Summary
The development of methods for identifying toxic chemicals has been the focus of substantial research efforts by the National Institute of Environmental Health Sciences (NIEHS), the National Toxicology Program (NTP), the Environmental Protection Agency (EPA), the Agency for Toxic Substances and Disease Registry (ATSDR), the Food and Drug Administration (FDA), and other agencies at various levels of government. This report summarize a workshop held in Washington, D.C., on December 13-15, 1989, on research to improve the methodology for predicting the long-term toxicity of chemicals.
The salient concepts indicated by a number of workshop participants are:
-
Developing improved methods for predicting the long-term toxicity of chemicals will depend on improving our understanding of the underlying science and on effective coordination and integration of the relevant clinical, epidemiological, and laboratory approaches. To achieve these goals and to mobilize needed resources, a national strategy for fostering and coordinating such activities in the public and private sectors is needed. It is encouraging that the National Institute of Environmental Health Sciences (NIEHS) has the beginning of such a strategy in place.
-
The existing methods for predicting long-term chemical toxicity—for example, analysis of structure-activity relationships (SAP,) and other correlational techniques, in vitro and in vivo short-term tests (STTs), and longer-term animal bioassays—all have valid, if different, uses at present and promise to become increasingly effective in the future, given appropriate research for their further development, validation, and integration. Understanding the mechanisms of action at relevant exposure concentrations and times should improve the accuracy of predictions from laboratory results W humans.
-
Computerized SAR analyses—ultimately ideal in terms of their relative economy and rapidity—have considerable potential but have been restricted largely to the prediction of mutagenicity and carcinogenicity of chemicals in structurally related classes. Such analyses can be expected to become more useful within the next 5 to 10 years through improvement of analytical models, better understanding of limitations in the models, lowered computer costs, and widening of the range of toxicological end points to which the models are applicable. These advances will depend to a large extent on: a) further research to validate SAR methodologies; b) expanded research on the use of the methodologies, with particular reference to end points other than mutagenicity and carcinogenicity; c) coordinated development of data bases of the richness and quality needed to support the application of SAR methodologies, including the pertinent metabolic, toxicokinetic, and functional data; d) investigation of the importance of the physical-chemical properties of substances in SAR models; e) studies to explain the molecular mechanisms underlying SAR; and f) provision of stable funding necessary to support the further development of SAR methodologies.
-
More than 180 in vitro and in vivo STTs for predicting mutagenicity and carcinogenicity have been developed, but few have been evaluated on a sufficiently broad spectrum of carcinogens and noncarcinogens to define their predictive value, and no single SST or combination of SSTs has been found to be adequately predictive of carcinogenicity. For toxicological end points other than mutagenicity and carcinogenicity, there has been relatively little development of STTs, although for certain life-cycle-dependent processes
-
such as reproduction, STTs might have advantages over long-term tests in the detection of toxic effects. In spite of their currently limited usefulness for predicting long-term chemical toxicity, STTs have increasingly wide use for screening purposes and for supplementing other methods of evaluation. Because they can done quickly and cheaply, such tests promise to become more important in the future. Thus, to expedite STT developments, the following research activities are suggested: a) further validation of STTs for specific uses; b) development on a battery of STTs that are predictive for carcinogens of all chemical classes; c) development of SITs applicable to toxicological end points other than mutagenicity and carcinogenicity; d) development of STT's capable of assessing potency as well as binary (positive or negative) responses; e) further development of STTs applicable to human cells, tissues, and body fluids (e.g., biological markers and DNA probes); f) establishment of an organizational and policy framework to coordinate relevant STT development and validation activities in government, industry, and academia; and g) establishment of training programs and other measures to increase the number of able investigators in the field.
-
Of all toxicological methods employing surrogate test systems to predict the long-term toxicity of chemicals for humans, those based on studies using whole animals have the widest applicability and appear to be the most reliable, although the predictability of many animal models remains to be validated. Especially deserving of further development are animal test methodologies for predicting neurotoxicity, reproductive toxicity, immunotoxicity, developmental toxicity, and the toxicological effects of chemical mixtures. To improve these methodologies, the following research activities are suggested: a) studies to elucidate the fundamental biology of toxicological effects on organ systems in different species and strains leading to the selection of the best predicting species and strains, knowledge of which is essential for confident extrapolation of animal data to humans; b) refinement of toxicokinetic and mathematical dose-response models for estimating human risks from animal data; c) investigation of the extent to which toxicological data on a subset of compounds in a given chemical class are predictive for other members of the same class; d) study of the extent to which the predictive reliability of animal models may be enhanced by optimizing the experimental protocols; e.g. through changes in the duration of exposure, age, or genetic background of the experimental animals (including the use of outbred animals or transgenic animals), and e) development of institutional and funding mechanisms to foster integration of studies on toxicological mechanisms into the design and conduct of long-term animal bioassays.
-
Because the principal utility of surrogate test systems depends on their ability to predict for the human species, the systematic collection and study of relevant human data are important. For purposes of test validation, therefore, the following research activities are needed: a) clinical, toxicological, and epidemiological investigation of the effects of human exposures to chemicals; b) international cooperation to exploit research opportunities arising out of accidental, occupational, or other types of high-level human exposures wherever they may occur in the world; c) research to improve short-term follow-up and hypothesis generation, in human exposure assessment, through government-university-private sector collaboration, computer linkage of poison control centers and occupational health clinics population registries, repositories for storage of appropriate biological specimens, studies of pertinent pharmacokinetics, and evaluation of relevant biological markers; d) improvement in the collection and recording of morbidity and mortality data on the general population; e) systematic study of the pharmacokinetics and toxicological responses of humans, compared with nonhuman species, through carefully designed studies on human volunteers, human tissues, and human cells, including human germ cells; and f) expansion of interdisciplinary training to increase the number of scientists wit the kinds and breadth of expertise needed.
Research To Improve Predictions Of Long-Term Chemical Toxicity
INTRODUCTION
This report summarizes a workshop held in Washington, D.C., on December 13-15, 1989, on research to improve the methodology for evaluating the long-term toxicity of chemicals. Workshop participants are listed at the end of this document.
The questions placed before the workshop participants were:
-
How can we get information on the toxicity of a material more efficiently, more accurately, and more quickly? This multisided question was addressed by the groups examining structure-activity relationships, short-term in vivo and in vitro tests, and whole animal tests.
-
How can we know that the data developed from nonhuman systems are meaningful for humans? This question was addressed by the group examining methods for validating predictive tests of chemical toxicity.
-
Do the answers to the first two questions most effectively tell regulators and the research workers where next to go scientifically? The group on overarching strategy, policy, and resource considerations attempted to answer question.
Workshop participants did not attempt an exhaustive scientific report, fully referenced and data rich. Rather, this report is a summary of the joint expert opinions of a collection of specialists in several fields. Not all participants necessarily agree with all aspects of this report.
Several consistent themes appear in the answers provided by the work groups. First, much more work is needed on the validation (in human populations, wherever possible) of the predictions made from the various testing and forecasting systems. For example, many participants in the group examining SSTs found a strong need for determining how well the existing tests perform as predictors. They did not look with any great enthusiasm on devoting equivalent energy to developing new tests. Several participants in each of the working groups expressed a need to develop a unified direction and coordinate research efforts as current programs are scattered. In addition, applied research programs are needed to answer the question, ''How could a research program oriented toward a specific publicly obvious goal be moved toward that goal efficiently and effectively?''
