SESSION 5
PHOTOCHEMICAL SYNTHESIS
David F. Eaton
The DuPont Company
Wilmington, Delaware
Rapporteur's Report
The purpose of this session of the workshop was to assess the current status of research in, and identify potential applications for, the use of concentrated solar photons in photochemical synthesis and to recommend research directions that could lead to them, or to other, applications.
Session Chairs, Professor Veronica Vaida and Dr. Jack St. Clair Kilby, arranged a program in which the two main, current themes of solar photochemistry were highlighted. Dr. John Connolly (Solar Energy Research Institute) covered the current status of research to mimic the fundamental steps of photosynthesis as a route to harness solar photons for use in conventional synthesis. Dr. James Yardley (Allied-Signal) addressed the potential industrial applications of solar photons. The discussion which followed the session probed the needs of researchers in the solar community for new facilities for experimental work and identified new research directions.
The main conclusions of the participants are presented here and elaborated upon below:
• Current State of Knowledge.
Research in the area of photochemical synthesis has been ongoing for nearly 100 years, though the majority of published work has appeared within the last 20. The development of the field has been evolutionary, not revolutionary. Many individual photochemical reactions are known which are capable of assembling complex organic molecules in a single photochemical event from simple precursors, but no single reaction is especially suited to solar applications.
• Potential Applications.
None are known which are attractive targets for near-term research. It is premature to seek practical demonstrations of the efficacy of solar photons in synthesis.
• Research Paths.
Basic research into models for charge separation during the photosynthetic processes should continue. Opportunities exist for significant new research directed at understanding the chemistry which could occur in solar concentrators: reactions which use both electronic quanta and thermal input should be scouted. Research which probes the spectral absorption shifts experienced by molecules under solar concentrator conditions should be initiated. Molecules may actually absorb considerably more solar radiation
under some circumstances (high temperature, adsorbed on surfaces, etc.) than is currently thought to be the case. Concentrated solar photons may have considerable utility in materials processing applications in which chemistry occurs at a surface or interface. Such opportunities should be sought.
There appears to be a need for a shared research facility to which the photochemical community could bring or submit samples for exposure to real solar concentrator conditions. While much useful and productive work could be (and should be) accomplished in laboratory scale solar concentrators, the existence of an accessible user facility would allow any photochemist to try a ''dumb'' experiment, and it would also serve to link the photochemical community more tightly to the solar energy community.
Models for Charge Separation in Photosynthesis
Solar photosynthesis is accomplished on an enormous scale each day. Photosynthetic reaction centers in green plants and in photosynthetic bacteria employ low energy photons in the solar spectrum to drive endothermic reactions for the production of high energy metabolic products. The driving force for the chemistry comes from the initial separation of charges (electrons and holes) along a charge transport chain initiated by light absorption in porphyrin pigments. During very rapid initial charge transfer events, electrons are driven away from the porphyrin site down an energy gradient to become trapped on a quinone acceptor some 20 Å away within about 200 ps. At this point in the process, the charge separation is effectively complete. Subsequent, slower events transport the electron further away from the initial site of excitation and initiate the chemical parts of the electron transport process. The cationic hole left behind on the once-excited porphyrin is also used in the redox chain of events.
Chemists have had considerable success building small molecule models for the initial events of the photosynthetic process. The current state of the modelers' art is provided by the latest linked carotenoid-porphyrinquinone "pentad" system devised by Gust, Moore, and coworkers, which can absorb a red photon and produce a charge separated ionic state containing 1 eV of potential energy, in which the initial electron-hole pair is separated by nearly 80 Å and which will live for about 10 µs before collapsing back to its ground state.1 This is a remarkable achievement which gives chemists confidence that one could design molecules capable of storing considerable electrochemical redox potential, and that one could use the redox sites to initiate energy storing chemical synthesis.
