Page 413
13
Tropospheric Ozone and Global Change
Introduction
This chapter addresses the scientific evidence that relates global change1 in atmospheric gases and climate to tropospheric ozone. Such a consideration of global changes is important because they are likely to continue and might hamper local efforts to meet the ozone National Ambient Air Quality Standards (NAAQS). Discussions in this chapter must be mostly qualitative, because few examples of research couple global changes with air quality. This lack of information points to the need for a long-term coordinated research program (see Chapter 14).
Global Change: Observations
Two recent reports on global change (WMO, 1990; IPCC, 1990) have presented detailed reviews of the observations and likely causes of the increases found in most long-lived atmospheric trace gases. Concentrations of gases such as carbon dioxide (CO2), trichlorofluoromethane (CFC13), dichlorodifluoromethane (CF2C12), methane (CH4), and nitrous oxide (N2O) are increasing at typical observation sites (see Table 13-1).
1Global change refers to changes in climate and changes in atmospheric chemistry.
Page 414
| TABLE 13-1 | |||||
| Species | Mean global concentration | Annual rate of increase during 1980s | |||
| Pre-industrial | Circa 1987 | ||||
| CO2 | ˜280 ppm | 348 ppm | 0.5% | ||
| CH4 | ˜600 ppb | 1680 ppb | 0.8% | ||
| N2O | ˜ 285 ppb | 307 ppb | 0.2% | ||
| CFCl3 | 0 | 240 ppt | 4% | ||
| CF2C12 | 0 | 415 ppt | 4% | ||
| CCl4 | 0 | 140 ppt | 1.5% | ||
| CH3CC13 | 0 | 150 ppt | 4% | ||
| CH3Cl | 600 ppt? | 600 ppt | ˜0% | ||
| CO | ? | 90 ppb | ˜1% (northern hemisphere) <1% (southern hemisphere) | ||
| Source: WMO (1990) | |||||
These gases act as greenhouse gases that contribute to the radiative forcing of the atmosphere, increasing the radiative forcing at the tropopause by about 0.5 watts/meter2 over the past decade. The record of global mean surface temperature exhibits fluctuations, but with an apparent increasing trend (Figure 13-1). Global mean surface air temperatures have increased by as much as 0.3ºC to 0.5ºC this century (Hansen and Lebedeff, 1988; Jones, 1988). The temperature trend for the United States is more ambiguous because of the smaller sampling area, but also shows a temperature increase, albeit smaller, about 0.1ºC to 0.3ºC (Hansen et al., 1989).
Column ozone has been decreasing over the past 2 decades in both hemispheres (see UNEP/WMO, 1990).2 The decrease in stratospheric ozone
2Column ozone is the abundance of ozone, predominantly stratospheric, that is obtained by integrating the amount of atmospheric ozone in the vertical direction.
Page 415
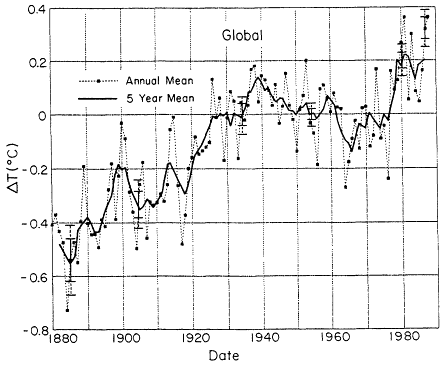
Figure 13-1
Observed trends in surface air temperatures. Source: Hansen and Lebedoff, 1988.
over Antarctica is clearly one of the largest anthropogenic perturbations to our planet. Ozone losses in the northern midlatitudes have been less dramatic but still important—as much as 8% over the past decade in winter, with smaller but significant losses in summer (Stolarski et al., 1991).
Increases in CH4 and CO, along with other photochemically active trace gases such as odd-nitrogen compounds and volatile organic compounds (VOCs), increase the potential for production of ozone throughout the troposphere.
