5
MICROWAVE APPLICATIONS
INTRODUCTION
Due in large part to the overwhelming success of microwave ovens for home use, microwave processing is seen by the unwary as a panacea for all heating applications. Microwave energy is perceived to provide a means for rapid, even heating, improved processing efficiencies, and heretofore unobtainable materials properties. However, as previous sections of this report have shown, not all materials and processes are amenable to microwave processing. Even for materials and processes where microwave heating is technically an option, additional technical and economic considerations must be evaluated, on a case-by-case basis, to determine whether it is the best alternative. This chapter provides examples of work accomplished in applications of microwaves in materials processing. The observations made in previous chapters on equipment selection, process design and evaluation, and application criteria will be amplified through the examples given.
Microwave energy has found general, commercial application in very few areas. These include food processing, analytical chemistry, and heating and vulcanization of rubber. Food processing and rubber manufacture involve relatively high-volume, continuous processing. Analytical chemistry applications are broad in scope and involve high-volume, repetitive, batch processing, often with long intermediate drying and reaction steps that can be shortened using microwave heating.
Much work has been undertaken to investigate the use of microwaves for the processing of a wide range of materials, including ceramics, polymers, composites (ceramic and polymer matrix), powders, and minerals. Microwaves have also been investigated in a broad range of plasma processes (surface modification, chemical vapor infiltration, powder processing), chemical synthesis and processing, and waste remediation. Despite the considerable effort that has been expended in microwave process development, there has been little industrial application to date, with most of the effort still in the laboratory stage. Some of the more significant problems that have inhibited industrial application of microwave processing include:
-
the cost of equipment;
-
limited applicability;
-
variation in dielectric properties with temperature; and
-
the inherent inefficiency of electric power.
Much of this work has been undertaken without the initial cross-disciplinary evaluation and processing system design approach emphasized in this report. Discussion of these results in light of this multidisciplinary approach will serve to highlight the limitations in terms of capabilities and scaling and will lead to identification of promising processes and needed research.
A broad range of applications will be discussed. Much work has been accomplished on ceramic, polymer, and plasma processing, and the lessons that can be learned from this work will help to identify promising applications for future development and will help processors avoid possible pitfalls. Emerging and innovative applications in microwave chemistry, minerals processing, and waste remediation are also reviewed.
CERAMICS/CERAMIC MATRIX COMPOSITES
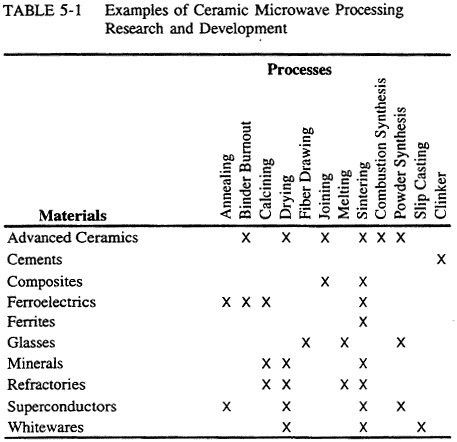
The use of microwave energy for processing ceramics and ceramic matrix composites has been the subject of a large amount of exploratory research. The range of materials and processes that have been investigated is shown in Table 5-1.
TABLE 5-1 Examples of Ceramic Microwave Processing Research and Development
 |
The potential advantages of microwave processes over conventional processes for ceramic processing include reduced processing time, improved product uniformity and yields, improved or unique microstructure, and the ability to synthesize new materials (Sutton, 1989). A number of review articles on ceramic microwave applications have been published (Sutton, 1989, 1993). Rather than providing a broad review of ceramic processes, this section will examine two important processing areas——sintering and powder processing——in light of the perceived advantages described above, comment on lessons to be learned from previous work in these areas, and suggest promising applications for the future. Microwave sintering is of interest because of the extensive exploratory work accomplished and because of the broad range of ceramic materials that have been investigated. Microwave processing of ceramic powders is a relatively new area with promise of broad applications in synthesis and processing.
Sintering
Microwave heating has been touted as a means of sintering ceramics since the early 1970s. Microwave sintering of a number of oxides and nonoxide ceramics ranging from low-loss materials like Al2O3 to relatively high-loss materials such as SiC, TiB2, and B4C has been reported. The perceived advantages of microwave sintering over conventional sintering include expectations for more-uniform heating, better properties of the product, greater throughput with resulting smaller plant size, and greater energy efficiency. It is generally assumed that since microwave energy is deposited in the bulk, significantly less time is required to heat the part to the sintering temperature than would be required to diffuse the heat from the exterior, particularly for large parts or large batches of small parts. The resulting rapid sintering may lead to smaller grain size at a given density, with consequently better mechanical properties. Although some advantageous application of microwave processing in sintering has been demonstrated, the perceived potential of the technology has gone largely unrealized on a production scale.
Oxides
Berteaud and Badot (1976) investigated the sintering of alumina and zirconia and the melting of silica at 2.45 GHz in a rectangular single-mode cavity. They recognized many of the potential advantages of microwave sintering, including high thermal efficiency as well as rapid processing, and also discovered many of the problems that have plagued the process, including difficulty in temperature measurement due to temperature gradients and the propensity for thermal runaway. Colomban and Badot (1978, 1979) investigated the sintering of β-alumina, again in a single-mode cavity, where they observed rapid sintering but not the expected small grain size.
Microwave sintering of alumina sparkplug insulators was investigated with goals to replace large (50-m long), gas-fired line kilns with significantly smaller equipment and to reduce process cycle-time (Schubring, 1983). Microwave sintering was found to be feasible, with cycle time reduction from 24 hours to 3—6 hours. The energy consumption was half that for gas
firing, but the energy costs were higher for microwave heating because of the relative costs of gas versus electric energy. Although part to part density variations were observed in the 186-part sagger, acceptable properties were obtained. A similar study of the sintering of ferrites resulted in similar conclusions regarding feasibility (Krage, 1981). However, neither process was carried to practice.
Large castable refractory crucibles have been successfully sintered in a microwave cavity (Sutton, 1988). Firing times were significantly reduced compared with conventional heating, because the penetration depth of microwaves allowed even heating throughout the thickness of the rather large sections involved.
Ultra-rapid sintering of β-alumina, Al2O3, and Al2O3/TiC rods and thin-walled tubes using a rapid pass-through, zone-sintering process in single-mode applicators, has been investigated (Johnson, 1991). Isostatically pressed rods, 4 mm in diameter, of β-alumina powder were sintered at specimen translation rates of up to 40 mm/min, with the final density independent of translation rate. The time from onset of heating to final density was on the order of 30 s at the highest translation rate. Attempts to sinter thin-wall β-alumina with a diameter of 15 mm tubes failed, because a small region of the tube would become hot and remain hot to the exclusion of the rest of the specimen, even though the tube was rotated in the cavity. The size of the spot, on the order of several millimeters in diameter, was sensitive to the power applied, was stable in time, and did not propagate around the circumference of the tube.
A single-mode cavity was used to sinter α-alumina rods with a diameter of 4 mm to high density and fine grain size (99.8 percent dense and 2 μm, respectively; Tian et al., 1988a). To avoid thermal runaway, applied power had to be carefully controlled as the sintering temperature was approached. Stable heating, again with high densities and fine grain size, was also observed in sintering Al2O3/TiC rods with a diameter of 4 mm (Tian et al., 1988b). Thermal runaway was avoided if the concentration of TiC was greater than about 20 percent by weight.
Of several reported attempts to sinter Al2O3 in multimode cavities, the experiments of Patterson et al. (1991) were among the most successful. Single and multiple specimens (19 mm diameter by 16 mm long) were sintered to greater than 98 percent density with three different alumina powders. If the heating rate was too high, nonuniform grain sizes resulted, with the largest grains in the center of the specimen. A 60-minute firing cycle that resulted in uniform grain sizes was developed. Few details about procedures, thermal insulation, or the oven configuration were given.
Sintering of a few other oxide materials with varying degrees of success has been reported. Some of these reports are listed in Table 5-2. In most cases, the procedures were not described well enough for the committee to determine whether sintering enhancement was observed.
Nonoxides
B4C and TiB2 have been successfully heated to very high temperatures using granular Y2O3 as the microwave-transparent primary insulation system (Holcombe and Dykes, 1991a, b). Increased density and improved mechanical properties of microwave-sintered B4C were reported
TABLE 5-2 Selected Microwave Sintering reports.
|
Material |
Insulation |
Coupling |
Reference |
|
Al2O3 |
None |
Self |
(Tian et al., 1988a) |
|
Al2O3 |
Al2O3 fiber |
Hybrid (insulation + self) |
(Patil et al., 1991) |
|
Al2O3 |
Un-named fiber |
Hybrid (SiC liner) |
(Dé et al., 1991b, c) |
|
Al2O3 |
Not given |
Not given |
(Patterson et al., 1991) |
|
Al2O3 |
Un-named fiber |
Hybrid (tubular receptors) |
(Brandon et al., 1992) |
|
Al2O3/MgO |
Al2O3-SiO2 fiber |
Not given |
(Cheng et al., 1992) |
|
Al2O3-TiC |
None |
Self |
(Tian et al., 1958b) |
|
Al2O3-ZrO4 |
Al2O3 and/or ZrO2 |
Hybrid (picket fence, 2.45 GHz); self at 28 GHz |
(Kimrey et al., 1991) |
|
Al2O3-ZrO4 |
Un-named fiber |
Hybrid (tubular susceptors) |
(Brandon et al., 1992) |
|
ß-alumina |
None |
Self |
(Johnson, 1991) |
|
B4C |
Y2O3 grain |
Self |
(Holcombe and Dykes, 1991a) |
|
BaTiO3 |
Y2O3 fiber and powder |
Probably self |
(Lauf et al., 1992) |
|
Hydroxyapatite |
Zr2O3 fiber |
Not given |
(Agrawal et al., 1992) |
|
LaCrO3 |
|
Self |
(Janney and Kimrey, 1992) |
|
Si3N4 |
ZrO2 and Safil fibers |
Hybrid (powder bed) |
(Patterson et al., 1992a) |
|
Si3N4 |
Not given |
Not given |
(Zhang et al., 1992) |
|
TiB2 |
Y grain |
Self |
(Holcombe and Dykes, 1991b) |
|
TiO2 nanophase |
ZrO2 fiber |
Not given |
(Eastman et al., 1991) |
|
YBa2Cu3Ox |
Al2O3-SiO2 |
Hybrid (SiC liner) |
(Ozzi et al., 1991) |
|
ZnO varistor |
Not given |
Probably self |
(Levinson et al., 1992; McMahon et al., 1991) |
|
ZrO2/12% CeO2 |
ZrO2 fiber |
Hybrid (insulation) |
(Janney et al., 1992b) |
|
ZrO2/8% Y2O3 |
ZrO2 fiber next to specimen |
Hybrid (insulation, picket fence; self at 28 GHz |
(Janney et al., 1991b, 1992b) |
compared with the conventionally sintered material. A barrier layer was required to preclude Y2O3 contamination of the TiB2.
