10
Endocrine and Immune System Responses to Stress
William R.Beisel1
INTRODUCTION
The interacting responses of the endocrine and immune systems characterize various forms of stress. Although only partially defined, these responses evolve in combination with stress-induced responses of the central nervous system (CNS).
To encompass the complexities of this neuroendocrine-immune axis, equally complex titles for the field are now emerging, as typified by the term psychoneuroendocrineimmunology. Recent reviews of this subject include a book edited by Chrousos et al. (1988), two conferences of the New York Academy of Sciences (see Bomberger and Haar [1988] and Goetzl et al. [1990]), and a review by Chrousos and Gold (1992).
Molecular participants in these responses to stress include traditional hormones, neuropeptides, immunologically generated cytokines, and the secondary and tertiary messengers formed within responding cells. These
participants in stress responses can act as autocrines, paracrines, or circulating endocrines (or endogenous mediators) with far-ranging effects.
Neuroendocrine-immune system responses to stress are characterized by multiple checks and balances and interacting feedback loops. Other prominent features include redundancy, which is most evident with cytokines, many of which have overlapping activities, plus the ability to enlist related cytokines. Many cytokines also exhibit pleiotropy, with multiple functions (Dantzer and Kelley, 1989).
Neural cells and lymphocytes are capable of producing some of the peptide hormones, and many have receptors that allow them to respond to hormonal stimuli.
Responses of the neuroendocrine-immune axis vary with the form, duration, and severity of the inciting stress. Different patterns of response may also evolve longitudinally, over time, if stress is protracted.
Because the molecular mediators involved in responses to stress show differences in the timing and magnitude of their endogenous production, it is not surprising that their physiologic, metabolic, and nutritional consequences are not consistent. Furthermore, the immunological consequences of stress may impair host defense mechanisms against infectious diseases and malignancies. Host defense mechanisms against infectious diseases are of special concern when military populations are under consideration.
Valuable insights have been obtained by studying responses to stress in laboratory animals. However, because of differences between species, such models may not accurately reflect the stress-induced responses that occur in humans.
HISTORICAL PERSPECTIVES
The first hormone to be discovered, just prior to the turn of the century, was adrenalin (epinephrine), a major contributor to immediate cardiovascular responses to stress. Several decades elapsed, however, before William Cannon, in 1929, summarized his theories of homeostasis and his easily understood concept of “fight or flight” (Kopin et al., 1988).
Contributions of Hans Selye
Two additional decades elapsed before Hans Selye divided stress reactions into three stages: an initial sympathoadrenomedullary “alarm reaction,” a subsequent “stage of resistance” with activation of the hypothalamic-pituitary-adrenocortical axis, and a final stage of exhaustion and death (Kopin et al., 1988).
Selye’s resistance stage included his general adaptation syndrome. This was characterized by adrenocortical secretion and hypertrophy, gastrointestinal ulceration, and thymic and lymphoid shrinkage. These concepts of Selye gained widespread acceptance, in part because they immediately preceded the clinical availability and use of cortisone and adrenocorticotropic hormone (ACTH).
Selye taught his students that “to measure is to know.” This dictum is reflected in the subsequent logarithmic growth in knowledge during the 1950s, 1960s, and 1970s about each hormone or hormone group. These knowledge bursts depended on advances in steroid and protein chemistry, which allowed hormones and their metabolic products to be assayed in body fluids. Use of radioisotopic iodine was also a major factor in allowing thyroid physiology to be deciphered.
Studies of Endogenous Pyrogen
Following Paul Beeson’s observation (1948) that endotoxin-free substances obtained from neutrophils were capable of inducing fever, many studies of endogenous pyrogen (EP) were initiated. This research consumed much of the professional lifetimes of individuals such as W.Barry Wood, Jr., Elisha Adkins, Phyllis Bodel, and Patrick A.Murphy.
Studies of Infectious Stress
In the early 1960s, Beisel (1991) initiated comprehensive prospective longitudinal studies of the endocrine, metabolic, physiological, and nutritional responses to the stress of infectious disease in research volunteers. These studies were superimposed on ongoing tests of new, experimental vaccines conducted at the U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID).
The USAMRIID group measured day-to-day changes in glucocorticoids and was the first to report increases in aldosterone, growth hormone, insulin, and glucagon during the stress of infectious illnesses and the depression of thyroid function. Similar changes have subsequently been found in individuals with other forms of stress.
However, hormonal changes failed to account for the metabolic and nutritional changes detected in plasma glycoproteins, amino acids, and trace elements or for other components of acute-phase reactions in volunteers (Beisel, 1991). The dilemma was solved, however, by USAMRIID’s discovery of leukocyte products that induced acute-phase reactions and that stimulated endocrine responses as well. These hormone-like substances were originally named leukocytic endogenous mediator(s) (LEMs) (Pekarek and Beisel, 1971);
they are now called interleukin-1, interleukin-6, and/or tumor necrosis factor, the three “proinflammatory” cytokines.
Studies in Ranger Trainees
Beginning in the late 1970s, a Norwegian group led by Aakvaag and coworkers (1978), Opstad and colleagues (1980, 1981, 1982, 1983, 1984, 1985, 1991, 1992), and Øektedalen (1982, 1983a,b,c) initiated a comprehensive (still ongoing) study of the endocrinological responses of Ranger trainees during 5-day exercises, which included the stresses of food and sleep deprivation as well as severe physical demands. Similar studies have recently been conducted in U.S. Army Rangers. U.S. Army Rangers, who also underwent comprehensive physiological and nutritional measurements as well as immunological studies (Moore et al., 1992)1
Immunological Progress
Logarithmic growth in immunological knowledge also accompanied new research techniques. Identification of populations and subpopulations of T and B lymphocytes in 1968 led to current concepts of the humoral and cell-mediated arms of the immune system and their interrelationships.
Another direction of immunological growth focused on the cytokines. The mutual identity of lymphocyte-activating factor, EP, and LEM was recognized, and in 1979, the three were renamed interleukin-1 (IL-1). The interleukin designation was then used for numerous other cytokines (Beisel, 1991). A few new cytokines may still be identified, but cytokine research still has other major objectives; that is, questions remain about how cytokine genes are regulated, how cytokine gene regulation relates to immune system functions and clinical disease, and how their effects are modulated by circulating cytokine receptors and receptor antagonists.
ENDOCRINE RESPONSES TO STRESS
Most traditional hormones have been implicated in responses to stress, although data on their concentrations in body fluids are relatively scarce and
data on production rates are scarcer still. Despite these shortcomings, major hormonal responses to military stresses are now fairly well defined. Hormonal responses to stress are not simply “all out” but are modulated and carefully controlled by feedback loops. Furthermore, CNS peptide mediators that normally function as neurotransmitters may reach concentrations in plasma that allow them to function as hormones (Geelhoed, 1987).
Catecholamines
Acute responses to stress, that is, Cannon’s “fight or flight” and Selye’s “alarm reaction,” focus on catecholamines. These are products of the CNS’s locus ceruleus and the sympathetic nervous system. A dense network of CNS neurons produces norepinephrine, and epinephrine is released from the adrenal medulla and sympathetic nerve terminals (Chrousos and Gold, 1992). These and other neurotransmitters initiate immediate responses to stress (Kopin et al., 1988), including tachycardia, hyperventilation, sweating, piloerection, dilation of pupils and bronchi, vasomotor changes, and altered gut motility.
Increased concentrations of epinephrine and norepinephrine occur during Ranger training (Opstad, 1991; Opstad et al., 1980), as an early component of trauma or surgical stress (Geelhoed, 1887), during intense physical exercise (Landmann et al., 1984), and in patients who suffer strokes (O’Neill et al., 1991) or severe lobar pneumonia (Feldman et al., 1989). Food restriction stress in mice induces gastric ulcers and increases plasma catecholamine values (Nakamura et al., 1990).
Catecholamine responses to infectious disease stress vary with the severity of illness. Although no changes may be detected in subjects with mild, brief infections, increased plasma epinephrine and norepinephrine concentrations urqwoccur in subjects with septic shock (Beisel, 1991) and critically severe infections (Feldman et al., 1989). It should also be noted that vitamin C is required for the production of these catecholamines. The highest concentrations of vitamin C found in the human body are in the catecholamine-producing areas of both the CNS and adrenal glands.
Adrenocortical Responses
Secretion of increased levels of adrenocortical hormones, central to Selye’s general adaptation syndrome, is initiated by corticotropin-releasing factor (CRF) from hypothalamic neurons; this is followed by the release of ACTH from the anterior pituitary gland. Thus, the central hormonal role in stress is
now often ascribed to CRF (Audhya et al., 1988; Koob et al., 1988; McEwen et al., 1988).
CRF and ACTH
In addition to its role in stimulating greater adrenocorticoid secretion, CRF appears to act in mediating the release of oxytocin, vasopressin, and vasoactive intestinal peptide (Koob et al., 1988; McEwen et al., 1988) and by initiating visceral and paracrine responses to stress (Audhya et al., 1988; Lenz, 1990; Murison and Badde, 1990).
