Appendix A
A Review of the Isotope Geochemistry of the Yucca Mountain, Nevada, Proposed Nuclear Waste Repository Site.
Prepared for the National Research Council's Panel on Coupled Hydrologic/Tectonic/Hydrothermal Systems at Yucca Mountain
Douglas Rumble
Geophysical Laboratory
Washington, D.C., 20015-1305
Revised March 19, 1992
SUMMARY
It has been proposed that calcite veins near the Yucca Mountain, Nevada, proposed nuclear waste repository site were precipitated from upwelling ground water (Szymanski, 1989). Testing the hypothesis is important because, if it is correct, future upwellings might flood the repository. Tests of the hypothesis carried out with isotope geochemistry show that the calcites did not precipitate from present-day ground water but, on the contrary, from surface waters. Changes in the isotopic compositions of ancient ground waters to bring them to parental compatibility with the calcites are larger than those inferred from available data. Additional information is needed, however, to reach definitive conclusions about the isotopic compositions of paleo-ground waters.
The methods of isotope geochemistry make it possible to test whether or not a particular calcite precipitated from a specific ground water, provided that analyses of both calcite and ground water have been performed. The efficacy of isotope geochemistry methods may be verified by considering the calcite veins of Devils Hole, Nevada, located 40 km SSE of the proposed repository site. Data on the stable isotopes of C and O and on the radioactive decay of Rb, U, and Th show that Devils Hole calcites precipitated from ground waters of the Ash Meadows flow system. Direct observation and sample collecting by SCUBA divers confirm the precipitation of calcite from ground water at Devils Hole.
The contrast between isotope relationships measured at Devils Hole and those at the Repository Site is striking and much greater in magnitude than could have been caused by chance analytical errors. Calcites at the Repository are too rich in 18O to have precipitated from analyzed ground water. The calcites are also too low in 234U/238U and too high in 87Sr/86Sr to have precipitated from analyzed ground water. The Szymanski hypothesis is directly contradicted by the available data of isotope geochemistry. The isotope data are consistent with precipitation of Repository Site calcites from surface derived waters in the unsaturated zone.
INTRODUCTION
Szymanski (1989) has proposed that calcite veins in the vicinity of the Yucca Mountain, Nevada, Nuclear Waste Repository Site were deposited from upwelling ground water. His hypothesis has significant implications because it forecasts possible flooding of the Repository Site. Testing the hypothesis is important in order to evaluate the probability of such an hazard befalling the Repository. Szymanski, himself, has suggested a number of tests to be made of his hypothesis including: (a) monitoring the stability of the water table at Yucca Mountain; (b) measurement of in-situ values of “closure pressure”; (c) establishing the origin and age of “mosaic breccias”; and (d) measurement of the strain rates of wall rock separation during formation of calcite veins (Szymanski, letter of transmittal to R. A. Levich dated July 26, 1989, p.4). The following review discussion addresses test (c), as listed above.
Isotope geochemistry provides an objective means for testing whether or not a specific calcite precipitated from a particular ground water. Note that testing is limited by the availability of data: one has to have analyses of both calcites and ground waters to make the tests. Pairs of heavy isotopes such as 87Sr and 86Sr or 234U and 238U are not
fractionated during calcite precipitation: they provide fingerprints of origin. Moreover, secular disequilibrium of 234U, 238U, and 230Th gives age dates for calcite that are valid up to 500,000 years before present. Pairs of light isotopes such as 13C and 12C or 18O and 16O are partitioned between ground water and calcite during precipitation: They make possible estimates of temperatures of formation as well as fingerprint origins.
The following review discussion is divided into three sections: (1) C-O-H isotopes; (2) U-Th isotopes; and (3) Sr isotopes. In each section, the efficacy of isotopic methods to fingerprint the origin of calcite is verified by comparing data on Devils Hole calcite veins with that on ground waters of the Ash Meadows flow system.
Devils Hole is a useful test case. It is located only 40 km SSE of the proposed repository site so environmental factors are similar. Ground water is currently flowing through the caverns at Devils Hole. The precipitation of calcite from ground water has been directly observed and sampled by SCUBA divers. Calcite is presently precipitating above the water table at Devils Hole in the Brown Room, an air-filled cavern isolated from the surface by a natural syphon. I did not find isotopic data on samples from Brown Room. Calcite that precipitated from ground water below the water table occurs in mammary structures of finely-laminated growth layers coating the walls of open fractures. The calcite deposits from below the water table are usually referred to as “vein calcites” to distinguish them from speleothems above the water table. Calcite is observed to precipitate on seed crystals suspended in ground water below the water table. The youngest vein calcite that has been dated so far is 20,000 years old (Winograd, personal communication); the oldest is 566,000 years old (Ludwig, et al. 1990). The vein calcite preserves a continuous record of climate from about 570,000 to 60,000 years ago (570 - 60 ka). A plot of δ18O vs. time for vein calcites closely resembles the marine δ18O curve as well as polar ice core records (Winograd et al., 1988).
Devils Hole is a good test case because there is abundant isotopic data on both ground waters and vein calcites. Isotopic methods can be evaluated with confidence because it is well established that calcite did, in fact, precipitate from ground water at Devils Hole.
The isotope values δ18O and δD are reported relative to VSMOW (Vienna Standard Mean Ocean Water, the isotope reference standard defined by the International Atomic Energy Agency, Vienna, Austria).
and
Values of δ13C are reported relative to PDB (Pee Dee Belemnite).
Values of 87Sr/86Sr are reported as atomic ratios. Values of 234U/238U and 230Th/234U are reported as activity ratios where the activity equals the number of atoms at time “t” multiplied by the decay constant.
Two ground-water flow systems dominate the hydrology of the Yucca Mountain area (see Chapter 3 of this report). The Ash Meadows system has its recharge in the Spring Mountains, near Las Vegas, Nevada, and discharges at Ash Meadows, Nevada, near the California state border. The aquifer is a Paleozoic limestone that is confined by aquitards both above and below in the stratigraphic succession. Owing to uplift and erosion, the aquitards have been breached in a number of fault block mountains throughout the region. The Alkali Flat/Furnace Creek flow system immediately underlies Yucca Mountain. Its recharge area is north and east of Yucca Mountain in the vicinity of Pahute Mesa and Fortymile Wash. Discharge is near Furnace Creek, Death Valley, California, and at Franklin Lake Playa, California. The aquifer beneath the Yucca Mountain area is composed of Tertiary and Quaternary volcanic tuffs and alluvium. Ground water flows primarily through fractures in the tuffs of Yucca Mountain.
