1
Introduction
Health care in the United States has improved markedly over the past five decades, in large measure because of the advances in health research that have been supported by a myriad of federal agencies, industry, the private nonprofit sector, and research institutions. Diverse teams of scientists composed of basic scientists, physicians, nurses, dentists, pharmacists, and other health professionals have been involved in research spanning a spectrum from fundamental biological discoveries about life processes, to behavioral and social research, to clinical and population-based studies, and to research on the organization, financing, and delivery of health care services. For example, imaging technology that allows investigators to peer into the human brain and observe the circuitry and biochemical reactions as humans construct thoughts and words is now available. Progress in genome mapping promises to provide monumental advances in understanding of genetic diseases and to aid investigators in finding biological therapies. Rational drug design is allowing researchers to custom design pharmacologic agents that can act on specific tissues, organs, or cell receptors and treat a broad spectrum of human maladies. Entirely new approaches to the treatment, cure, and prevention of human diseases are evolving with the availability of biological products and gene therapies. Research methods are being developed to permit investigators to evaluate the outcomes and effectiveness of health care practices. Research in these and other areas has formed a dynamic synergy that has positioned the United States at the forefront of innovation in medicine.
Despite all the advances, however, signs of stress are surfacing throughout the health care and health research systems. The soaring costs of health care and
the escalating number of uninsured and underinsured people in the United States have thrown health issues into the policy arena at all levels of government. In medicine, highly subspecialized medical training, a declining interest by U.S. medical students in primary care training, and shortages of physicians willing to practice in rural or inner-city areas are all cited as symptoms of a worsening problem. The emergence of human immunodeficiency virus infection has demonstrated that new diseases can arise unexpectedly, and that a multifaceted approach spanning a variety of fields of research and a range of professional research scientists is needed to develop fundamental knowledge about a disease process, diagnosis, effective therapies, and prevention strategies and to assess the subsequent outcomes of health care practices. This can only be accomplished with highly talented and well-trained researchers in all areas of research, from basic to clinical research to outcomes and health services research.
Research is a highly social and political process of communication, interpersonal relationships, and scientific exchange that seeks to describe, explain, and modify biological and pathological processes. Researchers develop hypotheses and test them by collecting and analyzing data. The results add to existing knowledge. The unique feature of clinical research that distinguishes it from laboratory research is the direct involvement of human subjects. Although both laboratory and clinical research employ the same scientific principles for experimental procedures, the use of human subjects increases the complexities of scientific investigations. Whereas laboratory studies can more easily control for as many variables as possible to yield reproducible results, clinical research involves more heterogeneous populations, often is more expensive, takes longer to develop, requires long periods of time for data collection, and may be difficult to reproduce (Kimes et al., 1991). To advance medical care in patients, however, research must be performed in populations of patients with diseases.
Many research activities performed by a broad spectrum of professionals fall under the rubric of clinical research. Whereas many kinds of clinical research require similar skills and abilities, others may require different tools to achieve research objectives. Examples of how earlier investigations have influenced today's medical care are well known. Present research studies will improve tomorrow's medical practice, while future clinical research opportunities will affect care in the twenty-first century. What is the scope of clinical research, and what are the settings for conducting clinical research and the opportunities for future research?
SCOPE OF CLINICAL RESEARCH
Clinical research is a relatively new discipline. Although the American Society for Clinical Investigation was formed in 1908, clinical advances prior to the 1950s were often based on imprecise observations by practicing clinicians
(Cadman, 1994; Fox et al., 1992). In 1948 the British published the first randomized clinical trial, evaluating streptomycin in the treatment of malaria (Medical Research Council, 1948); the first clinical trial published in the United States was a study evaluating the effectiveness of penicillin for treating pneumoccocal pneumonia (Austrian et al., 1951). As methods for large-scale clinical studies became more refined, investigators gained an appreciation for new study designs, methodological advances, and the power of statistical analysis that permitted the validation of small differences between treatment regimens. Clinical research quickly became accepted as scholarly work and as an academic discipline (Fye, 1991; Ledley, 1991).
