7.A INTRODUCTION
Within the broad theme of pollution prevention, earlier chapters of this book consider various management strategies to reduce the formation of waste during laboratory operations. These include reducing the scale of laboratory operations, cataloging and reusing excess materials, and recycling chemicals that can be recovered safely. Clearly, the best approach to laboratory waste is to not generate it. However, this ideal situation is seldom attained in the laboratory. Therefore, this chapter considers methods for dealing with the waste that is generated during laboratory operations and for accomplishing its ultimate disposal.
The earlier chapters are directed primarily at enhancing the safety of laboratory workers and visitors and focus on the laboratory environment. However, discussing prudent practices for disposal of waste requires a broader perspective. When waste is eventually removed from the laboratory, it affects individuals other than those who acquired or generated it, and, ultimately, society as a whole. Waste is disposed of by three routes: (1) into the atmosphere, either through evaporation or through the volatile effluent from incineration; (2) into rivers and oceans via the sewer system and wastewater treatment facilities; and (3) into landfills. Occasionally, waste has to be held indefinitely at the laboratory site or elsewhere until acceptable modes of disposal are developed. The laboratory worker who generates waste has an obligation to consider the ultimate fate of the materials resulting from his or her work. The high cost of disposal of many materials, the potential hazards to people outside the laboratory, and the impact on the environment are all important factors to be considered.
Because of the potential adverse impact on the public through pollution of the air, water, or land, society invariably regulates waste disposal. Disposal of household waste is usually regulated by municipalities, while hazardous waste disposal is regulated at the federal level and often also by states and municipalities. The focus in this chapter is on the disposal of waste that may present chemical hazards, as well as those multihazardous wastes that contain some combination of chemical, radioactive, and biological hazards. Many of the disposal solutions outlined in this chapter have been designed to take advantage of the fact that there is a normal stream of nonhazardous waste generated in the laboratory and other parts of the institution. In some instances, waste that is classified as hazardous can be modified to permit disposal as nonhazardous waste, which is usually a less expensive and less cumbersome undertaking. The scientist who generates hazardous waste must make decisions consistent with the institutional framework for handling such materials.
Generally, waste is defined as surplus, unneeded, or unwanted material. It is usually the laboratory worker or supervisor who decides whether to declare a given laboratory material a waste. However, specific regulatory definitions must be taken into account as well. Even the question of when an unwanted or excess material becomes a waste involves some regulatory considerations. Whereas some institutions have created glossaries of terms to label waste materials as co-products or surplus reagents, regulations state that a material may be declared a waste if it is ''abandoned" or is considered "inherently wastelike." Spilled materials, for example, often fall into these latter categories. Therefore, it is not necessarily up to the generator to decide whether or not a material is a waste.
Once material becomes a waste by a generator's decision or by regulatory definition, the first responsibility for its proper disposal rests with the laboratory worker. These experimentalists are in the best position to know the characteristics of the materials they have used or synthesized. It is their responsibility to evaluate the hazards and assess the risks associated with the waste and to choose an appropriate strategy to handle, minimize, or dispose of it. As discussed earlier in this volume (see Chapter 3, section 3.B), there are numerous sources of information available to the laboratory worker to guide in the decision making, including those required under various Occupational Safety and Health Administration (OSHA) regulations.
7.B CHEMICALLY HAZARDOUS WASTE
7.B.1 Characterization of Waste
Because proper disposal requires information about the properties of the waste, it is recommended that all chemicals used or generated be identified clearly. In general, they must be retained in clearly marked containers, and if they have been generated within the laboratory, their source must be defined clearly on the container and ideally in some type of readily available notebook record. In academic laboratories where student turnover is frequent, it is particularly important that the materials used or generated be identified. This practice can be as important for small quantities as it is for large quantities of material.
It is usually quite simple to establish the hazardous characteristics of clearly identified waste. Unidentified materials present a problem, however, because treatment disposal facilities are prohibited from accepting materials whose hazards are not known. In those cases when the identity of the material is not known, it is possible to carry out simple tests to determine the hazard class into which the material should be categorized. Because the generator may be able to apply some gen-
eral information, it is usually advisable to carry out the hazard categorization process before the materials are removed from the laboratory. Having the analysis done at the laboratory is also usually cheaper than having it performed by the treatment disposal facility or an outside contractor.
Generally, it is not necessary to determine the molecular structure of the unknown material precisely. Hazard classification information usually satisfies the regulatory requirements and those of the treatment disposal facility. However, it is important to establish that the disposal facility will accept the analytical data that are ultimately provided.
The first concern in identification of an unknown waste is safety. The laboratory worker who carries out the procedures should be familiar with the characteristics of the waste and any necessary precautions. Because the hazards of the materials being tested are unknown, it is imperative that proper personal protection and safety devices such as fume hoods and shields be employed. Older samples can be particularly dangerous because they may have changed in composition, for example, through the formation of peroxides. (See Chapter 3, section 3.D.3.2, and Chapter 5, section 5.G.3, for more information on peroxides.)
The following information is commonly required by treatment disposal facilities before they will consider handling unknown materials:
-
physical description,
-
water reactivity,
-
water solubility,
-
pH and possibly also neutralization information,
-
ignitability (flammability),
-
presence of oxidizer,
-
presence of sulfides or cyanides,
-
presence of halogens,
-
presence of radioactive materials,
-
presence of biohazardous materials, and
-
presence of toxic constituents.
The following test procedures should be readily accomplished by a trained laboratory worker. The overall sequence for testing is depicted in Figure 7.1 for liquid and solid materials.
-
Physical description. The physical description should include the state of the material (solid, liquid), the color, and the consistency (for solids) or viscosity (for liquids). For liquid materials, describe the clarity of the solution (transparent, translucent, or opaque). If an unknown material is a bi- or tri-layered liquid, describe each layer separately, giving an approximate percentage of the total for each layer.
-
After taking appropriate safety precautions for handling the unknown, including the use of personal protection devices, remove a small sample for use in the following tests.
-
Water reactivity. Carefully add a small quantity of the unknown to a few milliliters of water. Observe any changes, including heat evolution, gas evolution, and flame generation.
-
Water solubility. Observe the solubility of the unknown in water. If it is an insoluble liquid, note whether it is less or more dense than water (i.e., does it float or sink?). Most nonhalogenated organic liquids are less dense than water.
-
pH. Test the material with multirange pH paper. If the sample is water-soluble, test the pH of a 10% aqueous solution. It may also be desirable or even required to carry out a neutralization titration.
-
Ignitability (flammability). Place a small sample of the material (<5 milliliters (mL)) in an aluminum test tray. Apply an ignition source, typically a propane torch, to the test sample for one-half second. If the material supports its own combustion, it is a flammable liquid with a flash point of less than 60 °C. If the sample does not ignite, apply the ignition source again for one second. If the material burns, it is combustible. Combustible materials have a flash point between 60 and 93 °C.
-
Presence of oxidizer. Wet commercially available starch-iodide paper with 1 N hydrochloric acid, and then place a small portion of the unknown on the wetted paper. A change in color of the paper to dark purple is a positive test for an oxidizer. The test can also be carried out by adding 0.1 to 0.2 grams (g) of sodium or potassium iodide to 1 mL of an acidic 10% solution of the unknown. Development of a yellow-brown color indicates an oxidizer. To test for hydroperoxides in water-insoluble organic solvents, dip the test paper into the solvent, and then let it evaporate. Add a drop of water to the same section of the paper. Development of a dark color indicates the presence of hydroperoxides.
-
Presence of sulfide. The test for inorganic sulfides is carried out only when the pH of an aqueous solution of the unknown is greater than 10. Add a few drops of concentrated hydrochloric acid to a sample of the unknown while holding a piece of commercial lead acetate paper, wetted with distilled water, over the sample. Development of a brown-black color on the paper indicates generation of hydrogen sulfide. Because of the toxicity of the hydrogen sulfide formed during this test, only a small sample should be tested, and appropriate ventilation should be used.
-
Presence of cyanide. The test for inorganic cyanides is carried out only when the pH of an aqueous solution of the unknown is greater than 10. Prior to testing for
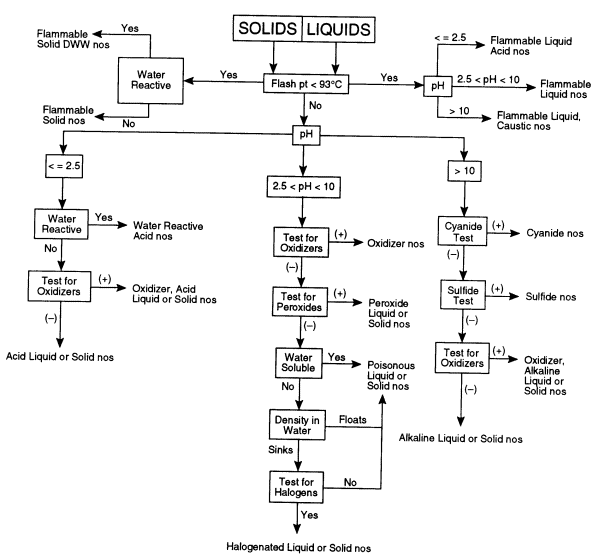
FIGURE 7.1 Flow chart for categorizing unknown chemicals. This decision tree shows the sequence of tests that may need to be performed to determine the appropriate hazard category of an unknown chemical. DWW, dangerous when wet; nos, not otherwise specified.
-
cyanides, the following stock solutions should be prepared: 10% aqueous sodium hydroxide (solution A), 10% aqueous ferrous sulfate (solution B), and 5% ferric chloride (solution C). Mix 2 mL of the sample with 1 mL of distilled water and 1 mL each of solutions A, B, and C. Add enough concentrated sulfuric acid to make the solution acidic. Development of a blue color (Prussian blue, from ferric ferrocyanide) indicates cyanide. Because of the toxicity of the hydrogen cyanide formed during this test, only a small sample should be tested, and appropriate ventilation should be used.
-
Presence of halogen. Heat a piece of copper wire until red in a flame. Cool the wire in distilled or deionized water, and then dip the wire into the unknown. Again heat the wire in the flame. The presence of halogen is indicated by a green color around the wire in the flame.
7.B.2 Regulated Chemically Hazardous Waste
An important question for planning within the laboratory is whether or not a waste is regulated as hazardous, because regulated hazardous waste must be handled and disposed of in rather specific ways. This determination has very important regulatory implications, which can lead to significant differences in disposal cost. The Environmental Protection Agency
(EPA) defines chemically hazardous waste under the Resource Conservation and Recovery Act (RCRA), and the U.S. Nuclear Regulatory Commission (U.S. NRC) defines radioactivity hazards. Biological hazards are generally not defined within federal regulations.
Although we must consider and pay close attention to the regulatory definitions and procedures that govern the handling and disposal of waste, primary importance must be given to the safe and prudent handling of this material. It is important to remember that the danger associated with a specific hazardous waste depends not only on the composition of the waste, but also on its quantity. In fact, regulations recognize quantity in many of their definitions for hazard compliance as well as in the definitions of waste generators. The concept of de minimis quantities, that is, very small amounts of material, though not defined clearly, is also a consideration in determining hazardous waste risk. Enlightened risk management dictates that the amount of material be one factor in the decisions on handling and disposal of waste.
Waste that is regulated as hazardous because of its chemical properties is defined by EPA in two ways: (1) waste that has certain hazardous characteristics and (2) waste that is on certain lists of chemicals. The first category is based on properties of materials that should be familiar to every laboratory worker. The second category is based on lists, established by EPA and certain states, of certain chemicals common to industry. These lists generally include materials that are widely used and recognized as hazardous. Chemicals are placed on these RCRA lists primarily on the basis of their toxicity. (To determine if waste is hazardous or not, see Chapter 9, section 9.D.2.)
Regardless of the regulatory definitions of hazard, understanding chemical characteristics that pose potential hazards should be a fundamental part of the education and training of any laboratory worker. These characteristics may be derived from knowledge of the properties and/or precursors of the waste. The characteristics may also be established by specific tests cited in the regulations.
(Regulatory issues, specifically RCRA, are discussed further in Chapter 9, section 9.D.)
7.B.2.1 Definition of Characteristic Waste
The properties that pose potential hazards are as follows:
-
Ignitability. Ignitable materials are defined as having one or more of the following characteristics:
-
Liquids that have a flash point of less than 60 °C or some other characteristic that has the potential to cause fire.
-
Materials other than liquids that are capable, under standard temperature and pressure, of causing fire by friction, adsorption of moisture, or spontaneous chemical changes and, when ignited, burn so vigorously and persistently as to create a hazard.
-
Flammable compressed gases, including those that form flammable mixtures.
-
Oxidizers that stimulate combustion of organic materials.
Ignitable materials include most common organic solvents, gases such as hydrogen and hydrocarbons, and certain nitrate salts.
-
-
Corrosivity. Corrosive liquids have a pH of 2 or less or 12.5 or greater or lead to corrosion of certain grades of steel. Most common laboratory acids and bases are corrosive. Solid corrosives, such as sodium hydroxide pellets and powders, are not legally considered by RCRA to be corrosive. However, laboratory workers must recognize that such materials can be extremely dangerous to skin and eyes and must be handled accordingly.
-
Reactivity. The reactivity classification includes substances that are unstable, react violently with water, are capable of detonation if exposed to some initiating source, or produce toxic gases. Alkali metals, peroxides and compounds that have peroxidized, and cyanide or sulfide compounds are classed as reactive.
-
Toxicity. Toxicity is established through the Toxicity Characteristic Leaching Procedure (TCLP), which measures the tendency of certain toxic materials to be leached (extracted) from the waste material under circumstances assumed to reproduce conditions of a landfill. The TCLP list includes a relatively small number of industrially important toxic chemicals and is based on the concentration above which a waste is considered hazardous. Failure to pass the TCLP results in classification of a material as a toxic waste.
7.B.2.2 Definition of Listed Waste
Although EPA has developed several lists of hazardous waste, three regulatory lists are of most interest to laboratory workers:
-
the F list: waste from nonspecific sources (e.g., spent solvents and process or reaction waste),
-
the U list: hazardous waste (e.g., toxic laboratory chemicals), and
-
the P list: acutely hazardous waste (e.g., highly
-
toxic laboratory chemicals, that is, chemicals having an LD50 of less than 50 mg/kg (oral; rat)).
These lists may be updated periodically by EPA.