ANCIENT GROUND WATERS VS. MODERN GROUND WATERS
The data set discussed below consists of isotopic analyses of ground waters sampled over the past several years and calcites that were precipitated during recent times to at least 566 ka. Some of the calcites in the volcanic rocks probably formed during hydrothermal activity associated with active volcanism some 10 to 15 million years ago (Ma). Clearly, calcite isotopic compositions predicted from analyses of modern ground waters are not directly comparable to ancient calcites.
In the discussion that follows it will be demonstrated that known surface calcite deposits at Yucca Mountain did not precipitate from analyzed present-day ground waters. Whether or not the calcites could have precipitated from ancient ground waters cannot be proven because
critical data on paleo-ground waters are lacking. The data that are available suggest that isotopic compositions of ancient ground waters have been similar to modern values for the past 300 to 500 ka.
The calcite veins at Devils Hole, Nevada (40 km SE of Yucca Mountain), preserve a record of calcite precipitation over the past 566 ka (Ludwig et al., 1990). The δ13CPDB values range from −2.8 to −1.5‰ (Coplen et al., 1990; Coplen, written communication, 1992) and δ18OVSMOW from +13.1 to +15.6‰ (Winograd et al., 1988). The range of 87Sr/86Sr is from 0.7123 to 0.7128 (Marshall et al., 1990) and that of initial 234U/ 238U activity ratios is from 2.6 to 2.8 over the time interval (Ludwig et al., 1990). Assuming that the temperature of ancient ground waters was 33.7°C, equal to the present value, the range of δ18OVSMOW of paleo-ground waters was −13.7 to −11.2‰ at Devils Hole over the past 566 ka. These changes are not large enough to justify the ground waters as parental to Yucca Mountain surficial calcite deposits. The Devils Hole data, however, are not directly applicable to Yucca Mountain because the two localities belong to different hydrologic flow systems, e.g. Ash Meadows and Alkali Flat/Furnace Creek, respectively.
Samples of ancient ground water are preserved as fluid inclusions in calcite veins at Furnace Creek, Death Valley, California (60 miles SW of Yucca Mountain). Modern springs at Furnace Creek are among the discharge points of the Alkali Flat/Furnace Creek flow system, the system that underlies Yucca Mountain. U and Th isotopes were analyzed from calcite vein laminae for purposes of age dating. The fluid inclusions were analyzed for D/H ratios (Winograd et al., 1985). The δDVSMOW average concentrations range from −93 to −87‰ over the past 300 ka. Samples from laminae 0.5 to 1.0 Ma average δDVSMOW = −75‰ and those older than 1.0 Ma range from −70 to −45‰. Owing to technical difficulties the analyses for D/H are not as precise as one might like: replicate analyses of fluid inclusions from the same vein lamina gave standard deviations as high as 11 ‰. Accepting, at least for the moment, the results as published, the variation of 6‰ in δD measured over the past 300 ka years corresponds to a change in δ18O of less than 1‰ along the meteoric water line. A change of 1‰ in δ18O of paleo-ground waters is not large enough to make it possible to derive Yucca Mountain surficial calcites from ancient ground waters.
C-O-H ISOTOPES
O and H:
Sensitive tests for the origins of ground waters may be made by analyzing for the stable isotopes of hydrogen and oxygen. Such pro-
cesses as evaporation and geothermal heating are also recorded by the isotopes. Figure 1 shows ground waters from the Yucca Mountain area compared to Craig 's (1961) meteoric water line. The ground-water isotopic contents occupy a narrow range overlapping and parallel to the meteoric water line. Both the Ash Meadows flow system and the ground waters of the Alkali Flat/Furnace Creek system are indistinguishable in terms of δ18O and δD. The δ18O and δD values of the Ash Meadows flow system are controlled primarily by the isotopic composition of winter-spring precipitation over recharge areas in the Spring Mountains. The similar values of the Alkali Flat/ Furnace Creek ground-water system suggest a similar control on isotopic content. Note that precipitation collected at stations on the Nevada Test Site (north and east of Yucca Mountain) has higher δD

Figure 1 Plot of δD vs δ18O for ground waters of the Ash Meadows and Alkali Flat/Furnace Creek (Alk./Furn.) flow systems. Also shown are precipitation on the Nevada Test Site, surface waters and the meteoric water line (“MWL”) of Craig (1961). “LMWL” is the local meteoric water line. Data and additional discussion in Claasen (1985, 1986); Benson and Klieforth (1989); Benson and McKinley (1985); Benson et al. (1983); Ingraham et al. (1990); and Lyles et al. (1990). Analytical uncertainty is ±0.2‰ for δ18O and ±2.5‰ for δD.
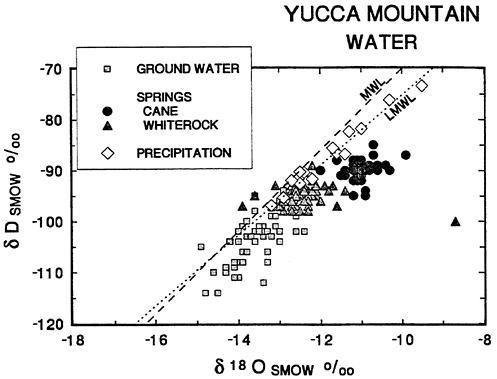
Figure 2 Plot of δD vs. δ18O of Yucca Mountain ground waters, springs and precipitation on the Nevada Test Site. The global meteoric water line is labeled “MWL”; the local meteoric water line is “LMWL”. The ground water samples are from the Ash Meadows and Alkali Flat/Furnace Creek regional aquifers. Cane Spring flows from a perched water table beneath the east end of Skull Mountain, 30 km east of Yucca Mountain. Whiterock Spring is 45 km northeast of Yucca Mountain and may be of the same origin as Cane Spring. Data as in Figure 1.
and δ18O concentrations than deep ground water in the Ash Meadows and Alkali Flat/Furnace Creek flow systems (Figure 1). The disparity between the composition of deep ground water and local meteoric water is illustrated in Figure 2. Comparison of water from springs fed from local, perched water tables with ground water from the deeper aquifers shows the springs to have both higher δ18O and higher δD, consistent with local precipitation (Figure 2). Cane Spring waters show the effects of local evaporation. The important point to remember in the following discussion is this: Both the surface waters at Yucca Mountain and the ground waters are of meteoric origin. Surface and ground waters differ in δ18O and δD because the recharge areas of the ground water flow system are at higher elevations
and therefore have lower temperatures than Yucca Mountain surface water.