Global tropospheric ozone is important as the primary source of tropospheric oxidation (mainly through OH) and as a greenhouse gas in the upper troposphere (but not in the atmospheric boundary layer, where it radiates at the same temperature as the surface). Tropospheric ozone is highly variable, but exhibits systematic patterns: generally increasing from the equator to the
Page 416
midlatitudes and from the surface to the tropopause (Chatfield and Harrison, 1977; Logan, 1985). Near the surface, average concentrations in the nonurban atmosphere (30-50 ppb) are greatest in spring and summer over northern midlatitudes. Interpretation of ozone trends is difficult because of the high variability in its concentrations. Reported trends (at the surface and from ozone sondes below 8 kilometers altitude) consistently show increases of about 1% per year over the past decade or two at northern midlatitudes (Angell and Korshover, 1983; Logan, 1985; Oltmans and Komhyr, 1986; Tiao et al., 1986; Feister and Warmbt, 1987; Bojkov, 1988; Crutzen, 1988)—a trend consistent with nineteenth-century measurements (Bojkov, 1986; Volz and Kley, 1988). Data for the Southern Hemisphere are scarce, but they indicate a small decline in two locations (Oltmans et al., 1989). The observed trends in tropospheric ozone should be considered as continental or possibly hemispheric in extent, but not global. As discussed below, it is not possible to make quantitative predictions regarding future trends in tropospheric ozone or its greenhouse effects.
Global Change: Expectations And Response
It is difficult to make quantitative predictions of global change. Moreover, it would be impossible to make accurate predictions about the response of regional tropospheric ozone to global-scale changes in climate or atmospheric composition, even if we knew what the conditions in the future global atmosphere were going to be (see general discussions: Bachmann, 1988; Bernabo, 1989). Accordingly, qualitative estimates of some of the trends in temperature and trace gases that might be expected over the next two decades are presented. Mechanisms by which these global changes are likely to perturb tropospheric oxidants and local air quality are discussed, but in some cases even the direction (increase or decrease) of the effect is uncertain. Table 13.2 summarizes the links between human activities, global chemical and climate changes, and regional ozone.
Greenhouse gases are expected to increase substantially in the next century: CO2 and CH4 could double, N2O could increase by 25%, and CFCs could more than double if not controlled under the Montreal Protocol (Prather and Watson, 1990). The sources of the CH4 and N2O increases are not yet fully understood, but the estimates are based on currently observed trends. Accurate prediction of CO2 is hampered by a lack of understanding of the net biospheric source and oceanic uptake (IPCC, 1990).
T, the mean tropospheric temperature (Table 13-2), is predicted by climate models to continue to rise globally in response to the increased greenhouse forcing (Hansen et al., 1988; Raval and Ramanathan, 1989), with greater
Page 417
increases in large urban areas than in rural ones (Oke, 1973; Viterito, 1989). Average global increases of 0.5ºC to 1.0ºC are predicted over the next two to three decades (IPCC, 1990), but there is considerable uncertainty in these calculations. Late in the twenty-first century, the increase could be as large as 5ºC (NRC, 1991).
There is an empirical relationship between worsened air quality and higher temperatures. As discussed in Chapter 2, a high temperature is generally a necessary but not sufficient condition for the occurrence of high ozone concentrations. This relationship is complex and cannot readily be extrapolated to a warmer climate because higher temperatures are often correlated empirically with sunlight and meteorology. Temperature increases will tend to destabilize peroxyacetyl nitrate (PAN) and related compounds, releasing more NOx into the urban environment. There is much uncertainty about the effect of temperature on anthropogenic VOC emissions as well as on the possible enhancement of biogenic emissions of VOCs. Temperature has a direct calculable effect on the photolysis of ozone and other kinetic rates, but the effect on ozone concentration is expected to be small.
Water-vapor concentrations will increase concurrently with temperature, probably maintaining the same relative humidity, and hence increase by 6% per degree Celsius rise in temperature. Water-vapor increases will increase tropospheric ozone loss and OH production through the reactions involving water and photolysis products of ozone.
On average, global tropospheric OH concentrations would increase about 10% for a 25% increase in H2O. The effect on aerosol chemistry and photochemical fogs is not known because the change in relative humidity is not known. The increase in water vapor would have a substantial effect on clean air chemistry, decreasing ozone, but should not significantly affect urban chemistry.