Excellent progress is being made to understand the factors which control the reactivity of such model systems. Energetics (molecular spectroscopy, electrochemistry, and reorganizational energies associated with various redox processes), structural factors (distances among chromophores or redox pairs, their relative orientations, and the flexibility of the linkages connecting them), and parameters relating to the medium in which the reaction occurs (dielectric constant, index of refraction, and viscosity) all are considerations which bear on the outcome of the photoinitiated event. They are all related by the theoretical framework of the Marcus treatment of electron transfer processes.
Much research remains to be done to develop molecular devices or membrane based organized assemblies which can truly mimic the photosynthetic process, but the goal is achievable. Research to these ends should continue. It is a highly interdisciplinary effort requiring the combined talents of synthetic chemists, spectroscopists, electrochemists, photophysicists and others. The research is synthesis limited. Opportunities for application of concentrated solar photons in this area are not apparent.
Industrial Applications of Concentrated Solar Photons
Industry has had a perverse interest in solar photochemistry for many years. If a dye fades in the sunlight, or an automobile paint changes color in the sun with time, customers get angry. Industry has worried about such deleterious solar photochemistry for many years. However, industrial scale photochemistry, much less solar driven photochemistry, has not proved feasible.2 There are several factors responsible for this condition. Economic factors may be dominant: high value products which can accommodate the relatively high cost of photons generated by conversion of electrical current (lamps) generally are produced for a limited, small volume market. Industrial experience is that high process and low volumes, while they generate significant revenues, do not create large market value for most large companies. On the other hand, high volume, lower cost products which create huge market opportunities attractive even to the largest companies, cannot accommodate the cost of photons. There are only a very few, niche market areas where use of photons is commercially attractive.
The analysis above, while appropriate for electrically generated photons, probably also holds for solar photons, since there is a direct cost associated with even "free" sunlight. It is not clear that solar concentration alters the argument sufficiently to recommend it.
Many technical factors mitigate against the use of photons—solar or electric. Many photochemical reactions have significant secondary photo-reactivity. There are also severe scale-up limitations with photochemical reactors, though new reactor designs might overcome this difficulty. A troubling difficulty is the normally observed decrease in photochemical reaction efficiency as the concentration of reagents is increased. Quantum yields obtained in dilute solution rarely can be obtained under the high concentration conditions compatible with industrial scale material transport considerations.
The lessons learned from the search for practical uses of laser photochemistry in industry are instructive here. Many scientist-years of effort were applied to the task of identifying and evaluating potential uses of lasers in chemical processes. Of the targets identified, only two appear to have any potential for commercial use. Many have none. Photochemical synthesis with lasers cannot compete even with conventional photochemical processes (e.g., synthesis of vitamin D). High value purification processes (removal of arsine from silane) and high value separation processes initiated by lasers (isotope separation, rare earth separations) have not proved feasible. Laser initiation of chain reaction chemical synthesis (vinyl chloride or addition of HBr to ethylene) also proved impractical. Only laser pyrolysis processes leading to fine, refractory powder synthesis (ceramics or catalysts) and several high value laser processing applications (laser lithographies, laser CVD processes, laser medicinal or surgical procedures, or materials processing functions) have become commercial. In these successful applications, the laser provides spatiotemporal advantages not obtainable in any other way.
Potential applications of concentrated solar photons must similarly seek out opportunities where their advantages over competitive methods are clear. The advantages of concentrated solar photons include their low cost (though that may be debatable when full amortization of facilities is included), their high continuous wave (cw) (when compared to pulsed lasers) fluences, and their ability to provide the high photon fluences in combination with high temperature. The latter characteristic appears to represent a unique research opportunity for concentrated solar photons. However, little is known today about the behavior of molecules under the simultaneous stimulation of both
photons and heat, especially when the photon fluence is high enough that sequential multiphoton excitation processes may occur in the reactor. Under the conditions of high solar concentration, it is likely that reactive intermediates (some excited states, radicals, carbenes, etc.) produced by initial photon absorption will be themselves subject to electronic excitation. It is therefore possible that highly energetic reaction paths could be followed. It is also conceivable that at the high temperatures that can be obtained in solar furnaces, the initially or subsequently excited states produced could react in thermally activated processes not observed to date in conventional photochemical experiments at room temperature. These possibilities merit exploratory research. Much basic information about the spectroscopy of one-photon produced excited states and intermediates now exists, but little information is available on the photo-or thermochemistry of these species.