CRF is produced by many CNS neurons in addition to those in the hypothalamus. Its production in the brain’s locus ceruleus gives it a role in stimulating norepinephrine production (Valentino, 1988). Because of its presence in the thymus and spleen, CRF can play a role in neuroimmunomodulation (Audhya et al., 1988; Irwin et al., 1990; Jain et al., 1991). Like CNS opioids, CRF may also have analgesic functions (Bianchi et al., 1991).
Neuronal production of CRF can be stimulated by IL-1 (Rothwell and Grimble, 1992) and platelet-activating factor (PAF) (Rougeot et al., 1990). In contrast, CRF is not stimulated by some stimuli that induce ACTH release, including epinephrine, norepinephrine, angiotensin II, oxytocin, arginine vasopressin, tumor necrosis factor (TNF), IL-2, and IL-6 (Plotsky, 1988; Rothwell and Grimble, 1992). ACTH concentrations in plasma are increased during stress (Chakraborti, 1989; Plotsky, 1988). The responsiveness of adrenocortical cells to ACTH is heightened by immune system activation (Torres-Aleman et al., 1988).
Adrenal Glucocorticoids
The principal glucocorticoids, cortisol in humans and corticosterone in rodents, show relatively modest responses to most stresses. Beisel and coworkers (1967, 1969) found that the normal circadian periodicity of plasma cortisol concentrations was lost during early febrile stages of infections induced in volunteers. Although morning cortisol concentrations were not elevated, the normal circadian decline in cortisol concentrations in the afternoon failed to occur. This combination resulted in a modest increase in 24-h urinary 17-hydroxy-corticosteroids (17-OHCS) excretion (Beisel, 1991).
Increased plasma cortisol concentrations may occur in patients with infections of great severity or terminally ill patients; in such instances, the increased cortisol concentrations can usually be explained by impairment of the hepatic enzymes that convert plasma cortisol to water-soluble metabolites (Beisel, 1991).
Some of the largest adrenocortical responses occur after trauma or surgical stress (Geelhoed, 1987). However, only modest elevations of plasma cortisol and urinary 17-OHCS have been reported with various other stresses such as heat (Armstrong et al., 1989), high-altitude and cold (Chakraborti, 1989), and in patients with strokes (Mulley et al., 1989; O’Neill et al., 1991).
The stress of Ranger training also is accompanied by a modest adrenocorticoid response (Opstad, 1991, 1992; Opstad and Aakvaag, 1983; Opstad et al., 1980) and a loss of circadian rhythm (Opstad and Aakvaag, 1981). Similar small increases in plasma cortisol concentrations were seen in U.S. Rangers (Moore et al., 1992). Elevations of plasma corticosterone concentrations occur during various forms of experimental stress in rodents (Flores et al., 1990; Kandil and Borysenko, 1988; Kant et al., 1987).
The cortisol response to stress in humans is always proportionally greater than the responses of any other, less potent adrenal glucocorticoids or the adrenal androgens (Beisel, 1991). In fact, adrenal androgen concentrations were found to decrease during Ranger training (Opstad, 1992).
Increased body temperatures caused by the stress of a hot, humid environment produced an adreno-cortical response, along with sizable losses of body nitrogen, electrolytes, and minerals (Beisel et al., 1968).
The physiologic effects (and side effects) of these combined glucocorticoid responses to stress are quite small when compared with those of highly potent synthetic adrenocortical steroids. Furthermore, production of adrenocortical steroids may fall below normal concentrations if disease or surgical stress is protracted (Beisel, 1991; Geelhoed, 1987).
ADRENAL MINERALOCORTICOIDS
The increases in aldosterone concentrations produced during the stress of febrile infections and surgical procedures explains the renal retention of sodium (Beisel, 1991). The increase in aldosterone concentrations may be accompanied by a seemingly inappropriate secretion of antidiuretic hormone, which occurs in some infections, especially those localized within the skull (Beisel, 1991). This combination may lead to retention of dangerous amounts of salt and water.
An increase in the amount of secreted aldosterone and renin has been noted during heat stress (Armstrong et al., 1989) and Ranger training (Opstad et al., 1985).
Growth Hormone
A stress-induced increase in plasma immunoreactive growth hormone (GH) concentrations was first reported during the stress of infectious disease (Beisel, 1991). Similar increases have been shown to occur in patients with severe pneumonia (Feldman et al., 1989) and have been reported in Norwegian Rangers (Aakvaag et al., 1978; Opstad, 1991; Opstad and Aakvaag, 1981, 1983; Opstad et al., 1980). Six- to 10-fold increases were found in U.S. Army Rangers by MAJ K.Friedl and colleagues (Moore et al., 1992).
Increased GH secretion by the pituitary could be due to growth hormone-releasing factor stimulation (Dieguez et al., 1988) or stress-induced increases in pituitary dopamine values (Aakvaag et al., 1978). It is also possible that lymphocyte secretion of GH could contribute to increased concentrations of GH in plasma (Kelley, 1990).
Thyroidal Responses
Stress-induced activation of the hypothalamic-pituitary-adrenal axis produces secondary suppression of thyroidal responses (Chrousos and Gold, 1992). The concentrations of plasma protein-bound iodine, thyroxine (T4), triiodothyronine (T3), and thyroid-stimulating hormone (TSH) fall during infectious illnesses (Beisel, 1991). The decrease in T3 concentrations can be explained, in part, by an increase in “reverse” T3. These altered values all rebound to normal concentrations during convalescence.
An identical pattern of thyroidal suppression was observed repeatedly during the stress of Ranger training (Opstad and Aakvaag, 1981, 1983; Aakvaag et al., 1978; Opstad et al., 1980, 1984). These Ranger data were compatible with an almost complete halt in thyroidal T4 release (Aakvaag et al., 1978).
Analogous thyroidal suppression occurs in older or poorly conditioned long-distance runners (Hesse et al., 1989). Low thyroid hormone concentrations also characterize traumatic or surgical stress (Geelhoed, 1987).
Other Pituitary Hormones
Like the thyroidal hormone responses, the reproductive hormone axis is inhibited at all levels by components of the hypothalamic-pituitary-adrenal axis (Chrousos and Gold, 1992). Initially, prolactin (PRL) concentrations were thought to be increased by acute stress (Aakvaag et al., 1978), and small increases in PRL concentrations have been found in patients with severe
pneumonia (Feldman et al., 1989). In contrast, a decline in plasma PRL concentrations was noted in each Ranger group studied (Aakvaag et al., 1978; Opstad and Aakvaag, 1982; Opstad et al., 1980, 1991). These declines in PRL concentrations were minimized by allowing extra sleep time, but not by increasing the daily food intake of the Rangers.
Luteinizing hormone concentrations have shown variable changes in Rangers, with increases and decreases being noted in different groups (Aakvaag et al., 1978; Opstad, 1992; Opstad and Aakvaag, 1983). Another pituitary gonadotropin, follicle-stimulating hormone, showed consistent declines in the plasma of Rangers (Aakvaag et al., 1978; Opstad, 1992).
Testosterone
Testosterone and other androgens share similar fates. Sharp and sustained declines in the concentrations of testosterone and other gonadal androgens occurred in the serum of all Ranger groups tested (Aakvaag et al., 1978; Opstad, 1992; Opstad and Aakvaag, 1983). Declines of 25 and 33 percent were also detected in U.S. Rangers (Moore et al., 1992). It is not known whether testosterone concentration declines were secondary to declines in PRL and the gonadotropin concentrations or whether they were due to some direct testicular effect of heavy exercise.
Pancreatic Hormones
Within 12 h after the onset of fever in infected volunteers, baseline concentrations of both insulin and glucagon in plasma became elevated; glucose tolerance curves and insulin responses also became abnormal, resembling those of adult-onset diabetics (Beisel, 1991). Simultaneous elevations of both insulin and glucagon are most unusual, because these hormones usually act reciprocally. These changes, plus the appearance of cellular insulin resistance, have been attributed to the effects of interleukin-1 (IL-1) (Beisel, 1991).
Increased plasma glucagon values were found in patients with recent strokes (O’Neill et al., 1991). An increase in the insulin concentrations in the plasma of fully fed Rangers was reversed when food restriction stress was added to their training (Opstad and Aakvaag, 1981).
Intestinal Hormones
Øektedalen et al. (1982, 1983a,b,c) reported three- to sixfold increases in plasma secretin values in fasting Ranger trainees and athletes who participated in long cross-country ski races. These high values rapidly returned to normal after a meal or oral glucose feeding.
Plasma vasoactive intestinal peptive and pancreatic peptide values were also elevated in these ski racers, whose gastric acid secretions showed a threefold increase.
Other Hormones and Neuroendocrines
Nussey et al. (1988) found increases in plasma oxytocin and arginine vasopressin concentrations during surgical stress in elderly patients.
Opioid peptides (endorphins, enkephalins, and dynorphin) are produced by lymphocytes and phagocytic cells as well as by the central nervous system (CNS) (Teschemacher et al., 1990). The stresses of severe exercise, surgery, hyperthermia, and severe pain all trigger the release of beta-endorphin but not methionine-enkephalin (Vescovi etal., 1990). Similar beta-endorphin increases are seen in critically ill children (Dindar et al., 1990).
