SESSION 3
MATERIALS PROCESSING AND SYNTHESIS
Bruce M. Clemens
Stanford University
Stanford, California
Rapporteur's Report
INTRODUCTION
This workshop was organized to assist the Committee on Potential Applications of Concentrated Solar Photons in achieving the goals of its study. These goals, as listed in the Committee's statement of work, are
-
assess the knowledge base for photon/matter interaction phenomena underlying the use of concentrated solar flux for prospective new non-electric applications;
-
critically evaluate the merits of potential new applications for both near-and long-term use of concentrated solar energy, particularly those applications that address national needs and have the potential for being cost-effective through research and development; and
-
recommend research paths and priorities to enhance the scientific basis of, and increase the potential for, the development of successful applications.
Due to strong absorption, solar radiation will interact with the surface region of most materials. Thus, in Session 3, Materials Processing and Synthesis, the technology area judged to be most amenable to application of concentrated solar photons was surface treatment and modification. The first two speakers in Session 3 addressed this area, with descriptions of current surface modification and thin film growth technologies. A summary of the various current techniques was made, and the features which make each viable were accentuated. The third speaker discussed current research at the Solar Energy Research Institute aimed at exploring the application of concentrated solar photons to surface treatment. Proof of concept experiments have been performed in several application areas. The fourth speaker discussed the economic analysis of solar furnaces versus conventional intense light sources. Points raised during the discussion included the unique aspects of solar light which might make applications feasible and recommendations for future work.
This report is organized in a manner analogous to the presentations. The first section concerns current surface modification and thin film growth technologies. The second section is a discussion of the possible applications of concentrated solar photons. The third section summarizes the results of an economic analysis, and the fourth has suggestions for future research and organizational needs.
PRESENT SURFACE TREATMENT TECHNIQUES
Surface treatment is used to impart desirable properties to the surface of a sample or part without the added expense in materials and energy required
to alter the bulk. This gives the ability to independently optimize the bulk and surface properties; for instance, a surface region may need to be hard or corrosion resistant, while mechanical toughness is required of the bulk. In the first talk of Session 3, William D. Sproul of Northwestern University, discussed several current surface modification technologies.
Surface Heating with Light
Since visible light is absorbed in about the first 30 nm in most metals and opaque materials, intense light fluxes can be used to selectively heat the surface region. Techniques which use light to heat surfaces can be classified according to the interaction time and energy density. In general, the processes which have a short interaction time (10-4-10-10 sec.) have a high energy density (104 -1010 W/m2). Short interaction time processes can achieve extremely high heating and cooling rates (1012 K/sec.), and thus can be used to produce nonequilibrium phases such as metallic glasses. Laser glazing of a previously applied thin film can heal pinholes and defects as well as promote adhesion and densification. Slower interaction time processes can be used to anneal or harden the surface region or melt a previously applied powder or film. Powder melting can produce a dense well-adhered film. These films can be several mils to over a hundred mils thick. One of the primary disadvantages is that the surface tends to be quite rough and requires a postmachining process. Lasers can be used to ablate material to pattern the surface on a very fine scale. Printing roles are patterned in this manner to achieve optimum inking characteristics.
Thermal Spray/Plasma Arc
In the techniques of thermal spray or plasma arc, powder is fed into a heat source (either a flame or electric arc) where it is melted and then accelerated as molten droplets into the surface where it condenses into a film. This is a relatively inexpensive and rapid process for forming a surface film, but the films which result have a high density of several types of defects, including unmelted particles, voids, and oxidized particles. The process is extremely complex, with many parameters that affect the final film properties in a complicated manner. Nonetheless, this is a widely used process, particularly in the aerospace industry where a typical gas turbine engine has 15 pounds of thermal spray coatings.
Chemical Vapor Deposition
Chemical vapor deposition (CVD) uses thermal energy to decompose precursor molecules which results in deposition of a film. This technique is widely used in the semiconductor and metallurgical coatings industries to produce Si, SiO2, TiN, and Al2O3, among others. of particular interest is photo-assisted CVD where light is used to not only heat the surface region, but to photolytically assist in breaking the precursor molecule bonds. Principal disadvantages are the high temperatures required and the toxicity of the precursor materials. Advantages are nondirectionality of deposition, which results in uniform coatings on complex shaped parts, and low cost.
Physical Vapor Deposition
In physical vapor deposition, films are produced by physical transport of atoms from source to substrate. Evaporation techniques use resistance or electron beam heated hearths to evaporate the source atoms, while sputter deposition forms films from atoms ejected from the source by bombardment by energetic particles, usually inert gas ions. Both techniques need a vacuum environment, and the film properties are strongly affected by the energy and surface mobility of the arriving species.
Ion Beam Techniques
George Fenske, of Argonne National Laboratory, discussed several techniques that utilize bombardment with energetic ions to alter the surface properties. These techniques can be classified as ion mixing, ion implantation, or ion-assisted deposition. In ion mixing, an ion beam is used to posttreat a film to achieve mixing at the interface resulting in improved adhesion. Ion implantation uses ion beams to directly enrich the surface region in the ion species. Both these techniques require expensive ion sources and are inherently slow. Ion-assisted deposition uses ion bombardment during vapor deposition to enhance mixing with the substrate and arriving species mobility, resulting in improved film qualities. In addition, ion bombardment can be used prior to film deposition to clean and roughen substrates and thus improve film adhesion. These later two uses of ion beams can utilize less expensive lower energy ion sources than ion beam mixing or implantation.
MATERIAL USES OF CONCENTRATED SOLAR PHOTONS
In order to assess the application of concentrated solar photons for materials, it is instructive to examine the properties obtainable in concentrated solar beams. J. Roland Pitts of the Solar Energy Research Institute discussed solar radiation, its concentration, and several possible applications. The solar spectra is (not coincidentally) mainly in the visible spectral range and has a penetration depth of about 20–50 nm in many metals and opaque materials. Thus highly concentrated solar beams are ideally suited for surface heating. Solar furnaces can obtain concentration factors of up to 10,000 resulting in fluxes of 1000 W/cm2. Solar light can be delivered at a fraction of the energy cost of conventional sources such as lasers or arc furnaces. During discussion, the relevant characteristics of solar light were proposed to be
-
its radiative nature, allowing its use for surface heating;
-
the ability to concentrate solar light to produce high flux-high area light sources; and
-
the spectral characteristics of solar light.
The possible applications which utilize these characteristics can be classified into thermal and photolytic.
Thermal Applications
This class of applications utilizes concentrated solar flux to heat sample surfaces. There are many technologies that can utilize this ability, and Pitts discussed many proof of concept experiments which have been performed at SERI. Included in these was powder melting or cladding, surface film glazing, including the initiating of self-propagating reactions of multilayer Ni/Al films, and hardening of steel surfaces. Concentrated solar photons were used to form the desired high-Tc, phase during rapid thermal annealing of a thin film in an oxygen atmosphere, as well as for thin film deposition by CVD. Films formed in this manner included diamond-like carbon and TiN, and SiC. In addition the high flux capability of a solar furnace could be used for rapid thermal chemical vapor deposition. Other applications suggested during the workshop included using solar energy as a heating source for evaporation. This could be used in environments where electron beams cannot be used, such as in evaporation in a partial vacuum to produce nanophase powders. Also suggested was the use of concentrated solar photons to heat a substrate either prior to or during deposition. This application is
attractive in cases where extremely high temperatures (~2000 K) are needed, as these are difficult or impossible to obtain in other manners.
Photolytic Applications
Photolytic applications would utilize solar light to directly break chemical bonds. Due to the broadband characteristics of the solar spectra, there is relatively little power at any given wavelength. Thus a photolytic application should take advantage of as much of the solar spectrum as possible. Furthermore, most absorption processes need shorter wavelength photons than are available in the solar spectrum, so some control of absorption processes is needed for these applications. This may be possible with absorption of molecules on surfaces. One area where these types of processes may be useful is in polymers, where bonds can be readily broken by solar radiation. No applications were suggested in the workshop, and a need for concepts in this area was expressed.
ECONOMIC ANALYSIS
Greg Kolb, of Sandia National Laboratories, presented the results of his study comparing the relative economic advantages of solar furnaces and high intensity arc lamps. Arc lamps are cheaper initially and are unaffected by weather. They perform better for assembly line continuous processes since they can be operated continuously. However, the energy costs of arc lamps are much higher, and as the cost of energy rises, the solar furnace will become more practical. In addition, for applications involving batch processing of parts which utilize 5000 suns or more, a solar furnace is more economical to run even with current energy prices. The solar furnace can also have a long distance between workpiece and optical elements, allowing for greater flexibility of process design, particularly in cases where the process generates dirt which can damage optical elements.
The outline of a second study by Walter Short comparing solar furnaces with CO2 lasers was also circulated. CO2 lasers, which are widely used for surface annealing, have several advantages over solar furnaces. These include high intensity, availability, and control, as well as independence from the weather and the ability to use an existing building. However, CO2 lasers are even more energy inefficient than arc lamps, and thus solar furnaces have an even greater advantage. They can also deliver high power over larger areas and have better absorption characteristics than CO2 laser light.
SUGGESTIONS FOR FUTURE RESEARCH AND DEVELOPMENT
Future research should concentrate on areas that take advantage of the unique characteristics of concentrated solar photons. The ability to deliver high flux over a large area suggests that a solar furnace could be used for rapid heating and cooling of large areas. The limits on cooling and heating rates for solar furnaces should be explored. The surface absorption of solar light also implies that high gradients can be produced. In other words, the surface can be heated to high temperatures without affecting the bulk. Of course this also leads to high cooling rates, and applications exploring the formation of metastable phases should be explored. In addition solar furnaces can possibly be used to obtain extremely high temperatures (~2000 K). The limits on this ability should be explored and applications which need high temperatures should be sought. Applications in the areas of polymers should be researched.