Stratospheric ozone is expected to decrease in response to the rise in atmospheric chlorine that is inevitable through the end of this century. The depletion in column ozone could be limited (no more than 5-10% at northern midlatitudes in spring) if an enhanced Montreal Protocol leads to a phaseout of CFCs (Prather and Watson, 1990; WMO, 1990). Depletion of stratospheric ozone leads to a direct increase in the penetration of solar ultraviolet light. The effect of increased ultraviolet light on tropospheric ozone formation depends on NOx concentrations and other conditions that vary with latitude (Liu and Trainer, 1988; Gery et al., 1988b; Derwent, 1989). Enhanced photochemical activity in the troposphere will lead to increased production of ozone over most continental regions of the northern hemisphere, but can lead to reductions of ozone in dean maritime conditions where photochemistry is a net sink for ozone (Liu and Trainer, 1988; Schnell et al., 1991).
Page 418
| TABLE 13-2 | ||
| Results of human activities | Expected atmospheric changes | Effect on regional ozone |
| Increased greenhouse gases (CO2, CH4, N2O, CFCs | Warmer tropospheric temperature | Increase |
| Increased tropospheric H2O | Local effects small (global decrease small) | |
| Altered global circulation; possible enhancement of stagnation episodes | Unknown; possible increase | |
| Possible increase in stratospheric turnover rate, with greater injection of O3 and NO3 into troposphere | Possible increase | |
| Increased CFCs and halons | Less stratospheric O3; more solar ultraviolet radiation reaching troposphere | Increase in polluted regions; decrease in remote global areas |
| Increased urbanization | Warmer local temperature | Increase |
| Possible enhancement of stagnation episodes | Unknown; possible increase | |
| Increased regional emissions of VOC, CO, and NOx | Increased regional production of O3 | Increase (local and global |
| Possible increased regional emissions of sulfur and aerosols | Enhanced cloud chemistry; possible increase in cloud cover | Unknown; possible decrease |
Page 419
Global tropospheric ozone is expected to respond to many aspects of climate change as outlined here. Current models cannot incorporate all the important components in the tropospheric ozone budget, but studies with simple models have shown that the response of ozone is complex and depends on the suite of changes in the global troposphere, including changes in methane and other VOCs, NOx, water vapor, and carbon monoxide (Isaksen and Hov, 1987; Liu et al., 1987; Isaksen et al., 1988; Liu and Trainer, 1988; Prather, 1989; Thompson et al., 1989, 1990). Moreover, there is observational evidence that biomass burning (Delany et al., 1985; Logan and Kirchhoff, 1986; Kirchhoff et al., 1989) and regional pollution (Cox et al., 1975; Fishman et al., 1985) create extended layers of air in the troposphere with increased concentrations of ozone (see Figure 13-2) and could be an important part of the current budget for tropospheric ozone. Available predictions for future tropospheric ozone are varied, but they show limited increases over the next 50-100 years under the most extreme scenarios (not more than 50%, increases of 60-120 ppb).
Generally, increases in global tropospheric ozone will be expected to lead to a proportional rise in the ozone abundance of air entering metropolitan regions. However, the expected rise in ozone at a specific metropolitan region is dependent upon the local photochemistry and the initial ozone concentrations. Based on currently observed trends we might expect a systematic increase in local ozone of 10 ppb over the next 20 years. The feedback relationship between global air quality and local air quality could become more important in the future.
The abundance of global OH determines the global oxidative capacity of the lower atmosphere. OH, hydrogen peroxide (H2O2) and other oxidants are local quantities that respond to daily variations in ultraviolet sunlight, CO, O3, CH4 and NOx (Levy, 1971; Sze, 1977; Thompson and Cicerone, 1986; Liu and Trainer, 1988; Thompson et al., 1990). The global average of OH is not directly influenced by the OH concentrations in the urban atmosphere because the air over cities constitutes such a small fraction of the global atmosphere. However, the abundance of OH in the nonurban atmosphere could be strongly affected by urban emissions of NOx, because tropospheric chemistry over much of the globe is in the low-NOx limit. When NOx concentrations are low, e.g., less than 0.1 ppb, the abundance of OH responds almost linearly to NOx, because HOx is effectively recycled to OH by reaction with NO. When NOx concentrations are high, on the other hand, e.g., greater than 1 ppb, NOx reactions such as formation of nitric acid reduce OH. A countering influence is the expected increase in CHx, CO, and long-lived VOCs (such as C2H6) because of the increase in the emissions of these gases in the future. These species are the sink for OH in the remote atmosphere, which has low concen-
Page 420
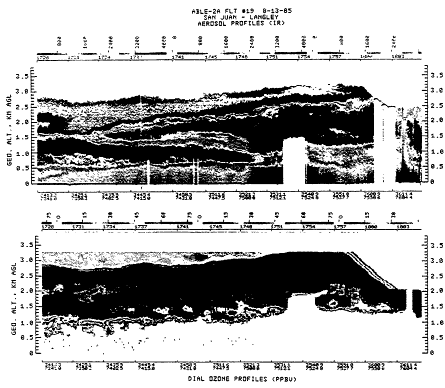
Figure 13-2
Vertical distribution of ozone in the troposphere immediately downwind
of the east coast of the United States. The data were obtained with an
airborn lidar. The high concentration of ozone at altitudes of 1-3 km are
typical of polluted air masses moving off the continental United States toward
the North Atlantic Ocean.
