18
Structured Lipids: An Overview and Comments on Performance Enhancement Potential
Ronald J.Jandacek1
INTRODUCTION
This examination of the role of structured lipids in performance enhancement defines structured lipids and compares their properties and metabolism with those of typical long-chain dietary fats. The potential use of structured lipids in diets intended to enhance performance is examined in this chapter in light of the current understanding of fat metabolism and the relatively small amount of information now available on the specific use of structured lipids in performance enhancement.
The primary focus of research with structured lipids has been in the areas of parenteral and enteral nutritional regimens for the stresses caused by surgery, burns, and trauma. A review of results of these uses of structured lipids is included in this chapter to give a complete picture of the metabolism of structured lipids.
Investigations into the use of structured lipids to enhance physical performance in normal subjects have been limited to the replacement of typical fats or carbohydrates by the significant fatty acid component of structured lipids: medium-chain fatty acids. These few studies are summarized, and their results are discussed.
This chapter first discusses the metabolism of typical long-chain triacylglycerol (LCT) fats. It then compares the metabolism and properties of medium-chain fatty acids and medium-chain triacylglycerols (MCTs) with those of LCTs. Structured lipids are then defined, and their uses in enteral and parenteral nutrition are reviewed. Finally, the data from the use of MCTs in exercise trials are reviewed and discussed.
THE METABOLISM OF TYPICAL LCT FATS
Most of the fats in the human diet are triacylglycerol compounds, which are glycerol esters of fatty acids with 12 to 18 carbon atoms. A class of triacylglycerols that occurs occasionally in natural fats and oils has been made synthetically by esterification of glycerol with a mixture of long-chain fatty acids (12–18 carbon atoms) and of medium-chain fatty acids (6–10 carbon atoms). The metabolism of these mixed fatty acid triacylglycerols, known as structured lipids, has been compared with that of typical LCTs (Figure 18–1).
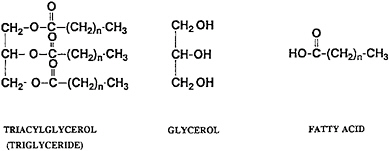
FIGURE 18–1 Structure of a typical long-chain triacylglycerol fatty acid.
To assess the metabolism of structured lipids, it is useful to review the areas of fat metabolism in which structured lipids may differ significantly from normal fats.
The utilization of typical LCT fats begins with ingestion and transit to the stomach where a gastric or lingual lipase begins to catalyze the hydrolysis of a small fraction of the fat to diacylglycerol and free fatty acid (Hamosh et al., 1975). This hydrolysis may be more important in the infant than in the adult.
Fat is released into the small intestine more slowly than water-miscible substances, and here most of the fat is digested and absorbed. The coarse emulsion of fat is converted into a much finer dispersion of oil droplets through mechanical mixing and the reduction of interfacial tension as bile salts, and phospholipids are introduced through gallbladder contraction.
This dispersion of fat greatly increases the interfacial area where pancreatic lipase can act to catalyze the hydrolysis of the triacylglycerol into the 2-monoacylglycerol and fatty acids from the 1 and 3 positions of the glycerol. These digestion products become part of mixed micelles of bile salts and phospholipid—molecular aggregates that are compatible with theaqueous milieu of the intestine but that contain lipophilic components. These mixed micelles are responsible for the transport of fatty acids and 2-monoacylglycerol through the aqueous medium to the intestinal mucosal cell. Micellar transport also facilitates the transport of the fat digestion products through an unstirred water layer that hinders the absorption of hydrophobic compounds (Figure 18–2).
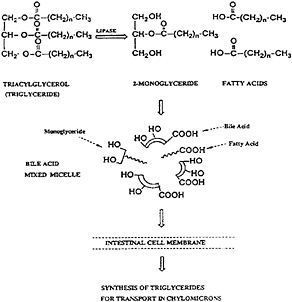
FIGURE 18–2 Lipase-catalyzed hydrolysis of triacylglycerol and the micellar dissolution and transport of the resulting fatty acids and 2-monoacylglycerol products.
It is important to note that the fat must be hydrolyzed before it can be absorbed. There is no absorption through the assimilation of intact oil droplets by pinocytosis (Cardell et al., 1967).
Although pancreatic lipase hydrolyzes fat only in the 1 and 3 positions of the molecule, it is nevertheless possible for fatty acids in the 2 position of the triacylglycerol to be hydrolyzed. This apparent violation of the specificity of pancreatic lipase occurs because of the relative instability of both the 2-monoacylglycerol and the 1,2-diacylglycerol (Crossley et al., 1959). These molecules rearrange by migration of the fatty acid in the 2 position to the 1 or 3 position, which is readily hydrolyzed by lipase (Figure 18–3). This rearrange-
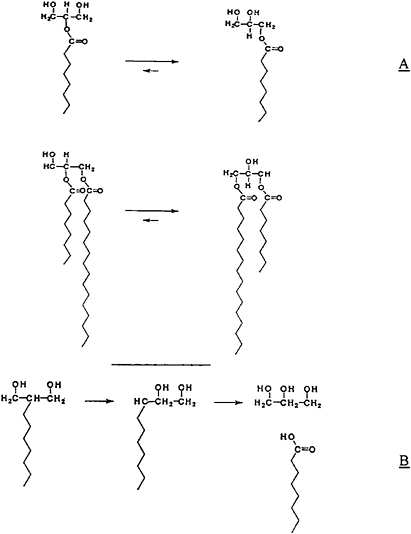
FIGURE 18–3 (A) Rearrangement of 2-monoacylglycerol and 1,2-diacylglycerol. (B) Effect of acyl migration on the hydrolysis of acylglycerols.
rangement is more rapid when the fatty acid is either a short-chain one or an unsaturated one, and a portion of the 2-position fatty acids may be absorbed as fatty acids rather than as monoacylglycerols (Benzonana et al., 1964).
In the enterocyte of the intestinal wall, high concentrations of fatty acids and monoacylglycerols are undesirable, and these compounds are rapidly converted into less toxic species, a triacylglycerol similar to that which was ingested. The 2 position of the ingested LCT is essentially maintained in the newly synthesized triacylglycerol. The fat is combined with a small amount of protein and phospholipid and is packaged in a chylomicron particle that is transported via the lymph into the blood circulation. These chylomicrons encounter lipoprotein lipase during the initial circulation, and their triacylglycerol compounds are hydrolyzed to form fatty acids that are available for utilization by the peripheral tissue. The chylomicron remnants and remaining fat are removed from the circulation by the liver for conversion into very-low-density lipoprotein.
A COMPARISON OF MEDIUM- AND LONG-CHAIN FATTY ACIDS
Medium-Chain Fatty Acids
Fat digestion and absorption change markedly with fatty acids that contain fewer than 12 carbon atoms. There is evidence that lauric acid, with 12 carbon atoms, partly follows the absorption pattern for long-chain fatty acids and partly follows that for the 6–8- and 10-carbon fatty acids, generally classified as medium-chain fatty acids (Bragdon and Karmen, 1960) (Figure 18–4). The atypical (compared with long-chain fatty acids) digestion, absorption, and metabolism of caproic (hexanoic), caprylic (octanoic), and capric (decanoic) acids are the source of the unique properties of structured lipids. A summary of these properties lays the foundation for a review of structured lipids.
