Page 379
12
Alternative Fuels
Introduction
Current estimates show that automobiles and trucks account for about 45% of the anthropogenic VOCs (volatile organic compounds), 50% of the NOx (oxides of nitrogen) (Figures 12-1, 12-2, 12-3, 12-4), and 90% of the CO (carbon monoxide) in dries where the ozone NAAQS is not met (OTA, 1989). This is by far the largest category of emissions, and recent studies indicate that these estimates could be low for VOCs and CO (Pierson et al., 1990; Lawson et al., 1990b). So, although strategies to improve air quality should consider all sources, it is dear that reductions in automotive emissions are necessary. Some reductions will occur as older, dirtier vehicles are taken off the road and replaced with new, well-controlled vehicles. However, these reductions will be small, and by the year 2004, the increase in vehicle use is expected to lead to an increase in VOC emissions (OTA, 1989). A longer term solution is necessary. One possibility is using alternative fuels. However, the use of alternative fuels alone will not solve the ozone problems of the most severely affected areas. Moreover, it will not necessarily alleviate the most critical problem associated with motor vehicle emissions, which is the increase in emissions that occurs over time with in-use vehicles (see Chapter 9).
The central role of VOCs in the formation of tropospheric ozone suggests that changing fuels could be effective by reducing the reactivity of the VOC emissions, the total mass emitted, or both. Gasoline and the exhaust from conventionally fueled vehicles are highly reactive in the atmosphere because
Page 380
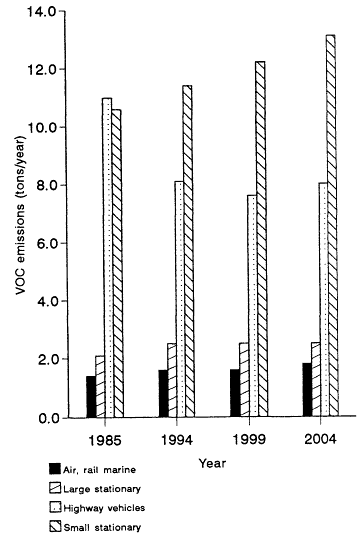
Figure 12-1
Estimated nationwide VOC emissions by source category, by year. Assumes no
regulations other than those in place in 1987. The estimates are representative of the emissions
on a typical nonattainment day, multiplied by 365 days per year, rather than estimates
of true annual emissions. The baseline does not include reductions due to the limit
on gasoline volatility of 10.5 psi Reid vapor pressure (RVP). Stationary sources that emit more than
50 tons per year of VOC are included in the "Large" category. Source: OTA, 1989.
Page 381
they are rich in aromatics and alkenes. Alternatively fueled vehicles, such as those that run on natural gas or methanol, would have less reactive emissions and hence reduce ozone. There is also the possibility of reducing the mass emission rates of VOCs, CO, and NOx when these fuels are used (Austin et al., 1989; Williams et al., 1989). Electric vehicles would run virtually without emissions. As discussed elsewhere in this report, the relative benefits of VOC and NOx controls vary by location, and this phenomenon is critical to determining the benefits (or lack thereof) of alternative fuel use. For example, reducing the reactivity of VOCs can reduce ozone in NOx-rich urban centers, such as downtown Los Angeles and New York, but would likely provide little help in regions with high VOC-to-NOx ratios, such as Houston and Atlanta. The degree to which the different alternative fuels could improve air quality is discussed below. It should be noted that the choice of alternative fuel cannot be made on environmental considerations alone, nor are different locations going to be affected similarly. Economics, politics, energy security and diversity issues, consumer acceptance, technological advances, and the relative prices of fuels will enter into the choice, but such considerations are beyond the scope of this report. Concern over global warming and the need to improve air quality suggest that the use of alternative fuels, and the choice of those fuels, will need to be considered thoroughly and carefully.
Fuel Choices
A variety of alternative fuels and technologies are or could become available for automotive use, including natural gas; methanol (and methanol blends); ethanol (and ethanol blends), liquid petroleum gas (LPG), including propane; hydrogen; electricity; and reformulated gasoline. Although much interest has focused on use in light-duty vehicles (cars and fight trucks), most of the fuels also can be used in heavy-duty vehicles. Different technologies also exist to use these fuels: the traditional internal combustion engine (using organic fuels and hydrogen) and electric motors coupled with battery storage or fuel cells (running on hydrogen or methanol). Each of the fuels and the associated technologies will have various environmental effects and could be viable at different times in the future.
In the near term (0-5 years) the only alternative fuel that can effect ozone concentrations is reformulated gasoline, whose potential is uncertain. No new technology or distribution system is required, although refining capacity for these fuels is limited. Most major oil companies have environmentally improved, reformulated gasolines for sale in limited markets, targeting those urban areas with the most severe air-quality problems. These fuels are formu-
Page 382
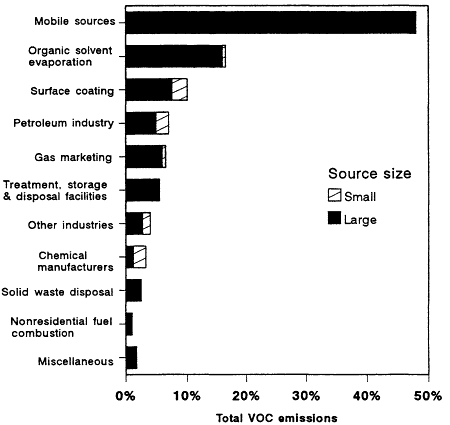
Figure 12-2
VOC emissions in nonattainment cities, by source category, 1985. Stationary sources that
emit more than 50 tons per year of VOCs are included in the ''Large'' category. Total emissions,
11 million tons/year. Source: OTA, 1989.
Page 383
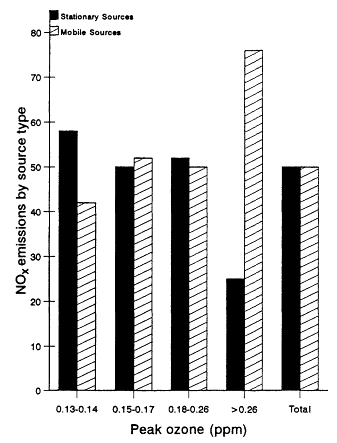
Figure 12-3
NOx emissions an peak concentrations of ozone in non-attainment
cities, 1985. Source: Adapted from OTA, 1989.
lated to have lower emissions of highly reactive or toxic organic compounds. These fuels are new, and their ability to improve air quality is not dear. Reformulated gasoline is obtained from refined petroleum. As such, it is not considered a true alternative, but it is discussed here because it is viewed as a candidate means for improving air quality.
In the middle term (5-20 years), likely candidates to replace gasoline- and diesel-powered vehicles are similar vehicles powered by internal combustion engines that run on methanol, natural gas, or reformulated gasoline. Electric vehicles are another middle-term possibility.
It is unclear which fuels and technologies have the greatest potential or will
Page 384
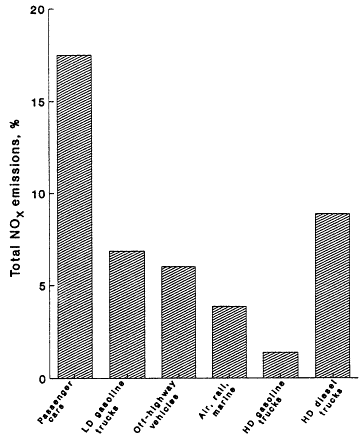
Figure 12-4
NOx emissions from mobile sources in 1985 as a percentage of total (mobile
plus stationary) emissions. LD refers to light duty; HD refuers to
heavy duty. Source: OTA, 1989.
even be viable for the long term. Reformulated gasoline, methanol, and natural gas would still be available. It is dear that there are ample supplies of both petroleum and natural gas worldwide (Sperling, 1991). Other fuels, in particular hydrogen and electricity, are more attractive from an environmental standpoint. Advances in battery technology could make electric vehicles an attractive alternative to vehicles with internal combustion engines.