Batches of Si3N4 cutting tools, with 90 parts per batch, were sintered using a cylindrical multimode cavity (Patterson et al., 1992a). Parts were arranged in six layers embedded and isolated from each other within a packing powder and were enclosed in a cylindrical alumina crucible. The packing powder, consisting of 40 percent SiC, 30 percent BN, and 30 percent Si3N4 by weight, served multiple purposes——providing a source for N2, providing high thermal conductivity, acting as a getter for O2, and acting as a microwave absorber. Three conductive rings were placed around the alumina crucible to shape the microwave field. The temperature increased with some degree of nonuniformity during a slow increase in microwave power. A locally high-temperature area would commence at one end of the load and gradually spread throughout the entire load. Thus, after 50 minutes the surface temperatures ranged from 536—1190 ºC, whereas after 140 minutes the range was from 1540—1610 ºC. After optimizing the process, uniform density among the parts was obtained. Energy consumption was estimated to be on the order of 80 percent less than experienced with conventional heating. In this case, electric heat is mandated for conventional processing because of the tendency of the material to oxidize in combustion gases.
Issues in Microwave Sintering
Microwave Enhancement Effects
There have been numerous reports of enhancement of sintering kinetics when using microwave processing. Probably the most startling is a report of as much as a 400 ºC reduction in sintering temperature along with a dramatically reduced activation energy for Al2O3 processed in a 28-GHz microwave cavity (Janney and Kimrey, 1988, 1990). As discussed in Chapter 3 of this report, significant errors in temperature measurement can lead to misleading processing results. Shielded and grounded thermocouples, as discussed in Chapter 3, as well as optical pyrometers, were used to minimize temperature-measurement errors, and carefully designed insulation systems were used in the studies referenced above to minimize temperature gradients. By switching the microwave power off and on, and observing thermocouple response, it was demonstrated that the microwave field did not bias the thermocouple output (Janney et al., 1991a). While temperature measurement may yet be a problem, it is difficult to imagine a 400 ºC error.
Significant reductions in sintering temperatures or enhancements in the diffusion coefficient for sintering have also been reported for Al2O3 (Patil et al., 1991; Cheng et al., 1992) and Al2O3 doped with MgO (Cheng et al., 1992). Reductions in sintering temperature and activation energy have been reported for the sintering of zirconia-toughened alumina (Kimrey et al., 1991) and zirconia (Janney et al., 1991b, 1992a). A variety of other ceramics were similarly sintered using microwaves, including B4C (Holcombe and Dykes, 1991a), LaCrO3 (Janney and Kimrey, 1992), and Si3N4 (Tiegs et al., 1991; Kiggans et al., 1991; Kiggans and Tiegs, 1992). These results, for a broad range of materials, indicated that the reduction in sintering temperature was observed in insulators and ionically conducting materials but not in
electronically conducting materials. Reductions in sintering temperature and activation energy were greater at 28 GHz than they were at 2.45 GHz.
Enhanced microwave plasma sintering of alumina and a few other oxides has been observed, but only the data for alumina were presented quantitatively (Bennett et al., 1968). A 200 ºC reduction in the sintering temperature of Linde A alumina was reported compared with the same temperature and time in a conventional furnace. Rapid pass-through sintering of thin-wall tubes and rods in the radio frequency (RF) induction coupled plasma and microwave plasmas has been investigated (Sweeney and Johnson, 1991). Densification times in thin-wall tubes in the RF induction coupled plasma were as low as 10 seconds from onset to completion of densification, with final densities as high as 99.7 percent for MgO-doped alumina. Similar sintering speeds were obtained with rods in a microwave plasma. Although the microwave plasma process has not yet been thoroughly characterized, a clear enhancement of sintering was observed in the 5-MHz induction coupled plasma sintering of alumina (with pains taken to correct for temperature measurement errors through extensive calibration procedures).
Microwave enhancement effects have not been observed universally. Patterson et al. (1991) saw a slight increase in the sintering rate of three alumina powders——the sintering time was cut in half at 1600 ºC relative to conventional sintering, with comparable densities and elastic modulus. Levinson et al. (1992) found no significant difference in the sintering of ZnO varistor materials in microwaves relative to that in conventional firing, and there was no difference in properties. The interdiffusion of Cr2O3 and Al2O3 under microwave heating was studied to determine if there was enhanced diffusion in ceramics heated by microwaves (Katz et al., 1991). Although a slight apparent enhancement was observed, it was concluded that this could be accounted for without resorting to a rate enhancement by microwaves.
Part of the controversy surrounding the ''microwave effect'' is that satisfactory physical explanations are missing. Booske et al. (1992) proposed a theory in which the enhanced sintering is attributed to enhancement of the phonon energy distribution in the high end of the distribution. The same research group later reported that further calculations showed the proposed effect was of insufficient magnitude to explain the observations (Booske et al., 1993). A satisfactory physical explanation of microwave effects must show why electronically insulating materials have shown the effect while conducting materials have not.
A series of careful experiments is needed to eliminate the doubts that remain about the "microwave effect." Since temperature measurement is often problematical, some method of internal calibration of the temperature is imperative.
Hybrid Heating
Electrically transparent (low-loss) materials, such as SiO2 and Al2O3, are difficult to heat at room temperature. Additionally, many materials that are hard to heat at room temperature possess electrical conductivity or dielectric loss factors that rise rapidly in magnitude as the temperature rises. Thus these materials will absorb microwave energy if they can be preheated to a suitable temperature using another source of heat. This has led to the development of passive hybrid heating using higher dielectric loss susceptors, insulation, or coatings that absorb incident microwave power more readily at low temperature.
The sintering of ZrO2-toughened Al2O3, ZrO2/8% Y2O3 and zirconia/12% CeO2 at 2.45 GHz provides an example of how a hybrid heating process can improve unstable heating (Janney et al., 1992b). Although these materials could be sintered readily at 28 GHz, attempts at 2.45 GHz, where equipment costs are more attractive, were frustrated until SiC rods were inserted into the insulation that surrounded the specimens in what was referred to as the "picket fence" arrangement. The microwave energy initially heated the SiC rods, which, in turn, transferred heat to the insulation and eventually to the specimens. As the specimen temperature increased, it more effectively coupled with the microwave energy and began to heat directly. Figure 5-1, part a shows the calculated field distribution (using finite-difference time-domain modeling) in a simulation of four ceramic samples surrounded by insulation inside the cavity, showing a concentration of fields at the sides of the insulation. Although the inclusion of four SiC rods, as shown in Figure 5-1, part b, reduced the overall field strength, it helped to concentrate the fields in areas of interest near the samples rather than in the insulation. With this arrangement, these materials were successfully sintered, albeit at fairly low heating rates (2 ºC/min).
Another hybrid microwave heating scheme involved applying a thin layer of SiC powder to the interior of the thermal insulation chamber that is placed within the microwave oven (Dé et al., 1991a, b, c). As in the picket fence arrangement, the silicon carbide is initially heated by the microwaves, transferring heat to the specimen. The silicon carbide layer is thin enough that significant penetration of the microwaves occurs. With this arrangement, a number of ceramic materials have been successfully sintered.
In yet another hybrid heating process, tubular susceptors of various sizes were inserted over relatively large sized compacts of alumina and zirconia toughened alumina (50 mm diameter by 60 mm long) which were then sintered to 1500 ºC at heating rates of about 10 ºC/min (Brandon et al., 1992). Compacts of this size could not be sintered using conventional processes at 10 or even 5 ºC/min without cracking.
Simulations have shown that increasing the ambient temperature through some form of hybrid heating can increase the critical temperature to as high as required for sintering. These results explain the success achieved with hybrid heating processes that is reported in the literature. The simulations also explain, qualitatively at least, the observed difficulties in sintering low-loss oxide materials by microwave heating (Spotz, et al., 1993).
Although some impressive results have been reported in the hybrid heating of alumina, controlled rapid heating of oxides with both low initial dielectric loss factors and high temperature dependence of dielectric loss factors is difficult to achieve. Success generally has been limited to single specimens of simple geometry in carefully designed sintering chambers.
Insulation
Unstable heating due to changing permittivity or thermal gradients caused by heat loss from part surfaces can be minimized using effective insulation. Almost all cases in which successful microwave sintering has been reported have necessitated carefully designed insulation systems. In many cases, packing powders are required, which would only be acceptable for products of very high value. Development of a workable insulating system has been identified
as "one of the most challenging tasks in the high temperature microwave processing of ceramics" (Janney et al., 1992b).
While temperature gradients within the specimen can be reduced by the presence of insulation, they can be eliminated only if all of the microwave energy is absorbed by the insulation, or a susceptor, and subsequently transferred to the specimen. In fact, the various hybrid heating approaches move in this direction. However, temperature gradients of a certain magnitude may be acceptable.
If the microwave power absorption increases sharply with increasing temperature, there always will be heating problems because of this volumetric heating and surface cooling of even well-insulated specimens. In some cases, surface thermal gradients may be overcome by slow heating, but, in other cases, microwave heating will be unstable, making uniform sintering impossible. This difficulty is particularly exacerbated in the case of multiple-part sintering, where a hotter part may preferentially absorb more microwave energy at the expense of cooler parts.
Materials with well-behaved heating behavior, such as the carbides, usually require very high sintering temperatures. This presents problems with regard to setters and insulation. There are no truly microwave-transparent insulation materials capable of operation in the 2000 ºC range, although granular Y2O3 has shown some promise.
The need for carefully controlled insulation has forced microwave sintering to be basically a batch process, often with only a single part being sintered at a time. Successful demonstration of large batches has only rarely been successful. Even in successful cases, part-to-part variations were common. In the extreme cases, some parts have undergone thermal runaway and others were not fully sintered. Unfortunately, these effects are exacerbated by large batch size and rapid heating, both of which are desirable from a manufacturing point of view. Further investigation is needed to discover the regimes of microwave-power absorption characteristics, batch size, heating rate, and other variables in which microwave sintering can be reproducible and uniform.
