HIV-1 PREVENTION: INTERDISCIPLINARY STUDIES AND REVIEWS ON EFFICACY OF BLEACH AND COMPLIANCE TO BLEACH PREVENTION PROTOCOLS
CLYDE B. MCCOY, Comprehensive Drug Research Center and Department of Epidemiology, University of Miami, Florida; PAUL SHAPSHAK, Comprehensive Drug Research Center and Departments of Psychiatry, Neurology, and Pathology, University of Miami, Florida; SYED M. SHAH, Comprehensive Drug Research Center, University of Miami, Florida; H. V. MCCOY, Comprehensive Drug Research Center, University of Miami, Florida, and Department of Public Health, Florida International University, Miami, Florida; JAMES E. RIVERS and J. BRYAN PAGE, Comprehensive Drug Research Center and Departments of Psychiatry and Epidemiology, University of Miami, Florida; DALE D. CHITWOOD, Comprehensive Drug Research Center and Department of Sociology, University of Miami, Florida; NORMAN L. WEATHERBY, Comprehensive Drug Research Center and Department of Epidemiology, University of Miami, Florida; JAMES A. INCIARDI, Comprehensive Drug Research Center, University of Miami, Florida, and Center for Drug and Alcohol Studies, University of Delaware, Newark; DUANE C. MCBRIDE, Department of Behavioral Sciences, Andrews University, Berrien Springs, Michigan; DEBORAH C. MASH, Comprehensive Drug Research Center and Department of Neurology, University of Miami, Florida; and JOHN K. WATTERS, Institute for Health Policy Studies, University of California, San Francisco
SUMMARY
A major federally-funded approach to human immunodeficiency virus (HIV-1) prevention for injecting drug users (IDUs) includes teaching them to always rinse their needles/syringes with household bleach and water before use. This report describes interdisciplinary studies and reviews of the extent to which HIV-1 can be found in injection equipment and the efficacy of bleach as a disinfectant, and the compliance of IDUs to bleach cleansing protocols, under simulated field conditions.
Bloody needle/syringe units collected from Miami, Florida, shooting galleries or from community outreach prevention participants were selected for these studies. Groups of needle/syringe units were cleansed with bleach using a standard technique taught to IDUs in community outreach programs. Cleansed and uncleansed groups of needles/syringe units were then tested for the presence of HIV-1.
Participants (450) in a NIDA federally funded intervention project were tested on the ability to recall and perform bleach cleansing protocols taught six months earlier. IDUs demonstrated high compliance on basic elements, but somewhat less so with each detailed steps of the protocol.
The data demonstrate the efficacy of bleach rinses in reducing the risks of HIV-1 infection from needle/syringe units and indicate that the teaching and self-demonstrations of a bleach cleansing method to IDUs should be part of a total AIDS prevention effort to increase efficacy and compliance.
INTRODUCTION
In their attempt to understand the transmission of HIV-1, and therefore the potential for reducing and preventing its spread, researchers have viewed the transmission in several ways, characterizing the spread of HIV-1 among high risk groups such as the gay population and injecting drug users (IDUs), prostitutes and sexual partners of IDUs. This characterization of high risk groups sometimes clouded the issue that transmission occurred because of high risk behaviors occurring between individuals, one of whom had already been infected with HIV-1 regardless of whether the individual was a member of such the risk group or not. These high risk behaviors therefore can be characterized as often being carried out in high risk environments such as bath houses, crack dens, shooting galleries, and places of prostitution. These high risk environments provide useful access points for the study of the transmission of HIV-1.
Since it was first described in 1981, acquired immune deficiency syndrome (AIDS) has been concentrated in the United States among people who engage in certain high-risk behaviors. Intravenous and other injecting drug users represent the second highest risk group after homosexual and bisexual men and comprise an increasing percentage of all new cases of AIDS.
RISKY PRACTICES
HIV-1 is transmitted among IDUs by the sharing and/or pooling of contaminated injecting apparatus and associated paraphernalia, as well as by the sharing of injection drugs themselves from contaminated equipment/paraphernalia. The intravenous administration of heroin, cocaine, and other drugs typically includes a practice known within injecting drug subcultures as "booting." The practice involves the aspiration of venous blood back into a syringe for the purpose of mixing the drug with blood, while the needle remains inserted in the vein. The mixed blood/drug solution is then injected back into the vein. Injecting drug users value the mixing of blood for several reasons: some repeated pumping and drawing back of the blood-drug mix allows the user to titrate the dose and avoid overdose or the full effects of potential contamination often present in drug preparations; the drawing of blood into the syringe also indicates that the needle has hit a usable vein; some believe that this "pre-mixing" enhances a drug's effects. Since injecting drug users often inject with needles and syringes previously used by others, particularly if they are administering the drugs in "shooting galleries" (places where injecting drug users gather to take drugs), booting increases the probability that HIV-1 will remain in a syringe to be transmitted to the next user.
"Frontloading" and "backloading" are IDU drug sharing practices conducted in group settings, possibly but not necessarily in the injecting equipment rented and shared in shooting galleries. These practices were recently reported in the professional literature by investigators of community outreach and intervention projects sponsored by the National Institute on Drug Abuse.8,9 In groups using these practices, all of the chosen or available drug(s) are pooled for mixing and distribution among the present agreed-upon participants. One participant will measure the prepared drug(s) solution by drawing it into his/her syringe and checking the number of cc units. After calculating each person's equal share by dividing the amount by the number of participants, each user receives his/her allocation by either "backloading" (ejecting it from the syringe of the mixer directly into the other users' open syringes) or "frontloading" (ejecting all but the mixer's own share back into the ''cooker" [or mixing container], with each user then drawing up their own share). In either method, if the mixer/distributor has HIV-contaminated injection equipment (which may even be his/her personal apparatus), each of the other users can be HIV-infected, even though they have not shared needle/syringes or even if they have used their own personal "works," sterile used or even new equipment.
RISKY ENVIRONMENTS
It is common knowledge that the likelihood of performing many behaviors is increased in certain physical environments that are identifiable by characteristic behavior of the group(s) who habituate that setting or locale; this is sometimes called a contextual effect. Therefore, the likelihood that individuals will engage in behavior representing, in general, high risk for HIV infection is increased when they are among many others engaging in such behavior and their risk is elevated even further when the rate of HIV seropositivity is high among the group being emulated. Environments such as bath houses, houses or areas of prostitution, and shooting galleries represent this scenario.10
Frequent injection in "shooting galleries" long has been considered to be particularly associated with HIV infection among IDUs.11,12,13 In these environments, individuals rent used needle and syringe units which typically are reconditioned to extend their usable life far beyond nine uses, which is the average number reported by injection drug users who reuse their own or share their personal injecting equipment with others.14 The risk-laden practices of "frontloading" and "backloading" are also more likely to occur in these group environments.8,9
Research and clinical observation suggest that "booting", the use of shooting galleries, and the sharing of needles combine to explain the increasing proportion of injecting drug users infected with HIV-1.26,27 The sharing of needle/syringe units has been well documented as a primary vector for the spread of the AIDS virus among IDUs.1,2,3 In particular, frequency of injection in shooting galleries has been associated with HIV-1 seropositivity among IDUs.4,5,6 Recent studies have identified the presence of HIV-1 antibody in a large sample of needle/syringe units collected from shooting galleries in Miami, Florida.7,8 In an effort to reduce exposure to HIV-1 among out-of-
treatment IDUs, a number of behavioral risk reduction programs has been initiated in communities with high rates of injecting drug use and AIDS.9,10 Instruction in the cleansing of needle/syringe units with full-strength household bleach is one of the practical skills taught to participants in these programs who choose to continue injecting drugs.11 This cleansing technique was first implemented on a largescale in 1986 in a San Francisco AIDS prevention program for out-of-treatment IDUs.12,13,14 Given the general lack of needle exchange programs in the United States, and internationally,15,16,31,32,33 such risk reduction efforts remain as one of the more significant modus operandi for reducing the spread of HIV-1 among IDUs.
Two important separate, but complementary, issues surround the utilization of bleach as a preventive measure to reduce the risk of transmitting the HIV-1 from contaminated needle/syringe units: 1) the efficacy or actual effectiveness of bleach in the decontamination of the needle/syringe units, and 2) the compliance to the cleansing protocol taught in these prevention programs-how reliably do IDU's actually carry out the procedures that are taught?
More recently, information presented at a public health workshop at Johns Hopkins in Baltimore prompted the release of two important and unique federal bulletins which resulted in additional questions about what the specific decontamination protocol should be.17,18 This series of events has inspired an attempt to accelerate related research and its dissemination, further examining both the effectiveness of bleach and the implications of these new findings for compliance to protocols.
A multi-disciplinary research team has been carrying out a series of collaborative studies on these issues for the past several years. This article presents the research procedures and findings of these interdisciplinary studies that attempt to establish empirical bases underlying revised guidelines and protocols that will increase the effectiveness of risk reduction strategies utilized in community prevention programs.
While household bleach has been shown to inactivate HIV-1 in clinical and laboratory settings,19 there have been few studies which have directly examined the efficacy of bleach disinfection of injection equipment under conditions which realistically approximate the field conditions faced by IDUs.21,22
Six separate, but interdependent, sets of experiments combining field, laboratory and clinical techniques and methodologies are presented in this paper as outlined below.
-
Prevalence of HIV-1 in field-collected needles and syringes.
-
Testing the efficacy of bleach in needles and syringes collected from field conditions and randomized for bleach cleansing.
-
Inactivation of HIV-1 with bleach: Results under laboratory conditions.
-
Inactivation of HIV-1 with bleach: Results under approximated field conditions
-
Results of tests utilizing diluted bleach
-
Bleach utilization and compliance among participants in a community prevention program.