Bemuse many variables affect which fuel is best, it is important to consider
Page 385
the attributes of each. Below, the environmental attributes of each fuel are discussed, followed by a summary of the studies that have looked at their effects on air quality. Results of the air-quality studies are then used to estimate the relative effectiveness of each fuel in lowering ozone. For each of the fuels, there are significant uncertainties in the likely environmental benefits. Still, some general conclusions can be developed, and these are discussed at the end of the chapter.
Attributes of Alternative Fuels
Reformulated Gasoline
As the name implies, reformulated gasoline is a refined petroleum product whose composition is similar to gasoline. It can be used in conventional engines with no modification, although achieving the greatest air-quality benefits could require modification of the automotive control system. The composition of the gasoline is altered to make exhaust products less photochemically reactive and toxic and to lower total emissions. This is accomplished by modifying the refining process and by adding oxygenates. The resulting fuel is lower in olefins and aromatics, and has a lower Reid vapor pressure (RVP). (RVP is the constrained vapor pressure of the fuel at 100ºF.) Oxygenates serve two purposes: First, they enhance the fuel's octane rating, which is lowered when the aromatic and olefin content is reduced. Second, they can improve the efficiency of combustion, and the presence of fuel oxygen tends to decrease CO emissions.
Commonly used oxygenates include ethers, particularly methyl t-butyl ether (MTBE) and ethyl t-butyl ether (ETBE), and alcohols (methanol and ethanol). The trend is to use the ethers, although ethanol is used extensively in the Midwest, especially to reduce CO emissions in the winter. The addition of alcohols can raise the vapor pressure of the gasoline, increasing evaporative emissions and decreasing the fuels' positive benefits during the summer. Oxygenates also can increase the rate of formaldehyde (HCHO) emissions (Anderson et al., 1989). Oxygenates may comprise a few to more than 15% of the reformulated fuel.
Reformulated gasolines have been introduced in limited markets—those that have severe photochemical smog problems. The potential for using reformulated gasoline to improve air quality is uncertain. First, reformulated gasolines are new, and their compositions could evolve further. Second, it needs to be determined to what degree automobile control systems and fuels can be matched to lower emissions; this would likely require standardization of fuel
Page 386
composition. Still to be fully explored is the possibility of reducing total mass emissions of VOCs and NOx by using different blends of reformulated gasolines. A major effort, the Auto/Oil Air Quality Improvement Research Program, is under way to help accomplish these goals (Burns et al., 1991).
Natural Gas
Natural gas is primarily methane (> 90%), with other light hydrocarbons, including ethane, ethene, propane, propene, and butane, as impurities. For use as an automotive fuel, it is compressed and stored at pressures up to 30 megapascals (4500 psi), or liquified. Because liquified natural gas (LNG) requires cryogenic cooling and storage, it has not been used as much as compressed natural gas (CNG). (In this section, reference is made to natural gas vehicles [NGVs.]. Unless stated otherwise, these are vehicles that use either CNG or LNG. For the most part, the advantages and disadvantages of the two fuels are similar.)
VOC emissions from NGVs mimic the fuel, and are largely methane (Table 12-1). Given its very low atmospheric reactivity, methane has great potential for reducing ozone formation. However, impurities in natural gas can greatly reduce its benefit. Ethane and propane contribute up to 25 times as much ozone on a mass basis (grams ozone/grams VOC), as does the less reactive methane, and the presence of alkenes would lead to even less benefit (Carter, 1990b). Assuring a low alkene content of the natural gas is crucial to achieving the maximum benefit of NGVs. Also, the products of incomplete combustion (aldehydes) are highly reactive, although the mass emission rates of these species are likely to be small (Alson, 1988; Austin et al., 1989; CARB, 1989a,b).
Emissions of CO from NGVs, which are usually operated under lean-bum conditions (i.e., more air is used than is required for complete combustion of the fuel), are generally much less than from gasoline-powered vehicles (Alson, 1988; Austin et al., 1989). Tests consistently show reductions on the order of 90%. In addition to lowering ambient CO, the decrease in CO emissions lowers, slightly, the ozone formation resulting from the vehicles. This is because CO oxidation produces hydroperoxyl (HO2), resulting in increased nitric oxide (NO) oxidation. It is difficult to state how NOx emissions will compare in an optimized NGV engine, and tests show results in both directions (Alson, 1988; Austin et al., 1989). Meeting the NOx emission standard limits the ability to use lean-burn engines in NGVs or methanol-fueled vehicles. Both fuels can be burned very lean, leading to increased fuel economy and lower engine-out VOC, CO, and NOx emissions. (Engine-out emissions are those
Page 387
| TABLE 12-1 | |||||||
| VOC | Indoline | Methanolc | Ethanol | Liquid petroleum gas | Compressed natural gasd | ||
| M85 | M100 | ||||||
| Alkanes | 0.632 | 0.224 | 0.023 | 0.077 | 0.797 | 0.170 | |
| Alkenes | 0.040 | 0.007 | 0.001 | 0.002 | 0.062 | 0.031 | |
| Formaldehyde | 0.021 | 0.067 | 0.050 | 0.010 | 0.041 | 0.023 | |
| Aldehydes | 0.004 | 0.004 | 0.001 | 0.050 | 0.005 | 0.005 | |
| Ethene | 0.031 | 0.005 | 0.001 | 0.034 | 0.082 | 0.017 | |
| Toluene | 0.199 | 0.032 | 0.009 | 0.023 | 0.007 | 0.007 | |
| Aromatics | 0.059 | 0.023 | 0.005 | 0.010 | 0.003 | 0.014 | |
| Methyl ethyl ketone | 0.015 | 0.005 | 0.001 | 0.002 | 0.003 | 0.009 | |
| Methanol | 0 | 0.633 | 0.911 | 0 | 0 | 0 | |
| Ethanol | 0 | 0 | 0 | 0. 791 | 0 | 0 | |
| aCompositions given in decimal form. | |||||||
| bThe tests relied on very few vehicles, and the individual tests are not likely to be representative of the actual fleet of vehicles operating on each fuel. | |||||||
| cM85, 85% methanol by volume; M100, 100% methanol. | |||||||
| dThe composition of exhaust is primarily methane, and the composition shown reflects that about 70% of the VOC is nonreactive. Methane comprises a much smaller fraction of the exhaust when other fuels are used. | |||||||
| Source: CARB, 1989a. | |||||||
Page 388
released prior to catalyst reduction by a catalytic converter.) However, the exhaust is so oxygen-rich and CO-lean that the performance of the NOx reduction catalyst is very poor, leading to higher exhaust NOx emissions. Although it could be possible to reduce the rate of emissions to 0.4 g NOx/mile, estimates indicate that California's standard of 0.2g NOx/mile would not be met using a lean-burn engine (Austin et al., 1989). Given the recent findings that further NOx reductions are effective for reducing ozone, using a lean-burn engine fueled by natural gas (or methanol) could be counterproductive in many environments.