The strong correlation of δD and δ18O along the meteoric water line is controlled primarily by temperature. Higher values of δD and δ18O correspond to warmer climates, lower elevations, lower latitudes, and to summer rather than winter precipitation at a given site. Lower values reflect colder climates, higher elevations, higher latitudes, and winter instead of summer precipitation.
Ground waters and surface waters of the Salton Sea Geothermal Field located in S. California 384 km south of Yucca Mountain show the effects of both evaporation and isotopic exchange between waters and wall rocks under conditions of geothermal heating (Figure 3). The isotopic signature of evaporation is illustrated by the linear array of surface water values aligned between local Colorado River

Figure 3 Plot of δD vs δ18O for Yucca Mountain ground waters in comparison to Salton Sea rainfall, surface, and geothermal waters. The meteoric water line (MWL) of Craig (1961) is shown. Data from Williams and McKibben (1989, Figure 5, p. 1910).
water and Salton Sea water. Evaporation leads to enrichment in both 18O and D in liquid water because of preferential loss of 16O and H to water vapor. Isotopic exchange between ground waters and wall rocks under geothermal or hydrothermal conditions shows small changes in δD but large increases in δ18O because wall rocks are usually dominated by oxygen-bearing rather than by hydrogen-bearing minerals. Geothermal waters at Salton Sea show enrichments of 12‰ in δ18O in comparison to surface waters. Compare the small range of variation in δD and δ18O from Yucca Mountain ground waters with the large variations measured at Salton Sea (Figure 3). There is no evidence for either evaporation or geothermal exchange controlling the isotopic composition of ground waters at Yucca Mountain, except locally, at Cane Springs.
C and O:
The stable isotopes of carbon and oxygen are important in deducing the source of calcites from Yucca Mountain. The δ18O of ground waters in the area is controlled by meteoric water in the recharge zones, as discussed above. The δ13C values, however, are subject to localized effects because the total amount of carbon dissolved in ground waters is small. Among the factors influencing the δ13C of ground waters are biomass in recharge areas including the proportion of C-3 to C-4 plants, soil pH and oxygen fugacity in recharge zones, and isotope exchange between ground waters and carbonate acquifers. Figure 4 shows a plot of analyses of ground waters of the Ash Meadows flow system. The values show a narrow range in δ18O (−15 to −13‰) but a large variation in δ13C (−2 to −10‰).
I will first consider the test case of Devils Hole calcite veins and the Ash Meadows flow system to verify that it is possible to accurately predict the δ13C and δ18O values of calcites precipitated from ground waters of known isotopic composition. The triangles on the right side of Figure 4 give the calculated values of calcite in isotopic equilibrium with reported ground waters at 25°C. It is assumed that calcite is in oxygen isotope exchange equilibrium with H20 and is in carbon isotope exchange equilibrium with bicarbonate ion.
The fractionation between calcite and water of 18O - 16O is large (roughly 29‰) but the fractionation of 13C - 12C is only about 2‰. For this reason, tie-lines connecting coexisting calcite and ground water are nearly horizontal in the plot. The composition of ground waters at Devils Hole is currently δ18O = −13.6‰ and δ13C = −5‰. The expected compositions of calcites precipitating from these waters are δ18O =+15‰ and δ13C = −3‰. Note that the calculated calcite

Figure 4 Plot of δ13C vs δ18O for ground water of Ash Meadows flow system (references given for Figure 1) and Devils Hole calcite (Coplen and Winograd, personal communication (1990); Coplen et al. (1990)). The values of calcite in equilibrium with analyzed ground waters (equilib. cc.) were calculated for 25 °C with 1000 In α (18O/16O) for CaCO3− H2O = 28.5 and (13C/12C) for HCO3- − CaCO3 = −2.2 (Friedman and O'Neil, 1977). The δ18O values of calcite in equilibrium with analyzed ground waters shift to 20-21‰ for water temperature of 0°C and to 9-10‰ at 50°C. Analytical uncertainty is ± 0.2‰ for both δ18O and δ13C.
values plot within the narrow range of measured values for Devils Hole calcite veins. The value of 25°C was chosen as a general reference. If the correct value of ground water temperature at Devils Hole is used in the calculation (e.g. 33.7°C) the calculated δ18O shifts to 13.2‰, close to the value of 14‰, measured for contemporary calcite. The test case shows that it is possible to accurately predict the isotopic composition of calcite precipitated from analyzed ground waters.
The next figure (Figure 5) includes the data from the previous figure but also gives the δ13C and δ18O analyses of ground waters from the Alkali Flat/Furnace Creek flow system, i.e. the ground-
water flow system in the Tertiary/Quaternary rocks that immediately underlies Yucca Mountain. The ground waters show a narrow range of δ18O, −15 to −12.5‰, but a large range in δ13C of −5 to −13‰. The δ18O values of both Ash Meadows and Tertiary/Quaternary ground waters are closely similar but the latter are typically more depleted in δ13C than Ash Meadows. Also shown are the calculated δ13C and δ18O values of calcites that would precipitate from Tertiary/ Quaternary ground waters.
I now show a plot (Figure 6) to demonstrate that the calcite veins of Yucca Mountain did not precipitate from known ground waters. Figure 6 includes data from previous figures but data on calcite veins from Trench 14 and Busted Butte as well as soil calcites have been added. Note that there is no overlap between the values of calculated

Figure 5 Plot of δ13C vs δ18O for ground waters of Ash Meadows (A.M.) and Alkali Flat/Furnace Creek (Alk./Furn. and A./F.) flow systems and Devils Hole calcites. Values of calcites in equilibrium with analyzed ground waters calculated as for Figure 4. Wells drilled at Yucca Mountain give ground-water values of δ18O from −14 to −12.8‰ and δ13C from −12.7 to −4.9‰.