A depression in the concentrations of insulin-like growth factor (somatomedin C) to 30–50 percent of the baseline concentrations in plasma was measured during all four phases of U.S. Army Ranger training (Moore et al., 1992), despite the concomitant rise in plasma growth hormone values.
IMMUNE SYSTEM RESPONSES TO STRESS
Stress and a variety of psychiatric illnesses, notably the affective disorders, may be associated with immunosuppression (Khansari et al., 1990). However, studies attempting to link various forms of stress to an increased susceptibility to infectious diseases or malignancies seldom include direct measurements of immune system competence.
Sophisticated tests of immune functions have generally not been available in clinical laboratories (although the Acquired Immunodeficiency Syndrome (AIDS) epidemic may be changing that, especially for the important task of counting of helper T-lymphocyte [CD4] numbers). Furthermore, interpretation of delayed dermal hypersensitivity skin tests as a measure of cell-mediated immunity (CMI) requires a competent, well-trained observer.
Nevertheless, the available data suggest that stress may reduce functional immune system competence, especially CMI. Emotional stresses such as the
death of a family member, divorce, and major depressions all have immunological links, that is, depressed lymphocyte counts and decreased responsiveness of lymphocytes to mitogens (Bonneau et al., 1990).
Changes in Lymphoid Cells and Tissues
Thymic involution, a component of Selye’s general adaptation syndrome, was first recognized in the early 1800s as a component of severe cachexia; it remains a major problem in malnourished children and in adults with disease-induced cachexia. Involution is most prominent in T-cell areas of the thymus and other lymphoid tissues. In contrast, B-cells and plasma cells are usually spared (Beisel, 1991). Stresses that induce cachexia or deficiencies in levels of body zinc can also induce a reversible state of thymic involution. Other stresses can also influence thymic cells; for example, auditory stress in mice inhibits migration of prethymic stem cells into the thymus (Bomberger and Haar, 1988).
Thymic involution is accompanied by reduced production of zinc-containing hormones by thymic epithelial cells. These peptides (thymosin, thymopoietin, thymopentin) have essential roles in the continued maintenance of T-cell functions throughout the body (Beisel, 1991). These thymic peptides can also increase the production of adrenocorticotropic hormone (ACTH) (Khansari et al., 1990) and may play a role in stressful situations.
Lymphocyte Counts in Stress
Reductions in lymphocyte counts, T-cell counts, and CD4/CD8 cell ratios are seen during some stresses (reduced CD4 cell counts in patients with AIDS are attributed to direct viral invasion of those cells). Lymphopenia caused by corticosteroid-induced lympholysis is a phenomenon of rodents but not of humans.
Simultaneous intravenous injections ofcortisol and epinephrine in healthy adult volunteers caused an initial increase in lymphocyte numbers (particularly suppressor CD8 cells and natural killer [NK] cells); these responses were followed by a decline in lymphocyte numbers and then a normalization within 24 h (Brohee et al., 1990).
Humoral Immunity and Stress
Paradoxically, concentrations of total serum immunoglobulins (IgM, IgG, and IgA) all tend to be increased in children with severe malnutrition. In contrast, secretory IgA is diminished. Antibody responses to a new vaccine also tend to be reduced, but not eliminated, in these children (Beisel, 1991).
U.S. Army Rangers showed diminished antibody responses to two standard vaccines when they were inoculated 2 weeks after beginning their stressful training (Bernton, 1992). Similarly, subjects experiencing major stresses tended to have lower baseline serum IgG values and a poorer 3-week response to immunization with a novel test antigen, keyhole limpet hemocyanin (KLH), than did a control population (Snyder et al, 1990).
Neonatal mice subjected to immobilization stress had diminished antibody responses to test antigens (Taylor and Ross, 1991). Rats subjected to electrical shocks had reduced IgG antibody responses to KLH immunization (Laudenslager et al., 1988). However, Korneva and Shkhinek (1990) found that the nature, intensity, and duration of a stress altered the humoral immune responses to standardized antigens: poor responses were seen after cold or traumatic stresses, average responses were seen after rotation or immobilization stresses, and enhanced responses were seen after repeated swimming stresses.
Peak antibody titers after immunization may coincide with a rise in circulating glucocorticoids in mice (Blalock, 1988).
Cell-Mediated Immunity and Stress
Cell-mediated immune responses to stress include poor responses of lymphocytes to mitogens, diminished delayed dermal hypersensitivity (DDH) responses, and decreased NK cell activity. Although DDH responses have widely been used to study immunological dysfunctions in patients with trauma-or disease-induced cachexia or other forms of malnutrition (Beisel, 1991), DDH has rarely been measured in humans with other types of stresses.
U.S. Army Rangers showed significant declines in DDH responses after 4 weeks of training, with a slight rebound in the sixth week, in a study conducted by LTC E.Bernton (Bernton, 1992). In another Ranger group, however, DDH responses were quite variable; an increase that coincided with a rebound in lymphocyte responsiveness to mitogens was noted during the last phase of training (Moore et al., 1992).
Lymphocyte Responses to Mitogens
In U.S. Army Rangers, lymphocytes showed diminished in vitro responses to mitogens during the second and third phases of training, with a rebound in the fourth and final phase (Moore et al., 1992). Walter Reed Army Institute of Research investigators found slight but progressive declines in lymphocyte numbers during a 6-week period in other trainees (Bernton, 1992). Stresses of avoidable electric shock or loud noise in volunteers each led to poor in vitro lymphocyte responses to mitogens (Weisse et al., 1990).
Sheep exposed to heat stress showed diminished in vitro lymphocyte mitogenesis, and their sera caused reduced mitogenesis of lymphocytes from unstressed sheep (Niwano et al., 1990). In contrast, the lymphocytes of rats subjected to involuntary treadmill running exercise periods (45 minutes per day, for 8 weeks) showed significant increases in mitogen responsiveness (Tharp and Pruess, 1991). This apparent difference may be explained by the study of Hoffman-Goetz et al. (1988), who subjected mice to treadmill running stress. Well-trained mice showed improved lymphocyte mitogenesis, whereas lympho-cytes from untrained novice mice showed decreased mitogenic activity.
Natural Killer Cell Activity
Both lymphocyte mitogenesis and NK cell numbers were found to be decreased in medical students taking final examinations (Bonneau et al., 1990). NK cell activity as well as NK cell surface markers were reduced in individuals categorized as “noncopers” in comparison with the findings for “copers” (Schlesinger and Yodfat, 1988).
Studies in animals showed a reduction in NK cell activity in mice subjected to rotation-induced stress (Kandil and Borysenko, 1988), but an increase in mice stressed by food restriction (Nakamura et al., 1990). A decrease in NK cell activity and NK cell recycling capacity occurred in rats subjected to surgical stress (Pollock et al., 1989). This impaired role of NK cells was shown most dramatically by Zoller et al. (1989), who observed survival in all rats subjected to leg amputation 7 days after footpad implants of adenocarcinoma cells; in contrast, 80 percent of rats died of metastases if a simple laparotomy was done 2 days before the leg amputation.
Cytokine Responses to Stress
The cytokines include interleukins, tumor necrosis factor (TNF; cachectin), interferons, lymphotoxin, colony-stimulating factors, and cell growth factors.
The number of identifiable cytokines continues to grow, and many are important participants in body responses to stress. In many types of stress, cytokines play a major role in triggering and modulating acute-phase reactions, which include hormonal responses and immune system activation.
Many cytokines have now been isolated and produced by recombinant DNA technology. Preclinical and clinical studies have employed several of the cytokines in immunocompromised hosts (Roilides and Pizzo, 1992). These studies have shown dangers as well as benefits from cytokines such as interleukins 1 and 2 (IL-1 and IL-2), TNF, and gamma interferon.
In studies in animals and trials in humans, high doses of these cytokines cause hypotensive shock, capillary leak syndrome, and multiorgan failure. This form of shock is due to the vasodilatory effects of nitric oxide (NO), which is formed when these cytokines activate NO synthase in vascular endothelial cells (Kilbourn and Belloni, 1990).
NO has thus been identified as the “vascular relaxing factor” of septic shock (Hibbs et al., 1992). NO-induced hypotension is not always reversible, even with the most potent pressor agents.
Microbicidal and Tumoricidal Effects of NO
Although the cytokine-induced generation of NO may have lethal consequences, it can also provide an alternative mechanism for the microbicidal and tumoricidal activities of macrophages and other body cells, a mechanism that is totally independent of the well-studied actions of free oxygen radicals generated during the respiratory burst of phagocytic cells.
Following its intracellular induction by cytokine actions, NO synthase produces NO from the terminal guanidino nitrogen atoms of arginine, an amino acid that might be a candidate for use as a nutritional supplement. NO aids in killing tumor cells and some infectious agents by functioning in arginine-dependent cell-mediated immune responses, and by interfering with DNA replication and iron-containing mitochondrial enzymes in tumor cells and microbes (Hibbs et al., 1992). In the process, NO is metabolized to nitrites and then to nitrates, the form in which it is excreted in urine.
During mild infections in humans or induced nonlethal experimental endotoxemia in rats, urinary nitrate excretion can jump 10-fold (Wagner and Tannenbaum, 1982). This newly defined, cytokine-induced microbicidal mechanism is totally dependent on the presence of arginine. Arginine is the only substrate for this NO- and citrulline-generating pathway in endothelial cells and certain phagocytes. Of note, as part of the “urea cycle” in hepatocytes, an analogous pathway proceeds in the opposite direction, that is
citrulline is processed into arginine, which, in turn, is converted to urea and ornithine.