Enthusiasts for solar photochemistry must also be aware of the limitations of their light source. While the large spectral bandwidth and resulting high infrared (heating) component of solar irradiation are often cited as disadvantages, the discussion above describing the opportunities for multiphoton excitation and thermal excitation processes at high degrees of solar concentration actually relies on the broadband nature of the solar spectrum. In other applications it may be a disadvantage. The solar light source is also intermittent, both diurnally and during bad weather. This can severely limit the productivity of any proposed solar industrial facility. Low productivity rates can allow competing technologies for the synthesis of the target material to acquire advantage. It must always be recalled that solar synthesis schemes compete against a mature synthesis industry which can probably make any synthetic target by some nonphotochemical process. This fact renders most solar synthesis schemes ultimately vulnerable to nonphotochemical competing routes.
Overall Assessment
Opportunities do exist for application of concentrated solar photons in synthetic chemistry, but they are limited and they are not readily apparent. Greater opportunity probably exists in preparation of inorganic materials than organic ones, especially for refractory particles. Specialized purification processes are also potential areas for application. For one-sun level irradiation, biomass conversions may present opportunities. The most probable area for potential application is in materials processing. All of these areas are premature for application. Much research is required to understand the processes which can occur under high solar fluences before firm prediction of applications can be made. The data base of industrial processes developed to seek applications of lasers in synthesis should be reexamined in the context of solar sources.
PHOTOPHYSICS AND PHOTOCHEMISTRY OF PORPHYRIN SYSTEMS
John S. Connolly
Photoconversion Research Branch
Solar Energy Research Institute
Golden, Colorado
This research program addresses some fundamental aspects of photoinduced electron-and charge-transfer in molecular systems leading to the design and assembly of a synthetic photoreaction center. Current investigations are concerned with tightly linked porphyrin-quinone molecules in which strong electronic coupling between the donor and acceptor moieties is highly sensitive to small changes in the dielectric properties of the host matrix. This permits us to probe the local dielectric environments in various media, including natural and synthetic polymers. We have also synthesized some new molecular systems to study the effects of distance, extended conjugation, and energetics on the rates of intramolecular electron and charge transfer, and the influence of substituents on conformational rigidity of the porphyrin ring.
We have previously reported the synthesis and characterization of a porphyrin-anthraquinone (PAQ) molecule (I) in which the quinone is attached at a meso position of tritolylporphyrin. The absorption spectra of both the free-base (H2PAQ,I) and zinc (ZnPAQ,II) forms show pronounced perturbations in the bands of the porphyrin and quinone groups that are independent of solvent polarity. The fluorescence spectrum of I is only slightly red-shifted, but the lifetimes and intensities depend markedly on dielectric constant, with the major changes occurring over the range: 4 < e, < 9.
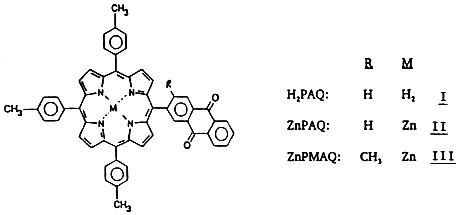
Reprinted with Permission from: Solar Energy Research Institute, Photoconversion Research Branch
We have extended these studies to the Zn analogue of H2PAQ (II) in which the energy gap between the S1 state of the porphyrin and the sum of the redox potentials (measured in benzonitrile) is -0.55 eV. Thus, if a two-sphere dielectric continuum treatment is applicable, the onset of CT interactions would be expected to occur at much lower solvent polarities than in the case of the free-base form. This has indeed been found to be the case. In fact,
we can resolve the emission of a CT state even in solvents such as benzene and toluene, compared with ZnTPP in these solvents as well as with ZnPAQ in lower dielectric media (e.g., cyclohexane). Unambiguous identification of the CT state has been provided by time-resolved microwave conductivity measurements, which reveal a transient dipole of >30 debye with a lifetime of ~1–2.5 ns, depending on solvent. It appears that "TICT" (i.e., twisted intramolecular charge-transfer) states are involved.