PREVALENCE OF HIV-1 IN FIELD COLLECTED NEEDLES AND SYRINGES
When the University of Miami researchers began their first series of studies, they reviewed studies of HIV-1 in needles and syringes and discovered that there were no reports that assessed the extent to which injection equipment owned by shooting galleries is positive for HIV-1 antibodies. A study of needle exchange programs in Sydney, Australia, had determined that antibodies for HIV-1 were present in 3.1 percent of 1,544 needle/syringe units exchanged at two exchange centers.22 More recently, studies of needle exchange programs in the U.S. also tested a large sample of needle/syringe units.31,32,33 The University of Miami researchers suspected that risk of exposure to HIV-1 probably is higher in shooting galleries. There, each individual rents works that may have been used by others and passed on to others later. Needle/syringe units are routinely reconditioned to extend their useful life far beyond the average nine uses reported by injecting drug users in Miami who reuse or share personal works.7
In order to examine the potential for HIV-1 transmission through the use of injection equipment available in these high-risk settings, needle/syringe units were collected from these shooting galleries frequented by parenteral drug users in Miami and were tested for antibodies to HIV-1 (see Table 1). "Fifteen of 148 needles (10.1 percent) tested positive for HIV-1 antibody. Seropositivity rates did not vary by the day of the week of collection, nor by shooting gallery from which they were collected. When the needle appeared to contain blood residue, 20.0 percent were positive versus 5.1 percent with no visible blood residue. These findings suggest that needles/syringes used in shooting galleries are likely to serve as reservoirs and/or vectors of transmission of the HIV-1 virus, and that although visual inspection of the needle/syringe may be useful in lessening the chance for transmission, even the visually "clean" needles may result in transmission of infection.7
Based upon this study, a probability matrix was constructed to determine the likelihood of infection in number of days given the two conditions of blood visibility and non-visibility in the syringes8 (see Table 2). The data indicate that given the assumptions of randomness and a 10.1% likelihood of needle/syringe contamination with seropositive blood, a user shooting up just once a day in a gallery would have a 90% chance of encountering an HIV-1 contaminated needle/syringe (or 10% likelihood of not encountering one) within 22 days. Shooting up 3 times a day in a gallery (and using a different needle/syringe each time) reduces the number of days to 7, and shooting up 5 times a day further reduces the time for a seropositive encounter to within 4 days. We should point out that, depending on the drug used, the personal schedule of the user, and the number of pooled needles versus clients, those who shoot more than once a day could be using the same needle/syringe more than once a day (which may or may not have been used by other individuals). A cocaine shooter, for example, may come in for
three quick hits on a single occasion, using the same house-issued needle/syringe consecutively all three times.
RANDOMIZED STUDY OF FIELD COLLECTED NEEDLES/SYRINGES
The second set of studies also focused upon the environment of shooting galleries, and in addition to estimating the extent of HIV-1 in needle/syringe units, addressed the effects of bleach cleansing. While household bleach has been shown to inactivate HIV-1 in clinical and laboratory settings, there have been few studies which have directly examined the efficacy of bleach disinfection of injection equipment under conditions which realistically approximate the field conditions faced by IDUs. Assessing the extent of HIV-1 in needle/syringe units and the efficacy of bleach in decontamination of any HIV-1 present followed the overall study design presented in Figure 1. The research design called for (a) the controlled collection of needle/syringe units from representative shooting galleries in Miami, (b) testing these in the laboratory for presence of HIV-1, and (c) testing the efficacy of bleach in the decontamination process.
In order to provide a more definitive validation of bleach cleansing methods, used needle/syringe units were collected from shooting galleries in Miami and randomized into two groups. One group was cleansed using the standard technique taught to National AIDS Demonstration Research (NADR) project participants in the Miami community outreach prevention program, while the other remained uncleansed. Both the cleansed and the uncleansed groups were then tested for antibodies to HIV-1.
Used needle/syringe units were collected from four separate shooting galleries in Miami. These were among the most frequently mentioned galleries patronized by IDUs enrolled in the Miami National AIDS Demonstration Research project. Shooting galleries again were chosen as the source of the needle/syringe units, based upon the extent of HIV-1 presence found in the study discussed above.7,8 In addition, collecting the needle/syringe units in a systematic manner from similar sources (the shooting galleries) provided a more controlled study approach that also permitted randomization.
Each gallery was located in a different inner-city area known for its high rates of drug use. Field observations at additional Miami shooting galleries indicated that the four sites from which the needle/syringe units were gathered were typical of area shooting galleries and were similar and from the same parts of the city as galleries reported in earlier studies.7 Access to each of the four galleries was gained through the efforts of a staff outreach worker who had established a network of contacts with the IDU population in Miami. All shooting gallery operators rented used injection equipment to their clients for $2. Each operator was paid a flat fee of $24 per specimen collection visit by the outreach worker and did not participate in the selection of the needle/syringe units.
During a three-month period in late 1991, needle/syringe units that recently had been used in the shooting galleries (up to 24 hours earlier) were collected each morning by the outreach worker and brought to the Comprehensive Drug Research Center at the
University of Miami. Approximately 15 to 20 needles/syringes were brought in each week.
Laboratory Control Study
Prior to conducting the study of the field-collected needle/syringe units, it was necessary to validate the laboratory measures which would be used. Blood from three seropositive IDUs (confirmed by p24 antigen capture assay and Western Blot) was drawn into 24 needle/syringe units. These seropositive subjects were in an early asymptomatic stage of HIV-1 infection based on interviews and physical examination. Eight needle/syringe units were filled with blood from each subject, emptied, and left undisturbed overnight.
Twelve needle/syringe units (four from each of the three subjects) were then cleansed with bleach using the standard National AIDS Demonstration Research (NADR) projects technique.11, 34 Full-strength household bleach (5.25% sodium hypochlorite, volume/volume), was drawn up through the needle to completely fill the syringe. After emptying, the needle/syringe unit was again filled with bleach and emptied a second time. It was then flushed twice with water. Time intervals for experiments with needles were measured by observing a precision laboratory clock.
Laboratory procedures were done as previously described23,24. After the cleansing, 0.5 ml volumes of phosphate-buffered saline (PBS) solution was then used to rinse each of the 12 bleach-cleansed and the 12 uncleansed needle/syringe units. Thin wires were used as catheter plungers to dislodge debris and promote the rinses when necessary. The PBS solutions were then tested for antibodies to HIV-1 using the Western Blot procedure. Western Blot detection of antibodies to HIV-1 was performed using licensed kits from Biorad Laboratories according to the manufacturer's instructions (Biorad Inc., Hercules, CA). The Western Blot procedure was sensitive down to 30 nanoliters of serum from HIV-1 positive individuals (data not shown).
The results of the laboratory control study revealed that the PBS rinses from all (100%) of the 12 HIV-1-contaminated needle/syringe units that were rinsed with bleach and water tested negative for HIV-1 antibodies. All (100%) of the PBS rinses from 12 needle/syringe units that were not rinsed with bleach and water tested positive (Table 3). These results validated the methodology for the simulated field cleansing of the needle/syringe units collected from the Miami shooting galleries.
Simulated Field Cleansing of Used Needles/Syringes
Using a method previously described,7 the needle/syringe units were graded as to appearance and only those showing visible blood were selected and numbered. These were then randomized into two groups using a table of random numbers and sent directly to the Retroviral Immunodiagnostic and Research Laboratory at the University of Miami where they were identified only by number.
A laboratory staff member cleansed the 121 needle/syringe units in the first group with household bleach using the method described above. The other group of 116 needle/syringe units remained uncleansed. All needle/syringe units were then rinsed with phosphate-buffered saline (PBS) solution and tested using the Western Blot procedure.
A total of 11 needle/syringe units (3 from the bleach cleansed group and 8 from the uncleansed group) tested indeterminate on Western Blot according to the specifications of the manufacturer. In an attempt to resolve the indeterminate classification, the volume of rinse solution tested in the Western Blot was increased three-fold. New test results remained indeterminate, nevertheless, and these needle/syringe units were excluded from the analysis.
The results of testing the needle/syringe units, collected from four Miami shooting galleries, are provided in Table 4. Of the 108 needle/syringe units not rinsed with bleach and water, more than half of these tested positive for HIV-1 antibodies and 44 percent tested negative. Of the 120 needle/syringe units, rinsed with bleach and water, none (0%) of these tested positive with Western Blot for HIV-1 antibodies.
These findings suggest that needle-syringe units used in shooting galleries are likely to serve as reservoirs and/or vectors of transmission of HIV-1. Further, they suggest that simple visual inspection of needle-syringe units might lessen individual risk of infection if the IDU were to react to observed residue by properly cleansing the dirty equipment with bleach and water. At the same time, the fact that even visually "clean" needle-syringe units tested HIV-1 antibody positive suggests that appearance is not a reliable indicator of the potential for contracting the virus.
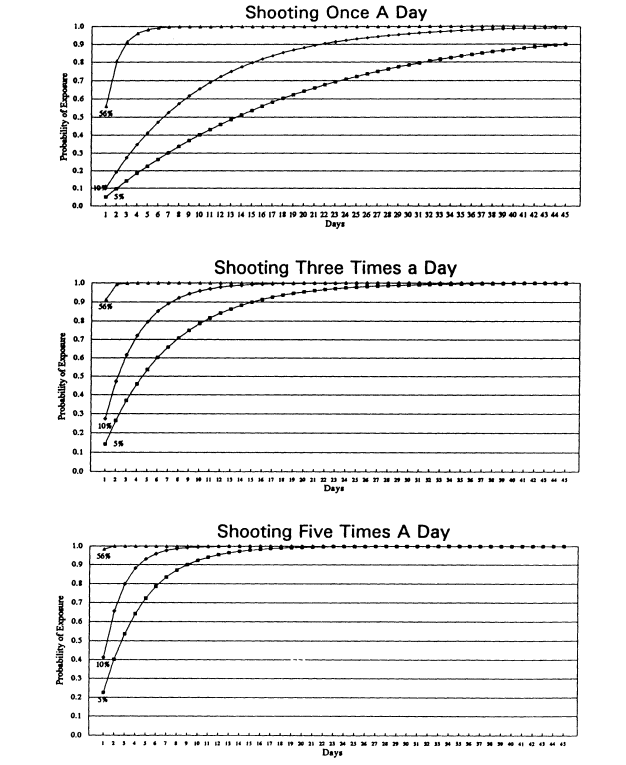
Given that the seropositivity rates of these visibly bloody needle/syringe units were much higher than in our earlier studies, we thought it would be instructive to update the probability matrix, including the 5%-10% seropositivity of the earlier studies with the 52% of the most recent study. The relative probability of exposure in number of days to a contaminated needle representing varying rates of seropositivity found in the shooting galleries is provided in Figure 3.
The Miami researchers constructed probability graphs, similar to the matrix discussed earlier, based upon rates observed from both studies, to determine the likelihood of exposure to HIV via a contaminated needle-syringe unit in shooting galleries (see Figure 2). Given the assumptions of randomness and a 10 percent likelihood of needle-syringe unit contamination, they estimate that a user "shooting up" just once per day in one of these galleries would have a 90 percent chance of encountering an HIV-contaminated unit within 22 days. More frequent injection events decreased the days-to-exposure: injecting three times per day (assuming a different unit were used each time) reduced the number of days to 7; shooting five times per day raises the risk to 4 days. When the assumption is changed to the 52 percent rate observed in the second study, the once-per-day shooting gallery user might have a 90 percent chance of exposure to HIV-contaminated equipment in just 3 days.
There are, of course, other variables which can effect these probabilities, such as the personal schedule of the user and the drug used. For example, a cocaine user may come in for three quick "hits" on a single occasion, using the same house injecting unit
each time. The investigators note that the more important points from their analysis are: the risk of infection stemming from injecting equipment reuse and rental practices in shooting galleries is extremely high, and explaining these 'odds' in understandable and persuasive terms should be an educational priority in any HIV risk reduction strategy.