The Immunological Role of Arginine
In addition to its immunological role as the precursor for NO production in body cells, there is growing evidence that arginine supplementation may have immunostimulatory activity, and that arginine becomes an indispensable amino acid after trauma or severe sepsis (Jeevanandam et al., 1993; Kirk and Barbul, 1992; Nirgiotis et al., 1991). Following trauma in rodents, arginine supplements enhance thymic size, prevent thymic involution, and stimulate lymphocyte functions, including the synthesis of IL-2 and the production of natural killer cells. In this latter role, arginine also promotes host antitumor responses in a number of tumor models (Kirk and Barbul, 1992).
The formation of NO and citrulline from arginine in various body cells is totally dependent on the action on intracellular NO synthase. Recent evidence shows that, unlike rodents, human mononuclear phagocytes (monocytes and macrophages) do not possess the enzyme, NO synthase, and under tightly controlled conditions, cannot be induced to produce NO following cellular activation by endotoxin or various cytokines (Schneemann et al., 1993). No reports have yet been forthcoming about similar studies in human granulocytes. Additional studies are also needed to evaluate the potential role of alanine supplements in severely stressed humans.
The Acute Phase Reaction
The triad of IL-1, TNF, and IL-6 initiates and sustains acute-phase reactions, with their attendant fever, myalgia, cephalalgia, somnolence, anorexia, hypermetabolism, immune system activation, hepatic production of acute-phase proteins, muscle protein catabolism, amino acid fluxes from muscle to liver, and the sequestration of iron and zinc (Beisel, 1991).
These responses are accompanied by activation of the pituitary-adrenal axis; release of other hormones such as growth hormone, aldosterone, insulin, and glucagon; alterations in lipid and carbohydrate metabolism; as well as losses of body weight, muscle mass, and body nutrient stores (Beisel, 1991; Parker, 1991).
The acute-phase reaction can be triggered by infectious microorganisms, trauma, or any stress that activates macrophages, thereby initiating the release of IL-1. This can include strenuous exercise. The magnitude and duration of
acute-phase reactions depend on the production and release of IL-1, TNF, and IL-6.
Although acute-phase reactions may have some beneficial aspects, an excess production of IL-1 or TNF can be quite detrimental (Moldawer and Lowry, 1992), in part because of increased cellular release of phospholipase A2, leukotrienes, and prostaglandins (Larson and Henson, 1983; Vadas, 1984). Acute-phase reactions during very severe illnesses or bacterial endotoxemias can be complicated by hypotensive shock attributed to the previously described cytokine-induced formation of nitric oxide (Kilbourn and Belloni, 1990).
Interleukin-1
IL-1 peptides have alpha or beta forms with dissimilar amino acid sequences but with apparently identical actions. These forms can have similar or dissimilar receptors with varying affinities for the many cell types that respond to IL-1 (Scarborough, 1990). Cytokine receptors can become soluble and circulate in plasma, where they may serve to modulate cytokine activities (Kern et al., 1992).
As illustrated in Figure 10–1, IL-1 or TNF, as prototype cytokines, initiate cellular responses when they contact cell receptors. This activates cell wall phospholipase A2 (in a process accompanied by calcium influx), causing the release of arachidonic acid, eicosapentanoic acid, or platelet-activating factor from cell wall phospholipids. Release of these lipids is blocked by glucocorticoid hormones (Claman, 1988).
The fatty acid composition of cell wall phospholipids, as influenced by dietary intake of unsaturated fatty acids of the omega-3 or omega-6 varieties, determines which second messenger acids will be released into the cell (Rothwell and Grimble, 1992). A dietary supplement of linoleic acid in healthy volunteers was shown to increase neutrophil phagocytosis, arachidonic acid release, and leukotriene B4 generation (Jannace et al., 1992).
Cellular responses to these second messenger acids are then governed by the specific enzymes that are contained in individual responding cells. These enzymes convert second messenger acids to third messenger leukotrienes (LTs) or prostaglandins (PGs). Cells that possess lipooxygenase enzymes convert arachidonic or eicosapentanoic acids to LTs, whereas cells that possess cyclooxygenase enzymes convert these acids to PGs (Beisel, 1991; Kinsella et al., 1990).
Arachidonic acid is converted to LTs of the 4 series or PGs of the 2 series. A diet rich in omega-3 fish oils increases eicosapentanoic acid levels in cell walls and leads to less active LTs of the 5 series and PGs of the 3 series (Rothwell and Grimble, 1992).
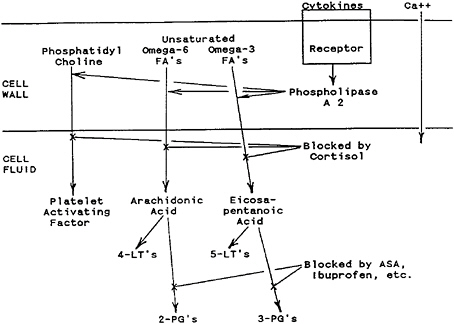
FIGURE 10–1 Cellular actions of prototype cytokines. Following the attachment of cytokines such as interleukin-1 (IL-1) or tumor necrosis factor (TNF) to cell wall receptors, phospholipase A2 is activated; this is accompanied by an influx of calcium. This enzymatic action on cell wall phospholipids creates platelet-activating factor from phosphatidylcholine, arachidonic acid from omega-6 fatty acids (FAs), and eicosapentanoic acid from omega-3 fatty acids. These effects are blocked by cortisol and other potent glucocorticoids. Arachidonic acid is converted to 4-series leukotrienes (LTs) or 2-series prostaglandins (PGs). Eicosapentanoic acid is converted to 5-series LTs or 3-series PGs. PG production is blocked by drugs such as ibuprofen or aspirin (ASA).
Importantly, drugs such as aspirin or ibuprofen can block IL-1-induced production of PGs in the hypothalamus, skeletal muscle, and other cells (Beisel, 1991). Such symptomatic therapy could be of importance for military personnel with minor infections, inflammation, trauma, or other common military stresses by minimizing decrements in the physical and mental performances of soldiers as well as by reducing losses of their body nutrients and muscle mass.
For example, in studies of groups of U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID) volunteers working 8 h/day on computer-generated problems, large performance decrements were shown to accompany illness produced by infectious diseases (Alluisi et al., 1973). However, symptomatic therapy with aspirin and propoxyphene hydrochloride (Darvon) markedly reduced fever and the symptoms of illness. At the same
time, this symptomatic therapy totally eliminated performance decrements (Beisel et al., 1974). Also, when the hypermetabolic effects of fever are minimized, losses of body nutrients are reduced, and skeletal muscle protein is preserved (Beisel, 1991).
Tumor Necrosis Factor
Like IL-1, TNF can be produced by many of the body’s cells. However, TNF is produced in greatest amounts by macrophages, which are stimulated by endotoxin from gram-negative bacteria. TNF is less potent than IL-1 in initiating fever and may even have antipyretic effects, as shown in rats subjected to stress (Long et al., 1990). Unlike IL-1, TNF inhibits cellular lipoproteinase enzymes, allowing triglycerides to accumulate in plasma (Beisel, 1991). TNF helps mediate the development of hypotensive septic shock, and TNF can lead to the development of chronic cachexia (Moldawer and Lowry, 1992; Tracey, 1992).
Concentrations of solable TNF receptors in serum were found to correlate with both malarial counts and severity of disease in African patients (Kern et al., 1988).
Interleukin-6
IL-6, the third member of the acute-phase reaction triad, is mainly responsible for stimulating hepatic production of acute-phase proteins. IL-6 also has antiviral properties, stimulates T cells, and augments antibody secretion by plasma cells (Rothwell and Grimble, 1992).
Epinephrine administration in rats causes marked increases in IL-6 concentrations in plasma (van Gool et al., 1990). Although little is known about IL-6 values in subjects under stress, Kramer and colleagues (Moore et al., 1992) detected an initial increase in IL-6 concentrations in plasma during Ranger training (National Academy of Sciences, 1993); this was followed by a progressive decrease to only 35 percent of the baseline level; IL-6 concenstrations in supernatants of whole blood cultures also fell to 40–80 percent of baseline concentrations during all four phases of Ranger training.
Potential Importance of Stress Proteins
Bacteria subjected to high temperatures or other stresses have long been known to manufacture unique new heat shock proteins that provide survival
value. However, stressed cells from higher species, including humans, produce a family of similar proteins now termed stress proteins (Lindquist, 1986).
Stress proteins from invading microorganisms are important immunologically because they can serve as antigens recognized by host T cells (Lamb and Young, 1990).
Stress proteins in humans have value in traumatized or glucose-starved cells because they can bind to damaged, partially unfolded, or denatured cellular proteins and assist in restoring them to their normal, prestress configuration. Stress proteins also help protect damaged cells from the free radicals generated by inflammatory reactions, and they protect cells against TNF and NK cells (Sugawara et al., 1990). Stress proteins also exist in healthy cells and may play a role in normal protein assembly (e.g., immunoglobulin heavy chain formation in lymphocytes).
