The Industrial Green Game. 1997. Pp. 37–47.
Washington, DC: National Academy Press.
Closing the Loop on Waste Materials
ROBERT A. FROSCH
Throughout history, human economic activity has been characterized by an open and linear system of materials flows, where materials are taken in, transformed, used, and thrown out. Tools, clothing, and other products were forged and fashioned from natural plant, animal, and mineral materials. Worn-out goods and materials left over from the production process were often dumped in backyards. Archaeologists find discarded reminders of the past—scrap stone, flints, and potsherds—in the rubbish dumps of the Neolithic period. People moved to new habitats when the old locations became unsuitable because of accumulated wastes.
Today, there are more of us and fewer new places to which to move. We face serious pollution in many locations and and have poisoned some areas into uninhabitability. As human populations grow, discarding waste material is becoming increasingly problematic.
These difficulties and the increasing public perception that we are drowning in a sea of garbage have resulted in considerable pressure regarding the freedom to throw things away. We fear that we are running out of places to discard or store waste materials, damaging the environmental life-support systems on which we depend, and running out of resources. We have not yet experienced any serious difficulties in finding new supplies of materials, although their extraction and the wastes from extraction have posed local and regional environmental problems. An industrial system with open, linear materials flows—one that takes in materials, and energy, creates products and waste materials, and then throws most of them away—probably cannot continue indefinitely.
Industrial ecology is the study of industrial systems (materials and energy flows) from the perspective of natural ecosystems. The natural ecosystem has
evolved so that any available source of useful material or energy is used by some organism in the system. Animals and plants live on each other and on each other's waste matter. Materials and energy tend to circulate in a complex web of interactions: Animal wastes and dead plant material are metabolized by microorganisms and turned into forms that are useful nutrients for plants. The plants, in turn, may be consumed by animals or simply die, decay, and cycle again through the system as nutrients and components of other flora and fauna. These systems do, of course, leave some waste materials, or fossil fuels would not exist. On the whole, the system regulates itself and consumes what it produces.
Can industrial systems be more self-sufficient and closed with regard to the flow of materials so that interactions with the environment are more compatible? The lessons of the planned economies of the former Soviet Union suggest that planning and controlling the industrial system is not the solution. Rather, we need to experiment with changes in policy and industrial practices to see what actions will nudge the system in the desired direction. These nudges must be regarded as experimental, to be adjusted in the light of experience (Frosch, 1996; Frosch and Gallopoulos, 1989; Frosch and Gallopoulos, 1992).
USING MATERIALS EFFICIENTLY
One way for industry to be more self-sufficient and closed is to improve the efficiency of materials use. Surprisingly, this obvious idea has not been much attempted. If material is bought at one end of the system and thrown away at the other, it is probably wasteful in the ordinary sense, and materials are not being used as efficiently as practicable.
In addition to the waste materials from production, the products themselves (which industrial firms generally do not regard as part of their system once they are sold) eventually become part of the waste stream of society. When products wear out or are replaced by newer models, they are usually thrown away. They may be used as landfill or incinerated or they may litter the landscape. It seems worthwhile to examine both production processes and product designs to see if the use of materials (and energy) can be improved.
Regulatory pressures and shifting public opinion have spurred the industrial and engineering community to initiate efforts aimed at closing the materials loops more effectively and improving energy-use efficiencies (Allenby and Richards, 1994; National Academy of Sciences, 1992; Richards and Frosch, 1994; Schmidheiny and the Business Council for Sustainable Development, 1992; Smart, 1992). Automobile manufacturers such as BMW and Volkswagen have designed cars for easy disassembly and recycling (Simon and Woodell, this volume). Companies such as Hewlett-Packard, Canon, and Xerox have begun to take back their own used components, such as toner cartridges, and to manufacture new ones using refurbished components and recycled materials from the old ones. By considering
their leased and sold products as assets, these companies are designing new products with reuse, remanufacture, and recycling in mind. Even before these newly designed products have come to market, the change has resulted in cost savings (Murray, 1994; Richards and Frosch, 1994). At the 3M Corporation, a simple measure—waste mass divided by the total output mass (the sum of product, by-product, and waste masses)—is used to encourage materials-use efficiency (Richards and Frosch, 1994). Enterprising entrepreneurs are using recycled materials in innovative new products, such as textiles.