Until recently, the major toxic end point examined in the various predictive systems has been cancer. The question asked was usually, "Did this material increase cancer incidence?" Other toxic end points are often harder to measure—but awareness of them exists—and developments are needed so that knowledge of toxic effects other than cancer can enter into a regulator's evaluation of a material. Several workshop participants questioned the adequacy of testing for neurotoxicity, teratogenicity, and related reproductive effects and birth defects, and general organ toxicity. As part of the emphasis on applied science, much more work needs to be done to develop knowledge of the mechanisms or the pathways leading to a toxic response to a xenobiotic.
The questions about the adequacy of testing recall the review Toxicity, Testing: Strategies to Determine Needs and Priorities (NRC, 1984). Several workshop participants believe there is strong reason for a new look at the problems raised in the report and the recommendations made in it.
The specific considerations and suggestions from workshop participants are given below.
Each group approached the problems in a similar way—defining the current status of the field; considering administrative needs, such as funding and training and paying substantial attention to unresolved problems, research needs, and potential for further development. Only one group found it useful to prepare an extended bibliography.
STRUCTURE-ACTIVITY RELATIONSHIPS
Status and Outlook
The potential for the study of SAR appears to lie in goal-oriented efforts to develop standardized published data for several toxicological end points in addition to cancer. Several workshop participants indicated that such work could provide the potential for identifying chemicals not known to pose a long-range health hazard. Once the methodologies have matured and have been validated, this could be done efficiently for a large number of chemicals, at reduced expense and without the need to sacrifice a large number of animals, thus making testing goal-oriented and cost-effective. Uses of SAR over the next 5 years call for progressive improvements driven by increased efficiency, lowered computer costs, improved models, and a greater range of biological effects for which structural relationships can be identified Workshop participants indicated that this approach could lead to greater acceptance, better understanding of the uses and limitations of the models, and increased confidence in their applications, not only to carcinogenicity but also to other areas of toxicology, during the next 5 years.
Research and Resource Needs
Review of SAR Methodologies
Current SAP, methodologies stem mainly from similar systematic studies of basic mechanistic research in chemistry. With the availability of computers, these studies have evolved into a promising field of research for evaluating toxicological end points. Although the field is still in its infancy, it provides the possibility of bringing a rational or even a mechanistic basis for understanding the experimentally observed toxic end points.
Problem specific to biological end points still need to be resolved. In contrast with chemical properties, biological end points often are the result of complex components and intertwined mechanistic paths. These complexities include transport properties, metabolic transformations, receptor-binding activity, and reactivity. Models exist for each of the properties and usually enter SAP. studies in one form or another. However, these components are considered in parallel, particularly in quantitative SARs. The consideration of hierarchy in the occurrence of biological events is one area in which progress might be made.
Within each of the current SAP, models, better understanding of molecular recognition and alternate representation of molecular properties might be considered as high priority. In addition, relevant models and mechanistic relationships than now are available could be identified.
Validation
Several workshop participants indicated that validation of predictions is a major area of concern. Ideally, a validation process should lead into an exploration of the specific deficiencies and defects in current methodologies. Some believe that validation should involve prospective prediction of specific activities (carcinogenicity, teratogenicity, etc.) for a group of chemicals of unknown activity. The process of validation is not a pass or fail examination, but rather part of an ongoing learning process; information derived from a validation effort could be used to improve the next attempts at prediction. Experts might be included in the validation process to attempt to predict long-term toxicity of new chemicals, such as those in EPA's premanufacturing notification process.
Availability of Relevant Toxicological Data
Although much SAP, development has concentrated on carcinogenicity (and to a lesser extent on mutagenicity and certain ecotoxicological end points), data are available for teratogenicity, acute toxicity, and other toxicological end points. These data have been
only sparsely used; however, a formal mechanism or clearinghouse to collect these data is not needed. Rather, mechanisms could be set up so that individual investigators can be guided toward these sources.
For example, results assembled on genotoxicity led several workshop participants to believe it is possible to develop a battery of SAR models for genotoxicological end points. Inasmuch as chemicals are not usually tested in logical sets, these participants believe it is desirable to use SAR models to identify chemical moieties that need to be tested to fill in gaps in toxicological knowledge.
Treatment of Physicochemical Properties in Models of Long-Term Toxicity
Most SAR models of long-term toxicity, including models of carcinogenicity, have used descriptors of chemical structure to explain the toxicological end points. There also seems to be a place for using physicochemical (p-c) factors, such as octanol-water partition coefficient (log P), solubility, melting and boiling point, dissociation constant (pKa), dipole moment, molar volume, molecular volume, and bond dissociation energy.
Several p-c factors have been used in models of acute toxicity, e.g., log P and bioconcentration factor in models of aquatic toxicity and (pKa) in models of skin and eye irritation. Substantial progress has also been made in the development of pharmacokinetic models. However, the connection between pharmacokinetics and long-term toxicity is lacking for most situations.
In many instances, the use of the substructural fragments that contribute to p-c factors is at least as effective in modeling toxicological end points as the factors themselves; for example, a particular value of log P can be obtained from many diverse structures. On the other hand, it is important to determine whether equally good SAP, models could be devised if the p-c factors themselves were used instead of their substructural components.
The Molecular Basis of Toxic Effects
Workshop participants indicated that research should be directed at the molecular mechanisms of action of carcinogenic and genotoxic compounds, as well as for other toxicological end points. This research might include the following:
-
Detailed investigations of the SARs of possible carcinogens and their metabolic intermediates.
-
Synthesis and biological evaluation of key compounds as predicted by SAR methods and mechanistic models to have identifiable toxic properties.
-
Detailed studies of the nature, rates, and mechanisms of critical reactions (e.g., alkylation) of electrophilic species and other intermediates.
-
Study of the role of pharmacokinetics in activation or detoxication.
-
Investigation of the chemical reactivity of "masked" electrophiles or other reactive intermediates that could be unleashed after a biological transformation, such as quinone methides, anion radicals, or cation radicals.
Incorporation of Functional Criteria Relating to Toxicological End Points
Reports or publications in toxicology should include all pertinent data on functional criteria that might aid in evaluation and interpretation of long-term toxic effects and facilitate extension of the results to SAP, evaluation of untested compounds. These data include results of short-term predictive tests, transpecies effects, dose/potency factors, chemical disposition, metabolic pathways, acute or subchronic toxicity, and effects on immune or endocrine systems. Such "supplementary" data are not published by many journals due to space restrictions, but several workshop participants believe efforts should be made to provide a microfilm record of such material. Data should be systematically organized on a chemical structural class basis to identify class-specific functional criteria, which would be useful for reinforcing or complementing SAR prediction within a specific chemical class.
Development of Methods for Identifying Nongenotoxic Carcinogens
Better methods are needed to identify what are now called "nongenotoxic" carcinogens. An
appreciable number of compounds are considered nongenotoxic carcinogens including most halogenated aliphatics, 2,3,7,8-tetrachlorodibenzodioxin (TCDD), some chlorinated pesticides (DDT and chlordane), 2,2,4-trimethylpentane, di(ethylhexyl)phthalate, benzene, and saccharin. More research is required to systematize knowledge of the factors involved in the ultimate mechanisms of action of nongenotoxic carcinogens and to elucidate the stages in the process. Such efforts will increase the likelihood for better recognition of epigenetic or nongenotoxic carcinogens.
Funding
Much SAP, development stems from studies conducted in universities. Workshop participants believe new approaches must be evaluated and encouraged and funding developed to bring new talent into this area. Research proposals submitted to granting agencies often are too narrowly focused to be funded. As a result, very few groups have been involved in such research. Collaborative or cooperative efforts between government and academic institutions should be encouraged to promote more efficient use of resources.