The stress proteins formed in cells traumatized during the rigors of military training have potential importance in the hormonal and immunological responses to stress. Their presence in lymphocytes is stimulated by IL-2. Stress proteins can bind to and temporarily inactivate intracellular glucocorticoid receptors (Lamb and Young, 1990). Stress proteins and other cellular proteins released from damaged cells may also serve as antigens that can initiate harmful autoantibody reactions (Lamb and Young, 1990; Leclere and Weryha, 1989).
Military Aspects of the Acute-Phase Reaction
The major knowledge gap in considering endocrine and immune system responses to military stresses is a deficiency of specific data concerning the possible occurrence of acute-phase reactions in soldiers. This reaction occurs with major wounds or infections as generalized nonspecific host responses that help to activate the immune system.
On the other hand, acute-phase reactions contribute to (or cause) the malaise, myalgia, headaches, somnolence, losses of body weight and muscle mass, and decrements in physical and mental performance that accompany military stresses in soldiers who do not have major wounds or infections. Indirect evidence suggests the occurrence of acute-phase reactions.
Data are needed to confirm or reject the possibility that acute-phase reactions are frequent companions of severe military stress. The data that are needed include those that can be obtained from assays of acute-phase reaction components such as acute-phase reactant glycoproteins, triad cytokines (IL-1, IL-6, and TNF) and their receptors, phospholipase A2, leukotrienes, and prostaglandins in plasma. The acute-phase glycoproteins include haptoglobin,
C-reactive protein, amyloid A, alpha-1 antitrypsin, ceruloplasmin, and alpha-1 acid globulin (orosomucoid).
Malnutrition and Immune System Dysfunctions
The immunological dysfunctions associated with malnutrition have been studied in some detail. Protein energy malnutrition (PEM) produces severe immunological problems. These include lymphoid tissue atrophy, depression of thymic hormone production, dysfunction of all aspects of cell-mediated immunity, and to a lesser extent, an impaired humoral response to new antigens (Beisel, 1991).
Individuals with cachexia caused by protracted semistarvation experience the disappearance of allergic and hypersensitivity reactions, and eosinophils are rarely found in their blood (Winick, 1979). The effects of other stresses on allergic illnesses, IgE values, eosinophils, basophils, mast cells, and histamine release have not been studied, however (Parker, 1991).
Many individual micronutrients are also important for proper immune system function, each in its own manner (Beisel, 1991). Important vitamins include A, beta carotene (as distinct from vitamin A), vitamin C, vitamin E, pyridoxine, folic acid, vitamin B12, and to a lesser extent, the other B vitamin group members, vitamin D, and vitamin K. Important trace element deficiencies (or excesses) include those of zinc, iron, copper, and selenium (Beisel, 1982, 1991).
Immune system dysfunctions associated with severe PEM and/or single-nutrient malnutrition have been termed nutritionally acquired immunodeficiency syndrome (NAIDS); unlike AIDS, NAIDS is potentially curable (Beisel, 1991).
U.S. Ranger trainees experienced a significant decline in serum retinol and smaller declines in erythrocyte indicators for several of the B vitamins (Moore et al., 1992).
Nutritional Support for the Immune System
Attempts to improve immune system functions in cachectic patients have included supplementation of diets with vitamins, minerals, unsaturated fatty acids, single amino acids (especially arginine, glutamine, and alanine), precursors of nucleotides, purines (adenine and guanine), pyrimidines (uracil, cytosine, and thymine), and yeast RNA (Balch, 1990).
Supplementation of military diets with single (or combinations) of nutrients such as these could have immunological consequences. Too little
information is available, however, to determine whether the immunological consequences would be helpful or harmful in healthy subjects subjected to severe military stresses.
For instance, polyunsaturated fatty acid supplements increase the fluidity of cell walls in lymphocytes, phagocytes, and other body cells (Kinsella et al., 1990); the omega-3 versus omega-6 composition of these fatty acid supplements helps determine the activities of the leukotrienes and protaglandins produced in response to cytokines (Rothwell and Grimble, 1992). Would such changes be helpful or harmful to the multiply stressed soldier?
On the other hand, infectious stress is known to accelerate the metabolic degradation or loss of body vitamins, but the administration of multivitamin supplement at the Recommended Dietary Allowance maintained virtually normal vitamin concentrations in the plasma of USAMRIID volunteers experiencing an experimental viral infection (Beisel et al., 1972).
HORMONAL AND IMMUNOLOGICAL INTERACTIONS DURING STRESS
Responses of both the immune and endocrine systems differ according to the nature of the stress, its intensity, and its duration (Korneva and Shkhineks, 1990; Landmann et al., 1984), with many interactions becoming apparent (Weigent et al., 1990).
Perhaps the most important response with regard to military personnel, is the complex interaction between cytokines and the central nervous system-pituitary-adrenal axis. IL-1, IL-2, and TNF can stimulate this axis to cause increased cortisol secretion, and at the same time, they activate the immune system. Cytokines and glucocorticoids stimulate gluconeogenesis. The cytokine-induced stimulation of new protein synthesis in hepatocytes requires the permissive presence of cortisol (Beisel, 1985).
Along with these synergistic relationships, however, are antagonistic ones. Cortisol blunts fever, inflammatory reactions, the formation of arachidonic acid, leukotrienes (LTs) and prostaglandins (PGs); and cortisol is immunosuppressive (Beisel, 1985).
Activated lymphocytes are capable of secreting small quantities of a number of hormones, including adrenocorticotropic hormone (ACTH), growth hormone (GH), somatostatin, vasoactive intestinal peptide (VIP), thyroid-stimulating hormone (TSH), prolactin (PRL), and many pain-reducing opioids. Lymphocytes also have cell surface receptors for ACTH, VIP, PRL, GH, catecholamines, and endorphins (Khansari et al., 1990). Releasing factors and cytokines of lymphocytic origin can increase the concentrations of many
hormones in plasma and help to mediate pituitary-adrenal axis activation (Blalock, 1988).
Conversely, lymphocytes have receptors for many hormones, including ACTH, GH, PRL, VIP, steroids, opioids, catecholamines, and releasing factors and can be influenced by these hormones (Khansari et al., 1990). Thymic involution follows hypophysectomy in rats (Kelley, 1990).
Hormonal Effects on Immune System Functions
Corticotropin-releasing factor (CRF) mediates the decrease in natural killer (NK) cell activity caused by stress (Irwin et al., 1990; Jain et al., 1991). CRF also leads to a 75 percent reduction in T-cell mitogenesis in rats stressed by electric shocks; this reduction can be blunted by CRF antagonists or antibodies (Jain et al., 1991). The glucocorticoids suppress NK cell activity and reduce the amounts of cytokines and antibodies produced by lymphocytes (Khansari et al., 1990). These steroids also block the intracellular production of arachidonic acid following interleukin-1 (IL-1) stimulation (Claman, 1988).
Catecholamines suppress lymphocyte mitogenesis (Khansari et al., 1990), stimulate the production of IL-6 in rats (van Gool et al., 1990), and participate in immunoregulatory functions (Besedovsky et al., 1979).
GH enhances the activities of NK and T cells; aids in the production of antibodies, TNF, and thymulin; modulates the effects of IL-2; and enhances macrophage activation (Khansari et al., 1990). GH also helps prime macrophages for superoxide anion production (Edwards et al., 1988) and thus augments the actions of gamma interferon (Kelley, 1990).
PRL stimulates T-cell gene transcription and mitogenesis, T-cell-dependent macrophage tumoricidal activity, and the production of gamma interferon and IL-2 (Bernton et al., 1988; Khansari et al., 1990; Yu-Lee et al., 1990).
The neuropeptide VIP has numerous immunological functions. It influences the homing and recycling of lymphocytes, NK cell activity, and production of antibodies and cytokines. VIP also functions in hypersensitivity reactions by conveying signals from mast cells, basophils, and eosinophils to lymphocytes (Goetzl et al., 1990; Wenger et al., 1990). A 5- to 10-fold increase in high-affinity VIP receptors occurs during stress (Wiik, 1990).
The endorphin opioids enhance NK cell, T-cell, B-cell, and macrophage activities; lymphocyte mitogenesis; and antibody production (Bonneau et al., 1990; Khansari et al., 1990).
Gonadal hormones, ACTH, melatonin, somatostatin, thyroxine, vasopressin, and oxytocin are other hormones that have immunomodulatory properties (Khansari et al., 1990).
CONCLUSIONS
Stress initiates many interacting endocrine, immune system, and central nervous system (CNS) responses. Responses to stress vary widely, depending on the nature, severity, and duration of the stress. Excellent physical conditioning may minimize the magnitude of stress responses.
Endocrine responses to stress have been studied in some detail. Many hormonal changes during stress are components of the acute-phase reaction and tend to be relatively stereotyped.
Acute emotional and physical stresses may evoke immediate CNS-catecholamine responses. Physical and disease-related stresses are generally accompanied by activation of the CNS-pituitary-adrenal axis and increased levels of secretion of aldosterone, growth hormone, and sometimes, insulin and glucagon. In contrast, thyroid hormones, gonadatropins, and androgens show decreased outputs.