A major paradigm shift in clinical research was initiated in the 1970s when human cells were grown in vitro. As a result, some forms of clinical research could be performed on human cell lines grown in culture. This initiated a period some refer to as reductionism in which patients were no longer used as the primary focus of clinical research. This idea was extended by using the techniques of molecular biology, which permitted the study of human nucleic acid alterations in disease instead of requiring the study of the entire patient. Yet, in the final analysis, the application of these discoveries to improve medical care requires that these findings be used on the whole patient.
In parallel, during the last decade the discipline of clinical research has undergone a remarkable evolution in the scope, sophistication, and power of its methodologies. Changes have occurred in the approach to data collection, experimental design, and data analysis, and these changes provide a stronger basis for clinical research. In addition, understanding of the pathogenesis of diseases has provided more precise concepts of preclinical and subclinical disease states. The application of molecular epidemiology is a prime example of these changes in clinical research. Now the results of new biology are ripe for application to improve medical care, but many fear that a talented cohort of clinical investigators has not been prepared to translate these fundamental advances into improved medical care.
The revolution of fundamental research discovery is expected to accelerate in the future, driven by the explosion of science in biotechnology, molecular biology, computer technology, diagnostic systems, decision modeling, and clinical measurements technology. The sophisticated methods for clinical research require investigators with the requisite talents to design excellent clinical studies, recruit adequate numbers of research subjects, and analyze the large amounts of data collected. The need for cross-disciplinary teams to accomplish the objectives of multicenter, complex clinical trials is readily apparent. It is clear that training for a career in clinical research must be as rigorous as training for a career in the traditional basic sciences. Understanding of both the basic sciences and the evaluative sciences is essential to the success of clinical researchers. Moreover, novice clinical investigators require the same mentoring and nurturing in a supportive environment as those engaged in fundamental
research disciplines if they are to develop into mature, independent scientists who remain competitive and productive over an extended period of time.
Numerous advances can be cited to describe opportunities in clinical research; the following allow one to comprehend their broad scope. One dramatic example of progress in fundamental research that has opened up immeasurable clinical research opportunities is the discovery in 1989 of the gene that is mutated in patients with cystic fibrosis. The gene was identified by using the advanced methodologies of positional gene mapping. Investigators delineated the nature of the mutation that leads to the production of a defective protein in the membranes of cells from patients with cystic fibrosis patients. Subsequent research demonstrated that this protein is associated with a membrane channel involved in the transport of chloride ions. This understanding has led to a number of chemical approaches to treat the disease. In addition, new efforts are under way to treat or cure the pulmonary manifestations of the disease by employing methods that are being developed in DNA transfer therapy. Research that is now being conducted in the laboratory will soon be carried over to use in patients with cystic fibrosis. Clinical investigators are crucial to the performance of this work and in bringing these novel therapies into common practice. Their participation will also be necessary to help determine how to deliver the technology efficiently, under what conditions and to which patients, and to assess the outcomes of these new therapies.
Hundreds, if not thousands, of other genetic disease are now being studied in the same fashion. As knowledge about the underlying genetic mechanisms for these diseases grows, new treatment approaches developed from basic laboratory techniques will be carried forward into clinical trials. In addition, genetic factors are being defined in diseases that have been regarded as multifactorial. For example, breast cancer scientists are on the threshold of discovering the genes that regulate its occurrence. Thus, approaches to modify the expression of these genes may be useful in the treatment of breast cancer. Genes that regulate the formation of atherogenic lesions in arteries, abnormalities that lead to coronary artery disease and heart disease have recently been identified. Blocking the activities of these genes using antisense gene therapy has been shown to block the progression of atherogenic lesions in arteries in animal models. On the basis of results of these promising studies, antisense gene therapies are being developed for use in humans. Novel therapies directed at blocking the genetic expression of the factors that determine at herogenesis as well as genetically directed products that can prevent or reverse these effects may be developed in the future and may lead to treatments or cures for ischemic heart disease and some forms of stroke. Clearly, clinical investigators will be critical for developing and testing these new therapies to determine their safety, efficacy, effectiveness, and cost-effectiveness in humans. Clinical investigators will also play a role in discussions regarding ethical considerations such as genetic testing and elucidating the behavioral or environmental factors influencing genetic diseases.