7.B.2.3 Determining the Status of a Waste
The EPA regulations place on the waste generator the burden of determining whether the waste is regulated as hazardous and in what hazard classification it falls. Testing is not necessarily required, and in most cases the laboratory worker should be able to provide sufficient information about the waste to allow the hazard classification to be assigned. If the waste is not a common chemical with known characteristics, enough information about it must be supplied to satisfy the regulatory requirements and to ensure that it can be handled and disposed of safely. Often, information on only the components present in amounts greater than 1% is required, but confirmation is needed from the treatment/disposal facility. The information needed to characterize a waste also depends on the method of ultimate disposal. (See the discussion of disposal methods in sections 7.B.6 to 7.B.8 below.)
7.B.3 Collection and Storage of Waste
7.B.3.1 At the Location of Generation
The first step in the disposal sequence usually involves the accumulation and temporary storage of waste in or near the laboratory (satellite accumulation). This step directly involves the laboratory workers who are familiar with the waste and its generation and is a most important part of ensuring that the disposal process proceeds safely and efficiently. It is often the time at which a decision can be made to recycle or reuse surplus materials rather than sending them for disposal. All of the costs and benefits of either decision should be evaluated here.
Again, safety considerations must be of primary concern. Waste should be stored in clearly labeled containers in a designated location that does not interfere with normal laboratory operations. Ventilated storage may be appropriate.
Federal regulations allow the indefinite accumulation of up to 55 gallons of hazardous waste or 1 quart of acutely hazardous waste at or near the point of generation. However, prudence dictates that the quantities accumulated should be consistent with good safety practices. Furthermore, satellite accumulation time must be consistent with the stability of the material. It is generally recommended that waste not be held for more than 1 year. Within 3 days of the time that the amount of waste exceeds the 55-gallon (or 1 quart) limit, it must be managed under the storage and accumulation time limits required at a central accumulation area. (See Chapter 9, section 9.D.4, for more information.)
Often, different kinds of waste can be accumulated within a common container. Such commingled waste must be chemically compatible to ensure that heat generation, gas evolution, or another reaction does not occur. (See the discussion of commingling in section 7.B.3.2 below.)
Packaging and labeling are a key part of this initial in-laboratory operation. Waste must be collected in dependable containers that are compatible with their contents. Glass containers have traditionally been the most resistant to chemical action, but they can break easily. Metal containers are sturdier than glass, but often are corroded by their contents. Various chemically resistant plastic containers are becoming preferable substitutes for containers of glass or metal. Safety cans, metal or plastic, should be considered for holding flammable solvents. It is advisable to use secondary containers, such as trays, in case of spills or leakage from the primary containers. Containers are required to remain closed except when their contents are being transferred. Containers of incompatible materials should be separated physically or otherwise stored in a protective manner.
Every container must be labeled with the material's identity and its hazard (e.g., flammable, corrosive). Although the identity need not be a complete listing of all chemical constituents, it should enable knowledgeable laboratory workers to evaluate the hazard. However, when compatible wastes are collected in a common container, it is advisable to keep a list of the components to aid in later disposal decisions. Labeling must be clear and permanent. Although federal regulations do not require posting the date when satellite accumulation begins, some states do require this. The institution may suggest that this information be recorded as part of its chemicals management plan.
7.B.3.2 At a Central Accumulation Area
The central accumulation area is an important component in the organization's chemicals management plan. In addition to being the primary location where waste management occurs, it may also be the location where excess chemicals are held for possible redistribution. Along with the laboratory, the central accumulation area is often where hazard reduction of waste takes place through allowable on-site treatment processes.
The central accumulation area is often the appro-
priate place to accomplish considerable cost savings by commingling (i.e., mixing) waste materials. This is the process where compatible wastes from various sources are combined prior to disposal. Commingling is particularly suitable for waste solvents because disposal of liquid in a 55-gallon drum is generally much less expensive than disposal of the same volume of liquid in small containers. Because mixing waste requires transfer of waste between containers, it is imperative that the identity of all materials be known and their compatibility be understood. Safety in carrying out the procedures, including the use of personal protective devices as well as engineering controls such as fume hoods, must be of high priority.
In some cases, the disposal method and ultimate fate of the waste may require that different wastes not be accumulated together. For example, if commingled waste contains significant amounts of halogenated solvents (usually above 1%), disposing of the mixture can be markedly more costly. In such cases, segregation of halogenated and nonhalogenated solvents is economically favorable.
Based on federal regulations, storage at a central accumulation area is normally limited to 90 days, although more time is allowed for small-quantity generators or other special situations (180 or 270 days). The count begins when the waste is brought to the central accumulation area from the laboratory or satellite accumulation area. It is important to know that a special permit is required for long-term storage, that is, storage beyond the limit of 90 days (or 180 or 270 days, depending on the particular situation). Obtaining such a permit is usually too expensive and too time-consuming for most laboratory operations. (See RCRA and Chapter 9, section 9.D.4, for more information.)
Waste materials stored within a central accumulation area should be held in appropriate and clearly labeled containers, separated according to chemical compatibility as noted in the previous section. The label must include the accumulation start date and the words "Hazardous Waste." Fire suppression systems, ventilation, and dikes to avoid sewer contamination in case of a spill should be considered when such a facility is planned. Training of employees in correct handling of the materials as well as contingency planning for emergencies is expected to be a part of the central accumulation area operations.
Transportation of waste between laboratories (satellite accumulation areas) and the central accumulation area also requires specific attention to safety. Materials transported must be held within appropriate and clearly labeled containers. There must be provision for spill control in case of an accident during transportation and handling. For larger institutions, it is advisable to have some kind of internal tracking system to follow the movement of waste. If public roads are used during the transportation process, additional Department of Transportation (DOT) regulations may apply.
Final preparations for off-site disposal usually occur at the central accumulation area. Decisions on disposal options are best made here, as the larger quantities of waste are gathered. Identification of unknown materials not carried out within the laboratory must be completed at this point because unidentified waste cannot be shipped to a disposal site.
Laboratory waste typically leaves the generator's facility commingled in drums as compatible wastes or within a Lab Pack. Lab Packs are containers, often 55-gallon drums, in which small containers of waste are packed with an absorbent material. Lab Packs had been used as the principal method for disposing of laboratory waste within a landfill. However, recent landfill disposal restrictions severely limit landfill disposal of hazardous materials. Thus, the Lab Pack has become principally a shipping container. Typically, the Lab Pack is taken to a disposal facility, where it is either incinerated or unpacked and the contents redistributed for safe, efficient, and legal treatment and disposal.
7.B.4 Records
Records are needed both to meet regulatory requirements and to help monitor the success of the hazardous waste management program. Because the central accumulation area is usually the last place where waste is dealt with before it leaves the facility, it is often the most suitable place for ensuring that all appropriate and required records have been generated.
For regulatory purposes, the facility needs to keep records for on-site activities that include
-
the quantities and identification of waste generated and shipped,
-
documentation of analyses of unknown materials if required,
-
manifests for waste shipping as well as verification of disposal, and
-
any other information required to ensure compliance and safety from long-term liability.
Records of costs, internal tracking, and so forth, can provide information on the success of the hazardous waste management program.
7.B.5 Hazard Reduction
Hazard reduction is part of the broad theme of pollution prevention that is addressed in previous chapters
of this book. From a chemical point of view, it is feasible to reduce the volume or the hazardous characteristics of many chemicals by reactions within the laboratory. In fact, it is becoming common practice to include such reactions as the final steps in an experimental sequence. Such procedures, as part of an academic or industrial experiment, usually involve small amounts of materials, which can be handled easily and safely by the laboratory worker. Chemical deactivation as part of the experimental procedure can have considerable economic advantage by eliminating the necessity to treat small amounts of surplus materials as hazardous waste. Furthermore, the handling and deactivation of potential waste by the laboratory worker benefit from the expertise and knowledge about the materials of the person who has generated them.
The question of what is considered treatment under RCRA regulations has posed a dilemma for laboratory workers. RCRA regulations define treatment as "any method ... designed to change the physical, chemical, or biological character or composition of any hazardous waste so as to neutralize such waste, or so as to recover energy or material resources from the waste, or so as to render the waste non-hazardous or less hazardous ... " (U.S. Congress, 1978). Under RCRA, treatment, with very limited exceptions, must be permitted by EPA.
The regulatory procedures and costs to be a "permitted" treatment facility are beyond the resources and mission of most academic and industrial laboratories. Yet it is prudent to carry out small-scale "treatment" as a part of laboratory procedures. This fact has been recognized by state agencies and some regional EPA offices through "permit-by-rule," that is, by allowing categorical or blanket permitting of certain small-scale treatment methods. For example, elementary acid-base neutralization is usually allowed, as is treatment that is the last step of a chemical procedure. Most EPA regions also allow treatment in the waste collection container. It is important to note that treatment restrictions apply only to wastes that are addressed by EPA regulations.
A bill has been promoted in Congress to allow small-scale treatment by laboratory personnel. However, specific legislation has not been enacted at this time. The fact that regional EPA offices have interpreted such small-scale reactions differently further complicates decisions at the laboratory level. Because illegal treatment can lead to fines of up to $25,000 per day, it is most important that, before carrying out any processes that could be considered treatment, the responsible laboratory worker or the institution's environmental health and safety office check with the local, state, or regional EPA to clarify its interpretation of the rules.
(Section 7.D below provides methods for small-scale treatment of common chemicals.)
7.B.6 Disposal Options
Decisions on the ultimate disposal method are an important part of the on-site planning for handling of waste. The method of collection has an impact on, for example, how waste will be stored so as to most efficiently accomplish its transfer to the treatment, storage, and disposal facility (TSDF). Waste generators often use several disposal options because each has its own advantages for specific wastes. Disposal in the sanitary sewer, though appropriate in some cases, is becoming an unacceptable option in many communities. At the same time the options for landfill disposal are also disappearing rapidly. Incineration is becoming the most common disposal method. However, the long-term outlook for this method may be limited by increasing environmental concerns as well as the difficulty in obtaining permits for commercial incineration facilities. Waste minimization is the management strategy of the future. (See Chapter 4, section 4.B, for step-by-step instructions on source reduction and Chapter 7, section 7.C, for general information on minimizing hazardous waste.)
7.B.6.1 Incineration
Incineration is becoming the disposal method of choice for several reasons. It promises to give the generator the best assurance of long-term safety from liability. It also leads to a minimum amount of residues that must be disposed of in landfills. However, at this time, incineration is still one of the more expensive disposal options. It is becoming increasingly difficult to obtain a permit to establish a commercial incinerator because of local opposition (the "not in my backyard" syndrome) and environmental concerns centering on questions regarding the effectiveness of the incineration process.
Nevertheless, most disposal companies are moving toward incineration disposal, particularly for the kinds of hazardous waste generated by laboratories. Their typical variety of different wastes, usually in small quantities, makes incineration a favorable option. Laboratory waste can often be incinerated in its shipping Lab Packs without any further handling. Commingled flammable solvents are commonly blended with the incinerator fuel and thus destroyed as they provide energy for the burning process.
Earlier editions of this book were optimistic that small laboratory incinerator systems would be developed for efficient destruction of waste at the point of
generation. That has not happened. Several factors, including the cost of development and concern about how regulatory agencies would view this kind of "treatment," surely have contributed to the lack of progress. A changing regulatory environment could provide a favorable basis for development of such thermal treatment systems.
7.B.6.2 Disposal in the Normal Trash
Laboratory workers may be surprised to learn the number of wastes they generate that can be disposed of in the normal trash. However, because the disposal of trash from households and businesses is normally controlled by the local municipality, the local agency should be approached to establish what is allowed.
When disposing of chemicals in the normal trash, certain precautions should be observed. Because custodians, who usually empty the trash containers, are not usually familiar with laboratory operations, no objects that could cause harm to them should be disposed of in those containers. Such objects include containers of chemicals, unless they are overpacked to avoid breakage, and powders, unless they are in closed containers. Free-flowing liquids are usually prohibited. Sharp metal and broken glassware, even though they may be considered nonhazardous trash, should be collected in specially marked containers, never in the normal trash baskets.
7.B.6.3 Disposal in the Sanitary Sewer
Disposal in the sewer system (down the drain) had been a common method of waste disposal until recent years. However, environmental concerns, the viability of publicly owned treatment works (POTW), and a changing disposal culture have changed that custom markedly. In fact, many industrial and academic laboratory facilities have completely eliminated sewer disposal. Again, like trash disposal, most sewer disposal is controlled locally, and it is therefore advisable to consult with the POTW to determine what is allowed. Yet, it is often reasonable to consider disposal of some chemical waste materials in the sanitary sewer. These include substances that are water-soluble, that do not violate the federal prohibitions on disposal of waste materials that interfere with POTW operations or pose a hazard, and that are allowed by the local sewer facility.
Chemicals that may be permissible for sewer disposal include aqueous solutions that readily biodegrade and low-toxicity solutions of inorganic substances. Water-miscible flammable liquids are frequently prohibited from disposal in the sewer system. Water-immiscible chemicals should never go down the drain.
Disposal of regulated hazardous waste into the sanitary sewer is allowed only in limited situations. The total wastewater must be a mixture of domestic sewage along with the waste whose amount and concentration meet the regulations and limits of the POTW. If approved of by the local district, it may be allowable to dispose of dilute solutions of metals and other hazardous chemicals into the sanitary sewer.
Under the Clean Water Act, some exemption from regulation as a hazardous waste for wastewater containing laboratory-generated listed waste is allowed. In 1993, this exemption was expanded to include corrosive and ignitable wastes. For the exemption to apply, these laboratory wastes must be 1% or less of the annual total wastewater quantity reaching the facility's headworks or have an annualized average concentration of no more than 1 part per million (ppm) of the wastewater generated by the facility.
Waste should be disposed of in drains that flow to a POTW, never into a storm drain and seldom into a septic system. Waste should be flushed with at least a 100-fold excess of water, and the facility's wastewater effluent should be checked periodically to ensure that concentration limits are not being exceeded.
7.B.6.4 Release to the Atmosphere
The release of vapors to the atmosphere, via, for example, open evaporation or fume hood effluent, is not an acceptable disposal method. Apparatus for operations expected to release vapors should be equipped with appropriate trapping devices. Although the disposition of laboratories under the Clean Air Act is not established at this time, it is reasonable to expect that releases to the atmosphere will be controlled.
Fume hoods, the most common source of laboratory releases to the atmosphere, are designed as safety devices to transport vapors away from the laboratory in case of an emergency, not as a routine means for volatile waste disposal. Units containing absorbent filters have been introduced into some laboratories, but have limited absorbing capacity. Redirection of fume hood vapors to a common trapping device can completely eliminate discharge into the atmosphere. (See Chapter 8, sections 8.C.11 and 8.C.12, for more detail.)