More information is needed about stress-induced changes in intestinal hormones and neuroendocrine hormones.
Impairments in both cell-mediated and humoral immunities have been noted during stress, but little is known about the effects of stress on allergic and hypersensitivity reactions. Additional data are needed to define changes in cell-mediated and humoral immunities and lymphocyte subsets (especially natural killer cells and CD4 and CD8 cells) during military stresses.
The major immune system response to stress is the activation of cells that release cytokines, including the triad of interleukins 1 and 6 and tumor necrosis factor, which combine to initiate acute-phase reactions. Military stresses that include strenuous and prolonged physical exercise, numerous cuts and bruises, dermal inflammations, and nagging minor infections are likely to trigger acute-phase reactions, but definitive laboratory data to document the occurrence of such reactions have not yet been obtained. This is an important knowledge gap.
Cytokine-induced acute-phase reactions during stress contribute to deterioration of cognitive and psychomotor performance. Additional studies are needed to determine whether the drugs that block the prostaglandin-releasing actions of the cytokines would prevent or minimize stress-induced (1) decrements of military performance, (2) catabolism of skeletal muscle protein, and (3) catabolic losses of essential micronutrients.
Immune system dysfunctions are caused by protein energy malnutrition and/or inadequacies of certain essential vitamins and minerals. Nutritional rehabilitation with vitamins, minerals, certain amino and fatty acids, and nucleotide precursors appears to hasten immune system recovery.
Similar supplements may have a role in military stresses that generate losses of body weight, muscle mass, and essential micronutrients. There is
little to suggest, however, that nutritional supplementation of healthy individuals can induce a state of supernormal immunity. Rather, some nutrient excesses can suppress immunological functions.
Studies in animals are needed to evaluate the possible immunological importance of stress proteins in military-type stresses.
RECOMMENDATIONS
-
First of all, do no harm.
-
Gather additional data during military stress situations to document the possible occurrence of acute-phase reactions, cell-mediated immune system and humoral immune system dysfunctions, changes in lymphocyte subsets, changes in essential micronutrients, and changes in intestinal hormones and hormonal neurotransmitters.
-
Conduct studies in animals to explore the possible role of stress proteins in military-type stresses.
-
If warranted by additional data, protect the immune system during military stress with a daily multivitamin, multimineral preparation. This should include all vitamins in the amounts specified in the Recommended Dietary Allowance (RDAs)plus beta carotene; and the minerals iron, zinc, copper, and selenium, also in RDA amounts.
-
If new data confirm the occurrence of acute-phase reactions during military stress, the value of drug prophylaxis with ibuprofen (or aspirin) should be tested in an attempt to reduce decrements in performance and the loss of body weight and skeletal muscle mass. The doses of these drugs should be sufficient to minimize the prostaglandin-related effects of acute-phase reactions without masking the symptoms of major infections.
-
Carefully consider the possible adverse immunological consequences of any nutritional supplement given to enhance military performance.
-
Remember that the value of nutritional supplements or pharmaceutical agents in preventing performance decrements may equal or exceed their potential value as performance enhancers.
REFERENCES
Aakvaag, A., T.Sand, P.K.Opstad, and F.Fonnum 1978 Hormonal changes in serum in young men during prolonged physical strain. Eur. J. Appl. Physiol. 39:283–291.
Alluisi, E.A., W.R.Beisel, P.J.Bartelloni, and G.D.Coates 1973 Behavioral effects of tularemia and sandfly fever in man. J. Infect. Dis. 128:710–717.
Armstrong, L.E., R.P.Francesconi, W.J.Kraemer, N.Leva, J.P.Deluca, and R.W.Hubbard 1989 Plasma cortisol, renin, and aldosterone during an intense heat acclimation program. J. Sports Med. 10:38–42.
Audhya, T., D.Zwickler, B.Hutchinson, C.Brown, and C.S.Hollander 1988 Stress-inducing changes in corticotropin-releasing factor (CRF) in immune tissues and hypothalamus: Studies toward defining a role for CRF in neuroimmunomodulation. Trans. Assoc. Am. Physicians 101:62–69.
Balch, F.H. 1990 Symposium proceedings: Update on immunonutrition. Nutrition 6:1–106.
Beeson, P.B. 1948 Temperature elevating effects of a substance obtained from polymorphonuclear leukocytes. J. Clin. Invest. 27:548.
Beisel, W.R. 1982 Single nutrients and immunity. Am. J. Clin. Nutr. 35(Feb. Suppl.):417–68.
1985 Evolving concepts of the roles of interleukin-1 (IL-1) and cortisol in stress: Similarities and differences. Pp. 3–12 in The Physiologic, Metabolic, and Immunologic Actions of Interleukin-1, M.J.Kluger, J.J.Oppenheim, and M.C.Powanda, eds. New York: Alan R.Liss, Inc.
1991 Nutrition and infection. Pp. 507–542 in Nutritional Biochemistry and Metabolism, 2nd ed., M.C.Linder, ed. New York: Elsevier.
Beisel, W.R., J.Bruton, K.D.Anderson, and W.D.Sawyer 1967 Adrenocortical responses during tularemia in human subjects. J. Clin. Endocrinol. Metab. 27:61–69.
Beisel, W.R., and M.I.Rapoport 1969 Interrelations between adrenocortical functions and infections illnesses. N. Engl. J. Med. 280:541–546, 594–604.
Beisel, W.R., R.F.Goldman, and R.J.T.Joy 1968 Metabolic balance studies during induced hyperthermia in man. J. Appl. Physiol. 24:1–10.
Beisel, W.R., B.B.Morgan, Jr., P.J.Bartelloni, G.D.Coates, F.R.DeRubertis, and E.A.Alluisi 1974 Symptomatic therapy in viral illness. A controlled study of effects on work performance. J. Am. Med. Assoc. 228:581–587.
Beisel, W.R., Y.F.Herman, H.E.Sauberlich, R.H.Herman, P.J.Bartelloni, and J.E.Canham 1972 Experimentally induced sandfly fever and vitamin metabolism in man. Am. J. Clin. Nutr. 25:1165–1173.
Bernton, E.W. 1992 Immunological aspects of the Walter Reed Army Institute of Research Study of Class 11–91. Unpublished paper presented at a Meeting of the Food and Nutrition Board Committee on Military Nutrition Research, Review of a Nutritional Assessment of Ranger Training Class 11–91, National Academy of Sciences, February 5, 1992 , Washington, D.C.
Bernton, E.W., M.S.Meltzer, and J.W.Holaday 1988 Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science 239:401–403.
Besedovsky, H.O., A.del Rey, E.Sorkin, M.DaPrada, and H.H.Keller 1979 Immunoregulation mediated by the sympathetic nervous system. Cell. Immunol. 48:346–355.
Bianchi, M., P.Sacerdote, L.Locatelli, P.Mantegazza, and A.E.Panerai 1991 Corticotropin releasing hormone, interleukin-1, and tumor necorsis factor share characteristics of stress mediators. Brain Res. 546:139–142.
Blalock, E.J. 1988 Immunologically-mediated pituitary-adrenal activation. Adv. Exp. Med. Biol. 2– 45:217–223.
Bomberger, C.L., and J.L.Haar 1988 Effect of sound stress on the migration of prethymic stem cells. Ann. N.Y. Acad. Sci. 540:700–701.
Bonneau, R.H., J.K.Kiecolt-Glaser, and R.Glaser 1990 Stress-induced modulation of the immune response. Ann. N.Y. Acad. Sci. 594:253–269.
Brohee, D., M.Vanhaeverbeek, B.Kennes, and P.Neve 1990 Leukocyte and lymphocyte subsets after a short pharmacological stress by intravenous epinephrine and hydrocortisone in healthy humans. Int. J. Neurosci. 53:53–62.
Chakraborti, S. 1989 Adrenocortical activity and plasma camp levels of humans under high altitude stress. Clin. Chim. Acta 184:329–332.
Chrousos, G.P., and P.W.Gold 1992 The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. J. Am. Med. Assoc. 267:1244–1252.
Chrousos, G.P., D.L.Loraiux, and P.W.Gold 1988 Mechanisms of physical and emotional stress. Adv. Exp. Med. Biol. 245:1–530.
Claman, H.N. 1988 Corticosteroids and the immune response. Adv. Exp. Med. Biol. 245:203–208.
Dantzer, R., and K.W.Kelley 1989 Stress and immunity: An integrated view of relationships between the brain and the immune system. Life Sci. 44:1995–2008.
Dieguez, C., M.D.Page, and M.F.Scanlon 1988 Growth hormone neuroregulation and its alterations in disease states. Clin. Endocrinol. 28:109–143.
Dindar, A., H.Gunoz, and O.Neyzi 1990 ß-Endorphin levels of children in acute stress. Diabetes Res. Clin. Pract. 9:245–249.
Edwards, III, C.K., S.M.Ghiasuddin, J.M.Schepper, L.M.Unger, and K.W.Kelly 1988 A newly defined property of somatotropin: Priming of macrophages for production of superoxide anion. Science 239:769–770.