Although new therapies are being developed rapidly and require extensive clinical testing, old or current therapies should be rigorously evaluated as well. During the past few years several groups have initiated studies to examine the outcomes of current therapies for particular diseases or conditions (Eddy, 1984; Roper et al., 1984). For example, a broad-based research team has been investigating the treatment for benign prostate hypertrophy in a patient population in Maine (Wennberg et al., 1988). By taking into consideration the behavioral and social attributes of patients, the outcomes of the various treatments have been assessed. Not all treatment regimens are viewed favorably by patients, who have various needs and desired outcomes. Thus, the outcomes of particular therapies require sophisticated scientific methods to determine the effectiveness of therapy in patients with different expectations and needs. Other examples of opportunities for outcomes research can be cited by examining the topics under investigation by the Patient Outcomes Research Teams funded by the Agency for Health Care Policy and Research, such as low back pain, joint replacement, incontinence, and others. The research methods and tools used by those investigators are every bit as sophisticated as those needed to clone genes or isolate and characterize proteins. Similar studies in other fields of medical practice using these novel methodologies will be critical in the future.
The diversity of the preceding examples is a small sampling from a field rich in opportunity for improving medical care for millions of people in this country and around the world. An important interface in bringing these technologies to patients is the clinical investigator—the bridging scientist. The remarkable progress that has been evidenced in fundamental biology brings with it parallel opportunities for investigations in human populations. The realm of biomedical research can be viewed as a spectrum, with fundamental research occurring throughout the spectrum, some of which uses humans to answer crucial questions about human health and behavior. Thus, there is no discontinuity between fundamental biological science and clinical investigation. Indeed, it is progress throughout this research spectrum that frames the opportunities for progress in clinical research.
Increasing levels of sophistication and the assurance of an ample supply of excellent clinical investigators to carry technological advances to medical practice remain critical issues if the country is to continue to improve its health care system. There is growing evidence, however, of a discontinuity in the process of translating new research discoveries into improved health care; the process is further threatened by a potential lack of well-trained clinical investigators to provide the bridge to bring these discoveries into improved medical care (Kelley, 1988).
In the 20 years following World War II, bountiful resources were provided by the federal government to support research, primarily at the nation's research universities and medical schools (U.S. Department of Health, Education, and Welfare, Public Health Service, 1976). This paradigm of peer-reviewed,
university-based research has been attributed to the wisdom and foresight of Vannevar Bush (Bush, 1945). Resources were not only plentiful for supporting research but numerous programs were also initiated to build the physical research infrastructure and train more highly talented scientists (Institute of Medicine, 1990). The biomedical research community responded, and the nation's health research capacity expanded significantly. During this period research that involved interactions with human subjects, possibly with the exception of psychological studies, was primarily the domain of physician-scientists. Many of these physician-scientists were motivated to pursue research careers because of the rapid advances in biomedicine and the potential to become critical players in medical discovery. Others may have pursued research to avoid military service in an unpopular war in Southeast Asia. Nonetheless, after completing their clinical training residencies, many physicians sought fellowships at the National Institutes of Health (NIH) and subsequently moved into academic and research positions around the country. Whatever their motivation, most of these scientists have contributed to the fount of knowledge that serves as the basis of modern health care.
In the late 1970s and early 1980s, many leaders in the medical research community expressed concern about a perceived decline in the participation rates of physicians engaged in all aspects of biomedical research (DiBona, 1979; Gill, 1984; Kelley, 1980 and 1985; Thier et al., 1980; Wyngaarden, 1979). This perception was supported by data demonstrating that the ratio of M.D.s to Ph.D.s successfully obtaining research grant awards from NIH was declining. More alarming was the notion that individuals who were highly trained in patient care and who were considered the technology transfer agents were not seeking rigorous scientific training, which widened the gap between basic research discoveries and application of these advances to improved health care (Glickman 1985; Healy, 1988). Furthermore, although some physicians were seeking training in the basic biological sciences, there was a perception that few were being trained to develop and test hypotheses in human subjects or populations (Forrest, 1980). Ironically, data show that the number of full-time faculty in medical schools has grown by more than 20,000 over the past decade, to nearly 65,000. (Data from the Association of American Medical Schools report that medical school faculty totaled 65,000 in 1990, whereas data collected for the Liaison Committee for Medical Education reports that faculty totals were nearly 80,000.) It has been hypothesized that this growth reflects a growing dependence on medical center profits to offset increasing constraints on research funds and shrinking subsidies for graduate medical education (Chin, 1985; Hughes et al., 1991). Although faculty members are required to perform scholarly activity, there appears to be an increasing demand on the clinical faculty to derive revenue through patient care. Furthermore, the growth in clinical faculty may have increased tensions between the faculty in basic science departments and those in the clinical departments. These tensions may arise because basic science faculty fear that their research
funding base is being eroded by growing research activities in the clinical departments, and the growing number of clinical faculty bringing in patient care dollars positions the latter on a firmer financial footing. There is also a perception that some academic clinicians pursue research as a secondary interest and are not serious investigators. Many of these clinicians also feel that they cannot obtain tenure by performing human subject research, where the results may not be realized for many years and funding is believed to be extremely difficult to obtain. Moreover, those clinicians who focus on human research fear that they are perceived as second-rate scientists by their colleagues who perform fundamental research in both clinical and basic science departments.