Medium-chain triacylglycerols (MCTs) were first introduced to human nutrition more than three decades ago by V.K.Babayen, who was exploring ways to utilize the medium-chain fatty acids that were a by-product of the production of lauric acid from coconut oil (Senior, 1968). MCTs were prepared with these fatty acids by esterification with glycerol. Caprylic and capric acids together make up 13 percent of the fatty acids in coconut oil, and triacylglycerols made only of caprylic and capric acids account for somewhat less than 1 percent of the total oil. Thus, small amounts of MCTs have been consumed by humans for many centuries, and MCTs prepared by esterification of medium-chain fatty acids and glycerol were assumed to be generally recognized as safe. Studies of MCTs have included the early investigations of
digestion and absorption and more recent experiments on parenteral nutrition (Bach and Babayen, 1982).
It was found that MCTs are well utilized in cases of pancreatic insufficiency that hinder the digestion and absorption of typical long-chain triacylglycerols (LCTs). Although there is some evidence that MCTs could be absorbed as intact molecules in the absence of pancreatic lipase (Clark, 1968), hydrolysis possibly takes place during the absorption of most MCTs. MCTs differ markedly from LCTs in intestinal hydrolysis in that they are hydrolyzed at a much higher rate than the LCTs (Jandacek, 1987). This rapid hydrolysis is the key event that provides the unique behavior both of MCTs and of structured lipids. MCTs are readily hydrolyzed, and their digestion products are caprylic acid, capric acid, and the 2-monoacylglycerols of these acids. One can postulate that a rapid rearrangement of the 2-monoacylglycerol to the 1-monoacylglycerol (Figure 18–3B) takes place in the small intestine so that lipase catalyzes the nearly complete hydrolysis of the MCTs. It is also possible that the 1,2-diacylglycerol produced in the stomach by gastric lipase is rearranged to the 1,3-diacylglycerol in the small intestine and is then completely hydrolyzed.
The rapid hydrolysis of MCTs may in part result from the ease of removal of reaction products from the oil-water interface where the lipase is active. This prevention of rate-slowing product buildup depends on the solubilization and transport of fatty acids and monoacylglycerols by bile salt micelles in the hydrolysis of LCTs in the intestine. Although micelles may be somewhat involved in the removal of MCT digestion products, many of these products
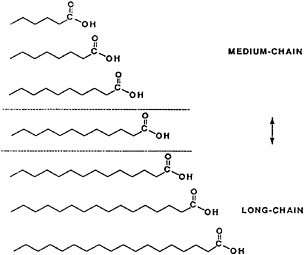
FIGURE 18–4 Description of fatty acids of various lengths. Medium-chain generally refers to fatty acids of 6, 8, or 10 carbon atoms. Long-chain fatty acids have 14 or more carbon atoms. Lauric acid (12 carbon atoms) can behave like both classes of fatty acids.
products are removed by a fast, nonmicellar dissolution in the aqueous medium of the small intestine (Figure 18–5). The products are transported to and through the unstirred water layer coating the intestinal cell membrane without dependence on micellar dissolution.
The ingestion of MCTs as a bolus does not stimulate contraction of the gallbladder, and it does not raise the plasma cholecystokinin level in the manner in which it occurs following LCT ingestion (Hopman et al., 1984; Isaacs et al., 1987). Intestinal cramping and diarrhea were observed after the MCT bolus (Hopman et al., 1984); however, the general acceptance of MCTs in mixed meals suggests amelioration by other nutrients or a development of tolerance.
MCTs are also quite distinct from LCTs in the next step in the metabolic pathway. After absorption into the intestinal mucosal cell, LCT digestion products are reassembled into newly synthesized LCTs, packaged into chylomicrons, and transported via the lymph into the blood. MCT fatty acids are only sparingly found in the lymph, and there they are found only as a trace component of a mixed triacylglycerol with two long-chain fatty acids and one medium-chain fatty acid. The aqueous solubility of the MCT fatty acids causes them to enter the portal circulation bound to albumin for direct transport to the liver (Hashim, 1968) (Figure 18–6). This first encounter of MCT fatty acids
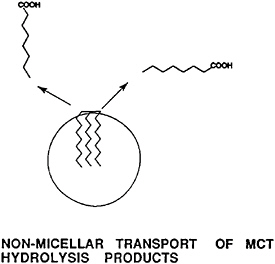
FIGURE 18–5 Nonmicellar (monomeric) dissolution and transport of medium-chain fatty acids contribute to the rapid digestion and absorption of fats containing medium-chain fatty acids.
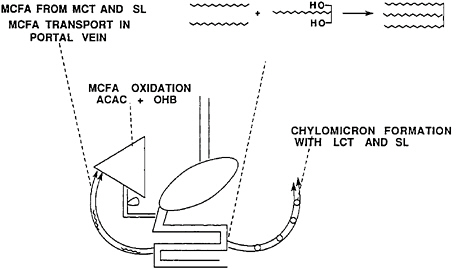
FIGURE 18–6 Medium-chain fatty acids (MCFA) are transported in the portal vein and oxidized to form acetoacetate (ACAC) and ß-hydroxybutyrate (OHB). Long-chain fatty acids and 2-monoacylglycerol form triacylglycerols in the enterocyte and are transported as chylomicrons in the lymph. (LCT, long-chain triacylglycerol; MCT, medium-chain triacylglycerol; SL, structured lipid; OH, hydroxyl).
with the liver is an important deviation from LCT metabolism, where the LCTs in chylomicrons reach the liver only after a significant portion has been hydrolyzed by lipoprotein lipase for delivery of fatty acids to peripheral tissues.
It is generally accepted that the oxidation of fatty acids is facilitated by carnitine, which transports the fatty acid as an acylcarnitine into mitochondria. It has also been widely accepted that carnitine does not play a significant role in the transport of medium-chain fatty acids; however, there is now some evidence for carnitine’s involvement in the metabolism of MCTs (Rossle et al., 1990). Measurements of the levels of short-chain and long-chain acylcarnitine in the blood after infusion of a mixed MCT-LCT emulsion showed an increase in short-chain carnitine. This increase is consistent with the fact that oxidation of medium-chain fatty acids depends in part on carnitine but does not imply carnitine’s role in their transport.
The difference between long-chain and medium-chain fatty acid metabolism continues in the mitochondria. There is a large body of evidence that shows medium-chain fatty acids to be preferentially oxidized in comparison with long-chain fatty acids. Scheig compared the oxidation of [1-14C] palmitic
acid and [1-14C]octanoic acid in rat tissues (Scheig, 1968). In the liver, kidney, diaphragm, heart, brain, and perirenal fat-pad, the extent of oxidation of octanoic acid to 14CO2 was higher than that of palmitic acid. The difference in the liver was approximately sevenfold. The epididymal fat-pad showed the same trend, but the difference was not significant. Complementary to this observation was the extent of lipid synthesis in each of these tissues. In all examined tissues except the perirenal fat-pad, more lipid was synthesized from palmitic acid than from octanoic acid, with more than a 10-fold difference seen in the liver.
The oxidation of MCTs results in the production of ketones (Yeh and Zee, 1976) when fat is the principal fuel for the body, as in those receiving very-low-carbohydrate diets. The level of ketone production seen after MCT ingestion is generally far less than the high levels that are cause for concern in patients with diabetic ketosis. It has been suggested that the production of ketone bodies from MCTs is of benefit in stress situations, since they can provide ketones as an energy source for extrahepatic tissue (Birkhahn, 1988; Maiz et al., 1984).
Comparison of the dietary thermogenesis resulting from meals containing LCTs and MCTs yielded results that further distinguished the metabolism of the fats on the basis of chain length. Hill and coworkers fed subjects liquid formula diets containing 150 percent of estimated energy requirements with 40 percent of calories as fat for 1 week in a double-blind crossover regimen (Hill et al., 1989). The thermal response to the MCTs was nearly twice that to the LCTs after 5 days of feeding (12±1.3 percent versus 6.6±1.0 percent) of ingested energy. The authors concluded that a principal component of this thermal effect of MCT-containing food was the energy expended in lipogenesis from the caloric excess of the MCTs. The lipogenesis from MCTs is an inefficient process with little elongation of the octanoate and, principally, the production of the acetate that is used in the de novo synthesis of fatty acids.