Feldman, C., B.Joffe, V.P.Panz, H.Levy, L.Walker, J.M.Kallenbach, and H.C.Stefel 1989 Initial hormonal and metabolic profile in critically ill patients with community-acquired lobar pneumonia. S. Afr. Med. J. 76:593–596.
Flores, C.M., M.C.Hernandez, K.M.Hargreaves, and B.M.Bayer 1990 Restraint stress-induced elevations in plasma corticosterone and ß-endorphin are not accompanied by alterations in immune functions. J. Neuroimmunol. 28:219–225.
Geelhoed, G.W. 1987 Endocrine emergencies in critical illness. Pp. 773–800 in Trauma: Emergency Surgery and Critical Care, J.H.Siegl, ed. New York: Churchill Livingstone.
Goetzl, E.J., T.Grotmol, R.W.Van Dyke, C.W.Turck, B.Wershil, S.J.Galli, and S.P. Sreedharan 1990 Generation and recognition of vasoactive intestinal peptide by cells of the immune system. Ann. N.Y. Acad. Sci. 594:34–44.
Hesse, V., C.Vilser, J.Scheibe, G.Jahreis, and T.Foley 1989 Thyroid hormone metabolism under extreme body exercises. Exp. Clin. Endocrinol. 94:82–88.
Hibbs, Jr., J.B., C.Westenfelder, R.Taintor, Z.Vavrin, C.Kabliz, R.L.Baranowski, J.H.Ward, R.L.Menlove, M.P.McMurry, J.P.Kushner, and W.E.Samlowski 1992 Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J. Clin. Invest. 89:867–877.
Hoffman-Goetz, L., R.J.Thorne, and M.E.Houston 1988 Splenic immune responses following treadmill exercise in mice. Can. J. Physiol. Pharmacol. 66:1415–419.
Irwin, M., W.Vale, and C.Rivier 1990 Central corticotropin-releasing factor mediates the suppressive effect of stress on natural killer cytotoxicity. Endocrinology 126:2837–2844.
Jain, R., D.Zwickler, C.S.Hollander, H.Brand, A.Saperstein, B.Hutchinson, B.Brown, and T.Audhya 1991 Corticotropin-releasing factor modulates the immune response to stress in the rat. Endocrinology 128:1329–1336.
Jannace, P.W., R.H.Lerman, J.I.Santos, and J.J.Vitale 1992 Effects of oral soy phosphatidylcholine on phagocytosis, arachidonate concentrations, and killing by human polymorphonuclear leukocytes. Am. J. Clin. Nutr. 56:599–603.
Jeevanandam, M., M.R.Ali, N.J.Holaday, and J.K.Weis 1993 Relative nutritional efficacy of arginine and ornithine salts of alpha-ketoisocaproic acid in traumatized rats. Am. J. Clin. Nutr. 57:889–896.
Kandil, O., and M.Borysenko 1988 Stress-induced decline in immune responsiveness in C3H/HeJ mice: Relation to endocrine alterations and tumor growth. Brain Behavior Immun. 2:32–49.
Kant, G.J., J.R.Leu, S.M.Anderson, and E.H.Mougey 1887 Effects of chronic stress of plasma corticosterone, ACTH, and prolactin. Physiol. Behavior 40:775–779.
Kelley, K.W. 1990 The role of growth hormone in modulation of the immune response. Ann. N.Y. Acad. Sci. 594:95–103.
Kern, P., C.J.Hemmer, H.Gallati, S.Neifer, P.Kremsner, M.Dietrich, and F.Porzsolt 1992 Soluble tumor necrosis factor receptors correlate with parasitemia and disease severity in human malaria. J. Infect. Dis. 166:930–934.
Khan, N.A., and V.Karla 1988 Urinary 5HIAA and VMA and their relationship with immunoregulatory cells in stress administered subjects. Arch. Immunol. Ther. Exp. 36:717–721.
Khansari, D.N., A.J.Murgo, and R.E.Faith 1990 Effects of stress on the immune system. Immunol. Today 11:170–175.
Kilbourn, R.G., and P.Belloni 1990 Endothelial cell production of nitrogen oxide in response to interferon gamma in combination with tumor necrosis factor, IL-1, or endotoxin. J. Natl. Cancer Inst. 82:772–776.
Kinsella, J.E., B.Lokesh, S.Broughton, and J.Whelan 1990 Dietary polyunsaturated fatty acids and eicosanoids: Potential effects on the modulation of inflammatory and immune cells: An overview. Nutrition 6:24–44.
Kirk, S.J., and A.Barbul 1992 Arginine and Immunity. Pp. 160–161 in Encyclopedia of Immunology, I.M.Roitt and P.J.Delves, eds. London: Academic Press.
Koob, G.F., K.Thatcher-Briton, A.Tazi, and M.LeMoal 1988 Behavioral pharmacology of stress: Focus on CNS corticotropin-releasing factor. Adv. Exp. Med. Biol. 245:25–34.
Kopin, I.J., G.Eisenhofer, and D.Goldstein 1988 Sympathoadrenal medullary system and stress. Adv. Exp. Med. Biol. 245:11–23.
Korneva, E.A., and E.K.Shkhinek 1990 Neuroendocrine mechanisms underlying stress-induced changes in immune reactions. Int. J. Neurosci. 51:225–226.
Lamb, J.R., and D.B.Young 1990 T cell recognition of stress proteins. A link between infectious and autoimmune disease. Mol. Biol. Med., 7:311–321.
Landmann, R.M.A., F.B.Muller, C.H.Perini, M.Wesp, P.Erne, and F.R.Buhler 1984 Changes of immunoregulatory cells induced by psychological and physical stress: Relationship to plasma catecholamines. Clin. Exp. Immunol. 58:127–135.
Larson, G.L., and P.M.Henson 1983 Mediators of inflammation. Annu. Rev. Immunol. 1:335–359.
Laudenslager, M.L., M.Fleshner, P.Hofstadter, P.E.Held, L.Simons, and S.F.Maier 1988 Suppression of specific antibody production by inescapable shock: Stability under varying conditions. Brain Behavior Immun. 2:92–101.
Leclere, J., and G.Weryha 1989 Stress and auto-immune endocrine diseases. Horm. Res. 31:90–93.
Lenz, H.J. 1990 Mediation of gastrointestinal stress responses by corticotropin-releasing factor. Ann. N.Y. Acad. Sci. 597:81–91.
Lindquist, S. 1986 The heat-shock response. Annu. Rev. Biochem. 55:1151–1191.
Long, N.C., A.J.Vander, S.L.Kunkel, and M.J.Kluger 1990 Antiserum against tumor necrosis factor increases stress hyperthermia in rats. Am. J. Physiol. 258:R591-R595.
McEwen, B.S., R.E.Brinton, and R.M.Sapolsky 1988 Glucocorticoid receptors and behavior: Implications for the stress response. Adv. Exp. Med. Biol. 245:35–45.
Moldawer, L.L., and S.F.Lowry 1992 Interactions between cytokine production and inflammation: Implications for therapies modulating the host defense to infection. Pp. 511–523 in Nutrient Modulation of the Immune Response, S.Cunningham-Rundles, ed., New York: Marcel Deckker, Inc.
Moore, R.J., K.E.Friedl, T.R.Kramer, L.E.Martinez-Lopez, R.W.Hoyt, R.T.Tulley, J.P. Delany, E.W.Askew and J.A.Vogel
1992 Technical Report 13–92, U.S. Army Research Institute of Environmental Medicine, Natick, Mass.
Mulley, G.P., R.C.Wilcox, and M.J.G.Harrison 1989 Plasma cortisol as a measure of stress response in acute stroke. Stroke 20:1593.
Murison, R., and H.K.Badde 1990 The role of corticotropin-releasing factor in rat gastric ulcerogenesis. Ann. N.Y. Acad. Sci. 597:71–80.
Nakamura, K., A.Aoike, T.Hosokaws, K.Rokutan, K.Koyana, Y.Nishi, A.Yoshida, and D.Kawai 1990 Effect of food-restriction stress on immune response in mice. J. Neuroimmunol. 30:23–29.
National Academy of Sciences 1992 A Nutritional Assessment of U.S. Ranger Training Class 11–91. A Brief Report of the Committee on Military Nutrition Research. B.M.Marriott, ed. Washington, D.C.: National Academy Press.
1993 Review of the Results of Nutritional Intervention, Ranger Training Class 11–92 (Ranger II). A Brief Report of the Committee on Military Nutrition Research. B.M.Marriott, ed. Washington, D.C.: National Academy Press.
Nirgiotis, J.G., P.J.Hennessey, and R.J.Andrassy 1991 The effects of an arginine-free enteral diet on wound healing and immune function in the post-surgical rat. J. Pediat Surg. 26:936–941.
Niwano, Y., B.A.Becker, R.Mitra, C.W.Caldwell, E.B.Abdalla, and H.D.Johnson 1990 Suppressed peripheral lymphocyte blastogenesis in pre- and postpartal sheep by chronic heat-stress, and suppressive property of heat-stressed sheep serum on lymphocytes. Dev. Comp. Immunol. 14:139–149.
Nussey, S.S., S.R.Page, V.T.Y.Ang, and J.S.Jenkins 1988 The response of plasma oxytocin to surgical stress. Clin. Endocrinol. 28:277–282.