As can be determined by viewing our overall research design (Figure 1), the initial intent was to culture any virus contained in the rinses from the needle/syringe units after processing in the laboratory. However, virus could not be consistently cultured from the rinses of the needle/syringe units collected from the field, as would be required in order to gain the precision and conclusive evidence needed by these types of experiments. When viruses were filtered using 0.2 micron pore-size filters, it was difficult to isolate the virus consistently from the subsequent cultures. Filtration was performed in order to eliminate microbial contamination in rinses of needle/syringe units from the field. In addition, filtration of rinses from needle/syringe units exposed to HIV-1 infected blood in the laboratory also resulted in a significant reduction in the ability to isolate viruses from the rinses (data not shown).
Therefore, in our attempt to determine bleach efficacy, our studies had to take a different direction and rely more strongly upon other laboratory experiments, as discussed below.
Inactivation of HIV-1 Pellets with Bleach
Due to the difficulties of consistently culturing the rinses directly from the needle/syringe units as described above to obtain the necessary precision, different laboratory techniques were utilized. We undertook another series of tests to derive a more precise estimate of the optimal dilution of bleach (e.g., 100% or 10% strength) and exposure time (15 seconds, 30 seconds, etc.) required to inactivate HIV-1.23
HIV-1 was pelleted from infected cell culture supernatants at 13,000 relative centrifugal force in micro-centrifuge tubes. These HIV-1 pellets were exposed to bleach for various periods of time, ranging from 15 seconds to 5 minutes. After gently removing the bleach from the tubes, the pellets were suspended immediately in cell culture medium and used to infect normal donor peripheral blood mononuclear cells (PBMNCs) using standard techniques23,24. The PBMNC cultures were maintained for up to 28 days and were assayed for virus production at weekly intervals by determination of HIV-1 p24 in the culture medium (using kits purchased from Abbott Laboratories, Inc., Chicago, IL., and according to manufacturer instructions).
The viability of normal PBMNCs was determined after treatment with bleach. After exposure to bleach for 15, 30, 45, 60 seconds, the suspensions of cells were immediately diluted to 50 ml (100-fold) using RPMI medium containing 500 units/ml each of penicillin and streptomycin, and 1% of fetal calf serum. Time intervals were measured with the use of a precision reverse count down laboratory timer. Cells were killed by exposure to 30 seconds or longer of undiluted bleach.
HIV-1 was completely and consistently inactivated by undiluted household bleach at all tested exposures of 30 seconds or longer. As summarized in Table 5, HIV-1 was not completely inactivated at shorter intervals of exposure to bleach. The p24 antigen concentrations reported in Tables 5 and 6 are averages for triplicate experiments at the indicated bleach exposure time periods. HIV-1 was not consistently inactivated at exposures to undiluted bleach durations less than 30 seconds such as 15 seconds.
INACTIVATION OF HIV IN BLOOD USING BLEACH APPROXIMATING FIELD CONDITIONS
The latest series of experiments uses three modifications of the above-reported experiments to improve the laboratory simulation of conditions under which IDUs are exposed to HIV-1. First, the most recent experiments used HIV-1 infected blood from human study drug users who were participants in federally funded community outreach projects. Second, blood was left in syringes for various periods of time up to 24 hours, to simulate needles utilized in shooting galleries. Third, the cleansing procedure taught in the Miami community HIV-1 risk reduction program was simulated in the laboratory when treating the needle/syringe units with bleach as described24. Specifically, blood from red top and (heparinized) green top tubes was drawn into needles/syringes and expelled leaving 50 ul of visible blood within the needle/syringe. After bleach treatments specified in Table 6 using the procedures described above,24 cultures were produced from the PBS rinses.
Results of these studies concluded that undiluted bleach treatment of the needle/syringe units inactivated HIV-1 consistently after an exposure of 30 seconds duration. Exposure to bleach for 15 seconds was unreliable for inactivation of HIV-1 in needle/syringe units left at room temperature. Without bleach treatment, virus was isolated from needles/syringes that remained up to 24 hours at room temperature.
TESTS UTILIZING DILUTED BLEACH
Earlier studies had shown some effectiveness using 10% volume/volume dilutions of bleach;19 however, others have questioned the effectiveness of 10% bleach at short time intervals of exposure.25 Although our primary results are that undiluted bleach is effective at inactivating HIV-1 in micro-pellets and in needle/syringes (clotted and unclotted blood) at 30 seconds of exposure, we also investigated the lack of effectiveness of 10% diluted bleach.
Ten percent bleach was shown to result in 10% viability of nPBMNCs after 24 hours of exposure whereas undiluted bleach killed cells within 30 seconds. Up to five minutes of exposure of micropellets of HIV-1 to 10% bleach resulted in no inactivation whereas undiluted bleach inactivated HIV-1 within 30 seconds. Exposure of clotted blood (from HIV-1 seropositive drug users) in needle/syringes simulating shooting gallery conditions as described above, to 10% bleach for 30 seconds did not result in
virus inactivation whereas the use of undiluted bleach did inactivate HIV-1 under these conditions (Tables 5 and 6).
BLEACH UTILIZATION AND COMPLIANCE
While there are many issues surrounding the efficacy of bleach per se in reducing the risks of infection from contaminated needle/syringe units, there are also issues surrounding the compliance by IDUs to any recommended protocols for cleansing of needle/syringe units.
These issues are germane to overall effectiveness and are similar for any prevention/intervention/therapeutic program. Overall effectiveness depends upon the two aspects of the intervention: efficacy of the bleach cleansing process and compliance to protocol. Although both efficacy or compliance have a theoretical range of total completeness to nothing being completed in most protocols, each is only completed with partial success (see Figure 3). Even if a protocol has proven to be highly effective under controlled conditions, whether it will be utilized faithfully and reliably depends upon a number of factors including its acceptability as well as its availability and accessibility. Total adherence in carrying out the specifics of the protocol also depends upon the knowledge, recall, and skills of the client or patient.
Compliance refers to how well IDUs heed the educational messages presented and/or reliably follow the cleansing procedures taught to them. Unfortunately, behavioral intervention programs that teach needle-syringe cleansing with bleach generally report poor or unreliable compliance by IDUs with the "works"-cleansing protocols being taught. Indeed, discussions of techniques to improve compliance are prominent in both the scientific and informal settings when representatives from risk reduction sites and sponsoring agencies gather.
The University of Miami research group availed itself of the opportunity to study compliance factors in its community outreach center wherein a variety of research assessments and prevention/intervention strategies that target drug users at high risk for HIV and related diseases. Our investigations focused on the specific compliance-influencing factors of protocol recall and knowledge, as demonstrated by skills performance.
Shortly following enrollment in an HIV risk reduction study, a cohort of IDUs were taught a bleach cleansing procedure that was "standard" among similar projects in a national program sponsored by the National Institute on Drug Abuse. Briefly, this procedure involves: filling the syringe with 100% bleach, twice; discarding the waste appropriately, i.e., not back into the bleach container; and, similarly following with two water rinses. As all project participants were sought for follow-up assessments for any possible changes in HIV status and/or risk behaviors, the Miami investigators decided also to study two aspects of the compliance issue: 1) whether the IDUs had sufficient recall of the protocol (procedures and materials), and 2) whether they could demonstrate retained knowledge by performing skills taught to them months earlier.
The recall/performance checklist was pretested and revised appropriately. Role play respondents assisted in assessing inter-rater reliability. Two objective examiners and four staff examiners who would conduct the tests took part in the evaluation. There was 100% agreement on all needle cleaning performance items. The consistency of rating of different raters for the same questions revealed 100% agreement of six raters on seven needle cleaning recall items, 67% agreement on one item, and 50% agreement on one item. Agreement was 100% on all seven condom use performance items. The raters were instructed on consistency of observing and marking the items on which there was disagreement prior to implementation of the instrument. Below we report only the results of the bleach cleaning items.
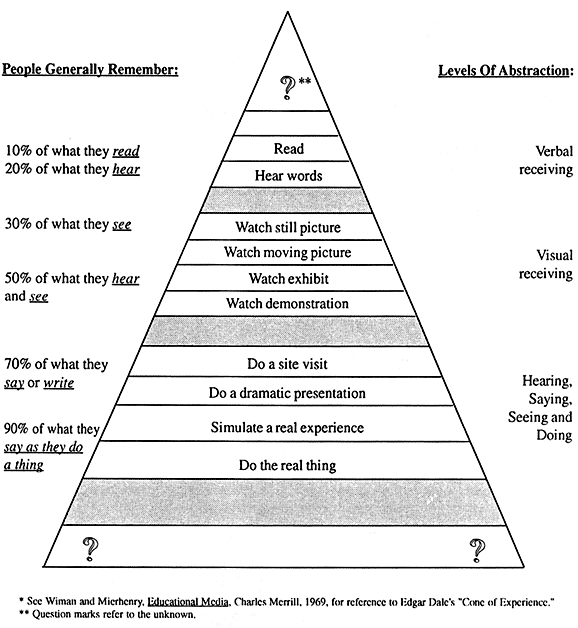
The intervention protocol was based on learning theory, drawing upon concepts similar to those of Edgar Dale (Figure 4). It presented the materials in written and verbal forms, where expectations generally are that only 10-20% of the material will be remembered, but also in visual form and by simulated situations and experiences, training techniques reported to have much greater (up to 90%) potential for yielding knowledge recall and skills retention.
The results of assessments for 450 IDU project participants revealed that more than 90 percent were able to demonstrate the basic elements of skills that had been taught to them six months earlier (Table 7). More than three-fourths of those who used the bleach flushed it twice. However, as one analyzes each step of the protocol as taught, greater variability and complexity of determining and gaining compliance are noted. For each step in the protocol, there is greater potential for non-compliance (Table 8). As the criteria of the protocol become more detailed, less adherence is noted. Although 83% used two or more bleach rinses, as opposed to one rinse, recall and performance of greater details of the protocol were not demonstrated as reliably. For example, only 43 percent of the assessed participants retracted the plunger fully to completely fill the syringe. Almost one-half (47.1%) of the participants only partially fill their works with bleach, and 28.3% of these participants go through this process only once rather than twice, as taught.
Although these data do not inform us of actual adherence in the field situation, they are instructive regarding those bleach cleansing steps which need special emphasis. They also remind us that, regardless of how effective bleach may be in killing the virus, the probability of reliable performance of protocol procedures is contingent upon the number of steps and precision required. These data also should be a reminder to policy makers, program planners, and practitioners to be attentive to all program requirements and components, avoiding those that are likely to complicate the protocol. This is a simple but important lesson from these data, as we all seek ways to improve efficacy, compliance, and utilization of HIV risk reduction efforts.
DISCUSSION
These interdisciplinary field, clinical and laboratory studies used needle/syringe units that were collected from the field or that were prepared simulating field conditions to determine the efficacy of bleach as an HIV-1 disinfectant and compliance to a bleach cleansing protocol. The studies demonstrate the difficulties involved in conducting research to confirm the efficacy of a bleach cleansing technique similar to those currently being taught in many AIDS risk reduction programs.