A cause and effect has been difficult, if not impossible, to prove. Determining the size of the cohort of clinical investigators is fraught with error, because no database currently exists to track these investigators. Moreover, there has been no systematic way to collect and analyze data on the number of individuals who choose to perform clinical investigations, the availability of training pathways, or the outcomes of those few programs that do exist. Although many believe that quantitative factors such as debt and economic status directly influence decisions to pursue academic and research careers, there appear to be no measures for factoring in personal considerations such as the effects of mentors and role models, the desire to spend time with one's family, or having leisure time to pursue other personal interests. The growing base of fundamental science, the increasing complexity of medical care and understanding outcomes or effectiveness research, the difficulty (real or perceived) in obtaining research funding, and countless demands on an investigator's time all seem to weigh heavily against pursuing a career in research, particularly research that involves interactions with human subjects. The many employment sectors that require this expertise, such as federal agencies and industry, are also obstacles to conducting a thorough analysis.
Although most attention has been focused on the plight of physician-scientists, many other professional groups are experiencing similar difficulties in the area of human research. As in medicine, training for research careers in other professions is often fragmented, and the career pathways that young trainees should pursue are not clearly delineated. Although many of these other professions also provide outstanding training for delivering care, their programs may not be specifically structured for developing research capabilities. Thus, the Institute of Medicine (IOM) sought to undertake an analysis of the problems affecting the career paths leading to clinical research.
ORIGINS OF THE STUDY
IOM has had an ongoing concern about the problems in the biomedical research arena, and particularly those problems confronting researchers who
perform studies that require human subject participation. In 1988, IOM was commissioned by NIH to conduct a study to assess the availability of resources for performing research using patients. The committee was asked to consider a series of issues, including the effects of changes in the health care system on the environment for clinical research; how to improve the recruitment of medical students and residents into clinical research careers; identification of barriers to translating basic research advances into clinical practice; how to improve the relationships among clinical researchers, federal sponsors, and industry; the organization of clinical research; and how to stimulate interest in evaluative clinical sciences. Whereas that committee was asked to examine clinical research in the narrow sense of human subject research, the data from NIH that were available to the committee included all research on humans or human materials approved by institutional review boards, as indicated on Public Health Service grant application form number 398. This included research on all human material such as DNA, RNA, proteins, cells, or body fluids for in use in vitro studies, not necessarily material related to a patient's disease or involving the patient. Moreover, the committee was not able to glean any information from the private sector, either for-profit or nonprofit, to construct a complete picture of the resource base for patient-oriented clinical research.
Following the release of its report, Resources for Clinical Investigation (Institute of Medicine, 1988), the IOM Board on Health Sciences Policy convened a working group to reexamine issues related to clinical research. The working group recognized that the heterogeneous nature of the research training pathways for physician-scientists and the broad spectrum of research questions pursued by those investigators had complicated earlier analyses. The working group met twice to develop a strategy for exploring problems associated with the clinical research training pathways, particularly for physician-scientists. The working group sought to refine an approach that would isolate only the small portion of physician-scientist training that it felt was in a particularly vulnerable stage—patient-oriented clinical research—and did not attempt to address all the problems associated with physicians engaging in basic or health services research.