In general, it is felt that there needs to be a mechanism established to find applications. The researchers involved in solar methods need to connect with researchers and technologists working in the application areas. It was suggested that perhaps the solar furnaces could be made available to outside researchers to foster this interaction.
APPLICATIONS OF ENERGETIC PARTICLES IN IMPROVING COATING ADHESION PROPERTIES*
George R. Fenske
Argonne National Laboratory
Argonne, Illinois
Introduction
In many engineering situations, material selection is often based on a compromise of bulk-mechanical properties and near-surface properties, with neither set of properties at its optimum value. Often the material of choice (e.g., high-Cr steel in corrosion applications, or TiN or WC in wear applications) cannot be fabricated into bulk components due to limitations based on cost, fabrication, and mechanical properties. In such cases, various processes are available to deposit coatings of the desired material on components fabricated from materials with desirable bulk properties. A key property of any coating process is adhesion of the coating to the substrate. Without adequate adhesion, the coating will be lost and thus can no longer protect the substrate.
Numerous surface-modification processes can be used to modify the surface properties of a wide range of materials. These processes range from surface heat treatments (e.g., thermal hardening, carburizing, nitriding, carbonitriding, boriding, and metalliding) that rely on thermal processes (primarily diffusion) to produce the desired property in near-surface regions, to surface coating processes (e.g., electro-and electroless chemical deposition, chemical vapor deposition [CVD], physical vapor deposition [PVD], spraying processes, and welding processes) in which material is formed or deposited on the surface. Adhesion is usually not a major concern with surface heat treatments because thermal diffusion produces a gradual change in composition. In surface coating treatments, however, the transition from the bulk material to the coating material is much more abrupt and thus adhesion is a very important factor that must be addressed in selecting the deposition process.
This paper addresses one approach (ion-beam-assisted deposition, or IBAD) that utilizes energetic ion beams to enhance the adhesion of metallic films to metallic and ceramic substrates. In one application, IBAD is used to improve the adhesion of silver films to ceramic substrates that are subjected to sliding wear conditions at elevated temperatures(1). In another application, the IBAD process is used to improve the adhesion and modify the microstructure of chromium films deposited on low-Cr steel[2]. Both applications suggest that adhesion can be increased by a number of mechanisms including (a) physical and chemical sputtering of surface contaminants (e.g., hydrocarbons and adsorbed water molecules); (b) preferential sputtering of a particular element of a compound, thus producing a surface enriched in a species that is chemically active with the depositing species; (c) activating chemical states; (d) mechanically toughening the surface, producing more surface area for bonding and sites to arrest surface cracks; and (e) recoil mixing during the initial stage of film deposition.
Ion-Beam-Assisted Deposition
A description of IBAD processes can be found elsewhere [3–5]. The main feature that separates IBAD from other PVD processes is that the film being deposited is also bombarded by energetic ions/atoms during deposition. This bombardment affects a number of phenomena (film nucleation and growth, film density, film crystallinity, and film orientation) that determine the adhesive strength of the film to the substrate.
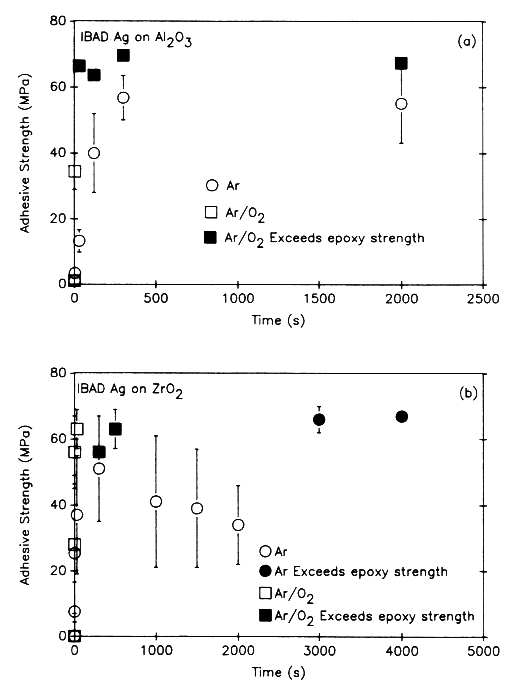
Figures 1A and 1B show the effect of sputter-cleaning on the adhesive strength of Ag films deposited on alumina and zirconia substrates, respectively[1,6]. The size of the error bars corresponds to one standard deviation in the measured values. In tests using an Ar beam only, the adhesion of Ag to Al2O3 increases with ion dose, rapidly at first and then reaching a steady-state value of approximately 55 MPa after presputtering for approximately 300 s, which corresponds to an ion dose of 5.6 × 1016/cm2. When an Ar + O beam was used, the adhesive strength increased at a faster rate as a function of time (compared to Ar ions only). Presputtering for 3 s was sufficient to raise adhesive strength to approximately 35 MPa. After 30 s of cleaning with Ar + O, the adhesive strength of the Ag to the Al2O3 exceeded the tensile strength of the epoxy bonding agent (approx. 60–70 MPa). In tests that used an Ar + O beam to sputter-clean and bombard the film during deposition[7], adhesion was higher than that for sputter-cleaning only with Ar and O.
For Ar sputter-cleaning of ZrO2 (Figure 1B), adhesive strength increased with ion dose (time), rapidly at first and then peaking at approximately 50 MPa after presputtering for 300 s to an ion dose of 7.5 × 1016/cm 2. Beyond 300 s, adhesive strength decreased to 34 MPa after 2000 s (5 × 1017/cm2) and then increased above 65–70 MPa (failure in the epoxy rather than at the Ag/ZrO2 interface) for cleaning times of 3000 s (7.5 × 1017/cm2). When Ar + O was used to sputter-clean the zirconia, adhesion increased very rapidly to values in excess of 65–70 MPa. As seen in Figure 1B, only 30 s (7.5 × 1015/cm2) of cleaning with Ar + O was required to exceed the tensile strength of the epoxy.
A number of mechanisms have been proposed to account for the increased adhesion observed in Figures 1A and 1B[8]. These include (a) physical and chemical sputtering of surface contaminants (e.g., hydrocarbons and adsorbed water molecules); (b) preferential sputtering of Al, Zr, or O that produces a surface enriched in a species that is chemically active with the depositing species; (c) activating chemical states; (d) mechanically roughening the surface, which produces more surface area for bonding and sites to arrest surface cracks; and (e) recoil mixing during the initial stage of film deposition.
Sputter-cleaning, either by Ar alone or with Ar + O ions, is effective in removing surface contaminants, particularly adsorbed water and hydrocarbons[9]. The substrates used in these tests were cleaned with a series of three organic solvents before insertion into the IBAD system[10]. Thus, part of the improved adhesion observed in Figures 1A and 1B can be associated with the removal of organic residues on the substrate surfaces. The higher rates of increases in adhesive strength with sputter time (or dose) seen with the Ar + O beams relative to those for Ar alone can be attributed to a chemical (or reactive) sputtering process in which volatile compounds (such as CO) may have been formed by the reaction of O ions with organic contaminants.
Preferential sputtering of one of the substrate species (such as O), leaving a surface layer enriched (and perhaps chemically active) with Al or Zr, is feasible. Monte Carlo TRIM calculations[11] of physical sputtering of Al2O3 by Ar + O indicate that O is preferentially sputtered. However, impingement of the sputtered surface by residual 02 in the vacuum chamber, particularly during the Ar + O sputter-cleaning (approximately 10-2 Pa of O2) probably negated the preferential sputtering effect, leaving a near-stoichiometric Al2O3 or ZrO2 surface.
Once a clean surface is established, continued bombardment of the surface could produce chemically active O atoms that react with depositing Ag atoms to form a stable compound. Silver is known to form stable oxides (such as Ag2O) at room temperature[12]; thus, it is feasible that the first monolayer of Ag reacts with O atoms to form a ternary Al-OAg compound across the interface similar to that found for Cu deposited on Al2O3[13].
Mechanical roughening due to sputtering is a viable process; however, the amount of material removed by sputtering is only about 40 nm, and measurements[14] of the center-line-average surface roughness before and after sputter-cleaning (from 17 nm before sputtering to 20 nm after sputtering) reveal that this is not a significant factor. Cross-sectional transmission electron microscopy (TEM) of IBAD silver-coated Al2O3 substrates[15], which shows a sharp interface between the deposited Ag and Al2O3 substrate, also indicates that dynamic mixing is not responsible for the improved adhesion of the Ag films tested.
For Cr films deposited on metallic substrates[2] the situation is different. Here, the deposited Cr atoms can be mixed into the substrate through a combination of dynamic mixing and radiation-enhanced mixing processes. The radiation-enhanced processes are dominant in this case because one is dealing with diffusion of Cr in a metallic substrate that already contains Cr, rather than with diffusion of Ag in ceramic substrates. Chromium films deposited by pure PVD or IBAD with 100 eV Ar ions exhibited a gap between the film and the substrate (indicative of degraded adhesion), as observed in cross-sectional TEM micrographs. In contrast, Cr films deposited by IBAD with 300 or 1000 eV Ar ions exhibited no gap between the film and the substrate. The 300 and 1000 eV IBAD films also had an intermixed Cr-enriched layer (approximately 30 to 50 nm deep) in the substrate material; this layer can act as a 'glue' to enhance adhesion of the film to the substrate.
Another difference between Ag and Cr deposition is the role of internal stresses on film adhesion. Cr films are known to delaminate from the substrate if large internal stresses exist. This situation is not as critical for Ag films because they are pliable and will easily undergo creep to alleviate high stress levels. Differences in the microstructures[2] and porosity of the IBAD films deposited with 100 to 1000 eV Ar ions suggest that internal stresses of the films can be controlled by proper selection of the Cr atom deposition rate, ion flux at the surface, and energy of the incident ions.