It is alarming that more than 50% of the cleansed needle/syringe units collected more recently from shooting galleries tested positive for antibody to HIV-1, illustrating the potential of exposing other IDUs to HIV-1. In the study conducted two years previously, the seropositivity of needle/syringe units with visible blood residue collected from three Miami shooting galleries was only 20%.7,8 These data emphasize the necessity of continuing to provide and expand prevention programs even in high prevalence settings. Existing public health agencies need to employ all possible intervention strategies in order to reduce the risk of transmission of HIV-1 from infected needles and syringes.
These must include new efforts to increase availability and utilization of personal sterile injection equipment and to decrease the amount of needle sharing among users who are not successful in abstention from drugs. While recognizing that these strategies are preferred, it also must be recognized that because of the difficulties of implementing these strategies for all IDUs, a new protocol for needle cleansing needs to be developed which utilizes all the new data available. Additional research is needed to fill in the gaps of information, especially concerning compliance issues.
However, the results of the Miami laboratory studies demonstrate that two bleach cleansing steps, totaling 30 seconds, followed by two water rinses, can be effective in the inactivation of HIV-1 in clotted and unclotted blood.
Our results underscore the appropriateness of ascertaining better whether IDUs who continue to inject are aware of this cleansing method, and whether they can actually perform the procedure correctly. Intervention programs for IDUs should conduct skills evaluation assessments in which participants go through the steps of the bleach cleansing technique in the presence of an interventionist or counselor using actual needle/syringe units. Incorrect practices can be noted to the IDU by the counselor, who then can demonstrate the correct technique and require the IDU to repeat the steps of the procedure. Also, direct observation of field situations should be conducted.
An additional consideration involves expanding the locations where bleach cleansing can be taught, going beyond demonstration projects to existing public health facilities so as to educate as many IDUs as possible in the use of recommended techniques. Alternative HIV-1 testing programs could demonstrate this cleansing method in their pre-test counseling sessions with IDUs. All of these discussions should begin with the importance of drug cessation and preferred use of sterile equipment.
As with other risk reducing behavioral changes, however, bleach cleansing must be practiced consistently, i.e., before every injection with a used needle and syringe. Perceived social norms within IDU networks can be powerful determinants of such
behavior.2 Norms supporting bleach cleansing, promulgated by outreach workers, who are often recovering addicts themselves, can bring about changes in injection practices, making bleach use or use of personal sterile needle/syringe units an intrinsic part of the injection ritual. Since bleach cleansing can take less than one minute to complete, success of the interventions may require very little alteration of existing patterns of needle use.
Based upon the research reported at a conference at Johns Hopkins and upon McCoy's and Shapshak's research reported in the February issue of Journal of AIDS, intervention programs have already changed their protocols to include instructions for the necessary exposure of 30 seconds.23,24 Since the exposure time was not an issue before this recent research, none of the protocols had previously emphasized the exposure time required. Therefore, the standard protocols that were being practiced among intervention programs needed to incorporate this new information into their procedures to give greater assurance that the HIV-1 will be inactivated.
Several examples illustrate differential responses of practical applications to attain the required 30 second exposure time. The Miami intervention site has maintained the standard protocol except to advise the clients to have minimal exposure of no less than 15 seconds with each of the two bleach flushes followed by two water rinses. The San Francisco site has opted for more bleach flushes, recommending five such bleach cleansing steps with the reminder that bleach must be inside the syringe and needle for at least 30 seconds. The additional bleach flushes might mechanically remove more residue and possibly provide greater exposure time of the bleach under the assumption that the clients will respond more to a number of flushes than holding the bleach longer in the syringes on fewer flushes. The Dayton, Ohio, and Denver, Colorado intervention sites recommend one bleach flush of at least 30 seconds, thinking that compliance would be greater with one such flush. Therefore, these three sites represent three different ways of carrying out the latest recommendations of 30 seconds of exposure to the bleach. Further research is required to determine whether any of these three different responses will bring about greater compliance than the others.
CONCLUSION
The exposure of HIV-1 infected blood to undiluted bleach, under varying conditions, including clotted blood, for durations of 30 seconds comprising two bleach rinses and followed by two water rinses, continues to be a laboratory defined standard for the inactivation of HIV-1. If this standard is implemented in a needle/syringe cleansing protocol which is correctly and reliably used by IDUs, the procedure will assist those who cannot abstain from drug use in reducing their risks of exposure to HIV-1 infection.
ACKNOWLEDGMENTS
Dr. T. Stephen Jones (Centers for Disease Control, Atlanta, Georgia) and Dr. Harry Haverkos (National Institute on Drug Abuse) are acknowledged for critical review of the manuscript. This work was supported in part by grants from the National Institute on Drug Abuse: DA04433, DA04787, DA06227, DA06910, DA07909 and from the National Institute of Mental Health: MH 50240.
REFERENCES
1. Des Jarlais DC, Friedman SR, Stoneburner RL. HIV-1 infection and intravenous drug use: critical issues in transmission dynamics, infection outcomes, and prevention. Rev Infect Dis. 1988;10:151-157.
2. Turner CF, Miller HG, Moses LE, eds. AIDS and IV drug use. In: AIDS, Sexual Behavior, and Intravenous Drug Use. Washington, DC: National Academy Press; 1989:186-255.
3. Schoenbaum EE, Hartel D, Selwyn PA et al. Risk factors for human immunodeficiency virus infection in intravenous drug users. N Engl J Med. 1989;321:874-879.
4. Chaisson RE, Bachetti P, Osmond D, Brodie B, Sande MA, Moss AR. Cocaine use and HIV-1 infection in intravenous drug users in San Francisco. JAMA. 1989;261:561-565.
5. McCoy CB, Khoury EL. Drug use and the risk of AIDS. Am Behav Sci. 1990;33:419-431.
6. Celentano DD, Vlahov D, Cohn S, Anthony JC, Solomon L, Nelson KE. Risk factors for shooting gallery use and cessation among intravenous drug users. Am J Public Health. 1991;81:1291-1295.
7. Chitwood DD, McCoy CB, Inciardi JA, McBride DC, Comerford M, Trapido E, McCoy V, Page B, Griffin J, Fletcher MA, Ashman MA. HIV-1 seropositivity of needles from shooting galleries in South Florida. Am J Public Health. 1990;80:150-152.
8. Inciardi JA, Page JB, McBride DC, Chitwood DD, McCoy, CB, McCoy HV, Trapido EJ. The risk of exposure to HIV-1-infected needles in shooting galleries. Chapter in: The American Drug Scene, Inciardi, JA . and Karen MacElrath (Edition), Roxbury Press, Los Angeles. (In press).
9. Chitwood DD, Inciardi JA, McBride DC, McCoy CB, McCoy HV, Trapido E. A Community Approach to AIDS Intervention: exploring the Miami Outreach Project for Injecting Drug Users and Other High Risk Groups. Westport, CT: Greenwood Press, 1991.
10. McCoy CB, Rivers JE, Khoury EL, et al. An emerging public health model for reducing AIDS related risk behavior among injecting drug users and their sexual partners. Drugs and Society. 1993;7(3/4):143-159.
11. McCoy CB, Chitwood DD, Khoury EL, Miles CE. The implementation of an experimental design in the evaluation of an intervention to prevent AIDS among IV drug users. J Drug Issues. 1990;20:215-222.
12. Watters JK, Newmeyer JA, Feldman HW, Biernacki P. Street-based AIDS Prevention for Intravenous Drug Users in San Francisco: Prospects, Options, and Obstacles. In: Community Epidemiology Work Group Proceedings: Volume II. Washington, DC: U.S. Government Printing Office 1986;I:37-42.
13. Watters JK, Downing M, Case P, Lorvick J, Cheng Y-T, Fergusson B. AIDS prevention for intravenous drug users in the community: Street-based education and risk behaviors. Am J Community Psychology. 1990;18:587-596.
14. Newmeyer JA. Why bleach? Fighting AIDS contagion among intravenous drug users: The San Francisco experience. J Psychoactive Drugs. 1988;20:159-163.
15. Wodak A, Dolan K, Imrie AA, Gold J, Whyte BM, Cooper DA. Antibodies to the HIV-1 in needles and syringes used by IV drug abusers. Med J Aust. 1987;147:275-276.
16. Ljungberg B, Christensson B, Tunving K, Andersson B, Landvall B, Lundberg M, Zall-Friberg AC. HIV-1 prevention among injecting drug users: three years of experience from a syringe exchange program in Sweden. J Acquir Immune Defic Syndr. 1991; 4:890-895.
17. Community Alert Bulletin, National Institute on Drug Abuse, March 25, 1993.
18. HIV/AIDS Bulletin CDC, CSAT, NIDA, March 25, 1993
19. Resnick L, Veren K, Salahuddin MS, Tondreau S, Markham P.D. Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. JAMA. 1986;255:1887-1891.
20. Newmeyer J, Drew L, Miner R. HIV-1 transmission in simulated conditions of sharing of hypodermic equipment. J Acquir Immune Defic Syndr. 1990;10:1019-1021.
21. Satter SA, Springthorpe VS. Survival and disinfectant inactivation of the Human Immunodeficiency Virus: a critical review. Rev Infect Dis. 1991;13:430-437.
22. Wolk J, Wodak A, Morlet A, et al: Syringe HIV-1 seroprevalence and behavioral and demographic characteristics of intravenous drug users in Sydney, Australia, 1987. AIDS 1988; 2:373-377.
23. Shapshak P, McCoy CB, Rivers JE, Chitwood DD, Mash DC, Weatherby NL, Inciardi JA, Shah SM, and Brown BS. Inactivation of human immunodeficiency virus-1 at short term intervals using undiluted bleach. J Acquir Immune Defic Syndr. 1993;6:218-219.
24. Shapshak P, McCoy CB, Shah SM, Page JB, Rivers JE, Weatherby NL, Chitwood DD, and Mash DC. Preliminary laboratory studies of inactivation of HIV-1 in needles and syringes containing infected blood using undiluted household bleach. J Acquir Immune Defic Syndr. 1994;7:754-759.
25. Aranda Anzaldo A, Viza D, and Busnel R.A., Chemical inactivation of HIV in Vitro, J of Viral Methods. 1992;37:71-82.
26. Chitwood, Dale D., Clyde B. McCoy, and Mary Comerford. ''Risk Behaviors of Intravenous Cocaine Users: Implications for Intervention." National Institute on Drug Abuse. 1989.
27. Curran, J., H. Jaffe, A. Hardy, W. Morgan, R. Selik, and T. Dondero. Epidemiology of HIV infection and AIDS in the United States. Science. 1988;239;610-616. 28.
28. Koester S. Copping, running and paraphernalia laws: Contextual variables and needle risk behavior among injection drug users in Denver. Human Organization. (in press, 1993).
29. Koester S. and Hoffer L. Indirect Sharing: additional risks of HIV transmission during drug preparation and injection. Scientific Meeting of NIDA Cooperative Agreement Projects, Flagstaff, AZ, Fall, 1993.