The feeding of MCTs to animals results in virtually no deposition of octanoic and decanoic acids in the carcass or adipose tissue (Jandacek et al., 1991). Rather, palmitic acid is the dominant species.
In addition to the metabolic differences between MCTs and LCTs, there are some noteworthy physical differences. The energy content (bomb calorimetry) of MCTs is approximately 8 kcal/g, in comparison with 9 kcal/g for LCTs because a higher fraction of the carbon atoms in MCTs is already bonded to oxygen and cannot be further oxidized. In the physical properties that are of importance in food preparation and consumption, MCTs are quite similar to unsaturated vegetable oils. They are, however, measurably less stable at frying temperatures and have a flash point that is markedly lower than those of typical vegetable oils (Yang, 1989). This difference results from the higher
volatility of the oil and the traces of lower-molecular-weight products produced during frying.
Given their palatability and their relatively high caloric content when compared with those of carbohydrate and protein, MCTs have been utilized as substitutes for LCTs in the diets of patients with metabolic disorders that limit the consumption of traditional LCT fats. MCTs can be used to provide the hedonic benefit and the caloric density of fat in a meal and have been successfully used in the nutritional management of pancreatic insufficiency (Holt, 1968; Huang, 1968). However, MCTs do not provide essential fatty acids, which are particularly crucial in children. MCTs are valuable in the nutritional treatment of alcoholic pancreatitis and cholestatic liver disease; both of which decrease the concentration of bile acids below their critical micellar concentration.
MCTs have also been used in patients with elevated blood triglycerides resulting from an inability to clear chylomicrons from their plasma. The MCTs can again provide the calories and palatability of fat, but they do not contribute to the formation of chylomicrons and therefore reduce the postprandial lipid levels in the blood (Furman, 1968).
MCTs are currently commercially available as an over-the-counter drug in neat form. MCTs are also included in infant formula that is formulated to provide well-absorbed energy to premature infants who have poorly developed systems of fat digestive enzymes. In Europe, MCTs are sold commercially as the fat component of a diet margarine and in neat form as a salad oil for special dietary purposes.
Other Dietary Fatty Acids
Some recent studies with structured lipids in the diets of patients experiencing trauma-induced stress have centered not only on the mediumchain fatty acid component of the structured lipid but also on the long-chain fatty acid moiety. In particular, structured lipids with omega-3 fatty acids have been synthesized and investigated, so a review of the properties of this class of fatty acids is relevant to an understanding of structured lipids.
The omega terminology for fatty acids is based on the number of carbon atoms from the terminal methyl carbon to the first double bond of the hydrocarbon chain. Omega-3 fatty acids, also called N-3 fatty acids, have a double bond three carbons from the terminal methyl group. The omega-3 fatty acids that have been the focus of hundreds of metabolic studies in the last decade are those found in marine oils, eicosapentaenoic and docosahexaenoic acids (EPA and DHA, respectively). The convenience of the omega terminology is seen in the elongation and desaturation pathways for the synthesis of
fatty acids in animals. The omega-3 fatty acids are precursors for other omega-3 fatty acids, and similarly, omega-6 fatty acids are precursors for elongated and desaturated omega-6 fatty acids. a-Linolenic acid is a precursor for DHA and EPA (Figure 18–7).
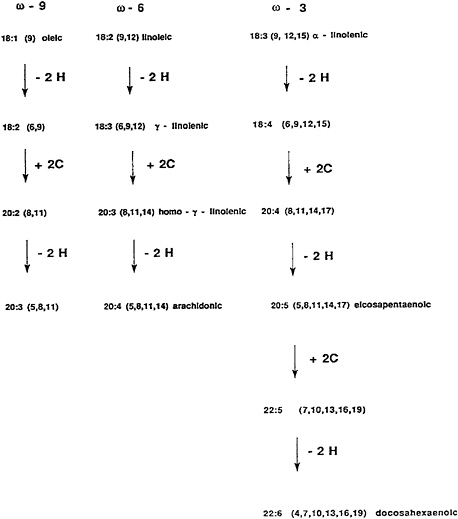
FIGURE 18–7 The metabolism of omega-9, omega-6, and omega-3 fatty acids.
The well-established effects of the addition of significant quantities of EPA and DHA to the diet are a reduction in plasma triglyceride levels and a decreased tendency for platelet aggregation. The latter effect has been shown to be a result of changing the eicosanoid levels that influence aggregation, in particular the levels of thromboxane and prostacyclin. This effect on eicosanoids presumably reflects the displacement of arachidonic acid by DHA in cell membrane phospholipids. Since arachidonic acid is the precursor for proaggregatory eicosanoids, a reduction in arachidonic acid levels and competition for enzymes by DHA alter prostaglandin and leukotriene levels. The inflammatory responses mediated by eicosanoids are also presumably altered by dietary DHA and EPA.
Since arachidonic acid is the precursor for most prostaglandins and leukotrienes, their production might also be altered by manipulation of the dietary level of the precursor to arachidonic acid, linoleic acid (both of these are omega-6 fatty acids). Linoleic acid is clearly established as an essential fatty acid that is a dietary requirement for good health (Holman, 1971; Innis, 1991). A level of linoleic acid that provides approximately 1 percent of energy is probably sufficient for normal growth and health. Relatively high levels of linoleic acid have generally been considered beneficial for health because of an association with reduced levels of total and low-density lipoprotein cholesterol. There is, however, a relatively recent concern that an excess of dietary linoleic acid may be detrimental because it raises the levels of arachidonic acid and its eicosanoid products. The essential fatty acid requirement has therefore been reexamined, with general acceptance of a requirement for omega-3 fatty acids as well as linoleic acid. There is also the possibility that under certain stress situations, minimization of linoleic acid’s activity through an appropriate dietary omega-3/omega-6 fatty acid ratio may be beneficial. Some studies with structured lipids have followed this general hypothesis (Teo et al., 1991).
DEFINITION OF STRUCTURED LIPIDS
The preceding review of the properties of medium-chain triacylglycerols (MCTs) is important to an understanding of the materials that have become known as structured lipids. Structured lipids are fats that are synthesized from mixtures of long-chain and medium-chain fatty acids (Figure 18–8), and indeed, it is the presence of the medium-chain fatty acids that differentiates structured lipids from typical long-chain triacylglycerols (LCTs). As discussed below, the features that differentiate MCTs from LCTs are included in part in the properties of the structured lipids. These differences include the rates of
lipase-catalyzed hydrolysis, oxidation, and lipogenesis. They have resulted in interesting therapeutic potentials for the structured lipids.
As mentioned earlier, structured lipids are not new to the human gastrointestinal system, since a calculation based on the approximation of a random distribution of medium- and long-chain fatty acids would indicate that approximately 16 percent of triacylglycerols in butterfat and 38 percent of triacylglycerols in coconut oil have the compositions of MML and MLL, where M and L signify medium- and long-chain fatty acids, respectively.