trations of NOx. Overall, the simple model studies mentioned above for tropospheric ozone tend to predict decreases in global OH over the next 50 years of order tens of percent. Regional air quality will not respond directly to changes in global OH, but the background abundances of biogenic VOCs may be altered and regional ozone thus affected (Chameides et al., 1988; Cardelino and Chameides, 1990).
Page 421
Stratospheric NOy will probably increase as aircraft emissions and N2O (stratospheric source of NOy) grow. Is this source of NOx important anywhere other than the upper troposphere? Does it affect global tropospheric ozone production? These questions need to be addressed with global three-dimensional models, as for tropospheric ozone.
Stratospheric exchange, the rate of turnover of stratospheric air, is likely to change in a world where the concentration of CO2 is doubled. If the rate of circulation in from the tropical tropopause, through the stratosphere, and out into the midlatitude troposphere were to speed up, then the stratospheric source of tropospheric ozone would increase. The only modeling study of this effect produced a measurable but modest increase of at most 15% in the residual circulation of the lower stratosphere (Rind et al., 1990). However, the implications for ozone and the lifetimes of other gases have not been examined.
Changes in atmospheric properties might lead to regional climate changes that could alter tropospheric concentrations (Hansen et al., 1989). Boundary layer exchange, in particular the rate of venting of the lowest atmospheric layers over the continents, has been predicted to change as temperatures rise (Rind, 1989). Storms, particularly the frequency and intensity of hurricanes, are hypothesized to increase as the sea surface temperature rises (Emanuel, 1987) and might lead to enhanced tropospheric mixing.
Fredicting Changes in Tropospheric Ozone
The global modeling of tropospheric ozone requires spatial and temporal resolution that can at best be achieved only with three-dimensional chemical transport models (CTMs). The scales of chemical heterogeneity critical to the modeling of global net production of tropospheric ozone occur over continental-maritime distances and on the much smaller scales of regional air pollution. In particular, the distributions of NOx and reactive VOCs are patchy and likely to be correlated; much of the net production of ozone will come from the highly perturbed regions and will not be related to the longitudinally averaged concentrations.
High-resolution regional three dimensional CTMs for tropospheric ozone have been developed in response to the need to study air pollution as discussed in Chapters 9 and 10 and elsewhere (McRae and Seinfeld, 1983; Liu et al., 1984; Carmichael et al., 1986; Chang et al., 1987; McKeen et al., 1990). These models have detailed photochemical mechanisms, but they are extremely limited in that they must be initialized, they depend on boundary conditions, and they are used only for brief simulations (generally, no more than three
Page 422
days). Their grids (5 km up to 80 km) cannot be readily expanded to global size (400 km), and they cannot be used to simulate the annual global climatology of ozone. (Climatology refers to the distributions of means, patterns, and variability.) Nevertheless, the problems addressed by these models—heterogeneous distribution of NOx and VOCs, and net ozone production—are important components of the ozone budget on a global scale (Liu et al., 1987). Even at the highest resolution, these regional models fail to resolve the individual pollution plumes associated with concentrated industrial sources that may represent an important nonlinear chemical processing of NOx emissions (Sillman et al., 1990a). At the other extreme, global models will not resolve some of the processes in the regional CTMs; these processes must be accounted for in terms of larger-scale calculated variables such as wind speed and temperature gradients.