30. McCoy CB, Inciardi JA. Sex drugs and the secondary spread of HIV. Roxbury Publications, Los Angeles, (in press).
31. New England Journal of Medicine, Vol. 327, No. 26, pp. 1883-1884, December 24, 1992.
32. Chance, Vol. 6, No.2, pp.9-14, July, 1993.
33. Needle Exchange Programs, GAO/HRD-93-60, March, 1993.
Table 1
SEROPOSITIVITY OF NEEDLE/SYRINGE UNITS WITHOUT AND WITH VISIBLE BLOOD
|
Appearance |
Total tested (n) |
Proportion HIV AB+ (%) |
|
No visible blood |
98 |
5.1 |
|
Visible blood |
50 |
20.0 |
|
Total |
148 |
10.1 |
|
Chitwood DD, McCoy CB, Inciardi JA, McBride DC, Comerford M, Trapido EJ, McCoy HV, Page JB, Griffin J, Fletcher MA and Ashman MA. "HIV Seropositivity of Needles from Shooting Galleries in South Florida": American Journal of Public Health. 1990;80(2): 150-152. |
||
Table 2
NUMBER OF DAYS BEFORE THERE IS A 90% PROBABILITY OF A SEROPOSITIVE NEEDLE/SYRINGE ENOUNTER AT VARYING FREQUENCIES OF INJECTION
|
Visible Blood |
|
No Visible Blood |
|
|
1x per day |
22 days |
1x per day |
44 days |
|
2x per day |
11 days |
2x per day |
22 days |
|
3x per day |
7 days |
3x per day |
15 days |
|
4x per day |
5 days |
4x per day |
11 days |
|
5x per day |
4 days |
5x per day |
9 days |
|
Inciardi JA, Page JB, McBride DC, Chitwood DD, McCoy CB, McCoy HV, Trapido EJ. "The Risk of Exposure to HIV-Contaminated Needles in Shooting Galleries." Chapter in: The American Drug Scene. Roxbury Press, Los Angeles (In Press). |
|||
Table 5
INACTIVATION OF HIV-1 BY UNDILUTED HOUSEHOLD BLEACH
|
Duration of Exposure |
Average concentration of p24 antigen in supernantants of cultures infected with virus treated for indicated time: pg/ml (standard deviation) |
|
0 |
≥100 |
|
15 seconds |
31(26) |
|
25 seconds |
13(13) |
|
30 seconds |
0 |
|
45 seconds |
0 |
|
60 seconds |
0 |
|
2.5 minutes |
0 |
|
5 minutes |
0 |
|
Shapshak P, McCoy CB, Rivers JE, Chitwood DD, Mash DC, Weatherby NL, Inciardi JA, Shah SM, Brown BS. "Inactivation of Human Immunodeficiency Virus-1 at Short Time Intervals Using Undiluted Bleach" Journal of Acquired Immune Deficiency Syndromes 6:218-219, 1993. |
|
Table 6
BLEACH INACTIVATES HIV-1-INFECTED BLOOD IN NEEDLES AT SHORT TIME INTERVALS
|
Treatment |
HIV-1 p24 (pg/ml) Treatments |
|||||
|
|
|
Undiluted Bleach |
|
|
|
|
|
Duration of Treatment |
15 Seconds |
Na |
30 Seconds |
N |
No Bleach |
N |
|
3 hours |
|
0 |
100 |
|||
|
6 hours |
100 |
3c |
0 |
100 |
3c |
|
|
18 hours |
|
|
0 |
3 |
100 |
3c |
|
24 hours |
0 |
3c |
0 |
3 |
100 |
|
|
a N: number of cultures at each indicated experimental condition. b: blank; not done. c: blood, clotted in needles, from red top tubes. d: blood from green top tubes. Shapshak P, McCoy CB, Shah SM, Page JB, Rivers JE, Weatherby NL, Chitwood DD, Mash DC, "Preliminary laboratory studies of inactivation of HIV-1 in needles and syringes containing infected blood using undiluted household bleach," J Acquir Immune Defic Syndr 1994;7:754-759. |
||||||
Table 7 SKILLS/COMPLIANCE EVALUATION NEEDLE CLEANING SKILLS DEMONSTRATION: BASIC ELEMENTS
|
|
Filled Needle/Syringe with Bleach % of Total (N) |
Filled Needle/Syringe with water % of Total (N) |
% of Those Using Bleach Disposing % of Total Appropriately (N) |
% of Those Using Water Disposing % of Total Appropriately (N) |
|
Yes |
90.2 |
94.8 |
91.9 |
92.0 |
|
(406) |
(427) |
(373) |
(393) |
|
|
No |
9.8 |
5.1 |
8.9 |
8.0 |
|
(44) |
(23) |
(33) |
(34) |
Table 8
SKILLS/COMPLIANCE EVALUATION NEEDLE CLEANING SKILL DEMONSTRATION: DETAILED
|
|
|
Number of Times Needle/Syringe Filled |
Discarded Waste Appropriately Row % (n) |
|
|
Filled Needle/Syringe With Bleach |
% of Total (n) |
One Row % (n) |
Two or More Row % (n) |
|
|
Completely* |
43.1 |
17.0 |
83.0 |
94.3 |
|
(194) |
(33) |
(161) |
(183) |
|
|
Partially |
47.1 |
28.3 |
71.7 |
89.6 |
|
(212) |
(60) |
(152) |
(190) |
|
|
Did not fill |
9.8 |
|
|
|
|
(44) |
|
|
|
|
|
Filled Needle/Syringe with Water |
||||
|
Completely* |
50.4 |
11.9 |
88.1 |
92.5 |
|
(227) |
(27) |
(200) |
(210) |
|
|
Partially* |
44.4 |
25.0 |
75.0 |
91.5 |
|
(200) |
(50) |
(150) |
(183) |
|
|
Did not fill |
5.1 |
|
|
|
|
(23) |
|
|
|
|
|
TOTAL |
100.0 |
|
|
|
|
(450) |
|
|
|
|
|
* The Plunger in the syringe had to be fully drawn back; otherwise, it was recorded as partially filled. |
||||
|
MEASUREMENT AND POLICY ISSUES OF PROTOCOL RECOMMENDATIONS/UTILIZATION |
|
|
|
INTERVENTION/THERAPEUTIC PROTOCOL |
|
|
None——-> Partial——-> Complete |
|
Effectiveness Compliance |
|
|
|
EFFECTIVENESS |
|
|
None——-> Partial——-> Complete |
|
Risk Reduction (Prevention) |
|
|
Curative Value |
|
|
|
Increase Survival |
|
|
Decrease Mortality |
|
|
Increase Satisfaction |
|
|
Quality of Life |
|
|
Decrease Pain |
|
|
Efficacy |
|
|
(Costs/Benefits) |
|
|
COMPLIANCE |
|
|
None—-> Partial——-> Complete |
|
Knowledge |
|
|
Recall |
|
|
Skills |
|
|
Adherence |
|
|
|
UTILIZATION |
|
|
None——-> Partial——-> Complete |
|
Availability |
|
|
Accessibility |
|
|
Acceptability |
|
Figure 3
INACTIVATION AND DISINFECTION OF HIV: A SUMMARY
LINDA S. MARTIN, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Atlanta, Georgia
In 1983 a retrovirus was isolated and classified as the causative agent of AIDS (1). Laboratories began research with the human immunodeficiency virus (HIV) soon after. Because of the serious consequences of infection with the virus, the susceptibility of the virus to chemicals and disinfectants was investigated (2,3,4).
Before discussing the results of these and other studies, it is necessary to review the principles of viral inactivation and the categories of disinfectants as they relate to microorganisms. The following definitions and tables are adapted from the third and fourth editions of Seymour S. Block's book entitled ''Disinfection, sterilization, and preservation" (5). Martin Favero and Walter Bond, Centers for Disease Control and Prevention (CDC) Hospital Infections Program, wrote the chapter on sterilization, disinfection and antisepsis in the hospital (6).
"Sterilization" is the use of physical or chemical procedures to destroy all microbial life, including large numbers of highly resistant bacterial endospores. The major process types include moist heat by steam autoclaving, ethylene oxide gas, and dry heat. A variety of chemical germicides have also been used (5,6).
"Disinfection" eliminates nearly all recognized pathogenic microorganisms, but not necessarily all microbial forms on inanimate objects. The effectiveness of a disinfection procedure is controlled by the nature and number of microorganisms, the amount of organic material, the type and condition of the materials to be disinfected, and the temperature (5,6).
"Decontamination" is a process of treatment that renders a device, instrument, or environmental surface safe to handle but does not necessarily mean that it is safe for patient use (5,6).
"Antisepsis" is the process of inhibiting or destroying microorganisms on skin or living tissue (5,6).
As shown in Table 1, bacterial spores are the most difficult form of microorganisms to inactivate or "kill" (6). The most resistant organisms in the vegetative state are in the class of Mycobacteria. The high level of resistance is due to the very waxy coating which confers more resistance to aqueous germicides than found with other vegetative bacteria. Following Mycobacteria, in descending order of resistance, are the nonlipid or small viruses, fungi, vegetative bacteria, and lipid or medium sized viruses, the least resistant organisms. HIV and hepatitis B virus (HBV) are both in the last category. Why are lipid viruses more susceptible to chemical germicides than other forms of microorganisms? Klein and Deforest outlined the principles of viral inactivation (7). Viruses and vegetative bacteria are generally similar in their susceptibility to physical agents such as heat, ionizing irradiation, and UV light. All viruses are generally
susceptible under appropriate conditions to broad spectrum germicides such as the halogens, some alcohols, and glutaraldehyde. Viruses do differ in their susceptibility to lipophilic germicides. These compounds have an affinity for lipid-containing viruses and some, but not all, nonlipid containing viruses (7).
Susceptibility to chemical disinfectants of most viruses can be predicted on the observation that the presence of lipid in a virus is uniformly associated with a high degree of susceptibility to all germicides. The absence of lipid and small size is associated with resistance to lipophilic germicides, but the absence of lipid and larger size is similar to lipid containing viruses (7,8).
E.H. Spaulding proposed three levels of germicidal action to properly carry out strategies for disinfection in hospitals. These include high, intermediate and low and are based on the fact that microorganisms can be categorized into several groups according to their innate resistance to a spectrum of physical or chemical germicidal agents (9). In Table 2, the Environmental Protection Agency (EPA) product label terminologies are compared with the CDC germicidal process terminology (6,9). Depending on the intended use, chemical germicides are regulated by the EPA, the Food and Drug Administration, or both. Germicides intended for use only on environmental surfaces are regulated only the EPA.
Does HIV react to disinfectants as predicted by Klein and Deforest? This question can be answered based on laboratory studies. First, the experimental laboratory conditions must be defined, then the results must be interpreted based on comparisons with the laboratory conditions and the conditions that may exist in body fluids such as blood. In the human body, cell free HIV enters the CD4+ lymphocyte and can either become latent in the cell or replicate resulting in new virus being released into the surrounding milieu where virus may infect another CD4+ cell. The number of infected cells/ml in an infected individual's blood is estimated to be about 100-1000. The titer of cell free virus is estimated to be about 100 or less. Both cell-free HIV and infected cells may be present in the circulating blood.