Among the first examinations of fat that was enriched with structured lipids was work reported in the mid-1950s and early 1960s. Mattson and coworkers showed that fats made from acetic acid and long-chain fatty acids (acetin fats) sustained growth in rats (Mattson et al., 1956). Fernandes and colleagues transesterified octanoic acid with olive oil to make a “capryl olive oil” in which octanoic acid was 46 percent of the fatty acids (Fernandes et al., 1962). Clement and coworkers showed that mixed butyrate and LCTs were hydrolyzed by human pancreatic lipase (Clement et al., 1962), and Jensen and colleagues studied the hydrolysis of triacylglycerols made with long-chain fatty acids and either butyrate or caproate (Jensen et al., 1962). In general, those studies showed that the hydrolysis of triacylglycerols comprising mixed short-or medium-chain fatty acids with long-chain fatty acids was similar to that of all LCTs. The absorption of the medium-chain fatty acids was found to be by a route other than the lymphatic system in those studies. No utility or benefit of dietary structured lipids was apparent from those early reports.
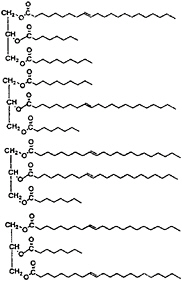
FIGURE 18–8 Triacylglycerols of structured lipids include randomly arranged medium- and long-chain structures. Examples of the principal components are shown.
STRUCTURED LIPIDS IN ENTERAL AND PARENTERAL NUTRITION
A second look at the mixed long- and medium-chain triacylglycerols that were to become known as structured lipids took place in the early 1980s. The potential application of these fats resulted from the experience with fat in total parenteral nutrition (TPN) formulations. Two decades of the use of fat in TPN formulations had demonstrated its advantages, including the provision of essential fatty acids and a high caloric density with a small osmotic load. Although the development of the process and the emulsifiers used for emulsification of soybean oil in these formulations allowed the use of a fat in TPN formulations, the metabolism of fat infused intravenously differs from that of orally ingested fat in some important ways. These differences are determined by the relationship of the physical chemistry of a fat dispersion to its metabolism.
An orally ingested LCT is hydrolyzed in the lumen of the small intestine and is reassembled in the enterocyte into a triacylglycerol that has approximately the same composition as and a structure similar to that of the ingested fat. This absorbed fat is made compatible with the aqueous phases of the lymphatic and blood systems by packaging the triacylglycerol into a chylomicron—a particle that is 75.0–1,000.0 nm in diameter with a hydrophilic shell of protein and phospholipid and a core of triacylglycerol and cholesterol ester. The chylomicron comprises 80–95 percent triacylglycerol by weight.
There is evidence that the emulsified fat in TPN formulations does not follow the same rates of hydrolysis and uptake by peripheral tissue as does the orally ingested fat in chylomicrons. The difference in the rates of uptake of infused chylomicrons and infused artificial emulsions by liver and adipose tissue has been shown in rats (Mattson and Jandacek, 1991; Waddell et al., 1954; Johnson et al., 1990). The reticuloendothelial system of the liver is affected by infused emulsions, as evidenced by the decreased clearance of injected particles in humans (Jensen et al., 1990).
Fats that are rapidly hydrolyzed by lipase, and in particular by lipoprotein lipase, might be expected to be advantageous for use in parenteral fat emulsions. Two approaches have been taken to provide a more nearly physiological rate of hydrolysis: formulations that blend MCTs with LCTs in a mixture of the two fats and formulations based on structured lipids. These formulations were originally intended to deliver essential fatty acids and provide well-utilized energy.
The most extensive exploration of the effects of structured lipids has been in TPN and enteral feeding formulations in trauma, burn, and infection stress models. Although such studies are not directly related to the potential use of structured lipids in the enhancement of performance, they are the principal
source of data related to the nutrition biochemistry of structured lipids. Since the end product of structured lipids in TPN or enteral feeding formulations or in a potentially performance-enhancing diet is the fatty acid, studies of medium chain fatty acids in enteral and TPN formulations are also of relevance.
A summary of studies that are representative of the application of structured lipids and MCTs in TPN and enteral feeding stress models is presented in Table 18–1. Investigations of the enteral and oral use of structured lipids have sought to utilize two potential benefits. The first is the end result of nitrogen sparing in stressed organisms. The nitrogen sparing presumably would result from the facile absorption of the fatty acids of the structured lipid, the relatively high caloric density (and low osmolality) of the nutrient formulation, and the utilization of the medium-chain fatty acids as energy sources. The production of ketones (acetoacetate and ß-hydroxybutyrate) from the medium-chain fatty acids could presumably directly deliver energy sources for utilization by muscle tissue. Three of these studies (DiMichele et al., 1988, 1989; Teo et al., 1989) support this possibility, although the advantage of a structured lipid over a physical mixture of MCTs and LCTs is not always evident, as shown in the nitrogen balance equivalence of the structured lipid and the MCT-LCT mix in the study of DiMichele and colleagues (1989).
The study by Teo and colleagues (1991) suggests that a structured lipid synthesized to include the omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), can be beneficial by reducing lactic acidosis. The authors suggest that the eicosanoid-mediated vasoactive effects of the omega-6 fatty acids were diminished by the omega-3 fatty acids in the structured lipid. In that study the omega-3 structured lipid was not compared with a structured lipid that was low in omega-6 fatty acids, such as one formulated from a vegetable oil with a high level of oleic acid.
The second potential benefit would apply in patients with insufficient lipase, as in the case of pancreatic obstruction in patients with cystic fibrosis. Structured lipids could hydrolyze as readily as MCTs and could reduce the level of pancreatic lipase required for hydrolysis and absorption of the fatty acids. The advantage of structured lipids over MCTs in this application would be the absorption of essential fatty acids as well as medium-chain fatty acids. Hubbard, McKenna, and colleagues compared the absorption of linoleic acid from structured lipids with that from safflower oil (Hubbard and McKenna, 1987; McKenna et al., 1985). The advantage of structured lipids was not clear in their study subjects, although there was a time lag in the appearance of linoleic acid in the subjects’ blood after ingestion of a safflower oil-containing meal but not after ingestion of a structured lipid with linoleic acid. Since lipase-catalyzed hydrolysis of triacylglycerol splits the 1- and 3-position fatty acids, a structured lipid with medium-chain fatty acids in these positions would seem appropriate for use in patients with pancreatic insufficiency. The hydrol-
TABLE 18–1 Summary of Studies Investigating the Potential of Structured lipids as a Source of Calories and Essential Fatty Acids in Enteral and Parenteral Nutrition
|
Route of Delivery and Study |
Species |
Method |
Type of Fat |
Results |
|
Enteral and oral delivery |
|
|||
|
McKenna et al. (1985) |
Cystic fibrosis patients |
Normal diet |
SL (25 percent 18:2), SL (40 percent 18:2), safflower oil, safflower oil emulsion |
Absorption of linoleate delayed after safflower oil compared with that after SL |
|
Hubbard and McKenna (1987) |
Cystic Fibrosis Patients |
Normal Diet |
SL (sunflower), safflower oil |
Absorption of linoleate delayed after safflower compared with that after SL |
|
Jandacek et al. (1987) |
Rat intestinal pancreatic insufficiency model |
Intestinal infusion |
Specific SL and LCT analog |
SL absorption higher than that of long-chain analog |
|
DiMichele et al. (1986) |
Rat, 30 percent burn |
Enteral (gastrostomy) |
MCT, LCT, SL (from dairy fat), SL (from safflower oil) |
Nitrogen balance positive and highest in both SL groups; protein synthesis highest in dairy SL; serum albumin lowest in LCT |
|
DiMichele et al. (1989) |
Rat, 30 percent burn |
Enteral (gastrostomy) |
MCT, LCT, SL, MCT-LCT mix |
Serum albumin higher in SL, MCT; Nitrogen balance higher in SL, mix |
|
Teo et al. (1989) |
Rat, 30 percent burn |
Enteral (gastrostomy) |
SL (fish oil), safflower oil |
SL increased nitrogen balance, liver protein synthesis |
|
Teo et al. (1991) |
Guinea pig, endotoxin infusion |
Oral feeding 6 weeks prior to infusion |
SL (MCT-fish oil), safflower oil |
Hyperlactatemia less with SL |
|
Parenteral delivery |
|
|||
|
Hamaway et al. (1985) |
Rat femoral fracture and Escherichia coli implant |
TPN, 4 days |
MCT-LCT mix, fat-free LCT |
Bacteria in blood highest with fat-free and LCT |
|
|
Rat injected with radio-labeled Escherichia coli |
TPN, 4 days |
MCT-LCT mix, fat-free LCT |
Liver uptake of label highest with MCT-LCT mix |
|
Sobrado et al. (1985) |
Guinea pig, burn, radio-labeled bacteria injected |
TPN |
LCT, MCT, SL |
At 75 percent of nonprotein energy, lung uptake of bacteria highest with LCT; at 50 percent, no difference |
|
Jensen et al. (1990) |
Human 99TC-sulfur colloid injection |
TPN |
Continuous LCT, intermittent LCT, intermittent MCT-LCT mix |
RES cleared colloid decreased by intermittent LCT |
|
Nakagawa et al. (1991) |
Rat |
TPN, fat as 10 and 20 percent of nonprotein energy |
Fat-free, MCT, LCT, SL (specific structure) MCT-LCT mix |
Hepatic lipid higher with fat-free and MCT; lowest was LCT and SL; nitrogen balance lowest in MCT |
|
Mitsuyoshi et al. (1992) |
Rat injected with streptozocin and given 14C-glucose |
TPN |
Fat-free, LCT, MCT, LCT-MCT mix, SL |
Highest 14CO2 and ketones in SL group |
|
NOTE: SL, structured lipids; LCT, long-chain triacylglycerols; MCT, medium-chain triacylglycerols; TPN, total parenteral nutrition; 99Tc, technetium-99; RES, reticuloendothelial system. |
||||
lysis rate and absorption of such a compound (2-linoleoyl-1,3-dioctanoyl glycerol) was found to be markedly higher than those of a typical LCT (Jandacek et al., 1987).