7.B.7 Disposal of Nonhazardous and Nonregulated Waste
Many laboratories do not distinguish between waste that is hazardous and waste that neither poses a hazard
nor is regulated as hazardous. If these different types of waste are combined, then the total must be treated as hazardous waste, and the price for disposal of the nonhazardous portion increases markedly. When safe and allowed by regulation, disposal of nonhazardous waste via the normal trash or sewer can substantially reduce disposal costs. This is the kind of waste segregation that makes economic as well as environmental sense.
It is wise to check the rules and requirements of the local solid waste management authority and develop a list of materials that can be disposed of safely and legally in the normal trash. This includes waste that is not regulated because it does not exhibit any of the hazardous characteristics (ignitability, corrosivity, reactivity, or toxicity) as defined by EPA and is not listed as hazardous. The common wastes usually not regulated as hazardous include certain salts (e.g., potassium chloride and sodium carbonate), many natural products (e.g., sugars and amino acids), and inert materials used in a laboratory (e.g., noncontaminated chromatography resins and gels).
7.B.8 Disposal of Spills
Most chemical spills can and should be cleaned up by laboratory workers themselves. In general, these are spills of known composition that do not involve injury, do not represent a fire or personal hazard, and are less than 1 gallon (or less for very toxic materials). Regulations allow laboratory workers to clean up such spills, although it is advisable that they have training to handle spills and adequate equipment to carry out the cleanup safely. Outside help, properly trained, should be requested if there is any doubt about the ability of the laboratory personnel to clean up the spill safely. But once help is requested from outside the immediate spill area, specific personnel training requirements and other regulatory control may apply.
General guidelines for cleaning up spills are as follows:
-
Assess the potential hazard presented by the spill to personnel within the work area as well as within other parts of the facility and the outside environment.
-
Remove possible sources of ignition if the spilled material is flammable:
-
Turn off hot plates, stirring motors, and flames.
-
Shut down equipment in the area that could increase danger.
-
-
Secure the area so that no one will walk through the spill or interfere with the cleanup efforts.
-
Choose appropriate personal protection devices:
-
Always wear protective gloves and goggles or a face shield.
-
If there is a chance of body contact with the spill, wear an apron or coveralls.
-
Wear rubber or plastic (not leather) boots if there is a chance of stepping into the spill.
-
Wear a respirator if there is danger of inhalation of toxic vapors, though only when proper training has preceded its use.
-
Note that protective devices must be chosen carefully to be appropriate for the anticipated hazard. Often training is appropriate or required (e.g., with respirators) prior to their use.
-
-
Locate a spill control kit or other appropriate absorbent and cleanup supplies.
-
Confine or contain the spill:
-
Do not let any of the spilled material enter the sewer system, for example, through a floor drain.
-
Cover the spill with an absorbent material; paper towels may be appropriate for small, unreactive materials.
-
Sweep up or in other ways collect the absorbed materials and place them in a container that can be securely closed.
-
If the spilled material is an acid or a base, use a neutralizing material; sodium bicarbonate is commonly used for acids, and sodium bisulfate for bases. Spill control kits are commercially available for the cleanup of many kinds of chemical spills. (Chapter 6, section 6.F.2.1 , has further information on spill control kits and spill absorbents.)
-
-
Dispose of the absorbed spill appropriately as hazardous or nonhazardous waste.
(See Chapter 5, sections 5.C.11.5 and 5.C.11.6, for more detail on spill cleanup.)
7.B.9 Monitoring of Off-Site Waste Disposal
The ultimate destination of waste is usually a treatment, storage, and disposal facility (TSDF). Here waste is held, treated (typically via chemical action or incineration), or actually disposed of. Although the waste has left the generator's facility, the generator retains the final responsibility for the long-term fate of the waste. It is imperative that the generator have complete trust and confidence in the TSDF, as well as in the transporter who carries the waste to the TSDF. In some cases the destination of waste is a recycler or reclaimer. The procedures for preparing and transporting the waste to such a facility are similar to those described above. (See section 7.B.3.)
7.B.9.1 Preparation for Off-site Disposal
How the waste has been handled at the generator site, usually at a central accumulation area, can have a significant impact on the cost of the off-site disposal operation. This usually depends on the method of preparation of the waste for disposal. Collecting containers of waste in a Lab Pack is usually much more expensive for ultimate disposal than is commingling of compatible wastes, partly because a 55-gallon Lab Pack only holds about 16 gallons of waste. However, factors other than economics may control those decisions. When small amounts of different wastes are generated, a situation typical of many academic laboratories, commingling compatible wastes may not be practical. The Lab Pack approach is usually chosen. On the other hand, waste solvents can often be combined advantageously, even in small operations.
On-site storage time limits also must be considered. For those generators that are governed by the 90-day limit, yet are relatively small operations, it is difficult to accumulate sufficient materials to make commingling a favorable option.
Safety can often be the determining factor. Using Lab Packs is quite simple. As described above, small containers of compatible waste materials are placed in a larger container, usually a 55-gallon drum, along with appropriate packing materials, as they are collected. When a drum is filled, it is sealed and ready for shipping. An inventory list of the contents of a Lab Pack is required for shipping and is usually requested by the TSDF.
In contrast, commingling requires opening of containers and transferring their contents from the smaller laboratory containers to a larger drum. Here the potential risk for workers is much greater. Furthermore, the containers should be rinsed before they are considered nonhazardous, and the rinsate must be treated as a hazardous waste. Drums of commingled waste usually require only a general hazard classification identification, although some TSDFs require an analysis or listing of contents.
7.B.9.2 Choice of Transporter and Disposal Facility
Because the long-term liability for the waste remains with the generator, it is imperative that the generator be thoroughly familiar with the experience and record of the transporter and TSDF. Economic factors alone should not govern choices, for the long-term consequences can be significant. The generator must obtain assurance, in terms of documentation, permits, records, insurance and liability coverage, and regulatory compliance history, that the chosen service provider is reliable.
There is often an advantage, particularly for smaller facilities, to contracting for all of the hazardous waste disposal operations. These include the packing and appropriate labeling of waste for off-site transportation and disposal, preparation of the shipping manifest, and arranging for the transporter and disposal facility. Again, the liability remains with the generator, and so the choice of such a contractor is critical.
In some states, Minnesota and Montana, for example, arrangements have been developed with local regulators to allow a large laboratory waste generator to handle the waste from very small laboratories such as those at small colleges and public schools. This plan results in informed assistance and cost savings for the smaller units. In Wisconsin, a statewide commercial contract that can be accessed by all state educational systems has been arranged. There is usually significant advantage to working with local and state agencies to develop acceptable plans for disposal methods that are environmentally and economically favorable for both large and small generators.
7.C MULTIHAZARDOUS WASTE
Multihazardous waste is waste that contains any combination of chemical, radioactive, or biological hazards. The combinations of these hazards are illustrated in Figure 7.2. Although many of the principles discussed for chemically hazardous waste earlier in this
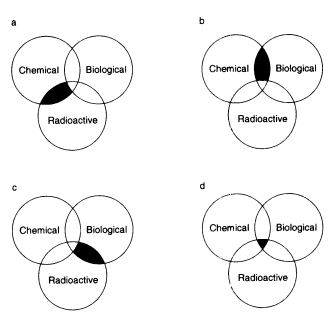
FIGURE 7.2 Multihazardous waste. (a) Chemical-radioactive waste, or 'mixed waste," (b) chemical-biological waste, (c) radioactive biological waste, and (d) chemical-radioactive biological waste.
chapter (section 7.B) apply here also, multihazardous waste requires special management considerations because the treatment method for one of the hazards may be inappropriate for the treatment of another. For example, if a waste that contains a volatile organic solvent and infectious agents is autoclaved, it may release hazardous levels of solvent into the atmosphere. (For more on multihazardous waste, refer to Chapter 9, section 9.D.2.)
Management of multihazardous waste is complicated further by local or state requirements that may be inconsistent with the relative risk of each hazard and with sound waste management practices. Chemically hazardous waste that contains short-half-life radionuclides may, for example, be best managed by holding the waste in storage for decay, which may require up to 2 years. However, the EPA and state rules usually limit storage of chemically hazardous waste to 90 days.
Commercial treatment or disposal facilities for multihazardous waste from laboratories are scarce. Many of these waste types are unique to laboratories and are generated in such small volumes that there is little incentive for the development of a commercial market for their management.
While multihazardous waste is currently difficult and expensive to manage, it is generated in medical, biochemical, and other types of critically important research, as well as in clinical and environmental laboratories. As interdisciplinary techniques, technologies, and studies become more widely used, multihazardous waste will be more widely generated. Legally acceptable protocols for dealing with multihazardous waste need to be developed. (See also Chapter 4, section 4.B.3.)
Radioactive hazardous waste generated by laboratories is usually limited to low-level radioactive waste from the use of by-product material and naturally occurring or accelerator-produced radioactive material (NARM). By-product material, as defined by the U.S. Nuclear Regulatory Commission (U.S. NRC), is reactor-produced radioactive material and includes most purchased radiolabeled chemicals; NARM includes uranium and thorium salts. The use and disposal of by-product material are regulated by the U.S. NRC and usually require a license. NARM waste is not regulated by the U.S. NRC but may be regulated to some extent by some states. Common waste management methods for low-level radioactive waste from laboratories include storage for decay and indefinite on-site storage, burial at a low-level radioactive waste site, incineration, and sanitary sewer disposal.
Waste is considered biohazardous or infectious if it contains agents of sufficient virulence and quantity that exposure of a susceptible host could cause transmission of an infectious disease. Unlike chemical and radioactive waste, infectious or medical waste is currently not subject to federal regulations that govern its treatment, storage, or disposal. OSHA regulates the collection and containment of certain laboratory waste that contains human blood or body fluids in order to prevent exposure of personnel to blood borne pathogens. Although OSHA does not regulate waste treatment or disposal, its standard is often the impetus for managing infectious waste in laboratories. Putrescible waste, such as tissue and carcasses of laboratory animals, is also classified as biological waste, although putrescible laboratory waste is usually not biohazardous or infectious. Hypodermic needles, lancets, scalpel blades, and other medical laboratory sharps are considered biohazardous because of their potential for being contaminated with pathogens and the likelihood of accidental skin puncture. Biological waste may also include whole animals or plants made transgenic via recombinant DNA technology or into which recombinant DNA has been introduced. (See Chapter 5, section 5.E, on biohazards and radioactivity.)
Common management methods for biological waste include disinfection, autoclaving, and, for liquids, disposal in the sanitary sewer. Putrescible waste is usually disposed of by incineration, which destroys the unpleasant nature of these materials. Needles and sharps require destruction, typically by incineration or grinding. In general, if all hazards cannot be removed in one step, the goal is to reduce a multihazardous waste to a waste that presents a single hazard. This single-hazard waste can then be managed by standard methods for that category.
Most management principles apply to all types of waste. These universal management methods include waste minimization, training of laboratory personnel and waste handlers, reviewing proposed procedures, keeping dissimilar waste materials separate, identification of waste materials, and labeling of waste containers. However, multihazardous waste, because of its combination of hazards and regulatory controls, requires more complex attention, as detailed in the following guidelines:
-
Assess the risk posed by the waste's inherent hazards. Laboratories often have flexibility to define those waste characteristics whose hazards are so low (de minimis) as to not present a significant risk. For example, the U.S. NRC or state authority may allow a licensee to propose limits below which laboratory waste can be designated as noncontaminated and disposed of as nonradioactive waste. (Some licensees have agreed with their regulators to consider some wastes nonradioactive if effluent concentrations are less than
-
what the U.S. NRC specifies for an unrestricted area.) As a result, some chemical-radioactive waste with a U.S. NRC-approved level of radioactivity can be managed as chemical waste. Likewise, laboratory personnel can specify which biological wastes require no special handling and should not be mixed with infectious or biohazardous waste. Laboratory personnel may also determine if their waste meets the definition of a chemically hazardous waste. If the waste component is not regulated within this category, then it may be possible to manage it as only a radioactive or biological waste.
-
Minimize the waste's hazards. Waste minimization methods specific to chemical, radioactive, or biological waste can be applied to multihazardous waste to mitigate or eliminate one hazard, which will then allow it to be managed as a single-hazard waste. For example, the substitution of nonignitable liquid scintillation fluid (LSF) for toluene-based LSF reduces a chemical-radioactive waste to a radioactive waste.
-
Determine options for managing the waste's hazards. Waste management options include laboratory methods, management at institutional waste facilities, and treatment and disposal at commercial sites. Options can vary considerably between laboratories owing to institutional capabilities and state and local laws. It may be appropriate to manage the waste in order of risk priority, from high to low risk. Options must be compatible with all hazards, and combinations of waste management methods may be limited by their order of application. Reject any combination or sequence of methods that may create an unreasonable risk to waste handlers or the environment, or that might increase the overall risk. If an option has a clear advantage in efficiency and safety, it should have highest priority. For example, if safe facilities are available on site, hold short-half-life radioactive waste for decay before managing it as a chemical or biological waste. However, remember that in most cases a waste that has chemically hazardous characteristics may not be held beyond 90 days.
-
When possible, select a single management option. Some waste management methods are appropriate for more than one waste hazard. Low-level radioactive animal tissue (a radioactive-biological waste) can often be incinerated on-site, which may be a satisfactory disposal option for both the radioactive and the biological characteristics of the waste. Some multihazardous waste can be disposed of safely in the sanitary sewer when allowed by the local publicly owned treatment works (POTW).
(See also Chapter 4 on chemical and waste management.)
7.C.1 Chemical-Radioactive (Mixed) Waste
''Mixed waste" is the regulatory term for multihazardous waste that contains chemical and radioactive hazards (see Figure 7.2a). Mixed waste is defined by EPA as "wastes that contain a chemically hazardous waste component regulated under Subtitle C of the Resource Conservation and Recovery Act of 1978 (RCRA) and a radioactive component consisting of source, special nuclear, or byproduct material regulated under the Atomic Energy Act of 1946 (AEA)" (U.S. EPA, 1986). The U.S. NRC defines mixed waste slightly differently. According to the U.S. NRC, mixed waste is a waste "that contains a chemically hazardous waste as defined in RCRA, and source, special nuclear, by-product material, low-level radioactive waste" (as defined in the Low-level Radioactive Waste Policy Act of 1980 (42 USC 2021(b) to 2021(j)), or "some types of naturally-occurring or accelerator-produced radioactive material (NARM), such as uranium and thorium." Regardless of the precise definition, well-informed laboratory workers should be prepared to deal with mixed waste in a prudent manner.
