Setting the Stage: Fetal Research, Fetal Tissue Research, and Historical Timeline of Regulation and Legislation1
FETAL RESEARCH
Fetal research is research done with living fetuses either inside the uterus (in utero) or outside the uterus (ex utero). Fetal research, in many contexts including this report, also refers to research with embryos. Some confusion about these terms is understandable, because they are used differently in various contexts. For example, although in legal writing the term "fetus" refers to all prenatal stages (see Figure 1 for various developmental stages), the term "embryo" is often used to denote the earliest stages following fertilization of an ovum by a sperm. Terms relating to different stages of embryonic development, however, are common and include zygote (the fertilized egg); early cleavage embryos produced by cell division up to the 50-to 60-cell stage, each cell of which is called a blastomere; and blastocyst (from the 60-cell stage to the point of implantation). Also commonly used are the terms ''preimplantation" and "postimplantation" embryo, relatively self-evident in meaning, and ''preembryo" referring to very early stages of development up to about an 8-or 16-cell mass.
Fetal research involves both invasive and noninvasive techniques (some of which are no longer used) and has led to improved techniques of in vitro fertilization and embryo transfer, and to major advances in the diagnosis and treatment of conditions that threaten the survival of fetuses and pregnant women. Some of these include
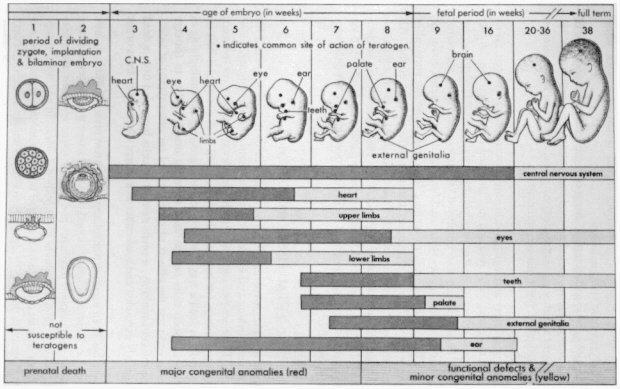
Figure 1
Schematic illustration of the critical periods in human development. During the first two weeks of development, the embryo is usually not susceptible to teratogens. During these predifferentiation stages, either a substance damages all or most of the cells of the embryo, resulting in its death, or it damages only a few cells, allowing the embryo to recover without developing defects. SOURCE: Moore, K.L., The Developing Human: Clinically Oriented Embryology, Third Edition, Philadelphia, W. B. Saunders Company, 1982. Reprinted with permission.
-
tests of efficacy of rubella (German measles) vaccine;
-
detection and treatment of Rh incompatibility (an immune system mismatch between the mother and fetus);
-
development of diagnostic techniques including amniocentesis, ultrasonography, and chorionic villi sampling;
-
detection of genetic and metabolic diseases in the fetus and assessment of other developmental problems, including fetal lung immaturity; and
-
development of better techniques for obstetrical anesthesia and treatment of maternal hypertension, heart disease, and diabetes.
Much of the regulatory and legislative history concerns fetal research, rather than fetal tissue research. Fetal research has attracted greater scrutiny largely because it emerged earlier than the very controversial use of fetal tissue for transplantation. Fetal research also employed technology that emerged earlier (although tissue culture techniques were used for fetal tissue research about the same time) and had wide applications ranging from improving the survival of fetuses to improving the fertility of couples that have difficulty conceiving. Although current controversy is more closely focused on fetal tissue research, especially fetal tissue transplantation, fetal research by its nature involves the complete spectrum of ethical, legal, and social issues that attend to experiments on living fetuses in utero, embryos produced by in vitro methods, and even the very ownership of those embryos.
FETAL TISSUE RESEARCH
Fetal tissue research involves cells from dead fetuses that are harvested for the purpose of establishing cell lines or for use as transplantation material and other purposes. There are two sources of such fetal tissue—elective (or induced) abortions and spontaneous (or natural) abortions. Cell lines are established by culturing fetal cells in such a way that they continue growing and multiplying in laboratory dishes. Such cells can be used to test a drug's ability to damage genetic material or to test the effects of specific viral (or other types) of infection. Because the cells multiply, a small number of cells harvested from a dead fetus can be greatly expanded and used either as a source of more cell lines or for transplants.
Fetal tissue has been used for transplantation for two reasons. First, certain fetal tissues lack cell-surface markers found in mature tissue that induce immune system reactions in transplant recipients and lead to tissue rejection and transplant failure. Thus, fetal tissue eludes these body defenses. In addition, groups of different kinds of fetal cells can be separated from one another in the laboratory to remove those cells that may trigger a recipient's immune system. Second, certain areas of the body do not regenerate after birth or after a few years of life, so the use of mature tissue for transplantation is not possible. Adult brain cells,
for example, regenerate slowly if at all, but when fetal brain cells are transplanted they will grow readily.