Figure 6 Plot of δ13C vs δ18O for ground waters of Alkali Flat/Furnace Creek flow system (Alk./Furn. and A./F.), and Yucca Mountain vein calcites and soil calcites. Yucca Mountain calcite data from Whelan and Stuckless (1990), Quade and Cerling (1990), and Quade et al. (1989). See caption for Figure 4 and Figure 5 for further explanation of values and data sources.
calcites expected to precipitate from analyzed ground waters and the measured calcite veins of Yucca Mountain. There is, however, a significant overlap between Yucca Mountain calcites and analyzed soil carbonates collected from localities surrounding the Repository Site in the southern Great Basin. These results demonstrate that calcite veins at Trench 14 and Busted Butte did not precipitate from analyzed ground waters but were deposited from local surface waters under soil conditions in the unsaturated zone, as deduced by Quade and Cerling (1990). In order to obtain the δ18O values of Trench 14 and Busted Butte calcites from analyzed ground waters, precipitation would have had to occur at the unreasonably low temperature of 0° to +8°C, hardly indicative of deep upwelling ground water.
The question arises: Are there absolutely no calcites from Yucca Mountain yet analyzed that bridge the gap between isotope values of
calcite precipitated from ground water and those deposited at surface conditions? The answer is that one sample of calcite recovered from a drill hole from a depth of 611 m. below the surface has an isotopic composition consistent with precipitation from ground water. Significantly, the sample comes from a depth 142m below the present ground water table. Analyses of drill hole calcites are shown in Figure 7 together with data on Devils Hole, Yucca Mountain, and soil calcites. The values of drill hole calcites overlap those of Yucca Mountain soil calcites but also extend towards δ18O values as low as 15.4‰.
Figure 8 gives an enlargement of δ13C and δ18O data on Yucca Mountain calcites. The drill hole calcites are labeled with either the depth below the water table from which they were collected (−142m) or the height above it (+186m.). Note that calcites collected as close as 186m. above the water table are essentially indistinguishable from
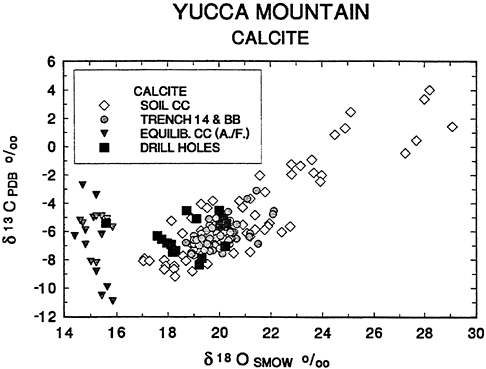
Figure 7 Plot of δ13C vs δ18O for Yucca Mountain vein calcites, soil calcites, and drill hole calcites enlarged from Figure 6, compared to calcites expected to precipitate from Alkali Flat/Furnace Creek (A./F.) ground waters. Data sources as in Figure 6 with data on drill hole calcites from Szabo and Kyser (1990). See caption for Figure 4 and Figure 5 for further explanation.
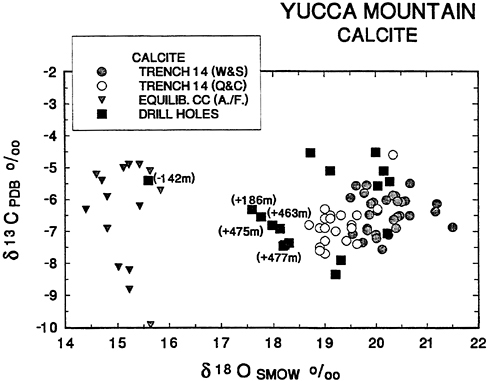
soil calcites (cf. Figure 7). The only calcite with isotopic values consistent with precipitation from analyzed ground waters was recovered 142 m below the water table. These data directly contradict the hypothesis that Yucca Mountain calcites were deposited from upwelling ground water. On the contrary, there is a strong implication that calcite isotopic compositions are dominated by surface waters to depths as close as 186m. above the water table, as concluded by Szabo and Kyser (1990).
If the reader will kindly continue to refer to Figure 8, I will conclude the discussion of stable isotope data with a brief consideration of interlaboratory standardization of analytical results. The separate analyses of Whelan and Stuckless (1990) and Quade and Cerling (1990) for calcites from Trench 14 are presented in Figure 8. The values occupy the same range in δ13C but Whelan and Stuckless results are about 0.7% enriched in δ18O relative to those of Quade and Cerling.
The results are not a formal interlaboratory comparison because the analysts did not exchange aliquots of the same samples. Nevertheless, there does appear to be a systematic difference in δ18O. An interlaboratory discrepancy of 0.7‰ is a little higher than one might like but is within the range observed in formal intercomparisons (Blattner and Hulston, 1978). In any case, the apparent discrepancy is smaller than the differences in δ18O discussed above and, thus, does not detract from the conclusions.
U AND TH ISOTOPES
The activity ratios of U and Th isotopes in ground waters and calcites of the Yucca Mountain area are shown in Figure 9. Values of 234U/238U for ground waters from both the Ash Meadows and the Alkali Flat/Furnace Creek flow systems are plotted in a stacked histogram on the left-hand side of the figure. The histogram has been rotated 90° to facilitate comparison of ground water with calcite data. The right-hand-side of the figure is a plot of 234U/238U vs. 230Th/234U for calcites. The dotted curves with gentle slopes are the trajectories followed by calcites as radioactive decay proceeds with increasing age. The steeply sloping solid lines connect calcites of equal age and are labeled with the age in years before present.
It is known that pure calcites precipitating from ground water have the same 234U/238U activity ratio as parental ground water but contain no Th. A recently precipitated calcite would plot along the vertical Y-axis at 230Th/234U = 0. Over the course of time, the isotopic composition of calcite shifts from left to right across the diagram and parallel to the dotted curves owing to the decay of 238U to 234U and of 234U to 230Th. The change in 234U/238U with age is small and, consequently, it is not a sensitive chronometer; it is, however, a reliable tracer of the composition of parental ground water. The activity ratio 230Th/234U changes greatly with age; it is a sensitive chronometer for ages up to 300,000 to 500,000 years before present. Ivanovich (1982) gives a clear and thorough discussion of U-Th systematics.