SHORT-TERM IN-VIVO AND IN-VITRO TESTS
The major effort to date in the development of short-term tests (STTs) has been in the area of genetic toxicity testing for the prediction of carcinogenicity after observation of mutagenicity. More than 180 short-term in vitro and in vivo tests have been developed or proposed to detect rodent carcinogens and, by implication, chemicals that would be carcinogenic in humans. Test combinations (or batteries) and sequential testing schemes have been proposed as ways to improve the predictability. In general, the batteries and combinations have not proved to be better predictors than the best of the single tests. Too often batteries of tests have increased sensitivity (finding more positives) at the cost of decreased specificity (labelling materials positive that were really negative).
STTs have been developed for other toxicological end points; however, the level of investigation for the majority of these has not been as extensive as that for carcinogenesis. Therefore, STTs for carcinogenesis can be used as the paradigm for all SSTs in general, and the mistakes takes made and lessons learned in this field can be used to guide development of comparable tests for other pathological end points.
Properties, Uses, and Limitations of STTs
STTs often are defined with respect to the long-term effect they are being used to identify or measure, rather than to a specific time component. For the purposes of this report, a precise definition of a STT is considered to be less important than the general principles that workshop participants believe could guide STT development, validation, and use. There are four reasons to perform an STT:
-
For screening purposes, to replace more costly long-term testing or to provide information that could be used to help design subsequent tests.
-
As an adjunct to other tests to aid in making regulatory or industrial decisions or to aid in the elucidation of mechanisms of toxicity.
-
To replace existing tests with more facile tests.
-
To take advantage of windows of opportunity for special toxicological responses involving life-cycle phenomena (e.g., embryonic development and fetal maturation) that might be missed by traditional testing in a standard population.
There are two types of STTs—those that are surrogates for other tests and measure the same toxicological event as the tests they replace and those that can be correlated with other toxicological end points, assuming little or no knowledge of mechanistic relationships. As with most biological systems, some STTs will not fall nearly into one or the other category but will have features of both.
STTs need to be validated. Like any other predictive tests, STTs will give false positives and false negatives. If STTs are part of a public-health-oriented scheme, attempts to develop a "no
false-negative" screen might be justified. Such a screen or test would have to be followed by more sophisticated tests capable of identifying false positives. Similarly, a materials development program (such as the discovery of an anticancer drug) might require that the test uncover all potentially useful materials with subsequent tests designed to weed out false leads.
Validation leads to the determination of the ability of a test to fulfill its intended purpose. The usefulness of a test often is constrained by its inter-and intralaboratory reproducibility of the test and the performance of the test under controlled conditions using coded chemicals. In addition, workshop participants believe that the effectiveness of a test for scrim ting between toxic and nontoxic chemicals could be determined, and the results of the validation studies published in the scientific literature. One important finding of the validation process might be that a test were accurate for some chemical classes and not for others.
Research and Resource Needs
Recruitment and Training of Personnel
The development of any technique, no matter how simple or straightforward, requires trained personnel. STTs usually are categorized as "applied research" and often are not seen as attractive by academic researchers who are the mentors of young research workers. However, for public-health concerns, research into the use of STTs might be as vital as basic research. Workshop participants believe programs should be developed and funds provided to interest researchers in STTs and prediction of long-term toxicity and to provide training in these fields.
Application to Nonbinary Toxicological End Points
STTs and long-term tests have been used to classify materials on a binary basis—yes/no, carcinogen/not a carcinogen. This has made comparisons among tests straightforward and relatively easy. However, it is obvious that many toxicological end points should not be expressed in a binary fashion, but rather as dose-response curves, dose ranges, or on a continuous response scale. For continuous responses, the determinations of sensitivity and specificity are similarly more complicated but are dearly possible. The continuous scales require scientific judgment to establish cutoff points below which a response is considered negative and above which is considered positive.
Coordination of Test Development and Validation
The development of predictive tests has usually lacked coordination. Workshop participants indicated that there is a need to devise an administrative framework to coordinate or direct the development and validation of new STTs. Among the tasks suitable for coordination are evaluating the tests (in use or proposed for use), assembling a centralized information data system that would be continually evaluated and updated and made accessible to investigators who are interested in test development and validation, and identifying areas of toxicology and public health that could benefit from the use of STTs. A coordinating group could identify model chemicals for study as well as develop STT data bases.
Assessment of Applicability to Regulatory Uses
One major use of STTs is to provide data in support of regulatory decisions. The use of STT data by different regulatory agencies should be examined, and the effect the tests have on regulatory decisions should be evaluated. The various regulatory agencies need to codify for public review their current requirements for accepting STTs and the minimum requirements they have for a new STT before the data are acceptable for regulatory purposes.
Application To Human Specimens
Because the ultimate organism of concern to humans is the human, the most relevant STTs would be those that could be performed using biological samples from exposed populations or populations otherwise at risk for chemically induced disease. Several workshop participants believe that STTs to identify and quantify human
risk should be developed. These tests could be used as markers of chemical exposure in humans or to identify early disease or organ damage. In addition, investigators developing or using SITs should examine the scientific basis for using cultured human cells in lieu of rodent cells or microbes. Special attention should be given to the identification and implementation of techniques derived from the new developments in biological technology that can be applied in humans and laboratory animal models (thereby reducing the uncertainty in the extrapolation to humans).
Broadening the Spectrum of Toxicological End Points
At present, many SITs are developed around one highly specific end point associated with a specific molecular or physiologic mechanism. Workshop participants believe that the development of short-term biological predictive systems that can be integrated with other systems, measure more than one end point, or respond to more than one mechanism of action should be encouraged.
Periodic Re-evaluation and Revalidation
For the purposes of evaluation, STTs are related to a "gold standard," that is, a specific toxicological effect of chemicals measured in rodents or humans. Workshop participants indicated that this standard, whether it is carcinogenesis in rodents or diminished fertility in humans, may be a poor one. Therefore, all SITs must be re-evaluated from time to time.
More Adequate Reporting of Relevant Data
To aid in the evaluation of STT performance, workshop participants suggested that individuals and laboratories reporting results should also provide quantitative data or make them available on request (including such estimated measures as the Lowest Effective Dose or Highest Ineffective Dose), Chemical Abstracts Services Registry Numbers, structures of test chemicals, and the source and purity of the chemicals tested.
Extrapolation to Humans
Research is needed on methodologies to extrapolate from in vitro to in vivo effects in laboratory animals and in humans without involving the unnecessary exposure of humans to potentially toxic materials.
Further Problems and Uses of STTs
Currently available tests can detect most genotoxic carcinogens. However, increasing evidence shows several types and classes of carcinogens are not detected using existing in vitro genotoxicity tests. SITs are needed that will effectively identify these carcinogens.
It is difficult to evaluate the toxicity of complex mixtures. In vivo, long-term experimental approaches to studying mixtures are severely limited by the high cost of multifactorial experimental designs. SITs have a significant advantage in investigating chemical interactions and the toxicity of complex mixtures. Research should be focused on developing and using SITs to identify chemical interactions and elucidate toxicological mechanisms involved.