Despite the close proximity between the P and AQ moieties (~1.4 Å edge to edge), the fluorescence data for both H2PAQ and ZnPAQ in more than 40 solvents and binary mixtures correlate surprisingly well with the solvent-dependent reaction energetics estimated using a two-sphere dielectric continuum model. These results have interesting implications for designing molecules to probe the dielectric environments in viscous media, e.g., vesicles and polymers.
Future Directions
Because these PAQ molecules are highly sensitive probes of the dielectric properties of nonpolar media, they should also be useful in characterizing the microphase dielectric of ordered structures such as vesicles and microemulsions. This information is particularly useful for understanding both the nature of structural perturbations caused by introducing dopant molecules into ordered fluids and the effect that microstructural organization might have on the intrinsic properties of the dopant, such as redox potentials.
The main question we wish to address regarding the behavior of polymers relates to the nature of the local dielectric environments in various regions. Spectroscopic and photophysical studies will be carried out on these PAQ probes codissolved in polymer films in order to understand their behavior in various environments. Key aspects will be to optimize the conditions for CT interactions at various temperatures, enhance the magnitude of the effect, and vary the emission wavelength in a systematic way in order to be able to deconvolve the observed kinetics in terms of specific rate constants for the stepwise processes involved.
Encapsulation of a relatively small molecule such as H2PAQ or ZnPAQ should also provide a highly sensitive probe of specific protein environments. Bowler and coworkers recently reported on long-range electron transfer from a pentaammineruthenium complex to the heme iron in a structurally engineered cytochrome c. Such studies are, in a sense, designed to sample the average dielectric environment of the ~15 Å pathway(s) between donor and acceptor, but cannot reveal any information about the local regions of either. We are exploring the feasibility of inserting a PAQ-like probe into such a preparation. For example, it may be possible to embed the porphyrin end of an appropriately substituted molecule in the heme cavity of an apomyoglobin without perturbing the surrounding protein.
The existence of CT states in simple linked donor-acceptor molecules demonstrates that it should be possible to devise more complex systems in which the fraction of excited-state energy that can be ultimately converted to stored redox energy can be maximized. Thus, such a CT state could be tuned to the medium and serve as a precursor to formation of a longer-lived RIP. This should be a major step in assembling a synthetic photoreaction center.
Acknowledgment
This work was supported by the Division of Chemical Sciences, Office of Basic Energy Sciences, U.S. Department of Energy.
POTENTIAL INDUSTRIAL APPLICATIONS OF PHOTONS
James T. Yardley
Allied Signal, Inc.
Research and Technology
Morristown, New Jersey
Abstract
Photons provide unique opportunity for chemical processing—a fact which has been well-utilized by nature, has been recognized by the ancients, and has received new significance with the development of laser technology. The maturity of solar energy control and management may open up even more unique and exciting opportunity areas. In this report, I will first present a very simple tutorial view of photochemical and photothermal processing.
I will next examine some of the experiences of scientists seeking to exploit the opportunities for lasers in chemical processing. In particular, I will present some simple economic factors which place severe limitations on the practical utility for lasers in photochemical synthesis. These realities include
-
the relatively high cost of photons,
-
market size considerations,
-
the difficulty of displacement of mature technologies with unproven ones, and
-
the need to consider not just presently used alternate technologies, but also other advanced technologies.
I will also explore some of the technical factors which have limited the use of lasers for chemical processing. These include
-
secondary photochemistry,
-
scale-up limitations,
-
quantum yield/density considerations,
-
mass and energy transport, and
-
the ability of chemists to accomplish the desired chemistry nonphotochemically.
I will show how the above considerations have shaped the evolution of the quest for viable photochemical processing.
Finally, I will discuss some opportunities for solar photochemical processing. I will first examine its particular gifts and limitations. I will then consider some possibilities in the areas of
-
organic synthesis,
-
inorganic synthesis,
-
purification,
-
biomass production, and
-
materials processing.