In December 1989, the National Research Council released the quadrennial report Biomedical and Behavioral Research Scientists: Their Training and Supply (National Research Council, 1989), which examined research training supported by the Public Health Service through National Research Service Awards (NRSAs). Although that report presented a detailed analysis of the doctoral biomedical and behavioral research workforce and recommended the numbers of NRSA trainees that should be supported, it paid scant attention to physician-scientists and largely ignored dentist- and nurse-scientists. The reasons for these omissions remain unclear, but they probably are the result of the inability to develop clearly defined populations of scientists in these professions. Whereas physicians, dentists, and nurses engage in a broad spectrum of fundamental research activities, they are critical players in clinical research. Although this
group of scientists has often been referred to as clinical researchers because of their clinical degrees, they might be more appropriately referred to as clinician-researchers. Furthermore, the population of doctoral scientists engaged in human research also has remained undefined.
Following the release of the 1989 NRSA study, IOM's Committee on Policies for Allocating Health Sciences Research Funds released a report in 1990, Funding Health Sciences Research: A Strategy to Restore Balance (Institute of Medicine, 1990). That committee also acknowledged that the limited understanding of the physician-scientist population and barriers to effective training of that population hampered the committee's attempts to recommend ways to overcome the barriers confronting those investigators. Thus, they recommended that a thorough analysis be performed on physician-scientists to clarify many of these issues.
CHARGE TO THE COMMITTEE
The Committee on Addressing Career Paths for Clinical Research was formed in 1991 and was charged with identifying and evaluating issues in the education and training pathways for individuals pursuing careers in clinical investigation. In particular, the committee was asked to investigate ways to improve the quality of training for clinical investigators and to delineate pathways for individuals pursuing careers in clinical investigation in nursing, dentistry, medicine, and other related health professions engaged in human research. The committee was charged with the following: defining clinical research, how to stimulate individuals to pursue careers in clinical investigation, how to define appropriate curricula for training, how to identify mechanisms to bridge the gap between the basic and clinical sciences, how to address funding mechanisms for clinical investigation, how to establish measures of success in clinical research other than obtaining R01 grant support, how to encourage academic and industrial institutions to protect and reward these valuable investigators, and how to ensure adequate support mechanisms for retaining clinical researchers. For comparison, the committee also examined the pathways that lead physicians toward careers in basic research. The study focused on how existing structures and mechanisms in the federal government, universities, and industry might be used in new and innovative ways to foster careers for these groups of researchers.
The chair of the National Research Council appointed a 16-member committee to address the questions posed in the committee's charge. The committee was composed of active researchers and research administrators with expertise in nursing, dentistry, evaluative clinical sciences, surgery, epidemiology, and various medical subspecialties. The committee viewed several areas as deserving special attention, and these were addressed by task forces,
including task forces in surgery, dentistry, nursing, and clinical psychology. The complete task force reports are included as appendixes to this report.
DEFINING CLINICAL RESEARCH
The first item on the committee's agenda was to derive a working definition of clinical research. Various definitions have been used to describe or inventory research and development activities. Many lexicons classify research and development expenditures into the following three general categories: (1) basic research, (2) applied research, and (3) development. Although this classification scheme is useful for describing various research activities for budgetary purposes, it becomes less appropriate for describing cross-disciplinary clinical research, which may encompass portions of each of these categories.
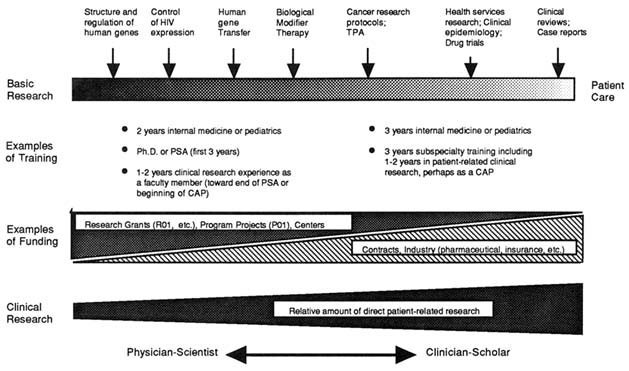
Classification schemes often portray a linear progression of scientific knowledge from basic biological research, to applied research and development, and to improved diagnosis, treatment, and prevention of human disease. Many would argue, however, that a broad spectrum of research activities, from the most basic discoveries of nature to the application of knowledge in humans to understand and treat disease, would more accurately portray biomedical research (Figure 1-1). Furthermore, research activity throughout the spectrum could be bidirectional or demonstrate circular feedback loops for generating new hypotheses. For example, many basic biomedical research questions arise from disease processes first observed in patients. Moreover, the boundaries between many of these subcategories are indistinct, with varying degrees of overlap and movement over time.