Discussion
The above examples illustrate the effects that energetic ions/atoms can have on the adhesion of coatings to substrates. The major effects observed were the (a) preparation or cleaning of the surface of the substrate prior to deposition, (b) promotion of strong chemical bonding at the interface, and (c) inducing mixing of deposited material into the substrate.
Concentrated solar beams provide an alternative approach for obtaining comparable effects via pyro-and photolytic reactions. With pyrolytic approaches, intense solar beams could be used to heat substrates and vapor sources to elevated temperatures. Solar heating of the substrates to elevated temperatures before deposition could be used to desorb common surface contaminants (adsorbed water and organic cleaning compounds), thereby providing a clean or chemically active surface for improved chemical bonding to films deposited in subsequent steps. In conventional PVD processes, where substrate heating is commonly used to improve adhesion and nucleation and growth characteristics[16], this step is typically performed in a vacuum because of the requirements of the vapor sources. With solar heating, however, this step could be performed in an inert atmosphere or, alternatively, in a reactive environment (e.g., halide gases) to chemically assist the formation of volatile compounds from the surface contaminants.
Concentrated solar beams could also be used to deposit films. In one approach, solar energy could directly heat and evaporate elements from a crucible. The power densities envisioned for intense solar beam systems (in
excess of several kW/cm2) exceed those typically found in electron beam evaporation sources that operate at power levels of 1 to 20 kW with a 5 cm diameter source (0. 05 to 1 kW/cm2) to evaporate pure elements. Alternatively, solar beam heating of the substrates could be used in CVD thin films in a manner similar to conventional CVD of coatings for electrical, decorative, and tribological applications.
Such applications of solar beams the beam energy to produce pyrolytic phenomena—processes that require elevated temperatures. In many instances, elevated processing temperatures are undesirable. For example, steel components are often heat treated to achieve a certain hardness and toughness. Exposing these components to temperatures above their annealing point degrades these properties. It is therefore difficult to coat many steel components at elevated temperatures without some type of postdeposition heat treatment (which often distorts the component) to return the component to its original specifications. Consequently, processes that can deposit coatings at low substrate temperatures (such as ion-beam techniques) are often desirable. The energy associated with the incident ions can impart sufficient energy to local regions of the coating and near-surface regions of the substrate to produce effects typically achieved at elevated temperatures. Another example of a low-temperature process—and more germane to this workshop—is the use of energetic photons to photolytically activate processes for both cleaning surfaces and depositing coatings.
A process known as photo-enhanced chemical vapor deposition (PHCVD) is receiving considerable attention in semiconductor applications as a low-temperature process for deposition of Si, SiO2, and GaAs[16]. The process is based on photodissociation of gas-phase molecules and typically uses UV light and/or excimer lasers to break the chemical bonds. PHCVD processes are strongly dependent on the absorption of photons with the appropriate wavelength to break the chemical bonds between atoms in the gaseous compound. A majority of the compounds used in PHCVD require UV light to dissociate the molecules, and thus a major obstacle to be overcome in implementing intense solar beams for PHCVD will be to identify compounds that photodissociate (either directly or through a catalytic reaction) at wavelengths that are more abundant in the solar spectrum.
Summary
Coatings are used in a wide number of applications where the properties of the underlying substrate will not suffice. Concentrated solar beams show potential as a viable energy source for a number of coating processes based on pyrolytic and photolytic reactions.
References
1. Fenske, G.R., R.A. Erck, A. Erdemir, V.R. Mori, and F.A. Nichols. 1990. Ion-Beam-Assisted Deposition of Adherent, Lubricious Coatings on Ceramics. Paper presented at 17th Leeds-Lyon Symposium on Tribology (Vehicle Tribology), Leeds, England, Sept. 4–7, 1990.
2. Cheng, C.C., R.A. Erck, and G.R. Fenske. 1989. Microstructural Studies of IAD and PVD Cr Coatings by Cross Section Transmission Electron Microscopy. Mat. Res. Soc. Proc., 140:177.
3. Smidt, F.A. 1990. Use of Ion Beam Assisted Deposition to Modify Microstructure and Properties of Thin Films. Int. Mat Rv. 35 (2):61.
4. Handbook of Ion Beam Processing Technology. Cuomo, J.J., S.M. Rossnagel, and H.R. Kaufman, eds. 1989. Noyes Publication, Park Ridge, N.J.
5. Fenske, G.R., A. Erdemir, R.A. Erck, C.C. Cheng, D.E. Busch, R.H. Lee, and F.A. Nichols. 1989. Ion-Assisted Deposition of High-Temperature Lubricious Surfaces. Presented at 35th STLE/ASME Tribology Conference, Ft. Lauderdale, Fla., Oct. 16–19, 1989, STLE preprint 89-TC-2E-2.
6. Erck, R.A., and G.R. Fenske. 1990. Adhesion of Silver Films to Ion-Bombarded Alumina. Mat. Res. Soc. Symp. Proc. 157:85.
7. Erck, R.A., A. Erdemir, and G.R. Fenske. 1990. Effect of Film Adhesion on Tribological Properties of Silver-Coated Alumina. Proceedings of 17th International Conference on Metallurgical Coatings. April 2–6, 1990, San Diego, Calif.
8. Baglin, J. 1989. Interface Structure and Thin Film Adhesion. In Handbook of Ion Beam Processing Technology. Cuomo, J.J., S.M. Rossnagel, and H.R. Kaufman, eds. Noyes Publication, Park Ridge, N.J., p. 279.
9. Beglin, J.E.E. 1985. Adhesion at Metal-Ceramic Interfaces: Ion Beam Enhancement and the Role of Contaminants. Mat. Res. Soc. Symp. Proc. 47:3.
10. Erdemir, A., G.R. Fenske, F.A. F.A. Nichols, and R.A. Erck. 1989. Solid Lubrication of Ceramic Surfaces by IAD-Silver Coatings for Heat Engine Applications. Presented at 35th STLE/ASME Tribology Conference, Ft. Lauderdale, Fla., Oct. 16–19, 1989, STLE preprint 89-TC-2E-1.
11. TRIM-89. The TRansport of Ions in Matter. Computer program provided courtesy of J.F. Ziegler, IBM Research, Yorktown, N.Y.
12. CRC Handbook of Chemistry and Physics, 60th ed., CRC Press, Boca Raton, Fla.
13. Schrott, A.G., R.D. Thompson, and K.N. Tu. 1986. Interaction of Copper with Single Crystal Sapphire. Mat. Res. Soc. Symp. Proc. 60:331.
14. Erck, R.A., and G.R. Fenske. 1990. Adhesion of Silver Films to Ion-Bombarded Zirconia, to be presented at STLE/ASME Tribology Conference, Oct. 7–10, 1990, Toronto, Ontario.
15. Erdemir, A., G.R. Fenske, R.A. Erck, and C.C. Cheng. 1990. Ion-Assisted Deposition of Silver Films on Ceramics for Friction and Wear Control. Lubr. Eng. 46:23.
16. Handbook of Thin-Film Deposition Processes and Techniques. 1988. K.K. Schugraf, ed. Noyes Publication, Park Ridge, N.J.
PULSED LASER PROCESSING OF SOLAR CELLS
Rajiv K. Singh
University of Florida
Gainesville, Florida
J. Narayan
North Carolina State University
Raleigh, North Carolina
Abstract
Pulsed nanosecond excimer lasers can be effectively used for fabrication of silicon based solar cells having controlled junction depths and sheet resistivities, and high minority carrier lifetimes. By using pulsed beams, the ion implanted layers are melted and the underlying ''defect-free crystal'' provides a seed for subsequent crystal growth, resulting in 100% electrical activation of the dopant atoms. The understanding of the laser-solid interactions throws insight into the thermal effects of solids irradiated with pulsed laser beams. For metallization of the p-n junctions, the excimer laser beams can also be used to deposit stoichiometric good quality TiN thin films by evaporation of bulk TiN targets.
Introduction
Pulsed excimer laser beams (wavelength λ = 0.193–308 µm, pulse duration τ = 15–45 × 10-9 sec) are ideally suited for fabrication of efficient (shallow junctions) solar cells [1,2]. High quality p-n junctions can be fabricated from polycrystalline silicon by ion implantation followed by laser annealing. By using pulsed nanosecond laser beams, the whole process (melting and resolidification) is completed within 200 × 10-9 sec [3,4]. Since the solidification velocities are typically 4 to 5 m/sec, and the specimens are subjected to very short times at high temperature, impurity segregation at the grain boundaries is completely eliminated. The depth of p-n junctions can be controlled by varying the laser and ion-implantation parameters. This process leads to formation of "defect-free" p-n junctions with response in the blue region close to that of p-n junctions fabricated from single crystals. In addition, since the substrate temperature remains close to the ambient value during laser annealing, minority carrier lifetime (MCL) of the substrate is not degraded as often happens during conventional furnace annealing. This factor leads to higher quantum efficiency for longer wavelengths in the red region. Thus, the overall efficiencies of solar cells are considerably improved. The next important step in the formation of solar cells, after the formation of p-n junctions, is the metallization process. This step assumes additional importance, particularly for shallow junctions. Recently, we have identified a pulsed laser evaporation technique [5–9] for TiN film fabrication at very low temperatures (25–400ºC). The TiN films are polycrystalline with grain size of ~100 Å, and electrical properties close to that of bulk TiN. This technique can be successfully used for metallization of shallow junctions without adversely affecting the junction characteristics.