In the laboratory, cell free HIV can be grown in CD4+ tissue culture cells. Generally, the virus containing supernatant fluid is harvested and the titer of cell free virus ranges from 104 to 106 per unit volume, titers higher than generally found in blood. The amounts of protein and other organic materials in laboratory tissue culture fluid are usually less than that found in blood.
Laboratory inactivation studies have been performed by mixing an equal volume of virus containing fluid and disinfectant for varying periods of time. Following the inactivation process, serial dilutions of each test disinfectant and controls were plated into CD4+ cells. After 7 days, the supernatant fluids from each dilution were harvested and tested for presence of virus using an ELISA. Additional incubation time may be needed to detect low levels of virus remaining following the inactivation step. The lack of viral replication indicated inactivation of the virus by the disinfectant. Controls must be done to determine if the test disinfectant killed the indicator CD4+ cells which would also result in no detection of viral replication (3).
Prior to the routine use of the ELISA assay to detect presence of virus, reverse transcripts (RT) assays were used to detect viral replication. Spire et al. reported the
first disinfection studies in 1984 using the RT assay (2). They reported that cell free virus was inactivated by 1:400 dilution of ß-propiolactone, .01% glutaraldehyde (95% inactivation), 30 mM sodium hydroxide, and .2% sodium hypochlorite. Inactivation with ethanol and formalin was variable.
Martin and McDougal (4), using the ID-50 immunoassay and ELISA, reported that HIV was inactivated by .3% hydrogen peroxide, 50% ethanol, .5% paraformaldehyde, .5% Lysol® (a proprietary mixture of phenolics and surfactants), and .1% household bleach (52.5 ppm NaOCl). Over time, many laboratories have performed inactivation studies with various compounds (Table 3) (2,3,4,10,11,12,13,14,15,16,17,18,19). Sattar summarized the results of many of these experiments (10). The predicted susceptibility of HIV to a vary wide variety of chemical disinfectants was confirmed.
Inactivation with NaOCl has been investigated in many laboratories (Table 4) (4,14,15,16,17,18,19,20). HIV inactivation has been studied for HIV in both the liquid and dried states. A range of dilutions of bleach and/or varying concentrations of serum or blood was not always tested in each laboratory. The concentrations of NaOCl reported to inactivate cell-free HIV ranged from a minimum of 52.5 ppm to 5000 ppm. The presence of blood or 50% serum increased the amount of available chlorine (Cl) required for complete inactivation.
Ingraham (8) wrote that the chlorine compounds are the most misunderstood and consequently the most abused disinfectants. Chlorine compounds can provide high degrees of disinfection. Chlorine works quickly as a bactericidal agent although its action is not fully understood. The toxicity can be due to a variety of factors including the pH, Cl ions, protein denaturation, nucleic acid inactivations, and oxidizing properties (8,21). Klein and Deforest reported that all of 25 viruses tested were inactivated in 10 minutes by a solution containing 0.02 % available chlorine (7). Available chlorine is a measurement of the total chlorine oxidizing potential in a given solution. Chlorine demand refers to the amount of Cl that is needed to react with impurities, both inorganic and organic. After this demand is met, any additional Cl is residual available Cl. Chlorine's disinfection ability is determined by the concentration of free and available chlorine in the solution. This is affected by the temperature, the presence of organic material, the pH, and the hardness of the water. Chlorine compounds usability may be limited by the corrosiveness and instability.
Serum proteins and other organic material in blood will react when mixed with chlorine compounds and reduce the chlorine available for microbial inactivation. For example, before using chlorine to clean up a blood spill, it is necessary to remove as much of the visible blood as possible with absorbent material. After this cleaning, material is appropriately discarded, the spill site may then be disinfected with bleach or other chlorine product. CDC recommends a 1:100 dilution of household bleach for cleaning blood-contaminated environmental surfaces that have been previously cleaned of visible material (22). The underlying principle is to remove as much of the organic material prior to using a Cl compound for disinfection.
The presence of infected cells in blood should also be considered when developing an inactivation protocol. Flynn (23) reported that whole blood is protective against disinfection of HIV. There was a step-wise increasing resistance to disinfection as the in
vivo situation was approached. That is, susceptibility to inactivation increased with cell-free HIV being the most sensitive, followed by cell associated, with cell-associated in blood being the most resistant. Dilute household bleach, 70% isopropyl alcohols and dilute liquid dish detergent were the most active of the tested disinfectants. Most experiments done with HIV-infected cells have focused on the testing of chemicals such as formaldehyde used to fix cells (both separated cells and in whole blood) for flow-cytometry and other laboratory procedures.
Moore (24) reported that HIV infected cells were inactivated in water, but this inactivation was not rapid. There was a 10-fold loss within 1 hour of tap-water exposure and a 100-fold loss after 8 hours. Attempts were also made to recover virus after the introduction of HIV-contaminated blood into tap water. Seropositive blood was diluted to a final total volume of 1 and 2 %. No virus was recovered after 1 minute in the 1% vol/vol experiment and after 5 minutes in the 2% vol/vol experiment.
Newmeyer (25) examined the transmission of HIV under simulated conditions of sharing of hypodermic equipment by intravenous drug users (IVDU). Hypodermic equipment was exposed to cell-free HIV in tissue culture medium and attempts were made to culture virus after different conditions of exposure. Viable HIV was cultured from the following conditions: contaminated needle and syringe left for an interval of 1 minute, exterior of needle exposed and left for 15 seconds, and decontamination twice with water. Viable HIV was not recovered from equipment under the following conditions: contaminated needle and syringe left for 60 minutes and decontamination once or twice with bleach. The researchers concluded that decontamination by bleach rinsing, but not water alone, appeared to be efficacious (25). Caution should be used in interpreting these experiments since whole blood was not tested.
As indicated, there are other disinfectants such as glutaraldehyde compounds, detergents, iodine, quaternary ammonium compounds, and alcohols which have been shown to inactivate HIV under certain conditions. Selection of another disinfectant, rather than NaOCl, to recommend for disinfection of needles and syringes designed for single use must take into consideration, ease of use, toxicity, effect on syringe and needle, disinfection capability in the presence of blood, and many other factors.
It is not surprising that the use of bleach for disinfection for syringes and needles contaminated with blood may not result in complete inactivation of cell-free HIV. The presence of organic material such as blood, liquid or dried, the absence of precleaning or rinsing with water, and the difficulty of cleaning a device not designed for reuse are all complicating factors. There is also the difficulty of documenting the effectiveness of disinfection strategies for IVDUs. Siegel (26) found that the greatest life-year savings were in areas of low HIV prevalence.
Clearly, the first course of action for drug users who are injecting drugs is to always use a sterile, never used needle and syringe obtained from a reliable source such as a pharmacy. This is the only safe recommendation for injecting drug users who cannot or will not stop injecting drugs. Disinfection procedures can reduce the amount of HIV or HIV-infected cells present in the injecting equipment, but can not guarantee complete inactivation every time.
ACKNOWLEDGMENT
The review by Walter Bond, CDC, is gratefully acknowledged.
REFERENCES
1. Barre-Sinoussi, F., Chermann, J.C., Rey, F. et al. 1983. Isolation of a Tlymphotropic retrovirus from a patient at risk for acquired immunodeficiency syndrome (AIDS). Science 220:868-71.
2. Spire B., Barre-Sinoussi, F., Montagnier, L., et al. 1984. Inactivation of lymphadenopathy associated virus by chemical disinfectants. Lancet ii:899-901.
3. McDougal, J.S., Cort, S.P., Kennedy, M.S., et al. 1985. Immunoassay for the Detection and Quantitation of Infectious Human Retrovirus, Lymphadenopathy Associated Virus (LAV), J Immun Meth 76:171-83.
4. Martin, L.S., McDougal, J.S., Loskoski, S.L. 1985. Disinfection and Inactivation of the Human T Lymphadenopathy Virus Type III/Lymphadenopathy-Associated Virus, J Infec Dis 152:400-3.
5. Block, S.S. 1983. Disinfection, Sterilization, and Preservation, 3 ed., Philadelphia, PA. Lea & Febiger.
6. Favero, M.S., Bond, W.W. 1991. Chemical Disinfection of Medical and Surgical Materials, In Block, SS. Disinfection, Sterilization, and preservation, 4th ed., Philadelphia, PA, Lea & Febiger.
7. Klein, M., Deforest A. 1983. Principles of viral inactivation. In: Block, SS. Disinfection, Sterilization, and Preservation. 3rd ed. Philadelphia, PA: Lea & Febiger. 422-34.
8. Ingraham, A.S., 1992. The Chemistry of Disinfectants and Sterilants. Cont Topics 31:18-23.
9. Spaulding, E.H. 1972. Chemical disinfection and antisepsis in the hospital. J Hosp Res 9:5-31.
10. Sattar, S.A., Springthorpe, V.S. 1991. Survival and Disinfectant Inactivation of the Human Immunodeficiency Virus: A Critical Review. Rev Infect Dis 13:430-47.
11. Quinnan, G.V. Jr., Wells, M.A., Wittek, A.E., et al. 1986. Inactivation of Human T-cell Lymphotropic Virus, Type III by Heat, Chemicals, and Irradiation., Transfusion 26(5); 481-3.
12. Hanson, P.J.V., Gor, D., Jeffries, D.J., et al. 1989. Chemical Inactivation of HIV on Surfaces. BMJ 298:862-4.
13. Tjotaa, E., Hungnes, O., Grinde, B., 1991. Survival of HIV-1 Activity After Disinfection, Temperature and pH Changes, or Drying. J Med Vir 35:223-227.
14. Resnick, L., Veren K., Salahuddin, S. Z., et al. 1986. Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. JAMA 255:53-68.
15. Bloomfield, S.F., Smith-Burchnell, C.A., Dalgleish, A.G. 1990. Evaluation of Hypochlorite-Releasing Disinfectants Against the Human Immunodeficiency Virus (HIV), J Hosp Inf 15:273-8.
16. Aranda-Anzaldo, A., Viza, D., Busnel, R.G. 1992. Chemical Inactivation of Human Immunodeficiency Virus in Vitro. J Viro Meth 37:71-82.
17. Busnel, R.G., Aranda-Anzaldo, A., Viza, D., and Hutchinson, S. A. 1990 Parameters of HIV inactivation by disinfectants. Int Conf AIDS Abstract 1076.
18. Van Bueren, J., Salman, H., Simpson, R.A., et al. 1992. Survival of HIV and Inactivation by Heat and Chemical Disinfectants. Int Conf AIDS, Jul 19-24; 8(2):A69
19. Prince, D.L., Prince, R.N., Prince, H.N. 1990. Inactivation of Human Immunodeficiency Virus Type 1 and Herpes Simplex Virus Type 2 by Commercial Hospital Disinfectants. Chem Times & Trends January: 13-17.
20. Shapshak, P., McCoy, C. B., Rivers, J.E. et al. 1993. Inactivation of human immunodeficiency virus-1 at short time intervals using undiluted bleach. JAIDS 6:218-219.