Thermal Runaway
As discussed in Chapter 2, the rapid rise in dielectric loss factor with temperature is the major issue in thermal runaway and temperature nonuniformity. Therefore, although microwave heating frequently is touted as providing more uniform heating, nonuniform heating is a reality in many oxides, often at nominal heating rates. The situation is worse when a multitude of parts are heated together, or for other than simple specimen geometry.
Some general observations can be made about factors relating to thermal runaway. First, if the temperature dependence of the power absorption is less than the temperature dependence of the heat dissipation at the surface of the specimen plus insulation system, stable heating should be observed. Second, hybrid heating using either lossy insulation or other susceptors that absorb a significant fraction of the microwave power and transmit it to the specimen by conduction or radiation is important in stable heating. Finally multiple specimens of differing size, or specimens with varying cross section or complex shapes, will be particularly difficult
to heat uniformly. Materials with lower temperature dependence of dielectric loss factor may be heated stably. However, the uniformity issues for complex shapes or differing sizes within a batch will persist. Further work is required to determine more fully the conditions under which stable heating of various materials can be achieved.
Property Enhancement
The final issue is the question of whether there are fundamental differences in the properties achievable by microwave sintering and those achievable by other methods. Microwave sintered and hot isostatically pressed Si3N4 cutting tools showed significantly improved performance compared with commercially available cutting tools (Patterson, 1992b). Dé et al. (1991b, c) investigated the effects of heating rate on the densification and microstructure of conventionally and microwave sintered materials in a hybrid system. They observed that higher heating rates result in higher density and smaller grains, just as with conventional fast firing. However, higher heating rates were achieved in the hybrid system than was possible with the same specimen size in a conventional furnace. It may be significant that the microwave sintered specimens had a smaller grain size at any given density during the densification process than did conventionally fast-fired specimens. Unfortunately, other researchers have not reported relationships of grain size versus density that would make it possible to determine whether this effect is widely realized with other materials.
Powder Processing
The synthesis and processing of powders is a key technology area affecting the future development of advanced ceramic materials. The application of microwaves to powder processing technology is relatively new and will be discussed briefly. Table 5-3 summarizes some of the areas where microwaves have been applied to ceramic powder processing.
Powder Synthesis
The characteristics of a starting powder (composition, size, structure, shape, etc.) strongly affect the control over the sintering behavior, microstructural development, improved properties, and reliability of the final product (Johnson, 1987). For this reason, there continues to be a significant effort to develop improved and tailorable powders to meet the increasing demands for a wide range of future, advanced ceramic products (Messing et al., 1987, 1988a, b).
The application of microwaves to the synthesis of ceramic (oxide and nonoxide) powders is a recent and emerging development and offers some unique benefits, especially with respect to producing particles of submicron (nano) size with controlled compositions. Microwave synthesis of ceramic powders offers greater process flexibility by taking advantage of several
TABLE 5-3 Microwave Applications in Ceramic Powder Processing
|
Powder Synthesis • Sol-gel Decomposition/Drying • Solution Evaporation/Decomposition • Gas-Phase Reactions • Gas-Solid Reactions • Solid-State Reactions • Ceramic Precursor Pyrolysis • Hydrothermal Reactions |
|
Powder Treatment • Dissolution • Drying • Calcining |
|
Powder Consolidation/Shaping • Sintering • Reaction and Sintering • Melting • Ignition |
combinations of volumetric, rapid, and selective heating conditions, which are not possible by conventional means. All of these heating advantages can be used to process and tailor extremely fine (less than 1-μm diameter) powders by controlled reactions in sol-gel processing, gas-phase synthesis, solution evaporation/decomposition, or hydrothermal reactions. Each of them, and other powder synthesis methods, will be described next.
Sol-Gel Decomposition/Drying
Microwaves have been used in several of the processing stages to synthesize BaTiO3 powders from a sol-gel precursor. A solution of barium and titanium acetate was decomposed to produce a dry gel, which was pyrolized to yield a brown product and then calcined to yield a colorless powder sample of BaTiO3 (Kladnig and Horn, 1990). Microwave energy was effectively utilized in all of these stages.
Fine crystalline mullite powders have been prepared from single-phase gels in a few minutes (Komarneni et al., 1988). The particle sizes of the dry powders were about 0.1 to 0.5 μm, with mullite crystallite sizes of 100—200 nm after microwave heating the gel for 5 minutes. At present, the mechanism for the microwave absorption of the aluminosilicate gels is not well understood. In other sol-gel studies, microwave absorption was also significant, and silica (Roy et al., 1985) and urania (Haas, 1979) gels could be rapidly dried and heated to their melting points.
Solution Evaporation/Decomposition
This method can also produce extremely fine powders of controlled (mono-or polyphased) compositions and high purity. A novel approach to this process used microwaves to decompose aqueous solutions of nitrates, nitrate-HF, and chlorine after the solutions had been sprayed into a microwave chamber (Kladnig and Horn, 1990). Water vapor and other volatiles were removed via a vacuum to pass into an absorption (condensing) system, which would regenerate the solvents (HNO3, HCL, HF, etc.). Depending on the composition of the starting solution, powders of ferrites, Al2O3, TiO2, and other oxides were produced. One advantage of this method is that it could be developed into a continuous process with the recovery of some of the starting solvents.
Microwave-generated plasmas have also been used to decompose atomized droplets of aqueous solutions containing the nitrates of Zr and Al, yielding very fine crystalline powders of γ-Al2O3 and ZrO2, respectively (Vollath et al., 1992). When nitrates of Zr, Al, and Y were atomized together, a mixed-oxide powder of ZrO2, Al2O3, and Y2O3 was produced. The chief advantages of microwave plasma processing were high efficiency (80 percent) in transferring thermal energy to the chemical reactions; formation of completely crystalline, spherical particles of ZrO2 (100—500 nm in diameter); and a capability to produce solid-solution particles.
Gas-Phase Reactions
Nonoxide powders of AlN, SiC, and Si3N4 have been synthesized by nonthermal microwave plasmas of precursor gases under conditions of laminar flow (Singh et al., 1991). The product particles were ultra-fine (~ 5 nm) and crystalline. AlN was stabilized in either the hexagonal or cubic phase, depending on the nitrogen concentration during the reactions. The SiC formed mostly cubic-3C, with other polytypic modifications, while the Si3N4 was formed as an α-phase modification.
Gas-Solid Reactions
This method has not received much attention from a microwave processing viewpoint. However, in a study of processing refractory ores in a microwave-induced cold plasma, Bullard and Lynch (1992) investigated the reduction of TiO2 powder in a hydrogen plasma under reduced pressure (16 Torr). They observed about a 60 percent conversion to the Ti2O3 phase in 11 minutes at low temperatures (below 735 K).
Solid-State Reactions
Microwaves have also been used to promote reactions between mixtures of solid particles to form powders of new (reacted) compositions.
In the case of oxide powders, a variety of oxides, such as KVO3, BaWO4, and YBa2Cu3O7-x have been produced via solid-state microwave synthesis (Mingos and Baghurst, 1992). These authors also used microwaves in the synthesis of borides by heating mixtures of boron with Cr, Fe, and Zr to 1000 ºC.
Ultra-fine SiC powders have also been synthesized by the carbothermal reduction of silica using microwave and conventional firing techniques (Kumar et al., 1991). Both techniques produced ß-SiC powders, but the crystallite size of the microwave-produced powders was 30—200 nm, versus 50—450 nm for the conventionally produced material. Microwaves were also used to synthesize SiC, TiC, NbC, and TaC from mixtures of the corresponding metal oxides and graphite powders. Temperatures of up to 1400—1500 ºC were obtained in 13 min, and the carbides were formed within 20 min (Kozuka and MacKenzie, 1991). This technique also appears to be a new means to produce SiC whiskers.
Ceramic Precursor Pyrolysis
A wide variety of ceramic powders have been produced by microwave heating of ceramic-precursor compounds or mixtures of such compounds without added solvents, thus avoiding the large volume of solvents to be removed in the solvent decomposition/evaporation process used in conventional processing (Willert-Porada et al., 1992). Control of powder properties is achieved through chemical modification of reaction mixtures, use of specially designed microwave applicators, and control over certain decomposition profiles. Fine powders were produced by using ceramic precursor alcoholates and acetylacetonates of Al, Zr, Ti, Si, Cu, and Mg. These compounds absorb microwaves readily. Single oxide powders, such as Al2O3 and ZrO2, were prepared by pyrolysis of Al-triisopropanolate (ATIP) or Zr-tetrapropylate (ZTP). Mixed-oxide powders, such as Al2O3 + ZrO2, Al2O3 + CuO or CuAlO2, or MgAl2O4, were prepared by the pyrolysis of appropriate precursor mixtures.
In addition, composite powders were also prepared by coating inert particles of Al2O3, BN, or SiC with a thin layer of a zirconia precursor (ZTP) or by coating reactive powders such as carbon with ZTP and other precursors to form carbide/oxide composite powders (Willert-Porada et al., 1992). As shown in Figure 5-2, by using microwave heating of metallorganic precursors, decomposition is enhanced and occurs at lower macroscopically measured temperatures than conventional thermal processing, so that a wide selection of mono-and polyphasic powders could be synthesized with reasonably high surface areas (10—700 m2/g).
Hydrothermal Reactions
Microwave-hydrothermal processing has been utilized in catalyzing the synthesis of crystalline, submicron powders of unary oxides such as TiO2, ZrO2, and Fe2O3 and binary oxides such as KNbO3 and BaTiO 3 (Komarneni et al., 1992). Also, a new layered alumina phase was
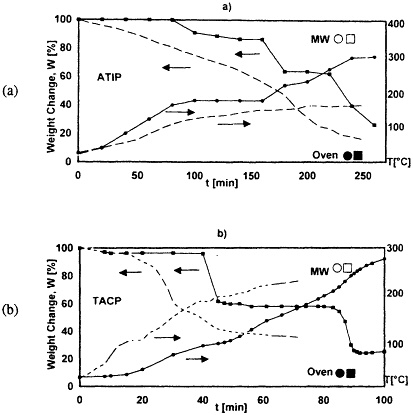
FIGURE 5-2 Oven versus microwave pyrolysis of grin alumina infiltrated with (a) Al(O-i-C 3H7)3 (ATIP), and with (b) Ti(O2C5H7)2(OC3H7)2 (TACP) (Willert-Porada, 1993).
synthesized, which can be intercalated with ethylene glycol. The system is controlled by pressure (which determines the temperature), and other variables such as time, concentration of metal solution, pH, etc., that are used to control the final composition, crystal size, morphology, and level of agglomeration. It was found that microwave-hydrothermal synthesis enhances the apparent kinetics of crystallization of the various oxides by one or two orders or magnitude over that of conventional (Parr bombs) methods. In some cases, the conventional methods, in addition to being much slower, did not lead to the crystallization of a pure oxide phase, as shown in Table 5-4 for TiO2.