The Devils Hole data on ground water and calcite provide a test case for the geochemical relationships discussed in the preceding paragraph. Devils Hole ground waters have an average 234U/238U activity ratio of 2.76 ±(0.09). Devils Hole calcite activity ratios trace out a curved line that lies parallel to and halfway between the decay curves for calcites with initial 234U/238U ratios of 2.5 and 3.0 (Figure 9). There is close agreement between the 234U/238U of ground water (2.76 ±0.09) and the average of calculated initial 234U/238U for calcites (2.70 ±0.07) over an age range of over 300 ka (Winograd et al., 1988). These data
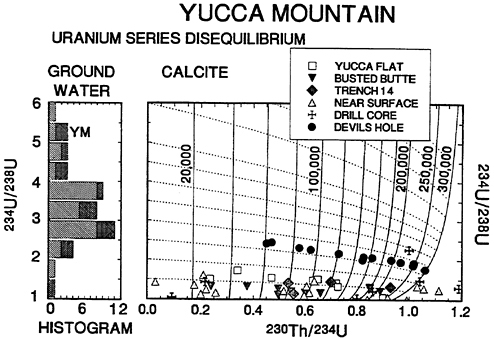
Figure 9 Left-hand side of diagram shows stacked histogram of 234U/238U activity ratios for ground waters of Ash Meadows (diagonal ruling) and Alkali Flat/Furnace Creek (cross hatched) flow systems. The symbol “YM” denotes ground waters sampled near Yucca Mountain. Histogram is rotated 90° to facilitate comparison with calcite data. Right-hand side of diagram shows plot of 234U/238U vs 230Th/234U for Yucca Mountain and Devils Hole calcites. Dashed lines are pathways of radioactive decay for calcites. Solid lines are isochrons labeled with age in years before present. Both dashed lines and solid lines are terminated at 300,000 years for clarity. Data sources are: Yucca Flat - Shroba et al. (1988); Busted Butte - Muhs and Whitney, personal communication (1990); Trench 14 - Muhs et al. personal communication (1990); near surface -Szabo et al. (1981); Szabo and O'Malley (1985); drill core - Szabo and Kyser (1990); Devils Hole - Winograd et al. (1988). Analytical uncertainty ±0.02 to ±0.04 for both 234U/238U and 230Th/234U.
validate the use of 234U/238U activity ratios to trace parental ground waters of pure calcite.
The calcite data from Yucca Mountain stand in strong contrast to those from Devils Hole. With but two exceptions, all calcites from near the Repository Site have measured 234U/238U less than 2.0. Note the frequency distribution of 234U/238U ratios in ground waters: there is only one sample out of a total of 16 analyzed Tertiary/Quaternary waters that is a permissible parent for the analyzed calcites. The anomalous ground water was collected from a well drilled at French-
man Flat, 60 km to the E of Yucca Mountain. Ground waters sampled in wells drilled near Yucca Mountain have 234U/238U activity ratios of 5 to 7 (one higher value of 6.9 is not shown in Figure 9). There are two calcites with high 234U/238U out of a total of 40 analyzed. One of these samples has 234U/238U = 2.26 and is from a depth of 63m. in drill hole USW G-3. The other sample has 234U/238U = 1.47 and is from drill hole UE - 25 a#1 at a depth of 283m (Szabo and Kyser, 1990). The projected initial activity ratios of these two samples are about 3.4 and 2.2, respectively. Neither of these values fall within the range (5-7) of ground waters sampled by drilling at Yucca Mountain.
There are two interesting discrepancies between δ18O data, and U-Th data measured for drill hole calcites. The sample from a depth of 611m in drill hole UE-25 a #1 has a δ18O value of +15.6‰ suggesting precipitation from ground water (the sample was collected 142m below the water table) but the 234U/238U activity ratio of 1.29 is compatible with surface calcites. A sample from a depth of 63m in drill hole USW G-3/Gu-3 (690 m above the ground water table) has 234U/238U = 2.26 suggesting a parent of mixed ground water and surface water but its δ18O value of +20.23 shows no evidence of a contribution from ground water.
The interpretation of raw U-Th data must be done with great care. I am indebted to my colleague Fouad Tera for raising this point very forcefully. A problem arises because of the difficulty in purifying surface calcite deposits such as soil calcites, caliche, and calcrete. The materials are heterogeneous mineralogically. Calcite is present as a cement binding mineral and rock fragments of the host rock or soil. Authigenic minerals such as clay, zeolites, and opaline silica may be present, as well. These impurities are of different ages and origins, thus their inclusion with calcite for analysis would scramble the U-Th isotopic signature. It is difficult to separate calcite from contaminants owing to the intimacy of the intergrowths and their fine-grained size. Acid leaching of samples yields a product that is mostly derived from calcite but that does contain some U and Th from the non-carbonate fraction.
Researchers in the field of U-series age dating are well aware of these problems. The isotope 232Th is routinely monitored to detect the presence of impurities. Isotopic analyses of acid soluble and insoluble residues of the same sample are compared and the activity of 232Th is used as an index of contamination to correct the acid soluble fraction for impurities. The advent of mass spectrometry instead of alpha spectrometry to measure activity ratios has alleviated the purification problem to a certain extent because the size of sample required for analysis has been reduced.
The problem of analysing impure surface calcite deposits has direct bearing on the interpretation of the Yucca Mountain and Devils Hole data. The Devils Hole vein calcites are very pure with less than 0.5 percent insoluble residues. The 230Th/232Th ratios show one sample with a value of 25 but in 12 other samples the ratio is greater than 100. The Devils Hole vein calcites, by this criteria, are ideally suited for U-Th series dating. The Yucca Mountain samples, in contrast, have 230Th/232Th values predominantly less than 100 with many values less than 10. The data reported in Figure 9 have had the 232Th correction applied but uncertainties remain. As stated by Szabo and Rosholt (1982, p. 260) “There are no absolutely certain ways to assess the correctness of the primary assumptions of this type of dating such as the assumption that the initial 230Th activity is negligibly small in the sample, or that the dated carbonate remained ideally closed with respect to the isotopes of interest.”
Despite the uncertainties discussed above, the coherence of the Yucca Mountain data, as shown in Figure 9, argues that the data should be given serious consideration. I conclude that the U-Th data are consistent with stable isotope data that shows Yucca Mountain calcite veins were not deposited from analyzed ground waters.
SR ISOTOPES
Strontium isotopes (87Sr and 86Sr) are similar in behavior to 234U and 238U in that they are not fractionated when calcite precipitates from ground water. Calcites do not accommodate 87Rb, the radioactive parent of 87Sr, in their crystal lattice. Consequently, once precipitated, the 87Sr/86Sr ratio of calcite is fixed. Faure (1986, Chap. 11) gives a clear and thorough discussion of these principles.