21. Van Houton, J., Hayre, M. D. 1991. Disinfectants and sterilants: Their chemistry, use and evaluation. AALAS Bulletin 30:24-27.
22. Centers for Disease Control and Prevention 1993 MMWR Morb Mortal Wkly Rep 42:418-490.
23. Flynn, N., Jain, S., Keddie, E., et al. 1990. Bleach is not enough: giving IV drug users a choice of disinfectants when they share needles and syringes.Int Conf AIDS Abstract SC761.
24. Moore, B. E. 1993. Survival of human immunodeficiency virus (HIV), HIV-infected lymphocytes, and poliovirus in water. Appl Env Micro 59:1437-1443.
25. Newmeyer, J., Drew, L., Minor, R., 1989 HIV transmission in simulated conditions of sharing of hypodermic equipment. Int Conf AIDS Abstract D.707
26. Siegel, J.E., Weinstein, M.C., Fineberg, H.V., 1991. Bleach Programs for Preventing AIDS Among IV Drug Users: Modeling the Impact of HIV Prevalence. AJPH 81:1273-78.
TABLE 1
Descending Order of Resistance to Germicidal Chemicals*
TABLE 2
Comparison of Environmental Protection Agency (EPA) Produce Table Terminology and Center for Disease Control and Prevention (CDC) Germicidal Process Terminology*
TABLE 3
Summary of susceptibility of cell-free HIV to chemical inactivation.*
TABLE 4
Inactivation of cell-free HIV with NaOCI: Summary of reported studies*
|
Cell-free HIV in liquid state |
|
|
|
52.5-5,250 ppm NaOCl (4)** |
|
|
262.5 ppm NaOCl (14) |
|
|
2,500 ppm available CL in 10% plasma (15) |
|
|
5,000 ppm NaOCl, equal volumes of virus and blood (complete killing after 2 minutes) (15) |
|
|
525 ppm NaOCl (16) |
|
|
262.5 ppm NaOCl inactivated 56ng of p24/ml (time must = 1 minute) (17) |
|
|
Efficacy of NaOCl was reduced considerably in the presence of blood or serum (18) |
|
HIV in dried state (19) |
|
|
|
2625 ppm NaOCl (inactivation in presence of 2.5-50% serum) |
|
|
1312 ppm NaOCl (inactivation in presence of 10% serum) |
|
|
263 ppm NaOCl (HIV titer reduced in presence of 2.5-5% serum) |
|
HIV in pelleted state (20) |
|
|
|
52,500 ppm NaOCl = Undiluted household bleach |
|
*Cell-free HIV was tested using laboratory cultured HIV. No viral replication was detected at concentrations/conditions indicated. Results are reported as given in references. A range of dilutions was not tested in every study. **( ) = Reference |
|
USE OF BLEACH BY INJECTION DRUG USERS
ALICE A. GLEGHORN, Department of Health Policy and Management, The Johns Hopkins University, Baltimore, Maryland
INTRODUCTION
In 1986, distribution of household bleach to injection drug users (IDUs) began in San Francisco in an effort to stem the spread of HIV1,2. Prior to that time, reported cleaning efforts by IDUs who shared their works were limited to rinsing the syringe with water before use; only a small portion reported rinsing with alcohol or using boiling water to clean the injection equipment (19 and 16 percent, respectively)3.
Instructions on "how to sterilise equipment"2 were developed through a combined effort of several public service agencies in San Francisco, which formed the Mid-City Consortium to Combat AIDS. The initial instructions were based on a report by Resnick and colleagues on in vitro use of diluted bleach which held that "viral infectivity is undetectable and reduced more than 7 log10TCID50 within one minute with 0.5% sodium hypochlorite"4. According to Newmeyer5, "The Mid-City strategists inferred from this data that a comparable reduction in virus activity could be accomplished by a few seconds' exposure to full-strength bleach.'' Froner6 further suggested that during the cleaning procedure, bleach may be in the syringe for 20-30 seconds, "ample time to kill any virus present".
The procedure that was developed and recommended by the Mid-City Consortium was to fill the syringe full ("flush") twice with full strength bleach, followed by rinsing the syringe twice with clean water7.
The use of these procedures for disinfection of syringes with bleach were widely and rapidly adopted throughout the United States and internationally.1,8,9,10,11 However, concerns were raised about how IDUs would interpret the instructions, and whether the actual performance of cleaning strategies by IDUs in their environment would be adequate5.
This concern resurfaced in Baltimore when Vlahov and colleagues found only a modest protective effect against HIV for reported use of chemical disinfection of injection equipment in an analysis of seroconverters and persistent seronegative IDUs.12 These findings were supported in a follow-up of this cohort,13 and further analysis controlling for sexual risk factors did not alter this result.14 Latkin et al. recently addressed concerns regarding the potential for a socially-desirable response bias by IDUs in this sample, and found that statistical adjustments for "self-deception" and "impression management" had an insignificant effect on the size of the relationship between risk behaviors and HIV serostatus.15
Insufficient contact time of disinfectant with the syringe was one of several factors hypothesized to be related to the apparent modest protective effect of disinfection.12 Several recent laboratory studies have noted that 30 seconds of continuous contact time
with bleach is the minimum necessary to inactivate HIV.16,17 Extended bleach contact time was also recommended in recently issued guidelines from a NIDA/CSAT/CDC Prevention Bulletin18, although the guidelines do not make specific recommendations for a minimum number of seconds of bleach contact or a minimum number of flushes with bleach.
METHODS
To obtain a more objective assessment of IDU syringe cleaning techniques than have been typically obtained through questionnaires, we recently conducted a study which videotaped 161 active IDUs demonstrating the cleaning strategies used during their last injection episode. Videotaping allowed us to obtain bleach contact time, and quantify techniques intrinsic to the IDU environment. Since the taping was conducted prior to the publication of the new NIDA/CSAT/CDC guidelines, the videotape analysis allowed us to evaluate the concordance of cleaning strategies with the original Mid-City guidelines, and to determine the proportion of IDUs whose current practices approximate the new NIDA/CSAT/CDC guidelines. Each cleaning segment was viewed and scored by a trained coder according to a standard protocol of decision rules. Multilap stopwatches were used to measure time variables. Detailed interviews were conducted in conjunction with the videotaping. A complete description of this study and results has been reported previously19
RESULTS
Among the 161 study participants, 144 (89%) were male, 150 (93%) were black, and the median age was 38.5 years old (range: 25.2 to 64.1); 79 (49%) had less than 12 years of education, and 134 (83%) reported < $2500 of legal income in the prior six months (Table 1). By HIV status, 110 (68%) were seronegative, 33 (21%) were seropositive, and 33 (21%) were known seroconverters since 1988. In terms of drug use, the median duration of drug use was 17.5 years (range: 4 to 47). All had injected during the past six months; the proportion who injected less than weekly was 20%, weekly was 22% and at least once a day was 58%.
Of the 161, 15 (9%) denied needle cleaning procedures the last time they injected because their needles were new; these subjects were excluded from further analysis. Of the 146 who reported any needle cleaning, 61 (42%) did not use full strength bleach. Of the 61, 55 (90%) used water alone, 5 (8%) used isopropyl alcohol, and 1 (2%) used diluted household bleach.
Among the 146 drug users who reported needle cleaning attempts the last time they injected, 85 (58%) used full strength household bleach. For the bleach users, 82% demonstrated the sequence of cleaning steps recommended by the Mid-City Consortium which was to repeat twice the procedure of drawing bleach into the syringe and squirting it out (mean number of flushes 2.2, s.d. = .95), followed by several water rinses
(mean 2.9, s.d. = 1.4) to clear the syringe of bleach; 18% of bleach users rinsed the syringe with water prior to flushing with bleach, the sequence recommended in the new NIDA/CSAT/CDC guidelines. A minority of bleach users (n =26, 30.6%) filled the syringe at least halfway. During the bleach cleaning step, 66% (n = 56) agitated the syringe, while 71% (n = 60) agitated the syringe at any point in the cleaning process. A small proportion (14%, n = 12) of bleach users dismantled the syringe during the cleaning process. Of the bleach users, 30 (35%) reported that they clean the cooker, however, none of the subjects were observed to clean the cooker during videotaping.
For the 85 bleach users, we calculated the draw and contact time per each single flush and then for total flushes (to allow for multiple flushes with bleach) (Table 2). The median draw time was 6.6 seconds (range: 1.4-33.6 seconds), the median contact time was 9.4 seconds (range: 1.4-138.3 seconds), and the median time per flush was 16.1 (range: 3.6-152.4). When all flushes were combined, the median total draw time was 12.4 seconds (range: 4.1-45 seconds), the median total contact time was 18.2 seconds (range: 4.3-138.2 seconds), and the median total flush time was 31.5 seconds (range: 8.8-152.4 seconds).
Based upon studies by Shapshak and colleagues (9), we used a flush time of 30 seconds to dichotomize the sample. Table 3 shows that 68 (80%) of the 85 bleach users had a total contact time of less than 30 seconds, and that 39 (46%) had a total flush time less than 30 seconds. Table 4 examines the characteristics that distinguish participants by bleach contact time. On univariate analyses, filling the syringe at least halfway full of bleach, (OR = 3.22), having less than 12 years of education, (OR = 3.65), and being older than 35 years of age, (OR = 3.18), were positively associated with bleach exposure times of at least 30 seconds. In our sample, bleach contact was not statistically associated with HIV serostatus. Small sample size precluded meaningful multivariate analysis.
Because videotaping is unlikely to be feasible in most field situations, we compared selected actual and reported times for the bleach users. Correlations of self-report and videotape were performed separately for the bleach step of the cleaning process, and then for the duration of the entire cleaning process. We observed a consistent pattern that the median time of self-reports was about twice as long as the duration measured from the videotapes (e.g., median time of self-reports for bleach step = 60.0 versus 33.0 seconds observed from videotape, Pearson r = -0.04, median total cleaning time self-report = 120.0 versus 78.2 second observed, Pearson r = 0.06).
Discussion
The results of this study suggest that the majority of injection drug users we videotaped flush their works with some solution in an attempt to clean their syringe. However, a high proportion of these used only water to clean the works, a strategy unlikely to provide protection against HIV. Furthermore, of those who used full strength bleach, even fewer used bleach with a minimum exposure in the syringe of 30 seconds. The results of this study suggest that the majority of cleaning techniques commonly practiced by IDUs are probably insufficient to achieve adequate levels of disinfection.
The results also suggest that the majority of those who used bleach followed the general sequence of steps suggested by the early Mid-City guidelines, the same strategy which had been disseminated by local AIDS prevention organizations in Baltimore. However, only a small proportion of bleach users practiced the more specific recommendation of filling the syringe all the way full. This indicates that while IDUs in this sample may have adopted general guidelines for bleach use, some specific practices important for adequate disinfection were not demonstrated by the majority of IDUs in this sample.