Laser-Solid Interactions
The understanding of the laser-solid interactions provides information on the effect of laser parameters including pulse energy density, wavelength, pulse duration on the maximum melt depths, melt lifetimes, surface temperatures, and solidification velocities. The photon energy of a
nanosecond excimer laser beam is absorbed by the semiconductor by electron-hole excitations and other absorption mechanisms resulting in thermal equilibrium of the carriers in less than 10-13 sec. The laser energy is transferred to the lattice by photon emission processes (heat) in times of the order of 10-12 sec, and thus for nanosecond irradiation regimes, the plasma effects of excited carriers are negligible [3]. The laser energy manifests itself in the form of heat, and the thermal effects of nanosecond laser irradiation can be determined by the solution of one-dimensional heat flow equation with appropriate boundary conditions, while taking into account the phase changes occurring in the material. A three-dimensional solution is not required because of the very short processing times (~200 ns) and the large transverse dimensions of the laser beam, which result in thermal gradients many orders of magnitude greater in the perpendicular direction to the surface than compared to the transverse directions [3,4]. The one-dimensional heat-flow equation is given by
where, x refers to the direction perpendicular to the plane of the sample and t is the time, and the subscripts i = 1,2 refer to the solid and liquid phase, respectively. The terms ρ, C, K, R, and α correspond to the temperature dependent mass density, specific heat capacity, thermal conductivity, reflectivity and absorption coefficient of the incident laser beam, respectively. Io corresponds to the time dependent laser intensity striking the surface. The boundary conditions at the front and back surface assume that there are no thermal losses which approximately hold for nanosecond processing times. The solid-liquid interface is assumed to be at the melt temperature, and the position of the interface S is determined by the heat balance equation at the interface given by
where Ks and K1 are the solid and liquid thermal conductivities at the interface, and L is the latent heat per unit volume of the material. The presence of a moving solid-liquid interface, and the temperature dependent thermophysical and optical properties make the analytical solution of the heat flow equation intractable, and thus approximate numerical techniques have to be applied. To solve Equation (1), we have adopted a higher order implicit finite difference method in which the thermal gradients at the interface are accurately determined for calculating the interfacial velocity[4]. The temperature dependent optical and thermal properties and the time dependent laser intensity have been taken into account in this solution.
The laser irradiation of ion-implanted samples leads to the removal of the implantation damage up to the distance of the maximum melt depth. The underlying defect-free substrate acts as a seed for subsequent crystal growths, resulting in removal of ion-implantation damage and electrical activation of the dopant atoms. To verify the working of the heat flow program the simulated values of the maximum melt depths were compared with the experimental values obtained by excimer laser irradiation of ion-implanted samples at various energy densities. Figure 1A shows TEM micrographs of boron-implanted samples which were irradiated with XeCl laser having a trapezoidal pulse shape and full width at half maximum (FWHM) of 25 and 70 × 10-9 sec, and energy density varying from 1.0 to 2.5 J/cm2. A complete removal of the dislocation loops in the annealed region together with a sharp transition between the annealed and unannealed regions is observed. The V-shaped dislocations are created because the melt front intersects the dislocation loops, and the two segments of the dislocation grow back to the surface because the dislocation cannot end inside the perfect lattice.
The kinetics of the melt front is crucial in understanding the nature of laser-solid interactions. Figure 2A shows the position of the solid-liquid interface of XeCl laser irradiated samples as a function of time for different pulse energy densities and durations. The slope of the curves corresponds to the velocity of the melt front at any instant. The figure shows that above a certain energy density and after a certain time interval, the surface starts to melt, and the melt front penetrates several thousands angstroms before heading back to the surface. The solidification velocity is approximately a few meters per second, which is about five orders of magnitude greater than the normal crystal growth velocity. The maximum solidification velocity dS/dt can be estimated as
where D corresponds to the thermal diffusivity, Tm the melt temperature, and C is constant factor between 1.5 to 2.0, the exact value depending on the shape of the laser pulse.
The maximum melt depths for excimer XeCl laser irradiation of silicon at different energy densities and pulse durations is shown in Figure 1B. For both 25 and 50 ns laser pulses, the maximum melt depth is found to be proportional to the energy density. The x intercept corresponds to the minimum energy required for propagation of the melt front into the substrate (Eth). The melting threshold can be estimated from energy balance considerations [4], and is approximately equal to
where R1 is the liquid reflectivity and τ correspond to the pulse duration. If we perform an energy balance, the maximum melt depth Δxt as a function of energy density is given by
where C1 = (1-R1)/(ρCpTm + L) is constant for a particular material and corresponds to the slope of the graph in Figure 1B. The value of C1 for silicon is 3800Å J-1 cm2, which is quite close to the value obtained from detailed heat flow calculations (3870Å J-1 cm2).
Another important aspect in the understanding of the laser-solid interactions are the temperature profiles generated during intense nanosecond laser irradiation. Figure 2B shows the surface temperature of silicon as a function of time for a 25 ns excimer laser pulse having different energy densities. It is seen from this figure that the surface temperature rises rapidly until it reaches the melting point of the material, where it pauses momentarily until the change in reflectivity upon melting of silicon is compensated, and then rises again until its maximum value. On cooling, the surface temperature quickly drops to the melt temperature and remains there until the interface recedes to the surface. From energy balance considerations, we can estimate the temperature rise as a function of energy density. The temperature rise above the melting temperature ΔT is equal to
This expression shows the parabolic nature of the surface temperature rise above the melt temperature with increasing pulse energy density. For silicon, the value of the constant in Equation (6) is equal to 242 K/(J cm-2)2 for a 25 ns pulse, which corresponds closely to the value of 242 K/(J cm-2)2 obtained from detailed heat flow calculations.
Electrical Properties of Laser Annealed Samples
The melt depth corresponds to the thickness to which the annealing of the implantation damage is observed. Laser annealing of ion-implanted damage yields significant improvement in the electrical properties important for photovoltaic applications. The number of electrically active implanted ions found to be present at the substitutional sites after laser annealing is much higher than conventional thermal annealing. After laser annealing, the ion-implanted specimens are completely free of extended defects, whereas a high density of loops and dislocations is observed in furnace-annealed specimens. Figure 3A shows a plot of the carrier concentration as a function of implanted dose after laser and conventional furnace annealing. A 100% electrical activation is realized after laser annealing compared to a less than 80% electrical activation after furnace annealing. Since pulsed melting involves subsequently non-equilibrium rapid quenching, the dopant concentrations far exceed the equilibrium solubility limits which can be incorporated into substitutional lattice sites via solute trapping phenomena[10]. It has also been observed that the laser annealing does not degrade, in contrast to thermal annealing, the minority carrier lifetime in the substrate or the base region because of the very short thermal diffusion lengths[1]. The laser annealing allows precise control of parameters especially junction depth, sheet resistivity, and MCL, which result in increased yield and improved cell performance.
The wavelength of the laser beam was found to strongly affect the solar cell characteristics. Under optimum annealing conditions, all the cells had open circuit voltage ranging from 600–610 mV and fill factors (FF) in the range of 0.77–0.80. However, there was noticeable difference in the short circuit current, Jsc. In general, the excimer laser annealed samples showed better Jsc than the ruby annealed ones; whereas among the excimer laser annealed samples, the BF3 implanted cells had higher Jsc than cells obtained by direct 5 kV implantation of boron. The results can be understood from internal quantum efficiency measurements before the application of antireflection coatings. The results shown in Figure 3B clearly indicate that the BF3-implanted, excimer laser annealed cells have the highest blue response. This can be attributed to the high quality shallow junctions formed from shallow implantation (1 kV) and extremely uniform annealing obtained with excimer laser. The best discharge implanted, excimer laser annealed solar cells obtained so far have the parameters Voc = 610 mV, Jsc = 34.7 mA/cm2, FF = 0.79, which result in an efficiency of 16.7%. This efficiency is comparable to the highest efficiency of Si solar cells made by more sophisticated conventional methods.
The excimer laser can be used effectively for the metallization of the solar cell by depositing thin layers of TiN using the pulsed laser evaporation technique (PLE). In this method, a pulsed excimer laser is used to evaporate thin a TiN target material in a high vacuum chamber, and the film is deposited on a substrate which is placed parallel to the target [6–8]. An important advantage of this technique is that under optimized laser conditions, the film retains its stoichiometry over a large area. Because of-the high kinetic energy and the mobility of the species, the TiN films can be fabricated in a temperature range of 25 to 400ºC. Figure 4A shows a typical Auger electron spectroscopy spectrum for a TiN film deposited on silicon at 400ºC and 5 J/cm2. The Auger spectrum of the films shows that the principal peaks corresponding to nitrogen and titanium are overlapped, thus complicating the analysis of the film. However, the Auger peaks are similar to the TiN bulk target, including the oxygen peak at 508 eV (as shown in Figure 4B. From the similarity of the two spectra, it is concluded that the material resistivity behavior with temperature, with a room temperature value of approximately 150 mW cm. These films are characterized by very small grains (~100 Å) which are randomly oriented to each other.
In conclusion, we have shown that laser processing of solar cells can be very effectively used to control junction depths, sheet resistivities, and
result in large minority carrier lifetimes. The excimer laser can be further used to evaporate stoichiometric nitride layers for metallization of the solar cells.
References
1. White, C.W., J. Narayan, and R.T. Young. 1979. Science 204:461.
2. Young, R.T., J. Narayan, W.H. Christie, G.A. Van der Leeden, J.I. Levatter, and L.J. Chen. 1983. Solid State Technol. 26:183.