The efficacy of 87Sr/86Sr ratios to fingerprint the parental ground waters of calcites may be verified by considering the Devils Hole data. The upper panel of Figure 10 is a stacked histogram of Ash Meadows ground water values and Devils Hole calcite values. The measured ground water value at Devils Hole is 0.7123. The calcite values are 0.7123 to 0.7128 for calcites with ages of 100-566 ka (Marshall et al., 1990). These results establish the reliability of Sr isotopes in calcite as tracers of parental ground waters and show that 87Sr/86Sr ratios have remained relatively constant in the Ash Meadows flow system for the past 566 ka.
The Yucca Mountain 87Sr/86Sr data are shown in the lower panel of Figure 10. Trench 14 calcites and soil calcites share, in part, the same range of values, but, the former are, on average, somewhat enriched in 87Sr. Ground waters sampled in drill holes at Yucca

Figure 10 Upper panel shows stacked histogram of 87Sr/86Sr values for ground waters of Ash Meadows flow system and Devils Hole calcites. Lower panel gives stacked histogram of 87Sr/86Sr for ground waters of Alkali Flat/Furnace Creek (Alk./Furn.) flow system and calcites from Trench 14, Busted Butte (BB), and soil calcites sampled from 1 m deep pits (Marshall et al., 1990). Analytical uncertainty is ±0.00005.
Mountain have 87Sr/86Sr values of 0.7100 to 0.7115. The analyzed ground waters overlap the lower end of the range of soil calcite values but do not overlap the range of Trench 14 and Busted Butte vein calcites. The ground water value of 0.7119 is from a well west of Bare Mtn., about 10km SE of Beatty ( Figure 10). It is concluded that vein calcites from Trench 14 and Busted Butte did not precipitate from analyzed ground waters.
NEW DATA ON CALCITE FROM DRILL HOLES
New isotopic data on calcite in core samples from drill holes USW-G1, -G2, -GU3, -G3, -G4, and UE25b#1 are currently being measured at USGS, Denver. Preliminary reports have been submitted for pre-
sentation at the Geological Society of America meeting in San Diego, October, 1991 (J. S. Stuckless, personal communication). Drill holes G2 and G1 bracket, from north to south, the hydrologic gradient at the north end of Yucca Mountain. The other holes lie 1 to 4 km south and east of the gradient. Core samples were obtained from the holes over a range of elevations from a height of +600m above the static water level to a depth of −1200m below it. The analyzed samples include secondary calcite from lithophysal cavities and cements in tuffs as well as from fracture walls.
The following comments are based on a graph showing δ13C, δ18O, and depth for some 80 samples, provided by J. S. Stuckless on June 6, 1991 (preprint of J. F. Whelan and J. S. Stuckless). Approximately 40 samples from above the static water level and 40 from below it were analyzed.
Values of δ18O of calcite show a decreasing trend with depth, similar to that noted by Szabo and Kyser (1990). Samples from above the static water level range from +14‰ to +21‰. Samples from below the static water level lie between +4‰ and +14‰. Many of the samples from the saturated zone appear to be in equilibrium with analyzed ground waters. Temperatures measured at depths of −500 to −1200 m below static water level range from 40°C to 60°C. Calcite precipitating from analyzed ground waters at these temperatures would have δ18O values of +8‰ to +12‰. There are two samples from below the water table, at −45 m and −130 m, however, that have values of 17.5‰, within the range of samples from the unsaturated zone.
Concentrations of δ13C of calcite show an increasing trend with depth. Calcites from the unsaturated zone give values of −10‰ to 0‰ similar to the range of values from Trench 14 and Busted Butte. Saturated zone calcites are −2‰ to +4‰. There are, however, two samples from the unsaturated zone, at a height of +430 m with values of +0.5‰ and +4.8‰. Furthermore, eight samples from the saturated zone, at depths of −300 m to −45 m below the static water level, lie in the range −10‰ to −5‰.
The existing data set shows a broad systematic trend but with obvious outliers. The question arises as to how much significance should be attached to outliers in an, as yet, incomplete data set. Another important consideration in interpreting the data is the age of calcites. Szabo and Kyser (1990) found a range of ages from 26 ka to over 400 ka in 29 calcites from drill cores. The two oxygen values from − 45 m and −130 m show possible evidence of recharge of water from above the static water level down into the saturated zone. The eight samples with δ13C of −10‰ to −5‰ support the δ18O evidence
of recharge. The two δ13C values of +0.5‰ and +4.8‰, however, may indicate a rise in the water table of as much as 430 m. An alternative explanation of the latter two δ13C values is that ancient surface waters may have been influenced by a biomass with a different ratio of C3/C4 plants, thus giving rise to high δ13C concentrations (Quade et al., 1989). The oxygen data is likely to be a more reliable hydrologic tracer because oxygen is a major constituent of water. Because carbon-bearing species are not abundant in ground water, their 13C/12C composition is subject to local wall rock control as well as contamination. In any event, additional data on the stable isotope composition of calcites from drill cores and, especially, more U-Th age dates are needed.
Preliminary Sr isotope values of some 16 calcites from drill cores show a clear demarcation between saturated and unsaturated zone samples (Z. E. Peterman, J. S. Stuckless, S. Mahan, E. D. Gutentag, and J. S. Downey, preprint). Calcites (9 samples) from +50m to +400m above the static water level are 0.7108 to 0.7128 87Sr/86Sr, overlapping the range of soil calcite and Trench 14. Values of seven samples from depths of −600 to −1200m below the static water level are 0.7086 to 0.7089 87Sr/86Sr, at the low end of the range for waters from the Tertiary/Quaternary aquifer.
The majority of presently available data for drill hole calcites suggests a stable elevation for the static water level, over the period of time during which secondary calcite was deposited.
CONCLUSIONS
Analytical data on both stable isotopes and radiogenic isotopes agree that the calcite vein deposits of Yucca Mountain did not precipitate from analyzed ground waters. The stable isotope data strongly imply that the calcites were deposited from surface waters under soil conditions in the unsaturated zone.
The hypothesis of rising ground water as the origin of the calcites in the Yucca Mountain area has failed the tests of isotope geochemistry and is, in fact, contradicted by the available data.