Whether or not this specific structure is advantageous will require further comparisons with randomly arranged structured lipids. The rapid migration of fatty acids in the 2 position of monoacylglycerols and diacylglycerols to the equilibrium 1, and 3 positions may result in a rapid hydrolysis rate for randomly arranged structured lipids as well as for those with the medium-chain fatty acids in the 1 and 3 positions. For example, the hydrolysis of a triacylglycerol molecule from a randomly arranged structured lipid such as 1-linoleoyl-2,3-dioctanoyl glycerol would first form the 1-linoleoyl-2-octanoyl glycerol (diacylglycerol). Diacylglycerols with short or unsaturated fatty acids in the 2 position rapidly rearrange to the 1,3-diacylglycerol. This rearrangement would create a molecule with the medium-chain fatty acid in the lipase-accessible 3 position. After hydrolysis of this molecule, the octanoic acid and the 1-monoacylglycerol would be available for absorption. The importance of this process occurring in the intestine (or in blood, with lipoprotein lipase) would depend on the relative rates of hydrolysis, absorption, and rearrangement.
In light of the topic of this chapter, it should be noted that the investigations of the enteral feeding of structured lipids have used stressed animals. No advantages have been demonstrated in healthy animals. The comparisons of the absorption of fats from the intestine have been made with animal models or patients with insufficient pancreatic lipase. There has not been an investigation or hypothesis that supports an advantage for structured lipids in the nutrition of normal subjects with a normal concentration of lipase, which is far in excess of that required for normal fat consumption (Kasper, 1970).
The studies of the use of structured lipids in parenteral nutrition have generally demonstrated that structured lipids do not overload the reticuloendothelial system when compared with LCTs (Jensen et al., 1990; Sobrado et al., 1985). It is not clear, however, that there is an advantage when structured lipids are compared with a physical mixture of LCT and MCT (Hamawy et al., 1985; Nakagawa et al., 1991). The MCT-LCT mix has been included in a total parenteral nutrition (TPN) formulation that is marketed in Europe.
MEDIUM-CHAIN FATTY ACIDS IN EXERCISE STUDIES
The studies that are of most relevance to the topic of performance enhancement have not utilized structured lipids but have utilized MCTs. A review of those studies is of value, however, since normal, healthy subjects readily change a structured lipid into its fatty components. The lumen of the
intestine experiences the intact structured lipid triacylglycerol, but the metabolic fate of the structured lipid will be the same as that of a mixture of MCTs with LCTs since lipase-catalyzed hydrolysis presents the enterocyte with the same products (Figure 18–9). Essential fatty acid absorption would be expected to be the same regardless of whether the dietary fat comprised a physical mixture of MCTs and safflower oil or a structured lipid made from MCTs and safflower oil.
The general hypothesis for the benefit of MCTs in performance enhancement is that of the possibility of sparing glycogen from utilization during exercise through rapid oxidation of the medium-chain fatty acids for fuel. After digestion and absorption, the medium-chain fatty acids become a fuel that quickly reaches the liver and provides energy through mitochondrial oxidation. The production of acetoacetate and ß-hydroxybutyrate from fatty acid oxidation would be delivered as an energy source for muscle and would potentially diminish glycogen utilization. An extrapolation of this hypothesis would be to predict increased endurance since the utilization of glycogen would be delayed during a continuous period of work.
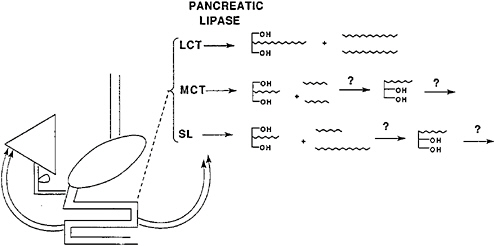
FIGURE 18–9 In the lumen of the small intestine, long-chain fatty acids and 2-monoacylglycerol are produced and absorbed. Medium-chain triacylglycerols (MCTs) and structured lipids (SLs) are hydrolyzed and probably rearranged to allow further hydrolysis. Structured lipids hydrolyze to give the same products that would be formed from mixtures of long-chain triacylglycerols (LCTs) and medium-chain triacylglycerols. OH, hydroxyl groups.
Another potential benefit of the use of MCTs in place of carbohydrate would be the avoidance of the insulin elevation and possible hypoglycemia during exercise that follows a high-carbohydrate meal. Again, the use of medium-chain fatty acids instead of glucose for fuel could result in this effect.
Although these hypotheses seem to be a reasonable mechanism for a metabolic benefit for medium-chain fatty acids resulting from MCTs or structured lipids, there has been no consistent experimental support for their validity. Table 18–2 summarizes the studies that have compared MCTs with other fuels during exercise.
These studies have consistently shown a modest increase in circulating ketone bodies after ingestion of MCT-containing meals. MCT was shown in one study to blunt the increased insulin levels that occurred after ingestion of an isocaloric amount of carbohydrate, but during exercise the carbohydrate was preferentially oxidized compared with the MCTs (Howald and Decombaz, 1983). Another study did not show a reduction of insulin levels when MCTs replaced carbohydrate (Ivy et al., 1980). There was no evidence that MCTs would spare glycogen, and as a result, there was also no evidence that MCTs would extend the time to exhaustion.