One advantage of NGVs, from an air-quality perspective, is that the emissions from a "super-emitting" NGV are not as likely to increase to the same levels as those from a super-emitting conventional vehicle. Engine-out emissions of VOCs, CO, and NOx from NGVs are substantially lower than from conventional vehicles; studies indicate that malfunctioning vehicles account for 10% of the conventional fleet and are responsible for 60% of fleet CO emissions (Lawson et al., 1990a). A second inherent advantage is that evaporative emissions from NGVs would be very small (if any) and only slightly reactive. This could be a substantial advantage if it turns out that evaporative emissions are the reason for the discrepancy between the inventories and ambient measurements discussed in Chapter 8. Refueling emissions also would be small.
Methanol
Methanol and methanol blends have recently been the subject of great interest. Methanol fuel is primarily methanol, although significant amounts (up to 15%) of other compounds are added for safety and performance reasons.
Pure methanol is a colorless, toxic liquid with low vapor pressure and high heat of vaporization. Although the latter two attributes can lower emissions when an engine is warm, they also make it difficult to start a vehicle running on pure methanol when cold, and high cold start emissions ensue. Also, the flame from a pure-methanol-based fire is nearly invisible in daylight, which may be a safety problem. Additives raise the vapor pressure, help cold start, and add color to the flame. The most common additive is unleaded gasoline, from 5 to 15% by volume. The resulting blends are designated by an "M" followed by the volume fraction of the methanol. Thus, M85, the most common blend, is 85% methanol by volume, but there is no standard blend. Dedicated methanol-fueled vehicles (MFVs) and flexibly fueled vehicles have been developed. The flexibly fueled vehicles are designed to operate on any combination of gasoline and M85 fuel, and in this way they ease the transition during periods when methanol is not widely available.
Page 389
On a mass basis, unburned methanol is the largest component of MFV exhaust whether M85 or M100 fuel is used (Table 12-1). The second most abundant species of methanol exhaust is formaldehyde (HCHO). Gasoline-like hydrocarbon components comprise 35-50% of the exhaust of M85-fueled vehicles (Horn and Hoekman, 1989; Snow et al., 1989; Gabele, 1990). HCHO is often cited as the most deleterious species emitted by MFVs because of its toxicity and high photochemical reactivity. Engine-out HCHO emissions are substantial, up to 600 mg/mile, and to derive any air-quality benefits the catalyst system must remove most of that. Tests of current vehicles show that after catalytic reduction, M85 vehicles emit 30-40 mg HCHO/mile—three to six times as much as is emitted by a conventional vehicle (Horn and Hoekman, 1989; Gabele, 1990), although amounts as high as 180 rag/mile have been reported for older (pre-1989) cars and trucks (Snow et al., 1989). Recent tests show that M100-fueled vehicles have 25% higher HCHO emissions than do vehicles with similar control systems fueled by M85 (Gabele, 1990). This is attributable to the poor cold start when using M100. During cold start, the catalyst takes about 30 seconds to light off (become hot enough to cause VOC oxidation), and engine-out HCHO is readily emitted during the transient warming. The high HCHO emissions, if not properly controlled, could negate any potential advantage of MFVs.
Cold start is responsible for a bulk of the emissions from MFVs, and various technologies are being studied to help solve this problem. One promising direction is a more closely coupled catalyst that is electrically heated before or during start-up. Tailpipe emissions of HCHO of as little as 4 mg/mile have been measured from an M100 vehicle with an electrically heated catalyst (Hellman, et al., 1989). This is a 55% decrease compared to emissions using an unheated catalyst, and this amount of HCHO emissions is comparable to that from a gasoline-fueled vehicle, although it has not been established that these results can be sustained in vehicles with higher mileage (more than 20,000 miles, for example). The resistively heated catalyst also decreases methanol, although NOx emissions increase. Tests consistently show that CO emissions from MFVs are lower than from their conventionally fueled counterparts (Williams et al., 1989; Gabele, 1990), because of the higher oxygen content of the fuel. Reductions of 50% could be realized. This is, however, a direct function of the control system used, and might not be realized in practice. This amount of CO reduction would lead to substantial decreases in ambient CO and slight decreases (0-2%) in ozone.
It is not dear whether methanol use would lead to lower NOx emissions. Limited tests on flexibly fueled vehicles show some NOx reductions, but a dedicated MFV would use a higher compression ratio, which could lead to higher NOx emissions. It is likely that the NOx emissions would be catalytical-
Page 390
ly controlled to meet emission standards, and there might be no substantial change in net NOx emissions.
Evaporative emissions from MFVs would be less reactive than those from a conventional vehicle. It is also possible that the mass emissions rate would be lower. To minimize evaporative emissions, the RVP of gasoline in California and in the northeastern United States is mandated to be less than 9.0 psi. Pure methanol has an RVP of 4.6 psi, and M85 has an RVP of 7.5-8.0 psi (Horn and Hoekman, 1989; Gabele, 1990). The lower RVP results in lower emissions. However, as the gasoline content of a methanol blend increases, so does the RVP, until the mixture is almost pure gasoline at which point RVP decreases rapidly (Figure 12-5). Thus, although using M100 or M85 could decrease evaporative emissions, a vehicle using mostly gasoline and small amounts of methanol would have higher evaporative emissions than would a vehicle running on pure gasoline. This suggests the need for some method to ensure that the fuel composition in an average commuter's tank is a high methanol blend, or that the evaporative control system is built to handle 11 RVP fuel. Dedicated vehicles would not have this problem.
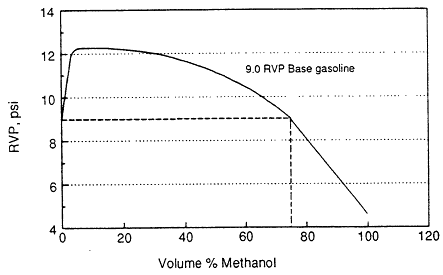
Figure 12-5
Approximate Reid vapor pressure dependence on fuel composition. From Black, 1991.
Page 391
Ethanol
Ethanol's use would be similar to methanol's, and flexibly fueled vehicles built to use methanol also can run on ethanol. The differences are in the sources of ethanol and in its smaller air-quality benefits. Ethanol is slightly more reactive than methanol, and it leads to peroxyacetyl nitrate (PAN) formation. Carter (1991) reports that ethanol produces about 15% more ozone than methanol does on a carbon-atom basis. There is considerably less information on the emission characteristics of well-controlled ethanol vehicles, and the limited tests show high emissions (CARB, 1989b). It should be noted that using ethanol as a blending agent in gasoline and simultaneously allowing for an increase in RVP would not achieve significant air-quality benefits, and in fact would likely be detrimental. The increased evaporative emissions and increased concentrations of ethanol and acetaldehyde in the atmosphere would lead to increases in such pollutants as PAN (Tanner et al., 1988). Ethanol is more reactive than methanol and other blending agents such as MTBE (Carter, 1990b, 1991; Japar et al., 1990)
Hydrogen
Hydrogen is the cleanest fuel that can be used in an internal combustion engine. In the longer term, the possibility of producing hydrogen from non-fossil electricity (solar, nuclear, or hydropower) is seen as a nearly emission-free source of transportation fuel. Hydrogen produced from solar or hydro-power would be renewable as well. Hydrogen-powered internal combustion vehicles would not be entirely clean. They emit NOx (DeLuchi, 1989) and tiny quantifies of carbon dioxide, CO, and VOCs (from burning oil).
Lpg And Propane
LPG, which is primarily propane, shares many of the attributes of compressed natural gas, with several disadvantages. The supply of LPG is limited, and it is a petroleum-refining byproduct (Sperling, 1988). Also, it would not provide as great a reduction in exhaust reactivity as CNG would (Carter, 1990b; Chang and Rudy, 1990). An advantage of LPG over CNG is its higher energy per unit volume, so a smaller fuel tank is required. LPG is not expected to be a major alternative transportation fuel outside of the current limited applications.