TABLE 5-4 X-ray Diffraction Analyses of Titania Powders Produced by Microwave-Hydrothermal and Conventional Hydrothermal Techniques (From Komarneni et al., 1992)
|
Concentration (M) |
|
Temperature (ºC) |
Duration (h) |
Reaction Products in order of abundance as determined by x-ray diffraction |
|
TICl4 |
HCl |
|
|
|
|
Microwave-hydrothermal |
||||
|
0.5 |
1 |
164* |
0.5 |
Rutile |
|
0.5 |
1 |
164* |
1 |
Rutile |
|
0.5 |
1 |
164* |
2 |
Rutile |
|
Conventional-hydrothermal |
||||
|
0.5 |
1 |
164 |
2 |
Anatase, small amount of rutile |
|
0.5 |
1 |
164 |
24 |
Rutile, small amount of anatase |
|
0.5 |
1 |
164 |
72 |
Rutile, small amount of anatase and amorphous |
|
* Based on autogenous pressure of 200 psi. |
||||
In summary, the microwave synthesis of powders is a new era of processing and provides many opportunities for future developments. Table 5-5 presents some of the ceramic powders that have been synthesized using microwave energy.
Powder Treatment
Drying
Because of the strong tendency of moisture to absorb microwaves, and because of the internal (volumetric) deposition of energy, polymeric, ceramic, and other powders can be efficiently dried, and desired residual moisture contents can be precisely controlled. This is an area where microwave processing is in common use (Chabinsky and Eves, 1986; Metaxas and Meredith, 1983).
Calcining
Powdered mixes have been calcined (reactively sintered) using microwaves; the mixes react to form compounds such as BaTiO3 and NaTiO3 (Oda and Balboa, 1988), Al6Si2O13 (mullite; Willert-Porada et al., 1992) and Al2TiO5 (Boch et al., 1992).
TABLE 5-5 Ceramic Powders Synthesized by Microwave Heating
|
Composition |
Process |
Composition |
Process |
|
Oxide |
|
Nonoxide |
|
|
Al2O3 |
Solution1 Pyrolysis2 Hydrothermal3 |
CrB Fe2B |
Solid-State7 Solid-State7 |
|
Fe2O3 |
Solution1 Hydrothermal3 |
ZrB2 |
Solid-State7 |
|
TiO2 |
Solution1 |
AlN |
Gas-Phase8 |
|
Ti2O3 |
Gas Solid4 |
Si3N4 |
Gas-Phase8 |
|
ZrO2 |
Solution5 |
SiC |
Gas-Phase8 |
|
MgAl2O4 |
Copyrolysis2 |
TiC |
Gas-Phase8 |
|
Al6Si2O13 |
Sol-gel6 Copyrolysis2 |
NbC |
|
|
CuAlO2 |
Copyrolysis2 |
TaC |
Gas-Phase8 |
|
BaTiO3 |
Sol-gel1 Hydrothermal3 |
Composite |
|
|
YBaCu3O7-x |
Solution1 Solid-State7 |
Al2O3/ZrO2/Y2O3 |
Solution5 |
|
Mn0.5Zr0.4Fe2O4 |
Solution1 |
SiC/SiO2 |
Particle + Coating Pyrolysis2 |
|
Mn0.6Zr0.4Fe2O4 |
Solution1 |
TiC/TiO2 |
Particle + Coating Pyrolysis2 |
|
KVO3 |
Solid-State7 |
ZrC/ZrO2 |
Particle + Coating Pyrolysis2 |
|
CuFe2O4 |
Solid-State7 |
ZrC/SiC |
Particle + Coating Pyrolysis2 |
|
BaWO4 |
Solid-State7 |
BN/ZrO2 |
Particle + Coating Pyrolysis2 |
|
La1.85Sr0.15CuO4 |
Solid-State7 |
SiC/ZrO2 |
Particle + Coating Pyrolysis2 |
|
|
|
Al2O3/ZrO2 |
Copyrolysis2 |
|
|
|
Al2O3/CuO |
Copyrolysis2 |
|
1 Kladnig and Horn, 1990 2 Willert-Porada et al., 1992 3 Komarneni et al., 1992 4 Bullard and Lynch, 1992 5 Vollath et al., 1992 6 Komameni et al., 1988 7 Mingos and Baghurst, 1992 8 Singh et al., 1991 9 Kumar et al., 1991 10 Kozuka and MacKenzie, 1991 |
|||
Enhancing Microwave Absorption
As mentioned in the previous section, many electrically insulating materials, such as oxides, are transparent to microwaves at room temperature. Powders of these materials can be made to couple readily by the addition and mixing of polar liquids or conducting particles. Many refractory oxides, such as alumina, mullite, zircon, MgO, or Si3N4, have been made to couple effectively with microwaves by the addition of electroconductive particles of SiC, Si, Mg, FeSi, and Cr2O3 (Nishitani, 1979). Oxides of Al2O3, SiO2 and MgO have also been effectively heated by the addition of lossy materials such as Fe3O4, MnO2, NiO, and calcium aluminate (Sutton and Johnson, 1980). Mixtures of conducting powders, such as Nb, TaC, SiC, MoSi2, Cu, and Fe, and insulators such as ZrO2, Y2O3, and Al2O3 have coupled well with microwaves (Sutton, 1989). Various materials in solution (zirconium oxynitrate, aluminum nitrate, and yttrium nitrate) that are good couplers have also been added to enhance microwave absorption of powdered insulating oxides (Sutton, 1989).
Powder Consolidation/Shaping
Reaction Sintering
Reaction sintering (or bonding) of oxides and silicon nitride using microwaves has been investigated. In the case of silicon nitride, porous powder compacts of silicon have been reacted with nitrogen at elevated temperatures of 1150—1450 ºC (Kiggans et al., 1991). The advantages of this promising process are discussed in more detail in a later section of this report.
Melting
Microwaves have been used to melt powdered materials to form coatings on various substrates by using focused millimeter-wavelength beams. Because of their shorter penetration characteristics, these beams have been used to selectively heat and fuse pore-free coatings, such as Al2O3, on lower-melting refractory substrates (Sklyarevich and Decker, 1991; Sklyarevich et al., 1992).
Ignition
Since microwaves create volumetric heating, they have been used to initiate internal ignition in mixtures of exothermic powder compacts (Ahmad et al., 1991). This provides thermal gradients and combustion fronts that move in directions opposite to those in powder compacts that are ignited by conventional (external) methods. The reverse thermal gradients and reaction fronts may enable the synthesis of new and unique structures, composition gradients,
and improved properties for this class of materials, which are produced by self-propagating high-temperature synthesis processing (Ahmad et al., 1991; Dalton et al., 1990).
Potential Advantageous Applications of Microwave Heating to Ceramics
Due to the strong absorption of microwaves by water, microwave drying of ceramics has been successful for both powders and bulk materials (Smith, 1974). While the high cost of microwave energy makes microwave drying inefficient at high moisture contents, at low moisture contents (less than 5 percent) the removal of water using conventional processes becomes inefficient, making microwave processes more competitive (Sutton, 1989). A hybrid system with both conventional and microwave heat sources may be the best solution for many drying applications (R. D. Smith, 1991). Due to the depth Of penetration, microwave drying is especially promising for removing low moisture contents from thick sections, including foundry cores (Schroeder and Hackett, 1971; Valentine, 1973), ladle linings (Ochiai et al., 1981), and plaster molds (Valentine, 1973; 1977).
Since temperature gradients are a given with microwave heating, processing schemes that take advantage of the temperature gradients may be attractive. Work is already under way in some of these areas, including microwave-assisted chemical vapor infiltration (CVI) (Evans and Gupta, 1991; Day et al., 1993) and microwave-assisted reaction-bonded silicon nitride (RBSN), (Tiegs et al., 1991; Kiggans et al., 1991; Kiggans and Tiegs, 1992; Thomas et al., 1993a, b).
In both CVI and RBSN, temperature-dependent chemical reactions take place to produce the ceramic material. The rate of reaction also depends upon the concentrations of reactants and any product species in the gas phase. Thus, isothermal processing results in preferential reaction at the surface, where the concentration of reactants is maximum. Moreover, the reaction in both CVI and RBSN tends to either seal the surface or otherwise dramatically inhibit gaseous diffusion, resulting in unreacted or uninfiltrated interior regions. Microwave heating in these cases allows the reaction to take place preferentially in the interior and work its way toward the surface, providing higher final density and greater percent conversion in the two processes. Significantly sized specimens have been successfully nitrided and subsequently sintered in a well-insulated microwave system (Tiegs et al., 1991; Kiggans et al., 1991; Kiggans and Tiegs, 1992). Thomas et al. (1993a, b) utilized a single-mode cavity to nitride disks and rods and demonstrated superior conversion than conventional processing. Finally, Day et al. (1993) used microwave-heating-assisted chemical vapor infiltration to make SiC/SiC and Al2O3/Al2O3 ceramic-matrix ceramic-fiber composites.
As described earlier in this section, the application of microwaves in ceramic powder synthesis offers some unique benefits, especially with respect to producing particles Of submicron (nano) size with controlled compositions. Extremely fine (less than 1 μm diameter) powders can be produced by controlled reactions in sol-gel processing, gas-phase synthesis, solution evaporation/decomposition, or hydrothermal reactions.
There is a growing interest in the use of microwaves to join ceramics (Silberglitt et al., 1993). Rapid, homogeneous joining can be accomplished using selective microwave heating in either a single-mode applicator (Palaith et al., 1988; Fukushima et al., 1990), by focussing the field at the interface, or a multimode (hybrid) applicator, by using susceptors (Al-Assafi and
Clark, 1992) and by using bonding agents with higher loss than the base material (Yiin et al., 1991; Yu et al., 1991). These studies have only shown the feasibility of joining processes. Applicator design to move the processes to production scale on more complex joints is required (Silberglitt et al., 1993).