These studies therefore do not provide support for the use of MCTs in the enhancement of work and exercise performance. The direct extrapolation of this conclusion to structured lipids is appropriate since the only unique fuel provided by structured lipids is the medium-chain fatty acid component. Medium-chain fatty acids from structured lipids follow the same digestion and absorption process as medium-chain fatty acids from MCTs (Webb and Sanders, 1991).
A clue to the inability of MCTs to spare glycogen is seen in a study in which dietary MCTs significantly enhanced dietary thermogenesis when MCTs were in excess of energy needs and compared with LCTs (Hill et al., 1989). That study raised the possibility that this thermogenesis represents energy that is uncoupled from phosphorylation so as to generate heat without work. The conversion of medium-chain fatty acids to long-chain fatty acids involves the energy-consuming process of the synthesis of fatty acids from acetate groups.
OTHER CONSIDERATIONS OF MCTs AND STRUCTURED LIPIDS
MCT, MCT-LCT mixes, and structured lipids may provide benefits in a nutritional regimen as part of the treatment for the stresses caused by burn injury, surgery, and infection, but there is currently little support for their use in performance enhancement. There are some other applications of the unique metabolic behaviors of precursors of medium-chain fatty acids.
With regard to the development of chronic illness, there is evidence that MCTs do not contribute to the promotion of tumors that is seen with long-chain fatty acid triacylglycerols in experimental animals that have been challenged with a carcinogen (Cohen and Thompson, 1987). This putative benefit may result from the lower caloric density of MCT or from the minimal deposition of fatty acids in the adipose tissues of MCT-fed animals.
The use of MCTs in the nutritional treatment of lipase insufficiency continues to be a viable regimen. In addition, the use of structured lipids with appropriate levels of essential fatty acids may ensure the maintenance or development of a healthful essential fatty acid status.
Another interesting property of structured lipids is the ability to combine desirable physical and metabolic properties in a triacylglycerol. The formulation of a structured lipid with medium-chain fatty acids and behenic acid (22 carbons, saturated), a high-melting-point fatty acid, yields a material (caprenin) that is similar in texture and melting behavior to cocoa butter (Webb and Sanders, 1991). This hedonic benefit is coupled with hydrolysis in the intestine to produce medium-chain fatty acids and a high-melting-point species of behenic acid—the fatty acid, its soap, or the monoglyceride. The behenic compounds, because of their insolubilities and high melting points, are poorly absorbed from the intestine and are excreted. Behenate absorption from caprenin has been shown to be about 30 percent of that ingested, so the caloric density of this structured lipid is approximately 5 kcal/g, or 55 percent of the 9 kcal of typical LCTs per g. The material is therefore a unique, low-calorie substitute for cocoa butter.
A technological development may produce another generation of structured lipids that have so far received little attention. Immobilized lipases have been successfully used in the synthesis of triacylglycerols with fatty acids in specific positions of the triacylglycerol structures. This process is presumably able to provide commercial quantities of specific structured triacylglycerols, and it is now possible to synthesize structured lipids with positional specificity (Jensen et al., 1987). Compounds such as the 1,3-dioctanoyl or 1,2-didecanoyl structured lipids should be available in quantity if research discloses some unique benefits attributable to these structures.
CONCLUSIONS AND RECOMMENDATIONS
Structured lipids are triacylglycerols synthesized from mixtures of long-chain fatty acids and medium-chain fatty acids. Structured lipids and medium-chain triacylglycerols (MCTs) have been shown to provide benefits in their applications in parenteral and enteral nutrition in the sparing of nitrogen and maintenance of reticuloendothelial system function in patients
TABLE 18–2 Summary of Studies of MCT Metabolism During Exercise
|
Study |
Method |
Diet |
Results |
|
Decombaz and Roux (1980) |
Rats on treadmill |
Corn oil, MCT, glucose, water |
MCT increased ketone levels; glycogen utilization was the same in all groups; time to exhaustion was the same in all groups |
|
Ivy et al. (1980) |
Humans at 70 percent of maximum oxygen consumption |
Fasting, MCT, LCT, carbohydrate |
Percentage of energy from lipid was the same with MCT, LCT, and carbohydrate; MCTs did not alter the pattern of preferential oxidation of carbohydrate during exercise; serum glucose and insulin levels were similar for all groups during exercise |
|
Decombaz et al. (1983) |
Humans at 60 percent of maximum oxygen consumption |
MCT, maltodextrins (carbohydrate) |
Glycogen decrease was the same for MCT and carbohydrate |
|
Howald and Decombaz (1983) |
Humans at 60 percent of maximum oxygen consumption |
MCT, maltodextrins |
Carbohydrate was oxidized more than MCT during exercise; MCT oxidized during rest period; MCTs reduced insulin peak |
|
Sabatin et al. (1987) |
Humans at 60 percent of maximum oxygen consumption |
MCT, LCT, glucose, fasting |
No change in time to exhaustion; glucose was 80 percent oxidized; MCT was 45 percent oxidized, and LCT was 9 percent oxidized |
|
Auclair et al. (1988) |
Rats on treadmill |
MCTs, LCTs, glucose, water |
MCT reduced insulin levels; ketone levels were lowest with glucose; muscle and liver glycogen levels were highest with glucose |
|
Sabatin et al. (1989) |
Rats on treadmill |
Carbohydrate, LCTs, MCTs, high protein |
MCT was equivalent to LCT for blood glucose, free fatty acids, and glycerol; MCT was greater than LCT for ketone body levels; MCT, LCT ketone bodies were greater than others; muscle glycogen levels were similar in all groups; liver glycogen of the MCT group was lowest |
|
Sabatin et al. (1991) |
Rats on treadmill |
Glucose, MCTs, LCTs |
Exercise decreased food intake in rats; food intake was decreased by LCT and MCT at 3–6 h and 0–3 h, respectively |
|
NOTE: MCT, medium-chain triacylglycerols; LCT, long-chain triacylglycerols. |
|||
stressed by burns, surgery, and trauma. These fats can also provide energy and essential fatty acids in an absorbable form to patients with lipase insufficiency. However, there is inadequate evidence to support the use of structured lipids or MCTs to enhance exercise performance.
Specific Recommendations
-
Examinations of MCTs in exercise studies have attempted to show a reduction in glycogen depletion by prefeeding of MCTs. There have apparently not been studies of the use of MCTs or structured lipids in glycogen repletion. Although there would not seem to be an advantage over dietary carbohydrate in this regard, there are no data to confirm or refute this possibility.
-
The studies with MCTs in exercise have generally utilized a high-exertion model. The possible sparing of glycogen by MCTs or structured lipids has not been addressed at moderate levels of work output.
-
It is possible that the thermogenesis provided by MCTs in overfeeding could have some utility in a low-temperature environment. If this application of MCTs is beneficial, then structured lipids may provide advantages over MCTs by lessening the gastrointestinal distress resulting from the MCT bolus.
-
Any benefits that may be discovered for MCTs in normal, healthy individuals may be better provided by structured lipids. The reduced gastrointestinal distress and the production of medium-chain fatty acids by the digestion of structured lipids suggest that structured lipids would provide the same nutrients in a more acceptable form.
-
The chronic ingestion of structured lipids with appropriate levels of omega-3 and omega-6 essential fatty acids may provide optimum health in terms of platelet aggregation, inflammatory reactions, and the promotion of cancer. Any studies of structured lipids in this context should include comparisons with other optimum fats such as those containing high levels of oleic acid or with low-fat diets.
-
If high-fat diets are desirable, as in the case of minimizing ration volume, then structured lipids might be advantageous compared with normal long-chain triacylglycerols (LCTs). The stomach emptying of structured lipids would be expected to be faster than that of LCTs and slower than that of MCTs. An optimum balance of caloric density and postprandial digestion might be provided.