Page 392
Electricity
Electricity-powered vehicles are the cleanest of the alternatives. Some of the benefits are obvious: Electric vehicles produce virtually no on-road emissions, and there is a wide variety of potential energy sources, including fossil fuels, nuclear and solar energy, and hydropower.
Use of electric vehicles would not eliminate all smog-producing emissions. In the near term, production of electricity would come largely from fossil fuel power plants that emit NOx, oxides of sulfur, and small quantities of VOCs and CO. These plants would contribute to urban or regional ozone formation. Depending on the source (coal, gas, nonfossil), the net change in NOx emissions could be a small increase (coal) or large decrease (nonfossil). Emissions of oxides of sulfur would likely increase. Direct emissions of particulate matter would increase, although the total attributable to increased electricity production has not been determined. Reductions in NOx would lower particulate nitrate, and increases in oxides of sulfur would increase particulate sulfate. Net CO and VOC emissions would be reduced by 95-99% (Krupnick et al., 1990; Sperling and DeLuchi, 1989).
Alternative Fuels for Heavy-Duty Vehicles
Most of the current discussion of alternative vehicles centers on light-duty vehicles (cars and small trucks), even though use in heavy-duty vehicles could be of more immediate benefit. There are demonstration fleets of alternatively fueled heavy-duty vehicles. Diesel vehicles are notorious for their particulate emissions and also have high emissions of HCHO, polynuclear aromatic hydrocarbons (PAHs), and NOx. Use of either methanol or CNG would virtually eliminate the particulate and PAH emissions. Evidence also suggests lower NOx emissions by a factor of about two. Heavy-duty vehicles, especially buses, are more commonly centrally fueled than are light-duty vehicles, making transition to an alternative fuel easier.
Alternative Fuels And Air Quality
Knowledge of the emissions characteristics of the different alternative fuels can be used to compare air-quality benefits. Very few detailed, extensive studies of any of the various fuels have been completed so far because interest in these fuels has only recently been renewed and the emissions data are
Page 393
extremely sparse (especially for vehicles under typical driving conditions and with high mileage). To date, the most extensively studied fuel is methanol, followed by electricity. There is some recent information on the potential air-quality effects of using reformulated gasolines, and extremely limited information on natural gas, LPG, and hydrogen.
Each of the fuels is targeted for its ability to reduce ozone, although all of them will affect concentrations of other pollutants. Accordingly, ozone has been the focus of most studies, and accompanying effects are sometimes given. Because ozone formation is the result of a complex interaction of photochemistry, transport, and emissions, the primary tools for testing the effects of alternative fuels are photochemical air-quality models; box, trajectory, and grid-based models have been used. In addition, smog chamber experiments have been used to test mathematical models. The results of air-quality studies are discussed below starting with those for methanol, for which the most information is available.
Methanol
Interest in using methanol as either a fuel additive or a base fuel arose in the late 1970s in response to the energy crisis. Since then, several studies, using mathematical models or smog chambers, have focused on the response of ozone to use of methanol (Bechtold and Pullman, 1980; O'Toole et al., 1983; Whitten and Hogo, 1983; Pefley et al., 1984; Balentine et al., 1985; Nichols and Norbeck, 1985; Jeffries et al., 1985; Carter et al., 1986b; Whitten et al., 1986; Chang et al., 1989; Chang and Rudy, 1989, 1990; Russell, 1989, 1990; Russell et al., 1989, 1990; Dunker, 1990). Although these studies generally agree that methanol use would decrease ozone levels, the amount of reduction predicted by the studies varied.
Experimental Studies
Carter et al. (1986b) performed a series of methanol-related smog chamber experiments in a 6400-liter indoor Teflon chamber (ITC) and in a 50,000-liter outdoor Teflon chamber (OTC). Compounds similar to those in a very dirty urban atmosphere were introduced into a chamber, and the pollutant concentrations were followed for periods of up to three days. This was done for pollutant mixes corresponding to current emissions and also for mixes corresponding to methanol-fueled engine emissions replacing one-third of the base mixture. On the first day, peak ozone levels were significantly lower for the
Page 394
methanol emissions mix than for the conventional emissions mix. However, this difference decreased over time, and by the third day ozone concentrations were similar. This result raised a question about the effects of methanol during multiday smog episodes. It was thought that methanol could build up, negating any benefits, and that a large fraction would react within three days. This study also showed that the ozone decreases were sensitive to the mount of formaldehyde emissions. In an earlier study, researchers at the University of North Carolina (Jeffries et al., 1985) conducted 29 smog chamber experiments that used methanol with varying fractions of formaldehyde. One-third of the base organic mixture was replaced with methanol or methanol-formaldehyde mixture, either a 90:10 CH3OH/HCHO ratio or an 80:20 ratio. Ozone reductions varied widely, from zero to 80%, depending on the HCHO content and amounts of VOC emissions.
Differences between smog chambers and the atmosphere make it difficult to use smog chamber results directly for predicting urban and regional air-quality changes that would result from methanol use. One difference is that a smog chamber does not replicate atmospheric diffusion and transport of chemical species. A second difference is that for the experiments conducted, all pollutants were present at the beginning of the smog chamber experiments. Fresh pollutants, however, are emitted throughout the day into the atmosphere. Consequently, the smog chambers had very low NOx concentrations on days two and three of the simulations, making ozone concentrations relatively insensitive to changes in VOCs. (This is also pertinent to understanding the benefits of reducing VOC in NOx-limited regions, such as rural and downwind areas.) A third difference is that even a very large smog chamber has a ratio of surface area to volume that is many orders of magnitude greater than the atmosphere's, and surface reactions have a much larger effect on pollution formation in the smog chamber than they do in the atmosphere. These experiments did, however, highlight issues that needed to be addressed further—especially the need for multiday simulations—and they have been crucial to the understanding of the chemical system.
Mathematical Modeling Studies
Atmospheric simulations of methanol have been accomplished by adapting existing photochemical models to include methanol chemistry. The chemistry of methanol is relatively simple, and it has been treated explicitly by addition of the following reaction,
Page 395
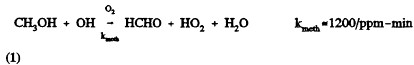
to the existing chemical mechanism. The input emissions are then adjusted accordingly to. approximate the change to methanol-fueled vehicles. Results of these studies are summarized in Table 12-2. Most of the early studies used single-day trajectory model simulations. Early studies that concentrated on Los Angeles showed relatively large reductions in ozone when methanol is substituted for conventional fuels (O'Toole et al., 1983; Whitten and Hogo, 1983). Later studies (e.g., Russell et al., 1990) showed less benefit from using methanol. This difference is due in part to the use in the earlier studies of trajectory or box models, which were very sensitive to initial conditions and had limitations in their formulation (see Chapter 10). Russell et al. (1989) found significant differences in the results of a trajectory model and grid model applied to the same period for methanol use. Much of the model response, or lack thereof, is the result of treatment of initial conditions. As noted above, the Carter et al. (1986b) study that used a smog chamber found that the relative ozone reductions on the second and third days of multiday experiments were less than on the first day. High ozone episodes are multiday events, and carryover of pollutants from one day to the next is critical. This too explains, in part, the higher ozone reductions found in early studies.