Several clinical research classification methodologies have been attempted, each with its own limitations. Clinical research encompasses a vast range of research activities that are conducted by investigators in numerous disciplines. Ahrens has categorized the disparate activities encompassed under the rubric of clinical research into the following seven areas (Ahrens, 1992, pp. 40-48):
-
Studies on the mechanisms of human disease
-
refinements in characterizations of disease processes
-
explorations of unresolved questions in human biology
-
-
Studies on management of disease
-
evaluations of new diagnostic and therapeutic techniques and devices
-
drug trials (phases II, III, IV)
-
studies of patient compliance and prevention measures
-
searches for accurate prognostic markers
-
-
In vitro studies on materials of human origin
-
Animal models of human health or disease
-
Field surveys
-
Development of new technologies
-
Assessment of health care delivery.
All seven categories of research are essential to the progress of medical care and, ultimately, to the prevention of disease. Because the boundaries between these areas are indistinct, individuals can be working in more than one category at any given time.
The committee sought to derive a definition of clinical research that would cut across artificial boundaries to describe the universe of clinical research in terms of research activities or goals. Although there is a large amount of basic biological research that is not directly relevant to specific human diseases, such laboratory-based preclinical bench research may have direct links to understanding normal human function and disease. For example, control of human or retroviral gene expression as well as animal or cellular models of normal or diseased biological processes in humans is often clinically relevant, and under some classification schemes it is defined as clinical research.
At the other end of the spectrum is research on human subjects and populations that have direct application for understanding the prevention, diagnosis, and treatment of human disease by exploiting disciplines such as health services research, clinical epidemiology, and outcomes assessment. Undoubtedly, clinical research includes phase I-III human clinical trials to assess the effectiveness of new methods of intervention or patient management in defined populations. A body of research is also directed at understanding motivational factors for disease prevention and screening and the social and emotional impacts of disease and treatment by employing the disciplines of psychosocial, behavioral, and educational research, which can be considered clinical research. Thus, the committee agreed that there is a continuum of research spanning a wide range of activities that can be regarded as clinical research.
The committee emphasizes that clinical research is not simply that research performed by physicians or other professionals holding clinical degrees. Clearly, many scientists holding doctorates in the basic sciences are performing research that is very clinical in nature; many physicians are also outstanding basic scientists. Although it is very difficult to arrive at an unambiguous definition that will be agreeable to all parties, the committee believes that clinical research should be directed toward the elucidation of human biology and disease, and the control thereof.
The committee emphasized that a common definition should be as broad and inclusive as possible to accurately reflect the population of biomedical scientists generating knowledge about human ''disease." Furthermore, the committee acknowledges that clinical researchers may be performing research in more than one category; they may move back and forth along the spectrum as their line(s) of investigation matures or new research questions evolve. Thus, the committee proposes the following definition:
Clinical investigation, broadly defined, includes all studies intended to produce knowledge valuable to understanding the prevention, diagnosis, prognosis, treatment, or cure of human disease. This includes biomedical and health services research carried out in humans, usually by health care professionals, as well as research in organs, tissues, cells, subcellular elements, proteins, and genes derived from humans. It may also include the study of micro-organisms as well as studies of other members of the animal kingdom when this research is directed toward human disease.
Whereas this definition is suitably inclusive for all the researchers engaged in clinical research in the broadest sense, the committee identified specific areas that it believes need particular attention. The evolution of the new biology has begun to erode the perceived boundaries among the various medical disciplines as well as the boundaries between basic and clinical research. Moreover, the importance of basic research or other training experiences for teaching research methodology and study design to young clinical investigators cannot be overstated. Thus, the committee felt compelled to develop a broad definition for clinical research and then to focus on areas that it believes need immediate remediation to foster continued progress in clinical research. The special theme and focus of this study was patient-oriented clinical research, defined as that which requires "hands-on" participation with a human subject as opposed to the entire spectrum of clinical research. Interpreting its charge, the committee recognized that many professions are engaged in clinical research, including dentistry, nursing, pharmacy, osteopathic medicine, and the behavioral sciences, among others, and sought to include the perspectives of members of those professions as well. Nevertheless, the committee reinforced the common theme of the study and posed the following global questions about clinical research and the clinical research workforce:
-
What can clinical research accomplish now and in the future to improve medical care?