More Effective Coordination of Test Development and Validation
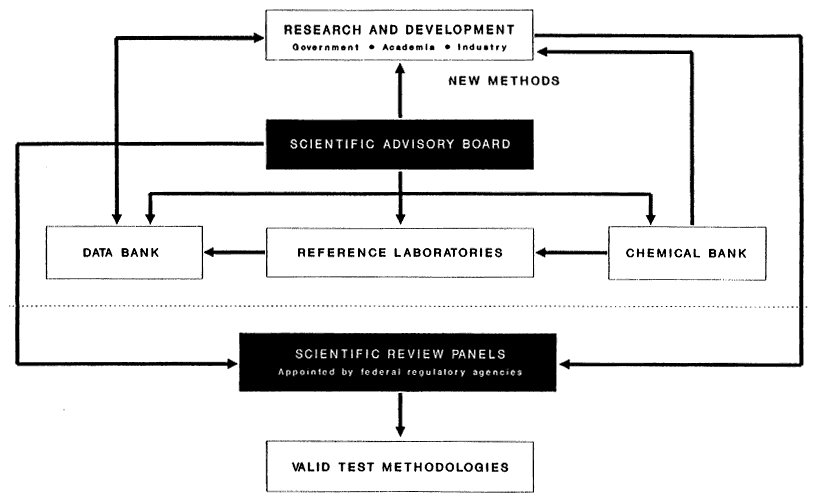
No formal science policy or framework organizes and coordinates the use and development of STTs. At present, methodology development is mainly driven by the goals of individual research laboratories. For a program to be efficient in the transfer of new technological developments from the research laboratory to routine toxicity testing, a framework capable of fostering this process is needed. Such a framework has been described by Drs. J. Frazier and A. Goldberg of the Johns Hopkins Center for Alternatives to Animal Testing and is briefly described below.
The overall scheme is divided into two major functional units (Figure 1). Above the dashed line in the figure, the structure describes a framework for test method development and validation. The components below the dashed line relate to judgments of whether a given methodology is acceptable for the purpose for which it is
proposed and to place a "seal of approval" on the tests. At the core of the methods development activity (above the dashed line) is a scientific advisory board (or boards). This board would:
-
Review the state of SSTs, identify gaps in scientific knowledge, and recommend research.
-
Recommend testing methodologies to a scientific review panel for evaluation and approval.
-
Advise and guide a chemical bank; select representative chemicals for test method validation.
-
Oversee a toxicity testing data bank and review data prior to its entry into a data bank.
-
Recommend activities for reference laboratories, including validation of developed methodologies and development of orphan methodologies.
The chemical bank is designed to address problems that retard test development and would be a repository for chemicals that could be used by research laboratories and reference laboratories to validate methods. Chemicals would be designated for different methodologies related to particular toxicities or pathologies (e.g., neurotoxicity, hepatotoxicity, and carcinogenicity) for validation and for calibration.
A toxicity testing data bank could collect and catalog data for methodology validation. Data on the performance of proposed and in-use test methods would be collected and classified. If a method were under consideration for validation, the data matrix could be searched for experimental data from all chemicals tested by the particular method. A scientific advisory panel would determine, on the basis of the available data, whether the method could be recommended for consideration by the scientific review panel.
Reference laboratories would serve two major functions. The first function relates to determining how readily a methodology could be transferred among laboratories. In the proposed framework, reference laboratories, under the direction of a scientific advisory panel, would implement the method under consideration and test sets of chemicals from a chemical bank. When sufficient data are collected, the method would be recommended to a scientific review panel for evaluation. The second function of reference laboratories would be to develop orphan methodologies—scientifically valuable tests identified by a scientific advisory panel which have not been fully developed for various reasons. Reference laboratories could be assigned to develop these methodologies further.
The actual evaluation and recommendation of methodologies for specific testing purposes would be the responsibility of a scientific review panel The panel could be either an interagency panel established by concerned regulatory agencies to evaluate methodologies for general use or individual panels established by each agency to evaluate methodologies in the context of a specific agency's needs. Implementation of such panels and their functions would require cooperation among all interested groups government, industry, and academe.
WHOLE-ANIMAL TESTS
In safety assessment and hazard evaluation of xenobiotics, what are the prospects of improving predictors of toxicity and their translation into risk? What are the relevant research needs and research opportunities to enhance the existing processes, and what are the strategy, resource, and policy considerations involved in enhancing predictability?
Workshop participants unanimously agreed that animal studies are the foundation of health risk assessment and will remain so for the foreseeable future. Assays have served well in protecting public health, but they can be improved. The results of such studies are used throughout the world to regulate xenobiotics in food, air, and water and to assess the safety of ingredients in pharmaceuticals, cosmetics, and pesticides.
The Strategies of Whole-Animal Tests
Historically, as studies in :animals have evolved, procedures have become increasingly sensitive and specific. The intact animal has provided the only source of data offering acceptable predictability as the integrator of dose, response, repair, and recovery, including: 1) biotransformation, pharmacodynamics, and pharmacokinetics; 2) compensatory and amplifying processes; 3) aging, including fetal life and geriatrics; 4) cumulative
toxicity at the molecular, cellular, tissue, and organ levels; 5) route as a determinant of toxicity, and 6) repair and reversibility.
Workshop participants ranked several toxicological end points to assess relative confidence in the three test systems for predicting adverse outcomes in humans. The systems compared were animal bioassays, in vitro assays, and SAR analyses. For all end points, intact animal studies coupled with available human data were considered to surpass the degree of predictive confidence generated by SAR and in vitro assays. The only area participants believed that SAR and in vitro assays resulted in greater than a 25% reduction in uncertainty was in determining potential carcinogenicity. In this case, the SAR and in vitro observations were judged to give consistent results within individual classes of chemicals (Table 1).
RESEARCH AND RESOURCE NEEDS
Validation by Appropriate Studies in Human Subjects
The art and essence of predicting teratological effects in humans from results of laboratory investigations have improved in recent years. However, in view of the recent activity in this field and conflicting statements, workshop participants were generally unwilling to make firm confidence statements.
Human data for validation are very hard to come by. Some workshop participants indicated that a strategy should be developed under strict ethical guidelines to study selected chemicals, under very stringent conditions, in single administration protocols, in human volunteers. The guidelines should be similar in intent to, but
TABLE 1 Confidence in the Test for Predicting Effects in Humans
|
Outcome |
Structure-Activity Relationships |
Short-Term Tests |
Animal Tests |
|
Biotransformation |
3* |
2 |
1 |
|
PK/PD |
|
|
|
|
Compensatory |
3 |
2 |
1 |
|
Processes |
|
|
|
|
Aging |
3 |
2 |
1 |
|
Cumulative Toxicity |
3 |
2 |
1 |
|
Route/Toxicity |
3 |
2 |
1 |
|
Repair/Reversibility |
3 |
2 |
1 |
|
Teratology |
3 |
3 |
1 |
|
Cancer |
2 |
2 |
1-2 |
|
* 1, highest; 3, lowest. |
|||
more rigid in control than, FDA guidelines for the protocols of Phase I experimental trials. Such observations of humans could provide essential information on the qualitative and quantitative aspects of human disposition of a xenobiotic and its metabolites. Human observations also allow laboratory animal models with the closest correspondence to human metabolism to be assigned to further investigations. Accidental exposures also provide an opportunity to gather applicable human data. With the decrease in emergency response time, several workshop participants believe that the scientific community should be prepared to collect samples from emergency incidents.
Funding
According to some workshop participants, research funding related to toxic chemicals is sometimes fragmented or duplicative. Furthermore, public monies are inappropriately spent on toxicological research for proprietary chemicals that are actively marketed. In addition, major flaws in funding priorities and mechanisms impede the application of integrative approaches involving all relevant disciplines. Current funding mechanisms actively encourage fragmentation of research approaches and tend to make toxicology a cottage industry.
Parkinsonism in young drug users. These and other instances of neurotoxicity provide an opportunity to examine questions typically not addressed in the conventional risk assessment scheme, e.g., 1) patterns of toxicity including the spectrum and sequence; 2) rate of progression and reversibility; and 3) latency.
Broadening of Risk Assessment End Points
According to several workshop participants, the risk assessment models for end points other than cancer, such as neurotoxicity, teratogenicity, and general organ toxicity have not been given adequate attention. Functional teratology provides an example of a need for a quantitative decrement model: environmental toxicants such as lead and methyl mercury have already been identified. Another example of chemically induced neurotoxicity is the case of MPTP, the "designer" drug that was found to induce rapid onset Parkinsonism in young drug users. These and other instances of neurotoxicity provide an opportunity to examine questions typically not addressed in the conventional risk assessment scheme, e.g., 1) patterns of toxicity including the spectrum and sequence; 2) rate of progression and reversibility; and 3) latency.