Examples of laboratory mixed waste include the following:
-
Used flammable (e.g., toluene) liquid scintillation cocktails.
-
Phenol-chloroform mixtures from extraction of nucleic acids from radiolabeled cell components.
-
Aqueous solutions containing more than 6 ppm chloroform (which exceeds the limit set by the Toxicity Characteristic Leaching Procedure (TCLP) test) and radioactive material (typically found in solutions generated by the neutralization of radioactive trichloroacetic acid solutions).
-
Certain gel electrophoresis waste (e.g., methanol or acetic acid containing radionuclides).
-
Lead contaminated with radioactivity.
Mixed waste produced at university, hospital, and medical research laboratories is typically a mixture of a low-level radioactive waste and chemically hazardous waste. Mixed waste from nuclear and energy research laboratories can include both low- and high-level (e.g., spent nuclear fuels) radioactive materials combined with chemically hazardous waste. Disposal options for mixed waste are usually very expensive. For many types of mixed waste, there are no management options other than indefinite storage on site, or at an approved facility, in the hope that treatment or disposal options will be created in the near future.
An example of the mixed waste problem and the importance of keeping waste separate is illustrated by
a researcher who accidentally combined a waste labeled as carbon-14 with 1 gallon of sulfuric acid-sodium dichromate solution. No disposal facility in the United States would accept the radioactive chromic acid waste. One option, the simple laboratory reduction of the dichromate, recovery of the chromium-containing precipitate, and neutralization of the acid, which would render the liquid waste only a radioactive hazard, may not be allowed without a permit in some states and EPA regions because it is considered to be treatment.
In large part, many of these regulatory dilemmas are unrelated to the real risks of laboratory mixed waste. Chief barriers to the safe and timely management of mixed waste include the following:
-
EPA regulations that inhibit laboratory and on-site minimization, storage, and treatment of mixed waste.
-
EPA regulations that discourage off-site minimization, treatment, storage, or disposal of mixed waste.
-
The U.S. NRC's reluctance at this time to establish a national policy that defines the de minimis level of contamination for all types of laboratory radioactive waste, below which the risk to health and the environment is not significant.
-
Community opposition to incinerators that could, with minimal risk, efficiently reduce hazard.
-
The low volume, unusual character, and great variety of this laboratory waste stream, which, together with the above barriers, discourage the development of commercial markets for mixed waste.
(For more complete information on regulations, see Chapter 9.)
7.C.1.1 Minimization of Mixed Waste
Rigorous application of waste minimization principles can often solve the problems of managing mixed waste. Minimization of mixed waste can be achieved by modifying laboratory processes, improving operations, or using substitute materials. Such efforts are most successful when scientists and environmental health and safety staff work together to evaluate laboratory processes. Examples include the following:
-
Use of 2.5-mL scintillation vials ("minivials") rather than 10-mL vials. Adapters are available for scintillation counters with 10-mL vial racks.
-
Counting of phosphorus-32 (32p) without scintillation fluid by the Cerenkov method on the tritium (3H) setting of a liquid scintillation counter (approximately 40% efficiency); iodine-125 (125I) can be counted without scintillation fluid in a gamma counter.
-
Use of microscale chemistry techniques.
-
Elimination of the methanol/acetic acid (chemical) and radioactive mixed hazards in gel electrophoresis work by skipping the gel fixing step if it is not required.
-
Prevention of lead contamination by radioactivity by lining lead containers with disposable plastic or by using alternative shielding materials.
-
Reducing the volume of dry waste by compaction of items such as contaminated gloves, absorbent pads, and glassware.
Some simple operational improvements can also help minimize mixed waste. Surpluses can be minimized by limiting the acquisition of chemicals and radioactive materials to immediate needs. Contaminated equipment can sometimes be reused within restricted areas or decontaminated. Establishing procedures for noncontaminated materials can enable generators to keep normal trash separate from contaminated waste.
When possible, a substitute can be used for either the chemical or the radioactive source of the mixed waste. With radioactivity, the experimenter should use the minimum activity necessary and select the radionuclide with the most appropriate decay characteristics. Examples include the following:
-
Use of nonignitable scintillation fluid (e.g., phenylxylylethane, linear alkylbenzenes, and diisopropylnaphthalene) instead of flammable scintillation fluid (e.g., toluene, xylene, and pseudocumene). Liquid scintillation fluid that is sold as being "biodegradable" or "sewer disposable" is more appropriately labeled as "nonignitable" because biodegradability in the sanitary sewer can vary considerably with the local treatment facility.
-
Use of nonradioactive substitutes such as scintillation proximity assays for 32P or sulfur-35 (35S) sequencing studies or 3H cation assays, and enhanced chemiluminescence (ECL) as a substitute for 32P and 35S DNA probe labeling and southern blot analysis.
-
Substitution of enriched stable isotopes for radionuclides in some cases. Mass spectrometry techniques, such as ICP-MS, are beginning to rival the sensitivity of some counting methods. Examples include use of oxygen-18 (18O) and deuterium (2H) with mass spectrometry detection as substitutes for 19O and 3H.
-
Substitution of shorter-half-life radionuclides such as 32P (t1/2 = 14 days) for 33P (t1/2 = 25 days) in orthophosphate studies, or 33P or 32P for 35S (t1/2 = 87 days) in nucleotides and deoxynucleotides. In many uses, 131I (t1/2 = 8 days) can be substituted for 125I (t1/2 = 60 days). Additional exposure precautions may be required.
7.C.1.2 Safe Storage of Mixed Waste
Waste containing short-half-life radionuclides should be stored for decay prior to subsequent waste management. The U.S. NRC refers to storage for decay as "decay-in-storage." Because on-site storage of low-level waste is very efficient and minimizes handling and transportation risks, laboratories and institutions should provide the space and safe storage facilities for decay-in-storage. In most cases, safe storage requires a designated room or facility that has been modified to contain the waste, protect workers, minimize the risk of a fire or spill, and minimize radiation levels outside the area. Proper ventilation and effluent trapping are critical needs for such a facility. Storage of mixed waste in the laboratory is not recommended because the required level of protection is difficult to achieve in a working area. Storage of such waste is not recommended when the waste may become more difficult to handle with age, such as with certain biological, putrescible, or reactive materials.
The specific U.S. NRC requirements for decay-in-storage of radioactive waste are usually detailed in the institution's license. Decay-in-storage is usually limited to half-lives of less than 65 days (although half-lives of up to 120 days are routinely approved by the U.S. NRC), but includes many of the radionuclides used in biomedical research. When the short-half-life radionuclides have decayed to background levels (the length of time depending on the initial radioactivity level but typically defined as a storage period of at least 10 half-lives), the chemical-radioactive waste can be managed as a chemical waste. After the decay period, U.S. NRC licenses usually require that the mixed waste be surveyed for external radiation prior to releasing it to the chemical waste stream.
EPA requirements for decay-in-storage of mixed waste have varied over time and by state and EPA region. Storage of mixed waste for more than 90 days, the period of time usually allowed for chemically hazardous waste, may require the approval of the state or regional EPA hazardous chemical waste authority. In permitted storage facilities, storage may be limited to 1 year for some types of mixed waste. Workers should contact their institution's environmental health and safety staff or local hazardous waste agency to determine their regulatory status and requirements for storing mixed waste for decay.
7.C.1.3 Hazard Reduction of Mixed Waste
Chemical hazards can be reduced by carrying out various common chemical reactions with the waste in the laboratory (see also section 7.B.5 and 7.D). However, "treatment" of chemically hazardous waste has regulatory implications that must be considered. Many of the same considerations apply to treatment of mixed waste.
Nevertheless, there are still justifiable and legal reasons to carry out such operations in the laboratory when hazards can be reduced safely. Neutralization, oxidation, reduction, and various other chemical conversions as well as physical methods of separation and concentration can be applied prudently to many laboratory-scale mixed wastes. However, the dual character of the hazard, chemical and radioactive, requires that additional precautions be exercised. Treatment for the chemical hazard must not create a radioactivity risk for personnel or the environment. For example, vapors or aerosols from a reaction, distillation, or evaporation must not lead to escape of unsafe levels of radioactive materials into the atmosphere. Fume hoods appropriate for such operations should be designed to trap any radioactive effluent. When mixed waste is made chemically safe for disposal into the sanitary sewer, the laboratory must ensure that the radioactivity hazard is below the standards set by the publicly owned treatment works (POTW). Several examples for reducing the hazard of mixed waste are described below:
-
The worker can reduce the chemical hazard to a safe level and then handle the material as only a radioactive hazardous waste. Many low-level radiation materials can then be allowed to decay to a safe level, following which simple disposal is allowable.
-
Some trichloroacetic acid (TCA) solutions contain chloroform in excess of 6 ppm. Such a solution is considered a hazardous chemical waste because it fails the TCLP test. If the neutralized solution is not acceptable to the sewage treatment plant because of the presence of chloroform, it may be possible to remove that component from the solution by filtration through activated charcoal. The resulting radioactive filtrate can usually be disposed of in the sanitary sewer, and the contaminated charcoal can usually be disposed of as a chemical waste.
-
Some radioactive methanol-acetic acid solutions from gel electrophoresis can be recycled via distillation and the methanol reused. The solution is neutralized prior to distillation to protect the distillation equipment from corrosion and to reduce the level of methyl acetate formed during the process.
-
The volume of waste phenol, chloroform, methanol, and water containing radionuclides can be reduced by separating the nonaqueous portion using a separatory funnel. After separation, the organic phase can be distilled to produce chloroform waste,
-
which may contain levels of radioactivity below license limits for radioactive waste. The still bottom and aqueous phase must be handled as a mixed waste.
-
High-performance liquid chromatography (HPLC), used to purify radiolabeled proteins and lipids, can generate a waste radioactive solution of acetonitrile, water, methanol, acetic acid, and often a small amount of dimethylformamide. When the solution is distilled by rotary flash evaporation, the distillate of acetonitrile, methanol, and water is nonradioactive and can be handled as a hazardous chemical waste. The radioactive still bottom, containing 1 to 5% methanol and acetic acid, can usually be neutralized, diluted, and disposed of in the sanitary sewer.
-
Aqueous solutions containing uranyl or thorium compounds can be evaporated to dryness and the residues disposed of as radioactive waste. Because of their toxicity, solidification may be necessary prior to burial at a low-level radioactive waste site.
-
Activated carbon, Molecular Sieves®, synthetic resins, and ion-exchange resins have been used with varying success in the separation of chemical and radioactive waste constituents. Activated carbon has been used to remove low concentrations of chloroform (less than 150 ppm) from aqueous mixed waste solutions. However, activated carbon is not suitable for high concentrations of phenol-chloroform or acetonitrile-water mixed waste. Amberlite® XAD resin, a series of Amberlite® polymeric absorbent resins used in chromatography, has been shown to be effective in removing the organic constituents from aqueous phenol, chloroform, and methanol solutions, leaving an aqueous solution that can be managed as a radioactive waste. Chemical constituents can be separated from mixed waste by using supercritical fluid extraction (e.g., carbon dioxide), which is now available commercially.
-
Surface contamination from radioactively contaminated lead can be removed by dipping the contaminated lead into a solution of 1 M hydrochloric acid. After rinsing the lead with water, it usually can be documented as nonradioactive. The acidic wash and rinse solutions contain radionuclides and lead and must be handled accordingly. However, decontaminating the lead results in a smaller mass of mixed waste and allows the decontaminated lead to be reused or recycled. Commercial rinse products are also available for this purpose.
-
Incineration is advantageous as a treatment for many types of chemical-radioactive waste, especially those that contain toxic or flammable organic chemicals. Incineration can destroy oxidizable organic chemicals in the waste. To comply with radionuclide release limits, U.S. NRC licensees need to control emissions and may need to restrict the incinerator's waste feed. Radioactive ash is typically managed as a radioactive waste. It is important to keep toxic metals (e.g., lead, mercury) out of the incinerable waste so that the ash is not chemically hazardous according to the TCLP test. On-site incineration minimizes handling and transportation risks; however, incineration of chemical waste is regulated by EPA and requires a permit, which is beyond the resources of most laboratory waste generators.
-
Procedures for the solidification and stabilization of inorganic compounds from mixed waste (using concrete or epoxy resin) to meet federal land ban restrictions have been outlined (40 CFR 268). This method may also abate the waste's chemical hazard and render a chemical-radioactive waste a radioactive waste. For example, waste lead citrate and uranyl acetate mixtures from electron microscopy can be solidified with port-land cement, which may be accepted for burial at a low-level radioactive waste site.
7.C.1.4 Commercial Disposal Services for Mixed Waste
Because of the great variety of laboratory mixed waste, it is often difficult to find a facility that can manage both the radioactive and the chemical hazards of the waste. In general, existing commercial disposal facilities are in business to manage mixed waste from the nuclear power industry, not waste from laboratories. Several commercial disposal facilities that accept mixed waste from off-site generators do exist in the United States. These sites have the capacity to manage liquid scintillation fluid, halogenated organics, and other organic waste. Treatment capacity exists for stabilization, neutralization, decontamination/ macroencapsulation of lead, and reduction of chromium waste.
In spite of this capacity, many types of laboratory mixed waste have no commercial repository. No commercial mixed waste disposal facilities exist for waste contaminated with most toxic metals (such as mercury) or for lead-contaminated oils. Commercial disposal capacity likewise does not exist for high concentrations of halogen-containing organics and other TCLP waste, such as waste that contains chloroform.
(See also sections 7.B.5 and 7.D.)
7.C.2 Chemical-Biological Waste
Laboratory waste that is both chemically hazardous and exhibits a biological characteristic (depicted in Figure 7.2b) merits special disposal procedures. Animal and medical waste incinerators are usually not licensed
to incinerate regulated chemical waste. Autoclaving or disinfection of chemical-biological waste usually does not destroy its chemically hazardous constituents, except for denaturing proteins and nucleic acids.
Conflicting laws are seldom a barrier to the safe management of chemical-biological waste. Although some states regulate the disposal of laboratory infectious waste, the federal government does not. Most laboratories using infectious agents abide by Centers for Disease Control and Prevention/National Institutes of Health (CDC/NIH) guidelines, which recommend on-site decontamination for agents in biosafety levels 3 and 4 (as defined in Chapter 5, section 5.E.1) but do not inhibit chemical waste storage or treatment (U.S. DHHS, 1993).