Additional cleaning activities included in the revised disinfection guidelines18 were observed here; the frequency of these practices has implications for the adoption and acceptance of the guidelines by IDUs. Since agitation of the syringe is a behavior intrinsic to drug use and it appears to have a mechanical "loosening" effect during cleaning, it is probably appropriate to include this activity as part of instructions to IDUs. Because agitation was practiced by a majority of bleach users in this sample, there should be a high level of compliance by IDUs with this recommendation. Other cleaning activities, such as dismantling equipment and a preliminary water rinse, occurred with less frequency in this sample, and may be adopted less readily. The discrepancy between the considerations recently disseminated in the NIDA/CSAT/CDC Prevention Bulletin18 and the actual practices observed here indicate the need for further education of IDUs on effective HIV prevention strategies.
Whether drug users are amenable to using bleach and lengthening the contact time with the disinfectant requires further study. Another interesting finding was the discrepancy between observed versus self-reported contact time of disinfectant. Self-reported times on average were two times longer than observed times. Thus, simple instruction on counting to 30 seconds may still result in insufficient contact times. Instructing users to rely on external timing measures rather than on subjective impressions seems prudent unless few have clocks nearby. Doubling counts is another approach, although the correlation obtained for this sample suggests that the relationship between self-reported and observed times is more complex, and such advice might be incorrect for some IDUs. A third approach derives from the data showing that the median contact time per flush was about 10 seconds, suggesting that a minimum contact time of 30 seconds might be achieved if drug users perform at least three flushes where the syringe is filled completely with bleach. An additional method currently taught in Baltimore involves letting the syringe sit with bleach inside it while the IDU performs other tasks, such as cleaning the cooker and getting fresh rinse water.
REFERENCES
1. Watters JK, Newmeyer JA, Feldman HW, Biernacki P. Street-based AIDS prevention for intravenous drug users in San Francisco: prospects, options, obstacles. In Community Epidemiology Work Group Proceedings, Vol. 2
2. Chaisson RE, Osmond D, Moss AR, Feldmen HW, Biernacki P. HIV, bleach, and needle-sharing. Lancet, June 20, 1987:14-30.
3. Chaisson RE, Moss AR, Onishi R, Osmond D, Carlson JR. Human immunodeficiency virus infection in heterosexual intravenous drug users in San Francisco. AJPH, 1987, 77:169-172.
4. Resnick L, Veren K Salahuddin SZ, et al: Stability and inactivation of HTLV/LAV under clinical and laboratory environments. JAMA 1986, 255:1887-1891.
5. Newmeyer JA. Why bleach? Development of a strategy to combat HIV contagion among San Francisco intravenous drug users. In Needle Sharing Among Intravenous Drug Abusers: National and International Perspectives (1988) (Eds. Battjes RJ and Pickens, RW) NIDA research monograph 80:151-159.
6. Froner, G. Disinfection of hypodermic syringes by IV drug users. AIDS 1987, 1:133-134.
7. Watters, JK. A street-based outreach model of AIDS prevention for intravenous drug users: preliminary evaluation. Contemp Drug Prob 1987, 14:411-23.
8. MMWR. Coordinated community programs for HIV prevention among intravenous-drug users-California, Massachusetts. June 2, 1989, 38, no. 21: 369-374.
9. Mckee MM, Caine VA & Bryson LM. Promoting safe injection technique and condom usage among injection drug users in Indianapolis, Indiana. Int Conf AIDS. 1992, Jul 19-24; 8 (2):D401 (abstract no. PoD 5085).
10. Loxley W, Marsh A & Hawks D. Differences in risk behaviour of injecting drug users between 1989 and 1990 in Perth, Australia . Int Conf AIDS, 1992, Jul 19-24; 8 (2):D447 (abstract no. PoD 5265).
11. Rodriguez Arenas MA, Donoghoe MC, Higueras I, Crosier A et. al. Differences in HIV prevalence and risk behaviour in injecting drug users in London and Madrid. Int Conf AIDS, 1992 Jul 19-24, 8 (3):184 (abstract no. PuC 8187).
12. Vlahov D, Muñoz A, Celentano DD, Cohn S, Anthony JC, Chilcoat H, Nelson KE. HIV seroconversion and disinfection of injection equipment among intravenous drug users, Baltimore, Maryland. Epidemiol 1991,2,6:444-446.
13. Vlahov D, Muñoz A, Celentano DD, Cohn S, Solomon L, Astemborski J, Nelson, KE. Field effectiveness of needle disinfection among injecting drug users. J Acquir Immune Defic Syndr 1994, 7(7):760-766.
14. Soloman L, Astemborski J, Warren D, Munoz A, Cohn S, Vlahov D, Nelson KE. Differences in risk factors for human immunodeficiency virus type 1 seroconversion among male and female intravenous drug users. Am J Epi 1993, 137:892-898.
15. Latkin CA, Vlahov D, Anthony JC. Socially desirable responding and self-reported HIV infection risk behaviors among intravenous drug users. Addiction 1993, 88: 517-526.
16. Shapshak P, McCoy CB, Rivers JE, Chitwood DD, Mash DC, Weatherby NL, Inciardi JA, Shah SM, Brown BS. Inactivation of human immunodeficiency virus-1 at short time intervals using undiluted bleach. J Acquir Immune Defic Syndr 1993;6:218-219.
17. Shapshak P, McCoy CB, Shah SM, et al. Preliminary studies of inactivation of HIV-1 in needles and syringes containing infected blood using undiluted household bleach. J Acquir Immune Defic Syndr 1994, 7(7):754-759.
TABLE 1
Demographic Characteristics of Injection Drug Users Enrolled in Study of Bleach Contact Times, Baltimore Maryland, 192
|
Characteristic |
N (161) |
Percent |
|
Gender |
||
|
Male |
144 |
89.4 |
|
Female |
17 |
10.6 |
|
Race |
||
|
Black |
150 |
93.2 |
|
Non-black |
11 |
7.8 |
|
Age |
||
|
<35 |
58 |
36.0 |
|
≥35 |
103 |
64.0 |
|
Median |
38.5 range |
(25.2-64.1) |
|
Education |
||
|
<12 years |
79 |
49.1 |
|
≥12 years |
82 |
50.9 |
|
Income |
||
|
≥$2500/6 month |
22 |
13.7 |
|
<$2500/6 months |
134 |
83.2 |
|
No Income |
4 |
2.5 |
|
Unknown |
1 |
0.6 |
|
HIV Status |
||
|
Negative |
110 |
68.3 |
|
Positive |
33 |
20.5 |
|
Seroconverters |
18 |
11.2 |
|
Duration of drug use (years) |
||
|
Median |
17.5 range |
(4-47) |
|
Frequency of Drug Use/6 Months |
||
|
Less than weekly |
32 |
19.9 |
|
Weekly |
33 |
21.7 |
|
At least once a day |
94 |
58.4 |
TABLE 2
Median Draw, Contact, Flush and Total Times in Seconds for Bleach Users (N=85)
|
Time in Seconds |
|||
|
Activity |
Median |
Minimum |
Maximum |
|
Draw Time |
6.6 |
1.4 |
33.6 |
|
Contact Time |
9.4 |
1.4 |
138.3 |
|
Flush Time |
16.1 |
3.6 |
152.4 |
|
(Draw + Contact) |
|||
|
Total Draw Time |
12.4 |
4.1 |
45.0 |
|
Total Contact Time |
18.2 |
4.3 |
138.3 |
|
Total Flush Time |
31.5 |
8.8 |
153.4 |
TABLE 3
Proportion of Bleach Users with Total Contact and Total Flush Times Less Than 30 Seconds and Greater Than or Equal to 30 Seconds
|
|
|
N (85) |
Percent |
|
Total Contact Time |
|||
|
|
< 30 seconds |
68 |
80.0 |
|
|
> = 30 seconds |
17 |
20.0 |
|
Total Flush Time (Draw + Contact) |
|||
|
|
< 30 seconds |
39 |
45.9 |
|
|
> = 30 seconds |
46 |
54.1 |
TABLE 4
Odds Ratios and 95% Confidence Limits for total bleach flush time less than 30 seconds or greater than or equal to 30 seconds by selected variables
|
Characteristic |
|||||
|
|
< 30 seconds (N = 39) |
= 30 seconds (N = 46) |
|
||
|
|
N (percent) |
N (percent) |
OR |
95% CI* |
|
|
Bleach level in Syringe |
< 50 units+ |
32 (82.0) |
27 (58.7) |
1.00 |
|
|
|
≥ 50 units |
7 (18.0) |
19 (41.3) |
3.22 |
[1.08,10.35] |
|
Education |
≥12 yrs |
23 (58.9) |
13 (28.3) |
1.00 |
|
|
|
< 12 yrs |
16 (41.0) |
33 (71.7) |
3.65 |
[1.49,8.92] |
|
Age |
< 35 yr |
17 (43.6) |
9 (19.6) |
1.00 |
|
|
|
≥ 35 yr |
22 (56.4) |
37 (80.4) |
3.18 |
[1.21,8.34] |
|
HIV Status |
Seronegative |
27 (69.2) |
31 (76.4) |
1.00 |
|
|
|
Seroconverters and Positive Combined |
12 (30.8) |
15 (32.6) |
1.09 |
[0.39,3.04] |
|
* Cochran-Mantel-Haenszel confidence bounds + 100 unit "u100" diabetic syringe-50 units = one half full |
|||||
DISCUSSION: BLEACH DISTRIBUTION PROGRAMS
T. STEPHEN JONES
T. Stephen Jones observed that the presentations in this workshop session and other recent research make clear the limitations of promoting the disinfection of needles with bleach as a means of preventing HIV transmission. He characterized it as a second-rank intervention: imagine, he said, that you are in a health care facility and about to receive an injection. Given the option of having the injection with a new, sterile syringe or a well-bleached one that has been recently used by someone else, who might well be HIV positive, it would not be difficult to decide what the safer choice would be. However, when there is no safer option, that is, no sterile syringe, then one that has been cleaned well with bleach takes on a different aspect.
He noted that one of the points that is probably clear by now is that there is no body of scientific data to indicate what we ought to tell drug injectors about how to use bleach to disinfect needles and syringes. It is disconcerting to be in the situation of trying to tell people what they should do, but not having a solid scientific basis for what you say. Boiling a syringe for 15 minutes will probably sterilize it. But that is not a practical option in the real world of injection drug use.
Some general principles about how to disinfect syringes with bleach are known, however: reducing the bioburden—the amount of blood or other material that will interfere with the effectiveness of the bleach—is important; contact time is very important (longer is better); agitating the syringe is beneficial because it mixes things up and increases the contact time; and multiple repetitions are also beneficial. About compliance: the larger the number of steps involved, the greater the chance of only partial compliance, suggesting that bleach protocols must be simplified into something that is teachable and do-able in the field.
Finally, Jones said, to the extent that there is not a solid base of information regarding how it should be used in the field, bleach will remain a second-rank intervention for preventing the spread of HIV among drug injectors. It this regard, it should be kept in mind that the more readily available sterile needles are, the less need there will be for bleach as a disinfectant.