Re-evaluation of Risk Assessment Models
Participants indicated that assumptions underlying risk assessment methods and models need periodic re-examination. Recent advances in toxicology and supporting disciplines show that the nonthreshold linearized multistage model for carcinogen risk assessment might need to be replaced by a more mechanism-oriented model for some carcinogens. The NAS/NRC Committee on Risk Assessment Methodology is making such a reassessment. Similar critical attention to the models used for risk assessment for other end points is needed (e.g., reproduction and development.)
Elucidation of Causative Mechanisms of Toxicity
The development of knowledge in toxicology has been an interactive process, usually commencing with an observation of an adverse effect in humans or laboratory animals, followed by in vitro or in vivo experiments to define the sites and mechanisms of action. Eventually, long-term studies axe conducted in intact animals. Predictive toxicology evolved as a modification of this process. The underlying assumption of predictive toxicology is that animal studies, properly designed, can predict biological effects in humans. To improve long-term bioassays, several workshop participants indicated that greater understanding of the roe ban of toxic effects is needed. Research to elucidate such mechanisms is critical to build confidence for the use of animal test results for human risk assessment. Research on mechanisms should exploit the latest concepts from the entire literature, not just the toxicology-related literature. For this purpose, a series of staggered-start, overlapped investigations might allow an
investigative team to describe lesions at the minimal toxic dose and to address the question of mechanisms in the whole animal.
Grouping of Chemicals by Mechanisms of Toxicity
The burden of testing might be reduced if the toxic effects of a group of xenobiotics could be explained by a common mechanism. This approach could limit the number of future tests needed. Further efforts should be made to identify categories or classes of chemicals that induce similar lesions. Representatives of these categories could then be tested in long-term assays to search for further commonalities; that might then further reduce testing.
Participants recommended testing categories for two reasons: First, the NAS Committee on Toxicity Testing Needs noted that fewer than 20% of chemicals in commerce have been tested adequately for toxicity. For some toxicities, life-time testing is not needed and involves unnecessary exposure to some compounds. Second, bioassays are expensive. If results from testing one or a few compound could be applied to an entire class, some workshop participants believe this would enhance the cost-effectiveness of testing and might provide a stronger basis for developing SAP, prediction systems.
Methodologies for Complex Mixtures
There is an acute need for methodologies to study complex mixtures (NRC 1988), including diets that contain naturally occurring carcinogens and anticarcinogens. Studies of food extracts are likely to lead to inappropriate conclusions regarding the toxic potential of fruits and vegetables. Complex mixtures are best studied within the milieu in which they are found (i.e., ''top down'') rather than as a summation of individual components (i.e., "bottom-up").
Studies in Animals with Special Phenotypes and Genotypes
To improve and widen the predictivity of animal assays, some participants called for studies in animals with specific phenotypes and genotypes. Examples include the aged animal (to coincide with the effect of an aging population); for developing and validating biological markers of aging, apart from chemically induced degenerative lesions (defined as structural and/or functional decrements); outbred strains for toxicity testing to mimic the heterogeneous human population; and transgenic mice with human genes, to study susceptibility of the human genes in situ to environmental toxicants, with emphasis on the role(s) of oncogenes, tumor-suppressor genes, and cellular receptors (such as the epidermal growth factor receptor and the hormone receptors) in the toxicity spectrum.
Integrated Analysis of Dose-Response Relationships
Some workshop participants believe that the design and interpretation of whole-organism dose-response studies should be coordinated with dose-response studies of cellular and molecular processes. Models involving possible precursors, such as cell proliferation, need to be further developed.
VALIDATING PREDICTIVE TESTS
Status and Outlook
The major question asked of laboratory tests is how well they predict what would happen to exposed humans. A good predictor is said to be valid test; a valid test will produce few false negatives and few false positives. Toxicological tests and procedures can be used to predict the risk of illness, e.g., the structure of biochloromethyl ether led to the prediction from structure-activity considerations that it was a likely carcinogen; tests in animal systems supported this conclusion, and cancer was found in exposed workers. Ideally, the results of a test should be used to prevent illness. However, when test results preclude the use of chemicals, there will be no human exposure, and true validation, particularly the identification of false positives, becomes impossible. When positive, a test should be used to eliminate or reduce exposure, and thus help to prevent illness. When test results are used
to eliminate all exposure, there will be no human data for comparison with the test data, and, as a consequence no opportunity for validation or for identifying false positives. False negatives can still be uncovered, but no formal system is designed to do so.
Technical attempts to reduce experimental variability of all tests used, in the sense of basic quality assurance, is essential How reproducible is the test? What are the intra-and inter-laboratory variations? What variation occurs over time? Validation of individual tests must be carried out before comparison between tests can be attempted. Validation can be quantitative or qualitative. The process is iterative. On the qualitative level a test, for example, might be able to identify carcinogens but be unable to quantify their dose-response relationships. Three levels of predictive testing have been identified in this report, ranging from simple to complex situations: level 1) chemical action: SARs; level 2) in vitro and short-term in vivo assays; and level 3) animal bioassays. The results of these may be compared to data from human epidemiology.
Several workshop participants indicated that validation needs to be carried out within and between levels of testing. Many in vitro and short-term in vive tests have been developed. One approach to improving prediction is to use a battery of tests and, by way of validation, to compare the results of the battery with some accepted standard—recognizing that at times the standard itself may be inadequate. In one study by NIEHS, 73 chemicals were run through four tests (Ames, sister chromatic exchange (SCE), chromosome aberration, and mouse lymphoma) and compared with the outcomes of rodent bioassays. The results did not support the intuitively appealing idea that a battery of tests would improve predictability. The battery was no more valid than the single Ames test.
Validation of the predictability between levels of testing especially for predicting results between species, presents the most difficult problems. Some laboratory results have, as yet, no meaning with respect to human outcomes. What are the consequences to human health of SCE, or somatic-and germ-cell mutation? Studies in which individuals (human and other animals) are followed for a lifetime are rarely carried out, e.g., following animals with known chromosome breaks to see if more tumors develop in these individuals than in those that are free of the defect.
Knowledge of mechanisms could help bridge the gap between testing levels. Lack of knowledge of exposure is often a stumbling block in attempting comparison of laboratory results with outcomes in humans. In tests carried out at levels 2 and 3, the exposure is known, but predictions between level 3 (animal bioassays) and human epidemiology often founder because human exposure is usually not quantified. Even with knowledge of the slope and shape of dose-response curve, knowledge of exposure is required before predictions can be made. Exposure may at times need to be estimated from knowledge of levels in food, air, and water from direct chemical measurement or from use of biological markers.
In some circumstances, validation of the results of animal experiments by comparison with effects on humans may not be possible given current experimental procedures. According to workshop participants, animal feeding studies are usually begun at a later relative age in the animal than the age at which exposure begins in the human. Exposures to the fetus are not mimicked in laboratory studies except rare multigenerational studies. Additional difficulties arise when the primary exposed individual is the sire and the material under suspicion is delivered indirectly, as a one-time event, to the egg cell (and thus to the embryo) during fertilization. Difficulties in validation between testing levels and the need to have accurate exposure information—which varies greatly with demographic factors such as age, occupation, and location-caused a number of workshop participants to suggest that a major effort should be made to improve epidemiology, particularly of accidentally or occupationally exposed persons. The genetic heterogeneity of the human species in comparison with the highly inbred experimental systems must always be keep in mind. The ultimate link in the validation of SAR, STTs, and animal bioassays is the relationship of the test results to the action of chemicals in humans. The greater the range of materials that have been well-studied in humans, the greater the opportunities to validate (or discard as invalid) toxicological tests.