Successful industrial implementation of microwave processing depends in large measure upon continuous processing schemes in which parts pass through the microwave cavity. Hybrid heating schemes may find important usage in this regard. In any event, application of microwave processing will probably be limited to materials that do not show a large temperature dependence of the dielectric loss factor and thus are susceptible to thermal runaway. Significant effort must be directed toward applicator design, specifically addressing the issue of openings in the microwave applicator for introduction and removal of the parts.
POLYMERS AND POLYMER-MATRIX COMPOSITES
Polymer Processing
There are increasing demands across broad product lines for new polymeric materials and processes that are cost-effective and environmentally safe. Over the past twenty years, research in the area of microwave processing has shown some potential advantages in the ability not only to process polymers at lower cost but to fabricate new materials and composites that may not be possible using conventional thermal treatments.
One of the first industrial applications of microwave radiation for the processing of polymeric materials was the vulcanization of rubber in the tire industry during the 1960s, with commercial application beginning late in that decade (Chabinsky, 1983a, b; Schwartz et al., 1975). The principal mechanism of coupling of the microwave radiation to the material occurred via carbon black fillers already present in many rubber formulations. Since different grades of carbon black had different coupling characteristics (Ippen, 1971), rubber compounders learned to control the heating patterns throughout the multilayered product through variation of carbon grade and concentration. Application of this processing technology was limited due to the nonuniformity of the microwave curing ovens that were available at that time and thermal runaway attributable to increases in dielectric loss with increasing temperature.
The importance of increased throughput and reduced operating costs, along with advances in microwave equipment, fueled a resurgence in robber processing in the 1980s. Microwave vulcanization of extruded robber weather stripping for the automotive and construction industries has found commercial application, with over 600 installations worldwide (Krieger, 1992). Microwave processing offered rubber processors significant advantages over conventional processing, including improved product uniformity; reduced extrusion-line length; reduced scrap; improved process control and automation; continuous vulcanization rather than conventional batch processes; and improved cleanliness and environmental compatibility compared with steam autoclaves, hot air, salt bath, or fluid bed heating processes.
There is significant interest in applying this technology to the processing of high-performance, high-cost materials, such as reinforced composite materials including carbon, glass, and ceramic-fiber reinforcement; ceramics; and high-temperature polymers.
Mechanism of Microwave Coupling in Polymers
The principal mechanism of microwave absorption in a polymer is the reorientation of dipoles in the imposed electric field. As in a home microwave, the materials with the greatest dipole mobilities will exhibit the most efficient coupling. Microwave heating, therefore, will couple most efficiently with the strongest dipole in a system and has the potential to selectively heat polar polymers in mixtures. The efficiency of microwave coupling with polymer materials is dependent on the dipole strength, its mobility and mass, and the matrix state of the dipole (Metaxus and Meredith, 1983). Microwave coupling to a given dipole will be greater in a liquid, less in a rubber, and even further reduced in a glassy or crystalline polymer.
Polymer dielectric constants can vary during a processing cycle or if a phase change occurs as temperature varies, solvent is removed, and the reaction proceeds changing the type and concentration of dipoles. Generally, several distinct dielectric relaxation processes are present in a solid polymeric material. This is shown in Figure 5-3, which is a scan of dielectric
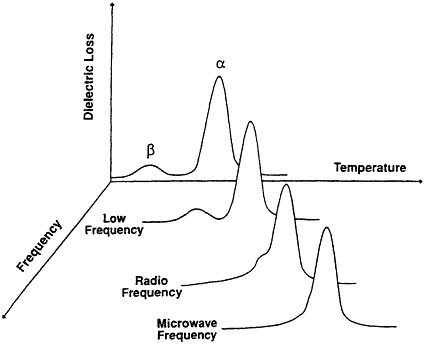
FIGURE 5-3 Schematic dielectric loss versus temperature and frequency for polymer materials (from Chen et al., 1991).
loss at constant frequency as a function of temperature. Similar relaxation processes are observed in dynamic mechanical properties of polymers, with analogous dispersions in real and imaginary components of viscoelastic response (Ward and Chen, 1992). An excellent review of the dielectric properties of polymers at microwave frequencies is presented by Bur (1985).
Polymers for Microwave Applications
Not all polymer materials are suitable for microwave processing. However, many polymers contain groups that form strong dipoles (e.g., epoxy, hydroxyl, amino, cyanate, etc.). Microwave processing can be used over a broad range of polymers and products, including thermoplastic and thermosetting resins, rubber, and composites.
Initially, thermosetting polymers are low-viscosity liquids that can flow into a mold or around fibers. During processing, thermosets react to increase molecular weight and viscosity, eventually becoming highly cross-linked, insoluble, infusible materials. Thermoset cure processes consist of three basic steps: (1) preheating of the components; (2) reaction, producing the corresponding exotherm; and (3) cooling of the cured materials (Van and Gourdenne, 1987). Permittivity and dielectric loss factor of thermosets generally increase with temperature and decrease with extent of cure (Jow et al, 1988). These polymers tend to be efficient absorbers of microwave radiation initially, with ![]() increasing as the resin is heated. As the cure reaction progresses, the temperature may be difficult to control due to the additional heat input caused by the exothermic reaction.
increasing as the resin is heated. As the cure reaction progresses, the temperature may be difficult to control due to the additional heat input caused by the exothermic reaction.
Thermoplastics are fully polymerized materials that melt and flow upon application of heat. They are processed well above their glass transition temperatures or melting points (if the material is semicrystalline) to reduce the melt viscosity and allow flow and to promote adhesion. High-performance, semicrystalline thermoplastic polymers, such as Polyetheretherketone (PEEK), can be difficult to heat using microwaves until a critical temperature is reached, where ![]() , and therefore the heating rate, increases significantly (Chen et al., 1989). This critical temperature is related to increased molecular mobility but may not be the same as the glass transition temperature of the polymer. The crystallinity of these materials is important; amorphous polymers heat more effectively than semicrystalline polymers (DeMeuse, 1992).
, and therefore the heating rate, increases significantly (Chen et al., 1989). This critical temperature is related to increased molecular mobility but may not be the same as the glass transition temperature of the polymer. The crystallinity of these materials is important; amorphous polymers heat more effectively than semicrystalline polymers (DeMeuse, 1992).
Functionally terminated thermoplastics combine the toughness of thermoplastics with the ease of processing and the creep resistance and solvent resistance of thermosets. These materials undergo a combination of thermoset and thermoplastic processing with the initial heating reducing viscosity and improving flow and ultimately reaction providing a cross-linked network. Microwave processing of functionally terminated thermoplastics offers advantages over conventional processing, particularly in reducing the processing time (Hedrick et al., 1989). One of the challenges in the microwave processing of these polymers is that the processing temperature is often very close to its thermal degradation temperature, making temperature control crucial. If the temperature is too high, the polymer undergoes undesirable cross-linking, scission, and oxidation, which can cause significant changes in the mechanical and optical properties of the material. This behavior places a strict requirement on the microwave system to provide very uniform temperature distributions throughout the part being processed and careful control of the temperature of the part.
Although the polymer systems that are candidates for microwave processing are typically not conductive, particles and fibers that are conductive, or have dielectric properties significantly different from the matrix polymer, may be included to aid processing or to modify the mechanical, physical, or optical properties. The presence of these inclusions can strongly influence the way in which the composite material interacts with the microwave radiation. Conductors also modify the electric field pattern in and around the composite, potentially resulting in very different heating profiles than with the neat resin. Some examples of these conductive additives include carbon black (used extensively in rubber formulation); carbon or metal fibers; and metal flakes, spheres, or needles with sizes ranging from 0.1 to 100 μm. Although the final composite is not necessarily conductive, the surfaces of the conducting inclusions interact strongly with the microwave radiation. The effect of conductive additives on microwave heating and skin depth of the composite depends on the size, shape, concentration and electrical resistivity of the inclusions and their distribution in the matrix (Lagarkov et al., 1992).
The presence of conducting fillers may inhibit microwave heating by decreasing skin depth. However, by controlling the nature, orientation, and concentration of the fillers, the microwave response of the material can be tailored over a broad range. For example, carbon fibers have a relatively high resistivity and heat the surrounding matrix very effectively; the thermal profile has a maximum at the surface of the fibers. This preferential heating has been shown to provide an enhancement of the interfacial adhesion between the fibers and the matrix resin (Agrawal and Drzal, 1989) and a subsequent improvement in the fracture properties of microwave-processed composite materials. Preferential heating of conducting fillers has also been utilized in the joining of polymers and polymeric composites (Varadan, et al., 1990). Baziard and Gourdenne (1988a, b) report an increased rate of cross-linking in a composite system of an aluminum powder and epoxy resin. The rate of cross-linking is attributed to the higher dielectric loss due to the presence of the filler. Similar results for carbon-black filled epoxy resin systems have been reported (Bouazizi and Gourdenne, 1988).
Nonconductive additives such as glass fibers and nonconducting metal oxides which are used as pigments (e.g., titanium dioxide), can also influence composite properties through preferential heating mechanisms, depending on their dielectric properties.
Enhanced Reaction Kinetics
In addition to the efficient coupling of microwave energy in polar materials and significant depth of penetration, nonthermal ''microwave effects,'' including accelerated apparent kinetics (Lewis et al., 1992, 1987, 1988; Hedrick et al., 1989), retarded kinetics (Mijovic and Wijaya, 1990), and dependencies of the heating rate (Gourdenne, 1992; Chan and Gourdenne, 1992) and structure (Thuillier et al., 1986) of the cured polymer structures formed on the pulse repetition frequency have been reported. The most prevalent reports of microwave effects have been acceleration of reaction rates. There have also been reports in which no effect of the radiation on the kinetics was observed (Mijovic et al., 1992a, b; Jullien and Petit, 1992; Jordan et al., 1992; Mijovic et al, 1991).
One of the difficulties in the comparison and rationalization of these effects is that the experimental conditions and the materials have differed from group to group. A number of the reports of microwave effects are for the curing of epoxy resins and simply measure conversion with time (Boey et al., 1992). Unfortunately, it is difficult to analyze these data further, since the curing reaction can behave autocatalytically. Even within the general class of epoxy resins on which a large amount of work has been performed, the reactivity can vary more than an order of magnitude depending on the resin constituents and formulations. Furthermore, as the reaction progresses, molecular weight and cross-link density increase, limiting molecular mobility (which limits reaction rate) and making comparison of reaction kinetics difficult, especially at high conversions. A meaningful kinetic analysis must account for the development of network structure and the resulting reduction in mobility of reactive groups (Wingard and Beatty, 1990; Woo and Seferis, 1990).