Øektedalen, O., P.K.Opstad, and O.B.Schaffalitzky de Muckadell 1982 Secretin—a new stress hormone? Regulatory Peptides 4:213–219.
Øektedalen, O., P.K.Opstad, and O.B.Schaffalitzky de Mukadell 1983a The plasma concentrations of secretin and vasoactive intestinal polypeptide (VIP) after long-term strenuous exercise. Eur. J. Appl. Physiol. 52:5–8.
Øektedalen, O., P.K.Opstad, O.B.Schaffalitzky de Mukadell, O.Fausa, and O.Flaten 1983b Basal hyperchlorhydria and its relation to the plasma concentrations of secretin, vasoactive intestinal polypeptide (VIP) and gastrin during prolonged strain. Regulatory Peptides 5:235–244.
Øektedalen, O., P.K.Opstad, H.Waldum, and R.Jorde 1983c The fasting levels and the post-prandial response of gastroenteropancreatic hormones before and after prolonged fasting. Scand. J. Gastroenterol. 18:555–560.
O’Neill, P.A., I.Davies, K.J.Fullerton, and D.Bennett 1991 Stress hormone and blood glucose response following acute stress in the elderly. Stroke 22:842–847.
Opstad, P.K. 1991 Alterations in the morning plasma levels of hormones and the endocrine responses to bicycle exercise during prolonged strain. The significance of energy and sleep deprivation. Acta Endocrinol. (Copenhagen) 125:14–22.
1992 Androgenic hormones during prolonged physical stress, sleep, and energy deprivation. J. Clin. Endocrinol. 74:1176–1183.
Opstad, P.K., and A.Aakvaag 1981 The effect of a high calorie diet on hormonal changes in young men during prolonged physical strain and sleep deprivation. Eur. J. Appl. Physiol. 46:31–39.
1982 Decreased serum levels of oestradiol, testosterone, and prolactin during prolonged physical strain and sleep deprivation, and the influence of a high calorie diet. Eur. J. Appl. Physiol. 49:343–348.
1983 The effect of sleep deprivation on the plasma levels of hormones during prolonged physical strain and calorie deficiency. Eur. J. Appl. Physiol. 51:97–107.
Opstad, P.K., A.Aakvaag, and T.O.Rognum 1980 Altered hormonal response to short-term bicycle exercise in young men after prolonged physical strain, caloric deficit, and sleep deprivation. Eur. J. Appl. Physiol. 45:51–62.
Opstad, P.K., D.Falch, O.Øktedalen, F.Fonnum, and R.Wergeland 1984 The thyroid function in young men during prolonged exercise and the effect of energy and sleep deprivation. Clin. Endocrinol. 20:657–669.
Opstad, P.K., O.Øktedalen, A.Aarvaag, F.Fonnum, and P.K.Lund 1985 Plasma renin activity and serum aldosterone during prolonged physical strain. Eur. J. Appl. Physiol. 54:1–6.
Parker, C.W. 1991 Environmental stress and immunity: Possible implications for IgE-mediated allergy. Perspect. Biol. Med., 34:197–212.
Parker, L.N., E.R.Levin, and E.T.Lifrak 1985 Evidence for adrenocortical adaptation to severe illness. J. Clin. Endocrinol. Metab. 60:947–952.
Pekarek, R.S., and W.R.Beisel 1971 Characterization of the endogenous mediator(s) of serum zinc and iron depression during infection and other stresses. Proc. Soc. Exp. Biol. Med. 138:728–732.
Plotsky, P.M. 1988 Hypophysiotropic regulation of stress-induced ACTH secretion. Adv. Exp. Med. Biol. 245:65–81.
Pollock, R.E., E.Lotzova, and S.D.Stanford 1989 Surgical stress impairment of murine natural killer cell cytotoxicity involves pre- and postbinding events. J. Immunol. 143:3396–3403.
Roilides, E., and P.A.Pizzo 1992 Modulation of host defenses by cytokines: Evolving adjuncts in prevention and treatment of serious infection in immunocompromised hosts. Clin. Infect. Dis. 15:508–524.
Rothwell, N.J., and R.F.Grimble 1992 Metabolic and nutritional effects of TNF. Pp. 237–253 in Tumor Necrosis Factors: The Molecules and Their Emerging Role in Medicine, B.Beutler, ed. New York: Raven Press.
Rougeot, C, M.P.Junier, J.Weidenfeld, P.Praquet, and F.Dray 1990 Intracerebroventricular injection of platelet-activating factor induces secretion of adrenocorticotropin, beta-endorphin and corticosterone in conscious rats: A possible link between the immune and nervous systems. Neuroendocrinology 51:267–275.
Scarborough, D.E. 1990 Cytokine modulation of pituitary hormone secretion. Ann. N.Y. Acad. Sci. 594:169–187.
Schneemann M., G.Schoedon, S.Hofer, N.Blau, L.Guerrero, and A.Schaffner 1993 Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J. Infect. Dis. 167:1358–1363.
Schlesinger, M., and Y.Yodfat 1988 Effect of psychosocial stress on natural killer cell activity. Cancer Detect. Prevent. 12:9–14.
Snyder, B.K., K.J.Roghmann, and L.H.Sigal 1990 Effect of stress and other biopsychosocial factors on primary antibody response. J. Adolesc. Health Care. 11:472–479.
Sugawara, S., M.Nowicki, S.Zie, H.J.Song, and G.Dennert 1990 Effects of stress on lysability of tumor targets by cytotoxic T cells and tumor necrosis factor. J. Immunol. 145:1991–1998.
Taylor, C.E., and L.L.Ross 1991 Alteration of antibody response to pneumococcal polysaccharide type III in rats by neonatal immobilization stress. Brain Behav. Immunol. 3:160–170.
Teschemacher, H., G.Koch, H.Scheffler, A.Hildebrand, and V.Brantl 1990 Opioid peptides immunological significance? Ann. N.Y. Acad. Sci. 594:66–88.
Tharp, G.D., and T.L.Pruess 1991 Mitogenic response of T-lymphocytes to exercise and stress. J. Appl. Physiol. 70:2535–2538.
Torres-Aleman, I., I.Barasoain, J.Borrell, and C.Guaza 1988 Immune activation and psychoneurogenic stress modulate corticosterone-releasing effects of lymphokines and ACTH. Am. J. Physiol. 255:R839-R845.
Tracey, K.J. 1992 The acute and chronic pathophysiologic effects of TNF: Mediation of septic shock and wasting (cachexia). Pp. 255–273 in Tumor Necrosis Factors: The Molecules and Their Emerging Role in Medicine, B.Beutler, ed. New York: Raven Press.
Vadas, P. 1984 Plasma phospholipase A2 levels correlate with the hemodynamic and pulmonary changes in Gram negative septic shock in man. J. Lab. Clin. Med. 104:873–881.
Valentino, R.J. 1988 CRH effects on central noradrenergic neurons: Relationship to stress. Adv. Exp. Med. Biol. 245:47–64.
van Gool, J., H.van Vugt, M.Helle, and L.A.Aarden 1990 The relation among stress, adrenalin, interleukin 6 and acute-phase proteins in the rat. Clin. Immunol. Immunopathol. 57:200–210.
Vescovi, P.P., G.Gerra, G.Pioli, M.Medrazzoni, L.Manninette, and M.Passeri 1990 Circulating opioid peptides during thermal stress. Horm. Metab. Res. 22:44–46.
Wagner, D.A., and S.R.Tannenbaum 1982 Enhancement of nitrate biosynthesis by Escherichia coli lipopolysaccharide. Pp. 437–441 in Nitrosamines and Human Cancer, P.N.MaGee, ed. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory.
Weigent, D.A., D.J.J.Caar, and J.E.Blalock 1990 Bidirectional communication between the neuroendocrine and immune systems. Ann. N.Y. Acad. Sci. 579:17–27.
Weisse, C.S., C.N.Pato, C.G.McAlister, R.Littman, A.Brier, S.M.Paul, and A.Baum 1990 Differential effects of controllable and uncontrollable acute stress on lymphocyte proliferation and leukocyte percentages in humans. Brain Behavior Immunol. 4:339–351.
Wenger, G.D., M.S.O’Dorisio, and E.J.Goetzl 1990 Vasoactive intestinal peptide. Messenger in a neuroimmune axis. Ann. N.Y. Acad. Sci. 594:140–119.
Wiik, P. 1990 VIP inhibition of monocyte respiratory burst ex vivo during prolonged strain and energy deficiency. Int. J. Neurosci. 51:195–196.
Winick, M., ed. 1979 Hunger Disease. Studies by the Jewish Physicians in the Warsaw Ghetto. New York: John Wiley & Sons.
Yu-Lee, L.-Y., A.M.Stevens, J.A.Hrachovy, and L.A.Schwarz 1990 Prolactin-mediated regulation of gene transcription in lymphocytes. Ann. N.Y. Acad. Sci. 594:146–155.
Zoller, M., U.Heumann, M.Betzler, H.Stimmel, and S.Matzka 1989 Depression of nonadaptive immunity after surgical stress: Influence on metastatic spread. Invasion Metastasis 9:46–68.
