Several studies by Russell and co-workers (Russell, 1989; Russell et al., 1989, 1990) use a grid-based airshed model to look at the air-quality effects methanol could have in the Los Angeles basin in two future years: 2000 and 2010. Russell et al. (1990) found peak ozone reductions of 9-17% in 2000, depending on the fuel type simulated, and of about 4% in 2010. In these simulations all light- and medium-duty vehicles, and some heavy-duty vehicles, were assumed to be converted. The 2010 ozone peak occurred far east of central Los Angeles and was found to be NOx-limited and relatively insensitive to methanol emissions. Central basin ozone was lowered by about 15%. Ozone exposures were predicted to be reduced by 12-20% in both years. When coupled with reductions in stationary-source emissions, a later study by Russell (1990) found that a 50% penetration of vehicles fueled with M85 would lead to a 9% reduction in peak ozone and a 19% reduction in population exposure. The reason for the larger reduction in exposure in both studies is that the population is more concentrated in regions with higher NOx concentrations, and ozone formation in those areas is more sensitive to VOC reactivity than is the ozone peak. Despite the limitations of trajectory models,
Page 396
| TABLE 12-2 | |||||||
| Application area | Model typea | Modeling period | Vehicles substituted | Base yearb | Composition of exhaustc | Peak ozone reduction in percent | Reference |
| Los Angeles | EKMA | 1 day | All gasoline | 1987 | 100/0/0 | 31 | Whitten and Hogo, 1983 |
| 90/10/0 | 22 | ||||||
| 90/20/0 | 13 | ||||||
| Los Angeles | Multilevel trajectory | 1 day | All gasoline | 2000 | 57/17/26 | 14 | O'Toole et al., 1983 |
| Los Angeles | Box | I day | All gasoline | 1987 | 90/10/0 | 18 | Pefley et al., 1984 |
| 20 Cities | EKMA | 1 day | Light-dutyl gasoline | 1982 | 100/0/0 | 13d | Nichols and Norbeck, 1985 |
| 90/10/0 | 3.5d | ||||||
| Philadelphia | Trajectory and othersh | 1 day | Mobile sources | 2000 | 100/0/0 | 10 | Whitten et al., 1986 |
| 110/6/0 | 7 | ||||||
(Table continued on next page)
Page 397
(Table continued from previous page)
| Application area | Model typea | Modeling period | Vehicles substituted | Base yearb | Composition of exhaustc | Peak ozone reduction in percent | Reference | |
| 20 Cities | EKMA | 1 day | Light-duty gasoline | 2000 | 90/10/0 | 1.9c | Chang et al., 1989 | |
| M85f | 1.3c | |||||||
| M100g | 2.6c | |||||||
| 100/0/0 | 2.9c | |||||||
| 4 Cities | EKMA | 1 day | Light-duty gasoline | 2000 | M85 | 2 | Chang et al., 1989 | |
| M100 | 3 | |||||||
| Los Angeles | Airshed | 3 days | Post-1990 mobile sources | 2000 | M85i | 9.3/13j | Russell et al., 1990 | |
| M100i | 17/20j | |||||||
| 2010 | M85i | 4/12j | ||||||
| M100i | 4.3/19j | |||||||
| Los Angeles | Airshed | 3 days | Clean Air Actk | 2010 | M85 | 9/19 | ||
| Specifics of each study can be found in the cited reference. This tabulation is a summary of those findings and follows the format of Chang et al. (1989). | ||||||||
| aMathematical models used include box, trajectory, and grid-based photochemical models. Some studies used more than one, in which case the most representative results are used. | ||||||||
| bBase year is the year of emissions inventory used. | ||||||||
| cComposition of exhaust is shown as percentage of nonmethane VOCs substituted by methanol, formaldehyde, or hydrocarbons. | ||||||||
| dAverage over 20 cities. Range was 1-36%. | ||||||||
| eAverage of four cities. | ||||||||
| fA complex mixture simulating M85 exhaust measurements used. | ||||||||
| gMixture simulating M100 exhaust used. | ||||||||
| hResults based on trajectory modeling.iComplex mixture of methanol and other organics used. Synergistic effects on evaporative emissions | ||||||||
| iComplex mixture of methanol and other organics used. Synergistic effects on evaporative emissions | ||||||||
(Table continued on next page)
Page 398
(Table continued from previous page)
| calculated. Formaldehyde emissions were 15-23 mg/mile from light-duty vehicles. Only post-1990 vehicles assumed to use methanol. |
| jThe two values reported are the reductions in peak ozone followed by the reduction in exposure to ozone concentrations over 120 ppb. |
| kIn this case, the 2010 base inventory included proposed emission reductions from stationary sources. The methanol case looked at the effect of introducing 300,000 vehicles per year (about half the fleet), as was proposed as part of the Clean Air Act amendments. The resulting act did not include specific targets for methanol fuel use. |
| lRefers primarily to passenger vehicles weighing less than 3,750 lb. |
Page 399
the results of the other studies are not terribly inconsistent with the grid-based modeling studies considering the different treatment of emissions.
Ozone reduction from methanol use in most other areas is not expected to be as great as it is for Los Angeles. This is attributable to higher VOC-to-NOx ratios in other cities and to lower traffic-related emissions. The Ford studies (Nichols and Norbeck, 1985; Chang and Rudy, 1989) show average reductions of 1-3% (depending on fuel) in the year 2000, although a few cities (Pittsburgh, Pennsylvania, for example) are predicted to see more substantial ozone reductions. Total removal of light-duty VOC emissions led to only a 7% ozone reduction in the dries studied. However, those calculations assumed that mobile sources in the year 2000 will account for only 13% of the total VOC, on average, or about one-third of the current mobile-source contribution. This is probably a low estimate. These results, along with those of Russell et al. (1990) and Dunker (1990), indicate that in NOx-rich areas, vehicles fueled with M100 would contribute 45-75% as much ozone as would equal mass emissions from conventional vehicles, and M85 vehicles would contribute 70-80% as much. In VOC-rich areas, little effect would be expected.
Russell et al. (1989) also looked at the effect that methanol substitution would have on other species. It was speculated that the increased direct emissions of (HCHO) from methanol fueled vehicles could lead to unacceptably high ambient concentrations of HCHO. It was found, however, that ambient HCHO concentrations change very little, and in some cases decrease, when methanol use is simulated. This is because most atmospheric formaldehyde is formed photochemically as the product of VOC oxidation (Grosjean, 1982; Rogozen and Ziskind, 1984). Methanol's low reactivity slows atmospheric production, offsetting the increase in direct emissions. Direct emissions of HCHO from methanol-burning vehicles could constitute a problem in closed areas (parking garages and tunnels) under extreme circumstances; CO buildup would be a similar problem in those cases (Machiele, 1987; Gold and Moulis, 1988). Chang and Rudy (1989) found that eye irritation in some individuals may result in the most severe tunnel exposures. A similar study is under way for parking garages. Ambient exposure to HCHO from methanol-fueled vehicles would be a small fraction of the total individual exposure (Gold and Moulis, 1988). Unlike many compounds found in gasoline, methanol is not a precursor to PAN or to higher organic nitrates, and those compound concentrations were predicted to decrease by 25% (Russell et al., 1990). Predictions indicate that the lower reactivity of methanol would slow oxidation of nitrogen compounds, leading to reductions in nitrogen dioxide (NO2), nitric acid (HNO3), and particulate nitrate of 20-40%. Other particulate components, such as carbon and sulfate, would decrease when methanol
Page 400
is used in place of diesel fuels. In the study by Russell et al. (1989), the use of methanol in place of diesel fuel was found to be particularly attractive because of the reduction in particulate matter and NOx emissions. It is expected that benzene concentrations, which are due predominantly to gasoline, also would decrease when methanol fuel is used (Gabele, 1990). Methanol-fueled vehicles will lead to increased atmospheric concentrations of methanol, although the predicted concentrations are likely to be less than the level of concern (e.g., the threshold limit value) (Chang and Rudy, 1990; Russell et al., 1990). Also, these vehicles will likely emit formic acid (Smith, 1982), which would contribute to acid deposition.