Some global two-dimensional, zonally averaged models for tropospheric ozone use realistic photochemical schemes but, by their nature, fail to resolve the continent-ocean differences in surface emissions and zonal transport (Isaksen and Hov, 1987; Hough and Derwent, 1990). Consequently, these models fail to account for the nonlinear dependence of ozone production on NOx concentrations. Regional box models for tropospheric chemistry are a subset of the two-dimensional transport models and also have been applied to ozone (Thompson et al., 1989).
Three-dimensional models are far more difficult to design, initialize, and evaluate. Global CTMs depend on working, general circulation models for a complete and consistent picture of the physical climate system. It is not surprising therefore that none of the three-dimensional CTMs has presented a global tropospheric ozone simulation with realistic photochemistry. Early work with CTMs focused on stratospheric ozone chemistry (Hunt, 1969; Cunnold et al., 1975; Mahlrnan et al., 1980) and did not include the complexities of tropospheric ozone chemistry. More recent CTM studies have studied the climatology of tropospheric ozone (Levy et al., 1985) but include only a stratospheric source with a surface sink and no in situ chemistry. These studies have contributed to the tropospheric CTMs by better defining the stratospheric source of tropospheric ozone. Nevertheless, none of these CTM studies has been able to include the photochemical sources of ozone from urban regions or from tropical biomass burning (Fishman et al., 1985; 1990).
What steps are needed to devise a global CTM for tropospheric ozone? For example, the research at NOAA's General Fluid Dynamics Laboratory (GFDL) began with a seminal paper defining its tracer model (Mahlman and Moxim, 1978) and continued with a sequence of numerical experiments applicable to trace species with more complex sources and sinks: ozonelike (Mahlman et al., 1980), N2O (Levy et al., 1982), tropospheric ozone with no chemis-
Page 423
try (Levy et al., 1985), and tropospheric NOx (Levy and Moxim, 1989). Similarly, the Goddard Institute for Space Studies/Harvard CTM began with a paper defining the model (Russell and Lerner, 1981) and then proceeded with a series of detailed studies that were meant to calibrate the model: CO2 (Fung et al., 1983), CFCs (Prather et al., 1987), krypton (Jacob et al., 1987), radon (Balkanski and Jacob, 1990; Jacob and Prather, 1990), and CH3CCl3 (Spivakovsky et al., 1990a). Research groups at the Max Planck Institute in Hamburg (Heimann et al., 1990; Brost and Heimann, 1991), the National Center for Atmospheric Research (Rasch and Williamson, 1991) and the Lawrence Livermore National Laboratory (Penner et al., 1991) are similarly pursuing the development of global CTMs in studies of the calibration of continental and global transport and mixing, as well as the chemistry of tropospheric NOx and hydrocarbons. The development of global CTMs will soon be applied to tropospheric ozone, and such tested and verified models will probably appear over the next five years.
A prediction of changes in global tropospheric ozone is needed by the U.S. Environmental Protection Agency (EPA) to study the effects of various policies on global chemistry and the climate. EPA has used results directly from the two-dimensional chemical or multibox budget models (Isaksen et al., 1988; Thompson et al., 1990) or has incorporated them into simple parameterized models that include a wider range of feedback couplings (e.g., Prather, 1989). Current models and emissions scenarios have not been consistently intercom-pared; however, most studies agree qualitatively, if not quantitatively (Isaksen and Hov, 1987; Liu et al., 1987; Isaksen et al., 1988; Liu and Trainer, 1988; Prather, 1989; Thompson et al., 1989, 1990). The response of ozone to global change is complex and will depend greatly on future global emissions. Under most circumstances, increases in tropospheric ozone are predicted by the middle of the twenty-first century, but they range from small to as much as 50%. A major limitation of these models is that they do not properly account for the nonlinear dependence of tropospheric chemistry on NOx and VOC concentrations.
Summary
The effect of global changes in the climate and atmospheric chemistry on tropospheric ozone are currently unpredictable, but they could lead to substantial increases in the number and duration of pollution episodes and in the size of the regions affected by high oxidant production. A warmer climate with less stratospheric ozone will enhance local photochemistry and probably local oxidant formation. Therefore, research efforts must elucidate the rela-
Page 424
tionships of such effects with attainment of the ozone National Ambient Air Quality Standard (NAAQS). For example, it will be important to continue to develop global chemical transport models (CTMs) to predict changes in tropospheric ozone concentrations. A major synergism is potentially available between the global and regional CTMs in the simulation of ozone.