Two general observations that can be made are that (1) slower-reacting systems tend to show a greater effect under microwave radiation than faster-reacting systems and (2) the magnitude of the observed effect decreases as the temperature of the reaction is increased. The manner in which temperature is measured and controlled is critical in kinetic analysis. The challenges associated with temperature measurement in a microwave field are discussed in Chapter 3.
A number of problems associated with kinetic analysis of a reacting system were avoided in a study of a solution imidization reaction. This reaction followed first-order kinetics, and the reactant and product remained in solution throughout the reaction (Lewis et al., 1992). Isothermal conditions were maintained by varying the microwave power or detuning the applicator. An 18- to 35-fold enhancement in the reaction rate was reported over the temperature range studied. The enhanced reaction rate corresponded to a reduction in the activation energy for the reaction from 105 kJ/mole to 55 kJ/mole.
A proposed mechanism for the "microwave effect" in polymers suggests a nonequilibrium, nonuniform energy distribution on the molecular level, which results in certain dipoles having a greater energy than the "average" energy of adjacent groups (Lewis et al., 1988, 1992). For the solution imidization of a poly(amic acid), this increased energy corresponded to an increase in an effective temperature of the reacting groups of approximately 50 ºC over the bulk temperature. The energy couples directly with a reactive polar group in this system and dissipates through adjacent groups by the usual mechanisms. However, if the energy is absorbed faster than it is transferred, at least initially, there will be a nonuniformity present. This mechanism is consistent with some of the recent pulsed-radiation studies in that the rate of energy transfer along the chain may be related to chain relaxations that occur on a similar time scale to the pulse repetition frequency.
Because of the range of materials studied, differences in temperature control and measurement methods, and variations in microwave applicators, based on available data it is impossible to determine the effect that microwave processing has on reaction kinetics. Consistent, controlled experiments, with careful measurement and control of temperature, that account for variations in resin chemistry and changes in reaction mechanisms during cure, are needed to investigate nonthermal microwave effects.
Polymer-Matrix Composites
High-performance polymeric composites, reinforced with carbon, glass, or aramid fibers, have been effectively used by the aerospace and electronics industries in applications requiring light weight, high specific strength and stiffness, corrosion and chemical resistance, and tailorable thermal-expansion coefficients. The dielectric properties of glass or high-performance, polymeric fiber-reinforced composites have made them attractive for printed circuit boards and in aviation, marine, and land-based systems radome applications.
More-general application of polymeric composites has been hindered by their high cost of orientation (layup) and forming (molding and curing) processes. Innovative processing, including automated lamination, rapid consolidation and curing, and out-of-autoclave processing, is being pursued in an attempt to reduce the costs associated with processing. Microwave processing shows promise for rapid, nonautoclave processing of composite structures.
The processing of very thick cross-section parts using conventional processing requires complex cure schedules with very slow thermal ramp rates and isothermal holds to control overheating due to cure reaction exotherms and poor thermal conductivity. Because of microwave penetration and rapid, even heating characteristics, thick composites were initially targeted as ideal applications for microwave processing. Early studies (Lee and Springer, 1984a, b) indicated that, while microwave curing of composites in wave-guide applicators was feasible, materials with conducting (carbon) fibers would be limited to unidirectional composites with less than about 32 plies (approximately 7—8 mm thick) due to the high reflectivity of the fibers and, hence, poor penetration depth of the radiation into the composite.
Tunable, single-mode resonant cavity applicators with feedback controls to allow the resonant frequency to be changed as material properties vary during processing have been developed to allow more-efficient coupling with composites (Asmussen et al., 1987, Asmussen, 1992). Much of the work accomplished in polymer and composite processing has utilized this type of cavity applicator.
The feasibility of curing thick cross-plied carbon fiber composites was shown when 36-and 72- ply composites were successfully cured using a single-mode resonant cavity (Wei et al., 1991). Heating was controlled through feedback on/off switching of microwave power based on sample temperature as measured using a fluoro-optic probe. The characteristic temperature excursion resulting from the exothermic reaction during epoxy cure was eliminated by using a pulsed system that allowed a higher temperature cure without thermal degradation (Jow et al., 1989; Jow, 1988). The mechanical properties of microwave-processed glass/vinyl ester composites were shown to be at least equivalent to those of conventionally processed materials, with indication that some property enhancement attributable to reduced void content occurred (Ramakrishna et al., 1993). Increased adhesion and improved mechanical properties at the fiber/matrix interface were observed for carbon-fiber composites due to preferential heating at the conductive fiber surface (Drzal et al., 1991). Although this process works well for flat parts, tuning of a single-mode cavity containing complex or large parts to provide uniform heating has not yet been accomplished (Fellows et al., 1993).
A tunable single-mode applicator was used to heat carbon-fiber reinforced PEEK thermoplastic (Lind et al., 1991). Enough power was absorbed to rapidly heat the PEEK matrix
to melt temperatures so that it could be bonded to a consolidated laminate. Based on these results, an applicator was designed and preliminary concepts were developed for an automated tape placement process for fabrication of composite parts (Figure 5-4). Feedback controls to adjust cavity resonance to account for panel curvature are required for scaling.
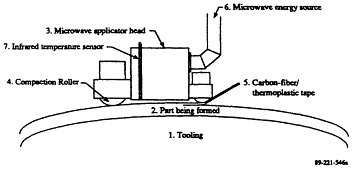
FIGURE 5-4 Concept drawing showing use of a microwave applicator in a tape-placement part-forming device (from Lind et al., 1991).
Potential Applications of Microwaves to Polymers and Composites
Although various studies have claimed that the microwave curing processes can have cost advantages over conventional processes (Simonian, 1979; Chabinsky, 1988; Akyel and Bilgen, 1989), microwave processing of polymers has not found widespread industrial application. However, there are polymer processes that are particularly promising for industrial application.
An area where microwave processing has shown promise is composite pultrusion. In pultrusion, a polymeric composite preform is pulled through a heated die, where the shape is molded and the matrix cured. In conventional processes, the processing chamber consists of a heated die, which is quite long due to the slow heat transfer to the polymer matrix and relatively long cure times. A single-mode resonance cavity has been used to rapidly heat the part using microwave radiation in a significantly shorter process chamber, resulting in less force required to pull the fiber bundle through the die (Methven and Ghaffairyan, 1992). Since the part configuration that the applicator sees is fixed for each shape, process control should be relatively simple.
In a process analogous to pultrusion, polymeric fibers are drawn through heated dies to increase their axial strength and stiffness through polymer chain orientation. When microwave radiation was utilized for drawing fibers, it was shown that the draw ratio could be increased from approximately 20:1 to 35:1 with a corresponding increase in the modulus from 35—40 GPa for conventional processing to 55—60 GPa for poly(oxymethylene) (Nakagawa et al., 1983; Takeuchi et al., 1985; Nakagawa et al., 1985), with similar results for other polymers (Amano and Nakagawa, 1987a, b). The significantly superior mechanical properties of microwave ultradrawn poly(oxymethylene) fibers over conventionally processed fibers (Nakagawa et al, 1983) were due to the increased orientation of the polymer chain in the fiber direction.
Because microwaves will couple selectively with materials that contain polar functionalities, it is possible to combine the efficiency and uniformity of heating with the selectivity of materials and accelerate and improve adhesion by coupling microwave energy directly into the adhesion interface. Recently, it has been shown that an intrinsically conducting organic polymer "self-heats" when it is exposed to electromagnetic radiation from a microwave, dielectric, or induction source or when a current is passed through it. The high dielectric loss tangent of a conducting polymer such as polyaniline (loss tangent greater than or equal to 10-1 at 6.5 GHz) is responsible for its microwave heating (Epstein et al., 1993). This heat is sufficient to locally melt and weld adjoining thermoplastic parts or cure thermoset polymers, but it does not heat the entire structure, which can result in softening or distortion. This phenomenon can be used to fabricate strong joints of plastics or composites either with each other or with metals. Extensive work has been done on the microwave welding of high density polyethylene (HDPE) using conductive gaskets made from a blend of HDPE and conducting polyaniline (Wu and Benatar, 1992). Under optimum welding conditions, the microwave-welded joint had a tensile strength equal to that of the bulk material.
MICROWAVE PLASMA PROCESSING OF MATERIALS
Microwave excitation readily forms plasmas at reduced gas pressures and, under some circumstances, at pressures in excess of 1 atm. Microwave plasmas are being utilized extensively for various applications in microelectronic processing, including deposition and etching for diamond film deposition; for surface modification; and, on an experimental basis, for sintering of ceramics. An important application of microwave plasmas, analytical spectroscopy, is outside the scope of this study.
Plasmas interact with surfaces in one of two ways beyond simply providing thermal energy for heating. Atomic or ionic species in the plasma may react with the substrate to form volatile constituents (etching), or species in the plasma may react to form solid materials, which are deposited on the substrate (plasma-enhanced chemical vapor deposition). Plasma surface modification processes may involve either of these interactions.
Microwave plasmas are generated in single-or multimode cavities, electron cyclotron resonance cavities, and coaxial torches. Coaxial torches find little use in materials processing. Microwave plasmas, in contrast to parallel plate RF plasmas, do not involve electrodes in contact with the plasma. This avoids contamination arising from sputtering from the electrodes. The specimen may be in direct contact with the plasma, or the effluent of the plasma may be utilized in the processing.
There are significant differences between microwave plasmas and the more common parallel-plate RF plasmas that are used for microelectronics processing. In RF plasmas, one or both of the electrode plates is excited at radio frequency, typically 13.56 MHz. A large DC bias is developed between the plasma and the electrode on which the specimen rests, causing bombardment of the specimen with directed high-energy ions. This phenomenon is utilized in the reactive ion etching RF systems. In a microwave plasma, a much smaller bias is developed between the plasma and the specimen than in RF plasmas. In addition, the degree of ionization is greater in the microwave plasma. These characteristics have significant consequences in
plasma processing. Depending on the process, the differences may be an issue in deciding whether to use a microwave plasma. An excellent review of microwave plasmas has appeared recently (Moisan and Pelletier, 1992).
The current literature on microwave plasma processing is heavily dominated by reports on diamond film formation. The growth of diamond films requires an abundance of atomic hydrogen, which etches graphitic nuclei in the deposit and leaves the diamond-like nuclei to grow. Plasmas generated by any means are, in general, good sources of this species. There are certain perceived advantages of microwave plasmas over other diamond film-forming methods. Cited examples include stability and reproducibility of the plasma, energy efficiency, availability of inexpensive magnetrons, and potential for scaling to larger sizes (NRC, 1990). A further advantage is that the microwave plasma can heat the substrate to the temperature required for good deposition conditions (greater than 500 ºC).