REFERENCES
Auclair, E., P.Satabin, E.Servan, and C.Y.Guezennac 1988 Metabolic effects of glucose, medium-chain triglyceride and long-chain triglyceride feeding before prolonged exercise in rats. Eur. J. Appl. Physiol. 57:126–131.
Bach, A.C., and V.K.Babayen 1982 Medium-chain Triglycerides; an update. Am. J. Clin. Nutr. 36:950–952.
Benzonana, G.B., B.Entressangles, G.Marchis-Mouren, L.Pasero, L.Sarda, and P.Desnuelle 1964 Further studies on pig pancreatic lipase. Pp. 141–154 in Metabolism and Physiological Significance of Lipids, R.M.C.Dawson and D.N.Rhodes, eds. New York: Wiley.
Birkhahn, R.H. 1988 Invited comment: The role of synthetic compounds in clinical nutrition. J. Parenter. Enteral Nutr. 9:559–565.
Bragdon, J.H., and A.Karmen 1960 The fatty acid composition of chylomicrons of chyle and serum following the ingestion of different oils. J. Lipid Res. 1:167–170.
Cardell, R.R., S.Badenhausen, and K.R.Porter 1967 Intestinal triglyceride absorption in the rat. J. Cell Biol 34:123–155.
Clark, S.B. 1968 Limiting factors in maximal steady state absorption of medium chain triglycerides. Pp. 69–95 in Medium Chain Triglycerides, J.R.Senior, ed. Philadelphia: University of Pennsylvania.
Clement, G., J.Clement, and J.Bezard 1962 Action of human pancreatic lipase on synthetic mixed symmetrical triglycerides of long-chain acids and butyric acid. Biochem. Biophys. Res. Commun. 8:238–242.
Cohen, L.A., and D.O.Thompson 1987 The influence of dietary medium chain triglycerides on rat mammary tumor development. Lipids 22:455–461.
Crossley, A., I.P.Freeman, B.J.F.Hudson, and J.H.Pierce 1959 Acyl migration in diglycerides. J. Chem. Soc. No volume:760–764.
Decombaz, J., and L.Roux 1980 Glycogen utilization in exercise after increased plasma fatty acids or ketone bodies. Int. J. Vitam. Nutr. Res. 50:210–211.
Decombaz, J., M.J.Arnaud, H.Milon, H.Moesch, G.Philippossian, A.L.Thelin, and H.Howard 1983 Energy metabolism of medium-chain triglycerides versus carbohydrate during exercise. Eur. J. Appl. Physiol. 52:9–14.
DiMichele, S.J., M.D.Karlstad, V.K.Babayan, N.Istfan, G.L.Blackburn, and B.R.Bistrian 1988 Enhanced skeletal muscle and liver protein synthesis with structured lipid in enterally fed burned rats. Metabolism 37:787–795.
DiMichele, S.J., M.D.Karlstad, B.R.Bistrian, N.Istfan, V.K.Babayan, G.L.Blackburn 1989 Enteral nutrition with structured lipid: Effect on protein metabolism in thermal injury. Am. J. Clin. Nutr. 50:1295–1302.
Fernandes, J., J.H.Van de Kamer, and H.A.Weijers 1962 The absorption of fats studied in a child with chylothorax. J. Clin. Invest. 34:1026–1036.
Furman, R.H. 1968 Effects of medium-chain length triglycerides on serum lipids. Pp. 51–61 in Medium Chain Triglycerides, J.R.Senior, ed. Philadelphia: University of Pennsylvania.
Hamawy, K.J., L.L.Moldawer, M.Georgieff, and A.J.Valicente 1985 The effect of lipid emulsions on reticuloendothelial system function in the injured animal. J. Parenter. Enteral Nutr. 9:559–565.
Hamosh, M., H.L.Klaeveman, R.O.Wolf, and R.O.Scow 1975 Pharyngeal lipase and digestion of dietary triglyceride in man. J. Clin. Invest. 55:908–913.
Hashim, S.A. 1968 Studies of medium chain fatty acid transport in portal blood. Pp. 81–90 in Medium Chain Triglycerides, J.R.Senior, ed. Philadelphia: University of Pennsylvania.
Hill, J.O., J.C.Peters, D.Yang, T.Sharp, M.Kaler, N.Abumrad, and H.L.Greene 1989 Thermogenesis in humans during overfeeding with medium-chain triglycerides. Metabolism 38:641–648.
Holman, R.T. 1971 Essential fatty acid deficiency. Prog. Chem. Fats Other Lipids 9:279–348.
Holt, P.R. 1968 Studies of medium chain triglycerides in patients with differing mechanisms for fat malabsorption. Pp. 97–107 in Medium Chain Triglycerides, J.R.Senior, ed. Philadelphia: University of Pennsylvania.
Hopman, W.P.M., J.B.M.J.Janasen, G.Rosenbusch, and C.Lamers 1984 Effect of equimolar amounts of long-chain triglycerides and medium-chain triglycerides on plasma cholecystokinin and gallbladder contraction. Am. J. Clin. Nutr. 39:356–359.
Howald, H., and J.Decombaz 1983 Nutrient intake and energy regulation in physical exercise. Experientia 44:77–88.
Huang, N.N. 1968 Medium chain triglycerides in cystic fibrosis. Pp. 207–217 in Medium Chain Triglycerides, J.R.Senior, ed. Philadelphia: University of Pennsylvania.
Hubbard, V.S., and M.C.McKenna 1987 Absorption of safflower oil and structured lipid preparations in patients with cystic fibrosis. Lipids 22:424–441.
Innis, S.M. 1991 Essential fatty acids in growth and developmnent. Prog. Lipid Res. 30:39–103.
Isaacs, P.E.T., S.Ladas, I.C.Forgacs, R.H.Dowling, S.V.Ellam, T.E.Adrian, and S.R.Bloom 1987 Comparison of effects of ingested medium- and long-chain triglycerides on gallbladder volume and release of cholecystokinin and other gut peptides. Dig. Dis. Sci. 32:481–486.
Ivy, J.L., D.L.Costill, W.J.Fink, and E.Maglischo 1980 Contribution of medium and long chain triglyceride intake to energy metabolism during prolonged exercise. Int. J. Sports Med. 1:15–20.
Jandacek, R.J., J.A.Whiteshide, B.N.Holcombe, R.A.Volpenhein, and J.D.Taulbee 1987 The rapid hydrolysis and efficient absorption of triglyceride with octanoic acid in the 1 and 3 position and long-chain fatty fatty acid in the 2 position. Am. J. Clin. Nutr. 45:940–945.
Jandacek, R.J., J.A.Whiteside, B.N.Holcombe, C.M.Kuehlthau, J.C.Peters, and J.D. 1991 Taulbee Reduced storage of eicosapentaenoic and docosahexaenoic acids in the weanling rat. J. Nutr. Biochem. 2:142–149.
Jensen, G.L., E.A.Mascioli, D.L.Seidner, N.W.Istfan, A.M.Domnitch, K.Selleck, V.K.Babayen, G.L.Blackburn, and B.R.Bistrian 1990 Parenteral infusion of long- and medium-chain triglycerides and reticuloendothelial function in man. J. Parenter. Enteral Nutr. 14:467–471.
Jensen, R.G., J.Sampugna, R.M.Parry, and T.L.Forster 1962 Absence of fatty acid specificity during lipolysis of some synthetic triglycerides by ß-esterase preparations from milk. J. Dairy Sci. 45:842–847.