Although EPA is proposing to regulate transgenic plants that express insecticidal proteins, most types of chemical-biological waste are not regulated by EPA as a hazardous (RCRA) chemical waste. Waste (or spent) formalin that has been used in a process such as tissue preservation is not a discarded commercial chemical product and therefore is not regulated federally. However, its handling should be consistent with personal and environmental safety and within the limits set by local regulation. Animal tissue is regulated as chemical waste only in the unlikely circumstance that it contains a toxic chemical and the waste fails the TCLP test, or if the animal had been exposed to polychlorinated biphenyls (PCBs) in concentrations greater than 50 ppm.
Disposal is most difficult for the very small amount of chemical-biological waste that is EPA-regulated as chemically hazardous or contains a chemical, such as lead, that is inappropriate for an animal or medical waste incinerator. Disposal of tissue specimens preserved in ethanol or another flammable solvent is also difficult. In most cases, storage of this waste is limited to 90 days and must be managed at an EPA-permitted chemical waste facility. However, few chemical waste facilities are prepared to handle waste that is putrescible, infectious, or biohazardous.
7.C.2.1 Disposal of Chemically Contaminated Animal Tissue
Animal carcasses and tissues that contain a toxic chemical may be the most prominent chemical-biological laboratory waste. Such waste includes biological specimens preserved in formalin and rodents that have been fed lead, mercury, or PCBs in toxicity studies. If storage of such putrescible waste is necessary, refrigeration is usually advisable. Freezing potentially infectious animal tissue at the point of generation can add a margin of safety during waste handling, transport, and prolonged storage. Infectious waste should be stored separately in a secure area.
Incineration is the most appropriate disposal method for this putrescible waste, and it can also destroy the infectious agents that such waste may contain. As discussed above (see beginning of section 7.C), federal law allows the incineration of most chemical-biological waste in an animal or medical waste incinerator. Most modern, efficiently run animal and medical waste incinerators can adequately destroy the small quantities of toxic organic chemicals present in chemically contaminated animal tissue. Large research institutions are likely to have an on-site animal incinerator. Medical waste incineration is also available through commercial waste haulers.
Incineration does not destroy lead and other inorganic chemicals, and they will be emitted or concentrated in the ash. In addition, some organic chemicals form products of incomplete combustion (PICs), which may be more toxic than the chemical contaminant. Incineration of PCBs and some other chlorinated aromatics, for example, can form extremely toxic polychlorinated dibenzo[p]dioxins and furans. Commercial disposal may be preferred for such waste.
(If animal or commercial incineration is unavailable, methods in section 7.C.3.3 below may be adaptable to chemical-biological waste.)
7.C.2.2 Sewer Disposal of Chemical-Biological Liquids
Laboratories that manipulate infectious agents, blood, or body fluids may generate waste that is contaminated with these materials and toxic chemicals. In most cases, blood and body fluids that contain toxic chemicals can be disposed of safely in a sanitary sewer, which is designed to accept biological waste. Approval for such disposal should be requested from the local wastewater treatment works. Chemical concentrations in such waste are typically low enough to be accepted by a local treatment works. OSHA recommends that a separate sink be used exclusively for disposal of human blood, body fluids, and infectious waste. It may be prudent to treat blood and body fluids with bleach (usually a 1:10 aqueous dilution of household bleach) prior to disposal in the sanitary sewer. The worker should take care to prevent personal exposure while waste is being discharged into the sewer.
7.C.2.3 Disinfection and Autoclaving of Contaminated Labware
Contaminated labware may include cultures, stocks, petri plates, and other disposable laboratory items
(e.g., gloves, pipettes, and tips). In many cases, the small quantities of infectious waste on such labware can be disinfected safely with bleach or other chemical disinfectant (e.g., by soaking overnight). The disinfected waste can then be treated as a chemical waste. The worker must check with the state or regional EPA office to determine if a treatment permit is required for chemical disinfection of chemical-biological waste.
Autoclaves can be used to steam-sterilize infectious waste but should be tested routinely for efficacy. Autoclaving does not require an EPA permit. Care must be taken because autoclaving of chemical-biological waste at 120 to 130 °C may result in the volatilization or release of the chemical constituent. Additional waste containment may be needed to minimize chemical releases, but it can interfere with steam penetration into the waste load and sterilization. Before autoclaving untested waste streams containing volatile chemicals, a small load should be processed while monitoring the air emissions.
Autoclaving waste containing flammable liquids may result in a fire or explosion. (It should also be noted that steam sterilization of waste that contains bleach may harm an autoclave.) To autoclave voluminous chemical-biological waste streams, it may be appropriate to dedicate an autoclave room with ample ventilation and to restrict access.
The autoclaved chemical-biological waste can be managed as a chemical waste. After autoclaving, the biohazard markings on the container should be defaced or the material overpacked in a second container to indicate that the waste has been sterilized.
Similar precautions should be observed when using microwaves for decontamination. Although still under development, ultraviolet peroxidation may have the capacity to both sterilize and destroy certain chemicals in the waste. Chemical treatment permits may be required.
7.C.2.4 Disposal of Chemically Contaminated Medical Waste and Sharps
Laboratories that work with human blood must adhere to OSHA's Standard for Occupational Exposure to Blood borne Pathogens (29 CFR 1910.1030), which requires waste containment, marking, and labeling. The OSHA standard also regulates waste disposal from laboratories that manipulate human immunodeficiency virus (HIV) or hepatitis B virus (HBV). In general, such waste that is chemically contaminated can be incinerated with other medical waste or can be autoclaved and managed as a chemical waste.
Waste hypodermic needles and other "sharps" (e.g, scalpels and razor blades) need to be contained in a puncture-resistant waste collection container. Sharps should be destroyed by incineration or by grinding as part of the disinfection treatment. Incineration of chemical or drug-contaminated needles in a medical waste incinerator is appropriate if the waste is not an EPA-regulated chemical waste and if the chemical's toxicity or contamination is low. Needles and other sharps that are contaminated with toxic chemicals and infectious agents or blood can be autoclaved or disinfected on site (see the precautions above), and then managed as a chemical waste. The waste container's biohazard symbol and markings should be defaced after autoclaving or disinfection to indicate that the waste has been sterilized. Noninfectious needles and sharps with high chemical toxicity or contamination are accepted by chemical incinerators.
Some biomedical research generates materials contaminated with blood and antineoplastic drugs. Incineration of these materials as medical waste is appropriate if the level of chemical contamination is low, which is typical. In some cases, chemical disinfection and treatment can be combined to destroy both infectious agents and the antineoplastic drug. It should be noted that unemptied source containers of some antineoplastic drugs are EPA-listed hazardous waste and must be managed as a regulated chemical waste.
7.C.2.5 Minimization Methods for Chemical-Biological Waste
Waste minimization methods used for chemical waste can be used to reduce or eliminate the chemical hazard of chemical-biological waste. Some laboratories that generate biohazardous waste have replaced disposable items with reusable supplies, which are disinfected between uses.
For biological waste, waste minimization can be accomplished best through careful source separation of biological waste from other waste streams. When state guidelines for defining infectious waste do not exist, it is important for laboratories to define carefully those biological wastes that can be disposed of safely as noninfectious within the framework of the CDC/ NIH guidelines (U.S. DHHS, 1993). Training workers to identify and separate biological waste will prevent its inadvertent mixing with other waste streams and normal trash.
7.C.3 Radioactive-Biological Waste
The management of radioactive-biological laboratory waste (shown in Figure 7.2c) can be difficult because of limited on- and off-site disposal options.
Basic principles for the management of radioactive-biological waste include the following:
-
Risk associated with the waste should be assessed. It may be prudent to disinfect highly biohazardous agents first to minimize handling risks and prevent growth of the waste's microbiological load. Appropriate containment, handling, and storage precautions should be taken prior to treatment.
-
Radioactive-biological waste containing short-half-life radionuclides can be held for decay. After decay-in-storage, most U.S. NRC licenses allow the waste to be managed as biological waste. If the waste supports the growth of an infectious agent that it contains, storage should be in a freezer to prevent the waste's infectious risk from increasing.
-
Refrigerated storage facilities or other preservation methods are necessary for putrescible waste.
7.C.3.1 On-site Incineration of Low-level Radioactive Waste
Laboratories that have an on-site radioactive waste incinerator have a great advantage in their ability to manage radioactive-biological waste. On-site incineration of radioactive-biological waste is practical and can be done with minimal impact to health or the environment. For waste that is putrescible or may be infectious, on-site incineration is ideal.
The institution's U.S. NRC license will identify which radionuclides can be incinerated and will set emission limits for those materials based on activity. It is usually beneficial to separate waste by radionuclide volatility and half-life. Incinerator emissions of waste containing short-half-life radionuclides can be minimized by refrigerated storage for decay prior to incineration. If the license allows, ash from the incineration of nonvolatile radionuclides that have short half-lives may be held for decay and disposed of as normal trash. Other ash must be disposed of at a radioactive waste site.
7.C.3.2 Off-site Management of Low-level Radioactive Waste
Many laboratories do not have an on-site incinerator for radioactive-biological waste. Communities tend to oppose waste incinerators, and on-site incineration is prohibitively costly for some radioactive-biological waste generators. Even institutions that have incinerators must usually rely on off-site disposal for some of their radioactive waste. For radioactive putrescible waste, off-site disposal requires special packaging, storage, and transport considerations.
Reliable access to off-site disposal will depend on the establishment of regional sites, which have been slow to develop under the Low-level Radioactive Waste Policy Act of 1980. Moreover, when established, regional low-level radioactive waste sites may not immediately accommodate laboratory radioactive-biological waste. As discussed earlier in this chapter, choice of off-site disposal must involve careful consideration of the safety record of the facility to ensure that the generator's long-term responsibility is liability-free.
7.C.3.3 Disposal of Radioactive Animal Carcasses and Tissue
Waste radioactive animal carcasses and tissue generated from biomedical research typically pose no significant infectious hazard, but they are putrescible. U.S. NRC regulations allow animal carcasses and tissue with less than 1.85 kilobecquerels per gram (kBq/g) of 3H or 14C to be disposed of without regard to radioactivity. Thus animal carcasses and tissue below this limit need not be managed as a radioactive-biological waste but only as a biological waste. Animal tissue with higher levels of activity or other radionuclides must be managed as a radioactive waste. As with all putrescible waste, waste should be either refrigerated, frozen, or otherwise preserved during accumulation, transport, and storage.
While on-site incineration is the preferred method of managing radioactive animal carcasses and tissue, several alternatives exist. Alkaline digestion of animal carcasses containing 3H, 14C, and formaldehyde, followed by neutralization, results in an aqueous radioactive stream that can usually be disposed of in the sanitary sewer. The process uses 1 M sodium hydroxide at 300 °C and pressures up to 150 psi. Commercial units are available for this process. Radioactive animal carcasses may be accepted at a low-level radioactive waste site when packed in lime.
Some institutions grind radioactive animal tissue for disposal in the sanitary sewer, although the U.S. NRC requires that all sewer-disposable waste be dispersible. Preventing contamination and exposure of waste handlers to dust or particles is an important safety measure in this operation.
Autoclaving of infectious animal carcasses is difficult because of the waste's high heat capacity and poor heat conductivity, and often unproductive because treated waste remains putrescible.
7.C.3.4 Disposal of Radioactive-Biological Contaminated Labware
Radioactive-biological contaminated labware (e.g., gloves and disposable laboratory articles) is generated
from biomedical research using radioactive materials with infectious agents, blood, and body fluids. On-site incineration, autoclaving, and off-site disposal are the management options for this waste. Chemical decontamination (e.g., soaking in bleach) may be appropriate if it can be done without risking personal exposure, increasing waste volumes, or creating a waste that is difficult to handle (e.g., wet waste). After disinfection, radioactive-biological waste can be managed as radioactive waste.
Infectious waste and needle boxes that contain radionuclides can be autoclaved safely if the following precautions are satisfied:
-
Monitor the air emissions of a test load to determine if the release of radioactive material is in compliance with U.S. NRC and license limits.
-
Wipe-test the autoclave interior for surface contamination regularly.
-
For ongoing treatment of this waste, dedicate an autoclave or autoclave room for this purpose. The room should have ample ventilation.
-
Restrict access during autoclaving.
-
Test the autoclave efficacy regularly.
Radioactive needles contaminated with infectious agents or blood should be autoclaved as described above, and then incinerated on site or shipped to a low-level radioactive waste site. To prevent injuries, it is important that hypodermic needles and other sharps be kept in waste containers that are puncture-resistant, leak-proof, and closable from the point of discard through ultimate disposal. To prevent airborne radioactive materials, destruction of needles by grinding or a similar means is not recommended.
7.C.3.5 Sewer Disposal of Radioactive-Biological Liquids
Radioactive blood, body fluids, and other sewer-compatible liquids may be disposed of in the sanitary sewer if quantities are within U.S. NRC license and treatment works limits. Precautions must be taken to prevent exposure of waste handlers. OSHA recommends that disposal of human blood and body fluids be done in a dedicated sink.
7.C.4 Chemical-Radioactive-Biological Waste
Chemical-radioactive-biological laboratory waste (depicted in Figure 7.2.d) is the most difficult multihazardous waste to manage. The strategies for managing the various other types of multihazardous waste described above are generally applicable to chemical-radioactive-biological waste. For example, toxicological research sometimes generates animal tissue that contains a radioactively labeled toxic chemical. However, the chemical toxicity of such waste is commonly inconsequential, both legally and in relation to the waste's other characteristics. It could be appropriate to dispose of such animal tissue as a radioactive-biological waste, without regard to its low toxic chemical content.
Reduction or elimination of one of the waste hazards through waste management methods is often an efficient first step. Decay-in-storage is a simple, low-cost way to reduce the radioactivity hazard of a waste with short-lived radionuclides. After decay, most U.S. NRC licenses allow the waste to be managed as a chemical-biological waste. Similarly, autoclaves are readily available to most laboratories for destruction of infectious agents. As described above, autoclaving multihazardous waste requires certain precautions, but renders a chemical-radioactive-biological waste a chemical-radioactive waste. Autoclaving or disinfection makes sense when any of the waste's characteristics (e.g., nutrient value) could support the growth of an infectious agent it contains and thus could increase the waste's risk.