-
Is the current clinical research community poised and prepared to accomplish these goals?
-
If the clinical research community is not prepared to accomplish these goals, what is the evidence that there is either inadequate clinical investigation or an inadequate number of well-trained clinical investigators to meet this need?
-
What are the best approaches or best vehicles for change to improveclinical investigation and ensure a supply of highly competent clinicalinvestigators to meet these needs and accomplish the research goals?
LIMITS ON THE SCOPE OF THE STUDY
Although the committee developed a broad definition of clinical research, the major focus of the study was clinical research in which patients serve as the research subjects, often referred to as patient-oriented, patient-related, or preferably, human research. This category of clinical research includes research activities such as the characterization of healthy and diseased human function; evaluation of new diagnostic, therapeutic, and prognostic techniques, approaches, and devices; medical decision making; patient compliance and disease prevention research; health education research; drug trials; and the assessment of health care practices on patient populations. Thus, the committee's deliberations focused on the issues surrounding the preparation and training of clinical researchers who are engaged in research that requires the direct participation of human subjects. Lastly, although the committee frequently mentions areas of potential clinical research opportunities, it was not charged with developing a research agenda in clinical research and uses the examples only for reference.
CONDUCT OF THE STUDY
During the course of the study, the committee held four meetings to develop strategies and to analyze data. The committee used a variety of approaches to expand its expertise by involving as many avenues of input as possible to achieve its objectives, including four subcommittees, three task forces, a workshop, 11 commissioned papers, and information gained through solicitations of written input and interviews.
Subcommittees
First, the committee divided its members into the following four subcommittees to identify problems along the career pathways of clinical researchers: (1) undergraduate and precollege science education and research training, (2) research training during health professional school, (3) postdoctoral clinical research training, and (4) nurturing clinical research faculty. These subcommittees were convened separately to identify issues confronting their respective portions of the pathways and to develop approaches to collecting and analyzing data that could be used to draw conclusions.
Task Forces
Three task forces were convened in the spring of 1992 to address clinical research issues specific to (1) nursing and clinical psychology, (2) dentistry, and (3) surgery. Each of these task forces was chaired by a member of the committee and the membership was selected from those in the profession. They were charged with the following:
-
Describe the clinical research performed by researchers in their respective professions and emphasize how it is different from that in other professions.
-
Determine how many researchers in their profession are engaging in clinical research and estimate how many are needed.
-
Identify the barriers to careers in clinical research in their profession, including the following:
-
Identify what needs to be enhanced or changed to encourage recruitment and the retention of clinical researchers in the profession.
-
Identify the funding sources for clinical research in their profession.
-
-
Assess research training for clinical research in their profession.
-
Explore the training backgrounds of the current cohort of clinical researchers in the profession.
-
Identify the education and training requirements for preparing clinical researchers in the profession.
-
Recommend changes necessary to address new clinical research questions for the profession in the future.
-
Describe how changes can be implemented or interwoven into existing organizational structures.
-
Identify the research training resources for individuals pursuing careers in clinical research for the profession.
-
Recommend possible solutions to improving the career pathways leading to clinical research.
The complete task force reports can be found in Appendixes A, B, and C at the end of this report.
Workshop
In June 1992 the committee sponsored a one-and-one-half-day workshop entitled "Clinical Research and Research Training: Spotlight on Funding." The overall goal of the workshop was to analyze training and research funding data and to explore innovative approaches to the training and support of clinical investigators. The first day of the workshop focused on the roles and responsibilities of research sponsors including the federal government, industry,
the private nonprofit sector, third-party payers, and academic health centers and research institutions. The second day concentrated on the organizational barriers to clinical research training as well as the funding available for training. A transcript of the meeting was made for the use of the committee in preparing this report, but the committee chose not to publish a separate workshop proceedings.
Commissioned Papers
The committee commissioned 11 background papers to analyze topics of particular importance to the committee's deliberations. Although the findings of the papers are incorporated into this report, the committee felt that the papers were of such high-quality and made such significant contributions toward a better understanding of clinical research careers that they encouraged the authors to publish them separately. The following is a list of the paper titles and authors:
-
"Early Exposure to Research: Opportunities and Effects," by Marsha Lake Matyas of the American Association for the Advancement of Science.