Jensen, R.G., D.R.Galluzo, and B.Huge-Jensen 1987 Specificity of free and immobilized lipases from Mucor miehi. J. Am. Oil Chem. Soc. 64:644.
Johnson, R.C., S.K.Young, R.Cotter, L.Lin, and W.B.Rowe 1990 Medium-chain-triglyceride lipid emulsion: Metabolism and tissue distribution. Am. J. Clin. Nutr. 52:502–508.
Kasper, H. 1970 Faecal fat excretion, diarrhea, and subjective complaints with highly dosed oral fat intake. Digestion 3:321–320.
Maiz, A., K.Yamazaki, J.Sobrado, V.K.Babayen, L.L.Moldawer, B.R.Bistrian, and G.L. Blackburn 1984 Protein metabolism during total parenteral nutrition (TPN) in injured rats using medium-chain triglycerides. Metabolism 33:901–915.
Mattson, F.H., and R.J.Jandacek 1991 Distribution among tissues of intravenously administered sucrose octaoleate. Lipids 26:750–753.
Mattson, F.H., J.C.Alexander, F.J.Baur, and H.H.Reller 1956 Short-term feeding studies on acetin fats. J. Nutr. 59:277–285.
McKenna, M.C., V.S.Hubbard, and J.G.Bieri 1985 Linoleic acid absorption from lipid supplements in patients with cystic fibrosis with pancreatic insufficiency and in control subjects. J. Pediatr. Gastroenterol. Nutr. 4:45–51.
Mitsuyoshi, K., Y.Hiramatsu, M.Nakagawa, M.Yamamura, K.Hioki, and M.Yamamota 1992 Effect of structured lipids as energy substrate after hepatectomy in rats with streptozocin-induced diabetes. Nutrition 8:41–46.
Nakagawa, M., Y.Hiramatsu, K.Mitsuyoshi, M.Yamamura, K.Hioki, and M.Yamamato 1991 Effect of various lipid emulsions on total parenteral nutrition-induced hepatosteatosis in rats. J. Parenter. Enteral Nutr. 15:137–143.
Rossle, C., Y.A.Caroentier, M.Richelle, W.Dahlan, N.P.D’attelis, P.Furst, and D.H.Elwyn 1990 Medium-chain triglycerides induce alterations in carnitine metabolism. Am. J. Physiol. 258:E944–E947.
Satabin, P., P.Portero, G.Defer, J.Brecout, and C.Guezennec 1987 Metabolic and hormonal responses to lipid and carbohydrate diets during exercise in man. Med. Sci. Sports Exerc. 19:218–223.
Satabin, P., B.Bois-Joyeux, M.Chanez, C.Y.Guezennec, and J.Peret 1989 Effects of long-term feeding of high-protein or high-fat diets on the response to exercise in the rat. Eur. J. Appl. Physiol. 58:583–590.
Sabatin, P., E.Auclair, E.Servan, C.L.Achagiotis and C.Y.Guezennec 1991 Influence of glucose, medium- and long-chain triglyceride gastric loads and forced exercise on food intake and body weight in rats. Physiol. Behav. 50:147–150.
Scheig, R. 1968 Hepatic metabolism of medium chain fatty acids. Pp. 39–49 in Medium Chain Triglycerides, J.R.Senior, ed. Philadelphia: University of Pennsylvania.
Senior, J.R. 1968 Introductory remarks by the chairman. Pp. 3–7 in Medium Chain Triglycerides, J.R. Senior, ed. Philadelphia: University of Pennsylvania.
Sobrado, J., M.L.Moldawer, J.J.Pomposelli, E.A.Mascioli, V.K.Babayen, B.R.Bistrian, and G.L. 1985 Blackburn Lipid emulsions and reticulo-endothelial function in healthy and burned guinea pigs. Am. J. Clin. Nutr. 42:855–863.
Teo, T.C., S.J.DeMichele, K.M.Selleck, V.K.Babayan, G.L.Blackburn, and B.R.Bristrian 1989 Administration of structured lipid composed of MCT and fish oil reduces net protein catabolism in enterally fed burned rats. Ann. Surg. 210:100–107.
Teo, T.C., K.M.Selleck, J.M.F.Wan, J.J.Pomposelli, V.K.Babayen, G.L.Blackburn, and B.R. Bristrian 1991 Long-term feeding with structured lipid composed of medium-chain and N-3 fatty acids ameliorates endotoxic shock in guinea pigs. Metabolism 40:1152–1159.
Waddell, W.R., R.P.Geyer, E.Clarke, and F.J.Stare 1954 Function of the reticuloendothelial system in removal of emulsified fat from the blood. J. Physiol. 177:747–750.
Webb, D.R., and R.A.Sanders 1991 Caprenin 1. Digestion, absorption, and rearrangement in thoracic duct-cannulated rats. J. Am. Coll. Toxicol. 10:325–340.
Yang, D.K. 1989 Tailored triglycerides having improved autoignition characteristics. U.S. Patent 4,832,975.
Yeh, Y.Y., and P.Zee 1976 Relationship of ketosis to metabolic changes induced by acute medium-chain triglyceride feeding in rats. J. Nutr. 106:58–67.
DISCUSSION
GILBERT LEVEILLE: The exercise phenomenon has not been greatly studied but I would think that during aerobic exercise, when tissues would be in a relatively anaerobic state, one would predict that medium-chain triglycerides (MCTs) would be no better and, possibly, could be worse than long-chain fatty acids. And this would not prevent the depletion of glycogen. That is what people found, but apparently, no one looked at the effect of medium-chain triglycerides or structured lipids in a situation with a more moderate exercise level.
RONALD JANDACEK: I have not seen that.
GILBERT LEVEILLE: That would be the situation in which it should have a beneficial effect.
ELDON ASKEW: On the effect of medium-chain triglycerides on thermogenesis, is this simply that the MCTs have a higher specific dynamic action? Is that what you are referring to there?
ELDON ASKEW: Nothing, though, on a substituted normal-calorie diet?
RONALD JANDACEK: No.
STEVEN ZEISEL: You touched in it during your talk, but could you go over the evidence that omega-3 fatty acid pretreatment seems to moderate the response to endotoxin? We know that if you are using MCT, it would be the same.
RONALD JANDACEK: Not MCT. There were two studies by Teo and the Harvard group. Again, I do not think that the mechanism is very clear, other than the mechanism for those with a vasoactive effect, these omega-3 fatty acids and also an effect on the eicosanoids, that the omega-3 fatty acids do reduce the eicosanoid production that is normally seen from omega-6 fatty acids or arachidonic acid.
CAROLE GREENWOOD: Question about cholesterol metabolism and structured lipids.
RONALD JANDACEK: I do no think that it is. There are a couple of studies with octanoic acid. G.L.Crozier showed that octanic acid is a substrate for cholesterol synthesis. But I do not know where the break in chain length is.
WILLIAM BEISEL: On the basis of what we predict from just Clyde Beany’s studies with acid, do you predict that the structured lipids will be able to deliver the omega-3 or omega-6 fatty acids to generate predictable changes in the cell wall, phagocytic cells, or lymphocytes. Has any of that been done?
RONALD JANDACEK: Not with structured lipids. I think the advantage is that if you make a specific structured lipid, you can define exactly what your omega-3 fatty acid level is. There is one patent by F.Mendy in France showing some oxidative stabilization of these long-chain, highly unsaturated fatty acids by putting the medium-chain in the 1 and 3 positions. I do not know whether he proved that or not, but if that is the case, that may be an advantage because certainly the lack of hedonic benefits of fish oil fatty acids is clear.






