RECOMMENDATIONS
The importance of deducing the isotopic contents of ancient ground waters at Yucca Mountain has been discussed above. The available data, however, do not support definitive conclusions. Additional data is needed, similar to that measured by Szabo and Kyser (1990) who determined δ18O, δ13C, and U/Th ages on aliquots of the same calcite
samples from drill holes. In order to avoid circular reasoning, independent estimates of the temperatures of paleo-ground waters should be made. Calcite veins intersected in drill cores should be searched for fluid inclusions. Microthermometry of the fluid inclusions will provide independent estimates of calcite precipitation temperatures.
The availability of high quality data on a number of unrelated isotope systems leads to more definitive conclusions than might be gained from considering one pair of isotopes, alone. Comparison of the behaviors of geochemically dissimilar isotopes gives cross reference points and provides strict hypothesis testing. It is strongly recommended that research on both stable and radioactive isotopes be diligently pursued.
An important caveat is implied by use of the word “analyzed” to modify the phrase “ground-waters”. One cannot test parental ground waters when data are lacking. There is need for additional isotopic data on ground waters so that the density of areal coverage will more closely match that now available for soil and vein calcites. Presumably, collecting additional ground water data will require drilling more wells. Additional isotopic data on calcite from localities such as the WT-7 well pad and the tufa at sample site 199 would be useful to extend mapping the importance of surface vs. ground waters.
The contrast in behavior between isotopes present in dominant amount in water such as 18O and 16O, or D and H and those present in small amounts such as 13C and 12C, 234U and 238U or 87Sr and 86Sr has been noted. It is recommended that additional data be gathered to more fully document the control of local wall rocks on the abundance of trace constituents in ground water. It would be interesting to drill deeply into the saturated zone of the Tertiary/Quaternary flow system and sample both ground waters and wall rocks at closely spaced intervals in the same drill hole. Such a detailed data set might shed light on the specific mechanisms of chemical and isotopic exchange of trace constituents between ground waters and wall rocks.
If it is accepted that Trench 14 calcites did not precipitate from upwelling ground water then how did they form? Additional isotopic data on the calcites should help to answer this question. Isotopic analysis of rainfall and soil moisture at sites where calcite has already been analyzed would directly test the hypothesis that surface calcites precipitated from surface waters in the unsaturated zone. Analysis of wind-blown dust would help to quantify its role in the origin of surficial calcite deposits. New data on drill hole calcites are needed to explore the possibility that some of them may have precipitated from mixtures of ground waters and surface waters. The increased use of mass spectrometry methods (Ludwig, et al., 1990;
Papanastassiou, et al., 1991) to improve U-Th series age dating is needed to provide chronological constraints on surface processes.
The brief discussion of interlaboratory standardization given above suggests that formal intercomparisons of analytical results should be carried out between different laboratories measuring the same isotopes. In cases where only one laboratory has been involved in analyzing a particular isotopic system, it is recommended that additional laboratories be brought in to verify analytical accuracy. I have no reason to doubt any of the data discussed above. The laboratories reporting the data are well known and reputable in the geochemistry community. Nevertheless, in a project of such importance to the public as the Yucca Mountain Repository Site Evaluation it would seem prudent to leave nothing to chance including the verification of analytical data by independent laboratories.
ACKNOWLEDGEMENTS
The task of gathering data for this review was greatly aided by the work of J. S. Stuckless who presented a review of the isotope geochemistry of Yucca Mountain to the panel on May 30, 1990 in Menlo Park, CA. I am very grateful to T. E. Cerling, T. B. Coplen, D. Muhs, J. S. Stuckless, and I. Winograd who provided preprints of data in advance of publication. My colleagues G. E. Bebout, G. Goodfriend, P. Koch, and F. Tera contributed helpful, constructive criticism.
REFERENCES
Benson, L. V. and H. Klieforth. 1989. Stable isotopes in precipitation and ground-water in the Yucca Mountain region, Nevada—paleoclimatic interpretation In Aspects of Climate Variability in the Pacific and W. Americas, D. H. Peterson, ed. American Geophysical Union. Monograph 55: 41-49.
Benson, L. V. and P. W. McKinley. 1985. Chemical composition of ground-water in the Yucca Mountain area, Nevada, 1971-84 U. S. Geological Survey Open-File Report. 85-484: 10 pp.
Benson, L. V., J. H. Robison, R. K. Blankennagel, and A. E. Ogard. 1983. Chemical composition of ground-water and the locations of permeable zones in the Yucca Mountain Area, Nevada U. S. Geological Survey Open-File Report. 83-484: 19 pp.
Blattner, P. and J. R. Hulston. 1978. Proportional variations of geochemical d18O scales—an interlaboratory comparison Geochim. Cosmochim. 42: 57-62.
Claasen, H. C. 1985. Sources and mechanisms of recharge for ground-water in the west central Amargosa Desert, Nevada: a geochemical interpretation, U. S. Geological Survey. Professional Paper. 712-F, F1-F31.
Claasen, H. C. 1986. Late-Wisconsin paleohydrology of the west-central Amargosa Desert, Nevada, USA Chem. Geol. 58: 311-323.
Coplen, T. B., I. J. Winograd, K. B. Ludwig, B. J. Szabo, J. M. Landwehr, P. T. Kolesar,
and R. J. Hofmann. 1990. Continuous 500,000 year climate record from Great Basin calcite −2: the δ13C time series Geol. Soc. Am. Abstract. 22: 1209.
Craig, H. 1961. Isotopic variations in meteoric waters. Science. 133: 1702-1703.
Faure, G. 1986. Principles of Isotope Geology. 2nd ed. John Wiley, New York, 589 pp.
Friedman, I., and J. R. O'Neil. 1977. Compilation of stable isotope fractionation factors of geochemical interest In Data of Geochemistry, 6th ed., M. Fleischer, ed. U. S. Geol. Surv. Prof. Paper. 440-KK.
Ingraham, N. L., R. L. Jacobson, J. W. Hess, and B. F. Lyles. 1990. Stable isotopic study of precipitation and spring discharge on the Nevada Test Site, Desert Research Institute Publication 45078. University of Nevada.
Ivanovich, M. 1982. Uranium series disequilibria: applications in geochronology, in Uranium Series Disequilibrium: Applications to Environmental Problems M. Ivanovich and R. S. Harmon, eds. Clarendon Press. Oxford. 56-78.