Certain waste treatments reduce multiple hazards in one step. For example, incineration can destroy oxidizable organic chemicals and infectious agents, waste feed rates can be controlled to meet emission limits for volatile radionuclides, and radioactive ash can be disposed of as a dry radioactive waste. Likewise, some chemical treatment methods (e.g., those using bleach) both oxidize toxic chemicals and disinfect biological hazards. Such treatment could convert a chemical-radioactive-biological waste to a radioactive waste.
7.C.5 Future Trends in Management of Multihazardous Waste
Multihazardous waste is becoming the focus of much attention by regulatory agencies, as well as by the laboratories that must deal with it. The U.S. NRC relieved much of the laboratory mixed waste problem by allowing liquid scintillation fluid (LSF) with less than 1.85 kBq/g of 3H or 14C to be disposed of without regard to radioactivity. Thus ignitable LSF below this limit need not be managed as a mixed waste but only as a hazardous chemical waste. As explained above, although U.S. NRC policy has not established a de minimis level for other types of laboratory radioactive waste, licensees can often propose a license-specific de minimis level, below which mixed waste can be released for management as a chemical waste.
Regional EPA offices and state and local hazardous waste authorities differ in their regulation of storage
and chemical treatment of mixed waste. Under certain conditions, regulators may allow such activities without a permit or may temporarily waive the storage or treatment permit requirements. Most regulators allow separation or treatment of chemical and radioactive components in the waste collection container or as part of a process without a RCRA permit.
Within the context of these changes, attention is being directed at promising technologies for the treatment of multihazardous waste. At this time, it cannot be determined if and when these technologies will be available, or if they will be developed for use in the laboratory or on a commercial scale. Nevertheless, they deserve careful attention, as do other approaches that will surely be developed in the near future, including the following:
-
Ultraviolet peroxidation is being reviewed by the National Institutes of Health as a method to treat laboratory aqueous mixed waste containing low concentrations of organics. The process, though still under development, is expected to treat a wide range of organics and sterilize the waste.
-
Wet oxidation and supercritical fluid oxidation (for aqueous solutions containing 1 to 10% organics) are being developed to destroy the chemical constituents in mixed waste.
-
Biodegradation has been used successfully to treat soil, sludge, and other contaminated waste streams containing up to 1% organic waste. Laboratory-scale bioreactors are commercially available.
-
Plasma torch, thermal desorption, molten salt pyrolysis, vitrification, and arc or hearth pyrolysis followed by incineration have all been used to treat mixed waste.
7.D PROCEDURES FOR THE LABORATORY-SCALE TREATMENT OF SURPLUS AND WASTE CHEMICALS
Concerns about environmental protection, bans on landfill disposal of waste, and limited access to sewer disposal have encouraged the development of strategies to reduce hazardous waste from laboratories. Many management methods are considered in earlier chapters of this book (see Chapter 4, section 4.B, and Chapter 5, section 5.B). The small-scale treatment and deactivation of products and by-products as part of the experiment plan is one approach that can be used to address the problem at the level of the actual generator, the laboratory worker. However, unless there is a significant reduction in risk by such action, there may be little benefit in carrying out a procedure that will simply produce another kind of waste with similar risks and challenges for disposal. Furthermore, the question of what constitutes "legal" treatment within the laboratory is still unresolved.
Nevertheless, there is often merit for such in-laboratory treatment. Below are some procedures of general use at the laboratory scale. Additional procedures can be found in the earlier edition of this book (Prudent Practices for Disposal of Chemicals from Laboratories; NRC, 1983) and other books listed in the bibliography. More specific procedures for laboratory treatment are increasingly being included in the experimental sections of chemical journals and in publications such as Organic Syntheses and Inorganic Syntheses.
Safety must be the first consideration before undertaking any of the procedures below. The procedures presented here are intended to be carried out only by, or under the direct supervision of, a trained scientist or technologist who understands the chemistry and hazards involved. Appropriate personal protection should be used. With the exception of neutralization, the procedures are intended for application to small quantities, that is, not more than a few hundred grams. Because risks tend to increase exponentially with scale, larger quantities should be treated only in small batches unless a qualified chemist has demonstrated that the procedure can be scaled up safely. The generator must ensure that the procedure eliminates the regulated hazard before the products are disposed of as nonhazardous waste. In addition, if the procedure suggests disposal of the product into the sanitary sewer, this strategy must comply with local regulations.
(See Chapter 6, section 6.F, for further information on protective clothing and also Chapter 5, section 5.C.2.6.)
7.D.1 Acids and Bases
Neutralization of acids and bases (corrosives) is generally exempt from a RCRA treatment permit. However, because the products of the reaction are often disposed of in the sanitary sewer, it is important to ensure that hazardous waste such as toxic metal ions is not a part of the effluent.
In most laboratories, both waste acids and waste bases are generated, and so it is most economical to collect them separately and then neutralize one with the other. If additional acid or base is required, sulfuric or hydrochloric acid and sodium or magnesium hydroxide, respectively, can be used.
If the acid or base is highly concentrated, it is prudent to first dilute it with cold water (adding the acid or base to the water) to a concentration below 10%. Then the acid and base are mixed, and the additional water is slowly added when necessary to cool and dilute the neutralized product. The concentration of neutral salts
disposed of in the sanitary sewer should generally be below 1%.
7.D.2 Organic Chemicals
7.D.2.1 Thiols and Sulfides
Small quantities of thiols (mercaptans) and sulfides can be destroyed by oxidation to a sulfonic acid with sodium hypochlorite. If other groups that can be oxidized by hypochlorite are also present, the quantity of this reagent used must be increased accordingly.
Procedure for oxidizing 0.1 mol of a liquid thiol:
Five hundred milliliters (0.4 mol, 25% excess) of commercial hypochlorite laundry bleach (5.25% sodium hypochlorite) is poured into a 5-L three-necked flask located in a fume hood. The flask is equipped with a stirrer, thermometer, and dropping funnel. The thiol (0.1 mol) is added dropwise to the stirred hypochlorite solution, initially at room temperature. A solid thiol can be added gradually through a neck of the flask or can be dissolved in tetrahydrofuran or other appropriate nonoxidizable solvent and the solution added to the hypochlorite. (The use of tetrahydrofuran introduces a flammable liquid that could alter the final disposal method.) Traces of thiol can be rinsed from the reagent bottle and dropping funnel with additional hypochlorite solution. Oxidation, accompanied by a rise in temperature and dissolution of the thiol, usually starts after a small amount of the thiol has been added. If the reaction has not started spontaneously after about 10% of the thiol has been added, addition is stopped and the mixture warmed to about 50 °C to initiate this reaction. Addition is resumed only after it is clear that oxidation is occurring. The temperature is maintained at 45 to 50 °C by adjusting the rate of addition and using an ice bath for cooling if necessary. Addition requires about 15 minutes. If the pH drops below 6 because of generation of the sulfonic acid, it may be necessary to add some sodium hydroxide or additional bleach because hypochlorite is destroyed under acidic conditions. Stirring is continued for 2 hours while the temperature gradually falls to room temperature. The mixture should be a clear solution, perhaps containing traces of oily by-products. The reaction mixture can usually be flushed down the drain with excess water. The unreacted laundry bleach need not be decomposed.
(Because sodium hypochlorite solutions deteriorate on storage, it is advisable to have relatively fresh material available. A 5.25% solution of sodium hypochlorite has 25 g of active chlorine per liter. If determination of the active hypochlorite content is justified, it can be accomplished as follows. Ten milliliters of the sodium hypochlorite solution is diluted to 100.0 mL, and then 10.0 mL of this diluted reagent is added to a solution of 1 g of potassium iodide and 12.5 mL of 2 M acetic acid in 50 mL of distilled water. Using a starch solution as indicator, titrate the solution with 0.1 N sodium thiosulfate. One milliliter of titrant corresponds to 3.5 mg of active chlorine. A 5.25% solution of sodium hypochlorite requires approximately 7 mL of titrant.)
Calcium hypochlorite may be used as an alternative to sodium hypochlorite and requires a smaller volume of liquid. For 0.1 mol of thiol, 42 g (25% excess) of 65% calcium hypochlorite (technical grade) is stirred into 200 mL of water at room temperature. The hypochlorite soon dissolves, and the thiol is then added as in the above procedure.
Laboratory glassware, hands, and clothing contaminated with thiols can be deodorized by a solution of Diaperene®, a tetraalkylammonium salt used to deodorize containers in which soiled diapers have been washed.
Small amounts of sulfides, RSR', can be oxidized to sulfones (RSO2 R') to eliminate their disagreeable odors. The hypochlorite procedure used for thiols can be employed for this purpose, although the resulting sulfones are often water-insoluble and may have to be separated from the reaction mixture by filtration.
Small amounts of the inorganic sulfides, sodium sulfide or potassium sulfide, can be destroyed in aqueous solution by sodium or calcium hypochlorite using the procedure described for oxidizing thiols.
7.D.2.2 Acyl Halides and Anhydrides
Acyl halides, sulfonyl halides, and anhydrides react readily with water, alcohols, and amines. They should never be allowed to come into contact with waste that contains such substances. Most compounds in this class can be hydrolyzed to water-soluble products of low toxicity.
Procedure for hydrolyzing 0.5 mol of RCOX, RSO2X, or (RCO)2O:
A 1-L three-necked flask equipped with a stirrer, dropping funnel, and thermometer is placed on a steam bath in a hood, and 600 mL of 2.5 M aqueous sodium hydroxide (1.5 mol, 50% excess) are poured
into the flask. A few milliliters of the acid derivative are added drop wise with stirring. If the derivative is a solid, it can be added in small portions through a neck of the flask. If reaction occurs, as indicated by a rise in temperature and dissolution of the acid derivative, addition is continued at such a rate that the temperature does not rise above 45 °C. If the reaction is sluggish, as may be the case with less soluble compounds such as p-toluenesulfonyl chloride, the mixture is heated before adding any more acid derivative. When the initial added material has dissolved, the remainder is added drop wise. As soon as a clear solution is obtained, the mixture is cooled to room temperature, neutralized to about pH 7 with dilute hydrochloric or sulfuric acid, and washed down the drain with excess water.
7.D.2.3 Aldehydes
Many aldehydes are respiratory irritants, and some, such as formaldehyde and acrolein, are quite toxic. There is sometimes merit in oxidation of aldehydes to the corresponding carboxylic acids, which are usually less toxic and less volatile.
Procedure for permanganate oxidation of 0.1 mol of aldehyde:
A mixture of 100 mL of water and 0.1 mol of aldehyde is stirred in a 1-L round-bottomed flask equipped with a thermometer, dropping funnel, stirrer, steam bath, and, if the aldehyde boils below 100 °C, a condenser. Approximately 30 mL of a solution of 12.6 g (0.08 mol, 20% excess) of potassium permanganate in 250 mL of water is added over a period of 10 minutes. If the temperature rises above 45 °C, the solution should be cooled. If this addition is not accompanied by a rise in temperature and loss of the purple permanganate color, the mixture is heated by the steam bath until a temperature is reached at which the color is discharged. The rest of the permanganate solution is added slowly at within 10 °C of this temperature. The temperature is then raised to 70 to 80 °C, and stirring continued for 1 hour or until the purple color has disappeared, whichever occurs first. The mixture is cooled to room temperature and acidified with 6 N sulfuric acid. (CAUTION: Do not add concentrated sulfuric acid to permanganate solution because explosive manganese oxide (Mn2O7) may precipitate.) Enough solid sodium hydrogen sulfite (at least 8.3 g, 0.08 mol) is added with stirring at 20 to 40 °C to reduce all the manganese, as indicated by loss of purple color and dissolution of the solid manganese dioxide. The mixture is washed down the drain with a large volume of water.
If the aldehyde contains a carbon-carbon double bond, as in the case of the highly toxic acrolein, 4 mol (20% excess) of permanganate per mol of aldehyde is required to oxidize the alkene bond and the aldehyde group.
Formaldehyde is oxidized conveniently to formic acid and carbon dioxide by sodium hypochlorite. Thus 10 mL of formalin (37% formaldehyde) in 100 mL of water is stirred into 250 mL of hypochlorite laundry bleach (5.25% NaOC1) at room temperature and allowed to stand for 20 minutes before being flushed down the drain. This procedure is not recommended for other aliphatic aldehydes because it leads to chloro acids, which are more toxic and less biodegradable than corresponding unchlorinated acids.
7.D.2.4 Amines
Acidified potassium permanganate efficiently degrades aromatic amines. Diazotization followed by hypophosphorus acid protonation is a method for deamination of aromatic amines, but the procedure is more complex than oxidation.
Procedure for permanganate oxidation of 0.01 mol of aromatic amine:
A solution of 0.01 mol of aromatic amine in 3 L of 1.7 N sulfuric acid is prepared in a 5-L flask; 1 L of 0.2 M potassium permanganate is added, and the solution allowed to stand at room temperature for 8 hours. Excess permanganate is reduced by slow addition of solid sodium hydrogen sulfite until the purple color disappears. The mixture is then flushed down the drain.
7.D.2.5 Organic Peroxides and Hydroperoxides
(CAUTION: Peroxides are particularly dangerous. These procedures should be carried out only by knowledgeable laboratory workers.) Peroxides can be removed from a solvent by passing it through a column of basic activated alumina, by treating it with indicating Molecular Sieves®, or by reduction with ferrous sulfate. Although these procedures remove hydroperoxides, which are the principal hazardous contaminants of peroxide-forming solvents, they do not remove dialkyl peroxides, which may also be present in low concentrations. Commonly used peroxide reagents, such as acetyl peroxide, benzoyl peroxide,
t-butyl hydroperoxide, and di-t-butyl peroxide, are less dangerous than the adventitious peroxides formed in solvents.
Removal of peroxides with alumina:
A 2 x 33 cm column filled with 80 g of 80-mesh basic activated alumina is usually sufficient to remove all peroxides from 100 to 400 mL of solvent, whether water-soluble or water-insoluble. After passage through the column, the solvent should be tested for peroxide content. Peroxides formed by air oxidation are usually decomposed by the alumina, not merely absorbed on it. However, for safety it is best to slurry the wet alumina with a dilute acidic solution of ferrous sulfate before it is discarded.
Removal of peroxides with Molecular Sieves®:
Reflux 100 mL of the solvent with 5 g of 4- to 8-mesh indicating activated 4A Molecular Sieves® for several hours under nitrogen. The sieves are separated from the solvent and require no further treatment because the peroxides are destroyed during their interaction with the sieves.