The industrial ecology perspective is beginning to influence designers of manufacturing processes to seriously consider waste streams. Designers of products are beginning to view their creations as transient embodiments of matter and energy with added value that can be recaptured and recreated within a continuing flow of materials extending beyond the point of sale. Products and the materials they contain are being designed so that they can be reused at the end of their lives.
The whole industrial process can be thought of as a closed cycle in which the manufacturer has overall custody for the material used. In this system, the manufacturer must consider the entire material and energy stream, from materials input and manufacturing through the life of the product and its eventual reuse or disposal. This concept has begun to be embodied in law (as in Germany), making manufacturers responsible for their products through to final disposition.
From a systems point of view, it is not clear whether it is most useful to consider the flow of materials at the level of an individual plant, a manufacturing firm, a group of firms engaged in producing a product, an industrial sector, or industrial activities as a whole. Suboptimization (i.e., optimization of a particular process or subsystem instead of the larger system in which the process or subsystem is embedded) may be less efficient than optimization of the larger-scale system. For example, a larger, more complex, more diverse system may offer a wider variety of opportunities for reuse of materials. More complete system closure may be attained by considering all of industry as the system to be optimized and closed rather than by considering a single firm, process or product.
Some of the complexity of the flow of materials through the U.S. industrial system is suggested by the case of lead (Figure 1). This attempt to follow the flow of materials through "industrial metabolism" (Ayres, 1989) shows that the recycling of spent consumer products already plays a major role in the U.S. lead industry.
PRODUCTS OR SERVICES?
Some firms have already begun to design their products and processes with a view to closing material loops as much as possible. However, if a product is the transient embodiment of materials to which a firm has already added value, then closing the loop on those value-added materials raises an important question for
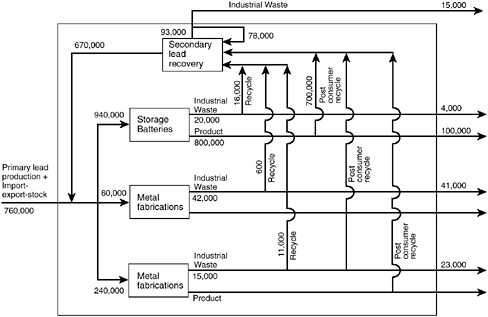
FIGURE 1 Simple model of the lead flows in the United States (metric tons/year). About 47 percent (670,000 tons) of the lead entering the U.S. market each year is from recycled sources. Almost 90 percent of the 800,000 tons per year that ends up in storage batteries is eventually recycled (16,000 tons of industrial waste from battery manufacture and 700,000 tons from post-consumer recycle). SOURCE: Allen and Behmanesh, 1994.
the firm: Is the product simply the hardware being sold, or is it rather the services that the product can provide? (Stahel, 1994; Stahel, this volume). There was a time when it was common practice to lease rather than sell many products outright. In a lease-based system, the manufacturer controls and therefore is responsible for the end of the product's life and is always prepared to take it back for recycling, reuse, or refurbishment.
Designing a product as a temporary provider of a service, to be used later in the creation of another product, is a novel idea in modern manufacturing and raises a new set of issues. A product is generally sold with the assumption that a consumer or sequence of consumers will use it until it cannot be used anymore. If the manufacturer thinks about taking it back for remanufacturing, the length of time the product spends in the customer's hands becomes an adjustable design variable. The maker may not want the product to wear out by being used for an indefinite time and so might choose to reclaim it at an optimum time for remanufacture. Thus, the notion of what a product is changes. Similarly, its life cycle may also change. The manufacturer may increasingly want to choose materials and designs that take serious account of eventual "demanufacture" and reuse. The various times involved in such a process can, of course, raise design conflicts.