3. Wood, R.F., and G.E. Giles. 1981. Phys. Rev. B23:2923.
4. Singh, R.K. and J. Narayan. 1989. Mat. Sci. and Engr. B3:217.
5. Biunno, N., J. Narayan, S.K. Hofmeister, A.R. Srivatsa. and R.K. Singh. 1990. Appl. Phys. Lett. 54:1519.
6. Narayan, J., N. Biunno, R.K. Singh, O.W. Holland, and O. Auchiello. 1987. Appl. Phys. Lett. 51:1845.
7. Singh, R.K. and J. Narayan. 1990. Phys. Rev. B41:8843.
8. Singh, R.K. and J. Narayan. 1990. J. Appl. Phys. 68:233.
9. Singh, R.K., N. Biunno and J. Narayan. 1988. Appl. Phys. Lett. 53:1013.
10. Narayan, J. R.T. Young and C.W. White. 1987. J. Appl. Phys. 49:3912.
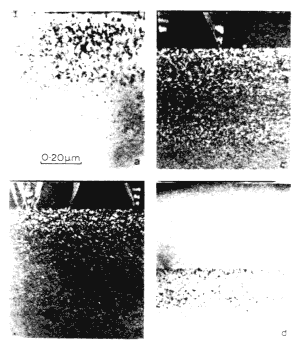
Figure 1A <110> XTEM micrographs showing depth of melting as a function of energy density for 25 and 70 ns laser pulses: (a) 1.0; (b) 1.5 J/cm2 with pulse duration t = 70ns; (c) 1.0; and (d) 2.0 J/cm2, with t = 25ns.
Reprinted with Permission from: Materials Sciences & Engineering Department, North Carolina State University
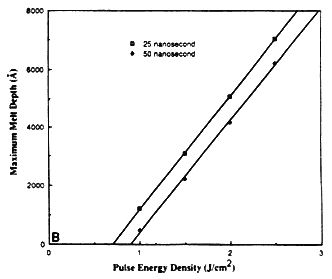
Figure 1B Calculated depth of melting as a function of energy density and pulse duration for XeCl laser irradiated Si samples.
Reprinted with Permission from: Materials Sciences & Engineering Department, North Carolina State University
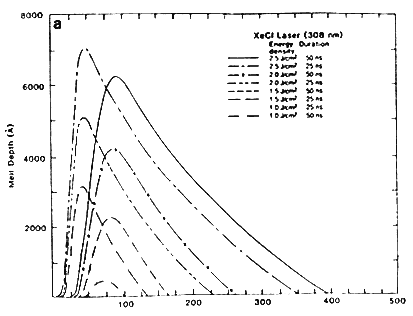
Figure 2A Melt from for 25 and 50 ns laser pulse.
Reprinted with Permission from: Materials Sciences & Engineering Department, North Carolina State University
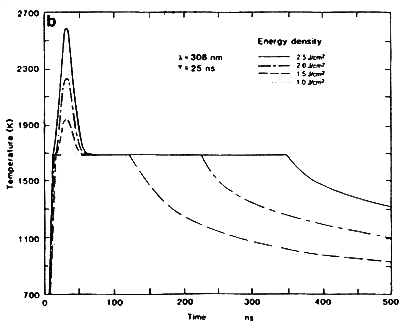
Figure 2B Surface temperature for 25 ns laser pulse as a function of time for Si samples irradiated with XeCl excimer lasers at different energy densities.
Reprinted with Permission from: Materials Sciences & Engineering Department, North Carolina State University
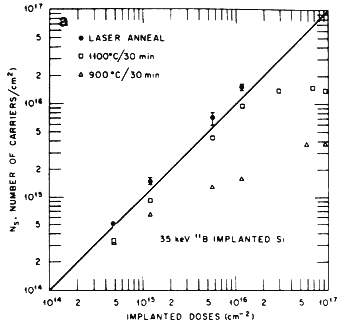
Figure 3A Carrier concentration as a function of implanted dose in thermally and laser annealed specimens.
Reprinted with Permission from: Materials Sciences & Engineering Department, North Carolina State University
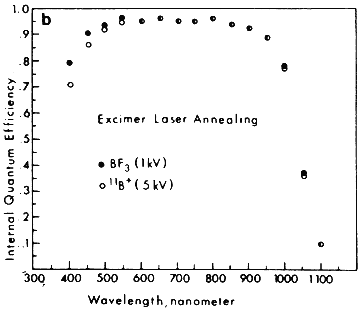
Figure 3B Comparison of the internal quantum efficiency between cells fabricated using excimer laser annealing after BF3+ (1kV) and 11B+ (5kV) implantations.
Reprinted with Permission from: Materials Sciences & Engineering Department, North Carolina State University
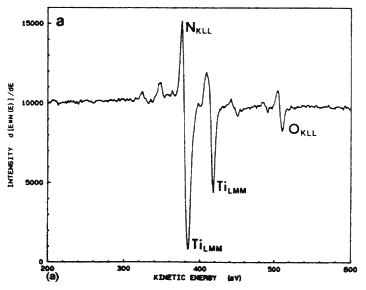
Figure 4A Auger electron spectrum taken from a TiN film grown at 5 J/cm2 and 400ºC.
Reprinted with Permission from: Materials Sciences & Engineering Department, North Carolina State University
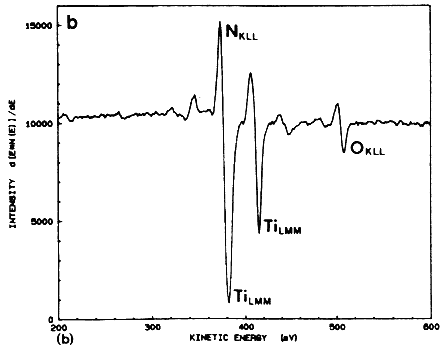
Figure 4B Auger electron spectrum taken from a bulk TiN target irradiated after 4 min Ar sputtering.
Reprinted with Permission from: Materials Sciences & Engineering Department, North Carolina State University
SURFACE MODIFICATION TECHNOLOGIES USING CONCENTRATED SOLAR RADIATION
J.R. Pitts, J.T. Stanley, and E. Tracy
Solar Energy Research Institute
Golden, Colorado
C.L. Fields
Dept. of Chemistry
University of Northern Colorado
Greeley, Colorado
Abstract
A recent report by the National Research Council, entitled Materials Science and Engineering for the 1990s: Maintaining Competitiveness in the Age of Materials, presents "a unified view of recent progress and new directions in materials science and engineering." In particular, it describes important new areas of research and development, the roles of the federal and private sectors as they relate to a balanced national materials effort, and areas of work deemed crucial to the strength of the U.S. economy and defense. Research conducted at the Solar Energy Research Institute (SERI) during the past three years addresses a number of the critical areas described in this report and has explored the possibility of using highly concentrated solar radiation to induce beneficial surface transformation. The principal goal is to develop new coatings and processes that improve the performance and lifetime of materials at reduced processing costs.
Highly concentrated radiant energy provides a controllable means of delivering large flux densities to solid surfaces, where the resulting thermal energy can cause phase changes, atomic migrations, and chemical reactions on a surface without greatly perturbing the bulk properties; alternatively, the photons may directly interact with species on the surface. These changes may result in improved properties of the materials by making the surface harder, more resistant to corrosion or wear, thermally resistant, or with lower coefficients of friction. In a solar furnace, this flux can be delivered in large quantities over large areas, or it can be tailored to match the demands of a particular process. Furthermore, this occurs without the environmental liability associated with providing power to more conventional light sources. Recent work at SERI has used fluxes in the range from 100 to 250 W/cm2 for inducing such beneficial surface transformations. Significant results have been obtained in the area of phase transformation hardening of steels and melting powders and preapplied coatings to form fully dense, well-bonded coatings on the surface. New directions in coating technology using highly concentrated solar beams to induce chemical vapor deposition processes are described here. Application areas that have not been researched in detail but would appear to be good matches to the solar technology are also reviewed.
INTRODUCTION
Several years ago, when the price of oil dropped below $20 per barrel, interest in generating electricity with alternative sources waned. However, a substantial technology base for concentrating solar energy existed. The natural question to ask was, "Is there anything unique or something that has commercial potential that can use highly concentrated solar radiation?" We embarked upon a study of this question and determined that there is no salient uniqueness to exploit but that there are commercial potentials in areas of technology that require the supply of large amounts of radiant energy[1]. Solar radiation can be directed and concentrated with reflectors that lose
only 5–10% of the radiation at each reflection. Lasers and arc lamps, on the other hand, operate with total conversion efficiencies in the range of 4–9%. Therefore, for processes that require large amounts of radiant energy and where the solar resource is compatible, substantial cost savings might be obtained by using solar furnace technology[2,3].
We have looked at performing surface modification processes with highly concentrated solar beams in some detail. Most of the work has used solar fluxes in the range from 100 to 250 W/cm2. Recently, however, there has been a push to raise the available flux using secondary concentrators, and fluxes up to 2000 W/cm2 have been produced[4]. The potential to go to even higher fluxes for some applications exists, but we have obtained interesting results at the relatively modest flux levels available for most of our experiments. The topical areas that we have experimented with include phase transformation hardening, cladding, radiant joining, initiating self-propagating high temperature synthesis (SHS) reactions, and rapid thermal film growth in controlled atmospheres. Other areas of interest that have been identified are rapid thermal annealing, zone melt recrystallization, rapid thermal processing of ceramic materials, metallization of ceramics, and joining ceramics. Progress in the former areas is described, and the potential of applications in the latter areas is outlined.
PROGRESS
Phase Transformation Hardening
Experiments using solar beams to modify the surface properties of metals have been relatively successful. Hardenable steels (A2, 4340, and nitride grade) have been pulsed and scanned to produce hardened zones on the surface[5]. In addition, we have performed some hardening and annealing procedures on some Cu alloys to demonstrate that near surface regions can be modified without affecting the bulk of the material. These experiments were accomplished on substrates varying in thickness from 1 mm to 1 cm and with pulses varying from 1 to 30 s. With fluxes in the range from 100 to 250 W/cm2, surface regions from 1 to 4 mm can be fully hardened. However, the heat affected zones (HAZ) are not sharp and extend to the back side in samples with longer exposures. Processing at 250 W/cm2 is not viable for most surface hardening tasks because of the depth of penetration of the HAZ. It is known that at flux levels 4 times greater, very nice surface hardening processes can be performed, and applications in selectively hardening plow blades are fully developed[6].