Removal of peroxides with ferrous sulfate:
A solution of 6 g of FeSO4 · 7H2O, 6 mL of concentrated sulfuric acid, and 11 mL of water is stirred with 1 L of water-insoluble solvent until the solvent no longer gives a positive test for peroxides. Usually only a few minutes are required.
Diacyl peroxides can be destroyed by this reagent as well as by aqueous sodium hydrogen sulfite, sodium hydroxide, or ammonia. However, diacyl peroxides with low solubility in water, such as dibenzoyl peroxide, react very slowly. A better reagent is a solution of sodium iodide or potassium iodide in glacial acetic acid.
Procedure for destruction of diacyl peroxides:
For 0.01 mol of diacyl peroxide, 0.022 mol (10% excess) of sodium or potassium iodide is dissolved in 70 mL of glacial acetic acid, and the peroxide added gradually with stirring at room temperature. The solution is rapidly darkened by the formation of iodine. After a minimum of 30 minutes, the solution is washed down the drain with a large excess of water.
Most dialkyl peroxides (ROOR) do not react readily at room temperature with ferrous sulfate, iodide, ammonia, or the other reagents mentioned above. However, these peroxides can be destroyed by a modification of the iodide procedure.
Procedure for destruction of dialkyl peroxides:
One milliliter of 36% (w/v) hydrochloric acid is added to the above acetic acid/potassium iodide solution as an accelerator, followed by 0.01 mol of the dialkyl peroxide. The solution is heated to 90 to 100 °C on a steam bath over the course of 30 minutes and held at that temperature for 5 hours.
7.D.3 Inorganic Chemicals
7.D.3.1 Metal Hydrides
Most metal hydrides react violently with water with the evolution of hydrogen, which can form an explosive mixture with air. Some, such as lithium aluminum hydride, potassium hydride, and sodium hydride, are pyrophoric. Most can be decomposed by gradual addition of (in order of decreasing reactivity) methyl alcohol, ethyl alcohol, n-butyl alcohol, or t-butyl alcohol to a stirred, ice-cooled solution or suspension of the hydride in an inert liquid, such as diethyl ether, tetrahydrofuran, or toluene, under nitrogen in a three-necked flask. Although these procedures reduce the hazard and should be a part of any experimental procedure that uses reactive metal hydrides, the products from such deactivation may be hazardous waste that must be treated as such on disposal.
Hydrides commonly used in laboratories are lithium aluminum hydride, potassium hydride, sodium hydride, sodium borohydride, and calcium hydride. The following methods for their disposal demonstrate that the reactivity of metal hydrides varies considerably. Most hydrides can be decomposed safely by one of the four methods, but the properties of a given hydride must be well understood in order to select the most appropriate method. (CAUTION: Most of the methods described below produce hydrogen gas, which can present an explosion hazard. The reaction should be carried out in a hood, behind a shield, and with proper safeguards to avoid exposure of the effluent gas to spark or flame. Any stirring device must be spark-proof.)
Decomposition of lithium aluminum hydride:
Lithium aluminum hydride (LiA1H4) can be purchased as a solid or as a solution in toluene, diethyl ether, tetrahydrofuran, or other ethers. Although drop-
wise addition of water to its solutions under nitrogen in a three-necked flask has frequently been used to decompose it, vigorous frothing often occurs. An alternative is to use 95% ethanol, which reacts less vigorously than water. A safer procedure is to decompose the hydride with ethyl acetate, because no hydrogen is formed.
To the hydride solution in a flask equipped with a stirrer, ethyl acetate is added slowly. The mixture sometimes becomes so viscous after the addition that stirring is difficult and additional solvent may be required. When the reaction with ethyl acetate has ceased, a saturated aqueous solution of ammonium chloride is added with stirring. The mixture separates into an organic layer and an aqueous layer containing inert inorganic solids. The upper, organic layer should be separated and disposed of as a flammable liquid. The lower, aqueous layer can often be disposed of in the sanitary sewer.
Decomposition of potassium or sodium hydride:
Potassium and sodium hydride (KH, NaH) in the dry state are pyrophoric, but they can be purchased as a relatively safe dispersion in mineral oil. Either form can be decomposed by adding enough dry hydrocarbon solvent (e.g., heptane) to reduce the hydride concentration below 5% and then adding excess t-butyl alcohol drop wise under nitrogen with stirring. Cold water is then added drop wise, and the resulting two layers are separated. The organic layer can be disposed of as a flammable liquid. The aqueous layer can often be neutralized and disposed of in the sanitary sewer.
Decomposition of sodium borohydride:
Sodium borohydride (NaBH4) is so stable in water that a 12% aqueous solution stabilized with sodium hydroxide is sold commercially. In order to effect decomposition, the solid or aqueous solution is added to enough water to make the borohydride concentration less than 3%, and then excess equivalents of dilute aqueous acetic acid are added drop wise with stirring under nitrogen.
Decomposition of calcium hydride:
Calcium hydride (CaH2), the least reactive of the materials discussed here, is purchased as a powder. It is decomposed by adding 25 mL of methyl alcohol per gram of hydride under nitrogen with stirring. When reaction has ceased, an equal volume of water is gradually added to the stirred slurry of calcium methoxide. The mixture is then neutralized with acid and disposed of in the sanitary sewer.
7.D.3.2 Inorganic Cyanides
Inorganic cyanides can be oxidized to cyanate using aqueous hypochlorite following a procedure similar to the oxidation of thiols. Hydrogen cyanide can be converted to sodium cyanide by neutralization with aqueous sodium hydroxide, and then oxidized.
Procedure for oxidation of cyanide:
An aqueous solution of the cyanide salt in an ice-cooled, three-necked flask equipped with a stirrer, thermometer, and dropping funnel is cooled to 4 to 10 °C. A 50% excess of commercial hypochlorite laundry bleach containing 5.25% (0.75 M) sodium hypochlorite is added slowly with stirring while maintaining the low temperature. When the addition is complete and heat is no longer being evolved, the solution is allowed to warm to room temperature and stand for several hours. The mixture can then be washed down the drain with excess water. The same procedure can be applied to insoluble cyanides such as cuprous cyanide (though copper salts should not be disposed of in the sanitary sewer). In calculating the quantity of hypochlorite required, the experimenter should remember that additional equivalents may be needed if the metal ion can be oxidized to a higher valence state, as in the reaction,
A similar procedure can be used to destroy hydrogen cyanide, but precautions must be taken to avoid exposure to this very toxic gas. Hydrogen cyanide is dissolved in several volumes of ice water. Approximately 1 molar equivalent of aqueous sodium hydroxide is added at 4 to 10 °C to convert the hydrogen cyanide into its sodium salt, and then the procedure described above for sodium cyanide is followed. (CAUTION: Sodium hydroxide or other bases, including sodium cyanide, must not be allowed to come into contact with liquid hydrogen cyanide because they may initiate a violent polymerization of the hydrogen cyanide.)
This procedure also destroys soluble ferrocyanides and ferricyanides. Alternatively, these can be precipitated as the ferric or ferrous salt, respectively, for possible landfill disposal.
(See Chapter 6, section 6.D, for details on working with hazardous gases.)
7.D.3.3 Metal Azides
Heavy metal azides are notoriously explosive and should be handled by trained personnel. Silver azide (and also fulminate) can be generated from Tollens reagent, which is often found in undergraduate laboratories. Sodium azide is explosive only when heated to near its decomposition temperature (300 °C), but heating it should be avoided. Sodium azide should never be flushed down the drain. This practice has caused serious accidents because the azide can react with lead or copper in the drain lines to produce an azide that may explode. It can be destroyed by reaction with nitrous acid:
Procedure for destruction of sodium azide:
The operation must be carried out in a hood because of the formation of toxic nitric oxide. An aqueous solution containing no more than 5% sodium azide is put into a three-necked flask equipped with a stirrer and a dropping funnel. Approximately 7 mL of 20% aqueous solution of sodium nitrite (40% excess) per gram of sodium azide is added with stirring. A 20% aqueous solution of sulfuric acid is then added gradually until the reaction mixture is acidic to litmus paper. (CAUTION: The order of addition is essential. Poisonous, volatile hydrazoic acid (HN3) will evolve if the acid is added before the nitrite.) When the evolution of nitrogen oxides ceases, the acidic solution is tested with starch iodide paper. If it turns blue, excess nitrite is present, and the decomposition is complete. The reaction mixture is washed down the drain.
7.D.3.4 Alkali Metals
Alkali metals react violently with water, with common hydroxylic solvents, and with halogenated hydrocarbons. They should always be handled in the absence of these materials. The metals are usually destroyed by controlled reaction with an alcohol. The final aqueous alcoholic material can usually be disposed of in the sanitary sewer.
Procedure for destruction of alkali metals:
Waste sodium is readily destroyed with 95% ethanol. The procedure is carried out in a three-necked, round-bottomed flask equipped with a stirrer, dropping funnel, condenser, and heating mantle. Solid sodium should be cut into small pieces with a sharp knife while wet with a hydrocarbon, preferably mineral oil, so that the unoxidized surface is exposed. A dispersion of sodium in mineral oil can be treated directly. The flask is flushed with nitrogen and the pieces of sodium placed in it. Then 13 mL of 95% ethanol per gram of sodium are added at a rate that causes rapid refluxing. (CAUTION: Hydrogen gas is evolved and can present an explosion hazard. The reaction should be carried out in a hood, behind a shield, and with proper safeguards (such as in Chapter 5, sections 5.G.4 and 5.G.5) to avoid exposing the effluent gas to spark or flame. Any stirring device must be spark-proof.) Stirring is commenced as soon as enough ethanol has been added to make this possible. The mixture is stirred and heated under reflux until the sodium is dissolved. The heat source is removed, and an equal volume of water added at a rate that causes no more than mild refluxing. The solution is then cooled, neutralized with 6 M sulfuric or hydrochloric acid, and washed down the drain.
To destroy metallic potassium, the same procedure and precautions as for sodium are used, except that the less reactive t-butyl alcohol is used in the proportion of 21 mL/g of metal. (CAUTION: Potassium metal can form explosive peroxides. Metal that has formed a yellow oxide coating from exposure to air should not be cut with a knife, even when wet with a hydrocarbon, because an explosion can be promoted.) If the potassium is dissolving too slowly, a few percent of methanol can be added gradually to the refluxing t-butyl alcohol. Oxide-coated potassium sticks should be put directly into the flask and decomposed with t-butyl alcohol. The decomposition will require considerable time because of the low surface/volume ratio of the metal sticks.
Lithium metal can be treated by the same procedure, but using 30 mL of 95% ethanol per gram of lithium. The rate of dissolution is slower than that of sodium.
7.D.3.5 Metal Catalysts
Metal catalysts such as Raney nickel and other fine metal powders can be slurried into water; dilute hydrochloric acid is then added carefully until the solid dissolves. Depending on the metal and on local regulations, the solution can be discarded in the sanitary sewer or with other hazardous or nonhazardous solid waste. Precious metals should be recovered from this process.
7.D.3.6 Water-Reactive Metal Halides
Liquid halides, such as TiCl4 and SnCl4, can be added to well-stirred water in a round-bottomed flask cooled by an ice bath as necessary to keep the exothermic
reaction under control. It is usually more convenient to add solid halides, such as AlCl3 and ZrCl4, to stirring water and crushed ice in a flask or beaker. The acidic solution can be neutralized and, depending on the metal and local regulations, discarded in the sanitary sewer or with other hazardous or nonhazardous solid waste.
7.D.3.7 Halides and Acid Halides of Nonmetals
Halides and acid halides such as PCl3, PCl5, SiCl4, SOCl2, SO2Cl2, and POCl3 are water-reactive. The liquids can be hydrolyzed conveniently using 2.5 M sodium hydroxide by the procedure described earlier for acyl halides and anhydrides. These compounds are irritating to the skin and respiratory passages and, even more than most chemicals, require a good hood and skin protection when handling them. Moreover, PCl3 may give off small amounts of highly toxic phosphine (PH3) during hydrolysis.
Sulfur monochloride (S2Cl2) is a special case. It is hydrolyzed to a mixture of sodium sulfide and sodium sulfite, so that the hydrolyzate must be treated with hypochlorite as described earlier for sulfides before it can be flushed down the drain.
The solids of this class (e.g., PCl5) tend to cake and fume in moist air and therefore are not conveniently hydrolyzed in a three-necked flask. It is preferable to add them to a 50% excess of 2.5 M sodium hydroxide solution in a beaker or wide-mouth flask equipped with a stirrer and half-filled with crushed ice. If the solid has not all dissolved by the time the ice has melted and the stirred mixture has reached room temperature, the reaction can be completed by heating on a steam bath, and then the acidic solution neutralized and disposed of in the sanitary sewer.
7.D.3.8 Inorganic Ions
Many inorganic wastes consist of a cation (metal or metalloid atom) and an anion (which may or may not contain a metalloid component). It is often helpful to examine the cationic and anionic parts of the substance separately to determine whether either possesses a hazard.
If a substance contains a ''heavy metal," it is often assumed that it is highly toxic. While salts of some heavy metals, such as lead, thallium, and mercury, are highly toxic, those of others, such as gold and tantalum, are not. On the other hand, compounds of beryllium, a "light metal," are highly toxic. In Table 7.1, cations of metals and metalloids are listed alphabetically in two groups: those whose toxic properties as described in the toxicological literature present a significant hazard, and those whose properties do not. The basis for separation is relative, and the separation does not imply that those in the second list are "nontoxic." Similarly, Table 7.2 lists anions according to their level of toxicity and other dangerous properties, such as strong oxidizing power (e.g., perchlorate), flammability (e.g., amide), water reactivity (e.g., hydride), and explosivity (e.g., azide).
Materials that pose a hazard because of significant radioactivity are outside the scope of this volume, although they may be chemically treated in a manner similar to the nonradioactive materials discussed in this chapter. Their handling and disposal are highly regulated in most countries. Low-level radioactive mixed waste is discussed in section 7.C above.
7.D.3.8.1 Chemicals in Which Neither the Cation nor the Anion Presents a Significant Hazard
Chemicals in which neither the cation nor the anion presents a significant hazard consist of those chemicals composed of ions from the right-hand columns of Tables 7.1 and 7.2. Those that are soluble in water to the extent of a few percent can usually be disposed of in the sanitary sewer. Only laboratory quantities should be disposed of in this manner, and at least 100 parts of water per part of chemical should be used. Local regulations should be checked for possible restrictions. Dilute slurries of insoluble materials, such as calcium sulfate or aluminum oxide, also can be handled in this way, provided the material is finely divided and not contaminated with tar, which might clog the piping. Some incinerators can handle these chemicals. If time and space permit, dilute aqueous solutions can be boiled down or allowed to evaporate to leave only a sludge of the inorganic solid for landfill disposal. However, appropriate precautions, including the use of traps, must be considered to ensure that toxic or other prohibited materials are not released to the atmosphere.