Microwave plasma processing has had a major impact in microelectronics device processing, where it is a mature art. A state-of-the-art review listed microwave plasma processing as a key technology that was sufficiently developed for imminent implementation in industry (NRC, 1986). The two major applications are plasma-enhanced chemical vapor deposition and etching, which includes the possibility of high-resolution etching of silicon (Moisan and Pelletier, 1992).
Deposition
Microwave plasma deposited materials include silicon films, which are amorphous or polycrystalline depending upon the substrate temperature, and silicon oxide and nitride. In addition, silicon can be oxidized to form silicon oxide films. The primary advantage of microwave plasma-enhanced chemical vapor deposition is reduction in radiation damage compared with conventional RF plasma chemical vapor deposition. This is because the microwave discharge results in a lower acceleration potential between the plasma and the substrate. The electron cyclotron resonance plasma technique is particularly useful in depositing silicon oxide and silicon nitride films on silicon for device processing. Films deposited at temperatures less than 150 ºC have chemical and physical properties equivalent to films deposited at 900 ºC using conventional chemical vapor deposition processes, and the low-energy ion bombardment does not damage the substrate. Similarly, silicon oxide films grown on silicon appear comparable to those grown by conventional thermal oxidation at 1000 ºC. Proper design of equipment, including positioning of feedstock injection, is important to avoid unwanted depositions on the walls of the reactor or other places.
The second major application of microwave plasmas is etching in electronic device processing. The principal advantage is that the microwave plasmas are more selective between photoresist and the underlying material. The second advantage is the lower intensity of radiation damage in reactive ion etching compared with conventional plasma etching because of the lower acceleration potential for ions. Finally, microwave plasma etching is reported to give highly anisotropic etching, although an RF bias is usually required to achieve the desired level of anisotropic etching (Moisan and Pelletier, 1992).
An RF bias to a microwave plasma not only increases the directionality of the etching, it also increases the rate of etching. Thus the microwave plasma is more selective than the RF plasma, whereas the RF plasma provides better directionality, so a combination of the two is required to obtain the desirable degree of both selectivity and directionality.
Surface Treatment
A third area of use of microwave plasmas is in surface treatment, where it has been applied to polymer fibers, as well as in the microelectronics industry. Chemical modification of the surface can be achieved with or without adding reactive components in the plasma. It has been demonstrated that treatment of polyamide fibers in a large microwave plasma system improves the bonding between the fiber and the matrix in composites (Wertheimer and Schreiber, 1981). This results in a dramatically different response to mechanical loads, providing for higher strength but at the same time a lower ballistic strength. Fiber mechanical properties can be degraded by the microwave plasma treatment.
Microwave plasmas are used also to promote adhesion of films in microelectronics processing. Advantage is taken here of the lower degree of radiation damage that is achievable with the microwave plasma than with other plasmas. By using a combination of microwave excitation and RF biasing, it is possible to independently control the relative contribution of the chemical component and the physical component (energetic ions, electrons, and photons).
In addition, microwave plasma sources have been used to passivate the surface of GaAs, resulting in superior device properties. The avoidance of direct ion bombardment of the surface was key to the success of this application.
The interactions among the physical and chemical components of a microwave plasma system are numerous and not well understood. Further work of a basic nature is required to better elucidate these interactions. Until then the industry and art probably will be dominated by solutions arrived at by trial and error. One of the aspects that should be explored in more detail is the effect of variable frequency on the chemical and physical processes occurring in the microwave plasma and on interactions with the substrate during deposition, etching, and surface modification.
MINERALS PROCESSING
The minerals and extractive metallurgy industry is a major consumer of energy and contributor to environmental degradation. For instance, about 4 percent of the carbon dioxide emitted to the atmosphere comes from the worldwide extractive metallurgy industry (Forrest and Szekely, 1991). Microwave processing may provide substantial benefits in reducing energy consumption and environmental impact by this industry.
In mineral processing, the extraction of values in an ore from the waste or gangue is an energy intensive and energy inefficient process. According to Walkiewicz et al., (1991) approximately 50—70 percent of the energy used for minerals extraction is consumed during
comminution (grinding) and separation. The energy efficiency of conventional grinding is about 1 percent, and most of the energy is wasted in heat generated in the material and equipment.
Microwave processing of ores provides a possible mechanism to induce fractures between the values in the ore and the waste material surrounding it, due to the differential in absorption of microwaves and the differences in thermal expansion among various materials. These differentials induce tensile fractures in the material (Figure 5-5), and as a consequence, substantially reduce the energy required in grinding to separate the values from the waste material.
A limited amount of work has been done in this area, and it is clear that microwaving of certain sulfide and oxide ores does result in fractures along the interface between the values and the waste material. Grindability tests show improved grindability (less energy is required to achieve a given mesh size for the ore) for a series of iron ores. However, it is not clear that the reduction in energy required in grinding will balance or exceed the energy expended in the microwave treatment of the ore. In iron ores the preliminary results indicate a deficit in the energy balance. To justify using microwave processing, it is also necessary to consider wear on grinding mills, cleaner liberation of the values in the ore, and lower chemical emission during the pyrometallurgy and hydrometallurgy processing steps.
Additional research needs to be done to determine the efficiency of coupling the microwave energy to the ore, the effect of particle size on susceptibility to cracking, and the effect on the cracking efficiency of using high power sources (the present work has been limited to maximum of about 3 kW).
MICROWAVE CHEMISTRY
Microwave chemistry is a rapidly growing field that has been gaining attention recently (IMPI, 1992; EPRI, 1993). The effect of microwave processing on chemical reactions or processes touches on most of the application areas emphasized elsewhere in this report. Some of these include ceramic sintering and synthesis, polymer curing, plasma processing, and waste remediation. In this section, applications in analytical and synthetic chemistry and extensions of these applications to the chemical industry are considered.
The most widespread use of microwaves in chemistry is in analytical laboratories. Microwave energy has been used in analytical chemistry since the mid-1970s, primarily for sample preparation. In that time, microwave ovens have become generally accepted tools in the modern analytical laboratory, increasing from a couple hundred units in 1975 to close to 10,000 units in 1992, while the annual expenditures for laboratory microwave systems increased from under $1 million in 1975 to close to $50 million (Neas, 1992a).
Applications span a wide range of sample preparation methods including drying, extractions, acid dissolution, decomposition, and hydrolysis. In these applications, microwave heating has been used as a replacement for conventional heating techniques. In general, analytical chemistry involves time-consuming sample preparation steps to get the samples in a suitable form for analysis.

FIGURE 5-5 Photomicrograph of pyrite ore (a) nonmicrowaved and (b) microwaved, showing stress cracking. The light phase is pyrite; and the dark phase is quartz (magnified 100×). (Courtesy of J. Walkiewicz, U.S. Bureau of Mines)
Microwave digestion of materials, such as minerals, oxides, glasses, and alloys, is used in laboratories worldwide to prepare samples for chemical analysis. The decomposition rate of many difficult-to-dissolve materials in closed-reaction vessels is greatly enhanced by using microwave energy; often only a few minutes are required as opposed to the several hours needed for conventional means (Kingston and Jassie, 1988a, b). In addition, volatile elements such as selenium and phosphorous can be quantitatively retained in a sealed vessel using microwave decomposition prior to instrumental analysis (Patterson et al., 1988).
Applications in the chemical laboratory generally use relatively simple ovens and controls. Most of the development work in equipment for these applications has involved improving the existing equipment to extend the operating range and to improve safety and reproducibility. Examples include improved turntables and sample fixtures, pressure vessels fabricated from glass and quartz to allow higher reaction temperatures and pressures than teflon vessels, and optimized pressure relief valves (Baghurst and Mingos, 1992b). The result of these advances has been the development of testing standards that are simple, reproducible, and automatable (Kingston, 1992). A wide variety of organic synthetic reactions have been shown to be enhanced by microwave processing (Bose et al., 1993; Majetich and Hicks, 1993). Using microwave processing, a number of fundamental organic reactions have shown accelerated reaction rates and increased yields over conventional techniques. While these processes have not yet been scaled to production, important advantages have been realized in education, where reactions that took too long to accomplish in a laboratory session using conventional heating can now be completed using microwave heating.
The primary motivation for use of microwave heating has been time savings through rapid heating, rather than any nonthermal effects. Penetrating radiation (and reverse thermal gradients), the ability to superheat polar solvents, and the ability to selectively heat reactive or catalytic compounds were responsible for time savings realized in chemical processes.
Microwave energy penetrates into the interior of the sample without relying on conduction from the surface required in conventional heating methods. This allows the entire sample temperature to be raised rapidly without overheating, and possibly degrading, the surface. Convective heat losses from the surface to the cooler surroundings allow processors to take advantage of reverse thermal gradients.
The reaction temperature of solvent diluents can be raised above the ambient boiling points of the diluents in both closed-and open-reaction vessels (Baghurst and Mingos, 1992a). This allows for significant increases in reaction rates in a variety of applications (Mingos, 1993). Reaction rate enhancements were attributable to Arrhenius rate effects due to increased reaction temperature or selective heating of reactants over diluents. There are no persuasive arguments to support nonthermal reaction enhancements attributable to the use of microwaves (Majetich, 1992; Mingos, 1992).
In closed vessels, the increased vapor pressure over the liquid suppresses further boiling. Microwave super-heating of volatile solvents can lead to significant acceleration of chemical processes compared with conventional reflux conditions. The development of microwave transparent glass and quartz reaction vessels and improved pressure-relief valves has been critical in allowing attainment of higher temperatures and pressures than was possible with low-loss teflon vessels (Baghurst and Mingos, 1992b).
In open vessels, most polar solvents have an inherent ability to be heated above their conventional boiling points. This effect has been observed by several researchers (Mingos, 1993; Baghurst and Mingos, 1992a; Neas, 1992b; Majetich, 1992). The phenomenon has been explained using a model of nucleation-limited boiling point (Baghurst and Mingos, 1992a). During a boiling process, bubbles nucleate preferentially at sites (cavities, pits, scratches) on the vessel wall, allowing growth of the vapor phase. With conventional heating, the vessel wall and liquid surface are generally hotter than the bulk. In microwave processes, however, the vessel wall is cooler than the bulk solution due to convective heat losses from the surface, allowing the
bulk to attain temperatures above the conventional boiling point before the boiling process commences. As shown in Table 5-7, the nucleation-limited boiling point varies with the solvent and with the ability of the solvent to wet the vessel surface, that is, the more effectively the solvent wets the vessel, the more difficult bubble nucleation becomes.