Methanol's low reactivity led to concern that it could be transported long distances and cause high ozone or formaldehyde concentrations downwind. Calculations by Russell (1990) and Sillman and Samson (1989) show that this is not the case. Reasons for this finding include the high VOC-to-NOx ratio during long-range transport and the slow atmospheric production of HCHO from methanol compared with other VOCs.
Both the modeling and experimental results indicate that methanol use can improve air quality by lowering nitrogen dioxide, aerosols, and some toxics (e.g., benzene), as well as ozone, although the expected ozone reductions are modest, especially from M85 fuels. If flexibly fueled vehicles are used, air-quality benefits likely will be achieved only if M85 or purer fuel is used consistently. If most vehicles were running on a more dilute blend (say M50 or M25), increased evaporative and other organic emissions could lead to increases in ozone. Use of methanol in heavy-duty applications has promise for reducing particulate matter and also could reduce ozone. Increased research on technologies to reduce methanol and HCHO emissions effectively is necessary to obtain these potential benefits.
Reformulated Gasoline
One outcome of the consideration of alternative transportation fuels has been an interest in how reformulating the composition of gasoline may improve air quality. This effort has been spearheaded by some individual companies, as well as the Auto/Oil Air Quality Improvement Research Program (AQIRP) (Burns et al., 1991). Ultimately the AQIRP provide extensive information about the air-quality impacts of reformulated gasoline and methanol blends, but only limited information is available at this time. In particular, only the predicted impacts of a limited variation in gasoline composition are available as used in 1989-model-year vehicles (Auto/Oil Air Quality Improvement Research Program, 1991; Hochhauser et al., 1991).
Page 401
The AQIRP involves testing the effect of varying fuel compositions on emissions composition and quantity, and modeling the changes in air quality that would accompany use of the various fuels. In particular, the AQIRP is investigating changes in aromatic, olefin, sulfur, and oxygenate (MTBE) content and in the fuel vapor pressure. A suite of late model (1989) light-duty vehicles are run on these reformulated fuels, and the resulting exhaust and evaporative emissions are measured for composition and mass. These test results are then used to speedy input data for air-quality models. An EKMA-type model is being used for screening purposes, and a grid model is used for more in-depth analysis. The grid model is being used to simulate air-quality impacts in Los Angeles, New York, and Dallas.
Airshed model results to date show that changing fuel composition can have an effect on air quality, due to changing the mass of emissions of VOC, NOx and CO, as well as the reactivity of the VOC. In future year simulations (2005 for New York and Dallas, 2010 for Los Angeles), peak ozone concentrations were predicted to be reduced only from about 1% (Dallas) to 3% (Los Angeles) in response to gasoline changes. These small changes, however, are about one-fourth of the predicted peak ozone reduction due to the complete removal of all light-duty vehicle emissions. Increasing vapor pressure and olefinic content were predicted to lead to increased ozone peaks and exposure. The aromatic and MTBE content did not have as a marked effect on predicted ozone.
Studies of more extensively reformulated gasolines than those tested in the AQIRP have found greater potential ozone decreases (Boekhaus et al., 1991; DeJovine et al., 1991). For one advanced reformulated gasoline reductions in VOC mass emissions of 31% were found and the reactivity of the organics also decreased leading to an effective ''reactivity-adjusted'' VOC reduction of 39%. NOx and CO also decreased by 26% each.
Air-Quality Benefits of Other Fuels
There have been few detailed studies of the likely air-quality benefits of alternative fuels other than methanol and reformulated gasoline. Carter's studies (1990b, 1991) provide the ability to estimate the likely benefits of using ethanol-based fuels, natural gas, and LPG. Krupnick et al. (1990) and Hempel et al. (1989) have conducted detailed studies of electric vehicle use in Los Angeles.
Carter's relative reactivity measure indicates that switching to ethanol-based fuel (both E85 and E95) would provide small decreases in reactivity, as compared with gasoline or diesel fuel. However, the ethanol-fueled vehicles tested
Page 402
had greater mass emission rates. (The CARB [1989a, b] vehicle tests relied on very few vehicles, and the individual tests are not likely to be representative of in-use vehicles optimized to run on ethanol.) The mass-weighted emissions are predicted to increase ozone compared with emissions from conventional vehicles. LPG emissions would lead to 47% as much ozone, and CNG about 25% as much, after accounting for reactively differences and reductions in mass emissions. Evaporative emissions from LPG and CNG vehicles are expected to be small and unreactive. When weighted by the mass emission rates measured, LPG vehicles would contribute about half as much ozone as conventional vehicles, and NGVs would contribute about one-fourth as much.
It has been proposed that gasoline using ethanol as a blending agent (gasohol) would be allowed to have an increased vapor pressure, and hence increased evaporative emissions. Given the minor reactivity reduction of ethanol compared with gasoline, the increased evaporative emissions would negate much of the ozone reduction, and actually could lead to increased ozone production. This is similar to the effect resulting from use of low methanol blends in flexibly fueled vehicles (Figure 12-3).
Although Carter and co-workers have not specifically considered the effects on production of pollutants other than ozone, the atmospheric chemistry of the emitted compounds provides much of the information needed to assess the likely response of other compounds. Acetaldehyde is the atmospheric oxidation product of ethanol, and it is also relatively abundant in ethanol-fueled vehicle emissions. Acetaldehyde is a precursor to peroxyacetyl radical and PAN formation. Increased PAN levels can be expected, as has been noticed in Brazil (Tanner et al., 1988; Grosjean et al., 1990b).
Natural Gas And Lpg
NGVs and LPG vehicles emit relatively small amounts of formaldehyde. Given the low reactivity of their emissions, it is likely their use would reduce atmospheric HCHO and PAN concentrations. It also is reasonable to expect slower NO oxidation to NO2 and HNO3, lowering those species' concentrations. Emissions of toxics (such as benzene) also would be substantially less from NGVs and LPG vehicles than from conventional vehicles.
Electricity
Electric vehicles are the cleanest of the alternative vehicles. Their emissions are essentially displaced to centralized electricity-generating stations.
Page 403
Utility boilers emit, in comparison to internal combustion engines, very little VOCs and CO. NOx emissions depend on the type of fuel and control technology used. Krupnick et al. (1990) used an airshed model to find the benefits of using 500,000 to 1.5 million electric vehicles in Los Angeles (1.5 million vehicles would be about 17% of the light-duty fleet). Peak ozone reductions of as much as 4.1% were found. This study also showed that using electric vehicles would lead to almost three times the reduction as that from using M85 vehicles. A study by Hempel et al. (1989) found similar results, assuming a much larger portion of electric vehicles in fleet.
Of the alternatives, electric vehicles are the only vehicles that, unequivocally, will lead to large NOx reductions in urban areas. Regional effects are less certain and will depend on how electricity is produced. Increased NOx emissions from electricity-generating stations could lead to increased regional ozone that can be transported into cities. The expected change in NOx emissions is an important consideration, given that mobile sources are the dominant source of NOx, and recent studies show that ozone formation in some areas is NOx-limited (Chameides et al., 1988; Milford et al., 1989). Also, concentrations of particulate matter and organic nitrates can be effectively reduced by lowering NOx emissions (Russell et al., 1988b; Milford et al., 1989).