Ludwig, K. R., K. R. Simmons, B. J. Szabo, and A. C. Riggs. 1990. Mass-spectrometric 230Th - 234U - 238U dating of the Devils Hole calcite vein: a precise record of continuous growth from ~566 ka to 60 ka Geol. Soc. Amer. Abstracts with Programs 1990 A310
Lyles, B. F., J. Edkins, R. L. Jacboson, and J. W. Hess. 1990. Time-series analysis of ion and isotope geochemistry of selected springs of the Nevada Test Site, Nye Co., Nevada. Desert Research Institute Publication 45068. University of Nevada.
Marshall, B. D., Z. E. Peterman, K. Futa, J. S. Stuckless, S. A. Mahan, J. S. Downey, and E. D. Gutentag. 1990. Origin of carbonate deposits in the vicinity of Yucca Mountain, Nevada: preliminary results of strontium-isotope analyses Proceedings of the American Nuclear Society International Meeting on High Level Radioactive Waste Management 2: 921-923.
Muhs, D. R., J. W. Whitney, R. R. Shroba, E. M. Taylor, and C. A. Bush. 1990. Uranium-series dating of secondary carbonates near Yucca Mountain, Nevada: applications to tectonic, paleoclimatic, and hydrologic problems Proceedings of the American Nuclear Society International Meeting on High Level Radioactive Waste Management 2: 924-929.
Osmond, J. K. and J. B. Cowart. 1982. Ground-water, in Uranium Series Disequilibrium: Applications to Environmental Problems M. Ivanovich and R. S. Harmon, eds. Clarendon Press. Oxford. 202-245.
Papanastassiou, D. A., J. H. Chen, and G. J. Wasserburg. 1991. The application of advances in thermal ionization mass spectrometry to U-Th decay series disequilibrium EOS. 72: 291.
Quade, J., T. E. Cerling, and J. R. Bowman. 1989. Systematic variations in the carbon and oxygen isotopic composition of pedogenic carbonate along elevation transects in the southern Great Basin, United States Geol. Soc. Am. Bull. 101: 464-47.
Quade, J., T. E. Cerling, and J. R. Bowman. 1989. Systematic variations in the carbon and oxygen isotopic composition of pedogenic carbonate along elevation transects in the southern Great Basin, United States Geol. Soc. Am. Bull. 101: 464-475.
Quade, J. and T. E. Cerling. 1990. Stable isotope evidence for a pedogenic origin of carbonates in Trench 14 near Yucca Mountain, Nevada Science. 250: 1549-1552.
Shroba, R. R., D. R. Muhs, and J. N. Rosholt. 1988. Physical properties and radiometric age estimates of surficial and fracture-fill deposits along a portion of the Carpetbag fault system Nevada Test Site, Nye County, Nevada. U. S. Geological Survey Report DOE/NV/10583-1. 34 pp.
Szabo, B. J., W. J. Carr, and W. C. Gottschall. 1981. U-Th dating of Quaternary carbonate accumulations in the Nevada Test Site region, southern Nevada U. S. Geological Survey Open-File Report. 81-119 : 35 pp.
Szabo, B. J. and T. K. Kyser. 1990. Ages and stable isotope compositions of secondary calcite and opal in drill cores from Tertiary volcanic rocks of the Yucca Mountain area, Nevada Geol. Soc. Am. Bull. 102: 1714-1719.
Szabo, B. J. and P. A. O'Malley. 1985. Uranium-series dating of secondary carbonate of silica precipitates relating to fault movements in the Nevada Test Site region and of caliche and travertine samples from the Amargosa Desert U. S. Geological Survey Open-File Report. 85-47 : 12 pp.
Szabo, B. J. and J. N. Rosholt. 1982. Surficial continental sediments. In Uranium Series Disequilibrium: Applications to Environmental Problems M. Ivanovich and R. S. Harmon, eds. Clarendon Press. Oxford. 246-267.
Szymanski, J. S. 1989 Conceptual considerations of the Yucca Mountain ground-water system with special emphasis on the adequacy of this system to accomodate a High-Level Nuclear Waste Repository DOE Internal report.
Whelan, J. F., and J. S. Stuckless. 1990. Reconnaissance δ13C and δ18O data from Trench 14, Busted Butte, and drill hole G-4, Yucca Mountain, Nevada Test Site Proceedings of the American Nuclear Society International Meeting on High Level Radioactive Waste Management 2: 930-933.
Williams, A. E., and M. A. McKibben. 1989. A brine interface in the Salton Sea Geothermal System, California: fluid geochemical and isotopic characteristics Goechim. Cosmochim. Acta 53: 1905-1920.
Winograd, I.J., and F. J. Pearson, Jr. 1976. Major 14C anomaly in a regional carbonate aquifer: Possible evidence for megascale channeling, South Central Great Basin Water Resources Research 12: 1125-1143.
Winograd, I.J., B. J. Szabo, T. B. Coplen, A. C. Riggs, and P. T. Kolesar. 1985. Two million year record of deuterium depletion in Great Basin waters Science 227: 519-522.
Winograd, I. J., B. J. Szabo, T. B. Coplen, and A. C. Riggs. 1989. A 250,000-Year climate record from Great Basin vein calcite: implications for Milankovitch theory Science 242: 1275-1280.
Zielinski, R. A., and J. N. Rosholt. 1978. Nevada Test Site, Nye County, Nevada Uranium in waters and acquifer rocks at the J. Res. U.S. Geol. Surv. 6: 489-498.
USEFUL REFERENCES NOT CITED IN THE TEXT
Marshall, B. D., Z. E. Peterman, K. Futa, and J. S. Stuckless. 1991. Strontium isotopes in carbonate deposits at Crater Flat, Nevada. High-Level Radioactive Waste Proceedings of the 2nd Annual International Conference, Las Vegas, Nevada April 28 - May 3, 1991 1423-1428.
Spengler, R. W. and Z. E. Peterman. 1991. Distribution of Rb, Sr, and Zr in tuff from two deep coreholes at Yucca Mountain, Nevada Ibid. 1416-1422.
Stuckless, J. S. 1991. An evaluation of evidence pertaining to the origin of vein deposits exposed in Trench 14, Nevada Test Site, Nevada Ibid. 1429-1438.
Stuckless, J. S. and J. F. Whelan. 1991. Isotopic discontinuities in ground water beneath Yucca Mountain, Nevada Ibid. 1410-1415.



