TABLE 5-7 Nucleation-Limited Boiling Points for a Range of Solvents
|
Solvent |
B.p./(ºc) |
Nucleation-Limited Boiling Point (ºC) |
Boiling Point Change (ºC) |
|
Water |
100 |
104 |
4 |
|
Ethanol |
79 |
103 |
24 |
|
Methanol |
65 |
84 |
19 |
|
Dichloromethane |
40 |
55 |
15 |
|
Tetrahydrofuran |
66 |
81 |
15 |
|
Acetonitrile |
81 |
107 |
26 |
|
Propan-2-ol |
82 |
100 |
18 |
|
Acetone |
56 |
81 |
25 |
|
Butanol |
118 |
132 |
14 |
|
1.2-Dimethoxyethane |
85 |
106 |
21 |
|
Diglyme |
162 |
175 |
13 |
|
Ethyl acetate |
78 |
95 |
17 |
|
Acetic anhydride |
140 |
155 |
15 |
|
iso-Pentyl alcohol |
130 |
149 |
19 |
|
Butan-2-one |
80 |
97 |
17 |
|
Chlorobenzene |
132 |
150 |
18 |
|
Trichloroethylene |
87 |
108 |
21 |
|
Dimethylformamide |
153 |
170 |
17 |
|
Chlorobutane |
78 |
100 |
22 |
|
iso-Propyl ether |
69 |
84 |
16 |
|
Source: Baghurst and Mingos, 1992a. |
|||
By using microwave heating, the processor is able to target compounds with high dielectric loss over less-lossy compounds. This characteristic has been shown to enhance a number of chemical processes, including catalytic reactions utilizing metallic or dielectric catalysts, gas-phase synthesis of metal halides and nitrides, and metal reduction processes (Bond et al., 1992).
The promising future of microwave chemistry to the chemical industry is just beginning to be realized. Advantages in the form of time savings, increased reaction yields, and new processes have been demonstrated in the laboratory using simple multimode ovens. Scaling to
widespread production applications requires development of applicators and handling systems to account for increased product throughput, automation, and large reactors.
Microwave processing for small-scale, custom organic synthesis looks promising due to the relatively modest equipment investment, broad applicability to a variety of reactions, and significantly reduced processing times (Bose et al., 1993). The availability of equipment and the long history of use in the laboratory makes these near-term applications low risk. Another area where microwaves can show an advantage is in producing products or intermediates that are needed in small quantities and may be hazardous and expensive to ship, store, and handle (Wan and Koch, 1993).
WASTE PROCESSING AND RECYCLING
The processing of industrial wastes is an area of tremendous promise for the application of microwave energy. The types of industrial waste that have been shown to be amenable to microwave processing, at least at laboratory scale, include hazardous waste (including toxic and radioactive) with high disposal, storage, or treatment cost and nonhazardous waste where the recovery or reuse of a raw material represents a significant cost or energy savings. Waste processing includes treatment or remediation of process wastes, detoxification or consolidation of stored waste, or cleanup of storage or disposal sites.
The application of microwave energy in the processing of industrial waste has seen significant progress in terms of process development and demonstration but limited commercial application. In varying degrees, applications in this area take advantage of unique features of microwave heating: rapid heating, selective coupling with lossy constituents, and reaction steps not possible or practical with other methods.
Process Waste Treatment
Potential applications of process waste treatment include microwave plasma hydrogen sulfide dissociation, detoxification of trichloroethane (TCE) through microwave plasma assisted oxidation, and microwave plasma regeneration of activated carbon.
The dissociation of hydrogen sulfide (H2S) in a microwave plasma was first described in Soviet literature (Balebanov et al., 1985). The potential for this process is in the refuting industry for the treatment of the sour gas resulting from hydrodesulfurization of hydrocarbon feedstocks. Subsequent work at Argonne National Laboratory has validated the applicability (Harkness et al., 1990) and economic viability (Daniels et al., 1992) of this process in the treatment of refining wastes. A schematic of the microwave dissociation process is shown in Figure 5-6. The attainable conversions, between 40 and 90 percent, were most sensitive to gas flow rate and power. Conversions up to 99 percent are achievable by cycling the residual H2S back through the process in multiple passes. Work is continuing to scale the process. The economic viability of the process depends on the sale of recovered hydrogen and is sensitive to required dissociation power.
FIGURE 5-6 Hydrogen sulfide waste-treatment process utilizing microwave plasma dissociation. (Courtesy of E. Daniels, Argonne National Laboratory)
TCE oxidation and activated carbon regeneration are accomplished through selective heating of lossy components (SiC or carbon) in a fluidized bed. These processes cause degradation of hazardous organic compounds at significantly lower overall temperatures than conventional heating methods. Additionally, severe corrosion of furnace components caused by the gases released in conventional high-temperature oxidation of chlorinated hydrocarbons is eliminated in the microwave process.
Stored Waste Treatment
Examples of microwave processing of stored waste include ''in-can'' evaporation of water and consolidation of low-level radioactive waste (Oda et al., 1991; White et al., 1991a). These processes heat low-level sludge wastes in the final storage containers by applying a microwave field using a slotted waveguide applicator. In-can processes use the rapid, selective heating of
water possible with microwaves and the portability of microwave equipment to reduce transfer and handling of hazardous materials that is necessary when using conventional drying approaches. This approach need not be limited to radioactive sludge but can be applied to any evaporative treatment of stored materials. Even though, as mentioned earlier, the efficiency of bulk drying using microwaves is questionable, the cost avoidance realized by reducing handling steps may justify the increased energy costs.
Waste-Site Cleanup
The cleanup of contaminated industrial, disposal, and storage sites is a formidable task due to the large number of waste sites and the complex chemistries and remediation requirements involved. Currently, the majority of site cleanup efforts require removal and incineration of the waste. Since removal and transfer of contaminated materials for incineration may represent an unacceptable risk, innovative processes to cleanup contaminated storage and disposal sites are being investigated. Microwave processing shows great promise for site cleanup applications, since microwaves can be applied in situ, avoiding costly and risky excavation and transportation, and can target compounds with high dielectric loss for selective heating, for example, moisture in soils (Dauerman, 1992).
Potential applications of microwave processes for cleanup of contaminated sites include removal of volatile organic compounds from soil (George et al., 1991; Windgasse and Dauerman, 1992) and remediation of soils contaminated with nonvolatile organic compounds, by causing reaction with bound indigenous organics (Zhu et al., 1992), and chromium, by causing conversion from the toxic hexavalent form to the nontoxic trivalent form (Sedhom et al., 1992). The feasibility of these processes has been demonstrated on a bench scale in a multimode oven. However, the challenge of bringing applicators and sufficient power to waste sites is formidable. If the promise of these applications is to be realized, additional work needs to be done to develop applicators for in situ processing and to show applicability and cost effectiveness on a larger scale in the field.
The cleanup of surface layers (0.5—5 cm depth) of concrete structures contaminated with radioactive or hazardous materials is a potentially costly problem affecting research laboratories, power plants, and processing and storage facilities. Mechanical removal methods create potentially hazardous dust, may drive contamination into the interior, or may create a large waste stream of contaminated water generated in dust amelioration efforts. Microwaves have been shown, in experiments in Japan (Yasunaka et al., 1987), Britain (Hills, 1989), and the United States (White et al., 1991b), to be effective in rapidly removing the outer layer of concrete in a dry process with reduced dust generation. Work is underway at Oak Ridge National Laboratory to scale-up the microwave concrete-removal process and to develop, build, and test a full-scale prototype. It is believed that removal rates exceeding those attainable through mechanical techniques are possible with optimized power, frequency, and applicator design. A schematic of the prototype apparatus is shown in Figure 5-7.
FIGURE 5-7 Schematic of prototype concrete-scabbing apparatus. (Courtesy of Dr. Terry L. White, Fusion Energy Division, Oak Ridge National Laboratory, Department of Energy).
SUMMARY
While a wide variety of materials have been processed using microwaves, including rubber, polymers, ceramics, composites, minerals, soils, wastes, chemicals, and powders, there are characteristics that make some materials very difficult to process. First, materials with significant ionic or metallic conductivity cannot be effectively processed due to inadequate penetration of the microwave energy. Second, insulators with low dielectric loss, including oxide ceramics and thermoplastic polymers, are difficult to heat from room temperature due to their low absorption of the incident energy. Since permittivity and loss factors often increase with temperature, hybrid heating may be used to process these types of materials by using alternate or indirect heating to raise the temperature of the parts to where they can be more effectively heated with microwaves. Finally, materials with permittivity or loss factors that increase rapidly during processing, such as alumina, can exhibit hot spots and thermal runaway. Although insulation or hybrid heating can improve the situation, stable microwave heating of these types of materials is problematic.
Enhanced apparent process kinetics due to microwave processing have been claimed for a range of materials, most notably ceramic sintering and polymer curing. However, in most cases, insufficient care was taken in temperature control and measurement and in measurement of critical process variables and material physical properties. A series of careful experiments with an internal calibration of the temperature is needed to eliminate the doubts that remain about the microwave enhancement effects.
Further investigation is needed to develop maps of the regimes of microwave-power absorption characteristics, batch size, heating rate, and other variables where microwave processing can be reproducible and uniform. This would allow processors to make informed decisions concerning microwave applications and process and equipment selection, while avoiding inefficient heating, uneven heating, and thermal runaway problems that have plagued earlier attempts.
Specific processes that show promise for future development include:
-
ceramic processes including drying, chemical vapor infiltration, reaction bonding of silicon nitride, powder synthesis, and joining;
-
polymeric composite pultrusion, ultradrawing of polymeric fibers, and adhesive bonding with intrinsically conducting organic polymers;
-
chemical processes, including custom organic synthesis, hazardous materials processing, solvent extraction, and drying; and
-
industrial waste processing, including treatment or remediation of process wastes, detoxification or consolidation of stored waste, and cleanup of storage or disposal sites.
In general, the elements required for successful application of microwave processing to industrial materials include selection of materials amenable to microwave processing; an understanding of the process requirements; an understanding of the process economics; characterization of material thermochemical properties; selection of equipment and design of applicators suitable for the application; an understanding of how the parts to be processed will interact with the microwave field; and adequate measurement and control of process variables such as incident power, part temperature, and field strength.