Research Needs and Possibilities
Studies of Heavily Exposed Populations
In an effort to expedite validation studies through comparison with effects in humans, several workshop participants indicated that efforts are needed to make the most of research opportunities arising from massive exposures to chemicals. Any special exposures to humans need to be brought to researchers' attention. Massive ingestion of a pure chemical, for example, for therapeutic reasons, by personal or occupational accident, or by suicidal intention, might provide comparative toxicologists a basis for developing information on biotransformation, tissue distribution, and pharmacokinetics that might guide interpretation of studies conducted in experimental systems. Interaction with clinical toxicologists, poison control centers, state health departments, the Agency for Toxic Substances and Disease Registry (ATSDR), and the National Toxicology Programs (NTP) would expedite examination of valuable research specimens and could improve medical management. For example, studies of reproduction in cancer patients who received well-documented, high doses of genotoxic drugs and radiation provide an opportunity to understand human germ-cell mutagenesis, while validating other measures of somatic cell mutation.
International Epidemiological Studies
Several workshop participants indicated that U.S. support should be encouraged for epidemiological studies wherever there are populations that receive substantial environmental or occupational exposures to chemicals of public-health interest. International studies should be done in collaboration with WHO and appropriate groups in this country (e.g., DHHS, EPA, and NAS-NRC). Exposures should be well characterized and of a magnitude at which observable effects would be expected. The use of biological markers for exposure, biological effects, and susceptibility should be encouraged. Living conditions in non-U.S. study populations should not be so different from those in the United States as to constitute a problem in extrapolating the data from country to country.
Registries of High-Level Exposures
Development of registries of individuals with high exposures, much in the manner of ATSDR, should be encouraged according to a number of workshop participants. Populations with large exposures—due to industrial accidents, for example—might provide the best groups to be followed. Most surveillance systems (e.g., epidemiologic studies, and disease registries) for human disease end points lack appropriate information on exposure. Registries of effects are most useful when they can be linked with exposure information. A problem inherent in following exposed populations for ultimate health effects is that industrial populations are often exposed to many potential toxicants, making the establishment of cancer-causing linkages to single agents difficult.
Improvement of Exposure Measurement and Quantification
To improve predictions of long-term chemical toxicity, priority must be given to developing effective programs in exposure assessment. These could include the following:
-
A computer-linked network of poison-control centers or occupational health clinics would identify individuals who have had biologically significant exposures to environmental or workplace chemicals. linking this system to a sample storage program that contained samples of body fluids or tissues from selected individuals would enable researchers to approach recently exposed (24-48 hrs) individuals or to obtain samples from individuals with less recent (e.g., up to 1 year prior) exposures. Workshop participants indicated that specific protocols should be developed to be used in the cases of chemical accidents. Such a network/sample storage program could be used to develop information in humans on: pharmacokinetics (metabolism, deposition, and tissue distribution) of an agent; biological markers of exposure; and biological markers of effect.
-
A collaborative program among the government, universities, and the private sector could be used to develop accurate exposure
-
records on high-exposure employees in targeted segments of selected industries.
Biological Markers of Exposure and Effect
Clinical measures of chemical exposures in humans (e.g., biological markers) need to be related to disease outcome.
Laboratory documentation of exposure to environmental agents has usually been correlated indirectly to suspected health outcomes. For example, various chromosomal defects have been seen as constitutional, and acquired abnormalities in peripheral lymphocytes and bone marrow cells following some chemical or radiation exposures. Some chromosome breakage states have been associated with an excess of malignant neoplasms. But, even among the Japanese survivors of the atomic bombs, direct documentation has not been made that individuals with excessive chromosomal defects are the individuals who later develop excess cancers. With international collaboration, especially where record linkage is excellent, it seems feasible to follow-up occupational cohorts that have been karyotyped to document the actual disease outcomes in individuals.
The best predictor of a received dose at present is measurement of the chemicals in human fluids and tissues, e.g., urine or blood. Measurements of chemicals in the environment (e.g., soft and water) are poor indexes of doses to humans. Based on tissue-response information, it might be possible to develop better models to assess exposure by indirect means for chemicals that cannot be measured in human samples either because analytical methods do not exist or because the chemicals are rapidly metabolized and excreted, leaving no traces.
Tissue data could lead to discovering which environmental pathways are important for human exposures. Characteristics of the chemicals (solubility, chemical form for metals, and reactivity), binding to media (e.g., soil, sediment, and particles), particle size, and environmental fate and transport must be considered.
Many chemicals are ubiquitous in our environment in low concentrations, and the general population is exposed to them. Unless subpopulations have received much higher doses, exposure registries are not likely to be useful for the study of disease outcomes. The usefulness of a registry will depend in part on the potency of the chemical, the range of doses received by different individuals, and the heterogeneity of the population. Therefore, several workshop participants suggested that criteria related to these concerns be developed and applied to the establishment of any future registries and to re-examine the usefulness of existing registries.
Molecular Approaches
Workshop participants believe molecular techniques must be developed for extension to epidemiological studies. As new molecular techniques become available for assessing health effects, they should be implemented in epidemiological studies. Two examples are given below.
-
Assays for genetic changes—Better methods are needed in humans for rapid analysis of genetic changes, including recombination, structural, and numerical chromosomal aberrations, as well as genetic mutations. New candidate methods include the use of antikinetochore antibody in the micronucleus assay and mutational spectra analysis. Efforts to implement the developing information on oncogenes and tumor suppressor genes for new epidemiologic assays should be encouraged.
-
Mechanistic markers for reproductive toxicity in humans—Molecular markers are needed for each of the successive stages of the reproductive process. For example, a marker for successful implantation of the fertilized egg (level of human chorionic gonadotrophin, hCG) permits the examination of the prevalence of postimplantation loss. An analogous measure is needed to assay for preimplantation loss. An assay for levels of the hormone relaxin is a candidate approach. The relationship between abnormal sperm factors (e.g., morphology and motility) and clinical outcomes needs to be determined. Linking sperm factors with reproductive risk and developing methods to detect preimplantation loss would enable to characterization of the major determinants of reproductive failure in humans.
As new methods are developed and validated, resources should be made available to permit the full use of these assays in epidemiological studies.
To illustrate the scarcity of resources, all research groups using the radioimmunoassay for hCG to monitor for reproductive outcomes in humans are dependent upon one laboratory for a supply of antibody and for continued development of the technique.
Information on Disease Incidence
Baseline information on the background frequencies of health effects or their molecular surrogates in human populations needs to be developed. Information is usually needed on the effects of age, diet, and other factors on the measured factor. The background frequencies of many clinical end points (e.g., spontaneous abortions and menstrual disorders) also need to be developed.
To improve our understanding of human disease, it is important to know the incidence of specific diseases in the general population and the trends in disease incidence. At present, U.S. record systems are not adequate to supply accurate information. This is particularly true for the less-common diseases, for diseases not usually fatal, for neurological diseases and for reproductive outcomes. Many birth defects are not recognized at birth and are therefore poorly recorded. The number of autopsies conducted has greatly decreased over the past few decades, suggesting that information on causes of death might be becoming less accurate.
Training
Progress in improving predictions of long-term chemical toxicity, as well as related environmental and toxicological issues, will be seriously slowed according to some workshop participants as long as there remains a dearth of scientists with relevant interdisciplinary training To illustrate, a review of the cytogenetic literature suggests that cytogeneticists often lack epidemiological training. The human studies they plan and conduct are at times undertaken without adequate attention to the sample size, the appropriateness of controls, or the potential for selection biases. Epidemiologists, on the other hand, often lack familiarity with genetic toxicology literature. Suspected mechanisms often are not known to epidemiologists, and the overall synthesis process is negatively affected. The need for incorporating current developments in oncogene research into epidemiology has already been noted. It would be appropriate for federal agencies to undertake traineeship or postdoctoral programs along the NIH model to develop the needed researchers with interdisciplinary skills.