Cladding
Experiments in cladding corrosion and wear resistant materials onto steel substrates have been quite successful. We have been able to clad a variety of powders to 4340, A2, and 1040 steels, as well as to pure iron and nickel substrates. The powders used to date are commercially available plasma spray powders: NiCr alloy (#761), 316 stainless steel, WC in a Co matrix, nickel aluminides, and chrome oxide. The principal difficulties in performing the cladding operations is not the flux levels available, but the control of the atmosphere during the cladding operation, and the horizontal geometry of the beam line in the available facilities. Powders absorb the radiation very well through multiple reflections and heat rapidly because of relatively poor conduction to the substrate. The geometry of the available furnaces dictated mounting the target surfaces vertically, and this meant that the powders had to be fixed to the surface with a binder. Gravity and the outgassing of the binder caused some difficulties, which had to be overcome by applying the appropriate thermal profile. For some samples, substrate oxidation at elevated temperatures prevented the melt from wetting the surface. In these cases, metallurgical bonding did not occur, and it was necessary to go to controlled atmospheres to prevent oxidation and get a good quality clad. The
results of these experiments and micrographs of the metallurgical cross-sections are presented elsewhere [7].
Radiative Joining
Certain aluminum/bronze alloys are under development for use in forms to lay up carbon fiber composite structures for aerospace vehicles. There are a variety of reasons that this material development is needed for the composite manufacturing. The most important are that the forms are easily designed by CAD/CAM procedures and cast in near net shape, and the materials may be selected such that they precisely match the coefficient of thermal expansion of the cured composite structure. Further, the expected lifetime of the forms is long compared to the monolithic graphite forms now in use, and they can be assembled from subunits so that the casting procedures are simple and inexpensive. The problem that is solved by radiative joining is that of making the seams gas-tight to 100 psig without warping the form. This requirement rules out any conventional means of brazing because the forms have an unusual honeycomb configuration that warps at low temperatures. So a radiative joining technique is the only one that works. Laser welding is effective but expensive compared to the other steps in the process, and it leaves a surface bead that must be postmachined. The material is highly reflective in the infrared, so laser brazing procedures are difficult to carry out. Using absorber coatings for the laser results in so much heat being put into the surface that the near surface melts or evaporates before penetration to braze alloy depth is achieved. A solar beam in the range of 300 W/cm2 provides the right heating rates and depth of penetration for an effective brazing procedure.
Experiments were carried out on small coupons at first, until the appropriate combination of flux and braze alloy were found. Successful joints were made with these coupons. Initial experiments with a pilot scale test piece using the full honeycomb structure resulted in severe warping of the plates. This was caused by the size of the beam at the Sandia Solar Furnace and the large total thermal delivery on target. With a water-cooled aluminum mask between the beam, and the target seam positioned such that only a strip 2 mm wide was illuminated, a good metallurgical joint was achieved, thereby demonstrating the viability of the process. Optimization of the process remains. This will require a substantial commitment in terms of R&D effort and equipment. About 2 kW total power is needed in a beam of 4 mm diameter, with a peak flux of about 500 W/cm2. Two axis flexibility in the light optics is also needed (perhaps a fiber optic delivery system!), as well as a three axis manipulator for the target piece. This would be a very nice engineering project with important implications in the future for applications in space [8].
Self-Propagating High Temperature Synthesis Reactions
The SHS reactions are characterized by being so exothermic that the heat released upon initiation of the reaction is sufficient to allow propagation throughout the bulk of the mixed reactants until all of the reactants are consumed. The classic example is the thermite reaction. When powders of starting materials or layers of thin films are used, it is sometimes found that the reactions do not propagate uniformly. Further, for some materials the reactions can only be sustained or the right product can be obtained only if the reaction mixture is maintained above a defined (high) temperature. Both of these situations can be addressed by supplying additional energy to the reaction mixture prior to and during the reaction. For the production of coatings, delivering radiant energy to the surface of the target is the most convenient and conservative method of supplying the extra energy required to produce the desired phase.
We have experimented with several SHS reactions designed to deposit high quality ceramic and intermetallic materials on ordinary metal substrates. The research is in a very early stage, but encouraging results can be reported.
Three types of experiments have been carried out. Mixtures of pure powders have been glued to Fe, Ni, Ti, Mo, and 4340 steel substrates. Exposure to a 1–6 a pulse in a solar furnace is sufficient to initiate the reactions, and coatings of various nickel aluminides, TiB2, and TiC have been formed. Because these exposures were performed in air, some of the film quality is poor. In some cases, parts of the films either lifted off or did not wet the substrate material. In other cases, so much energy was deposited on the surface of the substrate that substantial melting and surface roughening occurred during the reaction. Other experiments involved exposing multiple layers of vacuum-deposited thin films. In this case, the films were too thin to allow propagation over the whole surface and depended upon the radiative input of energy to drive the reaction. High quality nickel aluminide films were formed approximately 1 µm thick. The third type of experiment involves reacting preapplied plasma spray coatings with their substrate material to form a new alloy phase on the surface. The specific sample set used plasma sprayed aluminum on Fe, Ni, and Ti substrates. Exposures were conducted in air. Aluminides with the substrate materials were formed as a result of exposure in the solar beam.
Thin Film Growth in Controlled Atmospheres
Early experiments pointed out the need for inert atmospheres over most target materials to control oxidation. A high-vacuum-compatible target chamber was constructed to allow this. By modifying the chamber to allow us to control the flow of reactive gases over the target during exposure to the solar beam, we produced the capability of conducting rapid thermal chemical vapor deposition that results in thin film growth directly on the target. This system allows programmable control of gas constituents (up to four gases simultaneously), flow rate, pressure, and target temperature. The near-term objective for this research is to explore the growth of several ultrahard coating materials (hard carbon, TiN, SiC, and TiB2) on substrates of steel, Fe, Ni, and Si. Longer-term objectives include the growth of multilayer electronic materials, especially photovoltaic materials.
Thin films of carbon have been grown on Ni and Si substrates using CH4 and H2 as precursors. The carbon growth on Ni results in a graphitic phase. Experiments with Si substrates have produced thin films of SiC and diamond-like carbon films, as well as graphitic carbon, depending upon the growth conditions. The objective of this area of investigation is to define the process parameters required for diamond thin-film growth. Current technology requires using large amounts of energy to grow diamond thin films, and this may be an area where the solar-based technique can have a substantial advantage over the more conventional techniques employing microwave plasmas or hot filaments.
FUTURE AREAS OF RESEARCH AND DEVELOPMENT
When one considers the nature of the energy resource and the method of delivery, it becomes obvious that the areas of applicability of solar furnace technology are processes that require very high temperatures, or very high heating rates, or where the cost of energy is one of the major factors driving the cost of the process. Further advantage for the solar technology is gained when we can exploit either the large area capability or the ability to operate in remote locations more effectively than conventionally powered resources. These criteria suggest several candidate areas of future research and development:
-
Rapid thermal processing of ceramic materials — This would include selective structural refinement, metallization, joining, and bonding ceramics to metals. In addition, SHS reactions could be used to deposit ceramic coatings on either ceramic, composite, or metallic materials.
-
Zone melt recrystallization — This process remelts and recrystallizes materials that have been previously formed. The objective is to produce larger crystals or crystals with fewer defects.
-
Metalorganic deposition — In this process, substrate materials are dipped, sprayed, painted, or spin-coated with metalorganic materials that are dried and fired in a rapid thermal process to derive special properties in the coatings or at the interface, or to minimize interaction with the substrate. These processes can be used to deposit metal, optical, and electronic coatings.
-
Rapid thermal processing of electronic materials — This would include initiating and controlling diffusion processes and the rapid thermal chemical vapor deposition of multilayered electronic structures. Wide-area applications would be of primary interest and would include the growth of photovoltaic films and high temperature superconductor coatings.
-
Materials science and processing and construction in space — A number of extraterrestrial applications exist for providing radiative energy for materials processing, repair, or refurbishing, and construction in space.
References
1. Pitts, J.R., J.T. Stanley, and C.L. Fields. 1990. Solar Induced Surface Transformation of Materials (SISTM). Proceedings of the Fourth International Symposium on Solar Thermal Technology, Santa Fe, N.M., June 13–17. B.P. Gupta and W.H. Traugott, eds. Hemisphere Publishing Corp., New York, p. 459.
2. Kolb, G.J. 1990. A Comparison of the Economics of Materials Processing with Solar Furnaces and High-Intensity Arc Lamps. Sandia National Laboratories, Albuquerque, N.M., (in preparation).
3. Short, W. Solar Energy Research Institute, Golden, Colorado. Private communication .
4. Lewandowski, A. Solar Energy Research Institute, Golden, Colorado. Private communication.
5. Stanley, J.T., J.R. Pitts, and C.L. Fields. Solar Induced Surface Transformation of Steel Samples. Proceedings of the Fifth Annual Northeast Regional Meeting (TMS): Protective Coatings: Processing and Characterization, May 3–5, 1989, Stevens Institute of Technology, Hoboken, N.J. R.M. Yazici, ed. TMS, Warrendale, Pa. in press.
6. Tan, R.K., N.L. Arrison, D.A. Parfeniuk, and D.M. Camm. 1988. Surface Treatment Using Powerful White Light Sources. VORTEK Industries LTD, October 7.
7. Pitts, J.R., C.L. Fields, J.T. Stanley, and B.L. Pelton. 1990. Materials Processing Using Highly Concentrated Solar Radiation. Proceedings of the 25th Intersociety Energy Conversion Engineering Conference , Reno, Nev., August 12–17. P.A. Nelson, W.W. Schertz, and R.H. Till, eds. American Institute of Chemical Engineers, New York. 6:262.
8. Pitts, J.R., T. Wendelin, and J.T. Stanley. 1990. Applications of Solar Beams for Materials Science and Processing in Space. Ibid. 1:553.