-
"Advisers, Mentors, and Role Models in Graduate and Professional Education: Implications for the Recruitment, Training, and Retention of Physician-Investigators," by Judith P. Swazey of the Acadia Institute.
-
"The Effectiveness of Federally Supported Research Training in Preparing Clinical Investigators: Important Questions but Few Answers," by Georgine Pion of Vanderbilt University.
-
"Considerations of Educational Debt and the Selection of Clinical Research Careers," by Robert L. Beran of the Association of American Medical Colleges.
-
"Models of Postdoctoral Training for Clinical Research," by Thomas Lee and Lee Goldman of the Brigham and Women's Hospital.
-
"Models of Postdoctoral Training for Clinical Research," by David Atkins, Richard A. Deyo, Richard K. Albert, Donald J. Sherrard, and Thomas S. Inui of the University of Washington.
-
"Role of the GCRC in Establishing Career Paths in Clinical Research," by Charles Pak of the University of Texas Health Science Center.
-
"The Image of the Clinical Investigator," by Edwin Cadman of Yale University.
-
"University-Industry Relationships in Clinical Research: University Perspective," by David A. Blake of Johns Hopkins University.
-
"Roles and Responsibilities of Resident Review Committees and Certification Boards in Promoting Research Careers," by Linda Blank of the American Board of Internal Medicine.
-
"Clinical Research in Allied Health," by Leopold G. Selker of the University of Illinois.
Grants Analysis
The committee also undertook a detailed analysis of R01 grant awards that have been approved by institutional review boards to determine the fraction of awards that are truly patient-oriented, apart from those that use human materials or body fluids. Because the R01 pool represents about 55 percent of the total extramural funds awarded by NIH and because of the large time commitment required to read through grant files, the committee chose to limit the analysis to R01-type grant awards that were considered by initial review groups (study sections) in the Division of Research Grants (DRG). Of the more than 16,000 R01 grants active in fiscal year 1991, about 14,535 were reviewed by DRG study sections; of those, about 4,284 indicated the involvement of human subjects or materials. Of this 4,284, a random sample of 450 from 11 institutes was used for this analysis. The committee reviewed grants provided by the National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute of Deafness and Communicative Disorders, National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institute of Child and Human Development, National Institute of Neurological Diseases and Stroke, National Institute of Allergy and Infectious Diseases, National Institute of Aging, National Institute of General Medical Sciences, National Institute of Diabetes, Digestive and Kidney Diseases, and National Eye Institute. Since the committee convened task forces on nursing and dentistry that evaluated grants in these disciplines, it did not include grants from the National Institute of Nursing Research (formerly the National Center for Nursing Research at the time the task force was convened) or the National Institute for Dental Research in the analysis. Furthermore, because National Institute of Mental Health, National Institute of Drug Abuse, and National Institute of Alcohol and Alcohol Abuse were not officially part of NIH at the start of this project and the transfer of these institutes was not assured in mid-June of 1992, grants from these institutes also were not included in the analysis. The results of this analysis are presented in Chapter 3.
To supplement the information gleaned from each of the aforementioned mechanisms and to add breadth to the material available to the committee, IOM staff undertook several interviews of staff in various federal agencies, including NIH, the Food and Drug Administration, the Agency for Health Care Policy and Research, and Alcohol Drug Abuse and Mental Health Administration. Many of the data for the study came from NIH staff, to whom the committee is truly indebted. Because of the broad nature of the study, many sectors, public as well as private, contributed valuable information. Appendix E recognizes the many individuals who made important contributions to the report and are not cited elsewhere.
STRUCTURE OF THE REPORT
This report presents the findings from all the aforementioned methods of data collection and analysis. The following chapters elaborate on the issues the committee explored, presents its findings and conclusions, and offers its recommendations for improving clinical research career pathways. Chapter 2 examines the employment sectors and issues and obstacles confronting established clinical investigators, with an emphasis on academic clinical investigators. Chapter 3 discusses the available resources for funding clinical research. The obstacles and barriers to training pathways are presented in Chapter 4. Chapter 5 explores the academic-industry relationships and the roles and responsibilities of investigators in these alliances.