Incremental Voc Reactivity
A recognized limitation of the studies conducted so far is the uncertainty about the emission composition of exhaust from conventional and methanol-fueled vehicles in the future. The studies discussed above use forecast emissions data or data from limited measurements of prototype methanol-fueled and flexibly fueled vehicles. The limited availability of emissions data has led to an alternative approach to estimating air quality that is applicable not only to methanol but to the other fuels as well. Instead of using a simulated exhaust mixture, the sensitivity of the ozone-forming potential of increased emissions of individual compounds is predicted (Carter and Atkinson, 1989b; Carter, 1991; Russell, 1990). Then the contributions of each emission species, multiplied by the fraction of that species in the exhaust, can be added to find the net emissions reactivity. This allows the flexibility of rapidly estimating impacts of future vehicles as the data become available. This also can guide manufacturers and regulators toward what constitutes a cleaner fuel and how fuels compare. A shortcoming of this approach is that it does not fully account for the nonlinearities in the chemistry of ozone formation.
Carter (1991) and Carter and Atkinson (1989b) used an EKMA-type model
Page 404
with a chemically explicit mechanism to find the incremental reactivity (IR) of individual organic compounds. They define IR as
![]()
R is a measure of the ozone formation and DORG is the change in the organic gas input. Carter and Atkinson (1989b) defined R as the maximum of the difference between the ozone concentration and NO concentration during the simulation (see also Chapter 5). In essence, this is the local sensitivity of ozone formation to emissions of specific organic gases, and it is given as the maximum number of moles of ozone formed by a one-mole increase of carbon in VOCs. A summary of their calculation results is given in Table 12-3 for some species typically emitted from alternate and conventional vehicles. Note that the incremental reactivities of many compounds are sensitive to VOC/NOx. Some, such as toluene, have a negative sensitivity at high VOC/ NOx because of NOx and radical scavenging by oxidation products. Chang and Rudy (1990) found similar results from a similar study with a less explicit mechanism. An important finding of both studies was that the maximum incremental reactivity of the organics was found at a VOC-to-NOx ratio of about 6.
Two aspects of the above studies must be noted. First, they used predominantly single-day simulations, whose results depend on how initial conditions and emissions scheduling are treated. Second, the emissions and other inputs do not correspond to a particular air basin, and ozone responses to changes in VOCs are location specific. Russell et al. (1991; 1992) conducted a similar analysis using multiday airshed calculations for Los Angeles. However, the chemical mechanism employed was more lumped (see Chapter 5) than that used by Carter and Atkinson (1989b). Russell et al. (1991; 1992) calculated local sensitivities to lumped organic classes, methanol, and formaldehyde (Table 12-4), relative to ozone sensitivity to CO emissions. Individual compound sensitivities multiplied by the exhaust compositions given in Table 12-1 yield the relative reactivity of the exhaust. If the exhaust emissions rates also are used, the relative reactivities per mile can be calculated. These measures, for methanol and other fuels, are given in Table 12-5.
Comparison of the incremental reactivity method with the model results that look specifically at switching to methanol show general agreement. VOC emissions from M100 would be a little more than half as reactive in terms of ozone formation as would emissions from conventional fuels, and M85 would
Page 405
| TABLE 12-3 | |||
| Compound | VOC/NOx, ppbC/ppb | ||
| 4 | 10 | 20 | |
| Formaldehyde | 2.42 | 0.77 | 0.24 |
| Ethane | 0.024 | 0.031 | 0.015 |
| n-Butane | 0.10 | 0.031 | 0.052 |
| Ethene | 0.85 | 0.64 | 0.30 |
| Propene | 1.28 | 0.61 | 0.25 |
| Toluene | 0.26 | 0.04 | -0.058 |
| m-Xylene | 0.98 | 0.32 | 0.012 |
| Methanol | 0.12 | 0.12 | 0.055 |
| Ethanol | 0.18 | 0.14 | 0.038 |
| Carbon monoxide | 0.011 | 0.018 | 0.010 |
| Source: Carter and Atkinson, 1989b | |||
be about 70% as reactive. This assumes that the total emissions of VOCs are about the same, that HCHO emissions are not excessive (<30 mg/mile), and that emissions are limited to areas that are not NOx-limited (VOC/NOx < 10). Further air-quality benefits depend on lowering HCHO emissions.
Regulatory Implementation of Alternative Fuel Use
Because of the considerable contribution of mobile source emissions to
Page 406
| TABLE 12-4 | |||
| Airshed (Russell, 1990)a | |||
| Peak | Exposure | Carter (1989) | |
| CO | 1 | 1 | 1 |
| Aldehydes >C2 | 64 | 64 | 93b |
| Alkanes | 16 | 11 | 9.5c |
| Alkenes | 67 | 83 | 51d |
| Aromatics | 51 | 82 | 53e |
| Ethene | 71 | 67 | 78 |
| Formaldehyde | 119 | 148 | 180 |
| Toluene | 15 | 24 | 25 |
| Methanol | 17 | 14 | 17 |
| aAirshed model results were combined with the population distribution to find the sensitivities of both the peak ozone and ozone exposure as VOC emission rates are increased. Exposure, in this case, is defined as the ozone concentration multiplied by the population density for ozone concentrations in excess of the NAAQS concentration of 120 ppb. | |||
| bused incremental reactivity for acetaldehyde. | |||
| cUsed 50% C4-C5 alkanes, and 50% C6 + alkanes from Carter (1989) | |||
| dUsed equal portions of C4-C5 and C6 alkenes from Carter (1989) | |||
| eUsed equal portions of di- and tri-alkylbenzenes from Carter (1989) | |||
California's air-quality problems, controlling these emissions has been a key aspect of the overall air pollution strategy of the California Air Resources Board (CARB). In recent years, CARB has adopted various measures to reduce mobile source emissions through better control of in-use emissions and more stringent emission standards. This process was furthered in September, 1990 with CARB's approval of the "low-emission vehicles and dean fuels" regulations (Resolution 90-58), which were approved by the California Office of Administrative Law on August 30, 1991.
The low-emission vehicles and clean fuels regulations, an integral part of
Page 407
| TABLE 12-5 | ||||
| Fuel | Cartera (1991) | Dunkerc(1990) | Russell (1990)/Williams et al. (1989)b | |
| Reactivity/gram (exhaust + evap) nonmethane VOCc | Reactivity/mile Total emissionsc | |||
| Gasoline(indolene) | 1 | 1 | 1d | 1d |
| E95f | 0.84 | 2.0 | — | — |
| E85g | 0.81 | 2.1 | — | — |
| M85h | 0.73 | 0.63 | 0.93 | 0.73 (0.55)e |
| Methanol | 0.54 | 0.97 | 0.64 | 0.58 (0.48)e |
| Liquid propane gas | 0.83 | 0.47 | — | — |
| Compressed natural gas | 0.44 | 0.24 | — | — |
| aCarter (1991) used measurements conducted by CARB (1989a, b) to derive fuel-based reactivities;bThe measurements by Williams et al. (1989) are used for a flexibly fueled vehicle fueled on M85 and M100. The relative reactivities of the emissions components are taken from Russell. Dunker (1990) used the same emissions profiles and modeled the exact composition, but did not use relative reactivities.;cReactivities are relative to gasoline (or indolene) being 1. Indolene is commonly used as a test fuel;dCornposition profile developed by Sigsby et al. (1987);eThe first value is for exhaust formaldehyde emissions as measured; the second, in parentheses, is for a low-formaldehyde (5 mg/mile)-emitting vehicle;fE95, 95% ethanol and 5% gasoline;gE85, 85% ethanol and 15% gasoline;hM85, 85% methanol and 15% gasoline. | ||||
Page 408
CARB's Long-Range Motor Vehicle Plan, established stringent, reactivity-based exhaust emission standards for new passenger cars, light-duty trucks and medium-duty vehicles and required that any dean alternative fuels needed by these vehicles be made available to the public.