BARRIERS TO INDUSTRIAL RECYCLING
Automobiles, their components, and other metal products, especially those made of iron and steel, have a long history of being recycled without regulatory prodding. (It was a common lament after Pearl Harbor that the Japanese fleet had been built mostly with American scrap metal.) For other products and materials, progress has come later and been much slower.
For example, wastes with a higher metal content than is found in commercial ores are often not recovered. Allen and Behmanesh (1994) demonstrated this by comparing concentrations of metals in waste streams against the Sherwood plot. For various nonferrous metals that are regularly recovered from scrap and waste, Figure 2 relates the market price of the pure metal against the minimum concentration of metal wastes undergoing recycling. The diagonal line in Figure 2 is known as the Sherwood plot, after Thomas Sherwood who in the 1950s pointed out the relation between the price of a metal and its concentration in commercially viable virgin ore. The few points below the Sherwood line in Figure 2 indicate metals (such as chromium and vanadium) that are being vigorously recycled.
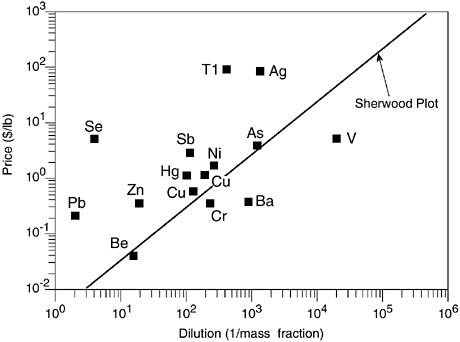
FIGURE 2 The Sherwood plot for waste streams. The diagonal line (Sherwood plot) correlates concentration of metal in commercial ores to market price of metals. Points above the Sherwood plot indicate metals in waste streams that are richer than typical virgin ores being disposed of. Points below the Sherwood plot indicate metals that are vigorously being recycled from industrial hazardous waste streams. SOURCE: Allen and Behmanesh, 1994.
Points far above the Sherwood line indicate underexploited metallic waste streams. Aluminum, although not shown in this Sherwood diagram, has been very heavily recycled in recent years. In the case of aluminum, the energy cost of electrolytic extraction from the ore is much higher than the energy cost of recycling used soda cans.
Why is there so much waste, especially of iron, steel, and precious metals, in the metal industry, which has such a long tradition of recycling? The barriers to industrial recycling of metals can be classified into six interrelated areas: technical hurdles, economic barriers, information barriers, organizational obstacles, regulatory issues, and legal concerns. When recycling is technically feasible, it may be economically unsound. When it is technically and economically satisfactory, a lack of information may block its adoption. Even when the requisite information is at hand, organizational problems can still stymie implementation. Finally, when all else is satisfactory, a recycling scheme can founder on the rocks of regulatory or other legal barriers.
Technical Hurdles
The suitability of the material for an intended reuse is a key technical concern. Metals, metal compounds, and organic materials make up a large fraction of industrial products. The metals are relatively easy to reprocess and reuse. In many cases, however, organic materials are best thought of as energy stored in chemical bonds (largely carbon bonds) rather than as reusable materials; they are generally exothermically oxidizable. The choice between recycling the material and burning it as fuel or otherwise extracting its chemical energy might be made on the basis of comparative market values.
Waste and product materials sometimes contain unwanted ''tramp" elements. These contaminants can ruin the reuse potential of the materials or make handling difficult or dangerous; purification is often problematic. As products are redesigned for newer more cyclical material use, some of the material problems may be eliminated through smarter design. However, it will not always be possible to "design out" problematic materials. For example, zinc is often used to coat steel to prevent corrosion. It can interfere with the desirable properties of new steel forged from melted recycled scrap steel. Steel mills therefore limit the permissible content of zinc in the scrap they buy or they pay less for scrap with more than a threshold concentration of zinc. Such matters are generally handled by scrap dealers, who blend zinc-free and zinc-coated scrap in the mix they sell to the mills. In the long run, this practice may lead to a zinc contamination problem in the steel-scrap recycling business, but there are no good, inexpensive substitutes for zinc as an anticorrosion coating. The blending process is sometimes regarded as a case of sham recycling, because the zinc is not being recycled. One could say that it is being disposed of in the steel. Such sham recycling, however, may be preferable to uncontrolled releases of zinc to the environment.
Economic Barriers
The manufacturing process tends to mix materials that are further mixed in the process of waste disposal. In remanufacturing, one generally wants to separate things into their original components and materials. There are costs involved in collecting, sorting, and transporting used-up products, scrap, and waste. Such separation requires information, effort, and energy, which must all be paid for. These costs must be compared with the costs of new materials.
Even when the operating costs of recycling are attractive, there may be capital costs that pose barriers. Heavy capital investment in existing systems may prevent a company from securing an easy source of new investment to start over. This obstacle may only introduce a time lag, postponing the decision to recycle until it is suitable to make a capital investment, such as when the machinery requires change for some other reason. Some companies that face competitive forces of ever-shorter product times, particularly those in the electronics industry, have introduced "design for the environment" techniques as a major impetus for reengineering their products and processes.
The cost of eliminating or reusing certain materials must be balanced against the cost of disposal. Disposal costs bring up the question of how companies should take account of indirect costs such as the effect of wastes on the environment. These issues have generally been handled by regulatory control of emissions but could equally be dealt with by including the costs of environmental damage in a firm's bookkeeping (Macve, this volume; Todd, 1994). The bookkeeping approach would provide an incentive to minimize such costs, and it might force a truer comparison of the costs of alternative schemes. However, it has proved very difficult to find suitable, agreed-upon measures for such costs.
Information Barriers
The requisite information about costs is not usually available to everyone in the firm who might be able to use it to good advantage. It is sometimes not clear who might need the information, and standard management and other accounting systems often do not track costs in a way that is useful to designers. Design engineers may not know of the real costs to the company of the materials they choose because costs usually appear as prices offered by the suppliers. Designers generally have no idea what waste problems will be posed by manufacturing with different materials. Better systems for acquiring and disseminating cost information within firms are needed.
In the larger economy, outside the firm, where waste and scrap materials may be transferred and used, information is needed about potential customers and suppliers of these materials. In traditional metal-recycling sectors, complex, multitier networks of scrap collectors, sorters, brokers, alloyers, refiners, and smelters serve as the information network. Business and trade organizations,
such as the Institute of Scrap Recycling Industries and the Steel Can Recyclers Institute, also provide information. In newer areas of recycling, such as plastics, adequate informational and organizational mechanisms are yet to evolve.
In materials sectors where traditional recycling networks do not exist, information may be difficult to find, especially if users and providers are in very different geographical areas or different parts of the industrial system. Existing waste exchanges and brokerage systems are generally small, local, and ineffective on a large scale. Attempts are being made to create better market arrangements. The Recycling Advisory Council and the Chicago Board of Trade, with the support of the U.S. Environmental Protection Agency, are seeking to create an exchange market on an electronic bulletin board. This market would initially trade in glass, polyethylene terephthalate, and high-density polyethylene. Some metal recyclers, stimulated by public opinion and the resulting anticipation of new recycling markets, are expanding into other materials to fill the gap (Kisser, 1994).
Organizational Obstacles
The internal organization of a firm can be difficult to change. Changing the whole concept of a product or adding new criteria for environmental compatibility to the design process may not fit the ideas on which the firm operates or its internal incentive system. The business-unit structure may make perception and solution of problems that cross organization lines very difficult.
External to a firm, the idea that anything secondhand must be second rate has become institutionalized in the distinction between dealers in new and the used materials and products. Organizational issues can plague not only the establishment of markets, but also the creation of new institutions such as information and waste exchanges and larger-scale brokerages.
Regulatory Issues
The U.S. regulatory system for industrial wastes has been designed around disposal, and the rules treat recycling and reuse as forms of disposal. The designation of a material as waste, as distinguished from scrap or hazardous material, can be crucial.
There are many inconsistencies in the Resource Conservation and Recovery Act. For example, the waste classification of a solvent-laden rag used to clean machinery depends on how it was used. If the solvent is poured first on the machinery and then wiped with a clean rag, the rag is a hazardous waste. However, if the solvent is poured first on the rag and then the rag is used to wipe the machinery clean, the rag is not considered a hazardous waste (Starr et al., 1994).
Recycling an industrial waste material is likely to require the recycler to become a legal disposer of that material under the regulations. Obtaining a permit
has significant time, financial, and bureaucratic costs attached, which are a nontrivial barrier to reuse of industrial waste materials (Scrap Processing and Recycling, 1994).
A characteristic tale from industry is illustrative of the problems facing those firms that attempt to use materials more efficiently. The corrosion coating of auto bodies is accomplished by passing the cars through a zinc phosphate bath. After a period of use, the bottom of the bath contains a slurry rich in zinc. At one plant, this slurry was for many years removed periodically when the tanks were cleaned and then sent to a zinc smelter, which processed it and put the resulting zinc metal back into the industry supply stream. In the course of regulatory actions not aimed at this material, the slurry became classified as a hazardous waste. When the smelter became acquainted with the regulations that would now apply, it refused to accept the material any longer. At the time this anecdote was told, the slurry was being sent to a landfill.
Such problems are frequently solved by getting waivers or exceptions to cover the particular case, but the process is very cumbersome. The details of such problems may differ from state to state, and state regulations can be even more restrictive than federal regulations.
Waste is regulated separately from new materials. The system for control of virgin toxic materials is not nearly as cumbersome as that for waste materials. It is difficult, for example, to dispose of waste cyanide or send it for reuse, but it is easy to purchase new cyanide from a chemical manufacturer. Such regulations were intended to encourage environmentally cleaner technology by making disposal difficult and expensive. Although it makes good sense to control through reuse and recycling hazardous waste materials, in practice the system inadvertently encourages the use of new materials and the disposal of old materials.
A recent initiative by the EPA administrator seeks to change the regulatory system toward coordinated regulation of an industry rather than regulation determined simply by the material or medium in question. Such a system, it is hoped, would cope better with the realities of manufacturing and industrial life (Environmental Protection Agency, 1994).
Legal Concerns
Under current legal practice, liability considerations for a hazardous material often favor its disposal over its sale or transfer for reuse. Liability is often targeted at the original seller of any material used in a product implicated in a damage suit, even if the material has been reused and remanufactured by several parties en route to that ultimate product. The trail of potential liability can be so long and so unpredictable as to be thoroughly unpalatable. Furthermore, under the current practice of "joint and several" liability, damages in a lawsuit may be distributed according to the depths of the pockets of the various parties rather than their responsibility for the harm.
A supplier of a generally harmless, minor component material in a product might be assessed high liability damages because the product caused harm, even if that supplier was not a party to the product design and the material was not at fault. This practice has serious implications for commerce generally, and it appears to explain why firms often choose to dispose of scrap and waste rather than seek users for them.
The following example from a glassmaker is illustrative. Certain nonhazardous wastes from glassmaking would make good additions to concrete, improving its properties. Nevertheless the glassmaker disposes of these wastes in a landfill, because the firm's legal counsel worries about potential liabilities if the concrete ends up in an apartment house or a highway. Such liability risks are hard to predict and quite unacceptable in comparison with the more predictable liabilities related to landfill disposal. Elsewhere in the same industry, however, glass scrap is routinely added to asphalt road-paving material, giving rise to the name "glasphalt".
A shift from selling goods to leasing the services they provide could raise antitrust issues, because leasing can imply greater control of the product market. Separation of wholesale and retail sales organizations was an issue in the IBM and AT&T antitrust actions of recent years, and in the separation of automotive manufacturers from their dealership systems.
CLOSING THE LOOP
Industrial ecology views industry's impact on the environment in terms of a comprehensive system that uses and disposes of materials. We can learn to close the materials loop more efficiently by thinking on a larger scale about the flow back into industry of materials that would otherwise be discarded into the environment. There are numerous means of protecting the environment from industrial wastes. We can, for example, forgo the benefits of a potentially harmful material or we can seek to replace it with a more benign substitute. We can redesign products with a view to reusing materials and components. It is not yet clear what mix of remedies will most economically minimize the impact of industrial materials on the environment. The various possibilities hold out great promise, but there are complex problems and barriers to be overcome as we develop and implement a new, ecologically sound model for the management of materials in industry.
REFERENCES
Allen, D., and N. Behmanesh, 1994. Wastes as Raw Materials. Pp. 69–89 in The Greening of Industrial Ecosystems, B. R. Allenby and D. J. Richards, eds. Washington, D.C.: National Academy Press.
Allenby, B. R., and D. J. Richards, eds. 1994. The Greening of Industrial Ecosystems. Washington, D.C.: National Academy Press.
Ayres, R. U. 1989. Industrial metabolism. Pp. 23–49 in Technology and Environment, J. H. Ausubel and H. E. Sladovich, eds. Washington, D.C.: National Academy Press.
Environmental Protection Agency. 1994. Browner names six industries in plan to improve environmental protection. Press release. Environmental Protection Agency: Washington, D.C.
Frosch, R. A. 1996. Toward the End of Waste. Reflections on a New Ecology of Industry. Pp. 165–175 in Technology Trajectories and the Human Environment, J. H. Ausubel, D. Langford, eds. Washington, D.C.: National Academy Press.
Frosch, R. A., and N. E. Gallopoulos. 1989. Strategies for manufacturing. Scientific American 260:144–152.
Frosch, R. A., and N. E. Gallopoulos. 1992. Towards an Industrial Ecology. Pp. 269–292 in The Treatment and Handling of Wastes. A. D. Bradshaw, R. Southwood, and F. Warner, eds. London: Chapman and Hall.
Kisser, K. 1994. Scrap Processing and Recycling 51(3):74.
Murray, F. E. S. 1994. Xerox: Design for the Environment. Harvard Business School case N9-794-022. Cambridge, Mass.: Harvard Business School.
National Academy of Sciences. 1992. Proceedings of the National Academy of Sciences. 89: 793–884.
Richards, D. J., and R. A. Frosch, eds. 1994. Corporate Environmental Practices: Climbing the Learning Curve. Washington, D.C.: National Academy Press.
Schmidheiny, S., and the Business Council for Sustainable Development. 1993. Changing course: A global business perspective on development and the environment. Boston: Massachusetts Institute of Technology Press.
Scrap Processing and Recycling. 1994. 51(3):11.
Smart, B. 1992. Beyond Compliance. Washington, D.C.: World Resources Institute.
Stahel, W. 1994. The utilization-focused service economy: Resource efficiency and product-life extension. Pp. 178–190 in The Greening of Industrial Ecosystems, B. R. Allenby and D. J. Richards, eds. Washington, D.C.: National Academy Press.
Starr, J. W., J. G. Block, and J. F. Cooney. 1994. Legal Times, May 31:6.
Todd, R. 1994. Zero-loss environmental accounting systems. Pp. 191–200 in The Greening of Industrial Ecosystems, B. R. Allenby and D. J. Richards, eds. Washington, D.C.: National Academy Press.