STRATEGY, POLICY, AND RESOURCES
Linking and Integrating Evaluative Approaches
All of the different toxicological approaches discussed in the workshop have value, and all have reached what might be considered adolescence, if not full maturity. The challenge now is to link them, evaluate their potential in predicting human-health effects, and integrate their selection and use in evaluating particular chemicals (see e.g., NRC, 1983; Anderson and Ehrlich, 1985; U.S. Department of Health and Human Services, 1986).
Several workshop participants indicated that this linking, evaluation, and integration needs to be conducted in perspective of the ultimate uses to be made of the information, for it is obvious that toxicological testing approaches and regimes should be oriented to providing data that satisfy dearly conceived objectives of human-health protection.
Procedure flexibility, interpretive judgment, and continual improvement of methods are essential-Regulators and other users of tests must be open to adopting improved methods and to applying judgment in making inferences about human-health effects from routine tests. The challenge is to embrace flexibility and methodological evolution within the standardized procedures that bureaucratic systems naturally require (NRC, 1984).
Two themes pervaded the workshop. First, wherever possible, methods should be extended to cover a wide variety of health-effect end points, not only acute injury, cancer, neurological, and reproductive effects, but also immunological, renal, hepatic, and other health effects. For each end point, this implies major research needs. Every effort should be made within existing
structures to broaden the reach of tests, rather than trying to develop entirely new administrative structures for the purpose.
Second, several workshop participants indicated that the ability of all of the methods to predict human health effects needs to be validated. Again, to do this properly implies major programmatic needs.
An important way to facilitate linkage and comparative evaluation might be through improving toxicological data bases and data-base systems. Data gaps have to be bridged, and throughout, data quality and relevance have to be established. In all of this activity, the power of the inferential logic that these methods provide is at issue (Hattis and Kennedy, 1986).
Structure-Activity Relationships
Clearly, SAR analyses are informative. But because they have been applied extensively to only a few compound classes, they lack confirmed predictive power for many classes of compounds. Also, they have been much more highly developed for carcinogenicity and a few acute health effects than for other health effects (Ashby et al., 1989).
Several workshop participants indicated that SAR techniques should be extended to many other molecular groups and health-end-point classes. This will require much more data gathering, evaluation in the SAP, framework, and validation of predictive power against data from other test systems and biological understanding.
SAR development would benefit from more active coordination among the relatively few SAP, research groups and more orientation to the needs of regulatory and other end users of the techniques. Some workshop participants indicated that SAP, practitioners should explore coordinative possibilities with the NRC, federal agencies, and other central organizations.
Short-Term In-Vitro and In-Vivo Tests
As with SAP, techniques, STTs have been successfully developed for mutagenicity (which can be taken as possible indication of carcinogenicity) and for some acute toxicities, notably neurotoxicity. But, in general, STTs vary in their analytical and predictive power (Ashby et al., 1989).
Given the rich portfolio of mutagenicity assays that now is available, little payoff is to be expected from further improving mutagenicity tests, unless perhaps human cell systems or human genetic materials can be incorporated into the assays. Attention still needs to be devoted to optimizing the selection of STT batteries for particular purposes, despite their poor performance to date.
STTs need to be developed for nongenetic health effects. These will need to be complemented or even driven by research on biological mechanisms and by other research insights that will make the empirical STTs understandable. In neurotoxicology, for instance, the STTs need to be linked to the understandings of experimental psychology. Many of the tests now widely in use urgently need to be validated as predictors of human-health effects.
As STTs move into much wider routine application, precautions will have to be taken against improper use and interpretation.
Whole-Animal Tests
Animal assays continue to be essential for predictions. They are necessary as guides for regulation, and they generate much useful basic biological information (Tomatis, 1988). Their predictive power continues to need to be evaluated against human health experience.
Like SAP, techniques and STTs, whole-animal tests have been better developed for carcinogenicity than for other toxic end points (Huff et al., 1988). They need to be extended to apply to other end points. As this extension is pursued, several workshop efforts should be made to explore how the tests will be interpreted and weighted for decision making. For example, in regulation, it might not be obvious what importance should be accorded reversible alteration of hormone levels, compared with irreversible damage of some other son. Thinking through these issues might generate implications for the scientific agenda (Kimmel et al., 1989).
Whole-animal tests are burdensome, and consideration should be given to the possibilities for optimizing bioassay test designs (even more than they have been improved over recent years) to obtain reliable information in shorter time and
at less expense. For example, in some instances 6-month animal assays might be just as informative as 2-year assays (Kimmel et al., 1989). Therefore, whole-animal tests should be conducted in such a way as to allow systematic observation of multiple health effects within an assay series and perhaps other research areas (such as pharmacokinetics) as well
The topic of optimal strategic and tactical design of whole-animal tests would be a subject for a future workshop.
Validation
Ultimately, the value of all toxicology testing depends on how well the tests predict human health effects. Therefore, a number of workshop participants indicated that all toxicological methods should be subjected to evaluation with respect to their reproducibility, specificity, sensitivity, and practical applicability as indicators of human health effects. This has been accomplished for some test methods to some extent, and much more is needed.
Despite the value of proxy information, there is no substitute for human data (Erdreich and Burnett, 1985). Therefore, epidemiological research continues to be a central component of health-effects evaluation. Epidemiological research must be supported and nourished as a complement to toxicological research. Coordinated exploration of unusually highly exposed human populations for study to complement particular toxicological research will be essential
Research Needs and Possibilities
Potency and Severity of Toxic Response
According to several workshop participants, attention needs to be given to the concept and expression of toxic potency and the rate of change of potency relative to dose. The notion of potency has been developed intensively for carcinogenesis assays (DeRosa et al., 1985). Single-value potency estimates are moving toward a more complicated index, as numbers of this kind are being generated by a diversity of assay methods whose biological interpretation must differ. Moreover, such biological variables as sex or metabolism may moderate a molecule's intrinsic potency (such as by attenuating its reactivity with target genetic or other molecules).
Increasingly, toxicological assessments need to recognize that biological effects may be graduated. Moreover, the assessments will have to include evaluations of widely differing severities of outcome (such as behavioral deficits as compared with other neural impairments or nonfatal cancers as compared with fatal cancers).
Exposure Assessment
Risk assessments are compound estimates of adverse health effects given toxicity, toxic potency, and exposure. Effective predictions of human-health risk, therefore, require good estimates of exposure. Improvement of exposure assessments and of the requisite methods should yield major payoffs in risk-assessment quality. Such methods as exposure markers, pollutant modelling, and the like should continue to be refined.
Evaluation of Mixtures
All human exposure to chemicals involves exposure to mixtures. But toxicological research has been pursued largely for single, well-described compounds. As demonstrated by the considerations of the health effects of exposure to gasoline vapors, evaluating mixtures cannot be avoided, and the methods for doing so need to be improved (Vouk et al., 1987).
In general, the evaluation of mixtures (either well-characterized mixtures or such highly variable environmental samples as exhausts, effluents, or soils) tends to be scientifically impure and imprecise. But it can be pragmatically illuminating, helping establish the relative risk of complex chemical hazards in a preliminary way and providing cues for further toxicological investigation.
Research Incentives
There is substantial concern about the problem of making it important and worthwhile for research-based industries to pursue toxicological investigation, exposure-assessment, and related investigations. The topic of incentive systems should be addressed according to several workshop participants in a future workshop, convened by the NRC or another such organization.