Under the low-emission vehicle regulations, four categories of low-emission vehicles, each certified to a particular set of exhaust emission standards, will be phased in during the mid-1990s. In order of increasing stringency, the vehicle categories are
• Transitional Low-Emission Vehicle ("TLEV")
• Low-Emission Vehicle ("LEV")
• Ultra-Low-Emission Vehicle ("ULEV")
• Zero-Emission Vehicle ("ZEV")
The standards for all four categories of low-emission vehicles represent significant reductions in emissions compared to previous standards. Table 12-6 summarizes the 50,000-mile certification standards for passenger cars and small light-duty trucks. The regulations also promulgated emission standards for light-duty trucks above 3750 pounds loaded vehicle weight (LVW) and for medium-duty vehicles and engines.
A regulatory problem that has plagued alternative fuels and reformulated gasoline has been how to treat all fuels equally, without apparent bias that is unwarranted on an air-quality basis. One strategy to account for the lower ozone-forming potential of alternative fuels, yet allow for all fuels and technologies to compete on an equal regulatory basis, is to use a "reactivity-adjusted" emission standard. CARB (1990) has adopted regulations that use the incremental reactivity of each compound in automobile exhaust (see Table 5-5) to calculate a Reactivity Adjustment Factor (RAF). The RAF is the reactivity of the alternative fuel exhaust compared to that of the baseline fuel. If the RAF is less than 1, proportionally more mass can be emitted, such that the total ozone-forming potential is equivalent to that from the baseline fuel. For example, if the RAF of the alternative fuel is 0.5, then 2 g of alternative fuel emissions would have the same ozone-forming potential as 1 g of baseline fuel emissions. At present, the method for calculating the reactivities of individual compounds involves using an EKMA-type box model, exercised for about 75 trajectories representing different days in different cities (Carter, 1991). The simulations are, in effect, less than one day long, which may cause the results not to reflect actual airshed behavior. As expressed elsewhere in this report, it is strongly suggested that multiday simulations using grid-based models be used for control strategy analysis. Application of a grid-based model over a three-day period found good agreement with the box model results, but the
Page 409
| TABLE 12-6 | ||||
| Category | NMOGa | COb | NOxc | HCHOd |
| Conventional | 0.25e | 3.4 | 0.4 | 0.015f |
| Transitional Low Emission Vehicle | 0.125 | 3.4 | 0.4 | 0.015 |
| Low- Emission Vehicle | 0.075 | 3.4 | 0.2 | 0.015 |
| Ultra-Low-Emission Vehicle | 0.040 | 1.7 | 0.2 | 0.008 |
| Zero-Emission Vehicle | zero | zero | zero | zero |
| aNMOG: Non-methane organic gases | ||||
| bCO: Carbon monoxide | ||||
| cNOx: Oxides of nitrogen | ||||
| dHCHO: Formaldehyde | ||||
| eStandard is for non-methane hydrocarbons | ||||
| fApplies to methanol vehicles only> | ||||
less reactive compounds were relatively more reactive over the three days in the grid-based model than in the box model (Russell et al., 1991a, b). The box model's simulations of less than a day do not fully account for the multiday buildup and carryover of the less reactive compounds. While there are differences in the results of the two models, the studies to date support the use of reactivity scaling. However, there are important areas for further investigation, such as the more widespread use of advanced models and mechanisms, studies of the impact of different meteorological conditions and locations, and the development of uncertainty estimates.
Summary
Alternative fuels have the potential to improve air quality, especially in
Page 410
some urban areas, by reducing concentrations of the precursors to ozone and other photochemical oxidants. However, alternative fuels alone will not solve the air quality problems experienced by major cities. They could be an effective addition to ozone abatement strategies, but they must be considered in combination with other possible controls. Also, there are significant uncertainties left to be resolved, many of which stem from the inability to predict what the emissions will be from alternatively fueled vehicles in the future. Also, it must be stressed that the use of alternative fuels other than electricity and natural gas may have little effect on the increase in emissions that occurs over time with in-use vehicles, particularly its "super-emitters." It is this emissions deterioration that is the most central aspect of the motor vehicle emissions problem (Chapter 9).
Alternatively fueled vehicles could reduce mass emissions of volatile organic compounds (VOCs) and oxides of nitrogen (NOx) and could decrease the atmospheric reactivity of the emissions (by using methanol or natural gas). There are some regions where alternative fuels could work effectively and others where little benefit would result. Furthermore, the effectiveness of these fuels could vary within a single airshed, depending on the VOC/NOx ratio. Their use must be considered for each location separately.
The expected air quality benefits from each of the choices are not equal. Estimates of the potential benefits can be derived from emissions composition and from recent studies on compound reactivities (or ozone-forming potential). The studies to date have been limited to a few cities under a few conditions, so increased study is warranted.
Electric vehicles would give the greatest improvement in air quality by virtually eliminating VOCs, CO, and NOx (net NOx emission changes would depend on the source). They would lead to ozone reductions in virtually any region.
The fossil fuel alternatives and reformulated gasolines should lead to ozone reductions in areas with low VOC/NOx, such as downtown Los Angeles and New York City. However, their effects in areas with high VOC/NOx (such as Houston, Atlanta, or regions downwind from urban centers) would be minimal, unless NOx emissions are also reduced. The benefits in intermediate regions must be explored further.
Natural gas is the cleanest of the fossil fuels, and natural gas vehicles appear to have the lowest reactivity VOC emissions. Vehicles in areas that have carbon monoxide (CO) problems would especially benefit from use of natural gas. However, those vehicles are usually designed to burn fuel-lean, and might not be able to meet proposed NOx standards.
If cold-start problems are overcome, burning essentially pure methanol gives substantial reductions in exhaust and evaporative emission reactivity, although the air quality benefits are degraded if M85 (or lower percent methanol) fuel is
Page 411
used or if formaldehyde emissions are not effectively controlled.
Flexibly fueled vehicles could provide an easy transition mechanism to extensive fleets of methanol-fueled vehicles, but they will not offer the air quality benefits of dedicated vehicles. Increased mass emissions from flexibly fueled vehicles using low-methanol-blend fuels could counter the decreased reactivity of those emissions. Also, formaldehyde emissions must be controlled over the life of the vehicle.
Reformulated gasolines offer the easiest transition to a cleaner fuel and studies in progress indicate that properly reformulated gasolines can meet or surpass reductions in the emission reactivity of methanol-gasoline blends (e.g. M85). Ethanol would provide less benefit and might even be detrimental (Table 12-5).
Recent studies indicate that mobile-source emissions have been seriously underestimated (Chapter 9). Also, if the excess emissions are from ''super-emitters,'' this could affect the choice of alternative fuels used. Not enough is known to assess this issue in detail, although these findings could support the use of naturally cleaner fuels, such as electricity and natural gas. On the other hand, if the inventories have underestimated the VOC emissions from stationary and biogenic sources, the benefits of using alternative fuels would be less than currently predicted.
Although the degree to which alternatively fueled, vehicles can improve air quality is not known, the use of alternative fuels can become part of an effective ozone control strategy. Certain applications now exist where these fuels can be used effectively. Heavy-duty-vehicle use of methanol or natural gas appears promising. It is not clear which choice is the best for light-duty vehicles, and fthat decision depends on location and goals.


