It is interesting that fetal tissue research has produced one of the major medical breakthroughs of our time, the development of polio vaccine through the use of fetal cell lines in the 1950s, but also some of greatest current controversy about the use of such cells for transplantation. The first use of fetal cells for transplantation occurred in 1982, when Swedish physicians transplanted fetal brain cells into a patient suffering from Parkinson's disease, a progressive degenerative disease in which cells containing the neurotransmitter dopamine begin to die. Since that time, animal and human experiments (many of the human experiments have been done outside the United States or with private funds) have examined the usefulness of transplanting fetal cells to cure or lessen the effects of diabetes, certain blood disorders, radiation poisoning, and a variety of neurological disorders.
HISTORICAL TIMELINE OF REGULATION AND LEGISLATION
Specific reference to fetal research in federal regulations or legislation began in the 1970s. Public debate about fetal research, however, has its roots in the development of policies governing human subjects research, a process that took center stage in 1972 when the abuses of the Tuskegee Syphilis Study were exposed and a panel was convened to report on this abuse.2 This panel also recommended that a permanent body be established to regulate human subjects research. Also, prior to the landmark Supreme Court decision in Roe v. Wade in 1973, fetal research was banned or regulated indirectly by many state abortion laws. Finally, for fetal tissue research in particular, regulation was applied under the Uniform Anatomical Gift Act that was ratified by the states in 1973 and regulated the use of human organs and tissues after death, prohibiting their sale for profit and their use for any but research or therapeutic reasons. Most likely, it was Roe v. Wade that opened the floodgates to federal regulation, however, because the legalization of abortion, although possibly increasing the potential supply of fetal tissue and cells, brought the debate into full view. The summary that follows provides an overview of that debate but, unless otherwise noted, pertains to fetal research only.
1973
Amendments were put forward in the U.S. Congress to ban research on a fetus outside the uterus, if that fetus had a beating heart. In addition, hearings were held by Senator Edward Kennedy of Massachusetts regarding the protec-
tion of human subjects in research. These hearings were in part fueled by controversy about reports of fetal research being done in Sweden.
1974
The National Research Act (Public Law 93-348) established a National Commission for the Protection of Human Subjects in Biomedical and Behavioral Research. This law further prohibited research on a fetus from an elective abortion until the commission had reported back to Congress. Just after passage of this law, a panel that had been convened in 1971 by the (then) Department of Health, Education, and Welfare (DHEW) issued its report. This report recommended that research resulting in no harm to the fetus be permitted, so long as that research might benefit other fetuses. The panel further recommended, as a protection to pregnant women, that no research be requested or initiated until after the abortion had been initiated. Despite the panel's report, the National Research Act took precedence and all such research was banned pending the National Commission report.
1975
After the National Commission issued its report (Report and Recommendations: Research on the Fetus), fetal research following abortion was permitted under subsequent DHEW regulations for therapeutic reasons, but otherwise held to the standard of "minimal risk." Minimal risk means that no more potential harm is tolerated than would be encountered in daily life. In the case of a fetus, almost all interventions exceed minimal risk, and the regulations did not distinguish between fetuses that were carried to term and those intended for abortion. The DHEW regulations, however, contained the possibility of waiver of the minimal risk standard on a project-by-project basis by a complicated procedure to be decided ultimately by an Ethics Advisory Board.
1978–1980
The first Ethics Advisory Board (EAB) was convened in 1978. The sole waiver issued by this body was to test the efficacy of using fetal blood samples for prenatal diagnosis of sickle cell anemia. The charter for the EAB expired in 1980, and despite publication of a draft charter in 1988, it has not been reactivated.
1985
The Health Research Extension Act was passed, reauthorizing the National Institutes of Health. This legislation contained two important additions. First, a three-year moratorium was imposed on issuing waivers for fetal research, so that
only research involving minimal risk or for therapeutic purposes would be allowed. Second, it called for the establishment of a Congressional Biomedical Ethics Board, comprised of members of Congress, to appoint and oversee a Biomedical Ethics Advisory Committee (BEAC).
1987
The Congressional Biomedical Ethics Board was established. Concurrently, the Uniform Anatomical Gift Act, governing fetal tissue research, was revised and submitted for ratification by the states.
1988–1989
In March 1988, Assistant Secretary for Health Robert Windom of the Department of Health and Human Services (formerly DHEW) imposed a moratorium on transplantation research with fetal tissue from induced abortions until an advisory committee could examine the ethical issues involved. The National Institutes of Health (NIH) convened an ad hoc committee, the Human Fetal Tissue Transplantation Research Panel, which issued a report in December 1988. The majority of panel members concluded that fetal tissue transplantation was acceptable and that the moratorium on use of the waiver provision should be lifted. There were a few panel members who, because of their views concerning abortion, disagreed strongly with the majority. This minority view prevailed when, in 1989, the Secretary of the Department of Health and Human Services extended the moratorium.
In 1988, legislation reauthorizing the NIH extended the fetal research moratorium, pending a report by the BEAC. Although scheduled to expire within a week, the BEAC met for the first time in September 1988 and addressed three topics, one of which was fetal research. In 1989, the BEAC became the victim of political disagreement within its governing Congressional Board and expired having issued no reports.
1993
President Clinton signed an executive order (58 FR 7468) lifting the moratorium and charging the National Institutes of Health to develop guidelines for fetal tissue transplantation research and for fetal research.