An alternative procedure is to precipitate the metal ion by the agent recommended in Table 7.1. The precipitate can often be disposed of in a secure landfill. The most generally applicable procedure is to precipitate the cation as the hydroxide by adjusting the pH to the range shown in Table 7.3.
7.D.3.8.2 Precipitation of Cations as Their Hydroxides
Because the pH range for precipitation varies greatly among metal ions, it is important to control it carefully. The aqueous solution of the metal ion is adjusted to the recommended pH (Table 7.3) by addition of a solu-
TABLE 7.1 High-and Low-Toxicity Cations and Preferred Precipitants
|
High Toxic Hazard |
Low Toxic Hazard |
||
|
Cation |
Precipitanta |
Cation |
Precipitanta |
|
Antimony |
OH-, S2- |
Aluminium |
OH- |
|
Arsenic |
S2- |
Bismuth |
OH-, S2- |
|
Barium |
SO42-, CO32- |
Calcium |
SO42-, CO32- |
|
Beryllium |
OH- |
Cerium |
OH- |
|
Cadmium |
OH-, S2- |
Cesium |
|
|
Chromium(III)b |
OH |
Copperc |
OH-, S2- |
|
Cobalt(II)b |
OH-, S2- |
Gold |
OH-, S2- |
|
Gallium |
OH- |
Ironc |
OH-, S2- |
|
Germanium |
OH-, S2- |
Lanthanides |
OH- |
|
Hafnium |
OH- |
Lithium |
|
|
Indium |
OH-, S2- |
Magnesium |
OH- |
|
Iridiumd |
OH-, S2- |
|
|
|
Lead |
OH-, S2- |
Niobium(V) |
OH- |
|
Manganese(II)b |
OH-, S2- |
Palladium |
OH-, S2- |
|
Mercury |
OH-, S2- |
Potassium |
|
|
Nickel |
OH-, S2- |
Rubidium |
|
|
OH-, S2- |
Scandium |
OH- |
|
|
Platinum(II)b |
OH-, S2- |
Sodium |
|
|
Rhenium(VII)b |
S2- |
Strontium |
SO42- CO32- |
|
Rhodium(III)b |
OH-, S2- |
Tantalum |
OH- |
|
Ruthenium(II)b |
OH-, S2- |
Tin |
OH-, S2- |
|
Selenium |
S2- |
Titanium |
OH- |
|
Silverd |
Cl, OH-, S2- |
Yttrium |
OH- |
|
Tellurium |
S2 |
Zincc |
OH-, S2- |
|
Thallium |
OH-, S2- |
Zirconium |
OH- |
|
|
|
|
|
|
Vanadium |
OH-, S2- |
|
|
|
a Precipitants are listed in order of preference: OH-, CO32- =base (sodium hydroxide or sodium carbonate), S2- = sulfide, SO42- = sulfate, and Cl- = chloride. b The precipitant is for the indicated valence state. c Very low maximum tolerance levels have been set for these low-toxicity ions in some countries, and large amounts should not be put into public sewer systems. The small amounts typically used in laboratories will not normally affect water supplies, although they may be prohibited by the local publicly owned treatment works (POTW). d Recovery of these rare and expensive metals may be economically favorable. e These ions are best precipitated as calcium molybdate(VI) or calcium tungstate(VI). f CAUTION: Osmium tetroxide, OSO4, a volatile, extremely poisonous substance, is formed from almost any osmium compound under acid conditions in the presence of air. Reaction with corn oil or powdered milk will destroy it. |
|||
tion of 1 M sulfuric acid, or 1 M sodium hydroxide or carbonate. The pH can be determined over the range 1 through 10 by use of pH test paper.
The precipitate is separated by filtration, or as a heavy sludge by decantation, and packed for disposal. Some gelatinous hydroxides are difficult to filter. In such cases, heating the mixture close to 100 °C or stirring with diatomaceous earth, approximately 1 to 2 times the weight of the precipitate, often facilitates filtration.
As shown in Table 7.1, precipitants other than a base may be superior for some metal ions, such as sulfuric acid for calcium ion. For some ions, the hydroxide precipitate will redissolve at a high pH (Table 7.3). For a number of metal ions the use of sodium carbonate will result in precipitation of the metal carbonate or a mixture of hydroxide and carbonate.
7.D.3.8.3 Chemicals in Which the Cation Presents a Relatively High Hazard from Toxicity
In general, waste chemicals containing any of the cations listed as highly hazardous in Table 7.1 can be precipitated as their hydroxides or oxides. Alternatively, many can be precipitated as insoluble sulfides by treatment with sodium sulfide in neutral solution (Table 7.4). Several sulfides will redissolve in excess sulfide ion, and so it is important that the sulfide ion concentration be controlled by adjustment of the pH.
Precipitation as the hydroxide is achieved as described above. Precipitation as the sulfide is accomplished by adding a 1 M solution of sodium sulfide to the metal ion solution, and then adjusting the pH to
TABLE 7.2 High- and Low-Hazard Anions and Preferred Precipitants
|
High-Hazard Anions |
|||
|
Ion |
Hazard Typea |
Precipitant |
Low-Hazard Anions |
|
Aluminium hydride, AlH4- |
F, W |
— |
Bisulfite, HSO3- |
|
Amide, NH2- |
F, Eb |
— |
Borate, BO33-, B4O72- |
|
Arsenate, AsO3-,AsO43- |
T |
Cu2+, Fe2+ |
Bromide, Br- |
|
Arsenite, AsO2-, AsO33- |
T |
Pb2+ |
Carbonate, CO32- |
|
Azide, N3- |
E, T |
— |
Chloride, Cl- |
|
Borohydride, BH4- |
F |
— |
Cyanate, OCN- |
|
Bromate, BrO3- |
O, F, E |
— |
Hydroxide, OH- |
|
Chlorate, ClO3- |
O, E |
— |
Iodide, I- |
|
Chromate, CrO42-, Cr2O72- |
T,O |
|
|
|
Oxide, O- |
|
|
|
|
Cyanide, CN- |
T |
— |
Phosphate, PO43- |
|
Ferricyanide, {Fe(CN)6}3- |
T |
Fe2+ |
Sulfate, SO42- |
|
Ferrocyanide, {FE(CN)6)4- |
T |
Fe3+ |
Sulfite, SO32- |
|
Fluoride, F- |
T |
Ca2+ |
Thiocyanate, SCN- |
|
Hydride, H- |
F, W |
— |
|
|
Hydroperoxide, O2H- |
O, E |
— |
|
|
Hydrosulfide, SH- |
T |
— |
|
|
Hypochlorite, OCl- |
O |
— |
|
|
Iodate, IO3- |
O, E |
— |
|
|
Nitrate, NO3- |
O |
— |
|
|
Nitrite, NO2- |
T, O |
— |
|
|
Perchlorate, ClO4- |
O, E |
— |
|
|
Permanganate, MnO4- |
T, O |
— |
|
|
Peroxide, O22- |
O, E |
|
|
|
Persulfate, S2O82- |
O |
— |
|
|
Selenate, SeO42- |
T |
Pb2+ |
|
|
Selenide, Se2- |
T |
Cu2+ |
|
|
Sulfide, S2- |
T |
|
|
|
a T = toxic; O = oxidant; F = flammable; E = explosive; and W = water reactive. b Metal amides readily form explosive peroxides on exposure to air. c Reduce and precipitate as Cr(III). d Reduce and precipitate as Mn(II); see Table 7.1. e See Table 7.4. |
|||
neutral with 1 M sulfuric acid. (CAUTION: Avoid acidifying the mixture because hydrogen sulfide could be formed.) The precipitate is separated by filtration or decantation and packed for disposal. Excess sulfide ion can be destroyed by the addition of hypochlorite to the clear aqueous solution.
The following ions are most commonly found as oxyanions and are not precipitated by base: As3+, As5+, Re7+, Se4+, Se6+, Te4+, and Te6+ . These elements can be precipitated from their oxyanions as the sulfides by the above procedure. Oxyanions of Mo6+ and W6+ can be precipitated as their calcium salts by the addition of calcium chloride. Some ions can be absorbed by passing their solutions over ion-exchange resins. The resins can be landfilled, and the effluent solutions poured down the drain.
Another class of compounds whose cations may not be precipitated by the addition of hydroxide ions are the most stable complexes of metal cations with Lewis bases, such as ammonia, amines, and tertiary phosphines. Because of the large number of these compounds and their wide range of properties, it is not possible to give a general procedure for separating the cations. In many cases, metal sulfides can be precipitated directly from aqueous solutions of the complexes by the addition of aqueous sodium sulfide. If a test-tube experiment shows that other measures are needed, the addition of hydrochloric acid to produce a slightly acidic solution will often decompose the complex by protonation of the basic ligand. Metal ions that form insoluble sulfides under acid conditions can then be precipitated by drop wise addition of aqueous sodium sulfide.
A third option for this waste is incineration, provided that the incinerator ash is to be sent to a secure landfill. Incineration to ash reduces the volume of
TABLE 7.4 Precipitation of Sulfides
|
Precipitated at pH 7 |
Not Precipitated at Low pH |
Soluble Complex at High pH |
|
Ag+ |
|
|
|
As3+a |
|
x |
|
Au+a |
|
x |
|
Bi3+ |
|
|
|
Cd2+ |
|
|
|
Co2+ |
x |
|
|
Cr3+a |
|
|
|
Cu2+ |
|
|
|
Fe2+a |
x |
|
|
Ge2+ |
|
x |
|
Hg2+ |
|
x |
|
In3+ |
x |
|
|
Ir4+ |
|
x |
|
Mn2+a |
x |
|
|
Mo3+ |
|
x |
|
Ni2+ |
x |
|
|
Os4+ |
|
|
|
Pb2+ |
|
|
|
Pd2+a |
|
|
|
pt2+a |
|
x |
|
Re4+ |
|
|
|
Rh2+a |
|
|
|
Ru4+ |
|
|
|
Sb3+a |
|
x |
|
Se2+ |
|
x |
|
Sn2+ |
|
x |
|
Te4+ |
|
x |
|
Tl+a |
x |
|
|
V4+a |
|
|
|
Zn2+ |
x |
|
|
NOTE: Precipitation of ions listed without an x is usually not pH-dependent. a Higher oxidation states of this ion are reduced by sulfide ion and precipitated as this sulfide. SOURCE: Swift and Schaefer (1961) |
||
waste going to a landfill. Waste that contains mercury, thallium, gallium, osmium, selenium, or arsenic should not be incinerated because volatile, toxic combustion products may be emitted.
7.D.3.8.4
Chemicals in Which an Anion Presents a Relatively High Hazard
The more common dangerous anions are listed in Table 7.2. Many of the comments made above about the disposal of dangerous cations apply to these anions. The hazard associated with some of these anions is their reactivity or potential to explode, which makes them unsuitable for landfill disposal. Most chemicals containing these anions can be incinerated, but strong oxidizing agents and hydrides should be introduced into the incinerator only in containers of not more than a few hundred grams. Incinerator ash from anions of chromium or manganese should be transferred to a secure landfill.
Some of these anions can be precipitated as insoluble salts for landfill disposal, as indicated in Table 7.2. Small amounts of strong oxidizing agents, hydrides, cyanides, azides, metal amides, and soluble sulfides or fluorides can be converted into less hazardous substances in the laboratory before being disposed of. Suggested procedures are presented in the following paragraphs.
7.D.3.8.5
Procedure for Reduction of Oxidizing Salts
Hypochlorites, chlorates, bromates, iodates, periodates, inorganic peroxides and hydroperoxides, persulfates, chromates, molybdates, and permanganates can be reduced by sodium hydrogen sulfite. A dilute solution or suspension of a salt containing one of these anions has its pH reduced to less than 3 with sulfuric acid, and a 50% excess of aqueous sodium hydrogen sulfite is added gradually with stirring at room temperature. An increase in temperature indicates that the reaction is taking place. If the reaction does not start on addition of about 10% of the sodium hydrogen sulfite, a further reduction in pH may initiate it. Colored anions (e.g., permanganate and chromate) serve as their own indicators of completion of the reduction. The reduced mixtures can often be washed down the drain. However, if large amounts of permanganate have been reduced, it may be necessary to transfer the manganese dioxide to a secure landfill, possibly after a reduction in volume by concentration or precipitation. Do not dispose of chromium salts in the sanitary sewer.
Hydrogen peroxide can be reduced by the sodium hydrogen sulfite procedure or by ferrous sulfate as described earlier for organic hydroperoxides. However, it is usually acceptable to dilute it to a concentration of less than 3% and dispose of it in the sanitary sewer. Solutions with a hydrogen peroxide concentration greater than 30% should be handled with great care to avoid contact with reducing agents, including all organic materials, or with transition metal compounds, which can catalyze a violent reaction.
Concentrated perchloric acid (particularly when stronger than 60%) must be kept away from reducing agents, including weak ones such as ammonia, wood, paper, plastics, and all other organic substances, because it can react violently with them. Dilute perchloric acid is not reduced by common laboratory reducing agents such as sodium hydrogen sulfite, hydrogen sulfide, hydriodic acid, iron, or zinc. Perchloric acid is most easily disposed of by stirring it gradu-
ally into enough cold water to make its concentration less than 5%, neutralizing it with aqueous sodium hydroxide, and washing the solution down the drain with a large excess of water.
Nitrate is most dangerous in the form of concentrated nitric acid (70% or higher), which is a potent oxidizing agent for organic materials and all other reducing agents. It can also cause serious skin burns. Dilute aqueous nitric acid is not a dangerous oxidizing agent and is not easily reduced by common laboratory reducing agents. Dilute nitric acid should be neutralized with aqueous sodium hydroxide before disposal down the drain; concentrated nitric acid should be diluted carefully by adding it to about 10 volumes of water before neutralization. Metal nitrates are generally quite soluble in water. Those of the metals listed in Table 7.1 as having a low toxic hazard, as well as ammonium nitrate, should be kept separate from oil or other organic materials because on heating such a combination, fire or explosion can occur. Otherwise, these can be treated as chemicals that present no significant hazard.
Nitrites in aqueous solution can be destroyed by adding about 50% excess aqueous ammonia and acidifying with hydrochloric acid to pH 1: