1.A INTRODUCTION
Over the past century, chemistry has made great contributions toward our understanding of and our ability to manipulate the physical and biological world. Most of the items we take for granted in our day-to-day life involve synthetic or natural chemical processing. Indeed, even our own bodies may be viewed as chemical machines, now that molecular biology has removed the traditional boundary between chemistry and biology. The chemical laboratory has become the center for acquiring knowledge and developing new materials for future use, as well as for monitoring and controlling those chemicals currently used routinely in thousands of commercial processes. Many of these chemicals are beneficial, but others have the potential to cause damage to human health and the environment, and therefore also to the public attitude toward the chemical enterprise on which we also heavily depend.
Since the age of alchemy, some chemicals have demonstrated dramatic and dangerous properties, which have required the development of special techniques for handling them safely. We also know now that many more are insidious poisons. Until recently, the chemical hazards in many laboratories were not accepted and taken into account by those working in them, and, accordingly, the necessity of putting "safety first" was not fully appreciated. During the "heroic age" of chemistry the notion of martyrdom for the sake of science was actually accepted widely, according to an 1890 address by the great chemist August Kekulé: ''If you want to become a chemist, so Liebig told me, when I worked in his laboratory, you have to ruin your health. Who does not ruin his health by his studies, nowadays will not get anywhere in Chemistry" (as quoted in Purchase, 1994). In sharp contrast, a growing recognition of moral responsibility and mounting public pressure have made institutions housing chemical laboratories accountable for providing safe working environments for those employed in them and complying with extensive regulation of the transport of chemicals to the laboratories and removal of waste from them. The "old days" of easygoing attitudes toward laboratory safety and down-the-sink disposal are over! Laboratories have become safe places to work.
1.B THE NEW CULTURE OF LABORATORY SAFETY
A new culture of safety consciousness, accountability, organization, and education has developed in the laboratories of the chemical industry, government, and academe. To a degree that could scarcely have been foreseen 25 years ago, programs have been implemented to train1 laboratory personnel and to monitor the handling of chemicals from the moment they are ordered until their departure for ultimate treatment or disposal.
Workers in many hazardous fields2 (e.g., seamen and construction workers) have developed traditions of working together for mutual protection and the maintenance of correct professional standards. In the same way, laboratory workers have come to realize that the welfare and safety of each individual depends on clearly defined attitudes of teamwork and personal responsibility. Learning to participate in this culture of habitual risk assessment, experiment planning, and consideration of worst-case possibilities for oneself and one's fellow workers is as much a part of a scientific education as learning the theoretical background of experiments or the step-by-step protocols for doing them in a professional and craftsmanlike manner.
Accordingly, a crucial component of chemical education at every level is to nurture basic attitudes and habits of prudent behavior in the laboratory so that safety is a valued and inseparable part of all laboratory activity. In this way, "safety first" becomes an internalized attitude, not just an external expectation driven by institutional rules. This process must be part and parcel of each person's chemical education throughout his or her scientific career. One aim of the present volume is to encourage academic institutions to address this responsibility effectively and cultivate their students' participation in the culture of laboratory safety as a solid basis for their careers as professional chemists.
1.C RESPONSIBILITY AND ACCOUNTABILITY FOR LABORATORY SAFETY
The culture of laboratory safety depends ultimately on the working habits of individual chemists and their
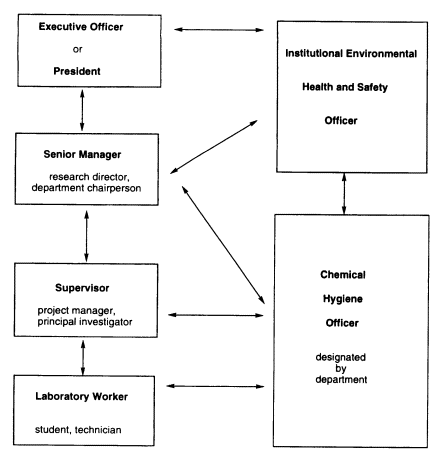
FIGURE 1.1 Pattern of interactions through which laboratory safety can be arranged within an institution.
sense of teamwork for protection of themselves, their neighbors, and the wider community and environment. However, safety in the laboratory also depends on well-developed administrative structures and supports that extend beyond the laboratory's walls within the institution. There are many ways that the detailed organization for laboratory safety can be arranged. Within a business, academic, or governmental institution, organizations often involve interactions such as those shown in Figure 1.1.
The protection of health and maintenance of safety constitute a moral obligation shared by everyone. Federal, state, and local laws and regulations make safety a legal requirement and an economic necessity as well. Laboratory safety, therefore, is not a purely voluntary function; it requires mandatory safety rules and programs and a commitment to them. A sound safety organization that is respected by all requires the wholehearted participation of laboratory administrators, employees, and students.
The ultimate responsibility for safety within any institution lies with its chief executive officer or president. That individual must provide the leadership to ensure that an effective safety program is in place so that all institutional officials will demonstrate a sincere and continuing interest in the program. Even a well-conceived safety program is apt to be treated casually by the workers if it is neglected by top management. Initiative and support for good safety programs, like most other institutional plans, usually come from the top down.
Although the responsibility for safety in a department or other administrative unit lies with its director or chairperson, the responsibility for the delineation of the appropriate safety procedures and the instruction of those who will carry out the operation lie with the project manager or principal investigator. The responsibility for safety during the execution of an operation lies with those technicians, students, and other workers who actually perform that operation. Nevertheless, the primary responsibility for maintaining safe behavior in a safe laboratory environment remains with the project manager or principal investigator. Each institution should develop policies that
help to determine who has accountability for accidents or safety violations.
While the school principal or college president is ultimately responsible for the safety of students in courses that involve laboratory activity, the laboratory instructor carries direct responsibility for what actually takes place under his or her direction. The instructor is responsible for developing the positive attitudes and habits of the culture of laboratory safety as well as the necessary skills for handling chemicals safely.
The expanding system of federal, state, and local regulations for the handling and disposal of chemicals has resulted in institutional infrastructures to oversee compliance with safety laws. Most industrial, governmental, and academic institutions that maintain laboratory operations have an environmental health and safety office made up of individuals with appropriate professional credentials. These individuals may have expertise in chemical safety, industrial hygiene, engineering, biological safety, environmental health, occupational medicine, health physics, fire safety, or toxicology. Functions of environmental health and safety offices generally include technical consultation, hazardous waste management, accident reviews, inspections and audits, compliance monitoring, training, record-keeping, and emergency response. These offices assist laboratory management in establishing safety policies and promoting high standards of laboratory safety. To be most effective, they should share in a genuine partnership with all department chairpersons or directors, principal investigators or managers, and laboratory workers in helping to design safety programs that provide technical guidance and training support that are relevant to the operations of the laboratory, are practical to carry out, and comply with the law. They should help technical and professional personnel to be aware of their legal responsibilities without being overwhelmed by a sense of unlimited liability from a mass of regulations.
In view of the importance of the environmental health and safety office to the whole safety enterprise, it should be directed by people who are truly knowledgeable about the operations. Safety directors should be given a high level of authority and responsibility for the development of a unified safety program. The safety director should also have direct access, when necessary, to people at the highest level in the institution who carry its ultimate accountability to the public through the media and the law. Department chairpersons need to deal directly with the safety officers, who are not only knowledgeable about safety regulations and consistent in enforcing them but also appreciate the unique problems of progressive training and prudent operations in academic teaching and research institutions.
1.D SPECIAL SAFETY CONSIDERATIONS IN ACADEMIC LABORATORIES
Academic laboratories, like industrial and government ones, are concerned with meeting the fundamental safety goals of minimizing accidents and injuries, but there are differences that should be recognized when developing prudent and realistic safety programs for teaching institutions. Forming the foundation for a lifelong attitude of safety consciousness, risk assessment, and prudent laboratory practice should be an integral part of every stage of scientific education—in the classroom, in textbooks, and in the laboratory from the earliest exposures in primary or secondary school through graduate and postdoctoral training. Teaching and academic institutions have this essential and unique responsibility. They are also faced with the special problems that go with a rapid turnover of young people. The manifold requirements for record-keeping and waste handling can be especially burdensome for overworked teachers in high school or college laboratories.
In addition to providing well-trained students, institutions with graduate programs also have the responsibility of discovering new knowledge through research programs, and these often involve unpredictable hazards. The safety goals and the allocation of resources to achieve them are sufficiently different for high school, undergraduate, and graduate teaching laboratories that they are discussed separately here. In research universities the goals for safety in teaching laboratories and in research laboratories usually overlap but may also compete for attention and funds.
1.D.1 High School Teaching Laboratories
Recognizing and evaluating hazards, assessing risks, selecting appropriate practices, and performing them proficiently are essential elements of laboratory safety. The training to lay the foundation for acquiring these skills begins with the student's first experience in the laboratory. Even the earliest chemical experiments should cover the proper approach to dealing with the principal hazardous properties of chemicals (e.g., flammability, reactivity, corrosiveness, and toxicity) as an introduction to laboratory safety, and should also begin to instill responsibility for sound environmental practice when managing chemical waste. Advanced high school chemistry courses should assume the same responsibilities for developing professional attitudes toward safety and pollution control as are expected of college and university courses.
1.D.2 Undergraduate Teaching Laboratories
Undergraduate chemistry courses are faced with the problem of introducing inexperienced people (frequently in enormous numbers) to the laboratory culture, including the handling of hazardous materials. Although many students come to their first undergraduate course with good preparation from their high school science courses, others may be "chemophobic," having a prejudice against chemicals of all kinds. They must learn to evaluate intelligently the wide range of hazards in laboratories and learn the techniques by which potential dangers can be controlled routinely with negligible risk.
In research universities the primary responsibility for undergraduate laboratory teaching is often assigned to teaching assistants, who may have widely different backgrounds and communication skills. At the same time that they are adapting to their first teaching experience, they are also trying to handle their first graduate courses. Some who are unfamiliar with safe laboratory practice and the proper disposal of chemical waste may have to learn new attitudes and habits at the same time that they are teaching them to the undergraduates. The supervision of teaching assistants and monitoring of their performance must be taken as a special departmental responsibility because the safe, meaningful operation of undergraduate laboratories depends so heavily on them.
In research universities the inherent problems of transmitting good laboratory training to undergraduates can be compounded by the conflicting demands for resources and attention between research and undergraduate teaching. Commitment of the entire faculty to laboratory safety and the responsible disposal of chemicals is a crucial factor in the initiation of all students into the laboratory culture at every level.
1.D.3 Academic Research Laboratories
Advanced training in safety is an important component of education through research. Unlike laboratory course work, where training comes primarily from repeating well-established procedures perfected through many years of experience, research often includes the production of new materials by unprecedented methods, which may involve unknown hazards. As a result, academic research laboratories may place an enormous range of processes and products in the hands of young investigators of widely varied scientific experience. Often the transition from undergraduate laboratory course work to the first experience of independent research is too abrupt. If high school and college laboratory work has placed all of the responsibility for safety planning on the teachers and graduate research leaves it all to the student, neophyte researchers will be ill-prepared to face the many real hazards of the research laboratory. Given these circumstances, heavy responsibility rests with the faculty to provide the safest possible environment for research by careful oversight and example. Faculty should pay particular attention to the introduction of first-year graduate students to the research laboratory. To further enhance a good safety environment, many chemistry departments now present regular courses on laboratory safety for incoming graduate students and require that postdoctoral associates show proficiency on a regular safety test before starting laboratory work. Such preparations contribute greatly to the complete professional training required by most chemical companies.
Safety training must be a continuing process; it should become an integral part of the daily activities of laboratory workers and those who are accountable for them. It need not be an arduous task. As a student or laboratory worker learns a new protocol, safe practices relevant to it should also be emphasized in the normal setting of the laboratory, with the careful guidance of a mentor and the shared responsibility of colleagues. Opportunities to encourage and enhance informal safety training through collegial interactions should be pursued vigorously as a valuable way to exchange safety information, convey meaningful guidance, and sustain an atmosphere in which colleagues reinforce each others' good work habits.
Formal safety education for advanced students and laboratory workers should be made as relevant to their work activities as possible. Training that is conducted simply to satisfy regulatory requirements tends to subordinate the relevant safety issues to details associated with compliance. Such bureaucratic safety management has actually worked against fostering positive safety attitudes in many well-experienced laboratory workers and has undermined the credibility of warnings about bona fide hazards by emphasizing pro forma violation of rules.
Although principal investigators and project managers are legally accountable for the maintenance of safety in laboratories under their direction, this activity, like much of the research effort, is distributable. Well-organized academic research groups develop hierarchical structures of experienced postdoctoral associates, graduate students at different levels, undergraduates, and technicians, which can be highly effective in transmitting the importance of safe, prudent laboratory operation. When the principal investigator offers leadership that demonstrates a deep concern for
safety, the university safety program thrives. However, if the principal investigator's attitude is laissez-faire or hostile to the university safety program, careless attitudes can take hold of the whole group and set the stage for accidents, costly litigation, and expensive reeducation for those who move on to a more responsible institution.
1.E THE SAFETY CULTURE IN INDUSTRY
Industrial laboratories that use chemicals engage in extremely varied activities. Some are at the heart of the chemical industry, with the complete complement of research, analytical, pilot plant, and production facilities engaged in making chemicals. In others the use of chemicals is more incidental to the production of special products.
Not surprisingly, the degree of commitment to environmental health and safety programs varies widely as well. Many chemical companies have recognized both their moral responsibility and their self-interest in developing the best possible safety programs. Others have done little more than is absolutely required by law and regulations, if that. Unfortunately, the bad publicity from a serious accident or violation in one carelessly operated laboratory or plant tarnishes the credibility of all those whose operations are above reproach. The public perception that chemical companies have "deep pockets" can place a high price on chemical accidents.
The industrial or government laboratory environment can provide strong corporate structure and discipline for maintaining a well-organized safety program where the safety culture is thoroughly understood, respected, and enforced from the highest level of management down. New employees coming from the more casual atmosphere of some academic research laboratories are often surprised to discover "a new world" of attention to the detailed planning and extensive checking that are required in preparation for running experiments. In return for their efforts, they enjoy the sense of security that goes with high professional standards.
Industrial safety programs can face several obstacles. Financial limitations to safety programs can prevent optimal development in business as well as academe. However, the short-sighted bottom-line thinking that can surface when management is separated from the laboratory by geography or by a lack of commitment to safety is a more common problem for industrial laboratories. Poor communication between laboratory workers and environmental health and safety officers can lead to adversarial relations when a perception develops that a bureaucracy is generating rules for the sake of rules. Compliance with institutional safety regulations does not guarantee a real acceptance of the culture of safety.
1.F FACTORS THAT ARE CHANGING THE CULTURE OF SAFETY
Over the past 20 years, several trends have emerged that are changing the shape of the safety enterprise in the chemical laboratories of industry, government, and academe. These factors include advances in technology and changes in cultural values and in the legal and regulatory climate.
1.F.1 Advances in Technology
Several recent advances in technology have begun to change the safety requirements in chemical laboratories. For example, in response to the increasingly high cost of handling chemicals through the whole cycle from purchase to waste disposal, there has been a steady movement toward miniaturizing chemical operations in both teaching and research laboratories. This trend not only has had a significant effect on laboratory design but also has reduced costs of acquiring, handling, and disposing of chemicals. Another trend—motivated at least partially by safety concerns—is the simulation of laboratory experiments by computer. Such programs are a valuable conceptual adjunct to laboratory training but are by no means a substitute for it. As mentioned above, only students who have been educated carefully through a well-graded series of hands-on experiments in the laboratory will have the confidence and expertise needed to handle real laboratory procedures in a safe manner as they move on to advanced courses or research work.
1.F.2 The Culture of Pollution Prevention
A recent and widely accepted cultural change that affects laboratory work is the concept of pollution prevention. The idea is simplicity itself: if one makes less waste, there is less waste to dispose of, and therefore less impact on the environment. A frequent, but not universal, corollary is that costs are also reduced.
The terms "waste reduction," "waste minimization," and "source reduction" are often used interchangeably with "pollution prevention." In most cases the distinction is not important. However, the term ''source reduction" may be used in a narrower sense than the other terms, and the limited definition has even been suggested as a regulatory approach that mandates pollution prevention. The narrow definition of source reduction includes only procedural and pro-
cess changes that actually produce less waste. The definition does not include recycling or treatment to reduce the hazard of a waste. For example, changing to microscale techniques is considered source reduction, but recycling a solvent waste is not.
1.F.2.1 Waste Management Hierarchy for Pollution Prevention
The distinctions made above are the result of an approach to waste management that incorporates a hierarchy of pollution prevention techniques. At the top of the hierarchy is source reduction, which is always the preferred technique. Source reduction can be achieved by using a smaller quantity of material or a less hazardous material or by making a process more efficient. However, while source reduction is highly desirable, it is not always technically or economically feasible.
The second level of the hierarchy is recycling/reuse/recovery. The distinction here is that the waste requires some input of energy (e.g., distillation) before it can be reused. Because of this additional waste-handling step, there is also an increased potential for spillage and other fugitive losses of material that would not occur had the waste not been generated in the first place. However, when source reduction techniques are not available or practical, recycling, reuse, or recovery can be important alternatives to disposal.
The third level is treatment. Generally, treatment of a waste renders it less hazardous or nonhazardous but does not allow reuse of the material. For materials that have no potential for recovery and are not amenable to source reduction, treatment—such as neutralization of acids, incineration of organic sludges, and oxidation of cyanides—becomes an important part of the waste management system.
The last level (and least desirable alternative) is land disposal. Certain hazardous wastes, particularly the heavy metals, cannot be rendered completely nonhazardous and cannot realistically be recovered. They can, however, be stabilized to reduce the likelihood of movement in the environment, and regulations require this procedure before land disposal is permitted. Whereas land disposal of laboratory waste (in "Lab Packs") was once the most common form of waste management, it is now rarely used, and then only in very specialized situations and locations.
1.F.2.2 Making Pollution Prevention Work
Many advantages can be gained by taking an active pollution prevention approach to laboratory work, and these are well documented throughout this book. Some potential drawbacks do exist, and these are discussed as well and should be kept in mind when planning activities. For example, dramatically reducing the quantity of chemicals used in teaching laboratories may leave the student with an unrealistic appreciation of their behavior when used on a larger scale. Also, certain types of pollution prevention activities, such as solvent recycling, may cost far more in dollars and time than the potential value of recovered solvent. Certain waste treatment procedures may even have regulatory strictures placed against them.
Perhaps the most significant impediment to comprehensive waste reduction in laboratories is the element of scale. Techniques that are practical and cost-effective on a 55-gallon or tank car quantity of material may be highly unrealistic when applied to a 50-gram (or milligram) quantity. Evaluating the costs of both equipment and time becomes especially important when dealing with very small quantities.
1.F.3 Changes in the Legal and Regulatory Climate
Many important changes in the legal and regulatory climate over the last 20 years have added to the changing culture of safety. Because of increased regulation, the collection and disposal of laboratory waste now constitute a major budget item in the operation of every chemical laboratory. Also, it is now widely recognized that protection of students and research personnel from toxic materials is not only a moral obligation but also an economic necessity-the price of accidents in terms of time and money spent on fines for regulatory violations and on litigation can be very high.
In response to the heightened concern for safety in the workplace, the OSHA Laboratory Standard (29 CFR 1910.1450) requires every institution that handles chemicals to develop a Chemical Hygiene Plan. This requirement has generated a greater awareness of safety issues at all educational science and technology departments and research institutions. Although the priority assigned to safety varies widely among personnel within chemistry departments and divisions, increasing pressure is coming from several other directions in addition to the regulatory agencies and accident litigation. In some cases, significant fines to principal investigators who have received citations for safety violations have increased the faculty's concern for laboratory safety. Boards of trustees or regents of educational institutions often include prominent industrial leaders who are highly aware of the increasing national concern with safety and environmental issues and are particularly sensitive to the possibility of institutional liability as a result of laboratory accidents. Academic and government labo-
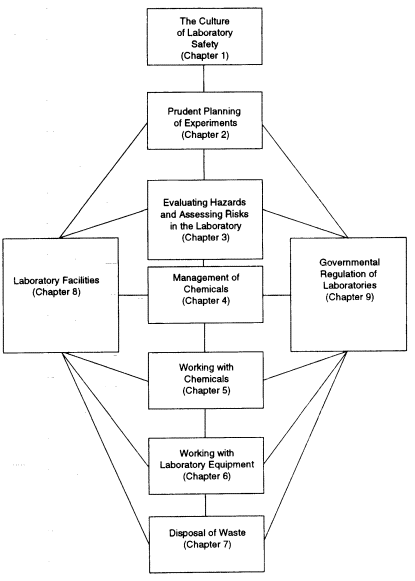
FIGURE 1.2 Protocol for planning and carrying out an experiment with chemicals in a laboratory.
ratories, like industrial ones, can be the targets of expensive lawsuits. The trustees can assist academic officers both by helping to develop an appropriate institutional safety system with an effective environmental health and safety office and by supporting departmental requests for modifications of facilities that are necessary for compliance with safety regulations.
Increasing concern for laboratory safety is also being engendered by federal granting agencies. The fact that negligent or cavalier treatment of laboratory safety regulations may jeopardize not only an individual investigator's but also a department's or an institution's ability to obtain funding may become a powerful incentive for improvement in this area.
1.G ORGANIZATION OF THIS BOOK
This book is organized around the protocol for planning and executing an experiment with chemicals in a laboratory. Figure 1.2 outlines the work flow. The chapters that follow represent the likely steps in this process, and guidelines on how to infuse the new culture of laboratory safety into each step are presented throughout the book.








