-AIDS |
|
1981 |
12,637 |
0 |
12,376 |
|
1982 |
12,689 |
27 |
12,662 |
|
1983 |
13,414 |
59 |
13,355 |
|
1984 |
13,661 |
168 |
13,493 |
|
1985 |
13,100 |
191 |
12,909 |
|
1986 |
12,540 |
235 |
12,305 |
|
1987 |
12,720 |
399 |
12,321 |
|
1988 |
13,249 |
537 |
12,712 |
|
1989 |
13,204 |
763 |
12,441 |
|
1990a |
13,214 |
887 |
12,327 |
|
1990b |
13,779 |
1,072 |
12,707 |
|
1991a |
14,133 |
1,183 |
13,031 |
|
aPresident's budget request. bRevised budget (January 1990). SOURCE: Division of Financial Management, National Institutes ofHealth, January 29, 1989. |
|||
REFERENCES
Ad Hoc Consultants to the NIH AIDS Executive Committee. 1986. Future Directions for AIDS Research: Report to Congress from the Ad Hoc Consultants to the National Institutes of Health AIDS Executive Committee. November. Bethesda, Md.: National Institutes of Health.
Balz, D. 1990. Raises set for federal executives. Washington Post, December 13.
Buehler, J. W. 1990. Table of estimates of years of potential life lost before age 65 for HIV/AIDS. Centers for Disease Control, Atlanta, Ga.
CDC (Centers for Disease Control). 1990. Fact Book FY 1990. Atlanta, Ga.: CDC.
IOM/NAS (Institute of Medicine/National Academy of Sciences). 1988. A Healthy NIH Intramural Program: Structural Change or Administrative Remedies? Washington, D.C.: National Academy Press.
IOM/NAS. 1990. Funding Health Sciences Research: A Strategy to Restore Balance. Washington, D.C.: National Academy Press.
Lee, P. R., and P. S. Arno. 1986. The federal response to the AIDS epidemic. Health Policy 6:259–267.
Maurer, B. A. 1990. Letter from Bruce A Maurer, Chief, Immunology, Virology, and Pathology Review Section, Division of Research Grants, to Michael McGeary, June 6, 1990.
Meier, G. W. 1990. Interview with Gilbert W. Meier, Executive Secretary, AIDS and Related Research Study Section 2 (epidemiology and population studies) and Section 6 (behavioral research), Division of Research Grants, by Michael McGeary, June 1, 1990.
NAS/IOM. 1990. Forum on Supporting Biomedical Research: Near-Term Problems and Options for Action. Summary. Washington, D.C.: National Academy Press.
NCI (National Cancer Institute). 1990. 1992 (By-Pass) Budget Estimate. September. Bethesda, Md.: National Cancer Institute.
NIH (National Institutes of Health). 1989a. AIDS planning session with the director. Office of AIDS Research. January.
NIH. 1989b. Orientation Handbook for Members of Scientific Review Groups. June. Bethesda, Md.: Division of Research Grants, NIH.
NIH. 1990a. AIDS briefing session with the director. Office of AIDS Research. February.
NIH. 1990b. NIH Advisory Committees: Authority, Structure, Function, Members. April. NIH Publication No. 90-11. Bethesda, Md.: NIH.
OTA (Office of Technology Assessment). 1985. Review of the Public Health Service's Response to AIDS. Technical Memorandum, OTA-TM-H-24. February. Washington, D.C.: U.S. Government Printing Office.
OTA 1990. How Has Federal Research on AIDS/HIV Disease Contributed to Other Fields? Staff Paper 5 in OTA's Series on AIDS-Related Issues. April. Washington, D.C.: Health Program, OTA.
Panem, S. 1988. The AIDS Bureaucracy. Cambridge, Mass.: Harvard University Press.
Presidential Commission on the Human Immunodeficiency Virus Epidemic . 1988. Report of the Presidential Commission on the Human Immunodeficiency Virus Epidemic. June 24. Washington, D.C.: U.S. Government Printing Office.
PHS (Public Health Service). 1989. Justification of Appropriation Estimates for Committee on Appropriations, Fiscal Year 1991. Vol. 6, National Institutes of Health, Research Resources through Office of the Director. Washington, D.C.: U.S. Government Printing Office.
PHS. 1990a. Recruitment and Retention: Scientific, Medical, and Engineering Positions . October. Washington, D.C.: Office of Personnel Management.
PHS. 1990b. The Role of the Public Health Service in Combatting the HIV/AIDS Epidemic (August 31 draft). Washington, D.C.: National AIDS Program Office.
Stoolmiller, A. C. 1990. Telephone interview with Allen C. Stoolmiller, Executive Secretary, Special Review Section for the first AIDS RFA, Division of Research Grants, by Michael McGeary, May 15, 1990.
U.S. Congress. 1988a. AIDS Issues (Part 3). Hearings before the Subcommittee on Health and the Environment of the House Committee on Energy and Commerce (hearing on “Needs for AIDS Research Activities”). March 15. Serial No. 100-140. Washington, D.C.: U.S. Government Printing Office.
U.S. Congress. 1988b. Hearings on Departments of Labor, Health and Human Services, Education, and Related Agencies Appropriations for 1989. Part 4A. National Institutes of Health. House Committee on Appropriations, Subcommittee on the Departments of Labor, Health and Human Ser. March. Washington, D.C.: U.S. Government Printing Office.
U.S. Congress. 1988c. Therapeutic Drugs for AIDS: Development, Testing, and Availability. Hearings before the Human Resources and Intergovernmental Relations Subcommittee of the House Committee on Government Operations. April 28 and 29, 1988. Committee Print. Washington, D.C.: U.S. Government Printing Office.
U.S. Congress. 1989. Report to Accompany H.R. 2990, Departments of Labor, Health and Human Services, and Education, and Related Agencies Appropriation Bill, 1990. House Report 101-172. 101st Cong., 1st sess. July 25.
U.S. Congress. 1990a. Hearings on Departments of Labor, Health and Human Services, Education, and Related Agencies Appropriations for 1991. Part 4A, National Institutes of Health. House Committee on Appropriations, Subcommittee on the Departments of Labor, Health and Human Ser. March. Washington, D.C.: U.S. Government Printing Office.
U.S. Congress. 1990b. Hearings on Departments of Labor, Health and Human Services, Education, and Related Agencies Appropriations for 1991. Part 4B, National Institutes of Health. House Committee on Appropriations, Subcommittee on the Departments of Labor, Health and Human Ser. March. Washington, D.C: U.S. Government Printing Office.
U.S. Congress. 1990c. Hearings on Departments of Labor, Health and Human Services, and Education, and Related Agencies Appropriations, Fiscal Year 1991. Part 2, Department of Health and Human Services. Senate Committee on Appropriations, Subcommittee on Departments of Labor, H. February. Washington, D.C.: U.S. Government Printing Office.
Winkenwerder, W., A. R. Kessler, and R. M. Stolec. 1989. Federal spending for illness caused by the human immunodeficiency virus. N. Engl. J. Med., 320(June):1598–1603.