A COMPARISON OF THE ECONOMICS OF MATERIALS PROCESSING WITH SOLAR FURNACES AND HIGH-INTENSITY ARC LAMPS*
Gregory J. Kolb
Sandia National Laboratories
Albuquerque, New Mexico
Abstract
The cost and performance of treating materials with a solar furnace were compared to similar treatment with high-intensity electric-arc lamps. Qualitative results indicate that because of the long focal length of the solar furnace, it is capable of performing much dirtier materials processing tasks than the arc lamp. Quantitative results indicate that if the furnace is located in a good solar region, the solar furnace can beat the economics of the lamp by as much as a factor of three under certain operating scenarios. In other scenarios, the lamp is more cost-effective. The scenario that appears most promising for the furnace is batch processing that employs flux levels near 500 W/cm2 or greater. At lower flux levels, or in assembly-line-type processing tasks, the arc lamp is preferred.
Introduction
High-intensity white light is finding increased usage within the materials processing community. White light sources with intensities ranging from 1000 to 22,000 times the terrestrial solar flux have been used by industry to perform transformation hardening of metals, rapid thermal processing of semiconductors, and advanced thermal testing of materials[1]. These applications are currently using an arc lamp developed by Vortek Industries Limited. The arc lamp is being used because it is a cost-effective alternative to processing with lasers. Concurrent with the deployment of these systems, the Solar Energy Research Institute (SERI) is conducting research sponsored by the U.S. Department of Energy to demonstrate materials processing using a high-intensity solar furnace. A furnace is being explored because it is much less dependent on conventional, nonrenewable energy resources and it may possess some unique capabilities. To date SERI's work has demonstrated case hardening of steel, powder coating and brazing of metals, as well as chemical vapor deposition[2].
Since the intensity and spectral content of the light emanating from the arc lamp and solar furnace are similar, it is feasible that these devices could perform similar materials processing tasks. The decision of which one to use would be determined by an economic comparison as well as other factors. If the processing factory is located in a poor solar region a furnace would not be practical and an arc lamp would be chosen. However, if the factory is located in a sunny region, such as the U.S. Southwest, a solar furnace could be the best choice.
The purpose of this paper is to compare the economics of energy delivery from an arc lamp with a solar furnace located in the Southwest. Qualitative features of the systems are also discussed.
Description of the Arc Lamp System
The arc lamp system chosen for study is Model 110-100c built by Vortek. The white light emanates from a single arc tube. Water and gas are injected into the arc tube with high angular momentum and spiral down inside the tube. The water forms a thin layer on the inside surface of the tube and the gas (argon, krypton, or xenon) forms a vortex within the liquid wall. The gas vortex stabilizes the arc along the tube axis and the Water-WallTM restricts the arc diameter. Electrodes are internally water cooled. The Water-WallTM cools the arc tube and reduces the amount of electrode debris that reaches the tube. The spiraling water over the inside surface of the tube improves cooling and permits input powers up to 30 kW/cm of arc length and currents over 1200 amps.
The Model 110-100c requires an electrical power input of 105 kWe. Uniform-focus and line-focus reflectors deliver approximately 31 and 20 kW of optical power to the target, respectively. By using the uniform reflector, the flux level at the target is 100 W/cm2, i.e., 1000 times terrestrial insolation or 1000X. This flux is uniformly distributed over a rectangle measuring 20 cm × 12 cm. By using the line reflector, the flux level at the target is 5000X with the peak occurring along a 20 cm line with a 50% reduction in flux 1.5 cm away from the line. The target is placed in the focal point which is a few centimeters away from the reflector/lamp assembly. To protect the assembly during a sputtering materials processing task, an air curtain is often installed between the focal point and the assembly. A typical Vortek arc lamp system is pictured in Figure 1.
Description of the Solar Furnace System
Many experimental solar furnaces exist throughout the world. These facilities are rated at power levels from a few kilowatts to several megawatts at flux levels ranging from 1000 to 1700X. Each of these systems is unique, and a commercial manufacturer of these systems does not exist. However, it is plausible that given a market need and favorable economics, a solar furnace industry could arise. Based on Sandia's experience with building two solar furnaces[3,4], we were able to make a reasonable projection of a commercial design.
One of the Sandia furnaces is pictured in Figure 2. Solar flux is initially reflected by a flat mirror assembly called a heliostat. The reflected beam passes through an attenuator to a secondary parabolic concentrator where it is redirected toward the target located several meters away from the concentrator. Due to the long focal length, the concentrator would be protected from damage that could occur during a sputtering materials processing task. A control system is used for heliostat tracking of the sun and to adjust power level via movement of the attenuator. (The attenuator controls light much like a common window blind.) The secondary concentrator is composed of several individual facets that can be aligned to produce a variety of flux shapes and peak flux values on the target. Optical power delivered to the target has a peak value of 16 kW.
The hypothetical commercial furnace chosen for study here consists of components representing newer and more simplified technology than was employed in the 16 kW furnace. For example, rather than using the glass heliostat, glass concentrator, and stand-alone attenuator depicted in Figure 2, a commercial furnace could be composed of a stretched-membrane heliostat[5] and a stretched-membrane concentrator[6], and could employ a simple attenuator that is incorporated into the side of the building. The newer technology is much more lightweight and is estimated to cost significantly less.
We performed an annual performance calculation using actual insolation data[7] recorded at 15 minute intervals to determine the optimum optical design of the commercial furnace. This analysis indicated that a circular heliostat with a diameter of 13.8 meters in combination with a 10-meter concentrator, gave the best annual performance. This design produced a peak
power of near 50 kW with a steady state power of 38 kW. This steady state power was chosen because it is in the same range as the 31 kW Vortek arc lamp and it was identified as the optimum that can be produced with a single large heliostat. To be comparable to the high quality of the arc lamp beam, we assumed that the flux level and shape of the beam from the furnace must remain relatively constant while in operation.1 This can be accomplished by adjusting the attenuator to maintain a constant power and by keeping the concentrator completely covered with light from the heliostat. If the concentrator is not completely covered, a skewed flux pattern could occur on the target. Because of these restrictions, a fraction of the solar power reflected early and late in the day is not usable because the power is too low or because the angle of the sun relative to the heliostat causes a heliostat image that is too small. Given the constraint of a high quality beam, we calculate that the commercial scale furnace would be able to operate an average of 6 hours per day over the course of a year.
Definition of Case Studies
We will compare the economics associated with materials processing tasks that require flux levels of either 1000 or 5000X with a total power of between 20 and 40 kW. An example of a task conducted at 1000X is rapid thermal annealing of semiconductors. We will also investigate assembly-line vs. batch processing tasks.
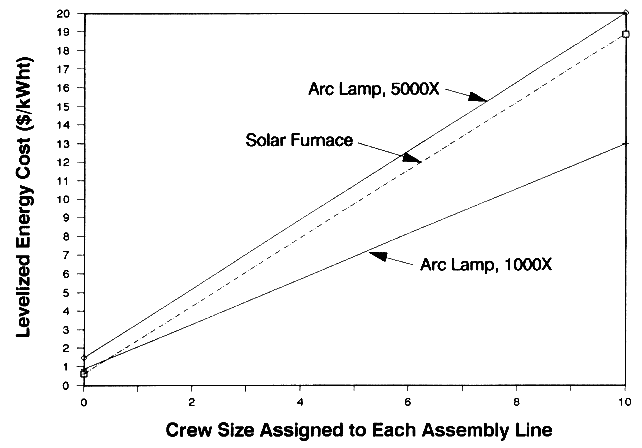
In an assembly-line application, the materials processing task performed by the lamp or furnace is only one task of an assembly line that operates continuously. For example, initial tasks in an assembly line could be to manufacture a metal part. This part would travel down the line and subsequently undergo surface treatment by the arc lamp or furnace. The assembly line is assumed to operate 24 hours a day when using a lamp and during the daytime when using the furnace. An operating crew is employed to only perform actions related to the assembly line. The crew size is assumed to vary depending on the complexity of the assembly line process. If the lamp or furnace is unavailable due to equipment failures, or if the furnace is unavailable due to bad weather, the crew does not perform other tasks. When comparing the economics of arc lamps and furnaces within an assembly line, we included costs related to the size of the crew because one technology may have an advantage related to optimum utilization of crew time. For example, in a poor solar region the arc lamp should beat the economics of a furnace because the crew would waste time waiting for sunny conditions.2
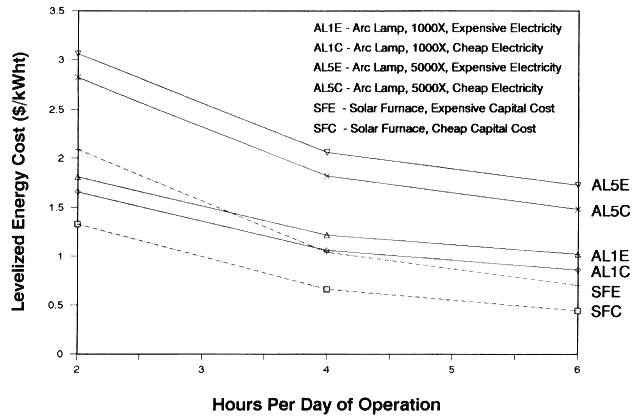
In a batch processing application, the lamp or furnace would only be operated a fraction of the work day. In this analysis we investigate situations in which they are operated an average of 2, 4, or 6 hours per day. When comparing the economics of arc lamps and furnaces within a batch process, we did not include costs related to the size of the crew because they can perform other constructive tasks when the lamps and furnaces are unavailable. It should be noted that most of the lamps Vortek has sold are used in a batch mode.
Economic Models
We compared the levelized energy cost (LEC) of delivering power to the target. Assuming that inflation and escalation do not occur during the life of the equipment (constant dollar analysis), the LEC can be calculated with the following equation:
The annualized capital cost is the fraction of the installed capital costs that must be paid every year during the life of the equipment (assumed to be 30 years) to repay the principal and interest on the loan and other smaller items. This fraction is reduced to include depreciation allowances. It is calculated by multiplying the installed capital costs by a fixed charge rate. In this analysis we used a typical fixed charge rate of 10.5% [8].