Coordination and Planning
From experience in the National Toxicology Program and elsewhere over the past decade, it is clear that it is possible for industrial groups (either commercial groups or such organizations as the Health Effects Institute and the Chemical Industry Institute for Toxicology) to work in coordinated fashion with federal research laboratories. There have been a number of successful consultations and complementary or parallel research programs, although there is some thought that the use of public funds to test proprietary materials already being marketed is inappropriate.
The nomination, selection, and toxicological evaluation of major chemicals for priority testing deserves continual re-examination, as does development of more useful data on industrial production of, use of, release of and potential human exposure to chemicals.
Throughout the workshop, concern was expressed that all approaches be coordinated to gain efficiency. The issues of consistency of approach among research and regulatory agencies also surfaced. A plan to accomplish these objectives did not emerge. According to a number of workshop participants, a formal plan should not be expected; the fields are too dispersed, and considerable disagreement surrounds many of the methods. But leaders and leading institutions should do all they can to coordinate pursuit of these efforts.
REFERENCES
Anderson, E.L., and A.M. Ehrlich. 1985. New risk assessment initiatives in EPA. Toxicol. Ind. Health 1:7-22.
Ashby, J., R.W. Tennant, E. Zeiger, and S. Stasiewicz. 1989. Classification according to chemical structure, mutagenicity to Salmonella and level of carcinogenicity of a further 42 chemicals tested for carcinogenicity by the U.S.National Toxicology Program. Mutat. Res. 223:73-103.
DeRosa, C.T., J.F. Stara, and P.R. Durkin. 1985. Ranking of chemicals based on chronic toxicity data. Toxicol. Ind. Health 1:177-192.
Erdreich, L.S., and C. Burnett. 1985. Improving the use of epidemiologic data in health risk assessment. Toxicol. Ind. Health. 1:65-81.
Hattis, D., and D. Kennedy. 1986. Assessing risks from health hazards: An imperfect science. Technol. Rev. 89:60-71.
Huff, J.E., E.E. McConnell, J.K. Haseman, G.A. Boorman, S.L. Eustis, B.A. Schwetz, G.N. Rao, C.W. Jameson, L.G. Hart, and D.P. Rall. 1988. Carcinogenesis studies: Results of 398 experiments on 104 chemicals from the U.S. National Toxicology Program. Ann. N.Y. Acad. Sci. 534:1-30.
Kimmell, C.A., D.G. Wellington, W. Farland, P. Ross, J.M. Manson, N. Chernoff, J.F. Young, S.G. Selevan, N. Kaplan, C. Chen, L.D. Chitlik, C.L. Siegel-Scott, G. Valaoras, and S. Wells. 1989. Overview of a workshop on quantitative models for developmental toxicity risk assessment. Environ. Health Perspect. 79:209-216.
NRC (National Research Council). 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1984. Toxicity Testing: Strategies to Determine Needs and Priorities. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1988. Complex Mixtures. Washington, D.C.: National Academy Press.
Tomatis, L. 1988. The contribution of the IARC Monographs Program to the identification of cancer risk factors. Ann. N.Y. Acad. Sci. 534:31-38.
U.S. Department of Health and Human Services. 1986. Determining Risks to Hear Federal Policy and Practice. Dover, Mass.: Auburn House Publishing.
Vouk, V.B., G.R. Butler, A.C. Upton, D.V. Parke, and S.C. Asher, eds. 1987. Methods for Assessing the Effects of Mixtures of Chemicals Scope. New York: John Wiley & Sons.
RELATED PUBLICATIONS
NRC (National Research Council). 1989. Biological Markers in Pulmonary Toxicology. Washington, D.C.: National Academy Press. NRC (National Research Council). 1988. Complex Mixtures. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1986. Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1985. Epidemiology and Air Pollutants. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1984. Toxicity Testing: Strategies to Determine Needs and Priorities. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1981. Committee on Indoor Air Pollutants. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1981. Committee on Medical and Biological Effects of Environmental Pollutants. Washington, D.C.: National Academy Press.
Workshop Participants
Research to Improve Predictions of Long-Term Chemical Toxicity Workshop Participants by Workgroup Washington, D.C. December 13-15, 1989
Structure-Activity-Relationship Analyses and Other Correlational Techniques
Dr. Elizabeth Weisburger, Chair, National Cancer Institute (retired), Bethesda, Maryland
Mr. Kurt Enslein, Health Design, Inc., Rochester
Dr. Gilles Klopman, Case Western Reserve University, Cleveland
Dr. Harold Moore, University of California, Irvine
Dr. Ray Tennant, NIEHS, Research Triangle Park
Dr. Yin-tak Woo, EPA, Washington, D.C.
Short-Term In Vitro and In Vivo Tests
Dr. Herbert Rosenkranz, Chair, University of Pittsburgh Graduate School of Public Health, Pittsburgh
Dr. John Frazier, Johns Hopkins University, Baltimore
Dr. Claude Hughes, Duke University Medical Center, Durham
Dr. Marvin Legator, University of Texas Medical Branch, Galveston
Dr. Vernon Ray, Pfizer, Inc., Groton, Connecticut
Dr. Michael Waters, Environmental Protection Agency, Research Triangle Park
Dr. Gary Williams, American Health Foundation, Valhalla, New York
Dr. Errol Zeiger, NIEHS, Research Triangle Park
Whole-Animal Tests
Dr. Michael Gallo, Chair, UMDNJ, R.W. Johnson Medical School, Piscataway
Dr. Deborah Barsotti, Agency for Toxic Substances and Disease Registry, Atlanta
Dr. Thomas Fuhremann, Monsanto Co., St. Louis
Dr. Richard Griesemer, NIEHS, Research Triangle Park
Dr. Emil Pfitzer, Hoffman-LaRoche, Inc., Nutley, New Jersey
Dr. Jerold M. Ward, National Cancer Institute, Frederick, Maryland
Dr. Bernard Weiss, University of Rochester Medical Center, Rochester
Validation
Dr. Roy Albert, Chair, University of Cincinnati Medical Center, Cincinnati
Dr. David Hoel, NIEHS, Research Triangle Park
Dr. Kim Hooper, State of California Department of Health, Berkeley
Dr. Renate Kimbrough, Environmental Protection Agency, Washington, D.C.
Dr. Peter Magee, Temple University School of Medicine, Philadelphia
Dr. Barry Margolin, University of North Carolina, Chapel Hill
Dr. John Mulvihill University of Pittsburgh Graduate School of Public Health, Pittsburgh
Dr. David Peakall, Canadian Wildlife Service, Ottawa
Strategy, Policy, and Resource Considerations
Dr. William Lowrance, Chair, Rockefeller University, New York
Dr. John Andrews, ATSDR, Atlanta
Dr. Irv Baumel, Health Effects Research Division, U.S. Army, Biomedical R&D Lab, Frederick, Maryland
Dr. Irina Cech, U.S. House of Representatives, Energy and Commerce Committee, Washington, D.C.
Dr. William Farland, Environmental Protection Agency, Washington, D.C.
Dr. James Huff, NIEHS, Research Triangle Park
Dr. Jack Moore, Institute for Evaluating Health Risks, Irvine, California
Dr. Andrew Sivak, Health Effects Institute, Cambridge
Dr. James Wilson, American Industrial Health Council, Washington, D.C.
Dr. John Young, Hampshire Research Associates, Inc., Alexandria, Virginia
Observers
Dr. Devra Lee Davis, Scholar in Residence, National Research Council, Washington, D.C.
Mr. Carl Gerber, Environmental Protection Agency, Washington, D.C.
Dr. Sid Siegel, National Library of Medicine, Bethesda