The annual operating and maintenance (O&M) costs include items such as parts replacement, electricity to run the equipment, and general repair and maintenance. The annual energy is the summation of the optical power delivered to the target over the course of a year and is expressed in kilowatt-hours.
In Table 1 and 2 we compare estimates for installed capital costs, O&M costs, annual energy, and LEC for the arc lamps and solar furnace for a batch mode operation in which they are operated 6 hours per day. Blank entries in the tables imply that the cost and performance item is identical to that listed in the column to the left.
In Table 1 arc lamp costs are given for the 1000X and 5000X systems by assuming two different prices of electric power. Since the arc lamp systems use a significant amount of electric power, this parameter was varied to gauge its effect on LEC. The cheap electric power case assumes $0.05/kWh and the expensive, $0.094. The former case is typical in many parts of the United States, and the latter is what is charged for power in Albuquerque, New Mexico, home of Sandia National Laboratories and an excellent solar region.
The anode and cathode within the lamp degrade after a few hundred hours of operation and must be replaced. In addition, we have assumed that the lamp and reflector must be changed once per lamp-year (8760 hours of operation). Since the target is only a few centimeters away from the lamp and reflector, it is plausible that a sputtering materials processing task or some other accident could damage these items. The costs related to the cooling tower and water are needed to cool the lamp, as described previously. The arc lamp system is also expected to have an electric hookup charge, beyond what would be needed by the furnace, because of the current and voltage ratings of the equipment.
It can be seen in Table 1 that each arc lamp reflector delivers different amounts of optical power to the target; the 1000X model furnishes nearly 31 kW and is more efficient than the 5000X model which supplies 19.2 kW. This performance penalty associated with increased concentration is not expected with the solar furnace system.
In Table 2 costs of solar furnaces are given for two different assumptions regarding the capital costs of the heliostat and concentrator. For the expensive case, the prices were estimated based on Sandia's experience with building our two experimental solar furnaces. These costs are high because they were specially built for us and we could not benefit from cost reductions due to mass production. For the cheap case, the prices were estimated by assuming a mass production scenario of approximately 2500 heliostats and 2500 concentrators per year. Such a scenario is predicted to
occur when solar central receive and Stirling dish power plants are built in the Southwest[8].3
Case Study Results
Figure 3 displays the results for batch processing of materials. The LEC is reduced as operating hours per day increase because the systems deliver more annual energy to the target, given a fixed capital cost; i.e., the systems are utilized more fully. We have limited the chart to 6 hours per day because we calculate the commercial scale furnace could operate up to 6 hours a day, on the average, over the course of a year in the U.S. Southwest. It can be seen that the economics of the furnace and the 1000X lamp are similar for batch operations less than 4 hours per day. Beyond 4 hours the furnace has the advantage. It is also seen that the furnace beats the economics of the 5000X lamp for every case. Depending on the combination of assumptions depicted in the Figure 3, the LEC from the furnace is from 25 to 75% less than the 5000X arc lamp. The furnace has a greater advantage over the 5000X lamp because, as stated earlier, the 5000X reflector is less efficient than the 1000X reflector. Finally, it should be noted that if Figure 3 is modified by replacing LEC with the present value of the capital and annual O&M costs, given a 30 year life and a 6.5% discount rate, the conclusions do not change.
Figure 4 displays the results for continuous processing of materials. These LEC curves are dominated by the wages paid to the crew members (the loaded salary was assumed to be $7000/man-years). The 1000X arc lamp is seen to take a significant advantage as the crew size associated with the process train becomes larger. The primary reason the furnace is not as good as the 1000X lamp is because the crew is not being used optimally. The process train and the crew are idle during cloudy periods, as well as during early morning and late afternoon when the insolation is poor. Operation of the lamp, on the other hand, is not affected by the weather. It can also be seen that the economics of the 5000X lamp is similar to the furnace. The 5000X lamp lost the advantage the 1000X lamp had over the furnace because of the efficiency drop described previously.
Summary and Conclusions
The arc lamp and solar furnace each have their own set of advantages. The advantages of each are summarized below.
Arc Lamp Advantages
-
Arc lamps are more convenient since they are not dependent on the weather. The materials processing business can precisely schedule their use.
-
The materials processing industry does not have to be located in a sunny region such as the Southwest.
-
Because of better utilization of the operating crew, the arc lamp is recommended for materials processing tasks located within a continuous process.
-
The capital cost of an arc lamp is lower than that of a solar furnace.
-
The arc lamp is much smaller than a solar furnace and would be easier to implement within an existing building.
Solar Furnace Advantages
-
Since the focal point is much further from the reflector, the furnace will be able to operate in conjunction with dirtier materials processing tasks.
-
The economics of the furnace is not dependent on the price of electricity.
-
For Southwest locations, the furnace is significantly more economical than the lamp for batch processing tasks requiring concentrations near 5000X or greater.
-
Operation of the furnace does not require cooling water.
-
The solar furnace has lower operating and maintenance costs.
-
The solar furnace will have less of an environmental impact than the are lamp because it uses an insignificant amount of electricity.
In closing, it should be noted that for applications requiring significant exposure times at very high concentrations (< 10,000X), the solar furnace should significantly beat the economics of the are lamp. Currently, the lifetimes of arc lamp components are short at such high-intensity levels.
Acknowledgements
I would like to thank Jim Pacheco of Sandia for developing the heliostat image equations used in the optical calculation for the solar furnace. I also appreciate the efforts of Gary Albach of Vortek Industries Limited. He provided cost and performance information regarding the arc lamp and performed a detailed technical review of the analysis.
References
1. Tan, R.K., H.L. Arrison, D.A. Parfeniuk, and D.M. Camm. 1988. Surface Treatment Using Powerful White Light Sources. Vortek Industries, Ltd., Vancouver, B.C. (October).
2. Pitts, J.R., J.T. Stanley, and C.L. Fields. 1990. Solar Induced Surface Transformation of Materials (SISTM). Solar Thermal Technology-Research, Development, and Applications. Proceedings of the Fourth International Symposium, B.M. Gupta, ed. Hemisphere Publishing Corporation.
3. Edgar, R.M., and J.T. Holmes. 1982. The Horizontal-Axis Solar Furnace at the Central Receiver Test Facility. High Temperature Technology . (November).
4. Cameron, C.P. 1991. 60 kW Horizontal-Axis Solar Furnace. (to be published).
5. Alpert, D.J., and R.M. Houser. 1989. Evaluation of the Optical Performance of a Prototype Stretched-Membrane Mirror Module for Solar Central Receivers. ASME Journal of Solar Energy Engineering. 111:37-43. (February).
6. Solar Kinetics, Inc. 1978. Development of a Stretched-Membrane Dish. SAND88-7031. (October).
7. Randall, C.M. 1978. Barstow Insolation and Meteorological Data Base. Aerospace Report No. ATR-78 (7695-05)-2. The Aerospace Corporation, El Segundo Calif. (March).
Table 1 Cost/Performance of Arc Lamps Operated 6 Hours per Day
|
|
1000X Reflector |
5000X Reflector |
||
|
|
Cheap Electricity |
Expensive Electricity |
Cheap Electricity |
Expensive Electricity |
|
Capital cost ($) |
|
|
|
|
|
Lamp system |
136,500 |
|
147,000 |
|
|
Cooling tower |
6,000 |
|
6,000 |
|
|
Electric hookup |
5,500 |
|
5,500 |
|
|
Building |
50,000 |
|
50,000 |
|
|
Total capital cost |
198,000 |
|
208,500 |
|
|
Annual operating cost ($) |
|
|
|
|
|
Electricity |
11,700 |
22,000 |
11,700 |
22,000 |
|
Water |
140 |
|
140 |
|
|
Lamp replacement |
70 |
|
70 |
|
|
Reflector replacement |
6,600 |
|
9,200 |
|
|
Argon gas |
120 |
|
120 |
|
|
Anode replacement |
9,500 |
|
9,500 |
|
|
Cathode replacement |
3,300 |
|
3,300 |
|
|
General maintenance |
5,000 |
|
5,000 |
|
|
Total O&M cost |
36,400 |
46,700 |
39,000 |
49,300 |
|
Annual performance |
|
|
|
|
|
Annual operating hours |
2,190 |
|
2,190 |
|
|
Power to target (kW) |
30.8 |
|
19.2 |
|
|
Annual energy to target (kWh) |
67,450 |
|
42,100 |
|
|
Levelixed Energy Cost |
|
|
|
|
|
($/kWh) |
0.86 |
1.01 |
1.47 |
1.72 |
Table 2 Cost/Performance of Solar Furnaces operated 6 Hour per Day
|
|
Cheap Capital Cost |
Expensive Capital Cost |
|
Capital cost ($) |
|
|
|
Building |
130,000 |
|
|
Attenuator |
50,000 |
|
|
Heliostat |
22,000 ($150/m2) |
100,000 ($670/m2) |
|
Concentrator |
16,000 ($230/m2) |
50,000 ($1700/m2) |
|
Test table |
25,000 |
|
|
Computer |
30,000 |
|
|
Clock |
2,000 |
|
|
Installation |
28,000 |
46,000 |
|
Total capital cost |
303,000 |
503,000 |
|
Annual operating cost ($) |
|
|
|
Electricity |
<100 |
|
|
General maintenance |
5,000 |
|
|
Total O&M cost |
5,100 |
|
|
Annual performance |
|
|
|
Annual operating hours |
2,190 |
|
|
Power to target (kW) |
38 |
|
|
Annual energy to target ($/kWh) |
83,000 |
|
|
Levelized energy cost (S/kWh) |
0.44 |
0.70 |